Abstract
Spermatogonial stem cells (SSCs) are the only adult stem cells capable of passing genes onto the next generation. SSCs also have the potential to provide important knowledge about stem cells in general and to offer critical in vitro and in vivo applications in assisted reproductive technologies. After century-long research, proof-of-principle culture systems have been introduced to support the in vitro differentiation of SSCs from rodent models into haploid male germ cells. Despite recent progress in organotypic testicular tissue culture and two-dimensional or three-dimensional cell culture systems, to achieve complete in vitro spermatogenesis (IVS) using non-rodent species remains challenging. Successful in vitro production of human haploid male germ cells will foster hopes of preserving the fertility potential of prepubertal cancer patients who frequently face infertility due to the gonadotoxic side-effects of cancer treatment. Moreover, the development of optimal systems for IVS would allow designing experiments that are otherwise difficult or impossible to be performed directly in vivo, such as genetic manipulation of germ cells or correction of genetic disorders. This review outlines the recent progress in the use of SSCs for IVS and potential in vivo applications for the restoration of fertility.
Keywords: spermatogonial stem cells, germline stem cells, male germ cells, in vitro culture, in vitro spermatogenesis, male infertility
1. Introduction
Spermatogonial stem cells (SSCs) are a small group of testis cells, residing at the basal membrane of seminiferous tubules. They can undergo measured mitotic divisions to balance self-renewal and formation of exponentially increasing numbers of differentiating germ cells. What makes SSCs unique among all other adult stem cells is their potential for contributing genes to the next generation. Due to their fundamental role in maintaining spermatogenesis, SSCs ensure a supply of millions to billions of sperm per day throughout the reproductive life of a male. SSCs produce cohorts of daughter germ cells to undergo synchronized, sequential, and extensive differentiation processes to be transformed from a typical spherical cell into self-propelling vehicle systems with only one mission, to deliver the male haplotype into the female counterpart, the haploid oocyte [1,2]. Considerable insight has been gained in the past three decades about the functional potential of SSCs in initiating spermatogenesis, and even their potential to be driven into pluripotency for use as an alternative to embryonic stem (ES) cells. However, we are still far from sufficient understanding of SSCs and realizing their full potential in assisted reproductive technologies and stem cell therapy. This is largely due the difficulty of unequivocal identification of SSCs and the complexity of replicating their differentiation properties and function in vitro [3,4].
Since SSCs are very rare in the testis, development of effective and efficient in vitro culture systems that support the maintenance and expansion of SSCs is crucial for their characterization and manipulation. Moreover, optimal conditions for the in vitro culture and propagation of SSCs are also needed to help boost the potential therapeutic application of SSCs in fertility preservation or restoration. Long-term culture of rodent SSCs is well demonstrated; however, relatively few in vitro culture conditions have been examined for primate SSCs [2]. Any potential in vivo application of cultured human SSCs, such as in restoration of fertility, requires extensive studies to ensure their safety and efficacy. The establishment of efficient culture conditions for human SSCs also necessitates the availability of proper animal models, for instance, xenotransplantation techniques to assess the quality and quantity of such cultured SSCs; these assays have so far been used sporadically for testing primate SSCs [4].
Spermatogenesis is a complex process of germ cell proliferation and differentiation that requires extensive interactions among different cell types, hormones, growth factors, and various other signals, making it difficult to be replicated in vitro. There are several objectives for establishing an optimal and efficient culture system to recapitulate the process of germ cell development in vitro. This includes the study of basic requirements of male germ cell development, proliferation, differentiation, and production of haploid germ cells in a controlled in vitro environment. Additionally, such culture systems could be potentially used to produce haploid male germ cells from undifferentiated germ cells isolated from the testis of infertile adult patients and/or testicular biopsies collected from prepubertal cancer patients before undergoing gonadotoxic treatment. The establishment of culture systems for in vitro spermatogenesis (IVS) would also enable experimentations that are otherwise difficult to be performed directly in vivo such as pharmaceutical or toxicological study of new drugs or potential toxicants on human spermatogenesis. Other applications include the study of mechanisms of testicular tumors, genetic causes of male infertility, or even correction of genetic disorders causing infertility. Current approaches to IVS can be divided into three general categories including organ/tissue culture, and two-dimensional (2D) or three-dimensional (3D) cell suspension culture systems. Moreover, developing an efficient IVS model can be of benefit not only from a research perspective but also from an animal ethics point of view by using non-animal models.
This article summarizes the progress made in offering efficient methods for SSC isolation, characterization, in vitro culture, and in vitro differentiation, particularly using primate models. Moreover, in this article we review some of the salient proposed approaches for the preservation and restoration of fertility in prepubertal and pubertal patients using currently-available and potential future SSC-driven biotechnological strategies.
2. Spermatogonial Stem Cells (SSCs)
Male germline stem cells include SSCs, as a better-known component, as well as two earlier cellular stages, namely primordial germ cells (PGCs) and gonocytes. All mammalian germ cells originate from PGCs which are the primary cells of the germline lineage in both male and female embryos. PGCs first appear as a small population of alkaline phosphatase-expressing cells at ~7.5–8 days post-coitum (dpc) in rodents and at ~three weeks gestation in humans. Drawn by chemotaxis, PGCs migrate to the genital ridge and reside in the indifferent gonad at ~11–13 dpc and 4–5 weeks gestation in rodent and human embryos, respectively. Once PGCs lose alkaline phosphatase expression, they become known as gonocytes at ~14.5 dpc in rodents and ~seven weeks gestation in human embryos. After birth, gonocytes transform into spermatogonia at ~5 days post-partum (dpp) in rodents and ~three months after birth in newborn boys [5,6].
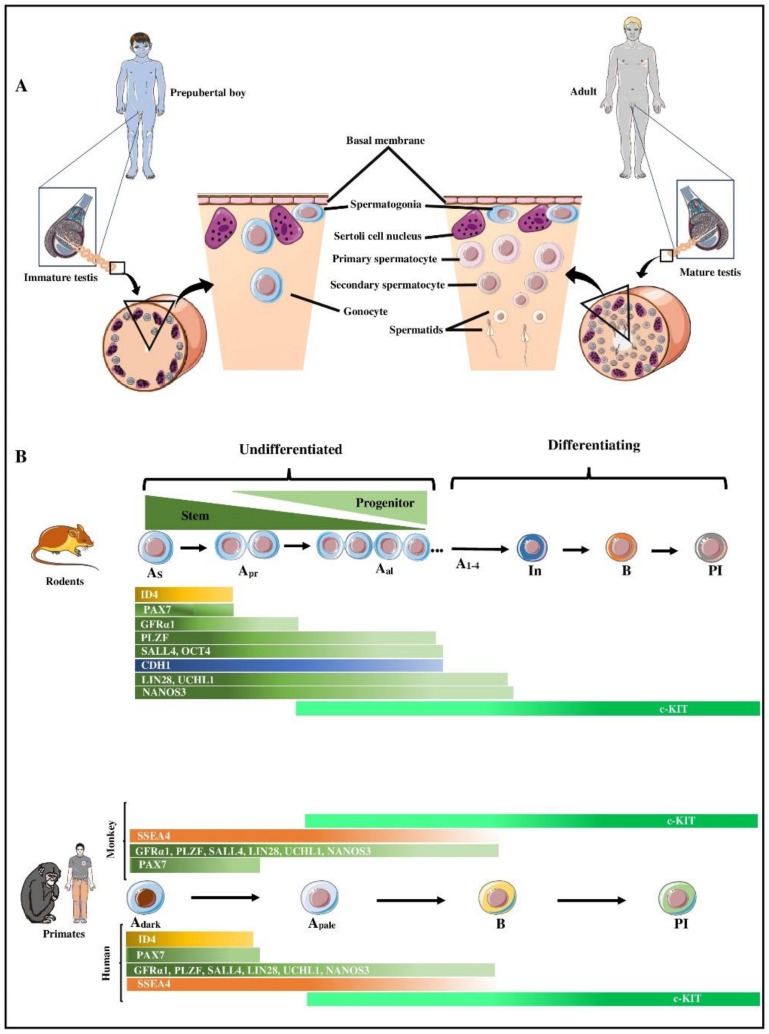
As depicted in Figure 1A, parenchyma of the postnatal testis tissue has a compartmentalized structure where germ cells along with their main supporting somatic, Sertoli, cells are enclosed in seminiferous tubules (or cords when they lack a lumen in immature testes), surrounded by peritubular myoid cells. The space between the tubules/cords is occupied by androgen producing Leydig cells, along with other components of the connective tissue. Spermatogenesis involves various interactions between somatic cells and germ cells. The process of spermatogenesis begins with spermatogonia that reside at the basal membrane which undergo a proliferation phase, comprised of a series of mitotic divisions to exponentially increase the number of germ cells, before undergoing meiosis to be transformed into haploid germ cells, spermatids and sperm. This process is repeated regularly at pre-determined intervals to ensure a continuous supply of sperm is present at any given time during the reproductive life of an adult male. Based on morphological studies using rodent models, spermatogonia in non-primate mammals have been proposed to include stem cell (Asingle), proliferative (Apaired, Aaligned), and differentiating (A1, A2, A3, A4, intermediate, and B) spermatogonia.
Figure 1.
Schematic representation of various spermatogenic cells present in the prepubertal and adult testis, as well as comparative representation of spermatogonial markers in rodents and primates. (A) A schematic pie slice of a seminiferous cord/tubule of a human prepubertal and an adult showing the different types of spermatogenic cells present. Gonocytes or spermatogonia (depending on age) are the only types of germ cells present in the testis of prepubertal boys, whereas in the adult testis all stages of spermatogenic cells can be present. Spermatogonia reside at the basal membrane and are intra-tubularly surrounded by Sertoli cells. Spermatogonia undergo differentiation into spermatocytes, spermatids, and ultimately sperm. (B) Comparative developmental patterns and expression profile of select spermatogonial markers in rodents and primates. Rodents and primates have several common spermatogonial markers (green color-coded) with few exceptions (blue, orange, and gold-coded). For a more complete list of the currently-known molecular markers of rodent and primate spermatogonia see Table 1. As: Type A-single spermatogonia; Apr: Type A-paired spermatogonia; Aal: Type A-aligned spermatogonia; Adark: Type A spermatogonia with dark nuclei; Apale: Type A spermatogonia with pale nuclei; In: intermediate spermatogonia; B: Type B spermatogonia; Pl: preleptotene spermatocytes.
Type Asingle spermatogonia are present as individual half-moon-shaped cells in close proximity to the basal membrane [1,7]. Being the most primitive spermatogonia, Asingle spermatogonia are believed to be SSCs which can undergo a symmetric division to produce two separate, but otherwise similar, self-renewing Asingle (SSCs) to increase their numbers and form a stem cell pool, for instance to regenerate steady-state spermatogenesis after cytotoxic insult. Alternatively, and during steady-state spermatogenesis, SSCs can undergo an asymmetric division to produce a self-renewing Asingle to maintain the stem cell pool and a progenitor Asingle, destined for differentiation. Each progenitor Asingle can then divide to produce two cells which remain connected, hence referred to as Apaired spermatogonia [8]. All subsequent generations of germ cells also remain connected through intercellular cytoplasmic bridges, thought to be important for the coordination of their progression in the differentiation process. Hence, clones of 4-16 or sometimes 32 germ cells are formed in a chain, known as Aaligned spermatogonia [1,7].
Type A spermatogonia that belong to the Asingle, Apaired, and Aaligned stages are collectively referred to as undifferentiated spermatogonia, but transplantation studies have concluded that they are indeed a heterogenous population. An adult mouse testis contains ~35,000 Asingle out of a total number of ~330,000 undifferentiated spermatogonia. Although Asingle are typically designated as ‘true’ SSCs, functional assays have shown that only an estimated 8.5%–17% of all Asingle have stem cell potential. In other words, >80% of Asingle are in fact not SSCs; however, it has been suggested that stem cell activity is strongest but not restricted to Asingle. Although an exact point of no-return has not been determined, it is believed that the SSC activity sharply declines as spermatogonia progress into Apaired, and especially into Aaligned. The same functional assays conclude that only ~3000–6000 ‘functional’ SSCs exist in an adult mouse testis, comprising 0.9%–1.8% of all undifferentiated spermatogonia or 0.01%–0.02% of total cells in the seminiferous tubules [9].
Larger clones of Aaligned spermatogonia differentiate without a cellular division to A1 spermatogonia and initiate synchronized further mitotic divisions to produce types A2, A3, A4, intermediate, and B spermatogonia. While under conventional histological preparations various undifferentiated spermatogonia show similar cell-nuclear morphology, transition of Aaligned into A1 signals the start of differentiation and involves major changes in morphology and mitotic behavior. Type B spermatogonia must physically pass through the Sertoli-cell barrier (also known as the blood-testis barrier), before dividing to produce primary spermatocytes which in turn undergo two meiotic divisions to give rise to secondary spermatocytes and round spermatids, before undergoing spermiogenesis to produce sperm [4,7,9,10].
In primate testes, categorization of spermatogonia also includes type A and B spermatogonia but especially for type A spermatogonia it differs and includes Adark and Apale. Designation as Adark is primarily based on their dark nuclear staining with hematoxylin. Adark are believed to comprise a population of ‘reserve’ stem cells since they show low proliferative activity during normal spermatogenic activity. In contrast, Apale are less densely stained during histological processing and are thought to proliferate continuously. Although, both Adark and Apale reside at the basal membrane, they have other morphological differences; Adark are characterized as being relatively small, round or slightly ovoid, while Apale are relatively larger, oval or almost round cells. Subpopulations of Adark have been shown to contain 1–3 chromatin-free vacuolar spaces known as nuclear rarefaction zones [11], which can be used as a background morphological indicator to help discriminate spermatogonial subtypes undergoing immunohistochemical analyses.
