Abstract
Background:
Obesity remains a relative contraindication for heart transplantation, and hence, obese patients with advanced heart failure receive ventricular assist devices (VAD) either as a destination or “bridge to weight loss” strategy. However, impact of obesity on clinical outcomes after VAD implantation is largely unknown. We sought to determine the clinical outcomes of obese patients with body mass index (BMI) ≥ 35 kg/m2) following contemporary VAD implantation.
Methods:
The INTERMACS registry was queried for patients who underwent VAD implantation. Patients were categorized into BMI groups based on World Health Organization classification.
Results:
Of 17,095 patients; 2620 (15%) had a BMI ≥ 35 kg/m2. Obese patients were likely to be young, nonwhite, females with dilated cardiomyopathy and undergo device implantation as destination. Survival was similar amongst BMI groups (p=0.058). Obese patients had significantly higher risk for infection (hazard ratio (HR): 1.215; p=<0.001), device malfunction or thrombosis (HR: 1.323; P=<0.001), cardiac arrhythmia (HR: 1.188; P=<0.001) and hospital readmissions (HR: 1.073; p=0.022), but lower risk of bleeding (HR: 0.906; p=<0.018). Significant weight loss (≥10%) during VAD support was achieved only by a small proportion (18.6%) of patients with BMI ≥ 35 kg/m2. Significant weight loss rates observed in obese patients with VAD implantation as destination and bridge to transplant strategy were comparable. Obese patients with significant weight loss were more likely to undergo cardiac transplantation. Weight loss worsened bleeding risk without altering risk for infection, cardiac arrhythmia and device complications.
Conclusions:
Obesity alone should not be considered a contraindication for VAD therapy in contemporary era. Given favorability towards transplantation, strategies should be developed to promote weight loss which occurs infrequently in obese patients. Impact of weight loss on clinical outcome of obese patients warrants further investigation.
Keywords: ventricular assist device, obesity, weight loss, device malfunction, device thrombosis, bleeding
Introduction
As a result of the obesity epidemic, an increasing number of obese patients with symptomatic heart failure (HF) need heart replacement therapy (1-4). Morbid obesity is generally considered a contraindication to heart transplantation due to decreased survival, increased graft failure, peri- and post-operative complications along with challenges with donor and recipient size matching (5-11). Therefore, the vast majority of obese patients who require heart replacement therapy undergo long-term ventricular assist device (VAD) implantation as either destination therapy or “bridge to weight loss” strategy for subsequent transplantation eligibility (11,12). Prior studies have suggested obesity as a risk factor for VAD complications; however, the majority of these studies were single-center and underpowered to assess potential impact of obesity on morbidity and mortality. In addition, the success rate of “bridge-to-weight loss” strategy and impact of weight loss on clinical outcomes with VAD support remains unknown (3,9,13-20). Although the recent review of the United Network for Organ Sharing (UNOS) database showed an increased risk of complications and lack of significant weight loss after VAD implantation in obese patients, these patients were listed as bridge to transplant (11).
In this context, the purpose of this study was to investigate the impact of obesity on clinical outcomes of patients undergoing contemporary long-term VAD support irrespective of initial implantation strategy in a large national VAD registry; and determine if the implantation of a VAD allowed for weight loss. Impact of weight loss on clinical outcomes was examined as well.
Materials and Methods:
Study Population
The study population for this retrospective investigation consisted of patients who underwent Left ventricular assist device (LVAD) implantation in the United States as reported in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). Current analyses were restricted to adult patients (≥19 years) who were prospectively enrolled in INTERMACS and received a continuous flow long-term LVAD with or without a right VAD between June 2006 and July 2014 with follow up data through September 2015.
Study Group
Using World Health Organization classification patients were grouped into five body mass index (BMI) categories: underweight (BMI <18.5 Kg/ m2), normal (BMI: 18.5 to 24.99 kg/m2), overweight (BMI: 25 to 29.99 Kg/ m2), obese class I (BMI: 30 to 34.99 Kg/ m2), and obese class II (BMI ≥35 kg/m2). Individual patient weight was recorded at the time of LVAD implantation and various other time periods during follow up. Groups were compared in terms of heart transplantation rates, survival, and adverse clinical events (including device malfunction or thrombosis, neurological events, infection, bleeding, cardiac arrhythmia and hospital readmissions).. We also examined the significant weight loss (>10%), and impact of weight loss on clinical outcomes during LVAD support in this study cohort.
Separately, we also examined the outcomes in sub group of patients with BMI ≥40 kg/m2 as an exploratory analysis as these patients are much less likely to become transplant eligible and stay on device longer. Complication end-points definitions were derived from the INTERMACS (https://www.uab.edu/medicine/intermacs/appendices/app-a-5-0).
Statistical Analysis
Demographic and clinical variables were summarized with standard descriptive statistics. Comparisons between BMI groups were made with ANOVA and chi-square tests where appropriate. Multivariable adjusted hazard ratio estimates for the risk of LVAD outcomes were calculated for BMI categories with normal BMI category being reference group. Variables adjusted for include age, gender, race, blood type, etiology of HF, intermacs class, implant strategy, device type (centrifugal- vs. axial-flow), right sided VAD use, serum albumin, and serum creatinine. Kaplan-Meier estimates were used to compare survival based on baseline BMI groups, censoring patients at the time of transplantation, explant for recovery, or study end. Clinical outcomes conditional upon survival at 1 year was comparatively analyzed using Kaplan-Meier estimates to determine the impact of weight loss on adverse events. For all comparisons, a p-value of 0.05 was considered statistically significant. Data were analyzed using SAS, version 9.4 (SAS Institute, Inc., Carey, North Carolina). This study was reviewed and approved by the INTERMACS Data Access & Analysis Committee.
Results
Baseline Clinical Characteristics
Of 17,095 patients supported with a CF- LVAD from the INTERMACS registry, 2620 (15.3%) had BMI ≥ 35 kg/m2 and 968 (5.6%) had BMI ≥ 40 kg/m2. Tables 1 and 2 display the baseline clinical characteristics, laboratory parameters and hemodynamics of the study population categorized by BMI groups. Obese patients with BMI ≥ 35 kg/m2 were significantly younger, more likely to be female and African American with predominantly non-ischemic cardiomyopathy. These patients were more likely to receive LVAD as destination therapy. A significantly higher proportion of obese patients received guideline directed medical therapy and implantable cardioverter defibrillators at the time of LVAD implantation. Obese patients had higher cardiac filling pressures despite lower plasma brain natriuretic peptide levels.
Table 1.
