Abstract
Background
Cognitive impairments, particularly memory problems, are a defining feature of the early stages of Alzheimer's disease (AD) and vascular dementia. Cognitive training and cognitive rehabilitation are specific interventional approaches designed to address difficulties with memory and other aspects of cognitive functioning. The present review is an update of previous versions of this review.
Objectives
The main aim of the current review was to evaluate the effectiveness and impact of cognitive training and cognitive rehabilitation for people with mild Alzheimer's disease or vascular dementia in relation to important cognitive and non‐cognitive outcomes for the person with dementia and the primary caregiver in the short, medium and long term.
Search methods
The CDCIG Specialized Register, ALOIS, which contains records from MEDLINE, EMBASE, CINAHL, PsycINFO, LILACS and many other clinical trial databases and grey literature sources, was most recently searched on 2 November 2012.
Selection criteria
Randomised controlled trials (RCTs), published in English, comparing cognitive rehabilitation or cognitive training interventions with control conditions, and reporting relevant outcomes for the person with dementia and/or the family caregiver, were considered for inclusion.
Data collection and analysis
Eleven RCTs reporting cognitive training interventions were included in the review. A large number of measures were used in the different studies, and meta‐analysis could be conducted for 11 of the primary and secondary outcomes of interest. Several outcomes were not measured in any of the studies. The unit of analysis in the meta‐analysis was the change from baseline score. Overall estimates of treatment effect were calculated using a fixed‐effect model, and statistical heterogeneity was measured using a standard Chi2 statistic. One RCT of cognitive rehabilitation was identified, allowing examination of effect sizes, but no meta‐analysis could be conducted.
Main results
Cognitive training was not associated with positive or negative effects in relation to any reported outcomes. The overall quality of the trials was low to moderate. The single RCT of cognitive rehabilitation found promising results in relation to a number of participant and caregiver outcomes, and was generally of high quality.
Authors' conclusions
Available evidence regarding cognitive training remains limited, and the quality of the evidence needs to improve. However, there is still no indication of any significant benefit derived from cognitive training. Trial reports indicate that some gains resulting from intervention may not be captured adequately by available standardised outcome measures. The results of the single RCT of cognitive rehabilitation show promise but are preliminary in nature. Further, well‐designed studies of cognitive training and cognitive rehabilitation are required to obtain more definitive evidence. Researchers should describe and classify their interventions appropriately using available terminology.
Keywords: Humans; Alzheimer Disease; Alzheimer Disease/rehabilitation; Cognitive Behavioral Therapy; Cognitive Behavioral Therapy/methods; Dementia, Vascular; Dementia, Vascular/rehabilitation; Randomized Controlled Trials as Topic
Plain language summary
Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia
Dementia due to Alzheimer’s and vascular disease is an enormous public health problem. Currently, an estimated 36 million people worldwide live with dementia, and this number is expected to increase to more than 115 million by the year 2050. Effective interventions to reduce the burden of disease are urgently needed. Cognitive training and cognitive rehabilitation are non‐pharmacological methods that aim to help people with early‐stage dementia make the most of their memory and cognitive functioning despite the difficulties they are experiencing. Cognitive training focuses on guided practice on a set of tasks that reflect particular cognitive functions, such as memory, attention or problem‐solving. Cognitive rehabilitation focuses on identifying and addressing individual needs and goals, which may require strategies for taking in new information or compensatory methods such as using memory aids.
This review included 11 trials of cognitive training and a single trial of cognitive rehabilitation. We found no evidence for the efficacy of cognitive training in improving cognitive functioning, mood or activities of daily living in people with mild to moderate Alzheimer's disease or vascular dementia; however the quality of the studies was generally not high. The single trial of cognitive rehabilitation provided preliminary indications of the potential benefits of individual cognitive rehabilitation in improving activities of daily living in people with mild Alzheimer's disease. More high‐quality trials of both cognitive training and cognitive rehabilitation are needed to establish their efficacy for people with early‐stage dementia.
Summary of findings
Summary of findings for the main comparison. Cognitive training compared to control in the short‐term (i.e. immediately post‐intervention) for early‐stage Alzheimer's disease and vascular dementia.
| Cognitive training compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia Settings: Intervention: Cognitive training Comparison: Control in the short term (i.e. post‐intervention) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short‐term (i.e. post‐intervention) | Cognitive training | |||||
| Change in a global measure of cognition MMSE, ADAS‐Cog, Mattis Dementia Rating Scale | The mean change in a global measure of cognition in the intervention groups was 0.10 higher (‐0.21 lower to 0.40 higher) | 173 (6 studies) | ⊕⊕⊝⊝ low1,2 | |||
| Change in participant's capacity for activities of daily living (Caregiver reported) | The mean change in participant's capacity for activities of daily living (caregiver reported) in the intervention groups was 0 standard deviations higher (0.38 lower to 0.38 higher) | 107 (4 studies) | ⊕⊕⊝⊝ low3,4,5 | SMD 0 (‐0.38 to 0.38) | ||
| Change in participant's mood (self‐reported) | The mean change in participant's mood (self‐reported) in the intervention groups was 0.03 standard deviations higher (0.34 lower to 0.41 higher) | 114 (4 studies) | ⊕⊕⊕⊝ moderate6 | SMD 0.03 (‐0.34 to 0.41) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in immediate verbal memory scores | The mean change in immediate verbal memory scores in the intervention groups was 0.1 standard deviations higher (0.18 lower to 0.38 higher) | 201 (7 studies) | ⊕⊕⊝⊝ low7,8 | SMD 0.1 (‐0.18 to 0.38) | ||
| Change in self‐reported burden of care | The mean change in self‐reported burden of care in the intervention groups was 1.16 lower (9.67 lower to 7.34 higher) | 80 (2 studies) | ⊕⊕⊕⊝ moderate9 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely. 2 All studies reporting this outcome suffered from risk of bias in several domains, including random sequence generation, allocation concealment, and lack of blinding. 3 No explanation was provided. 4 The direction of the effect in the four studies that reported this effect was inconsistent. 5 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely. 6 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely. 7 Several studies measuring this outcome were at high risk of bias because of lack of blinding of outcome assessment. 8 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely. 9 The confidence interval of the effect included a zero effect. Therefore, imprecision is likely.
Summary of findings 2. Cognitive rehabilitation compared to control in the short‐term (i.e. immediately post‐intervention) for early‐stage Alzheimer's disease and vascular dementia.
| Cognitive rehabilitation compared to control in the short‐term (i.e. post‐intervention) for early‐stage Alzheimer's disease and vascular dementia | ||||||
| Patient or population: participants with early‐stage Alzheimer's disease and vascular dementia Settings: Intervention: cognitive rehabilitation Comparison: control in the short term (i.e. post‐intervention) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control in the short term (i.e. post‐intervention) | Cognitive rehabilitation | |||||
| Change in a global measure of cognition-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in participant's self‐reported performance in relation to individual goals (COPM Performance, self‐reported) Canadian Occupational Performance Measure | The mean change in participant's capacity for activities of daily living (COPM Performance, self‐reported) in the intervention groups was 1.22 higher (0.09 to 2.35 higher) | 39 (1 study) | ⊕⊕⊕⊕ high | |||
| Change in participant's mood (Depression, self‐reported) Hospital Anxiety and Depression Scale | The mean change in participant's mood (depression, self‐reported) in the intervention groups was 0.24 standard deviations lower (0.86 lower to 0.37 higher) | 41 (1 study) | ⊕⊕⊕⊕ high | SMD ‐0.24 (‐0.86 to 0.37) | ||
| Change in rates of admission to residential care-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in measures of dementia severity-not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Change in self‐reported mood (Depression-caregiver) | The mean change in self‐reported mood (depression, caregiver) in the intervention groups was 1.08 lower (3.24 lower to 1.08 higher) | 18 (1 study) | ⊕⊕⊕⊕ high | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Cognitive impairment is a defining feature of dementia caused by neurodegenerative conditions such as Alzheimer's disease (AD) and cerebrovascular disease. In the milder stages of dementia, cognitive impairments are often the most disabling and distressing features for the individual and for the family. For the person with dementia, memory and other cognitive difficulties can have a major impact on self‐confidence and can lead to anxiety, depression and withdrawal from activities, which in turn can make the difficulties seem worse. This is an example of what has been termed 'excess disability' (Reifler 1990). Family caregivers are also affected by the practical impact of cognitive problems on everyday life and by the strain and frustration that can result. Interventions designed to assist with aspects of cognitive functioning such as memory problems are therefore important in the milder stages of dementia, as they may allow the person greater independence and can potentially minimise the risk of excess disability. The current review is an update of previous versions of this review (Clare 2003; Clare 2008).
Description of the condition
AD and cerebrovascular disease are the most common aetiologies underlying dementia among older individuals (Alzheimer's Disease International 2009). Dementia due to AD is generally characterised by an insidious onset; vascular dementia is often associated with a more rapid onset. However, both disorders have a progressive course that eventually culminates in global cognitive impairment and compromised functional independence. During milder stages, clinical signs typically include forgetfulness for recent events and other cognitive impairments such as word‐finding difficulties or increased confusion in navigating unfamiliar environments. These signs often precede the formal diagnosis by several years, but they can be difficult to distinguish from the common forgetfulness associated with normal ageing—a factor that often leads to delays in bringing the situation to medical attention. During this pre‐dementia phase, there is often no, or minimal, impairment in the ability of the individual to carry out most activities of daily living. With disease progression, difficulties develop in most other cognitive domains, such as semantics, praxis and executive functioning. Functional impairment also becomes increasingly evident. In more advanced dementia, most cognitive and functional abilities are profoundly impaired, and behavioural changes such as apathy, depression, aggression and agitation are frequently observed (Lyketsos 2002; Mortby 2011).
On neuropsychological examination, the earliest signs are almost invariably related to episodic memory function, particularly in the person with AD. Deficits in new learning and delayed recall of information precede the diagnosis by several years (Arnaiz 2003; Collie 2000). Studies have established that associative memory functions, particularly the ability to form arbitrary inter‐modal and intra‐modal associations, show a striking deficit very early in AD (Fowler 2002; Lowndes & Savage 2007). Although deficits noted on measures of episodic memory are central to vascular dementia, people with vascular dementia display a more striking deficit on executive and attention tasks, as well as on measures of semantic knowledge and visuospatial function (Graham 2004)
Pathologically, AD is characterised by the build‐up of extra‐cellular Aβ plaques and intra‐cellular neurofibrillary tangles, which spread in a predictable and well‐described manner through cortical and subcortical regions (Braak & Braak 2012). In the case of both Alzheimer's and vascular pathology, the pathological cascade commences years or even decades before the onset of obvious clinical symptoms, at which stage individuals are increasingly brought to clinical attention.
Description of the intervention
Cognition‐focused interventions as a group fall under the broader umbrella of non‐pharmacological interventions. Cognition‐focused interventions can be broadly defined as interventions that directly or indirectly target cognitive functioning as opposed to interventions that focus primarily on behavioural (e.g. wandering), emotional (e.g. anxiety) or physical (e.g. sedentary lifestyle) function. Several types of cognition‐based interventions have been described. The potential benefits of non‐specific stimulation of cognitive functioning for people with dementia have long been recognised. These interventions typically involve engaging the person with dementia in a range of general activities and discussions, are commonly conducted in groups and are aimed at general enhancement of cognitive and social functioning. A separate recent Cochrane Review, which focuses on interventions that fall under this category (collectively termed 'cognitive stimulation'), has concluded that general cognitive stimulation and reality orientation approaches consistently produce improvements in general cognition and, in some cases, in self‐reported quality of life and wellbeing, primarily for people with mild to moderate dementia (Woods 2012).
Progress in understanding the operation of memory and related cognitive functions and of the mechanisms underpinning learning has facilitated the development of more specific approaches designed to help maintain or enhance cognitive functioning and wellbeing for people with AD or vascular dementia-most commonly those in the milder stages. These more recent approaches to cognition‐based interventions are most commonly classified as either cognitive training (or 'retraining' or 'remediation' or ‘brain training’) or cognitive rehabilitation. These terms have been and continue to be applied somewhat interchangeably in the literature (e.g. Fernandez‐Prado 2012; Giordano 2010); therefore in previous versions of this review (Clare 2003; Clare 2008), we have offered the following broad definitions and descriptions with the aim of clarifying the nature of these two related but distinct forms of intervention.
Cognitive training
Cognitive training typically involves guided practice on a set of standardised tasks designed to reflect particular cognitive functions such as memory, attention or problem‐solving. Tasks may be presented in paper‐and‐pencil (Davis 2001; de Vreese 1998; Quayhagen 1995; Quayhagen 2000) or computerised (Heiss 1993; Hofmann 1996) form, or may involve analogues of activities of daily living (Farina 2002; Zanetti 1994; Zanetti 1997; Zanetti 2001; Loewenstein 2004; Neely 2009). Tailoring of task difficulty based on individual performance level and adaptive training (i.e. adjustment of task difficulty in response to changes in performance level) are becoming more available through computerised packages (e.g. Peretz 2011). One assumption underlying cognitive training is that practice has the potential to improve or at least maintain functioning in the given domain. An additional assumption is that any effects of practice will generalise beyond the immediate training context. Although this last assumption has not often been supported by the evidence (Owen 2010; Papp 2009), some have argued that failure to produce transferable benefits is related in part to problems with task design (Jaeggi 2010). Some authors have recently broadened the definition of cognitive training to include strategy training, which involves instruction in and practice of strategies designed to minimise cognitive impairment while enhancing performance (e.g. method of loci, visual imagery) and cognitive exercise (Gates 2011). Cognitive training may be offered through individual (Davis 2001; de Vreese 1998; Koltai 2001; Loewenstein 2004; Farina 2002) or group (Cahn‐Weiner 2003; Koltai 2001; Ermini Fuenfsch 1995; Kesslak 1997; Moore 2001) sessions or may be facilitated by family members (Quayhagen 1995; Quayhagen 2000; Neely 2009) with therapist support. In accordance with the suggestion that cognitive training may enhance the effects of pharmacological therapy (Newhouse 1997), some studies have evaluated the efficacy of cognitive training in combination with the use of acetylcholinesterase‐inhibiting (Cahn‐Weiner 2003; de Vreese 1998; Loewenstein 2004) or other (Yesavage 1981; Heiss 1993) medications. In addition, cognitive training for the person with dementia has sometimes been included as a component of supportive interventions for caregivers (Brodaty 1989; Brodaty 1997).
Cognitive rehabilitation
Historically, rehabilitation has been viewed as a process aimed at helping people achieve or maintain an 'optimal level of physical, psychological and social functioning' in the context of specific impairments arising from illness or injury (McLellan 1991), thus facilitating participation in preferred activities and valued social roles (WHO 2001). More recent views of rehabilitation include a deeper appreciation of the complex interplay between disease and ability to function: A disability may endure even once the disease that triggered it has been eliminated, and equally, disability can be reduced in the face of permanent injury or even chronic disease (Institute of Medicine 2011). Cognitive rehabilitation, originally developed mainly through work with younger brain‐injured people but equally applicable to progressive conditions, refers to the rehabilitation of people with cognitive impairments. Although the concept continues to evolve, cognitive rehabilitation generally refers to an individualised approach to helping people with cognitive impairments, by which those affected, and their families, work together with healthcare professionals to identify personally relevant goals and to devise strategies for addressing these (Wilson 2002). The emphasis is not on enhancing performance on cognitive tasks as such, but rather on improving functioning in the everyday context. Cognitive rehabilitation interventions aim to tackle directly those difficulties considered most relevant by the person with dementia and by his or her family members or supporters and to target everyday situations in the real‐life context. Cognitive rehabilitation approaches tend to be implemented in real‐world settings because there is no implicit assumption that changes instituted in one setting would necessarily generalise to another. Goals for intervention are selected collaboratively, and interventions are usually provided on an individual basis.
Both cognitive training and cognitive rehabilitation might be accompanied by (1) psychoeducational activities aimed at facilitating an understanding of cognitive strengths and difficulties, and (2) supportive discussion related to individual emotional reactions or other needs; where appropriate, links may be made to other possible sources of support. Table 1 summarizes the main differences between the attributes of cognitive training and cognitive rehabilitation.
| Table 1. Selected differences between cognitive training and cognitive rehabilitation | ||
| Cognitive training | Cognitive rehabilitation | |
| Target | Impairment | Participation restriction |
| Context | Structured tasks and environments | Real‐world setting |
| Focus of intervention | Isolated cognitive abilities and processes | Groups of cognitive abilities and processes required to perform everyday tasks |
| Format | Individualised or group | Individualised |
| Proposed mechanism of action | Mainly restorative; sometimes combined with psychoeducation and strategy training | A combination of restorative and compensatory approaches combined with psychoeducation and strategy training |
| Goals | Improved or maintained ability in specific cognitive domains | Performance and functioning in relation to collaboratively set goals |
How the intervention might work
Cognition‐based interventions for persons with acquired disorders of the central nervous system (including traumatic brain injury, stroke and neurodegenerative conditions) are driven by knowledge of brain‐behaviour relationships and mechanisms of injury, disease and recovery. Historically, such interventions have reflected two broad conceptual frameworks for the recovery of function after brain illness or injury: a traditional or restorative approach, and a contextualised or compensatory approach (Ylvisaker 2002). Techniques usually associated with cognitive training such as the repeated exercise of standardised cognitive tests of increasing difficulty, targeting specific cognitive domains, tend to reflect restorative principles and “thrive on the lure of neuroplasticity” (Rabipour & Raz 2012, p. 2). Evidence in support of this comes from a recent functional magnetic resonance imaging (fMRI) study that reported increased memory‐related brain activation following cognitive training in several brain regions of individuals at high risk of dementia due to mild cognitive impairment (Belleville 2011). Such increased brain activation may be the result of processes of synaptic growth and repair triggered by repeated practice on standardised tests. Techniques usually associated with cognitive rehabilitation, on the other hand, such as optimising residual cognitive abilities in impaired domains and making the most of unimpaired cognitive abilities, lend themselves more to compensatory approaches. For example, in relation to memory and learning, it is well established that the processes of memory encoding and consolidation, as well as the sub‐system of declarative memory, tend to be profoundly impaired even in the milder stages of AD (Christensen 1998). Nevertheless, research has shown that given appropriate conditions and support, and sufficient time, people with dementia can retain the ability to learn and can hold onto some information and skills despite their memory difficulties (Bäckman 1992; Bäckman 1996; Kopelman 1985; Little 1986). A cognitive rehabilitation approach may focus on helping people with dementia and their families make the most of residual memory ability, for example, by identifying the best ways of taking in important information (Bäckman 1991; Camp 1989; Camp 2000; Clare 1999; Clare 2000; Clare 2001; Hill 1987; Clare 2002; Anderson 2001) or by carrying out important real‐life practical skills (Josephsson 1993). Indeed, several learning principles and techniques (e.g. errorless learning, spaced retrieval) have been found to lead to improved rates of learning and memory among patients with mild dementia (Boudreaux 2011Clare, Wilson et al 2000; Dunn 2007). It is well documented that despite the severity of memory difficulties, certain memory systems and processes such as implicit memory (e.g. priming, procedural memory) are relatively preserved in the milder stages of AD and vascular dementia (Brandt 1995; Morris 1996). This profile suggests that interventions may aim to build on areas of relative strength reflected in preserved aspects of memory by helping patients develop strategies for learning information via less impaired components of the memory system. Finally, cognitive rehabilitation interventions also attempt to assist patients in developing ways to compensate for impairments in those aspects of memory that are significantly affected (e.g. using external memory aids, making environmental changes), so as to minimise the cognitive demands of various activities (Bird 2001; Bourgeois 1990; Clare 2000; Kurlychek 1983). Cognitive rehabilitation interventions use these and other techniques to enhance or maintain everyday functioning and wellbeing and to reduce excess disability for the person with dementia, while reducing strain for family caregivers.
Why it is important to do this review
Both pharmacological treatments with cholinesterase inhibitors and cognition‐based interventions can be defined as symptomatic treatments in that they do not target hypothesised disease mechanisms. Extensive efforts to develop disease‐modifying treatments continue; however, consistently disappointing results from drug trials of various agents have resulted in considerable doubt that disease‐modifying treatments can show a positive effect by the time dementia is fully developed (Salomone 2012), and efforts in this direction are increasingly being shifted to the pre‐dementia or even the pre‐symptomatic stage. In contrast, non‐pharmacological interventions, particularly cognition‐based interventions, are increasingly recognised as an important adjunct and in some cases as an alternative to pharmacological treatments for individuals with dementia and those at risk of dementia. Nevertheless, earlier studies suggested that cognition‐based interventions are not appropriate, as they are ineffective and result in frustration and depression among participants and caregivers (Small 1997). With growing emphasis on early detection and intervention in dementia care, the need for a clear evidence base for cognition‐focused interventions is becoming apparent (Woods & Clare 2006). As was already mentioned, a recent systematic review concluded that general cognitive stimulation and reality orientation provide benefit in terms of the overall cognitive status of patients and aspects of their wellbeing (Woods 2012). Whether or not more targeted approaches such as cognitive training and cognitive rehabilitation can produce similarly encouraging outcomes has not yet been determined.
The present review is an update of the original review and updated versions of this review (Clare 2003; Clare 2008). The latest update of this review included 9 randomised controlled trials (RCTs) of cognitive training and found no evidence for efficacy of cognitive training in relation to cognitive outcomes for the person with dementia. No RCTs of cognitive rehabilitation were found in searches for previous versions of this review; therefore no conclusions could be drawn regarding the efficacy of this type of intervention.
In selecting studies for this review, we have classified interventions on the basis of the ways in which they are described in relation to the definitions previously provided. In some cases, this led to classification of an intervention as ‘cognitive training’ even when the term ‘cognitive rehabilitation’ was used by the study authors. In other cases, an intervention described as ‘cognitive training’ might be deemed to fit more closely with the principles of ‘cognitive stimulation’, thus leading to exclusion from the current review. We acknowledge that the identified categories represent broad definitions and that some cases may reflect an overlap between techniques found in cognitive 'training' and those classified as cognitive 'rehabilitation', which in turn may have some commonalities with cognitive 'stimulation'. Therefore, although the current classification of cognition‐based interventions is gradually gaining some consensus among researchers, this classification should remain open to additional refinement in the future.
Objectives
To evaluate the effects of cognitive training and cognitive rehabilitation for people with mild AD or vascular dementia in relation to cognitive and non‐cognitive outcomes for individuals affected and for their caregivers.
To update previous versions of this review (Clare 2003; Clare 2008).
To consider the nature and quality of available evidence on this topic as derived from RCTs.
To assist in establishing the appropriateness of cognitive training and cognitive rehabilitation interventions offered to people with early‐stage dementia and, where relevant, to identify the factors associated with efficacy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials for which adequate information was provided or could be obtained from the researchers were considered for inclusion. For consistency with previous versions of the review, we decided to include only studies that were published in the English language. Although in some cases this may lead to an increased language bias, evidence suggests that the effects of language bias have diminished as a result of the continuous shift towards publishing of trial results in English (Sterne 2011). No study was excluded solely on the basis of language other than English, as whole non‐English studies that were screened beyond the title (n = 3) were found to fail other inclusion criteria (non‐randomised trials or trials of cognitive stimulation).
