Abstract
Purpose.
To characterize molecular and cellular changes induced by sustained expression of ciliary neurotrophic factor (CNTF) in the rds mutant mouse retina.
Methods.
Recombinant adeno-associated virus (rAAV) expressing CNTF was injected subretinally, for transduction of peripherin/rds+/− transgenic mice that carry the P216L mutation found in human retinitis pigmentosa. Characterization of retinal neurons and glia was performed by immunocytochemistry with cell-type–specific markers. Activation of signaling molecules was examined by Western blot and immunostaining. Alterations of gene transcription profiles were studied by microarray analyses.
Results.
CNTF viral transduction maintained rhodopsin expression in surviving rod photoreceptors, but greatly reduced both S- and M-opsin normally expressed in cones. In addition, CNTF treatment resulted in increased numbers and dispersion of Müller glia and Chx10-positive bipolar cells within the inner nuclear layer. Persistent CNTF signaling also caused enhanced phosphorylation of STAT1, STAT3, and p42/44 ERK, as well as their levels of expression. Moreover, altered transcription profiles were detected for a large number of genes. Among these, Crx and Nrl involved in photoreceptor differentiation and several genes involved in phototransduction were suppressed.
Conclusions.
Despite the rescue from cell death, continuous exposure to CNTF changed photoreceptor cell profiles, especially resulting in the loss of cone immunoreactivity. In addition, the Müller glia and bipolar cells became disorganized, and the number of cells expressing Müller and bipolar cell markers increased. Constitutive CNTF production resulted in sustained activation of cytokine signal transduction and altered the expression of a large number of genes. Therefore, stringent reg ulation of CNTF may be necessary for its therapeutic application in preventing retinal degeneration.
The neurocytokine ciliary neurotrophic factor (CNTF) displays a variety of effects during the development and maintenance of the nervous system. In the developing vertebrate retina, CNTF crucially influences the differentiation of developing photoreceptor cells as it suppresses rhodopsin expression in the rodent retina and enhances cone opsin expression in the chicken retina.1–5 In addition, CNTF induces late retinal progenitor cells to express bipolar cell markers and to specialize as Müller glia in postnatal rodent retinas.3,6,7 In the mature retina, CNTF is among the most potent neurotrophic factors protecting retinal neurons against degeneration.8 To date, CNTF has been shown to prolong photoreceptor survival in a variety of degeneration models including those caused by genetic mutations in different genes and light-induced injury.9–16 Furthermore, CNTF is effective in promoting retinal ganglion cell survival and axonal growth in several glaucoma and axotomy models.17–23
CNTF binds to a tripartite receptor complex that activates receptor associated Jak kinases, which in turn phosphorylate the intracellular portion of the receptors and activate multiple downstream signaling components.24–26 Activation of the Jak kinases leads to phosphorylation of two members of the signal transducer and activator of transcription (STAT) family: STAT1 and STAT3.27 In addition, CNTF triggers the extracellular signal–regulated kinase (p42/44 ERK)28–31 and the phosphatidylinositol 3 kinase-Akt pathways.32 In the developing mouse retina, CNTF stimulation causes differential activation of the Jak-STAT and ERK pathways among progenitor cells and postmitotic neurons.31 The suppression of rhodopsin expression by CNTF in postmitotic photoreceptor precursor cells involves the Jak-STAT, but not the ERK pathway.31,33,34 In contrast, promotion of a Müller glial cell fate in the neonatal retina by CNTF requires both STAT and ERK activation.7 In the mature rodent retina, intravitreal injection of CNTF or its analogue axokine similarly induces the phosphorylation of STAT and ERK proteins; however, this primarily occurs in retinal ganglion cells and Müller glia.29,30
Owing to the significant potential of CNTF as a broad neuroprotective agent for the retina, both viral vector– based and cell-based CNTF delivery approaches have been developed in animal models and were used recently in a clinical trial.35 Nonetheless, the cellular mechanisms of CNTF-dependent neural protection in various retinal degeneration models have not been elucidated. In a mouse model of retinitis pigmentosa (RP) caused by a dominant mutation (P216L) in the rds/peripherin gene,36,37 subretinal delivery of a recombinant adeno-associated virus (rAAV) that expresses a secreted CNTF resulted in effective long-term rescue of the photoreceptor cell layer.12 However, the surviving cells exhibited enlarged nuclei with increased euchromatin. In addition, rAAV-CNTF treated rds/peripherin mutant retinas showed decreased ERG amplitudes in the a-wave of rod photoreceptors and the b-wave of rod and cone photoreceptors, compared with untreated mutant retinas, thus indicating an adverse effect on retinal function.12,13 Similarly, a cellular rescue without functional restoration has been observed in rats carrying dominant rhodopsin mutations.38 Moreover, in normal rabbit retinas treated with high doses of CNTF, the photoreceptor nuclei were increased in size and accompanied by a significantly reduced cone b-wave ERG amplitude.39 To delineate the underlying causes of altered retinal morphology and function, we have performed molecular and cellular analyses of rAAV-CNTF treated rds/peripherin retinas. Our results demonstrate that viral-mediated CNTF expression in the mature retina results in altered retinal organization and abnormal gene expression, as well as persistent and elevated cytokine signaling events. These findings suggest that properly regulated CNTF expression may be essential for preventing retinal degeneration and achieving functional rescue.
Materials and Methods
Animals, Virus Preparation, Injection, and Electroretinographic Analysis
All procedures involving the use of mice were in accordance with the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research; the University of California, Los Angeles; and the University of California, San Francisco. A recombinant adeno-associated virus (rAAV) encoding the chicken β-actin promoter (CBA)/CMV enhancer followed by a modified human CNTF cDNA was prepared as described.12 This recombinant CNTF contains the human growth hormone signal peptide40 and S166D/G167H mutations that increase its affinity toward the CNTF receptor-α (CNTFRα).41 Transgenic mice (rds+/−P216L) of the line 130036 on a C57BL/6 background were injected subretinally with rAAV-CBA-sDH-CNTF (abbreviated as rAAV-CNTF hereafter) unilaterally between postnatal day (P)23 and P25. The injection procedure including anesthesia, viral titer, and injection volume were identical, as previously described in Bok et al.12 In total, 37 rds line 1300 animals were injected with rAAV-CNTF. Injected eyes were analyzed by ERG before harvesting at P44, P70 to P72, or P90 to assess retinal function as described.12 The contralateral noninjected rds eyes and wild-type adult C57BL/6 eyes were used as the control.
Immunohistochemistry and TUNEL Assay
Wild-type C57BL/6, noninjected and rAAV-CNTF injected rds line 1300 eyes were harvested at P70, P72, or P90 and processed as previously described31 with minor modifications. Antibodies used are summarized in Table 1. Mice killed with carbon dioxide were immediately fixed by transcardiac perfusion with 4% paraformaldehyde (PFA) in PBS. Enucleated eyes were further fixed in 4% PFA in PBS overnight at 4°C. The cornea and lens were removed before cryoprotection in 30% sucrose/PBS and embedding in OCT. Cryosections of 14-μm thickness were washed with PBS and incubated with a blocking solution containing DMEM, 10% fetal calf serum, 2% goat serum, 2% donkey serum, and 0.1% Triton X-100 for 1 hour at room temperature. For labeling with anti-phospho-STAT3 and anti-phospho-ERK, cryosections were first washed three times with PBS and then incubated in deionized 95% formamide, 1.5% 20× SSC (pH 7.0), for 10 minutes at 70°C before incubation with the blocking solution. For double staining with Chx10 and Cyclin D3, cryosections were pretreated in antigen retrieval reagent for 5 minutes at 95°C (R&D System, Minneapolis, MN). After blocking, primary antibodies were incubated at 4°C overnight and secondary antibodies at room temperature for 1 hour, each followed with four washes of PBS containing 0.1% Tween 20.31 Immunofluorescent signals were captured with a microscope (E800; Nikon, Tokyo, Japan) equipped with a digital camera (SPOT II; Diagnostic Instruments, Sterling Heights, MI). Confocal imaging was performed (LSM 510 or upgraded LSM 410 microscope; Carl Zeiss Meditec, Inc., Dublin, CA) with argon or helium-neon laser. For each antibody, two or more retinas from different individuals at P70, P72, or P90 were used.
TABLE 1.
Summary of Antibodies
| Cell Type | Antibody Name | Type | Dilution | Source | Catalog Number |
|---|---|---|---|---|---|
| Primary antibodies | |||||
| Amacrine cell | AP-α (3B5) | mAb | 1:10 | DSHB (Univ. of Iowa) | |
| Bipolar cell | 115A10 | mAb | 1:5 | DSHB | |
| Chx10 | shAb | 1:50 | Chemicon | AB9014 | |
| Ganglion cell | Brn3a | mAb | 1:200 | Chemicon | MAB1585 |
| Horizontal cell | Calbindin (D-28K) | rAb | 1:500 | Chemicon | AB1778 |
| Müller glia | Cyclin D3 | mAb | 1:100 | Cell Signaling Inc. | 2936 |
| Glial fibrillary acidic protein (GFAP) | rAb | 1:400 | Sigma-Aldrich | G 9269 | |
| Glutamine synthetase (GS) | mAb | 1:50 | Chemicon | MAB302 | |
| Photoreceptor | M-opsin | rAb | 1:200 | Chemicon | AB5405 |
| Mouse cone arrestin (mCAR) | rAb | 1:2000 | Cheryl M. Craft | Ref. 44 | |
| Peanut agglutinin (PNA) | 1:250 | Vector Laboratories | FL-1071 | ||
| Rhodopsin (Rho4D2) | mAb | 1:250 | Robert Molday | Ref. 42 | |
| S-opsin | rAb | 1:200 | Chemicon | AB5407 | |
| Signaling molecules | ERK2 (C-14) | rAb | 1:1000 (wt) | Santa Cruz Biotech. | sc-154 |
| p-ERK1/2 (Thr202/Tyr204) | rAb | 1:100, 1:1000 (wt) | Cell Signaling Inc. | 9101 | |
| p-STAT3 (Tyr705) | rAb | 1:100, 1:1000 (wt) | Cell Signaling Inc. | 9171 | |
| p-STAT1 (Tyr701) | rAb | 1:1000 (wt) | Cell Signaling Inc. | 9131 | |
| STAT1 (p84/p91) | rAb | 1:1000 (wt) | Santa Cruz Biotech. | sc-346 | |
| STAT3 (K-15) | rAb | 1:1000 (wt) | Cell Signaling Inc. | 9132 | |
| Secondary antibodies | |||||
| Alexa 488 conjugated | dAb | 1:500 | Invitrogen/Molecular Probes | A11015 | |
| Alexa 488 conjugated | gAb | 1:500 | Invitrogen/Molecular Probes | A11008 | |
| Alexa 568 conjugated | gAb | 1:500 | Invitrogen/Molecular Probes | A11004 | |
| HRP conjugated | shAb | 1:1000 (w) | GE Healthcare | NA931V | |
| HRP conjugated | dAb | 1:1000 (w) | GE Healthcare | NA934V | |
| Other | |||||
| Propidium iodide (PI) | 3.3 μg/mL | Invitrogen/Molecular Probes | P-3566 | ||
| 4′-6-Diamidino-2-phenylindole (DAPI) | 1.0 μg/mL | Sigma-Aldrich |
For quantification of cell markers, 1.7− or 2.5-μm confocal optical section images were used. A minimum of four eyes (n = 4), each with 4 fields derived from different cryosections (total of 16 fields) of the central 30% of the retina were quantified. Commercial software (ImagePro Plus; Media Cybernetics, Silver Spring, MD) was used to measure and analyze the fluorescent signal-positive cells, using the same cutoff thresholds for a given marker. Quantification of marker-positive cells per field is expressed as mean ± SEM, with P < 0.05 considered statistically significant according to the Student’s t-test.
