Abstract
Phytoestrogens are nonsteroidal plant compounds with similar chemical structures to mammalian estrogen capable of mimicking the effect of estrogen in selective tissues. A diet rich in phytoestrogens is associated with a variety of health benefits including decreased risks for heart disease, breast cancer, and osteoporosis. Obesity has long thought to be associated with improved bone density due to increased mechanical loading, but recent literature suggests obesity may actually decrease bone health. Daidzein, a soy-derived phytoestrogen, has been shown to improve parameters of bone health in lean animal models of osteoporosis but has not been tested in obese animals. Following a one-week acclimation to a standard AIN-93G diet, 19 five-week-old female obese Zucker rats (OZR) were randomly assigned to a modified AIN-93G diet containing either high daidzein (HD, 0.121g/kg feed) or low daidzein (LD, 0.01g/kg feed). After 8 weeks, tibias and femurs were removed to assess true density (Archimedes principal), mechanical strength (three-point bending test), and femoral osteogenic gene expression. Serum was collected to assess osteocalcin and deoxypyridinoline. Our results indicated that there were no significant differences between the measures for tibial or femoral true density or mechanical strength for the rats in the HD and LD diet groups. Similarly, there were no significant differences in gene expressions related to osteogenic pathways, or serum biomarkers of bone formation and resorption. Overall, an increased dose of daidzein from soy protein supplementation does not elicit an improvement in markers of bone health in obese Zucker rats.
Keywords: osteoporosis, bone, obesity, soy isoflavone, Zucker rats
INTRODUCTION
Obesity and osteoporosis are global health issues associated with significant morbidity and mortality. According to the most recent epidemiological data from the National Health and Nutrition Examination Survey (NHANES 2015–2016), greater than one-third (39.8%) of American adults are currently obese (body mass index, BMI ≥ 30 kg/m2).1 Additionally, obesity is now considered a disease, which independently increases risk for chronic diseases such as type 2 diabetes mellitus (T2DM), cardiovascular diseases, hypertension, stroke, and some types of cancer.2
Paradoxically, obesity has been historically associated with a protective effect against osteoporosis, presumably due to the link between obesity and increased bone mineral density (BMD), which is the primary predictor of osteoporosis.3–5 The relationship between BMD and excess body weight has been attributed to possible mechanisms involving the mechanical loading effect on bone from increased body weight, as well as increased levels of adipose tissue-derived hormones, such as estrone and leptin.6, 7 For example, estrone was shown to be positively associated with BMD in postmenopausal women.6 Additionally, early in vitro studies have demonstrated a role for leptin in increasing osteoblast differentiation.7 However, more recent analyses indicate that the detrimental effects of common obesity induced factors, such as increased inflammation and oxidative stress, may counteract the benefits of body weight on BMD.8, 9 In support of this hypothesis, evidence from the Global Longitudinal Study of Osteoporosis in Women revealed similar prevalence and incidence of fragility fractures, particularly in the ankle, upper leg, and vertebrae, in postmenopausal women who were obese compared to non-obese. However, postmenopausal obese women were also significantly less likely to be receiving bone protective treatment, possibly due to the aforementioned misconception of intrinsic protection.10
Hormone replacement therapy (HRT) has proved to be the most effective treatment of osteoporosis, but presents several health risks including those for coronary artery disease, stroke, and certain forms of cancer.11 The Women’s Health Initiative study raised concerns about the safety of HRT, and, subsequently, women’s usage of HRT was reported to significantly decrease.12, 13 As a response to these reports, search for natural alternatives to prescription medications have increased.14 Soy foods, in particular, have received considerable attention due to their rich source of health-promoting nutrients and phytochemicals, including isoflavones.15 Isoflavones are phytoestrogens, or estrogen-like plant compounds found in soybeans and other leguminous plants may structurally and functionally mimic 17 β-estradiol (E2), the most abundant form of human estrogen, by weakly binding to estrogen receptors (ERs) in estrogen responsive tissue.16
Several epidemiological studies support the observation that postmenopausal women with greater consumption of phytoestrogens have higher correlating values for BMD.17–19 Other studies have shown similar findings in postmenopausal women, but show no effect in premenopausal women.20 Clinical interventions with isoflavones by Huang, et al.21 and Chi, et al.22 at 100 mg/day and 90 mg/day, respectively, showed that supplementation for 6 months or 1 year could significantly increase BMD in postmenopausal women. However, in the former study, a treatment of 200 mg/day did not produce the same results, perhaps indicating a dose dependence to treatment effect.21 To date, there have not been any reported epidemiological or intervention studies observing the effects of isoflavones specifically on obese populations.
