Abstract
Background
Zinc is a vital micronutrient for humans and is essential for protein synthesis, cell growth, and differentiation. Severe zinc deficiency can lead to slower physical, cognitive and sexual growth, cause skin disorders, decrease immunity, increase incidence of acute illnesses in infants and children and contribute to childhood stunting. By estimation, 17.3% of the world population is at risk of inadequate zinc intake. Such nutritional impairment increases the risk of diarrhoea and pneumonia by 20%, as well as leads to a global loss of more than 16 million disability‐adjusted life years in children less than five years of age. Not only does zinc deficiency affect lives, it adds to the considerable financial burden on depleted resources in countries that are most affected. By preventing or curing this deficiency, we can improve childhood mortality, morbidity and growth.
Objectives
To assess the effectiveness of zinc supplementation for the promotion of growth, reduction in mortality, and the prevention of infections in infants less than six months of age.
Search methods
We used the standard search strategy of the Cochrane Neonatal Group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 4), MEDLINE via PubMed (1966 to 18 May 2018), Embase (1980 to 18 May 2018), and CINAHL (1982 to 18 May 2018). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials. An updated search from 1 January 2018 to 29 January 2020 was run in the following databases: CENTRAL via CRS Web, MEDLINE via Ovid, and CINAHL via EBSCOhost.
Selection criteria
All randomised controlled (individual and cluster randomised) and quasi‐randomised trials of zinc supplementation in healthy, term infants, less than six months of age comparing infant mortality, incidence of diarrhoea or respiratory illnesses, growth and/or serum zinc levels were eligible.
Data collection and analysis
Two review authors screened search results (title and abstracts) and relevant full texts. Studies fulfilling prespecified inclusion criteria were included with any disagreements resolved by consensus. Extraction and analysis were then conducted. We used the GRADE approach to assess the quality of evidence as indicated by certainty in effect estimates.
Main results
Eight studies (with 85,629 infants) were included and five studies were meta‐analysed, out of which four studies compared zinc with placebo, and one compared zinc plus riboflavin versus riboflavin. Certain growth outcomes after six months of intervention (Weight for Age Z‐scores (WAZ) (standardised mean difference) (SMD) 0.16, 95% CI 0.03 to 0.29; three studies, n = 955; fixed‐effect; heterogeneity Chi² P = 0.96); I² = 0%); change in WAZ (SMD 0.16, 95% CI 0.07 to 0.25; one study, n = 386; fixed‐effect); (Weight‐for‐Length Z‐score (WLZ) (SMD 0.15, 95% CI 0.02 to 0.28; three studies, n = 955; fixed‐effect; heterogeneity: Chi² P = 0.81); I² = 0%); (change in WLZ (SMD 0.17, 95% CI 0.06 to 0.28; one study, n = 386; fixed‐effect)) were positively affected by zinc supplementation compared to placebo. A single study reported no difference in the incidence of diarrhoea and lower respiratory tract infection with zinc supplementation. Zinc had no effect on mortality in children younger than 12 months.
When zinc plus riboflavin was compared to riboflavin only, significant improvement was observed in the incidence of wasting at 24 months (risk ratio (RR) 0.59, 95% CI 0.37 to 0.96; one study, n = 296; fixed‐effect), but significant worsening of incidence of stunting was present at 21 months (RR 1.53, 95% CI 1.09 to 2.16; one study, n = 298; fixed‐effect).
Authors' conclusions
There was a significant positive impact of zinc supplementation on WAZ and WLZ after six months of intervention in infants compared to placebo. When a combined supplement of zinc and riboflavin was compared to riboflavin, there was a significant reduction in wasting at 24 months, but stunting at 21 months was negatively affected. Although included trials were of good‐to‐moderate quality, evidence that could be meta‐analysed was based on a few studies which affected the overall quality of results. Regardless, there is a need for strong trials conducted in infants younger than six months before a strong recommendation can be made supporting zinc supplementation in this age group.
Keywords: Humans; Infant; Infant, Newborn; Growth; Body Weight; Infant Mortality; Infection Control; Infection Control/methods; Randomized Controlled Trials as Topic; Riboflavin; Riboflavin/administration & dosage; Trace Elements; Trace Elements/administration & dosage; Vitamin B Complex; Vitamin B Complex/administration & dosage; Wasting Syndrome; Wasting Syndrome/prevention & control; Zinc; Zinc/administration & dosage; Zinc/deficiency
Plain language summary
Zinc supplementation for improving the health and survival of infants less than six months old
Review question: does zinc supplementation reduce mortality, morbidity and increase growth in infants aged less than six months old?
Background: zinc deficiency is prevalent in the world. Adequate zinc levels are linked to decreased deaths, illnesses and improved growth in all populations particularly in developing countries. Zinc supplementation in older children has shown some positive effects.
Study characteristics: we searched for studies through January 2020 that enrolled healthy infants aged less than six months old and provided at least six months of zinc supplementation. We included eight studies that covered zinc supplementation compared with placebo, zinc and riboflavin compared with riboflavin only, zinc, riboflavin and B complex vitamins compared with riboflavin and B complex vitamins.
Key results and certainty of evidence: zinc had no effect on decreasing deaths or illnesses in children younger than 12 months. Moderate‐ to good‐certainty evidence from three studies suggested an increase in weight‐for‐age Z‐scores and weight‐for‐length Z‐scores after zinc supplementation for six months compared to placebo only. Only one study could be analysed for zinc and riboflavin supplementation compared to riboflavin only, and the results suggested a decrease in wasting after 24 months of supplementation and an increase in stunting after 21 months.
Conclusion: with this evidence, we cannot strongly recommend clinicians to prescribe zinc supplements to children under six months of age. We also encourage the development of trials that will provide zinc as well as other micronutrients in this age group and for such trials to evaluate outcomes such as death, disease, side effects and growth.
Summary of findings
Summary of findings for the main comparison. Zinc supplementation compared to placebo; no treatment for the promotion of growth and prevention of infections in infants less than six months of age.
| Zinc supplementation compared to placebo/ no treatment for the promotion of growth and prevention of infections in infants less than six months of age | |||||
| Patient or population: the promotion of growth and prevention of infections in infants less than six months of age Setting: India, Indonesia, Nepal Thailand, USA, Vietnam, Zanzibar Intervention: zinc supplementation before the age of 6 months Comparison: placebo/ no treatment | |||||
| Outcomes | № of participants (studies) Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo/ no treatment | Risk difference with Zinc supplementation | ||||
| Length (cm) after 6 months | 644 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ‐ | The mean length (cm) after 6 months was 0 SD | SMD 0.07 SD higher (0.08 lower to 0.23 higher) |
| Weight (g) after 6 months | 644 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | The mean weight (g) after 6 months; Country was 0 | SMD 0.16 higher (0 to 0.31 higher) |
| Weight for age Z‐score after 6 months | 955 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ‐ | The mean weight for age Z‐score after 6 months was 0 | SMD 0.16 higher (0.03 higher to 0.29 higher) |
| Length for age Z‐score after 6 months | 955 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ‐ | The mean length for age Z‐score after 6 months was 0 | SMD 0.06 higher (0.07 lower to 0.19 higher) |
| Weight for length z‐score after 6 months | 955 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ‐ | The mean weight for length z‐score after 6 months was 0 | SMD 0.15 higher (0.02 higher to 0.28 higher) |
| Stunted after 6 months | 955 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | RR 0.82 (0.65 to 1.03) | Study population | |
| 264 per 1,000 | 48 fewer per 1,000 (92 fewer to 8 more) | ||||
| Wasted after 6 months | 955 (3 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | RR 0.78 (0.48 to 1.26) | Study population | |
| 71 per 1,000 | 16 fewer per 1,000 (37 fewer to 19 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SD: standard deviation; RCT: randomised controlled trial; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Out of the three studies contributing to this outcome, there was a 19% loss of follow‐up in Dijkhuizen 2001 which may have affected outcomes; Heinig 2006 had a high risk of reporting bias due to the failure to report details of the difference in incidence or prevalence of various morbidities. Due to insufficient details it was impossible to determine whether or not Berger 2006 was free from detection or attrition bias due as details about blinding procedures were not provided
2 Effect sizes between Heinig 2006 and the 2 other studies are different in magnitude and direction. Heinig 2006 was conducted in a developed country (USA) with a very specific set of eligibility criteria that extended to the mother, included children who were between 4 to 10months and provided 5 mg/day of zinc compared to the 10 mg/day provided in the other 2 studies.
3 Out of the three studies contributing to this outcome, there was a 19% loss of follow‐up in Dijkhuizen 2001 which may have affected outcomes; Berger 2006 provided insufficient details about blinding procedures provided to determine whether or not there was detection or attrition biases and Fahmida 2007 provided insufficient details to make judgements about the presence of selection bias, performance bias and detection bias.
4 The number of events in Dijkhuizen 2001 are very small and the number of children enrolled was much smaller than the sample size calculated as being necessary to detect an effect (360 vs. 480). Also, the confidence interval is very wide and the effect size quite large and in the opposite direction to the other 2 studies.
Background
Description of the condition
Zinc deficiency is thought to be one of the most prevalent micronutrient deficiencies worldwide (Wieringa 2015), and is associated with a high global burden of morbidity in low‐ and middle‐income countries (LMICs). Black 2013 estimated that zinc deficiency is related with 116,000 deaths among children aged five years, which represents 1.7% of all deaths in this age group. This contributes to 3.1 million of global deaths of these children (45%) due to nutritional conditions. According to the 2013 Lancet series on diarrhoea and pneumonia, zinc deficiency increases the risk of these morbidities by 20% (Walker 2013). Previous studies have attributed a global loss of more than 16 million disability‐adjusted life years in < 5 years‐old children to zinc deficiency (Walker 2009). Because of diverse functions of zinc in vivo, it has been difficult to measure a single biomarker of zinc status. Plasma zinc concentrations as a biomarker has been used to rule out the deficiency, but this still is nonspecific (King 2011). Zinc deficiency can lead to childhood stunting. A high prevalence of stunting has been used as a useful proxy of the zinc deficiency at a population level (Mayo‐Wilson 2014; Wessells 2012), although its use is considered controversial by some authors (Wieringa 2015).
Until 2007, there was still no single accepted indicator or reliable biomarker to evaluate the zinc status in humans. A synthetic procedure was developed by the World Health Organization (WHO), the United Nations Children’s Fund (UNICEF), the International Atomic Energy Agency (IAEA), and the International Zinc Nutrition Consultative Group (IZiNCG) including biochemical, dietary, and functional indicators (Davidsson 2007). However, to our knowledge, only a few countries such as Pakistan, Cameroon, and Sri Lanka have collected complete information about zinc status as it is fairly costly and logistically challenging (Liu 2017).
It is now recognised that mild‐to‐moderate zinc deficiency due to inadequate dietary intake is prevalent in all parts of the world. The higher prevalence of zinc deficiency in developing countries is primarily due to low intake of zinc from animal sources, high dietary phytate content (found in cereals and legumes; phytates limit the bioavailability of zinc), and inadequate food intake (Mayo‐Wilson 2014). A population‐level analysis from national food balance sheets has estimated that 17.3% of the world population is at risk of inadequate zinc intake. Levels are even higher in less‐developed countries, reaching up to 29.6% in South Asia (six countries), 25.6% in Sub Saharan Africa (48 countries) and 22.1% in East and Southeast Asia and Pacific (21 countries) (Wessells 2012).
Zinc is a vital micronutrient for humans and is essential for protein synthesis, cell growth, and differentiation (Caulfield 2006). Zinc is not stored in the body, so the levels are determined by the balance between dietary intake, absorption, and losses. Severe zinc deficiency has been shown to be associated with stunting, hypogonadism, impaired immune function, skin disorders, cognitive dysfunction, anorexia and an increase in the number of episodes of acute illnesses both infants and children (Shankar 1998). Zinc deficiency also impairs immunocompetence with reduced cell‐mediated immune responses; decreased T lymphocytes; abnormal T helper or suppressor functions, or both; impaired macrophage function; and reduced killer cells and antibody‐dependent cytotoxicity (Ibs 2003).
Clinical and field studies have consistently observed an association between zinc deficiency and morbidity owing to infectious diseases, particularly diarrhoea in early childhood (Bhandari 1996; Black 2013). Regarding this, the WHO and UNICEF, currently, recommend provision of zinc supplements along with oral rehydration therapy for acute diarrhoea. Nevertheless, no routine supplementation recommendations currently exist for the prevention of zinc deficiency (Bailey 2015). Marginal zinc deficiency is associated with about a 50% increased risk and number of days with diarrhoea. Zinc deficiency results in higher rates of other infectious diseases as well, including skin infections, respiratory infections, malaria, and delayed wound healing (Aggett 1995, Yakoob 2011). Overall, zinc‐deficient children are at a three‐fold increased risk of an acute respiratory infection (Bhandari 1996).
Description of the intervention
The recommended dietary allowance (RDA) for infants under six months is 2.0 mg, and it is 3.0 mg per day for young children aged seven to 36 months (Bethesda 2006). However, the amount of zinc needed in young infants to maintain a positive zinc balance in areas with a high prevalence of zinc deficiency is unknown. The majority of published results of efficacy trials of zinc treatment have tested doses ranging from 10 mg (infants) to 20 mg (children under five years of age) of elemental zinc per day, a dosage that is safe in these children. Doses of up to 70 mg twice a week have been provided without any toxic effect (Bates 1993). A zinc sulphate 20 mg tablet (which is now known as 'Baby Zinc' in Bangladesh), is recommended by WHO and most widely used in developing countries. The tablet is placed in a spoon or a small cup and water added that leads it to disperse into a sweet, vanilla‐flavoured syrup that masks the taste of zinc.
Acute zinc toxicity due to excess administration (225 mg to 450 mg of zinc) includes gastrointestinal symptoms, such as nausea, vomiting, epigastric pain, abdominal cramps, and bloody diarrhoea (Fosmire 1990). Transient vomiting or nausea among adults at doses of 50 mg/day or higher are well‐known side effects of zinc (Weimar 1978). Whether similar effects at the lower dose of 10 mg/day to 20 mg/day will be observed in children is unclear. Strand 2002 reported a nearly two‐fold increase in vomiting when treating Nepalese children with 15 mg/day to 30 mg/day. Whether this was because of a direct side effect of zinc or because of inadequate masking of the metallic taste of zinc could not be differentiated. Other trials, including the effectiveness trial of Baqui 2002, did not report increased risks of vomiting.
How the intervention might work
Zinc deficiency appears to be widespread in low‐income countries because of a low dietary intake of zinc‐rich animal‐source foods and a high consumption of cereal grains and legumes, which inhibit the absorption of zinc (Sandstead 1991). Without intervention, a child whose diet does not provide him or her with enough zinc will eventually develop zinc deficiency and all consequences associated with it.
Moreover, innate immunity is the body’s first line of defence to pathogens, and its functions are also altered by zinc levels. Similarly, natural killer cell numbers and function are dependent on normal levels of serum zinc (Ravaglia 2000). Zinc is also required for the development and activation of T lymphocytes. When zinc supplements are given to individuals with zinc deficiency, the numbers of T‐cell lymphocytes circulating in the blood increase, and the ability of lymphocytes to fight against infection improves. The possible mechanisms of the effect of zinc treatment on the duration and severity of diarrhoea has been studied in depth and shows improved absorption of water and electrolytes by the intestines (Ghishan 1984), regeneration of gut epithelium (Bettger 1981), increased levels of enterocyte brush‐border enzymes (Jones 1981), and enhanced immunological mechanisms for the clearance of infection.
Lastly, zinc is also considered an essential micronutrient for the growth of children. Zinc, magnesium, and phosphorus have been referred to as type II nutrients (Golden 2004), which are essential for growth. In the absence of adequate amounts of these type II nutrients, cell function ceases. These type II nutrients are present in all tissues, and there is no clearly identifiable ‘storage’ compartment from which the nutrient can be mobilised. So, deficiency of any type II nutrient will lead to growth retardation (Wieringa 2015). Additionally, several nucleoproteins containing zinc are involved in gene expression of proteins important for growth and the production of insulin‐like growth factor‐1 is retarded in zinc deficiency, along with the cellular responsiveness to its hormone (Cole 2008).
Why it is important to do this review
Theoretically, breast milk supplies all the zinc needs for at least the first several months of life, although the period during which breast milk alone remains sufficient is uncertain (Brown 2009). Therefore, it remains to be seen if additional zinc provision will be of any benefit, especially in infants less than six months of age who are breast‐fed. Studies on the effect of zinc supplementation on diarrhoeal episodes in children have yielded varying results depending on age, with a clear beneficial effect in infants older than six months (Bhutta 2000; Haider 2009), but no effect before that (Brooks 2005; Fisher Walker 2006). A systematic review on zinc supplementation trials in infants less than six months of age is lacking. Brown 2009 has mentioned trials that have been conducted, but no pooled analysis has been done. A few trials are available from developing countries, but no systematic review has been done on the trials. Therefore, a systematic review is needed to summarise the effect of zinc supplementation on growth and in the prevention of infections among infants under six months of age.
Objectives
To assess the effectiveness and safety of zinc supplementation for the promotion of growth and the prevention of infections in infants less than six months of age.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled (individual‐ and cluster‐randomised) and quasi‐randomised trials of zinc supplementation in infants less than six months of age. We excluded observational studies and cross‐over trials.
