Abstract
Glaucoma is one of the leading causes of blindness worldwide, and as the proportion of those over age 40 increases, so will the prevalence of glaucoma. The pathogenesis of primary open angle glaucoma (POAG) is unclear and multiple ocular risk factors have been proposed, including intraocular pressure, ocular perfusion pressure, ocular blood flow, myopia, central corneal thickness, and optic disc hemorrhages. The purpose of this review was to analyze the association between systemic vascular risk factors (including hypertension, diabetes, age, and migraine) and POAG, based on major epidemiological studies. Reports presenting the association between POAG and systemic vascular risk factors included a total of over 50,000 patients. Several epidemiological studies confirmed the importance of vascular risk factors, particularly hypertension and blood pressure dipping, in the pathogenesis and progression of glaucomatous optic neuropathy. We found that diabetes mellitus is associated with elevated intraocular pressure, but has no clear association with POAG. No significant correlation between migraine and POAG was found, however, the definition of migraine varied between studies.
Keywords: primary open angle glaucoma, diabetes, hypertension, migraine, population based study
1. Introduction
Glaucoma is one of the leading causes of blindness worldwide [1]. As the proportion of those over age 40 increases, so will the prevalence of glaucoma. These demographics will challenge our resources and ingenuity [2]. Primary open angle glaucoma (POAG) is the most common form of the disease worldwide, particularly in Africa and in the Western countries [3,4,5]. POAG is defined as a progressive optic neuropathy with loss of ganglion cells and visual field deterioration in eyes with gonioscopically open angles, with or without elevated intraocular pressure (IOP). The pathogenesis of POAG is unclear. Multiple ocular risk factors have been proposed, including IOP, ocular perfusion pressure, ocular blood flow, myopia, central corneal thickness, and optic disc hemorrhages. Systemic risk factors include age, smoking, African ancestry, family history, genetic factors, systemic hypertension (HTN), low blood pressure (BP) (particularly a nocturnal drop in BP), atherosclerosis, lipid dysregulation, type 2 diabetes mellitus (DM), glucose intolerance, obesity, vasospasm, migraine, Raynaud syndrome, stress, and primary vascular dysregulation [5,6,7,8,9,10,11,12]. Genetic abnormalities are believed to initiate a cascade of events that lead to glaucomatous optic nerve injury and remodeling. Several studies have revealed a significant role of the myocilin, optineurin, and cytochrome CYP1B1 genes in glaucoma development [13,14,15,16]. Moreover, genome-wide association studies have shown associations of sequence variants [17,18,19,20,21] and single-nucleotide polymorphisms [22,23,24,25,26] with POAG. Notwithstanding the role of genetic mutations and variations on glaucoma onset, the role of different systemic risk factors remains debatable.
The purpose of this study was to analyze the association of systemic vascular risk factors (including HTN, diabetes, age, and migraine) and POAG, based on major epidemiological studies.
2. Materials and Methods
2.1. Literature Search
We used the PubMed database to identify studies that included the term glaucoma in association with the following keywords (Appendix A)—vascular risk factors, systemic risk factors, blood pressure, systemic hypertension, systemic hypotension, diabetes, dipping, vasospasm, age, and migraine. The search identified 270 unique articles. Only English-language articles were selected. Articles cited in the reference lists of other publications and review articles were also considered for inclusion. Studies were critically reviewed to create an overview and guidance for further search. No attempts to discover unpublished data were made. Articles providing original research were included. We also incorporated information from relevant reviews and meta-analyses.
2.2. Study Selection
We identified all studies evaluating the association between the selected risk factors and POAG. Articles were included in our meta-analyses if they met the following criteria—(1) the study design was a population-based study; (2) the association of the exposure of interest with POAG was evaluated; (3) the outcome of interest was POAG; and (4) risk ratio (RR), odds ratio (OR), hazard ratio (HR), or proportions and their corresponding 95% confidence interval (CI) (or data to calculate them) were reported. If more than one identified article reported on the same study population, we selected the study with the longest follow-up or the most recent study. We assessed the risk of bias using the methods described by Sanderson et al. [27]; we evaluated the methods for selecting study participants, various study designs, and criteria for defining exposures and outcomes. Studies were included in our meta-analyses if they provided quantitative estimates of POAG prevalence in population-based surveys. We excluded research evaluating association between vascular risk factors and normal tension glaucoma. Investigations were not excluded on the basis of clinical definitions of POAG or methods used to diagnose POAG cases; studies using self-reported diagnosis of glaucoma were excluded. Articles that did not meet the aforementioned criteria were not included in the meta-analysis, however, could be presented within the discussion.
2.3. Data Extraction
Two authors (A.G. and M.O.) separately reviewed all searched articles to determine the eligible studies and the extracted data from the selected results. Any disagreements were resolved by adjudication. The data extraction was performed using a standardized data-collection form. Information was extracted as follows—the first author’s last name; publication year; country location; characteristics of study population (age, number of participants); number of POAG patients; methods for identification of risk factors for glaucoma in question; RR (or OR) from the most fully adjusted models and its corresponding 95% CI; and statistical adjustments for the confounding factors.
2.4. Statistical Analysis
Statistical calculations were conducted with the Stata package (Special Edition, release 14.2, StatCorp LLC, College Station, TX, USA). The meta-analysis was performed as recommended by the Cochrane Collaboration [28]. In order to present the data visually, forest and funnels plots were created. Forest plots are routinely used in meta-analysis, while funnel plots are a good way to show publication bias; funnel plot asymmetry might reflect differences in the results between studies, or bias of particular studies. Inconsistency was measured with the I2 function [29,30]. A quantity of the I2 equal to 0% indicated no observed heterogeneity, while 100% indicated no consistency of the analyzed data.
3. Results
We collected and analyzed 88 articles dated from 1993 to 2019. Most of the included studies were conducted over the last decade. Studies presenting the association between POAG and systemic vascular risk factors included a total of over 50,000 patients (Table 1). Only one study assessed all of the analyzed risk factors [31]. Other studies investigated HTN (13 studies), diabetes (12 studies), age (5 studies), and migraine (4 studies). The meta-analyses encompassed eighteen original papers and the results were presented on forest plots.
Table 1.
Studies investigating systemic vascular risk factors in primary open angle glaucoma.
