Abstract
Bronchus-associated lymphoid tissue (BALT) is a constitutive mucosal lymphoid tissue adjacent to major airways in some mammalian species, including rats and rabbits, but not humans or mice. A related tissue, inducible BALT (iBALT), is an ectopic lymphoid tissue that is formed upon inflammation or infection in both mice and humans and can be found throughout the lung. Both BALT and iBALT acquire antigens from the airways and initiate local immune responses and maintain memory cells in the lungs. Here, we discuss the development and function of BALT and iBALT in the context of pulmonary immunity to infectious agents, tumors, and allergens as well as autoimmunity and inflammatory diseases of the lung.
1. Introduction
Bronchus-associated lymphoid tissue (BALT) is considered by early investigators to be a mucosal secondary lymphoid tissue embedded in the walls of the large airways (Sminia et al., 1989), similar to Peyer's patches in the small intestine. However, it is clear that BALT is not constitutively present in all mammalian species, notably mice and humans (Pabst, 1992, Pabst & Gehrke, 1990), and is induced in response to microbial exposure or other types of pulmonary inflammation (Tshering and Pabst, 2000). These inducible tissues may be more properly referred to as a tertiary or ectopic lymphoid tissue and we have coined the term inducible BALT (iBALT) to describe them (Moyron-Quiroz et al., 2004). In the context of this review, however, we do not attempt to make a distinction between BALT and iBALT and refer to both types of tissues as BALT.
The development of secondary lymphoid organs (Drayton et al., 2006, Randall et al., 2008) and tertiary lymphoid tissues (Carragher et al., 2007) has been recently reviewed. Thus, this review focuses primarily on features and functions specific to BALT and the role of BALT in pulmonary immunity. BALT was previously reviewed by Bienenstock and McDermott (2005) and by us in 2007 (Moyron-Quiroz et al., 2007). However, there have been significant advances since then, particularly in the way particular types of immune responses contribute to BALT formation. Therefore, this comprehensive review focuses first on the architecture and development of BALT, then on the functional properties of BALT, and finally on the role of BALT in response to respiratory infections, allergens, and autoantigens and the role of BALT in pulmonary malignancies.
2. Lymphoid Architecture of BALT
2.1. Basic architecture and placement in the lung
As originally described in rabbits and rats, which develop BALT independently of microbial stimulation (Pabst & Gehrke, 1990, Sminia et al., 1989), BALT is a densely packed cluster of lymphocytes with follicular structures enmeshed in a reticular network of stromal cells and underlying a specialized airway epithelium that lacks cilia (Sminia et al., 1989), much like Peyer's patches in the small intestine or the nasal-associated lymphoid tissue (NALT) in the nose (Kiyono and Fukuyama, 2004). These structures are described to be located along major bronchial airways embedded in the airway wall with extensive lymphocytic infiltration of the epithelial layer creating a classic dome epithelium (Sminia et al., 1989, van der Brugge-Gamelkoorn et al., 1986b). BALT is also described to occur at airway bifurcations, where it is placed to trap inhaled antigens. However, in other species, in which BALT is not constitutively present in the lung and instead develops in response to microbial stimulation or inflammation, BALT does not always have such a defined structure or rigorous placement in the lung. Instead, iBALT can be located throughout the lung, typically adjacent to small pulmonary arteries (Moyron-Quiroz et al., 2006).
In fact, an important site for the formation of iBALT is the perivascular space, which is filled with periarterial capillaries (Pabst and Tschernig, 2002). This space often becomes densely packed with lymphocytes upon pulmonary inflammation and is often classified as perivascular cuffing. Perivascular cuffing is not necessarily equivalent to BALT, as BALT requires additional architectural changes, including the development of a stromal cell network, separation of B and T cell areas, the formation of follicular dendritic cells (FDCs) in the B cell follicles, and the development of high endothelial venules (HEVs) as well as lymphatics to facilitate leukocyte entry and exit from BALT. Since pulmonary arteries are typically parallel to bronchial airways, the formation of BALT in the perivascular space also tends to place BALT next to airways. However, in many instances, the lymphocytes in BALT do not infiltrate the airway epithelium and a classic dome epithelium is not necessarily present. Thus, these structures are not absolutely analogous to classic mucosal lymphoid tissues, like Peyer's patches or NALT. In addition, lymphoid clusters can be found even in the small airspaces that are apparently not adjacent to an artery or airway. Some of these clusters can be organized with B and T cell areas and specialized stromal cells, whereas others are simply small clusters of mostly B cells with little obvious organization. Thus, the term BALT is often used to encompass a wide range of tissues in various locations throughout the lung. However, we feel that minimally a pulmonary lymphoid cluster should have a B cell follicular structure with histologically identifiable FDCs in order to be termed BALT (Pabst, 2007).
2.2. Organization of B cell follicles in BALT
The B cell follicle is the most prominent feature of BALT (Fig. 7.1 ), and in many cases, BALT consists almost exclusively of B cell follicles without adjacent T cell areas (Rangel-Moreno et al., 2006). Many B cell areas in BALT consist of follicles composed primarily of IgDhiIgMlo mature resting B cells (Kocks et al., 2007). However, B cell areas of BALT responding to infection or other antigens often contain germinal centers, which can be identified histologically as large blast cells in the center of the follicle that express Ki-67 or PCNA (Rangel-Moreno et al., 2006), and rapidly incorporate BrdU and bind peanut agglutinin (PNA) or the antibody GL7 (Moyron-Quiroz et al., 2004). Germinal center B cells also lose IgD and may have switched to alternative isotypes, such as IgG, IgA, or IgE. In classic BALT tissues that have a dome epithelium, the B cell follicle is found immediately below the epithelium (Sminia et al., 1989). B cell follicles also contain CD4 T cells (Fig. 7.1), particularly in reactive follicles with germinal centers (Woodland and Randall, 2004), but they rarely contain CD8 T cells. In addition, some dendritic cells (DCs) and macrophages are found within the follicle (Fig. 7.1), where they may present antigen or facilitate the clearance of apoptotic germinal center B cells. Plasma cells are not often found within the follicle and are instead located around the edge of the follicle or in the T cell zone (GeurtsvanKessel et al., 2009, Rangel-Moreno et al., 2006). Not all B cell clusters in the lung—even those that form in the perivascular space adjacent to airways—are follicles. Some of these areas lack FDCs and are not separated from T cells or other cell types (Fig. 7.2 ). These areas may eventually develop into follicles or may simply be loose clusters of lymphocytes. However, it is still not clear whether these different types of tissues represent a morphological spectrum of functionally similar structures or whether the different structures imply different functions.
Figure 7.1.
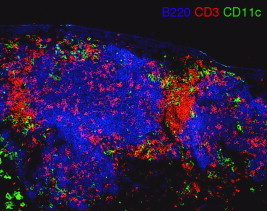
Organization of murine BALT. Lungs from mice infected with influenza 12 days previously were frozen in OCT medium and sectioned on a cryostat. Sections were probed with antibodies to B220 (blue), CD3 (red), and CD11c (green). Note the presence of three B220+ B cell follicles, two of which are underneath a major airway. CD3+ T cell zones are between the follicles and some T cells are scattered in the follicles. CD11c+ DCs are located primarily in the T cell zones.
Figure 7.2.
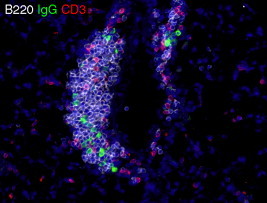
IgG-secreting cells in murine BALT. Lungs from mice infected with influenza 10 days previously were frozen in OCT medium and sectioned on a cryostat. Sections were probed with antibodies to B220 (white), IgG (green), and CD3 (red) and were counterstained with DAPI (blue). Note that the architecture is not well developed and the B220+ B cells, CD3+ T cells, and IgG+ plasma cells are intermixed.
Areas of BALT contain FDCs in the center of the B cell follicles (Fig. 7.3 ). FDCs in mice are identified by binding to antibodies against CD21/CD35 (Moyron-Quiroz et al., 2004), FDCM1, or FDCM2 (Chvatchko et al., 1996) and their ability to retain immune complexes (Haberman and Shlomchik, 2003). FDCs are dependent on the lymphotoxin (LT) signaling pathway for their differentiation in conventional lymphoid tissues as well as in BALT. Thus, although extensive areas of perivascular cuffing are often observed in the lungs of Lta −/− and LTβR −/− mice (Futterer et al., 1998, Moyron-Quiroz et al., 2004), these areas lack FDCs and fail to develop proper B cell follicles. FDCs also express CXCL13, which is responsible for the organization of the follicle and for recruiting B cells and some types of T cells to the B cell area (Ansel et al., 2000). In the absence of CXCL13, B cell areas are still formed, but they lack the densely packed structure of true follicles and fail to develop FDCs (Rangel-Moreno et al., 2007). Therefore, the major hallmark of true B cell follicles in BALT is the formation of FDCs.
Figure 7.3.
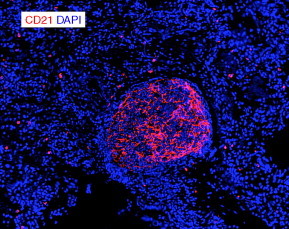
FDCs in human BALT. A formalin-fixed, paraffin-embedded lung biopsy from a patient with hypersensitivity pneumonitis was sectioned on a microtome and probed with an antibody to CD21 (red) and counterstained with DAPI (blue). Note the robust FDC network and the way the follicular areas are partially separated from the rest of the tissue.
2.3. T cell zones
T cell zones in BALT are typically found surrounding B cell follicles or between B cell follicles (Fig.7.1; Woodland and Randall, 2004). T cell areas are also home to DCs (Fig. 7.1; Woodland and Randall, 2004), plasma cells (GeurtsvanKessel et al., 2009), macrophages (Hiramatsu et al., 2003), and occasionally eosinophils (Lee et al., 1997) and neutrophils. HEVs and lymphatics are also found in the T cell zone or sometimes at the boundary between the B cell follicle and the T cell area (Moyron-Quiroz et al., 2004, Rangel-Moreno et al., 2006). In some cases, the T cell zone is minimally populated with T cells and those that are present are widely scattered (Rangel-Moreno et al., 2006). In these cases, the structure of BALT consists almost entirely of the B cell follicle. However, the T cell zone is often densely packed with T cells and DCs and appears similar to the intrafollicular T cell zone in Peyer's patches.
Reticular cells are found in T cell areas of BALT (Sminia et al., 1989) and given the separation of B and T cell areas, are likely to express T cell attracting chemokines, like CCL21 and CCL19. Both of these chemokines are expressed in the lung (Moyron-Quiroz et al., 2004, Rangel-Moreno et al., 2007), and in species like mice and humans, the mRNA expression of CCL19 in particular is increased following infection or inflammation (Khader et al., 2009). However, histological evidence for the stromal cell expression of CCL19 is lacking and CCL21 seems most prominently expressed on HEVs and lymphatics in BALT (Rangel-Moreno et al., 2007). In plt/plt mice, which lack CCL19 and CCL21 (Nakano & Gunn, 2001, Nakano et al., 1997), the T cell areas of BALT are significantly reduced (Rangel-Moreno et al., 2007). However, BALT is still formed and still contains some B and T cells, which are separated into B and T cell areas (Rangel-Moreno et al., 2007). Thus, CCL19 and CCL21 cannot be the only chemokines that promote BALT organization. Similarly, Ccr7 −/− mice also develop BALT and, according to several reports (Kahnert et al., 2007, Kocks et al., 2007), have more extensive and numerous areas of BALT than do normal mice. Again, B and T cells are separated into distinct regions, suggesting that additional chemokines, such as CXCL12 or others, help to define B and T cell areas in BALT.
2.4. HEVS and lymphocyte homing to BALT
A typical feature of BALT is the development of HEVs (Moyron-Quiroz et al., 2004, Rangel-Moreno et al., 2007), which are thought to recruit recirculating lymphocytes from the blood. HEVs in BALT are located around and between the B cell follicles in the T cell areas (Moyron-Quiroz et al., 2004). Interestingly, HEVs in BALT express peripheral lymph node addressin (PNAd) rather than mucosal addressin cell adhesion molecule (MAdCAM) (Moyron-Quiroz et al., 2004, Xu et al., 2003), suggesting that BALT recruits naïve peripheral lymphocytes rather than memory B and T cells that have been primed in mucosal sites. Interestingly, HEVs in BALT areas of mice express much higher levels of VCAM-1 than HEVs in other secondary lymphoid organs (Xu et al., 2003). Consistent with their expression patterns, l-selectin, PNAd, and LFA-1 are the most important homing molecules for the recruitment of B and T cells to BALT, whereas α4β7 integrin and MAdCAM are not involved (Xu et al., 2003).
Pulmonary inflammation or immunization appears to facilitate lymphocyte homing to BALT, as CFSE-labeled thoracic duct lymphocytes from TNP-KLH-immunized rats intravenously transferred to recipient rats that had also been intratracheally immunized with TNP-KLH were recruited more efficiently to the BALT than those transferred to nonimmunized rats (Sato et al., 2000). Interestingly, labeled cells were observed inside HEVs as well as in T cells areas within 12 h after injection (Sato et al., 2000), suggesting that T cell trafficked through the HEVs to enter BALT and subsequently entered the T cell area or follicles. Consistent with the idea that microbial stimulation promotes the homing of T cells to BALT, OTI TCR transgenic T cells home to BALT induced either by MTB infection (Day et al., 2010) or by infection with modified vaccinia Ankara virus (Halle et al., 2009) and proliferate in response to antigen. In both cases, BALT had to be generated by prior infection in order for naïve T cells to be recruited to the lung.
Prior inflammation is also important for T cell homing to BALT areas in a rat model of asthma (Schade et al., 2010). In these experiments, CFSE-labeled T cells were transferred to normal or CD26-deficient rats either before or after sensitization with aerosolized ovalbumin (OVA). Prior to pulmonary sensitization, there was no difference in T cell homing to the BALT areas of normal or CD26-deficient lungs (Schade et al., 2010). However, after sensitization, T cells preferentially homed to the BALT of CD26-defient animals (Schade et al., 2010). Given the peptidase activity of CD26, the authors concluded that CD26 normally degrades a T cell attracting chemokine that is normally induced after pulmonary inflammation and that in the absence of CD26, T cells were attracted to the BALT areas more efficiently (Schade et al., 2010). Thus, despite the fact that the chemokines involved in homeostatic and inflammatory homing to BALT via HEVs are not completely understood, it is clear that they play an important role in lymphocyte trafficking to this tissue.
The current model of lymphocyte recruitment to secondary lymphoid tissues involves multiple steps, including addressin-mediated rolling, integrin activation by chemokines, which stops rolling and mediates firm adhesion, and finally, the transendothelial migration of lymphocytes into the tissue following a chemokine gradient (Campbell et al., 1998). Thus, the expression of chemokines on HEVs is critical to proper homing of lymphocytes to lymphoid tissues. In the case of BALT, CCL21 is observed on HEVs (Rangel-Moreno et al., 2007) and probably serves to attract naïve or central memory T cells as well as B cells. Consistent with this idea, we found that mice lacking CCL19 and CCL21 poorly recruited T cells to BALT areas (Rangel-Moreno et al., 2007). However, BALT was still formed and still contained some B and T cells, suggesting that CCL21 is not the only chemokine that plays a role in recruiting T cells from the blood. Moreover, Ccr7 −/− mice also develop BALT and, according to several reports (Demoor et al., 2009b, Kahnert et al., 2007, Kocks et al., 2007), have more extensive BALT than do normal mice. In part, this may be due to the failure of regulatory mechanisms (Kocks et al., 2007). However, the data still suggest that signaling through CCR7 is not absolutely required for entry to BALT.
2.5. DCs in BALT
As mentioned above, DCs are present in BALT and are found in highest concentrations in the T cell zone (Fig.7.1; Woodland and Randall, 2004), but can be scattered throughout the B cell follicle as well. These cells are easily identified in the mouse using antibodies to CD11c (GeurtsvanKessel et al., 2009, Halle et al., 2009, Woodland & Randall, 2004) or CD205 (Kocks et al., 2007). In humans, DCs have been identified using antibodies to DC-LAMP and DC-SIGN (Marchal-Somme et al., 2007), S100 (Sarradell et al., 2003, Yoshinouchi et al., 1999), and MHC class II. Although numerous DC subsets have been identified in the lung, the DC subsets present in BALT have not been rigorously examined due to the difficulties in separating BALT areas from the rest of the lung tissue during preparation for flow cytometric analysis. However, plasmacytoid DCs (pDCs) are known to accumulate in the T cell areas of BALT and are more prevalent in patients with mild chronic obstructive pulmonary disease (COPD) than in patients with advanced disease (Van Pottelberge et al., in press). The ability of DCs to present antigen in BALT and to maintain BALT architecture is discussed in later sections.
3. Development and Maintenance of BALT
3.1. Role of homeostatic chemokines and LT
The field of lymphoid organ development has progressed significantly since the discovery that Lta −/− mice do not develop LNs or Peyer's patches (Banks et al., 1995, de Togni et al., 1994). The critical signal for lymphoid organ development comes through the LTβR on mesenchymal cells, which leads to the production of homeostatic chemokines, such as CCL19, CCL21, and CXCL13, that in turn recruit lymphocytes and promote lymphoid organ development (Mebius, 2003, Randall et al., 2008). The expression of these chemokines by stromal cells and the expression of LTαβ on recirculating lymphocytes, particularly B cells, are reinforced by a positive feedback loop (Ngo et al., 1999), in which LT controls chemokine expression by stromal cells and chemokine signaling on lymphocytes maintains surface expression of LTαβ on lymphocytes. Thus, the disruption of any part of this loop leads to impaired lymphoid organ development and architecture.
Similar events occur in the development and maintenance of BALT, although details are slightly different. For example, LT signaling is important for the maintenance of BALT (GeurtsvanKessel et al., 2009) and is important for the expression of homeostatic chemokines in models of chronic inflammation, such as in pulmonary exposure to cigarette smoke (Demoor et al., 2009a). The LT-dependent expression of CXCL13 and CCL19 in the lungs of smoke-exposed mice is consistent with data showing that CXCL13 and CCL19 are controlled by LT signaling in conventional lymphoid organs like the spleen (Ngo et al., 1999). However, it is not consistent with our data showing that these chemokines are induced to similar levels in normal and Lta −/− mice upon acute infection with influenza virus (Moyron-Quiroz et al., 2004). Thus, the expression of the so-called homeostatic chemokines seems to be controlled differently during acute and chronic inflammation of the lung.
