Abstract
Background
Coronavirus disease 2019 (COVID‐19) is a rapidly emerging disease that has been classified a pandemic by the World Health Organization (WHO). To support WHO with their recommendations on quarantine, we conducted a rapid review on the effectiveness of quarantine during severe coronavirus outbreaks.
Objectives
We conducted a rapid review to assess the effects of quarantine (alone or in combination with other measures) of individuals who had contact with confirmed cases of COVID‐19, who travelled from countries with a declared outbreak, or who live in regions with high transmission of the disease.
Search methods
An information specialist searched PubMed, Ovid MEDLINE, WHO Global Index Medicus, Embase, and CINAHL on 12 February 2020 and updated the search on 12 March 2020. WHO provided records from daily searches in Chinese databases up to 16 March 2020.
Selection criteria
Cohort studies, case‐control‐studies, case series, time series, interrupted time series, and mathematical modelling studies that assessed the effect of any type of quarantine to control COVID‐19. We also included studies on SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) as indirect evidence for the current coronavirus outbreak.
Data collection and analysis
Two review authors independently screened 30% of records; a single review author screened the remaining 70%. Two review authors screened all potentially relevant full‐text publications independently. One review author extracted data and assessed evidence quality with GRADE and a second review author checked the assessment. We rated the certainty of evidence for the four primary outcomes: incidence, onward transmission, mortality, and resource use.
Main results
We included 29 studies; 10 modelling studies on COVID‐19, four observational studies and 15 modelling studies on SARS and MERS. Because of the diverse methods of measurement and analysis across the outcomes of interest, we could not conduct a meta‐analysis and conducted a narrative synthesis. Due to the type of evidence found for this review, GRADE rates the certainty of the evidence as low to very low.
Modeling studies consistently reported a benefit of the simulated quarantine measures, for example, quarantine of people exposed to confirmed or suspected cases averted 44% to 81% incident cases and 31% to 63% of deaths compared to no measures based on different scenarios (incident cases: 4 modelling studies on COVID‐19, SARS; mortality: 2 modelling studies on COVID‐19, SARS, low‐certainty evidence). Very low‐certainty evidence suggests that the earlier quarantine measures are implemented, the greater the cost savings (2 modelling studies on SARS). Very low‐certainty evidence indicated that the effect of quarantine of travellers from a country with a declared outbreak on reducing incidence and deaths was small (2 modelling studies on SARS). When the models combined quarantine with other prevention and control measures, including school closures, travel restrictions and social distancing, the models demonstrated a larger effect on the reduction of new cases, transmissions and deaths than individual measures alone (incident cases: 4 modelling studies on COVID‐19; onward transmission: 2 modelling studies on COVID‐19; mortality: 2 modelling studies on COVID‐19; low‐certainty evidence). Studies on SARS and MERS were consistent with findings from the studies on COVID‐19.
Authors' conclusions
Current evidence for COVID‐19 is limited to modelling studies that make parameter assumptions based on the current, fragmented knowledge. Findings consistently indicate that quarantine is important in reducing incidence and mortality during the COVID‐19 pandemic. Early implementation of quarantine and combining quarantine with other public health measures is important to ensure effectiveness. In order to maintain the best possible balance of measures, decision makers must constantly monitor the outbreak situation and the impact of the measures implemented. Testing in representative samples in different settings could help assess the true prevalence of infection, and would reduce uncertainty of modelling assumptions.
This review was commissioned by WHO and supported by Danube‐University‐Krems.
Plain language summary
Does quarantine control coronavirus (COVID‐2019) either alone or in combination with other public health measures?
Background Coronavirus disease 2019 (COVID‐19) is caused by a new virus that has spread quickly throughout the world. COVID‐19 spreads easily between people who are in close contact, or through coughs and sneezes. Most infected people suffer mild, flu‐like symptoms but some become seriously ill and even die. There is no effective treatment or vaccine (a medicine that stops people catching a specific disease) for COVID‐19, so other ways of slowing (controlling) its spread are needed. One of the World Health Organization’s (WHO) recommendations for controlling the disease is quarantine. This means separating healthy people from other healthy people, in case they have the virus and could spread it. Other similar recommendations include isolation (like quarantine, but for people with COVID‐19 symptoms) and social distancing (where people without symptoms keep a distance from each other physically). What did we want to find out? We wanted to find out whether and how effectively quarantine stops COVID‐19 spreading and if it prevents death. We wanted to know if it was more effective when combined with other measures, such as closing schools. We also wanted to know what it costs. Study characteristics COVID‐19 is spreading rapidly, so we needed to answer this question as quickly as possible. This meant we shortened some steps of the normal Cochrane Review process. Nevertheless, we are confident that these changes do not affect our overall conclusions. We looked for studies that assessed the effect of any type of quarantine, anywhere, on the spread and severity of COVID‐19. We also looked for studies that assessed quarantine alongside other measures, such as isolation, social distancing, school closures and hand hygiene. COVID‐19 is a new disease, so, to find as much evidence as possible, we also looked for studies on similar viruses, such as SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). Studies measured the number of COVID‐19, SARS or MERS cases, how many people were infected, how quickly the virus spread, how many people died, and the costs of quarantine. Key results We included 29 studies. Ten studies focused on COVID‐19, 15 on SARS, two on SARS plus other viruses, and two on MERS. Most of the studies combined existing data to create a model (a simulation) for predicting how events might occur over time, for people in different situations (called modelling studies). The COVID‐19 studies simulated outbreaks in China, UK, South Korea, and on the cruise ship Diamond Princess. Four studies looked back on the effect of quarantine on 178,122 people involved in SARS and MERS outbreaks (called ‘cohort’ studies). The remaining studies modelled SARS and MERS outbreaks. The modelling studies all found that simulated quarantine measures reduce the number of people with the disease and the number of deaths. With quarantine, estimates showed a minimum reduction in the number of people with the disease of 44%, and a maximum reduction of 81%. Similarly, with quarantine, estimates of the number of deaths showed a minimum reduction of 31%, and a maximum reduction of 63%. Combining quarantine with other measures, such as closing schools or social distancing, is more effective at reducing the spread of COVID‐19 than quarantine alone. The SARS and MERS studies agreed with the studies on COVID‐19.
Two SARS modelling studies assessed costs. They found that the costs were lower when quarantine measures started earlier.
We cannot be completely certain about the evidence we found for several reasons. The COVID‐19 studies based their models on limited data and made different assumptions about the virus (e.g. how quickly it would spread). The other studies investigated SARS and MERS so we could not assume the results would be the same for COVID‐19. Conclusion
Despite limited evidence, all the studies found quarantine to be important in reducing the number of people infected and the number of deaths. Results showed that quarantine was most effective, and cost less, when it was started earlier. Combining quarantine with other prevention and control measures had a greater effect than quarantine alone. This review includes evidence published up to 12 March 2020.
Background
Coronavirus disease 2019 (COVID‐19) is a new, rapidly emerging zoonotic infectious disease (WHO 2020a). The first case was reported from Wuhan (Hubei province, China) on 31 December 2019. On 30 January 2020 the World Health Organization (WHO) declared the outbreak a global health emergency, on 11 March 2020, a pandemic (WHO 2020b).
COVID‐19 is caused by a novel coronavirus, SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus‐2), which is transmitted via droplets during close unprotected contact with an infector and fomites (WHO 2020a). Healthcare setting transmissions play an important role in the spread of the disease (del Rio 2020). The virus is genetically similar to the coronaviruses that caused severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), but SARS‐CoV‐2 appears to have greater transmissibility and lower pathogenicity than the aforementioned viruses. Preliminary estimates of the basic reproduction number (R0) of SARS‐CoV‐2, as a metric for transmissibility, range from 2.8 to 5.5, in the absence of intense quarantine and social distancing measures (Read 2020). In comparison, R0 for SARS was estimated at 3.0 (Bauch 2005), and at less than 1.0 for MERS in most regions (Park 2018). The average reproduction number for seasonal influenza viruses is about 1.8 (Biggerstaff 2014).
The pathogenicity of SARS‐CoV‐2 appears to be substantially lower than that of SARS and MERS. The majority (81%) of symptomatic COVID‐19 patients develop a mild form of the disease with dry cough, fever, or unspecific symptoms such as headache, myalgias, or fatigue. More severe cases suffer from dyspnoea and pneumonia, and about 5.0% to 6.0% of COVID‐19 patients are critically ill with respiratory failure, sepsis, or multi‐organ failure (WHO 2020a; Wu 2020a). The case‐fatality rate for COVID‐19 was high at the beginning of the outbreak in Wuhan but has declined over time to 0.7% for patients with symptom onset after 1 February 2020 (WHO 2020a), which is substantially lower than case‐fatality rate for SARS (9.6%; WHO 2020c), and MERS (34.4%; WHO 2020d), but higher than that for seasonal influenza pandemics (0.01%; Taubenberger 2006). The case‐fatality rate, especially at the beginning of an outbreak, has to be interpreted with caution since the denominator (number of infected people) is often not yet well known. In addition case‐fatality rates differ by location, time, and specific demographics like age or pre‐existing health conditions. Data, based on confirmed COVID‐19 cases in Mainland China from 11 February 2020 showed that while the case‐fatality rate for people aged 40 to 49 years was 0.4 it was 8.0 for those aged 70 to 79 years, and 14.8 for infected people of 80 years or older (China CDC 2020).
Currently, no effective pharmacological interventions or vaccines are available to treat or prevent COVID‐19. For this reason, nonpharmacological public health measures such as isolation, social distancing, and quarantine are the only effective ways to respond to the outbreak. Isolation refers to the separation of symptomatic patients whereas quarantine is the restriction of asymptomatic healthy people who have had contact with confirmed or suspected cases. Quarantine can be implemented on a voluntary basis or can be legally enforced by authorities and may be applied at an individual, group, or community level (community containment (Cetron 2005)). A recent rapid review reported that quarantine can have negative psychological effects such as post‐traumatic stress symptoms, confusion and anger, which can lead to adverse long‐term psychological effects (Brooks 2020). At this time, WHO and the US Center for Disease Control and Prevention (CDC) recommend 14 days of quarantine for individuals who were in close contact with a confirmed case, based on the estimated incubation period of SARS‐CoV‐2 (Jernigan 2020; WHO 2020e).
According to the International Health Regulations 2005 (WHO 2005), that govern the management of disease outbreaks in 196 countries, any public health measures must be based on scientific evidence and recommendations from WHO (Habibi 2020). At the beginning of February 2020, WHO requested the review authors to conduct a rapid review on the effectiveness of quarantine during serious coronavirus outbreaks to support recommendations on quarantine. We updated the rapid review in March 2020.
Objectives
To support WHO for their recommendations on quarantine, we conducted a rapid review on the effectiveness of quarantine during serious coronavirus outbreaks. We aimed to answer the following key questions (KQs). Figure 1 depicts the analytic framework.
1.
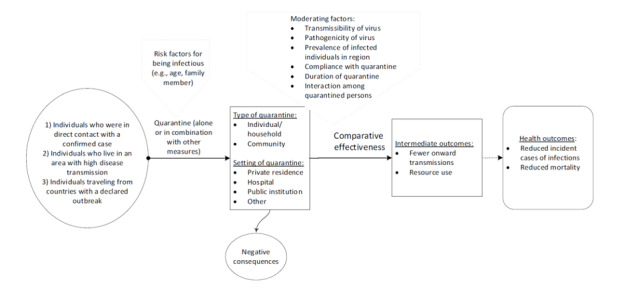
Analytic framework
-
KQ 1: is quarantine of asymptomatic individuals who were in contact with a confirmed or suspected case of COVID‐19, effective to control the COVID‐19 outbreak?
KQ1a: are there differences in the effectiveness of quarantine in different settings?
KQ1b: how effective is quarantine when combined with other interventions such as case isolation or school closures in reducing transmission, incidence of diseases, and mortality?
-
KQ 2: is quarantine of individuals coming from a country with a declared COVID‐19 outbreak, effective in controlling the COVID‐19 outbreak?
KQ2a: are there differences in the effectiveness of quarantine in different settings?
Methods
To conduct this rapid review, we employed abbreviated systematic review methods. Compared with the methods of a systematic review, the review team applied the following methodological shortcuts for this rapid review:
no specific searches of gray literature;
we dually screened only 30% of abstracts;
no dual independent 'Risk of bias' assessment and rating of the certainty of evidence; one review author conducted the ratings, a second review author checked the plausibility and correctness. We adhered to PRISMA throughout this manuscript (Moher 2009).
Criteria for considering studies for this review
The WHO expert panel selected four outcomes that they deemed relevant for their decision‐making process: incident cases, onward transmission, mortality, and resource use (see Table 1).
1. Inclusion and exclusion criteria of the rapid review.
| Inclusion | Exclusion | |
| Population |
KQ1
KQ2
|
|
| Intervention |
KQ1 and KQ2 Different types and locations of quarantinesb of individuals
KQ1b
|
|
| Control |
|
|
| Outcomes |
|
|
| Study designs |
|
|
| Languages |
|
|
| COVID‐19: coronavirus disease 2019; KQ: key question; MERS: Middle East respiratory syndrome; SARS: severe acute respiratory syndrome; WHO: World Health Organization | ||
aDefined by WHO as an "occurrence of disease cases in excess of normal expectancy. The number of cases varies according to the disease‐causing agent, and the size and type of previous and existing exposure to the agent." (WHO 2020f). bWe included studies combining isolation with quarantine because isolation of confirmed cases is a prerequisite for quarantine of individuals who were in contact with these cases
Types of studies
As randomization of quarantine is unethical and not feasible for the diseases in question, we considered non‐randomized studies of interventions to be the best potentially available empirical evidence. In addition, we also included modelling studies, because, especially for COVID‐19, we did not yet expect empirical studies to be available.