| Study | Country (Population) | Study Design | Participants (n) |
Patients’ Age | Statistically Significant Association with | ||||
|---|---|---|---|---|---|---|---|---|---|
| Systolic Hypertension | Low DBP | Diabetes Mellitus | Age | Migraine | |||||
| Barbados Eye Study [32] |
Barbados (African descent) | Population-based cohort study (prospective) |
3222 | 40–84 | No (p = 0.26) |
Yes | No (p = 0.49) |
Yes (p = 0.0001) |
N/A |
| Rotterdam Study [33,34] |
The Netherlands | Population-based cross-sectional study (prospective) |
4187 | 55+ | Yes | No | Yes | NA | NA |
| Egna Neumarkt study [35] |
Italy | Population-based cross-sectional study | 4297 | 40+ | Some association | Yes (for low DPP) | NA | NA | NA |
| Baltimore Eye Survey [36,37] |
USA | Population-based prevalence survey | 5308 | 40+ | Yes (SBP and DBP) |
Yes for low DPP |
No | NA | NA |
| Beaver Dam Study [38,39] |
USA | Population based study. | 4926 | 43–84 | NA | NA | Yes (p = 0.004) |
NA | No (p = 0.87) |
| Blue Mountains Eye Study [11,40,41] |
Australia | Population-based survey | 3654 | 49–96 | Yes | Some association (p = 0.05 when Age and Sex Adjusted) |
Yes | NA | Yes (in 70–79 years of age) |
| The Singapore Malay eye study [42] |
Singapore (Malay) | Population-based cross-sectional study | 3280 | 40–80 | No | No | No | NA | NA |
| The Beijing Eye Study [43,44] |
China | Population-based survey | 3251 | 40+ | No risk for glaucoma progression | No risk for progression of glaucoma | No | NA | NA |
| Tajimi Study [31] |
Japan | Population-based cross-sectional study | 2874 | 40+ | Yes (p = 0.0094) |
NA | No (p = 0.85) |
Yes (p < 0.0001) |
No (p > 0.99) |
| Early Manifest Glaucoma Trial [45] |
Sweden | Randomized clinical trial | 255 | 50–80 | No risk for progression of glaucoma (p = 0.1279) |
Yes (risk for progression of glaucoma in patients with low baseline IOP) |
NA | Yes (p = 0.0270) |
NA |
| Thessaloniki Eye trial [46] |
Greece | Population-based cross-sectional study | 1991 | 60+ | Yes (p = 0.033) |
NA | Yes (p = 0.003) |
Yes (p = 0.001) |
NA |
| Visual Impairment Project [47] |
Australia | Cohort study | 2415 | 40+ | NA | NA | NA | Yes | NA |
| African eyes [48] |
Nigeria | Hospital-based multicenter study (prospective) |
506 | 10+ | Yes | No | Yes | NA | NA |
| Optime study [49] |
Italy | Observational survey | 3852 | 55+ | Yes | No | Yes | NA | No |
| Los Angeles Latino Eye Study [50,51] |
USA (Latino) | Population-based cross-sectional study | 6130 | 40+ | Yes | No | Yes | NA | NA |
DBP, diastolic blood pressure; DPP, diastolic perfusion pressure; DPP = DBP − intraocular pressure. NA, not applicable; SBP, systolic blood pressure; p values are presented if provided in the study.
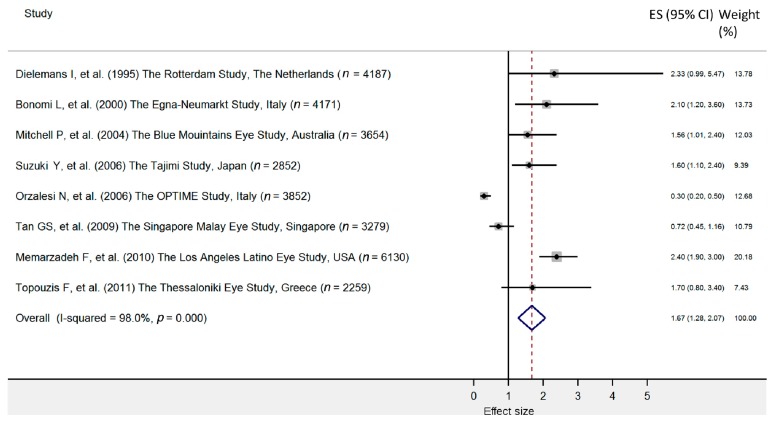
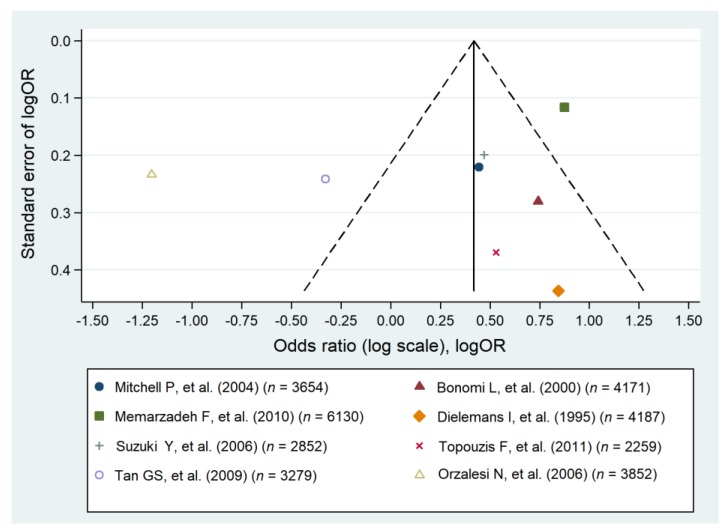
Most of the analyzed studies identified an association of HTN with glaucoma and optic nerve damage [31,33,35,40,46,48,49,50,52] (Table 1). The meta-analysis involving eight original studies on the prevalence of arterial HTN revealed a significant relationship with the occurrence of POAG (pooled OR = 1.67; 95% CI: 1.28–2.07) (Figure 1). Despite the high heterogeneity of our results (I2 = 98.0%), it could be concluded that hypertension is associated with POAG (Figure 2).
Figure 1.
Forest plot of cohort studies that evaluated arterial hypertension as a risk factor for primary open angle glaucoma. The effect size (square, with size proportional to the weights used) and 95% confidence intervals (horizontal lines) is presented for each study. The overall measure revealed by pooled analysis is marked with the center of the diamond, while the associated confidence intervals are indicated by the lateral tips of the diamond [31,33,35,40,42,46,49,50].
Figure 2.
Funnel plot of cohort studies that evaluated arterial hypertension as a risk factor for primary open angle glaucoma [31,33,35,40,42,46,49,50].
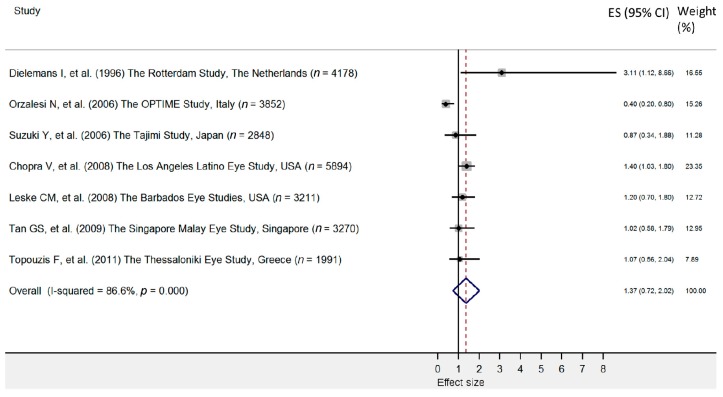
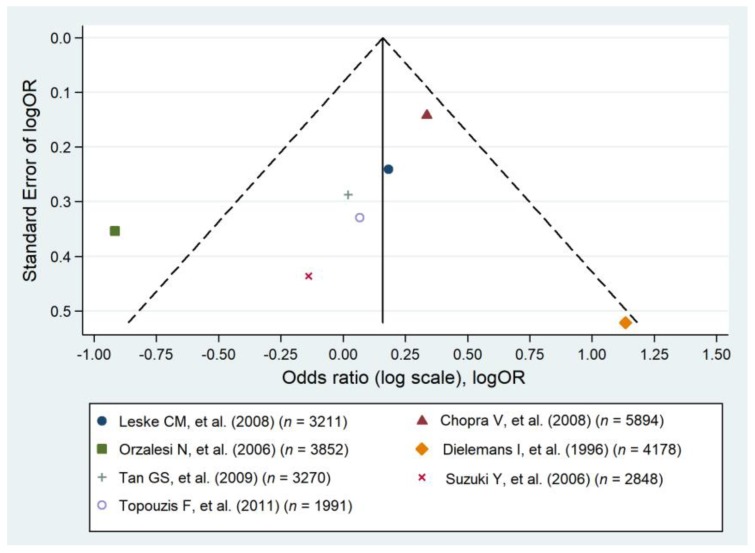
It was reported that DM is associated with an elevated IOP [53]; therefore, DM influence POAG by mediation of IOP. However, current evidence to support a direct relationship remains conflicting and is still not fully understood [6]. Seven population-based studies reported a statistically significant association between DM and POAG [34,38,41,46,48,49,51], but five studies did not confirm an association [31,32,36,42,43]. In the pooled analysis comprising seven original studies no significant relationship between the prevalence of POAG and DM was identified (pooled OR = 1.37; 95% CI: 0.72–2.02), and heterogeneity was high (I2 = 86.6%) (Figure 3 and Figure 4).
