Abstract
Objectives
To investigate the association between late-life blood pressure and the incidence of cognitive impairment in older adults.
Design
Prospective cohort study.
Setting
Community-living older adults from 22 provinces in China.
Participants
We included 12,281 cognitively normal (Mini-Mental State Examination [MMSE] ≥ 24) older adults (median age: 81 years) from the Chinese Longitudinal Healthy Longevity Survey. Eligible participants must have baseline blood pressure data and have one or more follow-up cognitive assessments.
Measurements
Baseline systolic (SBP) and diastolic blood pressure (DBP) were measured by trained internists. Cognitive function was evaluated by MMSE. We considered mild/moderate/severe cognitive impairment (MMSE < 24, and MMSE decline ≥ 3) as the primary outcome.
Results
The participants with hypertension had a significantly higher risk of mild/moderate/severe cognitive impairment (HR 1.17, 95% CI 1.10 to 1.24). Overall the associations with cognitive impairment seem to be hockey stick-shaped for SBP and linear for DBP, though the estimated effects for low SBP/DBP were less precise. High SBP was associated with a gradual increase in the risk of mild/moderate/severe cognitive impairment (P-trend < 0.001). Compared with SBP 120–129 mmHg, the adjusted HR was 1.17 (95% CI 1.07 to 1.29) for SBP 130–139 mmHg, increased to 1.54(95% CI 1.35 to 1.75) for SBP≥180 mmHg. Analyses for high DBP showed the same increasing pattern, with an adjusted HR of 1.09 (95% CI 1.01 to 1.18) for DBP 90–99 mmHg and 1.19 (95% CI 1.02 to 1.38) for DBP ≥110 mmHg, as compared with DBP 70–79 mmHg.
Conclusion
Late-life high blood pressure was independently associated with cognitive impairment in cognitively normal Chinese older adults. Prevention and management of high blood pressure may have substantial benefits for cognition among older adults in view of the high prevalence of hypertension in this rapidly growing population.
Keywords: blood pressure, cognitive impairment, older adults, cohort study
Introduction
Cognitive impairment in older adults represents a major and growing health problem worldwide. Globally over 46 million people were living with dementia in 2015 and this number is projected to reach 75 million by 2030.1 In the absence of effective treatments for dementia, identifying modifiable risk factors then reducing the risk is currently the fundamental strategy against this disease.
There is strong evidence that high blood pressure in midlife is associated with cognitive impairment,2, 3 but the association of late-life hypertension with cognition is less clear.4 Previous prospective studies have shown mixed results demonstrating either a harmful,5–8 protective,9 or null effect 10–12 of high blood pressure on cognition. The inconsistencies in these findings may result from the variations in analysis strategies, sample-size, adjustment for potential confounders, and participant characteristics such as age, sex, and ethnicity. Moreover, ethnicity may play a role in cognitive impairment,6, 13 but most published studies investigating the association between blood pressure and cognitive impairment were carried out in Caucasians.5–12 We have investigated the association in 7,144 Chinese older adults,14 but the causal relationship could not be confirmed due to the nature of cross-sectional design. We therefore carried out this prospective cohort study to evaluate the relationship between blood pressure and the incidence of cognitive impairment in Chinese older adults.
Methods
Study design and participants
This study is a prospective community-based cohort study of the participants from the Chinese Longitudinal Healthy Longevity Survey (CLHLS). The CLHLS is an ongoing longitudinal survey in 22 of 31 provinces in China. The investigation began in 1998 and follow-up surveys were conducted in 2000, 2002, 2005, 2008, 2011, and 2014, of which the response rate was approximately 90% for each wave. Because the death rate was high in older adults, new participants were recruited in the follow-up surveys to maintain a stable sample size. Approximately two third of the subjects in each wave were participants from the previous wave, and the rest were new recruits. Details of this survey have been described elsewhere.15 The study was approved by the Biomedical Ethics Committee of Peking University. Written consent was obtained from all participants.
Because the follow-up time varied among older adults due to death, we evaluated the cognitive impairment risk with survival analysis to make full use of the observed data as previous studies.16, 17 We included older adults (65 years or above) from the 1998 survey and the new recruits from the follow-up surveys. We included cognitive impairment-free older adults, which were defined as having a baseline Mini-Mental State Examination (MMSE) ≥ 24. We excluded participants without baseline blood pressure data (data missing rate: 4.5%) or with follow-up time less than 2 years. The participants without follow-up MMSE assessments were excluded as in previous studies16, 17 because these participants could not contribute to the evaluation of risk (see the flowchart of participant enrolment in Appendix Figure A1).
