Abstract
Background
Despite low plasma human immunodeficiency virus (HIV) RNA, HIV controllers have evidence of viral replication and elevated inflammation. We assessed the effect of antiretroviral therapy (ART) on HIV suppression, immune activation, and quality of life (QoL).
Methods
A5308 was a prospective, open-label study of rilpivirine/emtricitabine/tenofovir disoproxil fumarate in ART-naive HIV controllers (N = 35), defined as having HIV RNA <500 copies/mL for ≥12 months. The primary outcome measured change in %CD38+HLA-DR+ CD8+ T cells. Residual plasma viremia was measured using the integrase single-copy assay. QoL was measured using the EQ-5D questionnaire. Outcomes were evaluated using repeated measures general estimating equations models.
Results
Before ART, HIV controllers with undetectable residual viremia <0.6 HIV-1 RNA copies/mL had higher CD4+ counts and lower levels of T-cell activation than those with detectable residual viremia. ART use was effective in further increasing the proportion of individuals with undetectable residual viremia (pre-ART vs after 24–48 weeks of ART: 19% vs 94%, P < .001). Significant declines were observed in the %CD38+HLA-DR+CD8+ T cells at 24–48 (−4.0%, P = .001) and 72–96 (−7.2%, P < .001) weeks after ART initiation. ART use resulted in decreases of several cellular markers of immune exhaustion and in a modest but significant improvement in self-reported QoL. There were no significant changes in CD4+ counts or HIV DNA.
Conclusions
ART in HIV controllers reduces T-cell activation and improves markers of immune exhaustion. These results support the possible clinical benefits of ART in this population.
Keywords: HIV controllers, antiretroviral therapy, inflammation, immune activation, immune exhaustion
A5308 was a prospective, open-label study of rilpivirine/emtricitabine/tenofovir disoproxil fumarate in antiretroviral therapy (ART)-naive human immunodeficiency virus (HIV) controllers. ART further suppressed low-level viremia with reductions in T-cell activation and markers of immune exhaustion, suggesting possible clinical benefits for HIV controllers.
A small proportion of people living with human immunodeficiency virus (HIV) maintain low or even undetectable levels of HIV RNA without antiretroviral therapy (ART). These individuals are referred to as “HIV controllers”; those without detectable virus are referred to as “nonviremic” or “elite” controllers. Generally asymptomatic from the HIV perspective, HIV controllers usually have high CD4+ T-cell counts and are not at risk for AIDS-related opportunistic infections. Because few HIV controllers were enrolled in the pivotal START study of early vs deferred ART in patients with high CD4+ cell counts, the benefits of ART in HIV controllers remain uncertain [1], with this uncertainty also reflected in treatment guidelines [2].
Although these HIV controllers have low or even undetectable viremia by conventional assays, they harbor replication-competent virus and have evidence of viral evolution secondary to immune selection pressure [3–6]. The ongoing viral replication in HIV controllers may be associated with adverse consequences, including the progressive loss of CD4+ cells, increased T-cell activation, and inflammation [7–10]. Chronic immune activation and systemic inflammation have been associated with poor clinical outcomes in noncontrollers [11–14] but also in HIV controllers who are reported to have an increased risk of cardiovascular disease [15] and hospitalizations [16].
While ART is likely to be effective in suppressing HIV replication in HIV controllers [17], the effect that ART may have on immune activation and inflammation has not been confirmed. In HIV noncontrollers with high viral loads, the decline in viral load after ART initiation has been found to occur in at least 3 phases [18]. The evaluation of such viral decay rates has also not been performed in HIV controllers and can provide insights on the composition and half-life of HIV-infected cellular reservoirs.
We performed a prospective, open-label trial to assess the effect of ART on HIV suppression, levels of cellular immune activation and exhaustion, CD4+ cell dynamics, ART tolerability, and quality of life (QoL) in treatment-naive HIV controllers. We also evaluated the effect of ART on soluble markers of inflammation and the HIV reservoir. Finally, we compared the rates of ART-induced viral decay in HIV controllers vs noncontrollers.
METHODS
Study Design and Participants
A5308 was a prospective, open-label study of fixed-dose combination rilpivirine/emtricitabine/tenofovir disoproxil fumarate (RPV/FTC/TDF) in ART-naive HIV controllers conducted at 19 clinical research sites within the AIDS Clinical Trials Group (ACTG). Participants were aged ≥18 years diagnosed with HIV infection based on a positive screening antibody test and confirmed by a Western blot or a second antibody test. They were ART-naive (received ≤7 days of ART) with documented HIV-1 viral loads <500 copies/mL for 12 months or longer. Viral loads ≥500 HIV-1 copies/mL were permitted provided they represented fewer than half of the total measurements over the prior 12 months and the screening HIV-1 RNA was <500 copies/mL. Any measurement ≥1000 HIV-1 RNA copies/mL over the prior 12 months was exclusionary.
