Abstract
The Middle East respiratory syndrome (MERS) is a lethal zoonosis caused by MERS coronavirus (MERS-CoV) and poses a significant threat to public health worldwide. Therefore, a rapid, sensitive, and specific serologic test for detecting anti-MERS-CoV antibodies in both humans and animals is urgently needed for the successful management of this illness. Here, we evaluated various novel luciferase immunosorbent assays (LISA) based on nucleocapsid protein (NP) as well as fragments derived from spike protein (S) including subunit 1 (S1), N terminal domain (NTD), receptor-binding domain (RBD) and subunit 2 (S2) of S for the detection of MERS-CoV-specific IgG. Fusion proteins, including nanoluciferase (NLuc) and various fragments derived from the NP or S protein of MERS-CoV, were expressed in human embryonic kidney 293 T cells. LISAs that detected anti-MERS-CoV IgG were further developed using cell lysates expressing various fusion proteins. Panels of human or animal samples infected with MERS-CoV were used to analyze the sensitivity and specificity of various LISAs in reference to a MERS-CoV RT-PCR, commercial S1-based ELISA, and pseudovirus particle neutralization test (ppNT). Our results showed that the S1-, RBD-, and NP-LISAs were more sensitive than the NTD- and S2-LISAs for the detection of anti-MERS-CoV IgG. Furthermore, the S1-, RBD-, and NP-LISAs were more sensitive (by at least 16-fold) than the commercially available S1-ELISA. Moreover, the S1-, RBD-, and NP-LISA specifically recognized anti-MERS-CoV IgG and did not cross-react with samples derived from other human CoV (OC43, 229E, HKU1, NL63)-infected patients. More importantly, these LISAs proved their applicability and reliability for detecting anti-MERS-CoV IgG in samples from camels, monkeys, and mice, among which the RBD-LISA exhibited excellent performance. The results of this study suggest that the novel MERS-CoV RBD- and S1- LISAs are highly effective platforms for the rapid and sensitive detection of anti-MERS-CoV IgG in human and animal samples. These assays have the potential to be used as serologic tests for the management and control of MERS-CoV infection.
Keywords: Luciferase immunosorbent assay (LISA), MERS-CoV, Serological IgG detection, Samples of humans and animals
Highlights
-
•
Scientific question
This study evaluated novel luciferase immunosorbent assays (LISAs) based on nucleocapsid protein (NP) as well as fragments derived from spike protein (S) for detection of MERS-CoV-specific IgG in humans and animals.
-
•
Evidence before this study
Enzyme-linked immunosorbent assay (ELISA), microneutralization (MN), immunofluorescence assay (IFA), and pseudovirus particle neutralization test (ppNT) have been performed to detect serum antibodies against MERS-CoV. There remains a need to develop novel serological assays independent of protein purification, special secondary antibody, virus cultivation and Biosafety Level 3 (BSL-3) laboratory.
-
•
New findings
In this study, novel LISAs based on the MERS-CoV S fragments and NP were developed. Human and animal samples infected with MERS-CoV were measured by the newly developed LISAs as well as reference methods including commercial S1-ELISA and ppNT. The results showed that the S1-, RBD-, and NP-LISAs were able to specifically distinguish MERS-CoV-infected samples from samples infected by other HCoV as consistent as the reference methods. Comparing with the commercially available S1-ELISA, the S1- and RBD-LISAs were 64-folds more sensitive. Moreover, the applicability and reliability of the LISAs were verified by detecting anti-MERS-CoV IgG in samples from camels, monkeys, and mice. The RBD-LISA exhibited superior sensitivity and specificity.
-
•
Significance of the study
The novel MERS-CoV RBD- and S1-LISAs were developed independent of protein purification and special secondary antibody, and showed super specificity and efficiency for the detection of anti-MERS-CoV IgG in human and animal samples. These assays are recommended for serological diagnosis of MERS-CoV infection in the investigation of epidemic characteristic, origin tracing and vaccine study of MERS-CoV, they would contribute to the scientific control and prevention of MERS.
HIGHLIGHTS
HIGHLIGHTS
Scientific question
This study evaluated novel luciferase immunosorbent assays (LISAs) based on nucleocapsid protein (NP) as well as fragments derived from spike protein (S) for detection of MERS-CoV-specific IgG in humans and animals.
Evidence before this study
Enzyme-linked immunosorbent assay (ELISA), microneutralization (MN), immunofluorescence assay (IFA), and pseudovirus particle neutralization test (ppNT) have been performed to detect serum antibodies against MERS-CoV. There remains a need to develop novel serological assays independent of protein purification, special secondary antibody, virus cultivation and Biosafety Level 3 (BSL-3) laboratory.
New findings
In this study, novel LISAs based on the MERS-CoV S fragments and NP were developed. Human and animal samples infected with MERS-CoV were measured by the newly developed LISAs as well as reference methods including commercial S1-ELISA and ppNT. The results showed that the S1-, RBD-, and NP-LISAs were able to specifically distinguish MERS-CoV-infected samples from samples infected by other HCoV as consistent as the reference methods. Comparing with the commercially available S1-ELISA, the S1- and RBD-LISAs were 64-folds more sensitive. Moreover, the applicability and reliability of the LISAs were verified by detecting anti-MERS-CoV IgG in samples from camels, monkeys, and mice. The RBD-LISA exhibited superior sensitivity and specificity.
Significance of the study
The novel MERS-CoV RBD- and S1-LISAs were developed independent of protein purification and special secondary antibody, and showed super specificity and efficiency for the detection of anti-MERS-CoV IgG in human and animal samples. These assays are recommended for serological diagnosis of MERS-CoV infection in the investigation of epidemic characteristic, origin tracing and vaccine study of MERS-CoV, they would contribute to the scientific control and prevention of MERS.
Alt-text: Unlabelled Box
1. Introduction
The Middle East respiratory syndrome (MERS) is a lethal zoonosis caused by MERS coronavirus (CoV) [1,2]. MERS-CoV infection in humans causes asymptomatic or mild respiratory illness, severe pneumonia, multi-organ failure, and even death [[3], [4], [5]]. According to the World Health Organization (WHO), as of May 9, 2019, there were 2,419 laboratory-confirmed cases of MERS and 836 deaths (35% of case-fatality rate) reported from 27 countries (https://www.who.int/csr/don/09-may-2019-mers-saudi-arabia/en/). The majority of MERS cases occurred in the Middle East, especially in Saudi Arabia [1,3,[6], [7], [8], [9]]. The largest epidemic of MERS outside of the Arabian Peninsula occurred in South Korea in May 2015, and the index case was a traveler who visited the Middle East [10,11]. To date, all confirmed MERS cases have been linked to the Middle East. Human-to-human transmission of MERS-CoV has been well documented in family, community, and healthcare settings [5,[12], [13], [14]]. MERS-CoV poses a significant threat to public health worldwide because of the high fatality rate of MERS infection and potential outbreaks of MERS-CoV infection in healthcare facilities. Furthermore, there are no specific vaccines or therapeutics for the prevention or treatment of MERS-CoV infection [[15], [16], [17], [18], [19], [20], [21]].