Types of participants
The participants included:
infants less than six months of age;
infants who were more than 37 weeks' gestation at the time of birth (i.e. not preterm);
free from chronic diseases such as sickle cell disease, cystic fibrosis, or severe protein energy malnutrition;
breast‐fed infants;
not receiving parenteral nutrition.
Types of interventions
Orally administered zinc supplements in syrup form for a minimum of six months. Studies of zinc supplemented in a fortified form were excluded because the zinc dose does not meet WHO guidelines for daily dose or minimal treatment days.
The adjunct micronutrients considered were riboflavin (B2), vitamin A, vitamin C, vitamin D, copper, and calcium. The word micronutrient here means any formulation that does not include zinc.
Zinc versus placebo or no treatment or single/multiple micronutrients. Further subgroup analyses were done as follows:
zinc versus placebo/no treatment;
zinc plus single micronutrient versus single micronutrient;
zinc plus multi‐micronutrient versus multi‐micronutrient*;
zinc plus vitamin A versus vitamin A;
zinc plus riboflavin plus vitamin A versus riboflavin plus vitamin A;
zinc plus vitamin A plus vitamin C versus vitamin A plus vitamin C;
zinc plus riboflavin versus riboflavin;
zinc plus several B vitamins versus several B vitamins;
zinc plus vitamin C versus vitamin C;
zinc plus vitamin D versus vitamin D;
zinc plus calcium plus vitamin A versus calcium plus vitamin A;
zinc plus calcium versus calcium;
zinc plus copper versus copper only.
* Multi‐micronutrient contains vitamin A, vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12, folic acid, vitamin C, vitamin D, vitamin E, copper, selenium, iodine; with iron 30 mg and zinc 15 mg.
Studies that provided iron were excluded because iron is known to interfere with zinc absorption and iron‐containing formulations are not recommended for the treatment of diarrhoea (WHO 2010).
Types of outcome measures
Primary outcomes
Infant mortality defined as the number of infant deaths (one year of age or younger) among those infants enrolled in the trials.
*Growth measured as mean length; change in length; mean weight; change in weight; mean head circumference; change in head circumference; Weight for Age Z‐scores (WAZ); change in WAZ; Length for Age Z‐scores (LAZ); change in LAZ; Weight for Length Z‐scores (WLZ); change in WLZ; incidence of stunting, wasting or underweight children.
Incidence of diarrhoea (defined as three or more loose stools in a 24‐hour period).
Secondary outcomes
Diarrhoea‐specific mortality rate (mortality due to diarrhoea among those infants enrolled in the trials).
Prevalence of diarrhoea (defined as the number of days of diarrhoea).
Incidence of failure to thrive (defined as weight for chronological age < 5th percentile).
Serum or plasma zinc levels (as measured in trials at three, six, and 12 months of supplementation).
Incidence of sepsis (defined as a positive culture of the blood).
Incidence of lower respiratory tract infection (defined as: (1) clinical signs such as cough, difficulty breathing, tachypnoea (abnormally rapid breathing), respiratory distress, and signs of infection such as fever or rhinorrhoea ('runny nose'), or (2) clinical signs of infection with an abnormal chest radiography).
Incidence of severe malaria (acute falciparum malaria with signs of severity or evidence of vital organ dysfunction, or both) (WHO 2010).
Incidence of acute zinc toxicity (vomiting, loss of appetite, diarrhoea).
*The growth outcomes were measured at six weeks after the start of the supplementation. The outcomes of infections were surveyed within the time period of the duration of supplementation.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see Cochrane Neonatal's search strategy for specialized register (https://neonatal.cochrane.org/resources‐authors/author‐resources‐new‐reviews)).
Electronic searches
We conducted a comprehensive search in November 2015 (see Appendix 1). We updated the search in May 2018 in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 4) in the Cochrane Library; MEDLINE via PubMed (1966 to 18 May 2018); Embase (1980 to 18 May 2018); and CINAHL (1982 to 18 May 2018) using the following search terms: (zinc), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry).
We conducted a comprehensive update search in January 2020 in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 1) in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (01 January 2018 to 29 January 2020); and CINAHL (01 January 2018 to 29 January 2020). We have included the search strategies for each database in Appendix 3. We did not apply language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched The World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), and the U.S. National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry for any unique trials not found through the Cochrane CENTRAL search.
Searching other resources
We scrutinised the reference lists of identified trials and important review articles for possible trials missed by electronic searches. We did not impose any language or publication restrictions (published, unpublished, in press, and in progress).
Data collection and analysis
We followed standard review methods as outlined by the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.1.0) and the Cochrane Neonatal Group (Higgins 2011).
Selection of studies
Two review authors (AM and CO) independently assessed the eligibility of the trials. We selected studies as being potentially relevant by screening the titles and abstracts. We retrieved the full text of the article for review if the relevance could not be ascertained by screening the title and abstract. We retrieved full texts of all potentially relevant articles and assessed the eligibility independently by filling out eligibility forms designed in accordance with the specified inclusion criteria. We resolved any disagreements by discussion with third trial author (ZL and ZB), and consensus was reached. We documented the studies excluded from the review in the table Characteristics of excluded studies along with the reason of exclusion. An updated search was run in May 2018; two review authors (ZL and JK) screened the titles and abstracts for relevance and updated the list of studies excluded from the review.
Data extraction and management
Two review authors (AM and CO) independently extracted data from the included studies using standardised data extraction forms. We then compared the extracted data to correct errors and resolved any disagreements through discussion. We resolved any differences in data interpretation with the help of the trial author (ZL and ZB). We used Review Manager 5 (RevMan 2014), software to enter all the data or a sub‐sample of the data.
Assessment of risk of bias in included studies
Three review authors (AM, CO and JK) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011), for the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion or by a third assessor (ZL and ZB). See Appendix 4 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We presented results as summary of risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data. We used the mean difference (MD) if outcomes were measured in the same way between trials for continuous data. We used the standardised mean difference (SMD) to combine trials that measure the same outcome but used different methods. We expressed outcomes in terms of MD/SMD and 95% CIs.
Unit of analysis issues
We dealt with cluster‐randomised control trials as specified in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
There were four trials that could not be included in the analysis due to a lack of clarification on certain points (Castillo Durán 2001; Hong 1992; Locks 2016, NCT02319499). For Castillo Durán 2001, Locks 2016 and NCT02319499, we contacted trial authors for missing information. For Hong 1992, we did not have any method for contact. All of these studies are currently in awaiting classification (Characteristics of studies awaiting classification).
Assessment of heterogeneity
We applied tests for heterogeneity between trials, if appropriate, using the I² statistic or P value of Chi² test. Where we identified high levels of heterogeneity among the trials (exceeding 50%), we explored this by subgroup analysis, as specified below.
Assessment of reporting biases
We did not use funnel plots to assess reporting biases since no outcome was reported by more than 10 studies.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). In the absence of significant heterogeneity, where trials were sufficiently similar, we used a fixed‐effect meta‐analysis model for combining data. Otherwise, we used a random‐effects meta‐analysis model.
Certainty of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes: infant mortality, growth (length, weight, head circumference, Weight for Age Z‐scores (WAZ), Length for Age Z‐scores (LAZ), Weight for Length Z‐scores (WLZ), stunting, wasting and underweight) and incidence of diarrhoea.
Two review authors (ZL and JK) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence in one of four grades.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis on the basis of following (Table 2).
1. Study details.
|
Study ID |
Intervention | Dosage | Age of participants | Duration of supplementation | Baseline plasma zinc levels*1 | Outcomes | Country | |||
| Intervention *2 | Control | Primary outcomes | Secondary outcomes | |||||||
| Berger 2006 | Zinc vs placebo | 10 mg zinc as zinc sulphate | 4 to 7 months | 6 months | 14.41 umol/L | 14.59 umol/L | Height (cm), weight (kg), HAZ (z‐scored), WAZ (z‐scored), WHZ (z scored), stunting (%) HAZ ≤ 2 z‐scores, wasting (%) WHZ ≤ 2 z‐scores, change in HAZ, WAZ, WHZ | Serum zinc | Vietnam (developing) | |
| Dijkhuizen 2001 | Zinc vs placebo | 10 mg (not specified) | 4 months | 6 months | ‐ | ‐ | Weight (kg), length (cm), WAZ, HAZ, % stunted, WHZ, % wasted, knee‐heel length (cm) | Plasma zinc | Indonesia (developing) | |
| Fahmida 2007 | Zinc vs placebo*3 | 10 mg zinc sulphate |
3 to 6 months | 6 months | Zn: 15.3 (12.2 to 18.4) umol/L |
Placebo 15.3 (13.8 to 17.2) umol/L | Stunting (HAZ < ‐2.00), Wasting (WHZ < ‐2.00), Underweight (WAZ < ‐2.00), HAZ, WAZ, WHZ | Serum Zinc | Indonesia (developing) | |
| Heinig 2006 | Zinc vs placebo | 5 mg as zinc sulphate | 4 months | 6 months | 0.71 (0.51 to 0.82) ug/mL (10.86 umol/L) |
0.76 (0.62 to 0.97) ug/mL (11.63 umol/L) |
Length (cm), Weight (g), Head circumference (cm) and Change Morbidity data were coded and grouped into 5 major categories: respiratory illness, diarrhoea, otitis media, fever (without other symptoms), and other illnesses. |
Serum zinc | USA (developed) | |
| Radhakrishna 2013 | Zinc and riboflavin vs riboflavin | 5 mg (formulation not specified) | 4 months | 14 months | ‐ | ‐ | Weight(kg), length (cm), head circumference (cm), Under weight (WAZ < –2 SD), Stunting (HAZ < –2 SD), Wasting (WHZ < –2SD) Diarrheal and respiratory morbidity |
Serum zinc | India (developing) | |
| Sazawal 2007 | Zinc vs placebo | 10 mg as zinc sulphate (dispersible tablet given in solution) | 1 to 36 months | Variable duration, till 48 months of age | ‐ | ‐ | The primary outcome was overall mortality in participating children aged 1 to 48 months. Secondary outcomes were age‐specific, sex‐specific, and cause‐specific mortality. |
Zanzibar archipelago (developing) | ||
| Tielsh 2007 | Zinc vs placebo | 10 mg as zinc sulphate Ferrous sulphate (dispersible tablet given in solution for young children) |
1 to 35 months | 12 months | ‐ | ‐ | The primary outcome was all‐cause mortality; Secondary outcomes were: cause‐specific mortality; the incidence and severity of diarrhoea, dysentery as assessed in the morbidity sub study | The incidence and severity of acute respiratory illness as assessed in the morbidity sub study | Nepal (developing) | |
| Wasantwisut 2006 | Zinc vs placebo | 5 g/L (76.5 mmol/L) zinc as zinc sulphate | 4 to 6 months | 6 months | Zinc 11.5 + 2.9 umol/L zinc and iron 11.3 + 2.3 umol/L |
Placebo 11.1 + 2.7 umol/L Iron 10.4 + 2.5 umol/L |
Length (cm) , weight (kg), LAZ, WAZ, WLZ | Serum zinc | Thailand (developing) | |
|
1* Using as cut‐off: http://ajcn.nutrition.org/content/78/4/756/T1.expansion.html Normal serum zinc levels in 3‐9 years 82.5 +‐ 0.3 ug/dL, 12.63 umol/L 2*( ug/dl = umol/L http://www.endmemo.com/medical/unitconvert/Zinc.php ) 3* Also contains iron and zinc, iron, zinc and vitamin A groups but cannot be compared | ||||||||||
HAZ: Height for Age Z‐score; LAZ: Length for Age Z‐score; WAZ: Weight for Age Z‐scores; WLZ: Weight for Length Z‐scores
Dose of zinc (10 mg/day, > 10 mg/day). The zinc dosage subgroup analysis was not conducted since all studies reported the use of 10 mg of elemental zinc or less than this value in their interventions.
Country: developing or developed. We used World Bank criteria to define which group each country belonged to: low‐and middle‐income countries (LMICs) were categorised under developing countries, while high‐income countries (HICs) were categorised under developed countries (World Bank).
Age of participant (neonate, post‐neonate up to six months).
Baseline plasma zinc levels. We were unable to find an established guideline for a definitive normal serum or plasma zinc level for infants under six months of age. We found non‐fasting serum zinc concentration cutoffs for children under 10 years of age from the population studies (IZiNCG 2004). The majority of the studies that provided baseline zinc concentration did not mention the time when the blood sample was collected. Besides that, there are no cut‐offs available for fasting zinc levels (IZiNCG 2004). Therefore, we could not perform the subgroup analysis based on this factor.
Duration of supplementation subgroup analysis was not conducted since all studies included in the pooled analysis had an intervention duration of six months.
We performed subgroup analyses based on country classification and all children that were assessed were postneonatal.
Sensitivity analysis
Sensitivity analysis was not required since all included studies were of moderate to good quality with no study having a high risk of bias.
Results
Description of studies
Results of the search
After using the defined search strategy, 2505 articles were screened. Two review authors (CO and AM) independently reviewed the titles and abstracts of all potential articles. Discrepancies in relation to the papers and data to be included in the systematic review were solved by a third review author (ZL and ZB).
We identified 58 potential studies for inclusion. After reviewing the full text of these articles, eight studies were found eligible for inclusion in this review. The study selection process can be found in Figure 1. The details of these studies are reported in the Characteristics of included studies table.
1.

Study flow diagram.
Four studies (Castillo Durán 2001; Hong 1992; Locks 2016; NCT02319499), are in the awaiting classification list and details on these studies are provided in Characteristics of studies awaiting classification; 48 studies were excluded and reasons for exclusion are provided in Characteristics of excluded studies table.
Included studies
Eight studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007; Wasantwisut 2006), fulfilled our inclusion criteria. Five studies were included in the meta‐analysis (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Radhakrishna 2013), and three could not be pooled (Sazawal 2007; Tielsh 2007; Wasantwisut 2006). These studies, although eligible for inclusion, were not analysed because the data were either not presented in a form that allowed us to analyse (e.g. total number of participants per intervention group was not specified or standard deviations were not mentioned), included the data of participants older than six months or the study did not report this review's prespecified outcomes.
Setting
Studies were classified as "developed" or "developing" using income levels as defined by the World Bank; low‐ and medium‐income levels were classified as developing countries while those designated as high‐income countries classified as developed countries.
One study conducted by Heinig 2006 in the USA fell under the developed world category. The remaining studies were classified under developing world: Radhakrishna 2013 in India; Dijkhuizen 2001 and Fahmida 2007 in Indonesia; Berger 2006 in Vietnam; Wasantwisut 2006 in Thailand; Tielsh 2007 in Nepal and Sazawal 2007 in the Zanzibar.
Participants
The trials included a total of 85,629 children (intervention = 43,145, control = 42,484) at first follow‐up; age at recruitment of participants included ranged from one to 35 months (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007; Wasantwisut 2006). Six studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Wasantwisut 2006), reported on one‐ to seven‐month old infants (number of participants in intervention = 903 and control group = 904, respectively). Two studies (Sazawal 2007; Tielsh 2007), reported on participants aged from one month to under three years (number of participants in intervention = 42,242; number of participants in control = 41,580).
Five studies (Berger 2006; Fahmida 2007; Heinig 2006; Sazawal 2007; Wasantwisut 2006), reported a baseline zinc level of the participants. Berger 2006, and Fahmida 2007 reported levels above normal serum values of zinc; whereas Heinig 2006 and Wasantwisut 2006 reported lower than normal values.
Intervention
The eligible studies allocated participants into two groups.
Zinc alone versus placebo.
Zinc plus riboflavin versus riboflavin alone.
Seven studies reported zinc as the intervention group and placebo as the control group (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Wasantwisut 2006; Sazawal 2007; Tielsh 2007). One study reported zinc and riboflavin as the intervention group and riboflavin as the control group (Radhakrishna 2013).
Three studies included additional intervention arms comprising a combination of zinc and iron, and an iron‐alone arm (Berger 2006; Dijkhuizen 2001; Wasantwisut 2006). Two studies had an iron+folic acid intervention arm and an iron‐folic acid‐zinc in addition to a zinc‐only intervention arm a (Sazawal 2007; Tielsh 2007). One study had a zinc+iron and a zinc+iron+vitamin A intervention in addition to a zinc‐only intervention arm (Fahmida 2007).These additional interventions arms were not included in the analyses due to interference in zinc absorption posed by the presence of iron.
Experimental and control formulations were administered via oral route in the form of a syrup in six studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Wasantwisut 2006). Two studies (Sazawal 2007; Tielsh 2007), provided it in a dispersible tablet form that was dissolved in water or breast milk as appropriate to the age of the infant.
Seven studies provided the zinc supplement as zinc sulphate (Berger 2006; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007; Wasantwisut 2006). One study did not specify the type of zinc being used (Dijkhuizen 2001).
Overall, the doses used by the trials were between 5 mg to 10 mg of elemental zinc per day. Five studies (Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007; Wasantwisut 2006), provided 5 mg and three studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007), provided 10 mg of zinc per day per child.
