Publisher Summary
Chinese hamsters are small rodents with a grayish black coat and a black dorsal stripe. Adult animals weigh approximately 39–46 gm, and measure approximately 9 cm in length. This species has been shown to be susceptible to a number of experimentally induced viral, bacterial, and parasitic infections. In recent years, the Chinese hamster's contributions as a laboratory animal have been largely overshadowed by the focus on its cell lines and the role it plays in scientific research and biotechnology. The Chinese hamster used in biomedical research is traditionally classified as Cricetulus griseus. It has several biological features that have helped promote its use in biomedical research and these attributes include its small size, polyestrous cycle, short gestation period, and low chromosome number. The Chinese hamster has a low incidence of spontaneous and endogenous viral infections. This species has been shown to be susceptible to a number of experimentally induced viral, bacterial, and parasitic infections. Chinese hamster-derived cells have played a major role in cytogenetic toxicity assays and the production of glycosylated therapeutic proteins. The behavior, research uses, and general toxicology of the Chinese hamster are summarized in this chapter.
Keywords: Chinese hamster, striped-back hamster, gray hamster, laboratory animal, infectious disease, epidemiology research, metabolic disease, diabetes mellitus, mutagenicity, carcinogenicity studies, tissue culture, Chinese hamster ovary (CHO) cells, recombinant proteins, therapeutic proteins
The Chinese hamster, also known as the striped-back or gray hamster, is indigenous to Northern Asia. Its small size, polyestrous cycle, short gestation period, and low chromosome number are among the biological attributes that have made it an invaluable laboratory animal for biomedical research. The Chinese hamster first played a role in infectious disease research to type pneumococci strains. It was subsequently used in a variety of infectious disease and epidemiological studies. With early attempts at inbreeding, a hereditary form of diabetes mellitus was identified in the species. The Chinese hamster subsequently became a useful tool for characterizing the metabolic disorder and developing therapies for the human disease. Due to its low chromosome number, Chinese hamster tissue cultures have been a popular research tool for mutagenicity and carcinogenicity studies. During the past 20 years, Chinese hamster ovary (CHO) cells have been utilized to synthesize a wide array of recombinant protein products. Therapeutic proteins derived from CHO cells are currently used to treat numerous human diseases.
Introduction
The Chinese hamster used in biomedical research is traditionally classified as Cricetulus griseus (Honacki et al., 1982), but some taxonomists (Nowack and Paradiso, 1983) do not recognize this species and classify these animals as Cricetulus barabensis. For the purpose of this chapter, the Chinese hamster will be referred to as Cricetulus griseus the classification used by most authors. The Chinese hamster, also known as the striped-back hamster (Figure 35.1 ), is indigenous to Northern Asia, with a range extending from southern Siberia, Mongolia, and northeastern China, to the northern regions of North Korea. The Chinese hamster has had an important and much storied role in biomedical research. However in recent years, this hamster’s contributions as a laboratory animal have been largely overshadowed by the focus on its cell lines and the role they play in scientific research and biotechnology.
Figure 35.1.

An adult Chinese hamster, Cricetulus griseus. Note the dark stripe on the dorsal midline.
Photo courtesy of Dr. Don Holmes, Department of Veteran Affairs.
The Chinese hamster has several biological features that have helped promote its use in biomedical research. These attributes include its small size, polyestrous cycle, short gestation period and low chromosome number (Chang et al., 1987). In addition, the Chinese hamster has a low incidence of spontaneous and endogenous viral infections. This species has been shown to be susceptible to a number of experimentally induced viral, bacterial, and parasitic infections (Diani and Gerritsen, 1987).
The Chinese hamster was first used as a laboratory animal to type pneumococci obtained from human patients (Hsieh, 1919). Subsequently, the Chinese hamster became a valuable animal model in other infectious disease and epidemiology research studies. This hamster is a known carrier of the protozoal parasite Leishmania that causes the often deadly human disease leishmaniasis, also known as Kala-azar or black fever.
Efforts at domestication, in the mid-20th century, resulted in the serendipitous finding that some inbred lines of Chinese hamsters develop spontaneous hereditary diabetes mellitus. This finding fostered interest in the Chinese hamster as an animal model for diabetes and helped spur research interest in hamster genetics (Yerganian, 1959, 1985).
The Chinese hamster has been used as an animal model in radiobiological research and has been shown to be more resistant to the effects of radiation exposure than many other common laboratory rodents (Corbascio et al., 1962). In addition, the Chinese hamster has been utilized for the experimental induction of gastric and esophageal tumors (Baker et al., 1974).
Tijo and Puck (1958) isolated Chinese hamster ovary (CHO) cells (Figure 35.2 ) which have since been commonly used as an in vitro research tool for mutagenesis and carcinogenesis studies. Subsequent laboratory investigations have led to a series of elegant research studies directed at utilizing CHO cells for the production of recombinant therapeutic proteins (Oka and Rupp, 1990). During the past 20 years, CHO cells have been used to synthesize a wide array of recombinant proteins that have found clinical application in the treatment of a variety of human diseases (Chin, 2007, Jayapal et al., 2007).
Figure 35.2.
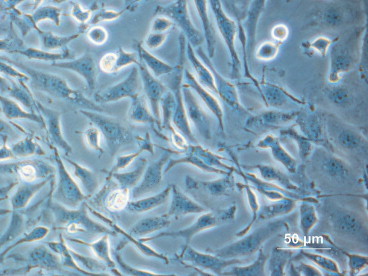
Chinese hamster ovary cell culture, phase contrast (positive) microscopy.
Photo courtesy of Wikimedia Commons and User Alcibindes.
Biology
General
Chinese hamsters are small rodents with a grayish black coat and a black dorsal stripe. Adult animals weigh approximately 39–46 grams, and measure approximately 9 cm in length. The average gestation length is 20.5 days, with a litter size of approximately 4–5 offspring. Newborn pups weigh approximately 1.5–2.5 grams. The average life span under laboratory conditions is 2.5–3.0 years (Harkness, 1984; Harkness and Wagner, 1985). An overview of physiological reproductive data is provided in Table 35.1 .
Table 35.1.
| Parameter | Mean ± SD |
|---|---|
| Erythrocytes (106/µl) | 7.1 ± 0.01 |
| Packed cell volume (%) | 42.1 ± 5.6 |
| Hemoglobin (g/dl) | 12.4 |
| Leukocytes (103/µl) | 5.5 |
| Neutrophils (%) | 19.3 ± 2.2 |
| Bands (%) | 0.2 ± 0.1 |
| Lymphocytes (%) | 76.1 ± 7.8 |
| Monocytes (%) | 2.1 ± 0.3 |
| Eosinophils (%) | 1.7 ± 0.7 |
| Basophils | 0.1 ± 0.04 |
| Sedimentation rate (mm/h) | 3.5 ± 1.7 |
| Bleeding time (s)b | 55 |
From Moore (1966).
From Yerganian et al. (1955).
The Chinese hamster, like the Syrian hamster, has a cheek pouch that can be used as an immunologically privileged site. Normal hematological parameters for the Chinese hamster are shown in Table 35.2 . In terms of distinctive anatomical features that have been quantitatively assessed, the brain and spleen in both sexes are relatively larger, with respect to body size, than those of the Syrian hamster (Festing, 1972). In addition, adult male Chinese hamsters have exceptionally large testicles.
Table 35.2.
Reproductive Physiological Data for the Chinese Hamstera
| Weaned | 21–25 daysb |
|---|---|
| Sexually mature | 8–12 weeksb |
| Type of estrous cycle | Polyestrusc |
| Duration of estrous cycle | 4 daysb |
| Length of estrus | 6–8 hoursb |
| Ovulation time | Immediately before estrusc |
| Copulation | 2–4 h after start of dark periodd |
| Implantation | 5–6 daysc |
| Gestation | 20.5 daysb |
| Average litter size | 4.5–5.2c |
| Number of mammae | 8c |
| Postpartum estrus | 4 daysb |
Table from Hankenson and Van Hoosier (2002); reprinted with permission from Academic Press.
From Yerganian (1958).
From Festing (1972).
From Moore (1965).
Behavior
Chinese hamsters, particularly sexually mature females, are bellicose and frequently fight with one another. Fights erupt frequently between females, and least often among males. The presence of females usually suppresses fighting behavior in males, whereas the presence of males has no effect on female aggression and fighting behavior.
The marking pattern used by the Chinese hamster involves vigorous scratching of the flank gland with a hindfoot, followed by pressing the anogenital region onto a substrate to transfer the scent-bearing secretion of the flank gland onto the substrate. In female–female encounters, marking frequency correlates with behavioral dominance. In female–male interactions, the male marks more frequently although the females are usually dominant in fights or chases. In typical female–female and male–male encounters, marking behavior follows rather than precedes engagement in fighting behavior. The lack of warning behaviors and the high level of food caching behavior observed in the Chinese hamster are suggestive that in their natural habitat this species lives a relatively solitary existence with infrequent encounters with conspecifics (Skirrow and Rysan, 1976).
Care and Husbandry
Housing
The Chinese hamster can be purchased commercially or bred under laboratory conditions. It does well under standard laboratory animal housing conditions. Since the Chinese hamster is comparable in size to the mouse, a similar type of caging and husbandry practices are commonly used, and have proven adequate for general housing. Hamsters with metabolic disorders such as diabetes are more susceptible to stress and special precautions must be taken in the provision of routine husbandry to optimize colony management for diabetic animals.
Feeding
There are no unique dietary requirements for the Chinese hamster. A standard, commercially available laboratory rodent diet provides adequate nutrition. Wheat germ may be used as a dietary supplement for breeders. Average daily water intake is 11.4 ml and 12.9 ml per 100 g body weight for male and female hamsters, respectively (Thompson, 1971).
Breeding
Early attempts to breed Chinese hamsters under laboratory conditions were unsuccessful. However, a reversed illumination schedule has been used to successfully establish a production colony for research purposes (Yerganian, 1958). Subsequently, several other schemes have been utilized to manage Chinese hamster breeding colonies due to the aggressiveness of the animals, particularly the females. Consequently, females with non-aggressive behavior (Calland, 1986) or sexually aggressive males (Moore, 1965) are selected as breeders. Modified test mating and monogamous mating schemes are used to breed the Chinese hamster (Camden et al., 1968, 1969). In one approach, a female is placed with a male for 7 days or longer, but the female is removed prior to delivery of the litter. In a second scheme, the male and female are left together during the entire reproductive life of the pair. Hand-mating and artificial insemination have been used with moderate success (Avery, 1968). A more practical and efficient approach to produce Chinese hamsters on a commercial scale has been reported (Cisar et al., 1972). In this system, three males are placed in a breeding cage with three to five female littermates at weaning; the authors reported a 3.5% loss of males per month.
Progesterone levels are markedly different during estrus and pregnancy. During the Chinese hamster’s 4-day estrous cycle, maximal progesterone synthesis occurs on day 3, in contrast to the low levels observed on day 3 of the Syrian hamster estrous cycle (Sato et al., 1984). During pregnancy, progesterone blood levels increase through day 12, with a general stabilization through day 18 of gestation. Progesterone blood levels peak on day 19 with a dramatic drop immediately prior to parturition (Sato et al., 1984). Breeding is confirmed by the presence of a vaginal plug. Pregnancy is indicated by a closed vagina, with dry, pale, and scaly perineal tissue at 4 days post-mating. In one study, an increase in ambient room temperature (above 82°F) has been shown to increase the number of runts in a litter (Yerganian, 1958).
