Abstract
Chondral and osteochondral defects in the knee are common and may lead to degenerative joint disease if treated inappropriately.
Conventional treatments such as microfracture often result in fibrocartilage formation and are associated with inferior results. Additionally, microfracture is generally unsuitable for the treatment of defects larger than 2–4 cm2.
The osteochondral autograft transfer system (OATS) has been shown to produce superior clinical outcomes to microfracture but is technically difficult and may be associated with donor-site morbidity. Osteochondral allograft use is limited by graft availability and failure of cartilage incorporation is an issue.
Autologous chondrocyte implantation (ACI) has been shown to result in repair with hyaline-like cartilage but involves a two-stage procedure and is relatively expensive.
Rehabilitation after ACI takes 12 months, which is inconvenient and not feasible for athletic patients.
Newer methods to regenerate cartilage include autologous stem cell transplantation, which may be performed as a single-stage procedure, can have a shorter rehabilitation period and is less expensive than ACI. Longer-term studies of these methods are needed.
Cite this article: EFORT Open Rev 2020;5:156-163. DOI: 10.1302/2058-5241.5.190031
Keywords: autologous chondrocyte implantation, cartilage repair/regeneration techniques, mesenchymal stem cells
Introduction
Articular cartilage is a highly specialized connective tissue that provides a smooth, lubricated, friction-reducing surface.1 Histologically, articular cartilage is ‘hyaline’ and consists of a dense extracellular matrix with chondrocytes derived from mesenchymal cells during development. The extracellular matrix contains predominantly water, collagen and proteoglycans, with smaller amounts of non-collagenous proteins. Type II collagen is the most abundant form of collagen and accounts for 90–95% of the collagen in the extracellular matrix.1,2 Cartilage is avascular, aneural and alymphatic and consequently has limited regenerative potential.3
Chondral and osteochondral lesions of the knee are common and may lead to significant pain and morbidity. Chondral lesions have been found in approximately 60% of patients undergoing knee arthroscopy.4 These lesions may lead to significant pain and morbidity.5,6 Chondral defects are associated with higher contact stresses in the adjacent intact cartilage.7–9 If left untreated, progressive cartilage degeneration and ultimately ‘early-onset’ osteoarthritis may occur.10
The management of chondral defects is challenging. Numerous surgical techniques have been used. This review discusses current treatment options with a focus on chronic osteochondral defects of the knee.
Bone marrow stimulation techniques
Bone marrow stimulation procedures are commonly used to treat osteochondral lesions and are usually performed arthroscopically. There are many proposed methods, the most commonly used being microfracture, subchondral drilling or abrasion of the subchondral bone, mosaicplasty and autologous matrix-induced chondrogenesis.
The aim of bone marrow stimulation is to penetrate the subchondral bone plate with an awl, allowing a fibrin clot containing mesenchymal stem cells to be produced in the defect.11 The mesenchymal stem cells are able to differentiate into fibrochondrocytes, leading to fibrocartilage formation and occasionally hyaline cartilage. Fibrocartilage consists of predominantly type I collagen and has inferior biochemical and biomechanical properties compared with hyaline cartilage.12
Microfracture
Microfracture is generally considered the first-line procedure for the treatment of osteochondral defects.13 Microfracture involves using an arthroscopic awl to make multiple holes approximately 3 to 4 mm apart and 4 mm in depth across the defect.14 A number of studies have shown that microfracture has good clinical outcomes for smaller lesions, i.e. less than 2 to 4 cm2 in size, in younger patients.15,16 Outcomes are less favourable in the long term, with failure expected after five years.16
Subchondral drilling or abrasion
Subchondral drilling is an alternative to microfracture that involves drilling multiple holes into the subchondral bone plate using either a surgical twist drill bit or a Kirschner wire.17 It is less popular than microfracture due to the theoretical risk of thermal necrosis, although it has been demonstrated in an animal model that subchondral drilling does not lead to greater osteocyte death due to thermal necrosis than microfracture.18 Choi and Lee compared subchondral drilling and microfracture for the treatment of small to mid-sized osteochondral lesions of the talus and found similar improvements in clinical outcomes at a mean follow-up of 43 months.19 Subchondral abrasion involves using a motorized burr to debride the defect. This technique is also less commonly used due to the risk of thermal necrosis as well as injury to the underlying bone, resulting in necrosis, hypertrophy or cysts.14
Autologous matrix-assisted chondrogenesis