Procedures
After study entry, there was a 12-week lead-in period during which participants remained off ART. All participants then initiated open-labeled ART with RPV/FTC/TDF for 48 weeks with on-ART evaluations at 4, 12, 24, 36, and 48 weeks after ART initiation (step 1). After 48 weeks, participants could be followed for an additional 48 weeks (weeks 60, 72, and 96 after initial ART initiation) either on or off ART, based on individual preference (step 2).
Study Endpoints
The primary endpoint was the change in CD8+ T-cell activation as measured by the %CD38+HLA-DR+ CD8+ cells after 24–48 weeks of ART. Secondary endpoints included changes after 24–48 and 72–96 weeks of ART in CD4+ counts, markers of immune activation/exhaustion, systemic inflammation, HIV viremia, reservoir levels, QoL, and safety. Safety was defined as grade ≥3 symptoms, diagnoses, or laboratory abnormalities.
Viral and Immune Characterization
Plasma viremia was quantified using the Abbott RealTime HIV-1 viral load assay. Levels of residual viremia were measured using the ultrasensitive integrase single-copy assay (iSCA) as previously described [19]. Levels of total cell-associated HIV-1 DNA in CD4+ cells were quantified by droplet digital polymerase chain reaction [20]. Immune phenotyping was performed with flow cytometry using an LSRFortessa (BD Biosciences) and analyzed using FlowJo software (Treestar, version 9.9.3). Levels of CD4+ and CD8+ cell activation were assessed by the percentage of cells expressing both CD38+ and HLA-DR+. Immune exhaustion was quantified by the percentage of CD4+ and CD8+ cells expressing PD-1+, TIGIT+, LAG-3+, Tim-3+, CD73+, and CD160+ (BD Biosciences). Levels of 6 soluble inflammatory markers were measured using enzyme-linked immunosorbent assay according to the manufacturer’s instructions: D-dimer (Diagnostica Stago), hs-CRP, hsIL-6, IP-10, sCD14, and sCD163 (R&D Systems). Additional details of the HIV DNA quantification and viral decay analysis are outlined in the Supplementary Methods.
QoL Measurements
QoL was measured using the EQ-5D questionnaire [21]. The QoL index at each time point was obtained by averaging the 5 responses from the questionnaire (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression).
Statistical Analyses
Analyses of change from baseline after 24–48 weeks and after 72–96 weeks of ART were based on the estimated treatment effect from a repeated measures analysis using general estimating equations. In assessing the effects of ART, analyses excluded time points after ART discontinuation. Pre-ART values were generally an average of 2 measurements prior to ART initiation, study entry, and study week 12. Rank-based (Spearman) correlations for continuous outcomes and exact Wilcoxon rank sum and Fisher tests for categorical outcomes were used to assess cross-sectional associations. No adjustments were made for multiple comparisons.
RESULTS
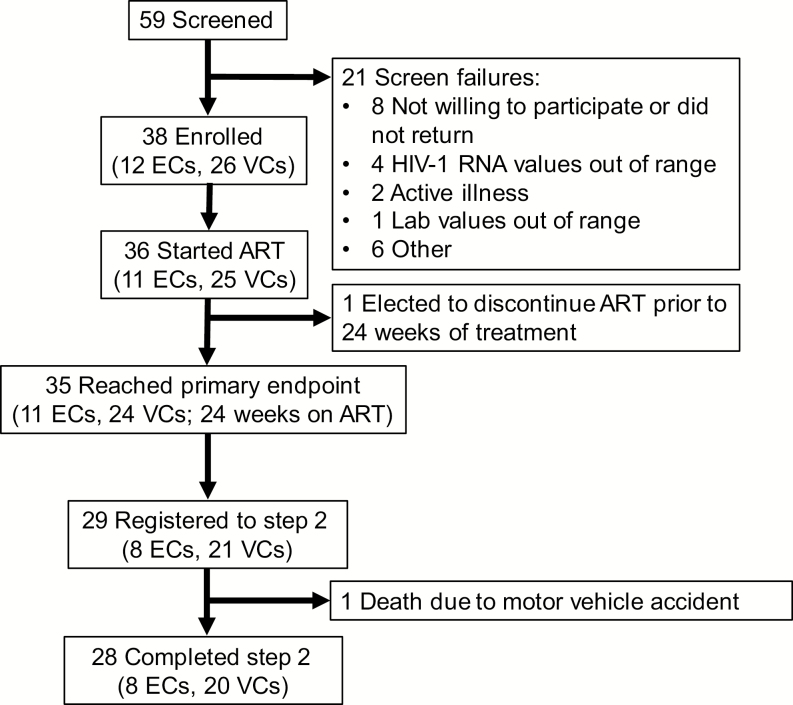
A total of 59 candidates from 19 ACTG clinical sites were screened for this study, and 38 were enrolled. Twenty-one were screen failures, primarily due to individuals who declined to participate or who had out-of-range HIV-1 RNA or other lab values (Figure 1). Thirty-six participants initiated ART, and 35 reached the first primary endpoint of 24 weeks on ART and were included in the analysis. Twenty-nine individuals elected to be followed for an additional 48 weeks in step 2. One individual died in a motor vehicle accident and the remaining 28 completed 96 total weeks of follow-up after ART initiation.