TUNEL assay was performed at P44, P70 to P72, or P90, using cryosections and the In Situ Cell Death Detection Kit (Fluorescein; Roche Diagnostics, Indianapolis, IN). The fluorescein-dUTP DNA labeling signals were captured with a microscope (E800; Nikon, Tokyo, Japan) equipped with a digital camera (SPOT II; Diagnostic Instruments).
Western Blot Analyses
Cell lysates of individual P90 retinas were extracted as previously described.31 For serial detection with different primary antibodies, nitrocellulose (NC) membranes were stripped by submerging in 50 mL of 100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-Cl (pH 6.7) at 50°C for 30 minutes. NC blots were washed twice for 10 minutes with PBS at room temperature and processed for another round of detection (Enhanced Chemiluminescence System; GE Healthcare, Piscataway, NJ).
Microarray Analysis
Total retinal RNA from four eyes injected with rAAV-CNTF virus at P23 and four noninjected contralateral eyes was extracted at P90 (RNeasy Protect Mini Kit; Qiagen Inc., Valencia, CA). Equal amounts of total RNA (2 μg) from each retina were processed for one round of linear amplification according to the instructions provided (RiboAmp RNA Amplification Kit; Arcturus Applied Genomics, Sunnyvale, CA). During amplification, a transcript labeling kit (BioArray High Yield RNA Transcript Labeling Kit; ENZO, Farmingdale, NY) was used in conjunction to produce Biotin-labeled single-stranded RNA probes. All RNA measurements were performed with a spectrophotometer (ND-1000; NanoDrop Technologies Inc., Wilmington, DE). The quality and integrity of RNA samples were evaluated by electrophoresis on 1.2% agarose-formaldehyde gels. Before hybridization, fractionation of each labeled probe was analyzed (2100 Bioanalyzer; Agilent Technologies, Foster City, CA). Each labeled RNA probe (10 μg) was individually hybridized (Mouse Genome 430–2.0 Arrays in a Gene Chip Hybridization Oven 640) followed by washes in a fluidics station (model 450; all from Affymetrix, Santa Clara, CA). Quadruplet arrays were scanned and data were acquired (Scanner 3000 and Gene Chip Operating Software 1.3; Affymetrix). Data analysis was then performed (ArrayAssist Analysis Software; Iobion/Stratagene, La Jolla, CA).
Results
CNTF Reduces Electroretinographic Responses
In previous work, the same rAAV-CNTF used in the present study resulted in reduced ERG amplitudes when injected subretinally between P23 and P25 and examined at P90.12 In the present study, of the 19 rds+/− mice carrying the P216L mutant transgene36 and injected subretinally, 18 showed significant depression of scotopic and photopic ERG responses (Table 2). The one mouse that had no recordable difference between the two eyes presumably had a failed subretinal injection and was not used to harvest retinas or for immunocytochemistry. Several mutant mice examined at P70 displayed similar reduced ERG amplitudes, but the scotopic a-waves were not as significantly reduced as the other ERG parameters (Table 2).
TABLE 2.
ERG Analysis
| % Reduction Compared with Control Eye* |
||||
|---|---|---|---|---|
| Age | n | Scotopic a-Wave | Scotopic b-Wave | Photopic b-Wave |
| P70–P72 | 4 | 16.4 ± 8.5 | 68.9 ± 8.0 | 70.8 ± 14.7 |
| P | NS | < 0.03 | < 0.02 | |
| P90 | 18 | 63.7 ± 9.4 | 81.3 ± 5.5 | 83.0 ± 4.3 |
| P | < 5 × 10−5 | < 5 × 10−8 | < 5 × 10−10 | |
Both eyes of mice were recorded simultaneously, and the differences between the two eyes were assessed using a two-tailed, paired Student’s t-test.
Mean ± SEM.
CNTF Sustains Rod but Not Cone Photoreceptor Opsin Expression
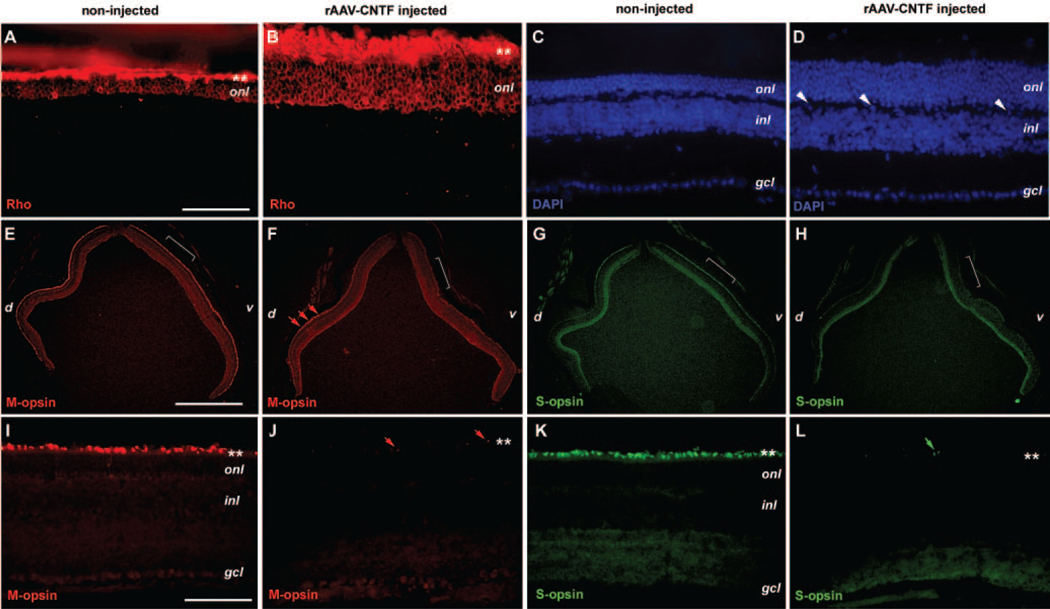
Subretinal delivery of rAAV expressing CNTF caused dramatic improvement of cell survival in rds retinas as indicated by the increased thickness of the outer nuclear layer (ONL).12 To investigate whether the surviving ONL cells maintained differentiated features of photoreceptors, we performed immunofluorescent staining for rod and cone opsins. At P90, the untreated control rds retinas had three to four rows of cell nuclei (Fig. 1C) in the ONL with rhodopsin (Rho4D242) immunostaining signals within the ONL as well as in the outer segments (Fig. 1A), indicating that the residual rod photoreceptor cells at this stage retained their rhodopsin expression. In P90 rds retinas treated with rAAV-CNTF at P23, the ONL contained seven to eight rows of cell nuclei (Fig. 1D), and rhodopsin immunostaining signals were detected in most of the ONL cells (Fig. 1B) with intense labeling of the inner and outer segments and aberrant perinuclear distribution in the ONL.
FIGURE 1.
Expression of photoreceptor opsins in the rds mutant retina treated with CNTF. Immunofluorescent images of P90 rds retinas treated with rAAV-CNTF at P23 (B, D, F, H, J, L) and untreated rds control retinas (A, C, E, G, I, K) labeled for rhodopsin (A, B), M-opsin (E, F, I, J), S-opsin (G, H, K, L), and 4′,6′-diamino-2-phenylindole (DAPI; C, D) are shown. (C) and (D) Corresponding sections to those shown in (A) and (B). (D, white arrowheads) Nuclei located in the outer plexiform layer. (E–H, brackets) Regions enlarged in (I–L), respectively. (F, J, red arrows) Residual M-opsin labeling; (L, green arrows) residual S-opsin signals. (A, B, I–L ✽) Position of photoreceptor inner and outer segments. d, dorsal; v, ventral; onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer. Scale bars: (A–D, I–L) 100 μm; (E–H) 1 mm.
In the untreated rds+/−P216L retinas, M-opsin immunostaining detected M-opsin-positive cells distributed in the dorsal and ventral retina (Figs. 1E, 1I). In contrast, S-opsin immunostaining of rds+/−P216L retinas showed a ventrally biased distribution of S-cone cells (Figs. 1G, 1K). These results were similar to the patterns of M-cone and S-cone photoreceptor cell distribution in the wild-type mouse retina,43 indicating that rds mutant retinas maintained cone photoreceptor cells as late as P90. In rAAV-CNTF treated rds+/−P216L retinas, however, the M-opsin positive cone cells were greatly reduced in the ventral retina, with only residual labeled cells detected in the dorsal retina (Figs. 1F, 1J). Similarly, rAAV-CNTF treated retinas were almost entirely devoid of S-opsin staining signals, suggesting the reduction of S-cone immunoreactivity (Figs. 1H, 1L). To examine further whether the CNTF treatment causes a reduction in the number of cone cells or altered expression of cone-specific genes, we also labeled P70 rds retinas with anti-cone arrestin antibody44 and fluorescence-conjugated peanut agglutinin (PNA). The untreated control rds retinas showed cone arrestin labeling in the outer segments, cell somata, and synapses, similar to that found in the wild-type retinas, whereas rAAV-CNTF-treated retinas displayed a reduced arrestin signal in the ONL and synaptic termini, but retained a low level of arrestin labeling in the outer segments (Supplementary Figs. S1A–S1D, online at http://www.iovs.org/cgi/content/full/48/3/1389/ DC1). The PNA labeling was also reduced, but was still detectable in rAAV-CNTF–treated retinas (Supplementary Figs. S1E–S1H).
