Abstract
Candida albicans, along with other closely related Candida species, are the primary causative agents of vulvovaginal candidiasis (VVC)—a multifactorial infectious disease of the lower female reproductive tract resulting in pathologic inflammation. Unlike other forms of candidiasis, VVC is a disease of immunocompetent and otherwise healthy women, most predominant during their child-bearing years. While VVC is non-lethal, its high global incidence and profound negative impact on quality-of-life necessitates further understanding of the host and fungal factors that drive disease pathogenesis. In this review, we cover the current state of our understanding of the epidemiology, host response, fungal pathogenicity mechanisms, impact of the microbiome, and novel approaches to treatment of this most prevalent human candidal infection. We also offer insight into the latest advancements in the VVC field and identify important questions that still remain.
Keywords: vaginitis, vulvovaginal, Candida, vagina, VVC, fungal, candidiasis
1. Pathology and Epidemiology of Vulvovaginal Candidiasis
Vulvovaginal candidiasis (VVC), is an exceedingly common mucosal infection of the lower female reproductive tract (FRT), caused mostly by the polymorphic opportunistic fungus Candida albicans. A member of the normal human microbiota, C. albicans commonly colonizes the vaginal lumen asymptomatically [1]. However, symptomatic infection can result from exuberant mucosal inflammation that is caused primarily by fungal overgrowth in the vagina and subsequent epithelial invasion and production of virulence effectors. Common disease symptoms include vaginal itching, burning, pain and redness. Often, these are accompanied by a vaginal discharge consisting of sloughed epithelium, immune cells, yeast, and vaginal fluid. VVC is the most prevalent human candidal infection, estimated to afflict approximately 75% of all women at least once in their lifetime [2]. Moreover, recurrent VVC (RVVC, defined as >3 episodes per year) affects nearly 8% of women globally [3]. Frequently, RVVC requires antifungal maintenance therapy with azole drugs to attenuate disease reemergence [1]. Static activity of the azoles and inadequate immune-mediated clearance are key drivers of disease recurrence. Risk factors for VVC are the use of antibiotics, sexual activity, high-estrogen containing oral contraceptives, pregnancy, use of sodium glucose cotransporter 2 (SGLT2) inhibitors, and uncontrolled diabetes mellitus [4,5]. Risk factors for RVVC are currently unknown, although genome-wide association studies have begun to unravel some genetic determinants of susceptibility (as discussed in depth below).
In contrast to invasive and oral candidiasis, R/VVC is a disease of immunocompetent and otherwise healthy women [6]. Thus, the global disease burden is much higher for VVC than these other infectious routes. Using rough estimates of susceptible global populations and incidence rates for each of these disease states, invasive candidiasis causes ~700,000 cases per year, oral candidiasis results in ~15.5 million infections per year, and RVVC alone causes approximately 140M cases per year (Table 1). The incidence rate for acute VVC is practically impossible to estimate, given that it is underreported to clinicians due to largely effective over-the-counter treatment options [7]. While VVC is non-lethal, the sheer enormity of disease burden results in ~$1.8B in medical costs each year and the economic impact due to lost work hours was recently extrapolated to approach an additional $1B per annum in the US alone [3,7].
Table 1.
Estimated annual global incidence of candidiasis.
| Candidiasis Route | Avg. Incidence | Approx. Target Population 1 | Approx. Individuals Impacted | Refs. |
|---|---|---|---|---|
| Invasive | 0.009% | 7,800,000,000 | 702,000 | [8] |
| Oropharyngeal | - | - | 15,337,200 | |
| HIV/AIDS 2 | 72.5% | 15,200,000 | 11,020,000 | [9,10] |
| Head/neck cancer | 40% | 650,000 | 260,000 | [11,12] |
| Is Organ transplant | 11.6% | 100,800 | 11,690 | [13] |
| Infants (< 6 mos.) | 6% | 67,425,000 3 | 4,045,500 | [14,15,16] |
| Recurrent vulvovaginal | 8% | 1,682,200,000 | 134,560,000 | [17] |
1 Based on references cited. 2 Based on global population living with HIV/AIDS and not currently on retroviral therapy. 3 Calculated as: (crude global birth rate - global infant mortality rate)/2.
While C. albicans is the causative agent of over 90% of VVC cases, other non-albicans Candida (NAC) species have been identified as etiological agents. In some instances, the prevalence of NAC species is disproportionately high, exceeding 50% [18]. Of the NAC species, C. glabrata is regarded as the second leading cause of VVC (~8% of cases), while C. krusei, C.parapsilosis, and C. tropicalis make up a majority of the remainder [18,19,20]. Vaginal symptoms resulting from infection with NAC species are often reported as being milder than those experienced during VVC caused by C. albicans [21]. However, inherent resistance to the azole drug class, as well as acquired resistance mechanisms, can complicate treatment of the NAC species [22,23]. Often, prolonged antifungal regimens or alternative treatment approaches (e.g., vaginal boric acid suppositories) are required for clearance [24,25]. Given reduced therapeutic efficacy, recent reports suggesting increased incidence rates of NAC-associated VVC are somewhat unsettling [26,27]. However, such reports should be taken with caution, as symptomatic VVC can mimic several disease states of the lower FRT and the ability to distinguish Candida as a true pathogen from an asymptomatic colonizer can be difficult. Many such reports identifying high proportions of NAC species come from tertiary vaginitis clinics, focused on patients who have failed conventional antifungal therapy [24,28]. Thus, these studies may overestimate the prevalence of NAC species in the general population. One explanation for increased NAC species causing VVC is the use of over-the-counter azole creams for VVC which could lead to the selection of these inherently antifungal resistant species, supporting the above observation [4,29].
Prior and recent work using an animal model of infection demonstrated that representative NAC species isolates are incapable of driving vaginal inflammation similar to that of C. albicans [30,31]. This was attributed to a lack of robust virulence mechanisms (as described in more detail below). However, it is somewhat difficult to reconcile these results with the relatively large incidence of C. glabrata vaginitis [18]. Thus, it is possible that significant strain-to-strain heterogeneity of virulence or metabolism exists amongst clinical isolates or differential mucosal sensitivity to fungi that could help explain NAC-associated VVC. Because the NAC species are comparatively understudied, the remainder of this review will focus largely on disease pathogenesis and therapy through the lens of C. albicans.
Lastly, the influences of host physiological processes on the development of VVC remain unclear. However, estrogen clearly plays a major role in governing disease susceptibility [32]. All animal models of VVC (mouse, rat, pig, macaque) require administration of exogenous estrogen to maintain the vaginal fungal burden, inducing keratinization and cornification of the upper squamous epithelial layer [33]. Estrogen susceptibility to VVC is also reflected clinically. Women in their child-bearing years and estrogenic-phases of their reproductive cycle are most susceptible to disease onset. However, prepubescent girls and post-menopausal women are infrequently afflicted, unless they undergo hormone replacement therapy [33]. Whether this susceptibility is exerted through effects on host immune function, vaginal epithelium stratification, or the fungus itself, are still unknown but all have been reported to potentially play a role [34,35].
2. Fungal Pathogenicity Mechanisms and Host Response
2.1. VVC as an Immunopathology
For decades, VVC was regarded by many as the result of a defective adaptive immune response. However, several cross-sectional clinical studies revealed that HIV+ women (with reduced CD4+ T-cell counts) were at no greater risk for developing VVC as compared to healthy controls [6,36,37,38]. This, coupled with animal model data demonstrating no robust role for cell-mediated or humoral immune responses, clearly pointed toward an alternative explanatory mechanism [39]. A groundbreaking study using human volunteers intravaginally challenged with live C. albicans revealed that VVC was actually mediated via innate immune responses, in which neutrophil recruitment into the vaginal lumen was positively correlated with reported disease symptoms [40]. Due to the negative impact of host immunity on disease progression, VVC was labeled as an immunopathology. Recognition of the importance of the innate response in driving inflammatory responses associated with VVC has led to many recent insights regarding disease pathogenesis.
As described above, C. albicans is polymorphic, adopting two major morphological forms: the ovoid yeast and elongated hypha [41]. It has long been observed that the capacity to transition between these morphologies is the primary virulence attribute of C. albicans, as strains unable to undergo this switch are severely attenuated in pathogenicity or colonization [42]. Surprisingly, the use of hypha-deficient transcriptional regulator mutants (e.g., Δ/Δefg1 and Δ/Δefg1/cph1) in a murine model of VVC revealed elevated colonization as compared to hypha-competent strains [43]. However, the capacity to elicit neutrophil recruitment, mucosal damage (measured by LDH release), innate inflammatory cytokine (e.g., Interleukin-1β), or alarmins (e.g., S100A8) was significantly reduced with these mutants. These results raised two questions: “Do neutrophils help clear the fungus?” and “Are recruited neutrophils themselves causative agents of host damage (as is observed for other common immunopathologies such as arthritis and asthma)?” However, depletion of neutrophils with neutralizing antibodies neither reduced fungal load nor vaginal LDH levels during infection, suggesting that neutrophils are non-protective under such conditions and that mucosal damage is mediated by the fungus [43]. These results were mirrored in a prior study demonstrating that neutrophil depletion indeed reduced vaginal inflammation during murine VVC, but ultimately did not impact colonization and the course of infection [44]. Clearly, C. albicans is capable of eliciting vaginal inflammation, but how?
2.2. Sensing Fungi at the Vaginal Mucosa
Prior to the initiation of an immune response, C. albicans must first be sensed at the mucosal interface. Primarily, this is mediated through pattern recognition receptors (PRR) that exist at the cellular surface of epithelial and innate immune cells. Ligation of these receptors to their cognate ligands (typically C. albicans cell wall constituents) induces pro-inflammatory cytokine signaling and the recruitment of both innate and adaptive immune cells to infectious foci via activation of downstream signaling cascades (Figure 1) [45]. Sensing of C. albicans is somewhat dependent on morphogenetic switching, as the challenge of vaginal epithelial cell cultures with hypha-defective Δ/Δefg1/Δ/Δcph1 mutants results in a significantly reduced release of pro-inflammatory cytokines and a delayed, alternative activation of the mitogen-activated protein kinase (MAPK) system [46].
