This cross-sectional study assesses the types of antibiotic resistance profiles and trends among bacterial isolates from ocular sources that were prevalent from 2009 to 2018.
Key Points
Question
What are the antibiotic resistance profiles and trends among common ocular pathogens across the United States?
Findings
In this cross-sectional study of more than 6000 ocular isolates of Staphylococcus aureus, coagulase-negative staphylococci, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae collected between 2009 and 2018, methicillin resistance and multidrug resistance were prevalent among staphylococci. Antibiotic resistance profiles were mostly unchanged during 10 years.
Meaning
These in vitro antibiotic resistance data may assist clinicians in selecting appropriate antibiotics for treatment of ocular infections.
Abstract
Importance
Antibiotic resistance in ocular infections can affect treatment outcomes. Surveillance data on evolving antibacterial susceptibility patterns inform the treatment of such infections.
Objective
To assess overall antibiotic resistance profiles and trends among bacterial isolates from ocular sources collected during 10 years.
Design, Setting, and Participants
This cross-sectional study of longitudinal data from the ongoing, nationwide, prospective, laboratory-based surveillance study, the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study, included clinically relevant isolates of Staphylococcus aureus, coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae cultured from patients with ocular infections at US centers from January 1, 2009, to December 31, 2018.
Main Outcomes and Measures
Minimum inhibitory concentrations were determined for various combinations of antibiotics and species. Odds ratios (ORs) were determined for concurrent antibiotic resistance; analysis of variance and χ2 tests were used to evaluate resistance rates by patient age and geographic region; Cochran-Armitage tests identified changing antibiotic susceptibility trends over time.
Results
A total of 6091 isolates (2189 S aureus, 1765 CoNS, 590 S pneumoniae, 767 P aeruginosa, and 780 H influenzae) from 6091 patients were submitted by 88 sites. Overall, 765 S aureus (34.9%) and 871 CoNS (49.3%) isolates were methicillin resistant and more likely to be concurrently resistant to macrolides (azithromycin: S aureus: OR, 18.34 [95% CI, 13.64-24.67]; CoNS: OR, 4.59 [95% CI, 3.72-5.66]), fluoroquinolones (ciprofloxacin: S aureus: OR, 22.61 [95% CI, 17.96-28.47]; CoNS: OR, 9.73 [95% CI, 7.63-12.40]), and aminoglycosides (tobramycin: S aureus: OR, 18.29 [95% CI, 13.21-25.32]; CoNS: OR, 6.28 [95% CI, 4.61-8.56]) compared with methicillin-susceptible isolates (P < .001 for all). Multidrug resistance was observed among methicillin-resistant S aureus (577 [75.4%]) and CoNS (642 [73.7%]) isolates. Antibiotic resistance among S pneumoniae isolates was highest for azithromycin (214 [36.3%]), whereas P aeruginosa and H influenzae isolates showed low resistance overall. Differences in antibiotic resistance were found among isolates by patient age (S aureus: F = 28.07, P < .001; CoNS: F = 11.46, P < .001) and geographic region (S aureus: F = 8.03, P < .001; CoNS: F = 4.79, P = .003; S pneumoniae: F = 8.14, P < .001; P aeruginosa: F = 4.32, P = .005). Small changes in antibiotic resistance were noted over time (≤2.5% per year), with decreases in resistance to oxacillin/methicillin (oxacillin: −2.16%; 95% CI, −3.91% to −0.41%; P < .001) and other antibiotics among S aureus isolates, a decrease in ciprofloxacin resistance among CoNS (−1.38%; 95% CI, −2.24% to −0.52%; P < .001), and an increase in tobramycin resistance among CoNS (0.71%; 95% CI, –0.29% to 1.71%; P = .03). Besifloxacin retained consistently low minimum inhibitory concentrations.
Conclusions and Relevance
Antibiotic resistance may be prevalent among staphylococcal isolates, particularly among older patients. In this study, a few small differences in antibiotic resistance were observed by geographic region or longitudinally.
Introduction
The finding of significant antibiotic resistance among ocular pathogens in recent decades is a concern.1,2,3,4,5,6,7 Antibiotic resistance among ocular bacteria can lead to treatment failure8,9,10,11,12,13 and complicate the choice of antibiotic in clinical practice.
In any infection, identification of causative pathogens and determination of their antibiotic resistance profiles should ideally precede initiation of antibiotic therapy.14 Although cultures are performed for vision-threatening ocular infections, they are seldom performed for routine eye infections, with physicians favoring empirical therapy to avoid treatment delays associated with the time required to obtain culture and sensitivity results and/or to avoid the costs of culturing.15,16 In the absence of culture and sensitivity results, antibiotic resistance data from surveillance studies can inform the choice of initial or empirical treatment. However, regardless of how the treatment decision is made, antibiotic resistance remains an important consideration in the treatment of eye infections.
The Ocular Tracking Resistance in US Today (Ocular TRUST) study6,17,18 was a nationwide surveillance program conducted from 2005 to 2008 to monitor antibiotic resistance specific to common ocular pathogens. Results showed high levels of methicillin resistance among staphylococci, with a predominance of concurrent resistance to other antibiotic classes.
The Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study is a multicenter, nationwide, prospective surveillance study initiated in 200919 and designed to extend on the Ocular TRUST study in surveying antibacterial resistance among clinically relevant isolates of Staphylococcus aureus, coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae. Periodic updates and various subset analyses of ARMOR data have provided clinicians with an understanding of antibiotic resistance patterns to aid in antibiotic selection and ultimately improve patient outcomes.19,20,21,22,23,24,25,26 To our knowledge, Ocular TRUST6,17,18 and ARMOR19,21,22,23 are the only prospective surveillance studies on ocular isolates, and ARMOR is the only ongoing study in the United States. Using data from ARMOR, we assessed the overall antibiotic resistance profiles and trends by age, geographic region, and over time for ocular isolates collected from January 1, 2009, to December 31, 2018.
Methods
ARMOR Study Design
This cross-sectional study used longitudinal data from the ARMOR surveillance study; the design and methods have been described previously.19 Community hospitals, academic or university hospitals, specialty or ocular centers, and reference laboratories in the United States were asked to provide isolates of S aureus, CoNS, S pneumoniae, P aeruginosa, and H influenzae from patients with ocular infections to a central laboratory for testing. From January 1, 2009, through December 31, 2013, up to 65 isolates, including S aureus (≤20), CoNS (≤20), S pneumoniae (≤5), P aeruginosa (≤15), and H influenzae (≤5) were requested from each site per collection year; from January 1, 2014, through December 31, 2018, a maximum of 50 (≤12 from any of the aforementioned species) were requested from each site per collection year. Sites were asked to submit isolates deemed to be clinically relevant based on their discretion; isolates could be sourced from any ocular tissue, although endophthalmitis isolates were excluded in earlier collection years (2009-2014). Site participation was inconsistent throughout all 10 years, and new sites were recruited yearly as needed. Initial ocular culture samples were not prospectively collected for ARMOR but were taken as part of routine medical care unrelated to the study and stored on site until shipment to the central laboratory. Because the study entailed laboratory research on samples already collected and there was no identifiable patient information provided with the isolates, the study was deemed to be exempt from formal institutional review board review activity per title 45 of the Code of Federal Regulations. However, the ARMOR study protocol deferred the final need for any institutional review board review activity to individual participating sites based on their own local institutional review board requirements. Institutional review board approval was not required for study conduct at any of the 88 participating ARMOR sites.
