Abstract
It is generally accepted that moderate amounts of exercise improve immune system functions and hence reduce the risk of infection whereas athletes engaged in regular prolonged and/or intensive training have a higher than “normal” incidence of minor infections, especially of the upper respiratory tract (URT, e.g., common cold and influenza). This is likely related to regular acute (and possibly chronic) periods of exercise-induced changes in immune function. URT infections can compromise performance directly if suffered shortly before or during competition or indirectly if suffered at other times via effects on training and/or physiological adaptations. This chapter covers the effects of exercise (acute and chronic), both positive and negative, on immune function and consequent infection risk, and considers the current state-of-the-art for monitoring and assessing this in athletes.
Keywords: Upper respiratory tract infection (URTI), endurance, training, athlete, performance
15.1. Introduction
It is generally accepted that moderate amounts of exercise improve immune system functions and hence reduce the risk of infection. However, there is strong evidence that athletes engaged in regular prolonged and/or intensive training have a higher than “normal” incidence of minor infections, especially of the upper respiratory tract (URT, e.g., common cold and influenza) (Gleeson and Walsh, 2012). This is particularly apparent in endurance athletes such as cyclists, runners, swimmers, and triathletes, but any athletes with a high training load and/or suboptimal recovery may be at increased risk. Such infections can compromise training and/or competition performance (Pyne et al., 2005).
15.2. Exercise and Upper Respiratory Illness
Upper respiratory tract infections (URTI) are among the most frequent presentations to general practitioners (Hasham and Hall, 2003). These do not usually require hospital admission, but such illnesses have a significant economic and social impact—e.g., absence from work, healthcare costs, increased morbidity, reduced feelings of well-being, health and quality of life, and reduced social interaction. Broadly, moderate amounts of exercise are associated with enhanced immunity and resistance to such infections whereas high amounts or prolonged and/or vigorous training may increase the risk. Indeed, URTI are also suggested to be the most common type of infection in the athletic population (Roberts, 1986, Cannon, 1993, Peters, 1997; Gleeson and Walsh, 2012). In fact they have shown to be a highly prevalent medical condition in athletes at clinics in both the summer and winter Olympic Games (e.g., Robinson and Milne; 2002; Engebretsen et al., 2010, Engebretsen et al., 2013).
15.2.1. Beneficial Effects with Moderate Exercise
Observational and experimental studies have investigated the proposed greater resistance to pathogens with moderately active lifestyles. Animal investigations have demonstrated that brief bouts of moderate physical activity (20–30 min treadmill running) compared to inactivity prior to or immediately following inoculation with pathogens leads to decreased mortality and morbidity from infection (Davis et al., 1997, Lowder et al., 2005). Early exercise training studies of older and obese humans also demonstrated that 12–15 weeks of moderate exercise [30 min walking at 60%–75% of maximal oxygen uptake ()] resulted in lower incidence or duration of self-reported URTI compared to sedentary individuals (Nieman et al., 1990b, Nieman et al., 1993, Nieman et al., 1998). These effects have been supported by several longitudinal studies of the wider general population (ages 18–85 years) where maintenance of a moderately active lifestyle leads to lower self-reported or laboratory confirmed URI/URTI episodes (Kostka et al., 2000, Matthews et al., 2002, Kohut and Senchina, 2004, Ciloglu, 2005, Kostka and Praczko, 2007, Kostka et al., 2008; Nieman, 2012); (Spence et al., 2007, Barrett et al., 2012).
15.2.2. Effects With Strenuous Training/in Athletes
Although patterns vary between sports, a review by Walsh et al. (2011b) suggested that athletes tend to report URTI either during the high-intensity and tapering period prior to competition (e.g., swimming, team sports) or in the period following competition (e.g., long distance running). It has long been hypothesized that a J-shaped relationship exists between exercise workload and susceptibility to URTI (Nieman, 1994). This model suggests that an individual involved in regular moderate exercise is less likely to contract URTI compared to a sedentary individual but prolonged high-intensity exercise or periods of strenuous exercise training are associated with an above-average risk of URTI (see Fig. 15.1 ). Indeed, the J-shaped model was initially based on findings of increased self-reporting of URTI in the 1–2 week period following participation in competitive endurance races (e.g., Nieman et al., 1990a).
Figure 15.1.
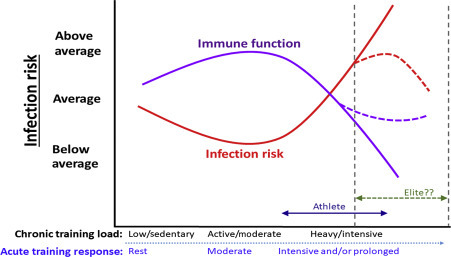
Stylized representation of the relationship between exercise, immune function, and infection risk: J-shaped and S-shaped models are depicted (area indicated and labeled “Elite??” shows S-shaped theory, as discussed further in the text).
Further support for an adverse effect of prolonged/strenuous exercise on susceptibility to URTI has come from animal studies (Davis et al., 1997, Gross et al., 1998, Folsom et al., 2001, Lowder et al., 2005, Murphy et al., 2008). Prolonged exercise (treadmill running for >2 h) has been shown to increase morbidity and mortality of mice inoculated with respiratory viruses (e.g., herpes simplex type 1 virus, influenza) prior to (Davis et al., 1997, Murphy et al., 2008) or following (Lowder et al., 2005) this type of exertion compared to resting and/or moderately exercised mice. In addition to acute exertion, equine studies have also demonstrated that intensified periods (5–28 days) of exercise training prior to or following inoculation with influenza leads to greater severity of infection in vaccinated (Folsom et al., 2001) and nonvaccinated horses (Gross et al., 1998). Generalizing these results to the human in vivo environment is questionable (Albers et al., 2005, Albers et al., 2013; Bermon et al., 2017) and such approaches (i.e., pathogen challenge) with human volunteers do have considerable ethical constraints (Hope and McMillan, 2004). Although not yet investigated in an exercise context, pathogen challenge studies in humans have demonstrated lower resistance to URTI due to other life stressors (e.g., psychological stress, sleep disturbance) (Cohen et al., 1991, Cohen et al., 2009, Prather et al., 2015). In addition to the potential exposure to these stressors in a training and competition environment (Coutts et al., 2007, Hausswirth et al., 2014), the relevance of these findings to athletes is emphasized by the impact of transient modulation in host resistance to URTI by a stressor.
Exercise immunology research (epidemiological and experimental) has grown substantially over the last few decades to investigate the relationship between exercise and URTI in humans (Shepard, 2010). In contrast to animal research, human studies (attempting to discern the effects of prolonged exercise/intense training on URTI) have mainly involved monitoring athletes following heavy exertion (i.e., relied on natural exposure to pathogens) but only a limited number of these have verified that symptoms are due to infectious agents (pathogens) (Spence et al., 2007, Schwellnus et al., 2010, Hanstock et al., 2016). This has raised concerns regarding the validity of URTI episodes (i.e., self-reported) in athletes that occur in and around competition or heavy periods of training (Bermon, 2007, Walsh et al., 2011b). Discrepancies between physician and laboratory diagnosed URTI has also highlighted the limitations with evaluation of URTI episodes (Cox et al., 2008). Upon presentation of symptoms at a sports medicine clinic, the study of Cox et al. (2008) observed that only 57% of cases were found to be indicative of infection with laboratory methods (e.g., identified pathogen) while 89% were diagnosed as URTI by physicians. In a surveillance study of a range of athletes (recreational and elite) and sedentary controls, Spence et al. (2007) demonstrated that the first two days of symptoms with infectious or noninfectious (see later) cases were similar but duration and severity of symptoms on subsequent days were greater with infectious cases. On the other hand, the common symptom scoring methods applied in self-report questionnaire studies usually require symptoms to be present for two or more days in order to be counted as an “episode” (e.g., Fricker et al., 2005; Gleeson et al., 2011), which may offer some protection against such limitations. It is also worthy of note that a subsequent UK-based study observed an 82% agreement rate between self-report and laboratory-confirmed pathogen detection (Hanstock et al., 2016). The difference to earlier studies could be due to time of year, location, or perhaps the sensitivity of detection methods has improved since the earlier studies (e.g., Spence et al., 2007). Nevertheless, for clarity the term upper respiratory tract symptoms or upper respiratory illness (URI) are generally accepted in this area unless infection has been clinically confirmed (when URTI can be used).
Although there were numerous early anecdotal reports and retrospective survey data to support the proposed J-shaped relationship (Simon, 1987, Shephard et al., 1995; Nieman, 2000), such observations alone cannot validate the influence of exercise on URI. However, further support to the J-shaped model (i.e., heavy exercise workload) was provided by a number of prospective and retrospective studies which suggested that marathon or ultra-marathon runners suffer from an increased risk of URI (e.g., 1–2 weeks following competitions) (Nieman et al., 1990a, Peters et al., 1993). The study of Peters and Bateman (1983) was a seminal study in highlighting this by randomly recruiting a sample of 140 runners who competed in the 1982 Two Oceans Marathon in Cape Town. In the 14 days following the 56 km event, 33% of runners reported URI compared to 15% of age-matched controls that did not participate in the marathon but shared living space with runners (i.e., to control for exposure to pathogens and other environmental factors). Nieman et al (1990a) went on to show that, compared to equally experienced runners who did not compete, there was a sixfold increase of URI in runners during the seven days following the 1987 Los Angeles Marathon. Taking into account other factors influencing risk of URI (age, stress levels, and illness at home), the likelihood of URI was doubled in those who ran >96 km compared to those who ran <32 km as part of their weekly training programmes leading up to the event. Heath et al. (1991) also highlighted running mileage as a significant risk factor for incidence of URI in a cohort of runners followed for a period of 12 months. More recently, Matthews et al. (2010) have also suggested that runners with higher training loads tend to be more prone to URI and that endurance athletes in particular suffer from longer episodes of URI than their recreational counterparts. However, such findings have not been demonstrated consistently as shorter observational studies have failed to observe any associations of URI with differences in training mileage, intensity, and load (Fricker et al., 2005).
Despite much interest, there remains more uncertainties than evidence based facts regarding the notion that high volumes of training are associated with an increase in the incidence of URI (Walsh et al., 2011b). One suggestion is that such inconsistent findings may be related to whether participants within studies are considered “elite” or “highly trained.” Malm (2006) suggests that a prerequisite to achieving elite athlete status is an immune system which can withstand the strenuous nature of training and competition as susceptibility to infections is incompatible with elite performance. For this reason, Malm (2006) proposed an S-shaped rather than a J-shaped curve to include elite training which is associated with a lower risk of infection compared to high exercise workload (see Fig. 15.1). However, it is highly likely that elite/professions athletes will have considerable support (financial, medical, sports science, and nutrition, etc.) and their support team may implement preventive and treatment strategies to reduce the risk and limit the effects of URI and this may also contribute to better management of other stressors. It could be, therefore, that rather than being naturally able to withstand strenuous training and infections, it is simply that they are better supported (compared to counterparts who lack such support mechanisms) to reduce controllable risk factors.
Nieman (2001) suggested most athletes may not report URI or suffer from an increased risk if they avoid periods of overreaching or overtraining (Nieman et al., 2000). It seems that an increase in URI may only be attributed to participation in acute prolonged exertion (e.g., marathon, ultra-marathon) or more importantly when athletes are exposed to a greater strain of training through exceeding individual training thresholds coupled with inadequate recovery or other life stressors (e.g., sleep disturbance) (Foster, 1998, Pyne and Gleeson, 1998, Hausswirth et al., 2014). In other words, training load or volume alone does not give full information on the level of stress that an athlete (and their immune system) is under. Indeed, the way training in distributed or periodized is of key important also. In support of this, Svendsen et al. (2016) have shown that rapid changes in training load (i.e., increasing too quickly) are better predictors of URI risk than total load alone, which goes some way to explaining the above mentioned discrepancies where only total load or volumes were considered.
15.3. Etiology of Upper Respiratory Illness
In line with the general population, when pathogen identification has been attempted, bacteria are rare causes of URTI in athletes, with viruses being responsible in most cases (in particular rhinovirus, adenovirus, and parainfluenza) (Roberts, 1986, Mäkelä et al., 1998). URTI of viral origin last approximately 3–14 days but clearance of the particular virus from the system may take longer (Winther et al., 1986, Heikkinen and Jarvinen, 2003). The route of entry into the body by most viruses is the respiratory tract where symptoms reflect the perturbations in function of infected cells and the attempts of the immune system to contain the infection (Roberts, 1986). As the nature of symptoms of some (i.e., infectious) URI may be similar to noninfectious inflammatory factors and/or presentation of allergic conditions, identification of antibody titers, and isolation of specific pathogens from body fluids of athletes have been recommended to clarify causes of symptoms and provide the most appropriate treatment or management strategies (Gleeson, 2006, Cox et al., 2008). It was generally believed that upper respiratory symptoms in athletes were due to an infective cause, however, it is only more recently that other causes (“noninfectious hypothesis”) have been proposed during training and competition (Bermon, 2007, Schwellnus et al., 2010).
Robson-Ansley et al. (2012) reported that a higher incidence of URI in runners following a marathon (47%) compared to nonrunners (19%) was significantly associated with positive responses in the Allergy Questionnaire for Athletes. As the prevalence of inhalant allergy may be as common as 16%–32% of highly trained athletes, it may partly provide an explanation to incidence of URI (Langdeau et al., 2000, Lumme et al., 2003, Schwellnus et al., 2010). Environmental influences have been considered relevant to certain groups of competitive athletes, in particular swimmers who are exposed to chlorine derivatives from swimming pool disinfectants while inhaling large amounts of air above the water surface (Piacentini et al., 2007, Bougault et al., 2009). In addition to inhaled irritants, the combination of high ventilation rate in heavy training and surrounding cold, dry air is another possible source of noninfectious, nonallergenic inflammatory stimuli to URI in some athletes (e.g., runners, cyclists) (Bermon, 2007, Cox et al., 2008).
There is also potential for direct damage in epithelial tissue of the URT and/or fibers of the contracting skeletal muscle following strenuous exercise to contribute to the noninfectious, local, and systemic inflammatory origin of URI (Peters, 2004). Schwellnus et al. (1997) reported that the use of an antiinflammatory agent reduced URI in participants following an ultra-marathon event, but as this nasal, buccopharyngeal spray also contained antimicrobial properties it does not provide any conclusive evidence to support a purely noninfectious inflammatory cause for URI. In contrast, administration of an antiinflammatory throat spray in the period leading up to and following a half-marathon event had no influence on the incidence of URI in runners but did reduce the severity of recorded symptoms (Cox et al., 2010). Although conflicting evidence here may purely reflect site-specific differences between agents, together these studies do provide evidence that noninfectious inflammation may not be the underlying cause of all URI, but may interact with infectious causes to potentiate the symptoms that occur as a result of responses to pathogen challenge.
In their surveillance study, Spence et al. (2007) demonstrated that the distribution of URI closely followed the J-shaped curve in terms of training status (i.e., both the control and elite athlete group suffered from greater days of illness than their recreational counterparts), but only 30% of reported illnesses were confirmed by identification of common respiratory pathogens. It is worthy to note that only specific pathogens were tested in Spence et al. (2007), thus URI were possibly caused by known pathogens not tested for, unknown pathogens and/or new strains of viruses which were yet to be identified (Bermon, 2007). Despite the low number of identified pathogens highlighted with laboratory evaluation of URI in some studies (i.e., Spence et al., 2007; Cox et al., 2008), it must be emphasized that this does not rule out infectious causes for these cases as such diagnostics procedures do have inherent limitations in identifying causative agents from an evolving diverse pool of pathogens (e.g., ~200 common cold viruses) (Heikkinen and Jarvinen, 2003, Eccles, 2005). Furthermore, a more recent (albeit smaller) study based in the United Kingdom during the winter months (i.e., typical URI season) observed that a much higher proportion (82%) of reported illnesses were confirmed by identification of URTI-causing pathogens (Hanstock et al., 2016). In this study, 33 recreational-level athletes completed the study, which included a 3-week monitoring period during which 11 subjects reported URI using the daily Jackson common cold questionnaire. Of these 11 subjects, URTI-causing pathogens were detected in 9 (all were positive for Rhinovirus and one was concurrently positive for coronavirus).
Not all cases of URI can present as a typical response to a primary viral infection of initial upper respiratory symptoms followed by local and systemic inflammation (e.g., fever, aches) (Gleeson et al., 2002). Occasionally symptoms may be minor and/or short-lasting (1–3 days) resulting in training being unaffected or be a reflection of persistent fatigue and recurrent infections as a result of chronic heavy exertion (Reid et al., 2004). In these cases it has been suggested that, in addition to exercise-induced inflammation, URI following exercise may be related to reactivation of latent viruses within the upper airways rather than the incidence of primary infections in the recovery period (Gleeson et al., 2002) or prior URT infections that have not been fully eliminated even after symptoms have subsided. Ekblom et al. (2006) found in a group of recreational runners that prerace URI was significantly associated with URI incidence following the marathon. This was supported by a longitudinal observational field study, where it was suggested that reactivation of prerace viruses and exercise-induced inflammatory responses were the primary causes of elevated URI incidence prior to and following an 86.5 km Marathon (Peters et al., 2010). The 57% of runners, who recorded incidence of URI during the 7–14 days following the race, also recorded symptoms in the time leading up to the race. These findings may support the aforementioned animal studies whereby participation in prolonged exercise may worsen symptom severity induced by existing infection (Malm, 2006). It has also been suggested that the reactivation of latent viruses (e.g., Epstein–Barr Virus, EBV) could be implicated in the etiology of URT infections (Gleeson et al., 2002). EBV is a herpes virus that typically infects ~80%–90% of the world’s adult population (Gleeson et al., 2002, Staras et al., 2006, Bate et al., 2010). After initial primary infection, these viruses lay dormant within the cells of the immune system and immunocompetent individuals are generally asymptomatic (Kano and Shiohara, 2000, Crawford, 2001). However, under significant physical and/or psychological stress the immune system’s ability to control these infections (keeping latent) may be lost and such latent viruses may become reactivated (Tingate et al., 1997, Glaser et al., 1999, Mehta et al., 2000b, Stowe et al., 2001). In an exercising population, EBV has received attention due to its ability to replicate continuously or intermittently from the oropharynx (Faulkner et al., 2000, Nadal et al., 2002). Gleeson et al. (2002) found a significant relationship between previous EBV infection and URI in elite swimmers: while all seronegative swimmers remained unaffected (no reported URI) during a 30 day period of intensive training 7 out of the 11 seropositive swimmers had EBV DNA detected in saliva during the study with 6 of these going on to develop URI which appeared 4–18 days following first detection of EBV DNA. It was postulated that EBV was implicated in the symptoms. However, Cox et al. (2004) treated a group of endurance runners with a herpes-virus-specific antiviral treatment and although this was able to significantly reduce EBV expression, this had no effect on URI. It is possible, therefore, that EBV is not directly involved/the cause of URI per se, but rather an in vivo indication of immunodepression/compromised immunity and, therefore, increased susceptibility to other URI-causing pathogens. Indeed, for the reactivation of EBV to occur, there must be a disturbance within certain parameters of the immune system which usually keep the virus tightly-regulated and latent. This reflection of immune perturbations within the host (Mehta et al., 2000a) occur in line with the most researched hypothesis (“open window”) behind an increased risk of primary infections in athletes who participate in prolonged exercise. This theory is still it its infancy however and requires further study, although it does seem to be supported by studies showing athletes to have greater levels of detectable EBV DNA compared to controls (Hoffmann et al., 2010). In a clinical investigation of elite athletes suffering recurrent episodes of URI, EBV viral shedding was detected in 22% of the cohort (Reid et al., 2004). Yamauchi et al. (2011) also found in an intensive training period with rugby players that salivary expression of EBV DNA was 1.5 times greater in participants with URI compared to those without URI.
The “open window” hypothesis suggests that prolonged/heavy exercise causes depression of the immune system which leaves the body less resistant to viruses and bacteria and thus increases the risk of subclinical and clinical infection for between 3 and 72 h (Pedersen and Ullum, 1994; Nieman, 2001), which reflects that the increased risk of URI with heavy exertion was due to a decrease in immunosurveillance (and vice versa, the decreased risk with moderate exercise was due to an increase in immunosurveillance). The immunological response to acute exercise is deemed a subset of stress immunology (Hoffman-Goetz and Pedersen, 1994), where responses have been likened to those caused by infection, sepsis, burns, or trauma (Pedersen and Hoffman-Goetz, 2000). Although exercise does share some similarities to the hormonal and immunological responses of these clinical physical stressors, there are also important distinct differences in the magnitude and temporal responses (Shephard, 2001, Shephard, 2002). Strenuous exercise induces an ordered sequence of modest changes in pro-inflammatory signaling followed predominantly by antiinflammatory responses which down-regulates immune function whereas some of the clinical stressors listed above (e.g., sepsis) trigger an excessive and overwhelming elevation in systemic pro-inflammatory responses (Shepard, 2002). Exercise immunologists have controlled and adapted the duration and intensity of the stress model of exercise to gain a deeper understanding into how alterations in immune function following acute exercise and training may lead to changes in susceptibility to pathogens.
