Abstract
Objective: Upper gastrointestinal (UGI) cancers, predominantly gastric cancer (GC) and esophageal cancer (EC), are malignant tumor types with high morbidity and mortality rates. Accumulating studies have focused on metabolomic profiling of UGI cancers in recent years. In this systematic review, we have provided a collective summary of previous findings on metabolites and metabolomic profiling associated with GC and EC.
Methods: A systematic search of three databases (Embase, PubMed, and Web of Science) for molecular epidemiologic studies on the metabolomic profiles of GC and EC was conducted. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included articles.
Results: A total of 52 original studies were included for review. A number of metabolites were differentially distributed between GC and EC cases and non-cases, including those involved in glycolysis, anaerobic respiration, tricarboxylic acid cycle, and protein and lipid metabolism. Lactic acid, glucose, citrate, and fumaric acid were among the most frequently reported metabolites of cellular respiration while glutamine, glutamate, and valine were among the most commonly reported amino acids. The lipid metabolites identified previously included saturated and unsaturated free fatty acids, aldehydes, and ketones. However, the key findings across studies to date have been inconsistent, potentially due to limited sample sizes and the majority being hospital-based case-control analyses lacking an independent replication group.
Conclusions: Studies on metabolomics have thus far provided insights into etiological factors and biomarkers for UGI cancers, supporting the potential of applying metabolomic profiling in cancer prevention and management efforts.
Keywords: Gastric cancer, esophageal cancer, metabolomics, Warburg effect, biomarkers
Introduction
Upper gastrointestinal (UGI) cancers, predominantly gastric cancer (GC) and esophageal cancer (EC), are major malignancies in China and worldwide1, with prognosis remaining poor in many countries without effective screening programs2,3. Holistic promotion of etiological research and identification of novel biomarkers is essential to ensure implementation of timely and appropriate preventive and treatment strategies. Developments in molecular biology, along with emergence of various new omics techniques, have provided powerful tools for advancement of molecular epidemiologic studies on UGI cancers.
Metabolic dysregulation has been shown to underlie carcinogenesis of UGI cancers4. In addition to the alterations in glucose metabolism, as indicated by the well-known Warburg effect, dysregulated metabolism of amino acids, lipids, and nucleotides has been demonstrated, both in vitro and in vivo5–7. Metabolites represent the end product of complex joint effects of intrinsic metabolism, environmental exposures, and genetic predisposition. High-throughput metabolomics techniques can facilitate comprehensive identification and quantitative profiling of the entire spectrum of endogenous low molecular weight metabolites (< 1000 Da) in a single sample8,9, which may not only aid in identifying promising novel biomarkers but also provide insights into cancer etiology, leading to the development of novel preventive approaches and therapeutic targets10.
Studies have been conducted to investigate the broad network of metabolites in UGI cancers based on various human biological samples, including tissue, plasma, and urine11. Although efforts have been made to review past literature on the metabolomics of UGI cancers4,11–14, these reports were simply narrative descriptions. Only one systematic review was available as of 2012, which included 20 references4. In view of the accumulating studies on metabolomic profiling of UGI cancers over the last 6 years, an updated systematic review is warranted to summarize the available literature for a clear understanding of the field of metabolomic studies on UGI cancers and identify specific metabolites and metabolic pathways consistently associated with these cancer types.
To address this issue, we conducted a systematic review of the currently available metabolomic studies on GC and EC. Given the described major interests and our long-standing top priority as cancer epidemiologists to promote cancer prevention and management at the population level, we focused on previous human molecular epidemiologic studies on metabolomic profiling of UGI cancers. Here, we present a summary of the latest advances in determining the individual metabolites and metabolic pathways associated with these cancers while highlighting the limitations of the available studies, with the aim of providing insights into future metabolomic approaches, promoting etiologic research and precision prevention and control of UGI cancers.
Materials and methods
This study was performed and presented following the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement)15 and Preferred Reporting Items for Systematic Review and Meta-Analysis protocols (PRISMA-P)16.
Search strategy and data extraction
We searched the literature for studies focusing on metabolomic profiling of human GC and EC as of September 4, 2019, using Embase, PubMed, and Web of Science databases. Multiple combinations of the keywords, including “mass spectrometry/nuclear magnetic resonance spectroscopy”, “metabolomic/metabonomic/metabolic profiling”, “gastric cancer/stomach cancer”, and “oesophageal/esophageal cancer”, were used (Supplementary Table S1). Articles in both English and Chinese were considered.
The identified literature was imported to EndNote, a standard software for publishing and managing bibliographies, citations, and references. Two researchers (S.H. and Y.G.) independently screened the title and abstract of each reference. Non-metabolomic (proteomic, glycomic and volatile organic compound-related) studies and conference abstracts were excluded. Studies comparing the metabolomic profiling of human biological specimens from GC/EC patients to those of control samples (either biological specimens from independent individuals or tumor-adjacent tissues) were included. Owing to our primary interest in the risk of UGI cancer development, studies concentrating on metabolomics associated with responses to cancer therapy and recurrence and metastasis of UGI cancers were additionally excluded.
For all selected articles, information on authors, publication year, sample type, analytical platform, sample size, and differentially distributed metabolites across comparison groups were independently extracted by two investigators (S.H. and Y.G.). In addition to individual metabolites, the two investigators independently reviewed findings on alterations in major metabolic pathways associated with UGI cancers.
Study quality assessment
The quality of included studies was assessed using the Newcastle–Ottawa Scale (NOS)17, which covers three key domains, including Selection (4 items), Comparability (1 item), and Exposure (3 items), with a total of 8 items. Studies were rated on each of the eight items using a star system, with the final scores for each study ranging from 0 to 9 stars. A maximum of 1 star could be awarded for each item within the Selection and Exposure categories and a maximum of 2 stars allowed for the one item within the Comparability category. Studies that scored more than 6 stars were classified as high quality, and any discrepancies between the findings of the two investigators (S.H. and Y.G.) were resolved by discussion. In addition to NOS, we applied a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS)18 as a supplement. BIOCROSS includes 10 items in 5 domains, including “Study rational”, “Design/Methods”, “Data analysis”, “Data interpretation” and “Biomarker measurement”, and has been proved to be reliable in facilitating comprehensive review of human biomarker studies18.
Results
Study characteristics
Following application of inclusion criteria, a total of 52 studies were enrolled, including 30 on GC, 21 on EC, and 1 on both GC and EC (Figure 1, Table 1). In the majority of studies, controls were described as healthy individuals. Several studies (n = 10) included cases of benign gastric or esophageal lesions as controls. Among these, 5 included subjects with precancerous gastric lesions, 3 of which reported metabolic changes in precancerous gastric lesions compared with less severe lesions or normal controls, and 4 included subjects with precancerous esophageal lesions displaying metabolic alterations. However, findings from these studies were inconsistent.
Figure 1.
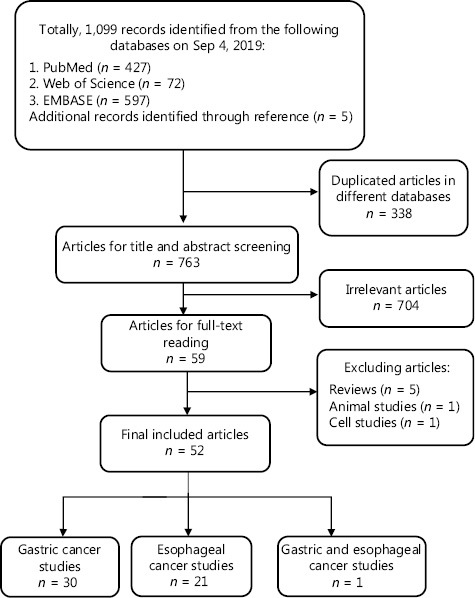
Flow chart of literature identification and the selection process.
Table 1.
