Abstract
The role of platelets in haemostasis has long been known, but understanding of these cells’ involvement in wound healing/tissue repair is more recent and has given rise to a multitude of translational studies. Tissue repair processes consist of complex, regulated interactions between cells modulated by biologically active molecules, most of which are growth factors released by activated platelets: this aspect represents the rationale on which the use of platelet derivatives for clinical purposes is based. In the last years, many in vitro studies have focused on the mechanisms of action by which these growth factors affect the biological activities of cells, thus supporting tissue healing. Although limited by some drawbacks (two-dimensional in vitro monocultures cannot replicate the tissue architecture and organisation of organs or the continuous interplay between different cell types), in vitro studies do have the advantages of giving rapid results and allowing precise control of platelet concentrations and other parameters.
This review offers an updated overview of the data obtained from the most recent bench-top studies focused on the effects of platelet derivatives on a wide variety of human cells, highlighting their possible impact for in vivo applications. The heterogeneity of the data obtained so far is very evident. This can be explained by the different experimental settings used in each study, which may be the cause of the variability in clinical outcomes. In fact, in vitro studies suggest that the composition of platelet derivatives and the method used for their production and activation (or not) and the platelet concentration used can have profound effects on the final results.
Keywords: regenerative medicine, blood platelets, wound healing, platelet-rich plasma (PRP)
TISSUE REPAIR PROCESSES
“Tissue repair” is a term that refers to those dynamic processes that normally occur in the body as a physiological response to tissue damage, aiming to restore the normal function and architecture of the damaged area. These processes consist of a complex set of cellular/molecular events that, regardless of the type of damage (acute or chronic) and the extent of tissue loss, is split into three overlapping stages: inflammatory, proliferative and remodelling1,2.
The first stage occurs soon after the tissue damage as a reaction to blood vessel injury; it begins with vasoconstriction, which lasts a few seconds, followed by platelet clotting. As the platelets form a cap to close the vessels temporarily, the coagulation system is activated and an insoluble fibrin matrix is formed to fill the lesion and to become the temporary scaffold for infiltrating cells. Very soon after, the influx of neutrophils begins: these white blood cells are attracted to the area of the wound by inflammatory cytokines released from activated platelets, such as interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), and interferon-γ (IFN-γ)2. Inflammatory cells play a crucial role in preventing infection and facilitating the clean-up of cellular debris and damaged tissue.
As inflammation moves toward resolution, the proliferative stage begins. This stage consists of new tissue formation and involves the proliferation and migration of several cell types, with endothelial cells and fibroblasts being among the most important. Endothelial cells are needed for angiogenesis, the coordinated process that consists in the formation of new vessels from pre-existing ones. Angiogenesis begins from the sprouting of intact blood vessels present at the edge of the lesion and is sustained by the proliferation of endothelial cells. Along with angiogenesis, vasculogenesis, the formation of new vessels from endothelial progenitor cells, can sustain this neovascularisation too. These processes are regulated mainly by vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF), extensively released by activated platelets3,4. Meanwhile, fibroblasts can migrate into the clot from the surrounding tissue using the fibrin network as a temporary matrix. Fibroblasts proliferate in the site of the wound in response to PDGF, transforming growth factor (TGF)-β and TNF, originating from leucocytes and platelets, and secrete cytokines and growth factors (GF) that stimulate healing. They also produce a “granulation tissue” secreting precursors of collagen (mainly type III), elastin, proteoglycans and other glycoproteins which then mature outside the cells restoring a three-dimensional extracellular matrix (ECM)2.
Tissue repair ends with a remodelling stage that aims to restore the normal tissue structure; this process needs reorganisation, degradation, and re-synthesis of the ECM and leads to a tissue that, at last, will be impoverished of cells and vessels but enriched in collagen fibres2: blood vessels are removed by apoptosis, the type III collagen is degraded by means of matrix metalloproteinases (MMP) and replaced by type I collagen, and most of the immune cells and fibroblasts disappear. Some of the fibroblasts transform into myofibroblasts, which are rich in smooth muscle actin and are responsible for the contraction of the wound’s edges toward the centre5,6. PDGF, FGF, and TGF-β are the main coordinators of these final events1,2.
PLATELET INVOLVEMENT IN TISSUE REPAIR
Although the role of platelets in haemostasis has long been known, the involvement in these cells in wound healing and tissue repair is more recent knowledge. The sequence of all the events leading to wound healing is finely regulated by an extensive communication that is established between the different cellular constituents involved and, as explained above, is mainly mediated by GF and cytokines. Some of these are released by activated platelets as well as by many other types of cells (Table I), such as macrophages, mast cells, T lymphocytes, endothelial cells, and keratinocytes; once stimulated, these cells synthesise GF and release them, some hours or days after receiving the stimulus. In contrast, platelets, which store GF inside their alpha-granules, quickly release these factors once activated, modulating many processes involved in wound healing, including:
Table I.
Main growth factors and cytokines involved in wound healing
| Growth factors and cytokines | Cell source | Effects |
|---|---|---|
| Ang-1 | Platelets, neutrophils | Induces angiogenesis stimulating migration and proliferation of endothelial cells. Supports and stabilises blood vessel development via the recruitment of pericytes3,5 |
| CTGF | Platelets, fibroblasts | Stimulates leucocyte migration; promotes angiogenesis; activates myofibroblasts stimulating ECM deposition and remodelling6–8 |
| EGF | Platelets, macrophages | Regulates epithelial migration, fibroblast/epithelial/endothelial proliferation; promotes M2 differentiation1,4,5,9 |
| FGF | Platelets, macrophages, mast cells, T lymphocytes, endothelial cells, fibroblasts | Calls macrophages, fibroblasts, endothelial cells; regulates fibroblast/monocyte/ epithelial/endothelial migration, fibroblast/epithelial/endothelial proliferation, collagenase synthesis; induces angiogenesis; contributes in wound contraction1,3,5,9 |
| HGF | Platelets, mesenchymal cells | Regulates cell growth and motility in epithelial/endothelial cells, supporting epithelial repair and neovascularisation during wound healing5,10,11 |
| IGF-I | Platelets and all tissues | Calls fibroblasts3,5,12 |
| KGF | Fibroblasts, mesenchymal cells | Regulates epithelial migration and proliferation1,9,13 |
| PDGF | Platelets, endothelial cells, macrophages, smooth muscle cells | Calls neutrophils, macrophages, fibroblasts, endothelial cells, mesenchymal stem cells; regulates fibroblast proliferation, collagen and collagenase synthesis; supports angiogenesis; aids in wound contraction; promotes M2 differentiation1,3,5,6,9 |
| PF-4 | Platelets | Calls leucocytes and regulates their activation. Microbiocidal activities3,5 |
| SDF-1α | Platelets, endothelial cells, fibroblasts | Calls CD34+ cells, induces their homing, proliferation and differentiation into endothelial progenitor cells stimulating angiogenesis. Calls mesenchymal stem cells and leucocytes3,5 |
| TGF-α | Macrophages, T lymphocytes, keratinocytes | Regulates fibroblast/epithelial proliferation, epithelial migration; involved in angiogenesis1 |
| TGF-β | Platelets, endothelial cells, T lymphocytes, keratinocytes, macrophages | Involved in fibroblast proliferation, fibroblast/monocyte migration, collagen and collagenase synthesis; modulates angiogenesis1,3,5,9 |
| TNF | Macrophages, mast cells, T lymphocytes | Regulates monocyte migration, fibroblast proliferation, macrophage activation, angiogenesis1,3,9 |
| VEGF | Platelets, macrophages, keratinocytes, endothelial cells | Induces angiogenesis stimulating migration and proliferation of endothelial cells; regulates collagenase synthesis and collagen secretion; calls macrophages and granulocytes1,3,5 |
Ang-1: angiopoietin-1; CTGF: connective tissue growth factor; ECM: extracellular matrix; EGF: epidermal growth factor; FGF: fibroblast growth factor; HGF: hepatocyte growth factor; IGF-I: insulin-like growth factor; KGF: keratinocyte growth factor; PDGF: platelet-derived growth factor; PF-4: platelet factor 4; SDF-1α: stromal cell-derived factor-1α; TGF: transforming growth factor; TNF: tumour necrosis factor; VEGF: vascular endothelial growth factor.
angiogenesis: GF stimulate endothelial cell proliferation, migration, and association in tubular structures, as well as the recruitment of perivascular cells providing the damaged tissue with new blood vessels;
renovation of connective tissue: GF orchestrate all those fibroblast activities needed to restore damaged tissue, from proliferation to migration to collagen synthesis;
restoration of tissue-specific cell types: GF modulate the proliferation and differentiation of tissue mesenchymal stem cells into tissue-specific cell types5.