Types of participants
Participants with a medical diagnosis of dementia, possibly further specified as Alzheimer's disease, vascular dementia or mixed Alzheimer's and vascular dementia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV), the International Classification of Diseases, Tenth Revision (ICD‐10), criteria of the National Institute of Neurological and Communicative Disorders-Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) or research diagnostic criteria of the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINDS‐AIREN) (APA 1995; WHO 1992; McKhann 1984; Roman 1993). Given common limitations of available data regarding specific diagnoses, we decided to consider these diagnostic categories together. We excluded data from participants for whom dementia was known to have an aetiology other than AD or cerebrovascular pathology (e.g. frontotemporal dementia, Lewy body dementia), as the format of cognitive training and rehabilitation in other dementia types is likely to differ substantially from that applied in AD or vascular dementia.
We included only studies that reported the severity of dementia through group mean scores, ranges of scores or individual scores on a standardized scale such as the Mini‐Mental State Examination (MMSE; Folstein 1975) or the Clinical Dementia Rating (CDR; Hughes 1982).
Studies targeting primarily people with minimal, mild or moderate dementia (MMSE score > 12 or CDR score < or = 2), although studies with a small proportion of participants in the more severe ranges (< 20%) were considered acceptable.
Qualifying participants were expected in the main to be residing at home, but interventions might be offered in a range of settings, and data from home, outpatient, day‐care and residential settings were considered acceptable for inclusion. However, it was considered appropriate to exclude data from long‐term residents of psychiatric hospitals, where pre‐existing psychiatric conditions were likely to occur.
No specific restrictions were set regarding age. Although it was previously planned to examine the potential moderation of age on observed outcomes, given the limited data available, it was decided not to examine the role of age at this point.
No restrictions were placed on current pharmacological treatment. Where available, we noted information about participants' use of cholinesterase inhibitors.
It was decided that data from family caregivers would be included where available, and where the relationship between the caregiver and the person with dementia was specified, including whether the two were co‐residents.
In previous versions, it was proposed that information regarding the use of coping strategies used by participants or caregivers to maintain or enhance cognitive function would be noted. However, no study provided this information.
Types of interventions
Experimental interventions
Interventions meeting our definition of cognitive training or cognitive rehabilitation were acceptable for inclusion. These might also be described as memory 'therapy', 'groups', 'retraining', 'support' or 'stimulation', or as cognitive 'training', 'retraining', 'remediation', 'support' or 'stimulation'.
Interventions were required to specifically address one or more target areas relevant to cognitive functioning, either singly or in combination with interventions directed at other targets (e.g. relieving anxiety or depression) or other cognitive functions (e.g. attention or problem‐solving).
When more than one experimental group was included in the study, the group that provided the treatment most similar to that described in other included studies was selected for analysis (e.g. individual interventions were selected over interventions delivered to dyads, and stand‐alone cognitive training or rehabilitation interventions were selected over interventions that combined pharmacological and non‐pharmacological components).
Comparator interventions
No treatment/standard treatment. Unless otherwise specified, whenever groups were described as 'no treatment' in individual studies, it was assumed that this referred to the usual/standard treatment, and not to withholding of treatment. 'Usual or standard treatment' refers to what would normally be provided in the study locality to participants with early‐stage Alzheimer's or vascular dementia, and might include provision of medication, clinic consultations, contact with a community mental health team, day care or support from voluntary organisations, but not cognitive training or rehabilitation interventions.
Wait‐list control. In studies of this kind, the experimental intervention was offered to the control group after the study had ended.
Active control condition. For example, active control conditions consisted of an equivalent number of sessions or visits in which general social support was provided, but during which no structured cognitive training or rehabilitation intervention was offered.
When more than one comparator intervention was included in the study, the group that was most similar to that included in other studies was selected for analyses. This was usually a 'no treatment' group.
All interventions
Interventions conducted in individual or group modalities, with or without involvement of family caregivers, were acceptable for inclusion.
Interventions included at least a baseline assessment and an immediate post‐intervention assessment, with or without follow‐up assessment.
No restrictions were imposed regarding duration of intervention or number of treatment sessions. It was decided to consider differences in these parameters when making comparisons between studies.
Types of outcome measures
Primary and secondary outcomes were examined in three categories:
Cognitive and non‐cognitive outcomes of the intervention for the person with dementia
Outcomes for the primary caregiver
The impact of the intervention on the course of the disorder
Outcomes for the person with dementia and for the primary caregiver were considered for inclusion in the meta‐analysis when they were assessed using scores on at least one standardised test or questionnaire measure. When more than one measure was used to assess a particular outcome (e.g. immediate memory), we included in the comparison the measure on which group differences were observed at post‐intervention or follow‐up assessments (if relevant), or the measure that ,most resembled the measures contributed by other studies. Behavioural observations and ad hoc measures were considered as additional information.
Rates of attrition and reasons for attrition were noted where available. Drop‐out rates in the context of progressive conditions may in part reflect changes in the needs of the individual that prompted a needed change in therapeutic approach. With a progressive condition, individual needs may change during the course of an intervention and follow‐up period, requiring implementation of a different approach, but this should not be interpreted as evidence that the approach itself is ineffective.
Outcome measures for the person with dementia seek to identify whether changes are observed after the intervention, and to determine the extent to which these can be attributed to the intervention itself. Given the progressive nature of dementia, improved performance may not necessarily be a goal. Instead, preserved performance on a trained task in the context of a decline in untrained tasks could be interpreted as evidence of efficacy. Differences in the trajectory of change between scores on intervention targets and standardised measures are as important as the overall level of change; for example, maintenance of functioning on a target task in the context of a decline in scores on standardised assessments might indicate that the intervention was effective in relation to the targeted area of functioning.
Primary outcomes
For each of the outcomes described previously, we intended to conduct separate comparisons for those measured short term (immediately post‐intervention), medium term (3 to 12 months post‐intervention) and long term (> 12 months post‐intervention). However, no study reported relevant outcomes beyond the medium term.
(A) Cognitive outcomes for the person with dementia
(A1) Change in scores on global cognitive screening measures (e.g. MMSE) and in orientation and self‐reported and caregiver‐reported cognitive abilities in the short term (i.e. immediately post‐intervention-A1.1), in the medium term (i.e. 3 months up to one year-A1.2) and in the long term (i.e. longer than a year-A1.3).
(A2) Change in performance on neuropsychological measures (immediate and delayed memory, working memory and attention, language, executive function) in the short term (i.e. immediately post‐intervention-A2.1), in the medium term (i.e. 3 months up to one year-A2.2) and in the long‐term (i.e. longer than a year-A2.3).
(B) Non‐cognitive outcomes for the person with dementia
Self‐reported or caregiver‐reported changes in mood, capacity for activities of daily living, behaviour, adjustment to disability, general health and quality of life in the short term (B1), in the medium term (B2) and in the long‐term (B3).
Secondary outcomes
(C) Outcomes regarding the course of dementia
Change in scores on measures of dementia severity (e.g. CDR) or rates of admission to residential care in the short term (C1), in the medium term (C2) and in the long term (C3).
(D) Outcomes for the family caregiver
Self‐reported changes in mood, wellbeing, burden of care and quality of life in the short term (D1), in the medium term (D2) and in the long term (D3).
(E) Outcomes for disease biomarkers of the person with dementia
(E1) Changes in in vivo measures of neuropathology (e.g. amyloid or tau pathology, brain atrophy) in the short term (E1.1), in the medium term (E1.2) and in the long term (E1.3).
(E2) Changes in measures of brain function (e.g. fluorodeoxyglucose positron emission tomography (FDG PET), fMRI) in the short term (E2.1), in the medium term (E2.2) and in the long term (E2.3).
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois)-the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register-on 2 November 2012.
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy individuals. Studies are identified from the following:
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and LILACS.
Monthly searches of a number of trial registers, including ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others).
Quarterly search of The Cochrane Central Register of Controlled Trials (CENTRAL).
Six‐monthly searches of a number of grey literature sources, including ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS Website.
Details of the search strategies used for retrieval of reports of trials from healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed using many of the sources previously listed to cover the time frame from the last searches performed for ALOIS, to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
Searches carried out in previous versions of the review can be viewed in Appendix 2 and Appendix 3.
Data collection and analysis
Selection of studies
The search results (covering the period April 2006-November 2012) were reviewed by one review author (AB‐F), who identified all relevant RCTs of cognition‐based interventions in mild AD or vascular dementia and retrieved the full texts. Two review authors (AB‐F and LC) then independently reviewed each article to determine whether inclusion criteria were met. There were no disagreements regarding the inclusion of studies.
Data extraction and management
All relevant data were extracted from the studies selected for inclusion, recorded on a data entry form and entered into Review Manager (RevMan). Additional information was sought from study authors as appropriate. Data extracted from each trial included characteristics of the experimental and control groups used in each study, as well as characteristics of the interventions provided. Mean scores and standard deviations from baseline, post‐intervention and, where available, follow‐up assessments on all relevant outcome measures for treatment and comparison groups were also extracted. Two studies (Koltai 2001; Beck 1988) directly reported the data in terms of change from baseline. In the remaining studies, changes from baseline statistics were calculated from group means and standard deviations at baseline, post‐intervention and follow‐up. Baseline was defined as the latest assessment available before randomisation, but no more than two months before.
Assessment of risk of bias in included studies
Assessment of risk of bias was conducted by AB‐F using The Cochrane Collaboration's Risk of Bias tool (Higgins 2011) and was subsequently reviewed by LC. Consistent with the risk of bias tool, study quality was assessed in the following domains: sequence generation, allocation concealment, blinding of participants and investigators, incomplete outcome data and selective reporting of outcomes. Studies were rated as 'low risk', 'high risk' or 'unclear risk' in each of these domains. There were no disagreements between review authors in ratings of risk of bias.
Measures of treatment effect
The meta‐analysis was conducted on change‐from‐baseline scores. A zero correlation between measurements at baseline and those at subsequent time points was assumed. This method overestimates the standard deviation of the change from baseline but provides a conservative approach considered to be preferable in a meta‐analysis. Outcome measures were treated as continuous measures. In some cases, outcomes were derived from ordinal rating scales; provided these contained a reasonably large number of categories (> 10), the data were treated as continuous variables arising from a normal distribution. There were no examples of binary outcome measures, which would have required an odds ratio calculation.
The mean difference (MD) with 95% confidence interval (CI) was used whenever studies used the same outcome measure, and the standardised mean difference (SMD), which is the absolute mean difference divided by the pooled standard deviation, was used when the same outcome was assessed with the use of different measures.
Unit of analysis issues
Three types of unit of analysis issues were encountered: cross‐over trial designs, multiple treatment groups and repeated assessments. For cross‐over trials, only data from the first treatment period were used. In the case of studies that compared more than two treatment groups, the analysis focused on the two groups providing the most pertinent data that most resembled conditions included in other studies. Wherever possible, a condition in which individual cognitive training or rehabilitation was delivered was compared with a condition that included no cognitive intervention. To address the issue of repeated assessments (more than one post‐intervention assessment), we intended to conduct separate comparisons to assess outcomes immediately post‐intervention (the first post‐intervention assessment), short‐term outcomes (up to 12 months post‐intervention) and longer‐term outcomes (more than 12 months post intervention).
Dealing with missing data
Numbers of participants who commenced and who completed the intervention in each group were noted where available, and these numbers contributed to the assessment of risk due to incomplete outcomes data. Studies generally provided minimal detail on the causes and impact of missing data. In general, it was assumed that data were missing at random, and analyses in individual studies were generally performed per protocol rather than on an intention‐to‐treat basis.
Assessment of heterogeneity
Statistical heterogeneity was assessed using a standard Chi2 statistic and an associated l2 statistic. Consistent with recommendations, heterogeneity was deemed to be present when the Chi2 statistic was significant at the P = 0.1 level, or when the l2 suggested that more than 40% of the variability in effect estimate was due to heterogeneity (Higgins 2011).
Data synthesis
As no evidence of statistical heterogeneity was found, all analyses were conducted using a fixed‐effect model and the inverse variance method.
Subgroup analysis and investigation of heterogeneity
As no heterogeneity was detected, no subgroup analyses were conducted.
Sensitivity analysis
Inflated estimates of the standard deviation of change scores, associated with the assumption of zero correlation between pre‐intervention and post‐intervention scores on outcome measures, can potentially obscure real effects of the interventions. To address this possibility, we re‐ran the meta‐analysis for some of the central outcome measures using post‐intervention scores only, thus avoiding the need to estimate the standard deviation of change scores. This sensitivity analysis did not lead to a change in any of the results reported here.
Results
Description of studies
Results of the search
Electronic searches conducted in November 2012, December 2011 and September 2009 retrieved a combined total of 1339 results. Following preliminary screening and removal of duplicate studies by Anna Noel‐Storr, Trial Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group, 495 records were forwarded to the review authors for further evaluation. After title and abstract review by one review author (AB‐F), 49 records were selected for closer assessment, and full records were retrieved and reviewed independently by two review authors (AB‐F, LC). Upon review and discussion, three trials were identified that met the inclusion criteria-two trials describing a cognitive training intervention (Galante 2007; Neely 2009) and one trial describing a cognitive rehabilitation intervention (Clare 2010). The two cognitive training studies were added to the nine studies that were included in the previous meta‐analysis, bringing the total number of studies in the meta‐analysis to 11. Because no previous trials on individualised cognitive rehabilitation had been undertaken, no meta‐analysis of cognitive rehabilitation could be performed. The flow of studies through the review process is shown in Figure 1.
1.

Study flow diagram.
RCT = randomised controlled trial.
CT = cognitive training.
CR = cognitive rehabilitation.
Additional information
Additional information was sought from study authors where necessary. With regard to de Vreese 1998, another abstract was published in 1999 (see under de Vreese 1998), additional data were reported in a later review article (see under de Vreese 1998) and further information including mean scores was kindly supplied by the author. Additional data related to Loewenstein 2004 were also kindly supplied by the author. Queries related to Koltai 2001, Beck 1988, Quayhagen 1995, Quayhagen 2000, Galante 2007 and Heiss 1993 were answered by the investigators. No responses were received to queries related to the studies by Davis 2001, Cahn‐Weiner 2003 and Neely 2009.
Included studies reported that a total of 117 measures (100 measuring patient outcomes, 17 measuring caregiver outcomes) were used to examine the 22 primary and secondary outcomes selected for examination in this review. For cognitive training interventions, data for meta‐analysis were available for 8 of the 14 primary outcomes and for 6 of the 8 secondary outcomes over the short term. Meta‐analysis could be performed on 2 of 14 primary outcome measures and on 2 of 8 secondary outcome measures over the medium term. No cognitive training studies reported an outcome measure over the long term. As only one study of cognitive rehabilitation met inclusion criteria for this review, no meta‐analysis of cognitive rehabilitation could be conducted.
Included studies
Significant diversity was noted among the 12 studies on a range of parameters. Seven studies included only participants diagnosed with AD, but the other four included participants diagnosed with AD, vascular dementia or mixed dementia. In one study (Quayhagen 2000), participants were included if they were diagnosed with dementia due to Parkinson's disease (PD) in addition to AD and vascular and mixed dementia, but it was not possible to ascertain how many of the included participants had PD, because data for all aetiologies were reported together. Severity of dementia varied in the included studies from very mild to moderate; this was generally determined on the basis of scores on a measure of dementia severity or global cognition (e.g. Clinical Dementia Rating, MMSE). Although not stated explicitly in most studies, it appears that in most cases, patients were recruited from the community; in a small number of studies, patients who resided in residential care homes were also included. The duration of interventions provided in the included studies varied considerably, ranging from 4 to 24 weeks. Four studies reported follow‐up assessments over the medium term; these occurred at 8 weeks, as well as at 3, 6 and 9 months, after the end of treatment. The content of the interventions also varied considerably, ranging from training in the use of compensatory strategies to practice on computerised tasks to working toward achieving collaboratively derived goals. Selected features of the included studies are further described here and are summarised in the Characteristics of included studies table.
Objectives of the studies
Beck 1988: Compared 'cognitive skills remediation training', delivered on a one‐to‐one basis, with a usual‐treatment control condition.
Heiss 1993: Compared computerised cognitive training alone with two conditions in which computerised cognitive training was combined with drug treatment (cognitive training plus pyritinol and cognitive training plus phosphatidylserine) and an active control condition (social support). The relevant comparison for this review is that between cognitive training alone and social support.
Quayhagen 1995: Compared cognitive training with active and wait‐list control conditions. The relevant comparison for this review is that between cognitive training and the wait‐list control condition.
de Vreese 1998: The study initially set out to compare cognitive training alone, acetylcholinesterase‐inhibiting medication (AChEI) alone, cognitive training plus AChEI and placebo. Raw data for the group receiving cognitive training alone were not reported in the 1998 paper and are no longer available. The design was subsequently amended as caregivers were dissatisfied with the possibility of receiving cognitive training alone, so the comparisons reported in 2001 and augmented with further information from the author involve three groups: AChEI alone, cognitive training plus AChEI and active control. For the purposes of this review, the comparison of interest lies in the difference between AChEI alone and cognitive training plus AChEI.
Quayhagen 2000: Compared four intervention approaches-cognitive training, dyadic counselling, dual supportive seminar groups and early‐stage day care with caregiver support-with a wait‐list control condition. For the purposes of this review, the comparison of interest is that between cognitive training and the wait‐list control condition.
Davis 2001: Compared cognitive training with a 'mock' (active control) intervention in a cross‐over design. The comparison of interest is that between training and active control groups following the initial intervention stage; cross‐over data are not considered here.
Koltai 2001: Compared a memory and coping programme, delivered in individual or group session format, with a wait‐list control condition. The results for individual and group training were analysed together in the trial report as no differences were observed between them.
Cahn‐Weiner 2003: Compared a memory training programme delivered in small‐group format with a control condition involving didactic presentation.
Loewenstein 2004: Compared 'cognitive rehabilitation training' with 'mental stimulation', delivered in one‐to‐one sessions.
Galante 2007: Compared individual computerised cognitive training with an active control condition.
Neely 2009: Compared collaborative cognitive training (dyadic), individual cognitive training and a no treatment control condition. The relevant comparison for this review is that between individual cognitive training and no treatment groups.
Clare 2010: Compared individual, goal‐oriented cognitive rehabilitation with relaxation therapy, and with a no treatment control condition. The relevant comparison for this review is that between the cognitive rehabilitation and no treatment groups.
Participant numbers and characteristics in the overall samples
Beck 1988: Participants included 20 individuals over 55 years of age with moderately impaired cognitive functioning (MMSE score of 15 to 20) and findings compatible with a diagnosis of Alzheimer's disease or mixed dementia, living in one of four nursing homes or in the geriatric unit of a Veterans Administration hospital.
Heiss 1993: Of 80 people who entered the study, data were available for 70. Included in this group were 37 men and 33 women with a diagnosis of possible or probable AD according to NINCDS‐ADRDA criteria and a modified Hachinski score of 3 or less, ranging in age from 48 to 79 years (average age 66.63 years), and with MMSE scores ranging from 13 to 26. On entry to the study, none were taking any medications known to affect the central nervous system. This study was carried out in Germany.
Quayhagen 1995: Of 135 care recipient/caregiver dyads initially assessed, 95 were eligible for inclusion, 79 completed the study and data were available for 78. These were families in which one person had a diagnosis of possible/probable AD and was in the mild or moderate stage with a Mattis Dementia Rating Scale (DRS) score of 90 or above. People with dementia included 51 men and 27 women, with an average age of 73.6 years (standard deviation (SD) 8.0) and an average education level of 12.6 years (SD 4.1). They were not participating in any clinical trials of anti‐dementia medication. Caregivers consisted of 18 men and 60 women, with an average age of 66.7 years (SD 10.8) and an average education level of 14.1 years (SD 2.7). Twenty‐nine percent of caregivers attended support groups periodically, and 14% had previously sought psychological help. This study was conducted in California, USA, and ethnicity within the whole sample was described as 85% white, 3% African American and 11% Hispanic.
de Vreese 1998: The 1998 paper reports the inclusion of 24 participants with a diagnosis of AD according to NINCDS‐ADRDA orDSM‐IV criteria and a CDR rating of 1 to 2. Average age was 72.6 years (range 61 to 83 years). The 2001 review paper reports the inclusion of 27 participants with early‐stage AD and MMSE scores ranging from 20 to 26, representing the removal of the 6 people in the original cognitive training alone condition and the addition of 3 more participants to each of the other groups. Participants were taking no concurrent medication known to affect the central nervous system. This study was undertaken in Italy.
Quayhagen 2000: Participants included 103 dyads consisting of a person with dementia and a caregiving spouse. The people with dementia had a diagnosis of possible or probable AD (more than 70% were in this category), vascular dementia or Parkinson's dementia, and were in the mild or moderate stages, scoring above 100 on the DRS. They included 65 men and 38 women, with an average age of 74.51 years (SD 7.11) and an average education level of 14.57 years (SD 3.05). The caregivers were 38 men and 65 women, with an average age of 71.83 years (SD 8.12) and an average education level of 14.42 years (SD 3.05). The study took place in California, USA, and the ethnic mix within the whole sample was described as 93% white, 2% African American, 1% Asian and 4% Hispanic.
Davis 2001: The participants were 37 individuals (16 men and 21 women) with a diagnosis of probable AD according to NINCDS‐ADRDA criteria. MMSE score range was 15 to 29 (average score 22.31). Average age in the sample was 70.62 years, and average level of education was 14.02 years. Mean score on the 30‐item Geriatric Depression Scale (5.02) was within the normal range. This study was carried out in Texas, USA.
Koltai 2001: The participants were 24 older people aged 60 to 84 with a diagnosis of AD and a CDR score of 0.5 or 1.0. Of the 25 people initially identified as eligible, one found the group treatment modality unacceptable and declined to take part. The study was carried out in North Carolina, USA.
Cahn‐Weiner 2003: The participants were 34 individuals (20 women and 14 men) with a diagnosis of probable AD according to NINCDS‐ADRDA criteria. The study was conducted in Rhode Island, USA.
Loewenstein 2004: The participants were 44 individuals (26 men and 18 women) with a diagnosis of probable or possible AD according to NINCDS‐ADRDA criteria. The authors note that those with a diagnosis of probable AD met DSM‐IV criteria for dementia, and those with possible AD did not show sufficient functional impairment to merit a DSM‐IV diagnosis of dementia. All participants had been on a stable dose of an acetylcholinesterase inhibitor for 8 weeks at the start of the study; 41 of these were taking donepezil (doses ranged from 5 to 15 mg). Approximately two‐thirds of participants were English speakers, and the remaining 14 were Spanish speakers, mostly of Cuban origin, for whom all components of the programme were conducted in Spanish. The study took place in Florida, USA.
