Abstract
Activating receptor tyrosine kinase RET (rarranged during transfection) gene alterations have been identified as oncogenic in multiple malignancies. RET gene rearrangements retaining the kinase domain are oncogenic drivers in papillary thyroid cancer, non–small-cell lung cancer, and multiple other cancers. Activating RET mutations are associated with different phenotypes of multiple endocrine neoplasia type 2 as well as sporadic medullary thyroid cancer. RET is thus an attractive therapeutic target in patients with oncogenic RET alterations. Multikinase inhibitors with RET inhibitor activity, such as cabozantinib and vandetanib, have been explored in the clinic for tumors with activating RET gene alterations with modest clinical efficacy. As a result of the nonselective nature of these multikinase inhibitors, patients had off-target adverse effects, such as hypertension, rash, and diarrhea. This resulted in a narrow therapeutic index of these drugs, limiting ability to dose for clinically effective RET inhibition. In contrast, the recent discovery and clinical validation of highly potent selective RET inhibitors (pralsetinib, selpercatinib) demonstrating improved efficacy and a more favorable toxicity profile are poised to alter the landscape of RET-dependent cancers. These drugs appear to have broad activity across tumors with activating RET alterations. The mechanisms of resistance to these next-generation highly selective RET inhibitors is an area of active research. This review summarizes the current understanding of RET alterations and the state-of-the-art treatment strategies in RET-dependent cancers.
INTRODUCTION
The receptor tyrosine kinase RET (rearranged during transfection) plays an important role in the development of the kidney and nervous system. When aberrantly activated, it can act as an oncogene in multiple malignancies. RET fusions retaining the kinase domain are drivers of papillary thyroid cancer (PTC), non–small-cell lung cancer (NSCLC), and other cancers. Activating RET mutations are associated with different phenotypes of multiple endocrine neoplasia type 2 (MEN2) as well as sporadic medullary thyroid cancer (MTC). RET is thus an attractive therapeutic target in patients with oncogenic RET alterations. Multikinase inhibitors (MKIs) with ancillary RET inhibitor activity, such cabozantinib and vandetanib, have been explored in the clinic for RET-driven cancers. The off-target adverse effects, such as hypertension and diarrhea, have restricted the dosing that patients can tolerate. In contrast, the recent discovery and clinical validation of next-generation highly potent selective RET inhibitors (pralsetinib/BLU667, selpercatinib/LOXO-292) demonstrating improved efficacy and a more favorable toxicity profile in registrational clinical trials are poised to alter the landscape of RET-altered cancers.1,2 This review summarizes the current understanding of RET alterations and the state-of-the-art treatment strategies in RET-aberrant cancers.
THE FUNCTION AND BIOLOGY OF RET
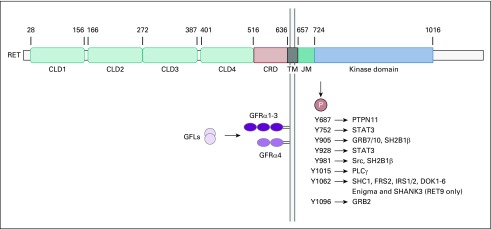
The proto-oncogene RET was identified in 1985 by Takahashi et al3 as a transforming gene that was derived by DNA rearrangement during transfection of mouse NIH3T3 cells with human lymphoma DNA. Therefore, it was designated RET. The RET gene encodes a receptor tyrosine kinase (RTK) that contains a large extracellular domain, a transmembrane domain, and an intracellular tyrosine kinase domain (Fig 1).4 Studies from molecular modeling,5 electron microscopy, and small-angle x-ray scattering6 revealed the structure of the RET extracellular domain, including four cadherin-like domains (CLD1-4), a calcium-binding side between CLD2 and CLD3, and a conserved cysteine-rich domain. After the transmembrane domain, a juxtamembrane segment lies at the beginning of the intracellular portion of RET and immediately adjacent to the kinase domain. The C-terminal tail of RET has two major forms, which diverge after residue G1063 because of alternative splicing—a short 9–amino acid one (RET9) and a long 51–amino acid one (RET51). Although the two isoforms share a largely common sequence and are coexpressed in many tissues, numerous studies have demonstrated differences in their temporal and spatial regulation of expression, cellular localization and trafficking, and biologic functions. It has been suggested that RET51 is the more prominent isoform in tumors. RET51 is more effective than RET9 at promoting cell proliferation, migration, and anchorage-independent growth.7,8 In addition, the transcripts of RET51 are more abundant than those of RET9 in some MEN2 tumors.9 In breast cancer cells, estrogen upregulates RET51 at a much greater level compared with RET9.10 RET51 expression is increased in 4 out of 5 stage IIB pancreatic tumors.11
FIG 1.
Schematic illustration of RET protein, its ligands, receptors, and signaling pathways. The numbers above the RET domains indicate amino acid positions. The main RET phosphorylation sites are listed together with their binding proteins. CLD, cadherin-like domain; CRD, cysteine-rich domain; GFLs, GDNF-family ligands; GFRα, GDNF-family receptor-α; JM, juxtamembrane; TM, transmembrane domain.
The RET ligands include glial cell line–derived neurotrophic factor (GDNF), neurturin, artemin, and persephin, all belonging to the GDNF family ligands (GFLs).12 These GFLs do not directly bind to RET and instead bind to GDNF family receptor-α (GFRα) coreceptors, which in turn recruit RET for dimerization.6,13 Subsequently, autophosphorylation on intracellular tyrosine residues of RET creates docking sites for downstream signaling adaptors, leading to the activation of multiple pathways (Fig 1).12 Phosphorylated Y1062 is the key docking site for several adaptor proteins, which can activate pathways such as Ras/MAPK, PI3K/AKT, and JNK.14,15 Autophosphorylation of Y1096 on the RET51 isoform (and not on RET9) also contributes to the activation of Ras/MAPK and PI3K/AKT pathways.14,16 Among other autophosphorylation sites, Y1015 is involved in the activation of protein kinase C signaling through binding of phospholipase Cγ (PLCγ).17 Y752 and Y928 are STAT3 docking sites.18 Phosphorylated Y687 and Y981 bind to tyrosine phosphatase Shp2 and Src kinase, respectively.19,20
In addition, RET plays important roles in the development of the kidney and nervous system. Studies in mouse models have shown that RET and the phosphorylation of its docking sites are critical for the growth and branching morphogenesis of ureteric bud cells from the metanephric mesenchyme.21,22 RET is expressed in neural crest cells and required for the proliferation, differentiation, and survival of these cells.21,23 RET is also involved in motoneuron survival and connectivity.24,25 In addition, RET signaling contributes to the regulation and function of hematopoietic cells and spermatogenesis.26,27 Loss-of-function RET mutations in humans have been linked to Hirschsprung disease, congenital anomalies of kidney or urinary tract, and congenital central hypoventilation syndrome.28
ONCOGENIC ACTIVATION OF RET
RET is activated in cancer mainly through chromosomal rearrangements that generate fusion genes containing the kinase domain of RET (Fig 2) and gain-of-function missense mutations in both the extracellular and cytoplasmic regions of RET protein (Fig 3). Apart from these mechanisms, the increased expression level of wild-type RET has been linked to the pathogenesis of several cancer types.28
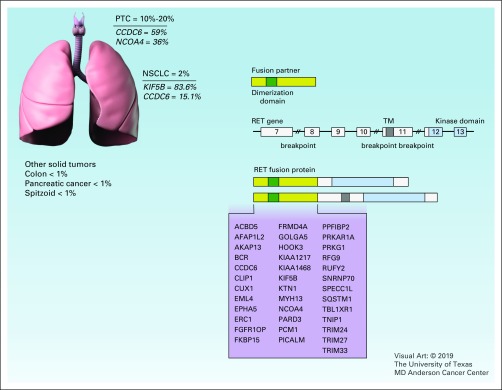
FIG 2.