The contents of alpha-granules not only contribute to tissue healing but also take part indirectly in antimicrobial activities since platelets release chemokines and cytokines that recruit and activate immune cells; upon activation they also release molecules with direct microbicidal properties such as reactive oxygen species, kinocidins (i.e. chemokines that exert direct antimicrobial activity such as platelet factor 4 [PF4]), defensins (e.g. β-defensin 2), thrombocidines (e.g. neutrophil-activating peptide-2 [NAP-2] and connective tissue-activating peptide-III [CTAP-III]) and proteases, playing important roles in the defence against pathogens3,14.
PLATELET DERIVATIVES
For many years now, the clinical use of platelet derivatives as an adjuvant to hard and soft tissue healing, in virtue of their GF content, has been widely adopted in various medical and surgical procedures, ranging from ophthalmology, skin ulcers, gynaecological and urogenital disorders to almost all fields of surgery - orthopaedic, oral and maxillofacial, cosmetic, cardiothoracic, vascular, otorhinolaryngological, and neurosurgery15,16.
Platelet derivatives include platelet-rich plasma (PRP), fibrin glue (FG), platelet gel (PG), plasma rich in growth factors (PRGF), platelet-rich fibrin (PRF), hyperacute serum (HAS), serum eye-drops (E-S), PRP eye-drops (E-PRP) and platelet lysates (PL)17.
Platelet derivatives can be autologous or allogeneic. The use of autologous platelet derivatives avoids any type of virus or prion contamination and immune reactions associated with allogeneic proteins. Although the volume of autologous platelets may be sufficient for clinical use, limitations of these types of products include wide variability in quality due to changes in platelet counts and GF content that are influenced by the patient’s age and biological conditions. In contrast, allogeneic platelet derivatives are prepared from healthy donor blood using standard working procedures that guarantee products enriched in platelets and GF, with minimal contamination from red blood cells and leucocytes than single-donor batches18,19.
There are no standardised protocols for the preparation of platelet derivatives in clinical practice: the parameters considered during the preparation include the number and concentration of platelets over baseline, centrifugation conditions and activation of platelets. All these parameters contribute to the composition of platelet derivatives and, ultimately, to their therapeutic effect20–22.
The general method to prepare platelet derivatives involves sequential steps: whole blood is collected with or without an anticoagulant (e.g. in acid-citrate-dextrose tubes), centrifuged to concentrate the platelets, then activated to allow the alpha-granules to release their biological molecules23. The platelets are concentrated according to protocols that include centrifugation steps with different speeds (100–300 g), times (4–20 minutes) and temperatures (12–26°C). The number of platelets in the final product is four to five times greater than the baseline value; all suspensions of platelets in plasma with a platelet count greater than the baseline count can be identified as PRP or platelet concentrates17,20–23.
To obtain a product with a higher concentration of GF, some protocols produce platelet concentrations up to ten times higher than the baseline value by combining low temperatures, high speeds, and various centrifugation cycles6,23,24. These conditions can, however, induce premature activation of the platelets, thereby altering the properties of the final product.
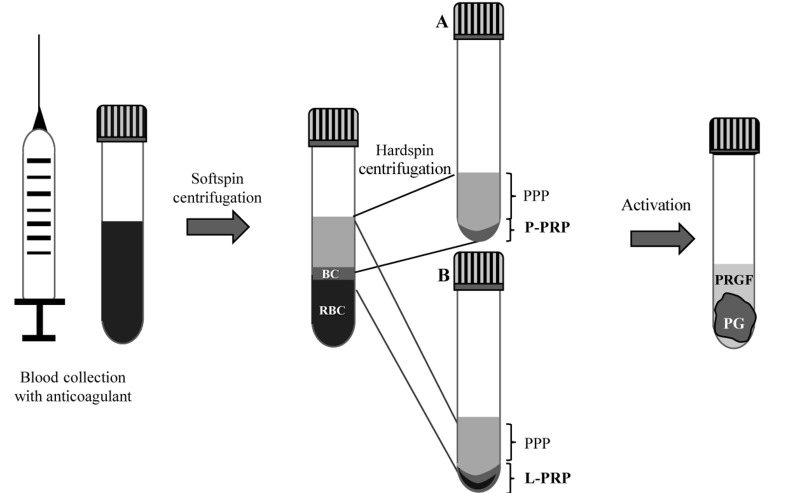
In order to produce pure platelet-rich plasma (P-PRP), also known as leucocyte-poor platelet-rich plasma (LP-PRP), the whole blood is collected and centrifuged at low speed to separate the red blood cells - which settle at the bottom of the tube - from white blood cells/platelets and a upper plasma layer, which sediment as an intermediate layer (called the buffy coat) and higher layer, respectively. The upper layer is composed of plasma and a gradient of platelets: poor on the surface, intermediate in the middle and rich near the buffy coat23. The upper layer and just the superficial layer of buffy coat are transferred into a sterile tube and then centrifuged at high speed to obtain the P-PRP, which consists of the small volume at the bottom of the tube (about the lower one-third) and is mainly composed of platelets; the resulting supernatant (about the upper two-thirds) constitutes platelet-poor plasma (PPP)25 (Figure 1A).
Figure 1.
Two-step centrifugation protocol to obtain pure platelet-rich plasma (P-PRP), leucocyte- and platelet-rich plasma (L-PRP), platelet gel (PG) and plasma rich in growth factors (PRGF)
The first step consists of “softspin” centrifugation of the whole blood that leads to three layers: an upper layer containing mostly platelets and white blood cells, an intermediate “buffy coat” (BC) layer rich in white blood cells and a bottom layer consisting mostly of red blood cells (RBC). To produce the P-PRP the upper layer and superficial BC are transferred to a new tube and “hardspin”-centrifuged to obtain P-PRP at the bottom of the tube and platelet-poor plasma (PPP) on the top (A). To obtain L-PRP, instead, the upper layer and the whole BC along with some RBC are transferred to a new tube and “hard spin”-centrifuged to obtain L-PRP at the bottom of the tube and PPP at the top (B). The PPP is removed and P-PRP or L-PRP eventually activated to induce clotting and produce the PG and PRGF. Fibrin glue (FG) is obtained from coagulation of PPP17,23,25.
PPP has a very low cellular content; after induction of the coagulation cascade, fibrinogen polymerises into fibrin monomers which finally form a three-dimensional network called FG that has a high content of fibrin along with a paucity of platelet-derived factors, except for insulin growth factor-1 (IGF-1) and hepatocyte growth factor (HGF)20,26,. In spite of this, in some animal models, FG was shown to be more effective than PG for the preservation of sockets with buccal dehiscence27. This may be because fibrin can act as a natural biomaterial scaffold, having a structure very similar to the native ECM and thus a good capacity to bind cells. It has also been proven that it is biocompatible and biodegradable, which are essential features for its use as a scaffold in regenerative medicine applications28.
In order to produce leucocyte- and platelet-rich plasma (L-PRP), after the low speed centrifugation of whole blood, the entire buffy coat (avoiding red blood cell contamination) along with the upper layer is transferred into a tube and then centrifuged to obtain the L-PRP (enriched in platelets and white blood cells) at the bottom of the tube and PPP above (Figure 1B).
The process of activation of the PRP, which is due to the action of thrombin and the generation of fibrin, is generally achieved by the addition of calcium chloride or calcium gluconate and leads to the production of the corresponding PG accompanied by release of the biologically active molecules contained in the alpha-granules29,30. The activation protocols define a time from 20 min to 1 hour at 37°C or room temperature30.
