Abstract
Objectives:
To report biopsy-related and oncologic outcomes in a large prospective active surveillance cohort that was initiated in the pre-MRI era and to additionally identify clinical factors associated with disease reclassification in order to inform future studies designed to improve enrollment and follow-up on AS.
Methods:
Patients were prospectively enrolled at a single institution from 2006 to 2014 and followed until 2016. Men with Gleason 6 or 7 disease were eligible, and those with >6 months follow-up were included in the analysis. Patients were risk stratified based on clinical/pathologic criteria, including based on a combination of baseline and confirmatory biopsy tumor characteristics. Reclassification-free survival, based on tumor volume increase or Gleason score increase, was analyzed using multivariable Cox proportional hazards models.
Results:
Of 825 enrolled patients, 682 met inclusion criteria. Median follow-up was 40 months (range 6.6–126.8). Disease was reclassified in 249 (36.5%), and 157 (23.0%) underwent treatment. A single positive core with a negative confirmatory biopsy was significantly associated with time to reclassification (median not met vs. 43 months, log rank test p<0.001). Composite tumor length, defined as the combined tumor length between baseline and confirmatory biopsies, was associated with shorter Gleason upgrade-free survival (hazard ratio 1.24, 95% confidence interval 1.11–1.40, p<0.001) in multivariable analysis.
Conclusions:
Baseline stratification using clinical factors including tumor length may refine risk stratification and offer the foundation on which new systems that incorporate modalities such as MRI may be based.
Keywords: Active surveillance, Prostate cancer, Stratification, Reclassification, Progression
Introduction
Active surveillance (AS) is an increasingly utilized strategy for the management of prostate cancer and has been established as a preferred strategy for the management of low-risk disease.1–4 In stringently selected patients, AS is a safe management strategy, with 15-year cancer-specific mortality as low as 0.1%.5
Presently, clinical determinations such as life expectancy, laboratory findings (e.g., prostate-specific antigen [PSA] measurements), and pathologic results (e.g., Gleason score [GS] and measures of tumor volume on prostate biopsy) are used to both stratify patients at baseline and to determine progression; however, there is great variation in the application of these criteria.6 Accurate risk stratification is a critical, ongoing challenge for clinicians given its implications in patient counseling, treatment decisions, and surveillance intensity.
In recent years, modalities such as magnetic resonance imaging (MRI) and tissue-based genomic tests have demonstrated improvement in the determination of aggressive prostate cancer and have been suggested for use in stratification for patients eligible for AS.7 However, their use has not been prospectively validated in this population and has an as yet-undetermined impact on cost.8 As the application of these tests increases, it is critical to combine their use with clinical factors that offer a robust baseline assessment of patient risk for disease aggression and subsequent progression while on AS.
In addition to reporting intermediate-term results from an inclusive, prospective AS protocol that enrolled in the pre-MRI era, we aimed to investigate clinically-based factors associated with reclassification. In doing so, we sought to identify clinical factors that may help provide the foundation for future studies incorporation of imaging and genomic technologies in AS stratification and monitoring, potentially identifying groups in whom additional testing may be warranted.
Methods
AS clinical trial
This study was conducted by a multidisciplinary team of urologic surgeons, radiation oncologists, and medical oncologists and was approved by The University of Texas MD Anderson Cancer Center (MDACC) Institutional Review Board. All patients signed informed consent. The trial was registered with clinical.trials.gov NCT00490763.
Protocol criteria were adhered to as previously reported.9 In summary, any patient with localized prostate cancer diagnosed within 6 months who had pathologic grade group (GG) 1 or 2 disease was eligible for enrollment. Select patients with GG3 were also eligible based on treating physician discretion. All outside pathology underwent a central review by genitourinary pathologists at MDACC. All patients had ECOG performance status 0 or 1 and were eligible for primary treatment. The study opened in February of 2006. Starting in July of 2007, all patients with outside diagnostic biopsies were required to undergo confirmatory biopsy within 6 months of enrollment.
