Abstract
Platelets are small, anucleated effector cells that play an important role in linking the hemostatic and inflammatory processes in the body. Platelet function is known to be altered under various inflammatory conditions including aging. A gain in platelet function during aging can increase the risk of thrombotic events, such as stroke and acute myocardial infarction. Anti-platelet therapy is designed to reduce risk of serious cerebrovascular and cardiovascular events, but the adverse consequences of therapy, such as risk for bleeding increases with aging as well. Age-associated comorbidities such as obesity, diabetes, and hyperlipidemia also contribute to increased platelet activity and thus can enhance the risk of thrombosis. Therefore, identification of unique mechanisms of platelet dysfunction in aging and in age-associated comorbidities is warranted to design novel antiplatelet drugs. This review outlines some of the current areas of research on aging-related mechanisms of platelet hyperactivity and addresses the clinical urgency for designing anti-platelet therapies toward novel molecular targets in the aging population.
Keywords: Aging, anti-platelets, platelet, thrombosis
Introduction
A greater life expectancy has led to an increasing proportion of elderly patients in the population that will pose a significant challenge to health-care systems in coming years. Thrombotic diseases, such as stroke and myocardial infarction (MI), are the leading causes of morbidity and mortality in the elderly [1–5]. Importantly, aging is associated with co-morbidities such as diabetes, hypertension, and hyperlipidemia, which are major triggers for thrombotic sequela [6,7]. Platelets play a crucial role in the initiation and progression of thrombosis. Anti-platelet drugs, such as aspirin and thienopyridine derivatives (clopidogrel and ticagrelor) are known to reduce the recurrence of ischemic stroke [8] and MI [9,10]. However, despite decades of use of anti-platelets for thrombotic diseases, controversy exists over their efficacy and safety, especially in the elderly [11,12]. Much of the controversy may be due to the knowledge gap between the molecular mechanisms of platelet activation and thrombosis across the ages and its modulation with other comorbidities.
In this critical review, we summarize findings from key studies that examined the effects of aging and age-associated comorbid factors on platelet function. We address how aging can affect the platelet micro-environment and interaction of platelets with other cells to increase the propensity for thrombosis. We discuss the challenges with current anti-platelet therapies in the aging population. Finally, with this foundation, we address the critical question of what key pathways may be altered in aging-associated platelet dysfunction, and that a mechanistic examination of these pathways may have potential to identify novel targets.
Aging and Platelet Function
The literature on the aspects of platelet function with increasing age is limited and the mechanisms of altered platelet activities are not well defined across the age-groups. Several studies beginning in the early 1980s reported changes in platelet function with age [13]. These studies used different measures of platelet activity, such as bleeding time [14], platelet aggregation [15,16], and plasma and urine markers of platelet activation [17,18], and suggested that platelet activity increases with age. However, these studies had several limitations, such as inadequate sample sizes [15,19], lack of rigorous exclusion of subjects with other comorbidities[16], and lack of consensus on what constitutes young, middle-aged, or elderly groups [15,16,19]. Overall, these early studies were descriptive and did not address the mechanisms of increased platelet activation. Secondly, several studies lacked platelet activity data from very elderly subjects such as those over 75 years of age [18,20]. Risk of bleeding increases in patients over age 75 on antiplatelet therapy [12]; therefore, this is a critical age where the effectiveness and safety of antiplatelet therapy needs to be improved. It is likely that mechanisms of platelet activation are different in this age-group and may represent an age-adjusted adaptation [21]. Therefore, a rigorous examination of critical pathways of platelet activation is needed across the age-groups and it is important to determine how they may modulate thrombotic outcomes in age-specific manner.
Effects of Aging-Mediated Inflammation on Platelet Function
A hallmark of the aging process is increased inflammation and oxidative stress [22]. Platelets are responsive to multiple circulating factors released from damaged or inflammatory cells and the current understanding is that platelet micro-environment of inflammation and oxidative stress can drive thrombotic phenotype. In this section, we discuss the inflammatory changes within platelets that may potentiate thrombotic conditions, and in the next section, we describe the role of oxidative stress pathways in platelet activation. Several of the findings discussed here and in subsequent sections were generated in aged mice or relatively younger mice challenged with age-associated comorbidities. It is pertinent to mention that considering the life-span of C57Bl6J mice is 27–29 months, the 4–6-, 12–14-, 18–20- and 24–27-month-old mice are roughly equivalent to young adults (20–30 years of age), middle aged (45–55 years of age), older (60–70 years of age) and very-old (>80 years of age) humans, respectively.
Platelets are known to serve an important role in hemostasis and inflammation[23]. Activated platelets secrete several procoagulant factors that are also inflammatory signals, such as platelet factor 4, plasminogen activator inhibitor (PAI-1), fibrinogen, and von Willebrand factor (VWF), and many of which are known to increase with age [24–26]. Platelets also release soluble CD40L [27] and P-selectin [28], which serve a dual-purpose of activating inflammatory cells and promoting thrombus formation. Platelet P-selectin has been shown to bind PSGL-1 on leukocytes to promote fibrin deposition [28] and tissue factor release [29,30]. This platelet-leukocyte cross-talk is important for thrombus formation and stabilization: P-selectin/PSGL-1 binding upregulates the leukocyte beta-integrin, Mac-1, and mediates stable platelet–leukocyte interactions [31,32]. CD40L, which is expressed on platelets and secreted, is a strong inflammatory stimulus that also aids in platelet-leukocyte aggregate formation [33]. Furthermore, platelet-neutrophil and platelet-monocyte aggregates have been observed in elderly patients with varying inflammatory and thrombotic conditions, such as stroke [34], myocardial infarction [35] and venous thromboembolism [36] and portend poorer outcome [37]. It remains to be seen if platelet–leukocyte interactions are increased in aging as a by-product of an already heighted inflammatory state or is a mechanism independent of changes in aging-related inflammatory milieu [38].
Like immune sensing cells (dendritic and other phagocytic cells), platelet also express several subtypes of toll-like receptors (TLRs), such as TLR2 and TLR4, which aid in the surveillance of foreign pathogens or damaged cells during infection or inflammation[39]. Expression of certain TLRs on platelets has been linked to the propensity for arterial thrombosis in mice models of atherosclerosis [40,41]. While studies have not examined the alterations in age-dependent platelet TLR expression per se, a study in platelets from middle-aged and elderly subjects (aged 50–75) with elevated BMI and CV risk factors demonstrated that mRNA for several platelet TLR isoforms, such as TLR2, 4, and 9 positively correlated with CV risk factors and inflammatory markers, CRP and IL-6 [42]. Furthermore, Freedman et al. [43] observed age-related changes in platelet expression of inflammatory genes associated with the NF-kB pathway, such as IL-1, IL-6, ICAM-1, COX-2, and TLR-4. In their study, platelet inflammatory gene expression was a better predictor of age and associated CV risk factors compared to leukocyte inflammatory gene expression. For example, elderly (60–69 yrs) subjects had greater platelet inflammatory gene expression compared to the younger (<60 years) age group despite similar inflammatory gene expression in leukocytes [43]. Whether these observations are generalizable to the healthy aging population remains to be determined. So, in future, a rigorous study design should address sex and age differences in TLR expression and expression of other genes linked to inflammatory pathways using a broader cohort of young, middle-aged, and elderly subjects.
Yet another recent study by Davizon-Castillo and colleagues [44] studying platelets from aged mouse (>18 month of age) and humans (mean age 79.5 years) reported that elevated systemic levels of TNFα in aging promotes platelet hyperactivity and increased platelet–leukocyte interaction and blockade of TNFα reverses the adverse effect of inflammation. Their studies implied changes in megakaryocyte programming as a possible link for the TNFα driven platelet activation, but underlying mechanisms are not completely defined. Overall, age-induced inflammation can modulate interaction of platelets with other cell types and platelet activation with a potential to drive thrombosis; however, in-depth mechanistic studies are required to clarify which of the platelet-inflammatory pathways are most critical and should be targeted for controlling thrombotic events in age-specific manner.