Early research hypothesized that isoflavones were likely responsible for the bone sparing effects of soy in ovariectomized (OVX) rat models of osteoporosis.23 However, a study by Picheret et, al. indicated that mixed isoflavone consumption was not effective at increasing bone density in obese Zucker (fa/fa) rats (OZR).24 Other research focused on identifying whether a single isoflavone could be responsible for the bone protective effects of soy. Daidzein, when compared directly to other major isoflavones or in combination, has been shown to be the most effective in preserving bone health in animal models of postmenopausal bone loss.25, 26 Daidzein, and its metabolite equol, have been shown to suppress bone loss in young27 and old rats25, positively affect the biological markers of bone formation, osteocalcin, and bone resorption, deoxypyridinoline (DPD)25, as well as preserve parameters of bone histomorphometry26, 28 in OVX rats. While studies have elucidated daidzein’s positive effects on bone health stratified by age, sex, and menopausal status, it remains unclear whether daidzein alone will exhibit a similar effect on an obese population. Therefore, the purpose of this study was to investigate whether a high or low daidzein diet will affect the bone density or osteogenic gene expression in female OZR. We hypothesized that a diet high in daidzein would results in greater bone density and osteogenic gene expression, relative to a low daidzein diet, in female OZR.
MATERIALS AND METHODS
Animal Procedures
All experiments were approved by the Institutional Animal Care and Use Committee (Protocol # 3642) at the University of Arkansas for Medical Sciences. Nineteen (n = 19) five-week-old female OZR were purchased from Harlan Industries (Indianapolis, IN), for use in this study. Rats acclimated to the housing facility for one week prior to beginning the 8-week diet intervention. Rats were individually housed in cages receiving 12-hour light and dark cycles and provided with ad libitum access to both feed and standard drinking water. Upon completion of the study, rats were anesthetized by exposure to carbon dioxide (CO2) and subsequently euthanized via decapitation. Right femurs and tibias were dissected, cleaned of muscle and connective tissue, and snap frozen in liquid nitrogen and stored at −80°C. Left femurs and tibias were dissected, wrapped in saline (0.9% NaCl) soaked gauze, and stored at −20°C.
Diets
OZR were randomly assigned to either a high daidzein (0.121 g/kg; n = 10) modified AIN-93G diet , or a low daidzein (0.01 g/kg; n = 10) modified AIN-93G diet, and continued with this diet for 8 weeks thereafter as previously described30. High daidzein (HD) and low daidzein (LD) modified AIN-93G diets were matched to levels of daidzein found in a high isoflavone soy protein isolate (HISPI, 0.121g daidzein/kg) diet or low isoflavone soy protein isolate (LISPI, 0.01g daidzein/kg) diet, accordingly. With the exception of the level of daidzein, the formulas for the diets were identical; thus, the low-daidzein diet group served as control. Each diet contained 3.8 kcal/g of feed. The percentage of kilocalories from macronutrients were as follows: 18.8% protein, 64% carbohydrates, and 17.2% fat. The compositions of both LD and HD diets are listed in Table 1.
Table 1.
Diet Formula
| Ingredient | g/kga |
|---|---|
| Casein | 200.0 |
| L-cystine | 3.0 |
| Corn starch | 397.5 |
| Maltodextrin | 132.0 |
| Sucrose | 100.0 |
| Corn oil | 70.0 |
| Cellulose | 50.0 |
| Mineral mix, AIN-93G-MX | 35.0 |
| Vitamin mix, AIN-93G-VX | 10.0 |
| Choline bitartrate | 2.5 |
| TBHQ, antioxidant | 0.014 |
| Low daidzein | 0.01 |
| High daidzein | 0.121 |
Grams of ingredient per kilogram of feed
Body Weight and Energy Intake
Following the acclimation period, rats were randomized to diet groups facilitating an equalization of baseline mean body weights between the groups, which has been reported elsewhere29. Rats were weighed twice weekly for the duration of the study. Feed intake was measured once weekly and occurred over two days. Body weight and energy intake were collected as previously described30.
Determination of Bone Mineral Density
Left femurs and tibias were first hydrated in distilled water at reduced atmospheric pressure (15 in/Hg) for one hour. After hydration, the weights of the bones were measured both submerged in and out of water using the Mettler Toledo Density Kit ME-DNY-4. Bone densities (g/cm3) were determined using Archimedes Principle and calculated by the following formula:
where A=weight of hydrated bone out of water, B=weight of hydrated bone submerged in water, and P=density of distilled water at 24°C.