Galante 2007: Participants were 12 individuals who met NINCDS‐ADRDA criteria for mild AD and scored 19 to 26 on the MMSE or 70 to 90 on the Milan Overall Dementia Assessment (MODA). All were treated with AChEI for at least 3 months. The mean age of the sample was 76 years (SD 6), and the mean educational level was 6.3 years (SD 2.2). One control participant was excluded from analysis "due to poor compliance". The study was conducted in Italy.
Neely 2009: Forty‐seven individuals who met DSM‐IV criteria for mild to moderate AD or vascular dementia and their spouses were approached for the study, and 30 patients (15 males, 15 females) consented to participate. All participants were diagnosed with dementia within the 8 months immediately before the study, were living at home with their spouses and were free from significant psychiatric disorders. The mean age of patients was 75.4 years (SD 6.4). The study was conducted in the Stockholm area of Sweden.
Clare 2010: Participants were 69 individuals (41 women, 28 men) with a mean age of 77.78 years (SD 6.32), and a mean education level of 10.64 years (SD 1.67). They were diagnosed with AD (n = 56) or mixed AD and vascular dementia (n = 13) according to NINCDS‐ADRDA criteria. The mean MMSE score was 23 (SD = 3.02), and all participants were on a stable dose of AChEIs. Forty‐four participants had family members involved, and in all but 4 cases, these individuals were living with the person with dementia. The study was conducted in the North Wales area of the UK.
Characteristics of participants in the treatment and comparison groups
Beck 1988: Characteristics of participants in the treatment and control groups are summarised in Table 3. No significant differences were found between the two groups. Each group comprised 7 white and 3 black participants. In the treatment group 2 had completed grade school, 6 high school and 2 college, and in the control group, 2 had completed grade school, 7 high school and 1 college. In the treatment group 6 people resided in nursing homes and 4 in hospital, and in the control group 9 people resided in nursing homes and 1 in hospital.
1. Summary characteristics of participants in cognitive training and control groups.
| Study | Condition |
n (completed baseline assessment) |
Age mean (SD), range |
Gender balance (m:f) | Years of education | Number taking AChE‐I |
Baseline MMSE score |
Discontinue rates |
| Beck 1988 | Cognitive training | 10 | 74 (range 68‐75) | 5:5 | Attended college = 2 | none | not reported | 0 |
| Control | 10 | 76 (range 70‐93) | 3:7 | Attended college = 1 | none | not reported | 0 | |
| Heiss 1993 | Cognitive training | not reported (18 completed the study) |
65.9 (6.28) | 9:9 | not reported | none | 20.55 (4.42) | not reported |
| Control | not reported (17 completed the study) |
66.6 (10.17) | 10:7 | not reported | none | 20.23 (4.10) | not reported | |
| Quayhagen 1995 | Cognitive training | 25 | not reported | not reported | not reported | not reported | not assessed | not reported |
| Control | 25 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| de Vreese 1998 | Cognitive training | 9 | not reported | not reported | not reported | all | 17.33 (3.39) | 0 |
| Control | 9 | not reported | not reported | not reported | all | 17 (3.2) | 0 | |
| Quayhagen 2000 | Cognitive training | 21 | not reported | not reported | not reported | not reported | not assessed | not reported |
| Control | 15 | not reported | not reported | not reported | not reported | not assessed | not reported | |
| Davis 2001 | Cognitive training | 19 | 68.67 (3.86) | 10:9 | 15.06 (3.86) | 5 | 21.84(4.03) | 0 |
| Control | 18 | 72.56 (7.62) | 6:12 | 12.97 (2.56) | 4 | 22.78 (4.45) | 0 | |
| Koltai 2001 | Cognitive training | 16 | 72.9 (6.7) | not reported | 15.0 (4.0) | not reported | 22.9 (3.6) | 2 |
| Control | 8 | 73.9 (7.2) | not reported | 15.0 (4.0) | not reported | 26.6 (2.5) | 0 | |
| Cahn‐Weiner 2003 | Cognitive training | 19 | 77. 8 (6.9) | 9:8 | 12.7 (2.1) | all | 24.3 (2.2) | 2 |
| Control | 20 | 76.0 (7.7) | 5:12 | 13.1 (3.5) | all | 25.1 (1.7) | 3 | |
| Loewenstein 2004 | Cognitive training | 28 | 78.12 (4.3) | 15:10 | 13.08 (4.1) | all | 23.4 (2.9) | 3 |
| Control | 21 | 74.74 (7.5) | 11:8 | 14.37 (3.0) | all | 24.53 (4.5) | 2 | |
| Galante 2007 | Cognitive training | 7 | not reported | not reported | not reported | all | 22.9 (3.1) | 0 |
| Control | 4 | not reported | not reported | not reported | all | 23.1 (1.8) | 1 | |
| Neely 2009 | Cognitive training | 10 | 74.8 (6.7) | 6:4 | not reported | not reported | 22.9 (4.15) | 0 |
| Control | 10 | 77.0 (6.6) | 6:4 | not reported | not reported | 18.6 (5.7) | 1 | |
| Clare 2010 | Cognitive rehabilitation | 22 | 76.3 (6.39), 64‐89 | 9:13 | 11.41 (2.81), 9‐19 | all | 23.14 (3.12), 18‐27 | 2 |
| Control | 22 | 78.1 (6.61), 56‐87 | 9:13 | 11.43 (2.99), 9‐19 | all | 22.32 (3.05), 18‐30 | 1 |
Date in the table are generally reported only for those participants who completed the interventions.
Heiss 1993: Mean ages and gender distributions for cognitive training and social support conditions are summarised in Table 3. No significant differences were noted between groups.
Quayhagen 1995: Details are not reported separately for the cognitive training and comparison groups, but the authors comment that no significant differences were observed between groups.
de Vreese 1998: In the 1998 paper, groups were reportedly matched on educational level and illness severity, although the cognitive training plus AChEI group had a significantly longer duration of illness. The 2001 paper reports that groups were matched on MMSE scores; mean MMSE scores are reported in Table 3.
Quayhagen 2000: Details are not reported separately for the cognitive training and comparison groups, but the authors comment that no significant differences were noted between groups.
Davis 2001: Characteristics of participants in each of the two groups are summarised in Table 3. No statistically significant differences were reported, but some trends were apparent; participants in the cognitive training group were on average younger and better educated and were more likely to be male and to be receiving anti‐depressant medication.
Koltai 2001: Characteristics of participants in training and control groups are summarised in Table 3. Participants in the control group had significantly higher MMSE scores at baseline (26.6 vs 22.9) and significantly lower relative rated levels of depression on the Geriatric Depression Scale (GDS) (8.3 vs 14.7).
Cahn‐Weiner 2003: Characteristics of participants in each of the two groups are summarised in Table 3. No statistically significant differences were noted between the groups on these parameters.
Loewenstein 2004: Characteristics of participants in each of the two groups are summarised in Table 3. No statistically significant differences were noted between the groups on these parameters, except that three‐month follow‐up was significantly later for the cognitive training group (13.67 weeks from post‐intervention, compared with 12.79 for the mental stimulation group).
Galante 2007: The authors provided the mean age and education level for the full sample, but no information regarding these patient characteristics was provided at the group level. In addition, the authors report in a table the means and SDs for the two groups on the cognitive measures at all time points, but significance levels are provided only for the Time × Group interaction. Therefore, it is not possible to ascertain whether the groups were equivalent at baseline. Visual inspection shows clear trends for group differences on a number of cognitive (e.g. prose memory), functional (e.g. instrumental activities of daily living (IADL)) and mood (e.g. Neuropsychiatric Inventory (NPI)) measures at baseline. Available characteristics of participants in the training and control groups are summarised in Table 3.
Neely 2009: Relevant characteristics of the treatment and control groups are summarised in Table 3. The groups did not differ in age, levels of depression, MMSE scores or subjective health, or on any of the cognitive measures included at baseline. Data on participants' education level was not reported.
Clare 2010: Characterisitcs of the intervention and control groups are summarised in Table 3. No group differences were found at baseline in any of the demographic, cognitive or functional measures or in the presence of comorbid medical conditions.
Description of the interventions
Beck 1988: Cognitive skills remediation training included exercises on attention and reading, concentration on detail and remembering. Exercises were graded for difficulty level, and participants were given assistance when they had problems with the tasks.
Heiss 1993: Computerised cognitive training for one hour, twice a week, with commercially available software designed for use in neurological rehabilitation (produced by Rigling Reha‐Service), running on a Commodore C64 computer. Participants had to solve memory, perceptual or motor tasks, selected according to the profile of cognitive impairment, of varying difficulty levels. Duration of training was 24 weeks.
Quayhagen 1995: One hour per day of active cognitive stimulation, six days per week, facilitated by the family caregiver in the home setting, using ecologically valid exercises addressing memory, problem‐solving and conversational fluency. A workbook provided for family caregivers contained exercises of varying difficulty levels from which they could select appropriate tasks. The exercises were continued for 12 weeks.
de Vreese 1998: Twice‐weekly, 45‐minute individual sessions with caregivers present, aimed at '(re)training memory (in particular autobiographical and implicit), language and executive abilities associated with reality orientation therapy, to be repeated at home by the caregiver'. The 2001 paper describes the sessions as 30 to 40 minutes in length and involving individually tailored memory training exercises that provided support for encoding (use of real‐life material, involvement of motor activity, self‐generation of cues) and for retrieval (provision of supplementary cues, use of forced‐choice recognition). The sessions were introduced after a 3‐month run‐in period on the drug treatment and were continued for 12 weeks.
Quayhagen 2000: As for Quayhagen 1995, but given 5 days per week for 8 weeks. Post‐treatment assessment was carried out at 12 weeks.
Davis 2001: Weekly individual one‐hour sessions at the clinic, covering (1) spaced retrieval training for personal information (although half recalled the information without training, so there was a ceiling effect); (2) the 'peg' task mnemonic strategy (for those who required little or no spaced retrieval training) and (3) face‐name association using mnemonics. Home attention exercises were carried out for 30 minutes per day, 6 days a week, and were directed by the caregiver. Duration of treatment was five weeks.
Koltai 2001: The memory and coping programme was delivered in individual or group modality. The group format consisted of five weekly, one‐hour sessions conducted in groups of four. The individual format consisted of a mean of six individual sessions. Caregivers joined the last 10 to 15 minutes of each session, where available. The programme involved training and practice in the following techniques: spaced retrieval, face‐name recall, verbal elaboration, concentration/overt repetition, external memory aids and coping strategies.
Cahn‐Weiner 2003: The memory training provided was a modified version of a manualised protocol, involving practice with memory strategies such as categorisation and visualisation and word list learning.
Loewenstein 2004: The cognitive rehabilitation training covered time and place orientation, face‐name association learning, object manipulation, attention and visuomotor training with a computer, making change for a purchase from a $20 bill and balancing a cheque book. Participants were encouraged to use a memory notebook and to practice what they had learned at home between sessions.
Galante 2007: The computerised cognitive training group was trained on a set of computerised exercises selected from a software package covering the domains of memory, language, perception, attention, spatial cognition and intelligence. Exercises were administered in a fixed sequence to all participants, and most exercises lasted 3 minutes.
Neely 2009: The cognitive training was conducted with the support of a research assistant. Participants were trained on a name‐face learning task and on a table‐setting activity. Spaced retrieval and the provision of letter cues were used to support training on the face‐name learning task, whereas a hierarchical cueing technique was used to support training on the table‐setting activity.
Clare 2010: The focus of the intervention was addressing personally meaningful goals; goals were collaboratively identified, and individualised interventions were developed. This was supported by the provision of practical aids and strategies, techniques for learning new information, practice in maintaining attention and concentration and techniques for stress management. Participants were encouraged to work on goals and practise strategies between intervention sessions, and caregivers were invited to participate in the final 15 minutes of each session to assist with between‐session implementation.
Length and duration of the interventions
A summary of the duration of interventions and the timing of assessments is shown in Table 4.
2. Summary of duration of interventions and timing of assessments.
| Study | Intervention length | Initial assessment | Interim assessment | Post‐interv assessment | Follow‐up assessments | Details of sessions | Format of sessions |
| Beck 1988 | 6 weeks | week 0 | n/a | week 6 | n/a | 18 × 30‐ to 40‐minute sessions | Individual |
| Heiss 1993 | 24 weeks | week 0 | weeks 8 and 16 (plus monthly physician appointments) | week 25 | n/a | 48 × 1‐hour sessions | Individual |
| Quayhagen 1995 | 12 weeks | week 0 | n/a | week 13 | week 38 | 72 × 1‐hour caregiver‐facilitated sessions | Individual |
| de Vreese 1998 | 12 weeks (after 12 weeks on drug) | weeks 0 and 13 | n/a | week 26 | n/a | 24 × 45‐minute sessions | Individual |
| Quayhagen 2000 | 8 weeks | week 0 | n/a | week 12 | n/a | 40 × 1‐hour caregiver‐facilitated sessions | Individual |
| Koltai 2001 | 5 to 6 weeks | weeks 0 to 2 | n/a | weeks 6 to 8 | n/a | 5 × 1‐hour sessions (group) or mean of 6 × 1‐hour sessions (group) | Group or individual |
| Davis 2001 | 5 weeks | week 0 | n/a | week 6 | week 12 (cross‐over) | 5 × 1‐hour sessions | Individual |
| Cahn‐Weiner 2003 | 6 weeks | week 0 | n/a | weeks 8 to 9 (mean 59 days post‐baseline) | week 16 (mean 114.5 days post‐baseline) | 6 × 45‐minute sessions | Group |
| Loewenstein 2004 | 12 to 16 weeks | week 0 | n/a | weeks 13 to 18 | weeks 25 to 31 | 24 × 45‐minute sessions | Individual |
| Galante 2007 | 4 weeks | week 0 | n/a | week 5 | 3, 6 & 9 months (MMSE only) post‐interventions | 12 × 60‐minute sessions 3 times per week | Individual |
| Neely 2009 | 8 weeks | week 0 | n/a | week 9 | n/a | 8 × 60‐minute sessions | Dyads or Individual |
| Clare 2010 | 8 weeks | week 0 | n/a | week 9 | 6 months | 8 × 60‐minute sessions | Individual |
Beck 1988: The intervention was delivered in one‐to‐one sessions lasting 30 to 40 minutes, held three times a week for six weeks.
Heiss 1993: Twice‐weekly, one‐hour individual sessions for 24 weeks.
Quayhagen 1995: One hour per day, 6 days per week, for 12 weeks, facilitated by caregiver, plus weekly session with member of research team.
de Vreese 1998: Twice‐weekly 45‐minute individual sessions for 12 weeks, supplemented by home practice.
Quayhagen 2000: One hour per day, 5 days per week, for 8 weeks, facilitated by caregiver, plus modelling of the intervention by member of the research team to assist the caregiver.
Davis 2001: Weekly individual one‐hour sessions for 5 weeks, supplemented by home practice, 30 minutes per day, 6 days per week. Koltai 2001: The group format consisted of five weekly, one‐hour sessions conducted in groups of four. The individual format consisted of a mean of six individual sessions. Caregivers joined the last 10 to 15 minutes of each session where available. As no differences were observed in results for group and individual training, the data were analysed together.
Cahn‐Weiner 2003: Weekly small‐group sessions lasting 45 minutes each, over a 6‐week period.
Loewenstein 2004: The interventions were delivered in 24 individual sessions, each lasting 45 minutes over a 12‐ to 16‐week period.
Galante 2007: The intervention was delivered individually, with each participant receiving twelve 60‐minute sessions, three times a week, over 4 weeks,
Neely 2009: Participants were offered a one‐hour session of home‐based training each week for a period of 8 weeks.
Clare 2010: Cognitive rehabilitation was delivered in eight weekly, 1‐hour individual sessions conducted in participants' homes.
Description of the comparison conditions
Beck 1988: Participants in the control condition received treatment as usual with no additional intervention.
Heiss 1993: The social support condition consisted of weekly, one‐hour individual sessions that included conversation about personal problems in managing daily life, as well as past experiences, sometimes assisted by games. It is not clear whether the sessions were carried out individually or in groups.
Quayhagen 1995: Placebo intervention consisted of similar types of caregiver‐facilitated exercises, offered for an equivalent length of time but designed to elicit only passive responses rather than active processing and engagement.
de Vreese 1998: Participants received AChEI medication alone for a 6‐month period.
Quayhagen 2000: Wait‐list control condition.
Davis 2001: 'Mock' intervention involved five weekly, one‐hour individual sessions comprising unstructured conversation, recitation of 'overlearned material' and watching of health‐related videos. Koltai 2001: Wait‐list control condition. Cahn‐Weiner 2003: The comparison condition was an educational group intervention in which didactic information about ageing and dementia was provided.
Loewenstein 2004: The mental stimulation intervention included individual sessions comprising computer games, exercises like 'hangman', word‐finding tasks and discussion of the 'topic of the day'. Galante 2007: Participants in the nonspecific treatment condition participated in a semi‐structured interview on current affairs and relevant events of their own life history. The neuropsychologist who conducted the sessions made use of audiovisual material and information received from participants and their relatives on the participant's life history, hobbies and favourite activities. The control condition was matched with the intervention group in terms of number, duration and frequency of sessions.
Neely 2009: Participant dyads in the control condition did not receive any intervention between the pretest and the post‐test.
Clare 2010: Participants in the 'no treatment' group had no contact with the research team between baseline and post‐intervention assessments.
Excluded studies
Characterisitcs of excluded studies are summarised in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias for individual studies, along with a justification for our ratings, is summarised in tables under the Characteristics of included studies section. Risk of bias for specific outcomes across studies is summarised in Figure 2 and Figure 3.
2.
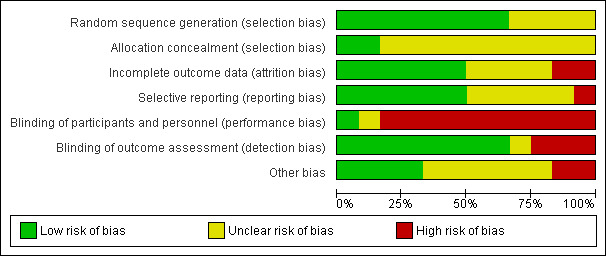
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most studies had a low risk of bias in relation to random sequence generation procedures, although lack of detail in four studies led to difficulty in evaluating whether random sequence generation was adequately performed. In addition, in all but two studies, insufficient detail was provided to ascertain whether concealment of the randomisation sequence was attempted.
Blinding
In most of the included studies (8/12), outcome assessments were conducted by individuals described as blind to participants' group allocation. However, most studies were classified as high risk in relation to blinding of participants and personnel. Although it is recognised that blinding of participants and personnel is difficult or impossible in trials of cognition‐based interventions, researchers can take steps to reduce the risk of bias due to lack of blinding of participants and research personnel (Higgins 2011).
Incomplete outcome data
Six studies (50%) were classified as low risk as the result of incomplete outcome data, two studies were classified as high risk and the remaining four were classified as unclear risk.
Selective reporting
Again, 6 of the 12 studies (50%) were classified as having low risk of bias as the result of selective reporting, one study was classified as having a high risk of bias and the remaining five as having unclear risk.
Effects of interventions
Cognitive training
The meta‐analysis revealed no differences between cognitive training and control conditions on any of the primary or secondary outcomes included in the analyses (Data and analyses). Table 1 shows the results for a selected number of central outcomes, and details of selected analyses of interest are shown in Figure 4, Figure 5 and Figure 6. As can be seen in the Table 1, longer‐term outcomes related to the trajectory of dementia (i.e. severity of dementia, rates of admission to residential care) have not been assessed in any of the included studies. Furthermore, evidence from cognitive training interventions to date has been found to be of low to moderate quality.
4.
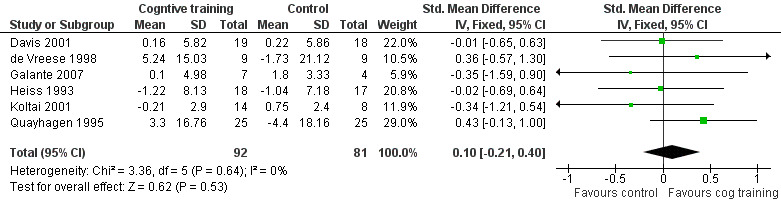
Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.1 A1.1 Change in a global measure of cognition.
5.
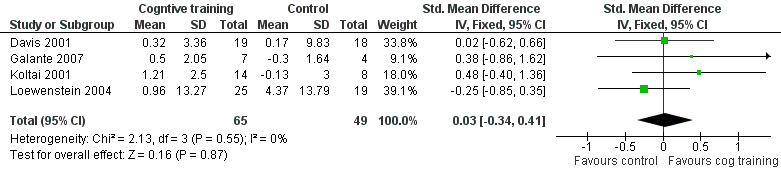
Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.13 B1.2 Change in participant's mood (self‐reported).
6.

Forest plot of comparison: 13 Cognitive training vs control in the short term (immediately post‐intervention) outcome: 13.15 B1.4 Change in participant's capacity for activities of daily living (Carer reported).
Cognitive rehabilitation
Because only a single trial of cognitive rehabilitation (Clare 2010) met criteria for inclusion in this review, no meta‐analysis could be conducted. Table 2 shows the results for a selected number of important outcomes in Clare 2010. As can be seen, cognitive rehabilitation was found to be superior to the control condition in the primary outcome of patient‐reported improvement in goal performance over the short term as measured by the Canadian Occupational Performance Measure (effect size Z = 2.11, P = 0.04). Cognitive rehabilitation was also found to be superior to the control condition in relation to outcomes not shown in Table 2, Specifically, participants in the cognitive rehabilitation group rated themselves as more satisfied with their ability to carry out meaningful activities of daily living compared with the control condition immediately following the intervention, and they were more satisfied with their memory performance six months after the intervention compared with the control group. A trend that approached significance suggested that six months after the intervention, participants in the cognitive rehabilitation group rated their overall quality of life as higher than that of participants in the control condition (Clare 2010). Evidence also indicated that caregivers of participants in the cognitive rehabilitation group had improved social relationships after the intervention relative to the control condition. Finally, in a subset of participants who underwent functional neuroimaging with fMRI while performing a learning task, cognitive rehabilitation was associated with an increase in brain activation relative to the control condition in the right fusiform face area-the right medial prefrontal cortex. In addition, although participants in the control condition showed lower brain activation after the intervention in the right parahippocampal cortex and in the right temporal parietal junction, no reduction in brain activity in these regions was noted for the group that underwent cognitive rehabilitation (Van Paasschen 2013). As can be seen in the Characteristics of included studies and Table 2, the evidence from Clare 2010 was generally regarded as of high quality. However, because the evidence comes from a single study carried out in one setting with a limited sample, the overall quality of evidence in relation to cognitive rehabilitation is best described as moderate.
