Abstract
The one-drug/one-target/one-disease approach to drug discovery is presently facing many challenges of safety, efficacy, and sustainability. Network biology and polypharmacology approaches gained appreciation recently as methods for omics data integration and multitarget drug development, respectively. The combination of these two approaches created a novel paradigm called network pharmacology (NP) that looks at the effect of drugs on both the interactome and the diseasome level. Ayurveda, the traditional system of Indian medicine, uses intelligent formulations containing multiple ingredients and multiple bioactive compounds; however, the scientific rationale and mechanisms remain largely unexplored. NP approaches can serve as a valuable tool for evidence-based Ayurveda to understand the medicines’ putative actions, indications, and mechanisms. This chapter discusses NP and its potential to explore traditional medicine systems to overcome the drug discovery impasse.
Keywords: Polypharmacology, network pharmacology, ayurveda, bioactive, drug discovery
Introduction
Drug discovery, the process by which new candidate medications are discovered, initially began with random searching of therapeutic agents from plants, animals, and naturally occurring minerals (Burger, 1964). For this, they depended on the materia medica that was established by medicine men and priests from that era. This was followed by the origin of classical pharmacology in which the desirable therapeutic effects of small molecules were tested on intact cells or whole organisms. Later, the advent of human genome sequencing revolutionized the drug discovery process that developed into target–based drug discovery, also known as reverse pharmacology. This relies on the hypothesis that the modulation of the activity of a specific protein will have therapeutic effects. The protein that the drug binds to or interacts with is also referred to as a “target.” In this reductionist approach, small molecules from a chemical library are screened for their effect on the target’s known or predicted function (Hacker et al., 2009). Once the small molecule is selected for a particular target, further modifications are carried out at the atomic level to ameliorate the lock-and-key interactions. This one-drug/one-target/one-therapeutic approach was followed for the last several decades.
The information technology revolution at the end of 20th century metamorphosed the drug discovery process as well (Clark and Pickett, 2000). Advancements in omics technologies during this time were used to develop strategies for different phases of drug research (Buriani et al., 2012). Computational power was implemented in the discovery process for predicting a drug-likeness of newly designed or discovered compounds and ligand-protein docking for predicting the binding affinity of a small molecule with a protein three-dimensional structure. In silico tools were developed to predict other pharmacological properties of the drug molecules such as absorption, distribution, metabolism, excretion, and toxicity—abbreviated together as ADMET (van de Waterbeemd and Gifford, 2003, Clark and Grootenhuis, 2002). The technological advancements triggered discovery efforts in a direction to discover more specific magic bullets that were completely against the holistic approach of traditional medicine. This magic bullet approach is currently in decline phase. The major limitations of this drug discovery approach are side effects and the inability to tackle multifactorial diseases. This is mainly due to the linearity of this approach.
During the peak, historical time of drug discovery and development of natural products–based drugs had played a significant role due to their superior chemical diversity and safety over synthetic compound libraries (Zimmermann et al., 2007). Currently, it is estimated that more than one hundred new, natural product–based leads are in clinical development (Harvey, 2008). Many active compounds (bioactives) from traditional medicine sources could serve as good starting compounds and scaffolds for rational drug design. Natural products normally act through modulation of multiple targets rather than a single, highly specific target. But in drug discovery and development, technology was used to synthesize highly specific mono-targeted molecules that mimic the bioactives from natural compounds rather than understanding the rationale behind their synergistic action and developing methods to isolate the bioactives from natural resources. Researchers understand that most diseases are due to dysfunction of multiple proteins. Thus, it is important to address multiple targets emanating from a syndrome-related, metabolic cascade, so that holistic management can be effectively achieved. Therefore, it is necessary to shift the strategy from one that focuses on a single-target, new chemical entity to one of a multiple-target, synergistic, formulation-discovery approach (Patwardhan et al., 2015). This tempted the research world to go back and extensively explore natural sources, where modern pharmacology had begun. This renewed research focus indicates the need to rediscover the drug discovery process by integrating traditional knowledge with state-of-the-art technologies (Patwardhan, 2014a).
Network Pharmacology
A new discipline called network pharmacology (NP) has emerged which attempts to understand drug actions and interactions with multiple targets (Hopkins, 2007). It uses computational power to systematically catalogue the molecular interactions of a drug molecule in a living cell. NP appeared as an important tool in understanding the underlying complex relationships between botanical formula and the whole body (Zhang et al., 2013, Berger and Iyengar, 2009). It also attempts to discover new drug leads and targets and to repurpose existing drug molecules for different therapeutic conditions by allowing an unbiased investigation of potential target spaces (Kibble et al., 2015). However, these efforts require some guidance for selecting the right type of targets and new scaffolds of drug molecules. Traditional knowledge can play a vital role in this process of formulation discovery and repurposing existing drugs. By combining advances in systems biology and NP, it might be possible to rationally design the next generation of promiscuous drugs (Cho et al., 2012, Hopkins, 2008, Ellingson et al., 2014). NP analysis not only opens up new therapeutic options, but it also aims to improve the safety and efficacy of existing medications.
Network Biology to Network Pharmacology
The postgenomic era witnessed a rapid development of computational biology techniques to analyze and explore existing biological data. The key aim of the postgenomic biomedical research was to systematically catalogue all molecules and their interactions within a living cell. It is essential to understand how these molecules and the interactions among them determine the function of this immensely complex machinery, both in isolation and when surrounded by other cells. This led to the emergence and advancement of network biology, which indicates that cellular networks are governed by universal laws and offer a new conceptual framework that could potentially revolutionize our view of biology and disease pathologies in the 21st century (Barabási and Oltvai, 2004). During the first decade of the 21st century, several approaches for biological network construction were put forward that used computational methods, and literature mining especially, to understand the relation between disease phenotypes and genotypes. As a consequence, LMMA (literature mining and microarray analysis), a novel approach to reconstructing gene networks by combining literature mining and microarray analysis, was proposed (Li et al., 2006, Huang and Li, 2010). With this, a global network was first derived using the literature–based, cooccurrence method and then refined using microarray data. The LMMA biological network approach enables researchers to keep themselves up to date with relevant literature on specialized biological topics and to make sense of the relevant large-scale microarray dataset. Also, LMMA serves as a useful tool for constructing specific biological network and experimental design. LMMA–like representations enable a systemic recognition for the specific diseases in the context of complex gene interactions and are helpful for studying the regulation of various complex biological, physiological, and pathological systems.
The significance of accumulated-data integration was appreciated by pharmacologists, and they began to look beyond the classic lock-and-key concept as a far more intricate picture of drug action became clear in the postgenomic era. The global mapping of pharmacological space uncovered promiscuity, the specific binding of a chemical to more than one target (Paolini et al., 2006). As there can be multiple keys for a single lock, in the same way, a single key can fit into multiple locks. Similarly, a ligand might interact with many targets and a target may accommodate different types of ligands. This is referred to as “polypharmacology.” The concept of network biology was used to integrate data from DrugBank (Re and Valentini, 2013) and OMIM (Hamosh et al., 2005), an online catalog of human genes and genetic disorders to understand the industry trends, the properties of drug targets, and to study how drug targets are related to disease-gene products. In this way, when the first drug-target network was constructed, isolated and bipartite nodes were expected based on the existed one-drug/one-target/one-disease approach. Rather, the authors observed a rich network of polypharmacology interactions between drugs and their targets (Yildirim et al., 2007). An overabundance of “follow-on” drugs that are drugs that target already targeted proteins was observed. This suggested a need to upgrade the single-target single-drug paradigm, as single-protein single-function relations are limited to accurately describing the reality of cellular processes.
Advances in systems biology led to the realization that complex diseases cannot be effectively treated by intervention at single proteins. This made the drug researchers accept the concept of polypharmacology which they previously thought as an undesirable property that needs to be removed or reduced to produce clean drugs acting on single-targets. According to network biology, simultaneous modulation of multiple targets is required for modifying phenotypes. Developing methods to aid polypharmacology can help to improve efficacy and predict unwanted off-target effects.
Hopkins (Hopkins, 2007, Hopkins, 2008) observed that network biology and polypharmacology can illuminate the understanding of drug action. He introduced the term “network pharmacology.” This distinctive new approach to drug discovery can enable the paradigm shift from highly specific magic bullet–based drug discovery to multitargeted drug discovery. NP has the potential to provide new treatments to multigenic complex diseases and can lead to the development of e-therapeutics where the ligand formulation can be customized for each complex indication under every disease type. This can be expanded in the future and lead to customized and personalized therapeutics. Integration of network biology and polypharmacology can tackle two major sources of attrition in drug development such as efficacy and toxicity. Also, this integration holds the promise of expanding the current opportunity space for druggable targets. Hopkins proposed NP as the next paradigm in drug discovery.
Polypharmacology expands the space in drug discovery approach. Hopkins had suggested three strategies to the designers of multitarget therapies: the first was to prescribe multiple individual medications as a multidrug combination cocktail. Patient compliance and the danger of drug–drug interactions would be the expected drawbacks of this method. The second proposition was the development of multicomponent drug formulations. The change in metabolism, bioavailability, and pharmacokinetics of formulation as well as safety would be the major concerns of this approach. The third strategy was to design a single compound with selective polypharmacology. According to Hopkins, the third method is advantageous, as it would ease the dosing studies. Also, the regulatory barriers for the single compound are fewer compared to a formulation. An excellent example of this is metformin, the first-line drug for Type II diabetes that has been found to have cancer-inhibiting properties (Leung et al., 2013).
The following years witnessed the application research of NP by integrating network biology and polypharmacology. A computational framework, based on a regression model that integrates human protein–protein interactions, disease phenotype similarities, and known gene–phenotype associations to capture the complex relationships between phenotypes and genotypes, has been proposed. This was based on the assumption that phenotypically similar diseases are caused by functionally related genes. A tool named CIPHER (Correlating protein Interaction network and PHEnotype network to pRedict disease genes) has been developed that predicts and prioritizes disease-causing genes (Wu et al., 2008). CIPHER helps to uncover known disease genes and predict novel susceptibility candidates. Another application of this study is to predict a human disease landscape that can be exploited to study the related genes for related phenotypes that will be clustered together in a molecular interaction network. This will facilitate the discovery of disease genes and help to analyze the cooperativity among genes. Later, CIPHER-HIT, a Hitting-Time-based method to measure global closeness between two nodes of a heterogeneous network, was developed (Yao et al., 2011). A phenotype–genotype network can be explored using this method for detecting the genes related to a particular phenotype. A network–based gene clustering and extension were used to identify responsive gene modules in a condition–specific gene network aimed to provide useful resources to understand physiological responses ( Gu et al., 2010 ).
NP was also used to develop miRNA–based biomarkers (Lu et al., 2011). For this, a network of miRNA and their targets was constructed and further refined to study the data for specific diseases. This process integrated with literature mining was useful to develop potent miRNA markers for diseases. NP was also used to develop a drug gene–disease comodule (Zhao and Li, 2012). Initially, a drug-disease network was constructed by information gathered from databases followed by the integration of gene data. The gene closeness was studied by developing a mathematical model. This network inferred the association of multiple genes for most of the diseases and target sharing of drugs and diseases. These kinds of networks give insight into new drug-disease associations and their molecular connections.
Network Ethnopharmacology
During the progression period of network biology, natural products were gaining importance in the chemical space of drug discovery, as these have been economically designed and synthesized by nature for the benefit of evolution (Wetzel et al., 2011). Researchers began analyzing the logic behind traditional medicine systems and devised computational ways to ease the analysis. A comprehensive herbal medicine information system that was developed integrates information of more than 200 anticancer herbal recipes that have been used for the treatment of different types of cancer in the clinic, 900 individual ingredients, and 8500 small organic molecules isolated from herbal medicines (Fang et al., 2005). This system, which was developed using an Oracle database and Internet technology, facilitates and promotes scientific research in herbal medicine. This was followed by the development of many databases that serve as a source of botanical information and a powerful tool that provides a bridge between traditional medicines and modern molecular biology. These kinds of databases and tools made the researchers conceive the idea of NP of botanicals and their formulations to understand the underlying mechanisms of traditional medicines. We refer to such networks as “ethnopharmacological networks” and the technique as “Network Ethnopharmacology (NEP)” (Patwardhan and Chandran, 2015). Shao Li pioneered this endeavor and proposed this network as a tool to explain the ZHENG (syndrome of traditional Chinese medicine (TCM)) and the multiple-targets’ mechanism of TCM (Li, 2007).