Figure 3.
Forest plot of cohort studies that evaluated diabetes as a risk factor for primary open angle glaucoma. The effect size (square, with size proportional to weights used) and 95% confidence intervals (horizontal lines) is presented for each study. The overall measure revealed by pooled analysis is marked with the center of the diamond, while the associated confidence intervals are marked by lateral tips of diamond [31,32,34,42,46,49,51].
Figure 4.
Funnel plot of cohort studies that evaluated diabetes as a risk factor for primary open angle glaucoma [31,32,34,42,46,49,51].
All studies that investigated the correlation between age and POAG confirmed this association [31,32,45,46,47]. Studies assessing the POAG prevalence and patient age approached the issue in very different ways. Various age ranges were assessed; some studies presented ORs [31,33,34,35,36,38,40,41,42,44,46,48,49,50,51], others provided RRs [32,47,54] or HRs [45], while some reported proportions and their corresponding 95% CIs [37,43]. A number of studies did not provide the effect sizes. There were also original articles in which age was not evaluated as an independent variable. In order to perform a reliable meta-analysis considering the prevalence of POAG versus age, the individual study results must be coherent, i.e., contain the same effect size measures (either OR or RR), and identical class intervals for the patients’ age. Given the variable quality and heterogeneity of the reviewed studies, and that the results focused upon the relationship bet ween age and prevalence of POAG, a meta-analysis was not performed.
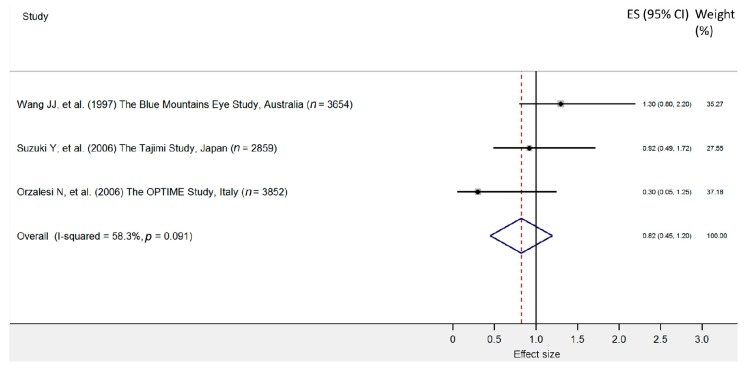
One study suggested an association between migraine and glaucoma among patients in the age range of 70 to 79 [11]. Three studies identified no association between glaucoma and migraine [31,39,49]. Our meta-analysis encompassing three original studies identified no association between glaucoma and migraine (pooled OR = 0.82; 95% CI: 0.45–1.20). As the heterogeneity was low, the results can be accepted with greater certainty (Figure 5 and Figure 6).
Figure 5.
Forest plot of cohort studies that evaluated migraine as a risk factor for primary open angle glaucoma. The effect size (square, with size proportional to weights used) and 95% confidence intervals (horizontal lines) is presented for each study. The overall measure revealed by pooled analysis is marked with the center of the diamond, while the associated confidence intervals are marked by the lateral tips of the diamond [11,31,49].
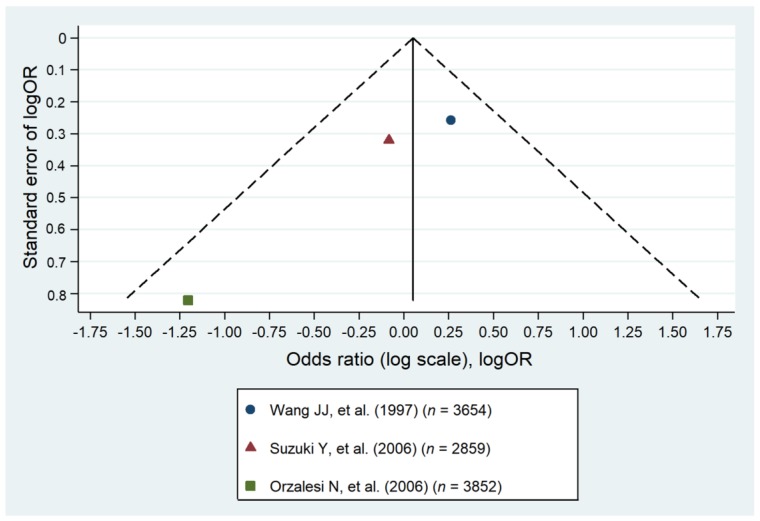
Figure 6.
Forest plot of the cohort studies that evaluated migraine as a risk factor for the primary open angle glaucoma [11,31,49].
4. Discussion
4.1. Systemic Hypertension and Hypotension
The majority of studies defined HTN as systolic BP (SBP) greater than 160 mm Hg, diastolic BP (DBP) greater than 95 mmHg, or the use of anti-HTN medications ascertained from interview with patients. The study by Topouzis et al. found that both treated HTN (with BP within normal limits), and high systolic or diastolic BP are risk factors for POAG, with an OR for the presence of POAG being 2.04 (95% CI: 0.88–4.73) and 1.43 (95% CI 0.70–2.91), respectively [31,46]. Moreover, the severity of glaucoma was found to be higher in patients with HTN than in glaucomatous individuals with a normal BP [55]. The associations of SBP and DBP with glaucomatous optic neuropathy depend on the specifics of the patients studied—age, ethnic origin, concurrent diseases, other risk factors, treatment of HTN, type of medication used, and vascular endothelial function. Several mechanisms have been suggested to explain the risk of the developing POAG in individuals with HTN [56]. Abnormal neuro-metabolic-endothelial mechanisms of the vascular tone control in glaucoma patients is related to the effects of vasoactive factors—endothelin–1 (ET-1), neuropeptide Y (NPY), nitric oxide (NO), prostacyclin (PGI 2), tumor necrosis factor (TNF-alpha), cyclooxygenase (COX-2), and metalloproteinases (MMP-9). Variation in the levels of these factors leads to endothelial dysfunction and to dysregulation of the autonomic nervous system. These abnormalities can produce vasoconstriction and optic nerve head ischemia in normal tension glaucoma (NTG) [3]. Theoretically, the duration of HTN and degree of vascular damage could be correlated with the risk of glaucoma; however, none of the studies analyzed such an association. A study by Jung et al. found that glaucomatous patients with multiple retinal nerve fiber layer (RNFL) defects had a higher prevalence of concomitant vascular diseases (including HTN, end-stage renal disease or cerebrovascular disease) compared to those with glaucoma and no RNFL defects [57]. Additionally, HTN was found to be a risk factor for reduced RNFL in healthy individuals in the European Eye Epidemiology study [58]. These results highlight the potential role of vascular insufficiency, not only as a risk factor of glaucoma, but also for potential glaucomatous damage [57]. Moreover, smoking was found to be a risk factor for POAG, and smoking presumably deteriorated ocular hypoxia caused by the ocular hypertension [59]. The analyzed studies did not determine smoking as a risk factor for POAG incidence [46] or progression [43]. However, heavy smoking, which was defined by a greater number of cigarette packs smoked daily was associated with higher odds of glaucoma [60]. With this, the role of cytochrome CYP1B1 was confirmed in primary congenital glaucoma, as it influenced the development of the trabecular meshwork [61,62]; CYP1B1 is also a potential regulator in energy homeostasis that promotes hypertension as well [63].