Aging, Oxidative Stress, and Pathways for Platelet Activation
While aging and oxidative stress have largely been studied in context of vascular inflammation [45], little is known about how platelets are affected by these interrelated mechanisms. What is known is that reactive oxygen species (ROS) production is critical for physiological platelet activation [46,47]. For example, intracellular ROS signaling is important for collagen and thrombin-induced platelet aggregation [48–50]. With increased oxidative stress in aging elevations of ROS can occur within platelets that can augment platelet activation and thrombotic susceptibility[51].
Several types of ROS including superoxide and hydrogen peroxide (H2O2) have been implicated in platelet activation. Superoxide, the key ROS is transient and is converted to H2O2 by the antioxidant enzyme superoxide dismutase (SOD1 or SOD2, expressed in platelet cytosol and mitochondria, respectively). In 18–20-month-old aged mice, we have demonstrated that mRNA for SOD1 is increased within platelets [51] suggesting that increased generation of platelet superoxide may be occurring during aging. Findings from past studies in humans and mice have suggested that platelet NADPH-oxidase containing Nox2 catalytic subunit is important for platelet superoxide generation and subsequent platelet activation [49,52–54]. Yet recent studies in our lab [55] and others [56,57] using young (3–4 month-old) mice deficient in Nox2 have observed that neither superoxide production and platelet activation, nor susceptibility to carotid artery thrombosis were different in these mice compared to wild type mice. While these studies suggest that Nox2-derived superoxide is not essential for platelet activation, other Nox-subunits or regulatory subunits of NADPH oxidase may be critical, particularly during aging. Consistent with this idea, in 18–20-month-old aged mice we have demonstrated upregulation of intra-platelet mRNA for a regulatory subunit p47Phox but not Nox2, and the increased activation of platelet was overcome by apocynin, an NADPH oxidase inhibitor [51]. Mitochondria are an alternative pathway for superoxide generation and thrombin is known to elicit a strong mitochondrial bioenergetics response in platelets [58], with the potential to generate mitochondrial ROS. Increased platelet-mitochondrial ROS is considered important in platelet activation in some disease state [59,60], but it is not known if mitochondrial superoxide is increased during aging and thus contribute to increased platelet activation.
Hydrogen peroxide, which serves as signaling molecule for several vascular process is also shown to trigger platelet activation [61,62]. A study from our lab in 18–20-month-old mice demonstrated an age-dependent increase in platelet H2O2 that drives platelet activation [51]. This harmful ROS is converted to water through the intracellular antioxidant, glutathione peroxidase (Gpx1). Overexpression of Gpx1 in 18–20-month-old mice reduced H2O2 levels within platelets and decreased the age-dependent platelet activation and thrombosis [51]. Studies in humans have also supported the role of Gpx1 in thrombotic cardiovascular (CV) events. A prospective study observed an inverse correlation between Gpx1 activity in red blood cells and risk for non-fatal MI or mortality due to CV causes, after adjusting for traditional CV risk factors[63]. Interestingly, SOD levels did not correlate with risk of cardiovascular events [63]. Similarly, plasma Gpx3 deficiency has been shown to increase platelet-dependent thrombosis in mice [64] and clinically increase the risk of thrombotic events, such as stroke [65,66], but, whether Gpx3 is altered with age is not known. These studies corroborate the notion that the aging-associated increase in platelet reactivity may result due to an imbalance of antioxidants and pro-oxidants.
Age-associated Comorbid Risk Factors and their Effects on Platelet Function
So far, our discussion has been on the effects of aging alone on platelet function. However, the prevalence of comorbid risk factors, such as obesity-related type 2 diabetes, hyperlipidemia, and hypertension tend to increase with age, especially in those over age 65 (CDC Faststats:Diabetes 2011–14; CDC Faststats:Obesity 2013–16; CDC Faststats:Hypetension 2015–16). All of these co-morbid conditions are also associated with an increased risk of CV disease (MI, stroke). Little is known, however, how age interacts with these risk factors to exacerbate platelet activation or whether certain comorbidities pose a relatively higher risk for platelet activation than others for precipitating thrombotic events in aging population.
Metabolic conditions like diabetes and obesity can affect platelet function through alterations in inflammatory signaling and oxidative stress. Both obesity and diabetes are associated with increased expression of platelet pro-inflammatory markers, such as P-selectin and CD40L [67,68]. Also, platelet-mitochondrial superoxide has been shown to be increased in diabetes [69] and may be further elevated with aging under this disease condition. Studies have shown that mean platelet volume (MPV), a marker of platelet reactivity, increases with diabetes and obesity [70–72]. Hyperglycemia has also been shown to activate platelets directly: Increased glucose in the blood can result in nonspecific glycation of circulating proteins, which has been shown to activate platelets via CD36 leading to arterial thrombosis [73]. Uptake of glucose by platelets can also affect platelet metabolism and function [74]. A recent study by Fidler et al. demonstrated that murine platelets depend on GLUT1 and GLUT4 transporters for glucose uptake and platelet activation and streptozotocin-induced young diabetic mice had higher glucose uptake and platelet hyperactivity compared to control mice [75]. Similarly, a study in human platelets from middle-aged diabetic patients (aged 50–60 years) reported an increase in glucose-mediated inflammatory NF-kB gene expression and heightened sensitivity to the agonist, ADP[76], demonstrating the link between platelet metabolism, inflammatory signaling, and platelet reactivity. These biochemical and functional changes in platelets with hyperglycemia might explain the pro-thrombotic state of diabetic patients [77] and the findings that diabetics have increased platelet activation despite being on antiplatelet therapy [78].
Other commonly observed co-morbid conditions with aging, such as hypertension and hyperlipidemia, affect platelet function as well. Elevated platelet P-selectin expression and platelet activation is reported in patients with uncontrolled severe hypertension [79]. Elevations in intracellular calcium in platelets are observed in hypertensive patients, which may potentiate heightened responses to platelet agonists, such as serotonin and epinephrine [80]. Another study in similar patient population reported that enhanced platelet superoxide production within platelets is mediated through AT1 receptors [81], and this increased superoxide generation has the potential to enhance platelet reactivity. In hyperlipidemia, elevated oxidized LDL (ox-LDL) can induce platelet aggregation via CD36 activation [82,83]. Hyperlipidemia is also associated with increased expression of platelet tissue factor, which is a potent pro-coagulant [84]. A recent study [21] demonstrated that age-associated increase in platelet activation is linked to decrease in platelet antioxidants in patients (age 40–79 years) with CV co-morbidities including diabetes, hypertension, and hyperlipidemia. This study suggested that diminishing antioxidant capacity with age leads to platelet activation under these conditions. Interestingly, they also reported that very elderly patients in the age group of 80–100 years developed adaptive increase in antioxidant levels and exhibited less severe platelet phenotype, which needs to be confirmed in other cohorts. Further studies are needed to determine how aging and one or more of the age-associated comorbidities affects the platelet-inflammatory micro-environment and exert imbalance between pro- and antioxidants.