15.4. Immune System and Exercise
The immune system has evolved to protect the human body from pathogens (viruses, bacteria, and parasites). It encompasses the ability to maintain homeostasis even when exposed to a wide range of foreign and self molecules (i.e., antigens). The components of the nonspecific innate system and the specific acquired system overlap to ensure that a state of immunity against infection is established.
15.4.1. Moderate Exercise
One of the main mechanisms responsible for changes in host defense with moderate activity seems to be a greater immunosurveillance associated with moderate activity. Regular bouts of moderate intensity activity generally induce transient improvements in the immune system (Woods et al., 1999). There are various factors which mediate these relationships, one of which is the fitness status of participants, although many studies assessing the immune response to moderate exercise have focused on interventions for previously sedentary individuals.
15.4.2. Strenuous or Intensive Exercise
Many components of the immune system are temporarily reduced (exercise-induced immunodepression) after strenuous and/or prolonged bouts of exercise (Gleeson and Walsh, 2012). This may persist for as little as a few hours or a long as a few days (depending on the nature of the exercise). In particular, if subsequent bouts are commenced too soon, before the immune system has fully recovered, then a progressive accumulation of immunodepression may ensue. Periods of depressed immunity, whether small acute periods or more chronic periods, are termed “Open Windows” (as discussed earlier) and this is a likely mechanism explaining increased susceptible. However, it is important to point out that this does not necessarily mean that changes in isolated immune markers alone can predict illness risk. There is considerable redundancy in the immune system, so it is important to note that changes in isolated in vitro and ex vivo markers may not give a good representation of the ability of the whole immune system to mount an effective response. Even if it was possible to measure every component of the immune system (in vitro) concurrently, it would still be virtually impossible to calculate how such results could be combined to predict the whole integrated (in vivo) immune response. For this reason in vivo measures or markers that represent the ability of the whole immune system to mount a coordinated, integrated response are the most useful and clinically relevant markers (see Albers et al., 2005, and Albers et al., 2013 for detailed reviews). It remains important to study such in vitro and ex vivo markers nonetheless as they provide mechanistic information and insight on the effects of exercise on immunity, but for most of these measures researchers (and practitioners) must exercise caution in their interpretation of such data, which should be used to supplement more clinically relevant markers and provide additional mechanistic insight.
15.4.2.1. In Vitro and Ex Vivo Markers
15.4.2.1.1. Leukocyte Count Changes and Acute Exercise
The number of circulating immune cells (leukocytes) is profoundly influenced by acute exercise with reports from over a century ago highlighting the exercise-induced mobilization following the Boston Marathon (Larrabbe, 1902). Circulating leukocytes consist of the granulocytes (neutrophils, eosinophils, and basophils; 60%–70% of total), monocytes (5%–15%), dendritic cells (less than 1%), and the lymphocytes (15%–25%) which can be divided into innate [natural killer (NK) cells] and acquired (T helper, T cytotoxic, and B cells) cells. It is now clear that leukocytosis, which is an increase in the total number of circulating leukocytes (mainly neutrophils and lymphocytes) occurs (up to 400%) during and immediately post exercise (Simpson, 2013). The observed changes are dependent on the exercise intensity and duration (Gleeson, 2007) with prolonged endurance exercise (>1.5 h) causing a greater leukocytosis (three- to fourfold increase) than brief (20–40 min) high-intensity exercise (Robson et al., 1999b).
At rest, it is estimated that an equal amount of leukocytes are circulating within the blood and located within marginated pools, adhered to blood vessel walls of the circulatory system (Athens et al., 1961, Berkow and Dodson, 1987). Foster et al. (1986) suggested the increased cardiac output during exercise and the subsequent shear stress (increased blood flow) within blood vessels induces leukocytes to enter circulation in a process known as demargination. However, it is anticipated that marginal pools within the liver, lung, spleen and other vital organs (e.g., bone marrow, intestines) also contribute to the large leukocytosis following exercise as pools in the lung alone possess lymphocytes which are present in 10 times larger amounts than the circulatory pool (Hogg and Doerschuk, 1995, Simpson, 2013).
Activation of the sympathetic nervous system (elevated concentrations of plasma catecholamines) and the hypothalamic–pituitary–adrenal (HPA) axis (cortisol release) during prolonged exercise also play an integral role in exercise-induced leukocytosis (Nieman, 2001, Atanackovic et al., 2006, Anane et al., 2009). The immediate leukocytosis, particularly neutrophilia (increased neutrophil count) upon onset of exercise is suggested to be due to the haemodynamic and catecholamine induced demargination of vascular and pulmonary pools but this is later followed by a cortisol-induced release of neutrophils from the bone marrow otherwise known as delayed leukocytosis (Allsop et al., 1992). This effect is seen simultaneously with the immediate leukocytosis during prolonged exercise of >1 h as cortisol level is sufficiently elevated and its action is prolonged, explaining the previously mentioned greater leukocytosis than following shorter, higher intensity exercise (Robson et al., 1999b). Neutrophils released from the bone marrow following stimulation by cortisol are suggested to include a greater proportion of immature cells (e.g., band cells) compared to neutrophils that demarginate from the endothelial walls upon onset of exercise which have similar maturity levels to those already in circulation (Hetherington and Quie, 1985, McCarthy et al., 1991). Although effects on dendritic cells remain unclear, the bone marrow is also a source of maturation for both monocytes and B cells where monocytes also increase proportionately with exercise duration and B cells are associated with limited redistribution (Pedersen et al., 1990, Shek et al., 1995, Nieman et al., 1998, Lancaster et al., 2005a, Okutsu et al., 2008).
NK and T cells (mostly cytotoxic) also increase proportionately with exercise intensity and duration unlike neutrophils where increases are predominantly influenced by duration (McCarthy and Dale, 1988, Gabriel et al., 1991, Shek et al., 1995, Campbell et al., 2009). Even though lymphocytes are present at numerous sites within the body, evidence suggests that mobilization of these cells during exercise mainly occurs from secondary lymphoid organs, for example, spleen, intestinal Peyer’s patches (PP) rather than primary lymphoid organs such as the thymus or bone marrow (Simpson et al., 2007, Simpson et al., 2008, Campbell et al., 2009). The spleen is considered to be an abundant source of the lymphocytes deployed during exercise (Baum et al., 1996, Nielsen et al., 1997), with shear stress and catecholamines being important release mechanisms (Kappel et al., 1991, Benschop et al., 1993, Shephard, 2003, Timmons and Cieslak, 2008, Dimitrov et al., 2010). There is a clear consensus developing that exercise triggers a redistribution of T cells with a longer history of antigen exposure rather than naive T cells (Campbell et al., 2009, Simpson, 2011).
Following the lymphocytosis during and upon completion of prolonged exercise, the high concentration of adrenaline and cortisol often cause lymphocytopenia (lymphocyte count below resting levels) (Nieman, 1994, Nieman, 2001) during the recovery. This biphasic response (lymphocytosis during exercise and lymphocytopenia during recovery) has been found with various protocols requiring heavy/prolonged exertion or exhaustive exercise (e.g., Fry et al., 1992; Shek et al., 1995), with lymphocyte count up to 60% below resting levels reported post exercise (Simpson, 2011). Lymphocytopenia is due to the selective extravasation of lymphocyte subsets, NK and T cytotoxic cells, from blood to the surrounding tissues (Gabriel et al., 1991, Simpson et al., 2006, Kruger et al., 2008). The return of total leukocyte count to resting levels generally begins immediately post exercise with diminishing activation of the sympathetic nervous system and HPA axis, but in the case of very intense exercise, leukocyte (primarily neutrophils) count may continue to increase as described above (McCarthy and Dale, 1988, Allsop et al., 1992). Given these leukocyte perturbations, an increase in neutrophil:lymphocyte ratio is suggested to be an indicator of the overall magnitude of the stress response induced by exercise (Nieman, 1998).
15.4.2.1.2. Innate Immune Cell Function and Acute Exercise
The immune system consists of many physical barriers (e.g., skin, mucus, cilia) which act to prevent entry of pathogens into the human body. If such attempts fail, infectious agents will be detected by receptors present on the cellular components of the innate immune system such as the granulocytes (mostly neutrophils, but include basophils and eosinophils), NK lymphocytes, monocytes (which mature into macrophages within tissue), and dendritic cells.
The recognition of foreign material is one way in which the innate and acquired immune systems differ from one another. Unlike acquired immunity, the innate system does not exhibit memory of previous encounters with antigens (i.e., foreign molecule), therefore a similar response takes place during any future exposure. Present on the cell surface of innate cells are pattern recognition receptors (PRR) that distinguish self from nonself material by recognizing molecules on microbes which have been conserved through evolution due to their essential function i.e., bacterial DNA, lipopolysaccharides (LPS), and other bacterial cell wall components (Kimbrell and Beutler, 2001, Beutler and Rietschel, 2003). The majority of these structures comprise of the Toll-like receptors (TLR) which are crucial components of the antigen presentation cells (APC) (discussed later in this section) of innate immunity (monocytes/macrophages).
Neutrophils also known as polymorphonuclear (PMN) cells (due to their multilobed nucleus) are the most abundant circulating leukocyte population of the immune system. These leukocytes are considered specialised short-lived cells with deficits in numbers and/or function of this subset being associated with increased risk of potentially fatal bacterial infections (Smith et al., 1996, Boxer, 2003, Viscoli et al., 2005). Once released from the bone marrow, neutrophils migrate out of the circulation within 4–10 h to marginated and tissue pools where they reside for a further 1–2 days (Smith, 1994, Summers et al., 2010).
Neutrophils respond to chemotactic stimuli (e.g., formylated peptides such as formyl methionyl leucyl phenylalanine, fMLP) from fragments of invading microorganisms and tissue damage at sites of infection and inflammation respectively where they engage in numerous intrinsic conformation adaptations during and following their migration (chemotaxis) (Smith and Pyne, 1997). Similar to other phagocytes (e.g., APC, discussed later in this section), the detection of pathogens or tissue damage through PRR (e.g., FPR1, a fMLP receptor) on the neutrophil surface leads to internalization (phagocytosis) and holding of these fragments within the cytoplasm (via a membrane bound vesicle known as phagosome) thereby activating a series of effector functions in the cell (Nathan, 2006, Borregaard, 2010). The mechanistic detail of engulfment by neutrophils may depend on the involvement of the specific ligand (i.e., antigen) attached to the receptors or whether fragments have been opsonised by soluble immune factors, but the process will ultimately involve internalization into a phagosome (Amulic et al., 2012). Soluble components which act as opsonins (promote attachment of antigen to phagocyte) include acute phase proteins, antibodies (see Section 1.3.3) and complement proteins (e.g., C3) (Thiel et al., 1992, Gordon, 2002, Brekke et al., 2007). It is worthy to note that elevations in circulating concentrations of acute phase proteins (c-reactive protein, CRP) in response (i.e., acute phase response) to perturbations in homeostasis (e.g., trauma, tissue damage) are considered one example of why the strenuous nature of prolonged exercise is likened to medical conditions (e.g., sepsis, burns) (Kushner and Rzewnicki, 1994, Pedersen and Hoffman-Goetz, 2000, Fallon, 2001).
Neutrophils are also known as granulocytes due to the characteristic release (degranulation) of primary azurophil [defensins, elastase, myeloperoxidase (MPO)] and secondary specific (lactoferrin, lysozyme) granule contents into the cytoplasm to fuse with the phagosome or the plasma membrane to create an antimicrobial milieu inside and outside of the cell. These granules do show varying readiness to mobilize in response to inflammatory signaling, with the azurophilic subset being the most difficult to mobilize (Amulic et al., 2012). The degradation of particles within the phagosome or extracellular space of infected or damaged tissue is aided further by the process of neutrophil oxidative burst involving the formation of reactive oxygen species (ROS) through the NADPH oxidase system (Weiss, 1989, Babior, 1999). Depending on the stimulus (see Section 2.4.3.1 for relevant stimuli of this thesis), the NADPH oxidase system may assemble on the membrane of the phagosome and/or the cell where the main ROS produced are the superoxide anions which react to form other intermediates including hydrogen peroxide, hypochlorous acid, hydroxyl radical, and singlet oxygen (Peake, 2002). It has been suggested that components of azurophilic (e.g., MPO) and specific (e.g., flavocytochrome b558) granules may regulate the activity of the NADPH oxidase (Tal et al., 1998, Amulic et al., 2012), highlighting that intracellular signaling cascades triggered by attachments of ligands to surface receptors, may lead to coordinated degranulation and oxidative burst responses to invading pathogens (Daniels et al., 1994, Pyne, 1994).
It has also been demonstrated that neutrophils also possess antimicrobial capacity independent of phagocytosis known as neutrophil extracellular traps (NETs) (Mantovani et al., 2011). Although mechanisms of NETs are not completely understood, it has been established that classical effector functions of neutrophils (degranulation and oxidative burst) play important roles in mediating the response (Fuchs et al., 2007, Patel et al., 2010, Metzler et al., 2011, Amulic et al., 2012). NETs are suggested to form upon an active form of cell death which results in the release of a network of nuclear filaments (DNA and histone) into the extracellular space from the degrading neutrophil (Brinkmann et al., 2004, Fuchs et al., 2007).
On the basis of the exercise-induced changes in neutrophil counts it is not surprising that exercise also affects functional responses, but due to the variation in study design the early evidence was conflicting (Peake, 2002). Nevertheless, it can be argued that when responses are controlled for on a per cell basis the effects of exercise duration (i.e., prolonged exercise) are clearer. Neutrophils may present on a continuum of state of activation from dormant to primed through to being fully activated (Smith, 1994). During exercise, there is a release of agents into the circulation which may prime (e.g., induce assembly of NADPH on membrane) or desensitize (internalization of receptors) the capacity of neutrophils for enhanced responsiveness to later stimulation or inhibit such functional responses respectively (Pyne, 1994, Peake, 2002, Amulic et al., 2012).
The number of neutrophils engaging in phagocytic activity is increased following prolonged exercise but the phagocytic capacity of each neutrophil is decreased within the circulation and the nasal cavity (Gabriel et al., 1994, Müns, 1994, Blannin et al., 1996a, Nieman et al., 1998, Chinda et al., 2003). Albers et al. (2005) suggested that neutrophil phagocytosis may have low suitability as a marker of the immunomodulatory effects of exercise and assessing the killing capacity (degranulation and oxidative burst) may be more sensitive to reflect a susceptibility to infection.
Simultaneous with neutrophilia following prolonged exercise there is an increase in unstimulated degranulation and oxidative burst responses (e.g., measured by total plasma elastase, spontaneous ROS production) (Blannin et al., 1996b, Suzuki et al., 1999, Bishop et al., 2003). These findings provide support that prolonged exercise induces neutrophil activation (possibly due to muscle damage) that may result in the cells entering a “refractory period” in the recovery from prolonged exercise whereby there is a transient inability to respond to subsequent stimulation (e.g., in vitro) (Peake, 2002).
Numerous studies have found significant decreases in neutrophil degranulation and/or oxidative burst responses to in vitro stimulation by bacterial peptides (fMLP and LPS) and/or synthetic stimuli (phorbol-12-myristate-13-acetate, PMA) during the recovery from prolonged (>1.5 h) exercise (Chinda et al., 2003, Suzuki et al., 2003, Davison and Gleeson, 2005, Davison and Gleeson, 2006, Davison and Gleeson, 2007, Davison et al., 2007, Laing et al., 2008, Davison and Diment, 2010). This decline in killing capacity has not been coupled to changes in cell surface receptors, suggesting that the modulatory effects of exercise on in vitro stimulants occur downstream in intracellular signal transduction pathways of neutrophils such as phosphorylation cascades or secondary messengers (e.g., cyclic adenosine monophosphate, calcium) (Mooren et al., 2001, Peake, 2002). The magnitude of the effects of prolonged exercise on neutrophil effector functions will depend on the balance of immunosuppressive (e.g., “refractory period”) and immunostimulating factors (e.g., priming agents) which largely depend on the extent of neutrophilia (Peake, 2002). As previously mentioned, prolonged exercise (i.e., elevations in stress hormones) induces a mobilization of immature neutrophils into the circulation which have been shown to exhibit reduced NADPH oxidase activity and granular content (Hetherington and Quie, 1985, Berkow and Dodson, 1986). The immuodepressive effects of cortisol and the subsequent release of subpopulations from the bone marrow was supported by Robson et al. (1999b) who found that the degree of exercise-induced decrease in stimulated degranulation per neutrophil and neutrophilia was greater following prolonged exercise (~3 h) compared to short, intense exercise (~40 min).
Although considered to be a major factor, activation of the HPA axis alone cannot wholly account for changes in the neutrophil function (Laing et al., 2008), as some of the noted inhibitory effects of cortisol on receptor mediated responses (e.g., fMLP) would not explain decreased responses to stimulants that activate neutrophils independent of surface receptors (e.g., PMA) (O’Flaherty et al., 1991, Tomchek et al., 1991, Peake, 2002). Thus other mechanisms have also been suggested to be involved in neutrophil dysfunction, where elevations in catecholamines, cyclic adenosine monophosphate, complement proteins (C5a), direct cellular oxidative damage and growth hormone may occur with the inflammatory response to prolonged exercise and have been shown to interfere with calcium signaling or other intermediates of intracellular pathways (Henson et al., 1978, Hack et al., 1994, Suzuki et al., 1999, Thibault et al., 2000, Tintinger et al., 2001, Robson et al., 2003, Laing et al., 2008).
The susceptibility of phagocytes to modulation by strenuous exertion are also demonstrated by the decreased expression of TLR on the cell surface of monocytes immediately and up to 2 h following prolonged exercise (Lancaster et al., 2005b, Oliveira and Gleeson, 2010) which are not explained by exercise-induced changes in monocyte numbers alone (Simpson et al., 2009). The phagocytic function of monocytes has been shown to increase following prolonged exercise which may be related to the increased pro-inflammatory phenotype observed with exercise in this leukocyte (Steppich et al., 2000, Hong and Mills, 2008). Once monocytes reach the tissues they will form mature macrophages, therefore the biological significance of changes in monocytes is unclear as it may not reflect exercise-induced changes in immunosurveillance within tissue (i.e., at sites of inflammation or infection) (Simpson et al., 2009, Walsh et al., 2011b). These macrophages along with other phagocytes (dendritic cells) are present in majority of body tissues where unlike the direct killing capacity of neutrophils, macrophages and dendritic cells mostly act as professional APC (Beutler, 2004, Iwasaki and Medzhitov, 2004). Investigations within exercise stress models thus far have been limited to animal studies which are difficult to generalize to the human response, but do suggest dysfunction in the capacity of these cells to present antigens following prolonged exercise (Davis et al., 1997, Woods et al., 1997, Ceddia and Woods, 1999, Ceddia et al., 2000, Woods et al., 2000, Murphy et al., 2004, Liao et al., 2006, Chiang et al., 2007).
NK cells are large granular cells that can sense structures of high-molecular weight glycoproteins expressed on virus-infected cells via PRR on their cell surface. They form up to 15% of the lymphocytes within the body and are vital in defense against viral infection. However, compared to other peripheral lymphocytes (B and T cells, see Section 1.3.3), prior sensitisation is not required (Cerwenka and Lanier, 2001). Thus upon activation NK cells trigger apoptosis or lysis of a virus-infected cell by releasing granule contents such as the pore forming proteins perforin and cytolysin. The initial investigations into NK cell activity (NKCA) showed that exercise-induced responses were largely mediated by the duration and intensity of the bout (Gannon et al., 1995). The NKCA was demonstrated to mirror the increases in NK cells following moderate or exhaustive exercise (Gannon et al., 1995, Woods et al., 1998). However, when the exercise bout is intense and prolonged, NCKA on a per cell basis has been shown to be reduced for several hours (Kappel et al., 1991, Nieman et al., 1993, McFarlin et al., 2004).
15.4.2.1.3. Acquired Immune Cell Function and Acute Exercise
Binding of microorganisms to PRR (e.g., TLR) of innate immune cells not only triggers activation of innate parameters but also induces the generation of protein messengers (e.g., cytokines) and other signaling components to stimulate acquired immunity (Takeda and Akira, 2005). However, compared to innate immunity, the acquired immune response to a pathogen is delayed due to a lag period for recognition by the vast range of antigen receptors within B and T lymphocyte populations, clonal selection/expansion of the pertinent lymphocytes and clonal elimination for tolerance of self to occur (Kimbrell and Beutler, 2001, Medzhitov, 2001).
T and B cells form 60%–80% and 5%–15% of the circulating pool of lymphocytes respectively, where the T cell population mature in the thymus gland and the B cells in the bone marrow. T lymphocytes are subdivided further into cytotoxic (Tc, CD8+), helper (Th, CD4+), and regulatory (Treg) where the Treg are formed from a naive CD4+ cell and modulate immune responses compared to the primary involvement in removal of pathogens by Tc and Th.