These results suggest that, despite the photoreceptor cell degeneration, the rds+/−P216L retinas retained both rod and cone photoreceptor cells. Viral-mediated CNTF expression was effective in sustaining cells with rod photoreceptor features but resulted in suppression of cone-specific genes, including M-opsin, S-opsin, and cone arrestin.
CNTF Causes Activation and Dispersion of Müller Glial Cells
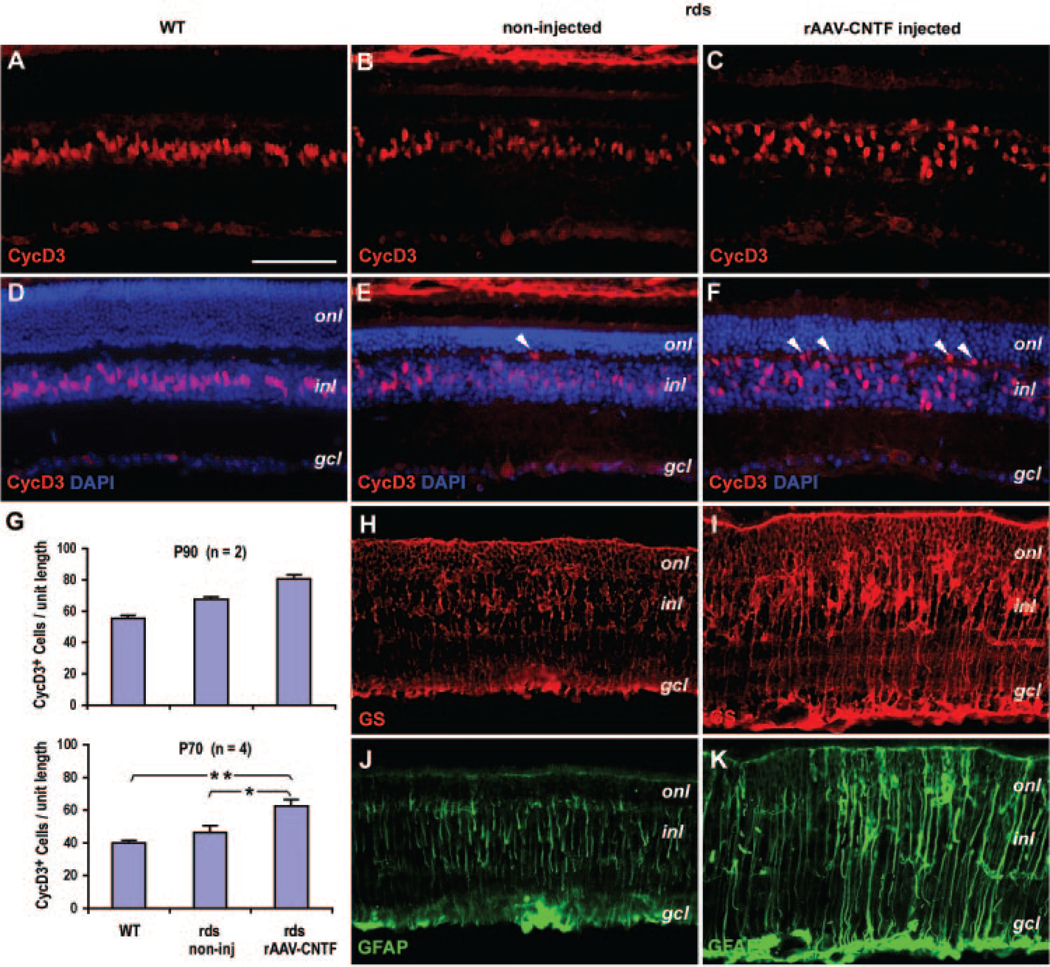
We next examined the influence of rAAV-mediated CNTF e pression on the Müller glial cells, which have been shown to contain phosphorylated STAT and ERK protein on transient CNTF stimulation in vivo.29,30 In the mature wild-type retina, immunostaining by a Müller glial marker cyclin D345 showed that Müller glial nuclei occupied the central stratum of the inner nuclear layer (INL; Figs. 2A, 2D). Compared with the wild-type retina, the P90 untreated rds+/−P216L retinas contained cyclin D3-positive Müller glial nuclei in a slightly dispersed pattern within the INL, with occasional nuclei ectopically located near the outer plexiform layer (OPL; Figs. 2B, 2E).
FIGURE 2.
Influence of CNTF on Müller glial cells in the rds retina. Immunofluorescent images of adult wild-type (WT) retina (A, D), P90 rds retinas treated with rAAV-CNTF at P23 (C, F, I, K), and the untreated rds control (B, E, H, J) labeled for CycD3 (A–C), CycD3 and 4′,6′-dia-mino-2-phenylindole (DAPI; D–F), GS (H, I), and GFAP (J, K) are shown. (E, F, white arrowheads) Ectopic cell nuclei residing in the outer plexiform layer. (G) Quantification of CycD3-positive cells at P90 (top, n = 2, 10 fields each) per unit length of retina (250 μm, at 14-μm thickness) and at P70 (bottom, n = 4, 16 fields each) per unit length of retina (317 μm, 1.7-μm-thick confocal optical sections). *Statistically significant sample pairs: P < 0.02 between untreated and CNTF-treated rds retinas, and P < 0.001 between wild-type (WT) and CNTF-treated rds retinas. onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer. Scale bar, 100 μm.
In P90 rAAV-CNTF treated rds+/−P216L retinas, cyclin D3-pos itive cell nuclei were broadly dispersed throughout the entire INL, with many positioned within the OPL, which is normally devoid of cell nuclei (Figs. 2C, 2F). Furthermore, immunostaining with Müller glial markers glutamine synthetase (GS)45 and glial fibrillary acidic protein (GFAP)46 revealed a low degree of Müller activation in untreated and a high degree of Müller activation in rAAV-CNTF treated retinas, as indicated by significantly expanded and swollen Müller glial processes throughout all laminar layers of the retina (Figs. 2H–K). In general, the rAAV-CNTF treated rds retinas showed an increased thickness in the ONL and INL compared with the untreated rds retina (Figs. 2D–F).
We also counted the numbers of cyclin D3-positive cells in the central region of the P90 retinas. Compared with the wild-type retinas, untreated and rAAV-CNTF treated rds retinas contained an average increase of 22% and 46%, respectively (Fig. 2G, n = 2, total 10 fields per retina). To obtain a more precise quantification, confocal images of noninjected wild-type retinas and P70 rds retinas (data not shown) injected with rAAV-CNTF at P23 were also analyzed (Fig. 2G, n = 4, 16 fields per retina). The results showed that cyclin D3-positive cells in rAAV-CNTF treated retinas (62.6 ± 3.7 per frame) had increased 56.5% (P = 0.001) over wild-type retinas (39.9 ± 1.7 per frame) and 35.6% (P = 0.027) over untreated rds retinas (46.2 ± 4.3 per frame).
These results suggest that the rds retina, while undergoing slow photoreceptor degeneration, harbors reactive Müller glia, and rAAV-CNTF treatment enhances Müller glial activation and mobilization and increases the number of Müller glial marker–positive cells.
CNTF Enhances Chx10-Positive Cell Population
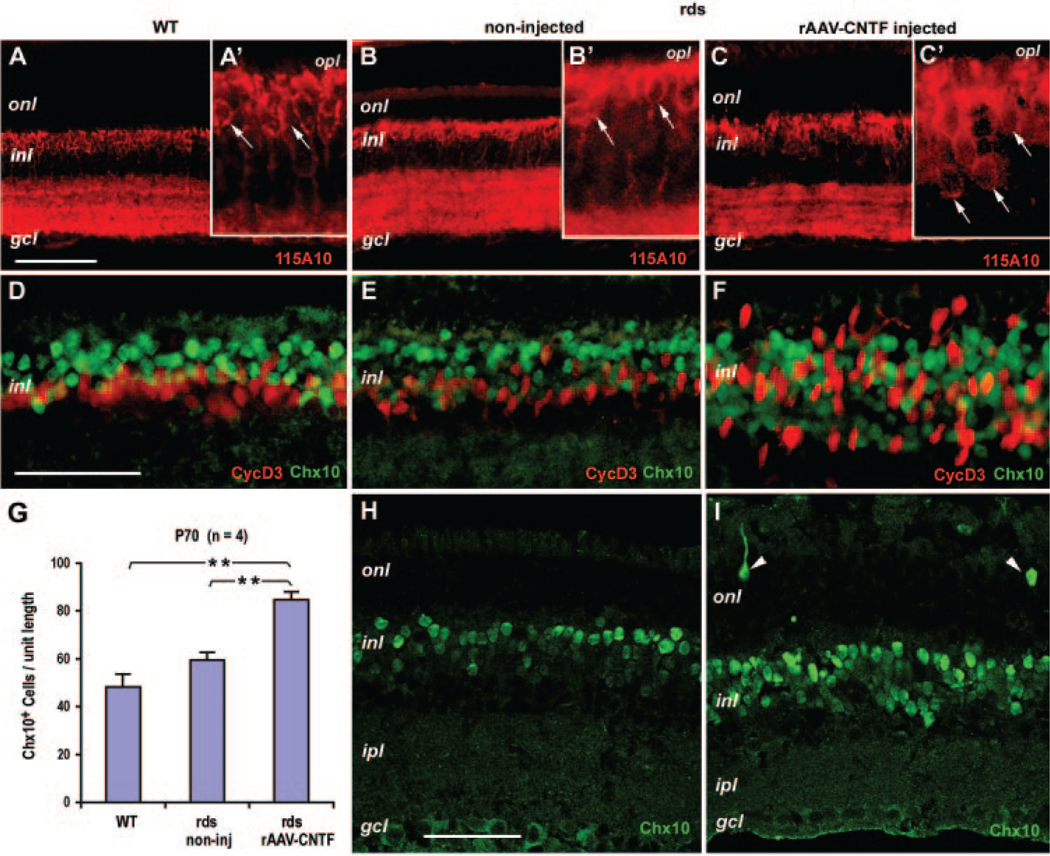
The reduction in ERG b-wave amplitudes caused by rAAV-CNTF injection suggests that CNTF affects cells in the INL. Immunostaining with the bipolar cell marker 115A1047 revealed that rAAV-CNTF treatment resulted in a slightly expanded zone occupied by 115A10-labeled cell bodies within the INL (Figs. 3A–C). We next analyzed the labeling pattern of the homeobox protein Chx10, which is expressed by retinal progenitor cells during development and by bipolar neurons in the mature retina.48 Chx10 staining patterns were similar between the wild-type and untreated rds+/−P216L retinas (Figs. 3D, 3E), whereas rAAV-CNTF treatment resulted in a wide dispersion of Chx10-positive cells in the INL (Fig. 3F). Since, under certain conditions, Müller glia may re-enter the proliferative cell cycle and generate new neurons,49,50 we tested whether the dispersed Chx10-positive cells co-label with Müller cell markers. Costaining of the two nuclear antigens Chx10 and cyclin D3 showed minimal overlapping but extensive intermingling between the two cell populations in rAAV-CNTF treated rds+/−P216L retinas (Fig. 3F). To determine whether there was also an increase in the number of Chx10-positive cells, we performed confocal imaging analyses of P70 to P72 rds retinas, which showed Chx10 expression patterns similar to those found at P90 (Figs. 3H, 3I). Quantification of the cells in the central retina (Fig. 3G, n = 4, 16 fields per retina) showed that Chx10-positive cells in rAAV-CNTF treated retinas (84.6 ± 3.4 per frame) had increased 75.2% (P = 0.001) over wild-type retinas (48.3 ± 5.3 per frame) and 42.2% (P = 0.001) over untreated rds retinas (59.5 ± 3.2 per frame), respectively. Interestingly, occasional ectopic Chx10-positive cells were detected in the ONL of rAAV-CNTF–treated retinas at this stage (Fig. 3I).
