Abstract
Background:
The male genital tract (MGT) is a viral sanctuary and likely HIV reservoir; understanding MGT pharmacokinetics (PK) of antiretrovirals (ARVs) used for curative strategies is critical to eradication and cure. Tenofovir alafenamide (TAF) is a tenofovir (TFV) formulation designed to maximize efficacy/minimize toxicity with unknown MGT PK.
Methods:
HIV-positive and HIV-negative men receiving TFV-based regimens provided 6 paired blood plasma (BP) and semen samples. Extracellular (TFV, TAF, emtricitabine [FTC]) drug concentrations in BP and seminal plasma (SP), and intracellular metabolite (IM) and endogenous nucleotide (EN) concentrations were measured in peripheral blood mononuclear cells (PBMCs) and seminal mononuclear cells (SMCs). Exposure ratios for SP:BP, SMC:PBMC, and IM:EN were calculated from PK parameters generated by noncompartmental analysis. HIV viral load was measured in BP and SP.
Results:
Sixteen HIV-positive (n=8, TDF/FTC; n=8, TAF/FTC) and eight HIV-negative (TDF/FTC) men provided samples. Median TFV SP:BP ratios differed between TDF and TAF (1.5 vs 7.4), due to lower TFV BP concentrations with TAF coupled with TFV SP concentrations similar to TDF. FTC SP:BP ratios were approximately 3. SMC concentrations of IMs and ENs were a fraction of PBMC concentrations (1–22%), though IM:EN ratios exceed a suggested protective threshold.
Conclusions:
TAF SP PK was unexpected. IM SMC concentrations were low relative to PBMC, as were EN concentrations, suggesting differences in cell phenotype and lineage in the MGT; these differences in phenotype and pharmacology may have an impact on selecting and dosing ARVs used in cure strategies.
INTRODUCTION
The blood-testes barrier acts to limit drug penetration into the male genital tract (MGT). P-glycoprotein in this barrier returns drug to the blood, limiting entry of substrates such as HIV protease inhibitors.(1) For nucleoside reverse transcriptase inhibitors (NRTIs), equilibrative nucleoside transporters facilitate entry into the MGT(2), generally resulting in high seminal plasma (SP) concentrations relative to blood.(1)
Several lines of investigation suggest viral compartmentalization in the MGT.(3–6) Its immune system mainly resides in the testes(7) and seminal vesicles(8), comprised mainly of monocyte-and macrophage-derived cells. These macrophages may constitute a viral reservoir and contribute to HIV persistence (8–10). For “kick and kill” cure strategies to be successful, drug penetration into the MGT and cell entry is necessary.(11)
Tenofovir/emtricitabine (TFV/FTC) are widely used NRTIs; both are intracellularly to active phosphorylated forms and compete with endogenous nucleotides for virologic activity.(12) The ratio of the metabolite to its corresponding endogenous nucleotide is a critical component of protecting vulnerable cells from HIV infection.(13, 14) Tenofovir is approved in two forms, tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF). Tenofovir diphosphate (TFVdp) is the active metabolite of both, though pharmacokinetics of TFV and TFVdp differ between the two.(15)
Here, our goal was to characterize TFVdp in seminal mononuclear cells (SMCs) following TAF administration and TDF administration. We hypothesized that SMC concentrations of TFVdp with TAF would reflect the increased PBMC concentrations observed with TAF administration, compared to TDF, and expected TFV exposure in BP and SP would be lower with TAF compared to TDF.
