Abstract
Context
Inflammation and insulin resistance are often present in polycystic ovary syndrome (PCOS).
Objective
We determined the effect of saturated fat ingestion on mononuclear cell (MNC) nuclear factor-κB (NFκB) activation; NFκB, inhibitory-κBα (IκBα), and tumor necrosis factor-α (TNFα) gene expression; and circulating C-reactive protein (CRP) in women with PCOS.
Design
Cross-sectional study.
Setting
Academic medical center.
Patients
Twenty reproductive-age women with PCOS (10 lean, 10 with obesity) and 20 ovulatory controls (10 lean, 10 with obesity).
Main Outcome Measures
Activated NFκB, NFκB heterodimer subunits, IκBα and TNFα messenger ribonucleic acid content and NFκB p65 and IκBα protein content were quantified in mononuclear cells (MNC), and CRP was measured in plasma from blood drawn fasting and 2, 3, and 5 h after saturated fat ingestion. Insulin sensitivity was derived from oral glucose tolerance testing (ISOGTT). Androgen secretion was assessed from blood drawn fasting and 24, 48, and 72 h after human chorionic gonadotropin (HCG) administration.
Results
In response to saturated fat ingestion, women with PCOS regardless of weight class exhibited lipid-induced increases in activated NFκB, NFκB, and TNFα gene expression and plasma CRP and decreases in IκBα protein compared with lean control subjects. Both PCOS groups exhibited lower ISOGTT and greater HCG-stimulated androgen secretion compared with control subjects. Lipid-stimulated NFκB activation was negatively correlated with ISOGTT, and positively correlated with HCG-stimulated androgen secretion.
Conclusion
In PCOS, increases in NFκB activation and circulating CRP and decreases in IκBα protein following saturated fat ingestion are independent of obesity. Circulating MNC and excess adipose tissue are separate and distinct contributors to inflammation in this disorder.
Keywords: hyperandrogenism, insulin resistance, polycystic ovary syndrome, saturated fat
As the most common female endocrinopathy, polycystic ovary syndrome (PCOS) is prevalent in 15% to 18% of reproductive age women (1,2). PCOS is diagnosed on the basis of hyperandrogenism, ovarian dysfunction, and polycystic ovarian morphology when at least 2 of these criteria are present, and other phenotypically similar disorders are excluded (2). Obesity, insulin resistance, and dyslipidemia are present in as many as 60% to 70% of women with PCOS (3‒5), although obesity per se is strongly linked to insulin resistance and dyslipidemia (6,7).
We have recently shown that in PCOS, saturated fat ingestion promotes oxidative stress and inflammation due to its ability to generate reactive oxygen species (ROS) and increase suppressor of cytokine-3 (SOCS-3) expression from peripheral mononuclear cells (MNC) (8,9). We have also reported lipid-induced increases in circulating levels of tumor necrosis factor-α (TNFα), a proinflammatory cytokine that is a known mediator of insulin resistance (9,10). Moreover, an increase in TNFα expression following ROS generation upregulates suppressor of cytokine signaling 3 (SOCS-3), which in turn truncates insulin signaling to attenuate facilitative glucose transport (11‒13). Thus, a prooxidant, proinflammatory state triggered by saturated fat ingestion may be an important contributor to insulin resistance in PCOS.
Activation of nuclear factor-κB (NFκB) is the cardinal inflammatory signal necessary for stimulating TNFα secretion from circulating MNC (14). NFκB is a transcription factor heterodimer typically consisting of the deoxyribonucleic acid (DNA)-binding subunits, p50 and p65 (15,16). The p50 subunit in particular is derived from p105, a larger precursor that is transcribed and translated before being processed to yield p50 (17). In resting MNC, NFκB is present in the cytoplasm complexed to its inhibitory protein known as inhibitory-κBα (IκBα). In response to lipid-induced oxidative stress, inhibitory-κB kinaseβ (IKKβ) is upregulated to phosphorylate IκBα (11,14,18). In the process, phosphorylated IκBα dissociates from NFκB and undergoes ubiquitination and degradation (19). Activated NFκB is freed to undergo nuclear translocation and subsequent DNA binding to promote the transcription of genes encoding its own subunits, along with those of IκBα and TNFα (11,20). Newly synthesized IκBα is unstable when not bound to NFκB, and is rapidly degraded with the exception of a small amount that escapes into the nucleus where it attaches to DNA-bound NFκB (20,21). The newly formed NFκB–IκBα complex detaches from DNA for return to the cytoplasm in its resting state (20). Thus, NFκB is autoregulated, and its activation following saturated fat ingestion may be the key molecular event for induction of insulin resistance in PCOS.
We examined the effect of saturated fat ingestion on MNC-derived NFκB activation in women with PCOS. We also examined this effect on the messenger ribonucleic acid (mRNA) content of the NFκB heterodimer subunits, IκBα and TNFα, the protein content of IKKβ, NFκB p65 and IκBα, and plasma C-reactive protein (CRP), a well-recognized circulating marker of chronic low-grade inflammation. We hypothesized that in response to saturated fat ingestion, activated NFκB, the NFκB subunits, IκBα and TNFα mRNA content, IKKβ and p65 protein content and plasma CRP are increased and IκBα protein content is decreased in women with PCOS compared with ovulatory controls of similar age and body mass index (BMI) and that there is an association between these markers of inflammation and adiposity, insulin sensitivity, levels of fasting lipids, and ovarian androgen secretion. We separately evaluated lean women with PCOS who represent the authentic syndrome and obese women with PCOS who may represent the superimposed effects of obesity on this disorder.
Materials and Methods
Participants
Twenty women with PCOS (10 lean, 10 with obesity) 18 to 37 years of age and 20 control subjects (10 lean, 10 with obesity) 19 to 39 years of age with a similar BMI served as study participants. Some subjects in the current study were involved in our previous work on lipopolysaccharide-mediated inflammation in PCOS (8). Lean subjects had a BMI between 18 and 25 kg/m2. Obesity was defined as a BMI between 30 and 40 kg/m2. The diagnosis of PCOS was based on the presence of oligo-amenorrhea and hyperandrogenemia after excluding nonclassic congenital adrenal hyperplasia, Cushing syndrome, hyperprolactinemia, and thyroid disease. Polycystic ovaries were present on ultrasound in all women with PCOS. Ovulation was documented in all control women on the basis of regular menses every 25 to 35 days and a luteal range serum progesterone level (>5 ng/mL). Serum androgen levels were normal in all control women, and they had no signs of androgen excess skin manifestations or polycystic ovaries on ultrasound.
Four women with PCOS (1 lean, 3 with obesity) met World Health Organization criteria for impaired glucose tolerance (22), and 2 of these women with PCOS and obesity also met Adult Treatment Panel III criteria for metabolic syndrome (23). Fourteen women with PCOS (5 lean, 9 with obesity) and 14 control subjects (8 lean, 6 with obesity) had a family history of type 2 diabetes. However, none of the subjects had diabetes or an inflammatory illness, nor did they smoke tobacco or use medications that would impact carbohydrate metabolism or immune function for at least 6 weeks before study entry. All subjects were weight stable within 5 pounds and were either sedentary or lightly active during the 6 months before study entry. The level of physical activity was similar among study groups. All subjects provided written informed consent in keeping with the Institutional Review Board guidelines for the protection of human subjects.
Study design
A cream challenge test (CCT) was performed on all study subjects between days 5 and 8 after the onset of a spontaneous menses, or in the case of 5 amenorrhoeic women with PCOS, after a progestin-induced withdrawal bleed. An oral glucose tolerance test (OGTT) was performed on the next day. An overnight fast for ~12 h was required before undergoing both tests. All subjects were provided with a healthy diet consisting of 50% carbohydrate, 35% fat, and 15% protein for 3 consecutive days before the CCT and on the day preceding the OGTT once they completed the CCT. An assessment of body composition was performed on the same day as the CCT. All subjects underwent a human chorionic gonadotropin stimulation test over 4 days beginning on the day of the OGTT.
Cream challenge test
As adapted from Deopurkar et al (24), all subjects consumed 100 mL of dairy cream (gourmet heavy whipping cream; Land O Lakes Inc, Arden Hills, MN) composed in volume of 70% saturated fat content, 28% unsaturated fat, <2% protein, and 0% glucose. All subjects underwent blood sampling in the fasting state and 2, 3, and 5 hours after cream ingestion to quantify molecular markers of inflammation from MNC isolated as described previously (25). Plasma was isolated from these same blood samples and stored at –80°C until assayed for CRP and fasting lipids.
Oral glucose tolerance test
All subjects consumed a 75-gram glucose beverage and underwent blood sampling in the fasting state and 30, 60, 90, 120, and 180 min after glucose ingestion. For all blood samples, glucose was measured right away, and insulin was measured later from plasma stored at –80°C. Insulin sensitivity was derived from the OGTT (ISOGTT) using the Matsuda index formula: 10 000 divided by the square root of the fasting glucose level multiplied by the fasting insulin level and that result multiplied by the product of the mean glucose level and mean insulin level (26).
