Supplemental Digital Content is available in the text.
Background.
The impact of acute-on-chronic liver failure (ACLF) defined by European Association for the Study of the Liver-Chronic Liver Failure in liver transplant (LT) recipients has not been well characterized. The aim of the study was to assess early posttransplant morbidity and survival of ACLF patients.
Methods.
Eight hundred twenty-five consecutive LT patients (04/2006–03/2013) were included in a retrospective analysis. Of the 690 evaluable patients, 589 had no ACLF, and the remaining 101 were grouped into ACLF Grades 1–3 (ACLF Grade 1: 50 [49.5%], ACLF Grade 2: 32 [31.7%], and ACLF Grade 3: 19 [18.8%]).
Results.
LT recipients transplanted in the context of ACLF had significantly increased serum creatinine (2.27 ± 1.16 versus 0.98 ± 0.32; P < 0.0001), and inferior 1-year graft (90% versus 78%; P < 0.0001) and patient survival (92% versus 82%; P = 0.0004) by Kaplan-Meier survival analysis; graft and patient survival correlated negatively with increasing severity of ACLF. One-year graft and patient survival were lower in those with high ACLF (Grade 2 and 3) irrespective of Model for End-Stage Liver Disease compared with other groups. The ACLF group had longer intensive care unit stays (10.6 ± 19.5 versus 4.2 ± 9; P < 0.0001), hospital stays (20.9 ± 25.9 versus 11.7 ± 11.4; P < 0.0001), and increased surgical re-exploration (26.7 % versus 14.6%, P = 0.002).
Conclusions.
Patients with ACLF undergoing LT have significantly higher resource utilization, inferior graft survival and patient survival, and renal dysfunction at 1 year. The combination of ACLF and Model for End-Stage Liver Disease can be considered when determining the suitability for potential transplantation.
Acute-on-chronic liver failure (ACLF) is an increasingly recognized entity encompassing acute deterioration of patients with chronic liver disease associated with multiple organ failures leading to increased mortality.1 Several definitions of ACLF have been proposed; however, currently the most accepted definition of ACLF was coined by the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) Consortium Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) study.2,3 The CANONIC study proposed diagnostic criteria based on patients with cirrhosis and acute decompensation who had organ failures and a high 28-day mortality rate.2,3 The precipitating events leading to acute deterioration of their liver function were either secondary to superimposed liver injury or extrahepatic factors such as infection or gastrointestinal hemorrhage.1
ACLF patients have been shown to have a high mortality according to the CANONIC study.3 Grade 1 ACLF had a 28-day and 90-day mortality of 22.1% and 40.7%, respectively. Grade 2 ACLF had a 28-day and 90-day mortality of 32.0% and 52.3%, respectively. Grade 3 ACLF had a 28-day and 90-day mortality of 76.7% and 79.1%, respectively. However, the posttransplant morbidity and mortality of patients transplanted for ACLF has not been thoroughly studied using consistent criteria. Although a few recent studies have attempted to address this issue,4,5,6,7 more studies with granular data are needed to better define survival outcomes in patients with ACLF undergoing liver transplant (LT).
Our primary aim was to determine posttransplant early survival outcomes at 90 days and 1-year in a single center based on pretransplant ACLF severity as defined by EASL-CLIF Consortium definition. We also aimed to assess several surrogate markers for resource utilization, such as length of hospital stay, early hospital readmissions, early surgical re-exploration/interventions, and intraoperative transfusion requirements.
MATERIALS AND METHODS
A retrospective review of 825 consecutive LT patients between April 2006 and March 2013 at Methodist University Hospital Transplant Institute was performed. Patients without chronic liver disease (n = 31), retransplant recipients (n = 53), combined liver/kidney transplant recipients (n = 35), and acute liver failure patients (n = 16) were excluded.
Of the remaining 690 evaluable patients, 589 had no ACLF. The final cohort of patients who met the EASL-CLIF Consortium definition included 101 LT recipients, whom were further subdivided into ACLF Grades 1–3 based on the CLIF-Sequential Organ Failure Assessment (SOFA) score.
We defined “ACLF” and “ non-ACLF” using the EASL-CLIF Consortium definition based on data at the time of transplant,8,9 as follows: Non-ACLF: (1) patients with no organ failure; or (2) patients with a single “nonkidney” organ failure who had a serum creatinine level <1.5 mg/dL and no hepatic encephalopathy, or (3) patients with single cerebral failure who had a serum creatinine level <1.5 mg/dL. ACLF Grade 1: (1) patients with single kidney failure; or (2) patients with single failure of the liver, coagulation, circulation or respiration who had a serum creatinine level ranging from 1.5 to 1.9 mg/dL and/or mild to moderate hepatic encephalopathy, or (iii) patients with single cerebral failure who had a serum creatinine level ranging from 1.5 to 1.9 mg/dL. ACLF Grade 2: patients with 2 organ failures. ACLF Grade 3: patients with 3 or more organ failures. The definition of organ failure was based on the CLIF-SOFA score.8 Liver failure was defined by a serum bilirubin level of ≥12.0 mg/dL. Kidney failure was defined by a serum creatinine level of ≥2.0 mg/dL or the use of renal replacement therapy. Cerebral failure was defined by grade III or IV hepatic encephalopathy, according to the West Haven classification. Coagulation failure was defined by an international normalized ratio (INR)>2.5. Circulatory failure was defined by the use of vasopressors. Respiratory failure was defined by the ratio of PaO2 to FiO2 of ≤200 or an SpO2 to FiO2 ratio of ≤214.
Demographic data were collected on all patients including age, race, sex, and cause of liver disease. Laboratory data including bilirubin, creatinine, and INR were collected to calculate the native Model for End-Stage Liver Disease (MELD) score at time of transplantation, as well as to categorize patients into Grades of ACLF. ACLF Grade 2 and 3 were considered high ACLF, and a MELD score ≥30 was considered a high MELD for the purpose of this study. In addition, donor and intraoperative characteristics including use of blood products were recorded.
Posttransplant data collected included liver enzymes, serial serum creatinine measurements, surgical re-exploration following LT, repeat LT due to graft failure, length of stay posttransplant, length of intensive care unit (ICU) stay posttransplant, and liver- and nonliver-related mortality. Considering the greatest impact on survival will be early after LT in the ACLF group, we analyzed survival up until 1-year post-LT separately from long-term survival (beyond 1 y).