Assessment of Blood Pressure
Baseline blood pressure was evaluated by trained internists with at least 3 years of work experience. Baseline arterial blood pressure was measured using a mercury sphygmomanometer placing on the right arm at heart level of a seated subject after he/she has rested for at least 5 minutes under supervision. The blood pressure of bedridden participants was measured in a recumbent position. Phase I and V Korotkoff sounds were designated as the SBP and DBP value respectively. We considered SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg as the definition for hypertension.18 We categorized SBP and DBP based on a 10 mmHg interval to reflect both the latest ACC/AHA19 and the Chinese classification 18, 20 of blood pressure in adults (SBP: <110 mmHg, 110–119 mmHg, 120–129 mmHg, 130–139 mmHg, 140–149 mmHg, 150–159 mmHg, 160–169 mmHg, 170–179 mmHg, and ≥ 180 mmHg; DBP: <70 mmHg, 70–79 mmHg, 80–89 mmHg, 90–99 mmHg, 100–109, and ≥ 110 mmHg).
Assessment of Cognitive Function
We evaluated cognitive function by the Chinese version of MMSE, which is a widely used 30-point assessment tool for testing global cognitive function.21, 22 The MMSE tests the cognitive function by examining orientation, registration, attention, memory, language, and visual construction skills. Cognitive function was repeatedly evaluated in the follow-up surveys.
The following cut-off levels were often used to classify cognitive impairment: 1) 24 ≤ MMSE ≤ 30: no cognitive impairment; 2) 18≤ MMSE <24: mild cognitive impairment; 3) 0 ≤ MMSE <18: moderate/severe cognitive impairment. 22, 23 We considered mild/moderate/severe cognitive impairment (follow-up MMSE score <24 points) as the primary outcome. Because a reliable change in MMSE should be at least 2–4 points,24 we applied an additional restriction of MMSE decline ≥ 3 points to our definition of the primary outcome. We considered moderate/severe cognitive impairment (follow-up MMSE score <18 points) as the secondary outcome. Additional restriction of MMSE decline ≥ 3 points was not needed for the secondary outcome because the decline was at least 6 points (24 minus 18). We defined the first time when a participant experienced cognitive impairment as the length of time for survival analyses. The participants who did not experience cognitive impairment were considered as censored observations and the censoring time was calculated from the baseline to the last cognitive assessment.
Covariates
We selected covariates that may confound the relationship between blood pressure and cognitive impairment based on the review of literature. Covariate information was obtained from the structured questionnaire for the baseline survey.25 The covariates for our analyses included sociodemographic characteristics (age, sex, height, weight, education, co-residence, and marital status), lifestyle behaviors (smoking, alcohol drinking, physical activity, fresh fruit and vegetable consumption), self-reported medical history (hypertension, diabetes mellitus, heart disease, cerebrovascular disease, bronchitis, emphysema, asthma, and pneumonia), activities of daily living (ADL),26 and depressive symptoms.
Statistical Analysis
To explore the shape of the relationship between blood pressure and cognitive impairment, we used additive Cox regression taking SBP/DBP as smoothed terms in the model.27 Penalized splines were used for smoothing. The choice of the degrees of freedom was determined by comparing the Akaike Information Criterion and residual deviance of different models.28 Because no single parameter values for the exposure were returned directly from additive Cox regression model, making the results difficult to interpret, we additionally evaluated the hazard ratios (HRs) of cognitive impairment by different SBP/DBP levels with regular Cox regression. We defined SBP 120–139 mmHg and DBP 80–89 mmHg as the reference group based on the definition of normal blood pressure for Chinese older adults.18, 20 We did not take SBP 110–119 mmHg and DBP 70–79 mmHg as the reference group because the numbers of participants in these groups were too small to calculate a precise estimate of hazard ratio for other groups. In order to evaluate the incremental risk of cognitive impairment, we analyzed the association by including blood pressure as a continuous variable in Cox regression models.
Multivariate Cox models were adopted to adjust for established and potential confounding factors. The basic model adjusted baseline age (continuous), sex (men or women), years of education (0 or ≥ 1 year), residence (urban or rural), and co-residence (live alone or with others). The fully adjusted model additionally adjusted smoking (current smoker, former smoker, or non-smoker), alcohol drinking (current drinker, former drinker, or non-drinker), frequent vegetable consumption (yes or no), frequent fruit consumption (yes or not), frequent physical activity (yes or no), ADL (restricted or normal), obesity (underweight, normal weigh, or overweight/obese, according to the criteria of body mass index for Chinese population),29 self-reported diabetes mellitus (yes or no), heart disease (yes or no), and cerebrovascular disease (yes or no).