FIGURE 3.
Influence of CNTF on bipolar cells in the rds retina. Immunofluorescent images of adult wild-type (WT) retinas (A, D), P90 (B, C, E, F) and P72 (H, I) rds retinas treated with rAAV-CNTF at P23 (C, F, I) and the untreated rds controls (B, E, H) labeled for 115A10 (A–C; A′–C′: higher magnifications of A–C), Chx10 and CycD3 (D–F), and Chx10 (H, I) are shown. (H, I) Examples of the confocal optical sections used for the quantification of Chx10 in (G). (A′–C′, white arrows) Cell bodies labeled by 115A10. (I, white arrowheads) Ectopic Chx10-positive cells in the ONL. (G) Quantification of Chx10-positive cells at P70 (n = 4, 16 fields each) per unit length (230 μm) of retina (1.7-μm-thick confocal optical sections). *Statistically significant sample pairs: P < 0.001 between untreated and CNTF-treated rds retinas, and between wild-type (WT) and CNTF-treated rds retinas. onl, outer nuclear layer; opl, outer plexiform layer; inl, inner nuclear layer; ipl, inner plexiform layer; gcl, ganglion cell layer. Scale bars: (A–C) 100 μm; (D–F, H, I) 50 μm; (A′–C′) magnification of (A–C) ×3.
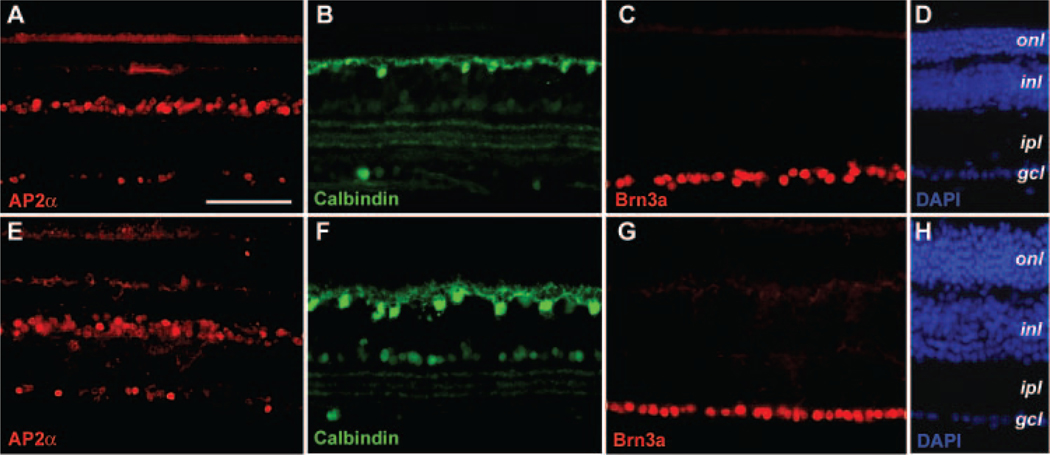
We also evaluated whether CNTF influences other retinal cell types. Immunostaining by an amacrine cell marker AP2α51 detected only a slight disorganization of the amacrine cells in the rAAV-CNTF–treated retinas (Figs. 4A, 4E). Labeling with calbindin revealed more robust immunostaining of cell somata and processes of horizontal cells in the treated retinas (Figs. 4B, 4F). No apparent change in the ganglion cells was detected at P90 with an antibody against Brn3a (Figs. 4C, 4G), a POU domain nuclear protein expressed by differentiated retinal ganglion cells.52 Quantitative analyses of confocal images derived from P70 to P72 rds retinas detected no significant change in the number of Brn3a labeled ganglion cells (n = 4, data not shown), confirming the immunostaining results of the P90 rds retinas.
FIGURE 4.
Effects of CNTF on other retinal cell types in the rds retina. Immunofluorescent images of P90 rds retinal sections treated with rAAV-CNTF at P23 (E–H) and the untreated control retinas (A–D) labeled for amacrine cell marker AP2α (A, E), horizontal cell marker calbindin (B, F), ganglion cell marker Brn3a (C, G), and 4′,6′-diamino-2-phenylindole (DAPI; D, H) are shown. Abbreviations as in Figure 3. Scale bar, (A) 100 μm.
Therefore, in addition to mobilization and activation of the Müller glia, persistent CNTF expression caused an increase in both the number and dispersion of Chx10-positive cells accompanied by only minor changes of other INL interneurons.
Persistent STAT and ERK Activation by rAAV-CNTF
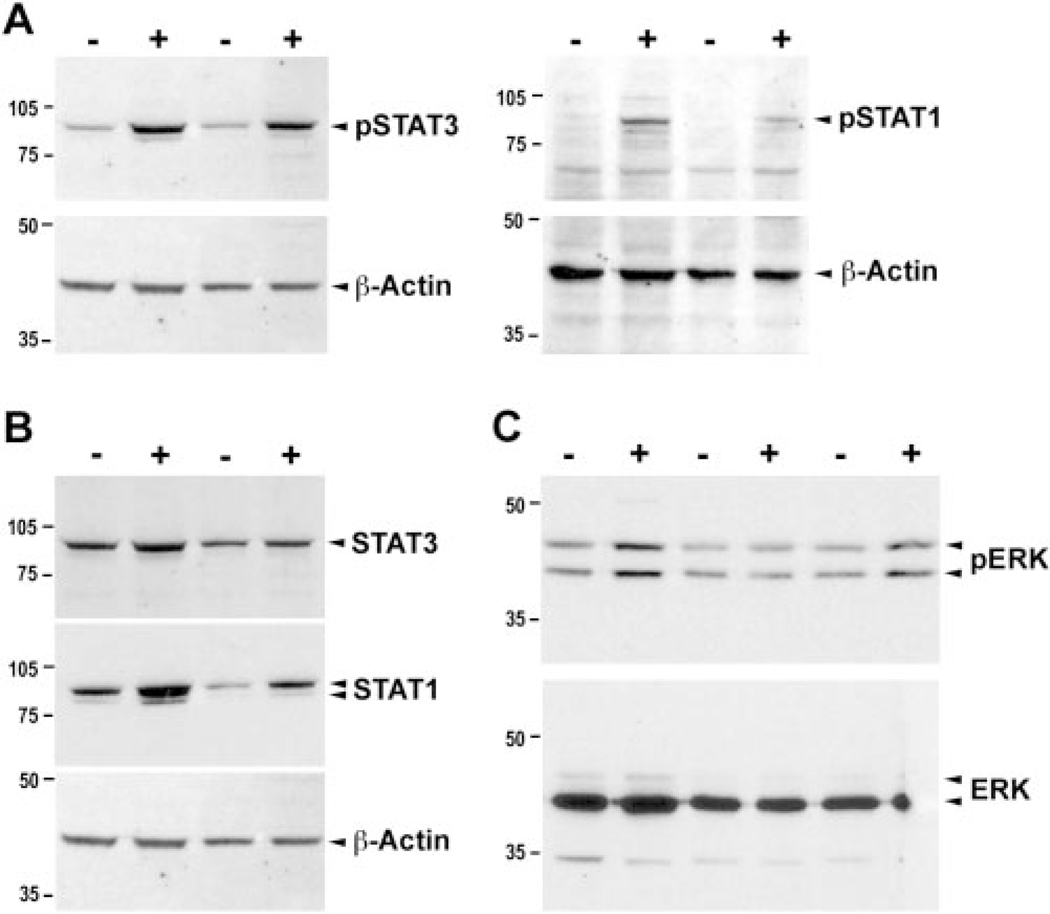
Previous studies have shown that intravitreal injection of CNTF protein into the adult rodent eye causes enhanced but transient STAT and ERK activation in retinal ganglion cells and Müller glia.29,30 To determine whether constitutive CNTF expression causes persistent signaling pathway activation, we examined the phosphorylated status of STAT and ERK proteins. Western blot analyses showed elevated phospho-STAT3 and phospho-STAT1 in rAAV-CNTF treated rds+/−P216L retinas compared with untreated control retinas (Fig. 5A). In addition, the total cellular STAT3 and STAT1 protein levels were increased (Fig. 5B). Furthermore, Western blots detected an increase of phospho-ERK1 and ERK2 (Fig. 5C).