In 1959, Clermont proposed that Adark are SSCs which undergo self-renewal to maintain their population and at the same time also give rise to Apale that subsequently generate differentiating B spermatogonia. Ten years later, Clermont revised his model based on the incorporation of 3H-thymidine in Apale instead of Adark spermatogonia of vervet monkeys, suggesting that Apale are the ‘active’ stem cells that maintain spermatogenesis in the adult testis [12]. In humans, a similar model has been proposed, where Apale act as active stem cells to maintain their population and produce differentiating B spermatogonia. However, some believe that the original model was more accurate, stating that Adark are indeed true SSC because of their low mitotic index, while nearly all Apale undergo regular divisions suggesting that these cells are the ‘renewing progenitors’ that amplify spermatogonial output to B1. Primate Apale and Adark spermatogonia are considered as counterparts of rodent undifferentiated spermatogonia (Asingle, Apaired, and Aaligned), and primate type B spermatogonia as equivalents of rodent differentiating spermatogonia (A1–A4, intermediate and B spermatogonia) (Figure 1B). Interestingly, the rhesus monkey testis contains approximately the same total number of Adark and Apale as in the human [13,14].
In humans, Apale divide every 16 days, the length of one seminiferous epithelium cycle, and repeat it ~4.5 times; hence the germ cells that resulted from the first division of Apale become sperm after ~75 days [15]. A low proliferative activity of SSCs is deemed beneficial [16], because it decreases the chance of errors in DNA duplication during the S phase. While the number of spermatogonial divisions from SSCs to spermatocytes is comparable between the monkey and non-primates, this number is lower in humans compared with many non-primates. In humans, there is only one spermatogonial generation between the A1 and B stages [17], while there are six divisions separating these stages in most non-primate mammals [18].
3. Characteristics of Spermatogonia in Primates
From the above discussion it can be concluded that SSCs are a rare and heterogenous subpopulation of undifferentiated spermatogonia that can only be defined by their function, not morphology. Hence, there is a need to identify specific biomarkers that allow isolation and propagation of SSCs to better gain insight into their biology and harness their potential. In response to this need, in 1994 using mouse models, a technique for transplantation of germ cells was developed which can be viewed as a unique bioassay to assess the stem cell activity of putative SSCs in any given population of testis cells.
Germ cell transplantation involves the microinjection of testis cells from a donor male into the seminiferous tubules of a recipient male. This transplantation technique has also been extended to various species, but performing primate-to-primate germ cell transplantation is more technically challenging. Although, primate-to-mouse germ cell transplantation has been shown to allow evaluation of primate spermatogonia, it provides limited information regarding the identity of primates SSCs. Nevertheless, this assay has ever since been used as a routine method for the identification, quantification, characterization, and confirmation of functional competence of SSCs. Our recent knowledge of the molecular signature and biomarkers of SSCs, especially in rodents, has allowed enrichment of testis cells for SSCs to tens- or even hundreds of folds; however, still no unequivocal and exclusive SSC marker is available. Therefore, identification of SSC markers in primates remains as one of the areas of intense research in male reproductive science and medicine [4,19,20,21].
Analysis of monkey spermatogonia demonstrated that they share certain germ cell and SSC markers with rodent spermatogonia (Figure 1B, Table 1). Some of these markers are expressed on the cell surface and hence can be potentially used for isolation and enrichment through cell sorting methods, such as fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS). Other spermatogonial markers are located intracellularly and can only be used for retrospective identification of SSCs.
Table 1.
Molecular markers of undifferentiated spermatogonia in primates and mice.
| Markers | Mouse | Monkey | Human |
|---|---|---|---|
| Cell Surface Markers | |||
| THY1 (CD90) | + [30] | + [44] | + [32] |
| β1-integrin (ITGB1, CD29) | + [29] | + [45] | + [46] |
| α6-Integrin (ITGA6, CD49f) | + [29] | + [44] | + [32] |
| PROM1 (CD133) | ? | ? | + [32] |
| GFRα1 | + [47] | + [28] | + [32] |
| CDH1 | + [39] | ? | ? |
| SSEA4 | ? | + [44] | + [48] |
| FGFR3 | + [49] | ? | + [50] |
| DSG2 | ? | ? | + [50] |
| LPPR3 | ? | ? | + [51] |
| TSPAN33 | ? | ? | + [51] |
| CD9 | + [31] | ? | + [52] |
| GPR125 | + [35] | ? | + [36] |
| Intracellular Markers | |||
| PAX7 | + [53] | + [53] | + [53] |
| DDX4 (VASA) | + [54] | + [25] | + [55] |
| DAZL | + [13] | + [25] | + [33] |
| CHK2 | ? | ? | + [43] |
| LIN28 | + [22] | + [23] | + [23] |
| MAGEA4 | ? | + [56] | + [57] |
| NANOS2 | + [58] | + [59] | + [60] |
| NANOS3 | + [60] | + [59] | + [60] |
| SALL4 | + [61] | + [27] | + [24] |
| UCHL1 (PGP9.5) | + [62] | + [24] | + [33] |
| PLZF | + [63] | + [25] | + [33] |
| POU5F1 (OCT4) | + [64] | + [56] | + [65] |
| ID4 | + [34] | ? | + [36] |
| NEUROG3 (NGN3) | + [66] | + [28] | ? |
| DMRT1 | + [67] | ? | + [68] |
| UTF1 | + [69] | + [23] | + [70,71] |
| TCF3 | ? | ? | + [33] |
| SPOCD1 | ? | ? | + [68] |
| ENO2 | ? | + [72] | + [70] |
| SNAP91 | ? | ? | + [50] |
| CBL | ? | ? | + [50] |
| PIWIL4 | ? | ? | + [51] |
| ZKSCAN2 | ? | ? | + [68] |
| RET | + [37] | ? | ? |
| STRA8 | + [38] | ? | ? |
| RBM | + [73] | ? | + [74] |
| TSPY | − [42] | ? | + [41] |
“+” or “−“ indicate that evidence is available on the presence or absence of these markers, respectively; “?” indicates that we are not aware of studies that have examined these markers.
Mouse gonocytes and spermatogonia express LIN28 throughout testicular development [22]. The analysis of newborn macaques (Macaca mulatta) testes also revealed that 100% of seminiferous tubules contained LIN28+ germ cells; however, the number of immune-positive cells decreased during testicular development. On the other hand, although several LIN28+ tubular cross-sections in adult mouse testes can be detected, only a few LIN28+ tubular cross-sections can be observed in adult marmoset, rhesus, or human testes [23]. Marmoset gonocytes and spermatogonia have also shown expression for SALL4, where the proportion of SALL4+ germ cells decreased during puberty and was restricted to Adark and Apale spermatogonia in pubertal and adult testes. The expression of SALL4 was demonstrated in the majority of gonocytes in fetal human testes and type A spermatogonia of 1-year-old boys [24]. Adult rhesus testicular tissues also showed the expression of DDX4 (VASA), DAZL, GFRα1, and PLZF [25]. Interestingly, the number of PLZF+ cells was calculated to be ~1.86 per cross-section, suggesting that the SSC population in monkey testes is a subset of either the Adark or Apale spermatogonia. Other known markers of non-human primate spermatogonia include DPPA4 [26], TRA-1-60, TRA-1-81, [27], and THY1 [28].
Similar to non-human primates, spermatogonia and their progenitors in humans and rodents also share some but not all markers (Figure 1B, Table 1). For instance, in mice, α6-integrin, β1-integrin [29], and THY1(CD90) [30] are well-known surface markers of SSCs/progenitor cells, while CD9 is a surface marker of both rat and mouse SSCs [31]. Surface markers including α6-integrin, CD90, GFRα1, and CD133 have also been successfully used to select human spermatogonia using MACS [32]. The expression of PLZF has also been observed in whole mounts of seminiferous tubules of human testes [33]. Additionally, ID4 [34] and GPR125 are considered markers for mouse spermatogonia and their progenitors [35], and their expression has also been observed in human spermatogonia [36]. In contrast, some other markers of rodent spermatogonia and their progenitors may not be conserved in humans. For example, it remains to be explored whether certain markers of rodent spermatogonia such as RET [37], STRA8 [38], CDH1 [39], and NEUROG3 (NGN3) [40] are also present in human spermatogonia. Conversely, certain specific markers of human spermatogonia have not been observed in rodents. For example, TSPY, a specific marker for human spermatogonia [41] is not expressed by rodent spermatogonia; however, elongated spermatids of rats are positive for TSPY [42]. Similarly, other markers of human spermatogonia such as CD133 [32] or CHK2 [43] are yet to be examined for expression in rodents. Such studies can further reveal similarities and differences between spermatogonia in rodents and primates.
4. Isolation and Enrichment of SSCs in Primates
Because SSCs are a very rare subpopulation of testis cells, their use in downstream applications requires optimal isolation and purification, as an important first step. In addition to the need for large numbers of SSCs for applications such as transplantation into recipient testes, access to additional SSCs is also warranted for critical analysis of cultured cells in terms of genetic and epigenetic stability as well as functionality.
Testis cells can be isolated using enzymatic digestion, usually involving the use of a combination of enzymes in two steps. In brief, after removing the tunica albuginea and excess connective tissue, the testis parenchyma is divided into smaller fragments to be first incubated with collagenase to disperse the seminiferous tubules, followed by the addition of trypsin to obtain a single-cell suspension. If necessary, DNase-I is also added to prevent adhesion of the resultant cells. Two-step enzymatic digestion protocols have been widely used for digestion of testis tissue from non-human primates [44] and humans [75]. Since this digestion method is not an optimized or cell-targeted process, it generates a mixed population of testis cells where SSCs are present at low numbers. Therefore, it would be ideal if modified protocols were to be developed for digestion of primate testis tissue to favor the isolation toward collecting more germ cells. This optimization can be similar to a three-step digestion protocol that we developed specifically for neonatal pig testes which yields ~40% highly-viable gonocytes within an hour [76]. In the absence of optimized digestion protocols, the obtained testis cell suspensions would typically need to go through further enrichment steps to reduce the number of contaminating somatic cells or advanced stages of germ cells.
Enrichment of germ cells can be achieved with or without the use of antibodies. Specific antibodies may be used to separate SSCs (positive selection) or to exclude contaminating cells (negative selection) using FACS or MACS. For instance, MACS with an anti-GFRα1 antibody has been used for the isolation of spermatogonial subpopulations from adult monkey and human testes. Gassei et al. (2010) reported that about two thirds of GFRα1-positive cells found in the sorted fractions resembled Adark spermatogonia, while one third resembled the Apale [77], suggesting that most of the isolated cells were SSCs. MACS has also been successfully employed for the enrichment of human spermatogonia using antibodies against GPR125 [33] and SSEA4 [48]. Nickkholgh et al. (2014) concluded that MACS for ITGA6+ and HLA−/GPR125+ can be used for efficient enrichment of human spermatogonia [78]. The use of FACS to select OCT4-positive cells has also resulted in obtaining 87% purity of human SSCs [79]. Compared to FACS, MACS is considered a more efficient, accurate, and easier method for enrichment of SSCs, especially since it does not require large numbers of cells. Thus, MACS is better suited for applications such as isolating SSCs from cells that result from small biopsies of human testis tissue.
Germ cell enrichment strategies that do not implement antibodies rely on the innate cellular characteristics that differentiate germ cells from somatic cells. Such strategies include the affinity of different cell types for adhesion to the culture plate or to extracellular matrices (ECM). Since Sertoli cells adhere to the culture plate faster than SSCs, differential planting is a widely-used technique for the separation of germ cells from somatic cells. Alternatively, differences in velocity sedimentation or density gradient centrifugation can be used to separate somatic and germ cells. Percoll density gradient for instance has been used for cell separation of human testis cells leading to ~87% pure population of SSCs [79]. However, adjusting the timing for cell sedimentation is critical to prevent the loss of large numbers of SSCs during the high activity of the sedimentation process [80].
5. In Vitro Culture of Primate Gonocytes/Spermatogonia
Culture of rodent gonocytes/spermatogonia is well demonstrated [81]; however, especially long-term culture and expansion of primate SSCs, and conclusive demonstration of their SSC potential has been challenging. Establishment of an efficient in vitro culture system to maintain both the self-renewal and proliferation capacity of human SSCs is crucial for their potential clinical applications. Culturing human SSCs, isolated from testicular biopsies of obstructive azoospermia patients (aged 22–35 years), in StemPro-34 SFM (serum free medium) containing growth factors (commonly referred to as a serum-free germline culture medium) and hydrogel, resulted in significant propagation of SSCs for two months. During the culture, expression of numerous SSC markers was maintained, suggesting that SSCs retained their undifferentiated status [82]. Moreover, similar culture conditions appear to also support the short-term in vitro culture of human SSCs from healthy individuals without altering their undifferentiated status [33].
The media initially used to culture human hematopoietic stem cells also supported the long-term culture of human SSCs [83]. The growth factors used in the latter study (i.e., GDNF, BFGF, EGF, and LIF) were previously reported to support the long-term culture of rodents SSCs [84]. In these culture conditions, human testicular cells formed two types of cell colonies; one had a flat morphology and an appearance similar to ES cells, while the other had rounded morphology. Morphologically, round cells showed an expression profile typical of spermatogonia (e.g., PLZF, ITGA6, and ITGB). The SSC clusters presented as clumps of individually visible cells, while colonies of ES-like cells were sharp-edged and compact. In these culture conditions, human SSCs could be propagated for 15 weeks, after which germline stem cell clusters no longer appeared, and ES-like cells and somatic cells started detaching from the dish. It was also found that sub-culturing human SSCs under feeder cell-free conditions in laminin-coated culture dishes could increase the duration of propagation (up to 20 weeks). Xenotransplantation assays showed an impressive 18,450-fold increase in human SSC numbers within a time frame of 64 days [83].