Antibiotic Susceptibility Testing
Bacterial isolates were sent via ESwab Collection/Transport Systems (Becton, Dickinson and Company) to an independent central laboratory (Eurofins Medinet [2009-2013]; International Health Management Associates Inc [2014-2018]) for species confirmation, and minimum inhibitory concentrations (MICs) were determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) methods with frozen antibacterial microtiter panels.27,28,29,30 The lowest drug concentrations that inhibited the growth of 90% of indicated isolates (MIC90s) were calculated. The following representative antibiotics from 10 different classes were tested as appropriate based on bacterial species: azithromycin (macrolide); clindamycin (lincosamide); besifloxacin, ciprofloxacin, gatifloxacin, levofloxacin, moxifloxacin, and ofloxacin (fluoroquinolones); chloramphenicol (amphenicol); oxacillin and penicillin (beta-lactams); polymyxin B (polypeptide); tetracycline (tetracycline); tobramycin (aminoglycoside); trimethoprim (dihydrofolate reductase inhibitor); and vancomycin (glycopeptide). Additional antibiotics were tested in earlier years (eg, ceftriaxone, imipenem) but are not reported because of CLSI changes in recommended antibiotics to be used for susceptibility determinations. Some nonophthalmic antibiotics were included to allow for a comparison with reported resistance rates for nonocular isolates of the same species. When available, MICs were interpreted as indicating antibiotic susceptible, intermediate, or resistant based on the CLSI interpretive criteria in use during the collection year for that antibiotic or species combination; because no systemic formulation exists for besifloxacin, interpretive criteria are not available for this drug.31,32,33,34,35,36,37,38,39,40 Staphylococci were categorized as methicillin resistant or methicillin susceptible based on oxacillin susceptibility; the break point for oral penicillin was used to determine susceptibility of S pneumoniae to penicillin. Unless otherwise indicated, break points for ciprofloxacin were used to determine resistance to the fluoroquinolone class. Calculations for the percentage of antibiotic resistance included antibiotic-intermediate and antibiotic-resistant isolates except where noted otherwise. Multidrug resistance (MDR) was defined as resistance to at least 3 classes of antibiotics.
Statistical Analysis
Odds ratios (ORs) for resistance to each antibiotic were based on sample proportions computed directly from the data. One-way analysis of variance was used to evaluate antibiotic resistance of isolates by age of the patient and by geographic region; age analysis involved categorization of isolates by decade of life, whereas geographical analysis involved categorization of isolates based on region of origin (Midwest, Northeast, South, and West) as previously described.20 Because not all antibiotic classes were tested each year, the analysis of variance used means of the percentage of drug classes to which each isolate of a species or species group was resistant based on the number of antibiotic classes tested. If P ≤ .05, the data provided evidence that the means were not equal. Subsequently, the Tukey honestly significant differences test was applied.41 Additional analyses of differences among staphylococci by methicillin resistance status were performed using χ2 tests followed by a multiple-comparisons test for proportions. Changes in antibiotic resistance over time were examined using a Cochran-Armitage test42 for linear trends in a proportion, with 2-tailed P < .05 values reported; weighted least squares regression analysis was conducted to estimate the magnitude of any change (ie, slope, 95% CI) over time. Statistical testing was performed using Statistix 10 (Analytical Software).
Results
Source of Isolates
A total of 6091 isolates (2189 S aureus, 1765 CoNS, 590 S pneumoniae, 767 P aeruginosa, and 780 H influenzae) were collected from 88 sites (47 community hospitals, 29 academic or university hospitals, 9 specialty or ocular centers, and 3 reference laboratories) across 41 states (2526 Midwest, 1300 Northeast, 783 South, and 1482 West). Of 6091 patients from whom isolates were obtained, 2735 (44.9%) were male, 2851 (46.8%) were female, and sex was not reported for 505 (8.3%). Patient age was known for 4988 isolates, with 1102 obtained from patients younger than 10 years. A total of 349 of 621 H influenzae isolates (56.2%) and 165 of 463 S pneumoniae isolates (35.6%) were from patients aged 0 to 9 years, whereas 313 of 1834 S aureus isolates (17.1%) came from patients with similar ages. The anatomical source (other than for ocular isolates with an unknown anatomical source) was known for 3132 isolates (51.4%) and included the conjunctiva (n = 1609), cornea (n = 1288), aqueous humor (n = 73), and vitreous humor (n = 162). Among the 460 P aeruginosa isolates from a known anatomical source, 325 (70.7%) were from corneal scrapings, and among the 328 H influenzae isolates from a known anatomical source, 287 (87.5%) were from the conjunctiva.
Cumulative Antibiotic Resistance Rates
Cumulative MIC90s and antibiotic resistance profiles for collected isolates, including those for staphylococci by methicillin-resistance phenotype, are presented by species or antibiotic combination in the Table. Of 2189 S aureus isolates, 765 (34.9%) were resistant to oxacillin/methicillin (methicillin-resistant S aureus [MRSA]). Resistance was observed to ciprofloxacin in 733 (33.5%) and to azithromycin in 1306 (59.7%) of S aureus isolates, although all were susceptible to vancomycin. Compared with methicillin-susceptible S aureus (MSSA) isolates, resistance to other antibiotics was more prevalent among MRSA isolates, with resistance greater than 70% for fluoroquinolones (not applicable for besifloxacin) and 92.9% for azithromycin. The later-generation fluoroquinolones (besifloxacin, gatifloxacin, and moxifloxacin) had lower MIC90s overall compared with the earlier ones (ciprofloxacin, levofloxacin, and ofloxacin). Besifloxacin showed the lowest MIC90s, and ciprofloxacin showed the highest.
Table. Antibiotic Resistance Profiles and MIC90s for Staphylococcus aureus, CoNS, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae Isolates.
| Organism, antibiotic | Isolates, No. | MIC90, μg/mL | Antibiotic resistance profile, %a | ||
|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | |||
| S aureus | |||||
| Besifloxacin | |||||
| All | 2189 | 1 | NA | NA | NA |
| MSSA | 1424 | 0.25 | NA | NA | NA |
| MRSA | 765 | 2 | NA | NA | NA |
| Moxifloxacin | |||||
| All | 2189 | 4 | 68.6 | 6.4 | 25.0 |
