Abstract
Programmed cell death-1 (PD-1) pathway inhibition in head and neck squamous cell carcinoma (HNSCC) has demonstrated inconsistent efficacy regarding human papillomavirus (HPV) status and PD-L1 expression. This study compared outcomes in HNSCC in the context of PD-L1 and HPV expression. Outcomes: PD-L1 and HPV expression; overall survival (OS), and tumor response (ORR). 1088 patients received PD-1/L1 inhibitors. Four methodologies were identified in determining PD-L1 expression, most commonly using the Dako PD-L1 IHC 22C3 pharmaDx assay. Using a 1% threshold, ORR was greater for PD-L1 expressers vs non-expressers (18.9%, CI 16.1–21.8 v 8.8% CI 5.3–13.7, P = 0.009), as was OS at 6 months (60.6%, CI 49.2–71.4 v 49.0%, CI 39.1–59.0, P = 0.04) but not at 12 or 18 months. No advantages were identified for HPV expressers. Patients expressing PD-L1 may have a better tumor response and OS. No impact on survival or response was observed based on HPV status.
Keywords: head and neck squamous cell carcinoma, HPV, immunotherapy, PD-L1, survival
1 |. INTRODUCTION
Head and neck cancers represent the sixth most prevalent malignancy and have an annual incidence of approximately 600,000 cases.1 It is estimated that up to 90% of these cancers are classified histologically as squamous cell carcinoma (HNSCC).2,3 Historically, HNSCC has been associated with several modifiable risk factors including tobacco use, alcohol consumption, and chewing betel quid.4–6 Despite a decrease in HNSCC cases caused by these risk factors, overall prevalence is still on the rise due to an increasing subset of cancers caused by human papillomavirus (HPV).7,8 Unfortunately, up to 66% of patients with HNSCC are diagnosed at advanced stages (III or IV), with about 10% of patients presenting with distant metastases.9 As a result, these patients often receive aggressive multimodal therapeutic regimens consisting of combinations of chemotherapy, radiation, and surgery.
The aggressive nature of solid tumors can be partly attributed to their ability to evade an immune response. One mechanism used to accomplish this is through tumor-cell expression of programmed cell death-ligand 1 (PD-L1), which interacts with its receptor (PD-1) on tumor-specific T cells and limits their antitumor activity.10 Novel PD-1/L1 targeting agents block this interaction, thereby permitting reinvigorated lymphocyte proliferation and effector function.11 PD-1 and PD-L1 inhibitors have recently changed the landscape of clinical oncology for patients with HNSCC.12,13 In 2016, pembrolizumab and nivolumab were approved by the U.S. Food and Drug Administration (FDA) for use in patients with recurrent or metastatic (R/M) HNSCC who progressed during or after platinum-based chemotherapy. Indications for pembrolizumab recently changed when the FDA granted approval as first-line treatment for patients with R/M HNSCC regardless of prior treatment; individuals whose tumors express PD-L1 can receive pembrolizumab monotherapy, but individuals without expressivity receive pembrolizumab in combination with other standard agents.14 This divergence in treatment regimen was based upon the finding that individuals whose tumors expressed PD-L1, defined by a Combined Positive Score (CPS) ≥1 (a measure of the extent of expressivity in tumor and immune cells),15 had enriched responses and a favorable overall survival compared with those whose tumors were non-expressive.14,16 However, whether this observed benefit is maintained across studies using various PD-1/L1 targeting antibodies, each with particular PD-L1 detecting techniques, is not well understood and was addressed herein.
Clinical trials of R/M HNSCC patients receiving monotherapy PD-1/L1 blockade have demonstrated variable outcomes among patients stratified by PD-L1 expression and HPV status.16–20 However, these trials have utilized a variety of methodologies for determining PD-L1 and HPV expression, and further reported various cutoff points for determining PD-L1 “positivity.” These inconsistencies are largely owing to the absence of a standardized method for quantifying PD-L1 expression and determining an appropriate cutoff level to dichotomize patients. To further understand the role of PD-L1 expression and HPV status in augmenting the response to PD-1/PD-L1 inhibitors in R/M HNSCC, we performed a systematic review to report the survival and tumor response stratified by tumor PD-L1 and HPV expression from published clinical trials.
2 |. METHODS
2.1 |. Search strategy
This study was designed with the PRISMA guidelines.21 PubMed (NLM NIH), Scopus (Elsevier), Embase (Elsevier), Web of Science (Clarivate), and Cochrane Library (Wiley) were searched from inception through May 10, 2019. Our search strategies used a combination of subject headings (eg, MeSH in PubMed) and keywords such as: head and neck cancer, head and neck squamous cell carcinoma, oral cancer, oropharyngeal cancer, immunotherapy, program cell death 1 receptor/antagonist and inhibitor, survival rate, disease free survival (Supporting Information Appendix A1). References were uploaded to EndNote (Clarivate Analytics, Philadelphia, Pennsylvania) and screened for relevance by authors JJP and DAL.
2.2 |. Selection criteria
Inclusion criteria required the use of a PD-1 or PD-L1 inhibitor monotherapy for HNSCC in prospective trials with a description noting the methods used to determine PD-L1 expression and/or HPV status. Furthermore, studies were required to stratify outcomes according to PD-L1 expression and/or HPV status. Studies not in English were excluded. Additional exclusion criteria included (a) insufficient data or data was not extractable; (b) ongoing project; (c) patients with cancer sites other than HNSCC and/or HNSCC data was not extractable; (d) subgroup analysis of patients from a larger study; (e) retrospective design; vi) article type was either review, letter to the editor, conference abstract, personal opinion, case report, or book chapter. Articles were critically appraised to assess level of evidence using the Oxford Center for Evidence-Based Medicine criteria.22
2.3 |. Data extraction
Several parameters related to PD-L1 and HPV status were extracted from each article. For PD-L1 status, specific methodology of quantifying patient PD-L1 expression was extracted, as well as the cutoff(s) used for dichotomization within each article. Methodology of HPV status was also determined, along with any information pertaining to subject selection for HPV testing. When explicitly reported, anatomic subsite of the primary tumor was extracted, as well as any relevant oncologic information such as number of prior treatments for R/M HNSCC. After stratifying by PD-L1 expressivity and HPV status, the primary outcomes were median overall survival (mOS), overall survival rate (OS), median progression free survival (mPFS), and progression free survival rate (PFS). Tumor response determined by Reporting the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was used to extract objective response rate (ORR; equal to the sum of patients who achieved complete response and partial response), stable disease (SD), and progressive disease (PD).23 Of note, when part or all of the patient populations were reported in more than one publication, only the most comprehensive and updated study was included in the final analysis.