Dromedary camels have been implicated as a zoonotic source of human infections [3,4,16,[22], [23], [24], [25], [26], [27], [28], [29], [30], [31]], and MERS-CoV RNA and viable virus have been isolated from them [7,22,32]. The seroprevalence of MERS-CoV antibodies is very high in dromedary camels within Eastern Africa and the Arabian Peninsula [22,24,25,29,[33], [34], [35], [36], [37], [38], [39]]. The continuing MERS epidemic in the Middle East is believed to be related to the failure to control the zoonotic sources, particularly dromedary camels, resulting in ongoing camel-to-human transmission [2,26,[40], [41], [42]]. Moreover, MERS-like CoVs have been detected in bat species globally, and thus, are thought to be the reservoirs of MERS-CoV [7,41,43,44]. However, the exact source of many primary MERS cases remains unknown, and the transmission mechanisms are poorly understood.
The MERS-CoV genome encodes 16 non-structural proteins (nsp1–16) and four structural proteins, including the spike (S), small envelope (E), membrane (M), and nucleocapsid (N) proteins. The viral structural proteins, S and N, have the highest immunogenicity [45]. The S protein undergoes protease cleavage into S1 and S2 subunits. The S1 and S2 subunits mediate coronavirus entry into host cells by binding to the receptor on the host-cell surface via the receptor-binding domain (RBD) of the S1 subunit [32,[46], [47], [48]]. Both the S and N proteins are the major immunodominant regions of MERS-CoV, and are often applied as target antigens for detection of a humoral immune response after infection.
Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) is a common method for the diagnosis of MERS-CoV infection [6,49]. However, it is not suitable for the detection of infection in some clinical units and large-scale epidemiological screening. Specific antibodies play important roles in determining the severity of human infection, and detection of these specific antibodies is used in the epidemiological investigation of MERS-CoV infection in animals and humans. Several methods can be used to detect serum antibodies against MERS-CoV, such as enzyme-linked immunosorbent assay (ELISA), microneutralization (MN), immunofluorescence assay (IFA), the plaque reduction neutralization test (PRNT), and the pseudovirus particle neutralization test (ppNT). Among these assays, MN, IFA, and PRNT depend on virus cultivation, and thus, require equipment and facilities located within a Biosafety Level 3 (BSL-3) laboratory [1,3,12,18,35,39,[50], [51], [52], [53], [54]]. While there are commercial anti-MERS-CoV serologic test kits for humans and animals including ELISA and IFA, rapid and sensitive serological assays for the detection of anti-MERS-CoV IgG independent of purified protein, specific secondary antibody, virus cultivation and BSL-3 facilities are still urgently needed.
An ultrasensitive and high-throughput luciferase immunosorbent assay (LISA) based on recombinant antigens fused with NanoLuc luciferase (NLuc) has been reported to detect IgG against several pathogens [[55], [56], [57], [58]]. This assay has the advantage of not requiring a BSL-3 laboratory facility and species-specific labeled secondary antibodies for detection. In this study, we developed various novel LISA platforms based on the MERS-CoV NP or fragments of S. The diagnostic performance of various MERS-CoV LISA were evaluated using serum panels from MERS-CoV-infected humans and animals, as well as sera from patients infected with other human CoV.
2. Materials and methods
2.1. Samples and ethics
Several panels for anti-MERS-CoV IgG detection were collected and stored at −70 °C before testing. The detailed information is described in Table 1, Table 2 . In brief, these panels included the following. 1) Normal serum samples from 40 healthy blood donors without MERS-CoV infection, which were stored in our laboratory. 2) Convalescent sera from five MERS-CoV infected patients. Sera from five MERS-CoV patients with high antibody titers and sera from five MERS-CoV patients with medium titers were mixed to prepare two pooled convalescent serum samples. Control sera from seven non-MERS-CoV patients were from individuals with antibodies to common human CoV (HCoV), namely, HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1, all of which were supplied by the WHO [59]. 3) Serum samples from confirmed MERS-CoV-infected camels from Saudi Arabia, were supplied by the University of Hong Kong. Serum samples from MERS-CoV-infected monkeys and mice were stored in our laboratory. Besides all the samples listed in Table 1, Table 2, one convalescent serum sample from the first imported MERS-CoV-infected patient in China was also detected in the study to characterize the LISAs. The present studies were approved by the Institutional Review Board of the National Institute for Viral Disease Control and Prevention, China CDC, and by the Ethics Committee of Southern Medical University (SMU No. 20160428).
Table 1.
Comparison of LISAs with ppNT and S1-ELISA.a
| Name | Description | Number | Expected result | ppNT | ELISA | LISA |
||
|---|---|---|---|---|---|---|---|---|
| S1 | RBD | NP | ||||||
| Convalescent sera | Individual serum from laboratory confirmed MERS patients | 5 | + | + | + | + | + | + |
| Pooled convalescent sera | Pooled serum samples from laboratory confirmed MERS patients | 2 | + | + | + | + | + | + |
| Non-MERS-CoV sera | Control sera from patients infected by common human coronaviruses (OC43, NL63, 229E, HKU1) |
7 | − | − | − | − | − | − |
| Normal sera (negative) control | Serum samples from 40 healthy blood donors | 40 | − | − | − | − | − | − |
+, positive; −, negative.
Table 2.
Summary of the detection results in animal samples.
| Samplea | RT-PCRb | ppNT | S1-LISA | RBD-LISA | NP-LISA |
|---|---|---|---|---|---|
| C1 | + | − | − | − | − |
| C2 | + | + | + | + | − |
| C3 | + | + | − | − | − |
| C4 | + | + | + | + | + |
| C5 | + | + | + | + | + |
| C6 | + | + | + | + | + |
| C7 | + | − | + | + | + |
| C8 | + | + | + | + | + |
| C9 | + | + | + | + | + |
| C10 | + | + | + | + | − |
| C11 | + | + | − | + | − |
| C12 | + | + | + | + | + |
| C13 | + | − | − | − | − |
| C14 | + | + | − | + | − |
| C15 | + | + | + | + | + |
| C16 | + | + | + | + | + |
| C17 | + | + | + | + | + |
| C18 | + | + | + | + | + |
| C19 | + | + | + | + | + |
| C20 | + | + | − | + | − |
| C21-C38 | − | − | − | − | − |
| R1 | ND | − | − | − | − |
| R2 | ND | + | + | + | + |
| R3 | ND | + | + | + | + |
| M1 | ND | + | + | + | + |
| M2 | ND | + | + | + | + |
C, camel serum; C21-C38, normal camel serum controls; R1, marmoset serum, infected 3 days; R2, rhesus serum, infected 14 days; R3, rhesus serum, infected 21 days; M, MERS-CoV-infected mice.
Detection of nucleic acid extracted from swab samples; ND, not determined; +, positive; −, negative.
2.2. Construction and characterization of recombinant proteins comprised of various fragments of MERS-CoV proteins fused with luciferase
As shown in Figure 1A, MERS-CoV NP (1–414 aa) and fragments of MERS-CoV S [S1 (1–734aa), N-terminal domain (NTD) (18–353 aa), RBD (367–606 aa), and S2 (788–1353 aa)] were amplified by RT-PCR. All of these gene fragments were cloned into the luciferase expression vector pNLF-1-N (Promega, Madison, WI, USA). All recombinant plasmids were confirmed by DNA sequencing. Human embryonic kidney (HEK) 293 T cells were seeded in a cell culture plate and cultured until the confluence reached >85%. Then recombinant plasmids were transfected into HEK 293 T cells using jetPRIME® transfection reagent (Polyplus, Illkirch, France) according to the manufacturer's instructions. After 48 h, cells were lysed on ice for 30 min in lysate buffer, and the supernatants were harvested after centrifugation at 12,000 × rpm for 5 min at 4 °C. The expression of NP or S fragments in the fusion proteins was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting (WB). Nluc activities in the supernatants were detected after reacting with the luciferase substrate mix (Promega). Relative fluorescence intensity (RFI) values were immediately determined using the Gaomax luminometer (Promega).