Based on prior work at our institution identifying a group that is generally low-risk for reclassification,10 we hypothesized that patients with a single disease-positive biopsy core, low PSA level, and negative confirmatory biopsy were at low risk of disease reclassification. The criteria for this low-risk group, termed “Group I,” were therefore established as follows: Baseline biopsy with GG1 in one core (tumor focus <3.0 mm) or GG2 in one core (tumor <2.0 mm) AND PSA <4 ng/mL AND negative confirmatory biopsy. Patients with a GG1 or GG2 tumor who did not meet the criteria for Group I were allocated to Group II. Group II was sub-stratified as follows: Group IIA (one positive core, PSA >4 ng/mL, Group IIB (GG1, >one core positive, any PSA or tumor length), and Group IIC (GG2 not meeting Group I criteria, including men with GG3 who were offered surveillance by their treating physician).
Following confirmatory biopsy performance, all patients were evaluated every 6 months by clinical examination (e.g., digital rectal examination) and laboratory studies (e.g., PSA). Prostate biopsy was repeated yearly; however, biopsy was omitted the year following a negative result. All biopsies were performed by a transrectal ultrasound–guided technique with an 11-core biopsy scheme.11 Patients who refused repeat biopsy during the study were allowed to remain on protocol provided the treating physician agreed. Patients otherwise remained on protocol unless they were treated, lost to follow-up, requested removal from study, were diagnosed with a second malignancy, developed disease metastases, or died. MRI was not standard at protocol inception and was not mandated by the protocol.
Disease reclassification and progression
Disease reclassification was based on biopsy findings following baseline AND confirmatory biopsy. The criteria included an increase in tumor length (greater than 1 mm) in a positive core beyond or GG increase. Men with a single positive core between both baseline and confirmatory biopsies (thus, a single core positive at diagnosis and a negative confirmatory biopsy) were additionally reclassified if they had >1 core positive on subsequent biopsy. Reclassification based on GG, alone, was additionally deemed of interested and was analyzed separately. PSA measurement, alone, was not a basis for reclassification, though a significant increase (>30%) could prompt further investigation, such as biopsy. Patients with disease reclassification were recommended to consider treatment, though were allowed to remain on study if treatment was declined.
Statistical analysis
Patient characteristics were summarized using descriptive statistics. Cox proportional hazards models were fit to evaluate associations between time to disease reclassification and baseline clinicopathologic characteristics that were expected to be associated with disease reclassification and GG upgrading based on prior analyses7: patient age,7 number of positive cores, PSA level, and GG. A separate model was used to investigate the association between characteristics and time to reclassification based on GG, alone. We hypothesized that a novel, clinically based characteristic termed “composite tumor length” would be independently associated in time to event analyses. Composite tumor length was defined as the sum of total tumor length measured from positive biopsy cores in BOTH the diagnostic (typically performed at outside facility) and confirmatory (MDACC) biopsies. Given this hypothesis, we also performed a time-to-event analysis stratified by composite tumor length using both the Kaplan Meier method and a Cox proportional hazards model.
Time to event analyses were also completed based on patient group status, and the Kaplan-Meier method was used to evaluate time to disease reclassification and GG upgrading based on group.
Patients were included in all analyses if they had 6 months or greater follow-up. P values <0.05 were deemed as statistically significant. All analyses were performed in SAS (Version 9.4 for Windows, SAS Institute, Cary, NC) and S-plus software (Version 8.2 for Windows, TiBCO Spotfire, Palo Alto, CA).
Results
From February 2006 through February 2014, 825 patients enrolled on the AS protocol, and 682 (82.7%) had >6 months of follow-up. Follow-up was censored at 12/31/2016 to allow for establishment of a thoroughly annotated cohort for analysis. Figure 1 shows the study participant flowchart and Table 1 shows baseline characteristics of the 682 included patients. Mean age was 64 years (standard deviation [SD] 8.2) and 588/682 (86.2%) patients had GG1 disease. Characteristics by group are summarized in Supplementary Tables 1a/1b. Patient age, prostate size, PSA level, PSA density (PSAD), time on study, and tumor length all differed significantly among groups (p<0.01 for all). Nine men with an average age of 75.8 (SD 7.6) years were enrolled who had a single core of GG3 disease following consultation with their treating physician. Five were reclassified at a median follow-up of 24 months with four electing treatment. None were diagnosed with advanced disease.
Fig. 1 – Study participant enrollment and outcomes.