HCG stimulation test
All subjects received an intramuscular injection of 5000 IU of human chorionic gonadotropin (HCG; Pregnyl®; Merck & Co., Whitehouse Station, NJ). Blood sampling was performed at 8 am after an overnight fast of ~12 h just before as a baseline and 24, 48, and 96 h after the HCG injection. Serum isolated from these samples was stored at –80°C until assayed for testosterone, androstenedione, and 17-hydroxyprogesterone (17-OHP). The area under the curve (AUC) for androgens and 17-OHP was calculated using the trapezoidal rule (27).
Body composition assessment
Height without shoes was measured to the nearest 1.0 cm. Body weight was measured to the nearest 0.1 kg. Dual-energy X-ray absorptiometry was performed in all subjects using a QDR 4500 Elite model scanner (Hologic Inc., Waltham, MA) to determine the percentage of total body fat and truncal fat as well as R1 central abdominal fat, the latter of which is defined as an area of 50 cm2 at the center of midline between the iliac crests and the lowest rib margin upon completing a normal expiration as described previously (28,29).
Electrophoretic mobility shift assay
Nuclear-bound NFκB was quantified by electrophoretic mobility shift assay as previously described (30). The supershift experiments were performed by preincubating a nuclear extract sample with antibodies against p50, p65, or early growth response-1, the latter of which served as a nonspecific antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The competition experiment was performed by preincubating a nuclear extract sample with either an unlabeled NFκB oligonucleotide consensus sequence (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) serving as a specific cold competitor or with an unlabeled activator protein-1 oligonucleotide consensus sequence (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) serving as a nonspecific cold competitor.
Real-time polymerase chain reaction
An RNAeasy kit (Quiagen, Germantown, MD) was used to isolate total ribonucleic acid from MNC that were previously stabilized in RNAlater® (Sigma-Aldrich, St. Louis, MO). Real-time polymerase chain reaction was used to quantify the mRNA content of p50, p105, IκBα, and TNFα as previously described (31). However, an ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) was used for the current study. PRIMER EXPRESS software (PE Biosystems, Foster City, CA) was used to select the primer sequences for p50, p105, and TNFα as previously described (32), and for IκBα (GenBank NM_020529, forward primer 5′-ACAATGGCCACACGTGTCTACA′, reverse primer 5′-CACCCAAGGACACCAAAAGC′). The rRNA signal for the housekeeping gene ribosomal protein L13a was used to normalize against differences in ribonucleic acid isolation and degradation and in reverse transcription and polymerase chain reaction efficiencies using the comparative cycle threshold method.
Western blotting
The protein content of IKKβ, p65, IκBα and actin was quantified by Western blotting as previously described using a monoclonal antibody against IKKβ (BD Pharmingen™, San Jose, CA) at a 1:350 dilution, and p65 (BD Transduction Laboratories™, San Jose, CA), IκBα (United States Biological Corp., Salem, MA) or actin (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:1000 dilution (33). Densitometry was performed on the scanned films of Western blots using molecular imaging software version 5.0.2.30 (Carestream Health, Rochester, NY), and all values for IKKβ, p65 and IκBα were corrected for loading using those obtained for actin.
Plasma and serum measurements
Plasma CRP was measured by a high sensitivity enzyme-linked immunosorbent assay (Alpha Diagnostics International, San Antonio, TX; sensitivity, 0.01 mg/L; intraassay coefficient of variation [CV] 6.3%; interassay CV 7.8%). Plasma levels of glucose, insulin, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and serum levels of luteinizing hormone (LH), androstenedione, dehydroepiandrosterone-sulfate (DHEA-S), and 17-OHP were measured as previously described (8). Serum testosterone levels were measured by a radioimmunoassay (Siemens, Los Angeles, CA; sensitivity, 5 ng/dL, 0.1 ng/mL; intraassay CV 6.8%; interassay CV 11.2%) that demonstrates good correlation with commercial liquid chromatography tandem mass spectrometry (34). All samples from each subject were measured in duplicate in the same assay at the end of the study.
Statistics
The StatView software package (SAS Institute, Cary, NC) was used to perform the statistical analysis. All values were initially examined graphically for departure from normality. The presence or absence of normality was subsequently confirmed using the Shapiro–Wilk test. The natural logarithm transformation was applied to total cholesterol and LH before the analysis since these values were not normally distributed. For each participant, the percentage change from baseline was used to determine treatment effects on inflammation markers due to inter-subject variability. The incremental AUC (iAUC) for each inflammation marker was also calculated using the trapezoidal rule (30). Since prior work by our group suggests that obesity increases inflammation and reduces insulin sensitivity in PCOS (8,9,25,35,36), analysis of variance was used to compare data from this study across groups (lean PCOS vs lean control vs obese PCOS vs obese control) followed by post hoc analyses using the Tukey’s honestly significant difference test to identify the source of significance. Differences across groups in the response of inflammation markers over time during the CCT were analyzed using repeated measures analysis of variance followed by post hoc analyses. Pearson product moment correlation coefficients were calculated initially for correlation analyses. Partial Pearson correlations with inflammation markers were subsequently calculated that separately adjusted for each measure of adiposity due to collinearity. Data are presented as mean ± standard error, and results with a 2-tailed α-level of .05 were considered to be significant. However, Pearson correlation results required an α level of .02 to be considered significant after determining the false discovery rate using the Bejamini–Hochberg approach to correct for multiple comparisons (37).
Results
Age, body composition, and blood pressure
Age, height, systolic and diastolic blood pressures were similar in all 4 groups (Table 1). Weight, BMI, percentage total body fat, percentage truncal fat, and R1 fat were significantly (P < .05) higher in subjects with obesity compared with lean subjects whether or not they had PCOS. However, these measures of body composition were similar when women with PCOS were compared with control subjects of similar weight class.
Table 1.
Age, body composition, endocrine, and metabolic parameters of subjects
| Controls | PCOS | |||
|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |
| Age, yr | 29 ± 3 | 31 ± 2 | 27 ± 1 | 28 ± 1 |
| Height, cm | 165.7 ± 1.3 | 163.6 ± 2.4 | 162.9 ± 1.9 | 160.9 ± 3.3 |
| Body weight, kg | 61.9 ± 2.4 | 92.6 ± 3.8a,b | 59.5 ± 2.2 | 88.3 ± 3.5c,d |
| Body mass index, kg/m2 | 22.5 ± 0.8 | 34.5 ± 0.7a,b | 22.4 ± 0.6 | 34.1 ± 0.9c,d |
| Total body fat, % | 29.9 ± 1.9 | 41.9 ± 0.9a,b | 31.8 ± 2.0 | 44.3 ± 1.2c,d |
| Truncal fat, % | 25.2 ± 2.2 | 41.4 ± 1.3a,b | 27.5 ± 2.4 | 43.0 ± 1.3c,d |
| Central fat (R1), gm | 776 ± 83 | 2088 ± 131a,b | 869 ± 104 | 2121 ± 115c,d |
| Systolic blood pressure, mmHg | 118 ± 3 | 126 ± 3 | 117 ± 5 | 119 ± 5 |
| Diastolic blood pressure, mmHg | 73 ± 2 | 74 ± 2 | 71 ± 3 | 75 ± 2 |
| CRP, mg/L | 0.3 ± 0.1 | 6.6 ± 1.5a,b | 1.8 ± 0.2 | 8.9 ± 2.0c,d |
| Fasting glucose, mg/dL | 91 ± 2 | 87 ± 3 | 88 ± 3 | 93 ± 2 |
| 2-h glucose, mg/dL | 99 ± 5 | 91 ± 9 | 100 ± 8 | 136 ± 8c,d,f |
| Fasting insulin, µU/mL | 5.4 ± 1.3 | 15.7 ± 2.7a,b | 7.4 ± 1.3 | 18.0 ± 3.9c,d |
| ISOGTT | 11.5 ± 2.0 | 5.3 ± 1.0a | 7.5 ± 1.1e | 3.3 ± 0.4c,d |
| Total cholesterol, mg/dL | 143 ± 5 | 149 ± 10b | 171 ± 6e | 184 ± 8c,f |
| Triglycerides, mg/dL | 59 ± 6 | 87 ± 12 | 65 ± 5 | 139 ± 32c,d,f |
| HDL – cholesterol, mg/dL | 55 ± 3 | 49 ± 3 | 54 ± 2 | 49 ± 3 |
| LDL – cholesterol, mg/dL | 76 ± 6 | 83 ± 9b | 104 ± 6e | 108 ± 8c,f |
| LH, mIU/mL | 5.8 ± 0.5 | 4.9 ± 0.8b | 14.3 ± 1.7e | 13.5 ± 1.8c,f |
| Testosterone, ng/dL | 29.3 ± 5.6 | 20.6 ± 3.7b | 59.5 ± 5.6e | 69.3 ± 7.8c,f |
| Androstendione, ng/mL | 2.0 ± 0.2 | 2.1 ± 0.2b | 3.9 ± 0.3e | 3.9 ± 0.2c,f |
| DHEA-S, µg/dL | 212 ± 23 | 151 ± 21b | 261 ± 35 | 205 ± 26 |
| Testosterone, AUC | 3152 ± 486 | 3683 ± 180b | 6088 ± 700e | 7639 ± 1135c,f |
| Androstendione, AUC | 293 ± 25 | 321 ± 34b | 473 ± 21e | 562 ± 48c,f |
| 17OH-progesterone, AUC | 10 136 ± 1291 | 9997 ± 552b | 19 336 ± 2767e | 21 450 ± 2971c,f |
Values are expressed as means ± standard error. Conversion factors to SI units: Testosterone ×3.467 (nmol/L), Androstenedione ×3.492 (nmol/L), DHEA-S ×0.002714 (µmol/L), Glucose x0.0551 (mmol/L), Insulin ×7.175 (pmol/L).
