Abstract
NAC (NAM, ATAF1,2, and CUC2) transcription factors are one of the largest transcription factor families found in the plants and are involved in diverse developmental and signalling events. Despite the availability of comprehensive genomic information from diverse plant species, the basic genomic, biochemical, and evolutionary details of NAC TFs have not been established. Therefore, NAC TFs family proteins from 160 plant species were analyzed in the current study. Study revealed, Brassica napus (410) encodes highest number and Klebsormidium flaccidum (3) encodes the lowest number of TFs. The study further revealed the presence of NAC TF in the Charophyte algae K. flaccidum. On average, the monocot plants encode higher number (141.20) of NAC TFs compared to the eudicots (125.04), gymnosperm (75), and bryophytes (22.66). Furthermore, our analysis revealed that several NAC TFs are membrane bound and contain monopartite, bipartite, and multipartite nuclear localization signals. NAC TFs were also found to encode several novel chimeric proteins and regulate a complex interactome network. In addition to the presence of NAC domain, several NAC proteins were found to encode other functional signature motifs as well. Relative expression analysis of NAC TFs in A. thaliana revealed root tissue treated with urea and ammonia showed higher level of expression and leaf tissues treated with urea showed lower level of expression. The synonymous codon usage is absent in the NAC TFs and it appears that they have evolved from orthologous ancestors and undergone vivid duplications to give rise to paralogous NAC TFs. The presence of novel chimeric NAC TFs are of particular interest and the presence of chimeric NAC domain with other functional signature motifs in the NAC TF might encode novel functional properties in the plants.
Introduction
Next-generation sequencing (NGS) has fostered the sequencing of many plant genomes. The availability of so many genomes has allowed researchers to readily identify genes, examine genetic diversity within a species, and gain insight into the evolution of genes and gene families. Gene expression is regulated in part by different families of proteins known as transcription factors (TFs) [1–4]. The TFs are involved in inducing the transcription of DNA into RNA [5–8]. They include numerous and diverse proteins, all of which contain one or more DNA-binding motifs [8–10]. The DNA-binding domain enables them to bind to the promoter or repressor sequence of DNA that is present either at the upstream, downstream, or within an intron region of a coding gene [11,12]. Some TFs bind to a DNA promoter region located near the transcription start site of a gene and help to form the transcription initiation complex [13–16]. Other TFs bind to regulatory enhancer sequences and stimulate or repress transcription of the related genes [17–19]. Regulating transcription is of paramount importance to controlling gene expression and TFs enable the expression of an individual gene in a unique manner, such as during different stages of development or in response to biotic or abiotic stress [20–22]. TFs act as a molecular switch for temporal and spatial gene regulation [23,24]. A considerable portion of a genome consists of genes encoding transcription factors. For example, there are at least 52 different TF families in the Arabidopsis thaliana, and the NAC (no apical meristem (NAM) TF family is one of them.
NAC TFs are characterised by the presence of a conserved N-terminal NAC domain comprising approximately 150 amino acids and a diversified C-terminal end. The DNA binding NAC domain is divided into five sub-domains designated A-E. Sub-domain A is apparently involved in the formation of functional dimers, while sub-domains B and E appear to be responsible for the functional divergence of NAC genes [25–28]. The dimeric architecture of NAC proteins can remain stable even at a concentration of 5M NaCl [28]. The dimerization is established by Leu14-Thr23, and Glu26-Tyr31 amino acid residues. The dimeric form is responsible for the functional unit of stress-responsive SNAC1 and can modulate DNA-binding specificity [28–30]. Sub-domains C and D contain positively charged amino acids that bind to DNA [28]. The crystal structure of the SNAC1 TF revealed the presence of a central semi-β-barrel formed from seven twisted anti-parallel β-strands with three α-helices [28]. The NAC domain is most responsible for DNA binding activity that lies between amino acids Val119-Ser183, Lys123-Lys126, with Lys79, Arg85, and Arg88 reside within different strands of β-sheets [26,31,32]. The remaining portion of the NAC domain contains a loop region composed of the amino acids, Gly144-Gly149 and Lys180-Asn183, which are very flexible in nature [28]. The loop region of SNAC1 is quite long and different from the loop region of ANAC, an abscisic-acid-responsive NAC, and could underlie the basis for different biological functions. NAC TFs possesses mono or bipartite nuclear localization signals which contain a Lys residue in sub-domain D [25,32–34]. In addition, NAC proteins, as part of a mechanism of self-regulation, also modulate the expression of several other proteins [32,35]. The D subunit of a few NAC TFs contain a hydrophobic negative regulatory domain (NRD), comprised of L-V-F-Y amino acids, which is involved in suppressing transcriptional activity [36]. For example, the NRD domain can suppress the transcriptional activity of Dof, WRKY, and APETALA 2/dehydration responsive elements (AP2/DRE) TFs [36].
Studies indicate that the diverse C-terminal domain contains a transcription regulatory region (TRR) which has several group-specific motifs that can activate or repress transcription activity [37–40]. The C-terminal region imparts differences in the function of individual NAC proteins by regulating the interaction of NAC TFs with various target proteins. Although the C-terminal region of NAC TFs is varied greatly, it also contains group-specific conserved motifs [41]. Although various aspects of NAC TFs have been studied [42,43], most studies were limited within a few plant species. For example, Zhu et al., (2012) has studied with only 16 species where in few cases they used expressed sequence tag (EST) as well [42] and Pereira-Santana et al., (2015) used 24 land plant species [43] where they were included the genome sequences of unicellular organisms including algae and bacteria. However, Pereira-Santana et al., (2015) did not find any NAC TFs in the algae and bacteria [43]. Therefore, a detailed comparative study of the genomic, molecular biology, and evolution of NAC TFs has across the lineage level of plant kingdom has not been conducted so far. Therefore, a comprehensive analysis of NAC TFs is presented in the current study. We analysed nucleotide and protein data of the NAC TFs to find out the genomic diversity, biochemical, evolutionary, and expression analysis of NAC TFs from 160 plant species.
Materials and methods
Identification of NAC TFs
NAC genes from 160 plant species (9 algae, 3 bryophytes, 1 pteridophyte, 5 gymnosperms, and 142 higher plants) were obtained from searches in the National Centre for Biotechnology Information (https://www.ncbi.nlm.nih.gov/), Phytozome, and Plant Genome databases [44,45]. BLASTP (E-value cut-off was 1E-5) and hidden Markov model were used to identify the NAC TFs in different species using AtNAC1 and AtNAC2 as the query sequences [46]. BLASTP analysis was conducted against the respected proteome of the individual species to find the best hit to minimize the error rate [44]. Protein and CDS sequences of each species were collected and further analysed. Protein sequences of the NAC TFs were subjected to BLASTP analysis against the reference databases NCBI, Phytozome, and Plant Genome Database [44,45] to reconfirm them as a NAC TF of the respective identified species. All of the NAC TF protein sequences in the examined species were also subjected to ScanProsite and InterProScan to confirm the presence of a NAC domain [47,48]. Sequences that were found to contain a NAC domain were considered as NAC TFs. The presence of multiple NAC domains, along with the presence of chimeric NAC domains, were determined through ScanProsite and InterProScans [47,48]. The presence of multiple functional sites in NAC TFs were also analysed using ScanProsite software [48].
Analysis of membrane attachment and nuclear localization signal sequences
The presence of transmembrane domains in NAC TFs of all of the examined species were identified using TMHMM server v. 2.0 [49]. Nuclear localization signal sequences in NAC TFs were identified using NLStradamus software, which uses a hidden Markov model for the prediction of nuclear localization signals [50]. The NAC TF protein sequences were uploaded in FASTA format to run the program. The parameters used to run the NLS analysis were; HMM state emission and transition frequencies, 2 state HMM static; prediction type Viterbi and posterior, prediction cut-off 0.4; prediction display, and image and graphic [50].
Interactome analysis of NAC TFs
A. thaliana NAC TFs were used to examine the complex interactome network of NAC TFs. The individual interaction network of each NAC TF in A. thaliana was searched in a string database that contains 9.6 million proteins from 2031 organisms [51,52]. The interactome network of each of NAC TF were noted and the results were later used to construct the interactome network of A. thaliana NAC TFs. The presented interactome network was based on an experimentally validated network, co-expressed network, and a mined network [52]. These outputs were used to construct the interactome network. The NAC TFs used to construct the interactome network were subjected to GO (gene ontology) and cellular process analyses [52].
Gene expression analysis
Differential gene expression of NAC TFs was analysed to elucidate their role in growth, development, and nitrogen assimilation. A. thaliana NAC TFs were used to examine differential gene expression. The transcriptome data from A. thaliana treated with ammonia, nitrate, and urea were utilized from the PhytoMine database in Phytozome [44]. The experimental conditions were as follows; the A. thaliana seeds were cold stratified in water for 3 days and sown in pots. The pots were placed in the growth chamber (22o C day/20o C night, 14 hrs light with flux density of 350 μmol m-2s-1) and later thinned one plant per pot. When rosette was achieved 7–8 leaves, treatment was conducted. The plants were watered with nutrient solution containing 5mM urea, 10 mM KNO3 (potassium nitrate), and 10 mM (NH4)3PO4 (ammonium phosphate) for each of individual experiment. The nutrient solutions were supplied at three days interval for four weeks. After four weeks, the leaf, stem, and root tissues were harvested for expression analysis. The expression pattern of NAC TFs for leaf and root tissues in the treated A. thaliana plants were analysed separately. The expression was measured in fragments per kilobase of exon per million fragments mapped (FPKM). Transcripts with a zero value were discarded from the study.
Construction of a phylogenetic tree
Two approaches were used to construct the phylogenetic trees. In the first approach, a phylogenetic tree was constructed using the NAC TFs of individual species. In the second approach, the NAC TFs of all of the examined species were combined to construct a phylogenetic tree. The phylogenetic tree for individual species was constructed to determine the deletion and duplication events in NAC TFs within individual species. We excluded the short sequences from the study those resulted in error during the alignment. Prior to the construction of the phylogenetic trees, a model selection was carried out in MEGA6 software [53]. The following parameters were used in the model, analysis, model selection; tree to use, automatic (neighbour joining), statistical method, maximum likelihood; substitution type, nucleotides; gaps/missing data treatment, partial deletion; site coverage cut-off (%), 95; codons included, 1st+2nd+3rd+non-coding. Based on the lowest BIC values of model selection, phylogenetic trees of NAC TFs were carried out using the neighbour joining method, a GTR statistical model, and 1000 bootstrap replicates.
Analysis of transition and transversion rates
Transition and transversion rates in NAC TFs within individual species were analysed using MEGA6 software [53]. The converted MEGA file format of individual species was used to determine the rate of transition and transversion. The following statistical parameters were used to study the transition/transversion rate: estimate transition/transversion bias; maximum composite likelihood estimates of the pattern of nucleotide substitution; substitution type, nucleotides; model/method, Tamura-Nei; gaps/missing data treatment, pairwise deletion; codon position, 1st, 2nd, 3rd, and non-coding sites.
Analysis of gene deletion and duplication
Prior to the analysis of deletion and duplication events in NAC TFs, a species tree was constructed in the NCBI taxonomy browser (https://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi). All of the studied species were used to construct the species tree. The resulting phylogenetic trees of individual species in a nwk file format were uploaded in Notung 2.9 software [54] as a gene tree and reconciled as a gene tree with the species tree to obtain duplicated and deleted genes. Deletion and duplication events were analysed in all of the studied species individually.
Results and discussion
NAC transcription factors exhibit diverse genomic and biochemical features
Advancements in genome sequencing technology have enabled the discovery of the genomic details of large number of plant species. The availability the genome sequence data allowed us to study the genomic details of NAC TFs in diverse plant species. The presence of NAC TFs in 160 species (18774 NAC sequences) was identified and served as the basis of the conducted analyses. Comparisons of NAC sequences revealed that Brassica napus has the highest number (410) of NAC TFs, while the pteridophyte plant, Marchantia polymorpha, was found to contain the lowest number (9) (Table 1). On average, monocot plants contain a higher (141.20) number of NAC TFs relative to dicot plants (125.56). Except for Hordeum vulgare (76), Saccharum officinarum (44), and Zostera marina (62) all other monocot species possess more than one hundred NAC TFs each (Table 1). Lower eukaryotic plants, bryophytes and pteridophytes also possess NAC TFs. In addition, the algal species, Klebsormidium flaccidum, also contains NAC TFs and this finding represents the first report of NAC TFs in algae (Table 1). A NAC TF in Trifolium pratense (Tp57577_TGAC_v2_mRNA14116) was found to be the largest NAC TF, comprising 3101 amino acids, while a NAC TF in Fragaria x ananassa (FANhyb_icon00034378_a.1.g00001.1) was found to be the smallest NAC TF, comprising only 25 amino acids. Although it only contains a 25 amino acid sequence, it still encodes a NAC domain. Typically, NAC TFs contain a single NAC domain located near the N-terminal region of the protein. The current analysis, however, also identified NAC TFs with two NAC domains. At least 77 of the 160 studied species were found to contain two NAC domains (Table 1).
Table 1. Genomic details of NAC TFs of plants.