Figure 1.
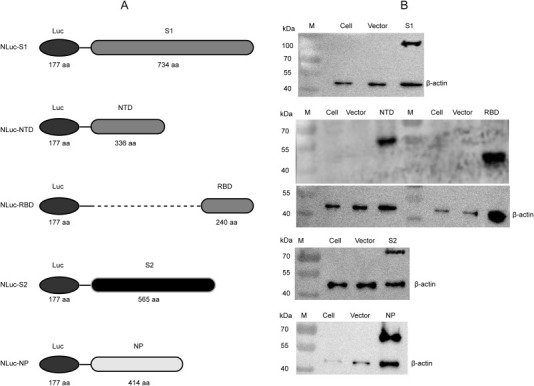
Design diagrams of NLuc-fusion proteins and their expression in mammalian HEK 293 T cells. (A) Design diagram of five NLuc-antigen fusion proteins. The NLuc we used can be secreted. We list the marked “linker” as linker sequence (GSSG). (B) Expressions of the recombinant NLuc-S1, NLuc-NTD, NLuc-RBD, NLuc-S2, and NLuc-NP proteins were identified by a specific monoclonal antibody via WB.
2.3. Development of the LISAs based on MERS-CoV NP and fragments of S
Briefly, 96-well Costar flat-bottomed luminometry plates were coated with protein G (5 μg/mL, 100 μL/well) in carbonate buffer (pH 9.6) overnight at 4 °C. After three washes with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T), the plates were incubated with blocking solution (PBS containing 5% non-fat milk) for 1 h at 37 °C. Then the wells were washed, and aliquots of serially diluted sera (100 μL) were added to the wells and incubated for 1 h at 37 °C. The plates were washed and incubated with diluted Nluc-fusion proteins (50 μL) for 30 min at 37 °C. Then the plates were washed again, and luciferase substrate (50 μL) (Promega) was added to each well. RFI values were determined using a Gaomax luminometer (Promega). Each sample was tested in duplicate. The cut-off values were determined as two-fold the average of the normal controls. Negative controls were an equal volume mixture of sera from healthy blood donors. Results were expressed as the mean RFI from duplicate wells, and values were corrected by subtracting the RFI value of the background wells incubated with HEK 293 T cell extracts in the absence of sera.
2.4. qRT-PCR screening assay
Detection of MERs-CoV-specific nucleic acid in the samples was performed using qRT-PCR targeting genes upstream of the envelope (upE) and NP (N) as previously reported [[60], [61], [62]].
2.5. Euroimmun S1-based ELISA for MERS-CoV
A commercially available anti-MERS-CoV ELISA IgG kit was based on the purified MERS-CoV spike protein S1 domain (S1-based ELISA, Euroimmun, Lübeck, Germany). The samples were serially diluted and tested according to the manufacturer's recommendations [63]. Euroimmun recommends interpreting results in a semiquantitative way as follows: a ratio of the extinction of the control or patient sample over the extinction of the calibrator <0.8, 0.8–1.1, and ≥1.1 were defined as negative, borderline, and positive, respectively.
2.6. MERS-CoV S-based ppNT
A MERS-CoV S-based ppNT was used to detect the neutralization activities of serum samples as previously reported [63]. The detection results were expressed as RLI. Serum neutralizing antibody activity was evaluated as the pseudovirus inhibition rate: Inhibition rate (%) = (RLInormal control − RLIsamples)/RLInormal control] × 100. An inhibition rate ≥ 50% was characterized as positive neutralization activity.
2.7. Statistical analysis
All data were analyzed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) and SPSS (IBM, New York, NY, USA). Pearson's correlation coefficients among different assays were calculated, and statistical significance was defined as a p value < 0.05.
3. Results
3.1. Expression of recombinant MERS-CoV NP and fragmented S proteins fused with luciferase
To establish a novel detection method for MERS-CoV IgG, five recombinant plasmids based on NLuc were constructed, which separately contained the full-length of MERS-CoV NP or S1, NTD, RBD, and S2 of S, (Figure 1A). These plasmids were confirmed by restriction endonuclease digestion, gel electrophoresis and DNA sequencing. The recombinant plasmids were used to transfect HEK 293 T cells, and expression of the target proteins in the supernatants of the cell lysates were determined by WB using murine polyclonal antibodies against MERS-CoV (Figure 1B). These results confirmed the construction of the recombinant plasmids and expression of the target fusion proteins.
3.2. The optimal antigen or antigenic domains needed for anti-MERS-CoV IgG detection
MERS-CoV NP and S1, NTD, RBD, and S2 of the S protein (Figure 1) were used to develop the LISA by characterizing the binding domains needed for anti-MERS-CoV IgG. In order to avoid the difference in transfection efficiency and protein expression from different preparations, we measured the luciferase activity of crude cell lysates to determine the RFI, which was usually between 108 and 1011. For individual antigens, cell lysates with a minimum of 107 RFI were added to each reaction in the LISA. In addition, we included positive and negative controls in each reaction plate to ensure that the results were consistent and reproducible. Normal (negative) controls of serum samples from 40 healthy blood donors were diluted by 1:100 and used to determine the background values and calculate the cut-off levels of anti MERS-CoV IgG detection by LISA. The cut-off values were determined to be 2-fold of the average RFI value of normal adult controls, i.e., 21,388, 17,344, 15,748, 18,398 and 15,259 for S1-, RBD-, NP-, NTD-, and S2-LISAs for MERS-CoV IgG, respectively. The serum sample from the first imported Figure 2 MERS-CoV-infected patient in China was serially diluted and detected. The S1- and RBD-LISAs confirmed the positivity of the sample at a dilution as low as 1:1600, which was slightly better than the NP-LISA. However, the NTD-LISA was unable to distinguish the positive sample from the normal control at the dilution of 1:400, and S2-LISA was not able to detect the difference at a dilution of 1:100 (Figure 2 ). This indicated that the S1-, RBD-, and NP-LISAs were superior to the NTD- and S2-LISAs for detecting MRES-CoV IgG from human samples.
Figure 2.
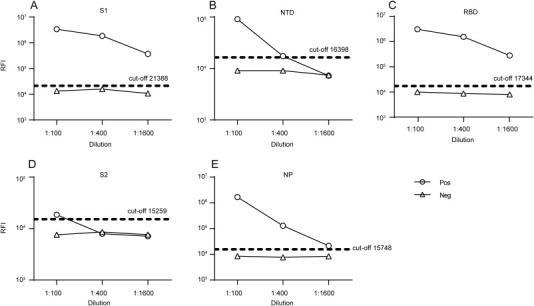
Sensitivities of the LISAs based on different NLuc-antigens. Serum samples from the first imported MERS patient in China collected 28 days after admission to the hospital (positive; Pos) were serially diluted and detected. One sample from a healthy blood donor was used as negative control (negative; Neg). Cut-off values were determined from serum samples from 40 healthy blood donors. RFI, relative fluorescence intensity.