Discussion
Summary of main results
The aim of this updated review was to evaluate current evidence regarding the efficacy of cognitive training and cognitive rehabilitation interventions for people with mild AD or vascular dementia. Eleven studies of cognitive training were identified for inclusion in the review (nine of which were included in the previous version of this review), and meta‐analysis could be conducted on 8 primary and 6 secondary outcomes in the short term, and on 2 primary and 2 secondary outcome measures in the medium term. No positive or adverse effects of cognitive training were detected in the meta‐analysis. The finding of no adverse effects of cognitive training is relevant in light of proposals from previous commentators (e.g. Small 1997) that cognitive training may have a negative impact, particularly on mood.
Only one RCT of individualised cognitive rehabilitation was identified (Clare 2010). Hence, no meta‐analysis could be conducted. Howevever, the results of this single, high‐quality trial are positive, indicating that cognitive rehabilitation is likely to provide some benefit for patients in the short term and in the medium term related to self‐rated competence and satisfaction in performing meaningful personal goals, memory capacity and general quality of life.
Overall completeness and applicability of evidence
Number of publications meeting inclusion criteria
Since the publication of the previous version of this review (Clare 2008), only two additional RCTs of cognitive training in participants with AD or vascular dementia were published that met the review criteria (Galante 2007; Neely 2009). In addition, only a single study met our inclusion criteria for individual cognitive rehabilitation (Clare 2010). Several factors appear to account for the small number of new studies that met criteria for the present review. First, insufficient methodological quality, namely, the absence of randomisation, led to several published trials (e.g. Bentwich 2011; Hwang 2012) not being included in the review. Second, several RCTs of cognition‐based interventions did not meet our definitions of cognitive training and cognitive rehabilitation, or they described multi‐component interventions (e.g. Graff 2006; Kurz 2012). Issues related to the inclusion criteria used in the current review are further discussed later. A third factor that may have contributed to inclusion of a smaller number of relevant studies in the literature is likely to be associated with the widely held belief that interventions, pharmacological and non‐pharmacological alike, have the greatest chance of success when applied in the earliest possible stage of AD or vascular dementia. Hence, in recent years studies have increasingly targeted individuals who do not meet criteria for dementia, but who nevertheless show significant cognitive decline-such as persons with amnestic mild cognitive impairment (MCI) (Albert 2011; Gauthier 2006). Indeed, many of the records that were retrieved in the updated literature search now exclusively focus on individuals with MCI, and separate reviews focusing on individuals with MCI are now available (Jean 2010Martin 2011).
Issues related to the inclusion of RCTs only
The original protocol for the current review (Clare, Woods, Moniz‐Cook et al 2001) stated that only RCTs would be included in the review. RCTs have been long regarded as the highest forms of evidence in medical research because of the lower risk of bias associated with them. However, most of the studies of cognitive training included in the present review have been rated as having substantial risk of bias in several domains, and the quality of evidence has been found to be low to moderate. Low‐quality RCTs can in principle be associated with a greater threat to internal validity of the study than high‐quality non‐randomised trials and even well conducted single‐case studies. Hence, although more recent studies are generally of a higher methodological quality, this trend is likely to continue, it might be justifiable to include, under strict conditions, high‐quality non‐randomised trials and single‐case studies in future versions of this review to increase the evidence base from which conclusions can be drawn. Several possible advantages are derived by including high‐quality non‐randomised trials in a systematic review, and pooled estimates of effect sizes from randomised and non‐randomised trials can be analysed separately (Reeves 2011).
Issues related to definitions of interventions and multi‐component interventions
Despite progress in the application of a clearer and more consistent terminology in referring to various cognition‐based interventions in mild dementia, interventions often continue to be inaccurately labelled. Specifically, studies continue to be published in which interventions are described as cognitive training or as cognitive rehabilitation, when in fact they appear to more closely reflect cognitive stimulation or reality orientation (e.g. Giordano 2010). This state of affairs means that, in reviewing the available literature and in choosing studies to include in the review, it was generally insufficient to examine the title used in the publication, and in many cases, the Methods section of a published trial had to be closely scrutinised to clarify whether the intervention actually provided was consistent with the one suggested by the title.
In addition, the present review excluded trials in which an intervention was described as a combination of elements from various approaches-such as cognitive behaviour therapy combined with elements of cognition‐focused intervention (e.g. Kurz 2012). This decision is related to the fact that different techniques are likely to have different mechanisms of action, and that it is generally not possible with such interventions to isolate the contributions of different components to the measured outcomes. The definitions of cognition‐based interventions provided in this review essentially reflect groups of intervention techniques that tend to go together, but some overlap has been noted in the techniques used in cognitive stimulation, training and rehabilitation (e.g. psychoeducation may be a component of each of these approaches). Because each of these broad approaches to intervention is likely to involve the use of more than one intervention technique with different mechanisms of action (e.g. setting goals, discovering effective ways to learn new information, using repeated practice), these approaches can also be regarded as essentially ‘multi‐component’ interventions. Additional work is required to better characterise the essential or core components of each of the broad approaches to intervention. It is possible that inclusion of studies based on their use of discrete intervention techniques (e.g. goal‐setting, practice of structured tasks, use of specific learning strategies such as errorless learning) rather than on whether they neatly fit into the definitions offered here might prove more informative.
Outcomes measured in included studies
A further issue influencing the completeness and applicability of the evidence is the range of outcomes reported in the included studies. Trials, particularly studies of cognitive training, have traditionally measured mainly cognitive outcomes in the form of performance on standardised cognitive measures. Very few studies have measured non‐cognitive outcomes for the person with dementia or for the primary caregiver (e.g. mood, quality of life, general health and wellbeing) or longer‐term outcomes that are likely to be of critical importance to policy‐makers, such as those related to the course of dementia, for example, dementia severity and rates of admission to residential care. Although obvious methodological constraints are applied to the measurement of longer‐term outcomes, such as admission to residential care, it is nonetheless important that future trials of cognition‐based interventions, particularly those found to be effective, routinely measure and report outcomes other than direct cognitive ones, and that attempts are made to capture outcomes related to the future trajectory of dementia. Given the nature and aims of individualised cognitive rehabilitation interventions, these approaches tend to emphasize individualised goals and activities of daily living over performance on standardised cognitive tests. Indeed, the single trial of cognitive rehabilitation included in the present review measured and reported several important outcomes other than cognitive outcomes that are of direct clinical relevance.
Methodological limitations of included studies
The lack of significant effects achieved in cognitive training studies must be interpreted in the context of methodological limitations that may have constrained the possibility of demonstrating significant gains, including issues related to power, choice of control condition and choice of outcome measures along with the impact of individual characteristics that may moderate treatment response.
Power to detect effects: Many of the included trials are likely to have suffered from limited statistical power to detect effects. Lack of power of individual studies to detect effects is commonly associated with small sample sizes, which is a frequent limitation in cognition‐based interventions for people with mild AD and vascular dementia. However, this explanation is unlikely to account for the lack of significant findings, as a meta‐analysis is designed to overcome limitations derived from individual studies associated with such factors as sample size. Indeed, not only was the size of the effects in individual studies small, but, and this is possibly of greater relevance, the direction of effects associated with some outcomes did not consistently favour cognitive training over the control condition. For example, in three out of five studies that reported the impact of cognitive training on a global measure of cognition in the short term, the direction of the effect favoured the control group, whereas in only one of the trials did the effect clearly favour the cognitive training condition (Figure 4). Indeed, such inconsistency in the direction of effects was found to be the case for a substantial number of outcomes reported by the studies, even when the same measures were used by different studies to evaluate a given outcome. Other factors that might contribute to the difficulty involved in detecting significant effects are difficulties in determining the right ‘dose’ of an intervention (i.e. frequency, intensity and duration of interventions), the presence of 'ceiling' or 'floor' effects, rendering it impossible to demonstrate improvements in a given domain, and baseline differences between treatment and control groups.
Choice of control condition: The difficulty of defining what constitutes an appropriate comparison condition is particularly important because in some studies (e.g. Cahn‐Weiner 2003; Loewenstein 2004) cognitive training may have been compared with other active treatments, thus masking potentially beneficial effects. Clinical practice requires the ability to distinguish which of a range of possible psychosocial interventions is most likely to be useful for a given individual, and the study designs used here do not allow this question to be addressed.
Use of neuropsychological tests as cognitive outcomes: The use of neuropsychological tests to measure cognitive outcomes effectively means that what is actually being assessed is transfer of benefit from trained to untrained tasks, rather than the effects of training on trained tasks. However, as was discussed in the introduction, very limited evidence has been found to support such transfer effects from trained to untrained tasks. When the trained tasks are analogous in some way to daily activities, however, improvement in such tasks may have direct relevance to daily functioning, but this would be missed if these benefits were not transferred to performance on standardised neuropsychological tests. For example, Davis 2001 noted improvement on tasks during training, such as recall of personal information and face‐name associations, but this was not captured by the neuropsychological measures selected to assess cognitive outcomes. A further problem with the use of standardised neuropsychological tests before and after the intervention to measure cognitive outcomes involves the potential for practice effects that may obscure possible effects of specific treatments. Finally, in some studies, more than one neuropsychological test or self‐report scale is used to measure the same outcome (e.g. executive function, general wellbeing). This leads to difficulties in choosing which is the most appropriate or relevant measure of the outcome under consideration for inclusion in the meta‐analysis.
Moderating role of patient characteristics in intervention outcomes: Investigators have learned that various patient characteristics have the potential to moderate response to the intervention, and as more evidence becomes available regarding important moderators, cognition‐focused interventions might be better able to control for the effects of such moderators. For example, Koltai 2001 retrospectively classified participants' level of awareness of their own impairments and found that a higher level of awareness was a predictor of a more successful outcome-a finding that has also been demonstrated in a prospective study of cognitive rehabilitation outcomes for a small group of people with mild AD (Clare 2004). The moderating impact of factors such as awareness of deficits or the presence of neuropsychiatric symptoms (e.g. apathy) may strengthen the rationale for inclusion of high‐quality single‐case studies in future versions of this review.
Study context: Non‐pharmacological studies are more likely than drug trials to be affected by the study context, including the healthcare setting, as well as by cultural and linguistic factors. Given that the studies reviewed have taken place in a variety of contexts, one cannot exclude the possibility that cognition‐based interventions are better suited for some contexts than others.
Quality of the evidence
As has been discussed, the generally low methodological quality of trials continues to limit the ability of researchers to evaluate the evidence base. The quality of most of the studies of cognitive training interventions included in the review was often compromised by significant risks of bias-particularly as a result of insufficient detail regarding the method used to generate a random group allocation sequence, concealment of this sequence from relevant members of the research team and attempts to blind participants, researchers or both to group allocation. Hence, the finding of no significant benefit (or harm) from cognitive training interventions needs to be interpreted with caution, and the estimate of effect sizes may vary in the future as the evidence comes from studies of greater quality. However, the methodological quality of trials is gradually improving, and this trend is expected to continue in coming years. Indeed, although only a single study of individualised cognitive rehabilitation was identified, this study was rated to be of high methodological quality and hence to have lower risk of bias-permitting the drawing of positive conclusions regarding the efficacy of this approach. Although effect estimates that are based on high‐quality evidence are generally regarded as estimates that are unlikely to change significantly with the publication of further studies, confidence in the positive outcomes of cognitive rehabilitation will nevertheless increase as evidence accumulates from further high‐quality, preferably multi‐site, trials of cognitive rehabilitation with larger sample sizes.
Agreements and disagreements with other studies or reviews
In recent years, two main systematic reviews that included an examination of the efficacy of cognitive training for people with mild dementia have been published. In reviewing the literature to 2004, Sitzer 2006 concluded that “cognitive training evidenced promise in the treatment of AD, with primarily medium effect sizes for learning, memory, executive functions, activities of daily living, general cognitive problems, depression, and self‐rated general functioning”. Closer examination of the methodology described in Sitzer 2006 reveals important differences that explain the inconsistency in the results of the current review. First, Sitzer 2006 applied inclusion criteria that were much less strict and included both randomised and non‐randomised trials (total 19), as well as studies that included participants with moderate to severe AD. Second, although Sitzer 2006 described their review as a review of cognitive training, of the 14 RCTs that met their inclusion criteria, 6 were in fact studies of other cognition‐based interventions (primarily reality orientation/cognitive stimulation) or multi‐component interventions. Indeed, in separate analyses, performed only on the five “high‐quality trials” (all of which were included in the current review)-the observed effects were very small and non‐significant. It is quite plausible that if studies of cognitive stimulation, training and rehabilitation for people with mild AD or vascular dementia were assessed together, some benefits would have been detected. However, given the important differences among the various cognition‐focused approaches to intervention, these should be treated separately. Indeed, although the current review did not identify any benefits associated with cognitive training, the results of a single, high‐quality trial of cognitive rehabilitation tentatively suggest that this approach may be associated with important benefits for the person with dementia and the primary caregiver. In addition, a separate Cochrane review of cognitive stimulation for mild AD has recently confirmed that this approach was associated with several positive outcomes for the person with dementia (Woods 2012).
More recently, Olazaran 2010 reviewed the general literature on the efficacy of 26 categories of non‐pharmacological interventions for people with dementia. In relation to cognitive training, these authors concluded that a Grade B recommendation (recommendation associated with low‐quality RCTs) can be given for the efficacy of individual and group cognitive training in improving cognitive functions. No effects of cognitive training on other outcomes were found. This conclusion seems to be different from that of the current review, and important differences are evident between this review and that of Olazaran 2010. Specifically, Olazaran 2010 included in their review participants with any kind of dementia, and in fact allowed for inclusion of a small proportion of participants with cognitive decline but without confirmed dementia. In addition, like Sitzer 2006, Olazaran 2010 used less strict inclusion criteria, leading to inclusion of several low‐quality studies. Finally, rather than examining different cognitive domains separately, Olazaran 2010 analysed cognition broadly, and studies contributed diverse measures of cognition to the evaluation of this outcome, whereas cognitive outcomes in the current study were evaluated separately against widely agreed cognitive domains. These methodological differences are most likely to account for the differences between the current review and the review by Olazaran 2010.
Authors' conclusions
Implications for practice.
Cognitive training: The review does not provide evidence to support the efficacy of cognitive training. Trial reports indicate that some gains resulting from intervention may not be captured adequately by available standardised outcome measures.
Cognitive rehabilitation: Data from a single, high‐quality trial provide preliminary positive results regarding the use of contextualised individual cognitive rehabilitation, emphasising collaborative goal‐setting to achieve better self‐rated competence and satisfaction with personally meaningful activities of daily living. Risk of harm or adverse effects of cognitive rehabilitation are unlikely. Given that the evidence to date comes from a single trial, the overall quality of evidence is best described as moderate. The capacity to make firmer treatment recommendations awaits the publication of additional trials of individual cognitive rehabilitation.
Implications for research.
Cognitive training: Further well‐designed single‐blind RCTs of cognitive training would help to provide more definitive evidence regarding efficacy. Future research would benefit from consideration of how to capture changes that are currently missed by the available standardised outcome measures, from development of greater consensus in the selection of specific outcome measures and from identification of the extent to which gains are clinically relevant and generalisable, and have the potential to make a difference for the person with dementia and the family caregiver in everyday life. Future research should continue the trend towards devising interventions that include personalised tasks or tasks based on analogues of daily activities. Future research also needs to consider outcomes beyond direct cognitive ones, to describe in greater detail the elements of the intervention used (preferably using manualised protocols) and to more accurately use existing classifications of cognition‐based interventions.
Cognitive rehabilitation: Additional RCTs of individualised cognitive rehabilitation are needed to provide further support for tentatively promising results.
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2013 | New citation required but conclusions have not changed | New lead author; conclusions unchanged |
| 2 November 2012 | New search has been performed | A pre‐publication search was performed for this review on 2 November 2012 |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 11 December 2011 | New citation required but conclusions have not changed | A search was conducted on December 11 2011 identifying several new references for assessment. |
| 28 January 2009 | New search has been performed | Update searches run on 7 January 2009; a number of results were sent to the authors for assessment |
| 5 June 2008 | Amended | Converted to new review format. |
| 14 February 2007 | New search has been performed | February 2007: 3 new studies were added (Cahn Weiner 2003, Loewenstein 2004, Beck 1988); analyses were completely re‐done; minor changes were made to background, method and discussion sections. The conclusions of the review have remained the same. This is a minor update. |
| 22 August 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
For the current version: We are grateful for the support of the Cochrane Dementia and Cognitive Improvement Group, particularly Ms. Anna Noel‐Storr for conducting the literature searches and for updating relevant search results sections of the manuscript. Alex Bahar‐Fuchs is grateful for the support of the Dementia Collaborative Research Centre-Early Detection and Prevention, especially Professor Kaarin Anstey and Dr Nicolas Cherbuin.
For previous versions: The review authors would like to thank Dr David Loewenstein and Dr Luc de Vreese for supplying additional information and data, and Dr Deborah Koltai‐Attix, Professor Cornelia Beck, Professor Dr Ruediger Mielke and Dr Mary Quayhagen for their helpful responses to queries. Christine Derrick, Patricia Hewitt and Christine Stockwell acted as consumer editors for the original version of the review (Clare 2003), and we were initially put in contact with them by Dr Julia Cream of the UK Alzheimer's Society. We are grateful to Dymphna Hermans for conducting literature searches and assisting with the progress of the review, and to Jacqueline Birks for statistical advice.