The fibrin clot produced following microfracture does not have adequate mechanical stability to withstand tangential forces.20 Autologous matrix-assisted chondrogenesis combines microfracture with a collagen scaffold. The aim is to improve the mechanical stability of the clot and to provide a stimulus for chondrogenic differentiation. Significant improvements in clinical scores have been demonstrated with this technique including for defects up to 12 cm2.20 A randomized controlled trial (RCT) evaluating the use of a chitosan-based scaffold combined with microfracture compared with microfracture alone has shown superior repair tissue quantity and quality over microfracture alone at five years but no difference in clinical outcomes.21
Mosaicplasty or osteochondral autograft transfer
Mosaicplasty or the osteochondral autograft transfer system (OATS) results in immediate filling of osteochondral defects with hyaline cartilage.13 The procedure involves harvesting multiple cylindrical osteochondral plugs from a non-weight-bearing portion of the joint. The plugs are then inserted into the defect site. The OATS procedure is a form of mosaicplasty. The plugs are usually larger and therefore fewer plugs are needed than for mosaicplasty. A systematic review of ten studies with a total of 610 patients who underwent osteochondral autograft transfer for knee osteochondral defects, with a mean defect size of 2.6 cm2, showed significantly improved clinical outcomes with an overall survival rate of 72% at a mean follow-up of 10.2 years. Increased age, previous surgery and larger defect size were associated with an increased failure rate.22 Mosaicplasty has been shown to result in better clinical outcomes than microfracture.23 The disadvantages of mosaicplasty or the OATS procedure include technical difficulty, donor-site morbidity, potential mismatch in the size and shape of the grafts compared with the defect and poorer results for larger defects.24,25
This is unsurprising since the gaps between the osteochondral plugs fill with fibrous tissue, which develops into fibrocartilage so that a homogeneous repair surface cannot be achieved.
A randomized controlled clinical trial has demonstrated the inferiority of mosaicplasty to autologous chondrocyte implantation (ACI) except for very small lesions of less than 2 cm2.25
Osteochondral allograft transplantation
Fresh osteochondral allograft (OCA) transplantation is an alternative to OATS and may be useful in cases where there has been a previous failed cartilage repair procedure.26,27 The advantage of this technique is that it avoids donor-site morbidity and may be used to treat Osteochondral defects (OCDs) that are too large for an autograft to be successful.26 Numerous studies have shown improved function with this technique for the treatment of both focal and diffuse lesions and in high-demand patients.26,28,29 Survivorship of 82% at 10 years and 74% at 15 years has been reported.30 Fresh osteochondral allografts stored at physiological temperatures are used due to high levels of viable donor chondrocytes, which is important for the success of OCA transplantation.13 The principle problem with this technique is that the graft induces a host-graft reaction, which can lead to graft failure. Furthermore, although thin bone grafts of 1 cm or less may incorporate, the cartilage graft does not incorporate with the articular cartilage around the defect. Additionally, graft availability may be a factor limiting the use of OCA transplantation; however, newer methods have been developed to improve the preservation of OCAs, which increases the availability of grafts.31
Autologous chondrocyte implantation (ACI) and matrix-assisted chondrocyte implantation (MACI)
Cell-based therapies have been developed by Brittberg et al32 based on the original animal studies of Bentley and Greer in the 1970s33 with the aim of achieving normal hyaline cartilage repair of OCDs.34 Autologous chondrocyte implantation (ACI) involves a two-stage approach. The first stage consists of harvesting cartilage from a non-weight-bearing portion of the joint. Enzymatic digestion is used to release chondrocytes from the cartilage. The cells are then culture-expanded in vitro for 4 to 6 weeks. In the second stage of the procedure, the chondrocytes are implanted into the defect. The original technique for ACI involved injection of the chondrocytes under a periosteal sleeve secured with fine sutures and sealed with fibrin glue (Fig. 1). This was commonly complicated by hypertrophy of the periosteum leading to painful clicking in approximately 25% of patients.35,36 Matrix-assisted chondrocyte implantation (MACI) is the second-generation technique and uses a type I/III collagen scaffold. The chondrocytes are cultured on the scaffold and then implanted into the defect and secured with fibrin glue.35,37 This technique has reduced the operative time and avoids the complications associated with the use of a periosteal patch. A further development is the application of chondrocytes cultured as small spheroids (chondrospheres).38
Fig. 1.