RET fusion. The chromosomal breakpoints of the RET gene often happen within intron 11 and occasionally in introns 7 and 10. The numbers indicate exons in RET gene. The resulted fusion protein contains the dimerization domain (green) from the fusion partner and the kinase domain (blue) of RET, or both the transmembrane (TM) domain (dark gray) and the kinase domain of RET. Reported fusion partner genes are listed in the figure. Frequencies are derived from COSMIC database. NSCLC, non–small-cell lung cancer; PTC, papillary thyroid cancer.
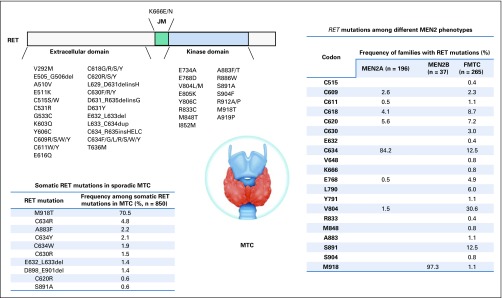
FIG 3.
RET mutation. Somatic RET mutations in sporadic medullary thyroid cancer (MTC) and among different multiple endocrine neoplasia type 2 (MEN2) phenotypes are shown. The frequencies of somatic mutations are derived from the COSMIC database. The frequencies of mutations in MEN2 are derived from published studies.44a-d FMTC, familial medullary thyroid carcinoma.
RET REARRANGEMENTS
Somatically occurring RET rearrangements involve the 3′ sequence of RET that encodes the kinase domain and the 5′ sequence of other partner genes. The chromosomal breakpoints of RET often occur within intron 11 and lead to fusions with only the cytoplasmic portion of RET. Occasionally, some breakpoints occur within introns 7 and 10, creating chimeric proteins containing the RET transmembrane domain (Fig 2).29 To date, more than 35 genes have been reported to form fusion genes with RET (Fig 2). These partner genes can contribute dimerization domains to the fusion proteins, such as the coiled-coil domain,30 the Lis1 homology (LisH) domain,31 and the sterile α motif (SAM) domain.32
RET fusions can activate downstream pathways through multiple means. By fusing to the kinase domain of RET, dimerization domains can mediate ligand-independent constitutive activation of the RET kinase33,34 (Fig 4). RET fusions can increase the expression of RET, as illustrated by Kohno et al,35 who showed that KIF5B-RET resulted in 2- to 30-fold higher transcription of RET than normal lung tissues. Altered function of the fusion partner may be another factor. One such partner, PRKAR1A, is a tumor suppressor gene that is inactivated in patients with Carney complex, an autosomal dominant syndrome with an increased risk of developing several types of tumors.36 PRKAR1A-RET fusion may not only activate RET but also inactivate PRKAR1A.
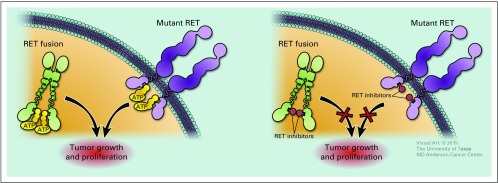
FIG 4.
Oncogenic RET signaling and RET inhibitors.
The molecular mechanism responsible for RET rearrangements is believed to be the unfaithful repair of DNA double-strand breaks through nonhomologous end joining, break-induced replication, and other complex rearrangements.29,37 Various noncellular and cellular causes can result in double-strand breaks, such as ionizing radiation and fragile site induction by genotoxic chemicals or stress factors (for instance, hypoxia and replication stress).38,39
In human cancers, RET rearrangement was initially identified in PTC in 1987.40 Recent clinical data suggest that RET rearrangements occur in up to 10%-20% of PTCs. The prevalence of RET rearrangements is much higher in radiation-induced PTCs. As an example, these alterations have been reported in approximately 50%-80% of patients with PTC who were previously exposed to the Chernobyl radioactive fallout or the atomic bomb in Japan.41-44 These rearrangements are more frequently identified in children than in adults with PTC, at least partially because of the high proliferation rate of thyroid follicular cells in children and consequently the increased susceptibility of these cells to DNA damage compared with adult cells. Among patients with PTC, CCDC6-RET and NCOA4-RET are the most common RET rearrangements,30 which are generated via reciprocal or nonreciprocal paracentric inversion on the long arm of chromosomal 10.45,46 RET rearrangements and BRAF mutations are largely mutually exclusive in PTCs.47,48 In addition to PTC, RET rearrangements have been identified at much lower prevalence in other types of thyroid cancer, such as anaplastic thyroid carcinoma, follicular thyroid carcinoma, and medullary thyroid carcinoma.49-51
During the past decades, RET rearrangements have been reported in a number of other cancer types, including, but not limited to, NSCLC,52 Spitz tumors and spitzoid melanomas,53 chronic myelomonocytic leukemia,54 colorectal cancer,55 and breast cancer.56 RET rearrangements are detected in approximately 1%-2% of NSCLCs, particularly adenocarcinoma.57,58 The patients with NSCLC with these rearrangements have shown unique clinicopathologic characteristics: they were relatively younger (≤ 60 years), had more poorly differentiated tumors, and had minimal or no prior history of smoking.59,60 RET fusions have been reported as a mechanism of acquired resistance to osimertinib in EGFR-mutant NSCLC.61 It has been shown clinically that this bypass track can be overcome by combining RET inhibitor to EGFR inhibitor. Interestingly, patients with RET-rearranged lung cancer generally showed low levels of PD-L1 expression and low tumor mutational burden and had poor outcome on immunotherapies.62 Another study demonstrated that RET-altered patients had shorter median time to progression with immune checkpoint inhibitors (ICIs) compared with non-ICI therapies.63
Despite that there is no universally accepted standard to detect RET rearrangements, several methods are used in the clinic. In general, immunohistochemistry is not reliable for the detection of RET rearrangement. Reverse transcription polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) are both sensitive and effective approaches. However, RT-PCR is insufficient to detect novel fusion partners or isoforms. FISH with dual color break-apart probe is unable to identify the specific fusion partner. Furthermore, it has been shown that next-generation sequencing (NGS) can simultaneously detect both gene fusions and somatic mutations in tumor samples. Targeted RNAseq is also complementary to DNA-based sequencing, as demonstrated by its ability to identify actionable alterations that were missed by DNA-based sequencing.64
ACTIVATING MUTATIONS OF RET
More than 60 activating RET mutations have been reported to date. Heritable activating mutations have been extensively studied in the MEN2 syndrome, an autosomal dominant multitumor syndrome that is characterized by a high risk of developing MTC (Fig 3).65 With novel detection technologies, especially NGS, somatic activating mutations of RET have been discovered in multiple other cancer types.66 These studies are outlined and discussed in the following sections.