The germline culture medium also supported the short-term in vitro culture of non-human primate SSCs [24], while Stem-Pro medium, supplemented with four growth factors (i.e., bFGF, GDNF, LIF, and EGF), actively maintained the SSCs isolated from prepubertal cynomolgus monkeys (aged 44–57 months) [85]. However, the lack of using functional assays to verify human SSCs in most studies, and similarities between SSC markers of human and rodents in other studies make the results controversial. Moreover, many markers used to identify human SSCs (e.g., PLZF, GFRA1, UCHL1, GPR125, and ITGA6) can also be observed in many testicular somatic cells, suggesting that these markers are not reliable. The only dependable protocol to access whether cultured SSCs are indeed functional germ cells is homologous transplantation of in vitro-cultured SSCs into a recipient testis (of another male of the same species) and subsequent generation of offspring. However, due to obvious ethical concerns, this approach is not practical in humans and difficult in monkeys. In non-human primates, different stage-specific germ cell markers have been better-defined compared with humans. Therefore, to validate the true nature of germ cells in the culture of non-human primates, monitoring of validated germ cell markers can be used to establish an efficient in vitro culture condition. Development of a definitive functional assay to evaluate the competency of human SSCs or to identify reliable SSC-specific markers would facilitate finding a culture condition for long-term in vitro maintenance of SSCs.
6. In Vitro Models of SSC Differentiation
Establishing efficient in vitro culture systems that can replicate the process of male germ cell development and spermatogenesis has several important applications. These applications include the study of basic requirements, mechanisms of action, and cell-to-cell interactions of male germ cells during development, proliferation, differentiation, and production of haploid germ cells in a controlled in vitro environment. For instance, the use of germ cells from infertile patients may help investigations into the various causes of male infertility due to stage-specific blockage of germ cell differentiation. Development of efficient culture systems for IVS would also allow experimentations that are otherwise difficult to be performed directly in vivo, such as genome editing of germ cells or correction of genetic causes of infertility. Such models can be of benefit not only from a research perspective but also from an animal ethics view by using non-animal models. Various research groups have taken different routes toward the goal of developing an in vitro culture system; these can be classified into one of three general approaches, namely organotypic culture of testicular tissue fragments, and two-dimensional or three-dimensional culture of testis cell suspensions.
6.1. Organotypic Culture of Testicular Tissue Fragments
The unique three-dimensional structure of seminiferous cords/tubules seems essential in facilitating the required interactions between germ cells and somatic cells. This partly includes providing a proper niche for SSCs to reside, and establish or maintain spermatogenesis. Thus, it makes sense to first try to culture small fragments of testis tissue or intact pieces of semiserious tubules; indeed, this is what many of the pioneering groups have done in the past and many current research labs are pursing. Organotypic culture of tissues and organs is, however, challenging because of the issues arising from the limited diffusion rates of the tissue, especially compared with monolayer cell cultures. As a result, a main hurdle in achieving IVS using testicular tissue fragments is maintenance of the tissue viability for the required length of culture.
In 1959, Trowell designed an organ culture system by employing a gas-liquid interface in which testicular fragments from adult rats could be maintained in Eagle’s minimum essential media (MEM) at 37 °C with 5% CO2 in air, albeit only for 6 days [86]. In 1964, another group using the same organ culture system was able to maintain testicular fragments of 4-day-old rats viable for four weeks, although cell differentiation was not observed [87], but in the same year they also reported differentiation of spermatogonia to spermatocytes within 2-3 weeks of culture [88]. The effects of temperature, pH, vitamins, hormones, sera, and tissue extracts were also examined; however, developmental progress was still limited up to pachytene spermatocyte [89]. These latter culture conditions were also used to maintain human testicular tissues in vitro for several weeks [90]. It was then shown that FSH is required for germline differentiation and more specifically in the conversion of type A spermatogonia into meiotic pachytene spermatocytes [91]. In 2003, Suzuki and Sato used a gas-liquid interface culture system that was developed decades earlier [92] with minimal changes to culture 5-day-old mouse testis tissue, but interestingly, round spermatids could be observed after two weeks of culture [93].
A dramatic turn in the long-standing efforts for replicating complete IVS took place when in 2011, Sato et al. reported successful production of offspring from in vitro produced haploid germ cells [94]. Testicular fragments of neonatal mice were cultured on agarose gel (half-socked in medium) in α-MEM supplemented with knockout serum replacement (KSR) or 40 mg/mL AlbuMAX instead of fetal bovine serum (FBS). Interestingly, this culture system was also successful for in vitro production of haploid male germ cells from the testis tissue of an infertile mutant mouse model [95] as well as after cryopreservation of the testis tissue [96]. The agarose gel-based organ culture system also led to the production of haploid male germ cells from adult mouse donors; however, the efficiency was far lower than that from neonatal donor mice [97].
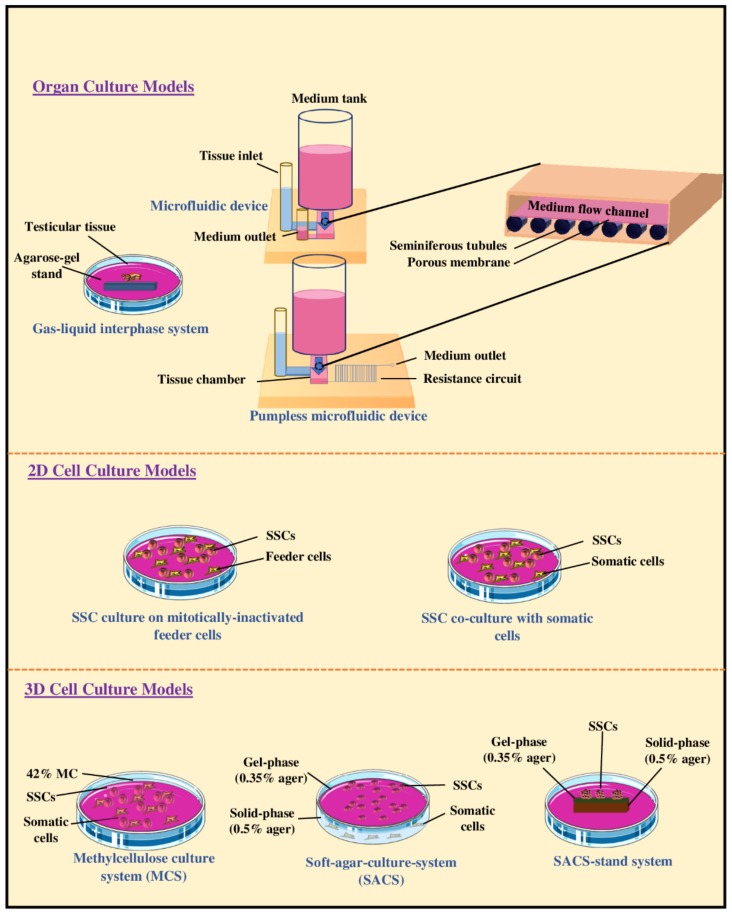
Subsequently, to improve the efficiency and duration of in vitro spermatogenesis (IVS), the agarose gel-based organ culture system was modified and a microfluidic technology was adopted for organ culture systems (Figure 2). The main rationale for using a microfluidic system was to encourage the exchange of substances such as gases, nutrients, and waste products by facilitating diffusion and creating conditions that are more representative of in vivo. Surprisingly, the microfluidic device allowed the maintenance of mouse testicular tissue for ~six months, prompted the induction of spermatogenesis, and led to the in vitro production of spermatids and sperm capable of giving rise to offspring after micro-insemination [98]. Ever since, pumpless microfluidic devices have also been developed (Figure 2). These systems use hydrostatic pressure and a resistance circuit to facilitate slow flow of the medium; this has led to the induction of efficient IVS for ~three months [99]. Taken together, these results suggest that the organotypic culture of testicular tissue or fragments is capable of maintaining the architecture and viability of germ cells, and induction of IVS. Moreover, the addition of a microfluidic device has shown the potential to improve organotypic culture systems, as it can lead to long-term ex vivo maintenance of testis tissues which is required for producing sperm.
Figure 2.
Schematic representation of organotypic culture of testicular tissue fragments, two-dimensional (2D), or three-dimensional (3D) cell suspension culture models for in vitro spermatogenesis. Three approaches to organotypic culture of testicular tissue fragments have been proven effective including using a gas-liquid interface system, a conventional microfluidic system, or a pumpless microfluidic device. 2D culture of cell suspensions can be achieved through two approaches, namely culturing isolated SSCs on mitotically-inactivated feeder cells (e.g., mouse embryonic fibroblast cells) or culturing on mixed populations of testicular cells (co-culturing of germ cells and somatic cells). 3D cell cultures can include using methylcellulose (MCS), a soft-agar culture system (SACS), or a SACS-stand system.
6.2. Two-Dimensional Culture of Testis Cell Suspensions
Two-dimensional (2D) culture systems using enzymatically-dispersed testis cell suspensions have also been widely used for both in vitro proliferation of SSCs and production of haploid male germ cells (Table 2, Figure 2). Compared with organotypic cultures of testicular tissue, the 2D culture systems for testis cell suspensions have innate advantages and disadvantages. A 2D cell culture system can be potentially used to expand SSC numbers in vitro for downstream applications such as in auto-transplantation to the individual’s testis following recovery from cancer. Using 2D culture systems of testis cells is also ideal when the objective is to investigate the role of individual cell types, putative factors, or candidate genes or gene-products on fate of germ cells. However, compared with organotypic testicular tissue culture systems, 2D culture of cells has at least two disadvantages. Firstly, it does not resemble or replicate the expected cellular interactions and structural conditions of intact seminiferous tubules, and secondly, in the absence of spatial positioning and typical cellular morphology, it is difficult to distinguish different types of cells without further analyses such as the use of flow cytometry.
Table 2.
Culture conditions for long-term in vitro maintenance of rodent or primate spermatogonial stem cells (SSCs).
| Culture Conditions | Mouse | Monkey * | Human | ||||
|---|---|---|---|---|---|---|---|
| Donor age | Newborn | 4.5–7.5-day-old | 8-day-old | 3.7–4.6 years (average age) | NA | 14, 34, and 45 years | 22–35 years |
| SSC isolation method | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion | Two-step enzymatic digestion |
| SSC enrichment method | Diff. plat. | MACS | MACS | Diff. plat./un enriched | Diff. plat. | Diff. plat. + MACS | Diff. plat. + MACS |
| Basic medium | StemPro-34 SFM | Alpha-MEM | StemPro-34 SFM | Alpha-MEM | StemPro-34 SFM | StemPro-34 SFM | StemPro-34 SFM |
| Additional supplements | StemPro supplement | Supplement | StemPro supplement | NA | StemPro supplement | StemPro supplement | StemPro supplement |
| Protein/serum source | FCS | BSA | Albumax II | FBS | FCS | BSA | FBS, BSA |
| Growth factors | GDNF, bFGF, EGF, LIF | GDNF, bFGF, GFRα1 | GDNF, bFGF, EGF | NA | GDNF, LIF, EGF, bFGF | GDNF, LIF, EGF, bFGF | GDNF, LIF, EGF, bFGF |
| Feeder cells | MEF | STO | NA | NA/Somatic cells | NA | NA | NA |
| Temperature | 37 oC | 37 oC | 37 oC | 35 oC | 37 oC | 37 oC | 37 oC |
| Subculture | 10–14 days | 5–7 days | 5–6 days | NA | 7–10 days | 10–15 days | NA |
| Max. culture duration | ∼160 days | ~180 days | ~178 days | ~11 days | ~196 days | ~120 days | ~60 days |
| Germ cell clump formation | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| SSC identification | Flow cytometry | Immunostaining | Flow cytometry | Immunostaining | Immunostaining | PCR | Immunostaining |
| Functional analyses | Yes | Yes | Yes | Yes | Yes | No | No |
| Reference | [108] | [109] | [110] | [111] | [83] | [112] | [82] |
* We are unaware of reports of longer SSC cultivation times than 11 days for non-human primates.
In 1983, it was reported that germ cells isolated from 6–15 wk-old mice cultured using a 2D system were able to differentiate into spermatocytes [100]. Interestingly, in 1989, haploid male germ cells were produced from testis cell suspensions of 14-day-old rats cultured on Type 1 collagen gels in a 1:1 mixture of Ham’s F12 medium and Leibovitz’s L15 medium with 10% FBS, epinephrine, and norepinephrine [101]. In 1993, there were two reports providing evidence that immature germ cells can be directed to form haploid male germ cells using 2D culture systems [102]; however, no follow-up studies using the same protocol have been published. In 1998, Durand’s group reported that they have produced haploid male germ cells from single-cell preparations of 23–25 day-old rats cultured in DMEM-F12 supplemented with 0.2% FBS, testosterone, and FSH [103]. They attributed their success to maintaining maximal interactions between Sertoli cells and spermatogenic cells. This raised awareness among researchers as to the importance of germ cell-Sertoli cell interactions and other groups tried to maintain such cellular interactions to encourage spermatogenesis in vitro. In 2000, the same group reported further in vitro differentiation of 2D cultured isolated advanced germ cells (i.e., leptotene spermatocytes), collected from 20, 22, or 28-day-old rats to haploid male germ cells [104].
The first report of successful production of offspring from in vitro produced early-stage haploid male germ cells (i.e., round spermatids) came in 2003 [105]. Germ cells isolated from 13–18 day-old mice were cultured with Sertoli cells in a serum-free, hormone/growth factor-supplemented medium, which after 7–10 days, led to the appearance of fertilization-competent round spermatids from pre-existing spermatocytes [105]. In 2006, the importance of cell contacts between germ cells and feeder cells for IVS was highlighted, when type A spermatogonia of an immature rat (7-day-old) co-cultured with Sertoli cells differentiated to spermatids [106]. More recently, different approaches have been proposed that could direct the differentiation of SSCs all the way to haploid male germ cells. Using isolated 6-day-old mouse SSCs, production of haploid germ cells was achieved by culturing testicular cells in 10% FBS-supplemented media for 3 days, changing the media into a retinoic acid (RA)-enriched medium to induce differentiation for 2 days, and followed by changing the media again to the first medium for 6–8 days [107].