The induction of a primary immune response to pathogens involves presentation of antigens to the cell surface receptor of T lymphocytes (T cell receptor) by dendritic cells (Lanzavecchia and Sallusto, 2001, Mellman and Steinman, 2001), while all other APC (e.g., monocytes, macrophages) are involved in the initiation of secondary immune responses via memory T cells which have encountered the antigen previously (Gallucci and Matzinger, 2001). T cells are able to recognize antigens via major histocompatibilty complex (MHC) class I and II molecules on the cell surface of an APC (Banchereau and Steinman, 1998). The number of antigens encountered by an individual will determine the proportion of naive (unactivated) or memory (activated) T cells circulating within the body.
Although structurally similar to the T cell receptor, the surface receptor of the B lymphocyte is a membrane-anchored immunoglobulin (Ig) (also termed antibody). Upon interaction of antigen and receptor, the B cell undergoes clonal expansion to form both short-lived plasma effector cells and long-lived memory cells or internalize the bound antigen and act as an APC to T cells (LeBien and Tedder, 2008). The short-lived plasma cells secrete Igs into the blood to aid destruction of the pathogen while the memory cells with their greater affinity to the antigens can remain in the circulation throughout life to rapidly differentiate into plasma effector cells if the same antigen is encountered again (Walsh et al., 2011b).
The circulating Igs fall into five major classes, IgM, IgG, IgA, IgD, and IgE where each of these classes can be divided further into subclasses. This diverse repertoire of Igs possess functional regions capable of binding to a vast range of antigen sites (epitopes) to form immune (antigen–antibody) complexes that neutralize the toxicity of certain antigens or common Ig regions which can activate phagocyte ingestion and soluble innate factors (e.g., complement) (Nieman and Nehlsen-Cannarella, 1991). Following a lag period for accumulation of Igs within the blood, the IgM class predominates during any primary response to an antigen whereas IgG predominates under normal resting conditions as well as in the rapid Ig response to secondary antigen exposure (McKune et al., 2005).
Therefore the acquired immune system consists of a cellular (i.e., T cells) and humoral component (i.e., Ig). Determining which component of the acquired immune system predominates, are the subpopulations of Th cells (Th1 and Th2) and the cytokine profile they produce and release (Walsh et al., 2011b). Cytokines act as protein messengers between one immune cell and other, where the cell receiving the signal may proliferate, secrete additional cytokines, migrate to the area of origin of the signal, differentiate into another type of cell or die (undergo apoptosis) (Curfs et al., 1997). The main cytokine group responsible for leukocyte communication is the interleukin (IL) family but other major cytokine groups are the colony stimulating factors (e.g., granulocyte macrophage colony stimulating factor, granulocyte-colony stimulating factor), tumor necrosis factors, and interferons (IFN) where their main roles are stimulation of cell growth, tumor cytoxicity, and inhibition of viral replication respectively (Curfs et al., 1997).
The stimulation of Th1 cells and production of cytokines such as IL-2, and IFN γ promotes cell-mediated immunity (Tc responses) for defense against intracellular pathogens while activation of Th2 cells (IL-4, IL-5, IL-6, and IL-13) coordinates humoral immunity (B cells) and release of Igs for defense against extracellular pathogens (Seder, 1994). It has been suggested that intense/prolonged exercise can modulate this balance by decreasing the proportion of Th1 cells in circulation whereas Th2 cells remain unaffected (Steensberg et al., 2001, Lancaster et al., 2004, Lancaster et al., 2005a). This shift in immunity has been proposed to provide an explanation for potential increases in the susceptibility to URI following prolonged exercise given the importance of type 1 responses towards viral infection (Steensberg et al., 2001, Fabbri et al., 2003).
Such effects of exercise are suggested to be mediated via the suppression of type 1 cytokine production by elevations in stress in hormones (adrenaline and cortisol) and the release of cytokines from contracting skeletal muscle (primarily IL-6) that favor production of type 2 cytokines (Gleeson, 2007). Supporting evidence for cytokine modulations following intense exercise has been provided by decreases in IL-2 and IFN and no changes in IL-4 shown with in vitro mitogen-stimulated isolated cells and whole blood culture (Tvede et al., 1993, Moyna et al., 1996, Smits et al., 1998, Starkie et al., 2001). These changes in cytokine production overlap with influences on other stages of T cell activation where decreases in mitogen-induced T cell proliferation have been observed following intense/prolonged exercise (Fry et al., 1992; Nieman et al., 1994, 1995; Henson et al., 1998; Bishop et al., 2005).
Despite these observations, the evidence suggesting that T cell activation is down-regulated by prolonged exercise is confounded by numerous methodological limitations (Walsh et al., 2011b). T cell proliferation assays generally use a fixed amount of total lymphocytes or whole blood so changes following exercise may only reflect changes in the proportion of lymphocyte subsets due to the greater increase in NK cells post exercise which do not respond to mitogen (Green and Rowbottom, 2003). Depletion of NK cells from cell culture has been found to remove the significance of changes in mitogen-induced proliferation following exercise (Green et al., 2002). Nieman et al. (1994) found that proliferative responses were substantially different based on adjustments for T cell populations, but there was still a significant fall in proliferation following intense exercise compared to preexercise. Nevertheless, the validity and sensitivity of mitogen-induced culture to assess T cell function have been questioned due to their nonspecific effects (activating other cell types, e.g., B cells) and lack of ability to detect subtle changes in individual T cell populations (Bishop et al., 2005).
Bishop et al. (2009) demonstrated that ex vivo migration of CD4+ and CD8+ cells to a human-rhinovirus infected bronchial (lung) epithelial cell line was decreased following a prolonged exercise bout (2 h of running). It is also difficult to extrapolate such observations on isolated cells from a circulating pool that is considerably lower than total lymphocyte mass within the complex in vivo environments at the skin, mucosa, and lymph nodes (Gleeson, 2007). Although investigations on whole blood maintains the proximity of leukocytes and the extracellular milieu of leukocytes compared to leukocyte isolation, the use of in vivo measures to assess response to antigenic challenge may be more clinically relevant (Albers et al., 2005, Albers et al., 2013, Walsh et al., 2011b, Bermon et al., 2017).
15.4.2.1.4. Mucosal Immunity and Acute Exercise
Both cell-mediated and adaptive parameters contribute to the largest component of the immune system, mucosal immunity (total surface area of 400 m2) (Brandtzaeg et al., 1999). The importance in host defense against pathogens becomes apparent when recognizing that the mucosal surfaces of the upper and lower respiratory tract account for ~50%–60% of total immune protection by the body and the small intestine along with the colon are responsible for 70% of all Igs produced (Kudsk, 2002). Increases in illness and morbidity have been attributed to impairment in mucosal immunity (Daele and Zicot, 2000), highlighting the importance that immune competence at mucosal surfaces has in the health and well-being of athletes (West et al., 2006). Although not functioning independently of the systemic immune system, mucosal immunity is also considered a distinct entity due to its autonomously regulated, localized defense mechanisms (Toy and Mayer, 1996).
The gut-associated lymphoid tissue, urogenital tracts, lacrimal glands, lactating mammary glands, and respiratory tracts which include the bronchus-associated lymphoid tissue (BALT), salivary glands and nasal-associated lymphoid tissue are all mucosal surfaces which fall under the network of immune structures known as the common mucosal immune system (CMIS) (Gleeson and Pyne, 2000). The immunological protection provided by this network may be via organized tissue with well-formed follicles (muscosa-associated lymphoid tissue) such as PP of the small intestine or as a diffuse accumulation of leukocytes (lymphocytes, plasma cells, and phagocytes) as found in the lung and the lamina propria (connective tissue) of the small intestine (Kyd and Cripps, 1999). These immune structures are pivotal for the mono-layered epithelial layer of mucosal surfaces which is continually exposed to a wide array of antigens or allergens including pathogenic bacteria or viruses, gut microflora and ingested food (Johansen et al., 2000). Indeed, the mucosae are considered to be the first line of defense as they are the sites where most pathogens enter the body (Macpherson et al., 2012).
The CMIS are differentiated into inductive and effector sites, where the induction sites (primarily PP) involve the sensitization of immune response following antigen presentation while the effector sites consist of interconnected distal sites (respiratory tract, lamina propria) where the array of activated B cells and plasma cells home and migrate to provide local protection (Kyd and Cripps, 1999, Kudsk, 2002). At least 80% of the body’s plasma cells (activated B cells) reside in the mucosal effector tissues, with local production of Igs representing a major immunological barrier at all mucosal surfaces and IgA being the predominant antibody (Brandtzaeg et al., 1999, Bishop and Gleeson, 2009).
Within the bloodstream, IgA under most circumstances is found as a monomeric peptide (Yel, 2010). However, in all mucosal secretions IgA exists as a dimeric protein covalently linked by a J chain containing another peptide termed the secretory component (Gleeson and Pyne, 2000). The secretory component is the cleaved segment of the polymeric Ig receptor (pIgR) that is produced by the mucosal and glandular epithelial cells and expressed on the basolateral membrane (Teeuw et al., 2004, Bishop and Gleeson, 2009). The proteolytic cleavage of pIgR occurs following its binding and induction of the active transport (exocytosis) of dimeric IgA through epithelial cells on to the mucosal surface (Strugnell and Wijburg, 2010). The remaining secretory component wrapped around the J chain-linked dimeric IgA forms secretory IgA (SIgA) which is considered to be resistant to proteases secreted at mucosal sites (e.g., intestinal mucosa) (Underdown and Dorrington, 1974, Lindh, 1975, Johansen et al., 2001, Strugnell and Wijburg, 2010). It is this local production of SIgA that forms the major effector function of mucosal immunity (Bishop and Gleeson, 2009). Only in the neonate or situations of IgA deficiency does IgM represent a significant defense at mucosal surfaces with IgG also being found in low quantities at mucosae (Brandtzaeg et al., 1999, Gleeson and Pyne, 2000).
IgA can be further divided into subclasses, where IgA2 is the most abundant in the distal gastrointestinal tract (60%), whereas IgA1 predominates in the salivary glands (60%–80%) and nasal lymphoid tissue (>90%) (Gleeson, 2000). Protection of mucosal surfaces via SIgA occurs through multiple mechanisms. One such mechanism known as immune exclusion involves the binding of antigens to regulate the commensal microorganisms (microbiota) and prevent the attachment and invasion of mucosal surfaces by pathogens (Strugnell and Wijburg, 2010, Sutherland and Fagarasan, 2012). Other mechanisms include the binding of antigens which have already crossed the mucosal barrier and actively transporting them back across the epithelial layer into the lumen or intracellular neutralisation of viruses when bound to pIgR within the mucosal epithelia (Lamm, 1988).
The presence of SIgA (formed by B cells adjacent to salivary ducts and glands) in saliva has tended to be the mucosal immune marker of choice due to the ease of collection (Korsrud and Brandtzaeg, 1980, Gleeson, 2000, Sari-Sarraf et al., 2006, Bishop and Gleeson, 2009). Most of the salivary fluid itself is formed by three pairs of major salivary glands (parotid, submandibular, sublingual) but production is supplemented by a vast amount of small submucosal glands that lie on and around the tissue (e.g., palate, tongue) within the oral cavity (Proctor and Carpenter, 2007). Although saliva drains from the acini (cluster of cells) of each of these glands into the mouth via striated and excretory ducts, the nature of the secretion differs whereby a serous (watery) fluid is produced by the parotid, mucous fluid by the submandibular and sero-mucous mixture by the sublingual (Aps and Martens, 2005). In unstimulated saliva secretion, the proportion of the fluid provided by parotid, submandibular, sublingual and the remaining submucosal glands are suggested to be 25%, 60%, 8%, and 8% respectively on average (Dawes, 2008).
In addition to the aforementioned transcytosis of SIgA into the mucosal secretion, saliva benefits from the import of several antimicrobial peptides (AMPs) that contribute to the first line of defense by providing innate defences compared to the specific nature of IgA (Bals, 2000). AMPs are categorized as being small cationic peptides (< 100 amino acids) that represent inducible, constituent factors of mucosal secretions (West et al., 2006). Although numerous AMPs require some form of enzymatic modification prior to their functional configuration, they act in synergy with other components of the innate immune system to prevent and aid clearance of infections (Wakabayashi et al., 2003, Bowdish et al., 2005, De Smet and Contreras, 2005, Ibrahim et al., 2005, Radek and Gallo, 2007). Several AMPs can modulate other immune processes such as leukocyte cytokine secretion, chemotaxis and remodeling of injured epithelia (Ganz, 2003, Bowdish et al., 2005, Tjabringa et al., 2005).
The most abundant AMPs in the secretions of the URT are lysozyme and lactoferrin (Singh et al., 2000). Lysozyme is a small protein (145 kDa) released into saliva by neutrophils, macrophages, and the submucosal glands that possesses bactericidal capacity through hydrolyzing the polysaccharide of bacterial cell walls (Jolles and Jolles, 1984, Travis et al., 2001, Bosch et al., 2002, West et al., 2006, Fabian et al., 2012). The antimicrobial properties of the smaller molecule lactoferrin (80 kDa) from neutrophils and submucosal glands are due to its ability to bind free iron, depriving bacteria of this nutrient that is essential for growth and multiplication (Legrand et al., 2004, Bowdish et al., 2005, Ward et al., 2005). Although not an inhibitor of the main causative agent of URTI (rhinovirus), lactoferrin is considered to be effective against other common respiratory viruses [adenovirus, respiratory syncytial virus (West et al., 2006)].
There is a wide variety of other AMPs, most of which have been grouped into three main families; cathelicidins (e.g., LL37), defensins (α and β subfamilies), and histatins (Bals, 2000). These are primarily released into the oral cavity by the epithelial cells, salivary glands, and/or neutrophils (De Smet and Contreras, 2005, Fabian et al., 2012). The AMPs work synergistically in low concentrations to destabilize cell walls of microorganisms and provide a broad spectrum of activity against gram-positive and gram-negative bacteria (Bals, 2000). Other salivary proteins that contribute an important line of defense via the inhibition of adherence and growth of specific bacteria (e.g., Streptococcus) at the oral mucosa include α-amylase (Scannapieco et al., 1993). In addition to influx of invading microorganisms from the external environment, the human mouth has a constant microbial presence that needs to be regulated (Bender et al., 2006).
Surfaces of the oral cavity are bathed with saliva, whereby the fluid is recognized to provide a “fingerprint” of the vast range of the resident microorganisms (Li and Gleeson, 2005, Boutaga et al., 2007, Fabian et al., 2008, Dewhirst et al., 2010). Reduced salivary flow rate can directly impact on the oral microbiome by inducing a shift towards colonization via pathogenic microorganisms (Meurman, 2012). Indeed, the entire surface of the respiratory tract is a source of commensal microorganisms which, similar to exogenous antigens, possess the ability to become pathogenic in the host (Watson et al., 2006, Bosch et al., 2013). AMPs not only play a crucial role in the protection against foreign pathogens, but also act as a synergic arsenal of molecules that regulate the response to commensal bacteria (Boman, 1995, Davison et al., 2009, Jones et al., 2014). Therefore the importance of AMPs is twofold; preventing disruption of the epithelial layer by acting as a critical first line of defense (biofilm) against pathogens, and maintaining homeostasis of the commensal community that act to outcompete invading microorganisms (Blaser and Falkow, 2009, Murphy et al., 2009, He et al., 2013).
The overlapping nature of these defenses is highlighted when reduction in expressions of AMPs in in vitro models lead to changes in bacterial colonization (Bals et al., 1999, Liu and Modlin, 2008). Subsequently, perturbations in the balance of the microbiota on the mucosal surface can lead to overgrowth and further amplification of microbes which may have direct effects locally (e.g., oral infection, URTI) or have indirect effects through predisposing to respiratory illness with the many established interactions between microorganisms (bacteria–bacteria, viral–bacteria) (Slots and Genco, 1984, Blaser and Falkow, 2009, Murphy et al., 2009, Meurman, 2012, Bosch et al., 2013). Such debilitating effects are likely to occur if the imbalance in microbiota occurred due to a lack of immune competence (e.g., decreased AMPs) (Bosch et al., 2013).
The resistance of the microbiota within the URT to the immune perturbations associated with prolonged exercise is currently unclear. The most popular parameter of mucosal immunity that has been investigated in an acute exercise setting has been salivary SIgA due to the notion that individuals who suffer from IgA deficiency contract URTI regularly (Gleeson and Pyne, 2000). Physiological changes (e.g., nervous stimulation, dehydration) during exercise can influence the secretion of saliva and its protein components (Walsh et al., 2004, Bishop and Gleeson, 2009). The salivary glands are innervated by both the parasympathetic and sympathetic nervous system, with changes in stimulation of either of these having an influence on the volume, viscosity, protein and mucin concentration (Aps and Martens, 2005). Parasympathetic nervous stimulation via vasodilation of the salivary glands is believed to trigger a high volume of watery saliva, low in protein concentration (Bishop and Gleeson, 2009). On the other hand, sympathetic stimulation produces salivary secretions which are low in volume but high in protein that is primarily due to enhanced active transport of proteins from salivary cells (Proctor and Carpenter, 2007). Thus similar to the other discussed immune parameters, salivary SIgA concentration is susceptible to effects (sympathetic and parasympathetic responses) of exercise intensity and duration (Walsh et al., 2011b).
The following discussion will consider those studies which have used the most reproducible collection method, unstimulated whole saliva collection (also termed passive drool), due to the potential for stimulated saliva flow (e.g., chewing) and other collection methods (e.g., swabs) to preferentially induce secretion from certain glands and/or influence saliva composition once secreted (Navazesh and Christensen, 1982, Navazesh, 1993, Proctor and Carpenter, 2001, Harmon et al., 2007, Bishop and Gleeson, 2009, Beltzer et al., 2010, Granger et al., 2012, Allgrove et al., 2014).
Although there is inconsistency in study design within the literature to draw a definitive conclusion, Walsh et al. (2011b) suggested that SIgA concentration in saliva generally decreases (e.g., Tomasi, et al., 1982; Nieman et al., 2002, Nieman et al., 2003; Palmer et al., 2003) or remains unchanged (e.g., MacKinnon and Hooper, 1994; Sari-Sarraf et al., 2006) following prolonged exercise (≥1.5 h at 50%–75% maximum oxygen uptake, ). It seems that the combination of high-intensity and exercise of a long duration has the most significant impact (i.e., depressive) on salivary SIgA concentration (Mackinnon, 1996, Nieman et al., 2002). The discrepancies in the literature are also believed to be partly due to the way in which salivary SIgA concentration is expressed relative to the exercise-induced changes in physiological responses (Walsh et al., 2002). In an attempt to account for such changes, salivary SIgA concentration has been expressed as a secretion rate or relative to total salivary protein/albumin/osmolality (Gleeson, 2000), but this makes the comparison between studies difficult (Bishop and Gleeson, 2009).
The secretion rate or expression relative to saliva osmolality are preferred over measures of SIgA as ratio to total protein (Blannin et al., 1998). One reason for this is that other salivary proteins (e.g., amylase) are known to increase with exercise without a change in SIgA (Walsh et al., 1999). Furthermore, expression as a secretion rate may better reflect the amount of available SIgA on the mucosal surface (MacKinnon et al., 1991). In contrast, it has been argued that salivary SIgA concentration is of greater significance as secretion rate may only provide an explanation to how salivary flow rate has changed (Bishop and Gleeson, 2009). Having said that, salivary SIgA when expressed as a secretion rate has been found to be the best predictor of URI incidence in athletes following a 160 km race (Nieman et al., 2003) or during intense training and competitive phases (Fahlman and Engels, 2005). Additionally, given that the majority of saliva is water, expression relative to secretion rate also accounts for the concentrating effect of other salivary components following any dehydration (Bishop et al., 2000, Oliver et al., 2007). As saliva osmolality reflects the inorganic electrolyte concentration (rather than protein content) and hence falls in proportion with decreases in flow rate, SIgA:osmolality provides an alternative method (Blannin et al., 1998, Bishop and Gleeson, 2009).
Although IgA is the dominant Ig in mucosal secretions, some have also attempted to identify changes in salivary IgG and IgM concentrations following exercise. Limited findings suggest IgG remains unchanged but IgM parallels decreases in salivary SIgA, highlighting the potential effects that acute strenuous exercise has on salivary immune parameters (Gleeson and Pyne, 2000, Bishop and Gleeson, 2009). Another aspect of mucosal immunity which has received little attention to date is the responses of AMPs to exercise (Walsh et al., 2011b). Despite the interest of the dentistry field in the role of AMPs in oral health and infection, (Putsep et al., 2002, Tanida et al., 2003, Tao et al., 2005, Dale et al., 2006), the relationship of AMPs with exercise-induced immune dysfunction is yet to be explored conclusively (Davison et al., 2009).