Cohort studies
Case‐control studies
Time series
Interrupted time series
Case series
Mathematical modelling studies
We excluded:
case reports
systematic reviews (used for reference list checking)
Language
English
Chinese (English‐language abstracts or, if available, English summaries provided by a Chinese WHO Collaborating Centre)
We did not include studies in other languages.
Types of participants
We included:
(KQ1) contacts of a confirmed or suspected case of COVID‐19 (SARS or MERS) or individuals who live in areas with high transmission rates;
(KQ2) individuals returning from countries with a declared outbreak of COVID‐19 (SARS or MERS), defined by WHO as an "occurrence of disease cases in excess of normal expectancy. The number of cases varies according to the disease‐causing agent, and the size and type of previous and existing exposure to the agent." (WHO 2020f).
We excluded:
symptomatic individuals of COVID‐19 (SARS or MERS) infections;
asymptomatic individuals exposed to other pathogens that can cause respiratory infections.
Types of interventions
Different types and locations of quarantines of individuals. We included studies combining isolation with quarantine because isolation of confirmed cases is a prerequisite for quarantine of individuals who were in contact with these cases.
(KQ1 and KQ2)
Voluntary quarantine (self‐quarantine)
Mandatory quarantine
-
Quarantine in
private residence
hospital
public institution
others (cruise ships, etc)
-
(KQ1b) Quarantine of individuals or a community in combination with other measures:
avoiding crowding
hand hygiene
isolation
personal protective equipment
school measures/closures
social distancing
workplace measures/closures
Control measures included the following.
No quarantine
Different types and locations of quarantines
Public health measures without quarantine to reduce the spread of the virus such as isolation, social distancing, personal protective equipment, hand hygiene, others
We excluded environmental measures and travel‐related measures (e.g. travel bans) as either an intervention or control measure.
Types of outcome measures
Primary outcomes
Incident cases (as reported by authors ‐ clinical diagnosis and/or laboratory confirmation)
Onward transmission
Mortality
Resource use (direct and/or indirect costs, cost effectiveness)
We focused on time points that studies reported for primary outcomes but also included time points that facilitated comparisons of effects across studies.
This rapid review did not examine the psychological impact of quarantine.
Search methods for identification of studies
An experienced information specialist conducted a systematic search of the literature published in English or German from 1 January 2002 to 12 March 2020 in Ovid MEDLINE, Embase (Elsevier), CINAHL (Cumulative Index to Nursing and Allied Health Literature; Ebsco) and the WHO Global Index Medicus. Appendix 1 presents the detailed search strategies.
In addition, WHO provided a list of citations and abstracts from articles published in Chinese‐language journals that was prepared by the WHO Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China. They conducted manual searches of selected international journals as well as searches of the following bibliographic databases: CNKI (China National Knowledge Infrastructure), Embase.com (Elsevier), PubMed.
For articles published in Chinese only, the Collaborating Centre prepared an English translation of the abstracts. If no abstract was available, the Collaborating Centre provided a ‘Brief Summary’ in English. This list was updated on a daily basis. The last date of the search considered for this review was 16 March 2020.
One team member of the WHO Collaborating Centre in Austria searched a WHO database (WHO 2020g), containing results of daily literature searches on COVID‐19 (up to 16 March 2020).
In addition, review authors screened reference lists of systematic reviews on quarantine in general, and included studies for additional relevant citations.
Data collection and analysis
Selection of studies
A team of experienced review authors screened all titles and abstracts based on predefined inclusion and exclusion criteria (Table 1). Two review authors independently screened the first 30% of records. In cases of disagreement about eligibility, the two review authors reached consensus by discussion or by involving a senior review author. A single review author screened the remaining 70% of titles and abstracts. The results of the updated and extended search from 12 March were screened dually.
The review author team retrieved the full texts of all included abstracts. Two review authors screened all full‐text publications independently. We resolved disagreements by consensus or by involving a third, senior review author. The team conducted literature screening using Covidence. One person checked the list of Chinese studies provided by WHO, and another person verified the decisions. WHO retrieved potentially relevant abstracts in full text and WHO collaborators from China translated them.
Data extraction and management
One experienced review author extracted data from the included studies into standardized tables; a second review author checked the data extraction for completeness and correctness. The data items for cohort studies included: author, publication year, country, study design, objective, characteristics of the study participants, description of the intervention, co‐interventions and comparison, important confounding factors, and results. For the modelling studies, the data items were: author, year, type of model, setting, time, data source and participants, interventions, and results. As different classifications for model types exist, we listed the model type as described by the study authors.
Assessment of risk of bias in included studies
The review author team assessed the risk of bias of the included cohort studies based on the Risk Of Bias In Non‐randomized Studies ‐ of Interventions (ROBINS‐I) tool (Sterne 2016). A single review author rated the risk of bias for each study; a second review author checked the ratings. As important confounding factors we considered co‐morbidities, health status, socio‐economic background, age, and sex. The risk of bias could be rated as low, moderate, serious, or critical. Due to time constraints, we omitted an independent, dual 'Risk of bias' assessment. As no validated 'Risk of bias' checklist for mathematical transmission models was available, we assessed whether the modelling and reporting followed the best practice recommendations of the International Society for Pharmacoeconomics and Outcomes (ISPOR) and the Society for Medical Decision making (SMDM) for dynamic mathematical transmission models. Dynamic transmission models allow for risk‐changes over time and can estimate direct and indirect effects of prevention and control measures on an infectious disease (Pitman 2012). We assessed whether the model was dynamic, whether the study authors conducted uncertainty analyses on key model parameters and assumptions, and whether the results provided estimates of the change in the burden of infection due to the intervention. We selected these three criteria because they best reflected methodological decisions that have an impact on results and conclusions. For modelling studies fulfilling all three criteria we had ‘no concerns to minor concerns’ concerning their quality; if one or more categories were unclear (e.g. because of incomplete reporting) we had ‘moderate concerns’, if one or more categories were not fulfilled we had ‘major concerns’. For the criterion 'estimates for burden of disease', we used the rating 'unclear' and also in cases where effects on the reproduction number or cases were reported, which is typical for mathematical modelling, but would not suffice for decision‐analytic modelling, where we expect outcomes such as mortality, or unintended harms. Two review authors rated the quality of modelling studies, a senior review author checked the ratings.
Data synthesis
We synthesized results narratively and in tabular form. Because of the heterogeneity of available primary studies, we did not consider quantitative analyses.
Assessment of the certainty of the evidence
We assessed the certainty of evidence for the four main outcomes. We have reported other patient‐relevant outcomes in the Results section, but we did not grade the certainty of evidence. One experienced review author assigned certainty of evidence ratings based on an approach developed by the GRADE Working Group (Guyatt 2008; Schünemann 2013; Schunemann 2019). For grading the certainty of evidence of modelling studies we followed the recent guidance from the GRADE Working Group (Brozek 2020). Modeling studies start at high‐certainty evidence and are downgraded according to assessments of risk of bias, indirectness, inconsistency, imprecision, and publication bias. GRADE uses four categories to classify the certainty of evidence. A high certainty rating of a body of evidence means that we were very confident that the estimated effect lies close to the true effect; moderate certainty means we assume the estimated effect is probably close to the true effect; a low certainty rating suggests that the estimated effect might substantially differ from the true effect; and very low certainty means that the estimated effect is probably markedly different from the true effect.
Table 2 summarizes the certainty of evidence for KQ1; Table 3 summarizes the certainty of evidence for KQ1b; and Table 4 presents the certainty of evidence ratings for KQ2.
2. Certainty of evidence ratings for the effectiveness of quarantine for individuals who were in contact with a confirmed COVID‐19 case.
| Outcome | Number of studies | Risk of bias | Indirectness | Imprecision | Inconsistency | Other considerations | Summary effect size/outcome | Certainty of the evidence |
| Incidence | 4 modelling studies (Cao 2020; Hsieh 2007; Rocklov 2020; Tang 2020) |
Very seriousa | Direct | Precise | Consistent | None |
COVID‐19
Cao 2020 simulated the effect of loosening quarantine measures that are already in place. They concluded that if 40% fewer people were quarantined (e.g. because of less strict follow‐ups of contacts), the peak number of cases would increase twofold compared to keeping a full quarantine in place. Rocklov 2020 estimated that isolation and quarantine prevented 2307 (67%) cases and lowered the reproduction number to 1.78 during the COVID‐19 outbreak on the Diamond Princess cruise ship. Tang 2020 estimated that without any measures, the number of confirmed COVID‐19 cases in Wuhan would be 7723 by the end of January 2020. They estimated that reduced contact by 50% could decrease the number of confirmed COVID‐19 cases from 7723 to 4335 (44% reduction); reduced contact by 90% to 2731 (65% reduction). SARS Hsieh 2007 state that quarantine is effective to reduce incident cases (461 SARS cases (81%) averted, with a low quarantine rate of 0.047 that equals quarantining 1 out of 21 people that should be quarantined) |
Low |
| Onward transmission | No evidence | |||||||
| Mortality | 2 modelling studies (Ferguson 2020; Hsieh 2007) | Very seriousa | Direct | Precise | Consistent | None | COVID‐19 Ferguson 2020 estimated that for a timeframe of 3 months, case isolation and household quarantine would decrease deaths in the UK by 31%–34%. SARS Effective to reduce mortality (62 SARS (63%) deaths averted, with a low quarantine rate of 0.047 in Taiwan; Hsieh 2007) | Low |
| Resource use | 2 modelling studies (Gupta 2005; Mubayi 2010) | Very seriousa | Indirectb | Precise | Consistent | None | SARS Gupta 2005 stated that at a transmission rate of 8%, the total savings of quarantine over isolation alone varies between CAD 279–232 million (reference year 2003). The earlier that effective quarantine measures are implemented, the greater the savings are. Mubayi 2010 came to similar conclusions and state that increasing the quarantine effort results in lower overall costs over the entire outbreak in all 3 assessed quarantine strategies. | Very low |
| COVID‐19: coronavirus disease 2019; SARS: severe acute respiratory syndrome | ||||||||
aDowngraded two steps for risk of bias because we had moderate to minor concerns regarding quality and model parameters are accompanied by uncertainties. bDowngraded one step for indirectness because studies were on SARS.
3. Certainty of evidence ratings for the effectiveness of quarantine in combination with other measures to contain COVID‐19.
| Outcome | Number of studies | Risk of bias | Indirectness | Imprecision | Inconsistency | Other considerations | Summary effect size/outcome | Certainty of the evidence |
| Incidence | 4 modelling studies (Choi 2020; Ferguson 2020; Wu 2020b; Zhao 2020a) | Very seriousa | Direct | Precise | Consistent | None |
COVID‐19
Choi 2020 stated that by reducing the transmission rate by 90% or 99% the proportion of COVID‐19 cases would only be 0.05% or 0.04% of the 5 million cases predicted for South Korea without any measures taken Ferguson 2020 stated: "Reduction of cases that require critical care beds compared with unmitigated COVID‐19 epidemic:b Case isolation + home quarantine + social distancing of those over 70 years of age: 67%" Wu 2020b stated that stronger control measures are more effective. By reducing the contact rate and infection efficiency by > 50% they predicted 3088 COVID‐19 cases within 3 months in Wuhan. By reducing it only by < 45% they predicted 4719 cases. Zhao 2020a predicted more than 800 million COVID‐19 cases for China (without Hubei) without the implementation of any measures and an epidemic duration of 477 days. With prevention and control measures (e.g. isolation, quarantine, travel restrictions) the number of cases could be only 13,322 and the duration could be only 45 days |
Low |
| Onward transmission | 2 modelling studies (Fang 2020; Geng 2020) | Very seriousa | Direct | Precise | Consistent | None |
COVID‐19
Fang 2020 stated that implementing a combination of containment measures including quarantine, school closures, travel restrictions, cancellation of mass gatherings, and strict exit screening reduced R0 from 2.9 to 2.3 starting at 2 weeks after implementation. Geng 2020 stated that quarantine and school closures in Wuhan reduced the peak of transmissions by 45.7% and 29.9% |
Low |
| Mortality | 2 modelling studies(Ferguson 2020; Wu 2020b) | Very seriousa | Direct | Precise | Consistent | None | COVID‐19 "Reduction of deaths compared with unmitigated COVID‐19 epidemic:b Case isolation + home quarantine + social distancing of those over 70 years of age: 49%" (Ferguson 2020) Wu 2020b stated that stronger control measures reduce mortality of COVID‐19. By reducing the contact rate and infection efficiency by > 50% they predicted 443 deaths out of 11.5 million inhabitants in Wuhan within 3 months, by reducing it only to < 45% they predicted 739 deaths. | Low |
| Resource use | No evidence | |||||||
| COVID‐19: coronavirus disease 2019 | ||||||||
aDowngraded two steps for risk of bias because we had moderate to minor concerns regarding quality and model parameters are accompanied by uncertainties. bNumbers based on unpublished manuscript, pre‐peer review; numbers of other combination strategies do not seem plausible (potential mislabeling of table); we contacted study authors but did not receive a response.