Baseline Clinical Characteristics
| Variable | Overall (n=17095) |
BMI < 18.5 (n=553) |
BMI ≥ 18.5 & < 25 (n=4723) |
BMI ≥ 25 & < 30 (n=5496) |
BMI ≥ 30 & < 35 (n=3703) |
BMI ≥ 35 (n=2620) |
p-value |
|---|---|---|---|---|---|---|---|
| Age > 60, (years), m(%) | 7863 (46.0) | 236 (42.7) | 2511 (53.2) | 2827 (51.4) | 1576 (42.6) | 713 (27.2) | <0.001 |
| Female Gender, n(%) | 3660 (21.4) | 175 (31.6) | 1037 (22.0) | 1025 (18.6) | 735 (19.8) | 688 (26.3) | <0.001 |
| Race | |||||||
| White, n(%) | 11560 (67.6) | 330 (59.7) | 3191 (67.6) | 3911 (71.2) | 2589 (69.9) | 1539 (58.7) | <0.001 |
| African American, n(%) | 4054 (23.7) | 147 (26.6) | 1042 (22.1) | 1126 (20.5) | 839 (22.7) | 900 (34.4) | <0.001 |
| Asian, n(%) | 249 (1.5) | 28 (5.1) | 115 (2.4) | 54 (1.0) | 33 (0.9) | 19 (0.7) | <0.001 |
| American Indian | 113 (0.7) | 3 (0.5) | 36 (0.8) | 34 (0.6) | 24 (0.6) | 16 (0.6) | 0.892 |
| Pacific Islander | 64 (0.4) | 5 (0.9) | 18 (0.4) | 14 (0.3) | 14 (0.4) | 13 (0.5) | 0.120 |
| Other | 748 (4.4) | 31 (5.6) | 221 (4.7) | 264 (4.8) | 145 (3.9) | 87 (3.3) | 0.006 |
| Unknown | 368 (2.2) | 12 (2.2) | 119 (2.5) | 111 (2.0) | 66 (1.8) | 60 (2.3) | 0.190 |
| Ethnicity | |||||||
| Hispanic | 1051 (6.1) | 34 (6.1) | 300 (6.4) | 359 (6.5) | 214 (5.8) | 144 (5.5) | 0.306 |
| Blood Type O, n(%) | 8021 (46.9) | 252 (45.6) | 2178 (46.1) | 2598 (47.3) | 1743 (47.1) | 1250 (47.7) | 0.040 |
| BMI (kg/m2), mean ± SD | 28.6 ± 7.0 | 15.2 ± 3.0 | 22.5 ± 1.7 | 27.4 ± 1.4 | 32.2 ± 1.4 | 40.0 ± 6.8 | <0.001 |
| BSA (m2), mean ± SD | 2.05 ± 0.29 | 1.49 ± 0.21 | 1.83 ± 0.17 | 2.03 ± 0.17 | 2.20 ± 0.19 | 2.42 ± 0.26 | <0.001 |
| Ischemic Etiology of HF | 7807 (45.7) | 232 (42.0) | 2111 (44.7) | 2734 (49.7) | 1758 (47.5) | 972 (37.1) | <0.001 |
| LVAD Configuration | 0.365 | ||||||
| LVAD | 16461 (96.3) | 526 (95.1) | 4557 (96.5) | 5282 (96.1) | 3563 (96.2) | 2533 (96.7) | |
| LVAD + RVAD | 634 (3.7) | 27 (4.9) | 166 (3.5) | 214 (3.9) | 140 (3.8) | 87 (3.3) | |
| LVAD type | <0.001 | ||||||
| Centrifugal | 2872 (16.8) | 149 (26.9) | 871 (18.4) | 919 (16.7) | 622 (16.8) | 311 (11.9) | |
| Axial | 14223 (83.2) | 404 (73.1) | 3852 (81.6) | 4577 (83.3) | 3081 (83.2) | 2309 (88.1) | |
| Device Strategy | <0.001 | ||||||
| Transplant listed | 4660 (27.3) | 171 (30.9) | 1279 (27.1) | 1613 (29.3) | 1124 (30.4) | 473 (18.1) | |
| Transplant eligible | 5267 (30.8) | 173 (31.3) | 1362 (28.8) | 1656 (30.1) | 1154 (31.2) | 922 (35.2) | |
| Destination Therapy | 7034 (41.1) | 204 (36.9) | 2048 (43.4) | 2190 (39.8) | 1390 (37.5) | 1202 (45.9) | |
| Bridge to recovery | 65 (0.4) | 2 (0.4) | 21 (0.4) | 15 (0.3) | 17 (0.5) | 10 (0.4) | |
| Other | 69 (0.4) | 3 (0.6) | 13 (0.3) | 22 (0.4) | 18 (0.5) | 13 (0.5) | |
| Comorbid Conditions | |||||||
| Diabetes, n(%) | 1121 (9.7) | 38 (9.2) | 191 (6.0) | 339 (9.1) | 313 (12.4) | 240 (13.7) | <0.001 |
| Active Smoker, n(%) | 826 (4.9) | 41 (7.5) | 262 (5.7) | 267 (4.9) | 177 (4.9) | 79 (3.1) | 0.001 |
| Pulmonary HTN, n(%) | 2681 (23.2) | 87 (21.1) | 677 (21.3) | 869 (23.4) | 625 (24.7) | 423 (24.2) | 0.020 |
| PVD, n(%) | 543 (4.7) | 19 (4.6) | 148 (4.7) | 195 (5.3) | 127 (5.0) | 54 (3.1) | 0.010 |
| Atrial Arrhythmia, n(%) | 2460 (21.2) | 84 (20.4) | 660 (20.8) | 789 (21.3) | 579 (22.8) | 347 (19.9) | 0.164 |
| CVA, n(%) | 452 (3.9) | 12 (2.9) | 125 (3.9) | 161 (4.3) | 98 (3.9) | 56 (3.2) | 0.258 |
| CKD, n(%) | 2626 (22.7) | 72 (17.5) | 642 (20.2) | 873 (23.6) | 614 (24.2) | 425 (24.3) | <0.001 |
| CHF Therapies | |||||||
| Beta Blocker | 8858 (51.8) | 256 (46.3) | 2212 (46.8) | 2816(51.2) | 2064 (55.7) | 1510 (57.6) | <0.001 |
| ACE Inhibitor | 4605 (26.9) | 144 (26.0) | 1121 (25.9) | 1464 (26.6) | 979 (26.4) | 797 (30.4) | 0.004 |
| Angiotensin Receptor Blocker | 1471 (8.6) | 34 (6.1) | 309 (6.5) | 477 (8.7) | 375 (10.1) | 276 (10.5) | <0.001 |
| Aldosterone antagonist | 6699 (39.2) | 216 (39.1) | 1795 (38.0) | 2083 (38.9) | 1471 (39.7) | 1134 (43.3) | <0.001 |
| Loop Diuretic | 14585 (85.3) | 471 (85.2) | 3955 (83.7) | 4645 (84.5) | 3188 (86.1) | 2326 (88.8) | <0.001 |
| AICD | 13641 (79.8) | 399 (72.2) | 3670 (77.7) | 4332 (78.8) | 3030 (81.8) | 2210 (84.4) | <0.001 |