Appendices
Appendix 1. Pre‐publication search: November 2012
|
Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Keyword search: "cognitive rehabilitation" OR "cognitive stimulation" OR "cognitive training" | 113 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. exp Dementia/ 2. Delirium, Dementia, Amnestic, Cognitive Disorders/ 3. dement*.mp. 4. alzheimer*.mp. 5. (lewy* adj2 bod*).mp. 6. (chronic adj2 cerebrovascular).mp. 7. ("organic brain disease" or "organic brain syndrome").mp. 8. (cerebr* adj2 deteriorat*).mp. 9. (cerebral* adj2 insufficient*).mp. 10. (pick* adj2 disease).mp. 11. or/1‐10 12. *Cognitive Therapy/ 13. (cognit* adj2 stimulation).ti,ab. 14. (cognit* adj2 rehabilitation).ti,ab. 15. (cognit* adj2 training).ti,ab. 16. (cognit* adj2 retrain*).ti,ab. 17. "cognitive support".ti,ab. 18. "memory function*".ti,ab. 19. (memory adj2 rehabilitation).ti,ab. 20. (memory adj2 therap*).ti,ab. 21. "memory aid*".ti,ab. 22. "memory group*".ti,ab. 23. "memory training".ti,ab. 24. ("memory retraining" or "memory re‐training").ti,ab. 25. "memory support".ti,ab. 26. "memory stimulation".ti,ab. 27. "memory strateg*".ti,ab. 28. "memory management".ti,ab. 29. or/12‐28 30. 11 and 29 31. randomized controlled trial.pt. 32. controlled clinical trial.pt. 33. randomized.ab. 34. placebo.ab. 35. randomly.ab. 36. trial.ab. 37. groups.ab. 38. or/31‐37 39. (animals not (humans and animals)).sh. 40. 38 not 39 41. 30 and 40 42. (201111* or 201112*).ed. 43. 2012*.ed. 44. 42 or 43 45. 41 and 44 |
53 |
| 3. EMBASE 1980‐2011 week 39 (Ovid SP) |
1. exp dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (lewy* adj2 bod*).mp. 5. (chronic adj2 cerebrovascular).mp. 6. ("organic brain disease" or "organic brain syndrome").mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. CADASIL.mp. 10. or/1‐9 11. (cognit* adj2 stimulation).ti,ab. 12. (cognit* adj2 rehabilitation).ti,ab. 13. (cognit* adj2 training).ti,ab. 14. (cognit* adj2 retrain*).ti,ab. 15. "cognitive support".ti,ab. 16. (memory adj2 rehabilitation).ti,ab. 17. (memory adj2 therap*).ti,ab. 18. "memory aid*".ti,ab. 19. "memory group*".ti,ab. 20. "memory training".ti,ab. 21. ("memory retraining" or "memory re‐training").ti,ab. 22. "memory support".ti,ab. 23. "memory stimulation".ti,ab. 24. "memory strateg*".ti,ab. 25. "memory management".ti,ab. 26. or/11‐25 27. 10 and 26 28. randomly.ab. 29. placebo*.ti,ab. 30. "double‐blind*".ti,ab. 31. randomized controlled trial/ 32. trial.ti,ab. 33. or/28‐32 34. 27 and 33 35. (2011* or 2012*).em. 36. 34 and 35 |
52 |
| 4. PSYCINFO 1806‐October week 5 2011 (Ovid SP) |
1. exp Dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (chronic adj2 cerebrovascular).mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. (cerebr* adj2 deteriorat*).mp. 7. (cerebral* adj2 insufficient*).mp. 8. or/1‐7 9. (cognit* adj2 stimulation).ti,ab. 10. (cognit* adj2 rehabilitation).ti,ab. 11. (cognit* adj2 training).ti,ab. 12. (cognit* adj2 retrain*).ti,ab. 13. "cognitive support".ti,ab. 14. (memory adj2 rehabilitation).ti,ab. 15. (memory adj2 therap*).ti,ab. 16. "memory aid*".ti,ab. 17. "memory group*".ti,ab. 18. "memory training".ti,ab. 19. ("memory retraining" or "memory re‐training").ti,ab. 20. "memory support".ti,ab. 21. "memory stimulation".ti,ab. 22. "memory strateg*".ti,ab. 23. "memory management".ti,ab. 24. or/9‐23 25. 8 and 24 26. randomly.ab. 27. randomi?ed.ab. 28. placebo*.ti,ab. 29. trial.ti,ab. 30. RCT.ti,ab. 31. groups.ab. 32. or/26‐31 33. 25 and 32 34. (2011* or 2012*).up. 35. 33 and 34 |
41 |
| 5. CINAHL (EBSCOhost) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 (MH "Rehabilitation, Cognitive") S21 TX (cognit* rehab*) S22 TX (cognit* train*) S23 TX (memory train*) S24 TX (memory support*) S25 TX (memory stimul*) S26 S20 or S21 or S22 or S23 or S24 or S25 S27 S19 and S26 S28 EM 2011 S29 EM 2012 S30 S28 or S29 S31 S27 and S30 |
67 |
| 6. Web of Science (1945‐present) and conference proceedings via Web of Knowledge | Topic=(dement* OR VCI OR "vascular cognitive impairment*" OR VaD OR alzheimer*) AND Topic=("cognit* train*" OR "cognit* rehab*" OR "memory aid*" OR "memory train*" OR "memory support*" OR "memory stimul*") AND Topic=(randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised) AND Year Published=(2011‐2012) Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH. Lemmatization=On |
86 |
| 7. LILACS (BIREME) | demenc$ OR dement$ OR alzheimer$ [Words] and memory [Words] and randomly OR randomised OR randomized OR trial OR ensaio clínico [Words] | 10 |
| 8. CENTRAL (The Cochrane Library) (Issue 2 of 4, 2011) | #1 MeSH descriptor Dementia explode all trees #2 dement* #3 alzheimer* #4 "chronic cerebrovascular" #5 "organic brain disease" or "organic brain syndrome" #6 "benign senescent forgetfulness" #7 "cerebr* deteriorat*" #8 "cerebral* insufficient*" #9 "pick* disease" #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 "cognit* rehab*" #12 "cognit* train*" #13 "cognit* stimul*" #14 "memory train*" #15 "memory support*" OR "memory aid*" #16 "memory therap*" #17 "memory group*" #18 "memory stimul*" OR "memory strateg*" #19 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 (#10 AND #19) #21 #20 AND (2011 OR 2012) |
9 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | dementia OR alzheimer OR alzheimers OR VCI OR vascular dementia OR VaD OR vascular cognitive impairment OR cadasil OR multi‐infarct OR binswanger | cognitive rehabilitaion OR cognitive training OR memory | Senior |
183 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry-India; Clinical Research Information Service-Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | Interventional Studies | dementia OR Alzheimer OR vascular impairment OR VCI OR Alzheimers | cognitive rehabilitaion OR cognitive training OR memory | received from 01/11/2011 to 02/11/2012 | 19 |
| TOTAL before de‐duplication | 633 | |
| TOTAL after de‐dupe and first‐assess | 123 | |
Appendix 2. Update search: December 2011
|
Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | ("cognitive training" OR "cognitive rehabilitation" OR "memory training") AND (dementia OR alzheimer) AND (2009 OR 2010 OR 2011) | 129 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. exp Dementia/ 2. Delirium, Dementia, Amnestic, Cognitive Disorders/ 3. dement*.mp. 4. alzheimer*.mp. 5. (lewy* adj2 bod*).mp. 6. (chronic adj2 cerebrovascular).mp. 7. ("organic brain disease" or "organic brain syndrome").mp. 8. (cerebr* adj2 deteriorat*).mp. 9. (cerebral* adj2 insufficient*).mp. 10. (pick* adj2 disease).mp. 11. or/1‐10 12. *Cognitive Therapy/ 13. (cognit* adj2 stimulation).ti,ab. 14. (cognit* adj2 rehabilitation).ti,ab. 15. (cognit* adj2 training).ti,ab. 16. (cognit* adj2 retrain*).ti,ab. 17. "cognitive support".ti,ab. 18. "memory function*".ti,ab. 19. (memory adj2 rehabilitation).ti,ab. 20. (memory adj2 therap*).ti,ab. 21. "memory aid*".ti,ab. 22. "memory group*".ti,ab. 23. "memory training".ti,ab. 24. ("memory retraining" or "memory re‐training").ti,ab. 25. "memory support".ti,ab. 26. "memory stimulation".ti,ab. 27. "memory strateg*".ti,ab. 28. "memory management".ti,ab. 29. or/12‐28 30. 11 and 29 31. randomized controlled trial.pt. 32. controlled clinical trial.pt. 33. randomized.ab. 34. placebo.ab. 35. randomly.ab. 36. trial.ab. 37. groups.ab. 38. or/31‐37 39. (animals not (humans and animals)).sh. 40. 38 not 39 41. 30 and 40 42. (2009* or 2010* or 2011*).ed. 43. 41 and 42 |
110 |
| 3. EMBASE 1980‐2011 week 49 (Ovid SP) |
1. exp dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (lewy* adj2 bod*).mp. 5. (chronic adj2 cerebrovascular).mp. 6. ("organic brain disease" or "organic brain syndrome").mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. CADASIL.mp. 10. or/1‐9 11. (cognit* adj2 stimulation).ti,ab. 12. (cognit* adj2 rehabilitation).ti,ab. 13. (cognit* adj2 training).ti,ab. 14. (cognit* adj2 retrain*).ti,ab. 15. "cognitive support".ti,ab. 16. (memory adj2 rehabilitation).ti,ab. 17. (memory adj2 therap*).ti,ab. 18. "memory aid*".ti,ab. 19. "memory group*".ti,ab. 20. "memory training".ti,ab. 21. ("memory retraining" or "memory re‐training").ti,ab. 22. "memory support".ti,ab. 23. "memory stimulation".ti,ab. 24. "memory strateg*".ti,ab. 25. "memory management".ti,ab. 26. or/11‐25 27. 10 and 26 28. randomly.ab. 29. placebo*.ti,ab. 30. "double‐blind*".ti,ab. 31. randomized controlled trial/ 32. trial.ti,ab. 33. or/28‐32 34. 27 and 33 35. (2009* or 2010* or 2011*).em. 36. 34 and 35 |
63 |
| 4. PsycINFO 1806‐December week 2 2011 (Ovid SP) |
1. exp Dementia/ 2. dement*.mp. 3. alzheimer*.mp. 4. (chronic adj2 cerebrovascular).mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. (cerebr* adj2 deteriorat*).mp. 7. (cerebral* adj2 insufficient*).mp. 8. or/1‐7 9. (cognit* adj2 stimulation).ti,ab. 10. (cognit* adj2 rehabilitation).ti,ab. 11. (cognit* adj2 training).ti,ab. 12. (cognit* adj2 retrain*).ti,ab. 13. "cognitive support".ti,ab. 14. (memory adj2 rehabilitation).ti,ab. 15. (memory adj2 therap*).ti,ab. 16. "memory aid*".ti,ab. 17. "memory group*".ti,ab. 18. "memory training".ti,ab. 19. ("memory retraining" or "memory re‐training").ti,ab. 20. "memory support".ti,ab. 21. "memory stimulation".ti,ab. 22. "memory strateg*".ti,ab. 23. "memory management".ti,ab. 24. or/9‐23 25. 8 and 24 26. randomly.ab. 27. randomi?ed.ab. 28. placebo*.ti,ab. 29. trial.ti,ab. 30. RCT.ti,ab. 31. groups.ab. 32. or/26‐31 33. 25 and 32 34. (2009* or 2010* or 2011*).up. 35. 33 and 34 |
48 |
| 5. CINAHL (EBSCOhost) | ||
| 6. ISI Web of Knowledge-all databases [includes Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] | Topic=(dement* OR VCI OR "vascular cognitive impairment*" OR VaD OR alzheimer*) AND Topic=("cognit* train*" OR "cognit* rehab*" OR "memory aid*" OR "memory train*" OR "memory support*" OR "memory stimul*") AND Topic=(randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised) AND Year Published=(2009‐2011) Timespan=2009‐2011. |
88 |
| 7. LILACS (BIREME) | memory [Words] and demenc$ OR dement$ OR alzheimer$ [Words] and randomly OR randomised OR randomized OR trial OR ensaio clínico [Words] | |
| 8. CENTRAL (The Cochrane Library) (Issue 4 of 4, Oct 2010) | #1 MeSH descriptor Dementia explode all trees #2 dement* #3 alzheimer* #4 "chronic cerebrovascular" #5 "organic brain disease" or "organic brain syndrome" #6 "benign senescent forgetfulness" #7 "cerebr* deteriorat*" #8 "cerebral* insufficient*" #9 "pick* disease" #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 "cognit* rehab*" #12 "cognit* train*" #13 "cognit* stimul*" #14 "memory train*" #15 "memory support*" OR "memory aid*" #16 "memory therap*" #17 "memory group*" #18 "memory stimul*" OR "memory strateg*" #19 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 (#10 AND #19) |
|
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | dementia | cognitive rehabilitaion OR cognitive training | Senior | received from 01/01/2009 to 12/14/2011 | 23 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry-India; Clinical Research Information Service-Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | Interventional Studies | dementia | cognitive rehabilitaion OR cognitive training | Senior | received from 01/01/2009 to 14/12/2011 | 18 |
| TOTAL before de‐duplication | 489 | |
| TOTAL after de‐dupe | 259 | |
Appendix 3. Update search: January 2006 to January 2009
| Source | Date Searched | Hits Retrieved |
| MEDLINE (PubMed) | January 7 | 27 |
| EMBASE (Ovid SP) | January 8 | 32 |
| PsycINFO (Ovid SP) | January 8 | 8 |
| CINAHL (Ovid SP) | January 8 | 7 |
| LILACS (bireme) | January 8 | 0 |
| CDCIG SR* | January 7 | 42 |
| CENTRAL (The Cochrane Library) | Issue 4 2008 | 48 |
| ISTP Conference Proceedings http://portal.isiknowledge.com/portal.cgi | January 8 | 32 |
| Australian Digital Theses Programme http://adt.caul.edu.au/ |
January 12 | 0 |
| Canadian Theses and Dissertations http://www.collectionscanada.ca/thesescanada/index‐e.html |
January 12 | 0 |
| WHO trials register | January 12 | 8 |
| Current Controlled trials: Meta Register of Controlled Trials (mRCT) http://www.controlled‐trials.com/ |
January 11 | 9 |
| ISRCTN Register |
January 11 | // |
| Nederlands Trial Register http://www.trialregister.nl/trialreg/index.asp | January 12 | 0 |
| ClinicalTrials.gov http://www.ClinicalTrials.gov |
Included in WHO portal | // |
| IPFMA Clinical Trials Register www.ifpma.org/clinicaltrials.html |
January 12 | 0 |
| UMIN Japan Trial Register http://www.umin.ac.jp/ctr/ |
January 12 | 2 |
| OPENsigle | January 12 | 2 |
Data and analyses
Comparison 13. Cognitive training vs. control in the short‐term (immediately post‐intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 A1.1 Change in a global measure of cognition | 6 | 173 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.21, 0.40] |
| 2 A1.2 Change in orientation | 2 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.13, 0.76] |
| 3 A1.3 Change in cognitive ability (self‐reported) | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.24, 0.74] |
| 4 A1.4 Change in cognitive ability (carer reported) | 3 | 100 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.17, 0.63] |
| 5 A2.1 Change in immediate verbal memory scores | 9 | 259 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.13, 0.37] |
| 6 A2.2 Change in delayed verbal memory scores | 3 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.27, 0.51] |
| 7 A2.3 Change in verbal memory recognition scores | 2 | 69 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.22, 0.73] |
| 8 A2.4 Change in executive function (sequencing) scores | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 7.47 [‐14.19, 29.14] |
| 8.1 Change in scores on Trails A | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 14.53 [‐9.35, 38.41] |
| 8.2 Change on Trails B | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐25.26 [‐76.70, 26.19] |
| 9 A2.5 Change in verbal letter fluency scores | 3 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.46, 0.42] |
| 10 A2.6 Change in verbal category fluency scores | 4 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.28, 0.42] |
| 11 A2.7 Change in attention and working memory scores | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.64, 0.72] |
| 12 B1.1 Change in participant's capacity for activities of daily living (self‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 B1.2 Change in participant's mood (self‐reported) | 4 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.34, 0.41] |
| 14 B1.3 Change in participant's general quality of life (self‐report) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 B1.4 Change in participant's capacity for activities of daily living (Carer reported) | 4 | 107 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.38, 0.38] |
| 16 B1.5 Change in participant's mood (carer reported) | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.38, 0.61] |
| 17 B1.6 Change in participant's general quality of life (carer‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 C1.1 Change in rates of admission to residential care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 C1.2 Change in measures of dementia severity | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 D1.1 Change in self‐reported mood (carer) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 D1.2 Change in self‐reported burden of care | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐9.67, 7.34] |
| 22 D1.3 Change in self‐reported overall wellbeing and quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 E1.1 Effect of cognitive training on biomarker evidence of brain function | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐3.67, 1.79] |
| 23.1 Change in glucose metabolism at rest (FDG PET) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐3.67, 1.79] |
| 23.2 Effects on glucose metabolism at activation (FDG PET task) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 E1.2 Effect of cognitive training on biomarker measures of neuropathology | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.1. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 1 A1.1 Change in a global measure of cognition.
13.2. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 2 A1.2 Change in orientation.
13.3. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 3 A1.3 Change in cognitive ability (self‐reported).
13.4. Analysis.
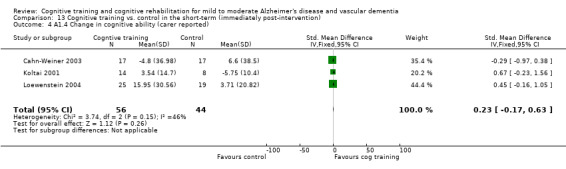
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 4 A1.4 Change in cognitive ability (carer reported).
13.5. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 5 A2.1 Change in immediate verbal memory scores.
13.6. Analysis.
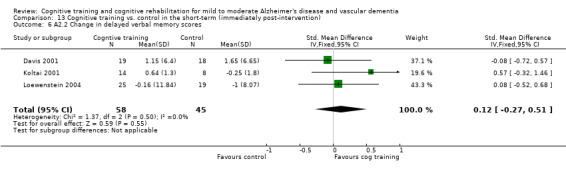
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 6 A2.2 Change in delayed verbal memory scores.
13.7. Analysis.
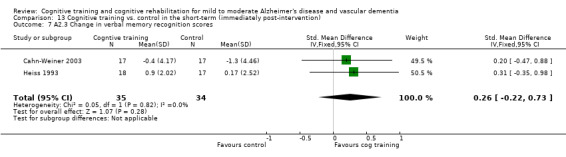
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 7 A2.3 Change in verbal memory recognition scores.
13.8. Analysis.
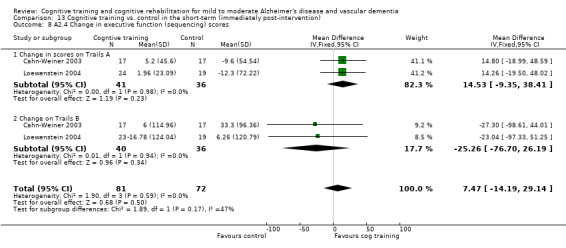
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 8 A2.4 Change in executive function (sequencing) scores.
13.9. Analysis.
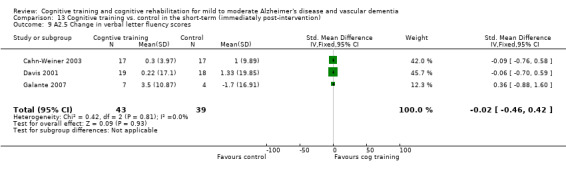
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 9 A2.5 Change in verbal letter fluency scores.
13.10. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 10 A2.6 Change in verbal category fluency scores.
13.11. Analysis.
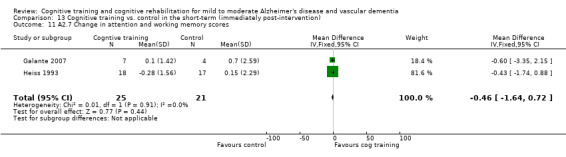
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 11 A2.7 Change in attention and working memory scores.
13.13. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 13 B1.2 Change in participant's mood (self‐reported).
13.15. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 15 B1.4 Change in participant's capacity for activities of daily living (Carer reported).
13.16. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 16 B1.5 Change in participant's mood (carer reported).
13.21. Analysis.

Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 21 D1.2 Change in self‐reported burden of care.
13.23. Analysis.
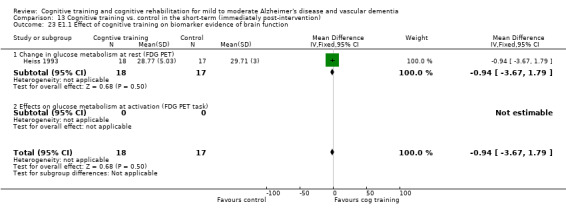
Comparison 13 Cognitive training vs. control in the short‐term (immediately post‐intervention), Outcome 23 E1.1 Effect of cognitive training on biomarker evidence of brain function.
Comparison 14. Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 A2.1.1 Change in a global measure of cognition | 2 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.01, 1.02] |
| 2 A2.1.2 Change in cognitive ability (self‐reported) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 A2.1.3 Change in cognitive ability (carer reported) | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.44, 0.45] |
| 4 A2.2.1 Change in immediate verbal memory scores | 3 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.48, 0.37] |
| 5 A2.2.2 Change in delayed verbal memory scores | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 A2.2.3 Change in executive function (sequencing) scores | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 9.38 [‐9.88, 28.65] |
| 6.1 Change in scores on Trails A | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 12.62 [‐7.98, 33.23] |
| 6.2 Change on Trails B | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.13 [‐67.45, 41.19] |
| 7 A2.2.4 Change in verbal letter fluency scores | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.18, 1.28] |
| 8 A2..2.5Change in verbal category fluency scores | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐1.28, 1.18] |
| 9 A2.2.6 Change in attention and working memory scores | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 B2.1 Change in participant's capacity for activities of daily living (self‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 B2.2 Change in participant's mood (self‐reported) | 1 | 11 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐1.34, 1.12] |
| 12 B2.3 Change in participant's general quality of life (self‐report) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 B2.4 Change in participant's capacity for activities of daily living (Carer reported) | 3 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.46, 0.38] |
| 14 B2.5 Change in participant's mood (carer reported) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 B2.6 Change in participant's general quality of life (carer‐reported) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 C2.1 Change in rates of admission to residential care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 C2.2 Change in measures of dementia severity | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 D2.1 Change in self‐reported mood | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 D2.2 Change in self‐reported burden of care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 D2.3 Change in self‐reported overall wellbeing and quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 E2.1 Effect of cognitive training on biomarker evidence of brain function | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Change in glucose metabolism at rest (FDG PET) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Effects on glucose metabolism at activation (FDG PET task) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 E2.2 Effect of cognitive training on biomarker measures of neuropathology | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 1 A2.1.1 Change in a global measure of cognition.
14.3. Analysis.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 3 A2.1.3 Change in cognitive ability (carer reported).
14.4. Analysis.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 4 A2.2.1 Change in immediate verbal memory scores.
14.6. Analysis.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 6 A2.2.3 Change in executive function (sequencing) scores.
14.7. Analysis.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 7 A2.2.4 Change in verbal letter fluency scores.
14.8. Analysis.

Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 8 A2..2.5Change in verbal category fluency scores.
14.11. Analysis.
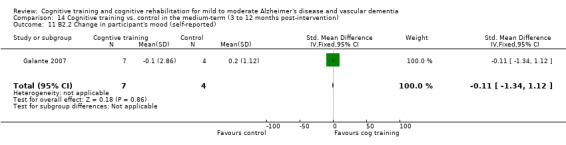
Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 11 B2.2 Change in participant's mood (self‐reported).
14.13. Analysis.
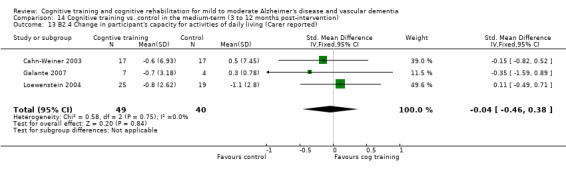
Comparison 14 Cognitive training vs. control in the medium‐term (3 to 12 months post‐intervention), Outcome 13 B2.4 Change in participant's capacity for activities of daily living (Carer reported).
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Beck 1988.
| Methods | RCT comparing cognitive training with a no‐treatment control condition. | |
| Participants | 20 participants with AD or mixed dementia and MMSE score of 15 to 20. | |
| Interventions |
Both groups continued to receive 'conventional' treatment during the intervention. |
|
| Outcomes | Cognitive outcomes immediately post‐intervention for the person with dementia were reported in the domains of attention (letter cCancellation), memory (recall of numbers, recall of details of a story) and visual perception (match‐to‐sample task). Differences in favour of the experimental group found on one measure of memory (recall of digits). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described. Beck (personal communication) indicates that the participants were randomised using random number tables. The equal number of participants in each group suggests that block randomisation was used. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment procedures were described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for all participants. |
| Selective reporting (reporting bias) | Low risk | No selective reporting of data was noted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants would have been aware of their group allocation. No data were provided regarding experimenter blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessments were conducted by assessors who were not involved in the cognitive training intervention. However, no information was given to reveal whether outcome assessments were conducted by assessors blind to group allocation; therefore this is unlikely to have been the case. |
| Other bias | Low risk | No other significant sources of bias were identified. |
Heiss 1993.
| Methods | RCT comparing four intervention conditions. | |
| Participants | 80 patients meeting NINCDS‐ADRDA criteria for probable AD of mild to moderate severity (MMSE 14 to 25). Data available for 70 of these. | |
| Interventions |
Six months' duration of treatment. For the purposes of this review, the CCT group was compared with the social support group. |
|
| Outcomes | Cognitive outcomes for the participant immediately post‐intervention were reported in the domains of global cognition (MMSE), orientation, reaction time (Go/NoGo), praxis, memory (verbal and visual selective reminding tasks), working memory (Corsi's Tapping Task), attention (concentration test for elderly people), language (verbal fluency, token test), executive function, visual perception (Gorlin's Incomplete Pictures Test) and motor function (finger‐tapping). Biomarker outcomes for the participant included measures of brain activation (quantitative electroencephalogram (EEG) and FDG PET). Improved cognitive and brain activation outcomes were reported for the groups that received cognitive training combined with pharmacological treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of randomisation was not described. Mielke (personal communication) indicates that the method used was ’randomisation by chance in blocks of four’. |
| Allocation concealment (selection bias) | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 80 participants who initially entered the study, complete data were available for only 70. It is not clear whether the attrition was evenly distributed between the studied groups. The authors reported that the attrition was attributable to technical insufficiencies in the PET or EEG data, or to side effects. Meilke (personal communication) comments that the authors considered the drop‐out rate to be in the normal range for clinical studies. |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcome data seem to have been reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants in the two groups of interest here would necessarily be aware of the intervention condition to which they were allocated. Whether research personnel were blind to group allocation was not indicated, but it is unlikely that this was the case. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The authors report that ’blinding was not attempted since...the tedious blinding procedures seemed unjustified in this exploratory pilot study’, but it is not clear whether this refers to participants alone or to both participants and assessors. |
| Other bias | Unclear risk | No other significant sources of bias were identified. |
Quayhagen 1995.
