INTRODUCTION:
Chronic abdominal pain (CAP) can arise from multiple conditions, including inflammatory disorders, trauma because of injury or surgery, or structural or functional causes. This prospective, single-arm study was designed to evaluate the safety and efficacy of 10-kHz spinal cord stimulation (SCS) in patients with intractable CAP over a 12-month follow-up period.
METHODS:
Subjects with CAP who had been refractory to conventional medical treatment for at least 3 months resulting in self-reported pain scores of ≥5 cm on a 10-cm visual analog scale were enrolled at 4 centers in the United States. Study subjects underwent a trial stimulation lasting up to 14 days with epidural leads implanted from the vertebral levels T4 through T8. Subjects who had ≥40% pain relief during the trial stimulation period were implanted with a Senza system (Nevro Corp., Redwood City, CA) and followed up to 12 months after surgery.
RESULTS:
Twenty-three of 24 subjects (95.8%) had a successful trial stimulation and proceeded to a permanent implant. After 12 months of treatment with 10-kHz SCS, 78.3% of subjects were responders (pain relief of ≥50%) and 14 of 22 subjects (63.6%) were remitters (sustained ≤3.0-cm visual analog scale scores). Secondary outcomes, including assessments of disability, mental and physical well-being, sleep quality, perception of improvement, and satisfaction, showed that 10-kHz SCS greatly improved the quality of life of patients with CAP. Observationally, most subjects also reported concurrent reduction or resolution of nausea and/or vomiting.
DISCUSSION:
10-kHz SCS can provide durable pain relief and improve the quality of life in patients with CAP.
INTRODUCTION
Abdominal pain is a very common condition that has been reported in up to a quarter of the adult population at any one time (1,2). Although most cases of abdominal pain are resolved, abdominal pain can become chronic, which is defined as lasting 3 months or longer (3).
Chronic abdominal pain (CAP) affects approximately 1%–2% of the adult population, and women are more frequently affected than men (3,4). Disorders that can produce CAP include structural/inflammatory disorders, such as chronic pancreatitis and inflammatory bowel disease, and functional disorders, such as irritable bowel syndrome, functional dyspepsia, gastroparesis, and functional abdominal pain syndrome (5). In addition, abdominal pain makes up 48% of chronic postsurgical pain cases (6). The surgical procedures most strongly associated with CAP include herniorrhaphy, adhesiolysis, and cholecystectomy. The incidence of painful postsurgical adhesions is the highest for open procedures (foreign bodies and contaminated surgical fields). Despite significantly better physical functioning, patients with CAP have been found to have significantly poorer perceptions of their overall health compared with those with chronic back pain using the Short-Form 36 Health Survey (7).
CAP is conventionally treated with pharmacotherapies, including nonsteroidal anti-inflammatory drugs, H2 antagonists, proton-pump inhibitors, gabapentinoids, and tricyclic antidepressants. Cognitive and behavioral therapies have also been effective in some patients (3,8). Injection of local anesthetic with or without corticosteroids, chemical neurolysis, and surgical correction are successful treatment options for CAP, but data on long-term effectiveness are lacking (9,10). Opioid analgesics are poor therapeutic options producing tolerance and dependency in many and can reduce gastrointestinal motility, which may directly exacerbate symptoms of CAP.
Spinal cord stimulation (SCS) is another minimally invasive, nonpharmacological, therapeutic option for controlling CAP. SCS therapy uses electrical leads implanted in the epidural space to deliver electrical impulses to the dorsal columns of the spinal cord, reducing pain in the trunk and limbs. Conventional SCS delivers electrical impulses at frequencies ranging from 2 to 1,200 Hz (but typically 40–60 Hz) and above sensory threshold amplitude producing paresthesia (11). Evidence from a published case series supports the effectiveness of conventional SCS for treating CAP because of chronic pancreatitis and other causes and shows that this treatment is associated with pain reduction lasting up to 1 year after implantation (12,13). Another small retrospective study found significant reductions in pain scores in patients after an average of 26 months of treatment with conventional SCS therapy for chronic visceral pain (14). A survey of more than 70 case reports using conventional SCS therapy to treat CAP caused by various conditions found a significant improvement in abdominal pain scores in patients who were permanently implanted with an SCS system after a successful trial stimulation (15).
These results indicate that conventional SCS offers a better treatment alternative to patients with CAP, but sensory paresthesia in the abdomen can be perceived as an uncomfortable or unpleasant sensation by patients, and an alternative treatment that does not produce paresthesia would be a valuable tool for treating this patient population. High-frequency SCS therapy that delivers electrical stimulation at 10 kHz and lower amplitude (1–5 mA) than conventional SCS is able to produce pain relief in patients without paresthesia (16,17). Moreover, 10-kHz SCS was approved by the Food and Drug Administration (FDA) with a pivotal study showing equivalent safety and superior back and leg pain relief as compared to conventional SCS (16) with sustained efficacy over a 2-year study period (18). The paresthesia-independent nature of high-frequency SCS also eliminates the need for paresthesia mapping during lead implantation or revision surgery because of lead migration, reducing patients' exposure to risk and discomfort. Research is being performed to elucidate the mechanism of action for the pain relief achieved with 10-kHz SCS despite the lack of paresthesia. Preclinical studies suggest that afferent pain signal reduction may result from inhibition of superficial dorsal horn circuits by activating inhibitory interneurons with a low-intensity 10-kHz SCS (19).
The objective of this prospective study was to evaluate the feasibility of 10-kHz SCS as a safe and effective treatment for CAP. This study represents the first step of determining clinical feasibility in what is a novel patient group for SCS therapy.
