Abstract
Global exposures to air pollution and cigarette smoke are novel in human evolutionary history and are associated with about 16 million premature deaths per year. We investigate the history of the human exposome for relationships between novel environmental toxins and genetic changes during human evolution in six phases. Phase I: With increased walking on savannas, early human ancestors inhaled crustal dust, fecal aerosols, and spores; carrion scavenging introduced new infectious pathogens. Phase II: Domestic fire exposed early Homo to novel toxins from smoke and cooking. Phases III and IV: Neolithic to preindustrial Homo sapiens incurred infectious pathogens from domestic animals and dense communities with limited sanitation. Phase V: Industrialization introduced novel toxins from fossil fuels, industrial chemicals, and tobacco at the same time infectious pathogens were diminishing. Thereby, pathogen-driven causes of mortality were replaced by chronic diseases driven by sterile inflammogens, exogenous and endogenous. Phase VI: Considers future health during global warming with increased air pollution and infections. We hypothesize that adaptation to some ancient toxins persists in genetic variations associated with inflammation and longevity.
Keywords: exposome, human evolution, genes, toxins, infections
Introduction
As human ancestors diverged from great apes, they encountered additional environmental hazards: increased savanna mineral dust and fecal aerosols; pathogens from decaying carrion; smoke from domestic fire; new pathogens from domesticated animals in the Neolithic; and, in the Industrial Age, airborne toxins from fossil fuels and tobacco. During these phases, humans also evolved larger brains and extended life histories with prolonged maturation and longer life spans, as discussed below. Genetic adaptations acquired during these six million years are analyzed in terms of the novel toxins from exogenous and endogenous sources. Table 1 outlines sequential phases of the expanding human exposome, in which new environmental hazards are cumulatively added to those from prior phases. These new exposures need not have occurred at the same time in all human populations, and should not be considered as hard boundaries for the phases.
TABLE 1.
Phases in the human exposome
| Exposome phase, species/life expectancy, age | Exposome cumulative progression | Chemistry |
|---|---|---|
| IA. Pre-Homo/25 y 5–2.5 MYA | Dust (mineral), pollen; endotoxins from herd animals; increased carrion pathogens | Iron and other toxic metals |
| IB. Early Homo/30 y 2.5–1 MYA | ||
| II. Early Homo/30 y 1–0.3 MYA | Dust, pollen, endotoxins, carrion pathogens; plus domestic biomass smoke and charred meat | Toxic metals; plus PAH |
| III. H. sapiens/35–45 y Paleo- to pre-Neolithic 0.3 MYA-10,000 BP | Dust, feces, endotoxins, smoke, charred meat; plus human feces | Toxic metals, PAH; plus endotoxins, infections |
| IV. H. sapiens/35–45 y Neolithic 10,000–200 YA | Dust, smoke, charred meat, human feces; plus high-density populations, domestic animal feces, new infections | Toxic metals, PAH; plus new endotoxins, antigens |
| V. H sapiens/50–85 y Industrial 1820–2020 | Dust, smoke, charred meat, human and animal feces, infections; plus fossil fuels, industrial toxins, sugar, tobacco | Toxic metals, NH4, PAH, endotoxins, infections; plus adiposity, CO, O3, NOx, SOx |
| VI. Future 21st-22nd centuries H. sapiens/35–90 y global warming and coastal inundation | Dust, smoke, charred meat, feces, infections, fossil fuels, industrial toxins; plus higher O3, crustal dust, insect-borne infections, migrations, water shortages | Transition metals, PAH, NH4, endotoxins, infections, O3, NOx, SOx; plus increased O3, glycoxidation, PAH, temperature |
New factors in each phase are italicized.
The exposome concept was introduced by Wild (2005, 2012) for comprehensive analysis of environmental and lifestyle factors in cancer. The exposome extends prior epidemiologic approaches that characterized individual factors, “one by one” and has become widely adapted to approach interactions of multiple exogenous and endogenous toxins across the lifetime (National Academies of Sciences, Engineering, and Medicine 2017). Wild (2012) identified three domains: the exogenous macrolevel (rural versus urban; social stratification); the exogenous individual (diet, infections); and the endogenous (biomes, fat depots, injuries). The exposome includes all stages of life history, from prefertilization gametes to development and later life. We focus on genes of host defense and brain development during the evolution of the long human life span with its uniquely prolonged postreproductive phase.
Evolutionary inquiry of the human exposome illuminates unexplored domains of inflammatory processes in the evolution of the lungs and brain that may inform the future of human health and longevity during global warming. Inflammatory responses are near ubiquitous in human adaptations to these exposures. Many inflammatory responses to airborne toxins from cigarettes and fossil fuels are shared with the pathophysiology of chronic diseases associated with modern air pollution. We hypothesize that adaptation to ancient airborne toxins may be recognized in modern genetic variations, including the genotypes of cigarette survivors who may have genetic resistance to cigarette aerosols.
Inflammation has become an environmental by word because inflammatory responses are broadly stimulated by molecular damage. We discriminate two broad classes of inflammatory stimulae: pathogen-driven inflammation from infectious viruses, microbes, and parasites versus sterile inflammation from noninfectious toxins and stressors such as cigarette smoke or fat depots (Crimmins and Finch 2006; Finch and Kulminski 2019; Phase V). Some inflammatory responses are shared by infectious pathogenic and sterile inflammogens, as in the toll-like receptor (TLR4) pathway responses to bacterial lipopolysaccharides (LPSs) and urban air pollution particles (Woodward et al. 2017). The many TLR pathways are critical to innate immune responses (“911 standby”), but also to the slower adaptive immune responses targeting specific antigens. Innate immune genes are prominent among the evolved genetic accommodations in the context of adaptive resistance to pathogens and survival of injury. Furthermore, neurodevelopmental processes employ innate immune mechanisms during brain maturation. Building from these established findings, we suggest how evolved immune genes may have interacted with new brain genes (Figure 1).
Figure 1. Novel Environmental Exposures During Human Evolution.

MYA: million years ago. Phases I–V are summarized in Table 1. The time trends are approximations. Dust/silica, based on deMenocal 1995, and Martίnez-Garcia et al. 2011; ozone, from U.S. Environmental Protection Agency 1980–2012; industrial coal/oil, U.S. data, see Figure 6. See text for background on other curves.
Exposome Phase I: Savanna Aerosols
Expanding Exposure To Dust, Pollen, Endotoxins, And Carrion Pathogens
The African environment has undergone major changes in the last 10 million years throughout its vast area (Cerling et al. 2011). The shrinking of the Tethys Sea 7–11 MYA caused major shifts in the African summer monsoon (Larrasoaña et al. 2013; Zhang et al. 2014). The resulting aridification of northern Africa eventually formed the Sahara desert 7 MYA (Zhang et al. 2014). As many diverse forests gradually became wooded grasslands and savannas, those major changes in landscape altered diet, behavior, and foraging territories (Larrasoaña et al. 2013). Sahelanthropus tchadensis (6–7 MYA), which lived in diverse environments near the southern edge of the Sahara, showed early evidence of bipedalism (Brunet et al. 2002). Later hominins, including Ardipithicus ramidus (approximately 4.4 MYA), inhabited a primarily forest and wooded grassland paleoecology (White et al. 2009).
Major aridification in East Africa during the last three million years has particular relevance to the emergence of Homo (Finch 2012). Early habitats gradually shifted from closed canopy forest to open grass-and shrub-land savannas (Feakins et al. 2005; Bonnefille 2010; Cerling et al. 2011). Savanna grasses generally rely on wind pollination, and thus produced more pollen than tropical trees that rely on insect or animal pollination (Dupont and Wyputta 2003). Thus, novel sources of pollen exposure may have increased as grasslands expanded. Arid areas are also major sources of dust (Prospero et al. 2002). The aridification the East African hominin sites is amply documented by an increase in windblown dust reaching marine sediments (deMenocal 1995) and by the carbon isotope ratios in paleosols that distinguish woodlands and grasslands (Sikes 1994; Cerling et al. 2011; Rowan and Reed 2015; Lüdecke et al. 2018). These changes in foliage were complex and regionally diverse, involving Southern Africa and the upper Rift Valley (Levin et al. 2011). Most hominid sites were within extensive woodlands (approximately 40%; Reed 1997; Cerling et al. 2011; Rowan and Reed 2015). Poor preservation of bones in forests limits knowledge of our early ancestors and their environmental conditions.
After 3 MYA in the Rift Valley, hominins were exposed to seasonal surges in airborne dust and pollen (Wood and Lonergan 2008; White et al. 2009). Fossil evidence suggests expanding populations of bovines and rodents 2.7–1.7 MYA with evidence of arid-adaptation as early as 2.7 MYA (Vrba et al. 1995; Bobe and Behrensmeyer 2004). The expanding savanna bovine population would have increased exposure to airborne fecal bacteria and endotoxins. Although simultaneously increasing aerobic capacity with shifting lung morphology, early hominids were exposed to novel aerosols, including dust, seasonal pollen, and fecal endotoxins from herbivore herds. The greater daily locomotion in the increasingly arid environments would have also exposed early hominids to novel levels of aerosols from seasonal dust, pollen, and airborne fecal microbiota of herd animals. Airborne dust in Asia and Africa has speciose microbiota with viable bacteria at densities up to 106/m3 (Hara and Zhang 2012; Yahya et al. 2019).
Bipedality, Lung Evolution, And Aerosol Exposures
These environmental changes increased demands on many physiological functions associated with foraging and predator avoidance, from locomotion to thermoregulation and new toxins in the respiratory and digestive systems (Kaplan et al. 2000; Finch 2012; Wessling et al. 2018). Besides novel levels of aerosols, we note potential increased exposure to xenobiotics from tubers, as evidenced by modern chimpanzee use of tools for tuber digging (Hernandez-Aguilar et al. 2007).
During these major shifts in ecology and food niches, hominids were evolving bipedality (Passey et al. 2010). We propose that bipedalism and increasing lung capacity added further inflammatory challenges from inhaled particles during these environmental shifts.
Quadrupedal movement is efficient for short distances and especially for arboreal movement. However, as woodlands gave way to savannas, the energetic and time costs of slow-moving quadrupedal gait would have increased risk of predation for chimpanzee ancestors less able to escape to trees. Large felids, hyena, and wild dogs are known to prey on chimpanzees on the ground (Tutin et al. 1981; Tsukahara 1993; Boesch and Boesch-Achermann 2000; Zuberbühler and Jenny 2002). Slow-moving inefficient quadrupeds are easy prey in open savanna.
Bipedal movement in chimps requires more O2 than quadrupedal movement shown for running (Pontzer et al. 2014). The chimpanzee quadrupedal gait imposed anatomical constraints on diaphragm and lung size (Schmid et al. 2013; Latimer et al. 2016). This constraint was overcome by the early bipedal Australopithecus sediba (Schmid et al. 2013) and A. afarensis (Latimer et al. 2016). The larger human lung volume is commensurate with the fourfold or greater daily walking distance of indigenous humans (Hadza, Tsimané) than wild chimpanzees (rainforest, savannas; Table 2). Most organs scale to body size: allometry predicts the human lung should be larger than the chimpanzee, but cannot explain shape changes in rib cage size and volume. Chimpanzees have a more funnel-shaped rib cage than humans. Our barrel-shaped rib cage with greater caudal width evolved, together with a more powerful diaphragm (Bastir et al. 2017). The larger ratio of upper to lower thorax may indicate higher arterial oxygen partial pressure in hominids than chimpanzees (Chan 2014). A. afarensis had an intermediate chest shape of greater caudal width than chimpanzee, and closer to Homo (Haile-Selassie et al. 2010).
TABLE 2.
Respiratory characteristics of chimpanzees versus humans and bipedal ancestor
| Human | Australopithecus sediba | Chimpanzee | |
|---|---|---|---|
| Tidal volume, mL | 596 ± 81.4 | 420 ± 63.4a | |
| Lung weight, g | 1117± 314 | ||
| Respiratory area index | 30.5 ± 1.6 | 32.9d | 27.2 ± 1.5d |
| Daily walking | Hadza 12.2km/db | Forest 2.1 + 0.06 km/de | |
| Tsimané, 18 km/dc | Savanna 3.3 + 0.1 km/df |
Respiratory area index: size-standardized to 4th rib respiratory area.
Pontzer and Wrangham 2004, wild chimpanzees Kanyawara/Kibale community, Uganda.
Wessling 2011; Jill D. Preutz (pers. comm.), Fongoli savanna, Senegal.
Ergonomic analysis of fossils suggests that the skeletal capacity for long-stride endurance running may be unique to the genus Homo: no great ape or other primate has the capacity for endurance running or extended walking (Carrier et al. 1984; Bramble and Lieberman 2004; Pontzer et al. 2010; Ruxton and Wilkinson 2011). The evolution of long-distance running is considered adaptive for human scavenging and hunting (Zhang et al. 2014). The larger human lung may have coevolved with more efficient bipedal locomotion as antipredator defenses as well as freeing hands and increasing daily range (Kaplan et al. 2000). Increased breathing capacity in the hominin lineage may have supported long-stride walking and running: humans have five- to tenfold more eccrine glands on major body surfaces than chimp and macaque (Kamberov et al. 2018), together with higher capillary density and eccrine gland glycogen (Best and Kamilar 2018). Hair follicle density, however, does not differ between human and chimpanzee in most body surfaces (Kamberov et al. 2018). Lacking archeological evidence for eccrine gland density in early hominins, it may still be possible to identify the timing of origins for genes of species-specific sweat gland development (Lu et al. 2016; Yao et al. 2019). The greater aerobic throughput also exposed early hominids to novel aerosols, including dust, seasonal pollen, and fecal endotoxins from herbivore herds. These aerosols can cause pulmonary inflammation and infections in modern populations. Specifically, silica dust inhalation can cause chronic lung inflammation and pulmonary fibrosis, as well as autoimmune disorders (Thakur et al. 2008).
Seasonal dust causes significant respiratory distress. For example, during desert dust episodes in Greece, an increase of 10 μg/m3 of PM10 (particulate matter smaller than 10 μm diameter) was associated with a doubling of emergency room visits for respiratory conditions (Trianti et al. 2017). Fecal aerosols from herd animals involve different hazards, which may be modeled by the loss of lung volume (vital capacity) in California dairy workers in proportion to cattle fecal aerosol density, measured as endotoxin per m3 (Mitchell et al. 2015). Dairy workers exposed to high levels of cattle feces faced a 50% higher exposure to small aerosolized particles (PM2.5), as well as twofold more exposure to endotoxins than the control group. Those in the highest quartile of endotoxin exposure per work shift had a 10% loss in lung capacity (24.5 mL reduction; Mitchell et al. 2015). Such high endotoxin levels likely exceed those of ancient savanna aerosols; however, agricultural workers frequently incur chronic bronchitis and airflow limitation (Guillien et al. 2019). As noted above, dust of African origin has high levels of viable microbes (Yahya et al. 2019).
Environmental variability and aridification also brought changes in flora resulting in novel exposures to pollens (Reed 1997; Potts 1998; Lüdecke et al. 2018). Pollen is generally considered in the class of coarse particles (PM10 to PM2.5; Kelly and Fussell 2012). Although we cannot know the species or pollen load (Carrión and Scott 1999), major variations in pollen density are documented during Phase I (Potts 1998; Domínguez-Rodrigo et al. 2001).
Seasonal droughts also required novel behavioral strategies, including migration for water. For example, seasonal migration occurred in both contemporary East Africa (Afifi et al. 2014) and precontact Australia (Webb 2009). Seasonal pursuit of water seems likely to increase dust inhalation together with increased walking.
In summary, the exposome of the common human-chimpanzee ancestor was less complex than ancient humans because the wooded environments were less exposed to savanna mineral dust and endotoxins and particulate matter from ungulate feces (Finch 2012). Increasing presence on savannas would have brought novel exposures to aerobic toxins. We discuss below how the immune system gene may have evolved to cope with the increased inhalation of dust, pollen, and bacteria in fecal aerosols.
Besides inhaled materials, Phase I exposed early humans to additional hazards from carrion scavenging and increased meat-eating. Human adaptations to these novel toxicants may be represented in the numerous gene mutations recently identified from genomic comparisons of modern and ancient humans with chimpanzees (Figure 2; Table 3). Genes of host defense and innate immunity are discussed for the successive exposome phases. Some of these genes are expressed in brain tissues and may have interacted with brain evolution. These genes were chosen because of plausible roles during specific phases of the exposome and because estimates were available for their time in evolution. Discussion of these genes is necessarily speculative. Few of these mutations can be proven as adaptive in the strict sense recognized for the recently evolved malarial resistance genes.
Figure 2. Genetic Changes in Exposome Phases I–III.

Superscripts identify gene function. B: brain-behavior; I: immunity; M: metabolism. Genes are briefly described in Table 3.
TABLE 3.
Gene timelines
| Abbreviation | Full gene name; species; function | Phase, MYA* |
|---|---|---|
| AMY1 | α-amylase 1, salivary enzyme; expanded gene copy number after split from Homo heidelbergensis | II, undated |
| ApoE | Apolipoprotein E, lipid transport in blood and brain; three common alleles: ApoE2,-E3,-E4; ApoE4 is ancestral and undated; Alzheimer disease risk | Pre-III, undated |
| ApoE3, early H. sapiens, 0.25 MYA | III, 0.23 | |
| ApoE2, spread after 0.1 MYA; not in Neandertals and Denisovans | III, 0.1 | |
| AHR | Aryl hydrocarbon receptor, detoxification of polycyclic aromatic hydrocarbons (PAHs); human-specific mutation V381A, not in Neandertals | III, post-0.5 |
| CD33M | Immune cell membrane protein, alternate name, Siglec-3; human, not Neandertal; Alzheimer’s disease risk | III, 0.5 |
| CD88 (C5) | Complement factor C5, innate immunity, anaphylactic peptides | IA, 5.2 |
| CFG | “Cooked food genes”: rat liver genes with differential expression for cooked versus raw food: MARCO and tnfrs11a; pre-Neandertals and Denisovans | II, 0.28–0.77 |
| CMAH | Cytidine monophosphate-N-acetylneuraminic acid hydroxylase; innate immunity; gene was inactivated pre-Homo | IB, 2.5–3 |
| CYP1A1, CYP1B1 | Cytochrome P450 family of enzymes, catabolize steroids and PAHs; gene is downstream of AHR; chimps, Neandertals, Denisovans, and humans | Pre-I, undated |
| DPEP1 | Dipeptidase 1, regulates blood homocysteine; Neandertal introgression | II, undated |
| FCGR1B, C, D | Fc fragment of immunoglobulin (IgG) receptor (high affinity), also CD64 | |
| FCGR1B | IA, 5.0 | |
| FCGR1C | IB, 2.4 | |
| FCGR1D | II, 1.2 | |
| FY*O | Malarial resistance gene of Duffy blood group antigens; alternate name, ACKR1 antigen; encodes chemokine receptors used by malarial parasites | III, 0.042 |
| HTR2A | Serotonin (5-HT) receptor; influences foraging behavior | II, 0.33 |
| HbS | Hemoglobin, sickle cell variant | III, 0.022 |
| KIR | Natural killer cell receptors distinct from KIR ion channels; six KIR are human-specific; KIR2DP1 inactivated pre-Neandertals | II, pre-0.5 |
| MARCO | Macrophage receptor with collagenous domain; alternate names Class A scavenger receptor (SCARA2) and CD204; phagocytosis of dust and pathogens; human substitution (F282S) absent in Neandertals and Denisovans; 452Q is shared with humans, Neandertals, and Denisovans; see CFG | II, pre-0.5 |
| NAT1,2 | N-acetyltransferases-1,−2; detoxify PAHs | III, 0.02 |
| OAS1,2,3 | Oligonucleotide adenylate synthase 1,2,3; innate immune, degrade viral RNA; Neandertal introgressed | III, 0.13 |
| PRNP | Prion protein; variants alter infectious prion transmission between species | II, 0.5 |
| ΔPtERV1 | Deletion of genomic retrovirus PtERV1 | IA, 4.7 |
| Siglec-3 | See CD33M | |
| Siglec-16 | Sialic acid-binding immunoglobulin-like protein-16; inactivated | IB, 3 |
| Siglec-16 activated by gene conversion | II, 0.8 | |
| SCARA2 | Scavenger receptor A2, alternate name for MARCO | |
| SLC6A4 | Serotonin (5-HT) receptor (38 kb); new alleles VNRT, LPR-S; aggressive-impulsive behaviors | III, 0.22–0.27 |
| SRGAP2B, SRGAP2C | Slit-Robo Rho GTPase activating proteins, regulate neuron spine density; human-specific gene duplications | IB-II, 2.4–1 |
| TCAF1,2 | TRPM8 channel-associated factors 1 and 2; detection of cold by somatosensory neurons; bind to TRPM8 ion channel; cancer metastasis | III, 0.3 |
| TLR1,6,10 | Toll-like receptors, innate immunity; TLR1 and TLR6 bind gram-positive bacteria for phagocytosis by macrophages; TLR10, orphan receptor; introgressed archaic alleles from Neandertals and Denisovans | III, undated |
| tnfrs11a | TNFα-receptor superfamily 11a; cooked food gene (CFG); pre-Neandertals and Denisovans | II, 0.3–0.7 |
| TPE | Tropoelastin; deletion of exons 35, 36; skin elasticity | Pre-I |
| TRPM8 | Transient receptor potential melastatin member 8; response to moderate cold; new allele | III, 0.024 |
| VIP | Virus-interacting proteins; broadly defined, inhibit microbial and viral infections; include Neandertal introgressed sequences | II |
MYA, million years ago.
Chimpanzee life spans are shorter than humans in modern and preindustrial populations. Although menopause occurs at about the same age (Hawkes and Smith 2010; Herndon et al. 2012), the chimpanzee postreproductive life span is much shorter than in all human populations. Although one chimpanzee community (Ngogo, 1996–20) is noted for much lower mortality rates approaching those of indigenous people (Wood et al. 2017), nonetheless, its 1.5-year life expectancy at age 65 was still much below that of the Tsimané of Bolivia at 8.5 years (Gurven et al. 2007). Another key species difference is the young adult mortality rate per year of chimpanzees, which is 35% greater than for traditional humans living under limited hygiene (Finch 2010; Gurven and Davison 2019). This human advantage may derive from evolved immune functions and stronger nurturing behaviors.
A limitation to understanding environmental hazards in human evolution is the unknown burden of infections and chronic disease in hominin ancestors—few physical remains of soft tissues allow the study of ancient infections. Alternatively, we can learn from infections of feral chimpanzees in comparison with indigenous people living under traditional preindustrial conditions of hygiene and medicine. Infections cause more than 50% of adult deaths of feral chimpanzees and traditional humans (Table 4). Chimpanzee infections and mortality patterns vary widely in association with ecological variations, human intrusions, and infectious episodes (Wood et al. 2017). Most mortality of young adult chimpanzees is attributed to infections, but it is unknown how their infections compare with traditional humans without modern medications and limited hygiene.
TABLE 4.
Mortality patterns of chimpanzee and human
| Chimpanzee, wild | Human, traditional (Tsimané) | Human, 21st century (U.S.) | |
|---|---|---|---|
| % Deaths by cause | |||
| Respiratory | 15 | 22 | 6 |
| Other infections | 22 | 28 | <1 |
| Accidents | 3 | 5 | 5 |
| Homicide + warfare | 15 | 11 | <1 |
| Cardiovascular | No data | Low levels | >50 |
| Life expectancy years | |||
| at birth | 13–33 | 30–45 | >75 |
| young adult | 24–27 | 42 | >50 |
| at menopause | 11 | 20 | 35 |
| Mortality young adult/yr (minimum adult mortality) | 0.03–0.05 | <0.02 | <0.001 |
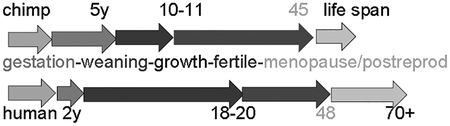
Cause of Death: Chimpanzees of Gombe, Mahale, and Taï; seveen groups of hunter-gatherers and forager-horticulturalists (Gurven and Kaplan 2007; Gurven and Gomes 2017). Chronic degenerative diseases of aging (cancer, heart disease) are major causes of death in industrialized humans, but are not well defined for chimpanzees or traditional humans. Postmortem findings for older chimpanzees are from captives; there are almost no autopsy data for traditional preindustrial humans. Heart failure of aging chimpanzees is generally nonischemic, based on captives maintained on a healthy diet (Varki et al. 2009). Although coronary artery atherosclerosis is unstudied in wild chimpanzees, aortic atherosclerosis was reported in a small sample of adult wild chimps killed in the Congo (Vastesaeger and Delcourt 1961). However, some early studies noted ischemic deaths in association with elevated chlolesterol (Finch and Stanford 2004:Table 3A, Appendix 4). Tsimané also have negligible ischemic deaths, with slower cardiovascular aging than industrial populations (Kaplan et al. 2017).
Life expectancy: Young adult chimpanzee (Bronikowski et al. 2016; Wood et al. 2017), human Tsimané (Gurven et al. 2007), and U.S. (National Center for Health Statistics 2016).
Mortality young adult: Feral chimpanzee (Bronikowski et al. 2011; Muller and Wrangham 2014; Gurven and Davison 2019); and human traditional and preindustrial (Finch and Crimmins 2004; Finch 2010; Beltrán-Sánchez et al. 2012). Mortality is minimal at age 10–20 years in humans (Finch and Crimmins 2004; Beltrán-Sánchez et al. 2012) and great apes (Gurven and Davison 2019).
Data are limited because sampling is largely based on fecal analysis, which requires consecutive samplings of the same individual (Muehlenbein and Watts 2010) and because of infections introduced by human intrusions and habitat degradation with expanded farming and exposure to domestic animals (McLennan et al. 2017). We must assume that all sampled chimpanzee populations have had direct or indirect exposure to transmissible pathogens from humans and domestic animals.
Several examples show the potential for bidirectional cross-species pathogen transmission. In samples of nearly 300 for each host species, Entamoeba enteric species were detected in 66% of chimpanzees and 60% of humans within the Gombe ecosystem, while the diarrhea-causing Entamoeba histolytica was in 34% of chimpanzees and 12% of humans (Deere et al. 2019). Respiratory viruses in Kibale chimpanzees show human origins: metapneumovirus (MPV), respiratory syncytial virus (RSV), and rhinovirus-C (RV-C; Emery Thompson et al. 2018). Parasitic gastrointestinal worms in chimpanzees also infect neighboring humans (Enterobius, Trichuris; Ebbert et al. 2015). We lack population-based data for parasite prevalence in these local human populations. In more comprehensive samples of the Bolivian Tsimané, most adults carried at least one helminth or protozoan parasite, averaging 1.3 species per person (Vasunilashorn et al. 2010; Blackwell et al. 2013, 2015). In Tsimané, diarrhea is also common (Blackwell et al. 2013, 2016; Rosinger and Tanner 2015). Chimpanzee exposure to their own feces is minimized by daily changes of the night nests that are typically abandoned each morning (Goodall 1986:208; Llorente Caño 2004; Stewart et al. 2011; Finch 2012), and adults fastidiously avoid contact with excreta (Goodall 1986:208). Even so, diarrhea is “common” (Goodall 1986:93–96). There are no quantitative measures of fecal contact in chimpanzees and in traditional subsistence cultures with limited hygiene practices.
Because soft tissues are rarely preserved, we know little about the chronic diseases of human ancestors. Skeletal abnormalities are frequent in Pleistocene remains by comparison with modern samples, and include curvatures and developmental defects (Trinkaus 2018). This sample of 66 individuals, mostly adults from Middle to Late Paleolithic, had defined 75 abnormalities, considered a vastly unprecedented excess. Although one-third of the abnormalities are common today, another third are rare, and the remaining unknown. The etiology may be in part be attributable to developmental stress, also indicated by frequent dental enamel hypoplasias in Pleistocene samples (Guatelli-Steinberg et al. 2013).
Bone cancer was reported for several Australopithecus species and early Homo (Rifkin et al. 2017), but was not mentioned by Trinkaus (2018). Bone cancers are rare in the fossil record until the Bronze Age (Nerlich et al. 2006; Lieverse et al. 2014). Although cancers may have occurred in human ancestors, as in the premodern world, their prevalence cannot be estimated from these haphazard specimens. The same conclusion holds for heart disease, which our team detected as arterial calcification in mummies from ancient Egyptian and other preindustrial populations (Thompson et al. 2013). Again, these samples are not population-based. Contemporary indigenous Tsimané have slowly progressing coronary calcification, but negligible ischemic disease (Kaplan et al. 2017). Provisionally we conclude that the typical modern killers of cancer and atherosclerosis were present throughout human evolution, but caused much less mortality than infections until the end of Phase V.
Scavenger Receptors Associated With Inhaled Particulate Matter
The changing of the forest to savanna landscape in East Africa increased the exposure to inhaled dust particles. Two groups of innate immunity genes are particularly relevant to the novel aerosol exposures of Phase I: cell surface scavenger receptors (SRs) and enzymes of Siglecs, cell surface glycoproteins. The SRs in lung epithelial cells bind to a wide range of inert particles, as well as bacteria. Eight subclasses are recognized by differences in structure and function (classes A–H). Class A scavenger receptors, particularly SR-A I/II (newly designated as CD204) and Macrophage Receptor with Collagenous Domain (MARCO; see Figure 2 and Table 3). MARCO mediates phagocytosis of a broad range of substrates by respiratory tract macrophages and epithelial cells (Thakur et al. 2008). Microbial substrates are degraded by subcellular processes, whereas silicates and other minerals resist degradation and accumulate during life (Hamilton et al. 2006; Novakowski 2018).
Ape-hominin comparisons for MARCO show two novel substitutions (Novakowski 2018). Residue 282 is exclusively serine (S) in chimpanzees, while humans have two alleles, the ancestral 282S and the derived allele phenylalanine 282F, which is more prevalent in modern populations. Denisovans and Neandertals had only 282S, like great apes. The apparent absence of 282F in Neandertals or Denisovans suggests its origin by 0.55 MYA; more specimens may show when humans, Neandertals, and Denisovans acquired glutamine (G) in substitution for chimpanzee histidine (G452Q). Both 282 and 452 sites show evidence of positive selection. These coding substitutions altered MARCO functions for ligand binding and phagocytosis in cell assays (Novakowski 2018), in correspondence to the associations of MARCO variants with pulmonary tuberculosis (Bowdish et al. 2013; Thuong et al. 2016). MARCO variants are associated with Streptococcus pneumoniae and respiratory syncytial virus.