4. Certainty of evidence ratings for the effectiveness of quarantine for travelers from regions with high transmission rates.
| Outcome | Number of studies | Risk of bias | Indirectness | Imprecision | Inconsistency | Other considerations | Summary effect size/outcome | Certainty of the evidence |
| Modeling studies | ||||||||
| Incidence | 2 modelling studies (Hsieh 2007; Yip 2007) | Very seriousa | Indirectb | Imprecisec | Inconsistentd | None | Small reduction of incidence | Very low |
| Onward transmission | No evidence | |||||||
| Mortality | 2 modelling studies (Hsieh 2007; Yip 2007) | Very seriousa | Indirectb | N/A | Inconsistentd | None | Small reduction of mortality | Very low |
| Resource use | No evidence | |||||||
| COVID‐19: coronavirus disease 2019; N/A: not applicable | ||||||||
aDowngraded two steps for risk of bias because we had moderate to minor concerns regarding quality and model parameters are accompanied by uncertainties. bDowngraded one step for indirectness because the studies used severe acute respiratory syndrome (SARS) data, which does not reflect the presymptomatic infectiousness of coronavirus disease 2019 (COVID‐19). cDowngraded one step because only two cases of SARS out of more than 95,000 quarantined. dDowngraded one step because a retrospective study (Hsieh 2005), not specifically reporting incidence of new cases but number of quarantined travellers who developed SARS, reported 0 SARS cases within more than 95,000 quarantined travellers. This differs slightly from the data used by Hsieh 2007: 2 SARS cases out of more than 95,000 quarantined travellers.
Results
Description of studies
Our searches identified 29 relevant studies (Becker 2005; Cao 2020; Chau 2003; Choi 2020; Day 2006; Fang 2020; Ferguson 2020; Fraser 2004; Geng 2020; Gumel 2004; Gupta 2005; Hsieh 2005; Hsieh 2007; Lloyd‐Smith 2003; Mubayi 2010; Nishiura 2004; Pang 2003; Park 2020; Peak 2017; Pourbohloul 2005; Rocklov 2020; Tang 2020; Wang 2004; Wang 2007; Wu 2020b; Yip 2007; Yue 2020; Zhang 2017; Zhao 2020a). Of these, 10 focused on COVID‐19 (Cao 2020; Choi 2020; Fang 2020; Ferguson 2020; Geng 2020; Rocklov 2020; Tang 2020; Yue 2020; Wu 2020b; Zhao 2020), 15 focused on SARS (Becker 2005; Chau 2003; Day 2006; Fraser 2004; Gumel 2004; Gupta 2005; Hsieh 2005; Hsieh 2007; Lloyd‐Smith 2003; Mubayi 2010; Nishiura 2004; Pang 2003; Wang 2004; Wang 2007; Yip 2007), two focused on SARS and other infectious diseases caused by other viruses (e.g. influenza) (Peak 2017; Pourbohloul 2005), and two focused on MERS (Park 2020; Zhang 2017).
The 10 studies addressing COVID‐19 were all modelling studies simulating outbreak scenarios for China, UK, South Korea, and the cruise ship Diamond Princess (Cao 2020; Choi 2020; Fang 2020; Ferguson 2020; Geng 2020; Rocklov 2020; Tang 2020; Yue 2020; Wu 2020b; Zhao 2020a). From the studies focusing on SARS or MERS, four were cohort studies from China, South Korea, and Taiwan that included data on 178,122 individuals (Hsieh 2005; Pang 2003; Park 2020; Wang 2007). The other 15 studies on SARS or MERS were modelling studies using data from outbreaks in Canada, China, Hong Kong, Japan, South Korea, Singapore, and Taiwan (Becker 2005; Chau 2003; Day 2006; Fraser 2004; Gumel 2004; Gupta 2005; Hsieh 2007; Lloyd‐Smith 2003; Mubayi 2010; Nishiura 2004; Peak 2017; Pourbohloul 2005; Wang 2004; Yip 2007; Zhang 2017).
Of the non‐randomized studies of interventions, we rated three as having a moderate risk of bias (Pang 2003;Wang 2007;Hsieh 2005), and one as having serious risk of bias (Park 2020). Regarding quality for eight of the modelling studies we had no concerns to minor concerns (Day 2006; Ferguson 2020; Gumel 2004; Mubayi 2010; Nishiura 2004; Rocklov 2020; Tang 2020; Zhang 2017), for 10 modelling studies we had moderate concerns (Becker 2005; Cao 2020; Fang 2020; Fraser 2004; Gupta 2005; Hsieh 2007; Lloyd‐Smith 2003; Peak 2017; Pourbohloul 2005; Zhao 2020a), and for seven modelling studies we had major concerns (Chau 2003; Choi 2020; Geng 2020; Wang 2004; Wu 2020b; Yip 2007; Yue 2020).
The PRISMA flow diagram in Figure 2 provides an overview of the study selection process; the characteristics of the included observational and modelling studies are in Characteristics of included studies tables. Table 5 presents the results of each individual study.
2.
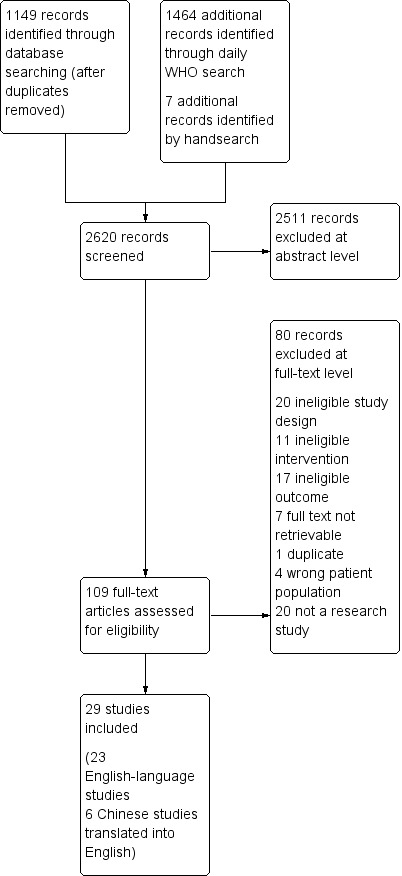
Study flow diagram
5. Results reported in individual studies.
| Study | Study design | Results |
| Hsieh 2005 | Retrospective cohort study | Level A (quarantine of close contacts): out of 55,632 quarantined individuals, 24 confirmed SARS cases Level B (quarantine of travellers): out of 95,828 quarantined individuals, 0 confirmed SARS cases Onset‐to‐diagnosis: significantly shorter in quarantined individuals (1.20 vs 2.89 days, P = 0.006) Diagnosis‐to‐classification: numerically shorter in quarantined individuals (6.21 vs 7.34 days, P = 0.7864) Onset‐to‐diagnosis time from period 1 to periods 2 and 3: significantly longer for period 1 (no intervention measures implemented) than period 2 (interventions include the implementation of a level B quarantine) (3.64 vs 2.10 days, P < 0.0001); no significant difference between periods 2 and 3 (expedited classification procedures in place) (2.10 vs 2.60 days, P = 0.072) Diagnosis‐to‐classification time from period 1 to periods 2 and 3: no statistically significant difference between periods 1 and 2 (9.18 vs 8.24 days); the time from period 2 to period 3 was significantly shortened (8.24 vs 5.65 days, P < 0.001) |
| Pang 2003 | Retrospective cohort study | Overall attack rate for becoming a probable case among close contacts: 6.3% (95% CI 5.3 to 7.3) Attack rate by demographics in % (95% CI)
Among 206 close contacts whose last contact with a patient with SARS was before the patient’s symptom onset; 4 (1.9%) developed SARS. Some interventions, such as the quarantine of low‐risk contacts and fever checks at transportation sites, seemed to have less direct impact in curbing the outbreak. |
| Park 2020 | Retrospective cohort study | Of all 116 quarantined people, 0% become confirmed cases during average quarantine duration of 15 days Overall survival rate: 104/116 (90% survived 2 years); no statistically significant difference between groups (P = 0.849) |
| Wang 2007 | Retrospective cohort study | Level A (quarantine of close contacts): out of 52,255 quarantined individuals:102 probable/suspected/laboratory‐confirmed SARS cases Level B (quarantine of travellers): out of 95,271 quarantined individuals: 56 probable/suspected/laboratory‐confirmed SARS cases Advanced age (> 60 years) was identified as a risk factor for SARS in both level A and level B quarantine. For level A quarantine, the odds ratio for developing SARS in this age group was 2.7; for level B quarantine, the odds ratio was 10.5. The probabilities for contracting SARS for the referent group (age < 20 years) were different (0.09% vs 0.02% for level A vs level B quarantine). Quarantining only those with known SARS exposure could have reduced the number of people quarantined by approximately 64% |
| Study | Type of model used | Results |
| Becker 2005 | Transmission model | Quarantine of households of a confirmed case is more efficient if transmission rate is high and time to diagnosis is long. It reduces the reproduction number below 1 if every case is diagnosed within 8.8 days. Quarantine of households combined with contact tracing and quarantining of contacts of confirmed cases reduces the reproduction number from its base value of 6 to below 1 when cases are diagnosed within about 5 days of the onset of infectivity. |
| Cao 2020 | SEIR model | With a combination of strict prevention and control measures (cancelling events, quarantine, social distancing) the peak in Hubei was modelled to be at about 50,000 cases on 19 February 2020. Without prevention and control measures, twice as many people would be infected; the peak would be earlier and higher, resulting in greater loss of life. Assuming the quarantine ratio drops to 0.6, the peak number of cases will double compared to keeping full prevention and control measures in place. |
| Chau 2003 | Back‐projection method | Quarantining the contacts of confirmed and suspected SARS cases seems to be more effective than quarantining only the contacts of confirmed cases due to the diagnosis time lag. Infections within hospitals can be reduced by better isolation measures and protective equipment. |
| Choi 2020 | Susceptible‐exposed‐infected‐hospitalized‐recovered model | Assuming that the effect of the epidemic prevention measures starts on 5 March, when the transmission rate is reduced by 90% or 99%, the epidemic peak will be advanced to 7 March and 6 March. The total number of patients will be reduced to 26,634 and 19,426 instead of 4,992,000 without any measures. With the decrease in transmission rate, the total epidemic time, the size of the epidemic focus, and the total number of patients will all be reduced. If the transmission time of infection is reduced from 4 days to 2 days, the total epidemic time will be reduced, but the size of epidemic point will be larger. Specific effect of quarantine = NR |
| Day 2006 | Probabilistic models | When isolation is ineffective, the use of quarantine will be most beneficial when there is significant asymptomatic transmission, and if the asymptomatic period is neither very long nor very short. Provided that isolation is effective, the number of infections averted through the use of quarantine is expected to be very low. |
| Fang 2020 | SEIR model | The declines in the dynamic trend of the effective reproduction number indicated the effectiveness of stringent government measures (early detection, isolation and quarantine, enough medical supplies, patients admitted to hospitals, therapeutic strategies). More rigorous government control policies are associated with a slower increase of the infected population. Quarantine and protective procedures are less effective as more cases accrue, so the optimization of a treatment plan and the development of specific drugs is of more importance. Specific effect of quarantine = NR |
| Ferguson 2020 | Modified, individual‐based simulation model | Without doing anything, the model predicts 510,000 deaths in UK For a timeframe of 3 months, home isolation and household quarantine would decrease the death rate by 31%‐34%. However, most effective is the combination of interventions (case isolation + home quarantine + social distancing). This combination reduces the critical care demand by two‐thirds and halves the number of deaths. |
| Fraser 2004 | Model of infectious disease outbreak dynamics of several pathogens | SARS and smallpox are easier to control than pandemic influenza and HIV using simple public health measures (i.e. isolation and quarantine). Influenza is very difficult to control even with 90% quarantining and contact tracing because of the high level of presymptomatic transmission and very short incubation (2 days) and infectious (3‐4 days) periods. |
| Geng 2020 | SEIR model | The model shows that a further reducing of the number of susceptible people in contact with the exposed and the sick people by travel restriction and work/school closure will slow down the development of the epidemic and reduce the peak of the exposed and the infected people by 45.71% and 29.90%, respectively. |
| Gumel 2004 | Deterministic model | Both isolation and quarantine seem to be effective means for controlling the spread of SARS. Reduction of the time to quarantine or isolation resulted in the greatest reduction of cumulative deaths. If limited resources are available, the study authors recommend investing all resources in 1 intervention rather than partially investing in both. |
| Gupta 2005 | Mathematical and health economic model | The results indicate that quarantine is effective in containing newly emerging infectious diseases and is also cost saving when compared to not implementing a widespread containment mechanism. Primary wave: infected = 1, quarantined = 100, averted infections = 4672 Secondary wave: infected = 8, quarantined = 900, averted infections = 4608 Tertiary wave: infected = 64, quarantined = 7400, averted infections = 4096 |
| Hsieh 2007 | Susceptible‐infective‐recovered model with additional compartments for Level A and Level B quarantine | Level A quarantine prevented approximately 461 additional SARS cases and 62 additional deaths. The effect of a Level B quarantine was comparatively minor; quarantined cases prevented 29 additional cases and 5 deaths. The combined impact of the 2 quarantine levels reduced the case number and deaths by almost half. |
| Lloyd‐Smith 2003 | Stochastic model | Contact tracing and quarantine can, to some extent, compensate for inadequate isolation facilities, making an increasingly significant contribution as the basic reproduction number rises. If contact tracing is delayed such that no individuals are quarantined until 5 days following exposure, the quarantine’s contribution is considerably reduced. Delays in initiating quarantine or isolation undermine the effectiveness of other control measures, particularly in high‐transmission settings. Healthcare workers are exposed to a prevalence much higher than that in the community at large. Measures that reduce transmission within hospitals have the greatest impact on the epidemic’s reproduction number. Combined strategy of contact tracing and case‐management measures (quarantine and isolation) led to rapid containment of the outbreak in 85% of simulations. |
| Mubayi 2010 | Dynamic model, cost‐effectiveness model | The effect of the combination of quarantine and contact tracing depends on infectiousness of the virus, susceptibility of the population and resource availability. The study authors concluded that increases in the quarantine rates have the same qualitative effect (but different quantitative effects) on each random tracing strategy, and that the total numbers of new cases, deaths, and time to extinction decrease monotonically. Results suggest that the greatest reduction in cases, deaths and isolated individuals can be obtained by the use of the control policy when the contact‐tracing rate assumes a maximum effort independent of the outbreak size. |
| Nishiura 2004 | Deterministic mathematical model | The possible trajectories of a SARS epidemic depend on the levels of public health interventions, as quarantine and precautionary measures greatly affect the transmissibility. It is shown that either 100% effective precautionary public health measures or quarantine would lead to decline in the incidence, but the combination of them reduce the reproduction number in a linear way unlike the practice of isolation. In the absence of precautionary public health measures, at least 66.7% of susceptible people, traced latent or traced uninfected contacts, should be quarantined to suppress the epidemic. Precautionary public measures should be undertaken by a high proportion of susceptible people (75% or 90%) to decrease the number of newly infected cases when no quarantine was carried out. |