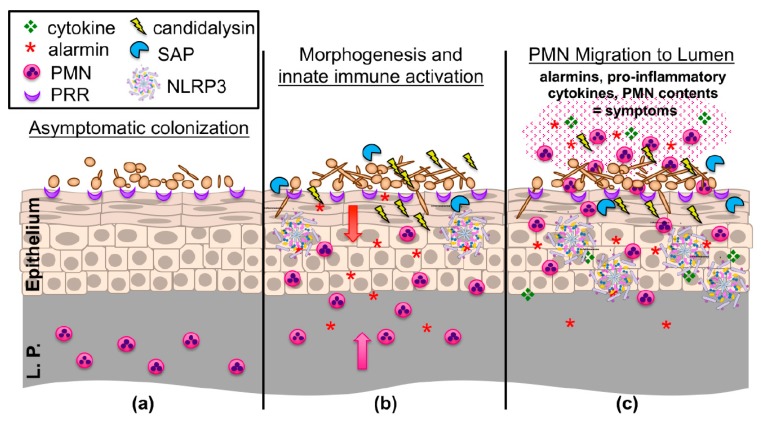
Figure 1.
An updated working model of the immunopathogenesis of C. albicans vaginitis. (a) Yeast forms of C. albicans asymptomatically colonize the vaginal epithelium despite the presence of numerous pattern recognition receptors (PRR) on the epithelial surface. (b) C. albicans begins to undergo the yeast-to-hypha switch under morphogenesis-inducing conditions (e.g., increases in estrogen, elevated vaginal pH, and microbiome disruption). Augmented recognition by PRRs, increased hyphal biomass, expression of hypha-associated virulence factors (candidalysin, secreted aspartyl proteinases (SAPs)) activates NLRP3 inflammasome signaling, eliciting inflammatory cytokines and chemokines (e.g., IL-1β, S100A8/9 alarmins) in the vaginal epithelium, resulting in initial migration of polymorphonuclear leukocytes (PMNs) from the lamina propria (L.P.) to the vaginal lumen. (c) Failure to adequately reduce immunopathological triggers results in the continued expression of innate immune effectors by the vaginal epithelium. These initial signals, coupled with the secondary amplification of immune effectors by recruited PMNs, contribute to symptomatic infection and characteristic immunopathology. Figure adapted from: PLoS Pathog. 2014 Apr; 10(4): e1003965 [47].
The role for Toll-like receptor (TLR) and C-type lectin receptor (CLR) families are the most well described with regard to VVC. Despite its clear protective role against invasive infection, discrepant results exist regarding the role for the CLR Dectin-1 that recognizes fungal β-glucan [48]. Attempts to determine the genetic linkage of RVVC with Dectin-1 deficiency (Y238X mutation) in women have yielded disparate results [49,50]. Using Dectin-1-/- mice, one study demonstrated an elevated susceptibility to VVC in C57BL/6 mice, yet a reduced susceptibility in the BALC/c background; these effects were purportedly due to Th17 effectors [51]. Expression of murine Dectin-1 was not found on the vaginal epithelium, suggesting that if Dectin-1 does play a role during VVC, it likely exerts its effects through the hematopoietic lineage [52]. However, a simultaneous blockade of TLR4 and SIGNR1 (Dendritic-Cell-Specific intercellular adhesion molecule-3-Grabbing Non-integrin) reduced the expression of alarmins during fungal challenge in vivo, demonstrating their important role during early inflammatory events [52]. Although strong clinical evidence exists that CARD9-deficiency (an adaptor protein important for broad CLR signaling) confers susceptibility to mucosal fungal infection, including RVVC, mechanistic studies to assess its role in animal models are lacking [53]. Similarly, the loss of all TLR signaling via MyD88 deletion has not been interrogated comprehensively during VVC. Defects in mannose receptor (MR) and mannose-binding lectin (MBL) signaling, that sense the carbohydrate mannan, have also been proposed to mediate susceptibility to fungal infection. A series of clinical studies identified a polymorphism (codon 54) in MBL-2 that associated with RVVC susceptibility [54,55]. Challenge of epithelial cells derived from MR-/- mice resulted in similar levels of inflammatory markers as those from wild-type mice [52]. Thus, more detailed mechanistic studies are required to fully elucidate the role of mannose recognition in the pathogenesis of VVC.
Using a comprehensive systemics genetics approach, a recent study by Jaeger and colleagues identified a polymorphism (rs2919643) in SIGLEC15, a sialic acid-binding lectin present on immune cells and vaginal mucosa, as a susceptibility allele for RVVC [56]. Siglec15 was shown to strongly bind sialic acid on the surface of a variety of Candida species and cleavage of sialic acid from the fungal surface using neuraminidase-attenuated immune activation during challenge of blood monocytes. In vivo knockdown of SIGLEC15 transcript resulted in a higher fungal burden and inflammation as compared to control animals, suggesting an important role in controlling infection. However, the polymorphism identified confers a hyper-inflammatory, pathologic phenotype, driving elevated inflammatory markers, including neutrophil recruitment to the vaginal lumen during murine VVC. Thus, attempts to pharmacologically attenuate this pathway to alleviate symptoms in the RVVC population would have to be carefully controlled as to further exacerbate inflammation or prevent fungal clearance.
2.3. Invasion and Secreted Virulence Effectors
Aside from signaling through PRRs, the capacity for C. albicans to simultaneously elicit tissue damage is another key piece to understanding the full activation of cellular immune responses [57]. C. albicans (as well as the NAC species) exert their damage primarily through two major mechanisms: direct invasion by hyphal filaments or secretion of virulence effectors (Figure 1). Hyphae are crucial for breaching mucosal barriers and causing tissue damage. Host membranes can be weakened by degradative enzymes secreted at the hyphal tip and pressure exerted by the elongating filament is then sufficient to penetrate the host cell [41,58]. Wächtler and colleagues demonstrated C. albicans attaches to and begins invading vaginal epithelial cells within 3 h and that continual hyphal growth is required for damage [59]. A number of hypha-associated adhesins help anchor C. albicans to the mucosal epithelium and are important for mature biofilm formation in the vagina as deletion of the master adhesin regulator BCR1 impaired biofilm formation in vivo [60]. Aside from binding to host E-cadherin (and other epithelial receptors), Agglutinin-like sequence 3 (Als3p) also acts as an invasin that induces clathrin-dependent endocytosis of C. albicans into epithelial cells, further contributing to its invasiveness [61]. The precise role of Als3 during VVC has not yet been established but a majority of vaginal clinical isolates were found to express this gene [62].
Regarding secreted effectors, the secreted aspartyl proteinase (SAP) and lipase gene families are the most studied. The SAPs are a collection of ~10 genes that degrade extracellular proteins to be used as metabolic nitrogen sources [63,64]. Extensive studies have revealed that SAPs demonstrate morphological specificity and pH optima: SAPs 1–3 are largely associated with expression in the yeast cell and at pH 2–5, while SAPs 4-6 are expressed during hyphal growth and are most active at pH 5–7 [65]. Mice intravaginally challenged with a Δsap456 triple mutant or Δsap5 single mutant demonstrated moderate but significantly reduced immunopathological markers early during infection [66]. Other studies using rodent models of VVC demonstrate that recombinant Sap2 and Sap6 are capable of driving vaginal inflammation and that a Δsap2 mutant is attenuated in pathogenicity [67,68]. However, overexpression of SAP1-6 in hypha-defective Δ/Δefg1/Δ/Δcph1 mutants did not elicit mucosal damage or inflammation during murine VVC [69]. Thus, at this time, the role of the SAPs in contributing to VVC immunopathogenesis is somewhat inconclusive and may be dependent on discrepancies in animal model systems, fungal genetic approaches, and protease doses utilized.
While the pathogenic role of SAPs has been extensively studied in C. albicans, the lipases have received less attention. Secreted lipases are encoded by a family of 10 genes (LIP1-10) and serve an important role in the hydrolysis and synthesis of triaclglycerols [70]. Much like the SAPs, they exhibit differential regulation and temporal patterns of expression [71,72]. Heterologous expression of lipases in Saccharomyces cervevisiae revealed that LIP4, 6, 8, and 10 exhibited true lipolytic activity [73]. Deletion of LIP8 in C. albicans or LIP1 and LIP2 in C. parapsilosis significantly reduced fungal burden and damage biomarkers, while enhancing survival in murine models of systemic infection [74,75]. However, the role of lipases during VVC has not yet been interrogated.
2.4. An Indispensable Role for Candidalysin
Recently, the ECE1 (extent of cell elongation) locus of C. albicans has received much attention, as its gene product is crucially important for pathogenicity [76]. Elegant work by the Moyes, Naglik, and Hube labs have demonstrated that the hypha-specific ECE1 transcript is translated into a 271 amino acid polypeptide, interspersed by seven lysine-arginine (KR) repeats [77]. Modeling and biochemical analyses have revealed that these KR sites are cleavable by the secretory Kex2p protease, resulting in eight peptides of variable length [77,78]. Disruption of these cleavage sites by alanine scanning and use of a Δ/Δkex2 mutant confirmed this suspected secretion mechanism [79]. While the role of seven of these peptides remains enigmatic, peptide three (N-SIIGIIMGILGNIPQVIQIIMSIVKAFKGNK) exhibits lytic activity reminiscent of bacterial cytolysins and the capacity to elicit innate inflammatory pathways (e.g., MAPK) in epithelial and endothelial cells. Thus, it was termed ‘candidalysin’ [77].
Disruption of the ECE1 locus or the region specifically encoding for candidalysin results in severe attenuation of pathogenicity in multiple infection models, including murine invasive and oral candidiasis, zebrafish swim bladder infection, and a variety of cell culture systems [77,80,81,82,83]. Similarly, deletion of ECE1 or candidalysin significantly reduces immunopathologic markers of infection (neutrophils, pro-inflammatory cytokines, alarmins) and tissue damage during experimental VVC (Figure 1) [81]. Importantly, these deletion mutants displayed no defect in filamentation or altered colonization in vivo, demonstrating that hyphae are insufficient, and that candidalysin is required for driving immunopathogenicity during VVC. Moreover, treatment of A431 vaginal epithelial cells with various concentrations of candidalysin dose-dependently elicited the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, and IL-8, among others, which was largely reflected in the mouse vagina using candidalysin deletion mutants and revertant strains [81]. Therefore, candidalysin has been established as a crucial virulence determinant driving murine VVC using the reference isolate SC5314. However, its role in clinical isolates and during human infection has not yet been resolved. This, along with the absolute requirement of filamentous or pseudofilamentous morphology in driving immunopathology, remain important, unanswered clinical questions [84].