Given the induced expression of CXCL13 and CCL19 (Moyron-Quiroz et al., 2004), we examined the role for these chemokines in the development of BALT (Rangel-Moreno et al., 2007). Interestingly, BALT was still formed in Cxcl13 −/− mice after influenza infection and had separated B and T cell areas, HEVs, and lymphatics (Rangel-Moreno et al., 2007). However, the B cell areas contained only loose collections of B cells and FDCs did not develop. Thus, CXCL13 is necessary for proper B cell follicle formation in BALT. In contrast, plt/plt mice, which lack CCL19 and CCL21, formed BALT with large B cell follicles, but much smaller T cell zones (Rangel-Moreno et al., 2007). HEVs were also smaller in the animals, possibly due to the lack of CCL21 expression and reduced traffic through the endothelium (Rangel-Moreno et al., 2007). Interestingly, Cxcl13 −/−×plt/plt mice failed to form detectable BALT following influenza infection (Rangel-Moreno et al., 2007). Thus, both B- and T cell attracting chemokines are important for BALT formation and play different roles in its architecture. Similar studies used these same mice to examine the formation and function of BALT surrounding tuberculosis granulomas and came to similar conclusions.
Other studies examined BALT formation using Ccr7 −/− mice (Demoor et al., 2009b, Kahnert et al., 2007, Kocks et al., 2007). Surprisingly, these animals developed BALT spontaneously and formed larger areas of BALT after infection than their normal counterparts. The overall architecture of BALT was apparently normal in Ccr7 −/− mice, with separated B and T cell zones, lymphatics, HEVs, and DCs (Kocks et al., 2007). The BALT hypertrophy in these mice was attributed to a failure of Tregs to home to conventional lymphoid organs and prevent autoimmune reactions (Kocks et al., 2007). Thus, BALT in Ccr7 −/− mice may be partly supporting autoreactive T and B cell responses, but CCR7 is not required for BALT development or organization.
CCR7 is also important for the recovery of CCR7+ T cells from peripheral nonlymphoid tissues via attraction to CCL21-expressing lymphatic vessels (Bromley et al., 2005, Debes et al., 2005). Therefore, in the absence of CCL21 or CCR7, CCR7-expressing cells, including T cells, B cells, and DCs, are unable to return to the circulation and may simply pile up in peripheral tissues (Bromley et al., 2005, Debes et al., 2005). If enough T and B cells are stuck in peripheral tissues, they may begin to spontaneously form organized lymphoid tissues. This possibility is proposed by some investigators to explain the development of ectopic follicles, such as BALT, in Ccr7 −/− mice (Hopken et al., 2007). In fact, a similar mechanism is proposed to explain the formation of BALT in normal animals at sites of inflammation or infection in normal individuals (Carragher et al., 2007, Thaunat et al., 2006). In this model, infection or inflammation leads to rerouting of the lymphatic drainage toward newly formed tertiary lymphoid tissues (Thaunat et al., 2006). As a result, the developing lymphoid tissue is maintained by the continuous influx of antigen and DCs, which leads to sustained local immune activation. Regardless of the final explanation, altered draining of pulmonary lymphatics is likely to play a role in the development and maintenance of BALT.
In addition to its role in the expression of homeostatic chemokines, LT signaling also controls the differentiation of HEVs (Browning et al., 2005) and is important for the expression of various adhesion molecules on vascular endothelial cells and stromal cells. For example, the expression of MAdCAM, PNAd, VCAM-1, and ICAM-1 is controlled, in part, by the activities of LTα, LTαβ, and TNF signaling through LTβR and TNFR1 (Browning et al., 2005, Cuff et al., 1998, Cuff et al., 1999). Similar pathways control the expression of the sulfotransferases that generate the PNAd epitope as well as the expression of GlyCAM and MAdCAM (Drayton et al., 2004, Pablos et al., 2005). Moreover, the development of FDCs is controlled by LT signaling (Endres et al., 1999) and some DC subsets are dependent on LT signaling for their homeostatic maintenance (Kabashima et al., 2005). Given that the LT signaling pathway controls so many aspects of lymphoid tissue architecture and the differentiation of so many cell types important for the function of lymphoid tissues, it is difficult to imagine that BALT can develop normally in the absence of LT signaling. Nevertheless, many publications claim to observe BALT and B cell follicles in Lta −/− mice (Day et al., 2010, Demoor et al., 2009a, Kashino et al., 2010). In fact, we also find perivascular clusters of B cells and even T cells in Lta −/− mice (Moyron-Quiroz et al., 2004). However, these clusters have very little organization and lack FDCs and HEVs. Despite this lack of organization, however, pulmonary immune responses can be generated in splenectomized Lta −/− mice (Constant et al., 2002, Day et al., 2010, Demoor et al., 2009a, Kashino et al., 2010, Lund et al., 2002), suggesting that lymphoid clusters in the lung do have some function, even in the absence of apparent organization.
Lymphoid tissue inducer (LTi) cells are instrumental for the initiation of conventional lymphoid organ development (Cupedo et al., 2002, Eberl & Littman, 2003). LTi cells are first generated in the fetal liver and although they express CD4, they do not express CD3 and are not T cells or B cells (Mebius et al., 2001). LTi cells express LTαβ and are the first cells to arrive at the developing LN anlagen, where they initiate LT signaling and promote the first stages of lymphoid organ development (Randall et al., 2008). Given their role in the development of conventional lymphoid organs, one might assume that they would be involved in the development of ectopic lymphoid tissues, such as BALT. In fact, transgenic mice that overexpress IL-7 have abnormally high numbers of LTi cells and develop a much higher number of LNs and Peyer's patches than normal mice and also develop ectopic lymphoid tissues in pancreas and salivary gland (Meier et al., 2007). We have also observed the development of BALT in IL-7 transgenic mice (J. Rangel-Moreno, T. D. Randall, and D. Finke, unpublished data), suggesting that LTi cells can lead to the development of BALT. Importantly, IL-7 transgenic mice crossed to Rorc −/− mice, which lack LTi cells (Sun et al., 2000), do not develop any secondary lymphoid organs or ectopic lymphoid tissues (Meier et al., 2007). Thus, in this model, the development of ectopic follicles is dependent on LTi cells. However, we have also observed that nontransgenic Rorc −/− mice and Id2 −/− mice infected with influenza do develop BALT (J. Rangel-Moreno and T. D. Randall, unpublished data). Since these mice fail to develop LTi cells, it seems unlikely that they play an essential role on BALT development. Thus, LTi cells may be sufficient, but not necessary for the development of ectopic tissues like BALT.
3.2. Dendritic cells
In addition to their antigen-presenting ability, DCs are also implicated in the maintenance of BALT (GeurtsvanKessel et al., 2009, Halle et al., 2009). For example, CD11bhiMHCIIhi DCs accumulate in the lungs after influenza infection and continue to increase in numbers after infection is cleared (GeurtsvanKessel et al., 2009), correlating with the formation of BALT. Importantly, the depletion of pulmonary DCs triggers the dissolution of BALT (GeurtsvanKessel et al., 2009). A similar study showed that pulmonary infection with modified vaccinia Ankara infection leads to the development of long-lasting BALT areas (Halle et al., 2009). As in the previous study, the depletion of DCs from the lungs led to a significant reduction in BALT size, although the numbers of BALT areas remained similar. Thus, it seems that DCs are required to support the ongoing maintenance of BALT. This is an intriguing idea, since it suggests that unlike conventional lymphoid organs, BALT areas must be actively maintained by immune-stimulating DCs.
How might DCs be important for BALT maintenance? One possibility is that DCs maintain BALT by expressing homeostatic chemokines, including CXCL12 and CXCL13, as well as the cytokine LTβ (GeurtsvanKessel et al., 2009). In fact, LTβR signaling is required for the maintenance of BALT (GeurtsvanKessel et al., 2009), similar to its role in the maintenance of other ectopic lymphoid tissues (Gatumu et al., 2009). However, given that the depletion of pulmonary DCs only modestly reduces LTβ expression and has no effect on either CXCL13 or CCL21 expression (GeurtsvanKessel et al., 2009), it seems that DCs are performing some other function, such as presenting IL-15 to CD8 T cells (McGill et al., 2010) that leads to the maintenance of BALT. Since CD8 T cells are essential for the maintenance of germinal centers in ectopic follicles in other locations (Wagner et al., 1998), they may also support the maintenance of BALT.
3.3. Neuromodulation of BALT
New data suggest that neuronal stimulation is important for secondary lymphoid organ development via the retinoic acid-dependent production of CXCL13 by nerve fibers adjacent to the developing LN anlagen (van de Pavert et al., 2009). Interestingly, areas of BALT contain numerous cholinergic neurons (Cavallotti et al., 2004, Cavallotti et al., 2005), suggesting a possible role for neuromodulation in BALT development or function. Consistent with this idea, one study showed that sensory nerve stimulation in the airways following respiratory syncytial virus (RSV) infection in rats leads to leukocyte recruitment to the airways and BALT hyperplasia (Auais et al., 2003). Interestingly, T cells in BALT expressed high levels of the high-affinity substance P receptor, neurokinin 1. Moreover, inhibitors of neurokinin 1 blocked lymphocyte recruitment to the airways (Auais et al., 2003). Based on these data, the authors of this study concluded that cells from BALT were directly being recruited to the airways, because lymphocytes in peripheral blood lack the neurokinin 1 receptor. However, neuronal stimulation could be indirectly promoting the recruitment of lymphocytes by increasing the production of CXCL13 or other chemokines that in turn recruit lymphocytes in areas of BALT (van de Pavert et al., 2009). Further study is required to clarify any potential role of neuronal stimulation in BALT development or function.
4. Antigen Acquisition
4.1. Microfold cells
Most mucosal lymphoid tissues are located directly beneath a specialized epithelial layer that is infiltrated by lymphocytes and DCs and contains microfold cells (M cells) (Man et al., 2004, Miller et al., 2007). M cells are specialized mucosal epithelial cells that lack cilia, do not express the polymeric Ig receptor, and appear flattened with surface microfolds on the luminal surface (Man et al., 2004, Miller et al., 2007). M cells are thought to transport antigens from the mucosal lumen to DCs that are in close contact with the dome epithelium. M cells are easily observed in NALT and Peyer's patches and are also observed on villous epithelium in the small intestine that is distant from organized lymphoid tissues (Jang et al., 2004) and in the nasal passages away from NALT (Kunisawa et al., 2005). Importantly, some studies provide clear evidence of M cells in the epithelial layer over BALT using electron microscopy (Tango et al., 2000), standard microscopy (Sminia et al., 1989), staining with the lectin, Ulex europaeus agglutinin 1 (UEA1) (Tango et al., 2000), and functionally by the transport of antigens (Gregson et al., 1982, van der Brugge-Gamelkoorn et al., 1985c). Changes in the epithelial layer over BALT consistent with the differentiation of M cells also occur upon pulmonary inflammation or antigen stimulation (van der Brugge-Gamelkoorn et al., 1986a, van der Brugge-Gamelkoorn et al., 1986b). Thus, it seems that epithelial M cells are one way in which BALT acquires antigen from the lumen of the airways. However, not all areas of BALT have a well-defined dome epithelium. Moreover, some areas of BALT appear to be exclusively perivascular or are found in the lower airspaces and lack an association with either airways or arterioles. Thus, these areas of BALT seem to lack M cells and must acquire antigens locally (such as autoantigens or infectious agents) or acquire pulmonary antigens via some other mechanism, such as afferent lymphatics.
4.2. Lymphatics and DC migration
BALT areas have lymphatic vessels that are identified using the antibodies LyVE1 in mice (Carragher et al., 2007) or M2A in humans (Rangel-Moreno et al., 2006). Lymphatics are observed surrounding the B cell follicle and in the T cell area (Carragher et al., 2007, Kocks et al., 2009, Rangel-Moreno et al., 2006). However, it is difficult to assess histologically whether these are afferent lymphatics that bring antigens and cells from distal regions of the lung to BALT or whether they are efferent lymphatics that collect lymphocytes from BALT and return them to the circulation. The idea that DCs acquire antigen in the airways and then migrate to BALT via afferent lymphatics is supported by evidence of DCs and possibly macrophages filled with diesel exhaust particles (DEPs) (Hiramatsu et al., 2003), silica particles (Lee and Kelly, 1993), cigarette smoke particles (van der Strate et al., 2006), and other antigens (Halle et al., 2009) in BALT. In each of these cases, the antigen-bearing DCs are located in the T cell zone of BALT (Fig. 7.1) near lymphatics. However, it is not clear whether antigen gets to BALT via some undefined mechanism, where it is engulfed by resident DCs or whether the DCs obtained the antigens in the airways.
An exciting new study resolves this issue using live imaging of ex vivo lung tissue to show that DCs can migrate from the lung airways into BALT (Halle et al., 2009). This study implies that DCs either migrate directly across the epithelium or enter afferent lymphatics that lead to BALT (Halle et al., 2009). In either case, these data demonstrate that pulmonary antigens can be acquired by BALT via mechanisms other than transfer across the epithelium by M cells (Kiyono and Fukuyama, 2004). Importantly, this study also showed that antigen-pulsed DCs transferred to the airways prime T cell responses in the BALT. Thus, DC migration to BALT is an important mechanism by which antigen is acquired for local immune responses.
4.3. In situ antigens
In cases in which BALT is formed in response to autoantigens, such as in rheumatoid arthritis (RA) (Rangel-Moreno et al., 2006), antigens could be expressed directly in the follicle—perhaps by DCs or other cell types. In addition, in cases where BALT is formed in response to infection, the infectious agent may actually be present in BALT. For example, BALT may form at sites of influenza virus infection and may encompass virally infected cells (Moyron-Quiroz et al., 2004). Thus, antigens would be present in BALT with no need to acquire antigen via M cells or lymphatics. Similarly, some viruses, such as Epstein Barr virus (EBV) directly infect B cells and may be present in the B cell follicles of BALT (Kocks et al., 2009). Moreover, in the case of MTB, BALT essentially forms around the tuberculosis granuloma (Kahnert et al., 2007). Again, MTB antigens may directly incorporated into BALT with the need for specialized mechanisms of antigen transport. Thus, there are many ways for BALT to acquire or be exposed to pulmonary antigens.
5. B Cell Responses in BALT
5.1. B cell responses and germinal centers
Germinal centers are oligoclonal clusters of B cells responding to antigen, typically in T cell-dependent immune responses (Thorbecke et al., 1994). B cells undergo intense clonal expansion in germinal centers, where they ultimately differentiate into long-lived plasma cells or memory B cells. Germinal centers are commonly observed in BALT and can be visualized by staining for markers of proliferating cells (PCNA or Ki-67) (Rangel-Moreno et al., 2006) or for cell surface markers of germinal center B cells, such as PNA and GL7 (GeurtsvanKessel et al., 2009, Moyron-Quiroz et al., 2004). We have also used BrdU labeling to identify germinal centers in mouse BALT and showed that nearly half of the B cells in the germinal centers can be labeled with BrdU in a few hours (Moyron-Quiroz et al., 2004). This rate of proliferation is consistent with B cell proliferation in the germinal centers of conventional lymphoid organs.
The presence of germinal centers in BALT suggests that B cell responses can be initiated and sustained locally. Consistent with this idea, antigen-specific antibody secreting cells are often observed in BALT following pulmonary immunization (GeurtsvanKessel et al., 2009, van der Brugge-Gamelkoorn et al., 1985b, van der Brugge-Gamelkoorn et al., 1986a, van der Brugge-Gamelkoorn et al., 1986b). Moreover, we find that preexisting BALT accelerates B cell responses to influenza in mice with preexisting BALT and leads to reduced morbidity and mortality in response to a number of viruses, including influenza, SARS corona virus, and mouse pneumovirus (Wiley et al., 2009). We observe similar results using mice lacking conventional lymphoid organs (Moyron-Quiroz et al., 2004), suggesting that on its own, BALT is able to initiate pulmonary immune responses that are faster and more protective than those initiated in systemic sites. Another study shows that influenza nucleoprotein-specific plasma cells as well as germinal centers are found in BALT after influenza infection (GeurtsvanKessel et al., 2009). This response is dependent on DCs, as germinal centers are reduced in size and IgA-secreting plasma cells are reduced in number when CD11c+ cells are depleted. Interestingly, long-lived nucleoprotein-specific plasma cells in the bone marrow are also reduced by the elimination of iBALT via DC depletion in the lung (GeurtsvanKessel et al., 2009), suggesting that many of the long-lived plasma cells in the bone marrow are derived from precursors in the lungs rather than in the LN. Moreover, the dissolution of iBALT following DC depletion results in reduced IgA responses in the lung and lower hemagglutinin inhibiting activity in the serum following challenge infection (GeurtsvanKessel et al., 2009). Thus, both local and systemic antibody responses are enhanced by the presence of BALT.
Interestingly, despite the fact that CXCL13 is responsible for organizing the B cell follicle, B cell responses in BALT are not dramatically disrupted in Cxcl13 −/− mice (Rangel-Moreno et al., 2007), since germinal centers are formed and antibody production appears normal following influenza infection. However, germinal centers are severely disrupted and antibody is dramatically reduced in the absence of CCL19 and CCL21, possibly due to defects in T cell responses or to poor DC recruitment to BALT. Thus, the homeostatic chemokines are important for BALT-dependent B cell responses in unexpected ways.
5.2. Isotype switching and somatic mutation
Mature resting B cells initially express surface IgM and IgD. However, when stimulated by antigen, particularly in T cell-dependent responses, B cells undergo isotype switching to various IgG isotypes, IgA and IgE (Stavnezer et al., 2008). In conventional lymphoid organs, switching can take place in extrafollicular foci or in germinal centers (Jacob and Kelsoe, 1992). Switching to particular isotypes depends on the type of immune response. For example, systemic Th1 responses typically trigger switching to IgG2a, whereas Th2 responses promote switching to IgG1 and IgE (Stavnezer et al., 2008). In contrast, mucosal immune responses typically lead to switching to IgA (Briere et al., 1995, Shikina et al., 2004), which is easily transported across mucosal epithelium via the polymeric Ig receptor (Johansen et al., 1999, Shimada et al., 1999). Based on the concept that BALT is a mucosal lymphoid tissue, one might expect that isotype switching in BALT would result in IgA-producing B cells. However, B cells in BALT appear to switch to the full spectrum of isotypes, depending on the type of immune response. For example, we find that immune responses to influenza lead to IgG-secreting cells in BALT (Fig. 7.2). Many studies find that IgG-secreting cells predominate over IgA-secreting cells in BALT (Plesch et al., 1983, Sminia et al., 1987, Sminia et al., 1989), suggesting that BALT does not act like a typical mucosal lymphoid tissue. However, IgA can be easily observed under a variety of conditions (Kolopp-Sarda et al., 1994), including after influenza infection (GeurtsvanKessel et al., 2009). In addition, IgE is often found in BALT areas of patients with allergies (Slavin et al., 1992) or in experimental animals that have been sensitized to antigens under Th2 inducing conditions (Chvatchko et al., 1996, Gajewska et al., 2001). Thus, BALT does not preferentially promote isotype-switching to IgA, but does promote switching to isotypes appropriate for the type of immune response.
Another function of germinal centers is to support somatic hypermutation and affinity selection of B cells (Kelsoe, 1996). Current thinking suggests that B cells proliferate in the dark zone of the germinal center and are selected for high-affinity antigen receptors by immune complex-bearing FDCs in the light zone (Allen et al., 2007, Schwickert et al., 2007). Most areas of BALT in patients or animals with active immune responses have germinal centers and FDCs (Fig. 7.3). Moreover, we find that germinal centers in patients with RA had distinct light and dark zones (Rangel-Moreno et al., 2006). Since extensive FDC networks are observed in the RA patients and since the FDCs in BALT are capable of binding immune complexes, it stands to reason that high-affinity B cells are being selected in BALT germinal centers and will likely produce both long-lived plasma cells and memory B cells. In the case of B cell responses to pulmonary infections, this type of response is probably beneficial. However, in response to autoantigens in patients with RA, this response is likely to be pathologic.