Li et al. tried to provide a molecular basis for 1000-year-old concept of ZHENG using a neuro-endocrine-immune (NEI) network (Li et al., 2007). ZHENG is the basic unit and key concept in TCM theory. It is also used as a guideline in disease classification in TCM. The HOT (HANS ZHENG in Mandarin) and COLD (RE ZHENG) are the two statuses of ZHENG which therapeutically directs the use of herbs in TCM. Chinese herbs are classified as HOT–cooling and are used to remedy HOT ZHENG and COLD–warming herbs that are used to remedy COLD ZHENG. According to the authors, hormones may be related to HOT ZHENG, immune factors may be related to COLD ZHENG, and they may be interconnected by neurotransmitters. This study provides a methodical approach to understand TCM within the framework of modern science. Later they reconstructed the NEI network by adding multilayer information including data available on the KEGG database related to signal transduction, metabolic pathways, protein–protein interactions, transcription factor, and micro RNA regulations. They also connected drugs and diseases through multilayered interactions. The study of COLD ZHENG emphasized its relation to energy metabolism, which is tightly correlated with the genes of neurotransmitters, hormones, and cytokines in the NEI interaction network (Zhang et al., 2008, Ma et al., 2010).
Another database, TCMGeneDIT, provides information about TCMs, genes, diseases, TCM effects, and TCM ingredients mined from a vast amount of biomedical literature. This would facilitate clinical research and elucidate the possible therapeutic mechanisms of TCMs and gene regulations (Fang et al., 2008). To study the combination rule of TCM formulae, an herb network was created using 3865 collaterals-related formulae (Li et al., 2010). They developed a distance-based, mutual-information model (DMIM) to uncover the combination rule. DMIM uses mutual-information entropy and “between herb distance” to measure the tendency of two herbs to form an herb pair. They experimentally evaluated the combination of a few herbs for angiogenesis. Understanding the combination rule of herbs in formulae will help the modernization of traditional medicine and also help to develop a new formulae based on the current requirement. A network target–based paradigm was proposed for the first time to understand the synergistic combinations (Li et al., 2011), and an algorithm termed “NIMS” (network target–based identification of a multicomponent synergy) was also developed. This was a step that facilitated the development of multicomponent therapeutics using traditional wisdom. An innovative way to study the molecular mechanism of TCM was proposed during this time by integrating the TCM experimental data with microarray gene expression data (Wen et al., 2011). As a demonstrative example, Si-Wu-Tang’s formula was studied. Rather than uncovering the molecular mechanism of action, this method would help to identify new health benefits of TCMs.
The initial years of the second decade of the 21st century witnessed the network ethnopharmacological exploration of TCM formulations. The scope of this new area attracted scientists, and they hoped NEP could provide insight into multicompound drug discoveries that could help overcome the current impasse in drug discovery (Patwardhan, 2014b, Li et al., 2012). NEP was used to study the antiinflammatory mechanism of Qingfei Xiaoyan, a TCM (Cheng et al., 2013). The predicted results were used to design experiments and analyze the data. Experimental confirmation of the predicted results provides an effective strategy for the study of traditional medicines. The potential of TCM formulations as multiple compound drug candidates has been studied using TCM formulations based NP. TCM formulations studied in this way are listed in Table 5.1 . Construction of a database containing 19,7201 natural product structures, followed by their docking to 332 target proteins of FDA-approved drugs, shows the amount of space shared in the chemical space between natural products and FDA drugs (Gu et al., 2013a). Molecular-docking technique plays a major role in NP. The interaction of bioactives with molecular targets can be analyzed by this technique. Molecular docking–based NEP can be a useful tool to computationally elucidate the combinatorial effects of traditional medicine to intervene disease networks (Gu et al., 2013c). An approach that combines NP and pharmacokinetics has been proposed to study the material basis of TCM formulations (Pei et al., 2013). This can be extrapolated to study other traditional medicine formulations as well.
Table 5.1.
TCM Formulations That Were Explored Using Network Pharmacology
| Formulation Name | Observations About Bioactive Compounds | References |
|---|---|---|
| QiShenYiQi | Shows antiapoptosis, antiinflammation, antioxidant, anticoagulation, energy utilization facilitation and angiogenesis promotion against myocardial infarction | Li et al. (2014c) |
| Useful against acute myocardial ischemia, and it might gain enhanced drug effect by regulating apoptosis and inflammation related pathways together | Wu et al. (2014) | |
| Fufang xueshuantong | Ameliorate the activation of coagulation system in thrombosis | Sheng et al. (2014) |
| Gansui banxia tang | Modulates Hsp90α, ATP1A1, and STAT3 and combats hepatocellular carcinoma, intestinal tuberculosis, and gastrointestinal inflammation | Zhang et al. (2013) |
| Bushenhuoxue formula | Useful in chronic kidney disease, as it regulates the coagulation and fibrinolytic balance, expression of inflammatory factors, and inhibits abnormal ECM accumulation | Shi et al. (2014) |
| Ge-genqin-lian decoction | Useful in Type 2 diabetes, as it increases the insulin secretion in RIN-5F cells and improves insulin resistance | Li et al. (2014b) |
| Liu-Wei-Di-Huang pill | Deals with Yin deficiency of chen through PPAR signaling, progesterone-mediated oocyte maturation, adipocytokine signaling, and aldosterone-regulated sodium reabsorption | Liang et al. (2014) |
| Si-Wu-Tang | Useful in primary dysmenorrhea of gynecology blood stasis syndrome, as it regulates lipid metabolism (Shaofu Zhuyu decoction), amino acid metabolism (Xiangfu SWT), carbohydrate metabolism (THSWT), ErbB, and VEGF signal transduction pathway (Qinlian SWT) | Liu et al. (2014) |
| Useful in climacteric syndrome, blood deficiency, as it regulates TGFβ signaling, pathway, oxidative stress–induced gene expression via Nrf2, and upregulates VEGFα expression | Fang et al. (2013) | |
| Useful in women’s diseases through regulation of Nrf2-mediated oxidative stress response pathways, upregulation of Nrf2-regulated genes, increases an antioxidant-response element activity, phytoestrogenic effect | Wen et al. (2011) | |
| Taohong Siwu decoction | Useful in osteoarthritis, as it inhibits MMP expression, reduces local ILs, ADAMTS-4, TNFα, iNOS, COX, VDR, PPARγ, CDK2, HO-1 pathways | Zheng et al. (2013) |
| Qingfei-Xiaoyan Wan | Useful in inflammation of respiratory system, asthma through reduction in the infiltration of cytokines through ERK1, and five inflammatory pathways | Cheng et al. (2013) |
| Buchang Naoxintong | Deals with coronary heart disease and stroke by targeting APOB, APOE, APOA1, LPL, LDLR | Chen et al. (2013) |
| Bushen Zhuanggu formula | Used against metastatic breast cancer, as it regulates OPG/RANKL/RANK system, TGFβ, COX-2, EGFR pathway | Pei et al. (2013) |
| Qing-Luo-Yin | Used against rheumatoid arthritis, as it regulates angiogenesis, inflammatory responses, and immune response pathways | Zhang et al. (2013) |
| Used against rheumatoid arthritis with cold patterns, as it regulates nitrogen metabolism, PXR/RXR activation, linoleic acid metabolism, and metabolism of xenobiotics by CYP | Li et al. (2012) | |
| Zhike Chuanbei Pipa Dropping Pill | Useful in airway inflammation and asthma, as it regulates the Toll-like receptor, TGFβ, MAPK, HSP 90-α pathways, and inhibits NF-κB | Yang et al., (2012) |
| Fufang Danshen formula | Useful in cardiovascular diseases, as it regulates PPARγ, ACE, KCNJ11, KCNQ1, ABCC8 pathways | Li et al. (2011) |
| Realgar-Indigo naturalis formula | Used against acute promyelocytic leukemia, as it regulates ubiquitination/degradation of promyelocytic leukemia–retinoic acid receptor α oncoprotein, stronger reprogramming of myeloid differentiation regulators, and enhanced G1/G0 arrest in APL cells | Wang et al. (2008) |
| Panax notoginseng | Useful in cardiovascular disease, as it targets various receptors and transcriptional factors that influence various types of cells in their proliferation, differentiation, migration and secretion, and prevents or inhibits early events of CVDs | Liu et al. (2014) |
| Xuesaitong injection | Used against myocardial infarction, as it modulates ErbB, MAPK, VEGF, and Wnt pathways | Wang et al. (2013a) |
| Panax notoginseng/Salvia miltiorrhiza | Useful in cardiovascular disease | Liu (2013) |
| Shenmai injection | Used against myocardial ischemia, as it upregulates SPP1, TNC, FST, ITGA11, COMP and downregulates INHBC, ACTN3, PPARα, FGF7, and GP5 | Wu et al. (2013) |
| Da Chuanxiong formula | Useful in migraine and nervous headache, as it dispels wind pathogens and dissipates blood stasis | Wang et al. (2013b) |
| Danggui/Chuanxiong | Maintains blood stasis by nourishing and tonifying blood, activates blood circulation and dissolves blood stasis, regulates menstruations, and relieves pain | Li et al. (2012) |
| Zhi-Zi-Da-Huang decoction | Antioxidant effect helps to treat alcoholic liver disease through regulation of enzymes, cytochrome P450 2E1 (CYP2E1), and xanthine oxidase (XO) | An and Feng (2015) |
| Diesun Miaofang | Used in treatment of traumatic injury and activates blood, removes stasis, promotes qi circulation, and relieves pain | Zheng et al. (2015) |
| Buyang Huanwu decoction | Qi deficiency and blood-stasis diseases targeted through COX-2 and PPAR-gamma; potentially useful in cancer treatment | Ding et al. (2014) |
| Modified Simiaowan | Useful in gout diseases and acts through 30 core ingredients in MSW and 25 inflammatory cytokines and uric acid synthetase or transporters | Zhao et al. (2015) |
| 8 formulations for CHD | 1588 ingredients from 36 herbs used in 8 core formulae for the treatment of coronary heart disease | Ding et al. (2015) |
| herb Folium Eriobotryae | Useful in inflammation, and acts through regulation of 43 inflammation-associated proteins, including especially COX2, ALOX5, PPARG, TNF, and RELA | Zhang et al. (2015) |
| Rhubarb on renal fibrosis | Useful in renal fibrosis through bioactives like rhein, emodin, catechin, and epicatechin | Xiang et al. (2015) |
| Fructus Schisandrae chinensis | Shows protective activity toward hepatocyte injury by targeting GBA3/SHBGin hepatocytes | Wang et al. (2015) |
| Ejiao slurry | Regulates cancer cell differentiation, growth, proliferation, and apoptosis, and shows an adjuvant therapeutic effect that enriches the blood and increases immunity | Xu et al. (2014b) |
| Xiao-Chaihu Decoction and Da Chaihu-Decoction | XCHD treats diseases accompanying symptoms of alternating fever and chills, no desire for food or drink, and dry pharynx, while DCHD treats those with symptoms of fullness, pain in abdomen, and constipation. | Li et al. (2014a) |
| Dragon’s blood | Used in colitis and acts through interaction with 26 putative targets | Xu et al. (2014a) |
In cancer research, numerous natural products have been demonstrated to have anticancer potential. Natural products are gaining attraction in anticancer research, as they show a favorable profile in terms of absorption and metabolism in the body with low toxicity. In a study all of the known bioactives were docked for their property to interact with 104 cancer targets (Luo et al., 2014). It was inferred that many bioactives are targeting multiple protein targets and thus are linked to many types of cancers. NP coupled to sophisticated spectroscopical analysis such as ultra-performance liquid chromatography–electrospray, ionization–tandem mass spectroscopy (UPLC-ESI-MS/MS) is a useful approach to study the absolute molecular mechanism of action of botanical formulations based on their constituent bioactives (Xu et al., 2014a). Bioactive–target analysis has shown that some of the botanical formulations are more effective than their corresponding marketed drug–target interactions (Zhang et al., 2014). This indicates the potential of NP to better understand the power of botanical formulations and to develop efficient and economical treatment options. The holistic approach of botanical formulations can be better explained by NP. A study has reported this property by exemplifying a TCM formulation against viral infectious disease (Zhang et al., 2014). Not only does the formulation target the proteins in the viral infection cycle, but it also regulates the proteins of the host defense system; thus, it acts in a very distinctive manner. This unique property of formulations is highly efficient for strengthening the broad and nonspecific antipathogenic actions. Thus, network-based, multitarget drugs can be developed by testing the efficacy of the formulation, identifying, and isolating the major bioactives and redeveloping a multicomponent therapeutic using the major bioactives based on synergism (Leung et al., 2013).