In conclusion, even a 2D cell suspension of testis cells under optimal culture conditions can induce spermatogenic differentiation; however, for this to work, cell-to-cell contact between germ cells and feeder cells appears to be important. Although, intensive research over the years has finally led to the emergence of culture conditions suitable for IVS of rodents, considerable gaps in knowledge remain in our understanding of the developmental processes involved.
6.3. Three-Dimensional Culture of Testis Cell Suspensions
The knowledge gained through intensive research on organotypic and 2D cell culture systems directed some of the research on testis cell suspensions toward the development of artificially constructed three-dimensional (3D) structures. In 2006, testicular cells isolated from 18-day-old rats were cultured on collagen gels to mimic the composition of basal membrane of seminiferous tubules. Not only did this 3D culture system increase the viability of testicular cells but it also directed the differentiation of germ cells, suggesting that cell-to-cell interactions in a 3D culture system sufficiently support the differentiation of germ cells [113]. In 2008, a soft-agar culture system (SACS) was introduced for IVS (Figure 2), which consisted of two phases based on agar concentrations; a gel phase containing enriched SSCs, and a solid phase comprising supporting cells (e.g., Sertoli cells). The expression of meiotic stage-specific markers was observed when testicular cells obtained from 10-day-old mice were mixed with the gel-agar medium (0.35%) and incubated on a solid-agar base (0.5%) [114]. In 2012, the production of haploid germ cells from undifferentiated germ cells, isolated from 7-day-old mice, was reported using the 3D SACS; however, the fertility of in vitro produced mature germ cells was not determined [115]. This latter condition also directed the differentiation of undifferentiated germ cells isolated from 13–33 month-old rhesus monkeys; an age range corresponding to the immature through pubertal and young adulthood ages [116]. A methylcellulose culture system (MCS) has also been proposed as yet another 3D culture system which appears to support the differentiation of immature germ cells into haploid male germ cells [117].
In order to artificially reproduce the in vivo form and function of the seminiferous epithelium, a 3D engineered blood-testis barrier (eBTB) system was introduced in 2010. In essence, the eBTB is aimed at providing a condition that can maintain the interactions between somatic and germ cells, deemed crucial in achieving spermatogenic differentiation. Testicular peritubular myoid cells were first cultured on the underside of culture inserts 3 days prior to adding a mixture of Sertoli and germ cells on top of the inserts, which after an additional 22 days of culture led to the formation of haploid male germ cells [118]. Yokonishi et al. (2013) reported that under these new 3D culture conditions, dissociated immature testicular cells reconstructed into structures resembling their original tissue architecture. Their new system included using testicular cells isolated from neonatal mice to be cultured in V-shaped plate wells for 2 days to allow aggregate formation, followed by placing the aggregates on top of agarose gel blocks. Interestingly, the aggregated cells formed tubule-like structures, and haploid germ cells were observed after 30–51 days of incubation [119]. Recently, a modified 3D culture method, three-layer gradient system (3-LGS), was also introduced for in vitro generation of testicular organoids using rat testis cell suspensions. In this approach, rat testis cells were suspended in Matrigel and placed between two cell-free layers of Matrigel, which after 7 days led to the formation of testicular organoids. Additionally, self-organization of testicular cells also led to eBTB formation and Sertoli cell epithelization; however, complete differentiation of germ cells was not observed [120]. These results collective suggest that maintaining the microenvironment of testicular cells even in the form of a 3D-culture system is crucial in achieving spermatogenesis ex vivo.
7. Progress of In Vitro Spermatogenesis in Primates
The first attempt at achieving IVS using human testicular biopsies was reported in 1967, in which after three weeks of culture, differentiation of primary spermatocytes from the preleptotene to pachytene stage was observed [121]. Later in 1971, testicular tissues obtained by orchidectomy due to prostate cancer were cultured, and round spermatids were the most advanced germ cells observed in the supernatant of the media used for culturing the tissue samples at 27 day of culture [90]. However, in 1981, it was reported that the latter culture system does not support complete differentiation of spermatogonia, rather only cells already committed to meiosis at the time of culture initiation can proceed through spermatogenesis [122].
The limited success of organotypic culture systems using human testis tissue in achieving IVS diverted some of the research focus toward the use of testicular cell suspensions for in vitro differentiation of germ cells. Culturing spermatids, isolated from testicular biopsies of obstructive azoospermia patients, in a culture medium containing FSH showed evidence of maturational process in the form of morphological changes in the spermatid nuclei and flagellar growth after only 48 h [123]. These results presented new hopes for treating infertilities related to germ cell maturation arrest. A year later, when in 1999, spermatids isolated from non-obstructive azoospermia patients were cultured on Vero cells for 5 days, round spermatids differentiated into elongated spermatids and a single mature spermatozoon was also observed [124]. Vero cell-conditioned medium supplemented with hormones also supported the in vitro meiosis and spermiogenesis of co-cultured somatic and germ cells isolated from testicular biopsies of non-obstructive azoospermic patients [125]. In 2003, the differentiation efficiency of primary spermatocytes, from azoospermic men with spermatogenic arrest, was tested after co-culture with Vero cells using various culture conditions. Primary spermatocytes co-cultured with Vero cells in MEM-based media, supplemented with 50% boar rete testicular fluid or in human synthetic oviduct fluid and 10% human serum, resulted in the generation of round spermatids at a rate of 10%. Moreover, the presence of 23 chromosomes and chromatids confirmed that the in vitro produced cells were indeed haploid [126]. In 2012, SSCs isolated from testicular biopsies of obstructive and nonobstructive azoospermic patients were co-cultured with Sertoli cells in a KSR-supplemented medium for 5 days, when fluorescence in situ hybridization (FISH) analysis of chromosomes confirmed the presence of haploid male germ cells [127]. However, a 5-day period of culture seems far too short to allow completion of the complex developmental process of differentiation from primary spermatocytes to round spermatids.
More recently, using an organotypic culture system, in vitro production of haploid male germ cells from immature human testicular tissue was also reported. Frozen-thawed 1-mm3 testicular fragments collected from prepubertal boys (aged 2–12 years) were cultured in a KSR-based medium supplemented with 5 IU/L of FSH, which after 16 days resulted in the formation of round spermatids. Interestingly, increasing the concentration of FSH from 5 to 50 IU/L failed to induce germ cell differentiation, perhaps due to desensitization of FSH receptors or adenyl cyclase in Sertoli cells [128]. However, it should be noted that currently such in vitro produced haploid male germ cells only serve as a proof-of-principle and would need be further analyzed for their fecundability and epigenetic characteristics before suggesting these techniques for potential clinical trials.
Using non-human primates, 3D culture models of IVS have also shown promising results. Among non-human primates, the juvenile rhesus monkey is considered a suitable model for prepubertal boys, because in both species the phase of prepubertal development is characterized by a protracted hypogonadotropic and hypoandrogenic state. These characteristics provide an ideal baseline for examining putative factors involved in the initiation of spermatogenesis. To check the efficiency of SACS and MCS culture systems in supporting the differentiation of primate germ cells, testicular cells isolated form juvenile rhesus monkeys (aged 13–33 months) were cultured in either culture system for 4–8 weeks. The MCS culture system supported the differentiation of immature testicular germ cells and after 30 days of culture, haploid male germ cells were observed [116]. However, the fertilizing potential and epigenetic characteristics of the in vitro produced haploid male germ cells were not determined.
8. Limitations of In Vitro Spermatogenesis in Non-Rodents
Despite the long history of experimentations targeting IVS, an optimal and efficient system capable of inducing complete differentiation of immature human testis cells or testicular tissue to form haploid male germ cells has not yet been developed. The gradual and incremental success using rodent models of IVS; however, is encouraging and suggest that recapitulation of human spermatogenesis will be eventually possible in the future. However, when such an IVS system is provided for use with human germ cells, extensive additional safety and ethical reviews would have to be completed before such tools can be considered for potential use in reproductive medicine.
Limited availability of basic knowledge about primate SSCs and their in vitro requirements, along with the long prepubertal period in primates compared with rodents, makes achieving IVS for primates even more challenging. Therefore, as a first step toward achieving complete IVS in primates, it is crucial to maintain the structural integrity of testicular tissue and extend the viability of isolated germ cells in long-term cultures. Moreover, obtaining sufficient amounts of donor primate testis tissue for various studies is difficult, if not impossible. Hence, the use of donor testes from other suitable non-rodent animal models such as neonatal pigs may provide an attractive alternative, given that pigs share considerably more anatomical and physiological similarities with humans than do rodents. Moreover, optimized in vitro and in vivo culture conditions for development of testicular cells and tissue from immature donor pigs have been developed to facilitate such studies [129,130,131,132].
Although, there are a few reports of in vitro production of haploid male germ cells in non-rodents, it has not yet been possible to produce morphologically normal, viable, motile, and fertilization-competent sperm from undifferentiated germ cells. This implies that cells at different stages of spermatogenesis have different culture requirements, which so far have not been met. Immotile in vitro produced male haploid germ cells are infertile when used in classical in vitro fertilization (IVF) or in artificial insemination (AI). Therefore, if the ultimate goal of primate IVS is to use the resultant sperm in IVF, then the current situation is still far from ideal. Additionally, even though round spermatids, elongated spermatids, or even sperm can theoretically be produced in vitro and individually used for fertilizing oocytes using intracytoplasmic injection (as discussed below), these potential haploid cells are not always suitable for forming a zygote. Therefore, even after successful development of an efficient system for primate IVS, confirmation of normalcy and fertilization-competence of the in vitro produced haploid germ cells are necessary important steps before experimental applications or potential clinical trials can be recommended.
In order to improve the in vitro culture system for non-rodents, a growing number of factors are being explored which have traditionally been neglected in the past. This includes, for example, the potential role of extracellular matrices or paracrine factors secreted by somatic components of the testis. For instance, paracrine factors secreted by Leydig and peritubular myoid cells (e.g., peritubular factor that modulates Sertoli cell function, PModS) have been shown to modulate the effects of testosterone on Sertoli cells, in turn altering the secretion of transferrin and inhibin [133]. These factors and other novel elements may play an important role in spermatogenesis; therefore, investigating the significance of putative factors is warranted and would likely help in defining optimal culture systems for achieving IVS in primates.
9. Future Prospects of In Vitro Spermatogenesis for Fertility Preservation and Restoration
IVS has many important potential clinical applications and in certain circumstances is the only currently conceivable safe option for preserving fertility and upholding biological fatherhood. At the present time, human IVS to allow differentiation from immature stages to fully-formed sperm has not been achieved; however, given the promising preliminary results and gradual improvements in research methodologies, this technique will hopefully be applicable in future to resolve many of the problems currently linked with the male factors of infertility.
9.1. Fertility Preservation of Prepubertal Cancer Patients for Subsequent Fertility Restoration
With advances in oncotherapy, thankfully >80% of childhood cancer patients survive but, due to the gonadotoxic side effects of cancer treatments, a significant number will experience subfertility or permanent sterility as adults [134,135]. Fertility has an important impact on the post-treatment quality of life. Although cryopreservation of semen prior to cancer therapy is an established method for adult male patients wishing to preserve their future fertility, semen collection is obviously not an option for pre-adolescent boys whose testes have not started sperm production. For such immature patients, cryopreservation of testicular biopsies collected prior to the start of gonadotoxic therapies is the only conceivable option to preserve their fertility potential. This is because the testis of prepubertal individuals contains SSCs, which can be potentially used to restore spermatogenesis and ultimately produce haploid germ cells [136,137].
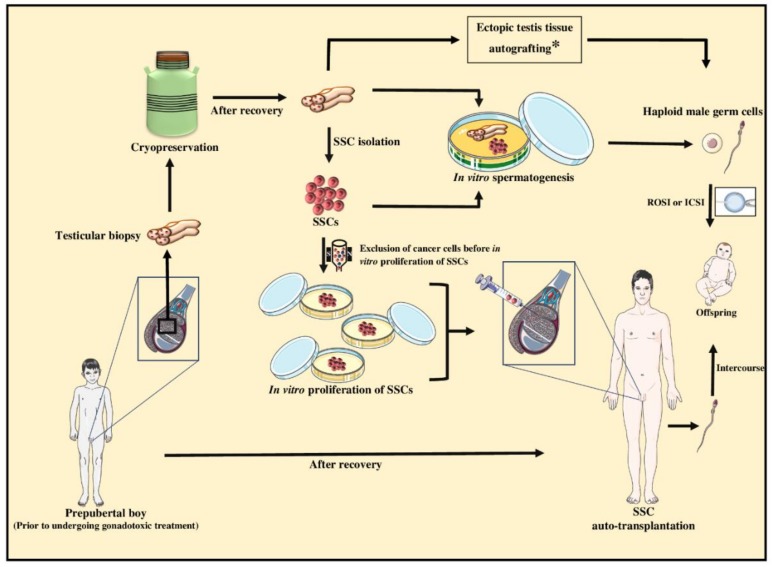
The pre-treatment testicular biopsies from immature boys can be cryopreserved and maintained in liquid nitrogen almost indefinitely, or until such time when the patients have recovered and been diagnosed with infertility as adults. Even if no sperm is found in the recovered individual’s semen, frequently haploid germ cells can still be retrieved from the testes, using established advanced reproductive technologies. This for instance includes microscopic testicular sperm extraction (micro-TESE) to be used for intracytoplasmic sperm injection (ICSI) or round-spermatid injection (ROSI). However, if retrieval of haploid germ cells is not an option, such as when there is spermatogenesis arrest at earlier stages, then the cryopreserved testicular biopsies can be thawed and used in technologies such as IVS, which hopefully by then would be optimized and proven safe. The various scenarios and theoretical options are summarized in Figure 3 and include the potential use of testis tissue fragments or isolated cell suspensions for IVS to produce haploid germ cells, followed by their use for ICSI or ROSI. Alternatively, the cryopreserved tissue fragments can be processed to obtain testis cells containing SSCs, which could include the exclusion of potentially residual malignant cells. This is then followed by subsequent expansion of SSC numbers in culture, before offering to perform auto-transplantation of SSCs for re-colonization of the individual’s testes, which can potentially lead to sufficient production of sperm to restore fertility [136,137].