Human-specific MARCO alleles that influence susceptibility to pulmonary tuberculosis arose by 0.5 MYA. Silica-induced lung fibrosis also depends on MARCO expression (Yang et al. 2019), but individual variations in fibrosis have not been examined for a relationship to MARCO variants. MARCO gene regulation is sensitive to crosstalk with diverse stimulae. In the mouse lung, the MARCO receptor mRNA was induced fifteenfold by endotoxin (LPS) and twenty-fivefold by combined exposure to LPS plus cigarette smoke, but only threefold by cigarette smoke alone (Meng et al. 2006). Lung cancer risk also shows super-additivity (positive synergy) of cigarette smoke with air pollution aerosols (Turner et al. 2014; Forman and Finch 2018). These examples of immune response crosstalk and synergies to xenobiotics suggest ancient complexities of the expanding human exposome that go beyond single factor effects on health. The seasonal surges of silica dust in the Rift Valley would have increased pulmonary phagocytic demands, with potential consequences to host defense because silica SiO2 inhibits responses of scavenger receptors to microbial receptors in alveolar macrophages (Beamer et al. 2016). Moreover, the SR and MARCO enable the phagocytosis of pollen, another seasonal demand. These and other synergies of inhaled environmental toxins (Forman and Finch 2018) suggest that we must consider multiple interactions in evolution of gene responses to the novel scale of inhaled particles by human ancestors.
Phase II–III: Where There Is Smoke, There Is Fire
Dust, Pollen, Endotoxins, And Carrion Pathogens; Plus Novel Toxins From Domestic Smoke And Cooking
Fire was “probably the greatest ever [discovery] made by man, excepting language” (Darwin 1890:54; Wrangham and Carmody 2010). We do not know the earliest controlled use of fire either for warmth, toolmaking, or cooking: hearths do not preserve well, and it is hard to differentiate naturally occurring wild fires from controlled fire use (Gowlett and Wrangham 2013). Controlled and routine use of fire was established by 0.5–0.75 MYA in Europe and Western Asia (Thieme 1997; Goren-Inbar et al. 2004; Wrangham and Carmody 2010; Gowlett and Wrangham 2013). However, earlier controlled use of fire is suggested from archeological sites and gut morphology in Africa between 1–2 MYA (Bellomo 1994; Wrangham and Carmody 2010; Gowlett and Wrangham 2013).
Fire brings many nutritional dietary benefits. Not only does cooking increase the digestibility of cooked food (Carmody and Wrangham 2009; Carmody et al. 2011), but cooking reduces demands for mastication. The MYH16 myosin masticatory gene in jaw muscles was lost before 0.6 MYA (Perry et al. 2015). Cooking and smoke-drying meat also increases its durability during storage by killing bacteria (Smith et al. 2015) and parasites (Perry 2014). However, meat and fish can also be stored safely after bacterial putrefaction that also generates additional nutritional benefits of vitamin C and other micronutrients (Speth 2017). Rotted meat and fish are staples in many traditional diets that may have originated in the Middle Paleolithic.
Cooking fires also produce potentially toxic byproducts of airborne particulate matter and polycyclic aromatic hydrocarbons (PAHs) in smoke, as well as advanced glycation end products (AGEs) from Maillard reaction chemistry, discussed below. Smoke from wild fires became an intermittent exposure in some environments approximately 0.35–0.4 MYA (Doerr and Santín 2016).
Smoke And Cooking: Detoxification By AHR-CYP and N-acetyltransferase Genes
The increasing use of controlled fire for cooking and warmth also brings airborne smoke with inevitably increased exposure to smoke particles and novel chemical toxins. Mortality from domestic smoke exposure is attributed to chronic lung infections (Smith et al. 2000; Fullerton et al. 2008; Phillips et al. 2018). Exposure to wood smoke can impair antiviral responses of nasal mucosa cells (Rebuli et al. 2019). Domestic smoke exposure during the Pleistocene may have mediated the emergence of tuberculosis, by promoting chronic lung inflammation (Chisholm et al. 2016). Smoke toxins include the large group of polycyclic aromatic hydrocarbons produced by partial (incomplete) combustion of biomass. PAHs are generally rare in the environment except during sporadic brush or forest fires. The PAHs include benzo(a)pyrene and other proven human carcinogens. Some PAHs are considered neurotoxic from epidemiological and clinical associations of impaired brain development (Peterson et al. 2015; Finch 2018) from rodent models exposed during gestation to benzo(a)pyrene (McCallister et al. 2008, 2016; Sheng et al. 2010; Geier et al. 2018; Slotkin et al. 2019).
After inhalation or ingestion, PAHs are detoxified by the aryl hydrocarbon receptor protein (AHR), a xenobiotic sensor. In turn, the PAH-activated (ligand-bound) AHR moves to the cell nucleus to alter transcription of catabolic pathway genes including the cytochromes CYP1A1 and CYP1B1. The multifunctional AHR protein is also a xenobiotic barrier against PAH in gut (Liu et al. 2018); prenatally, placental AHR and CYP1A1 proteins are elevated by maternal smoking (Huuskonen et al. 2008). Some AHR products are are fully detoxified, while others are carcinogenic. Adaptive responses to PAHs and other xenobiotics include gene variants that detoxify pesticides, shown in fish populations exposed to dioxins and other halogenated aryl hydrocarbons (Aarts et al. 2016; Hubbard et al. 2016; Hahn et al. 2017).
Comparisons of AHR gene evolution in hominids raise further questions. In benchmark experiments, Hubbard et al. (2016) compared the AHR protein of human and Neandertals with cell and biochemical studies of recombinant AHR engineered from bone fossil DNA sequence. The Neandertal AHR protein caused a hundredfold more ligand-mediated induction of downstream detoxifying gene CYP1A1 by benzo(a)pyrene. The human AHR gene uniquely substitutes valine for alanine at amino acid 381 (A381V); Neandertals and Denisovans share the primate AHR variant A381, which has reduced affinity for some PAH ligands. Structural modeling shows that A381V alters the ligand-binding pocket for benzo(a)pyrene. Because the AHR-CYP catabolism of PAH produces toxic intermediates, Hubbard et al. (2016) suggest that early H. sapiens were more resistant to domestic smoke toxicity than Neandertals. AHR is important in detoxifying response to modern domestic smokes, including responses to cigarette smoke and to domestic dung burning in poor households (see below). The aryl hydrocarbon repressor gene (AHRR) merits further evolutionary study. Although human-specific adaptations to smoke inhalation are retained as common gene variants in modern populations, there is extensive genetic variation within and across populations (Sudmant et al. 2015), and many ancestral alleles are still maintained in populations despite increased risk—for example, cooking-related genes (Phase II) and cigarette smoking survivor-related genes (Phase V).
Other PAH detoxification genes that retain modern variants were identified by Aarts et al. (2016) through bioinformatics analysis. For 80% of the variants, Neandertals and Denisovans had the same ancestral hominid genes (were homozygous for) as the chimpanzee and gorilla. Strikingly, for most genes (23/29), the ancestral allele was considered more protective for toxins related to smoke and cooked food than the modern human alleles. For example, Hanna et al. (2000) showed that the main CYP1B1 variant protein of modern humans has one-third less enzyme activity than the ancestral isoform variant shared with chimpanzees, Neandertals, and Denisovans. Caveat: their estrogen hydroxylation assay may not be generalizable to PAHs: although steroids are polycyclic, they differ chemically from PAHs by oxygen content. Another 20% of CYP1B1 polymorphisms are evolutionarily novel: of these, three are associated with lower cancer risks from cigarettes, while the other four increased cancer risk. Data from the 1000 Genomes Project showed that the high- and low-risk alleles in different genes were concurrent. High-risk alleles existed at least 45,000 years ago in H. sapiens DNA from Ust’-Ishim (Siberia).
N-acetyltransferases (NAT1,NAT2) are important for xenobiotic catabolism of modern drugs and ancient carcinogens such as PAHs in smoke from burning wood and tobacco (Zhou et al. 2013; Aarts et al. 2016; Vangenot et al. 2019). Because the NAT variants alter cancer risk from cigarette smoke (Matejcic et al. 2015; Sabbagh et al. 2018) and red meat (Wang et al. 2015), we suggest that NAT variants also modulate toxicity of domestic smoke from biomass burning used for cooking and warmth. Human NAT2 has single nucleotide polymorphisms (SNPs) at three coding sites with wide global variations (Lakkakula et al. 2014). Their association with diet, especially red meat, suggests a role in Neolithic diet transitions with increased exposure to xenobiotics (Luca et al. 2008; Sabbagh et al. 2018). The low cancer risk variants of human NAT1 and NAT2 are homozygous in Neandertals and Denisovans, as well as chimpanzees and gorillas, indicative of the ancestral alleles (Aarts et al. 2016). Humans and chimpanzees show opposite trends in allelic diversity for NAT1 (human:chimp, 0.2-fold less) versus NAT2 (human:chimp four- to nine-fold more). This divergence frustrates conclusions about the timing of divergence and whether parallel mutations arose independently. Several DNA samples from the Neolithic and Bronze Age had the modern high-risk alleles, suggesting recent origins. The major genetic instability of NAT genes across vertebrate phyla (Sabbagh et al. 2018) anticipates discovery of new variants from ongoing deep DNA sequencing.
Cooking, Calories, And Charred Meat
Cooking introduced further tradeoffs for domesticated fire. On the positive side, cooked foods (plant and animal) are more readily digested, which Carmody and Wrangham (2009) hypothesized was important to the evolution of our energy-demanding brain (Figure 3). Elegant experiments showed that young rats grew faster on isocaloric diets of briefly roasted food compared to raw food (Carmody et al. 2011). Six hepatic genes were identified as cooking-related for both meat and tubers, of which we note two: MARCO (alternatively named, scavenger receptorSCARA2) and tnfrs11a (TNFa-receptor family), which mediate air pollution inflammatory pathways, discussed below. Cooking-responsive genes diverged before the Neandertal and Denisovan lineages, circa 0.275 to 0.765 MYA, from comparisons in the 1000 Genomes Project with hominin fossil DNA.
Figure 3. Evolution of Bone Density-Strength, Body Height-Weight.
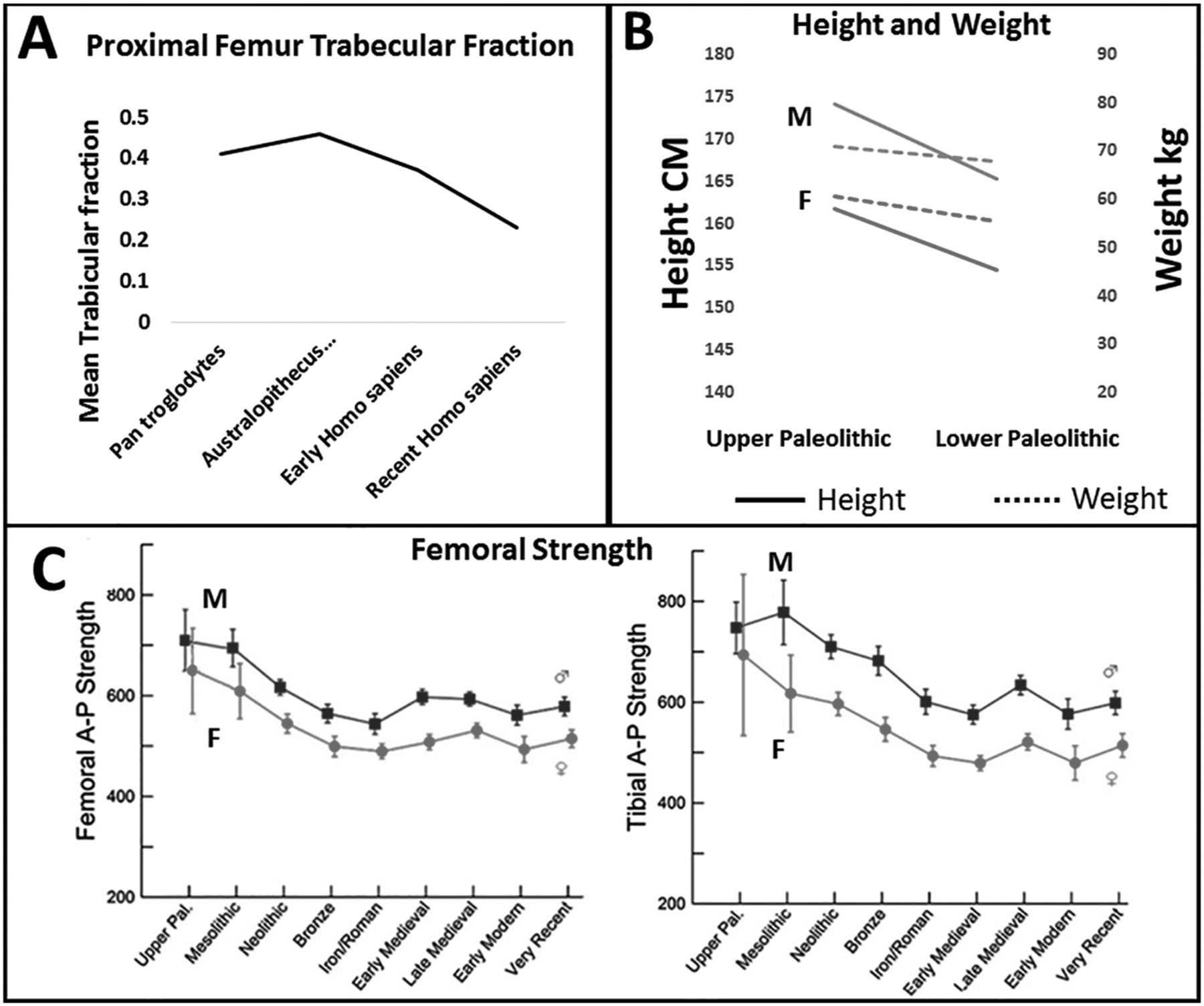
Panel A: Femoral trabecular fraction (Chirchir et al. 2015). Panel B: Body height and weight (Holt and Formicola 2008). Panel C: Femoral strength (Ruff et al. 2015). M, male; F, female.
Offsetting benefits of cooking to digestibility and taste, the browning and charring of foods generate evolutionarily novel toxins of the advanced glycation end products (Tamanna and Mahmood 2015; Delgado-Andrade and Fogliano 2018; Yu et al. 2018). The heat-driven spontaneous (nonenzymatic) Maillard reactions of amino acids and reducing sugars generate myriad chemical modifications, from simple adducts (carboxymethyl lysine, CML) to complex heterocyclic amines(HCAs). Cooking and browning also increase benzo(a)pyrene and other PAHs (Lintas et al. 1979; Rose et al. 2015) to which are added PAHs present in the smoke arising from the combustion of fat (Lee et al. 2016). The 10-year NIH-AARP Diet and Health Study, with over 500,000 participants, showed pancreatic cancer risks that correlated with dietary intake of CML, but only in men (Jiao et al. 2015). Breast cancer risk was 1.6-fold more frequent for carriers of CYP1A1 and CYP1B1 who ate more grilled and smoked meat in a population-based study of 2000 individuals with matched controls (Parada et al. 2017). This association further supports the broad role of CYP in detoxifying PAHs and other xenobiotics generated by burning of wood and by cooking. Together, the PAHs and Maillard products from domestic and fires cooking comprise an evolutionarily novel Combustion Exposome. The CYP and NAT gene variants discussed may also have arisen in response to other unknown xenobiotics. Another unknown is how xenobiotics interacted with biomes of the gut (Clarke et al. 2019) and airways (Hosgood et al. 2014).
Exposome Phases II–III
Dust, Fecal Aerosol Endotoxins, Smoke, And Charred Meat Plus Human Feces
Together with the changing sub-Saharan environments, the hominid dietary niche diversified from lower skill and lower calorie foods to high-quality and skill-intensive food sources (Kaplan et al. 2000). We focus on genetic changes that are meat-adaptive, as the ancestral diet shifted away from chimpanzee-like diet toward big game specialization (Finch and Stanford 2004; Lüdecke et al. 2018). Chimpanzees prioritize easily acquired foods with low nutritional value (Kaplan et al. 2000), as well as a few high-value, but hard-to-acquire foods such as hunted meat. About 2% of feral fecal samples contain evidence of vertebrate prey, comprising a small but important portion of the overall chimpanzee diet (Gilby 2006; Moore et al. 2017). In contrast, most of the Neandertal diet was meat-based (Sistiaga et al. 2014), while extant hunter-gatherers average 45–65% of their calories from meat (Cordain et al. 2000). Because of major evolved increase of meat consumption, much research has focused on changes in gut morphology (Aiello and Wheeler 1995). Additionally, recent evidence indicates genetic adaptations linked to meat-eating that are protective for exposure to parasites.
The early evidence of hominin butchery is 3.4 MYA (McPherron et al. 2010), followed by strong evidence of butchery with stone tools by 2.5 MYA (de Heinzelin et al. 1999). We recognize uncertainties on the significance of the site (Domínguez-Rodrigo et al. 2010). Greater exposure to the tissues of other vertebrates in correspondence with hunting suggests the parasite load experienced by early hominids likely exceeded that of chimpanzees. Coprolites provide evidence for parasite paleoecology (Spyrou et al. 2019), but are extremely rare prior to 50 KYA (Sistiaga et al. 2014). The earliest well-dated hominin coprolite is 1.8 MYA (Ferring et al. 2011), but apparently was not assessed for parasites. The first coprolite showing helminths is from a Neandertal site 50 KYA (Sistiaga et al. 2014). This lack of data frustrates assessment of the total disease burden across early hominid species. Fecal parasite analyses have many caveats: depending upon host immune system and the life-history stage for each given parasite, even individuals with high helminth or protozoal loads may not shed many eggs or parasites (Stear et al. 1995; Sithithaworn et al. 2009).
Chimpanzees and other primates often carry multiple species of helminths and protozoa (Muehlenbein 2005), and use behavioral strategies to ameliorate infections (Wrangham 1995; Huffman and Caton 2001). Current indigenous subsistence populations also carry many parasitic infections in adults (Hurtado et al. 2008). For example, the Tsimané often are infected by helminths at a rate of 30–72% (Blackwell et al. 2013, 2015, 2016) and protozoans at 30% (Blackwelletal. 2013, 2016).
The coevolution of early hominids and helminths appears to have been a lengthy process. Human-specific tapeworms survive at high temperatures (greater than 53°C) that would not have been encountered without cooking fires (Allen 1947; Perry 2014). Taenia solium, a porcine tapeworm that causes morbidity in humans, has 35% more copies of heat shock proteins than other tapeworm species (Tsai et al. 2013; Perry 2014), suggesting coevolution of tapeworms with cooking.
Chimpanzees eat all parts of their prey, with special focus on brain and other calorie-dense tissues (Gilby and Wawrzyniak 2018). Ingestion of brain and peripheral nervous tissue increases risk for novel zoonoses (infections from other species), including bacteria, viruses, and prions. The virus exposure of human ancestors is obscure. Two exogenous retroviruses are known to cause current human disease, HIV (lentivirus) and HTLV (delta retrovirus), and both are ubiquitous in primates. Humans are unusual among hominoids in their lack of endemic infections from simian foamy viruses (SFVs) and other spumaviruses, which are ubiquitous in nonhuman primates, but rarely pathological (Switzer et al. 2005; Peeters et al. 2014). Yet, we are susceptible to SFV infections transmitted from nonhuman primates by bites or from eating bushmeat. Fortunately, accidental transmission of SFV to other humans is rare (Switzer et al. 2005; van der Kuyl 2012).
Meat-Related Prion Transmission
The pathogens of carrion may have included infectious prions (Finch and Stanford 2004). The prion gene PRNP influences transmission of infectious prions between species and latency of disease onset (Telling et al. 1996; Prusiner 1998). The extensive human prion variants confer resistance in heterozygotes (Mead et al. 2003, 2008). Prions also interact with the human leukocyte antigen (HLA) system described above: HLADQ7 is 75% less frequent in those with variant Creutzfeldt-Jakob disease (vCJD) than in healthy controls (Jackson et al. 2001). The extensive diversity of prion variants suggests that kuru and other prion-caused neurodegenerative diseases were an ancient hazard of meat-eating and of ritual endocannibalism, an ancient practice continued until recently by the Fore people of New Guinea (Mead et al. 2008). Human PRNP gene polymorphisms may have evolved though multiple episodes of balancing selection, during the divergence of ancestral M and V lineages about 0.5 MYA (Mead et al. 2003). Primates are relatively vulnerable to prion infections (Cervenáková et al. 1994; Schätzl et al. 1995). Chimpanzee PRNP differs from humans at six sites (two coding, four noncoding; Mead et al. 2003) with unknown pathogenicity (Soldevila et al. 2004). The evolution of prion resistance in the Canidae (Fernández-Borges et al. 2018) may be a model for variations of PRNP allele frequency associated with levels of meat-eating and cannibalism in modern populations (Mead et al. 2003).
Siglecs At Cell Surfaces And Meat Consumption
Siglecs are sialic acid-binding, immunoglobulin-like proteins that mediate infections that enter cells by binding to host cell membrane sialic acids, particularly the neuraminic acids, Neu5Gc and Neu5Ac. These membrane constituents are made by the enzyme CMAH (cytidine monophosphate-N-acetylneuraminic acid hydroxylase), which converts N-acetylneuraminic acid (Neu5Ac) to N-glycolylneuraminic acid (Neu5Gc). Although other primates retain an active CMAH and Neu5Gc synthesis, the ancestral human CMAH gene was inactivated 2.5–3 MYA, possibly just before the emergence of the genus Homo (Okerblom and Varki 2017). The timing of the CMAH loss roughly approximates the major transitions to bipedalism and meat scavenging using Oldowan tools, and related adaptations.
Neu5Gc loss may have had broader effects, including altered immune responses and sensitivity to the gut biome that could alter resistance to infectious pathogens. However, human cells can acquire Neu5Gc from eating red meat (Samraj et al. 2015). Only Neu5Ac was detected in poultry meat and eggs, salmon, and shellfish; Neu5Gc is primarily found in red meats and also caviar. Although anti-Neu5Gc immunoglobulins are found at high levels in some individuals, anti-Neu5Gc antibodies have not shown anticipated correlation between levels of red meat consumption (Alisson-Silva et al. 2016). Varki and colleagues propose that cancer-risk is also a tradeoff of meat-eating (Samraj et al. 2015; Alisson-Silva et al. 2016). The relation of Neu5Gc to cancer was modeled with a mouse knockout of CMAH; these mice developed fivefold more hepatocarcinomas when fed Neu5GC and immunized with Neu5Gc to model the proposed xeno-autoantibodies from self-reaction to ingested Neu5Gc.
Neu5Ac is targeted by human influenza virus and bacterial pathogens including Salmonella typhi and Plasmodium falciparum. Other pathogens that prefer the Neu5Gc of chimpanzees include Plasmodium reichenowi, which may have diverged 5–7 MYA concurrent with the shared chimp-human ancestor. The loss of CMAH may have protected against infections by Plasmodium species that targeted Neu5Gc. In direct comparisons, human macrophages had greater phagocytic activity than macrophages of chimpanzees and of normal mice (Okerblom et al. 2017). As a model for the evolutionary deletion, mice with an engineered gene deletion of CMAH had increased macrophage phagocytosis.
Humans are unique among hominids in expressing Siglec-11 and Siglec-16 in brain microglia (Hayakawa et al. 2017). The Siglec-16 gene locus includes Siglec-16P, an inactive pseudogene found globally, which is absent in great apes (Cao et al. 2008; Wang et al. 2012). The initial inactivation of Siglec-16 by 3 MYA may predate the genus Homo (Angata et al. 2002; Cao et al. 2008). Subsequent gene conversions from the contiguous Siglec-11 about 1.1 MYA preceded the shared ancestor of humans, Neandertals, and Denisovans (Wang et al. 2012). Siglec-16 is also expressed in cervical epithelia (Wang et al. 2012; Landig et al. 2019), where it may mediate resistance to Neisseria gonorrhoeae; gonorrhea is uniquely human as a naturally transmitted infection (Landig et al. 2019). Because gonorrhea can reduce fertility from pelvic inflammation, Siglec-16 reactivation may have been adaptive for widely interbreeding human ancestors (Landig et al. 2019).
By living longer, humans are exposed to greater cumulative hazards, and developed novel gene adaptations that benefit later cognitive health. Siglec-3 is multiallelic; the evolved variant CD33m associated with late-onset Alzheimer’s disease (LOAD; Schwarz et al. 2016; Siddiqui et al. 2017). The baseline CD33m gene produces two transcripts and is associated with a SNP that influences exon 2 splicing. The AD-protective CD33m isoform is unique among Siglecs by its deletion of a ligand-binding domain that lacks sialic acid binding (Siddiqui et al. 2017). By shifting the normal location of Siglec-3/CD33m from the cell outer membrane to subcellular peroxisomes, the AD-protective CD33m is proposed to enhance clearance of the amyloid-β peptide by microglia. Humans uniquely have the protective allele, CD33m, which evolved after the common ancestor shared with Neandertals and Denisovans (Schwarz et al. 2016). Killer whales (Orcinus orca) also independently evolved this peroxisome-targeting motif, from which it is suggested that Siglec-3/CD33m evolved convergently in concert with the prolonged postreproductive phases observed in killer whales. Like humans, older killer whales have extensive social interactions with younger generations. Unlike humans, chimpanzee brains express the CD33m isoform although at 80% lower levels. The extreme neurodegeneration of human AD is apparently absent in chimpanzees (Austad and Finch 2014). The CD33 gene is close to another AD-protective locus on chromosome 19, the apolipoprotein E (ApoE3) gene that also evolved in modern humans.
Serotonin Genes Related To Foraging-Scavenging Behaviors
Serotonin (5-HT), a major neurotransmitter and neuromodulator, is relevant to the early exposome by influencing foraging, scavenging, and other exploratory behaviors (Elipot et al. 2013; Lottem et al. 2018). We suggest that serotonin system genes facilitated foraging and exploration in the spread of early H. sapiens. Genetic variants of several serotonin system genes emerged at about the same time as H. sapiens (Claw et al. 2010): based on mutation rate clocks (not fossil DNA), it appears that the 5-HT receptor allele HTR2A (5-hydroxytrptamine receptor 2A) emerged by 0.33 MYA and was followed by introduction of alleles of the 5-HT transporter SLC6A4 (solute carrier family 6 member 4), then the variable number tandem repeat of intron 2 (VNRT) at 0.27 MYA and, lastly, at 0.22 MYA the short allele of the linked polymorphic region (LPR-S). In modern populations, these alleles vary widely in prevalence (Claw et al. 2010; Iurescia et al. 2016). Serotonergic gene variants have expanding behavioral associations that included anxiety, aggressivity, and impulsivity (Backström and Winberg 2017; Wong-Lin et al. 2017). By favoring expanded exploration, these behaviors would have also expanded the diversity of environmental exposures. Other primates independently evolved variants in 5-HT receptors and transporters, including chimpanzees (Claw et al. 2010), macaques (Shattuck et al. 2014a,b), and baboons (Kalbitzer et al. 2016). These and other monoamine transmitter gene variants are associated with behavioral differences within and between populations and kindred species.
Introgressions Of Neandertal And Denisovan Genes
A new group of gene candidates in human evolution are the human-specific segmental duplications (HSDs) identified by Eichler and colleagues from comparisons of great apes with modern and archaic humans (Dennis et al. 2017). HSDs are defined as copy number increases of at least 2.5 copies in 90% of humans, expansions that are absent in great ape genomes. In our interpretation (Appendix Table 1), nearly one-half of the HSD segments included genes associated with brain functions; of these, most arose before 0.6 MYA, preceding the divergence of Neandertal, Denisovan, and modern humans. Immunity-related genes are the next largest class in HSD. Figure 2 maps the timing of these changes, which are briefly identified in Table 3.
We hypothesize that adaptation to ancient pathogens and airborne toxins may in some cases be protecting us today from novel airborne pollutants such as cigarettes and diesel smoke. However, we must also consider potentially deleterious effects because our environments are changing faster than our gene pools. Next we discuss known genetic differences between humans and archaic lineages across six major categories, including: innate immunity; scavenger receptors; immunoglobulin receptors; retroviral sequences; thermosensitivity and domestic fire for heat; and apolipoprotein E.
Neandertal-Derived Innate Immunity-Related Genes
Adaptive benefits of human interbreeding with Neandertal and Denisovan lineages are indicated for innate immune genes, particularly those responding to viruses (Enard and Petrov 2018).
Oligoadenylate Synthetase (OAS)
The OAS locus includes three genes (OAS1,2,3) that mediate antiviral defenses of innate immunity by activating the latent RNase L (ribonuclease L) that degrades invading viral RNA. OAS haplotypes alter resistance to hepatitis C virus and tick-borne encephalitis, among other flaviviruses. The ancestral R haplotype includes six sites attributable to Neandertal introgression at about 0.125 MYA (Mendez et al. 2013). The introgressed OAS1 from the Denisovan may be restricted to Southeast Asia. The OAS2 haplotype R lacks eight amino acids of other haplotypes; it is common in Eurasians, but rare in Africans. The haplotypes differ in OAS gene expression, as shown in macrophages infected with Salmonella, herpes simplex viruses (HSV-1, HSV-2), and influenza virus (Sams et al. 2016). The Neandertal introgressed OAS1 had higher bioactivity and showed evidence of positive selection. Moreover, OAS gene expression in different tissues was modified by archaic allele introgression (Dolgova and Lao 2018).
Toll-Like Receptors (TLR6, TLR1, TLR10)
For microbial resistance, a cluster of TLR genes (TLR6-TLR1-TLR10) shows introgression from the Neandertal and Denisovan genomes. The subclasses of TLR receptors recognize bacterial and fungal pathogens. Danneman et al. (2016) identified 61 archaic-like SNPs in a 143 kb region in the 1000 Genomes Project Phase III dataset. Three introgressed archaic haplotypes were identified: two were closest to Neandertals, and the other closer to Denisovans. These alleles were absent from modern African samples and evidenced positive selection in Asians and Europeans. One haplotype showed a twofold longitudinal gradient in Europe. The archaic SNPs fall mostly in noncoding regions of the 143 kb segment, which includes transcription factor-binding sites. Some archaic SNPs increased expression of the three TLR genes in lymphocytes. Archaic-like haplotypes comprise one-third of the TLR SNPs associated with lower sero-prevalence of Helicobacter pylori. The preservation of these ancient haplotypes in high frequencies suggests their continuing role in host defense during the last 50,000 years in Eurasia.