NAC TFs have not undergone conditional duplication and none of a NAC TF gene has lost. In addition, transfer of NAC TFs was not observed from one species to another.
| Sl. No | Name of the species | No. of double domain NAC TF | No. of Novel chimeric NAC TFs | Total No. of NAC TFs | No. of duplicated genes | No. of paralogous genes |
|---|---|---|---|---|---|---|
| Monocots | ||||||
| 1 | Aegilops tauschii | 4 | 117 | 114 | 114 | |
| 2 | Brachypodium distachyon | 2 | 1 | 137 | 135 | 135 |
| 3 | Brachypodium stacei | 1 | 1 | 128 | 127 | 127 |
| 4 | Hordeum vulgare | 76 | 76 | 76 | ||
| 5 | Leersia perrieri | 5 | 2 | 163 | 162 | 162 |
| 6 | Oropetium thomaeum | 1 | 118 | 103 | 103 | |
| 7 | Oryza barthii | 4 | 134 | 138 | 138 | |
| 8 | Oryza brachyantha | 1 | 1 | 118 | 110 | 110 |
| 9 | Oryza glaberrima | 1 | 116 | 110 | 110 | |
| 10 | Oryza glumipatula | 2 | 140 | 139 | 139 | |
| 11 | Oryza longistaminata | 1 | 6 | 125 | 98 | 98 |
| 12 | Oryza meridionalis | 2 | 2 | 127 | 123 | 123 |
| 13 | Oryza nivara | 4 | 1 | 146 | 130 | 130 |
| 14 | Oryza punctata | 6 | 1 | 135 | 133 | 133 |
| 15 | Oryza rufipogon | 4 | 3 | 136 | 129 | 129 |
| 16 | Oryza sativa subsp. indica | 1 | 3 | 157 | 156 | 156 |
| 17 | Oryza sativa subsp. japonica | 1 | 139 | 138 | 138 | |
| 18 | Panicum hallii | 3 | 6 | 139 | 126 | 126 |
| 19 | Panicum virgatum | 9 | 6 | 310 | 309 | 309 |
| 20 | Phoenix dactylifera | 3 | 1 | 124 | 123 | 123 |
| 21 | Phyllostachys edulis | 125 | 124 | 124 | ||
| 22 | Phyllostachys heterocycla | 2 | 2 | 125 | 124 | 124 |
| 23 | Saccharum officinarum | 44 | 33 | 33 | ||
| 24 | Setaria italica | 4 | 139 | 134 | 134 | |
| 25 | Setaria viridis | 1 | 135 | 118 | 118 | |
| 26 | Sorghum bicolor | 1 | 141 | 134 | 134 | |
| 27 | Spirodela polyrhiza | 55 | 48 | 48 | ||
| 28 | Triticum aestivum | 2 | 2 | 263 | 209 | 209 |
| 29 | Triticum urartu | 1 | 103 | 74 | 74 | |
| 30 | Zea mays | 1 | 1 | 130 | 119 | 119 |
| 31 | Zostera marina | 1 | 62 | 55 | 55 | |
| 32 | Zoysia japonica | 4 | 176 | 160 | 160 | |
| 33 | Zoysia matrella | 1 | 3 | 313 | 230 | 230 |
| 34 | Zoysia pacifica | 1 | 2 | 205 | 183 | 183 |
| Dicots | ||||||
| 35 | Actinidia chinensis | 1 | 5 | 167 | 166 | 166 |
| 36 | Aethionema arabicum | 3 | 85 | 84 | 84 | |
| 37 | Amaranthus hypochondriacus | 1 | 44 | 37 | 37 | |
| 38 | Amborella trichopoda | 46 | 45 | 45 | ||
| 39 | Ananas comosus | 1 | 73 | 72 | 72 | |
| 40 | Aquilegia coerulea | 80 | 79 | 79 | ||
| 41 | Arabidopsis halleri | 2 | 94 | 93 | 93 | |
| 42 | Arabidopsis lyrata | 4 | 1 | 122 | 121 | 121 |
| 43 | Arabidopsis thaliana | 5 | 113 | 112 | 112 | |
| 44 | Arabis alpina | 1 | 82 | 81 | 81 | |
| 45 | Arachis duranensis | 82 | 81 | 81 | ||
| 46 | Arachis hypogaea | 162 | 161 | 161 | ||
| 47 | Arachis ipaensis | 83 | 81 | 81 | ||
| 48 | Artemisia annua | 28 | 27 | 27 | ||
| 49 | Azadirachta indica | 183 | 182 | 182 | ||
| 50 | Beta vulgaris | 53 | 52 | 52 | ||
| 51 | Boechera stricta | 2 | 123 | 122 | 122 | |
| 52 | Brassica napus | 10 | 7 | 410 | 409 | 409 |
| 53 | Brassica oleracea | 4 | 3 | 271 | 270 | 270 |
| 54 | Brassica rapa | 4 | 2 | 256 | 255 | 255 |
| 55 | Cajanus cajan | 96 | 95 | 95 | ||
| 56 | Camelina sativa | 17 | 3 | 341 | 330 | 330 |
| 57 | Cannabis sativa | 58 | 57 | 57 | ||
| 58 | Capsella grandiflora | 2 | 95 | 94 | 94 | |
| 59 | Capsella rubella | 5 | 119 | 118 | 118 | |
| 60 | Capsicum annum | 96 | 95 | 95 | ||
| 61 | Carica papaya | 82 | 81 | 81 | ||
| 62 | Castanea mollissima | 4 | 91 | 78 | 78 | |
| 63 | Catharanthus roseus | 2 | 121 | 120 | 120 | |
| 64 | Chenopodium quinoa | 1 | 96 | 95 | 95 | |
| 65 | Cicer arietinum | 96 | 95 | 95 | ||
| 66 | Citrullus lanatus | 80 | 79 | 79 | ||
| 67 | Citrus clementina | 129 | 128 | 128 | ||
| 68 | Citrus sinensis | 2 | 145 | 143 | 143 | |
| 69 | Coffea canephora | 63 | 62 | 62 | ||
| 70 | Cucumis melo | 92 | 91 | 91 | ||
| 71 | Cuccumis sativus | 83 | 80 | 80 | ||
| 72 | Daucus carota | 2 | 96 | 95 | 95 | |
| 73 | Dianthus caryophyllus | 79 | 77 | 77 | ||
| 74 | Dichanthelium oligosanthes | 8 | 2 | 131 | 100 | 100 |
| 75 | Dorcoceras hygrometricum | 2 | 83 | 76 | 76 | |
| 76 | Elaeis guineensis | 2 | 1 | 170 | 167 | 167 |
| 77 | Eragrostis tef | 8 | 3 | 172 | 165 | 165 |
| 78 | Eucalyptus camaldulensis | 200 | 124 | 124 | ||
| 79 | Eucalyptus grandis | 164 | 150 | 150 | ||
| 80 | Eutrema salsugineum | 2 | 122 | 104 | 104 | |
| 81 | Fragaria vesca | 3 | 6 | 127 | 123 | 123 |
| 82 | Fragaria x ananassa | 2 | 1 | 98 | 97 | 97 |
| 83 | Genlisea aurea | 1 | 45 | 42 | 42 | |
| 84 | Glycine max | 180 | 175 | 175 | ||
| 85 | Glycine soja | 1 | 173 | 166 | 166 | |
| 86 | Gossypium arboreum | 150 | 146 | 146 | ||
| 87 | Gossypium hirsutum | 1 | 2 | 306 | 296 | 296 |
| 88 | Gossypium raimondii | 153 | 145 | 145 | ||
| 89 | Helianthus annuus | 21 | 20 | 20 | ||
| 90 | Humulus lupulus | 74 | 68 | 68 | ||
| 91 | Ipomoea trifida | 1 | 2 | 131 | 123 | 123 |
| 92 | Jatropha curcas | 1 | 97 | 93 | 93 | |
| 93 | Juglans regia | 3 | 92 | 81 | 81 | |
| 94 | Kalanchoe laxiflora | 166 | 165 | 165 | ||
| 95 | Kalanchoe marnieriana | 179 | 178 | 178 | ||
| 96 | Lactuca sativa | 54 | 52 | 52 | ||
| 97 | Linum usitatissimum | 1 | 1 | 191 | 187 | 187 |
| 98 | Lotus japonicus | 2 | 98 | 92 | 92 | |
| 99 | Malus domestica | 2 | 9 | 253 | 232 | 232 |
| 100 | Manihot esculenta | 130 | 128 | 128 | ||
| 101 | Medicago truncatula | 1 | 97 | 90 | 90 | |
| 102 | Mimulus guttatus | 114 | 113 | 113 | ||
| 103 | Morus notabilis | 2 | 78 | 77 | 77 | |
| 104 | Musa acuminata | 1 | 1 | 170 | 164 | 164 |
| 105 | Nelumbo nucifera | 88 | 79 | 79 | ||
| 106 | Nicotiana benthamiana | 2 | 2 | 227 | 185 | 185 |
| 107 | Nicotiana sylvestris | 156 | 149 | 149 | ||
| 108 | Nicotiana tabacum | 280 | 279 | 279 | ||
| 109 | Nicotiana tomentosiformis | 172 | 162 | 162 | ||
| 110 | Ocimum tenuiflorum | 2 | 1 | 110 | 82 | 82 |
| 111 | Petunia axillaris | 3 | 131 | 108 | 108 | |
| 112 | Petunia inflata | 157 | 147 | 147 | ||
| 113 | Phaseolus vulgaris | 85 | 84 | 84 | ||
| 114 | Populus euphratica | 2 | 3 | 155 | 149 | 149 |
| 115 | Populus trichocarpa | 1 | 169 | 149 | 149 | |
| 116 | Prunus mume | 1 | 129 | 128 | 128 | |
| 117 | Prunus persica | 1 | 1 | 115 | 114 | 114 |
| 118 | Pyrus bretschneideri | 1 | 5 | 185 | 183 | 183 |
| 119 | Raphanus raphanistrum | 4 | 3 | 207 | 206 | 206 |
| 120 | Raphanus sativus | 5 | 1 | 217 | 197 | 197 |
| 121 | Ricinus communis | 95 | 87 | 87 | ||
| 122 | Salix purpurea | 175 | 152 | 152 | ||
| 123 | Salvia miltiorrhiza | 1 | 2 | 87 | 81 | 81 |
| 124 | Sesamum indicum | 105 | 104 | 104 | ||
| 125 | Sisymbrium irio | 2 | 2 | 121 | 118 | 118 |
| 126 | Solanum lycopersicum | 101 | 94 | 94 | ||
| 127 | Solanum melongena | 1 | 3 | 95 | 85 | 85 |
| 128 | Solanum pennellii | 2 | 102 | 98 | 98 | |
| 129 | Solanum pimpinellifolium | 97 | 90 | 90 | ||
| 130 | Solanum tuberosum | 1 | 129 | 115 | 115 | |
| 131 | Spinacia oleracea | 45 | 43 | 43 | ||
| 132 | Tarenaya hassleriana | 1 | 178 | 177 | 177 | |
| 133 | Thellungiella halophila | 2 | 122 | 121 | 121 | |
| 134 | Thellungiella parvula | 1 | 92 | 91 | 91 | |
| 135 | Theobroma cacao | 132 | 131 | 131 | ||
| 136 | Trifolium pratense | 2 | 2 | 97 | 76 | 76 |
| 137 | Utricularia gibba | 1 | 74 | 73 | 73 | |
| 138 | Vigna angularis | 98 | 97 | 97 | ||
| 139 | Vigna radiata | 2 | 82 | 81 | 81 | |
| 140 | Vigna unguiculata | 20 | 19 | 19 | ||
| 141 | Ziziphus jujuba | 101 | 100 | 100 | ||
| 142 | Vitis vinifera | 1 | 70 | 79 | 79 | |
| Gymnosperms | ||||||
| 143 | Picea abies | 1 | 100 | 73 | 73 | |
| 144 | Picea glauca | 32 | 31 | 31 | ||
| 145 | Picea sitchensis | 16 | 15 | 15 | ||
| 146 | Pinus taeda | 31 | 27 | 27 | ||
| 147 | Pseudotsuga menziesii | 5 | 3 | 196 | 195 | 195 |
| Pteridophyte | ||||||
| 148 | Selaginella moellendorffii | 22 | 21 | 21 | ||
| Bryophytes | ||||||
| 149 | Marchantia polymorpha | 9 | ||||
| 150 | Physcomitrella patens | 33 | 32 | 32 | ||
| 151 | Sphagnum fallax | 26 | 25 | 25 | ||
| Algae | ||||||
| 152 | Bathycoccus prasinos | 0 | 0 | 0 | ||
| 153 | Chlamydomonas reinhardtii | 0 | 0 | 0 | ||
| 154 | Chlorella sp. NC64A | 0 | 0 | 0 | ||
| 155 | Coccomyxa sp. | 0 | 0 | 0 | ||
| 156 | Dunaliella salina | 0 | 0 | 0 | ||
| 157 | Klebsormidium flaccidum | 3 | 0 | 0 | ||
| 158 | Micromonas pusilla | 0 | 0 | 0 | ||
| 159 | Ostreococcus lucimarinus | 0 | 0 | 0 | ||
| 160 | Volvox carteri | 0 | 0 | 0 | ||
Multiple sequence alignment revealed the presence of a conserved consensus sequence at the N-terminus. The major conserved consensus sequences are P-G-F-R-F-H-P-T-D-D/E-L-I/V, Y-L-x2-K, D-L-x-K-x2-P-W-x-L-P, E-W-Y-F-F, G-Y-W-K-A/T-T-G-x-D-x 1-2-I/V, G-x-K-K-x-L-V-F-Y, and T-x-W-x-M-H-E-Y. Among these consensus sequences, D-D/E-L-I/V, E-W-Y-F-F, G-Y-W-K, and M-H-E-Y are the conserved motifs most observed. The D-D/E-L motif is a characteristic feature of the calcium-binding motifs present in the EF-hand of calcium-dependent protein kinases and the presence of this motif in NAC TFs indicates that they have the potential to regulate Ca2+ signalling events in cells [55]. The D-D-E/E motif is located in the β’ sheet whereas the Y-L-x2-K motif is in the α1a/b chain. Except for G-F-R-F-H-P-T-D-D/E-L-I/V, the conserved consensus sequences contain the positively charged amino acids Lys (L) and Arg (K) that can bind to negatively charged DNA. Welner et al. (2012) published the crystal structure of ANAC019 and reported that Y94-W-K-A-T-G-T-D in β3, I11-K-K-A-L-V-F-Y of β4, K123-A-P-K-G-T-K-T-N-W in the loop between β4 and β5, and I133-M-H-E-Y-R of β5 and Y160-K-K-Q at the C-terminal end are located close to the bound DNA and are associated with DNA binding activity [56]. They reported that Y94-W-K-A-T-G-T-D is responsible for the specific recognition of DNA and binds at the major groove within DNA, whereas I11-K-K-A-L-V-F-Y, K123-A-P-K-G-T-K-T-N-W, I133-M-H-E-Y-R, and Y160-K-K-Q bind to the backbone of the DNA molecule and provide affinity for DNA binding activity [56]. In the present analysis of 160 plant species, the identification of the conserved consensus sequences G-Y-W-K-A/T-T-G-x-D-x1-2-I/V, G-x-K-K-x-L-V-F-Y, and T-x-W-x-M-H-E-Y is in agreement with Welner et al (2012); suggesting that NAC TFs contain conserved consensus sequences for specific DNA recognition and increasing the affinity for DNA binding.
Hao et al., (2010) reported that the D subunit of NAC TFs contain a hydrophobic L-V-F-Y amino acid motif that partially suppresses the WRKY, Dof, and APETALA2 transcriptional regulators [36]. This suggests that NAC TFs function as a negative regulator of transcription for WRKY, Dof, and APETALA 2/ dehydration responsive element. The sequence alignment, however, revealed the presence L-V-F-Y transcriptional repressor motif in NAC TF family proteins in diverse plant species. If all the NAC TF with L-V-F-Y motif will supress the transcriptional activity of WRKY, Dof, and APETALA 2, it will be challenging for the plants to sustain its cellular and biological activities.
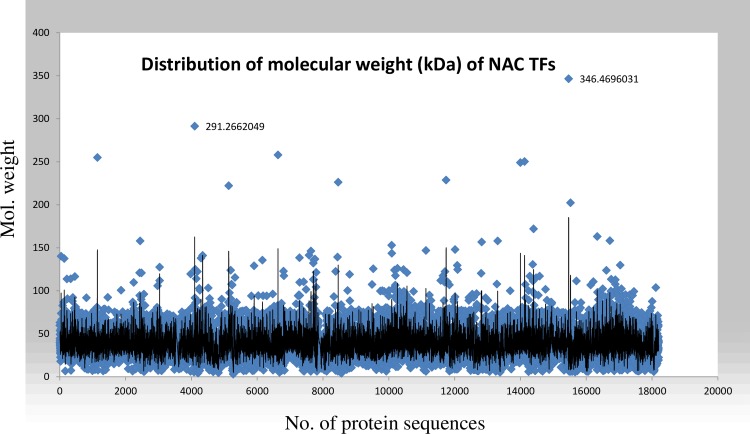
The molecular weight of NAC TFs ranged from 2.94 kDa (Fragaria x ananassa_FANhyb_icon00034378_a.1.g00001.1) to 346.46 kilodaltons (kDa) (Trifolium pratense_Tp57577_TGAC_v2) (Fig 1). Among the studied NAC TFs, only 10 NAC proteins have a molecular weight (MW) more than 200 kDa and 99 are between 100 to 200 kDa. The MW of the majority of the NAC proteins range between 40 to 55 kDa (Fig 1). The average molecular weight of the plant proteins falls in the same range (average 48.256 kDa) as found in the case of A. thaliana proteome) [57].