Figure 3.
Schematic representation of potential fertility preservation options for prepubertal cancer patients. Testicular biopsies can be collected from prepubertal boys before commencing gonadotoxic cancer treatments. The testicular biopsies can be then cryopreserved as a source of spermatogonial stem cells (SSCs) in case the patient indeed become infertile. To preserve the fertility potential, different theoretical or experimental approaches using testicular biopsies or isolated SSCs can be used to produce haploid male germ cells. Advanced assisted reproductive technologies that can be considered after recovery from cancer include in vitro spermatogenesis, ectopic testicular tissue autografting, or auto-transplantation of SSCs into the donor testis to induce spermatogenesis. The resultant haploid male germ cells can be used for fertilization through natural conception or with the help of intracytoplasmic sperm injection (ICSI) or round spermatid injection (ROSI). Asterisk (*) in the boxed text is to emphasize that even though testis cells can be potentially processed to eliminate malignant cells using flow cytometry, this is not applicable with tissue fragments. Therefore, the option of autografting (testicular fragments) is only applicable if the patient was not suffering from testicular cancer or metastatic tumors due to high risk of reintroducing the pre-existing malignant cells back into the patient.
Proof-of-principle studies for a number of the steps outlined in Figure 3 have been shown using various animal models, but at this time, none of the proposed approaches have been adequately tested using non-human primates to warrant even experimental use for humans. For instance, we and others have expanded the technique for male germ cell transplantation, a technique that was originally developed in mice, by showing the feasibility of autologous and homologous transplantations in various large animal models [138,139] and non-human primates, which also included successful production of fertile sperm [132]. Therefore, auto-transplantation of germ cells (containing SSCs) isolated from cryopreserved testicular biopsies is theoretically possible but not before other technical difficulties are overcome.
Firstly, it has been shown that an average testicular biopsy from prepubertal boys weighs ~31 mg and provides ~390,000 total cells, of which only ~11,700 are estimated to be spermatogonia [140]. Given the heterogenous nature of spermatogonia (as discussed above), a much smaller number of these cells would actually be expected to be functional SSCs; hence, it is unlikely that single biopsies will provide sufficient numbers of SSCs to repopulate the testis without further in vitro expansion of SSCs [140]. Therefore, an important first step toward the use of cryopreserved biopsies, as a source for auto-transplantation of testis cells, is exponential expansion of resultant SSC numbers in culture, which would take extensive additional research to optimize such an efficient culture system.
Secondly, in the process of in vitro culture, testicular cells are exposed to a number of ‘unnatural’ conditions including physical and chemical manipulations (e.g., enzymatic digestion, pH changes), and known and unknown factors (e.g., growth factors, cytokines), that may cause them to undergo oxidative stress or DNA methylation and/or histone post-translational modifications. Therefore, careful assessment of genetic and epigenetic modifications should be part of the further work on testis cell culture to ensure their safety for potential use in clinical applications [141,142,143].
Thirdly, because the biopsies are taken from immature patients before undergoing cancer treatment, they may carry malignant cells. This is especially the case for children with testicular cancer, lymphomas, leukemia, or other tumors that metastasize to the testis. In such cases, the auto-transplantation approach should not be considered due to the high risk of reintroducing malignant cells, and certainly not before the suggested methods for elimination of cancer cells using cell sorting (e.g., MACS [144]) have been optimized further and proven 100% efficient and safe [145].
Autologous grafting of the testicular biopsies has also been proposed as a potential approach for restoration of fertility in childhood cancer survivors. Auto-grafting, either in the form of orthotopic (placed within the scrotum) or ectopic (placed subcutaneously outside the scrotum), relies on the ability of the frozen-thawed immature testicular fragments in undergoing extensive development, complete differentiation, and formation of sperm or other haploid germ cells to be extracted and used in ICSI or ROSI [146]. However, because of the same reasons cited above against the use of auto-transplantation of cells, autografting of intact testicular biopsies should not be considered, if there is any chance that malignant cells may still be present in the biopsies. Nevertheless, the feasibility of auto-grafting has been shown using both ectopic and orthotopic grafting of cryopreserved immature testicular tissues in non-human primates, leading to production of sperm and even recently in the birth of a healthy monkey [146,147].
It should be noted that while the use of these testicular biopsies for xenografting into host mice has also been proposed by some fellow researchers and/or clinicians, we have deliberately refrained from including this approach as a potential option for restoration of fertility in our figures. In fact, we were first to show that fresh and cryopreserved immature testicular tissue fragments from various donor species can be grafted under the back skin of immunodeficient recipient mice where they completely develop and even produce large numbers of fertile xenogeneic sperm [148,149]. We were also among the first groups to expand the application of this testicular tissue xenografting system into primates by grafting testis tissue fragments from immature donor monkeys and mature humans [132,150,151]. Therefore, we are well-informed of the various potential applications of testicular tissue xenografting and can envision that current technical hurdles in achieving full spermatogenesis from immature human testis xenografts [152] can be sufficiently addressed. We also believe that it may even become theoretically possible to show that xenogeneic sperm from pre-treatment testicular biopsies would not carry a risk of reintroducing malignancy when used in ICSI. However, we strongly object to offering human testis tissue xenografting as a potential route to produce human sperm if it is exclusively meant to be used for human fertilization. This is because of the serious safety concerns, for instance in terms of the potential transfer of endogenous species-specific retroviral transcripts from the host mouse, as well as grave ethical and even legal limitations of using such xenogeneic sperm for human fertilization. On the other hand, if the goal is not to use the potential xenogeneic sperm for human fertilization, then further research on human testicular tissue xenografting is actually encouraged. For instance, such a system can provide a highly meritorious, novel, and unique assay for the basic research into factors that affect human testis development and spermatogenesis. It would also be a previously-unavailable tool for toxicological and pharmacological investigations into environmental toxicants or new medications that can affect human testis development or testis function [132].
Therefore, currently, the only plausibly safe approach to produce haploid male germ cells from immature human testicular biopsies for the listed potential future clinical applications is through IVS, because it does not carry a risk of reintroducing cancer cells. However, as outlined above, complete IVS has so far been only achieved using donor mice [94]. Although a similar organotypic culture system with some modifications has also been adopted to culture prepubertal human tissue, progression of spermatogonia has not been observed [153], suggesting that culture conditions developed for mouse may not directly or easily be applicable for primate IVS and would require extensive further research.
9.2. Upholding the Biological Fatherhood of Adult Infertile Patients
Meta-analysis of infertility data from various countries and continents concludes that globally an estimated 15% of couples experience infertility. Although the rate differs in different regions, overall about half (range 20%–70%) of the cases are thought to be due to the male factor [154]. Infertility in male also seems to show an uptrend, which can be due to various reasons including varicoceles, medications, obstruction, genetic disorders, or exposure to known and unknown environmental toxicants [155]. A number of infertility cases can be treated using experimental or clinical assisted reproductive techniques, as schematically shown in Figure 4. The currently applicable options include the use of ICSI for men with low numbers of sperm or the use of ICSI or ROSI following microsurgery for testicular sperm or spermatid extractions (micro-TESE) from men with obstructive azoospermia. However, in cases where the patient’s testis does not contain haploid male germ cells, these latter techniques cannot be applied.
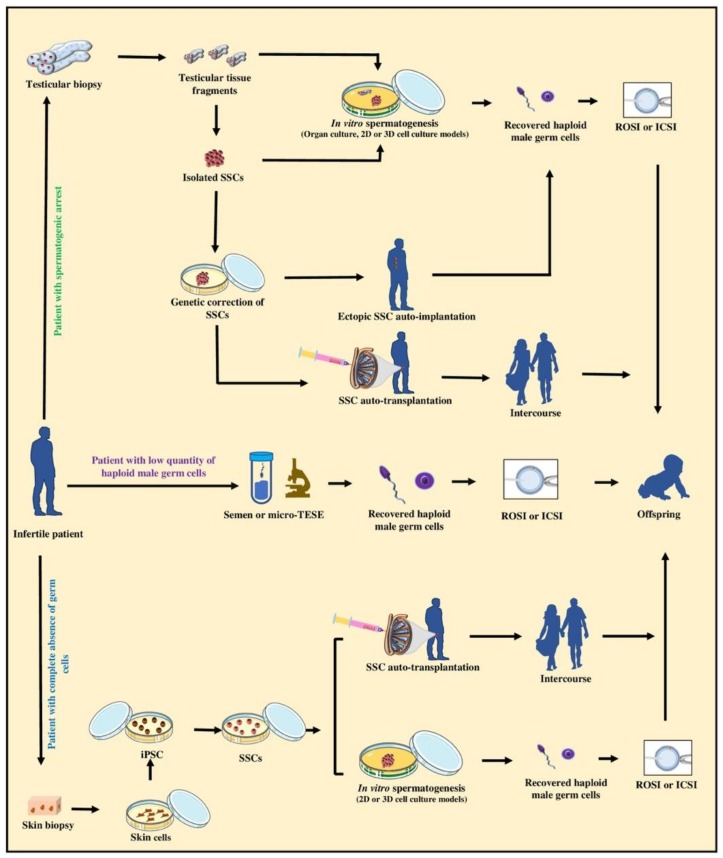
Figure 4.
Schematic representation of standard, experimental, and theoretical options for upholding the biological fatherhood of adult infertile patients. The standard practice for upholding the biological fatherhood of an adult infertile patient is to use sperm from ejaculated semen or to use testicular sperm obtained by microsurgical extraction (TESE) for in vitro fertilization such as using intracytoplasmic sperm injection (ICSI) or round spermatid injection (ROSI). However, this option is applicable only when the patient’s testis contains haploid male germ cells, such as in men with obstructive azoospermia. When infertility is due to spermatogenic arrest, testicular biopsies containing SSCs can be potentially obtained and used either for in vitro spermatogenesis (i.e., organotypic culture, 2D, or 3D cell culture models) in optimized culture conditions to produce haploid male germ cells. Moreover, theoretically genetic correction of isolated SSCs can also be applied in vitro before SSCs can be used for ectopic SSC auto-implantation or auto-transplantation into the patient testis to regenerate spermatogenesis and possibly lead to natural fertility. If the testis of the infertile patients does not contain normal germ cells, skin biopsies can be collected from the patient to theoretically produce induced pluripotent stem cells (iPSC), followed by differentiation of iPSC into SSCs. Such SSCs can then be potentially used for in vitro spermatogenesis (i.e., 2D or 3D cell culture models) to produce haploid male germ cells that can be used to fertilize eggs by ICSI or ROSI. Alternatively, auto-transplantation of SSCs can be potentially used to regenerate spermatogenesis and possibly natural fertility. It is important to emphasize that extensive further research is required to fully examine the various technical, safety, ethical, and legal aspects of the proposed experimental and theoretical options depicted here before they can be recommended for experimental or potential clinical trials.
Patients lacking haploid germ cells in the testis can be divided into those with complete absence of germ cells and those with meiotic-stage, or pre-meiotic spermatogenic arrest [156]. At times, the spermatogenic arrest is treatable using conventional andrology treatments and interventions such as hormonal therapy. To uphold the biological fatherhood of the remaining patients, however, the options are currently limited and novel biotechnology approaches may need to be developed or considered in the future. These theoretical proposed options include the use of testis tissue biopsies or cells in organotypic tissue culture, or 2D/3D cell suspension culture systems, as outlined above. Using such culture systems, the cause of the spermatogenetic arrest can also be explored further and potentially may even be overcome for instance by supplying the missing factors [95] to produce haploid germ cells in vitro for subsequent use in ICSI or ROSI.
Alternatively, testicular biopsies from patients who inherently have very few germ cells can be used as a source of SSCs to be expanded in vitro, followed by auto-transplantation to colonize their testes, and potentially initiate spermatogenesis to allow natural fertility. Theoretically, resultant SSCs can also be implanted ectopically under the skin of the patient for regeneration of testicular tissue to provide haploid germ cells for microsurgical extraction from the regenerated implants, followed by ICSI or ROSI. We and other have shown that testis cells collected after enzymatic digestion of immature donors of various species indeed maintain an unexpected de novo organogenic potential to regenerate functional testis tissue when cell aggregates are implanted under the skin of recipient animal models [132,157]. Interestingly, to overcome spermatogenic arrest at the level of meiotic cells using a mouse model, secondary spermatocytes have been injected into oocytes for the production of viable embryos and even offspring [158]. Intracytoplasmic injection of secondary spermatocytes has also been applied in human; however, the efficiency was reported to be low [159].
Yet, in situations where the patient’s testis does not support the differentiation of germ cells or does not have any type of germ cells due to genetic or chromosomal mutations, at least theoretically, they can still be a candidate for future potential interventions. This includes the in vitro use of gene therapy or genetic correction techniques to overcome the spermatogenic arrest if in the future safe genome editing tools become available. Proof-of-principle studies using a mouse model of infertility have shown the feasibility of such approaches. For instance, due to a mutation of the c-kit ligand (KITL), spermatogenesis is completely arrested and only few abnormal SSCs are present in the testes of Steel (Sl) mice. However, providing the missing component, in the form of recombinant KIT, to the cultured testicular fragments resulted in a dose-dependent increase in the number of SSCs and ultimately in complete IVS [95]. Emergence of induced pluripotent stem cells (iPSC) from somatic cells, which can also be driven to form germ cells, has opened new possibilities. Although the iPSC technology in its current form cannot be used in humans, if similar but safe future alternative technologies become available, theoretically they may provide another unique opportunity to allow fatherhood for individuals who have no other options. Such newly-derived SSCs may be used in IVS or in autologous transplantation to allow parenthood (Figure 4).