GERMLINE MUTATIONS
Germline activating RET mutations are pathognomonic in MEN2 (Table 3), which can be classified into three subtypes depending on clinical features—MEN2A, MEN2B, and familial medullary thyroid carcinoma (FMTC). MEN2A is the most common subtype and affects 60%-90% of MEN2 families. MEN2A is characterized by MTC in all patients, pheochromocytoma in approximately 50% of patients, and hyperparathyroidism and/or lichen planus amyloidosis in up to one-third of patients.67,68 MEN2B makes up 5% of MEN2 cases. It is the most aggressive subtype and has a very early onset of MTC.69 In addition to MTC (100% of cases) and pheochromocytoma (50% of cases), patients with this subtype have no hyperparathyroidism but present with extraendocrine features, including intestinal and mucosal ganglioneuromatosis, marfanoid habitus, skeletal abnormalities, and delayed puberty.70,71 FMTC is the most indolent subtype of MEN2 and is characterized by a later onset and MTC being the only consistent clinical feature.69 It has been proposed that FMTC should be considered a variant of MEN2A.72 Notably, prophylactic thyroidectomy on the basis of genetic screening of germline RET mutations has shown significant impact on MEN2 families.73,74
Mutation hotspots in patients with MEN2A cluster within the cysteine-rich domain of RET extracellular region. Substitutions at these cysteines (codons 609, 611, 618, and 620 in exon 10, and 630 and 634 in exon 11) occur in > 95% of patients with the MEN2A subtype. Particularly, patients with C634 mutations account for approximately 85% of the population.75,76 These mutations replace cysteines with other amino acids and decrease the formation of intramolecular disulfide bonds, promoting the formation of RET homodimer through intermolecular disulfide bonds between RET monomers. This results in ligand-independent constitutive activation of RET.77,78
The MEN2B subtype is associated with the kinase domain mutation M918T in > 95% of cases.75,79 Other mutations identified in patients with MEN2B include A883F, which is also located in the kinase domain of RET,79 and co-occurring RET mutations involving V804M.80 These mutations can change protein conformation, increase ATP binding affinity, and decrease autoinhibition.77,81 A883F is linked with less-aggressive phenotypes compared with M918T.82
Mutations of FMTC are found at not only the cysteine residues but also other noncysteine residues in both the extracellular and intracellular regions, such as G533, E768, L790, V804, and S891.83,84 In addition, cysteine substitutions occur at different frequencies in FMTC, with a much lower frequency of C634 substitutions and higher frequencies of substitutions from other cysteines.75 Furthermore, many of the noncysteine mutations are also identified in patients with MEN2A.85
However, these genotype-phenotype correlations can be confounded in some cases. For example, the polymorphism RET G691S, a modifying variant, enhances the oncogenic activity of RET S891A in vitro. Patients with FMTC harboring both variants demonstrated a trend toward an earlier age of diagnosis.86 G691S is associated with earlier onset of sporadic MTC.87 Several tandem mutations involving V804M, such as V804M and Q781R, are found in patients with an MEN2B phenotype instead of FMTC and MEN2A.80 Although two homozygous carriers were diagnosed with MTC, other family members bearing heterozygous A883T were not affected.88 Moreover, a few cysteine variants at codons 609, 611, 618, and 620 can cause both gain-of-function and loss-of-function in different tissues, thereby resulting in cosegregation of Hirschsprung disease and MEN2 in some families.89-91
SOMATIC MUTATIONS
Point mutations, small deletions, and/or insertions involving RET have been reported in both sporadic and familial MTC. Somatic RET mutation is a hallmark of sporadic MTC.92,93 Among these alterations, M918T is the most frequently reported mutation (Table 4). Other less-common somatic mutations occur at residues C634, A883, C630, and others.94 RET mutations have been found to be mutually exclusive with HRAS and KRAS mutations in sporadic MTC, indicating RAS activation as a driver pathway in MTC.95,96 A recent study used NGS to identify RET mutations in tumors from 4,871 patients.66 The result showed that somatic RET point mutations exist in a variety of cancer types, such as breast carcinoma (C634R), colorectal adenocarcinoma (V804M), GI stromal tumor (V804M), Merkel cell carcinoma (E511K), and paraganglioma (M918T). However, the functional effect of RET mutations on tumorigenesis in these tumors remains to be elucidated.
TARGETED THERAPIES FOR RET
MKIs
As a tyrosine kinase receptor, RET shares similarities in the sequence and structure of the kinase domain with other tyrosine kinases.65,97 Many MKIs have demonstrated activity against RET, such as cabozantinib, lenvatinib, sorafenib, vandetanib, ponatinib, sunitinib, and alectinib.28,98 Among them, cabozantinib and vandetanib are approved for advanced MTC by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), although RET mutation is not required as a selective biomarker. Data from the phase III trial of vandetanib in advanced MTC (ZETA; ClinicalTrials.gov identifier: NCT00410761)99 showed a predicted median progression-free survival (PFS) of 30.5 months by the Weibull model in the treatment group and a median PFS of 19.3 months in the placebo group. The objective response rates (ORRs) were 45% in the treatment group and 13% in the placebo group. Although the subgroup analysis based on RET mutation status is inconclusive, the patients whose cancers harbored a RET M918T mutation had a higher ORR with vandetanib than M918T-negative patients. In the phase III trial of cabozantinib (EXAM; ClinicalTrials.gov identifier: NCT00704730), the median PFS/ORR for cabozantinib and placebo are 11.2 months/28% and 4 months/0%, respectively.100 Retrospective analysis of the EXAM trial demonstrated that cabozantinib had increased benefit in patients with RET M918T than in patients without this mutation in term of PFS, ORR, and overall survival (OS).101,102 However, cabozantinib was favored for both OS and PFS regardless of RET mutation status.102 The clinical benefits were also not associated with RET mutation status in a phase II trial of lenvatinib in MTC.103 The ongoing observation study evaluating vandetanib in patients with MTC with or without RET mutations (ClincalTrials.gov identifier: NCT01945762), as well as future RET-directed prospective trials, will further shed light on this matter.
Despite the fact that lenvatinib and sorafenib are approved for radioactive iodine-refractory differentiated thyroid cancer (DTC) by the FDA and EMA, neither phase III trial that led to their approval investigated the correlation between RET rearrangement and the efficacy of the drugs.104,105 Several phase II trials involving sunitinib,106 dovitinib,107 and vandetanib108 in DTC did not explore this drug-biomarker relationship.