Characteristics of the included studies
| Study | Region | Sample type | Analytical platform | Cancer group |
Control group |
||
|---|---|---|---|---|---|---|---|
| Cancer | Sample size | Control | Sample size | ||||
| Lee (Anal Chim Acta.) 201919 | South Korea | Plasma | nUHPLC–MS/MS | GC | 20 | Healthy subjects | 20 |
| Xiu (Acad J Second Military Medical Univ.) 201820 | Mainland China | Plasma | UHPLC–MS/MS | GC | 104 | Healthy subjects | 50 |
| Corona (Int J Mol Sci.) 201821 | Italy | Serum | LC–MS/MS | GC Training set (n = 49) Validation set (n = 22) |
71 | First-degree relatives Training set (n = 37) Validation set (n = 17) |
54 |
| Tokunaga (Int J Oncol.) 201822 | Japan | Tissue | CE–TOFMS | EC | 35 | Tumor-adjacent tissues | 35 |
| Jing (Iubmb Life.) 201823 | Mainland China | Plasma | LC–MS/MS | EC | 84 | Gastric ulcer | 82 |
| Ma (J Pharm Biomed Anal.) 201824 | Mainland China | Serum | 2D LC–MS | EC | 34 | Healthy subjects | 32 |
| Lario (Sci Rep.) 201725 | Spain | Plasma | LC–MS | GC | 20 | NAG- (n = 19); CAG+ (n = 20); PLGC- (n = 19) |
60 |
| Zhang (Biochem Biophys Res Commun.) 201726 | Mainland China | Tissue | LC/MS | EC Training set (n = 35) Validation set (n = 5) |
40 | Tumor-adjacent tissues Training set (n = 35) Validation set (n = 5) |
40 |
| Cheng (Biochem Biophys Res Commun.) 201727 | Mainland China | Serum | UPLC–MS/MS | EC (n = 38) Metastatic EC (n = 38) |
76 | Healthy subjects | 28 |
| Cheng (Comb Chem High Throughput Screen.) 201728 | Mainland China | Serum | LC–MS/MS | EC Test set (n = 5) Training set (n = 35) |
40 | Healthy subjects Test set (n = 5) Training set (n = 22) |
27 |
| Zhu (Gastroenterol Res Pract.) 201729 | Mainland China | Tissue | GC/TOF–MS | EC Serum (n = 24) Tissue (n = 19) |
43 | Healthy subjects (serum, n = 21) and tumor-adjacent tissues (tissue, n = 19) | 40 |
| Reed (Neoplasia.) 201730 | UK | Tissue | 1H NMR | EC | 46 | BO, n = 7); patients undergoing upper gastrointestinal endoscopy for dyspeptic symptoms but without endoscopic abnormalities (controls, n = 68) | 75 |
| Wang (Oncotarget.) 201731 | Mainland China | Serum | HPLCESI/Q-TOFMS | GC Test group (n = 24) Validation group (n = 14) Additional group (n = 87) |
125 | Healthy subjects Test group (n = 24) Validation group (n = 14) |
38 |
| Choi (Biomed Chromatogr.) 201632 | South Korea | Serum and gastric juice | LC–MS/MS | GC | 35 | Gastritis (same race and same geo-graphic area) | 17 |
| Wang (BMC Cancer.) 201633 | Mainland China | Tissue | 1H NMR | GC | 125 | Healthy subjects | 54 |
| Chan (Br J Cancer.) 201634 | Canada | Urine | 1H-NMR | GC | 43 | Benign gastric disease (n = 40) and healthy subjects (n = 40) | 80 |
| Kuligowski (J Proteome Res) 201635 | Spain | Plasma | UPLC–TOFMS | GC | 33 | Dyspepsia | 110 |
| Xu (Sci Rep.) 201636 | Mainland China | Urine | LC–MS | EC | 62 | Healthy subjects | 62 |
| Liang (Appl Biochem Biotechnol.) 201537 | Mainland China | Urine | LC–MS | GC | 13 | Healthy subjects | 9 |
| Mir (J Proteomics.) 201538 | India | Serum | LC–MS | EC | 40 | Healthy subjects | 10 |
| Jung (Ann Surg Oncol.) 201439 | South Korea | Urine and tissue | 1H NMR and HR-MAS NMR | GC Urine (n = 50) Tissue (n = 30) |
80 | Healthy subjects Urine (n = 50) Tissue (n = 30) |
80 |
| Lo (Clin Chim Acta.) 201440 | Taiwan | Urine | HPLC/ESI–MS/MS | GC | 49 | Healthy subjects | 40 |
| Chen (Electrophoresis.) 201441 | Mainland China | Urine | MRB–CE–MS | GC | 26 | Healthy subjects | 14 |
| Hur (PLoS One.) 201442 | South Korea | Tissue | GC–MS | GC | 45 | Tumor-adjacent tissue | 45 |
| Yang (Se Pu.) 201443 | Mainland China | Serum | LC–MS | GC | 20 | Healthy subjects | 40 |
| Kwon (Open Proteomics J.) 201444 | South Korea | Tissue | MALDI MS | GC | 12 | Tumor-adjacent tissue | 12 |
| Yang (Anal Bioanal Chem.) 201345 | Mainland China | Tissue | 1H NMR | EC | 17 | Tumor-adjacent tissue | 14 |
| Zhang X (Biochim Biophys Acta) 201346 | Mainland China | Serum | 1H NMR and UHPLC | EC | 25 | Healthy subjects | 25 |
| Liu (Int J Mol Sci.) 201347 | Mainland China | Plasma | UPLC/TOF/MS | EC | 53 | Healthy subjects | 53 |
| Wang (Molecular Cancer.) 201348 | Mainland China | Tissue | 1H NMR | EC | 89 | Tumor-adjacent tissue | 26 |
| Song (Chinese J Clin Nutrition.) 201349 | Mainland China | Serum and tissue | GC–MS | GC Tissue (n = 40) Serum (n = 40) |
80 | Tumor-adjacent tissue (n = 40) and serum from healthy subjects (n = 40) |
80 |
| Ikeda (Biomed Chromatogr.) 201250 | Japan | Serum | GC–MS | GC (n = 11) and EC (n = 15) | 26 | Healthy subjects | 12 |
| Song (Braz J Med Biol Res.) 201251 | Mainland China | Serum | GC–MS | GC | 30 | Healthy subjects | 30 |
| Aa (Metabolomics.) 201252 | Mainland China | Serum and tissue | GC/TOFMS | GC (n = 17) and postoperative GC (n = 15) | 32 | Chronic superficial gastritis | 20 |
| Hasim (Mol Biol Rep.) 201253 | Mainland China | Plasma and urine | NMR | EC | 108 | Healthy subjects | 40 |
| Zhang (PLoS One.) 201254 | US | Serum | LC-MS and NMR | EC | 67 | Healthy subjects (n = 34), BE, n = 3), and high-grade dysplasia (HGD, n = 9) | 46 |
| Davis (World J Surg Oncol.) 201255 | Canada | Urine | 1H NMR | EC | 44 | Healthy subjects (n = 75) and BE (n = 31), | 106 |
| Song (Chin Med J.) 201256 | Mainland China | Tissue | GC/MS | GC | 30 | Tumor-adjacent tissues | 30 |
| Sun (Chinese J Gastroenterol.) 201157 | Mainland China | Tissue and plasma | GC/TOF–MS | GC tissue (n = 17), GC plasma (n = 15), and postoperative GC plasma (n = 15) | 47 | Chronic superficial gastritis tissue (n = 20) and plasma (n = 15) | 35 |
| Yu (J Gastroenterol Hepatol.) 201158 | Mainland China | Plasma | GC/TOF–MS | GC | 22 | Chronic superficial gastritis (n = 19), chronic atrophic gastritis (n = 13), intestinal metaplasia (n = 10), and dysplasia (n = 15) | 57 |
| Song (Oncol Rep.) 201159 | Mainland China | Tissue | GC–MS | GC | 30 | Tumor-adjacent tissue | 30 |
| Zhang (J Thorac Cardiovasc Surg.) 201160 | US | Serum | NMR | EC | 68 | Healthy subjects (n = 34), BE (n = 5), and HGD (n = 11) | 50 |
| Wu (Anal Bioanal Chem.) 201061 | Mainland China | Tissue | GC–MS | GC | 18 | Tumor-adjacent tissue | 18 |
| Yakoub (Cancer Res.) 201062 | UK | Tissue | NMR | EC | 52 | Healthy subjects | 35 |
| Cai (Mol Cell Proteomics.) 201063 | Mainland China | Tissue | GC–MS | GC | 65 | Tumor-adjacent tissue | 65 |
| Djukovic (Rapid Commun Mass Spectrom.) 201064 | US | Serum | HPLC/TQMS | EC | 14 | Healthy subjects | 12 |
| Ayshamgul (Chin J Oncol.) 201065 | Mainland China | Plasma | 1H NMR | EC | 109 | Healthy subjects | 50 |
| Hirayama (Cancer Res.) 200966 | Japan | Tissue | CE–MS | GC | 12 | Tumor-adjacent tissue | 12 |
| Wu (J Chromatogr B.) 200967 | Mainland China | Tissue | GC/MS | EC | 20 | Tumor-adjacent tissues | 20 |
| Calabrese (Cancer Epidemiol Biomarkers Prev.) 200868 | Italy | Tissue | HR-MAS MRS | GC | 5 | Healthy subjects (n = 12), autoimmune atrophic gastritis (n = 5), and H. pylori infection (n = 5) | 22 |
| Tugnoli (Oncol Rep.) 200669 | Italy | Tissue | HR-MAS NMR | GC | 5 | Healthy subjects | 11 |
| Mun (Magn Reson Imaging.) 200470 | South Korea | Tissue | 1H MRS | GC | 13 | Tumor-adjacent tissue | 22 |
BO, Barrett’s esophagus; CAG, chronic active gastritis; GC, gastric cancer; EC, esophageal cancer; HGD, high grade dysplasia; NAG, non-active gastritis; PLGC, precursor lesions of gastric cancer; H. pylori, Helicobacter pylori; 1H NMR, proton nuclear magnetic resonance; HR-MAS–NMR, high resolution-magic angle spinning-nuclear magnetic resonance spectroscopy; GC–MS, gas chromatography–mass spectrometry; CE–MS, capillary electrophoresis–mass spectrometry; HPLC–MS, high-performance liquid chromatography–mass spectrometry; LC–MS, liquid chromatography–mass spectrometry
The sample sizes of included studies ranged from 16 to 179, with a median of 81. Previous reports assayed tissue (n = 23), blood (n = 27), urine (n = 8) and gastric juice (n = 1), with 6 studies involving two or more types of biological specimens. The analytical platforms for measurement of metabolites also differed across studies, including nuclear magnetic resonance (n = 14), liquid chromatography–mass spectrometry (n = 20), gas chromatography–mass spectrometry (n = 13), capillary electrophoresis–mass spectrometry (n = 4), magnetic resonance spectroscopy (n = 2), and matrix-assisted laser desorption/ionization mass spectrometry (n = 1).