The intervention period was six months in five studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Wasantwisut 2006), and 12 months in Radhakrishna 2013. It was of a variable duration in Sazawal 2007 (where children were supplemented until age four years and outcomes were only reported at six‐monthly intervals from the start of intervention), and Tielsh 2007 (where children received supplementation until they were discharged at 36 months of age) .
Participants in two studies (Berger 2006; Fahmida 2007), were provided a dose of 100,000 IU of vitamin A to all the infants at study initiation, while participants of Sazawal 2007 were provided 100,000 IU of vitamin A to all infants aged six to 11 months and 200,000 IU to children older than 12 months every six months. Tielsh 2007 provided 200,000 IU to children 12 months or older, 100,000 IU to children between six to 12 months but none to children under six months. Wasantwisut 2006 provided all children with an equivalent of 1500 μg of retinol at baseline.
Excluded studies
We excluded 48 studies in total after full‐text review. Some studies had more than one reason for exclusion.
Inappropriate intervention
Twelve studies (Alam 2011; Aminisani 2011; Ashworth 1998; Coles 2008; Hamadani 2001; Jimenez 2007; Kumar 2012; Lira 1998; Ninh 1996; Osendarp 2002; Rana 2011; Walker 2007), were excluded because the intervention duration was less than six months. One study (Bilenko 2010), reported an inadequate amount of zinc used in the intervention. Three studies (Bhandari 2007; Castillo‐Durán 1995; Wieringa 2003), were excluded as they provided iron as an additional intervention to certain participants.
Four studies used an inappropriate form of supplementation: Gibson 2011 used a meal form, Salmenpera 1994 and Walravens 1976 used fortified milk and Zlotkin 2003 used sprinkles. NCT00133419 provided infants with a micronutrient mixture containing vitamins A, B,C, D, E and K and was thus excluded. Olney 2006 was excluded because interventions included iron+folic acid+zinc and iron+folic acid.
Inappropriate participants
Eleven studies were excluded because of the age of the child: five studies (Bates 1993; Dewey 2017; Hess 2017; Mallard 2014; Menon 2007), had children older than six months; three studies (Manno 2012; Mullen 2013; Muller 2001), involved children exactly six months old; two studies (Friel 1993; Terrin 2013), reported on preterm neonates. Yang 2002 was excluded because growth‐retarded pre‐schoolers were enrolled in the study.
Six studies (Beuno 2008; Bhatnagar 2012; Mehta 2012; Schlesinger 1993; Simmer 1988; Walravens 1989), reported on ill children and were also excluded. Four studies were excluded because they included low‐birth weight children (El‐Farghali 2015; Mahalanabis 2011; Sur 2003; Taneja 2009). Black 2004 was excluded because infants in the study were chronically undernourished, while Sazawal 2001 was excluded because small‐for‐gestational‐age children were enrolled.
Inappropriate interventions and participants
Two studies (Roy 2007; Krebs 2011), had more than one reason for exclusion. Roy 2007 reported on ill children with the intervention duration being less than six months long. Krebs 2011 included children older than six months as well as using a meal form of supplementation.
Inappropriate study design
Nissensohn 2016 was excluded because it was a systematic review.
Risk of bias in included studies
Risk of bias of each study is reported in the 'Risk of bias' section of the Characteristics of included studies table and Figure 2 and Figure 3 can be viewed for a summary of the overall quality of the trials included in this review.
2.
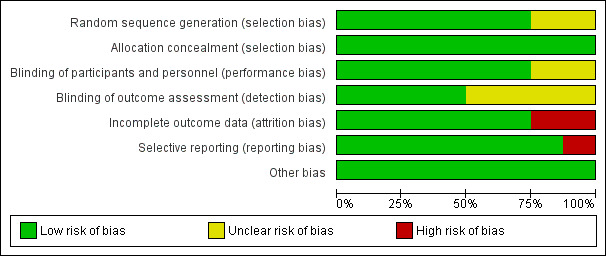
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
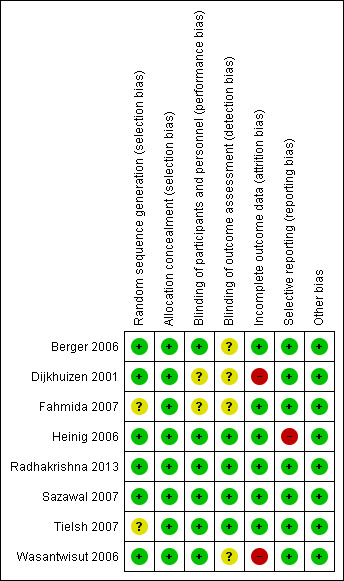
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
The following description includes details on risk of bias for the eight studies included in the data analysis.
Allocation
Sequence generation
We evaluated six trials (Berger 2006; Dijkhuizen 2001; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Wasantwisut 2006), as having adequate methods for generation a random sequence. Two trials (Fahmida 2007; Tielsh 2007), did not report clearly on methods used to ensure adequate randomisation.
Four trials (Berger 2006; Dijkhuizen 2001; Sazawal 2007; Tielsh 2007), used block randomisation, two trials (Fahmida 2007; Heinig 2006), stratified by sex, and Wasantwisut 2006 stratified by both age and sex .
Allocation Concealment
All eight included studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007; Wasantwisut 2006), were judged to have employed adequate methods for allocation concealment.
Blinding
Blinding of participants and staff (performance bias)
Blinding of participants and staff was adequately carried out in six trials (Berger 2006; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007; Wasantwisut 2006), by use of indistinguishable placebos and labelling. Two studies (Dijkhuizen 2001 and Fahmida 2007) did not clearly describe the methods used to minimize performance and detection bias.
Blinding of outcome assessors (detection bias)
Detection bias was unlikely to be present in four trials (Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007). These studies had adequate blinding with coding of intervention and control syrups or tablets which ensured masking of personnel involved in evaluating participants. Staff taking blood samples was separate from staff conducting laboratory analysis and therefore posed no risk to laboratory specimens.
The remaining four studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Wasantwisut 2006), did not clearly describe their methods of blinding.
Incomplete outcome data
Trials were assessed as being at high risk of attrition bias if total loss to follow‐up was greater than 20% of all participants, or there was a large difference between the groups (Dijkhuizen 2001; Wasantwisut 2006). For example, overall loss to follow‐up in Dijkhuizen 2001 was reported to be 19% but differences in attrition between zinc and placebo groups (17.6% zinc versus. 24.4% placebo) may have affected outcomes thus impacting the results of the meta‐analysis as well. For the remaining trials, the risk of bias was low (Berger 2006; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007). For three studies (Fahmida 2007; Heinig 2006; Radhakrishna 2013), follow‐up was good (ranging from 80% to greater than 90%) and therefore attrition bias in these studies was minimal). Although four studies (Berger 2006 11.8% in the intervention group versus 12.3% in the control Wasantwisut 2006 13.7% in the intervention group versus 5.56% in the control group; Sazawal 2007 17% in the intervention group versus 15.8% in the control group, and Tielsh 2007 17.3% in the intervention group versus 10.3% in the control group) had low total attrition rates, differences in attrition between intervention and control groups may have affected outcomes.
Selective reporting
Selective reporting was evaluated on protocols where available and from published studies where protocol was not available.
For most of the included studies, the protocol was not available and judgement of this bias was therefore based on all outcomes mentioned in the published study. Only one study (Heinig 2006), was judged to have selective reporting as it did not show all the outcomes mentioned in the methods.
Other potential sources of bias
All studies were free from other biases.
Effects of interventions
See: Table 1
1. Zinc supplementation versus placebo/no intervention (Comparison 1)
From the eight studies identified (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006; Radhakrishna 2013; Sazawal 2007; Tielsh 2007; Wasantwisut 2006), four (Berger 2006; Dijkhuizen 2001; Fahmida 2007; Heinig 2006), compared oral zinc versus placebo or no intervention and were included in the meta‐analysis. Three studies of the studies included in the meta‐analysis were conducted in developing countries (Berger 2006; Dijkhuizen 2001; Fahmida 2007), and one study was conducted in a developed country (Heinig 2006). All studies were conducted in the community setting. All included studies used 10 mg or less of zinc as zinc sulphate and provided supplementation for a period of at least six months.
Primary outcomes
Infant mortality
The studies by Sazawal 2007 and Tielsh 2007 were conducted in developing countries (Zanzibar and Nepal, respectively) and assessed the impact of zinc supplementation on infant mortality.
Sazawal 2007 evaluated approximately 43,000 children and reported a non‐significant 7% (95% confidence interval (CI) –6% to 19%; P = 0·29) reduction in the risk ratio (RR) of all‐cause infant mortality associated with zinc supplementation. In the study performed in Nepal (Tielsh 2007), there was no significant difference in mortality between the zinc and placebo groups in children under 35 months (316 versus 333 deaths; hazard ratio (HR) 0.92, 95% CI 0.75 to 1.12). Zinc had no effect on mortality in children younger than 12 months (181 versus 168 deaths; 1.04, 0.83 to 1.31); mortality was lower, but not statistically significantly.
Both the studies were carried out on children under 36 months and therefore were not included in the meta‐analysis.
Growth outcomes (Outcomes 1.1 to 1.21)
The parameters for growth outcomes we reported in this review were change or absolute length, weight and head circumferences as well as Z‐scores (WAZ, LAZ, WLZ). We also included incidence of stunting, wasting and underweight in infants.
Length
Heinig 2006 reported 'change in length after six months' in 70 infants. It was not significantly different between the two groups (mean difference (MD) 0.00cm, 95% CI ‐0.49 to 0.49; one study, n = 70; fixed‐effect) (Analysis 1.1). This study was conducted in a developed country (USA) on infants aged four months. Zinc was administered in a dose of 5 mg as zinc sulphate to all participants.
1.1. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 1 Change in length (cm) after 6 months.
'Length after six months' was reported in three studies (Berger 2006; Dijkhuizen 2001; Heinig 2006), with the overall estimated SMD suggesting no difference between intervention or control (standardised mean difference (SMD) 0.07, 95% CI ‐0.08 to 0.23; three studies, n = 644; fixed‐effect, heterogeneity: Chi² P = 0.93; I² = 0%; moderate certainty of evidence using GRADE assessments) (Analysis 1.2; Table 1), with a low heterogeneity. Subgroup analysis by country classification was also carried out; both studies in developed and developing countries showed no statistically significant difference within and between them.
1.2. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 2 Length after 6 months.
Weight
Heinig 2006 reported 'change in weight' at six months and the estimated MD (MD ‐77g, 95% CI ‐0.302 to 148.25; one study, n = 70; fixed‐effect) (Analysis 1.3), suggested no significant difference between intervention and control groups.
1.3. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 3 Change in weight (g) after 6 months.
The estimated SMD for 'weight after six months' suggested a significant difference between the two groups with low heterogeneity (SMD 0.16, 95% CI 0.00 to 0.31; three studies, n = 644; random‐effects, heterogeneity: Chi² P = 0.70; I² = 0%; low certainty of evidence using GRADE assessments) (Analysis 1.4; Table 1). All participants were greater than one month of age. Subgroup analysis by country classification revealed no effect on weight in the study performed in a developed country (USA) (SMD ‐0.02, 95% CI ‐0.49 to 0.45; one study, n = 70; random‐effects). On the other hand, the studies carried by Berger 2006 and Dijkhuizen 2001 in developing countries (Vietnam and Indonesia) reported a statistically significant increment in weight of 18 units among children who consumed the zinc compared to those in the placebo group (SMD 0.18, 95% CI 0.01 to 0.34; two studies, n = 574; random‐effects, heterogeneity: Chi² P = 0.79; I² = 0%).
1.4. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 4 Weight after 6 months; country.
Head circumference
Heinig 2006 reported no 'change in head circumference' after six months (MD 0.00cm, 95% CI ‐0.21 to 0.21; one study, n = 70; fixed‐effect) (Analysis 1.5). 'Head circumference' after six months was also not affected significantly by the intervention (MD 0.10cm, 95% CI ‐0.51 to 0.71; one study, n = 70; fixed‐effect).
1.5. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 5 Change in head circumference (cm) after 6 months.
Weight for age Z‐score (WAZ)
'Change in WAZ after six months' was reported by Berger 2006 in a study carried out in Vietnam.The estimated MD suggested an increase in 'change in WAZ' (MD 0.16, 95% CI 0.07 to 0.25; one study, n = 386; fixed‐effect) (Analysis 1.7). Infants received 10 mg of zinc as zinc sulphate daily over a period of six months.
1.7. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 7 Change in WAZ at 6 months.
The estimated SMD for 'WAZ' after six months suggested a significant increase in this outcome after zinc supplementation (SMD 0.16, 95% CI 0.03 to 0.29; three studies, n = 955; fixed‐effect, heterogeneity: Chi² P = 0.96; I² = 0%; moderate certainty of evidence using GRADE assessments) (Analysis 1.8; Figure 4; Table 1). Weight gain was higher in the zinc group. All participants were greater than one month of age and came from developing countries (Berger 2006; Dijkhuizen 2001; Fahmida 2007).
1.8. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 8 Weight for age Z score after 6 months.
4.
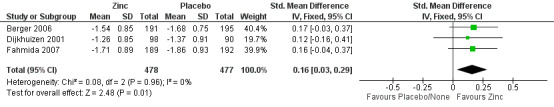
Forest plot of comparison: 1 Zinc supplementation vs placebo/ no treatment, outcome: 1.8 Weight for age Z‐score after 6 months.
'WAZ' after 12 months was reported in Indonesian infants by Fahmida 2007 and the estimated MD was not significantly different in either group (MD ‐0.03, 95% CI ‐0.24 to 0.18; one study, n = 353; fixed‐effect) (Analysis 1.9). It was provided to three‐ to six‐month old infants, with 10 mg of zinc as zinc sulphate, in a developing country.
1.9. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 9 Weight for age Z score after 12 months.
Length for age Z‐scores (LAZ)
'Change in LAZ after six months' was reported by Berger 2006 and the estimated MD was not significantly affected by the intervention (MD 0.05, 95% CI ‐0.04 to 0.14; one study, n = 386; fixed‐effect) (Analysis 1.10). This study dispensed 10 mg of zinc sulphate for a six‐month period to healthy, breast‐fed infants aged four to seven months .
1.10. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 10 Change in LAZ after 6 months.
The estimated SMD for 'LAZ' after six months showed no significant impact after the intervention (SMD 0.06, 95% CI ‐0.07 to 0.19; three studies, n = 955; fixed‐effects, heterogeneity: Chi² P = 0.92; I² = 0%; moderate certainty of evidence using GRADE assessments) (Analysis 1.11; Figure 5; Table 1). All participants came from developing countries (Vietnam and Indonesia).
1.11. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 11 Length for age Z score after 6 months.
5.
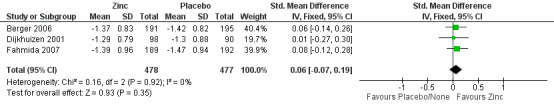
Forest plot of comparison: 1 Zinc supplementation vs placebo/ no treatment, outcome: 1.11 Length for age Z‐score after 6 months.
'LAZ' after 12 months was reported by Fahmida 2007 for Indonesian infants and the estimated MD was not significantly different in either group (SMD 0.07, 95% CI ‐0.12 to 0.26, one study, n = 353; fixed‐effect) (Analysis 1.12).
1.12. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 12 Length for age Z score after 12 months.
Weight for Length Z‐scores (WLZ)
'Change in WLZ after six months' was reported by Berger 2006 and the estimated MD (MD 0.17, 95% CI 0.06 to 0.28; one study, n = 386; fixed‐effect) (Analysis 1.13), suggested a significant increase post intervention.
1.13. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 13 Change in WLZ after 6 months.
The estimated SMD for 'WLZ' after six months reported by three studies in developing country settings did not suggest a significant increase after the intervention (SMD 0.15, 95% CI 0.02 to 0.28; three studies, n = 955; fixed‐effect, heterogeneity: Chi² P = 0.81; I² = 0%; moderate certainty of evidence using GRADE assessments) (Analysis 1.14; Figure 6; Table 1).
1.14. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 14 Weight for length Z score after 6 months.
6.
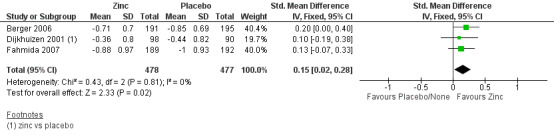
Forest plot of comparison: 1 Zinc supplementation vs placebo/ no treatment, outcome: 1.14 Weight for length Z‐score after 6 months.
'WLZ at 12 months' was by Fahmida 2007 and the estimated MD was not statistically significant between the groups (MD ‐0.11, 95% CI ‐0.38 to 0.16; one study, n = 353; fixed‐effect) (Analysis 1.15). In this study, Indonesian infants three to six months old received 10 mg of zinc sulphate for a period of six months.
1.15. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 15 Weight for length Z score after 12 months.
Incidence of stunting/ wasting/ underweight
Incidences of stunting, wasting and underweight were reported by three studies (Berger 2006; Dijkhuizen 2001; Fahmida 2007), after six months of intervention and by Fahmida 2007 after 12 months. All trials were performed in developing countries.
