Abstract
Stem cell treatment outside of studied and approved medical indications can have unforeseen adverse consequences. Here, we present a 74-year-old male that underwent such therapy. The patient presented to our institution with progressive lower extremity weakness and urinary incontinence. He had previously undergone intrathecal stem cell therapy in Moscow, Russia for weakness and fatigue. Magnetic resonance imaging of his thoracic and lumbar spine showed marked enlargement of the cauda equina nerve roots and abnormal mass-like soft tissue involving the thoracolumbar thecal sac. Surgical biopsy of the intrathecal soft tissue showed polyclonal lymphocytic and glial cell proliferation. The patient’s symptoms did not improve with medical treatment or radiation, and he is currently under observation after multidisciplinary evaluation. Our patient’s experience illustrates one of the potential risks of “stem cell tourism” and exemplifies the imaging and histopathologic features of this rare entity. We also compare our patient’s treatment with other similar examples of stem cell treatments in our institution and others. These have had a wide spectrum of results. In some instances, intrathecal stem cells have caused abnormal imaging findings without any associated patient symptoms. In extreme examples, however, stem cell treatments have resulted in central nervous system neoplasms. Our patient’s lesion is quite unique, with only one similar lesion having been previously published.
Keywords: glial cell proliferation, intrathecal, stem cell, stem cell tourism
Introduction
Exogenous administration of stem cells outside of medically approved indications is performed at various institutions, often located outside of the United States. These include intravenous, intrathecal, and other routes of administration. Regrettably, patients that seek these treatments often have symptoms or diseases that are not well understood. The phenomenon of such patients seeking unproven stem cell treatments, often abroad, is colloquially known as “stem cell tourism.”
Because stem cells are undifferentiated, they have the ability to replace deficient cell lines in various diseases processes.1 While there are many evidence-based therapeutic uses of stem cells, such as transplantation of bone marrow hematopoietic stem cells in leukemia therapy, those meeting the definition of “stem cell tourism” typically do not have documented medical benefits.2,3 In addition to often being ineffective in treating patients’ symptoms, these procedures are known to pose significant risks, including development of infections and neoplasia. Here, we report on a patient who received intrathecal stem cell treatment that resulted in formation of an intrathecal mass and cauda equina nerve root thickening, resulting in substantial neurologic deficits.
Presentation and work up
A 74-year-old man from the United States who had been experiencing fatigue and decreased exercise tolerance for years received intravenous and intrathecal stem cell injections in Moscow, Russia for treatment of his chronic symptoms. He subsequently developed progressive symmetric bilateral lower extremity weakness and underwent L1–L2 laminectomies at an outside institution. Intraoperatively, micro-dissection of the thecal sac revealed thickened arachnoid tissue with no definite abnormality of the underlying spinal cord parenchyma. The patient then also developed urinary incontinence. His symptoms were refractory to sequential treatments with corticosteroids, intravenous immunoglobulin, high-dose methotrexate, and radiation therapy.
The patient subsequently presented to our institution for further management. Based on his known prior symptoms of weakness and urinary incontinence, as well as previous intraoperative findings, magnetic resonance imaging (MRI) of the thoracic and lumbar spine was obtained. MRI demonstrated massively thickened cauda equina nerve roots (Figures 1 and 2). There was additional abnormal signal in the thecal sac extending cranially to involve the lower thoracic spine, which was felt to represent intrathecal soft tissue proliferation. There was clinical concern that these findings may have represented a neoplastic process given the history of intrathecal stem cell administration. Therefore, fluorodeoxyglucose positron emission tomography (FDG PET) with computed tomography (CT) were performed, which showed intense FDG avidity throughout the thecal sac from the lower thoracic spine to the conus, as well as FDG avidity of the cauda equina nerve roots (Figure 3). There was no remote FDG uptake to suggest metastatic disease or a widespread lymphoproliferative disorder.
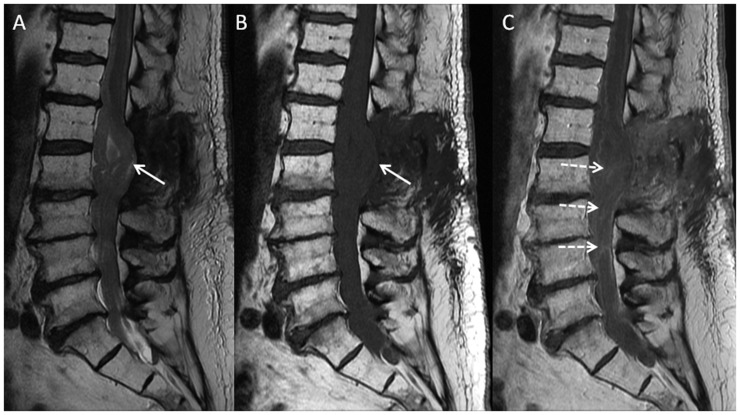
Figure 1.