2.5. A Controversial Role for IL-17 Signaling?
Cleary, candidalysin causes excessive damage at mucosal surfaces, further amplifying innate immune signaling. In vitro and in vivo work suggests that MAPK signaling, engaged via epithelial growth factor receptor (EGFR) phosphorylation, is important for candidalysin-triggered immune activation [85]. Candidalysin also plays a significant role in eliciting IL-17 responses at the oral epithelium, driving a feed-forward inflammatory loop characterized by IL-1α/IL-β responses [86]. These signaling events have not been entirely elucidated yet for VVC. However, an unbiased global transcriptomic approach using RNA-seq to detect differential gene expression in the murine vagina during infection revealed a gene signature that was strongly suggestive of an IL-17 response [66]. Moreover, this gene signature was remarkably similar to that observed during oral candidiasis [87]. Administration of halofuginone, a pharmacologic inhibitor of IL-17 T-helper cell development, resulted in elevated vaginal fungal burdens during murine infection [88]. Despite these strong indicators for a role of IL-17, the use of IL-17RA-/-, IL-22-/-, and IL-23p19-/- (major mediators of the IL-17 axis) knockout mice demonstrated that neither fungal burden nor neutrophil influx differed between those observed in wild-type mice during murine VVC [89]. Because the aforementioned effects of estrogen may impair IL-17 signaling as previously reported, similar experiments using IL-17RA-/-, ACT1-/-, and IL-22-/- mice were conducted in the absence of estrogen. Regardless of estrogen administration, susceptibility to VVC was not altered in knockout compared to wild-type animals, although less consistent colonization was observed in the absence of exogenous estrogen [87]. While the precise role for IL-17 during human infection is not known, congenital or acquired immunodeficiency affecting IL-17 or Th17 cells seemingly does not predispose women to VVC [90,91]. Collectively, these data support an emerging consensus that, despite the strong induction of the Th17 axis, it is dispensable for the immunopathogenesis of VVC.
2.6. The NLRP3 Inflammasome: A Key Role in VVC Pathogenesis
A closer look at transcriptional regulator analysis from RNA-seq studies revealed that the NLRP3 inflammasome plays an important role during disease pathogenesis, as IL-1R pathway genes were increased during VVC [66]. The inflammasomes are a collection of cytosolic innate pattern recognition receptors (PRR) that respond via a two-step activation process involving priming of the response via ligation of cell surface PRRs and sensing endogenous and exogenous danger signals [92]. Damage induction ultimately drives inflammasome oligomerization and activation of Caspase-1, leading to cleavage and secretion of IL-1β—the major inflammasome effector. Signaling via IL-1R can overlap with MAPK activation and subsequent pro-inflammatory cytokine production, followed by robust neutrophil recruitment [86,93]. Given these properties and the capacity for candidalysin to induce damage, and a demonstrable role for protection against oral candidiasis, the NLRP3 inflammasome as a partial driver of innate inflammatory events during VVC was plausible [94]. Indeed, independent experiments from different research teams confirmed that neutrophil influx, cytokine, and alarmin secretion were significantly attenuated in NLRP3-/- animals as compared to controls [66,95]. Interestingly, unlike most models of candidiasis where NLRP3 mediates protection, fungal burden in knockouts was not altered during infection, suggesting that, in the case of VVC, NLRP3 drives immunopathogenic inflammation (Figure 1).
A series of studies have further established the NLRP3 inflammasome as an important mediator of innate immune responses induced by C. albicans. Prior work established that Δ/Δefg1/Δ/Δcph1 mutants, incapable of undergoing the yeast-to-hypha transition, fail to activate IL-1β secretion in macrophage cell lines [96]. More recent work has established that candidalysin is a potent activator of the NLRP3 inflammasome, as treatment of NLRP3-/- or Casp1-/- macrophages with synthetic candidalysin fails to induce IL-1β secretion [80]. Similarly, challenge of wild-type macrophages with Δ/Δece1 or candidalysin deletion mutants abrogated IL-1β processing and secretion [82,97]. These results are in line with those prior, using hypha-defective strains that consequently also do not secrete candidalysin [96]. Work comparing the relative capacity of various Candida species to activate NLRP3 revealed that all, aside from C. albicans, are relatively poor inducers of these responses likely because they infrequently form hyphae (if at all) and demonstrate significantly reduced ECE1 expression [31]. This is supported by other work demonstrating that either a high multiplicity of infection or extended infectious time courses are required for the robust inflammasome responses elicited by NAC species [98]. This data could explain why NAC species make up a minority of VVC infections.
Aside from the role of candidalysin in eliciting inflammation via inflammasome signaling, the SAPs, particularly Sap2 and Sap6 have also been observed to activate NLRP3. Work by the Cassone group has shown that treatment of vaginal epithelial cells with recombinant Sap2p is sufficient to activate Caspase-1, induce IL-1β, and drive neutrophil migration, while its blockade with a neutralizing antibody or enzymatic inhibition with pepstatin A is sufficient to reduce these effects [68]. Similar results were also found for Sap6, where vaginal administration of SAPs to mice up-regulated neutrophil chemoattractants and drove sterile inflammation. Moreover, treatment of human monocytes with either rSap2p or rSap6p elicited high levels of NLRP3 inflammasome effectors IL-1β and IL-18, which was strongly attenuated by knockdown of NLRP3 transcripts [99]. While data suggest that rSAPs are capable of eliciting inflammasome signaling during VVC, this has not yet been conclusively demonstrated using NLRP3-/- mice. That said, C. albicans possesses multiple, redundant pathogenicity mechanisms for triggering inflammatory processes within the host.
Roles for inflammasome signaling during cell culture infection and VVC are clear and an increasing number of studies highlighting the relevancy of this pathway during human infection are starting to emerge. A large-scale clinical study revealed that women with RVVC exhibited significantly higher IL-1β and lower IL-1RA (IL-1R antagonist) levels in vaginal lavage fluid as compared to healthy controls, suggesting that the NLRP3 inflammasome may be more highly active in the RVVC population and driving exacerbated immunopathology [95]. A much smaller clinical study similarly revealed that NLRP3 and CASP1 expression was higher in vaginal epithelial cells of women with acute symptomatic VVC as compared to asymptomatic or uncolonized control subjects [100]. Interestingly, expression of ECE1 was significantly higher in the symptomatic as compared to the asymptomatic group, suggesting that candidalysin may be important for eliciting inflammasome signaling and subsequent inflammation. A genetic survey of over 800 patients revealed that increased VVC susceptibility associated with a unique 12/9 genotype that includes distinct polymorphisms in NLRP3 [101]. Inflammatory markers in vaginal fluid demonstrated increased IL-1β and decreased IL-1RA with this genotype under both asymptomatic and symptomatic states, suggesting that host genetic variation in inflammasome (or other pathways) likely influences VVC susceptibility. Higher powered, more comprehensive, genome-wide association studies are desperately needed to help identify genetic markers of vaginitis risk and novel intervention points.
2.7. Estrogen-Dependent Immunomodulation
Despite the robust inflammation and neutrophil recruitment observed during VVC, fungal burdens remain high. This begs the question as to why these cells are recruited to the vaginal lumen if they are, in fact, not protective? The answers may lie in hormonal effects exerted on host immunity. Mice, like humans, progress through a cyclical reproductive cycle characterized by waves of waxing and waning hormones [102]. However, unlike humans, mice do not menstruate but instead recruit neutrophils to the vaginal lumen to debride the endometrium. Under high estrogen conditions, neutrophil migration is arrested in the vaginal stroma, unless C. albicans elicit their chemotaxis by re-establishing chemokine gradients [103]. One theory suggests that estrogen creates an immunologically tolerant vaginal environment, to abrogate responsiveness to foreign antigens (e.g., sperm protein) encountered during peak mating receptiveness [104]. Thus, estrogen may promote a transient, lower FRT-specific immunosuppression, allowing for fungal overgrowth.
Fidel and colleagues presented a novel hypothesis referred to as “neutrophil anergy” to help explain neutrophil inactivity in the vagina [105]. C57BL/6 mice are highly susceptible to estrogen-dependent VVC, while CD-1 mice are relatively resistant to vaginal colonization [106,107]. Interestingly, supplementation of cell culture medium with vaginal fluid obtained from C57BL/6 mice significantly impeded neutrophil-mediated killing of C. albicans in vitro, while fluid from CD-1 mice was similar to untreated control medium. Moreover, fluid from C57BL/6 mice lost neutrophil inhibition when mice were not administered estrogen. The active compound in C57BL/6 fluid was identified as heparan sulfate, and its effects on PMN activity could be mitigated by degradation with heparanase. Evidently, heparan sulfate is a competitive inhibitor of Mac1, present on neutrophils and interferes with recognition of the fungal surface ligand Pra1p, allowing C. albicans to evade killing [107]. Given that heparan sulfate is estrogen-inducible, this could provide a rationale as to why neutrophils are ineffective at clearing C. albicans in vivo. Clinical studies are needed to survey heparan sulfate levels in RVVC, symptomatic, and control groups of women in order to further establish this interesting hypothesis.
3. A Role for the Microbiome?
A balanced and healthy vaginal microbiome is considered to be important for maintaining female health, further influencing the health of their sexual partners and newborns, as well as the success of pregnancy and delivery [108]. Alteration in vaginal microbiome is a significant risk factor for developing diseases of the FRT, including Candida vaginitis [109]. Comprehensive studies on healthy human vaginal microbiome have identified Lactobacillus spp. to be the dominant bacterial species by both culture-dependent and -independent methods [110,111,112,113]. Although absolute numbers vary, due to different endogenous and exogenous conditions (age, lifestyle, pregnancy, hormone levels, etc.), the relative abundance of Lactobacillus spp. in the human vagina is >70% [114]. Among the five groups of human vaginal communities from reproductive-age women, I, II, III, and V were dominated by Lactobacillus crispatus, L. gasseri, L. iners, and L. jensenii, respectively [113]. Through the excretion of metabolic byproducts (as discussed below) and subsequent acidification of the vaginal microenvironment, Lactobacillus spp. are considered to help maintain homeostasis by preventing the colonization or overgrowth of pathogens.