Additional evidence for affinity selection in the germinal centers of BALT comes from patients with COPD. BALT from these patients contains B cells that predominantly expressed CD27, which is a memory marker on B cells (van der Strate et al., 2006). BALT from these patients also contains numerous germinal centers and a molecular analysis of B cells from these germinal centers shows evidence of ongoing somatic hypermutation (van der Strate et al., 2006). Thus, it is likely that high-affinity B cells are being selected in these patients, although the antigen specificity of these B cells is unknown.
5.3. Maintenance of plasma and memory B cells
As mentioned in previous sections, isotype-switched plasma cells are often found in the T cell zone of BALT, similar to the placement of plasma cells in the T zone of the spleen. Given the dependence of BALT plasma cells on DCs (GeurtsvanKessel et al., 2009), it is possible that DCs or other myeloid cells in the T zone are producing plasma cell survival factors, such as IL-6 and APRIL (Mohr et al., 2009). Thus, the depletion of DCs may lead to reductions in local plasma cell numbers via impairment of the plasma cell niche. Moreover, CXCL12, which is thought to be important for the recruitment of long-lived plasma cells to the bone marrow (Hargreaves et al., 2001, Hauser et al., 2002), is also expressed in the T zones of BALT. Therefore, BALT may serve as a reservoir of long-lived plasma cells that were generated locally in response to pulmonary antigens.
6. T Cell Responses
6.1. T cell priming and maintenance of memory
Despite years of accumulating evidence that reactive BALT can be observed in experimentally immunized or environmentally exposed animals and the observations that antigen-specific B cell responses can occur in BALT, direct evidence for antigen-specific T cell responses in BALT has been lacking. We showed that in mice lacking conventional secondary lymphoid organs, CD8 T cell responses to influenza are primed with normal kinetics (Moyron-Quiroz et al., 2004) and that antigen-specific CD8 T cells can be found in regions of BALT (Moyron-Quiroz et al., 2006). Thus, we concluded that CD8 T cells could be primed in BALT, but we could not definitively exclude the (unlikely) possibility that they were actually primed in systemic sites like the bone marrow. Similar studies using splenectomized Lta −/− mice show that immune responses to pulmonary infection with MTB (Day et al., 2010, Kashino et al., 2010) and Leishmania major antigens (Constant et al., 2002) are intact in the absence of conventional lymphoid organs and antigen presentation appears to occur directly in the lung. These studies suggest that BALT is likely to be a priming site for naïve T cells responding to pulmonary antigens.
However, more recent studies directly demonstrate that BALT can recruit and prime naïve T cells. For example, following the induction of BALT by infection with MTB, adoptively transferred OVA-specific OTII TCR transgenic T cells are primed in the lungs by pulmonary exposure to OVA (Day et al., 2010). Importantly, T cell priming is intact in the lungs of MTB-infected mice that lack conventional secondary lymphoid organs, but does not occur if BALT is not previously induced by infection with MTB (Day et al., 2010). Thus, T cell priming in the lung to pulmonary antigens requires the presence of BALT. Similar studies showed that after BALT is induced in response to modified vaccinia virus, adoptively transferred OVA-specific naïve OTI cells are recruited to BALT and proliferate locally in response to intratracheally administered OVA-pulsed DCs (Halle et al., 2009). This study also examined the dynamics of T cells in BALT explants and showed that long-lasting interactions between T cells and antigen-bearing DCs occur in the T cell zone of BALT between 24 and 48 h after transfer (Halle et al., 2009). By 48 h after transfer, the T cells appeared blast-like and had probably begun to proliferate. In contrast, antigen-nonspecific T cells did not exhibit stable interactions with DCs and had much higher motility coefficients (Halle et al., 2009). Thus, antigen presentation and T cell priming can occur directly in BALT in response to antigens derived from the airways.
Memory T cells can also be maintained in BALT. We found that memory T cell responses to influenza were maintained in the lungs of mice lacking conventional secondary lymphoid organs (Moyron-Quiroz et al., 2006). BALT was present in these animals and was maintained for at least 3 months after infection (Moyron-Quiroz et al., 2006). Using in situ staining with influenza-specific tetramers, we showed that BALT had antigen-specific CD8 memory T cells and that these cells could react to secondary influenza infections in situ (Moyron-Quiroz et al., 2006). Importantly, T cell-dependent memory responses to influenza were intact in these mice and virus clearance was accelerated. Thus, BALT supports T cell priming and expansion as well as memory T cell maintenance.
6.2. Tregs in BALT
Mucosal lymphoid organs, such as the Peyer's patches, tend to elicit T cell responses with a mucosal phenotype that produce IL-5, TGFβ, or IL-10 rather than IFNγ or IL-4 (Kiyono and Fukuyama, 2004). In addition, mucosal immune responses to commensal or food antigens often elicit Tregs (Fujihashi et al., 2001). These data suggest that BALT may bias CD4 T cell differentiation toward mucosal type responses or Treg responses. Consistent with this idea, we find that in mice lacking conventional lymph nodes (Moyron-Quiroz et al., 2004) and in mice that have BALT preinduced with protein cage nanoparticles (Wiley et al., 2009), immune responses to subsequent pulmonary infection with influenza and other viruses lead to substantially reduced morbidity and mortality. Since morbidity in response to viral infection is often due to overexuberant T cell responses, it is possible that BALT primes T cells with an anti-inflammatory or mucosal phenotype. Unfortunately, the presence of Tregs was not examined in these studies.
Interestingly, IgA responses are often observed in BALT (Breel et al., 1988, GeurtsvanKessel et al., 2009, Sminia et al., 1987, Suda et al., 1999), and based on other data, may be linked to Tregs. For example, the transfer of naturally occurring Tregs to recipient mice results in their recruitment to Peyer's patches and their differentiation into T follicular helper (Tfh) cells that promote IgA production (Tsuji et al., 2009). Similarly, other studies report that Tregs facilitate germinal center reactions (Marinova et al., 2007). Thus, the presence of Tregs in BALT may reflect the presence of IgA-helper activity rather than suppressor activity.
Two studies have looked at the role of Tregs suppressor activity in BALT. In the first study, FOXP3+ Tregs were preferentially found in areas of BALT, rather than in nonlymphoid areas of the lungs (Heier et al., 2008). However, the presence or frequency of Tregs in BALT did not correlate with either atopy or clinical symptoms of asthma in a cohort of children less than 2 years of age. Another study showed a more direct effect of Tregs on BALT and demonstrated that Tregs normally suppressed the development of BALT (Kocks et al., 2007). This study showed that Ccr7 −/− mice have very few Tregs in their LNs and also develop iBALT shortly after birth. Furthermore, the development of iBALT is prevented by the adoptive transfer of Ccr7 +/+ Tregs to Ccr7 −/− recipients (Kocks et al., 2007). Surprisingly, Ccr7 −/− mice actually have higher numbers of Tregs in peripheral tissues, including the lung, suggesting that it is the activity of Tregs in the LNs rather than in the lungs that prevents iBALT formation (Kocks et al., 2007). Thus, Tregs normally suppress the formation of BALT, probably by suppressing the activity of autoreactive T cells. Given these data, then it should be instructive to examine the numbers and functions of Tregs in the BALT of patients with RA, as these patients often develop extensive areas of BALT.
7. Role of BALT in Resistance to Infectious Disease
7.1. Viral infections
The respiratory tract is a common portal of entry for viruses and it is a challenge for the immune system to effectively eliminate viruses and virally infected cells, without causing so much damage and inflammation that pulmonary function is compromised. This balancing act is achieved through an intricate network of innate and adaptive immune mechanisms as well as through immunoregulatory and anti-inflammatory mechanisms. BALT is likely to be one of the mechanisms that both helps to facilitate viral clearance by initiating immune responses and by reducing inflammatory responses. For example, we found that Lta −/− mice, which lack LNs and Peyer's patches, are more sensitive to influenza virus and, although they do generate immune responses, both B and T cell responses are delayed (Lund et al., 2002). At the time, we questioned where immune responses might be initiated in Lta −/− mice and concluded that both B and T cell responses were probably generated in the lungs based on the flow cytometric identification of germinal center B cells in the lung (Lund et al., 2002). Since germinal centers are exclusively formed in secondary lymphoid tissues, we suggested that BALT was formed in the lungs of Lta −/− mice and initiated immune responses to influenza locally.
We later tested this hypothesis by generating mice that lacked all secondary lymphoid organs, but retained the LT signaling pathway (splenectomized Lta −/− mice that had been irradiated and reconstituted with normal bone marrow—SLP mice; Moyron-Quiroz et al., 2004). Upon influenza infection, SLP mice rapidly formed BALT with easily identified germinal centers, separated B and T cell areas, HEVs, and other hallmarks of secondary lymphoid tissues (Moyron-Quiroz et al., 2004). Interestingly, homeostatic chemokines such as CCL21 and CXCL13 were induced in the lungs following influenza infection independently of the LT or TNF signaling pathways (Moyron-Quiroz et al., 2004). Given that LT signaling is essential for homeostatic chemokine expression in conventional lymphoid organs (Ngo et al., 1999), it seems that acute infection leads to an alternative pathway for the expression of these chemokines.
SLP mice also generated B and T cell responses with no delay and cleared virus with the same kinetics as normal mice (Moyron-Quiroz et al., 2004). Surprisingly, the SLP mice exhibited less morbidity in response to influenza infection and could survive doses of virus that killed normal mice (Moyron-Quiroz et al., 2004). Thus, it seems that immune responses initiated in BALT are less inflammatory than systemic immune responses. In fact, a more recent study showed that BALT can be induced by exposure to inert protein cage nanoparticles that are assembled from 24 subunits of the small heat shock protein (sHsp 16.5) of Methanococcus jannaschii (Wiley et al., 2009). Once induced, the presence of BALT led to accelerated immunity in response to influenza, mouse-adapted SARS coronavirus, and mouse pneumovirus (Wiley et al., 2009). Reduced morbidity and increased survival were also observed (Wiley et al., 2009). Thus, BALT seems to be generally protective against respiratory viruses.
Once BALT is formed, it seems to persist in the lungs for months following viral clearance (Moyron-Quiroz et al., 2006). Although the areas of BALT became smaller overtime, they can be easily identified for up to 3 months (Moyron-Quiroz et al., 2006). The long-lived areas of BALT harbored influenza-specific memory CD8 T cells that were maintained locally by homeostatic proliferation. Long-lived BALT was also home to influenza-specific plasma cells that probably contributed to the persistence of influenza-specific neutralizing IgG in the serum and BAL. Importantly, both long-lived antibody production and memory T cells were functional and could neutralize a viral challenge with the same strain of influenza (antibody) or accelerate the clearance of a different serotype of influenza (T cells) (Moyron-Quiroz et al., 2006). Thus, once formed, BALT probably plays an important role in combating successive rounds of the same infection and may even help initiate local immunity to unrelated viruses or pathogens.
The idea that successive viral infections alter subsequent immune responses is not new (Goulding et al., 2007). Some investigators have shown that the specific order of different viral infections leads to different types of pulmonary pathology, including the formation of BALT (Chen et al., 2003). Interestingly, in this study, acute infection with lymphocytic choriomeningitis virus (LCMV), murine cytomegalovirus (MCMV), or influenza led to moderate to severe interstitial pneumonia, but no discernable BALT areas. However, large areas of BALT developed following subsequent infection with vaccinia virus (Chen et al., 2003). Moreover, whereas influenza immune mice developed small areas of BALT even after challenge with LCMV, they developed extensive areas of BALT after challenge with MCMV (Chen et al., 2003). Interestingly, viral titers to secondary infection were decreased in the mice that developed BALT during primary infection (Chen et al., 2003), consistent with our observations that preexisting BALT facilitate the clearance of multiple respiratory viruses (Moyron-Quiroz et al., 2004, Wiley et al., 2009).
Two studies shed light on how viral infection leads to the formation and persistence of BALT (GeurtsvanKessel et al., 2009, Halle et al., 2009). The first study showed that pulmonary influenza virus infection leads to DC recruitment to the lungs and that DCs are required to maintain BALT structures (GeurtsvanKessel et al., 2009). When DCs are depleted using CD11c-diptheria toxin receptor transgenic (CD11c-DTR) mice, BALT areas are dramatically reduced in size. This study also demonstrated the involvement of BALT areas in local immune responses to influenza as they showed that nucleoprotein-specific plasma cells were formed in BALT and that influenza-specific antibody titers were decreased upon DC depletion (GeurtsvanKessel et al., 2009). The second study used infection with modified vaccinia Ankara virus to trigger BALT formation (Halle et al., 2009). Again, the maintenance of iBALT was dependent on CD11c+ cells, as depletion of CD11c+ cells from the lungs of infected CD11c-DTR mice lead to the rapid dissolution of iBALT. Functionally, this study showed that pulmonary DCs transferred to the airways of recipient mice rapidly accumulate in BALT areas where they interact with T cells and present antigen (Halle et al., 2009). Thus, DCs are an important component of BALT, not only for the presentation of antigen to T cells, but also for the maintenance of BALT integrity.
In addition to acute viral infection, some viruses, such as those in the herpesvirus family, become latent in a variety of cells types. For example, murine γ-herpesvirus 68 (γHV-68) is a natural rodent pathogen that is related to the human pathogens, EBV, and Kaposi's sarcoma virus (KSHV) (Efstathiou et al., 1990). Pulmonary infection of mice with γHV-68 leads to the development of BALT, which is exacerbated in Ccr7 −/− mice (Kocks et al., 2009). Despite the extensive areas of BALT in Ccr7 −/− mice, peak viral titers are higher and the clearance of lytic infection from the lungs is delayed (Kocks et al., 2009). These changes are likely due to poor priming of γHV-68-specific T cells in Ccr7 −/− mice. Interestingly, latent virus was detected in BALT areas of both normal and Ccr7 −/− mice. This is not too surprising since latent gHV-68 is known to be harbored in B cells (Flano et al., 2000). However, the authors of this study showed that genes from both lytic and latent phase of the virus were expressed in areas of BALT even at late timepoints after infection (Kocks et al., 2009), suggesting that virus was continuously undergoing reactivation in areas of BALT. If so, then it is possible that the reactivity of BALT to environmental antigens is leading to viral reactivation or conversely that viral reactivation is promoting immune activity in BALT and promoting BALT persistence. A similar possibility has been suggested for both EBV and KSHV in ectopic follicles in the CNS of patients with multiple sclerosis (Franciotta et al., 2008).
7.2. Tuberculosis
Mycobacterium tuberculosis (Mtb) remains one of the major health threats throughout the world and accounts for the deaths of over two million people per year (North and Jung, 2004). M. tuberculosis infection is typically restricted to the lungs and local immune mechanisms are primarily responsible for M. tuberculosis containment (North and Jung, 2004). Once Mtb is established in the lungs, it is difficult to eradicate through immune mechanisms alone and drug treatment is required for sterilizing clearance. Mtb becomes established in granulomas, which, in humans, consist of a central core that is often necrotic, surrounded by macrophages, multinucleate giant cells, and lymphocytes (Actor et al., 1999). CD4 T lymphocytes, particularly Th1 cells are essential for containment (Flynn and Chan, 2001). These cells are required to produce IFNγ and to activate macrophages, which are the main effector cell type that kills Mtb (North and Jung, 2004). Surrounding the granulomas in humans are clusters of lymphocytes, particularly B cells, which form structures similar to BALT (Ulrichs et al., 2004). These granuloma-associated BALT areas have B cell follicles with occasional Ki-67+ germinal center-like foci (Ulrichs et al., 2004). T cell areas are also observed around the outside edge of the follicles. Macrophages and DCs are also observed in the T cells, although not to the extent that they are found in the granuloma itself. Interestingly, granulomas had to reach a minimal size to trigger the formation of BALT areas, but the BALT areas did not expand as the granuloma size increased (Ulrichs et al., 2004).
Similar areas of BALT were found in murine models of Mtb infection, in which multiple B cell clusters were also observed surrounding the granuloma (Kahnert et al., 2007). Well-defined B cell areas with FDCs were formed as early as day 42 after pulmonary infection and were maintained to at least day 90 postinfection (Kahnert et al., 2007). Mtb infection progressively induced the expression of both CCL19 and CXCL13 (Kahnert et al., 2007, Khader et al., 2009), homeostatic chemokines implicated in the structural integrity of conventional secondary lymphoid organs (Ansel et al., 2000, Forster et al., 1999) as well as ectopic lymphoid tissues like BALT (Moyron-Quiroz et al., 2004, Rangel-Moreno et al., 2005). This study also examined Ccr7 −/− mice, which are unable to respond to the homeostatic chemokines CCL21 and CCL19 (Forster et al., 1999). Although the mycobacterial load in the lungs of Ccr7 −/− mice was no different than that in normal mice, the follicular structure of the B cell areas was disrupted and the overall size of the inflammatory lesions in the lung were increased (Kahnert et al., 2007). Moreover, the burden of Mtb in the spleen was increased in Ccr7 −/− mice (Kahnert et al., 2007), suggesting that the follicular structures in the lung may facilitate containment of Mtb.
Given the link between B follicular structures surrounding the granuloma and Mtb containment, another study examined the role for B cells in response to Mtb (Maglione et al., 2007). Although previous studies showed that B cells played a limited role in resistance to Mtb in mice (Turner et al., 2001), a newer study demonstrated that the B cell follicles formed around tuberculosis lesions in mice developed extensive germinal centers (Maglione and Chan, 2009), suggesting that the B cells were responding to antigen. In addition, B cell-deficient animals displayed more extensive lung lesions and higher mycobacterial burdens in the lung, and ultimately died earlier than their intact counterparts (Maglione and Chan, 2009), despite apparently normal Th1 responses. Thus, B cells do contribute to immunity to Mtb, possibly via their ability to form local immune aggregates in the lung.
Homeostatic chemokines also play a role in the containment of Mtb as shown in a recent study that examined pulmonary immune responses to Mtb in Cxcl13 −/− mice and in plt/plt mice as well as in Cxcl13 −/−×plt/plt mice (Khader et al., 2009). All three mutant strains of mice exhibited delayed granuloma formation and severely disrupted BALT architecture, despite clusters of iNOS-expressing activated macrophages in granulomatous lesions (Khader et al., 2009). Interestingly, the Cxcl13 −/− mice and plt/plt mice displayed slightly different immunological abnormalities. For example, the spatial arrangement of granulomas was disrupted in Cxcl13 −/− mice and T cells tended to accumulate in perivascular areas rather than near infected macrophages. In contrast, the generation of Th1 cells was delayed in plt/plt mice (Khader et al., 2009). Importantly, mycobacterial titers were higher in the lungs of both Cxcl13 −/− mice and in plt/plt mice and were higher still in the lungs of Cxcl13 −/−×plt/plt mice, demonstrating that the expression of homeostatic chemokines in the lungs were functionally important for priming local immune responses to Mtb (Khader et al., 2009).