Systemic BP has a circadian variation [8] and a growing number of studies analyzed the ocular perfusion pressure, defined as the difference between systemic pressure and IOP. Current evidence suggests that ocular perfusion pressure and IOP variations have a detrimental effect in the glaucomatous eye [64]. In patients with glaucoma and well-controlled HTN, a nocturnal BP fall of more than 10% was associated with a greater visual field defect and a greater degeneration of optic nerve fibers [65]. Nocturnal hypotension, in the presence of other vascular risk factors, might reduce the optic nerve head blood flow below a critical level and might play a role in the pathogenesis of glaucomatous optic neuropathy [66,67]. Another study also suggested that non-physiological nocturnal BP dipping and wider circadian fluctuations in ocular perfusion pressure were linked with the development and progression of glaucoma [8]. In the Maracaibo Aging Study, extreme decreases in nighttime systolic and diastolic BP (defined as a BP decrease greater than 20%, compared to daytime levels) were significant risk factors for glaucomatous damage (with the odds ratio 19.78 and 5.55, respectively) [68]. On the other hand, Yoshikawa et al. found that individuals with glaucoma have higher nighttime systolic BP (by 4.1 mmHg) than the healthy controls [69]. It was suggested that glaucoma progression in such cases is associated with insufficient vascular autoregulation [70]. Patients with POAG and those with NTG exhibit similar increases in nocturnal systemic BP variability, peripheral arterial stiffness, and carotid intima-media thickness, as well as reduced ocular perfusion pressure [71]. According to Flammer et al. both excessive nocturnal BP drop (over-dipping), or absent BP drop (non-dipping) are risk factors for glaucoma progression [70]. Moreover, low systemic DBP itself was defined as a risk factor for glaucoma progression [42,50].
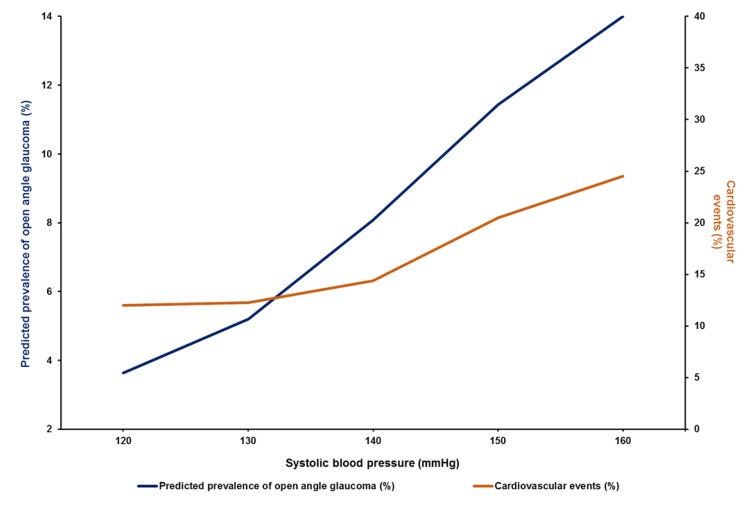
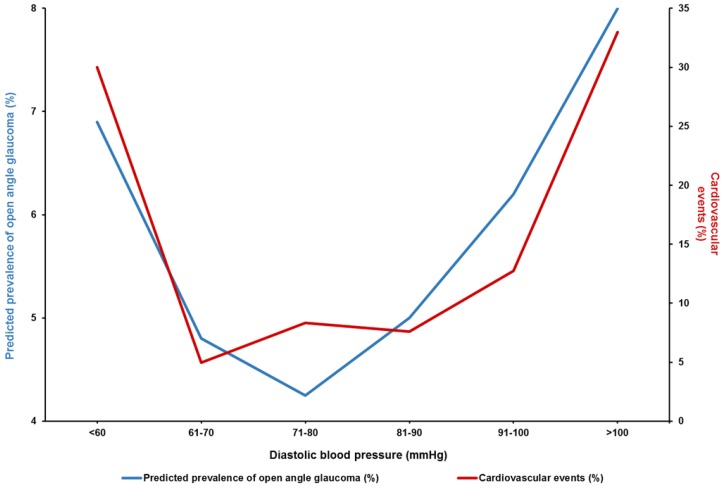
Abnormally low diastolic double product (dDP = DBP × heart rate) can be another marker of cardiovascular autonomic dysregulation, resulting in low ocular perfusion in NTG patients [72]. A positive correlation between SBP and the predicted prevalence of open angle glaucoma was reported in the Los Angeles Latino Eye Study [50]. A similar influence of SBP on cardiovascular risk factors was shown by the cardiovascular Ontarget trial (Figure 7) [73]. This study aimed to analyze the impact of systemic BP on cardiovascular events in well-treated, high-risk patients, enrolled in a large clinical trial. The analysis revealed that very high SBP was associated with glaucoma; moreover, a clear relationship between DBP and the predicted prevalence of OAG was observed (Figure 8) [50]. A very similar relation between DBP and cardiovascular risk (a so-called J-curve) was also observed in Coronary Artery Disease (CAD) patients, in the TNT study [74]. The TNT study showed that in patients with CAD, high DBP (>90–100 mmHg) and low DBP (<60–70 mmHg) portend an increased risk of future cardiovascular events. Randomized controlled trials of antihypertensive treatment provide strong evidence for J-shaped relationships between both DBP and SBP, and mortality (including all-cause mortality, cardiovascular mortality, nonfatal and fatal myocardial infarct, heart failure, stroke) in high-risk cardiovascular populations, including patients with CAD, DM, and left ventricular hypertrophy [75]. A similar J-shaped association was found between BP and glaucoma progression; patients with both high and low BP were at risk of glaucoma progression [76]. The treatment of HTN could also influence the risk of POAG; oral treatment with beta-blockers was shown to decrease the risk of POAG [77], while intake of calcium channel blockers (and particularly amlodipine) was associated with glaucoma severity and was found to increase the risk of POAG that required surgical intervention [78].
Figure 7.
The relationship between SBP and the predicted prevalence of primary open angle glaucoma (POAG) in participants in the Los Angeles Latino Eye Study and cardiovascular events in the Ontarget study [50,73].
Figure 8.
The relationship between diastolic BP (DBP) and the predicted prevalence of POAG in participants of the Los Angeles Latino Eye Study and cardiovascular events in the TNT study [50,74].
4.2. Diabetes Mellitus
DM is associated with microvascular damage and might affect vascular autoregulation of the retina and the optic nerve [51]. DM is not a homogenous condition. Study populations have varied with respect to diabetes characteristics—the time since diagnosis, type of treatment (diet, oral medication, insulin) as well as the presence of various complications from diabetes. The aforementioned studies did not control for the type of diabetes or the treatments used [31,32,34,36,38,41,42,43,46,48,49,51].
Many hypotheses have been offered to explain the correlation between DM and POAG. The first theory suggests that long-standing hyperglycemia and lipid dysregulation might increase the risk of neuronal stress damage [79]. A second theory proposes that diabetic eyes have a dysregulation in blood flow due to retinal vascular endothelial cell dysfunction; this hypothesis was confirmed both in animal studies and subsequent clinical trials [80]. Another explanation for the association between DM and POAG might be a remodeling of the connective tissue of the optic nerve head and dysregulation at the trabecular meshwork and the lamina cribrosa, with increased IOP and greater mechanical stress on the optic nerve head. Diabetes can exacerbate connective tissue remodeling and amplify these biomechanical changes [81,82]. Vascular endothelial cell dysfunction and loss of retinal pericytes have been described in diabetic retinopathy and are associated with hypoxia [83]. Thus, the function of the endothelium could theoretically be a marker of proper nourishment of the optic nerve cells. Moreover, the anatomical and functional status of the vessels can influence the structure and function of the optic nerve. Additionally, metabolic disturbances of the carbohydrate metabolism could play a role in glaucoma damage and pathogenesis [84]. Finally, diabetes is associated with an increased IOP, and this association is not through the influence of diabetes of central corneal thickness [85]. The aforementioned factors could highlight the importance of vascular risk factors in the pathogenesis of POAG. Interestingly, patients with POAG and treated DM tend to have significantly lower rates of RNFL thinning compared to those without diagnosed DM [86].