Nevertheless, insights have been provided by an array of clinical studies in RET-rearranged NSCLC. A phase II trial evaluating cabozantinib was conducted in patients with NSCLC with RET rearrangement. The ORR, median PFS, and OS among 25 patients were 28%, 5.5 months, and 9.9 months.109 Vandetanib was subsequently assessed in a Japanese phase II trial (LURET) and a Korean phase II trial on NSCLC with RET rearrangements. The analysis of the LURET trial showed 53% ORR, median PFS of 4.7 months, and median OS of 11.1 months.110 Even though the 18% ORR of a separate South Korean phase II trial was lower than that in LURET, the median PFS (4.5 months) and median OS (11.6 months) were comparable with LURET trial.111 In another phase II trial testing lenvatinib, the ORR was 16% and the median PFS was 7.3 months.112 In addition, a global registry of RET-rearranged NSCLC (GLORY) retrospectively reported 53 patients who were treated with one or more MKIs. Within these patients, the ORRs for cabozantinib, vandetanib, and sunitinib were 37%, 18%, and 22%, respectively. The median PFS and median OS were 2.3 months and 6.8 months.113 A recent phase I/Ib trial with RXDX-105 in RET fusion-positive NSCLC showed that the ORR with RXDX-105 was 19%.114 Interestingly, what was observed was a striking divergence in response to RXDX-105 dependent on the gene fusion partner, as responses were observed only in non-KIF5B upstream partners.114 The analysis of fusion partner in the aforementioned trials of cabozantinib, vandetanib, and RXDX-105 suggested a tendency toward worse clinical outcomes (ORR and PFS) in cancers with KIF5B-RET compared with cancers with other known RET fusion.109-111,115 Additional investigation is needed, considering that the sample sizes were small in these trials, and the results of GLORY study showed no significantly different clinical outcomes in patients bearing different RET fusions.113
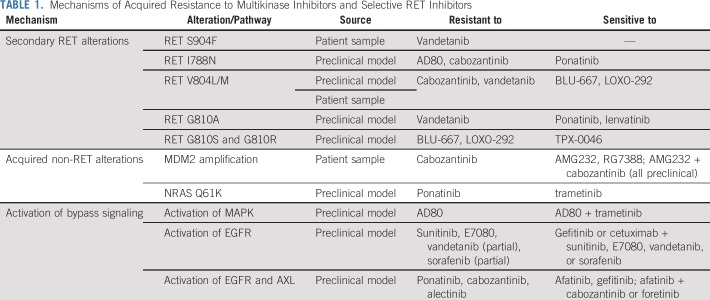
Although the data of MKIs have demonstrated their clinical utility in RET-driven cancers, the ORRs (16%-53%) and median PFSs (2.3-7.3 months) in RET-rearranged NSCLCs are lower than those seen in other patients with oncogene-driven NSCLC receiving targeted tyrosine kinase inhibitors. As an example, patients with NSCLC with EGFR mutation, ALK rearrangement, or ROS1 rearrangement have ORRs of 56%-85% and median PFS of 8-34.8 months with targeted therapies.116-119 The retrospective analysis of the EXAM trial showed no difference of OS between cabozantinib and placebo.102 The achievement of complete response was also rarely reported in all clinical trials mentioned previously. The limited efficacy of MKIs on RET-driven cancers can be at least partially attributed to the off-target activity of these inhibitors. MKIs can usually target a wide spectrum of kinases besides RET. Particularly, because of the high homology of the kinase domain between RET and VEGFR2, many VEGFR2 inhibitors can also target RET with a lower affinity, such as cabozantinib, vandetanib, and lenvatinib.85,120-122 The off-target effect contributes to inferior inhibition of RET, as well as drug-related toxicities, which can in turn result in drug discontinuation and dose reduction, further compromising the efficacy of these drugs. Moreover, an inhibitor may have different efficacies against various RET mutations and RET rearrangements with different fusion partners. For instance, cabozantinib and vandetanib can effectively block the activity of RET M918T but fail to inhibit the gatekeeper mutations RET V804M and V804L.123,124 The two gatekeeper mutations and other mutations like S904F, G810R, and I788N may emerge as mechanisms of acquired resistance to the MKIs.125-127 In addition, acquired genomic changes in other genes, such as NRAS Q61K or MDM2 amplification, can lead to resistance to these inhibitors.128,129 Another mechanism of acquired resistance is through the activation of bypass signaling, including MAPK, EGFR, and AXL pathways.125,128,130
The complexity of genomic changes in RET-driven cancers also underlines the need for combination therapies. Activation or genomic alterations of other pathways can co-occur with RET rearrangement. For example, concomitant activating BRAF, KRAS, and NRAS mutations have been identified in some RET-rearranged PTCs.131,132 AKT2 amplification was found to coexist with RET rearrangement in a patient with lung adenocarcinoma, who responded to the combination of vandetanib and everolimus with a decrease in the intracranial disease burden.133 This combination is being tested in a phase I trial (ClincalTrials.gov identifier: NCT01582191) and has demonstrated antitumor activity in patients with both RET fusions and mutations.134,135 In the aforementioned treatment-activated bypass signaling, preclinical data have shown that the resistant cells remain sensitive to strategies combining MKIs and MEK or EGFR inhibitors.125,128,130
Selective RET Inhibitors
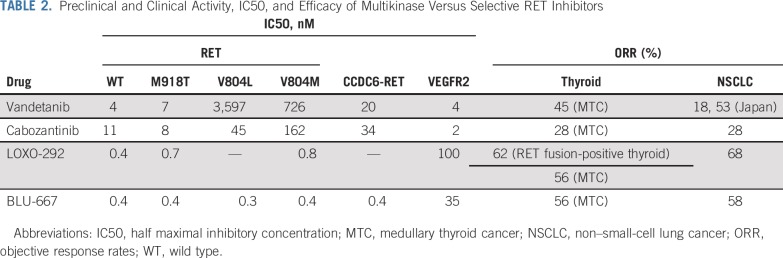
In recent years, selective RET inhibitors have been developed to achieve higher potency and less toxicity. Two such next-generation small molecular inhibitors, namely pralsetinib (BLU-667) and selpercatinib (LOXO-292), have been rapidly translated to clinic1,2 (Table 1). The functional studies using various in vitro and in vivo models showed that both inhibitors are capable of inhibiting a wide spectrum of RET alterations, including M918T, C634W, gatekeeper mutations V804L and V804M, KIF5B-RET, and CCDC6-RET (Table 1).1,2 Importantly, LOXO-292 and BLU-667 have much less activity against VEGFR2 relative to RET alterations, potentially reducing toxicity.
TABLE 1.
Mechanisms of Acquired Resistance to Multikinase Inhibitors and Selective RET Inhibitors
The preliminary results from early-phase trials have demonstrated such superior activity and tolerability with these agents compared with MKIs that these agents have received US FDA breakthrough designation and are on track for registration (Table 1). In the recent update of the ARROW trial (ClincalTrials.gov identifier: NCT03037385), pralsetinib showed an ORR of 56% in RET-mutated MTC136,137 and 58% in RET fusion-positive NSCLC.138 Among these patients, the ORRs were 60% in patients with post-platinum RET-fusion NSCLC and 63% in patients with RET-mutant MTC previously treated with MKIs treated at the 400-mg daily dosing. According to the registrational dataset analysis of a phase I/II LIBRETTO-001 trial (ClincalTrials.gov identifier: NCT03157128), selpercatinib showed an ORR of 68% in RET fusion-positive NSCLC (85% in treatment-naïve patients), 62% in RET fusion-positive thyroid cancer, and 56% in RET-mutant MTC (59% in cabozantinib/vandetanib-naïve patients).2,139-141 The median duration of response (DOR) and PFS were 20.3 months and 18.4 months in patients with RET fusion-positive NSCLC but not reached in treatment-naïve patients with NSCLC.140 The median DOR and PFS were not reached in RET-mutant MTC and RET fusion-positive thyroid cancer.141 In the both trials, most adverse events were grade 1 or 2, and only a few patients had treatment discontinued because of treatment-related adverse events (1.7% in NCT03157128, 2.9% in NCT03037385).136,138,140,141 This favorably compares with other MKIs such as vandetanib, cabozantinib, or lenvatinib that showed a drug discontinuation rate of 21%, 8%, and 20%, respectively. Notably, antitumor activity was observed in patients with brain metastases for both the selective RET inhibitors.139-142
The “RET+ all-comer basket arms” of these clinical trials are in active recruitment, and results from these arms may inform tissue-agnostic development potential. Beyond lung cancers and thyroid cancers, clinical activity of selective RET inhibitors has been seen in patients with GI cancers (pancreatic cancer and intrahepatic bile duct carcinoma)138,139 as well as in pediatric patients with RET-altered cancers (PTC, MEN2A MTC, infantile myofibroma, congenital mesoblastic nephroma, infantile fibrosarcoma, and lipofibromatosis).143
Newer Selective RET Inhibitors and Acquired Mechanisms of Resistance
Several other selective RET inhibitors, BOS172738, TPX-0046, and TAS0953/HM06, are also in early stages of development.144,145 In addition to the RET V804M gatekeeper mutation, several other acquired resistance mechanisms to MKIs have been reported (Table 2). Resistance mechanisms to selective RET remains an active area of research. A preclinical study has shown that novel solvent front mutation KIF5B-RET G810R may develop as on-target resistance to selpercatinib and pralsetinib but remains sensitive to another selective RET inhibitor, TPX-0046, designed with a macrocyclic structure targeting active RET confirmation (Table 2).145
TABLE 2.