METHODS
Study design and population
This prospective, single-arm, multicenter study was designed to evaluate the safety and efficacy of high-frequency 10-kHz SCS therapy in patients with CAP. SCS is considered an off-label indication for CAP, so an investigational device exemption approval was obtained from the FDA before the enrollment of subjects. The investigational plan and informed consent forms were reviewed and approved by the appropriate investigational review board at each study site (Novant Health, Winston-Salem, NC, and Western Institutional Review Board, Puyallup, WA) before implementation, and the study was conducted in compliance with the U.S. Code of Federal Regulations and recommendations guiding physicians in biomedical research by the 18th World Medical Assembly, Helsinki, Finland.
Potential subjects were identified from the pool of patients affiliated with, or referred to, the 4 clinical investigation sites. Key inclusion criteria included having a diagnosis of CAP (from T12 ribs to the inguinal crease) that was refractory to conservative therapy for at least 3 months, with a mean pain intensity of ≥5 cm on a 10-cm visual analog scale (VAS) over the 7 days before screening. Full inclusion criteria are listed in Supplemental Table S1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A189). Key exclusion criteria included the presence of a medical condition (such as fibromyalgia) or pain in another area, not intended to be treated with SCS, that could interfere with study procedures, accurate pain reporting, and/or confound evaluation of study end points. Full exclusion criteria are listed in Supplemental Table S2 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A189).
Procedures
Enrolled subjects were screened to confirm eligibility, and all subjects who met all inclusion criteria and none of the exclusion criteria underwent a trial stimulation, including the temporary implantation of a 10-kHz SCS system (Senza system; Nevro Corp., Redwood City, CA). The flow of patients through the study protocol is illustrated in Figure 1a. Stimulation leads were positioned from the vertebral levels T4–T8 (Figure 1b). Trial stimulation lasted up to 14 days, and those producing pain relief of at least 40% were deemed successful. Stimulation parameters were set to 10-kHz frequency and 30-μs pulse width, and amplitudes were adjusted to maximize the subject's pain relief. Follow-up visits were performed at 3, 6, and 12 months after the permanent implant. Study subjects who underwent a successful trial received a permanent implant of the 10-kHz SCS system. The intent-to-treat population was composed of those subjects who received a permanent implant, and the per-protocol population consisted of subjects who received a permanent implant and were available for outcome assessment at the 3-month follow-up visit.
Figure 1.
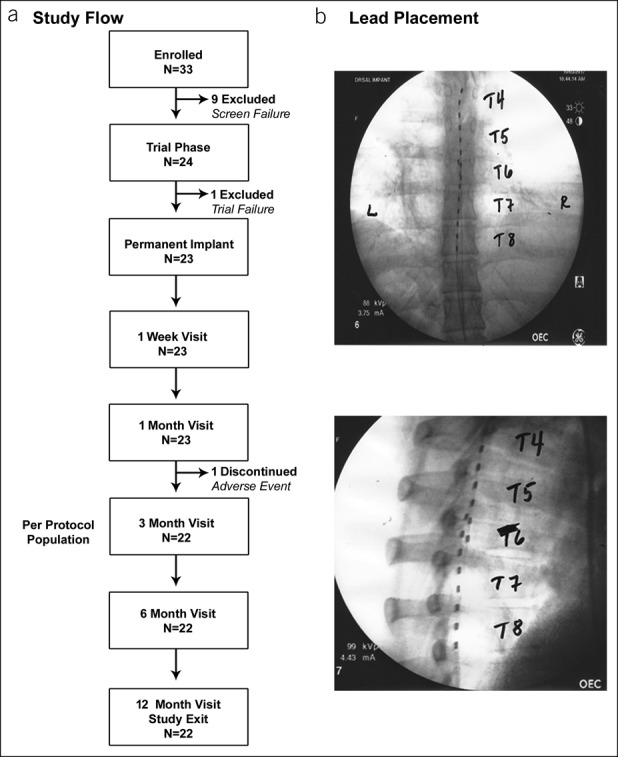
(a) Study subject flow chart. (b) X-ray images showing typical positioning of spinal cord stimulation leads.
Clinical outcome assessments
Clinical outcomes assessed at baseline and follow-up visits included pain intensity using the VAS scores, pain characteristics using the short-form McGill Pain Questionnaire 2 (SF-MPQ-2) (20), impairment of normal functioning using the Pain Disability Index (PDI) (21), mental health status using the Global Assessment of Functioning (GAF) (22), overall well-being using the 12-item Short-Form Health Survey (SF-12) (23), patient- and clinician-reported Global Impression of Change (24), sleep quality using the 3-item Pain and Sleep Questionnaire (PSQ-3) Index, and patient satisfaction.
Safety assessment
An assessment of adverse events was made by investigators starting at the enrollment and continued through study completion. Neurological examinations were conducted to monitor the possible deficits associated with stimulation, including assessments of motor, sensory, and reflex functions as is standard clinical practice. Investigators characterized the findings at follow-up visits as improved, maintained, or a deficit compared with baseline status.
Statistics
Continuous variables were reported as means and SDs or 95% confidence intervals (CIs) as appropriate, and categorical variables were reported as counts and percentages where possible. All the outcomes were analyzed by reporting descriptive statistics. Normality tests were performed on all measures, and appropriateness of parametric vs nonparametric testing was evaluated. To determine the statistical significance of longitudinal results for the VAS, SF-MPQ-2, SF-12, PDI, and PSQ-3 Index, baseline measures were compared with follow-up values using a mixed-model analysis of repeated measures with a fixed variable (postimplant measurement time) and a random variable (subject). The Tukey test was used to determine significance for pairwise comparisons. All statistical analyses were performed using Minitab 17.2.1 (Minitab, LLC, State College, PA) (25).
RESULTS
Study subjects
A total of 33 subjects were enrolled at 4 sites in the United States between June 1, 2016, and November 21, 2017, and follow-up continued through 12 months for each subject with the last visit on January 8, 2019. After the initial screening, 24 participants were deemed eligible to trial stimulation with 10-kHz SCS (Figure 1). Baseline demographic data for subjects who underwent a trial stimulation are summarized in Table 1. The study participants had a mean age of nearly 48.0 ± 13.1 years (±SD) and had been diagnosed an average of 7.8 ± 8.5 years (±SD) before the study. The study group included 23 white subjects (95.8%) and 19 women (79.2%). The most frequent diagnoses among the study population were gastroparesis/dysmotility (15/24; 62.5%) and postsurgical/post-traumatic abdominal pain (8/24; 33.3%).