METHODS
Clinical Study Design
A full description of the study protocol is available in the Supplemental Material. Briefly, men receiving either TDF/FTC (HIV-negative [Arm 1] and HIV-positive [Arm 2]) or TAF/FTC (HIV-positive only, Arm 3) participated in a study protocol approved by the UNC Biomedical Institution Review Board. All provided written informed consent prior to study procedures. Eight men per arm were enrolled. Paired blood and semen samples were obtained at 6 times post-dose; semen samples were self-collected. Detailed sample processing and analytical chemistry information are provided in Supplemental Material. TFV, FTC and TAF were measured in BP and SP; TFVdp, FTC triphosphate (FTCtp), and the endogenous nucleotides dATP and dCTP were measured in PBMCs and SMCs; SMC samples with <300,000 cell/mL were pooled within a participant. HIV RNA in BP and SP was measured using the Abbott RealTime HIV-1 Viral Load Assay (Abbott Laboratories. Abbott Park, Illinois, US).
Pharmacokinetic and Statistical Analysis
The six BP and SP concentrations provided a composite concentration-time profile, and noncompartmental analysis (Phoenix Win Nonlin v6.3, Certara Inc, Princeton, NJ) was performed to calculate the area-under-the-curve (AUC) over a dosing interval.
For PBMC and non-pooled SMC concentrations, AUC was calculated as above, and then divided by 24 hours to obtain an average steady-state concentration. Concentrations below the limit of quantification (BLQ) were imputed as one-half of the sample-specific LLOQ (1/2 LLOQ). For pooled samples, the concentration was used as the average steady-state concentration; BLQ pooled samples were also imputed as 1/2 LLOQ. Ratios between SMC and PBMC (SMC:PBMC), and ratios of drug metabolite to its endogenous nucleotide in each matrix (TFVdp:dATP, FTCtp:dCTP) were calculated.
These outcomes were compared among the three groups of men using nonparametric statistics (R version 3.5.1, r-project.org). A Kruskal-Wallis test was performed, followed by Dunn’s test for any p-value <0.05. No adjustments for multiple comparisons were made.
RESULTS
Participant Characteristics and Blood/Semen Viral Loads
The demographics and viral loads of the participants are shown in Table 1. All had normal renal function and most were African-American. Background regimen varied for the HIV-positive men.
Table 1.
Demographics of participants, by tenofovir (TFV) formulation and serostatus. Data are reported as median (25th, 75th percentile) or number. TAF: Tenofovir alafenamide; TDF: tenofovir disoproxil fumarate; BMI: body mass index; eGFR: estimated glomerular filtration rate; EVG: elvitegravir; COBI: cobicistat; DRV: darunavir; RAL: raltegravir; RPV rilpivirine; EFV: efanvirenz; ATV/r: atazanavir/ritonavir
| HIV+ TAF (n=8) | HIV+ TDF (n=8) | HIV− TDF (n=8) | |
|---|---|---|---|
| Age, years | 45.5 (34 – 52) | 36.5 (33 – 41) | 30.5 (24 – 39) |
| BMI, kg/m2 | 28.8 (25 – 31) | 29.8 (27 – 33) | 28.3 (24 – 32) |
| eGFR, mL/min | 109 (97 – 122) | 133 (117 – 154) | 115 (109 – 149) |
| African-American | 5 | 7 | 5 |
| Caucasian | 2 | 0 | 3 |
| Mixed Race | 1 | 1 | 0 |
| Other ARVs in Regimen | 3: EVG/COBI* | 3: EVG/COBI | N/A |
| Blood Plasma Viral Load | 8/8: undetectable | 6/8: undetectable | N/A |
| Seminal Plasma Viral Load | 5/5: undetectable# | 6/6: undetectable## | N/A |
TAF dose: 10mg with 200mg emtricitabine
TAF dose: 25mg with 200mg emtricitabine
2 men did not have sufficient sample volume for virology; 1 man had a low-volume sample that resulted in machine error and were unmeasurable
2 men had low-volume samples that resulted in machine error and were unmeasurable
Seminal Plasma Tenofovir Exposure
The median concentration-time profiles for BP and SP by dosage form are presented in Figure 1; here, TDF men were combined (n = 16). Four SP samples lacked sufficient volume to measure TFV/FTC concentrations.
Figure 1.