To date, the few investigations that have been conducted suggest in accordance with other immunological measures, responses of AMPs may be dependent on the intensity and duration of the exercise bout (Hoffman-Goetz and Pedersen, 1994). A 2 h cycling bout at ~65% resulted in a significant decrease in salivary lysozyme (sLys) concentration, sLys secretion rate and sLys:osmolality which recovered after 1 h of rest (Davison and Diment, 2010). In contrast, expressions of other AMPs (LL37 and defensins: human neutrophil peptide 1–3) following similar stressors (2.5 h at ~60% ) have been shown to significantly increased immediately post exercise. In the recovery period (0–1.5 h post) following a 50 km mountain trail race (mean running time ~8 h) there was a significant decrease in salivary lactoferrin (sLac) concentration and nonsignificant decreases in sLac and sLys secretion rate (Gillum et al., 2013).
The mechanism behind the effects of prolonged exercise on salivary parameters (AMPs, SIgA) remains unclear (Walsh et al., 2011b). The flow rate of saliva is considered to be the major source of variation in concentration of mucosal parameters (Gleeson and Pyne, 2000). In general, saliva flow rate decreases in response to a prolonged exercise bout (Walsh et al., 1999, Bishop et al., 2000). Decreases have been attributed to a withdrawal of parasympathetic stimulation rather than sympathetic-induced vasoconstriction of salivary glands (Bishop and Gleeson, 2009). Parasympathetic withdrawal associated with sensations of a dry mouth in response to other acute stressors (e.g., psychological) supports such proposals as does the lack of effect on saliva flow rate with interventions aimed to increase sympathetic stimulation (Bosch et al., 2002, Bishop et al., 2006).
A crucial determinant of SIgA release into saliva is the presence of pIgR to permit transport across the epithelial layer (Bosch et al., 2002). Evidence from animal studies suggests that increased mobilization of pIgR occurs only above a certain threshold of increased sympathetic stimulation (Proctor et al., 2003). This may explain why a brief bout of high-intensity exercise leads to increases in salivary SIgA (e.g., Davison, 2011). However, this does not explain the decrease found with prolonged exercise bouts (Bishop and Gleeson, 2009). It has been speculated that this nervous stimulation over a longer period (i.e., prolonged exercise) may deplete the available IgA (Proctor et al., 2003, Allgrove et al., 2008) or there may be a further threshold or interaction with duration where pIgR mobilization is down-regulated (Walsh et al., 2011b).
As brief bouts of maximal effort exercise result in increased sLac and sLys, it is reasonable to suggest that mobilization of AMPs during exercise is also influenced by sympathetic stimulation in relation to certain thresholds (Allgrove et al., 2008, West et al., 2010, Usui et al., 2011). However, the reductions in sLys following prolonged exercise may rather be related to an increased stimulation of the HPA axis and hence increases in salivary cortisol concentration, which have been associated (note, not cause, and effect) with reduced concentration of sLys (Perera et al., 1997, West et al., 2010). Additionally, it is worthy to note that the source of sLac and sLys (phagocytes, epithelial cells) is different to SIgA. Therefore it is possible that increases in AMPs during high-intensity exercise may be as a result of the effects of hyperventilation (e.g., drying of the mucosal surfaces) given that some of these changes are lost or reduced when expressed relative to osmolality or as a secretion rate (Davison, 2011).
Additionally, the exercise-induced damage to the epithelial layer could induce an inflammatory response whereby epithelial cells increase release of AMPs and other sources of AMPs are recruited (e.g., neutrophils) (West et al., 2010). The relative contribution of AMPs from each of these sources to saliva levels at both rest and following exercise is unclear. However, it is reasonable to suggest that an airway inflammatory response partly accounts for the increases in AMPs identified immediately following prolonged exercise (Davison et al., 2009). Certain AMPs (α defensins) have been suggested to make up 50% of the protein found in neutrophil azurophilic granules (Radek and Gallo, 2007). Müns (1994) demonstrated a significant (twofold) increase in the neutrophil count of mucosal secretions (nasal lavage fluid) following a prolonged exercise bout. Although neutrophils continually migrate into saliva from the circulation via gingival crevices (Lukac et al., 2003, Bender et al., 2006), the extent of neutrophila that occurs in the circulation following exercise may lead to the presence of neutrophils and their contents in saliva (e.g., AMPs) being substantially increased (Davison et al., 2009). Increased levels of AMPS (e.g., α defensins) have been observed in other body fluids (e.g., plasma) following a circulating neutrophila (Shiomi et al., 1993). The variation in the responses of different AMPs to prolonged exercise (Davison et al., 2009, Davison and Diment, 2010), therefore, may be due to the changes in the maturity of circulating neutrophils (as expressions of AMPs can vary throughout the maturation of the neutrophil in the bone marrow) or decreases in some AMPs may merely reflect neutrophils undergoing a refractory period post exercise (Cowland et al., 1995, Borregaard and Cowland, 1997, Gullberg et al., 1997, Nagaoka et al., 1997, Sorensen et al., 1997, Nagaoka et al., 1998, Nagaoka et al., 2000, Peake, 2002).
15.4.3. Exercise Training and Immune Function
Based on the evidence presented to date regarding acute prolonged exercise, it may be expected that the highly trained athletes demonstrate lower immune function at rest compared to nonexercising controls. However, numerous cross-sectional studies have attempted to discern such differences and found that when measures of immune function are gathered in a “resting state” (at least 24 h following the previous bout) there seems to be very little difference between athletes and controls (Gleeson, 2007). A review of early investigations in the area (Nieman, 2000) suggested even when significant immune perturbations had been observed in athletes participating in strenuous exercise, investigators had limited success in identifying such measures that influence alteration in rates of URI (Nieman et al., 1990b, Mackinnon et al., 1991, Nieman et al., 1998, Nieman et al., 2000). This actually adds further support to the Open Window theory, whereby these periods of depressed immunity are transient. However, for athletes who train many times per week, they will experience multiple open windows and the total time of increased susceptibility will be greater, meaning a higher overall risk of contracting an illness.
Despite the large inter-individual variation in concentration of salivary SIgA, (Walsh et al., 2011b) some of the early work (salivary SIgA responses to training) reported lower concentrations in endurance athletes compared to sedentary counterparts (Tomasi et al., 1982). In contrast, most of the subsequent investigations support evidence of other parameters by showing salivary SIgA to be broadly similar in the two populations (Gleeson and Pyne, 2000, Bishop and Gleeson, 2009). Conversely, findings in elite rowers had 50%–60% lower sLac concentration than controls at the start and mid-way through a 5 month training period (West et al., 2010), suggesting investigations of the mucosal immune compartment in a “resting state” between these populations requires further attention. Inverse relationships between salivary SIgA concentration/secretion and URI incidence in some studies of athletes undergoing periods of intensive training (i.e., athletes not necessarily in “resting state”) (Fahlman and Engels, 2005, Neville et al., 2008) further supports the need for longitudinal research on mucosal immunity with large athletic populations (Nieman, 2001). The common observations of this mucosal parameter within intensive training studies represents a narrow range that have established a link between an immune measure and URI in athletes (Gleeson, 2007, Walsh et al., 2011b).
Given the complexity of the immune system, it is unlikely that salivary SIgA alone would explain URI risk for all athletes, thus other factors in combination with the immune measure need to be determined (Nieman and Bishop, 2006). Investigators have begun attempts to address this by examining differences between illness prone and healthy athletes with early evidence suggesting that the blood obtained from the illness prone athletes has greater ex vivo production of antiinflammatory cytokines (e.g., IL-4 and IL-10) in response to multiantigen challenge, indicative of a downregulation of cell-mediated immunity (Gleeson et al., 2012, Gleeson and Walsh 2012, Gleeson and Bishop, 2013).
Multifactorial mechanisms beyond salivary SIgA alone in URI incidence become more apparent when athletes intensify their training over a short period of time (Gleeson, 2007). Indeed, 1–3 weeks of intensified training has been shown to induce marked reductions in neutrophil and monocyte function, lymphocyte proliferation and the circulating number of T cells (Verde et al., 1992, Robson et al., 1999a, Lancaster et al., 2003, Lancaster et al., 2004). In addition to intensive experimental protocols, longitudinal monitoring over a competitive seasons showed that team sports (rugby) and endurance athletes (cyclists) are sensitive to the intensive training periods with decreases in T cell counts, sLys, neutrophil oxidative burst, and IL-2 production (Baj et al., 1994, Cunniffe et al., 2011). Indeed, rapid changes (increases) in training load/stimulus may be the key risk factor (more so than total training load) for URI occurrence in athletes (e.g., Svendsen et al., 2016).
These findings suggest that there is a cumulative effect of repeated bouts of strenuous exercise due to an inadequate recovery time for the immune system (Papacosta and Gleeson, 2013). Furthermore, it may be that responses to acute prolonged exercise are more relevant than resting immunity in athletes where the immediate recovery period represents the “open window” when athletes are most vulnerable to infection (Nieman et al., 1990b, 1994; Pedersen and Bruunsgaard, 1995; Abbasi et al., 2013), since the chronic effects represent the accumulation of each acute response. Despite the lack of clear difference in measures at rest, as mentioned earlier, as athletes are exposed to more frequent acute bouts (“open windows”) of immunodepression, the overall risk may be considered greater than nonexercising controls and each acute response dictates the size and duration of each individual “open window.” The presence of any other risk factors (e.g., dietary deficiency, sleep disturbance, psychological stress) will have additive effects on the transient immune responses and increase the risk of illness following prolonged exercise as seen in many studies (Gleeson, 2007). Although underlying causes of URI remain uncertain, decrements in performance as a result of URI have been reported (Reid et al., 2004, Pyne et al., 2005). The monitoring of the risk status of an athlete is paramount in order to determine the appropriate preventive or therapeutic interventions required during stages of strenuous training/competition.
15.4.3.1. In Vitro and Ex Vivo Markers: Moderate Exercise
Regular moderate activity in both sedentary and active populations is considered to promote an antiinflammatory environment. This is an important underlying mechanism in the protection against chronic inflammatory conditions gained from physical activity in older populations (e.g., cardiovascular disease, type 2 diabetes, obesity) (Walsh et al., 2011a). Despite moderate activity induced decreases in resting concentrations of inflammatory mediators (e.g., acute phase reactants, CRP) (McFarlin et al., 2006), evidence of an influence of regular moderate activity on resting leukocyte function remains equivocal however. It is likely that additional mechanisms are also responsible for the benefits of physical activity on overall immunity and infection risk. Moderate activity (cycling) of 1 h duration has been shown to increase the capacity of blood neutrophils to respond to both receptor independent and dependent in vitro stimulation (Smith et al., 1996). This exercise-induced priming of neutrophil microbiocidal capacity has been suggested to be due to an increased presence of immunostimulating factors in the circulation and a greater proportion of responsive cells. Dhabar (2002) suggests that the immunopotentiation from moderate exercise is due to the bidirectional effect of stress hormones on immunity where subtle elevations are beneficial whereas significant and sustained elevations (as seen with prolonged and/or intensive exertion) are detrimental to the host. It is these short-lived changes in cell-mediated immunity that occur during and shortly after each acute moderate exercise bout itself that are proposed to contribute to the lower risk of URTI (Nieman et al., 2005).
To further investigate the effect of moderate activity on cell-mediated immunity, animal and human experimental studies have monitored the production of cytokines by T lymphocytes. It is well established that intracellular pathogens trigger a (type 1) cell-mediated immune response resulting in the differentiation of naive CD4+ and CD8+ T cells into T helper and T cytotoxic type 1 lymphocytes (Th1/Tc1) characterized by a phenotype of interferon (IFN)-γ and IL-2 production (Seder, 1994). In contrast, extracellular pathogens, induce a humoral (type 2) immune response resulting in the differentiation of naive CD4+ and CD8+ T cells into Th2/Tc2 lymphocytes characterized by a phenotype of IL-4, IL-5, IL-10, and IL-13 production (Seder, 1994). Type 1 and type 2 phenotypes are mutually inhibitory, whereby up-regulation or downregulation of either leads to an imbalance in immune responses (Zhao et al., 2012). For example, it has been suggested that moderate activity can induce heightened type 1 responses and, given the importance of type 1 responses towards viral infection (Steensberg et al., 2001, Fabbri et al., 2003), contribute to decreased URTI risk. Baum et al. (1997) showed that an acute bout of moderate cycling caused increases in IFN-γ production by peripheral blood lymphocytes of young adults where concentrations remained elevated compared to a control group 24 h post exercise. Longitudinal studies (0.5–4 years) of moderate activity (cycling or walking for 30 min each day) have to date only been investigated in the older population where an age-associated decline in type 1 lymphocytes and cytokines have been reversed (Ogawa et al., 2003, Shimizu et al., 2008). Although a type 1 response is critical for immmunosurveillance and early viral clearance, a sustained or excessive shift towards this phenotype has been implicated in tissue damage within lung pathology (Van Reeth, 2000). Martin et al. (2009) have hypothesized, albeit from animal models, that moderate activity also potentiates immunoregulatory mechanisms to skew towards a Th2 phenotype and antiinflammatory environment during responses to infection. Further research is warranted to determine the role of type 1/type 2 balance and cell-derived cytokines towards infection risk.
Longitudinal interventions (~16 weeks) also suggest that aerobic activities of a moderate intensity mediate the resting levels of other immune parameters in the circulatory and mucosal (saliva) compartments. This may include increasing (“restoring”) plasma Ig or preventing further age-related decline in salivary concentrations (secretory IgA, SIgA) in the elderly or increasing levels (e.g., SIgA secretion rate) in the younger adult population (Nehlsen-Cannarella et al., 1991, Klentrou et al., 2002, Akimoto et al., 2003, Martins et al., 2009, Sloan et al., 2013).
SIgA of the mucosal immune system expressed as a saliva secretion rate has previously been found to be the best predictor of URTI incidence (risk) during exposure to intense, prolonged exertion (Nieman et al., 2003). As a reduction in incidence of URTI symptoms during 12 weeks of moderate activity has been associated with an increase in SIgA (Klentrou et al., 2002), it is likely that this parameter plays an important role in the differential dose–response relationship of physical activity and infection risk. The underlying mechanisms of these changes are unclear but evidence from animal studies suggest that moderate activity may mediate an up-regulation of SIgA production by stimulating the expression of cytokines involved in the synthesis of the Ig (Drago-Serano et al., 2012). Based on the available evidence, regular aerobic moderate activity should be promoted as a preventive strategy against URTI due to a combination of short-term acute changes after each bout and a summation effect over a longer period, with the exact temporal pattern being dependent on the investigated immune parameter.
Resistance exercise has been studied less comprehensively in the context of its effects on immunity. The majority or research in this area has focused on the postexercise changes in leukocyte counts (Freidenrick and Volek, 2012). Following an acute bout of resistance exercise, NK cells, monocytes and neutrophils increase in the circulation whereby the number of some of these subsets (monocytes, neutrophils) remain elevated for up to 2 h post exercise (Ramel et al., 2003). The duration and magnitude of the postexercise leukocytosis is diminished in the older population as a consequence of age-related decline in leukocytes (Freidenrick and Volek, 2012). Differentiation of monocytes into macrophages and their infiltration into muscle tissue with neutrophils is essential for the regeneration and repair of muscle tissue after resistance exercise induced damage (Arnold et al., 2007; Tidball et al., 2010). This leukocyte redistribution has been shown to be dependent on individual components of the resistance exercise (e.g., rest length, training load) (Mayhew et al., 2005, Carlson et al., 2008). The extent of this damage depends largely on the eccentric component of the exercise bout (Sorichtere et al., 2006). The infiltration of immune cells and release of chemokines and other inflammatory mediators associated with this muscle damage creates a localized pro-inflammatory environment (Edwards et al., 2007). Although focus of investigations with resistance exercise has not been on changes in infection risk per se, this heightened inflammatory environment has been proposed to be an effective adjuvant that may enhance responses to vaccination (Edwards et al., 2010) as discussed in the vaccination section below.
15.4.3.2. In Vivo Measures
As discussed earlier, in vitro and ex vivo markers can help understanding of how the immune system responds to exercise but the most valuable (clinically relevant) measures are those which assess the whole integrated immune response (Albers et al., 2005, Albers et al., 2013). In the exercise immunology literature, such measures include challenge-type tests [such as delayed type hypersensitivity (DTH) or contact hypersensitivity (CHS) tests], response to vaccination, and possibly the reactivation of latent viruses (as discussed earlier).
15.4.3.2.1. Strenuous or Intensive Exercise
DTH is a complex local immunological reaction to an intracutaneous antigen (following previous encounter/sensitisation) that is primarily T-cell-mediated but involves interaction of various cell types and chemical mediators of the immune system to induce a cell-mediated immune response (Albers et al., 2005). Although the antigen presentation to T cells within a DTH reaction is similar to the response to intracellular pathogens (Kaufmann, 1995), it can be considered an amplified reflection of the stages that occur with a “conventional” antigen which allows for the cell-mediated immune response to be noninvasively quantified (Kobayashi et al., 2001). The DTH reaction is characterized by an infiltration of predominantly peripheral blood mononuclear cells [T cells (antigen-specific) and monocytes/macrophages] which release cytokines and other inflammatory mediators that present as eczematous plaques at the site (i.e., skin) (Bruunsgaard et al., 1997, Schwarz, 2003, Kaplan et al., 2012, Malajian and Belsito, 2013). Such epidermal indurations (edema and erythema) that peak in the 24–48 h following antigen reexposure can then provide the means by which cell-mediated immune response (the outcome) can be measured (Albers et al., 2005).
Bruunsgaard et al. (1997) showed that triathletes who completed prolonged exercise prior to intradermal injections of seven previously encountered antigens had significantly reduced skin responses compared to resting controls, indicating immunodepression. This method, however, is limited to the recall (elicitation) of preexisting immunological memory and does not provide any evidence on the effects of prolonged exercise on the primary immune (induction) response to a novel antigen (Harper-Smith et al., 2011). Contact sensitisation which involves topical skin exposure to novel synthetic chemicals provides an experimental model where both the elicitation and induction of T-cell-mediated responses can be measured (Palmer and Friedmann, 2004, Albers et al., 2005, Friedmann, 2007).
Harper-Smith et al. (2011) found that participating in prolonged exercise (2 h of running) compared with seated rest prior to cutaneous sensitisation with a novel antigen (diphenylcyclopropenone, DPCP) reduced the recall (elicitation) of the immune response (skin erythema and edema) to that same antigen four weeks later. When participants completed the same prolonged exercise immediately prior to the elicitation phase (recall at four weeks), there was also a reduction in skin responses to DPCP compared to resting controls. However, the magnitude of reduction in response to DPCP at this phase (elicitation, 20%) was considerably lower than the reduction observed when prolonged exercise was completed prior to DPCP sensitisation (induction, 50%). This suggests that the primary immune response to antigens (APC and induction of T cell specific memory) is more susceptible to the perturbations induced by prolonged exercise than responses to recall (previously exposed) antigens, which may have relevance regarding the susceptibility of athletes to new/novel compared to nonnovel pathogens. A number of follow-up studies using the CHS model have since demonstrated that this is a controllable, reproducible, sensitive, and valid in vivo marker of exercise-induced immunodepression (Diment et al., 2015; Davison et al., 2016; Jones et al., 2017). The study by Diment et al. (2015) also demonstrated no effect of the same exercise (2 h of treadmill running) on the skin’s response to the irritant croton oil, which provided strong evidence that the observed decrease in in vivo immunity assessed by this CHS method of immune induction to DPCP is an antigen-specific, T-cell-mediated, response.
Simultaneously to the aforementioned assessment of cell-mediated immunity, Bruunsgaard et al. (1997) also vaccinated all groups with previously encountered antigens that would stimulate B cell function. There were no differences between the prolonged exercise and control group in the ability of B cells to generate antibody responses to these recall antigens in the 14 days following vaccination. This may provide further supporting evidence to proposals that primary immune responses are more susceptible to the effects of exercise or it may suggest that perturbations are more pronounced in the immediate period following prolonged exercise (0–48 h, i.e., proposed open window). The response of circulating Igs to prolonged exercise has not been extensively researched with the studies that have investigated the predominant Igs in blood (A, G, M) reporting conflicted findings (decreases, increases, and no change) (Nieman and Nehlsen-Cannarella, 1991, Peters et al., 2010, Walsh et al., 2011b, Bishop, 2013). This may not be surprising given the small proportions of B cells (5%–15%) in the circulating lymphocyte pool and little redistribution of this subset following exercise. For the investigation of activated B cells and Ig responses to exercise, studies have focused primarily on other immune compartments which may have greater relevance to URTI (i.e., mucosa).