Table 1.
Baseline demographic data and clinical characteristics
Trial stimulation results
Of the 24 participants who underwent a trial stimulation with 10-kHz SCS, all but 1 subject experienced pain relief of at least 70% compared with the baseline VAS scores, easily meeting the 40% success criteria, resulting in a success rate of 95.8%. These 23 subjects each received an implantable pulse generator followed through 12 months.
Safety
A total of 3 study-related serious adverse events were reported in 2 subjects (8.3%) during the study period (see Supplemental Table 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A189). One subject required a surgical revision of the implantable pulse generator to resolve a postoperative wound infection. The other subject experienced aspiration during implantation surgery, which was resolved, but had an infection before the 3-month follow-up visit, which made explant of the SCS system necessary and led to discontinuation of the subject from the study. The subject recovered fully after the explant. The remaining 22 subjects continued treatment and follow-up through 12 months (Figure 1). Neural assessments at the 6- and 12-month follow-up visits showed no changes compared with baseline measurements.
Pain relief
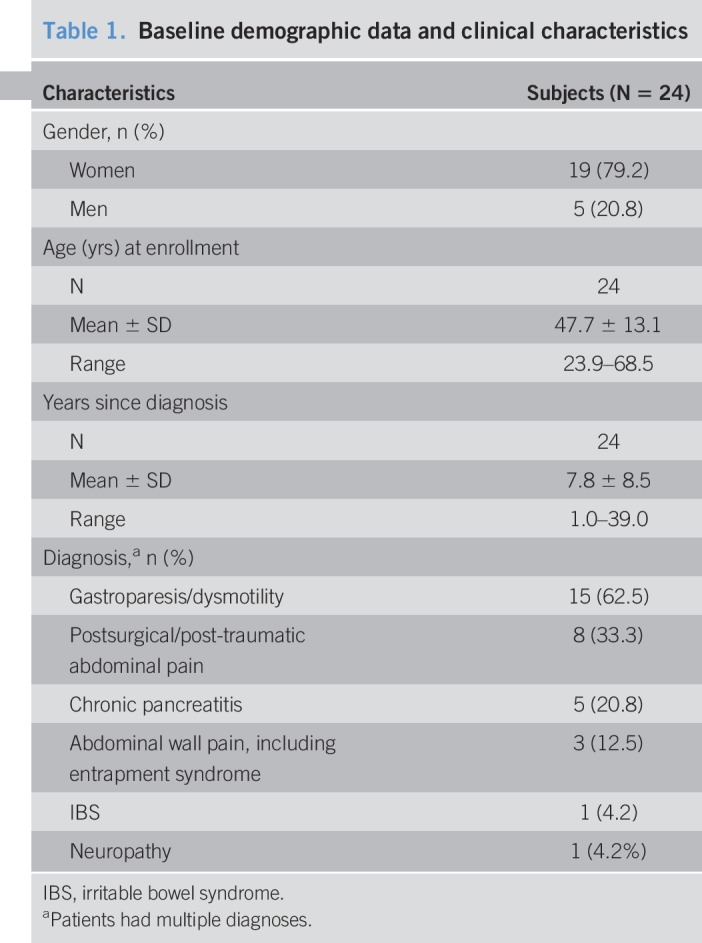
The results for pain are shown in Figure 2. In the 22 subjects who completed the study, the mean VAS scores decreased significantly from 8.3 cm (95% CI 7.5–9.5) at baseline to 2.3 cm (95% CI 0.7–2.8) at 3 months (P < 0.001), with an average of 73.3% pain relief, and these reductions were maintained through 6 and 12 months of treatment (Figure 2a,b). Study subjects who reported pain relief of ≥50% were considered responders, and responder rates during the study were 81.8% at the 3- and 12-month visits and 77.3% at the 6-month visit (Figure 2c,d). Subjects with VAS scores of ≤3.0 cm sustained for 6 months were classified as remitters (26), and 12 subjects (54.5%) were in remission after 6 months of treatment with 10-kHz SCS, increasing to 63.6% (14/22) after 12 months of stimulation (Figure 2e).
Figure 2.
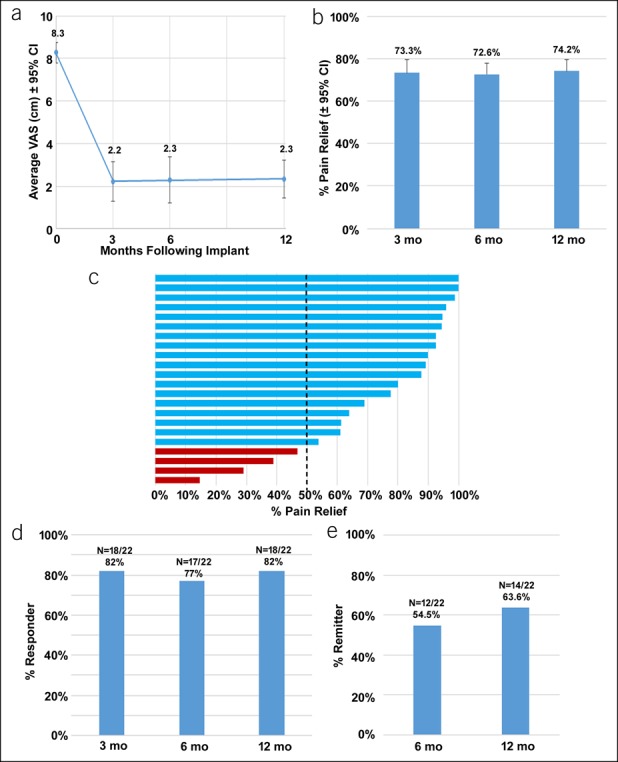
Abdominal pain is reduced with 10-kHz spinal cord stimulation, and pain relief is sustained over time. (a) Mean longitudinal abdominal pain VAS (±95% CI) scores from baseline (time 0) to 12 months after implant, where 0 represents no pain and 10.0 cm. (b) Mean percentage of pain relief (±95% CI) reported by subjects relative to baseline scores at 3, 6, and 12 months after implant. (c) Tornado plot of reported percent pain relief for all individual subjects; those reporting ≥50% relief were considered responders. (d) The response rate at 3, 6, and 12 months after implant. (d) The remitter rate (≤3.0-cm VAS) at 3, 6, and 12 months after implant. CI, confidence interval; EoT, end of trial; VAS, visual analog scale.