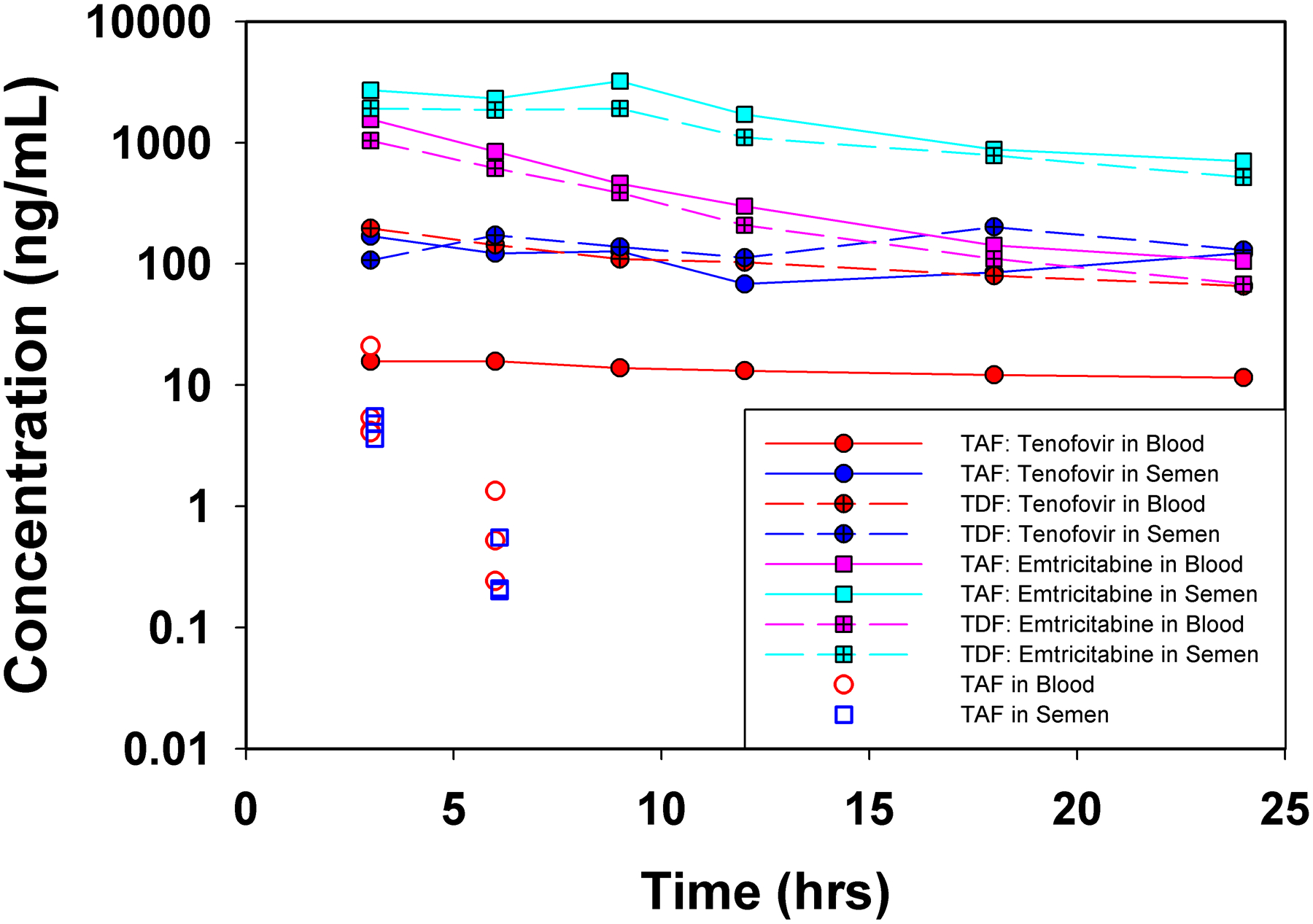
Median/IQR TFV concentrations in the blood plasma and seminal plasma following TDF and TAF administration
BP AUC of TFV is lower in men receiving TAF, compared to both HIV+ and HIV− men receiving TDF (p = 0.001 for both). For SP, TFV concentrations and AUC are similar regardless of dosage form (p=0.44). Owing to the lower BP AUC of TFV in the TAF men, the SP:BP AUC ratio is significantly higher, at 7.4 in TAF men, compared to median ratios near 1 for those receiving TDF (p = 0.006 for HIV−, p = 0.03 for HIV+). AUC values are provided in Supplemental Materials.