| Peak 2017 | Agent‐based branching model | The interventions are not equivalent, and the choice of which intervention to implement to achieve optimal control depends on the infectious disease’s natural history, its inherent transmissibility, and the intervention feasibility in the particular healthcare setting. The benefit of quarantine over symptom monitoring is maximized for fast‐course diseases (short duration of infectiousness and a short latent period compared with the incubation period) and in settings where isolation is highly effective, a large proportion of contacts is traced, or there is a long delay between symptom onset and isolation. |
| Pourbohloul 2005 | Urban contact network model | For a mildly contagious disease, an outbreak can be controlled with a combination of isolation, which reduces the infectious period by 25%, and quarantine, which successfully sequesters 30% of all case‐patient contacts. Much more rigorous isolation and quarantine are required for a more contagious disease. |
| Rocklov 2020 | SEIR model | The basic reproduction rate on board (the Diamond Princess cruise ship) was initially 4 times higher compared to the basic reproduction number in Wuhan, but the countermeasures lowered it substantially. Based on the modelled initial basic reproduction number of 14.8, it was estimated that without any interventions 2920 out of 3700 people (79%) would have been infected from 21 January to 19 February 2020. Isolation and quarantine therefore prevented 2307 cases and lowered the basic reproduction number to 1.78. |
| Tang 2020 | Deterministic, compartmental SEIR model | Reducing the contact rate persistently by isolation and quarantine decreases the peak value but may either delay or accelerate the peak. Increasing the quarantine rate by 10 or 20 times will accelerate the peak by 6.5 or 9 days and will lead to a reduction of the peak value by 87% or 93% in terms of the number of infected individuals. This indicates that enhancing quarantine and isolation following contact tracing and reducing the contact rate can significantly lower the peak and reduce the cumulative number of predicted reported cases. With travel restrictions in Wuhan, in 7 days the number of infected individuals in Beijing would decrease by 91.14%. Without travel restrictions, in 7 days, the number of infected individuals in Beijing would decrease by 88.84% only if the quarantine rate is increased by 100,000 times. This means that the effect of a travel restriction in Wuhan on the infection in Beijing is almost equivalent to increasing quarantine by a baseline value of 100,000. |
| Wang 2004 | General, deterministic model simplified to a 2‐compartment suspect‐probable model and a single‐compartment probable model | The incidence rate is characterized by 2 stages. The first stage is the process of developing protection measures and quarantine policy, and the second stage coincides with the process of maintaining control measures. The study showed the necessity of implementing maximal control measures in the second stage for a certain period to eradicate the disease. Furthermore, the control measures in the second stage should be implemented before a threshold for the number of probable cases is reached. When protection measures are taken, and the maximal control measures are maintained (quarantine, isolation, and various protection measures), the study authors predicted there will be 41 infected individuals if 1 infected person is introduced into a susceptible population, and the number of infective individuals returns to 1 after 61 days and dies out as time evolves thereafter. If the maximal control measures are not maintained, the disease will be persistent at a level of 688 infective individuals, and there will be 1000 infective individuals on the 43rd day. |
| Wu 2020b | Susceptible‐infected‐recovered model | Predicted infection numbers without control measures compared to actual infection numbers with control measures (timeframe 23 January‐31 January 2020): 23 January: 952 vs 495 to 31 January: 9801 vs 3215) Under weak prevention and control measures that only succeed in reducing the contact rate and infection efficiency by ≤ 45% the study authors predict 4719 cases with 739 deaths within 3 months out of 11.5 million inhabitants in Wuhan. Under strong prevention and control measures (defined as measures that succeed to reduce contact rate and infection efficiency by ≥ 50%) the number of infected people would be about 3088 and the death toll about 443. |
| Yip 2007 | Back‐projection method | The overall downward trend of the infection curve corresponds well to the date when changes in the review and classification procedure were implemented by the SARS Prevention and Extrication Committee. The start of large‐scale border control and home quarantine turned out to be the major turning point for ending the outbreak in Taiwan. |
| Yue 2020 | Dynamic infectious disease model | The study authors assume a worsening of the epidemic’s severity if the government relaxes control measures (e.g. allows travelling), while the situation can be controlled by putting strict control measures in place such as the close‐down in Wuhan. |
| Zhang 2017 | Transmission dynamics model | Quarantining close contacts and informing the public of the actual outbreak situation could be the main countermeasures. The most effective combination of interventions is characterized by the increased quarantine in designated hospitals, self‐protection of the public to reduce the contact rate, and the quick response to symptom onset for confirmation test with implementation of appropriate isolation procedures. |
| Zhao 2020a | Susceptible‐unquarantined infected‐quarantined infected‐confirmed infected model | Without any prevention and control measures the model predicts 802,606,289 cases in China (without Hubei) and a duration of 477 days of the epidemic. With prevention and control measures (e.g. quarantine, travel restrictions), the number of cases can decrease to 13,322 and the duration to 45 days. |
| CI: confidence interval; COVID‐19: coronavirus disease 2019; MERS: Middle East respiratory syndrome; SARS: severe acute respiratory syndrome; n: number of participants; NR: not reported; R0: basic reproduction number; SEIR: susceptible‐exposed‐infected‐recovered; vs: versus | ||
Risk of bias in included studies
Appendix 2 presents the assessment of risk of bias for the four cohort studies and Appendix 3 presents the quality rating of the included modelling studies.
Effects of interventions
1. Effectiveness of quarantine for individuals who were in contact with a confirmed COVID‐19 case (KQ1)
Direct evidence: COVID‐19
We did not identify any observational studies. We found four modelling studies that addressed the effectiveness of quarantine for individuals who were in close contact with a confirmed COVID‐19 case (in combination with isolation of cases) for China, UK, and the cruise ship Diamond Princess (Cao 2020; Ferguson 2020; Rocklov 2020; Tang 2020). One study used a modified individual‐based model (Ferguson 2020), the other three employed a susceptible–exposed–infected–recovered (SEIR) cohort model (Cao 2020; Rocklov 2020; Tang 2020). We report the evidence narratively.
The main objective of Ferguson 2020 was to compare two strategies intended to reduce transmission by limiting contacts in the general population (a mitigation versus suppression strategy). For each strategy, the study modelled a range of nonpharmaceutical interventions in different combinations and assessed their impacts on mortality and critical care bed requirements. The results showed that with an assumed reproduction number of 2.4, a combination of case isolation and voluntary quarantine for three months could prevent 31% of deaths compared with a scenario without any control measures for the epidemic Ferguson 2020.
Two modelling studies simulated the situation in China (Cao 2020; Tang 2020). Cao 2020 used data from the Hubei Province collected from 23 January to 24 February 2020 to build a SEIR model. The study authors did not explicitly assess the effectiveness of quarantine alone but the impact of loosening quarantine measures that had already been in place. They concluded that if 40% fewer people were quarantined (e.g. because of less strict contact tracing), the peak number of cases would have increased twofold compared to keeping a full quarantine in place. The simulation by Tang 2020 aimed to estimate the basic reproduction number of SARS‐CoV‐2 and infer the required effectiveness of isolation and quarantine to contain the outbreak. Their SEIR model was based on data of confirmed cases from Wuhan collected from 10 to 20 January 2020, before the community quarantine in Wuhan. They calculated a basic reproduction number (R0) of 6.47 and estimated that without action the number of confirmed cases in Wuhan would be at 7723 by the end of January 2020. They calculated that a 50% reduced contact rate (achieved by isolation and quarantine) would decrease the confirmed cases by 44%; reducing contacts by 90% would decrease the number of cases by 65%. Retrospectively, we know that by the end of January 2020 there were about 9000 cases, despite the community quarantine in place that started on 23 January 2020 (WHO 2020h).
In their modelling study, Rocklov 2020 used data from the Diamond Princess cruise ship. Due to the very dense population on board, the basic reproduction rate was four times higher than in Wuhan. Isolation (i.e. removal of confirmed cases from the ship to hospitals) and quarantine of passengers that needed to stay in their cabins prevented 2307 (67%) cases, and lowered the basic reproduction number from 14.8 to 1.78. However, the study authors also state that early evacuation of all passengers and crew members would have prevented the most infections.
Indirect evidence: MERS, SARS
Overall, we included three retrospective cohort studies (Hsieh 2005; Pang 2003; Wang 2007), and 15 modelling studies that provided indirect evidence for KQ 1 (Becker 2005; Chau 2003; Day 2006; Fraser 2004; Gumel 2004; Gupta 2005; Hsieh 2007; Lloyd‐Smith 2003; Mubayi 2010; Nishiura 2004; Peak 2017; Pourbohloul 2005; Wang 2004; Yip 2007; Zhang 2017). The cohort studies used data from Beijing and Taiwan during the SARS outbreaks in 2003. The modelling studies relied on data from SARS and MERS outbreaks in Canada, China, Hong Kong, Japan, Korea, Singapore, and Taiwan.
Effectiveness
One retrospective analysis of the SARS outbreak in Taiwan showed that out of 55,632 individuals quarantined due to contact with confirmed or probable cases, only 24 (0.04%) developed confirmed SARS (Hsieh 2005). The time from symptom onset to diagnosis was statistically significantly shorter in quarantined than in non‐quarantined people (1.20 versus 2.89 days, P = 0.0061; Hsieh 2005).
The other two retrospective data analyses from the SARS outbreaks in Beijing (Pang 2003), and Taiwan (Wang 2007), analyzed the risk of actually developing a SARS infection for different subgroups who were quarantined because they had close contact with confirmed or suspected SARS cases. In Beijing, more than 30,000 close contacts were quarantined for 14 days. The majority were quarantined at home (60%), the rest at designated sites. In this cohort of quarantined individuals, the overall attack rate was 6.3%. The attack rates were highest among spouses (15.4%), other household members (8.8%), and nonhousehold relatives (11.6%). In these groups, the attack rates increased with the age of the close contact individual. Children younger than 10 years had an attack rate of 5.0%; adults aged 60 to 90 years had an attack rate of 27.6%. The attack rates among work and school contacts were low (0.36%; Pang 2003). The Taiwanese study confirmed the results of the analyses of the Beijing data. Among more than 55,000 quarantined people who had close contact with a SARS case, advanced age (> 60: adjusted odds ratio (aOR) 2.7, 95% confidence interval (CI) 1.2 to 5.9) or being a family member or relative (aOR 4.7, 95% CI 2.0 to 11.0) were the main risk factors for developing SARS. Unprotected healthcare workers had the highest risk among all groups (aOR 17.5, 95% CI 6.9 to 44.1). By comparison, classmates, teachers, coworkers, and friends had no significant increase in the risk of developing SARS (aOR, 1.0 for all groups) (Wang 2007). The modelling studies combined epidemiological data from SARS and MERS outbreaks with different community characteristics. Continuous‐time or discrete‐time compartmental models were used in addition to back‐projection models and contact network models. Some studies considered multiple aspects of transmissibility, such as presymptomatic transmission, the contact intensity between individual people and households, the duration of infectiousness, and the host’s susceptibility to the infectious disease. Overall, the modelling studies consistently reported that quarantine was an effective measure to control SARS and MERS outbreaks. One study provided estimates of the impact of quarantine based on data from the 2003 SARS outbreak in Taiwan, where more than 55,000 individuals were quarantined because of contact with confirmed SARS cases (Hsieh 2007). The average quarantine rate in Taiwan during the outbreak, however, was estimated to be only 0.047. In other words, only one out of 21 asymptomatic individuals who should have been quarantined was indeed quarantined. Based on the study authors’ model, an increase of the quarantine rate to 0.1 would have averted 214 SARS cases and 33 deaths; an increase to 0.6 would have averted 477 SARS cases and 80 deaths. Nevertheless, even the low quarantine rate of 0.047 prevented 461 cases and 62 deaths (Hsieh 2007). Only three of these studies considered the effectiveness of quarantine in hypothetical examples that also modelled presymptomatic infectiousness (Day 2006; Fraser 2004; Peak 2017). Day 2006 used probabilistic models to determine the conditions under which quarantine is most useful. Their results indicated that the effectiveness of quarantine to reduce the number of infections depends on three main requirements: 1) that despite the implementation of isolation, a large disease reproduction number persists; 2) that a large proportion of infections generated by an individual could be prevented by quarantine; and 3) that there is a high probability (with a process in place such as contact tracing) that an asymptomatic individual will be quarantined before they develop symptoms. In the second study considering presymptomatic infectiousness, Peak 2017 found that the effectiveness of quarantine critically depends on the infectious disease’s biological dynamics (e.g. latent and infectious periods) and transmissibility. When the transmissibility is relatively low (reproduction number < 2.5), quarantine can control a disease, even when infectiousness precedes symptoms by several days. When transmissibility is high, and symptoms emerge long after infectiousness, quarantine will be insufficient. Using a different transmission model, Fraser 2004 reported findings consistent with those of Day 2006 and Peak 2017.