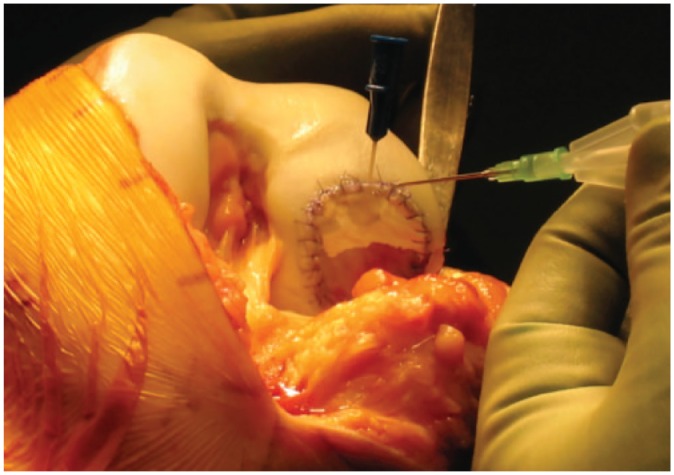
Intra-operative image of autologous chondrocyte implantation. Reproduced with permission from Professor George Bentley.
Autologous chondrocyte implantation (ACI) and MACI have been shown in histological studies to result in hyaline or hyaline-like cartilage formation.32,35 Graft survival of approximately 78% at five years and 51% beyond ten years as well as improved functional outcomes compared with mosaicplasty have been demonstrated with ACI and MACI in a large prospective study involving 831 patients.37,39 ACI/MACI has been used successfully for large defects up to 22 cm2 in size.37,40,41 Minas et al demonstrated ACI graft survivorship of 71% at 10 years and improved function in 75% of 210 patients with knee osteochondral defects with a mean size of 8.4 cm2.42 ACI/MACI is approved by the National Institute for Health and Care Excellence (NICE) in the UK and is recommended as a first-line procedure in appropriate patients.41 The main disadvantage of ACI/MACI is that it requires a two-stage approach, has a long rehabilitation period, and is associated with moderately high financial costs.
Stem cell transplantation
Stem cells have the ability to divide and differentiate into many different cell types in the body. Mesenchymal stem cells are multipotent cells that can differentiate into mesenchymal phenotypes including chondrocytes and are known to be capable of self-renewal as well as immune-modulatory and anti-inflammatory action.43–46 Bone-marrow-derived mesenchymal stem cell transplantation does not require a period of in vitro culture expansion and may be performed as a single-stage procedure, which makes it significantly less expensive than ACI/MACI.