Figure 1.
Consort diagram. ECs had pre-ART viral loads consistently below 40 HIV-1 RNA copies/mL. Abbreviations: ART, antiretroviral therapy; EC, elite controller; HIV-1, human immunodeficiency virus type 1; VC, viremic controller.
For the 35 HIV controllers included in the analysis, 43% were female, with a median pre-ART CD4+ cell count of 682 cells/mm3 (Table 1). Eleven HIV controllers (31%) had pre-ART viral loads consistently below the limit of quantification (<40 HIV-1 RNA copies/mL; Figure 1). Longitudinal viral load results by the iSCA were available for 31 participants, 19% of whom had pre-ART residual viremia below the limit of quantification (<0.6 HIV-1 RNA copies/mL). Before ART, participants with viral load <0.6 copies/mL by iSCA had higher CD4+ counts and CD4/CD8 ratios than those with detectable viral load (median CD4+ count, 1128 vs 659 cells/mm3, P = .03; CD4/CD8 ratio, 1.38 vs 0.82, P = .08). In addition, those with iSCA <0.6 copies/mL had lower levels of both CD8+ (median, 19.4% vs 26.5%; P = .04) and CD4+ cell activation (2.3% vs 2.9%; P = .04). There were no significant differences in levels of soluble inflammatory markers or HIV DNA categorized by iSCA viral load detectability.
Table 1.
Study Participant Demographics
| Characteristic | % or Median (Q1, Q3) (N = 35) |
|---|---|
| Female, % | 43 |
| Age, y | 47 (32, 54) |
| Race/ethnicity, % | |
| White, non-Hispanic | 17 |
| Black, non-Hispanic | 74 |
| Hispanic | 9 |
| Pre-ART CD4+ count, cells/mm3 | 682 (564, 1003) |
| Pre-ART human immunodeficiency virus type 1 RNA <40 copies/mL, %a | 31 |
| HLA*B27/57, % | 57 |
Abbreviations: ART, antiretroviral therapy.
aAt both pre-ART time points.
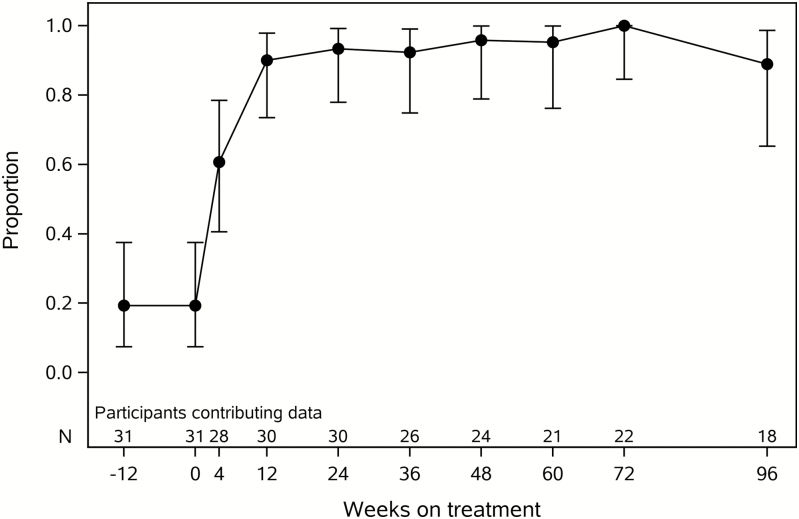
ART was effective in further reducing low-level viremia as measured by both commercial and iSCA viral load assays. The percentage of measurements with HIV-1 RNA <40 copies/mL increased from 37% at ART initiation to ≥96% starting at week 4 after ART initiation. Before ART, 19% of HIV controllers had an iSCA viral load <0.6 HIV-1 RNA copies/mL compared to 94% of measurements after 24–48 weeks of ART (Figure 2; P < .001). There was no significant change in HIV DNA levels with ART (Supplementary Figure 1).
Figure 2.