FIGURE 5.
Effects of CNTF on activation and expression of STATs and ERK proteins. Western blot analysis of cell extracts derived from P90 rAAV-CNTF treated (+) and untreated control rds retinas (−) are shown. The levels of phospho-STAT1 and phospho-STAT3 (A), the levels of total STAT1 and STAT3 (B), and the levels of phosphorylated p42/44 ERK (C) were all elevated in treated compared with untreated control retinas. Bottom panels show β-actin or total ERK as protein loading controls.
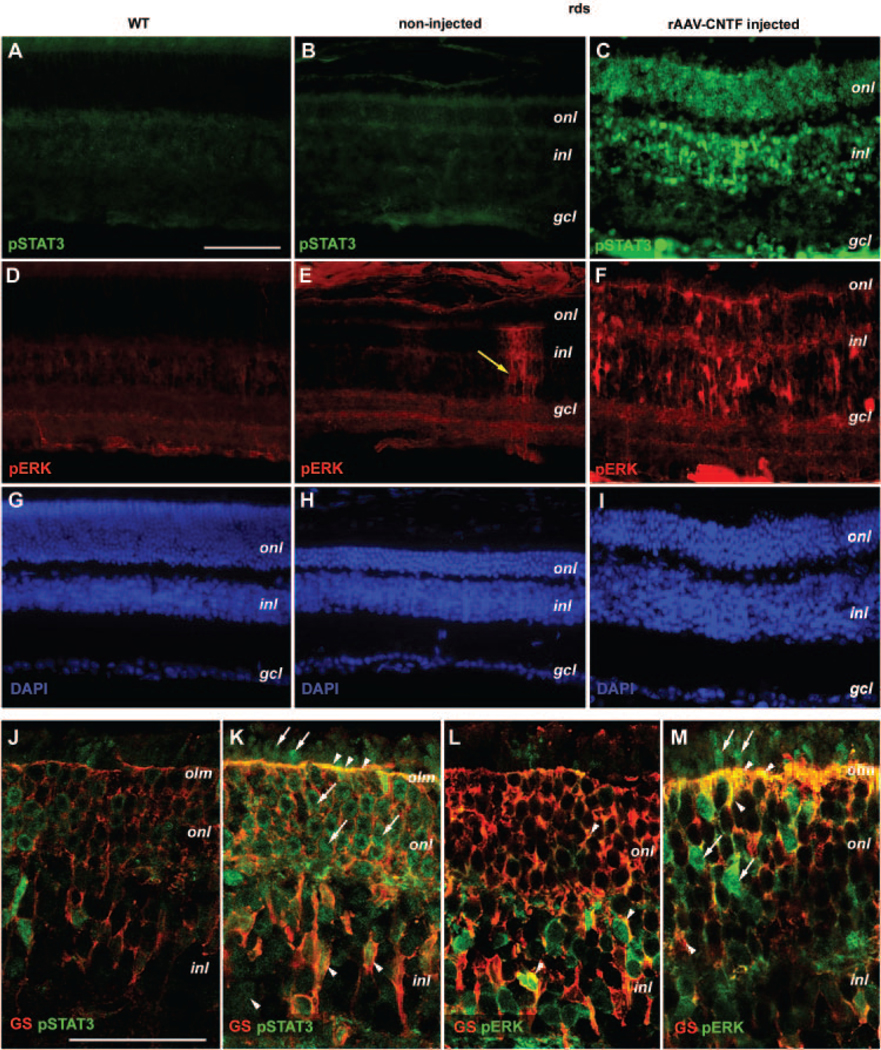
In P90 wild-type and untreated rds retinas, phospho-STAT3 immunolabeling did not detect significant levels of signals (Figs. 6A, 6B). However, in contrast to the wild-type retina, anti-phospho-ERK labeling detected dispersed clusters of cells as radial columns in untreated rds+/−P126L retinas (Figs. 6D, 6E). This pattern of labeling was also observed for phospho-ERK and phospho-STAT3 at P70 in untreated rds mutant (data not shown), probably representing spontaneous activation of ERK and STAT3 in a subset of Müller glia. Consistent with Western blot analyses, high levels of phospho-STAT3 and phospho-ERK labeling were detected in rAAV-CNTF-treated P90 retinas in all three cellular layers (Figs. 6C, 6F).
FIGURE 6.
Distribution of CNTF-induced phospho-STAT3 and phospho-ERK protein in the rds retina. Immunofluorescent images (A–I) of adult wild-type retina (A, D, G), P90 rds retinas treated with rAAV-CNTF (C, F, I), and the untreated rds control retinas (B, E, H) colabeled for phospho-STAT3 (A–C), phospho-ERK1/2 (D–F), and 4′,6′-diamino-2-phenylindole (DAPI; G–I) are shown. Merged confocal images (J–M; 0.8 μm) of P70 rds retinas treated with rAAV-CNTF (K, M) and the untreated control retinas (J, L) colabeled with GS and pSTAT3 (J, K) or pERK (L, M) are shown. (E, yellow arrow) Isolated phospho-ERK positive column in an untreated rds retina. (K–M) Arrowheads: colabeling of GS and pSTAT3 or pERK; arrows: pSTAT3 or pERK signals alone. Abbreviations as in Figure 3. olm, outer limiting membrane. Scale bar: (A–I) 100 μm; (J–M) 50 μm.
To delineate which cell-type contained activated STAT3 and ERK in rAAV-CNTF-treated retinas, we performed confocal imaging of co-labeled P70 to P72 rds retinas. In noninjected retinas, a low level of phospho-STAT3 signal was detected (Fig. 6J). Consistent with results obtained from P90 retinas, rAAV-CNTF treatment greatly enhanced the levels of phospho-STAT3 in all three cellular layers as well as in the plexiform layers at P70 (Fig. 6K). Merging of 0.8-μm optical sections demonstrated extensive colocalization of the Müller marker GS and phospho-STAT3 in Müller cell cytoplasm and nuclei within the INL and in Müller glial processes surrounding the photoreceptor cell bodies and terminating at the outer limiting membrane (OLM; Fig. 6K). In addition, phospho-STAT3 labeling signals were also distributed in photoreceptor cells including the inner and outer segments (Fig. 6K). The phospho-ERK signals were mostly detected in the INL and IPL in untreated rds retinas, especially in the Müller glial cell bodies labeled positive for GS (Fig. 6L). CNTF stimulation resulted in elevated ERK phosphorylation among Müller glia, as indicated by strong labeling of their processes in the ONL and OLM (Fig. 6M). In addition, a subset of cells in the ONL contained high levels of phospho-ERK (Fig. 6M).
Together, these results demonstrate a persistent activation of cytokine signaling caused by rAAV-mediated CNTF expression The nuclei and processes of Müller glia contained phosphorylated STAT3 and ERK proteins. Most of the photoreceptor cells also contained phospho-STAT3, whereas a subset of ONL cells accumulated high levels of phospho-ERK.
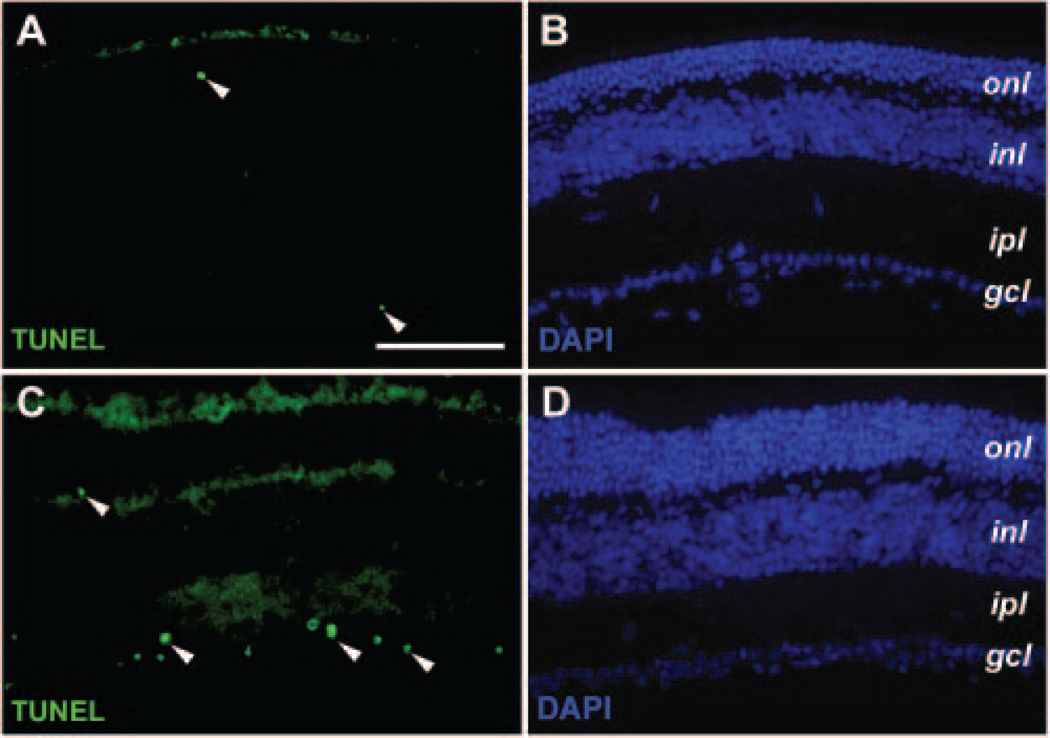
Effects of CNTF on Retinal Cell Death
The increased thickness of the ONL in rAAV-CNTF treated rds retina implied a reduction of photoreceptor cell death. Cell apoptosis TUNEL assays revealed very low levels of cell death in the ONL of treated and untreated rds retinas at P44, P70, and P90 (Figs. 7A–D, and data not shown). Instead, increased apoptosis was detected in the ganglion cell layer of rAAV-CNTF–treated P90 samples (Figs. 7A, 7C). However, no significant cell death was detected in the ganglion cell layer at P44 or P70.
FIGURE 7.