Cytogenetics and Fetal Development
The Chinese hamster has been frequently selected for studies of chromosomal abnormalities because of a low spontaneous mutation rate and a low diploid chromosome number (2N = 22). The ten large pairs of autosomes and the two sex chromosomes can be readily differentiated. Chinese hamster chromosomes can be divided into four morphologically distinct subgroups (Vistorin et al., 1977). Research investigations suggest that cleavage of embryos with chromosome abnormalities is delayed compared with karyotypically normal zygotes (Sonta et al., 1984). A survey of 226 Chinese hamster preimplantation embryos indicates a low incidence (0.9%) of genome mutations (Binkert and Schmid, 1977). The low spontaneous chromosome mutation rate has made the Chinese hamster a good animal model for mutagen testing.
Characterization of the gametes and in vivo fertilization of the Chinese hamster have been reported (Yanagimachi et al., 1983). Fertilization begins approximately 2–3 hours after ovulation and is completed within the next 4–5 hours. At 20–26 hours following ovulation, the first three cleavages occur. Four days post-ovulation, the fertilized egg reaches the blastocyst stage and enters the uterus. Implantation occurs at approximately day 5 or 6 post-ovulation (Pickworth et al., 1968). Embryonic and fetal development of the Chinese hamster have been extensively characterized. Prenatal developmental stages are similar to the developmental stages in the mouse (ten-Donkelaar et al., 1979).
Diseases
Infectious Diseases
A Chinese hamster colony was evaluated for the presence of murine viral antibodies and intestinal/cecal microflora by Schiff et al. (1973). The presence of antibodies against several murine viruses was reported including: Pneumonia virus of mice (RNA virus, family Paramyxoviridae), Kilham rat virus (DNA, family Parvoviridae), Theiler’s murine encephalomyelitis virus (GDVII ; RNA virus, family Picornaviridae), H-1 (DNA virus; family Parvoviridae), and Reovirus-3 (RNA virus, family Reoviridae). No animals were found to have detectable antibody levels to Sendai virus (RNA virus, family Paramyxoviridae), Polyoma virus (DNA virus; family Papovaviridae), mouse adenovirus (DNA virus; family Adenoviridae), mouse hepatitis virus (RNA virus, family Coronaviridae), or lymphocytic choriomeningitis virus (RNA virus, family Arenaviridae). On assessment of 30 Chinese hamsters aged 2, 3, or 4 months from a colony of 200, Lactobacillus predominates as the major bacterial group identified following quantitative recovery from the intestinal tract. On examination of the ceca, several bacterial genera have been routinely identified including: Bacillus, Bacteroides, Escherchia, Lactobacillus, Micrococcus, and Streptococcus.
A Mycoplasma species (M. cricetula) was isolated from the conjunctiva and nasopharynx of 55 animals in one Chinese hamster colony by Hill (1974). The pathogenic significance of this organism has not been determined.
Zook et al. (1977) diagnosed Tyzzer’s disease in a colony of Chinese hamsters. Affected animals exhibit clinical signs of lethargy, diarrhea, rough hair coat, and hunched posture. Gross necropsy findings include multiple, disseminated white foci in the liver and distension of the large intestine with semi-liquid yellow feces. On microscopic examination, there is focal necrosis of hepatic cells with no significant cellular inflammatory response. Giemsa, toluidine blue, and methenamine silver stains reveal clusters of pleomorphic bacilli in the cytoplasm of hepatic cells adjacent to necrotic foci. The histopathological lesions are consistent with a diagnosis of Tyzzer’s disease (Clostridium piliformis).
There are few reports of spontaneous parasitic diseases in the Chinese hamster. In one survey, Schiff et al. (1973) consistently found Trichomonas spp. in 2-, 3-, and 4-month-old animals from a group of 200. An investigation by Benjamin and Brooks (1977) suggests a low incidence of demodectic mange in the Chinese hamster. Desch and Hurley (1997) identified a new species of mite, Demodex sinocricetuli, in the Chinese hamster. In their study, histopathologic evidence of demodectic mange is found in only two of 157 animals. The clinical significance of this organism has not been determined.
Neoplastic Diseases
Chinese hamsters have a low incidence of spontaneous tumors; when tumors occur the most common association is involvement with the liver or reproductive tract. The rarity of spontaneous and induced tumors has been postulated to be attributable to the absence of tumor-associated viruses in this species.
Uterine and Ovarian Tumors
Ward and Moore (1969) described spontaneous uterine adenocarcinomas in 30 of 120 aged female Chinese hamsters; approximately 10% of the affected hamsters had lung metastases. Benjamin and Brooks (1977) found similar uterine neoplastic lesions in 11 of 77 animals, however, no pulmonary metastases were observed. Brownstein and Brooks (1980) characterized and classified uterine neoplasms in 21 of 93 multiparous, non-inbred Chinese hamsters ranging in age from 3 to 4 years.
Animals with malignant uterine neoplasms frequently exhibit clinical signs of vaginal hemorrhage. Gross necropsy findings include hemorrhage within the peritoneal cavity and tumor spread by direct extension to other organs. On gross examination, the tumor tissue is usually white to yellow with a nodular firm surface. Brownstein and Brooks (1980) developed a histological classification system for uterine neoplasms of the Chinese hamster (Table 35.3 ). The most frequently observed uterine tumor in the aged Chinese hamster is endometrial adenocarcinoma. The Chinese hamster might be a useful model for studying endomyometrial neoplasms of women since the relative frequency and classification of uterine tumors in the two species is similar.
Table 35.3.
Classification of Endomyometrial Neoplasms in 21 Chinese Hamstersa
| Site | Type | Occurrenceb | Metastasis |
|---|---|---|---|
| Endomyometrium | Adenocarcinoma | 13 | Abdominal spread (10) |
| Mixed adeno-squamous carcinoma | 3 | Abdominal spread (3) | |
| Mixed Mullerian carcinosarcoma | 2 | Abdominal spread (1) | |
| Lung metastasis (1) | |||
| Mixed mesodermal | 1 | Abdominal spread | |
| Myometrium | Leiomyoma | 1 | |
| Leiomyosarcoma | 1 | Abdominal spread |
From Brownstein and Brooks (1980); reprinted with permission from the Journal of the National Cancer Institute.
From a group of 93 nulliparous non-inbred females with a median survival time of 1040 days.
Kohn and Guttman (1964) report that the incidence of ovarian tumors is significantly increased following X-ray irradiation under several different exposure regimens, however tumors are rarely observed in control animals.
Hepatocellular Hyperplasia and Adenoma
Kohn and Guttman (1964) describe a benign liver lesion, hepatocellular adenoma, in five of 112 non-irradiated control animals but did not report any non-neoplastic liver lesions. Ward and Moore (1969) observed hepatocellular adenoma in 18 of 50 non-irradiated control animals; they found a similar incidence in animals receiving 131I. They also reported hepatocellular hyperplasia in some animals which they postulate might be a pre-neoplastic lesion. Nodular hepatic hyperplasia, a non-neoplastic lesion, is reported in 111 of 157 aging animals in a survey conducted by Benjamin and Brooks (1977). They found evidence of hepatic adenoma in only one of the 157 animals. Grossly, the two conditions appear similar with multiple nodules ranging in size from less than 1 mm to greater than 1 cm in diameter, and lighter in color than the surrounding tissue. Histologically, these are nodular hyperplastic lesions ranging from small nodules with normal sinusoid and central vein structure to coalescing masses with disrupted tissue architecture. Parenchymal cells are often enlarged with increased eosinophilic and sometimes vacuolated cytoplasm. Cell nuclei are enlarged but otherwise had a benign appearance. Nodular hyperplasia was distinguished from hepatocellular adenoma by the appearance of abundant hyperchromatic nuclei containing multiple nucleoli. Ward and Moore (1969) also distinguished between nodular hyperplasia and hepatic adenomas. In their investigation they found a much higher incidence of hepatic adenomas. Since the Chinese hamster is used in toxicology studies, it is important to distinguish spontaneous hepatic lesions from experimentally induced lesions.
Myeloproliferative Disease and Leukemia
Benjamin and Brooks (1977) report hematopoietic hyperplasia in 49 of 157 Chinese hamsters. Bone marrow hyperplasia occurred in 36 animals. In most cases, the hyperplasia is granulocytic. In approximately half the cases, the hematopoietic hyperplasia is associated with degenerative or inflammatory changes; in the other cases no underlying lesions are observed. Splenomegaly and a pale bone marrow are observed grossly in all clinical cases. On microscopic examination, the marrow is hypercellular and granulocytic cells replace erythroid cells. A diagnosis of leukemia is made in three cases; myelocytes and myeloblasts with leukemic infiltrates are found in liver, kidney, brain, spinal cord, and spleen. The Chinese hamster might provide a useful model for the study of myelogenous leukemia.
Other Neoplasms
Table 35.4 provides a summary of some infrequently occurring, spontaneous malignancies that have been reported for the Chinese hamster.
Table 35.4.
Infrequently Occurring Spontaneous Malignancies of the Chinese Hamstera
| Tumor type | Occurrence | Reference |
|---|---|---|
| Adrenal cortical carcinoma | 1/1574/112 | Benjamin and Brooks (1977)Kohn and Gultman (1964) |
| Pancreatic adenocarcinoma | 2/3 | Poel and Yerganian (1961) |
| Sweat gland adenocarcinoma | 1/157 | Benjamin and Brooks (1977) |
| Squamous cell carcinoma | 1/157 | Benjamin and Brooks (1977) |
| Subcutaneous fibrosarcoma | 1/157 | Benjamin and Brooks (1977) |
| Undifferentiated sarcoma | 1/157 | Benjamin and Brooks (1977) |
| Gastric adenocarcinoma | 1/112 | Kohn and Guttman (1964) |
| Bile duct carcinoma | 1/112 | Kohn and Guttman (1964) |
| Ovarian adenoma | 1/77 | Benjamin and Brooks (1977)b |
From Ladiges (1987); reprinted with permission from Elsevier.
Kohn and Guttman (1964) reported the occurrence of ovarian tumors in 18 of 74 irradiated females, but none in 69 control animals.
Metabolic and Genetic Disease
Spontaneous Diabetes Mellitus
Spontaneous diabetes mellitus was observed in Chinese hamsters during early attempts to establish inbred breeding colonies. This metabolic condition is a suitable model for studying various aspects of the human disease, and will be discussed in more detail in the section on research uses. Since diabetes is a spontaneous disease in the Chinese hamster, the occurrence of this metabolic disorder might potentially confound experimental results in animals used for research protocols unrelated to diabetes. In addition, the use of Chinese hamsters with spontaneous diabetes mellitus in cytogenetic experiments might have a confounding influence on experimental results due to metabolic aberrations associated with the diabetic state.
Primary Chromosomal Abnormalities
Mikamo and Kamiguchi (1983) studied the occurrence of spontaneous chromosomal aberrations in Chinese hamster oocytes and zygotes, and classified the lesions according to their causal mechanism. A summary of this classification is provided in Table 35.5 . The overall incidence of chromosomal anomalies has been determined to be 8%. This incidence is lower than that estimated for the human population; the lower incidence has been postulated to be due to the lack of mutagen influence in a controlled laboratory environment. Since the Chinese hamster is frequently used as an animal model for cytogenetic studies, it is important to be cognizant of the incidence of spontaneous chromosomal defects in this species.