Proportion of human immunodeficiency virus (HIV) controllers with HIV-1 RNA <0.6 copies/mL by the ultrasensitive integrase single-copy assay. Error bars denote 95% confidence intervals.
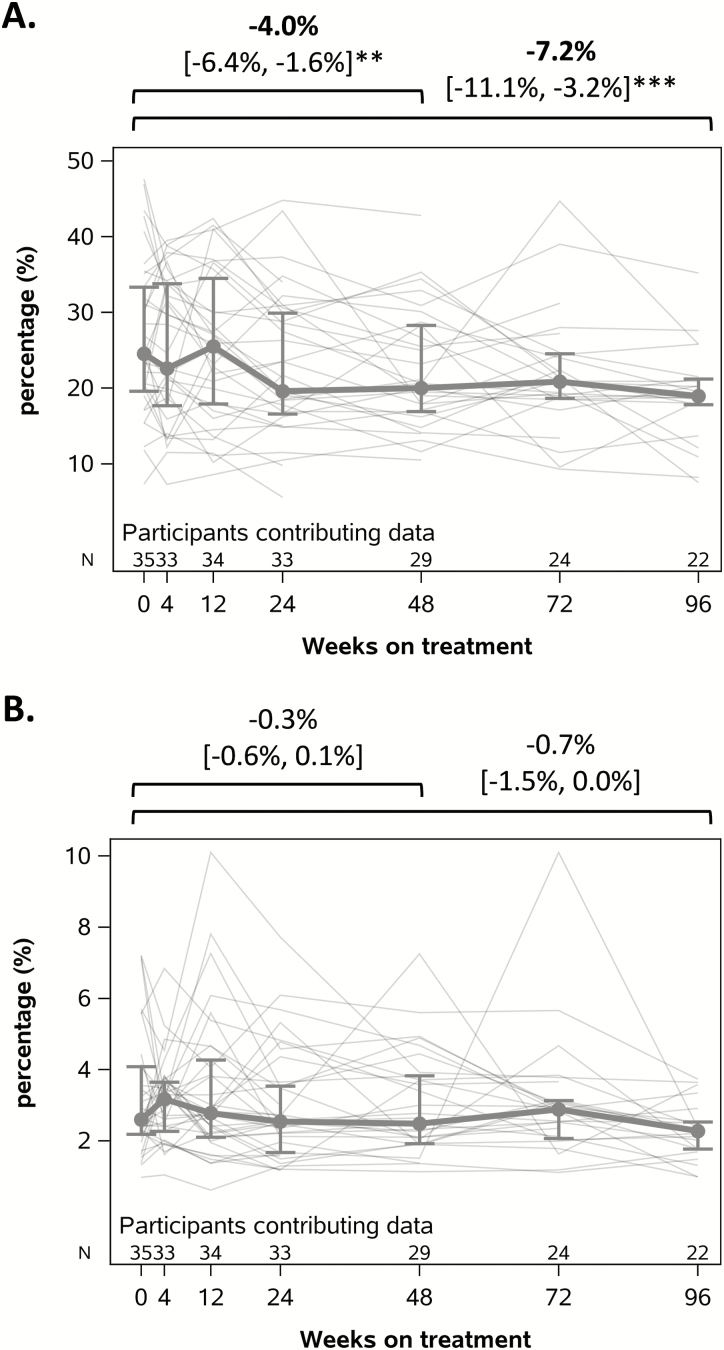
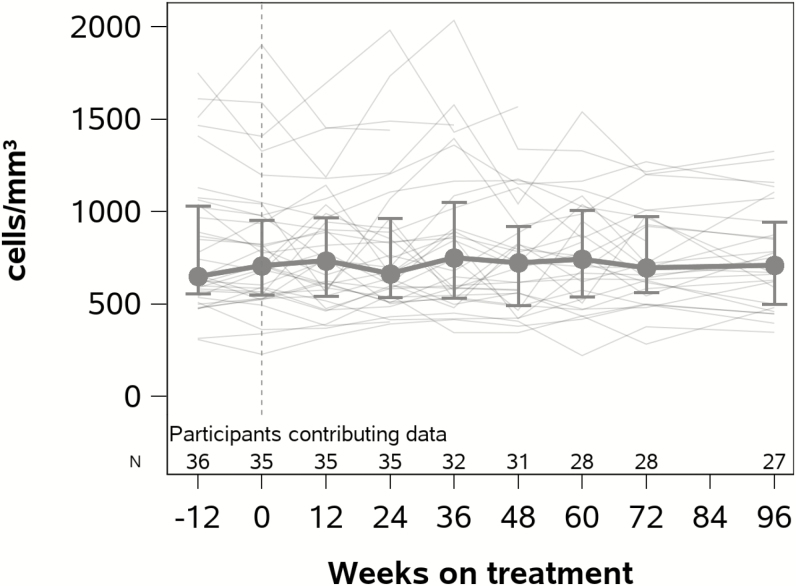
ART resulted in a significant decline in CD8+ cell activation by the %CD38+HLA-DR+ CD8+ cells at 24–48 (−4.0%; 95% confidence interval [CI], −6.4%, −1.6%; P = .001) and 72–96 (−7.2%; 95% CI, −11.1%, −3.2%; P < .001) weeks after ART initiation (Figure 3). This represented a 15% and 26% relative reduction compared to pre-ART levels at 24–48 and 72–96 weeks after ART initiation, respectively. Although not statistically significant, a modest decline in CD4+ cell activation was also observed, representing an 8% and 21% relative reduction compared to pre-ART levels at 24–48 and 72–96 weeks after ART initiation, respectively. ART initiation did not significantly increase CD4+ cell counts (Figure 4) but was associated with significant increases in the CD4/CD8 ratio at both 24–48 (increase of 0.18, P < .001) and 72–96 weeks of ART (increase of 0.29, P < .001).