Studies attempting to define the contribution of the microbiome to Candida vaginitis have resulted in disparate conclusions, mainly regarding the role of Lactobacillus spp. in preventing pathogen (Candida spp.) colonization. Interestingly, the vaginal microbiomes of women suffering from VVC, Chlamydia trachomatis, or bacterial vaginosis exhibit distinct profiles that further differ from those of otherwise healthy women [109]. Several studies have suggested vaginal dysbiosis during VVC, with reduced Lactobacillus colonization rates or patterns [109,115,116,117]. Although Lactobacillus remained dominant at the genus level, reduced rates (<67.5%) and altered species (L. crispatus progressively replaced by L. iners) were found during disease states [109,113]. The vaginal microbiome of VVC patients following antifungal treatment were also distinct, demonstrating similarities to both healthy and dysbiotic microbiomes, which might represent a transition between these states [116]. However, the protective role of Lactobacillus spp. against Candida spp. has been questioned, as high densities of lactobacilli often accompany Candida in the vagina during active vaginitis [117,118]. In fact, even elevated levels of Lactobacillus spp. (L. iners and L. crispatus) can be observed during VVC, broadly suggesting the failure of Lactobacillus spp. to suppress Candida colonization [118]. Other clinical studies have failed to provide evidence of an altered or abnormal vaginal microbiota in women suffering from Candida vaginitis, despite the maintenance of Lactobacillus abundance [119,120]. Discrepancies among studies may be due to inherent variables (e.g., patient geography, age, symptoms), as well as approaches used for sample collection, storage, culturing and identification.
Despite the unclear effect of Lacotbacillus in vivo, a majority of in vitro and animal studies have revealed that Lactobacillus spp. exerts an inhibitory effect on the growth, morphological transition, virulence, and biofilm formation of C. albicans [121,122,123]. The metabolites of Lactobacillus spp. including organic acids, hydrogen peroxide, bacteriocins, and biosurfactants, all contribute to these antifungal effects [124,125]. However, only specific Lactobacillus strains are capable of producing these effectors in quantities required for antifungal activity, which possibly explains the failure of some vaginal lactobacilli to suppress Candida colonization. The transition of C. albicans from yeast to hypha is inhibited at low pH (< 4.5) [126]. Thus, the secretion of lactic and other organic acids is believed to be the main mechanism by which lactobacilli impede candidal virulence. In support of this, down-regulation of hypha-specific transcriptional regulatory genes (EFG1, BCR1, and CPH1, etc.) and virulence related genes (HWP1, ALS3, ECE1, and SAP5) were observed during co-culture with lactobacilli or treatment with spent culture supernatant [125,127]. Lactobacilli were also reported to compete with the attachment of C. albicans to host cells, resulting in reduced adhesion to epithelial surfaces [128]. Lactobacillus strains originating from sites other than the vagina have also been frequently observed to exhibit strong anti-Candida properties, suggesting more global growth-inhibitory effects [129,130]. While the clinical data regarding Lactobacillus colonization and its role in preventing VVC are unclear, some clinical studies using lactobacilli as probiotic colonizers have shown promising protective effects [131,132].
4. Established and Novel Treatment Modalities
There are numerous current therapeutic options available for the treatment of VVC that vary based on uncomplicated or complicated (e.g., RVVC, NAC species) presentation. Treatments mainly consist of prescription oral dosage forms, or over-the-counter topical preparations, or vaginal suppositories [133]. Azoles are the most common class of antifungal drugs used to treat vaginal candidiasis due to their good bioavailability, antifungal efficacy, and relative safety. However, the azole class exhibits static activity, thus requiring efficient host immune function to aid in the resolution of symptoms. Common over-the-counter azoles and treatments are: clotrimazole 1%–4% topical cream for daily usage between 1–7 days, miconazole 2%–4% cream for 3–7 days or 100–1000 mg suppository for 1–7 days, and tioconazole 6.5% intravaginal ointment in a single application [134]. Prescription azole agents include butoconazole 2% topical cream applied intravaginally, terconazole 0.4%–0.8% daily for 7 days or 80 mg vaginal suppository (one every day for 3 days) and fluconazole 150 mg single oral dose [134]. Patients with NAC-related or recurrent VVC are often prescribed a longer duration of initial topical (e.g., 7–14 days) or oral (100–200 mg every 72 h for three doses) therapy [133]. Women suffering from severe or highly recurrent disease may take azole drugs weekly to prevent symptomatic episodes [135]. While oral and systemic isolates of C. albicans can exhibit high levels of azole resistance, treatment of VVC with this antifungal class is largely effective and overall resistance rates remain low [136]. In the event that resistance is detected, less conventional therapies including boric acid suppositories and creams containing nystatin, amphotericin B, or flucytosine, are indicated [17].
Topical products containing local anesthetics (5% benzocaine) and antiseptics (2% resorcinol) to relieve itching and burning also exist, but many women complain that they actually induce stinging and burning during application [137]. Thus, new treatment modalities are desperately needed to resolve disease symptoms in a timelier fashion. Given that the NLRP3 inflammasome appears to play a major role in driving murine and clinical immunopathology, strategies to attenuate the activation of this molecular complex are appealing. Administration of the IL-1R antagonist anakinra, to interfere with signaling of the inflammasome effector IL-1β, significantly reduced neutrophil recruitment and pro-inflammatory cytokines in a mouse model of VVC [95]. Administration of the anti-diabetic sulfonylurea glyburide (and established NLPR3 inhibitor) to mice attenuated neutrophil recruitment during VVC [66]. Moreover, treatment of macrophage-like cells with various second-generation sulfonylurea drugs and the highly potent NLRP3 antagonist MCC-950 (glyburide, glisoxepide, gliquidone, and glimepiride) dose-dependently inhibited NLRP3-dependent IL-1β secretion, suggesting that these anti-diabetics could be repurposed as potential anti-inflammatory agents [97]. Collectively, these data suggest that targeting the NLRP3 inflammasome during VVC may be a rational therapeutic option for disease management.
Probiotic therapy, or the administration of beneficial microbes to the mucosal surface, has been suggested for the treatment or prevention of Candida vaginitis. A study by Pericolini and colleagues has found that introduction of S. cerevisiae to the murine vagina could help protect against C. albicans challenge [138]. Purportedly, anti-candidal effects included adherence inhibition, accelerated clearance, blockade of the yeast-to-hypha transition, and reduced expression of established virulence effectors (e.g., SAPs). Unsurprisingly, this was correlated with reduced immunopathological markers of infection. Vaginal epithelial cells were also found to exhibit less exfoliation and reduced damage biomarkers when S. cerevisiae was introduced intravaginally. Whether these effects are strain-specific or effective against a broad array of Candida species or isolates remains unknown. Given that S. cerevisiae has been shown to cause vaginitis-like symptoms in a limited number of women, significant clinical testing would be required to rule out any potential negative consequence of abundant vaginal yeast colonization [139].
Lastly, the holy grail of reducing the incidence of VVC lies in the development and deployment of a safe, efficacious, and long-lasting vaccine. A significant amount of work by Edwards and colleagues has made remarkable strides in this arena with the advent of the NDV-3 vaccine [140]. NDV-3 is composed of a recombinant epitope of Als3p delivered in alum adjuvant. It has demonstrated excellent efficacy in dose-dependently reducing fungal burdens several logs in the mouse model of VVC [141]. NDV-3 exerts its protective effects by priming both humoral and adaptive immune responses, as protection was lost in both B- and T-cell-deficient mice. Importantly, reduced fungal burdens correlated with decreased neutrophils levels, indicating that NDV-3 administration should also temper symptomatic episodes. Administration in human volunteers established that the vaccine was well-tolerated and highly immunogenic, generating long-lasting memory B- (IgG, IgA1) and T-cell (IFNy, IL-17A) responses that were enhanced with a booster dose [140]. Recent data from a Phase 1b/2a clinical trial offered significant protection against RVVC in women < 40 years of age [142]. Serum recovered from immune subjects that remained VVC-free, as compared to those experiencing relapse, exhibited high Als3-specific IgG titers, which, when pre-absorbed to C. albicans, inhibited its filamentation, epithelial cell invasiveness, and biofilm formation. Aside from NDV-3, a truncated rSAP2 protein incorporated into influenza virosomes, termed PEV7, was also shown to confer protection against VVC in rats, presumably via antibody-mediated effects [143]. While these collective data are promising, important studies to delineate boosting strategies, include other antigenic determinants, or alter adjuvant choice, will help optimize the efficacy of a globally protective vaginitis vaccine.
5. Conclusions and Burning Questions
Candida vaginitis is a universally important disease with wide-reaching effects on the overall physical and mental health of women, coupled with significant impacts on the medical and work-force economy. Recent advances in our understanding of VVC as an immunopathology and the mechanistic roles of candidalysin, inflammasome, and PRR-mediated signaling at the vaginal mucosa have opened up multiple avenues for scientific exploration. While progress has been impressive, several important questions and challenges remain.
Our understanding of the host and fungal molecular mechanisms contributing to VVC pathogenesis have largely been identified using the C. albicans reference isolate SC5314. However, work with various clinical isolates, especially those obtained from women who are asymptomatically colonized or from vaginitis cases, should be a high priority to determine the relevancy of signaling pathways and effector mechanisms. For example, SC5314 expresses very high levels of ECE1, but is this representative of the majority of clinical isolates? Relatedly, how much candidalysin does C. albicans actually secrete, and are the relatively large concentrations used in cell culture assays physiologically representative? As the propensity to undergo morphogenetic switching across clinical isolates varies considerably, surely this, too, will impact pathogenicity [144]. Delineating genetic mechanisms that underlie vaginal strain-to-strain virulence, through the use of large-scale whole genome sequencing and genome-wide association studies, may identify pivotal regulons and novel therapeutic fungal targets to reduce VVC incidence.