| MSSA | 1424 | 0.5 | 90.0 | 2.9 | 7.1 |
| MRSA | 765 | 16 | 28.8 | 12.9 | 58.3 |
| Gatifloxacin | |||||
| All | 1989 | 4 | 68.2 | 2.4 | 29.4 |
| MSSA | 1302 | 1 | 89.2 | 1.4 | 9.4 |
| MRSA | 687 | 16 | 28.2 | 4.4 | 67.4 |
| Ciprofloxacin | |||||
| All | 2189 | 128 | 66.5 | 1.3 | 32.2 |
| MSSA | 1424 | 4 | 88.6 | 1.1 | 10.4 |
| MRSA | 765 | 256 | 25.5 | 1.8 | 72.7 |
| Levofloxacin | |||||
| All | 1989 | 16 | 67.9 | 1.6 | 30.5 |
| MSSA | 1302 | 2 | 89.1 | 1.0 | 9.9 |
| MRSA | 687 | 128 | 27.8 | 2.8 | 69.4 |
| Ofloxacin | |||||
| All | 1989 | >8 | 67.6 | 0.4 | 32.0 |
| MSSA | 1302 | 4 | 88.8 | 0.5 | 10.8 |
| MRSA | 687 | 16 | 27.5 | 0.3 | 72.2 |
| Azithromycin | |||||
| All | 2189 | >512 | 40.3 | 1.1 | 58.6 |
| MSSA | 1424 | >512 | 58.2 | 1.6 | 40.2 |
| MRSA | 765 | >512 | 7.1 | 0 | 92.9 |
| Chloramphenicol | |||||
| All | 1989 | 8 | 94.3 | 5.1 | 0.6 |
| MSSA | 1302 | 8 | 96.3 | 3.5 | 0.2 |
| MRSA | 687 | 8 | 90.4 | 8.3 | 1.3 |
| Clindamycin | |||||
| All | 2189 | >2 | 85.3 | 1.0 | 13.7 |
| MSSA | 1424 | 0.25 | 93.1 | 1.1 | 5.8 |
| MRSA | 765 | >2 | 71.0 | 0.8 | 28.2 |
| Oxacillin/methicillin | |||||
| All | 2189 | >2 | 65.1 | 0 | 34.9 |
| MSSA | 1424 | 0.5 | 100 | 0 | 0 |
| MRSA | 765 | >4 | 0 | 0 | 100 |
| Tetracycline | |||||
| All | 913 | 0.5 | 93.9 | 0.4 | 5.7 |
| MSSA | 666 | 0.5 | 97.1 | 0.3 | 2.6 |
| MRSA | 247 | 16 | 85.0 | 0.8 | 14.2 |
| Tobramycin | |||||
| All | 2189 | 128 | 84.4 | 1.0 | 14.6 |
| MSSA | 1424 | 0.5 | 96.7 | 0.2 | 3.1 |
| MRSA | 765 | 256 | 61.6 | 2.4 | 36.1 |
| Trimethoprim | |||||
| All | 1989 | 4 | 95.7 | 0 | 4.3 |
| MSSA | 1302 | 4 | 96.3 | 0 | 3.7 |
| MRSA | 687 | 2 | 94.5 | 0 | 5.5 |
| Vancomycin | |||||
| All | 2189 | 1 | 100 | 0 | 0 |
| MSSA | 1424 | 1 | 100 | 0 | 0 |
| MRSA | 765 | 1 | 100 | 0 | 0 |
| CoNS b | |||||
| Besifloxacin | |||||
| All | 1765 | 2 | NA | NA | NA |
| MSCoNS | 894 | 0.25 | NA | NA | NA |
| MRCoNS | 871 | 4 | NA | NA | NA |
| Moxifloxacin | |||||
| All | 1765 | 16 | 69.1 | 6.4 | 24.5 |
| MSCoNS | 894 | 1 | 89.1 | 2.8 | 8.1 |
| MRCoNS | 871 | 32 | 48.6 | 10.1 | 41.3 |
| Gatifloxacin | |||||
| All | 1621 | 16 | 67.5 | 4.6 | 27.9 |
| MSCoNS | 826 | 1 | 89.2 | 2.1 | 8.7 |
| MRCoNS | 795 | 32 | 44.9 | 7.3 | 47.8 |
| Ciprofloxacin | |||||
| All | 1765 | 64 | 65.8 | 2.0 | 32.2 |
| MSCoNS | 894 | 4 | 88.0 | 0.5 | 11.5 |
| MRCoNS | 871 | 64 | 43.1 | 3.6 | 53.4 |
| Levofloxacin | |||||
| All | 1621 | 128 | 67.2 | 3.0 | 29.8 |
| MSCoNS | 826 | 2 | 89.2 | 1.1 | 9.7 |
| MRCoNS | 795 | 256 | 44.3 | 5.0 | 50.7 |
| Ofloxacin | |||||
| All | 1621 | >8 | 66.7 | 0.4 | 32.9 |
| MSCoNS | 826 | 8 | 88.7 | 0.4 | 10.9 |
| MRCoNS | 795 | 32 | 43.8 | 0.5 | 55.7 |
| Azithromycin | |||||
| All | 1765 | >512 | 38.6 | 0.1 | 61.3 |
| MSCoNS | 894 | >512 | 55.5 | 0.1 | 44.4 |
| MRCoNS | 871 | >512 | 21.4 | 0 | 78.6 |
| Chloramphenicol | |||||
| All | 1621 | 8 | 98.8 | 0.6 | 0.7 |
| MSCoNS | 826 | 4 | 99.3 | 0.2 | 0.5 |
| MRCoNS | 795 | 8 | 98.2 | 0.9 | 0.9 |
| Clindamycin | |||||
| All | 1765 | 16 | 72.4 | 5.3 | 22.3 |
| MSCoNS | 894 | >2 | 82.2 | 7.4 | 10.4 |
| MRCoNS | 871 | >64 | 62.3 | 3.2 | 34.4 |
| Oxacillin/methicillin | |||||
| All | 1765 | >2 | 50.7 | 0 | 49.3 |
| MSCoNS | 894 | 0.25 | 100 | 0 | 0 |
| MRCoNS | 871 | >2 | 0 | 0 | 100 |
| Tetracycline | |||||
| All | 671 | 16 | 87.6 | 1.3 | 11.0 |
| MSCoNS | 341 | 2 | 90.6 | 2.6 | 6.7 |
| MRCoNS | 330 | >16 | 84.5 | 0 | 15.5 |
| Tobramycin | |||||
| All | 1765 | 16 | 82.5 | 6.9 | 10.7 |
| MSCoNS | 894 | 4 | 93.9 | 3.9 | 2.2 |
| MRCoNS | 871 | 32 | 70.8 | 9.9 | 19.3 |
| Trimethoprim | |||||
| All | 1621 | >128 | 72.1 | 0 | 27.9 |
| MSCoNS | 826 | 128 | 84.6 | 0 | 15.4 |
| MRCoNS | 795 | >256 | 59.1 | 0 | 40.9 |
| Vancomycin | |||||
| All | 1765 | 2 | 100 | 0 | 0 |
| MSCoNS | 894 | 2 | 100 | 0 | 0 |
| MRCoNS | 871 | 2 | 100 | 0 | 0 |
| S pneumoniae | |||||
| Besifloxacin | 590 | 0.06 | NA | NA | NA |
| Moxifloxacin | 590 | 0.25 | 99.8 | 0.2 | 0 |
| Gatifloxacin | 515 | 0.25 | 99.8 | 0.2 | 0 |
| Ciprofloxacin | 590 | 1 | NA | NA | NA |
| Levofloxacin | 515 | 1 | 100 | 0 | 0 |
| Ofloxacin | 515 | 2 | 99.2 | 0.6 | 0.2 |
| Azithromycin | 590 | >128 | 63.7 | 0.3 | 35.9 |
| Chloramphenicol | 590 | 4 | 96.9 | 0 | 3.1 |
| Penicillin | 590 | 1 | 67.8 | 24.4 | 7.8 |
| Tetracycline | 208 | 0.5 | 91.3 | 0 | 8.7 |
| P aeruginosa | |||||
| Besifloxacin | 767 | 4 | NA | NA | NA |
| Moxifloxacin | 767 | 4 | NA | NA | NA |
| Gatifloxacin | 667 | 1 | 94.3 | 2.4 | 3.3 |
| Ciprofloxacin | 767 | 0.5 | 92.8 | 1.4 | 5.7 |
| Levofloxacin | 667 | 1 | 93.9 | 1.0 | 5.1 |
| Ofloxacin | 667 | 2 | 93.1 | 1.9 | 5.0 |
| Polymyxin B | 667 | 2 | 92.4 | 5.7 | 1.9 |
| Tobramycin | 767 | 1 | 97.1 | 0.3 | 2.6 |
| H influenzae | |||||
| Besifloxacin | 780 | 0.03 | NA | NA | NA |
| Moxifloxacin | 780 | 0.03 | 99.9 | 0 | 0.1 |
| Gatifloxacin | 707 | 0.015 | 99.9 | 0 | 0.1 |
| Ciprofloxacin | 780 | 0.015 | 99.9 | 0 | 0.1 |
| Levofloxacin | 707 | 0.03 | 99.9 | 0 | 0.1 |
| Ofloxacin | 707 | 0.03 | 99.9 | 0 | 0.1 |
| Azithromycin | 780 | 2 | 99.6 | 0 | 0.4 |
| Chloramphenicol | 780 | 1 | 99.6 | 0 | 0.4 |
| Tetracycline | 354 | 0.5 | 98.6 | 0 | 1.4 |
Abbreviations: CoNS, coagulase-negative staphylococci; MIC90, minimum inhibitory concentration that inhibits the growth of 90% of indicated isolates; MRCoNS, methicillin-resistant CoNS; MRSA, methicillin-resistant S aureus; MSCoNS, methicillin-susceptible CoNS; MSSA, methicillin-susceptible S aureus; NA, Clinical and Laboratory Standards Institute interpretive break points currently not available or applicable.
Percent susceptible, intermediate, and resistant may not add to 100 because of rounding.
The 1765 CoNS isolates included Staphylococcus arlettae (1), Staphylococcus auricularis (2), Staphylococcus capitis (79), Staphylococcus caprae (14), Staphylococcus cohnii (3), Staphylococcus condimenti (1), Staphylococcus epidermidis (1349), Staphylococcus equorum (1), Staphylococcus hemolyticus (33), Staphylococcus hominis (96), Staphylococcus lugdunensis (31), Staphylococcus pasteuri (11), Staphylococcus pettenkoferi (9), Staphylococcus saprophyticus (3), Staphylococcus schleiferi (2), Staphylococcus simulans (8), Staphylococcus warneri (66) Staphylococcus xylosus (1), and unspeciated CoNS (55).
Antibiotic resistance profiles among CoNS isolates were similar to those observed for S aureus isolates, although oxacillin/methicillin resistance was found to be higher (871 [49.3%] were methicillin-resistant CoNS). As with S aureus isolates, higher rates of resistance to other antibiotics were seen among methicillin-resistant CoNS compared with methicillin-susceptible CoNS isolates, and the later-generation fluoroquinolones demonstrated generally lower MIC90s overall against CoNS isolates.