2.4 |. Statistical analysis
Meta-analysis of included studies evaluated RECIST data, OS and PFS among patients who were PD-L1 expressers or non-expressers, and HPV-positive or HPV-negative. Repeated dichotomization and analyses were performed stratifying patients according to the various reported cutoffs for PD-L1 expression: 1%, 5%, 10%, and 25%. Weighted paired comparisons were performed between cohorts with expression levels above and below each cutoff (eg, <1% vs ≥1%, <5% vs ≥5%, etc). Additionally, weighted paired comparisons were performed between PD-L1 positive groups with different cutoffs to determine the effect of intermediary levels of expression (eg, ≥1% vs ≥5%, ≥1% vs ≥ 10%, ≥5% vs ≥10%, etc). The effect of HPV was assessed by comparing outcomes (weighted proportions) from HPV-positive and HPV-negative subgroups.
Categorical variables were summarized by frequency and percentage. Continuous variables were summarized by mean ± SD (or range for means of median values) or median and interquartile range (IQR: 25th and 75th) where appropriate. A meta-analysis of proportions was performed using MedCalc 19.0.4 (MedCalc Software bvba, Belgium). This program lists the proportions (expressed as a percentage), with their 95% confidence intervals (CIs), found in the included studies. MedCalc uses a Freeman-Tukey transformation to calculate the weighted summary proportion under the fixed and random effects model. Under the fixed effects model, it is assumed that all studies come from a common population, and that the effect size (proportions) is not significantly different among trials. This assumption is tested by the “Heterogeneity test.” If this test yields a low P value (P < .05), then the fixed effects model may be invalid. In this case, the random effects model may be more appropriate, in which both the random variation within the studies and the variation between the different studies is incorporated. Both the fixed effects model and the random effects model were used in this study. Finally, the Sterne and Egger tests were performed to further assess risk of publication bias.24,25 Potential publication bias was evaluated by visual inspection of the funnel plot and Egger’s regression test, which statistically examines the asymmetry of the funnel plot. In a funnel plot, treatment effect is plotted on the horizontal axis and MedCalc plots the SE on the vertical axis.26 The vertical line represents the summary estimated derived using fixed-effect meta-analysis. Two diagonal lines represent (pseudo) 95% confidence limits (effect ± 1.96 SE) around the summary effect for each SE on the vertical axis. These show the expected distribution of studies in the absence of heterogeneity or selection bias. In the absence of heterogeneity, 95% of the studies should lie within the funnel defined by these diagonal lines. Publication bias results in asymmetry of the funnel plot. If publication bias is present, the smaller studies will show the larger effects. A P value of <.05 was considered to indicate a statistically significant difference for all statistical tests.
3 |. RESULTS
3.1 |. Results of the search methodology
A literature search identified 1125 publications following the removal of duplicates. After review by title and abstract, 58 articles remained. A total of 46 studies were excluded for the following reasons: retrospective design (n = 2); ongoing project (n = 10); SSHNSCC data not extractable (n = 4); subgroup analysis of a larger dataset (n = 10); insufficient data (n = 9); review, letter or conference abstract (n = 11). Twelve articles remained for analysis16–20,27–33 (Figure 1—PRISMA). Of the 12 references, five references were categorized as level 1b and seven were categorized as level 2b based upon the Oxford Center for Evidence Based Medicine definition. To investigate the presence of publication bias, inspection of the funnel plot of effects calculated from individual studies was performed. According to funnel plots and the Egger’s test, there was no indication of publication bias among the set of studies included in this meta-analysis (Figure S1).
FIGURE 1.
PRISMA diagram
3.2 |. Summary of included data
Twelve studies met inclusion criteria (Table 1), nine reporting outcomes from PD-1 inhibitors (pembrolizumab [6] and nivolumab [3]) and three used PD-L1 inhibitors (durvalumab [2] and atezolizumab [1]). The following data were extracted for analysis: OS, PFS, and RECIST outcomes. Three studies reported on the same population from the CheckMate-141 clinical trial. The primary CheckMate-141 study reported outcomes in 201633 and had two subsequent one27 and two32 year follow-ups. These studies were deemed appropriate for inclusion because they collectively presented a complete dataset on one unique cohort. When appropriate, the most recent study was used to report the outcomes of interest, however, if the most recent study did not report the outcome of interest, data from the older study were used. Furthermore, one study16 reported collective long-term follow-up data on the PD-L1 positive and biomarker-unselected expansion cohorts of KEYNOTE-012.17 Data from the individual studies was reported from the pooled analysis when appropriate, or as separate studies if the pooled analysis did not explicitly report the outcome of interest.
TABLE 1.