Pain scores calculated using the SF-MPQ-2 also significantly decreased from baseline to the 3- and 12-month follow-up visits. The mean total pain score decreased from 4.0 (95% CI 3.4–4.5) at baseline to 1.5 (95% CI 0.9–2.2) at 12 months (P < 0.001; Figure 3). In addition, the 4 individual factors comprising the total score, including continuous, intermittent, neuropathic, and affective descriptors of pain, were significantly decreased after both 3 and 12 months of treatment compared with baseline measures (P < 0.001).
Figure 3.
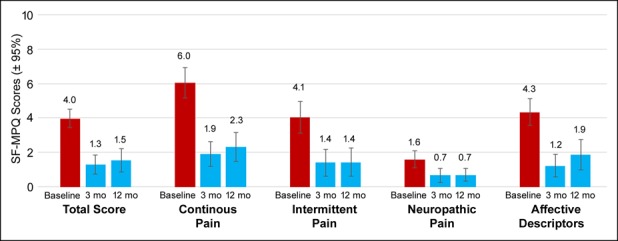
SF-MPQ scores are reduced after 10-kHz spinal cord stimulation. Data shown include mean ± 95% confidence interval at baseline and 3 and 12 months after implant for the overall score and the 4 components comprising the total score. SF-MPQ score represents an average of the participants' rating of 22 pain descriptors with an 11-point numeric rating scale (0 = “none” to 10 = “worst possible”). SF-MPQ, short-form McGill Pain Questionnaire.
Functional capacity and quality of life
Although the primary clinical outcome assessed in this study was pain reduction, the effects of 10-kHz SCS on patients' functional capacity and quality of life were also evaluated at baseline and follow-up using multiple assessments, as shown in Figure 4. The overall level of disability, as assessed by the mean PDI score, decreased from 48.5 (95% CI 43.0–53.9) at baseline to 21.0 (95% CI 13.6–28.3) after 3 months of treatment (P < 0.001), with a mean improvement of 54.8%, and after 12 months of treatment, the mean PDI was 21.0 (95% CI 14.3–27.7; P < 0.001 compared with baseline), with a mean improvement of 54.2% (Figure 4a). The median GAF scores increased significantly from 36.0 at baseline to 80.0 after 3 months of treatment and 90.0 after 12 months of treatment (Figure 4b). Subjects' overall well-being, as assessed by the SF-12 questionnaire, increased over the course of the study in both the physical and mental components (Figure 4c). The mean physical component subscale score increased from 30.1 (95% CI 26.6–33.5) at baseline to 39.9 (95% CI 35.8–44.0) after 12 months of treatment, which was statistically significant (P < 0.001), and the mean mental component subscale score increased from 43.8 (95% CI 39.7–47.9) at baseline to 50.5 (95% CI 46.8–54.2) after 12 months (P = 0.02). After 12 months of treatment, clinicians reported Global Impression of Change (GIC) as “a great deal better,” “better,” or “moderately better” in 20 of 22 subjects (90.9%; Figure 4d), whereas 19 of 22 subjects (86.4%) reported GIC as “a great deal better,” “better,” or “moderately better” (Figure 4e).
Figure 4.
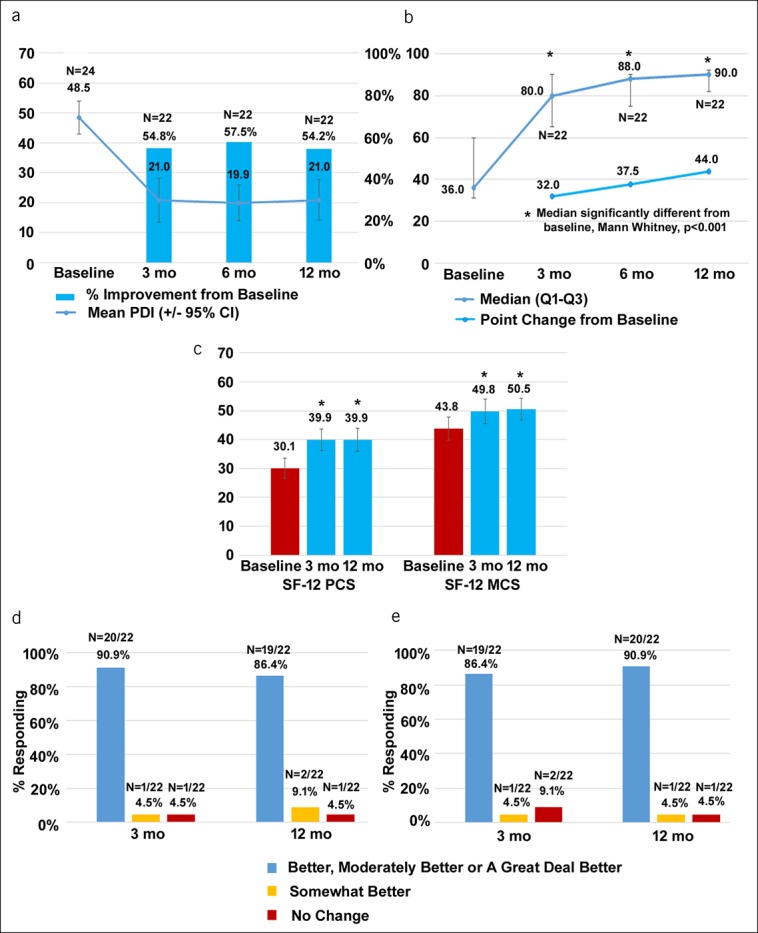