Controversy Related to Beneficial Effects of Anti-platelet Therapy in Elderly
Current conventional anti-platelet therapies, such as aspirin (a cyclooxygenase inhibitor) and clopidogrel (an ADP-receptor antagonist), have been widely used in the treatment of CVDs, but their efficacy in the elderly for the prevention of CVDs has been recently called into question [11]. For example, it has been shown that use of low dose aspirin for the primary prevention of CV events, such as stroke or MI, led to a higher risk of major bleeding in patient’s age >70 years, and did not decrease incidence of CV events compared to placebo group [11]. Efficacy and safety of other ADP-receptor antagonists such as prasugrel and ticagrelor has been also assessed in the elderly with acute coronary syndrome (ACS). There is a growing list of trials that have not found a clear benefit of prasugrel in the elderly (>74 years) compared to clopidogrel but it seems to confer a modest risk for bleeding [85–87]. Further, a substudy of the large PLATO trial showed no clear clinical benefit or difference in bleeding risk of ticagrelor over clopidogrel in the elderly [88] and head-to-head trial of prasugrel vs. ticagrelor showed similar bleeding risks [89]. Additional trials are underway comparing ticagrelor, prasugrel, and clopidogrel in patients with ACS > 70 years [90].
Elderly patients are often prescribed dual antiplatelet therapy (DAPT) after a stroke or stent procedure to target different platelet activation pathways: these combined treatments may also be ineffective and increase the risk of bleeding [91,92]. In older patient’s age >65 years, long term (>12 months) use of clopidogrel with aspirin was associated with higher risk of major bleeding compared to clopidogrel alone [93]. Further, a higher percentage of residual platelet activity was observed in very elderly (>70 or 75 years) despite use of DAPT [94,95]. This suggests an unidentified age-related factor affecting platelet activity [96]. Recent studies by Jain et al. [21] observed that age-associated adaptation occurs within platelets in the very elderly, leading to paradoxical increase in antioxidant capacity and protection from platelet activation. Such adaptation may predispose the very elderly to higher risk for bleeding if anti-platelets are prescribed.
In an attempt to further reduce bleeding risk of conventional therapies, other FDA-approved drugs have been tested and shown promise in reducing stroke and major bleeding risk but studies in the elderly are scarce. A Cochrane review identified two RCTs (CASISP [97] and CSPS2 [98]) in the Asian population (mean age 60 years) that compared aspirin to cilostazol, a phosphodiesterase-3 inhibitor which increases cAMP levels to inhibit platelet aggregation. These studies found a lower recurrence of stroke and intracranial bleeding with cilostazol compared to aspirin. Furthermore, cilostazol, in combination with DAPT, reduced in-stent restenosis with no increase in major bleeding [99,100]. Future trials are required with a broader demographic group including elderly and very elderly (age >75) patients to assess the efficacy of cilostazol compared to conventional therapy. Combined treatment strategies with anti-platelets and anticoagulants (i.e., coagulation factor inhibitor) have been also explored. The potential benefit of rivaroxaban (factor Xa inhibitor) was previously studied in the ATLAS ACS2-TIMI trial which found a reduction in acute cardiovascular-related death in patients with acute coronary syndrome but patients had a higher rate of bleeding [101]. The COMPASS trial expanded on this to include patients with stable coronary artery disease and showed that the rivaroxaban + aspirin group had lower cardiovascular-related death, stroke or MI but a higher GI bleeding risk, particularly among the very elderly (age > 75), suggesting such combined therapies may have an age-appropriate window.
Novel strategies targeting other platelet-dependent hemostatic pathways are currently in clinical trials [102]. Inhibitors of protease-activating receptors (PAR1 and 4), thrombin binding receptors, and GPVI, a collagen binding receptor, have shown effective platelet inhibition [103] and reduced thrombi formation in animal models [104]; These novel inhibitors may offer attractive targets in aging since these receptors are modulated by inflammatory, oxidative and hemostatic signals, all of which are altered with aging (Figure 1). However, there are contraindications: Vorapaxer, a platelet PAR-1 inhibitor, has been evaluated in clinical trials (TRA2P and TRACER) in patients with stable ACS: Though it effectively lowered the rate of ischemic events, the incidence of hemorrhagic stroke was higher than placebo in the elderly patient [105]. Interestingly, these patients were already on DAPT with aspirin and ADP-receptor antagonist, suggesting an increased bleeding risk with triple therapy. In another study of patients with ACS prescribed vorapaxar as an add on to standard antiplatelet therapy (aspirin with and without clopidogrel) showed no difference in ischemic events, such as stroke, MI, or death and no difference in major intracranial bleeding risk [106], suggesting voraxpar as an alternative to clopidogrel may be as effective, but further head-to-head trials comparing vorapaxar with clopidogrel and other P2Y12 antagonists are needed.
Figure 1.
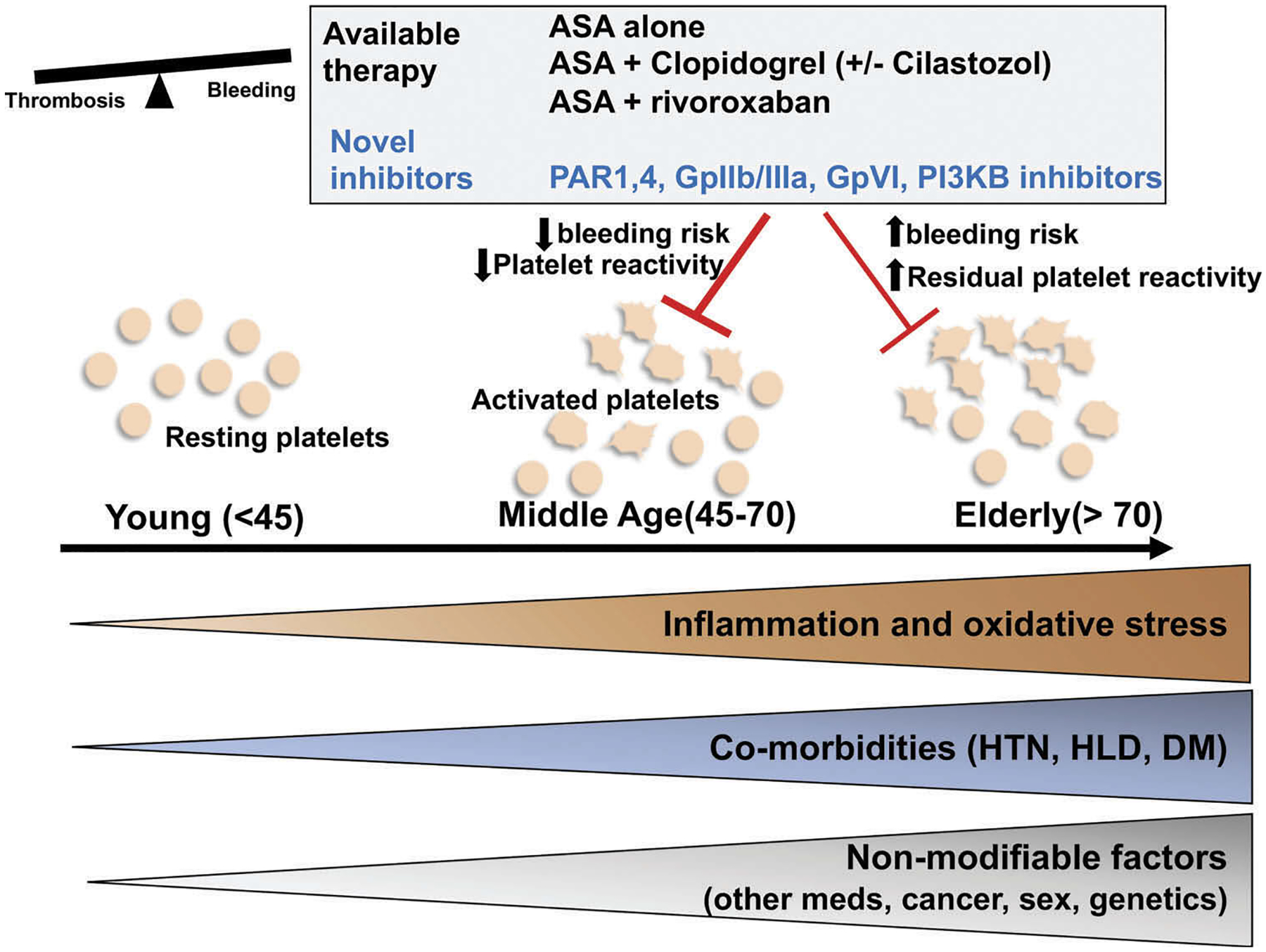
Risk factors associated with aging driving platelet activation and increased thrombotic susceptibility: therapeutic alternatives. Aging is associated with increased inflammation and oxidative stress that can affect platelet activation and aggregation. Other age-associated co-morbidities, such as hypertension, diabetes, and hypercholesterolemia can affect platelet metabolism and can heighten the inflammatory micro-environment. Less modifiable risk factors, such as sex, presence of malignancy, genetics, and multiple medication use for age-associated comorbidities can affect platelets through yet undescribed mechanisms. This can culminate in a reduced threshold for platelet activation. The use of antiplatelet medications requires balancing the risk of bleeding with the risk of thrombosis. Current therapies, such as aspirin (ASA) and clopidogrel target the COX-2/thromboxane and ADP-mediated pathways, respectively. These may be ineffective in the elderly population as platelets become more sensitive to agonists and have a higher bleeding risk. Other approved but less studied treatments include combination of ASA and cilostazol or factor Xa inhibitors (rivaroxaban). Other novel inhibitors targeting other platelet activation pathways that focus on inflammatory and hemostatic signaling are in clinical trials and may offer more beneficial therapy options to the aging population. Future trials should focus on the elderly population where the risks of bleeding and anti-thrombotic benefits need careful assessment (HTN = hypertension, HLD = hyperlipidemia, DM = diabetes mellitus, PAR = protease-activating receptor, PI3KB = phosphoinositide 3 kinase, ASA = aspirin).