Fig 1. The distribution of the molecular weight of NAC TFs.
The molecular weight of NAC TFs ranged from 2.94 kDa (Fragaria x ananassa, FANhyb_icon00034378_a.1.g00001.1) to 346.46 kDa (Trifolium pratense, Tp57577_TGAC_v2_mRNA14116). The average molecular weight of NAC TFs was 38.72 kDa. In total, 17158 NAC TFs were utilized in the analysis of molecular weight. The analysis was conducted using a protein isoelectric point calculator (http://isoelectric.org/).
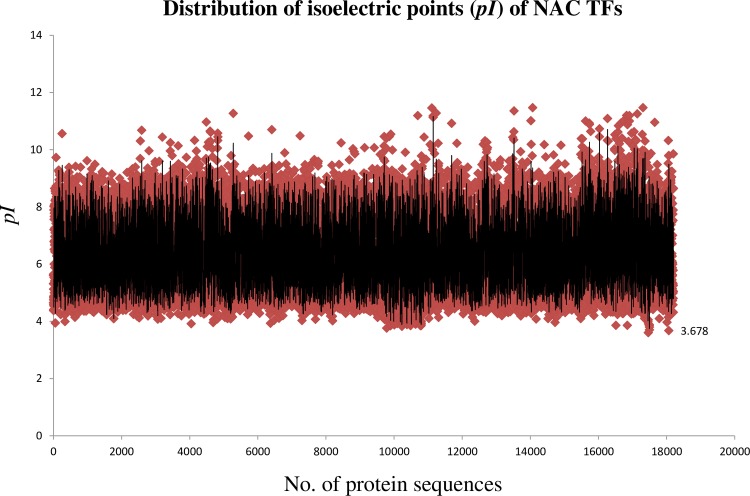
The Isoelectric point (pI) of the NAC proteins ranged from 11.47 (Brast01G304500.1.p, (Brachypodium stacei) to 3.60 (ObartAA03S_FGP19036, Oryza barthii). The majority of the NAC TFs fell within a pI rage of 5–8 (Fig 2). Among the 18774 analysed NAC TFs, the pI of 99 proteins were ≥ 10. Approximately 69.28% of the NAC TFs had a pI that was in an acidic range, whereas the remaining 30.72% had a pI within in a basic range. A protein with a pH below the pI carries a net positive charge, whereas a protein with a pH above the pI carries a net negative charge. The pI of a protein determines its transport, solubility, and sub-cellular localization [57–60]. Biomembranes, such as those surrounding the nucleus, are negatively charged; as a result, positively charged (acidic pI) NAC TFs are readily attracted to the nuclear membrane and subsequently transported into the nucleus to function in transcriptional regulation. There are, however, approximately 30.72% NAC TFs that possess a basic pI; suggesting that they are localized in the cytosol or plasma membrane of the cell. The major role of the TFs is to bind to specific DNA sequences to regulate transcription. The majority of the proteins have either an acidic or basic pI and those with a neutral pI close to 7.4 are few because proteins tend to be insoluble, unreactive, and unstable at a pH close to its pI. This is the main reason why among the 18774 NAC TFs analysed, only two (XP_010925972.1, Elaeis guineensis; Lus10008200, Linum usitatissimum) had a pI 7.4. The existence of NAC proteins with a pI above 10 led us to speculate whether these TFs function while attached to a transmembrane domain. Therefore, additional analyses were conducted to determine if NAC TFs also have the potential to bind to the transmembrane domain or if the NAC TFs with a basic pI remain within the cytosol.
Fig 2. The distribution of the isoelectric point of NAC TFs.
The isoelectric point of NAC TFs ranged from pI 3.78 (OB07G17140.1, Oryza brachyantha) to pI 11.47 (Sevir.3G242500, Setaria viridis). The average isoelectric point of NAC TFs was 6.38. A total of 17158 NAC TFs were utilized in the analysis of the pI of NAC TFs. The analysis of pI was conducted using a protein isoelectric point calculator (http://isoelectric.org/).
NAC TF proteins are membrane bound
Transcription factors regulate diverse cellular events at transcriptional, translational, and posttranslational levels. They are also involved in nuclear transport and posttranslational modifications. In several cases, TFs are synthesized but remain inactive in the cytoplasm and are only induced into activity through non-covalent interactions [61,62]. TFs are able to remain inactive through their physical association with intracellular membranes and are released by proteolytic cleavage. NAC TFs are a family of proteins whose numbers are in the hundreds in the majority of plant species. The fact that NAC TFs are such a large protein family, it is not surprising that NAC TFs have evolved diverse functional roles. Therefore, it is plausible that NAC TFs may be associated with sub-cellular organelle other than the nucleus to fulfil their diverse functional roles. It is essential, however, to confirm if NAC TFs contain signalling sequences for transmembrane localization. Therefore, we analysed the NAC gene sequences to determine if the signalling sequences present in NAC TFs possess a transmembrane domain.
Results indicated that at least 2190 (8.57%) NAC TFs possess a transmembrane domain (S1 Fig, S1 File). Transmembrane domains were found at both the N- and C-terminal ends of NAC proteins. In the majority of the cases, however, the transmembrane domain was located towards the C-terminal end. Seo et al., (2008) indicated the presence of a transmembrane domain in TFs and suggested that transmembrane domain functions through two proteolytic mechanisms, commonly known as regulated ubiquitin/proteasome-dependent (RUP) and regulated intramenbrane proteolysis (RIP) [63,64]. The bZIP plant TF is present as an integral membrane protein associated with stress response in the endoplasmic reticulum (ER) [65–68]. Studies suggest that the majority of membrane bound TFs are associated with the ER and a membrane bound TF was also found to be involved in cell division [69,70]. At least 10% of the TFs in Arabidopsis thaliana have been reported to be transmembrane bound [70]. The collective evidence clearly indicates that membrane-mediated transcriptional regulation is a common stress response and that NAC TFs play a vital role in stress resistance in the ER. Therefore, these membrane-bound NAC TFs can be of great importance for the manipulation of stress resistance using biotechnology.
NAC TF contain monopartite, bipartite, non-canonical, and nuclear export signal sequences
The import of NAC TFs into the nucleus is mediated by nuclear membrane-bound importins and exportins that form a ternary complex consisting of importin α, importin β1, and a cargo molecule. Importin α serve as an adaptor molecule of importin β1 and recognises the nuclear localization signal (NLS) of the cargo protein needing to be imported. Importin β1 and β2, however, also recognize the NLS directly and bind to the cargo protein. Although the NLS of TFs have been widely studied in the animal kingdom, their study in plants has been more restricted. Therefore, the NLS of NAC TFs was examined in the current study. Results indicate that NAC TFs contain diverse NLS. The NLS were found in the N- and C-terminal regions of NAC TF proteins. Some NAC TFs were found to contain only one NLS whereas other contain multiple NLS. At least 3579 of the total NAC TFs analysed were found to contain either one or multiple NLS (S2 Fig, S2 File). More specifically, 2604 NAC TFs were found to possess only one NLS at the N-terminal end of the NAC protein, whereas 975 were found to possess two NLS, 254 possess three NLS, and 48 were possess four NLS. The NLS were located towards the N-terminal end in the majority of NAC proteins.
NLS motifs are rich in positively charged amino acids and bind to importin α to be imported into the nucleus. The NLS motifs are classified as monopartite or bipartite. A monopartite NLS contains a single cluster of positively charged amino acids and are grouped into two subclasses, class-I and class-II. Class-I possesses four consecutives positively charged amino acids and class-II contains three positively charged amino acids, represented by K(K/R)-x-K/R; where x represents any amino acid that is present after two basic amino acids. Bipartite NLS motifs contain two clusters of positively charged amino acids separated by a 10–12 amino acid linker sequence. Bipartite NLS motifs are characterised by the consensus sequence K-R-P-A-A-T-K-K-A-G-Q-A-K-K-K-K. In addition to monopartite and bipartite NLS motifs, importin α also recognises non-canonical NLS motifs. Non-canonical NLS motifs are longer and considerably variable relative to monopartite and bipartite NLS motifs and are classified as class-III and class-IV NLS. Non-canonical NLS motifs are usually present in the C-terminal end and bind with importin β2. Class-III and class-IV NLS motifs contain K-R-x(W/F/Y)-x2-A-F and (P/R)-x2-K-R-(K/R) consensus sequences, respectively. We identified at least 1702 unique NLS consensus sequences in the N-terminal region of NAC TFs. The monopartite class I NLS motifs were found to contain more than four consecutive basic amino acids with the number of their consecutive basic amino acids ranging from four to fourteen (K-K-K-K-K-K-K-K-K-K-K-K-K-K-K). The bipartite NLS motifs contain two clusters of consecutive basic amino acids separated by up to twenty-four linker amino acids (K-K-K-x3-R- x2-R- x4-K- x3-K- x3-K-x-K- x2-R-K-K).
The non-canonical NLS motifs contain at least six centrally-located, positively charged amino acids (K-x-R-R-R-P-R-R-x2-R-K) flanked by positively charged amino acids on both sides. Our analysis of the N-terminal NLS of NAC TFs, however, did not identify any NAC TFs containing this consensus sequence. Instead, several new variants of this consensus sequence were identified with multiple clusters of positively charged amino acids. These NLS were designated as multipartite NLS motifs (Table 2, S2 Fig, S2 File). Much of the diversity of NLS motifs is associated with the sequence of the variable linker amino acids. In our analysis, we removed the linker amino acid sequences, represented as x, to obtain a more concise picture of NLS diversity. Removing the linker amino acids present in monopartite, bipartite, and multipartite NLS motifs resulted in the identification of 97 different NLS consensus sequences in the N-terminal region of NAC TFs (S2 Fig, S2 File). The R-K-R-R-K consensus sequence was found to be present 347 times, K-K-K 297 times, K-R-K 185 times, K-K-R 165 times, K-R-R 153 times, R-R-R 96 times, R-K-K 95 times, R-K-R 83 times, K-K-K-K 75 times, R-R-K 74 times, R-R-R-R 58 times, K-K-R-K 49 times, K-K-R-K-R 49 times, and K-R-K-R 40 times. At least 27 NLS amino acid consensus sequences were only found once among the 160 studied species (S2 File).
Table 2. Putative multipartite nuclear localization signal sequences of NAC transcription factor proteins.
The underlined amino acids are designated as NLS and letter x denoted as any amino acid.
| C-terminal multipartite NLS | N-terminal multipartite NLS |
|---|---|
| R-K-R-x-R-x-R-K-K-x4-K-x-K-K-K-R-x3-K-x3-K-K-x3-R-R-K-x2-K | K-K-K-K-x7-K-K-K-K-x7-K-K-K-K |
| R-R-R-x4-K-K-x6-R-x2-R-x2-R-R-x4-R-R-R-x6-R-x2-R-R-x9-R-R-R-R-R-R-R-x2-R-R | K-K-K-K-x-K-x5-K-x-K-K-x7-K-K-K-K-x2-K-K-K |
| K-K-K-x4-K-K-x-K-x5-K-x4-K-K-K-R-x-K-R-K-x-K-x4-K-K-K-R-K-K | K-K-K-x2-K-K-x-K-x5-K-x4-K-K-K-R-x-K-R-K-x-K-x4-K-K-K-R-K-K |
| K-K-R-x4-K-x2-K-x-K-x2-K-K-R-x-R-K-x4-K-x2-K-x-K-K-R-x-R-K-x4-K-x2-K-x-K-x-R | K-K-R-x-R-K-x2-K-x-K-x2-K-K-K-x-RK-x2-K-R-R-x2-K-K-K-x-R |
| K-K-R-x-R-K-x2-K-x-K-x2-K-K-K-x-R-K-x2-K-R-R-x2-K-K-K-x-R | K-K-R-x-R-K-x2-K-x-K-x2-K-K-R-x-R-K-x2-K-x-K-x2-K-K-R-x-R-K-x2-K-x2-K-x-K-x-R |
| K-K-R-x-R-K-x2-K-x-K-x2-K-K-R-x-R-K-x2-K-x-K-x2-K-K-R | K-x2-K-K-K-x3-K-K-K-K-K-x-K-x8-K-x9-K-x2-K-K-R-x2-K-K-K-K-x-K |
| R-K-R-x-R-x3-K-K-R-R-x2-K-x9-K-x4-R-x-K-x2-R-x-R-R-x5-K-K-R | K-x2-K-K-K-x3-K-x-K-K-K-x-K-K-K-x2-K-K-K-x-K |
| R-K-R-x-R-x-R-x5-K-x-K-K-K-R-x3-K-x4-K-R-x2-R-R-K | R-K-R-x-R-x-R-K-K-x2-K-x-K-K-K-R-x2-K-x2-KK-x2-R-R-K-x2-K |
| R-R-x-R-R-R-x-R-R-x8-R-x6-R-R-x5-R-R-R-x-R-x5-R-x8-R-R-R-R | R-K-R-x-R-x-R-x2-K-x-K-K-K-R-x2-K-x4-K-R-x2-R-R-K-x-K-x2-R |
| R-R-x-R-R-x-R-x-R-R-R-x9-R-x2-R-R-K-R-K-x-R-x4-R-R-R-R-R-R-x4-R-K | |
| R-x-R-R-R-R-x6-R-x11-R-x8-R-R-x3-R-R-R-x2-R-R-x-R-x-R-x6-R-R-R-R-R-x4-R-R-x2-R | |
| R-x-R-R-x3-K-R-R-R-x2-R-x-R-R-x-R-x-R-x7-R-x3-R-R-R-x7-R-x2-R-R-R-R | |
| R-x-R-x-R-R-R-x3-R-R-R-x3-R-x-R-x2-R-x4-R-R-R-x5-R-K-x-R-x3-R-R- x13-R-R-x-K-x5-R-R-x6-K-R-R |
The C-terminal end of NAC TF proteins also contain monopartite, bipartite, and multipartite NLS motifs (Table 2, S2 Fig, S2 File). Removal of the linker amino acids present in between the consecutive basic amino acids, resulted in the identification of 94 unique consensus sequences. Some of the important NLS found in the C-terminal end were K-K-K (144), K-K-R (83), R-R-R (65), K-R-K (60), K-K-R-K-R (58) and others (Table 2, S2 Fig, S2 File). A comparison of the 97 NLS consensus sequence present in N-terminal region with the 94 NLS sequences present in the C-terminal region indicated that 84 NLS consensus sequences were shared between the N-terminal and C-terminal regions. This indicates that there is a close relationship between the NLS sequences in these two regions. An analysis of the unique NLS consensus sequence in the N-and C-terminal regions indicated that 13 NLS consensus sequences were unique to the N-terminal region whereas nine NLS consensus sequences were unique to the C-terminal region (Table 2, S2 Fig, S2 File). Up to six classes of NLS have been reported to be associated with importin α subunit [71]. To the best of our knowledge, this is the first report describing such a high level of diversity and dynamism in the NLS consensus sequences of NAC TFs and plant transcription factors in general. This is also the first report of the presence of unique NLSs in the N-and C-terminal regions of NAC TFs.