PG is a platelet derivative that is mostly used to treat ulcers and wounds. Forty minutes after its activation, a liquid exudate forms. This exudate, also called PRGF (Figure 1), contains plasma proteins and the molecules released by activated platelets17.
PRF products are second-generation platelet derivatives resulting from coagulation induced during centrifugation. The advantages of these products compared to the PRP are due to the autologous process of platelet degranulation that occurs physiologically without biochemical modifications: this allows the creation of a fibrin network that can better support the release of cytokines and cell migration31,32.
A further activation method, leading to the “photo-activated PRP”, consists of exposing platelets to ultraviolet (UV) light irradiation. Although the mechanism is not well understood, there is evidence of positive effects of intra-articular injections of UV-activated PRP in the orthopaedic field33.
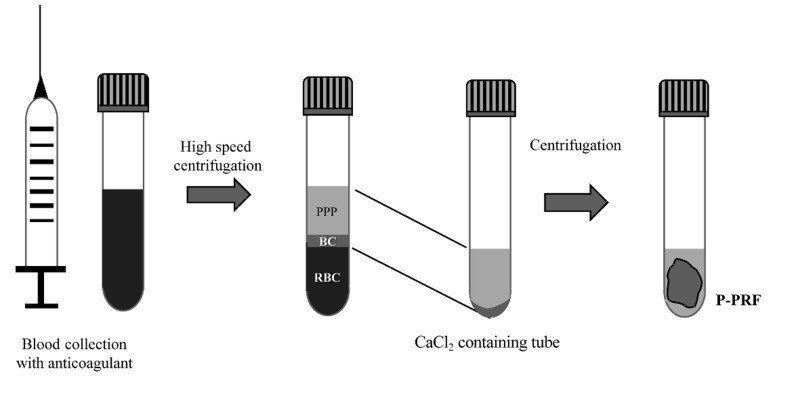
To obtain pure PRF (P-PRF), Fibrinet PRFM tubes are used; whole blood is collected in the presence of anticoagulant (tri-sodium citrate) and a separator gel and centrifuged. After transferring the buffy coat and the upper layer into a second tube, calcium chloride is added to induce clotting and the tube is centrifuged immediately, leading to the formation of a fibrin-rich and very stable clot (Figure 2). The separator gel removes mostly white blood cells enabling P-PRF to be obtained31.
Figure 2.
Schematic description of the procedure to obtain pure platelet-rich fibrin (P-PRF)
Whole blood is collected into specific Fibrinet PRFM tubes (Cascade Medical Enterprises Inc, Wajne, NJ, USA) and centrifuged at high speed. After transferring the buffy coat (BC) and upper layer of plasma into a second tube, CaCl2 is added and centrifuged immediately, allowing the formation of a fibrin-rich and very stable clot31. PPP: platelet-poor plasma; RBC: red blood cells.
Leucocyte- and platelet-rich fibrin (L-PRF), on the other hand, is obtained from whole blood collected without anticoagulant and centrifuged: platelet activation and fibrin formation take place immediately. After centrifugation three phases are formed: the red blood cells at the bottom, the plasma on the surface and the L-PRF clot in the middle17,31. L-PRF is mainly composed of a dense fibrin matrix that enables enmeshment of platelets and leucocytes and is used as a three-dimensional scaffold for tissue regeneration. Unlike the other platelet derivatives PRF incorporates all the cells that remain entrapped in fibrin clots and also contains several molecules and GF of therapeutic interest (FGF-2, VEGF, PDGF, TGF-β1) other than fibrin, making it very useful as a scaffold to support wound healing34.
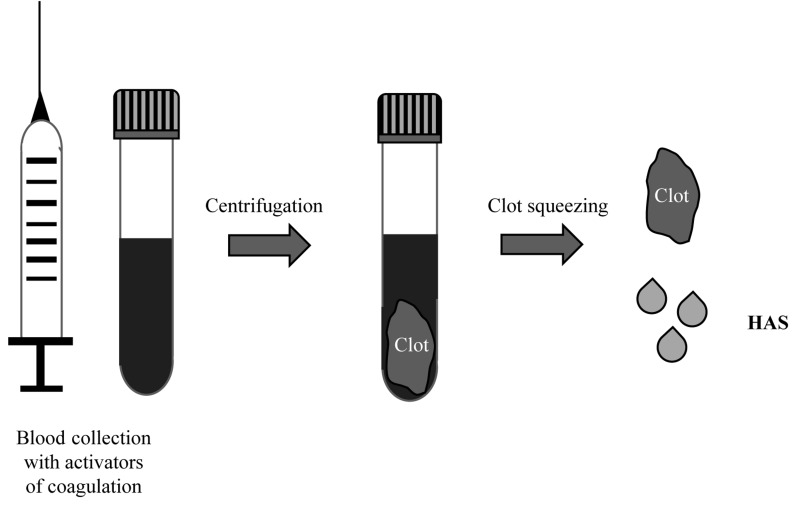
If at the end of the PRF clotting the serum is squeezed out from the PRF clot, hyperacute serum (HAS) is obtained, which, despite its method of preparation method being very similar to that of PRP, has a peculiar ionic and protein composition that could be beneficial for some cell functions (Figure 3)35.
Figure 3.
Schematic procedure to obtain hyperacute serum (HAS)
In order to prepare HAS, blood is collected into specific tubes containing coagulation activators (VACUETTE® Z Serum C/A tubes, Greiner Bio-One International GmbH, Kremsmünster, Austria) and centrifuged. Once the fibrin clot has been formed, it is placed in a new container and squeezed to obtain the serum portion, resulting in HAS35.
E-S and E-PRP are two further formulations used in ophthalmology because GF and proteins promote proliferation, migration, and adhesion of corneal epithelial cells36.
E-S derives from the spontaneous coagulation of whole blood; after centrifugation the clot separates from the serum, which is diluted with saline solution or antibiotics to constitute E-S. E-PRP derives from PRP: after the first centrifugation of whole blood the upper portion is aspirated with a syringe taking care to avoid contamination by red blood cells. The syringes thus prepared are stored at 4°C until used as normal eye drops36.
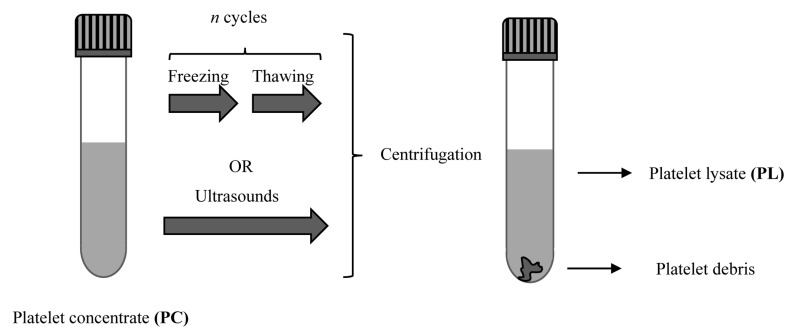
PRP can be used to prepare PL: alpha-granules are disrupted by freezing-thawing cycles or by ultrasound and thus release their content of biologically active molecules. The temperatures used in the treatment range from −80°C to 37°C, then centrifugation follows to separate the cellular debris; the supernatant containing the GF constitutes the PL17,37 (Figure 4). The GF contained in PL include VEGF, TGF-β1, and FGF-2 at levels comparable to those in other platelet derivatives; the level of PDGF in PL is, however, significantly lower than that in PRGF38. PL has the advantage that it can preserved without the levels of GF being changed; it has been demonstrated that the stability of the GF in PL is maintained during storage for 5 months at −20°C39 or for up to 9 months at 4°C, if previously lyophilised, with the GF maintaining their biological activities40.
Figure 4.
Schematic procedure to obtain platelet lysate (PL) from platelet concentrate or platelet-rich plasma
Platelets and alpha granules are broken using freezing-thawing cycles or by ultrasound; centrifugation is performed to separate the cellular debris from the supernatant rich in growth factors (i.e. the PL)37.