Sagittal T2 (a), and T1 pre- (b) and post-contrast (c) images of the lumbar spine demonstrate massive thickening of the cauda equina nerve roots, with scattered heterogeneous enhancement seen on post-contrast images (dashed arrows). The conus and several thickened nerve roots are dorsally deviated, likely tethered at the L1–2 laminectomy site (solid arrows).
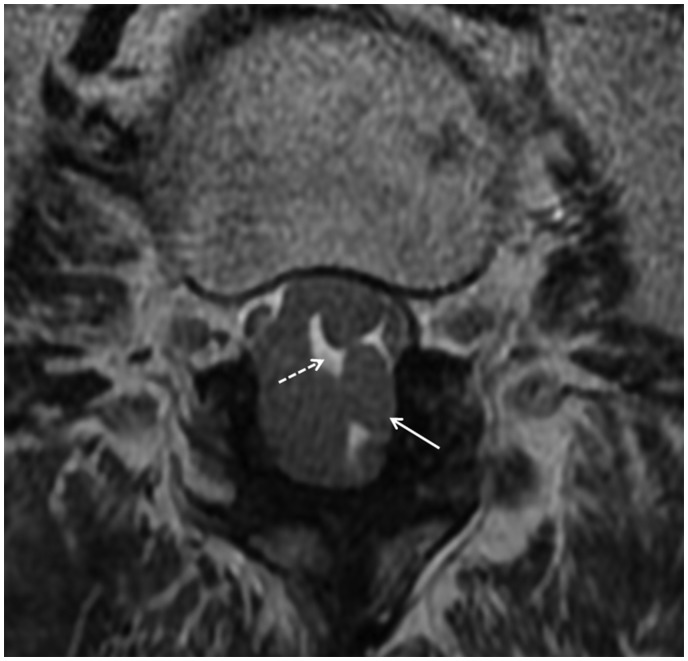
Figure 2.
Axial T2 weighted image shows severity of the cauda equina nerve root thickening (solid arrow) with interspersed pockets of bright CSF (dashed arrow).
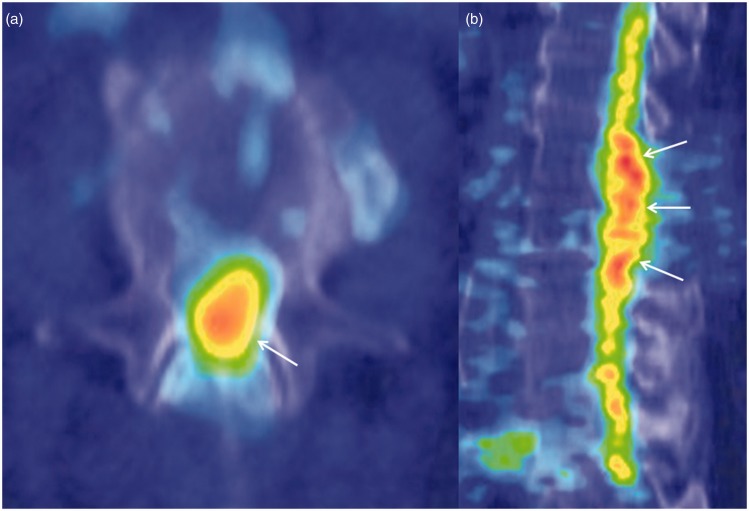
Figure 3.
Axial (a) and sagittal (b) FDG PET/CT images shows diffuse FDG avidity involving the lower thoracic cord, conus medullaris, and enlarged cauda equina (solid arrows).
Review of the patient’s prior biopsy showed fibrotic arachnoid with chronic inflammation, composed of predominantly polyclonal T-lymphocytes and macrophages. In addition, there was densely gliotic tissue harboring a small population of morphologically atypical glial cells (Figure 4), which showed loss of chromosome 10 by fluorescence in-situ hybridization. There was no evidence of a conventional neoplastic process, and the findings were considered to morphologically be in keeping with the previously described glioproliferative lesion reported in association with intrathecal stem cell therapy.4 The patient was referred to neurology for further management.