Overall, a combined treatment with these novel drugs in addition to de-escalation or modification of conventional therapies after assessing individual risk, may offer a better alternative to conventional therapy alone in elderly.
Pathways of Platelet Activation in Aging and Consideration for Novel Targets
One of the future goals of studying mechanisms of platelet-dependent thrombosis in the elderly is to elucidate newer molecular pathways for targeted therapy. From studies in our lab and others, elevated platelet ROS has been implicated as a mediator of platelet function in aging [51,107]. Generalized anti-oxidant supplementation, such as with vitamin E, has been shown to reduce platelet aggregation in a dose-dependent manner [108] but clinical trials have not shown its effectiveness in reducing CV events [109] suggesting a more targeted anti-oxidant approach is needed. Our studies in aged mice have suggested that overexpression of the anti-oxidant enzyme Gpx 1 protects from aging-associated platelet activation and thrombosis [51]. Gpx is selenium (Se)-dependent enzyme and supplementation with Se has been shown to increase platelet Gpx activity in the healthy population [110], but whether it will reduce oxidative stress-mediated platelet activation in aging is not known. Another alternative is supplementation with N-acetyl cysteine (NAC), a potential donor of sulfydryl groups in the biosynthesis of glutathione, the critical substrate for Gpx activity. A study using NAC has demonstrated some benefit in improving vascular function in patients with coronary artery disease [111]. Similarly, high-dose intravenous NAC administered with low-dose intravenous nitroglycerin is associated with reduced infarct size in patients with MI undergoing percutaneous coronary intervention [112]. In platelets, NAC protects against oxidative stress-induced activation and apoptosis [55,113] and could be considered as a potential therapy in addition to conventional antiplatelet therapy. Trials are needed to determine whether NAC- or Sesupplementation will reduce platelet hyperactivity in the elderly and thus protect from platelet-mediated thrombosis.
In addition to increasing anti-oxidants, a clear understanding of oxidative-pathways that generate ROS within platelets might also provide a novel therapeutic target. We previously mentioned that mitochondria had been implicated as a source of ROS in healthy platelets [55] and in disease states [59,114], but its role in aging is not yet defined. A key regulator of mitochondrial oxidative stress is the family of sirtuins (SIRT1 and SIRT3) and p66shc adapter protein. Sirtuins are histone deacetylases that modify the function of various proteins, including those involved in mitochondrial oxidative stress [115]. Deficiency of SIRT1 is known to accelerate aging and oxidative stress [116] whereas loss of p66shc in mice has been shown to lower the levels of ROS in endothelial cells [117] and protect from atherosclerosis [118]. SIRT1 is also thought to regulate p66shc acetylation and thus modulate mitochondrial ROS and apoptosis [119]. In platelets, it has been shown that p66shc expression was increased in mice on high-fat diet and correlated with increased P-selectin expression and aggregation [120]. Suppression of p66shc with short-hairpin interference RNA blunted the pro-inflammatory, hyper-reactive platelet phenotype [120], suggesting that p66shc or its regulator sirtuin may be a target to modulate ROS production in platelets. In platelets, a predominant form of sirtuin is SIRT3 [121], which is a mitochondrial-specific sirtuin, and is known to deacetylate and activate transcription of mitochondrial genes, such as the antioxidant enzyme SOD2. Recently, SIRT3 loss of function was shown to accelerate arterial thrombosis in mouse models [122]. While this study focused on neutrophil SIRT3 and SOD2 levels, SIRT3 (or SIRT1) may be an attractive target in platelets as it provides an important link to inflammatory and oxidative pathways for platelet activation.
Targeting mitochondrial metabolism within platelets is another growing area of interest. While resting platelets rely on oxidative phosphorylation and aerobic glycolysis to generate adenosine triphosphate, during activation platelets switch their energy metabolism to aerobic glycolysis, suggesting the existence of metabolic flexibility in platelets[58]. Two separate groups [123,124] recently showed that inhibitors of aerobic glycolysis attenuated the agonist-induced platelet responses. These studies suggested that reversing metabolic adaptations of platelets could be an effective alternative to conventional anti-platelet approaches. Though change in mitochondrial metabolism has been reported with aging in several tissues [125,126], data is lacking in platelets. A very recent study [44] did demonstrate increases in mitochondrial mass, oxygen consumption and ATP-linked respiration in platelets from mice over 18 months of age. It remains to be seen whether these changes mediate platelet activation and thrombosis and has distinct molecular pathway for targeted therapy.
Finally, aging is complicated by environmental and genetic factors that lead to cellular and molecular alterations. Many factors are modifiable, such as diet and lifestyle, but other aging-related factors, such as the presence of malignancy [127], menopause [128,129], and multiple medication use for other underlying conditions cannot be easily controlled and have an effect on platelet function (Figure 1). These factors may pose limitations while designing studies in the aging population and future work should consider these factors during examination of mechanistic pathways for platelet activation in human aging.
Summary
Platelets are key players in the thrombotic processes during aging. With the increase in aging population and prevalence of age-related comorbid conditions, such as diabetes, obesity, hyperlipidemia and hypertension, the thrombotic sequela such as stroke, MI, and deep vein thrombosis will continue to rise. Current literature provides minimal mechanistic insight in platelet activation at different stages of aging and its alteration with comorbidities. Therefore, incorporating aging populations for platelet studies in a broader age-groups and with varied comorbidities can help provide better mechanistic understanding of unique risk and protective factors at different stages of aging and help tailor age-appropriate anti-platelet therapies.
Footnotes
Declaration of interest statement
Authors have no conflict of interests.