TAF was also measured in BP and SP for TAF recipients (Figure 1); 11/48 SP samples lacked sufficient volume to measure TAF after TFV/FTC measurement, with 80% of these at ≥9 hours post-dose. No TAF concentrations were detectable after 6 hours post-dose. In 6/8 men, BP and SP concentrations were similar at 3 and 6 hours post-dose.
Seminal Mononuclear Cell Tenofovir Diphosphate Exposure
Given low SMC recovery, samples for 8/8 men in the TAF arm were pooled within a participant. Four pooled samples were imputed at 1/2 LLOQ for TFVdp, though FTCtp concentrations were quantifiable. In the TDF arms, 5/16 men’s samples were pooled and returned BLQ values for TFVdp, with measurable FTCtp concentrations.
For TFVdp in PBMCs, concentrations in TAF men were significantly higher than the TDF men, as expected (p = 0.004 for HIV−, p =0.03 for HIV+). Consistent with similar SP concentrations, TFVdp in SMCs were similar between dosage forms, ranging from 3–22% of PBMCs. Table 2 reports the average concentration over a dosage interval for TFVdp by dosage form and HIV serostatus, as well the SMC:PBMC ratios.
Table 2.
Average Steady-State Concentrations and Comparative Ratios for Intracellular Analytes, by Regimen and Serostatus. Data are reported as median (25th, 75th percentile). Eight men contributed data to each group. TFV: tenofovir; BP: blood plasma; SP: seminal plasma; AUC: area-under-the-concentration-time curve; TFVdp: tenofovir diphosphate; dATP: deoxyadenosine triphosphate; PBMC: peripheral blood mononuclear cells; SMC: seminal mononuclear cells; Css,ave: average steady-state concentration, calculated as AUC/dosing interval (PBMCs) or reported directly from pooled specimens (SMCs). TAF: Tenofovir alafenamide; TDF: tenofovir disoproxil fumarate. FTC: emtricitabine; FTCtp: emtricitabine triphosphate; dCTP: deoxycytidine triphosphate
| TFVdp PBMC Css, ave (fmol/ 106 cells) |
TFVdp SMC Css, ave (fmol/ 106 cells) |
TFVdp SMC: PBMC Css, ave Ratio |
dATP PBMC Css,ave (fmol/ 106 cells) |
dATP SMC Css, ave (fmol/ 106 cells) |
dATP SMC: PBMC Css, ave Ratio |
TFVdp:dATP PBMC Css,ave ratio |
TFVdp:dATP SMC Css,ave ratio |
|
|---|---|---|---|---|---|---|---|---|
| HIV− TDF/ FTC | 116 (108, 164) | 21 (4.2, 39.3) | 0.22 (0.04, 0.32) | 123 (111, 140)** | 64.6 (3.82, 82.7) | 0.54 (0.035, 0.78) | 0.96 (0.81, 1.45) | 0.656 (0.317, 1.21) |
| HIV+ TDF/ FTC | 192 (156, 237) | 21 (6.1, 58) | 0.09 (0.04, 0.39) | 192 (119, 224) | 33.3 (11.6, 55.4) | 0.20 (0.054, 0.35) | 1.1 (0.86, 1.14) | 0.99 (0.43, 1.54) |