Virus-Interacting Proteins (VIPs)
The broad category of virus-interacting proteins (VIPs) may comprise 30% of all adaptive amino acid changes in human proteome shared among mammals (Enard and Petrov 2018). Neandertal origins are defined for a subset of VIPs longer than 100 kb. Most long Neandertal-derived VIPs show recombination rates, with evidence for positive selection in Asian and European populations. Notably, Europeans had more Neandertal-derived VIPs than Asians, suggesting the introgressions post-dated their divergence. Various VIPs interact with human immunodeficiency virus (HIV-1), influenza virus A (IFA), and hepatitis virus C (HCV). Their gene ontology (GO) categories include “viral genome replication” and “immune effector processes”; the latter includes the toll-like receptor (TLR2), an HIV-bind ing protein that has increased expression in Neandertal-derived expression of quantitative trait loci (eQTL). The growing list of VIPs suggests viral arms races that contributed adaptive mutations to each species.
Natural Killer Cell Immunoglobulin-Like Receptors (KIRs)
Natural killer cells have immunoglobulin-like receptors (KIRs) expressed on embryonic trophoblast cells that differ remarkably between humans and chimpanzees (Hilton and Parham 2017). Since divergence, humans lost most of the KIR lineage diversity that had evolved in simians since 15 MYA, while generating six human-specific KIR genes. Gene inactivation of KIR2DP1 was present in Neandertals and Denisovans (Hilton et al. 2017). Elegant research in genetic engineering uncovered the immune cell consequences of these genetic changes: evolving KIRs incurred more bottlenecks in humans than chimpanzees. Southeast Asian populations show evidence for a selective sweep in a KIR ligand (HLAB*46) about 60,000 YA, soon after modern humans arrived (Abi-Rached et al. 2011; Abi-Rached and Raoult 2016). KIR haplotypes of Group B show correlation with reproductive success, suggesting balancing selection (Parham et al. 2012). Unlike the well-documented malaria-resistance genes, we lack definitive evidence for the pathogens that selected KIR haplotypes, including the pressures driving the extensive major histocompatability complex (MHC) variations within chimpanzee and bonobo populations(Maibach et al. 2017).
Scavenger Receptors For Microbes Differing From the MARCO Receptor
In lung macrophages and epithelial cells, the MARCO receptor mediates antigen presentation. Common MARCO alleles influence resistance to pulmonary pathogens that are human adapted: respiratory syncytial virus (RSV), Mycobacterium tuberculosis, and Streptococcus pneumoniae (Novakowski 2018). Human-specific MARCO alleles that influence susceptibility to pulmonary tuberculosis arose by 0.5 MYA (Figure 1). Neandertal immune-related genes are discussed below.
TLR and Expression of Quantitative Trait Loci (eQTL)
Several TLR inflammatory pathways of innate immunity differ quantitatively between European and African populations in expression of quantitative trait loci (eQTL) of white blood cells associated with Neandertal DNA sequences (Quach et al. 2016). The recent term eQTL represents quantitative differences in gene expression that are associated with single-nucleotide gene variations in genome-wide association studies (GWASs). These eQTLs accounted for 50% or more of population differences in response to bacterial and viral stimulae by TLR4 and TLR1/2, respectively. Neandertal-derived eQTLs that influenced gene expression in European samples were absent from African ones. Notably, most eQTLs were regulatory, rather than altering the amino acid sequence. Two loci represent 88% of trans-eQTLs associated with 794 trans-regulated genes: interferon beta 1 (IFNB1) and TLR1/2. These findings anticipate other archaic trans-regulatory “hotspots” relevant to lung inflammation and other immune interactions.
FGCR1 (FcγRI)
FGCR1 (FcγRI), a high-affinity immunoglobulin receptor, has major roles in defense against pathogenic bacteria and nematodes. For example, bacterial meningitis infections of neonatal mice depend on binding of the gut bacterium Escherichia coli to FGCR1 on macrophages (Mittal et al. 2010). Data are lacking to indicate how FGCR1 duplications during Phases I and II (Table 3) correspond to resistance to specific pathogens (Dennis et al. 2017).
Loss of Proretroviral Sequences
The deletion of the endogenous chimpanzee gene for the retrovirus PtERV1 (Pan troglodytes endogenous retrovirus-1) suggests shifting host defense mechanisms. Although chimpanzees carry more than 100 copies of PtERV1, humans have none (Yohn et al. 2005; Polavarapu et al. 2006). The loss of PtERV1 occurred to about 4.7 MYA, with an estimated 95% range of 7.2–1.9 MYA (Kronenberg et al. 2018), which places its deletion in early Homo, or in a shared ancestor before the lines separated. The genomic loss of PtERV1 was attributed to incomplete lineage sorting (Kronenberg et al. 2018). Overall, the human genome carries much fewer endogenous retroviruses than chimpanzees (Magiorkinis et al. 2005).
Thermosensitivity And Expansion To Colder Climes
The expanding range of humans to cold latitudes may have been facilitated by changes in TCAF and TRPM8 (transient receptor potential melastatin 8), two genes that mediate sensory perception of cold. About 0.3 MYA, TCAF1-TCAF2 evolved from a segmental duplication (Dennis et al. 2017). The TCAF proteins (TRPM8 channel-associated factors) detect coldness by their binding to the ion channel receptor TRPM8 (Gkika et al. 2015). TRPM8 is the only receptor that responds to moderate cold, and is expressed in somatosensory neurons and airway epithelial cells (Bautista et al. 2007; Knowlton et al. 2013; Liu et al. 2018). The TCAF genes have multiple copies in modern and archaic humans, while Neandertals and Denisovans had but one (Dennis et al. 2017). Although research is absent on extinct populations, the pleiotropies of TCAFs in modern humans include influences on cancer metastasis in prostate (Gkika et al. 2015) and glioblastoma (Klumpp et al. 2017).
The TRPM8 SNP, rs10166942, C/T varies more than tenfold between populations, with highest frequencies in northern Europe (Key et al. 2018). This SNP is 1 kb upstream of the coding sequences, implying its role in gene expression. The new T allele increases risk of migraine headache, with environmental sensitivity: migraines can increase sensitivity to cold temperature, while drinking cold water can trigger a migraine. Analysis of archaic DNA suggests that the T allele was prevalent 3000–8000 YA in Europe, and had spread about 26,000 YA, approximating the last glacial maximum. With expansion into colder climates, exothermic heat sources such as controlled fire likely became a critical part of daily life, above and beyond cooking behavior.
Dipeptidase
Genetic changes in dipeptidase (DPEP), an intestinal digestive enzyme, may have benefited resistance to domestic smoke exposure. Introgression of Neandertal DPEP1 (rs460879-T) in East Asians (Hu et al. 2015) has implications for stress responses to environmental smoke. DPEP1 enzyme variants influence blood homocysteine, a derived amino acid in the methionine cycle that is normally maintained at low levels. The elevation of homocysteine in cigarette smokers (Chen et al. 2015; Al Rifai et al. 2017) predicts systemic oxidative responses to domestic smoke. Because non-Neandertal DPEP1 alleles are common outside of East Asia, Hu et al. (2015) suggest that modern prevalence of the Neandertal East Asian allele represents its reintroduction from Altai Neandertal sources. Lower blood homocysteine may have lowered the risk of fetal neural tube defects, ischemic disease, and dementia associated with hyperhomocysteinemia (Perla-Kaján and Jakubowski 2019). Human chimpanzee comparisons for metabolism of glutathione and other antioxidants may in part explain why aging chimpanzees have low incidence of cardiovascular disease (Table 4) and Alzheimer’s dementia (see below).
Apolipoprotein E (ApoE)
ApoE is a multifunctional protein in lipid metabolism, immunity, and brain synapses. It was first known as a key transporter of cholesterol in the blood to the liver and in the brain to neurons. Humans evolved multiple ApoE isoform proteins differing in binding affinities for cholesterol and for lipoprotein receptors of the LDLR family (low-density lipoprotein receptor; Mahley et al. 2009). In all populations, ApoE3 is the most frequent allele, followed by the widely varying ApoE4 (Corbo and Scacchi 1999; Stengård et al. 2006; Reales et al. 2017). In Europe, ApoE4 has a strong latitudinal gradient, with a more than twofold excess in Nordic over Mediterranean populations. ApoE2 is generally the least prevalent, with some evidence that it may even be absent in some American indigenous groups (Reales et al. 2017). The higher prevalence of ApoE4 in northern Europe was hypothesized as adaptive for vitamin D needs in high latitudes with limited exposure to ultraviolet B (Gerdes 2003), discussed further in diet interactions below.
ApoE4 is associated with shorter life span in several populations (Drenos and Kirkwood 2010; Nygaard et al. 2014; Raichlen and Alexander 2014; Kulminski et al. 2016; Wolters et al. 2019) and is a major risk factor in Alzheimer’s disease (AD). Moreover, ApoE4 worsens recovery from head trauma (Lawrence et al. 2015), which itself is an AD risk factor (Mendez et al. 2015). Worse yet, ApoE4 increases dementia risk from air pollution (Cacciottolo et al. 2017). In contrast, ApoE2 lowers the risks of AD and mortality at later ages (Schächter et al. 1994; Drenos and Kirkwood 2010).
The global persistence of ApoE4 suggests adaptive advantages in prior generations. Despite its strong association with AD and premature memory declines at later ages, ApoE4 has shown cognitive advantage to younger ages. In some studies, young adults carrying ApoE4 had slightly higher IQ and educational achievement (Tuminello and Han 2011). As a model for developmental memory, mice carrying human ApoE4 had stronger hippocampal long-term potentiation as sub-adults (age 2 months), but not as 6-month-old adults (Kitamura et al. 2004). Recent findings show health advantages of ApoE4 in several highly infected environments where ApoE4 enhances growth and survival. Brazilian slum children carrying ApoE4 had less diarrhea and better cognitive development (Oriá et al. 2007; Mitter et al. 2012). Moreover, in rural Ghana, we found increased survival of ApoE4 carriers as children and adults (van Exel et al. 2017). Furthermore, in the Tsimané, older adults with high parasite burdens had better cognition if they were ApoE4 versus E3 carriers (Trumble et al. 2017). In transgenic mice, human ApoE4 increased resistance to cryptosporidial enteric infections (Azevedo et al. 2014). Experimental malarial infections of human erythrocytes also showed protection by E4/E4 versus E3/E3 (Fujioka et al. 2013). Risk of leprosy, a bacterial infection of skin, is associated with noncoding sites in ApoE, e.g., SNP rs405509 in the ApoE promotor influences levels of ApoE protein in skin cells (Wang et al. 2018). ApoE4 may favor some infections, e.g., Chlamydia pneumoniae attached preferentially to host cells transfected with ApoE4 versus E3 (Gérard et al. 2008), consistent with excess ApoE4 in a group of arthritic patients with synovial infections of C. pneumoniae (Gérard et al. 1999), but again these patients have low pathogen burdens.
Chimpanzee ApoE is considered the ancestral hominin prototype because it resembles ApoE4 at the two amino acid sites that distinguish ApoE3 and ApoE4 (arginine, R112. and R158; Table 5). Chimpanzees apparently have only one ApoE isoform, in samples from Eastern and Western subspecies (Hanlon and Rubinsztein 1995; Fullerton et al. 2000; McIntosh et al. 2012). Another critical site of human ApoE is R61 versus T61 in chimps. The Denisovan ApoE resembled human ApoE4 at these three amino acids (Table 5; McIntosh et al. 2012). Frustratingly, published Neandertal genomes do not provide data for site 112. We do not know how these other ApoE differ from human ApoE4 in in binding lipids and cellular lipoprotein receptors (Finch 2010). Uncertainties stem from differences in the amino acid at site 61, which is critical for lipid binding (Raffaï et al. 2001) and because chimp-human ApoE differ in 11 nonsynonymous sites (Huebbe and Rimbach 2017). Pilot studies suggest that chimp ApoE may function more like ApoE4: transgenic chimp ApoE expressed in mouse astrocytes had neurotrophic activity equivalent to human ApoE4 rather than E3 (Mafalda Cacciottolo, pers. comm.).
TABLE 5.
ApoE amino acids in hominins
| ApoE isoform, prevalence range | Site 61 | 112 | 158 |
|---|---|---|---|
| Human ApoE2, 1–19% | R | C | C |
| ApoE3, 55–90% | R | C | R |
| ApoE4, 5–40% | R | R | R |
| Denisovan | R | R | R |
| Neandertal | K | R | |
| Chimpanzee 100% | T | T | T |
C: cysteine; K: lysine; R: arginine; T: threonine; McIntosh et al. 2012. The Neandertal K61 was confirmed in the current ENSEMBL chimpanzee genome by Arvis Sulovari (Eichler Laboratory, University of Washington, pers. comm.).
These species comparisons, while limited by incomplete sequence data, support suggestions that ApoE4 was the ancestral isoform (Hanlon and Rubinsztein 1995; Fullerton et al. 2000; Mahley et al. 2009). ApoE has a deeper history within the ApoA/ApoC/ApoE gene family, which diversified during vertebrate evolution (Huebbe and Rimbach 2017). DNA estimates indicated that ApoE3 spread about 0.225 MYA (95% CI, 0.176–0.579 MYA), which approximates the definitive emergence of H. sapiens (Harvati et al. 2019). ApoE2 emerged later, about 0.08 MYA (Fullerton et al. 2000; Huebbe and Rimbach 2017). Possibly, ApoE3 was established 100,000 years before modern humans emigrated northeast to Eurasia.
Further studies of ApoE evolution must consider other genes that are closely linked to ApoE on chromosome 19q13.2. Its neighbor TOMM40 encodes a mitochondrial outer membrane protein (Lutz et al. 2016; Roses et al. 2016; Larsen et al. 2017). Alzheimer risk factors in TOMM40 coexist with ApoE alleles in haplotypes differing widely between Africans, Asians, and Europeans. The TOMM40 gene includes 16 Alu retroposon elements, which imply genetic unstability (Larsen et al. 2017). However, chimps and other great apes have the same Alu suite as human TOMM40, a remarkable stability over 15 million years (Arvis Sulovari and Evan Eichler, pers. comm.). Other neighboring genes on chromosome 19q13.2 have been associated with AD risk (Kulminski et al. 2018).
Modern ApoE alleles are associated with blood cholesterol levels (Sing and Davignon 1985). For example, human ApoE3 carriers had 22% lower plasma triglycerides after a fatty meal than carriers ApoE4 (Carvalho-Wells et al. 2010). ApoE3 was hypothesized to have evolved as a “meat-adaptive” gene by favoring lower blood cholesterol (Finch and Stanford 2004), and plays critical roles in immune function that may be beneficial in high pathogen environments. Among indigenous South Americans, the ancestral ApoE4 SNP (rs7412T) was more than fivefold enriched in hunter-gatherers versus horticulturalists (Reales et al. 2017). With high pathogen load, and little evidence of cardiovascular disease in South American indigenous populations, the advantages of low blood cholesterol in cardiovascular disease may have been outweighed by the immune benefits of the ApoE4 allele(Kaplanet al. 2017; Trumble et al. 2017). The pathophysiological impact of ApoE4 may depend on infections carried by the population as noted above.
Higher blood vitamin D levels were associated ApoE4 in two studies: in a large sample from northern Germany and in mice transgenic for human ApoE alleles (Huebbe et al. 2011). Diet ApoE-interactions are being considered for obesity and insulin resistance in the metabolic syndrome. In a randomized control trial, E4 carriers had stronger associations of insulin resistance with elevated levels of palmitate (C16:0), a saturated fatty acid (Caslake et al. 2008). These scattered findings suggest a deep involvement of ApoE alleles in the evolution of human diets.
Synthesis, Exposome Phases I–III
We propose that the brain and immune systems coevolved for pathogen resistance and for brain development. Most of the genetic variants outlined affect functions in the brain and immune system. Systemic inflammation endangers the brain during pre- and postnatal development (Jiang et al. 2018). During pregnancy, for example, third trimester elevations of blood IL-6 and C-reactive protein (CRP), which are induced by bacterial infections, showed strong correlation with impaired postnatal cognitive development and brain circuit connectivity in medial prefrontal cortex (Spann et al. 2018). The vulnerability of children’s brains to maternal infections would entail selective pressures on immune genes. The Fc gamma receptor gene (FCGR), which mediates defense against bacterial meningitis infections, underwent two events of duplication (Table 2). The MHC class I and class II proteins that mediate antigen presentation are active in brain development. For example, MHC class I-H2-Kb and H2-Db are expressed in neurons during normal brain development, and mediate synapse remodeling (Adelson et al. 2016). Neuronal stem cells also express MHC class II proteins in normal human embryos without pathogens or inflammation (Vagaska et al. 2016). MHC class I expression in dopaminergic neurons mediates reward-seeking behaviors (Murakami et al. 2018), while the natural killer cell receptor KIR has variants of MHC class I ligands that are associated with autism (Guerini et al. 2014; Torres et al. 2016). Complement factors also mediate synapse pruning (Györffy et al. 2018; Tenner et al. 2018), adding to the diverse co-option of ancient immune functions in brain evolution. Lastly, recall that ApoE4, a lipid transport gene, enhanced cognitive development and reduced diarrhea in children living in slums, a highly infectious environment. In healthy environments, ApoE4 carriers have smaller-sized frontal cortex regions as neonates, children, and young adults (Shaw et al. 2007; Piers 2018).
The ensemble of derived brain and immune genes in humans (Figure 2; Table 3) anticipates networks of brain-immune interactions that favored human expansion into environments with different pathogens and zoonotic possibilities. The evolution of longer postreproductive phases (Table 4) may have revealed novel frailties from the progressive slowing of information processing and neurotransmitter receptor loss during normal aging in modern humans that emerges in middle age without neuron cell losses of Alzheimer’s disease (Finch 2009).
Many of the exposures and adaptations noted in Phase I are still relevant in Phases II–III. Exposure to dust and novel pollens would only have increased as individuals entered into a mosaic of novel environments during the diaspora from Africa. Although many of the Phase I exposures were still relevant, the advent of fire and cooking likely limited carrion pathogen exposure. Parasites and pathogens were also evolving, e.g., Taenia solium, hominin specific parasites, evolved heat resistance that increased its survival to cooking.
Phase IV: Holocene-Neolithic to Industrial 12,000–200 Years bp
Plus High-Density Populations, Domestic Animal Feces, And New Infections
Rapid changes in lifestyle and environmental exposures occurred for humans between 12,000 years ago and 1820 ce, with extensive changes to social life, diet, physical activity, population densities, and exposure to novel anthropogenic chemicals and toxins. Although 10,000 years is brief in evolutionary time, the major selective pressures entailed have left genetic signatures of selection (Nesse and Williams 2012). Global population estimates from the early Holocene range widely, from one to ten million (Thomlinson 1975; Klein Goldewijk et al. 2011; Pala et al. 2012). By 1820, more than one million people lived in London, and the world population is approximated at one billion (Maddison 2003). These huge population expansions during shifts from hunting-foraging to settled farming to cities arose rapidly in evolutionary time. Ongoing studies of modern and fossil genomes anticipate many genomic changes in coding sequences relevant to pathogens, as is the case with resistance to malaria, but also in noncoding variants that alter gene expression with cell-type specificity (eQTLs).
Animal Domestication And Agriculture
The spread of agriculture and animal domestication must have increased exposure to animal pathogens and dust. Archeological and genetic evidence is consistent for the domestication of sheep, goats, pigs, and cows by 10–11 KYA (Germonpré et al. 2009; Larson et al. 2014; MacHugh et al. 2017). Animal domestication had multiple impacts on humans from major changes in diet, daily physical activity, and increased exposure to pathogens from high human density habitation with human and animal feces.
Changing Diets: Expanding Amylase Genes And Lactase Persistence
Animal and plant domestication introduced major changes in diet by increasing access to starchy foods, animal milk, and meat (Popkin 2006; Kraft et al. 2018). Although “no single diet… represented all hunter-gatherer societies” (Cordain et al. 2000:688), most hunter-foragers derived at least 50% of their diets from hunting and fishing (Cordain et al. 2000, 2005; Pontzer et al. 2018). Starchy foods are associated with gene evolutionary changes for the salivary enzymes α-amylase (AMY1,2,3) mediate predigestion of starches. Chimpanzees have one AMY1 gene, while humans average seven copies (Perry et al. 2015; Inchley et al. 2016; Pajic et al. 2019), with a broad range of 2–18 copies. Siberians with low-starch diets have fewer AMY1 and more AMY2A deletions (Inchley et al. 2016). The AMY1 gene expansion arose after the split with Neandertals from a selective sweep during the Middle Pleistocene, possibly in association with increased starch consumption. More generally, animals closely associated with human starchy foods have a thousandfold higher salivary amylase activity in association with lineage-specific AMY1 gene multiplication, e.g., domestic dogs have multiple copies of AMY1 unlike wolves (Pajic et al. 2019). Modern populations have genetic changes in the regulation of lactase, the enzyme of intestinal cells that yields glucose from lactose, the milk sugar.
Before animal husbandry, adults had negligible exposure to lactose after weaning. Unsurprisingly, genetic variants for lactase persistence (continued activity of the lactase enzyme past childhood) have not been detected in Neolithic European farmers (Burger et al. 2007). Lactase persistence (C-14010) had a selective sweep in sub-Saharan Africa within the last 7000 years (Tishkoff et al. 2006; Enattah et al. 2007). Moreover, lactase persistence shows convergence in several SNPs of sub-Saharan Africans (G/C-14010, T/G-13915, C/G-13907) and Europeans C/T-13910 (Tishkoff et al. 2006; Ranciaro et al. 2014). The individual duration of lactase expression into adulthood differs by levels of DNA methylation in intestinal cells for epigenetic haplotypes (Labrie et al. 2016). The rapid spread of these genes may have benefited from larger body mass and energy reserves that favored survival from acute and chronic infections, as well as increasing fertility (Montalva et al. 2019). Durable milk products such as cheese are found by 8000 years ago and gave dual benefits: longer storage and a reduction in lactose, which made dairy accessible to those lacking lactase persistence genes (Salque et al. 2012).
Fats, Fecal Aerosols, And Fiber Content
Besides increasing milk for those with lactase persistence genes, selective breeding of livestock increased the availability of dietary fat. The primary storage of subcutaneous fat in wild mammals is as saturated fatty acids, while fatty acids stored in muscle are polyand monounsaturated fats (Cordain et al. 2002). Typical wild prey mammals and birds only have abundant subcutaneous fat for a few months per year prior to reproduction, and thus during much of the year have low levels of saturated fats. Neolithic animals were bred to maintain subcutaneous fat year round. Consequently, grain-fed beef has severalfold more saturated fat than game, but less polyunsaturated fat (Cordain et al. 2002). Paralleling animals, plant domestication improved crop yields and caloric value, while narrowing the diversity of plant species consumed (Cordain 1999; Cordain et al. 2005). In addition to dietary exposure to lactose and higher fat concentration, domestication may have greatly increased overall exposure to potential pathogens from bacteria, fungi, worms, prions, and viruses, for which there is early evidence (Table 3). Infections have a huge range of vectors that include ectopara-sites (fleas, ticks) and insects (flies, mosquitoes) that are associated with domestic animals and agriculture (Zuckerman and Armelagos 2014; Ross et al. 2018; Asante et al. 2019; Davidson et al. 2019). The majority of our 2100 infectious pathogens are considered zoonotic (Lloyd-Smith et al. 2009). Domestic animals were often maintained in close proximity to cooking, eating, and sleeping. In Çatalhöyük on the Anatolian plateau 9000 YBP, domestic animal feces and parasite eggs were adjacent to the densely inhabited apartments (Larsen et al. 2019). Until recently, many traditional farmers kept livestock close to or within their living quarters for protection and warmth. Much is obscure about the contribution of pathogenic infections from natural versus domestic animal populations. Some evidence suggests bidirectional exchange of tuberculosis Mycobacterium species between humans and livestock: cattle, M. bovis; goats, M. caprae (Hershokovitz et al. 2015). We do not know how the gradual reduction of wild populations from expanding farming and urbanization altered zoonotic infections.
Sugar Cultivation
The saccharide content of many cultivars was increased over wild species (Hardy et al. 2015; Schnorr et al. 2015). Sugar cane was first domesticated 5–8 KYA in New Guinea (Denham 2011), long before molasses and other sugar products contributed to global diets. However, honey, one of the most energy dense foods in nature, is common across many contemporary subsistence populations (Hill and Hurtado 1996; Cordain et al. 2005; Crittenden 2011; Marlowe et al. 2014; Trumble et al. 2014; Kraft et al. 2018). Crystalized sugars were produced by 500 bc (Kuti and Galloway 1994). The use of sugar expanded rapidly in the last 400 years. By 2010 in the U.S., added sugars comprised than 14% of the diet (Drewnowski and Rehm 2014). Excessive intake of calories, particularly sugar and other carbohydrates, is a widely recognized risk factor for type 2 diabetes (T2DM).
Less is known about interactions of lifestyle, genetics, and T2DM. More than 150 SNPs are associated with T2DM in different populations, e.g., NIDDM1 in Mexican Americans, CAPN10 in northern Europeans (Horikawa et al. 2000), and SLC30A8 TCF7L2 IDE–KIF11–HHEX and EXT2–ALX4 in France (Sladek et al. 2007). Some SNPs associated with T2DM risk could have additional roles.
The capacity to rapidly store calories in fat is postulated as beneficial throughout human evolution in the thrifty phenotype hypothesis (Hales and Barker 2001). Traditional life prior to agriculture had high levels of physical activity, high parasite and pathogen load, and less caloric abundance. Under these conditions, rapid energy storage as fat would be advantageous (Hales and Barker 2001; Cordain et al. 2005; Gurven et al. 2016b, 2017). Nonetheless, in current sedentary urban environments with low parasites and pathogens and easily available foods, a “thrifty phenotype” is less advantageous. Moreover, T2DM shows intergenerational transmission in association with DNA methylation (Barrès and Zierath 2016).
In summary, the increased calories from domesticated crops and animals provided both increased calories that supported higher population densities and the availability of weaning foods decreased interbirth intervals resulting in faster population growth. In addition to higher population densities, the stationary nature of agriculture and some types of animal domestication meant that traditionally nomadic peoples settled, setting the stage for the first cities.
Population Density And Epidemics
The birth intervals are longer in hunter-gatherers than horticulturalists (Bentley et al. 2001; Helle et al. 2014): three to four years in the forest Aché (Hill and Hurtado 1996), Gainj (Wood 1994), and !Kung (Jones 1986) to 2.5 years in Tsimané forager-horticulturalists (Gurven et al. 2016a), and horticultural Aché postcontact (Hill and Hurtado 1996). Although fertility increased during this transition from foraging to farming (Bentley et al. 1993; Page et al. 2016), it is unknown how much of the increased fertility was due to consistent availability of calories year round (Kramer and Greaves 2007), access to weaning foods (Larsen 1995), or other lifestyle changes (Gage and DeWitte 2009). Although hunter-gatherers were infected by helminths and other long-lived lower virulence pathogens (Bennett et al. 1970; Hill and Hurtado 1996), the low population densities of hunter-gatherers gave some protection against the spread of high virulence pathogens causing acute infections that have limited protection by slow responding adaptive immune responses (Table 1; Henn et al. 2012; Karlsson et al. 2014). Extant horticulturalists may have higher parasite and pathogen loads than hunter-foragers due to higher population densities and sedentary communities with poor sanitation. Information is limited: we need more cross-population studies with consistent protocols (Bennett et al. 1970; Blackwell et al. 2011, 2013, 2016; London and Hruschka 2014).
Adult body size progressively decreased during the Paleolithic and Neolithic expansion of settled populations, assessed by height and weight (Holt and Formicola 2008), and bone mass density (Chirchir et al. 2015; Ruff et al. 2015; Figure 3). This combination suggests energetic tradeoffs in maintenance of skeletal tissues in higher pathogen contexts. Extant horticultural populations with high parasite and pathogen loads and high total fertility rates tend to have low bone mass density (Stieglitz et al. 2015, 2017, 2019), and shorter stature (Blackwell et al. 2017; Urlacher et al. 2018). These tradeoffs are consistent with the recent increase of European adult height in association with reduced early infections, and are discussed below.
Epidemics were favored by increasingly dense populations. Pathogens of most early plagues and ordinary infections are unknown; some parasitic infections can be identified by paleo-DNA (Table 6) of Variola, Yersinia pestis, Vibrio cholerae, and other highly virulent pathogens with fast reproductive rates (R0; Dobson and Carper 1996; Keeling and Gilligan 2000; Karlsson et al. 2014).
TABLE 6.
Early evidence of infectious disease and pathogen-resistance genes
| Disease | Place, date | Species | Protective gene candidates |
|---|---|---|---|
| Choleraa | Ganges delta, 5 KYA | Vibrio cholerae | NF-κB, cAMP-mediated chloride secretion blood group |
| Leprosyb | Sub-Sahara, >10 KYA | Mycobacterium leprae | TLR1 gene, CYLD |
| Nematodesc,d | Ancient Europe-Western Asia | Soil and meat-borne: whipworm Ascaris; roundworm Trichuris | ApoE4 |
| Plaguee | Eurasia, 5–3.5 KYA | Yersinia pestis, modern; many candidate pathogens | CCRΔ32 |
| Small poxb | Africa, 15–70 KYA | Variola virus | CCRΔ32 |
| Gonorrhea | Africa, >100 KYA | Neisseria gonorrhoeae | Siglec-16g |
| Malariab | Africa, >100 KYA | Plasmodium falciparum | Duffy, G6PD deficiency, α+ thalassemia, hemoglobin C, CGYPA/B/C-deficient erythrocytes |
| Malariaf | Africa, 22 KYA | P. falciparum | Hemoglobin βS, sickle cell mutant |
Little is known about ancient pathogenic infections. Helicobacter pylori may have been present in humans as early as 100,000 YPB in Africa, followed by clonal diversification after 60,000 YBP (Achtman 2016). Humans are the sole host, giving rise to H. acinonychis in large African cat species (Eppinger et al. 2006). The earliest evidence for M. tuberculosis comes from Atlit Yam, 9000 YBP near Haifa, Israel (Hershkowitz et al. 2015). The absence of tuberculosis in contemporary Çatalhöyük (Larsen et al. 2019) suggests uneven prevalence in the Neolithic, preceding its global spread (Achtman 2016; Saelens et al. 2019). The zoonotic origins of M. tuberculosis are unresolved; dating of the seven clonal families of obligate human-adapted pathogens is sensitive to molecular clock assumptions (Saelens et al. 2019). Malaria origins are discussed below. Small pox, “one of humankind’s greatest scourges” (Barquet and Domingo 1997:635), decimated many civilizations, including the Roman Empire, Hittites, and the 90% depopulation of Mexico after Spanish conquests of the 1500s (McNeill 1989). Smallpox has retained high mortality: case-fatality rates range from 30% for ordinary smallpox to 90–100% for the flat-type or hemorrhagic (Henderson et al. 1999). Mutation of the chemokine receptor gene CCRΔ32 mutation shows signs of positive selection (Galvani and Slatkin 2003), and originated from a single mutation event, 700–2000 years ago (Libert et al. 1998; Stephens et al. 1998; Galvani and Novembre 2005). Although CCRΔ32 protects against Yersinia pestis and some other major epidemic pathogens, epidemiological models suggest that the repeated smallpox epidemics had more impact on positive selection for CCRΔ32 than bubonic plague; Yersinia pestis alone could account for only 10% of the CCRΔ32 increase in European populations (Galvani and Slatkin 2003).