The estimated RR suggested no difference in incidence of stunting after six months (RR 0.82, 95% CI 0.65 to 1.03; three studies, n = 955; fixed‐effect, heterogeneity: Chi² P = 0.90; I² = 0% ; moderate certainty of evidence using GRADE assessments) (Analysis 1.16; Table 1), or 12 months (RR 0.94, 95% CI 0.79 to 1.11; one study, n = 349, fixed‐effect) (Analysis 1.17).
1.16. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 16 Stunted after 6 months.
1.17. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 17 Stunted after 12 months.
The estimated RR suggested no difference in incidence of wasting after six months (RR 0.78, 95% CI 0.48 to 1.26; three studies, n = 955; fixed‐effect, heterogeneity: Chi² P = 0.73; I² = 0%; low certainty of evidence using GRADE assessments) (Analysis 1.18; Table 1), or 12 months (RR 0.98, 95% CI 0.52 to 1.86; one study, n = 349; fixed‐effect) (Analysis 1.19).
1.18. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 18 Wasted after 6 months.
1.19. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 19 Wasted at 12 months.
The estimated RR suggested no difference in incidence of underweight infants after six months (RR 0.94, 95% CI 0.74 to 1.20; one study, n = 381; fixed‐effect) (Analysis 1.20), or 12 months (RR 0.98, 95% CI 0.77 to 1.26; one study, n = 349; fixed‐effect) (Analysis 1.21).
1.20. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 20 Underweight after 6 months.
1.21. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 21 Underweight after 12 months.
Incidence of diarrhoea (defined as three or more loose stools in a 24‐hour period) (Outcome 1.22)
Berger 2006 reported the number of new cases of diarrhoea over six months defined as quote: "three or more unformed stools per day" (52/195 with zinc supplementation and 51/197 without zinc) which was not statistically significant (RR 1.03, 95% CI 0.74 to 1.44, one study, n = 392; fixed‐effect) (Analysis 1.22). The cumulative incidence rates (number of new cases/total population at risk) for diarrhoea were 26.7% versus 25.9% in the zinc and placebo groups, respectively. This study provided 10 mg of zinc sulphate over six months to infants aged four to seven months.
1.22. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 22 Incidence of diarrhoea.
Secondary outcomes
Serum zinc at six months
Berger 2006 reported geometric means of serum zinc values in infants aged four to seven months, provided with 10 mg of oral zinc or placebo, over six months in a developing country. The intervention group (n = 161) had a mean (CI) serum zinc level of 23.07 umol/L (22.23 to 23.95 umol/L) compared to the control group (n = 155) value of 15.79 umol/L (15.20 to 16.40) umol/L, with a P value of less than 0.0001 (Table 3).
2. Additional Data.
| Study ID | Outcomes | Intervention | Control | Significance |
| Berger 2006 | Geometric means (CI) for serum zinc | Zinc: 23.07 (22.23 to 23.95) umol/L N = 161 |
Placebo: 15.79 (15.20 to 16.40) umol/L N = 155 |
P < 0.0001 |
| Dijkhuizen 2001 | Serum Zinc (median, IQR) |
Zinc 16.1 (13.4 to 20.3) umol/L N = 97 |
Placebo: 13 (10.7 to 15.3) umol/L N = 87 |
P < 0.01 |
| Fahmida 2007 | Serum Zinc (median, IQR) |
Zinc: 18.4 (16.8 to 23) umol/L N = 25 |
Placebo: 15.3 (13.8 to 16.8) umol/L N = 34 |
P < 0.001 |
| Radhakrishna 2013 | Median (range) number of episodes of diarrhoea per child per 100 days follow‐up Diarrhoeal duration (days per 100 days follow‐up) Number of episodes of respiratory infection per child per 100 days follow‐up Duration of respiratory infection (days for 100 days follow‐up) |
1.4 (0.0 to 10.5) 6.1 (0.0 to 98.6) days 0.7 (0.0 to 4.4) episodes 13.1 (0.0 to 13.9) |
1.4 (CI 0.0 to 5.6) 7.0 (0.0 to 32.9) 0.9 (0.0 to 4.9) 13.6 (0.0 to 46.0) |
P > 0.05 P = 0.10 P > 0.05 P > 0.05 |
| Sazawal 2007 | Overall mortality Rate per 100 child years Mortality Age 0 to 11 Rate per 100 child years |
401 1.42 204 3.55 |
433 1.53 192 3.36 |
RR 0.93 (95% CI: 0.81 to 1.06). P = 0.294 RR 1.06 (95% CI: 0.87 to 1.29). P = 0.566 |
| Tielsh 2007 | Overall mortality (1 to 35 months) mortality under 12 months of age |
316 181 |
333 168 |
HR 1.04 (95% CI: 0.83 to 1.31) HR 0.92 (95% CI: 0.75 to 1.12) |
IQR: interquartile range
Dijkhuizen 2001 reported median and interquartile ranges (IQR) of serum zinc values in infants aged four months, provided with 10 mg of oral zinc or placebo over six months in a developing country. The intervention group (n = 97) had a median (IQR) serum zinc level of 16.1 (13.4 to 20.3) umol/L compared to the control group (n = 87) value of 13 (10.7 to 15.3) umol/L, with a P value of less than 0.01 (Table 3).
Fahmida 2007 reported median and interquartile ranges of serum zinc values in infants aged three to six months, provided with 10 mg of oral zinc or placebo over six months in a developing country. The intervention group (n = 25) had a median (IQR) serum zinc level of 18.4 (16.8 to 23) umol/L compared to the control group (n = 34) value of 15.3 (13.8 to 16.8) umol/L, with a P value of less than 0.001 (Table 3).
Incidence of lower respiratory tract infection (Outcome 1.23)
Berger 2006 reported number of new cases of acute respiratory infection (ARI) defined as quote: "presence of cough or/and difficulty to breathe and/or elevated respiratory rate (an elevated respiratory rate was higher than 50/min in infant and higher than 40/min in children > one year of age)" (102/195 with zinc and 105/197 without zinc) and cough (83/195 with zinc and 83/197 without zinc) over six months. The estimated RR for this morbidity did not suggest a significant impact of the intervention over control (ARI = (RR 0.96, 95% CI 0.65 to 1.43; one study, n = 392; fixed‐effect) (Analysis 1.23).
1.23. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 23 Incidence of lower respiratory tract infection.
We could not estimate the subgroup analysis according to dose of zinc since all studies reported use of 10 mg of elemental zinc or less than this value in their interventions. Likewise, we did not find an established guideline for a definitive normal serum or plasma zinc level for infants under six months of age, only non‐fasting serum zinc concentration cut‐offs for children under 10 years of age in the trials. Most of the studies that presented baseline zinc serum information did not mention the time when the blood samples were obtained and there are no cut‐offs available for fasting zinc level (IZiNCG 2004). Additionally, the duration of supplementation subgroup analysis was not performed since all studies included in the pooled analysis had an intervention duration of six months.
The estimates related to mortality due to diarrhoea, prevalence of diarrhoea, incidence of failure to thrive, incidence of sepsis, severe malaria and acute zinc toxicity were not performed as the studies assessed did not evaluate these outcomes.
2. Zinc supplementation plus riboflavin versus riboflavin only (Comparison 2)
Radhakrishna 2013 reported on oral zinc with riboflavin in comparison to riboflavin only. This study was conducted in a developing country, on babies aged four months for a duration of 12 months with five mg of zinc in provided as zinc sulphate.
Primary outcomes
Growth (Outcome 2.1 to 2.12)
Length and weight
Length (Analysis 2.1), and weight (Analysis 2.2), were reported with no significant impact after the intervention at six months (MD ‐0.30 cm, 95% CI ‐0.90 to 0.30; one study, n = 324; fixed‐effect) (Analysis 2.1); (MD 0.00 g, 95% CI ‐0.20 to 0.20; one study, n = 324; fixed‐effect) (Analysis 2.2), respectively); 9 months (MD 0.10 cm, 95% CI ‐0.51 to 0.71; one study, n = 324; fixed‐effect); (MD ‐0.01 g, 95% CI ‐0.23 to 0.21; one study, n = 324; fixed‐effect), respectively); 12 months (MD ‐0.10 cm, 95% CI ‐0.73 to 0.53; one study,n = 318; fixed‐effect); (MD 0.00 g, 95% CI ‐0.24 to 0.24; one study, n = 318; fixed‐effect, respectively); 15 months (MD ‐0.20 cm, 95% CI ‐0.89 to 0.49; one study, n = 311; fixed‐effect); (MD 0.11 g, 95% CI ‐0.11 to 0.33; one study, n = 311; fixed‐effect), respectively); 18 months (MD ‐0.10 cm, 95% CI ‐0.82 to 0.62; one study, n = 301; fixed‐effect); (MD 0.06 g, 95% CI ‐0.17 to 0.29; one study, n = 301; fixed‐effect, respectively); 21 months (MD 0.10 cm, 95% CI ‐0.64 to 0.84; one study, n = 299; fixed‐effect); (MD 0.21 g, 95% CI ‐0.00 to 0.42; one study, n = 299; fixed‐effect), respectively); and 24 months (MD ‐0.40 cm, 95% CI ‐1.11 to 0.31; one study, n = 296; fixed‐effect); (MD 0.08 g, 95% CI ‐0.13 to 0.29; one study, n = 296, respectively).
2.1. Analysis.

Comparison 2 Zinc plus riboflavin vs riboflavin, Outcome 1 Length (cm).
2.2. Analysis.

Comparison 2 Zinc plus riboflavin vs riboflavin, Outcome 2 Weight (g).
Stunting
Incidence of children stunted at 21 months (RR 1.53, 95% CI 1.09 to 2.16; one study, n = 298; fixed‐effect) (Analysis 2.3), and children wasted at 24 months was significantly affected (RR 0.59, 95% CI 0.37 to 0.96; one study, n = 296; fixed‐effect) (Analysis 2.4), but not at 18 months for stunting (RR 1.00. 95% CI 0.83 to 1.19; one study, n = 301; fixed‐effect) or wasting (RR 0.64, 95% CI 0.30 to 1.37; one study, n = 301; fixed‐effect). Neither was there a significant effect on wasting at 21 months (RR 0.62, 95% CI 0.36 to 1.07; one study, n = 299; fixed‐effect). There was also no significant effect on incidence of stunting at 24 months (RR 1.16, 95% CI 0.80 to 1.68; one study, n = 296; fixed‐effect).
2.3. Analysis.

Comparison 2 Zinc plus riboflavin vs riboflavin, Outcome 3 Stunted.
2.4. Analysis.

Comparison 2 Zinc plus riboflavin vs riboflavin, Outcome 4 Wasted.
The incidence of children underweight was not significantly affected at 18 months (RR 1.01, 95% CI 0.75 to 1.34; one study, n = 301; fixed‐effect), 21 months (RR 0.80, 95% CI 0.59 to 1.07; one study, n = 299; fixed‐effect) or 24 months (RR 0.90, 95% CI 0.66 to 1.23; one study, n = 296; fixed‐effect).
The outcomes infant mortality and incidence of diarrhoea were not reported by the trials included in our systematic review.
Secondary outcomes
Prevalence of diarrhoea
Radhakrishna 2013 reported medians and ranges of diarrhoeal morbidity in infants aged four months old, provided with 5 mg of oral zinc and riboflavin or only riboflavin, for a duration of 12 months in a developing country. There was no difference between intervention and control groups in number of episodes per child per 100 days follow‐up (intervention: median 1.4, range (0.0 to 10.5); control: median 1.4, range 0.0 to 5.6, P > 0.5) and diarrhoeal duration (intervention: median 6.1 days, range 0.0 to 98.6); control: median 7 days, range (0.0 to 32.9, P = 0.1) of diarrhoeal episodes as days for 100 days of follow‐up (Table 3).
Incidence of lower respiratory tract infection
There was no difference between intervention and control groups in number of episodes per child per 100 days follow‐up (intervention: median 0.7, range 0.0 to 4.4; control: median 0.9, range 0.0 to 4.9, P > 0.5) and duration of respiratory infections (intervention: median 13.1 days, range 0.0 to 13.9; control: median 13.6 days, range 0.0 to 46.0, P > 0.5, respectively) as reported by Radhakrishna 2013 (Table 3).
Serum zinc at 18 months (Outcome 2.21.1)
Radhakrishna 2013 reported serum zinc values in 77 patients. The estimated MD was not significantly affected (MD 3.30 μg/dL, 95% CI ‐4.42 to 11.02; one study, n = 68; fixed‐effect) (Analysis 2.6).
2.6. Analysis.

Comparison 2 Zinc plus riboflavin vs riboflavin, Outcome 6 Serum Zinc (ug/dL).
The outcomes diarrhoea‐specific mortality rate, incidence of failure to thrive, incidence of sepsis, severe malaria and acute zinc toxicity were not described by any studies included in our systematic review.
Discussion
Summary of main results
Overall, eight studies were included but only five studies were included in the analysis, with four studies providing zinc or placebo and one study providing zinc and riboflavin or riboflavin only.
We looked at mortality, growth outcomes, morbidity and biochemical indicators for zinc levels. Significant effects were found for mortality by zinc versus placebo, however only one study covered this outcome.
Growth at six months was significantly affected as demonstrated by the statistically significant differences in weight, Weight for Age Z‐scores (WAZ) and Weight for Length Z‐scores (WLZ) between zinc supplementation and placebo groups; however, certainty that the true effect lies close to the estimated effect was moderate for length, WAZ and WLZ and low for weight after six months. When compared to riboflavin, supplementation with a combination of zinc and riboflavin was found to have a significant positive impact on the incidence of wasting at 24 months but a significant negative impact on the incidence of stunting at 21 months; however it is important to note that evidence for this comes from just one study. Moreover, since this comparison only included one trial, no subgroup analysis was conducted.
Some relevant outcomes could not be included in the analysis since they were not reported in an adequate manner, they can be found in Table 3.
Overall completeness and applicability of evidence
We believe the final studies included were covering most populations. Primarily breast‐fed, children from birth to three years old were included from both developing and developed country settings, received supplementation for periods ranging from six months to 12 months in the form of zinc sulphate at a doses between 5 mg to 10 mg. The majority of included trials also provided children with Vitamin A at baseline or periodically.
Our review was restricted to healthy, term babies and therefore cannot be applied to preterm or unhealthy children. However, we did include low birth weight neonates which may have affected the results.
Furthermore, we did not include outcomes that are also used to evaluate nourishment levels in a child including skinfold thickness. Certain studies showed a positive impact on skinfold thickness but not on linear growth. We did not evaluate cognitive or motor growth indicators.
Quality of the evidence
Overall, studies were of moderate to good quality with only a few studies having a high rate of attrition or selective reporting. Most issues found were with the way data were presented in papers, which was difficult to use in analysis. In terms of certainty of evidence on outcomes, we have moderate confidence in effect estimates for length, WAZ, LAZ, WLZ, and incidence of stunting based on GRADE assessments; we have low confidence in the effect estimates for weight after six months and incidence of wasting.
Potential biases in the review process
Review was conducted in concordance with the Cochrane guidelines and should not include potential biases.
Agreements and disagreements with other studies or reviews
Linear growth in infants under six months was positively affected as demonstrated by statistically significant results in change in WLZ after six months and WLZ scores in zinc groups versus placebo. The positive findings are supported by one review with 36 studies (Imdad 2011), which found increased linear growth with zinc alone as well as zinc and iron, with a dose of 10 mg zinc sulphate given for 24 weeks for children under five years of age from developing countries. However another review conducted in Latin America (Jimenez‐Morán 2013), with six studies, disagreed finding no impact on linear growth. Although stronger evidence supports linear growth, our data did not show consistent gains in length which may be due to the low number of studies included or inadequate zinc doses used.
We found that supplementing babies under six months of age for at least six months with zinc alone, increased serum zinc levels. However our analysis was limited to treatment durations of six months with only one study with a duration of 12 months (zinc plus riboflavin versus only riboflavin) and doses under or equal to 10 mg of zinc. A meta‐analysis conducted on 13 randomised controlled trials (RCTs) found supporting evidence with supplementation leading to increased serum zinc levels in infants, specially with durations from four to 20 weeks, and more as well as with doses ranging from 8.1 mg to 12 mg per day (Nissensohn 2013).
Our analysis included infants greater than one month of age. Current reviews conducted focus mostly on children under five with no stratification for neonatal age groups or above six months. This restricts the amount of available evidence with which we can compare our findings.
Studies included in the analysis were mostly from developing countries and presentation of data prevented pooling of data from developed and developing countries. Evidence from Krebs 2014 states that maternal factors such as young age, poor diet, prematurity and environmental enteropathy (prevalent in poor countries) can decrease zinc absorption or increase zinc losses. When provided with supplemental zinc, this population therefore showed the most impact, but also needed higher zinc doses to achieve healthy levels.
Authors' conclusions
Implications for practice.
ZInc alone or in addition to riboflavin maybe of benefit to growth in children aged under six months. However, data are limited on mortality and morbidity rates of included participants as well as on adverse effects of such interventions. This limits the applicability of zinc supplementation in current practice, and such practice should be conducted with caution.
Implications for research.
Although this review identified a few studies that fulfilled our inclusion criteria, more full‐scale RCTs which are powered to address mortality, morbidity and adverse events need to be conducted. When adverse events are recorded with current dose levels of zinc and an estimated safety profile can be created, researchers may also evaluate higher doses in relevant populations.