Figure 3.
Decline in T-cell activation with antiretroviral therapy as measured by the percentage of (A) CD8+ cells and (B) CD4+ cells coexpressing CD38 and HLA-DR. Mean absolute change in percent-activated T cells with 95% confidence values is shown above each graph. Within each graph, values for each individual are graphed in gray; mean and interquartile ranges are shown in bold. **P < .01; ***P < .001.
Figure 4.
CD4+ counts in human immunodeficiency virus controllers after initiation of antiretroviral therapy (ART). Vertical dotted line represents timing of ART initiation. Median values are shown with the interquartile range.
Several T-cell markers of immune exhaustion declined with ART. After 24–48 weeks of therapy, significant decreases were observed in the absolute percentages of CD8+ cells expressing PD1 (−1.8%; 95% CI, −3.2%, −0.4%; P = .02), TIGIT (−3.6%; 95% CI, −6.5%, −0.6%; P = .02), and CD160 (−4.2%; 95% CI, −6.7%, −1.7%; P = .001). There were no significant changes in CD4+ cell markers of immune exhaustion after 24–48 weeks of ART. After 72–96 weeks of ART, there were significant decreases in the absolute percentages of both CD8+ and CD4+ cells expressing CD160 (Supplementary Figure 2). Among the soluble inflammatory markers, ART use was associated with decreased IP-10 levels but increased levels of sCD163 (Supplementary Figure 3).
The effects of ART were also categorized by levels of viremia and age. At 24–48 weeks after ART initiation, the reduction in levels of both CD8+ and CD4+ cell activation observed in participants with pre-ART viral loads <40 HIV-1 RNA copies/mL (CD8+, −1.9%; P = .03 and CD4+, −0.4%; P = .02) did not significantly differ from those observed in HIV controllers with ≥40 HIV-1 RNA copies/mL (CD8+, −4.9%; P = .003 and CD4+, −0.2%; P = .45; Supplementary Figure 4). However, HIV controllers with ≥40 HIV-1 RNA copies/mL did show greater declines in markers of CD8+ cell exhaustion at 24–48 weeks compared to participants with pre-ART viral load <40 copies/mL (%PD-1 by pre-ART viral loads <40 vs ≥40 HIV-1 RNA copies/mL: −0.3% vs −2.4%, P = .05; %TIGIT: −0.2% vs −5.0%, P = .04; %CD160: 0.8% vs −6.3%, P = .003). No significant differences in levels of T-cell activation decline were detected between individuals aged <50 or ≥50 years. Among the immune exhaustion markers, individuals aged ≥50 years had significantly greater declines only in the %LAG-3+ CD8+ cells (<50 vs ≥50 years: 1.9% vs −1.8%, P = .005) and CD4+ cells (1.0% vs −0.5%, P = .02) after 24–48 weeks of ART.
RPV/FTC/TDF was well tolerated, with 2 adverse events categorized as possibly related to the treatment. One individual had a transient low phosphorus value of 1.4 mg/dL 4 weeks after initiating ART that resolved at week 8 of ART. A second participant experienced grade 2 nausea 5 days after initiating ART that resolved after RPV/FTC/TDF was withheld for 2 days. After 48 weeks of ART, participants had the option of continuing to be followed in the study for an additional 48 weeks either on or off ART. Of the 29 individuals who agreed, 97% decided to continue ART. In addition, ART resulted in a modest but significant improvement in self-reported QoL (absolute change from pre-ART to 24–48 weeks of ART was −0.07 (95% CI, −0.12, −0.02; P < .05).