Earlier meta-analyses suggested that diabetic patients are at a significantly increased risk of developing POAG [87,88,89]. In a study by Zhao et al. the risk of glaucoma increased by 5% for each year since diabetes diagnosis; their pooled analysis presented a 0.18 mmHg difference between IOP in patients with diabetes, compared to those without diabetes [90]. Our study identified no significant relationship between the prevalence of POAG and diabetes mellitus; the high heterogeneity among studies and, thus, the conclusion, must be interpreted with caution. Results for case-control studies could be different, e.g., in a Korean study, diabetes was associated with POAG in all age groups (the HR was 1.2 for individuals aged 40–59 years, and 1.18 for those aged 60–79 years) [91]. However, it is known that case-control designs are potentially viable to selection bias [92].
4.3. Age
The prevalence of glaucoma varies substantially by geographical region, ethnicity, and age group. The incidence of POAG ranges from 0.8% (in the Caucasian population in the Barbados Eye Study) [93] to 4.4% (African-descent population in the Barbados Eye Study) [32]. The prevalence of glaucoma is the highest in elderly patients. Even greater prevalence rates in the elderly were reported in people of African descent—11% in those aged over 80 years in the Baltimore Eye Survey [37], 23.2% in the patients over 75 years of age in the Salisbury Eye Evaluation Glaucoma Study [94], and 24.8% in the male population aged 80 to 86 years in the Barbados Eye Study [93]. Additionally, a recent database analysis study confirmed a strong relationship between age and the prevalence of POAG, however, the incidence rates increased until the age of 85–89 years, and then slightly decreased in those over 90 years of age [95].
There could be several explanations why the prevalence of glaucoma increases with age. First, experimental studies have shown that with age there is a reduction in trabecular lumen area and a subsequent decrease in aqueous outflow [96]. Second, biomechanics of the eye and optic nerve changed considerably with aging; it was proposed that the aged optic nerve has a more stiffened peripapillary scleral and lamina cribrosa connective tissue, and thus, the optic nerve head is more susceptible to axonal damage [97]. Moreover, as there is a high density of mitochondria around the optic disc, age-related mitochondrial dysfunction would reduce the energy supply [98,99]. Finally, aging-induced retrobulbar hemodynamic changes are similar to those seen in glaucoma; thus vascular changes could contribute to an increased risk for glaucoma in older age [9].
4.4. Migraine
Both migraine and glaucoma are associated with systemic vascular dysregulation or vasospasm [7]. Moreover, as migraine is accompanied with persistent blood flow changes in several brain regions [100], similarly poor blood flow at the optic nerve was reported in glaucoma [101]. Our meta-analysis demonstrated no significant relationship between migraine and the prevalence of POAG. However, the Collaborative Normal-Tension Glaucoma Study Group analyzed the risk factors for progression of visual field abnormalities in NTG and found migraine to be an independent risk factor for more rapid deterioration [102]. Moreover, migraineurs are more likely to develop POAG compared to subjects without migraine (95% confidence interval of POAG incidence: 1.20–2.36) [103]. Age, hyperlipidemia, and DM were noted as three significant risk factors for developing POAG in migraineurs [103]. Our meta-analysis encompassing three original studies identified no association of glaucoma and migraine. Similar results were reported in a recent meta-analysis by Xu et al.; however, one part of their analysis which included case-control studies found a significant association between migraine and POAG [104].
It should be underlined that the definition of migraine differed among studies. For example, in the Beaver Dam Eye Study, which presented no association between glaucoma and migraine, the question used for migraine diagnosis might have been overly restrictive—“Have you ever had migraine headaches (with vomiting, light flashes, or severe enough to keep you in bed) [39]?” Moreover, in the included studies migraine was only analyzed in a post-hoc fashion.
5. Conclusions
Several epidemiological studies confirmed the importance of vascular risk factors in the pathogenesis and progression of glaucomatous optic neuropathy, including hypertension and BP dipping. The pooled analysis confirmed hypertension as the most significant risk factor for primary open angle glaucoma. The association between POAG and diabetes mellitus type 2 or migraine still needs to be evaluated in future studies.
Appendix A. Method of Literature Search
The literature was searched on the MEDLINE database using PubMed, for articles in English, published between the 1990 and 2019, using the keywords: “vascular risk factors”, “systemic risk factors”, “blood pressure”, “systemic hypertension”, “systemic hypotension”, “diabetes”, “dipping”, “vasospasm”, “age”, and “migraine”. All returned articles, as well as their references, were screened for relevant data on the association between the selected risk factors and POAG. We included only population-based studies in which the exposure of interest and POAG was evaluated. We excluded studies using self-reported diagnosis of glaucoma. The reference lists of relevant papers were reviewed to identify additional papers, this process was iterated several times.
Funding
This research received no external funding.
Conflicts of Interest
The authors report no commercial or proprietary interest in any product or concept discussed in this article. Grzybowski reports grants, personal fees, and non-financial support from Bayer; grants, non-financial support from Novartis; non-financial support from Alcon; personal fees and non-financial support from Valeant; grants and non-financial support from Allergan; grants and non-financial support from Pfizer; grants and financial support from Santen. Och has nothing to disclose. Kanclerz reports non-financial support from Visim. No conflicting relationship exists for any author. Leffler and De Moraes has nothing to disclose.
References
- 1.Quigley H.A. Number of people with glaucoma worldwide. Am. J. Ophthalmol. 1996;122:460. doi: 10.1016/S0002-9394(14)72088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreb R.N., Khaw P.T. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 4.Tatham A.J., Weinreb R.N., Zangwill L.M., Liebmann J.M., Girkin C.A., Medeiros F.A. The relationship between cup-to-disc ratio and estimated number of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2013;54:3205–3214. doi: 10.1167/iovs.12-11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EGS Foundation European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition—Part 1. Supported by the EGS Foundation. Br. J. Ophthalmol. 2017;101:1–72. doi: 10.1136/bjophthalmol-2016-EGSguideline.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagi M., Kawasaki R., Wang J.J., Wong T.Y., Crowston J., Kiuchi Y. Vascular risk factors in glaucoma: A review. Clin. Experiment. Ophthalmol. 2011;39:252–258. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 7.Pache M., Flammer J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Surv. Ophthalmol. 2006;51:179–212. doi: 10.1016/j.survophthal.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Werne A., Harris A., Moore D., BenZion I., Siesky B. The circadian variations in systemic blood pressure, ocular perfusion pressure, and ocular blood flow: Risk factors for glaucoma? Surv. Ophthalmol. 2008;53:559–567. doi: 10.1016/j.survophthal.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Harris A., Harris M., Biller J., Garzozi H., Zarfty D., Ciulla T.A., Martin B. Aging affects the retrobulbar circulation differently in women and men. Arch. Ophthalmol. 2000;118:1076–1080. doi: 10.1001/archopht.118.8.1076. [DOI] [PubMed] [Google Scholar]