| CRT | 3204 (18.7) | 108 (19.5) | 837 (17.7) | 1000 (18.2) | 759 (20.5) | 500 (19.1) | 0.003 |
| IV Inotropes | 13835 (80.9) | 465 (84.1) | 3955 (83.7) | 4470 (81.3) | 2894 (78.2) | 2051 (78.3) | <0.001 |
| INTERMACS Profile | <0.001 | ||||||
| INTERMACS 1 | 2778 (16.4) | 113 (20.7) | 779 (16.6) | 918 (16.8) | 594 (16.1) | 374 (14.4) | |
| INTERMACS 2 | 6175 (36.3) | 209 (38.2) | 1857 (39.5) | 2000 (36.6) | 1222 (33.2) | 887 (34.1) | |
| INTERMACS 3 | 5197 (30.6) | 163 (29.8) | 1405 (29.9) | 1661 (30.4) | 1156 (31.4) | 812 (31.2) | |
| INTERMACS 4-7 | 2838 (16.7) | 62 (11.3) | 658 (14.0) | 882 (16.2) | 709 (19.3) | 527 (20.3) | |
| NYHA Class IV | 12899 (75.5) | 433 (78.3) | 3614 (76.5) | 4122 (75.0) | 2785 (75.2) | 1945 (74.2) | 0.084 |
| IABP | 3288 (19.2%) | 126 (22.8) | 958 (20.3) | 1119 (20.4) | 646 (17.4) | 439 (16.8) | <0.001 |
| ECMO | 568 (3.3%) | 25 (4.4) | 139 (2.9) | 193 (3.5) | 134 (3.6%) | 77 (2.9%) | 0.116 |
| Mechanical Ventilation | 822 (4.8%) | 22(4.0) | 216 (4.6) | 268 (4.9) | 194 (5.2%) | 122 (4.7%) | 0.543 |
| Dialysis | 461 (2.7) | 18 (3.9) | 117 (2.5%) | 146 (2.7) | 112 (3.0) | 68 (2.6) | 0.529 |
Table 2:
Pre-Implant Laboratory Values and Hemodynamics
| Variable | Overall (n=17095) |
BMI < 18.5 (n=553) |
BMI ≥ 18.5 & < 25 (n=4723) |
BMI ≥ 25 & < 30 (n=5496) |
BMI ≥ 30 & < 35 (n=3703) |
BMI ≥ 35 (n=2620) |
P-value |
|---|---|---|---|---|---|---|---|
| Laboratory | |||||||
| WBC (x10/uL) | 8.7 ± 4.1 | 8.5 ± 4.4 | 8.4 ± 3.9 | 8.6 ± 4.0 | 8.8 ± 3.9 | 9.1 ± 4.7 | <0.001 |
| Hemoglobin (g/dL) | 11.3 ± 2.1 | 10.8 ± 1.9 | 11.1 ± 2.0 | 11.2 ± 2.1 | 11.4 ± 2.1 | 11.5 ± 2.1 | <0.001 |
| Platelet (x10/uL) | 196.5 ± 80.5 | 194.8 ± 80.8 | 194.4 ± 83.4 | 193.1 ± 79.0 | 198.3 ± 78.2 | 205.4 ± 81.1 | <0.001 |
| Sodium (mEq/dL), mean ± SD | 134.9 ± 4.8 | 134.8 ± 4.8 | 134.5 ± 4.9 | 135.0 ± 4.9 | 135.3 ± 4.7 | 135.1 ± 4.8 | <0.001 |
| BUN (mg/dL), mean ± SD | 29.5 ± 18.1 | 29.1 ± 17.6 | 29.0± 18.0 | 29.7 ± 18.2 | 29.6 ± 18.1 | 29.8 ± 18.2 | 0.279 |
| Creatinine (mg/dL), mean ± SD | 1.41 ± 0.72 | 1.29 ± 0.72 | 1.35 ± 0.74 | 1.42 ± 0.70 | 1.44 ± 0.71 | 1.48 ± 0.74 | <0.001 |
| Albumin (g/dL), mean ± SD | 3.39 ± 0.66 | 3.35 ± 0.62 | 3.35 ± 0.65 | 3.37 ± 0.69 | 3.44 ± 0.65 | 3.45 ± 0.65 | <0.001 |
| Total Bilirubin (mg/dL), mean ± SD | 1.43 ± 2.02 | 1.49 ± 2.64 | 1.50 ± 2.12 | 1.41 ± 1.87 | 1.40 ± 1.83 | 1.40 ± 2.26 | 0.102 |
| AST(u/L) | 62.3 ± 245 | 67.5 ± 260 | 63.4 ± 229 | 64.4 ±263 | 55.9 ± 175 | 63.6 ±309 | 0.527 |
| ALT (u/L) | 72.4 ± 246 | 82.1 ± 290 | 76.6 ± 235 | 77.3 ± 265 | 64.5 ± 198 | 63.9 ± 272 | 0.032 |
| BNP (pg/mL) | 1146 ± 1079 | 1463 ± 1194 | 1125 ± 993 | 989 ± 1009 | 765 ± 844 | 765 ± 844 | <0.001 |
| Hemodynamics | |||||||
| CVP (mmHg) | 13.3 ± 8.3 | 12.2 ± 7.6 | 12.5 ± 8.4 | 13.1 ± 8.2 | 13.5 ± 8.0 | 15.3 ± 8.7 | <0.001 |
| HR (bpm) | 88.8 ± 17.7 | 90.0 ± 17.4 | 88.7 ± 17.6 | 88.4 ± 17.6 | 88.4 ± 17.7 | 90.0 ± 17.8 | <0.001 |
| SBP (mmHg) | 104.5 ± 16.1 | 101.0 ± 15.9 | 101.5 ± 15.6 | 104.7 ± 16.0 | 106.6 ± 16.1 | 107.4 ± 16.3 | <0.001 |
| DBP (mmHg) | 64.6 ± 11.5 | 63.4 ± 10.6 | 63.2 ± 11.4 | 64.6 ± 11.4 | 65.4 ± 11.5 | 65.9 ± 11.8 | <0.001 |
| PA Systolic (mmHg) | 50.0 ± 14.7 | 47.0 ± 13.5 | 48.6 ± 14.0 | 49.9 ± 14.8 | 51.0 ± 15.1 | 52.5 ± 15.1 | <0.001 |
| PA Diastolic (mmHg) | 25.1 ± 8.8 | 23.5 ± 8.1 | 24.0 ± 8.4 | 24.8 ± 8.5 | 25.7 ± 9.1 | 27.2 ± 9.5 | <0.001 |
| PCWP (mmHg) | 24.8 ± 9.1 | 23.9 ± 8.3 | 23.8 ± 8.8 | 24.6 ± 8.9 | 24.9 ± 9.1 | 26.8 ± 9.7 | <0.001 |
| CO (L/min) | 4.24 ± 1.46 | 3.72 ± 1.34 | 3.81 ± 1.31 | 4.19 ± 1.38 | 4.54 ± 1.46 | 4.82 ± 1.62 | <0.001 |
| LVEDD (cm) | 6.8 ± 1.1 | 6.7 ± 1.2 | 6.7 ± 1.1 | 6.7 ± 1.1 | 6.9 ± 1.1 | 7.1 ± 1.2 | <0.001 |
| CVP/PCWP | 0.56 ± 0.44 | 0.53 ± 0.36 | 0.54 ± 0.44 | 0.55 ± 0.40 | 0.57 ± 0.53 | 0.60 ± 0.38 | 0.001 |
| PAPi | 2.94 ± 3.05 | 3.23 ± 3.67 | 3.22 ± 3.24 | 3.02 ± 3.13 | 2.78 ± 2.88 | 2.45 ± 2.52 | <0.001 |
Survival and Adverse Events
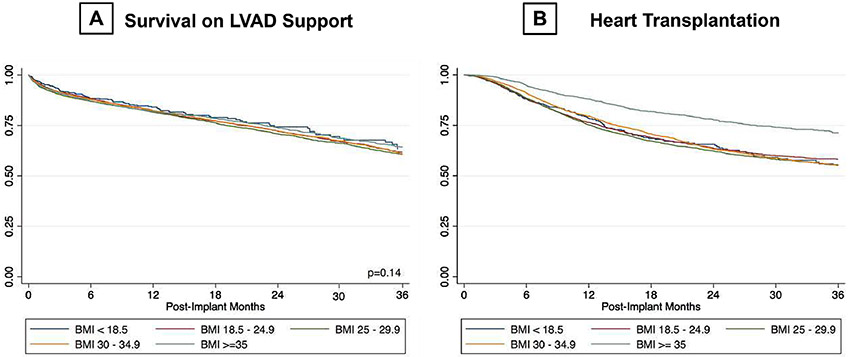
The impact of BMI on clinical outcomes is displayed on Table 3 and Figures 1-4. Overall, survival was similar between the BMI groups (Figure 1A). However, patients with BMI ≥ 35 kg/m2 were significantly less likely to undergo heart transplantation (HR: 0.571, p=<0.001) as opposed to lean groups (Figure 1B).