IN VITRO EVIDENCE
Platelet derivatives are now used widely in most clinical fields. Over time, many in vitro studies have been conducted to elucidate the biological processes triggered by platelets in cell types involved in in vivo wound healing, providing, mostly but not always, indications of their supportive effect for clinical applications. Numerous cell lines, sometimes of animal origin, have been used as the target of platelet derivatives. The derivatives have been tested in activated or not-activated forms, prepared by different techniques and starting from different platelet concentrations. For these reasons, opposing outcomes have often been obtained, making the interpretation of results very difficult. The information gained from rigid, controlled, reproducible in vitro studies is useful for a clearer understanding of the biological mechanisms triggered, to give indications on the most effective doses and conditions and, ultimately, to support or discourage the clinical use of platelet derivatives.
Fibroblasts are among the cells most extensively studied in vitro because they are involved in the healing of almost all damaged tissues in the body. They are dispersed in connective tissue contributing to tissue architecture and function by secreting ECM, especially type I/III collagen; they play pivotal roles in all three stages of wound healing, being involved in wound contraction, in the deposition of ECM and its subsequent remodelling41. When a tissue is injured, the surrounding fibroblasts proliferate, migrate into the wound and produce ECM. As such, they are involved in many clinical conditions related to wound healing; for example, fibroblasts from chronic wounds exhibit decreased proliferation, altered patterns of cytokine release, as well as decreased release of MMP-1 and active MMP-2 associated with increased levels of tissue inhibitor of metalloproteinases-1 and −2 (TIMP-1 and TIMP-2)42. In contrast, fibroblasts from keloid display increased proliferation and resistance to apoptosis associated with alterations in collagen production and degradation43. Some studies have highlighted how platelet derivatives could be of support in regenerative medicine, promoting cell growth and collagen biosynthesis44, and a potential therapeutic tool for those diseases in which fibrosis plays a major aetiological role, activating negative feedback signalling for TGF-β1 which, in turn, downregulates connective tissue growth factor (CTGF) expression45,46. In fact, excessive collagen synthesis and deposition by fibroblasts are regulated by cytokines, especially TGF-β and its downstream mediator CTGF47.
Fibroblasts also represent one of the precursor cells for myofibroblast differentiation. Myofibroblasts, being able to produce ECM and exert contractile forces, are among the most important cells in the creation of provisional scar tissue, a crucial step in the healing process. They usually disappear once the tissue has been repaired; the temporary scar is degraded and replaced by regenerated tissue. In contrast, in fibrotic diseases, myofibroblasts persist in an activated state, producing excessive ECM that can damage tissue architecture and function46. PRP has been considered as one of the possible therapeutic options to oppose fibrosis, but studies have produced contrasting results. Some of them indicated that PRP induces cell differentiation of fibroblast-like cells to myofibroblast-like cells48, while others suggested that PRP can prevent and inhibit TGF-β1-induced differentiation49. The contradiction in these findings probably stems from the different concentrations of platelets in the PRP used or the heterogeneity in the procedures employed to prepare the PRP, which yield formulations containing different doses of GF that can have pro-fibrotic (TGF-β) or anti-fibrotic (FGF-2) effects. For these reasons, further in vitro studies are needed to generate clear evidence on the role of PRP in fibrosis.
Fibroblasts also play an important role in photo-aging, a term used to indicate the changes to skin induced by exposure to solar radiation: indeed, UV irradiation induces a senescence-like phenotype. In photo-aged skin, collagen bundles undergo structural changes partly due to decreased collagen synthesis accompanied by increased degradation by MMP, and partly due to the reduced proliferation of fibroblasts50. For these reasons, many in vivo and in vitro studies have tried to determine the possible benefits deriving from the use of PRP, highlighting that this treatment counteracts the negative effects of UV irradiation51.
PRP is also extensively used in dentistry, as a boost to grafting materials to increase regeneration of bone and periodontal tissues; nevertheless, many researchers have found no benefits from its use. These observations generated controversy about the effectiveness of PRP in clinical procedures, but also encouraged research to better understand, using gingival fibroblasts in vitro, the biological basis for the use of PRP52,53.
Musculoskeletal cells have also been extensively studied. Platelet derivatives are widely used in orthopaedic procedures to facilitate wound haemostasis and to treat several musculoskeletal injuries and disorders, including tendinopathies and rotator cuff disease54,55. Tendons, in fact, have poor regenerative capacity because of their limited vascularisation and low cell density, making tendon injuries a difficult clinical problem and encouraging studies to evaluate the effect of PRP on their healing.
The healing of injured muscle, on the other hand, involves many cells, including muscle satellite cells, quiescent stem cells with very important features for muscle healing. These cells contribute to muscle regeneration because, once activated by an injury, they proliferate, undergo self-renewal, migrate to the damaged site and differentiate to generate new myofibres56. Over the last years many in vivo and in vitro studies have been conducted to test the effect of PRP on stimulating musculoskeletal tissue healing, supported by the hypothesis that the GF contained in the products could enhance regeneration, for example by modulating cell migration, proliferation, differentiation and acting on satellite cells. It must, however, be highlighted that evidence of the efficacy of PRP has been highly variable and the products studied have led to very heterogeneous outcomes54,57–60.
In the past few years, great interest has also arisen regarding the effects of platelet derivatives on stem cells. Due to their differentiation potential and high capacity for self-renewal and in vitro expansion, mesenchymal stem cells have been considered for possible use in wound healing processes. The main function of mesenchymal stem cells in wound healing relies on their ability to release cytokines and GF that act in a paracrine way and modulate several processes: they activate dermal fibroblasts, their proliferation, and migration; promote collagen production; activate keratinocytes; and increase angiogenesis and neovascularisation. Mesenchymal stem cells can also contribute to wound healing by differentiating into other cells such as fibroblasts, keratinocytes, and epithelial cells61. Many studies, to date, have explored the effects of PRP on various biological activities of mesenchymal stem cells, highlighting the ability of the products to stimulate proliferation and preserve multipotency62–64. Numerous studies have also been conducted to investigate how to use platelet derivatives in order to improve the performance of in vitro expansion and differentiation potential of stem cells and thereby obtain a number of cells sufficient for subsequent in vivo transplantation65,66. Stem cells isolated from synovium, for example, can be harvested for clinical applications rather easily without causing donor site morbidity and have been confirmed to have chondrogenic, osteogenic, and adipogenic potential; however, it is not possible to harvest enough cells for immediate use and prior in vitro expansion of the cells is always necessary.
In the case of cartilage, platelet derivatives have not only been considered to induce the healing/regeneration of injured tissue directly67, but also to provide a scaffold to support cartilage regeneration68 and to be carriers of biochemical stimuli able to overcome the state of dedifferentiation of chondrocytes cultured for autologous chondrocyte implantation. Articular cartilage, in fact, is not able to regenerate itself sufficiently to repair injuries or defects that occur after a trauma. One clinical treatment available to overcome this problem is autologous chondrocyte implantation: a small piece of the patient’s cartilage is removed and the chondrocytes are isolated, grown in the laboratory and re-implanted, with the hope that they will be able to repair the damaged area, restoring a new layer of articular cartilage69.
Table II reports a summary of some of the most recent in vitro studies, performed on a wide variety of human cell types involved in the repair/regeneration of different tissues and organs after treatment with platelet derivatives. It can be noted that many preparation methods, activation strategies, and platelet concentrations have been explored37,44–46,51–53,63,70–81.
Table II.