Among S pneumoniae isolates, resistance rates were less than 10% to all antibiotics tested except for azithromycin (214 [36.3%]) and penicillin (190 [32.2%]). Rates of antibiotic resistance among P aeruginosa and H influenzae were low overall. Against P aeruginosa isolates, ciprofloxacin demonstrated the lowest MIC90s, and moxifloxacin and besifloxacin had the highest; against H influenzae, the lowest MIC90s were observed among the fluoroquinolones.
Concurrent Antibiotic Resistance and MDR
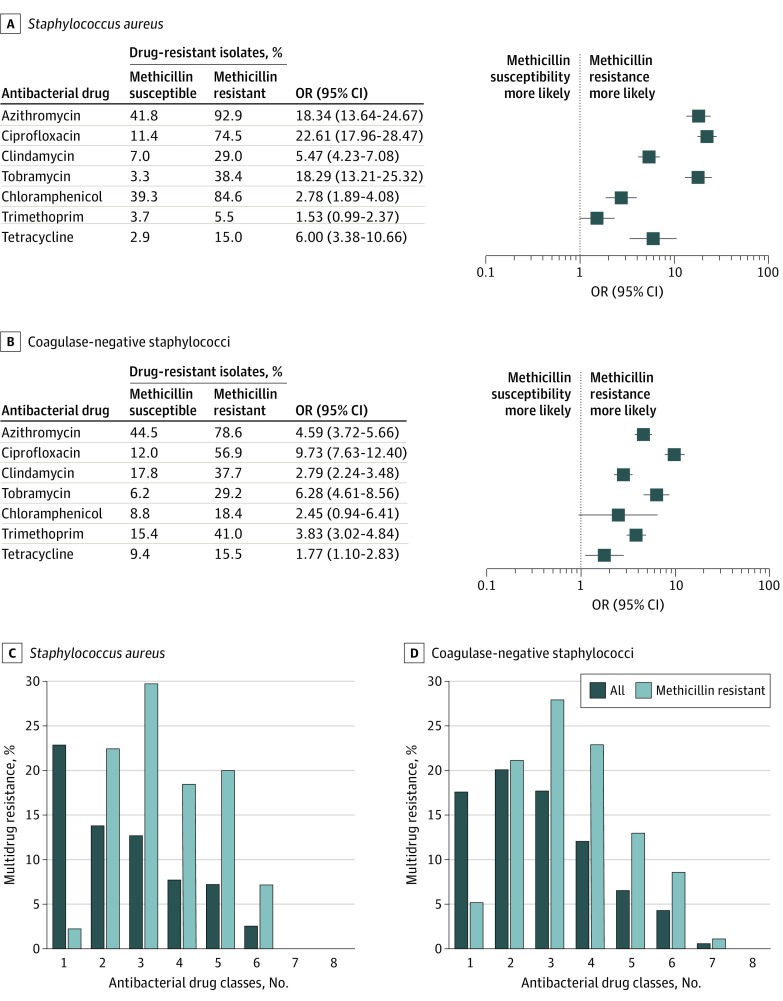
With the exception of trimethoprim, MRSA isolates were significantly more likely than MSSA isolates to be concurrently resistant to another antibacterial drug class: azithromycin (92.9 vs 41.8; OR, 18.34 [95% CI, 13.64-24.67]; P < .001), ciprofloxacin (74.5 vs 11.4; OR, 22.61 [95% CI, 17.96-28.47]; P < .001), clindamycin (29.0 vs 7.0; OR, 5.47 [95% CI, 4.23-7.08]; P < .001), tobramycin (38.4 vs 3.3; OR, 18.29 [95% CI, 13.21-25.32]; P < .001), chloramphenicol (84.6 vs 39.3; OR, 2.78 [95% CI, 1.89-4.08]; P < .001), and tetracycline (15.0 vs 2.9; OR, 6.00 [95% CI, 3.38-10.66]; P < .001) (Figure 1A). Similarly, with the exception of chloramphenicol, methicillin-resistant CoNS isolates were more likely to be concurrently resistant to another antibacterial drug class compared with methicillin-susceptible CoNS isolates: azithromycin (78.6 vs 44.5; OR, 4.59 [95% CI, 3.72-5.66]; P < .001), ciprofloxacin (56.9 vs 12.0; OR, 9.73 [95% CI, 7.63-12.40]; P < .001), clindamycin (37.7 vs 17.8; OR, 2.79 [95% CI, 2.24-3.48]; P < .001), tobramycin (29.2 vs 6.2; OR, 6.28 [95% CI, 4.61-8.56]; P < .001), trimethoprim (41.0 vs 15.4; OR, 3.83 [95% CI, 3.02-4.84]; P < .001), and tetracycline (15.5 vs 9.4; OR, 1.77 [95% CI, 1.10-2.83]; P = .02) (Figure 1B). Figure 1C and D summarize the percentage of MDR among staphylococcal isolates. The rate of MDR among all S aureus isolates was 30.1% and all CoNS isolates was 41.2%, whereas the rate among MRSA isolates was 75.4% and among methicillin-resistant CoNS isolates was 73.7%.
Figure 1. Concurrent Resistance to Other Antibiotics by Methicillin Resistance Phenotype and Multidrug Resistance Among Staphylococcal Isolates.
Isolates were tested against ciprofloxacin, azithromycin, chloramphenicol, clindamycin, oxacillin, tetracycline, tobramycin, trimethoprim, and vancomycin.
Antibiotic Resistance Rates by Patient Age or Geographic Region
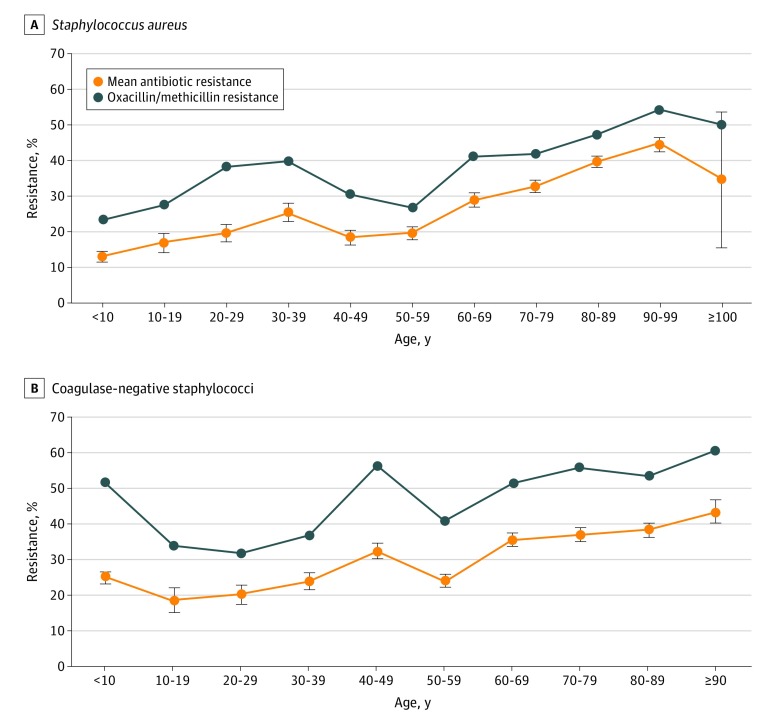
Analysis of variance of the mean percentage of resistance by patient age (categorized by decade of life) showed differences among S aureus (F = 28.07, P < .001) (Figure 2A), CoNS (F = 11.46, P < .001) (Figure 2B), and S pneumoniae isolates (F = 2.08, P = .03) but not among P aeruginosa and H influenzae isolates. Pairwise differences were found for S aureus isolates from younger patients compared with older patients, reflecting an increase in antibiotic resistance with patient age; similar results were obtained for CoNS isolates. For both S aureus and CoNS isolates, oxacillin/methicillin resistance also differed by patient age and paralleled that of mean percentage of resistance, again with higher methicillin resistance among isolates from older patients and significant pairwise differences across the decades (Figure 2). No significant pairwise differences were found for S pneumoniae isolates.
Figure 2. Mean Percentage of Antibiotic Resistance and Methicillin Resistance Among Staphylococcal Isolates by Patient Age.
P values were calculated using analysis of variance for mean percentage of resistance and the χ2 test for oxacillin/methicillin resistance (all P < .001). Pairwise differences are given in eTable 1 and eTable 2 in the Supplement. Error bars indicate standard error of the mean.