More trials are needed to be conducted which provide zinc in addition to other micronutrients as well.
Trials need to be clearer in terms of their goals and objectives, and would do well to further stratify study populations if large groups are included, such as stratifying children from nought to 3, 3 to 6, 6 to 12 months, to allow for better analysis as well as to distinguish whether children are deficient at initiation of study.
Additional reviews also need to include more outcomes such as skinfold thickness, additional serum biochemical indicators and cost‐effectiveness in babies aged six months or younger, that may affect final decisions on preventive or curative zinc supplementation for children under six months of age.
Furthermore, normal or deficient levels need to clearly elucidated for future research on zinc supplementation.
What's new
| Date | Event | Description |
|---|---|---|
| 15 April 2020 | Amended | We amended the Acknowledgements section. It now refers to both the protocol and review. |
Acknowledgements
We would like to thank Dr Roger F. Soll, Dr Jeffrey Horbar, Prof Michael Bracken, Dr Gautham Suresh, and Dr Arne Ohlsson for commenting on the draft protocol; Dr William McGuire for feedback on the review; Dr Stephen Allen for peer review; and María Victoria Leo Rosas for translation.
We acknowledge the work of Carol Friesen, Information Specialist with Cochrane Neonatal, in conducting the literature searches for the update of this review, and of Colleen Ovelman, Managing Editor with Cochrane Neonatal, in peer reviewing the Ovid MEDLINE search strategy.
Appendices
Appendix 1. 2015 Search methods
We searched the following databases on November 1st, 2015:
the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library);
MEDLINE;
Embase;
LILACS.
CINAHL
Clinical trials Registry (Clinicaltrials.gov),
Controlled trials Registry (controlled‐trials.com),
WHO’s International Clinical Trials Registry Platform (ICTRP)
The search terms were combined with the highly sensitive search strategy devised by Boluyt 2008 to identify child studies.
The terms were adapted to search EMBASE and LILACS. The search terms were as follows:
Zinc
zinc.tw.
infant/neonate/baby
growth/weight for age/height for age
diarrhoea [and diarrhoea]
morbidity
mortality
failure to thrive
multi‐nutrients
randomised controlled trial.pt.
controlled trial.pt
sepsis
lower respiratory tract infection/lrti/pneumonia
malaria
Appendix 2. 2018 Search methods
PubMed: (zinc AND (infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: zinc AND (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: zinc AND (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: zinc AND (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. 2020 Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2019). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
CENTRAL via CRS Web:
Date ranges: 01 January 2018 to 29 January 2020 Terms: 1 MESH DESCRIPTOR Zinc EXPLODE ALL AND CENTRAL:TARGET 2 zinc AND CENTRAL:TARGET 3 #1 OR #2 4 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 5 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 6 MESH DESCRIPTOR Infant EXPLODE ALL AND CENTRAL:TARGET 7 #4 OR #5 OR #6 8 #3 AND #7 9 2018 TO 2020:YR AND CENTRAL:TARGET 10 #8 AND #9
MEDLINE via Ovid:
Date ranges: 01 January 2018 to 29 January 2020 Terms: 1. exp Zinc/ 2. zinc.mp. 3. 1 or 2 4. exp infant, newborn/ or exp Infant/ 5. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 6. 4 or 5 7. randomized controlled trial.pt. 8. controlled clinical trial.pt. 9. randomized.ab. 10. placebo.ab. 11. drug therapy.fs. 12. randomly.ab. 13. trial.ab. 14. groups.ab. 15. or/7‐14 16. exp animals/ not humans.sh. 17. 15 not 16 18. 6 and 17 19. randomi?ed.ti,ab. 20. randomly.ti,ab. 21. trial.ti,ab. 22. groups.ti,ab. 23. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 24. placebo*.ti,ab. 25. 19 or 20 or 21 or 22 or 23 or 24 26. 5 and 25 27. limit 26 to yr="2018 ‐Current" 28. 18 or 27 29. 3 and 28 30. limit 29 to yr="2018 ‐Current"
CINAHL via EBSCOhost:
Date ranges: 01 January 2018 to 31 January 2020 Terms: (zinc) AND (infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Limiters ‐ Published Date: 20180101‐20200131
ISRCTN:
Date ranges: 2018 to 2020 Terms: Interventions: Zinc AND Participant age range: Neonate Condition: Infant* OR newborn* AND Interventions: Zinc (infant* OR newborn* OR neonat*) AND zinc
Appendix 4. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the table Characteristics of included studies. We evaluated the following issues and entered the findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Zinc supplementation vs placebo/ no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in length (cm) after 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.49, 0.49] |
| 2 Length after 6 months | 3 | 644 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.08, 0.23] |
| 2.1 Developed country | 1 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.43, 0.51] |
| 2.2 Developing country | 2 | 574 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.09, 0.24] |
| 3 Change in weight (g) after 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐77.0 [‐302.25, 148.25] |
| 4 Weight after 6 months; country | 3 | 644 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.00, 0.31] |
| 4.1 Developed | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.49, 0.45] |
| 4.2 Developing | 2 | 574 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.01, 0.34] |
| 5 Change in head circumference (cm) after 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.21, 0.21] |
| 6 Head circumference (cm) at 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.51, 0.71] |
| 7 Change in WAZ at 6 months | 1 | 386 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.07, 0.25] |
| 8 Weight for age Z score after 6 months | 3 | 955 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.03, 0.29] |
| 9 Weight for age Z score after 12 months | 1 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 10 Change in LAZ after 6 months | 1 | 386 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.04, 0.14] |
| 11 Length for age Z score after 6 months | 3 | 955 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.07, 0.19] |
| 12 Length for age Z score after 12 months | 1 | 353 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.12, 0.26] |
| 13 Change in WLZ after 6 months | 1 | 386 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.06, 0.28] |
| 14 Weight for length Z score after 6 months | 3 | 955 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.02, 0.28] |
| 15 Weight for length Z score after 12 months | 1 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.38, 0.16] |
| 16 Stunted after 6 months | 3 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.03] |
| 17 Stunted after 12 months | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 18 Wasted after 6 months | 3 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.48, 1.26] |
| 19 Wasted at 12 months | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.52, 1.86] |
| 20 Underweight after 6 months | 1 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.20] |
| 21 Underweight after 12 months | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.26] |
| 22 Incidence of diarrhoea | 1 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.44] |
| 23 Incidence of lower respiratory tract infection | 1 | 392 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.43] |
1.6. Analysis.

Comparison 1 Zinc supplementation vs placebo/ no treatment, Outcome 6 Head circumference (cm) at 6 months.
Comparison 2. Zinc plus riboflavin vs riboflavin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 6 months | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.90, 0.30] |
| 1.2 at 9 months | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.51, 0.71] |
| 1.3 at 12 months | 1 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.73, 0.53] |
| 1.4 at 15 months | 1 | 311 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.89, 0.49] |
| 1.5 at 18 months | 1 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.82, 0.62] |
| 1.6 at 21 months | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.64, 0.84] |
| 1.7 at 24 months | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.11, 0.31] |
| 2 Weight (g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 at 6 months | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.20, 0.20] |
| 2.2 at 9 months | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.23, 0.21] |
| 2.3 at 12 months | 1 | 318 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.24, 0.24] |
| 2.4 at 15 months | 1 | 311 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.11, 0.33] |
| 2.5 at 18 months | 1 | 301 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.17, 0.29] |
| 2.6 at 21 months | 1 | 299 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.00, 0.42] |
| 2.7 at 24 months | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.13, 0.29] |
| 3 Stunted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 at 18 months | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.19] |
| 3.2 at 21 months | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.09, 2.16] |
| 3.3 at 24 months | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.80, 1.68] |
| 4 Wasted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 at 18 months | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.30, 1.37] |
| 4.2 at 21 months | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| 4.3 a 24 months | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.37, 0.96] |
| 5 Underweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 at 18 months | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.34] |
| 5.2 at 21 months | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.07] |
| 5.3 at 24 months | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.23] |
| 6 Serum Zinc (ug/dL) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐4.42, 11.02] |
| 6.1 at 18 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐4.42, 11.02] |
2.5. Analysis.

Comparison 2 Zinc plus riboflavin vs riboflavin, Outcome 5 Underweight.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berger 2006.
| Methods | RCT | |
| Participants | Singleton breast‐fed infants, between 4 and 7 months of age. 24 communes of Que Vo, a rural and poor district, 50 km northwest of Hanoi in the Red River Delta in Vietnam. March to November 1998. |
|
| Interventions | Zn‐group a daily dose of 10 mg zinc as zinc sulphate, Placebo group a placebo. Fe–Zn group a daily dose of 10 mg iron as ferrous sulphate with 10 mg zinc, Fe‐group received a daily dose of 10 mg of iron as ferrous sulphate, Duration: the supplements were given 7 days/week during 6 months. Additional intervention: to avoid vitamin A deficiency becoming a limiting factor to infant health during the study period, a dose of 100,000 IU of vitamin A was given to all infants at the start of the study. |
|
| Outcomes | Anthropometrics, serum haemoglobin, serum ferritin, serum zinc. | |
| Notes | The financial contribution by UNICEF and the constant support of M. Tolvanen from UNICEF to this study are greatly acknowledged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomly assigned, following a computer‐generated block randomised group allocation." Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The supplements were coded with a letter at production and the code‐allocation kept secret until the end of the statistical analysis" Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Supplements were presented in similar coded bottles avoiding participants and health workers to differentiate between treatments". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14.3% total loss to follow‐up: for the zinc vs placebo: 11.8 vs 12.3; for zinc plus iron vs iron:18.8 vs 14.1. Comment: attrition rate unlikely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Low risk | Study seems to be free from other biases. |
Dijkhuizen 2001.
| Methods | RCT | |
| Participants | 4‐month old infants were identified by the village health volunteers, and mothers were invited to participate in the study. The study was carried out in a rural area of Bogor District, West Java, Indonesia. Time period between October 1997 and March 1999. |
|
| Interventions | Four groups of infants were supplemented with a syrup containing:
Formulation not specified Duration of six months |
|
| Outcomes | Plasma Hb, ferritin, zinc; anthropometrics. | |
| Notes | No funding source mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were assigned to one of the four supplementation groups by individual randomization, using a block randomised group allocation list, which was computer‐generated before the study began." Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The supplements were coded with a letter at production and the code‐allocation was safe‐kept at the Wageningen University, The Netherlands. The codes were not known at the study site in Indonesia. The code was revealed only after all subjects had completed the trial." Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Supplementation was double‐blind". Comment: insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Supplementation was double‐blind". Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19% loss to follow‐up: for the zinc vs placebo: 17.6 vs 24.3; for zinc plus iron vs iron: 35 vs 21.7. Comment: attrition rate may affect outcomes. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Low risk | Study seems to be free from other biases. |
Fahmida 2007.
| Methods | RCT (part of UNICEF’s Multi‐centre Study for a Trial of Iron and Zinc Supplementation during Infancy). | |
| Participants | The participants were selected on the basis of the following criteria: aged 3 to 6 months old at enrolment, predominantly breast‐fed, and free from apparent congenital abnormalities. The study was carried out in East Lombok district, West Nusa Tenggara province, Indonesia. Time period from July 1998 to March 1999. A follow‐up anthropometric measurement was conducted 6 months after the end of supplementation (September 1999). |
|
| Interventions | There were four supplementation groups in the study:
Supplementation was given for 6 months To avoid vitamin A deficiency becoming a limiting factor to child health, and to iron metabolism during the study period, vitamin A at a dose of 100,000 IU was given at the start of the study to all children. |
|
| Outcomes | Serum zinc, Hb, ferritin, retinol, anthropometrics, anaemia. | |
| Notes | UNICEF and Deustche Gesellchaft für Technische Zusammenarbeit (GTZ) funded this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation to supplementation groups was conducted using systematic random sampling in each sex group". Comment: unclear; Insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Low risk | Quote: "The supplements were coded with a letter and the code was safe‐kept at the SEAMEO laboratory by a laboratory assistant. Neither the investigator nor field assistants knew the codes until all subjects had completed the trial." Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.6% total loss to follow‐up: Zinc vs placebo: 8.1 vs 11.33; Zinc plus iron plus vitamin A vs zinc plus iron: 12.2 vs 9.1. Comment: attrition rate unlikely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Low risk | Study seems to be free from other biases. |
Heinig 2006.
| Methods | RCT | |
| Participants | Selection criteria included:
Conducted in USA. Time period between November 1994 to August 1997. |
|
| Interventions | Infants were randomly assigned to receive: zinc: 5 mg elemental zinc (as zinc sulphate); Placebo: placebo in drops given each day. Duration of six months |
|
| Outcomes | Anthropometry, serum zinc Hb, haematocrit,iron, copper, ferritin, Ig G2 and G4, dietary intake; morbidity data were coded and grouped into 5 major categories: respiratory illness, diarrhoea, otitis media, fever (without other symptoms), and other illnesses. Each month, infant motor development was assessed by using the Alberta Infant Motor Scale (AIMS). | |
| Notes | Supported by grant no 94‐37200‐2536 from the US Department of Agriculture. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were stratified by sex, and random assignment to groups was done by using the Moses‐Oakford algorithm, as described by Meinert and Tonascia" Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "an assistant, who was not in contact with the study subjects, labelled the bottles with 1 of 4 colours (2 colours were assigned to each group to reduce the chance that a group assignment would accidentally be revealed)." Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each mother‐infant pair was assigned to a colour group so that neither the primary investigator nor the mothers would know whether their infants received the zinc supplement." Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each mother‐infant pair was assigned to a colour group so that neither the primary investigator nor the mothers would know whether their infants received the zinc supplement." Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Comment:attrition rate unlikely to affect outcomes. |
| Selective reporting (reporting bias) | High risk | Quote: "In addition, the incidence (Figure 6) or prevalence (data not shown) of diarrhoea, otitis media, respiratory illness, fever only, and total illness over the study period did not differ significantly between groups". Comment: selective reporting present. |
| Other bias | Low risk | Study seems to be free from other bias. |
Radhakrishna 2013.
| Methods | RCT | |
| Participants | Selection criteria included term healthy infants who would stay in the study area until the child attains 2 years of age. 475 full‐term (gestational age > 37 weeks) normal pregnant women were contacted and enrolled in the last month of pregnancy from a low‐income urban community. After delivery, new born babies were assessed for confirmation of eligibility. located in the Secunderabad city of the South India. |
|
| Interventions | The mothers were instructed to give 0.5 mL of the preparation to children every morning after the initial feed. Zinc plus riboflavin: the intervention group received 5 mg of zinc plus riboflavin (0.5 mg/day) Riboflavin: the control groups of children received only riboflavin (0.5 mg/day) Duration of 14 months. |
|
| Outcomes | Maternal and child anthropometrics; diarrhoeal and respiratory morbidity; Hb, zinc, copper and vitamin A serum levels; dietary intake. | |
| Notes | The study was funded by the authors’ institute: National Institute of Nutrition, ICMR, Government of India. The funding party had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer generated simple randomisation was used to allocate the study children to either control or intervention group." Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation list and the supplements were given to a senior scientist at the institute who had no knowledge of the codes. After recruitment, the study children were given an identification number and were assigned treatment code by the senior scientist supervising the randomisation. After completion of the study analysis, the groups were decoded. Thus, all the investigators involved in data collection, analysis and interpretation were blind to allocation." Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The zinc and placebo were prepared and supplied by Biological Evans limited, in a syrup base, which were of similar colour, consistency and flavour; in two sets of identical looking bottles, labelled 1 and 2. The parents were also blind to the treatment given to their child." Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the investigators, including the medical doctor collecting clinical data and those collecting anthropometric measurement, as well as the statistician, were blind to the treatment." Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6.8% total loss to follow‐up: zinc plus riboflavin vs riboflavin: 8 versus 9.31. Comment: attrition rate unlikely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Low risk | Study seems to be free from other biases. |
Sazawal 2007.