| HIV+ TAF/ FTC | 935 (684, 2024)* | 35 (24, 78) | 0.03 (0.01, 0.07) | 184 (138, 262) | 43.4 (32.6, 71.1) | 0.17 (0.11, 0.35) | 5.95 (5.02, 7.34)*** | 1.10 (0.42, 1.70) |
| FTCtp PBMC Css, ave (fmol/ 106 cells) |
FTCtp SMC Css, ave (fmol/ 106 cells) |
FTCtp SMC:PBMC Css, ave Ratio |
dCTP PBMC Css,ave (fmol/ 106 cells) |
dCTP SMC Css, ave (fmol/ 106 cells) |
dCTP SMC: PBMC Css, ave Ratio |
FTCtp: dCTP PBMC Css,ave ratio |
FTCtp: dCTP SMC Css,ave ratio |
|
| HIV− TDF/ FTC | 6460 (5060, 7837) | 88.1 (62.9, 156.7) | 0.016 (0.0051, 0.032) | 585 (563, 698) | 115 (7.5, 224) | 0.22 (0.012, 0.29) | 10.8 (8.27 (13.4) | 1.85 (0.85, 3.31) |
| HIV+ TDF/ FTC | 6397 (5263, 9408) | 83.7 (52.5, 137.4) | 0.013 (0.0051, 0.020) | 590 (462, 825) | 71 (34, 107) | 0.13 (0.035, 0.17) | 10.1 (8.07, 12.5) | 1.40 (0.48, 1.82) |
| HIV+ TAF/ FTC | 7756 (7161, 14530) | 179 (135, 356) | 0.014 (0.011, 0.033) | 758 (712, 945) | 83 (39, 172) | 0.063 (0.044, 0.21) | 11.3 (10.4, 12.7) | 3.81 (2.30, 3.98) |
p = 0.004 for comparison to HIV-; p = 0.03 for comparison to HIV+
p =0.007 for TDF and TAF comparisons
p = 0.001 for comparison to HIV-; p=0.002 for comparison to HIV+
Emtricitabine and Emtricitabine Triphosphate Exposures in the MGT
FTCtp average concentrations in PBMCs and SMCs, and the SMC:PBMC ratios are presented in Table 2. Median FTC BP and SP concentration-time profiles are shown in Figure 1. FTC SP:BP AUC ratios were approximately 3 across the groups (Supplemental Material). FTCtp concentrations in SMCs were substantially lower than those observed in PBMCs (SMC:PBMC ratios < 2%). No statistically significant differences between groups were observed for FTC-and FTCtp-related parameters (p >0.05).
Endogenous Nucleotide Concentrations in the MGT
dATP and dCTP (Table 2) concentrations in PBMCs and SMCs were compared by matrix (SMC:PBMC ratio) and by concentrations of the drug metabolite with which they compete (TFVdp:dATP, FTCtp:dCTP). dCTP concentrations were similar across arms; dATP PBMC concentrations were significantly higher in HIV+ men, compared to HIV− (p =0.007 for TDF and TAF), though not in SMCs or in the SMC:PBMC ratio (p>0.05). For both dATP and dCTP, SMC concentrations were 6–54% lower than PBMC concentrations. The TFVdp:dATP ratio was ~1 for PBMC and SMC, except for men receiving TAF. The ratio of 5.95 for TAF was due to the significantly increased PBMC TFVdp concentrations compared to both HIV+ (p=0.002) and HIV− (p =0.001) men receiving TDF. For FTC, FTCtp:dCTP PBMC ratios were approximately 11; in SMCs, ratios ranged from 1.40 to 3.81 and did not differ by group.