NP also serves to document and analyze the clinical prescriptions of traditional medicine practitioners (Li et al., 2015). A traditional medicine network that links bioactives to clinical symptoms through targets and diseases is a novel way to explore the basic principles of traditional medicines (Luo et al., 2015).
Traditional Medicine Inspired Ethnopharmacological Networks
The network-based approaches provide a systematic platform for the study of multicomponent traditional medicine and has applications for its beneficial modernization. This platform not only recovers traditional knowledge, but it also provide new findings that can be used for resolving current problems in the drug industry (Zhang et al., 2013). This section explains a handful of ethnopharmacological networks that were developed to understand the scientific rationale of traditional medicine.
Dragon’s blood (DB) tablets, which are made of resins from Dracaena spp., Daemonorops spp., Croton spp., and Pterocarpus spp., is an effective TCM for the treatment of colitis. In a study, an NP-based approach was adopted to provide new insights relating to the active constituents and molecular mechanisms underlying the effects of DB (Xu et al., 2014a). The constituent chemicals of the formulation were identified using an ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry method. The known targets of those identified 48 compounds were mined from literature and putative targets that were predicted with the help of computational tools. The compounds were further screened for bioavailability followed by the systematic analysis of the known and putative targets for colitis. The network evaluation revealed the mechanism of action of DB bioactives for colitis through the modulation of the proteins of the NOD-like receptor signaling pathway (Fig. 5.1 ).
Figure 5.1.
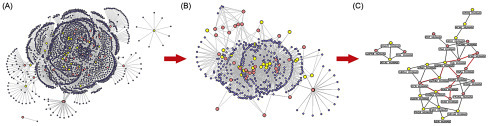
Putative DB targets-known colitis therapeutic targets protein–protein interaction (PPI) network: (A) The network between all targets and other human proteins. (B) The network of hub proteins in network (A). (C) The network of the major putative DB targets and the major known colitis therapeutic targets in network (B). Yellow spherical nodes indicate the putative targets; pink spherical nodes indicate the known therapeutic targets; purple spherical nodes indicate other human proteins that interact with putative targets or known therapeutic targets. Red edges in (C) indicate the PPIs of targets involved in the NOD–like receptor signaling pathway.
Source: From Xu H, Zhang Y, Lei Y, Gao X, Zhai H, Lin N, et al. A systems biology-based approach to uncovering the molecular mechanisms underlying the effects of dragon’s blood tablet in colitis, involving the integration of chemical analysis, ADME prediction, and network pharmacology. PLoS One. 2014;9:e101432.
The antioxidant mechanism of Zhi-Zi-Da-Huang decoction as an approach to treat alcohol liver disease was elucidated using NP (Li et al., 2011, An and Feng, 2015). An endothelial cell proliferation assay was performed for an antiangiogenic alkaloid, sinomenine, to validate the Network target–based Identification of Multicomponent Synergy (NIMS) predictions. The study was aimed at evaluating the synergistic relationship between different pairs of therapeutics, and sinomenine was found to have a maximum inhibition rate with matrine, both through the network and in vitro studies. The discovery of bioactives and elucidation of the mechanism of action of the herbal formulae, Qing-Luo-Yin and the Liu-Wei-Di-Huang pill, using NP, has given insight to the design validation experiments that accelerated the process of drug discovery (Li and Zhang, 2013). Validation experiments based on the network findings regarding Cold ZHENG and Hot ZHENG on a rat model of collagen–induced arthritis showed that the Cold ZHENG–oriented herbs tend to affect the hub nodes in the Cold ZHENG network, and the Hot ZHENG-oriented herbs tend to affect the hub nodes in the Hot ZHENG network (Li et al., 2007).
NP was used to explain the addition and subtraction theory of TCM. Two decoctions: Xiao Chaihu and Da Chaihu were studied using NP approach to investigate this theory. According to the addition and subtraction theory, the addition or removal of one or more ingredients from a traditional formulation resulted in a modified formula that plays a vital role in individualized medicine. Compounds from additive herbs were observed to be more efficient on disease–associated targets (Fig. 5.2 ). These additive compounds were found to act on 93 diseases through 65 drug targets (Li et al., 2014a). Experimental verification of the antithrombotic network of Fufang Xueshuantong (FXST) capsule was done through in vivo studies on lipopolysaccharide–induced disseminated intravascular coagulation (DIC) rat model. It was successfully shown that FXST significantly improves the activation of the coagulation system through 41 targets from four herbs (Sheng et al., 2014). NP analysis of the Bushenhuoxue formula showed that six components—Rhein, Tanshinone IIA, Curcumin, Quercetin and Calycosin—Acted through 62 targets for the treatment of chronic kidney disease. These predictions were validated using unilateral ureteral obstruction models, and it was observed that even though the individual botanicals showed a significant decrease in creatinine levels, the combination showed lower blood creatinine and urea nitrogen levels (Shi et al., 2014). The antidiabetic effects of Ge-Gen-Qin-Lian decoction were investigated using an insulin secretion assay, and an insulin–resistance model using 13 of the 19 ingredients showed antidiabetic activity using NP studies (Li et al., 2014b). To confirm the predictions of the network of Liu-Wei-Di-Huang pill, four proteins—PPARG, RARA, CCR2, and ESR1—that denote different functions and are targeted by different groups of ingredients were chosen. The interactions between various bioactives and their effect on the expression of the proteins showed that the NP approach can accurately predict these interactions, giving hints regarding the mechanism of action of the compounds (Liang et al., 2014). Experimental results confirmed that the 30 core ingredients in Modified Simiaowan, obtained through network analysis, significantly increased HUVEC viability and attenuated the expression of ICAM-1 and proved to be effective in gout treatment (Zhao et al., 2015). The role of anthraquinone and flavanols (catechin and epicatechin) in the therapeutic potential of rhubarb in renal interstitial fibrosis was examined using network analysis and by conventional assessment involving serum biochemistry, histopathological, and immunohistochemical assays (Xiang et al., 2015). In silico analysis and experimental validation demonstrated that compound 11/12 of fructus Schisandrae chinensis targets GBA3/SHBG (Wang et al., 2015).
Figure 5.2.
Drug–target network depicting the addition and subtraction theory of TCM. Drug–target interactions are shown as connecting lines between drugs (compounds, triangles) and targets (circles). The black nodes (circles) represent targets that are targeted by all the herbs of the formulation. Drugs belonging to individual herbs are highlighted in purple and green backgrounds.
Source: From Li B, Tao W, Zheng C, Shar PA, Huang C, Fu Y, et al. Systems pharmacology-based approach for dissecting the addition and subtraction theory of traditional Chinese medicine: An example using Xiao-Chaihu-Decoction and Da-Chaihu-Decoction. Comput. Biol. Med. Elsevier; 2014;53C:19–29.
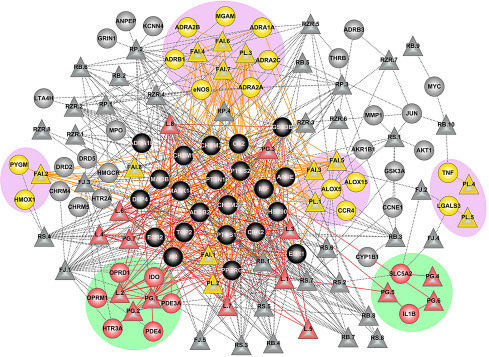
NP is a valuable method to study the synergistic effects of bioactives of traditional medicine formulation. This was experimentally shown on the Sendeng-4 formulation for rheumatoid arthritis (Fig. 5.3 ). Data and network analysis have shown that the formulation acts synergistically through nine categories of targets (Zi and Yu, 2015). Another network that studied three botanicals, Salviae miltiorrhizae, Ligusticum chuanxiong, and Panax notoginseng for Coronary Artery Disease (CAD), displayed their mode of action through 67 targets, out of which 13 are common among the botanicals (Fig. 5.4 ). These common targets are associated with thrombosis, dyslipidemia, vasoconstriction, and inflammation (Zhou and Wang, 2014). This gives insight to how these botanicals are managing CAD.
Figure 5.3.
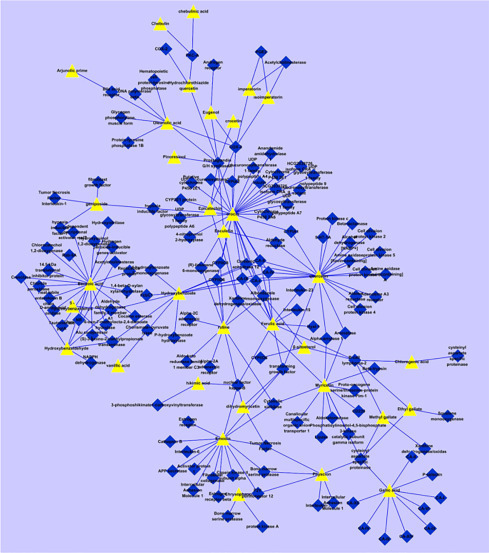
The chemical composition-target interaction network of Sendeng-4. The yellow nodes represent chemical components and the blue nodes represent targets. The edges represent interactions.
Source: From Zi, T., Yu, D., 2015. A network pharmacology study of Sendeng-4, a Mongolian medicine. Chin. J. Nat. Med. 13, 108–118.
Figure 5.4.
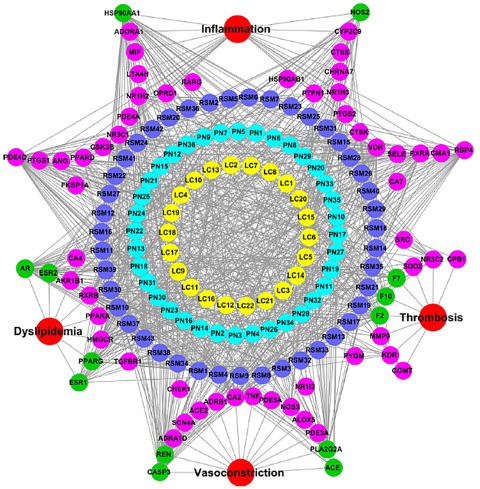
Network of three botanicals for Coronary Artery Disease (CAD).
Source: From Zhou, W., Wang, Y., 2014. A network-based analysis of the types of coronary artery disease from traditional Chinese medicine perspective: potential for therapeutics and drug discovery. J. Ethnopharmacol. 151, 66–77.
Another approach using NP is the construction of networks based on experimental data followed by literature mining. This method is very effective for large space data analysis, which will help to derive the mechanism of action of the formulation. A network of QiShenYiQi formulation having cardioprotective effects, constructed based on the microarray data and the published literature, showed that 9 main compounds were found to act through 16 pathways, out of which 9 are immune and inflammation-related (Li et al., 2014c). The mechanism of action for the Bushen Zhuanggu formulation was proposed based on LC-MS/MS standardization, pharmacokinetic analysis, and NP (Pei et al., 2013). The efficacy of Shenmai injection was evaluated using a rat model of myocardial infarction, genome-wide transcriptomic experiment, and then followed by a NP analysis. The overall trends in the ejection fraction and fractional shortening were consistent with the network–recovery index (NRI) from the network (Wu et al., 2013).