Several nuclear-associated proteins contain NLS, as well as nuclear export signals (NESs). Proteins that perform their function within the nucleus need to be exported out of the nucleus and into the cytoplasm to undergo proteosomal degradation. Therefore, a NES is required in addition to an NLS. A Ran-GTP complex binds directly to an NES and mediates the nuclear export process of the cargo molecules [72]. NES sequences contain a hydrophobic, conserved L-V-F-Y (substitute L-V/I-F-M) motif separated by variable linker amino acids at both ends [73]. The presence of an L-V-F-Y motif in all NAC proteins, suggests that all NAC proteins have the potential to be exported out of the nucleus. Hao et al. (2010), however, reported that the hydrophobic L-V-F-Y motif functions as a transcriptional repressor of WRKY, Dof, and APETALA TFs. If the L-V-F-Y motif (S3 Fig) acts as a transcriptional repressor, then the transcriptional activity of these TFs would be affected; resulting supressed transcriptional activities. Therefore, we feel that the L-V-F-Y motifs might not function as a transcriptional repressor for WRKY, Dof, and APETALA 2 transcription factor. Instead it act as a nuclear export signal sequence as reported by Kosugi et al. (2008) [73].
NAC TFs possess a complex interactome network
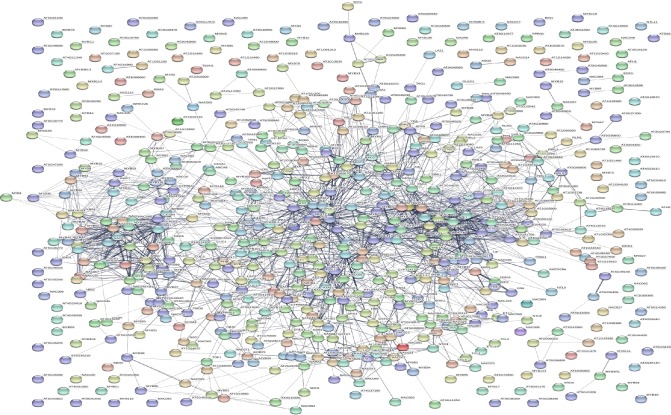
The interacting partner of a protein can provide significant information about its potential function and an entire protein-protein interactome network can greatly assist in unravelling the signalling cascade of the proteins. Different cascades are interlinked in signalling systems and form intricate constellations that provide information about cell response and function. Thus, the interactome network of NAC TFs in A. thaliana were explored. The presence of a dynamic network was revealed, and a diverse set of interacting protein partners of NAC TFs were identified (Fig 3, Table 3). The NAC TFs frequently interact with ABI (ABSCISIC ACID INSENSITIVE), VND7 (VASCULAR RELATED NAC DOMAIN), MYB (MYELOBLASTOSIS), DREB2A (DEHYDRATION RESPONSIVE ELEMENT BINDING), DREB2G, WRKY, JMJ (JUMONJI), LEA (LATE EMBRYOGENESIS ABUNDANT), KNAT (KNOX TAIL), CUC (CUP SHAPED COTYLEDON), MC5 (METACASPASES 5) and other important genes involved in plant growth, development, and stress responses (Table 3). In addition, NAC TFs was also found to interact with other NAC TFs as well (Table 3).
Fig 3. Interactome network of NAC TFs.
The interactome network of NAC TF reflects a diverse complex of interacting proteins. The NAC TFs of A. thaliana were utilized in the interactome network analysis. The interactome map of A. thaliana was determined using the string database (https://string-db.org).
Table 3. Interacting partners of NAC TFs in plants.
A. thaliana NAC TFs was used to construct the interactome network. Asterisk indicates no interaction.
| NAC TFs | Experimental Interactions | Co-expression | Text mining Interactions |
|---|---|---|---|
| NAC1 | RNS1, AT3G10260, AT1G17080 | NAC024, NAC095, ARV1, AT2G01410, AT1G60380, AT1G60340 | |
| NAC2 | ERD14 | NAC32, NAC102, DREB2A | NAC32, NAC102 |
| NAC3 | *** | **** | NTL |
| NAC4 | *** | **** | NTL, PLP transferase |
| NAC5 | **** | **** | CYP96A2, MYB |
| NAC7 | VND7 | XCP1, XCP2 | VND7, MYB46 |
| NAC8 | *** | ATM, ATR | ATM, ATR |
| NAC10 | *** | MYB83, MYB63 | MYB83, MYB85, MYB46, MY63, MYB58, MYB52, MYB69, KNAT |
| NAC11 | **** | **** | NAC95 |
| NAC12 | * | IRX1 | MYB46, MYB83, MYB58, MYB63, IRX9, APL, KNAT7 |
| NAC13 | RCD1 | AOX1A, RCD1 | AOX1A, RCD1, NAC88 |
| NAC14 | ASG2 | HB4, LZF1, NTL, BZIP61, MYB30, RSW3 | |
| NAC16 | NYE, NYC1, EEL, ABF2, PAP20, UTR1, TAG1 | ||
| NAC17 | TAG1, UTR1, UTR3, WRKY15, RGF6, FRU, AOX1A, NTL | ||
| NAC18 | GAI | NAM, NAC | |
| NAC19 | ZFHD1, TCP20, CPL1, TCP8, NAC32, RHA1A, RHA2A | NAC32, ERD1 | ZFHD1, TCP20, CPL1, TCP8, NAC32, RHA1A, RHA2A, ERD1 |
| NAC20 | AT3G43430, SHR, PHB, PLT2, MYB59, HB23, HB30 | TMO6, DOF6, SHR, PLT2 | TMO6, DOF6, SHR, PLT2, AT1G64620, AT3G43430 |
| NAC23 | **** | ***** | NAC95, AT3G01030, AT5G27880, AT5G01860, MYB64 |
| NAC24 | **** | ***** | NAC95, NAC47 |
| NAC25 | **** | At1g75910, GRP20, CYP86C4 | At1g75910, GRP20, CYP86C4 |
| NAC26 | VND7 | VND7, MYB83, XCP1, AT4G08160 | VND7, MYB46, MYB85, MYB83, XCP1 |
| NAC028 | ***** | ******* | TOM2A, TOM2B, TOM3, ARLA1C, ARLA1D, DBP1, PDLP2, OBE2 |
| NAC29 | NAC6, GRL, IAA14, | NAC6, HAI1 | NAC6, HAI1, SAG12, PI |
| NAC32 | HAI1, NAC019, ABI1, NAM, RVE2, PYL4 | ATAF1, HAI1, NAC019, GSTU7, | NAC102, NAM, NAC19ATAF1 |
| NAC36 | ***** | AT5G52760, XBAT34, AT5G52750, SOBIR1, RING1, WRKY53, WRKY46, SARD1, | AT5G42050 |
| NAC38 | BRM | MYB69, CIPK4, ABCA8 | AT4G29770, AIP2, SDE3 |
| NAC40 | NTL, MEE59, NPX, SCP2, SCO1, PUB18, PUB19, LB20 | ||
| NAC41 | NAC83 | NAC83, AT1G12810 | NAC83, GSTF3, AT1G12810 |
| NAC42 | **** | CYP71A12, GSTU10, AT5G38900, CYP71B6 | CYP71A12, GSTU10, AT5G38900 |
| NAC44 | **** | **** | AT1G54890, NAC90 |
| NAC45 | HB52, NAC97 | NAC97 | CYP71B34, WAK5, NAC97 |
| NAC46 | RCD1, BRM | CYP89A9, AT4G11910 | RCD1, AT1G78040, bHLH11, |
| NAC47 | *** | HAI1, Rap2.6L, NAC6 | NAC5, NAC24, HAI1, AT1G60380 |
| NAC48 | **** | ***** | CYP89A9, STAY-GREEN2 |
| NAC49 | **** | ***** | ERF115, WOX5, LBD19 |
| NAC50 | JMJ14, NAC052, GAI, TPL | NAC52, JMJ14 | JMJ14, PPR, NAC52, AT5G41650, CYP71A25 |
| NAC52 | JMJ14, NAC50 | JMJ14, PPR, UBP14 | JMJ14, NAC50, PPR, CRCK2, PPD6, MFDX1, CYP71A25 |
| NAC53 | **** | BZIP60, UGT73B, DREB2A, MYB27 | NTL, PUM4, MYB103, |
| NAC55 | ZFHD1, HAI1, F2P16.14 | ERD1, AT2G31945, MYB2 | ZFHD1, ERD1, HAI1, ABF2, bZIP, MYC2 |
| NAC57 | ***** | ***** | MYB19, AT3G58090, AT1G07730, AT4G13580, AT3G13650 |
| NAC58 | ***** | RWP1, ABCG6, CYP86A1 | PPR, RWP1, ABCG6, MYB86, MYB26 |
| NAC60 | **** | ABI4, DREB2G, WOX12 | NACA5, NTL, SCP2, SCO1, ZFP3, GRF7 |
| NAC61 | **** | NAC90, ACS4, | NAC44, LEA, NAC85, NAC95, NAC90, |
| NAC62 | **** | BZIP60, CZF, WRKY33, TIP, SZF1, CPK32, CPK28, TET8, | BZIP60, WRKY33, TIP |
| NAC63 | ***** | ****** | LRR, NAC95, ATPMEPCRD, |
| NAC64 | ***** | ***** | AT3G59880, AT5G50540, AT2G44010, sks16, SKS6 |
| NAC66 | ***** | ***** | MYB26, MYB46, MYB83, MYB85, MYB63, MYB58, KNAT7, WRKY12 |
| NAC67 | ***** | **** | NAM, AT1G78040, NAC95 |
| NAC68 | ***** | BZIp60, NAC62 | NTL, LPP gamma, LINC2, DEG9, S1P, ENODL17, RPL23AB |
| NAC69 | **** | NAC95 | NTL, IAA30, RIN3, SPT16, RLP18 |
| NAC71 | **** | WNK, TM6, AT1G64625 | Rap2.6L, AT2G41870, RAP2.4 |
| NAC73 | **** | MYB46, MYB83, IRX1, IRX3, CESA4 | MYB46, MYB83, IRX1, IRX3, MYB63, CESA4 |
| NAC74 | F2P16.14, TOPLESS, BRM | DSEL, scpl31, HXXXD type | SCRL20, F-ox/LLR, sks11 |
| NAC75 | ***** | RING/U-box | GATA5, LBD15, GATA12, JLO, scpl48, RNS3, EIF3E, SHM7 |
| NAC76 | VND7, NAC83 | **** | VND7, NAC83, UBQ, MYB46 |
| NAC77 | ****** | ****** | DOT5, NAC23, LBD10, NF-YB7, MYB84, GRF5, GRF7, RR8 |
| NAC78 | ****** | PIP-3 | NTL, MAYB27, MYB103, PUM4, KNAT2, KNAT6, SUF4, GH9B8 |
| NAC80 | BRM | ***** | PPR, TT7, 4CL3, BRM |
| NAC82 | SRO1, RCD1 | ***** | UBX, WW |
| NAC83 | VND7, NAC41, CUC2, VND1, NAC105, NAC76, NAC101, NAC1 | ***** | VND7, NAC41, CUC2, VND1, NAC105, NAC76, MYB83, MYB46 |
| NAC84 | **** | EDF3 | ZFP10, Delta9, EDF3, SPT16, GS1 |
| NAC85 | **** | **** | LEA, PUP4, NAC90, NAC61, XERO1 |
| NAC87 | **** | **** | SWAP, WRKY36, TIR-NBS, NBS-LRR, BHLH11 |
| NAC88 | **** | **** | UBC18, NAC17, NAC13, NAC53 |
| NAC89 | VAP27-1, TSPO, TI1, | ***** | BZIP28, BZIP60, MC5 |
| NAC90 | ***** | AT3G57460, MPK11 | DTA4, CHI, NAC44, NAC85, LEA |
| NAC94 | ***** | ***** | MC5, D111, RML, BAG6, LCAT3, AATP1, BZIP28 |
| NAC95 | ***** | NAC24, NAM | NAC23, NAM, NAC24, MAY64, NAC69 |
| NAC96 | T21F11.18 | ***** | ABF2, Dna-J, TOPLESS, |
| NAC97 | NAC45, LRR, BRM | ***** | ****** |
| NAC100 | ***** | ***** | AT4G27850, AT1G26410, GRP20, TT7, 4CL3, |
| NAC101 | RPA2, VND7, VR-NAC, NAC83 | ***** | NVD7, NAC83, XCP1, UBQ, RNS3 |
| NAC102 | **** | ATAF1, tolB, NAC32, RHL41, ZAT6, UGT73B2 | ATAF1, NAC32 |
| NAC103 | **** | ***** | BZIP60, BZIP28, D111, CLPTM1, NAC44 |
| NAC105 | VND7, NAC83, | ***** | VND7, GH, NAC83, UBQ, LAC1, MYB46, RIC4 |
The expression of several of NAC genes are either up- or down-regulated by auxin, ethylene, or ABA, suggesting that NAC TFs play a role in plant hormonal signalling [74–76]. One of the most challenging aspects of a protein-protein interactome network is that the interaction can vary depending upon the cell and its environment [77]. Therefore, it is necessary to investigate the dynamic interactions of proteins in different cells and environmental conditions to completely understand their interacting partner and the cellular function of the TF. NAC TFs regulate ERD and NCED (ABA biosynthesis) genes through a direct interaction with their promoters [78,79]. NAC TFs (ANAC019, ANAC055, and ANAC072) interact with ERD1 which encodes a Clp protease regulatory subunit [80]. The overexpression of one of these three NAC TFs, however, did not induce the up-regulation of ERD1 because the induction of ERD1 depends on the co-expression of a zinc finger homeodomain TF, ZFHD1 [80]. ANAC019 and ANAC055 interact with ABI (abscisic acid insensitive), and at least five MYB TFs can bind to the NAC TF promoter region [81,82]. In this case, the NAC DNA binding domain mediates the interaction with RHA2A and ZFHD1 [82].