Resource use
Two modelling studies assessed the resource use associated with quarantine during SARS outbreaks (Gupta 2005; Mubayi 2010). Gupta 2005 compared the costs of two scenarios. In scenario A, SARS could be transmitted throughout the population without major public health interventions in place (only infected people are isolated). In scenario B, the early quarantine of first‐degree contacts of the index case was implemented to contain the virus. The model used data from the SARS outbreak in Toronto, Canada. To assess the economic impact of both scenarios, they considered direct costs (e.g. hospitalization, administrative effort) and indirect costs (e.g. lost productivity). Depending on the transmission rate (8% to 25%), the costs of an epidemic without implementing quarantine vary. A transmission rate of 8% means that out of 100 contacts, eight get the infection; a transmission rate of 25% means that 25 contacts are infected. Aggregating primary, secondary, tertiary, and quaternary infections results in 4681 (with an 8% rate) to 406,901 infections (with a 25% rate). The direct and indirect costs of the disease would then range from CAD 72.0 to 25.4 million (reference year 2003). The study authors concluded that at a transmission rate of 8%, the quarantine costs would range between CAD 12.2 to 17.0 million, depending on the timing with which the quarantine measurements were effectively implemented. The total savings varied between CAD 279 to 232 million. The earlier effective quarantine measures are implemented, the greater the savings are.
Mubayi 2010 developed a general contact‐tracing model for the transmission of an infectious disease similar to SARS. They performed a cost‐analysis for various quarantine strategies combined with a fixed isolation strategy. They focused on direct costs allocated by public health authorities and present their analysis as incremental costs per infection prevented and lives saved. In strategy 1, a maximum quarantine effort at a per‐capita rate independent of the number of infected cases is in place. In strategy 2, the quarantine effort was proportional to the outbreak size, while in strategy 3, the quarantine process depended on the outbreak size, but was constrained by resource limitations. Contact tracing is assumed to happen randomly in the model, while in reality, this would depend on having contact with confirmed or suspected cases, so the model might overestimate the quarantine costs.
The study authors recommend using a combination of quarantine and isolation. Although isolation alone might be sufficient to control a SARS outbreak, it is too expensive and resource‐intensive, as isolation costs more than quarantine and it takes time to build isolation facilities. Therefore, a combination of quarantine and isolation is more beneficial than a single control measure. The optimal approach depends on available resources and the ability to quickly identify epidemiological factors, such as infectiousness or susceptibility during an outbreak to determine what quarantine and isolation combination is the best. Quarantine becomes less important the faster infectious patients are detected and isolated. Conversely, simulations show that the total cost is dominated by quarantine costs for a low contact‐tracing efficiency and by isolation at a high contact‐tracing efficiency. This means increasing the quarantine effort always results in lower overall costs over the entire outbreak. Strategy 1 was the most effective in decreasing the time to extinction but led to more cases, deaths, and people being isolated, though fewer were quarantined. Strategy 2 was the most cost‐effective strategy when comparing the cost of achieving a unit of health benefit (e.g. reduction of a case) and the cost of the quarantine/isolation strategies. The study authors stress that the greatest need for resources is early in the outbreak.
Table 2 summarizes the certainty of evidence for KQ1.
2. Comparative effectiveness of different types of quarantine (KQ1a)
A prospective cohort study from Korea followed 116 haemodialysis patients who had to be quarantined because they were exposed to individuals with confirmed MERS infections (Park 2020). For a mean of 15 days, they underwent different types of quarantine: single‐room quarantine (n = 54), cohort quarantine (n = 46), and self‐imposed quarantine (n = 16). None of the patients developed MERS symptoms, and no secondary transmission occurred. Because of the study’s small sample size, we are unable to draw any conclusions about the comparative effectiveness of the different quarantine types.
3. Effectiveness of quarantine in combination with other measures to control a COVID‐19 outbreak (KQ1b)
Direct evidence: COVID‐19
Seven modelling studies addressed the effectiveness of quarantine in combination with other measures to contain the COVID‐19 outbreak (Choi 2020; Fang 2020; Ferguson 2020; Geng 2020; Wu 2020b; Yue 2020; Zhao 2020a; see Table 5). One study used an individual‐based transmission model developed for pandemic influenza to explore the effectiveness of different social distancing measures for the UK (Ferguson 2020), one study used a susceptible‐exposed‐infected‐hospitalized‐recovered model to simulate the situation for South Korea (Choi 2020). The other five modelling studies were based on data from China: one study used a susceptible, unquarantined infected, quarantined infected, confirmed infected model (Zhao 2020a), one used a dynamic disease model (Yue 2020), the other three studies used SEIR models (Fang 2020; Geng 2020; Wu 2020b). The main objective of the modelling study from the UK was to compare two strategies intended to reduce transmission by reducing contacts in the general population (mitigation versus suppression strategy). For each strategy, the study modelled a range of nonpharmaceutical interventions in different combinations and assessed their impact on mortality and critical care bed requirements. Results showed that with an assumed reproduction number of 2.4, a combination of case isolation and voluntary quarantine for three months and social distancing of people 70 years or older for four months could prevent 49% of deaths. The need for critical care beds could be reduced by 67% with a combination of case isolation, voluntary quarantine, and social distancing of people 70 years or older. The combination of case isolation, household quarantine, social distancing of the entire population, and school and university closures would achieve the greatest effect (data in the pre‐publication manuscript not plausible) and could reduce the reproduction number close to 1. Effects would become apparent approximately three weeks after implementation and as long as measures are in place. Study authors point out, however, that the more successful a strategy is at temporary suppression, the larger the later epidemic is predicted to be because of the lesser build‐up of herd immunity (Ferguson 2020). One study simulated the outbreak for South Korea and estimated that there would be nearly 5 million COVID‐19 cases without any measures. By implementing prevention and control measures that are able to reduce the transmission rate by 90% or 99% the number of COVID‐19 cases would be only a fraction, at 0.5% or 0.4%, respectively (Choi 2020). The five Chinese modelling studies concluded that the key to controlling COVID‐19 is to focus on early and strict prevention and control measures. According to the studies, only comprehensive measures can achieve a reduction of transmission of SARS‐CoV‐2 (Fang 2020; Geng 2020; Yue 2020; Wu 2020b; Zhao 2020a). One of these studies predicted that without implementation of any measures, China (without Hubei) would have had more than 800 million COVID‐19 cases and an epidemic duration of 477 days. With prevention and control measures such as isolation, quarantine, and travel restrictions in place, not only could the number of cases be reduced but the duration of the outbreak could also be reduced (Zhao 2020a). The only identified study that specifically assessed the effects of community quarantine was based on data from Wuhan. That study reported that community quarantine and school closures reduced the peak of transmissions by 45.7% and 29.9%, respectively (Geng 2020).
Indirect evidence: SARS
Modeling studies also combined epidemiological data from MERS or SARS outbreaks with different community characteristics. Mostly, they used static models that assumed a constant risk of infection and did not consider the effects of disease control programs. Some studies considered multiple aspects of transmissibility, such as presymptomatic transmission, the contact intensity between individual people and households, the duration of infectiousness, and the host’s susceptibility to the infection. In general, they confirmed that a combination of quarantine with other interventions is effective to reduce the transmission of MERS and SARS.
Table 3 summarizes the certainty of evidence for KQ1b.
4. Effectiveness of quarantine for individuals travelling from a country with a declared COVID‐19 outbreak (KQ2)
Direct evidence: COVID‐19
We did not identify any study on quarantine for individuals travelling from a country with a declared COVID‐19 outbreak.
Indirect evidence: SARS
We identified two observational studies (Hsieh 2005; Wang 2007), and two modelling studies (Hsieh 2007; Yip 2007), assessing the effectiveness of quarantine for people travelling from countries with a declared SARS outbreak.
Effectiveness
Two retrospective analyses (Hsieh 2005; Wang 2007), and two modelling studies (Hsieh 2007; Yip 2007), addressed the effectiveness of quarantine to reduce transmissions from individuals who travelled from regions with high transmission rates. Hsieh 2005, Hsieh 2007 and Wang 2007 used data from the 2003 SARS outbreak in Taiwan during which the Taiwanese government home‐quarantined more than 95,000 travellers arriving at the borders from affected regions. Most quarantined people were confined to their homes for 10 to 14 days. While Wang 2007 reported that 56 of 95,271 quarantined people developed SARS, Hsieh 2005 reported that 0 out of 95,828 quarantined travellers developed SARS, indicating some inconsistency in the used data. Hsieh 2007 employed a susceptible‐infective‐recovered model with an estimated case fatality rate of 14.1%; the mean time of symptom onset to diagnosis were 1.20 days for the quarantined individuals and 2.89 days for those unquarantined. The results of the model showed that in the hypothetical scenario in which no one had been quarantined after arrival from a high‐transmission region, 511 additional SARS cases with 70 additional deaths would have occurred in Taiwan. In the database, 17 unquarantined imported cases could be traced (missed cases and cases before the quarantine was implemented). If all 17 unquarantined imported cases had been quarantined, 280 SARS cases and 48 deaths could have been averted. Based on their data source, out of the more than 95,000 quarantined people, only two developed SARS. If these two individuals had not been quarantined, 29 additional cases and five deaths would have occurred. The study authors acknowledge that caution should be exercised when viewing the numbers because the model did not account for the super‐spreading events that occurred in Taiwan.
Using data from Taiwan, Yip 2007 employed a back‐projection model without providing effect estimates for quarantine. The study authors state that the model confirms the effectiveness of quarantine measures in Taiwan, including the implementation of quarantine for travellers from regions with high transmission rates.
Resource use
We did not identify any studies assessing resource use of quarantine for travellers from regions with high transmission rates.
Table 4 presents the certainty of evidence ratings for KQ2.
Comparative effectiveness of quarantine of travellers (KQ2a)
We did not identify any studies assessing comparative effectiveness of diverse types of quarantine for travellers from regions with high transmission rates.
Discussion
To the best of our knowledge, this is the first rapid systematic evidence synthesis on the effectiveness of quarantine measures for COVID‐19. The evidence base is limited because all 10 studies on COVID‐19 are mathematical modelling studies based on limited data sets that make different assumptions on important model parameters. The other 19 included studies are on SARS and MERS and contribute only indirect evidence. Modeling studies on COVID‐19 consistently reported a benefit of the simulated quarantine measures, for example, quarantine of people exposed to confirmed cases averted high proportions of infections and deaths compared to no measures (low‐certainty evidence). Very low‐certainty of evidence indicated that the earlier that quarantine measures are implemented the greater cost savings are, and that the effect of quarantine of travellers from a country with a declared outbreak to avert transmission and deaths was small. However, this evidence is based on the SARS outbreak and generalizability to COVID‐19 is very limited. In general, the combination of quarantine with other prevention and control measures such as school closings, travel restrictions, social distancing, and others had a greater effect on the reduction of transmissions, cases that required critical care beds, and deaths than individual measures (low‐certainty evidence). Studies on SARS and MERS are consistent with findings from the studies on COVID‐19. Ferguson 2020 (also known as the Imperial College Study) illustrated the greater effect of combinations of measures in their model for the UK. They showed that a combination of case isolation and voluntary quarantine for three months could prevent 31% of deaths compared with an epidemic without any control measures. Adding social distancing of people 70 years or older for four months would increase the proportion of prevented deaths to 49% (R0 = 2.4 assumed). They deemed the combination of case isolation, household quarantine, social distancing of the entire population, and school and university closures as the most effective combination that could reduce the reproduction number close to 1 (Ferguson 2020).
Although more comprehensive and strict prevention and control measures are more effective in containing the COVID‐19 outbreak, at some point the incremental effect of adding another restrictive measure is only minimal and must be contrasted with the unintended negative effects that accompany it. The possible negative social and economic consequences on communities that have been subject to extended periods of social distancing and other prevention and control measures might also lead to an increase in the burden on health overall. In order to maintain the best possible balance of measures, decision makers must constantly monitor the outbreak situation and the impact of the measures implemented.
Quarantine alone is an important component of outbreak control but seems not to be enough to contain COVID‐19. Preliminary estimates of the basic reproduction number of SARS‐CoV‐2 range from 2.8 to 5.5 (Read 2020; Zhao 2020; Zhou 2020). Models have shown that the effectiveness of quarantining individuals during outbreaks of diseases with presymptomatic infectiousness and a basic reproduction number of greater than 2.5 is limited. Based on estimates of a basic reproduction number of 3.11 (95% CI 2.39 to 4.13), Read 2020 state in their preprint, that to stop the increase of COVID‐19 infections, 58% to 76% of the transmissions must be averted by control measures for COVID‐19 infections to stop increasing. In a situation with pre‐ or even asymptomatic infectiousness it is difficult to identify and isolate all cases and to place contacts of cases under quarantine early enough to reduce transmission markedly.
Limitations in the body of evidence
We did not identify any observational study that directly assessed the effects of quarantine alone or in combination with other measures to contain COVID‐19. The best available evidence at the present time is from 10 mathematical modelling studies that used current, but still highly variable estimates of the transmissibility, incubation period, and pathogenicity of SARS‐CoV‐2 to simulate the epidemic, determine the transmission dynamics and the effects of various interventions to control the outbreak. The basic reproduction number in these models, for example, ranged from 0.5 to 7.2. Important parameters that are still largely unknown but have a substantial impact on results of models, are the time of asymptomatic infectiousness and the proportion of unidentified infected individuals. Ferguson 2020, for example, assumed that infectiousness occurs only 12 hours prior to the onset of symptoms and that two‐thirds of cases are symptomatic. In other models, study author assumptions were substantially different. For example, Cao 2020 and Zhang 2017 assumed no asymptomatic infectiousness at all before onset of symptoms.
The majority of included studies in our review are on SARS and MERS. The applicability of the results from SARS and MERS studies is likely to be limited because transmission dynamics are different, e.g. many models assumed that infectiousness starts with symptom onset while COVID‐19 is associated with asymptomatic transmission. However, they support the findings from modelling studies for COVID‐19.