Knowledge Bases for Network Ethnopharmacology
In order to develop an ethnopharmacological network, exploring the existing databases to gather information regarding bioactives and targets is the first step. Further information such as target-related diseases, tissue distribution and pathways are also to be collected depending on the type of study that is going to be undertaken. The Universal Natural Products Database (UNPD) (Gu et al., 2013a) is one of the major databases that provides bioactives information. Other databases that provide information regarding bioactives include CVDHD (Gu et al., 2013b), TCMSP (Ru et al., 2014), TCM@Taiwan (Sanderson, 2011), SuperNatural (Banerjee et al., 2015), and Dr. Dukes’s phytochemical and ethnobotanical database (Duke and Beckstrom-Sternberg, 1994). The molecular structures of bioactives are usually stored as “SD” files and chemical information as smiles and inchkeys in these databases. Any of these file formats can be used as inputs to identify the targets in protein information databases. Binding database or “Binding DB” (Liu et al., 2007) and ChEMBL (Bento et al., 2014) are databases for predicting target proteins. Binding DB searches the exact or similar compounds in the database and retrieves the target information of those compounds. The similarity search gives the structurally similar compounds with respect to the degree of similarity as scores to the queried structure. The information regarding both annotated and predicted targets can be collected in this way. This database is connected to numerous databases, and these connections can be used to extract further information regarding the targets. The important databases linked to binding DB are UniProt (Bairoch et al., 2005), which gives information related to proteins and genes; Reactome, a curated pathway database (Croft et al., 2011); and the Kyoto Encyclopedia of Genes and Genomes (KEGG), a knowledge base for systematic analysis of gene functions and pathways (Ogata et al., 1999).
Therapeutic Targets Database (TTD) (Zhu et al., 2012) gives fully referenced information of targeted diseases of proteins, their pathway information, and the corresponding drug directed to each target. Disease and Gene Annotation (DGA), a database that provides a comprehensive and integrative annotation of human genes in disease networks, is useful in identifying the disease type that each indication belongs to (Peng et al., 2013). The human protein atlas (HPA) database (Pontén et al., 2011) is an open database showing the spatial distribution of proteins in 44 different normal human tissues. The information of the distribution of proteins in tissues can be gathered from HPA. The database also gives information regarding subcellular localization and protein class. An overall review of the methods to implement NP for herbs and herbal formulations is also available, including a systematic review of the databases that one could use for the same (Kibble et al., 2015, Lagunin et al., 2014).
Integration of knowledge bases helps data gathering for network pharmacological studies, and its knowledge base shows the inter-relationships among these databases (Fig. 5.5 ) (Yang et al., 2013). The counts of entities, such as bioactives, targets, and diseases, can vary based on the knowledge bases that are relied on for data collection. An integration of knowledge bases can overcome this limitation. Another factor that affects the counts of these entities is the time frame for data collection. This change occurs due to the ongoing, periodic updates of the databases.
Figure 5.5.
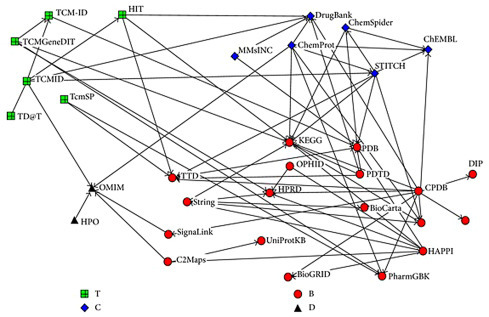
Database relationship network.
Source: From Yang, M., Chen, J.-L., Xu, L.-W., Ji, G., 2013. Navigating traditional Chinese medicine network pharmacology and computational tools. Evid. Based Complement Alternat. Med. 2013: 731969.
Network Construction
A network is the schematic representation of the interaction among various entities called nodes. In pharmacological networks, the nodes include bioactives, targets, tissue, tissue types, disease, disease types, and pathways. These nodes are connected by lines termed edges, which represent the relationship between them (Morris et al., 2012). Building a network involves two opposite approaches: a bottom-up approach on the basis of established biological knowledge and a top-down approach starting with the statistical analysis of available data. At a more detailed level, there are several ways to build and illustrate a biological network. Perhaps the most versatile and general way is the de novo assembly of a network from direct experimental or computational interactions, e.g., chemical/gene/protein screens. Networks encompassing biologically relevant nodes (genes, proteins, metabolites), their connections (biochemical and regulatory), and modules (pathways and functional units) give an authentic idea of the real biological phenomena (Xu and Qu, 2011).
Cytoscape, a Java-based open source software platform (Shannon et al., 2003), is a useful tool for visualizing molecular interaction networks and integrating them with any type of attribute data. In addition to the basic set of features for data integration, analysis, and visualization, additional features are available in the form of apps, including network and molecular profiling analysis and links with other databases. In addition to Cytoscape, a number of visualization tools are available. Visual network pharmacology (VNP) (Hu et al., 2014), which is specially designed to visualize the complex relationships among diseases, targets, and drugs, mainly contains three functional modules: drug-centric, target-centric, and disease-centric VNP. This disease-target-drug database documents known connections among diseases, targets, and the USFDA-approved drugs. Users can search the database using disease, target, or drug name strings; chemical structures and substructures; or protein sequence similarity, and then obtain an online interactive network view of the retrieved records. In the obtained network view, each node is a disease, target, or drug, and each edge is a known connection between two of them. The Connectivity Map, or the CMap tool, allows the user to compare gene-expression profiles. The similarities or differences in the signature transcriptional expression profile and the small molecule transcriptional response profile may lead to the discovery of the mode of action of the small molecule. The response profile is also compared to response profiles of drugs in the CMap database with respect to the similarity of transcriptional responses. A network is constructed and the drugs that appear closest to the small molecule are selected to have better insight into the mode of action.
Other software, such as Gephi, an exploration platform for networks and complex systems, and Cell Illustrator, a Java-based tool specialized in biological processes and systems, can also be used for building networks (Hu et al., 2014).
Ayurveda and Network Ethnopharmacology
Ayurveda, the Indian traditional medicine, offers many sophisticated formulations that have been used for hundreds of years. The Traditional Knowledge Digital Library (TKDL, http://www.tkdl.res.in) contains more than 36,000 classical Ayurveda formulations. Approximately 100 of these are popularly used at the community level and also as over-the-counter products. Some of these drugs continue to be used as home remedies for preventive and primary health care in India. Until recently, no research was carried out to explore Ayurvedic wisdom using NP despite Ayurveda holding a rich knowledge of traditional medicine equal to or greater than TCM. Our group examined the use of NP to study Ayurvedic formulations with the well-known Ayurvedic formulation Triphala as a demonstrable example (Chandran et al., 2015a, Chandran et al., 2015b).
In this chapter, we demonstrate the application of NP in understanding and exploring the traditional wisdom with Triphala as a model.
Network Ethnopharmacology of Triphala
Triphala is one of the most popular and widely used Ayurvedic formulations. Triphala contains fruits of three myrobalans: Emblica officinalis (EO; Amalaki) also known as Phyllanthus emblica; Terminalia bellerica (TB; Vibhitaka); and Terminalia chebula (TC; Haritaki).
Triphala is the drug of choice for the treatment of several diseases, especially those of metabolism, dental, and skin conditions, and treatment of cancer (Baliga, 2010). It has a very good effect on the health of heart, skin, eyes, and helps to delay degenerative changes, such as cataracts (Gupta et al., 2010). Triphala can be used as an inexpensive and nontoxic natural product for the prevention and treatment of diseases where vascular endothelial growth factor A–induced angiogenesis is involved (Lu et al., 2012). The presence of numerous polyphenolic compounds empowers it with a broad antimicrobial spectrum (Sharma, 2015).
Triphala is a constituent of about 1500 Ayurveda formulations and it can be used for several diseases. Triphala combats degenerative and metabolic disorders possibly through lipid peroxide inhibition and free radical scavenging (Sabu and Kuttan, 2002). In a phase I clinical trial on healthy volunteers, immunostimulatory effects of Triphala on cytotoxic T cells and natural killer cells have been reported (Phetkate et al., 2012). Triphala is shown to induce apoptosis in tumor cells of the human pancreas, in both in vitro and in vivo models (Shi et al., 2008). Although the anticancer properties of Triphala have been studied, the exact mechanism of action is still not known. The beneficial role of Triphala in disease management of proliferative vitreoretinopathy has also been reported (Sivasankar et al., 2015). One of the key ingredients of Triphala is Amalaki. Some studies have already shown the beneficial effect of Amalaki Rasayana to suppress neurodegeneration in fly models of Huntington’s and Alzheimer’s diseases (Dwivedi et al., 2012, Dwivedi et al., 2013). Triphala is an effective medicine to balance all three Dosha. It is considered as a good rejuvenator Rasayana, which facilitates nourishment to all tissues, or Dhatu.
Here we demonstrate the multidimensional properties of Triphala using human proteome, diseasome, and microbial proteome targeting networks.
Triphala Bioactives
The botanicals of Triphala—EO, TB, and TC—contain 114, 25, and 63 bioactives, respectively, according to UNPD data collected during June 2015. Of these, a few bioactives are common among the three botanicals. Thus, Triphala formulation as a whole contains 177 bioactives. Out of these, 36 bioactives were Score-1, based on Binding DB search carried out during June 2015. EO, TB, and TC contain 20, 4, and 20 Score-1 bioactives, respectively (Fig. 5.6 ). The Score-1 bioactives that are common among three plants are chebulanin, ellagic acid, gallussaeure, 1,6-digalloyl-beta-d-glucopiranoside, methyl gallate, and tannic acid. This bioactive information is the basic step toward constructing human proteome and microbial proteome targeting networks.
Figure 5.6.
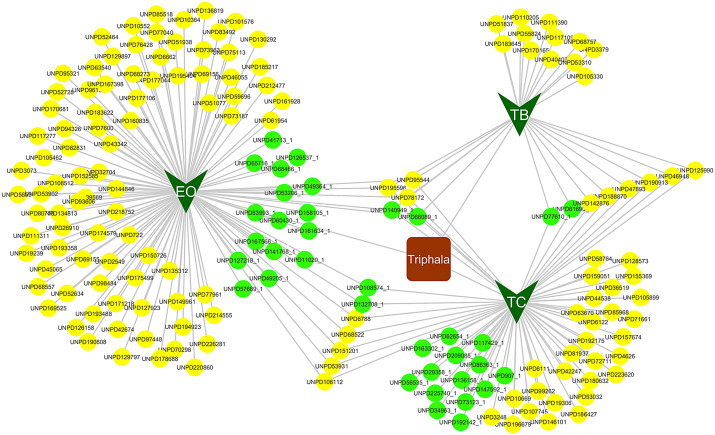
Bioactive network of Triphala. Dark green versus are the botanicals of Triphala and oval nodes are the bioactives where green represents Score 1 bioactives.
Human Proteome and Diseasome Targeting Network of Triphala
Thirty-six Score-1 bioactives of Triphala are shown to interact with 60 human protein targets in 112 combinations (Fig. 5.7 ). Quercetin, ellagic acid, 1,2,3,4,6-pentagalloylglucose and 1,2,3,6-tetrakis-(O-galloyl)-beta-d-glucose are the four bioactives that interact with the maximum number of targets: 21, 16, and 7, respectively. The other major bioactives that have multitargeting property include catechin; epicatechin; gallocatechin; kaempferol; and trans-3,3’,4’,5,7-pentahydroxylflavane. The major protein targets of Triphala include alkaline phosphatase (ALPL); carbonic anhydrase 7 (CA7); coagulation factor X (F10), DNA repair protein RAD51 homolog 1 (RAD51); GSTM1 protein (GSTM1); beta-secretase 1 (BACE1); plasminogen activator inhibitor 1 (SERPINE1), prothrombin (F2); regulator of G-protein signaling (RGS) 4, 7, and 8, tissue-type plasminogen activator (PLAT); and tyrosine-protein phosphatase nonreceptor type 2 (PTPN2).
Figure 5.7.