Heterogenous virulence among Candida isolates likely plays a role in distinguishing asymptomatic colonization from symptomatic infection. However, there are equally likely host genetic mechanisms at play that intertwine with hormonal regulation, lifestyle, pharmacologic influence, and microbiome composition. Appropriately controlling for all such factors, even in well-controlled clinical studies, is incredibly difficult. Moreover, early immunologic or pathogenic events may be overlooked in such studies, as the symptomatic episode commands focus. Race may also play a factor in disease severity, as African American women report symptoms more frequently and with higher incidence than Caucasian women [145,146]. These disparities further complicate accurate modeling of the natural history of VVC pathogenesis. Although it is difficult to recruit volunteers for a sufficiently powered study, more live-challenge trials with controlled inocula and isogenic strains are needed to comprehensively elucidate the mechanisms leading to human VVC.
While the animal model of VVC has been an indispensable tool for dissecting mechanisms contributing to VVC, it does not come without caveats [147]. Firstly, the vaginal pH of women is generally acidic (<4.5), while the pH of mice is more neutral (~7.0). Mice, unlike women, do not naturally harbor C. albicans in the vagina, so introduction of fungal inocula represents a first-time encounter that may result in exacerbated immune reactions. Moreover, the delivery of a bolus dose of fungus is artificial and does not represent the outgrowth of endogenous Candida flora occurring in women. Mice also do not exhibit clinical signs of VVC (scratching, itching, vaginal redness). Thus, it is imperative to establish whether these responses are merely correlates of infection or actually drive disease symptoms. Caveats aside, the excellent availability of genetic knockouts, immunological reagents, and low cost will keep the mouse as the standard model system for studying VVC. However, improvements can be made. Identifying a mouse line(s) that more accurately presents with disease symptoms would be immensely useful. Perhaps the use of humanized mice produced to maintain human immune cells would provide novel insights into disease pathogenesis. The CRISPR/Cas9 revolution should also make engineering genetic variants, especially those identified with high frequency in RVVC subjects, more amenable to reflect functional differences in human alleles. Lastly, a model of recurrent disease has been a long-time need; unfortunately, until the factors that contribute to RVVC are better understood, this will likely be unattainable.
Candida vaginitis is a complicated disease, whose symptoms are governed by the intersection of host physiology, fungal biology, and the immunological response (Figure 2). The recognition of VVC as an immunopathology was a paradigm-shift that cannot be overstated [40]. While we have gained significant understanding of both host and fungal factors driving pathogenesis through this lens, there is still much progress to be made. As the global incidence of VVC is predicted to rise by tens of millions in the following decade, novel treatment modalities, improved diagnostics, and broad-scale pathogenomics studies will be required to lessen the impact of this currently unmet clinical need [3].
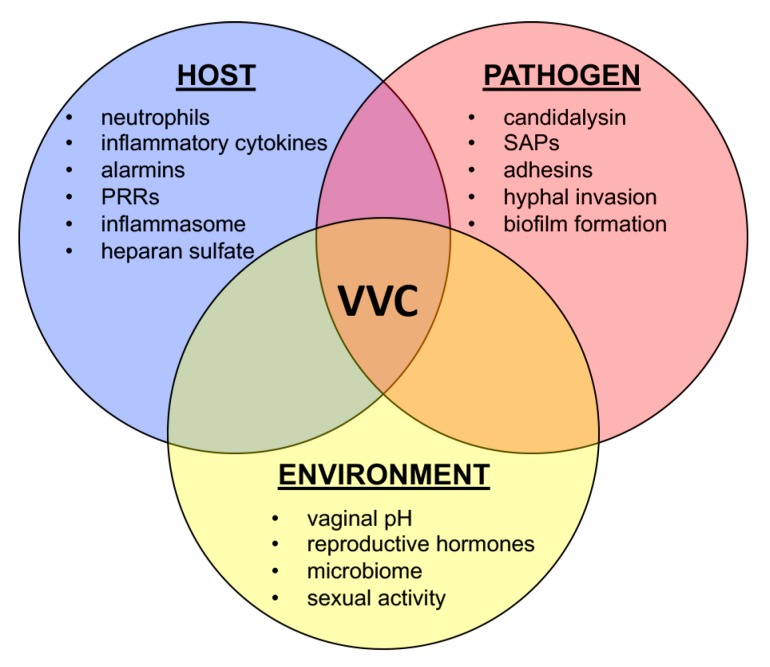
Figure 2.
Vulvovaginal candidiasis (VVC) is a multifactorial disease. Multiple inputs from host (blue), pathogen (red), and environment (yellow) are required to drive disease onset and symptomatic infection. Each circle represents major contributing factors to the immunopathogenesis of VVC.
Author Contributions
H.M.E.W., S.S.A., J.L., and B.M.P. contributed to manuscript synthesis. All authors participated in editing and revision. All authors have read and agreed to the published version of the manuscript.
Funding
Authors were supported by extramural funds provided by the National Institutes of Health – National Institute of Allergy and Infectious Disease by grants AI141829 and AI134796 awarded to BMP.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Achkar J.M., Fries B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010;23:253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobel J.D. Vaginitis. N. Engl. J. Med. 1997;337:1896–1903. doi: 10.1056/NEJM199712253372607. [DOI] [PubMed] [Google Scholar]
- 3.Denning D.W., Kneale M., Sobel J.D., Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018;18:e339–e347. doi: 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- 4.Sobel J.D., Faro S., Force R.W., Foxman B., Ledger W.J., Nyirjesy P.R., Reed B.D., Summers P.R. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 1998;178:203–211. doi: 10.1016/S0002-9378(98)80001-X. [DOI] [PubMed] [Google Scholar]
- 5.Nyirjesy P., Zhao Y., Ways K., Usiskin K. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr. Med. Res. Opin. 2012;28:1173–1178. doi: 10.1185/03007995.2012.697053. [DOI] [PubMed] [Google Scholar]
- 6.Fidel P.L., Jr. Distinct protective host defenses against oral and vaginal candidiasis. Med. Mycol. 2002;40:359–375. doi: 10.1080/714031126. [DOI] [PubMed] [Google Scholar]
- 7.Foxman B., Barlow R., D‘Arcy H., Gillespie B., Sobel J.D. Candida vaginitis: Self-reported incidence and associated costs. Sex. Transm. Dis. 2000;27:230–235. doi: 10.1097/00007435-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dave S., Peter T., Fogarty C., Karatzas N., Belinsky N., Pant Pai N. Which community-based HIV initiatives are effective in achieving UNAIDS 90-90-90 targets? A systematic review and meta-analysis of evidence (2007–2018) PLoS ONE. 2019;14:e0219826. doi: 10.1371/journal.pone.0219826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabeneck L., Crane M.M., Risser J.M., Lacke C.E., Wray N.P. A simple clinical staging system that predicts progression to AIDS using CD4 count, oral thrush, and night sweats. J. Gen. Intern. Med. 1993;8:5–9. doi: 10.1007/BF02600284. [DOI] [PubMed] [Google Scholar]
- 11.Redding S.W., Zellars R.C., Kirkpatrick W.R., McAtee R.K., Caceres M.A., Fothergill A.W., Lopez-Ribot J.L., Bailey C.W., Rinaldi M.G., Patterson T.F. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J. Clin. Microbiol. 1999;37:3896–3900. doi: 10.1128/JCM.37.12.3896-3900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh G.K., Capoor M.R., Nair D., Bhowmik K.T. Spectrum of fungal infection in head and neck cancer patients on chemoradiotherapy. J. Egypt. Natl. Canc. Inst. 2017;29:33–37. doi: 10.1016/j.jnci.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Patel R., Paya C.V. Infections in solid-organ transplant recipients. Clin. Microbiol. Rev. 1997;10:86–124. doi: 10.1128/CMR.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil S., Rao R.S., Majumdar B., Anil S. Clinical appearance of oral Candida infection and therapeutic strategies. Front. Microbiol. 2015;6:1391. doi: 10.3389/fmicb.2015.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WorldBank . Birth Rate, Crude (Per 1000 People) The World Bank Group; Washington, DC, USA: 2017. [(accessed on 22 January 2020)]. Available online: https://data.worldbank.org/indicator/SP.DYN.CBRT.IN. [Google Scholar]
- 16.WHO . Global Health Observatory. World Heatlh Organization; Geneva, Switzerland: 2017. [(accessed on 22 January 2020)]. Infant Mortality. Available online: https://www.who.int/gho/child_health/mortality/neonatal_infant_text/en/ [Google Scholar]
- 17.Sobel J.D., Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother. 2018;19:971–977. doi: 10.1080/14656566.2018.1476490. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy M.A., Sobel J.D. Vulvovaginal candidiasis caused by non-albicans Candida Species: New insights. Curr. Infect. Dis. Rep. 2010;12:465–470. doi: 10.1007/s11908-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 19.Parazzini F., Di Cintio E., Chiantera V., Guaschino S. Determinants of different Candida species infections of the genital tract in women. Sporachrom Study Geoup. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000;93:141–145. doi: 10.1016/S0301-2115(00)00289-X. [DOI] [PubMed] [Google Scholar]
- 20.Richter S.S., Galask R.P., Messer S.A., Hollis R.J., Diekema D.J., Pfaller M.A. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J. Clin. Microbiol. 2005;43:2155–2162. doi: 10.1128/JCM.43.5.2155-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dan M., Poch F., Levin D. High rate of vaginal infections caused by non-C. albicans Candida species among asymptomatic women. Med. Mycol. 2002;40:383–386. doi: 10.1080/mmy.40.4.383.386. [DOI] [PubMed] [Google Scholar]
- 22.Bitew A., Abebaw Y. Vulvovaginal candidiasis: Species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health. 2018;18:94–104. doi: 10.1186/s12905-018-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choukri F., Benderdouche M., Sednaoui P. In vitro susceptibility profile of 200 recent clinical isolates of Candida spp. to topical antifungal treatments of vulvovaginal candidiasis, the imidazoles and nystatin agents. J. Mycol. Med. 2014;24:303–307. doi: 10.1016/j.mycmed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Powell A.M., Gracely E., Nyirjesy P. Non-albicans Candida Vulvovaginitis: Treatment experience at a tertiary care vaginitis center. J. Low. Genit.Tract Dis. 2016;20:85–89. doi: 10.1097/LGT.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 25.Ray D., Goswami R., Banerjee U., Dadhwal V., Goswami D., Mandal P., Sreenivas V., Kochupillai N. Prevalence of Candida glabrata and its response to boric acid vaginal suppositories in comparison with oral fluconazole in patients with diabetes and vulvovaginal candidiasis. Diabetes Care. 2007;30:312–317. doi: 10.2337/dc06-1469. [DOI] [PubMed] [Google Scholar]
- 26.Hamad M., Kazandji N., Awadallah S., Allam H. Prevalence and epidemiological characteristics of vaginal candidiasis in the UAE. Mycoses. 2014;57:184–190. doi: 10.1111/myc.12141. [DOI] [PubMed] [Google Scholar]