Summary of some of the most recent in vitro studies performed using different platelet derivatives to treat a wide variety of human cell types involved in tissue repair/regeneration processes of different tissues
| Cell type | Experimental setting | Main results | Possible impact from in vivo application |
|---|---|---|---|
| Foreskin fibroblasts | 10% activated PRP | No promotion of proliferation, slight stimulation of motility | No effects70 |
| Hypertrophic scar dermal fibroblasts | 5% activated PRP | Activation of negative feedback signalling for TGF-β1 which, in turn, downregulates connective tissue growth factor expression | Improvement of hypertrophic scars45 |
| Skin fibroblasts | 1% and 5% PRP, not activated or Ca2+ -activated PRP supernatant | Increase of collagen synthesis and stimulation of prolidase activity; increase of β1-integrin receptor, focal adhesion kinase and phosphorylated mitogen-activated protein kinases. | Promotion of cell growth and collagen biosynthesis, which could be of support in regenerative medicine; PRP was the most effective platelet derivative among those analysed44 |
| Dermal fibroblasts | Activated PRP | Negative regulation of fibroblast-to-myofibroblast transition inhibiting TGF-β1/Smad3 signalling | PRP could be a potential therapy in those diseases in which fibrosis plays a major aetiological role46 |
| Dermal fibroblasts | Chronic UVA irradiation followed by 25% and 50% platelet-rich fibrin lysate treatment | UVA irradiation decreased the biological activities of fibroblasts (collagen deposition and migration rate). Treatment with platelet-rich fibrin lysate lessened this negative effect. | Platelet-rich fibrin lysate could be a good candidate for treating UVA-induced photo-aging of skin51 |
| Keratinocytes | PRGF (1:10–1:20–1:50) | Decrease of keratins-1 and −10 (early markers) and increase of involucrin and transglutaminase-1 (late markers). Induction of antimicrobial peptides human β-defensins-2 and -3 and psoriasin |
PRGF induces keratinocyte differentiation, which is an important step, being the endpoint in wound reepithelisation. It offers broad antimicrobial effects leading to a greater ability to promote wound closure71,72 |
| Gingival fibroblasts | 10%, 25%, 50%, 75% Caactivated and non-activated PRP | Increase in proliferation rate, with the strongest stimulation reached with the 10% activated PRP | Activated PRP has greater efficacy compared to nonactivated PRP52 |
| Gingival fibroblasts | 1%, 2%, 5% Ca-activated PRP | Increase in proliferation and migration | Stimulation by PRP could be very important in gingival tissue repair and wound healing53 |
| Fibroblast-like tenocytes | Activated PRP combined or not with IL-1β (which simulates tendon inflammation) | In the absence of IL-1β, PRP induced expression of pro-inflammatory cytokines and MMP (stimulating an inflammatory state) whilst in IL-1β-induced inflammation it improved inflammation, downregulating proinflammatory cytokines and MMP and upregulating some anti-inflammatory cytokines and inhibitors. | PRP has pleiotropic effects on tenocyte biology, depending on the presence or absence of an inflammatory state induced by IL-1β: it has an antiinflammatory effect on tenocytes only in inflammatory states typical of tendinopathic conditions while, otherwise, it stimulates a pro-inflammatory state73 |
| Fibroblasts from rotator cuff tendons | LP-PRP | Induction of proliferation. | LP-PRP has a positive effect on tendon wound healing and could be useful to treat tissue degeneration74 |
| Tenocytes | AlloPL, PRP, PC, PL | PC and alloPL, characterised by a higher content of growth factors, were not the products stimulating greatest tenocyte viability or expression of ECM proteins but did have the strongest effects on HGF expression and downregulation of COX-1 expression. | Both HGF and COX-1 are pain-associated molecules: HGF is a pain antagonist and COX-1 is a pain marker, thus, their modulation could result in pain reduction when using PRP in clinical applications37 |
| Tenocytes | Co-culture of tenocytes with mesenchymal stromal cells adding 10% fresh/frozen CaCl2-activated PRP | MSC alone could increase tenocyte migration and ECM production (fibronectin, collagen I and aggrecan); PRP acts as an adjuvant inducing greater effects, with the fresh PRP being more effective than the frozen one | PRP combined with MSC could lead to better tendon wound healing75 |
| Synoviocytes | 0%, 5%, or 10% PRGF with or without TNF-α (to create an in vitro model of rheumatoid arthritis). | Increase of cell viability by addition of PRGF; reduction of TNF-α, IL-6 and IL-1β release and increase of VEGF and IL-10 secretion when cells were pre-stimulated with TNF-α | Since TNF-α and IL-6 are usually increased in inflammatory states of synovia whereas IL-10 and VEGF are reduced, the data suggest an anti-inflammatory, and thus anti-arthritic, effect of PRGF76 |
| Fibroblast-like synoviocytes | 20% PPP or activated PRP in a cell model of rheumatoid arthritis induced by LPS. | PRP, more than PPP, counteracted the LPS-induced expression of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6). LPS also stimulated cell viability and reduced the percentage of apoptotic cells but PRP reversed this trend | Synovial tissue hyperplasia is typical of rheumatoid arthritis and synoviocyte growth plays a key role in this pathology, thus their inhibition by PRP is reassuring for in vivo applications77 |
| Articular chondrocytes | 10% PRP or HAS | Proliferation increased compared with HAS but not with PRP supplementation. PRP was capable of re-differentiating the de-differentiated chondrocytes while FCS or HAS led to further de-differentiation | Although HAS has positive effects on chondrocyte proliferation, PRP could be a better choice than standard FCS for chondrocyte expansion for clinical use in autologous chondrocyte implantation, being able to stimulate both proliferation and re-differentiation78 |
| Endometrial stromal fibroblasts and MSC | 5% PRP or PPP, in their activated and not activated forms | Increase in cell viability and migration after treatment with activated PRP; not activated PRP and PPP stimulated these processes but to a lesser extent compared to controls. No evidence of mesenchymal-to-epithelial transition. Activated PRP treatment upregulated several MMP (MMP-1, -3, -7, -26) and inflammatory markers (IL1A, IL1B, IL1R2, CCL5, CCL7, CXCL13), modulating inflammation and chemotaxis | PRP could be used to promote endometrial regeneration in clinical situations with compromised endometrial growth (such as Asherman's syndrome and atrophic/thin endometrial lining in infertile patients)79 |
| Adipose-derived stem cells | 10% PRP | No promotion of proliferation or migration | No effects70 |
| Adipose-derived stem cells | 5% PRP | Increased chondrogenic/osteogenic differentiation | Support for translational applications for the management of osteochondral defects in the field of regenerative medicine80 |
| BM-MSC | 1:50 and 1:100 PRP | BM-MSC viability and proliferation were positively regulated. The PRP/BM-MSC combination was more effective than PRP alone in sustaining the proliferation and myogenic differentiation of myoblasts | PRP alone or, to a greater extent, in combination with BM-MSC, may favour repair mechanisms in damaged skeletal muscle tissue, promoting myogenic differentiation63 |
| MSC isolated from amniotic fluid | 10% PRP | Migration and proliferation rate increased | PRP could act as a regulator of processes involved in in vivo wound healing81 |
AlloPL: autologous platelet lysate from pooled donors; BM-MSC: bone marrow-derived mesenchymal stromal/stem cells; MSC: mesenchymal stem cells; CCL5: chemokine (C-C motif) ligand 5; CCL7: chemokine (C-C motif) ligand 7; CXCL13: chemokine (C-X-C motif) ligand 13; COX-1: cyclo-oxygenase 1; ECM: extracellular matrix; FCS: foetal calf serum; HAS: hyperacute serum; HGF: hepatocyte growth factor; IL: interleukin; LPS: lipolysaccharide; LP-PRP: leucocyte-poor platelet-rich plasma; MMP: matrix metalloprotease; PC: platelet concentrate; PL: platelet lysate; PPP: platelet-poor plasma; PRGF: plasma rich in growth factors; PRP: platelet-rich plasma; TGF-β1: transforming growth factor-β1; TNF-α: tumour necrosis factor-α; VEGF: vascular endothelial growth factor; UVA: ultraviolet A.