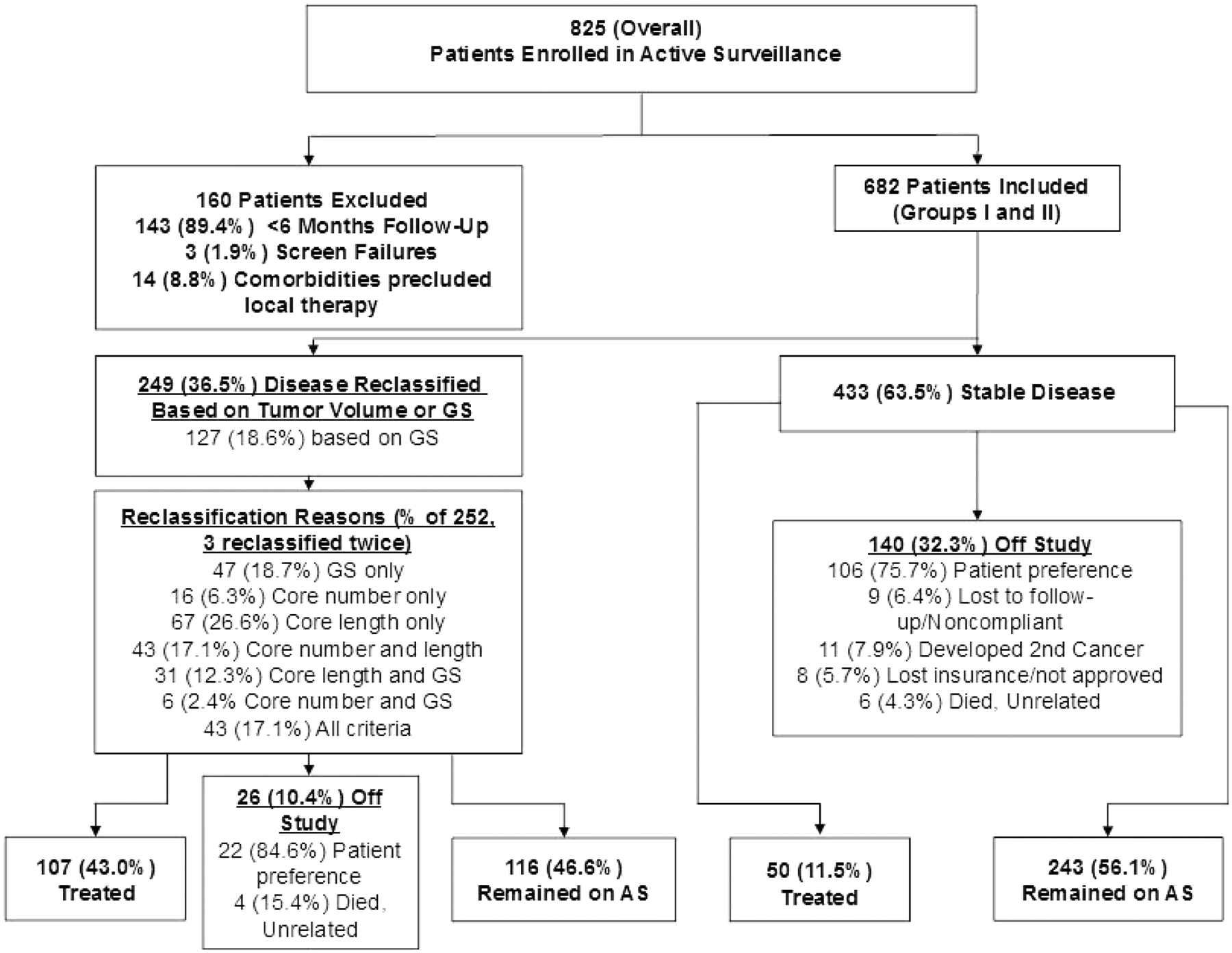
Flowchart showing patients included in the active surveillance cohort, reclassification rates, reasons for reclassification, and numbers who were treated or left the study for other reasons. GS, Gleason score; AS, active surveillance.
Table 1.