| Methods | RCT comparing cognitive training with placebo and wait‐list control. | |
| Participants | Out of 95 eligible families, 79 community‐dwelling persons with mild to moderate AD (scoring at least 90 on the Mattis Dementia Rating Scale) and their family caregiver completed the intervention. Data were available for 78 of these (51 male and 27 female patients; 18 male and 60 female caregivers). | |
| Interventions |
For the purposes of the current review, the experimental condition was compared with the wait‐list control condition. |
|
| Outcomes | Cognitive outcomes for person with dementia were reported immediately after the 12‐week intervention and at a 6‐month follow‐up in the domains of global cognition (Mattis Dementia Rating Scale), memory (Logical Memory‐I, Figural Memory, Visual Reproduction), language (letter and semantic fluency), problem‐solving (Geriatric Coping Measure), and attention (Block Span, Digit‐Span). Non‐cognitive outcomes for the person with dementia included the Memory and Behaviour Problems Checklist (caregiver reported). At the follow‐up assessment, participants in the experimental condition were at or around baseline on cognitive and behavioural measures, whereas the control group showed further decline. | |
| Notes | Comparison of the training programme with a shortened version used in subsequent work is covered in Quayhagen & Quayhagen 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of randomisation was not described. Quayhagen (personal communication) advises that the method used was ’stratified randomisation across groups with the strata representing severity of dementia as measured by the DRS’. The unit of randomisation was the caregiver/care recipient dyad, but randomisation was done on the basis of the level of severity of dementia of the care recipient. |
| Allocation concealment (selection bias) | Unclear risk | No information was available to reveal whether group allocations were concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Out of 95 eligible families, 79 completed the nine‐month project (83%). The 16 drop‐outs were attributed mainly to ’non‐project‐related mortality or morbidity’. One further family was excluded from the analysis as the result of ’data inconsistency’. The authors note that of 25 families in the wait‐list control group, only 5 opted to take cognitive training at the end of the waiting period. No information was provided comparing participants who completed the study with those who had not. |
| Selective reporting (reporting bias) | High risk | The authors declined to supply mean scores on individual measures for use in this review. Results were reported only for selected outcome measures rather than for all measures identified in the Methods section of the paper. It is possible, therefore, that some non‐significant results have not been reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The placebo condition provided a control for therapist attention and level of interaction between caregiver and care recipient. It is not clear, however, whether participants were aware of the condition to which they were assigned. The authors note that some members of the placebo group appeared to initiate active processing of the tasks, and this may have led to a confound between the two conditions. It is not clear who exactly carried out the interventions and whether the same individuals delivered interventions in the experimental and placebo groups. It is unlikely that those carrying out the interventions were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were carried out by research assistants who ’with rare exceptions’ were blind to the condition. |
| Other bias | Unclear risk | The authors did not provide baseline characteristics for the cognitive training and comparison groups, but the authors commented that no differences were noted between the groups at baseline. |
de Vreese 1998.
| Methods | RCT comparing three intervention groups with placebo control. | |
| Participants | 24 patients with mild to moderate AD (Clinical Dementia Rating score 1 to 2) according to NINCDS‐ADRDA criteria. | |
| Interventions |
For the purposes of this review, the cognitive training plus acetylcholinesterase (AChE) condition was compared with the AChE‐I‐only condition. |
|
| Outcomes | Cognitive outcomes for person with dementia were assessed in the domains of global cognition (MMSE, Alzheimer's Disease Assessment Scale, Cognitive subscale (ADAS‐Cog)). Non‐cognitive outcome measures for the person with dementia were reported in the domains of behaviour (Interview of Spontaneous Behaviour) and activities of daily living (Instrumental Activities of Daily Living Scale). Benefits for both cognitive and non‐cognitive outcomes were observed in the group that received a combination of AChE‐I and cognitive training. | |
| Notes | The authors state that the groups did not differ on demographic and baseline characteristics. However, group‐level data were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Whether allocation concealment was attempted was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention is made of attrition. |
| Selective reporting (reporting bias) | Unclear risk | Published results were reported in terms of change from baseline, but mean scores for each assessment point were provided by the author (de Vreese, personal communication). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were necessarily aware of whether they were in the AChE‐I plus cognitive training group as opposed to AChE‐I alone (and this presumably also indicated to them that they were receiving the active drug and not the placebo). The comparison does not incorporate any control for therapist attention or other placebo effects arising from the training condition. No information was provided regarding blinding of experimental personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blind to the condition. |
| Other bias | Low risk | No other significant sources of bias were identified. |
Quayhagen 2000.
| Methods | RCT comparing four treatment conditions with a wait‐list control. | |
| Participants | 103 persons (65 men, 38 women) with dementia (AD, vascular dementia or Parkinson's dementia) in the mild or moderate stage (scoring over 100 on the Mattis Dementia Rating Scale), together with their spouse caregivers. | |
| Interventions |
For the purposes of this review, cognitive training and wait‐list control conditions were compared. |
|
| Outcomes | Cognitive outcomes for the person with dementia were reported in the domains of memory (Logical Memory, Visual Reproduction), language (Verbal Fluency) and problem‐solving (Geriatric Coping Schedule). Non‐cognitive outcomes for the person with dementia were reported in the domain of behaviour (Memory and Behaviour Problems Checklist, caregiver‐rated). Outcomes for caregiver were reported in the domains of marital satisfaction (Marital Needs Satisfaction Scale), emotional status (Brief Symptom Inventory), morale (Geriatric Centre Morale Scale), physical health status (Health Assessment Scale), perceived stress (Memory and Behaviour Problems Checklist, Part B), coping (Coping Strategies Inventory-Revised), social support (Social Support Questionnaire) and satisfaction with intervention. Outcome for the course of the disease was measured using the Dementia Rating Scale. The cognitive stimulation group had better cognitive outcomes at 3 months post‐intervention. Caregivers of patients in this group had lower depressive symptoms. |
|
| Notes | Although the authors report in the article that treatment groups did not differ in terms of age, education or racial distribution, demographic data were not provided at the group level. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As in the 1995 trial, the method of randomisation was not described. Quayhagen (personal communication) advised that the method used was ’stratified randomisation across groups with the strata representing severity of dementia as measured by the DRS’. The unit of randomisation was the caregiver/care recipient dyad, but randomisation was done on the basis of the level of severity of dementia of the care recipient. |
| Allocation concealment (selection bias) | Unclear risk | No information was available to indicate whether group allocations were concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The participant sample reported appears to include only those who completed the study (n = 103), but the authors mention in the discussion that attrition did occur, and that this was higher in the early‐stage day care group, possibly because of transportation issues.The authors also reported that only 15 members of the wait‐list control group agreed to proceed to their allocated intervention after their waiting period, which is the total number given for the wait‐list group in the analyses. No other information was provided to clarify the risk of attrition bias, but the relative lack of change in most outcome measures within the early‐stage care group suggests that attrition may not have biased the results. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective data reporting was noted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of the intervention type to which they were allocated. It is not clear whether the therapists contributed to more than one intervention type. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blind to the condition to which participants were randomly assigned. |
| Other bias | Unclear risk | The authors did not provide baseline characteristics for the cognitive training and comparison groups, but the authors commented that no differences were noted between the groups at baseline. |
Davis 2001.
| Methods | RCT comparing intervention and placebo in cross‐over design: participants in placebo condition crossed over to receive intervention. | |
| Participants | 37 patients (16 men, 21 women) with probable AD and a mean (M) MMSE score of 22.78 (SD 4.45) for control, and M = 21.84 (SD 4.03) for intervention. | |
| Interventions | Intervention condition: one hour of individual training weekly for five weeks on face‐name associations and recall using spaced retrieval, plus home practice (0.5 hours/d for 6 days/wk) on attention‐training exercises. Placebo condition: 'mock' intervention consisting of one‐hour clinic visit weekly for unstructured conversation and questioning with examiner and viewing of health‐related videos. | |
| Outcomes | Cognitive outcomes for the person with dementia were reported in the domains of global cognitive functioning (MMSE), memory (Logical Memory, Visual Reproduction), working memory and attention (Digit‐Span, Verbal Series Attention Test), language (verbal fluency) and psychomotor ability (finger‐tapping). Non‐cognitive outcomes for the person with dementia were reported in the domains of depressive symptoms (Geriatric Depression Scale-self‐rated) and quality of life (Quality of Life Assessment-rated by the caregiver). Although participants in the cognitive training group improved in trained measures, no differences between this and the control group were observed in any of the untrained outcome measures. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | The method of randomisation was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition was reported for the initial phase. However, four participants who initially received the placebo condition decided to discontinue at the cross‐over point because of loss of interest in the trial. Because the analyses focused on the initial group allocation (before the cross‐over), at which no participants were lost, risk of attrition bias was unlikely at this point. |
| Selective reporting (reporting bias) | Unclear risk | The authors did not report the means and SDs of the placebo group at the third time point, that is, after they crossed over to receive the intervention. However, these data were not required for the meta‐analyses, as they focus only on the first phase (before cross‐over); therefore it is unlikely that selective reporting would have introduced a reporting bias into the analyses. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Once the intervention began, participants were aware of which condition they were receiving. It is not clear whether training and placebo conditions were provided by the same therapists, and it is not possible to evaluate the likelihood of contamination between conditions. The placebo condition, while controlling for length and duration of clinic visits and therapist attention, did not provide a control for the use of home practice. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blind to conditions at initial and post‐intervention assessments but not at the third assessment point for the initial placebo group after cross‐over. Random sequence generation. |
| Other bias | High risk | A trend towards differences favoured the cognitive training group in baseline characteristics that are likely to have an impact on outcomes (age, education, anti‐depressant medication use). |
Koltai 2001.
| Methods | RCT comparing two intervention conditions and wait‐list control. | |
| Participants | 24 participants (22 completed the study) with mild/moderate dementia (scoring 0.5 to 1.0 on the Clinical Dementia Rating). | |
| Interventions |
The programme included training and practice in strategies of spaced retrieval, face‐name recall, verbal elaboration, concentration/overt repetition, use of external memory aids and ways of coping. Caregivers joined the last 10 to 15 minutes of each session where available. As no differences in outcome were found between individual and group formats, the results for these two conditions were analysed together and were compared with those for the wait‐list control group. |
|
| Outcomes | Cognitive outcomes for the person with dementia included global cognition (MMSE), memory (List Learning from the Consortium to Establish a Registry for Alzheimer's Disease test battery), language (abbreviated Boston Naming Test, Category Fluency), apraxia (Rosen Figures of Constructional Praxis) and perceived memory problems (Everyday Memory Questionnaire-self‐ and caregiver‐rated). Non‐cognitive outcomes for the person with dementia included depressive symptoms (Geriatric Depression Scale-self‐ and caregiver‐rated). Trends favouring the cognitive training group were observed, but no comparison reached statistical significance. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of randomisation was not described. Koltai (personal communication) indicated that randomisation was done by roll of dice. Given the different sizes of the two groups, simple (consecutive) randomisation would have been used. |
| Allocation concealment (selection bias) | Unclear risk | No data were available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two participants from the Memory and Coping group did not complete the intervention because of serious illness (8% attrition). It was not stated whether this serious illness was related or unrelated to dementia severity, and therefore it is not clear whether these two patients would have had poorer outcomes had they remained in the study. If this had been the case, it may have contributed to the appearance of the intervention as more effective or of anosognosia as having greater impact. |
| Selective reporting (reporting bias) | Low risk | Raw data were not reported in the paper, as data were reported in terms of change from baseline scores. In addition, not all measures that were included in the assessment were reported in the results. Specifically, the Boston Naming Test, Category Fluency, and Rosen Figures were all included in the assessment but were not reported in the results. Given that the authors have stated that the intervention was not effective in relation to objective cognitive measures, it is assumed that no change was observed on these measures as well. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of their group allocation. Koltai conducted all therapeutic interventions and therefore would not have been blinded to group allocation. Whether other research personnel were blinded to group allocation was not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Koltai (personal communication) confirmed that assessments were conducted blind to the condition. Given that Koltai conducted all interventions, it is not clear who conducted the assessments. |
| Other bias | High risk | Baseline imbalances between the groups (favouring the control group) on a global measure of cognition (MMSE) and on the severity of depressive symptoms (rated by the caregiver) may have biased the outcomes. |
Cahn‐Weiner 2003.
| Methods | RCT comparing memory training with a no memory training control. | |
| Participants | 34 participants with mild probable AD and a mean MMSE score of 25.1 (SD 1.7) for control, and M = 24.3 (SD 2.2) for intervention. | |
| Interventions |
|
|
| Outcomes | Cognitive outcomes for the person with dementia immediately post‐intervention and at short‐term follow‐up were reported in the domains of global cognition (MMSE), memory (Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised), language (Boston Naming Test, Controlled Oral Word Association Test), executive function (Trail Making Test), perception (Judgement of Line Orientation Test) and self‐ and other‐reported memory function (Everyday Memory Functioning). Non‐cognitive outcomes for the person with dementia immediately following the intervention and at short‐term follow‐up included informant‐reported ADLs (Instrumental Activities of Daily Living and Physical Self‐Maintenance Scale). No group differences were observed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by coin‐toss. This type of simple randomisation tends to yield different group sizes with small samples (N < 100). Given that the intervention and control groups were of equal size (n = 17), it is unlikely that simple randomisation was used, and randomisation was most likely done in blocks. In addition, the authors state that the coin‐toss was performed at the time consent was obtained, which would have been before the baseline assessment. This approach is generally more prone to bias than when the randomisation sequence is performed after baseline assessment has been completed. |
| Allocation concealment (selection bias) | Unclear risk | Coin‐toss was performed at the time consent was signed. However, the authors provided no detail regarding who performed the coin‐toss. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants who were initially enrolled withdrew from the study because of difficulties involving transportation to the clinic. Three participants were from the control group, and two were from the cognitive training group. One of these attended a single intervention session, and the rest participated only in the baseline assessment. Five participants were excluded from all analyses. They had on average scores on the MMSE that were 1 point lower than those of the intervention groups and 2 points lower than those of the control group. However, the similar number of withdrawn participants in the two groups and the fact that all reportedly withdrew as the result of transportation problems are likely to lead to low attrition bias. |
| Selective reporting (reporting bias) | Low risk | No selective reporting of data was noted. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors report that participants and family caregivers were unaware of which condition they had been assigned to. However, it is questionable whether blinding of participants is genuinely possible in studies of this kind. A neuropsychologist blinded to the results of the baseline assessment conducted both experimental and control interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | A psychometrist blind to group allocation conducted the assessments at all three time points. One participant was assessed at the final time point by the group leader. |
| Other bias | Low risk | No other significant sources of bias were identified. |
Loewenstein 2004.
| Methods | RCT comparing cognitive training with placebo control. | |
| Participants | 44 participants meeting NINCDS‐ADRDA criteria for dementia and on stable dose of an AChE‐I and with a mean baseline MMSE score of 24.5 (SD 4.5) for control and M = 23.4 (SD 2.9) for intervention. | |
| Interventions |
|
|
| Outcomes | Cognitive outcomes for the person with dementia were reported in the domains of memory (List Learning from the CERAD battery, Logical Memory), attention and working memory (Digit‐Span), language (Category Fluency), executive function (Trail Making Test) and reported cognitive function (Informant Questionnaire of the Cognitive Decline in the Elderly Scale, self‐ and caregiver‐rated). Scores on measures analogous to tasks used in the training sessions were also used (e.g. Face‐Name Learning Task). Non‐cognitive outcome measures for the person with dementia were reported in the domains of behaviour (Revised Memory and Behaviour Problems Checklist, caregiver‐rated), activities of daily living (Bayer Activities of Daily Living Scale, self‐ and caregiver‐rated) and depressive symptoms (Centre for Epidemiological Studies-Depression Scale). Participants in the cognitive training group improved in their performance on tasks analogous to the ones used during training to a greater extent than the mental stimulation group. No group differences were reported on any of the untrained tasks. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described |
| Allocation concealment (selection bias) | Low risk | Loewenstein (personal communication) indicates that randomisation was done by envelope selection after baseline evaluation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants-three from the cognitive training group and two from the mental stimulation group-were not included in the statistical analyses because they did not complete all the required sessions. No information was provided on whether these sessions were intervention sessions or outcome assessments, and no reasons for these incomplete data were provided. Given the small and similar rates of incomplete data between groups, is it unlikely that this would have introduced substantial attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | No information was provided on the means and SDs of untrained cognitive tasks at the various assessment points. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Two neuropsychologists trained an equal number of participants in both treatment groups and would have been therefore aware of group allocation. Although not explicitly stated, participants would have known the group to which they were allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Baseline, post‐intervention, and follow‐up assessments of all outcomes were conducted by the same assessor, who was blind to the treatment condition. |
| Other bias | Low risk | No other significant sources of bias were identified. |
Galante 2007.
| Methods | Single‐blind RCT comparing the efficacy of computerised cognitive training (CT) versus a no treatment (NT) condition. | |
| Participants | 12 participants who met criteria for mild AD (according to NINCDS‐ADRDA criteria, with MMSE 19 to 26 or MODA 70 to 90) and who were treated with AChE‐I for at least 3 months. | |
| Interventions |
|
|
| Outcomes | Cognitive outcomes for the person with dementia were reported in the domains of global cognition (MMSE, MODA), memory (prose memory), visual working memory (Corsi's Block Tapping), attention (digit cancellation task, Bisyllabic Word Repetition Test), problem‐solving (Raven's Coloured Progressive Matrices), language (verbal fluency) and constructional and ideomotor apraxia. Non‐cognitive outcomes for the person with dementia were reported in the domains of mood (Neuropsychiatric Inventory, caregiver‐rated; Geriatric Depression Scale, self‐rated) and activities of daily living (Basic and Instrumental Activities of Daily Living Scales). Investigators found that although participants in the control group declined in terms of MMSE scores over the 9 months of the study, participants receiving cognitive training remained stable by the end of the study period. | |
| Notes | No information was provided on the extent to which the groups were matched on relevant variables before the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment or control group by simple randomisation, leading to unequal group sizes. The method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | No data were available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One participant, assigned to the control condition, was "excluded from the study for poor compliance". It is not clear at what point this participant was excluded (i.e. before or after completion of the intervention). Given that the control group had only four participants, it is quite possible that exclusion of this participant would have biased the results. |
| Selective reporting (reporting bias) | Low risk | All relevant outcome data were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No data were provided, but participants would have been aware of their group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were evaluated by a neuropsychologist blinded to patient group allocation. |
| Other bias | Unclear risk | Separate details for the groups at baseline were not provided. Visual inspection suggests trends towards baseline imbalances on cognitive and affective measures that are likely to be related to outcomes. The significance of these trends is, however, unclear. |
Neely 2009.
| Methods | RCT comparing collaborative CT (dyadic) with individual CT and no treatment control. | |
| Participants | Participants with mild to moderate AD or vascular dementia (n = 47 couples invited to participate, n = 30 agreed and randomly assigned), with a mean MMSE score of 18.6 (SD 5.7) for control and M = 22.9 (SD 4.1) for intervention. | |
| Interventions |
|
|
| Outcomes | Cognitive outcomes for the person with dementia were reported in the domain of memory (categorisable and non‐categorisable word‐list recall; random and clustered object recall task). Outcomes for caregiver were reported in the domains of caregiver burden (Zarit Caregiver Burden Interview) and depression (Beck Depression Inventory). |
|
| Notes | Two treatment groups were compared in the study: collaborative (dyadic) CT and individual CT. Outcomes included in the current review focus on the individual CT group. No differences between the individual training group and the control group were observed in any of the outcome measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors state that the first 30 couples to have consented for the study were randomly assigned to the collaborative programme, the individual programme, or the control group. Given the equal number of participants in each of the three groups, randomisation would have been done in blocks. No details on the method of randomisation were provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided to allow assessment of this risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data for one couple in the control group were noted, but no reasons were provided, and data analysis included the remaining nine dyads. Given the small sample size (n = 10 per group), missing data have the potential to bias the results. |
| Selective reporting (reporting bias) | Unclear risk | The authors reported a main effect of test occasion on caregiver‐rated depression, showing that across groups, an increase in depression followed the intervention. However, no detail is provided regarding an interaction between group and time in relation to depression, and unlike other measures, no means and SDs were provided in the article. In addition, no means and SDs were provided for the Zarit Caregiver Burden Scale, but the authors reported no main effect of caregiver burden. Whether the authors assessed for an interaction between group and test occasion on caregiver burden was not explicitly stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided in the article. However, participants would have been aware of their group allocation. In addition, although not stated explicitly, the person or persons delivering the intervention would have been aware of group allocation. Support for this is found in the description of the individual training group: "...the training was conducted without any involvement of the caregiver. Instead, the support strategies were provided by a research assistant, rather than by the spousal caregiver". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information was provided by the authors, and it is probable that no blinding of outcome assessment was carried out. |
| Other bias | Unclear risk | Data on baseline educational levels in the two groups were not reported. It is not clear whether differences existed that could bias relevant outcomes. |
Clare 2010.