Limitations of the review
Because of time constraints, we conducted a rapid review and abbreviated certain methodological steps of the review process. Specifically, we dually screened only 30% of the titles and abstracts; for the rest, we used single screening. A recent study showed that single abstract screening misses up to 13% of relevant studies (Gartlehner 2020). In addition, single review authors rated risk of bias, conducted data extraction and rated certainty of evidence. A second review author checked the plausibility of decisions and the correctness of data. Because these steps were not conducted dually and independently, we introduced some risk of error to this rapid review. Nevertheless, we are confident that none of these methodological limitations would change the overall conclusions of this review. Furthermore, we limited publications to English and Chinese languages. Because COVID‐19 has become a rapidly evolving pandemic, we might have missed recent publications in languages of countries that have become heavily affected in the meantime (e.g. Italian or Spanish).
This review focused on transmission, mortality reduction and resource use of quarantine, because the WHO expert panel selected these outcomes. We did not include psychological impact of quarantine on individuals, because this was addressed recently in a rapid review (Brooks 2020). However, there may be other health and economic adverse effects resulting from quarantine that have not been assessed by this review (e.g. quality of life, unemployment, domestic violence). For these reasons, our review is unable to address the question of when quarantining and other public health measures aiming to reduce the spread of COVID‐19 should be relaxed or lifted. It is also important to highlight that we did not subject the two modelling studies reporting on resource use to specific critical appraisal for economic evaluations and we did not attempt to draw conclusions regarding the relative costs or efficiency of quarantine alone or in combination with other public health measures compared to no such interventions or single public health measures.
Authors' conclusions
Implications for practice
Despite the limited evidence on quarantine to control COVID‐19, studies consistently concluded that quarantine is an important public health measure to reduce the number of people infected and the number of deaths. For both effectiveness and resource use, early and efficient implementation of quarantine seems to be key. Combination of quarantine with other prevention and control measures showed the greatest effect in reducing transmissions, incident cases, and mortality. In order to maintain the best possible balance of measures, decision makers must constantly monitor the outbreak situation and the impact of the measures implemented.
Implications for research
Further research on important disease dynamics of COVID‐19 such as prevalence, or case‐fatality rate are crucial. Testing in representative samples in different settings could help assess the true prevalence of infection, which would greatly reduce uncertainty of model simulations. Consequently, modelling studies simulating the effectiveness of control measures to contain COVID‐19 could be updated as soon as new knowledge on important parameters is available. Non‐randomized studies of interventions that assess the effectiveness of quarantine alone or in combination with other public health measures to control COVID‐19 are also needed. Different countries across the world have been implementing combinations of prevention of control measures at different intensity and speed. Comparing the effectiveness of these strategies will help us gain more evidence for future pandemics.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2020 | Amended | Corrected funding source name to University for Continuing Education Krems ‐ Danube University Krems |
History
Review first published: Issue 4, 2020
| Date | Event | Description |
|---|---|---|
| 29 June 2020 | Amended | Amended funding statement and added disclaimer statement to the Acknowledgements section |
| 7 May 2020 | Amended | Minor typographical amendments. Addition of affiliation to Acknowledgements. |
Acknowledgements
We would like to thank Susan Norris from the World Health Organization (WHO) for valuable feedback on the manuscript. Furthermore, we would like to thank Helen Wakeford (Managing Editor, Editorial and Methods Department, Cochrane) and Toby Lasserson (Deputy Editor in Chief, Cochrane) for editing the review, Robin Featherstone for peer review of the search and Denise Mitchell for transferring the document from Word to Review Manager 5, drafting a Plain Language Summary, and copy‐editing the manuscript on completion. We would also like to thank Beate Jahn, and Gaby Sroczynski from UMIT ‐ University for Health Sciences, Medical Informatics and Technology, Hall in Tirol, Austria for advice on how to classify modelling studies, Petra Wellemsen for administrative support throughout this project, and Genevieve Iseult Eldredge and Dawn Gartlehner for editing and proofreading the manuscript. We also want to thank Xuan Yu of the Evidence‐based Medicine Centre, Lanzhou University, China, for translating the Chinese‐language studies into English. In addition, we would like to thank Cochrane Editors Sarah Hodgkinson and Lisa Bero, and peer referees Ian Shemilt, Muh Yong Yen and Dale Fisher, for their valuable comments.
This rapid review was undertaken by the Department for Evidence‐based Medicine and Evaluation at University for Continuing Education Krems ‐ Danube University Krems, which is a WHO Collaborating Centre, and it is not a publication of the WHO. The review authors are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the WHO.
Appendices
Appendix 1. Search strategies: WHO Collaborating Centre in Austria
The information specialist of the WHO Collaborating Centre in Austria searched the following evidence sources.
Ovid MEDLINE(R) ALL
1946 to 12 March 2020
1. exp Coronavirus/
2. exp Coronavirus Infections/
3. (Coronavir* or nCov or covid or Middle East Respiratory Syndrome or MERS or Severe Acute Respiratory Syndrome or SARS).ti,ab,kf.
4. or/1‐3
5. quarantine/
6. patient isolation/
7. Hospitals, Isolation/
8. quarantin*.ti,ab,kf.
9. (isolat* adj2 (exposed or contact? or suspected or healthy or human? or people or person? or men or man or wom?n or child* or community or case? or infected or patient?)).ti,ab,kf.
10. (isolation adj2 (ward? or unit? or hospital? or room? or cohort or facilit* or area? or practice? or strateg* or procedure? or precaution?)).ti,ab,kf.
11. (lockdown? or lock‐down?).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
12. ((travel* or mobility or movement) adj2 restrict*).mp.
13. travel ban?.mp.
14. cordon? sanitaire?.mp.
15. sanitary barrier?.mp.
16. (contain* adj3 (communit* or geograph* or area* or local* or urban or region*)).mp.
17. or/5‐16
18. 4 and 17
19. limit 18 to "humans only (removes records about animals)"
20. limit 19 to yr="2002 ‐Current"
21. (english or german).lg.
22. 20 and 21
Embase.com (Elsevier)
12 March 2020
1. 'coronaviridae'/de OR 'coronavirinae'/exp
2. 'coronavirus infection'/exp
3. coronavir*:ti,ab,kw OR ncov:ti,ab,kw OR covid:ti,ab,kw OR 'middle east respiratory syndrome':ti,ab,kw OR mers:ti,ab,kw OR 'severe acute respiratory syndrome':ti,ab,kw OR sars:ti,ab,kw
4. #1 OR #2 OR #3
5. 'quarantine'/exp
6. 'isolation'/exp
7. quarantin*:ti,ab,kw
8. (isolat* NEAR/2 (exposed OR contact OR suspected OR healthy OR human$ OR people OR person$ OR men OR man OR wom?n OR child* OR community OR case$ OR infected OR patient$)):ti,ab,kw
9. (isolation NEAR/2 (ward$ OR unit$ OR hospital$ OR room$ OR cohort OR facilit* OR area$ OR practice$ OR strateg* OR procedure$ OR precaution$)):ti,ab,kw
10. lockdown:ti,ab,kw OR 'lock down':ti,ab,kw
11. ((travel* OR mobility OR movement) NEAR/2 restrict*):ti,ab,kw
12. "travel ban$":ti,ab,kw
13. "cordon$ sanitaire$":ti,ab,kw
14. "sanitary barrier$":ti,ab,kw
15. (contain* NEAR/3 (communit* OR geograph* OR area* OR local* OR urban OR region*)):ti,ab,kw
16. #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
17. #7 AND #16
18. ('animal experiment'/de OR 'animal'/exp) NOT 'human'/exp
19. #17 NOT #18
20. #19 AND ([english]/lim OR [german]/lim)
21. #20 NOT [conference abstract]/lim
22. #21 AND [2002‐2020]/py
CINAHL (Ebsco)
12 March 2020
#QueryLimiters/Expanders
S1 (MH "Coronavirus+")Search modes ‐ Boolean/Phrase
S2 (MH "Coronavirus Infections+")Search modes ‐ Boolean/Phrase
S3 Coronavir* OR nCov OR covid OR "Middle East Respiratory Syndrome" OR MERS OR "Severe Acute Respiratory Syndrome" OR SARSSearch modes ‐ Boolean/Phrase
S4 S1 OR S2 OR S3Search modes ‐ Boolean/Phrase
S5 (MH "Patient Isolation") OR (MH "Quarantine")Search modes ‐ Boolean/Phrase
S6 quarantin*Search modes ‐ Boolean/Phrase
S7 isolationSearch modes ‐ Boolean/Phrase
S8 isolat* N1 (exposed OR contact OR suspected OR healthy OR human# OR people OR person# OR men OR man OR wom#n OR child* OR community OR case# OR infected OR patient#)Search modes ‐ Boolean/Phrase
S9 lockdown OR lock‐downSearch modes ‐ Boolean/Phrase
S10 (travel* OR mobility OR movement) N1 restrict*Search modes ‐ Boolean/Phrase
S11 travel ban#Search modes ‐ Boolean/Phrase
S12 cordon# sanitaire#Search modes ‐ Boolean/Phrase
S13 sanitary barrier#Search modes ‐ Boolean/Phrase
S14 contain* N2 (communit* OR geograph* OR area* OR local* OR urban OR region*)Search modes ‐ Boolean/Phrase
S15 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 Search modes ‐ Boolean/Phrase
S16 S4 AND S15Search modes ‐ Boolean/Phrase
S17 S16Limiters ‐ Language: English, German
S18 S17Limiters ‐ Published Date: 20020101‐20201231
WHO Global Index Medicus (search.bvsalud.org/gim/)
12 March 2020 tw:((tw:(coronavir* OR ncov OR covid OR "Middle East Respiratory Syndrome" OR mers OR "Severe Acute Respiratory Syndrome" OR sars)) AND (tw:(quarantin* OR isolat* OR cordon* OR lockdown OR "lock down" OR cordon* OR "community containment" OR "containment area"))) NOT (mj:(animals))) AND ( la:("en")) AND (year_cluster:[2002 TO 2020])
Appendix 2. Risk of bias assessment of observational studies based on ROBINS‐I
| Author and year | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Hsieh 2005 | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Pang 2003 | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Park 2020 | Serious | Low | Low | Low | Moderate | Moderate | Low | Serious |
| Wang 2007 | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
Appendix 3. Quality rating of the modeling studies based on three best practice recommendations from ISPOR
| Author and year | Was the model a dynamic (transmission) model? | Did the authors conduct uncertainty analyses on key assumptions that may have had an impact of the conclusions? | Do the results provide estimates of the change in the burden of infection due to the intervention? | Quality |
| Becker 2005 | Yes | Unclear | Unclear | Moderate concerns |
| Cao 2020 | Yes | Unclear | Unclear | Moderate concerns |
| Chau 2003 | Yes | No | Unclear | Major concerns |
| Choi 2020 | Unclear | No | Unclear | Major concerns |
| Day 2006 | Yes | Yes | Yes | No concerns to minor concerns |
| Fang 2020 | Yes | Yes | Unclear | Moderate concerns |
| Ferguson 2020 | Yes | Yes | Yes | No concerns to minor concerns |
| Fraser 2004 | Yes | Unclear | Unclear | Moderate concerns |
| Geng 2020 | Yes | No | Yes | Major concerns |
| Gumel 2004 | Yes | Yes | Yes | No concerns to minor concerns |
| Gupta 2005 | Unclear | Yes | Yes | Moderate concerns |
| Hsieh 2007 | Yes | Unclear | Yes | Moderate concerns |
| Lloyd‐Smith 2003 | Yes | Yes | Unclear | Moderate concerns |
| Mubayi 2010 | Yes | Yes | Yes | No concerns to minor concerns |
| Nishiura 2004 | Yes | Yes | Yes | No concerns to minor concerns |
| Peak 2017 | Yes | Unclear | Yes | Moderate concerns |
| Pourbohloul 2005 | Yes | Yes | Unclear | Moderate concerns |
| Rocklov 2020 | Yes | Yes | Yes | No concerns to minor concerns |
| Tang 2020 | Yes | Yes | Yes | No concerns to minor concerns |
| Wang 2004 | Yes | Unclear | No | Major concerns |
| Wu 2020b | Yes | No | Yes | Major concerns |
| Yip 2007 | Yes | No | Unclear | Major concerns |
| Yue 2020 | Yes | No | Yes | Major concerns |
| Zhang 2017 | Yes | Yes | Yes | No concerns to minor concerns |
| Zhao 2020a | Yes | Yes | Unclear | Moderate concerns |
Appendix 4. Search strategies: WHO Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China
The WHO Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China searched the CNKI (China National Knowledge Infrastructure), Embase.com (Elsevier) and PubMed. The search strategy for CNKI is shown below. #1. 冠状病毒 #2. 新型冠状病毒 #3. 2019‐nCoV #4. 武汉病毒 #5. 武汉2019 #6. #1 OR #2 OR #3 OR #4 OR #5
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Becker 2005.
| Study characteristics | ||
| Study design | Modeling study Transmission model R0 = 6; infectious period 9 days; incubation period 6.5 days |
|
| Objectives | To determine to which extent the interventions reduce the effective reproduction number and which intervention requirements are necessary to achieve elimination of the disease | |
| Study details | Data from SARS outbreak 2003 in Singapore and Hong Kong and the Australian census 2001 n = NR | |
| Interventions |
|
|
| Notes | ||
Cao 2020.
| Study characteristics | ||
| Study design | Modeling study SEIR model R0 = 2; considers transmission risk during incubation period; does not consider asymptomatic patients |
|
| Objectives | To simulate the effect of the decrease in the proportion of follow‐up quarantine on the development of the epidemic after governmental prevention and control measures have been in place | |
| Study details | Data from COVID‐19 outbreak in the Hubei Province from 23 January 2020 to 24 February 2020 n = 59.17 million | |
| Interventions |
|
|
| Notes | ||
Chau 2003.