With the rise of concentrated populations in towns, cities, and megacities in the Industrial Age, waterborne disease would have become a significant cause of mortality and selective pressure before sanitation and water purification was prevalent (Cutler and Miller 2005). For example, Vibrio cholerae from the Ganges River causes high mortality (Karlsson et al. 2013, 2014). The nearly twofold higher rate of cholera infections for blood group O (Harris et al. 2008) may underlie the low prevalence of the O blood group in Bangladesh, an ancient epicenter of cholera (Karlsson et al. 2013). Other genes over-represented in Bangladesh include nuclear factor-κB (NF-κB) signaling, and cyclic AMP-mediated chloride secretion, related to cholera resistance (Karlsson et al. 2013).
Climate changes also favored mosquito-borne infections, particularly in the Green Sahara during the Holocene Wet Phase(Neolithic Subpluvial) 7500–3000 bce, with concurrent expansion of Plasmodium parasites with agriculture and animal domestication (Shriner and Rotimi 2018; Laval et al. 2019). Haldane hypothesized 70 years ago that heterozygotes for heritable thalassemia are resistant to malaria (Lederberg 1999). Sickle cell hemoglobin (HbS) became the iconic example of balancing selection, followed by many more genes conferring malarial resistance. A short list includes FY*O mutation of Duffy antigen receptor, a glycoprotein that facilitates infections (DARC; atypical chemokine receptor 1, ACKR1 gene); G6PD (glucose-6-phosphate dehydrogenase deficiency; glycophorin genes, and the 5q31–q33 immune gene cluster; Tishkoff et al. 2001; Malaria Genomic Epidemiology Network 2015; Marquet 2018). Epistatic gene interactions and DNA methylation further expand the complexity of malarial resistance (Arama et al. 2018; Marquet 2018).
Hemoglobin S has a single origin in Africa, which is dated differently according to models and assumptions as early as 7300 YBP (Shriner and Rotimi 2018) or pre-Neolithic 22,000 YBP (Laval et al. 2019). The Duffy blood group mutation FY*O may be even older at 42,000 YBP (McManus et al. 2017). FY*O is absent from Neandertals and Denisovans, which had the ancestral FY*B. This frameshift mutation causes premature termination of the ACKR1 transcript and dys-function of the glycoprotein mediator of Plasmodium vivax infection; FY*O has nearly replaced other DARC alleles in equatorial Africa in selective sweeps with extreme fitness coefficients of 4% (McManus et al. 2017). However, fixation is incomplete in sub-Saharan Africa (Howes et al. 2011). In Southeast Asia malarial prone regions, a novel 838 DARC mutant emerged independently of the African (Shimizu et al. 2000), but also has not reached fixation. Moreover, P. vivax has evolved a novel red cell invasion mechanism in Madagascar and Senegal in Duffy mutation carriers (Niang et al. 2018).
Ancient origins of malaria are being clarified. Six Plasmodium species naturally infect chimpanzee or gorilla (Otto et al. 2018). Of these, the gorilla P. falciparum became adapted to human by zoonotic transfer about 50,000 YBP. The pathogenicity of P. falciparum arises from adhesion domains in the var gene family that allow entrapment of infected red cells in the microcirculation; the var genes originated before their great ape hosts, suggesting ancient evolutionary battles of host resistance and Plasmodium genes that modify red cell adhesion and virulence (Brazier et al. 2017).
Toxic Metals
Most data are from the Bronze and Iron ages, when technological advancements introduced evolutionarily novel exposure to heavy metals. The earliest evidence of lead exposure was found in teeth of two Neanderthal children in the Rhône valley 140–300 KYA, showing tenfold higher lead above basal levels (Smith et al. 2018). These exposures appear to be isolated events following weaning. In the mid-Paleolithic, heavy metals nickel, zinc, and copper were found in Iberian cave sediments (Monge et al. 2015). Metallurgical lead exposure is documented by 3600 bce in the Balkans (Longman et al. 2018). Greenland Ice Cores record lead emissions far from industrial centers, corresponding to the Phoenician and Western Roman expansions (McConnell et al. 2018). Ice core data likely underestimate the local exposure from the lead water pipes commonly in Roman cities. Other data are from sediments in Roman harbors (Delile et al. 2014, 2017). The lead content of Roman bones was a thousandfold higher than of ancient Peruvians not exposed to lead smelting (Patterson et al. 1987). Lead exposure is unlikely to have triggered the collapse of the Western Roman Empire, because lead contamination was decreasing during its decline (Delile et al. 2017).
Air Pollution
Increased exposure to airborne pollutants followed the transition from seminomadic hunting and gathering to more sedentary horticultural and agrarian lifestyles, which is likely to have increased mortality: from 1.6 to 3.8 million excess deaths per year are attributed to indoor (household) air pollution (Landrigan et al. 2018) that arise from burning dried dung and other low-grade fuels (Table 7, Phase V, below), and represent about one-half of the total mortality from ambient air pollution and cigarettes. Permanent dwellings, particularly in cold climates, were typically built of higher quality than nomadic dwellings that allows less air exchange, and would retain more indoor airborne particulate matter. For example, indoor air modeling of 17th-century Danish farmhouses predicted carbon monoxide and PM2.5 levels above WHO guidelines (Ryhl-Svendsen et al. 2010). Besides wood as a domestic fuel, coal was increasingly used after 1500 in Europe; by the mid-16th century, coal provided 10% of the energy in England, rising to 80% by 1800 (Wrigley 2013). Increased household coal burning caused the smogs of London and other cities that were infamous long before the Industrial Revolution (Evelyn 1661; Brimblecombe and Grossi 2009). Although data before 1900 are lacking, pollution-related morbidity and mortality exposure were likely high for centuries in Europe’s smoky cities, which we infer from the 12,000 excess deaths attributed to the London Smog of 1952 (Bell et al. 2004; Finch 2018).
TABLE 7.
Mortality from airborne toxins
Synthesis Phase IV
Major changes in lifestyle during Holocene resulted from agriculture and animal domestication led to dense settlements with increased exposure to anthropogenic pollutants and favored epidemic disease. These exposures were compounded by increasing population densities and pollution of ground and river water from human and animal feces, as urban centers increased in density. Phase IV was associated with a suite of new selective pressures to infection pathogens, illustrated by candidate genes for resistance to cholera and malaria. Many of these factors would interact: air pollution combined with high population density and higher sugar and higher fat diets.
Exposome Phase V: Industrial Age, 1820–2020
Plus Sterile Inflammogens From Air Pollution, Fossil Fuel, Tobacco, And Adiposity With Reduced Mortality From Infectious Pathogens
In Exposome Phases I–IV, mortality was driven by pathogens from recurring acute bacterial and viral infections on the background of chronic infections of bacteria, viruses, and parasites. With understanding of hygiene and infectious diseases that emerged long before vaccinations and antibiotics, acute infections as a primary cause of death began to diminish. The initial reductions of infant mortality are attributed to improved hygiene, water quality, and food availability (Fogel and Costa 1997; Drevenstedt et al. 2008). However, with industrialization came new sources of chronic disease from environmental pollutants, industrial toxicants, and tobacco. Thereby, chronic pathogen-driven infections as a cause of adult mortality was gradually replaced by diseases of aging that are accelerated by sterile inflammogens from cigarette smoke and air pollution. There are important interactions of noncommunicable diseases with infections. Chronic inflammatory lung conditions from smoking and air pollution increase vulnerability to infections. For example, smoking increases the risk of pneumococcal infections fourfold over nonsmokers, and even secondhand exposure increased pneumonia risk by 2.5-fold over nonsmokers (Nuorti et al. 2000).
During Phase V, life spans in developing countries doubled in less than 10 generations during the diminution of infectious pathogens as the main cause of morbidity and mortality. Paradoxically, this mortality transition was fostered by the Industrial Revolution. In The Modern Rise of Population, McKeown (1976) argued that population growth during the Industrial Revolution was mainly attributable to improved food and better food distribution. Two decades later, the “techno-physio revolution” by Fogel and Costa (1997) argued for an equal or greater role of advances in hygiene, medicine, and technology in addition to food availability. These landmark studies do not, in our view, sufficiently recognize the role of reduced morbidity from infections in increasing healthspan and longevity (Finch and Crimmins 2004; Crimmins and Finch 2006; Finch 2007). Here we further the importance of the shift from pathogen-driven chronic inflammation to inflammation of chronic diseases that is driven by sterile inflammogens that include air pollution, cigarette smoke, and fat depots.
These complex changes emerged with major differences in life span and burden of chronic disease by gender and socioeconomic status (SES). Examples from the huge body of relevant work are chosen to illustrate major features of longevity increases in association with decreasing mortality from infections.
Mortality And Longevity
Mortality trends are known for a few countries as mortality from infections gradually diminished in the transition to industrialization. Sweden had the first household-based nationwide record of mortality across the life span in mid-18th century (Sköld 2004). The 19th-century survival curves dip sharply from early life mortality (Figure 4A). In 1800 the life expectancy at birth (Eo) was about 35 years, within the range of other preindustrial indigenous peoples in the 20th century (Gurven and Kaplan 2007; Phase IV). Survivors to age 20 in 1800 had remaining life expectancy of about 45 years. Major further reductions of early-age mortality lengthened survival curves into the 20th century (Figure 4A). As early age mortality dropped, later age survival also increased. Life expectancy at age 70 (LE70) has more than doubled since 1850 (Finch and Crimmins 2005; Figure 4B).
Figure 4. Swedish Mortality Since 1800.

Panel A: Survival curves (Human Mortality Database). Redrawn from Finch 2018. Panel B: Life expectancy at age 70. Redrawn from Crimmins and Finch 2006.
More broadly, the developing countries in Europe and North America share consistent increases toward LE0 of about 80. Centenarians have become the fastest growing age group (Robine and Cubaynes 2017; Gavrilov and Gavrilova 2019). In effect, the health-rich populations have more than doubled the LE0 of early hominid evolution in Exposome Phases I–III. Infant mortality declined sharply (Figure 4A). Although much of the increase in LE0 during the last few centuries was due to reduced infant mortality, survivorship was increased across the life span, including older ages (Figure 4B). We hypothesized that the surviving infants in highly infectious environments before 1900 carried determinants of earlier onset mortality, the cohort morbidity hypothesis (Finchand Crimmins 2004; Crimmins and Finch 2006). As early mortality from infections declined, successive birth cohorts had improved growth (Figure 5A), with increasing adult height shown for France (Figure 5B); other European countries have similar trends (Crimmins and Finch 2006).
Figure 5. Cohort Effects on Mortality and Adult Height.

Panel A: Mortality in Sweden by birth cohort, showing parallel shifts in mortality curves across the life span with declining early age mortality from infection. Redrawn from Finch 2018:Figure 5.10; Human Mortality Database, Eileen Crimmins (pers. comm.). Panel B: Adult height at age 21 varied inversely with neonatal mortality, 1806–1895, in French cohorts; England and Sweden showed similar relationships. Redrawn from Crimmins and Finch 2006.
Infections likely played an important role in both early age mortality to impaired growth in these historical cohorts (Crimmins and Finch 2006; Finch 2007). Chronic childhood gastrointestinal infections are associated with growth impairments, e.g., the number of diarrhea days experiences by rural Guatemalan children predicted their adult height (Martorell et al. 1975). Inflammatory (host defense) responses to chronic infections are metabolically demanding, while also critical for survival. Infections during development cause energy reallocation at the expense of growth (Finch 2007). In adults, fever associated with severe infections increases resting metabolic rates up to 100% (Waterlow 1984; Lochmiller and Deerenberg 2000). Moreover, fever during respiratory and diarrheal diseases is often accompanied by anorexia that can reduce food intake up to 20% (Martorell et al. 1980; Butte et al. 1989). Although we know less about the energetics of host defense in children, the effects of chronic infections on growth stunting are consistent with the concept of energy reallocation.
Birth Weight And Fetal Growth Retardation
Maternal health is also associated with birth-weight, postnatal growth, and later life health. These well-documented links are represented in the Barker hypothesis, also known as the fetal origins of adult disease (Barker 1995; Finch 2007:241). Birth cohorts showed correlations for cardiovascular disease (CVD) mortality with neonatal mortality (Barker and Osmond 1986) and for systolic blood pressure at age 50 with birthweight (Martyn et al. 1995). These English birth cohorts preceded the availability of antibiotics. Neonatal deaths from bronchitis and pneumonia correlated with later CVD mortality in cohort survivors (r = 0.68; Barker and Osmond 1986). These findings are broadly confirmed. A meta-analysis of 23 prospective studies calculated a 10–20% lower risk of CVD per 1 kg of higher birthweight (Wang et al. 2014). Birthweight is more strongly influenced by environmental factors than genetics (57,613 twins from 16 countries; Yokoyama et al. 2018). Low birth-weights also predict shorter adult height; adult height increased 1.14–4.25 cm per 1 kg increase of birthweight (41,852 twin pairs from 16 countries, CODATwins Project; Jelenkovic et al. 2018).
Twin birth weights reveal further complexity. In developed countries, twin birth weights are typically 2500 g or lower than singleton birth weights of 3000 g (Finch 2007:252). However, twins did not differ from singleton births for adult mortality and CVD (19,986 Danish twins, born 1870–1930; Christensen et al. 2001). The exception of twins to the strong birthweight-CVD relationship for singletons warns that fetal growth retardation does not inevitably cause fetal reprogramming for higher CVD risk.
Although maternal infections have diminished in the developed world, developing countries are incurring increased maternal malaria (50 million pregnancies) and impaired fetal growth (Kalanda et al. 2005; Soma-Pillay and Macdonald 2012). Maternal HIV infections are more prevalent than malaria in some developing countries, with increased risk of low birthweight (Xiao et al. 2015). Even brief infections from influenza can impact fetal health. The 2009 H1N1 pandemic increased risk of small-for-gestational-age births by 60% and lowered birth weight by 45 gm in Kaiser Permanente patients (Hansen et al. 2012; Richards et al. 2013). The exceptionally virulent 1918 influenza pandemic had lifelong impacts. Birth cohorts exposed in midgestation were slightly shorter at World War II enlistment, and ≥ 20% excess CVD deaths after age 60; the most affected cohort was born in the first quarter of 1919 and had 25% excess CVD mortality; rheumatic or hypertensive heart disease mortality was unaffected (Mazumder et al. 2010). This analysis was based on findings from our collaborators that these maternal exposure cohorts had lifetime lower economic productivity and more work disability (Almond and Mazumder 2005). Although total mortality from infections has declined in developed countries, malaria and other chronic infections remain globally important.
Maternal and childhood obesity are new risk factors for later life CVD (Bjerregaard et al. 2019). From 1989 to 2007, maternal BMI greater than 30 Kg/m2 (Heslehurst et al. 2010) in association with childhood obesity (Heslehurst et al. 2019). Maternal smoking also increases risk of childhood obesity by 60% (meta-analysis of 109,838 mother-child pairs; Riedel et al. 2014). Moreover, children have higher BMI in households with adult smokers or that are near major roadways (McConnell et al. 2015). The combined effect of roadway pollution and secondhand smoke was superadditive (synergistic) in this and another study (Kim et al. 2014). The developmental toxicity of air pollution and cigarette smoke is globally documented (Vieira 2015; Finch 2018).
More broadly, the Human Developmental Exposome comprises maternal and childhood environmental factors in three levels (Wild 2012): exogenous macrolevel factors (e.g., air pollution, climate, socioeconomic status); exogenous individual factors (e.g., maternal diet, infection, psychosocial stress, smoking); and endogenous individual factors (biomes of gut, lung, and mouth; gender and disease risk genotypes). Most of these factors also engage inflammatory responses that are part of chronic degenerative diseases of arteries, bones, and brain.
The Inflammatory Exposome Across The Life Span
Inflammatory processes of innate immunity are active in chronic diseases of aging that are the main causes of adult mortality: arterial degeneration, cancer, and dementia. Each disease develops slowly and interactions of inflammatory factors external to the organ. We outline several major factors in the Inflammatory Exposome.
Adipose Tissue
Obesity itself contributes to systemic inflammation, based on two lines of evidence (Johnson et al. 2012; Ellulu et al. 2017). First, fat tissues secrete inflammatory factors directly into the blood. In obese individuals, the venous blood effluent from fat depots is higher than from arterial blood for several acute phase inflammatory proteins including IL-6 and CRP (Calabro et al. 2005; Fontana et al. 2007; Madani et al. 2009). These findings include visceral and subcutaneous fat depots. Second, macrophage cells accumulate around adipocytes during obesity (Johnson et al. 2012). Although these obese subjects lacked clinical indications of infections, low-grade bacterial and viral infections could still contribute elevated cytokine production in adipose-embedded macrophages. With or without infections, we suggest that obesity be recognized as a major proinflammatory factor in the modern exposome that contributes to major diseases of aging, including cancer (Kompella and Vasquez 2019), cardiovascular disease (Packer 2018), and dementia (Finch and Kulminski 2019), among other pathogenic processes of aging (Pérez et al. 2016).
Air Pollution And Cigarettes In Aging
Airborne toxins have global impacts on health throughout the life span, causing 12–20 million excess deaths per year worldwide (Table 7; Li et al. 2019b). The World Health Organization recognizes three main sources of mortality from airborne toxins: ambient air pollution (AAP), household air pollution (HAP), and cigarette smoke (CS; Table 7). AAP is a mixture of aerosols from natural and industrial origins with carbonaceous particles, minerals and salts, gases, and volatile chemicals. AAP from combustion of fossil fuels and biomass is the single largest environmental health risk worldwide (Landrigan et al. 2018). Indoor or indoor air pollution includes ingressing AAP, plus cigarette smoke, and smoke from household (domestic) burning of wood and dung for cooking and heating. Myriad chemicals also enter indoor air from paint, cleaning and household products, and fabrics (Finch 2018; Anonymous 2019; Madruga et al. 2019).
Mortality From Airborne Toxins
AAP from airborne particles and gases have an unstable composition: at most sites, the sources and composition of AAP vary widely during the day and by season. The primary emissions of particles and gases comingle and interact with other anthropogenic and natural sources, such as erosion of vehicular tires, body paint, and brake linings; dust from roads, agricultural fields, and deserts; chemical industries; and cigarette smoke and biomass smokes from brush fires and household cooking (Finch 2018; Forman and Finch 2018).
Three size classes of airborne particulate matter (PM) are defined by their aerodynamic diameter in microns (μ): coarse PM10, fine PM2.5, and ultrafine PM0.1. Each size class includes all of the smaller sizes. Because most coarse PM are removed in the upper airways, the United States Environmental Protection Agency (EPA) has focused on PM2.5, which penetrate deeply into lung alveoli. The EPA standards for PM2.5 aim for an upper limit of 12 μg/m3, averaged over three years.
Until the 1860s, wood was the major fuel source in the U.S. when coal was increasingly available, soon succeeded by petroleum (Figure 6). From 1990 to 2015, the burden of disease due to airborne toxins increased: smoking remained the second leading cause, while ambient air pollution moved up to fifth (Cohen et al. 2017). Cattle also suffer air pollution, with up to 3% increased mortality, 10 μg/m3 of PM10 and ozone (Cox et al. 2016).
Figure 6. Energy Use in the United States Since 1775.

After 1900, wood fuels were progressively replaced by coal, and then to varying extents by natural gas, petroleum, and nuclear energy. United States Energy Information Administration 2011, 2016.
Although associations of mortality with AAP are now widely accepted, it took three decades after the London Smog of 1952 before cardiovascular mortality was definitively linked to air pollution of category PM2.5 by the longitudinal U.S. Six Cities Study (Dockery et al. 1993; Rajagopalan et al. 2018). Local ambient PM2.5 levels are strongly associated with risk of most major conditions of aging, including cardiovascular disease, Alzheimer’s disease, and lung cancer (Table 8; Finch 2018). Cigarette smoke exposure increases these same chronic conditions. Moreover, AAP, cigarette smoke, and infections have superadditive synergies on cardiovascular mortality, lung cancer, and cognitive decline.
TABLE 8.
Ambient air pollution (AAP), cigarette smoke (CS), and diseases of aging
| AAP | CS | Synergy | |
|---|---|---|---|
| Atherosclerosis carotid intimal medial thickness (CIMT) | Aguilera et al. 2016; Liu et al. 2015 | Hansen et al. 2016; Huang et al. 2016 | |
| coronary (CVD mortality) | Hartiala et al. 2016; Kaufman et al. 2016 | Benziger et al. 2016 | 1.1-fold excess Turner et al. 2017 |
| Cancer of the lung | Hamra et al. 2014; Cui et al. 2015 | Doll et al. 2004; Chen et al. 2015 | 2.2-fold excess Turner et al. 2014 |
| Dementia, including Alzheimer’s disease | Cacciottolo et al. 2017; Chen et al. 2017 |
Nunez et al. 2016; Barnes and Yaffee 2011 |
|
| Cognitive decline | Cacciottolo et al. 2017; Zhang et al. 2018 | 1.9-fold excess Ailshire and Crimmins 2014 |
|
| Obesity, BMI children | McConnell et al. 2015 | Kim et al. 2014 |
Gene-Environment Interactions
Increasing Female Advantages
During the great mortality declines of the 19th century, life spans of women began to expand faster than for men, starting from baselines of 1.1 F:M across Europe and North America (Beltrán-Sánchez et al. 2015). By the early 20th century, male mortality was twofold greater than for women. Our cohort analysis showed that, after 1870, mortality of women aged 40 to 80 years declined 70% faster than men, which progressively expanded the gap in life expectancy (Figure 7). Most women aged 40–80 are postreproductive, limiting the mortality contribution from childbirth. Pathogen-driven mortality was gradually replaced by accelerated cardiovascular disease from cigarette smoking that caused 35% of excess male mortality.
Figure 7. Historical Cohort Sex Differences in Mortality for Ages 40–90 Years.

Average of birth cohorts from eight European countries; Beltrán-Sánchez et al. 2015:Supplemental Figure 1. After 1870, mortality rates of women improved faster than men. A) Mortality rate averages. B) Life expectancy at age 40.
Infant mortality also shifted to greater male excess in these same countries, from baselines of 1.1 to greater than 1.25 by 1950 (Drevenstedt et al. 2008). Famine-related infant mortality had male excess (Zarulli et al. 2018). Although adult mortality is strongly influenced by lifestyle factors, infant mortality is dominated by biological factors. At the cell level, sex differences in stress resistance are well defined for neonatal and adult immune cells (Jaillon et al. 2019; Schurz et al. 2019), cardiomyocytes (Ross and Howlett 2012; Murphy et al. 2017), and vascular endothelia cells (Cattaneo et al. 2017). Correspondingly, women are less vulnerable to TB and other infections, and in autoimmune disorders (Jaillon et al. 2019; Schurz et al. 2019). The X chromosome has numerous immune-related genes, which undergo random inactivation for dose compensation in early embryogenesis, according to the Lyon hypothesis (1961). Schurz et al. (2019) suggest that female immune advantages may arise by gene mosaicism during X-inactivation. Expanding on the X-inactivation hypotheses of female immune function, the pregnancy compensation hypothesis suggests that females must be able to downregulate some aspects of immunity during pregnancy in order to facilitate placentation and fetal survival (Natri et al. 2019). Trends for fewer pregnancies in highly developed countries may have exacerbated higher rates of female autoimmune disorders (Natri et al. 2019).
ApoE
The ancestral ApoE4 allele adversely impacts the life span, and arterial and brain aging in populations with low levels of infections (Phase III). However, as discussed above, in several modern populations with high loads of infection and parasites, ApoE4 benefits cognition (Trumble et al. 2017) and survival of children and older adults (Oriá et al. 2007; Mitter et al. 2012; van Exel et al. 2017). ApoE2, the minor allele, lowers Alzheimer’s disease risk and favors longevity relative to the majority ApoE3 allele (Kulminski et al. 2016; Abondio et al. 2019). ApoE alleles may have interacted with gender differences in mortality during the decline of infections, as mortality increased from cardiovascular disease of ApoE4 carriers who smoked (Talmud et al. 2005).
Cigarette-Resistance Genes
Gene variants that increase resistance to cigarette smoke may be relevant to gene network adaptive for cooking and meat-eating. Recognizing that “not all smokers die young,” Levine and Crimmins (2014) identified gene variants (SNPs, GWAS) in cigarette survivors to age 80 in the U.S. National Health and Nutrition Examination Study (NHANES III). Not only did 22% more carriers of this SNP set reach ages 90–99 and 300% more reached age 100, they had 10% less cancer. Moreover, older cigarette survivors have the same mortality rates as same age never-smokers, and also had strong biomarkers for health (lung function, inflammation, blood HDL). The network of 215 genes included stress-resistance and longevity genes: FOXO, a transcription factor; insulin-like growth factors; and PI3K/AKT/mTOR of cell cycle and cancer. Frustratingly, ApoE alleles were not yet available for NHANES III.
Could cigarette-resistant genes also be gene candidates in the Evolutionary Exposome? FOXO3 and mTOR are among the subset of genes shared with cigarette-survivor SNPs that are also associated with longevity in multiple populations (Riera et al. 2016; Bae et al. 2018). In another approach, we examined brain mRNA responses in mice to air pollution nanoscale particles (PM0.2; A. Haghani and C. E. Finch, unpublished). A subset of 25 brain mRNA responses to PM0.2 was shared with cigarette survivors SNPs. Induction of the Siglec gene CMAH in the brain by air pollution parallels its induction in liver by cooked food (Table 3; Carmody et al. 2011). These pilot data anticipate that other longevity genes will increase resistance to airborne toxicants in fossil fuels, cigarettes, and burning biomass.
Epigenetics Of Pollution
Epigenetic impact on DNA and histone methylation from maternal exposures to diet and toxins can persist into adult life and future generations (Li et al. 2019a), illustrated by select examples. Prenatal maternal smoking altered DNA methylation of a small group of genes. Effects persisted from birth into middle age for four genes that included the detoxifying enzymes CYP1A1 and AHRR, discussed in Phase II (1220 adults, prospective study of five birth cohorts; Wiklund et al. 2019). Offspring of mice from mothers fed a fatty diet had increased histone methylation of the adipokine gene and reduced mRNA in adipose tissue; these effects persisted on normal diets into the F1 (next) generation, disappearing by F3 (Masuyama et al. 2015). Conversely, early life exposure to the Chinese Famine of 1959–1961 increased DNA methylation of the insulin-related IF2G gene, observed in adults together with proportionately increased blood cholesterol (Shen et al. 2019). Cognition was slightly impaired in young adults of mothers who experienced this famine (Li et al. 2015).
Lead exposure epigenetic modifications can persist into the F2 generation. Grandchildren of mothers from Detroit with high neonatal blood levels of lead still showed hypermethylation of six genes (Sen et al 2015). These F2 generation effects on DNA methylation support the prezygotic hypothesis for environmental influences: because the egg we came from was present in our mothers’ ovaries before her birth, we were exposed to grand-maternal environmental influences (Finch and Loehlin 1998). Beyond grandchildren, maternal exposure of rats to the fungicide vinclozolin altered DNA methylation and obesity into the F3 generation (Nilsson et al. 2018). Further studies are needed to resolve cost-benefits of epigenetic responses. Toxin-induced changes may be adaptive for detoxification, while metabolic responses may be adaptive for diet uncertainty (Li et al. 2019a,b).
Longevity Genes And GxE
ApoE may be the first example of a longevity gene with documented GxE (gene-environment interactions). Identification of further longevity genes will depend on understanding their GxE interactions, as proposed by Hook et al. (2018), and also discussed for Alzheimer’s disease (Finch and Kulminski 2019). Many longevity genes are shared broadly across short- and long-lived animals (Longo and Finch 2003; Hook et al. 2018; Khan et al. 2019). Curiously, chimpanzee genomes have not been considered for genes that might contribute to the human-evolved postreproductive phase of life span that is unique among hominids (Table 4).
Has the human gene pool changed during these major reductions of infant mortality and increased adult survival? Modern medicine has increased the survival of many with inherited diseases (Crocco et al. 2019; Ewald and Ewald 2019; Prohaska et al. 2019). For malarial sickle cell disease, autopsies showed that nearly one-half of 300 had died of infections (Manci et al. 2003). Effective treatments for sickle cell allele carriers (Maksoud et al. 2018) will help maintain these and other adverse genes. Moreover, the expansion of neonatal intensive care units (NICUs) after 1950 has increased survival of low-birth weight babies (Drevenstedt et al. 2008). Modern medicine, sanitation, and birth control may have relaxed natural selection for these and other special cases, but we lack population-based genetic data to show the impact on reproductive success.
Synthesis Phase V
In the last 200 years, human life expectancy has more than doubled from minimization of mortality from pathogenic infections throughout the life span. For the first time in human demographic history, 90% of births can reach age 70 in some populations with minimal infections. In contrast to these “health elites,” one-half or more of the world still suffers from malaria, HIV, and other infections that shorten life. The environmental impact of infections and malnutrition begins early. Historical cohort analysis shows the persisting impact of early life environmental insults on later life morbidity represented in two hypotheses: the cohort morbidity hypothesis that links early age and later age mortality trends (Finch and Crimmins 2004) and in fetal origins of adult disease that links fetal impairments to later chronic disease (Barker 1995). The Maternal Exposome gradually shifted from infectious pathogens and nutritional deficits to sterile inflammogens from maternal obesity and airborne toxins. The shift also altered GxE interactions, exemplified by the ApoE4 allele, which benefits early and later age survival and cognitive function with a high pathogen load, but has negative impact in healthy populations. The Future Exposome (Phase VI) portends regressive steps backward with global climate change to a worsening exposome with increased infections and airborne toxins.