Effects of rAAV-CNTF treatment on retinal cell death. Immunofluorescent images of P90 rds retinas treated with rAAV-CNTF at P23 (C, D) and untreated control retinas (A, B) labeled by TUNEL (A, C) and 4′,6′-diamino-2-phenylindole (DAPI; B, D) are shown. (A, C, arrowheads) Cells undergoing apoptosis. Increased apoptosis was detected in the ganglion cell layers of the treated P90 rds retinas (C). Abbreviations as in Figure 3. Scale bar, 100 μm.
Altered Gene Expression at the Transcriptional Level Due to CNTF
To obtain a more comprehensive assessment of the influence of chronic CNTF expression, we performed microarray analyses to examine changes of gene transcription profiles in the retina. Probes derived from RNAs of untreated control and rAAV-CNTF treated rds+/−P216L retinas (n = 4 each) were individually hybridized to gene microarrays (Mouse Genome 430 2.0 Arrays; Affymetrix), which contain 45,000 probe sets corresponding to 39,000 transcripts from 34,000 well-characterized mouse genes. Microarray data showed that compared with untreated rds retinas, CNTF consistently caused altered expression of a large number of genes. In at least three of four independent trials, 119 genes showed more than a 25% reduction in their transcript levels, whereas 152 genes showed more than a 25% increase in their transcript levels.
Table 3 summarizes 50 selected known genes that showed an increase or decrease in at least three of four individual trials. Among these, several genes involved in phototransduction were downregulated, including S-opsin, cone transducin α-sub-unit, rod transducin β1-subunit, and rod phosphodiesterase-β. In addition, genes implicated in photoreceptor function and diseases such as the crumbs homologue and retinitis pigmentosa-9 homologue also showed reduced transcript levels. Consistent with the observed nuclear morphologic changes in the rAAV-CNTF treated rds retinas, altered expression of genes that function in chromatin remodeling and transcription activation such as nucleolin and histone deacetylase were observed. Furthermore, the homeobox protein Crx required for photoreceptor differentiation and maintenance53–55 and the leucine zipper protein Nrl required for rod specification56 were decreased. As expected, GFAP transcription was enhanced in rAAV-CNTF treated rds retinas. In agreement with the rescuing effects of CNTF treatment, we also detected changes in transcript levels toward blocking cell death and promoting the cell cycle. Moreover, CNTF treatment affected the expression of a considerable number of signaling molecules. Among these, STAT1, STAT3, and ERK1 were augmented, whereas Jak1 was suppressed.
TABLE 3.
Representative Gene Expression Changes in rAAV-CNTF-Treated rds Retina*
| Potential or Known Function |
Gene Name | GenBank Acc. Number |
Ratios (Mean ± SEM)† (rAAV-CNTF inj./Non-inj.) |
|---|---|---|---|
| Phototransduction | S-opsin | NM 007538 | 0.40 ± 0.11 |
| Cone transducin alpha-subunit (Gnat2) | NM 008141 | 0.51 ± 0.01 | |
| Rod phosphodiesterase b (pdeb) | NM 008806 | 0.67 ± 0.09 | |
| Rod transducin beta1-subunit (Gnb1) | NM 008142 | 0.39 ± 0.13 | |
| Guanylate cyclase activator 1α (Guca1a) | NM 008189 | 0.47 ± 0.12 | |
| Retinal structure and Function | Crumbs homolog 1 (crb1) | NM 133239 | 0.66 ± 0.07 |
| Elongation of long chain fatty acids, ELOVL6 | BC 100576 | 0.58 ± 0.08 | |
| Synapsin II (Syn2) | NM 013680 | 0.66 ± 0.02 | |
| Retinitis pigmentosa 9 homolog (RP9) | BC 048480 | 0.55 ± 0.07 | |
| Retinol dehydrogenase 11 (Rdh11) | NM 021557 | 0.60 ± 0.001 | |
| Interphotoreceptor matrix proteoglycan 1 (Impg1) | NM 022016 | 1.47 ± 0.10 | |
| Nestin | NM 016701 | 1.71 ± 0.13 | |
| GFAP | NM 010277 | 2.60 ± 0.98 | |
| Chromatin | Nucleolin (Ncl) | NM 010880 | 0.64 ± 0.05 |
| Retinoblastoma binding protein 9 (Rbbp9) | NM 015754 | 0.59 ± 0.10 | |
| Early growth response 1 (egr1) | NM 007913 | 0.45 ± 0.03 | |
| Histone deacetylase 11 (Hdac11) | NM 144919 | 1.28 ± 0.02 | |
| Transcription | Cone rod homeobox gene (Crx) | NM 007770 | 0.71 ± 0.08 |
| Neural retina leucine zipper gene (Nrl) | NM 008736 | 0.66 ± 0.08 | |
| Trans-acting transcription factor 1 (SP1) | NM 013672 | 0.59 ± 0.02 | |
| Kruppel-like factor 4 (Klf4) | NM 010637 | 0.48 ± 0.08 | |
| Interleukine enhancer binding factor 2 (Ilf2) | NM 026374 | 0.53 ± 0.07 | |
| Hairy/enhancer-of-split related with YRPW2 (Hey2) | NM 013904 | 1.77 ± 0.07 | |
| Cell death/survival | Programmed cell death protein 7 (pdcd7) | BC 022772 | 0.66 ± 0.07 |
| Programmed cell death protein 4 (pdcd4) | BC 055739 | 0.61 ± 0.04 | |
| Bcl2-like 10 (Bcl2110) | NM 013479 | 1.54 ± 0.06 | |
| Cell cycle | Annexin A7 (Anxa7) | NM 009674 | 0.62 ± 0.08 |
| Transducer of ERBB2, 2 (Tob2) | NM 020507 | 0.66 ± 0.04 | |
| Wee 1 homolog | NM 009516 | 1.68 ± 0.20 | |
| G1 to phase transition 1 (Gspt1) | NM 146066 | 1.57 ± 0.12 | |
| Neuroblastoma myc-related oncogene 1 (Nmyc) | NM 008709 | 1.56 ± 0.10 | |
| Cyclin-dependent kinase-like 3 (Cdkl3) | NM 153785 | 1.35 ± 0.07 | |
| Signal transduction | Janus kinase 1 (Jak1) | NM 146145 | 0.65 ± 0.03 |
| Ephrin B2 (Efnb2) | NM 010111 | 0.48 ± 0.07 | |
| SH2 domain binding protein 1 (Sh2bp1) | BC 071247 | 0.56 ± 0.06 | |
| Cellular repressor of E1A-stimulated genes (Creg1) | NM 011804 | 0.67 ± 0.06 | |
| Protein tyr phosphatase, receptor type, E (Ptpre) | NM 011212 | 0.59 ± 0.03 | |
| Protein tyr phosphatase, non-receptor 21 (Ptpn21) | NM 011877 | 0.69 ± 0.03 | |
| STAT1 | NM 009283 | 1.65 ± 0.14 | |
| Smoothened (Smo) | BC 048091 | 0.65 ± 0.02 | |
| STAT3 | NM 011486 | 1.60 ± 0.40 | |
| MAPK1 | NM 011949 | 1.78 ± 0.25 | |
| MAPK-interacting ser/threo kinase 1 (Mknk1) | BC 021369 | 1.48 ± 0.12 | |
| Serum/glucocorticoid regulated kinase 2 (Sgk2) | NM 013731 | 1.47 ± 0.14 | |
| Neuropilin 1 (Nrp1) | NM 008737 | 1.52 ± 0.17 | |
| Growth hormone receptor (Ghr) | NM 010284 | 1.65 ± 0.36 | |
| Platelet derived growth factor, alpha (Pdgfa) | NM 008808 | 1.66 ± 0.22 | |
| Chemokine (C-X-C motif) ligand 13 (Cxcl13 | NM 018866 | 2.84 ± 0.37 | |
| Immunoresponse | Complement component factor I (Cfi) | NM 007686 | 1.42 ± 0.06 |
| Complement component 4 (C4) | M11789 | 1.66 ± 0.66 |
A representative 50 genes with transcript levels altered by more than 25% after rAAV-CNTF treatment are shown. Genes with decreased transcripts are listed before genes with increased transcripts in each functional category.
The ratios of rAAV-CNTF injected rds retina and control noninjected rds retina represent values from three or four independent array experiments (mean ± SEM).
Discussion
In this study, we investigated the molecular and cellular changes associated with viral mediated CNTF expression in a mouse model of RP caused by a mutation in the rds/peripherin gene. Sustained CNTF expression induced a persistent elevation of both the STAT and ERK signaling pathways in the mutant retina. Despite the protection of the photoreceptor cells in the ONL from the effects of the mutant transgene, CNTF caused significant alteration of cellular phenotypes and reorganization of several retinal cell types. Moreover, CNTF treatment resulted in altered levels of transcripts of a large number of genes in the retina.
Several studies to date have shown that CNTF treatment affects normal and mutant retinal functions. Expression of CNTF by rAAV rescues photoreceptors from cell death in the dominant peripherin/rds+/−P216L mutant mouse12 and dominant rhodopsin mutant rats,38 but causes attenuated ERG amplitudes. Furthermore, delivery of CNTF in combination with wild-type peripherin in Prph2 (Rd2/Rd2) mice negates the effect of gene-replacement therapy.13 Moreover, a cell-based delivery study in normal rabbits showed that the cone ERG is diminished at higher doses of CNTF.39 Results of immunocytochemical characterization presented here indicate that most of the surviving ONL cells in the rds retina have retained the rod photoreceptor identity with rAAV-CNTF treatment. The rescued rods show robust rhodopsin expression by immunocytochemistry, albeit with an aberrant cellular distribution compared with the wild-type. In contrast to the rod opsin, rAAV-CNTF–transduced rds retinas display a dramatic reduction of both the short wavelength and medium/long wavelength opsins. This reduction of cone opsin immunoreactivity most likely reflects altered gene expression within surviving cone photoreceptors rather than cone-specific cell death (see note added in proof) since the untreated rds retinas at an equivalent age contain an apparently normal distribution of cones, and CNTF-treated retinas still have measurable photopic ERG responses, although the amplitudes are reduced compared with untreated retinas (Table 2; Bok et al.12). Furthermore, the rAAV-CNTF treated retinas showed reduced but detectable levels of cone arrestin as well as PNA antigen associated with the cones. In the developing mouse and rat retinas, CNTF can strongly suppress rhodopsin expression in postmitotic photoreceptor precursor cells. However, once differentiating rod photoreceptor cells reach the stage of rhodopsin expression, they are no longer responsive to CNTF inhibition.3 The lack of CNTF suppression of rhodopsin expression in the rds mutant indicates that even persistent CNTF at high levels does not significantly alter the identity of mature rod photoreceptors, consistent with the observed responses of rods during differentiation.3 The suppression of the rod ERG in rAAV-CNTF treated rds retinas (Table 2; Bok et al.12) may be attributable to abnormal expression of other phototransduction genes expressed by rods. In contrast, specific effects of CNTF on S- and M-opsin expression in differentiating mammalian retinas have not been reported. In the chicken retina, CNTF has been shown to promote cone opsin expression in a small fraction of developing photoreceptor cells in vitro.1,57 The strong inhibitory effect of virally mediated CNTF expression on both cone opsins in the mature retina suggests that perhaps the established cone photoreceptor programs are more susceptible to cytokine influence, at least in the rds mutant mouse model. In fact, even in normal rats, the intravitreal injection of CNTF and the subretinal injection of rAAV-CNTF produce a greater reduction in photopic than in scotopic ERG responses, and these rats show concomitant reduction in visual performance in behavioral tasks in photopic light levels (McGill TJ et al. IOVS 2006; 47:ARVO E-Abstract 4815).