Patient characteristics at baseline
| Characteristic (categorical) | Strata | N (%) |
|---|---|---|
| Ethnicity | White | 554 (81.7%) |
| African American | 58 (8.6%) | |
| Hispanic | 52 (7.7%) | |
| Other/no response | 18 (2.6%) | |
| Clinical stage | ≤cT1c | 608(89.1%) |
| cT2a | 66(9.7%) | |
| cT2b | 3(0.4%) | |
| cT2c | 5(0.7%) | |
| Total biopsy number (including diagnostic and confirmatory) | 1, 2 | 44(6.5%) |
| 3 | 90(13.2%) | |
| 4 | 179(26.2%) | |
| ≥5 | 369(54.1%) | |
| Composite tumor length (diagnostic and confirmatory sum) | <2mm | 399(59.6%) |
| 2 - <5mm | 178(26.6%) | |
| ≥5mm | 93(13.9%) | |
| Gleason score (highest from confirmatory or diagnostic) | GG1 | 588(86.2%) |
| GG2 or GG3 | 94(13.8%) | |
| Positive core number (sum confirmatory and diagnostic | 1 | 476(69.8%) |
| >1 | 206(30.2%) | |
| Group stratification | Group I | 236(34.6%) |
| Group IIA | 64(9.4%) | |
| Group IIB | 291(42.7%) | |
| Group IIC | 91(13.3%) | |
| Characteristic (continuous) | Mean +/−SD | Median (range) |
| Age, years | 64 +/− 8.2 | 64 (36–88) |
| BMI | 28.8 +/− 4.4 | 28.4 (17.6–62.7) |
| TRUS volume, total, mm | 44.1 +/− 21.2 | 39.7 (13.5–196) |
| PSA | 4.6 +/− 3.1 | 4.1 (0.2–34) |
| PSAD | 0.1 +/− 0.1 | 0.1 (0.01–1.1) |
| Follow-up duration, months | 45.3 +/− 27.7 | 40 (6–126.8) |
SD, standard deviation; BMI, body mass index; GG, grade group; Group stratification as defined in the text, TRUS, transrectal ultrasound–guided biopsy; PSA, prostate-specific antigen; PSAD, PSA density
The median follow-up duration of included patients was 40 months (range 6.6–126.8). During follow-up, 369/682 (54.1%) patients underwent >4 biopsies. Of note, 24/682 (3.5%) patients had an MRI within 6 months of enrollment.
Overall, 249/682 (36.5%) patients were reclassified (three were reclassified twice); 127 of the 252 (51.0%) reclassification events were due to GG, only (Supplementary Figure). Among reclassification events, 123/252 (48.8%) occurred in the first year. Among men reclassified by any definition, 43% elected to undergo treatment. Group-stratified Kaplan-Meier curves demonstrating time to reclassification based on tumor size or GG and GG, only are shown in Figure 2. Median time to reclassification based on tumor size or GS in patients stratified to Group I or IIA was not reached, while median time to reclassification was 43 months for Group IIB/IIC (p<0.001, log rank test).