References
- 1.Howard G, Manolio TA, Burke GL, Wolfson SK, O’Leary DH. Does the association of risk factors and atherosclerosis change with age? An analysis of the combined ARIC and CHS cohorts. The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health Study (CHS) investigators. Stroke 1997;28(9):1693–1701. doi: 10.1161/01.str.28.9.1693 [DOI] [PubMed] [Google Scholar]
- 2.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis 2003;12(3):119–126. doi: 10.1016/S1052-3057(03)00042-9 [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, Kottke TE, Yawn BP, Frye RL. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med 2002;136(5):341–348. doi: 10.7326/0003-4819-136-5-200203050-00005 [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 2009;119(3):e21–181.doi: 10.1161/CIRCULATIONAHA.108.191261 [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019;139(10): e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 6.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120(3):439–448. doi: 10.1161/CIRCRESAHA.116.308413 [DOI] [PubMed] [Google Scholar]
- 7.Jaffer FA, O’Donnell CJ, Larson MG, et al. Age and sex distribution of subclinical aortic atherosclerosis: a magnetic resonance imaging examination of the framingham heart study. Arterioscler Thromb Vasc Biol 2002;22(5):849–854.doi: 10.1161/01.atv.0000012662.29622.00 [DOI] [PubMed] [Google Scholar]
- 8.Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack. Stroke 2019;50(3):773–778. doi: 10.1161/STROKEAHA.118.023954 [DOI] [PubMed] [Google Scholar]
- 9.Antithrombotic Trialists C. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (clinical Research Ed) 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Storey E, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 2018;379(16):1519–1528. doi: 10.1056/NEJMoa1803955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379(16):1509–1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Geraghty OC, Mehta Z, Rothwell PM, Oxford Vascular S. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017;390 (10093):490–499. doi: 10.1016/S0140-6736(17)30770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CI. Platelet function and ageing. Mamm Genome 2016;27 (7–8):358–366. doi: 10.1007/s00335-016-9629-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen KA, Dyerberg J, Olesen AS, Stoffersen E. Acetylsalicylic acid, bleeding time and age. Thromb Res 1980;19(6):799–805. doi: 10.1016/0049-3848(80)90007-9 [DOI] [PubMed] [Google Scholar]
- 15.Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation. Nature 1975;253(5490):355–357.doi: 10.1038/253355a0 [DOI] [PubMed] [Google Scholar]
- 16.Kasjanovova D, Balaz V. Age-related changes in human platelet function in vitro. Mech Ageing Dev 1986;37(2):175–182. doi: 10.1016/0047-6374(86)90074-6 [DOI] [PubMed] [Google Scholar]
- 17.Zahavi J, Jones NA, Leyton J, Dubiel M, Kakkar VV. Enhanced in vivo platelet “release reaction” in old healthy individuals. Thromb Res 1980;17(3–4):329–336. doi: 10.1016/0049-3848(80)90067-5 [DOI] [PubMed] [Google Scholar]
- 18.Bastyr EJ 3rd, Kadrofske MM, Vinik AI. Platelet activity and phosphoinositide turnover increase with advancing age. Am J Med 1990;88(6):601–606. doi: 10.1016/0002-9343(90)90525-i [DOI] [PubMed] [Google Scholar]
- 19.Gleerup G, Winther K. The effect of ageing on platelet function and fibrinolytic activity. Angiology 1995;46(8):715–718. doi: 10.1177/000331979504600810 [DOI] [PubMed] [Google Scholar]
- 20.Cowman J, Dunne E, Oglesby I, Byrne B, Ralph A, Voisin B, Mullers S, Ricco AJ, Kenny D. Age-related changes in platelet function are more profound in women than in men. Sci Rep 2015;5:12235. doi: 10.1038/srep12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain K, Tyagi T, Patell K, Xie Y, Kadado AJ, Lee SH, Yarovinsky T, Du J, Hwang J, Martin KA, et al. Age associated non-linear regulation of redox homeostasis in the anucleate platelet: implications for CVD risk patients. EBioMedicine 2019;44:28–40. doi: 10.1016/j.ebiom.2019.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging 2018;13:757–772. doi: 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JL, Zarbock A, Hidalgo A. Platelets as autonomous drones for hemostatic and immune surveillance. J Exp Med 2017;214 (8):2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hager K, Setzer J, Vogl T, Voit J, Platt D. Blood coagulation factors in the elderly. Arch Gerontol Geriatr 1989;9(3):277–282. [DOI] [PubMed] [Google Scholar]
- 25.Mehta J, Mehta P, Lawson D, Saldeen T. Plasma tissue plasminogen activator inhibitor levels in coronary artery disease: correlation with age and serum triglyceride concentrations. J Am Coll Cardiol 1987;9(2):263–268. doi: 10.1016/s0735-1097(87)80373-x [DOI] [PubMed] [Google Scholar]
- 26.Conlan MG, Folsom AR, Finch A, Davis CE, Sorlie P, Marcucci G, Wu KK. Associations of factor VIII and von Willebrand factor with age, race, sex, and risk factors for atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) study. Thromb Haemost 1993;70(3):380–385. [PubMed] [Google Scholar]
- 27.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Plateletderived CD40L: the switch-hitting player of cardiovascular disease. Circulation 2002;106(8):896–899. doi: 10.1161/01.cir.0000028962.04520.01 [DOI] [PubMed] [Google Scholar]
- 28.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, Sajer SA, Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 1992;359(6398):848–851. doi: 10.1038/359848a0 [DOI] [PubMed] [Google Scholar]
- 29.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost 2005;3(8):1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x [DOI] [PubMed] [Google Scholar]
- 30.Zwicker JI, Trenor CC 3rd, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol 2011;31(4):728–733. doi: 10.1161/ATVBAHA.109.200964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon DI, Chen Z, Xu H, Li CQ, Dong JF, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med 2000;192 (2):193–204. doi: 10.1084/jem.192.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Gao H, Shi C, Erhardt PW, Pavlovsky A, D AS, Bledzka A, Ustinov V, Zhu L, Qin J, et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbalpha. Nat Commun 2017;8:15559. doi: 10.1038/ncomms15559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lievens D, Zernecke A, Seijkens T, Soehnleim O, Beckers L, Munnix ICA, Wijnands E, Goossens P, vanKruchten R, Thevissen L, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 2010;116 (20):4317–4327. doi: 10.1182/blood-2010-01-261206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukasik M, Dworacki G, Kufel-Grabowska J, Watala C, Kozubski W. Upregulation of CD40 ligand and enhanced monocyte-platelet aggregate formation are associated with worse clinical outcome after ischaemic stroke. Thromb Haemost 2012;107(2):346–355. doi: 10.1160/TH11-05-0345 [DOI] [PubMed] [Google Scholar]
- 35.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001;104(13):1533–1537. doi: 10.1161/hc3801.095588 [DOI] [PubMed] [Google Scholar]