Major effects of persistent CNTF expression in the rds mutant retina also include the cellular alteration and reorganization of the INL, where the cell body of retinal interneurons and Müller glia reside. It has been shown previously that Müller glia become reactive in response to mechanical injury, retinal degeneration, and stimulation by growth factors.46,58 In the absence of exogenous CNTF, the mutant rds retinas show a mild Müller gliosis, as indicated by increased GFAP expression compared with the wild-type retinas. In contrast, rAAV-CNTF–treated retinas exhibit high levels of GFAP and glutamine synthetase labeling in the activated Müller cell body and processes. Intriguingly, labeling by cyclin D3 also revealed an increased number of Müller glial cells dispersed throughout the INL. Another INL retinal cell type greatly influenced by constitutive CNTF expression appears to be bipolar cells. Similar to its effect on cyclin D3–positive cells, CNTF causes an increase in Chx10-positive cells as well as their dispersion within the INL, and even into the ONL. The disorganization and repositioning of the Müller glia and bipolar cells within rAAV-CNTF–treated rds retinas are striking and may explain the reduced ERG b-waves originating from the INL (Table 2, Bok et al.12). The mechanism(s) underlying CNTF-dependent increases of Müller and bipolar marker–positive cells is currently not understood. Of interest, CNTF has been shown to enhance bipolar cell marker expression,3,6 stimulate proliferation of the late retinal progenitors,7,58 and promote Müller gliogenesis in the postnatal developing rodent retina in vitro.7 However, in transgenic mice, constitutive expression of leukemia inhibitory factor (LIF), a CNTF family of cytokine, does not seem to increase the proportion of bipolar and Müller cells at the expense of rod photoreceptors.59 Accumulating evidence to date suggests that in vertebrate retinas, Müller glia have the potential to become a source of neural stem cells, which can be triggered to reenter the cell cycle and generate new neurons when the retina is injured or is challenged by certain growth factors.48,49 It is plausible that CNTF or other growth factors released under the influence of CNTF can potentiate endogenous retinal stem cells. However, the slow onset of CNTF expression from the current AAV vector12 renders the detection of proliferative events difficult. In addition, current data cannot rule out the possibility of retinal cell transdifferentiation.
Sustained CNTF expression does not alter the normal laminar distribution of amacrine and horizontal cells within the INL. Despite elevated cell death in the ganglion cell layer at P90 in CNTF-treated retinas, we did not detect statistically significant changes in the number of ganglion cells in P70 rds retinas. Since Brn3a is an authentic ganglion cell marker, it is possible that the apoptotic cells seen in the ganglion cell layer were displaced amacrine cells or newly generated cells without proper connections. A recent study has shown that CNTF did not prevent ganglion cell death in a dog model for RP.60 Therefore, whether CNTF is neuroprotective for ganglion cells under all retinal degenerative conditions remains to be further determined.
In the wild-type mature retina, phospho-STAT3 and phospho-ERK1/2 are at nearly undetectable levels by immunocytochemistry.29,31 In the untreated rds mutant retina, antibodies against phospho-ERK and phospho-STAT3 occasionally labeled radial columns that resembled the morphology of a few clustered Müller glia. Such higher than basal level activation of signaling molecules is probably due to the release of endogenous growth factors in the rds retinas. In the CNTF-treated rds retinas, high levels of phospho-STAT3 and phospho-ERK are present throughout the retina. These distribution patterns of activated signaling molecules are very distinct from the transient STAT and ERK phosphorylation reported in the retinal ganglion cells and Müller glia after a single intravitreal CNTF injection.29 Furthermore, both transient and persistent CNTF expression causes not only the phosphorylation of STAT1 and STAT3, but also the elevation of total STATs at the RNA and protein levels. Cytokine signaling is normally subjected to rapid negative feedback controls, in part mediated by the cytokine induced Jak-STAT inhibitor SOCS (suppressors of cytokine signaling) proteins.61 The high levels of signaling molecules present in the rAAV-treated retinas perhaps reflect the renewable source of the CNTF ligand, which signals constitutively despite the induction of the negative inhibitors.
One important question regarding the mechanism of CNTF-mediated neural protection is whether CNTF directly targets the photoreceptor cells in the mature retina, or acts through intermediate factors. We have previously demonstrated that during development, CNTF directly triggers rapid STAT3 phosphorylation in The suppression of rhodopsin photoreceptor precursors.31 To date, several published studies have investigated the presence of CNTFRα in the ONL of various vertebrate retinas.62–66
However, the results have been inconsistent. We show that rAAV-CNTF treatment leads to elevated phospho-STAT3 and phospho-ERK in the ONL among other locations. Phospho-ERK is predominantly distributed in Müller glia and a subset of ONL cells, whereas phospho-STAT3 is localized not only in Müller glial cell bodies and processes but also in most of the photoreceptor cells in the ONL. Although we can conclude that vector-mediated CNTF expression causes accumulation of phospho-STAT3 and phospho-ERK among ONL cells, the presence of phosphorylated signaling proteins in the ONL of the rds retina does not necessarily confirm that mature photoreceptor cells respond directly to CNTF. The involvement of intermediary growth factors in triggering signal transduction in photoreceptors cannot be excluded at this time.
The cellular phenotypes in the rds retina exposed to chronic CNTF treatment are likely to be downstream events resulting from elevated signal transduction and altered gene expression. Indeed, our genomewide survey using mouse gene microarrays allowed us to detect a large number of genes with transcript levels affected by the long-term CNTF treatment. The numbers of genes affected are likely to be underestimated under the experimental conditions, since only those showing consistent changes in at least three of four independent trials are included. Even though the thresholds used (i.e., transcriptratios of <0.75 and >1.25), are not overtly stringent, the detected up- and downregulations are likely to be statistically significant, because each known mouse gene (i.e., probe set) is represented by 8 to 16 distinct hybridization data points on each array. Moreover, immunocytochemistry and Western blots, as well as semiquantitative PCRs (data not shown) have confirmed the expression status of a number of genes. Of interest, after constitutive CNTF exposure, genes associated with both rod and cone phototransduction or structures were reduced, correlating with the observed decrease in both rod and cone ERGs. The downregulation of Crx and Nrl is consistent with that observed in LIF-overexpressing mice67 and may be the underlying cause for changes seen in numerous photoreceptor-specific genes. Our results also agree with a recent study in normal and rod– cone degeneration canine models in which CNTF protected degenerating photoreceptors but reduced expression of some phototransduction proteins, as well as leading to an increased thickness of the inner retina.60 The changes in genes associated with chromatin remodeling and transcription activation in part reflect the altered nuclear morphology found in the ONL of several species after CNTF treatment. Moreover, CNTF also alters expression profiles of genes involved in the cell cycle and apoptosis, and a large number of signaling molecules, thus suggesting a broad impact of persistent cytokine signaling on retinal transcription and, consequently, on cellular function.
In conclusion, we have demonstrated that sustained, high-level CNTF treatment causes a multitude of cellular and molecular changes in a mouse model of retinitis pigmentosa. These results suggest that the mature retina is plastic and susceptible to cytokine influences. Therefore, when considering human application, careful regulation of the dosage and duration of CNTF treatment may be needed to achieve pro-longed optimal therapeutic efficacy.
Supplementary Material
Acknowledgment
The authors thank Douglas Yasumara, Michael T. Matthes, Marcia Lloyd, Xian-Mei Zhang, Vince Chiodo, Kate Donohue-Rolfe, and Carolyn Graybeal for excellent technical support; Cheryl M. Craft for the gift of cone arrestin antibodies; and Robert Molday for the rhodopsin antibody.
Supported by the National Eye Institute (XJY, DB, MML, JLD, WWH); Research to Prevent Blindness (XJY, JLD); the Karl Kirchgessner Foundation (XJY); the Foundation Fighting Blindness (XJY, DB, MML, JLD, WWH); the Macula Vision Research Foundation (WWH); and the Bernard A. Newcomb Fund (JLD).