Review of the methods used for data analysis showed that half (n = 26) of the previous studies only conducted univariate tests (Supplementary Figure S1). Only six studies corrected for multiple comparisons, with calculation of the false discovery rate in all cases. A receiver operating characteristic (ROC) curve was plotted to delineate the performance of biomarkers, with area under the receiver operating characteristic (AUC), sensitivity, and specificity reported in 40.4% (n = 21) studies.
Quality assessment of studies
Quality assessment with NOS revealed a mean score of 5.37 (ranging from 4 to 8) for studies on GC and 5.30 (ranging from 4 to 7) for studies on EC (Figure 2 and Supplementary Table S2). The majority of studies involved hospital-based subject selection but the comparability of groups was not adequately described. Around 59.6% (31/52) of the studies considered 1 or 2 important confounding factors (mostly age or sex) during study design or statistical analysis. Quality assessment using BIOCROSS disclosed similar results to those obtained with NOS assessment, raising possible concerns on study population representativeness, study limitations, and biomarker data modeling (Supplementary Figure S2).
Figure 2.
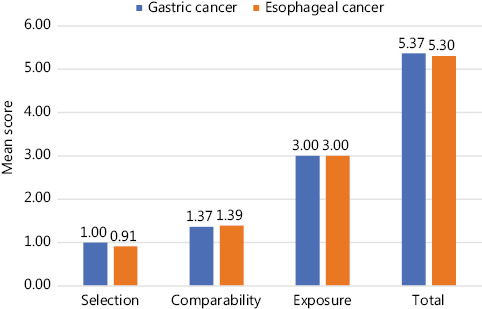
Quality assessment of included studies using the Newcastle–Ottawa Scale (NOS) (maximum scores of 4, 2, and 3 given in selection, comparability, and exposure categories, respectively).
Carbohydrate metabolism
Metabolites of carbohydrate metabolism have been previously associated with UGI cancers (Table 2). Several metabolites involved in cellular respiration, including lactic acid, glucose, citrate, and fumaric acid, have been frequently reported, but these results are not consistent across studies. Moreover, opposite associations of some metabolites with UGI cancers are documented by different research groups. For example, lactic acid was found to be upregulated in tissue and urine samples of GC in 8 studies33,39,41,42,49,52,63,66, while one group reported upregulation in tissue and conversely, downregulation in plasma57. Upregulation of citrate in EC was reported in 5 studies29,54,55,60,65 and downregulation in one study22. A separate study showed upregulation of citrate in plasma but downregulation in urine53. The findings also support distinct associations of different carbohydrate metabolites with GC and EC. Analysis of earlier data revealed upregulation of α-ketoglutaric acid in both GC42,49,59 and EC29 while isocitric acid was upregulated in GC63 and downregulated in EC22. Upregulation of glyceraldehyde in GC was reported by a number of studies63,66 but its association with EC is currently unclear.
Table 2.
Changes in carbohydrate metabolites in GC and EC, compared with controls
| Study | Sample type | Analytical platform | Glycolysis |
Anaerobic respiration lactic acid/lactate | TCA cycle |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Glyceraldehyde | Pyruvic acid | Citrate | Isocitric acid | α-ketoglutaric acid/α-ketoglutarate | Succinate | Fumaric acid/fumarate | Malate | ||||
| GC | |||||||||||||
| Hirayama (2009)66 | Tissue | CE–MS | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ||||
| Cai (2010)63 | Tissue | CE–MS | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | |||||
| Sun (2011)57 | Tissue Plasma | GC/TOF-MS | ↓ ↓ |
↓ | ↑ ↓ |
↑ | ↓ | ↓ | ↓ | ||||
| Song (2011)59 | Tissue | GC–MS | ↑ | ↑ | |||||||||
| Song (2012)51 | Serum | GC–MS | ↓ | ||||||||||
| Aa (2012)52 | Serum Tissue | GC/TOFMS | ↓ ↓ |
↑ | ↑ | ↓ | ↓ | ↓ ↑ |
|||||
| Ikeda (2012)50 | Serum | GC–MS | ↓ | ||||||||||
| Song (2013)49 | Serum Tissue | GC–MS | ↑ | ↑ | ↓ ↑ |
||||||||
| Jung (2014)39 | Urine Tissue |
1H NMR; HR-MAS NMR |
↑ ↑ |
↑ | |||||||||
| Chen (2014)41 | Urine | MRB-CE-MS | ↑ | ↓ | ↓ | ↓ | |||||||
| Hur (2014)42 | Tissue | GC-MS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||
| Liang (2015)37 | Urine | LC-MS | ↑ | ↓ | ↑ | ||||||||
| Wang (2016)33 | Tissue | 1H NMR | ↓ | ↑ | ↑ | ↑ | |||||||
| Wang (2017)31 | Serum | HPLCESI/Q-TOFMS | ↑ | ||||||||||
| EC | |||||||||||||
| Ayshamgul (2010)65 | Plasma | 1H NMR | ↑ | ↓ | ↑ | ||||||||
| Zhang (2011)60 | Serum | NMR | ↑ | ↑ | ↑ | ||||||||
| Zhang (2012)54 | Serum | LC-MS and NMR | ↑ | ↑ | ↑ | ||||||||
| Hasim (2012)53 | Urine Plasma | 1H NMR | ↑ ↑ |
↑ | ↓ | ↓ ↑ |
|||||||
| Davis (2012)55 | Urine | 1H NMR | ↓ | ↓ | ↑ | ↓ | |||||||
| Ikeda (2012)50 | Serum | GC–MS | ↓ | ||||||||||
| Zhang (2013)46 | Serum | 1H NMR; UHPLC | ↓ | ↑ | |||||||||
| Liu (2013)47 | Plasma | ||||||||||||
| Wang (2013)48 | Tissue | 1H NMR | ↓ | ||||||||||
| Zhu (2017)29 | Serum Tissue |
GC/TOF-MS | ↓ ↓ |
↑ ↓ |
↑ ↓ |
↑ ↑ |
↑ ↑ |
↓ ↑ |
↑ ↑ |
↑ ↑ |
|||
| Reed (2017)30 | Tissue | 1H NMR | ↓ | ||||||||||
| Tokunaga M (2018)22 | Tissue | CE-TOFMS | ↓ | ↑ | ↓ | ↓ | ↓ | ||||||
EC, esophageal cancer; GC, gastric cancer; TCA, tricarboxylic acid; 1H NMR, proton nuclear magnetic resonance; HR-MAS–NMR, High resolution-magic angle spinning-nuclear magnetic resonance spectroscopy; GC–MS, gas chromatography–mass spectrometry; CE–MS, capillary electrophoresis–mass spectrometry; HPLC–MS, high-performance liquid chromatography–mass spectrometry; LC–MS, liquid chromatography–mass spectrometry
We additionally attempted to provide a systematic summary of reports on metabolic pathways or profiles within the scope of carbohydrate metabolism but uncovered no direct findings on pathway-level associations or profiles.