Characteristics of included studies
| Author (year) | Study design | OLE | N | Male (%) | Median age, y (range) | Investigational drug | Drug target | Setting |
|---|---|---|---|---|---|---|---|---|
| Cohen (2019) | RCT | 1b | 247 | 207 (84) | 60 (55–66) | Pembrolizumab | PD1 | Multinational |
| Zandberg (2019) | Prospective single-arm | 2b | 112 | 80 (71) | 60 (24–84) | Durvalumab | PD-L1 | Multinational |
| Colevas (2018) | Prospective single-arm | 2b | 32 | 27 (84) | 62 (32–78) | Atezolumab | PD-L1 | Multinational |
| Ferris (2018)a | RCT | 1b | 240 | 197 (82) | 59 (29–83) | Nivolumab | PD1 | Multinational |
| Gillison (2018)a | RCT | 1b | - | - | - | Nivolumab | PD1 | Multinational |
| Mehra (2018)b | NAb | 2b | 192 | 159 (83) | 60 (20–84) | Pembrolizumab | PD1 | Multinational |
| Siu (2018) | RCT | 1b | 67 | 54 (81) | 62 (23–82) | Durvalumab | PD-L1 | Multinational |
| Bauml (2017) | Prospective single-arm | 2b | 171 | 138 (81) | 61 (33–90) | Pembrolizumab | PD1 | c |
| Hsu (2017) | Phase Ib | 2b | 27 | 21 (78) | 52 (18–68) | Pembrolizumab | PD1 | Multinational |
| Chow (2016) | Prospective single-arm | 2b | 132 | 110 (82) | 60 (25–84) | Pembrolizumab | PD1 | USA and Israel |
| Ferris (2016) | RCT | 1b | - | - | - | Nivolumab | PD1 | Multinational |
| Seiwert (2016) | Phase Ib | 2b | 60 | 49 (82) | 63 (20–83) | Pembrolizumab | PD1 | USA and Israel |
Abbreviations: mOS, median overall survival; mPFS, median progression-free survival; NA, not applicable; NCT, National Clinical Trial; OLE, Oxford level of evidence; PD-1, program death receptor 1; PD-L1, program death ligand 1; RCT, randomized control trial; RECIST, reporting the response evaluation criteria in solid tumors; TRAE, treatment-related adverse events.
This trial is a follow-up study of Ferris (2016) and does not present data on new subjects.
This trial is a follow-up study of both Seiwert (2016) and Chow (2016) and does not present data on new subjects.
Not available.
Stratification by PD-L1 expressivity was performed in all included studies, with variable assays and cutoff levels. The following assays were utilized in the included studies: Dako PD-L1 immunohistochemistry (IHC) 22C3 (four pembrolizumab studies) and 28–8 (one nivolumab study) pharmaDx assays (Agilent Technologies, Carpinteria, California), and Ventana PD-L1 SP263 (two durvalumab studies) and SP142 (one atezolizumab study) assays (Ventana Medical Systems, Inc., Oro Valley, Arizona). One study28 did not specify the assay utilized for PD-L1 expression, but rather reported the laboratory where analysis occurred (QualTek Molecular Laboratories, Goleta, California). This lab confirmed the use of an in-house protocol that is similar to the Dako PD-L1 IHC 22C3 pharmaDx assay kit for determining PD-L1 expression. Five studies quantified expression using CPS,16,19,28,31,33 while other reported methods were tumor cell (TC) expression,20,29 as well as tumor infiltrating immune cell (IC) expression.30 Various cutoffs were used to dichotomize patients as expressers or non-expressers, with several papers reporting outcomes based on more than one cutoff level: 1% (four studies), 5% (two studies), 10% (one studies), 25% (2 studies), and 50% (one study).
HPV status was reported in all but one study.28 Three studies utilized p16 IHC, with a 70% cutoff as surrogacy for HPV positivity.18,31,33 One study determined HPV status through PCR quantification of HPV-6, HPV-11, HPV-16, and HPV-18 genotypes.30 Four studies did not explicitly report the methodology of determining HPV status17,19,20,29 with two studies reporting HPV negativity in all sites other than the oropharynx17,19 (Table 2).
TABLE 2.
Methodologies for determining PD-L1 expression and HPV status
| HPV status |
PD-L1 expression |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (year) | N | Method | Evaluated | + | − | Method | Cutoffb | + | − |
| Cohen (2019) | 247 | OP Ventana p16 IHC, >70% is pos | a | 61 (a) | a | Agilent PD-L1 IHC 22C3 pharmDx | CPS > 1% | 196 (79) | 50 (20) |
| Zandberg (2019) | 112 | p16 IHC or PCR | 99 | 34 (34) | 65 (65) | Ventana PD-L1 (SP263) Assay | TC > 25% | 111 | 0 |
| Colevas (2018) | 32 | PCR | 28 | 13 (46) | 12 (43) | Ventana PD-L1 (SP142) Assay | IC > 5% | 25 (78) | 7 (22) |
| Ferris (2018)c | 240 | OP p16 IHC, >70% is pos | 120 | 64 (53) | 56 (47) | Dako PD-L1 IHC 28-8 pharmDx | CPS > 1% | 96 (40) | 76 (31) |
| Siu (2018) | 67 | a | 67 | 18 (27) | 49 (73) | Ventana PD-L1 (SP263) Assay | TC < 25% | 0 | 65 |
| Bauml (2017) | 171 | Institutional protocold | 168 | 37 (22) | 131 (78) | Dako PD-L1 IHC 22C3 pharmDx | CPS > 1% | 140 (82) | 26 (15) |
| Hsu (2017) | 27 | a | a | a | a | QualTek Molecular Labs | CPS > 1% | 27 | a |
| Chow (2016) | 132 | a | a | 28 (21) | 104 (79) | Agilent Dako PD-L1; Merck 22C3 pharmDx | CPS > 1%, TPS > 1% | a | a |
| Seiwert (2016) | 60 | p16 IHC, >70% is pos | 60 | 23 (38) | 37 (62) | Agilent Dako PD-L1; Merck 22C3 pharmDx | CPS > 1% | 60 | 0 |
Abbreviations: OP, oropharyngeal; TC, tumor cell staining; IC, area of tumor area positive for immune cells; CPS, Combined Positive Score; IHC, immunohistochemistry; PCR, polymerase chain reaction.
Not reported or not tested.
Various methodologies reported in the included studies, if more than one methodology was reported, CPS ≥1 was determined to indicated positivity.
Data from most recent study are presented here; if data in most recent study were not specifically reported, then data from prior studies are reported.
Non-oropharyngeal tumors were assumed to be HPV negative.