Footnotes
Disclosure: K.D. Rhee, None; A. Ruiz, None; J.L. Duncan, None; W.W. Hauswirth, Applied Genetic Technologies, Corp. (I, P, C); M.M. LaVail, None; D. Bok, None; X.-J. Yang, None
References
- 1.Fuhrmann S, Kirsch M, Hofmann HD. Ciliary neurotrophic factor promotes chick photoreceptor development in vitro. Development. 1995;121:2695–2706. [DOI] [PubMed] [Google Scholar]
- 2.Kirsch M, Fuhrmann S, Wiese A, Hofmann HD. CNTF exerts opposite effects on in vitro development of rat and chick photoreceptors. Neuroreport. 1996;7:697–700. [DOI] [PubMed] [Google Scholar]
- 3.Ezzeddine ZD, Yang XJ, DeChiara T, Yancopoulos G, Cepko CL. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997; 124:1055–1067. [DOI] [PubMed] [Google Scholar]
- 4.Neophytou C, Vernallis AB, Smith A, Raff MC. Müller-cell-derived leukaemia inhibitory factor arrests rod photoreceptor differentiation at a postmitotic pre-rod stage of development. Development. 1997;124:2345–2354. [DOI] [PubMed] [Google Scholar]
- 5.Schulz-Key S, Hofmann HD, Beisenherz-Huss C, Barbisch C, Kirsch M. Ciliary neurotrophic factor as a transient negative regulator of rod development in rat retina. Invest Ophthalmol Vis Sci. 2002; 43:3099–3108. [PubMed] [Google Scholar]
- 6.Bhattacharya S, Dooley C, Soto F, Madson J, Das AV, Ahmad I. Involvement of Ath3 in CNTF-mediated differentiation of the late retinal progenitors. Mol Cell Neurosci. 2004;27:32–43. [DOI] [PubMed] [Google Scholar]
- 7.Goureau O, Rhee KD, Yang XJ. Ciliary neurotrophic factor promotes Müller glia differentiation from the postnatal retinal progenitor pool. Dev Neurosci. 2004;26:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther. 1997;8: 423–430. [DOI] [PubMed] [Google Scholar]
- 10.Cayouette M, Behn D, Sendtner M, Lachapelle P, Gravel C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang FQ, Dejneka NS, Cohen DR, et al. V-mediated delivery of ciliary neurotrophic factor prolongs photoreceptor survival in the rhodopsin knockout mouse. Mol Ther. 2001;3:241–248. [DOI] [PubMed] [Google Scholar]
- 12.Bok D, Yasumura D, Matthes MT, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735. [DOI] [PubMed] [Google Scholar]
- 13.Schlichtenbrede FC, MacNeil A, Bainbridge JW, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–427. [DOI] [PubMed] [Google Scholar]
- 14.Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:3292–3298. [PubMed] [Google Scholar]
- 15.Adamus G, Sugden B, Shiraga S, Timmers AM, Hauswirth WW. Anti-apoptotic effects of CNTF gene transfer on photoreceptor degeneration in experimental antibody-induced retinopathy. J Autoimmun. 2003;21:121–129. [DOI] [PubMed] [Google Scholar]
- 16.Huang SP, Lin PK, Liu JH, Khor CN, Lee YJ. Intraocular gene transfer of ciliary neurotrophic factor rescues photoreceptor degeneration in RCS rats. J Biomed Sci. 2004;11:37–48. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. [DOI] [PubMed] [Google Scholar]
- 18.Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- 19.Jo SA, Wang E, Benowitz LI. Ciliary neurotrophic factor is an axogenesis factor for retinal ganglion cells. Neuroscience. 1999; 89:579–591. [DOI] [PubMed] [Google Scholar]
- 20.Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000;11:3999–4002. [DOI] [PubMed] [Google Scholar]
- 21.Cui Q, Yip HK, Zhao RC, So KF, Harvey AR. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003;22:49–61. [DOI] [PubMed] [Google Scholar]
- 22.van Adel BA, Kostic C, Deglon N, Ball AK, Arsenijevic Y. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum Gene Ther. 2003;14:103–115. [DOI] [PubMed] [Google Scholar]
- 23.Maier K, Rau CR, Storch MK, et al. Ciliary neurotrophic factor protects retinal ganglion cells from secondary cell death during acute autoimmune optic neuritis in rats. Brain Pathol. 2004;14: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ip NY, McClain J, Barrezueta NX, et al. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993; 10:89–102. [DOI] [PubMed] [Google Scholar]
- 25.Davis S, Aldrich TH, Stahl N, et al. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–1808. [DOI] [PubMed] [Google Scholar]
- 26.Stahl N, Boulton TG, Farruggella T, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. [DOI] [PubMed] [Google Scholar]
- 27.Bonni A, Frank DA, Schindler C, Greenberg ME. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993;262:1575–1579. [DOI] [PubMed] [Google Scholar]
- 28.Boulton TG, Stahl N, Yancopoulos GD. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269:11648–11655. [PubMed] [Google Scholar]
- 29.Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Müller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- 31.Rhee KD, Goureau O, Chen S, Yang XJ. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J Neurosci. 2004;24:9779–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh H, Fujio Y, Kunisada K, et al. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273:9703–9710. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa Y, Nakao K, Shimazaki T, et al. Downregulation of STAT3 activation is required for presumptive rod photoreceptor cells to differentiate in the postnatal retina. Mol Cell Neurosci. 2004;26: 258–270. [DOI] [PubMed] [Google Scholar]
- 34.Zhang SS, Wei J, Qin H, et al. STAT3-mediated signaling in the determination of rod photoreceptor cell fate in mouse retina. Invest Ophthalmol Vis Sci. 2004;45:2407–2412. [DOI] [PubMed] [Google Scholar]
- 35.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci. 1997;38:498–509. [PubMed] [Google Scholar]
- 37.Kedzierski W, Nusinowitz S, Birch D, et al. Deficiency of rds/peripherin causes photoreceptor death in mouse models of digenic and dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 2001;98:7718–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV: CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001;4:461–472. [DOI] [PubMed] [Google Scholar]
- 39.Bush RA, Lei B, Tao W, et al. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: dose-dependent effects on ERG and retinal histology. Invest Ophthalmol Vis Sci. 2004;45:2420–2430. [DOI] [PubMed] [Google Scholar]
- 40.Rade JJ, Schulick AH, Virmani R, Dichek DA. Local adenoviral-mediated expression of recombinant hirudin reduces neointima formation after arterial injury. Nat Med. 1996;2:293–298. [DOI] [PubMed] [Google Scholar]
- 41.Saggio I, Gloaguen I, Poiana G, Laufer R. CNTF variants with increased biological potency and receptor selectivity define a functional site of receptor interaction. EMBO J. 1995;14:3045–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laird DW, Molday RS. Evidence against the role of rhodopsin in rod outer segment binding to RPE cells. Invest Ophthalmol Vis Sci. 1988;29:419–428. [PubMed] [Google Scholar]
- 43.Applebury ML, Antoch MP, Baxter LC, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. [DOI] [PubMed] [Google Scholar]
- 44.Zhu X, Brown B, Li A, Mears AJ, Swaroop A, Craft CM. GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina. J Neurosci. 2003;23:6152–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hojo M, Ohtsuka T, Hashimoto N, Gradwohl G, Guillemot F, Kageyama R. Glial cell fate specification modulated by the bHLH gene Hes5 in mouse retina. Development. 2000;127:2515–2522. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Smith SB, Ogilvie JM, McCool DJ, Sarthy V. Ciliary neurotrophic factor induces glial fibrillary acidic protein in retinal Müller cells through the JAK/STAT signal transduction pathway. Curr Eye Res. 2002;24:305–312. [DOI] [PubMed] [Google Scholar]
- 47.Onoda N, Fujita SC. A monoclonal antibody specific for a subpopulation of retinal bipolar cells in the frog and other vertebrates. Brain Res. 1987;416:359–363. [DOI] [PubMed] [Google Scholar]
- 48.Burmeister M, Novak J, Liang MY, et al. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet. 1996; 12:376–384. [DOI] [PubMed] [Google Scholar]
- 49.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001; 4:247–252. [DOI] [PubMed] [Google Scholar]
- 50.Ooto S, Akagi T, Kageyama R, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA. 2004;101:13654–13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West-Mays JA, Zhang J, Nottoli T, et al. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206:46–62. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Khare SL, Liang X, et al. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development. 2000;127: 3237–3247. [DOI] [PubMed] [Google Scholar]
- 53.Chen S, Wang QL, Nie Z, et al. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. [DOI] [PubMed] [Google Scholar]
- 54.Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. [DOI] [PubMed] [Google Scholar]
- 55.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. [DOI] [PubMed] [Google Scholar]
- 56.Mears AJ, Kondo M, Swain PK, et al. A Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. [DOI] [PubMed] [Google Scholar]
- 57.Xie HQ, Adler R. Green cone opsin and rhodopsin regulation by CNTF and staurosporine in cultured chick photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:4317–4323. [PubMed] [Google Scholar]
- 58.Dyer MA, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3:873–880. [DOI] [PubMed] [Google Scholar]
- 59.Sherry DM, Mitchell R, Li H, Graham DR, Ash JD. Leukemia inhibitory factor inhibits neuronal development and disrupts synaptic organization in the mouse retina. J Neurosci Res. 2005;82:316–332. [DOI] [PubMed] [Google Scholar]
- 60.Zeiss CJ, Allore HG, Towle V, Tao W. CNTF induces dose-dependent alterations in retinal morphology in normal and rcd-1 canine retina. Exp Eye Res. 2006;82:395–404. [DOI] [PubMed] [Google Scholar]
- 61.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. [DOI] [PubMed] [Google Scholar]
- 62.Rhee KD, Yang XJ. Expression of cytokine signal transduction components in the postnatal mouse retina. Mol Vis. 2003;9:715–722. [PMC free article] [PubMed] [Google Scholar]
- 63.Beltran WA, Zhang Q, Kijas JW, et al. Cloning, mapping, and retinal expression of the canine ciliary neurotrophic factor receptor α (CNTFRα). Invest Ophthalmol Vis Sci. 2003;44:3642–3649. [DOI] [PubMed] [Google Scholar]
- 64.Valter K, Bisti S, Stone J. Location of CNTFRalpha on outer segments: evidence of the site of action of CNTF in rat retina. Brain Res. 2003;985:169–175. [DOI] [PubMed] [Google Scholar]
- 65.Beltran WA, Rohrer H, Aguirre GD. Immunolocalization of ciliary neurotrophic factor receptor alpha (CNTFRalpha) in mammalian photoreceptor cells. Mol Vis. 2005;11:232–244. [PubMed] [Google Scholar]
- 66.Wahlin KJ, Lim L, Grice EA, Campochiaro PA, Zack DJ, Adler R. A method for analysis of gene expression in isolated mouse phot receptor and Müller cells. Mol Vis. 2004;10:366–375. [PubMed] [Google Scholar]
- 67.Graham DR, Overbeek PA, Ash JD. Leukemia inhibitory factor blocks expression of Crx and Nrl transcription factors to inhibit photoreceptor differentiation. Invest Ophthalmol Vis Sci. 2005; 46:2601–2610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.