The University of Tennessee Health Science Center Institutional Review Board approved the study a priori.
Statistical Considerations
Descriptive statistics were calculated for all of the key variables. Continuous variables were expressed as means with SD and categorical variables as counts with percentages. Statistical significance was set a priori at the conventional P ≤ 0.05. Independent sample t-tests were used to compare the differences between mean values with Wilcoxon 2-Sample Test applied as indicated. We used the normal approximation method and reported 2-sided P-values. Categorical variables were evaluated by Yates corrected chi-squared tests with Fisher exact tests applied as indicated. Survival was analyzed using Kaplan-Meier survival curve using the log-rank tests. Survival time was counted from time of transplant until death or retransplant. Cox proportional hazard model was used to assess predictors of survival including variables significant on univariate analysis and those deemed clinically significant. We examined the Variance Inflation Factor (VIF) in determining which variables may be involved in multicollinearity. For the ith independent variable, the variance inflation factor is defined as 1/(1 − Ri2), where Ri is the coefficient of determination for the regression of the ith independent variable on all other independent variables. Any variables associated with a VIF value exceeding 1/(1 − Ri2) are more closely related to other independent variables than they are related to the dependent variable. If high bivariate correlations are present, and if “multicollinearity” was detected, one of the two variables was deleted and tested in separate models. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
Clinical and Demographic Characteristics
One hundred and one LT recipients (14.6%) with ACLF based on the EASL-CLIF Consortium definition were compared with 589 LT recipients who did not met the criteria for ACLF but had underlying chronic liver disease (Figure 1). The demographic and clinical characteristics of these patients are shown in Table 1. LT recipients were categorized into 3 categories of severity per EASL-CLIF Consortium definition for ACLF: 50 (49.5%) under ACLF Grade 1, 32 (31.7%) under ACLF Grade 2, and 19 (18.8%) under ACLF Grade 3 category. Of the included ACLF patients, 46 (45.5%) had liver failure, 65 (64.4%) had kidney failure, 42 (41.6%) had a coagulation failure, 13 (12.9%) had cerebral failure, 4 (4%) had circulatory failure, and 10 (9.9%) had respiratory failure. Overall mean CLIF-SOFA score in the ACLF group was 10.1 ± 2.1. In the ACLF grade 1, ACLF grade 2, and ACLF grade 3 groups, the mean CLIF SOFA score was 9.1 ± 1, 11.5 ± 0.9, and 13.7 ± 1.5, respectively.
FIGURE 1.
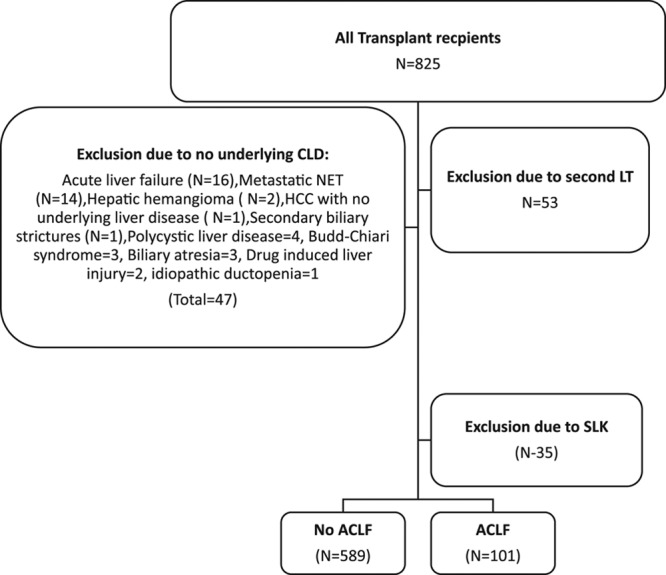