DISCUSSION
We determined the disposition of TFV in the MGT, in men receiving TDF or TAF. We did not observe differences in disposition of TFV following TDF administration in HIV-positive men receiving TDF/FTC (with a 3rd agent) for treatment and HIV-negative men receiving TDF/FTC for prophylaxis. We confirmed previous findings from our group and others that TFV and FTC penetrate SP at concentrations ≥BP, which is typical of the NRTI class.(1)
Unexpectedly, we observed TFV SP concentrations that were similar regardless of dosage form. SMC concentrations of TFVdp following both TDF and TAF were similar, and <20% of those in PBMCs. This was also unexpected, as we expected TFVdp concentrations in SMCs following TAF administration to be higher than for TDF administration, as seen in PBMCs. However, this observation is consistent with similar extracellular SP TFV concentrations. Other investigators have recently reported similar findings.(16) Several mechanisms for our observations are possible, ranging from the increased plasma stability of TAF, increased presence of cathepsin A in the MGT, and differences in transporter expression on SMCs vs PBMCs.
The low metabolite concentrations in SMC for both TFVdp and FTCtp is consistent with data for lamivudine and zidovudine.(17) Further, the concentrations of the endogenous nucleotides dATP and dCTP in SMCs demonstrate this same phenomenon. Many of the SMCs may derive from monocyte-derived lineages,(7–9) which have different biology than PBMCs.(18) These SMC dATP and dCTP concentrations are consistent with slower cellular growth and replication, providing further support for this theory. NRTI concentrations needed to inhibit HIV replication are lower in macrophage-and monocyte-derived cell lines,(19) and NRTI resistance may manifest differently in these cells.(20) Nevertheless, TFVdp:dATP and FTCtp:dCTP ratios exceed those thought to protect uninfected cells against HIV infection in an in vitro model of blood-derived white cell populations.(13)
Our findings are limited by the small sample size in each group; statistical comparisons should be interpreted cautiously. Our initial aim to characterize SMC TFVdp concentrations dictated our sampling scheme, which limited our ability to detect differences in drug absorption/bioavailability and to describe the full time-course of TAF. Semen sample processing for cells was complicated by low sample volumes and low cell yields, resulting in samples with concentrations below the limit of quantification and pooling of SMC samples.
In conclusion, TAF and TDF demonstrated similar extracellular TFV concentrations in semen, despite the lower blood concentrations with TAF. Intracellular TFVdp concentrations in SMCs were similar, and markedly less than PBMC concentrations. Regardless of TFV formulation with which it was administered, FTC penetrated SP at high concentrations relative to BP, and exhibited low SMC FTCtp concentrations compared to PBMCs. The decreased dATP and dCTP SMC concentrations are consistent with reports that cells recovered from semen are derived from slowly replicating cells, which requires further exploration to ensure the most effective use of these drugs in a “kick and kill” cure strategy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank the clinical study participants, the staff of the UNC Healthcare Infectious Diseases Clinic for assistance with recruitment, and the staff of the NC TraCS Institute Clinical and Translational Research Center. We also thank Dr. Mackenzie Cottrell, Ms. Kaitlyn Maffuid, and Ms. Kara Compliment for laboratory support during the conduct of the clinical study.
Phoenix Win Nonlin software is generously provided to the Division of Pharmacotherapy and Experimental Therapeutics at the UNC Eshelman School of Pharmacy by Certara, INC, through designation as a Certara Center of Excellence.
This work was funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases (5R01DK108424 to MSC; JBD, HMAP, JAEN are also supported by this award).
CS, APS, KHB, and ADMK are supported by the UNC Center for AIDS Research through the National Institutes of Allergy and Infectious Diseases, National Institutes of Health (5P30AI050410-20).
We also acknowledge the NC TraCS Institute for its support through the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR002489).
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
A version of this work has been previously presented at the 2017 Conferences on Retroviruses and Opportunistic Infections, February 13–16, Seattle, WA, Abstract 406.
At the time of the work, Dr. Jingxian Chen and Dr. Brian Mass were employees of the UNC Eshelman School of Pharmacy; both are currently employed at Merck.
AK has received research support from Gilead Sciences.
CLG has received research support from Bristol-Myers Squibb, Gilead Sciences, Abbott, Tibotec Therapeutics, Janssen, ViiV Healthcare and Merck.
The remaining authors declare no competing interests.