15.4.3.2.2. Moderate Exercise
The role of moderate exercise on in vivo immune markers has received relatively little attention in the literature. The majority of relevant research has studied the response to vaccination. However, most of these studies were designed to assess the utility of exercise as a vaccine adjuvant (e.g., in individuals who are less responsive to vaccination such as the elderly or those with certain chronic diseases) rather than exploring the effects of exercise on in vivo immunity per se. Such studies can provide some insight into the benefits of exercise to immunity but the extent to which this can be translated to other populations requires much further study. Long-term physical activity in such populations (pre and post vaccination) has been shown to improve antibody responses and immunogenicity to influenza, pneumococcus and meningococus vaccination (Kohut et al., 2002, Kohut et al., 2004, Keylock et al., 2007, Yang et al., 2007, Grant et al., 2008, Woods et al., 2009, Bachi et al., 2013), which support the beneficial effects of moderate exercise on in vivo immunity. However, there are a number of potential confounding factors (such as social interaction and support, psychological factors etc, from the exercise interventions) that may contribute to the benefit but are difficult to control in such studies.
The acute effects of various types of exercise has also been investigated in a number of studies (Edwards et al., 2006, Edwards et al., 2008, Campbell et al., 2010, Edwards et al., 2010, Edwards et al., 2012, Long et al., 2012, Ranadive et al., 2014). Generally, these studies have been conducted with younger rather than older subjects and in most of these cases no benefits are observed. This could be because younger populations generally mount a strong (optimal) response to vaccination anyway, leaving little scope for benefit. As such, in this context, response to vaccination may not be the best model of in vivo immune response since it does not completely mimic a real infective challenge (i.e., as the virus is usually not live, or is attenuated). Nevertheless, more work is needed in this area and other in vivo markers are desirable.
15.5. Conclusions
Athletes and individuals involved in heavy training programmes and/or prolonged bouts of exercise appear to have an increased risk of contracting URI. This is likely related to regular acute (and possibly chronic) periods of exercise-induced immunodepression (open windows). Regular moderate exercise, on the other hand, appears to have the opposite effect and reduces infection risk. Although in vitro and ex vivo measures and markers can contribute mechanistic information to the understanding of these relationships, they should not be considered as a substitute for clinically relevant/in vivo immune markers as most in vitro/ex vivo markers do not predict infection risk well. This highlights the importance of studying clinically relevant outcomes and the complex nature of the immune system. Hence, future research in this area requires more “clinically” relevant outcomes, and in vivo assessment of immune function.
References
- Abbasi A., Fehrenbach E., Hauth M., Walter M., Hudemann J., Wank V. Changes in spontaneous and LPS-induced ex vivo cytokine production and mRNA expression in male and female athletes following prolonged exhaustive exercise. Exerc. Immunol. Rev. 2013;19:8–28. [PubMed] [Google Scholar]
- Akimoto T., Kumai Y., Akama T. Effect of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br. J. Sports Med. 2003;37:76–79. doi: 10.1136/bjsm.37.1.76. 12547749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers R., Bourdet-Sicard R., Braun D., Calder P.C., Herz U., Lambert C. Monitoring immune modulation by nutrition in the general population: identifying and substantiating effects on human health. Br. J. Nutr. 2013;110:S1–S30. doi: 10.1017/S0007114513001505. [DOI] [PubMed] [Google Scholar]
- Albers R., Antoine J.M., Bourdet-Sicard R., Calder P.C., Gleeson M., Lesourd B. Markers to measure immunomodulation in human nutrition intervention studies. Br. J. Nutr. 2005;94:452–481. doi: 10.1079/bjn20051469. [DOI] [PubMed] [Google Scholar]
- Allgrove J.E., Gomes E., Hough J., Gleeson M. Effects of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. J. Sports Sci. 2008;26:653–661. doi: 10.1080/02640410701716790. [DOI] [PubMed] [Google Scholar]
- Allgrove J.E., Oliveira M., Gleeson M. Stimulating whole saliva affects the response of antimicrobial proteins to exercise. Scand. J. Med. Sci. Sports. 2014;24:649–655. doi: 10.1111/sms.12056. [DOI] [PubMed] [Google Scholar]
- Allsop P., Peters A.M., Arnot R.N., Stuttle A.W., Deenmamode M., Gwilliam M.E. Intrasplenic blood cell kinetics in man before and after brief maximal exercise. Clin. Sci. 1992;83:47–54. doi: 10.1042/cs0830047. [DOI] [PubMed] [Google Scholar]
- Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- Anane L.H., Edwards K.M., Burns V.E., Drayson M.T., Riddell N.E., van Zanten J.J. Mobilization of gammadelta T lymphocytes in response to psychological stress, exercise, and beta-agonist infusion. Brain Behav. Immun. 2009;23:823–829. doi: 10.1016/j.bbi.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Aps J.K., Martens L.C. Review: the physiology of saliva and transfer of drugs into saliva. Forensic. Sci. Int. 2005;150:119–131. doi: 10.1016/j.forsciint.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Arnold L., Henry A., Poron F. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. 17485518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D., Schnee B., Schuch G., Faltz C., Schulze J., Weber C.S. Acute psychological stress alerts the adaptive immune response: stress-induced mobilization of effector T cells. J. Neuroimmunol. 2006;176:141–152. doi: 10.1016/j.jneuroim.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Athens J.W., Haab O.P., Raab S.O., Mauer A.M., Ashenbrucker H., Cartwright G.E. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J. Clin. Invest. 1961;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B.M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Bachi A.L.L., Suguri V.M., Ramos L.R., Mariano M., Vaisberg M., Lopes J.D. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol. 2013;3:10–16. doi: 10.1016/j.rinim.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj Z., Kantorski J., Majewska E., Zeman K., Pokoca L., Fornalczyk E. Immunological status of competitive cyclists before and after the training season. Int. J. Sports Med. 1994;15:319–324. doi: 10.1055/s-2007-1021067. [DOI] [PubMed] [Google Scholar]
- Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 2000;1:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R., Weiner D.J., Moscioni A.D., Meegalla R.L., Wilson J.M. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 1999;67:6084–6089. doi: 10.1128/iai.67.11.6084-6089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Barrett B., Hayney M.S., Muller D., Rakel D., Ward A., Obasi C.N. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Ann. Fam. Med. 2012;10:337–346. doi: 10.1370/afm.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate S.L., Dollard S.C., Cannon M.J. Cytomegalovirus seroprevalence in the United States: The National Health and Nutrition Examination Surveys, 1988–2004. Clin. Infect. Dis. 2010;50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M., Geitner T., Liesen H. The role of the spleen in the leucocytosis of exercise: consequences for physiology and pathophysiology. Int. J. Sports Med. 1996;17:604–607. doi: 10.1055/s-2007-972902. [DOI] [PubMed] [Google Scholar]
- Baum M., Müller-Steinhardt M., Liesen H., Kirchner H. Moderate and exhaustive endurance exercise influences the interferon-gamma levels in whole-blood culture supernatants. Eur. J. Appl. Physiol. 1997;76:165–169. doi: 10.1007/s004210050229. [DOI] [PubMed] [Google Scholar]
- Beltzer E.K., Fortunato C.K., Guaderrama M.M., Peckins M.K., Garramone B.M., Granger D.A. Salivary flow and alpha-amylase: collection technique, duration, and oral fluid type. Physiol. Behav. 2010;101:289–296. doi: 10.1016/j.physbeh.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Bender J.S., Thang H., Glogauer M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. J. Periodontal Res. 2006;41:214–220. doi: 10.1111/j.1600-0765.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- Benschop R.J., Oostveen F.G., Heijnen C.J., Ballieux R.E. Beta 2-adrenergic stimulation causes detachment of natural killer cells from cultured endothelium. Eur. J. Immunol. 1993;23:3242–3247. doi: 10.1002/eji.1830231230. [DOI] [PubMed] [Google Scholar]
- Berkow R.L., Dodson R.W. Purification and functional evaluation of mature neutrophils from human bone marrow. Blood. 1986;684:853–860. [PubMed] [Google Scholar]
- Berkow R.L., Dodson R.W. Functional analysis of the marginating pool of human polymorphonuclear leukocytes. Am. J. Hematol. 1987;24:47–54. doi: 10.1002/ajh.2830240107. [DOI] [PubMed] [Google Scholar]
- Bermon S. Airway inflammation and upper respiratory tract infection in athletes: is there a link? Exerc. Immunol. Rev. 2007;13:6–14. [PubMed] [Google Scholar]
- Bermon S., Castell L.M., Calder P.,C., Bishop N.C., Blomstrand E., Mooren F.C. Consensus statement immunonutrition and exercise. Exerc. Immunol. Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- Beutler B. Innate immunity: an overview. Mol. Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Beutler B., Rietschel E.T. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- Bishop N.C. Effects of exercise on mucosal immunity. In: Gleeson M., Bishop N., Walsh N., editors. Exercise Immunology. Routledge; London: 2013. pp. 142–157. [Google Scholar]
- Bishop N.C., Gleeson M. Acute and chronic effects of exercise on markers of mucosal immunity. Front. Biosci. 2009;14:4444–4456. doi: 10.2741/3540. [DOI] [PubMed] [Google Scholar]
- Bishop N.C., Blannin A.K., Armstrong E., Rickman M., Gleeson M. Carbohydrate and fluid intake affect the saliva flow rate and IgA response to cycling. Med. Sci. Sports Exerc. 2000;32:2046–2051. doi: 10.1097/00005768-200012000-00013. [DOI] [PubMed] [Google Scholar]
- Bishop N.C., Walsh N., Scanlon G.A. Effect of prolonged exercise and carbohydrate on total neutrophil elastase content. Med. Sci. Sports Exerc. 2003;35:1326–1332. doi: 10.1249/01.MSS.0000078927.08049.A8. [DOI] [PubMed] [Google Scholar]
- Bishop N.C., Walker G.J., Bowley L.A., Evans K.F., Molyneux K., Wallace F.A. Lymphocyte responses to influenza and tetanus toxoid in vitro following intensive exercise and carbohydrate ingestion on consecutive days. J. Appl. Physiol. 2005;99:1327–1335. doi: 10.1152/japplphysiol.00038.2005. [DOI] [PubMed] [Google Scholar]
- Bishop N.C., Walker G.J., Scanlon G.A., Richards S., Rogers E. Salivary IgA responses to prolonged intensive exercise following caffeine ingestion. Med. Sci. Sports Exerc. 2006;38:513–519. doi: 10.1249/01.mss.0000187412.47477.ee. [DOI] [PubMed] [Google Scholar]
- Bishop N.C., Walker G.J., Gleeson M., Wallace F.A., Hewitt C.R. Human T lymphocyte migration towards the supernatants of human rhinovirus infected airway epithelial cells: influence of exercise and carbohydrate intake. Exerc. Immunol. Rev. 2009;15:127–144. [PubMed] [Google Scholar]
- Blannin A.K., Chatwin L.J., Cave R., Gleeson M. Effects of submaximal cycling and long-term endurance training on neutrophil phagocytic activity in middle aged men. Br. J. Sports Med. 1996;30:125–129. doi: 10.1136/bjsm.30.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blannin A.K., Gleeson N., Brooks S., Cave R. Acute effect of exercise on human neutrophil degranulation. J. Physiol. 1996;495:140. [Google Scholar]
- Blannin A.K., Robson P.J., Walsh N.P., Clark A.M., Glennon L., Gleeson M. The effect of exercising to exhaustion at different intensities on saliva immunoglobulin A, protein and electrolyte secretion. Int. J. Sports Med. 1998;19:547–552. doi: 10.1055/s-2007-971958. [DOI] [PubMed] [Google Scholar]
- Blaser M.J., Falkow S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H.G. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Cowland J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- Bosch J.A., Ring C., de Geus E.J.C., Veerman E.C.I., Amerongen A.V.N. Stress and secretory immunity. Neurobiol. Immun. Syst. 2002;52:213–253. doi: 10.1016/s0074-7742(02)52011-0. [DOI] [PubMed] [Google Scholar]
- Bosch A.A., Biesbroek G., Trzcinski K., Sanders E.A., Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS. Pathog. 2013;9:e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougault V., Turmel J., Levesque B., Boulet L.P. The respiratory health of swimmers. Sports Med. 2009;39:295–312. doi: 10.2165/00007256-200939040-00003. [DOI] [PubMed] [Google Scholar]
- Boutaga K., Savelkoul P.H., Winkel E.G., van Winkelhoff A.J. Comparison of subgingival bacterial sampling with oral lavage for detection and quantification of periodontal pathogens by real-time polymerase chain reaction. J. Periodontol. 2007;78:79–86. doi: 10.1902/jop.2007.060078. [DOI] [PubMed] [Google Scholar]
- Bowdish D.M.E., Davidson D.J., Hancock R.E.W. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- Boxer L.A. Neutrophil abnormalities. Pediatr. Rev. 2003;24:52–62. doi: 10.1542/pir.24-2-52. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Baekkevold E.S., Farstad I.N., Jahnsen F.L., Johansen F.E., Nilsen E.M. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol. Today. 1999;20:141–151. doi: 10.1016/s0167-5699(98)01413-3. [DOI] [PubMed] [Google Scholar]
- Brekke O.L., Christiansen D., Fure H., Fung M., Mollnes T.E. The role of complement C3 opsonization, C5a receptor, and CD14 in E. coli-induced up-regulation of granulocyte and monocyte CD11b/CD18 CR3, phagocytosis, and oxidative burst in human whole blood. J. Leukoc. Biol. 2007;81:1404–1413. doi: 10.1189/jlb.0806538. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H., Hartkopp A., Mohr T., Konradsen H., Heron I., Mordhorst C.H. In vivo cell-mediated immunity and vaccination response following prolonged, intense exercise. Med. Sci. Sports Exerc. 1997;29:1176–1181. doi: 10.1097/00005768-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Campbell J.P., Riddell N.E., Burns V.E., Turner M., van Zanten J.J., Drayson M.T. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav. Immun. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Campbell J.P., Edwards K.M., Ring C. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain Behav. Immun. 2010;242:236–242. doi: 10.1016/j.bbi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Cannon J.G. Exercise and resistance to infection. J. Appl. Physiol. 1993;74:973–978. doi: 10.1152/jappl.1993.74.3.973. [DOI] [PubMed] [Google Scholar]
- Carlson L.A., Headley S., DeBruin J., Tuckow A.T., Koch A.J., Kenefick R.W. Carbohydrate supplementation and immune responses after acute exhaustive resistance exercise. Int. J. Sport. Nutr. Exerc. Metab. 2008;18:47–259. doi: 10.1123/ijsnem.18.3.247. [DOI] [PubMed] [Google Scholar]
- Ceddia M.A., Woods J.A. Exercise suppresses macrophage antigen presentation. J. Appl. Physiol. 1999;87:2253–2258. doi: 10.1152/jappl.1999.87.6.2253. [DOI] [PubMed] [Google Scholar]
- Ceddia M.A., Voss E.W., Jr., Woods J.A. Intracellular mechanisms responsible for exercise-induced suppression of macrophage antigen presentation. J. Appl. Physiol. 2000;88:804–810. doi: 10.1152/jappl.2000.88.2.804. [DOI] [PubMed] [Google Scholar]
- Cerwenka A., Lanier L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Chiang L.M., Chen Y.J., Chiang J., Lai L.Y., Chen Y.Y., Liao H.F. Modulation of dendritic cells by endurance training. Int. J. Sports Med. 2007;28:798–803. doi: 10.1055/s-2007-964914. [DOI] [PubMed] [Google Scholar]
- Chinda D., Nakaji S., Umeda T., Shimoyama T., Kurakake S., Okamura N. A competitive marathon race decreases neutrophil functions in athletes. Luminescence. 2003;18:324–329. doi: 10.1002/bio.744. [DOI] [PubMed] [Google Scholar]
- Ciloglu F. The effect of exercise on salivary IgA levels and the incidence of upper respiratory tract infections in postmenopausal women. Kulak. Burun. Bogaz. Ihtis. Derg. 2005;15:112–116. [PubMed] [Google Scholar]
- Cohen S., Tyrrell D.A., Smith A.P. Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Cohen S., Doyle W.J., Alper C.M., Janicki-Deverts D., Turner R.B. Sleep habits and susceptibility to the common cold. Arch. Intern. Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts A.J., Wallace L.K., Slattery K.M. Monitoring changes in performance, physiology, biochemistry, and psychology during overreaching and recovery in triathletes. Int. J. Sports Med. 2007;28:125–134. doi: 10.1055/s-2006-924146. [DOI] [PubMed] [Google Scholar]
- Cowland J.B., Johnsen A.H., Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- Cox A.J., Gleeson M., Pyne D.B., Saunders P.U., Clancy R.L., Fricker P.A. Valtrex therapy for Epstein–Barr virus reactivation and upper respiratory symptoms in elite runners. Med. Sci. Sports Exerc. 2004;36:1104–1110. doi: 10.1249/01.mss.0000131957.40985.2b. [DOI] [PubMed] [Google Scholar]
- Cox A.J., Gleeson M., Pyne D.B., Callister R., Hopkins W.G., Fricker P.A. Clinical and laboratory evaluation of upper respiratory symptoms in elite athletes. Clin. J. Sport Med. 2008;18:438–445. doi: 10.1097/JSM.0b013e318181e501. [DOI] [PubMed] [Google Scholar]
- Cox A.J., Gleeson M., Pyne D.B., Saunders P.U., Callister R., Fricker P.A. Respiratory symptoms and inflammatory responses to Difflam throat-spray intervention in half-marathon runners: a randomised controlled trial. Br. J. Sports Med. 2010;44:127–133. doi: 10.1136/bjsm.2008.048298. [DOI] [PubMed] [Google Scholar]
- Crawford D.H. Biology and disease associations of Epstein–Barr virus. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356:461–473. doi: 10.1098/rstb.2000.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunniffe B., Griffiths H., Proctor W., Davies B., Baker J.S., Jones K.P. Mucosal immunity and illness incidence in elite rugby union players across a season. Med. Sci. Sports Exerc. 2011;43:388–397. doi: 10.1249/MSS.0b013e3181ef9d6b. [DOI] [PubMed] [Google Scholar]
- Curfs J.H., Meis J.F., Hoogkamp-Korstanje J.A. A primer on cytokines: sources, receptors, effects, and inducers. Clin. Microbiol. Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daele J., Zicot A.F. Humoral immunodeficiency in recurrent upper respiratory tract infections. Some basic, clinical and therapeutic features. Acta Otorhinolaryngol Belg. 2000;54:373–390. [PubMed] [Google Scholar]
- Dale B.A., Tao R., Kimball J.R., Jurevic R.J. Oral antimicrobial peptides and biological control of caries. BMC Oral Health. 2006;6:13. doi: 10.1186/1472-6831-6-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R.H., Elmore M.A., Hill M.E., Shimizu Y., Lackie J.M., Finnen M.J. Priming of the oxidative burst in human neutrophils by physiological agonists or cytochalasin B results from the recruitment of previously non-responsive cells. Immunology. 1994;82:465–472. [PMC free article] [PubMed] [Google Scholar]
- Davis J.M., Kohut M.L., Colbert L.H., Jackson D.A., Ghaffar A., Mayer E.P. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J. Appl. Physiol. 1997;83:1461–1466. doi: 10.1152/jappl.1997.83.5.1461. [DOI] [PubMed] [Google Scholar]
- Davison G. Innate immune responses to a single session of sprint interval training. Appl. Physiol. Nutr. Metab. 2011;36:395–404. doi: 10.1139/h11-033. [DOI] [PubMed] [Google Scholar]
- Davison G., Gleeson M. Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int. J. Sport. Nutr. Exerc. Metab. 2005;15:465–479. doi: 10.1123/ijsnem.15.5.465. [DOI] [PubMed] [Google Scholar]
- Davison G., Gleeson M. The effect of 2 weeks vitamin C supplementation on immunoendocrine responses to 2.5 h cycling exercise in man. Eur. J. Appl. Physiol. 2006;97:454–461. doi: 10.1007/s00421-006-0196-7. [DOI] [PubMed] [Google Scholar]
- Davison G., Gleeson M. The effects of acute vitamin C supplementation on cortisol, interleukin-6, and neutrophil responses to prolonged cycling exercise. Eur. J. Sports Sci. 2007;7:15–25. [Google Scholar]
- Davison G., Diment B.C. Bovine colostrum supplementation attenuates the decrease of salivary lysozyme and enhances the recovery of neutrophil function after prolonged exercise. Br. J. Nutr. 2010;103:1425–1432. doi: 10.1017/S0007114509993503. [DOI] [PubMed] [Google Scholar]