LEUCOCYTE CONTENT AND ANTIMICROBIAL ACTIVITIES
Although the use of platelet derivatives is justified by the release of platelet-derived GF leading to an acceleration of wound healing processes, many researchers have started to highlight the importance of leucocyte content in relation to antimicrobial activity. The use of platelet derivatives enriched in leucocytes leads to a decreasing number of infections in many clinical applications, which is an important aspect considering that, despite all the advances in surgical techniques and antibiotic use, the treatment of infected wounds is still associated with a high rate of complications82. Platelets are, in fact, an important source of antibacterial peptides (such as fibrinopeptide A and B, thymosin beta 4, platelet basic protein, connective tissue-activating protein 3, RANTES [regulated upon activation, normal T-cell expressed, and secreted] and PF4), but their antimicrobial role is not yet fully understood and requires further studies. Just as one example, Cieślik-Bielecka et al. found that L-PRP had an in vitro microbiocidal effect on some of the tested strains83. As far as concerns healing processes, it is still a subject of debate whether leucocytes contained in platelet derivatives have a positive or negative effect. On the one hand, leucocytes should hypothetically have beneficial effects, sustaining the immune response against infections and increasing GF release (thus contributing to angiogenesis, matrix production, and hypercellularity)84. On the other hand, they may release inflammatory cytokines (TNF-α and reactive oxygen species) exacerbating the inflammatory response, thus delaying tissue healing85, and inducing an increase in MMP levels, which could cause excessive matrix degradation and, consequently, inferior repair of wounded tissues and scar formation86. In fact, while several studies, mainly conducted in the orthopaedic field, have suggested that LP-PRP could induce more effective tissue healing when compared to leucocyte-rich, platelet-rich plasma (LR-PRP)86,87, other studies found no significant differences between them88,89. Given that the issue is not yet completely settled, clinicians should consider using LP-PRP or LR-PRP according to the specific pathology in order to achieve better clinical results from PRP therapy.
CONCLUSIONS
The heterogeneity of the results obtained from in vitro experiments on cells treated with platelet derivatives is mirrored by the variability of clinical outcomes and can be explained by the different experimental settings used each time. In fact, the type of platelet derivative and the method used for its production can have profound effects on the final results.
For example, an important parameter to consider is whether or not the platelets are activated. Some authors reported that the maximum effect on cells was achieved after the activation of PRP52,90. Furthermore, the very method chosen to activate platelets could affect the availability of GF, indeed, it has been shown that PRP activation performed with different protocols influences the amount and kinetics of GF release91. This suggests that the PRP activation strategy should be chosen according to the pursued biological effects in the tissue to be healed. Another, often underestimated but very important, parameter is the platelet concentration, which is partly dependent on marked differences in baseline platelet counts between individual patients, leading to variability in PRP composition and, therefore, concentrations of GF. Several studies have demonstrated, in vitro, that cells respond in a dose-dependent manner, but that very high concentrations of GF are not necessarily a prerequisite for optimal stimulation of cell processes, and may in fact be counterproductive. Numerous studies have shown that high GF concentrations can have a detrimental effect and can be more an obstacle than an advantage53,58,92–94. It is possible that the quantity of receptors on the cell surface is limited and thus, once the levels of GF are too high for available receptors, they became excessive and affect cell function negatively53.
For example, in human primary tenocytes an excessively high concentration of platelets was shown to have an inhibitory effect on proliferation, migration, and the production of collagen type I. In contrast, MMP production increased with increasing platelet concentration, which could be detrimental because excessive proteolysis may impair the mechanical stability of tendons58. Similarly, we showed that PG supernatant was able, in vitro, to stimulate all the necessary mechanisms for fibroblasts to restore normal tissue during wound healing in vivo, such as proliferation, migration, and invasion, but that in this case, too, excessively high concentrations had an inhibitory effect on the processes92. Many other studies have indicated similar repercussions. Choi et al. reported a similar effect on the viability and proliferation of alveolar bone cells95. Graziani et al. demonstrated that the maximum effect on cell proliferation was achieved with a 2.5× concentration of of activated PRP, while higher concentrations resulted in a reduction of cell proliferation96. Kakudo et al. observed that 5% activated PRP maximally promoted cell proliferation of human dermal fibroblasts and adipose-derived stem cells, but that activated PRP at 10% or 20% had a lesser effect90. Creeper et al. demonstrated that PRP could exert a positive effect on osteoblast and periodontal ligament cell migration, proliferation, and differentiation, but that the effects were concentration-specific with the maximal concentration of 100% being less effective than the 50% concentration97. Tavassoli-Hojjati et al. observed that 0.1% or 5% PRP supplementation was significantly more effective than 50% PRP supplementation in inducing fibroblast proliferation98. Klatte-Schulz et al. demonstrated that the higher concentrations of GF in two different platelet derivatives did not result in greater cell viability when compared with that induced by platelet derivatives containing lower levels of GF37.
These data strongly underscore that a high platelet or GF concentration is not necessarily related to a strong stimulatory effect but, instead, can have stagnating or inhibitory effects. Thus, in vitro studies have been and continue to be very useful for revealing how not all concentrations are equally functional to wound healing: maximal concentrations do not necessarily result in optimal clinical outcomes. Moreover, the inhibitory effects observed in vitro suggest that high concentrations could be counterproductive for wound healing in vivo too, prompting careful consideration of the clinical settings in which the products are to be used. The clinical effectiveness of different concentrations of platelet derivatives on different cell types still warrants further investigation to reach full standardisation.
It should not be forgotten that in vitro studies, although having many advantages (such as precise control of parameters and rapid results), also have some drawbacks. In fact, due to their architecture and organisation of all organs, there is a continuous interplay between different types of cells within an organ which it would be difficult to replicate in a two-dimensional monoculture in vitro: for example, cell density in the in vitro setting is usually less than 1% of the tissue situation (which affects cell signalling) and cell contacts with ECM are lacking. The accumulation of waste products, paralleled by a continues consumption of nutrients, is typical of culture conditions but is not a homeostatic condition, nor is the oxygen supply or the sudden exchange of media typical of in vitro cultures. Nonetheless, as highlighted by the large number of published papers in this field, in vitro studies remain important to provide indications on the biological processes sustained or hampered in vivo by using platelet derivatives99 and to support their clinical use.
Footnotes
The Authors declare no conflicts of interest.