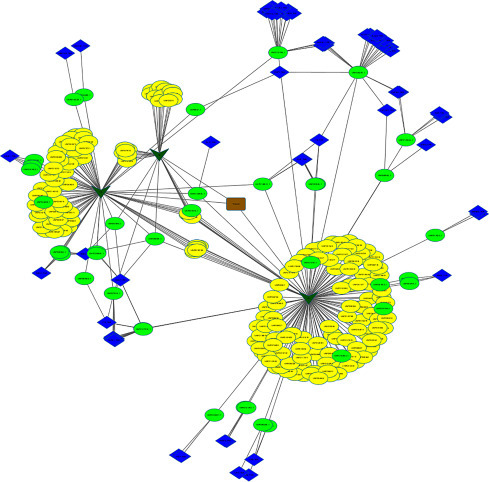
Bioactive–target network of Triphala. Dark green versus are the botanicals of Triphala and oval nodes are the bioactives where green represents score 1 bioactives. Blue diamonds denote targets.
The 60 targets of Triphala are associated with 24 disease types, which include 130 disease indications (Fig. 5.8 ). The major disease types in which Triphala targets are associated include cancers, cardiovascular diseases, nervous system diseases, and metabolic diseases. Analysis of existing data indicates that targets of Triphala bioactives are involved in the 40 different types of cancers making it the largest group of diseases, involving Triphala targets. This linkage is through the interaction of 25 bioactives and 27 target proteins in 46 different bioactive–target combinations. The types of cancers which are networked by Triphala include pancreatic, prostate, breast, lung, colorectal and gastric cancers, tumors, and more. Quercetin, ellagic acid, prodelphinidin A1, and 1,2,3-benzenetriol are the important bioactives; and RAD51, BACE1, F2, MMP2, IGF1R, and EGFR are the important targets that play a role in cancer.
Figure 5.8.
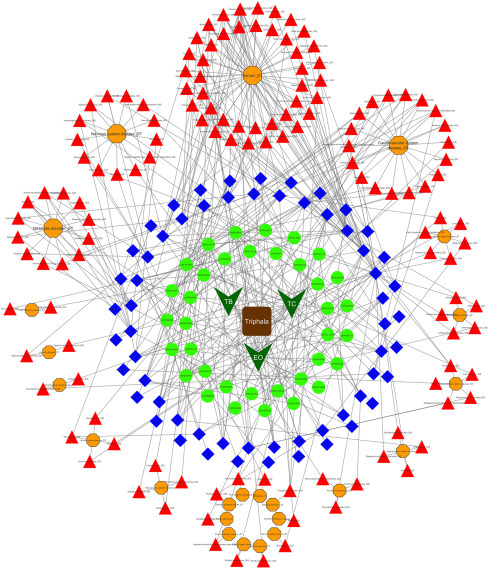
The human proteome and diseasome targeting network of Triphala. Dark green versus are the botanicals of Triphala and oval green nodes are the score1 bioactives. Targets are represented by blue diamond nodes, red triangle nodes depict diseases, and orange octagons indicate disease types.
Triphala shows links to 18 indications of cardiovascular diseases through 12 bioactives and 11 targets. The cardiovascular diseases that are covered in the Triphala network include atherosclerosis, myocardial ischemia, infarction, cerebral vasospasm, thrombosis, and hypertension. The bioactives playing a major role in cardiovascular diseases are quercetin, 1,2,3,4,6-pentagalloyoglucose, 1,2,3,6-tetrakis-(O-galloyl)-beta-d-glucose, bellericagenin A1, and prodelphinidin A1, whereas the targets playing an important role are SERPINE1, F10, F2, and FABP4.
Triphala’s network to nervous system disorders contains 13 diseases in which the significant ones are Alzheimer’s disease, Parkinson’s disease, diabetic neuropathy, and retinopathy. In this subnetwork, 14 bioactives interact with 11 targets through 21 different interactions. Quercetin, 1,2,3,4,6-pentagalloyoglucose, 1,2,3,6-tetrakis-(O-galloyl)-beta-d-glucose, and epigallocatechin-3-gallate are the most networked bioactives whereas the most networked targets are BACE1, SERPINE1, PLAT, ALDR, CA2.
The association of Triphala with metabolic disorders is determined by six bioactives that interact with seven targets. The major metabolic diseases come in this link are obesity, diabetic complications, noninsulin-dependent diabetes, hypercholesterolemia, hyperlipidemia, and more. The bioactives having more interactions with targets are ellagic acid, quercetin, and bellericagenin A1, whereas the highly networked targets are IGF1R, FABP5, ALDR, and AKR1B1. Triphala bioactives are also linked to targets of other diseases comprising autoimmune diseases, ulcerative colitis, McCune–Albright syndrome, psoriasis, gout, osteoarthritis, endometriosis, lung fibrosis, glomerulonephritis, and more.
The proteome-targeting network of Triphala, thus, shows its ability to synergistically modulate 60 targets that are associated with 130 disease indications. This data is generated with the available information that included only one-fifth of the total number of bioactives. Further logical analysis and experimental studies based on the network result are needed to explore the in-depth mechanism of action of Triphala. For researchers in this area, these kind of networks can give an immense amount of information that can be developed further to reveal the real mystery behind the actions of traditional medicine.
Microbial Proteome Targeting Network of Triphala
Triphala is also referred to as a “tridoshic rasayana,” as it balances the three constitutional elements of life. It tonifies the gastrointestinal tract, improves digestion, and is known to exhibit antiviral, antibacterial, antifungal, and antiallergic properties (Sharma, 2015, Amala and Jeyaraj, 2014, Sumathi and Parvathi, 2010). Triphala Mashi (mashi: black ash) was found to have nonspecific antimicrobial activity, as it showed a dose-dependent inhibition of Gram-positive and Gram-negative bacteria (Biradar et al., 2008). Hydroalcoholic, aqueous, and ether extracts of the three fruits of Triphala were reported to show antibacterial activity against uropathogens with a maximum drug efficacy recorded by the alcoholic extract (Bag et al., 2013, Prasad et al., 2009). The methanolic extract of Triphala showed the presence of 10 active compounds using GC-MS and also showed potent antibacterial and antifungal activity (Amala and Jeyaraj, 2014).
Triphala has been well studied for its antimicrobial activity against Gram-positive bacteria, Gram-negative bacteria, fungal species, and different strains of Salmonella typhi (Amala and Jeyaraj, 2014, Sumathi and Parvathi, 2010, Gautam et al., 2012, Srikumar et al., 2007). Triphala showed significant antimicrobial activity against Enterococcus faecalis and Streptococcus mutans grown on tooth substrate thereby making it a suitable agent for prevention of dental plaque (Prabhakar et al., 2010, Prabhakar et al., 2014). The application of Triphala in commercial antimicrobial agents has been explored. A significant reduction in the colony forming units of oral streptococci was observed after 6% Triphala was incorporated in a mouthwash formulation (Srinagesh et al., 2012). An ointment prepared from Triphala (10% (w/w)) showed significant antibacterial and wound healing activity in rats infected with Staphylococcus aureus, Pseudomonas aeruginosa, and Streptococcus pyogenes (Kumar et al., 2008). The antiinfective network of Triphala sheds light on the efficacy of the formulation in the simultaneous targeting of multiple microorganisms. Also, this network provides information regarding some novel bioactive–target combinations that can be explored to combat the problem of multidrug resistance.
Among the bioactives of Triphala, 24 Score-1 bioactives target microbial proteins of 22 microorganisms. The botanicals of Triphala-EO, TB, and TC- contain 19, 3, and 8 Score1 bioactives respectively which showed interactions with microbial proteins. They act through modulation of 35 targets which are associated with diseases such as Leishmaniasis, malaria, tuberculosis, hepatitis C, acquired immunodeficiency syndrome (AIDS), cervical cancer, candidiasis, luminous vibriosis, yersiniosis, skin and respiratory infections, severe acute respiratory syndrome (SARS), avian viral infection, bacteremia, sleeping sickness, and anthrax (Fig. 5.9 ). The microorganisms captured in the Triphala antiinfective network includes candida albicans, hepatitis C virus, human immunodeficiency virus 1, human papillomavirus type 16, human SARS coronavirus leishmania amazonensis, Mycobacterium tuberculosis, staphylococcus aureus, Plasmodium falciparum, and Yersinia enterocolitica.
Figure 5.9.
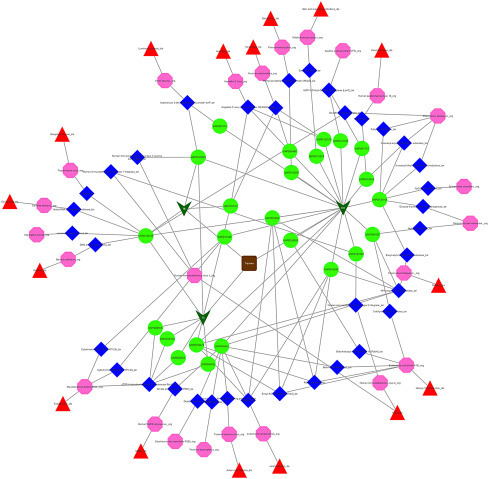
The microbial proteome–targeting network of Triphala. Dark green verus are the botanicals of Triphala and oval green nodes are the Score1 bioactives. Targets, diseases, and microorganisms are represented by blue diamond nodes, red triangle nodes, and pink octagon nodes, respectively.
In Mycobacterium tuberculosis, dTDP-4-dehydrorhamnose 3,5-epimerase RmlC is one of the four enzymes involved in the synthesis of dTDP-l-rhamnose, a precursor of l-rhamnose (Giraud et al., 2000). The network shows that Triphala has the potential to modulate the protein through four bioactives such as punicalins, terflavin B, 4-O-(S)-flavogallonyl-6-O-galloyl-beta-d-glucopyranose, and 4,6-O-(S,S)-gallagyl-alpha/beta-d-glucopyranose. Research on new therapeutics that target the mycobacterial cell wall is in progress. Rhamnosyl residues play a structural role in the mycobacterial cell wall by acting as a linker connecting arabinogalactin polymer to peptidoglycan and are not found in humans, which gives them a degree of therapeutic potential (Ma et al., 2001). Triphala can be considered in this line to develop novel antimycobacterial drugs.
The network shows the potential of gallussaeure and 3-galloylgallic acid to modulate human immunodeficiency virus type 1 reverse transcriptase. Inhibition of human immunodeficiency virus at the initial stage itself is crucial and thus, targeting human immunodeficiency virus type 1 reverse transcriptase, at the preinitiation stage is considered to be an effective therapy. Protein E6 of human papillomavirus 16 (HPV16) prevents apoptosis of infected cells by binding to FADD and caspase 8 and hence being targeted for development of antiviral drugs (Yuan et al., 2012). Kaempferol of Triphala is found to target protein E6 of HPV16, which is a potential mechanism to control the replication of the virus.
The network also shows Triphala’s potential to act on Plasmodium falciparum. Enoyl-acyl carrier protein reductase (ENR) has been investigated as an attractive target due to its important role in membrane construction and energy production in Plasmodium falciparum (Nicola et al., 2007) while the parasite interacts with human erythrocyte spectrin and other membrane proteins through protein M18 aspartyl aminopeptidase (Lauterbach and Coetzer, 2008). Trans-3,3’,4’,5,7-pentahydroxylflavane, epigallocatechin, and epicatechin can modulate both while epigallocatechin 3-gallate can regulate Enoyl-acyl carrier protein reductase and, Quercetin and vanillic acid can act on M18 aspartyl aminopeptidase. Epigallocatechin 3-gallate can also target 3-oxoacyl-acyl-carrier protein reductase which is a potent therapeutic target because of its role in type II fatty acid synthase pathway of Plasmodium falciparum (Karmodiya and Surolia, 2006).