- 36.Shih L, Kaplan D, Kraiss LW, Casper TC, Pendleton RC, Peters CL, Supiano MA, Zimmerman GA, Weyrich AS, Rondina MT. Platelet-monocyte aggregates and C-reactive protein are associated with VTE in older surgical patients. Sci Rep 2016;6:27478. doi: 10.1038/srep27478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rondina MT, Carlisle M, Fraughton T, Brown SM, Miller RR 3rd, Harris ES, Weyrich AS, Zimmerman GA, Supiano MA, Grissom CK. Platelet-monocyte aggregate formation and mortality risk in older patients with severe sepsis and septic shock. J Gerontol A Biol Sci Med Sci 2015;70(2):225–231. doi: 10.1093/gerona/glu082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohebali D, Kaplan D, Carlisle M, Supiano MA, Rondina MT. Alterations in platelet function during aging: clinical correlations with thromboinflammatory disease in older adults. J Am Geriatr Soc 2014;62(3):529–535. doi: 10.1111/jgs.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cognasse F, Nguyen KA, Damien P, McNicol A, Pozzetto B, Hamzeh-Cognasse H, Garraud O. The inflammatory role of platelets via their TLRs and siglec receptors. Front Immunol 2015;6:83. doi: 10.3389/fimmu.2015.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash P, Kulkarni PP, Lentz SR, Chauhan AK. Cellular fibronectin containing extra domain A promotes arterial thrombosis in mice through platelet Toll-like receptor 4. Blood 2015;125 (20):3164–3172. doi: 10.1182/blood-2014-10-608653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswas S, Zimman A, Gao D, Byzova TV, Podrez EA. TLR2 plays a key role in platelet hyperreactivity and accelerated thrombosis associated with hyperlipidemia. Circ Res 2017;121 (8):951–962. doi: 10.1161/CIRCRESAHA.117.311069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koupenova M, Mick E, Mikhalev E, Benjamin EJ, Tanriverdi K, Freedman JE. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2015;35(4):1030–1037. doi: 10.1161/ATVBAHA.114.304954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman JE, Larson MG, Tanriverdi K, O’Donnell CJ, Morin K, Hakanson AS, Vasan RS, Johnson AD, Iafrati MD, Benjamin EJ. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the Framingham heart study. Circulation 2010;122(2):119–129. doi: 10.1161/CIRCULATIONAHA.109.928192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davizon-Castillo P, McMahon B, Aguila S, Bark D, Ashworth K, Allawzi A, Campbell RA, Montenont E, Nemkov T, D’Alessandro A, et al. TNF-alpha driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood 2019. doi: 10.1182/blood.2019000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Assar M, Angulo J, Rodriguez-Manas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 46.Wachowicz B, Olas B, Zbikowska HM, Buczynski A. Generation of reactive oxygen species in blood platelets. Platelets 2002;13 (3):175–182. doi: 10.1080/09533710022149395 [DOI] [PubMed] [Google Scholar]
- 47.Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol 2017;14:126–130. doi: 10.1016/j.redox.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang JY, Min JH, Chae YH, Baek JY, Wang SB, Park SJ, Oh GT, Lee S-H, Ho Y-S, Chang T-S. Reactive oxygen species play a critical role in collagen-induced platelet activation via SHP-2 oxidation. Antioxid Redox Signal 2014;20(16):2528–2540. doi: 10.1089/ars.2013.5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood 2005;106 (8):2757–2760. doi: 10.1182/blood-2005-03-1047 [DOI] [PubMed] [Google Scholar]
- 50.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 1998;91(2):484–490. [PubMed] [Google Scholar]
- 51.Dayal S, Wilson KM, Motto DG, Miller FJ Jr., Chauhan AK, Lentz SR. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation 2013;127 (12):1308–1316. doi: 10.1161/CIRCULATIONAHA.112.000966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delaney MK, Kim K, Estevez B, Xu Z, Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J, Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol 2016;36(5):846–854. doi: 10.1161/ATVBAHA.116.307308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pignatelli P, Carnevale R, Di Santo S, Bartimoccia S, Sanguigni V, Lenti L, Finnochi A, Mendolicchio L, Soresina AR, Plebani A, et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler Thromb Vasc Biol 2011;31(2):423–434. doi: 10.1161/ATVBAHA.110.217885 [DOI] [PubMed] [Google Scholar]
- 54.Pignatelli P, Sanguigni V, Lenti L, Ferro D, Finocchi A, Rossi P, Violi F. gp91phox-dependent expression of platelet CD40 ligand. Circulation 2004;110(10):1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55 [DOI] [PubMed] [Google Scholar]
- 55.Sonkar VK, Kumar R, Jensen M, Wagner BA, Sharathkumar AA, Miller FJ Jr., Fasano M, Lentz SR, Buettner GR, Dayal S. Nox2 NADPH oxidase is dispensable for platelet activation or arterial thrombosis in mice. Blood Adv 2019;3(8):1272–1284. doi: 10.1182/bloodadvances.2018025569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dharmarajah J, Arthur JF, Sobey CG, Drummond GR. The anti-platelet effects of apocynin in mice are not mediated by inhibition of NADPH oxidase activity. Naunyn Schmiedebergs Arch Pharmacol 2010;382(4):377–384. doi: 10.1007/s00210-010-0552-3 [DOI] [PubMed] [Google Scholar]
- 57.Walsh TG, Berndt MC, Carrim N, Cowman J, Kenny D, Metharom P. The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation. Redox Biol 2014;2:178–186. doi: 10.1016/j.redox.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravi S, Chacko B, Sawada H, Kramer PA, Johnson MS, Benavides GA, O’Donnell V, Marques MB, Darley-Usmar VM. Metabolic plasticity in resting and thrombin activated platelets. PLoS One 2015;10(4):e0123597. doi: 10.1371/journal.pone.0123597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardenes N, Corey C, Geary L, Jain S, Zharikov S, Barge S, Novelli EM, Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood 2014;123(18):2864–2872. doi: 10.1182/blood-2013-09-529420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY, Sack MN. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp Clin Endocrinol Diabetes 2012;120(4):248–251. doi: 10.1055/s-0031-1285833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, Di Paola J. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood 2008;111(3):1257–1265. doi: 10.1182/blood-2007-05-092684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pratico D, Iuliano L, Ghiselli A, Alessandri C, Violi F. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis 1991;21 (3):169–174. doi: 10.1159/000216222 [DOI] [PubMed] [Google Scholar]
- 63.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. New England J Med 2003;349 (17):1605–1613. doi: 10.1056/NEJMoa030535 [DOI] [PubMed] [Google Scholar]
- 64.Jin RC, Mahoney CE, Coleman Anderson L, Ottaviano F, Croce K, Leopold JA, Zhang Y-Y, Tang S-S, Handy DE, Loscalzo J. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation 2011;123(18):1963–1973. doi: 10.1161/CIRCULATIONAHA.110.000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freedman JE, Loscalzo J, Benoit SE, Valeri CR, Barnard MR, Michelson AD. Decreased platelet inhibition by nitric oxide in two brothers with a history of arterial thrombosis. J Clin Invest 1996;97(4):979–987. doi: 10.1172/JCI118522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenet G, Freedman J, Shenkman B, Regina E, Brok-Simoni F, Holzman F, Vavva F, Brand N, Michelson A, Trolliet M, et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler Thromb Vasc Biol 1999;19(8):2017–2023. doi: 10.1161/01.atv.19.8.2017 [DOI] [PubMed] [Google Scholar]
- 67.De Pergola G, Pannacciulli N, Coviello M, Scarangella A, Di Roma P, Caringella M, Venneri MT, Quaranta M, Giorgino R. sP-selectin plasma levels in obesity: association with insulin resistance and related metabolic and prothrombotic factors. Nutr Metab Cardiovasc Dis 2008;18(3):227–232. doi: 10.1016/j.numecd.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 68.Pretorius L, Thomson GJA, Adams RCM, Nell TA, Laubscher WA, Pretorius E. Platelet activity and hypercoagulation in type 2 diabetes. 2018;17(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 2001;50 (6):1491–1494. doi: 10.2337/diabetes.50.6.1491 [DOI] [PubMed] [Google Scholar]