We undertook subgroup analyses of the association between hypertension and the primary outcome by age, sex, residence, education time, obesity, smoking status, and alcohol drinking status. The interaction effects were tested by including an interaction term in the Cox regression model. Subgroup analyses were undertaken with the fully-adjusted model.
We conducted a series of sensitivity analyses to check the robustness of the primary results: defining of hypertension based on the 2017 ACC/AHA guideline (SBP/DBP ≥ 130/80 mmHg),19 additionally adjusting for depressive symptoms; adjusting for marital status; adjusting for self-reported bronchitis, emphysema, asthma, and pneumonia; adjusting for the time of recruitment to clarify potential period-effects; and restricting participants with at least 2 or more follow-up MMSE assessments. Analyses were completed using Stata version12.0 (StataCorp LP, College Station, TX, USA) and R software version 3.4.1 (R Development Core Team, 2017). Two-sided P<0.05 was considered statistically significant for all analyses.
Results
Baseline characteristics
This study included 12,281 older adults (median age: 81 years). Table 1 presents the characteristics of included participants. A total of 6,850 participants (55.8%) were classified as having hypertension. The participants with hypertension were likely to be older, have a higher rate of women, overweight/obesity, former smoker and drinker, frequent vegetable consumption, taking physical activity regularly, restricted ADL, heart disease, and cerebrovascular disease. The mean follow-up time for included participants was 6.0 (standard deviation = 3.2) years, ranging from 2 to 16.4 years.
Table 1.
Characteristics of included participants
| Hypertension (SBP/DBP ≥ 140/90 mmHg) |
|||
|---|---|---|---|
| Yes | No | P value * | |
| No. of participants | 6,850 | 5,431 | |
| Median (IQR) age, years | 81(65–109) | 80(65–108) | 0.001 |
| Men, n(%) | 3,425(50.0) | 2,820(51.9) | 0.034 |
| Residence, n(%) | |||
| Urban | 2,847(41.6) | 2,283(42.0) | 0.60 |
| Rural | 4,003(58.4) | 3,148(58.0) | |
| Education time, years | |||
| 0 | 3,512(51.4) | 2,725(50.3) | 0.22 |
| ≥1 | 3,324(48.6) | 2,696(49.7) | |
| Co-residence | |||
| Live alone | 5,906(86.3) | 4,719(86.9) | 0.32 |
| Live with others | 938(13.7) | 711(13.1) | |
| Obesity, n(%) | |||
| Under weight | 2,025(30.1) | 1,914(35.8) | <0.001 |
| Normal weight | 3,274(48.7) | 2,575(48.2) | |
| Overweight/obese | 1,426(21.2) | 857(16.0) | |
| Smoking, n(%) | |||
| Non-smoker | 4,239(61.9) | 3,341(61.6) | 0.017 |
| Current smoker | 1,592(23.3) | 1,329(24.5) | |
| Former smoker | 1,014(14.8) | 757(13.9) | |
| Alcohol drinking, n(%) | |||
| Non-drinker | 4,524(66.1) | 3,583(66.0) | 0.001 |
| Current drinker | 1,636(23.9) | 1,400(25.8) | |
| Former drinker | 680(9.9) | 446(8.2) | |
| Frequent vegetable consumption, n(%) | 4,655(68.0) | 3,580(65.9) | 0.02 |
| Frequent fruit consumption, n(%) | 992(14.5) | 728(13.4) | 0.09 |
| Frequent physical activity, n(%) | 3,539(51.7) | 2,380(43.8) | <0.001 |
| Impaired activity of daily living, n(%) | 631(9.2) | 349(6.4) | <0.001 |
| Self-reported diabetes mellitus, n(%) | 159(2.3) | 104(1.9) | 0.11 |
| Self-reported heart disease, n(%) | 706(10.4) | 363(6.7) | <0.001 |
| Self-reported cerebrovascular disease, n(%) | 295(4.3) | 153(2.8) | <0.001 |
| Median(IQR) baseline MMSE score | 29(24–30) | 29(24–30) | 0.01 |
The differences between groups were tested by Kruskal-Walhs test or χ2 test.