Epigallocatechin 3-gallate and Quercetin are the bioactives that have shown maximum antimicrobial targets interaction. While Epigallocatechin 3-gallate shows interaction with 3-oxoacyl-(Acyl-carrier protein) reductase, CpG DNA methylase, Enoyl-acyl-carrier protein reductase, Glucose-6-phosphate 1-dehydrogenase, hepatitis C virus serine protease, NS3/NS4A and YopH of Plasmodium falciparum, Saccharomyces cerevisiae, and Spiroplasma monobiae; Quercetin acts on 3C-like proteinase (3CL-PRO), arginase, beta-lactamase AmpC, glutathione reductase, M18 aspartyl aminopeptidase, malate dehydrogenase and tyrosine-protein kinase transforming protein FPS of Escherichia coli, Fujinami sarcoma virus, human SARS coronavirus (SARS-CoV), Leishmania amazonensis, Plasmodium falciparum, Saccharomyces cerevisiae, and Thermus thermophiles.
Applications of Network Pharmacology
NP has gained impetus as a novel paradigm for drug discovery. This approach using in silico data is fast becoming popular due to its cost efficiency and comparably good predictability. Thus, network analysis has various applications and promising future prospects with regard to the process of drug discovery and development. Table 5.2 lists the important applications of NP.
Table 5.2.
Applications of Network Pharmacology
| Traditional medicine |
|
| Pharmacology |
|
| Drug research |
|
Limitations and Solutions
NP has proven to be a boon for drug research, and that helps in the revival of traditional knowledge. Albeit there are a few limitations of using NP for studying traditional medicine that would hopefully get resolved in the future. The major limitations and possible solutions are listed:
-
1.
NEP currently relies on various databases for literature and bioactive mining. Databases, though curated, may show discrepancies due to numerous sources of information, theoretical, and experimental data. Moreover, the botanicals that undergo certain preparatory procedures during the formulation of the medicine may have its constituents that have chemically changed due to the procedures; like boiling, acid/alkali reactions, interactions between the bioactives, etc. A way to navigate around this problem is to make use of modern, high-throughput chemical identification techniques like ultra-performance liquid chromatography–electrospray ionization–tandem mass spectroscopy (UPLC-ESI-MS/MS). This technique will help to identify the exact bioactives or the chemical constituents of the formulation, and will enrich the subsequent NEP studies. This is because the bioactives form the foundation of any traditional medicine network.
-
2.
Absorption, distribution, metabolism, excretion, and toxic effects (ADMET) parameters associated with the bioactives/formulation when they are administered in the form of the medicine need to be considered in order to extrapolate in silico and cheminformatics data to in vitro and in vivo models. In silico tools that offer the prediction of these parameters can be depended on for this. But traditional medicines are generally accompanied by a vehicle for delivery of the medicine. These vehicles, normally various solvents—water, milk, lemon juice, butter, ghee (clarified butter), honey—that alter the solubility of the bioactives, play a role in regulating ADMET parameters. Experimental validation studies are required to evaluate this principle of traditional medicine.
-
3.
Target identification usually relies on a single or a few databases due to the limited availability of databases with free access. This can occasionally give incomplete results. Also, there may be novel targets waiting to be discovered that could be a part of the mechanism of action of the bioactives. To deal with this discrepancy in the network, multiple databases should be considered for target identification. Integration of databases serving similar functions can also be a solution for this problem. In addition to this, experimental validation of the target molecules using protein–protein interaction studies or gene expression studies will provide concrete testimony to the network predictions.
-
4.
A number of traditional medicines act through multiple bioactives and targets. Synergy in botanical drugs helps to balance out the extreme pharmacological effects that individual bioactives may have. The interactions of bioactives with various target proteins, their absorption into the body after possible enzyme degradation, their transport, and finally their physiological effect are a crucial part of traditional medicine (Gilbert and Alves, 2003). However, in vitro assays or in silico tools are unable to give a clear idea as to the complete and exact interactions in a living organism. NP is only the cardinal step toward understanding the mechanism of bioactives/formulations. But this gives an overview of the action of traditional medicine which can be used to design in vivo experiments and clinical trials. This saves time and cost of research and inventions.
-
5.
It is observed that formulations are working by simultaneous modulation of multiple targets. This modulation includes activation of some targets and inhibition of other. In order to understand this complex synergistic activity of formulation, investigative studies regarding the interactions of ligands with targets are to be carried out. This can be achieved by implementing high-throughput omics studies based on the network data.
Conclusion
Network pharmacological analysis presents an immense scope for exploring traditional knowledge to find solutions for the current problems challenging the drug discovery industry. NEP can also play a key role in new drug discovery, drug repurposing, and rational formulation discovery. Many of the bioactive–target combinations have been experimentally studied. The data synthesis using NP provides information regarding the mode of action of traditional medicine formulations, based on their constituent bioactives. This is a kind of reverse approach to deduce the molecular mechanism of action of formulations using modern, integrated technologies. The current network analysis is based on the studies that have been conducted and the literature that is available. Hence, the data is inconclusive as a number of studies are still underway and novel data is being generated continuously. Despite its limitations, this still is a favorable approach, as it gives insight into the hidden knowledge of our ancient traditional medicine wisdom. NP aids the logical analysis of this wisdom that can be utilized to understand the knowledge as well as to invent novel solutions for current pharmacological problems.
References
- Amala V.E., Jeyaraj M. Determination of antibacterial, antifungal, bioactive constituents of Triphala by FT-IR and GC-MS analysis. Int. J. Pharm. Pharm. Sci. 2014;6:13–16. [Google Scholar]
- An L., Feng F. Network pharmacology-based antioxidant effect study of Zhi-Zi-Da-Huang decoction for alcoholic liver disease. Evid. Based Complement Alternat. Med. 2015;2015:1–6. doi: 10.1155/2015/492470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag A., Bhattacharyya S.K., Pal N.K. Antibacterial potential of hydroalcoholic extracts of Triphala components against multidrug-resistant uropathogenic bacteria--a preliminary report. Ind. J. Exp. Biol. 2013;51:709–714. [PubMed] [Google Scholar]
- Bairoch A., Apweiler R., Wu C.H., Barker W.C., Boeckmann B., Ferro S. The universal protein resource (UniProt) Nucl. Acids Res. 2005;33:D154–D159. doi: 10.1093/nar/gki070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga M.S. Triphala, ayurvedic formulation for treating and preventing cancer: a review. J. Altern. Compl. Med. 2010;16:1301–1308. doi: 10.1089/acm.2009.0633. [DOI] [PubMed] [Google Scholar]
- Banerjee P., Erehman J., Gohlke B.-O., Wilhelm T., Preissner R., Dunkel M. Super natural II--a database of natural products. Nucl. Acids Res. 2015;43:D935–D939. doi: 10.1093/nar/gku886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabási A.-L., Oltvai Z.N. Network biology: understanding the cell’s functional organization. Nat. Rev. Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- Bento A.P., Gaulton A., Hersey A., Bellis L.J., Chambers J., Davies M. The ChEMBL bioactivity database: an update. Nucl. Acids Res. 2014;42 doi: 10.1093/nar/gkt1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S.I., Iyengar R. Network analyses in systems pharmacology. Bioinformatics. 2009;25:2466–2472. doi: 10.1093/bioinformatics/btp465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biradar Y.S., Jagatap S., Khandelwal K.R., Singhania S.S. Exploring of antimicrobial activity of Triphala mashi-an ayurvedic formulation. Evidence-based complement. Altern. Med. 2008;5:107–113. doi: 10.1093/ecam/nem002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A. Approaches to drug discovery. N Engl. J. Med. 1964;270:1098–1101. doi: 10.1056/NEJM196405212702106. [DOI] [PubMed] [Google Scholar]
- Buriani A., Garcia-Bermejo M.L., Bosisio E., Xu Q., Li H., Dong X. Omic techniques in systems biology approaches to traditional Chinese medicine research: present and future. J. Ethnopharmacol. 2012;140:535–544. doi: 10.1016/j.jep.2012.01.055. [DOI] [PubMed] [Google Scholar]
- Chandran U., Mehendale N., Tillu G., Patwardhan B. Network pharmacology: an emerging technique for natural product drug discovery and scientific research on ayurveda. Proc. Ind. Natl. Acad. Sci. 2015;81:561–568. [Google Scholar]
- Chandran U., Mehendale N., Tillu G., Patwardhan B. Network pharmacology of ayurveda formulation Triphala with special reference to anti-cancer property. Comb. Chem. High Throughput Screen. 2015;18:846–854. doi: 10.2174/1386207318666151019093606. [DOI] [PubMed] [Google Scholar]
- Chen D., Lu P., Zhang F.-B., Tang S.-H., Yang H.-J. Molecular mechanism research on simultaneous therapy of brain and heart based on data mining and network analysis. Zhongguo Zhong Yao Za Zhi. 2013;38:91–98. [PubMed] [Google Scholar]
- Cheng B.F., Hou Y.Y., Jiang M., Zhao Z.Y., Dong L.Y., Bai G. Anti-inflammatory mechanism of qingfei xiaoyan wan studied with network pharmacology. Yao Xue Xue Bao. 2013;48:686–693. [PubMed] [Google Scholar]
- Cho D.-Y., Kim Y.-A., Przytycka T.M. Chapter 5: network biology approach to complex diseases. PLoS Comput. Biol. 2012;8:e1002820. doi: 10.1371/journal.pcbi.1002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.E., Grootenhuis P.D.J. Progress in computational methods for the prediction of ADMET properties. Curr. Opin. Drug Discov. Devel. 2002;5:382–390. [PubMed] [Google Scholar]
- Clark D.E., Pickett S.D. Computational methods for the prediction of “drug-likeness.”. Drug Discov. Today. 2000;5:49–58. doi: 10.1016/s1359-6446(99)01451-8. [DOI] [PubMed] [Google Scholar]
- Croft D., O’Kelly G., Wu G., Haw R., Gillespie M., Matthews L. Reactome: a database of reactions, pathways and biological processes. Nucl. Acids Res. 2011;39 doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F., Zhang Q., Hu Y., Wang Y. Mechanism study on preventive and curative effects of buyang huanwu decoction in Qi deficiency and blood stasis diseases based on network analysis. Zhongguo Zhong Yao Za Zhi. 2014;39:4418–4425. [PubMed] [Google Scholar]
- Ding F., Zhang Q., Ung C.O.L., Wang Y., Han Y., Hu Y. An analysis of chemical ingredients network of Chinese herbal formulae for the treatment of coronary heart disease. PLoS One. 2015;10:e0116441. doi: 10.1371/journal.pone.0116441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke J.A., Beckstrom-Sternberg S.M. Dr. Duke’s phytochemical and ethnobotanical databases. ARS/USDA; 1994. [Google Scholar]
- Dwivedi V., Anandan E.M., Mony R.S., Muraleedharan T.S., Valiathan M.S., Mutsuddi M. In vivo effects of traditional ayurvedic formulations in drosophila melanogaster model relate with therapeutic applications. PLoS One. 2012;7:e37113. doi: 10.1371/journal.pone.0037113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi V., Tripathi B.K., Mutsuddi M., Lakhotia S.C. Ayurvedic Amalaki Rasayana and Rasa-Sindoor suppress neurodegeneration in fly models of Huntington’s and Alzheimer’s diseases. Curr. Sci. 2013;105(12):1711–1723. [Google Scholar]
- Ellingson S.R., Smith J.C., Baudry J. Polypharmacology and supercomputer-based docking: opportunities and challenges. Mol. Simul. 2014;40:848–854. [Google Scholar]