Although the studies above suggested that BALT areas, B cells, and pulmonary expression of homeostatic chemokines were important for immunity to Mtb, it is often difficult to distinguish the effects of these mutations on local events in the lung, from their well-established effects in conventional secondary lymphoid organs. Thus, in order to convincingly demonstrate that BALT areas in the lung can play a role in initiating local immune responses to Mtb, two studies involved infected mice that lacked conventional secondary lymphoid organs and examined pulmonary immunity (Kashino et al., 2010). The first study showed that splenectomized Lta −/− mice could generate IFNγ-producing CD4 T cells in the lungs in response to pulmonary challenge (Kashino et al., 2010), although it was not clear whether this response was similar or reduced compared to that in normal mice. In addition, BALT was much more rapidly formed in splenectomized Lta −/− mice than in normal mice following Mtb infection (Kashino et al., 2010), similar to what we have observed in response to influenza virus.
In the second, much more detailed study, the authors found that splenectomized LTβR −/− mice could generate immune responses to Mtb, but that the CD4 T cell response was slower to get started and was reduced in magnitude in the absence of conventional lymphoid organs (Day et al., 2010). CD8 T cell responses were also slower to get started in mice lacking conventional lymphoid organs. Delayed and reduced immune responses resulted in high mycobacterial titers in the lungs (Day et al., 2010). Granulomatous lesions with associated BALT areas were observed in all groups of mice, although they were slower to appear in the lungs of mice lacking conventional lymphoid organs (Day et al., 2010). Again these data are suggestive that immune responses can be initiated in the BALT areas of the lungs. Importantly, the authors went on to show that adoptively transferred OVA-specific OTII TCR transgenic cells could be primed directly in the lungs of mice lacking conventional lymphoid organs, but only after the granulomatous response had initiated BALT development (Day et al., 2010). Thus, these results clearly demonstrate the utility of BALT initiating local pulmonary immune responses. The major drawback with both of these studies is that they used mice with defective LT signaling pathways and they did not look at BALT organization (Day et al., 2010, Kashino et al., 2010). Given the importance of LT signaling in the organization of BALT (Moyron-Quiroz et al., 2004), the development of FDCs (Endres et al., 1999) and the expression of PNAd on HEVs (Browning et al., 2005), it is very possible that immunity to Mtb may not be impaired at all in mice lacking conventional lymphoid organs and that BALT may be entirely sufficient to initiate local pulmonary immune responses.
7.3. Other bacterial infections
The presence of BALT in some species, such as mice and humans, is typically associated with infection (Gould and Isaacson, 1993). In other species, such as rats and rabbits, BALT seems to be constitutively present (Pabst and Gehrke, 1990), but is increased in number, size, and architectural complexity in response to microbial stimulation. In pigs, BALT is not present at birth or under germ-free conditions (Pabst and Gehrke, 1990), but is present in about 50% of pigs housed under conventional conditions by 2 months after birth (Pabst and Gehrke, 1990). In another study, 100% of 4-month-old pigs had BALT even prior to infection. However, infection with Actinobacillus pleuropneumoniae by aerosol leads to increases in the number of BALT areas and dramatic increases were observed if the pigs had been previously immunized against the bacteria (Delventhal et al., 1992). Moreover, pigs naturally infected with Mycoplasma hyopneumoniae have extensive BALT areas that are considerably expanded compared to controls (Sarradell et al., 2003). IgA-producing plasma cells are also associated with BALT expansion in response to infection with M. hyopneumoniae (Sarradell et al., 2003). In addition, IL-2, IL-4, and TNFα-producing cells were observed in BALT areas of M. hyopneumoniae-infected pigs (Rodriguez et al., 2004), suggesting that ongoing immune responses were occurring in these sites. Similarly, surfactant protein D (SPD) was observed to accumulate in DCs in the BALT areas of pigs responding to A. pleuropneumoniae and Staphylococcus aureus (Soerensen et al., 2005), suggesting that either DCs acquired SPD as they migrated from the airways to the BALT or that SPD-coated bacteria were transported by M cells to the underlying DCs in BALT. In either case, these data demonstrate that BALT efficiently collects antigens from the airways.
In humans, chronic bacterial stimulation is a common insult that leads to BALT development or hypertrophy, particularly, during development. For example, BALT was commonly observed in the lungs of fetuses with chorioamnionitis (31%) or chorioamnionitis with intrauterine pneumonia (69%) compared to fetal lungs showing no signs of infection (3%) (Gould and Isaacson, 1993). However, BALT was also observed at a high frequency (84%) in samples from infants who succumbed to sudden infant death syndrome (SIDS), even though only two of the infants with BALT showed evidence of pulmonary infection (Gould and Isaacson, 1993). Similarly, another study showed that BALT was present in 100% (66/66) of fetuses with chorioamnionitis, whereas BALT was present in only 10% (8/75) fetuses who died as a result of other causes (Ersch et al., 2005). Thus, pulmonary exposure to bacteria often leads to the development of BALT, which facilitates local immune responses.
8. Role of BALT in Pulmonary Responses to Allergens and Environmental Antigens
8.1. Endotoxin exposure
Endotoxin or lipopolysaccharide (LPS) is a component of gram-negative bacteria and is a classic T cell independent B cell antigen and mitogen. LPS engages the TLR4 signaling pathway (Hoshino et al., 1999, Takeuchi et al., 1999) and triggers B cell activation, proliferation, and differentiation to antibody secreting cells (Pike et al., 1987). TLR4 signaling also activated macrophages and DCs, epithelial cells, and even fibroblasts to produce inflammatory cytokines and chemokines (Kawai et al., 2001, Takeda & Akira, 2001). In the case of DCs, LPS induces them to mature into potent antigen-presenting cells that effectively prime T cells. Endotoxin is also common in the environment and exposure to significant levels of LPS is associated with the development or exacerbation of asthma (Becker et al., 2002, Murakami et al., 2007), bronchitis, and COPD (Droemann et al., 2005, Mizutani et al., 2009). Experimentally, pulmonary exposure of rats to endotoxin leads to the expansion of preexisting BALT and increases in pulmonary plasma cells and ultimately the formation of germinal centers (van der Brugge-Gamelkoorn et al., 1985a). Given that germinal centers are dependent on T cells, LPS in this case is probably acting to activate DCs, which migrate from the airways to BALT and prime B and T cells to environmental antigens. Interestingly, LPS does not have to be administered via the airways in order to lead to BALT hyperplasia (Banfi et al., 2009). In this study, the authors intraperitoneally administered LPS to rats as a model of sepsis-related lung injury and observed BALT hyperplasia, signs of acute respiratory distress syndrome (ARDS), diffuse alveolar damage, and emphysema (Banfi et al., 2009).
In mice, which lack BALT prior to pulmonary inflammation, persistent dosing with LPS leads to the development of BALT along major airways with the accumulation of B cells, T cells, and macrophages in the lungs—even in areas that lacked BALT (Vernooy et al., 2002). Macrophages in the lungs of LPS-exposed mice expressed very high levels of MHC class II, consistent with an activated phenotype. Marked changes in the airways were also observed with an increase in goblet cell numbers and enlargement of airspaces suggesting of emphysema (Vernooy et al., 2002).
Although the above studies experimentally administered LPS as an inflammatory agent, environmental exposures to LPS also induce changes in BALT (Charavaryamath et al., 2005). In one study, rats were exposed to swine barn air (containing very high concentrations of LPS) or ambient air for up to 20 days. Barn air-exposed mice exhibited an increase in airway hyper-reactivity, which correlated with increases in lymphocytes, neutrophils, and eosinophils (Charavaryamath et al., 2005). BALT was hyperplastic and contained reactive germinal centers and plasma cells in rats exposed to barn air (Charavaryamath et al., 2005). Thus, even environmental exposures to LPS, often with additional antigenic or inflammatory components, lead to BALT reactivity and alterations in pulmonary physiology.
8.2. Allergy and asthma
Given the role of pulmonary inflammation in asthma, one might expect that the development of BALT would correlate with asthma or at least with the severity of asthma. However, several studies suggest that BALT formation is not necessarily associated with asthma. For example, one study examined endobronchial biopsies from nonsmoking cross-country skiers and control subjects and found that although a higher frequency of skiers had areas of BALT than controls, the presence of BALT was not associated with either a history of respiratory allergy or asthma (Sue-Chu et al., 1998). In both groups, the areas of BALT consisted of small B cell follicles surrounded by T cells and macrophages or DCs, but did not appear reactive and did not contain germinal centers (Sue-Chu et al., 1998). Similarly, a study of 45 infants from 4 to 23 months of age concluded that BALT structures were present in about half the samples, but their presence did not correlate with lung function, atopy, or (surprisingly) rhinovirus positivity (Heier et al., 2008). Interestingly, in addition to the standard description of B cell follicles surrounded by T cells and DCs, the authors of this study found that FOXP3+ Tregs as well as pDCs were preferentially found in areas of BALT (Heier et al., 2008). Thus, there is no obvious connection between the presence of BALT and the development of asthma.
However, another study suggests that while the presence of BALT may not correlate with asthma, the reactivity of BALT increased in asthma patients (Elliot et al., 2004). In this study, a postmortem comparison of lung tissue from nonsmokers, smokers, nonfatal asthma, and fatal asthma revealed minimal differences in the frequency of patients in each group with BALT, the number of BALT areas, or the location of BALT areas (Elliot et al., 2004). However, the total area of BALT observed in each group was significantly different, with large BALT observed in both asthma groups compared to either nonsmokers or smokers (Elliot et al., 2004). In addition, there is evidence that exposure to particular allergens, such as Aspergillus fumigatus, can lead to pulmonary allergies reminiscent of asthma (Slavin et al., 1992). For example, extensive areas of BALT were observed in a patient with allergic bronchopulmonary aspergillosis, in which germinal centers were prevalent and in every case stained with IgE (Slavin et al., 1992). These data suggested that IgE was being generated locally, presumably in response to Aspergillus. Thus, BALT has potential to participate in allergic responses.
Similar results have been observed using experimental systems. For example, using the mouse model of allergic airway disease, in which mice are initially sensitized to OVA via the peritoneal cavity and then repeatedly challenged by intratracheal instillation of OVA, one study demonstrated that BALT was formed in response to OVA and that OVA-specific IgM-, IgG1-, and IgA-producing plasma cells could be found in the lung (Chvatchko et al., 1996). Moreover, germinal centers containing IgE-expressing B cells were observed and IgE was produced locally in the lung (Chvatchko et al., 1996). Thus, pulmonary antigen exposure in the context of a Th2 response can lead to local IgE responses in the BALT areas of the lung.
Eosinophilia is another hallmark of asthma. Eosinophils are recruited by Th2 responses and IL-5, one of the canonical cytokines made by Th2 cells (Maggi, 1998), is a growth and survival factor for eosinophils (Coffman et al., 1989, Sher et al., 1990). To determine a role for eosinophils in asthma, one study generated transgenic mice that expressed IL-5 in lung epithelial cells (Lee et al., 1997). The transgenic mice exhibited pulmonary pathology that was reminiscent of asthma, including a dramatic eosinophilia, goblet cell hyperplasia, and epithelial cell hypertrophy (Lee et al., 1997). However, the mice also developed extensive areas of BALT. Interestingly, eosinophils were clustered around the B cell follicles, in the same location where T cells and DCs are normally located (Lee et al., 1997). These mice also exhibited airway hyper-responsiveness, independently of any antigenic challenge, suggesting that the pathologic changes induced by IL-5 overexpression directly triggered this asthma-like condition (Lee et al., 1997).
The transgenic overexpression of IL-6 in the lung epithelium also induces the formation of BALT (Goya et al., 2003), but without the accumulation of eosinophils. BALT areas in IL-6 transgenic mice consisted primarily of primary B cell follicles without germinal centers. T cells, particularly CD4 T cells, were present surrounding and between B cell follicles and plasma cells were also observed (Goya et al., 2003). Although pulmonary function was not measured, there were no visible changes in the overall lung architecture (other than the accumulation of BALT) that might be suggestive of an asthma phenotype. Thus, the development of BALT per se does not directly lead to asthma or even lung disease, but the presence of BALT may participate in ongoing Th2 responses and exacerbate preexisting asthma.
An interesting question in asthma and allergic airway disease is where does Th2 priming occur? It seems likely that asthmatic individuals are exposed to allergens via the airways, but it is unclear whether T cells can be primed in situ in the lung or whether antigen must traffic to draining LNs. Two studies shed light on this issue in the context of Th2 responses and show that mice lacking draining LNs are fully competent to prime Th2 cells and promote allergic airways disease (Constant et al., 2002, Gajewska et al., 2001). Both studies used Lta −/− mice, which lack LNs and Peyer's patches and, after splenectomy, also lack the spleen. In the first study, the authors used an adenovirus to overexpress GM-CSF in the lungs prior to repeated aerosol OVA exposure (Gajewska et al., 2001). They observed robust Th2 responses in both normal and Lta −/− mice, with Th2 cytokine expression and the accumulation of eosinophils. However, the overall inflammatory response in the lungs of Lta −/− mice was dramatically higher than in normal mice (Gajewska et al., 2001), consistent with our observation using influenza infected Lta −/− mice (Lund et al., 2002). Interestingly, splenectomy of the Lta −/− mice completely abrogated the Th2-induced allergic response, suggesting that conventional secondary lymphoid tissues are essential for priming Th2 responses to pulmonary antigens (Gajewska et al., 2001). The authors of this study did not specifically examine BALT, but perivascular and peribronchiolar lymphoid aggregates could be observed in both normal and Lta −/− mice (Gajewska et al., 2001). However, in our experience, the lack of LT signaling in Lta −/− mice would have prevented BALT organization and may dramatically impair the ability of BALT to prime local Th2 responses (Lund et al., 2002, Moyron-Quiroz et al., 2004).
In the second study (Constant et al., 2002), the authors intranasally administered L. major parasites to normal and splenectomized Lta −/− mice and then intranasally challenged the primed mice a week later with another dose of L. major (Constant et al., 2002). Robust Th2 responses were observed in both groups, with the production of IL-5 and IL-13 and the accumulation of eosinophils (Constant et al., 2002). The authors did not specifically examine the lungs for BALT, but they did note that large numbers of antigen-bearing DCs remained in the lung after challenge leading to their conclusion that Th2 responses could be primed directly in the lung (Constant et al., 2002). The difference between the conclusions of these studies is striking and is likely due to the differences in the antigens used to prime Th2 responses. Certainly, one would expect that challenge with live L. major parasites to be a stronger stimulus than aerosol OVA. Nevertheless, we can conclude that spleen and LNs are not required for some pulmonary Th2 responses, but certainly facilitate normal responses, particularly, in the systemic absence of LT.
8.3. Hypersensitivity pneumonitis
Hypersensitivity pneumonitis is an inflammatory disease of the alveoli caused by hypersensitivity to inhaled organic antigens (Mohr, 2004). Exposure is commonly occupational and farmers, in particular, are often exposed to molds and fungi in barns leading to the term “Farmer's Lung” (Seal et al., 1968). The most effective treatment is avoidance of exposure, which can cause acute symptoms. Unlike asthma, hypersensitivity pneumonitis targets alveoli rather than airways.
Given that hypersensitivity pneumonitis is driven by chronic pulmonary exposure to antigen, it is not surprising that patients may develop BALT. In fact, we observed well-developed BALT areas with extensive germinal centers and FDC networks in lung biopsies of some hypersensitivity pneumonitis patients (Fig. 7.3; Rangel-Moreno et al., 2006), although other patients showed only smaller B cell clusters that lacked FDCs and germinal centers. Another study showed similar results with three of five patients showing well-defined BALT with FDCs and germinal centers (Suda et al., 1999). This study also showed that some areas of BALT were similar to classic mucosal type tissues with a dome epithelium containing subepithelial DCs and peripheral T cell areas (Suda et al., 1999). Experimentally, hypersensitivity pneumonitis can be mimicked by first immunizing animals with protein antigens and then chronically exposing them to the same antigen via the pulmonary route. For example, chronic pulmonary exposure of rats leads to BALT hypertrophy and histologic parameters of hypersensitivity pneumonitis (de Dios Escolar et al., 1994).
Given the antigen-driven development and exacerbation of hypersensitivity pneumonitis, it seems reasonable to conclude that pulmonary immune responses to the sensitizing antigen will be pathologic and not beneficial in this disease. Thus, one would like to conclude that the development or hyperplasia of BALT would contribute to pathology. However, despite the correlation between chronic hypersensitivity pneumonitis and BALT development, there is no direct causal link in either direction that establishes any functional relationship.
9. Role of BALT in Response to Particulates and Cigarette Smoke
9.1. Particulate exposure and BALT formation
The lung is exposed to a wide variety of particulates, many of which are intrinsically inflammatory because they cannot be metabolized and persist in phagocytes or because their components engage particular receptors that trigger an inflammatory response. For example, silicosis is a chronic diffuse parenchymal lung disease that results from prolonged exposure to inhaled crystalline silica particles. Pulmonary silica exposure results in nodules of mononuclear cell infiltration at site of silica accumulation that ultimately leads to pulmonary fibrosis. Alveolar macrophages are the major cells that respond initially by producing inflammatory mediators that recruit additional cells such as cytokine-producing lymphocytes. IFNγ-producing lymphocytes are important in the pathogenesis of silicosis and intriguingly, the majority of IFNγ-producing lymphocytes in the lung are found in regions of BALT that contain silica-laden macrophages, rather than in areas of diffuse inflammation (Davis et al., 1999). Interestingly, the pulmonary exposure of rats to silica prompted silica-laden alveolar macrophages to transmigrate across the epithelium and accumulate in the BALT (Lee and Kelly, 1993), similar to the kinetic observation of virus-activated DCs in the airways transmigrating across the epithelium into BALT (Halle et al., 2009). Additional silica-laden macrophages accumulated in the draining LN (Lee and Kelly, 1993). Silicosis also leads to persistent increases in TNFα and IL1β, both of which are expressed in BALT areas. However, any link between BALT formation or activity and the pathology of silicosis remains to be identified.
Another particulate to which humans are commonly exposed is DEPs. DEPs are a major component of air pollution in urban areas and exposure to these particles is thought to exacerbate asthma (Matsumoto et al., 2006) and COPD (Anderson et al., 1997) and lead to decreased pulmonary function. Interestingly, pulmonary exposure to high levels of DEPs correlated with the formation of BALT with central accumulations of B cells and peripheral accumulations of T cells (Hiramatsu et al., 2003). Cells filled with black DEPs could be clearly observed both in interstitial areas without organized lymphoid tissue as well as in areas of BALT (Hiramatsu et al., 2003), although areas of BALT appeared to accumulate a higher frequency of DEP-laden cells. These cells were claimed to be alveolar macrophages, which is certainly possible. However, they could easily be DEP-containing DCs that migrate into BALT areas as well. In either case, these cells are likely to initiate inflammatory responses and alter local immune responses to other pulmonary antigens and pathogens (Anderson et al., 1997, Matsumoto et al., 2006). Whether BALT has any effect on the pulmonary inflammatory response to DEP remains to be determined.