| Methods | RCT | |
| Participants | Parents of eligible children, those aged 1 to 35 months, who were likely to (U1) remain resident on the island and did not have severe malnutrition needing rehabilitation (defined as Kwashiorkor, noted by the enrolment worker), were invited to participate in the study. The study was undertaken in Pemba, the smaller of the 2 islands of the Zanzibar archipelago. |
|
| Interventions | The study was a double‐blind randomised trial with 4 arms: iron, folic acid, and zinc (IFAZ); iron and folic acid (IFA); zinc; and placebo. On recommendation of the Data and Safety Monitoring Board (DSMB), the IFAZ and IFA groups were stopped on Aug 19, 2003 because of overwhelming evidence of increased hospital admissions and a trend for increased mortality associated with iron supplementation; the results from these groups were subsequently reported. The supplement, a dispersible tablet was dissolved in 5mL to 10 mL of water or breast milk Zinc: 10 mg of elemental zinc sulphate to children aged 12 months or older were given one tablet a day; children aged younger than 12 months were given a half tablet a day. Placebo: plain tablet in water or breast milk Children received zinc or placebo supplements until they were 48 months of age. All children aged 12 months or older were given 200,000 IU of vitamin A every 6 months; children aged 6 to 11 months were given 100 000 IU. |
|
| Outcomes | The primary outcome was overall mortality in participating children aged 1 to 48 months. Secondary outcomes were age‐specific, sex‐specific, and cause‐specific mortality. | |
| Notes | Not extracted due to age 1 to 11 months in data This study was supported by a grant from WHO, Department of Child and Adolescent Health and Development with funds from United Nations Foundation, from the Family Health and Child Survival and Global Research Activity, Cooperative Agreements between Johns Hopkins Department of International Health and the United States Agency for International Development, and from the Bill & Melinda Gates Foundation through its support for micronutrient research at the Johns Hopkins Bloomberg School of Public Health. Quote: "The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was by household, by an allocation sequence (permuted block randomisation with block length of 16) computer‐generated by WHO. The blister strips were coded with one of the letter codes, and every child was assigned a letter on enrolment on the basis of randomisation sequence." Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The trial initially used 16‐letter codes (four‐letter codes assigned to the individual supplementation groups). The four‐letter codes for every group were known only to WHO and the manufacturer; the pharmacy dispensing the supplements knew only which letter code was assigned to each child, and the study worker and family knew neither. At the time of switch, WHO and the DSMB statistician provided an alternative letter code for all of the redundant eight‐letter codes." Comment: allocation concealment probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The zinc‐containing tablets and the placebo tablets were provided in blister strips. Tablets of both groups were similar in packaging, appearance, taste, and inactive ingredients." Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Three teams that consisted of two physicians and a medical assistant, independently assigned one primary and two secondary causes of death. Any disagreements were resolved by discussion. These teams did not include investigators and were masked to supplement allocation." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15.5% total loss to follow‐up: Zinc and vitamin A vs Vitamin A: 17% vs. 15.8%. Comment: attrition rate unlikely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Low risk | Study seems to be free from other biases. |
Tielsh 2007.
| Methods | Cluster‐RCT (NNIPS‐4) |
|
| Participants | All children who were 1 to 35 months of age living in households in the study area. 4 independent samples, each of about 1200 children younger than 24 months of age who were enrolled in the trial, were randomly selected for participation in a morbidity sub study. A further stratified random sample of children of 24 months or older was selected for assessment of zinc status after 12 months of supplementation Conducted in rural southern Nepal. Time period between October, 2001, and January 2006. |
|
| Interventions | The treatment groups were placebo, iron and folic acid, zinc, and iron and folic acid with zinc. The doses of the nutrients used were given daily in tablets that were placebos or that contained 12·5 mg elemental iron as ferrous sulphate and 50 μg folic acid, 10 mg elemental zinc as zinc sulphate, or both. The iron and folic acid arms of the trial were stopped because there was no effect on mortality or morbidity; All children enrolled after November, 2003, received only either placebo or zinc supplementation. Zinc: 10 mg elemental zinc as zinc sulphate. Children under 12 months received half a tablet. Placebo: dispersible tablet Children were discharged from the study when they reached 36 months of age. All children received vitamin A supplementation (200,000 IU for children 12 months or older, 100,000 IU for those 6 to 12 months, and none for children under 6 months) twice per year, through the national programme distribution system or from study staff. |
|
| Outcomes | The primary outcome was all‐cause mortality; secondary outcomes were: cause‐specific mortality; the incidence and severity of diarrhoea, dysentery, and acute respiratory illness as assessed in the morbidity sub study; growth; and motor and cognitive development as assessed in a subgroup of the population. | |
| Notes | This study was funded by grants from the National Institutes of Health, Bethesda, MD, USA (HD 38753), the Bill & Melinda Gates Foundation, Seattle, WA, USA (810‐2054), and a Cooperative Agreement between Johns Hopkins University and the Office of Health and Nutrition, US Agency for International Development, Washington, DC, USA (HRN‐A‐00‐97‐00015‐00). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sectors were randomly assigned to treatment groups in blocks of four, stratified by VDC and geographical area. Randomisation at the sector level protected the study from unintentional crossover because workers within a sector dispensed only one type of coded supplement. All possible orders of the four treatment groups were written on 107 paper slips, with roughly equal numbers of slips for each order. One slip was randomly drawn to assign treatment codes to four sectors within a VDC. This continued until all sectors were assigned". Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment codes were kept by the Department of Child and Adolescent Health and Development." Comment: probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All investigators, study staff, and participants were unaware of the assigned treatment" Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All investigators, study staff, and participants were unaware of the assigned treatment" Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Zinc vs placebo: 17.3% vs 10.3% after 36 months Comment: attrition rate unlikely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Low risk | Study seems to be free from other biases. |
Wasantwisut 2006.
| Methods | RCT | |
| Participants | Eligibility criteria were that infants were predominantly breast‐fed and free from apparent congenital abnormalities and that their parents agreed to participate in the study. When eligible infants reached the age of 4 to 6 months, a verbal informed consent was obtained from the mothers or caretakers. A total of 675 infants (339 boys and 336 girls) aged 4 to 6 months were enrolled in the study. Located in 3 rural districts of Khon‐Kaen Province, located 450 km northeast of Bangkok. |
|
| Interventions | Infants received iron‐only, zinc‐only, iron plus zinc supplements, or a placebo. Zinc: 10 mg of zinc (as zinc sulphate) Placebo: only placebo Zinc plus iron: 5 grams/L (76.5 mmol/L) zinc (as zinc sulphate) with 5 g/L (89.5 mmol/L) iron (as ferrous sulphate) Iron only: only iron The supplement was given daily for a duration of 6 months. In the present study, infants were measured at 4 to 6 months at baseline, and at 10 months 12 months at the end point. |
|
| Outcomes | Anthropometry Hb, serum ferritin, zinc, anaemia. | |
| Notes | Supported by UNICEF and the Thrasher Research Fund. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were stratified by age and sex and then random numbers were used to assign individual infants to supplemental groups. The randomisation was done by a statistician who was not involved in the study." Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The bottles of syrup were coded at the production site. The code allocation was kept at UNICEF, Jakarta, until the end of data analysis." Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The 4 types of supplements were indistinguishable in appearance, colour, taste, or odour." Comment: blinding of participants adequately done, for personnel probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9.64% total loss to follow‐up: for the zinc vs placebo: 13.7 vs 5.56; for zinc plus iron vs iron: 11.1 vs 7.8. Comment: attrition rate may affect outcomes. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Low risk | Study seems to be free from other biases. |
FE: iron; HB: haemoglobin; IU: international units; RCT: randomised controlled trial; Zn: zinc.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alam 2011 | Duration of treatment was 5 or 10 days. |
| Aminisani 2011 | Duration of treatment was 5 months (20 weeks). |
| Ashworth 1998 | Duration was 8 weeks. |
| Bates 1993 | Aged more than 6 months. |
| Beuno 2008 | Study was excluded because participants all had IUGR (intrauterine growth retardation). |
| Bhandari 2007 | Study was excluded because iron was provided to participants. |
| Bhatnagar 2012 | Trial included ill infants. |
| Bilenko 2010 | Multiple micronutrient supplementation used with inadequate amount of zinc. |
| Black 2004 | Study with vulnerable sample of infants, many of them were chronically undernourished. |
| Castillo‐Durán 1995 | Study was excluded because trial provided iron to all participants. |
| Coles 2008 | Duration of treatment was just till discharge which was less than six months. |
| Dewey 2017 | Wrong patient population. |
| El‐Farghali 2015 | All children included in the study were low‐birth‐weight neonates. |
| Friel 1993 | Mean gestational age less than 29 weeks, as well as supplementation provided via milk. |
| Gibson 2011 | Meal made of multiple nuts and micronutrients was used instead of syrup. |
| Hamadani 2001 | Duration was 5 months. |
| Hess 2017 | Wrong patient population. |
| Jimenez 2007 | Duration of intervention was 5 months. |
| Krebs 2011 | Age greater than six months and supplement was a cereal mix. |
| Kumar 2012 | Duration was 2 months. |
| Lira 1998 | Duration was 2 months. |
| Mahalanabis 2011 | Infants were born low birth weight. |
| Mallard 2014 | Micronutrient fortified, no amount given for zinc. Infants older than required age (6 months +/‐ 2 weeks). |
| Manno 2012 | Aged six months. |
| Mehta 2012 | Ppopulation had probable neonatal sepsis. |
| Menon 2007 | Age 9 to 12 months. |
| Mullen 2013 | Age 6 to 18 months. |
| Muller 2001 | Age 6 to 31 months. |
| NCT00133419 | Micronutrient mixture containing vitamins A, B, C, D, E, K. |
| Ninh 1996 | Duration 5 months. |
| Nissensohn 2016 | Not a study but a systematic review. |
| Olney 2006 | Include iron, folic acid, with and without zinc. |
| Osendarp 2002 | The study provided zinc supplementation for 24 weeks (5 months). |
| Rana 2011 | Duration 7 days. |
| Roy 2007 | Duration 2 weeks in acutely ill children. |
| Salmenpera 1994 | Fortified form used in milk. |
| Sazawal 2001 | The study included children small for gestational age. |
| Schlesinger 1993 | Chronically ill children (marasmus). |
| Simmer 1988 | Severely malnourished children. |
| Sur 2003 | The study included a birth cohort of 100 LBW infants. |
| Taneja 2009 | Study was excluded because it included low‐birth weight infants. |
| Terrin 2013 | Preterm neonates as participants. |
| Walker 2007 | Dispersible tablet for 2 weeks. |
| Walravens 1976 | Uses milk. |
| Walravens 1989 | Chronic PE malnutrition. |
| Wieringa 2003 | Iron was provided as an additional intervention to all anaemic participants. |
| Yang 2002 | Children enrolled were growth retarded and pre‐schoolers. |
| Zlotkin 2003 | Uses sprinkles on top of food as fortification. |
LBW: low birth weight
Characteristics of studies awaiting assessment [ordered by study ID]
Castillo Durán 2001.
| Methods | RCT |
| Participants | Term neonates of low socioeconomic status. |
| Interventions | Supplemental: zinc 5 mg/day (SG) Placebo: a lactose placebo (PG) Duration = unclear |
| Outcomes | Anthropometry measured monthly, psychomotor development (PDI), mental development (MDI), and behaviour including motor quality factor were assessed by Bayley Scales at 6 and 12 months. |
| Notes | Duration is unclear. Author has been emailed for clarification. |
Hong 1992.
| Methods | RCT |
| Participants | Newborns of high‐risk pregnancies. |
| Interventions | 102 neonates were divided into 3 groups. 1 group was breast‐fed, 1 group was formula fed with supplementary zinc and 1 group was formula fed with supplementary Vitamin B complex as placebo. Test group: vitamin B complex solution containing 1% zinc sulphate equivalent to 1.14 mg/kg/day to 2.28 mg/kg/day elemental zinc. Control group: vitamin B complex solution 0.5 mL/kg/day to 1.0 mL/kg/day. Duration: 3 days of age till 6 months of age. |
| Outcomes | Weight (g), crown to heel length (cm), serum zinc (umol/L) reported as 0 to 3, 3 to 6 and 0 to 6 month increments. |
| Notes | Unclear as to how children were administered solution. |
Locks 2016.
| Methods | RCT |
| Participants | Infants born to HIV‐negative mothers from 6 weeks of age for a period of 18 months, assigned in the trial at 5 to 7 week of age in Tanzania. |
| Interventions | 2400 infants were randomly assigned to receive one of the following 4 treatment regimens:
Duration of intervention: 18 months Study start date: September 2007 Study completion date: October 2012. |
| Outcomes | Reduction of symptoms of diarrhoea and lower respiratory infection. The mean change in height‐for‐age z‐score (HAZ) and in weight for‐age z‐scores (WAZs) and weight‐for‐height z‐scores (WHZs) among treatment groups. |
| Notes | The Eunice Kennedy Shriver National Institute of Child Health and Human Development (grants R01 HD048969‐01 and K24 DK104676) supported this study. Authors were contacted to provide data on infants who were not supplemented with iron. |
NCT02319499.
| Methods | RCT |
| Participants | Infants aged 3 to 6 months Location: Indonesia |
| Interventions | Experimental: zinc alone, zinc sulphate (10 mg Zn/day) Experimental: iron, zinc and vitamin A, ferrous sulphate, zinc sulphate and vitamin A (10 mg/day of each zinc and iron, plus 1000 IU vitamin A/day). Placebo Comparator: Placebo, No minerals/vitamin. |
| Outcomes | Primary outcome measures: change in Length‐for‐Age Z‐scores, Length‐for‐Age Z‐score, change in Weight‐for‐Length Z‐scores , Weight‐for‐Length Z‐score, change in Weight‐for‐Age Z‐scores, Weight‐for‐Age Z‐scores, changes in Mental Development Index, MDI of Bayley Scale of Infant Development II, changes in Psychomotor Development Index, PDI of Bayley Scale of Infant Development II. Secondary outcome measures: changes in haemoglobin, changes in serum zinc, changes in serum ferritin, changes in serum retinol. |
| Notes | Not published. |
IU: international units; RCT: randomised controlled trial.
Differences between protocol and review
We made the following changes to the published protocol (Hassan 2012).
We were unable to find an established guideline for a definitive normal serum or plasma zinc level for infants under six months of age. We found non‐fasting serum zinc concentration cut‐offs for children under 10 years of age from the population studies (IZiNCG 2004). Additionally, we have considered population‐based studies where children with prematurity, small of gestational age or low birth weight makes part of the population and, therefore, can not be excluded of the studies. We have specifically excluded efficacy trials that have included only theses subgroups of the population.
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see How CENTRAL is created). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL.
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs on the following platforms: ClinicalTrials.gov or from The World Health Organization’s International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see How CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN (at www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
For the 2020 update, we ran searches in the following databases: CENTRAL via CRS Web, MEDLINE via Ovid, and CINAHL via EBSCOhost. The search strategies are available in Appendix 3. The previous search methods are available in Appendix 2 and Appendix 1.
Contributions of authors
Zohra S Lassi, Anoosh Moin, Jaameeta Kurji and Cristieli Sérgio de Oliveira reviewed the search results, included and extracted study data, conducted the analyses as well as wrote the final draft under the supervision of Zulfiqar A Bhutta.
Sources of support
Internal sources
The Aga Khan University, Pakistan.
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
ZS has no interest to declare.
AM has no interest to declare.
JK has no interest to declare.
CO has no interest to declare.