Intra-articular injections of stem cells from various sources have been used to treat osteochondral defects and although the results are variable, some studies have shown improvements in clinical outcomes for patients with OCDs and osteoarthritis. Case series of intra-articular injections of autologous adipose-derived mesenchymal stem cells have shown improvements in clinical scores, magnetic resonance imaging (MRI) findings, arthroscopic appearances and/or histological evidence of hyaline-like cartilage formation.47–49 Allogeneic bone-marrow-derived mesenchymal stem cells have been injected into knees with osteoarthritis and demonstrated improvements in pain, quality of life and MRI appearances of cartilage quality compared with a control group receiving an intra-articular hyaluronic acid injection.50 However, the number of stem cells obtainable from bone marrow is known to be limited51,52 and there may be advantages in using alternative stem cell sources. Adipose tissue may be obtained from abdominal liposuction but may be associated with donor-site morbidity. The infrapatellar fat pad is being investigated as an alternative source of adipose-derived stem cells.53,54 Synovium is another source of stem cells being studied. A shortcoming of bone-marrow-derived stem cells is that they may differentiate into fibrous-like tissue instead of hyaline cartilage.55 It is hypothesized that synovium-derived stem cells may have the ability to enhance chondrogenic potential as the synovial membrane is attached at the surface edges of articular cartilage.55,56 Sekiya et al injected synovial stem cells cultured in autologous human serum into isolated cartilage defects under arthroscopic control and found improved clinical and MRI outcomes.57
Autologous mesenchymal stem cells within a scaffold have been transplanted into osteochondral defects as a single-stage procedure in a small number of studies so far. The majority of these studies have been case series. For example, Buda et al transplanted stem cells within a hyaluronic acid membrane into medial or lateral femoral condyle defects in 20 patients.58 They demonstrated significant clinical improvements at two-year follow-up. Nejadnik et al conducted a cohort study comparing ACI and stem cell transplantation with 36 patients in each group. Overall, they found comparable results in both groups with an improved quality of life. Physical role functioning improved to a greater degree over time in the patients who had undergone stem cell transplantation.59 There is currently an ongoing prospective assessment of stem cell transplantation into knee OCDs at the Royal National Orthopaedic Hospital, Stanmore, UK, which has demonstrated improvements in pain and functional scores in 100 patients at one year post-operatively (unpublished). A collagen type I scaffold with autologous bone-marrow-derived mesenchymal stem cells is transplanted into the OCDs and autologous fibrin glue, which is rich in growth factors, is used the secure the graft (Fig. 2).60 This is a modification of the technique described by Buda et al.58 Longer-term outcomes and randomized control trials are needed before stem cell transplantation is likely to be recommended by NICE as a first-line treatment for osteochondral defects instead of ACI/MACI.
Fig. 2.
Stem cell transplantation. Reproduced with permission from Professor George Bentley.
Growth factors, scaffolds and gene therapy
Growth factors
Growth factors are polypeptides that regulate cellular differentiation and proliferation, e.g. bone morphogenetic proteins (BMP), fibroblast growth factors (FGF), platelet-derived growth factors (PDGF). A variety of growth factors have been shown, both in vitro and in animal models, to have the potential to augment cartilage regeneration. For example, BMP-2 has been shown in small animal studies to enhance cartilage repair.61,62 Platelet-rich plasma (PRP), which contains growth factors, has been shown in clinical studies to result in a greater improvement in clinical and functional outcomes when injected into osteoarthritic knees when compared with hyaluronic acid and saline.63,64 There have been no long-term randomized studies of these methods.
Scaffolds
A variety of scaffolds have been used to deliver stem cells to OCDs and enhance cartilage regeneration. Scaffold materials include collagen type I/III, hyaluronan, polymers and fibrin. They may be fabricated as a sponge, foam, membrane or a gel. Scaffolds should be biocompatible and bioactive while providing mechanical support as well as support of cell proliferation and viability. The ideal scaffold would be enzymatically resorbable or biodegradable, would give way to newly formed tissue and would not hinder the growth of newly formed cartilage or generate any anti-inflammatory reaction. Further basic science investigation is underway to determine which scaffold materials are the most effective as well as to modify scaffold materials to improve the bioactivity and the mechanical and biochemical properties.65
Cell-free scaffolds are being evaluated as a treatment option for cartilage regeneration. Pre-clinical and early clinical studies have shown that type I collagen cell-free scaffolds implanted into cartilage defects may produce improved clinical and radiological results as well as histological appearance of articular cartilage formation with the presence of type II collagen.66–68 A cell-free scaffold fills the defect with a matrix for chondrocyte migration and proliferation. The source of chondrocytes is unclear. Several theories suggest that mesenchymal stem cells migrate into the defect from the subchondral bone, that chondrocytes may migrate from the surrounding articular cartilage or that mesenchymal progenitor cells from synovial fluid may become integrated into the graft and differentiate into chondrocytes.67 A prospective clinical trial of a cell-free collagen type I scaffold for cartilage repair showed clinical failure in 18% of cases at five-year follow-up, necessitating revision. The remaining patients had good to excellent clinical results, improved radiological appearances and histological evidence of articular cartilage-like repair tissue.69
Gene therapy
Gene therapy is another method being investigated to enhance cartilage regeneration. It involves the delivery of genetic material using a gene transfer vector to alter cell synthesis or function.70 Preclinical studies have shown enhanced cartilage regeneration following gene therapy with viral vectors using the basic fibroblast growth factor, insulin-like growth factor, transforming growth factor beta and bone morphogenetic proteins.70–73 There has been limited clinical translation to date. Ha et al conducted a Phase I dose-escalating clinical trial to evaluate the safety and biological activity of a cell-mediated gene therapy technique to deliver allogeneic chondrocytes expressing transforming growth factor beta (TGFb1) directly into the knees of 12 patients with full-thickness chondral defects. No serious adverse events occurred and although there was a dose-dependent trend suggesting clinical improvements, the differences observed were not statistically significant.74 Further research is required before conclusions can be drawn regarding the clinical efficacy of gene therapy.