The exposure to cigarette smoke is another major hazard to pulmonary health and function and is by far the most important risk factor for developing COPD (Pauwels and Rabe, 2004). COPD is the fifth leading cause of death in the world and, due to its chronic and debilitating nature, represents a major burden on the global economy (Pauwels and Rabe, 2004). The global initiative for chronic obstructive lung disease (GOLD) defines COPD as “a disease state characterized by airflow limitation that is not fully reversible, and that is usually both progressive and associated with abnormal inflammatory response of the lungs to noxious particles or gases” (Pauwels et al., 2001, Pauwels et al., 2004). Given the association between COPD and cigarette smoke exposure, several studies have examined how smoking might affect the formation of BALT. For example, an early study showed that smokers with significant airway obstruction had increased numbers of B cell areas (BALT) relative to smokers without airway obstruction (Bosken et al., 1992), whereas other studies showed that similar frequencies of smoking and nonsmoking patients had at least some areas of BALT, but that the frequency of BALT within the lungs of smokers was higher than in nonsmokers and the frequency of BALT areas with reactive germinal centers was also higher in smokers than in nonsmokers (Elliot et al., 2004). A study in rats (which typically have preexisting BALT) showed that exposure to cigarette smoke increased the BALT area by nearly fourfold and increased the length of the epithelium that was in contact with BALT by nearly threefold (Escolar Castellon et al., 1992). The measurements of BALT area and length of epithelial contact with BALT were measured in two-dimensional sections. Thus, the actual differences in BALT volume and epithelial surface area in contact with BALT are likely to be of much larger magnitude.
Attention to the possible role of BALT in the lungs of smokers was increased significantly by a study showing that the development of BALT was associated with severe COPD, particularly, patients classified with GOLD stages 3 and 4 disease (Hogg et al., 2004). BALT in these patients consisted of dense B cell follicles, often with germinal centers (Hogg et al., 2004). CD4 T cells were found within and surrounding the follicles and also scattered around the airways in areas that lacked BALT (Hogg et al., 2004). Importantly, changes in the forced expiratory volume in one second (FEV1) correlated very strongly with the percentage of airways with lymphoid follicles, the percentage of airways with B cells, and the accumulated volume of B cells in the lungs (Hogg et al., 2004), suggesting the possibility that BALT was playing an important role in COPD or that patients with advanced COPD were being colonized by opportunistic pathogens, which were leading to the development of BALT due to immune stimulation.
Given the association of B cells and BALT with advanced COPD, several studies examined how B cells might be involved in disease. One study showed that in surgical sections of lung from COPD patients undergoing either lung transplant or volume reduction surgery, B cell follicles were present and surrounded by T cell areas (van der Strate et al., 2006). Interestingly, this study also showed that cells filled with black pigment—presumed to be from smoke exposure—were clustered around the edge of the T cell area (van der Strate et al., 2006). These cells likely correspond to macrophages or DCs that have phagocytosed cigarette smoke particles in the airways and then migrated to the BALT areas. Importantly, the B cells in the follicles predominantly expressed CD27, which is a memory marker on B cells and some follicles showed evidence of proliferating germinal center cells (van der Strate et al., 2006). This study also showed that the B cells were likely responding to antigen as the authors found clonal B cell populations in seven of eight patients and also found evidence of ongoing somatic hypermutation (van der Strate et al., 2006). Thus, the authors conclude that the B cells in BALT areas are clearly responding to antigens, even though a direct link with ongoing COPD could not be established. In contrast, another study compared BALB/c and scid mice and showed that although cigarette smoke exposure induced BALT areas in normal mice, scid mice completely lacked identifiable BALT (D'Hulst et al., 2005). Nevertheless, both groups of mice developed pulmonary emphysema to a similar extent and expressed inflammatory cytokines to the same degree (D'Hulst et al., 2005). Thus, B cell reactivity and visible BALT formation do not appear to be necessary for the development of lung disease following cigarette exposure.
Given the well-established role of homeostatic chemokines and LT in the development of conventional lymphoid tissues as well as in BALT (Khader et al., 2009, Randall et al., 2008, Rangel-Moreno et al., 2007), it made sense to test the role of these molecules in mouse models of cigarette smoke-induced COPD. For example, one study found that 4 weeks of cigarette smoke exposure induced the expression of both CXCL13 and CCL19 in the lung tissue, but that this increase was abrogated in Lta −/− mice (Demoor et al., 2009a). This is consistent with data showing that CXCL13 and CCL19 are controlled by LT signaling in conventional lymphoid organs like the spleen (Ngo et al., 1999). However, it is inconsistent with data showing that these chemokines are induced to similar levels in mice upon acute infection with influenza virus (Moyron-Quiroz et al., 2004). However, despite the dependence of homeostatic chemokines on LT signaling in this model, more B cells accumulated in the lungs of Lta −/− mice and the formation of BALT-like areas were even more extensive, despite the lack of apparent organization. These data are consistent with previous studies showing that B cells accumulate normally in the lungs of Lta −/− and Cxcl13 −/− mice following influenza infection (Rangel-Moreno et al., 2007), but fail to form well-defined follicles. In addition, BALT-like areas were also observed in air-exposed Lta −/− mice, but not in air-exposed normal mice (Demoor et al., 2009a). These data are consistent with previous studies showing pulmonary inflammation in unmanipulated Ltbr −/− mice (Futterer et al., 1998). The BALT-like areas in Ltbr −/− mice were not organized into B cell follicles, did not have FDCs or separated B and T cell areas, and lacked detectable HEVs (Futterer et al., 1998), similar to the ones observed in cigarette smoke-exposed Lta −/− mice (Demoor et al., 2009a). IgA production in the BALF of smoke-exposed Lta −/− mice was also dramatically decreased compared to controls (Demoor et al., 2009a) again consistent with the previous data showing that IgA responses are impaired in the absence of LTβR signaling (Kang et al., 2002). Thus, although LT signaling controls homeostatic chemokine expression in the lung in response to cigarette smoke, the lack of LT signaling does not impede the accumulation of lymphocytes in BALT-like clusters in the lung. Unfortunately, there was no evaluation of pulmonary function in these animals so the impact of impaired LT signaling on COPD was not determined (Demoor et al., 2009a).
Similar results were observed using Ccr7 −/− mice (Demoor et al., 2009b), which lack the receptor for CCL19 and Ccl21 (Forster et al., 1999). In these experiments, the authors found that BALT-like areas were already present in untreated Ccr7 −/− mice and increased in size upon smoke exposure, whereas untreated normal animals completely lacked BALT and developed smaller areas upon smoke exposure (Demoor et al., 2009b). Interestingly, DC accumulation was impaired in the lungs of Ccr7 −/− mice 4 weeks after cigarette smoke exposure, but not at later times. However, B and T cell accumulation in the lungs was normal in the absence of CCR7 (Demoor et al., 2009b). Interestingly, the development of cigarette smoke-induced emphysema, as measured by airspace enlargement, was seemingly abrogated in the Ccr7 −/− mice (Demoor et al., 2009b). However, the airspaces in control Ccr7 −/− mice were already the same size as those in the cigarette-smoke-exposed normal mice, even though they did not enlarge as much after smoke exposure (Demoor et al., 2009b). Thus, the starting point was already higher in the Ccr7 −/− mice, perhaps because of the preexisting accumulations of BALT-like areas or perhaps because of other underlying immune defects in Ccr7 −/− mice, such as autoimmunity (Kocks et al., 2007).
In contrast to the results with Ccr7 −/− mice and Lta −/− mice, cigarette-smoke-exposed CCR6−/− mice developed slightly fewer and smaller lymphoid aggregates in their lungs (Bracke et al., 2006). Given that CCR6 is the receptor for CCL20 (Rossi and Zlotnik, 2000), which is a chemokine typically expressed in mucosal epithelium such as the dome epithelium of Peyer's patches (Iwasaki and Kelsall, 2000) or NALT (Rangel-Moreno et al., 2005), it makes sense that CCR6 could be involved in the recruitment or placement of DCs or lymphocytes in areas of BALT. In fact, CCL20 expression is increased on cigarette smoke exposure and the recruitment of DCs and T cells to the lungs of cigarette-smoke-exposed CCR6−/− mice is reduced (Bracke et al., 2006). Importantly, the reductions in lymphocytes and DC recruitment to the lungs correlated with less severe COPD in CCR6−/− mice, suggesting a possible link between the CCR6-mediated cellular recruitment and COPD (Bracke et al., 2006).
In addition to the role of cigarette smoke-induced homeostatic chemokines and their receptors in the formation of BALT, the role of some inflammatory chemokines has also been investigated. For example, cigarette smoke exposure of Ccr5 −/− mice led to decreases in the recruitment of neutrophils, DCs, and T cells to the lung as well as reduced expression of TNFα and IL-2 (Bracke et al., 2007). In addition, there were fewer lymphoid aggregates observed in the lungs of smoke-exposed Ccr5 −/− mice and those observed were smaller and less organized than those in smoke-exposed control mice (Bracke et al., 2007). Importantly, Ccr5 −/− mice were partially protected from cigarette smoke-induced emphysema (Bracke et al., 2007). Thus, as with the previous studies, it is difficult to firmly make a connection between the extent of BALT formation and the development of COPD. However, in this case, poor quality BALT correlated with reductions in some aspects of COPD-related pathology.
pDCs were the focus of another study of the role of lymphoid follicles and COPD (Van Pottelberge et al., in press). In this paper, the authors found that pDCs were located in the T cell areas of BALT in COPD patients (Van Pottelberge et al., in press), similar to where conventional DCs have been observed in previous studies (Woodland and Randall, 2004). Interestingly, the number of pDCs goes up in the airway walls and in BALT in patients with GOLD stages I and II disease, but not in GOLD stages III and IV disease (Van Pottelberge et al., in press). In addition, they found that cigarette smoke extract impaired the maturation of pDCs such that they produced less IFNα (Van Pottelberge et al., in press), suggesting that patients with the most severe disease were less capable of dealing with viral infections. However, pDC from patients with COPD produced higher amounts of TNFα and IL-8 compared to pDCs from healthy controls (Van Pottelberge et al., in press), suggesting that cigarette smoke exposure alters the type of inflammatory response triggered by viruses, which may lead to viral exacerbations of COPD (De Serres et al., 2009).
As the studies above clearly demonstrate, exposure to cigarette smoke clearly promotes the formation of BALT in experimental model systems and BALT is associated with severe COPD in human patients. However, the causal link between BALT and COPD remains nebulous. For example, BALT could be formed and COPD induced in response to cigarette smoke, but be independent of one another as suggested by the studies in scid mice (D'Hulst et al., 2005). Alternatively, cigarette smoke could be inducing the formation of lymphoid tissues that promote autoreactive or locally inflammatory responses that contribute to the pathogenesis of COPD (van der Strate et al., 2006). In another scenario, BALT is formed as a consequence of exposure to opportunistic pathogens in patients with severe COPD. In this case, BALT would be presumed to be beneficial due to its immune-promoting function that would facilitate pathogen clearance or containment (Khader et al., 2009, Moyron-Quiroz et al., 2004). Thus, it is not clear whether BALT is beneficial, harmful, or neutral to the progression of COPD (Brusselle et al., 2009).
10. Contribution of BALT to Autoimmunity and Pulmonary Fibrosis
10.1. RA and Sjogren's syndrome
RA and Sjogren's syndrome (SS) are two autoimmune diseases that are associated with the development of ectopic lymphoid follicles in affected target tissues. For example, RA patients often exhibit ectopic lymphoid follicles in their joints (Takemura et al., 2001), whereas SS patients often have ectopic follicles in their salivary and lacrimal glands (Gatumu et al., 2009). These follicles contain separated B and T cell areas, germinal centers, FDCs, and HEVs and are thought to be involved in autoimmunity by generating high-affinity autoreactive B cells and by maintaining autoreactive effector T cells. In addition, there is a subset of patients with RA and SS that develop pulmonary disease. Some of these patients develop pulmonary disease prior to other manifestations of RA or SS and are often diagnosed in a pulmonary clinic (Rangel-Moreno et al., 2006). This subset of patients often has much more severe disease with a worse long-term prognosis (Turesson et al., 2002, Turesson et al., 2003). We examined lung biopsies from several of these patients and consistently observed BALT. The BALT areas varied from rather small isolated lymphoid follicles to large, highly organized clusters of B cell follicles (Rangel-Moreno et al., 2006). Germinal centers were almost always present and often occupied almost the entire follicle and were supported by extensive FDC networks. CXCL13 was expressed in the B cell follicles as expected and CCL21 was observed on both lymphatics and HEVs (Rangel-Moreno et al., 2006). Lymphatics typically surrounded the B cell follicles. Other studies showed similar results and confirmed that both B cell follicles (Atkins et al., 2006) and CD4 T cell infiltrations of lymphoid follicles (Turesson et al., 2005) were much higher in patients with RA than in patients with interstitial pneumonia not related to RA. Additional studies showed that BALT areas in RA patients looked very much like mucosal lymphoid tissues, with prominent dome epithelium and IgA+ B cells (Hayakawa et al., 1998, Sato et al., 1996). However, the idea that BALT is truly a mucosal lymphoid tissue is at odds with our finding that most of the BALT areas in RA patients were not associated with a dome epithelium and in some cases, seemed to be encapsulated by lymphatic endothelium (Rangel-Moreno et al., 2006).
The B cell follicles of RA and SS patients seemed to be participating in local disease as we found plasma cells producing antibodies specific to citrullinated proteins in areas surrounding BALT. Thus, BALT areas in RA and SS disease seem to contribute to pathology (Rangel-Moreno et al., 2006). Interestingly, there is a strong association between RA and smoking (Klareskog et al., 2004), which may be explained by the induction of the citrullination process by cigarette smoke (Klareskog et al., 2006). Moreover, citrullination has been observed in lymphoid areas of the lungs of RA patients (Bongartz et al., 2007), suggesting that autoantigens are located inside BALT areas. Thus, in susceptible individuals, cigarette smoke may actively promote the development of RA, particularly with pulmonary involvement.
A final complication concerning any possible role of BALT in rheumatoid lung disease is that one of the drugs commonly used to treat RA, methotrexate, can itself induce pulmonary inflammation (Kelly and Saravanan, 2008). In fact, methotrexate pneumonitis occurs with an incidence of 1–7%, depending on the study (Saravanan and Kelly, 2004). Moreover, patients with preexisting lung disease were more susceptible to developing methotrexate pneumonitis (Saravanan and Kelly, 2004). Thus, the combination of rheumatoid lung disease with reactivity to methotrexate can exacerbate pulmonary pathology.
10.2. Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease that involves excessive collagen deposition or fibrosis in the pulmonary interstitium (Selman et al., 2001). IPF is characterized by a diagnosis of usual interstitial pneumonia (UIP), which includes interstitial scarring, interstitial fibrosis in a patchwork pattern, honeycomb changes, and fibroblast foci with minimal associated inflammation (American Thoracic Society, 2002, Selman et al., 2004). Given that there is minimal inflammation associated with this disease, it is not surprising that it is not often associated with the formation or hyperplasia of BALT. In our study of samples from 10 IPF patients, we found small lymphoid aggregates that contained B cells and occasionally T cells, but never contained FDCs, the hallmark feature of B cell follicles (Rangel-Moreno et al., 2006). However, other studies have suggested a more extensive formation of BALT, including the presence of B cell follicles (albeit without examining FDCs), T cell zones, HEVs, DC-LAMP+ DCs, and the expression of a variety of chemokines (Marchal-Somme et al., 2006). Studies from this same group showed that DC-Lamp+ DCs accumulate in lymphoid areas of fibrotic lungs and coexpress CD83 and CD86 (Marchal-Somme et al., 2007). Given the association of DCs with the formation or at least the maintenance of BALT as discussed in previous sections (GeurtsvanKessel et al., 2009, Halle et al., 2009), and the documented presence of DCs in the T cell zones of BALT (Moyron-Quiroz et al., 2004, Woodland & Randall, 2004), it is not too surprising that DCs are present in BALT areas. Nevertheless, based on the limited data in the literature, the incidence and involvement of BALT in IPF seem minimal, despite the dramatic fibrotic changes in the lungs of these patients.
11. Role of BALT in Pulmonary Malignancy
11.1. Lung cancer
Given that the formation of BALT is clearly associated with local immune responses to pulmonary pathogens and antigens, one might predict that the development of BALT adjacent to pulmonary malignancies would be beneficial for antitumor immune responses. In fact, such effects have been observed using a pulmonary metastasis model of melanoma in which an LT:antitumor antibody fusion protein was used to simultaneously engage an antigen on the surface of the tumor and the TNFR1 (Gillies et al., 1991). In this situation, LT is thought to be directly cytotoxic to tumor cells and to activate local immune cells and stromal cells, which promoted the accumulation of lymphocytes surrounding the tumors (Reisfeld et al., 1996). Antitumor immunity appeared to require asialo-GM1+ cells (probably NK cells) as well as B cells, but not T cells (Reisfeld et al., 1996). Subsequent studies by this same group showed that the LT:antitumor antibody fusion protein induced tertiary lymphoid tissue neogenesis—with accumulations of both CD4 and CD8 T cells, B cells, and HEVs that expressed PNAd (Schrama et al., 2001). The formation of lymphoid tissues was accompanied by changes in the T cell repertoire in local sites, suggesting that clonal expansions of tumor-specific T cells were occurring locally—a finding that was confirmed using ELISPOT and cytotoxicity assays (Schrama et al., 2001). This same group also demonstrated that targeting LTa to the tumor site was effective in Lta −/− mice, which completely lack lymph nodes. Thus, they reasoned that the immune response responsible for tumor eradication was generated locally in tertiary lymphoid tissues (Schrama et al., 2008). Together, these results suggest that the local induction of BALT surrounding pulmonary metastases could be beneficial for the induction of antitumor immunity and tumor regression, although it was difficult to distinguish the direct effects of LT signaling on the tumor from the indirect effects of BALT induction on antitumor immunity.
Other studies have also successfully targeted TNF family members, such as LIGHT, directly to tumors and shown an increase in immune cell infiltration to the tumor site and tumor regression (Yu et al., 2004, Yu et al., 2007). Again, the beneficial effects of LIGHT signaling through either LTβR or HVEM could be mediated directly on tumor cells or via environmental influences. In fact, more recent studies have shown that the formation of lymphoid-like stromal elements surrounding tumors may be detrimental to antitumor immune and may in fact, lead to tolerance (Shields et al., 2010). In these studies, the expression of high levels of CCL21 by tumor cells promoted the formation of a lymph node T zone-like environment surrounding the tumors. The formation of this structure correlated with the recruitment of LTi-like cells (Shields et al., 2010). Importantly, the lymphoid-like structure surrounding the tumors recruited both DCs and T cells via CCR7-dependent mechanisms and led to the differentiation or recruitment of Tregs that appeared to contribute to immunological tolerance and accelerated tumor outgrowth (Shields et al., 2010). Thus, the development of a lymphoid environment surrounding tumors may lead to antitumor immunity or immune tolerance, depending on some currently unknown factors.