Table 3:
Multivariable Hazard Ratio Estimates for the Risk of Left Ventricular Assist Device Outcomes by BMI Categories
| Mortality on LVAD support | Heart Transplantation | |||||
|---|---|---|---|---|---|---|
| HR | 95.0% CI for HR |
p-value | HR | 95.0% CI for HR |
p-value | |
| BMI | ||||||
| Underweight | 0.927 | 0.755 – 1.138 | 0.467 | 0.927 | 0.774 – 1.110 | 0.410 |
| Normal BMI (reference) | 1.000 | 1.000 | ||||
| Overweight | 1.049 | 0.966 – 1.139 | 0.256 | 0.997 | 0.922 – 1.079 | 0.940 |
| Moderately Obese | 1.030 | 0.938 – 1.130 | 0.538 | 0.886 | 0.811 – 0.968 | 0.007 |
| Severely Obese | 1.105 | 0.997 – 1.226 | 0.058 | 0.571 | 0.509 – 0.642 | <0.001 |
| Infection | Device Malfunctkan or Thrombosis | |||||
| HR | 95.0% CI for HR |
p-value | HR | 95.0% CI for HR |
p-value | |
| BMI | ||||||
| Underweight | 1.069 | 0.929 – 1.230 | 0.351 | 1.005 | 0.820 – 1.232 | 0.960 |
| Normal BMI(reference) | 1.000 | 1.000 | ||||
| Overweight | 1.060 | 0.997 – 1.128 | 0.061 | 1.060 | 0.972 – 1.157 | 0.189 |
| Moderately Obese | 1.109 | 1.037 – 1.187 | 0.003 | 1.166 | 1.062 – 1.280 | 0.001 |
| Severely Obese | 1.215 | 1.128 – 1.309 | <0.001 | 1.323 | 1.198 – 1.462 | <0.001 |
| Bleeding | Cardiac Arrhythmia | |||||
| HR | 95.0% CI for HR |
p-value | HR | 95.0% CI for HR |
p-value | |
| BMI | ||||||
| Underweight | 1.037 | 0.896 – 1.200 | 0.626 | 1.113 | 0.932 – 1.328 | 0.238 |
| Normal BMI (reference) | 1.000 | 1.000 | ||||
| Overweight | 0.952 | 0.894 – 1.015 | 0.132 | 1.067 | 0.986 – 1.154 | 0.109 |
| Moderately Obese | 0.894 | 0.833 – 0.961 | 0.002 | 1.217 | 1.079 – 1.309 | <0.001 |
| Severely Obese | 0.906 | 0.836 – 0.983 | 0.018 | 1.188 | 1.097 – 1.277 | <0.001 |
| Neurological Event | Hospital Readmission | |||||
| HR | 95.0% CI for HR |
p-value | HR | 95.0% CI for HR |
p-value | |
| BMI | ||||||
| Underweight | 1.054 | 0.858 – 1.297 | 0.615 | 1.180 | 1.056 – 1.317 | 0.003 |
| Normal BMI (reference) | 1.000 | 1.000 | ||||
| Overweight | 1.093 | 0.999 – 1.197 | 0.053 | 1.044 | 0.995 – 1.096 | 0.078 |
| Moderately Obese | 1.007 | 0.910 – 1.115 | 0.888 | 1.027 | 0.974 – 1.084 | 0.323 |
| Severely Obese | 1.037 | 0.926 – 1.163 | 0.527 | 1.073 | 1.010 – 1.140 | 0.022 |
Figure 1:
A) Survival; B) Heart transplantation on LVAD support as per BMI categories. BMI: body mass index; LVAD: left ventricular assist device.
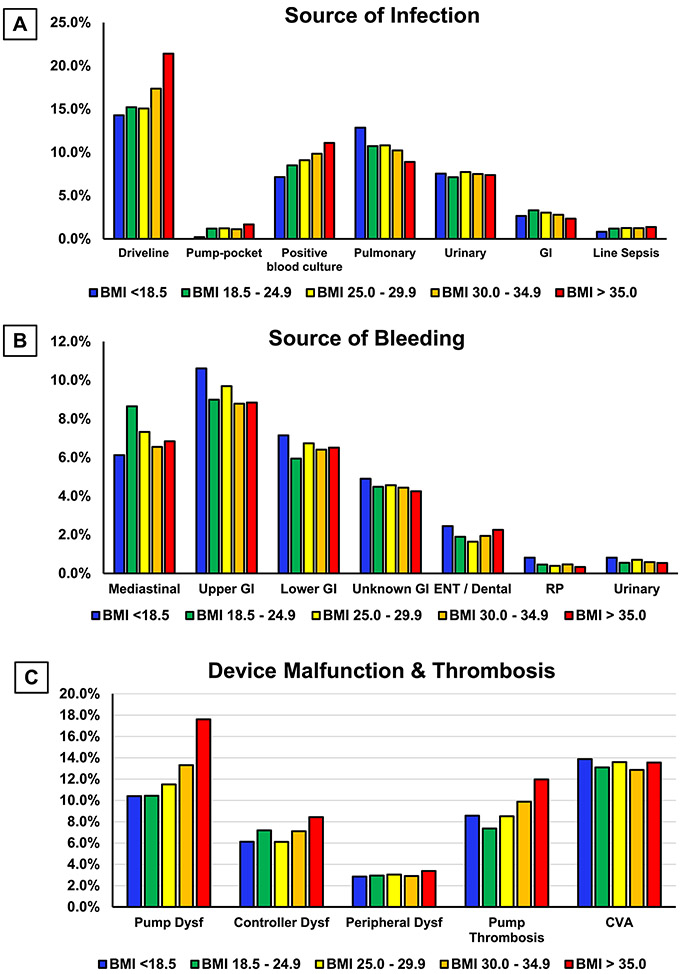
Figure 4:
A) Source of infection; B) Source of bleeding; C) Cause of device malfunction and thrombosis as per BMI categories after LVAD implant.