Fig. 2 -. Kaplan Meier survival analysis of grade group progression by composite tumor length tertiles.
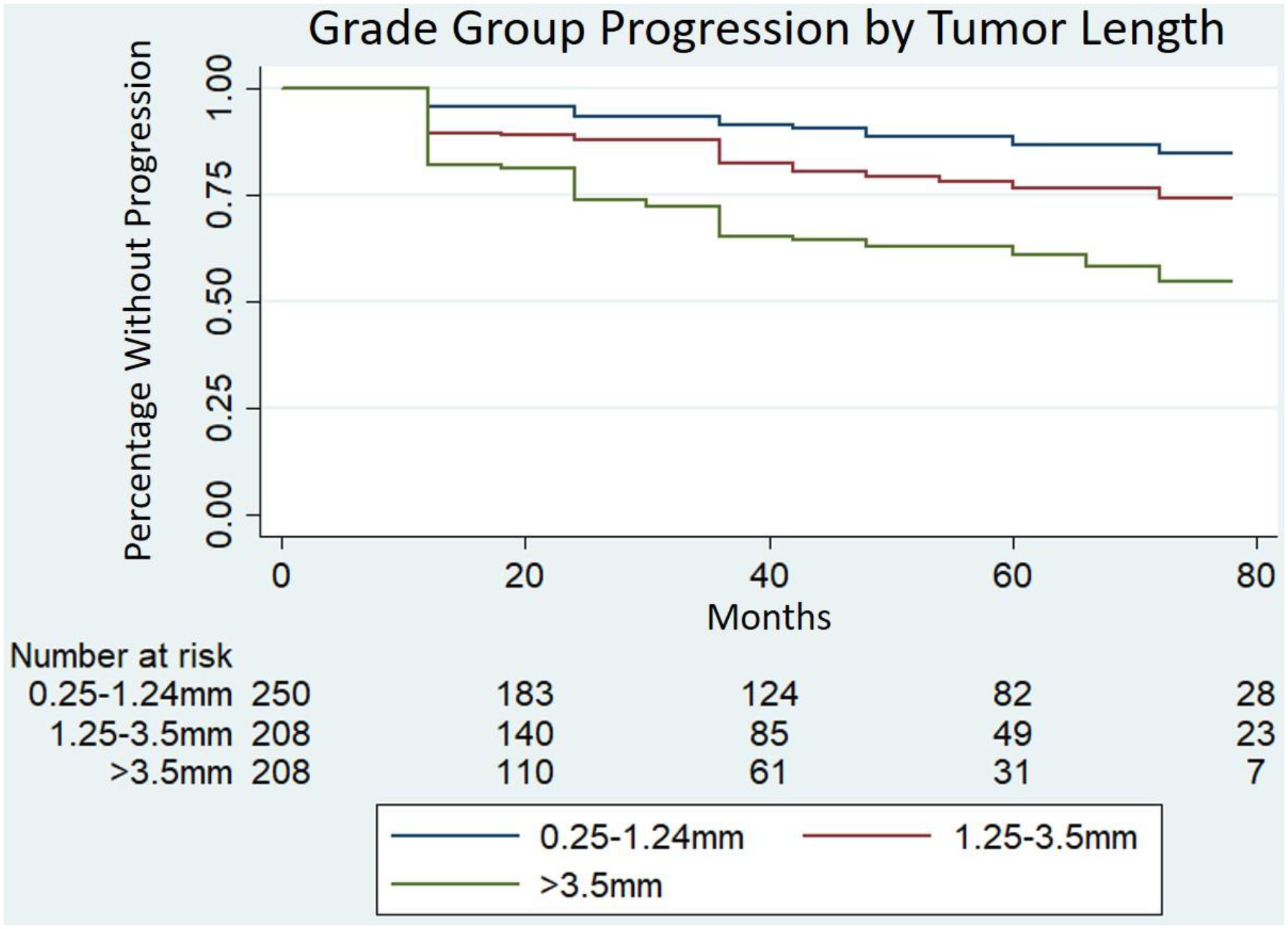
Cox proportional hazard multivariate model including composite tumor length, age, PSA and total number of positive cores revealed worsened progression-free survival for composite tumor length of 1.25–3.5mm (HR 1.73, 95CI 1.01–2.96, P=0.05) and >3.5mm (HR 3.03, 95CI 1.60–5.75, P<0.01) compared to the reference length of 0.25–1.24mm.
Table 2 shows associations of selected patient and pathologic characteristics with disease reclassification or progression in multivariable Cox models. No factor, including composite tumor length (hazard ratio [HR] 1.09, 95% CI 1.00–1.18, p=0.06) was associated with time to reclassification based on tumor size or GG; however, composite tumor length was significantly associated with time to reclassification based on GG, alone (HR 1.24 for each 1mm increase, 95% CI 1.11–1.40, p<0.001).
Table 2.
Cox proportional hazards models for time to reclassification based on tumor volume and grade group or grade group, alone
| Model of Disease Reclassification by grade group or tumor volume | ||||
|---|---|---|---|---|
| Covariate | Hazard Ratio | 95% CI | p-value | |
| Age | 1.01 | 0.99 | 1.02 | 0.33 |
| PSA | 1.01 | 0.97 | 1.04 | 0.80 |
| Composite tumor length | 1.09 | 1.00 | 1.18 | 0.06 |
| GG2 (vs. GG1) | 1.00 | 0.66 | 1.54 | 0.99 |
| No. of positive cores | 1.09 | 0.94 | 1.28 | 0.26 |
| Model of Reclassification by grade group, alone | ||||
| Covariate | Hazard Ratio | 95% CI | p-value | |
| Age | 1.01 | 0.99 | 1.03 | 0.30 |
| PSA | 1.02 | 0.97 | 1.07 | 0.38 |
| Composite tumor length | 1.24 | 1.11 | 1.40 | 0.0002 |
| No. of positive cores | 1.03 | 0.85 | 1.26 | 0.75 |
GG – grade group
Figure 2 shows Kaplan-Meier curves demonstrating time to GG progression based on composite tumor length strata in addition to multivariable Cox proportional hazards models. The highest tertile (>3.5mm of tumor length) was associated with worsened GG progression-free survival compared to the lowest tertile (0.25–1.24mm; HR 3.03, 95CI 1.6–5.75, P<0.01).