- Davison G., Gleeson M., Phillips S. Antioxidant supplementation and immunoendocrine responses to prolonged exercise. Med. Sci. Sports Exerc. 2007;39:645–652. doi: 10.1249/mss.0b013e318031303d. [DOI] [PubMed] [Google Scholar]
- Davison G., Allgrove J., Gleeson M. Salivary antimicrobial peptides LL-37 and alpha-defensins HNP1-3, antimicrobial and IgA responses to prolonged exercise. Eur. J. Appl. Physiol. 2009;106:277–284. doi: 10.1007/s00421-009-1020-y. [DOI] [PubMed] [Google Scholar]
- Davison G., Kehaya C., Diment B.C., Walsh N.P. Carbohydrate supplementation does not blunt the prolonged exercise-induced reduction of in vivo immunity. Eur. J. Nutr. 2016;55(4):1583–1593. doi: 10.1007/s00394-015-0977-z. [DOI] [PubMed] [Google Scholar]
- Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dental Assoc. 2008;139:18–24. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- De Smet K., Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol. Lett. 2005;27:1337–1347. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S. Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav. Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Diment B.C., Fortes M.B., Edwards J.P., Hanstock H.G., Ward M.D., Dunstall H.M. Exercise Intensity and Duration Effects on In Vivo Immunity. Med Sci Sports Exerc. 2015;47:1390–1398. doi: 10.1249/MSS.0000000000000562. [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Lange T., Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010;184:503–511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- Drago-Serrano M.E., Godínez-Victoria M., Lara-Padilla E. Moderate exercise enhances expression of SIgA in mouse ileum. Int. J. Sports Med. 2012;33:1–6. doi: 10.1055/s-0032-1312607. [DOI] [PubMed] [Google Scholar]
- Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K.M., Burns V.E., Reynolds T., Carroll D., Drayson M., Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav. Immun. 2006;20:159–168. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Burns V.E., Allen L.M. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav. Immun. 2007;21:209–217. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Burns V.E., Adkins A.E., Carroll D., Drayson M., Ring C. Meningococcal A vaccination response is enhanced by acute stress in men. Psychosom. Med. 2008;702:147–151. doi: 10.1097/PSY.0b013e318164232e. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Campbell J.P., Ring C. Exercise intensity does not influence the efficacy of eccentric exercise as a behavioural adjuvant to vaccination. Brain Behav. Immun. 2010;24:623–630. doi: 10.1016/j.bbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Pung M.A., Tomfohr L.M. Acute exercise enhancement of pneumococcal vaccination response: a randomised controlled trial of weaker and stronger immune response. Vaccine. 2012;30(45):6389–6395. doi: 10.1016/j.vaccine.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom B., Ekblom O., Malm C. Infectious episodes before and after a marathon race. Scand. J. Med. Sci. Sports. 2006;16:287–293. doi: 10.1111/j.1600-0838.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- Engebretsen L., Steffen K., Alonso J.M., Aubry M., Dvorak J., Junge A. Sports injuries and illnesses during the Winter Olympic Games 2010. Br. J. Sports Med. 2010;44:772–780. doi: 10.1136/bjsm.2010.076992. [DOI] [PubMed] [Google Scholar]
- Engebretsen L., Soligard T., Steffen K., Alonso J.M., Aubry M., Budgett R. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br. J. Sports Med. 2013;47(7):407–414. doi: 10.1136/bjsports-2013-092380. [DOI] [PubMed] [Google Scholar]
- Fabbri M., Smart C., Pardi R. T lymphocytes. Int. J. Biochem. Cell Biol. 2003;35:1004–1008. doi: 10.1016/s1357-2725(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Fabian T.K., Fejerdy P., Csermely P. Salivary genomics, transcriptomics and proteomics: the emerging concept of the oral ecosystem and their use in the early diagnosis of cancer and other diseases. Curr. Genomics. 2008;9:11–21. doi: 10.2174/138920208783884900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian T.K., Hermann P., Beck A., Fejerdy P., Fabian G. Salivary defense proteins: their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012;13:4295–4320. doi: 10.3390/ijms13044295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlman M.M., Engels H.J. Mucosal IgA and URTI in American college football players: a year longitudinal study. Med. Sci. Sports Exerc. 2005;37:374–380. doi: 10.1249/01.mss.0000155432.67020.88. [DOI] [PubMed] [Google Scholar]
- Fallon K.E. The acute phase response and exercise: the ultramarathon as prototype exercise. Clin. J. Sport Med. 2001;11:38–43. doi: 10.1097/00042752-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Faulkner G.C., Krajewski A.S., Crawford D.H. The ins and outs of EBV infection. Trends Microbiol. 2000;8:185–189. doi: 10.1016/s0966-842x(00)01742-x. [DOI] [PubMed] [Google Scholar]
- Folsom R.W., Littlefield-Chabaud M.A., French D.D., Pourciau S.S., Mistric L., Horohov D.W. Exercise alters the immune response to equine influenza virus and increases susceptibility to infection. Equine Vet. J. 2001;33:664–669. doi: 10.2746/042516401776249417. [DOI] [PubMed] [Google Scholar]
- Foster C. Monitoring training in athletes with reference to overtraining syndrome. Med. Sci. Sports Exerc. 1998;30:1164–1168. doi: 10.1097/00005768-199807000-00023. [DOI] [PubMed] [Google Scholar]
- Foster N.K., Martyn J.B., Rangno R.E., Hogg J.C., Pardy R.L. Leukocytosis of exercise: role of cardiac output and catecholamines. J. Appl. Physiol. 1986;61:2218–2223. doi: 10.1152/jappl.1986.61.6.2218. [DOI] [PubMed] [Google Scholar]
- Freidenreich D.J., Volek J.S. Immune responses to resistance exercise. Exerc. Immunol. Rev. 2012;18:8–41. [PubMed] [Google Scholar]
- Fricker P.A., Pyne D.B., Saunders P.U., Cox A.J., Gleeson M., Telford R.D. Influence of training loads on patterns of illness in elite distance runners. Clin. J. Sport Med. 2005;15:246–252. doi: 10.1097/01.jsm.0000168075.66874.3e. [DOI] [PubMed] [Google Scholar]
- Friedmann P.S. The relationships between exposure dose and response in induction and elicitation of contact hypersensitivity in humans. Br. J. Dermatol. 2007;157:1093–1102. doi: 10.1111/j.1365-2133.2007.08162.x. [DOI] [PubMed] [Google Scholar]
- Fry R.W., Morton A.R., Crawford G.P., Keast D. Cell numbers and in vitro responses of leucocytes and lymphocyte subpopulations following maximal exercise and interval training sessions of different intensities. Eur. J. Appl. Physiol. Occup. Physiol. 1992;64:218–227. doi: 10.1007/BF00626284. [DOI] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel H., Urhausen A., Kindermann W. Circulating leucocyte and lymphocyte subpopulations before and after intensive endurance exercise to exhaustion. Eur. J. Appl. Physiol. Occup. Physiol. 1991;63:449–457. doi: 10.1007/BF00868077. [DOI] [PubMed] [Google Scholar]
- Gabriel H., Muller H.J., Urhausen A., Kindermann W. Suppressed PMA-induced oxidative burst and unimpaired phagocytosis of circulating granulocytes one week after a long endurance exercise. Int. J. Sports Med. 1994;15:441–445. doi: 10.1055/s-2007-1021085. [DOI] [PubMed] [Google Scholar]
- Gallucci S., Matzinger P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Gannon G.A., Shek P.N., Shephard R.J. Natural killer cells: modulation by intensity and duration of exercise. Exerc. Immunol. Rev. 1995;1:26–48. [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Gillum T.L., Kuennen M., Gourley C., Schneider S., Dokladny K., Moseley P. Salivary Antimicrobial Protein Response to Prolonged Running. Biol. Sport. 2013;30:3–8. doi: 10.5604/20831862.1029814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Friedman S.B., Smyth J., Ader R., Bijur P., Brunell P. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behav. Immun. 1999;13:240–251. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Mucosal immunity and respiratory illness in elite athletes. Int. J. Sports Med. 2000;21:33–43. doi: 10.1055/s-2000-1450. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Immune system adaptation in elite athletes. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:659–665. doi: 10.1097/01.mco.0000247476.02650.18. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Immune function in sport and exercise. J. Appl. Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Pyne D.B. Special feature for the Olympics: effects of exercise on the immune system: exercise effects on mucosal immunity. Immunol. Cell Biol. 2000;78:536–544. doi: 10.1111/j.1440-1711.2000.t01-8-.x. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Bishop N.C. URI in athletes: are mucosal immunity and cytokine responses key risk factors? Exerc. Sport Sci. Rev. 2013;41:148–153. doi: 10.1097/JES.0b013e3182956ead. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Pyne D.B., Austin J.P., Lynn Francis J., Clancy R.L., McDonald W.A. Epstein–Barr virus reactivation and upper-respiratory illness in elite swimmers. Med. Sci. Sports Exerc. 2002;34:411–417. doi: 10.1097/00005768-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Bishop N.C., Oliveira M., Tauler P. Daily probiotic’s Lactobacillus casei Shirota reduction of infection incidence in athletes. Int. J. Sport. Nutr. Exerc. Metab. 2011;21:55–64. doi: 10.1123/ijsnem.21.1.55. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Bishop N., Oliveira M., McCauley T., Tauler P., Muhamad A.S. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand. J. Med. Sci. Sports. 2012;22:410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Walsh N.P. The BASES expert statement on exercise, immunity, and infection. J. Sports Sci. 2012;30:321–324. doi: 10.1080/02640414.2011.627371. [DOI] [PubMed] [Google Scholar]
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Granger D.A., Fortunato C.K., Beltzer E.K., Virag M., Bright M.A., Out D. Focus on methodology: salivary bioscience and research on adolescence: an integrated perspective. J. Adolesc. 2012;35:1081–1095. doi: 10.1016/j.adolescence.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Grant R.W., Mariani R.A., Vieira V.J. Cardiovascular exercise intervention improves the primary antibody response to keyhole limpet hemocyanin KLH in previously sedentary older adults. Brain Behav. Immun. 2008;226:923–932. doi: 10.1016/j.bbi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.J., Rowbottom D.G. Exercise-induced changes to in vitro T-lymphocyte mitogen responses using CFSE. J. Appl. Physiol. 2003;95:57–63. doi: 10.1152/japplphysiol.00011.2003. [DOI] [PubMed] [Google Scholar]
- Green K.J., Rowbottom D.G., Mackinnon L.T. Exercise and T-lymphocyte function: a comparison of proliferation in PBMC and NK cell-depleted PBMC culture. J. Appl. Physiol. 2002;92:2390–2395. doi: 10.1152/japplphysiol.00926.2001. [DOI] [PubMed] [Google Scholar]
- Gross D.K., Hinchcliff K.W., French P.S., Goclan S.A., Lahmers K.K., Lauderdale M. Effect of moderate exercise on the severity of clinical signs associated with influenza virus infection in horses. Equine Vet. J. 1998;30:489–497. doi: 10.1111/j.2042-3306.1998.tb04524.x. [DOI] [PubMed] [Google Scholar]
- Gullberg U., Andersson E., Garwicz D., Lindmark A., Olsson I. Biosynthesis, processing and sorting of neutrophil proteins: insight into neutrophil granule development. Eur. J. Haematol. 1997;58:137–153. doi: 10.1111/j.1600-0609.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Hack V., Strobel G., Weiss M., Weicker H. PMN cell counts and phagocytic activity of highly trained athletes depend on training period. J. Appl. Physiol. 1994;77:1731–1735. doi: 10.1152/jappl.1994.77.4.1731. [DOI] [PubMed] [Google Scholar]
- Hanstock H.G., Walsh N.P., Edwards J.P., Fortes M.B., Cosby S.L., Nugent A. Tear fluid SIgA as a noninvasive biomarker of mucosal immunity and common cold risk. Med. Sci. Sports Exerc. 2016;483:569–577. doi: 10.1249/MSS.0000000000000801. [DOI] [PubMed] [Google Scholar]
- Harmon A.G., Hibel L.C., Rumyantseva O., Granger D.A. Measuring salivary cortisol in studies of child development: watch out—what goes in may not come out of saliva collection devices. Dev. Psychobiol. 2007;49:495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Harper Smith A.D., Coakley S.L., Ward M.D., Macfarlane A.W., Friedmann P.S., Walsh N.P. Exercise-induced stress inhibits both the induction and elicitation phases of in vivo T-cell-mediated immune responses in humans. Brain Behav. Immun. 2011;25:1136–1142. doi: 10.1016/j.bbi.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Hashem M., Hall C.B. Respiratory syncytial virus in healthy adults: the cost of a cold. J. Clin. Virol. 2003;271:14–21. doi: 10.1016/s1386-6532(03)00047-7. [DOI] [PubMed] [Google Scholar]
- Hausswirth C., Louis J., Aubry A., Bonnet G., Duffield R., Le Meur Y. Evidence of disturbed sleep and increased illness in overreached endurance athletes. Med. Sci. Sports Exerc. 2014;46:1036–1045. doi: 10.1249/MSS.0000000000000177. [DOI] [PubMed] [Google Scholar]
- He C.S., Handzlik M., Fraser W.D., Muhamad A., Preston H., Richardson A. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 2013;19:86–101. [PubMed] [Google Scholar]
- Heath G.W., Ford E.S., Craven T.E., Macera C.A., Jackson K.L., Pate R.R. Exercise and the incidence of upper respiratory tract infections. Med. Sci. Sports Exerc. 1991;23:152–157. [PubMed] [Google Scholar]
- Heikkinen T., Jarvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P.M., Zanolari B., Schwartzman N.A., Hong S.R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin. Br. J. Immunol. 1978;121:851–855. [PubMed] [Google Scholar]
- Henson D.A., Nieman D.C., Parker J.C., Rainwater M.K., Butterworth D.E., Warren B.J. Carbohydrate supplementation and the lymphocyte proliferative response to long endurance running. Int. J. Sports Med. 1998;19:574–580. doi: 10.1055/s-2007-971962. [DOI] [PubMed] [Google Scholar]
- Hetherington S.V., Quie P.G. Human polymorphonuclear leukocytes of the bone marrow, circulation, and marginated pool: function and granule protein content. Am. J. Hematol. 1985;20:235–246. doi: 10.1002/ajh.2830200305. [DOI] [PubMed] [Google Scholar]
- Hoffman-Goetz L., Pedersen B.K. Exercise and the immune system: a model of the stress response? Immunol. Today. 1994;15:382–387. doi: 10.1016/0167-5699(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Wolfarth B., Horterer H.G., Halle M., Reichhuber C., Nadas K. Elevated Epstein–Barr virus loads and lower antibody titers in competitive athletes. J. Med. Virol. 2010;82:446–451. doi: 10.1002/jmv.21704. [DOI] [PubMed] [Google Scholar]
- Hogg J.C., Doerschuk C.M. Leukocyte traffic in the lung. Annu. Rev. Physiol. 1995;57:97–114. doi: 10.1146/annurev.ph.57.030195.000525. [DOI] [PubMed] [Google Scholar]
- Hong S., Mills P.J. Effects of an exercise challenge on mobilization and surface marker expression of monocyte subsets in individuals with normal vs. elevated blood pressure. Brain Behav. Immun. 2008;22:590–599. doi: 10.1016/j.bbi.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T., McMillan J. Challenge studies of human volunteers: ethical issues. J. Med. Ethics. 2004;30:110–116. doi: 10.1136/jme.2003.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim H.R., Inazaki D., Abdou A., Aoki T., Kim M. Processing of lysozyme at distinct loops by pepsin: a novel action for generating multiple antimicrobial peptide motifs in the newborn stomach. Biochim. Biophys. Acta. 2005;1726:102–114. doi: 10.1016/j.bbagen.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Johansen F.E., Braathen R., Brandtzaeg P. Role of J chain in secretory immunoglobulin formation. Scand. J. Immunol. 2000;52:240–248. doi: 10.1046/j.1365-3083.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- Johansen F.E., Braathen R., Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J. Immunol. 2001;167:5185–5192. doi: 10.4049/jimmunol.167.9.5185. [DOI] [PubMed] [Google Scholar]
- Jolles P., Jolles J. Whats new in lysozyme research—always a model system, today as yesterday. Mol. Cell. Biochem. 1984;63:165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Jones A.W., Cameron S.J., Thatcher R., Beecroft M.S., Mur L.A., Davison G. Effects of bovine colostrum supplementation on upper respiratory illness in active males. Brain Behav. Immun. 2014;39:194–203. doi: 10.1016/j.bbi.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Jones A.W., March D.S., Thatcher R., Diment B., Walsh N.P., Davison G. The effects of bovine colostrum supplementation on in vivo immunity following prolonged exercise: a randomised controlled trial. Eur. J. Nutr. 2017 doi: 10.1007/s00394-017-1597-6. https://doi.org/10.1007/s00394-017-1597-6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Shiohara T. Current understanding of cytomegalovirus infection in immunocompetent individuals. J. Dermatol. Sci. 2000;22:196–204. doi: 10.1016/s0923-1811(99)00085-7. [DOI] [PubMed] [Google Scholar]
- Kaplan D.H., Igyarto B.Z., Gaspari A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012;12:114–124. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel M., Tvede N., Galbo H., Haahr P.M., Kjaer M., Linstow M. Evidence that the effect of physical exercise on NK cell activity is mediated by epinephrine. J. Appl. Physiol. 1991;70:2530–2534. doi: 10.1152/jappl.1991.70.6.2530. [DOI] [PubMed] [Google Scholar]
- Kaufmann S.H. Immunity to intracellular microbial pathogens. Immunol. Today. 1995;16:338–342. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- Keylock K.T., Lowder T., Leifheit K.A. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J. Appl. Physiol. 2007;1023:1090–1098. doi: 10.1152/japplphysiol.00790.2006. [DOI] [PubMed] [Google Scholar]
- Kimbrell D.A., Beutler B. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2001;2:256–267. doi: 10.1038/35066006. [DOI] [PubMed] [Google Scholar]
- Klentrou P., Cieslak T., MacNeil M., Vintinner A., Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur. J. Appl. Physiol. 2002;87:153–158. doi: 10.1007/s00421-002-0609-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Kaneda K., Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- Kohut M.L., Senchina D.S. Reversing age-associated immunosenescence via exercise. Exerc. Immunol. Rev. 2004;10:6–41. [PubMed] [Google Scholar]
- Kohut M.L., Cooper M.M., Nickolaus M.S., Russell D.R., Cunnick J.E. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J. Gerontol. A. Biol. Sci. Med. Sci. 2002;579:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- Kohut M.L., Arntson B.A., Lee W. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22(17–18):2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Korsrud F.R., Brandtzaeg P. Quantitative immunohistochemistry of immunoglobulin- and J-chain-producing cells in human parotid and submandibular salivary glands. Immunology. 1980;39:129–140. [PMC free article] [PubMed] [Google Scholar]
- Kostka T., Praczko K. Interrelationship between physical activity, symptomatology of upper respiratory tract infections, and depression in elderly people. Gerontology. 2007;53:187–193. doi: 10.1159/000100017. [DOI] [PubMed] [Google Scholar]
- Kostka T., Berthouze S.E., Lacour J., Bonnefoy M. The symptomatology of upper respiratory tract infections and exercise in elderly people. Med. Sci. Sports Exerc. 2000;32:46–51. doi: 10.1097/00005768-200001000-00008. [DOI] [PubMed] [Google Scholar]
- Kostka T., Drygas W., Jegier A., Praczko K. Physical activity and upper respiratory tract infections. Int. J. Sports Med. 2008;29:158–162. doi: 10.1055/s-2007-965806. [DOI] [PubMed] [Google Scholar]
- Kruger K., Lechtermann A., Fobker M., Volker K., Mooren F.C. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav. Immun. 2008;22:324–338. doi: 10.1016/j.bbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kudsk K.A. Current aspects of mucosal immunology and its influence by nutrition. Am. J. Surg. 2002;183:390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- Kushner I., Rzewnicki D.L. The acute phase response: general aspects. Bailliere’s Clin. Rheumatol. 1994;8:513–530. doi: 10.1016/s0950-3579(05)80113-x. [DOI] [PubMed] [Google Scholar]