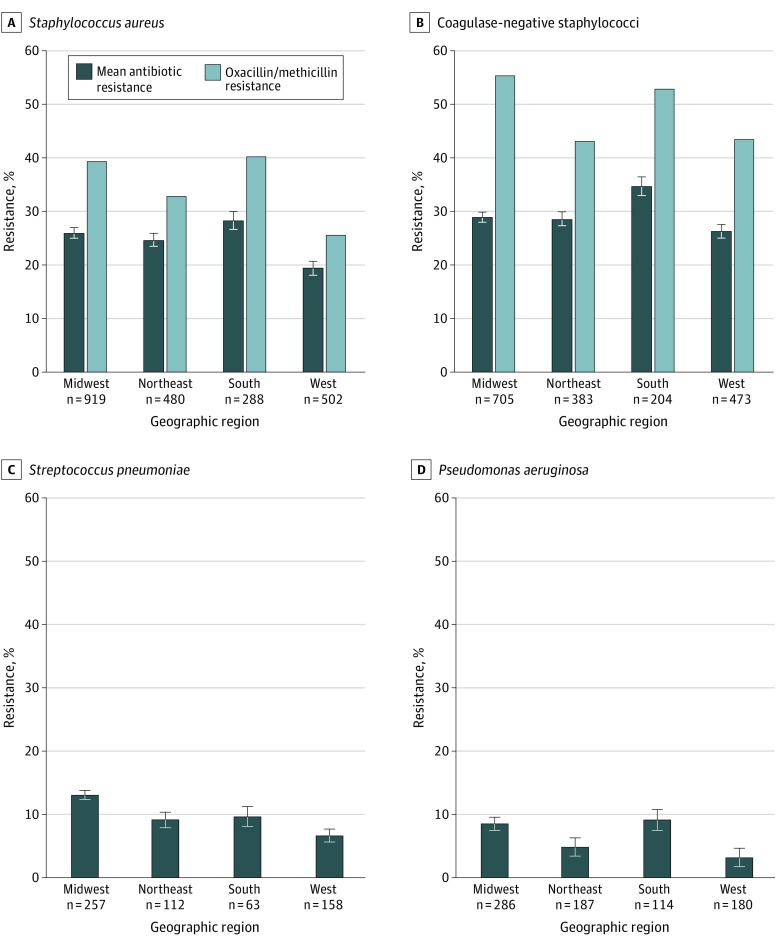
Analysis of variance showed small differences in the percentage of resistance by geographic region for S aureus (F = 8.03, P < .001), CoNS (F = 4.79, P = .003), S pneumoniae (F = 8.14, P < .001), and P aeruginosa isolates (F = 4.32, P = .005) (Figure 3). Compared with other regions, mean percentage of antibiotic resistance was significantly lower in the West for S aureus isolates and highest in the South for CoNS isolates. The mean percentage of resistance among S pneumoniae isolates was higher in the Midwest than in the Northeast and West, whereas for P aeruginosa isolates, antibiotic resistance in the Midwest and South was higher compared with the West. Oxacillin/methicillin resistance in S aureus isolates paralleled that for the mean percentage of resistance, with significant pairwise differences between the West and both the South and the Midwest. Among CoNS isolates, pairwise differences were observed between the Midwest and both the West and the Northeast.
Figure 3. Mean Antibiotic Resistance and Methicillin Resistance Among Isolates by Geographic Region.
P values were calculated using analysis of variance (ANOVA) for mean resistance and the χ2 test for oxacillin/methicillin resistance. A, P < .001 by ANOVA and χ2 test. B, P = .003 by ANOVA and P < .001 by χ2 test. C, P < .001 by ANOVA. D, P = .005 by ANOVA. There was insufficient evidence to conclude that regions had different mean resistance percentages for the Midwest, Northeast, and South in A; the Midwest, Northeast, and West in B; the Midwest and South and the Northeast, South, and West in C; and the Midwest, Northeast and South and the Northeast and West in D. Error bars indicate standard error of the mean.
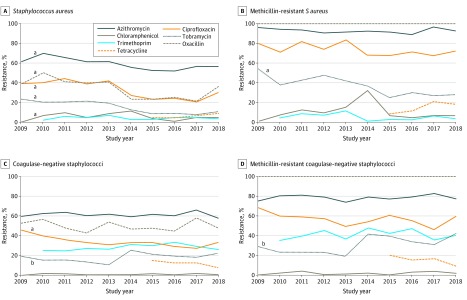
Antibiotic Resistance Rates Over Time
Figure 4 shows antibiotic resistance rates by year during the study period. Oxacillin/methicillin resistance decreased slightly among S aureus isolates but did not change among CoNS isolates. Small decreases over time were also observed in resistance to azithromycin, ciprofloxacin, chloramphenicol, and tobramycin among S aureus isolates and in ciprofloxacin among CoNS isolates. The mean changes per year in percent of antibiotic resistance were −2.16% (95% CI, −3.91% to −0.41%; P < .001) for oxacillin/methicillin, −1.44% (95% CI, −2.35% to −0.52%; P < .001) for azithromycin, −2.24% (95% CI, −3.59% to −0.89%; P < .001) for ciprofloxacin, −0.54% (95% CI, −1.28% to 0.21%; P = .005) for chloramphenicol, −1.84% (95% CI, −2.53% to −1.14%; P < .001) for tobramycin among S aureus isolates, and −1.38% (95% CI, −2.24% to −0.52%; P < .001) for ciprofloxacin among CoNS isolates. A small increase over time was noted in CoNS resistance to tobramycin, with a mean change in percent resistance per year of 0.71% (95% CI, −0.29% to 1.71%; P = .03). Among MRSA isolates, there was a decrease in resistance to tobramycin (mean change, −2.53%; 95% CI, −3.91% to −1.14%; P < .001), whereas methicillin-resistant CoNS isolates showed an increase in resistance to tobramycin (mean change, 1.85; 95% CI, 0.19 to 3.50; P < .001). No changes in antibiotic resistance were observed among P aeruginosa and S pneumoniae isolates (eFigure 1 in the Supplement).
Figure 4. Antibiotic Resistance Among Isolates Over Time.
P values were calculated using a Cochran-Armitage test to identify significant linear trends in antibiotic resistance.
aDecreasing trend in antibiotic resistance.
bIncreasing trend in antibiotic resistance.