The Future Human Exposome VI: 21st–22nd Centuries
Plus Climate Warming, Higher Ozone, Crustal Dust, and Insect-Borne Infections
Air pollution will increase into the 21st century as fossil fuel use continues. Fossil fuel production is predicted to increase another 30% through 2040 (Figure 6), with correspondingly increased CO2 emissions. Even with renewable energy, fossil fuels will remain the dominant global energy source beyond 2040 (U.S. Energy Information Administration 2016). Global climate changes will increase infections from redistribution of rainfall and water resources (Table 9).
TABLE 9.
Climate change and health
| 1. Warming and insect-borne diseases | The expansion of insect-borne infections with global warming is described in rigorous models (Wang and Zou 2018), e.g., dengue, yellow fever, and Zika by Aedes aegypti. Malaria is expanding globally (Flahault et al. 2016; Hundessa et al. 2018). The number of mosquito Disease Danger Days has increased in the U.S. since 1970, e.g., by 47 days in San Francisco (Langer et al. 2018). Arctic warming is associated with faster mosquito development (Culler et al. 2015). Lyme disease is expanding across temperate zones with the tick Ixodes scapularis (McPherson et al. 2017; Bouchard et al. 2018). At least one malaria resistance gene has become less effective: Plasmodium vivax has recently evolved a novel red cell invasion mechanism allowing it to infect FY*O gene carriers via other red cell proteins in two African populations (Niang et al. 2018; see Phase IV). |
| 2. Rising seas, mosquito-borne infections | Insect populations are fostered by the expanding coastal brackish waters from rising seas (Ramasamy and Surendran 2016; Diem et al. 2017). In Sri Lanka, mosquito-borne dengue fever was linked to larval density in brackish waters (Ramasamy and Surendran 2012). |
| 3. Dust from deforestation | Ambient dust is associated with surges of infectious diseases. African and Asian dust can carry a high density of viable bacteria (Phase I). In the southwestern U.S., Valley fever is associated with dust storms that increased 2.4-fold since 1990 (Tong et al. 2017), while the African “meningitis belt” is strongly associated with seasonal surges of Saharan dust (Pérez García- Pando et al. 2014; Woringer et al. 2018). Saharan dust is seasonally transported to the Caribbean and southeastern U.S. (Conway et al. 2019). |
| 4. Migrations from drought and heat | Refugee camps are vulnerable to infections, fostered by antibiotic resistance, poor hygiene, malnutrition, and limited medical services (Nellums et al. 2018; Schwartz and Morris 2018). African and Asian refugee camps are rife with cholera (Golicha et al. 2018) and respiratory viruses (Wu et al. 2019). Asylum seekers in Germany had severalfold more antibiotic resistance genes than native controls, including two genes absent from controls (Häsler et al. 2018). |
| 5. Ozone and respiratory disease | Ozone elevations are strongly associated with asthma. In the Central Valley of California, interquartile elevations of ozone increased emergency room visits for asthma in children (OR 1.22) and adults (OR 1.1) by 10% (Gharibi et al. 2018). Ozone levels also surge during heat events, with particular impact to urban inhabitants (Diem et al. 2017). Global warming will inevitably increase ozone because its chemical production responds linearly to temperature (Steiner et al. 2010), e.g., Los Angeles ozone levels varied daily by twofold across the ambient temperatures of 22 to 32°C. |
| 6. Mortality from heat stress | Cities are “urban heat islands” with ambient temperatures above rural areas with more foliage and lower density traffic. Extreme temperatures are predicted to increase mortality from heat stress by up to twentyfold in Africa and the Middle East (Diem et al. 2017; Ahmadalipour and Moradkhani 2018), with lesser but still major increases of mortality predicted for temperate zones (Eisenman et al. 2016; McLean et al. 2018). Air conditioning (AC) needs will increase, with corresponding demand for electrical power from fossil fuels and increasing air pollution. AC for vulnerable populations is already insufficient, but building design and urban planning can partly ameliorate needs for AC (Lundgren-Kownacki et al. 2018). |
In Table 9–1, warming promotes expansion of insect populations, particularly mosquito vectors of malaria and viruses. The expanding tick season is also increasing Lyme disease. In Table 9–2, rising sea levels may synergize with warming for mosquito-borne diseases in coastal zones of brackish waters. For Table 9–3, Decreased rainfall and deforestation are increasing; decreasing water needed for agriculture and human hygiene. In Table 9–4, major human migrations are anticipated, as drought reduces water for farmers and extreme heat limits habitability. Refugees are vulnerable to infections, fostered by poor hygiene, antibiotic resistance, malnutrition, and limited medical services. For Table 9–5, warming is increasing ozone levels that cause lung disease. And, finally, in Table 9–6, higher mortality from heat stress is a concern for children and the elderly. The need for air conditioning will increase demands for electrical power. We anticipate that health disparities will further widen with environmental degradation, between rich economies and their health elites versus rest of the world.
Summary of the Human Exposome: From Dust to Diesel
Throughout hominin evolution, our ancestors encountered and adapted to diverse environmental hazards, many of which left genetic signatures. The evolutionary exposome analysis gives a new conceptualization of ecological exposures, beginning with inhaled natural aerosols of savanna mineral dust (PM10), followed by anthropogenic smaller inhaled smoke particles (PM2.5), and eventually airborne toxins from tobacco and fossil fuels. We hypothesize that adaptations to ancient pathogens and airborne toxins may still be protective for some evolutionarily novel airborne pollutants from fossil fuels and cigarettes, while some genes such as ApoE4 became deleterious as our environments changed faster than our genetics (Figure 8).
Figure 8. Shifts of Mortality From Pathogen-Driven Inflammation to Sterile Inflammogens During Industrialization.

Panel A: Industrialization was associated with shifts from mortality caused by infections and pathogen-driven inflammation to chronic low-grade inflammation from sterile inflammogens. Panel B: Health changes during industrialization shifted endogenous sites of inflammation: premodern sites above the dotted line included chronic infections of the mouth (periodontal disease), lung, and gastrointestinal tract. Modern sites of sterile inflammogens include fat depots and atheromas. Under most conditions of sterile inflammogens, ApoE4 is maladaptive.
The inflammatory responses to airborne toxins from cigarettes and fossil fuels are shared with the pathophysiology of chronic diseases currently associated with air pollution, cigarette smoke, and infection, which cause about 16 million excess deaths per year (range 12–20 million). We hypothesize that genetic adaptations to ancient airborne toxins may play important roles in ameliorating the effects of exposures today, including survival of some elderly lifetime cigarette smokers. This synthesis summarizes known exposures to pathogens, diet, and chemicals in relation to specific gene changes during human evolution, and hypothesizes about potential exposures and hazards that humans will face in the coming century. We anticipate that interactions between infection, indoor and outdoor air pollution, cigarette smoke, and other environmental exposures will result in long-term damage to human health, with additive if not synergistic effects. ApoE4 might regain adaptive value with recurrence of global infections. Other ancient genes that were adaptive to xenotoxins in dust, smoke, and other environmental exposures may still be adaptive for current and future generations. Understanding the full breadth and history of the human exposome will inform the future of human health and longevity during the emerging ecological shifts from dust to diesel and beyond.
Acknowledgments
We thank the reviewers for their detailed comments and suggestions on this extremely long manuscript. Valuable comments were given by Angela Garcia, Arvis Sulovari, Michael Gurven, and Timothy Webster. Troy Palmer developed the excellent graphics. We are grateful for support from the National Institutes of Health (Benjamin C. Trumble: AG054442; Caleb E. Finch: AG051521, AG055367, AG054442), Arizona State University Center for Evolution and Medicine (Benjamin C. Trumble), Cure Alzheimer’s Fund (Caleb E. Finch), and Center for Academic Research and Training in Anthropogeny (Caleb E. Finch).
APPENDIX TABLE 1. Genes in human-specific DNA segment duplications (HSD)
| Brain | Immunity | Other | |
|---|---|---|---|
| Phase IA, Pre-Homo 5.3–4.7 MYA |
HIST2H2BE | CD88, Rock1B, FCGR1B | Cholesterol (HDL) PDZK1B |
| Phase IB, Pre-Homo 3.3–2.5 MYA |
SRGAP, FAM72 (4), HYDIN2 | FCGR1C | |
| Phase II, Homo 2.4–0.6 MYA |
SRGAP2C, GPRIN2B, GRIN2B gluR NPY4RB food intake TCAF2, CHR FAM7A, CHR FAM72 HIST2HD |
FCGRD1 | Vascular (OCLN) |
| Phase III, 0.3 MYA |
NAIP-C, DUSP22B SMN2, SERF1B, TCAF2B |
DUSP22B, NCF1C TCF1B |
Muscle SERF1 |
Data from Dennis et al. 2017, Figure 2. We defined three clusters in the timing of HSD: 5.3–4.7 MYA (seven genes); 3.3–2.3 MYA (12 genes); and 1.9–0.3 MYA (39 genes). These duplicated genes did not show disruptive mutations that would block transcription or translation, but expression by cell type is not fully characterized.
Contributor Information
Benjamin C. Trumble, School of Human Evolution & Social Change and Center for Evolution and Medicine, Arizona State University Tempe, Arizona 85287 USA
Caleb E. Finch, Leonard Davis School of Gerontology and Dornsife College, University of Southern California Los Angeles, California 90089-0191 USA
References
- Aarts JMMJG, Alink GM, Scherjon F, MacDonald K, Smith AC, Nijveen H, Roebroeks W 2016. Fire usage and ancient hominin detoxification genes: protective ancestral variants dominate while additional derived risk variants appear in modern humans. PLOS ONE 11:e0161102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Rached L, Raoult D 2016. Paleogenetics and past infections: the two faces of the coin of human immune evolution. Microbiology Spectrum 4:PoH-0018–2015. [DOI] [PubMed] [Google Scholar]
- Abi-Rached L,. Jobin MJ, Kulkarni S, et al. 2011. The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 334:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abondio P, Sazzini M, Garagnani P, Boattini A, Monti D, Franceschi C, Luiselli D, Giuliani C 2019. The genetic variability of APOE in different human populations and its implications for longevity. Genes 10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M 2016. How old are bacterial pathogens? Proceedings of the Royal Society B: Biological Sciences 283:20160990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson JD, Sapp RW, Brott BK, Lee H, Miyamichi K, Luo L, Cheng S, Djurisic M, Shatz CJ 2016. Developmental sculpting of intracortical circuits by MHC Class I H2-Db and H2-Kb. Cerebral Cortex 26:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi T, Liwenga E, Kwezi L 2014. Rainfall-induced crop failure, food insecurity and out-migration in Same-Kilimanjaro, Tanzania. Climate and Development 6:53–60. [Google Scholar]
- Aguilera I, Dratva J, Caviezel S, Burdet L, de Groot E, Ducret-Stich RE, Eeftens M, Keidel D, Meier R, Perez L, Rothe T, Schaffner E, Schmit-Trucksäss A, Tsai M-Y, Schindler C, Künzli N, Probst-Hensch N 2016. Particulate matter and subclinical atherosclerosis: associations between different particle sizes and sources with carotid intima-media thickness in the SAPALDIA study. Environmental Health Perspectives 124:1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadalipour A, Moradkhani H 2018. Escalating heat-stress mortality risk due to global warming in the Middle East and North Africa (MENA). Environment International 117:215–225. [DOI] [PubMed] [Google Scholar]
- Aiello LC, Wheeler P 1995. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Current Anthropology 36:199–221. [Google Scholar]
- Ailshire JA, Crimmins EM 2014. Fine particulate matter air pollution and cognitive function among older US adults. American Journal of Epidemiology 180:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Rifai M, DeFilippis AP, McEvoy JW, Hall ME, Acien AN, Jones MR, Keith R, Magid HS, Rodriguez CJ, Barr GR, Benjamin EJ, Robertson RM, Bhatnagar A, Blaha MJ 2017. The relationship between smoking intensity and subclinical cardiovascular injury: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 258:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisson-Silva F, Kawanishi K, Varki A 2016. Human risk of diseases associated with red meat intake: analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Molecular Aspects of Medicine 51:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RW 1947. The thermal death point of cysticerci of Taenia saginata. Journal of Parasitology 33:331–338. [PubMed] [Google Scholar]
- Almond D, Mazumder B 2005. The 1918 influenza pandemic and subsequent health outcomes: an analysis of SIPP data. American Economic Review 95:258–262. [DOI] [PubMed] [Google Scholar]
- Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A 2002. Cloning and characterization of human Siglec-11: a recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. Journal of Biological Chemistry 277:24466–24474. [DOI] [PubMed] [Google Scholar]
- Anonymous. 2019. Regulators must work on indoor air pollution. Lancet 394:94. [DOI] [PubMed] [Google Scholar]
- Arama C, Quin JE, Kouriba B, Östlund Farrants A-K, Troye-Blomberg M, Doumbo OK 2018. Epigenetics and malaria susceptibility/protection: a missing piece of the puzzle. Frontiers in Immunology 9:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante J, Noreddin A, El Zowalaty ME 2019. Systematic review of important bacterial zoonoses in Africa in the last decade in light of the “One Health” concept. Pathogens 8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad S, Finch CE 2014. Primate models for human brain aging and neurological diseases. Annual Review of Gerontology and Geriatrics 34:139–170. [Google Scholar]
- Azevedo OGR, Bolick DT, Roche JK, Pinkerton RF, Lima AAM, Vitek MP, Warren CA, Oriá RB, Guerrant RL 2014. Apolipoprotein E plays a key role against cryptosporidial infection in transgenic undernourished mice. PLOS ONE 9: e89562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backström T, Winberg S 2017. Serotonin coordinates responses to social stress—what we can learn from fish. Frontiers in Neuroscience 11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H, Gurinovich A, Malovini A, Atzmon G, Andersen SL, Villa F, Barzilai N, Puca A, Perls TT, Sebastiani P 2018. Effects of FOXO3 polymorphisms on survival to extreme longevity in four centenarian studies. Journals of Gerontology A: Biological Science and Medical Science 73:1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP 1995. The Wellcome Foundation Lecture, 1994: the fetal origins of adult disease. Proceedings of the Royal Society B: Biological Sciences 262: 19950173. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Osmond C 1986. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 327:1077–1081. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K 2011. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurology 10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquet N, Domingo P 1997. Smallpox: the triumph over the most terrible of the ministers of death. Annals of Internal Medicine 127:635–642. [DOI] [PubMed] [Google Scholar]
- Barrès R, Zierath JR 2016. The role of diet and exercise in the transgenerational epigenetic landscape of T2DM. Nature Reviews Endocrinology 12:441–451. [DOI] [PubMed] [Google Scholar]
- Bastir M, García-Martínez D, Williams SA, Recheis W, Torres-Sánchez I, García Río F, Oishi M, Ogihara N 2017. 3D geometric morphometrics of thorax variation and allometry in Hominoidea. Journal of Human Evolution 113:10–23. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt S-E, Julius D 2007. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208. [DOI] [PubMed] [Google Scholar]
- Beamer GL, Seaver BP, Jessop F, Shepherd DM, Beamer CA 2016. Acute exposure to crystalline silica reduces macrophage activation in response to bacterial lipoproteins. Frontiers in Immunology 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Davis DL, Fletcher T 2004. A retrospective assessment of mortality from the London smog episode of 1952: the role of influenza and pollution. Environmental Health Perspectives 112:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo RV 1994. Methods of determining early hominid behavioral activities associated with the controlled use of fire at FxJj 20 Main, Koobi Fora, Kenya. Journal of Human Evolution 27:173–195. [Google Scholar]
- Beltrán-Sánchez H, Crimmins EM, Finch CE 2012. Early cohort mortality predicts the rate of aging in the cohort: a historical analysis. Journal of Developmental Origins of Health and Disease 3:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Sánchez H, Finch CE, Crimmins EM 2015. Twentieth century surge of excess adult male mortality. Proceedings of the National Academy of Sciences of the United States of America 112:8993–8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FJ, Kagan IG, Barnicot NA, Woodburn JC 1970. Helminth and protozoal parasites of the Hadza of Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene 64:857–880. [DOI] [PubMed] [Google Scholar]
- Bentley GR, Goldberg T, Jasieńska G 1993. The fertility of agricultural and non-agricultural traditional societies. Population Studies 47:269–281. [Google Scholar]
- Bentley GR, Paine RR, Boldsen JL 2001. Fertility changes with the prehistoric transition to agriculture: perspectives from reproductive ecology and paleodemography Pages 203–232 in Reproductive Ecology and Human Evolution, edited by Ellison PT. New York: Aldine de Gruyter. [Google Scholar]
- Benziger CP, Roth GA, Moran AE 2016. The global burden of disease study and the preventable burden of NCD. Global Heart 11:393–397. [DOI] [PubMed] [Google Scholar]
- Best A, Kamilar JM 2018. The evolution of eccrine sweat glands in human and nonhuman primates. Journal of Human Evolution 117:33–43. [DOI] [PubMed] [Google Scholar]
- Bjerregaard LG, Adelborg K, Baker JL 2019. Change in body mass index from childhood onwards and risk of adult cardiovascular disease. Trends in Cardiovascular Medicine 10.1016/j.tcm.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Gurven MD, Sugiyama LS, Madimenos FC, Liebert MA, Martin MA, Kaplan HS, Snodgrass JJ 2011. Evidence for a peak shift in a humoral response to helminths: age profiles of IgE in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLOS Neglected Tropical Diseases 5:e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Martin M, Kaplan H, Gurven M 2013. Antagonism between two intestinal parasites in humans: the importance of co-infection for infection risk and recovery dynamics. Proceedings of the Royal Society B: Biological Sciences 280:20131671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, Martin M, Kaplan H, Gurven M 2015. Helminth infection, fecundity, and age of first pregnancy in women. Science 350: 970–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Trumble BC, Maldonado Suarez I, Stieglitz J, Beheim B, Snodgrass JJ, Kaplan H, Gurven M 2016. Immune function in Amazonian horticulturalists. Annals of Human Biology 43:382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Urlacher SS, Beheim B, von Rueden C, Jaeggi A, Stieglitz J, Trumble BC, Gurven M, Kaplan H 2017. Growth references for Tsimane forager-horticulturalists of the Bolivian Amazon. American Journal of Physical Anthropology 162:441–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe R, Behrensmeyer AK 2004. The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeography, Palaeoclimatology, Palaeoecology 207: 399–420. [Google Scholar]
- Boesch C, Boesch-Achermann H 2000. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. Oxford (United Kingdom): Oxford University Press. [Google Scholar]
- Bonnefille R 2010. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global and Planetary Change 72:390–411. [Google Scholar]
- Bouchard C, Aenishaenslin C, Rees EE, Koffi JK, Pelcat Y, Ripoche M, Milord F, Lindsay LR, Ogden NH, Leighton PA 2018. Integrated social-behavioral and ecological risk maps to prioritize local public health responses to Lyme disease. Environmental Health Perspectives 126:047008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish DME, Sakamoto K, Lack NA, Hill PC, Sirugo G, Newport MJ, Gordon S, Hill AVS, Vannberg FO 2013. Genetic variants of MARCO are associated with susceptibility to pulmonary tuberculosis in a Gambian population. BMC Medical Genetics 14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble DM, Lieberman DE 2004. Endurance running and the evolution of Homo. Nature 432:345–352. [DOI] [PubMed] [Google Scholar]
- Brazier AJ, Avril M, Bernabeu M, Benjamin M, Smith JD 2017. Pathogenicity determinants of the human malaria parasite Plasmodium falciparum have ancient origins. mSphere 2:e00348–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe P, Grossi CM 2009. Millennium-long damage to building materials in London. Science of the Total Environment 407:1354–1361. [DOI] [PubMed] [Google Scholar]
- Bronikowski AM, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A, Stoinski T, Morris WF, Strier KB, Alberts SC 2011. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 331:1325–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronikowski AM, Cords M, Alberts SC, Altmann J, Brockman DK, Fedigan LM, Pusey A, Stoinski T, Strier KB, Morris WF 2016. Female and male life tables for seven wild primate species. Scientific Data 3:160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet M, Guy F, Pilbeam D, et al. 2002. A new hominid from the Upper Miocene of Chad, Central Africa. Nature 418:145–151. [DOI] [PubMed] [Google Scholar]
- Burger J, Kirchner M, Bramanti B, Haak W, Thomas MG 2007. Absence of the lactase-persistence-associated allele in early Neolithic Europeans. Proceedings of the National Academy of Sciences of the United States of America 104:3736–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett R, Chen H, Szyszkowicz M, et al. 2018. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proceedings of the National Academy of Sciences of the United States of America 115:9592–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte NF, Wong WW, Garza C 1989. Energy cost of growth during infancy. Proceedings of the Nutrition Society 48:303–312. [DOI] [PubMed] [Google Scholar]
- Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, Serre ML, Vizuete W, Sioutas C, Morgan TE, Gatz M, Chui HC, Shumaker SA, Resnick SM, Espeland MA, Finch CE, Chen J-C 2017. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Translational Psychiatry 7:e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro P, Chang DW, Willerson JT, Yeh ETH 2005. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. Journal of the American College of Cardiology 46:1112–1113. [DOI] [PubMed] [Google Scholar]
- Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J, Barrow AD 2008. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. European Journal of Immunology 38:2303–2315. [DOI] [PubMed] [Google Scholar]
- Carmody RN, Wrangham RW 2009. The energetic significance of cooking. Journal of Human Evolution 57:379–391. [DOI] [PubMed] [Google Scholar]
- Carmody RN, Weintraub GS, Wrangham RW 2011. Energetic consequences of thermal and nonthermal food processing. Proceedings of the National Academy of Sciences of the United States of America 108: 19199–19203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier DR, Kapoor AK, Kimura T, Nickels MK, Satwanti, Scott EC, So JK, Trinkaus E 1984. The energetic paradox of human running and hominid evolution [and comments and reply]. Current Anthropology 25:483–495. [Google Scholar]
- Carrión JS, Scott L 1999. The challenge of pollen analysis in palaeoenvironmental studies of hominid beds: the record from Sterkfontein caves. Journal of Human Evolution 36:401–408. [DOI] [PubMed] [Google Scholar]
- Carvalho-Wells AL, Jackson KG, Gill R, Olano-Martin E, Lovegrove JA, Williams CM, Minihane AM 2010. Interactions between age and apoE genotype on fasting and postprandial triglycerides levels. Atherosclerosis 212:481–487. [DOI] [PubMed] [Google Scholar]
- Caslake MJ, Miles EA, Kofler BM, Lietz G, Curtis P, Armah CK, Kimber AC, Grew JP, Farrell L, Stannard J, Napper FL, Sala-Vila A, West AL, Mathers JC, Packard C, Williams CM, Calder PC, Minihane AM 2008. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. American Journal of Clinical Nutrition 88:618–629. [DOI] [PubMed] [Google Scholar]
- Cattaneo MG, Vanetti C, Decimo I, Di Chio M, Martano G, Garrone G, Bifari F, Vicentini LM 2017. Sex-specific eNOS activity and function in human endothelial cells. Scientific Reports 7:9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerling TE, Wynn JG, Andanje SA, Bird MI, Korir DK, Levin NE, Mace W, Macharia AN, Quade J, Remien CH 2011. Woody cover and hominin environments in the past 6 million years. Nature 476:51–56. [DOI] [PubMed] [Google Scholar]
- Cervenáková L, Brown P, Goldfarb LG, Nagle J, Pettrone K, Rubenstein R, Dubnick M, Gibbs CJ Jr., Gajdusek DC 1994. Infectious amyloid precursor gene sequences in primates used for experimental transmission of human spongiform encephalopathy. Proceedings of the National Academy of Sciences of the United States of America 91:12159–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LK 2014. The thoracic shape of hominoids. Anatomy Research International 2014:324850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, Hystad P, Martin RV, Murray BJ, Jessiman B, Wilton AS, Kopp A, Burnett RT 2017. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 389:718–726. [DOI] [PubMed] [Google Scholar]
- Chen S, Wu P, Zhou L, Shen Y, Li Y, Song H 2015. Relationship between increase of serum homocysteine caused by smoking and oxidative damage in elderly patients with cardiovascular disease. International Journal of Clinical Experimental Medicine 8:4446–4454. [PMC free article] [PubMed] [Google Scholar]
- Chirchir H, Kivell TL, Ruff CB, Hublin J-J, Carlson KJ, Zipfel B, Richmond BG 2015. Recent origin of low trabecular bone density in modern humans. Proceedings of the National Academy of Sciences of the United States of America 112:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm RH, Trauer JM, Curnoe D, Tanaka MM 2016. Controlled fire use in early humans might have triggered the evolutionary emergence of tuberculosis. Proceedings of the National Academy of Sciences of the United States of America 113:9051–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Wienke A, Skytthe A, Holm NV, Vaupel JW, Yashin AI 2001. Cardiovascular mortality in twins and the fetal origins hypothesis. Twin Research 4:344–349. [DOI] [PubMed] [Google Scholar]
- Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP 2019. Gut reactions: breaking down xenobiotic-microbiome interactions. Pharmacological Reviews 71:198–224. [DOI] [PubMed] [Google Scholar]
- Claw KG, Tito RY, Stone AC, Verrelli BC 2010. Haplotype structure and divergence at human and chimpanzee serotonin transporter and receptor genes: implications for behavioral disorder association analyses. Molecular Biology and Evolution 27:1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, et al. 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway TM, Hamilton DS, Shelley RU, Aguilar-Islas AM, Landing WM, Mahowald NM, John SG 2019. Tracing and constraining anthropogenic aerosol iron fluxes to the north Atlantic Ocean using iron isotopes. Nature Communications 10:2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo RM, Scacchi R 1999. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a “thrifty” allele? Annals of Human Genetics 63:301–310. [DOI] [PubMed] [Google Scholar]
- Cordain L 1999. Cereal grains: humanity’s double-edged sword Pages 19–73 in Evolutionary Aspects of Nutrition and Health: Diet, Exercise, Genetics, and Chronic Disease, Volume 84, edited by Simopoulos AP. Basel (Switzerland): Karger Publishers. [DOI] [PubMed] [Google Scholar]
- Cordain L, Miller JB, Eaton SB, Mann N, Holt SHA, Speth JD 2000. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. American Journal of Clinical Nutrition 71:682–692. [DOI] [PubMed] [Google Scholar]
- Cordain L, Watkins BA, Florant GL, Kelher M, Rogers L, Li Y 2002. Fatty acid analysis of wild ruminant tissues: evolutionary implications for reducing diet-related chronic disease. European Journal of Clinical Nutrition 56:181–191. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J 2005. Origins and evolution of the Western diet: health implications for the 21st century. American Journal of Clinical Nutrition 81:341–354. [DOI] [PubMed] [Google Scholar]
- Cox B, Gasparrini A, Catry B, Fierens F, Vangronsveld J, Nawrot TS 2016. Ambient air pollution-related mortality in dairy cattle: does it corroborate human findings? Epidemiology 27:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Finch CE 2006. Infection, inflammation, height, and longevity. Proceedings of the National Academy of Sciences of the United States of America 103:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden AN 2011. The importance of honey consumption in human evolution. Food and Foodways 19:257–273. [Google Scholar]
- Crocco P, Montesanto A, Dato S, Geracitano S, Ian-none F, Passarino G, Rose G 2019. Inter-individual variability in xenobiotic-metabolizing enzymes: implications for human aging and longevity. Genes 10:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui P, Huang Y, Han J, Song F, Chen K 2015. Ambient particulate matter and lung cancer incidence and mortality: a meta-analysis of prospective studies. European Journal of Public Health 25:324–329. [DOI] [PubMed] [Google Scholar]
- Culler LE, Ayres MP, Virginia RA 2015. In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proceedings of the Royal Society B: Biological Sciences 282:20151549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler D, Miller G 2005. The role of public health improvements in health advances: the twentieth-century United States. Demography 42:1–22. [DOI] [PubMed] [Google Scholar]
- Dannemann M, Andrés AM, Kelso J 2016. Introgression of Neandertal- and Denisovan-like haplotypes contributes to adaptive variation in human toll-like receptors. American Journal of Human Genetics 98:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C 1890. The Descent of Man, and Selection in Relation to Sex. Reprinted from the Second English Edition, Revised and Augmented. New York: A. L. Burt. [Google Scholar]
- Davidson G, Chua TH, Cook A, Speldewinde P, Weinstein P 2019. The role of ecological linkage mechanisms in Plasmodium knowlesi transmission and spread. EcoHealth 10.1007/s10393-019-01395-6. [DOI] [PubMed] [Google Scholar]
- Deere JR, Parsons MB, Lonsdorf EV, Lipende I, Kamenya S, Collins DA, Travis DA, Gillespie TR 2019. Entamoeba histolytica infection in humans, chimpanzees and baboons in the greater Gombe ecosystem, Tanzania. Parasitology 146:1116–1122. [DOI] [PubMed] [Google Scholar]
- de Heinzelin J, Clark JD, White T, Hart W, Renne P, WoldeGabriel G, Beyene Y, Vrba E 1999. Environment and behavior of 2.5-million-year-old Bouri hominids. Science 284:625–629. [DOI] [PubMed] [Google Scholar]
- Delgado-Andrade C, Fogliano V 2018. Dietary advanced glycosylation end-products (dAGEs) and melanoidins formed through the Maillard reaction: physiological consequences of their intake. Annual Review of Food Science and Technology 9:271–291. [DOI] [PubMed] [Google Scholar]
- Delile H, Blichert-Toft J, Goiran J-P, Keay S, Albarède F 2014. Lead in ancient Rome’s city waters. Proceedings of the National Academy of Sciences of the United States of America 111:6594–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delile H, Keenan-Jones D, Blichert-Toft J, Goiran J-P, Arnaud-Godet F, Albarède F 2017. Rome’s urban history inferred from Pb-contaminated waters trapped in its ancient harbor basins. Proceedings of the National Academy of Sciences of the United States of America 114: 10059–10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deMenocal PB 1995. Plio-Pleistocene African climate. Science 270:53–59. [DOI] [PubMed] [Google Scholar]
- Denham T 2011. Early agriculture and plant domestication in New Guinea and Island Southeast Asia. Current Anthropology 52:S379–S395. [Google Scholar]