SBP: systolic blood pressure; DBP: diastolic blood pressure; IQR: interquartile range; MMSE: mini-mental state examination
Association between hypertension and cognitive impairment
During a total of 66,619.9 person-years of observation, we documented 4,413 cases of mild/moderate/severe cognitive impairment and 2,092 cases of moderate/severe cognitive impairment. The crude HR for mild/moderate/severe cognitive impairment between participants with and without hypertension was 1.26 (95% CI 1.18 to 1.34). The association remained significant after multivariate adjustment (HR 1.17, 95% CI 1.10 to 1.24) (Appendix Table A1). The adjusted effect for moderate/severe cognitive impairment was similar (HR 1.18, 95% CI 1.08 to 1.29).
Appendix Table A2 shows subgroup analyses of the association between hypertension and the primary outcome. There were no significantly differences in the estimated effects between different age groups (65 to 85 years: HR 1.14, 95%CI 1.11 to 1.35; ≥ 85 years: HR 1.13, 95%CI 1.04 to 1.23) and between men and women (men: HR 1.22, 95%CI 1.02 to 1.34; women: HR 1.13, 95%CI 1.05 to 1.22). We did not identify any significant differences across strata according to residence (P = 0.19), education (P = 0.71), obesity (P = 0.54), smoking (P = 0.84), and alcohol drinking (P = 0.86).
There was almost no change in the association when we reclassified hypertension according to the 2017 ACC/AHA definition (SBP/DBP ≥130/80 mmHg) (HR 1.18, 95% CI 1.08 to 1.28) (Appendix Table A3). The association did not change after additionally adjustment for depressive symptoms, marital status, time of recruitment, and self-reported bronchitis, emphysema, asthma, and pneumonia. The restriction of participants with at least 2 or more follow-up MMSE assessments did not change the primary result.
Risk of cognitive impairment according to SBP/DBP level
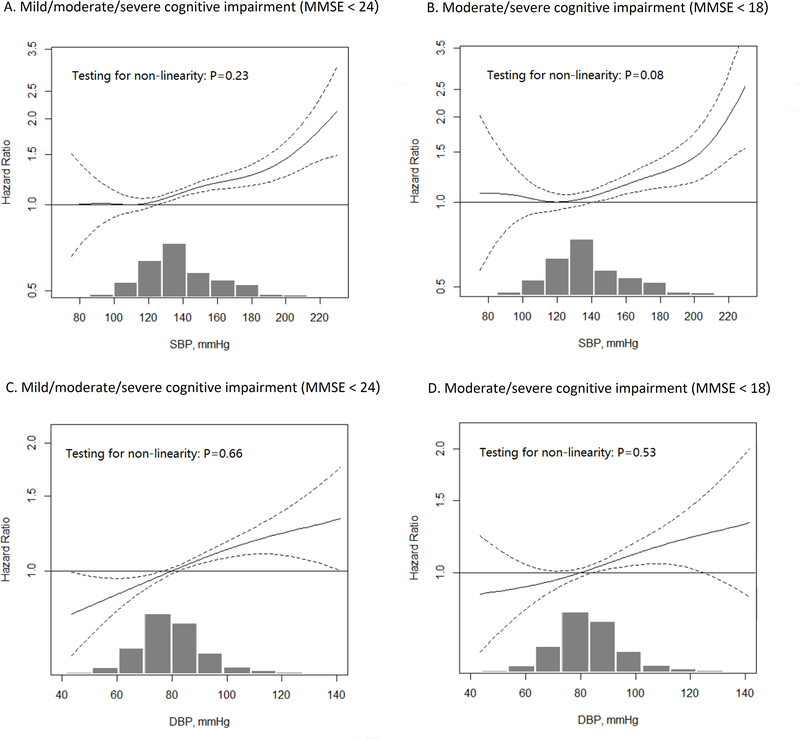
We explored the shape of the associations between SBP/DBP and cognitive impairment using additive Cox models (Figure 1). Overall the associations seem to be hockey stick-shaped for SBP (flat then increasing), though the estimated effects for low SBP were less precise. The inflection points with minimum HRs were 110 mmHg for mild/moderate/severe cognitive impairment and 120 mmHg for moderate/severe cognitive impairment. There was a clear trend that higher SBP than these inflection points was associated with a gradual increase in the risk of cognitive impairment. The association between DBP and cognitive impairment was linear. Appendix Table A4 presents the HRs of cognitive impairment for each 10 mmHg increase in SBP/DBP. Each 10 mmHg increase in SBP was associated with a 5% increase in the risk of mild/moderate/severe cognitive impairment. The adjusted HR for each 10 mmHg increase in DBP was 1.06(95% CI 1.03 to 1.08).