ZB has no interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Berger 2006 {published data only}
- Berger J, Ninh NX, Khan NC, Nhien NV, Lien DK, Trung NQ, et al. Efficacy of combined iron and zinc supplementation micronutrient status and growth in Vietnamese infants. European Journal of Clinical Nutrition 2006;60(4):443‐54. [DOI: 10.1038/sj.ejcn.1602336; PUBMED: 16306925] [DOI] [PubMed] [Google Scholar]
- Dijkhuizen MA, Winichagoon P, Wieringa FT, Wasantiwisut W, Utomo B, Ninh NX. Zinc supplementation improved length growth only in anemic infants in a multi country trial of iron and zinc supplementation in South‐East Asia. Journal of Nutrition 2008;138(10):1969‐75. [DOI: 10.1093/jn/138.10.1969; PUBMED: 18806109] [DOI] [PubMed] [Google Scholar]
Dijkhuizen 2001 {published data only}
- Dijkhuizen MA, Wieringa FT, West CE, Martuti S, Muhilal. Zinc supplementation in Indonesian Infants on micronutrient status and growth. Journal of Nutrition 2001;131(11):2860‐5. [DOI: 10.1093/jn/131.11.2860; PUBMED: 11694609] [DOI] [PubMed] [Google Scholar]
- Dijkhuizen MA, Winichagoon P, Wieringa FT, Wasantiwisut W, Utomo B, Ninh NX, et al. Zinc supplementation improved length growth only in anemic infants in a multi country trial of iron and zinc supplementation in South‐East Asia. Journal of Nutrition 2008;138(10):1969‐75. [DOI: 10.1093/jn/138.10.1969; PUBMED: 18806109] [DOI] [PubMed] [Google Scholar]
Fahmida 2007 {published data only}
- Fahmida U, Rumawas JS, Utomo B, Patmonodewo S, Schultink W. Zinc‐iron, but not zinc‐alone supplementation, increased linear growth of stunted infants with low haemoglobin. Asia Pacific Journal of Clinical Nutrition 2007;16(2):301‐9. [PUBMED: 17468087] [PubMed] [Google Scholar]
Heinig 2006 {published data only}
- Heinig MJ, Brown KH, Lönnerdal B, Dewey KG. Zinc supplementation does not affect growth, morbidity, or motor development of US term breast‐fed infants at 4‐10 months. American Journal of Clinical Nutrition 2006;84(3):594‐601. [DOI: ] [DOI] [PubMed] [Google Scholar]
Radhakrishna 2013 {published data only}
- Radhakrishna KV, Hemalatha R, Geddam JJ, Kumar PA, Balakrishna N, Shatrugna V. Effectiveness of zinc supplementation to full term normal infants: a community based double blind, randomized, controlled, clinical trial. PLOS One 2013;8(5):e61486. [DOI: 10.1371/journal.pone.0061486; PUBMED: 23737940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sazawal 2007 {published data only}
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Dutta A, Dhingra U, et al. Effect of zinc supplementation on mortality in children aged 1–48 months: a community‐based randomised placebo controlled trial. Lancet 2007;369(9565):927‐34. [DOI: 10.1016/S0140-6736(07)60452-8; PUBMED: 17368154] [DOI] [PubMed] [Google Scholar]
Tielsh 2007 {published data only}
- Siegel EH, Kordas K, Stoltzfus RJ, Katz J, Khatry SK, LeClerq SC, et al. Inconsistent effects of iron‐folic acid and/or zinc supplementation on the cognitive development of infants. Journal of Health Population and Nutrition 2011;29(6):593‐604. [PUBMED: 22283033] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Charles MK, Katz J, Siegel EH, Khatry SK, LeClerq SC, et al. The role of zinc and iron‐folic acid supplementation on early child temperament and eating behaviors in rural Nepal: a randomized controlled trial. PLOS One 2013;10(3):e0114266. [DOI: 10.1371/journal.pone.0114266; PUBMED: 25821959] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, Adhikari R, et al. Effect of daily zinc supplementation on child mortality in southern Nepal: a community‐based, cluster randomised, placebo controlled trial. Lancet 2007;370(9594):1230‐9. [DOI: 10.1016/S0140-6736(07)61539-6; PUBMED: 17920918] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, Adhikari R, et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in Southern Nepal: community‐based, cluster‐randomized, placebo‐controlled trial. Lancet 2006;367(9505):144–52. [DOI: 10.1016/S0140-6736(06)67963-4; PUBMED: 16413878] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasantwisut 2006 {published data only}
- Dijkhuizen MA, Winichagoon P, Wieringa FT, Wasantiwisut W, Utomo B, Ninh NX, et al. Zinc supplementation improved length growth only in anemic infants in a multi country trial of iron and zinc supplementation in South‐East Asia. Journal of Nutrition 2008;138(10):1969‐75. [DOI: 10.1093/jn/138.10.1969; PUBMED: 18806109] [DOI] [PubMed] [Google Scholar]
- Pongcharoen T, DiGirolamo AM, Ramakrishnan U, Winichagoon P, Flores R, Martorell R. Long term effects of Iron and zinc supplementation during infancy on cognitive function at 9 years of age in Northeast Thai children: a follow up study. American Journal of Clinical Nutrition 2011;93(3):636‐43. [DOI: 10.3945/ajcn.110.002220; PUBMED: 21270383] [DOI] [PubMed] [Google Scholar]
- Wasantwisut E, Winichagoon P, Chitchumroonchokchai C, Yamborisut U, Boonpraderm A, Pongcharoen T, et al. Iron and zinc supplementation improved iron and zinc status, but not physical growth, of apparently healthy, breast‐fed infants in rural communities of Northeast Thailand. Journal of Nutrition 2006;136(9):2405‐11. [DOI: 10.1093/jn/136.9.2405; PUBMED: 16920862] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alam 2011 {published data only}
- Alam DS, Yunus M, Arifeen SE, Chowdury HR, Larson CP, Sack DA. Zinc treatment for 5 or 10 days is equally efficacious in preventing diarrhea in the subsequent 3 months among Bangladeshi children. Journal of Nutrition 2011;141(2):312‐5. [DOI: 10.3945/jn.110.120857; PUBMED: 21147907] [DOI] [PubMed] [Google Scholar]
Aminisani 2011 {published data only}
- Aminisani N, Barak M, Shamshirgaran SM. Effect of zinc supplementation on growth of low birth weight infants aged 1–6 month in Ardabil, Iran. Indian Journal of Pediatrics 2011;78(10):1239–43. [DOI: 10.1007/s12098-011-0541-7; PUBMED: 21858548] [DOI] [PubMed] [Google Scholar]
Ashworth 1998 {published data only}
- Ashworth A, Morris SS, Lira PI, Grantham‐McGregor SM. Zinc supplementation, mental development and behaviour in low‐birth weight term infants in Northeast Brazil. European Journal Clinical Nutrition 1998;52(3):223‐7. [PUBMED: 9537309] [DOI] [PubMed] [Google Scholar]
Bates 1993 {published data only}
- Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn PG, Northrop‐Clewes CA, et al. A trial of zinc supplementation in young rural Gambian children. British Journal of Nutrition 1993;69(1):243‐55. [PUBMED: 8457531] [DOI] [PubMed] [Google Scholar]
Beuno 2008 {published data only}
- Bueno O, Bueno G, Moreno LA, Nuviala RJ, Pérez‐González JM, Bueno M. Zinc supplementation in infants with asymmetric intra uterine growth retardation [Effect on growth, nutritional status and leptin ecretion]. Nutricion Hospitalaria 2008;23(3):212‐9. [PUBMED: 18560697] [PubMed] [Google Scholar]
Bhandari 2007 {published data only}
- Bhandari N, Taneja S, Mazumder S, Bahl R, Fontaine O, Bhan MK, et al. Adding zinc to supplemental iron and folic acid does not affect mortality and severe morbidity in young children. Journal of Nutrition 2007;137(1):112‐7. [DOI: 10.1093/jn/137.1.112; PUBMED: 17182810] [DOI] [PubMed] [Google Scholar]
Bhatnagar 2012 {published data only}
- Bhatnagar S, Wadhwa N, Aneja S, Lodha R, Kabra SK, Natchu UC, et al. Zinc as adjunct treatment in infants aged between 7 and 120 days with probable serious bacterial infection:a randomised, double‐blind, placebo‐controlled trial. Lancet 2012;379(9831):2072‐8. [DOI: 10.1016/S0140-6736(12)60477-2; PUBMED: 22656335] [DOI] [PubMed] [Google Scholar]
Bilenko 2010 {published data only}
- Bilenko N, Belmaker I, Vardi H, Fraser D. Efficacy of multiple micronutrient supplementations on child health: study design and baseline characteristics. Israel Medical Association Journal 2010;12(6):342–7. [PUBMED: 20928987] [PubMed] [Google Scholar]
Black 2004 {published data only}
- Black MM, Sazawal S, Black RE, Khosla S, Kumar J, Menon V. Cognitive and motor development among small‐for‐gestational‐age infants: impact of zinc supplementation, birth weight, and caregiving practices. Pediatrics 2004;113(5):1297‐305. [PUBMED: 15121945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castillo‐Durán 1995 {published data only}
- Castillo‐Durán C, Rodriguez A, Venegas G, AIvarez P, Icaza G. Zinc supplementation and growth of infants born small for gestational age. Journal of Pediatrics 1995;27(2):206‐11. [PUBMED: 7636643] [DOI] [PubMed] [Google Scholar]
Coles 2008 {published data only}
- Coles CL, Sherchand JB, Khatry SK, Katz J, LeClerq SC, Mullany LC, et al. Zinc modifies the association between nasopharyngeal streptococcus pneumoniae carriage and risk of acute lower respiratory infection among young children in rural Nepal. Journal of Nutrition 2008;138(12):2462‐7. [DOI: 10.3945/jn.108.095422; PUBMED: 19022973] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dewey 2017 {published data only}
- Dewey KG. Heterogeneity in response to nutrition interventions during the first 1000 days: evidence from randomized controlled trials using lipid‐based nutrient supplements for mothers and infants. Annals of Nutrition and Metabolism 2017;71 (Suppl 2):12. [Google Scholar]
El‐Farghali 2015 {published data only}
- El‐Farghali O, El‐Wahed MA, Hassan NE, Imam S, Alian K. Early zinc supplementation and enhanced growth of the low‐birth weight neonate. Оpen Аccess Macedonian Journal of Medical Sciences 2015;3(1):63‐8. [DOI: 10.3889/oamjms.2015.007; PUBMED: 27275198] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friel 1993 {published data only}
- Friel JK, Andrews WL, Matthew JD, Long DR, Cornel AM, Cox M. Zinc supplementation in very low birth weight infants. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):97‐104. [PUBMED: 8350219] [DOI] [PubMed] [Google Scholar]
Gibson 2011 {published data only}
- Gibson RS, Kafwembe E, Gosset S, Bailey KB, Mullen A, Baisley KB, et al. A micronutrient‐fortified food enhances iron and selenium status of Zambian infants but has limited efficacy on zinc. Journal of Nutrition 2011;141(5):935‐43. [DOI: 10.3945/jn.110.135228; PUBMED: 21411608] [DOI] [PubMed] [Google Scholar]
Hamadani 2001 {published data only}
- Hamadani JD, Fuchs GJ, Osendarp SJ, Khatun F, Huda SN, Grantham‐McGregor SM. Randomized controlled trial of the effect of zinc supplementation on the mental development of Bangladeshi infants. American Journal of Clinical Nutrition 2001;74(3):381‐6. [DOI: 10.1093/ajcn/74.3.381; PUBMED: 11522564] [DOI] [PubMed] [Google Scholar]
Hess 2017 {published data only}
- Hess SY, Peerson JM, Becquey E, Abbeddou S, Ouedraogo CT, Some JW, et al. Differing growth responses to nutritional supplements in neighboring health districts of Burkina Faso are likely due to benefits of small‐quantity lipid‐based nutrient supplements (LNS). PLOS One 2017;12(8):e01817702017. [DOI: 10.1371/journal.pone.0181770; PUBMED: 28771493] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jimenez 2007 {published data only}
- Jimenez R, Martinez M, Penalver R. Zinc effects on growth and development of infants with low birth weight [Efecto del zinc sobre el crecimiento y desarrollo del nino con bajo peso al nacer]. Colombia Médica 2007;38 (Suppl 1):6‐13. [Google Scholar]
Krebs 2011 {published data only}
- Krebs NF, Hambidge KM, Mazariegos M, Westcott J, Goco N, Wright LL. Complementary feeding: a global network cluster randomized controlled trial. BMC Pediatrics 2011;11:4. [DOI: 10.1186/1471-2431-11-4; PUBMED: 21232139] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF, Mazariegos M, Chomba E, Sami N, Pasha O, Tshefu A. Randomized controlled trial of meat compared with multimicronutrient‐fortified cereal in infants and toddlers with high stunting rates in diverse settings. American Journal of Clinical Nutrition 2012;96(4):840‐7. [DOI: 10.3945/ajcn.112.041962; PUBMED: 22952176] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2012 {published data only}
- Kumar TV, Ramji S. Effect of zinc supplementation on growth in very low birth weight infants. Journal of Tropical Pediatrics 2012;58(1):50‐4. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lira 1998 {published data only}
- Lira PI, Ashworth A, Morris SS. Effect of zinc supplementation on the morbidity, immune function,and growth of low‐birth‐weight, full‐term infants in Northeast Brazil. American Journal of Clinical Nutrition 1998;68 (Suppl 2):418S‐24. [DOI: 10.1093/ajcn/68.2.418S; PUBMED: 9701155] [DOI] [PubMed] [Google Scholar]
Mahalanabis 2011 {published data only}
- NCT00495690. Impact of daily zinc supplementation to infants born with low birth weight on death and severe disease. clinicaltrials.gov/ct2/show/NCT00495690?term=%28zinc+supplementation%29+AND+%28newborn+OR+neonate+OR+child+OR+infant%29&rank=3 (first received 3 July 2007).
Mallard 2014 {published data only}
- Mallard SR, Houghton LA, Filteau S, Mullen A, Nieuwelink J, Chisenga M, et al. Dietary diversity at 6 months of age is associated with subsequent growth and mediates the effect of maternal education on infant growth in urban Zambia. Journal of Nutrition 2014;144(11):1818‐25. [DOI: 10.3945/jn.114.199547; PUBMED: 25332481] [DOI] [PubMed] [Google Scholar]
Manno 2012 {published data only}
- Manno D, Kowa PK, Bwalya HK, Siame J, Grantham‐McGregor S, Baisley K, et al. Rich micronutrient fortification of locally produced infant food does not improve mental and motor development of Zambian infants: a randomised controlled trial. British Journal of Nutrition 2012;107(4):556‐66. [DOI: 10.1017/S0007114511003217; PUBMED: 21733297] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2012 {published data only}
- Mehta K, Bhatta NK, Majhi S, Shrivastava MK, Singh RR. Oral zinc supplementation for reducing mortality in probable neonatal sepsis: a double blind randomized placebo controlled trial. Indian Pediatrics 2012;50(4):390‐3. [PUBMED: 23255688] [DOI] [PubMed] [Google Scholar]
Menon 2007 {published data only}
- Menon P, Ruel MT, Loechl CU, Arimond M, Habicht JP, Pelto G, et al. Micronutrient sprinkles reduce anemia among 9‐ to 24‐mo‐old children when delivered through an integrated health and nutrition program in rural Haiti. Journal of Nutrition 2007;137(4):1023‐30. [DOI: 10.1093/jn/137.4.1023; PUBMED: 17374671] [DOI] [PubMed] [Google Scholar]
Mullen 2013 {published data only}
- Mullen A, Gosset L, Larke N, Manno D, Chisenga M, Kasonka L, et al. The effects of micronutrient‐fortified complementary/replacement food on intestinal permeability and systemic markers of inflammation among maternally HIV‐exposed and unexposed Zambian infants. British Journal of Nutrition 2012;107(6):893‐902. [DOI: 10.1017/S0007114511003734; PUBMED: 21899803] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2001 {published data only}
- Müller O, Becher H, Zweeden AB, Ye Y, Diallo DA, Konate AT, et al. Effect of zinc supplementation on malaria and other causes of morbidity in West African children: randomised double blind placebo controlled trial. BMJ 2001;322(7302):1‐6. [PUBMED: 11431296] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00133419 {published data only}
- NCT00133419. Micronutrients and enteric infections in African children. clinicaltrials.gov/ct2/show/NCT00133419?term=NCT00133419&rank=1 (first received 23 August, 2005).