- 27.Spinillo A., Capuzzo E., Gulminetti R., Marone P., Colonna L., Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am. J. Obstet. Gynecol. 1997;176:138–141. doi: 10.1016/S0002-9378(97)80026-9. [DOI] [PubMed] [Google Scholar]
- 28.Kalaiarasan K., Singh R., Chaturvedula L. Fungal profile of vulvovaginal candidiasis in a tertiary care hospital. J. Clin. Diagn. Res. 2017;11:DC06–DC09. doi: 10.7860/JCDR/2017/23578.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinillo A., Nicola S., Colonna L., Marangoni E., Cavanna C., Michelone G. Frequency and significance of drug resistance in vulvovaginal candidiasis. Gynecol. Obstet. Invest. 1994;38:130–133. doi: 10.1159/000292465. [DOI] [PubMed] [Google Scholar]
- 30.Moyes D.L., Murciano C., Runglall M., Kohli A., Islam A., Naglik J.R. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med. Microbiol. Immunol. 2012;201:93–101. doi: 10.1007/s00430-011-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willems H.M.E., Lowes D.J., Barker K.S., Palmer G.E., Peters B.M. Comparative analysis of the capacity of the candida species to elicit vaginal immunopathology. Infect. Immun. 2018;86 doi: 10.1128/IAI.00527-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong E., Dixit S., Fidel P.L., Bradford J., Fischer G. Vulvovaginal candidiasis as a chronic disease: Diagnostic criteria and definition. J. Low. Genit. Tract Dis. 2013 doi: 10.1097/LGT.0b013e318287aced. [DOI] [PubMed] [Google Scholar]
- 33.Hamad M., Abu-Elteen K.H., Ghaleb M. Estrogen-dependent induction of persistent vaginal candidosis in naive mice. Mycoses. 2004;47:304–309. doi: 10.1111/j.1439-0507.2004.00994.x. [DOI] [PubMed] [Google Scholar]
- 34.Cheng G., Yeater K.M., Hoyer L.L. Cellular and molecular biology of Candida albicans estrogen response. Eukaryot. Cell. 2006;5:180–191. doi: 10.1128/EC.5.1.180-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wira C.R., Fahey J.V., Sentman C.L., Pioli P.A., Shen L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 36.Fidel P.L., Jr., Lynch M.E., Redondo-Lopez V., Sobel J.D., Robinson R. Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis. J. Infect. Dis. 1993;168:1458–1465. doi: 10.1093/infdis/168.6.1458. [DOI] [PubMed] [Google Scholar]
- 37.Fong I.W., McCleary P., Read S. Cellular immunity of patients with recurrent or refractory vulvovaginal moniliasis. Am. J. Obstet. Gynecol. 1992;166:887–890. doi: 10.1016/0002-9378(92)91356-F. [DOI] [PubMed] [Google Scholar]
- 38.Mendling W., Koldovsky U. Investigations by cell-mediated immunologic tests and therapeutic trials with thymopentin in vaginal mycoses. Infect. Dis. Obstet. Gynecol. 1996;4:225–231. doi: 10.1002/(SICI)1098-0997(1996)4:4<225::AID-IDOG5>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fidel P.L., Jr., Lynch M.E., Sobel J.D. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect. Immun. 1995;63:2403–2408. doi: 10.1128/IAI.63.7.2403-2408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fidel P.L., Jr., Barousse M., Espinosa T., Ficarra M., Sturtevant J., Martin D.H., Quayle A.J., Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect. Immun. 2004;72:2939–2946. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudbery P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 42.Lo H.J., Kohler J.R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G.R. Nonfilamentous, C. Albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 43.Peters B.M., Palmer G.E., Nash A.K., Lilly E.A., Fidel P.L., Jr., Noverr M.C. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 2014;82:532–543. doi: 10.1128/IAI.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black C.A., Eyers F.M., Russell A., Dunkley M.L., Clancy R.L., Beagley K.W. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect. Immun. 1998;66:1273–1275. doi: 10.1128/IAI.66.3.1273-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng S.C., Joosten L.A., Kullberg B.J., Netea M.G. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012;80:1304–1313. doi: 10.1128/IAI.06146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moyes D.L., Murciano C., Runglall M., Islam A., Thavaraj S., Naglik J.R. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE. 2011;6:e26580. doi: 10.1371/journal.pone.0026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters B.M., Yano J., Noverr M.C., Fidel P.L., Jr. Candida vaginitis: When opportunism knocks, the host responds. PLoS Pathog. 2014;10:e1003965. doi: 10.1371/journal.ppat.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown G.D., Taylor P.R., Reid D.M., Willment J.A., Williams D.L., Martinez-Pomares L., Wong S.Y., Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferwerda B., Ferwerda G., Plantinga T.S., Willment J.A., van Spriel A.B., Venselaar H., Elbers C.C., Johnson M.D., Cambi A., Huysamen C., et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usluogullari B., Gumus I., Gunduz E., Kaygusuz I., Simavli S., Acar M., Oznur M., Gunduz M., Kafali H. The role of human dectin-1 Y238X gene polymorphism in recurrent vulvovaginal candidiasis infections. Mol. Biol. Rep. 2014;41:6763–6768. doi: 10.1007/s11033-014-3562-2. [DOI] [PubMed] [Google Scholar]
- 51.Carvalho A., Giovannini G., De Luca A., D’Angelo C., Casagrande A., Iannitti R.G., Ricci G., Cunha C., Romani L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell. Mol. Immunol. 2012;9:276–286. doi: 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yano J., Palmer G.E., Eberle K.E., Peters B.M., Vogl T., McKenzie A.N., Fidel P.L., Jr. Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect. Immun. 2014;82:783–792. doi: 10.1128/IAI.00861-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosentul D.C., Plantinga T.S., Oosting M., Scott W.K., Velez Edwards D.R., Smith P.B., Alexander B.D., Yang J.C., Laird G.M., Joosten L.A., et al. Genetic variation in the Dectin-1/CARD9 recognition pathway and susceptibility to candidemia. J. Infect. Dis. 2011;204:1138–1145. doi: 10.1093/infdis/jir458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babula O., Lazdane G., Kroica J., Ledger W.J., Witkin S.S. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin. Infect. Dis. 2003;37:733–737. doi: 10.1086/377234. [DOI] [PubMed] [Google Scholar]
- 55.Donders G.G., Babula O., Bellen G., Linhares I.M., Witkin S.S. Mannose-binding lectin gene polymorphism and resistance to therapy in women with recurrent vulvovaginal candidiasis. BJOG. 2008;115:1225–1231. doi: 10.1111/j.1471-0528.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 56.Jaeger M., Pinelli M., Borghi M., Constantini C., Dindo M., van Emst L., Puccetti M., Pariano M., Ricano-Ponce I., Bull C., et al. A systems genomics approach identifies SIGLEC15 as a susceptibility factor in recurrent vulvovaginal candidiasis. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aar3558. [DOI] [PubMed] [Google Scholar]
- 57.Jabra-Rizk M.A., Kong E., Tsui C., Nguyen M., Clancy C.J., Fidel P.L., Jr., Noverr M. Candida albicans pathogenesis: Fitting within the “host-microbe damage response framework”. Infect. Immun. 2016;84:2724–2739. doi: 10.1128/IAI.00469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lew R.R. How does a hypha grow? The biophysics of pressurized growth in fungi. Nat. Rev. Microbiol. 2011;9:509–518. doi: 10.1038/nrmicro2591. [DOI] [PubMed] [Google Scholar]
- 59.Wachtler B., Wilson D., Hube B. Candida albicans adhesion to and invasion and damage of vaginal epithelial cells: Stage-specific inhibition by clotrimazole and bifonazole. Antimicrob. Agents Chemother. 2011;55:4436–4439. doi: 10.1128/AAC.00144-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harriott M.M., Lilly E.A., Rodriguez T.E., Fidel P.L., Noverr M.C. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156:3635–3644. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phan Q.T., Myers C.L., Fu Y., Sheppard D.C., Yeaman M.R., Welch W.H., Ibrahim A.S., Edwards J.E., Jr., Filler S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roudbarmohammadi S., Roudbary M., Bakhshi B., Katiraee F., Mohammadi R., Falahati M. ALS1 and ALS3 gene expression and biofilm formation in Candida albicans isolated from vulvovaginal candidiasis. Adv. Biomed. Res. 2016;5:105. doi: 10.4103/2277-9175.183666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaller M., Korting H.C., Borelli C., Hamm G., Hube B. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect. Immun. 2005;73:2758–2765. doi: 10.1128/IAI.73.5.2758-2765.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaller M., Schackert C., Korting H.C., Januschke E., Hube B. Invasion of Candida albicans correlates with expression of secreted aspartic proteinases during experimental infection of human epidermis. J. Investig. Dermatol. 2000;114:712–717. doi: 10.1046/j.1523-1747.2000.00935.x. [DOI] [PubMed] [Google Scholar]
- 65.Borg-von Zepelin M., Beggah S., Boggian K., Sanglard D., Monod M. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 1998;28:543–554. doi: 10.1046/j.1365-2958.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 66.Bruno V.M., Shetty A.C., Yano J., Fidel P.L., Jr., Noverr M.C., Peters B.M. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. MBio. 2015;6 doi: 10.1128/mBio.00182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Bernardis F., Arancia S., Morelli L., Hube B., Sanglard D., Schafer W., Cassone A. Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for Candida vaginitis. J. Infect. Dis. 1999;179:201–208. doi: 10.1086/314546. [DOI] [PubMed] [Google Scholar]