Figure 1.
Association between systolic/diastolic blood pressure and the incidence of cognitive impairment
The results were based on additive Cox-regression model taking SBP/DBP as a smoothing term. The models have adjusted for age, sex, education, co-residence, and residence, smoking, drinking, frequent vegetable consumption, frequent fruit consumption, frequent physical activity, impaired activity of daily living, obesity, activities of daily living, self-reported diabetes mellitus, self-reported heart disease, and self-reported cerebrovascular disease.
The histograms at the bottom of each panel present the distribution of SBP/DBP of included participants. For the estimated hazard ratio for SBP (Panel A & B), the hazards at inflection point with the lowest hazard (Panel A: SBP = 110 mmHg, Panel B: SBP = 120 mmHg) were considered as the reference. Because no inflection points for DBP (Panel C & D) were identified, the hazards at DBP 80 mmHg, the definition of normal blood pressure for Chinse adults, were considered as the reference.
MMSE: mini-mental state examination; SBP: systolic blood pressure; DBP: diastolic blood pressure.
We evaluated the association between SBP/DBP and cognitive impairment with regular Cox regression models for better interpretation (Table 2). The regression model suggested that high SBP was associated with a gradual increase in the risk of mild/moderate/severe cognitive impairment (P-trend < 0.001). Compared with the reference group (SBP 120–129 mmHg), the fully-adjusted HR was 1.17(95% CI 1.07 to 1.29) for SBP 130–139 mmHg, increased to 1.54 (95% CI 1.35 to 1.75) for SBP ≥180 mmHg. Analyses for high DBP and the risk of mild/moderate/severe cognitive impairment showed the same pattern, with an adjusted HR of 1.09 (95% CI 1.01 to 1.18) for DBP 90–99 mmHg, and 1.19 (95% CI 1.02 to 1.38) for DBP ≥110 mmHg.
Table 2.
Risk of cognitive impairment by systolic/diastolic blood pressure level
| HR [95% CI] for mild/moderate/severe cognitive impairment (MMSE<24) |
HR [95% CI] for moderate/severe cognitive impairment (MMSE<18) |
|||||
|---|---|---|---|---|---|---|
| Unadjusted model | Basic model † | Fully adjusted model ‡ | Unadjusted model | Basic model † | Fully adjusted model ‡ | |
| SBP | ||||||
| No. of participants | 12,271 | 12,241 | 11,946 | 12,271 | 12,241 | 11,946 |
| No. of events | 4,413 | 4,401 | 4,280 | 2,092 | 2,089 | 2,034 |
| No. of person years | 61,805.7 | 61,672.9 | 60,446.1 | 66,619.9 | 66,472.8 | 65,167.7 |
| <110 mmHg | 1.21[1.04, 1.41]* | 1.10[0.94, 1.28] | 1.11[0.95, 1.30] | 1.45[1.17, 1.79]*** | 1.32[1.06, 1.63]* | 1.32[1.06, 1.64]* |
| 110–119 mmHg | 1.02[0.90, 1.15] | 1.06[0.94, 1.20] | 1.07[0.94, 1.21] | 0.96[0.80, 1.17] | 1.02[0.85, 1.24] | 1.03[0.85, 1.25] |
| 120–129 mmHg | 1.00(referent) | 1.00(referent) | 1.00(referent) | 1.00(referent) | 1.00(referent) | 1.00(referent) |
| 130–139 mmHg | 1.19[1.08, 1.30]*** | 1.17[1.06, 1.28]*** | 1.17[1.07, 1.29]*** | 1.25[1.09, 1.44]** | 1.22[1.06, 1.40]** | 1.23[1.07, 1.42]** |
| 140–149 mmHg | 1.26[1.14, 1.39]*** | 1.21[1.10, 1.34]*** | 1.21[1.10, 1.34]*** | 1.26[1.09, 1.45]** | 1.18[1.02, 1.36]* | 1.19[1.03, 1.38]* |
| 150–159 mmHg | 1.37[1.22, 1.54]*** | 1.22[1.09, 1.37]*** | 1.24[1.10, 1.39]*** | 1.48[1.25, 1.74]*** | 1.29[1.09, 1.51]** | 1.30[1.10, 1.54]** |