Abbreviations: AUC, HCG-stimulated area under the curve; CRP, C-reactive protein; DHEA-S, dehydroepiandrosterone-sulphate; ISOGTT, insulin sensitivity derived from an oral glucose tolerance test; PCOS, polycystic ovary syndrome.
aObese control vs lean control, P < .008.
bObese control vs lean PCOS, P < .04.
cObese PCOS vs lean control, P < .003.
dObese PCOS vs. lean PCOS, P < .03.
eLean PCOS vs lean control, P < .05.
fObese PCOS vs obese control, P < .02.
Inflammation markers in MNC and plasma
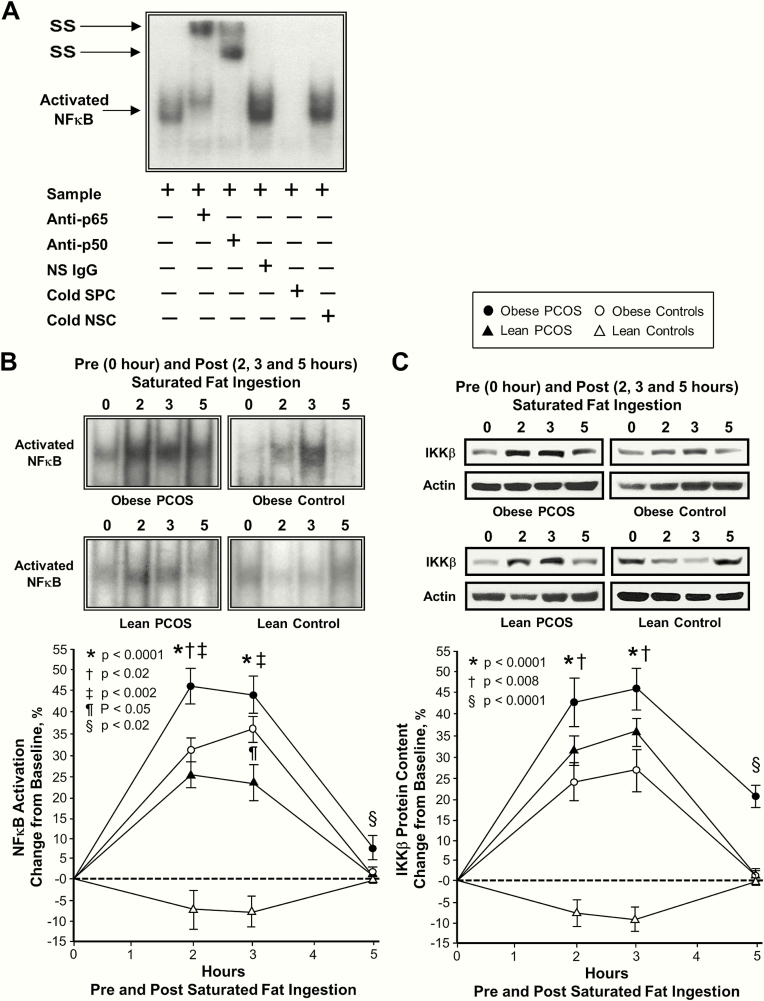
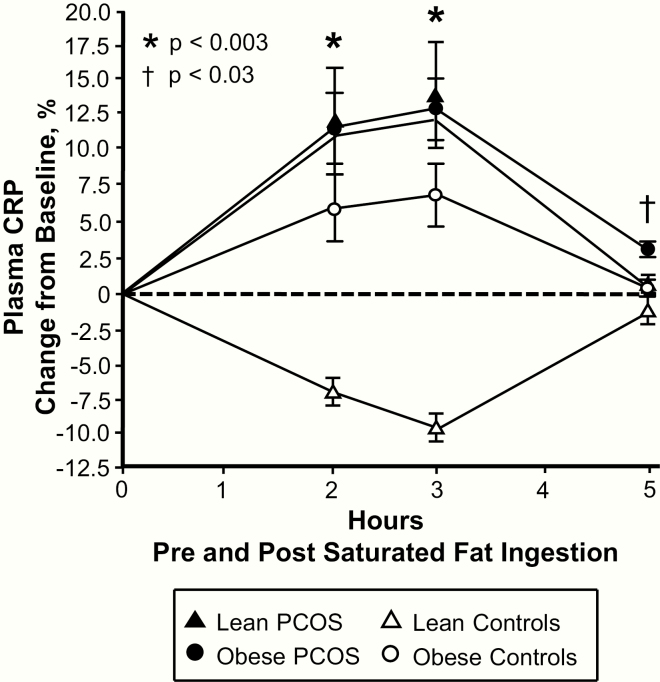
Plasma CRP levels were significantly (P < .05) higher in subjects with obesity compared with those who were lean regardless of PCOS status and in lean women with PCOS compared with lean control subjects (Table 1). Supershift and cold competition experiments verified the specificity of the electrophoretic mobility shift assay bands representing intranuclear activated NFκB (Fig. 1A).
Figure 1.
Comparison of the 4 study groups of the change from baseline (%) in mononuclear cell (MNC)-derived (A) inhibitory κB kinase-β (IKKβ) protein content and (B) activated nuclear factor κB (NFκB) from blood samples collected while fasting and 2, 3, and 5 h after saturated fat ingestion. (A) Representative western blots show the change in quantity of IKKβ in MNC homogenates and (B) representative electrophoretic mobility shift assay (EMSA) bands show the change in quantity of NFκB in nuclear extracts from MNC in samples collected during each time point. The samples used to quantify IKKβ and NFκB by densitometry were run on the same gel. Data are presented as mean ± standard error. (C) EMSA showing NFκB in nuclear extracts from MNC. A supershift of the NFκB band occurred during incubation with specific antibodies against NFκB subunits but not during incubation with nonspecific (NS) IgG. Neutralization of the NFκB band occurred during incubation with a specific cold competitor (SPC) of the oligonucleotide consensus sequence, but not during incubation with a nonspecific cold competitor (NSC). *Response in women with PCOS and obesity, lean women with PCOS, and control subjects with obesity was significantly different compared with lean control subjects; P < .0001. †Response in women with PCOS and obesity was significantly higher compared with control subjects with obesity; (A) P < .008 and (B) P < .02. ‡Response in women with PCOS and obesity was significantly different compared with lean women with PCOS; P < .002. ¶Response in lean women with PCOS was significantly different compared with control subjects with obesity; P < .05. §Residual response in women with PCOS and obesity was significantly different compared with the other three groups; (A) P < .0001 and (B) P < .02.