3.3 |. Patient characteristics
A total of 1088 patients were included of which 1007 (93%) were evaluated for PD-L1 status. Patients were predominately male (n = 883, 81.2%) and the mean of the median ages was 59.9 years (range 18–90; Table 1). Each patient had advanced, recurrent and/or metastatic disease. The number of prior treatments received for R/M HNSCC varied, with reports ranging from 0 to >5 prior treatments. Primary tumor site was reported in 826 (75.9%) individuals. The pharynx was the most common site (n = 420, 50.5%) with specifically reported subsites of nasopharynx (n = 39, 4.7%), oropharynx (n = 259, 31.1%), and hypopharynx (n = 29, 3.5%). HPV status was reported in 732 (67%) patients with 278 of these (38%) testing positive and 454 (62%) negative (148 positive, 93 negative by p16 IHC, 13 positive, 12 negative by PCR, 117 positive, 349 negative, exact method not known). Of the 259 oropharyngeal tumors, only 20% had HPV status determination, of which 60% were positive (Table 3).
TABLE 3.
Oncologic characteristics
| Primary tumor site, n (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oropharyngeal |
||||||||||
| Author (year) | N | Pharyngeal | All | Eval | HPV+ | HPV− | Nasal cavity | Oral cavity | Laryngeal | Other |
| Cohen (2019) | 247 | a | NA | NA | NA | NA | a | a | a | a |
| Zandberg (2019) | 112 | 40 (36) | 40 | 37 | 20 | 17 | 0 | 47 (39) | 15 (13) | 1 (1) |
| Colevas (2018) | 32 | 23 (72) | 18 | 16 | 12 | 4 | 0 | 7 (22) | 2 (6) | 0 |
| Ferris (2018)b | 240 | 92 (38) | NA | NA | NA | NA | 0 | 108 (45) | 34 (14) | 6 (3) |
| Mehra (2018)b | 192 | 97 | 76 | NA | NA | NA | 12 | 28 | 18 | 37 |
| Siu (2018) | 67 | 33 (49) | 25 | NA | NA | NA | 0 | 15 (22) | 17 (25) | 0 |
| Bauml (2017) | 171 | 108 (63) | 100 | NA | NA | NA | 1 (1) | 28 (16) | 30 (18) | 0 |
| Hsu (2017) | 27 | 27 (100) | NA | NA | NA | NA | 0 | 0 | 0 | 0 |
Abbreviations: CPS, Combined Positive Score; if more than one methodology was reported, Combined Positive Score ≥ 1 was determined to indicated positivity; Eval, evaluable; IC, area of tumor area positive for immune cells; NA, not available; TC, tumor cell staining.
Not reported or not tested.
Data from most recent study are presented here; if data in most recent study were not specifically reported, then data from prior studies are reported.
For PD-L1 expression, cutoff levels of 1%, 5%, 10%, and 25% were used to dichotomize patients. Using a cutoff of 1%, 747 patients were positive and 193 negative (804 evaluated with CPS, 111 with TC and 25 with IC); with a cutoff of 5%, 238 patients were positive, while 231 were negative (326 evaluated by CPS, 111 by TC, and 32 by IC); at 10% 202 were positive and 242 were negative (326 evaluated with CPS, 111 as TC and 7 as IC); lastly, at a 25% cutoff, 159 tested positive while 307 were negative (283 CPS, 176 TC and 7 IC). Of the evaluable PD-L1 patients, PD1 inhibitors were used in 799 (79%) patients (pembrolizumab, n = 627; nivolumab, n = 172), and 208 (19.4%) received a PD-L1 inhibitor (durvalumab, n = 176; atezolizumab, n = 32).
3.4 |. PD-L1 and HPV expression and associated clinical activity
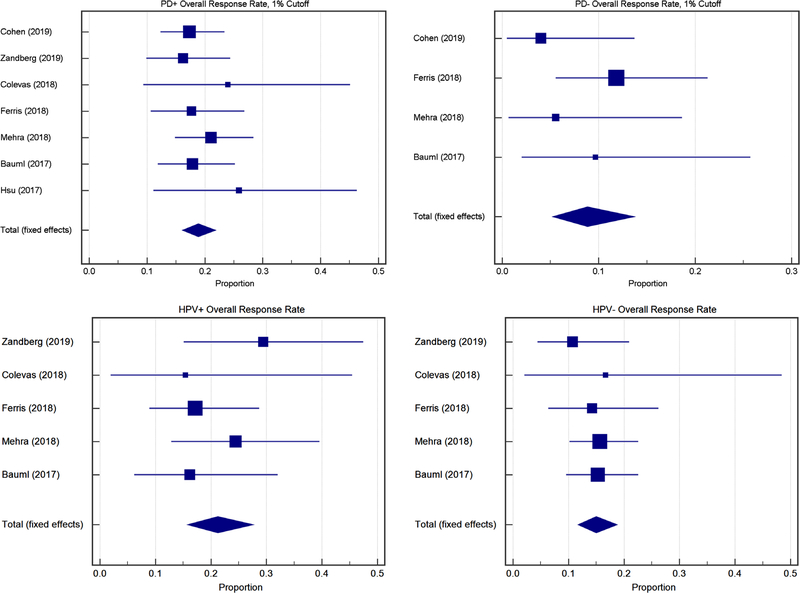
From the overall population, the mean of the median response time to treatment was 2.65 months (range 1.4–17; Table S1). Using a 1% cutoff, expressers had a similar SD rate compared to non-expressers (19.9%, CI 10.2–31.9 vs 20.4%, CI 12.4–30.7, P = .91) but a higher ORR (18.9%, CI 16.1–21.8 vs 8.8% CI 5.3–13.7, P < .009) (Figure 3). There was a similar divergent trend of higher ORR in expressers compared to non-expressers at 5% (20.9% CI 15.9–26.6 vs 9.2%, CI 5.9–13.7, P = .004), 10% (18.9%, CI 14.6–23.8 vs 8.8%, CI 5.6–13.1, P = .003) and 25% (19.7%, CI 13.8–26.6 vs 9.0%, CI 6.1–12.8, P = .001). The rates of SD were significantly different for non-expressers at cutoffs of 5% (9.5%, CI 5.7–14.7 vs 22.3%, CI 14.2–32.2, P = .004), 10% (11.3%, CI 3.5–22.8 vs 22.3%, CI 14.2–32.2, P = .01), and 25% (9.0%, CI 5.0–14.5 vs 18.3%, CI 7.9–31.7, P = .02) but not at 1%. There were no differences in PD rate using any cutoff level.