10. Conclusions
SSCs are uniquely positioned among adult stem cells because of their potential for passing on genes to the progeny. However, our current knowledge of their biology is limited and so is our ability to harness their full potential for in vitro and in vivo applications in assisted reproductive technologies. Recent progress in organotypic testicular tissue culture as well 2D and 3D cell suspension culture systems may eventually lead to complete IVS using human testicular biopsies to allow preservation and restoration of fertility potential of prepubertal cancer patients undergoing gonadotoxic treatment. Moreover, such optimal IVS systems may also offer unprecedented new perspectives in the study of infertility causes and even approaches to overcome some of the currently untreatable infertility cases in men. However, it has to be emphasized that the proposed potential options discussed here are purely theoretical or are still at early experimental stages, and any recommendation for applying them in the future would require extensive safety, feasibility, and bioethical considerations.
Acknowledgments
We thank the current and former trainees in the lab for their contribution to the published work presented here, and Shawn Honar for proof-reading the final version of the manuscript. We also thank the animal care personnel at the Prairie Swine Centre, the Animal Care Unit of the Western College of Veterinary Medicine, and the Laboratory Animal Services Unit of the College of Medicine.
Abbreviations
| Definition | Abbreviation |
| Artificial insemination | AI |
| Cadherin-1 | CDH1 |
| Casitas B-lineage lymphoma | CBL |
| Checkpoint kinase 2 | CHK2 |
| c-Kit ligand | KITL |
| Cluster of differentiation 9 | CD9 |
| Cluster of differentiation 90 | CD90 |
| Days post-coitum | dpc |
| Days post-partum | dpp |
| DEAD-box helicase 4 | DDX4 |
| Deleted in azoospermia-like | DAZL |
| Desmoglein 2 | DSG2 |
| Developmental pluripotency associated 4 | DPPA4 |
| Doublesex and mab-3 related transcription factor 1 | DMRT1 |
| Eagle’s minimum essential media | MEM |
| Embryonic stem | ES |
| Engineered blood-testis barrier | eBTB |
| Enolase 2 | ENO2 |
| Extracellular matrices | ECM |
| Fetal bovine serum | FBS |
| Fibroblast growth factor receptor 3 | FGFR3 |
| Fluorescence in situ hybridization | FISH |
| Fluorescence-activated cell sorting | FACS |
| GDNF family receptor alpha-1 | GFRα1 |
| G-protein coupled receptor 125 | GPR125 |
| In vitro fertilization | IVF |
| In vitro spermatogenesis | IVS |
| Induced pluripotent stem cells | iPSC |
| Intracytoplasmic sperm injection | ICSI |
| Knockout serum replacement | KSR |
| Lin-28 homology A | LIN28 |
| Magnetic-activated cell sorting | MACS |
| Melanoma-associated antigen 4 | MAGEA4 |
| Methylcellulose | MCS |
| Microscopic testicular sperm extraction | micro-TESE |
| Nanos C2HC-type zinc finger | NANOS |
| Neurogenin 3 | NGN3 |
| Octamer-binding transcription factor 4 | OCT4 |
| Paired box 7 | PAX7 |
| Peritubular factor that modulates Sertoli cell function | PModS |
| Phospholipid phosphatase related 3 | PLPPR3 |
| Piwi like RNA-mediated gene silencing 4 | PIWIL4 |
| POU class 5 homeobox 1 | POU5F1 |
| Primordial germ cells | PGCs |
| Prominin 1 (Cluster of differentiation 133) | PROM1 (CD133) |
| Promyelocytic leukemia zinc finger protein | PLZF |
| Ret proto-oncogene | RET |
| Retinoic acid | RA |
| RNA binding motif | RBM |
| Round-spermatid injection | ROSI |
| Sal-like protein 4 | SALL4 |
| Serum free medium | SFM |
| Soft-agar culture system | SACS |
| Spermatogonial stem cells | SSCs |
| SPOC domain containing 1 | SPOCD1 |
| Stage-specific embryonic antigen-4 | SSEA4 |
| Steel | SL |
| Stimulated by retinoic acid 8 | STRA8 |
| Synaptosome associated protein 91 | SNAP91 |
| Testis-specific Y-encoded protein | TSPY |
| Tetraspanin 33 | TSPAN33 |
| Three-dimensional | 3D |
| Three-layer gradient system | 3-LGS |
| Transformer-1 | TRA-1 |
| Two-dimensional | 2D |
| Ubiquitin C-terminal hydrolase L1 | UCHL1 |
| Undifferentiated embryonic cell transcription factor 1 | UTF1 |
| Vasa gene-encoded RNA binding protein | VASA |
| Zinc finger with KRAB and SCAN domains 2 | ZKSCAN2 |
Author Contributions
F.I. contributed to conceiving the concept, wrote the first draft of the manuscript, summarized tables, and prepared the figures. A.H. contributed to conceiving the concept, wrote parts of the manuscript, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants to A.H. from the Natural Sciences and Engineering Research Council (NSERC) of Canada, and the Saskatchewan Health Research Foundation (SHRF). Scholarships were provided to F.I. by the University of Saskatchewan’s Colleges of Graduates and Postdoctoral Studies, and Western College of Veterinary Medicine.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Russell L.D., Ettlin R.A., Hikim A.P.S., Clegg E.D. Histological and Histopathological Evaluation of the Testis. Int. J. Androl. 1993;16:83. doi: 10.1111/j.1365-2605.1993.tb01156.x. [DOI] [Google Scholar]
- 2.Martin L.A., Seandel M. Propagation of adult SSCs: From mouse to human. Biomed. Res. Int. 2013;2013:384734. doi: 10.1155/2013/384734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oatley J.M., Brinster R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012;92:577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubota H., Brinster R.L. Spermatogonial stem cells. Biol. Reprod. 2018;99:52–74. doi: 10.1093/biolre/ioy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jan S.Z., Hamer G., Repping S., de Rooij D.G., Van Pelt A.M.M., Vormer T.L. Molecular control of rodent spermatogenesis. Biochim. Biophys. Acta. 2012;1822:1838–1850. doi: 10.1016/j.bbadis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Hutson J.M., Li R., Southwell B.R., Petersen B.L., Thorup J., Cortes D. Germ cell development in the postnatal testis: The key to prevent malignancy in cryptorchidism? Front. Endocrinol. 2013;3:176. doi: 10.3389/fendo.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griswold M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2015;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griswold M.D., Oatley J.M. Concise review: Defining characteristics of mammalian spermatogenic stem cells. Stem Cells. 2013;31:8–11. doi: 10.1002/stem.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagano M.C., Yeh J.R. The identity and fate decision control of spermatogonial stem cells: Where is the point of no return? Curr. Top. Dev. Biol. 2013;102:61–95. doi: 10.1016/B978-0-12-416024-8.00003-9. [DOI] [PubMed] [Google Scholar]
- 10.De Rooij D.G., Russell L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;6:776–798. [PubMed] [Google Scholar]
- 11.Fayomi A.P., Orwig K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018;29:207–214. doi: 10.1016/j.scr.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clermont Y. Two classes of spermatogonial stem cells in the monkey (Cercopithecus aethiops) Am. J. Anat. 1969;126:57–71. doi: 10.1002/aja.1001260106. [DOI] [PubMed] [Google Scholar]
- 13.Ibtisham F., Wu J., Xiao M., An L., Banker Z., Nawab A., Zhao Y., Li G. Progress and future prospect of in vitro spermatogenesis. Oncotarget. 2017;8:66709–66727. doi: 10.18632/oncotarget.19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boitani C., Di Persio S., Esposito V., Vicini E. Spermatogonial cells: Mouse, monkey and man comparison. Semin. Cell Dev. Biol. 2016;59:79–88. doi: 10.1016/j.semcdb.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Amann R.P. The cycle of the seminiferous epithelium in humans: A need to revisit? J. Androl. 2008;29:469–487. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- 16.Lajtha L. Stem cell concepts. Differentiation. 1979;14:23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 17.Heller C.G., Clermont Y. Spermatogenesis in man: An estimate of its duration. Science. 1963;140:184–186. doi: 10.1126/science.140.3563.184. [DOI] [PubMed] [Google Scholar]
- 18.De Rooij D.G. The nature and dynamics of spermatogonial stem cells. Development. 2017;144:3022–3030. doi: 10.1242/dev.146571. [DOI] [PubMed] [Google Scholar]
- 19.Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagano M., Brinster R.L. Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. APMIS. 1998;106:47. doi: 10.1111/j.1699-0463.1998.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 21.Honaramooz A., Yang Y. Recent advances in application of male germ cell transplantation in farm animals. Vet. Med. Int. 2010;2011:1–9. doi: 10.4061/2011/657860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng K., Wu X., Kaestner K.H., Wang P.J. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aeckerle N., Eildermann K., Drummer C., Ehmcke J., Schweyer S., Lerchl A., Bergmann M., Kliesch S., Gromoll J., Schlatt S., et al. The pluripotency factor LIN28 in monkey and human testes: A marker for spermatogonial stem cells? Mol. Hum. Reprod. 2012;18:477–488. doi: 10.1093/molehr/gas025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eildermann K., Gromoll J., Behr R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Hum. Reprod. 2012;27:1754–1767. doi: 10.1093/humrep/des091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermann B.P., Sukhwani M., Lin C.-C., Sheng Y., Tomko J., Rodriguez M., Shuttleworth J.J., McFarland D., Hobbs R.M., Pandolfi P.P., et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Z.Y.-C., Hirano T., Shibata S., Seki N.M., Kitajima R., Sedohara A., Siomi M.C., Sasaki E., Siomi H., Imamura M., et al. Gene expression ontogeny of spermatogenesis in the marmoset uncovers primate characteristics during testicular development. Dev. Biol. 2015;400:43–58. doi: 10.1016/j.ydbio.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Muller T., Eildermann K., Dhir R., Schlatt S., Behr R. Glycan stem-cell markers are specifically expressed by spermatogonia in the adult non-human primate testis. Hum. Reprod. 2008;23:2292–2298. doi: 10.1093/humrep/den253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermann B.P., Sukhwani M., Simorangkir D.R., Chu T., Plant T.M., Orwig K.E. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum. Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinohara T., Avarbock M.R., Brinster R.L. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubota H., Avarbock M.R., Brinster R.L. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc. Natl. Acad. Sci. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanatsu-shinohara M., Toyokuni S., Shinohara T. CD9 Is a Surface Marker on Mouse and Rat Male Germline Stem Cells. Biol. Reprod. 2004;70:70–75. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- 32.Conrad S., Renninger M., Hennenlotter J., Wiesner T., Just L., Bonin M., Aicher W., Buhring H.-J., Mattheus U., Mack A., et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 33.Sakashita A., Yeh Y.-H.V., Namekawa S.H., Lin S.-P. Epigenomic and single-cell profiling of human spermatogonial stem cells. Stem Cell Investig. 2018;5:11. doi: 10.21037/sci.2018.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan F., Oatley M.J., Kaucher A.V., Yang Q.-E., Bieberich C.J., Shashikant C.S., Oatley J.M. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014;28:1351–1362. doi: 10.1101/gad.240465.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seandel M., James D., Shmelkov S.V., Falciatori I., Kim J., Chavala S., Scherr D.S., Zhang F., Torres R., Gale N.W., et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachs C., Robinson B.D., Andres Martin L., Webster T., Gilbert M., Lo H.-Y., Rafii S., Ng C.K., Seandel M. Evaluation of candidate spermatogonial markers ID4 and GPR125 in testes of adult human cadaveric organ donors. Andrology. 2014;2:607–614. doi: 10.1111/j.2047-2927.2014.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naughton C.K., Jain S., Strickland A.M., Gupta A., Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 38.Giuili G., Tomljenovic A., Labrecque N., Oulad-Abdelghani M., Rassoulzadegan M., Cuzin F. Murine spermatogonial stem cells: Targeted transgene expression and purification in an active state. EMBO Rep. 2002;3:753–759. doi: 10.1093/embo-reports/kvf149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokuda M., Kadokawa Y., Kurahashi H., Marunouchi T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol. Reprod. 2007;76:130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- 40.Makela J.-A., Hobbs R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction. 2019;158:R169–R187. doi: 10.1530/REP-18-0476. [DOI] [PubMed] [Google Scholar]
- 41.Honecker F., Stoop H., de Krijger R.R., Chris Lau Y.-F., Bokemeyer C., Looijenga L.H.J. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J. Pathol. 2004;203:849–857. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- 42.Kido T., Lau Y.-F.C. The rat Tspy is preferentially expressed in elongated spermatids and interacts with the core histones. Biochem. Biophys. Res. Commun. 2006;350:56–67. doi: 10.1016/j.bbrc.2006.08.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartkova J., Falck J., Rajpert-De Meyts E., Skakkebaek N.E., Lukas J., Bartek J. Chk2 tumour suppressor protein in human spermatogenesis and testicular germ-cell tumours. Oncogene. 2001;20:5897–5902. doi: 10.1038/sj.onc.1204746. [DOI] [PubMed] [Google Scholar]
- 44.Maki C.B., Pacchiarotti J., Ramos T., Pascual M., Pham J., Kinjo J., Anorve S., Izadyar F. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum. Reprod. 2009;24:1480–1491. doi: 10.1093/humrep/dep033. [DOI] [PubMed] [Google Scholar]