Eighteen HIV controllers agreed to the additional viral decay visits. Fourteen (78%) had entry iSCA values above the limit of quantification and were included in estimation of phase 1 viral decay (Supplementary Figure 5). Seven (39%) had sufficient detectable iSCA results for estimation of phase 2 viral decay. The median pre-ART viral load by iSCA was 81 HIV-1 RNA copies/mL for the HIV controllers and 56 550 copies/mL for the historical comparator group of noncontrollers. Phase 1 viral decay did not differ between HIV controllers and noncontrollers (median 0.58 vs 0.66 loge HIV-1 RNA copies/day, P = .81). Phase 2 decay rates were significantly shorter in HIV controllers compared to noncontrollers (0.069 vs 0.040 loge HIV-1 RNA copies/day, P = .04), corresponding to a half-life of 10 vs 17 days.
DISCUSSION
Despite the clinical stability of most HIV controllers, there is evidence that this condition is not entirely benign. Some controllers will experience a loss of viral control, and even among those who maintain low-level viremia, CD4+ cells may decline over time [22]. HIV controllers also have increased levels of immune activation and inflammation compared to both ART-treated and HIV-uninfected individuals [9, 23]. There is evidence that persistent low-level viral replication and inflammation in HIV controllers are associated with disease progression and immune dysfunction [22, 24, 25], as well as increased risk of cardiovascular disease [15] and hospitalizations [16]. Based on these concerning data regarding HIV controllers, we sought to evaluate the potential benefits of ART in this population.
Prior studies in HIV controllers have reported that ART can further decrease the HIV reservoir and reduce detectable low-level viremia [17, 26], as well as induce modest CD4+ cell gains [27, 28] or immune benefits [29]. However, these studies were limited by small sample sizes and incomplete evaluation of systemic inflammation and lacked an assessment of viral dynamics. The largest of the prospective studies was also limited by a relatively short duration of follow-up [17]. Recruiting from 19 clinical sites within the ACTG, we enrolled the largest study to date of ART for HIV controllers, with longitudinal follow-up for up to 2 years.
Before ART, HIV controllers with lower viral loads were found to have higher CD4+ cell counts and lower levels of T-cell activation, demonstrating important differences between elite and viremic controllers. One year of ART reduced T-cell activation and markers of immune exhaustion, in some cases with further decreases after 2 years of ART; these benefits were seen in both elite and viremic controllers, with the latter group having a greater response. The level of reduction in T-cell activation through 1 year (8% for CD4+ cells and 15% for CD8+ cells) was less dramatic than that reported in the A5321 study of HIV noncontrollers who initiated ART (49% and 57% reduction in CD4+ and CD8+ cell activation, respectively) [30] but not unexpected given the lower baseline pre-ART levels of immune activation in HIV controllers compared to ART-untreated noncontrollers [9]. ART was also effective in further suppressing already low levels of viremia, such that 94% of HIV controllers on ART had viremia <0.6 copies/mL by the ultrasensitive iSCA assay compared to 19% before ART.
In our study, the benefits of ART in HIV controllers did not extend to all viral or immunologic markers. While we detected significant improvements in the CD4/CD8 ratio, there were no significant changes in absolute CD4+ counts. The lack of CD4+ improvement with ART may reflect the relatively preserved CD4+ cell counts and is consistent with prior studies that demonstrated modest immune recovery after ART initiation in those with very low viral loads [17]. While pre-ART viral load has been associated with HIV DNA levels in noncontrollers [30], we detected no difference in pre-ART HIV DNA levels in HIV controllers with or without detectable residual viremia. After ART initiation, we found no significant decline in levels of total HIV DNA, potentially pointing to the presence of long-lived or clonally expanded populations of HIV-infected cells. Additional characterization of the intact and defective HIV proviral reservoir will be needed to further explore this finding. While ART resulted in significant reductions in levels of T-cell activation and exhaustion, there were no significant improvements in levels of several soluble inflammatory markers (D-dimer, hs-CRP, hsIL-6, and sCD14) and an unexpected increase in levels of sCD163. A study of ART in HIV noncontrollers found that higher baseline viral loads were associated with higher levels of soluble inflammatory factors and that pre-ART levels of soluble inflammatory markers predicted eventual levels while on ART [30]. These findings suggest that the lack of discernible impact of ART on soluble markers of inflammation may potentially be due to their relatively low levels before ART.