Discussion
The first analysis of ARMOR study data, based on ocular isolates collected in its inaugural year, showed high rates of methicillin resistance and MDR among S aureus and CoNS isolates.19 Analyses in subsequent years suggested that rates of MRSA could be declining; however, MDR, especially among methicillin-resistant staphylococci, remained high.21,22,23
The present report expands on previous ARMOR study reports and describes antibiotic resistance profiles for the current cumulative data set of more than 6000 clinical isolates collected during 10 years. Overall, 1 in 3 S aureus isolates and 1 in 2 CoNS isolates were resistant to methicillin and approximately 3 in 4 methicillin-resistant staphylococcal isolates were MDR. Concurrent antibiotic resistance was higher among methicillin-resistant compared with methicillin-susceptible staphylococcal isolates, with MRSA isolates being 3 to 23 times more likely to be resistant to other commonly used antibiotics except trimethoprim and with methicillin-resistant CoNS isolates being 2 to 10 times more likely to be resistant to other antibiotics. However, susceptibility to vancomycin remained high for all species. In contrast with staphylococcal isolates, low resistance was observed among S pneumoniae, P aeruginosa, and H influenzae isolates, although 1 in 3 S pneumoniae isolates were resistant to azithromycin and penicillin. Overall, these findings align with previous ARMOR reports19,21,22,23 and retrospective reviews of ocular isolates from single US clinical centers.1,43,44,45,46
Differences in MIC90s were found within the fluoroquinolone class of antibiotics. Among S aureus and CoNS isolates overall and by methicillin resistance phenotype, MIC90s were generally lower for the later-generation fluoroquinolones compared with the older ones, with besifloxacin demonstrating the lowest MIC90s. Besifloxacin also had the lowest MIC90 against S pneumoniae, whereas ciprofloxacin had the lowest MIC90 against P aeruginosa. Older fluoroquinolones may have acquired increased in vitro resistance because of longer systemic use47; resistance against older fluoroquinolones may result from a single mutation in DNA gyrase or DNA topoisomerase IV, whereas resistance against later-generation fluoroquinolones requires mutations in both enzymes.1,48,49 The MIC90s reported here for besifloxacin are similar to those obtained during clinical trials of besifloxacin treatment for bacterial conjunctivitis conducted from 2004 through 200719 and suggest negligible resistance development to this fluoroquinolone to date. These findings are notable given reporting in ocular infections—albeit limited, to date, to bacterial keratitis—of a correlation between low fluoroquinolone MICs and improved treatment outcomes,13,50,51,52 together with the established understanding in systemic infections that antibiotics with lower MICs are associated with higher rates of treatment response than those with higher MICs.53
Higher rates of mean percentage of resistance and methicillin resistance were seen among staphylococcal isolates obtained from older patients, consistent with previous reporting.19,21,24,45,47,54,55 These findings are likely related to older patients spending more time in hospitals and/or nursing homes, which is a known risk factor for exposure to antibiotic-resistant bacteria.56,57,58 Thus, the findings have implications for older patients presenting with ocular infections and/or undergoing ocular surgery. For example, dropless cataract surgery may need reconsideration in this age group because it is unclear whether intraocular moxifloxacin levels are maintained above the MIC sufficiently long enough to fully eradicate any antibiotic-resistant staphylococci that may breach the surgical wound59,60; in this regard, there have been reports of endophthalmitis after dropless cataract surgery with moxifloxacin.61,62,63,64 Of importance, of methicillin-resistant staphylococci sourced from vitreous or aqueous humor in this study, 91% of MRSA isolates and 71% of methicillin-resistant CoNS isolates had concurrent resistance to moxifloxacin (eAppendix, eFigure 2, and eFigure 3 in the Supplement). An increasing prevalence of resistance to antibiotics, especially fluoroquinolones, with patient age has also been reported by other researchers.24,65,66 In contrast to older patients, antibiotic resistance findings for pediatric patients were lower. However, even in this age group, the mean percentage of antibiotic resistance, including methicillin resistance among staphylococcal isolates, was notable (Figure 2) and should be considered in the context of an increasing recognition of the polybacterial nature of conjunctivitis and keratitis infections.67,68,69
Small differences were found in antibiotic resistance by geographic region for each species, with higher mean percentages of resistance (and methicillin resistance among staphylococcal isolates) in the South and/or Midwest and lower rates in the West. The findings for S aureus isolates are consistent with previous ARMOR reporting20,21 and results from Blanco and colleagues,70 who observed higher rates of MRSA infection in intensive care units in the southern region of the United States. Small changes, mostly decreases, were found in antibiotic resistance over time and likely reflect improved antibiotic stewardship. The small decrease in methicillin resistance suggested in earlier ARMOR reports22,23 was supported for the 10-year study period, although no such change was seen among CoNS isolates. Not observed before was the increase in CoNS resistance to tobramycin, whereas a previous increase to trimethoprim among CoNS isolates and previous decreases to ciprofloxacin and tobramycin among P aeruginosa isolates were not confirmed. Although an approximately 2-fold increase in resistance to azithromycin over time was observed among S pneumoniae isolates in the 7-year analysis,22 no changes in resistance over time to azithromycin, penicillin, moxifloxacin, chloramphenicol, or tetracycline among S pneumoniae isolates were evident over the extended 10-year time frame.
In addition, antibiotic resistance trends among ocular isolates were similar when evaluated by anatomical source (eg, conjunctiva, cornea, and intraocular) (eAppendix and eFigures 4-7 in the Supplement). In parallel with this finding, antibiotic resistance trends in the 10-year ARMOR data set were also similar to those reported for nonocular isolates from systemic infections in both US and global surveillance studies, such as Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE),58,71,72 Linezolid Experience and Accurate Determination of Resistance (LEADER),73,74 Tigecycline Evaluation and Surveillance Trial (TEST),75,76 and the SENTRY Antimicrobial Surveillance Program.77,78,79 For instance, recent reporting for more than 190 000 S aureus isolates collected worldwide over 20 years in SENTRY demonstrated a decrease in the incidence of MRSA infection from 44.2% (2005-2008) to 39.0% (2013-2016), with the highest proportion of MRSA infection observed among patients older than 80 years; 80% of MRSA isolates were concurrently resistant to ciprofloxacin.77 Likewise, penicillin resistance among more than 65 000 S pneumoniae isolates fluctuated by region over the 20-year study period, with global resistance rates of 34.2% in the last years (2015-2016)78; however, ciprofloxacin resistance among P aeruginosa isolates was higher in SENTRY79 than in our study. These findings suggest that ARMOR study results, exclusive to ocular isolates and independent of ocular source, may be reflective of global antibiotic resistance patterns in systemic infections.
Limitations
Limitations of our study include sampling biases owing to the relatively infrequent culturing of ocular pathogens during routine practice and the site’s selection of isolates for submission; therefore, the resistance rates may be overestimated and the generalizability of these data are unknown. Other limitations include the inconsistency of obtaining patient age, patient sex, and ocular tissue source for each isolate; the choice of antibiotics tested; and the lack of ophthalmic break points to interpret MIC data. Because empirical therapy is common when treating ocular infections, the bacteria cultured may have originated from more severe infections or represent treatment failures. Relevant ophthalmic antibiotics were included; however, some, such as bacitracin, could not be tested because of formulation characteristics or other practical considerations. In addition, the validity of applying systemic break points for interpreting the antibiotic resistance of ocular isolates has not been established and may also result in overreporting of resistance given higher concentrations of antibiotics achievable on the eye after topical instillation49; moreover, in the case of besifloxacin, no systemic break points were available for interpretation of the MIC data.
Conclusions
The findings suggest that methicillin resistance and MDR is prevalent among ocular S aureus and CoNS isolates in the United States and should be considered when treating staphylococcal ocular infections, especially in older patients. The small decreases in antibiotic resistance among S aureus isolates are encouraging but require further monitoring.
eAppendix. Web-based Interactive Data Visualization Tool
eTable 1. Resistance among Ocular Isolates of S aureus by Patient Age
eTable 2. Resistance among Ocular Isolates of Coagulase-Negative Staphylococci by Patient Age
eFigure 1. Resistance among Ocular Isolates of S pneumoniae (A) and P aeruginosa (B) by Antibiotic Over Time
eFigure 2. Resistance to Moxifloxacin Among Methicillin-Resistant S aureus
eFigure 3. Resistance to Moxifloxacin Among Methicillin-Resistant Coagulase-Negative Staphylococci
eFigure 4. Resistance among S aureus by Source
eFigure 5. Resistance among Coagulase-Negative Staphylococci by Source
eFigure 6. Resistance among S pneumoniae by Source
eFigure 7. Resistance among P aeruginosa by Source
References
- 1.Chang VS, Dhaliwal DK, Raju L, Kowalski RP. Antibiotic resistance in the treatment of Staphylococcus aureus keratitis: a 20-year review. Cornea. 2015;34(6):698-703. doi: 10.1097/ICO.0000000000000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grzybowski A, Brona P, Kim SJ. Microbial flora and resistance in ophthalmology: a review. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):851-862. doi: 10.1007/s00417-017-3608-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller D. Update on the epidemiology and antibiotic resistance of ocular infections. Middle East Afr J Ophthalmol. 2017;24(1):30-42. doi: 10.4103/meajo.MEAJO_276_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wurster JI, Bispo PJM, Van Tyne D, Cadorette JJ, Boody R, Gilmore MS. Staphylococcus aureus from ocular and otolaryngology infections are frequently resistant to clinically important antibiotics and are associated with lineages of community and hospital origins. PLoS One. 2018;13(12):e0208518. doi: 10.1371/journal.pone.0208518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adebayo A, Parikh JG, McCormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial conjunctival isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol. 2011;249(1):111-119. doi: 10.1007/s00417-010-1426-6 [DOI] [PubMed] [Google Scholar]
- 6.Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145(6):951-958. doi: 10.1016/j.ajo.2008.01.025 [DOI] [PubMed] [Google Scholar]
- 7.Blanco C, Núñez MX. Antibiotic susceptibility of staphylococci isolates from patients with chronic conjunctivitis: including associated factors and clinical evaluation. J Ocul Pharmacol Ther. 2013;29(9):803-808. doi: 10.1089/jop.2013.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amato M, Pershing S, Walvick M, Tanaka S. Trends in ophthalmic manifestations of methicillin-resistant Staphylococcus aureus (MRSA) in a northern California pediatric population. J AAPOS. 2013;17(3):243-247. doi: 10.1016/j.jaapos.2012.12.151 [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee S, Agrawal D. Multi-drug resistant Pseudomonas aeruginosa keratitis and its effective treatment with topical colistimethate. Indian J Ophthalmol. 2016;64(2):153-157. doi: 10.4103/0301-4738.179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology. 1999;106(7):1319-1323. doi: 10.1016/S0161-6420(99)00717-4 [DOI] [PubMed] [Google Scholar]
- 11.Moshirfar M, Mirzaian G, Feiz V, Kang PC. Fourth-generation fluoroquinolone-resistant bacterial keratitis after refractive surgery. J Cataract Refract Surg. 2006;32(3):515-518. doi: 10.1016/j.jcrs.2005.12.108 [DOI] [PubMed] [Google Scholar]
- 12.Segreti J, Jones RN, Bertino JS Jr. Challenges in assessing microbial susceptibility and predicting clinical response to newer-generation fluoroquinolones. J Ocul Pharmacol Ther. 2012;28(1):3-11. doi: 10.1089/jop.2011.0072 [DOI] [PubMed] [Google Scholar]
- 13.Wilhelmus KR, Abshire RL, Schlech BA. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. 2003;121(9):1229-1233. doi: 10.1001/archopht.121.9.1229 [DOI] [PubMed] [Google Scholar]
- 14.Goff DA, Jankowski C, Tenover FC. Using rapid diagnostic tests to optimize antimicrobial selection in antimicrobial stewardship programs. Pharmacotherapy. 2012;32(8):677-687. doi: 10.1002/j.1875-9114.2012.01137.x [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Ophthalmology . Bacterial keratitis preferred practice pattern–2018. https://www.aao.org/preferred-practice-pattern/bacterial-keratitis-ppp-2018. Published November 2018. Accessed March 5, 2019.