- 45.Husen B., Giebel J., Rune G. Expression of the integrin subunits alpha 5, alpha 6 and beta 1 in the testes of the common marmoset. Int. J. Androl. 1999;22:374–384. doi: 10.1046/j.1365-2605.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 46.Lim J.J., Sung S.-Y., Kim H.J., Song S.-H., Hong J.Y., Yoon T.K., Kim J.K., Kim K.-S., Lee D.R. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43:405–417. doi: 10.1111/j.1365-2184.2010.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadokoro Y., Yomogida K., Ohta H., Tohda A., Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech. Dev. 2002;113:29–39. doi: 10.1016/S0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 48.Maki C., Yuen C., Zhao H.H., Wong J., Pacchiarotti J., Howerton K., Chow M., Greilach S., Ramos T., Izadyar F., et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum. Reprod. 2011;26:1296–1306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- 49.Lai M.-S., Wang C.-Y., Yang S.-H., Wu C.-C., Sun H.S., Tsai S.-J., Chuang J.-I., Chen Y.-C., Huang B.-M. The expression profiles of fibroblast growth factor 9 and its receptors in developing mice testes. Organogenesis. 2016;12:61–77. doi: 10.1080/15476278.2016.1171448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Kopylow K., Kirchhoff C., Jezek D., Schulze W., Feig C., Primig M., Steinkraus V., Spiess A.-N. Screening for biomarkers of spermatogonia within the human testis: A whole genome approach. Hum. Reprod. 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- 51.Sohni A., Tan K., Song H.-W., Burow D., de Rooij D.G., Laurent L., Hsieh T.-C., Rabah R., Hammoud S.S., Vicini E., et al. The neonatal and adult human testis defined at the single-cell level. Cell Rep. 2019;26:1501–1517.e4. doi: 10.1016/j.celrep.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zohni K., Zhang X., Tan S.L., Chan P., Nagano M. CD9 is expressed on human male germ cells that have a long-term repopulation potential after transplantation into mouse testes. Biol. Reprod. 2012;87:27. doi: 10.1095/biolreprod.112.098913. [DOI] [PubMed] [Google Scholar]
- 53.Aloisio G.M., Nakada Y., Saatcioglu H.D., Pena C.G., Baker M.D., Tarnawa E.D., Mukherjee J., Manjunath H., Bugde A., Sengupta A.L., et al. PAX7 expression defines germline stem cells in the adult testis. J. Clin. Invest. 2014;124:3929–3944. doi: 10.1172/JCI75943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakai Y., Noce T., Yamashina S. Cleavage-like cell division and explosive increase in cell number of neonatal gonocytes. Dev. Growth Differ. 2004;46:15–21. doi: 10.1111/j.1440-169X.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 55.Altman E., Yango P., Moustafa R., Smith J.F., Klatsky P.C., Tran N.D. Characterization of human spermatogonial stem cell markers in fetal, pediatric, and adult testicular tissues. Reproduction. 2014;148:417–427. doi: 10.1530/REP-14-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell R.T., Cowan G., Morris K.D., Anderson R.A., Fraser H.M., Mckenzie K.J., Wallace W.H.B., Kelnar C.J.H., Saunders P.T.K., Sharpe R.M. Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum. Reprod. 2008;23:2755–2765. doi: 10.1093/humrep/den295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajpert-De Meyts E., Jacobsen G.K., Bartkova J., Aubry F., Samson M., Bartek J., Skakkebaek N.E. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology. 2003;42:217–226. doi: 10.1046/j.1365-2559.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 58.Sada A., Suzuki A., Suzuki H., Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 59.Yamauchi K., Hasegawa K., Chuma S., Nakatsuji N., Suemori H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLoS ONE. 2009;4:e5338. doi: 10.1371/journal.pone.0005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jorgensen A., Nielsen J.E., Blomberg Jensen M., Graem N., Rajpert-De Meyts E. Analysis of meiosis regulators in human gonads: A sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol. Hum. Reprod. 2012;18:523–534. doi: 10.1093/molehr/gas030. [DOI] [PubMed] [Google Scholar]
- 61.Gassei K., Orwig K.E. SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS ONE. 2013;8:e53976. doi: 10.1371/journal.pone.0053976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y.-L., Liu W., Sun Y.-J., Kwon J., Setsuie R., Osaka H., Noda M., Aoki S., Yoshikawa Y., Wada K. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 arrests spermatogenesis in transgenic mice. Mol. Reprod. Dev. 2006;73:40–49. doi: 10.1002/mrd.20364. [DOI] [PubMed] [Google Scholar]
- 63.Buaas F.W., Kirsh A.L., Sharma M., McLean D.J., Morris J.L., Griswold M.D., de Rooij D.G., Braun R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 64.Pesce M., Wang X., Wolgemuth D.J., Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 1998;71:89–98. doi: 10.1016/S0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 65.Bhartiya D., Kasiviswanathan S., Unni S.K., Pethe P., Dhabalia J. V., Patwardhan S., Tongaonkar H.B. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J. Histochem. Cytochem. 2010;58:1093–1106. doi: 10.1369/jhc.2010.956870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida S., Takakura A., Ohbo K., Abe K., Wakabayashi J., Yamamoto M., Suda T., Nabeshima Y.-I. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev. Biol. 2004;269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 67.Zhang T., Oatley J., Bardwell V.J., Zarkower D. DMRT1 is required for mouse spermatogonial stem cell maintenance and replenishment. PLoS Genet. 2016;12:e1006293. doi: 10.1371/journal.pgen.1006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo J., Grow E.J., Yi C., Mlcochova H., Maher G.J., Lindskog C., Murphy P.J., Wike C.L., Carrell D.T., Goriely A., et al. Chromatin and single-cell rna-seq profiling reveal dynamic signaling and metabolic transitions during human spermatogonial stem cell development. Cell Stem Cell. 2017;21:533–546.e6. doi: 10.1016/j.stem.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Bragt M.P.A., Roepers-Gajadien H.L., Korver C.M., Bogerd J., Okuda A., Eggen B.J.L., de Rooij D.G., van Pelt A.M.M. Expression of the pluripotency marker UTF1 is restricted to a subpopulation of early A spermatogonia in rat testis. Reproduction. 2008;136:33–40. doi: 10.1530/REP-07-0536. [DOI] [PubMed] [Google Scholar]
- 70.Valli H., Sukhwani M., Dovey S.L., Peters K.A., Donohue J., Castro C.A., Chu T., Marshall G.R., Orwig K.E. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil. Steril. 2014;102:566–580.e7. doi: 10.1016/j.fertnstert.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang M., Liu X., Chang G., Chen Y., An G., Yan L., Gao S., Xu Y., Cui Y., Dong J., et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. 2018;23:599–614.e4. doi: 10.1016/j.stem.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Fayomi A.P. Stem Cells and Spermatogenic Lineage Development in the Primate Testis. University of Pittsburgh; Pittsburgh, PA, USA: 2018. [(accessed on 1 March 2020)]. Available online: http://dscholarship.pitt.edu/34319/1/fayomiadetunjip_etd2018.pdf. [Google Scholar]
- 73.Jarvis S., Elliott D.J., Morgan D., Winston R., Readhead C. Molecular markers for the assessment of postnatal male germ cell development in the mouse. Hum. Reprod. 2005;20:108–116. doi: 10.1093/humrep/deh565. [DOI] [PubMed] [Google Scholar]
- 74.Elliott D.J., Millar M.R., Oghene K., Ross A., Kiesewetter F., Pryor J., McIntyre M., Hargreave T.B., Saunders P.T., Vogt P.H., et al. Expression of RBM in the nuclei of human germ cells is dependent on a critical region of the Y chromosome long arm. Proc. Natl. Acad. Sci. 1997;94:3848–3853. doi: 10.1073/pnas.94.8.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kossack N., Meneses J., Shefi S., Nguyen H.N., Chavez S., Nicholas C., Gromoll J., Turek P.J., Reijo-Pera R.A. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y., Yarahmadi M., Honaramooz A. Development of novel strategies for the isolation of piglet testis cells with a high proportion of gonocytes. Reprod. Fertil. Dev. 2010;22:1057–1065. doi: 10.1071/RD09316. [DOI] [PubMed] [Google Scholar]
- 77.Gassei K., Ehmcke J., Dhir R., Schlatt S. Magnetic activated cell sorting allows isolation of spermatogonia from adult primate testes and reveals distinct GFRa1-positive subpopulations in men. J. Med. Primatol. 2010;39:83–91. doi: 10.1111/j.1600-0684.2009.00397.x. [DOI] [PubMed] [Google Scholar]
- 78.Nickkholgh B., Sefika Canan M., Korver C., van Saskia K.D., Andreas M. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil. Steril. 2014;102:558–565. doi: 10.1016/j.fertnstert.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 79.Liu S., Tang Z., Xiong T., Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod. Biol. Endocrinol. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Y., Chen X., Zhu H., Wang D. Developments in techniques for the isolation, enrichment, main culture conditions and identification of spermatogonial stem cells. Cytotechnology. 2015;67:921–930. doi: 10.1007/s10616-015-9850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibtisham F., Zhao Y., Wu J., Nawab A., Mei X., Li G., Lilong A. The optimized condition for the isolation and in vitro propagation of mouse spermatogonial stem cells. Biol. Futur. 2019;70:79–87. doi: 10.1556/019.70.2019.10. [DOI] [PubMed] [Google Scholar]
- 82.Guo Y., Liu L., Sun M., Hai Y., Li Z., He Z. Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways. Exp. Biol. Med. 2015;240:1112–1122. doi: 10.1177/1535370215590822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sadri-Ardekani H., Mizrak S.C., van Daalen S.K.M., Korver C.M., Roepers-Gajadien H.L., Koruji M., Hovingh S., de Reijke T.M., de la Rosette J.J.M.C.H., van der Veen F., et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 84.Kanatsu-Shinohara M., Miki H., Inoue K., Ogonuki N., Toyokuni S., Ogura A., Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol. Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y.-H., Kang H.-G., Kim B.-J., Jung S.-E., Karmakar P.C., Kim S.-M., Hwang S., Ryu B.-Y. Enrichment and In vitro culture of spermatogonial stem cells from pre-pubertal monkey testes. Tissue Eng. Regen. Med. 2017;14:557–566. doi: 10.1007/s13770-017-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trowell O.A. The culture of mature organs in a synthetic medium. Exp. Cell Res. 1959;16:118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- 87.Steinberger E., Steinberger A., Perluff W.H. Initiation of spermatogenesis in vitro. Endocrinology. 1964;74:788–792. doi: 10.1210/endo-74-5-788. [DOI] [PubMed] [Google Scholar]
- 88.Steinberger E., Steinberger A., Perloff W.H. Studies in growth in organ culture of testicular tissue from rats of various age. Anat. Rec. 1964;148:581–589. doi: 10.1002/ar.1091480409. [DOI] [PubMed] [Google Scholar]
- 89.Steinberger A., Steinberger E. Stimulatory effect of vitamins and glutamine on the differentiation of germ cells in rat testes organ culture grown in chemically defined media. Exp. Cell Res. 1966;44:429–435. doi: 10.1016/0014-4827(66)90449-6. [DOI] [PubMed] [Google Scholar]
- 90.Matte R., Sasaki M. Autoradiographic evidence of human male germ-cell differentiation in vitro. Cytologia. 1971;36:298–303. doi: 10.1508/cytologia.36.298. [DOI] [PubMed] [Google Scholar]
- 91.Boitani C., Politi M.G., Menna T. Spermatogonial cell proliferation in organ culture of immature rat testis. Biol. Reprod. 1993;48:761–767. doi: 10.1095/biolreprod48.4.761. [DOI] [PubMed] [Google Scholar]
- 92.Steinberger A., Steinberger E., Perloff W.H. Mammalian testes in organ culture. Exp. Cell Res. 1964;36:19–27. doi: 10.1016/0014-4827(64)90156-9. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki S., Sato K. The fertilising ability of spermatogenic cells derived from cultured mouse immature testicular tissue. Zygote. 2003;11:307–316. doi: 10.1017/S0967199403002363. [DOI] [PubMed] [Google Scholar]
- 94.Sato T., Katagiri K., Gohbara A., Inoue K., Ogonuki N., Ogura A., Kubota Y., Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 95.Sato T., Yokonishi T., Komeya M., Katagiri K., Kubota Y., Matoba S., Ogonuki N., Ogura A., Yoshida S., Ogawa T. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc. Natl. Acad. Sci. 2012;109:16934–16938. doi: 10.1073/pnas.1211845109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yokonishi T., Sato T., Komeya M., Katagiri K., Kubota Y., Nakabayashi K., Hata K., Inoue K., Ogonuki N., Ogura A., et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat. Commun. 2014;5:4320. doi: 10.1038/ncomms5320. [DOI] [PubMed] [Google Scholar]
- 97.Sato T., Katagiri K., Kojima K., Komeya M., Yao M., Ogawa T. In vitro spermatogenesis in explanted adult mouse testis tissues. PLoS ONE. 2015;10:e0130171. doi: 10.1371/journal.pone.0130171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Komeya M., Kimura H., Nakamura H., Yokonishi T., Sato T., Kojima K., Hayashi K., Katagiri K., Yamanaka H., Sanjo H., et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016;6:21472. doi: 10.1038/srep21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komeya M., Hayashi K., Nakamura H., Yamanaka H., Sanjo H., Kojima K., Sato T., Yao M., Kimura H., Fujii T., et al. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci. Rep. 2017;7:15459. doi: 10.1038/s41598-017-15799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dietrich A.J., Scholten R., Vink A.C., Oud J.L. Testicular cell suspensions of the mouse in vitro. Andrologia. 1983;15:236–246. doi: 10.1111/j.1439-0272.1983.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 101.Nagao Y. Viability of meiotic prophase spermatocytes of rats is facilitated in primary culture of dispersed testicular cells on collagen gel by supplementing epinephrine or norepinephrine: Evidence that meiotic prophase spermatocytes complete meiotic divisions in vitro. Cell. Dev. Biol. 1989;25:1088–1098. doi: 10.1007/BF02621259. [DOI] [PubMed] [Google Scholar]