Preclinical and Clinical Activity, IC50, and Efficacy of Multikinase Versus Selective RET Inhibitors
In conclusion, the role of RET activating mutations and rearrangements in tumorigenesis has been established during the past three decades. There is considerable excitement in the RET field with the advent of highly selective RET inhibitors. The next-generation selective RET inhibitors selpercatinib and pralsetinib have demonstrated remarkable clinical efficacy and safety in preliminary phase I/II trials. Both agents have received US FDA breakthrough designations. Unanswered questions remain as to what the PFS, DOR, and OS with these agents would be; if all RET-aberrant cancers respond similarly for a tissue-agnostic indication; and what the acquired resistance mechanisms to the potent RET inhibitors would be. In addition, combination therapies exploring the concurrent inhibition of RET and related pathways will provide insight into the clinical utility of such strategies.
ACKNOWLEDGMENT
We thank the patients and their families for participating in RET inhibitor clinical trials.
SUPPORT
Supported in part by Cancer Prevention and Research Institute of Texas Grant No. RP1100584; Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy Grant No. 1U01 CA180964; National Center for Advancing Translational Sciences Grant No. UL1 TR000371; and MD Anderson Cancer Center Support Grant No. P30 CA016672.
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Vivek Subbiah
Financial support: Vivek Subbiah
Administrative support: Vivek Subbiah
Provision of study material or patients: Vivek Subbiah
Collection and assembly of data: Vivek Subbiah
Data analysis and interpretation: Vivek Subbiah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
State-of-The-Art Strategies for Targeting RET-Dependent Cancers
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharma-US
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), MultiVir (Inst), Blueprint Medicines (Inst), Loxo (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Amgen (Inst), TPX Therapeutics (Inst), Boston Pharmaceuticals (Inst).
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
Dong Yang
Employment: Qiagen
Travel, Accommodations, Expenses: Qiagen
Vamsidhar Velcheti
Consulting or Advisory Role: Genentech, Bristol-Myers Squibb, Merck, Foundation Medicine, AstraZeneca/MedImmune, Alkermes, Reddy Labs, Nektar, Novartis, Boston Scientific, Eli Lilly
Research Funding: Genentech (Inst), Trovagene (Inst), Eisai (Inst), OncoPlex Diagnostics (Inst), Alkermes (Inst), NantOmics (Inst), Genoptix (Inst), Altor BioScience (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Atreca (Inst), Heat Biologics (Inst), Leap Therapeutics (Inst), RSIP Vision (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Eisai, Foundation Medicine, Merck
Alexander Drilon
Honoraria: AbbVie, Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, PeerView
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Ariad/Millenium, BerGenBio, MORE Health, Lilly, Verastem
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
Funda Meric-Bernstam
Honoraria: Sumitomo Group, Dialectica
Consulting or Advisory Role: Genentech, Inflection Biosciences, Pieris Pharmaceuticals, Clearlight Diagnostics, Darwin Health, Samsung Bioepis, Spectrum Pharmaceuticals, Aduro Biotech, Origimed, Xencor, Debiopharm Group, Mersana Therapeutics, Seattle Genetics, Silverback Therapeutics, Immunomedics, IBM Watson Health, F. Hoffman-LaRoche, IBM Watson Health (I)
Speakers' Bureau: Chugai Biopharmaceuticals
Research Funding: Novartis, AstraZeneca, Taiho Pharmaceutical, Genentech, Calithera Biosciences, Debiopharm Group, Bayer, Aileron Therapeutics, Puma Biotechnology, CytomX Therapeutics, Jounce Therapeutics, Zymeworks, Curis, Pfizer, eFFECTOR Therapeutics, AbbVie, Boehringer Ingelheim (I), Guardant Health (Inst), Daiichi Sankyo, GlaxoSmithKline, Seattle Genetics
Travel, Accommodations, Expenses: Taiho Pharmaceutical, Seattle Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8:836–849. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- 2.Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M, Buma Y, Iwamoto T, et al. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene. 1988;3:571–578. [PubMed] [Google Scholar]
- 5.Anders J, Kjar S, Ibáñez CF. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem. 2001;276:35808–35817. doi: 10.1074/jbc.M104968200. [DOI] [PubMed] [Google Scholar]
- 6.Goodman KM, Kjær S, Beuron F, et al. RET recognition of GDNF-GFRα1 ligand by a composite binding site promotes membrane-proximal self-association. Cell Reports. 2014;8:1894–1904. doi: 10.1016/j.celrep.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Rossel M, Pasini A, Chappuis S, et al. Distinct biological properties of two RET isoforms activated by MEN 2A and MEN 2B mutations. Oncogene. 1997;14:265–275. doi: 10.1038/sj.onc.1200831. [DOI] [PubMed] [Google Scholar]
- 8.Lian EY, Maritan SM, Cockburn JG, et al. Differential roles of RET isoforms in medullary and papillary thyroid carcinomas. Endocr Relat Cancer. 2017;24:53–69. doi: 10.1530/ERC-16-0393. [DOI] [PubMed] [Google Scholar]
- 9.Le Hir H, Charlet-Berguerand N, Gimenez-Roqueplo A, et al. Relative expression of the RET9 and RET51 isoforms in human pheochromocytomas. Oncology. 2000;58:311–318. doi: 10.1159/000012118. [DOI] [PubMed] [Google Scholar]
- 10.Griseri P, Garrone O, Lo Sardo A, et al. Genetic and epigenetic factors affect RET gene expression in breast cancer cell lines and influence survival in patients. Oncotarget. 2016;7:26465–26479. doi: 10.18632/oncotarget.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veit C, Genze F, Menke A, et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291–5300. doi: 10.1158/0008-5472.CAN-04-1112. [DOI] [PubMed] [Google Scholar]
- 12.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16:441–467. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Worby CA, Vega QC, Chao HH, et al. Identification and characterization of GFRalpha-3, a novel co-receptor belonging to the glial cell line-derived neurotrophic receptor family. J Biol Chem. 1998;273:3502–3508. doi: 10.1074/jbc.273.6.3502. [DOI] [PubMed] [Google Scholar]
- 14.Besset V, Scott RP, Ibáñez CF. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H, Ichihara M, Iwashita T, et al. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Vega QC, Decker RA, et al. Oncogenic RET receptors display different autophosphorylation sites and substrate binding specificities. J Biol Chem. 1996;271:5309–5312. doi: 10.1074/jbc.271.10.5309. [DOI] [PubMed] [Google Scholar]
- 17.Borrello MG, Alberti L, Arighi E, et al. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol Cell Biol. 1996;16:2151–2163. doi: 10.1128/mcb.16.5.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuringa JJ, Wojtachnio K, Hagens W, et al. MEN2A-RET-induced cellular transformation by activation of STAT3. Oncogene. 2001;20:5350–5358. doi: 10.1038/sj.onc.1204715. [DOI] [PubMed] [Google Scholar]
- 19.Perrinjaquet M, Vilar M, Ibáñez CF. Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase. J Biol Chem. 2010;285:31867–31875. doi: 10.1074/jbc.M110.144923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Encinas M, Crowder RJ, Milbrandt J, et al. Tyrosine 981, a novel ret autophosphorylation site, binds c-Src to mediate neuronal survival. J Biol Chem. 2004;279:18262–18269. doi: 10.1074/jbc.M400505200. [DOI] [PubMed] [Google Scholar]