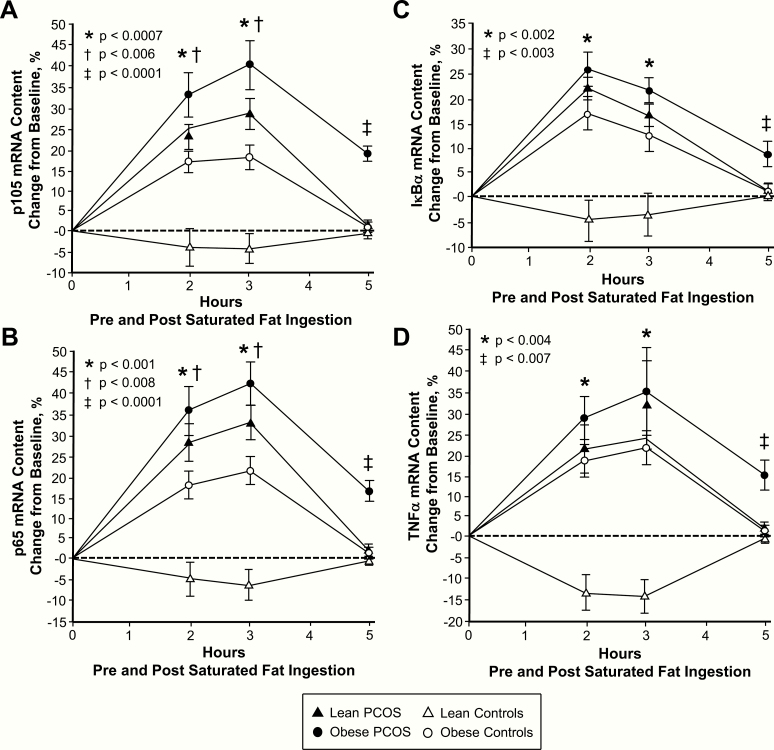
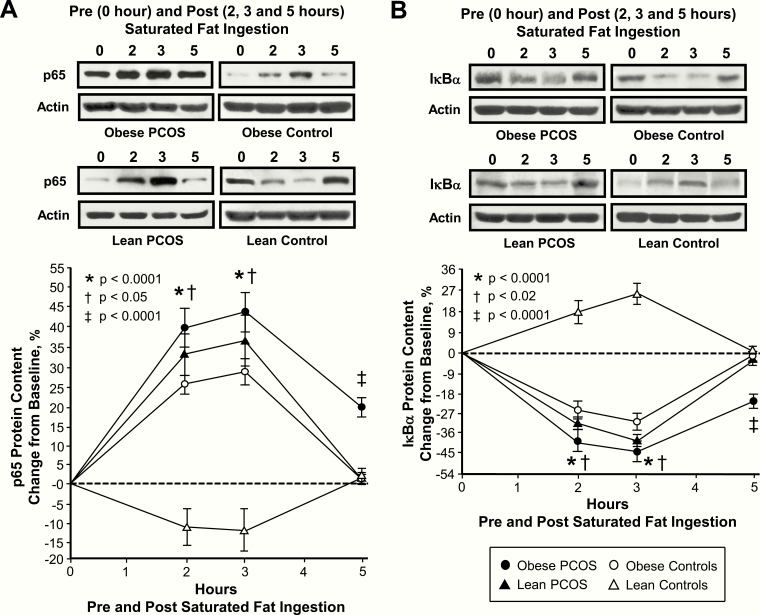
In response to saturated fat ingestion, the change from baseline in activated NFκB, p105, p65, IκBα and TNFα mRNA content, IKKβ and p65 protein content, and plasma CRP decreased in lean control subjects and was significantly (P < .004) different compared with the increase observed in women with PCOS regardless of weight class and control subjects with obesity after 2 and 3 h (Figs 1B‒C, 2‒4). In contrast, the change from baseline in IκBα protein content increased in lean control subjects and was significantly (P < .002) different compared with the decrease observed in the other 3 groups after 2 and 3 h. The maximum response in activated NFκB was reached at 2 h in both groups of women with PCOS and at 3 h in both groups of control subjects. In all 4 groups, the maximum response in IκBα mRNA content was reached at 2 h, and the maximum response in p105, p65 and TNFα mRNA content, IKKβ, p65 and IκBα protein content, and plasma CRP was reached at 3 h.
Figure 2.
Comparison of the 4 study groups of the change from baseline (%) in mononuclear cell (MNC)-derived (A) p105, (B) p65, (C) inhibitory κBα (IκBα) and (D) TNFα mRNA content from blood samples collected while fasting and 2, 3, and 5 h after saturated fat ingestion. Data are presented as mean ± standard error. *Response in women with PCOS and obesity, lean women with PCOS, and control subjects with obesity was significantly different compared with lean control subjects; (A) P < .0007, (B) P < .001, (C) P < .002, and (D) P < .004. †Response in women with PCOS and obesity was significantly different compared with control subjects with obesity; (A) P < .006 and (B) P < .008. ‡Residual response in women with PCOS and obesity was significantly different compared with the other three groups; (A) and (B) P < .0001, (C) P < .003, and (D) P < .007.
Figure 4.
Comparison of the 4 study groups of the change from baseline (%) in plasma C-reactive protein (CRP) blood samples collected while fasting and 2, 3, and 5 h after saturated fat ingestion. Data are presented as mean ± standard error. *Response in women with PCOS and obesity, lean women with PCOS, and control subjects with obesity was significantly different compared with lean control subjects; P < .003. †Residual response in women with PCOS and obesity was significantly different compared with the other three groups; P < .03.
Figure 3.
Comparison of the 4 study groups of the change from baseline (%) in mononuclear cell (MNC)-derived (A) p65 and (B) inhibitory κBα (IκBα) protein content from blood samples collected while fasting and 2, 3, and 5 h after saturated fat ingestion. (A) Representative western blots show the change in quantity of p65 and IκBα in MNC homogenates in samples collected during each time point. The samples used to quantify p65 and IκBα by densitometry were run on the same gel. Data are presented as mean ± standard error. *Response in women with PCOS and obesity, lean women with PCOS, and control subjects with obesity was significantly different compared with lean control subjects; P < .0001. †Response in women with PCOS and obesity was significantly different compared with control subjects with obesity; (A) P < .05 and (B) P < .02. ‡Residual response in women with PCOS and obesity was significantly different compared with the other three groups; P < .0001.
In women with PCOS and obesity, the responses of activated NFκB, p105 and p65 mRNA content, and IKKβ and p65 protein content were significantly (P < .05) higher, and the IκBα protein response was significantly (P < .02) lower compared with control subjects with obesity after 2 h. The activated NFκB response in women with PCOS and obesity was also significantly (P < .002) higher compared with lean women with PCOS after 2 and 3 h. All 9 inflammation markers returned to baseline in both lean groups and in control subjects with obesity after 5 h. In contrast, women with PCOS and obesity exhibited significantly (P < .03) higher residual responses in all 9 inflammation markers compared with the other 3 groups after 5 h.
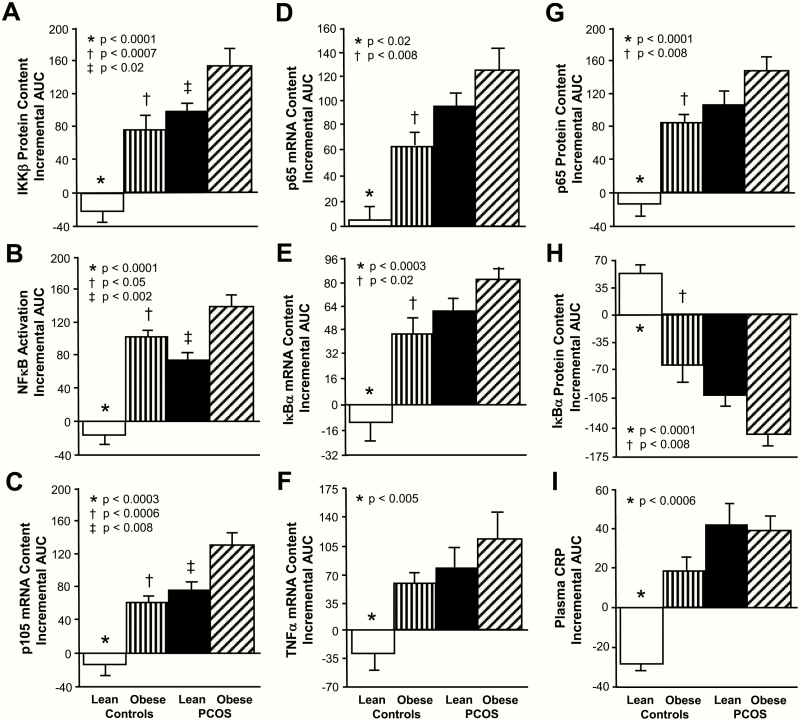
The iAUC for activated NFκB, p105, p65, IκBα and TNFα mRNA content, IKKβ and p65 protein content, and plasma CRP decreased in lean control subjects and was significantly (P < .02) different compared with the increase observed in women with PCOS regardless of weight class and control subjects with obesity (Fig. 5). In contrast, the iAUC for IκBα protein content increased in lean control subjects and was significantly (P < .004) different compared with the decrease observed in the other 3 groups.
Figure 5.
Comparison of the 4 study groups of the incremental area under the curve (iAUC) in response to saturated fat ingestion for mononuclear cell (MNC)-derived (A) inhibitory κB kinase-β (IKKβ) protein content, (B) activated nuclear factor κB (NFκB) (C) p105, (D) p65, (E) inhibitory κBα (IκBα) and (F) TNFα mRNA content, (G) p65 and (H) IκBα protein content, and (I) plasma C-reactive protein (CRP) levels. Data are presented as mean ± standard error. *The iAUC in lean control subjects was significantly different compared with the other 3 groups; (A, B, E, G, and H) P < .0001, (C) P < .0003, (D) P < .02, (F) P < .0003, (F) P < .005, and (I) P < .0006. †The iAUC in control subjects with obesity was significantly different compared with women with PCOS and obesity; (A) P < .0007, (B) P < .05, (C, D, G, and H) P < .008, and (E) P < .02. ‡The iAUC in lean women with PCOS was significantly different compared with women with PCOS and obesity; (A) P < .02, (B) P < .002, and (C) P < .008.
In women with PCOS and obesity, the iAUC for activated NFκB and p105 mRNA content was significantly (P < .05) higher compared with lean women with PCOS and control subjects with obesity. Women with PCOS and obesity also exhibited significantly (P < .03) higher iAUC for p65, IκBα mRNA and protein content, and plasma CRP and significantly (P < .02) lower iAUC for IκBα protein content compared with control subjects and obesity.