- 10.Flammer J., Pache M., Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog. Retin. Eye Res. 2001;20:319–349. doi: 10.1016/S1350-9462(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 11.Wang J.J., Mitchell P., Smith W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology. 1997;104:1714–1719. doi: 10.1016/S0161-6420(97)30075-X. [DOI] [PubMed] [Google Scholar]
- 12.Costa V.P., Harris A., Anderson D., Stodtmeister R., Cremasco F., Kergoat H., Lovasik J., Stalmans I., Zeitz O., Lanzl I., et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014;92:e252–e266. doi: 10.1111/aos.12298. [DOI] [PubMed] [Google Scholar]
- 13.Tamm E.R. Myocilin and glaucoma: Facts and ideas. Prog. Retin. Eye Res. 2002;21:395–428. doi: 10.1016/S1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 14.Sarfarazi M., Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol. Clin. North Am. 2003;16:529–541. doi: 10.1016/S0896-1549(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti S., Ghanekar Y., Kaur K., Kaur I., Mandal A.K., Rao K.N., Parikh R.S., Thomas R., Majumder P.P. A polymorphism in the CYP1B1 promoter is functionally associated with primary congenital glaucoma. Hum. Mol. Genet. 2010;19:4083–4090. doi: 10.1093/hmg/ddq309. [DOI] [PubMed] [Google Scholar]
- 16.Fingert J.H. Primary open-angle glaucoma genes. Eye. 2011;25:587–595. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorleifsson G., Walters G.B., Hewitt A.W., Masson G., Helgason A., DeWan A., Sigurdsson A., Jonasdottir A., Gudjonsson S.A., Magnusson K.P., et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazawa A., Fuse N., Mengkegale M., Ryu M., Seimiya M., Wada Y., Nishida K. Association between primary open-angle glaucoma and WDR36 DNA sequence variants in Japanese. Mol. Vis. 2007;13:1912–1919. [PubMed] [Google Scholar]
- 19.Lopez-Martinez F., Lopez-Garrido M.-P., Sanchez-Sanchez F., Campos-Mollo E., Coca-Prados M., Escribano J. Role of MYOC and OPTN sequence variations in Spanish patients with primary open-angle glaucoma. Mol. Vis. 2007;13:862–872. [PMC free article] [PubMed] [Google Scholar]
- 20.Alward W.L.M., Kwon Y.H., Kawase K., Craig J.E., Hayreh S.S., Johnson A.T., Khanna C.L., Yamamoto T., Mackey D.A., Roos B.R., et al. Evaluation of optineurin sequence variations in 1048 patients with open-angle glaucoma. Am. J. Ophthalmol. 2003;136:904–910. doi: 10.1016/S0002-9394(03)00577-4. [DOI] [PubMed] [Google Scholar]
- 21.He J.N., Lu S., Chen L.J., Tam P.O.S., Zhang B.N., Leung C.K.S., Pang C.P., Tham C.C.Y., Chu W.K. Coding Region Mutation Screening in Optineurin in Chinese Normal-Tension Glaucoma Patients. Dis. Markers. 2019;2019:5820537. doi: 10.1155/2019/5820537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Lin Y., Vithana E.N., Jia L., Zuo X., Wong T.Y., Chen L.J., Zhu X., Tam P.O.S., Gong B., et al. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat. Genet. 2014;46:1115–1119. doi: 10.1038/ng.3078. [DOI] [PubMed] [Google Scholar]
- 23.Bailey J.N.C., Loomis S.J., Kang J.H., Allingham R.R., Gharahkhani P., Khor C.C., Burdon K.P., Aschard H., Chasman D.I., Igo R.P., Jr., et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat. Genet. 2016;48:189–194. doi: 10.1038/ng.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M., Yu X., Xu J., Ma J., Chen X., Chen B., Gu Y., Wang K. Association of Gene Polymorphisms with Primary Open Angle Glaucoma: A Systematic Review and Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2019;60:1105–1121. doi: 10.1167/iovs.18-25922. [DOI] [PubMed] [Google Scholar]
- 25.Rong S.S., Chen L.J., Leung C.K.S., Matsushita K., Jia L., Miki A., Chiang S.W.Y., Tam P.O.S., Hashida N., Young A.L., et al. Ethnic specific association of the CAV1/CAV2 locus with primary open-angle glaucoma. Sci. Rep. 2016;6:27837. doi: 10.1038/srep27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan B.J., Wang D.Y., Fan D.S.P., Tam P.O.S., Lam D.S.C., Tham C.C.Y., Lam C.Y., Lau T.C., Pang C.P. SNPs and interaction analyses of myocilin, optineurin, and apolipoprotein E in primary open angle glaucoma patients. Mol. Vis. 2005;11:625–631. [PubMed] [Google Scholar]
- 27.Sanderson S., Tatt I.D., Higgins J.P.T. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. Int. J. Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 28.Meta-Analysis: What, Why, and How. [(accessed on 14 February 2020)]; Available online: https://uk.cochrane.org/news/meta-analysis-what-why-and-how.
- 29.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.2 Identifying and Measuring Heterogeneity. [(accessed on 14 February 2020)]; Available online: https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm.
- 31.Suzuki Y., Iwase A., Araie M., Yamamoto T., Abe H., Shirato S., Kuwayama Y., Mishima H., Shimizu H., Tomita G. Risk Factors for Open-Angle Glaucoma in a Japanese Population. The Tajimi Study. Ophthalmology. 2006;113:1613–1617. doi: 10.1016/j.ophtha.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 32.Leske M.C., Wu S.-Y., Hennis A., Honkanen R., Nemesure B. BESs Study Group Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Dielemans I., Vingerling J.R., Algra D., Hofman A., Grobbee D.E., de Jong P.T.V.M. Primary Open-angle Glaucoma, Intraocular Pressure, and Systemic Blood Pressure in the General Elderly Population. The Rotterdam Study. Ophthalmology. 1995;102:54–60. doi: 10.1016/S0161-6420(95)31054-8. [DOI] [PubMed] [Google Scholar]
- 34.Dielemans I., de Jong P.T., Stolk R., Vingerling J.R., Grobbee D.E., Hofman A. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population. The Rotterdam Study. Ophthalmology. 1996;103:1271–1275. doi: 10.1016/S0161-6420(96)30511-3. [DOI] [PubMed] [Google Scholar]
- 35.Bonomi L., Marchini G., Marraffa M., Bernardi P., Morbio R., Varotto A. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–1293. doi: 10.1016/S0161-6420(00)00138-X. [DOI] [PubMed] [Google Scholar]
- 36.Tielsch J.M., Katz J., Quigley H.A., Javitt J.C., Sommer A. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey. Ophthalmology. 1995;102:48–53. doi: 10.1016/S0161-6420(95)31055-X. [DOI] [PubMed] [Google Scholar]
- 37.Tielsch J.M., Sommer A., Katz J., Royall R.M., Quigley H.A., Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374. doi: 10.1001/jama.1991.03470030069026. [DOI] [PubMed] [Google Scholar]
- 38.Klein B.E., Klein R., Jensen S.C. Open-angle glaucoma and older-onset diabetes. The Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–1177. doi: 10.1016/S0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 39.Klein B.E., Klein R., Meuer S.M., Goetz L.A. Migraine headache and its association with open-angle glaucoma: The Beaver Dam Eye Study. Investig. Ophthalmol. Vis. Sci. 1993;34:3024–3027. [PubMed] [Google Scholar]
- 40.Mitchell P., Lee A.J., Rochtchina E., Wang J.J. Open-angle glaucoma and systemic hypertension: The blue mountains eye study. J. Glaucoma. 2004;13:319–326. doi: 10.1097/00061198-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell P., Smith W., Chey T., Healey P.R. Open-angle glaucoma and diabetes: The Blue Mountains eye study, Australia. Ophthalmology. 1997;104:712–718. doi: 10.1016/S0161-6420(97)30247-4. [DOI] [PubMed] [Google Scholar]