- 70.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract 2005;59 (8):981–982. doi: 10.1111/j.1742-1241.2005.00500.x [DOI] [PubMed] [Google Scholar]
- 71.Ranjith MP, Divya R, Mehta VK, Krishnan MG, KamalRaj R, Kavishwar A. Significance of platelet volume indices and platelet count in ischaemic heart disease. J Clin Pathol 2009;62 (9):830–833. doi: 10.1136/jcp.2009.066787 [DOI] [PubMed] [Google Scholar]
- 72.Kodiatte TA, Manikyam UK, Rao SB, Jagdish TM, Reddy M, Lingaiah HK, Lakshmaiah V. Mean platelet volume in Type 2 diabetes mellitus. J Lab Physicians 2012;4(1):5–9. doi: 10.4103/0974-2727.98662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu W, Li W, Silverstein RL. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood 2012;119(25):6136–6144. doi: 10.1182/blood-2011-10-387506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kimura M, Ishizawa M, Miura A, Itaya S, Kanoh Y, Yasuda K, Uno Y, Morita H, Ishizuka T. Platelet protein kinase C isoform content in type 2 diabetes complicated with retinopathy and nephropathy. Platelets 2001;12(3):138–143. doi: 10.1080/09537100120039343 [DOI] [PubMed] [Google Scholar]
- 75.Fidler TP, Marti A, Gerth K, Middleton EA, Campbell RA, Rondina MT, Weyrich AS, Abel ED. Glucose metabolism is required for platelet hyperactivation in a murine model of type 1 diabetes mellitus. Diabetes 2019;68(5):932–938.db180981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu L, Chang L, Zhang Y, Zhai L, Zhang S, Qi Z, Yan H, Yan Y, Luo X, Zhang S, et al. Platelets express activated P2Y12 receptor in patients with diabetes mellitus. Circulation 2017;136 (9):817–833. doi: 10.1161/CIRCULATIONAHA.116.026995 [DOI] [PubMed] [Google Scholar]
- 77.Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications 2001;15(1):44–54. [DOI] [PubMed] [Google Scholar]
- 78.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, hernandez R, Moreno R, Escaned J, Alfonso F, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 2005;54 (8):2430–2435. doi: 10.2337/diabetes.54.8.2430 [DOI] [PubMed] [Google Scholar]
- 79.Preston RA, Coffey JO, Materson BJ, Ledford M, Alonso AB. Elevated platelet P-selectin expression and platelet activation in high risk patients with uncontrolled severe hypertension. Atherosclerosis 2007;192(1):148–154. doi: 10.1016/j.atherosclerosis.2006.04.028 [DOI] [PubMed] [Google Scholar]
- 80.Erne P, Resink TJ, Burgisser E, Buhler FR. Platelets and hypertension. J Cardiovasc Pharmacol 1985;7(Suppl 6):S103–108. doi: 10.1097/00005344-198500076-00017 [DOI] [PubMed] [Google Scholar]
- 81.Germano G, Sanguigni V, Pignatelli P, Caccese D, Lenti L, Ragazzo M, Lauro R, Violi F. Enhanced platelet release of superoxide anion in systemic hypertension: role of AT1 receptors. J Hypertens 2004;22(6):1151–1156. doi: 10.1097/00004872-200406000-00016 [DOI] [PubMed] [Google Scholar]
- 82.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res 2008;102 (12):1512–1519. doi: 10.1161/CIRCRESAHA.108.172064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magwenzi S, Woodward C, Wraith KS, Aburima A, Raslan Z, Jones H, McNeil C, Wheatcroft S, Yuldasheva N, Febbriano M, et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015;125(17):2693–2703.doi: 10.1182/blood-2014-05-574491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panes O, Gonzalez C, Hidalgo P, Valderas JP, Acevedo M, Contreras S, Sanchez X, Pereira J, Rigotti A, Mezzano D. Platelet tissue factor activity and membrane cholesterol are increased in hypercholesterolemia and normalized by rosuvastatin, but not by atorvastatin. Atherosclerosis 2017;257:164–171. doi: 10.1016/j.atherosclerosis.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 85.Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, Bossi I, Sganzerla P, Tortorella G, Cacucci M, et al. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation 2018;137 (23):2435–2445. doi: 10.1161/CIRCULATIONAHA.117.032180 [DOI] [PubMed] [Google Scholar]
- 86.Roe MT, Goodman SG, Ohman EM, Stevens SR, Hochman JS, Gottlieb S, Martinez F, Dalby AJ, Boden WE, White HD, et al. Elderly patients with acute coronary syndromes managed without revascularization: insights into the safety of long-term dual anti-platelet therapy with reduced-dose prasugrel versus standard-dose clopidogrel. Circulation 2013;128(8):823–833. doi: 10.1161/CIRCULATIONAHA.113.002303 [DOI] [PubMed] [Google Scholar]
- 87.Bangalore S. Prasugrel in the Elderly. Circulation 2018;137 (23):2446–2449. doi: 10.1161/CIRCULATIONAHA.118.033952 [DOI] [PubMed] [Google Scholar]
- 88.Husted S, James S, Becker RC, Horrow J, Katus H, Storey RF, Cannon CP, Heras M, Lopes RD, Morais J, et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes 2012;5(5):680–688. doi: 10.1161/CIRCOUTCOMES.111.964395 [DOI] [PubMed] [Google Scholar]
- 89.Motovska Z, Hlinomaz O, Miklik R, Hromadka M, Varvarovsky I, Dusek J, Knot J, Jarkovsky J, Kala P, Rokyta R, et al. Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: multicenter randomized PRAGUE-18 study. Circulation 2016;134 (21):1603–1612. doi: 10.1161/CIRCULATIONAHA.116.024823 [DOI] [PubMed] [Google Scholar]
- 90.Qaderdan K, Ishak M, Heestermans AA, de Vrey E, Jukema JW, Voskuil M, de Boer MJ, van’t Hof AW, Groeneneijer BE, Vos GJ, et al. Ticagrelor or prasugrel versus clopidogrel in elderly patients with an acute coronary syndrome: optimization of anti-platelet treatment in patients 70 years and older–rationale and design of the POPular AGE study. Am Heart J 2015;170(5):981–985.e981. doi: 10.1016/j.ahj.2015.07.030 [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369 (1):11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 92.Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007;49(19):1982–1988. doi: 10.1016/j.jacc.2007.03.025 [DOI] [PubMed] [Google Scholar]
- 93.Berger PB, Bhatt DL, Fuster V, Steg PG, Fox KA, Shao M, Brennan DM, Hacke W, Montalescot G, Steinhubal SR, et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010;121(23):2575–2583. doi: 10.1161/CIRCULATIONAHA.109.895342 [DOI] [PubMed] [Google Scholar]
- 94.Silvain J, Cayla G, Hulot JS, Finzi J, Kerneis M, O’Connor SA, Bellemain-Appaix A, Barthelemy O, Beygui F, Collet JP, et al. High on-thienopyridine platelet reactivity in elderly coronary patients: the SENIOR-PLATELET study. Eur Heart J 2012;33 (10):1241–1249. doi: 10.1093/eurheartj/ehr407 [DOI] [PubMed] [Google Scholar]
- 95.Verdoia M, Pergolini P, Rolla R, Nardin M, Schaffer A, Barbieri L, Marino P, Bellomo G, Suryapranata H, De Luca G. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J Thromb Haemost 2016;14(1):57–64. doi: 10.1111/jth.13177 [DOI] [PubMed] [Google Scholar]
- 96.Frelinger AL 3rd, Bhatt DL, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, Lampa M, Brooks JK, Barnard MR, et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J Am Coll Cardiol 2013;61(8):872–879. doi: 10.1016/j.jacc.2012.11.040 [DOI] [PubMed] [Google Scholar]
- 97.Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, Li Z, Zhang W, Ding M, Gao X, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol 2008;7(6):494–499. doi: 10.1016/S1474-4422(08)70094-2 [DOI] [PubMed] [Google Scholar]
- 98.Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010;9(10):959–968. doi: 10.1016/S1474-4422(10)70198-8 [DOI] [PubMed] [Google Scholar]
- 99.Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim DS, Kim DK, Seol SH, Kim DI, Cho KI, et al. A meta-analysis of randomized controlled trials appraising the efficacy and safety of cilostazol after coronary artery stent implantation. Cardiology 2012;122 (3):133–143. doi: 10.1159/000339238 [DOI] [PubMed] [Google Scholar]