Amino acid metabolism
Alterations in essential and non-essential amino acids were reported for UGI cancers (Table 3), the most frequent being valine, glutamate, and glutamine. Increased valine was consistently detected in studies on GC based on tissue, plasma, serum, and urine samples while inconsistent findings were obtained from studies on EC. Increased glutamate in tissue, blood and urine samples was reported in the majority of available studies on GC (5/6) and EC (8/9). Earlier studies on glutamine reported variable findings from different biological specimens of GC and EC. In addition, alterations in tryptophan were frequently reported in UGI cancers. Decreased tryptophan in tissue and blood samples was observed in the majority of available studies on GC (5/6 studies) while results for EC differed based on the biosample type.
Table 3.
Changes in amino acid metabolites in GC and EC, compared with controls
| Study | Sample type | Analytical platform | Essential amino acid |
Non-essential amino acid |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Val | His | Phe | Thr | Leu | Met | Trp | Ile | Lys | Tyr | Gln | Glu | Ser | Asn | Gly | Ala | Arg | Asp | Cys | Pro | |||
| GC | ||||||||||||||||||||||
| Tugnoli (2006)69 | Tissue | HR-MAS NMR | ↑ | |||||||||||||||||||
| Calabrese (2008)68 | Tissue | HR-MAS MRS | ↑ | ↑ | ||||||||||||||||||
| Hirayama (2009)66 | Tissue | CE–MS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Wu (2010)61 | Tissue | GC–MS | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| Yu (2011)58 | Plasma | GC/TOF MS | ↑ | ↑ | ||||||||||||||||||
| Song (2012)51 | Serum | GC–MS | ↑ | ↓ | ||||||||||||||||||
| Aa (2012)52 | Serum Tissue |
GC/TOFMS | ↓ | ↓ | ↓ ↑ |
|||||||||||||||||
| Jung (2014)39 | Urine Tissue |
1H NMR; HR-MAS NMR |
↑↑ | ↑ | ↑ ↑ |
↑ ↑ |
↑ | ↑ | ↑ | ↑ ↑ |
↑ | ↑ | ↑ ↑ |
↑ | ↑ | |||||||
| Chen (2014)41 | Urine | MRB-CE-MS | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ||||||||||||
| Yang (2014)43 | Serum | LC–MS | ↑ | ↑ | ||||||||||||||||||
| Liang (2015)37 | Urine | LC–MS | ↓ | ↑ | ↑ | |||||||||||||||||
| Wang (2016)33 | Tissue | 1H NMR | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | |||||||||
| Chan (2016)34 | Urine | 1H-NMR | ↑ | |||||||||||||||||||
| Kuligowski (2016)35 | Plasma | UPLC–TOFMS | ↓ | ↑ | ||||||||||||||||||
| Wang (2017)31 | Serum | HPLCESI/Q-TOFMS | ↑ | ↑ | ↑ | |||||||||||||||||
| Lario (2017)25 | Plasma | LC–MS | ↓ | ↓ | ↑ | |||||||||||||||||
| Xiu (2018)20 | Plasma | UHPLC–MS/MS | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ||
| Jing (2018)23 | Plasma | LC–MS/MS | ↓ | ↓ | ↓ | ↓ | ||||||||||||||||
| EC | ||||||||||||||||||||||
| Wu (2009)67 | Tissue | GC/MS | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||
| Yakoub (2010)62 | Tissue | NMR | ↑ | |||||||||||||||||||
| Ayshamgul (2010)65 | Plasma | 1H NMR | ↓ | ↓ | ↓ | ↓ | ||||||||||||||||
| Zhang (2011)60 | Serum | NMR | ↑ | ↑ | ||||||||||||||||||
| Zhang (2012)54 | Serum | LC–MS and NMR | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ | |||||||||||||
| Hasim (2012)53 | Urine Plasma |
1H NMR | ↓ ↓ |
↓ | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ | ||||||||||||
| Davis (2012)55 | Urine | 1H NMR | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | |||||||||||||
| Ikeda (2012)50 | Serum | GC–MS | ↑ | |||||||||||||||||||
| Yang (2013)45 | Tissue | 1H NMR | ↑ | ↑ | ||||||||||||||||||
| Zhang (2013)46 | Serum | 1H NMR; UHPLC | ↑ | ↑ | ↑ | ↓ | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | |||||||||
| Wang (2013)48 | Tissue | 1H NMR | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ||||||||||
| Xu (2016)36 | Urine | LC–MS | ↑ | ↑ | ||||||||||||||||||
| Zhang (2017)26 | Tissue | LC–MS | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | ||||||||||||||
| Cheng (2017)27 | Serum | UPLC-MS/MS | ↑ | |||||||||||||||||||
| Cheng (2017)28 | Serum | LC-MS/MS | ↓ | |||||||||||||||||||
| Zhu (2017)29 | Serum Tissue |
GC/TOF-MS | ↓ ↑ |
↑ | ↓ ↑ |
↓ ↑ |
↓ ↑ |
↓ ↑ |
↓ ↑ |
↓ ↑ |
↑ ↑ |
↑ | ↑ ↓ |
↓ ↑ |
↓ ↑ |
↓ ↑ |
↓ ↑ |
↓ ↑ |
↑ | ↑ ↑ |
↓ ↑ |
|
| Reed (2017)30 | Tissue | 1H NMR | ↓ | ↓ | ||||||||||||||||||
| Tokunaga (2018)22 | Tissue | CE-TOFMS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Ma (2018)24 | Serum | 2D LC–MS | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||
EO, esophageal cancer; GC, gastric cancer; Val, valine; His, histidine; Phe, phenylalanine; Thr, threonine; Leu, leucine; Met, methionine; Trp, tryptophan; Ile, isoleucine; Lys, lysine; Tyr, tyrosine; Gln, glutamine; Glu, glutamic acid; Ser, serine; Asn, asparagine; Gly, glycine; Ala, alanine; Arg, arginine; Asp, aspartic acid; Cys, cystine; Pro, proline; 1H NMR, proton nuclear magnetic resonance; HR-MAS-NMR, high-resolution magic angle spinning-nuclear magnetic resonance spectroscopy; GC–MS, gas chromatography–mass spectrometry; CE–MS, capillary electrophoresis–mass spectrometry; HPLC–MS, high-performance liquid chromatography–mass spectrometry; LC–MS, liquid chromatography–mass spectrometry
Several studies additionally reported altered levels of primary derivatives of amino acids in UGI cancers. Upregulation of kynurenine, anthranilic acid, and nicotinic acid was observed in tissue, plasma, serum and gastric juice of GC and (or) EC patients26,32,35. In addition, kynurenic acid was upregulated in tissue of EC and gastric juice of GC patients29,32 but downregulated in serum of GC patients32.
Although a number of studies briefly discussed the potential biological mechanisms of related amino acids24,37,42,46,48,54,59,66, none directly examined the pathways or profiles of amino acids associated with UGI cancers using statistical approaches.
Lipid metabolism
All studies based on tissues, blood, and urine samples demonstrated lipid dysregulation in UGI cancers (Table 4), among which sphingomyelins, phosphatidylcholines, and phosphatidylethanolamine were the most frequently reported. However, findings on these lipid metabolites were not consistent across studies. In contrast, data obtained for several less commonly reported metabolites were generally consistent across studies, including upregulated triacylglycerides (2/2 studies)68,69 and downregulated arachidonic acid (2/2 studies)56,59 in GC, as well as downregulated unsaturated lipids (4/4 studies)21,43,44,48, low-density lipoprotein (3/3 studies) and very low-density lipoprotein (3/3 studies) in EC21,43,44.
Table 4.