NAC TFs encodes chimeric proteins and contain multiple binding sites
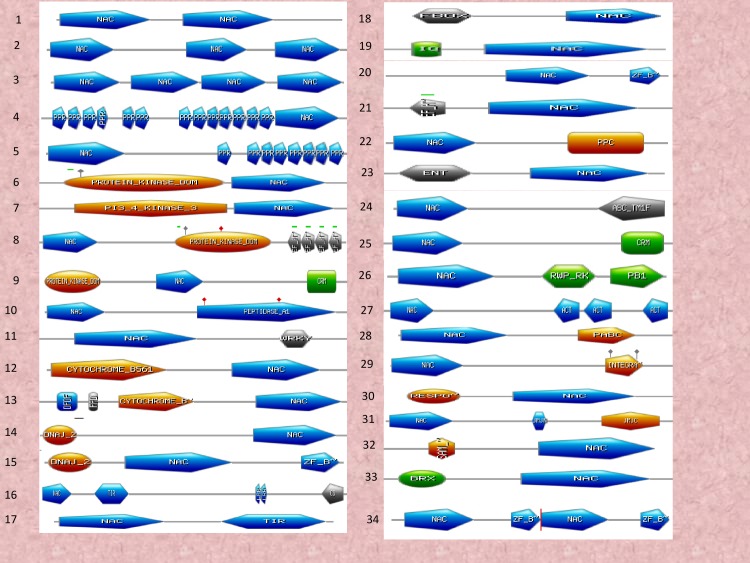
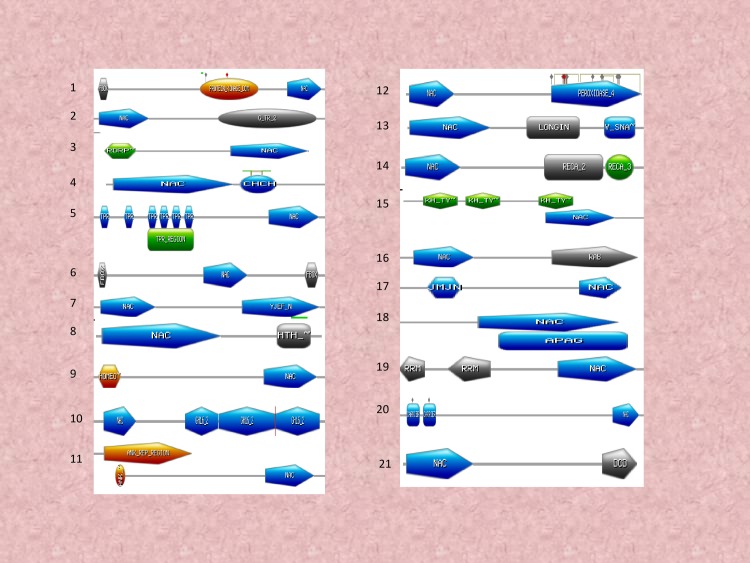
NAC TFs are characterised by the presence of a DNA binding domain. Several NAC TFs, however, contain more than one NAC domain. Chimeric NAC TFs have also been identified. At least 45 variants of chimeric NAC TFs were identified in our analysis (Fig 4). Several of the NAC TFs were also found to possess as many as three or four NAC DNA binding domains. Furthermore, the NAC domains were found to be associated with PPR (pentatricopeptide), protein kinase, PI3_4_kinase_3, EF-hands (elongation factor), CRM, peptidase A1, WRKY, cytochrome B561, OFOF, FFO, Dna_J2, ZF_B, TIR, LRR, CS, F-box, IQ, PPC, ENT, ABC_TM1F, RWP_RK, PB1, PABC, ACT, INTEGRA, RESPO, JMJC, SAM, BRX, G_TR_2, RORP, CHCH, TPR, YJEF_N, HTH, HOMEO, GH16, ANK_REP_REGION, Peroxidase, LONGIN, V_SNA, RECA_2, KH_TY, APAG, RRM, carrier, and a DCO domain. At least four NAC TFs from A. thaliana, ten from B. napus, four from B. rapa, two from M. domestica, four from P. virgatum, 17 from C. sativa, eight from D. oligosanthes, eight from E. tef, and five from L. perrieri were found to possess 2 NAC domains (S1 Table). NAC TFs in several other species were also found to contain two NAC domains (S1 Table). When two NAC domains were present, both domains were located towards the N-terminal end. NAC TFs of at least three species, O. rufipogon, B. stacei, and Camelina sativa were found to possess three NAC domains whereas the NAC TFs in A. lyrata (gene id: 338342), C. sativa (Csa16g052260.1), and E. tef (462951506) were found to possess four NAC domains (Fig 4). Other chimeric domains were also identified in different regions of the NAC protein (Fig 4). The F-box and protein kinase domain was followed by a NAC domain and the NAC domain was followed by a G_TR_2 domain (Fig 5).
Fig 4. Chimeric NAC domains.
NAC TFs possess chimeric NAC domains with at least 34 diverse chimeric NAC domains identified in the studied species. (1) two NAC domain (2) three NAC domain (3) four NAC domain (4) 13 PPR repeats followed by a NAC (5) NAC domain followed by eight PPR repeats (6) protein kinase domain followed by NAC (7) PI3_kinase_3 domain followed by NAC (8) NAC domain followed by kinase and EF-hand domain (9) protein kinase domain followed by NAC and CRM domain (10) NAC domain followed by peptidase A1 domain (11) NAC domain followed by WRKY domain (12) cytochrome B561 domain followed by NAC (13) two DFDF domain followed by cytochrome B and NAC (14) DNA_J2 domain followed by NAC (15) DNA_J2 domain followed by NAC and ZF_B domain (16) NAC domain followed by a TIR, two LRR and a CS domain (17) NAC followed by TIR domain (18) F-box domain followed by NAC (19) IQ domain followed by NAC (20) NAC domain followed by ZF_B domain (21) EF-hand domain followed by NAC (22) NAC domain followed by PPC domain (23) ENT domain followed by NAC (24) NAC domain followed by ABC_TM1F domain (25) NAC domain followed by CRM domain (26) NAC domain followed by RWP_RK and PB1 domain (27) NAC domain followed by three ACT domain (28) NAC domain followed by PABC domain (29) NAC domain followed by INTEGRA domain (30) RESPO domain followed by NAC (31) NAC domain followed by JMJN and JMJC domain (32) SAM domain followed by NAC (33) BRX domain followed by NAC and (34) repeat of NAC and ZF_domain. The identification of chimeric NAC domain sequences was determined using the ScanProsite and InterProScan server. The details regarding the presence of chimeric NAC TF in different taxa can be found in S1 Table.
Fig 5. Chimeric NAC domains NAC TFs possess chimeric NAC domains with at least 21 diverse chimeric NAC domains identified in the studied species.
(1) F-box domain followed by protein kinase and NAC domain (2) NAC domain followed by G_TR_2 domain (3) RDRP domain followed by NAC (4) NAC domain followed by CHCH domain (5) TPR repeats followed by NAC domain (6) F-box domain followed by NAC and F-box domain (7) NAC domain followed by YJEF_N domain (8) NAC domain followed by HTH domain (9) Homeobox domain followed by NAC domain (10) NAC domain followed by three GH6.2 domain (11) ANK repeat domain followed by NAC domain (12) NAC domain followed by peroxidase domain (13) NAC domain followed by LONGIN and V_SNA domain (14) NAC domain followed by RECA_2 and RECA_3 domain (15) KH_TY repeats followed by NAC domain (16) NAC domain followed by RAB domain (17) JMJN domain followed by NAC domain (18) NAC domain followed by APAG domain (19) two RRM domain followed by NAC domain (20) carrier domain followed by NAC domain and (21) NAC domain followed by DCO domain. The identification of chimeric NAC domain sequences was determined using the ScanProsite and InterProScan server. The details regarding the presence of chimeric NAC TF in different taxa can be found in S1 Table.
The presence of chimeric domains within NAC TFs is of particular interest, especially for understanding why they are there and how they impact the function of a specific NAC TF. The most common domains, such as PPR, TIR, WRKY, protein kinase, ZF_B, EF-hands, cytochrome B, DNAJ, F-box, peroxidase, and GH16 are involved in diverse cellular processes, including transcriptional regulation of plant development and stress response [83–91]. The association of a TIR domain with an NBS-LRR domain is an example of the association of TF domains with other domains to form chimeric proteins [92]. The presence of different domains with the NAC domain could potentially enable the NAC domain to assist in the function of the associated domains and vice versa. For example, NAC TFs could have the potential to regulate peroxidase by possessing a peroxidase domain within the NAC TF, instead of regulating it separately with another TF. The presence of multiple domains can enable the co-regulation of diverse functional sites within the NAC TFs. The presence of chimeric TFs has been recently reported in WRKY TFs as well [93,94]. Therefore, the presence of chimeric domains in NAC TFs can impart a significant dynamic aspect to the ability of NAC TFs to regulate gene expression.
In addition to the presence of multiple chimeric domains, NAC TFs were also found to contain diverse active/binding motifs for several other proteins. It is possible that NAC TFs may play a dual role as a transcription factor and as an enzyme. At least 404 NAC TFs were found to possess other functional motifs comprising 101 unique functional sequences (S2 Table). Some of the highly abundant functional motifs of NAC TFs were 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase signature, aldehyde dehydrogenase glutamic acid active site, lipocalin signature, phosphopantetheine attachment site, cysteine protease inhibitor, ATP synthase alpha and beta subunit signature, aminotransferase class II-pyridoxal-phosphate attachment site and others (S2 Table). This is the first study to report the presence of such a diverse number of functional sites and signature motifs in NAC TFs. Although majority of the functional domains are associated with a specific function in plants, the presence of a histocompatibility complex and a translationally controlled tumour protein (TCTP) sequence are of very interesting. These proteins are specifically found in animal systems and the histocompatibility complex is the major contributing factor regulating the binding of antigens. More specifically, TCTP is a highly conserved protein that is involved in microtubule stabilization, calcium binding, and apoptosis and is associated with the early growth phase of tumours [95]. The presence of MHC and TCTP in association with NAC domains suggests that this combination may be playing a crucial role in the plant immune system and in uncontrolled cell growth. The presence of diverse functional sites in NAC TFs indicates that NAC TFs are involved in diverse cellular functions and metabolic pathways. This statement is supported by the large number of NAC TFs that are present in plant genomes.
NAC TFs are involved in diverse cellular processes
NAC TFs are known to possess diverse chimeric domains, as a result, it is more than likely that NAC TFs are also involved in the regulation of diverse cellular pathways and cellular processes. To help substantiate this premise, the interactome associated with NAC TFs in A. thaliana was analysed. Results indicated that NAC TFs are potentially involved in a least 289 different cellular processes and pathways (S3 Table). The majority are related to cell, tissue, and organ (root, stem, meristem) development, as well as signalling processes. Several NAC TFs also appear to be associated with phytohormone signalling, including auxin, gibberellin, jasmonic acid, and salicylic acid signalling pathways. NAC TFs were also found to be associated with pathways involved in the response to bacterial, fungal, UV, heat and other biotic and abiotic stresses (S3 Table). At least 202 genes in the NAC TF interactome network were found to be associated with pathways related to the nucleus, 239 were associated with intracellular membranes, and 241 were associated with intracellular organelles, 20 with the endoplasmic reticulum, and 3 with the nuclear matrix. If the association is designated based on the description of a pathway, 127 genes were found to be associated with transcription factor activity and sequence-specific DNA binding, 143 with DNA binding, 146 with nucleic acid binding, 220 with organic cyclic compound binding, 220 with heterocyclic compound binding, 65 with ATP binding, 49 with macromolecular complex binding, 48 with chromatin binding, 35 with ADP binding, 25 with sequence-specific DNA binding, 18 with transcription regulatory region binding, 8 with structural constituents of the cell wall, 11 with auxin transport activity, 2 with LRR binding, and 2 with bHLH transcription factor binding. These data clearly indicate that NAC TFs are involved in diverse cellular processes. The identification of LRR protein in the pathway description of NAC TFs agrees with the presence of an LRR domain in a chimeric NAC domain of NAC TFs.
NAC TFs are expressed in a spatiotemporal manner
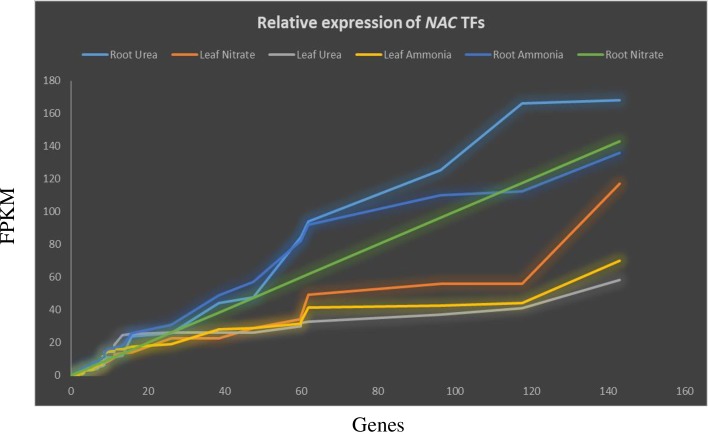
Plant uses ammonia, nitrate, and urea as the source of nitrogen for its growth and development. Nitrogen is also associated with an increased rate of photosynthesis. Therefore, the role of ammonia source in the growth and development of the plants is very important. Nitrate is readily available as nitrogen source for plants and the uptake of nitrate is high in the acidic pH whereas the uptake of ammonia is high in the neutral pH. Studying the expression pattern of NAC TFs in nitrate and ammonia treated plant can explains how different nitrogen source modulate the expression of NAC TFs and give the glimpse of their role in plants growing in the acidic and neutral pH soil. Urea is applied as an artificial nitrogen sources for the plants when there is a lack of nitrate or ammonia in the soil. Therefore, patterns of NAC TF gene expression were analysed in leaf and root tissues of A. thaliana treated with ammonia, nitrate, or urea (Fig 6). Among a total of 120 NAC TFs, 95, 97, and 98 were differentially expressed in leaf tissue treated with ammonia, nitrate, or urea, respectively. Leaf tissues treated with ammonia, nitrate and urea exhibited 70.14, 117.11, and 58.35 FPKM expression values for AtNAC1 (AT1G01010.1), AtNAC4 (AT1G02230.1), and AtNAC1 (AT1G01010.1), respectively. At least 46 genes in leaves exhibited expression of more than one FPKM in response to ammonia, 54 in response to nitrate, and 44 in response to urea. AtNAC1 was highly expressed in ammonia and urea treated leaves. At least 24, 26, and 25 NAC TFs did not exhibit any expression in leaf tissues treated with ammonia, nitrate, or urea. The AtNAC1 is involved in auxin signaling and modulates lateral root formation [74,96,97]. The higher expression of AtNAC1 with response to treatment of nitrogenous compound reflects it role in plant development. AtNAC4 is reported to be involved in nitrate transport and its higher expression in nitrate treated plant directly indicate its active role nitrogen transport and assimilation [98].
Fig 6. Differential expression of NAC TFs in leaves and roots of A. thaliana plants treated with ammonia, nitrate, and urea.
The expression of A. thaliana NAC TFs was analysed to determine their response to different sources of nitrogen. Urea and ammonia in root tissue show higher expression level whereas urea treated leaf tissue showed low level of NAC expression. The expression data were obtained from the PhytoMine database in Phytozome and presented as FPKM (Fragments per Kilobase of transcripts per million mapped reads). The X-axis represents the NAC TF genes and Y-axis represent the Fragments per Kilobase of transcripts per million mapped reads.
Relative to leaf tissues, the expression of NAC TFs in root tissues was more dynamic. Root tissue treated with urea exhibited the highest expression of NAC TFs relative to leaves treated with ammonia or nitrate (Fig 6). The number of AtNAC TFs whose expression was one or more FPKM in response to ammonia, nitrate, or urea were 75, 71, and 70, respectively. AtNAC8 (AT5G08790.1) was highly expressed in ammonia-treated roots, whereas, AtNAC91 (AT5G24590.2) was highly expressed in nitrate- and urea-treated roots. Urea, ammonia and nitrate (UAN) commonly serve as a source of nitrogen (N) for plants. Analysis of the levels of gene expression indicate that ammonia and nitrate modulate the expression of NAC TFs more than urea. A study utilizing Pinus taeda revealed that fertilization with ammonium, nitrate, or urea produces different effects on growth and drought tolerance [99]. Results of the current analysis indicate that AtNAC8 and AtNAC91 are the major NAC TFs involved in nitrogen assimilation during plant growth. The TaNAC8 was reported to be associated with strip rust and abiotic stress responses [100,101].