REFERENCES
- 1.Krafts KP. Tissue repair: the hidden drama. Organogenesis. 2010;6:225–33. doi: 10.4161/org.6.4.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez AC, Cost TF, Andrada ZA, Medrado AR. Wound healing. A literature review. An Bras Dermatol. 2016;91:614–20. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nurden AT. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front Biosci (Landmark Ed) 2018;23:726–51. doi: 10.2741/4613. [DOI] [PubMed] [Google Scholar]
- 4.Wallace HA, Basehore BM, Zito PM. StatPearls [Internet] Treasure Island: [Accessed on 02/07/2019]. Wound Healing Phases. [Updated 2019 Aug 10] Available at: https://www.ncbi.nlm.nih.gov/books/NBK470443/ [Google Scholar]
- 5.Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29:556–68. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- 6.De Pascale MR, Sommese L, Casamassimi A, Napoli C. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev. 2015;29:52–61. doi: 10.1016/j.tmrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodelling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5( Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnylal S, Shi-wen X, Leoni P, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–32. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117:1–32. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 10.Li JF, Duan HF, Wu CT, et al. HGF accelerates wound healing by promoting the dedifferentiation of epidermal cells through β1-integrin/ILK pathway. Biomed Res Int. 2013;2013 doi: 10.1155/2013/470418. 470418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyagi H, Thomasy SM, Russell P, Murphy CJ. The role of hepatocyte growth factor in corneal wound healing. Exp Eye Res. 2018;166:49–55. doi: 10.1016/j.exer.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botusan IR, Zheng X, Narayanan S, et al. Deficiency of liver-derived insulin-like growth factor-I (IGF-I) does not interfere with the skin wound healing rate. PLoS One. 2018;13:e0193084. doi: 10.1371/journal.pone.0193084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen TT, Thao DT, Thuoc TL. An overview on keratinocyte growth factor: from the molecular properties to clinical applications. Protein Pept Lett. 2014;21:306–17. doi: 10.2174/09298665113206660115. [DOI] [PubMed] [Google Scholar]
- 14.Sut C, Tariket S, Aubron C, et al. The non-hemostatic aspects of transfused platelets. Front Med (Lausanne) 2018;5:42. doi: 10.3389/fmed.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Zapata MJ, Martí-Carvajal A, Solà I, et al. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: systematic review. Transfusion. 2009;49:44–56. doi: 10.1111/j.1537-2995.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 16.Lang S, Loibl M, Herrmann M. Platelet-rich plasma in tissue engineering: hype and hope. Eur Surg Res. 2018;59:265–75. doi: 10.1159/000492415. [DOI] [PubMed] [Google Scholar]
- 17.Mendes BB, Gómez-Florit M, Babo PS, et al. Blood derivatives awaken in regenerative medicine strategies to modulate wound healing. Adv Drug Deliv Rev. 2018;129:376–93. doi: 10.1016/j.addr.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Word Health Organization. Guidelines on good manufacturing practices for blood establishments. World Health Organ Tech Rep Ser. 2011;961( Annex 4):148–214. [Google Scholar]
- 19.European Directorate for the Quality of Medicines. Guide to the preparation, use and quality assurance of blood components: recommendation no R(95) 15. 18th ed. Strasbourg: Council of Europe Publishing; 2015. [Google Scholar]
- 20.Amable PR, Carias RB, Teixeira MV, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez M, Anitua E, Andia I. Poor standardization in platelet-rich therapies hampers advancement. Arthroscopy. 2010;26:725–26. doi: 10.1016/j.arthro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 23.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg. 2014;7:189–97. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24:173–82. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 25.Piccin A, Di Pierro AM, Canzian L, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15:333–40. doi: 10.2450/2016.0038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Hatakeyama I, Marukawa E, Takahashi Y, Omura K. Effects of platelet-poor plasma, platelet-rich plasma, and platelet-rich fibrin on healing of extraction sockets with buccal dehiscence in dogs. Tissue Eng Part A. 2014;20:874–82. doi: 10.1089/ten.tea.2013.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khodakaram-Tafti A, Mehrabani D, Shaterzadeh-Yazdi H. An overview on autologous fibrin glue in bone tissue engineering of maxillofacial surgery. Dent Res J (Isfahan) 2017;14:79–86. [PMC free article] [PubMed] [Google Scholar]
- 29.Lacoste E, Martineau I, Gagnon G. Platelet concentrates: effects of calcium and thrombin on endothelial cell proliferation and growth factor release. J Periodontol. 2003;74:1498–507. doi: 10.1902/jop.2003.74.10.1498. [DOI] [PubMed] [Google Scholar]
- 30.Mazzucco L, Balbo V, Cattana E, Borzini P. Platelet rich plasma and platelet gel preparation using Plateltext. Vox Sang. 2008;94:202–8. doi: 10.1111/j.1423-0410.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 31.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Choukroun J, Diss A, Simonpieri A, et al. Platelet rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord. 2016;17:67. doi: 10.1186/s12891-016-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimoto S, Fujita K, Sotsuka Y, et al. Growth factor measurement and histological analysis in platelet rich fibrin: a pilot study. J Maxillofac Oral Surg. 2015;14:907–13. doi: 10.1007/s12663-015-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kardos D, Simon M, Vácz G, et al. The composition of hyperacute serum and platelet-rich plasma is markedly different despite the similar production method. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030721. pii: E721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada C, King KE, Ness PM. Autologous serum eyedrops: literature review and implications for transfusion medicine specialists. Transfusion. 2008;48:1245–55. doi: 10.1111/j.1537-2995.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 37.Klatte-Schulz F, Schmidt T, Uckert M, et al. Comparative analysis of different platelet lysates and platelet rich preparations to stimulate tendon cell biology: an in vitro study. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19010212. pii: E212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardi M, Agostini F, Chieregato K, et al. The production method affects the efficacy of platelet derivatives to expand mesenchymal stromal cells in vitro. J Transl Med. 2017;15:90. doi: 10.1186/s12967-017-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauch C, Feifel E, Amann EM, et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28:305–16. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 40.Notodihardjo SC, Morimoto N, Kakudo N, et al. Comparison of the efficacy of cryopreserved human platelet lysate and refrigerated lyophilized human platelet lysate for wound healing. Regen Ther. 2018;10:1–9. doi: 10.1016/j.reth.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.desJardins-Park HE, Foster DS, Longaker MT. Fibroblasts and wound healing: an update. Regen Med. 2018;13:491–5. doi: 10.2217/rme-2018-0073. [DOI] [PubMed] [Google Scholar]
- 42.Cook H, Davies KJ, Harding KG, Thomas DW. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP-1, TIMP-2, and MMP-2 activity. J Invest Dermatol. 2000;115:225–33. doi: 10.1046/j.1523-1747.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang C, Murphy GF, Akaishi S, Ogawa R. Keloids and hypertrophic scars: update and future directions. Plast Reconstr Surg Glob Open. 2013;1:e25. doi: 10.1097/GOX.0b013e31829c4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guszczyn T, Surażyński A, Zaręba I, et al. Differential effect of platelet-rich plasma fractions on β1-integrin signaling, collagen biosynthesis, and prolidase activity in human skin fibroblasts. Drug Des Devel Ther. 2017;11:1849–57. doi: 10.2147/DDDT.S135949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nam SM, Kim YB. The effects of platelet-rich plasma on hypertrophic scars fibroblasts. Int Wound J. 2018;15:547–54. doi: 10.1111/iwj.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chellini F, Tani A, Vallone L, et al. Platelet-rich plasma prevents in vitro transforming growth factor-β1-induced fibroblast to myofibroblast transition: involvement of vascular endothelial growth factor (VEGF)-A/VEGF receptor-1-mediated signaling. Cells. 2018;7 doi: 10.3390/cells7090142. pii E142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–8. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos-Torrecillas J, Luna-Bertos E, Manzano-Moreno FJ, et al. Human fibroblast-like cultures in the presence of platelet-rich plasma as a single growth factor source: clinical implications. Adv Skin Wound Care. 2014;27:114–20. doi: 10.1097/01.ASW.0000443266.17665.19. [DOI] [PubMed] [Google Scholar]