Table 35.5.
Primary Incidence of Spontaneous Chromosomal Anomalies, Classified According to Their Origins and Causal Mechanisms in the Chinese Hamstera
| Chromosomal Anomaly | Origin and Causal Mechanism | Incidence (%) |
|---|---|---|
| Aneuploidy | Non-disjunction and anaphase lagging | |
| During oogenesis | 2.1 | |
| During spermatogenesis | 0.7 | |
| Mosaic | Non-disjunction and anaphase lagging | |
| During first somatic division | 0.5 | |
| Triploidy | Diandry | 0.9 |
| Digyny | 0.3 | |
| Uncertain | 0.3 | |
| Haploidy | Parthenogenesis | 0.5 |
| Structural anomaly | Breakage during oogenesis | 1.3 |
| Breakage during spermatogenesis | 1.4 | |
| Total | 8.0 |
From Mikamo and Kamiguchi (1983); reprinted with permission from Elsevier.
Traumatic Diseases
Female littermates can become very aggressive as they reach sexual maturity. Fighting and severe bite wounds may lead to death. In addition, females can be very aggressive following mating with resultant death of male breeders. Separation of litters before fighting occurs, and removal of the male following mating are among the strategies used to prevent serious injury and death. Selection of non-aggressive female breeders through genetic selection has also been utilized as a strategy to reduce fighting and traumatic injuries in Chinese hamster breeding colonies.
Reproductive Disorders
Avery (1968) reported infertility in young female Chinese hamsters housed in open-bottom cages. Infertility was believed to be attributable to excess hair growth around the vulva that prevented penile penetration during copulation. Infertility has also been associated with diabetic females in inbred colonies. Parkening (1982) studied aging changes and the effect on reproductive performance. Clinical signs include failure to conceive or decreased conception rates. Cage type and colony health status have been shown to have an impact on conception rates. Progesterone administration, immediately following mating, has been used with some success to increase conception in inbred and/or diabetic breeders, and in aged animals (Avery, 1968; Parkening, 1982). Testicular degeneration and atrophy has been observed in 14 of 80 male Chinese hamsters in an aging colony of outbred hamsters (Benjamin and Brooks, 1977).
Miscellaneous Diseases
Pulmonary Granulomas
Pulmonary granulomas have been observed in 54 of 157 aging Chinese hamsters (Benjamin and Brooks, 1977). On gross examination, the lesions are subpleural, 1–3-mm yellow-gray foci with variable degrees of lung involvement. Histologically, mild lesions consist of lipid-filled macrophages, while more severe lesions include well-developed granulomas containing lipid-filled macrophages, inflammatory cells, focal septal fibrosis, and occasional cholesterol clefts. Several animals exhibit severe alveolar and bronchiolar epithelial hyperplasia. The cause of the pulmonary granulomas has not been determined. The significance of this spontaneous lesion should be differentiated from pathology attributable to experimentally induced causes.
Cerebral Hemorrhage
Cerebral hemorrhage is observed in 20% of both control and experimental animals in a 131I chronic toxicity study (Ward and Moore, 1969). Sporadic deaths are observed in 1–2-year-old animals. The cause of the cerebral hemorrhage has not been determined but is postulated to involve an inflammatory or degenerative change in the anterior cerebral artery. On gross examination, hemorrhage is observed between the cerebral hemispheres with blood often present in the lateral ventricles. On histological examination, the lesions are restricted to the anterior cerebral artery. In acute cases, the vascular changes include an increased arterial lumen diameter, and thickened arterial wall with the presence of inflammatory cells. An amorphous eosinophilic material is often present in the media of the arterial wall. The eosinophilic material is periodic acid-Schiff (PAS)-reagent-positive but fibrin-negative. In chronic cases, the arterial wall is greatly thickened with numerous macrophages surrounding the lesions. In affected animals, no vascular changes are found elsewhere in the brain or in other organs. The incidence of cerebral hemorrhage is not known since this clinical condition has not been reported in other Chinese hamster colonies.
Degenerative Renal Disease
Intercapillary Glomerulosclerosis
Intercapillary glomerulosclerosis has been reported in Chinese hamsters in association with diabetes mellitus (Meier and Yerganian, 1961). It has also been reported as a progressive condition associated with aging (Guttman and Kohn, 1960; Kohn and Guttman, 1964).
Nephrosclerosis
Nephrosclerosis is described in 46 of 157 Chinese hamsters in one colony of animals older than 600 days (Benjamin and Brooks, 1977). Incidence and severity of the condition are age-dependent. Early changes include tubule degeneration, mild interstitial fibrosis, and cortical atrophic foci. Progressive pathologic changes consist of glomerular sclerosis, severe interstitial fibrosis, and severe tubular degeneration and atrophy. Severely affected kidneys are small in size, pale tan, and have a pitted surface.
Periodontitis
Cohen et al. (1961) describe periodontitis in an inbred strain of Chinese hamsters with hereditary diabetes mellitus. Incidence correlate with the presence and severity of the underlying metabolic disorder. The periodontitis in the Chinese hamster corresponds closely to the development of the clinical condition in humans affected by diabetes mellitus.
Spondylosis
Silberberg and Gerritsen (1976) report an increased incidence of spondylosis in an inbred strain of Chinese hamsters with spontaneous diabetes mellitus compared to non-diabetic control animals.
Research Uses
Infectious Diseases
There are few reports of spontaneously occurring infectious diseases in Chinese hamsters. However, this species has been experimentally shown to be susceptible to a variety of different infectious agents and has been used extensively in viral, bacterial, and parasitological disease investigations. Table 35.6 provides a list of some infectious disease agents that have been studied experimentally using the Chinese hamster as an animal model.
Table 35.6.
Representative Experimental Infectious Diseases in the Chinese Hamster
| Disease agent | Reference |
|---|---|
| Viral | |
| Rabies virus | Yen (1936) |
| Influenza | Yen (1940) |
| Viral encephalitis virus | Chang et al. (1951) |
| Scrapie virus | Chandler and Turfrey (1972) |
| Rous sarcoma virus | Hillova et al. (1985) |
| Transmissible mink encephalopathy virus | Kimberlin (1987) |
| Corona virus | Luo (2007) |
| Bacteria | |
| Pneumococcus | Hsieh (1919) |
| Corynebacterium diphtheria | Lu and Zia (1935) |
| Mycobacterium tuberculosis | Wang (1951) |
| Leptospira spp. | Plesko (1977) |
| Parasites | |
| Leishmania sp. | Hindle et al. (1926) |
| Monila sp. | Kurotchkin and Lin (1930) |
| Trichinella spiralis | Ritterson (1968) |
| Hymenolepsis microstoma | Ritterson (1971) |
| Babesia microti | Krampitz and Baumler (1978) |
| Toxoplasma gondii | Fuji et al. (1983) |
| Schistosoma mansoni | Crabtree and Wilson (1984) |
| Trichinella pseudospiralis | Stewart and Larsen (1989) |
| Acanthamoeba sp. | Van Klink et al. (2007) |
Metabolic Disorders
Spontaneous Diabetes Mellitus
Spontaneous hereditary diabetes mellitus, with similarities to the human disease, was first described in Chinese hamster inbred lines by Meier and Yerganian (1959). The diabetic Chinese hamster has been studied extensively to elucidate the pathophysiology associated with the disease and to develop effective clinical therapies for treatment of the human disease. Numerous reviews of the spontaneously diabetic Chinese hamster model have been published (Sirek and Sirek, 1967, Sirek and Sirek, 1971, Wappler and Fiedler, 1972, Hunt et al., 1976, Chang, 1978, Chang, 1981, Gerritsen, 1982, Diani and Gerritsen, 1987).
Pathophysiology
The diabetic Chinese hamster has been described as an inbred, lean animal that exhibits glucose intolerance, mild to severe hyperglycemia, hypoinsulinemia, polyuria, glycosuria, possible ketonuria, and high free fatty acid levels (Dulin and Gerritsen, 1967, Gerritsen and Dulin, 1967). Several genetic and environmental factors have been demonstrated to play a role in this metabolic disturbance in the hamster and influence the extent of beta cell death. These factors include the amount of food consumed, dietary composition, and uterine environment of the dam (Dulin and Gerritsen, 1967, 1972; Gerritsen and Dulin, 1967, Gerritsen and Dulin, 1972, Gerritsen et al., 1970, Gerritsen et al., 1974, Gerritsen et al., 1976, Gerritsen et al., 1981). Plasma insulin and glucagon levels have been shown to be altered in chronically diabetic animals. In general, diabetic Chinese hamsters exhibit low, non-fasting plasma insulin levels (Gerritsen and Dulin, 1967, Gerritsen and Blanks, 1974, Gerritsen, 1982). Plasma insulin levels of newly diagnosed diabetic Chinese hamsters are usually within normal limits or slightly elevated (Gerritsen and Blanks, 1974, Gerritsen, 1982). Plasma glucagon levels are usually elevated with chronic disease (Chang et al., 1977a). The chronic absolute deficiency of plasma insulin and the high plasma glucagon levels in the diabetic Chinese hamster are similar to endocrinologic changes observed for human type I diabetes. Pancreatic hormone levels are markedly altered in the chronic diabetic state. Pancreatic insulin levels are decreased, whereas pancreatic glucagon levels are generally elevated, particularly with chronic disease. Pancreatic somatostatin concentrations are significantly decreased in diabetic Chinese hamsters (Petersson et al., 1977).
Pathology
A number of studies using the Chinese hamster have focused on structural changes in the islets of Langerhans. Primary morphological alterations are observed in the β cells, which degranulate as the disease progresses (Boquist, 1969, Like et al., 1974a, Like et al., 1974b, Malaisse et al., 1967). The defective β cells in the Chinese hamster are characterized by proliferation of rough endoplasmic reticulum, expansion of the Golgi apparatus, and glycogen deposition (Boquist, 1969, Luse et al., 1967, Soret et al., 1974). The islets are reduced in size due to β-cell death (Carpenter et al., 1967, Carpenter et al., 1970). Some peripheral tissues and organs have been studied by light and/or electron microscopy, to better characterize the diabetic Chinese hamster. Glycogen deposition in the renal tubules is the most consistent change in the kidney (Soret et al., 1973, Soret et al., 1974). Examination of the eyes of diabetic Chinese hamsters documents the presence of retinal lesions. The most consistent finding involves glycogen deposition within Müller cells of the outer nuclear layer (Soret et al., 1973, Soret et al., 1974). Structural changes have been identified in the central, peripheral, and autonomic nervous systems of diabetic Chinese hamsters. Alterations of the hypothalamic–hypophyseal axis may provide the basis for the development of diabetic syndrome in this animal model (Bestetti and Rossi, 1982, Deslex and Rossi, 1976, Luse et al., 1970). Extensive studies of the autonomic nervous system, including both qualitative and morphometric, have been conducted in the diabetic Chinese hamster. Aberrant myelination and axonal degeneration of pelvic viscera may contribute to urinary bladder distension in the diabetic Chinese hamster (Dail et al., 1977). Reduction of myelinated and unmyelinated fiber size in the abdominal sympathetic trunk and ventral abdominal vagus nerve might be a causative factor underlying gastrointestinal malfunction in the diabetic Chinese hamster (Diani et al., 1981, Diani et al., 1984).