Codon usage in NAC TF is dynamic
Codon usage bias in NAC TFs of the examined species were studied. separately. Among 61 sense codons, only 14 were found in the all species. These included AAG (K), ACU (R), AGA (R), AGG (R), UCU (S), AUC (I), AUG (M), CAA (Q), CCU (P), GAA (E), GCU (A), GGA (G), UGG (0), and UUC (F) (Table 4). The most abundant codon was UCU (S), which was found 30 times in in Humulus lupulus NAC TFs (Table 4). The codons CGA (R), CGC (R), CGG (R), CGU (R) were absent in 127 of the 160 examined species. ACG (T), UCG (S), CAG (Q), CAC (H), CCA (P), CCC (P), CCG (P), and GCG (A) were absent in 126 of the examined species (S4 File). The highest relative synonymous codon usage bias (RSCU) was found to be 1.35, 1.23, 1.29 for the codon AAA (K) in Ocimum tenufolium, Picea sitchensis, and Ipomea trifida. Synonymous codon-usage was not observed in NAC TFs. Relative codon usage is determined by dividing the ratio of observed frequency of codons by the expected frequency, provided that all of the synonymous codons for the same amino acids are used equally. Relative Synonymous Codon Usage (RSCU), however, is not related to the usage of amino acids. An RSCU > 1 indicates the occurrence of codons more frequently than expected, while an RSCU < 1 indicates that the codon occurs less frequently than expected [102,103]. Non-synonymous substitution in organisms is subject to natural selection [104,105]. Genes with lower non-synonymous selection leads to functional diversity of a gene. The presence of a low level of nonsynonymous codon usage in NAC TFs indicates that they are functional and have evolved from paralogous ancestors.
Table 4. Codon usage of NAC TFs in plants.
| Codons | Codon present in No. of species | Codon absent in No. of species | Average abundance of codons | Highest no. of codons | Name of the species with highest no. of codons |
|---|---|---|---|---|---|
| AAA (K) | 126 | 20 | 4.77 | 9.9 | Glycine soja |
| AAG (K) | 146 | 0 | 10.75 | 24.2 | Sphagnum fallax |
| AAC (N) | 144 | 2 | 3.66 | 14.2 | Beta vulgaris |
| AAU (N) | 127 | 19 | 9.25 | 20.5 | Spinacia oleracea |
| ACA (T) | 139 | 7 | 2.33 | 15.2 | Citrus sinensis |
| ACC (T) | 137 | 9 | 2.4 | 17 | Amborella trichopoda |
| ACG (T) | 20 | 126 | 5.91 | 13 | Dorcoceras hygrometricum |
| ACU (T) | 146 | 0 | 7.42 | 16.6 | Sesamum indicum |
| AGA (R) | 146 | 0 | 10.92 | 24.3 | Klebsormidium flaccidum |
| AGG (R) | 146 | 0 | 4.12 | 18.8 | Amborella trichopoda |
| CGA (R) | 19 | 127 | 5.22 | 13.9 | Linum usitatissimum |
| CGC (R) | 19 | 127 | 2.47 | 6 | Linum usitatissimum |
| CGG (R) | 19 | 127 | 3.93 | 8.6 | Citrullus lanatus |
| CGU (R) | 19 | 127 | 2.06 | 4.7 | Linum usitatissimum |
| AGC (S) | 143 | 3 | 3.54 | 24.2 | Beta vulgaris |
| AGU (S) | 144 | 2 | 1.83 | 5.2 | Dorcoceras hygrometricum |
| UCC (S) | 141 | 5 | 4.51 | 12.3 | Aegilops tauschii |
| UCG (S) | 20 | 126 | 2.64 | 6.4 | Dorcoceras hygrometricum |
| UCU (S) | 146 | 0 | 4.65 | 30.5 | Humulus lupulus |
| UCA (S) | 139 | 7 | 5.09 | 15.1 | Morus notabilis |
| AUA (I) | 124 | 22 | 4.80 | 15.3 | Sphagnum fallax |
| AUC (I) | 146 | 0 | 5.10 | 16.7 | Sphagnum fallax |
| AUU (I) | 126 | 20 | 8.71 | 15.9 | Spinacia oleracea |
| AUG (M) | 146 | 0 | 7.81 | 22.8 | Sphagnum fallax |
| CAA (Q) | 146 | 0 | 5.31 | 15.4 | Fragaria vesca |
| CAG (Q) | 20 | 126 | 13.3 | 22.6 | Linum usitatissimum |
| CAC (H) | 20 | 126 | 6.64 | 10.9 | Beta vulgaris |
| CAU (H) | 144 | 2 | 4.45 | 9.7 | Setaria viridis |
| CCA (P) | 20 | 126 | 11.09 | 16.3 | Dorcoceras hygrometricum |
| CCC (P) | 20 | 126 | 14.18 | 19.2 | Amborella trichopoda |
| CCG (P) | 20 | 126 | 5.10 | 11.1 | Dorcoceras hygrometricum |
| CCU (P) | 146 | 0 | 8.00 | 24.7 | Klebsormidium flaccidum |
| CUA (L) | 143 | 3 | 5.83 | 28.3 | Sphagnum fallax |
| CUC (L) | 123 | 23 | 5.74 | 23.6 | Sphagnum fallax |
| CUG (L) | 142 | 4 | 5.87 | 43.9 | Sphagnum fallax |
| CUU (L) | 145 | 1 | 5.94 | 32.6 | Sphagnum fallax |
| UUG (L) | 125 | 21 | 5.94 | 24.4 | Sphagnum fallax |
| UAA (L) | 124 | 22 | 5.37 | 17.2 | Sphagnum fallax |
| GAA (E) | 146 | 0 | 4.62 | 27 | Klebsormidium flaccidum |
| GAG (E) | 145 | 1 | 5.54 | 18.1 | Sphagnum fallax |
| GAC (D) | 145 | 1 | 5.05 | 14.9 | Beta vulgaris |
| GAU (D) | 144 | 2 | 5.86 | 21.7 | Spinacia oleracea |
| GCA (A) | 135 | 11 | 5.49 | 18.5 | Citrus sinensis |
| GCC (A) | 130 | 16 | 5.05 | 15 | Amborella trichopoda |
| GCG (A) | 20 | 126 | 4.64 | 11.2 | Dorcoceras hygrometricum |
| GCU (A) | 146 | 0 | 4.65 | 31.1 | Setaria viridis |
| GGA (G) | 146 | 0 | 4.63 | 27.5 | Setaria viridis |
| GGC (G) | 141 | 5 | 5.41 | 17.1 | Amborella trichopoda |
| GGG (G) | 145 | 1 | 2.7 | 6.7 | Elaeis guineensis |
| GGU (G) | 145 | 1 | 2.40 | 5.9 | Elaeis guineensis |
| GUA (V) | 140 | 6 | 1.46 | 3.5 | Sphagnum fallax |
| GUC (V) | 123 | 23 | 0.93 | 2 | Morus notabilis |
| GUG (V) | 142 | 4 | 4.35 | 11.8 | Beta vulgaris |
| GUU (V) | 143 | 3 | 5.38 | 16.6 | Klebsormidium flaccidum |
| UAC (Y) | 138 | 8 | 3.84 | 10.1 | Morus notabilis |
| UAU (Y) | 126 | 20 | 6.23 | 14.8 | Solanum melongena |
| UGG (W) | 147 | 0 | 3.89 | 14.5 | Vitis vinifera |
| UGC (C) | 143 | 3 | 5.14 | 15.6 | Oropetium thomaeum |
| UGU (C) | 145 | 1 | 3.9 | 9.6 | Zoysia matrella |
| UUC (F) | 146 | 0 | 4.60 | 25.4 | Picea glauca |
| UUU (F) | 126 | 20 | 10.67 | 19.2 | Sphagnum fallax |
Rate of transition of NAC TFs is higher than the rate of transversion
Nucleotide mutation is an integral part of the evolution of a genome and leads to the acquisition of required traits and the elimination of detrimental traits from the genome. It is a regular process and hundreds of thousands of nucleotides have undergone addition or deletion events in the evolution of a genome. The alteration or conversion of a nucleotide occurs either through a transition or a transversion. A transition event involves the interchange of two-ring purines (A and G) or of one-ring pyrimidines (C and T). Transversion events the exchange of a purine for a pyrimidine or vice versa. The rate at which these two events occur is important to understanding of the evolution of a gene. Therefore, the rate of nucleotide substitution in NAC TFs was analysed. Results indicated that the rate of transition in NAC TFs is higher than the rate of transversion. The substitution of adenine with guanine was found to be highest in Linum usitatissimum (15.82), while the substitution of guanine to adenine was found to be the highest in Lotus japonicas (19.07). The lowest rate of substitution from adenine to guanine and vice versa was found in Trifolium pratense (9.73) and Amborella trichopoda (10.8), respectively (S4 Table). The highest rate of substitution from thiamine to cytosine and vice versa was found in Klebsormidium flaccidum (7.19) and Pseudotsuga menziesii (11.59), respectively. The lowest rate of substitutions from thiamine to cytosine and vice versa was found in Capsella grandiflora (2.41) and Cicer arietinum (1.62), respectively (S4 Table). These data make it evident that the rates of transition of purine (adenine and guanine) nucleotides are higher than the rates of pyrimidines. The highest rate of transversion from adenine to thiamine and vice versa was found in Capsella grandiflora (12.34 for adenine to thiamine and 9.91 for thiamine to adenine) (S4 Table). The rate of substitution by transversion is slower relative to the rate of substitution by transition.
Capsella grandiflora is a close relative of Arabidopsis thaliana and is predicted to be the progenitor of Capsella bursa-pastoris. Capsella grandiflora is a self-pollinating plant and is used as a model organism in evolutionary studies and the change from self-incompatibility into self-compatibility. The genomic consequences of the evolution of selfing, however, is poorly understood. Capsella rubella, a close relative of Capsella grandiflora, that evolved self-compatibility 200,000 years ago [106] also exhibits a high rate of transversion from adenine to thiamine (11.19). Thus, the higher rate of transversion from adenine to thiamine in Capsella grandiflora and Capsella rubella may be a possible factor in the evolution of self-pollination. Higher rates of transversion were also found in Solanum pimpinellifolium (11.4) and Castanea mollissima ((11.31) Chinese chestnut). Solanum pimpinellifolium is self-pollinating and exhibits high levels of stress tolerance [107]. Castanea mollissima has evolved over a period of time in coexistence with chestnut blight and is resistant to the pathogen. This indicates that higher rates of transversion from adenine to thiamine and vice versa are associated with self-pollination and stress tolerance in plants. The highest rate of substitution from guanine to cytosine and vice versa was found in Arachis hypogaea (11.07), and Camelina sativa (11.46), respectively (S4 Table). The lowest rate of substitution from adenine to thiamine and vice versa was found in Linum usitatissimum (3.72) and Klebsormidium flaccidum (6.67), respectively. Notably, the highest rate of substitution from thiamine to cytosine was found in Klebsormidium flaccidum and the highest rate of substitution from adenine to guanine was found in Linum usitatissimum. This indicates that organisms which exhibit the highest rate of transition possess the lowest rate of transversion.
NAC TFs evolved from orthologous ancestors
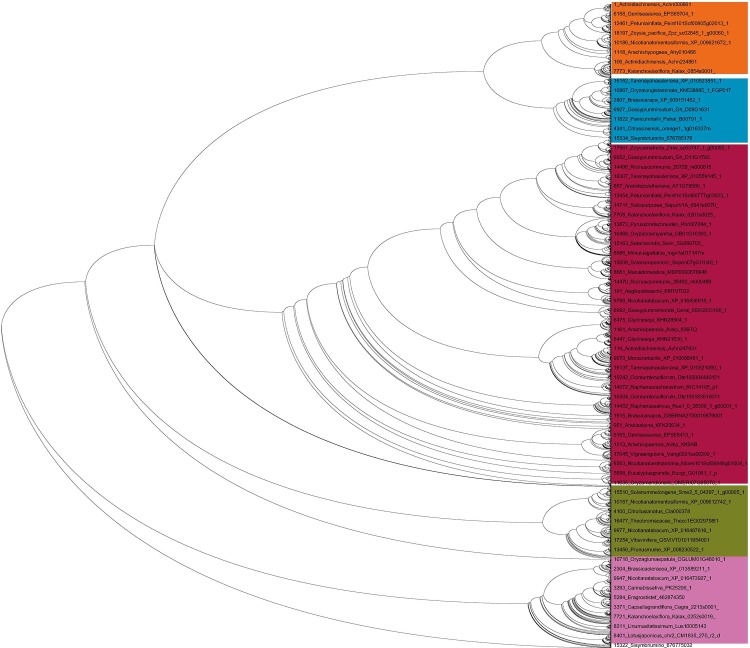
A phylogenetic tree of NAC TFs was constructed to understand their evolutionary relationships. A model selection was conducted before constructing the phylogenetic tree using the maximum likelihood statistical method. The phylogenetic tree revealed the presence of at least seven phylogenetic clustered orthologous groups (COGs) originating from a common, orthologous ancestor (Fig 7). Each phylogenetic cluster was further divided into two or more sub-groups. A phylogenetic tree of each individual species was subsequently constructed to examine the duplication and loss events in NAC TFs. The phylogenetic tree of each species was independently reconciled with the collective species tree. This analysis indicated that NAC TFs in all of the species were duplicated and none of a NAC TFs was found to be lost. This suggest that NAC TFs evolved from common ancestors (orthology) and underwent numerous duplication events during the divergence and speciation (paralogy) events, which gave rise to diverse gene functions in plant development and growth. The NAC TFs of K. flaccidum might be the most possible common ancestors of some plant species and the NAC TFs of other algal species could have contributed towards the evolution of other NAC TFs in plants. If the duplication would have disrupted the normal functioning of the cell, the organism might have reduced its reproductive fitness and would have been died. However, the duplication of NAC TFs possesses beneficial character thus providing the fitness advantage. Gene duplication contribute to the evolution that provides new genetic content for mutation, selection, and drift to act and to create new evolutionary opportunities [108]. Genome duplication is a common event in plants and multiple event of genome duplication have occurred during the diversification of angiosperms [109]. Genome duplication sometimes followed by the increased rate of evolution of some important genes [109]. The duplicated genes is responsible for the functional divergence and may play role in escaping the extinction [109,110]. In addition, duplication can lead to decreased probabilities of extinction, increase genetic variation, mutational robustness, and tolerance to changing environmental conditions [109]. The genetic variation incurred by duplication contribute to selection pressure and provide the opportunities for survival diverse environmental stress. Being, NAC TFs are highly duplicated, they might be providing such genetic variability in the plant kingdom to evade diverse environmental responses.
Fig 7. Phylogenetic tree of NAC TFs.
A phylogenetic tree of NAC TF reveals the presence of seven clustered orthologous groups (COGs). Each group also possesses two or more sub-groups. The phylogenetic tree shows lineage (monocot/dicot) specific grouping of NAC TFs. The phylogenetic tree was constructed using the neighbour-joining method with 1000 bootstrap replicates.
We also checked for the presence of potential foreign or homologous sequences (xenologs) in NAC TFs. No primary xenologs, sibling donor xenologs, sibling recipient xenologs, incompatible xenologs, autoxenologs, or paraxenologs were identified in NAC TFs. Although the phylogenetic tree indicates the evolution NAC TFs from common ancestors, none of the NAC genes in the examined species were found to have been transferred from one species to another. Previous studies of NAC TFs in six plant species also reported a high level of duplication and divergent evolution [111]. The expansion of TF families was associated with an increase in the structural complexity of the organism [112]. Previous studies reported the lineage-specific grouping of transcription factors [93,111]. The phylogenetic tree of NAC TFs also revealed the presence of lineage-specific clustering as well. In a few cases, however, order-specific clustering of NAC TFs was also observed. For example, NAC TFs in dicot species of the Brassica lineage, including A. thaliana, A. halleri, B. napus, B. rapa, R. sativus, R. raphanistrum, C. rubella, A. alpine, and others, grouped together. Similarly, NAC TFs in monocot plant species, including O. sativa, O. nivara, B. distachyon, and others, also grouped together.