Algorithm showing methods of inclusion of the LT recipients with and without ACLF. ACLF, acute-on-chronic liver failure; LT, liver transplant.
TABLE 1.
Clinical and demographic profile of the LT recipients with and without ACLF
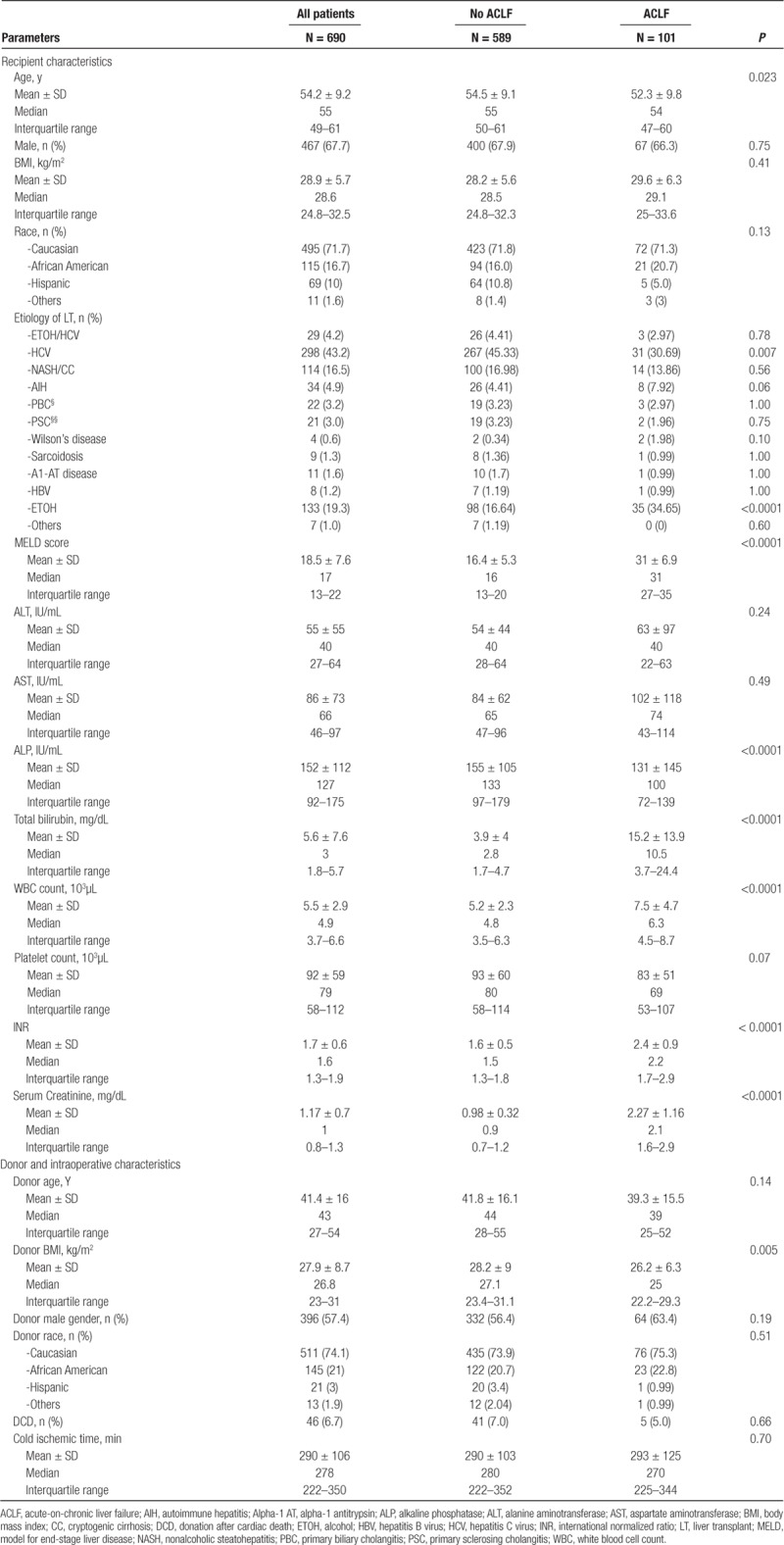
LT recipients with ACLF were younger, but no significant differences were noted in sex, race, or ethnicity. The mean MELD score was significantly higher in the ACLF group when compared with the non-ACLF group (31 ± 6.9 versus 16.4 ± 5.3, P < 0.0001). Etiology of underlying chronic liver disease in the ACLF group was significantly more likely to be alcohol and hepatitis C accounting for one-third each, whereas chronic hepatitis C was the most common underlying liver disease in the non-ACLF group accounting for nearly half of the group. No significant differences were noted in donor characteristics, except patients with ACLF received allografts from recipients with lower BMI.
The mean volume of blood transfused was not significantly different in the ACLF group [4.2 ± 4.8 units, median, 3 units, interquartile range, 0–7 units] compared with the non-ACLF group (3.9 ± 4.8 units, median 3 units, interquartile range, 0–6 units, P = 0.44). In addition, the mean volume of fresh frozen plasma (5.4 ± 6.0 versus 5.6 ± 6.8 units, P = 0.87) and platelets (10.2 ± 9.8 versus 11.2 ± 10.5 units, P = 0.31) was not significantly different.
Early Posttransplant Clinical and Laboratory Profile
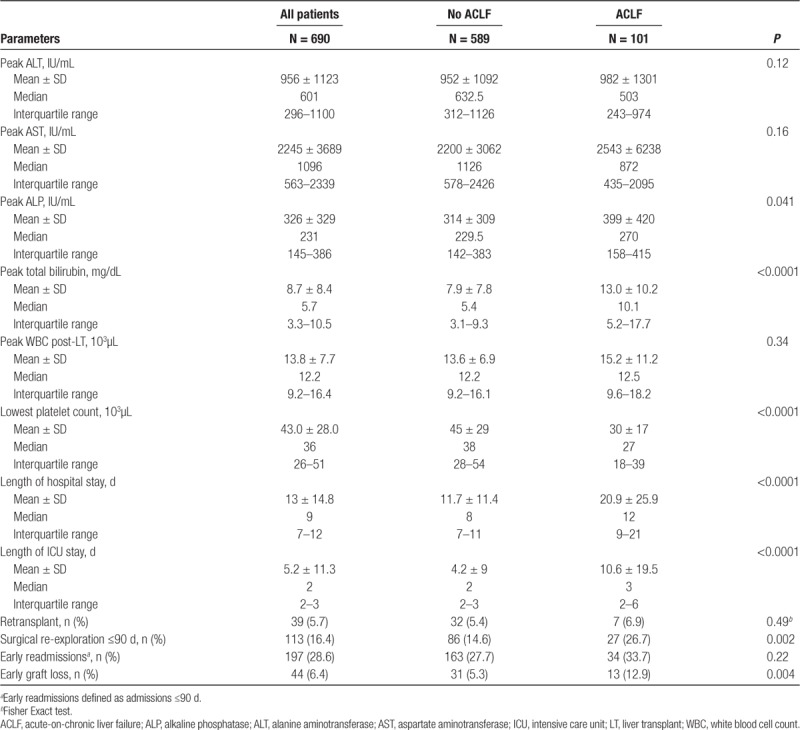
During the early post-LT period, significant differences were noted in the laboratory profile of ACLF and non-ACLF group (Table 2). LT recipients with ACLF not only needed prolonged hospitalization (20.9 ± 25.9 versus 11.7 ± 11.4 d, P < 0.0001) but also had a prolonged length of ICU stay (10.6 ± 19.5 versus 4.2 ± 9 d, P < 0.0001). There was a greater percentage of early (defined as <90 d) hospital readmissions (>1 admissions) in the ACLF group, although the differences were not statistically different. Of the 113 early surgical re-explorations, 27 (26.7%) occurred in the ACLF group compared with 86 (14.6%) in the non-ACLF group; these differences were statistically significant (P = 0.002). The type of early surgical complications/interventions are noted in Table S1 (SDC, http://links.lww.com/TXD/A246).
TABLE 2.
Early posttransplant profile of the LT recipients with and without ACLF
Graft Survival by ACLF
Early graft loss defined as graft loss within 90 days was noted in 44 (6.4%) LT recipients. In the non-ACLF group, 31 (5.3%) had early graft loss as opposed to 13 (12.9%) in the ACLF group (P = 0.004). Further stratification revealed an incremental graft loss with ACLF grades: 31 of 589 (5.3%) in the non-ACLF group, 3 of 50 (6%) with ACLF Grade 1, 6 of 32 (18.8%) with ACLF Grade 2, and 4 of 19 (21%) with ACLF Grade 3 (P < 0.003).