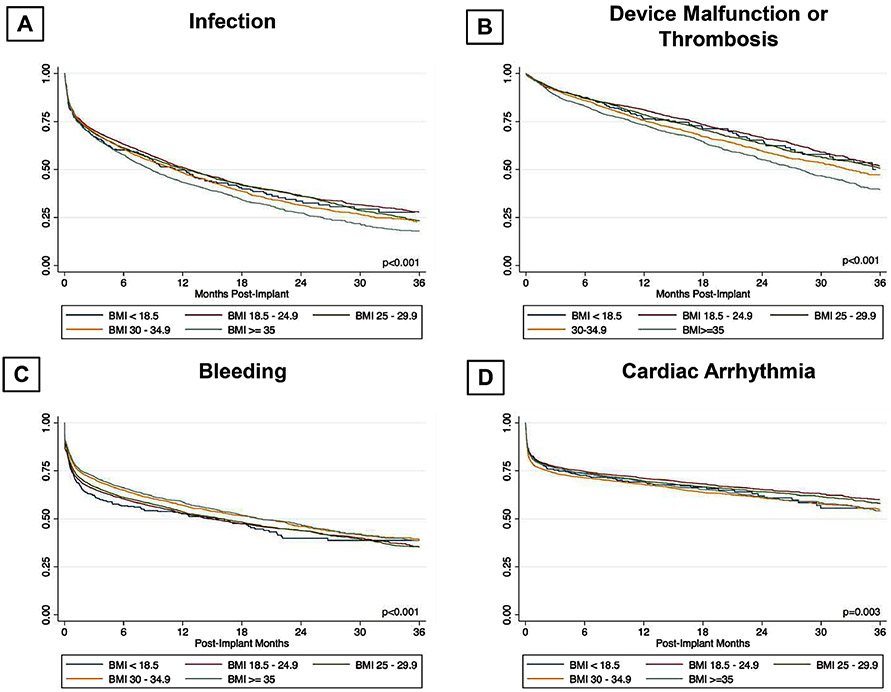
In regards to adverse events, patients with a BMI ≥ 35 kg/m2 had significantly higher incidence of device malfunction or thrombosis (HR: 1.323, p=<0.001), infection (HR: 1.215, p=<0.001), cardiac arrhythmia (HR: 1.118, p=<0.001) and hospital readmissions (HR: 1.073, p=0.022) (Table 3). Conversely, this group had significantly lower incidence of bleeding (HR: 0.906, p=0.018). Higher infection rate (54.2% vs. 47.2%) noted in patients with BMI ≥ 35 kg/m2 most likely resulted from higher driveline (21.4% vs. 15.2%) and pump-pocket (1.7% vs. 1.2%) infections compared to patients with lean BMI groups (as shown in figure 4). Higher rates of positive blood cultures were also noted in higher BMI categories. Similarly, higher rates of pump dysfunction (17.6% vs. 10.4%), controller dysfunction (8.4% vs. 7.2%) and pump thrombosis (12.0 % vs. 7.4%) were observed in patients with BMI ≥ 35 kg/m2 compared to patients with BMI ≥ 18.5 & < 25.0 Kg/m2 (Figure 4).
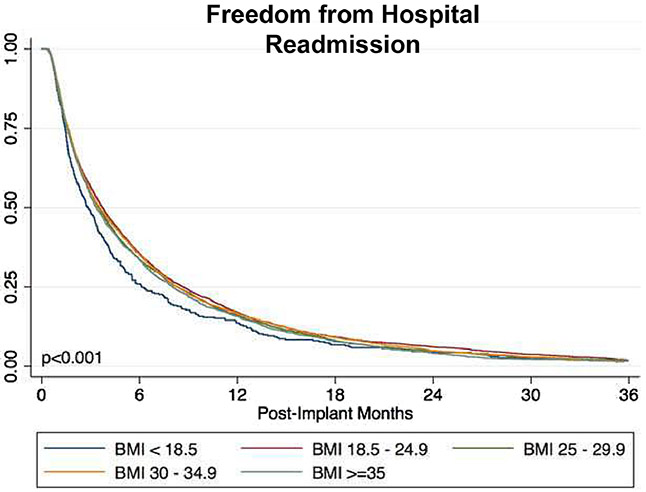
In addition, similar to patients with a BMI ≥ 35 kg/m2, we also noted significantly higher infection, device malfunction or thrombosis, cardiac arrhythmia, and lower bleeding risk in patients with BMI >30 & <35 Kg/m2. Patients with BMI <18.5 Kg/m2 sustained high rehospitalization risk as well (HR: 1.18, p=0.003).
Weight Trends on LVAD Support
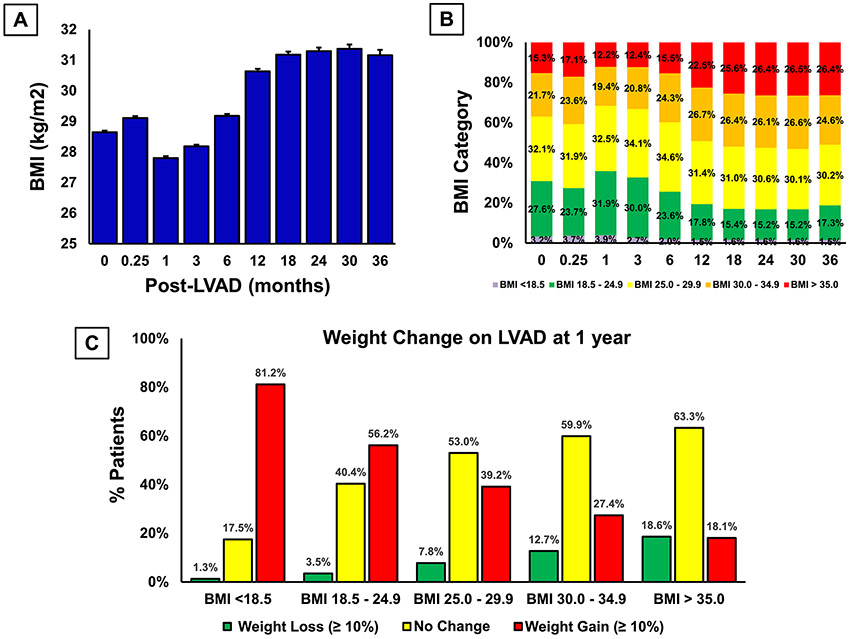
VAD support resulted in an early reduction in weight which was reversed by 12 months of support (Figure 5A). Mean BMI of the study population increased from 28.6 kg/m2 at pre-implantation to 30.6 kg/m2 at 1 year, and 31.2 kg/m2 at 2 year of device support. Overall, weight gain of the study population at one year and beyond resulted in more patients in BMI ≥ 35 kg/m2 category compared to baseline (>26.4% vs. 15.3%) (Figure 5B). Significant weight loss (≥10%) and shift into a lower BMI group were infrequent after LVAD implantation.