- 102.Rassoulzadegan M., Paquis-Flucklinger V., Bertino B., Sage J., Jasin M., Miyagawa K., van Heyningen V., Besmer P., Cuzin F. Transmeiotic differentiation of male germ cells in culture. Cell. 1993;75:997–1006. doi: 10.1016/0092-8674(93)90543-Y. [DOI] [PubMed] [Google Scholar]
- 103.Hue D., Staub C., Perrard-Sapori M.H., Weiss M., Nicolle J.C., Vigier M., Durand P. Meiotic differentiation of germinal cells in three-week cultures of whole cell population from rat seminiferous tubules. Biol. Reprod. 1998;59:379–387. doi: 10.1095/biolreprod59.2.379. [DOI] [PubMed] [Google Scholar]
- 104.Staub C., Hue D., Nicolle J.C., Perrard-Sapori M.H., Segretain D., Durand P. The whole meiotic process can occur in vitro in untransformed rat spermatogenic cells. Exp. Cell Res. 2000;260:85–95. doi: 10.1006/excr.2000.4998. [DOI] [PubMed] [Google Scholar]
- 105.Marh J., Tres L.L., Yamazaki Y., Yanagimachi R., Kierszenbaum A.L. Mouse round spermatids developed in vitro from preexisting spermatocytes can produce normal offspring by nuclear injection into in vivo-developed mature oocytes1. Biol. Reprod. 2003;69:169–176. doi: 10.1095/biolreprod.102.015099. [DOI] [PubMed] [Google Scholar]
- 106.Iwanami Y., Kobayashi T., Kato M., Hirabayashi M., Hochi S. Characteristics of rat round spermatids differentiated from spermatogonial cells during co-culture with Sertoli cells, assessed by flow cytometry, microinsemination and RT-PCR. Theriogenology. 2006;65:288–298. doi: 10.1016/j.theriogenology.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 107.Wang P., Suo L.-J., Shang H., Li Y., Li G.-X., Li Q.-W., Hu J.-H. Differentiation of spermatogonial stem cell-like cells from murine testicular tissue into haploid male germ cells in vitro. Cytotechnology. 2014;66:365–372. doi: 10.1007/s10616-013-9584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 109.Kubota H., Avarbock M.R., Brinster R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanatsu-Shinohara M., Inoue K., Ogonuki N., Morimoto H., Ogura A., Shinohara T. Serum- and Feeder-Free Culture of Mouse Germline Stem Cells1. Biol. Reprod. 2011;84:97–105. doi: 10.1095/biolreprod.110.086462. [DOI] [PubMed] [Google Scholar]
- 111.Langenstroth D., Kossack N., Westernstroer B., Wistuba J., Behr R., Gromoll J., Schlatt S. Separation of somatic and germ cells is required to establish primate spermatogonial cultures. Hum. Reprod. 2014;29:2018–2031. doi: 10.1093/humrep/deu157. [DOI] [PubMed] [Google Scholar]
- 112.Kokkinaki M., Djourabtchi A., Golestaneh N. Long-term culture of human ssea-4 positive spermatogonial stem cells (SSCs) J. Stem Cell Res. Ther. 2011;2:2488. doi: 10.4172/2157-7633.S2-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee J.H., Kim H.J., Kim H., Lee S.J., Gye M.C. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006;27:2845–2853. doi: 10.1016/j.biomaterials.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 114.Stukenborg J.-B., Wistuba J., Luetjens C.M., Elhija M.A., Huleihel M., Lunenfeld E., Gromoll J., Nieschlag E., Schlatt S. Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J. Androl. 2008;29:312–329. doi: 10.2164/jandrol.107.002857. [DOI] [PubMed] [Google Scholar]
- 115.Abu Elhija M., Lunenfeld E., Schlatt S., Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J. Androl. 2012;14:285–293. doi: 10.1038/aja.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huleihel M., Nourashrafeddin S., Plant T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta) Asian J. Androl. 2015;17:972–980. doi: 10.4103/1008-682X.154994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stukenborg J.-B., Schlatt S., Simoni M., Yeung C.-H., Elhija M.A., Luetjens C.M., Huleihel M., Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol. Hum. Reprod. 2009;15:521–529. doi: 10.1093/molehr/gap052. [DOI] [PubMed] [Google Scholar]
- 118.Legendre A., Froment P., Desmots S., Lecomte A., Habert R., Lemazurier E. An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials. 2010;31:4492–4505. doi: 10.1016/j.biomaterials.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 119.Yokonishi T., Sato T., Katagiri K., Komeya M., Kubota Y., Ogawa T. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol. Reprod. 2013;89:1–6. doi: 10.1095/biolreprod.113.108613. [DOI] [PubMed] [Google Scholar]
- 120.Alves-Lopes J.P., Soder O., Stukenborg J.-B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials. 2017;130:76–89. doi: 10.1016/j.biomaterials.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 121.Steinberger A., Steinberger E. Factors affecting spermatogenesis in organ cultures of mammalian testes. J. Reprod. Fertil. 1967;(Suppl.):117–124. [Google Scholar]
- 122.Curtis D. In vitro differentiation of diakinesis figures in human testis. Hum. Genet. 1981;59:406–411. doi: 10.1007/BF00295480. [DOI] [PubMed] [Google Scholar]
- 123.Tesarik J., Greco E., Rienzi L., Ubaldi F., Guido M., Cohen-Bacrie P., Mendoza C. Differentiation of spermatogenic cells during in-vitro culture of testicular biopsy samples from patients with obstructive azoospermia: Effect of recombinant follicle stimulating hormone. Hum. Reprod. 1998;13:2772–2781. doi: 10.1093/humrep/13.10.2772. [DOI] [PubMed] [Google Scholar]
- 124.Cremades N., Bernabeu R., Barros A., Sousa M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum. Reprod. 1999;14:1287–1293. doi: 10.1093/humrep/14.5.1287. [DOI] [PubMed] [Google Scholar]
- 125.Sousa M., Cremades N., Alves C., Silva J., Barros A. Developmental potential of human spermatogenic cells co-cultured with Sertoli cells. Hum. Reprod. 2002;17:161–172. doi: 10.1093/humrep/17.1.161. [DOI] [PubMed] [Google Scholar]
- 126.Tanaka A., Nagayoshi M., Awata S., Mawatari Y., Tanaka I., Kusunoki H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil. Steril. 2003;79(Suppl. 1):795–801. doi: 10.1016/S0015-0282(02)04833-1. [DOI] [PubMed] [Google Scholar]
- 127.Riboldi M., Rubio C., Pellicer A., Gil-Salom M., Simon C. In vitro production of haploid cells after coculture of CD49f+ with Sertoli cells from testicular sperm extraction in nonobstructive azoospermic patients. Fertil. Steril. 2012;98:580–590.e4. doi: 10.1016/j.fertnstert.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 128.de Michele F., Poels J., Vermeulen M., Ambroise J., Gruson D., Guiot Y., Wyns C. Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front. Physiol. 2018;9:1413. doi: 10.3389/fphys.2018.01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Awang-Junaidi A.H., Honaramooz A. Optimization of culture conditions for short-term maintenance, proliferation, and colony formation of porcine gonocytes. J. Anim. Sci. Biotechnol. 2018;9:8. doi: 10.1186/s40104-017-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Awang-Junaidi A.H., Singh J., Honaramooz A. Regeneration of testis tissue after ectopic implantation of porcine testis cell aggregates in mice: Improved consistency of outcomes and in situ monitoring. Reprod. Fertil. Dev. 2020 doi: 10.1071/RD19043. [DOI] [PubMed] [Google Scholar]
- 131.Fayaz M.A., Awang-Junaidi A.H., Singh J., Honaramooz A. Validation of ultrasound biomicroscopy for the assessment of xenogeneic testis tissue grafts and cell implants in recipient mice. Andrology. 2020 doi: 10.1111/andr.12771. [DOI] [PubMed] [Google Scholar]
- 132.Ibtisham F., Awang-Junaidi A.H., Honaramooz A. The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res. 2020 doi: 10.1007/s00441-020-03212-x. [DOI] [PubMed] [Google Scholar]
- 133.Albrecht M. Insights into the nature of human testicular peritubular cells. Ann. Anat. 2009;191:532–540. doi: 10.1016/j.aanat.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 134.Hudson M.M. Reproductive outcomes for survivors of childhood cancer. Obstet. Gynecol. 2010;116:1171–1183. doi: 10.1097/AOG.0b013e3181f87c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thomson A.B., Campbell A.J., Irvine D.C., Anderson R.A., Kelnar C.J.H., Wallace W.H.B. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: A case-control study. Lancet. 2002;360:361–367. doi: 10.1016/S0140-6736(02)09606-X. [DOI] [PubMed] [Google Scholar]
- 136.Goossens E., Van Saen D., Tournaye H. Spermatogonial stem cell preservation and transplantation: From research to clinic. Hum. Reprod. 2013;28:897–907. doi: 10.1093/humrep/det039. [DOI] [PubMed] [Google Scholar]
- 137.Gassei K., Orwig K.E. Experimental methods to preserve male fertility and treat male factor infertility. Fertil. Steril. 2016;105:256–266. doi: 10.1016/j.fertnstert.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Honaramooz A., Megee S.O., Dobrinski I. Germ cell transplantation in pigs. Biol. Reprod. 2002;66:21–28. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- 139.Honaramooz A., Behboodi E., Blash S., Megee S.O., Dobrinski I. Germ cell transplantation in goats. Mol. Reprod. Dev. 2003;64:422–428. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- 140.Wu X., Schmidt J.A., Avarbock M.R., Tobias J.W., Carlson C.A., Kolon T.F., Ginsberg J.P., Brinster R.L. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. 2009;106:21672–21677. doi: 10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Patra T., Gupta M.K. Cryopreservation of murine testicular Leydig cells by modified solid surface vitrification with supplementation of antioxidants. Cryobiology. 2019;88:38–46. doi: 10.1016/j.cryobiol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 142.Oblette A., Rondeaux J., Dumont L., Delessard M., Saulnier J., Rives A., Rives N., Rondanino C. DNA methylation and histone post-translational modifications in the mouse germline following in-vitro maturation of fresh or cryopreserved prepubertal testicular tissue. Reprod. Biomed. Online. 2019;39:383–401. doi: 10.1016/j.rbmo.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 143.Weissbein U., Plotnik O., Vershkov D., Benvenisty N. Culture-induced recurrent epigenetic aberrations in human pluripotent stem cells. PLoS Genet. 2017;13:e1006979. doi: 10.1371/journal.pgen.1006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang J., Wu Y., Gao W., Li F., Bo Y., Zhu M., Fu R., Liu Q., Wen S., Wang B. Identification and characterization of CD133(+)CD44(+) cancer stem cells from human laryngeal squamous cell carcinoma cell lines. J. Cancer. 2017;8:497–506. doi: 10.7150/jca.17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lau G.A., Schaeffer A.J. Current standing and future directions in pediatric oncofertility: A narrative review. Transl. Androl. Urol. 2018;7:S276–S282. doi: 10.21037/tau.2018.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jahnukainen K., Ehmcke J., Nurmio M., Schlatt S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res. 2012;72:5174–5178. doi: 10.1158/0008-5472.CAN-12-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fayomi A.P., Peters K., Sukhwani M., Valli-Pulaski H., Shetty G., Meistrich M.L., Houser L., Robertson N., Roberts V., Ramsey C., et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363:1314–1319. doi: 10.1126/science.aav2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Honaramooz A., Snedaker A., Boiani M., Scholer H., Dobrinski I., Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- 149.Honaramooz A. Potential and challenges of testis tissue xenografting from diverse ruminant species. Biosci. Proc. 2019;8 doi: 10.1530/biosciprocs.8.019. [DOI] [Google Scholar]
- 150.Honaramooz A., Li M.-W., Penedo M.C.T., Meyers S., Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol. Reprod. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- 151.Schlatt S., Honaramooz A., Ehmcke J., Goebell P.J., Rübben H., Dhir R., Dobrinski I., Patrizio P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum. Reprod. 2006;21:384–389. doi: 10.1093/humrep/dei352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van Saen D., Goossens E., Bourgain C., Ferster A., Tournaye H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum. Reprod. 2011;26:282–293. doi: 10.1093/humrep/deq321. [DOI] [PubMed] [Google Scholar]
- 153.de Michele F., Poels J., Weerens L., Petit C., Evrard Z., Ambroise J., Gruson D., Wyns C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2017;32:32–45. doi: 10.1093/humrep/dew300. [DOI] [PubMed] [Google Scholar]
- 154.Agarwal A., Mulgund A., Hamada A., Chyatte M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nordkap L., Joensen U.N., Blomberg Jensen M., Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Mol. Cell. Endocrinol. 2012;355:221–230. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 156.Hamada A., Esteves S.C., Nizza M., Agarwal A. Unexplained Male infertility: Diagnosis and Management. Int. Braz J. Urol. 2012;38:576–594. doi: 10.1590/S1677-55382012000500002. [DOI] [PubMed] [Google Scholar]
- 157.Honaramooz A., Megee S.O., Rathi R., Dobrinski I. Building a testis: Formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol. Reprod. 2007;76:43–47. doi: 10.1095/biolreprod.106.054999. [DOI] [PubMed] [Google Scholar]
- 158.Kimura Y., Yanagimachi R. Development of normal mice from oocytes injected with secondary spermatocyte nuclei. Biol. Reprod. 1995;53:855–862. doi: 10.1095/biolreprod53.4.855. [DOI] [PubMed] [Google Scholar]
- 159.Sofikitis N., Mantzavinos T., Loutradis D., Yamamoto Y., Tarlatzis V., Miyagawa I. Ooplasmic injections of secondary spermatocytes for non-obstructive azoospermia. Lancet. 1998;351:1177–1178. doi: 10.1016/S0140-6736(05)79121-2. [DOI] [PubMed] [Google Scholar]