Conclusion
NAC TFs are present in higher plants, as well as in a few species of algae. The number of NAC TFs per genome and their structural and functional properties increased with the complexity of the organism. The algae Klebsormidium flaccidum, a charophyte, was also found to possess NAC TFs; suggesting that the evolution of NAC TFs was associated with the adaptation of plant life from an aquatic to a terrestrial form. The paralogous evolution of NAC TFs underlies their diverse functional role in plant growth and development. Duplication events in NAC TFs were greater than deletion events and the absence of any loss of NAC TFs in different plant species indicates their evolution in recent times. As NAC TFs play a pivotal role within the nucleus regulating gene expression, the presence of bipartite and multipartite nuclear localization signals is of particular interest and provides the basis for further investigation of their functional roles.
Supporting information
(DOCX)
(PDF)
(PDF)
(DOCX)
(XLSX)
Sheet 1 of the file show all the raw N-terminal NLS consensus sequences, unique NLS with linker amino acids, and unique NLS post removal of linker amino acids. Sheet 2 represents the number of occurrences of N-terminal NLS and sheet 3 represents C-terminal NLS, number of occurrences, C-terminal unique NLS, and N-and C-terminal unique NLS.
(XLSX)
(XLSX)
(XLSX)
(XZ)
(XZ)
(RAR)
Acknowledgments
The author would like to extend their sincere thanks to the Natural and Medical Sciences Research Center, University of Nizwa to facilitate the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author would like to extend their sincere thanks to the Natural and Medical Sciences Research Center, University of Nizwa to facilitate the study. The authors would also like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2019/134), King Saud University, Riyadh, Saudi Arabia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yanagisawa S. Transcription factors in plants: Physiological functions and regulation of expression. J Plant Res [Internet]. 1998;111:363–71. Available from: 10.1007/BF02507800 [DOI] [Google Scholar]
- 2.Nuruzzaman M, Sharoni AM, Kikuchi S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front Microbiol [Internet]. 2013;4:248 Available from: https://www.frontiersin.org/article/10.3389/fmicb.2013.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan X, Stege J, Kim M, Dahmani Z, Fan N, Heifetz P, et al. Heritable endogenous gene regulation in plants with designed polydactyl zinc finger transcription factors. Proc Natl Acad Sci [Internet]. National Academy of Sciences; 2002;99:13296–301. Available from: https://www.pnas.org/content/99/20/13296 10.1073/pnas.192412899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortigosa A, Fonseca S, Franco-Zorrilla JM, Fernández-Calvo P, Zander M, Lewsey MG, et al. The JA-pathway MYC transcription factors regulate photomorphogenic responses by targeting HY5 gene expression. Plant J [Internet]. John Wiley & Sons, Ltd; 2019;n/a. Available from: 10.1111/tpj.14618 [DOI] [PubMed] [Google Scholar]
- 5.Franco-Zorrilla JM, López-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci [Internet]. National Academy of Sciences; 2014;111:2367–72. Available from: https://www.pnas.org/content/111/6/2367 10.1073/pnas.1316278111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todeschini A-L, Georges A, Veitia RA. Transcription factors: specific DNA binding and specific gene regulation. Trends Genet [Internet]. Elsevier; 2014;30:211–9. Available from: 10.1016/j.tig.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 7.Geertz M, Maerkl SJ. Experimental strategies for studying transcription factor–DNA binding specificities. Brief Funct Genomics [Internet]. 2010;9:362–73. Available from: 10.1093/bfgp/elq023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badis G, Berger MF, Philippakis AA, Talukder S, Gehrke AR, Jaeger SA, et al. Diversity and Complexity in DNA Recognition by Transcription Factors. Science (80-) [Internet]. American Association for the Advancement of Science; 2009;324:1720–3. Available from: https://science.sciencemag.org/content/324/5935/1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burley SK. DNA-binding motifs from eukaryotic transcription factors. Curr Opin Struct Biol [Internet]. 1994;4:3–11. Available from: http://www.sciencedirect.com/science/article/pii/S0959440X94900531 [Google Scholar]
- 10.Zamanighomi M, Lin Z, Wang Y, Jiang R, Wong WH. Predicting transcription factor binding motifs from DNA-binding domains, chromatin accessibility and gene expression data. Nucleic Acids Res [Internet]. Oxford University Press; 2017;45:5666–77. Available from: 10.1093/nar/gkx358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morett E, Cannon W, Buck M. The DNA-binding domain of the transcriptional activator protein NifA resides in its carboxy terminus, recognises the upstream activator sequences of nif promoters and can be separated from the positive control function of NifA. Nucleic Acids Res [Internet]. 1988;16:11469–88. Available from: https://pubmed.ncbi.nlm.nih.gov/3062575 10.1093/nar/16.24.11469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizutani A, Tanaka M. Regions of GAL4 critical for binding to a promoter in vivo revealed by a visual DNA-binding analysis. EMBO J [Internet]. Oxford University Press; 2003;22:2178–87. Available from: https://pubmed.ncbi.nlm.nih.gov/12727884 10.1093/emboj/cdg220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaschka C, Hantsche M, Dienemann C, Burzinski C, Plitzko J, Cramer P. Transcription initiation complex structures elucidate DNA opening. Nature [Internet]. 2016;533:353–8. Available from: 10.1038/nature17990 [DOI] [PubMed] [Google Scholar]
- 14.Nikolov DB, Burley SK. RNA polymerase II transcription initiation: A structural view. Proc Natl Acad Sci [Internet]. National Academy of Sciences; 1997;94:15–22. Available from: https://www.pnas.org/content/94/1/15 10.1073/pnas.94.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pufall MA, Kaplan CD. Mechanisms of eukaryotic transcription. Genome Biol [Internet]. 2013;14:311 Available from: 10.1186/gb-2013-14-9-311 10.1186/gb-2013-14-9-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugh BF, Tjian R. Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev. 1991;5:1935–45. 10.1101/gad.5.11.1935 [DOI] [PubMed] [Google Scholar]
- 17.Dao LTM, Spicuglia S. Transcriptional regulation by promoters with enhancer function. Transcription [Internet]. 2018/06/25. Taylor & Francis; 2018;9:307–14. Available from: https://pubmed.ncbi.nlm.nih.gov/29889606 10.1080/21541264.2018.1486150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabidi MA, Stark A. Regulatory Enhancer–Core-Promoter Communication via Transcription Factors and Cofactors. Trends Genet [Internet]. 2016;32:801–14. Available from: http://www.sciencedirect.com/science/article/pii/S0168952516301214 10.1016/j.tig.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahnamoun H, Orozco P, Lauberth SM. The role of enhancer RNAs in epigenetic regulation of gene expression. Transcription [Internet]. Taylor & Francis; 2020;11:19–25. Available from: 10.1080/21541264.2019. 1698934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann K, Airoldi CA. Master Regulatory Transcription Factors in Plant Development: A Blooming Perspective In: Yamaguchi N, editor. Plant Transcr Factors Methods Protoc [Internet]. New York, NY: Springer New York; 2018. p. 3–22. Available from: 10.1007/978-1-4939-8657-6_1 [DOI] [PubMed] [Google Scholar]
- 21.Singh KB. Transcriptional Regulation in Plants: The Importance of Combinatorial Control. Plant Physiol [Internet]. American Society of Plant Biologists; 1998;118:1111–20. Available from: http://www.plantphysiol.org/content/118/4/1111 10.1104/pp.118.4.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal Behav [Internet]. Taylor & Francis; 2016;11:e1117723 Available from: 10.1080/15592324.2015.1117723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leng P, Zhao J. Transcription factors as molecular switches to regulate drought adaptation in maize. Theor Appl Genet [Internet]. 2019; Available from: 10.1007/s00122-019-03494-y [DOI] [PubMed] [Google Scholar]
- 24.Pajerowska-Mukhtar KM, Wang W, Tada Y, Oka N, Tucker CL, Fonseca JP, et al. The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defense transition. Curr Biol [Internet]. 2012/01/12. 2012;22:103–12. Available from: https://pubmed.ncbi.nlm.nih.gov/22244999 10.1016/j.cub.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puranik S, Sahu PP, Srivastava PS, Prasad M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. Elsevier Ltd; 2012;17:369–81. 10.1016/j.tplants.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 26.Ernst HA, Nina Olsen A, Skriver K, Larsen S, Lo Leggio L. Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors. EMBO Rep. 2004;5:297–303. 10.1038/sj.embor.7400093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen M, Kjaersgaard T, Nielsen M, Galberg P, Petersen K, O’ Shea C, et al. The Arabidopsis thaliana NAC transcription factor family: structure-function relationships and determinants of ANAC019 stress signalling. Biochem J. 2009;426:183–96. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Wang Q, Xiong L, Lou Z. A structural view of the conserved domain of rice stress-responsive NAC1. Protein Cell. Beijing: Higher Education Press; 2011;2:55–63. 10.1007/s13238-011-1010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Li X, Jin S, Liu X, Zhu L, Nie Y, et al. Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS One [Internet]. Public Library of Science; 2014;9:e86895–e86895. Available from: https://pubmed.ncbi.nlm.nih.gov/24489802 10.1371/journal.pone.0086895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An X, Liao Y, Zhang J, Dai L, Zhang N, Wang B, et al. Overexpression of rice NAC gene SNAC1 in ramie improves drought and salt tolerance. Plant Growth Regul [Internet]. 2015;76:211–23. Available from: 10.1007/s10725-014-9991-z [DOI] [Google Scholar]
- 31.Duval M, Hsieh T-F, Kim SY, Thomas TL. Molecular characterization of AtNAM: a member of theArabidopsis NAC domain superfamily. Plant Mol Biol. 2002;50:237–48. 10.1023/a:1016028530943 [DOI] [PubMed] [Google Scholar]
- 32.Olsen AN, Ernst HA, Leggio L Lo, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. Elsevier; 2005;10:79–87. 10.1016/j.tplants.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 33.Le DT, Nishiyama R, Watanabe Y, Mochida K, Yamaguchi-Shinozaki K, Shinozaki K, et al. Genome-Wide Survey and Expression Analysis of the Plant-Specific NAC Transcription Factor Family in Soybean During Development and Dehydration Stress. DNA Res An Int J Rapid Publ Reports Genes Genomes. Oxford University Press; 2011;18:263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen AN, Ernst HA, Leggio L Lo, Skriver K. DNA-binding specificity and molecular functions of NAC transcription factors. Plant Sci. 2005;169:785–97. [DOI] [PubMed] [Google Scholar]
- 35.Greve K, La Cour T, Jensen MK, Poulsen FM, Skriver K. Interactions between plant RING-H2 and plant-specific NAC (NAM/ATAF1/2/CUC2) proteins: RING-H2 molecular specificity and cellular localization. Biochem J. 2003;371:97–108. 10.1042/BJ20021123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao Y-J, Song Q-X, Chen H-W, Zou H-F, Wei W, Kang X-S, et al. Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation. Planta. 2010;232:1033–43. 10.1007/s00425-010-1238-2 [DOI] [PubMed] [Google Scholar]
- 37.Fang Y, You J, Xie K, Xie W, Xiong L. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol Genet Genomics. 2008;280:547–63. 10.1007/s00438-008-0386-6 [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi M, Ohtani M, Mitsuda N, Kubo M, Ohme-Takagi M, Fukuda H, et al. VND-INTERACTING2, a NAC Domain Transcription Factor, Negatively Regulates Xylem Vessel Formation in Arabidopsis. Plant Cell. American Society of Plant Biologists; 2010;22:1249–63. 10.1105/tpc.108.064048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho SK, Byung OP, Jae HY, Mi SJ, Sang ML, Hay JH, et al. Identification of a calmodulin-binding NAC protein as a transcriptional repressor in Arabidopsis. J Biol Chem. 2007;282:36292–302. 10.1074/jbc.M705217200 [DOI] [PubMed] [Google Scholar]
- 40.Christian D, Kemal K, WI W., Van Der SD, John M, DE S., et al. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. Plant J. Wiley/Blackwell (10.1111); 2005;43:745–57. 10.1111/j.1365-313X.2005.02488.x [DOI] [PubMed] [Google Scholar]
- 41.Shen H, Yin Y, Chen F, Xu Y, Dixon RA. A Bioinformatic Analysis of NAC Genes for Plant Cell Wall Development in Relation to Lignocellulosic Bioenergy Production. BioEnergy Res. 2009;2:217. [Google Scholar]
- 42.Zhu T, Nevo E, Sun D, Peng J. PHYLOGENETIC ANALYSES UNRAVEL THE EVOLUTIONARY HISTORY OF NAC PROTEINS IN PLANTS. Evolution (N Y) [Internet]. John Wiley & Sons, Ltd (10.1111); 2012;66:1833–48. Available from: 10.1111/j.1558-5646.2011.01553.x [DOI] [PubMed] [Google Scholar]
- 43.Pereira-Santana A, Alcaraz LD, Castaño E, Sanchez-Calderon L, Sanchez-Teyer F, Rodriguez-Zapata L. Comparative Genomics of NAC Transcriptional Factors in Angiosperms: Implications for the Adaptation and Diversification of Flowering Plants. PLoS One [Internet].; 2015;10:e0141866 Available from: 10.1371/journal.pone.0141866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–86. 10.1093/nar/gkr944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong Q, Schlueter SD, Brendel V. PlantGDB, plant genome database and analysis tools. Nucleic Acids Res [Internet]. Oxford University Press; 2004;32:D354–9. Available from: https://pubmed.ncbi.nlm.nih.gov/14681433 10.1093/nar/gkh046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boratyn GM, Schäffer AA, Agarwala R, Altschul SF, Lipman DJ, Madden TL. Domain enhanced lookup time accelerated BLAST. Biol Direct. BioMed Central; 2012;7:12 10.1186/1745-6150-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. Oxford University Press; 2014;30:1236–40. 10.1093/bioinformatics/btu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, et al. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–5. 10.1093/nar/gkl124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes11Edited by F. Cohen. J Mol Biol. 2001;305:567–80. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 50.Nguyen Ba A, Pogoutse A, Provart N, Moses A. NLStradamus: a simple Hidden Markov Model for nuclear localization signal prediction. BMC Bioinformatics. BioMed Central; 2009;10:202 10.1186/1471-2105-10-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. Oxford University Press; 2017;45:D362–8. 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. Oxford, UK: Oxford University Press; 2003;31:258–61. 10.1093/nar/gkg034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamura K, Filipski A, Peterson D, Stecher G, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol [Internet]. 2013;30:2725–9. Available from: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K, Durand D, Farach-Colton M. NOTUNG: A Program for Dating Gene Duplications and Optimizing Gene Family Trees. J Comput Biol [Internet]. Mary Ann Liebert, Inc., publishers; 2000;7:429–47. Available from: 10.1089/106652700750050871 [DOI] [PubMed] [Google Scholar]