- 16.American Academy of Ophthalmology . Conjunctivitis preferred practice pattern–2018. https://www.aao.org/preferred-practice-pattern/conjunctivitis-ppp-2018. Published November 2018. Accessed March 5, 2019.
- 17.Asbell PA, Sahm DF. Longitudinal nationwide antimicrobial susceptibility surveillance in ocular isolates: results from Ocular TRUST 2. Presented at: American Society of Cataract and Refractive Surgery Annual Meeting; April 28, 2008; San Diego, CA. [Google Scholar]
- 18.Asbell PA, Sahm DF, Shedden A. Ocular TRUST 3: ongoing longitudinal surveillance of antimicrobial susceptibility in ocular isolates. Presented at: American Society of Cataract and Refractive Surgery Annual Meeting; April 7, 2009; San Francisco, CA. [Google Scholar]
- 19.Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 Surveillance Study. Am J Ophthalmol. 2011;152(4):567.e3-574.e3. doi: 10.1016/j.ajo.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 20.Asbell PA, Pandit RT, Sanfilippo CM. Antibiotic resistance rates by geographic region among ocular pathogens collected during the ARMOR Surveillance Study. Ophthalmol Ther. 2018;7(2):417-429. doi: 10.1007/s40123-018-0141-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States: five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015;133(12):1445-1454. doi: 10.1001/jamaophthalmol.2015.3888 [DOI] [PubMed] [Google Scholar]
- 22.Asbell PA, Sanfilippo CM. Antibiotic resistance trends among ocular pathogens in the US—cumulative results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. US Ophthalmic Rev. 2017;10(1):35-38. doi: 10.17925/USOR.2017.10.01.35 [DOI] [Google Scholar]
- 23.Thomas RK, Melton R, Asbell PA. Antibiotic resistance among ocular pathogens: current trends from the ARMOR Surveillance Study (2009-2016). Clin Optom (Auckl). 2019;11:15-26. doi: 10.2147/OPTO.S189115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter SJ, Sanfilippo CM, Asbell PA, DeCory HH. Antibiotic resistance among pediatric-sourced ocular pathogens: 8-year findings from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. Pediatr Infect Dis J. 2019;38(2):138-145. doi: 10.1097/INF.0000000000002206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asbell PA, Mah FS, Sanfilippo CM, DeCory HH. Antibiotic susceptibility of bacterial pathogens isolated from the aqueous and vitreous humor in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. J Cataract Refract Surg. 2016;42(12):1841-1843. doi: 10.1016/j.jcrs.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 26.Asbell PA, DeCory HH. Antibiotic resistance among bacterial conjunctival pathogens collected in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. PLoS One. 2018;13(10):e0205814. doi: 10.1371/journal.pone.0205814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. CLSI document M07–A8. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 9th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. CLSI document M07–A9. [Google Scholar]
- 29.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 10th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. CLSI document M07–A10. [Google Scholar]
- 30.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 11th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. CLSI document M07–A11. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 19th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. CLSI document M100–S19. [Google Scholar]
- 32.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. CLSI document M100–S20. [Google Scholar]
- 33.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 21st Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. CLSI document M100–S21. [Google Scholar]
- 34.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 22nd Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. CLSI document M10–S22. [Google Scholar]
- 35.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 23rd Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. CLSI document M100–S23. [Google Scholar]
- 36.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 24th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2014. CLSI document M100–S24. [Google Scholar]
- 37.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 25th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. CLSI document M100–S25. [Google Scholar]
- 38.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: 26th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. CLSI document M100–S26. [Google Scholar]
- 39.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. CLSI document M100–S27. [Google Scholar]
- 40.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; 27th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. CLSI document M100–S28. [Google Scholar]
- 41.Tukey JW. Comparing individual means in the analysis of variance. Biometrics. 1949;5(2):99-114. doi: 10.2307/3001913 [DOI] [PubMed] [Google Scholar]
- 42.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11(3):375-386. doi: 10.2307/3001775 [DOI] [Google Scholar]
- 43.Truong DT, Bui MT, Memon P, Cavanagh HD. Microbial keratitis at an urban public hospital: a 10-year update. J Clin Exp Ophthalmol. 2015;6(6):498. doi: 10.4172/2155-9570.1000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin H, Parker WT, Law NW, et al. Evolving risk factors and antibiotic sensitivity patterns for microbial keratitis at a large county hospital. Br J Ophthalmol. 2017;101(11):1483-1487. doi: 10.1136/bjophthalmol-2016-310026 [DOI] [PubMed] [Google Scholar]
- 45.Oydanich M, Dingle TC, Hamula CL, Ghisa C, Asbell P. Retrospective report of antimicrobial susceptibility observed in bacterial pathogens isolated from ocular samples at Mount Sinai Hospital, 2010 to 2015. Antimicrob Resist Infect Control. 2017;6:29. doi: 10.1186/s13756-017-0185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37(1):84-87. doi: 10.1097/ICO.0000000000001417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fintelmann RE, Hoskins EN, Lietman TM, et al. Topical fluoroquinolone use as a risk factor for in vitro fluoroquinolone resistance in ocular cultures. Arch Ophthalmol. 2011;129(4):399-402. doi: 10.1001/archophthalmol.2011.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hooper DC. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin Infect Dis. 2000;31(suppl 2):S24-S28. doi: 10.1086/314056 [DOI] [PubMed] [Google Scholar]
- 49.Kowalski RP. Is antibiotic resistance a problem in the treatment of ophthalmic infections? Expert Rev Ophthalmol. 2013;8(2):119-126. doi: 10.1586/eop.13.7 [DOI] [Google Scholar]
- 50.Chen A, Prajna L, Srinivasan M, et al. Does in vitro susceptibility predict clinical outcome in bacterial keratitis? Am J Ophthalmol. 2008;145(3):409-412. doi: 10.1016/j.ajo.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaye S, Tuft S, Neal T, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51(1):362-368. doi: 10.1167/iovs.09-3933 [DOI] [PubMed] [Google Scholar]
- 52.Lalitha P, Srinivasan M, Manikandan P, et al. Relationship of in vitro susceptibility to moxifloxacin and in vivo clinical outcome in bacterial keratitis. Clin Infect Dis. 2012;54(10):1381-1387. doi: 10.1093/cid/cis189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doern GV, Brecher SM. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J Clin Microbiol. 2011;49(9)(suppl):S11-S14. doi: 10.1128/JCM.00580-11 [DOI] [Google Scholar]
- 54.Chiquet C, Maurin M, Altayrac J, et al. Correlation between clinical data and antibiotic resistance in coagulase-negative Staphylococcus species isolated from 68 patients with acute post-cataract endophthalmitis. Clin Microbiol Infect. 2015;21(6):592.e1-592.e8. doi: 10.1016/j.cmi.2015.01.028 [DOI] [PubMed] [Google Scholar]