- 21.Schuchardt A, D’Agati V, Larsson-Blomberg L, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 22.Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 24.Gould TW, Yonemura S, Oppenheim RW, et al. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J Neurosci. 2008;28:2131–2146. doi: 10.1523/JNEUROSCI.5185-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer ER, Knott L, Su F, et al. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron. 2006;50:35–47. doi: 10.1016/j.neuron.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Jain S, Naughton CK, Yang M, et al. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development. 2004;131:5503–5513. doi: 10.1242/dev.01421. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca-Pereira D, Arroz-Madeira S, Rodrigues-Campos M, et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature. 2014;514:98–101. doi: 10.1038/nature13498. [DOI] [PubMed] [Google Scholar]
- 28.Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15:151–167. doi: 10.1038/nrclinonc.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizukami T, Shiraishi K, Shimada Y, et al. Molecular mechanisms underlying oncogenic RET fusion in lung adenocarcinoma. J Thorac Oncol. 2014;9:622–630. doi: 10.1097/JTO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 30.Santoro M, Carlomagno F. Central role of RET in thyroid cancer. Cold Spring Harb Perspect Biol. 2013;5:a009233. doi: 10.1101/cshperspect.a009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossi D, Carlomagno F, Pallavicini I, et al. Functional characterization of a novel FGFR1OP-RET rearrangement in hematopoietic malignancies. Mol Oncol. 2014;8:221–231. doi: 10.1016/j.molonc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi HQ, Wu XS, Wei B, et al. Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med. 2012;16:2894–2909. doi: 10.1111/j.1582-4934.2012.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Li Y, Liu C, et al. Identification of a novel KIF13A-RET fusion in lung adenocarcinoma by next-generation sequencing. Lung Cancer. 2018;118:27–29. doi: 10.1016/j.lungcan.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 34. doi: 10.1186/1476-4598-13-176. Qian Y, Chai S, Liang Z, et al: KIF5B-RET fusion kinase promotes cell growth by multilevel activation of STAT3 in lung cancer. Mol Cancer 13:176, 2014 [Erratum: Mol Cancer 18:164, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boikos SA, Stratakis CA. Carney complex: Pathology and molecular genetics. Neuroendocrinology. 2006;83:189–199. doi: 10.1159/000095527. [DOI] [PubMed] [Google Scholar]
- 37.Seki Y, Mizukami T, Kohno T. Molecular process producing oncogene fusion in lung cancer cells by illegitimate repair of DNA double-strand breaks. Biomolecules. 2015;5:2464–2476. doi: 10.3390/biom5042464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi M, Dillon LW, Pramanik S, et al. DNA breaks at fragile sites generate oncogenic RET/PTC rearrangements in human thyroid cells. Oncogene. 2010;29:2272–2280. doi: 10.1038/onc.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ameziane-El-Hassani R, Boufraqech M, Lagente-Chevallier O, et al. Role of H2O2 in RET/PTC1 chromosomal rearrangement produced by ionizing radiation in human thyroid cells. Cancer Res. 2010;70:4123–4132. doi: 10.1158/0008-5472.CAN-09-4336. [DOI] [PubMed] [Google Scholar]
- 40.Fusco A, Grieco M, Santoro M, et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature. 1987;328:170–172. doi: 10.1038/328170a0. [DOI] [PubMed] [Google Scholar]
- 41.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123:4935–4944. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: Biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 43.Elisei R, Romei C, Vorontsova T, et al. RET/PTC rearrangements in thyroid nodules: Studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab. 2001;86:3211–3216. doi: 10.1210/jcem.86.7.7678. [DOI] [PubMed] [Google Scholar]
- 44.Hamatani K, Eguchi H, Ito R, et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;68:7176–7182. doi: 10.1158/0008-5472.CAN-08-0293. [DOI] [PubMed] [Google Scholar]
- 44a.Nguyen L, Niccoli-Sire P, Caron P, et al. Pheochromocytoma in multiple endocrine neoplasia type 2: A prospective study. Eur J Endocrinol. 2001;144:37–44. doi: 10.1530/eje.0.1440037. [DOI] [PubMed] [Google Scholar]
- 44b.Frank-Raue K, Hoppner W, Frilling A, et al. Mutations of the ret protooncogene in German multiple endocrine neoplasia families: Relation between genotype and phenotype. German Medullary Thyroid Carcinoma Study Group. J Clin Endocrinol Metab. 1996;81:1780–1783. doi: 10.1210/jcem.81.5.8626834. [DOI] [PubMed] [Google Scholar]
- 44c.Romei C, Tacito A, Molinaro E, et al. Twenty years of lesson learning: How does the RET genetic screening test impact the clinical management of medullary thyroid cancer? Clin Endocrinol (Oxf) 2015;82:892–899. doi: 10.1111/cen.12686. [DOI] [PubMed] [Google Scholar]
- 44d.Romei C, Mariotti S, Fugazzola L, et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): Results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur J Endocrinol. 2010;163:301–308. doi: 10.1530/EJE-10-0333. [DOI] [PubMed] [Google Scholar]
- 45.Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60:557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- 46.Santoro M, Dathan NA, Berlingieri MT, et al. Molecular characterization of RET/PTC3; a novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–516. [PubMed] [Google Scholar]
- 47.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 48.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mochizuki K, Kondo T, Nakazawa T, et al. RET rearrangements and BRAF mutation in undifferentiated thyroid carcinomas having papillary carcinoma components. Histopathology. 2010;57:444–450. doi: 10.1111/j.1365-2559.2010.03646.x. [DOI] [PubMed] [Google Scholar]
- 50.Grubbs EG, Ng PK, Bui J, et al. RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab. 2015;100:788–793. doi: 10.1210/jc.2014-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 52.Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballerini P, Struski S, Cresson C, et al. RET fusion genes are associated with chronic myelomonocytic leukemia and enhance monocytic differentiation. Leukemia. 2012;26:2384–2389. doi: 10.1038/leu.2012.109. [DOI] [PubMed] [Google Scholar]
- 55.Le Rolle AF, Klempner SJ, Garrett CR, et al. Identification and characterization of RET fusions in advanced colorectal cancer. Oncotarget. 2015;6:28929–28937. doi: 10.18632/oncotarget.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 58. doi: 10.1038/nature13385. Cancer Genome Atlas Research Network: Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543-550, 2014 [Erratum: Nature 514:262, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 60.Tsuta K, Kohno T, Yoshida A, et al. RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571–1578. doi: 10.1038/bjc.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov. 2018;8:1529–1539. doi: 10.1158/2159-8290.CD-18-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Offin M, Guo R, Wu SL, et al: Immunophenotype and response to immunotherapy of RET-rearranged lung cancers. JCO Precis Oncol 10.1200/PO.18.00386. [DOI] [PMC free article] [PubMed]
- 63. Hegde A, Huang L, Liu S, et al: Abstract 4997: Responsiveness to immune checkpoint inhibitors in RET dependent cancers. Cancer Res 79, 2019 (abstr 49997)
- 64. Benayed R, Offin M, Mullaney K, et al: High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res 25:4712-4722, 2019. [DOI] [PMC free article] [PubMed]
- 65.Plaza-Menacho I. Structure and function of RET in multiple endocrine neoplasia type 2. Endocr Relat Cancer. 2018;25:T79–T90. doi: 10.1530/ERC-17-0354. [DOI] [PubMed] [Google Scholar]