Conclusions
The current methods commonly used clinically to treat osteochondral lesions mainly result in fibrocartilage formation and are only suitable for the treatment of small defects less than 2–4 cm2 in diameter. Cell-based methods, especially ACI and MACI have resulted in impressive functional outcomes for periods of up to 20 years in several large studies resulting in hyaline-like cartilage formation in larger lesions. Such methods are now accepted for these lesions and are undergoing trials for treatment of early osteoarthritis. Promising results for stem cell transplantation have been demonstrated but more high-level, larger and longer-term studies are needed to prove its effectiveness. Augmentation of cartilage regeneration procedures with the use of growth factors and improved scaffold materials requires long-term randomized studies to evaluate these methods for general clinical use, especially for osteoarthritis.
Footnotes
ICMJE Conflict of interest statement: GB reports that he is the Scientific Editor for EFORT Open Reviews, outside the submitted work.
The other authors report no conflict of interest relevant to this work.
Funding statement
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article. In addition, benefits have been or will be directed to a research fund, foundation, educational institution, or other non-profit organization with which one or more of the authors are associated.
References
- 1. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health 2009;1:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med 2005;33:295–306. [DOI] [PubMed] [Google Scholar]
- 3. Oldershaw RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol 2012;93:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 2002;18:730–734. [DOI] [PubMed] [Google Scholar]
- 5. Moyad TF. Cartilage injuries in the adult knee: evaluation and management. Cartilage 2011;2:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med 2004;32:317–320. [DOI] [PubMed] [Google Scholar]
- 7. Gratz KR, Wong BL, Bae WC, Sah RL. The effects of focal articular defects on cartilage contact mechanics. J Orthop Res 2009;27:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med 2004;32:1451–1458. [DOI] [PubMed] [Google Scholar]
- 9. Wong BL, Sah RL. Effect of a focal articular defect on cartilage deformation during patello-femoral articulation. J Orthop Res 2010;28:1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strauss EJ, Fonseca LE, Shah MR, Yorum T. Management of focal cartilage defects in the knee: is ACI the answer? Bull NYU Hosp Jt Dis 2011;69:63–72. [PubMed] [Google Scholar]
- 11. Chawla A, Twycross-Lewis R, Maffulli N. Microfracture produces inferior outcomes to other cartilage repair techniques in chondral injuries in the paediatric knee. Br Med Bull 2015;116:93–103. [DOI] [PubMed] [Google Scholar]
- 12. Gill TJ, Asnis PD, Berkson EM. The treatment of articular cartilage defects using the microfracture technique. J Orthop Sports Phys Ther 2006;36:728–738. [DOI] [PubMed] [Google Scholar]
- 13. Richter DL, Schenck RC, Jr, Wascher DC, Treme G. Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health 2016;8:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozmeriç A, Alemdaroğlu KB, Aydoğan NH. Treatment for cartilage injuries of the knee with a new treatment algorithm. World J Orthop 2014;5:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller BS, Briggs KK, Downie B, Steadman JR. Clinical outcomes following the microfracture procedure for chondral defects of the knee: a longitudinal data analysis. Cartilage 2010;1:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy 2013;29:1579–1588. [DOI] [PubMed] [Google Scholar]