FIGURE 3.
Forest plots of PD-L1+, 1% cutoff, ORR RECIST Outcomes. Top: ORR PD-L1+ vs PD-L1−at a 1% cutoff. Bottom: ORR HPV+ vs HPV−
SD and ORR were compared posthoc between different groups of expressers based on their levels of expression. Patients with ≥1% expression had a higher rate of SD (19.9%, CI 10.2–31.9) compared to patients with ≥5% expression (9.5%, CI 5.7–14.7, P = .001), ≥10% expression (11.3%, CI 3.5–22.8, P = .003), and ≥25% expression (9.0%, CI 5.0–14.5, P = .002). ORR was similar between all groups of expressers, and PD was similar between all groups of expressers except between ≥1% and ≥10% (43.5%, CI 33.4–53.8 vs 52.2%, CI 45.8–58.5, P = .03). There was no difference in tumor response for HPV positive vs negative patients in terms of SD (17.8%, CI 10.8–26.8, vs 18.9% 14.6–23.9, P = .8) and PD (47.4%, CI 37.2–57.8 vs 50.0% CI 44.1–55.9, P = .6); however, ORR did approach significance in favor of HPV positive patients (21.2% CI 15.8–27.6, vs 15.0% CI 11.7–18.8, P = .06) (Figure 3) (Table 4).
TABLE 4.
Comparison of outcomes
| SD | PD | ORR | SD | PD | ORR | ||||
|---|---|---|---|---|---|---|---|---|---|
| PD-L1+ 1% | % effect | 19.9 | 43.5 | 18.8 | PD-L1+ 10% | 11.3 | 52.2 | 18.9 | |
| N | 499 | 474 | 747 | 251 | 251 | 294 | |||
| 95% CI | 10.2–31.9 | 33.4–53.8 | 16.1–21.8 | 3.5–22.8 | 45.8–58.5 | 14.6–23.8 | |||
| PD-L1− 1% | % effect | 20.4 | 52.8 | 8.8 | PD-L1− 10% | 22.3 | 54.3 | 8.8 | |
| N | 81 | 81 | 193 | 88 | 81 | 242 | |||
| 95% CI | 12.4–30.7 | 33.8–81.3 | 5.3–13.7 | 14.2–32.2 | 43.0–65.3 | 5.6–13.1 | |||
| Comparison | % Diff | 0.5 | 9.3 | 10.0 | Comparison | 11 | 2.1 | 8.7 | |
| P value | 0.9 | 0.1 | 0.0009a | 0.01a | 0.7 | 0.003a | |||
| PD-L1+ 5% | % effect | 9.5 | 47.8 | 20.9 | PD-L1+ 25% | 9 | 47.8 | 19.7 | |
| N | 184 | 159 | 238 | 159 | 159 | 159 | |||
| 95% CI | 5.7–14.7 | 39.9–55.8 | 15.9–26.6 | 5.0–14.5 | 39.9–55.8 | 13.8–26.6 | |||
| PD-L1− 5% | % effect | 22.3 | 54.3 | 9.2 | PD-L1− 25% | 18.3 | 58.8 | 9 | |
| N | 88 | 81 | 321 | 153 | 146 | 307 | |||
| 95% CI | 14.2–32.2 | 43.0–65.3 | 5.9–13.7 | 7.9–31.7 | 50.5–66.8 | 6.1–12.8 | |||
| Comparison | % Diff | 12.7 | 6.5 | 11.7 | Comparison | 9.3 | 11 | 10.7 | |
| P value | 0.004a | 0.34 | 0.004a | 0.02a | 0.05 | 0.001a | |||
| HPV+ | % effect | 17.8 | 47.4 | 21.2 | HPV Comparison | % Diff | 1.3 | 2.8 | 6.2 |
| N | 95 | 95 | 193 | ||||||
| 95% CI | 10.8–26.8 | 37.2–57.8 | 15.8–27.6 | ||||||
| HPV− | % effect | 18.9 | 50 | 15 | P value | 0.8 | 0.6 | 0.06 | |
| N | 290 | 290 | 411 | ||||||
| 95% CI | 14.6–23.9 | 44.1–55.9 | 11.7–18.8 | ||||||
| PD-L1+ 1% vs 5% | % Diff | 10.4 | 4.3 | 2.1 | PD-L1+ 5% vs 10% | 1.7 | 4.7 | 2 | |
| P value | 0.001a | 0.3 | 0.5 | 0.6 | 0.4 | 0.6 | |||
| PD-L1+ 1% vs 10% | % Diff | 8.6 | 8.7 | 0.05 | PD-L1+ 5% vs 25% | 0.5 | 6.5 | 1.2 | |
| P value | 0.003a | 0.03a | 1.0 | 0.9 | 0.3 | 0.8 | |||
| PD-L1+ 1% vs 25% | % Diff | 10.9 | 4.4 | 0.9 | PD-L1+ 10% vs 25% | 0.8 | 4.4 | 0.8 | |
| P value | 0.002a | 0.3 | 0.8 | 0.8 | 0.4 | 0.8 |
Abbreviations: ORR, overall response rate; PD, progressive disease; SD, stable disease.
Significant difference.