Measures of subjects' functional capacity and quality of life improve after treatment with 10-kHz spinal cord stimulation. (a) Mean PDI scores (±95% CI) are shown by the line, and the bars represent mean %PDI improvement from baseline at 3, 6, and 12 months after implant. The participant enters a score between 0 (no disability) and 10 (complete disability) with respect to 7 functional areas, and those scores are summed to create a total score that ranges between 0 and 70. (b) Median GAF scores and IQR are shown by the line, and the bars represent median GAF point change ± IQR from baseline at 3, 6, and 12 months after implant. The GAF score is a participant self-rating of global symptoms and functioning between 0 and 100, with descriptors provided for each 10-pt interval, 0 being the worst assessment described as persistent danger of severely hurting self and others and 100 as superior functioning, positive outlook, and no symptoms. (c) SF-12 scores ± 95% CI at baseline and after 3 and 12 months of treatment for both the MCS and PCS. The participant provides categorical answers to 12 items that represent quality of health and mental and physical functioning. These answers are weighted and used to create an overall mental and physical subscale, where a higher value represents higher health status. (d) Clinician responses to the CGIC at 3 and 12 months after implant. (e) Patient responses to the PGIC at 3 and 12 months after implant. CI, confidence interval; CGIC, clinician-reported Global Impression of Change; GAF, Global Assessment of Functioning; IQR, interquartile range; MCS, mental component subscale; PCS, physical component subscale; PDI, Pain Disability Index; PGIC, patient-reported Global Impression of Change; SF-12, 12-item Short-Form Health Survey.
Sleep and subject satisfaction
Patient sleep quality improved on the initiation of 10-kHz SCS, as shown by the results from the PSQ-3 Index (Figure 5a,b). The median PSQ-3 scores decreased from 23.5 (95% CI 19.6–27.5) at baseline to 5.2 (95% CI 0.3–11.6) at the 12-month follow-up visit, a statistically significant decrease (P < 0.001). The PSQ-3 scores declined by an average of 76.6% at 12 months, and scores were significantly reduced in all 3 subdomains of the PSQ-3, including trouble falling asleep and being awakened by pain at night and in the morning (Figure 5a). Surveys of study participants showed that 20 of 22 (90.9%) were either “satisfied” or “very satisfied” with treatment using 10-kHz SCS after 3 months, and 19 of 22 (86.4%) continued to be satisfied or very satisfied after 12 months (Figure 5c).
Figure 5.
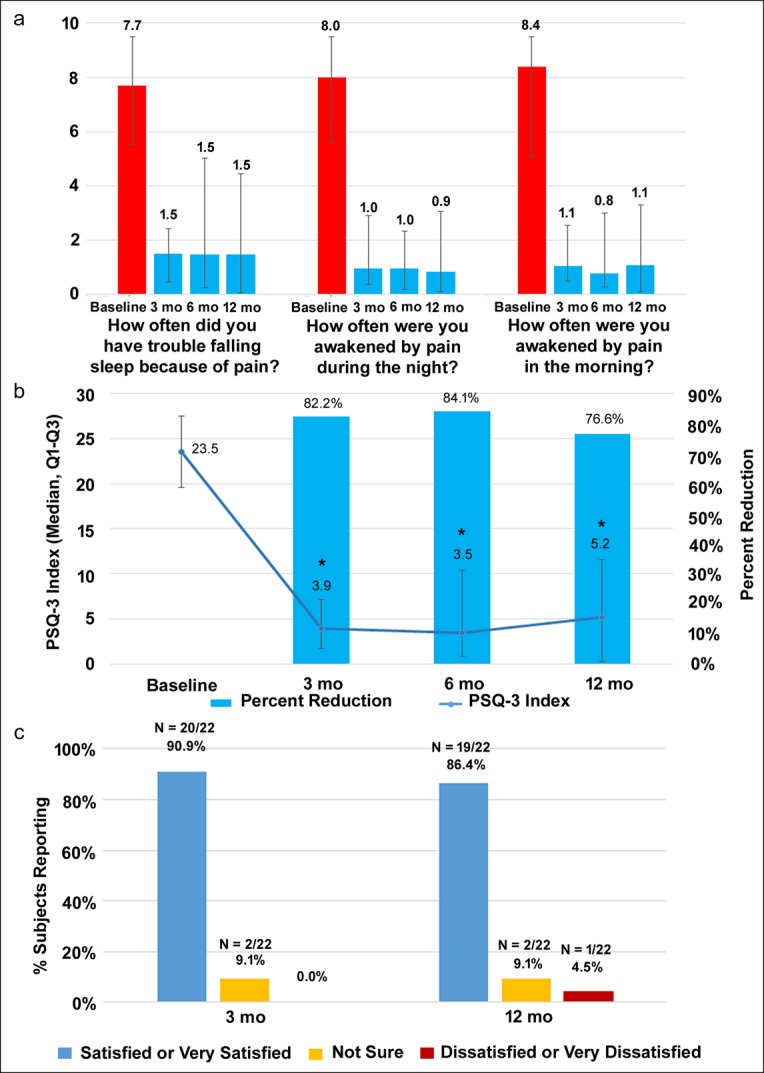
10-kHz SCS increases sleep quality and patient satisfaction. (a) Median score ± interquartile range for the 3 individual subdomains of the PSQ-3 at baseline and after 3, 6, and 12 months of treatment. (b) Median PSQ-3 overall scores are shown by the line, and the median reduction in PSQ-3 scores are shown by the bars. The individual PSQ-3 items are based on a visual analog scale, with a range from 0 (always) to 10 cm (never), and the index is calculated by summing the 3 items. (c) Patient satisfaction responses after 3 and 12 months of treatment with 10-kHz SCS. CI, confidence interval; PSQ-3, 3-item Pain and Sleep Questionnaire; SCS, spinal cord stimulation.