- 100.Tamhane U, Meier P, Chetcuti S, Chen KY, Rha SW, Grossman MP, Gurm H. Efficacy of cilostazol in reducing restenosis in patients undergoing contemporary stent based PCI: a meta-analysis of randomised controlled trials. EuroIntervention 2009;5(3):384–393. [DOI] [PubMed] [Google Scholar]
- 101.Mega JL, Braunwald E, Wiviott SD, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366(1):9–19. doi: 10.1056/NEJMoa1112277 [DOI] [PubMed] [Google Scholar]
- 102.Majithia A, Bhatt DL. Novel antiplatelet therapies for atherothrombotic diseases. Arterioscler Thromb Vasc Biol 2019;39 (4):546–557. doi: 10.1161/ATVBAHA.118.310955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ungerer M, Li Z, Baumgartner C, Vogelmann J, Holthoff HP, Gawaz M, Munch G. The GPVI-Fc fusion protein Revacept reduces thrombus formation and improves vascular dysfunction in atherosclerosis without any impact on bleeding times. PLoS One 2013;8(8):e71193. doi: 10.1371/journal.pone.0071193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson SJ, Ismat FA, Wang Z, Cerra M, Narayan H, Raftis J, Gray TJ, Connell S, Garonzik S, Ma X, et al. PAR4 (Protease-Activated Receptor 4) antagonism with BMS-986120 inhibits human ex vivo thrombus formation. Arterioscler Thromb Vasc Biol 2018;38(2):448–456. doi: 10.1161/ATVBAHA.117.310104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ungar L, Clare RM, Rodriguez F, Kolls BJ, Armstrong PW, Aylward P, Held C, Moliterno DJ, Strony J, Van de werf F, et al. Stroke outcomes with vorapaxar versus placebo in patients with acute coronary syndromes: insights from the TRACER trial. J Am Heart Assoc 2018;7(24):e009609. doi: 10.1161/JAHA.118.008528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tricoci P, Lokhnygina Y, Huang Z, Van de Werf F, Cornel JH, Chen E, Wallentin L, Held C, Aylward PE, Moliterno DJ, et al. Vorapaxar with or without clopidogrel after non-ST-segment elevation acute coronary syndromes: results from the thrombin receptor antagonist for clinical event reduction in acute coronary syndrome trial. Am Heart J 2014;168(6):869–877.e861. doi: 10.1016/j.ahj.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 107.Fuentes E, Palomo I. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci 2016;148:17–23. doi: 10.1016/j.lfs.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 108.Calzada C, Bruckdorfer KR, Rice-Evans CA. The influence of antioxidant nutrients on platelet function in healthy volunteers. Atherosclerosis 1997;128(1):97–105. doi: 10.1016/s00219150(96)05974-6 [DOI] [PubMed] [Google Scholar]
- 109.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative group of the primary prevention project. Lancet 2001;357(9250):89–95. doi: 10.1016/s0140-6736(00)03539-x [DOI] [PubMed] [Google Scholar]
- 110.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol 1995;9(2):65–73.doi: 10.1016/S0946-672X(11)80013-1 [DOI] [PubMed] [Google Scholar]
- 111.Andrews NP, Prasad A, Quyyumi AA. N-acetylcysteine improves coronary and peripheral vascular function. J Am Coll Cardiol 2001;37(1):117–123. doi: 10.1016/s0735-1097(00)01093-7 [DOI] [PubMed] [Google Scholar]
- 112.Pasupathy S, Tavella R, Grover S, Raman B, Procter NEK, Du YT, Mahadavan G, Stafford I, Heresztyn T, Holmes A, et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute myo-cardial infarction]). Circulation 2017;136(10):894–903. doi: 10.1161/CIRCULATIONAHA.117.027575 [DOI] [PubMed] [Google Scholar]
- 113.Wang B, Yee Aw T, Stokes KY. N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes. Redox Biol 2017;14:218–228. doi: 10.1016/j.redox.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baaten C, Moenen F, Henskens YMC, Swieringa F, Wetzels RJH, van Oerle R, Heijnen HFG, Ten Cate H, Holloway GP, Beckers EAM, et al. Impaired mitochondrial activity explains platelet dysfunction in thrombocytopenic cancer patients undergoing chemotherapy. Haematologica 2018;103(9):1557–1567. doi: 10.3324/haematol.2017.185165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang BL. Sirt1 and the Mitochondria. Mol Cells 2016;39 (2):87–95. doi: 10.14348/molcells.2016.2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang L, Wu Q, Fan Z, Xie R, Wang Z, Lu Y. Platelet mitochondrial dysfunction and the correlation with human diseases. Biochem Soc Trans 2017;45(6):1213–1223. doi: 10.1042/BST20170291 [DOI] [PubMed] [Google Scholar]
- 117.Oshikawa J, Kim SJ, Furuta E, Caliceti, Chen GF, McKinney RD, Kuhr F, Levitan I, Fukai T, Ushio-Fukai M Novel role of p66Shc in ROS-dependent VEGF signaling and angiogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 2012;302(3): H724–732. doi: 10.1152/ajpheart.00739.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early ather-ogenesis in mice fed a high-fat diet. Proc Natl Acad Sci U S A 2003;100(4):2112–2116. doi: 10.1073/pnas.0336359100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trinei M, Berniakovich I, Beltrami E, Migliaccio E, Fassina A, Pelicci P, Giorgio M P66Shc signals to age. Aging 2009;1 (6):503–510. doi: 10.18632/aging.100057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kumar S, Vikram A, Kim YR, Jacobs SJ, Irani K. P66Shc mediates increased platelet activation and aggregation in hypercholesterolemia. Biochem Biophys Res Commun 2014;449(4):496–501. doi: 10.1016/j.bbrc.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 121.Kumari S, Chaurasia SN, Nayak MK, Mallick RL, Dash D. Sirtuin inhibition induces apoptosis-like changes in platelets and thrombocytopenia. J Biol Chem 2015;290 (19):12290–12299. doi: 10.1074/jbc.M114.615948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gaul DS, Weber J, van Tits LJ, Sluka S, Pasterk L, Reiner MF, Calatayud N, Lohmann C, Klingenberg R, Pahla J, et al. Loss of Sirt3 accelerates arterial thrombosis by increasing formation of neutrophil extracellular traps and plasma tissue factor activity. Cardiovasc Res 2018;114(8):1178–1188. doi: 10.1093/cvr/cvy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kulkarni PP, Tiwari A, Singh N, Gautam D, Sonkar VK, Agarwal V, Dash D Aerobic glycolysis fuels platelet activation: small-molecule modulators of platelet metabolism as anti-thrombotic agents. Haematologica 2019;104 (4):806–818. doi: 10.3324/haematol.2018.205724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nayak MK, Dhanesha N, Doddapattar P, Roodriguez O, Sonkar VK, Dayal S, Chauhan AK. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv 2018;2 (15):2029–2038. doi: 10.1182/bloodadvances.2018022392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yin F, Boveris A, Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal 2014;20 (2):353–371. doi: 10.1089/ars.2012.4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 2008;294(5):H2121–2128. doi: 10.1152/ajpheart.00012.2008 [DOI] [PubMed] [Google Scholar]
- 127.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev 2014;33(1):231–269. doi: 10.1007/s10555-014-9498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aldrighi JM, Oliveira RL, D’Amico E, Rocha T, Gebara OE, Rosano GM, Ramires JA. Platelet activation status decreases after menopause. Gynecol Endocrinol 2005;20(5):249–257. doi: 10.1080/09513590500097549 [DOI] [PubMed] [Google Scholar]
- 129.Roshan TM, Normah J, Rehman A, Naing L. Effect of menopause on platelet activation markers determined by flow cytometry. Am J Hematol 2005;80(4):257–261. doi: 10.1002/ajh.20472 [DOI] [PubMed] [Google Scholar]