- 66.Kato S, Subbiah V, Marchlik E, et al. RET aberrations in diverse cancers: Next-generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23:1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- 67.Verga U, Fugazzola L, Cambiaghi S, et al. Frequent association between MEN 2A and cutaneous lichen amyloidosis. Clin Endocrinol (Oxf) 2003;59:156–161. doi: 10.1046/j.1365-2265.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- 68.Sipple JH. Multiple endocrine neoplasia type 2 syndromes: Historical perspectives. Henry Ford Hosp Med J. 1984;32:219–221. [PubMed] [Google Scholar]
- 69.Romei C, Pardi E, Cetani F, et al. Genetic and clinical features of multiple endocrine neoplasia types 1 and 2. J Oncol. 2012;2012:705036. doi: 10.1155/2012/705036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vasen HF, van der Feltz M, Raue F, et al. The natural course of multiple endocrine neoplasia type IIb. A study of 18 cases. Arch Intern Med. 1992;152:1250–1252. [PubMed] [Google Scholar]
- 71.Carney JA, Sizemore GW, Lovestedt SA. Mucosal ganglioneuromatosis, medullary thyroid carcinoma, and pheochromocytoma: Multiple endocrine neoplasia, type 2b. Oral Surg Oral Med Oral Pathol. 1976;41:739–752. doi: 10.1016/0030-4220(76)90187-0. [DOI] [PubMed] [Google Scholar]
- 72.Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frank-Raue K, Buhr H, Dralle H, et al. Long-term outcome in 46 gene carriers of hereditary medullary thyroid carcinoma after prophylactic thyroidectomy: Impact of individual RET genotype. Eur J Endocrinol. 2006;155:229–236. doi: 10.1530/eje.1.02216. [DOI] [PubMed] [Google Scholar]
- 74.Skinner MA, Moley JA, Dilley WG, et al. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. 2005;353:1105–1113. doi: 10.1056/NEJMoa043999. [DOI] [PubMed] [Google Scholar]
- 75.Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276:1575–1579. [PubMed] [Google Scholar]
- 76.Mulligan LM, Eng C, Healey CS, et al. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet. 1994;6:70–74. doi: 10.1038/ng0194-70. [DOI] [PubMed] [Google Scholar]
- 77.Santoro M, Carlomagno F, Romano A, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 78.Asai N, Iwashita T, Matsuyama M, et al. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol. 1995;15:1613–1619. doi: 10.1128/mcb.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gimm O, Marsh DJ, Andrew SD, et al. Germline dinucleotide mutation in codon 883 of the RET proto-oncogene in multiple endocrine neoplasia type 2B without codon 918 mutation. J Clin Endocrinol Metab. 1997;82:3902–3904. doi: 10.1210/jcem.82.11.4508. [DOI] [PubMed] [Google Scholar]
- 80.Nakao KT, Usui T, Ikeda M, et al. Novel tandem germline RET proto-oncogene mutations in a patient with multiple endocrine neoplasia type 2B: Report of a case and a literature review of tandem RET mutations with in silico analysis. Head Neck. 2013;35:E363–E368. doi: 10.1002/hed.23241. [DOI] [PubMed] [Google Scholar]
- 81.Gujral TS, Singh VK, Jia Z, et al. Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2B. Cancer Res. 2006;66:10741–10749. doi: 10.1158/0008-5472.CAN-06-3329. [DOI] [PubMed] [Google Scholar]
- 82.Jasim S, Ying AK, Waguespack SG, et al. Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid. 2011;21:189–192. doi: 10.1089/thy.2010.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berndt I, Reuter M, Saller B, et al. A new hot spot for mutations in the ret protooncogene causing familial medullary thyroid carcinoma and multiple endocrine neoplasia type 2A. J Clin Endocrinol Metab. 1998;83:770–774. doi: 10.1210/jcem.83.3.4619. [DOI] [PubMed] [Google Scholar]
- 84.Hofstra RM, Fattoruso O, Quadro L, et al. A novel point mutation in the intracellular domain of the ret protooncogene in a family with medullary thyroid carcinoma. J Clin Endocrinol Metab. 1997;82:4176–4178. doi: 10.1210/jcem.82.12.4439. [DOI] [PubMed] [Google Scholar]
- 85.Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12:192–202. doi: 10.1038/nrendo.2016.11. [DOI] [PubMed] [Google Scholar]
- 86.Colombo C, Minna E, Rizzetti MG, et al. The modifier role of RET-G691S polymorphism in hereditary medullary thyroid carcinoma: Functional characterization and expression/penetrance studies. Orphanet J Rare Dis. 2015;10:25. doi: 10.1186/s13023-015-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cardot-Bauters C, Leteurtre E, Leclerc L, et al. Does the RET variant G691S influence the features of sporadic medullary thyroid carcinoma? Clin Endocrinol (Oxf) 2008;69:506–510. doi: 10.1111/j.1365-2265.2008.03230.x. [DOI] [PubMed] [Google Scholar]
- 88.Elisei R, Cosci B, Romei C, et al. Identification of a novel point mutation in the RET gene (Ala883Thr), which is associated with medullary thyroid carcinoma phenotype only in homozygous condition. J Clin Endocrinol Metab. 2004;89:5823–5827. doi: 10.1210/jc.2004-0312. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi M, Iwashita T, Santoro M, et al. Co-segregation of MEN2 and Hirschsprung’s disease: The same mutation of RET with both gain and loss-of-function? Hum Mutat. 1999;13:331–336. doi: 10.1002/(SICI)1098-1004(1999)13:4<331::AID-HUMU11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 90.Arighi E, Popsueva A, Degl’Innocenti D, et al. Biological effects of the dual phenotypic Janus mutation of ret cosegregating with both multiple endocrine neoplasia type 2 and Hirschsprung’s disease. Mol Endocrinol. 2004;18:1004–1017. doi: 10.1210/me.2003-0173. [DOI] [PubMed] [Google Scholar]
- 91.Chappuis-Flament S, Pasini A, De Vita G, et al. Dual effect on the RET receptor of MEN 2 mutations affecting specific extracytoplasmic cysteines. Oncogene. 1998;17:2851–2861. doi: 10.1038/sj.onc.1202202. [DOI] [PubMed] [Google Scholar]
- 92.Romei C, Cosci B, Renzini G, et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC) Clin Endocrinol (Oxf) 2011;74:241–247. doi: 10.1111/j.1365-2265.2010.03900.x. [DOI] [PubMed] [Google Scholar]
- 93.Blaugrund JE, Johns MM, Jr, Eby YJ, et al. RET proto-oncogene mutations in inherited and sporadic medullary thyroid cancer. Hum Mol Genet. 1994;3:1895–1897. doi: 10.1093/hmg/3.10.1895. [DOI] [PubMed] [Google Scholar]
- 94.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 95.Moura MM, Cavaco BM, Leite V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr Relat Cancer. 2015;22:R235–R252. doi: 10.1530/ERC-15-0070. [DOI] [PubMed] [Google Scholar]
- 96.Agrawal N, Jiao Y, Sausen M, et al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab. 2013;98:E364–E369. doi: 10.1210/jc.2012-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knowles PP, Murray-Rust J, Kjaer S, et al. Structure and chemical inhibition of the RET tyrosine kinase domain. J Biol Chem. 2006;281:33577–33587. doi: 10.1074/jbc.M605604200. [DOI] [PubMed] [Google Scholar]
- 98.Wells SA., Jr Advances in the management of MEN2: From improved surgical and medical treatment to novel kinase inhibitors. Endocr Relat Cancer. 2018;25:T1–T13. doi: 10.1530/ERC-17-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sherman SI, Clary DO, Elisei R, et al. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer. 2016;122:3856–3864. doi: 10.1002/cncr.30252. [DOI] [PubMed] [Google Scholar]
- 102.Schlumberger M, Elisei R, Müller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28:2813–2819. doi: 10.1093/annonc/mdx479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schlumberger M, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res. 2016;22:44–53. doi: 10.1158/1078-0432.CCR-15-1127. [DOI] [PubMed] [Google Scholar]