- 55.Mohanta T, Mohanta N, Mohanta Y, Bae H. Genome-Wide Identification of Calcium Dependent Protein Kinase Gene Family in Plant Lineage Shows Presence of Novel D-x-D and D-E-L Motifs in EF-Hand Domain. Front Plant Sci. Frontiers Media S.A.; 2015;6:1146 10.3389/fpls.2015.01146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welner DH, Lindemose S, Grossmann JG, Møllegaard NE, Olsen AN, Helgstrand C, et al. DNA binding by the plant-specific NAC transcription factors in crystal and solution: a firm link to WRKY and GCM transcription factors. Biochem J. 2012;444:395–404. 10.1042/BJ20111742 [DOI] [PubMed] [Google Scholar]
- 57.Mohanta TK, Khan AL, Hashem A, Abd_Allah EF, Al-Harrasi A. The Molecular Mass and Isoelectric Point of Plant Proteomes. BMC Genomics [Internet]. 2019;20:631 Available from: http://biorxiv.org/content/early/2019/02/10/546077.abstract 10.1186/s12864-019-5983-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw KL, Grimsley GR, Yakovlev GI, Makarov AA, Pace CN. The effect of net charge on the solubility, activity, and stability of ribonuclease Sa. Protein Sci. Cold Spring Harbor Laboratory Press; 2001;10:1206–15. 10.1110/ps.440101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pergande MR, Cologna SM. Isoelectric Point Separations of Peptides and Proteins. Coorssen JR, Yergey AL, Wisniewski JR, editors. Proteomes. MDPI; 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunningham J, Estrella V, Lloyd M, Gillies R, Frieden BR, Gatenby R. Intracellular Electric Field and pH Optimize Protein Localization and Movement. Csermely P, editor. PLoS One. San Francisco, USA: Public Library of Science; 2012;7:e36894 10.1371/journal.pone.0036894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoppe T, Rape M, Jentsch S. Membrane-bound transcription factors: regulated release by RIP or RUP. Curr Opin Cell Biol. 2001;13:344–8. 10.1016/s0955-0674(00)00218-0 [DOI] [PubMed] [Google Scholar]
- 62.Vik Å, Rine J. Membrane biology: Membrane-regulated transcription. Curr Biol. Elsevier; 2000;10:R869–71. [DOI] [PubMed] [Google Scholar]
- 63.Hoppe T, Matuschewski K, Rape M, Schlenker S, Ulrich HD, Jentsch S. Activation of a Membrane-Bound Transcription Factor by Regulated Ubiquitin/Proteasome-Dependent Processing. Cell. Elsevier; 2000;102:577–86. [DOI] [PubMed] [Google Scholar]
- 64.Seo PJ, Kim SG, Park CM. Membrane-bound transcription factors in plants. Trends Plant Sci. 2008;13:550–6. 10.1016/j.tplants.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 65.Shen J, Chen X, Hendershot L, Prywes R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev Cell. Elsevier; 2002;3:99–111. 10.1016/s1534-5807(02)00203-4 [DOI] [PubMed] [Google Scholar]
- 66.Liu J-X, Srivastava R, Che P, Howell SH. An Endoplasmic Reticulum Stress Response in Arabidopsis Is Mediated by Proteolytic Processing and Nuclear Relocation of a Membrane-Associated Transcription Factor, bZIP28. Plant Cell. American Society of Plant Biologists; 2007;19:4111–9. 10.1105/tpc.106.050021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J-X, Srivastava R, Che P, Howell SH. Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. Blackwell Publishing Ltd; 2007;51:897–909. 10.1111/j.1365-313X.2007.03195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwata Y, Koizumi N. An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc Natl Acad Sci U S A. National Academy of Sciences; 2005;102:5280–5. 10.1073/pnas.0408941102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y-S, Kim S-G, Park J-E, Park H-Y, Lim M-H, Chua N-H, et al. A Membrane-Bound NAC Transcription Factor Regulates Cell Division in Arabidopsis. Plant Cell. American Society of Plant Biologists; 2006;18:3132–44. 10.1105/tpc.106.043018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim S-Y, Kim S-G, Kim Y-S, Seo PJ, Bae M, Yoon H-K, et al. Exploring membrane-associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucleic Acids Res. Oxford University Press; 2007;35:203–13. 10.1093/nar/gkl1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kosugi S, Hasebe M, Matsumura N, Takashima H, Miyamoto-Sato E, Tomita M, et al. Six classes of nuclear localization signals specific to different binding grooves of importin α. J Biol Chem. 2009;284:478–85. 10.1074/jbc.M807017200 [DOI] [PubMed] [Google Scholar]
- 72.Mattaj IW, Englmeier L. Nucleocytoplasmic Transport: The Soluble Phase. Annu Rev Biochem. Annual Reviews; 1998;67:265–306. 10.1146/annurev.biochem.67.1.265 [DOI] [PubMed] [Google Scholar]
- 73.Kosugi S, Hasebe M, Tomita M, Yanagawa H. Nuclear export signal consensus sequences defined using a localization-based yeast selection system. Traffic. 2008;9:2053–62. 10.1111/j.1600-0854.2008.00825.x [DOI] [PubMed] [Google Scholar]
- 74.Xie Q, Frugis G, Colgan D, Chua N-H. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes Dev. Cold Spring Harbor Laboratory Press; 2000;14:3024–36. 10.1101/gad.852200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He X, Mu R, Cao W, Zhang Z, Zhang J, Chen S. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. Wiley/Blackwell (10.1111); 2005;44:903–16. 10.1111/j.1365-313X.2005.02575.x [DOI] [PubMed] [Google Scholar]
- 76.Sperotto RA, Ricachenevsky FK, Duarte GL, Boff T, Lopes KL, Sperb ER, et al. Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta. 2009;230:985–1002. 10.1007/s00425-009-1000-9 [DOI] [PubMed] [Google Scholar]
- 77.Bonetta L. Interactome under construction. Nature. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2010;468:851 10.1038/468851a [DOI] [PubMed] [Google Scholar]
- 78.Tran L-SP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, et al. Isolation and Functional Analysis of Arabidopsis Stress-Inducible NAC Transcription Factors That Bind to a Drought-Responsive <em>cis</em>-Element in the <em>early responsive to dehydration stress 1</em> Promoter. Plant Cell. 2004;16:2481 LP–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jensen MK, Lindemose S, Masi F de, Reimer JJ, Nielsen M, Perera V, et al. ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana. FEBS Open Bio. Elsevier; 2013;3:321–7. 10.1016/j.fob.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kazuo N, Tomohiro K, Kazuko Y-S, Kazuo S. A nuclear gene, erd1, encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. Plant J. Wiley/Blackwell (10.1111); 2003;12:851–61. [DOI] [PubMed] [Google Scholar]
- 81.Reeves WM, Lynch TJ, Mobin R, Finkelstein RR. Direct targets of the transcription factors ABA-Insensitive(ABI)4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol Biol. Dordrecht: Springer Netherlands; 2011;75:347–63. 10.1007/s11103-011-9733-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jensen MK, Skriver K. NAC transcription factor gene regulatory and protein-protein interaction networks in plant stress responses and senescence. IUBMB Life. 2014;66:156–66. 10.1002/iub.1256 [DOI] [PubMed] [Google Scholar]
- 83.Jingjing J, Shenghui M, Nenghui Y, Ming J, Jiashu C, Jianhua Z. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. Wiley/Blackwell (10.1111); 2016;59:86–101. [DOI] [PubMed] [Google Scholar]
- 84.Pandey SP, Somssich IE. The Role of WRKY Transcription Factors in Plant Immunity. Plant Physiol. American Society of Plant Biologists; 2009;150:1648–55. 10.1104/pp.109.138990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saha D, Prasad AM, Srinivasan R. Pentatricopeptide repeat proteins and their emerging roles in plants. Plant Physiol Biochem. 2007;45:521–34. 10.1016/j.plaphy.2007.03.026 [DOI] [PubMed] [Google Scholar]
- 86.Barkan A, Small I. Pentatricopeptide Repeat Proteins in Plants. Annu Rev Plant Biol. Annual Reviews; 2014;65:415–42. 10.1146/annurev-arplant-050213-040159 [DOI] [PubMed] [Google Scholar]
- 87.Nandety RS, Caplan JL, Cavanaugh K, Perroud B, Wroblewski T, Michelmore RW, et al. The role of TIR-NBS and TIR-X proteins in plant basal defense responses. Plant Physiol. 2013;162:1459–72. 10.1104/pp.113.219162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wan H, Yuan W, Ye Q, Wang R, Ruan M, Li Z, et al. Analysis of TIR- and non-TIR-NBS-LRR disease resistance gene analogous in pepper: characterization, genetic variation, functional divergence and expression patterns. BMC Genomics. 2012;13:502 10.1186/1471-2164-13-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohanta TK, Occhipinti A, Atsbaha Zebelo S, Foti M, Fliegmann J, Bossi S, et al. Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS One. 2012;7:e32822 10.1371/journal.pone.0032822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohanta TK, Arora PK, Mohanta N, Parida P, Bae H. Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genomics. 2015;16 10.1186/s12864-014-1191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohanta TK, Mohanta N, Mohanta YK, Bae H. Genome-Wide Identification of Calcium Dependent Protein Kinase Gene Family in Plant Lineage Shows Presence of Novel D-x-D and D-E-L Motifs in EF-Hand Domain. Front Plant Sci. 2015;6:1146 10.3389/fpls.2015.01146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Bernoux M, Bentham AR, Newman TE, Ve T, Casey LW, et al. Multiple functional self-association interfaces in plant TIR domains. Proc Natl Acad Sci. 2017;114:E2046 LP–E2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohanta TK, Park Y-H, Bae H. Novel Genomic and Evolutionary Insight of WRKY Transcription Factors in Plant Lineage. Sci Rep. 2016;6 10.1038/s41598-016-0015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rinerson CI, Rabara RC, Tripathi P, Shen QJ, Rushton PJ. The evolution of WRKY transcription factors. BMC Plant Biol. 2015;15:1–18. 10.1186/s12870-014-0410-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holger T, Mario B, Angela S, Bernd-Joachim T. Expression of the gene and processed pseudogenes encoding the human and rabbit translationally controlled tumour protein (TCTP). Eur J Biochem. Wiley/Blackwell (10.1111); 2003;267:5473–81. [DOI] [PubMed] [Google Scholar]
- 96.Takada S, Hibara KI, Ishida T, Tasaka M. The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development. 2001;128:1127–35. [DOI] [PubMed] [Google Scholar]
- 97.Guo H-S, Xie Q, Fei J-F, Chua N-H. MicroRNA Directs mRNA Cleavage of the Transcription Factor NAC1 to Downregulate Auxin Signals for Arabidopsis Lateral Root Development. Plant Cell [Internet]. American Society of Plant Biologists; 2005;17:1376–86. Available from: http://www.plantcell.org/content/17/5/1376 10.1105/tpc.105.030841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vidal EA, Álvarez JM, Gutiérrez RA. Nitrate regulation of AFB3 and NAC4 gene expression in Arabidopsis roots depends on NRT1.1 nitrate transport function. Plant Signal Behav [Internet]. Taylor & Francis; 2014;9:e28501 Available from: 10.4161/psb.28501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faustino LI, Moretti AP, Graciano C. Fertilization with urea, ammonium and nitrate produce different effects on growth, hydraulic traits and drought tolerance in Pinus taeda seedlings. Tree Physiol. 2015;35:1062–74. 10.1093/treephys/tpv068 [DOI] [PubMed] [Google Scholar]
- 100.Xia N, Zhang G, Sun Y-F, Zhu L, Xu L-S, Chen X-M, et al. TaNAC8, a novel NAC transcription factor gene in wheat, responds to stripe rust pathogen infection and abiotic stresses. Physiol Mol Plant Pathol [Internet]. 2010;74:394–402. Available from: http://www.sciencedirect.com/science/article/pii/S0885576510000470 [Google Scholar]
- 101.Chen L, Ren J, Shi H, Chen X, Zhang M, Pan Y, et al. Physiological and Molecular Responses to Salt Stress in Wild Emmer and Cultivated Wheat. Plant Mol Biol Report [Internet]. 2013;31:1212–9. Available from: 10.1007/s11105-013-0584-1 [DOI] [Google Scholar]
- 102.Kirkpatrick CL, Martins D, Redder P, Frandi A, Mignolet J, Chapalay JB, et al. Growth control switch by a DNA-damage-inducible toxin–antitoxin system in Caulobacter crescentus. Nat Microbiol. Macmillan Publishers Limited; 2016;1:16008 10.1038/nmicrobiol.2016.8 [DOI] [PubMed] [Google Scholar]
- 103.Xu X, Liu Q, Fan L, Cui X, Zhou X. Analysis of synonymous codon usage and evolution of begomoviruses. J Zhejiang Univ Sci B. Hangzhou: Zhejiang University Press; 2008;9:667–74. 10.1631/jzus.B0820005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu T, Banzhaf W. Nonsynonymous to Synonymous Substitution Ratio k a / k s : Measurement for Rate of Evolution in Evolutionary Computation. PPSNX, LNCS 5199. 2008. p. 448–57. [Google Scholar]
- 105.Tomoko O. Synonymous and nonsynonymous substitutions in mammalian genes and the nearly neutral theory. J Mol Evol. 1995;40:56–63. 10.1007/bf00166595 [DOI] [PubMed] [Google Scholar]
- 106.Slotte T, Hazzouri KM, Ågren JA, Koenig D, Maumus F, Guo Y-L, et al. The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat Genet. Nature Publishing Group; 2013;45:831–5. [DOI] [PubMed] [Google Scholar]
- 107.Bolger A, Scossa F, Bolger ME, Lanz C, Maumus F, Tohge T, et al. The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat Genet. The Author(s); 2014;46:1034 10.1038/ng.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang J. Evolution by gene duplication: an update. Trends Ecol Evol [Internet]. 2003;18:292–8. Available from: http://www.sciencedirect.com/science/article/pii/S0169534703000338 [Google Scholar]
- 109.Crow KD, Wagner GP. What Is the Role of Genome Duplication in the Evolution of Complexity and Diversity? Mol Biol Evol [Internet]. 2005;23:887–92. Available from: 10.1093/molbev/msj083 [DOI] [PubMed] [Google Scholar]
- 110.Zhang J, Zhang Y, Rosenberg HF. Adaptive evolution of a duplicated pancreatic ribonuclease gene in a leaf-eating monkey. Nat Genet [Internet]. 2002;30:411–5. Available from: 10.1038/ng852 [DOI] [PubMed] [Google Scholar]
- 111.Jin X, Ren J, Nevo E, Yin X, Sun D, Peng J. Divergent Evolutionary Patterns of NAC Transcription Factors Are Associated with Diversification and Gene Duplications in Angiosperm. Front Plant Sci. 2017;8:1156 10.3389/fpls.2017.01156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmitz JF, Zimmer F, Bornberg-Bauer E. Mechanisms of transcription factor evolution in Metazoa. Nucleic Acids Res. Oxford University Press; 2016;44:6287–97. 10.1093/nar/gkw492 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(PDF)
(DOCX)
(XLSX)
Sheet 1 of the file show all the raw N-terminal NLS consensus sequences, unique NLS with linker amino acids, and unique NLS post removal of linker amino acids. Sheet 2 represents the number of occurrences of N-terminal NLS and sheet 3 represents C-terminal NLS, number of occurrences, C-terminal unique NLS, and N-and C-terminal unique NLS.
(XLSX)
(XLSX)
(XLSX)
(XZ)
(XZ)
(RAR)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.