| Study characteristics | ||
| Study design | Modeling study Back‐projection method R0 = NR; incubation period 6.37 days (SD 4.09), time from onset of clinical symptoms to admission to hospital differs from time to time (3.67‐4.85 days) |
|
| Objectives | To estimate the SARS infection curve and assess the effectiveness of interventions | |
| Study details | Data from the SARS outbreak in Hong Kong, 1 March 2003 to 24 June 2003 n = NR | |
| Interventions |
|
|
| Notes | ||
Choi 2020.
| Study characteristics | ||
| Study design | Modeling study Susceptible‐exposed‐infected‐hospitalized‐recovered model R0 = 3.48‐3.54; transmission period 2‐4 days |
|
| Objectives | To estimate and evaluate the effectiveness of preventive measures using mathematical modelling | |
| Study details | Data from COVID‐19 outbreak in South Korea (Daegu and North Gyeongsang) between 20 January and 4 March 2020 n = NR | |
| Interventions | Package of epidemic prevention measures implemented in South Korea (e.g. isolation, quarantine, social distancing) | |
| Notes | ||
Day 2006.
| Study characteristics | ||
| Study design | Modeling study Probabilistic model R0 = 3; infectious period 8 days; latent period 6.4 days; transmission rate 0.375 |
|
| Objectives | To determine factors that make quarantine an effective control measure for some diseases but not for others | |
| Study details | Data based on other mathematical models and epidemiological studies of SARS n = NR | |
| Interventions |
|
|
| Notes | ||
Fang 2020.
| Study characteristics | ||
| Study design | Modeling study SEIR model R0 = NR; probability of infection per exposure 10%; frequency of exposure 10; incubation period 7 days; average recovery time 10.25 days |
|
| Objectives | To simulate the spread dynamics of a COVID‐19 outbreak and the impact of different control measures, to conduct a sensitivity analysis to identify the key factor(s), to plot the trend curve of the effective reproduction number, and to perform data fitting after the simulation | |
| Study details | Data from COVID‐19 outbreak in China and former studies concerning COVID‐19 n = 1,000,000 Population consistent with the size of Wuhan city | |
| Interventions |
|
|
| Notes | ||
Ferguson 2020.
| Study characteristics | ||
| Study design | Modeling study Modified, individual‐based simulation model R0 = 2–2.6 (2.4 at baseline); incubation period 5.1 days; mean infectious period 6.5 days; symptomatic individuals are 50% more infectious than asymptomatic individuals |
|
| Objectives | To assess the impact of nonpharmaceutical interventions on the death rate and the peak healthcare demand during a COVID‐19 epidemic. | |
| Study details | Setting: UK Data based on census data to define age and household distribution size, data on average class sizes and staff‐student ratios, data on the distribution of workplace size n = NR |
|
| Interventions |
|
|
| Notes | ||
Fraser 2004.
| Study characteristics | ||
| Study design | Modeling study Model of infectious disease outbreak dynamics of several pathogens Various R0 and incubation period depending on the pathogen |
|
| Objectives | To identify the general properties of emerging infectious agents that determine the likely success of isolating symptomatic individuals and tracing and quarantining their contacts | |
| Study details | Data based on other mathematical models, the analysis of clinical patient records and case studies of 4 known pathogens: SARS, HIV, pandemic influenza, smallpox n = NR | |
| Interventions |
|
|
| Notes | ||
Geng 2020.
| Study characteristics | ||
| Study design | Modeling study SEIR model R0 ≈ 2.38–2.72 |
|
| Objectives | To model the transmission process of SARS‐CoV‐2 | |
| Study details | Data from National Bureau of Statistics of the People’s Republic of China (population of Wuhan at the end of 2018) n = 8.8 million | |
| Interventions |
|
|
| Notes | ||
Gumel 2004.
| Study characteristics | ||
| Study design | Modeling study Deterministic model R0 = 4.8, 3.6, 5.04, and 4.91; rate of development of clinical symptoms 0.1 and 0.125 |
|
| Objectives | To examine the impact of isolation and quarantine on the control of SARS and cumulative deaths | |
| Study details | Data from WHO and epidemiological studies (outbreaks in Toronto, Beijing, Hong Kong, Singapore) n = NR | |
| Interventions |
|
|
| Notes | ||
Gupta 2005.
| Study characteristics | ||
| Study design | Modeling study Mathematic and health economic model R0 = NR; transmission rate of infection 0.08, 0.15 and 0.25; incubation period 10 days |
|
| Objectives | To estimate the economic effects of an epidemic, the number of averted infections, the direct and indirect costs of quarantine, and the total savings | |
| Study details | Data from other researchers, the popular press, and interviews about the SARS outbreak in Toronto 2003 n = NR | |
| Interventions |
|
|
| Notes | ||
Hsieh 2005.
| Study characteristics | ||
| Study design | Retrospective cohort study | |
| Objectives | To evaluate the effectiveness of quarantine in reducing the time from onset to diagnosis and the time from diagnosis to classification | |
| Study details | SARS‐positive patients, previously quarantined or not quarantined during the 2003 outbreak Setting: Taiwan n = 480 | |
| Interventions |
|
|
| Notes | Case definition: confirmed cases had clinical diagnosis and positive laboratory test for SARS‐CoV | |
Hsieh 2007.
| Study characteristics | ||
| Study design | Modeling study Susceptible–infective–recovered model with additional compartments R0 = NR; daily infection rate 0.347 (95% CI 0.3108–0.3837) |
|
| Objectives | To assess the impact of quarantine on prevented additional SARS cases and additional deaths | |
| Study details | Data from Taiwan Center for Disease Control, SARS database (SARS outbreak in Taiwan 2003) n = 151,460 | |
| Interventions |
|
|
| Notes | ||
Lloyd‐Smith 2003.
| Study characteristics | ||
| Study design | Modeling study Stochastic model R0 ranging from 1.5‐5; mean incubation period 4.5 days; mean symptomatic period 16.3 days (SD of 7.3 days) |
|
| Objectives | To address the relative benefits of case isolation, quarantine, hospital‐wide contact precautions and reduced HCW‐community mixing | |
| Study details | Data source = NR n = 100,000 individuals and a hospital of 3000 individuals | |
| Interventions |
|
|
| Notes | ||
Mubayi 2010.
| Study characteristics | ||
| Study design | Modeling study Dynamical model, cost‐effectiveness model R0 = 3.22; mean infectious period 28.4 days; mean incubation period 6.37 days |
|
| Objectives | To compare three different quarantine strategies implemented alongside a single isolation strategy, with resource allocation modelled in terms of simple cost functions | |
| Study details | Data from SARS outbreaks in Hong Kong (census data from 2001–2004 in Hong Kong City) and related studies n = NR | |
| Interventions |
|
|
| Notes | ||
Nishiura 2004.
| Study characteristics | ||
| Study design | Modeling study Deterministic mathematical model R0 = 3; in sensitivity analysis various R0 (depending on the effectiveness of the quarantine and of the precautionary measures) |
|
| Objectives | To predict the epidemiological outcomes and assess the effect of any specified control strategy on SARS | |
| Study details | Data from SARS outbreak in Hong Kong and epidemiological data from other countries n = NR | |
| Interventions |
|
|
| Notes | ||
Pang 2003.
| Study characteristics | ||
| Study design | Retrospective cohort study | |
| Objectives | To describe and evaluate measures undertaken to control the SARS outbreak: quarantine among other things | |
| Study details | Individual with close contact to a SARS patient who were quarantined Setting: Beijing n = 30,000 | |
| Interventions | Quarantine of individuals who were in close contact with infected people | |
| Notes | Case definition: cases had to meet one of the three following categories:
|
|
Park 2020.
| Study characteristics | ||
| Study design | Prospective cohort study | |
| Objectives | To evaluate the effects of different quarantine strategies on the prevention and rate of secondary viral transmission | |
| Study details | Patients from 3 haemodialysis units exposed to MERS during the 2015 outbreak Setting: Korea n = 116 | |
| Interventions | Quarantine of exposed individuals | |
| Notes | Case definition: to confirm cases serologic analysis were performed Potential bias: allocation to type of quarantine was based on disease severity |
|
Peak 2017.
| Study characteristics | ||
| Study design | Modeling study Agent‐based branching model of several pathogens Parameters depend on the type of disease, e.g. SARS: R0 = 2.9 (95% CI 2.2 to 3.6) Incubation period 4.01 days; Maximum duration of infectiousness 21.6 days |
|
| Objectives | To identify which disease characteristics and intervention attributes are most critical in deciding between quarantine and symptom monitoring and to provide a general framework for understanding the consequences of isolation policies during emerging epidemics | |
| Study details | Data from other case studies of 7 known pathogens (Ebola, hepatitis A, influenza A, MERS, pertussis, SARS, smallpox) n = NR | |
| Interventions |
|
|
| Notes | ||
Pourbohloul 2005.
| Study characteristics | ||
| Study design | Modeling study Urban contact network model Mildly contagious disease R0 = 1.545 Moderately contagious disease R0 = 5.047 |
|
| Objectives | To assess a population’s vulnerability to an infectious disease based on the structure of the network and on the average transmissibility of the disease | |
| Study details | Publicly available data from sources such as Statistics Canada n = 10,308 (2000 households) | |
| Interventions |
|
|
| Notes | ||
Rocklov 2020.
| Study characteristics | ||
| Study design | Modeling study SEIR model Initial R0 = 14.8; infectious period 10 days; incubation period 5 days |
|
| Objectives | To estimate the basic reproduction number under cruise ship conditions and the response effectiveness of the quarantine and removal interventions, and to compare scenarios of an earlier and later evacuation of the ship | |
| Study details | Based on confirmed cases of COVID‐19 on the Diamond Princess cruise ship 21 January 2020–20 February 2020 n = 3700 | |
| Interventions |
|
|
| Notes | ||
Tang 2020.
| Study characteristics | ||
| Study design | Modeling study Deterministic, compartmental SEIR model R0 = 6.47; incubation period 7 days |
|
| Objectives | To estimate the basic reproduction number of SARS‐CoV‐2 and infer the required effectiveness of isolation and quarantine to prevent an outbreak | |
| Study details | Based on confirmed COVID‐19 cases in Wuhan, China from 10 January 2020–22 January 2020 n = 11,081,000 | |
| Interventions |
|
|
| Notes | ||
Wang 2004.
| Study characteristics | ||
| Study design | Modeling study General, deterministic model, simplified to a 2‐compartment suspect‐probable model and a single‐compartment probable model R0 = varies from 1.1–3.3 |
|
| Objectives | To predict future incidence and simulate the impact of additional control strategies by studying the transmission dynamics of the spread of SARS in Beijing | |
| Study details | Daily reported cases by the Ministry of Health of the People's Republic of China n = NR | |
| Interventions |
|
|
| Notes | ||
Wang 2007.
| Study characteristics | ||
| Study design | Retrospective cohort study | |
| Objectives | To identify risk factors for the development of SARS among quarantined people | |
| Study details | Individuals with known or suspected (travellers coming from SARS‐affected areas) exposure to infected people during the 2003 outbreak Setting: Taiwan n = 147,526 | |
| Interventions |
|
|
| Notes | Case definition: cases were classified according to the WHO case definition in suspected, probable, and laboratory‐confirmed cases | |
Wu 2020b.
| Study characteristics | ||
| Study design | Modeling study Susceptible–infected–recovered model R0 = 2.9 and 3.6; infectious period 14 days |
|
| Objectives | To predict the outcome of prevention and control measures of diverse intensity in Wuhan | |
| Study details | Official data from COVID‐19 outbreak in Wuhan n = 1,500,000 (inhabitants of Wuhan) | |
| Interventions | Combination and different intensity of
|
|
| Notes | ||
Yip 2007.
| Study characteristics | ||
| Study design | Modeling study Back‐projection method R0 = 2.9 and 3.6; infectious period 14 days |
|
| Objectives | To reconstruct the infection curve for the 2003 SARS epidemic in Taiwan and to ascertain the temporal changes in the mean daily number of infections that occurred during the course of the outbreak | |
| Study details | Taiwan Center for Disease Control and the WHO n = NR | |
| Interventions |
|
|
| Notes | ||
Yue 2020.
| Study characteristics | ||
| Study design | Modeling study Dynamic infectious disease model R0 = NR |
|
| Objectives | To develop a model to predict the future trend of the epidemic, introducing a quarantine rate parameter to the model | |
| Study details | Numbers of confirmed cases and cures published by the Chinese National Health Committee n = NR | |
| Interventions |
|
|
| Notes | ||
Zhang 2017.
| Study characteristics | ||
| Study design | Modeling study Transmission dynamics model R0 ranged from 2.5‐7.2; incubation period 7.5 days; no asymptomatic transmission |
|
| Objectives | To estimate the transmissibility of MERS and identify the effective countermeasures that stopped its spread | |
| Study details | Outbreak data released by Korea Centers for Disease Control and Prevention n = NR | |
| Interventions |
|
|
| Notes | ||
Zhao 2020a.