3.3. Comparison of the characteristics of MERS-CoV LISAs and the commercial S1-ELISA and ppNT for the detection of anti-MERS-CoV IgG
To further confirm the efficiency of S1-, RBD-, and NP-LISAs, a panel of samples supplied by the WHO (Table 1) were used to compare the efficacy of various LISAs in detecting anti-MERS-CoV IgG, together with the commercially available Euroimmune S1-ELISA and ppNT kits as controls. The results are summarized in Table 1 and illustrated in Figure 3 . The NP-, S1-, and RBD-LISAs showed high consistency with S1-ELISA and ppNT. All of these methods were able to specifically distinguish MERS-CoV-infected samples from other HCoV-infected samples (Table 1 and Figure 3). Subsequently, correlations of S1-, RBD-, and NP-based LISAs with that based on the S1-ELISA were analyzed (Figure 4 ). The scatter plots showed excellent correlations between the S1-ELISA and the S1-, RBD-, NP-LISAs, with Pearson's correlation coefficients of 0.8096, 0.8218, and 0.8731, respectively. To further evaluate the sensitivity of the S1-ELISA and S1-, RBD-, NP-LISAs, several serum samples supplied by the WHO were diluted serially and detected by each of these assays (Figure 5 ). The RBD-LISA confirmed the positivity of all samples and S1-LISA confirmed two of four (the volume of the fifth serum was not enough for the detection) at a dilution as low as 1:25,600. RBD-LISA, and S1-LISA were highly superior to that of NP, which detected all samples as positive at a dilution of 1:1600. However, all four positive samples were not detected by the S1-ELISA, even at a 1:400 dilution, indicating that the lower limits of detection of the MERS-CoV LISAs were 16–64-fold higher than those of the S1-ELISA. Moreover, a receiver operating characteristic (ROC) analysis was conducted to determine the sensitivity and specificity of the S1-ELISA, S1-, RBD-, and NP-LISAs (Figure 6 ). When the serum was diluted to 1:100, the area under the curve (AUC) was calculated to be 1.0 for all four assays, and both the percentage of sensitivity and specificity for each assay was estimated to be 100% at the given cut-off values. When the serum was diluted to 1:400, 1:1600, and even 1:6400, the AUC for the S1-LISA and RBD-LISA was calculated to be 1.0, while the AUC for the NP-LISA and S1-ELISA declined slightly to 0.98 and 0.88, respectively. Additionally, the percentage of sensitivity and specificity for the S1-LISA and RBD-LISA was maintained at 100% at the given cut-off values; however, the sensitivity for NP-LISA and S1-ELISA declined to about 80% and 70%, respectively, even though the specificity was still 100%. These results indicated that the S1- and RBD-LISAs were superior to the NP-LISA and S1-ELISA in sensitivity and specificity for detecting anti-MERS-CoV antibodies in human serum samples.
Figure 3.
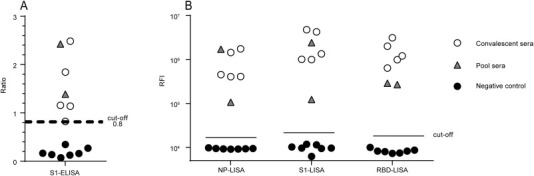
Specificity and cross-reactivity of a LISA and commercial S1-ELISA were tested with a panel of sera. The panel included single and pooled convalescent sera from confirmed MERS patients and negative control serum. The details of the sample descriptions are shown in Table 1.
Figure 4.
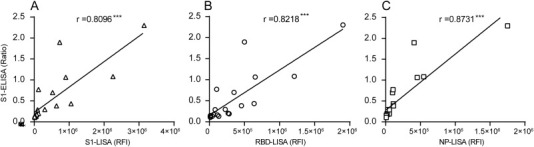
Correlation of the MERS-CoV LISAs (RFI) with the S1-ELISA (ratio) for detecting IgG antibody. Ratio of the S1-ELISA results was plotted against the RFI of the S1-LISA, RBD-LISA, and NP-LISA. The samples used in the plots included five convalescent serum samples from a MERS-CoV-infected patient with multiple dilutions and three sera from normal (negative) controls listed in Table 1.
Figure 5.
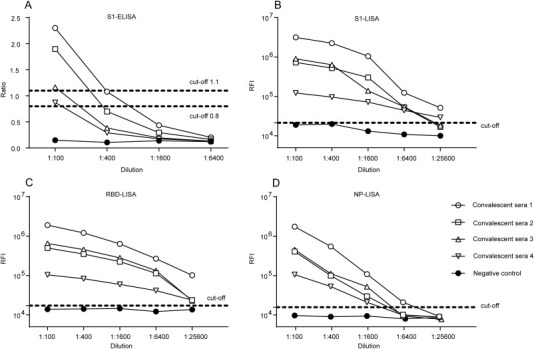
Analysis of the sensitivity of MERS-CoV S1-ELISA and LISAs. Five samples (four convalescent sera from MERS-CoV-infected patients and one normal serum used as negative control, the volume of the fifth convalescent serum was not enough for detection) were serially diluted and dispensed into the wells of the same plate. Each sample was tested in duplicate. Data are representatively from at least two independent experiments.
Figure 6.
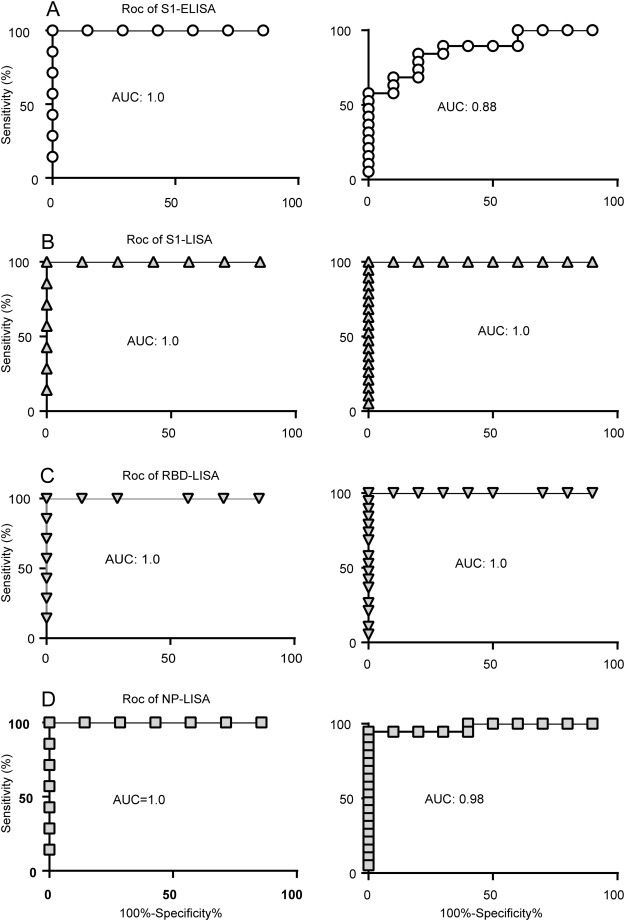
ROC analysis. AUC values for the WHO antibody panel were determined using a 1:100 dilution (left) or 1:100, 1:400, and 1:1600 to 1:6400 dilution (right) using commercial Euroimmun S1-ELISA (A), in-house S1-LISA (B), RBD-LISA (C), and NP-LISA (D).