| Methods | Single‐blind RCT comparing cognitive rehabilitation (CR) with relaxation therapy (RT) and with no treatment. | |
| Participants | 69 people (28 men, 41 women) with mild AD (MMSE > 18). | |
| Interventions |
|
|
| Outcomes | Cognitive outcomes for the person with dementia were reported in the domains of memory (Rivermead Behavioural Memory Test), language (verbal fluency), attention (Map Search, Elevator Counting, Elevator Counting With Distraction) and perceived memory functioning (Memory Awareness Rating Scale, self‐ and caregiver‐rated). Non‐cognitive outcomes for the person with dementia were reported in the domains of goal performance and satisfaction (Canadian Occupational Performance Measure), functional abilities (Independent Living Scale-Health and Safety subset), mood (Hospital Anxiety and Depression Scale) and quality of life (Quality of Life in Alzheimer's Disease, self‐ and caregiver‐rated). fMRI was reported as a biomarker outcome for a subset of persons with dementia. Outcomes of the caregiver were reported in the domains of quality of life (World Health Organisation Quality of Life Assessment-Short Version), general health (General Health Questionnaire‐12), mood (Hospital Anxiety and Depression Scale) and stress (Relatives' Stress Scale). Participants in the cognitive rehabilitation group have shown significant improvement in their rating of goal performance and satisfaction, as well as increased or preserved activation in several brain regions. |
|
| Notes | Clare 2010 was the only study found in the search that satisfied the definition of 'cognitive rehabilitation' used in this review. Therefore no meta‐analysis of cognitive rehabilitation outcomes could be conducted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted by an independent trials unit using a computer algorithm and was stratified for gender, age (up to 69 years vs 70 years and older), and geographical location (western, central, or eastern district of the catchment area)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants’ details were passed to the therapist, an experienced occupational therapist, who contacted the trials unit to initiate randomisation. Thus, only the therapist was aware of the identities of the participants allocated to each condition." Given the use of a centralised randomisation procedure, allocation concealment was more than likely achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were not available for four participants who either withdrew from the study or died. Two of these participants were allocated to the cognitive rehabilitation group. An additional participant withdrew from the relaxation therapy group, and one participant from the control group died. In addition, one participant who was initially allocated to the cognitive rehabilitation group was later deemed to have met criteria for mild cognitive impairment rather than dementia and was therefore excluded from the study, but this was reported in a separate publication. Given the small quantity of incomplete data and the relatively even spread, the results are not likely to have been biased by incomplete data. Given that the participant excluded from the CR group as the result of a changed diagnosis had results in the same direction as that reported for the CR group, his exclusion is unlikely to have biased the results. |
| Selective reporting (reporting bias) | Low risk | No evidence suggested selective reporting of data. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants would have been aware of the group to which they were allocated, and one therapist conducted all interventions and was therefore not blinded to group allocation. The study authors were blinded to participants' group allocation (Clare, personal communication). Although the primary outcome measure in the study (Canadian Occupational Performance Measure (COPM)) is based on subjective ratings of performance and satisfaction, and this could therefore be biased by lack of participant blinding, it is unlikely to have biased outcomes in the current study given the substantial memory loss in this population, who would have not been able to recall their previous ratings. Although the therapist conducting the interventions was not blinded, the authors state that treatments were conducted using a "structured treatment protocol" and that "adherence to therapy protocols was monitored through supervision and review of session and home‐practice records", thereby reducing the risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessments were conducted by research personnel blinded to participant group allocation. |
| Other bias | Unclear risk | No other significant sources of bias were identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2001 | Not RCT. |
| Arkin 1997 | Not RCT. |
| Barban 2012 | Conference abstract. |
| Bentwich 2011 | Not RCT. |
| Bernhardt 2002 | Not RCT. |
| Bottino 2005 | Describes cognitive stimulation. |
| Breuil 1994 | Describes cognitive stimulation. |
| Brinkmann 1982 | Not RCT. |
| Brodaty 1989 | Not RCT. |
| Brodaty 1997 | Long‐term outcome from Brodaty 1989 (see above). |
| Ceccato 2012 | This study describes a cognition‐based music therapy intervention. Music therapy is recognised as a separate form of intervention; therefore it was decided that this study would best fit into a review of music therapy interventions. An additional factor leading to the exclusion of this trial is that the type of dementia was not specified, so it is not possible to verify that participants were diagnosed only with AD or vascular dementia. |
| Chapman 2004 | Describes cognitive stimulation. |
| Clare 2002 | Not RCT. |
| Diesfeldt 1991 | No standardised outcome measures employed; not available in English. |
| Dunlosky 2003 | Participant criteria not met. |
| Ermini Fuenfsch 1995 | Not RCT. |
| Farina 2006 | Not RCT. |
| Fernandez 2006 | A single case study. |
| Fernandez‐Calvo 2010 | Article published in Spanish and seems to fit better with a cognitive stimulation approach. |
| Forster 2011 | Although participants with MCI underwent cognitive training, the intervention delivered to participants with dementia was weighted much more toward cognitive stimulation. |
| Gaugler 2011 | The intervention is best described as a memory support group. |
| Giordano 2010 | Describes reality orientation. Not RCT. |
| Goudour 2011 | Study not published in English. |
| Graessel 2011 | The intervention described in this study is a multi‐component intervention that included motor stimulation, practice in ADLs and cognitive stimulation. Hence, the active ingredients of the intervention cannot be identified. |
| Graff 2006 | The study describes a multi‐component intervention following an occupational therapy clinical guideline. |
| Guenther 1991 | Participant criteria not met. |
| Haslam 2011 | Not RCT. |
| Hwang 2012 | Not RCT. |
| Israel 1987 | Participant criteria not met. |
| Israel 1989 | Participant criteria not met. |
| Jelcic 2012 | Conference paper. |
| Jobe 2001 | Participant criteria not met. |
| Kixmiller 2002 | Not RCT. |
| Kurz 2012 | The intervention was a structured, multi‐component intervention that has previously been variously described by the authors as 'cognitive resource‐oriented therapy' or as 'neuropsychologically informed behaviour therapy'. The intervention was said to draw on principles from psychotherapy and neurorehabilitation, and included a range of elements such as reminiscence, activity scheduling, use of memory aids and coping skills for managing memory difficulties. Hence the active ingredients could not be separated out, and the study was excluded. |
| Lam 2010 | Intervention was an individualised functional enhancement programme and did not meet criteria for CT or CR. |
| Mate‐Kole 2007 | Not RCT. |
| Mayer 2012 | Single case study of attention process training in an individual with CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy). |
| Onor 2007 | Study protocol describes reality orientation and reminiscence therapy-therefore not CT or CR. |
| Oresnik 2008 | Not RCT. |
| Panza 1996 | Not RCT. |
| Raggi 2007 | Not RCT. |
| Requena 2004 | Describes cognitive stimulation. |
| Schreiber 1999 | Not RCT. |
| Sheikh 1986 | Participant criteria not met. |
| Spector 2003 | Describes cognitive stimulation. |
| Talassi 2007 | Not RCT. |
| Tarraga 2006 | Describes cognitive stimulation. |
| Van Tilborg 2011 | Not RCT. Intervention does not appear to meet criteria for CT or CR. |
| Viola 2011 | The intervention was described as a multi‐component intervention including elements of cognitive training, physical activity and expressive activities. The active ingredients could not be separated, and the study was excluded. |
| Yesavage 1981 | Not RCT. |
| Zarit 1982 | Participant criteria not met. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611001173987.
| Trial name or title | Effectiveness of self‐management skills enhancing rehabilitation on patients with dementia and their spousal caregivers. |
| Methods | Parallel group RCT, no blinding will be attempted. |
| Participants | 160 patients with dementia and their spouses. |
| Interventions | Separate group‐based interventions for the person with dementia and the primary caregiver. Groups will meet for four hours once a week for 8 weeks. Group sessions will focus on empowering patients, increasing the sense of agency and developing strategies to enhance their self‐management skills. |
| Outcomes | Primary outcomes only:
|
| Starting date | 10/11/2011. |
| Contact information | Marja‐Liisa Laakkonen Helsinki Health Center Laakso Hospital P.O. Box 6600 00099 The City of Helsinki e: marja‐liisa.laakkonen@kolumbus.fi |
| Notes | Study set in Helsinki, Finland. It is unlikely to meet criteria for the current review. |
ISRCTN21027481.
| Trial name or title | Goal‐oriented cognitive Rehabilitation in Early‐stage Alzheimer's disease: multi‐centre single‐blind randomised controlled Trial (GREAT). |
| Methods | RCT of cognitive rehabilitation vs usual care. |
| Participants | AD patients (MMSE 14 to 26). Target N = 480. |
| Interventions | Participants will be randomised to cognitive rehabilitation or treatment as usual. The cognitive rehabilitation intervention protocol will consist of 10 weekly sessions followed by four maintenance sessions spread over a six‐month period. |
| Outcomes | Bangor Goal‐Setting Interview (Primary outcome measure to be assessed at three and nine months post‐randomisation). |
| Starting date | 1/10/2012. |
| Contact information | Mrs Aleksandra Kudlicka Trial Manager School of Psychology Bangor University Bangor, Gwynedd LL57 2AS, UK a.kudlicka@bangor.ac.uk |
| Notes | The trial is expected to conclude on 31/1/2017. |
NCT01329484.
| Trial name or title | Computerised personal interventions for Alzheimer's patients. |
| Methods | RCT. |
| Participants | N = 159 (estimated). |
| Interventions |
|
| Outcomes | Cognitive function measured by Mindstreams (NeuroTrax Corp, Bellaire, TX) computerised neuropsychological assessment instrument at baseline and at 1, 3 and 6 months post‐intervention. |
| Starting date | March 2011. |
| Contact information | Tzvi Dwolatzky, MD, Mental Health Center, Beer‐Sheva, Israel. |
| Notes |
NCT01689948.
| Trial name or title | Alzheimer disease: Rehabilitation's Intervention at Home (pré‐MATAPA). |
| Methods | |
| Participants | Patients with AD or mixed dementia, living at home and with an available family caregiver (N = 30, estimated). |
| Interventions | Experimental: home rehabilitation therapy-12 weekly rehabilitation sessions in the home. |
| Outcomes | Primary outcome: scores on instrumental activities of daily living (IADLs) at 27 weeks. |
| Starting date | September 2012. |
| Contact information | Gilles BERRUT, Pr, e: gilles.berrut@chu‐nantes.fr. |
| Notes |
Vidovich 2011.
| Trial name or title | Cognitive activity for the treatment of older adults with mild Alzheimer's disease (AD)‐‐PACE AD: study protocol for a randomised controlled trial. |
| Methods | RCT. |
| Participants | 128 community‐dwelling men and women with probable AD. |
| Interventions | (1) Participants with mild AD and their companions together. (2) Companions of participants with mild AD alone. The intervention will consist of a twelve‐week programme of cognitive stimulation. Seven weeks of the programme will involve 90‐minute group sessions delivered once per week; the remaining weeks of the programme will involve structured home‐based activities with telephone support. |
| Outcomes | The primary outcome measure of the study is the change from baseline in total score on the Alzheimer Disease Assessment Scale-Cognitive (ADAS‐Cog). Secondary outcomes of interest include changes in health‐related quality of life, mood, memory, language, executive functions, independent living abilities and psychiatric symptoms for participants with mild AD. Primary endpoints will be collected 13 and 26 weeks after the baseline assessment. |
| Starting date | 1/1/2010. |
| Contact information | Mandy Vidovich Western Australia Centre for Health and Ageing (WACHA) Level 6 Ainslie House 48 Murray Street Perth WA 6000 vidovichm@meddent.uwa.edu.au |
| Notes | It is not clear whether the described intervention is cognitive stimulation or cognitive training. |
Contributions of authors
For the current version:
ABF: drafting and revising of the review, correspondence, review of search records and selection of trials, extraction and entry of data, assessment of risk of bias, data analyses, interpretation of data analyses.
LC: correspondence, selection of trials, interpretation of data analyses, commenting on drafts of the review.
BW: commenting on drafts of the review.
Searches: Anna Noel‐Storr.
Contact editor: Sascha Köpke.
For previous versions
LC: correspondence, drafting of review versions, selection of trials, extraction of data, entry of data, data analysis, interpretation of data analyses, updating of review. RW: selection of trials, interpretation of data analyses, updating of review.
Searches: Dymphna Hermans and Vittoria Lutje (2009). Statistical advice: Jacqueline Birks.
Consumer editors: Christine Derrick, Patricia Hewitt and Christine Stockwell.
Contact editor: Frans Verhey.
Sources of support
Internal sources
Bangor University, UK.
Dementia Collaborative Research Centre-Early Detection and Prevention, The Australian National University, Canberra, Australia.
External sources
The Alzheimer's Society, UK.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Beck 1988 {published data only}
- Beck C, Heacock P, Mercer S, Thatcher R, Sparkman C. The impact of cognitive skills remediation training on persons with Alzheimer's disease or mixed dementia. Journal of Geriatric Psychiatry 1988;21:73‐88. [PubMed] [Google Scholar]
Cahn‐Weiner 2003 {published data only}
- Cahn‐Weiner DA, Malloy PF, Rebok GW, Ott BR. Results of a placebo‐controlled study of memory training for mildly‐impaired Alzheimer's disease patients. Applied Neuropsychology 2003;10:215‐23. [DOI] [PubMed] [Google Scholar]
Clare 2010 {published data only}
- Clare L, Evans S, Parkinson C, Woods R, Linden D. Goal‐setting in cognitive rehabilitation for people with early stage Alzheimer's disease. Clinical Gerontologist 2011;34(3):220‐36. [Google Scholar]
- Clare L, Linden DEJ, Woods R, Whitaker R, Evans SJ, Parkinson CH, et al. Goal‐oriented cognitive rehabilitation for people with early‐stage Alzheimer disease: a single‐blind randomized controlled trial of clinical efficacy. American Journal of Geriatric Psychiatry 2010;18:928‐39. [DOI] [PubMed] [Google Scholar]
Davis 2001 {published data only}
- Davis RN, Massman PJ, Doody RS. Cognitive intervention in Alzheimer disease: a randomized placebo‐controlled study. Alzheimer Disease and Associated Disorders 2001;15(1):1‐9. [DOI] [PubMed] [Google Scholar]
de Vreese 1998 {published and unpublished data}
- Vreese LP, Neri M. Ecological impact of combined cognitive training programs (CTP) and drug treatment (ChE‐I) in AD. International Psychogeriatrics 1999;11 Suppl:S91. [Google Scholar]
- Vreese LP, Neri M, Fioravanti M, Belloi L, Zanetti O. Memory rehabilitation in Alzheimer's disease: a review of progress. International Journal of Geriatric Psychiatry 2001;16:794‐809. [DOI] [PubMed] [Google Scholar]
- Vreese LP, Verlato C, Emiliani S, Schioppa S, Belloi L, Salvioli G, et al. Effect size of a three‐month drug treatment in AD when combined with individual cognitive retraining: preliminary results of a pilot study. European Archives of Psychiatry and Clinical Neuroscience 1998;248:41‐2. [Google Scholar]
Galante 2007 {published data only (unpublished sought but not used)}
- Galante E, Venturini G, Fiaccadori C. Computer‐based cognitive intervention for dementia: preliminary results of a randomized clinical trial. Giornale Italiano di Medicina del Lavoro ed Ergonomia 2007;29(3):26‐32. [PubMed] [Google Scholar]
Heiss 1993 {published data only}
- Heiss W‐D, Kessler J, Mielke R, Szelies B, Herholz K. Long‐term effects of phosphatidylserine, pyritinol and cognitive training in Alzheimer's disease: a neuropsychological, EEG, and PET investigation. Dementia 1994;5(2):88‐98. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Slansky I, Mielke R, Szelies B, Herholz K. Activation PET as an instrument to determine therapeutic efficacy in Alzheimer's disease. Annals of the New York Academy of Sciences 1993;695:327‐31. [DOI] [PubMed] [Google Scholar]
Koltai 2001 {published data only}
- Koltai DC, Welsh‐Bohmer KA, Smechel DE. Influence of anosognosia on treatment outcome among dementia patients. Neuropsychological Rehabilitation 2001;11(3/4):455‐75. [Google Scholar]
Loewenstein 2004 {published and unpublished data}
- Loewenstein DA, Acevedo A, Czaja SJ, Duara R. Cognitive rehabilitation of mildly impaired Alzheimer's disease patients on cholinesterase inhibitors. American Journal of Geriatric Psychiatry 2004;12:395‐402. [DOI] [PubMed] [Google Scholar]
Neely 2009 {published data only}
- Neely AS, Vikstrom S, Josephsson S. Collaborative memory intervention in dementia: caregiver participation matters. Neuropsychological Rehabilitation 2009;19(5):696‐715. [DOI] [PubMed] [Google Scholar]
Quayhagen 1995 {published data only}
- Corbeil RR, Quayhagen MP, Quayhagen M. Intervention effects on dementia caregiving interaction: a stress‐adaptation modeling approach. Journal of Aging and Health 1999;11(1):79‐95. [DOI] [PubMed] [Google Scholar]
- Quayhagen MP, Quayhagen M, Corbeil RR, Roth PA, Rodgers JA. A dyadic remediation program for care recipients with dementia. Nursing Research 1995;44(3):153‐9. [PubMed] [Google Scholar]
Quayhagen 2000 {published data only}
- Quayhagen MP, Quayhagen M, Corbeil RR, Hendrix RC, Jackson JE, Snyder L, et al. Coping with dementia: evaluation of four nonpharmacologic interventions. International Psychogeriatrics 2000;12(2):249‐65. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2001 {published data only}
- Anderson J, Arens K, Johnson R, Coppens P. Spaced retrieval vs memory tape therapy in memory rehabilitation for dementia of the Alzheimer's type. Clinical Gerontologist 2001;24(1‐2):123‐39. [Google Scholar]
Arkin 1997 {published data only}
- Arkin Sharon M. Alzheimer memory training: quizzes beat repetition, especially with more impaired. American Journal of Alzheimer's Disease 1997; Vol. 12, issue 4:147‐58.
Barban 2012 {published data only}
- Barban F, Annicchiarico R, Perri R, Fadda L, Carlesimo GA, Pantelopoulos S, et al. Randomized clinical trial of a computer‐based cognitive treatment for healthy elderly, clinical and preclincal Alzheimer's disease. The SOCIABLE project. Proceedings of the 7th Congresso Sindem of the Italian Association for the Study of Dementia Linked to the Italian Neurological Society; Naples, Italy; March 22‐24, 2012. 2012, issue var.pagings.
Bentwich 2011 {published data only}
- Bentwich J, Dobronevsky E, Aichenbaum S, Shorer R, Peretz R, Khaigrekht M, et al. Beneficial effect of repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer's disease: a proof of concept study. Journal of Neural Transmission 2011;118(3):463‐71. [DOI] [PubMed] [Google Scholar]
Bernhardt 2002 {published data only}
- Bernhardt T, Maurer K, Froelich L. Influence of a memory training program on attention and memory performance of patients with dementia [Der Einfluss eines alltagsbezogenen kognitiven Trainings auf die Aufmerksamkeits‐und Gedaechtnisleistung von Personen mit Demenz]. Zeitschrift fuer Gerontologie und Geriatrie 2002;35:32‐8. [DOI] [PubMed] [Google Scholar]
Bottino 2005 {published data only}
- Bottino CM, Carvalho IA, Alvarez AM, et al. Cognitive rehabilitation combined with drug treatment in Alzheimer's disease patients: a pilot study. Clinical Rehabilitation 2005;19(8):861‐9. [DOI] [PubMed] [Google Scholar]
Breuil 1994 {published data only}
- Breuil V, Rotrou J, Forette F, Tortrat D, Ganansia Ganem A, Frambourt A, et al. Cognitive stimulation of patients with dementia: preliminary results. International Journal of Geriatric Psychiatry 1994; Vol. 9, issue 3:211‐7.
Brinkmann 1982 {published data only}
Brodaty 1989 {published data only}
- Brodaty H, Gresham M. Effect of a training programme to reduce stress in carers of patients with dementia [see comments]. Comment in: BMJ 1990 Feb 3;300(6720):330. British Medical Journal 1989;299(6712):1375‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodaty 1997 {published data only}
- Brodaty H, Gresham M, Luscombe G. The Prince Henry Hospital dementia caregivers' training programme. International Journal of Geriatric Psychiatry 1997;12(2):183‐92. [DOI] [PubMed] [Google Scholar]
Ceccato 2012 {published data only}
- Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, Crociani S, et al. STAM protocol in dementia: a multicenter, single‐blind, randomized, and controlled trial. American Journal of Alzheimer's Disease and Other Dementias 2012; Vol. 27, issue 5:301‐10. [DOI] [PMC free article] [PubMed]
Chapman 2004 {published data only}
- Chapman SB, Weiner MF, Rackley A, Hynan LS, Zientz J. Effects of cognitive‐communication stimulation for Alzheimer's disease patients treated with donepezil. Journal of Speech, Language and Hearing Research 2004;47(5):1149‐63. [DOI] [PubMed] [Google Scholar]
Clare 2002 {published data only}
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Relearning of face‐name associations in early‐stage Alzheimer's disease. Neuropsychology 2002;16(4):538‐47. [DOI] [PubMed] [Google Scholar]
Diesfeldt 1991 {published data only}
- Diesfeldt HF, Smits JC. Face‐name learning in psychogeriatric patients [Gezichten krijgen namen-een cognitieve training voor psychogeriatrische patienten voor het onthouden van namen en gezichten]. Tijdschr Gerontol Geriatr 1991;22:221‐7. [PubMed] [Google Scholar]
Dunlosky 2003 {published data only}
- Dunlosky J, Kubat Silman AK, Hertzog C. Training monitoring skills improves older adults' self‐paced associative learning. Psychology of Aging 2003;18(2):340‐5. [DOI] [PubMed] [Google Scholar]
Ermini Fuenfsch 1995 {published data only}
- Ermini Fuenfschilling D, Meier D. Memory training: an important part of a "milieu therapy" for patients with senile dementia [Gedaechtnistraining: Wichtiger Bestandteil der Milieutherapie bei seniler Demenz]. Zeitschrift fuer Gerontologie und Geriatrie 1995; Vol. 28, issue 3:190‐4. [PubMed]
Farina 2006 {published data only}
- Farina E, Mantovani F, Fioravanti R, Rotella G, Villanelli F, Imbornone E, et al. Efficacy of recreational and occupational activities associated to psychologic support in mild to moderate Alzheimer disease: a multicenter controlled study. Alzheimer Disease & Associated Disorders 2006;20(4):275‐82. [DOI] [PubMed] [Google Scholar]
Fernandez 2006 {published data only}
- Fernandez AL, Manoiloff LMV, Monti AA. Long‐term cognitive treatment of Alzheimer's disease: a single case study. Neuropsychological Rehabilitation 2006;16(1):96‐109. [DOI] [PubMed] [Google Scholar]
Fernandez‐Calvo 2010 {published data only}
- Fernandez‐Calvo B, Contador I, Serna A, de LVM, Ramos F. The effect of an individual or group intervention format in cognitive stimulation of patients with Alzheimer's disease. [Spanish]. Revista de Psicopatologia y Psicologia Clinica 2010;15(2):115‐23. [Google Scholar]
Forster 2011 {published data only}
- Buschert VC. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer's disease: a pilot study. Journal of Alzheimer's Disease 2011;25(4):679‐94. [DOI] [PubMed] [Google Scholar]
- Forster S, Buschert VC, Teipel SJ, Friese U, Buchholz H G, Drzezga A, et al. Effects of a 6‐month cognitive intervention on brain metabolism in patients with amnestic MCI and mild Alzheimer's disease. Journal of Alzheimers Disease 2011; Vol. 26:337‐48. [DOI] [PubMed]
Gaugler 2011 {published data only}
- Gaugler JE, Gallagher‐Winker K, Kehrberg K, Lunde AM, Marsolek CM, et al. The Memory Club: providing support to persons with early‐stage dementia and their care partners. American Journal of Alzheimer's Disease & Other Dementias 2011; Vol. 26, issue 3:218‐26. [DOI] [PMC free article] [PubMed]
Giordano 2010 {published data only}
- Giordano M, Dominguez LJ, Vitrano T, Curatolo M, Ferlisi A, Prima A, et al. Combination of intensive cognitive rehabilitation and donepezil therapy in Alzheimer's disease (AD). Archives of Gerontology and Geriatrics 2010;51(3):245‐9. [DOI] [PubMed] [Google Scholar]
Goudour 2011 {published data only}
Graessel 2011 {published data only}
- Graessel E, Stemmer R, Eichenseer B, Pickel S, Donath C, Kornhuber J, et al. Non‐pharmacological, multicomponent group therapy in patients with degenerative dementia: a 12‐month randomizied, controlled trial. BMC Medicine 2011; Vol. 9, issue 129. [DOI] [PMC free article] [PubMed]
Graff 2006 {published data only}
- Graff MJL, Vernooij‐Dassen MJM, Thijssen M, Dekker J, Hoefnagels WHL, Rikkert MGM. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. British Medical Journal 2006;333(7580):1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guenther 1991 {published data only}
- Guenther V, Fuchs D, Schett P, Meise U, Rhomberg HP. Cognitive training in organic psychogeriatric syndromes [Kognitives Training bei organischem Psychosyndrom]. Deutsche Medizinische Wochenschrift 1991;116(22):846‐51. [DOI] [PubMed] [Google Scholar]
Haslam 2011 {published data only}
- Haslam C, Hodder KI, Yates PJ. Errorless learning and spaced retrieval: how do these methods fare in healthy and clinical populations?. Journal of Clinical and Experimental Neuropsychology 2011;33(4):432‐47. [DOI] [PubMed] [Google Scholar]
Hwang 2012 {published data only}
- Hwang HR, Choi SH, Yoon DH, Yoon BN, Suh YJ, Lee D, et al. The effect of cognitive training in patients with mild cognitive impairment and early Alzheimer's disease: a preliminary study. Journal of Clinical Neurology 2012; Vol. 8, issue 3:190‐7. [DOI] [PMC free article] [PubMed]
Israel 1987 {published data only}
- Israel L, Dell'Accio E, Martin G, Hugonot R. [[Extrait de Ginkgo biloba et exercises d'entrainement de la memoire: evaluation comparative chez des personnes agees ambulatoires]]. Psychologie Medicale 1987;19(8):1431‐9. [Google Scholar]
Israel 1989 {published data only}
- Israel L, Dell'Accio E, Hugonot R. [[Action combinee de Praxilene et d'exercises d'entrainement mental sur les troubles de la memoire de sujets ages]]. Psychologie Medicale 1989;21(8):1219‐34. [Google Scholar]
Jelcic 2012 {published data only}
- Jelcic N, Meneghello F, Bonetto N, Ermani M, Dam M, Cagnin A. Effects of lexical‐semantic treatment on memory in early Alzheimer disease: an observer‐blinded randomized controlled trial. Proceedings of the 7th Congresso Sindem: Italian Association for the Study of Dementia Linked to the Italian Neurological Society; Naples, Italy; March 22‐24, 2012. 2012, issue var.pagings.