| 160–169 mmHg | 1.50[1.32, 1.69]*** | 1.27[1.12, 1.44]*** | 1.29[1.13, 1.46]*** | 1.51[1.26, 1.81]*** | 1.22[1.01, 1.46]* | 1.22[1.01, 1.47]* |
| 170–179 mmHg | 1.75[1.50, 2.05]*** | 1.33[1.14, 1.55]*** | 1.34[1.14, 1.56]*** | 2.14[1.73, 2.64]*** | 1.52[1.23, 1.88]*** | 1.58[1.27, 1.96]*** |
| >180 mmHg | 2.01[1.77, 2.28]*** | 1.48[1.30, 1.69]*** | 1.54[1.35, 1.75]*** | 2.24[1.87, 2.69]*** | 1.57[1.31, 1.89]*** | 1.62[1.35, 1.96]*** |
| P-trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| DBP | ||||||
| No. of participants | 12,258 | 12,228 | 11,934 | 12,258 | 12,228 | 11,934 |
| No. of events | 4,408 | 4,396 | 4,276 | 2,091 | 2,088 | 2,033 |
| No. of person years | 61,730.8 | 61598 | 60,375.3 | 66,541.9 | 66,394.8 | 65,093.7 |
| <70 mmHg | 0.94[0.83, 1.06] | 0.83[0.73, 0.94]** | 0.84[0.74, 0.96]** | 1.12[0.94, 1.33] | 0.98[0.82, 1.16] | 0.99[0.83, 1.18] |
| 70–79 mmHg | 1.03[0.95, 1.11] | 0.98[0.90, 1.06] | 0.97[0.89, 1.05] | 1.07[0.95, 1.21] | 1.01 [0.89, 1.13] | 1.00[0.88, 1.12] |
| 80–89 mmHg | 1.00(referent) | 1.00(referent) | 1.00(referent) | 1.00(referent) | 1.00(referent) | 1.00(referent) |
| 90–99 mmHg | 1.10[1.02, 1.19]* | 1.09[1.01, 1.18]* | 1.09[1.01, 1.18]* | 1.18[1.05, 1.32]** | 1.15[1.02, 1.28]* | 1.12[1.00, 1.26]* |
| 100–109 mmHg | 1.30[1.16, 1.47]*** | 1.21[1.08, 1.37]*** | 1.20[1.06, 1.35]** | 1.38[1.16, 1.63]*** | 1.21[1.01, 1.43]* | 1.18[0.99, 1.41] |
| >110 mmHg | 1.16[1.00, 1.35]* | 1.20[1.04, 1.39]* | 1.19[1.02, 1.38]* | 1.25[1.01, 1.54]* | 1.31[1.06, 1.62]* | 1.25[1.01, 1.56]* |
| P-trend | <0.001 | <0.001 | <0.001 | 0.01 | 0.001 | 0.003 |
The results were based on regular cox regression taking SBP/DBP level as a categorical variable in the model.
Basic model: adjusted for age, sex, education, co-residence, and residence;
Fully adjusted model: additionally adjusted for smoking, drinking, frequent vegetable consumption, frequent fruit consumption, frequent physical activity, impaired activity of daily living, obesity, activities of daily living, self-reported diabetes mellitus, self-reported heart disease, and self-reported cerebrovascular disease.
HR: hazard ratio; CI: confidence interval; MMSE: mini-mental state examination; SBP: systolic blood pressure; DBP: diastolic blood pressure.
0.01 ≤ P < 0.05
0.001 ≤ P < 0.01
P < 0.001
HR: hazard ratio; CI: confidence interval; MMSE: mini-mental state examination; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Due to relatively small sample size for older adults with low SBP, our evaluation of the cognitive impairment risk for this group was less precise. There was insufficient evidence that the risk of mild/moderate/severe cognitive impairment in this group was higher than the reference group (adjusted HR 1.11, 95% CI 0.95 to 1.30). The participants with a SBP < 110 mmHg tended to have higher risk of moderate/severe cognitive impairment (adjusted HR 1.32, 95% CI 1.06 to 1.64).
Discussion
This community-based cohort study showed that hypertension, either defined as SBP/DBP ≥ 140/90 mmHg or ≥130/80 mmHg, was associated with a significantly increased risk of cognitive impairment in cognitively normal Chinese older adults. The association was not modified by age, sex, residence, education, obesity, smoking, and alcohol drinking. Though the estimated effects for lower blood pressure were less precise, the associations with cognitive impairment were likely to be hockey stick-shaped for SBP and linear for DBP.