Genetics
The original hypothesis for diabetes in the Chinese hamster proposed that a single recessive gene and certain modifier genes are responsible for the syndrome (Meier and Yerganian, 1959, Meier and Yerganian, 1961a, Meier and Yerganian, 1961b, Yerganian, 1964). However, based upon epidemiologic examination of diabetic animals from other colonies, a polygenic hypothesis has been proposed for the disease. This hypothesis states that the Chinese hamster possesses a minimum of four recessive diabetogenic genes. In the homozygous state, any two of the genes produce a glycosuric hamster. Extensive genetic studies involving backcrosses have now shown that certain sublines of hamsters possess a single recessive gene for diabetes, whereas other sublines possess two recessive genes (Gerritsen et al., 1984). The onset of glycouria in the diabetic hamsters is regulated by a multi-gene system. Inbred non-diabetic Chinese hamsters appear to lack any recessive genes for diabetes.
Effect of Diet
Dietary modification has been shown to have a pronounced effect on diabetes development in this animal model. In prediabetic hamsters, hyperphagia is often observed. By limiting feed consumption to that of a non-diabetic hamster, a delayed onset of glycosuria and prevention of urinary ketones is achieved (Gerritsen and Blanks, 1974, Gerritsen, 1975, Gerritsen and Dulin, 1972). In another study, dietary restriction was shown to delay the development of glycosuria and hyperglycemia. Hyperinsulinemia is typically not observed in non-hyperphagic prediabetic animals (Gerritsen et al., 1981). Metabolic complications associated with diabetes are reduced or prevented by changes in dietary composition. A reduction in dietary fat and replacement of animal fat with vegetable fat in the diet ameliorates or prevents ketonuria (Gerritsen et al., 1981, Grodsky et al., 1974).
Therapy
The diabetic Chinese hamster is an insulin-deficient model of diabetes mellitus. Treatment with exogenous insulin has a beneficial effect on the metabolic alterations. Severely diabetic animals treated with Neutral Protamine Hagedorn (NPH) insulin exhibit a substantial reduction in glycosuria (Gerritsen and Dulin, 1966). In other investigations, continuous insulin infusion for 7 or 14 days was demonstrated to elicit improvements in blood glucose levels and endogenous insulin release from diabetic animals (Bringer et al., 1981; Frankel et al., 1979). However, insulin treatment of prediabetic Chinese hamsters does not delay the onset or the severity of glycosuria or hyperglycemia (Frankel and Grodsky, 1979).
Chemically Induced Diabetes Mellitus
Several chemical agents have been used to induce the metabolic and pathologic complications of diabetes mellitus in hamsters which do not possess the genes for diabetes. Experimentally induced clinical abnormalities include hyperglycemia, glucosuria, abnormal plasma insulin levels, and β cell abnormalities. These abnormalities are similar to those observed in chemically induced diabetic rats and mice. Diabetogenic substances include: streptozotocin, N-nitrosomethylurea, alloxan, monosodium glutamate, cortisone, and zinc deficiency.
Streptozotocin
Streptozotocin, an antibiotic derived from Streptomyces achromogenes, has a direct cytotoxic effect on pancreatic islet β cells of the Chinese hamster. A single intravenous streptozotocin injection (75 mg/kg) into fasted animals causes a decrease in insulin levels, hyperglycemia, an increase in serum free fatty acids, and an elevation in hepatic glucose-6-phosphatase activity. There is no observed concurrent degranulation and degeneration of islet β cells (Losert et al., 1971; Richter et al., 1971). A transient glycosuria is produced with low-dose streptozotocin (25 mg/kg) administered in concert with NPH insulin therapy (Berman et al., 1973). In several studies, streptozotocin was administered to adult animals as a single dose (175 or 200 mg/kg). Changes indicative of diabetogenesis included hyperglycemia, glycosuria, and pancreatic islet changes affecting both α and β cells. Further, pancreatic insulin and glucagon levels were significantly reduced (Dulin and Soret, 1977). Others have shown that, following treatment with streptozotocin, the β cells undergo severe degeneration characterized by cytoplasmic vacuolization, nuclear pyknosis, and cell necrosis (Wilander and Boquist, 1972).
Alloxan
Alloxan, a pyrimidine nucleic acid, has a cytotoxic effect on cells and has been used to induce diabetes in the Chinese hamster. Intramuscular or intraperitoneal injection of a single alloxan dose (300 mg/kg) is shown to cause a temporary, modest rise in blood glucose levels in the Chinese hamster (Boquist, 1967). Alloxan is considered a transitory diabetogenic agent in the Chinese hamster (Boquist, 1968, Boquist and Falkmer, 1970). Normalization of blood glucose and regeneration of islet β cells are shown to occur within several weeks of alloxan administration.
Monosodium Glutamate
A single subcutaneous injection of monosodium glutamate (4 mg/kg) into newborn Chinese hamsters causes a marked change in the hypothalamus including severe necrosis and loss of nerve cell bodies in the arcuate and ventromedial hypothalamic nuclei (Komeda et al., 1980). These pathological changes are suggestive that the hypothalamus plays a central role in the development of monosodium glutamate-induced diabetes.
General Toxicology
The non-diabetic Chinese hamster has received considerable attention as a rodent model for the toxicological evaluation of a wide range of agents. Its usefulness is attributable to its small size, relatively long lifespan, and ease of care and handling. The Chinese hamster has been utilized to evaluate a wide range of chemicals and physical agents including radioisotopes, X-irradiation, microwaves, environmental pollutants, therapeutic drugs, pesticides, and potent tumorigenic agents. Table 35.7 provides an overview of some select general toxicology studies conducted in the Chinese hamster.
Table 35.7.
Chemical Agents (Environmental Pollutants and Therapeutic Drugs) Which have been Tested for their Ability to Produce Genetic Aberrations in Chinese Hamsters
| Agent | Genetic Aberration | Reference |
|---|---|---|
| 3,4-benzopyrene | Sister chromatid exchange in bone marrow cells | Bayer et al. (1981) |
| 7,12-dimethyl-benzanthracene | Chromosome breakage in bone marrow cells | Kato et al. (1969) |
| Formaldehyde | Chromatid gaps, breaks and exchanges of ovary cells | Natarajan et al. (1983) |
| Phenanthrene | Sister chromatid exchange in bone marrow cells | Bayer et al. (1978) |
| Styrene | None | Norppa et al. (1980) |
| Vinyl chloride | Chromatid breaks, fragments, gaps, deletions and sister chromatid exchange in bone marrow cells | Basler and Rohrborn (1980) |
| Acyclovir | Chromosome breakage in bone marrow cells | Clive et al. (1983) |
| Benzodiazepine | None | Schmid and Staiger (1969) |
| Cyclophosphamide | Chromosome gaps and exchanges in bone marrow cells and spermatogonia | Miltenburger et al. (1981) |
| Phenylbutazone | None | Muller and Strasser (1971) |
| Praziquantel | None | Machemer and Lorke (1978) |
| Quinine | None | Munzer and Renner (1983) |
| Sulfonylureas | Sister chromatid exchange in bone marrow cells | Renner and Munzer (1980) |
Genetic Toxicology
The Chinese hamster has been widely used for cytogenetic testing. It is an ideal candidate for genetic toxicology studies, due to its well-defined, stable karyotype, short gestation period, large chromosomes, and low chromosome number. Cytogenetic testing requires careful attention to a number of factors including: growth conditions, controls, doses, treatment conditions, and the time interval between treatment and sample analysis. In vivo assays for chromosomal aberrations involve treatment of intact animals and later collection of intact cells for cytogenetic analysis (Kirkland et al., 1990; Tice et al., 1994). The main advantage of an in vivo cytogenetic assay is that mammalian metabolism, DNA repair, and pharmacodynamics can be evaluated. The target is tissue from which large dividing cells can be easily prepared. Bone marrow from rats, mice, or Chinese hamsters has been most commonly used.
Cytogenetic assays have been developed utilizing mammalian cell lines to identify cells that have experienced genetic damage (e.g., chromosomal aberrations, micronuclei, sister chromatid exchanges, or changes in chromosome number). A large body of literature exists on the effects of a variety of chemical insults on chromosome composition in Chinese hamster cells. These insults include radiation, environmental pollutants, therapeutic drugs, pesticides, mycotoxins, and other agents. Metaphase analysis is used in conventional cytogenetics to detect chromosomal anomalies, especially unstable chromosomes or chromosomal aberrations.
Two in vitro assays for chromosomal damage have been developed in cloned Chinese hamster ovary cells to identify chemicals capable of inducing chromosomal aberrations or sister chromatid exchanges in mammalian cells. Both assays are described in detail by Galloway et al., 1985, Galloway et al., 1987.
Chromosomal Aberration (CA) Test
Since many birth defects and most cancers are associated with abnormal chromosome complements, it is important to identify chemicals that can induce chromosome damage. To assess induction of chromosomal aberrations, cells are harvested during the first mitotic division after initiation of chemical exposure. The CA assay only detects structural chromosomal damage; it does not detect changes in chromosome number (aneuploidy) induced by chemical exposure.
Sister Chromatid Exchange (SCE) Test
Sister chromatid exchanges are a measure of DNA damage. Increased levels of DNA damage are associated with mutation induction and cancer. Sister chromatid exchange assays require examination of cells that have entered their second mitotic division following the initiation of chemical exposure, and assessment of the number of chromatid exchanges that have occurred.
Cytogenetics of Germ and Somatic Cells
A large body of cytogenetic literature exists for the Chinese hamster due to the small number of chromosomes present in this rodent species as well as a low rate of spontaneous chromosomal anomalies. The Chinese hamster has a small number of chromosomes compared with humans and other vertebrates, which has permitted in-depth analysis of banding patterns and systematic analysis of mitosis, meiosis, spermatogenesis, and oogenesis. Table 35.8, Table 35.9 summarize select cytogenetic studies performed on germ and somatic cells of the Chinese hamster.
Table 35.8.
Cytogenetic Studies of Male and Female Germ Cells in Chinese Hamstersa
| Study | Reference(s) |
|---|---|
| Chromosome activities during meiosis of male germ cells | Jhanwar et al. (1981) |
| Chromosome activities during meiosis of female germ cells | Mikamo and Sugawara (1980) |
| Chromosome anomalies of oocytes | Mikamo and Kamiguchi (1983) |
| Chromosome activities during spermatogenesis | Murkherjee and Ghosal (1969); Barcellona and Brinkley (1973) |
| Chromosome banding patterns of sperm | Utakoji (1966); Pathak et al. (1976); Vistorin et al. (1982) |
| Cytochemistry and behavior of dense bodies in sperm | Takanari et al. (1982) |
| Karyotyping of synaptonemal complex in sperm | Dresser and Moses (1980) |
From Diani and Gerritsen(1987); reprinted with permission of Elsevier.
Table 35.9.