Graft survival was significantly lower in the ACLF group compared with the non-ACLF group by Kaplan-Meier survival analysis at 1-year follow-up (P < 0.0001; Figure 2A). Overall graft survival at 90, 180, 270, 360 days in the non-ACLF and ACLF groups based on ACLF severity grades are displayed in Figure 2B. Among those with ACLF, there was no difference in graft survival based on number of organ failures (Figure S1, SDC, http://links.lww.com/TXD/A246). Graft survival in the non-ACLF group was significantly better than graft survival in those with ACLF Grade 1 (P = 0.003), ACLF Grade 2 (P = 0.0008), and ACLF Grade 3 (P < 0.0001) after adjusting for multiple comparisons using the log-rank test.
FIGURE 2.
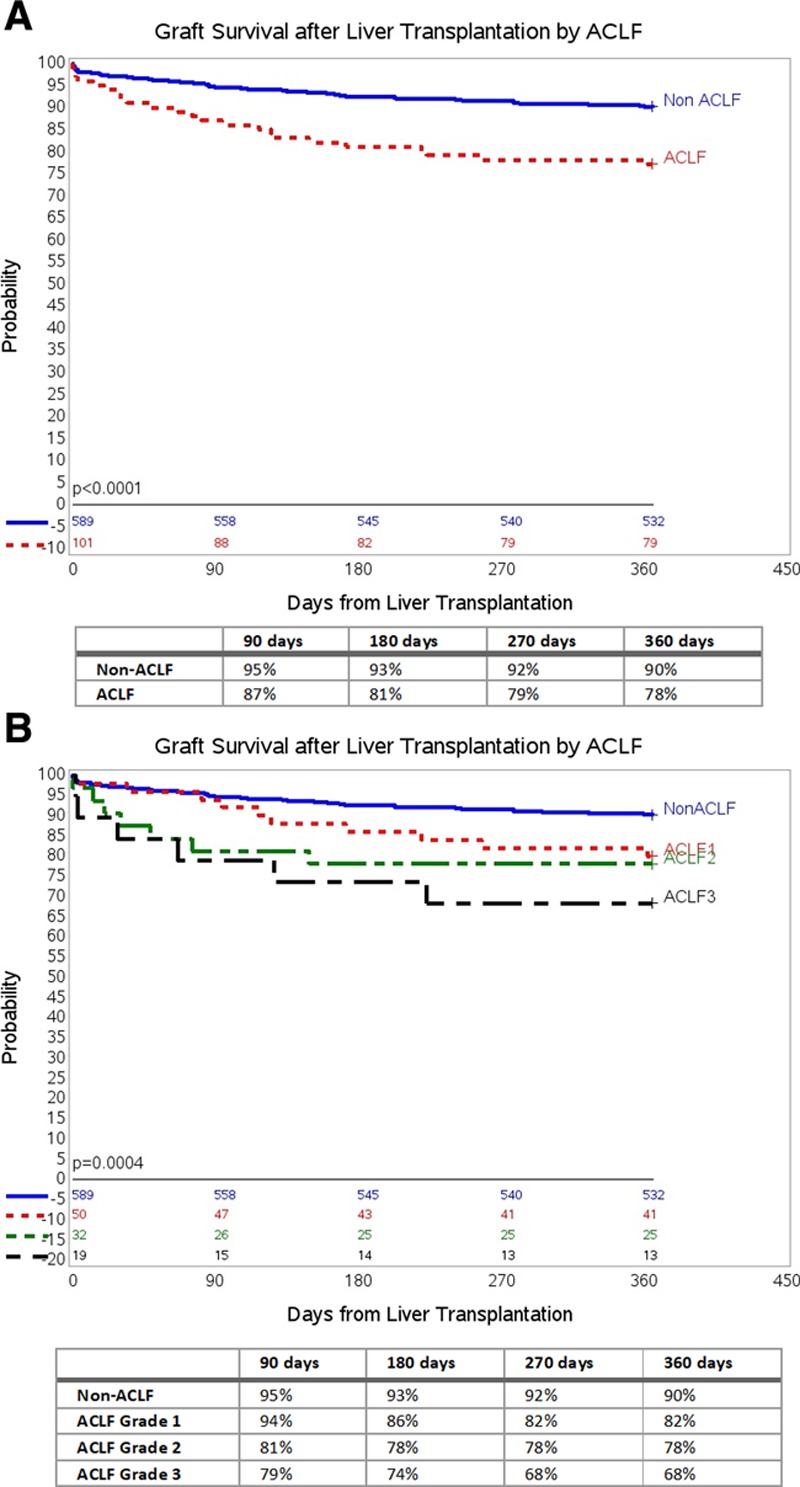
Cumulative graft survival (death/retransplant) in ACLF and non-ACLF LT recipients at 1-y follow-up. A, One-y graft survival by ACLF using Kaplan-Meier survival analysis. B, One-y graft survival by ACLF Grades using Kaplan-Meier survival analysis. ACLF, acute-on-chronic liver failure; LT, liver transplant.
Patient Survival by ACLF
Early death defined as death within 90 days was noted in 39 (5.7%) LT recipients. In the non-ACLF group, 27 (4.6%) had early graft loss as opposed to 12 (11.9%) in the ACLF group (P = 0.003). Further stratification revealed an incremental increase in mortality with severity of ACLF grades: 27 of 589 (4.6%) in the non-ACLF group, 3 of 50 (6%) with ACLF Grade 1, 5 of 32 (15.6%) with ACLF Grade 2, and 4 of 21 (19%) with ACLF Grade 3 (P < 0.003).
Overall patient survival within 1-year in the non-ACLF group was higher compared with the ACLF group of LT recipients (P = 0.0004; Figure 3A). Patient survival within 1-year based on ACLF severity grades is shown in Figure 3B. Patient survival in the non-ACLF group was significantly better than those with ACLF Grade 1 (P = 0.01), ACLF Grade 2 (P = 0.002), and ACLF Grade 3 (P = 0.005) after adjusting for multiple comparisons using the log-rank test.
FIGURE 3.