We also assessed viral dynamics after starting treatment. In HIV noncontrollers with high pre-ART viral loads, the decline in viral loads after ART initiation occurs initially in 3 phases. The first 2 phases are relatively rapid and occur with a half-life of 1–2 days for the first phase and several weeks for the second phase [31, 32]. However, the third phase of viral decay is much slower (estimated t1/2 = 39 weeks) and likely reflects the clearance of a latently infected cellular compartment or viral reservoir [18]. In the viral dynamics component of A5308, HIV controllers were found to have similar rates of first-phase viral decay but faster second-phase viral decay. Subsequent viral loads were too low to assess the presence of additional phases of viral decay despite the use of an ultrasensitive assay. These results reveal the extremely low numbers of medium- and long-lived HIV-expressing cells in ART-treated HIV controllers. Furthermore, the second phase decay rates suggest differences between HIV controllers and noncontrollers in the composition or turnover of the cellular populations that support active viral replication. These findings highlight additional potential directions for future HIV reservoir studies.
There are several limitations to our study. Despite being the largest prospective study of treatment in HIV controllers to date, our sample size was still limited and included relatively few elite controllers. Many of the elite controllers at our study sites, even those who had actively participated in research studies for years, were reluctant to begin ART, mostly due to concerns about drug toxicity. We selected RPV/FTC/TDF because of its simplicity and low incidence of side effects and because RPV/FTC/TAF is currently one of the “recommended initial regimens in certain clinical situations” in the latest guidelines by the US Department of Health and Human Services [2]. We do not know if our results would be similar if a different drug class had been used; previous studies have found a more pronounced effect of integrase strand-transfer inhibitors on soluble inflammatory markers [30, 33]. Finally, the single-arm study design, sample size, and follow-up period do not permit us to make any definitive conclusions about the clinical benefits of ART or improvements in QoL for HIV controllers.
These limitations notwithstanding, the results of the A5308 show that ART given to HIV controllers further reduced viremia and decreased both T-cell activation and markers of immune exhaustion. These virologic and immunologic benefits were observed in both HIV elite and viremic controllers. Treatment was well tolerated and associated with a modest improvement in QoL. Overall, the results of the study suggest possible benefits of initiating ART, even in individuals with very low or undetectable plasma HIV RNA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
A5308 ACKNOWLEDGEMENT APPENDIX
Cornelius Van Dam MD and Kim Epperson RN BSN–Greensboro CRS (Site 3202) grant A1068636. Michael C Keefer MD and Catherine A Bunce RN MS–University of Rochester (Site 31787) grant UM1AI069511. Beverly E Sha MD and Mark Mall RN–Rush University Medical Center (Site 2702) grant U01 AI069471. 4651, Institute of Human Virology Baltimore Treatment CRS. Paul Sax MD and Cheryl Keenan RN BC–Brigham and Women’s Hospital (Site 107) grant 5UM1AI068636-11. Benigno Rodriguez MD and Jane Baum RN–Case CRS (Site 2501, 2503) grant AI69501. Baiba Berzins MPH and Donna McGregor NP–Northwestern University CRS (Site 2701) grant 2UM1 AI068636. 31469, Bronx-Lebanon Hospital Center CRS. Victoria A Johnson and Michael Messer–Alabama CRS (Site 31788) grant UM1 AI069452. David Hardy, MD and Lynsay MacLaren–31791, Whitman Walker Health CRS. Joseph Eron, MD, Mina Hosseinipour, MD, and Becky Straub–3201, Chapel Hill CRS. Anandi Sheth MD and Ericka R Patrick RN MSN–Emory-CDC PONCE CRS (Site 5802) grant UM1A1069418-08, Emory CFAR# P30AI050409. Sara Onesi RN and Renee Weinman MPPM–University of Pittsburgh (Site 1001) grant AI069494. Teri Flynn MSN ANP and Amy Sbrolla BSN RN–MGH CRS (Site 101) grant UM1 AI069412-08. Eva Whitehead RN BSN and Carl J Fichtenbaum MD–Cincinnati CRS (Site 2401) grant UM1-AI069501. Karen Tashima and Pamela Poethke–2951, The Miriam Hospital (TMH) CRS. Roberto C Arduino MD and Aristoteles E Villamil MD–HART (Site 31473) grant 5 UM1 AI069503-13, 5 UM1 AI068636-13. Pablo Tebas, MD and Eileen Donaghy–6201, Penn Therapeutics CRS. Diane Havlir, MD and Jay Dwyer–801, University of California, San Francisco HIV/AIDS CRS.
Notes
Acknowledgments. The authors thank the A5308 study participants, the A5308 study team, and the site staff at all of the AIDS Clinical Trials Group (ACTG) clinical research sites.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), Frederick National Lab, Center for Cancer Research.
Financial support. This study was funded in part by NIH grants U01 AI068636 (ACTG), Gilead CO-US-264-0116, AI068634 (Statistical and Data Analysis Center of the AIDS Clinical Trials Group), a subcontract from UM1 AI068636 to the Harvard Virology Support Laboratory, and UM1 AI106701 to the Rush Immunology Support Laboratory. Antiretroviral medications were provided by Gilead. This project has been funded in part with federal funds from the Frederick National Laboratory for Cancer Research (under contract HHSN261200800001E).