- Dennis MY, Harshman L, Nelson BJ, et al. 2017. The evolution and population diversity of human-specific segmental duplications. Nature Ecology and Evolution 1:0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem JE, Stauber CE, Rothenberg R 2017. Heat in the southeastern United States: characteristics, trends, and potential health impact. PLOS ONE 12:e0177937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AP, Carper ER 1996. Infectious diseases and human population history: throughout history the establishment of disease has been a side effect of the growth of civilization. BioScience 46:115–126. [Google Scholar]
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr., Speizer FE 1993. An association between air pollution and mortality in six U.S. cities. New England Journal of Medicine 329:1753–1759. [DOI] [PubMed] [Google Scholar]
- Doerr SH, Santín C 2016. Global trends in wildfire and its impacts: perceptions versus realities in a changing world. Philosophical Transactions of the Royal Society B: Biological Sciences 371:20150345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgova O, Lao O 2018. Evolutionary and medical consequences of archaic introgression into modern human genomes. Genes 9:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I 2004. Mortality in relation to smoking: 50 years’ observations on male British doctors. British Medical Journal 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Rodrigo M, Lopez-Saez JA, Vincens A, Alcala L, Luque L, Serrallonga J 2001. Fossil pollen from the Upper Humbu Formation of Peninj (Tanzania): hominid adaptation to a dry open Plio-Pleistocene savanna environment. Journal of Human Evolution 40:151–157. [DOI] [PubMed] [Google Scholar]
- Domínguez-Rodrigo M, Pickering TR, Bunn HT 2010. Configurational approach to identifying the earliest hominin butchers. Proceedings of the National Academy of Sciences of the United States of America 107: 20929–20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenos F, Kirkwood TBL 2010. Selection on alleles affecting human longevity and late-life disease: the example of apolipoprotein E. PLOS ONE 5:e10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevenstedt GL, Crimmins EM, Vasunilashorn S, Finch CE 2008. The rise and fall of excess male infant mortality. Proceedings of the National Academy of Sciences of the United States of America 105:5016–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Rehm CD 2014. Consumption of added sugars among US children and adults by food purchase location and food source. American Journal of Clinical Nutrition 100:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont LM, Wyputta U 2003. Reconstructing pathways of aeolian pollen transport to the marine sediments along the coastline of SW Africa. Quaternary Science Reviews 22:157–174. [Google Scholar]
- Ebbert MA, McGrew WC, Marchant LF 2015. Differences between chimpanzee and baboon gastrointestinal parasite communities. Parasitology 142: 958–967. [DOI] [PubMed] [Google Scholar]
- Eisenman DP, Wilhalme H, Tseng C-H, Chester M, English P, Pincetl S, Fraser A, Vangala S, Dhaliwal SK 2016. Heat death associations with the built environment, social vulnerability and their interactions with rising temperature. Health & Place 41:89–99. [DOI] [PubMed] [Google Scholar]
- Elipot Y, Hinaux H, Callebert J, Rétaux S 2013. Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network. Current Biology 23:1–10. [DOI] [PubMed] [Google Scholar]
- Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y 2017. Obesity and inflammation: the linking mechanism and the complications. Archives of Medical Science 13:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TL, Chapman CA, Wrangham RW 2018. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Royal Society Open Science 5:180840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard D, Petrov DA 2018. Evidence that RNA viruses drove adaptive introgression between Neanderthals and modern humans. Cell 175:360–371.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enattah NS, Trudeau A, Pimenoff V, et al. 2007. Evidence of still-ongoing convergence evolution of the lactase persistence T−13910 alleles in humans. American Journal of Human Genetics 81:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger M, Baar C, Linz B, Raddatz G, Lanz C, Keller H, Morelli G, Gressmann H, Achtman M, Schuster SC 2006. Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLOS Genetics 2:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evelyn J 1661. Fumifugium, Or the Inconvenience of the Aer and Smoak of London Dissipated Together with Some Remedies Humbly Proposed. London (United Kingdom): W. Godbid. [Google Scholar]
- Ewald PW, Ewald HAS 2019. Genetics and epigenetics Pages 77–130 in Oxford Handbook of Evolutionary Medicine, edited by Brüne M and Schiefenhövel W. Oxford (United Kingdom): Oxford University Press. [Google Scholar]
- Feakins SJ, deMenocal PB, Eglinton TI 2005. Bio-marker records of late Neogene changes in northeast African vegetation. Geology 33:977–980. [Google Scholar]
- Fernández-Borges N, Di Bari MA, Eraña H, Sánchez-Martín M, Pirisinu L, Parra B, Elezgarai SR, Vanni I, López-Moreno R, Vaccari G, Venegas V, Charco JM, Gil D, Harrathi C, D’Agostino C, Agrimi U, Mayoral T, Requena JR, Nonno R, Castilla J 2018. Cofactors influence the biological properties of infectious recombinant prions. Acta Neuropathologica 135:179–199. [DOI] [PubMed] [Google Scholar]
- Ferring R, Oms O, Agustí J, Berna F, Nioradze M, Shelia T, Tappen M, Vekua A, Zhvania D, Lordkipanidze D 2011. Earliest human occupations at Dmanisi (Georgian Caucasus) dated to 1.85–1.78 Ma. Proceedings of the National Acadamy of Sciences of the United States of America 108:10432–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE 2007. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Burlington (Massachusetts): Academic Press. [Google Scholar]
- Finch CE 2009. The neurobiology of middle-age has arrived. Neurobiology of Aging 30:515–520; discussion, 530–533. [DOI] [PubMed] [Google Scholar]
- Finch CE 2010. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proceedings of the National Academy of Sciences of the United States of America 107(Supplement 1):1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE 2012. Evolution of the human lifespan, past, present, and future: phases in the evolution of human life expectancy in relation to the inflammatory load. Proceedings of the American Philosophical Society 156:9–44. [PubMed] [Google Scholar]
- Finch CE 2018. The Role of Global Air Pollution in Aging and Disease: Reading Smoke Signals. London (United Kingdom): Elsevier. [Google Scholar]
- Finch CE, Crimmins EM 2004. Inflammatory exposure and historical changes in human life-spans. Science 305:1736–1739. [DOI] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM 2005. Response to comment on “Inflammatory exposure and historical changes in human life-spans.” Science 308:1743. [DOI] [PubMed] [Google Scholar]
- Finch CE, Kulminski AM 2019. The Alzheimer’s disease exposome. Alzheimer’s & Dementia 15:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Loehlin JC 1998. Environmental influences that may precede fertilization: a first examination of the prezygotic hypothesis from maternal age influences on twins. Behavioral Genetics 28:101–106. [DOI] [PubMed] [Google Scholar]
- Finch CE, Stanford CB 2004. Meat-adaptive genes and the evolution of slower aging in humans. Quarterly Review of Biology 79:3–50. [DOI] [PubMed] [Google Scholar]
- Flahault A, de Castaneda RR, Bolon I 2016. Climate change and infectious diseases. Public Health Reviews 37:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RW, Costa DL 1997. A theory of technophysio evolution, with some implications for forecasting population, health care costs, and pension costs. Demography 34:49–66. [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S 2007. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56:1010–1013. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Finch CE 2018. A critical review of assays for hazardous components of air pollution. Free Radical Biology and Medicine 117:202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka H, Phelix CF, Friedland RP, Zhu X, Perry EA, Castellani RJ, Perry G 2013. Apolipoprotein E4 prevents growth of malaria at the intraerythrocyte stage: implications for differences in racial susceptibility to Alzheimer’s disease. Journal of Health Care for the Poor and Underserved 24(Supplement):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller GW, Font A 2019. Keeping air pollution policies on track. Science 365:322–323. [DOI] [PubMed] [Google Scholar]
- Fullerton DG, Bruce N, Gordon SB 2008. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Transactions of the Royal Society of Tropical Medicine and Hygiene 102:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengård JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF 2000. Apolipoprotein E variation at the sequence haplotype level:implications for the origin and maintenance of a major human polymorphism. American Journal of Human Genetics 67:881–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage TB, DeWitte S 2009. What do we know about the agricultural demographic transition? Current Anthropology 50:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani AP, Novembre J 2005. The evolutionary history of the CCR5-Δ32 HIV-resistance mutation. Microbes and Infection 7:302–309. [DOI] [PubMed] [Google Scholar]
- Galvani AP, Slatkin M 2003. Evaluating plague and smallpox as historical selective pressures for the CCR5-Δ32 HIV-resistance allele. Proceedings of the National Academy of Sciences of the United States of America 100:15276–15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS 2019. New trend in oldage mortality: gompertzialization of mortality trajectory. Gerontology 65:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Tobacco Collaborators. 2017. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389:1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier MC, Minick DJ, Truong L, Tilton S, Pande P, Anderson KA, Teeguardan J, Tanguay RL 2018. Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicology and Applied Pharmacology 354: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard HC, Wang GF, Balin BJ, Schumacher HR, Hudson AP 1999. Frequency of apolipoprotein E (APOE) allele types in patients with Chlamydia-associated arthritis and other arthritides. Microbial Pathogenesis 26:35–43. [DOI] [PubMed] [Google Scholar]
- Gérard HC, Fomicheva E, Whittum-Hudson JA, Hudson AP 2008. Apolipoprotein E4 enhances attachment of Chlamydophila (Chlamydia) pneumoniae elementary bodies to host cells. Microbial Pathogenesis 44:279–285. [DOI] [PubMed] [Google Scholar]
- Gerdes LU 2003. The common polymorphism of apolipoprotein E: geographical aspects and new pathophysiological relations. Clinical Chemistry and Laboratory Medicine 41:628–631. [DOI] [PubMed] [Google Scholar]
- Germonpré M, Sablin MV, Stevens RE, Hedges REM, Hofreiter M, Stiller M, Després VR 2009. Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: osteometry, ancient DNA and stable isotopes. Journal of Archaeological Science 36:473–490. [Google Scholar]
- Gharibi H, Entwistle MR, Ha S, Gonzalez M, Brown P, Schweizer D, Cisneros R 2018. Ozone pollution and asthma emergency department visits in the Central Valley, California, USA, during June to September of 2015: a time-stratified case-crossover analysis. Journal of Asthma 56:1037–1048. [DOI] [PubMed] [Google Scholar]
- Gilby IC 2006. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Animal Behaviour 71:953–963. [Google Scholar]
- Gilby IC, Wawrzyniak D 2018. Meat eating by wild chimpanzees (Pan troglodytes schweinfurthii): effects of prey age on carcass consumption sequence. International Journal of Primatology 39:127–140. [Google Scholar]
- Gkika D, Lemonnier L, Shapovalov G, Gordienko D, Poux C, Bernardini M, Bokhobza A, Bidaux G, Degerny C, Verreman K, Guarmit B, Benahmed M, de Launoit Y, Bindels RJM, Fiorio Pla A, Prevarskaya N 2015. TRP channel-associated factors are a novel protein family that regulates TRPM8 trafficking and activity. Journal of Cell Biology 208:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golicha Q, Shetty S, Nasiblov O, et al. 2018. Cholera outbreak in Dadaab refugee camp, Keny—November 2015–June 2016. Morbidity and Mortality Weekly Report (MMWR) 67:958–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J 1986. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge (Massachusetts): Belknap Press of Harvard University Press. [Google Scholar]
- Goren-Inbar N, Alperson N, Kislev ME, Simchoni O, Melamed Y, Ben-Nun A, Werker E 2004. Evidence of hominin control of fire at Gesher Benot Yaàqov, Israel. Science 304:725–727. [DOI] [PubMed] [Google Scholar]
- Gowlett JAJ, Wrangham RW 2013. Earliest fire in Africa: towards the convergence of archaeological evidence and the cooking hypothesis. Azania: Archaeological Research in Africa 48:5–30. [Google Scholar]
- Guatelli-Steinberg D, Buzhilova AP, Trinkaus E 2013. Developmental stress and survival among the mid Upper Paleolithic Sunghir children: dental enamel hypoplasias of Sunghir 2 and 3. International Journal of Osteoarchaeology 23:421–431. [Google Scholar]
- Guerini FR, Bolognesi E, Chiappedi M, Manca S, Ghezzo A, Agliardi C, Zanette M, Littera R, Carcassi C, Sotgiu S, Clerici M 2014. Activating KIR molecules and their cognate ligands prevail in children with a diagnosis of ASD and in their mothers. Brain, Behavior, and Immunology 36:54–60. [DOI] [PubMed] [Google Scholar]
- Guillien A, Soumagne T, Dalphin J-C, Degano B 2019. COPD, airflow limitation and chronic bronchitis in farmers: a systematic review and meta-analysis. Occupational and Environmental Medicine 76:58–68. [DOI] [PubMed] [Google Scholar]
- Gurven MD, Davison RJ 2019. Periodic catastrophes over human evolutionary history are necessary to explain the forager population paradox. Proceedings of the National Academy of Sciences of the United States of America 116:12758–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Gomes CM 2017. Mortality, senescence, and life span Pages 181–216 in Chimpanzees and Human Evolution, edited by Muller MN et al. Cambridge (Massachusetts): Belknap Press of Harvard University Press. [Google Scholar]
- Gurven MD, Kaplan H 2007. Longevity among hunter-gatherers: a cross-cultural examination. Population and Development Review 33:321–365. [Google Scholar]
- Gurven MD, Kaplan H, Supa AZ 2007. Mortality experience of Tsimane Amerindians of Bolivia: regional variation and temporal trends. American Journal of Human Biology 19:376–398. [DOI] [PubMed] [Google Scholar]
- Gurven MD, Jaeggi AV, Kaplan H, Cummings D 2013. Physical activity and modernization among Bolivian Amerindians. PLOS ONE 8:e55679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Costa M, Trumble B, Stieglitz J, Beheim B, Eid Rodriguez D, Hooper PL, Kaplan H 2016a. Health costs of reproduction are minimal despite high fertility, mortality and subsistence lifestyle. Scientific Reports 6:30056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Trumble BC, Stieglitz J, Blackwell AD, Michalik DE, Finch CE, Kaplan HS 2016b. Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths? Evolution, Medicine, and Public Health 2016: 338–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Stieglitz J, Trumble B, Blackwell AD, Beheim B, Davis H, Hooper P, Kaplan H 2017. The Tsimane Health and Life History Project: integrating anthropology and biomedicine. Evolutionary Anthropology: Issues, News, and Reviews 26:54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györffy BA, Kun J, Török G, Bulyáki É, Borhegyi Z, Gulyássy P, Kis V, Szocsics P, Micsonai A, Matkó J, Drahos L, Juhász G, Kékesi KA, Kardos J 2018. Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proceedings of the National Academy of Sciences of the United States of America 115:6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Merson RR 2017. Diversity as opportunity: insights from 600 million years of AHR evolution. Current Opinion in Toxicology 2:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile-Selassie Y, Latimer BM, Alene M, Deino AL, Gibert L, Melillo SM, Saylor BZ, Scott GR, Lovejoy CO 2010. An early Australopithecus afarensis postcranium from Woranso-Mille, Ethiopia. Proceedings of the National Academy of Sciences of the United States of America 107:12121–12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJP 2001. The thrifty phenotype hypothesis: type 2 diabetes. British Medical Bulletin 60:5–20. [DOI] [PubMed] [Google Scholar]
- Hamilton RF Jr., Thakur SA, Mayfair JK, Holian A 2006. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. Journal of Biological Chemistry 281:34218–34226. [DOI] [PubMed] [Google Scholar]
- Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P, Yorifuji T, Loomis D 2014. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environmental Health Perspectives 122:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CS, Rubinsztein DC 1995. Arginine residues at codons 112 and 158 in the apolipoprotein E gene correspond to the ancestral state in humans. Atherosclerosis 112:85–90. [DOI] [PubMed] [Google Scholar]
- Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF 2000. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Research 60:3440–3444. [PubMed] [Google Scholar]
- Hansen C, Desai S, Bredfeldt C, Cheetham C, Gallagher M, Li D-K, Raebel MA, Riedlinger K, Shay DK, Thompson M, Davis RL 2012. A large, population-based study of 2009 pandemic influenza A virus subtype H1N1 infection diagnosis during pregnancy and outcomes for mothers and neonates. Journal of Infectious Diseases 206:1260–1268. [DOI] [PubMed] [Google Scholar]
- Hansen K, Östling G, Persson M, Nilsson PM, Me-lander O, Engström G, Hedblad B, Rosvall M 2016. The effect of smoking on carotid intima-media thickness progression rate and rate of lumen diameter reduction. European Journal of Internal Medicine 28:74–99. [DOI] [PubMed] [Google Scholar]
- Hara K, Zhang D 2012. Bacterial abundance and viability in long-range transported dust. Atmospheric Environment 47:20–25. [Google Scholar]
- Hardy K, Brand-Miller J, Brown KD, Thomas MG, Copeland L 2015. The importance of dietary carbohydrate in human evolution. Quarterly Review of Biology 90:251–268. [DOI] [PubMed] [Google Scholar]
- Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque ASG, Ryan ET, Qadri F, Calderwood SB 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLOS Neglected Tropical Diseases 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartiala J, Breton CV, Tang WHW, Lurmann F, Hazen SL, Gilliland FD, Allayee H 2016. Ambient air pollution is associated with the severity of coronary atherosclerosis and incident myocardial infarction in patients undergoing elective cardiac evaluation. Journal of the American Heart Association 5:e003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvati K, Röding C, Bosman AM, Karakostis FA, Grün R, Stringer C, Karkanas P, Thompson NC, Koutoulidis V, Moulopoulos LA, Gorgoulis VG, Kouloukoussa M 2019. Apidima Cave fossils provide earliest evidence of Homo sapiens in Eurasia. Nature 571:500–504. [DOI] [PubMed] [Google Scholar]
- Häsler R, Kautz C, Rehman A, et al. 2018. The antibiotic resistome and microbiota landscape of refugees from Syria, Iraq and Afghanistan in Germany. Microbiome 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, Smith KR 2010. Do women stop early? Similarities in fertility decline in humans and chimpanzees. Annals of the New York Academy of Sciences 1204:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Khedri Z, Schwarz F, Landig C, Liang S-Y, Yu H, Chen X, Fujito NT, Satta Y, Varki A, Angata T 2017. Coevolution of Siglec-11 and Siglec-16 via gene conversion in primates. BMC Evolutionary Biology 17:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Effects Institute. 2019. State of Global Air 2019: Air Pollution a Significant Risk Factor Worldwide. Boston (Massachusetts): Health Effects Institute. [Google Scholar]
- Helle S, Brommer JE, Pettay JE, Lummaa V, Enbuske M, Jokela J 2014. Evolutionary demography of agricultural expansion in preindustrial northern Finland. Proceedings of the Royal Society B: Biological Sciences 281:20141559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, Hauer J, Layton M, McDade J, Osterholm MT, O’Toole T, Parker G, Perl T, Russell PK, Tonat K, for the Working Group on Civilian Biodefense. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127–2137. [DOI] [PubMed] [Google Scholar]
- Henn BM, Cavalli-Sforza LL, Feldman MW 2012. The great human expansion. Proceedings of the National Academy of Sciences of the United States of America 109:17758–17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Aguilar RA, Moore J, Pickering TR 2007. Savanna chimpanzees use tools to harvest the underground storage organs of plants. Proceedings of the National Academy of Sciences of the United States of America 104:19210–19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Paredes J, Wilson ME, Bloomsmith MA, Chennareddi L, Walker ML 2012. Menopause occurs late in life in the captive chimpanzee (Pan troglodytes). Age 34:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz I, Donoghue HD, Minnikin DE, May H, Lee OY-C, Feldman M, Galili E, Spigelman M, Rothschild BM, Bar-Gal GK 2015. Tuberculosis origin: the Neolithic scenario. Tuberculosis 95: S122–S126. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD 2010. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. International Journal of Obesity 34:420–428. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, Pemu A, Rankin J 2019. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLOS Medicine 16:e1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Hurtado AM 1996. Aché Life History: The Ecology and Demography of a Foraging People. New York: Aldine de Gruyter. [Google Scholar]
- Hilton HG, Parham P 2017. Missing or altered self: human NK cell receptors that recognize HLA-C. Immunogenetics 69:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton HG, Blokhuis JH, Guethlein LA, Norman PJ, Parham P 2017. Resurrecting KIR2DP1: a key intermediate in the evolution of human inhibitory NK cell receptors that recognize HLA-C. Journal of Immunology 198:1961–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt BM, Formicola V 2008. Hunters of the Ice Age: the biology of Upper Paleolithic people. American Journal of Physical Anthropology 137(Supplement):70–99. [DOI] [PubMed] [Google Scholar]
- Hook M, Roy S, Williams EG, Bou Sleiman M, Mozhui K, Nelson JF, Lu L, Auwerx J, Williams RW 2018. Genetic cartography of longevity in humans and mice: current landscape and horizons. Biochimica Biophysica Acta—Molecular Basis of Disease 1864:2718–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa Y, Oda N, Cox NJ, et al. 2000. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nature Genetics 26:163–175. [DOI] [PubMed] [Google Scholar]
- Hosgood HD III, Sapkota AR, Rothman N, Rohan T, Hu W, Xu J, Vermeulen R, He X, White JR, Wu G, Wei F, Mongodin EF, Lan Q 2014. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environmental and Molecular Mutagenesis 55:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, Zimmerman PA, Barnadas C, Beall CM, Gebremedhin A, Ménard D, Williams TN, Weatherall DJ, Hay SI 2011. The global distribution of the Duffy blood group. Nature Communications 2:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ding Q, He Y, Xu S, Jin L 2015. Reintroduction of a homocysteine level-associated allele into East Asians by Neanderthal introgression. Molecular Biology and Evolution 32:3108–3113. [DOI] [PubMed] [Google Scholar]
- Huang L-C, Lin R-T, Chen C-F, Chen C-H, Juo S-HH, Lin H-F 2016. Predictors of carotid intima-media thickness and plaque progression in a Chinese population. Journal of Atherosclerosis and Thrombosis 23:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Bisson WH, Sullivan AP, Sebastian A, Perry GH, Jablonski NG, Perdew GH 2016. Divergent Ah receptor ligand selectivity during hominin evolution. Molecular Biology and Evolution 33:2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebbe P, Rimbach G 2017. Evolution of human apolipoprotein E (APOE) isoforms: gene structure, protein function and interaction with dietary factors. Ageing Research Reviews 37:146–161. [DOI] [PubMed] [Google Scholar]
- Huebbe P, Nebel A, Siegert S, Moehring J, Boesch-Saadatmandi C, Most E, Pallauf J, Egert S, Müller MJ, Schreiber S, Nöthlings U, Rimbach G 2011. APOE ε4 is associated with higher vitamin D levels in targeted replacement mice and humans. FASEB Journal 25:3262–3270. [DOI] [PubMed] [Google Scholar]
- Huffman MA, Caton JM 2001. Self-induced increase of gut motility and the control of parasitic infections in wild chimpanzees. International Journal of Primatology 22:329–346. [Google Scholar]
- Hundessa S,Williams G,Li S,Liu DL,Cao W, Ren H, Guo J, Gasparrini A, Ebi K, Zhang W, Guo Y 2018. Projecting potential spatial and temporal changes in the distribution of Plasmodium vivax and Plasmodium falciparum malaria in China with climate change. Science of the Total Environment 627:1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado AM, Frey MA, Hurtado I, Hill K, Baker J 2008. The role of helminthes in human evolution: implications for global health in the 21st century Pages 153–180 in Medicine and Evolution: Current Applications, Future Prospects, edited by Elton S and O’Higgins P. Boca Raton (Florida): CRC Press/Taylor & Francis Group. [Google Scholar]
- Huuskonen P, Storvik M, Reinisalo M, Honkakoski P, Rysä J, Hakkola J, Pasanen M 2008. Microarray analysis of the global alterations in the gene expression in the placentas from cigarette-smoking mothers. Clinical Pharmacology & Therapeutics 83:542–550. [DOI] [PubMed] [Google Scholar]
- Inchley CE, Larbey CDA, Shwan NAA, et al. 2016. Selective sweep on human amylase genes post-dates the split with Neanderthals. Scientific Reports 6:37198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurescia S, Seripa D, Rinaldi M 2016. Role of the 5-HTTLPR and SNP promoter polymorphisms on serotonin transporter gene expression: a closer look at genetic architecture and in vitro functional studies of common and uncommon allelic variants. Molecular Neurobiology 53:5510–5526. [DOI] [PubMed] [Google Scholar]
- Jackson GS, Murray I, Hosszu LLP, Gibbs N, Waltho JP, Clarke AR, Collinge J 2001. Location and properties of metal-binding sites on the human prion protein. Proceedings of the National Academy of Sciences of the United States of America 98:8531–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon S, Berthenet K, Garlanda C 2019. Sexual dimorphism in innate immunity. Clinical Reviews in Allergy and Immunology 56:308–321. [DOI] [PubMed] [Google Scholar]
- Jelenkovic A, Yokoyama Y, Sund R, et al. 2018. Associations between birth size and later height from infancy through adulthood: an individual based pooled analysis of 28 twin cohorts participating in the CODATwins Project. Early Human Development 120:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang NM, Cowan M, Moonah SN, Petri WA Jr. 2018. The impact of systemic inflammation on neuro-development. Trends in Molecular Medicine 24:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L, Stolzenberg-Solomon R, Zimmerman TP, Duan Z, Chen L, Kahle L, Risch A, Subar AF, Cross AJ, Hollenbeck A, Vlassara H, Striker G, Sinha R 2015. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. American Journal of Clinical Nutrition 101:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Milner JJ, Makowski L 2012. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunological Reviews 249:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NB 1986. Bushman birth spacing: a test for optimal interbirth intervals. Ethology and Sociobiology 7: 91–105. [Google Scholar]
- Kalanda BF, van Buuren S, Verhoeff FH, Brabin BJ 2005. Catch-up growth in Malawian babies, a longitudinal study of normal and low birthweight babies born in a malarious endemic area. Early Human Development 81:841–850. [DOI] [PubMed] [Google Scholar]
- Kalbitzer U, Roos C, Kopp GH, Butynski TM, Knauf S, Zinner D, Fischer J 2016. Insights into the genetic foundation of aggression in Papio and the evolution of two length-polymorphisms in the promoter regions of serotonin-related genes (5-HTTLPR and MAOALPR) in Papionini. BMC Evolutionary Biology 16:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamberov YG, Guhan SM, DeMarchis A, Jiang J, Wright SS, Morgan BA, Sabeti PC, Tabin CJ, Lieberman DE 2018. Comparative evidence for the independent evolution of hair and sweat gland traits in primates. Journal of Human Evolution 125: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado AM 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evolutionary Anthropology 9: 156–185. [Google Scholar]
- Kaplan H, Thompson RC, Trumble BC, et al. 2017. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet 389:1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EK, Harris JB, Tabrizi S, Rahman A, Shlyakhter I, Patterson N, O’Dushlaine C, Schaffner SF, Gupta S, Chowdhury F, Sheikh A, Shin OS, Ellis C, Becker CE, Stuart LM, Calderwood SB, Ryan ET, Qadri F, Sabeti PC, LaRocque RC 2013. Natural selection in a Bangladeshi population from the cholera-endemic Ganges River delta. Science Translational Medicine 5:192ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EK, Kwiatkowski DP, Sabeti PC 2014. Natural selection and infectious disease in human populations. Nature Reviews Genetics 15:379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JD, Spalt EW, Curl CL, Hajat A, Jones MR, Kim S-Y, Vedal S, Szpiro AA, Gassett A, Sheppard L, Daviglus ML, Adar SD 2016. Advances in understanding air pollution and CVD. Global Heart 11:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling MJ, Gilligan CA 2000. Metapopulation dynamics of bubonic plague. Nature 407:903–906. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Fussell JC 2012. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmospheric Environment 60:504–526. [Google Scholar]
- Key FM, Abdul-Aziz MA, Mundry R, Peter BM, Sekar A, D’Amato M, Dennis MY, Schmidt JM, Andrés AM 2018. Human local adaptation of the TRPM8 cold receptor along a latitudinal cline. PLOS Genetics 14:e1007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AH, Zou Z, Xiang Y, Chen S, Tian X-L 2019. Conserved signaling pathways genetically associated with longevity across the species. Biochimicaet Biophysica Acta—Molecular Basis of Disease 1865:1745–1755. [DOI] [PubMed] [Google Scholar]
- Kim H-W, Kam S, Lee D-H, 2014. Synergistic interaction between polycyclic aromatic hydrocarbons and environmental tobacco smoke on the risk of obesity in children and adolescents: the U.S. National Health and Nutrition Examination Survey 2003–2008. Environmental Research 135:354–360. [DOI] [PubMed] [Google Scholar]
- Kitamura HW, Hamanaka H, Watanabe M, Wada K, Yamazaki C, Fujita SC, Manabe T, Nukina N 2004. Age-dependent enhancement of hippocampal long-term potentiation in knock-in mice expressing human apolipoprotein E4 instead of mouse apolipoprotein E. Neuroscience Letters 369:173–178. [DOI] [PubMed] [Google Scholar]
- Klein Goldewijk K, Beusen A, van Drecht G, de Vos M 2011. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Global Ecology and Biogeography 20: 73–86. [Google Scholar]
- Klumpp D, Frank SC, Klumpp L, Sezgin EC, Eckert M, Edalat L, Bastmeyer M, Zips D, Ruth P, Huber SM 2017. TRPM8 is required for survival and radioresistance of glioblastoma cells. Oncotarget 8:95896–95913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD 2013. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. Journal of Neuroscience 33:2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompella P, Vasquez KM 2019. Obesity and cancer: a mechanistic overview of metabolic changes in obesity that impact genetic instability. Molecular Carcino-genesis 58:1531–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft TS, Stieglitz J, Trumble BC, Martin M, Kaplan H, Gurven M 2018. Nutrition transition in 2 lowland Bolivian subsistence populations. American Journal of Clinical Nutrition 108:1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KL, Greaves RD 2007. Changing patterns of infant mortality and maternal fertility among Pumé foragers and horticulturalists. American Anthropologist 109:713–726. [Google Scholar]