- 68.Pericolini E., Gabrielli E., Amacker M., Kasper L., Roselletti E., Luciano E., Sabbatini S., Kaeser M., Moser C., Hube B., et al. Secretory Aspartyl Proteinases cause vaginitis and can mediate vaginitis caused by Candida albicans in mice. MBio. 2015;6:e00724. doi: 10.1128/mBio.00724-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willems H.M.E., Bruner W.S., Barker K.S., Liu J., Palmer G.E., Peters B.M. Overexpression of Candida albicans Secreted Aspartyl Proteinases 2 or 5 is not sufficient for exacerbation of immunopathology in a murine model of vaginitis. Infect. Immun. 2017 doi: 10.1128/IAI.00248-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaller M., Borelli C., Korting H.C., Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 71.Schofield D.A., Westwater C., Warner T., Balish E. Differential Candida albicans lipase gene expression during alimentary tract colonization and infection. FEMS Microbiol. Lett. 2005;244:359–365. doi: 10.1016/j.femsle.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Schofield D.A., Westwater C., Warner T., Nicholas P.J., Paulling E.E., Balish E. Hydrolytic gene expression during oroesophageal and gastric candidiasis in immunocompetent and immunodeficient gnotobiotic mice. J. Infect. Dis. 2003;188:591–599. doi: 10.1086/377182. [DOI] [PubMed] [Google Scholar]
- 73.Roustan J.L., Chu A.R., Moulin G., Bigey F. A novel lipase/acyltransferase from the yeast Candida albicans: Expression and characterisation of the recombinant enzyme. Appl. Microbiol. Biotechnol. 2005;68:203–212. doi: 10.1007/s00253-005-1896-3. [DOI] [PubMed] [Google Scholar]
- 74.Gacser A., Stehr F., Kroger C., Kredics L., Schafer W., Nosanchuk J.D. Lipase 8 affects the pathogenesis of Candida albicans. Infect. Immun. 2007;75:4710–4718. doi: 10.1128/IAI.00372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trofa D., Soghier L., Long C., Nosanchuk J.D., Gacser A., Goldman D.L. A rat model of neonatal candidiasis demonstrates the importance of lipases as virulence factors for Candida albicans and Candida parapsilosis. Mycopathologia. 2011;172:169–178. doi: 10.1007/s11046-011-9429-3. [DOI] [PubMed] [Google Scholar]
- 76.Birse C.E., Irwin M.Y., Fonzi W.A., Sypherd P.S. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect. Immun. 1993;61:3648–3655. doi: 10.1128/IAI.61.9.3648-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moyes D.L., Wilson D., Richardson J.P., Mogavero S., Tang S.X., Wernecke J., Hofs S., Gratacap R.L., Robbins J., Runglall M., et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bader O., Krauke Y., Hube B. Processing of predicted substrates of fungal Kex2 proteinases from Candida albicans, C. glabrata, Saccharomyces cerevisiae and Pichia pastoris. BMC Microbiol. 2008;8:116. doi: 10.1186/1471-2180-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richardson J.P., Mogavero S., Moyes D.L., Blagojevic M., Kruger T., Verma A.H., Coleman B.M., De La Cruz Diaz J., Schulz D., Ponde N.O., et al. Processing of Candida albicans ece1p is critical for candidalysin maturation and fungal virulence. MBio. 2018;9 doi: 10.1128/mBio.02178-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kasper L., Konig A., Koenig P.A., Gresnigt M.S., Westman J., Drummond R.A., Lionakis M.S., Gross O., Ruland J., Naglik J.R., et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018;9:4260. doi: 10.1038/s41467-018-06607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richardson J.P., Willems H.M.E., Moyes D.L., Shoaie S., Barker K.S., Tan S.L., Palmer G.E., Hube B., Naglik J.R., Peters B.M. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect. Immun. 2017 doi: 10.1128/IAI.00645-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rogiers O., Frising U.C., Kucharikova S., Jabra-Rizk M.A., van Loo G., Van Dijck P., Wullaert A. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. MBio. 2019;10 doi: 10.1128/mBio.02221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swidergall M., Khalaji M., Solis N.V., Moyes D.L., Drummond R.A., Hube B., Lionakis M.S., Murdoch C., Filler S.G., Naglik J.R. Candidalysin is required for neutrophil recruitment and virulence during systemic candida albicans infection. J. Infect. Dis. 2019 doi: 10.1093/infdis/jiz322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roselletti E., Monari C., Sabbatini S., Perito S., Vecchiarelli A., Sobel J.D., Cassone A. A role for yeast/pseudohyphal cells of Candida albicans in the correlated expression of NLRP3 inflammasome inducers in women with acute vulvovaginal Candidiasis. Front. Microbiol. 2019;10:2669. doi: 10.3389/fmicb.2019.02669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ho J., Yang X., Nikou S.A., Kichik N., Donkin A., Ponde N.O., Richardson J.P., Gratacap R.L., Archambault L.S., Zwirner C.P., et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat. Commun. 2019;10:2297. doi: 10.1038/s41467-019-09915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verma A.H., Richardson J.P., Zhou C., Coleman B.M., Moyes D.L., Ho J., Huppler A.R., Ramani K., McGeachy M.J., Mufazalov I.A., et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017;2 doi: 10.1126/sciimmunol.aam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peters B.M., Coleman B.M., Willems H.M.E., Barker K.S., Aggor F.E.Y., Cipolla E., Verma A.H., Bishu S., Huppler A.H., Bruno V.M., et al. The IL-17R/IL-22R signaling axis is dispensable for vulvovaginal candidiasis regardless of estrogen status. J. Infect. Dis. 2019 doi: 10.1093/infdis/jiz649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pietrella D., Rachini A., Pines M., Pandey N., Mosci P., Bistoni F., D‘Enfert C., Vecchiarelli A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS ONE. 2011;6:e22770. doi: 10.1371/journal.pone.0022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yano J., Kolls J.K., Happel K.I., Wormley F., Wozniak K.L., Fidel P.L., Jr. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS ONE. 2012;7:e46311. doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boisson B., Wang C., Pedergnana V., Wu L., Cypowyj S., Rybojad M., Belkadi A., Picard C., Abel L., Fieschi C., et al. A biallelic ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39:676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J., Casanova J.L., Puel A. Mucocutaneous IL-17 immunity in mice and humans: Host defense vs. excessive inflammation. Mucosal. Immunol. 2017 doi: 10.1038/mi.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 93.Altmeier S., Toska A., Sparber F., Teijeira A., Halin C., LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12:e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hise A.G., Tomalka J., Ganesan S., Patel K., Hall B.A., Brown G.D., Fitzgerald K.A. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borghi M., De Luca A., Puccetti M., Jaeger M., Mencacci A., Oikonomou V., Pariano M., Garlanda C., Moretti S., Bartoli A., et al. Pathogenic NLRP3 inflammasome activity during Candida infection is negatively regulated by IL-22 via activation of NLRC4 and IL-1Ra. Cell Host Microbe. 2015;18:198–209. doi: 10.1016/j.chom.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 96.Joly S., Ma N., Sadler J.J., Soll D.R., Cassel S.L., Sutterwala F.S. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J. Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lowes D.J., Hevener K.E., Peters B.M. The second-generation anti-diabetic sulfonylureas inhibit Candida albicans and Candidalysin mediated activation of the NLRP3 inflammasome. Antimicrob. Agents Chemother. 2019 doi: 10.1128/AAC.01777-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toth A., Zajta E., Csonka K., Vagvolgyi C., Netea M.G., Gacser A. Specific pathways mediating inflammasome activation by Candida parapsilosis. Sci. Rep. 2017;7:43129. doi: 10.1038/srep43129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pietrella D., Pandey N., Gabrielli E., Pericolini E., Perito S., Kasper L., Bistoni F., Cassone A., Hube B., Vecchiarelli A. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur. J. Immunol. 2013;43:679–692. doi: 10.1002/eji.201242691. [DOI] [PubMed] [Google Scholar]
- 100.Roselletti E., Perito S., Gabrielli E., Mencacci A., Pericolini E., Sabbatini S., Cassone A., Vecchiarelli A. NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans. Sci. Rep. 2017;7:17877. doi: 10.1038/s41598-017-17649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jaeger M., Carvalho A., Cunha C., Plantinga T.S., van de Veerdonk F., Puccetti M., Galosi C., Joosten L.A., Dupont B., Kullberg B.J., et al. Association of a variable number tandem repeat in the NLRP3 gene in women with susceptibility to RVVC. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:797–801. doi: 10.1007/s10096-016-2600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Byers S.L., Wiles M.V., Dunn S.L., Taft R.A. Mouse estrous cycle identification tool and images. PLoS ONE. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lasarte S., Samaniego R., Salinas-Munoz L., Guia-Gonzalez M.A., Weiss L.A., Mercader E., Ceballos-Garcia E., Navarro-Gonzalez T., Moreno-Ochoa L., Perez-Millan F., et al. Sex hormones coordinate neutrophil immunity in the vagina by controlling chemokine gradients. J. Infect. Dis. 2016;213:476–484. doi: 10.1093/infdis/jiv402. [DOI] [PubMed] [Google Scholar]
- 104.Relloso M., Aragoneses-Fenoll L., Lasarte S., Bourgeois C., Romera G., Kuchler K., Corbi A.L., Munoz-Fernandez M.A., Nombela C., Rodriguez-Fernandez J.L., et al. Estradiol impairs the Th17 immune response against Candida albicans. J. Leukoc. Biol. 2012;91:159–165. doi: 10.1189/jlb.1110645. [DOI] [PubMed] [Google Scholar]