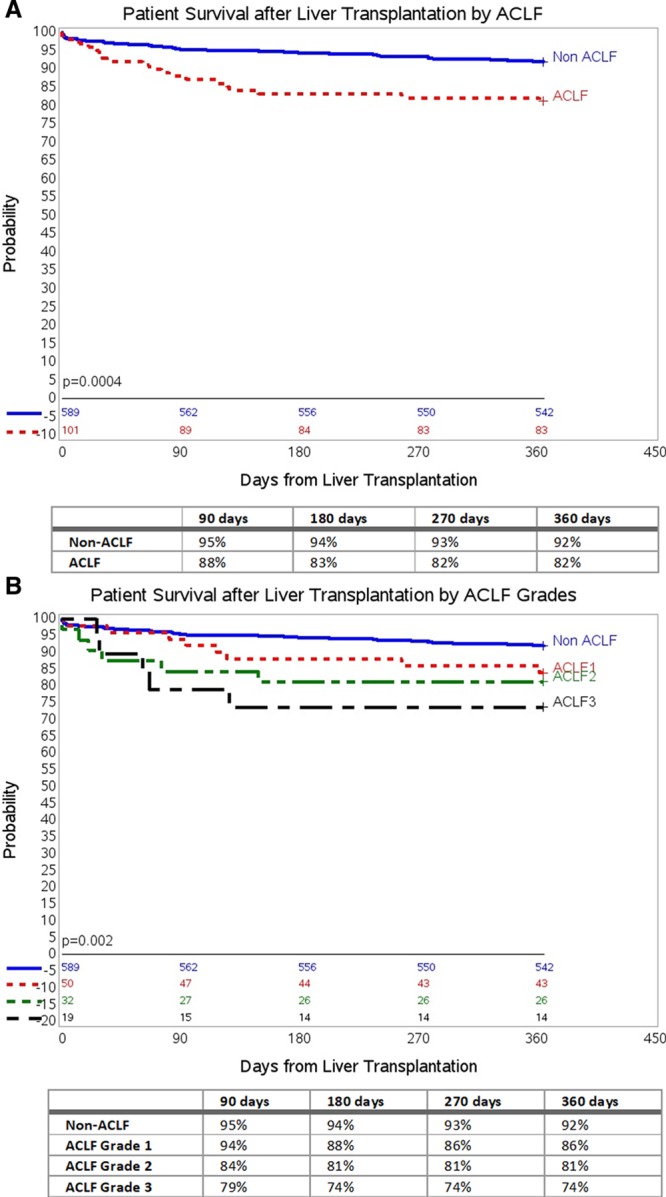
Cumulative patient survival (death) in ACLF and non-ACLF LT recipients at 1-y follow-up. A, One-y patient survival by ACLF using Kaplan-Meier survival analysis. B, One-y patient survival by ACLF Grades using Kaplan-Meier survival analysis. ACLF, acute-on-chronic liver failure; LT, liver transplant.
Graft and Patient Survival by ACLF and MELD
We further analyzed the combined effect of MELD and ACLF to estimate survival probability. We categorized the patients into the following combinations: high ACLF with high MELD, high ACLF with low MELD, low ACLF with high MELD, and low ACLF with low MELD. Using the low ACLF with low MELD group as control, we noted an inferior graft survival in the high ACLF with high MELD group (P = 0.001), high ACLF with low MELD group (P < 0.0001) as well as the low ACLF with high MELD group (P = 0.006) at 1-year follow-up (Figure 4A).
FIGURE 4.
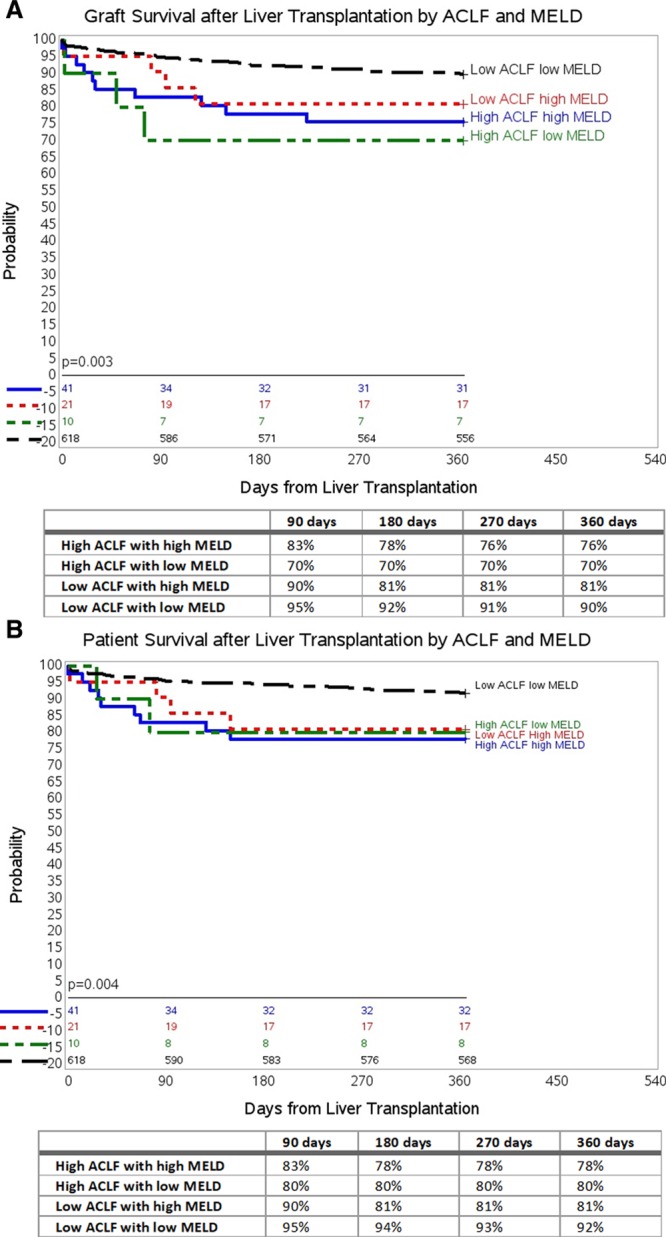
Cumulative graft (death/retransplant) and patient survival (death) in all recipients by ACLF and MELD at 1-y follow-up. A, One-y graft survival by ACLF and MELD using Kaplan-Meier survival analysis. B, One-y patient survival by ACLF and MELD using Kaplan-Meier survival analysis. ACLF, acute-on-chronic liver failure; MELD, Model for End-Stage Liver Disease.
Overall patient survival in these combined categories is shown in Figure 4B. Using the low ACLF with low MELD group as control, we noted an inferior patient survival in the high ACLF with high MELD group (P = 0.001), high ACLF with low MELD group (P = 0.001) as well as the low ACLF with high MELD group (P = 0.003) at 1-year follow-up.