Changes in lipid metabolites in gastric cancer and esophageal cancer, compared with controls
| Study | Sample type | Analytical platform | Un-saturated fatty acid |
Saturated fatty acid |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palmitoleic acid | Cervonic acid | Linoleic acid | Oleic acid | Linolenic acid | Vaccenic acid | Gondoic acid | Arachidonic acid | Myristic acid | Enanthic acid | Margaric acid | Caprylic acid | |||
| GC | ||||||||||||||
| Song (2011)59 | Tissue | GC–MS | ↓ | ↑ | ↓ | ↓ | ||||||||
| Yu (2011)58 | Plasma | GC/TOF MS | ↑ | |||||||||||
| Song (2012)51 | Serum | GC–MS | ↓ | ↓ | ||||||||||
| Aa (2012)52 | Serum Tissue |
GC/TOFMS | ↑ | ↑ | ↑ ↑ |
|||||||||
| Song (2012)56 | Tissue | GC–MS | ↓ | ↓ | ||||||||||
| Mun (2004)70 | Tissue | 1H NMR | Decrease in lipids | |||||||||||
| Tugnoli (2006)69 | Tissue | HR-MAS NMR | Increase in TAG | |||||||||||
| Calabrese (2008)68 | Tissue | HR-MAS MRS | Increase in TAG | |||||||||||
| Hirayama (2009)66 | Tissue | CE–MS | Increase in glyceraldehyde-3P | |||||||||||
| Cai (2010)63 | Tissue | CE–MS | Increase in glyceraldehyde | |||||||||||
| Jung (2014)39 | Urine Tissue |
1H NMR; HR-MAS NMR |
Urine: increase in acetone Tissue: decrease in lipid I and lipid II |
|||||||||||
| Yang (2014)43 | Serum | LC-MS | Increase in PE, FFA, PC, SM, dihydrocholesterol Decrease in lyso-PC, lyso-PE, FFA, PC, choline, SM |
|||||||||||
| Kwon (2014)44 | Tissue | MALDI MS | Increase in SM Decrease in PC |
|||||||||||
| Wang (2016)33 | Tissue | 1H NMR | Decrease in lipids, VLDL, acetone, β-hydroxybutyrate | |||||||||||
| Corona (2018)21 | Serum | LC-MS/MS | Increase in acylcarnitines derivatives (C2, C16, C18:1) Decrease in hydroxylated SM (SM(OH)22:1, SM(OH)22:2, SM(OH)24:1) and PC (PC ae 40:1, PC ae 42:2, PC ae 42:3) |
|||||||||||
| Lee (2019)19 | Plasma | nUHPLC-MS/MS | Increase in LPC18:2; LPE16:0 Decrease in PI; PE; LPE18:1; LPE18:0; LPC16:0; LPA18:2; PC |
|||||||||||
| EC | ||||||||||||||
| Wu (2009)67 | Tissue | GC/MS | ↑ | ↑ | ||||||||||
| Zhang (2012)54 | Serum | LC-MS and NMR | ↓ | ↓ | ↓ | ↑ | ||||||||
| Zhang (2017)26 | Tissue | LC-MS | ↑ | ↑ | ||||||||||
| Zhu (2017)29 | Serum Tissue |
GC/TOF-MS | ↓ ↑ |
↑ | ||||||||||
| Ayshamgul (2010)65 | Plasma | 1H NMR | Decrease in unsaturated lipid, VLDL and LDL, acetone | |||||||||||
| Zhang (2011)60 | Serum | NMR | Increase in β-hydroxybutyrate | |||||||||||
| Davis (2012)55 | Urine | 1H NMR | Decrease in acetone | |||||||||||
| Hasim (2012)53 | Urine Plasma |
1H NMR | Decrease in unsaturated lipids, VLDL and LDL, acetone | |||||||||||
| Zhang (2013)46 | Serum | 1H NMR; UHPLC | Increase in β-hydroxybutyrate Decrease in unsaturated lipids, VLDL and LDL |
|||||||||||
| Liu (2013)47 | Plasma | UPLC/TOF/MS | Increase in PI, PA, PC, PE, sphinganine-1-phosphate, PS (16:0/14:0) | |||||||||||
| Wang (2013)48 | Tissue | 1H NMR | Increase in short-chain fatty acids, acetone Decrease in unsaturated lipids |
|||||||||||
| Mir (2015)38 | Serum | LC–MS | Increase in PC (20:4/0:0), PC (16:0/18:2), PC(18:1/18:1) Decrease in PC (18:2/0:0), PC (18:1/18:2), PC(O-18:1/18:2), PC(16:0/h18:2) |
|||||||||||
| Ma (2018)24 | Serum | 2D LC–MS | Decrease in fatty acids, PC, and FFA | |||||||||||
| Tokunaga (2018)22 | Tissue | CE-TOFMS | Increase in betaine aldehyde Decrease in dihydroxyacetone |
|||||||||||
EC, esophageal cancer; GC, gastric cancer; TAG, triacylglycerides; PE, phosphatidylethanolamine; FFA, free fatty acids; PC, phosphatidylcholine; SM, sphingomyelin; VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; PI, phosphatidylinositol; PA, phosphatidic acid; PS, phosphatidylserine; 1H NMR, proton nuclear magnetic resonance; HR-MAS-NMR, high resolution-magic angle spinning-nuclear magnetic resonance spectroscopy; GC–MS, gas chromatography–mass spectrometry; CE–MS, capillary electrophoresis–mass spectrometry; HPLC–MS, high-performance liquid chromatography–mass spectrometry; LC–MS, liquid chromatography–mass spectrometry
Metabolites of free fatty acid (FFA) oxidation are additionally known to be associated with UGI cancers. Three studies demonstrated increased aldehyde levels in UGI cancer tissues, i.e., glyceraldehyde in GC63,66 and betaine aldehyde in EC22. In addition, 2 of the 3 endogenous ketones, acetone22,33,39,48,53,55,65 and β-hydroxybutyrate33,46,52,54,60, were reported to be associated with GC and EC, although these findings were not consistent across studies.
Despite several investigations on the mechanisms underlying altered lipid metabolism in association with UGI cancers37,42,46,48,54,59, we uncovered no direct findings in terms of metabolic pathway-level associations with UGI cancers.
Nucleotide metabolism
Several studies focused on metabolites of nucleotides associated with GC and EC. Review of the data showed upregulation of pyrimidine nucleotides67, adenine48,62, and uridine-containing compounds62 and downregulation of uracil48 in EC tissues, compared with controls. In addition, one study reported upregulation of guanosine, cytidine and adenosine-containing compounds, along with downregulation of uridine in serum of EC patients64. In GC patients, increased levels of cytidine-containing compounds40 in urine and uracil in tissues and decreased uridine in tissues52 were documented. Although dysregulation of pathways of nucleotide metabolism in UGI cancers have been demonstrated66, direct evidence on the metabolic pathways of nucleotides related to UGI cancers is still unavailable.
Performance of metabolites as potential biomarkers
A total of 21 studies assessed the performance of specific metabolites as potential biomarkers for predicting the risk of UGI cancers, the majority of which showed area under ROC curve (AUC) values ≥ 0.80 (19/21 studies) for individual metabolites or metabolite set. However, among the predictive models reported, we identified no overlapping metabolite biomarkers for risk of UGI cancers.
Discussion
Metabolomic profiling has been increasingly applied for comprehensive characterization of the functional phenotypes of metabolic changes in cancers. Here, we systematically reviewed 52 molecular epidemiologic studies on metabolomic profiling of human UGI cancers and summarized key findings on the dysregulation of major metabolic pathways (glucose, amino acid, lipid, and nucleotide) associated with UGI cancers.
Metabolic reprogramming is a hallmark of cancer71. During tumor development and progression, metabolic pathways are reprogrammed to maintain cancer cell proliferation and survival, which involves large demands for adenosine triphosphate (ATP), nicotinamide adenine dinucleotide phosphate, nicotinamide adenine dinucleotide, carbon skeletons, and other molecules72. Metabolic alterations have been shown to be closely associated with GC and EC, raising the profile of metabolomics as a promising tool for etiologic research and biomarker screening of UGI cancers4,12.
Alterations in carbohydrate metabolism have been reported in a number of earlier metabolomic studies on UGI cancers. Detection of dysregulated pivotal intermediates of glycolysis, such as glucose, fructose, glyceraldehyde, and pyruvic acid, in the UGI cancers GC and EC29,53,63,66 corroborates the well-known Warburg effect22,29,33, which highlights the phenomenon that most cancer cells avidly consume glucose to generate energy mainly by glycolysis instead of oxidative phosphorylation through the tricarboxylic acid (TCA) cycle, even under aerobic conditions73. This less efficient method of generating ATP by glycolysis is subsequently rationalized through diverting available glycolytic intermediates into biosynthetic pathways critical for the synthesis of amino acids, lipids and nucleosides to produce new cells74. The Warburg effect can also enhance generation of pyruvic acid and subsequent lactic acid fermentation catalyzed by lactate dehydrogenase, which may partly explain the upregulation of lactic acid observed particularly in GC.