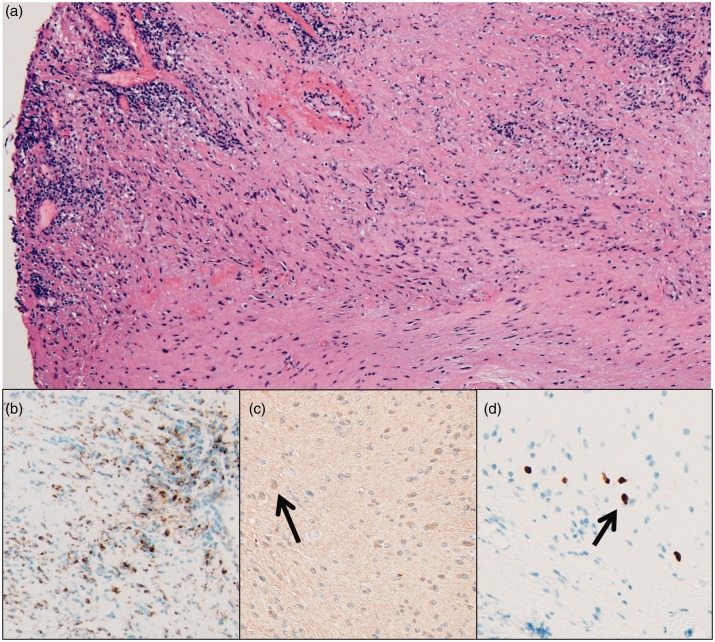
Figure 4.
Hematoxylin and eosin stained photomicrograph of a thoracolumbar spinal cord biopsy demonstrates dense fibrosis with admixed hypercellular areas (a, scaled from 100×). There is a significant population of CD68-positive macrophages (b, scaled from 100×). There are scattered foci of atypical glial cells (arrows) which are GFAP positive (c, scaled from 200×) and Olig2 positive (d, scaled from 200×).
After multidisciplinary evaluation, the patient remains under observation. Follow-up MRI after a period of several months demonstrated a slight decrease in cauda equina enlargement but was otherwise unchanged. The patient’s symptoms have remained stable.
Discussion
This report illustrates the dangers of unregulated intrathecal stem cell treatment, in which such therapy resulted in significant neurologic deficits that were refractory to medical and surgical therapy. Based on the imaging features and pathology, this was possibly due to a leptomeningeal proliferation of glial cells of unclear origin, as well as associated chronic inflammation through an uncertain mechanism. Although this lesion may be considered “neoplastic,” it is in fact distinct from the unregulated proliferation of self/host cells in the classical definition of a neoplasm.4 The imaging findings of this type of lesion are likely variable. Our patient’s MRI showed an intrathecal mass with cauda equina nerve root thickening (Figures 1 and 2), while a similar previously reported stem cell-induced glioproliferative lesion showed a predominantly intradural mass.4 Our patient’s FDG PET scan also demonstrated hypermetabolism associated with the lesion (Figure 3), though FDG PET findings are likely variable.
Though scantly reported, a spectrum of other abnormalities has been described following unregulated intrathecal stem cell administration, and this example joins a growing body of research highlighting the dangers of such therapy.5 More severe complications have been documented as well, including a donor-derived brain tumor resulting from intrathecal stem cell injections.6 Similar ramifications have also been reported following stem cell administration into other parts of the body. For instance, hematopoietic stem cell injections into the kidneys resulted in angiomyeloproliferative renal lesions in a patient undergoing treatment for lupus nephritis.7
Treatment of stem cell-induced proliferative lesions remains poorly studied due to their rarity and novelty. However, it is known that proliferative processes such as these have unique characteristics that may make them particularly difficult to treat. For example, neoplasms associated with dedifferentiated cells may be less susceptible to radiotherapy.8 In addition, certain stem cells have key molecular signaling pathways that potentiate their oncogenic capabilities.9 The patient presented here exemplified such treatment challenges, as he remained symptomatic despite surgery, medical therapy, and radiation.
Outside of stem cell tourism, regulated and well-researched stem cell therapy is well established for a wide variety of clinical applications, with the most salient example being hematopoietic stem cell transplantation in the setting of hematologic malignancies. Moreover, research continues to discover further applications of stem cell therapy. Stem cell therapy has recently been used in treating Lyme disease with central nervous system involvement.10 There is also promising research demonstrating the value of stem cells in patients with spinal cord injury.10,11 Moreover, stem cell treatments have been shown to benefit sickle cell disease patients.12 Stem cell lines have also been used extensively in research geared toward developing tumor vaccines as prophylactic measures for ovarian cancer and other neoplasms.13 In our institution, physicians recently explored the potential use of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis and multiple system atrophy (MSA), which has thus far been safe except for some instances of transient back and radicular leg pain.14,15 Many of the MSA patients receiving intrathecal stem cell treatment were noted to have lumbar spine MRI abnormalities such as cauda equina nerve root thickening, clumping, and enhancement, though very few of these patients had any neurologic symptoms. However, there have been reports of symptomatic patients with similar imaging findings after intrathecal stem cell treatments.16
In summary, it is important to differentiate unsanctioned experimental use of stem cell therapy from genuine research and proven applications. Our patient’s experience is along a spectrum of complications that can occur with intrathecal stem cell treatments and illustrated MRI and FDG PET findings helping to explain his clinical picture. Until this field is better understood, patient education and appropriate regulation will help curb the recent rise of stem cell tourism. For now, it is important to share insight into diagnosis and treatment strategies for patients who do experience adverse effects from unregulated stem cell therapy.