Cytogenetic Studies of Somatic Cells in the Chinese Hamstera
| Study | Reference(s) |
|---|---|
| Banding pattern of chromosomes | Raicu (1970); Kakati and Sinha (1972); Ray and Mohandis (1976); Gamperl et al. (1978) |
| Chromosome activities during mitosis | Bregman (1972); Vig and Miltenburger (1976); Jhanwar and Chaganti (1981) |
| Chromosome analysis of Rous sarcoma virus | Kato (1968) |
| Cytotaxonomy | Yerganian (1972) |
| DNA replication | Utakoji and Hsu (1965) |
| Effect of bromodeoxyuridine on chromosomes | Lin et al. (1984) |
From Diani and Gerritsen (1987); reprinted with permission of Elsevier.
Cell Lines Derived from Chinese Hamster Tissues and Organs
Puck et al. (1958) obtained a female Chinese hamster from Yerganian’s laboratory and used it to derive the original Chinese hamster ovary (CHO) cell line. Cell lines derived from Chinese hamster tissues and organs have been utilized for in vitro experimentation in a wide range of scientific disciplines including cytogenetics, toxicology, immunology, and cell physiology. The Chinese hamster ovary (CHO) and V-79 lung fibroblast are two cell lines derived from the Chinese hamster that have been used extensively in biomedical research (Bradley et al., 1981).
The adaptive ability of CHO cells and their ease of maintenance have been utilized in many basic biomedical research disciplines. G-coupled protein receptors and their signaling pathways have been studied by stable expression in CHO cells, and have contributed to advances in the fields of anesthesiology and pharmacology, and ultimately to patient therapy (Figler et al., 2003, Hornigold et al., 2003, Schulte and Fredholm, 2003). CHO cells serve as a tool for understanding cytoskeletal structure, adhesion, and motility, and have provided a better understanding of cancer biology and treatment (Hari et al., 2003, Zeng et al., 2003). CHO cells are relatively easy to synchronize, which has facilitated their use in cell cycle studies (Fiore et al., 2002). In addition, CHO cells are used for studies to assess the effects of various agents on mammalian cells (Cai et al., 2001, Fernandez et al., 2003, Soloneski et al., 2002). CHO cell mutants have been utilized in DNA repair and radiation research studies (Dunkern and Kaina, 2002, Batista et al., 2006).
Some early cell biology studies conducted in CHO cells involved mutagenesis and isolation of certain auxotrophs (Puck and Kao, 1967) that facilitate cell growth and viability in cell culture. Cell mutants with deficiencies in metabolic enzymes such as adenosine phosphoribosyl transferase (APRT) and dihydrofolate reductase (DHFR) have been isolated and utilized as research tools (Taylor et al., 1977; Urlaub and Chasin, 1980). In addition, mutants defective in transcription, translation, and amino acid/polyamine biosynthesis have been isolated. The isolation of cells deficient in DHFR enzyme has led to an effective means for selection of stable clones and amplification of genes (Puck, 1985). DHFR is an enzyme that catalyzes the conversion of folic acid to tetrahydrofolate (THF). THF is a carrier for one-carbon molecules required in the biosynthesis of glycine, purine, and thymidine. In the early 1980s, Chasin et al. isolated two DHFR-deficient mutants (DXB11 and DG44), that are common parent cell lines used today (Graf and Chasin, 1982, Urlaub and Chasin, 1980, Urlaub et al., 1983). Both DHFR-deficient mutants are derivatives of a proline-requiring auxotroph established by Kao and Puck (1968).
Summary
The Chinese hamster has played an invaluable role in biomedical research from rigorous in vivo scientific investigations of its unique biological features to cell-culture-based experimentation. With the advent of cell culture techniques developed within the past 50 years, Chinese hamster-derived cells have played a major role in cytogenetic toxicity assays and the production of glycosylated therapeutic proteins. Numerous recombinant cell lines have been produced using Chinese hamster cells transfected with genes derived from human or other mammalian cells. Advances in cell culture techniques have obviated the need for performing many in vivo studies, and Chinese hamster cell culture research has led to the prolific production of recombinant proteins through bioengineering. During the past 20 years, CHO cells have been utilized to produce numerous recombinant therapeutic proteins to treat a wide array of human diseases. A list is provided in Table 35.10 of some select therapeutic proteins derived from Chinese hamster cell cultures. Currently, greater than 50% of all recombinant therapeutic proteins are derived from cells of Chinese hamster origin, with annual world-wide revenues exceeding 30 billion dollars (Jayapal et al., 2007). The current focus on the use of Chinese hamster cells in cell culture research exemplifies animal welfare initiatives to refine, reduce, and replace the use of animals in research. The history of the Chinese hamster as a laboratory animal is replete with studies clearly demonstrating its value as a unique animal model in biomedical research.
Table 35.10.
Selected List of Biologics Produced in Chinese Hamster Ovary (CHO) Cell Linesa
| Product | Type | Therapeutic Use | Manufacturer | FDA Approval |
|---|---|---|---|---|
| Vectibix | Anti-EGFR mAb | Metastatic colorectal cancer | Amgen | 2006 |
| Myozyme | α-Glucosidase | Pompe disease | Genzyme | 2006 |
| Aldurazyme | Laronidase | Mucopolysaccharidosis I | Genzyme | 2006 |
| Orencia | lg-CTLA4 fusion | Rheumatoid arthritis | Bristol-Myers Squibb | 2005 |
| Naglazyme | N-AGA-4-sulfatase | Mucopolysaccharidosis VI | Biomarin Pharm | 2005 |
| Luveris | Luteinizing hormone | Infertility | Serono | 2004 |
| Avastin | Anti-VEGF mAb | Metastatic colorectal & lung cancer | Genentech | 2004 |
| Advate | Factor VIII | Hemophilia A | Baxter | 2003 |
| Xolair | Anti-IgE mAb | Moderate/severe asthma | Genentech | 2003 |
| Raptiva | Anti-CD11a mAb | Chronic psoriasis | Genentech | 2003 |
| Fabrazyme | α-galactosidase | Fabray disease | Genzyme | 2003 |
| Rebif | Interferon-β | Relapsing multiple sclerosis | Serono | 2002 |
| Humira | Anti-TNFα mAb | Rheumatoid arthritis | Abbott | 2002 |
| Aranesp | Erythopoietin | Anemia | Amgen | 2001 |
| Campath | Anti-CD52 mAb | Chronic lymphocytic leukemia | Genzyme, Bayer | 2001 |
| ReFacto | Factor VIII | Hemophilia A | Wyeth | 2000 |
| Tenectaplase | Tissue plasminogen activator | Myocardial infarction | Genentech | 2000 |
| Herceptin | Anti-HER2 mAb | Metastatic breast cancer | Genentech | 1998 |
| Enbrel | TNF α receptor fusion | Rheumatoid arthritis | Amgen, Wyeth | 1998 |
| Benefix | Factor IX | Hemophilia B | Wyeth | 1997 |
| Follistim/Gonal-F | Follicle stimulating hormone | Infertility | Serono/NV Organon | 1997 |
| Rituxan | Anti-CD20 mAb | Non-Hodgkin’s lymphoma | Genentech, Biogen Idec | 1997 |
| Avonex | Interferon-β | Relapsing multiple sclerosis | Biogen Idec | 1996 |
| Cerezyme | β-glucocerebrosidase | Gaucher’s disease | Genzyme | 1994 |
| Pulmozyme | Deoxyribonuclease I | Cystic fibrosis | Genentech | 1993 |
| Epogen/Procrit | Erythropoietin | Anemia | Amgen/Ortho | 1989 |
| Activase | Tissue plasminogen activator | Acute myocardial infarction | Genentech | 1987 |
From Jayapal et al. (2007). Reprinted with permission from the American Institute of Chemical Engineers.
References
- Baker J.R., Mason M.M., Yerganian G., Weisburger E.K., Weisburger J.H. Induction of tumors of the stomach and esophagus in inbred Chinese hamsters by oral diethylnitrosamine. Proc. Soc. Exp. Biol. Med. 1974;146:291–293. doi: 10.3181/00379727-146-38090. [DOI] [PubMed] [Google Scholar]
- Batista L.F., Chiganças V., Brumatti G., Amarante-Mendes G.P., Menck C.F. Involvement of DNA replication in ultraviolet-induced apoptosis of mammalian cells. Apoptosis. 2006;11:1139–1148. doi: 10.1007/s10495-006-7109-4. [DOI] [PubMed] [Google Scholar]
- Barcellona W.J., Brinkley B.R. Effects of actinomycin D on spermatogenesis in the Chinese hamster. Biol. Reprod. 1973;8:335–349. doi: 10.1093/biolreprod/8.3.335. [DOI] [PubMed] [Google Scholar]
- Basler A., Rohrborn G. Vinyl chloride: an example for evaluating mutagenic effects in mammals in vivo after exposure to inhalation. Arch. Toxicol. 1980;45:1–7. doi: 10.1007/BF00303288. [DOI] [PubMed] [Google Scholar]
- Bayer U. In vivo induction of sister chromatid exchanges by 3 polyaromatic hydrocarbons. Carcino.-Compr. Surv. 1978;3:423–428. [Google Scholar]
- Bayer U., Siegers C.P., Younes M. Enhancement of benzo(a)pyrene-induced sister chromatid exchanges as a consequence of glutathione deplrtion in vivo. Toxicol. Lett. 1981;9:339–344. doi: 10.1016/0378-4274(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Benjamin S.A., Brooks A.L. Spontaneous lesions in Chinese hamsters. Vet Pathol. 1977;14:449–462. doi: 10.1177/030098587701400504. [DOI] [PubMed] [Google Scholar]
- Berman L.D., Hayes J.A., Sibay T.M. Effect of streptozotocin in the Chinese hamster (Cricetulus griseus) J. Natl. Cancer Inst. 1973;51:1287–1294. doi: 10.1093/jnci/51.4.1287. [DOI] [PubMed] [Google Scholar]
- Bestetti G., Rossi G.L. Hypothalamic changes in diabetic Chinese hamsters. A semi-quantitative, light and electrom microscopic study. Lab. Invest. 1982;47:516–522. [PubMed] [Google Scholar]
- Boquist L. Some aspects on the blood glucose regulation and the glutathione content of the non-diabetic adult Chinese hamster, Cricetulus griseus. Acta. Soc. Med. Ups. 1967;72:358–375. [PubMed] [Google Scholar]
- Boquist L. Alloxan administration in the Chinese hamster. Virchows Arch. 1968;B 1:157–168. [Google Scholar]