Insulin sensitivity and fasting lipids
ISOGTT was significantly lower (P < .05) in subjects with obesity regardless of PCOS status compared with lean control subjects, in women with PCOS and obesity compared with lean women with PCOS, and in lean women with PCOS compared with lean control subjects (Table 1). Plasma cholesterol and LDL were significantly (P < .05) higher in women with PCOS compared with control subjects regardless of body composition status. Plasma triglycerides was significantly (P < .03) higher in women with PCOS and obesity compared with those of the other 3 groups. Plasma HDL was similar in all 4 groups. Nevertheless, the only clinically elevated mean plasma lipid was the LDL level (>100 mg/dL) in women with PCOS regardless of weight class.
Basal hormone levels and HCG-stimulated androgen and 17-OHP responses
Serum levels of LH, testosterone, and androstenedione were significantly (P < .05) higher in women with PCOS compared with control subjects regardless of body composition status. Serum DHEA-S levels were significantly (P < .04) higher in lean women with PCOS compared with control subjects with obesity (Table 1).
The AUC for the HCG-stimulated responses of testosterone, androstenedione, and 17-OHP were significantly (P < .05) higher in women with PCOS compared with control subjects regardless of weight class.
Correlations
Adiposity vs inflammation.
BMI, percentage total body fat, percentage truncal fat, and R1 fat were positively correlated with the iAUC for activated NFκB, p105, IκBα and TNFα mRNA content, IKKβ and p65 protein content, and plasma CRP and was negatively correlated with the iAUC for IκBα protein content for the combined groups and separately for control subjects (Table 2). In women with PCOS, percentage total body fat, percentage truncal fat, and R1 fat were positively correlated with the iAUC for activated NFκB and p105 and IκBα mRNA content and negatively correlated with the iAUC for IκBα protein content.
Table 2.
Pearson correlations of inflammation markers iAUC during the cream challenge test with measures of adiposity and insulin sensitivity
| IKKβ Protein Content iAUC | Activated NFκB iAUC | p105 mRNA Content iAUC | p65 mRNA Content iAUC | IκBα mRNA Content iAUC | TNFα mRNA Content iAUC | p65 Protein Content iAUC | IκBα Protein Content iAUC | Plasma CRP iAUC | |
|---|---|---|---|---|---|---|---|---|---|
| Combined group | |||||||||
| BMI (kg/m2) | |||||||||
| r | 0.451 | 0.645 | 0.471 | 0.318 | 0.488 | 0.409 | 0.433 | –0.488 | 0.474 |
| P | 0.004* | 0.0001* | 0.002* | 0.046 | 0.002* | 0.009* | 0.005* | 0.002* | 0.003* |
| Total body fat (%) | |||||||||
| r | 0.391 | 0.558 | 0.446 | 0.352 | 0.399 | 0.359 | 0.337 | –0.510 | 0.489 |
| P | 0.013* | 0.0002* | 0.004* | 0.026 | 0.012* | 0.017* | 0.004* | 0.0008* | 0.002* |
| Truncal fat (%) | |||||||||
| r | 0.470 | 0.640 | 0.485 | 0.333 | 0.450 | 0.367 | 0.375 | –0.493 | 0.459 |
| P | 0.002* | 0.0001* | 0.002* | 0.036 | 0.004* | 0.019* | 0.017* | 0.001* | 0.003* |
| Central fat, R1 (gm) | |||||||||
| r | 0.482 | 0.677 | 0.488 | 0.320 | 0.462 | 0.423 | 0.391 | –0.444 | 0.559 |
| P | 0.002* | 0.0001* | 0.002* | 0.044 | 0.003* | 0.007* | 0.013* | 0.004* | 0.0003* |
| ISOGTT | |||||||||
| r | –0.556 | –0.532 | –0.466 | –0.491 | –0.393 | –0.356 | –0.595 | 0.531 | –0.539 |
| P | 0.0002* | 0.0004* | 0.002* | 0.001* | 0.013* | –0.019* | 0.0001* | 0.0004* | 0.0005* |
| PCOS | |||||||||
| BMI (kg/m2) | |||||||||
| r | 0.517 | 0.684 | 0.560 | 0.261 | 0.606 | 0.336 | 0.303 | –0.310 | 0.010 |
| P | 0.019* | 0.009* | 0.012* | 0.267 | 0.006* | 0.160 | 0.208 | 0.196 | 0.967 |
| Total body fat (%) | |||||||||
| r | 0.343 | 0.562 | 0.430 | 0.148 | 0.565 | 0.305 | 0.020 | –0.578 | 0.100 |
| P | 0.151 | 0.012* | 0.018* | 0.534 | 0.018* | 0.205 | 0.935 | 0.015* | 0.676 |
| Truncal fat (%) | |||||||||
| r | 0.531 | 0.654 | 0.670 | 0.134 | 0.677 | 0.289 | 0.036 | –0.609 | 0.018 |
| P | 0.019* | 0.002* | 0.002* | 0.544 | 0.002* | 0.231 | 0.883 | 0.007* | 0.941 |
| Central fat, R1 (gm) | |||||||||
| r | 0.523 | 0.654 | 0.572 | 0.290 | 0.653 | 0.296 | 0.178 | –0.616 | 0.018 |
| P | 0.022 | 0.002* | 0.013* | 0.215 | 0.003* | 0.218 | 0.466 | 0.007* | 0.939 |
| ISOGTT | |||||||||
| r | –0.460 | –0.575 | –0.457 | –0.294 | –0.583 | –0.012 | –0.765 | 0.524 | –0.075 |
| P | 0.048 | 0.008* | 0.042 | 0.222 | 0.011* | 0.962 | 0.0003* | 0.031 | 0.754 |
| Controls | |||||||||
| BMI (kg/m2) | |||||||||
| r | 0.684 | 0.792 | 0.728 | 0.584 | 0.745 | 0.660 | 0.787 | –0.709 | 0.763 |
| P | 0.0009* | 0.0001* | 0.0003* | 0.007* | 0.0002* | 0.002* | 0.0001* | 0.0005* | 0.0001* |
| Total body fat (%) | |||||||||
| r | 0.571 | 0.677 | 0.521 | 0.575 | 0.544 | 0.734 | 0.640 | –0.664 | 0.693 |
| P | 0.009* | 0.001* | 0.019* | 0.008* | 0.016* | 0.0005* | 0.002* | 0.001* | 0.0007* |
| Truncal fat (%) | |||||||||
| r | 0.661 | 0.791 | 0.566 | 0.571 | 0.587 | 0.776 | 0.713 | –0.718 | 0.741 |
| P | 0.002* | 0.0001* | 0.009* | 0.009* | 0.008* | 0.0002* | 0.0004* | 0.0004* | 0.0002* |
| Central fat, R1 (gm) | |||||||||
| r | 0.727 | 0.818 | 0.677 | 0.549 | 0.649 | 0.908 | 0.700 | –0.700 | 0.735 |
| P | 0.0003* | 0.0001* | 0.001* | 0.015* | 0.003* | 0.0001* | 0.0006* | 0.0006* | 0.0002* |
| ISOGTT | |||||||||
| r | –0.599 | –0.753 | –0.649 | –0.664 | –0.636 | –0.672 | –0.652 | 0.641 | –0.542 |
| P | 0.007* | 0.0005* | 0.004* | 0.002* | 0.011* | 0.002* | 0.002* | 0.003* | 0.014* |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; iAUC, incremental area under the curve; IκBα, inhibitor κBα; IKKβ, inhibitor of nuclear factor κB kinase β; ISOGTT, insulin sensitivity index derived from an oral glucose tolerance test; mRNA, messenger ribonucleic acid; NFκB, nuclear factor κB; TNFα, tumor necrosis factor-α.
*P < .02.
Insulin sensitivity vs inflammation.
ISOGTT was negatively correlated with BMI (r = –0.52, P < .0006), percentage total body fat (r = 0.51, P < .0009), percentage truncal fat (r = –0.55, P < 0.0004), and R1 fat (r = –0.55, P < .0004) for the combined groups. ISOGTT was also negatively correlated with the iAUC for activated NFκB, p105, p65, IκBα and TNFα mRNA content, IKKβ and p65 protein content, and plasma CRP and negatively correlated with the iAUC for IκB protein content for the combined groups and in control subjects (Table 2). In women with PCOS, ISOGTT was negatively correlated with the iAUC for activated NFκB and IκBα mRNA content and positively correlated with the iAUC for IκBα protein content.
Fasting lipids vs inflammation.
For the combined groups, plasma total cholesterol, triglycerides, and LDL were positively correlated with the iAUC for activated NFκB, p105 mRNA content, and IKKβ protein content (Table 3). Plasma total cholesterol and LDL were also positively correlated with the iAUC for plasma CRP and negatively correlated with the iAUC for IκBα protein content. Plasma HDL was negatively correlated with the iAUC for activated NFκB, p105 and IκBα mRNA content, IKKβ protein content, and plasma CRP and positively correlated with the iAUC for IκBα protein content.