Conclusion
We describe the experience of a patient who presented to our institution with an intrathecal mass-like polyclonal lymphocytic and glial cell proliferation resulting from intrathecal stem cell treatments outside of typically approved indications. This resulted in significant neurologic deficits that were unresponsive to initial medical or surgical intervention. Our patient’s experience illustrates one of the potential risks of “stem cell tourism” and demonstrates the multimodality imaging and histopathologic features of this rare entity.
Supplemental Material
Supplemental material, NEU902451 Supplemental Material for Polyclonal lymphocytic infiltrate with arachnoiditis resulting from intrathecal stem cell transplantation by Ajay A. Madhavan, Dan Summerfield, Christopher H. Hunt, Dong K. Kim, Karl N. Krecke, Aditya Raghunathan and John C. Benson in The Neuroradiology Journal
Acknowledgements
The authors thank Sonia Watson, PhD for her assistance with formatting the above manuscript and references listed below.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ajay A. Madhavan https://orcid.org/0000-0003-1794-4502
References
- 1.Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: Applications and warnings. J Exp Clin Cancer Res 2011; 30: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Master Z, Resnik DB. Stem-cell tourism and scientific responsibility. Stem-cell researchers are in a unique position to curb the problem of stem-cell tourism. EMBO Rep 2011; 12: 992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aytan P, Yeral M, Korur A, et al. Factors associated with overall survival in acute myeloid leukemia patients before and after hematopoietic stem cell transplant. Exp Clin Transplant 2019. Epub ahead of print 2019/08/20. DOI: 10.6002/ect.2018.0352. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz AL, Miller MB, Mir SA, et al. Glioproliferative lesion of the spinal cord as a complication of “Stem-Cell Tourism”. N Engl J Med 2016; 375: 196–198. [DOI] [PubMed] [Google Scholar]
- 5.Carannante G. Press release. “Stem Cell Implants to Treat Spinal Cord Injury: No Scientific Evidence, Beware of Hope Thieves”. Neuroradiol J 2010; 23: 253. [DOI] [PubMed] [Google Scholar]
- 6.Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 2009; 6: e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer G, Elsallab M, Abou-El-Enein M. Concise review: A comprehensive analysis of reported adverse events in patients receiving unproven stem cell-based interventions. Stem Cells Transl Med 2018; 7: 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SY, Jeong EK, Ju MK, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer 2017; 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tandon I, Waghmode A, Sharma NK. Cancer stem cells equipped with powerful hedgehog signaling and better epigenetic memory: Avenues to look for cancer therapeutics. Curr Cancer Drug Targets 2019. Epub ahead of print 2019/08/09. DOI: 10.2174/1568009619666190808155432. [DOI] [PubMed] [Google Scholar]
- 10.Shroff G. Single-photon emission tomography imaging in patients with Lyme disease treated with human embryonic stem cells. Neuroradiol J 2018; 31: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shroff G. Magnetic resonance imaging tractography as a diagnostic tool in patients with spinal cord injury treated with human embryonic stem cells. Neuroradiol J 2017; 30: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastings B, Patil C, Gallo AM. The experience and health-related quality of life after haploidentical stem cell transplantation for adults with sickle cell disease. West J Nurs Res 2019; 41: 1829–1844. [DOI] [PubMed] [Google Scholar]
- 13.Wu D, Yu X, Wang J, et al. Ovarian cancer stem cells with high ROR1 expression serve as a new prophylactic vaccine for ovarian cancer. J Immunol Res 2019; 2019: 9394615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staff NP, Madigan NN, Morris J, et al. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 2016; 87: 2230–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer W, Dietz AB, Zeller AD, et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology 2019; 93: e77–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst RW, Bosch EP, Morris JM, et al. Inflammatory hypertrophic cauda equina following intrathecal neural stem cell injection. Muscle Nerve 2013; 48: 831–835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, NEU902451 Supplemental Material for Polyclonal lymphocytic infiltrate with arachnoiditis resulting from intrathecal stem cell transplantation by Ajay A. Madhavan, Dan Summerfield, Christopher H. Hunt, Dong K. Kim, Karl N. Krecke, Aditya Raghunathan and John C. Benson in The Neuroradiology Journal