- 104.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 106.Ravaud A, de la Fouchardière C, Caron P, et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: Mature data from the THYSU study. Eur J Cancer. 2017;76:110–117. doi: 10.1016/j.ejca.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 107.Lim SM, Chung WY, Nam KH, et al. An open label, multicenter, phase II study of dovitinib in advanced thyroid cancer. Eur J Cancer. 2015;51:1588–1595. doi: 10.1016/j.ejca.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 108.Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 109.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5:42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- 111.Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: A phase II clinical trial. Ann Oncol. 2017;28:292–297. doi: 10.1093/annonc/mdw559. [DOI] [PubMed] [Google Scholar]
- 112. Velcheti V, Hida T, Reckamp KL, et al: Phase 2 study of lenvatinib (LN) in patients (Pts) with RET fusion-positive adenocarcinoma of the lung. Ann Oncol 27:1204PD, 2016 (Suppl 6)
- 113.Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: Results from the Global, Multicenter RET Registry. J Clin Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drilon A, Fu S, Patel MR, et al. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov. 2019;9:384–395. doi: 10.1158/2159-8290.CD-18-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Drilon AE, Liu S, Doebele R, et al: LBA19A phase 1b study of RXDX-105, a VEGFR-sparing potent RET inhibitor, in RETi-naïve patients with RET fusion-positive NSCLC. Ann Oncol 28:mdx440.012, 2017 (suppl 5)
- 116.Castellanos E, Feld E, Horn L. Driven by mutations: The predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12:612–623. doi: 10.1016/j.jtho.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 117.Caccese M, Ferrara R, Pilotto S, et al. Current and developing therapies for the treatment of non-small cell lung cancer with ALK abnormalities: Update and perspectives for clinical practice. Expert Opin Pharmacother. 2016;17:2253–2266. doi: 10.1080/14656566.2016.1242578. [DOI] [PubMed] [Google Scholar]
- 118.Facchinetti F, Rossi G, Bria E, et al. Oncogene addiction in non-small cell lung cancer: Focus on ROS1 inhibition. Cancer Treat Rev. 2017;55:83–95. doi: 10.1016/j.ctrv.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 119. doi: 10.1016/j.jtho.2019.03.007. Camidge DR, Dziadziuszko R, Peters S, et al: Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol 14:1233-1243, 2019 [Erratum: J Thorac Oncol 14:2023, 2019] [DOI] [PubMed] [Google Scholar]
- 120.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 121.Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62:7284–7290. [PubMed] [Google Scholar]
- 122.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]
- 123.Mologni L, Redaelli S, Morandi A, et al. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol Cell Endocrinol. 2013;377:1–6. doi: 10.1016/j.mce.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 124.Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004;23:6056–6063. doi: 10.1038/sj.onc.1207810. [DOI] [PubMed] [Google Scholar]
- 125. doi: 10.1126/scitranslmed.aah6144. Plenker D, Riedel M, Brägelmann J, et al: Drugging the catalytically inactive state of RET kinase in RET-rearranged tumors. Sci Transl Med 9:eaah6144, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang Q, Schneeberger VE, Luetteke N, et al. Preclinical modeling of KIF5B-RET fusion lung adenocarcinoma. Mol Cancer Ther. 2016;15:2521–2529. doi: 10.1158/1535-7163.MCT-16-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakaoku T, Kohno T, Araki M, et al. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat Commun. 2018;9:625. doi: 10.1038/s41467-018-02994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nelson-Taylor SK, Le AT, Yoo M, et al. Resistance to RET-inhibition in RET-rearranged NSCLC is mediated by reactivation of RAS/MAPK signaling. Mol Cancer Ther. 2017;16:1623–1633. doi: 10.1158/1535-7163.MCT-17-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Somwar R, Smith R, Hayashi T, et al. MDM2 amplification (Amp) to mediate cabozantinib resistance in patients (Pts) with advanced RET-rearranged lung cancers. J Clin Oncol. 2016;34(15_suppl; abstr 9068) [Google Scholar]
- 130.Chang H, Sung JH, Moon SU, et al. EGF induced RET inhibitor resistance in CCDC6-RET lung cancer cells. Yonsei Med J. 2017;58:9–18. doi: 10.3349/ymj.2017.58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zou M, Baitei EY, Alzahrani AS, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014;24:1256–1266. doi: 10.1089/thy.2013.0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guerra A, Zeppa P, Bifulco M, et al. Concomitant BRAF(V600E) mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid. 2014;24:254–259. doi: 10.1089/thy.2013.0235. [DOI] [PubMed] [Google Scholar]
- 133.Subbiah V, Berry J, Roxas M, et al. Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases. Lung Cancer. 2015;89:76–79. doi: 10.1016/j.lungcan.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cascone T, Hess KR, Piha-Paul SA, et al. Safety, toxicity and activity of multi-kinase inhibitor vandetanib in combination with everolimus in advanced solid tumors. J Clin Oncol. 2016;34(15_suppl; abstr 9073) [Google Scholar]
- 135.Cascone T, Subbiah V, Hess KR, et al. Significant systemic and CNS activity of RET inhibitor vandetanib combined with mTOR inhibitor everolimus in patients with advanced NSCLC with RET fusion. J Clin Oncol. 2016;34(15_suppl; abstr 9069) [Google Scholar]
- 136.Taylor MH, Gainor JF, Hu MI-N, et al. Activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients with advanced RET-altered thyroid cancers. J Clin Oncol. 2019;37(15_suppl; abstr 6018) [Google Scholar]
- 137. doi: 10.1158/2159-8290.CD-NB2019-084. BLU-667 Controls RET-Altered Thyroid Cancers. Cancer Discov 9:OF5, 2019. [DOI] [PubMed] [Google Scholar]
- 138. Gainor JF, Lee DH, Curigliano G, et al: Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 37, 2019 (abstr 9008) [Google Scholar]
- 139.Drilon AE, Subbiah V, Oxnard GR, et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J Clin Oncol. 2018;36(15_suppl; abstr 102) [Google Scholar]
- 140.Drilon A, Oxnard G, Wirth L, et al. PL02.08 Registrational results of LIBRETTO-001: A phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J Thorac Oncol. 2019;14:S6–S7. [Google Scholar]
- 141. Wirth L, Sherman E, Drilon A, et al: LBA93 Registrational results of LOXO-292 in patients with RET-altered thyroid cancers. Ann Oncol 30, 2019 (suppl 5; abstr) [Google Scholar]
- 142. Subbiah V, Taylor M, Lin J, et al: Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase I study of advanced, RET-altered solid tumors. Cancer Res 78, 2018 (abstr CT043) [Google Scholar]
- 143. Gerdemann U, Lee YA, Henry D, et al: First experience of LOXO-292 in the management of pediatric patients with RET-altered cancers. J Clin Oncol 37, 2019 (15_suppl; abstr 10045) https://doi.org/10.1200/JCO.2019.37.15_suppl.10045.
- 144.Schoffski P, Aftimos PG, Massard C, et al. A phase I study of BOS172738 in patients with advanced solid tumors with RET gene alterations including non-small cell lung cancer and medullary thyroid cancer. J Clin Oncol. 2019;37(15_suppl; abstr TPS3162) [Google Scholar]
- 145. Drilon A, Rogers E, Zhai D, et al: 506PTPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven cancers. Ann Oncol 30, 2019 (suppl 5; abstr) [Google Scholar]