- 49.Anitua E, de la Fuente M, Muruzabal F, et al. Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp Eye Res. 2015;135:118–26. doi: 10.1016/j.exer.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz E, Cruickshank FA, Christensen CC, et al. Collagen alterations in chronically sun-damaged human skin. Photochem Photobiol. 1993;58:841–4. doi: 10.1111/j.1751-1097.1993.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 51.Wirohadidjojo YW, Budiyanto A, Soebono H. Platelet-rich fibrin lysate can ameliorate dysfunction of chronically UVA-irradiated human dermal fibroblasts. Yonsei Med J. 2016;57:1282–5. doi: 10.3349/ymj.2016.57.5.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vahabi S, Yadegari Z, Mohammad-Rahimi H. Comparison of the effect of activated or non-activated PRP in various concentrations on osteoblast and fibroblast cell line proliferation. Cell Tissue Bank. 2017;18:347–53. doi: 10.1007/s10561-017-9640-7. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen PA, Pham TAV. Effects of platelet-rich plasma on human gingival fibroblast proliferation and migration in vitro. J Appl Oral Sci. 2018;26:e20180077. doi: 10.1590/1678-7757-2018-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624–34. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assirelli E, Filardo G, Mariani E, et al. Effect of two different preparations of platelet-rich plasma on synoviocytes. Knee Surg Sports Traumatol Arthrosc. 2015;23:2690–703. doi: 10.1007/s00167-014-3113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang S, Wang Z. Influence of platelet-rich plasma on proliferation and osteogenic differentiation of skeletal muscle satellite cells: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:453–62. doi: 10.1016/j.tripleo.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Navani A, Li G, Chrystal J. Platelet rich plasma in musculoskeletal pathology: a necessary rescue or a lost cause? Pain Physician. 2017;20:E345–56. [PubMed] [Google Scholar]
- 58.Giusti I, D’Ascenzo S, Mancò A, et al. Platelet concentration in plateletrich plasma affects tenocyte behavior in vitro. Biomed Res Int. 2014;2014 doi: 10.1155/2014/630870. 630870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984–99. doi: 10.1007/s00167-016-4261-4. [DOI] [PubMed] [Google Scholar]
- 60.Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6:211–20. doi: 10.5312/wjo.v6.i2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahmoudian-Sani MR, Rafeei F, Amini R, Saidijam M. The effect of mesenchymal stem cells combined with platelet-rich plasma on skin wound healing. J Cosmet Dermatol. 2018;17:650–9. doi: 10.1111/jocd.12512. [DOI] [PubMed] [Google Scholar]
- 62.Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 2014;4:52–62. [PMC free article] [PubMed] [Google Scholar]
- 63.Sassoli C, Vallone L, Tani A, et al. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018;372:549–70. doi: 10.1007/s00441-018-2792-3. [DOI] [PubMed] [Google Scholar]
- 64.Changsuo X, Hui H, Chao W, et al. Platelet-rich plasma affects proliferation and collagen production in mesenchymal stem cells. J Biomaterials Tissue Eng. 2018;8:750–5. [Google Scholar]
- 65.Gersch RP, Glahn J, Tecce MG, et al. Platelet rich plasma augments adipose-derived stem cell growth and differentiation. Aesthet Surg J. 2017;37:723–9. doi: 10.1093/asj/sjw235. [DOI] [PubMed] [Google Scholar]
- 66.Lai F, Kakudo N, Morimoto N, et al. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res Ther. 2018;9:107. doi: 10.1186/s13287-018-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartilage. 2013;21:1627–37. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP -derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008–18. doi: 10.1016/j.biomaterials.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 69.Mistry H, Connock M, Pink J, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21:1–294. doi: 10.3310/hta21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.López JF, Sarkanen JR, Huttala O, et al. Adipose tissue extract shows potential for wound healing: in vitro proliferation and migration of cell types contributing to wound healing in the presence of adipose tissue preparation and platelet rich plasma. Cytotechnology. 2018;70:1193–204. doi: 10.1007/s10616-018-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bayer A, Tohidnezhad M, Lammel J, et al. Platelet-released growth factors induce differentiation of primary keratinocytes. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/5671615. 5671615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bayer A, Lammel J, Tohidnezhad M, et al. The antimicrobial peptide human beta-defensin-3 is induced by platelet-released growth factors in primary keratinocytes. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/6157491. 6157491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jo CH, Lee SY, Yoon KS, et al. Allogenic pure platelet-rich plasma therapy for rotator cuff disease: a bench and bed study. Am J Sports Med. 2018;46:3142–54. doi: 10.1177/0363546518800268. [DOI] [PubMed] [Google Scholar]
- 74.Noh K, Liu XN, Zhuan Z, et al. Leukocyte-poor platelet-rich plasma-derived growth factors enhance human fibroblast proliferation in vitro. Clin Orthop Surg. 2018;10:240–7. doi: 10.4055/cios.2018.10.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veronesi F, Pagani S, Torricelli P, et al. PRP and MSCs on tenocytes artificial wound healing: an in vitro study comparing fresh and frozen PRP. Histol Histopathol. 2018;3:1323–34. doi: 10.14670/HH-18-018. [DOI] [PubMed] [Google Scholar]
- 76.Tohidnezhad M, Bayer A, Rasuo B, et al. Platelet-released growth factors modulate the secretion of cytokines in synoviocytes under inflammatory joint disease. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/1046438. 1046438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tong S, Liu J, Zhang C. Platelet-rich plasma inhibits inflammatory factors and represses rheumatoid fibroblast-like synoviocytes in rheumatoid arthritis. Clin Exp Med. 2017;17:441–9. doi: 10.1007/s10238-017-0449-2. [DOI] [PubMed] [Google Scholar]
- 78.Jeyakumar V, Niculescu-Morzsa E, Bauer C, et L. Platelet-rich plasma supports proliferation and redifferentiation of chondrocytes during in vitro expansion. Front Bioeng Biotechnol. 2017;5:75. doi: 10.3389/fbioe.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aghajanova L, Houshdaran S, Balayan S, et al. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J Assist Reprod Genet. 2018;35:757–70. doi: 10.1007/s10815-018-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scioli MG, Bielli A, Gentile P, et al. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng Regen Med. 2017;11:2398–410. doi: 10.1002/term.2139. [DOI] [PubMed] [Google Scholar]
- 81.Roubelakis MG, Trohatou O, Roubelakis A, et al. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. 2014;10:417–28. doi: 10.1007/s12015-013-9494-8. [DOI] [PubMed] [Google Scholar]
- 82.Cieślik-Bielecka A, Glik J, Skowroński R, Bielecki T. Benefit of leukocyte- and platelet-rich plasma in operative wound closure in oral and maxillofacial surgery. Biomed Res Int. 2016;2016 doi: 10.1155/2016/7649206. 7649206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cieślik-Bielecka A, Bold T, Ziółkowski G, et al. Antibacterial activity of leukocyte- and platelet-rich plasma: an in vitro study. Biomed Res Int. 2018;2018 doi: 10.1155/2018/9471723. 9471723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dohan Ehrenfest DM, Bielecki T, Jimbo R, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF) Curr Pharm Biotechnol. 2012;13:1145–52. doi: 10.2174/138920112800624382. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Y, Zhang J, Wu H, et al. The differential effects of leukocytecontaining and pure plateletrich plasma (PRP) on tendon stem/progenitor cellsimplications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173. doi: 10.1186/s13287-015-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cross JA, Cole BJ, Spatny KP, et al. Leukocytereduced plateletrich plasma normalizes matrix metabolism in torn human rotator cuff tendons. Am J Sports Med. 2015;43:2898906. doi: 10.1177/0363546515608157. [DOI] [PubMed] [Google Scholar]
- 87.Yin W, Qi X, Zhang Y, et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J Transl Med. 2016;14:73. doi: 10.1186/s12967-016-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yerlikaya M, Talay Çaliş H, Tomruk Sütbeyaz S, et al. Comparison of effects of leukocyte-rich and leukocyte-poor platelet-rich plasma on pain and functionality in patients with lateral epicondylitis. Arch Rheumatol. 2017;33:73–9. doi: 10.5606/ArchRheumatol.2018.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giusti I, Di Francesco M, D’Ascenzo S, et al. Leukocyte depletion does not affect the in vitro healing ability of platelet rich plasma. Exp Ther Med. 2018;15:4029–38. doi: 10.3892/etm.2018.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kakudo N, Minakata T, Mitsui T, et al. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352–60. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 91.Cavallo C, Roffi A, Grigolo B, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016;2016 doi: 10.1155/2016/6591717. 6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giusti I, Rughetti A, D’Ascenzo S, et al. The effects of platelet gel-released supernatant on human fibroblasts. Wound Repair Regen. 2013;21:300–8. doi: 10.1111/wrr.12025. [DOI] [PubMed] [Google Scholar]
- 93.Giusti I, Rughetti A, D’Ascenzo S, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–8. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 94.Rughetti A, Giusti I, D›Ascenzo S, et al. Platelet gel-released supernatant modulates the angiogenic capability of human endothelial cells. Blood Transfus. 2008;6:12–7. doi: 10.2450/2008.0026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi BH, Zhu SJ, Kim BY, et al. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg. 2005;34:420–4. doi: 10.1016/j.ijom.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 96.Graziani F, Ivanovski S, Cei S, et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212–9. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 97.Creeper F, Lichanska AM, Marshall RI, et al. The effect of platelet-rich plasma on osteoblast and periodontal ligament cell migration, proliferation and differentiation. J Periodontal Res. 2009;44:258–65. doi: 10.1111/j.1600-0765.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 98.Tavassoli-Hojjati S, Sattari M, Ghasemi T, et al. Effect of platelet-rich plasma concentrations on the proliferation of periodontal cells: an in vitro study. Eur J Dent. 2016;10:469–74. doi: 10.4103/1305-7456.195165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghallab A. In vitro test systems and their limitations. EXCLI J. 2013;12:1024–6. [PMC free article] [PubMed] [Google Scholar]