At time of censoring, 359/682 (52.6%) patients remained on AS and were active in the program, and 157/682 (23.0%) had undergone treatment. Of the treated patients, 107/157 (68.2%) had undergone reclassification. Overall, 81/157 (51.6%) treated patients underwent prostatectomy.
Regarding oncologic outcomes, among prostatectomy patients, 70 had evaluable final pathology. 23/70 (32.9%) cases were upgraded on final pathology, while 10/70 (13.3%) were downgraded. In total, 1 (1.4%) had GG1 disease, 43 (61.4%) had GG2, 20 (28.6%) had GG3, 2 (2.9%) had GG4, and 4 (5.7%) had GG5 (Supplementary Table 2). Two patients had lymph node–positive disease and 11/73 with evaluable margins (15.1%) had a positive margin (7/11 pT2, 3/11 pT3a and 1/11 pT3b disease).
Among 79 men who had prostatectomy and available postoperative data, the median follow-up duration was 28 months (range 2–91). 4/79 (5.1%) had a biochemical recurrence, and one patient developed distant metastatic (M1a) disease (Supplementary table 3). In all, 4/682 (0.6%) patients in the AS cohort developed advanced and/or metastatic disease (Supplementary Table 3).
Discussion
We report an intermediate follow-up assessment of a large cohort of men enrolled on an AS protocol for localized prostate cancer at a tertiary cancer center. Overall, 36.5% of patients were reclassified, with 51% of these being due to GG upgrading, alone. In addition, we identified a novel marker of disease aggression, composite tumor length, was shown to be independently associated with GG upgrading. Finally, we demonstrated that a clinically risk-based stratification system, comprised of PSA, GS, tumor volume and biopsy positivity, is significantly associated with time to reclassification.
Since study inception, AS has transitioned from a clinically acceptable option for low-risk prostate cancer to the preferred option for men with very-low-risk and a recommended option for low-risk disease.4 The patient grouping strategy used in our cohort yielded multiple findings that may be applied in the future to both refine risk stratification and optimize surveillance frequency. Building on prior results that identified a group at low risk for progression based on a low volume single core of tumor with a negative confirmatory biopsy,9 we showed that inclusion of essentially all patients with a single positive core and a negative confirmatory biopsy (Groups I and IIA), results in a low rate of volume- and tumor-based reclassification at follow-up (Figure IIA).
Perhaps the most important finding in this analysis is the independent association of composite tumor length, defined as the sum tumor length detected in both diagnostic and confirmatory biopsy, with GG upgrading. Prior studies have shown that patient age, PSA kinetics, biopsy results, and core positivity are associated with progression, though none have examined composite tumor length.5,7,12–14 Multiple studies have shown that tumor volume based on a summation of positive tumor locations on multiple biopsies is associated with clinical outcomes in surveillance.15,16 A report on the Royal Marsden cohort showed that percentage of core positivity was associated with progression,17 while a study by Ng et al. showed similar findings (albeit not significant in multivariable analysis).18 Recent work from Johns Hopkins additionally demonstrated that core positivity (in terms of number and percentage) was associated with increased risk of pathologic upgrading.19 However, while percentage of core positivity appears to be independently useful in risk stratification, it may not offer an accurate picture of overall tumor bulk, given that short cores can sample a small-volume tumor with a resultant high percentage of core positivity (and vice versa). We therefore submit that composite tumor length, defined as the total sum of two initial biopsies, may be an important component of risk stratification.