Despite the apparent contradictions in the literature, the ability of BALT to promote immunity to lung tumors is an intriguing idea. Importantly, BALT has been observed to be generated spontaneously in apparent response to the development of pulmonary tumors or the metastasis of other tumor to the lung. For example, we previously observed the presence of BALT in some samples of human pulmonary carcinoma (Rangel-Moreno et al., 2006). However, of the five patient samples that we observed, only one had evidence of BALT despite extensive tumor infiltration of the lung tissue (Rangel-Moreno et al., 2006). These data suggest that the development of pulmonary carcinoma does not always lead to BALT formation and, conversely, that BALT is not a prerequisite for the development of carcinoma (Rangel-Moreno et al., 2006). Intriguingly, a much more comprehensive study showed that the formation of tertiary lymphoid structures (BALT) surrounding nonsmall-cell lung cancer lesions was associated with a better long-term prognosis over 50 months (Dieu-Nosjean et al., 2008). The areas of BALT in these samples were well-formed and consisted of DC-Lamp+ DCs, separated B and T cell areas, FDC-containing B cell follicles and, in some cases, well-defined germinal centers (Dieu-Nosjean et al., 2008). However, when these features were correlated with prognostic value, the density of DC-Lamp DCs in the BALT areas was the only feature to correlate significantly with long-term survival. However, the density of DCs did correlate with the infiltration of B cells and T cells and the presence of T-bet+ cells, suggesting that the presence of BALT promotes local immune responses to lung tumors. However, there are a number of questions that remain. Was BALT induced independently of tumorigenesis and did it develop prior to tumor formation? If so, then BALT may be responding to some other inflammation-inducing stimuli that helps initiate both BALT and antitumor immunity. Alternatively, was BALT generated in response to the tumor itself and is there something different about the tumors that promoted BALT formation—perhaps high expression of inflammatory cytokines or tumor antigens? These questions need to be answered in order to understand the potential role of BALT in antitumor immunity.
Although the studies above suggest that BALT may contribute to the initiation of antitumor immune responses and promote tumor regression, other studies suggest that the presence of BALT may actually contribute to the formation of lung tumors or promote the metastasis of tumors to the lung. For example, prostate tumor cells were observed to metastasize to BALT regions in a rat model of pulmonary metastasis, in which monodispersed tumor cells were injected intravenously (Geldof and Rao, 1989). Importantly, BALT existed in control rats prior to tumor inoculation and tumor cells rapidly accumulated in BALT areas adjacent to the major artery in BALT (Geldof and Rao, 1989). Thus, these results suggest that alterations in homing molecules and specialized vasculature in BALT areas may recruit tumor cells to the lung and promote metastasis. These data are consistent with other results showing that inflammatory stimuli, such as cigarette smoke, can increase the efficiency of pulmonary metastases (Lu et al., 2007). Although this study showed that the primary effect of cigarette smoke was to impair NK cell function (Lu et al., 2007), other studies have shown that BALT formation is associated with cigarette smoking (Bosken et al., 1992, Escolar Castellon et al., 1992) and is present in patients with severe COPD (Hogg et al., 2004), a pulmonary disease often associated with cigarette smoking.
As mentioned in previous sections, the formation of BALT is often associated with pulmonary inflammation and exposure to a wide variety of inflammatory stimuli. Thus, it is not particularly surprising that the pulmonary administration of Benzo[a]pyrene (B[a]P) leads to the development of BALT hyperplasia in Wistar rats (Silva et al., 2007). However, B[a]P is also associated with tumorigenesis and can induce pulmonary adenocarcinoma (Silva et al., 2007). Thus, inflammatory responses in the lung can simultaneously promote BALT and neoplasia. In fact, given the links between chronic inflammation and the development of cancer (Marx, 2004), it is possible that BALT formation precedes tumorigenesis in this case. Alternatively, BALT formation and adenocarcinoma formation could be independently linked to B[a]P exposure and unrelated to each other. Nevertheless, the link between chronic inflammation and tumorigenesis is intriguing and is discussed further in Section 11.2.
11.2. BALT lymphoma
Pulmonary non-Hodgkin lymphomas are identified as monoclonal lymphoid malignancies that are initially confined to the lungs. Isaacson was the first to identify these low-grade pulmonary lymphomas as a subset of mucosa-associated lymphoid tissue (MALT) lymphomas based on their apparent origins from centrocyte-like or marginal zone-like B cell precursors (Isaacson and Wright, 1983). Given their presence in the lung, they are referred to as BALT lymphomas. These malignancies present diagnostic problems for pathologists due to their heterogeneous appearance and similarity to reactive lymphoid tissue in the lung, including conventional BALT (Koss, 1995, Koss, 2004). Most BALT lymphomas are of low histologic grade and have a relatively benign clinical behavior, typically leading to good clinical prognosis (Stefanovic et al., 2008).
Morphologically, the BALT lymphomas often form dense lymphoid clusters of B cells (Fiche et al., 1995, Koss, 2004, Kurtin et al., 2001). These are often organized into structures reminiscent of B cell follicles in conventional BALT, with most lymphoid cells being positive for CD20 and BCL2 (Koss, 2004, Li et al., 1990) and mixed with nonlymphoid cells that are positive for CD21/CD35 (presumably, FDCs) (Koss, 2004). Some BALT lymphomas even contain histologically defined germinal centers (Koss, 2004, Kurtin et al., 2001, Li et al., 1990). T cells can be found around the outside of peribronchial lymphoid nodules (Koss, 2004), just as they would be around the B cell follicles of conventional BALT. Most BALT lymphomas interact extensively with the bronchial epithelium and form small clusters of lymphoma cells inside polyp-like bulges in the epithelium (Fiche et al., 1995, Kurtin et al., 2001). These interactions are similar to the interactions of B cells in the dome epithelium of typical mucosal lymphoid tissues (Kiyono and Fukuyama, 2004). Thus, many of the histologic features of BALT lymphoma have some correspondence with the architecture of nonmalignant areas of BALT.
The most common type of MALT lymphomas affect the stomach (Isaacson, 1999), where they are often associated with Helicobacter pylori infection (Go & Smoot, 2000, Lochhead & El-Omar, 2007). In the case of gastric lymphoma, lymphomagenesis is directly associated with infection as antibiotics can often induce tumor regression (Isaacson, 1999). Thus, chronic inflammation as well as antigen stimulation and T cell help seem to cooperate to trigger tumorigenesis (Isaacson, 1999). Similar events are likely to occur in BALT lymphoma, however, the etiology of the disease is not as clear-cut (Koss, 2004). For example, some BALT lymphomas are associated with underlying autoimmune diseases, such as SS (Imai et al., 2009, Ingegnoli et al., 2008), systemic lupus erythematosus (SLE) (Gayed et al., 2009), and RA (Bende et al., 2005). In contrast, the development of some BALT lymphomas is associated with chronic mycobacterial infections, including infection with Mycobaterium avium and Mycobacterium tuberculosis (Gaur et al., 2004, Inadome et al., 2001, Klein et al., 2007). This may not be altogether surprising, given the association with pulmonary mycobacterial infection with the development of BALT in both mice and humans (Kahnert et al., 2007, Tsai et al., 2006, Ulrichs et al., 2004). Interestingly, a recent study used an analysis of 16S-RNA to determine the entire bacterial population found in samples from nine BALT lymphomas (Adam et al., 2008). In eight of the nine samples, the authors found RNA from bacteria of the Alcaligenaceae family. These gram-negative bacteria are widespread in the environment and are not typically considered to be pathogenic. Thus, further studies are required to confirm this link and establish any connection between pulmonary exposure to bacteria of the Alcaligenaceae family and lymphomagenesis. Nevertheless, there is likely to be some connection between chronic inflammation from some source and the development of BALT lymphoma.
12. Summary and Perspectives
Based on the accumulating data, it is clear that term “BALT” encompasses a wide range of lymphoid tissues in the lung from small disorganized clusters of lymphocytes and DCs, to highly organized lymphoid tissues with B cell follicles, germinal centers, FDCs, HEVs lymphatics, and a well-developed dome epithelium. All of these tissues seem to be functional to some degree, although there are likely to be functional differences that are not currently appreciated. In any case, we now know that BALT can be a fully functional lymphoid tissue that collects antigens from the pulmonary airways, primes B and T cell responses, and contributes to the clearance of infectious diseases. In addition, the development or expansion of BALT is also associated with a wide variety of chronic inflammatory diseases. However, the role of BALT in these diseases is less well established. Thus, future research should be directed toward understanding how BALT may contribute (both positively and negatively) to chronic lung diseases.
Acknowledgments
This work was supported by NIH grants HL69489, AI61511, and AI72689 and by the Sandler Program for Asthma Research.
References
- Actor J.K., Olsen M., Jagannath C., Hunter R.L. Relationship of survival, organism containment, and granuloma formation in acute murine tuberculosis. J. Interferon Cytokine Res. 1999;19:1183. doi: 10.1089/107999099313136. [DOI] [PubMed] [Google Scholar]
- Adam P., Gernert C., Schmitt S., Haralambieva E., Ott G., Muller-Hermelink H.K., Hentschel U. The spectrum of microbiological agents causing pulmonary MALT-type lymphomas. A 16S rRNA-based analysis of microbial diversity. Pathologe. 2008;29(Suppl. 2):290. doi: 10.1007/s00292-008-1068-1. [DOI] [PubMed] [Google Scholar]
- Allen C.D., Okada T., Tang H.L., Cyster J.G. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias (2002). This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002;165:277. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- Anderson H.R., Spix C., Medina S., Schouten J.P., Castellsague J., Rossi G., Zmirou D., Touloumi G., Wojtyniak B., Ponka A., Bacharova L., Schwartz J., Katsouyanni K. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: Results from the APHEA project. Eur. Respir. J. 1997;10:1064. doi: 10.1183/09031936.97.10051064. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., Ngo V.N., Hayman P.L., Luther S.A., Forster R., Sedgwick J.D., Browning J.L., Lipp M., Cyster J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Atkins S.R., Turesson C., Myers J.L., Tazelaar H.D., Ryu J.H., Matteson E.L., Bongartz T. Morphologic and quantitative assessment of CD20+ B cell infiltrates in rheumatoid arthritis-associated nonspecific interstitial pneumonia and usual interstitial pneumonia. Arthritis Rheum. 2006;54:635. doi: 10.1002/art.21758. [DOI] [PubMed] [Google Scholar]
- Auais A., Adkins B., Napchan G., Piedimonte G. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L105. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- Banfi A., Tiszlavicz L., Szekely E., Petak F., Toth-Szuki V., Barati L., Bari F., Novak Z. Development of bronchus-associated lymphoid tissue hyperplasia following lipopolysaccharide-induced lung inflammation in rats. Exp. Lung Res. 2009;35:186. doi: 10.1080/01902140802495862. [DOI] [PubMed] [Google Scholar]
- Banks T.A., Rouse B.T., Kerley M.K., Blair P.J., Godfrey V.L., Kuklin N.A., Bouley D.M., Thomas J., Kanangat S., Mucenski M.L. Lymphotoxin a deficient mice: Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 1995;155:1685. [PubMed] [Google Scholar]
- Becker S., Fenton M.J., Soukup J.M. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am. J. Respir. Cell Mol. Biol. 2002;27:611. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- Bende R.J., Aarts W.M., Riedl R.G., de Jong D., Pals S.T., van Noesel C.J. Among B cell non-Hodgkin's lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J. Exp. Med. 2005;201:1229. doi: 10.1084/jem.20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., McDermott M.R. Bronchus- and nasal-associated lymphoid tissues. Immunol. Rev. 2005;206:22. doi: 10.1111/j.0105-2896.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Bongartz T., Cantaert T., Atkins S.R., Harle P., Myers J.L., Turesson C., Ryu J.H., Baeten D., Matteson E.L. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2007;46:70. doi: 10.1093/rheumatology/kel202. [DOI] [PubMed] [Google Scholar]
- Bosken C.H., Hards J., Gatter K., Hogg J.C. Characterization of the inflammatory reaction in the peripheral airways of cigarette smokers using immunocytochemistry. Am. Rev. Respir. Dis. 1992;145:911. doi: 10.1164/ajrccm/145.4_Pt_1.911. [DOI] [PubMed] [Google Scholar]
- Bracke K.R., D'Hulst A.I., Maes T., Moerloose K.B., Demedts I.K., Lebecque S., Joos G.F., Brusselle G.G. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J. Immunol. 2006;177:4350. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- Bracke K.R., D'Hulst A.I., Maes T., Demedts I.K., Moerloose K.B., Kuziel W.A., Joos G.F., Brusselle G.G. Cigarette smoke-induced pulmonary inflammation, but not airway remodelling, is attenuated in chemokine receptor 5-deficient mice. Clin. Exp. Allergy. 2007;37:1467. doi: 10.1111/j.1365-2222.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- Breel M., Van der Ende M., Sminia T., Kraal G. Subpopulations of lymphoid and non-lymphoid cells in bronchus-associated lymphoid tissue (BALT) of the mouse. Immunology. 1988;63:657. [PMC free article] [PubMed] [Google Scholar]
- Briere F., Defrance T., Vanbervliet B., Bridon J.M., Durand I., Rousset F., Banchereau J. Transforming growth factor beta (TGF beta) directs IgA1 and IgA2 switching in human naive B cells. Adv. Exp. Med. Biol. 1995;371A:21. doi: 10.1007/978-1-4615-1941-6_4. [DOI] [PubMed] [Google Scholar]
- Bromley S.K., Thomas S.Y., Luster A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005;6:895. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- Browning J.L., Allaire N., Ngam-Ek A., Notidis E., Hunt J., Perrin S., Fava R.A. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Brusselle G.G., Demoor T., Bracke K.R., Brandsma C.A., Timens W. Lymphoid follicles in (very) severe COPD: Beneficial or harmful? Eur. Respir. J. 2009;34:219. doi: 10.1183/09031936.00150208. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Hedrick J., Zlotnick A., Siani M.A., Thompson D.A., Butcher E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Carragher D.M., Rangel-Moreno J., Randall T.D. Ectopic lymphoid tissues and local immunity. Semin. Immunol. 2007;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallotti C., Tonnarini G.F., Tranquilli Leali F.M. Cholinergic innervation of BALT (bronchus associated lymphoid tissue) in rat. Lung. 2004;182:27. doi: 10.1007/s00408-003-1042-x. [DOI] [PubMed] [Google Scholar]
- Cavallotti C., Tonnarini G., D'Andrea V., Cavallotti D. Cholinergic staining of bronchus-associated lymphoid tissue. Neuroimmunomodulation. 2005;12:141. doi: 10.1159/000084845. [DOI] [PubMed] [Google Scholar]
- Charavaryamath C., Janardhan K.S., Townsend H.G., Willson P., Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir. Res. 2005;6:50. doi: 10.1186/1465-9921-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.D., Fraire A.E., Joris I., Welsh R.M., Selin L.K. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am. J. Pathol. 2003;163:1341. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatchko Y., Kosco-Vilbois M.H., Herren S., Lefort J., Bonnefoy J.-Y. Germinal center formation and local immunoglobulin E (IgE) production in the lung after and airway antigenic challenge. J. Exp. Med. 1996;184:23. doi: 10.1084/jem.184.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R.L., Seymour B.W., Hudak S., Jackson J., Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- Constant S.L., Brogdon J.L., Piggott D.A., Herrick C.A., Visintin I., Ruddle N.H., Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J. Clin. Invest. 2002;110:1441. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff C.A., Schwartz J., Bergman C.M., Russell K.S., Bender J.R., Ruddle N.H. Lymphotoxin alpha3 induces chemokines and adhesion molecules: Insight into the role of LT alpha in inflammation and lymphoid organ development. J. Immunol. 1998;161:6853. [PubMed] [Google Scholar]
- Cuff C.A., Sacca R., Ruddle N.H. Differential induction of adhesion molecule and chemokine expression by LTalpha3 and LTalphabeta in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J. Immunol. 1999;162:5965. [PubMed] [Google Scholar]
- Cupedo T., Kraal G., Mebius R.E. The role of CD45+CD4+CD3- cells in lymphoid organ development. Immunol. Rev. 2002;189:41. doi: 10.1034/j.1600-065x.2002.18905.x. [DOI] [PubMed] [Google Scholar]
- D'Hulst A.I., Maes T., Bracke K.R., Demedts I.K., Tournoy K.G., Joos G.F., Brusselle G.G. Cigarette smoke-induced pulmonary emphysema in scid-mice. Is the acquired immune system required? Respir. Res. 2005;6:147. doi: 10.1186/1465-9921-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G.S., Pfeiffer L.M., Hemenway D.R. Expansion of interferon-gamma-producing lung lymphocytes in mouse silicosis. Am. J. Respir. Cell Mol. Biol. 1999;20:813. doi: 10.1165/ajrcmb.20.4.3407. [DOI] [PubMed] [Google Scholar]
- Day T.A., Koch M., Nouailles G., Jacobsen M., Kosmiadi G.A., Miekley D., Kuhlmann S., Jorg S., Gamradt P., Mollenkopf H.J., Hurwitz R., Reece S.T., Kaufmann S.H., Kursar M. Secondary lymphoid organs are dispensable for the development of T-cell-mediated immunity during tuberculosis. Eur. J. Immunol. 2010;40:1663. doi: 10.1002/eji.201040299. [DOI] [PubMed] [Google Scholar]
- de Dios Escolar J., Alfaro E., Roche P.A., Almajano C., Gallego B. Pulmonary response to bovine albumin. A morphometric study in rats. Histol. Histopathol. 1994;9:15. [PubMed] [Google Scholar]
- De Serres G., Lampron N., La Forge J., Rouleau I., Bourbeau J., Weiss K., Barret B., Boivin G. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J. Clin. Virol. 2009;46:129. doi: 10.1016/j.jcv.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Togni P., Goellner J., Ruddle N.H., Streeter P.R., Fick A., Mariathasan S., Smith S.C., Carlson R., Shornick L.P., Strauss-Schoenberger J., Russell J.H., Karr R., Chaplin D.D. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Debes G.F., Arnold C.N., Young A.J., Krautwald S., Lipp M., Hay J.B., Butcher E.C. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005;6:889. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delventhal S., Hensel A., Petzoldt K., Pabst R. Effects of microbial stimulation on the number, size and activity of bronchus-associated lymphoid tissue (BALT) structures in the pig. Int. J. Exp. Pathol. 1992;73:351. [PMC free article] [PubMed] [Google Scholar]