Predictors of 1-year Graft and Patient Survival
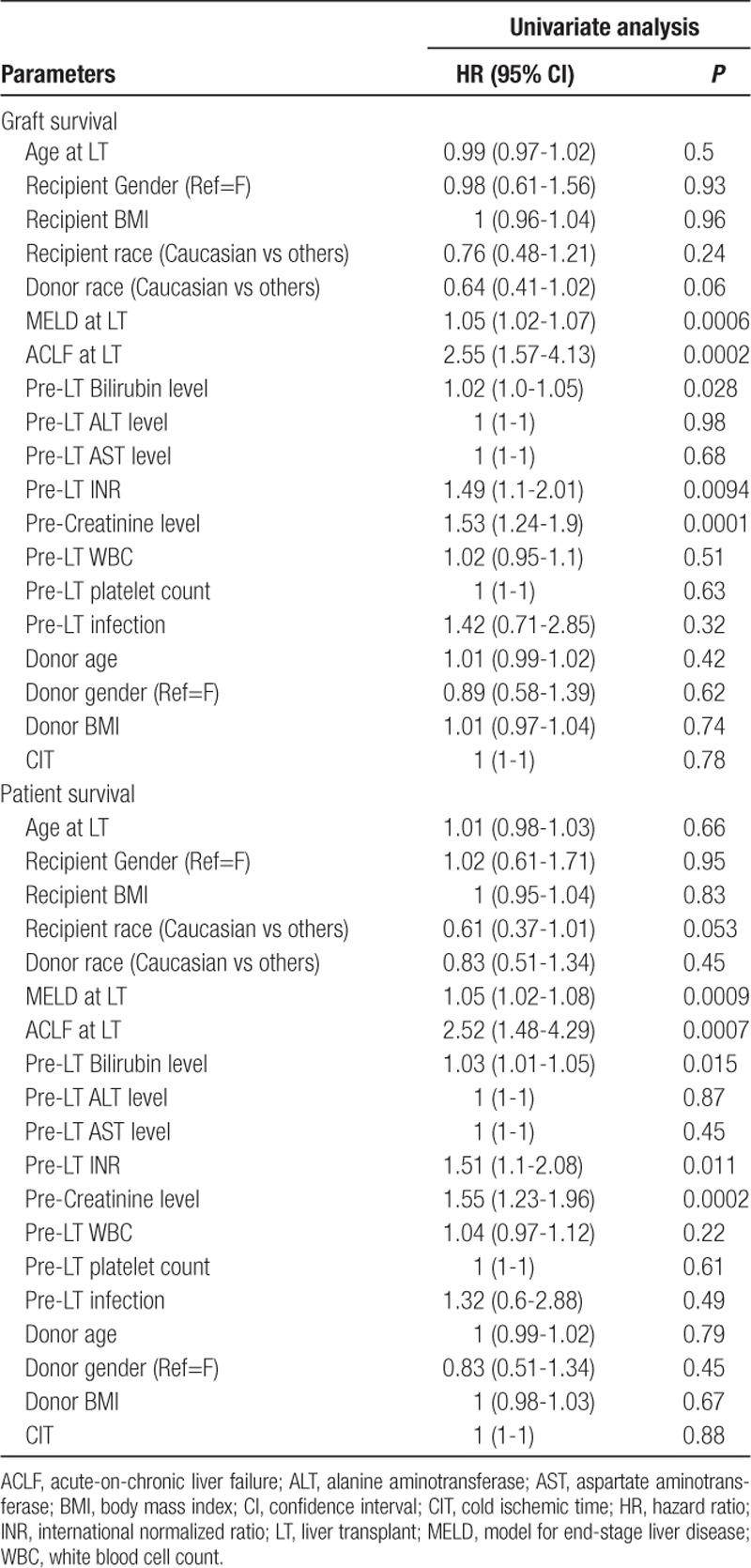
Predictors of 1-year graft and patient survival in the ACLF group of patients are shown in Table 3. On univariate analysis using the cox-proportional hazard model, MELD score at LT, ACLF at LT, pre-LT INR, pre-LT bilirubin, and pre-LT serum creatinine were significantly associated with 1-year graft and patient survival.
TABLE 3.
Predictors of 1-y graft and patient survival by univariate cox proportional hazards model patients
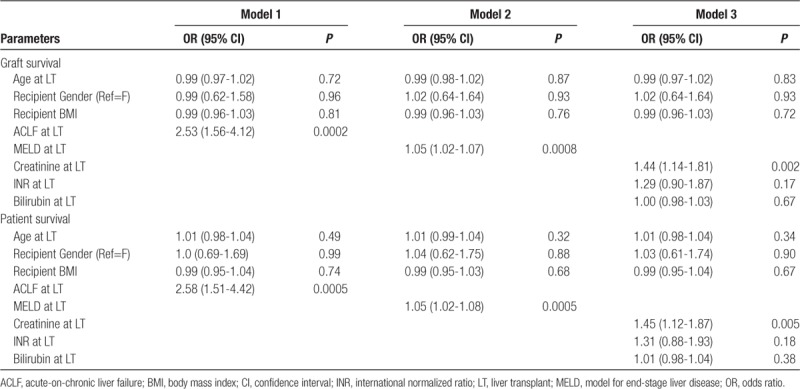
After adjusting for age at transplant, recipient BMI, and sex of the patient, multivariate analysis was conducted including variables significant on univariate analysis and those deemed clinically significant (Table 4). We tested these variables in 3 independent models: Model 1 included ACLF at LT, Model 2 included MELD at LT, and Model 3 included INR, bilirubin, and serum creatinine, all at LT. ACLF at LT (Model 1), MELD score at LT (Model 2), and serum creatinine at LT (Model 3) were significant predictors of graft as well as patient survival at 1-year after LT. We did not include MELD and ACLF in the same model due to “multicollinearity.”
TABLE 4.
Predictors of 1-y graft and patient survival by multivariate cox proportional hazards model
The most common cause of graft loss in all groups was sepsis with 6 (5.9%) in the ACLF group losing their allograft compared with 16 (2.7%) in the non-ACLF group (Table S2, SDC, http://links.lww.com/TXD/A246). The number of retransplants was also significantly higher in the ACLF group compared with the non-ACLF group (7 [6.9%] versus 32[5.4%]).