REFERENCES
- 1.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antiviral therapy. 2011;16(8):1149–67. [DOI] [PubMed] [Google Scholar]
- 2.Klein DM, Evans KK, Hardwick RN, Dantzler WH, Wright SH, Cherrington NJ. Basolateral uptake of nucleosides by Sertoli cells is mediated primarily by equilibrative nucleoside transporter 1. The Journal of pharmacology and experimental therapeutics. 2013;346(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JA, Ping LH, Dibben O, Jabara CB, Arney L, Kincer L, et al. HIV-1 Populations in Semen Arise through Multiple Mechanisms. PLoS pathogens. 2010;6(8):e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaillon A, Gianella S, Wertheim JO, Richman DD, Mehta SR, Smith DM. HIV migration between blood and cerebrospinal fluid or semen over time. J Infect Dis. 2014;209(10):1642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunnari G, Leto D, Sullivan J, Xu Y, Mehlman KE, Kulkosky J, et al. Seminal reservoirs during an HIV type 1 eradication trial. AIDS research and human retroviruses. 2005;21(9):768–75. [DOI] [PubMed] [Google Scholar]
- 6.Ghosn J, Viard JP, Katlama C, de Almeida M, Tubiana R, Letourneur F, et al. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. AIDS (London, England). 2004;18(3):447–57. [DOI] [PubMed] [Google Scholar]
- 7.Roulet V, Satie AP, Ruffault A, Le Tortorec A, Denis H, Guist’hau O, et al. Susceptibility of human testis to human immunodeficiency virus-1 infection in situ and in vitro. The American journal of pathology. 2006;169(6):2094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deleage C, Moreau M, Rioux-Leclercq N, Ruffault A, Jegou B, Dejucq-Rainsford N. Human immunodeficiency virus infects human seminal vesicles in vitro and in vivo. The American journal of pathology. 2011;179(5):2397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, et al. Semen CD4+ T cells and macrophages are productively infected at all stages of SIV infection in macaques. PLoS pathogens. 2013;9(12):e1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, et al. Macrophages sustain HIV replication in vivo independently of T cells. The Journal of clinical investigation. 2016;126(4):1353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashiri K, Rezaei N, Nasi M, Cossarizza A. The role of latency reversal agents in the cure of HIV: A review of current data. Immunology letters. 2018;196:135–9. [DOI] [PubMed] [Google Scholar]
- 12.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. The Journal of antimicrobial chemotherapy. 2011;66(2):240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis. 2016;214(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Lerma JG, Aung W, Cong ME, Zheng Q, Youngpairoj AS, Mitchell J, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. Journal of virology. 2011;85(13):6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral research. 2016;125:63–70. [DOI] [PubMed] [Google Scholar]
- 16.Imaz AJN, Cottrell ML, Perez E, Kashuba AD, Tiraboschi J, et al. Seminal Tenofovir Concentrations, Viral Suppression, and Semen Quality with TAF vs TDF. Conference on Retroviruses and Opportunistic Infections; Boston, MAMarch 4–7, 2018. [Google Scholar]
- 17.Dumond JB, Reddy YS, Troiani L, Rodriguez JF, Bridges AS, Fiscus SA, et al. Differential extracellular and intracellular concentrations of zidovudine and lamivudine in semen and plasma of HIV-1-infected men. Journal of acquired immune deficiency syndromes (1999). 2008;48(2):156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavegnano C, Kennedy EM, Kim B, Schinazi RF. The Impact of Macrophage Nucleotide Pools on HIV-1 Reverse Transcription, Viral Replication, and the Development of Novel Antiviral Agents. Molecular biology international. 2012;2012:625983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antiviral chemistry & chemotherapy. 2009;20(2):63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Bercoff D, Wurtzer S, Compain S, Benech H, Clavel F. Human immunodeficiency virus type 1: resistance to nucleoside analogues and replicative capacity in primary human macrophages. Journal of virology. 2007;81(9):4540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