Although not fully understood, high blood pressure may affect the risk of cognitive impairment through several mechanisms. It has been suggested that high blood pressure related functional and structural changes in cerebral blood vessels could adversely impact brain circulation, cognitive dysfunction may develop subsequently.30, 31 Additionally, high blood pressure may cause white matter lesions 32 and cortical thickness reduction,31 which are closely related to the cognitive performance in older adults. Other possible mechanisms include blood-brain barrier dysfunction,33 the accumulation of beta amyloid protein in the brain,34 and the activation of the renin-angiotensin-aldosterone system.35
Our results were consistent with a number of prospective studies.5–8, 36 For example, the English Longitudinal Study on Aging (n = 8,780) showed that SBP ≥160 mmHg was associated with lower global cognitive and specific memory scores at 8-year follow-up.5 Analyses of 3,657 participants aged 65 or above from the EPESE and the HDFP project suggested the older adults with a SBP ≥160 mmHg had 7% more errors on cognitive tests as compared with the reference group (SBP 130–139 mmHg).36 To the contrary, one population-based study of 559 participants aged 90 or above showed that those who developed hypertension after age 80 were associated with lower risk of dementia when compared with those without hypertension.9 The inconsistencies in these results may be explained by the differences in analysis strategies, sample-size, and adjustment for potential confounders. Another explanation is that the study suggesting a protective effect 9 was carried out in survivors of the targeted population, so the participants may have different characteristics from general older population.
Our study was unable to give a precise estimate of the effect for low blood pressure due to small sample size in this group. The regression analysis suggested that low SBP was likely to associate with higher risk of moderate/severe impairment. Our results were consistent with the Duke Populations Studies of the Elderly, which indicated that extreme low SBP was associated with decline in cognitive function over 3 years.6 The present study, unlike our previous cross-sectional study,14 did not find sufficient evidence of increased cognitive impairment risk for low DBP. The inconsistency may be caused by the variations in study design and participant characteristics (we only included cognitively normal older adults in the present study).
This study is by far the largest cohort study investigating the association between blood pressure and cognitive impairment in Asian older adults. The strength of this study included large sample size, unique study population, prospective community-based study design, survival analysis to make full use of the observed data, careful adjustment for established and potential risk factors, and robust sensitivity analysis results.
This study has a number of limitations. First, although we carefully adjusted many established and potential risk factors for cognitive impairment, residual confounding by other unmeasured or unknown factors was still possible. Many factors that may influence the relationship, such as duration of hypertension, treatment for hypertension, and plasma glucose, were not collected in CLHLS and therefore could not be analyzed. Second, we were unable to thoroughly investigate the relationship between low blood pressure and cognitive impairment due to small sample size. Third, we restricted eligible participants to those with at least one follow-up MMSE assessment. Such restriction enabled us to include most participants in analyses. However, those died or lost to follow-up before the first follow-up survey were not included and they might have different characteristics from the included participants. Last, the estimated effects for moderate/severe cognitive impairment were likely to be influenced by low event rate (16.8%); however, the influence would be minor because moderate/severe cognitive impairment was the secondary outcome and the results were consistent with the primary outcome.
Conclusions
This community-based cohort study showed that hypertension was independently associated with cognitive impairment among cognitively normal Chinese older adults. The associations with cognitive impairment seem to be hockey stick-shaped for SBP and linear for DBP. Our findings support a role of late-life hypertension in the development of cognitive impairment. Prevention and management of high blood pressure may have substantial benefits for cognition among older adults in view of the high prevalence of hypertension in this rapidly growing population.
Supplementary Material
Acknowledgments
Sponsor’s Role:
The sponsors did not play a role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Funding
This work was supported by National Natural Sciences Foundation of China [grant numbers 71233001, 71490732 and 81573247], The U.S. National Institute of Aging / United Nations Fund for Population Activities[grant number 2P01AG031719], and Claude D. Pepper Older Americans Independence Centers grant [grant number 5P30 AG028716].
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Reference
- 1.World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incid ence, cost and trends. 2015. Available at: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf.