Ninh 1996 {published data only}
- Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Keteislegers JM. Zinc supplementation increases growth and circulating insulin‐like growth factor I (IGF‐l) in growth‐retardedVietnamese children. Journal of Clinical of Nutrition 1996;63(4):514‐9. [DOI: 10.1093/ajcn/63.4.514; PUBMED: 8599314] [DOI] [PubMed] [Google Scholar]
Nissensohn 2016 {published data only}
- Nissensohn M, Sánchez‐Villegas A, Lugo DF, Sánchez PH, Alonso JD, Quintana LP, et al. Effect of zinc intake on growth in infants: a meta‐analysis. Critical Reviews in Food Science and Nutrition 2016;56(3):350‐63. [DOI: 10.1080/10408398.2013.802661; PUBMED: 25365524] [DOI] [PubMed] [Google Scholar]
Olney 2006 {published data only}
- Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, et al. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5‐ to 11‐mo old. Journal of Nutrition 2006;136(9):2427–34. [DOI: 10.1093/jn/136.9.2427; PUBMED: 16920865] [DOI] [PubMed] [Google Scholar]
Osendarp 2002 {published data only}
- Osendarp SJ, Santosham M, Black RE, Wahed MA, Raaij JM, Fuchs GJ. Effect of zinc supplementation between 1 and 6 mo of life on growth and morbidity of Bangladeshi infants in urban slums. American Journal of Clinical Nutrition 2002;76(6):1401‐8. [DOI: 10.1093/ajcn/76.6.1401; PUBMED: 12450909] [DOI] [PubMed] [Google Scholar]
Rana 2011 {published data only}
- Rana N, Mishra S, Bhatnagar S, Paul V, Deorari AK, Agarwal R. Efficacy of zinc in reducing hyperbilirubinemia among at‐risk neonates: a randomized, double‐blind, placebo‐controlled trial. Indian Journal of Pediatrics 2011;78(9):1073‐8. [DOI: 10.1007/s12098-011-0407-z; PUBMED: 21455724] [DOI] [PubMed] [Google Scholar]
Roy 2007 {published data only}
- Roy SK, Tomkins AM, Akramuzzaman SM, Chakraborty B, Ara G, Biswas R, et al. Impact of zinc supplementation on subsequent morbidity and growth in Bangladeshi children with persistent diarrhoea. Journal of Health, Population, and Nutrition 2007;25(1):67‐74. [PUBMED: 17615905 ] [PMC free article] [PubMed] [Google Scholar]
- Roy SK, Tomkins AM, Haider R, Behren RH, Akramuzzaman SM, Mahalanabis D, et al. Impact of zinc supplementation on subsequent growth and morbidity in Bangladeshi children with acute diarrhoea. European Journal of Clinical Nutrition 1999;53(7):529‐34. [PUBMED: 10452407] [DOI] [PubMed] [Google Scholar]
Salmenpera 1994 {published data only}
- Salmenpera L, Perheentupa J, Pakarinen P, Siimes MA. Zinc supplementation of infant formula. American Journal of Clinical Nutrition 1994;59(5):985‐9. [DOI: 10.1093/ajcn/59.5.985; PUBMED: 8172105] [DOI] [PubMed] [Google Scholar]
Sazawal 2001 {published data only}
- Sazawal S, Black RE, Menon VP, Dinghra P, Caulfield LE, Dhingra U, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics 2001;108(6):1280‐6. [PUBMED: 11731649] [DOI] [PubMed] [Google Scholar]
Schlesinger 1993 {published data only}
- Schlesinger L, Arevalo M, Arredondo S, Lonnerdal B, Stekel A. Zinc supplementation impairs monocyte function. Acta Paediatrica 1993;82(9):734‐8. [PUBMED: 8241668] [DOI] [PubMed] [Google Scholar]
Simmer 1988 {published data only}
- Simmer K, Khanum S, Carlsson L, Thompson RP. Nutritional rehabilitation in Bangladesh‐the importance of zinc. Journal of Clinical Nutrition 1988;47(6):1036‐40. [DOI: 10.1093/ajcn/47.6.1036; PUBMED: 3132034] [DOI] [PubMed] [Google Scholar]
Sur 2003 {published data only}
- Sur D, Gupta DN, Mondal SK, Ghosh S, Manna B, Rajendran K, et al. Impact of zinc supplementation on diarrheal morbidity and growth pattern of low birth weight infants in Kolkata, India: a randomized, double‐blind, placebo‐controlled, community‐based study. Pediatrics 2003;112(6 (Pt 1)):1327‐32. [PUBMED: 14654605] [DOI] [PubMed] [Google Scholar]
Taneja 2009 {published data only}
- Taneja S, Bhandari N, Rongsen‐Chandola T, Mahalanabis D, Fontaine O, Bhan MK. Effect of zinc supplementation on morbidity and growth in hospital‐born, low‐birth‐weight infants. American Journal of Clinical Nutrition 2009;90(2):385‐91. [DOI: 10.3945/ajcn.2009.27707; PUBMED: 19553296] [DOI] [PubMed] [Google Scholar]
Terrin 2013 {published data only}
- Terrin G, Canani RB, Passariello A, Messina F, Conti MG, Caoci S, et al. Zinc supplementation reduces morbidity and mortality in very‐low birth‐ weight preterm neonates: a hospital‐based randomized, placebo‐controlled trial in an industrialized country. American Journal of Clinical Nutrition 2013;98(6):1468‐74. [DOI: 10.3945/ajcn.112.054478; PUBMED: 24025633] [DOI] [PubMed] [Google Scholar]
Walker 2007 {published data only}
- Walker CL, Bhutta ZA, Bhandari N, Teka T, Shahid F, Taneja S, et al. Zinc during and in convalescence from diarrhea has no demonstrable effect on subsequent morbidity and anthropometric status among infants 6 mo of age. American Journal of Clinical Nutrition 2007;85(3):887‐94. [DOI: 10.1093/ajcn/85.3.887; PUBMED: 17344513] [DOI] [PubMed] [Google Scholar]
Walravens 1976 {published data only}
- Walravens PA, Hambidge KM. Growth of infants fed a zinc supplemented formula. American Journal of Clinical Nutrition 1976;29(10):1114‐21. [DOI: 10.1093/ajcn/29.10.1114; PUBMED: 788494] [DOI] [PubMed] [Google Scholar]
Walravens 1989 {published data only}
- Walravens PA, Hambidge KM, Koepfer DM. Zinc supplementation in infants with a nutritional pattern of failure to thrive: a double‐blind, controlled study. Pediatrics 1989;83(4):532‐8. [PUBMED: 2927993] [PubMed] [Google Scholar]
Wieringa 2003 {published data only}
- Dijkhuizen MA, Winichagoon P, Wieringa FT, Wasantiwisut W, Utomo B, Ninh NX. Zinc supplementation improved length growth only in anemic infants in a multi country trial of iron and zinc supplementation in South‐East Asia. Journal of Nutrition 2008;138(10):1969‐75. [DOI: 10.1093/jn/138.10.1969; PUBMED: 18806109] [DOI] [PubMed] [Google Scholar]
- Wieringa FT, Dijkhuizen MA, West CE, Thurnham DI, Muhilal, Mee JW. Redistribution of vitamin A after iron supplementation in Indonesian Infants. American Journal of Clinical Nutrition 2003;77(3):651‐7. [DOI: 10.1093/ajcn/77.3.651; PUBMED: 12600856] [DOI] [PubMed] [Google Scholar]
Yang 2002 {published data only}
- Yang YX, Han JH, Shao XP, He M, Bian LH, Wang Z, et al. Effect of micronutrient supplementation on the growth of preschool children in China. Biomedical Environmental Science 2002;15(3):196‐202. [PUBMED: 12500659] [PubMed] [Google Scholar]
Zlotkin 2003 {published data only}
- Zlotkin S, Arthur P, Schauer C, Antwi KY, Yeung G, Piekarz A. Home‐fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. Journal of Nutrition 2003;133(4):1075‐80. [DOI: 10.1093/jn/133.4.1075; PUBMED: 12672922] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Castillo Durán 2001 {published data only}
- Castillo‐Durán C, Perales CG, Hertrampf ED, Marín VB, Rivera FA, Icaza G. Effect of zinc supplementation on development and growth of Chilean infants. Journal of Pediatrics 2001;138(2):229‐35. [PUBMED: 11174621] [DOI] [PubMed] [Google Scholar]
Hong 1992 {published data only}
- Hong Z, Zhang Y, Xu J, Zhou J, Gao X, Liu X, et al. Growth promoting effect of Zinc supplementation in infants of high risk pregnancies. Chinese Medical Journal 1992;105(10):844‐8. [PUBMED: 1291203] [PubMed] [Google Scholar]
Locks 2016 {published data only}
- Locks 2016. Effect of zinc and multiple micronutrient supplements on growth in Tanzanian children. FASEB Journal. 2015; Vol. 29 Suppl 1.
- Locks LM, Manji KP, McDonald CM, Kupka R, Kisenge R, Aboud S, et al. Effect of zinc and multivitamin supplementation on the growth of Tanzanian children aged 6–84 wk: a randomized, placebo‐controlled, double‐blind trial. American Journal of Clinical Nutrition 2016;103(3):910–8. [DOI: 10.3945/ajcn.115.120055; PUBMED: 26817503] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locks LM, Manji KP, McDonald CM, Kupka R, Kisenge R, Aboud S, et al. The effect of daily zinc and/or multivitamin supplements on early childhood development in Tanzania: results from a randomized controlled trial. Maternal and Child Nutrition 2017;13(2):1‐19. [DOI: 10.1111/mcn.12306; PUBMED: 27189038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CM, Manji KP, Kisenge R, Aboud S, Spiegelman D, Fawzi WW, et al. Daily zinc but not multivitamin supplementation reduces diarrhea and upper respiratory infections in Tanzanian infants: a randomized, double‐blind, placebo‐controlled clinical trial. Journal of Nutrition 2015;145(9):2153‐60. [DOI: 10.3945/jn.115.212308; PUBMED: 26203094] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02319499 {published data only}
- NCT02319499. Zinc, iron, vitamin A and psychosocial care for child growth and development. clinicaltrials.gov/show/NCT02319499 (first received 18 December 2014).
Additional references
Aggett 1995
- Aggett PJ, Comerford JG. Zinc and human health. Nutrition Reviews 1995;53(9 Pt 2):S16‐22. [PUBMED: 8577412] [PubMed] [Google Scholar]
Bailey 2015
- Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Annals of Nutrition Metabolism 2015;66(12):22‐3. [DOI: 10.1159/000371618; PUBMED: 26045325] [DOI] [PubMed] [Google Scholar]
Baqui 2002
- Baqui AH, Black RE, Arifeen S, Yunus M, Chakraborty J, Ahmed S, et al. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: community randomised trial. BMJ 2002;325(7372):1059. [PUBMED: 12424162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bethesda 2006
- National Institutes of Health. Health information: zinc. ods.od.nih.gov/factsheets/list‐all/Zinc/ (accessed 24 September 2012).
Bettger 1981
- Bettger WJ, O'Dell BL. A critical physiological role of zinc in the structure and function of biomembranes. Life Sciences 1981;28(13):1425‐38. [PUBMED: 7017326] [DOI] [PubMed] [Google Scholar]
Bhandari 1996
- Bhandari N, Bahl R, Hambidge KM, Bhan MK. Increased diarrhoeal and respiratory morbidity in association with zinc deficiency ‐ a preliminary report. Acta Paediatrica 1996;85(2):148‐50. [PUBMED: 8640039] [DOI] [PubMed] [Google Scholar]
Bhutta 2000
- Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, Hidayat A, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. American Journal of Clinical Nutrition 2000;72(6):1516‐22. [DOI: 10.1093/ajcn/72.6.1516; PUBMED: 1110148] [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382(9890):427‐51. [DOI: 10.1016/S0140-6736(13)60937-X; PUBMED: 23746772] [DOI] [PubMed] [Google Scholar]
Boluyt 2008
- Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatric Adolescent Medicine 2008;162(2):111‐6. [DOI: 10.1001/archpediatrics.2007.40; PUBMED: 18250233] [DOI] [PubMed] [Google Scholar]
Brooks 2005
- Brooks WA, Santosham M, Roy SK, Faruque AS, Wahed MA, Nahar K, et al. Efficacy of zinc in young infants with acute watery diarrhea. American Journal of Clinical Nutrition 2005;82(3):605‐10. [DOI: 10.1093/ajcn.82.3.605; PUBMED: 16155274] [DOI] [PubMed] [Google Scholar]
Brown 2009
- Brown KH, Stone RE, Krebs NF, Peerson JM. Dietary intervention strategies to enhance zinc nutrition: promotion and support of breastfeeding for infants and young children. Food Nutrition Bulletin 2009;30(1 Suppl):S144‐71. [DOI: 10.1177/15648265090301S108; PUBMED: 19472605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caulfield 2006
- Caulfield L, Richard S, Rivera J, Musgrove P, Black R. Stunting, wasting, and micronutrient deficiency disorders. In: Jamison D, Breman J, Measham A, Alleyne G, Claeson M, Evans DB, et al. editor(s). Disease Control Priorities in Developing Countries. 2nd Edition. Washington, DC: Oxford University Press, 2006:551‐67. [Google Scholar]
Cole 2008
- Cole CR, Lifshitz F. Zinc nutrition and growth retardation. Pediatric Endocrinology Reviews : PER 2008;5(4):889‐96. [PUBMED: 18552751] [PubMed] [Google Scholar]
Davidsson 2007
- Davidsson L, Fontaine O, Hotz C. Conclusions of the joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutrition Bulletin 2007;28(3):S480‐2486. [DOI: 10.1177/15648265070283S306; PUBMED: 17988008] [DOI] [PubMed] [Google Scholar]
Fisher Walker 2006
- Fischer Walker CL, Bhutta ZA, Bhandari N, Teka T, Shahid F, Taneja S, et al. Zinc Study Group. Zinc supplementation for the treatment of diarrhea in infants in Pakistan, India and Ethiopia. Journal of Pediatric Gastroenterology and Nutrition 2006;43(3):357‐63. [DOI: 10.1097/01.mpg.0000232018.40907.00; PUBMED: 16954960] [DOI] [PubMed] [Google Scholar]
Fosmire 1990
- Fosmire GJ. Zinc toxicity. American Journal of Clinical Nutrition 1990;51(2):225‐7. [DOI: 10.1093/ajcn/51.2.225; PUBMED: 2407097] [DOI] [PubMed] [Google Scholar]
Ghishan 1984
- Ghishan FK. Transport of electrolytes, water, and glucose in zinc deficiency. Journal of Pediatric Gastroenterology and Nutrition 1984;3(4):608‐12. [PUBMED: 6090631] [DOI] [PubMed] [Google Scholar]
Golden 2004
- Golden, MH. Malnutrition. In Textbook of Pediatric Gastroenterology and Nutrition. In: Guandalini S editor(s). Malnutrition.. London: Taylor & Francis, 2004:489–524. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 5 June 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haider 2009
- Haider BA, Bhutta ZA. The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food Nutrition Bulletin 2009;30(1 Suppl):S41‐59. [DOI: 10.1177/15648265090301S104; PUBMED: 19472601] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ibs 2003
- Ibs KH, Rink L. Zinc‐altered immune function. Journal of Nutrition 2003;133(5 Suppl 1):1452S‐6S. [DOI: 10.1093/jn/133.5.1452S; PUBMED: 12730441] [DOI] [PubMed] [Google Scholar]
Imdad 2011
- Imdad A, Bhutta ZA. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta‐analysis of studies for input to the lives saved tool. BMC Public Health 2011;11 Suppl 3:S22. [DOI: 10.1186/1471-2458-11-S3-S22; PUBMED: 21501440] [DOI] [PMC free article] [PubMed] [Google Scholar]
IZiNCG 2004
- International Zinc Nutrition Consultative Group (IZiNCG), Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, et al. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutrition Bulletin 2004;25(1 Suppl 2):S130–62. [PUBMED: 18046856] [PubMed] [Google Scholar]
Jimenez‐Morán 2013
- Jiménez‐Morán E, Bacardí‐Gascón M, Jiménez‐Cruz A. Efficacy of zinc on lineal growth on Latin American children younger than 5; systematic review [Efecto del zinc sobre el crecimiento lineal en menores de cinco años de Latinoamérica; revisión sistemática]. Nutricion Hospitalaria 2013;28(5):1574‐9. [DOI: 10.3305/nh.2013.28.5.6771; PUBMED: 24160218] [DOI] [PubMed] [Google Scholar]
Jones 1981
- Jones PE, Peters TJ. Oral zinc supplements in non‐responsive coeliac syndrome: effect on jejunal morphology, enterocyte production, and brush border disaccharidase activities. Gut 1981;22(3):194‐8. [PUBMED: 7227852] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2011
- King JC. Zinc: an essential but elusive nutrient. American Journal of Clinical Nutrition 2011;94(2):679S–84S. [DOI: 10.3945/ajcn.110.005744; PUBMED: 21715515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2014
- Krebs NF, Miller LV, Hambidge KM. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatric Internaitonal Child Health 2014;34(4):279‐88. [DOI: 10.1179/2046905514Y.0000000151; PUBMED: 25203844] [DOI] [PubMed] [Google Scholar]
Liu 2017
- Liu X, Piao J, Zhang Y, He Y, Li W, Yang L, et al. Assessment of zinc status in school‐age children from rural areas in China nutrition and health survey 2002 and 2012. Biological Trace Element Research 2017;178(2):194‐200. [DOI: 10.1007/s12011-016-0922-x; PUBMED: 28101714] [DOI] [PubMed] [Google Scholar]
Mayo‐Wilson 2014
- Mayo‐Wilson E, Junior JA, Imdad A, Dean S, Chan XH, Chan ES, et al. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD009384.pub2] [DOI] [PubMed] [Google Scholar]
Nissensohn 2013
- Nissensohn M, Villegas AS, Lugo DF, Sánchez PH, Alonso JD, NM Lowe, et al. Effect of zinc intake on serum/plasma zinc status in infants: a meta‐analysis. Maternal & Child Nutrtrition 2013;9(3):285‐98. [DOI: 10.1111/mcn.12045; PUBMED: 23647725] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravaglia 2000
- Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, Mariani E, et al. Effect of micronutrient status on natural killer cell immune function in healthy free‐living subjects aged ≥ 90 years. American Journal of Clinical Nutrition 2000;71(2):590‐8. [DOI: 10.1093/ajcn/71.2.590; PUBMED: 10648276] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandstead 1991
- Sandstead HH. Zinc deficiency: a public health problem?. American Journal of Diseases in Children 1991;145(8):853‐9. [PUBMED: 1858720] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shankar 1998
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. American Journal of Clinical Nutrition 1998;68(2 Suppl):447S‐63S. [DOI: 10.1093/ajcn/68.2.447S; PUBMED: 9701160] [DOI] [PubMed] [Google Scholar]
Strand 2002
- Strand TA, Chandyo RK, Bahl R, Sharma PR, Adhikari RK, Bhandari N, et al. Effectiveness and efficacy of zinc for the treatment of acute diarrhea in young children. Pediatrics 2002;109(5):898‐903. [PUBMED: 11986453] [DOI] [PubMed] [Google Scholar]
Walker 2009
- Walker FC, Ezzati M, Black RE. Global and regional child mortality and burden of disease attributable to zinc deficiency. European Journal of Clinical Nutrition 2009;63(5):591‐7. [DOI: 10.1038/ejcn.2008.9; PUBMED: 18270521] [DOI] [PubMed] [Google Scholar]
Walker 2013
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405‐16. [DOI: 10.1016/S0140-6736(13)60222-6; PUBMED: 23582727] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weimar 1978
- Weimar VM, Puhl SC, Smith WH, tenBroeke JE. Zinc sulfate in acne vulgaris. Archives of Dermatology 1978;114(12):1776‐8. [PUBMED: 153730] [PubMed] [Google Scholar]
Wessells 2012
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLOS One 2012;7(11):e50568. [DOI: 10.1371/journal.pone.0050568; PUBMED: 23209782] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Guidelines for the treatment of malaria, 2010. whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf (accessed 28 September 2012).
Wieringa 2015
- Wieringa FT, Dijkhuizen MA, Fiorentino M, Laillou A, Berger J. Determination of zinc status in humans: which indicator should we use?. Nutrients 2015;7(5):3252‐63. [DOI: 10.3390/nu7053252; PUBMED: 25954900] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank
- World Bank. World Bank country and lending groups. data.worldbank.org/about/country‐and‐lending‐groups (accessed prior to 14 March 2019).
Yakoob 2011
- Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, et al. Preventive zinc supplementation in developing countries: Impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11 Suppl 3:S23. [DOI: 10.1186/1471-2458-11-S3-S23; PUBMED: 21501441] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hassan 2012
- Hassan A, Lassi ZS, Bhutta ZA. Zinc supplementation for the promotion of growth and prevention of infections in infants less than six months of age. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010205] [DOI] [PMC free article] [PubMed] [Google Scholar]