Figure 5:
Temporal trend of A) BMI; B) BMI categories; C) weight loss (≥10%) in different baseline BMI categories.
Moreover, significant weight loss rate in obese bridge to transplant patients was comparable to obese destination therapy patients (17.8% vs. 19.1%, p=0.572). Although 27% patients with baseline BMI ≥ 35 kg/m2 lost enough weight to move to lower BMI groups; the loss was not sustainable and majority of these patients gained weight subsequently, and only 19% of these patients remained at lower BMI categories at one, two and three years after device implantation (Supplemental Figure 1). In fact, weight gain after LVAD implementation is likely in patients who are non-obese at the time of LVAD evaluation (Figure 5C). Moreover, 18% patients with baseline BMI ≥ 35 kg/m2 gained significant weight (≥10%) (Figure 5C). Similarly, despite an initial weight loss, patients with BMI ≥ 40 kg/m2 at the time of device implantation subsequently gained weight resulting in no change in their BMI category (Supplemental Figure 1).
Patients with older age, female and sicker clinical profile at the time of device implantation were more likely to lose significant weight after LVAD implantation (Supplemental Table 1).
Weight loss and clinical outcomes
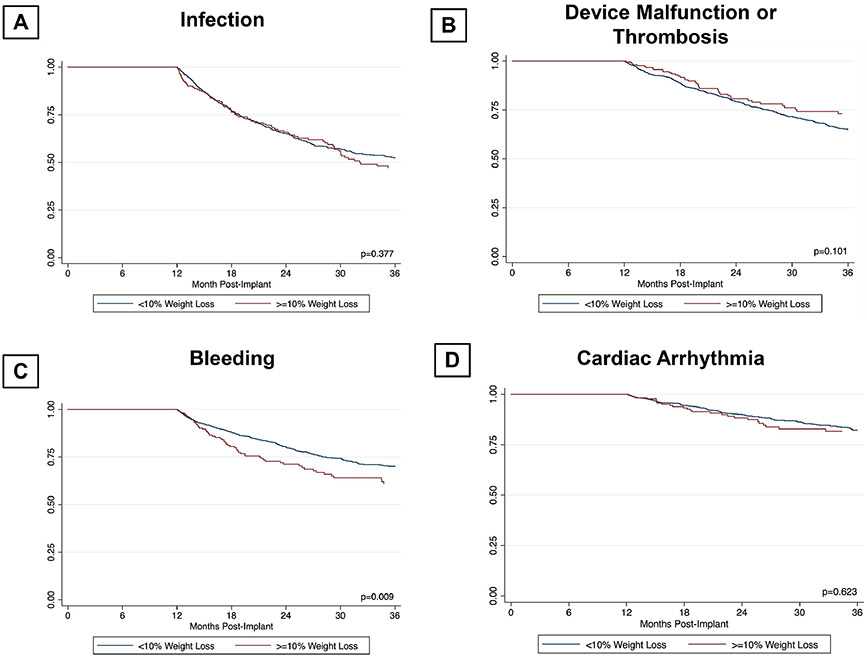
In patients with BMI ≥ 35 kg/m2 significant weight loss (≥10%) resulted in higher likelihood of undergoing cardiac transplantation (Supplemental Figure 2). However, weight loss did not result in decrease in infection, device malfunction or thrombosis, and cardiac arrhythmia rates (Figure 6). In fact, weight loss in these patients was associated with higher bleeding risk.
Figure 6:
Interaction of weight loss and complications in patients with baseline BMI ≥ 35 kg/m2
Discussion
Obesity is a growing epidemic and independent risk factor for heart failure. Although the impact of obesity on clinical outcomes after VAD implantation has been reported in studies with limited population of patients, confirmation of results awaits a large multicenter randomized placebo controlled clinical trial. However, the well-known benefits of long-term VAD raise important ethical issues, and question the feasibility of a large placebo controlled trial. In the absence of randomized studies the outcome of such patients is unclear and practice varies among centers which participate in heart transplantation and long-term mechanical circulatory support.
In this context, the current study reviewed the INTERMACS - a comprehensive nationwide VAD registry seeking to determine clinical outcomes of obese patients after LVAD implantation, if the device implantation allowed for significant weight loss, and impact of weight loss on clinical outcomes. Major findings of this analysis are; 1) Obesity does not impact mortality on LVAD support but reduces chances of cardiac transplantation, 2) Obese patients on LVAD support are at higher risk for infection, device malfunction and thrombotic events, hospital readmissions, but lower risk for bleeding complications, 3) Significant weight loss (≥10%) is achieved by a minority of obese patients after LVAD implantation, 4) Weight loss increases the likelihood of cardiac transplantation in obese patients, 5) Weight loss did not result in decrease in complication rates in obese patients. Taken together, these findings suggest that BMI alone should not be a contraindication for LVAD therapy. Given the higher risk of complications and lower chances of cardiac transplantation, research efforts should focus on developing weight loss strategies and assessing impact of weight loss on clinical outcomes for these obese patients supported by long-term LVAD.
Contrary to increased mortality observed in obese patients after heart transplantation and other cardiac surgery (20-22), we did not find an association between elevated BMI and mortality after LVAD implantation. This could be attributed to several contributing factors. First, as advanced HF is catabolic state, obese patients with end stage HF may have more metabolic reserve (23-25). In fact, patients with low BMI seem to have worse prognosis, which could possibly be due to HF-related cachexia (15,26). Indeed, we observed higher hospital readmission rates among patients in underweight group. Second, our obese patients were younger with less comorbidities and higher rates of non-ischemic cardiomyopathy. This observation also raises the possibility of underlying obesity associated cardiomyopathy. While independent association between obesity and cardiomyopathy remains controversial, the coronary artery risk development in young adults (CARDIA) study showed early LV dysfunction in young cohort with obesity (27,28). Moreover, these early signs of LV dysfunction were associated with incident heart failure (29,30). In the Framingham heart study, every unitary increment in BMI was independently associated with a 5% and a 7% increased risk of heart failure in men and women, respectively (1). Third, relatively good survival in obese LVAD recipients could be another example of “risk factor paradox” where higher blood pressure and weight are associated with improved outcomes in HF (31-34). Indeed, patients with BMI ≥ 35 kg/m2 had significantly higher systolic and diastolic blood pressures in this study. In addition, elevated blood pressure might have allowed frequent use of guideline directed medical treatment which, in turn, might have attenuated the detrimental effects of sympathetic and renin-angiotensin aldosterone responses. Finally, obese patients who underwent LVAD placement may have survival bias since these patients survived through the lengthy and specific selection process and their characteristics may not reflect that of obese HF patients in general population. It is noteworthy that other studies have demonstrated similar lack of increased mortality in obese patients after LVAD implantation albeit in significantly smaller cohort (11,14-16,19,20,35). The current study extends the finding of UNOS database which only included obese patients with LVAD as bridge to transplant strategy (11).