- Boquist L. Pancreatic islet morphology in diabetic Chinese hamsters. A light microscopic and electron microscopic study. Acta Pathol. Microbiol. Scand. 1969;75:399–414. [PubMed] [Google Scholar]
- Boquist L., Falkmer S. Morphologic changes in the pancreatic islets of the Chinese hamster in spontaneous diabetes and some experimental conditions. Z. Versuchstierkd. 1970;12:96–99. [PubMed] [Google Scholar]
- Bregman A.A. Asynchronous centromere division in the Chinese hamster. Cytogenetics. 1972;11:102–112. doi: 10.1159/000130180. [DOI] [PubMed] [Google Scholar]
- Brownstein D.G., Brooks A.L. Spontaneous endomyometrial neoplasms in aging Chinese hamsters. J. Natl. Cancer Inst. 1980;64:1209–1214. [PubMed] [Google Scholar]
- Butler L., Gerritsen G.C. A comparison of the modes of inheritance of diabetes in the Chinese hamster and KK mouse. Diabetologia. 1970;6:163–167. doi: 10.1007/BF01212224. [DOI] [PubMed] [Google Scholar]
- Cai Y., Ludeman S.M., Wilson L.R., Chung A.B., Dolan M.E. Effect of O6-benzylguanine on nitrogen mustard-induced toxicity, apoptosis, and mutagenicity in Chinese hamster ovary cells. Mol. Cancer Ther. 2001;1:21–28. [PubMed] [Google Scholar]
- Calland C.J., Wightman S.R., Neal S.B. Establishment of a Chinese hamster breeding colony. Lab. Anim. Sci. 1986;36:183–185. [PubMed] [Google Scholar]
- Carpenter A.M., Gerritsen G.C., Dulin W.E., Lazarow A. Islet and beta cell volumes in offspring of diabetic Chinese hamsters and their nondiabetic siblings. Diabetologia. 1967;6:168–176. doi: 10.1007/BF01212225. [DOI] [PubMed] [Google Scholar]
- Carpenter A.M., Gerritsen G.C., Dulin W.E., Lazarow A. Islet and beta cell volumes in offspring of severely diabetic ketotic Chinese hamsters. Diabetologia. 1970;6:168–176. doi: 10.1007/BF01212225. [DOI] [PubMed] [Google Scholar]
- Chandler R.L., Turfrey B.A. Inoculation of voles, Chinese hamsters, gerbils and guinea pigs with scrapie brain material. Res. Vet. Sci. 1972;13:219–224. [PubMed] [Google Scholar]
- Chang A.Y. Spontaneous diabetes in animals. Gen. Pharmacol. 1978;9:447–450. doi: 10.1016/0306-3623(78)90033-2. [DOI] [PubMed] [Google Scholar]
- Chang A.Y. Biochemical abnormalities in the Chinese hamster (Cricetulus griseus) with spontaneous diabetes. Int. J. Biochem. 1981;13:41–43. doi: 10.1016/0020-711x(81)90134-8. [DOI] [PubMed] [Google Scholar]
- Chang A., Diani A., Connell M. The Chinese hamster,biology and care. In: Van Hoosier G.L. Jr., McPherson C.W., editors. Laboratory Hamsters. Academic Press; San Diego: 1987. pp. 305–320. [Google Scholar]
- Chang A.Y., Noble R.E., Wyse B.M. Comparison of highly inbred diabetic and nondiabetic lines in the Upjohn colony of Chinese hamsters. Diabetes. 1977;26:1963–1971. doi: 10.2337/diab.26.11.1063. [DOI] [PubMed] [Google Scholar]
- Chang N., Liu Y., Lin F. Experimental encephalitis virus infection in Chinese hamsters. Chin. Med. J. 1951;69:420–426. [PubMed] [Google Scholar]
- Chin K. From Chinese hamsters to therapeutic proteins. Society for biological engineers (SBE) special section. Chem. Eng. Prog. 2007;103:33–52. [Google Scholar]
- Cisar C.F., Gumperz E.P., Nicholson F.S., Moore W., Jr. A practical method for breeding of Chinese hamsters (Cricetulus griseus) Lab. Anim. Sci. 1972;22:725–727. [PubMed] [Google Scholar]
- Clive D., Turner N.T., Hozier J., Batson A.G., Tucker W.E. Preclinical toxicology studies with acyclovir: genetic toxicity tests. Fundam. Appl. Toxicol. 1983;3:587–602. doi: 10.1016/s0272-0590(83)80109-2. [DOI] [PubMed] [Google Scholar]
- Cohen M.M., Shklar G., Yerganian G. Perioddontal pathology in a strain of Chinese hamster, Cricetulus griseus, with hereditary diabetes mellitus. Am. J. Med. 1961;31:864–867. doi: 10.1016/0002-9343(61)90027-4. [DOI] [PubMed] [Google Scholar]
- Corbascio A.N., Kohn H.I., Yerganian G. Acute X-ray lethality in the Chinese hamster after whole- body and partial-body irradiation. Radiat. Res. 1962;17:521–530. [PubMed] [Google Scholar]
- Crabtree J.E., Wilson R.A. Schistosoma mansoni, cellular reactions to challenge infections in the cheek pouch skin of chronically infected Chinese hamsters. Parasitology. 1984;84:59–69. doi: 10.1017/s003118200000113x. [DOI] [PubMed] [Google Scholar]
- Dail W.G., Evan A.P., Gerritsen G.C., Dulin W.E. Abnormalities in pelvic visceral nerves: a basis for neurogenic bladder in the diabetic Chinese hamster. Invest. Urol. 1977;15:161–166. [PubMed] [Google Scholar]
- Desch C.E., Jr., Hurley R.J. Demodex sinocricetuli: new species of hair follicle mite (Acari: Demodicidae) from the Chinese form of the striped hamster, Cricetulus barabensis (Rodentia: Muridae) J. Med. Entomol. 1997;34:317–320. doi: 10.1093/jmedent/34.3.317. [DOI] [PubMed] [Google Scholar]
- Deslex P., Rossi G.L. Quantitative evaluation of somatotropic cells in the adenohypophysis of normal and diabetic male Chinese hamsters. Diabetologia. 1976;12:489–493. doi: 10.1007/BF01219513. [DOI] [PubMed] [Google Scholar]
- Diani A., Gerritsen G. The Chinese hamster, use in research. In: Van Hoosier G.L. Jr., McPherson C.W., editors. Laboratory Hamsters. Academic Press; San Diego: 1987. pp. 329–347. [Google Scholar]
- Diani A.R., Davis D.E., Fix J.D., Swartzman J., Gerritsen G.C. Morphometric analysis of autonomic neuropathology in the abdominal sympathetic trunk of the ketonuric diabetic Chinese hamster. Acta Neuropathol. 1981;53:293–298. doi: 10.1007/BF00690371. [DOI] [PubMed] [Google Scholar]
- Diani A.R., West C., Vidmar T.J., Peterson T., Gerritsen G.C. Morphometric analysis of the vagus nerve in nondiabetic and ketonuric diabetic Chinese hamsters. J. Comp. Pathol. 1984;94:495–504. doi: 10.1016/0021-9975(84)90053-7. [DOI] [PubMed] [Google Scholar]
- Dresser M.E., Moses M.J. Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griseus). IV. Light and electron microscopy of synapsis and nucleolar development by silver staining. Chromosoma. 1980;76:1–22. doi: 10.1007/BF00292222. [DOI] [PubMed] [Google Scholar]
- Dulin W.E., Gerritsen G.C. Summary of biochemical, physiological and morphological changes associated with diabetes in the Chinese hamster. Int. Congr. Ser.-Excerpta Med. 1967;172:806–812. [Google Scholar]
- Dulin W.E., Soret M.G. Interaction of genetics and environment on diabetes in the Chinese hamster as compared with human and other diabetic animal species. Acta. Diabetol. Lat. 1972;9:48–84. [Google Scholar]
- Dulin W.E., Soret M.G. Chemically and hormonally induced diabetes. In: Volk B.W., Wellman K.F., editors. The Diabetic Pancreas. Plenum; New York: 1977. pp. 425–465. [Google Scholar]
- Dunkern T.R., Kaina B. Cell proliferation and DNA breaks are involved in ultraviolet light-induced apoptosis in nucleotide excision repair-deficient Chinese hamster cells. Mol. Biol. Cell. 2002;13:348–361. doi: 10.1091/mbc.01-05-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M.J., López A., Santa-Maria A. Apoptosis induced by different doses of caffeine on Chinese hamster ovary cells. J. Appl. Toxicol. 2003;23:221–224. doi: 10.1002/jat.910. [DOI] [PubMed] [Google Scholar]
- Festing M. Hamsters. In: University Federation for Animal Welfare, editor. The U.F.A.W. Handbook on the Care and Management of Laboratory Animals. Churchill Livingstone; Edinburgh: 1972. pp. 242–256. [Google Scholar]
- Figler H., Olsson R.A., Linden J. Allosteric enhancers of A1 adenosine receptors increase receptor-G protein coupling and counteract guanine nucleotide effects on agonist binding. Mol. Pharmacol. 2003;64:1557–1563. doi: 10.1124/mol.64.6.1557. [DOI] [PubMed] [Google Scholar]
- Fiore M., Zanier R., Degrassi F. Reversible G(1) arrest by dimethyl sulfoxide as a new method to synchronize Chinese hamster cells. Mutagenesis. 2002;11:419–424. doi: 10.1093/mutage/17.5.419. [DOI] [PubMed] [Google Scholar]
- Frankel B.J., Grodsky G.M. Effect of continuous low-dose insulin treatment on subsequent incidence of diabetes in genetically pre-diabetic Chinese hamsters. Diabetes. 1979;28:544–547. doi: 10.2337/diab.28.6.544. [DOI] [PubMed] [Google Scholar]
- Frankel B.J., Schmid F.G., Grodsky G.M. Effect of continuous insulin infusion with an implantable seven-day minipump in the diabetic Chinese hamster. Endocrinology. 1979;104:1532–1539. doi: 10.1210/endo-104-5-1532. [DOI] [PubMed] [Google Scholar]
- Fuji H., Kamiyama T., Hagiwara T. Species and strain differences in sensitivity to Toxoplasma infection among laboratory rodents. Jpn. J. Med Sci. Biol. 1983;36:343–346. doi: 10.7883/yoken1952.36.343. [DOI] [PubMed] [Google Scholar]
- Galloway S.M., Armstrong M.J., Reuben C., Colman S., Brown B., Cannon C. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ. Mol. Mutagen. 1987;10(Suppl. 10):1–175. doi: 10.1002/em.2850100502. [DOI] [PubMed] [Google Scholar]
- Galloway S.M., Bloom A.D., Resnick M., Margolin B.H., Nakamura F., Archer P. Development of a standard protocol for in vitro cytogenetic testing with Chinese hamster ovary cells: comparison of results for 22 compounds in two laboratories. Environ. Mutagen. 1985;7:1–51. doi: 10.1002/em.2860070102. [DOI] [PubMed] [Google Scholar]
- Gamperl R., Vistorin G., Rosenkranz W. Comparison of chromosome banding patterns in 5 members of Cricetinae with comments on possible relationships. Caryologia. 1978;31:343–354. [Google Scholar]
- Gerritsen G.C. Experimental prevention of diabetes in prediabetics: studies of Chinese hamsters. Compr. Ther. 1975;1:25–29. [PubMed] [Google Scholar]
- Gerritsen G.C. The Chinese hamster as a model for the study of diabetes mellitus. Diabetes. 1982;31:14–23. doi: 10.2337/diab.31.1.s14. [DOI] [PubMed] [Google Scholar]