Survival Outcomes Based on Delta MELD at Transplant in ACLF Patients
We analyzed the impact of delta MELD (d-MELD) on graft and patient survival in the 101 patients with ACLF. We defined d-MELD as the difference in the MELD at the time of LT and the highest MELD within 4 weeks before LT. We grouped patients into 3 groups based on d-MELD: negative d-MELD, positive d-MELD, and unchanged d-MELD. Overall graft and patient survival in these categories is shown in Figure S2A and S2B (SDC, http://links.lww.com/TXD/A246), respectively. While there was a noticeable change in graft survival, it was not statistically significant (P = 0.065), but patient survival was noted to be significant (P = 0.012). In summary, patients with unchanged MELD score had the best posttransplant survival, and those with a negative d-MELD (incremental increase in MELD) had inferior graft and patient survival. Additionally, those with some improvement (positive d-MELD), reflecting recent ACLF events, still had inferior survival compared with those with unchanged MELD.
Renal Outcomes by Presence of ACLF
The median estimated glomerular filtration rate in the ACLF group at LT (42 ± 33 versus 85 ± 33 mL/min/1.73m2, P < 0.0001), 90 days post-LT (55 ± 28 versus 67 ± 28 mL/min/1.73m2, P < 0.0001), and at 1-year post-LT (57 ± 24 versus 70 ± 24 mL/min/1.73m2, P = 0.008) was significantly lower at all time points compared with the non-ACLF group. Although the ACLF group recovered significantly from their baseline by 90 days post-LT, the median estimated glomerular filtration rate never reached the median level of the non-ACLF group by 1-year. Additionally, there was a numerically higher need for long-term dialysis support post-LT in the ACLF group compared with non-ACLF group (5 [5.1%] versus 12 [2.1%], P = 0.08).
DISCUSSION
This is one of the first single-center studies examining LT outcomes and resource utilization among those transplanted for ACLF in the United States using the CANONIC study criteria. It has been challenging to compare previous studies such as those using the Asian Pacific Association for the Study of the Liver Consensus Meeting10,11 or change in MELD from ACLF,5 due to the heterogeneity of definitions. The CLIF-SOFA score and subsequent grading classification serve to standardize the definition of this syndrome for clinical prognosis.
Our study follows a large cohort of ACLF LT recipients providing insightful perspective regarding outcomes in this population, who if not transplanted would otherwise have had a high mortality. We demonstrated that 1-year graft and patient survival rates were significantly lower in recipients transplanted with and among the varying grades of ACLF compared with those transplanted without ACLF. Despite the lower 1-year graft (78%) and patient survival (82%) rate among those transplanted in the context of ACLF, this may be acceptable in patients who otherwise would have died without an LT. When comparing graft and patient survival among patients with or without ACLF, among the different grades of ACLF, and among the combined effect of ACLF and MELD, the trend at 90 days held fairly constant at subsequent time intervals. This highlights that transplant outcomes in those transplanted with ACLF are determined early on in the posttransplant setting.
We also note both ACLF Grade 2 and ACLF Grade 3 have a significantly lower survival compared with the group without ACLF which is supported by the literature demonstrating lower survival among LT recipients with increasing grades of ACLF.12 This is in contrast to the study by Artru et al, which showed that LT recipients with pretransplant ACLF Grade 3 had similar survival compared with recipients with lower grades of ACLF.4 The discrepancy in survival when our study is compared with that of Artru et al could be due to timing of LT. The concept of a “transplantation window” has been raised, suggesting a narrow clinical time frame during the dynamic ACLF process whereby patients with ACLF can be stabilized and transplanted with acceptable morbidity and mortality; once this transplant opportunity closes, invasive interventions become futile.4 It has been proposed that ACLF grade evaluated between day 3 and day 7 of admission is a better indicator than admission ACLF for determining prognosis.13 It is uncertain if some of the LT recipients in our study were outside of this window, as we did not assess changes in ACLF grade during admission.
The effect of MELD score on posttransplant survival is unclear with some studies suggesting that patients with higher MELD scores at time of transplant may have lower survival,14,15 while others show no association between MELD score and posttransplant outcomes.16,17 We observed MELD score at LT is a significant predictor for graft survival both on univariate and multivariate analysis. When evaluating the combined effect of ACLF and MELD scores, we found that the 90-day graft and patient survival of the group with low ACLF (no ACLF or ACLF 1) and high MELD (MELD ≥30) was not far off from those patients with low ACLF and low MELD scores (90% versus 95% for both graft and patient survival, respectively).
Overall, this suggests that the presence and severity of ACLF, specifically the presence of multiple organ failures, drives morbidity and mortality in this population. Thus, factors that are not included in the MELD scoring system may play a pivotal role in influencing outcomes in this critically ill population. The occurrence of nonhepatic organ dysfunction-shock, respiratory failure, cerebral failure, and other factors that are not captured in the MELD score, negatively impact the natural history of these patients early in the posttransplant period. Organ failures captured by the MELD scoring system may be rapidly reversible with transplantation, but recovery from the established extrahepatic organ failures reflected in ACLF may be slower and somewhat independent of liver function. It is no wonder that we found patients with high ACLF regardless of MELD had a significantly lower 90-day graft and patient survival compared with patients with any range of MELD score with low ACLF. Interestingly, the lowest 90-day graft and patient survival was in those with high ACLF and low MELD scores, although this group was small in number. Larger, prospective studies are needed to further clarify the combined effect of MELD and ACLF on survival.
Another important observation noted was the significant renal dysfunction at 1-year follow-up in the ACLF group compared with non-ACLF group. Additionally, the need for dialysis was also significantly higher in the ACLF patients at 1-year post-LT. This finding is novel and has not been reported in earlier studies. Clearly, patients with ACLF need closer monitoring during their post-LT period, and strategies should be devised to prevent worsening of renal dysfunction and need for renal replacement therapy in this vulnerable group.
Nationwide, there has been a 3-fold increase in patients with liver disease who meet the criteria for ACLF and consequently a 5-fold increase in costs associated with admission for ACLF, now reaching $1.7 billion dollars annually.18 In this study, we observed LT recipients with ACLF to have higher healthcare resource utilization after transplant, which is similar to that found in other studies.4,12,15 This group had increased operative blood transfusion, longer length of ICU stay posttransplant, longer overall hospital stay, and increased readmissions. Readmissions, particularly those in the first 6 months after LT, have been shown to predict mortality.19 The relatively higher surgical re-exploration in the ACLF cohort (26.7%) compared with the non-ACLF LT recipients (14.6%) may reflect the challenging nature of any surgical intervention in this critically ill group.