Higher complication rates after device implantation observed in obese patients in current study are in accordance with previous reports (11,36-39). Higher rates of diabetes and prediabetes might contribute to increased surgical site infections in obese patients (40). In addition, impaired immune surveillance, chemotaxis and macrophage function associated with obesity could increase infection risk (41). Moreover, post-operative hyperglycemia even in non-diabetic obese after cardiac surgery can predispose to risk of infection and sepsis (42).
Similarly, increased device thrombosis could be due to pro-inflammatory state, impaired thrombolysis and increased platelet activity associated with obesity (43,44). Furthermore, cardiac surgery itself may worsen prothrombotic state by shortening clotting time, partial thromboplastin time and increasing platelet count and fibrinogen in obese patients with BMI≥ 35 Kg/m2 (45). Lower gastrointestinal bleeding rate in obese patients is intriguing and warrants further investigation. A review of 233 patients on LVAD support from Minnesota University reported an inverse relation with BMI as well (46). Similarly, a post hoc analysis of patients enrolled in the HeartMate II trials showed a lower bleeding rate in obese patients with BMI ≥35 kg/m2 (16). Although exact explanation is unclear, several factors might have been involved. Young obese patients in this study might have lower rates of degenerative lesions like vascular malformation and angiodysplasias, which are more prevalent in elderly (47). Moreover, defective synthesis of ADAMTS 13 (A disintergrin and metalloproteinase with a thrombospondin type 1 motif, member 13) and the presence of anti-ADAMTS13 antibodies in obese patients may cause higher thromboembolism and lower bleeding rates (48). The impact of new LVAD with smaller profile in reducing device related complications in obese cohort requires investigation and may present positive outcomes (49).
Although weight loss on LVAD support is possible, it was infrequent. In our study cohort, while 18.6% patients with BMI ≥ 35 kg/m2 lost significant weight (≥10%), an equal proportion of such patients (18.1%) gained significant weight. Similar findings were reported from the UNOS database which showed that only 15% patients with BMI≥ 35 kg/m2 lost enough weight to move to a lower BMI group (11). Furthermore, our analysis shows that weight gain after LVAD implementation is common in patients who are non-obese at the time of LVAD evaluation. In fact, >25% patients with BMI≥ 30-34.9 kg/m2 gained significant weight. Of note, LVAD implantation allowed for significant weight gain in 80% of underweight (BMI<18.5 kg/m2) patients suggesting reversal of cardiac cachexia. This finding is in accordance with prior similar reports and strengthens the case for LVAD as first line of therapy in patients with advanced heart failure and cardiac cachexia (50). Observed increased hospital readmission among patients in this group in our analysis could have been driven by sub group of such patients who failed to gain weight after LVAD therapy.
Weight loss resulted in higher likelihood of cardiac transplantation in our obese patients. However, weight loss in obese patients did not result in reduction of adverse events. Contrarily, bleeding risk was worsened with weight loss in obese patients. While the reasons behind this observation remain unclear, we reflect that anticoagulant dosing and loss of obesity related protection from bleeding might have contributed. Obese patients require higher doses of warfarin to maintain a therapeutic anticoagulation, and subsequent weight reduction might have resulted in higher than therapeutic range anticoagulation and an increased bleeding risk.
Cardiac rehabilitation participation among overweight and obese patients for weight loss have produced modest results at best. A combination of behavioral weight loss counseling and an approach to exercise that maximizes exercise-related caloric expenditure has been explored to achieve significant weight loss (51). In addition, some centers have implemented a strategy of combined approach of LVAD implantation and bariatric surgery to increase chances of cardiac transplantation 52,53). Whether weight loss strategies such as gastric bypass will have positive impact on clinical outcomes in obese patients with LVAD support is unclear and merits further investigation.
Limitations
Although INTERMACS represents a high-quality registry database, limitations inherent to the retrospective analyses apply to this study and causality is impossible to demonstrate. As such, survival analyses should be interpreted as hypothesis-generating. Furthermore, determination of destination therapy was made at the discretion of the implanting center rather than upon standardized criteria, and not all patients had a strategy assigned at the time follow up.
In conclusion, patients with BMI ≥ 35 kg/m2 had comparable survival to that of other patients in different BMI groups while on LVAD support despite higher complication rates. This suggests that while the care of obese patients with LVAD is clearly more complex, evidence supports that these patients can safely undergo LVAD implantation with comparable survival to their non-obese counterparts. Greater care must be exercised to prevent device complications in obese. Significant weight loss is uncommon after device implantation. Weight loss facilitates cardiac transplantation in obese patients. The role of weight loss strategies (prior and after LVAD support) on clinical outcomes in such patients need further exploration.
Supplementary Material
Figure 2:
Freedom from: A) Infection; B) Device malfunction or thrombosis; C) Bleeding; D) Cardiac arrhythmia as per BMI categories after LVAD implant.
Figure 3:
Freedom from hospital readmissions as per BMI categories after LVAD implant.
Acknowledgements:
The authors would like to acknowledge all the participating centers, investigators, coordinators, patients who are part of the INTERMACS and the INTERMACS research committee.
Financial Support: This project has been funded in whole or in part with federal funds by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center, and the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), and the Department of Health and Human Services (HHS) under Contract Number HHSN268201100025C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Industry Relationships: All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Kenchaiah S, Evans JC, Levy D et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 4.Ng M, Fleming T, Robinson M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grady KL, White-Williams C, Naftel D et al. Are preoperative obesity and cachexia risk factors for post heart transplant morbidity and mortality: a multi-institutional study of preoperative weight-height indices. Cardiac Transplant Research Database (CTRD) Group. J Heart Lung Transplant 1999;18:750–63. [DOI] [PubMed] [Google Scholar]
- 6.Lietz K, John R, Burke EA et al. Pretransplant cachexia and morbid obesity are predictors of increased mortality after heart transplantation. Transplantation 2001;72:277–83. [DOI] [PubMed] [Google Scholar]
- 7.Mehra MR, Kobashigawa J, Starling R et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant 2006;25:1024–42. [DOI] [PubMed] [Google Scholar]
- 8.Russo MJ, Hong KN, Davies RR et al. The effect of body mass index on survival following heart transplantation: do outcomes support consensus guidelines? Ann Surg 2010;251:144–52. [DOI] [PubMed] [Google Scholar]
- 9.Zahr F, Genovese E, Mathier M et al. Obese patients and mechanical circulatory support: weight loss, adverse events, and outcomes. Ann Thorac Surg 2011;92:1420–6. [DOI] [PubMed] [Google Scholar]