- 42.Tan G.S., Wong T.Y., Fong C.-W., Aung T. Singapore Malay Eye Study Diabetes, metabolic abnormalities, and glaucoma. Arch. Ophthalmol. 2009;127:1354–1361. doi: 10.1001/archophthalmol.2009.268. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y.X., Hu L.N., Yang H., Jonas J.B., Xu L. Frequency and associated factors of structural progression of open-angle glaucoma in the Beijing Eye Study. Br. J. Ophthalmol. 2012;96:811–815. doi: 10.1136/bjophthalmol-2011-301224. [DOI] [PubMed] [Google Scholar]
- 44.Wang S., Xu L., Jonas J.B., Wong T.Y., Cui T., Li Y., Wang Y.X., You Q.S., Yang H., Sun C. Major Eye Diseases and Risk Factors Associated with Systemic Hypertension in an Adult Chinese Population. Ophthalmology. 2009;116:2373–2380. doi: 10.1016/j.ophtha.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 45.Leske M.C., Heijl A., Hyman L., Bengtsson B., Dong L., Yang Z. EMGT Group Predictors of Long-term Progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Topouzis F., Wilson M.R., Harris A., Founti P., Yu F., Anastasopoulos E., Pappas T., Koskosas A., Salonikiou A., Coleman A.L. Risk factors for primary open-angle glaucoma and pseudoexfoliative glaucoma in the Thessaloniki eye study. Am. J. Ophthalmol. 2011;152:219–228. doi: 10.1016/j.ajo.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 47.Le A., Mukesh B.N., McCarty C.A., Taylor H.R. Risk factors associated with the incidence of open-angle glaucoma: The visual impairment project. Investig. Ophthalmol. Vis. Sci. 2003;44:3783–3789. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 48.Omoti A.E., Enock M.E., Okeigbemen V.W., Akpe B.A., Fuh U.C. Vascular risk factors for open angle glaucoma in african eyes. Middle East Afr. J. Ophthalmol. 2009;16:146–150. doi: 10.4103/0974-9233.56229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orzalesi N., Rossetti L., Omboni S., OPTIME Study Group (Osservatorio sulla Patologia glaucomatosa, Indagine Medico Epidemiologica) CONPROSO (Collegio Nazionale dei Professori Ordinari di Scienze Oftalmologiche) Vascular risk factors in glaucoma: The results of a national survey. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:795–802. doi: 10.1007/s00417-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 50.Memarzadeh F., Ying-Lai M., Chung J., Azen S.P., Varma R., Los Angeles Latino Eye Study Group Blood pressure, perfusion pressure, and open-angle glaucoma: The Los Angeles Latino Eye Study. Investig. Ophthalmol. Vis. Sci. 2010;51:2872–2877. doi: 10.1167/iovs.08-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chopra V., Varma R., Francis B.A., Wu J., Torres M., Azen S.P., Los Angeles Latino Eye Study Group Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–232. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tielsch J.M., Katz J., Sommer A., Quigley H.A., Javitt J.C. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch. Ophthalmol. 1995;113:216–221. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 53.Klein B.E.K., Klein R., Moss S.E. Intraocular Pressure in Diabetic Persons. Ophthalmology. 1984;91:1356–1360. doi: 10.1016/S0161-6420(84)34142-2. [DOI] [PubMed] [Google Scholar]
- 54.Gramer G., Weber B.H.F., Gramer E. Migraine and Vasospasm in Glaucoma: Age-Related Evaluation of 2027 Patients with Glaucoma or Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2015;56:7999–8007. doi: 10.1167/iovs.15-17274. [DOI] [PubMed] [Google Scholar]
- 55.Khatri A., Shrestha J.K., Thapa M., Khatri B.K., Kharel M. Severity of primary open-angle glaucoma in patients with hypertension and diabetes. Diabetes Metab. Syndr. Obes. 2018;11:209–215. doi: 10.2147/DMSO.S160978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tham Y.-C., Cheng C.-Y. Associations between chronic systemic diseases and primary open angle glaucoma: An epidemiological perspective. Clin. Experiment. Ophthalmol. 2016;45:24–32. doi: 10.1111/ceo.12763. [DOI] [PubMed] [Google Scholar]
- 57.Jung K.I., Kim S.J., Park C.K. Systemic Vascular Risk Factors for Multiple Retinal Nerve Fiber Layer Defects. Sci. Rep. 2018;8:7797. doi: 10.1038/s41598-018-26160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mauschitz M.M., Bonnemaijer P.W.M., Diers K., Rauscher F.G., Elze T., Engel C., Loeffler M., Colijn J.M., Ikram M.A., Vingerling J.R., et al. Systemic and Ocular Determinants of Peripapillary Retinal Nerve Fiber Layer Thickness Measurements in the European Eye Epidemiology (E3) Population. Ophthalmology. 2018;125:1526–1536. doi: 10.1016/j.ophtha.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Y., Zhu W., Wang C. The effect of smoking on the risk of primary open-angle glaucoma: An updated meta-analysis of six observational studies. Public Health. 2016;140:84–90. doi: 10.1016/j.puhe.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Law S.M., Lu X., Yu F., Tseng V., Law S.K., Coleman A.L. Cigarette smoking and glaucoma in the United States population. Eye. 2018;32:716–725. doi: 10.1038/eye.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teixeira L.B.C., Zhao Y., Dubielzig R.R., Sorenson C.M., Sheibani N. Ultrastructural abnormalities of the trabecular meshwork extracellular matrix in Cyp1b1-deficient mice. Vet. Pathol. 2015;52:397–403. doi: 10.1177/0300985814535613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y., Wang S., Sorenson C.M., Teixeira L., Dubielzig R.R., Peters D.M., Conway S.J., Jefcoate C.R., Sheibani N. Cyp1b1 mediates periostin regulation of trabecular meshwork development by suppression of oxidative stress. Mol. Cell. Biol. 2013;33:4225–4240. doi: 10.1128/MCB.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah B.R., Xu W., Mraz J. Cytochrome P450 1B1: Role in health and disease and effect of nutrition on its expression. RSC Advances. 2019;9:21050–21062. doi: 10.1039/C9RA03674A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quaranta L., Katsanos A., Russo A., Riva I. 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Surv. Ophthalmol. 2013;58:26–41. doi: 10.1016/j.survophthal.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Krasińska B., Karolczak-Kulesza M., Krasiński Z., Pawlaczyk-Gabriel K., Niklas A., Głuszek J., Tykarski A. A marked fall in nocturnal blood pressure is associated with the stage of primary open-angle glaucoma in patients with arterial hypertension. Blood Press. 2011;20:171–181. doi: 10.3109/08037051.2010.538964. [DOI] [PubMed] [Google Scholar]
- 66.Hayreh S.S., Zimmerman M.B., Podhajsky P., Alward W.L. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am. J. Ophthalmol. 1994;117:603–624. doi: 10.1016/S0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 67.Graham S.L., Drance S.M., Wijsman K., Douglas G.R., Mikelberg F.S. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102:61–69. doi: 10.1016/S0161-6420(95)31053-6. [DOI] [PubMed] [Google Scholar]
- 68.Melgarejo J.D., Lee J.H., Petitto M., Yépez J.B., Murati F.A., Jin Z., Chávez C.A., Pirela R.V., Calmón G.E., Lee W., et al. Glaucomatous Optic Neuropathy Associated with Nocturnal Dip in Blood Pressure: Findings from the Maracaibo Aging Study. Ophthalmology. 2018;125:807–814. doi: 10.1016/j.ophtha.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshikawa T., Obayashi K., Miyata K., Saeki K., Ogata N. Increased Nighttime Blood Pressure in Patients with Glaucoma: Cross-sectional Analysis of the LIGHT Study. Ophthalmology. 2019;126:1366–1371. doi: 10.1016/j.ophtha.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Flammer J., Konieczka K., Bruno R.M., Virdis A., Flammer A.J., Taddei S. The eye and the heart. Eur. Heart J. 2013;34:1270–1278. doi: 10.1093/eurheartj/eht023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mroczkowska S., Benavente-Perez A., Negi A., Sung V., Patel S.R., Gherghel D. Primary open-angle glaucoma vs normal-tension glaucoma: The vascular perspective. JAMA Ophthalmol. 2013;131:36–43. doi: 10.1001/2013.jamaophthalmol.1. [DOI] [PubMed] [Google Scholar]