3.4. Application of LISA in detecting anti-MERS-CoV IgG from animal samples
To further evaluate the efficiency of MERS-CoV-LISA, serum samples from uninfected camels (controls), MERS-CoV-infected camels (confirmed to be positive by RT-PCR), monkeys, and mice were detected using the above-mentioned LISAs, with ppNT as the control. The results are shown in Table 2. All 18 normal camel samples (C21–C38) were confirmed to be negative by all the methods tested. A total of 17 of 20 MERS-CoV-infected camel samples were positive by ppNT, while C1, C7, and C13 were negative, which was inconsistent with RT-PCR results. Notably, C7 was positive for anti-MERS-CoV IgG based on the S1-, RBD-, and NP-LISAs, while C3 was detected as positive by ppNT but as negative for anti-MERS-CoV IgG based on S1-, RBD-, and NP-LISAs. Moreover, samples C11, C14, and C20 were determined to be positive by ppNT and RBD-LISA, but were negative by S1- and NP-LISA. Our results also showed that the two samples from MERS-CoV-infected marmoset monkeys (R2 and R3) and the two samples from MERS-CoV infected mice (M1 and M2) were positive via ppNT, as well as S1-, RBD-, and NP-LISAs. The results showed that if RT-PCR results served as the standard, the sensitivity of S1-, RBD-, and NP-LISA was 70%, 85%, and 60% respectively, and the specificity was 100% for all assays. When ppNT results served as the standard, the sensitivity was 81%, 95%, and 71%, respectively, and the specificity was 95% for all assays. The results showed that S1-, RBD-, and NP-LISAs were highly consistent with the ppNT results. In conclusion, MERS-CoV S1-, RBD-, and NP-LISAs showed high consistency with ppNT and RT-PCR. The RBD-LISA showed the highest consistency with ppNT (Table 2).
4. Discussion
MERS is an emerging infectious disease with a high fatality rate [64]. Diagnosis of MERS-CoV infection using anti-MERS-CoV IgG is typical for post-exposure epidemiologic studies or seroprevalence investigations [18,48,53,54,63]. Although anti-MERS-CoV serologic test kits are commercially available, such as ELISA and IFA developed by Euroimmun (Lübeck, Germany), the performance (especially sensitivity as a screening test) of these kits needs to be optimized. In this study, we reported various novel MERS-CoV recombinant antigen-based LISAs that do not require a BSL-3 laboratory or species-specific secondary antibodies. Furthermore, these LISA-based assays are potentially useful for the rapid, specific, and sensitive detection of anti-MERS-CoV IgG in both humans and animals.
Traditionally, PRNT and MN have been the standards for detecting MERS-CoV-specific antibodies. However, these assays are time-consuming, laborious, and require high-level biosafety facilities [53,60]. The performance of the commercial MERS-CoV S1-based ELISA has been assessed by different groups [18,35,51,65,66], all of which have shown that the sensitivity of this assay needs to be improved, as it shows high cut-off values from the optical density (OD) ratio (the manufacturer's instructions provided a higher breakpoint for ELISA IgG to warrant specificity, with a borderline OD ratio cut-off of 0.8–1.1 and positive ≥1.1). LISA is characterized by rapid preparation, ultra-sensitivity, high-throughput, and simple operation [55,56]. Cell lysates are directly used in the detection assay, which avoids purification and post-expression modifications [55]. In the current study, we developed LISAs for the detection of anti-MERS-CoV IgG in human and animal sera based on novel and ultrasensitive NLuc approaches. We compared LISAs with the commercially available S1-based ELISA kit using a panel of anti-MERS-CoV IgG sera. Our data indicated that several LISAs (RBD-, S1-, and NP-LISAs) were highly correlated and more sensitive (by at least 16-fold) than the S1-based ELISA in detecting anti-MERS-CoV IgG in patient samples. We also confirmed that the RBD-, S1-, and NP-LISAs were able to specifically recognize anti-MERS-CoV IgG without cross-reactiondy with samples derived from other HCoV (OC43, 229E, HKU1, NL63)-infected patients.
Recently, Alagaili et al. [33] and Okba et al. [67] reported a luciferase immunoprecipitation system (LIPS) and detected MERS-CoV antibody with improved sensitivity and specificity. Compared to LIPS, LISA does not need pre-incubation of Ruc-antigen and samples and transfer of the mixture to a special 96-well filter plate containing the protein A/G beads. Moreover, LISA does not need a special plate washer with vacuum to capture the antigen-antibody-beads complex [55,68]. In the LISA system, Nluc replaced the Renilla luciferase used in LIPS. NLuc has been shown to generate a glow-type luminescence (signal half-life >2 h) with 150-fold greater activity than Renilla luciferase. It is also brighter than Firefly luciferase [56]. Lastly, it is easy to construct fusion proteins based on Nluc enzyme with the full length of 171 amino acids, which makes it an excellent reporter protein [69].
CoV structural proteins, especially S and NP proteins, play important roles in CoV infection and detection. In addition, NP is probably the most abundant antigen after CoV infection [45], and antibodies to NP have been used as serological diagnostic biomarkers. To optimize the antigens for anti-MERS-CoV IgG detection, we constructed various NLuc fusion proteins based on MERS-CoV NP protein or fragments of S protein. Our results showed that S1-, RBD-, and NP-LISAs were more sensitive than the NTD- and S2-LISA for detecting anti-MERS-CoV IgG. Moreover, ROC analysis showed that S1- and RBD-LISAs were superior to the NP-LISA and S1-ELISA in regard to the sensitivity and specificity of detecting anti-MERS-CoV IgG in serum samples.
MERS-CoV is a zoonotic disease that causes global public health concerns. Dromedary camels, bats, and other animals are potentially involved in MERS-CoV transmission [1,12,35,42,60]. Antibodies against MERS-CoV in domestic livestock (including dromedary camels) in the Middle-East and Africa were investigated using ppNT, MN, and IFA assays [7,27,29,[34], [35], [36],48,53,60,70]. MERS-CoV IgG seropositivity gradually increased in dromedary calves from the Middle East with increasing age based on the S1-ELISA. Currently, there are several knowledge gaps regarding the zoonotic origin and animal-human interface of MERS-CoV infection due to limited tools and resources [12,49]. It is very important to establish a platform to screen anti-MERS-CoV IgG among different species in endemic areas and conduct a traceable investigation of animal epidemic foci. Theoretically, samples from vectors, such as mites and mosquitoes, as well as other animals, including bats, mice, camels, and monkeys, could be detected by LISA; however, these may not be detected using a typical ELISA due to the lack of suitable enzyme-labeled secondary antibodies. In this study, the commercial S1 ELISA did not detect positive sera samples from camels, monkeys, or mice. However, the positive samples were successfully detected by several LISAs established in this study, which proved the applicability and reliability of LISAs. Additionally, anti-MERS-CoV IgG detected by LISAs demonstrated a strong correlation with ppNT and RT-PCR from several animal hosts including camels, mice, and monkeys. Furthermore, RBD-LISA was the most sensitive LISA developed in this study. In conclusion, we have developed several novel LISAs for the detection of anti-MERS-CoV IgG in humans and animals. These assays have the potential to be used for large-scale screening of anti-MERS-CoV IgG and study of serological epidemiology of MERS-CoV infection among all species.