- Demoor T., Bracke K.R., Maes T., Vandooren B., Elewaut D., Pilette C., Joos G.F., Brusselle G.G. Role of lymphotoxin-alpha in cigarette smoke-induced inflammation and lymphoid neogenesis. Eur. Respir. J. 2009;34:405. doi: 10.1183/09031936.00101408. [DOI] [PubMed] [Google Scholar]
- Demoor T., Bracke K.R., Vermaelen K.Y., Dupont L., Joos G.F., Brusselle G.G. CCR7 modulates pulmonary and lymph node inflammatory responses in cigarette smoke-exposed mice. J. Immunol. 2009;183:8186. doi: 10.4049/jimmunol.0902015. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean M.C., Antoine M., Danel C., Heudes D., Wislez M., Poulot V., Rabbe N., Laurans L., Tartour E., de Chaisemartin L., Lebecque S., Fridman W.H., Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008;26:4410. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- Drayton D.L., Bonizzi G., Ying X., Liao S., Karin M., Ruddle N.H. I kappa B kinase complex alpha kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal-associated lymphoid tissue. J. Immunol. 2004;173:6161. doi: 10.4049/jimmunol.173.10.6161. [DOI] [PubMed] [Google Scholar]
- Drayton D.L., Liao S., Mounzer R.H., Ruddle N.H. Lymphoid organ development: From ontogeny to neogenesis. Nat. Immunol. 2006;7:344. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Droemann D., Goldmann T., Tiedje T., Zabel P., Dalhoff K., Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir. Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G., Littman D.R. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol. Rev. 2003;195:81. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Ho Y.M., Hall S., Styles C.J., Scott S.D., Gompels U.A. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 1990;71(Pt. 6):1365. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- Elliot J.G., Jensen C.M., Mutavdzic S., Lamb J.P., Carroll N.G., James A.L. Aggregations of lymphoid cells in the airways of nonsmokers, smokers, and subjects with asthma. Am. J. Respir. Crit. Care Med. 2004;169:712. doi: 10.1164/rccm.200308-1167OC. [DOI] [PubMed] [Google Scholar]
- Endres R., Alimzhanov M.B., Plitz T., Futterer A., Kosco-Vilbois M.H., Nedospasov S.A., Rajewski K., Pfeffer K. Mature follicular dendritic cell networks depend on expression of lymphotoxin b receptor by radioresistant stromal cells and of lymphotoxin b and tumor necrosis factor by B cells. J. Exp. Med. 1999;189:159. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersch J., Tschernig T., Stallmach T. Frequency and potential cause of bronchus-associated lymphoid tissue in fetal lungs. Pediatr. Allergy Immunol. 2005;16:295. doi: 10.1111/j.1399-3038.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- Escolar Castellon J.D., Escolar Castellon A., Roche Roche P.A., Minana Amada C. Bronchial-associated lymphoid tissue (BALT) response to airway challenge with cigarette smoke, bovine antigen and anti-pulmonary serum. Histol. Histopathol. 1992;7:321. [PubMed] [Google Scholar]
- Fiche M., Caprons F., Berger F., Galateau F., Cordier J.F., Loire R., Diebold J. Primary pulmonary non-Hodgkin's lymphomas. Histopathology. 1995;26:529. doi: 10.1111/j.1365-2559.1995.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Flano E., Husain S.M., Sample J.T., Woodland D.L., Blackman M.A. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 2000;165:1074. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- Flynn J.L., Chan J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001;19:93. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- Forster R., Schubel A., Breitfeld D., Kremmer E., Renner-Muller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Franciotta D., Salvetti M., Lolli F., Serafini B., Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- Fujihashi K., Dohi T., Rennert P.D., Yamamoto M., Koga T., Kiyono H., McGhee J.R. Peyer's patches are required for oral tolerance to proteins. Proc. Natl. Acad. Sci. USA. 2001;98:3310. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer A., Mink K., Luz A., Kosco-Vilbois M.H., Pfeffer K. The lymphotoxin b receptor controls organogenisis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Gajewska B.U., Alvarez D., Vidric M., Goncharova S., Stampfli M.R., Coyle A.J., Gutierrez-Ramos J.C., Jordana M. Generation of experimental allergic airways inflammation in the absence of draining lymph nodes. J. Clin. Invest. 2001;108:577. doi: 10.1172/JCI12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatumu M.K., Skarstein K., Papandile A., Browning J.L., Fava R.A., Bolstad A.I. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjogren's syndrome in salivary glands of non-obese diabetic mice. Arthritis Res. Ther. 2009;11:R24. doi: 10.1186/ar2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S., Trayner E., Aish L., Weinstein R. Bronchus-associated lymphoid tissue lymphoma arising in a patient with bronchiectasis and chronic Mycobacterium avium infection. Am. J. Hematol. 2004;77:22. doi: 10.1002/ajh.20136. [DOI] [PubMed] [Google Scholar]
- Gayed M., Bernatsky S., Ramsey-Goldman R., Clarke A., Gordon C. Lupus and cancer. Lupus. 2009;18:479. doi: 10.1177/0961203309102556. [DOI] [PubMed] [Google Scholar]
- Geldof A.A., Rao B.R. Metastasis of prostate tumor cells to bronchus associated lymphoid tissue. In Vivo. 1989;3:87. [PubMed] [Google Scholar]
- GeurtsvanKessel C.H., Willart M.A., Bergen I.M., van Rijt L.S., Muskens F., Elewaut D., Osterhaus A.D., Hendriks R., Rimmelzwaan G.F., Lambrecht B.N. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 2009;206:2339. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S.D., Young D., Lo K.M., Foley S.F., Reisfeld R.A. Expression of genetically engineered immunoconjugates of lymphotoxin and a chimeric anti-ganglioside GD2 antibody. Hybridoma. 1991;10:347. doi: 10.1089/hyb.1991.10.347. [DOI] [PubMed] [Google Scholar]
- Go M.F., Smoot D.T. Helicobacter pylori, gastric MALT lymphoma, and adenocarcinoma of the stomach. Semin. Gastrointest. Dis. 2000;11:134. [PubMed] [Google Scholar]
- Gould S.J., Isaacson P.G. Bronchus associated lymphoid tissue (BALT) in human fetal and infant lung. J. Pathol. 1993;169:229. doi: 10.1002/path.1711690209. [DOI] [PubMed] [Google Scholar]
- Goulding J., Snelgrove R., Saldana J., Didierlaurent A., Cavanagh M., Gwyer E., Wales J., Wissinger E.L., Hussell T. Respiratory infections: Do we ever recover? Proc. Am. Thorac. Soc. 2007;4:618. doi: 10.1513/pats.200706-066TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya S., Matsuoka H., Mori M., Morishita H., Kida H., Kobashi Y., Kato T., Taguchi Y., Osaki T., Tachibana I., Nishimoto N., Yoshizaki K., Kawase I., Hayashi S. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J. Pathol. 2003;200:82. doi: 10.1002/path.1321. [DOI] [PubMed] [Google Scholar]
- Gregson R.L., Edmondson N.A., Plesch B. Preferential uptake of soluble antigen by respiratory tract epithelium overlying bronchus-associated lymphoid tissue in the rat. Adv. Exp. Med. Biol. 1982;149:499. doi: 10.1007/978-1-4684-9066-4_70. [DOI] [PubMed] [Google Scholar]
- Haberman A.M., Shlomchik M.J. Reassessing the function of immune-complex retention by follicular dendritic cells. Nat. Rev. Immunol. 2003;3:757. doi: 10.1038/nri1178. [DOI] [PubMed] [Google Scholar]
- Halle S., Dujardin H.C., Bakocevic N., Fleige H., Danzer H., Willenzon S., Suezer Y., Hammerling G., Garbi N., Sutter G., Worbs T., Forster R. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 2009;206:2593. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves D.C., Hyman P.L., Lu T.T., Ngo V.N., Bidgol A., Suzuki G., Zou Y.R., Littman D.R., Cyster J.G. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 2001;194:45. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A.E., Debes G.F., Arce S., Cassese G., Hamann A., Radbruch A., Manz R.A. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 2002;169:1277. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- Hayakawa H., Sato A., Imokawa S., Todate A., Chida K., Suzuki K., Iwata M. Diffuse panbronchiolitis and rheumatoid arthritis-associated bronchiolar disease: Similarities and differences. Intern. Med. 1998;37:504. doi: 10.2169/internalmedicine.37.504. [DOI] [PubMed] [Google Scholar]
- Heier I., Malmstrom K., Pelkonen A.S., Malmberg L.P., Kajosaari M., Turpeinen M., Lindahl H., Brandtzaeg P., Jahnsen F.L., Makela M.J. Bronchial response pattern of antigen presenting cells and regulatory T cells in children less than 2 years of age. Thorax. 2008;63:703. doi: 10.1136/thx.2007.082974. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K., Azuma A., Kudoh S., Desaki M., Takizawa H., Sugawara I. Inhalation of diesel exhaust for three months affects major cytokine expression and induces bronchus-associated lymphoid tissue formation in murine lungs. Exp. Lung Res. 2003;29:607. doi: 10.1080/01902140390240140. [DOI] [PubMed] [Google Scholar]
- Hogg J.C., Chu F., Utokaparch S., Woods R., Elliott W.M., Buzatu L., Cherniack R.M., Rogers R.M., Sciurba F.C., Coxson H.O., Pare P.D. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350:2645. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Hopken U.E., Wengner A.M., Loddenkemper C., Stein H., Heimesaat M.M., Rehm A., Lipp M. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 2007;109:886. doi: 10.1182/blood-2006-03-013532. [DOI] [PubMed] [Google Scholar]
- Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749. [PubMed] [Google Scholar]
- Imai H., Sunaga N., Kaira K., Kawashima O., Yanagitani N., Sato K., Tomizawa Y., Hisada T., Ishizuka T., Hirato J., Saito R., Nakajima T., Mori M. Clinicopathological features of patients with bronchial-associated lymphoid tissue lymphoma. Intern. Med. 2009;48:301. doi: 10.2169/internalmedicine.48.1438. [DOI] [PubMed] [Google Scholar]
- Inadome Y., Ikezawa T., Oyasu R., Noguchi M. Malignant lymphoma of bronchus-associated lymphoid tissue (BALT) coexistent with pulmonary tuberculosis. Pathol. Int. 2001;51:807. doi: 10.1046/j.1440-1827.2001.01272.x. [DOI] [PubMed] [Google Scholar]
- Ingegnoli F., Sciascera A., Galbiati V., Corbelli V., D'Ingianna E., Fantini F. Bronchus-associated lymphoid tissue lymphoma in a patient with primary Sjogren's syndrome. Rheumatol. Int. 2008;29:207. doi: 10.1007/s00296-008-0645-1. [DOI] [PubMed] [Google Scholar]
- Isaacson P.G. Mucosa-associated lymphoid tissue lymphoma. Semin. Hematol. 1999;36:139. [PubMed] [Google Scholar]
- Isaacson P., Wright D.H. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Kelsall B.L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)3a, MIP-3b, and secondary lymphoid organ chemokine. J. Exp. Med. 2000;191:1381. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J. Exp. Med. 1992;176:679. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M.H., Kweon M.N., Iwatani K., Yamamoto M., Terahara K., Sasakawa C., Suzuki T., Nochi T., Yokota Y., Rennert P.D., Hiroi T., Tamagawa H., Iijima H., Kunisawa J., Yuki Y., Kiyono H. Intestinal villous M cells: An antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA. 2004;101:6110. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen F.E., Pekna M., Norderhaug I.N., Haneberg B., Hietala M.A., Krajci P., Betsholtz C., Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 1999;190:915. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K., Banks T.A., Ansel K.M., Lu T.T., Ware C.F., Cyster J.G. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kahnert A., Hopken U.E., Stein M., Bandermann S., Lipp M., Kaufmann S.H. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J. Infect. Dis. 2007;195:46. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- Kang H.S., Chin R.K., Wang Y., Yu P., Wang J., Newell K.A., Fu Y.X. Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production. Nat. Immunol. 2002;3:576. doi: 10.1038/ni795. [DOI] [PubMed] [Google Scholar]
- Kashino S.S., Vallerskog T., Martens G., Troudt J., Keyser A., Taylor J., Izzo A., Kornfeld H., Campos-Neto A. Initiation of acquired immunity in the lungs of mice lacking lymph nodes after infection with aerosolized Mycobacterium tuberculosis. Am. J. Pathol. 2010;176:198. doi: 10.2353/ajpath.2010.090446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Takeuchi O., Fujita T., Inoue J., Muhlradt P.F., Sato S., Hoshino K., Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001;167:5887. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- Kelly C., Saravanan V. Treatment strategies for a rheumatoid arthritis patient with interstitial lung disease. Expert Opin. Pharmacother. 2008;9:3221. doi: 10.1517/14656560802591430. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. The germinal center: A crucible for lymphocyte selection. Semin. Immunol. 1996;8:179. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- Khader S.A., Rangel-Moreno J., Fountain J.J., Martino C.A., Reiley W.W., Pearl J.E., Winslow G.M., Woodland D.L., Randall T.D., Cooper A.M. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J. Immunol. 2009;183:8004. doi: 10.4049/jimmunol.0901937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H., Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004;4:699. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Alfredsson L., Rantapaa-Dahlqvist S., Berglin E., Stolt P., Padyukov L. What precedes development of rheumatoid arthritis? Ann. Rheum. Dis. 2004;63(Suppl. 2):ii28. doi: 10.1136/ard.2004.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Stolt P., Lundberg K., Kallberg H., Bengtsson C., Grunewald J., Ronnelid J., Harris H.E., Ulfgren A.K., Rantapaa-Dahlqvist S., Eklund A., Padyukov L., Alfredsson L. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- Klein T.O., Soll B.A., Issel B.F., Fraser C. Bronchus-associated lymphoid tissue lymphoma and Mycobacterium tuberculosis infection: An unusual case and a review of the literature. Respir. Care. 2007;52:755. [PubMed] [Google Scholar]
- Kocks J.R., Davalos-Misslitz A.C., Hintzen G., Ohl L., Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J. Exp. Med. 2007;204:723. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks J.R., Adler H., Danzer H., Hoffmann K., Jonigk D., Lehmann U., Forster R. Chemokine receptor CCR7 contributes to a rapid and efficient clearance of lytic murine gamma-herpes virus 68 from the lung, whereas bronchus-associated lymphoid tissue harbors virus during latency. J. Immunol. 2009;182:6861. doi: 10.4049/jimmunol.0801826. [DOI] [PubMed] [Google Scholar]
- Kolopp-Sarda M.N., Bene M.C., Massin N., Moulin J.J., Faure G.C. Immunohistological analysis of macrophages, B cells and T cells in the mouse lung. Anat. Rec. 1994;239:150. doi: 10.1002/ar.1092390205. [DOI] [PubMed] [Google Scholar]
- Koss M.N. Pulmonary lymphoid disorders. Semin. Diagn. Pathol. 1995;12:158. [PubMed] [Google Scholar]
- Koss M.N. Malignant and benign lymphoid lesions of the lung. Ann. Diagn. Pathol. 2004;8:167. doi: 10.1016/j.anndiagpath.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Kunisawa J., Fukuyama S., Kiyono H. Mucosa-associated lymphoid tissues in the aerodigestive tract: Their shared and divergent traits and their importance to the orchestration of the mucosal immune system. Curr. Mol. Med. 2005;5:557. doi: 10.2174/1566524054863924. [DOI] [PubMed] [Google Scholar]
- Kurtin P.J., Myers J.L., Adlakha H., Strickler J.G., Lohse C., Pankratz V.S., Inwards D.J. Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am. J. Surg. Pathol. 2001;25:997. doi: 10.1097/00000478-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Kelly D.P. Translocation of particle-laden alveolar macrophages and intra-alveolar granuloma formation in rats exposed to Ludox colloidal amorphous silica by inhalation. Toxicology. 1993;77:205. doi: 10.1016/0300-483x(93)90161-k. [DOI] [PubMed] [Google Scholar]
- Lee J.J., McGarry M.P., Farmer S.C., Denzler K.L., Larson K.A., Carrigan P.E., Brenneise I.E., Horton M.A., Haczku A., Gelfand E.W., Leikauf G.D., Lee N.A. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J. Exp. Med. 1997;185:2143. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Hansmann M.L., Zwingers T., Lennert K. Primary lymphomas of the lung: Morphological, immunohistochemical and clinical features. Histopathology. 1990;16:519. doi: 10.1111/j.1365-2559.1990.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Lochhead P., El-Omar E.M. Helicobacter pylori infection and gastric cancer. Best Pract. Res. Clin. Gastroenterol. 2007;21:281. doi: 10.1016/j.bpg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Lu L.M., Zavitz C.C., Chen B., Kianpour S., Wan Y., Stampfli M.R. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J. Immunol. 2007;178:936. doi: 10.4049/jimmunol.178.2.936. [DOI] [PubMed] [Google Scholar]
- Lund F.E., Partida-Sanchez S., Lee B.O., Kusser K.L., Hartson L., Hogan R.J., Woodland D.L., Randall T.D. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J. Immunol. 2002;169:5236. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology. 1998;3:233. doi: 10.1016/s1380-2933(97)10005-7. [DOI] [PubMed] [Google Scholar]
- Maglione P.J., Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur. J. Immunol. 2009;39:676. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione P.J., Xu J., Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J. Immunol. 2007;178:7222. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]
- Man A.L., Prieto-Garcia M.E., Nicoletti C. Improving M cell mediated transport across mucosal barriers: Do certain bacteria hold the keys? Immunology. 2004;113:15. doi: 10.1111/j.1365-2567.2004.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal-Somme J., Uzunhan Y., Marchand-Adam S., Valeyre D., Soumelis V., Crestani B., Soler P. Cutting edge: Nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J. Immunol. 2006;176:5735. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- Marchal-Somme J., Uzunhan Y., Marchand-Adam S., Kambouchner M., Valeyre D., Crestani B., Soler P. Dendritic cells accumulate in human fibrotic interstitial lung disease. Am. J. Respir. Crit. Care Med. 2007;176:1007. doi: 10.1164/rccm.200609-1347OC. [DOI] [PubMed] [Google Scholar]
- Marinova E., Han S., Zheng B. Germinal center helper T cells are dual functional regulatory cells with suppressive activity to conventional CD4+ T cells. J. Immunol. 2007;178:5010. doi: 10.4049/jimmunol.178.8.5010. [DOI] [PubMed] [Google Scholar]
- Marx J. Cancer research. Inflammation and cancer: The link grows stronger. Science. 2004;306:966. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Hiramatsu K., Li Y., Azuma A., Kudoh S., Takizawa H., Sugawara I. Repeated exposure to low-dose diesel exhaust after allergen challenge exaggerates asthmatic responses in mice. Clin. Immunol. 2006;121:227. doi: 10.1016/j.clim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- McGill J., van Rooijen N., Legge K.L. IL-15 trans-presenting pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J. Exp. Med. 2010 doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 2003;3:292. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- Mebius R.E., Miyamoto T., Christensen J., Domen J., Cupedo T., Weissman I.L., Akashi K. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3-cells, as well as macrophages. J. Immunol. 2001;166:6593. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- Meier D., Bornmann C., Chappaz S., Schmutz S., Otten L.A., Ceredig R., Acha-Orbea H., Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Miller H., Zhang J., Kuolee R., Patel G.B., Chen W. Intestinal M cells: The fallible sentinels? World J. Gastroenterol. 2007;13:1477. doi: 10.3748/wjg.v13.i10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani N., Fuchikami J., Takahashi M., Nabe T., Yoshino S., Kohno S. Pulmonary emphysema induced by cigarette smoke solution and lipopolysaccharide in guinea pigs. Biol. Pharm. Bull. 2009;32:1559. doi: 10.1248/bpb.32.1559. [DOI] [PubMed] [Google Scholar]