The question remains, however, as to the utility of clinical stratification factors and risk grouping in the current era of prostate MRI and genomic data. Prostate MRI with targeted fusion has been shown to increase the sensitivity of prostate biopsy for clinically significant disease20 and may decrease the diagnosis of GG1 tumors when systematic biopsy is omitted in a screening population.21 Cohort-based evidence also exists that MRI has a high negative predictive value20,22 and may improve detection of ≥GG2 disease.23 However, a recent randomized trial examining the utility of MRI-guided fusion biopsy in conjunction with systematic biopsy as confirmatory biopsy upon enrollment to AS demonstrated that rates of upgrading were not significantly improved in the fusion group, and omission of 12 core biopsy would have resulted in a lower rate of upgrading.24 Despite multiple expert-based guidelines that recommend MRI use at surveillance enrollment,25,26 these data call into question the effectiveness of this approach. Other markers of disease progression, including genomic classifiers and other assays performed on biopsy tissue, may aid in risk stratification in men enrolled on surveillance22; however, they, too, add additional cost and remain to be prospectively validated in AS patients.7 As shown in our study and others, clinically based factors, alone, perform well in the intermediate term at detecting aggressive prostate cancer and enabling treatment prior to metastases. It is therefore likely that stratification using clinical factors, including risk grouping and/or composite tumor length described here, may accurately determine which patients would benefit from performance of MRI (or other studies) at AS enrollment. Further studies are needed, however, to prospectively evaluate the stratified use imaging-based or other risk predictors.
Strengths of the current study include prospective follow-up of a large group of AS patients using a standardized protocol that included frequent prostate biopsy. Study limitations include the fact that it was performed at a single tertiary care facility (MDACC) with a broad referral base. In addition to possibly differing in makeup from a primary urology practice, patients may be prone to elective study removal over time given geographic constraints. While this study represents an increasingly rare investigation of AS enrollment during the pre-MRI era, MRI was ordered at the discretion of the treating physician during patient follow-up. These results may be confounded by later MRI use, as at least one MRI was ordered on over 1/3 of patients who were followed on protocol during the study (though the majority of which was ordered years after study enrollment). Finally, the issue of whether disease reclassification or GG upgrading is clinically meaningful in patients on AS is a limitation of all studies of contemporary AS cohorts. While prevention of metastases and death are clear goals in the treatment of all prostate cancer patients, these rates are very low in AS populations.5 Further, randomized data has shown that, in predominantly low risk patients, localized treatment did not improve 10-year cancer-specific mortality when compared to AS (though it did improve time to metastatic disease).27 Nonetheless, as evidenced here and in other cohorts, the current methods of AS initiation and follow-up do not eliminate cancer-related events. While difficult to accomplish, only further prospective studies of risk-based surveillance application with long-term follow-up will determine the best methods to combine clinical factors with newer modalities such as MRI to prevent the development of advanced prostate cancer while limiting rates of overtreatment.
Conclusions
We demonstrate the safety and intermediate reclassification rates and other outcomes in a large AS cohort. We additionally identified clinically-based risk factors for reclassification, including a risk group score incorporating tumor length, core number, PSA and confirmatory biopsy results. A novel clinical marker, composite tumor length, defined as the total length of tumor in mm between baseline and confirmatory biopsy was shown to be independently associated with time to GG upgrading. These factors help form a foundation from which future studies investigating the use of MRI and other novel markers to risk stratify and monitor patients who elect AS following diagnosis of localized prostate cancer.
Supplementary Material
Acknowledgements
We would like to acknowledge the MD Anderson Department of Scientific Publications for help in editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67(1):44–50. doi: 10.1016/j.eururo.2014.08.024 [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314(1):80–82. doi: 10.1001/jama.2015.6036 [DOI] [PubMed] [Google Scholar]