- Kyd J., Cripps A. Nontypeable Haemophilus influenzae: challenges in developing a vaccine. J. Biotechnol. 1999;73:103–108. doi: 10.1016/s0168-1656(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Laing S.J., Jackson A.R., Walters R., Lloyd-Jones E., Whitham M., Maassen N. Human blood neutrophil responses to prolonged exercise with and without a thermal clamp. J. Appl. Physiol. 2008;104:20–26. doi: 10.1152/japplphysiol.00792.2007. [DOI] [PubMed] [Google Scholar]
- Lamm M.E. The IgA mucosal immune system. Am. J. Kidney Dis. 1988;125:384–387. doi: 10.1016/s0272-6386(88)80030-1. [DOI] [PubMed] [Google Scholar]
- Lancaster G.I., Halson S., Kahn Q., Drysdale P., Jeukendrup A.E., Drayson M.T. Effect of acute exhaustive exercise and a 6-day intensified period of intensified training on immune function in cyclists. J. Physiol. 2003;548:96. [Google Scholar]
- Lancaster G.I., Halson S.L., Khan Q., Drysdale P., Wallace F., Jeukendrup A.E. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc. Immunol. Rev. 2004;10:91–106. [PubMed] [Google Scholar]
- Lancaster G.I., Khan Q., Drysdale P.T., Wallace F., Jeukendrup A.E., Drayson M.T. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J. Appl. Physiol. 2005;98:565–571. doi: 10.1152/japplphysiol.00754.2004. [DOI] [PubMed] [Google Scholar]
- Lancaster G.I., Khan Q., Drysdale P., Wallace F., Jeukendrup A.E., Drayson M.T. The physiological regulation of toll-like receptor expression and function in humans. J. Physiol. 2005;563:945–955. doi: 10.1113/jphysiol.2004.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdeau J.B., Turcotte H., Bowie D.M., Jobin J., Desgagne P., Boulet L.P. Airway hyperresponsiveness in elite athletes. Am. J. Respir. Crit. Care. Med. 2000;161:1479–1484. doi: 10.1164/ajrccm.161.5.9909008. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Larrabee R.C. Leucocytosis after violent Exercise. J. Med. Res. 1902;7:76–82. [PMC free article] [PubMed] [Google Scholar]
- LeBien T.W., Tedder T.F. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D., Elass E., Pierce A., Mazurier J. Lactoferrin and host defence: an overview of its immuno-modulating and anti-inflammatory properties. Biometals. 2004;17:225–229. doi: 10.1023/b:biom.0000027696.48707.42. [DOI] [PubMed] [Google Scholar]
- Li T.L., Gleeson M. The effects of carbohydrate supplementation during the second of two prolonged cycling bouts on immunoendocrine responses. Eur. J. Appl. Physiol. 2005;95:391–399. doi: 10.1007/s00421-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Liao H.F., Chiang L.M., Yen C.C., Chen Y.Y., Zhuang R.R., Lai L.Y. Effect of a periodized exercise training and active recovery program on antitumor activity and development of dendritic cells. J. Sports Med. Phys. Fitness. 2006;46:307–314. [PubMed] [Google Scholar]
- Lindh E. Increased risistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J. Immunol. 1975;114:284–286. [PubMed] [Google Scholar]
- Liu P.T., Modlin R.L. Human macrophage host defense against Mycobacterium tuberculosis. Curr. Opin. Immunol. 2008;20:371–376. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Long J.E., Ring C., Drayson M. Vaccination response following aerobic exercise: can a brisk walk enhance antibody response to pneumococcal and influenza vaccinations? Brain Behav. Immun. 2012;264:680–687. doi: 10.1016/j.bbi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Lowder T., Padgett D.A., Woods J.A. Moderate exercise protects mice from death due to influenza virus. Brain Behav. Immun. 2005;19:377–380. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lukac J., Mravak-Stipetic M., Knezevic M., Vrcek J., Sistig S., Ledinsky M. Phagocytic functions of salivary neutrophils in oral mucous membrane diseases. J. Oral Pathol. Med. 2003;32:271–274. doi: 10.1034/j.1600-0714.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Lumme A., Haahtela T., Ounap J., Rytila P., Obase Y., Helenius M. Airway inflammation, bronchial hyperresponsiveness and asthma in elite ice hockey players. Eur. Respir. J. 2003;22:113–117. doi: 10.1183/09031936.03.00112403. [DOI] [PubMed] [Google Scholar]
- Mackinnon L.T. Immunoglobulin, antibody, and exercise. Exerc. Immunol. Rev. 1996;2:1–34. [Google Scholar]
- Mackinnon L.T., Hooper S. Mucosal secretory immune system responses to exercise of varying intensity and during overtraining. Int. J. Sports Med. 1994;15:179–183. doi: 10.1055/s-2007-1021134. [DOI] [PubMed] [Google Scholar]
- Mackinnon L.T., Ginn E., Seymour G.J. Temporal relationship between exercise-induced decreases in salivary IgA concentration and subsequent appearance of upper respiratory illness in elite athletes. Med. Sci. Sports Exerc. 1991;23:45. [Google Scholar]
- Macpherson A.J., Geuking M.B., McCoy K.D. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33:160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Mäkelä M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimaki M. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malajian D., Belsito D.V. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2013;69:232–237. doi: 10.1016/j.jaad.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Malm C. Susceptibility to infections in elite athletes: the S-curve. Scand. J. Med. Sci. Sports. 2006;16:4–6. doi: 10.1111/j.1600-0838.2005.00499.x. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Martin S.A., Pence B.D., Woods J.A. Exercise and respiratory tract viral infections. Exerc. Sport Sci. Rev. 2009;37:157–164. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R.A., Cunha M.R., Neves A.P., Martins M., Teixeira-Veríssimo M., Teixeira A.M. Effects of aerobic conditioning on salivary IgA and plasma IgA, IgG and IgM in older men and women. Int. J. Sports Med. 2009;30:906–912. doi: 10.1055/s-0029-1237389. [DOI] [PubMed] [Google Scholar]
- Matthews C.E., Ockene I.S., Freedson P.S., Rosal M.C., Merriam P.A., Hebert J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med. Sci. Sports Exerc. 2002;34:1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Matthews A., Pyne D., Saunders P., Fallon K., Fricker P. A self-reported questionnaire for quantifying illness symptoms in elite athletes. J. Open Access J. Sports Med. 2010;1:15–22. doi: 10.2147/oajsm.s7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew D.L., Thyfault J.P., Koch A.J. Rest-interval length affects leukocyte levels during heavy resistance exercise. J. Strength Cond. Res. 2005;19:16–22. doi: 10.1519/R-14113.1. [DOI] [PubMed] [Google Scholar]
- McCarthy D.A., Dale M.M. The leucocytosis of exercise. A review and model. Sports Med. 1988;6:333–363. doi: 10.2165/00007256-198806060-00002. [DOI] [PubMed] [Google Scholar]
- McCarthy D.A., Grant M., Marbut M., Watling M., Wade A.J., Macdonald I. Brief exercise induces an immediate and a delayed leucocytosis. Br. J. Sports Med. 1991;25:191–195. doi: 10.1136/bjsm.25.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin B.K., Flynn M.G., Stewart L.K., Timmerman K.L. Carbohydrate intake during endurance exercise increases natural killer cell responsiveness to IL-2. J. Appl. Physiol. 2004;96:271–275. doi: 10.1152/japplphysiol.00585.2003. [DOI] [PubMed] [Google Scholar]
- McFarlin B.K., Flynn M.G., Campbell W.W. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J. Gerontol. A. Biol. Sci. Med. Sci. 2006;61:388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- McKune A.J., Smith L.L., Semple S.J., Wadee A.A. Influence of ultra-endurance exercise on immunoglobulin isotypes and subclasses. Br. J. Sports Med. 2005;39:665–670. doi: 10.1136/bjsm.2004.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Mehta S.K., Pierson D.L., Cooley H., Dubow R., Lugg D. Epstein–Barr virus reactivation associated with diminished cell-mediated immunity in antarctic expeditioners. J. Med. Virol. 2000;61:235–240. doi: 10.1002/(sici)1096-9071(200006)61:2<235::aid-jmv10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Mehta S.K., Stowe R.P., Feiveson A.H., Tyring S.K., Pierson D.L. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J. Infect. Dis. 2000;182:1761–1764. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- Mellman I., Steinman R.M. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Metzler K.D., Fuchs T.A., Nauseef W.M., Reumaux D., Roesler J., Schulze I. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurman J.H. Functional foods/ingredients and oral mucosal diseases. Eur. J. Nutr. 2012;51:31–38. doi: 10.1007/s00394-012-0324-6. [DOI] [PubMed] [Google Scholar]
- Mooren F.C., Lechtermann A., Pospiech S., Fromme A., Thorwesten L., Volker K. Decoupling of intracellular calcium signaling in granulocytes after exhaustive exercise. Int. J. Sports Med. 2001;22:323–328. doi: 10.1055/s-2001-15647. [DOI] [PubMed] [Google Scholar]
- Moyna N.M., Acker G.R., Fulton J.R., Weber K., Goss F.L., Robertson R.J. Lymphocyte function and cytokine production during incremental exercise in active and sedentary males and females. Int. J. Sports Med. 1996;17:585–591. doi: 10.1055/s-2007-972899. [DOI] [PubMed] [Google Scholar]
- Müns G. Effect of long-distance running on polymorphonuclear neutrophil phagocytic function of the upper airways. Int. J. Sports Med. 1994;15:96–99. doi: 10.1055/s-2007-1021027. [DOI] [PubMed] [Google Scholar]
- Murphy E.A., Davis J.M., Brown A.S., Carmichael M.D., Mayer E.P., Ghaffar A. Effects of moderate exercise and oat beta-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J. Appl. Physiol. 2004;97:955–959. doi: 10.1152/japplphysiol.00252.2004. [DOI] [PubMed] [Google Scholar]
- Murphy E.A., Davis J.M., Carmichael M.D., Gangemi J.D., Ghaffar A., Mayer E.P. Exercise stress increases susceptibility to influenza infection. Brain Behav. Immun. 2008;22:1152–1155. doi: 10.1016/j.bbi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Murphy T.F., Bakaletz L.O., Smeesters P.R. Microbial interactions in the respiratory tract. Pediatr. Infect. Dis. J. 2009;28:S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- Nadal D., Blasius M., Niggli F.K., Meier G., Berger C. Epstein–Barr virus EBV DNA levels in palatine tonsils and autologous serum from EBV carriers. J. Med. Virol. 2002;67:54–58. doi: 10.1002/jmv.2192. [DOI] [PubMed] [Google Scholar]
- Nagaoka I., Tsutsumi-Ishii Y., Yomogida S., Yamashita T. Isolation of cDNA encoding guinea pig neutrophil cationic antibacterial polypeptide of 11 kDa CAP11 and evaluation of CAP11 mRNA expression during neutrophil maturation. J. Biol. Chem. 1997;272:22742–22750. doi: 10.1074/jbc.272.36.22742. [DOI] [PubMed] [Google Scholar]
- Nagaoka I., Hirata M., Sugimoto K., Tsutsumi-Ishii Y., Someya A., Saionji K. Evaluation of the expression of human CAP18 gene during neutrophil maturation in the bone marrow. J. Leukoc. Biol. 1998;64:845–852. doi: 10.1002/jlb.64.6.845. [DOI] [PubMed] [Google Scholar]
- Nagaoka I., Hirota S., Yomogida S., Ohwada A., Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Navazesh M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- Navazesh M., Christensen C.M. A Comparison of whole mouth resting and stimulated salivary measurement procedures. J. Dent. Res. 1982;61:1158–1162. doi: 10.1177/00220345820610100901. [DOI] [PubMed] [Google Scholar]
- Nehlsen-Cannarella S.L., Nieman D.C., Jessen J. The effects of acute moderate exercise on lymphocyte function and serum immunoglobulin levels. Int. J. Sports Med. 1991;12:391–398. doi: 10.1055/s-2007-1024700. [DOI] [PubMed] [Google Scholar]
- Neville V., Gleeson M., Folland J.P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med. Sci. Sports Exerc. 2008;40:1228–1236. doi: 10.1249/MSS.0b013e31816be9c3. [DOI] [PubMed] [Google Scholar]
- Nielsen H.B., Secher N.H., Kristensen J.H., Christensen N.J., Espersen K., Pedersen B.K. Splenectomy impairs lymphocytosis during maximal exercise. Am. J. Physiol. 1997;272:1847–1852. doi: 10.1152/ajpregu.1997.272.6.R1847. [DOI] [PubMed] [Google Scholar]
- Nieman D.C. Exercise, infection, and immunity. Int. J. Sports Med. 1994;15:131–141. doi: 10.1055/s-2007-1021128. [DOI] [PubMed] [Google Scholar]
- Nieman D.C. Exercise immunology: nutritional countermeasures. Can. J. Appl. Physiol. 2001;26:45–55. doi: 10.1139/h2001-041. [DOI] [PubMed] [Google Scholar]
- Nieman D.C. Clinical implications of exercise immunology. J. Sport Health Sci. 2012;11:12–17. [Google Scholar]
- Nieman D.C., Nehlsen-Cannarella S.L. The effects of acute and chronic exercise on immunoglobulins. Sports Med. 1991;11:183–201. doi: 10.2165/00007256-199111030-00003. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Bishop N.C. Nutritional strategies to counter stress to the immune system in athletes, with special reference to football. J. Sports Sci. 2006;24:763–772. doi: 10.1080/02640410500482982. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Johanssen L.M., Lee J.W., Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fitness. 1990;30:316–328. [PubMed] [Google Scholar]
- Nieman D.C., Nehlsen-Cannarella S.L., Markoff P.A., Balk-Lamberton A.J., Yang H., Chritton D.B. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int. J. Sports Med. 1990;11:467–473. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Miller A.R., Henson D.A., Warren B.J., Gusewitch G., Johnson R.L. Effects of high- vs moderate-intensity exercise on natural killer cell activity. Med. Sci. Sports Exerc. 1993;25:1126–1134. [PubMed] [Google Scholar]
- Nieman D.C., Simandle S., Henson D.A., Warren B.J., Suttles J., Davis J.M. Lymphocyte proliferative response to 2.5 hours of running. Int. J. Sports Med. 1995;16:404–409. doi: 10.1055/s-2007-973028. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Nehlsen-Cannarella S.L., Henson D.A., Koch A.J., Butterworth D.E., Fagoaga O.R. Immune response to exercise training and/or energy restriction in obese women. Med. Sci. Sports Exerc. 1998;30:679–686. doi: 10.1097/00005768-199805000-00006. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Nehlsen-Cannarella S.L., Fagoaga O.R., Henson D.A., Shannon M., Hjertman J.M. Immune function in female elite rowers and non-athletes. Br. J. Sports Med. 2000;34:181–187. doi: 10.1136/bjsm.34.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman D.C., Henson D.A., Fagoaga O.R., Utter A.C., Vinci D.M., Davis J.M. Change in salivary IgA following a competitive marathon race. Int. J. Sports Med. 2002;23:69–75. doi: 10.1055/s-2002-19375. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Dumke C.I., Henson D.A., McAnulty S.R., McAnulty L.S., Lind R.H. Immune and oxidative changes during and following the Western States Endurance Run. Int. J. Sports Med. 2003;24:541–547. doi: 10.1055/s-2003-42018. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Henson D.A., Austin M.D., Brown V.A. Immune response to a 30-minute walk. Med. Sci. Sports Exerc. 2005;37:57–62. doi: 10.1249/01.mss.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- O’Flaherty J.T., Rossi A.G., Redman J.F., Jacobson D.P. Tumor necrosis factor-alpha regulates expression of receptors for formyl-methionyl-leucyl-phenylalanine, leukotriene B4, and platelet-activating factor. Dissociation from priming in human polymorphonuclear neutrophils. J. Immunol. 1991;147:3842–3847. [PubMed] [Google Scholar]
- Ogawa K., Oka J., Yamakawa J., Higuchi M. Abitual exercise did not affect the balance of type 1 and type 2 cytokines in elderly people. Mech. Ageing. Dev. 2003;124:951–956. doi: 10.1016/s0047-6374(03)00167-2. [DOI] [PubMed] [Google Scholar]
- Okutsu M., Suzuki K., Ishijima T., Peake J., Higuchi M. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav. Immun. 2008;22:1066–1071. doi: 10.1016/j.bbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Oliveira M., Gleeson M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur. J. Appl. Physiol. 2010;109:251–257. doi: 10.1007/s00421-009-1350-9. [DOI] [PubMed] [Google Scholar]
- Oliver S.J., Laing S.J., Wilson S., Bilzon J.L., Walters R., Walsh N.P. Salivary Immunologyoglobulin A response at rest and after exercise following a 48 h period of fluid and/or energy restriction. Br. J. Nutr. 2007;97:1109–1116. doi: 10.1017/S0007114507682919. [DOI] [PubMed] [Google Scholar]
- Palmer R.A., Friedmann P.S. Ultraviolet radiation causes less immunosuppression in patients with polymorphic light eruption than in controls. J. Investig. Dermatol. 2004;122:291–294. doi: 10.1046/j.0022-202X.2004.22213.x. [DOI] [PubMed] [Google Scholar]
- Palmer F.M., Nieman D.C., Henson D.A., McAnulty S.R., McAnulty L., Swick N.S. Influence of vitamin C supplementation on oxidative and salivary IgA changes following an ultramarathon. Eur. J. Appl. Physiol. 2003;89:100–107. doi: 10.1007/s00421-002-0756-4. [DOI] [PubMed] [Google Scholar]
- Papacosta E., Gleeson M. Effects of intensified training and taper on immune function. Braz. J. Phys. Educ. Sport. 2013;27:159–176. [Google Scholar]
- Patel S., Kumar S., Jyoti A., Srinag B.S., Keshari R.S., Saluja R. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide. 2010;22:226–234. doi: 10.1016/j.niox.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Peake J.M. Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action. Exerc. Immunol. Rev. 2002;8:49–100. [PubMed] [Google Scholar]
- Pedersen B.K., Ullum H. NK cell response to physical activity: possible mechanisms of action. Med. Sci. Sports Exerc. 1994;262:140–146. doi: 10.1249/00005768-199402000-00003. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Bruunsgaard H. How physical exercise influences the establishment of infections. Sports Med. 1995;19:393–400. doi: 10.2165/00007256-199519060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K., Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Tvede N., Klarlund K., Christensen L.D., Hansen F.R., Galbo H. Indomethacin in vitro and in vivo abolishes post-exercise suppression of natural killer cell activity in peripheral blood. Int. J. Sports Med. 1990;11:127–131. doi: 10.1055/s-2007-1024776. [DOI] [PubMed] [Google Scholar]
- Perera S., Uddin M., Hayes J.A. Salivary lysozyme: a noninvasive marker for the study of the effects of stress on natural immunity. Int. J. Behav. Med. 1997;4:170–178. doi: 10.1207/s15327558ijbm0402_5. [DOI] [PubMed] [Google Scholar]
- Peters E.M. Exercise, immunology and upper respiratory tract infections. Int. J. Sports Med. 1997;18:69–77. doi: 10.1055/s-2007-972702. [DOI] [PubMed] [Google Scholar]
- Peters E.M. Postrace upper respiratory tract ‘infections’ in ultramarathoners—Infection, allergy or inflammation? S. Afr. J. Sports Med. 2004;16:3–9. [Google Scholar]
- Peters E.M., Bateman E.D. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S. Afr. Med. J. 1983;64:582–584. [PubMed] [Google Scholar]
- Peters E.M., Goetzsche J.M., Grobbelaar B., Noakes T.D. Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am. J. Clin. Nutr. 1993;57:170–174. doi: 10.1093/ajcn/57.2.170. [DOI] [PubMed] [Google Scholar]
- Peters E.M., Shaik J., Kleinveldt N. Upper respiratory tract infection symptoms in ultramarathon runners not related to immunoglobulin status. Clin. J. Sports Med. 2010;20:39–46. doi: 10.1097/JSM.0b013e3181cb4086. [DOI] [PubMed] [Google Scholar]
- Piacentini G.L., Rigotti E., Bodini A., Peroni D., Boner A.L. Airway inflammation in elite swimmers. J. Allergy Clin. Immunol. 2007;119:1559–1560. doi: 10.1016/j.jaci.2007.01.050. author reply 1560-1. [DOI] [PubMed] [Google Scholar]
- Prather A.A., Janicki-Deverts D., Hall M.H., Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;389:1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor G.B., Carpenter G.H. Chewing stimulates secretion of human salivary secretory immunoglobulin A. J. Dent. Res. 2001;80:909–913. doi: 10.1177/00220345010800031201. [DOI] [PubMed] [Google Scholar]
- Proctor G.B., Carpenter G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Proctor G.B., Garrett J.R., Carpenter G.H., Ebersole L.E. Salivary secretion of immunoglobulin A by submandibular glands in response to autonomimetic infusions in anaesthetised rats. J. Neuroimmunol. 2003;136:17–24. doi: 10.1016/s0165-5728(02)00466-6. [DOI] [PubMed] [Google Scholar]
- Putsep K., Carlsson G., Boman H.G., Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- Pyne D.B. Regulation of neutrophil function during exercise. Sports Med. 1994;17:245–258. doi: 10.2165/00007256-199417040-00005. [DOI] [PubMed] [Google Scholar]
- Pyne D.B., Gleeson M. Effects of intensive exercise training on immunity in athletes. Int. J. Sports Med. 1998;19:183–194. doi: 10.1055/s-2007-971991. [DOI] [PubMed] [Google Scholar]