| Study characteristics | ||
| Study design | Modeling study Susceptible, unquarantined infected, quarantined infected, confirmed infected model R0 ranged between 0.48 and 5.93 |
|
| Objectives | To characterize the dynamics of COVID‐19 in China and whether control and prevention measures are effective | |
| Study details | Model uses data on COVID‐19 confirmed cases from China, 20 January‐21 February 2020 n = 187,009 | |
| Interventions | Combination of control and prevention measures (quarantine) implemented in China after the COVID‐19 outbreak | |
| Notes | ||
COVID‐19: corona virus disease 2019; HCW: healthcare workers; MERS: Middle East respiratory syndrome; NR: not reported; SARS: severe acute respiratory syndrome; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus‐2; SD: standard deviation; SEIR: susceptible‐exposed‐infected‐recoveredWHO: World Health Organization
Contributions of authors
Study design: BN, GG, IK Literature search: IK Study selection, data collection: AD, AC,VM, EP, BN, GG, GW, CC, CZ Quality assessment of studies: AD, AC, BN, CC, CZ, EP, GG, GW, US, VM GRADEing the certainty of evidence: GG, BN Narrative synthesis and data interpretation: BN, GG Figures and tables: AD, AC, BN, CC, CZ, EP, GG, GW, US, VM Writing the manuscript: BN Supervision: GG Critical revision and feedback to the manuscript: AD, AC, CC, CZ, EP, GG, GW, IK, US, VM
Sources of support
Internal sources
No sources of support supplied
External sources
-
University for Continuing Education Krems ‐ Danube University Krems, Austria
This review was performed by the Department for Evidence‐based Medicine and Evaluation, University for Continuing Education Krems ‐ Danube University Krems in its capacity as a WHO Collaborating Center for evidence‐based medicine
Declarations of interest
Barbara Nussbaumer‐Streit: no conflicts of interest with respect to the topic of this manuscript Verena Mayr: no conflicts of interest with respect to the topic of this manuscript Andreea Iulia Dobrescu: no conflicts of interest with respect to the topic of this manuscript Andrea Chapman: no conflicts of interest with respect to the topic of this manuscript Emma Persad: no conflicts of interest with respect to the topic of this manuscript Irma Klerings: no conflicts of interest with respect to the topic of this manuscript Gernot Wagner: no conflicts of interest with respect to the topic of this manuscript Uwe Siebert: no conflicts of interest with respect to the topic of this manuscript Claudia Christof: no conflicts of interest with respect to the topic of this manuscript Casey Zachariah: no conflicts of interest with respect to the topic of this manuscript Gerald Gartlehner: no conflicts of interest with respect to the topic of this manuscript
Edited (no change to conclusions)
References
References to studies included in this review
Becker 2005 {published data only}
- Becker NG, Glass K, Li Z, Aldis GK. Controlling emerging infectious diseases like SARS. Mathematical Biosciences 2005;193(2):205-21. [DOI] [PubMed] [Google Scholar]
Cao 2020 {published data only}
- Cao S, Feng P, Shi P. Study on the epidemic development of coronavirus disease-19 (COVID-19) in Hubei Province by a modified SEIR model. Journal of Zhejiang University (Medical Sciences) 2020. [DOI: 10.3785/j.issn.1008-9292.2020.02.05] [DOI] [PMC free article] [PubMed]
Chau 2003 {published data only}
- Chau PH, Yip PS. Monitoring the severe acute respiratory syndrome epidemic and assessing effectiveness of interventions in Hong Kong Special Administrative Region. Journal of Epidemiology and Community Health 2003;57(10):766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2020 {published data only}
- Choi SC, Ki M. Estimating the reproductive number and the outbreak size of novel coronavirus disease (COVID-19) using mathematical model in Republic of Korea. Epidemiology and Health 2020:e2020011. [DOI] [PMC free article] [PubMed]
Day 2006 {published data only}
- Day T, Park A, Madras N, Gumel A, Wu J. When is quarantine a useful control strategy for emerging infectious diseases? American Journal of Epidemiology 2006;163(5):479-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fang 2020 {published data only}
- Fang Y, Nie Y, Penny M. Transmission dynamics of the COVID-19 outbreak and effectiveness of government interventions: a data-driven analysis. Journal of Medical Virology 2020;06:06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson 2020 {published data only}
- Ferguson NM, Laydon D, Nedjati-Gilani G, Imai N, Ainslie K, Baguelin M, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. Available from www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf 2020. [DOI] [PMC free article] [PubMed]
Fraser 2004 {published data only}
- Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proceedings of the National Academy of Sciences of the United States of America 2004;101(16):6146-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geng 2020 {published data only}
- Geng H, Xu A, Wang X, Zhang Y, Yin X, Mao MA, et al. Analysis of the role of current prevention and control measures in the epidemic of new coronavirus based on SEIR model. Journal of Jinan University (Natural Science & Medicine Edition) 2020.
Gumel 2004 {published data only}
- Gumel AB, Ruan S, Day T, Watmough J, Brauer F, Van den Driessche P, et al. Modelling strategies for controlling SARS outbreaks. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. Royal Society (Great Britain) 2004;271(1554):2223-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2005 {published data only}
- Gupta AG, Moyer CA, Stern DT. The economic impact of quarantine: SARS in Toronto as a case study. Journal of Infection 2005;50(5):386-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsieh 2005 {published data only}
- Hsieh YH, King CC, Chen CW, Ho MS, Lee JY, Liu FC, et al. Quarantine for SARS, Taiwan. Emerging Infectious Diseases 2005;11(2):278-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsieh 2007 {published data only}
- Hsieh YH, King CC, Chen CW, Ho MS, Hsu SB, Wu YC. Impact of quarantine on the 2003 SARS outbreak: a retrospective modeling study. Journal of Theoretical Biology 2007;244(4):729-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd‐Smith 2003 {published data only}
- Lloyd-Smith JO, Galvani AP, Getz WM. Curtailing transmission of severe acute respiratory syndrome within a community and its hospital. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. Royal Society (Great Britain) 2003;270(1528):1979-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mubayi 2010 {published data only}
- Mubayi A, Zaleta CK, Martcheva M, Castillo-Chavez C. A cost-based comparison of quarantine strategies for new emerging diseases. Mathematical Biosciences and Engineering : MBE 2010;7(3):687-717. [DOI] [PubMed] [Google Scholar]
Nishiura 2004 {published data only}
- Nishiura H, Patanarapelert K, Sriprom M, Sarakorn W, Sriyab S, Ming Tang I. Modelling potential responses to severe acute respiratory syndrome in Japan: the role of initial attack size, precaution, and quarantine. Journal of Epidemiology and Community Health 2004;58(3):186-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pang 2003 {published data only}
- Pang X, Zhu Z, Xu F, Guo J, Gong X, Liu D, et al. Evaluation of control measures implemented in the severe acute respiratory syndrome outbreak in Beijing, 2003. JAMA 2003;290(24):3215-21. [DOI] [PubMed] [Google Scholar]
Park 2020 {published data only}
- Park HC, Lee SH, Kim J, Kim DH, Cho A, Jeon HJ, et al. Effect of isolation practice on the transmission of Middle East respiratory syndrome coronavirus among hemodialysis patients: a 2-year prospective cohort study. Medicine 2020;99(3):e18782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peak 2017 {published data only}
- Peak CM, Childs LM, Grad YH, Buckee CO. Comparing nonpharmaceutical interventions for containing emerging epidemics. Proceedings of the National Academy of Sciences of the United States of America 2017;114(15):4023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pourbohloul 2005 {published data only}
- Pourbohloul B, Meyers LA, Skowronski DM, Krajden M, Patrick DM, Brunham RC. Modeling control strategies of respiratory pathogens. Emerging Infectious Diseases 2005;11(8):1249-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rocklov 2020 {published data only}
- Rocklov J, Sjodin H, Wilder-Smith A. COVID-19 outbreak on the Diamond Princess cruise ship: estimating the epidemic potential and effectiveness of public health countermeasures. Journal of Travel Medicine 2020;28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2020 {published data only}
- Tang B, Wang X, Li Q, Bragazzi NL, Tang S, Xiao Y, et al. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. Journal of Clinical Medicine 2020;9(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang W, Ruan S. Simulating the SARS outbreak in Beijing with limited data. Journal of Theoretical Biology 2004;227(3):369-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang TH, Wei KC, Hsiung CA, Maloney SA, Eidex RB, Posey DL, et al. Optimizing severe acute respiratory syndrome response strategies: lessons learned from quarantine. American Journal of Public Health 2007;97 Suppl 1:S98-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2020b {published data only}
- Wu W-T, Li D-N, Li L. Analysis of the role of different intensity prevention and control measures in the current epidemic of novel coronavirus (2019-nCoV) infected pneumonia in Wuhan based on SIR model. New Medicine 2020;30(1):78-82. [Google Scholar]
Yip 2007 {published data only}
- Yip PS, Hsieh YH, Xu Y, Lam KF, King CC, Chang HL. Assessment of intervention measures for the 2003 SARS epidemic in Taiwan by use of a back-projection method. Infection Control and Hospital Epidemiology 2007;28(5):525-30. [DOI] [PubMed] [Google Scholar]
Yue 2020 {published data only}
- Yue Y, Chen Y, Liu K, Luo X, Xu B, Jiang Y, et al. Modeling and prediction of new coronavirus pneumonia based time-delay dynamic system. Scientia Sinica Mathematica 2020;50(3):1-8.
Zhang 2017 {published data only}
- Zhang XS, Pebody R, Charlett A, Angelis D, Birrell P, Kang H, et al. Estimating and modelling the transmissibility of Middle East respiratory syndrome coronavirus during the 2015 outbreak in the Republic of Korea. Influenza and Other Respiratory Viruses 2017;11(5):434-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2020a {published data only}
- Zhao S, Chen H. Modeling the epidemic dynamics and control of COVID-19 outbreak in China. Quantitative Biology 2020:1-9. [DOI] [PMC free article] [PubMed]
Additional references
Bauch 2005
- Bauch CT, Lloyd-Smith JO, Coffee MP, Galvani AP. Dynamically modeling SARS and other newly emerging respiratory illnesses: past, present, and future. Epidemiology 2005;16(6):791-801. [DOI] [PubMed] [Google Scholar]
Biggerstaff 2014
- Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infectious Diseases 2014;14(1):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 2020
- Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Available from SSRN: ssrn.com/abstract=3532534 2020. [DOI] [PMC free article] [PubMed]
Brozek 2020
- JL, Canelo-Aybar C, Akl EA, Bowen JM, Butcher J, Chiu WA, Cronin M. GRADE Guidelines 30: the GRADE approach to assessing the certainty of modelled evidence - an overview in the context of health decision-making. Draft article. [DOI] [PMC free article] [PubMed]
Cetron 2005
- Cetron M, Landwirth J. Public health and ethical considerations in planning for quarantine. Yale Journal of Biology and Medicine 2005;78(5):329-34. [PMC free article] [PubMed] [Google Scholar]
China CDC 2020
- Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chinese Journal of Epidemiology 2020;41(2):145-51. [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed March 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
del Rio 2020
Gartlehner 2020
- Gartlehner G, Affengruber L, Titscher V, Noel-Storr A, Dooley G, Ballarini N, et al. Single-reviewer abstract screening missed 13 percent of relevant studies: a crowd-based, randomized controlled trial. Journal of Clinical Epidemiology 2020;21:20-8. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Habibi 2020
- Habibi R, Burci GL, Campos TC, Chirwa D, Cinà M, Dagron S, et al. Do not violate the International Health Regulations during the COVID-19 outbreak. Lancet 2020;395(10225):664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jernigan 2020
- Jernigan DB. Update: Public Health response to the coronavirus disease 2019 outbreak — United States. MMWR. Morbidity and Mortality Weekly Report 2020;69:216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Park 2018
- Park J-E, Jung S, Kim A. MERS transmission and risk factors: a systematic review. BMC Public Health 2018;18(1):574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitman 2012
- Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group–5. Medical Decision Making 2012;32(5):712-21. [DOI] [PubMed] [Google Scholar]
Read 2020
- Read JM, Bridgen JR, Cummings DA, Ho A, Jewell CP. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. medRxiv 2020;version 2. [DOI: 10.1101/2020.01.23.20018549] [DOI] [PMC free article] [PubMed]
Schunemann 2019
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2019;111:105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taubenberger 2006
- Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerging Infectious Diseases 2006;12(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization (WHO). International Health Regulations. Available from apps.who.int/iris/bitstream/handle/10665/246107/9789241580496-eng.pdf;jsessionid=8E87192BD7F98D81317FD117AC6B43E4?sequence=1 (accessed after 12 March 2020).
WHO 2020a
- World Health Organization (WHO). Report of the WHO-China Joint Mission on coronavirus disease 2019 (COVID-19). Available from www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed 29 February 2020).
WHO 2020b
- World Health Organization (WHO). Rolling updates on coronavirus diseases (COVID-19). Available from www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed 3 April 2020).
WHO 2020c
- World Health Organization (WHO). Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS). Available from www.who.int/csr/sars/country/en/ (accessed 28 February 2020).
WHO 2020d
- World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV). Available from www.who.int/emergencies/mers-cov/en/ (accessed 28 February 2020).
WHO 2020e
- World Health Organization (WHO). Considerations for quarantine of individuals in the context of containment for coronavirus disease (COVID-19). Available from www.who.int/internal-publications-detail/considerations-for-quarantine-of-individuals-in-the-context-of-containment-for-coronavirus-disease-(covid-19) (accessed 29 February 2020).
WHO 2020f
- World Health Organization (WHO). Environmental health in emergencies. www.who.int/environmental_health_emergencies/disease_outbreaks/en/ (accessed 12 February 2020).
WHO 2020g
- World Health Organization (WHO). Global research on coronavirus disease (COVID-19). Available from www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov;(accessed 12 February 2020).
WHO 2020h
- World Health Organization (WHO). Novel Coronavirus (2019-nCoV) Situation Report-11. Available from www.who.int/docs/default-source/coronaviruse/situation-reports/20200131-sitrep-11-ncov.pdf?sfvrsn=de7c0f7_4 (accessed 2 April 2020).
Wu 2020a
Zhao 2020
- Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. International Journal of Infectious Diseases 2020;92:214-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2020
- Zhou T, Liu QH, Yang ZM. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. Chinese Journal of Evidence-based Medicine (preprint) 2020;20(3):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