Table 3.
Pearson correlations of inflammation markers iAUC during the cream challenge test with circulating lipids
| IKKβ Protein Content iAUC | Activated NFκB iAUC | p105 mRNA Content iAUC | p65 mRNA Content iAUC | IκBα mRNA Content iAUC | TNFα mRNA Content iAUC | p65 Protein Content iAUC | IκBα Protein Content iAUC | Plasma CRP iAUC | |
|---|---|---|---|---|---|---|---|---|---|
| Combined group | |||||||||
| Total cholesterol (mg/dL) | |||||||||
| r | 0.514 | 0.485 | 0.403 | 0.397 | 0.408 | 0.387 | 0.344 | –0.442 | 0.398 |
| P | 0.0007* | 0.002* | 0.011* | 0.011* | 0.011* | 0.014* | 0.029 | 0.004* | 0.011* |
| Triglycerides (mg/ dL) | |||||||||
| r | 0.454 | 0.400 | 0.458 | 0.325 | 0.421 | 0.297 | 0.400 | –0.340 | 0.330 |
| P | 0.003* | 0.011* | 0.003* | 0.048 | 0.008* | 0.074 | 0.013* | 0.032 | 0.046 |
| HDL cholesterol (mg/dL) | |||||||||
| r | –0.377 | –0.429 | –0.335 | –0.251 | –0.400 | –0.226 | –0.104 | 0.424 | –0.392 |
| P | 0.019* | 0.006* | 0.035 | 0.151 | 0.013* | 0.161 | 0.525 | 0.007* | 0.016* |
| LDL cholesterol (mg/dL) | |||||||||
| r | 0.431 | 0.542 | 0.333 | 0.320 | 0.343 | 0.411 | 0.277 | –0.523 | 0.405 |
| P | 0.006* | 0.0003* | 0.036 | 0.044 | 0.035 | 0.008* | 0.084 | 0.005* | 0.009* |
| PCOS | |||||||||
| Total cholesterol (mg/dL) | |||||||||
| r | 0.137 | 0.262 | 0.152 | 0.086 | 0.280 | 0.236 | 0.133 | –0.047 | 0.116 |
| P | 0.534 | 0.265 | 0.523 | 0.719 | 0.232 | 0.316 | 0.575 | 0.846 | 0.625 |
| Triglycerides (mg/ dLl) | |||||||||
| r | 0.473 | 0.236 | 0.619 | 0.060 | 0.527 | 0.078 | 0.564 | –0.065 | 0.217 |
| P | 0.048 | 0.316 | 0.006* | 0.802 | 0.017* | 0.744 | 0.018* | 0.785 | 0.358 |
| HDL cholesterol (mg/dLl) | |||||||||
| r | –0.541 | –0.617 | –0.601 | –0.536 | –0.030 | –0.563 | –0.544 | 0.517 | –0.106 |
| P | 0.021 | 0.005* | 0.005* | 0.017* | 0.901 | 0.015* | 0.024 | 0.028 | 0.658 |
| LDL cholesterol (mg/dLl) | |||||||||
| r | 0.127 | 0.528 | 0.025 | 0.530 | 0.025 | 0.524 | 0.530 | –0.588 | 0.142 |
| P | 0.595 | 0.020 | 0.917 | 0.029 | 0.917 | 0.021 | 0.024 | 0.013* | 0.551 |
Abbreviations: HDL, high-density lipoprotein; IKKβ, inhibitor of nuclear factor κB kinase β; NFκB, nuclear factor κB; IκBα, inhibitor κBα; TNFα, tumor necrosis factor-α; CRP, C-reactive protein; iAUC, incremental area under the curve; LDL, low-density lipoprotein.
*P < .02.
In women with PCOS, plasma triglycerides were positively correlated with the iAUC for p105 and IκBα mRNA content and p65 protein content, and plasma LDL was negatively correlated with the iAUC for IκBα protein content. Plasma HDL was negatively correlated with the iAUC for activated NFκB, p105, p65 and TNFα mRNA content, and IKKβ protein content.
LH and androgens vs inflammation.
For the combined groups, basal levels of LH, testosterone, and androstenedione and the HCG-stimulated AUC for testosterone, androstenedione, and 17OHP were positively correlated with the iAUC for activated NFκB, p105 and IκBα mRNA content, IKKβ protein content, and plasma CRP and negatively correlated with the iAUC for IκBα protein content (Table 4). Basal androstenedione levels and the HCG-stimulated AUC for 17OHP were also positively correlated with the iAUC for p65 mRNA and protein content.
Table 4.
Pearson correlations of inflammation markers iAUC during the cream challenge test with circulating LH and androgens
| IKKβ Protein Content iAUC | Activated NFκB iAUC | p105 mRNA Content iAUC | p65 mRNA Content iAUC | IκBα mRNA Content iAUC | TNFα mRNA Content iAUC | p65 Protein Content iAUC | IκBα Protein Content iAUC | Plasma CRP iAUC | |
|---|---|---|---|---|---|---|---|---|---|
| Combined groups | |||||||||
| LH (iU/mL) | |||||||||
| r | 0.543 | 0.395 | 0.433 | 0.213 | 0.470 | 0.328 | 0.468 | –0.477 | 0.527 |
| P | 0.0008* | 0.016* | 0.005* | 0.188 | 0.003* | 0.044 | 0.003* | 0.002* | 0.0005* |
| Testosterone (ng/dL) | |||||||||
| r | 0.421 | 0.375 | 0.439 | 0.355 | 0.497 | 0.357 | 0.324 | –0.454 | 0.606 |
| P | 0.007* | 0.019* | 0.005* | 0.026 | 0.001* | 0.028 | 0.047 | 0.004* | 0.0001* |
| Androstenedione (ng/mL) | |||||||||
| r | 0.631 | 0.388 | 0.479 | 0.514 | 0.576 | 0.591 | 0.548 | –0.665 | 0.604 |
| P | 0.0001* | 0.015* | 0.002* | 0.008* | 0.0002* | 0.0001* | 0.0008* | 0.0001* | 0.0001* |
| DHEA-S (ug/dL) | |||||||||
| r | 0.075 | 0.081 | 0.089 | 0.082 | 0.014 | 0.029 | 0.016 | –0.082 | 0.111 |
| P | 0.645 | 0.618 | 0.585 | 0.614 | 0.933 | 0.857 | 0.924 | 0.616 | 0.494 |
| Testosterone AUC | |||||||||
| r | 0.583 | 0.525 | 0.448 | 0.275 | 0.436 | 0.360 | 0.465 | –0.478 | 0.594 |
| P | 0.0002* | 0.0005* | 0.004* | 0.086 | 0.006* | 0.022 | 0.004* | 0.002* | 0.0001* |
| Androstenedione AUC | |||||||||
| r | 0.605 | 0.500 | 0.707 | 0.354 | 0.551 | 0.587 | 0.449 | –0.601 | 0.701 |
| P | 0.0001* | 0.001* | 0.0001* | 0.025 | 0.0003* | 0.0001* | 0.004* | 0.0001* | 0.0001* |
| 17OH-progesterone AUC | |||||||||
| r | 0.486 | 0.437 | 0.417 | 0.504 | 0.457 | 0.526 | 0.412 | –0.487 | 0.380 |
| P | 0.002* | 0.005* | 0.010* | 0.001* | 0.003* | 0.0008* | 0.008* | 0.002* | 0.019* |
| PCOS | |||||||||
| LH (iU/mL) | |||||||||
| r | 0.187 | 0.512 | 0.031 | 0.512 | 0.116 | 0.278 | 0.077 | –0.103 | 0.28 |
| P | 0.431 | 0.036 | 0.896 | 0.021 | 0.625 | 0.236 | 0.748 | 0.665 | 0.273 |
| Testosterone (ng/dL) | |||||||||
| r | 0.078 | 0.751 | 0.013 | 0.168 | 0.493 | 0.047 | 0.278 | –0.135 | 0.771 |
| P | 0.744 | 0.0005* | 0.956 | 0.479 | 0.027 | 0.845 | 0.235 | 0.571 | 0.0002* |
| Androstenedione (ng/ mL) | |||||||||
| r | 0.511 | 0.577 | 0.013 | 0.489 | 0.446 | 0.274 | 0.006 | –0.631 | 0.077 |
| P | 0.030 | 0.015* | 0.957 | 0.047 | 0.049 | 0.242 | 0.978 | 0.005* | 0.746 |
| DHEA-S (ug/dL) | |||||||||
| r | 0.159 | 0.325 | 0.060 | 0.311 | 0.188 | 0.175 | 0.202 | –0.003 | 0.150 |
| P | 0.504 | 0.162 | 0.800 | 0.181 | 0.427 | 0.461 | 0.393 | 0.989 | 0.528 |
| Testosterone AUC | |||||||||
| r | 0.099 | 0.634 | 0.081 | 0.180 | 0.235 | 0.088 | 0.134 | –0.023 | 0.638 |
| P | 0.677 | 0.005* | 0.732 | 0.448 | 0.319 | 0.711 | 0.575 | 0.923 | 0.004* |
| Androstenedione AUC | |||||||||
| r | 0.565 | 0.636 | 0.562 | 0.008 | 0.473 | 0.476 | 0.103 | –0.167 | 0.174 |
| P | 0.015* | 0.006* | 0.015* | 0.974 | 0.035 | 0.046 | 0.666 | 0.481 | 0.464 |
| 17OH-progesterone AUC | |||||||||
| r | 0.217 | 0.573 | 0.140 | 0.479 | 0.343 | 0.140 | 0.025 | –0.008 | 0.263 |
| P | 0.359 | 0.016* | 0.556 | 0.048 | 0.139 | 0.557 | 0.917 | 0.973 | 0.263 |
Abbreviations: AUC, HCG-stimulated area under the curve. CRP, C-reactive protein; iAUC, incremental area under the curve; IκBα, inhibitor κBα; IKKβ, inhibitor of nuclear factor κB kinase β; LH, luteinizing hormone; NFκB, nuclear factor κB; TNFα, tumor necrosis factor-α.