Abdominal symptoms
All subjects in this study reported intense pain at baseline; however, many of the subjects also reported additional abdominal symptoms at baseline. Some of these abdominal symptoms also improved in subjects after 12 months of 10-kHz SCS treatment, including vomiting, which was reduced or resolved in 9 of 11 subjects (90.9%), and nausea, which was reduced or resolved in 16 of 20 subjects (80.0%). The proportion of patients who reported improvement varied widely depending on the specific abdominal symptom; more detailed results are shown in Supplemental Figure S1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A189).
DISCUSSION
The current treatment options for patients with CAP are limited, and new treatment modalities are urgently needed. CAP is associated with impaired functioning and depression (7). The substantial emotional and mental burden associated with CAP is demonstrated by increased rates of suicidal behavior in patients compared with healthy controls or subjects with chronic pain in other locations (27). In addition, annual per-patient costs for treating abdominal pain are significantly greater than those associated with other gastrointestinal conditions, such as irritable bowel syndrome and diarrhea, and these costs are higher in more severe cases (28). A recent study of the Dutch healthcare system showed that approximately half of all patients with CAP have a functional disorder and that patients with CAP because of functional disorders have especially high diagnostic and treatment costs (29).
Currently, little evidence is available regarding the safety and effectiveness of using SCS to treat CAP. The FDA considered SCS as an off-label indication for SCS, and therefore, investigational device exemption was obtained for this feasibility study. There are no randomized controlled trial data available, and all currently published studies are retrospective in nature. A case series published in 2005 showed that conventional SCS had reduced VAS pain scores by 4.9 cm and narcotic analgesic use by half in 9 patients with CAP from various causes (30). In 2 reviews of patients with CAP arising primarily from chronic pancreatitis, patients had successful trials of SCS (≥50% pain relief) in 30 of 35 subjects (86%) (13) and 24 of 30 subjects (80%) (12). Patients treated with conventional SCS reported VAS pain score decreases of 4.3–5.1 cm and significant decreases in opioid medication use. Another retrospective study of 26 patients with CAP likewise found significantly reduced pain scores and opiate use after 26 months of stimulation (14). Finally, a review of 70 case reports of CAP from 23 pain specialists reported successful trial stimulations in 66 patients (94%) and significant reductions in pain scores and opioid use (15).
This is the first prospective study of 10-kHz SCS therapy (or any other form of SCS) in patients with treatment refractory CAP, and the results show that this treatment significantly reduced VAS pain scores by 6 cm from baseline to 12 months after the initiation of treatment. This reduction in pain intensity was greater than the magnitude of effect reported in previous studies examining the use of conventional SCS in patients with CAP for 12–26 months, which ranged from 4.3 cm to 5.5 cm (12–15). The magnitude of pain reduction and responder rate in this study was also comparable with the results reported by a randomized controlled trial of 10-kHz SCS in patients with chronic low back and leg pain. Participants in this study reported pain intensity reductions of 4.9 cm for back pain and 5.0 cm for leg pain, response rates of 78.7% for both types of pain, and remitter rates of 68.5% for back pain and 67.4% for leg pain (16). The significant reductions in all 4 components of the SF-MPQ-2 assessments at 12 months after surgery further supported the overall pain results. The magnitude of pain relief and responder rate found in these subjects with CAP is comparable with previous studies of 10-kHz SCS in the treatment of chronic widespread pain (31), chronic low back pain with or without previous surgery (32–34), and in real-world settings (35).
As secondary outcomes, the current study used assessments of functional impact and quality of life–related outcomes, such as the PDI, GAF, SF-12, GIC, and patient satisfaction (21,36–38). The median PDI score was significantly reduced by 32 pts after 12 months of treatment, and 20 of 22 subjects (90.9%) met or exceeded the minimal clinically important difference of 8.5–9.5 pts (37). Likewise, assessments of patient functioning, quality of life, sleep quality, and satisfaction after treatment with 10-kHz SCS showed improvement in all these areas, most of which were not reported in previous studies of conventional SCS. Baranidharan et al. published a retrospective case review that was the only study of conventional SCS in CAP to assess quality-of-life measures. The results showed statistical improvements in GIC, daily activities, and mood, but no improvement in sleep (14), in contrast to the decreased PSQ-3 scores found in the present study using 10-kHz SCS.
In addition to these predefined outcomes, some study subjects reported improvement in gastrointestinal symptoms other than pain, such as nausea and vomiting. It has been previously hypothesized that gastrointestinal symptoms may be related to dysfunction of bidirectional, integrated signaling between motor, sensory, and autonomic circuits in the gut and cortical centers in the brain (the “brain–gut” axis) (30), and the possibility of treating both pain and other gastrointestinal symptoms with 10-kHz SCS is an intriguing possibility for further research. The pain relief mechanism may be both activation of inhibitory dorsal horn cells and autonomic modulation. Published research suggests that SCS can reduce sympathetic tone and thereby increase gastric emptying and gastric accommodation, improving the dysfunction, which may be the cause of the gastrointestinal symptoms (39–41).