The metabolite intermediates of the TCA cycle, such as citrate, α-ketoglutaric acid, and fumaric acid, have also been identified in UGI cancers22,29,30,33,37,39,41,42,49,51–55,57,59,60,63,65,66. Consistent with the findings of a previous systematic review4, fumaric acid and citrate were determined as the most frequently reported metabolites of the TCA cycle. However, results from different studies were inconsistent, supporting the necessity for further investigation. Although cancer cells favor glycolysis over oxidative phosphorylation, the increase in TCA cycle metabolites may rely on the process of anaplerosis, which refers to replenishment of TCA metabolites via generation of α-ketoglutarate from the source of glutamate by deaminating glutamine72,75. The present review revealed frequent aberrant metabolism of glutamine and glutamate in UGI cancer patients, supporting the importance of the pathway in this cancer type.
Along with glutamine and glutamate, valine is another frequently reported amino acid dysregulated in UGI cancer 20,22,26,29,33,39,48. Elevated levels of valine can be converted into TCA intermediates to generate energy12. The collective data from studies in the literature clearly demonstrate that valine is upregulated in GC20,33,39,41,51,61,66 although findings in EC are inconsistent22,26,29,48,53,55,65,67. It remains to be established whether these results reflect differential metabolic profiling for GC and EC.
Prior studies have also reported alterations in tryptophan and kynurenine in UGI cancers26,32,35,46,54, indicating that potential metabolic perturbations of the tryptophan/kynurenine catabolism pathway are associated with development of EC and GC. Considerable evidence supports the theory that molecules in this pathway are involved in the immune regulation of tumor cells. For example, the tryptophan-catabolizing enzyme, indoleamine-2, 3-dioxygenase (IDO), may alter tumor microenvironment to favor cancer progression76,77. IDO is proposed to function as an immune suppressor and induce immune tolerance76,78, and its increased expression in the tumor microenvironment is correlated with immunosuppression in UGI cancers32,79. A number of studies have reported upregulation of lysine, serine or arginine22,24,39,66,67 and downregulation of isoleucine, tyrosine or glycine20,23,52,55,65 in UGI cancers, although the findings were not all consistent20,29,54. Increased levels of amino acids in tissues and other biological specimens may be generated from various sources, such as environmental degradation of the extracellular matrix and autophagic degradation of preexisting intracellular proteins66, while amino acid overutilization in tumor tissues may have contributed to the decreased levels of amino acids (such as methionine, histidine, and leucine) observed in some studies33,57.
While previous investigations have highlighted changes in fatty acid metabolic pathways associated with UGI cancers, mixed findings were reported on the levels of unsaturated and saturated FFA in UGI cancers48,59. Low levels of FFA or lipids have been attributed to increased consumption by tumors due to their anabolic metabolism while metabolic reprogramming in cancer is related to fatty acid increase in the tumor environment80. In addition, systemic lipolysis secondary to cancer cachexia or de novo fatty acid synthesis may contribute to FFA accumulation4,81. Although the mechanisms underlying lipid synthesis in cancer are not fully understood at present, it is proposed that de novo lipid synthesis leads to the formation of structural lipids for cell membrane production, provides energy through β-oxidation, and affects fundamental cellular processes, such as signal transduction81,82.
β-Oxidation of fatty acids is a main source of energy generation83. Dysregulation of aldehydes and ketones, the metabolic products of β-oxidation, has been consistently reported in UGI cancer patients22,33,39,46,48,52–55,60,63,65,66. Altered ketone body synthesis and degradation have also been documented in relation to UGI cancers42,46,54. Fatty acid β-oxidation not only efficiently produces energy but also promotes reactive oxygen species generation84, facilitating lipid peroxidation and aldehyde production85.
Several studies have shown alterations in nucleotide metabolites in UGI cancers40,48,52,62,64,66,67. Nucleotide synthesis and metabolism are required for adequate energy generation and proposed to be critical for proliferation and differentiation of cancer cells12. The growth superiority of cancer cells gradually switching to anaerobic glycolysis may partly explain the mixed findings on nucleotide metabolites.
Review of the collective data from the literature suggests that consistent findings on UGI cancer-associated metabolites based on metabolomic studies are limited. Moreover, discrepancies in results even exist among studies on the same types of biospecimens. While different analytical platforms and biospecimens may partly explain these inconsistencies, discrepant findings also reflect the heterogeneity in study design and subject selection across studies. The majority of prior studies were hospital-based cross-sectional or case-control analyses with a modest sample size and may have led to high false-positive probability due to negligence of potential multiple comparisons and lack of an independent replication group. Under representativeness of specific study populations may have restricted the extrapolation of findings across studies. Multivariate adjustments were not possible for most studies due to the unavailability of detailed information on UGI cancer risk factors and potential residual confounding may have distorted these findings.
Conclusions
In conclusion, a total of 52 molecular epidemiologic studies on metabolomics have been conducted for human UGI cancers over the years. Studies on metabolomics have thus far facilitated effective biomarker detection in GC and EC, supporting the potential of applying metabolomic profiling in cancer prevention and management efforts. Although a number of metabolites have been identified for GC and EC, identification of putative metabolomic biomarkers has been inadequate. Application of metabolomic profiling to molecular epidemiologic studies on UGI cancers may provide insights into the biological significance of crucial metabolites and metabolic pathways but there is no actual information on the underlying mechanisms. Given the multi-stage progression of UGI carcinogenesis, it is necessary to identify metabolic biomarkers associated with both precancerous and early UGI cancers, which would benefit screening of high-risk populations and early diagnosis. Limited studies to date have focused on metabolomic profiling for the cascade of precancerous lesions and UGI cancers.
To fulfill the potential of effectively applying metabolomics for UGI cancer prevention and control in public health and clinical practices, major gaps need to be filled with the aid of well-designed molecular epidemiologic studies. Studies with large sample sizes, clearly defined study population and independent validation samples are warranted to identify metabolomic biomarkers and define the critical metabolomic pathways and patterns. Prospective follow-up of subjects covering a cascade of precancerous lesions and subsequent cancers would also be advantageous in identifying metabolomics biomarkers for efficient assessment of the risk of UGI cancer development and progression.