*P < .02.
In women with PCOS, basal levels of testosterone and androstenedione and the HCG-stimulated AUC for testosterone, androstenedione, and 17OHP were positively correlated with the iAUC for activated NFκB. The basal and HCG-stimulated AUC for testosterone were also positively correlated with the iAUC for plasma CRP, and basal androstenedione was negatively correlated with the iAUC for IκBα protein content.
None of the inflammation markers were correlated with fasting lipids and hormones in control subjects (data not shown). All of the significant relationships between inflammation markers and these variables for the combined groups and in women with PCOS were maintained after adjusting for adiposity (data not shown).
Discussion
Our data clearly show for the first time that in PCOS, NFκB activation is a central molecular event in the inflammatory response from MNC triggered by saturated fat ingestion and is independent of obesity. Lean women with PCOS exhibit lipid-induced increases in NFκB activation and decreases in IκBα protein compared with lean control subjects. Women with PCOS and obesity exhibit similar findings compared with control subjects with obesity. In women with PCOS, NFκB activation in response to saturated fat ingestion is negatively associated with insulin sensitivity and positively associated with basal and HCG-stimulated androgen secretion providing strength to the notion that lipid-stimulated inflammation plays an important role in the development of insulin resistance and hyperandrogenism in PCOS. Molecular and circulating inflammation markers are also positively associated with measures of adiposity suggesting that in PCOS excess adipose tissue is an additional source of inflammation that can modulate insulin action.
Lean ovulatory women of reproductive-age demonstrate an attenuated inflammatory response to saturated fat ingestion, which may be the in vivo norm in this population. The responses of IKKβ protein, NFκB activation and gene expression, IκBα and TNFα mRNA, and plasma CRP decrease while the IκBα protein response increases in lean control subjects. Our previous studies have shown that in lean young women, TNFα secretion from MNC and other MNC-derived inflammation markers respond in similar fashion following glucose ingestion (25,32,35,36,38). These findings are important because resident macrophages emanating from MNC present in excess adipose tissue and muscle exert a paracrine effect on insulin action (39,40). Indeed, resident macrophage ROS generation activates NFκB to promote TNFα secretion that reduces insulin signaling to impede glucose uptake (41). In contrast, insulin sensitivity increases when macrophages are eradicated in muscle of insulin resistant animals (42). Thus, attenuation of NFκB activation to downregulate TNFα gene expression after saturated fat ingestion may be a safeguard for maintaining insulin signaling to optimize glucose disposal in lean young women.
Women with PCOS demonstrate a proinflammatory response to saturated fat ingestion. In the present study, MNC-derived IKKβ protein, NFκB activation, and gene expression increases and IκBα protein decreases following saturated fat ingestion in women with PCOS compared with control subjects, regardless of weight class. Furthermore, IκBα and TNFα mRNA and plasma CRP increase in lean women with PCOS compared with lean control subjects. The paradoxical increase in IκBα mRNA in relation to the decrease in IκBα protein most likely reflects NFκB autoregulation in which lipid-induced NFκB activation upregulates IκBα gene expression as unstable cytoplasmic IκBα protein is rapidly degraded (19,20). NFκB is eventually removed from DNA as it is deactivated when bound by the small fraction of IκBα protein that escapes to the nucleus (20,21). These findings are consistent with our previous studies showing that in PCOS, MNC are preactivated in the fasting state and glucose ingestion activates NFκB in MNC (35,36,38,43). In particular, they showcase the key role of circulating MNC in establishing a proinflammatory state in PCOS culminating in lipid-induced increases in circulating TNFα and MNC-derived SOCS-3 expression (8). A prooxidant, proinflammatory response from MNC is also evoked following protein ingestion (44). Thus, insulin resistance in PCOS can be mediated by an acute inflammatory response to nutrient ingestion even when excess adiposity is absent. Additional evidence is furnished by a report of reduced inflammation in normal humans after a 2-day fast, along with the inverse link between inflammation markers and insulin sensitivity in the present study (45).
Inflammation in response to saturated fat ingestion and adiposity are linked in PCOS. Lipid-stimulated inflammation markers are positively associated with measures of adiposity for the combined groups and in women with PCOS. BMI, total body fat, and abdominal fat in women with PCOS are positively associated with the iAUC for activated NFκB and p105 and IκBα mRNA and negatively associated with the iAUC for IκBα protein following saturated fat ingestion. Hypoxia-related adipocyte death incites oxidative stress during phagocytosis by resident macrophages emanating from MNC that migrate to the stromal-vascular compartment of excess adipose tissue (39,46). NFκB is activated in the process causing an increase in TNFα production in macrophages culminating in paracrine stimulation of TNFα production in adipocytes to mediate insulin resistance (47). Indeed, all of the adiposity measures in the present study including abdominal adiposity are negatively associated with insulin sensitivity. Thus, when obesity is present in PCOS, both circulating MNC and excess adipose tissue play a part in promoting systemic inflammation and insulin resistance.
Inflammation in response to saturated fat ingestion and dyslipidemia are linked in PCOS. In the present study, total cholesterol and LDL are positively associated with the iAUC for activated NFκB, p105 mRNA, IKKβ protein, and plasma CRP and negatively associated with the iAUC for IκBα protein for the combined groups. Triglycerides are positively associated with the iAUC for p105 mRNA, and HDL is negatively associated with the iAUC for activated NFκB, p105 mRNA, and IKKβ protein in the combined groups and in women with PCOS. TNFα and other cytokines regulate synthesis of fatty acids in the liver and lipolysis in adipose tissue and transport of fatty acids to the liver, all of which yield substrates for hepatic triglyceride and triglyceride-rich very low-density lipoprotein (VLDL) production and secretion into peripheral blood (48). Increases in VLDL alone enhance the transfer of triglycerides from VLDL to LDL. Subsequent hydrolysis of LDL by hepatic lipase forms small dense LDL, which is extremely atherogenic because it can pass into the blood vessel subendothelium with ease. Small dense LDL is also swiftly oxidized for improved uptake by foamy macrophages of atherosclerotic lesions (49). TNFα can decrease circulating HDL levels and promote changes in HDL structure that lowers its capacity to remove cholesterol from foamy macrophages (50). In the present study, cholesterol and LDL are higher in women with PCOS regardless of weight class, while triglycerides are higher in women with PCOS and obesity in particular. Thus, lipid-induced inflammation may be a powerful stimulator of dyslipidemia in PCOS especially when obesity is present, with the combined effects of inflammation and derangements in lipids leading to the early onset of atherogenesis.
Inflammation in response to saturated fat ingestion and hyperandrogenism are also linked in PCOS. In the present study, basal LH and androgen levels along with the HCG-stimulated androgen secretion are positively associated with the panel of lipid-stimulated inflammation markers for the combined groups and in women with PCOS. Our previous studies have reported similar findings in response to both glucose and saturated fat ingestion (8,9,25,35,36,37). While the connection with LH suggests a central impact of inflammation on ovarian androgen production, direct effects of inflammation within the ovary are well documented. An increase in MNC-derived macrophages within the ovary has been reported in response to saturated fat ingestion (51). Macrophage TNFα secretion may enhance serine phosphorylation to increase the activity of the 17,20-lyase arm of CYP17 (53). TNFα also stimulates proliferation of ovarian theca cells (52, 53). In corroboration, proinflammatory stimuli such as lipopolysaccharide and interleukin 1 beta increase thecal cell androgen production in vitro (54), and antioxidant treatment using resveratrol decreases circulating androgens in women with PCOS (55). Our own preliminary communication shows that long-term suppression of lipid-induced NFκB activation and TNFα secretion from MNC with salicylate treatment decreases basal and HCG-stimulated androgen secretion in lean women with PCOS who are not insulin resistant (56,57). Thus, the trafficking of MNC into the polycystic ovary after saturated fat ingestion may stimulate a local inflammatory response characterized by NFκB activation and subsequent TNFα secretion to increase the proliferation and steroidogenic activity of thecal cells, thereby increasing ovarian androgen production.