- Pyne D.B., Hopkins W.G., Batterham A.M., Gleeson M., Fricker P.A. Characterising the individual performance responses to mild illness in international swimmers. Br. J. Sports Med. 2005;39:752–756. doi: 10.1136/bjsm.2004.017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek K., Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin. Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Ramel A., Wagner K.H., Elmadfa I. Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J. Sports Sci. 2003;21:1001–1008. doi: 10.1080/02640410310001641395. [DOI] [PubMed] [Google Scholar]
- Ranadive S.M., Cook M., Kappus R.M. Effect of acute aerobic exercise on vaccine efficacy in older adults. Med. Sci. Sports Exerc. 2014;463:455–461. doi: 10.1249/MSS.0b013e3182a75ff2. [DOI] [PubMed] [Google Scholar]
- Reid V.L., Gleeson M., Williams N., Clancy R.L. Clinical investigation of athletes with persistent fatigue and/or recurrent infections. Br. J. Sports Med. 2004;38:42–45. doi: 10.1136/bjsm.2002.002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.A. Viral illnesses and sports performance. Sports Med. 1986;3:298–303. doi: 10.2165/00007256-198603040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Milne C. Medicine at the 2000 Sydney Olympic Games: the New Zealand health team. Br. J. Sports Med. 2002;36:229. doi: 10.1136/bjsm.36.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P.J., Blannin A.K., Walsh N.P., Bishop N.C., Gleeson M. The effect of an acute period of intense interval training on human neutrophil function and plasma glutamine in endurance-trained male runners. J. Physiol. 1999;515:84–85. [Google Scholar]
- Robson P.J., Blannin A.K., Walsh N.P., Castell L.M., Gleeson M. Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int. J. Sports Med. 1999;20:128–135. doi: 10.1055/s-2007-971106. [DOI] [PubMed] [Google Scholar]
- Robson P.J., Bouic P.J., Myburgh K.H. Antioxidant supplementation enhances neutrophil oxidative burst in trained runners following prolonged exercise. Int. J. Sport. Nutr. Exerc. Metab. 2003;13:369–381. doi: 10.1123/ijsnem.13.3.369. [DOI] [PubMed] [Google Scholar]
- Robson-Ansley P., Howatson G., Tallent J., Mitcheson K., Walshe I., Toms C. Prevalence of allergy and upper respiratory tract symptoms in runners of the London marathon. Med. Sci. Sports Exerc. 2012;44:999–1004. doi: 10.1249/MSS.0b013e318243253d. [DOI] [PubMed] [Google Scholar]
- Sari-Sarraf V., Reilly T., Doran D.A. Salivary IgA response to intermittent and continuous exercise. Int. J. Sports Med. 2006;27:849–855. doi: 10.1055/s-2006-923777. [DOI] [PubMed] [Google Scholar]
- Scannapieco F.A., Torres G., Levine M.J. Salivary alpha-amylase: role in dental plaque and caries formation. Crit. Rev. Oral. Biol. Med. 1993;4(3–4):301–307. doi: 10.1177/10454411930040030701. [DOI] [PubMed] [Google Scholar]
- Schwarz T. Skin immunity. Br. J. Dermatol. 2003;149:2–4. doi: 10.1046/j.0366-077x.2003.05624.x. [DOI] [PubMed] [Google Scholar]
- Schwellnus M.P., Kiessig M., Derman W., Noakes T.D. Fusafungine reduces symptoms of upper respiratory tract infections URTI in runners after a 56 km race. Med. Sci. Sports Exerc. 1997;29:296. [Google Scholar]
- Schwellnus M.P., Lichaba M., Derman E.W. Respiratory tract symptoms in endurance athletes—a review of causes and consequences. Curr. Allergy Clin. Immunol. 2010;23:52–57. [Google Scholar]
- Seder R.A. Acquisition of lymphokine-producing phenotype by CD4+ T cells. J. Allergy Clin. Immunol. 1994;94:1195–1202. doi: 10.1016/0091-6749(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Shek P.N., Sabiston B.H., Buguet A., Radomski M.W. Strenuous exercise and immunological changes: a multiple-time-point analysis of leukocyte subsets, CD4/CD8 ratio, immunoglobulin production and NK cell response. Int. J. Sports Med. 1995;16:466–474. doi: 10.1055/s-2007-973039. [DOI] [PubMed] [Google Scholar]
- Shephard R.J. Sepsis and mechanisms of inflammatory response: is exercise a good model? Br. J. Sports Med. 2001;35:223–230. doi: 10.1136/bjsm.35.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard R.J. Cytokine responses to physical activity, with particular reference to IL-6: sources, actions, and clinical implications. Crit. Rev. Immunol. 2002;22:165–182. [PubMed] [Google Scholar]
- Shephard R.J. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 2003;33:261–284. doi: 10.2165/00007256-200333040-00002. [DOI] [PubMed] [Google Scholar]
- Shephard R.J. Development of the discipline of exercise immunology. Exerc. Immunol. Rev. 2010;16:194–222. [PubMed] [Google Scholar]
- Shephard R.J., Kavanagh T., Mertens D.J., Qureshi S., Clark M. Personal health benefits of Masters athletics competition. Br. J. Sports Med. 1995;29:35–40. doi: 10.1136/bjsm.29.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Kimura F., Akimoto T. Effect of moderate exercise training on T-helper cell subpopulations in elderly people. Exerc. Immunol. Rev. 2008;14:24–37. [PubMed] [Google Scholar]
- Shiomi K., Nakazato M., Ihi T., Kangawa K., Matsuo H., Matsukura S. Establishment of radioimmunoassay for human neutrophil peptides and their increases in plasma and neutrophil in infection. Biochem. Biophys. Res. Commun. 1993;195:1336–1344. doi: 10.1006/bbrc.1993.2190. [DOI] [PubMed] [Google Scholar]
- Simon H.B. Exercise and infection. Phys. Sports Med. 1987;15:134–141. [Google Scholar]
- Simpson R.J. Ageing, persistent viral infections, and immunosenescence: can exercise “make space”? Exerc. Sport Sci. Rev. 2011;39:23–33. doi: 10.1097/JES.0b013e318201f39d. [DOI] [PubMed] [Google Scholar]
- Simpson R.J. The effects of exercise on blood leukocyte numbers. In: Gleeson M., Bishop N., Walsh N., editors. Exercise Immunology. Routledge; London: 2013. pp. 207–238. [Google Scholar]
- Simpson R.J., Florida-James G.D., Whyte G.P., Guy K. The effects of intensive, moderate and downhill treadmill running on human blood lymphocytes expressing the adhesion/activation molecules CD54 ICAM-1, CD18 beta2 integrin and CD53. Eur. J. Appl. Physiol. 2006;97:109–121. doi: 10.1007/s00421-006-0146-4. [DOI] [PubMed] [Google Scholar]
- Simpson R.J., Florida-James G.D., Cosgrove C., Whyte G.P., Macrae S., Pircher H. High-intensity exercise elicits the mobilization of senescent T lymphocytes into the peripheral blood compartment in human subjects. J. Appl. Physiol. 2007;103:396–401. doi: 10.1152/japplphysiol.00007.2007. [DOI] [PubMed] [Google Scholar]
- Simpson R.J., Cosgrove C., Ingram L.A., Florida-James G.D., Whyte G.P., Pircher H. Senescent T-lymphocytes are mobilised into the peripheral blood compartment in young and older humans after exhaustive exercise. Brain Behav. Immun. 2008;22:544–551. doi: 10.1016/j.bbi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Simpson R.J., McFarlin B.K., McSporran C., Spielmann G., ó Hartaigh B., Guy K. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav. Immun. 2009;23:232–239. doi: 10.1016/j.bbi.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Singh P.K., Tack B.F., McCray P.B., Welsh M.J. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- Sloan C.A., Engels J.J., Fahlman M.M., Yarandi H.E., Davis J.E. Effects of exercise on S-IGA and URS in postmenopausal women. Int. J. Sports Med. 2013;34:81–86. doi: 10.1055/s-0032-1314817. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R.J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J. Dent. Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Smith J.A. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- Smith J.A., Pyne D.B. Exercise, training, and neutrophil function. Exerc. Immunol. Rev. 1997;3:96–116. [PubMed] [Google Scholar]
- Smith J.A., Gray A.B., Pyne D.B., Baker M.S., Telford R.D., Weidemann M.J. Moderate exercise triggers both priming and activation of neutrophil subpopulations. Am. J. Physiol. 1996;270:838–845. doi: 10.1152/ajpregu.1996.270.4.R838. [DOI] [PubMed] [Google Scholar]
- Smits H.H., Grunberg K., Derijk R.H., Sterk P.J., Hiemstra P.S. Cytokine release and its modulation by dexamethasone in whole blood following exercise. Clin. Exp. Immunol. 1998;111:463–468. doi: 10.1046/j.1365-2249.1998.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Arnljots K., Cowland J.B., Bainton D.F., Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- Sorichter S., Martin M., Julius P. Effects of unaccustomed and accustomed exercise on the immune response in runners. Med. Sci. Sports Exerc. 2006;38:1739–1745. doi: 10.1249/01.mss.0000230213.62743.fb. [DOI] [PubMed] [Google Scholar]
- Spence L., Brown W.J., Pyne D.B., Nissen M.D., Sloots T.P., McCormack J.G. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med. Sci. Sports Exerc. 2007;39:577–586. doi: 10.1249/mss.0b013e31802e851a. [DOI] [PubMed] [Google Scholar]
- Staras S.A., Dollard S.C., Radford K.W., Flanders W.D., Pass R.F., Cannon M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Starkie R.L., Rolland J., Febbraio M.A. Effect of adrenergic blockade on lymphocyte cytokine production at rest and during exercise. Am. J. Physiol. Cell. Physiol. 2001;281:1233–1240. doi: 10.1152/ajpcell.2001.281.4.C1233. [DOI] [PubMed] [Google Scholar]
- Steensberg A., Toft A.D., Bruunsgaard H., Sandmand M., Halkjaer-Kristensen J., Pedersen B.K. Strenuous exercise decreases the percentage of type 1 T cells in the circulation. J. Appl. Physiol. 2001;91:1708–1712. doi: 10.1152/jappl.2001.91.4.1708. [DOI] [PubMed] [Google Scholar]
- Steppich B., Dayyani F., Gruber R., Lorenz R., Mack M., Ziegler-Heitbrock H.W. Selective mobilization of CD14+CD16+ monocytes by exercise. Am. J. Physiol. Cell Physiol. 2000;279:C578–C586. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- Stowe R.P., Pierson D.L., Barrett A.D.T. Elevated stress hormone levels relate to Epstein–Barr virus reactivation in astronauts. Psychosom. Med. 2001;63:891–895. doi: 10.1097/00006842-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Strugnell R.A., Wijburg O.L. The role of secretory antibodies in infection immunity. Nat. Rev. Microbiol. 2010;8:656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- Summers C., Rankin S.M., Condliffe A.M., Singh N., Peters A.M., Chilvers E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D.B., Fagarasan S. IgA synthesis: a form of functional immune adaptation extending beyond gut. Curr. Opin. Immunol. 2012;24:261–268. doi: 10.1016/j.coi.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Totsuka M., Nakaji S., Yamada M., Kudoh S., Liu Q. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999;87:1360–1367. doi: 10.1152/jappl.1999.87.4.1360. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Nakaji S., Yamada M., Liu Q., Kurakake S., Okamura N. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003;35:348–355. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- Svendsen I.S., Taylor I.M., Tønnessen E., Bahr R., Gleeson M. Training-related and competition-related risk factors for respiratory tract and gastrointestinal infections in elite cross-country skiers. Br. J. Sports Med. 2016;5013:809–815. doi: 10.1136/bjsports-2015-095398. [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tal T., Sharabani M., Aviram I. Cationic proteins of neutrophil azurophilic granules: protein-protein interaction and blockade of NADPH oxidase activation. J. Leukoc. Biol. 1998;63:305–311. doi: 10.1002/jlb.63.3.305. [DOI] [PubMed] [Google Scholar]
- Tanida T., Okamoto T., Okamoto A., Wang H., Hamada T., Ueta E. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J. Oral Pathol. Med. 2003;32:586–594. doi: 10.1034/j.1600-0714.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- Tao R., Jurevic R.J., Coulton K.K., Tsutsui M.T., Roberts M.C., Kimball J.R. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob. Agents Chemother. 2005;49:3883–3888. doi: 10.1128/AAC.49.9.3883-3888.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeuw W., Bosch J.A., Veerman E.C., Amerongen A.V. Neuroendocrine regulation of salivary IgA synthesis and secretion: implications for oral health. Biol. Chem. 2004;385:1137–1146. doi: 10.1515/BC.2004.147. [DOI] [PubMed] [Google Scholar]
- Thibault N., Harbour D., Borgeat P., Naccache P.H., Bourgoin S.G. Adenosine receptor occupancy suppresses chemoattractant-induced phospholipase D activity by diminishing membrane recruitment of small GTPases. Blood. 2000;95:519–527. [PubMed] [Google Scholar]
- Thiel S., Holmskov U., Hviid L., Laursen S.B., Jensenius J.C. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin. Exp. Immunol. 1992;90:31–35. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J.G., Villalta S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons B.W., Cieslak T. Human natural killer cell subsets and acute exercise: a brief review. Exerc. Immunol. Rev. 2008;14:8–23. [PubMed] [Google Scholar]
- Tingate T.R., Lugg D.J., Muller H.K., Stowe R.P., Pierson D.L. Antarctic isolation: immune and viral studies. Immunol. Cell Biol. 1997;75:275–283. doi: 10.1038/icb.1997.42. [DOI] [PubMed] [Google Scholar]
- Tintinger G.R., Theron A.J., Anderson R., Ker J.A. The anti-inflammatory interactions of epinephrine with human neutrophils in vitro are achieved by cyclic AMP-mediated accelerated resequestration of cytosolic calcium. Biochem. Pharmacol. 2001;61:1319–1328. doi: 10.1016/s0006-2952(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Tjabringa G.S., Vos J.B., Olthuis D., Ninaber D.K., Rabe K.F., Schalkwijk J. Host defense effector molecules in mucosal secretions. FEMS. Immunol. Med. Microbiol. 2005;45:151–158. doi: 10.1016/j.femsim.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Tomasi T.B., Trudeau F.B., Czerwinski D., Erredge S. Immune parameters in athletes before and after strenuous exercise. J. Clin. Immunol. 1982;2:173–178. doi: 10.1007/BF00915219. [DOI] [PubMed] [Google Scholar]
- Tomchek L.A., Hartman D.A., Lewin A.C., Calhoun W., Chau T.T., Carlson R.P. Role of corticosterone in modulation of eicosanoid biosynthesis and antiinflammatory activity by 5-lipoxygenase 5-LO and cyclooxygenase CO inhibitors. Agents Actions. 1991;34:20–24. doi: 10.1007/BF01993226. [DOI] [PubMed] [Google Scholar]
- Toy L.S., Mayer L. Basic and clinical overview of the mucosal immune system. Semin. Gastrointest. Dis. 1996;7:2–11. [PubMed] [Google Scholar]
- Travis S.M., Singh P.K., Welsh M.J. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr. Opin. Immunol. 2001;13:89–95. doi: 10.1016/s0952-7915(00)00187-4. [DOI] [PubMed] [Google Scholar]
- Tvede N., Kappel M., Halkjaer-Kristensen J., Galbo H., Pedersen B.K. The effect of light, moderate and severe bicycle exercise on lymphocyte subsets, natural and lymphokine activated killer cells, lymphocyte proliferative response and interleukin 2 production. Int. J. Sports Med. 1993;14:275–282. doi: 10.1055/s-2007-1021177. [DOI] [PubMed] [Google Scholar]
- Underdown B.J., Dorrington K.J. Studies on the structural and conformational basis for the relative resistance of serum and secretory immunoglobulin A to proteolysis. J. Immunol. 1974;112:949–959. [PubMed] [Google Scholar]
- Usui T., Yoshikawa T., Orita K., Ueda S.Y., Katsura Y., Fujimoto S. Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur. J. Appl. Physiol. 2011;111:2005–2014. doi: 10.1007/s00421-011-1830-6. [DOI] [PubMed] [Google Scholar]
- Van Reeth K. Cytokines in the pathogenesis of influenza. Vet. Microbiol. 2000;74:109–116. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- Verde T., Thomas S., Shephard R.J. Potential markers of heavy training in highly trained distance runners. Br. J. Sports Med. 1992;26:167–175. doi: 10.1136/bjsm.26.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli C., Varnier O., Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin. Infect. Dis. 2005;40:240–245. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H., Takase M., Tomita M. Lactoferricin derived from milk protein lactoferrin. Curr. Pharm. Des. 2003;9:1277–1287. doi: 10.2174/1381612033454829. [DOI] [PubMed] [Google Scholar]
- Walsh N.P., Blannin A.K., Clark A.M., Cook L., Robson P.J., Gleeson M. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J. Sports Sci. 1999;17:129–134. doi: 10.1080/026404199366226. [DOI] [PubMed] [Google Scholar]
- Walsh N.P., Bishop N.C., Blackwell J., Wierzbicki S.G., Montague J.C. Salivary IgA response to prolonged exercise in a cold environment in trained cyclists. Med. Sci. Sports Exerc. 2002;34:1632–1637. doi: 10.1097/00005768-200210000-00015. [DOI] [PubMed] [Google Scholar]
- Walsh N.P., Montague J.C., Callow N., Rowlands A.V. Saliva flow rate, total protein concentration and osmolality as potential markers of whole body hydration status during progressive acute dehydration in humans. Arch. Oral. Biol. 2004;49:149–154. doi: 10.1016/j.archoralbio.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Walsh N.P., Gleeson M., Pyne D.B., Nieman D.C., Dhabhar F.S., Shephard R.J. Position statement. Part two: maintaining immune health. Exerc. Immunol. Rev. 2011;17:64–103. [PubMed] [Google Scholar]
- Walsh N.P., Gleeson M., Shephard R.J., Woods J.A., Bishop N.C., Fleshner M. Position statement. Part one: immune function and exercise. Exerc. Immunol. Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Ward P.P., Paz E., Conneely O.M. Multifunctional roles of lactoferrin: a critical overview. Cell. Mol. Life Sci. 2005;62:2540–2548. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K., Carville K., Bowman J., Jacoby P., Riley T.V., Leach A.J. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatr. Infect. Dis. J. 2006;25:782–790. doi: 10.1097/01.inf.0000232705.49634.68. [DOI] [PubMed] [Google Scholar]
- Weiss S.J. Tissue destruction by neutrophils. N Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- West N.P., Pyne D.B., Renshaw G., Cripps A.W. Antimicrobial peptides and proteins, exercise and innate mucosal immunity. FEMS Immunol. Med. Microbiol. 2006;48:293–304. doi: 10.1111/j.1574-695X.2006.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West N.P., Pyne D.B., Kyd J.M., Renshaw G.M., Fricker P.A., Cripps A.W. The effect of exercise on innate mucosal immunity. Br. J. Sports Med. 2010;44:227–231. doi: 10.1136/bjsm.2008.046532. [DOI] [PubMed] [Google Scholar]
- Winther B., Gwaltney J.M., Jr., Mygind N., Turner R.B., Hendley J.O. Sites of rhinovirus recovery after point inoculation of the upper airway. J. Am. Med. Assoc. 1986;256:1763–1767. [PubMed] [Google Scholar]
- Woods J.A., Ceddia M.A., Kozak C., Wolters B.W. Effects of exercise on the macrophage MHC II response to inflammation. Int. J. Sports Med. 1997;18:483–488. doi: 10.1055/s-2007-972668. [DOI] [PubMed] [Google Scholar]
- Woods J.A., Evans J.K., Wolters B.W., Ceddia M.A., McAuley E. Effects of maximal exercise on natural killer NK cell cytotoxicity and responsiveness to interferon-alpha in the young and old. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 1998;53:430–437. doi: 10.1093/gerona/53a.6.b430. [DOI] [PubMed] [Google Scholar]
- Woods J.A., Davis J.M., Smith J.A., Nieman D.C. Exercise and cellular innate immune function. Med. Sci. Sports Exerc. 1999;31:57–66. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- Woods J., Lu Q., Ceddia M.A., Lowder T. Special feature for the Olympics: effects of exercise on the immune system: exercise-induced modulation of macrophage function. Immunol. Cell Biol. 2000;78:545–553. doi: 10.1111/j.1440-1711.2000.t01-9-.x. [DOI] [PubMed] [Google Scholar]
- Woods J.A., Keylock K.T., Lowder T. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: the immune function intervention trial. J. Am. Geriatr. Soc. 2009;5712:2183–2191. doi: 10.1111/j.1532-5415.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi R., Shimizu K., Kimura F., Takemura M., Suzuki K., Akama T. Virus activation and immune function during intense training in rugby football players. Int. J. Sports Med. 2011;32:393–398. doi: 10.1055/s-0031-1271674. [DOI] [PubMed] [Google Scholar]
- Yang Y., Verkuilen J., Rosengren K.S. Effects of a Taiji and Qigong intervention on the antibody response to influenza vaccine in older adults. Am. J. Chin. Med. (Gard. City. N. Y.). 2007;354:597–607. doi: 10.1142/S0192415X07005090. [DOI] [PubMed] [Google Scholar]
- Yel L. Selective IgA deficiency. J. Clin. Immunol. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Zhou S., Davie A., Su Q. Effects of moderate and high intensity exercise on T1/T2 balance. Exerc. Immunol. Rev. 2012;18:98–114. [PubMed] [Google Scholar]