- 2.Iadecola C, Yaffe K, Biller J, et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 2014;71:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Yaffe K, Biller J, et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 2016;68:E67–E94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dregan A, Stewart R, Gulliford MC. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing 2013;42:338–345. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon AD, Fillenbaum GG, Pieper CF, et al. Relationship of race/ethnicity and blood pressure to change in cognitive function. J Am Geriatr Soc 2002;50:424–429. [DOI] [PubMed] [Google Scholar]
- 7.Yasar S, Ko JY, Nothelle S, et al. Evaluation of the effect of systolic blood pressure and pulse pressure on cognitive function: the Women’s Health and Aging Study II. PLoS One 2011;6:e27976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reitz C, Tang MX, Manly J, et al. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007;64:1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrada MM, Hayden KM, Paganini-Hill A, et al. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+Study. Alzheimers & Dementia 2017;13:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert LE, Scherr PA, Bennett DA, et al. Blood pressure and late-life cognitive function change: a biracial longitudinal population study. Neurology 2004;62:2021–2024. [DOI] [PubMed] [Google Scholar]
- 11.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology 2004;63:1882–1891. [DOI] [PubMed] [Google Scholar]
- 12.Yaffe K, Haan M, Blackwell T, et al. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc 2007;55:758–762. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council (US) Panel on Race, Ethnicity, and Health in Later Life; Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington (DC): National Academies Press (US); 2004. 4, Ethnic Differences in Dementia and Alzheimer’s Disease. Available from: https://www.ncbi.nlm.nih.gov/books/NBK25535/. [PubMed] [Google Scholar]
- 14.Lv YB, Zhu PF, Yin ZX, et al. A U-shaped Association Between Blood Pressure and Cognitive Impairment in Chinese Elderly. J Am Med Dir Assoc 2017;18:193 e197–193 e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Y Towards Deeper Research and Better Policy for Healthy Aging --Using the Unique Data of Chinese Longitudinal Healthy Longevity Survey. China Economic J 2012;5:131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doody RS, Massman P, Dunn JK. A method for estimating progression rates in Alzheimer disease. Arch Neurol 2001;58:449–454. [DOI] [PubMed] [Google Scholar]
- 17.Uc EY, McDermott MP, Marder KS, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 2009;73:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Committee of Cardio-Cerebro-Vascular Diseases of Gerontological Society of C, Chinese College of Cardiovascular Physicians of Chinese Medical Doctor A. [Chinese expert consensus on the diagnosis and treatment of hypertension in the elderly(2017)]. Zhonghua Nei Ke Za Zhi 2017;56:885–893. [DOI] [PubMed] [Google Scholar]
- 19.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. [DOI] [PubMed] [Google Scholar]
- 20.Liu LS, Writing Group of Chinese Guidelines for the Management of H. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579–615. [PubMed] [Google Scholar]
- 21.Katzman R, Zhang MY, Ouang Ya Q, et al. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol 1988;41:971–978. [DOI] [PubMed] [Google Scholar]
- 22.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–935. [DOI] [PubMed] [Google Scholar]
- 23.An RP, Liu GG. Cognitive impairment and mortality among the oldest-old Chinese. International Journal of Geriatric Psychiatry 2016;31:1345–1353. [DOI] [PubMed] [Google Scholar]
- 24.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry 2007;78:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y, Vaupel J, Xiao Z,Liu Y, Zhang CY. Chinese Longitudinal Healthy Longevity Survey (CLHLS), Data Collection Instrument 1998–2012. Available at: http://www.icpsr.umich.edu/cgi-bin/file?comp=none&study=36179&ds=1&file_id=1194492&path=NACDA; Accessed at: 1 Sep. 2017.
- 26.Katz S Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983;31:721–727. [DOI] [PubMed] [Google Scholar]
- 27.Hastie T, Tibshirani R. Exploring the nature of covariate effects in the proportional hazards model. Biometrics 1990;46:1005–1016. [PubMed] [Google Scholar]
- 28.Hurvich CM, Simonoff JS, Tsai CL. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. Journal of the Royal Statistical Society Series B-Statistical Methodology 1998;60:271–293. [Google Scholar]
- 29.Chen C, Lu FC, Department of Disease Control Ministry of Health PRC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 2004;17 Suppl:1–36. [PubMed] [Google Scholar]
- 30.Gasecki D, Kwarciany M, Nyka W, et al. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep 2013;15:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alosco ML, Gunstad J, Xu X, et al. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens 2014;8:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002;125:765–772. [DOI] [PubMed] [Google Scholar]
- 33.Skoog I The relationship between blood pressure and dementia: a review. Biomed Pharmacother 1997;51:367–375. [DOI] [PubMed] [Google Scholar]
- 34.Carnevale D, Mascio G, D’Andrea I, et al. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 2012;60:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajjar I, Hart M, Mack W, et al. Aldosterone, cognitive function, and cerebral hemodynamics in hypertension and antihypertensive therapy. Am J Hypertens 2015;28:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynn RJ, Beckett LA, Hebert LE, et al. Current and remote blood pressure and cognitive decline. JAMA 1999;281:438–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.