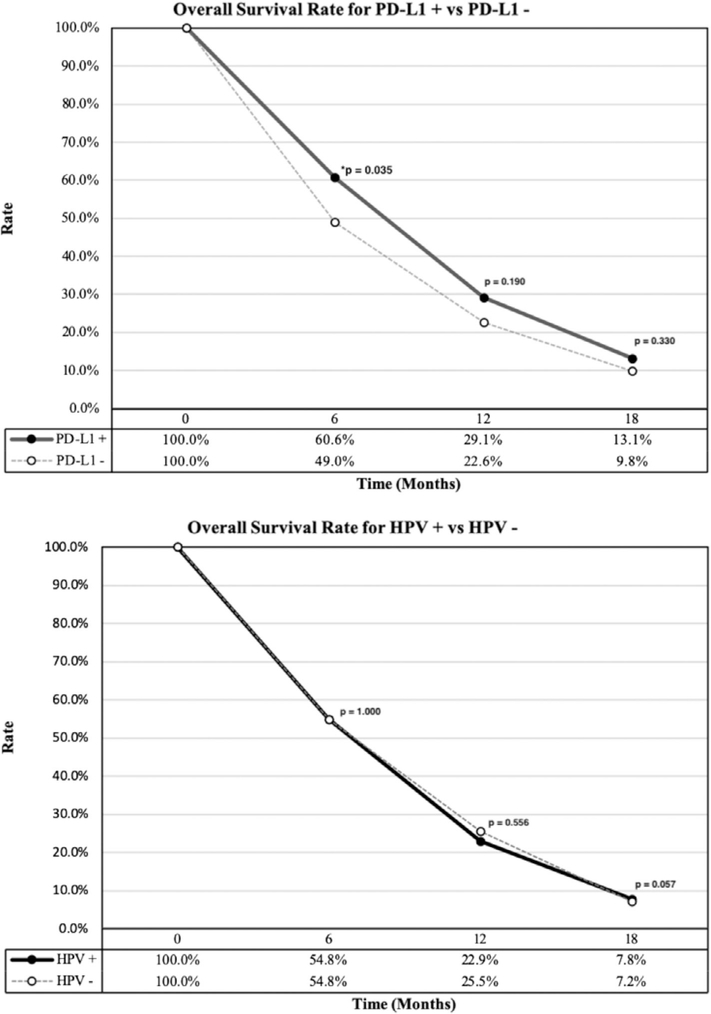
The median follow-up time was reported by seven studies with a weighted average of 10.07 months (range 0.0–32.0). The weighted mOS was 7.97 months (range 6.0–16.5). When stratified based on the expression of PD-L1, patients with expression ≥1% had a weighted mOS of 8.67 months (range 4.9-NR) and patients <1% had 4.88 months (range 1.8–11.7). Comparison of the weighted OS of patients with ≥1% PD-L1 expression, compared to those with <1% expression was significant at 6 months (60.6%, CI 49.2–71.4 vs 49.0%, CI 39.1–59.0, P = .04) but not at 12 months (29.1%, CI 10.3–52.8 vs 22.6%, CI 2.1–56.2, P = .19) or 18 months (13.1%, CI 1.2–34.9, vs 9.8%, CI 1.0–45.5, P = .33). The weighted OS of HPV positive patients compared to HPV negative patients was 54.8% (CI 47.0–62.5) vs 54.8% (CI 48.0–61.4; P = 1.0) at 6 months, 22.9% (CI 9.9–39.3) vs 25.5% (CI 3.4–58.8; P = .56) at 12 months and 7.8% (CI 0.1–26.2) vs 7.2% (CI 0.4–32.4; P = .05) at 18 months (Figure 2). The weighted mPFS of all the studies included was 2.84 months (range 1.9–6.5). PD-L1 positive patients with ≥1% expression had a weighted mPFS of 3.34 months (range 1.9–13.4), however, data on <1% PD-L1 expression were not sufficient for analysis. Similarly, mOS and mPFS data were not sufficient for a pooled analysis based on HPV status.
FIGURE 2.
Comparisons of overall survival rate. Top: Overall survival for PD-L1+ (solid line) vs PD-L1−(dashed line). Bottom: Overall survival for HPV+ (solid line) vs HPV−(dashed line)
4 |. DISCUSSION
The utility of PD-L1 expression as a predictive biomarker has been widely debated by clinicians in a variety of different cancers.34–39 Studies in non-small-cell lung cancer40–42 and melanoma43 have shown that while patients with PD-L1 expressivity generally have improved survival vs PD-L1 negative cohorts, patients with PD-L1 negative tumors can still derive benefit from therapy targeting this axis. For HNSCC, the predictive capacity of PD-L1 is less understood and the immune landscape of HPV+ vs HPV−tumors may further impact the response of these tumors to immunotherapy. In light of the recent FDA approval of pembrolizumab and nivolumab for R/M HNSCC,14,44,45 our meta-analysis sought to illustrate the potential of either PD-L1 or HPV status as predictive biomarkers for survival rate and tumor response post PD-1/L1 inhibition in HNSCC patients.
Initially, KEYNOTE-012 demonstrated the safety and efficacy of body weight-dosed pembrolizumab in patients with PD-L1 positive HNSCC.18 The follow-up expansion cohort assessed a fixed dosing regimen for biomarker unselected patients with R/M HNSCC, showing that the clinical benefit (ORR, mOS, median duration of response) in these patients was similar to the initial cohort of PD-L1 positive patients.17 However, the authors concluded that PD-L1 positive patients had an enriched response compared to the PD-L1 negative patients from the expansion cohort. This analysis compared patients using both CPS and the tumor proportion score (TPS), which quantifies the number of tumors cells expressing membranous PD-L1.46 Interestingly, comparison based on TPS scoring did not reveal a difference between expressers and non-expressers, whereas comparison using the CPS system demonstrated a significant difference with an advantage in ORR for expressors.17 KEYNOTE-05947 also demonstrated the superior predictability of the CPS scoring system with a cutoff of 1% when compared to TPS.15 Although the TPS system was first utilized to receive FDA approval for pembrolizumab treatment in NSCLC, these studies highlight that this scoring system may have different predictive abilities than other systems such as CPS.17,48 As a result, several HNSCC trials utilized the CPS scoring system to determine PD-L1 expressivity and may represent the optimal choice for determining expression for this population.17,19,28,31,33 Clinically, a standardized scoring system and consistent method of determining PD-L1 expression are critical for more concordant conclusions regarding PD-L1 predictive capacity especially given new treatment guidelines for R/M HNSCC based on tumor PD-L1 expression.