RNA Isolation and Purification
A Spex 6700 freezer mill (SPEX Sample Prep, Metuchen, NJ) was used to pulverize the heads of right femurs, and extraction of RNA was executed by use of TRI Reagent (Sigma-Aldrich, Bellefonte, PA) solution per manufacturers protocols. After RNA extraction, purity and concentration were assessed by reading the absorbance at A260 nm and the ratio of A260/280 nm on a Nano-Drop 1000A Spectrophotometer (Fisher Scientific LLC, Hanover Park, IL).
Analysis of Osteogenic Gene Expression
Reverse transcription of femoral RNA to cDNA was performed using an iScript cDNA Synthesis Kit, according to the manufacturer’s protocol. Genes of interest included TNF superfamily member 11 (Tnfsf11), TNF receptor superfamily member 11A (Tnfrsf11a), TNF receptor superfamily member 11B (Tnfrsf11b), NADPH oxidase 4 (Nox4), bone gamma-carboxyglutamate protein (Bglap), sclerostin (Sost), dickkopf WNT signaling pathway inhibitor 1 (Dkk1), 5’ nucleotidase, ecto (Nt5e), Wnt family member 3A (Wnt3a), axin 1 (Axin1), runt-related transcription factor 2 (Runx2), and LDL receptor related protein 5 (Lrp5). Genes were normalized to the housekeeping gene, Glyceraldehyde-3-phosphate-dehydrogenase (Gapdh). For each gene being investigated, a master-mix consisting of nuclease free water, the corresponding forward and reverse primer, and SsoAdvanced Universal SYBR Green Supermix was prepared. cDNA samples were then diluted to a concentration of 230 ng/μl based on calculations enabling two technical replicates. In a 384 well plate, 1μl of diluted cDNA and 9 μl of corresponding master-mix were added per well. After covering with MicroAMP Optical Adhesive Film and centrifuging at 1000 rpm for 1 minute, the samples were loaded into a ViiA Real-Time quantitative polymerase chain reaction (qPCR) Detection System set for 40 cycles. Each cycle was performed under the following conditions: denaturation of cDNA strands at 95ºC for 15 seconds, and annealing of the primer and extension of the complementary DNA at 60°C for 1 minute. Calculation of the Ct values and analysis of gene expression amplification plots was performed with the ViiA 7 Software Version 1.1. Differences in gene expression between diet groups were calculated using the delta-delta-CT method.
Analysis of Blood Biomarkers
Blood was collected and serum was separated by centrifuging tubes at 3000 × g at 4ºC and stored at −20ºC until time of analysis. Serum concentration values of deoxypyridinoline (DPD) and osteocalcin were measured with a MicroVue Bone tDPD Assay (Quidel, San Diego, CA; Catalog # 8032) and Rat Osteocalcin enzyme-linked immunosorbent assay (ELISA) Kit (Immunotopics, Inc., San Clemente, CA; Catalog # 60–1505), respectively. All assay procedures were administered according to manufacturers’ protocols.
Analysis of Mechanical Strength
Left femurs and tibias were thawed at room temperature, fully hydrated, and measured by caliper to ascertain dimensions for length, width, and height. A TA.XT Plus texture analyzer (Manufacture, City, State) with a 50-kg load cell and a three-point bending rig was used to analyze texture and mechanical properties of bone as an estimation for diaphysis strength and fracturability. Supported horizontally at both ends with a gap distance of 20 mm, femurs were placed with the posterior side facing down and the tibiae were placed on their lateral side. A 3mm width blade travelling 15mm downwards at 1mm/s compressed and fractured the bones on the anterior side at the midpoint between the supports. Force and displacement data, including ultimate load (N), displacement-to-ultimate (mm), stiffness (N/mm), and energy-to-ultimate (mJ) were recorded at 200 Hz by the Exponent software (Version 6, 1, 10, 0; Stable Micro System; Godalming, United Kingdom). In order to account for size variation in samples, Young’s modulus of bend (GPa) was calculated as follows:
where L = bone length, F = breaking force, w = bone width, h = bone thickness, d = breaking distance.
Statistical Analysis
All statistical analyses were conducted with IBM SPSS Statistics Version 22 (IBM, Armonk, NY). Statistical significance was determined at a p-value of p ≤ 0.05. Student’s t-tests were conducted to compare LD and HD mean body weights, bone mineral density, and osteogenic gene expression of femurs. Results are expressed in terms of mean ± standard deviation (SD).
RESULTS
Body Weight
Mean body weights between groups followed similar trends but began to differentiate over the course of the experiment29. At the end of the experiment (age 98 days), body weights (means ± SD) per group were 486 ± 30 g and 476 ± 24 g for LD and HD, respectively. Parametric testing on end-point mean body weights revealed that the difference between the LD and HD diet group means was not statistically significant (p = 0.408).