The safety results from this study suggest that the device was safe and well tolerated. A single patient required explant of the SCS system, but 2 other serious adverse events encountered during the course of the study were successfully resolved without discontinuing the treatment. This is in contrast to higher rates of explant observed in studies of conventional SCS for treating CAP, which ranged from 1 of 9 cases reported by Khan et al. (30) to 5 of 27 in patients with chronic pancreatitis (12), and the primary reasons for these explants were lead migration, infection, and ineffectiveness (15). The largest study of conventional SCS in CAP also reported 8 implant revisions in 70 cases (11%) (15), a procedure not often needed with 10-kHz SCS, because lead placement is dictated by anatomical position rather than paresthesia coverage of pain areas. Finally, no neurological deficits were observed in the subjects treated with 10-kHz SCS, and overall, these results were consistent with the good safety results reported for 10-kHz SCS in the SENZA-RCT study (16), open-label studies out to 2 years (18,31), and retrospective review of extensive postmarket experience (35).
The primary limitations of this study are the lack of a control group and randomization, which could lead to selection bias in the patient population. It needs to be noted that the current study is a feasibility study with the objective of exploring the safety and efficacy of 10-kHz SCS in the treatment of CAP. Sponsors had no role in selection of subjects and multicenter design of the study aimed to partly address the selective enrichment of the subject population. To address the lack of control arm, extensive literature search was performed to mine the historical data, which were used to compare with the findings from the current study. However, a larger randomized controlled study might be needed to further confirm the results from the current feasibility study.
This study is the first prospective trial of SCS in patients with CAP and the first published trial of 10-kHz SCS in this patient population. However, 10-kHz SCS was able to produce clinically meaningful paresthesia-free pain relief in most study subjects. The magnitude of pain relief was greater than that reported for conventional SCS in patients with CAP, and response and remission rates, as well as pain relief, were comparable with previously published trials of 10-kHz SCS in patients with back and limb pain. The data also suggest that gastric symptoms in addition to pain, such as nausea and vomiting, were improved by 10-kHz SCS treatment of CAP, although a prospective study using validated gastrointestinal symptom outcome measures is needed to confirm this potential benefit of high-frequency SCS. These results demonstrate the promise of 10-kHz SCS as a new treatment option for patients with intractable CAP.
CONFLICTS OF INTEREST
Guarantor of the article: Leonardo Kapural, MD, PhD, accepts full responsibility for the conduct of the study and has full access to the data.
Specific author contributions: L.K.: planning and conducting study, collecting and interpreting data, and revision of the manuscript. M.G.: conducting study, collecting and interpreting data, and revision of the manuscript. R.P., W.S., K.E.V., and C.G.: conducting study, collecting data, and review of manuscript. R.P.-A.: interpreting data and drafting the manuscript. A.R.: interpreting data and drafting the manuscript. J.S. and B.G.: planning study and revision of the manuscript.
Financial support: This study was funded by Nevro Corp. Nevro Corp. had the final control of study design under advisory of L.K. The authors R.P.-A, A.R., J.S., and B.G. are employees of Nevro Corp. and contributed to the report as described above.
Potential competing interests: L.K., M.G., and R.P. received a research grant from Nevro Corp.; L.K., M.G., and C.G. serve as consultants to Nevro Corp.; L.K. and M.G. received lecture fees from Nevro Corp.; B.G., A.R., J.S., and R.P.-A. are employees of Nevro Corp. W.S. and K.E.V. declare that they have no conflict of interest.
Study Highlights.
WHAT IS KNOWN
✓ CAP has few treatment options and opioids do not work.
✓ Patients with CAP have a high rate of emergency department visits.
✓ Retrospective case series show that SCS reduces pain and disability in CAP.
WHAT IS NEW HERE
✓ We prospectively investigate the efficacy and safety of SCS to treat CAP.
✓ The SCS we are investigating is nontraditional 10-kHz frequency.
✓ Pain, disability, and gastrointestinal symptoms reduced at 12 months with 10-kHz SCS.
TRANSLATIONAL IMPACT
✓ Provides support for initiation of randomized controlled clinical trial.
✓ Provides information relevant to adding specific language for chronic abdominal or visceral pain to labeling for spinal cord stimulators.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Erik J. MacLaren, PhD, Galen Medical Writing, LLC, for drafting the manuscript and Dr Madhuri Bhandaru, PhD, for assistance in preparation of illustrations.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A189
REFERENCES
- 1.Almario CV, Ballal ML, Chey WD, et al. Burden of gastrointestinal symptoms in the United States: Results of a nationally representative survey of over 71,000 Americans. Am J Gastroenterol 2018;113:1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandler RS, Stewart WF, Liberman JN, et al. Abdominal pain, bloating, and diarrhea in the United States: Prevalence and impact. Dig Dis Sci 2000;45:1166–71. [DOI] [PubMed] [Google Scholar]
- 3.Greenberger N. Chronic Abdominal Pain and Recurrent Abdominal Pain. Merck Manual: Professional Version. Merck & Co., Inc: Kenilworth, NJ, 2018. [Google Scholar]
- 4.Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med 2008;9:803–12. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA. Chronic functional abdominal pain. Am J Gastroenterol 1996;91:2270–81. [PubMed] [Google Scholar]
- 6.Crombie IK, Davies HT, Macrae WA. Cut and thrust: Antecedent surgery and trauma among patients attending a chronic pain clinic. Pain 1998;76:167–71. [PubMed] [Google Scholar]
- 7.Townsend CO, Sletten CD, Bruce BK, et al. Physical and emotional functioning of adult patients with chronic abdominal pain: Comparison with patients with chronic back pain. J Pain 2005;6:75–83. [DOI] [PubMed] [Google Scholar]