- Fang X., Shao L., Zhang H., Wang S. CHMIS-C: a comprehensive herbal medicine information system for cancer. J. Med. Chem. 2005;48:1481–1488. doi: 10.1021/jm049838d. [DOI] [PubMed] [Google Scholar]
- Fang Y.-C., Huang H.-C., Chen H.-H., Juan H.-F. TC;MGeneDIT: a database for associated traditional Chinese medicine, gene and disease information using text mining. BMC Compl. Altern. Med. 2008;8:58. doi: 10.1186/1472-6882-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Lu B., Liu M., Zhang M., Yi Z., Wen C. Evaluating the pharmacological mechanism of Chinese medicine Si-Wu-Tang through multi-level data integration. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0072334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A.K., Avasthi S., Sharma A., Bhadauria R. Antifungal potential of Triphala churna ingredients against Aspergillus species associated with them during storage. Pakistan J. Biol. Sci. 2012;15:244–249. doi: 10.3923/pjbs.2012.244.249. [DOI] [PubMed] [Google Scholar]
- Gilbert B., Alves L.F. Synergy in plant medicines. Curr. Med. Chem. 2003;10:13–20. doi: 10.2174/0929867033368583. [DOI] [PubMed] [Google Scholar]
- Giraud M.F., Leonard G.A., Field R.A., Berlind C., Naismith J.H. RmlC, the third enzyme of dTDP-L-rhamnose pathway, is a new class of epimerase. Nat. Struct. Biol. 2000;7:398–402. doi: 10.1038/75178. [DOI] [PubMed] [Google Scholar]
- Gu J., Chen Y., Li S., Li Y. Identification of responsive gene modules by network-based gene clustering and extending: application to inflammation and angiogenesis. BMC Syst. Biol. 2010;4:47. doi: 10.1186/1752-0509-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gui Y., Chen L., Yuan G., Lu H.Z., Xu X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0062839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gui Y., Chen L., Yuan G., Xu X. CVDHD: a cardiovascular disease herbal database for drug discovery and network pharmacology. J. Cheminform. 2013;5:51. doi: 10.1186/1758-2946-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Yin N., Pei J., Lai L. Understanding traditional Chinese medicine anti-inflammatory herbal formulae by simulating their regulatory functions in the human arachidonic acid metabolic network. Mol. Biosyst. 2013;9:1931–1938. doi: 10.1039/c3mb25605g. [DOI] [PubMed] [Google Scholar]
- Gupta S.K., Kalaiselvan V., Srivastava S., Agrawal S.S., Saxena R. Evaluation of anticataract potential of Triphala in selenite-induced cataract: in vitro and in vivo studies. J. Ayurveda Integr. Med. 2010;1:280–286. doi: 10.4103/0975-9476.74425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker M., Messer W.S., II, Bachmann K.A. Pharmacology: Principles and Practice. Elsevier/Academic Press; 2009. [Google Scholar]
- Hamosh A., Scott A.F., Amberger J.S., Bocchini C.A., McKusick V.A. Online mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucl. Acids Res. 2005;33:D514–D517. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A.L. Natural products in drug discovery. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Hopkins A.L. Network pharmacology. Nat. Biotechnol. 2007;25:1110–1111. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Hu Q.-N., Deng Z., Tu W., Yang X., Meng Z.-B., Deng Z.-X. VNP: interactive visual network pharmacology of diseases, targets, and drugs. CPT Pharmacometrics Syst. Pharmacol. 2014;3:e105. doi: 10.1038/psp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Li S. Detection of characteristic sub pathway network for angiogenesis based on the comprehensive pathway network. BMC Bioinformatics. 2010:11. doi: 10.1186/1471-2105-11-S1-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmodiya K., Surolia N. Analyses of co-operative transitions in plasmodium falciparum beta-ketoacyl acyl carrier protein reductase upon co-factor and acyl carrier protein binding. FEBS J. 2006;273:4093–4103. doi: 10.1111/j.1742-4658.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- Kibble M., Saarinen N., Tang J., Wennerberg K., Mäkelä S., Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015;32:1249–1266. doi: 10.1039/c5np00005j. [DOI] [PubMed] [Google Scholar]
- Kumar M.S., Kirubanandan S., Sripriya R., Sehgal P.K. Triphala promotes healing of infected full-thickness dermal wound. J. Surg. Res. 2008;144:94–101. doi: 10.1016/j.jss.2007.02.049. [DOI] [PubMed] [Google Scholar]
- Lagunin A.A., Goel R.K., Gawande D.Y., Pahwa P., Gloriozova T.A., Dmitriev A.V. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: a critical review. Nat. Prod. Rep. 2014;31(11):1585–5611. doi: 10.1039/c4np00068d. [DOI] [PubMed] [Google Scholar]
- Lauterbach S.B., Coetzer T.L. The M18 aspartyl aminopeptidase of plasmodium falciparum binds to human erythrocyte spectrin in vitro. Malar. J. 2008;7:161. doi: 10.1186/1475-2875-7-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E.L., Cao Z.-W., Jiang Z.-H., Zhou H., Liu L. Network-based drug discovery by integrating systems biology and computational technologies. Brief. Bioinform. 2013;14:491–505. doi: 10.1093/bib/bbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Tao W., Zheng C., Shar P.A., Huang C., Fu Y. Systems pharmacology-based approach for dissecting the addition and subtraction theory of traditional Chinese medicine: an example using xiao-chaihu-decoction and da-chaihu-decoction. Comput. Biol. Med. 2014;53C:19–29. doi: 10.1016/j.compbiomed.2014.05.007. Elsevier. [DOI] [PubMed] [Google Scholar]
- Li H., Zhao L., Zhang B., Jiang Y., Wang X., Guo Y. A network pharmacology approach to determine active compounds and action mechanisms of Ge-Gen-Qin-Lian decoction for treatment of type 2 diabetes. Evid. Based Complement Altern. Med. 2014;2014 doi: 10.1155/2014/495840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lu C., Jiang M., Niu X., Guo H., Li L. Traditional Chinese medicine-based network pharmacology could lead to new multicompound drug discovery. Evid. Based. Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/149762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Framework and practice of network-based studies for Chinese herbal formula. J. Chinese Integr. Med. 2007;5:489–493. doi: 10.3736/jcim20070501. [DOI] [PubMed] [Google Scholar]
- Li S., Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin. J. Nat. Med. 2013;11:110–120. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- Li S., Wu L., Zhang Z. Constructing biological networks through combined literature mining and microarray analysis: a LMMA approach. Bioinformatics. 2006;22:2143–2150. doi: 10.1093/bioinformatics/btl363. [DOI] [PubMed] [Google Scholar]
- Li S., Zhang Z.Q., Wu L.J., Zhang X.G., Li Y.D., Wang Y. Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst. Biol. 2007;1:51–60. doi: 10.1049/iet-syb:20060032. [DOI] [PubMed] [Google Scholar]
- Li S., Zhang B., Jiang D., Wei Y., Zhang N. Herb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulae. BMC Bioinformatics. 2010;11 doi: 10.1186/1471-2105-11-S11-S6. BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang B., Zhang N. Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst. Biol. 2011;5 doi: 10.1186/1752-0509-5-S1-S10. BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Tang Y., Shang E., Guo J., Huang M., Qian D. Analysis on correlation between general efficacy and chemical constituents of danggui-chuanxiong herb pair based on artificial neural network. Zhongguo Zhong Yao Za Zhi. 2012;37:2935–2942. [PubMed] [Google Scholar]
- Li X., Wu L., Fan X., Zhang B., Gao X., Wang Y. Network pharmacology study on major active compounds of fufang danshen formula. Zhongguo Zhong Yao Za Zhi. 2011;36:2911–2915. [PubMed] [Google Scholar]
- Li X., Wu L., Liu W., Jin Y., Chen Q., Wang L. A network pharmacology study of Chinese medicine QiShenYiQi to reveal its underlying multi-compound, multi-target, multi-pathway mode of action. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0095004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li R., Ouyang Z., Li S. Herb network analysis for a famous TCM doctor’ s prescriptions on treatment of rheumatoid arthritis. Evidence-based complement. Altern. Med. 2015;2015 doi: 10.1155/2015/451319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Li H., Li S. A novel network pharmacology approach to analyse traditional herbal formulae: the Liu-Wei-Di-Huang pill as a case study. Mol. Biosyst. 2014;10:1014–1022. doi: 10.1039/c3mb70507b. [DOI] [PubMed] [Google Scholar]
- Liu T., Lin Y., Wen X., Jorissen R.N., Gilson M.K. BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucl. Acids Res. 2007;35:198–201. doi: 10.1093/nar/gkl999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Duan J.-A., Bai G., Su S.-L. Network pharmacology study on major active compounds of siwu decoction analogous formulae for treating primary dysmenorrhea of gynecology blood stasis syndrome. Zhongguo Zhong Yao Za Zhi. 2014;39:113–120. [PubMed] [Google Scholar]
- Liu X. Computational pharmacological comparison of Salvia miltiorrhiza and Panax notoginseng used in the therapy of cardiovascular diseases. Exp. Ther. Med. 2013:1163–1168. doi: 10.3892/etm.2013.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K., Chakroborty D., Sarkar C., Lu T., Xie Z., Liu Z., Basu S. Triphala and its active constituent chebulinic acid are natural inhibitors of vascular endothelial growth factor-a mediated angiogenesis. PLoS One. 2012;7:e43934. doi: 10.1371/journal.pone.0043934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Li Y., Li S. Computational identification of potential microRNA network biomarkers for the progression stages of gastric cancer. Int. J. Data Min. Bioinform. 2011;5:519–531. doi: 10.1504/ijdmb.2011.043031. [DOI] [PubMed] [Google Scholar]
- Luo F., Gu J., Chen L., Xu X. Systems pharmacology strategies for anticancer drug discovery based on natural products. Mol. Biosyst. 2014;10:1912–1917. doi: 10.1039/c4mb00105b. [DOI] [PubMed] [Google Scholar]
- Luo F., Gu J., Zhang X., Chen L., Cao L., Li N. Multiscale modeling of drug-induced effects of ReDuNing injection on human disease: from drug molecules to clinical symptoms of disease. Sci. Rep. 2015;5:10064. doi: 10.1038/srep10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Stern R.J., Scherman M.S., Vissa V.D., Yan W., Jones V.C. Drug targeting mycobacterium tuberculosis cell wall synthesis: genetics of dTDP-rhamnose synthetic enzymes and development of a microtiter plate-based screen for inhibitors of conversion of dTDP-glucose to dTDP-rhamnose. Antimicrob. Agents Chemother. 2001;45:1407–1416. doi: 10.1128/AAC.45.5.1407-1416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Tan C., Zhang H., Wang M., Ding W., Li S. Bridging the gap between traditional Chinese medicine and systems biology: the connection of cold syndrome and NEI network. Mol. Biosyst. 2010;6:613–619. doi: 10.1039/b914024g. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Ph D., Kuchinsky A., Pico A., Institutes G. Analysis and Visualization of Biological Networks with Cytoscape. UCSF; 2012. p. 65. [Google Scholar]
- Nicola G., Smith C.A., Lucumi E., Kuo M.R., Karagyozov L., Fidock D.A. Discovery of novel inhibitors targeting enoyl-acyl carrier protein reductase in plasmodium falciparum by structure-based virtual screening. Biochem. Biophys. Res. Commun. 2007;358:686–691. doi: 10.1016/j.bbrc.2007.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H., Goto S., Sato K., Fujibuchi W., Bono H., Kanehisa M. KEGG: kyoto encyclopedia of genes and genomes. Nucl. Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini G.V., Shapland R.H.B., van Hoorn W.P., Mason J.S., Hopkins A.L. Global mapping of pharmacological space. Nat. Biotechnol. 2006;24:805–815. doi: 10.1038/nbt1228. [DOI] [PubMed] [Google Scholar]
- Patwardhan B. Rediscovering drug discovery. Comb. Chem. High Throughput Screen. 2014;17:819. doi: 10.2174/138620731710150108230829. [DOI] [PubMed] [Google Scholar]
- Patwardhan B. The new pharmacognosy. Comb. Chem. High Throughput Screen. 2014;17:97. doi: 10.2174/138620731702140119160627. [DOI] [PubMed] [Google Scholar]
- Patwardhan B., Chandran U. Network ethnopharmacology approaches for formulation discovery. Ind. J. Tradit. Knowl. 2015;14:574–580. [Google Scholar]
- Patwardhan B., Mutalik G., Tillu G. Integrative Approaches For Health: Biomedical Research, Ayurveda and Yoga. Academic Press, Elsevier Inc; 2015. [Google Scholar]