Energy Intake
The weakly mean ± SD kilocalorie intakes were 267 ± 97 kcal kg−1 for the LD group and 265.2 ± 93 kcal kg−1 for the HD group, which were not statistically significantly different according to parametric testing (p = 0.9). However, similar to the slight differentiation observed with average feed intake, the end-point mean kcal kg−1 for LD versus HD was 190 ± 13 kcal kg−1 and 176 ± 13.9 kcal kg−1 , respectively. Because the data in the LD group were not normally distributed, a Mann–Whitney U test was performed to compare the difference between the end-point kcal kg−1 values of the diet groups. Distributions of kcal kg−1 intake for LD and HD groups were not similar, as assessed by visual inspection. Energy intake for the LD group (mean rank = 12.67) trended towards being significantly higher than for the HD group (mean rank = 7.6): U = 21.0, z = −1.96, p = 0.053. Overall, kcal kg−1 intake between the diet groups did not significantly differ throughout the experiment.
Bone Mineral Density
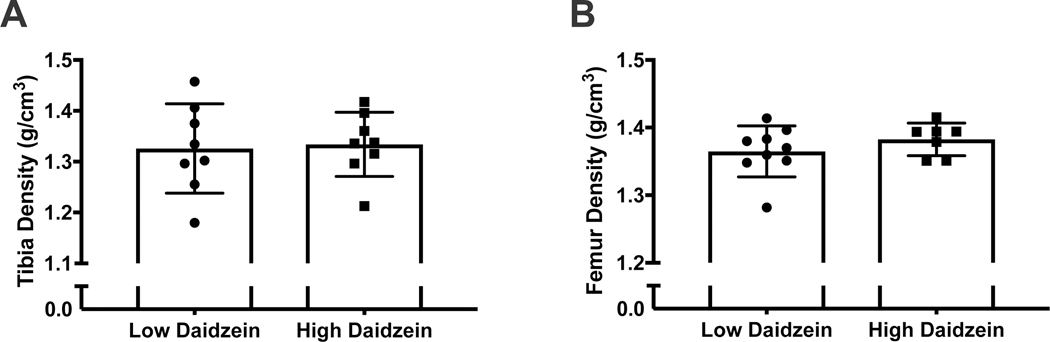
True density measurements (g/cm3) of tibias were 1.33±0.09 and 1.33 ±0.06 for the LD and HD groups, respectively (p = 0.0835) (Figure 1, A). Mean true density measurements of femurs were 1.36 ±0.04 for the LD group and 1.38±0.02 for the HD group (p = 0.30) (Figure 1, B).
figure 1.
dietary daidzein concentration does not influence tibia and femur density. a) tibia and b) femur density in low (0.01 g/kg) and high daidzein (0.121 g/kg) fed ozr.
Gene Expression
Gene expression results were expressed in terms of fold change in gene expression from a low to high daidzein treatment normalized to Gapdh, as well as by statistical differences in delta-delta CT values between the groups. No differences in gene expression were found for either measure (see Table 2).
Table 2.
Fold Change and Relative Significance of Osteogenic Gene Expression Between Obese Zucker Rats Fed Low and High Daidzein Diets
| Gene | Fold Change | P value |
|---|---|---|
| RankL | 1.038 | 0.439 |
| Rank | 0.946 | 0.645 |
| OPG | 1.038 | 0.363 |
| Nox4 | 0.911 | 0.429 |
| Bglap | 1.115 | 0.173 |
| Sost | 0.992 | 0.909 |
| Dkk1 | 0.989 | 0.502 |
| Ctnnb1 | 1.001 | 0.931 |
| Wnt3a | 0.999 | 0.973 |
| Axin1 | 0.870 | 0.485 |
| Runx2 | 1.046 | 0.761 |
| LRP5 | 0.996 | 0.99 |
Fold Change = fold increase or decrease between low and high daidzein gene expression as measured by rt-qPCR.
Mechanical Strength
Biomechanical properties of strength were measured by the three-point bending test and expressed in terms of ultimate force, displacement, and energy-to-ultimate. Mean measurements for mechanical properties, including ultimate force in tibias (p = 0.64) or femurs (p = 0.49), displacement for tibias (p = 0.19) or femurs (p = 0.16), and energy-to-ultimate in tibias (p = 0.36) and femurs (p = 0.09), were not statistically significant between the LD and HD groups (see Table 3).
Table 3.