Currently, Agilent PD-L1 IHC 22C3 pharmaDx assay is the only FDA approved diagnostic tool in evaluating PD-L1 expression following its proven efficacy in clinical trials of pembrolizumab as a treatment option for NSCLC.15 In this meta-analysis, we demonstrated the methodologic heterogeneity in determining PD-L1 expression among the included studies. In this pooled analysis, PD-L1 expressing patients demonstrated a significant benefit in ORR when compared to non-expressers, irrespective of their expression threshold. This suggests a possible benefit of PD-L1 expression in patients receiving PD-1 pathway blockade. Nonetheless, a head-to-head analysis of PD-L1 expressers defined at different thresholds demonstrated no significant benefit in ORR at greater thresholds, which questions the utility of using any cut-off other than 1%. However, preliminary data from the KEYNOTE-048 trial of R/M HNSCC demonstrated that patients with a CPS ≥20% had a superior OS than those with a CPS ≥1% with pembrolizumab monotherapy.14 Therefore, further research that compares groups of patients using exclusively predetermined ranges of expression will help establish the effect of greater expressivity.
Similarly, among PD-L1 expressers, the rate of RECIST SD was greater in the ≥1% cutoff in comparison to ≥5%, ≥10%, and ≥25%, but not when ≥5%, ≥10%, and ≥25% were compared against one another. The exact reason for this variance remains elusive as this may represent a statistical aberration due to the 1% cutoff population representing a larger subset of the population. Interestingly, when comparing expressers vs non--expressers the rate of SD was significantly better for patients without PD-L1 expression at thresholds greater than 5%. This divergence may be explained as PD-L1 expressers are having a better ORR, resulting in more SD for the non-expressers. An analysis on the rate of PD suggests that regardless of the expression of PD-L1, patients have similar outcomes in terms of PD. The head-to-head comparison supports this finding, except at the ≥1% to ≥10% cutoff, possibly another aberration. Thus, PD-L1 non-expressers gain some tumor response benefits from PD-1 immunotherapy likely representing the therapeutic value of treating biomarker unselected patients.
The analysis of OS based on PD-L1 expression demonstrated higher OS at 6 months for PD-L1 expressers. This statistical difference did not track further as survival became similar between the groups at 12 and 18 months, likely making this observation at 6 months clinically insignificant. The weighted mOS for PD-L1 expressers was also found to be greater, however, we are unable to statistically compare these values without additional data. These findings agree with previous literature on the benefits of PD-L1 expression for OS in other cancers.49,50 However, the data available for the present analysis was limited and is subjected to poor statistical power. Thus, caution should be exercised when interpreting the pooled analysis on OS benefits from PD-L1 expression.
HPV-associated tumors have traditionally been reported to have better outcomes than their non-associated counterparts in oropharyngeal SCC.51 With respect to inhibiting the PD-1 pathway, this difference remains controversial as our study did not identify any statistically significant difference in ORR, SD, PD, or OS when patients were stratified according to HPV status. Previous studies have identified a response benefit for HPV positive patients,16,17,20 while conflicting results were observed in other studies.18,19,33,52 Our analysis has demonstrated that the difference in ORR is approaching statistical significance (P = .06), thus, it remains an interesting front for future investigators to explore if HPV-positive patients do indeed receive enhanced treatment benefits with PD1 inhibitors. Unfortunately, our data does not account for the variability in which HPV status was assessed or in determining which patients were selected for testing. As of 2018, the NCCN guidelines now require determination of HPV expression in oropharyngeal cancers by p16 immunohistochemistry, with an option to confirm with PCR or in situ hybridization.53 While this study is subjected to heterogeneity in this measure, hopefully this change in guidelines will yield more uniform data in the future that would remove obscurity in published data. Furthermore, hopefully this can be adapted to the realm of PD-L1 expression, as uniform methods would enable more accurate cross-trial comparisons. Henceforth, future studies should investigate the prognostic value of HPV-driven tumors by concurrently analyzing the PD-L1 expressivity to further delineate the value of HPV expression in a PD-L1 stratified population.
4.1 |. Limitations
There are several limitations to the current study. First, the study design mandates the use of group data, precluding our ability to confirm individual outcomes and hindering the quality of the reported data. Essential metrics such as tumor stage, location, biopsy confirmation of HNSCC, and classification of the location of disease were inconsistently reported; duration, treatment dosage, and extent of surgeries were also not included for individual patients. The substantial variability of number and types of previous treatments plays an unclear role in this population. Tumors previously treated may harbors mutational burdens or resistance mechanisms secondary to cytotoxic therapies. The generalizability of the present study’s findings is also narrow as only advanced or R/M HNSCC patients were studied, thus restricting our ability to comment on other head and neck neoplasms such as salivary gland or cutaneous malignancies. The heterogeneity in primary tumor sites presents another complexity in this study.
Dichotomization of PD-L1-expressivity reported herein fails to capture the dynamic nature of this metric. Comparing patient outcomes based on PD-L1 expression is further limited based on the variability of detection assays. Four different assays using distinct antibody clones were utilized in determining PD-L1 expression among the included studies. In NSCLC, it was reported that the SP142 antibody stains PD-L1 with less intensity, resulting in lower expressivity scores by blinded pathologists.54 Direct comparison of these assays in HSNCC tumors is important for proper interpretation of expression profiles across studies. Beyond the detection methods, inconsistent measures of expressivity were used, such as tumor staining only, tumor and immune cell staining, or tumor, immune, or stromal cell staining. Clinically, it remains uncertain whether these tests must be paired with their companion therapeutic antibody. If so, use of PD-L1 as a biomarker would rely on therapy selection prior to assessment for PD-L1 status, which could ultimately require more than one test on limited tumor tissue to determine best treatment choice in a personalized manner.