Jobe 2001 {published data only}
- Jobe JB, Smith DM, Ball K, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials 2001;22(4):453‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kixmiller 2002 {published data only}
- Kixmiller JS. Evaluation of prospective memory training for individuals with mild Alzheimer's disease. Brain and Cognition 2002;49(2):237‐41. [PubMed] [Google Scholar]
Kurz 2012 {published data only}
- Kurz A, Thone‐Otto A, Cramer B, Egert S, Frolich L, Gertz H‐J, et al. CORDIAL: Cognitive rehabilitation and cognitive‐behavioral treatment for early dementia in alzheimer disease: a multicenter, randomized, controlled trial. Alzheimer Disease and Associated Disorders 2012; Vol. 26, issue 3:246‐53. [DOI] [PubMed]
Lam 2010 {published data only}
- Lam CW, Lui WC, Luk NY, Chau SC, Poon V, Tam P, et al. Effectiveness of an individualized functional training program on affective disturbances and functional skills in mild and moderate dementia-a randomized control trial. International Journal of Geriatric Psychiatry 2010;25(2):133‐41. [DOI] [PubMed] [Google Scholar]
Mate‐Kole 2007 {published data only}
- Mate‐Kole CC, Fellows RP, Said PC, McDougal J, Catayong K, Dang V, et al. Use of computer assisted and interactive cognitive training programmes with moderate to severely demented individuals: a preliminary study. Aging & Mental Health 2007;11(5):485‐95. [DOI] [PubMed] [Google Scholar]
Mayer 2012 {published data only}
Onor 2007 {published data only}
- Onor ML, Trevisiol M, Negro C, Alessandra S, Saina M, Aguglia E. Impact of a multimodal rehabilitative intervention on demented patients and their caregivers. American Journal of Alzheimer's Disease and Other Dementias 2007;22(4):261‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oresnik 2008 {published data only}
- Oresnik M. The influence of cognitive rehabilitation on cognitive competence in patients with Alzheimer's disease. Psychiatria Danubina 2008;20(2):174‐8. [PubMed] [Google Scholar]
Panza 1996 {published data only}
- Panza F, Solfrizzi V, Mastroianni F, Nardo GA, Cigliola F, Capurso A. A rehabilitation program for mild memory impairments. Archives of Gerontological Geriatrics 1996; Suppl 5:51‐5:51‐5. [DOI] [PubMed] [Google Scholar]
Raggi 2007 {published data only}
- Raggi A, Iannaccone S, Marcone A, Ginex V, Ortelli P, Nonis A, et al. The effects of a comprehensive rehabilitation program of Alzheimer's disease in a hospital setting. Behavioural Neurology 2007;18(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Requena 2004 {published data only}
- Requena C, Lopez Ibor MI, Maestu F, Campo P, Lopez Ibor JJ, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia. Dementia and Geriatric Cognitive Disorders 2004;18(1):50‐4. [DOI] [PubMed] [Google Scholar]
- Requena C, Maestu F, Campo P, Fernandez A, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia: two‐year follow up. Dementia and Geriatric Cognitive Disorders 2006;22(4):339‐45. [DOI] [PubMed] [Google Scholar]
Schreiber 1999 {published data only}
- Schreiber M, Schweizer A, Lutz K, Kalveram KT, Jaencke Lutz. Potential of an interactive computer‐based training in the rehabilitation of dementia: an initial study. Neuropsychological Rehabilitation 1999; Vol. 9, issue 2:155‐67.
Sheikh 1986 {published data only}
- Sheikh JI, Hill RD, Yesavage JA. Long‐term efficacy of cognitive training for age‐associated memory impairment: a six‐month follow‐up study. Developmental Neuropsychology 1986; Vol. 2, issue 4:413‐21.
Spector 2003 {published data only}
- Knapp M, Thorgrimsen L, Patel A, et al. Cognitive stimulation therapy for people with dementia: cost‐effectiveness analysis. British Journal of Psychiatry 2006;188:574‐80. [DOI] [PubMed] [Google Scholar]
- Orrell M, Spector A, Thorgrimsen L, Woods B. A pilot study examining the effectiveness of maintenance cognitive stimulation therapy (MCST) for people with dementia. International Journal of Geriatric Psychiatry 2005;20(5):446‐51. [DOI] [PubMed] [Google Scholar]
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence‐based stimulation therapy programme for people with dementia. British Journal of Psychiatry 2003;183(24):248‐54. [DOI] [PubMed] [Google Scholar]
Talassi 2007 {published data only}
- Talassi E, Guerreschi M, Feriani M, Fedi V, Bianchetti A, Trabucchi M. Effectiveness of a cognitive rehabilitation program in mild dementia (MD) and mild cognitive impairment (MCI): a case control study. Archives of Gerontology and Geriatrics 2007;44:391‐9. [DOI] [PubMed] [Google Scholar]
Tarraga 2006 {published data only}
- Tarraga L, Modinos G, et al. A randomised pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry 2006;77:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Tilborg 2011 {published data only}
- Tilborg Ilse ADA, Kessels RPC, Hulstijn W. How should we teach everyday skills in dementia? A controlled study comparing implicit and explicit training methods. Clinical Rehabilitation 2011;25(7):638‐48. [DOI] [PubMed] [Google Scholar]
Viola 2011 {published data only}
- Viola LF, Nunes PV, Yassuda MS, Aprahamian I, Santos FS, Santos GD, et al. Effects of a multidisciplinary cognitive rehabilitation program for patients with mild Alzheimer's disease. Clinics (Sao Paulo, Brazil) 2011; Vol. 66, issue 8:1395‐400. [DOI] [PMC free article] [PubMed]
Yesavage 1981 {published data only}
Zarit 1982 {published data only}
References to ongoing studies
ACTRN12611001173987 {published data only}
- ACTRN12611001173987. Effectiveness of self‐management skills enhancing group rehabilitation on dementia patients' and their spousal caregivers' quality‐of‐life. ANZCTR: http://www.anzctr.org.au/ACTRN12611001173987.aspx.
ISRCTN21027481 {published data only}
- ISRCTN21027481. Goal‐oriented cognitive Rehabilitation in Early‐stage Alzheimer's disease: multi‐centre single‐blind randomised controlled Trial (GREAT). ISRCTN: http://isrctn.org/ISRCTN21027481. [DOI] [PMC free article] [PubMed]
NCT01329484 {published data only}
- NCT01329484. Computerized personal interventions for Alzheimer's patients. http://www.clinicaltrials.gov/ct2/show/study/NCT01329484?term=NCT01329484&rank=1.
NCT01689948 {published data only}
- NCT01689948. Alzheimer disease: rehabilitation's intervention at home. A preliminary pilot study. ClinicalTrials.gov: http://clinicaltrials.gov/show/NCT01689948.
Vidovich 2011 {published data only}
- Vidovich MR, Shaw J, Flicker L, Almeida OP. Cognitive activity for the treatment of older adults with mild Alzheimer's disease (AD)-PACE AD: study protocol for a randomised controlled trial. Trials 2011; Vol. 12:47. [DOI] [PMC free article] [PubMed]
Additional references
Albert 2011
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging and Alzheimer's Association workgroup. Alzheimer's and Dementia 2011;7(3):270‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alzheimer's Disease International 2009
- Alzheimer's Disease International. World Alzheimer's Report. 2009. [http://www.alz.co.uk/research/files/WorldAlzheimerReport‐ExecutiveSummary.pdf]
APA 1995
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. IV. Washington, DC: American Psychiatric Association, 1995. [Google Scholar]
Arnaiz 2003
- Arnaiz E, Almkvist O. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease.. Acta Neurologica Scandinavica 2003; Suppl 179:34‐41. [PubMed] [Google Scholar]
Belleville 2011
Bird 2001
- Bird M. Behavioural difficulties and cued recall of adaptive behaviour in dementia: experimental and clinical evidence. Neuropsychological Rehabilitation 2001;11:357‐75. [Google Scholar]
Boudreaux 2011
Bourgeois 1990
- Bourgeois M. Enhancing conversation skills in patients with Alzheimer's disease using a prosthetic memory aid. Journal of Applied Behavior Analysis 1990;23:29‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braak & Braak 2012
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurologica Scandinavica 2012;94(S165):3‐12. [DOI] [PubMed] [Google Scholar]
Brandt 1995
- Brandt J, Rich JB. Memory disorders in the dementias. In: Baddeley AD, Wilson BA, Watts FN editor(s). Handbook of Memory Disorders. Chichester: John Wiley & Sons Ltd, 1995. [Google Scholar]
Bäckman 1991
- Bäckman L, Josephsson S, Herlitz A, Stigsdotter A, Viitanen M. The generalisability of training gains in dementia: effects of an imagery‐based mnemonic on face‐name retention in dementia. Psychology and Aging 1991;6(3):489‐92. [DOI] [PubMed] [Google Scholar]
Bäckman 1992
- Bäckman L. Memory training and memory improvement in Alzheimer's disease: rules and exceptions. Acta Neurologica Scandinavica 1992; Suppl 139:84‐9. [DOI] [PubMed] [Google Scholar]
Bäckman 1996
- Bäckman L. Utilizing compensatory task conditions for episodic memory in Alzheimer's disease. Acta Neurologica Scandinavica 1996; Suppl 165:109‐13. [DOI] [PubMed] [Google Scholar]
Camp 1989
- Camp CJ. Facilitation of new learning in Alzheimer's disease. In: Gilmore G, Whitehouse P, Wykle M editor(s). Memory and Aging: Theory, Research and Practice. New York: Springer, 1989. [Google Scholar]
Camp 2000
- Camp CJ, Bird MJ, Cherry KE. Retrieval strategies as a rehabilitation aid for cognitive loss in pathological aging. In: Hill RD, Bäckman L, Stigsdotter Neely A editor(s). Cognitive Rehabilitation in Old Age. Oxford: Oxford University Press, 2000. [Google Scholar]
Christensen 1998
- Christensen H, Kopelman MD, Stanhope N, Lorentz L, Owen P. Rates of forgetting in Alzheimer dementia. Neuropsychologia 1998;36:547‐57. [DOI] [PubMed] [Google Scholar]
Clare 1999
- Clare L, Wilson BA, Breen K, Hodges JR. Errorless learning of face‐name associations in early Alzheimer's disease. Neurocase 1999;5:37‐46. [Google Scholar]
Clare 2000
- Clare L, Wilson BA, Carter G, Gosses A, Breen K, Hodges JR. Intervening with everyday memory problems in early Alzheimer's disease: an errorless learning approach. Journal of Clinical and Experimental Neuropsychology 2000;22:132‐46. [DOI] [PubMed] [Google Scholar]
Clare 2001
- Clare L, Wilson BA, Carter G, Hodges JR, Adams M. Long‐term maintenance of treatment gains following a cognitive rehabilitation intervention in early dementia of Alzheimer type: a single case study. Neuropsychological Rehabilitation 2001;11:477‐94. [Google Scholar]
Clare 2004
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Awareness in early‐stage dementia: relationship to outcome of cognitive rehabilitation intervention. Journal of Clinical and Experimental Neuropsychology 2004;26:215‐26. [DOI] [PubMed] [Google Scholar]
Clare, Wilson et al 2000
- Clare L, Wilson BA, Carter G, Breen K, Gosses A, Hodges JR. Intervening with everyday memory problems in dementia of Alzheimer type: an errorless learning approach. Journal of Clinical and Experimental Neuropsychology 2000 Feb;22(1):132‐46. [DOI] [PubMed] [Google Scholar]
Collie 2000
- Collie A, Marruf P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment.. Neuroscience and Biobehavioural Reviews 2000;24:365‐74. [DOI] [PubMed] [Google Scholar]
Dunn 2007
- Dunn J, Clare L. Learning face‐name associations in early‐stage dementia: comparing the effects of errorless learning and effortful processing. Neuropsychological Rehabilitation 2007 Dec;17(6):735‐54. [DOI] [PubMed] [Google Scholar]
Farina 2002
- Farina E, Fioravanti R, Chiavari L, Imbornone E, Alberoni M, Pomati S, et al. Comparing two programs of cognitive training in Alzheimer's disease: a pilot study. Acta Neurologica Scandinavica 2002;105:365‐71. [DOI] [PubMed] [Google Scholar]
Fernandez‐Prado 2012
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. 'Mini‐mental state': a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Fowler 2002
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT.. Journal of the International Neuropsychological Society 2002;8(1):58‐71. [PubMed] [Google Scholar]
Gates 2011
- Gates N, Sachdev P, Singh MF, Valenzuela M. Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatrics 2011;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gauthier 2006
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet 2006 Apr 15;367(9518):1262‐70. [DOI] [PubMed] [Google Scholar]
Graham 2004
- Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. Journal of Neurololgy Neurosurgery and Psychiatry 2004;75(1):61‐71. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. [Google Scholar]
Hill 1987
- Hill RD, Evankovich KD, Sheikh JI, Yesavage JA. Imagery mnemonic training in a patient with primary degenerative dementia. Psychology and Aging 1987;2:204‐5. [DOI] [PubMed] [Google Scholar]
Hofmann 1996
- Hofmann M, Hock C, Kuhler A, Muller‐Spahn F. Interactive computer‐based cognitive training in patients with Alzheimer's disease. Journal of Psychiatric Research 1996;30:493‐501. [DOI] [PubMed] [Google Scholar]
Hughes 1982
- Hughes C, Berg L, Danziger W, Coben L, Martin R. A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982;140:566‐72. [DOI] [PubMed] [Google Scholar]
Institute of Medicine 2011
- Koehler R, Wilhelm E, Shoulson I. Cognitive Rehabilitation Therapy for Traumatic Brain Injury. Cognitive Rehabilitation Therapy for Traumatic Brain Injury: Evaluating the Evidence. Washington, DC: The National Academies Press, 2011. [Google Scholar]
Jaeggi 2010
- Jaeggi SM, Studer‐Luethi B, Buschkuehl M, Su YF, Jonides J, Perrig WJ. The relationship between n‐back performance and matrix reasoning—implications for training and transfer. Intelligence 2010;38(6):625‐35. [Google Scholar]
Jean 2010
- Jean L, Bergeron MÈ, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. American Journal of Geriatric Psychiatry 2010;18(4):281. [DOI] [PubMed] [Google Scholar]
Josephsson 1993
- Josephsson S, Backman L, Borell L, Bernspang B, Nygard L, Ronnberg L. Supporting everyday activities in dementia: an intervention study. International Journal of Geriatric Psychiatry 1993;8:395‐400. [Google Scholar]
Kesslak 1997
- Kesslak JP, Nackoul K, Sandman CA. Memory training for individuals with Alzheimer's disease improves name recall. Behavioural Neurology 1997;10:137‐42. [DOI] [PubMed] [Google Scholar]
Kopelman 1985
- Kopelman MD. Rates of forgetting in Alzheimer‐type dementia and Korsakoff's syndrome. Neuropsychologia 1985;23:623‐38. [DOI] [PubMed] [Google Scholar]
Kurlychek 1983
- Kurlychek RT. Use of a digital alarm chronograph as a memory aid in early dementia. Clinical Gerontologist 1983;1:93‐4. [Google Scholar]
Little 1986
- Little AG, Volans PJ, Hemsley DR, Levy R. The retention of new information in senile dementia.. British Journal of Clinical Psychology 1986;25:71‐2. [DOI] [PubMed] [Google Scholar]
Lowndes & Savage 2007
- Lowndes G, Savage G. Early detection of memory impairment in Alzheimer’s disease: a neurocognitive perspective on assessment.. Neuropsychology Review 2007;17(3):193‐202. [DOI] [PubMed] [Google Scholar]
Lyketsos 2002
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288(12):1475‐83. [DOI] [PubMed] [Google Scholar]
Martin 2011
- Martin M, Clare MM, Altgassen AM, Cameron MH, Zehnder F. Cognition‐based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurology 1984;34:939‐44. [DOI] [PubMed] [Google Scholar]
McLellan 1991
- McLellan DL. Functional recovery and the principles of disability medicine. In: Swash M, Oxbury J editor(s). Clinical Neurology. Vol. 1, London: Churchill Livingstone, 1991:768‐90. [Google Scholar]
Moore 2001
- Moore S, Sandman CA, McGrady K, Kesslak JP. Memory training improves cognitive ability in patients with dementia. Neuropsychological Rehabilitation 2001;11(3/4):245‐61. [Google Scholar]
Morris 1996
- Morris RG. The neuropsychology of Alzheimer's disease and related dementias. In: Woods RT editor(s). Handbook of the Clinical Psychology of Ageing. Chichester: John Wiley & Sons Ltd, 1996. [Google Scholar]
Mortby 2011
- Mortby ME, Maercker A, Forstmeier S. Apathy: a separate syndrome from depression in dementia? A critical review. Aging Clinical and Experimental Research 2011;24(4):305‐16. [DOI] [PubMed] [Google Scholar]
Newhouse 1997
- Newhouse PA, Potter A, Levin ED. Nicotinic system involvement in Alzheimer's and Parkinson's diseases: implications for therapeutics. Drugs and Aging 1997;11:206‐28. [DOI] [PubMed] [Google Scholar]
Olazaran 2010
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña‐Casanova J, Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dementia and Geriatric Cognitive Disorders 2012;30(2):161‐78. [DOI] [PubMed] [Google Scholar]
Owen 2010
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, et al. Putting brain training to the test. Nature 2010;465(7299):775‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papp 2009
- Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimer's and Dementia 2009;5(1):50‐60. [DOI] [PubMed] [Google Scholar]
Peretz 2011
- Peretz C, Korczyn AD, Shatil E, Aharonson V, Birnboim S, Giladi N. Computer‐based, personalized cognitive training versus classical computer games: a randomized double‐blind prospective trial of cognitive stimulation.. Neuroepidemiology 2011;36(2):91‐9. [DOI] [PubMed] [Google Scholar]
Quayhagen & Quayhagen 2001
- Quayhagen M, Quayhagen M. Testing of a cognitive stimulation intervention for dementia caregiving dyads. Neuropsychological Rehabilitation 2001;11(3‐4):319‐332. [Google Scholar]
Rabipour & Raz 2012
- Rabipour S, Raz A. Training the brain: fact and fad in cognitive and behavioral remediation. Brain and Cognition 2012;79(2):159‐79. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Reifler 1990
- Reifler BV, Larson E. Excess disability in dementia of the Alzheimer's type. In: Light E, Lebowitz BD editor(s). Alzheimer's Disease Treatment and Family Stress. New York: Hemisphere, 1990. [Google Scholar]
Roman 1993
- Roman GC, Tatemichi MD, Erkinjuntti MD, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43:250‐60. [DOI] [PubMed] [Google Scholar]
Salomone 2012
- Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer's disease: focus on disease modifying drugs. British Journal of Clinical Pharmacology 2012;73(4):504‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sitzer 2006
- Sitzer D, Twamley E, Jeste D. Cognitive training in Alzheimer's disease: a meta‐analysis of the literature. Acta Psychiatrica Scandinavica 2006;114(2):75‐90. [DOI] [PubMed] [Google Scholar]
Small 1997
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association and the American Geriatric Society. JAMA 1997;278:1363‐71. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (Eds). Chapter 10: Addressing reporting biases. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. [Google Scholar]
Van Paasschen 2013
- Paasschen J, Clare L, Yuen K, Woods R, Evans S, Parkinson C, et al. Cognitive rehabilitation changes memory‐related brain activity in people with Alzheimer’s disease. Neurorehabilitation and Neural Repair 2013. [DOI: 10.1177/1545968312471902] [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organisation. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organisation, Division of Mental Health, 1992. [Google Scholar]
WHO 2001
- World Health Organisation. International Classification of Functioning, Disabilitiy and Health (ICF). http://www.who.int/classifications/icf/en/ Accessed on May 14th 2013.
Wilson 2002
- Wilson BA. Towards a comprehensive model of cognitive rehabilitation. Neuropsychological Rehabilitation 2002;12(2):97‐110. [Google Scholar]
Woods & Clare 2006
- Woods RT, Clare L. Cognition‐based therapies and mild cognitive impairment. In: Tuokko H, Hultsch D editor(s). Mild Cognitive Impairment: International Perspectives. New York: Taylor & Francis, 2006:245‐64. [Google Scholar]
Woods 2012
- Woods B, Aguirre E, Spector A, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI] [PubMed] [Google Scholar]
Ylvisaker 2002
- Ylvisaker M, Hanks R, Johnson‐Greene D. Perspectives on rehabilitation of individuals with cognitive impairment after brain injury: rationale for reconsideration of theoretical paradigms. The Journal of Head Trauma Rehabilitation 2002;17(3):191‐209. [DOI] [PubMed] [Google Scholar]
Zanetti 1994
- Zanetti O, Magni E, Binetti G, Bianchetti A, Trabucchi M. Is procedural memory stimulation effective in Alzheimer's disease?. International Journal of Geriatric Psychiatry 1994;9:1006‐7. [Google Scholar]
Zanetti 1997
- Zanetti O, Binetti G, Magni E, Rozzini L, Bianchetti A, Trabucchi M. Procedural memory stimulation in Alzheimer's disease: impact of a training programme. Acta Neurologica Scandinavica 1997;95:152‐7. [DOI] [PubMed] [Google Scholar]
Zanetti 2001
- Zanetti O, Zanieri G, Giovanni G, Vreese LP, Pezzini A, Metitieri T, et al. Effectiveness of procedural memory stimulation in mild Alzheimer's disease patients: a controlled study. Neuropsychological Rehabilitation 2001;11:263‐72. [Google Scholar]
References to other published versions of this review
Clare 2003
- Clare L, Woods RT, Moniz‐Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early‐stage Alzheimer's disease and vascular dementia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003260] [DOI] [PubMed] [Google Scholar]
Clare 2008
- Clare L, Woods B. Cognitive rehabilitation and cognitive training for early‐stage Alzheimer’s disease and vascular dementia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI] [PubMed] [Google Scholar]
Clare, Woods, Moniz‐Cook et al 2001
- Clare L, Woods RT, Moniz ‐ Cook ED, Orrell M, Spector A. Cognitive rehabilitation interventions to improve memory functioning in early‐stage Alzheimer'sdisease and vascular dementia. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI] [PubMed] [Google Scholar]