- Gerritsen G.C., Blanks M.C. Characterization of Chinese hamsters by metabolic balance, glucose tolerance and insulin secretion. Diabetologia. 1974;10:493–500. doi: 10.1007/BF01221979. [DOI] [PubMed] [Google Scholar]
- Gerritsen G.C., Dulin W.E. Characterization of diabetes in the Chinese hamster. Diabetologia. 1967;3:74–84. doi: 10.1007/BF01222182. [DOI] [PubMed] [Google Scholar]
- Gerritsen G.C., Dulin W.E. Effect of diet restriction on onset of development of diabetes in prediabetic Chinese hamsters. Acta Diabetol. Lat. 1972;9:597–613. [Google Scholar]
- Gerritsen G.C., Blanks M.C., Miller R.L., Dulin W.E. Effect of diet limitation on the development of diabetes in prediabetic Chinese hamsters. Diabetologia. 1974;10:559–565. doi: 10.1007/BF01221987. [DOI] [PubMed] [Google Scholar]
- Gerritsen G.C., Blanks M.C., Schmidt F.L., Dulin W.E. Environmental influences on the manifestation of diabetes mellitus in Chinese hamsters. In: Creutzfeldt W., Kobberling J., Neel J.V., editors. The Genetics of Diabetes Mellitus. Springer-Verlag; Berlin: 1976. pp. 165–187. [Google Scholar]
- Gerritsen G.C., Connell M.A., Blanks M.C. Effect of environmental factors on genetically determined diabetes of Chinese hamsters. Proc. Nutr. Soc. 1981;40:237–245. doi: 10.1079/pns19810034. [DOI] [PubMed] [Google Scholar]
- Gerritsen G.C., Needham L.B., Schmidt F.L., Dulin W.E. Studies on the prediction and development of diabetes in offspring of diabetic Chinese hamsters. Diabetologia. 1970;6:158–162. doi: 10.1007/BF01212223. [DOI] [PubMed] [Google Scholar]
- Graf L.H., Jr., Chasin L.A. Direct demonstration of genetic alterations at the dihydrofolate reductase locus after gamma irradiation. Mol. Cell Biol. 1982;2:93–96. doi: 10.1128/mcb.2.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G.M., Frankel B.J., Gerich K.E., Gerritsen G.C. The diabetic Chinese hamster: in vitro insulin and glucagon release; the “chemical diabetic”; and the effect of diet on ketonuria. Diabetologia. 1974;10:521–528. doi: 10.1007/BF01221982. [DOI] [PubMed] [Google Scholar]
- Hankenson F.C., Van Hoosier G.L., Jr. Biology and diseases of hamsters. In: Fox J.G., Anderson L.C., Loew F.M., Quimby F.W., editors. Laboratory Animal Medicine. Academic Press; San Diego: 2002. pp. 167–202. [Google Scholar]
- Hari M., Wang Y., Veeraraghavan S., Cabral F. Mutations in alpha- and beta-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol. Cancer Ther. 2003;2:597–605. [PubMed] [Google Scholar]
- Harkness J. Small rodents. Vet. Clin. North Am. Small Anim. Pract. 1994;21:89–102. doi: 10.1016/s0195-5616(94)50004-4. [DOI] [PubMed] [Google Scholar]
- Harkness J.E., Wagner J.E. The Biology and Medicine of Rabbits and Rodents. Williams & Wilkins; Media, PA: 1995. [Google Scholar]
- Hillova J., Hiu M., Mariages R., Belehrad J. RSV provirus with some flanking sequences is formed on different size classes of Chinese hamster chromosomes. Intervirology. 1985;23:29–43. doi: 10.1159/000149564. [DOI] [PubMed] [Google Scholar]
- Hindle E., Hon P.C., Patton W.S. Serological studies on Chinese Kala-azar. Proc. R. Soc, London, Ser B. 1926;100:368–373. [Google Scholar]
- Hornigold D.C., Mistry R., Raymond P.D., Blank J.L., Challiss R.A. Evidence for cross-talk between M2 and M3 muscarinic acetlcholine receptors in the regulation of second messenger and extracellular signal-regulated kinase signaling pathways in Chinese hamster ovary cells. Br. J. Pharmacol. 2003;138:1340–1350. doi: 10.1038/sj.bjp.0705178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C.E., Lindsey S.R., Walkley S.U. Animal models of diabetes and obesity including the PBB/L1 mouse. Fed Proc., Fed. Am. Soc. Ex. Biol. 1976;35:1206–1217. [PubMed] [Google Scholar]
- Hsieh E.T. A new laboratory animal, Cricetulus griseus. Natl. Med. J. China. 1919;5:20–24. [Google Scholar]
- Jayapal K.P., Wlaschin K.F., Yap M.G.S., Hu W.-S. Recombinant protein therapeutics from CHO cells – 20 years and counting. Chem. Eng. Prog. 2007;103:40–47. [Google Scholar]
- Jhanwar S.C., Chaganti R.S.K. Pachytene chromomere maps of Chinese hamster autosomes. Cytogen. Cell Genet. 1981;31:70–76. doi: 10.1159/000131627. [DOI] [PubMed] [Google Scholar]
- Jhanwar S.C., Prensky W., Chaganti R.S.K. Localization and metabolic activity of ribosomal genes in Chinese hamster meiotic and mitotic chromosomes. Cytogenet. Cell Genet. 1981;30:39–46. doi: 10.1159/000131586. [DOI] [PubMed] [Google Scholar]
- Kakati S., Sinha A.K. Banding patterns of Chinese hamster chromosomes. Genetics. 1972;72:357–362. doi: 10.1093/genetics/72.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R. The chromosomes of forty-two primary Rous sarcomas of the Chinese hamster. Hereditas. 1968;59:63–119. doi: 10.1111/j.1601-5223.1968.tb02166.x. [DOI] [PubMed] [Google Scholar]
- Kato R., Bruze M., Tegner Y. Chromosome breakage induced in vivo by a carcinogenic hydrocarbon in bone marrow cells of the Chinese hamster. Hereditas. 1969;61:1–8. doi: 10.1111/j.1601-5223.1969.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Kohn H.I., Guttman P.H. Life span, tumor incidence, and intercapillary glomerulosclerosis in the Chinese hamster (Cricetulus griseus) after whole-body and partial-body exposure to X-rays. Radiat. Res. 1964;21:622–643. [PubMed] [Google Scholar]
- Kao F.T., Puck T.T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc. Natl. Acad. Sci. 1968;60:1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda K., Yokote M., Oki Y. Diabetic syndrome in the Chinese hamster induced with monosodium glutamate. Experientia. 1980;36:232–234. doi: 10.1007/BF01953751. [DOI] [PubMed] [Google Scholar]
- Krampitz H.E., Baumler W. Occurrence, host range and seasonal prevalence of Babesia microti (Fran Ca, 1912)in rodents of southern Germany. Z. Parastitenkd. 1978;58:15–33. doi: 10.1007/BF00930788. [DOI] [PubMed] [Google Scholar]
- Kurotchkin T.J., Lin L.E. The effect of animal passage upon pathogenic monilia. Natl. Med. J. China. 1930;16:337. [Google Scholar]
- Ladiges W.C. The Chinese hamster, diseases. In: Van Hoosier G.L. Jr., McPherson C.W., editors. Laboratory Hamsters. Academic Press; San Diego: 1987. pp. 321–328. [Google Scholar]
- Like A.A., Gerritsen G.C., Dulin W.E., Gandreau P. Studies in the diabetic Chinese hamster: light microscopy and autoradiography of pancreatic islets. Diabetologia. 1974;10:501–508. doi: 10.1007/BF01221980. [DOI] [PubMed] [Google Scholar]
- Like A.A., Gerritsen G.C., Dulin W.E., Gandreau P. Studies in the diabetic Chinese hamster: electron microscopy of pancreatic islets. Diabetologia. 1974;10:509–520. doi: 10.1007/BF01221981. [DOI] [PubMed] [Google Scholar]
- Lin M.S., Takabayashi T., Wilson M.G., Marchese C.A. An in vitro and in vivo study of a bromodeoxyuridine-sensitive fragile site in the Chinese hamster. Cytogenet. Cell Genet. 1984;38:211–215. doi: 10.1159/000132062. [DOI] [PubMed] [Google Scholar]
- Losert W., Rilke A., Loge O., Richter K.D. Comparative biochemical studies on the diabetogenic action of streptozotocin in mice, rats, Chinese hamsters and guinea pigs. Arzneim.-Forsch. 1971;21:1643–1653. [PubMed] [Google Scholar]
- Lu K.J., Zia S.H. Use of the Chinese hamster for testing the virulence of C. diptheriae. Proc. Soc. Exp. Biol. Med. 1935;33:334–337. [Google Scholar]
- Luse S.A., Caramia F., Gerritsen G., Dulin W.G. Spontaneous diabetes mellitus in the Chinese hamster: an electron microscopic study of the islets of Langerhans. Diabetologia. 1967;3:97–108. doi: 10.1007/BF01222185. [DOI] [PubMed] [Google Scholar]
- Luse S.A., Gerritsen G.C., Dulin W.G. Cerebral abnormalities in diabetes mellitus: an ultrastructural study of the brain in early-onset diabetes mellitus in the Chinese hamster. Diabetologia. 1970;6:192–198. [Google Scholar]
- Machemer L., Lorke D. Mutagenicity studies with praziquantel, a new anthelmintic drug, in mammalian systems. Arch. Toxicol. 1978;39:187–197. doi: 10.1007/BF00368227. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Gerritsen G.C., Dulin W.E. Insulin secretion in vitro by the pancreas of the Chinese hamster. Diabetologia. 1967;3:109–114. doi: 10.1007/BF01222186. [DOI] [PubMed] [Google Scholar]
- Meier H.M., Yerganian G. Spontaneous hereditary diabetes mellitus in the Chinese hamster (Cricetulus griseus). I. Pathological findings. Proc. Exp. Biol. Med. 1959;100:810–815. doi: 10.3181/00379727-100-24786. [DOI] [PubMed] [Google Scholar]
- Meier H.M., Yerganian G. Spontaneous diabetes mellitus in the Chinese hamster (Cricetulus griseus). III. Maintenance of a diabetic hamster colony with the aid of hypoglycemic therapy. Diabetes. 1961;10:19–21. doi: 10.2337/diab.10.1.19. [DOI] [PubMed] [Google Scholar]
- Meier H., Yerganian G.A. Spontaneous hereditary diabetes mellitus in Chinese hamster. II. Findings in the offspring of diabetic parents. Diabetes. 1961;10:12–18. doi: 10.2337/diab.10.1.12. [DOI] [PubMed] [Google Scholar]
- Mikamo K., Kamiguchi Y. Primary incidences of spontaneous chromosomal anomalies and their origins and causal mechanisms in the Chinese hamster. Mutat. Res. 1983;108:265–278. doi: 10.1016/0027-5107(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Mikamo K., Sugawara S. Colchicine-induced abnormal meiotic chromosomal segregation in primary oocytes of the Chinese hamster 2. Anaphase lagging. Jpn. J. Hum. Genet. 1980;25:241–248. doi: 10.1007/BF01997702. [DOI] [PubMed] [Google Scholar]
- Miltenburger H.G., Engelhardt G., Rohrborn G. Differential chromosomal damage in Chinese hamster bone marrow cells and in spermatogonia after mutagenic treatment. Mutat. Res. 1981;81:117–122. doi: 10.1016/0027-5107(81)90092-0. [DOI] [PubMed] [Google Scholar]