- 17. Gao L, Goebel LKH, Orth P, Cucchiarini M, Madry H. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech 2018;11:dmm034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H, Sun J, Hoemann CD, et al. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res 2009;27:1432–1438. [DOI] [PubMed] [Google Scholar]
- 19. Choi JI, Lee KB. Comparison of clinical outcomes between arthroscopic subchondral drilling and microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 2016;24:2140–2147. [DOI] [PubMed] [Google Scholar]
- 20. Gille J, Behrens P, Volpi P, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg 2013;133:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shive MS, Stanish WD, McCormack R, et al. BST-CarGel® treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage 2015;6:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pareek A, Reardon PJ, Maak TG, Levy BA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy 2016;32:1174–1184. [DOI] [PubMed] [Google Scholar]
- 23. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15–17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med 2018;46:826–831. [DOI] [PubMed] [Google Scholar]
- 24. Patil S, Tapasvi SR. Osteochondral autografts. Curr Rev Musculoskelet Med 2015;8:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bentley G, Biant LC, Carrington RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br 2003;85:223–230. [DOI] [PubMed] [Google Scholar]
- 26. Thomas D, Shaw KA, Waterman BR. Outcomes after fresh osteochondral allograft transplantation for medium to large chondral defects of the knee. Orthop J Sports Med 2019;7:2325967119832299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res 2010;468:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Briggs DT, Sadr KN, Pulido PA, Bugbee WD. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage 2015;6:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy 2013;29:575–588. [DOI] [PubMed] [Google Scholar]
- 30. Levy YD, Görtz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res 2013;471:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook JL, Stoker AM, Stannard JP, et al. A novel system improves preservation of osteochondral allografts. Clin Orthop Relat Res 2014;472:3404–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889–895. [DOI] [PubMed] [Google Scholar]
- 33. Bentley G, Greer RB., III Homotransplantation of isolated epiphyseal and articular cartilage chondrocytes into joint surfaces of rabbits. Nature 1971;230:385–388. [DOI] [PubMed] [Google Scholar]
- 34. Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc 2017;25:3786–3799. [DOI] [PubMed] [Google Scholar]
- 35. Gikas PD, Bayliss L, Bentley G, Briggs TW. An overview of autologous chondrocyte implantation. J Bone Joint Surg Br 2009;91:997–1006. [DOI] [PubMed] [Google Scholar]
- 36. Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee 2006;13:203–210. [DOI] [PubMed] [Google Scholar]
- 37. Nawaz SZ, Bentley G, Briggs TW, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am 2014;96:824–830. [DOI] [PubMed] [Google Scholar]
- 38. Becher C, Laute V, Fickert S, et al. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J Orthop Surg Res 2017;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br 2012;94:504–509. [DOI] [PubMed] [Google Scholar]
- 40. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 2005;87:640–645. [DOI] [PubMed] [Google Scholar]
- 41. National Institute for Health and Care Excellence (NICE) guidance: Autologous chondrocyte implantation for treating symptomatic articular cartilage defects of the knee. https://www.nice.org.uk/guidance/ta508 (last accessed 7 March 2018).