In conclusion, women with PCOS display increases in MNC-derived IKKβ protein, NFκB activation and gene expression, IκBα and TNFα mRNA, and plasma CRP along with decreases in IκBα protein in response to saturated fat ingestion that are independent of obesity. Insulin resistance, dyslipidemia, and hyperandrogenism in PCOS may be the consequence of a lipid-induced proinflammatory state. The link between lipid-induced inflammation markers and measures of adiposity supports the role of excess adipose tissue as an additional instigator of inflammation in this disorder. Thus, circulating MNC and excess adipose tissue provide separate and distinct contributions to metabolic aberration and endocrine dysfunction in PCOS.
Acknowledgments
We thank the nursing staff of the Indiana Clinical and Translational Sciences Institute Clinical Research Center for supporting the implementation of the study and assisting with data collection. We gratefully acknowledge Merck Sharp & Dohme for generously donating the Pregnyl® used in this study. This paper was presented in part at the 69th meeting of the American Society for Reproductive Medicine, Boston, MA, October 12–17, 2013, and the 97th meeting of the Endocrine Society, San Diego, CA, March 5–8, 2015.
Financial Support: This research was supported by grant R01 DK107605 to FG from the National Institutes of Health, the Indiana Clinical and Translational Sciences Institute Clinical Research Center which is funded, in part by grant UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and the Indiana University Center for Diabetes and Metabolic Diseases funded by grant P30 DK097512 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical Trial Registration Number: ClinicalTrials.gov NCT01489319 (registered December 9, 2011).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 2. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 3. Lim SS, Norman RJ, Davies MJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. [DOI] [PubMed] [Google Scholar]
- 4. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607–613. [DOI] [PubMed] [Google Scholar]
- 6. Ciaraldi TP, Kolterman OG, Olefsky JM. Mechanism of the postreceptor defect in insulin action in human obesity. Decrease in glucose transport system activity. J Clin Invest. 1981;68(4):875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. González F, Considine RV, Abdelhadi OA, Acton AJ. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(3):934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. González F, Considine RV, Abdelhadi OA, Acton AJ. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(11):5360–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor α inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91(11):4854‒4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165(2):1013–1021. [DOI] [PubMed] [Google Scholar]
- 12. Emanuelli B, Peraldi P, Filloux C, et al. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276(51):47944–47949. [DOI] [PubMed] [Google Scholar]
- 13. Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277(44):42394–42398. [DOI] [PubMed] [Google Scholar]
- 14. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622. [DOI] [PubMed] [Google Scholar]
- 15. Baldwin AS., Jr The transcription factor NFκB and human disease. J Clin Invest. 2001;107(1):3‒ 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan CM, Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature. 1991;354(6352):395–398. [DOI] [PubMed] [Google Scholar]
- 18. Ortego M, Gómez-Hernández A, Vidal C, et al. HMG-CoA reductase inhibitors reduce I kappa B kinase activity induced by oxidative stress in monocytes and vascular smooth muscle cells. J Cardiovasc Pharmacol. 2005;45(5):468–475. [DOI] [PubMed] [Google Scholar]
- 19. Hay RT, Vuillard L, Desterro JM, Rodriguez MS. Control of NF-kappa B transcriptional activation by signal induced proteolysis of I kappa B alpha. Philos Trans R Soc Lond B Biol Sci. 1999;354(1389):1601–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cogswell PC, Scheinman RI, Baldwin AS Jr. Promoter of the human NF-kappa B p50/p105 gene. Regulation by NF-kappa B subunits and by c-REL. J Immunol. 1993;150(7):2794–2804. [PubMed] [Google Scholar]
- 21. Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298(5596):1241–1245. [DOI] [PubMed] [Google Scholar]
- 22. Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples. Diabetes. 1989;38(12):1630–1635. [DOI] [PubMed] [Google Scholar]
- 23. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 24. Deopurkar R, Ghanim H, Friedman J, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33(5):991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. González F, Kirwan JP, Rote NS, Minium J. Glucose ingestion stimulates atherothrombotic inflammation in polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2013;304(4):E375–E383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 27. Yeh ST. Using a trapezoidal rule for the area under a curve calculation – SAS advanced tutorial. In: Proceedings of the 27th Annual Conference of SAS Users Group International Orlando, FL; 2002: Abstract 229. [Google Scholar]
- 28. González F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-alpha release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(9):5336–5342. [DOI] [PubMed] [Google Scholar]
- 29. Carmina E, Bucchieri S, Esposito A, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92(7):2500–2505. [DOI] [PubMed] [Google Scholar]
- 30. González F, Rote NS, Minium J, O’Leary VB, Kirwan JP. Obese reproductive age women exhibit a proatherogenic inflammatory response during hyperglycemia. Obesity. 2007;15(10):2436‒ 2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab. 2008;294(2):E336–E344. [DOI] [PubMed] [Google Scholar]
- 32. González F, Nair KS, Daniels JK, Basal E, Schimke JM. Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. Am J Physiol Endocrinol Metab. 2012;302(3):E297–E306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aljada A, Ghanim H, Dandona P. Translocation of p47phox and activation of NADPH oxidase in mononuclear cells. Methods Mol Biol. 2002;196:99–103. [DOI] [PubMed] [Google Scholar]
- 34. Legro RS, Schlaff WD, Diamond MP, et al. ; Reproductive Medicine Network Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95(12):5305–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(4):1508–1512. [DOI] [PubMed] [Google Scholar]
- 36. González F, Sia CL, Shepard MK, Rote NS, Minium J. Inflammation in response to glucose ingestion is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(11):4071–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289–300. [Google Scholar]
- 38. González F, Sia CL, Shepard MK, Rote NS, Minium J. The altered mononuclear cell-derived cytokine response to glucose ingestion is not regulated by excess adiposity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(11):E2244–E2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Varma V, Yao-Borengasser A, Rasouli N, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab. 2009;296(6):E1300–E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91(11):4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. González F, Rote NS, Minium J, Kirwan JP. Insulin sensitivity and hyperandrogenism in polycystic ovary syndrome are related to activated nuclear factor κ B from mononuclear cells in the fasting state. In: Program of the 89th Meeting of the Endocrine Society Toronto, ON;June 12–16, 2007. [Google Scholar]
- 44. Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 2002;75(4):767–772. [DOI] [PubMed] [Google Scholar]
- 45. Dandona P, Mohanty P, Hamouda W, et al. Inhibitory effect of a two day fast on reactive oxygen species (ROS) generation by leucocytes and plasma ortho-tyrosine and meta-tyrosine concentrations. J Clin Endocrinol Metab. 2001;86(6):2899–2902. [DOI] [PubMed] [Google Scholar]
- 46. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. [DOI] [PubMed] [Google Scholar]
- 47. Fain JN, Bahouth SW, Madan AK. TNFα release by nonfat cells of adipose tissue. Int J Obes. 2004;28(4):616–622. [DOI] [PubMed] [Google Scholar]
- 48. Brewer HB., Jr Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. Am J Cardiol. 1999;83(9B):3F–12F. [DOI] [PubMed] [Google Scholar]
- 49. Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–1196. [DOI] [PubMed] [Google Scholar]
- 50. Feingold KR, Hardardottir I, Memon R, et al. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J Lipid Res. 1993;34(12):2147–2158. [PubMed] [Google Scholar]
- 51. Thornton K, Asemota O, Jindal S, Charron M, Buyuk E. High fat diet and aging are associated with macrophage infiltration in mice ovaries. In: Program of the 71st meeting of the American Society for Reproductive Medicine Baltimore, MD; October 17–21, 2015: Abstract O-273. 10.1016/j.fertnstert.2015.07.322. [DOI] [Google Scholar]
- 52. Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20 lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1995;92(23):10619–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61(4):993–998. [DOI] [PubMed] [Google Scholar]
- 54. Fox CW, Zhang L, Sohni A, et al. Inflammatory stimuli trigger increased androgen production and shifts in gene expression in theca-interstitial cells. Endocrinology. 2019;160(12):2946–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4322–4328. [DOI] [PubMed] [Google Scholar]
- 56. González F, Mather KJ, Considine RV, Pardue SL, Acton AJ. Suppression of nutrient-induced inflammation with a nonsteroidal anti-inflammatory agent ameliorates ovarian dysfunction in lean insulin-sensitive women with polycystic ovary syndrome. In: Program of the 71st Meeting of the American Society for Reproductive Medicine Baltimore, MD; October 17–21, 2015: Abstract O-51. 10.1016/j.fertnstert.2015.07.064. [DOI] [Google Scholar]
- 57. González F, Abdelhadi OA, Considine RV, Acton AJ. Anti-inflammatory therapy suppresses proinflammatory cytokine secretion from mononuclear cells and reduces hyperandrogenism in lean women with polycystic ovary syndrome. In: Program of the 72nd Meeting of the American Society for Reproductive Medicine Salt Lake City, UT; October 15–19, 2016: Abstract O-81. 10.1016/j.fertnstert.2016.07.104. [DOI] [Google Scholar]