- Pei L., Bao Y., Liu S., Zheng J., Chen X. Material basis of Chinese herbal formulas explored by combining pharmacokinetics with network pharmacology. PLoS One. 2013;8:e57414. doi: 10.1371/journal.pone.0057414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K., Xu W., Zheng J., Huang K., Wang H., Tong J. The disease and gene annotations (DGA): an annotation resource for human disease. Nucl. Acids Res. 2013;41:553–560. doi: 10.1093/nar/gks1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetkate P., Kummalue T., U-Pratya Y., Kietinun S. Significant increase in cytotoxic T lymphocytes and natural killer cells by Triphala: a clinical phase i study. Evid. Based Complement Alternat. Med. 2012;2012 doi: 10.1155/2012/239856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén F.K., Schwenk J.M., Asplund A., Edqvist P.H.D. The human protein atlas as a proteomic resource for biomarker discovery. J. Intern. Med. 2011;270:428–446. doi: 10.1111/j.1365-2796.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- Prabhakar J., Senthilkumar M., Priya M.S., Mahalakshmi K., Sehgal P.K., Sukumaran V.G. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against enterococcus faecalis biofilm formed on tooth substrate: an in vitro study. J. Endod. 2010;36:83–86. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Prabhakar J., Balagopal S., Priya M., Selvi S., Senthilkumar M. Evaluation of antimicrobial efficacy of Triphala (an Indian Ayurvedic herbal formulation) and 0.2% chlorhexidine against Streptococcus mutans biofilm formed on tooth substrate: an in vitro study. Ind. J. Dent. Res. 2014;25:475. doi: 10.4103/0970-9290.142539. [DOI] [PubMed] [Google Scholar]
- Prasad C.P., Rath G., Mathur S., Bhatnagar D., Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: modulation of Wnt/beta-catenin signaling. Chem. Biol. Interact. 2009;181:263–271. doi: 10.1016/j.cbi.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Re M., Valentini G. Network-based drug ranking and repositioning with respect to DrugBank therapeutic categories. IEEE/ACM Trans. Comput. Biol. Bioinform. 2013;10:1359–1371. doi: 10.1109/TCBB.2013.62. [DOI] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabu M.C., Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J. Ethnopharmacol. 2002;81:155–160. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. Databases aim to bridge the East-West divide of drug discovery. Nat. Med. 2011;17:1531. doi: 10.1038/nm1211-1531a. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. [DOI] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. Triphala powder: a wonder of ayurveda. Int. J. Recent Res. Asp. 2015;2:107–111. [Google Scholar]
- Sheng S., Wang J., Wang L., Liu H., Li P., Liu M. Network pharmacology analyses of the antithrombotic pharmacological mechanism of Fufang Xueshuantong Capsule with experimental support using disseminated intravascular coagulation rats. J. Ethnopharmacol. 2014;154:735–744. doi: 10.1016/j.jep.2014.04.048. [DOI] [PubMed] [Google Scholar]
- Shi S.H., Cai Y.P., Cai X.J., Zheng X.Y., Cao D.S., Ye F.Q. A network pharmacology approach to understanding the mechanisms of action of traditional medicine: bushenhuoxue formula for treatment of chronic kidney disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Sahu R.P., Srivastava S.K. Triphala inhibits both in vitro and in vivo xenograft growth of pancreatic tumor cells by inducing apoptosis. BMC Cancer. 2008;8:294. doi: 10.1186/1471-2407-8-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankar S., Lavanya R., Brindha P., Angayarkanni N. Aqueous and alcoholic extracts of Triphala and their active compounds chebulagic acid and chebulinic acid prevented epithelial to mesenchymal transition in retinal pigment epithelial cells, by inhibiting SMAD-3 phosphorylation. PLoS One. 2015;10:e0120512. doi: 10.1371/journal.pone.0120512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumar R., Parthasarathy N.J., Shankar E.M., Manikandan S., Vijayakumar R., Thangaraj R. Evaluation of the growth inhibitory activities of Triphala against common bacterial isolates from HIV infected patients. Phytother. Res. 2007;21:476–480. doi: 10.1002/ptr.2105. [DOI] [PubMed] [Google Scholar]
- Srinagesh J., Krishnappa P., Somanna S.N. Antibacterial efficacy of Triphala against oral streptococci: an in vivo study. Ind. J. Dent. Res. 2012;23:696. doi: 10.4103/0970-9290.107423. [DOI] [PubMed] [Google Scholar]
- Sumathi P., Parvathi A. Antibacterial potential of the three medicinal fruits used in Triphala: an ayurvedic formulation. J. Med. Plants. 2010;4:1682–1685. [Google Scholar]
- van de Waterbeemd H., Gifford E. ADMET in silico modelling: towards prediction paradise? Nat. Rev. Drug Discov. 2003;2:192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhou G.-B., Liu P., Song J.-H., Liang Y., Yan X.-J. Dissection of mechanisms of Chinese medicinal formula realgar-indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li Z., Zhao X., Liu W., Liu Y., Yang J. A network study of Chinese medicine xuesaitong injection to elucidate a complex mode of action with multicompound, multitarget, and multipathway. Evid. Based Complement Alternat. Med. 2013;2013 doi: 10.1155/2013/652373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang J., Hong Y., Feng Y., Chen M., Wang Y. Phytochemical and pharmacological review of da Chuanxiong formula: a famous herb pair composed of chuanxiong rhizoma and gastrodiae rhizoma for headache. Evid. Based Complement Alternat. Med. 2013;2013 doi: 10.1155/2013/425369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Y., Fu L.L., Zhang S.Y., Tian M., Zhang L., Zheng Y.X. In silico analysis and experimental validation of active compounds from fructus Schisandrae chinensis in protection from hepatic injury. Cell Prolif. 2015;48:86–94. doi: 10.1111/cpr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Wang Z., Wang S., Ravula R., Yang L., Xu J. Discovery of molecular mechanisms of traditional Chinese medicinal formula Si-Wu-Tang using gene expression microarray and connectivity map. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel S., Bon R.S., Kumar K., Waldmann H. Biology-oriented synthesis. Angew. Chemie-Int. Ed. 2011;50:10800–10826. doi: 10.1002/anie.201007004. [DOI] [PubMed] [Google Scholar]
- Wu L., Wang Y., Nie J., Fan X., Cheng Y. A network pharmacology approach to evaluating the efficacy of Chinese medicine using genome-wide transcriptional expression data. Evid. Based Complement. Alternat. Med. 2013;2013:915343. doi: 10.1155/2013/915343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Wang Y., Li Z., Zhang B., Cheng Y., Fan X. Identifying roles of “Jun-Chen-Zuo-Shi” component herbs of QiShenYiQi formula in treating acute myocardial ischemia by network pharmacology. Chin. Med. 2014;9:24. doi: 10.1186/1749-8546-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Jiang R., Zhang M.Q., Li S. Network-based global inference of human disease genes. Mol. Syst. Biol. 2008;4:189. doi: 10.1038/msb.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z., Sun H., Cai X., Chen D., Zheng X. The study on the material basis and the mechanism for anti-renal interstitial fibrosis efficacy of rhubarb through integration of metabonomics and network pharmacology. Mol. Biosyst. 2015;11:1067–1078. doi: 10.1039/c4mb00573b. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhang Y., Lei Y., Gao X., Zhai H., Lin N. A systems biology-based approach to uncovering the molecular mechanisms underlying the effects of dragon’s blood tablet in colitis, involving the integration of chemical analysis, ADME prediction, and network pharmacology. PLoS One. 2014;9:e101432. doi: 10.1371/journal.pone.0101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.-Y., Wang S.-S., Yang H.-J., Bian B.-L., Tian S.-S., Wang D.-L. Study on action mechanism of adjuvant therapeutic effect compound Ejiao slurry in treating cancers based on network pharmacology. Zhongguo Zhong Yao Za Zhi. 2014;39:3148–3151. [PubMed] [Google Scholar]
- Xu Q., Qu F., Pelkonen O. Network Pharmacology and Traditional Chinese Medicine. In: Hiroshi Sakagami., editor. Alternative medicine. InTech; 2011. pp. 277–297. [Google Scholar]
- Yang H., Xing L., Zhou M.-G. Network pharmacological research of volatile oil from Zhike Chuanbei Pipa Dropping Pills in treatment of airway inflammation. Chinese Traditional and Herbal Drugs. 2012;43:1129–1135. [Google Scholar]
- Yang M., Chen J.-L., Xu L.-W., Ji G. Navigating traditional Chinese medicine network pharmacology and computational tools. Evid. Based Complement. Alternat. Med. 2013;2013:731969. doi: 10.1155/2013/731969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Hao H., Li Y., Li S. Modularity-based credible prediction of disease genes and detection of disease subtypes on the phenotype-gene heterogeneous network. BMC Syst. Biol. 2011;5:79. doi: 10.1186/1752-0509-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M.A., Goh K., Cusick M.E., Barabási A., Vidal M. Drug–target network. Nat. Biotechnol. 2007;25:1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- Yuan C.H., Filippova M., Tungteakkhun S.S., Duerksen-Hughes P.J., Krstenansky J.L. Small molecule inhibitors of the HPV16-E6 interaction with caspase 8. Bioorg. Med. Chem. Lett. 2012;22:2125–2129. doi: 10.1016/j.bmcl.2011.12.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wang X., Li S. An integrative platform of TCM network pharmacology and its application on a herbal formula, Qing-Luo-Yin. Evid. Based Complement Alternat. Med. 2013:2013. doi: 10.1155/2013/456747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.-P., Pan J.-B., Zhang C., Ji N., Wang H., Ji Z.-L. Network understanding of herb medicine via rapid identification of ingredient-target interactions. Sci. Rep. 2014;4:3719. doi: 10.1038/srep03719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ma T., Li Y., Li S. dbNEI2.0: building multilayer network for drug-NEI-disease. Bioinformatics. 2008;24:2409–2411. doi: 10.1093/bioinformatics/btn388. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li Y., Chen S.-S., Zhang L., Wang J., Yang Y. Systems pharmacology dissection of the anti-inflammatory mechanism for the medicinal herb folium eriobotryae. Int. J. Mol. Sci. 2015;16:2913–2941. doi: 10.3390/ijms16022913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Gu J., Cao L., Li N., Ma Y., Su Z. Network pharmacology study on the mechanism of traditional Chinese medicine for upper respiratory tract infection. Mol. Biosyst. 2014;10 doi: 10.1039/c4mb00164h. [DOI] [PubMed] [Google Scholar]
- Zhao F., Guochun L., Yang Y., Shi L., Xu L., Yin L. A network pharmacology approach to determine active ingredients and rationality of herb combinations of modified-simiaowan for treatment of gout. J. Ethnopharmacol. 2015;168:1–16. doi: 10.1016/j.jep.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Zhao S., Li S. A co-module approach for elucidating drug-disease associations and revealing their molecular basis. Bioinformatics. 2012;28:955–961. doi: 10.1093/bioinformatics/bts057. [DOI] [PubMed] [Google Scholar]
- Zheng C., Fu C., Pan C., Bao H., Chen X., Ye H. Deciphering the underlying mechanisms of diesun miaofang in traumatic injury from a systems pharmacology perspective. Mol. Med. Rep. 2015:1–8. doi: 10.3892/mmr.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C.-S., Xu X.-J., Ye H.-Z., Wu G.-W., Li X.-H., Xu H.-F. Network pharmacology-based prediction of the multi-target capabilities of the compounds in Taohong Siwu decoction, and their application in osteoarthritis. Exp. Ther. Med. 2013;6:125–132. doi: 10.3892/etm.2013.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Wang Y. A network-based analysis of the types of coronary artery disease from traditional Chinese medicine perspective: potential for therapeutics and drug discovery. J. Ethnopharmacol. 2014;151:66–77. doi: 10.1016/j.jep.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Zhu F., Shi Z., Qin C., Tao L., Liu X., Xu F. Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucl. Acids Res. 2012;40:1128–1136. doi: 10.1093/nar/gkr797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi T., Yu D. A network pharmacology study of Sendeng-4, a Mongolian medicine. Chin. J. Nat. Med. 2015;13:108–118. doi: 10.1016/S1875-5364(15)60014-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann G.R., Lehár J., Keith C.T. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov. Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]