Supporting Information
Acknowledgments
This work was supported by grants from the Michigan Medicine-PKUHSC Joint Institute for Translational and Clinical Research (Grant No. BMU2020JI004), Capital’s Funds for Health Improvement and Research (CFH).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388:2606. doi: 10.1016/S0140-6736(16)32226-7. [DOI] [PubMed] [Google Scholar]
- 4.Abbassi-Ghadi N, Kumar S, Huang J, Goldin R, Takats Z, Hanna GB. Metabolomic profiling of oesophago-gastric cancer: a systematic review. Eur J Cancer. 2013;49:3625–37. doi: 10.1016/j.ejca.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Gu J, Hu X, Shao W, Ji T, Yang W, Zhuo H, et al. Metabolomic analysis reveals altered metabolic pathways in a rat model of gastric carcinogenesis. Oncotarget. 2016;7:60053–73. doi: 10.18632/oncotarget.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KB, Yang JY, Kwack SJ, Park KL, Kim HS, Ryu DH, et al. Toxicometabolomics of urinary biomarkers for human gastric cancer in a mouse model. J Toxicol Environ Health A. 2010;73:1420–30. doi: 10.1080/15287394.2010.511545. [DOI] [PubMed] [Google Scholar]
- 7.Matsunaga S, Nishiumi S, Tagawa R, Yoshida M. Alterations in metabolic pathways in gastric epithelial cells infected with Helicobacter pylori. Microb Pathog. 2018;124:122–9. doi: 10.1016/j.micpath.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Di Gialleonardo V, Tee SS, Aldeborgh HN, Miloushev VZ, Cunha LS, Sukenick GD, et al. High-throughput indirect quantitation of 13C enriched metabolites using 1H NMR. Anal Chem. 2016;88:11147–53. doi: 10.1021/acs.analchem.6b03307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–7. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016;15:473–84. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 11.Chan AW, Gill RS, Schiller D, Sawyer MB. Potential role of metabolomics in diagnosis and surveillance of gastric cancer. World J Gastroenterol. 2014;20:12874–82. doi: 10.3748/wjg.v20.i36.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao S, Zhou L. Gastric cancer: metabolic and metabolomics perspectives (Review) Int J Oncol. 2017;51:5–17. doi: 10.3892/ijo.2017.4000. [DOI] [PubMed] [Google Scholar]
- 13.Yuan LW, Yamashita H, Seto Y. Glucose metabolism in gastric cancer: the cutting-edge. World J Gastroenterol. 2016;22:2046–59. doi: 10.3748/wjg.v22.i6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayavelu ND, Bar NS. Metabolomic studies of human gastric cancer: review. World J Gastroenterol. 2014;20:8092–101. doi: 10.3748/wjg.v20.i25.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA -P) 2015: elaboration and explanation. Br Med J. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle–Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Wirsching J, Grassmann S, Eichelmann F, Harms LM, Schenk M, Barth E, et al. Development and reliability assessment of a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS) BMC Med Res Methodol. 2018;18:122. doi: 10.1186/s12874-018-0583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee GB, Lee JC, Moon MH. Plasma lipid profile comparison of five different cancers by nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2019;1063:117–26. doi: 10.1016/j.aca.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Xiu FM, Zhang F, Jiao JP, Zhang YC, Pei B, Zhao J, et al. Variation of 24 plasma amino acid metabolite levels in patients with gastric cancer. Acad J Second Milit Med Univ. 2018;39:62–7. [Google Scholar]
- 21.Corona G, Cannizzaro R, Miolo G, Caggiari L, De Zorzi M, Repetto O, et al. Use of metabolomics as a complementary omic approach to implement risk criteria for first-degree relatives of gastric cancer patients. Int J Mol Sci. 2018;19:750. doi: 10.3390/ijms19030750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokunaga M, Kami K, Ozawa S, Oguma J, Kazuno A, Miyachi H, et al. Metabolome analysis of esophageal cancer tissues using capillary electrophoresis-time-of-flight mass spectrometry. Int J Oncol. 2018;52:1947–58. doi: 10.3892/ijo.2018.4340. [DOI] [PubMed] [Google Scholar]
- 23.Jing F, Hu X, Cao Y, Xu M, Wang Y, Jing Y, et al. Discriminating gastric cancer and gastric ulcer using human plasma amino acid metabolic profile. IUBMB Life. 2018;70:553–62. doi: 10.1002/iub.1748. [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Wang S, Zhang T, Zhang EY, Zhou L, Hu C, et al. Activation of choline kinase drives aberrant choline metabolism in esophageal squamous cell carcinomas. J Pharm Biomed Anal. 2018;155:148–56. doi: 10.1016/j.jpba.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 25.Lario S, Ramirez-Lazaro MJ, Sanjuan-Herraez D, Brunet-Vega A, Pericay C, Gombau L, et al. Plasma sample based analysis of gastric cancer progression using targeted metabolomics. Sci Rep. 2017;7:17774. doi: 10.1038/s41598-017-17921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Wang L, Hou Z, Ma H, Mamtimin B, Hasim A, et al. Metabolomic profiling reveals potential biomarkers in esophageal cancer progression using liquid chromatography-mass spectrometry platform. Biochem Biophys Res Commun. 2017;491:119–25. doi: 10.1016/j.bbrc.2017.07.060. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J, Jin H, Hou X, Lv J, Gao X, Zheng G. Disturbed tryptophan metabolism correlating to progression and metastasis of esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2017;486:781–7. doi: 10.1016/j.bbrc.2017.03.120. [DOI] [PubMed] [Google Scholar]
- 28.Cheng J, Zheng G, Jin H, Gao X. Towards tyrosine metabolism in esophageal squamous cell carcinoma. Comb Chem High Throughput Screen. 2017;20:133–9. doi: 10.2174/1386207319666161220115409. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Wang K, Liu G, Wang Y, Xu J, Liu L, et al. Metabolic perturbation and potential markers in patients with esophageal cancer. Gastroenterol Res Pract. 2017;2017:5469597. doi: 10.1155/2017/5469597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed MA, Singhal R, Ludwig C, Carrigan JB, Ward DG, Taniere P, et al. Metabolomic evidence for a field effect in histologically normal and metaplastic tissues in patients with esophageal adenocarcinoma. Neoplasia. 2017;19:165–74. doi: 10.1016/j.neo.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang DG, Li W, Zou Q, Yin L, Du YC, Gu JK, et al. Serum metabolomic profiling of human gastric cancer and its relationship with the prognosis. Oncotarget. 2017;8:110000–15. doi: 10.18632/oncotarget.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JM, Park WS, Song KY, Lee HJ, Jung BH. Development of simultaneous analysis of tryptophan metabolites in serum and gastric juice – an investigation towards establishing a biomarker test for gastric cancer diagnosis. Biomed Chromatogr. 2016;30:1963–74. doi: 10.1002/bmc.3773. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Zhang H, Deng P, Liu C, Li D, Jie H, et al. Tissue metabolic profiling of human gastric cancer assessed by 1H NMR. BMC Cancer. 2016;16:371. doi: 10.1186/s12885-016-2356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan AW, Mercier P, Schiller D, Bailey R, Robbins S, Eurich DT, et al. 1H-NMR urinary metabolomic profiling for diagnosis of gastric cancer. Br J Cancer. 2016;114:59–62. doi: 10.1038/bjc.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuligowski J, Sanjuan-Herraez D, Vazquez-Sanchez MA, Brunet-Vega A, Pericay C, Ramirez-Lazaro MJ, et al. Metabolomic analysis of gastric cancer progression within the Correa’s cascade using ultraperformance liquid chromatography-mass spectrometry. J Proteome Res. 2016;15:2729–38. doi: 10.1021/acs.jproteome.6b00281. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Chen Y, Zhang R, He J, Song Y, Wang J, et al. Global metabolomics reveals potential urinary biomarkers of esophageal squamous cell carcinoma for diagnosis and staging. Sci Rep. 2016;6:35010. doi: 10.1038/srep35010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Q, Wang C, Li B. Metabolomic analysis using liquid chromatography/mass spectrometry for gastric cancer. Appl Biochem Biotechnol. 2015;176:2170–84. doi: 10.1007/s12010-015-1706-z. [DOI] [PubMed] [Google Scholar]
- 38.Mir SA, Rajagopalan P, Jain AP, Khan AA, Datta KK, Mohan SV, et al. LC–MS-based serum metabolomic analysis reveals dysregulation of phosphatidylcholines in esophageal squamous cell carcinoma. J Proteomics. 2015;127:96–102. doi: 10.1016/j.jprot.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Jung J, Jung Y, Bang EJ, Cho SI, Jang YJ, Kwak JM, et al. Noninvasive diagnosis and evaluation of curative surgery for gastric cancer by using NMR-based metabolomic profiling. Ann Surg Oncol. 2014;21(Suppl 4):S736–42. doi: 10.1245/s10434-014-3886-0. [DOI] [PubMed] [Google Scholar]
- 40.Lo WY, Jeng LB, Lai CC, Tsai FJ, Lin CT, Chen WT. Urinary cytidine as an adjunct biomarker to improve the diagnostic ratio for gastric cancer in taiwanese patients. Clin Chim Acta. 2014;428:57–62. doi: 10.1016/j.cca.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Chen JL, Fan J, Lu XJ. CE-MS based on moving reaction boundary method for urinary metabolomic analysis of gastric cancer patients. Electrophoresis. 2014;35:1032–9. doi: 10.1002/elps.201300243. [DOI] [PubMed] [Google Scholar]