Acknowledgments
Acknowledgements
This work was supported by the following grants: the National Major Project for Control and Prevention of Infectious Disease in China (No. 2018ZX10101002 and 2018ZX10732401), and the National Key Research and Development Program of China (No. 2016YFD0500301 and 2017YFC1200503). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Conception and design of the study: W. Wang, T. Wang, W. Tan and S. Tang; Performance of experiment: T. Wang, W. Wang, Y. Deng, P. Niu, R. A and M. Peiris; Providing samples: W. Wang, J. Zhao and M. Peiris; Statistical analysis: W. Wang, T. Wang and W. Tan; Drafting the article: W. Wang, T. Wang and W. Tan; Revision of the draft: J. Zhao, M. Peiris and S. Tang; Final approval of the version to be submitted: All authors.
Contributor Information
Shixing Tang, Email: tamgshixing@smu.edu.cn.
Wenjie Tan, Email: tanwj@ivdc.chinacdc.cn.
References
- 1.Al-Tayib O.A. An overview of the most significant zoonotic viral pathogens transmitted from animal to human in Saudi Arabia. Pathogens. 2019;8:25. doi: 10.3390/pathogens8010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gossner C., Danielson N., Gervelmeyer A., Berthe F., Faye B., Aaslav K.K., Adlhoch C., Zeller H., Penttinen P., Coulombier D. Vol. 63. 2016. Human – Dromedary Camel Interactions and the Risk of Acquiring Zoonotic Middle East Respiratory Syndrome Coronavirus Infection; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Kahlout R.A., Nasrallah G.K., Farag E.A., Wang L., Lattwein E., Müller M.A., El Zowalaty M.E., Al Romaihi H.E., Graham B.S., Al Thani A.A., Yassine H.M. Comparative Serological Study for the Prevalence of Anti-MERS Coronavirus Antibodies in High- and Low-Risk Groups in Qatar. J. Immunol. Res. 2019;2019:1–8. doi: 10.1155/2019/1386740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-taw J.A., Gautret P. Asymptomatic Middle East respiratory syndrome coronavirus (MERS-CoV ) infection: Extent and implications for infection control: a systematic review. Travel Med. Infect. Dis. 2019;27:27–32. doi: 10.1016/j.tmaid.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-tawfiq J.A., Zumla A., Memish Z.A. Vol. 27. 2014. Coronaviruses: Severe Acute Respiratory Syndrome Coronavirus and Middle East Respiratory Syndrome Coronavirus in Travelers; pp. 411–417. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A.E., Al-jahdali H., Alshukairi A.N., Alaqeel M., Siddiq S.S., Alsaab H., Sakr E.A., Alyahya H.A., Alandonisi M.M., Subedar A.T., Aloudah N.M., Baharoon S., Alsalamah M.A., Al S., Alghamdi M.G. Early identification of pneumonia patients at increased risk of Middle East respiratory syndrome coronavirus infection in Saudi Arabia. Int. J. Infect. Dis. 2018;70:51–56. doi: 10.1016/j.ijid.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omrani A.S., Al-tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathog. Glob. Health. 2016;109:354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabaan A.A., Bazzi A.M., Al-Ahmed S.H., Al-Tawfiq J.A. Molecular aspects of MERS-CoV. Front. Med. 2017;11:365–377. doi: 10.1007/s11684-017-0521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spanakis N., Tsiodras S., Haagmans B.L., Raj V.S., Pontikis K., Koutsoukou A., Koulouris N.G., Osterhaus A.D.M.E., Koopmans M.P.G., Tsakris A. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int. J. Antimicrob. Agents. 2014;44:528–532. doi: 10.1016/j.ijantimicag.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang H.J. Estimation of basic reproduction number of the Middle East respiratory syndrome coronavirus (MERS-CoV) during the outbreak in South Korea, 2015. Biomed. Eng. Online. 2017;16:1–11. doi: 10.1186/s12938-017-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.Y., Kim Y.J., Chung E.H., Kim D.W., Jeong I., Kim Y., Yun M. Ran, Kim S.S., Kim G., Joh J.S. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect. Dis. 2017;17:1–10. doi: 10.1186/s12879-017-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly-Cirino C., Mazzola L.T., Chua A., Oxenford C.J., Van Kerkhove M.D. An updated roadmap for MERS-CoV research and product development: focus on diagnostics. BMJ Glob. Heal. 2019;4 doi: 10.1136/bmjgh-2018-001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui D.S., Azhar E.I., Kim Y., Memish Z.A., Oh M., Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect. Dis. 2018;18:e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Guan W., Mok C.K.P., Chen Z.L., Feng L.Q., Li Z.T., Huang J.C., Ke C.W., Deng X., Ling Y., Wu G., Niu X.F., Perera R.A., Da Xu Y., Zhao J., Zhang L.Q., Li Y.M., Chen R.C., Peiris M., Chen L., Zhong N.S. Characteristics of traveler with Middle East respiratory syndrome, China, 2015. Emerg. Infect. Dis. 2015;21:2278–2280. doi: 10.3201/eid2112.151232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y., Yang Y., Huang J., Jiang S., Du L. Advances in MERS-CoV vaccines and therapeutics based on the receptor-binding domain. Viruses. 2019;11:1–18. doi: 10.3390/v11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui D.S., Zumla A. Advancing priority research on the Middle East respiratory syndrome coronavirus. J. Infect. Dis. 2014;209:173–176. doi: 10.1093/infdis/jit591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uyeki T.M., Erlandson K.J., Korch G., O’Hara M., Wathen M., Hu-Primmer J., Hojvat S., Stemmy E.J., Donabedian A. Development of medical countermeasures to Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. 2016;22:e1–e11. doi: 10.3201/eid2207.160022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K., Ko H.L., Lee E.Y., Park H.J., Kim Y.S., Kim Y.S., Cho N.H., Park M.S., Lee S.M., Kim J., Kim H., Seong B.L., Nam J.H. Development of a diagnostic system for detection of specific antibodies and antigens against Middle East respiratory syndrome coronavirus. Microbiol. Immunol. 2018;62:574–584. doi: 10.1111/1348-0421.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y., Lei C., Hu D., Dimitrov D.S. Human monoclonal antibodies as candidate therapeutics against emerging viruses. Front. Med. 2017;11:462–470. doi: 10.1007/s11684-017-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Bao L., Chen C., Zou T., Xue Y., Li F., Lv Q., Gu S., Gao X. Human neutralizing monoclonal antibody inhibition of Middle East respiratory syndrome coronavirus replication in the common marmoset. J. Infect. Dis. 2017;215:1807–1815. doi: 10.1093/infdis/jix209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skariyachan S., Challapilli S.B., Packirisamy S. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for Middle East respiratory syndrome coronavirus infections. Front. Microbiol. 2019;10:569. doi: 10.3389/fmicb.2019.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dighe A., Jombart T., Van Kerkhove M.D., Neil F. A systematic review of MERS-CoV (Middle East respiratory syndrome coronavirus) seroprevalence and viral RNA prevalence in dromedary camels: implications for animal vaccination. Epidemics. 2019:100350. doi: 10.1016/j.epidem.2019.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Doremalen N., Miazgowicz K.L., Milne-price S., Bushmaker T., Robertson S., Scott D., Kinne J., Mclellan J.S., Zhu J., Munster J. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J. Virol. 2014;88:9220–9232. doi: 10.1128/JVI.00676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamau E., Ongus J., Gitau G., Galgalo T., Lowther S.A., Bitek A., Munyua P. Knowledge and practices regarding Middle East respiratory syndrome coronavirus among camel handlers in a slaughterhouse, Kenya, 2015. Zoonoses Public Heal. 2019;66:169–173. doi: 10.1111/zph.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gikonyo S., Kimani T., Matere J., Kimutai J., Kiambi S.G., Bitek A.O., Ngeiywa K.J.Z.J., Makonnen Y.J., Tripodi A., Morzaria S., Lubroth J., Rugalema G., Fasina F.O. Mapping potential amplification and transmission hotspots for MERS-CoV. Kenya, Ecohealth. 2018;15:372–387. doi: 10.1007/s10393-018-1317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexandersen S., Kobinger G.P., Soule G., Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound. Emerg. Dis. 2014;61:105–108. doi: 10.1111/tbed.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David D., Rotenberg D., Khinich E., Erster O., Bardenstein S., Van Straten M., Okba N.M.A., Raj S.V., Haagmans B.L., Miculitzki M., Davidson I. Middle East respiratory syndrome coronavirus specific antibodies in naturally exposed Israeli llamas, alpacas and camels. One Heal. 2018;5:65–68. doi: 10.1016/j.onehlt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein S.A., Weiss S.R. Origins and pathogenesis of Middle East respiratory syndrome-associated coronavirus: recent advances [version 1; referees: 3 approved] F1000Res. 2017;6:1628. doi: 10.12688/f1000research.11827.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zohaib A., Saqib M., Athar M.A., Chen J., Sial A. ur R., Khan S., Taj Z., Sadia H., Tahir U., Tayyab M.H., Qureshi M.A., Mansoor M.K., Naeem M.A., Hu B.J., Khan B.A., Ujjan I.D., Li B., Zhang W., Luo Y., Zhu Y., Waruhiu C., Khan I., Yang X.L., Sajid M.S., Corman V.M., Yan B., Shi Z.L. Countrywide survey for MERS-coronavirus antibodies in dromedaries and humans in Pakistan. Virol. Sin. 2018;33:410–417. doi: 10.1007/s12250-018-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Memish Z.A., Cotten M., Meyer B., Watson S.J., Alsahafi A.J., Al Rabeeah A.A., Corman V.M., Sieberg A., Makhdoom H.Q., Assiri A., Al Masri M., Aldabbagh S., Bosch B.J., Beer M., Müller M.A., Kellam P., Drosten C. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg. Infect. Dis. 2014;20:1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta V., Mason-sharma A., Caty S.N., Kerry V. Adapting global health aid in the face of climate change. Lancet Glob. Heal. 2016;5:e133–e134. doi: 10.1016/S2214-109X(17)30002-5. [DOI] [PubMed] [Google Scholar]
- 32.Gierer S., Bertram S., Kaup F., Wrensch F., Heurich A., Kramer-Kuhl A., Welsch K., Winkler M., Meyer B., Drosten C., Dittmer U., von Hahn T., Simmons G., Hofmann H., Pohlmann S. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J. Virol. 2013;87:5502–5511. doi: 10.1128/JVI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alagaili A., Briese T., Mishra N., Kapoor V., Sameroff S., de Wit E., Munster V., Hensley L., Zalmout I., Kapoor A., Epstein J., WB K., Daszak P., Mohammed O., Lipkin W. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5:e00884–00814. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrath R., Abu Duhier F.M. Sero-prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) specific antibodies in dromedary camels in Tabuk, Saudi Arabia. J Med Virol. 2018;90:1285–1289. doi: 10.1002/jmv.25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wernery U., El Rasoul Ih., Wong E.Y.M., Joseph M., Chen Y., Jose S., Tsang A.K.L., Patteril N.A.G., Chen H., Elizabeth S.K., Yuen K.Y., Joseph S., Xia N., Wernery R., Lau S.K.P., Woo P.C. A phylogenetically distinct Middle East respiratory syndrome coronavirus detected in a dromedary calf from a closed dairy herd in Dubai with rising seroprevalence with age. Emerg. Microbes Infect. 2015;4:e74. doi: 10.1038/emi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harcourt J.L., Rudoler N., Tamin A., Leshem E., Giladi R.M., Haynes L.M. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Public Heal. 2018;65:749–754. doi: 10.1111/zph.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller M.A., Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Bosch B., Lattwein E., Hilali M., Musa B.E., Bornstein S. MERS coronavirus neutralizing antibodies in camels, eastern Africa, 1983–1997. Emerg. Infect. Dis. 2014;20:2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu D.K.W., Hui K.P.Y., Perera R.A.P.M., Miguel E., Niemeyer D., Zhao J., Channappanavar R., Dudas G., Oladipo J.O., Traoré A., Fassi-Fihri O., Ali A., Demissié G.F., Muth D., Chan M.C.W., Nicholls J.M., Meyerholz D.K., Kuranga S.A., Mamo G., Zhou Z., So R.T.Y., Hemida M.G., Webby R.J., Roger F., Rambaut A., Poon L.L.M., Perlman S., Drosten C., Chevalier V., Peiris M. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc. Natl. Acad. Sci. 2018;115:3144–3149. doi: 10.1073/pnas.1718769115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aly M., Elrobh M., Alzayer M., Aljuhani S., Balkhy H. Occurrence of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) across the Gulf corporation council countries: four years update. PLoS One. 2017;12:1–11. doi: 10.1371/journal.pone.0183850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan J.F.W., Lau S.K.P., K.K.W. To, Cheng V.C.C., Woo P.C.Y., Yuen K. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau S.K.P., Li K.S.M., Tsang A.K.L., Lam C.S.F., Ahmed S., Chen H., Chan K.-H., Woo P.C.Y., Yuen K.-Y. Genetic characterization of betacoronavirus lineage C viruses in bats reveals marked sequence divergence in the spike protein of Pipistrellus bat coronavirus HKU5 in Japanese Pipistrelle: implications for the origin of the novel Middle East respiratory Sy. J. Virol. 2013;87:8638–8650. doi: 10.1128/jvi.01055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fehr A.R., Channapannavar R., Perlman S. Middle East respiratory syndrome: emergence of a pathogenic human coronavirus. Annu. Rev. Med. 2017;68:387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong A.C.P., Li X., Lau S.K.P., Woo P.C.Y. Global epidemiology of bat coronaviruses. Viruses. 2019;11:1–17. doi: 10.3390/v11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee A., Falzarano D., Rapin N., Lew J., Misra V. Interferon regulatory factor 3-mediated signaling limits middle-east respiratory syndrome (MERS) insectivorous bat. Viruses. 2019;11:1–21. doi: 10.3390/v11020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao X., Zhou H., Wu C., Xiao Y., Ren L., Paranhos-Baccalà G., Guo L., Wang J. Antibody against nucleocapsid protein predicts susceptibility to human coronavirus infection. J. Inf. Secur. 2015;71:599–602. doi: 10.1016/j.jinf.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y., Rajashankar K.R., Yang Y., Agnihothram S.S., Liu C., Lin Y.-L., Baric R.S., Li F. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J. Virol. 