Likewise, the methodology of HPV status determination is an added limitation. Studies either determined HPV status with p16 surrogacy or utilized PCR for high-risk strains. In several instances, studies did not analyze the HPV status of tumors outside the oropharynx as HPV infection is less prevalent in other subsites, yet still classified these tumors as non-HPV related.55,56 This may inaccurately represent the results of the HPV negative individuals by possibly including HPV positive patients, and may further introduce variability by reporting patients that were deemed positive by different standards. Thus, a prospective study with unification of the methodology utilized in assessing PD-L1 and HPV status is pivotal in teasing apart the intricate interactions of these variables within the HNSCC population.
5 |. CONCLUSION
This study supports the premise that PD-L1 expression enhances ORR in R/M HNSCC, although the clinical significance of this finding remains unclear. The CPS system has proven its efficacy in several different cancers and may represent the optimal system for evaluating the membranous expression of PD-L1 in HNSCC. Moreover, HPV status was not associated with any difference in survival or tumor response among these patients receiving PD-L1 blockade. Further investigation is required with concurrent analysis of PD-L1 expression and HPV status to analyze the benefits of each respective biomarker.
Supplementary Material
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96(2): 99–106. [DOI] [PubMed] [Google Scholar]
- 5.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988; 48(11):3282–3287. [PubMed] [Google Scholar]
- 6.Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2004;85: 1–334. [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza G, Kreimer AR, Viscidi R, et al. Case–control study of human papillomavirus and Oropharyngeal cancer. New Engl J Med. 2007;356(19):1944–1956. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Castellsagué X, Chaturvedi A, et al. Eurogin roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancer. 2014;134(3):497–507. [DOI] [PubMed] [Google Scholar]
- 9.Cohen N, Fedewa S, Chen AY. Epidemiology and demographics of the head and neck cancer population. Oral Maxillofac Surg Clin North Am. 2018;30(4):381–395. [DOI] [PubMed] [Google Scholar]
- 10.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling DC, Bakkenist CJ, Ferris RL, Clump DA. Role of immunotherapy in head and neck cancer. Semin Radiat Oncol. 2018; 28(1):12–16. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, Liu TJ, Qu Y, et al. Progresses and perspectives of anti-PD-1/PD-L1 antibody therapy in head and neck cancers. Front Oncol. 2018;8:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rischin D, Harrington KJ, Greil R, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. 2019;37(15_suppl):6000–6000. [Google Scholar]
- 15.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of Pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330–337. [DOI] [PubMed] [Google Scholar]
- 16.Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119(2):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of Pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. [DOI] [PubMed] [Google Scholar]
- 19.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and Cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35 (14):1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with >/=25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142–152. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howick J, Chalmers I, Glasziou P, et al. Explanation of the 2001 Oxford Centre for Evidence-based medicine (OCEBM) levels of evidence (background document). 2016. Oxford, England: The Centre of Evidence Based Medicine (CEBM). [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54 (10):1046–1055. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 27.Gillison ML, Blumenschein G, Fayette J, et al. CheckMate 141: 1-year update and subgroup analysis of Nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist. 2018;23(9):1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of Pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–4056. [DOI] [PubMed] [Google Scholar]
- 29.Siu LL, Even C, Mesia R, et al. Safety and efficacy of Durvalumab with or without Tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. 2018;29(11):2247–2253. [DOI] [PubMed] [Google Scholar]
- 31.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. The Lancet. 2019;393(10167):156–167. [DOI] [PubMed] [Google Scholar]
- 32.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. [DOI] [PubMed] [Google Scholar]
- 35.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson RH, Webster WS, Cheville JC, et al. B7-H1 glycoprotein blockade: a novel strategy to enhance immunotherapy in patients with renal cell carcinoma. Urology. 2005;66(5 Suppl):10–14. [DOI] [PubMed] [Google Scholar]
- 37.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):31673175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le DT, Uram JN, Wang H, et al. PD-1 blockade in Tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):25092520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in Resectable lung cancer. New Engl J Med. 2018;378(21): 1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. New Engl J Med. 2018;378(24):2288–2301. [DOI] [PubMed] [Google Scholar]
- 42.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New Engl J Med. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 43.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus placebo in resected stage III melanoma. New Engl J Med. 2018;378(19):1789–1801. [DOI] [PubMed] [Google Scholar]
- 44.Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Head and Neck Cancers. 2019; Version 2.2019:https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf Accessed July 19, 2019.
- 46.PD-L1 IHC 22C3 pharmDx for Autostainer Link 48. 2019; https://www.agilent.com/en/product/pharmdx/pd-l1-ihc-22c3-pharmdx/pd-l1-ihc-22c3-pharmdx-for-autostainer-link-4894449 Accessed July 19, 2019.
- 47.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of Pembrolizumab Monotherapy in patients with previously treated advanced gastric and Gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5): e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016; 17(6):717–726. [DOI] [PubMed] [Google Scholar]
- 49.Kim KH, Choi KU, Kim A, et al. PD-L1 expression on stromal tumor-infiltrating lymphocytes is a favorable prognostic factor in ovarian serous carcinoma. J Ovarian Res. 2019;12(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Gu J. PD-L1 expression and EGFR status in advanced non-small-cell lung cancer patients receiving PD-1/PD-L1 inhibitors: a meta-analysis. Future Oncol. 2019;15(14):1667–1678. [DOI] [PubMed] [Google Scholar]
- 51.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang BC, Cao RB, Li PD, Fu C. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: a systematic review and meta-analysis. Cancer Med. 2019; 8(13):5969–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Head and Neck Cancers Version 1.2018. 2018; Version 1.2018 Accessed August 6th, 2019.
- 54.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non–small cell lung CancerAssessment of 4 assays for PD-L1 expression in NSCLC Assessment of 4 assays for PD-L1 expression in NSCLC. JAMA Oncol. 2017;3(8):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Modern Pathol. 2011;24(10): 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2013;2(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.