- 42.Hur H, Paik MJ, Xuan Y, Nguyen DT, Ham IH, Yun J, et al. Quantitative measurement of organic acids in tissues from gastric cancer patients indicates increased glucose metabolism in gastric cancer. PLoS One. 2014;9:e98581. doi: 10.1371/journal.pone.0098581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang T, Luo P, Li Y, Hua R, Yin P, Xu G. [A serum metabolomics study of gastric cancer based on pseudotargeted liquid chromatography-mass spectrometry approach] Se Pu = Chin J Chromatogr. 2014;32:126–32. doi: 10.3724/sp.j.1123.2013.11050. [DOI] [PubMed] [Google Scholar]
- 44.Kwon SY, Choi SH, Park YS, Park DY, Park YI, Hwang I, et al. Lipid MALDI MS profiles of gastric cancer. Open Proteomics J. 2014;7:1–4. [Google Scholar]
- 45.Yang Y, Wang L, Wang S, Liang S, Chen A, Tang H, et al. Study of metabonomic profiles of human esophageal carcinoma by use of high-resolution magic-angle spinning 1H NMR spectroscopy and multivariate data analysis. Anal Bioanal Chem. 2013;405:3381–9. doi: 10.1007/s00216-013-6774-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Xu L, Shen J, Cao B, Cheng T, Zhao T, et al. Metabolic signatures of esophageal cancer: NMR-based metabolomics and UHPLC-based focused metabolomics of blood serum. Biochim Biophys Acta. 2013;1832:1207–16. doi: 10.1016/j.bbadis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Liu R, Peng Y, Li X, Wang Y, Pan E, Guo W, et al. Identification of plasma metabolomic profiling for diagnosis of esophageal squamous-cell carcinoma using an UPLC/TOF/MS platform. Int J Mol Sci. 2013;14:8899–911. doi: 10.3390/ijms14058899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Chen J, Chen L, Deng P, Bu Q, Xiang P, et al. 1H-NMR based metabonomic profiling of human esophageal cancer tissue. Mol Cancer. 2013;12:25. doi: 10.1186/1476-4598-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song H, Xu W, Song J, Xu YX, Fu W, Zheng JN, et al. Changes of glucose metabolites in gastric tissue and serum in patients with gastric cancer. Chin J Clin Nutr. 2013;21:209–12. [Google Scholar]
- 50.Ikeda A, Nishiumi S, Shinohara M, Yoshie T, Hatano N, Okuno T, et al. Serum metabolomics as a novel diagnostic approach for gastrointestinal cancer. Biomed Chromatogr. 2012;26:548–58. doi: 10.1002/bmc.1671. [DOI] [PubMed] [Google Scholar]
- 51.Song H, Peng JS, Dong-Sheng Y, Yang ZL, Liu HL, Zeng YK, et al. Serum metabolic profiling of human gastric cancer based on gas chromatography/mass spectrometry. Braz J Med Biol Res. 2012;45:78–85. doi: 10.1590/S0100-879X2011007500158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aa J, Yu L, Sun M, Liu L, Li M, Cao B, et al. Metabolic features of the tumor microenvironment of gastric cancer and the link to the systemic macroenvironment. Metabolomics. 2012;8:164–73. [Google Scholar]
- 53.Hasim A, Ma H, Mamtimin B, Abudula A, Niyaz M, Zhang LW, et al. Revealing the metabonomic variation of EC using 1H-NMR spectroscopy and its association with the clinicopathological characteristics. Mol Biol Rep. 2012;39:8955–64. doi: 10.1007/s11033-012-1764-z. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Bowers J, Liu L, Wei S, Gowda GA, Hammoud Z, et al. Esophageal cancer metabolite biomarkers detected by LC-MS and NMR methods. PLoS One. 2012;7:e30181. doi: 10.1371/journal.pone.0030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis VW, Schiller DE, Eurich D, Sawyer MB. Urinary metabolomic signature of esophageal cancer and Barrett’s esophagus. World J Surg Oncol. 2012;10:271. doi: 10.1186/1477-7819-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song H, Peng JS, Yao DS, Liu DL, Yang ZL, Du YP, et al. Metabolic disorders of fatty acids and fatty acid amides associated with human gastric cancer morbidity. Chin Med J (Engl) 2012;125:757–63. [PubMed] [Google Scholar]
- 57.Sun M, Wang H, Xu J, A J, Shi R, Qiu H, et al. Study on saccharometabolism of gastric cancer tissues and plasma based upon metabolomics. Chin J Gastroenterol. 2011;16:609–12. [Google Scholar]
- 58.Yu L, Aa J, Xu J, Sun M, Qian S, Cheng L, et al. Metabolomic phenotype of gastric cancer and precancerous stages based on gas chromatography time-of-flight mass spectrometry. J Gastroenterol Hepatol. 2011;26:1290–7. doi: 10.1111/j.1440-1746.2011.06724.x. [DOI] [PubMed] [Google Scholar]
- 59.Song H, Wang L, Liu HL, Wu XB, Wang HS, Liu ZH, et al. Tissue metabolomic fingerprinting reveals metabolic disorders associated with human gastric cancer morbidity. Oncol Rep. 2011;26:431–8. doi: 10.3892/or.2011.1302. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Liu L, Wei S, Nagana Gowda GA, Hammoud Z, Kesler KA, et al. Metabolomics study of esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:469–75. doi: 10.1016/j.jtcvs.2010.08.025. 475.e1-4. [DOI] [PubMed] [Google Scholar]
- 61.Wu H, Xue R, Tang Z, Deng C, Liu T, Zeng H, et al. Metabolomic investigation of gastric cancer tissue using gas chromatography/mass spectrometry. Anal Bioanal Chem. 2010;396:1385–95. doi: 10.1007/s00216-009-3317-4. [DOI] [PubMed] [Google Scholar]
- 62.Yakoub D, Keun HC, Goldin R, Hanna GB. Metabolic profiling detects field effects in nondysplastic tissue from esophageal cancer patients. Cancer Res. 2010;70:9129–36. doi: 10.1158/0008-5472.CAN-10-1566. [DOI] [PubMed] [Google Scholar]
- 63.Cai Z, Zhao JS, Li JJ, Peng DN, Wang XY, Chen TL, et al. A combined proteomics and metabolomics profiling of gastric cardia cancer reveals characteristic dysregulations in glucose metabolism. Mol Cell Proteomics. 2010;9:2617–28. doi: 10.1074/mcp.M110.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Djukovic D, Baniasadi HR, Kc R, Hammoud Z, Raftery D. Targeted serum metabolite profiling of nucleosides in esophageal adenocarcinoma. Rapid Commun Mass Spectrom. 2010;24:3057–62. doi: 10.1002/rcm.4739. [DOI] [PubMed] [Google Scholar]
- 65.Ayshamgul H, Batur M, Ilyar S. 1H-MRS metabonomic analysis of plasma samples of esophageal cancer patients based on different pattern recognition. Chin J Oncol. 2010;32:681–4. [PubMed] [Google Scholar]
- 66.Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–25. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- 67.Wu H, Xue R, Lu C, Deng C, Liu T, Zeng H, et al. Metabolomic study for diagnostic model of oesophageal cancer using gas chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3111–7. doi: 10.1016/j.jchromb.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 68.Calabrese C, Pisi A, Di Febo G, Liguori G, Filippini G, Cervellera M, et al. Biochemical alterations from normal mucosa to gastric cancer by ex vivo magnetic resonance spectroscopy. Cancer Epidemiol Biomarkers Prev. 2008;17:1386–95. doi: 10.1158/1055-9965.EPI-07-2676. [DOI] [PubMed] [Google Scholar]
- 69.Tugnoli V, Mucci A, Schenetti L, Righi V, Calabrese C, Fabbri A, et al. Ex vivo hr-mas magnetic resonance spectroscopy of human gastric adenocarcinomas: a comparison with healthy gastric mucosa. Oncol Rep. 2006;16:543–53. doi: 10.3892/or.16.3.543. [DOI] [PubMed] [Google Scholar]
- 70.Mun CW, Cho JY, Shin WJ, Choi KS, Eun CK, Cha SS, et al. Ex vivo proton MR spectroscopy (1H-MRS) for evaluation of human gastric carcinoma. Magn Reson Imaging. 2004;22:861–70. doi: 10.1016/j.mri.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Romero-Garcia S, Lopez-Gonzalez JS, Baez-Viveros JL, Aguilar-Cazares D, Prado-Garcia H. Tumor cell metabolism: an integral view. Cancer Biol Ther. 2011;12:939–48. doi: 10.4161/cbt.12.11.18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–73. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 76.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985–91. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 77.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 78.Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic t-cell responses in vitro and in vivo. Transpl Int. 2005;18:95–100. doi: 10.1111/j.1432-2277.2004.00031.x. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Lu G, Tang F, Liu Y, Cui G. Localization of indoleamine 2,3-dioxygenase in human esophageal squamous cell carcinomas. Virchows Arch. 2009;455:441–8. doi: 10.1007/s00428-009-0846-3. [DOI] [PubMed] [Google Scholar]
- 80.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–23. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 81.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 82.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369–72. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khasawneh J, Schulz MD, Walch A, Rozman J, Hrabe de Angelis M, Klingenspor M, et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci U S A. 2009;106:3354–9. doi: 10.1073/pnas.0802864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J, Ellis JM, Wolfgang MJ. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep. 2015;10:266–79. doi: 10.1016/j.celrep.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