- Kronenberg ZN, Fiddes IT, Gordon D, et al. 2018. High-resolution comparative analysis of great ape genomes. Science 360:eaar6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Rothman DS, Turner S, Solórzano J, Hughes B 2016. Beyond attributable burden: estimating the avoidable burden of disease associated with household air pollution. PLOS ONE 11:e0149669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Raghavachari N, Arbeev KG, Culminskaya I, Arbeeva L, Wu D, Ukraintseva SV, Christensen K, Yashin AI 2016. Protective role of the apolipoprotein E2 allele in age-related disease traits and survival: evidence from the Long Life Family Study. Biogerontology 17:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Huang J, Wang J, He L, Loika Y, Culminskaya I 2018. Apolipoprotein E region molecular signatures of Alzheimer’s disease. Aging Cell 17:e12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuti JO, Galloway CM 1994. Sugar composition and invertase activity in prickly pear fruit. Journal of Food Science 59:387–388. [Google Scholar]
- Labrie V, Buske OJ, Oh E, et al. 2016. Lactase non-persistence is directed by DNA-variation-dependent epigenetic aging. Nature Structural & Molecular Biology 23:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkakula S, Pathapati RM, Chaubey G, Munirajan AK, Lakkakula BVKS, Maram R 2014. NAT2 genetic variations among South Indian populations. Human Genome Variation 1:14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landig CS, Hazel A, Kellman BP, Fong JJ, Schwarz F, Agarwal S, Varki N, Massari P, Lewis NE, Ram S, Varki A 2019. Evolution of the exclusively human pathogen Neisseria gonorrhoeae: human-specific engagement of immunoregulatory siglecs. Evolutionary Applications 12:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Hu H, Caravanos J, Cropper ML, Hanrahan D, Sandilya K, Chiles TC, Kumar P, Suk WA 2018. Pollution and global health–an agenda for prevention. Environmental Health Perspectives 126:084501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer J, Dufoe A, Brady J 2018. U.S. Faces a Rise in Mosquito “Disease Danger Days.” Princeton (New Jersey): Climate Central; http://assets.climatecentral.org/pdfs/August2018_CMN_Mosquitoes.pdf?pdf=Mosquitoes-Report. [Google Scholar]
- Larrasoaña JC, Roberts AP, Rohling EJ 2013. Dynamics of green Sahara periods and their role in hominin evolution. PLOS ONE 8:e76514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CS 1995. Biological changes in human populations with agriculture. Annual Review of Anthropology 24:185–213. [Google Scholar]
- Larsen CS, Knüsel CJ, Haddow SD, Pilloud MA, Milella M, Sadvari JW, Pearson J, Ruff CB, Garofalo EM, Bocaege E, Betz BJ, Dori I, Glencross B 2019. Bioarchaeology of Neolithic Çatalhöyük reveals fundamental transitions in health, mobility, and lifestyle in early farmers. Proceedings of the National Academy of Sciences of the United States of America 116:12615–12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PA, Lutz MW, Hunnicutt KE, Mihovilovic M, Saunders AM, Yoder AD, Roses AD 2017. The Alu neurodegeneration hypothesis: a primate-specific mechanism for neuronal transcription noise, mitochondrial dysfunction, and manifestation of neurodegenerative disease. Alzheimer’s & Dementia 13: 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson G, Piperno DR, Allaby RG, et al. 2014. Current perspectives and the future of domestication studies. Proceedings of the National Academy of Sciences of the United States of America 111:6139–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer BM, Lovejoy CO, Spurlock L, Haile-Selassie Y 2016. The thoracic cage of KSD-VP-1/1 Pages 143–153 in The Postcranial Anatomy of Australopithecus afarensis: New Insights from KSD-VP-1/1, edited by Haile-Selassie Y and Su DF. Dordrecht (The Netherlands): Springer. [Google Scholar]
- Laval G, Peyrégne S, Zidane N, Harmant C, Renaud F, Patin E, Prugnolle F, Quintana-Murci L 2019. Recent adaptive acquisition by African rainforest hunter-gatherers of the late Pleistocene sickle-cell mutation suggests past differences in malaria exposure. American Journal of Human Genetics 104:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DW, Comper P, Hutchison MG, Sharma B 2015. The role of apolipoprotein E episilon (ε)-4 allele on outcome following traumatic brain injury: a systematic review. Brain Injury 29:1018–1031. [DOI] [PubMed] [Google Scholar]
- Lederberg J 1999. J. B. S. Haldane (1949) on infectious disease and evolution. Genetics 153:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-G, Kim S-Y, Moon J-S, Kim S-H, Kang D-H, Yoon H-J 2016. Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chemistry 199:632–638. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A, Münzel T 2019. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Europian Heart Journal 40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE 2011. Paleosol carbonates from the Omo Group: isotopic records of local and regional environmental change in East Africa. Palaeogeography, Palaeoclimatology, Palaeoecology 307:75–89. [Google Scholar]
- Levine M, Crimmins E 2014. Not all smokers die young: a model for hidden heterogeneity within the human population. PLOS ONE 9:e87403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Na L, Ma H, Zhang Z, Li T, Lin L, Li Q, Sun C, Li Y 2015. Multigenerational effects of parental prenatal exposure to famine on adult offspring cognitive function. Scientific Reports 5:13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen M, Li Y, Tollefsbol TO 2019a. Prenatal epigenetics diets play protective roles against environmental pollution. Clinical Epigenetics 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jin L, Kan H 2019b. Air pollution: a global problem needs local fixes. Nature 570:437–439. [DOI] [PubMed] [Google Scholar]
- Libert F, Cochaux P, Beckman G, et al. 1998. The Δccr5 mutation conferring protection against HIV-1 in Caucasian populations has a single and recent origin in northeastern Europe. Human Molecular Genetics 7:399–406. [DOI] [PubMed] [Google Scholar]
- Lieverse AR, Temple DH, Bazaliiskii VI 2014. Paleopathological description and diagnosis of meta-static carcinoma in an early Bronze Age (4588+34 cal. BP) forager from the Cis-Baikal region of Eastern Siberia. PLOS ONE 9:e113919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas C, de Matthaeis MC, Merli F 1979. Determination of benzo[a]pyrene in smoked, cooked and toasted food products. Food and Cosmetics Toxicology 17:325–328. [DOI] [PubMed] [Google Scholar]
- Liu X, Lian H, Ruan Y, Liang R, Zhao X, Routledge M, Fan Z 2015. Association of exposure to particular matter and carotid intima-media thickness: a systematic review and metaanalysis. International Journal of Environmental Research and Public Health 12:12924–12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang H, Zhang H, Niu Y, Fu Y, Nie J, Yang A, Zhao J, Yang J 2018. Mediation effect of AhR expression between polycyclic aromatic hydrocarbons exposure and oxidative DNA damage among Chinese occupational workers. Environmental Pollution 243:972–977. [DOI] [PubMed] [Google Scholar]
- Llorente Caño M 2004. Nesting behaviour of the Kanyawara community of chimpanzees. Kibale National Park, Uganda: PhD diss., University of Barcelona. [Google Scholar]
- Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT 2009. Epidemic dynamics at the human-animal interface. Science 326:1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98. [Google Scholar]
- London D, Hruschka D 2014. Helminths and human ancestral immune ecology: what is the evidence for high helminth loads among foragers? American Journal of Human Biology 26:124–129. [DOI] [PubMed] [Google Scholar]
- Longman J, Veres D, Finsinger W, Ersek V 2018. Exceptionally high levels of lead pollution in the Balkans from the Early Bronze Age to the Industrial Revolution. Proceedings of the National Academy of Sciences of the United States of America 115:E5661–E5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Finch CE 2003. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299:1342–1346. [DOI] [PubMed] [Google Scholar]
- Lottem E, Banerjee D, Vertechi P, Sarra D, oude Lohuis M, Mainen ZF 2018. Activation of serotonin neurons promotes active persistence in a probabilistic foraging task. Nature Communications 9: 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CP, Polak L, Keyes BE, Fuchs E 2016. Spatio-temporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 354:aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca F, Bubba G, Basile M, Brdicka R, Michalodimitrakis E, Rickards O, Vershubsky G, Quintana-Murci L, Kozlov AI, Novelletto A 2008. Multiple advantageous amino acid variants in the NAT2 gene in human populations. PLOS ONE 3:e3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke T, Kullmer O, Wacker U, Sandrock O, Fiebig J, Schrenk F, Mulch A 2018. Dietary versatility of Early Pleistocene hominins. Proceedings of the National Academy of Sciences of the United States of America 115:13330–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren-Kownacki K, Hornyanszky ED, Chu TA, Olsson JA, Becker P 2018. Challenges of using air conditioning in an increasingly hot climate. International Journal of Biometeorology 62:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MW, Crenshaw D, Welsh-Bohmer KA, Burns DK, Roses AD 2016. New genetic approaches to AD: lessons from APOE-TOMM40 phylogenetics. Current Neurology and Neuroscience Reports 16:48. [DOI] [PubMed] [Google Scholar]
- Lyon MF 1961. Gene action in the X-chromosome of the mouse (Mus musculus l.). Nature 190:372–373. [DOI] [PubMed] [Google Scholar]
- MacHugh DE, Larson G, Orlando L 2017. Taming the past: ancient DNA and the study of animal domestication. Annual Review of Animal Biosciences 5: 329–351. [DOI] [PubMed] [Google Scholar]
- Madani R, Karastergiou K, Ogston NC, Miheisi N, Bhome R, Haloob N, Tan GD, Karpe F, Malone-Lee J, Hashemi M, Jahangiri M, Mohamed-Ali V 2009. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. American Journal of Physiology, Endocrinology and Metabolism 296:E1262–E1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison A 2003. Development Centre Studies the World Economy: Historical Statistics. Paris (France): OECD Publishing. [Google Scholar]
- Madruga DG, Ubeda RM, Terroba JM, dos Santos SG, García-Cambero JP 2019. Particle-associated polycyclic aromatic hydrocarbons in a representative urban location (indoor-outdoor) from South Europe: assessment of potential sources and cancer risk to humans. Indoor Air 29:817–827. [DOI] [PubMed] [Google Scholar]
- Magiorkinis EN, Magiorkinis GN, Paraskevis DN, Hatzakis AE 2005. Re-analysis of a human hepatitis B virus (HBV) isolate from an East African wild born Pan troglodytes schweinfurthii: evidence for interspecies recombination between HBV infecting chimpanzee and human. Gene 349:165–171. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y 2009. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. Journal of Lipid Research 50:S183–S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maibach V, Hans JB, Hvilsom C, Marques-Bonet T, Vigilant L 2017. MHC class I diversity in chimpanzees and bonobos. Immunogenetics 69:661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksoud E, Koehl B, Facchin A, Ha P, Zhao W, Kaguelidou F, Benkerrou M, Mariani P, Faye A, Lorrot M, Jacqz-Aigrain E 2018. Population pharmacokinetics of cefotaxime and dosage recommendations in children with sickle cell disease. Antimicrobial Agents and Chemotherapy 62:e00637–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaria Genomic Epidemiology Network. 2015. A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature 526:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manci EA, Culberson DE, Yang Y-M, Gardner TM, Powell R, Haynes J Jr., Shah AK, Mankad VN, Investigators of the Cooperative Study of Sickle Cell Disease. 2003. Causes of death in sickle cell disease: an autopsy study. British Journal of Haematology 123: 359–365. [DOI] [PubMed] [Google Scholar]
- Marlowe FW, Berbesque JC, Wood B, Crittenden A, Porter C, Mabulla A 2014. Honey, Hadza, hunter-gatherers, and human evolution. Journal of Human Evolution 71:119–128. [DOI] [PubMed] [Google Scholar]
- Marquet S 2018. Overview of human genetic susceptibility to malaria: from parasitemia control to severe disease. Infection Genetics and Evolution 66:399–409. [DOI] [PubMed] [Google Scholar]
- Martínez-Garcia A, Rosell-Melé A, Jaccard SL, Geibert W, Sigman DM, Haug GH 2011. Southern Ocean dust-climate coupling over the past four million years. Nature 476:312–325. [DOI] [PubMed] [Google Scholar]
- Martorell R, Yarbrough C, Lechtig A, Habicht J-P, Klein RE 1975. Diarrheal diseases and growth retardation in preschool Guatemalan children. American Journal of Physical Anthropology 43:341–346. [DOI] [PubMed] [Google Scholar]
- Martorell R, Yarbrough C, Yarbrough S, Klein RE 1980. The impact of ordinary illnesses on the dietary intakes of malnourished children. American Journal of Clinical Nutrition 33:345–350. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Barker DJ, Jespersen S, Greenwald S, Osmond C, Berry C 1995. Growth in utero, adult blood pressure, and arterial compliance. British Heart Journal 73:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H, Mitsui T, Nobumoto E, Hiramatsu Y 2015. The effects of high-fat diet exposure in utero on the obesogenic and diabetogenic traits through epigenetic changes in Adiponectin and Leptin gene expression for multiple generations in female mice. Endocrinology 156:2482–2491. [DOI] [PubMed] [Google Scholar]
- Matejcic M, Vogelsang M, Wang Y, Parker IM 2015. NAT1 and NAT2 genetic polymorphisms and environmental exposure as risk factors for oesophageal squamous cell carcinoma: a case-control study. BMC Cancer 15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder B, Almond D, Park K, Crimmins EM, Finch CE 2010. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. Journal of Developmental Origins of Health and Disease 1:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister MM, Maguire M, Ramesh A, Aimin Q, Liu S, Khoshbouei H, Aschner M, Ebner FF, Hood DB 2008. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology 29:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister MM, Li Z, Zhang T, Ramesh A, Clark RS, Maguire M, Hutsell B, Newland MC, Hood DB 2016. Revealing behavioral learning deficit phenotypes subsequent to in utero exposure to benzo(a)pyrene. Toxicological Sciences 149:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell JR, Wilson AI, Stohl A, Arienzo MM, Chellman NJ, Eckhardt S, Thompson EM, Pollard AM, Steffensen JP 2018. Lead pollution recorded in Greenland ice indicates European emissions tracked plagues, wars, and imperial expansion during antiquity. Proceedings of the National Academy of Sciences of the United States of America 115: 5726–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang C-C, Lurmann F, Berhane K 2015. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environmental Health Perspectives 123:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Bennett C, Dickson D, Anestis SF, Watts DP, Webster TH, Fontenot MB, Bradley BJ 2012. The apolipoprotein E (APOE) gene appears functionally monomorphic in chimpanzees (Pan troglodytes). PLOS ONE 7:e47760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown T 1976. The Modern Rise of Population. London (United Kingdom): Edward Arnold. [Google Scholar]
- McLean KE, Stranberg R, MacDonald M, Richardson GRA, Kosatsky T, Henderson SB 2018. Establishing heat alert thresholds for the varied climatic regions of British Columbia, Canada. International Journal of Environmental Research and Public Health 15:2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan MR, Hasegawa H, Bardi M, Huffman MA 2017. Gastrointestinal parasite infections and self-medication in wild chimpanzees surviving in degraded forest fragments within an agricultural landscape mosaic in Uganda. PLOS ONE 12:e0180431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus KF, Taravella AM, Henn BM, Bustamante CD, Sikora M, Cornejo OE 2017. Population genetic analysis of the DARC locus (Duffy) reveals adaptation from standing variation associated with malaria resistance in humans. PLOS Genetics 13: e1006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill WH 1989. Plagues and Peoples. New York: Anchor Books. [Google Scholar]
- McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Béarat HA 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466:857–860. [DOI] [PubMed] [Google Scholar]
- McPherson M, García-García A, Cuesta-Valero FJ, Beltrami H, Hansen-Ketchum P, MacDougall D, Ogden NH 2017. Expansion of the Lyme disease vector Ixodes scapularis in Canada inferred from CMIP5 climate projections. Environmental Health Perspectives 125:057008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead S, Stumpf MPH, Whitfield J, Beck JA, Poulter M, Campbell T, Uphill JB, Goldstein D, Alpers M, Fisher EMC, Collinge J 2003. Balancing selection at the prion protein gene consistent with prehistoric kurulike epidemics. Science 300:640–643. [DOI] [PubMed] [Google Scholar]
- Mead S, Whitfield J, Poulter M, Shah P, Uphill J, Beck J, Campbell T, Al-Dujaily H, Hummerich H, Alpers MP, Collinge J 2008. Genetic susceptibility, evolution and the kuru epidemic. Philosophical Transactions of the Royal Society B: Biological Sciences 363:20080087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez FL, Watkins JC, Hammer MF 2013. Neandertal origin of genetic variation at the cluster of OAS immunity genes. Molecular Biology and Evolution 30:798–801. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Paholpak P, Lin A, Zhang JY, Teng E 2015. Prevalence of traumatic brain injury in early versus late-onset Alzheimer’s disease. Journal of Alzheimer’s Disease 47:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QR, Gideon KM, Harbo SJ, Renne RA, Lee MK, Brys AM, Jones R 2006. Gene expression profiling in lung tissues from mice exposed to cigarette smoke, lipopolysaccharide, or smoke plus lipopolysaccharide by inhalation. Inhalation Toxicology 18:555–568. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Armitage TL, Schenker MB, Bennett DH, Tancredi DJ, Langer CE, Reynolds SJ, Dooley G, Mehaffy J, Mitloehner FM 2015. Particulate matter, endotoxin, and worker respiratory health on large California dairies. Journal of Occupational and Environmental Medicine 57:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PD 2015. Human parasites in medieval Europe: lifestyle, sanitation and medical treatment. Advances in Parasitology 90:389–420. [DOI] [PubMed] [Google Scholar]
- Mitchell PD 2017. Human parasites in the Roman world: health consequences of conquering an empire. Parasitology 144:48–58. [DOI] [PubMed] [Google Scholar]
- Mittal R, Sukumaran SK, Selvaraj SK, Wooster DG, Babu MM, Schreiber AD, Verbeek JS, Prasadarao NV 2010. Fcγ receptor I alpha chain (CD64) expression in macrophages is critical for the onset of meningitis by Escherichia coli K1. PLOS Pathogens 6:e1001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter SS, Oriá RB, Kvalsund MP, Pamplona P, Joventino ES, Mota RMS, Gonçalves DC, Patrick PD, Guerrant RL, Lima AAM 2012. Apolipoprotein E4 influences growth and cognitive responses to micronutrient supplementation in shantytown children from northeast Brazil. Clinics 67:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge G, Jimenez-Espejo FJ, García-Alix A, Martínez-Ruiz F, Mattielli N, Finlayson C, Ohkouchi N, Sánchez MC, de Castro JMB, Blasco R, Rosell J, Carrión J, Rodríguez-Vidal J, Finlayson G 2015. Earliest evidence of pollution by heavy metals in archaeological sites. Scientific Reports 5:14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalva N, Adhikari K, Liebert A, Mendoza-Revilla J, Flores SV, Mace R, Swallow DM 2019. Adaptation to milking agropastoralism in Chilean goat herders and nutritional benefit of lactase persistence. Annals of Human Genetics 83:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J, Black J, Hernandez-Aguilar RA, Idani G, Piel A, Stewart F 2017. Chimpanzee vertebrate consumption: savanna and forest chimpanzees compared. Journal of Human Evolution 112:30–40. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP 2005. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. American Journal of Primatology 65:167–179. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Watts DP 2010. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. BioPsychoSocial Medicine 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW 2014. Mortality rates among Kanyawara chimpanzees. Journal of Human Evolution 66:107–114. [DOI] [PubMed] [Google Scholar]
- Murakami G, Edamura M, Furukawa T, Kawasaki H, Kosugi I, Fukuda A, Iwashita T, Nakahara D 2018. MHC class I in dopaminergic neurons suppresses relapse to reward seeking. Science Advances 4:eaap7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Amanakis G, Fillmore N, Parks RJ, Sun J 2017. Sex differences in metabolic cardiomyopathy. Cardiovascular Research 113:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2017. Using 21st Century Science to Improve Risk-Related Evaluations. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- National Center for Health Statistics. 2016. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville (Maryland): National Center for Health Statistics, U.S. Department of Health and Human Services; Available at: http://www.cdc.gov/nchs/data/hus/hus16.pdf. [PubMed] [Google Scholar]
- Natri H, Garcia AR, Buetow KH, Trumble BC, Wilson MA 2019. The pregnancy pickle: evolved immune compensation due to pregnancy underlies sex differences in human diseases. Trends in Genetics 35:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellums LB, Thompson H, Holmes A, Castro-Sánchez E, Otter JA, Norredam M, Friedland JS, Hargreaves S 2018. Antimicrobial resistance among migrants in Europe: a systematic review and meta-analysis. Lancet Infectious Diseases 18:796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlich AG, Rohrbach H, Bachmeier B, Zink A 2006. Malignant tumors in two ancient populations: an approach to historical tumor epidemiology. Oncology Reports 16:197–202. [PubMed] [Google Scholar]
- Nesse RM, Williams GC 2012. Why We Get Sick: The New Science of Darwinian Medicine. New York: Vintage. [Google Scholar]
- Niang M, Sane R, Sow A, Sadio BD, Chy S, Legrand E, Faye O, Diallo M, Sall AA, Menard D, Toure-Balde A 2018. Asymptomatic Plasmodium vivax infections among Duffy-negative population in Kedougou, Senegal. Tropical Medicine and Health 46:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, King SE, McBirney M, Kubsad D, Pappalardo M, Beck D, Sadler-Riggleman I, Skinner MK 2018. Vinclozolin induced epigenetic transgenerational inheritance of pathologies and sperm epimutation biomarkers for specific diseases. PLOS ONE 13:e0202662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Mori F, Hanida S, Kumahata K, Ishikawa S, Samarat K, Miyabe-Nishiwaki T, Hayashi M, Tomonaga M, Suzuki J, Matsuzawa T, Matsuzawa T 2016. Impaired air conditioning within the nasal cavity in flat-faced Homo. PLOS Computational Biology 12:e1004807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakowski KE 2018. Identification and functional characterization of conserved residues and domains in the macrophage scavenger receptor MARCO. PhD diss., McMaster University. [Google Scholar]
- Nunez K, Kay J, Krotow A, Tong M, Agarwal AR, Cadenas E, de la Monte SM 2016. Cigarette smoke-induced alterations in frontal white matter lipid profiles demonstrated by MALDI-imaging mass spectrometry: relevance to Alzheimer’s disease. Journal of Alzheimer’s Disease 51:151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF, and the Active Bacterial Core Surveillance Team. 2000. Cigarette smoking and invasive pneumococcal disease. New England Journal of Medicine 342:681–689. [DOI] [PubMed] [Google Scholar]
- Nygaard M, Lindahl-Jacobsen R, Soerensen M, Mengel-From J, Andersen-Ranberg K, Jeune B, Vaupel JW, Tan Q, Christiansen L, Christensen K 2014. Birth cohort differences in the prevalence of longevity-associated variants in APOE and FOXO3A in Danish long-lived individuals. Experimental Gerontology 57: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okerblom J, Varki A 2017. Biochemical, cellular, physiological, and pathological consequences of human loss of N-glycolylneuraminic acid. ChemBioChem 18: 1155–1171. [DOI] [PubMed] [Google Scholar]
- Okerblom JJ, Schwarz F, Olson J, Fletes W, Ali SR, Martin PT, Glass CK, Nizet V, Varki A 2017. Loss CMAH during human evolution primed the monocyte-macrophage lineage toward a more inflammatory and phagocytic state. Journal of Immunology 198:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriá RB, Patrick PD, Blackman JA, Lima AAM, Guerrant RL 2007. Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Medical Hypotheses 68:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto TD, Gilabert A, Crellen T, Böhme U, Arnathau C, Sanders M, Oyola SO, Okouga AP, Boundenga L, Willaume E, Ngoubangoye B, Moukodoum ND, Paupy C, Durand P, Rougeron V, Ollomo B, Renaud F, Newbold C, Berriman M, Prugnolle F 2018. Genomes of all known members of a Plasmodium subgenus reveal paths to virulent human malaria. Nature Microbiology 3:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M 2018. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. Journal of the American College of Cardiology 71:2360–2372. [DOI] [PubMed] [Google Scholar]
- Page AE, Viguier S, Dyble M, Smith D, Chaudhary N, Salali GD, Thompson J, Vinicius L, Mace R, Migliano AB 2016. Reproductive trade-offs in extant hunter-gatherers suggest adaptive mechanism for the Neolithic expansion. Proceedings of the National Academy of Sciences of the United States of America 113:4694–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajic P, Pavlidis P, Dean K, Neznanova L, Romano R-A, Garneau D, Daugherity E, Globig A, Ruhl S, Gokcumen O 2019. Independent amylase gene copy number bursts correlate with dietary preferences in mammals. eLife 8:e44628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala M, Olivieri A, Achilli A, et al. 2012. Mitochondrial DNA signals of late glacial recolonization of Europe from near Eastern Refugia. American Journal of Human Genetics 90:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada H Jr., Steck SE, Cleveland RJ, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD 2017. Genetic polymorphisms of phase I metabolizing enzyme genes, their interaction with lifetime grilled and smoked meat intake, and breast cancer incidence. Annals of Epidemiology 27:208–214.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P, Norman PJ, Abi-Rached L, Guethlein LA 2012. Human-specific evolution of killer cell immunoglobulin-like receptor recognition of major histocompatibility complex class I molecules. Philosophical Transactions of the Royal Society B: Biological Sciences 367:20110266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passey BH, Levin NE, Cerling TE, Brown FH, Eiler JM 2010. High-temperature environments of human evolution in East Africa based on bond ordering in paleosol carbonates. Proceedings of the National Academy of Sciences of the United States of America 107:11245–11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CC, Shirahata H, Ericson JE 1987. Lead in ancient human bones and its relevance to historical developments of social problems with lead. Science of the Total Environment 61:167–200. [DOI] [PubMed] [Google Scholar]
- Peeters M, D’Arc M, Delaporte E 2014. Origin and diversity of human retroviruses. AIDS Reviews 16: 23–34. [PMC free article] [PubMed] [Google Scholar]
- Pérez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Gálvez BG 2016. “Adi-paging”: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. Journal of Physiology 594:3187–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez García-Pando C, Stanton MC, Diggle PJ, Trzaska S, Miller RL, Perlwitz JP, Baldasano JM, Cuevas E, Ceccato P, Yaka P, Thomson MC 2014. Soil dust aerosols and wind as predictors of seasonal meningitis incidence in Niger. Environmental Health Perspectives 122:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perla-Kaján J, Jakubowski H 2019. Dysregulation of epigenetic mechanisms of gene expression in the pathologies of hyperhomocysteinemia. International Journal of Molecular Sciences 20:3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry GH 2014. Parasites and human evolution. Evolutionary Anthropology: Issues, News, and Reviews 23: 218–228. [DOI] [PubMed] [Google Scholar]
- Perry GH, Kistler L, Kelaita MA, Sams AJ 2015. Insights into hominin phenotypic and dietary evolution from ancient DNA sequence data. Journal of Human Evolution 79:55–63. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, Semanek D, Perera F 2015. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 72:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DIW, Osmond C, Southall H, Aucott P, Jones A, Holgate ST 2018. Evaluating the long-term consequences of air pollution in early life: geographical correlations between coal consumption in 1951/1952 and current mortality in England and Wales. BMJ Open 8:e018231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piers RJ 2018. Structural brain volume differences between cognitively intact ApoE4 carriers and non-carriers across the lifespan. Neural Regeneration Research 13:1309–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polavarapu N, Bowen NJ, McDonald JF 2006. Identification, characterization and comparative genomics of chimpanzee endogenous retroviruses. Genome Biology 7:R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H, Wrangham RW 2004. Climbing and the daily energy cost of locomotion in wild chimpanzees: implications for hominoid locomotor evolution. Journal of Human Evolution 46:315–333. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Rolian C, Rightmire GP, Jashashvili T, Ponce de León MS, Lordkipanidze D, Zollikofer CPE 2010. Locomotor anatomy and biomechanics of the Dmanisi hominins. Journal of Human Evolution 58:492–504. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Raichlen DA, Rodman PS 2014. Bipedal and quadrupedal locomotion in chimpanzees. Journal of Human Evolution 66:64–82. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Raichlen DA, Wood BM, Emery Thompson M, Racette SB, Mabulla AZP, Marlowe FW 2015. Energy expenditure and activity among Hadza hunter-gatherers. American Journal of Human Biology 27:628–637. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Wood BM, Raichlen DA 2018. Hunter-gatherers as models in public health. Obesity Reviews 19 (Supplement):24–35. [DOI] [PubMed] [Google Scholar]
- Popkin BM 2006. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. American Journal of Clinical Nutrition 84:289–298. [DOI] [PubMed] [Google Scholar]
- Potts R 1998. Variability selection in hominid evolution. Evolutionary Anthropology 7:81–96. [Google Scholar]
- Prohaska A, Racimo F, Schork AJ, Sikora M, Stern AJ, Ilardo M, Allentoft ME, Folkersen L, Buil A, Moreno-Mayar JV, Korneliussen T, Geschwind D, Ingason A, Werge T, Nielsen R, Willerslev E 2019. Human disease variation in the light of population genomics. Cell 177:115–131. [DOI] [PubMed] [Google Scholar]
- Prospero JM, Ginoux P, Torres O, Nicholson SE, Gill TE 2002. Environmental characterization of global sources of atmospheric soil dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Reviews of Geophysics 40:2–1–2–31. [Google Scholar]
- Prusiner SB 1998. Prions. Proceedings of the National Academy of Sciences of the United States of America 95: 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach H, Rotival M, Pothlichet J, et al. 2016. Genetic adaptation and Neandertal admixture shaped the immune system of human populations. Cell 167: 643–656.E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaï RL, Dong L-M, Farese RV Jr., Weisgraber KH 2001. Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proceedings of the National Academy of Sciences of the United States of America 98:11587–11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Alexander GE 2014. Exercise, APOE genotype, and the evolution of the human lifespan. Trends in Neuroscience 37:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Al-Kindi SG, Brook RD 2018. Air pollution and cardiovascular disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology 72:2054–2070. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Surendran SN 2012. Global climate change and its potential impact on disease transmission by salinity-tolerant mosquito vectors in coastal zones. Frontiers in Physiology 3:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Surendran SN 2016. Mosquito vectors developing in atypical anthropogenic habitats: global overview of recent observations, mechanisms and impact on disease transmission. Journal of Vector Borne Diseases 53:91–98. [PubMed] [Google Scholar]