- 42. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res 2014;472:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E. Stem cells in articular cartilage regeneration. J Orthop Surg Res 2016;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 2007;262:509–525. [DOI] [PubMed] [Google Scholar]
- 45. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc 2013;21:1717–1729. [DOI] [PubMed] [Google Scholar]
- 46. Manferdini C, Maumus M, Gabusi E, et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum 2013;65:1271–1281. [DOI] [PubMed] [Google Scholar]
- 47. Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 2014;32:1254–1266. [DOI] [PubMed] [Google Scholar]
- 48. Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013;29:748–755. [DOI] [PubMed] [Google Scholar]
- 49. Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2015;23:1308–1316. [DOI] [PubMed] [Google Scholar]
- 50. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 2015;99:1681–1690. [DOI] [PubMed] [Google Scholar]
- 51. Freitag J, Shah K, Wickham J, Boyd R, Tenen A. The effect of autologous adipose derived mesenchymal stem cell therapy in the treatment of a large osteochondral defect of the knee following unsuccessful surgical intervention of osteochondritis dissecans: a case study. BMC Musculoskelet Disord 2017;18:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng L, Jia Z, Yin X, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 2008;17:761–773. [DOI] [PubMed] [Google Scholar]
- 53. Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012;19:902–907. [DOI] [PubMed] [Google Scholar]
- 54. do Amaral RJFC, Almeida HV, Kelly DJ, O’Brien FJ, Kearney CJ. Infrapatellar fat pad stem cells: from developmental biology to cell therapy. Stem Cells Int 2017;2017:6843727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee WY, Wang B. Cartilage repair by mesenchymal stem cells: clinical trial update and perspectives. J Orthop Translat 2017;9:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kubosch EJ, Lang G, Furst D, et al. The potential for synovium-derived stem cells in cartilage repair. Curr Stem Cell Res Ther 2018;13:174–184. [DOI] [PubMed] [Google Scholar]
- 57. Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res 2015;473:2316–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am 2010;92:2–11. [DOI] [PubMed] [Google Scholar]
- 59. Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 2010;38:1110–1116. [DOI] [PubMed] [Google Scholar]
- 60. Chimutengwende-Gordon M, Mudussar A, Bentley G, Donaldson J, Miles J, Carrington R. A prospective study of bone marrow-derived stem cell transplantation for the treatment of osteochondral defects of the knee. Perth, WA, Australia: Australian and New Zealand Orthopaedic Research Society, 2018. [Google Scholar]
- 61. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res 2011;469:2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage 1998;6:306–317. [DOI] [PubMed] [Google Scholar]
- 63. Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy 2017;33:659–670.e1. [DOI] [PubMed] [Google Scholar]
- 64. Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol 2008;26:910–913. [PubMed] [Google Scholar]
- 65. Rai V, Dilisio MF, Dietz NE, Agrawal DK. Recent strategies in cartilage repair: a systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A 2017;105:2343–2354. [DOI] [PubMed] [Google Scholar]
- 66. Efe T, Theisen C, Fuchs-Winkelmann S, et al. Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results. Knee Surg Sports Traumatol Arthrosc 2012;20:1915–1922. [DOI] [PubMed] [Google Scholar]
- 67. Schüettler KF, Struewer J, Rominger MB, Rexin P, Efe T. Repair of a chondral defect using a cell free scaffold in a young patient: a case report of successful scaffold transformation and colonisation. BMC Surg 2013;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schneider U, Schmidt-Rohlfing B, Gavenis K, Maus U, Mueller-Rath R, Andereya S. A comparative study of 3 different cartilage repair techniques. Knee Surg Sports Traumatol Arthrosc 2011;19:2145–2152. [DOI] [PubMed] [Google Scholar]
- 69. Schüttler KF, Götschenberg A, Klasan A, et al. Cell-free cartilage repair in large defects of the knee: increased failure rate 5 years after implantation of a collagen type I scaffold. Arch Orthop Trauma Surg 2019;139:99–106. [DOI] [PubMed] [Google Scholar]
- 70. Cucchiarini MMH. Advances in gene therapy for cartilage repair. Ann Joint 2018;3:97. [Google Scholar]
- 71. Cucchiarini M, Madry H, Ma C, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther 2005;12:229–238. [DOI] [PubMed] [Google Scholar]
- 72. Cucchiarini M, Madry H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther 2014;21:811–819. [DOI] [PubMed] [Google Scholar]
- 73. Cucchiarini M, Asen AK, Goebel L, et al. Effects of TGF-β overexpression via rAAV gene transfer on the early repair processes in an osteochondral defect model in minipigs. Am J Sports Med 2018;46:1987–1996. [DOI] [PubMed] [Google Scholar]
- 74. Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 2012;14:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]