- 8.Bharucha AE, Chakraborty S, Sletten CD. Common functional gastroenterological disorders associated with abdominal pain. Mayo Clin Proc 2016;91:1118–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koop H, Koprdova S, Schürmann C. Chronic abdominal wall pain. Dtsch Arztebl Int 2016;113:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapural L, Cheng J. Abdominal pain. In: Cheng J, Rosenquist RW. (eds). Fundamentals of Pain Medicine. Springer International Publishing: Cham, Switzerland, 2018, pp 261–9. [Google Scholar]
- 11.Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: Clinical efficacy and potential mechanisms. Pain Pract 2018;18:1048–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapural L, Cywinski JB, Sparks DA. Spinal cord stimulation for visceral pain from chronic pancreatitis. Neuromodulation 2011;14:423–6; discussion 426–7. [DOI] [PubMed] [Google Scholar]
- 13.Kapural L, Nagem H, Tlucek H, et al. Spinal cord stimulation for chronic visceral abdominal pain. Pain Med 2010;11:347–55. [DOI] [PubMed] [Google Scholar]
- 14.Baranidharan G, Simpson KH, Dhandapani K. Spinal cord stimulation for visceral pain—A novel approach. Neuromodulation 2014;17:753–8; discussion 758. [DOI] [PubMed] [Google Scholar]
- 15.Kapural L, Deer T, Yakovlev A, et al. Technical aspects of spinal cord stimulation for managing chronic visceral abdominal pain: The results from the national survey. Pain Med 2010;11:685–91. [DOI] [PubMed] [Google Scholar]
- 16.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: The SENZA-RCT randomized controlled trial. Anesthesiology 2015;123:851–60. [DOI] [PubMed] [Google Scholar]
- 17.De Carolis G, Paroli M, Tollapi L, et al. Paresthesia-independence: An assessment of technical factors related to 10 kHz paresthesia-free spinal cord stimulation. Pain Physician 2017;20:331–41. [PubMed] [Google Scholar]
- 18.Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016;79:667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KY, Lee DC, Kagan Z, et al. High Frequency kHz Spinal Cord Stimulation (SCS) Differently Affects Rodent Superficial Dorsal Horn Cell Types. North American Neuromodulation Society: Las Vegas, NV, 2018. [Google Scholar]
- 20.Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009;144:35–42. [DOI] [PubMed] [Google Scholar]
- 21.Chibnall JT, Tait RC. The pain disability index: Factor structure and normative data. Arch Phys Med Rehabil 1994;75:1082–6. [DOI] [PubMed] [Google Scholar]
- 22.Aas IH. Global assessment of functioning (GAF): Properties and frontier of current knowledge. Ann Gen Psychiatry 2010;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: Can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med 1997;19:179–86. [DOI] [PubMed] [Google Scholar]
- 24.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- 25.Abdi H, Williams L. Tukey's honestly significant difference (HSD) test. In: Salkind N. (ed). Encyclopedia of Research Design. Sage: Thousand Oaks, CA, 2010. [Google Scholar]
- 26.Amirdelfan K, Gliner BE, Kapural L, et al. A proposed definition of remission from chronic pain, based on retrospective evaluation of 24-month outcomes with spinal cord stimulation. Postgrad Med 2019;131:278–86. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel B, Schoenfeld P, Naliboff B. Systematic review: The prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment Pharmacol Ther 2007;26:183–93. [DOI] [PubMed] [Google Scholar]
- 28.Nyrop KA, Palsson OS, Levy RL, et al. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther 2007;26:237–48. [DOI] [PubMed] [Google Scholar]
- 29.Jansen LA, Knauff EA, Tan SS, et al. Estimated hospital health costs of chronic abdominal pain in the Netherlands. Neth J Med 2014;72:102–6. [PubMed] [Google Scholar]
- 30.Khan YN, Raza SS, Khan EA. Application of spinal cord stimulation for the treatment of abdominal visceral pain syndromes: Case reports. Neuromodulation 2005;8:14–27. [DOI] [PubMed] [Google Scholar]
- 31.Salmon J. High-frequency spinal cord stimulation at 10 kHz for widespread pain: A retrospective survey of outcomes from combined cervical and thoracic electrode placements. Postgrad Med 2019;131:230–8. [DOI] [PubMed] [Google Scholar]
- 32.Al-Kaisy A, Van Buyten JP, Smet I, et al. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med 2014;15:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Kaisy A, Palmisani S, Smith TE, et al. 10 kHz high-frequency spinal cord stimulation for chronic axial low back pain in patients with No history of spinal surgery: A preliminary, prospective, open label and proof-of-concept study. Neuromodulation 2017;20:63–70. [DOI] [PubMed] [Google Scholar]
- 34.Rapcan R, Mlaka J, Venglarcik M, et al. High-frequency—Spinal cord stimulation. Bratisl Lek Listy 2015;116:354–6. [DOI] [PubMed] [Google Scholar]
- 35.Stauss T, El Majdoub F, Sayed D, et al. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol 2019;6:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soer R, Koke AJ, Speijer BL, et al. Reference values of the pain disability index in patients with painful musculoskeletal and spinal disorders: A cross-national study. Spine (Phila Pa 1976) 2015;40:E545–51. [DOI] [PubMed] [Google Scholar]
- 37.Soer R, Reneman MF, Vroomen PC, et al. Responsiveness and minimal clinically important change of the pain disability index in patients with chronic back pain. Spine (Phila Pa 1976) 2012;37:711–5. [DOI] [PubMed] [Google Scholar]
- 38.Tait RC, Chibnall JT, Krause S. The pain disability index: Psychometric properties. Pain 1990;40:171–82. [DOI] [PubMed] [Google Scholar]
- 39.Lind G, Winter J, Linderoth B, et al. Therapeutic value of spinal cord stimulation in irritable bowel syndrome: A randomized crossover pilot study. Am J Physiol Regul Integr Comp Physiol 2015;308:R887–94. [DOI] [PubMed] [Google Scholar]
- 40.Song GQ, Sun Y, Foreman RD, et al. Therapeutic potential of spinal cord stimulation for gastrointestinal motility disorders: A preliminary rodent study. Neurogastroenterol Motil 2014;26:377–84. [DOI] [PubMed] [Google Scholar]
- 41.Goudman L, Brouns R, Linderoth B, et al. Effects of spinal cord stimulation on heart rate variability in patients with failed back surgery syndrome. PLoS One 2019;14:e0219076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