- Mohr L.C. Hypersensitivity pneumonitis. Curr. Opin. Pulm. Med. 2004;10:401. doi: 10.1097/01.mcp.0000135675.95674.29. [DOI] [PubMed] [Google Scholar]
- Mohr E., Serre K., Manz R.A., Cunningham A.F., Khan M., Hardie D.L., Bird R., MacLennan I.C. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J. Immunol. 2009;182:2113. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz J.E., Rangel-Moreno J., Kusser K., Hartson L., Sprague F., Goodrich S., Woodland D.L., Lund F.E., Randall T.D. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004;10:927. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz J.E., Rangel-Moreno J., Hartson L., Kusser K., Tighe M.P., Klonowski K.D., Lefrancois L., Cauley L.S., Harmsen A.G., Lund F.E., Randall T.D. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz J., Rangel-Moreno J., Carragher D.M., Randall T.D. The function of local lymphoid tissues in pulmonary immune responses. Adv. Exp. Med. Biol. 2007;590:55. doi: 10.1007/978-0-387-34814-8_4. [DOI] [PubMed] [Google Scholar]
- Murakami D., Yamada H., Yajima T., Masuda A., Komune S., Yoshikai Y. Lipopolysaccharide inhalation exacerbates allergic airway inflammation by activating mast cells and promoting Th2 responses. Clin. Exp. Allergy. 2007;37:339. doi: 10.1111/j.1365-2222.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- Nakano H., Gunn M.D. Gene duplications at the chemokine locus on mouse chromosome 4: Multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 2001;166:361. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- Nakano H., Tamura T., Yoshimoto T., Yagita H., Miyasaka M., Butcher E.C., Nariuchi H., Kakiuchi T., Matsuzawa A. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur. J. Immunol. 1997;27:215. doi: 10.1002/eji.1830270132. [DOI] [PubMed] [Google Scholar]
- Ngo V.N., Korner H., Gunn M.D., Schmidt K.N., Riminton D.S., Cooper M.D., Browning J.L., Sedgewick J.D., Cyster J.G. Lymphotoxin a/b and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 1999;189:403. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R.J., Jung Y.J. Immunity to tuberculosis. Annu. Rev. Immunol. 2004;22:599. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- Pablos J.L., Santiago B., Tsay D., Singer M.S., Palao G., Galindo M., Rosen S.D. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-alpha in cultured endothelial cells. BMC Immunol. 2005;6:6. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R. Is BALT a major component of the human lung immune system? Immunol. Today. 1992;13:119. doi: 10.1016/0167-5699(92)90106-H. [DOI] [PubMed] [Google Scholar]
- Pabst R. Plasticity and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunol. Lett. 2007;112:1. doi: 10.1016/j.imlet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Pabst R., Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am. J. Respir. Cell Mol. Biol. 1990;3:131. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- Pabst R., Tschernig T. Perivascular capillaries in the lung: An important but neglected vascular bed in immune reactions? J. Allergy Clin. Immunol. 2002;110:209. doi: 10.1067/mai.2002.126836. [DOI] [PubMed] [Google Scholar]
- Pauwels R.A., Rabe K.F. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- Pauwels R.A., Buist A.S., Calverley P.M., Jenkins C.R., Hurd S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001;163:1256. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Calverley P., Buist A.S., Rennard S., Fukuchi Y., Stahl E., Lofdahl C.G. COPD exacerbations: The importance of a standard definition. Respir. Med. 2004;98:99. doi: 10.1016/j.rmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Pike B.L., Alderson M.R., Nossal G.J. T-independent activation of single B cells: An orderly analysis of overlapping stages in the activation pathway. Immunol. Rev. 1987;99:119. doi: 10.1111/j.1600-065x.1987.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Plesch B.E.C., van der Brugge-Gamelkoorn G.J., van de Ende M.B. Development of bronchus associated lymphoid tissue (BALT) in the rat, with special reference to T and B cells. Dev. Comp. Immunol. 1983;7:79. doi: 10.1016/0145-305x(83)90066-6. [DOI] [PubMed] [Google Scholar]
- Randall T.D., Carragher D.M., Rangel-Moreno J. Development of secondary lymphoid organs. Ann. Rev. Immunol. 2008;26:627. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J., Moyron-Quiroz J., Kusser K., Hartson L., Nakano H., Randall T.D. Role of CXC chemokine ligand 13, CC chemokine ligand (CCL) 19, and CCL21 in the organization and function of nasal-associated lymphoid tissue. J. Immunol. 2005;175:4904. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- Rangel-Moreno J., Hartson L., Navarro C., Gaxiola M., Selman M., Randall T.D. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J. Clin. Invest. 2006;116:3183. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J., Moyron-Quiroz J.E., Hartson L., Kusser K., Randall T.D. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc. Natl. Acad. Sci. USA. 2007;104:10577. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R.A., Gillies S.D., Mendelsohn J., Varki N.M., Becker J.C. Involvement of B lymphocytes in the growth inhibition of human pulmonary melanoma metastases in athymic nu/nu mice by an antibody–lymphotoxin fusion protein. Cancer Res. 1996;56:1707. [PubMed] [Google Scholar]
- Rodriguez F., Ramirez G.A., Sarradell J., Andrada M., Lorenzo H. Immunohistochemical labelling of cytokines in lung lesions of pigs naturally infected with Mycoplasma hyopneumoniae. J. Comp. Pathol. 2004;130:306. doi: 10.1016/j.jcpa.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Rossi D., Zlotnik A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000;18:217. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Saravanan V., Kelly C.A. Reducing the risk of methotrexate pneumonitis in rheumatoid arthritis. Rheumatology (Oxford) 2004;43:143. doi: 10.1093/rheumatology/keg466. [DOI] [PubMed] [Google Scholar]
- Sarradell J., Andrada M., Ramirez A.S., Fernandez A., Gomez-Villamandos J.C., Jover A., Lorenzo H., Herraez P., Rodriguez F. A morphologic and immunohistochemical study of the bronchus-associated lymphoid tissue of pigs naturally infected with Mycoplasma hyopneumoniae. Vet. Pathol. 2003;40:395. doi: 10.1354/vp.40-4-395. [DOI] [PubMed] [Google Scholar]
- Sato A., Hayakawa H., Uchiyama H., Chida K. Cellular distribution of bronchus-associated lymphoid tissue in rheumatoid arthritis. Am. J. Respir. Crit. Care Med. 1996;154:1903. doi: 10.1164/ajrccm.154.6.8970384. [DOI] [PubMed] [Google Scholar]
- Sato J., Chida K., Suda T., Sato A., Nakamura H. Migratory patterns of thoracic duct lymphocytes into bronchus-associated lymphoid tissue of immunized rats. Lung. 2000;178:295. doi: 10.1007/s004080000033. [DOI] [PubMed] [Google Scholar]
- Schade J., Schmiedl A., Stephan M., Pabst R., von Horsten S. Transferred T cells preferentially adhere in the BALT of CD26-deficient recipient lungs during asthma. Immunobiology. 2010;215:321. doi: 10.1016/j.imbio.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Schrama D., thor Straten P., Fischer W.H., McLellan A.D., Brocker E.B., Reisfeld R.A., Becker J.C. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 2001;14:111. doi: 10.1016/s1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- Schrama D., Voigt H., Eggert A.O., Xiang R., Zhou H., Schumacher T.N., Andersen M.H., thor Straten P., Reisfeld R.A., Becker J.C. Immunological tumor destruction in a murine melanoma model by targeted LTalpha independent of secondary lymphoid tissue. Cancer Immunol. Immunother. 2008;57:85. doi: 10.1007/s00262-007-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert T.A., Lindquist R.L., Shakhar G., Livshits G., Skokos D., Kosco-Vilbois M.H., Dustin M.L., Nussenzweig M.C. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- Seal R.M., Hapke E.J., Thomas G.O., Meek J.C., Hayes M. The pathology of the acute and chronic stages of farmer's lung. Thorax. 1968;23:469. doi: 10.1136/thx.23.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M., King T.E., Pardo A. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 2001;134:136. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Selman M., Thannickal V.J., Pardo A., Zisman D.A., Martinez F.J., Lynch J.P., 3rd Idiopathic pulmonary fibrosis: Pathogenesis and therapeutic approaches. Drugs. 2004;64:405. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R.L., Hieny S., Scott P., Cheever A.W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc. Natl. Acad. Sci. USA. 1990;87:61. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields J.D., Kourtis I.C., Tomei A.A., Roberts J.M., Swartz M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- Shikina T., Hiroi T., Iwatani K., Jang M.H., Fukuyama S., Tamura M., Kubo T., Ishikawa H., Kiyono H. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J. Immunol. 2004;172:6259. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- Shimada S., Kawaguchi-Miyashita M., Kushiro A., Sato T., Nanno M., Sako T., Matsuoka Y., Sudo K., Tagawa Y., Iwakura Y., Ohwaki M. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J. Immunol. 1999;163:5367. [PubMed] [Google Scholar]
- Silva B.A., Silva I.S., Pereira D.M., Aydos R.D., Carvalho Pde T., Facco G.G. Experimental model of pulmonary carcinogenesis in Wistar rats. Acta Cir. Bras. 2007;22(Suppl. 1):16. doi: 10.1590/s0102-86502007000700005. [DOI] [PubMed] [Google Scholar]
- Slavin R.G., Gleich G.J., Hutcheson P.S., Kephart G.M., Knutson A.P., Tsai C.C. Localization of IgE to lung germinal lymphoid follicles in a patient with allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 1992;90:1006. doi: 10.1016/0091-6749(92)90479-l. [DOI] [PubMed] [Google Scholar]
- Sminia T., van de Ende M.B., Jeurissen S.H. Specific antibody formation in mucosa associated lymphoid tissue after intratracheal and intra-intestinal administration of TNP-KLH; differences between BALT and GALT. Adv. Exp. Med. Biol. 1987;216:B:981. [PubMed] [Google Scholar]
- Sminia T., van der Brugge-Gamelkoorn G.J., Jeurissen S.H. Structure and function of bronchus-associated lymphoid tissue (BALT) Crit. Rev. Immunol. 1989;9:119. [PubMed] [Google Scholar]
- Soerensen C.M., Holmskov U., Aalbaek B., Boye M., Heegaard P.M., Nielsen O.L. Pulmonary infections in swine induce altered porcine surfactant protein D expression and localization to dendritic cells in bronchial-associated lymphoid tissue. Immunology. 2005;115:526. doi: 10.1111/j.1365-2567.2005.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Guikema J.E., Schrader C.E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic A., Morgensztern D., Fong T., Lossos I.S. Pulmonary marginal zone lymphoma: A single centre experience and review of the SEER database. Leuk. Lymphoma. 2008;49:1311. doi: 10.1080/10428190802064933. [DOI] [PubMed] [Google Scholar]
- Suda T., Chida K., Hayakawa H., Imokawa S., Iwata M., Nakamura H., Sato A. Development of bronchus-associated lymphoid tissue in chronic hypersensitivity pneumonitis. Chest. 1999;115:357. doi: 10.1378/chest.115.2.357. [DOI] [PubMed] [Google Scholar]
- Sue-Chu M., Karjalainen E.M., Altraja A., Laitinen A., Laitinen L.A., Naess A.B., Larsson L., Bjermer L. Lymphoid aggregates in endobronchial biopsies from young elite cross-country skiers. Am. J. Respir. Crit. Care Med. 1998;158:597. doi: 10.1164/ajrccm.158.2.9711012. [DOI] [PubMed] [Google Scholar]
- Sun Z., Unutmaz D., Zou Y.-R., Sunshine M.J., Pierani A., Brenner-Morton S., Mebius R.E., Littman D.R. Requirement for RORg in thymocyte survival and lymphoid organ development. Science. 2000;288:2369. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. Regulation of innate immune responses by Toll-like receptors. Jpn J. Infect. Dis. 2001;54:209. [PubMed] [Google Scholar]
- Takemura S., Braun A., Crowson C., Kurtin P.J., Cofield R.H., O'Fallon W.M., Goronzy J.J., Weyand C.M. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 2001;167:1072. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Tango M., Suzuki E., Gejyo F., Ushiki T. The presence of specialized epithelial cells on the bronchus-associated lymphoid tissue (BALT) in the mouse. Arch. Histol. Cytol. 2000;63:81. doi: 10.1679/aohc.63.81. [DOI] [PubMed] [Google Scholar]
- Thaunat O., Kerjaschki D., Nicoletti A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006;27:441. doi: 10.1016/j.it.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Thorbecke G.J., Amin A.R., Tsiagbe V.K. Biology of germinal centers in lymphoid tissue. FASEB J. 1994;8:832. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- Tsai M.C., Chakravarty S., Zhu G., Xu J., Tanaka K., Koch C., Tufariello J., Flynn J., Chan J. Characterization of the tuberculous granuloma in murine and human lungs: Cellular composition and relative tissue oxygen tension. Cell. Microbiol. 2006;8:218. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- Tshering T., Pabst R. Bronchus associated lymphoid tissue (BALT) is not present in normal adult lung but in different diseases. Pathobiology. 2000;68:1. doi: 10.1159/000028109. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Komatsu N., Kawamoto S., Suzuki K., Kanagawa O., Honjo T., Hori S., Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- Turesson C., O'Fallon W.M., Crowson C.S., Gabriel S.E., Matteson E.L. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J. Rheumatol. 2002;29:62. [PubMed] [Google Scholar]
- Turesson C., O'Fallon W.M., Crowson C.S., Gabriel S.E., Matteson E.L. Extra-articular disease manifestations in rheumatoid arthritis: Incidence trends and risk factors over 46 years. Ann. Rheum. Dis. 2003;62:722. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesson C., Matteson E.L., Colby T.V., Vuk-Pavlovic Z., Vassallo R., Weyand C.M., Tazelaar H.D., Limper A.H. Increased CD4+ T cell infiltrates in rheumatoid arthritis-associated interstitial pneumonitis compared with idiopathic interstitial pneumonitis. Arthritis Rheum. 2005;52:73. doi: 10.1002/art.20765. [DOI] [PubMed] [Google Scholar]
- Turner J., Frank A.A., Brooks J.V., Gonzalez-Juarrero M., Orme I.M. The progression of chronic tuberculosis in the mouse does not require the participation of B lymphocytes or interleukin-4. Exp. Gerontol. 2001;36:537. doi: 10.1016/s0531-5565(00)00257-6. [DOI] [PubMed] [Google Scholar]
- Ulrichs T., Kosmiadi G.A., Trusov V., Jorg S., Pradl L., Titukhina M., Mishenko V., Gushina N., Kaufmann S.H. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J. Pathol. 2004;204:217. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- van de Pavert S.A., Olivier B.J., Goverse G., Vondenhoff M.F., Greuter M., Beke P., Kusser K., Hopken U.E., Lipp M., Niederreither K., Blomhoff R., Sitnik K. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat. Immunol. 2009;10:1193. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G., van de Ende M., Sminia T. Uptake of antigens and inert particles by bronchus associated lymphoid tissue (BALT) epithelium in the rat. Cell Biol. Int. Rep. 1985;9:524. doi: 10.1016/0309-1651(85)90011-6. [DOI] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G.J., Plesch B.E., Sminia T., Langevoort H.L. Histological changes in rat bronchus-associated lymphoid tissue after administration of five different antigens. Respiration. 1985;48:29. doi: 10.1159/000194795. [DOI] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G.J., Plesch B.E., Sminia T., Langevoort H.L. Specific antibody-forming cells in bronchus-associated lymphoid tissue (BALT) and lung of the rat after intratracheal challenge with horseradish peroxidase. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1985;49:269. doi: 10.1007/BF02912104. [DOI] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G.J., Claassen E., Sminia T. Anti-TNP-forming cells in bronchus-associated lymphoid tissue (BALT) and paratracheal lymph node (PTLN) of the rat after intratracheal priming and boosting with TNP-KLH. Immunology. 1986;57:405. [PMC free article] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G.J., van de Ende M., Sminia T. Changes occurring in the epithelium covering the bronchus-associated lymphoid tissue of rats after intratracheal challenge with horseradish peroxidase. Cell Tissue Res. 1986;245:439. doi: 10.1007/BF00213952. [DOI] [PubMed] [Google Scholar]
- van der Strate B.W., Postma D.S., Brandsma C.A., Melgert B.N., Luinge M.A., Geerlings M., Hylkema M.N., van den Berg A., Timens W., Kerstjens H.A. Cigarette smoke-induced emphysema: A role for the B cell? Am. J. Respir. Crit. Care Med. 2006;173:751. doi: 10.1164/rccm.200504-594OC. [DOI] [PubMed] [Google Scholar]
- Van Pottelberge G.R., Bracke K.R., Van den Broeck S., Reinartz S.M., van Drunen C.M., Wouters E.F., Verleden G.M., Vermassen F.E., Joos G.F., Brusselle G.G. Plasmacytoid dendritic cells in pulmonary lymphoid follicles of patients with COPD. Eur. Respir. J. 2010 doi: 10.1183/09031936.00140409. PMID 20351031 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Vernooy J.H., Dentener M.A., van Suylen R.J., Buurman W.A., Wouters E.F. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am. J. Respir. Cell Mol. Biol. 2002;26:152. doi: 10.1165/ajrcmb.26.1.4652. [DOI] [PubMed] [Google Scholar]
- Wagner U.G., Kurtin P.J., Wahner A., Brackertz M., Berry D.J., Goronzy J.J., Weyand C.M. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J. Immunol. 1998;161:6390. [PubMed] [Google Scholar]
- Wiley J.A., Richert L.E., Swain S.D., Harmsen A., Barnard D.L., Randall T.D., Jutila M., Douglas T., Broomell C., Young M. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS ONE. 2009;4:e7142. doi: 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland D.L., Randall T.D. Anatomical features of anti-viral immunity in the respiratory tract. Semin. Immunol. 2004;16:163. doi: 10.1016/j.smim.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Wagner N., Pham L.N., Magno V., Shan Z., Butcher E.C., Michie S.A. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by l-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J. Exp. Med. 2003;197:1255. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinouchi T., Ohtsuki Y., Ueda R., Sato S., Ueda N. Myofibroblasts and S-100 protein positive cells in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated interstitial pneumonia. Eur. Respir. J. 1999;14:579. doi: 10.1034/j.1399-3003.1999.14c16.x. [DOI] [PubMed] [Google Scholar]
- Yu P., Lee Y., Liu W., Chin R.K., Wang J., Wang Y., Schietinger A., Philip M., Schreiber H., Fu Y.X. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat. Immunol. 2004;5:141. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- Yu P., Lee Y., Wang Y., Liu X., Auh S., Gajewski T.F., Schreiber H., You Z., Kaynor C., Wang X., Fu Y.X. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J. Immunol. 2007;179:1960. doi: 10.4049/jimmunol.179.3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