Our study’s strengths include its large sample size, as we were able to evaluate demographic and clinical information and outcomes of 690 LT recipients at a single transplant center. Limitations include those inherent in any retrospective study in which confounding, and selection biases can occur. This is particularly true as we did not examine the course of ACLF in patients who were not transplanted. We did not have information on the evolution of ACLF during the pretransplant phase of each recipient’s or transplant candidate’s admission. Also, patients' clinical and demographic characteristics, as well as clinical and immunosuppression practices vary among transplant centers and could influence results. As such, results of this study needs external validation.
In summary, although LT is an effective treatment with ACLF, 1-year mortality and graft loss is higher for LT recipients with ACLF compared with that of non-ACLF LT recipients. The severity of ACLF correlated significantly with posttransplant mortality and graft loss. Moreover, there are higher rates of complications and resource utilization among transplanted patients with ACLF.
The groups with both high ACLF regardless of MELD had the lowest graft and patient survival. Larger and more controlled prospective studies are needed to assess the combined effect of MELD score and ACLF in determining prognosis after transplant in these critically ill patients. Our findings highlight that MELD score for the most part is just a number indicating severity of liver damage and is quickly reversible with organ transplant, but the presence of multiple nonliver organ failures reflected in ACLF may independently affect the viability of the new liver as well as the patient in the immediate posttransplant period. Given the very high mortality without LT in those with advanced ACLF and the significantly worse posttransplant graft and patient survival compared with those without ACLF, centers will need to carefully weigh the risks and benefits before proceeding with transplantation in these select patients. Future research should focus on prognostication with and without LT in those with ACLF, early diagnosis and aggressive management to improve ACLF scores with medical therapy, and determination of the optimal timing of LT in this high-risk population. Meanwhile, given the scarcity of liver grafts and lower, albeit acceptable graft and survival outcomes in this high-risk population, it is advisable to carefully risk-stratify this population. Further, when transplant is pursued in this population, we should anticipate increased costs and resource utilization to support this group during the challenging posttransplant period where complications are likely to develop.
Supplementary Material
Footnotes
Published online 18 March, 2020.
The authors declare no funding or conflicts of interest.
A.S. and S.K.S. conceptualized and designed the study. A.S., S.K., P.P., and W.G. collected the data. S.K.S. analyzed the data and supervised the study. U.A. and S.K.S. interpreted the data and wrote the initial draft of the article. U.A., M.Y., S.K.S., and B.M. revised the article with the intellectual input from A.S., J.M.V., H.G., J.E., M.Z.M., and S.N. All authors were involved in additional discussion and revision of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol. 2012; 57:1336–1348 [DOI] [PubMed] [Google Scholar]
- 2.Arroyo V, Moreau R, Jalan R, et al. ; EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol. 2015; 621 SupplS131–S143 [DOI] [PubMed] [Google Scholar]
- 3.Jalan R, Saliba F, Pavesi M, et al. ; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014; 61:1038–1047 [DOI] [PubMed] [Google Scholar]
- 4.Artru F, Louvet A, Ruiz I, et al. Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017; 67:708–715 [DOI] [PubMed] [Google Scholar]
- 5.Bahirwani R, Shaked O, Bewtra M, et al. Acute-on-chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation. 2011; 92:952–957 [DOI] [PubMed] [Google Scholar]
- 6.Hernaez R, Kramer JR, Liu Y, et al. Prevalence and short-term mortality of acute-on-chronic liver failure: a national cohort study from the USA. J Hepatol. 2019; 70:639–647 [DOI] [PubMed] [Google Scholar]
- 7.Thuluvath PJ, Thuluvath AJ, Hanish S, et al. Liver transplantation in patients with multiple organ failures: feasibility and outcomes. J Hepatol. 2018; 69:1047–1056 [DOI] [PubMed] [Google Scholar]
- 8.Silva PE, Fayad L, Lazzarotto C, et al. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015; 35:1516–1523 [DOI] [PubMed] [Google Scholar]
- 9.Moreau R, Jalan R, Gines P, et al. ; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144:1426–37, 1437.e1 [DOI] [PubMed] [Google Scholar]
- 10.Chan AC, Fan ST, Lo CM, et al. Liver transplantation for acute-on-chronic liver failure. Hepatol Int. 2009; 3:571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkenstedt A, Nachbaur K, Zoller H, et al. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013; 19:879–886 [DOI] [PubMed] [Google Scholar]
- 12.Levesque E, Winter A, Noorah Z, et al. Impact of acute-on-chronic liver failure on 90-day mortality following a first liver transplantation. Liver Int. 2017; 37:684–693 [DOI] [PubMed] [Google Scholar]
- 13.Gustot T, Fernandez J, Garcia E, et al. ; CANONIC Study Investigators of the EASL-CLIF Consortium. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015; 62:243–252 [DOI] [PubMed] [Google Scholar]
- 14.Saab S, Wang V, Ibrahim AB, et al. MELD score predicts 1-year patient survival post-orthotopic liver transplantation. Liver Transpl. 2003; 9:473–476 [DOI] [PubMed] [Google Scholar]
- 15.Panchal HJ, Durinka JB, Patterson J, et al. Survival outcomes in liver transplant recipients with model for end-stage liver disease scores of 40 or higher: a decade-long experience. HPB (Oxford). 2015; 17:1074–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karvellas CJ, Lescot T, Goldberg P, et al. ; Canadian Liver Failure Study Group. Liver transplantation in the critically ill: a multicenter Canadian retrospective cohort study. Crit Care. 2013; 17:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umgelter A, Lange K, Kornberg A, et al. Orthotopic liver transplantation in critically ill cirrhotic patients with multi-organ failure: a single-center experience. Transplant Proc. 2011; 43:3762–3768 [DOI] [PubMed] [Google Scholar]
- 18.Allen AM, Kim WR, Moriarty JP, et al. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016; 64:2165–2172 [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Goodrich NP, Schaubel DE, et al. National assessment of early hospitalization after liver transplantation: risk factors and association with patient survival. Liver Transpl. 2017; 23:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