- 3.Barocas DA, Cowan JE, Smith JA, Carroll PR, CaPSURE Investigators. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008;180(4):1330–1334; discussion 1334–1335. doi: 10.1016/j.juro.2008.06.019 [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Cancer Netw JNCCN. 2016;14(1):19–30. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(30):3379–3385. doi: 10.1200/JCO.2015.62.5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iremashvili V, Pelaez L, Manoharan M, Jorda M, Rosenberg DL, Soloway MS. Pathologic Prostate Cancer Characteristics in Patients Eligible for Active Surveillance: A Head-to-Head Comparison of Contemporary Protocols. Eur Urol. 2012;62(3):462–468. doi: 10.1016/j.eururo.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 7.Loeb S, Bruinsma SM, Nicholson J, et al. Active Surveillance for Prostate Cancer: A Systematic Review of Clinicopathologic Variables and Biomarkers for Risk Stratification. Eur Urol. 2015;67(4):619–626. doi: 10.1016/j.eururo.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson R, Lotan Y. Cost consideration in utilization of multiparametric magnetic resonance imaging in prostate cancer. Transl Androl Urol. 2017;6(3):345–354. doi: 10.21037/tau.2017.01.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis JW, Ward JF, Pettaway CA, et al. Disease reclassification risk with stringent criteria and frequent monitoring in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int. 2016;118(1):68–76. doi: 10.1111/bju.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochiai A, Troncoso P, Chen ME, Lloreta J, Babaian RJ. THE RELATIONSHIP BETWEEN TUMOR VOLUME AND THE NUMBER OF POSITIVE CORES IN MEN UNDERGOING MULTISITE EXTENDED BIOPSY: IMPLICATION FOR EXPECTANT MANAGEMENT. J Urol. 2005;174(6):2164–2168. doi: 10.1097/01.ju.0000181211.49267.43 [DOI] [PubMed] [Google Scholar]
- 11.Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163(1):152–157. [PubMed] [Google Scholar]
- 12.Alam R, Carter HB, Landis P, Epstein JI, Mamawala M. Conditional Probability of Reclassification in an Active Surveillance Program for Prostate Cancer. J Urol. 2015;193(6):1950–1955. doi: 10.1016/j.juro.2014.12.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welty CJ, Cowan JE, Nguyen H, et al. Extended Followup and Risk Factors for Disease Reclassification in a Large Active Surveillance Cohort for Localized Prostate Cancer. J Urol. 2015;193(3):807–811. doi: 10.1016/j.juro.2014.09.094 [DOI] [PubMed] [Google Scholar]
- 14.Ankerst DP, Xia J, Thompson IM, et al. Precision Medicine in Active Surveillance for Prostate Cancer: Development of the Canary–Early Detection Research Network Active Surveillance Biopsy Risk Calculator. Eur Urol. 2015;68(6):1083–1088. doi: 10.1016/j.eururo.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong JY, Capella C, Teplitsky S, et al. Impact of Tumor Regional Involvement on Active Surveillance Outcomes: Validation of the Cumulative Cancer Location Metric in a US Population. Eur Urol Focus. May 2019. doi: 10.1016/j.euf.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 16.Tan GH, Finelli A, Ahmad A, et al. A novel predictor of clinical progression in patients on active surveillance for prostate cancer. Can Urol Assoc J J Assoc Urol Can. 2019;13(8):250–255. doi: 10.5489/cuaj.6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkitaraman R, Norman A, Woode-Amissah R, et al. Predictors of histological disease progression in untreated, localized prostate cancer. J Urol. 2007;178(3 Pt 1):833–837. doi: 10.1016/j.juro.2007.05.038 [DOI] [PubMed] [Google Scholar]
- 18.Ng MK, Van As N, Thomas K, et al. Prostate-specific antigen (PSA) kinetics in untreated, localized prostate cancer: PSA velocity vs PSA doubling time. BJU Int. 2009;103(7):872–876. doi: 10.1111/j.1464-410X.2008.08116.x [DOI] [PubMed] [Google Scholar]
- 19.Tosoian JJ, Mamawala M, Patel HD, et al. Tumor Volume on Biopsy in Low-Risk Prostate Cancers Managed on Active Surveillance. J Urol. October 2017. doi: 10.1016/j.juro.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–397. doi: 10.1001/jama.2014.17942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378(19):1767–1777. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornberg Z, Cowan JE, Westphalen AC, et al. Genomic Prostate Score, PI-RADSv2, and Progression in Men with Prostate Cancer on Active Surveillance. J Urol. September 2018. doi: 10.1016/j.juro.2018.08.047 [DOI] [PubMed] [Google Scholar]
- 23.Schoots IG, Nieboer D, Giganti F, Moore CM, Bangma CH, Roobol MJ. Is MRI-targeted biopsy a useful addition to systematic confirmatory biopsy in men on active surveillance for low-risk prostate cancer? A systematic review and meta-analysis. BJU Int. April 2018. doi: 10.1111/bju.14358 [DOI] [PubMed] [Google Scholar]
- 24.Klotz L, Loblaw A, Sugar L, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): Results of a Randomized Multicenter Prospective Trial. Eur Urol. July 2018. doi: 10.1016/j.eururo.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 25.Briganti A, Fossati N, Catto JWF, et al. Active Surveillance for Low-risk Prostate Cancer: The European Association of Urology Position in 2018. Eur Urol. 2018;74(3):357–368. doi: 10.1016/j.eururo.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management. 2014. https://www.nice.org.uk/guidance/cg175. Accessed September 8, 2018. [PubMed]
- 27.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