- Ranciaro A, Campbell MC, Hirbo JB, Ko W-Y, Froment A, Anagnostou P, Kotze MJ, Ibrahim M, Nyambo T, Omar SA, Tishkoff SA 2014. Genetic origins of lactase persistence and the spread of pastoralism in Africa. American Journal of Human Genetics 94:496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reales G, Rovaris DL, Jacovas VC, Hünemeier T, Sandoval JR, Salazar-Granara A, Demarchi DA, Tarazona-Santos E, Felkl AB, Serafini MA, Salzano FM, Bisso-Machado R, Comas D, Paixão-Côrtes VR, Bortolini MC 2017. A tale of agriculturalists and hunter-gatherers: exploring the thrifty genotype hypothesis in native South Americans. American Journal of Physical Anthropology 163:591–601. [DOI] [PubMed] [Google Scholar]
- Rebuli ME, Speen AM, Martin EM, Addo KA, Pawlak EA, Glista-Baker E, Robinette C, Zhou H, Noah TL, Jaspers I 2019. Wood smoke exposure alters human inflammatory responses to viral infection in a sex-specific manner. A randomized, placebo-controlled study. American Journal of Respiratory and Critical Care Medicine 199:996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KE 1997. Early hominid evolution and ecological change through the African Plio-Pleistocene. Journal of Human Evolution 32:289–322. [DOI] [PubMed] [Google Scholar]
- Richards JL, Hansen C, Bredfeldt C, Bednarczyk RA, Steinhoff MC, Adjaye-Gbewonyo D, Ault K, Gallagher M, Orenstein W, Davis RL, Omer SB 2013. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clinical Infectious Diseases 56:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel C, Schönberger K, Yang S, Koshy G, Chen Y-C, Gopinath B, Ziebarth S, von Kries R 2014. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared with paternal smoking—a meta-analysis. International Journal of Epidemiology 43:1593–1606. [DOI] [PubMed] [Google Scholar]
- Riera CE, Merkwirth C, De Magalhaes Filho CD, Dillin A 2016. Signaling networks determining life span. Annual Review of Biochemistry 85:35–64. [DOI] [PubMed] [Google Scholar]
- Rifkin RF, Potgieter M, Ramond J-B, Cowan DA 2017. Ancient oncogenesis, infection and human evolution. Evolutionary Applications 10:949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine JM, Cubaynes S 2017. Worldwide demography of centenarians. Mechanisms of Ageing and Development 165:59–67. [DOI] [PubMed] [Google Scholar]
- Rose M, Holland J, Dowding A, Petch SRG, White S, Fernandes A, Mortimer D 2015. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food and Chemical Toxicology 78:1–9. [DOI] [PubMed] [Google Scholar]
- Roses A, Sundseth S, Saunders A, Gottschalk W, Burns D, Lutz M 2016. Understanding the genetics of APOE and TOMM40 and role of mitochondrial structure and function in clinical pharmacology of Alzheimer’s disease. Alzheimer’s & Dementia 12:687–694. [DOI] [PubMed] [Google Scholar]
- Rosinger A, Tanner S 2015. Water from fruit or the river? Examining hydration strategies and gastrointestinal illness among Tsimané adults in the Bolivian Amazon. Public Health Nutrition 18:1098–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AA, Müller KM, Weese JS, Neufeld JD 2018. Comprehensive skin microbiome analysis reveals the uniqueness of human skin and evidence for phylosymbiosis within the class Mammalia. Proceedings of the National Academy of Sciences of the United States of America 115:E5786–E5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Howlett SE 2012. Age and ovariectomy abolish beneficial effects of female sex on rat ventricular myocytes exposed to simulated ischemia and reperfusion. PLOS ONE 7:e38425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan J, Reed KE 2015. The paleoclimatic record and Plio-Pleistocene paleoenvironments Pages 465–491 in Handbook of Paleoanthropology, edited by Henke W and Tattersall I. Berlin (Germany): Springer-Verlag. [Google Scholar]
- Ruff CB, Holt B, Niskanen M, Sladek V, Berner M, Garofalo E, Garvin HM, Hora M, Junno J-A, Schuplerova E, Vilkama R, Whittey E 2015. Gradual decline in mobility with the adoption of food production in Europe. Proceedings of the National Academy of Sciences of the United States of America 112: 7147–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruxton GD, Wilkinson DM 2011. Thermoregulation and endurance running in extinct hominins: Wheeler’s models revisited. Journal of Human Evolution 61:169–175. [DOI] [PubMed] [Google Scholar]
- Ryhl-Svendsen M, Clausen G, Chowdhury Z, Smith KR 2010. Fine particles and carbon monoxide from wood burning in17th–19th century Danish kitchens: measurements at two reconstructed farm houses at the Lejre Historical–Archaeological Experimental Center. Atmospheric Environment 44:735–744. [Google Scholar]
- Sabbagh A, Darlu P, Vangenot C, Poloni ES 2018. Arylamine N-acetyltransferases in anthropology Pages 165–193 in Arylamine N-Acetyltransferases in Health and Disease: From Pharmacogenetics to Drug Discovery and Diagnostics, edited by Lauieri N and Sim E. Hackensack (New Jersey): World Scientific Publishing. [Google Scholar]
- Saelens JW, Viswanathan G, Tobin DM 2019. Mycobacterial evolution intersects with host tolerance. Frontiers in Immunology 10:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salque M, Bogucki PI, Pyzel J, Sobkowiak-Tabaka I, Grygiel R, Szmyt M, Evershed RP 2012. Earliest evidence for cheese making in the sixth millennium bc in northern Europe. Nature 493:522–525. [DOI] [PubMed] [Google Scholar]
- Samraj AN, Pearce OMT, Läubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bing-man AE, Secrest P, Diaz SL, Varki NM, Varki A 2015. A red meat-derived glycan promotes inflammation and cancer progression. Proceedings of the National Academy of Sciences of the United States of America 112:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams AJ, Dumaine A, Nédélec Y, Yotova V, Alfieri C, Tanner JE, Messer PW, Barreiro LB 2016. Adaptively introgressed Neandertal haplotype at the OAS locus functionally impacts innate immune responses in humans. Genome Biology 17:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schächter F, Faure-Delanef L, Guénot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D 1994. Genetic associations with human longevity at the APOE and ACE loci. Nature Genetics 6:29–32. [DOI] [PubMed] [Google Scholar]
- Schätzl HM, Da Costa M, Taylor L, Cohen FE, Prusiner SB 1995. Prion protein gene variation among primates. Journal of Molecular Biology 245: 362–374. [DOI] [PubMed] [Google Scholar]
- Schmid P, Churchill SE, Nalla S, Weissen E, Carlson KJ, de Ruiter DJ, Berger LR 2013. Mosaic morphology in the thorax of Australopithecus sediba. Science 340:1234598. [DOI] [PubMed] [Google Scholar]
- Schnorr SL, Crittenden AN, Venema K, Marlowe FW, Henry AG 2015. Assessing digestibility of Hadza tubers using a dynamic in-vitro model. American Journal of Physical Anthropology 158:371–385. [DOI] [PubMed] [Google Scholar]
- Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M 2019. The X chromosome and sex-specific effects in infectious disease susceptibility. Human Genomics 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz KL, Morris SK 2018. Travel and the spread of drug-resistant bacteria. Current Infectious Disease Reports 20:29. [DOI] [PubMed] [Google Scholar]
- Schwarz F, Springer SA, Altheide TK, Varki NM, Gagneux P, Varki A 2016. Human-specific derived alleles of CD33 and other genes protect against postreproductive cognitive decline. Proceedings of the National Academy of Sciences of the United States of America 113:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Heredia N, Senut M-C, Hess M, Land S, Qu W, Hollacher K, Dereski MO, Ruden DM 2015. Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots. Epigenomics 7:379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck MR, Satkoski-Trask J, Deinard A, Tito RY, Smith DG, Melnick DJ, Malhi RS 2014a. Patterns of genetic variation and the role of selection in HTR1A and HTR1B in macaques (Macaca). BMC Genetics 15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck MR, Satkoski-Trask J, Deinard A, Tito RY, Smith DG, Malhi RS 2014b. The evolutionary history of SLC6A4 and the role of plasticity in Macaca. American Journal of Physical Anthropology 153:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN 2007. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurology 6:494–500. [DOI] [PubMed] [Google Scholar]
- Shen L, Li C, Wang Z, Zhang R, Shen Y, Miles T, Wei J, Zou Z 2019. Early-life exposure to severe famine is associated with higher methylation level in the IGF2 gene and higher total cholesterol in late adulthood: the Genomic Research of the Chinese Famine (GRECF) study. Clinical Epigenetics 11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Ding X, Ferguson M, McCallister M, Rhoades R, Maguire M, Ramesh A, Aschner M, Campbell D, Levitt P, Hood DB 2010. Prenatal polycyclic aromatic hydrocarbon exposure leads to behavioral deficits and downregulation of receptor tyrosine kinase, MET. Toxicological Sciences 118:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Ao H, Soemantri A, Tiwawech D, Settheetham-Ishida W, Kayame OW, Kimura M, Nishioka T, Ishida T 2000. Sero- and molecular typing of Duffy blood group in Southeast Asians and Oceanians. Human Biology 72:511–518. [PubMed] [Google Scholar]
- Shriner D, Rotimi CN 2018. Whole-genome-sequence-based haplotypes reveal single origin of the sickle allele during the Holocene Wet Phase. American Journal of Human Genetics 102:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui SS, Springer SA, Verhagen A, Sundaramurthy V, Alisson-Silva F, Jiang W, Ghosh P, Varki A 2017. The Alzheimer’s disease-protective CD33 splice variant mediates adaptive loss of function via diversion to an intracellular pool. Journal of Biological Chemistry 292:15312–15320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes NE 1994. Early hominid habitat preferences in East Africa: paleosol carbon isotopic evidence. Journal of Human Evolution 27:25–45. [Google Scholar]
- Sing CF, Davignon J 1985. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. American Journal of Human Genetics 37:268–285. [PMC free article] [PubMed] [Google Scholar]
- Sistiaga A, Mallol C, Galván B, Summons RE. 2014. The Neanderthal meal: a new perspective using faecal biomarkers. PLOS ONE 9:e101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Tesana S, Pipitgool V, Kaewkes S, Pairojkul C, Sripa B, Paupairoj A, Thaiklar K 2009. Relationship between faecal egg count and worm burden of Opisthorchis viverrini in human autopsy cases. Parasitology 102:277–281. [DOI] [PubMed] [Google Scholar]
- Sköld P 2004. The birth of population statistics in Sweden. History of the Family 9:5–21. [Google Scholar]
- Sladek R, Rocheleau G, Rung J, et al. 2007. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Ko A, Levin ED, Seidler FJ 2019. The developmental neurotoxicity of tobacco smoke can be mimicked by a combination of nicotine and benzo[a]pyrene: effects on cholinergic and serotonergic systems. Toxicological Sciences 167:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Carmody RN, Dutton RJ, Wrangham RW 2015. The significance of cooking for early hominin scavenging. Journal of Human Evolution 84:62–70. [DOI] [PubMed] [Google Scholar]
- Smith KR, Samet JM, Romieu I, Bruce N 2000. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax 55:518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM, Austin C, Green DR, Joannes-Boyau R, Bailey S, Dumitriu D, Fallon S, Grün R, James HF, Moncel M-H, Williams IS, Wood R, Arora M 2018. Wintertime stress, nursing, and lead exposure in Neanderthal children. Science Advances 4: eaau9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søe MJ, Nejsum P, Seersholm FV, Fredensborg BL, Habraken R, Haase K, Hald MM, Simon-sen R, Højlund F, Blanke L, Merkyte I, Willerslev VE, Kapel CMO 2018. Ancient DNA from latrines in Northern Europe and the Middle East (500 BC–1700 AD) reveals past parasites and diet. PLOS ONE 13:e0195481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldevila M, Andrés AM, Blancher A, Calafell F, Ordoñez M, Pumarola M, Oliva B, Aramburu J, Bertranpetit J 2004. Variation of the prion gene in chimpanzees and its implication for prion diseases. Neuroscience Letters 355:157–160. [DOI] [PubMed] [Google Scholar]
- Soma-Pillay P, Macdonald AP 2012. Malaria in pregnancy. Obstetric Medicine 5:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann MN, Monk C, Scheinost D, Peterson BS 2018. Maternal immune activation during the third trimester is associated with neonatal functional connectivity of the salience network and fetal to toddler behavior. Journal of Neuroscience 38:2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth JD 2017. Putrid meat and fish in the Eurasian middle and upper Paleolithic: are we missing a key part of Neanderthal and modern human diet? Paleo-Anthropology 2017:44–72. [Google Scholar]
- Spyrou MA, Tukhbatova RI, Wang C-C, Valtueña AA, Lankapalli AK, Kondrashin VV, Tsybin VA, Khokhlov A, Kühnert D, Herbig A, Bos KI, Krause J 2018. Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nature Communications 9:2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyrou MA, Bos KI, Herbig A, Krause J 2019. Ancient pathogen genomics as an emerging tool for infectious disease research. Nature Reviews Genetics 20:323–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stear MJ, Bishop SC, Doligalska M, Duncan JL, Holmes PH, Irvine J, McCrire L, McKellar QA, Sinski E, Murray M 1995. Regulation of egg production, worm burden, worm length and worm fecundity by host responses in sheep infected with Ostertagia circumcincta. Parasite Immunology 17:643–652. [DOI] [PubMed] [Google Scholar]
- Steiner AL, Davis AJ, Sillman S, Owen RC, Michalak AM, Fiore AM 2010. Observed suppression of ozone formation at extremely high temperatures due to chemical and biophysical feedbacks. Proceedings of the National Academy of Sciences of the United States of America 107:19685–19690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengård JH, Kardia SLR, Hamon SC, Frikke-Schmidt R, Tybjærg-Hansen A, Salomaa V, Boerwinkle E, Sing CF 2006. Contribution of regulatory and structural variations in APOE to predicting dyslipidemia. Journal of Lipid Research 47:318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JC, Reich DE, Goldstein DB, et al. 1998. Dating the origin of the CCR5-Δ32 AIDS-resistance allele by the coalescence of haplotypes. American Journal of Human Genetics 62:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FA, Piel AK, McGrew WC 2011. Living archaeology: artefacts of specific nest site fidelity in wild chimpanzees. Journal of Human Evolution 61:388–395. [DOI] [PubMed] [Google Scholar]
- Stieglitz J, Beheim BA, Trumble BC, Madimenos FC, Kaplan H, Gurven M 2015. Low mineral density of a weight-bearing bone among adult women in a high fertility population. American Journal of Physical Anthropology 156:637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz J, Trumble BC, Kaplan H, Gurven M 2017. Horticultural activity predicts later localized limb status in a contemporary pre-industrial population. American Journal of Physical Anthropology 163:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz J, Trumble BC, HORUS Study Team, Finch CE, Li D, Budoff MJ, Kaplan H, Gurven MD 2019. Computed tomography shows high fracture prevalence among physically active forager-horticulturalists with high fertility. eLife 8:e48607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudmant PH, Rausch T, Gardner EJ, et al. 2015. An integrated map of structural variation in 2,504 human genomes. Nature 526:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer WM, Salemi M, Shanmugam V, Gao F, Cong M. e., Kuiken C, Bhullar V, Beer BE, Vallet D, Gautier-Hion A, Tooze Z, Villinger F, Holmes EC, Heneine W 2005. Ancient co-speciation of simian foamy viruses and primates. Nature 434:376–380. [DOI] [PubMed] [Google Scholar]
- Talmud PJ, Stephens JW, Hawe E, Demissie S, Cupples LA, Hurel SJ, Humphries SE, Ordovas JM 2005. The significant increase in cardiovascular disease risk in APOEε4 carriers is evident only in men who smoke: potential relationship between reduced antioxidant status and ApoE4. Annals of Human Genetics 69:613–622. [DOI] [PubMed] [Google Scholar]
- Tamanna N, Mahmood N 2015. Food processing and Maillard reaction products: effect on human health and nutrition. International Journal of Food Science 2015:526762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082. [DOI] [PubMed] [Google Scholar]
- Tenner AJ, Stevens B, Woodruff TM 2018. New tricks for an ancient system: physiological and pathological roles of complement in the CNS. Molecular Immunology 102:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur SA, Hamilton RF Jr., Holian A 2008. Role of scavenger receptor A family in lung inflammation from exposure to environmental particles. Journal of Immunotoxicology 5:151–157. [DOI] [PubMed] [Google Scholar]
- Thieme H 1997. Lower Palaeolithic hunting spears from Germany. Nature 385:807–810. [DOI] [PubMed] [Google Scholar]
- Thomlinson R 1975. Demographic Problems: Controversy Over Population Control. Second Edition Encino (California): Dickenson Publishing Company. [Google Scholar]
- Thompson RC, Allam AH, Lombardi GP, Wann LS, Sutherland ML, Sutherland JD, Soliman MA-T, Frohlich B, Mininberg DT, Monge JM, Vallodolid CM, Cox SL, Abd el-Maksoud G, Badr I, Miyamoto MI, el-Halim Nur el-din A, Narula J, Finch CE, Thomas GS 2013. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet 381: 1211–1222. [DOI] [PubMed] [Google Scholar]
- Thuong NTT, Tram TTB, Dinh TD, Thai PVK, Heemskerk D, Bang ND, Chau TTH, Russell DG, Thwaites GE, Hawn TR, Caws M, Dunstan J 2016. MARCO variants are associated with phagocytosis, pulmonary tuberculosis susceptibility and Beijing lineage. Genes and Immunity 17: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff SA, Varkonyi R, Cahinhinan N, Abbes S, Argyropoulos G, Destro-Bisol G, Drousiotou A, Dangerfield B, Lefranc G, Loiselet J, Piro A, Stoneking M, Tagarelli A, Tagarelli G, Touma EH, Williams SM, Clark AG 2001. Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Science 293:455–462. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS, Powell K, Mortensen HM, Hirbo JB, Osman M, Ibrahim M, Omar SA, Lema G, Nyambo TB, Ghori J, Bumpstead S, Pritchard JK, Wray GA, Deloukas P 2006. Convergent adaptation of human lactase persis tence in Africa and Europe. Nature Genetics 39:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong DQ, Wang JXL, Gill TE, Lei H, Wang B 2017. Intensified dust storm activity and Valley fever infection in the southwestern United States. Geophysical Research Letters 44:4304–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A, Westover J, Benson M, Johnson R, Dykes A 2016. A killer immunoglobulin-like receptor gene-content haplotype and a cognate human leukocyte antigen ligand are associated with autism. Autism Open Access 6:1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trianti S-M, Samoli E, Rodopoulou S, Katsouyanni K, Papiris SA, Karakatsani A 2017. Desert dust outbreaks and respiratory morbidity in Athens, Greece. Environmental Health 16:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkaus E 2018. An abundance of developmental anomalies and abnormalities in Pleistocene people. Proceedings of the National Academy of Sciences of the United States of America 115:11941–11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Smith EA, O’Connor KA, Kaplan HS, Gurven MD 2014. Successful hunting increases testosterone and cortisol in a subsistence population. Proceedings of the Royal Society B: Biological Sciences 281:20132876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Stieglitz J, Blackwell AD, Allayee H, Beheim B, Finch CE, Gurven M, Kaplan H 2017. Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden. FASEB Journal 31:1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai IJ, Zarowiecki M, Holroyd N, et al. 2013. The genomes of four tapeworm species reveal adaptations to parasitism. Nature 496:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T 1993. Lions eat chimpanzees: the first evidence of predation by lions on wild chimpanzees. American Journal of Primatology 29:1–11. [DOI] [PubMed] [Google Scholar]
- Tuminello ER, Han SD 2011. The apolipoprotein E antagonistic pleiotropy hypothesis: review and recommendations. International Journal of Alzheimer’s Disease 2011:726197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Cohen A, Jerrett M, Gapstur SM, Diver WR, Pope CA III, Krewski D, Beckerman BS, Samet JM 2014. Interactions between cigarette smoking and fine particulate matter in the risk of lung cancer mortality in Cancer Prevention Study II. American Journal of Epidemiology 180:1145–1149. [DOI] [PubMed] [Google Scholar]
- Turner MC, Cohen A, Burnett RT, Jerrett M, Diver WR, Gapstur SM, Krewski D, Samet JM, Pope CA III. 2017. Interactions between cigarette smoking and ambient PM2.5 for cardiovascular mortality. Environmental Research 154:304–310. [DOI] [PubMed] [Google Scholar]
- Tutin CEG, McGrew WC, Baldwin PJ 1981. Responses of wild chimpanzees to potential predators Pages 136–141 in Primate Behavior and Sociobiology, edited by Chiarelli AB and Corruccini RS. Berlin (Germany): Springer. [Google Scholar]
- United States Energy Information Administration. 2011. History of Energy Consumption in the United States, 1775–2009. Washington (DC): U.S. Energy Information Administration; Available at: https://www.eia.gov/todayinenergy/detail.php?id=10. [Google Scholar]
- United States Energy Information Administration. 2016. International Energy Outlook 2016. Washington (DC): U.S. Energy Information Administration; Available at: https://www.eia.gov/outlooks/archive/ieo16/. [Google Scholar]
- United States Environmental Protection Agency. 2019. Report on the Environment: Indoor Air Quality. Washington (DC): U.S. Environmental Protection Agency; Available at: http://www.epa.gov/report-environment/indoor-air-quality. [Google Scholar]
- Urlacher SS, Ellison PT, Sugiyama LS, Pontzer H, Eick G, Liebert MA, Cepon-Robins TJ, Gild-ner TE, Snodgrass JJ 2018. Tradeoffs between immune function and childhood growth among Amazonian forager-horticulturalists. Proceedings of the National Academy of Sciences of the United States of America 115:E3914–E3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagaska B, New SEP, Alvarez-Gonzalez C, D’Acquisto F, Gomez SG, Bulstrode NW, Madrigal A, Ferretti P 2016. MHC-class-II are expressed in a subpopulation of human neural stem cells in vitro in an IFNγ-independent fashion and during development. Scientific Reports 6:24251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kuyl AC 2012. HIV infection and HERV expression: a review. Retrovirology 9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Exel E, Koopman JJE, van Bodegom D, Meij JJ, de Knijff P, Ziem JB, Finch CE, Westendorp RGJ 2017. Effect of APOE e4 allele on survival and fertility in an adverse environment. PLOS ONE 12:e0179497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangenot C, Gagneux P, de Groot NG, Baumeyer A, Mouterde M, Crouau-Roy B, Darlu P, Sanchez-Mazas A, Sabbagh A, Poloni ES 2019. Humans and chimpanzees display opposite patterns of diversity in arylamine N-acetyltransferase genes. G3 9:2199–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki N, Anderson D, Herndon JG, Pham T, Gregg CJ, Cheriyan M, Murphy J, Strobert E, Fritz J, Else JG, Varki A 2009. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evolutionary Applications 2:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastesaeger MM, Delcourt R 1961. Spontaneous atherosclerosis and diet incaptive animals. Nutritio et Dieta 3:174–188. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn S, Crimmins EM, Kim JK, Winking J, Gurven M, Kaplan H, Finch CE 2010. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. American Journal of Human Biology 22:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira SE 2015. The health burden of pollution: the impact of prenatal exposure to air pollutants. International Journal of Chronic Obstructive Pulmonary Disorder 10:1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrba ES, Denton GH, Partridge TC, Burckle LH 1995. Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven (Connecticut): Yale University Press. [Google Scholar]
- Wang D, Zhang D-F, Li G-D, Bi R, Fan Y, Wu Y, Yu X-F, Long H, Li Y-Y, Yao YG 2018. A pleiotropic effect of the APOE gene: association of APOE polymorphisms with multibacillary leprosy in Han Chinese from southwest China. British Journal of Dermatology 178:931–939. [DOI] [PubMed] [Google Scholar]
- Wang H, Iwasaki M, Haiman CA, Kono S, Wilkens LR, Keku TO, Berndt SI, Tsugane S, Le Marchand L 2015. Interaction between red meat intake and NAT2 genotype in increasing the risk of colorectal cancer in Japanese and African Americans. PLOS ONE 10:e0144955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-F, Shu L, Sheng J, Mu M, Wang S, Tao X-Y, Xu S-J, Tao F-B 2014. Birth weight and risk of coronary heart disease in adults: a meta-analysis of prospective cohort studies. Journal of Developmental Origins of Health and Disease 5:408–419. [DOI] [PubMed] [Google Scholar]
- Wang X, Zou X 2018. Threshold dynamics of a temperature-dependent stage-structured mosquito population model with nested delays. Bulletin of Mathematical Biology 80:1962–1987. [DOI] [PubMed] [Google Scholar]
- Wang X, Chow R, Deng L, Anderson D, Weidner N, Godwin AK, Bewtra C, Zlotnik A, Bui J, Varki A, Varki N 2011. Expression of Siglec-11 by human and chimpanzee ovarian stromal cells, with uniquely human ligands: implications for human ovarian physiology and pathology. Glycobiology 21:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mitra N, Cruz P, Deng L, NISC Comparative Sequencing Program, Varki N, Angata T, Green ED, Mullikin J, Hayakawa T, Varki A 2012. Evolution of Siglec-11 and Siglec-16 genes in hominins. Molecular Biology and Evolution 39:2073–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterlow JC 1984. Protein turnover with special reference to man. Quarterly Journal of Experimental Physiology 69:409–438. [DOI] [PubMed] [Google Scholar]
- Webb S 2009. Palaeopathology of Aboriginal Australians: Health and Disease Across a Hunter-Gatherer Continent. Cambridge (United Kingdom): Cambridge University Press. [Google Scholar]
- Wessling EG 2011. Rank-related differences in the travel patterns of savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal. Master’s thesis, Iowa State University. [Google Scholar]
- Wessling EG, Kühl HS, Mundry R, Deschner T, Pruetz JD 2018. The costs of living at the edge: seasonal stress in wild savanna-dwelling chimpanzees. Journal of Human Evolution 121:1–11. [DOI] [PubMed] [Google Scholar]
- White TD, Ambrose SH, Suwa G, et al. 2009. Macro-vertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science 326:67–93. [PubMed] [Google Scholar]
- Wiklund P, Karhunen V, Richmond RC, Parmar P, Rodriguez A, De Silva M, Wielscher M, Rezwan FI, Richardson TG, Veijola J, Herzig K-H, Holloway JW, Relton CL, Sebert S, Järvelin M-R 2019. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clinical Epigenetics 11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP 2005. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiology, Biomarkers & Prevention 14:1847–1850. [DOI] [PubMed] [Google Scholar]
- Wild CP 2012. The exposome: from concept to utility. International Journal of Epidemiology 41:24–32. [DOI] [PubMed] [Google Scholar]
- Wolters FJ, Yang Q, Biggs ML, Jakobsdottir J, Li S, Evans DS, Bis JC, Harris TB, Vasan RS, Zihao NR, Ghanbari M, Ikram A, Launer L, Psaty BM, Tranan GJ, Kulminski AM, Gudnason V, Seshadri S, for the E2-CHARGE Investigators. 2019. The impact of APOE genotype on survival: results of 38,537 participants from six population-based cohorts (E2-CHARGE). PLOS ONE 14:e0219668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Lin K, Wang D-H, Moustafa AA, Cohen JY, Nakamura K 2017. Toward a multiscale modeling framework for understanding serotonergic function. Journal of Psychopharmacology 31:1121–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B, Lonergan N 2008. The hominin fossil record: taxa, grades and clades. Journal of Anatomy 212:354–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood BM, Watts DP, Mitani JC, Langergraber KE 2017. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. Journal of Human Evolution 105:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JW 1994. Dynamics of Human Reproduction: Biology, Biometry, Demography. New York: Aldine de Gruyter. [Google Scholar]
- Woodward NC, Levine MC, Haghani A, Shirmohammadi F, Saffari A, Sioutas C, Morgan TE, Finch CE 2017. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. Journal of Neuroinflammation 14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woringer M, Martiny N, Porgho S, Bicaba BW, Bar-Hen A, Mueller JE 2018. Atmospheric dust, early cases, and localized meningitis epidemics in the African meningitis belt: an analysis using high spatial resolution data. Environmental Health Perspectives 126:097002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2018. Burden of Disease From Household Air Pollution For 2016. Geneva (Switzerland): World Health Organization; Available at: https://www.who.int/airpollution/data/HAP_BoD_results_May2018_final.pdf. [Google Scholar]
- Wrangham RW 1995. Relationship of chimpanzee leaf-swallowing to a tapeworm infection. American Journal of Primatology 37:297–303. [DOI] [PubMed] [Google Scholar]
- Wrangham R, Carmody R 2010. Human adaptation to the control of fire. Evolutionary Anthropology: Issues, News, and Reviews 19:187–199. [Google Scholar]
- Wrigley EA 2013. Energy and the English Industrial Revolution. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 371:20110568. [DOI] [PubMed] [Google Scholar]
- Wu X, Lu X, Schneider E, Ahmed JA, Njenga MK, Breiman RF, Eidex R, Erdman DD 2019. Reassessment of high prevalence human adenovirus detections among residents of two refugee centers in Kenya under surveillance for acute respiratory infections. Journal of Medical Virology 91:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P-L, Zhou Y-B, Chen Y, Yang M-X, Song X-X, Shi Y, Jiang Q-W 2015. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy and Childbirth 15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahya RZ, Arrieta JM, Cusack M, Duarte CM 2019. Airborne prokaryote and virus abundance over the Red Sea. Frontiers in Microbiology 10:1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Qian X, Wang N, Ding Y, Li H, Zhao Y, Yao S 2019. Inhibition of MARCO ameliorates silica-induced pulmonary fibrosis by regulating epithelial-mesenchymal transition. Toxicology Letters 301: 64–72. [DOI] [PubMed] [Google Scholar]
- Yao B, Xie J, Liu N, Hu T, Song W, Huang S, Fu X 2019. Direct reprogramming of epidermal cells toward sweat gland-like cells by defined factors. Cell Death & Disease 10:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn CT, Jiang Z, McGrath SD, Hayden KE, Khaitovich P, Johnson ME, Eichler MY, McPherson JD, Zhao S, Pääbo S, Eichler EE 2005. Lineage-specific expansions of retroviral insertions within the genomes of African great apes but not humans and orangutans. PLOS Biology 3: e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama Y, Jelenkovic A, Hur Y-M, et al. 2018. Genetic and environmental factors affecting birth size variation: a pooled individual-based analysis of secular trends and global geographical differences using 26 twin cohorts. International Journal of Epidemiology 47:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, He S, Tang M, Zhang Z, Zhu Y, Sun H 2018. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chemistry 243:249–257. [DOI] [PubMed] [Google Scholar]
- Zarulli V, Barthold Jones JA, Oksuzyan A, Lindahl-Jacobsen R, Christensen K, Vaupel JW 2018. Women live longer than men even during severe famines and epidemics. Proceedings of the National Academy of Sciences of the United States of America 115: E832–E840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen X, Zhang X 2018. The impact of exposure to air pollution on cognitive performance. Proceedings of the National Academy of Sciences of the United States of America 115:9193–9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ramstein G, Schuster M, Li C, Contoux C, Yan Q 2014. Aridification of the Sahara desert caused by Tethys Sea shrinkage during the Late Miocene. Nature 513:401–404. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ma Z, Dong D, Wu B 2013. Arylamine N-acetyltransferases: a structural perspective. British Journal of Pharmacology 169:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbühler K, Jenny D 2002. Leopard predation and primate evolution. Journal of Human Evolution 43: 873–886. [DOI] [PubMed] [Google Scholar]
- Zuckerman MK, Armelagos GJ 2014. The hygiene hypothesis and the second epidemiologic transition Pages 301–320 in Modern Environments and Human Health: Revisiting the Second Epidemiologic Transition, edited by Zuckerman MK. Hoboken (New Jersey): Wiley-Blackwell. [Google Scholar]