2013;87:10777–10783. doi: 10.1128/JVI.01756-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haagmans B.L., Al Dhahiry S.H.S., Reusken C.B.E.M., Raj V.S., Galiano M., Myers R., Godeke G.J., Jonges M., Farag E., Diab A., Ghobashy H., Alhajri F., Al-Thani M., Al-Marri S.A., Al Romaihi H.E., Al Khal A., Bermingham A., Osterhaus A.D.M.E., AlHajri M.M., Koopmans M.P.G. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Sousa R., Reusken C., Koopmans M. MERS coronavirus: data gaps for laboratory preparedness. J. Clin. Virol. 2014;59:4–11. doi: 10.1016/j.jcv.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaoka Y., Matsuyama S., Fukushi S., Matsunaga S., Matsushima Y., Kuroyama H., Kimura H., Takeda M., Chimuro T., Ryo A. Development of monoclonal antibody and diagnostic test for Middle East respiratory syndrome coronavirus using cell-free synthesized nucleocapsid antigen. Front. Microbiol. 2016;7:509. doi: 10.3389/fmicb.2016.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko J.H., Müller M.A., Seok H., Park G.E., Lee J.Y., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H., Kang J.M., Kim Y.J., Jo I.J., Chung C.R., Hahn M.J., Drosten C., Kang C.I., Chung D.R., Song J.H., Kang E.S., Peck K.R. Suggested new breakpoints of anti-MERS-CoV antibody ELISA titers: performance analysis of serologic tests. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:2179–2186. doi: 10.1007/s10096-017-3043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trivedi S.U., Miao C., Sanchez J.E., Caidi H., Tamin A., Haynes L., Thornburg N.J. Development and evaluation of a multiplexed immunoassay for simultaneous detection of serum IgG antibodies to six human coronaviruses. Sci. Rep. 2019;9:1–8. doi: 10.1038/s41598-018-37747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perera R.A., Wang P., Gomaa M.R., El-Shesheny R., Kandeil A., Bagato O., Siu L.Y., Shehata M.M., Kayed A.S., Moatasim Y., Li M., Poon L.L., Guan Y., Webby R.J., Ali M.A., Peiris J.S., Kayali G. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.36.20574. pii=20574. [DOI] [PubMed] [Google Scholar]
- 54.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H., Cai Q., Liang Y., Shui J., Tang S. A simple and high-throughput luciferase immunosorbent assay for both qualitative and semi-quantitative detection of anti-HIV-1 antibodies. Virus Res. 2019;263:9–15. doi: 10.1016/j.virusres.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 56.England C.G., Ehlerding E.B., Cai W. NanoLuc: a small luciferase is brightening up the field of bioluminescence. Bioconjug. Chem. 2016;27:1175–1187. doi: 10.1021/acs.bioconjchem.6b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang T., Zhan Y., Wu D., Chen Z., Wu W., Deng Y., Wang W., Tan W., Tang S. Development and evaluation of a universal and supersensitive NS1- based luciferase immunosorbent assay to detect Zika virus-specific IgG. Virol. Sin. 2019 doi: 10.1007/s12250-019-00160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nath N., Flemming R., Godat B., Urh M. Development of NanoLuc bridging immunoassay for detection of anti-drug antibodies. J. Immunol. Methods. 2017;450:17–26. doi: 10.1016/j.jim.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Harvey R., Mattiuzzo G., Hassall M., Sieberg A., Müller M.A., Drosten C., Rigsby P., Oxenford C.J. Comparison of serologic assays for Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. 2019;25:1878–1883. doi: 10.3201/eid2510.190497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kandeil A., Gomaa M., Shehata M., El-Taweel A., Kayed A.E., Abiadh A., Jrijer J., Moatasim Y., Kutkat O., Bagato O., Mahmoud S., Mostafa A., El-Shesheny R., Perera R.A., Ko R.L., Hassan N., Elsokary B., Allal L., Saad A., Sobhy H., McKenzie P.P., Webby R.J., Peiris M., Ali M.A., Kayali G. Middle East respiratory syndrome coronavirus infection in non-camelid domestic mammals. Emerg. Microbes Infect. 2019;8:103–108. doi: 10.1080/22221751.2018.1560235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu R., Wu J., Niu P., Zhao Y., Zhou W., Zou L., Wang H., Li H., Ke C., Guizhen W., Tan W. Comparison of laboratory methods used for identification of the first imported case of Middle East respiratory syndrome infection in China, 2015. Chin. J. Exp. Clin. Virol. 2015;29:193–195. [Google Scholar]
- 62.Lu X., Whitaker B., Sakthivel S.K., Kamili S., Rose L.E., Lowe L., Mohared E., Elassal E.M., Al-sanouri A., Haddadin D.D.E. Real-time reverse transcription-PCR assay panel for Middle East respiratory syndrome coronavirus. J. Clin. Microbiol. 2014;52:67–75. doi: 10.1128/JCM.02533-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W., Wang H., Deng Y., Song T., Lan J., Wu G., Ke C., Tan W. Characterization of anti-MERS-CoV antibodies against various recombinant structural antigens of MERS-CoV in an imported case in China. Emerg. Microbes Infect. 2016;5:e113. doi: 10.1038/emi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dawson P., Malik M.R., Parvez F., Morse S.S. What have we learned about Middle East respiratory syndrome coronavirus emergence in humans? A systematic literature review. Vector Borne Zoonotic Dis. 2019;19:174–192. doi: 10.1089/vbz.2017.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y., Chan K.H., Kang Y., Chen H., Luk H.K.H., Poon R.W.S., Chan J.F.W., Yuen K.Y., Xia N., Lau S.K.P., Woo P.C.Y. A sensitive and specific antigen detection assay for Middle East respiratory syndrome coronavirus. Emerg. Microbes Infect. 2015;4:e26. doi: 10.1038/emi.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W.L., Wang H.J., Deng Y., Song T., Lan J.M., Wu G.Z., Ke C.W., Tan W.J., Ling W.W., Juan W.H., Yao D., Tie S., Ming L.J., Zhen W.G., Wen K.C., Jie T.W. Serological study of an imported case of middle east respiratory syndrome and his close contacts in China, 2015. Biomed. Environ. Sci. 2016;29:219–223. doi: 10.3967/bes2016.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okba N.M.A., Raj V.S., Widjaja I., Geurts van Kessel C.H., de Bruin E., Chandler F.D., Park W.B., Kim N.-J., Farag E.A.B.A., Al-Hajri M., Bosch B.-J., Oh M., Koopmans M.P.G., Reusken C.B.E.M., Haagmans B.L. Sensitive and specific detection of low-level antibody responses in mild Middle East respiratory syndrome coronavirus infections. Emerg. Infect. Dis. 2019;25:22–25. doi: 10.3201/eid2510.190051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burbelo P., Ching K., Klimavicz C., Iadarola M. Antibody profiling by luciferase immunoprecipitation systems (LIPS) J. Vis. Exp. 2009;32:1–3. doi: 10.3791/1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall M., Unch J., Binkowski B., Valley M., Butler B., Wood M., Otto P., Zimmerman K., Vidugiris G., MacHleidt T., Robers M.B., Benink H.A., Eggers C.T., Slater M.R., Meisenheimer P.L., Klaubert D.H., Fan F., Encell L.P., Wood K.V. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012;7:1848–1857. doi: 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Islam A., Epstein J.H., Rostal M.K., Islam S., Rahman M.Z., Enayet M., Uzzaman M.S., Munster V.J., Peiris M., Flora M.S., Rahman M. Middle East respiratory syndrome coronavirus antibodies in dromedary camels, Bangladesh, 2015. 2018;24:926–928. doi: 10.3201/eid2405.171192. [DOI] [PMC free article] [PubMed] [Google Scholar]