- 105.Yano J., Peters B.M., Noverr M.C., Fidel P.L., Jr. Novel mechanism behind the immunopathogenesis of vulvovaginal candidiasis: “Neutrophil Anergy”. Infect. Immun. 2018;86 doi: 10.1128/IAI.00684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clemons K.V., Spearow J.L., Parmar R., Espiritu M., Stevens D.A. Genetic susceptibility of mice to Candida albicans vaginitis correlates with host estrogen sensitivity. Infect. Immun. 2004;72:4878–4880. doi: 10.1128/IAI.72.8.4878-4880.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yano J., Noverr M.C., Fidel P.L., Jr. Vaginal heparan sulfate linked to neutrophil dysfunction in the acute inflammatory response associated with experimental vulvovaginal candidiasis. MBio. 2017;8 doi: 10.1128/mBio.00211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma B., Forney L.J., Ravel J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012;66:371–389. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ceccarani C., Foschi C., Parolin C., D‘Antuono A., Gaspari V., Consolandi C., Laghi L., Camboni T., Vitali B., Severgnini M., et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019;9:14095. doi: 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen C., Song X., Wei W., Zhong H., Dai J., Lan Z., Li F., Yu X., Feng Q., Wang Z., et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017;8:875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong K.H., Hong S.K., Cho S.I., Ra E., Han K.H., Kang S.B., Kim E.C., Park S.S., Seong M.W. Analysis of the vaginal microbiome by next-generation sequencing and evaluation of its performance as a clinical diagnostic tool in vaginitis. Ann. Lab. Med. 2016;36:441–449. doi: 10.3343/alm.2016.36.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rampersaud R., Randis T.M., Ratner A.J. Microbiota of the upper and lower genital tract. Semin. Fetal Neonatal Med. 2012;17:51–57. doi: 10.1016/j.siny.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller E.A., Beasley D.E., Dunn R.R., Archie E.A. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2016;7:1936. doi: 10.3389/fmicb.2016.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Auger P., Joly J. Microbial flora associated with Candida albicans vulvovaginitis. Obstet. Gynecol. 1980;55:397–401. doi: 10.1097/00006250-198003000-00029. [DOI] [PubMed] [Google Scholar]
- 116.Liu M.B., Xu S.R., He Y., Deng G.H., Sheng H.F., Huang X.M., Ouyang C.Y., Zhou H.W. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS ONE. 2013;8:e79812. doi: 10.1371/journal.pone.0079812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McClelland R.S., Richardson B.A., Hassan W.M., Graham S.M., Kiarie J., Baeten J.M., Mandaliya K., Jaoko W., Ndinya-Achola J.O., Holmes K.K. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J. Infect. Dis. 2009;199:1883–1890. doi: 10.1086/599213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swidsinski A., Guschin A., Tang Q., Dorffel Y., Verstraelen H., Tertychnyy A., Khayrullina G., Luo X., Sobel J.D., Jiang X. Vulvovaginal candidiasis: Histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am. J. Obstet. Gynecol. 2019;220 doi: 10.1016/j.ajog.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 119.Pramanick R., Mayadeo N., Warke H., Begum S., Aich P., Aranha C. Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: Are they different from normal microbiota? Microb. Pathog. 2019;134:103599. doi: 10.1016/j.micpath.2019.103599. [DOI] [PubMed] [Google Scholar]
- 120.Zhou X., Westman R., Hickey R., Hansmann M.A., Kennedy C., Osborn T.W., Forney L.J. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect. Immun. 2009;77:4130–4135. doi: 10.1128/IAI.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Gregorio P.R., Silva J.A., Marchesi A., Nader-Macias M.E.F. Anti-Candida activity of beneficial vaginal lactobacilli in in vitro assays and in a murine experimental model. FEMS Yeast Res. 2019;19 doi: 10.1093/femsyr/foz008. [DOI] [PubMed] [Google Scholar]
- 122.Dos Santos C.I., Franca Y.R., Lima Campos C.D., Quaresma Bomfim M.R., Melo B.O., Assuncao Holanda R., Santos V.L., Gomes Monteiro S., Buozzi Moffa E., Souza Monteiro A., et al. Antifungal and antivirulence activity of vaginal Lactobacillus Spp. Products against Candida vaginal isolates. Pathogens. 2019;8:150. doi: 10.3390/pathogens8030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jang S.J., Lee K., Kwon B., You H.J., Ko G. Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci. Rep. 2019;9:8121. doi: 10.1038/s41598-019-44579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fuochi V., Cardile V., Petronio Petronio G., Furneri P.M. Biological properties and production of bacteriocins-like-inhibitory substances by Lactobacillus sp. strains from human vagina. J. Appl. Microbiol. 2019;126:1541–1550. doi: 10.1111/jam.14164. [DOI] [PubMed] [Google Scholar]
- 125.Wang S., Wang Q., Yang E., Yan L., Li T., Zhuang H. Antimicrobial compounds produced by vaginal Lactobacillus crispatus are able to strongly inhibit Candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front. Microbiol. 2017;8:564. doi: 10.3389/fmicb.2017.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buffo J., Herman M.A., Soll D.R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984;85:21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- 127.Ribeiro F.C., de Barros P.P., Rossoni R.D., Junqueira J.C., Jorge A.O. Lactobacillus rhamnosus inhibits Candida albicans virulence factors in vitro and modulates immune system in Galleria mellonella. J. Appl. Microbiol. 2017;122:201–211. doi: 10.1111/jam.13324. [DOI] [PubMed] [Google Scholar]
- 128.Coudeyras S., Jugie G., Vermerie M., Forestier C. Adhesion of human probiotic Lactobacillus rhamnosus to cervical and vaginal cells and interaction with vaginosis-associated pathogens. Infect. Dis. Obstet. Gynecol. 2008;2008:549640. doi: 10.1155/2008/549640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martinez R.C., Seney S.L., Summers K.L., Nomizo A., De Martinis E.C., Reid G. Effect of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiol. Immunol. 2009;53:487–495. doi: 10.1111/j.1348-0421.2009.00154.x. [DOI] [PubMed] [Google Scholar]
- 130.Wagner R.D., Johnson S.J. Probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J. Biomed. Sci. 2012;19:58. doi: 10.1186/1423-0127-19-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Seta F., Parazzini F., De Leo R., Banco R., Maso G.P., De Santo D., Sartore A., Stabile G., Inglese S., Tonon M., et al. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: A retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;182:136–139. doi: 10.1016/j.ejogrb.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 132.Murina F., Graziottin A., Vicariotto F., De Seta F. Can Lactobacillus fermentum LF10 and Lactobacillus acidophilus LA02 in a slow-release vaginal product be useful for prevention of recurrent vulvovaginal candidiasis?: A clinical study. J. Clin. Gastroenterol. 2014;48:S102–S105. doi: 10.1097/MCG.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 133.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., Reboli A.C., Schuster M.G., Vazquez J.A., Walsh T.J., et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Owen M.K., Clenney T.L. Management of vaginitis. Am. Fam. Physician. 2004;70:2125–2132. [PubMed] [Google Scholar]
- 135.Sobel J.D., Wiesenfeld H.C., Martens M., Danna P., Hooton T.M., Rompalo A., Sperling M., Livengood C., III, Horowitz B., Von Thron J., et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004;351:876–883. doi: 10.1056/NEJMoa033114. [DOI] [PubMed] [Google Scholar]
- 136.Marchaim D., Lemanek L., Bheemreddy S., Kaye K.S., Sobel J.D. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol. 2012;120:1407–1414. doi: 10.1097/AOG.0b013e31827307b2. [DOI] [PubMed] [Google Scholar]
- 137.Sobel J.D. Factors involved in patient choice of oral or vaginal treatment for vulvovaginal candidiasis. Patient Prefer. Adherence. 2013;8:31–34. doi: 10.2147/PPA.S38984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pericolini E., Gabrielli E., Ballet N., Sabbatini S., Roselletti E., Cayzeele Decherf A., Pelerin F., Luciano E., Perito S., Justen P., et al. Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis. Virulence. 2017;8:74–90. doi: 10.1080/21505594.2016.1213937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sobel J.D., Vazquez J., Lynch M., Meriwether C., Zervos M.J. Vaginitis due to Saccharomyces cerevisiae: Epidemiology, clinical aspects, and therapy. Clin. Infect. Dis. 1993;16:93–99. doi: 10.1093/clinids/16.1.93. [DOI] [PubMed] [Google Scholar]
- 140.Schmidt C.S., White C.J., Ibrahim A.S., Filler S.G., Fu Y., Yeaman M.R., Edwards J.E., Jr., Hennessey J.P., Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine. 2012;30:7594–7600. doi: 10.1016/j.vaccine.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ibrahim A.S., Luo G., Gebremariam T., Lee H., Schmidt C.S., Hennessey J.P., Jr., French S.W., Yeaman M.R., Filler S.G., Edwards J.E., Jr. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine. 2013;31:5549–5556. doi: 10.1016/j.vaccine.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uppuluri P., Singh S., Alqarihi A., Schmidt C.S., Hennessey J.P., Jr., Yeaman M.R., Filler S.G., Edwards J.E., Ibrahim A.S. Human anti-Als3p antibodies are surrogate markers of NDV-3A vaccine efficacy against recurrent vulvovaginal candidiasis. Front. Immunol. 2018;9:1349. doi: 10.3389/fimmu.2018.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Bernardis F., Amacker M., Arancia S., Sandini S., Gremion C., Zurbriggen R., Moser C., Cassone A. A virosomal vaccine against candidal vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine. 2012;30:4490–4498. doi: 10.1016/j.vaccine.2012.04.069. [DOI] [PubMed] [Google Scholar]
- 144.Cavalieri D., Di Paola M., Rizzetto L., Tocci N., De Filippo C., Lionetti P., Ardizzoni A., Colombari B., Paulone S., Gut I.G., et al. Genomic and phenotypic variation in morphogenetic networks of two Candida albicans isolates subtends their different pathogenic potential. Front. Immunol. 2017;8:1997. doi: 10.3389/fimmu.2017.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Geiger A.M., Foxman B. Risk factors for vulvovaginal candidiasis: A case-control study among university students. Epidemiology. 1996;7:182–187. doi: 10.1097/00001648-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 146.Foxman B., Marsh J.V., Gillespie B., Sobel J.D. Frequency and response to vaginal symptoms among white and African American women: Results of a random digit dialing survey. J. Womens Health. 1998;7:1167–1174. doi: 10.1089/jwh.1998.7.1167. [DOI] [PubMed] [Google Scholar]
- 147.Cassone A., Sobel J.D. Experimental models of vaginal candidiasis and their relevance to human candidiasis. Infect. Immun. 2016;84:1255–1261. doi: 10.1128/IAI.01544-15. [DOI] [PMC free article] [PubMed] [Google Scholar]