- 10.Nagendran J, Moore MD, Norris CM et al. The varying effects of obesity and morbid obesity on outcomes following cardiac transplantation. Int J Obes (Lond) 2016;40:721–4. [DOI] [PubMed] [Google Scholar]
- 11.Clerkin KJ, Naka Y, Mancini DM, Colombo PC, Topkara VK. The Impact of Obesity on Patients Bridged to Transplantation With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail 2016;4:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhesi P, Simsir SA, Daneshvar D, Rafique A, Phan A, Schwarz ER. Left ventricular assist device as 'bridge to weight loss' prior to transplantation in obese patients with advanced heart failure. Ann Transplant 2011;16:5–13. [PubMed] [Google Scholar]
- 13.Raymond AL, Kfoury AG, Bishop CJ et al. Obesity and left ventricular assist device driveline exit site infection. ASAIO J 2010;56:57–60. [DOI] [PubMed] [Google Scholar]
- 14.Coyle LA, Ising MS, Gallagher C et al. Destination therapy: one-year outcomes in patients with a body mass index greater than 30. Artif Organs 2010;34:93–7. [DOI] [PubMed] [Google Scholar]
- 15.Butler J, Howser R, Portner PM, Pierson RN 3rd. Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg 2005;79:66–73. [DOI] [PubMed] [Google Scholar]
- 16.Brewer RJ, Lanfear DE, Sai-Sudhakar CB et al. Extremes of body mass index do not impact mid-term survival after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2012;31:167–72. [DOI] [PubMed] [Google Scholar]
- 17.Drakos SG, Janicki L, Horne BD et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030–5. [DOI] [PubMed] [Google Scholar]
- 18.Slaughter MS, Rogers JG, Milano CA et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–51. [DOI] [PubMed] [Google Scholar]
- 19.Mohamedali B, Yost G, Bhat G. Obesity as a Risk Factor for Consideration for Left Ventricular Assist Devices. J Card Fail 2015;21:800–5. [DOI] [PubMed] [Google Scholar]
- 20.Musci M, Loforte A, Potapov EV et al. Body mass index and outcome after ventricular assist device placement. Ann Thorac Surg 2008;86:1236–42. [DOI] [PubMed] [Google Scholar]
- 21.van Straten AH, Bramer S, Soliman Hamad MA et al. Effect of body mass index on early and late mortality after coronary artery bypass grafting. Ann Thorac Surg 2010;89:30–7. [DOI] [PubMed] [Google Scholar]
- 22.Healy AH, Stehlik J, Edwards LB, McKellar SH, Drakos SG, Selzman CH. Predictors of 30-day post-transplant mortality in patients bridged to transplantation with continuous-flow left ventricular assist devices--An analysis of the International Society for Heart and Lung Transplantation Transplant Registry. J Heart Lung Transplant 2016;35:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anker SD, Negassa A, Coats AJ et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 2003;361:1077–83. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol 2004;43:1439–44. [DOI] [PubMed] [Google Scholar]
- 25.Lavie CJ, Mehra MR, Milani RV. Obesity and heart failure prognosis: paradox or reverse epidemiology? Eur Heart J 2005;26:5–7. [DOI] [PubMed] [Google Scholar]
- 26.Davos CH, Doehner W, Rauchhaus M et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail 2003;9:29–35. [DOI] [PubMed] [Google Scholar]
- 27.Kishi S, Armstrong AC, Gidding SS et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail 2014;2:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan MF, Movahed MR. Obesity cardiomyopathy and systolic function: obesity is not independently associated with dilated cardiomyopathy. Heart Fail Rev 2013;18:207–17. [DOI] [PubMed] [Google Scholar]
- 29.Choi EY, Rosen BD, Fernandes VR et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol 2009;54:618–24. [DOI] [PubMed] [Google Scholar]
- 31.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 2001;38:789–95. [DOI] [PubMed] [Google Scholar]
- 32.Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail 2002;8:216–24. [DOI] [PubMed] [Google Scholar]
- 33.Poole-Wilson PA, Uretsky BF, Thygesen K et al. Mode of death in heart failure: findings from the ATLAS trial. Heart 2003;89:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009;53:1925–32. [DOI] [PubMed] [Google Scholar]
- 35.Yanagida R, Czer LS, Mirocha J et al. Left ventricular assist device in patients with body mass index greater than 30 as bridge to weight loss and heart transplant candidacy. Transplant Proc 2014;46:3575–9. [DOI] [PubMed] [Google Scholar]
- 36.John R, Aaronson KD, Pae WE et al. Drive-line infections and sepsis in patients receiving the HVAD system as a left ventricular assist device. J Heart Lung Transplant 2014;33:1066–73. [DOI] [PubMed] [Google Scholar]
- 37.Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943–9. [DOI] [PubMed] [Google Scholar]
- 38.Boyle AJ, Jorde UP, Sun B et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 2014;63:880–8. [DOI] [PubMed] [Google Scholar]
- 39.Kirklin JK, Naftel DC, Kormos RL et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant 2014;33:12–22. [DOI] [PubMed] [Google Scholar]
- 40.Bryan CS, Yarbrough WM. Preventing deep wound infection after coronary artery bypass grafting: a review. Tex Heart Inst J 2013;40:125–39. [PMC free article] [PubMed] [Google Scholar]
- 41.Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37:333–40. [DOI] [PubMed] [Google Scholar]
- 42.Kuznetsova LA, Iavorovskii AG, Petunina NA, Morozov A, Eremenko AA, Ziuliaeva TP. [Predictive value of body mass index for perioperative hyperglycemia occurrence in cardio-surgical patients without diabetes mellitus]. Anesteziol Reanimatol 2014:11–3. [PubMed] [Google Scholar]
- 43.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood 2013;122:3415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blokhin IO, Lentz SR. Mechanisms of thrombosis in obesity. Curr Opin Hematol 2013;20:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kindo M, Minh TH, Gerelli S et al. The prothrombotic paradox of severe obesity after cardiac surgery under cardiopulmonary bypass. Thromb Res 2014;134:346–53. [DOI] [PubMed] [Google Scholar]
- 46.Harvey L, Holley CT, John R. Gastrointestinal bleed after left ventricular assist device implantation: incidence, management, and prevention. Ann Cardiothorac Surg 2014;3:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boley SJ, Sammartano R, Adams A, DiBiase A, Kleinhaus S, Sprayregen S. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology 1977;72:650–60. [PubMed] [Google Scholar]
- 48.Lombardi AM, Fabris R, Scarda A et al. Presence of anti-ADAMTS13 antibodies in obesity. Eur J Clin Invest 2012;42:1197–204. [DOI] [PubMed] [Google Scholar]
- 49.Mehra MR, Naka Y, Uriel N et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med 2017;376:440–450. [DOI] [PubMed] [Google Scholar]
- 50.Emani S, Brewer RJ, John R et al. Patients with low compared with high body mass index gain more weight after implantation of a continuous-flow left ventricular assist device. J heart lung transplant 2013;32:31–35. [DOI] [PubMed] [Google Scholar]
- 51.Ades PA, Savage PD, harvey-Berino J et al. High-calorie-expenditure exercise: a new approach to cardiac rehabilitation for overweight coronary patients. Circulation. 2009;119:2671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah SK, Gregoric ID, Nathan SS et al. Simultaneous left ventricular assist device placement and laparoscopic sleeve gastrectomy as a bridge to transplant for morbidly obese patients with severe heart failure. J Heart Lung Transplant 2015;34:1489–91. [DOI] [PubMed] [Google Scholar]
- 53.Chaudhry UI, Kanji A, Sai-Sudhakar CB, Higgins RS, Needleman BJ. Laparoscopic sleeve gastrectomy in morbidly obese patients with end-stage heart failure and left ventricular assist device: medium-term results. Surg Obes Relat Dis 2015;11:88–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.