- Moore W., Jr. Observations on the breeding and care of the Chinese hamster, Cricetulus griseus. Lab. Anim. Care. 1965;15:95–101. [PubMed] [Google Scholar]
- Moore W., Jr. Hemogram of the Chinese hamster. Am. J. Vet. Res. 1966;27:608–610. [PubMed] [Google Scholar]
- Müller D., Strasser F.F. Comparative studies on the Chinese hamster bone marrow after treatment with phenylbutazone and cyclophosphamide. Mutat. Res. 1971;13:377–382. doi: 10.1016/0027-5107(71)90049-2. [DOI] [PubMed] [Google Scholar]
- Munzer R., Renner H.W. Mutagenicity testing of quinine with submammalian and mammalian systems. Toxicology. 1983;26:173–178. doi: 10.1016/0300-483x(83)90068-9. [DOI] [PubMed] [Google Scholar]
- Murkherjee B.B., Ghosal S.K. Replicative differentiation of mammalian sec chromosomes during spermatogenesis. Exp. Cell Res. 1969;54:101–106. doi: 10.1016/0014-4827(69)90298-5. [DOI] [PubMed] [Google Scholar]
- Natarajan A.T., Darroudi F., Bussman C.J.M., Van Kesteren-Van Leeuwen A.C. Evaluation of the mutagenicity of formaldehyde in mammalian cytogenetic assays in vivo and in vitro. Mutat. Res. 1983;122:355–360. doi: 10.1016/0165-7992(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Norppa H., Sorsa M., Vainio H. Chromosomal aberrations in bone marrow of Chinese hamsters exposed to styrene and ethanol. Toxicol. Lett. 1980;5:241–244. doi: 10.1016/0378-4274(80)90066-1. [DOI] [PubMed] [Google Scholar]
- Oka M.S., Rupp R.G. Large-scale cell culture: a biological perspective. Bioprocess Tech. 1990;10:71–92. [PubMed] [Google Scholar]
- Pathak S., Hsu T.C., Markvong A. Pachytene mapping of the male Chinese hamster. Cytogenet. Cell Genet. 1976;17:1–8. doi: 10.1159/000130681. [DOI] [PubMed] [Google Scholar]
- Petersson B., Elde R., Efendic S., Hokfelt T., Johansson O., Luft R. Somatostatin in the pancreas, stomach and hypothalamus of the diabetic Chinese hamster. Diabetologia. 1977;13:463–466. doi: 10.1007/BF01234497. [DOI] [PubMed] [Google Scholar]
- Plesko I. Experimental leptospirosis in Chinese hamsters. Biologia (Bratislava) 1977;32:164–172. [Google Scholar]
- Poel W.E., Yerganian G. Adenocarcinoma of the pancreas in diabetes-prone Chinese hamsters. Am. J. Med. 1961;31:861–863. doi: 10.1016/0002-9343(61)90026-2. [DOI] [PubMed] [Google Scholar]
- Puck T.T. Development of the Chinese hamster ovary (CHO) cell. In: Gottesman M.M., editor. Molecular Cell Genetics. John Wiley; New York: 1985. pp. 37–64. [Google Scholar]
- Puck T.T., Ciecura S.J., Robinson A. Genetics of somatic mammalian cells III. Long- term cultivation of euploid cells from human and animal subjects. J. Exp. Med. 1958;108:945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T.T., Kao F.T. Genetics of somatic mammalian cells. V. Treatment with 5-bromodeoxyuridine and visible light for isolation of nutritionally deficient mutants. Proc. Natl. Acad. Sci. 1967;58:1227–1234. doi: 10.1073/pnas.58.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raicu P., Nicolaescu M., Kirillova M. The sex chromatin in 5 species of hamsters. Chromosoma. 1970;31:61–67. doi: 10.1007/BF00321156. [DOI] [PubMed] [Google Scholar]
- Ray M., Mohandis T. Proposed banding nomenclature for the Chinese hamster chromosomes (Cricetulus griseus) Cytogenet. Cell Genet. 1976;16:83–91. doi: 10.1159/000130559. [DOI] [PubMed] [Google Scholar]
- Renner H.W., Munzer R. Mutagenecity of sulphonylureas. Mutat. Res. 1980;77:349–355. doi: 10.1016/0165-1218(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Richter K.D., Loge O., Losert W. Comparative morphological studies on the diabetogenic activity of streptozotocin in rats, Chinese hamsters, guinea pigs and rabbits. Arzneim.-Forsch. 1971;21:1654–1656. [PubMed] [Google Scholar]
- Ritterson A.L. Resistance of Chinese hamsters to Hymenolepsis microstoma and its reversal by immunosuppression. J. Parasitol. 1971;57:1247–1250. [PubMed] [Google Scholar]
- Ritterson A.L. Effect of immunosuppressive drugs (6-mercaptopurine and methotrexate) on resistance of Chinese hamsters to the tissue phase of Trichinella spiralis. J. Infect. Dis. 1968;118:365–369. doi: 10.1093/infdis/118.4.365. [DOI] [PubMed] [Google Scholar]
- Sato T., Komeda K., Shirama K. Plasma progesterone concentrations during the estrous cycle, pregnancy, and lactation in the Chinese hamster, Cricetulus griseus. Jikken Dobutsu. 1984;33:501–508. doi: 10.1538/expanim1978.33.4_501. [DOI] [PubMed] [Google Scholar]
- Schmid W., Staiger G.R. Chromosome studies on bone marrow from Chinese hamsters treated with benzodiazepine tranquilizers and cyclophosphamide. Mutat. Res. 1969;7:99–108. doi: 10.1016/0027-5107(69)90053-0. [DOI] [PubMed] [Google Scholar]
- Schulte G., Fredholm B.B. The G(s)-coupled adenosine A(2B) receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp. Cell Res. 2003;290:168–176. doi: 10.1016/s0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- Silberberg R., Gerritsen G.C. Aging changes in intervertebral discs and spondylosis in Chinese hamsters. Diabetes. 1976;25:477–483. doi: 10.2337/diab.25.6.477. [DOI] [PubMed] [Google Scholar]
- Sirek O.V., Sirek A. The colony of Chinese hamsters of the C.H. Best Institute. A review of Experimental work. Diabetologia. 1967;3:65–73. doi: 10.1007/BF01222181. [DOI] [PubMed] [Google Scholar]
- Sirek O.V., Sirek A. Spontaneous diabetes in animals. In: Ellenberg M., Aifkin H., editors. Diabetes. McGraw-Hill; New York: 1971. pp. 256–260. [Google Scholar]
- Soloneski S. Effect of dithiocarbamate pesticide zineb and its commercial formulation, azzurro. III. Genomic evaluation on Chinese hamster ovary (CHO) cells. Mutat. Res. 2002;514:201–212. doi: 10.1016/s1383-5718(01)00337-0. [DOI] [PubMed] [Google Scholar]
- Soret M.G., Dulin W.E., Gerritsen G.C. Microangiopathy in animals with spontaneous diabetes. In: Camerini-Davalos R.A., Cole H., editors. Vascular and Neurological Changes in Early Diabetes. Academic Press; San Diego: 1973. pp. 291–298. [Google Scholar]
- Soret M.G., Dulin W.E., Mathews J., Gerritsen G.C. Morphologic abnormalities observed in retina, pancreas and kidney of diabetic Chinese hamsters. Diabetologia. 1974;10:567–579. doi: 10.1007/BF01221988. [DOI] [PubMed] [Google Scholar]
- Takanari H., Pathak S., Hsu T.C. Dense bodies in silver-stained spermatocytes of the Chinese hamster. Behavior and cytochemical nature. Chromosoma. 1982;86:359–373. doi: 10.1007/BF00292263. [DOI] [PubMed] [Google Scholar]
- Tijo J.H., Puck T.T. Genetics of somatic mammalian cells. II. Chromosomal constitution of cells in tissue culture. J. Exp. Med. 1958;108:259–268. doi: 10.1084/jem.108.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L.A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc. Natl. Acad. Sci. 1980;77:4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Käs E., Carothers A.M., Chasin L.A. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell. 1983;33:405–412. doi: 10.1016/0092-8674(83)90422-1. [DOI] [PubMed] [Google Scholar]
- Utakoji T. On the homology between the X and Y chromosomes of the Chinese hamster. Chromosoma. 1966;18:449–454. doi: 10.1007/BF00332548. [DOI] [PubMed] [Google Scholar]
- Utakoji T., Hsu T.C. DNA replication patterns in somatic and germ-line cells of the male Chinese hamster. Cytogenetics. 1965;4:295–315. doi: 10.1159/000129865. [DOI] [PubMed] [Google Scholar]
- Vig B.K., Miltenburger H.G. Sequence of centromere separation of mitotic chromosomes in Chinese hamster. Chromosoma. 1976;55:75–80. doi: 10.1007/BF00288329. [DOI] [PubMed] [Google Scholar]
- Vistorin G., Gamperl R., Rosenkrantz W. Studies on sex chromosomes of four hamster species: cricetus cricetus, Cricetulus griseus, Mesocricetus auratus, and Phodopus sungorus. Cytogenet. Cell Genet. 1977;18:24–32. doi: 10.1159/000130745. [DOI] [PubMed] [Google Scholar]
- Wang U.F.L. The use of Chinese hamsters (Cricetulus griseus) in a study of Mycobacterium tuberculosis. II. For differentiation of human and bovine types. Chin. Med. J. 1951;69:155–159. [PubMed] [Google Scholar]
- Wappler E., Fiedler H. The Chinese hamster Cricetulus griseus, a spontaneously diabetic animal. A review. Z. Versuchstierkd. 1972;14:1–16. [PubMed] [Google Scholar]
- Ward B.C., Moore W.J., Jr. Spontaneous lesions in a colony of Chinese hamsters. Lab Anim. Care. 1969;19:516–521. [PubMed] [Google Scholar]
- Wilander E., Boquist L. Streptozotocin diabetes in the Chinese hamster: blood glucose and structural changes during the first 24 hours. Horm. Metab. Res. 1972;4:426–433. doi: 10.1055/s-0028-1094000. [DOI] [PubMed] [Google Scholar]
- Yerganian G. The striped-back or Chinese hamster. J. Natl. Cancer Inst. 1958;20:705–727. [PubMed] [Google Scholar]
- Yerganian G. History and cytogenetics of hamsters. Pathology of the Syrian Hamster. In: Homburger F., editor. Vol 16. S. Karger, Basel; 1972. pp. 2–41. (Progress in Experimental Tumor Research). [DOI] [PubMed] [Google Scholar]
- Yerganian G. The biology and genetics of the Chinese hamster. In: Gottesman M., editor. Molecular Cell Genetics. John Wiley & Sons, Inc.; New York: 1985. pp. 3–36. [Google Scholar]
- Yerganian G.A. Spontaneous diabetes mellitus in the Chinese hamster, Cricetulus griseus. IV. Genetics aspects. In: Cameron M.P., O’Connor M., editors. Aetiology of Diabetes Mellitus and Its Complications. Churchill; London: 1964. pp. 25–41. [Google Scholar]
- Yerganian G., Klein E., Roy A. Physiological studies on Chinese hamster, Cricetulus griseus. II. A method for study of bleeding time in hamsters. Fed. Proc., Fed. Am. Soc. Exp. Biol. 1955;14:424. [Google Scholar]
- Zeng Q., Dong J.M., Guo K., Li J., Tan H.X., Koh V. PRL-3 and PRL-1 promote cell migration, invasion and metastasis. Cancer Res. 2003;63:2716–2722. [PubMed] [Google Scholar]