- 72.Mallick J., Devi L., Malik P.K., Mallick J. Update on Normal Tension Glaucoma. J. Ophthalmic Vis. Res. 2016;11:204–208. doi: 10.4103/2008-322X.183914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sleight P., Redon J., Verdecchia P., Mancia G., Gao P., Fagard R., Schumacher H., Weber M., Böhm M., Williams B., et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J. Hypertens. 2009;27:1360–1369. doi: 10.1097/HJH.0b013e32832d7370. [DOI] [PubMed] [Google Scholar]
- 74.Bangalore S., Messerli F.H., Wun C.-C., Zuckerman A.L., DeMicco D., Kostis J.B., LaRosa J.C. Treating to New Targets Steering Committee and Investigators J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur. Heart J. 2010;31:2897–2908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- 75.Banach M., Aronow W.S. Blood pressure j-curve: Current concepts. Curr. Hypertens. Rep. 2012;14:556–566. doi: 10.1007/s11906-012-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung H.J., Hwang H.B., Lee N.Y. The Association between Primary Open-Angle Glaucoma and Blood Pressure: Two Aspects of Hypertension and Hypotension. Biomed Res. Int. 2015;2015:827516. doi: 10.1155/2015/827516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu A., Khawaja A.P., Pasquale L.R., Stein J.D. A review of systemic medications that may modulate the risk of glaucoma. Eye. 2019;34:12–28. doi: 10.1038/s41433-019-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng W., Dryja T.P., Wei Z., Song D., Tian H., Kahler K.H., Khawaja A.P. Systemic Medication Associations with Presumed Advanced or Uncontrolled Primary Open-Angle Glaucoma. Ophthalmology. 2018;125:984–993. doi: 10.1016/j.ophtha.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 79.Kong G.Y.X., Van Bergen N.J., Trounce I.A., Crowston J.G. Mitochondrial dysfunction and glaucoma. J. Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- 80.Clermont A.C., Bursell S.-E. Retinal blood flow in diabetes. Microcirculation. 2007;14:49–61. doi: 10.1080/10739680601072164. [DOI] [PubMed] [Google Scholar]
- 81.Roberts M.D., Grau V., Grimm J., Reynaud J., Bellezza A.J., Burgoyne C.F., Downs J.C. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2009;50:681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Francis-Sedlak M.E., Uriel S., Larson J.C., Greisler H.P., Venerus D.C., Brey E.M. Characterization of type I collagen gels modified by glycation. Biomaterials. 2009;30:1851–1856. doi: 10.1016/j.biomaterials.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 83.Song B.J., Aiello L.P., Pasquale L.R. Presence and Risk Factors for Glaucoma in Patients with Diabetes. Curr. Diab. Rep. 2016;16:124. doi: 10.1007/s11892-016-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elisaf M., Kitsos G., Bairaktari E., Kalaitzidis R., Kalogeropoulos C., Psilas K. Metabolic abnormalities in patients with primary open-angle glaucoma. Acta Ophthalmol. Scand. 2001;79:129–132. doi: 10.1034/j.1600-0420.2001.079002129.x. [DOI] [PubMed] [Google Scholar]
- 85.Luo X.-Y., Tan N.Y.Q., Chee M.-L., Shi Y., Tham Y.-C., Wong T.Y., Wang J.J., Cheng C.-Y. Direct and Indirect Associations between Diabetes and Intraocular Pressure: The Singapore Epidemiology of Eye Diseases Study. Investig. Ophthalmol. Vis. Sci. 2018;59:2205. doi: 10.1167/iovs.17-23013. [DOI] [PubMed] [Google Scholar]
- 86.Hou H., Shoji T., Zangwill L.M., Moghimi S., Saunders L.J., Hasenstab K., Ghahari E., Manalastas P.I.C., Akagi T., Christopher M., et al. Progression of Primary Open-Angle Glaucoma in Diabetic and Nondiabetic Patients. Am. J. Ophthalmol. 2018;189:1–9. doi: 10.1016/j.ajo.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonovas S., Peponis V., Filioussi K. Diabetes mellitus as a risk factor for primary open-angle glaucoma: A meta-analysis. Diabet. Med. 2004;21:609–614. doi: 10.1111/j.1464-5491.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhou M., Wang W., Huang W., Zhang X. Diabetes mellitus as a risk factor for open-angle glaucoma: A systematic review and meta-analysis. PLoS ONE. 2014;9:e102972. doi: 10.1371/journal.pone.0102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y.X., Chen X.W. Diabetes and risk of glaucoma: Systematic review and a Meta-analysis of prospective cohort studies. Int. J. Ophthalmol. 2017;10:1430. doi: 10.18240/ijo.2017.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao D., Cho J., Kim M.H., Friedman D.S., Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: A meta-analysis. Ophthalmology. 2015;122:72–78. doi: 10.1016/j.ophtha.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 91.Rim T.H., Lee S.Y., Bae H.W., Seong G.J., Kim S.S., Kim C.Y. Increased risk of open-angle glaucoma among patients with diabetes mellitus: A 10-year follow-up nationwide cohort study. Acta Ophthalmol. 2018;96:e1025–e1030. doi: 10.1111/aos.13805. [DOI] [PubMed] [Google Scholar]
- 92.Kopec J.A., Esdaile J.M. Bias in case-control studies. A review. J. Epidemiol. Community Health. 1990;44:179–186. doi: 10.1136/jech.44.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leske M.C., Connell A.M., Schachat A.P., Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch. Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 94.Friedman D.S., Jampel H.D., Muñoz B., West S.K. The prevalence of open-angle glaucoma among blacks and whites 73 years and older: The Salisbury Eye Evaluation Glaucoma Study. Arch. Ophthalmol. 2006;124:1625–1630. doi: 10.1001/archopht.124.11.1625. [DOI] [PubMed] [Google Scholar]
- 95.Kreft D., Doblhammer G., Guthoff R.F., Frech S. Prevalence, incidence, and risk factors of primary open-angle glaucoma—A cohort study based on longitudinal data from a German public health insurance. BMC Public Health. 2019;19:166. doi: 10.1186/s12889-019-6935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y., Wolf N.S. Effects of age and long-term caloric restriction on the aqueous collecting channel in the mouse eye. J. Glaucoma. 1997;6:18–22. doi: 10.1097/00061198-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Burgoyne C.F., Downs J.C. Premise and prediction-how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J. Glaucoma. 2008;17:318–328. doi: 10.1097/IJG.0b013e31815a343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barron M.J., Griffiths P., Turnbull D.M., Bates D., Nichols P. The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. Br. J. Ophthalmol. 2004;88:286–290. doi: 10.1136/bjo.2003.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kroemer G., Reed J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 100.Charles A. The evolution of a migraine attack—A review of recent evidence. Headache. 2013;53:413–419. doi: 10.1111/head.12026. [DOI] [PubMed] [Google Scholar]
- 101.Cull G., Told R., Burgoyne C.F., Thompson S., Fortune B., Wang L. Compromised Optic Nerve Blood Flow and Autoregulation Secondary to Neural Degeneration. Investig. Ophthalmol. Vis. Sci. 2015;56:7286–7292. doi: 10.1167/iovs.15-17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drance S., Anderson D.R., Schulzer M. Collaborative Normal-Tension Glaucoma Study Group Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 2001;131:699–708. doi: 10.1016/S0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 103.Huang J.-Y., Su C.-C., Wang T.-H., Tsai I.-J. Migraine and increased risk of developing open angle glaucoma: A population-based cohort study. BMC Ophthalmol. 2019;19:50. doi: 10.1186/s12886-019-1062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu C., Li J., Li Z., Mao X. Migraine as a risk factor for primary open angle glaucoma: A systematic review and meta-analysis. Medicine. 2018;97:e11377. doi: 10.1097/MD.0000000000011377. [DOI] [PMC free article] [PubMed] [Google Scholar]