- 55.Olson R, Donnenfeld E, Bucci FA Jr, et al. Methicillin resistance of Staphylococcus species among health care and nonhealth care workers undergoing cataract surgery. Clin Ophthalmol. 2010;4:1505-1514. doi: 10.2147/OPTH.S14333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynolds C, Quan V, Kim D, et al. Methicillin-resistant Staphylococcus aureus (MRSA) carriage in 10 nursing homes in Orange County, California. Infect Control Hosp Epidemiol. 2011;32(1):91-93. doi: 10.1086/657637 [DOI] [PubMed] [Google Scholar]
- 57.Mody L, Kauffman CA, Donabedian S, Zervos M, Bradley SF. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin Infect Dis. 2008;46(9):1368-1373. doi: 10.1086/586751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sader HS, Mendes RE, Jones RN, Flamm RK. Antimicrobial susceptibility patterns of community- and hospital-acquired methicillin-resistant Staphylococcus aureus from United States hospitals: results from the AWARE Ceftaroline Surveillance Program (2012-2014). Diagn Microbiol Infect Dis. 2016;86(1):76-79. doi: 10.1016/j.diagmicrobio.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 59.Lindstrom RL, Galloway MS, Grzybowski A, Liegner JT. Dropless cataract surgery: an overview. Curr Pharm Des. 2017;23(4):558-564. doi: 10.2174/1381612822666161129150628 [DOI] [PubMed] [Google Scholar]
- 60.Rhee MK, Mah FS. Cataract drug delivery systems (dropless vs. nondropless cataract surgery). Int Ophthalmol Clin. 2016;56(3):117-136. doi: 10.1097/IIO.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 61.Chang VS, Schwartz SG, Davis JL, Flynn HW Jr. Endophthalmitis following cataract surgery and intracameral antibiotic: moxifloxacin resistant Staphylococcus epidermidis. Am J Ophthalmol Case Rep. 2018;13:127-130. doi: 10.1016/j.ajoc.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kishore K, Brown JA, Satar JM, Hahn JM, Bond WI. Acute-onset postoperative endophthalmitis after cataract surgery and transzonular intravitreal triamcinolone-moxifloxacin. J Cataract Refract Surg. 2018;44(12):1436-1440. doi: 10.1016/j.jcrs.2018.07.055 [DOI] [PubMed] [Google Scholar]
- 63.Tye A, Witkin AJ. The safety, efficacy, and potential complications of intracameral antibiotics. Curr Ophthalmol Rep. 2018;6(2):152-156. doi: 10.1007/s40135-018-0174-3 [DOI] [Google Scholar]
- 64.Ng C, Chalam KV. Endophthalmitis after dropless (Tri-Moxi injection) cataract surgery. [published online June 3, 2019]. Eur J Ophthalmol. 2019. doi: 10.1177/1120672119852505 [DOI] [PubMed] [Google Scholar]
- 65.Delorme T, Rose S, Senita J, Callahan C, Nasr P. Epidemiology and susceptibilities of methicillin-resistant Staphylococcus aureus in northeastern Ohio. Am J Clin Pathol. 2009;132(5):668-677. doi: 10.1309/AJCPQ46ZPQXVHHNC [DOI] [PubMed] [Google Scholar]
- 66.Garcia A, Delorme T, Nasr P. Patient age as a factor of antibiotic resistance in methicillin-resistant Staphylococcus aureus. J Med Microbiol. 2017;66(12):1782-1789. doi: 10.1099/jmm.0.000635 [DOI] [PubMed] [Google Scholar]
- 67.Blondeau JM, Sanfilippo CM, DeCory HH. Incidence of polybacterial infections in three bacterial conjunctivitis studies and outcomes with besifloxacin ophthalmic suspension 0.6%. Invest Ophthalmol Vis Sci. 2019;60(9):245. [Google Scholar]
- 68.Aoki R, Fukuda K, Ogawa M, et al. Identification of causative pathogens in eyes with bacterial conjunctivitis by bacterial cell count and microbiota analysis. Ophthalmology. 2013;120(4):668-676. doi: 10.1016/j.ophtha.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 69.Tuft S. Polymicrobial infection and the eye. Br J Ophthalmol. 2006;90(3):257-258. doi: 10.1136/bjo.2005.084095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blanco N, Perencevich E, Li SS, et al. ; CDC Prevention Epicenter Program . Effect of meteorological factors and geographic location on methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci colonization in the US. PLoS One. 2017;12(5):e0178254. doi: 10.1371/journal.pone.0178254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfaller MA, Mendes RE, Duncan LR, Flamm RK, Sader HS. In vitro activities of ceftaroline and comparators against Streptococcus pneumoniae isolates from US hospitals: results from seven years of the AWARE Surveillance Program (2010 to 2016). Antimicrob Agents Chemother. 2018;62(2):e01555-e17. doi: 10.1128/AAC.01555-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sader HS, Mendes RE, Streit JM, Flamm RK. Antimicrobial susceptibility trends among Staphylococcus aureus isolates from US hospitals: results from 7 years of the Ceftaroline (AWARE) Surveillance Program, 2010 to 2016. Antimicrob Agents Chemother. 2017;61(9):e01043-e17. doi: 10.1128/AAC.01043-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfaller MA, Mendes RE, Streit JM, Hogan PA, Flamm RK. Five-year summary of in vitro activity and resistance mechanisms of linezolid against clinically important gram-positive cocci in the United States from the LEADER Surveillance Program (2011 to 2015). Antimicrob Agents Chemother. 2017;61(7):e00609-e00617. doi: 10.1128/AAC.00609-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flamm RK, Mendes RE, Hogan PA, Streit JM, Ross JE, Jones RN. Linezolid surveillance results for the United States (LEADER Surveillance Program 2014). Antimicrob Agents Chemother. 2016;60(4):2273-2280. doi: 10.1128/AAC.02803-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z, Chen M, Yu Y, Pan S, Liu Y. Antimicrobial susceptibility among gram-positive and gram-negative blood-borne pathogens collected between 2012-2016 as part of the Tigecycline Evaluation and Surveillance Trial. Antimicrob Resist Infect Control. 2018;7:152. doi: 10.1186/s13756-018-0441-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giammanco A, Calà C, Fasciana T, Dowzicky MJ. Global assessment of the activity of tigecycline against multidrug-resistant gram-negative pathogens between 2004 and 2014 as part of the Tigecycline Evaluation and Surveillance Trial. mSphere. 2017;2(1):e00310-e00316. doi: 10.1128/mSphere.00310-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY Antimicrobial Surveillance Program. Open Forum Infect Dis. 2019;6(suppl 1):S47-S53. doi: 10.1093/ofid/ofy270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sader HS, Mendes RE, Le J, Denys G, Flamm RK, Jones RN. Antimicrobial susceptibility of Streptococcus pneumoniae from North America, Europe, Latin America, and the Asia-Pacific Region: results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997-2016). Open Forum Infect Dis. 2019;6(suppl 1):S14-S23. doi: 10.1093/ofid/ofy263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shortridge D, Gales AC, Streit JM, Huband MD, Tsakris A, Jones RN. Geographic and temporal patterns of antimicrobial resistance in Pseudomonas aeruginosa over 20 years from the SENTRY Antimicrobial Surveillance Program, 1997-2016. Open Forum Infect Dis. 2019;6(suppl 1):S63-S68. doi: 10.1093/ofid/ofy343 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Web-based Interactive Data Visualization Tool
eTable 1. Resistance among Ocular Isolates of S aureus by Patient Age
eTable 2. Resistance among Ocular Isolates of Coagulase-Negative Staphylococci by Patient Age
eFigure 1. Resistance among Ocular Isolates of S pneumoniae (A) and P aeruginosa (B) by Antibiotic Over Time
eFigure 2. Resistance to Moxifloxacin Among Methicillin-Resistant S aureus
eFigure 3. Resistance to Moxifloxacin Among Methicillin-Resistant Coagulase-Negative Staphylococci
eFigure 4. Resistance among S aureus by Source
eFigure 5. Resistance among Coagulase-Negative Staphylococci by Source
eFigure 6. Resistance among S pneumoniae by Source
eFigure 7. Resistance among P aeruginosa by Source