Potential conflicts of interest. J. Z. L. has received research support and consulted for Gilead Sciences and Merck. F. P. S. is a full-time employee at the Novartis Institutes for BioMedical Research. A. L. has consulted for Gilead and Merck. X. G. Y. has received research support from Gilead Sciences. P. E. S. has received research support from Gilead, Merck, and ViiV and is on the scientific advisory boards of Gilead, Janssen, Merck, and ViiV. C. N. V. D. has received research support from Gilead and ViiV, has been on scientific advisory boards for Gilead and ViiV, and served as a speaker for Janssen and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lundgren JD, Babiker AG, Gordin F, et al. ; INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services; Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed 20 December 2018. [Google Scholar]
- 3. Blankson JN, Bailey JR, Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol 2007; 81:2508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol 2010; 84:7018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey JR, Brennan TP, O’Connell KA, Siliciano RF, Blankson JN. Evidence of CD8+ T-cell-mediated selective pressure on human immunodeficiency virus type 1 nef in HLA-B*57+ elite suppressors. J Virol 2009; 83:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med 2006; 203:1357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mavigner M, Delobel P, Cazabat M, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One 2009; 4:e7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostrowski SR, Katzenstein TL, Pedersen BK, Gerstoft J, Ullum H. Residual viraemia in HIV-1-infected patients with plasma viral load <or=20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol 2008; 68:652–60. [DOI] [PubMed] [Google Scholar]
- 9. Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis 2009; 200:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18:332–40. [DOI] [PubMed] [Google Scholar]
- 12. Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999; 179:859–70. [DOI] [PubMed] [Google Scholar]
- 13. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 2003; 17:1881–8. [DOI] [PubMed] [Google Scholar]
- 15. Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012; 26:2409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowell TA, Gebo KA, Blankson JN, et al. ; HIV Research Network Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis 2015; 211:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hatano H, Yukl SA, Ferre AL, et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog 2013; 9:e1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2008; 105:3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cillo AR, Vagratian D, Bedison MA, et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol 2014; 52:3944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014; 20:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu AW, Hanson KA, Harding G, et al. Responsiveness of the MOS-HIV and EQ-5D in HIV-infected adults receiving antiretroviral therapies. Health Qual Life Outcomes 2013; 11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leon A, Perez I, Ruiz-Mateos E, et al. ; EC and Immune Pathogenesis Working Group of the Spanish AIDS Research Network Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 2016; 30:1209–20. [DOI] [PubMed] [Google Scholar]
- 23. Li JZ, Arnold KB, Lo J, et al. Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis 2015; 2:ofu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boufassa F, Saez-Cirion A, Lechenadec J, et al. ; ANRS EP36 HIV Controllers Study Group CD4 dynamics over a 15 year-period among HIV controllers enrolled in the ANRS French observatory. PLoS One 2011; 6:e18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Groves KC, Bibby DF, Clark DA, et al. Disease progression in HIV-1-infected viremic controllers. J Acquir Immune Defic Syndr 2012; 61:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chun TW, Shawn Justement J, Murray D, et al. Effect of antiretroviral therapy on HIV reservoirs in elite controllers. J Infect Dis 2013; 208:1443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okulicz JF, Grandits GA, Weintrob AC, et al. CD4 T cell count reconstitution in HIV controllers after highly active antiretroviral therapy. Clin Infect Dis 2010; 50:1187–91. [DOI] [PubMed] [Google Scholar]
- 28. Boufassa F, Lechenadec J, Meyer L, et al. ; ANRS CO18 HIV Controllers Cohort; Cascade Collaboration in Eurocoord; SCOPE Cohort; International HIV Controllers Study Blunted response to combination antiretroviral therapy in HIV elite controllers: an international HIV controller collaboration. PLoS One 2014; 9:e85516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim CJ, Kovacs C, Chun TW, et al. Antiretroviral therapy in HIV-infected elite controllers: impact on gut immunology, microbial translocation, and biomarkers of serious non-AIDS conditions. J Acquir Immune Defic Syndr 2014; 67:514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuritzkes DR, Ribaudo HJ, Squires KE, et al. ; ACTG A5166s Protocol Team Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis 2007; 195:1169–76. [DOI] [PubMed] [Google Scholar]
- 32. Haubrich RH, Riddler SA, Ribaudo H, et al. ; AIDS Clinical Trials Group (ACTG) A5160 and A5142 Study Teams Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, nonnucleoside reverse transcriptase inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS 2011; 25:2269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hileman CO, Kinley B, Scharen-Guivel V, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.