Measurements of Mechanical Strength of Femurs and Tibias Between Low and High Daidzein Groups
| Measurement | LD Femurs | HD Femurs | LD Tibias | HD Tibias |
|---|---|---|---|---|
| Length (cm) | 3.2 ± 0.13 | 3.1 ± 0.13 | 3.5 ± 0.13 | 3.6 ± 0.13 |
| Ultimate Load (N) | 91.8 ± 8.97 | 89.1 ± 6.62 | 57.2 ± 5.26 | 55.9 ± 5.27 |
| Displacement to Ultimate (mm) | 1.4 ± 0.37 | 1.7 ± 0.39 | 2.1 ± 0.71 | 1.6 ± 0.36 |
| Energy to Ultimate (mJ) | 68.8 ± 16.83 | 56.0 ± 11.80 | 30.8 ± 11.48 | 36.28 ± 10.94 |
All values expressed as mean ± standard deviation.
Serum Biomarkers
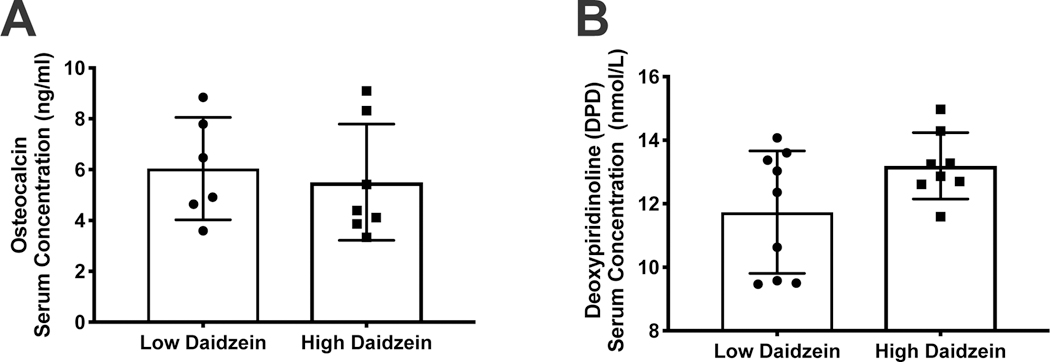
Serum markers of bone turnover, osteocalcin and DPD, were analyzed for comparison between the low and high daidzein diet groups. The mean concentration for serum osteocalcin was measured at 6.04±2.01 ng/ml in the LD and 5.50±2.29 ng/ml in the HD group. Mean concentration for serum DPD was measured at 11.73±1.93 nmol/L in the LD group and 13.19±1.04 nmol/L in the HD group. No statistically significant differences between the groups was observed for either osteocalcin or DPD. (Figures 2 A, B)
figure 2.
dietary daidzein concentration does not influence serum concentrations of osteocalcin and deoxypiridinoline. serum concentration of a) osteocalcin and b) deoxypiridinoline in low (0.01 g/kg) and daidzein (0.121 g/kg) fed ozr.
DISCUSSION
Although obesity is a disease and a chronic disease risk factor, historically obesity has been viewed as advantageous to bone health due to the protective effect of mechanical loading.2, 3 Conversely, several epidemiological studies have shown similar risks for fragility fractures in obese populations, leading to the hypothesis that bone protection may be overridden by common obesity-induced factors such as oxidative stress and hormone dysregulation.8, 10 The reported dangers of HRT have led many to seek natural alternatives to bone protective treatments, including isoflavones, of which daidzein (10 μg/[gram body weight] for 12 weeks and 50 μg/[gram body weight] for 4 weeks) has been shown to be the most effective in various animal models of bone loss.25–27 However, it remained to be determined whether daidzein treatment could be beneficial to an obese population. Therefore, the objective of this study was to observe the effects of a low and high daidzein diet on the bone density and osteogenic gene expression in OZR. Importantly, the high daidzein dose (~ 10 μg/gram body weight/day) was chosen to mimic the lowest beneficial dose reported in previous studies25.
Although previous studies have demonstrated a bone-protective effect of daidzein (10–50 μg/gram body weight/day) 25–27, and an increase in osteogenic gene expression in in vitro osteoblast cell cultures30, the present study suggests there is no difference in bone density and osteogenic gene expression in groups treated with low (~ 1 μg/[gram body weight] ) and high doses (~ 10 μg/[g body weight]) of daidzein. Unlike previous studies, in which daidzein increased bone density in OVX rat models, the results of our study indicate that the true bone densities of tibias and femurs were not significantly different between obese rats following LD and HD daidzein treatment. Similarly, we did not find any significant differences between the LD and HD groups regarding the expression of several genes involved in the maintenance of bone health. Lastly, daidzein supplementation did not significantly affect serum markers of bone formation, osteocalcin and bone resorption.
However, several results of this study support previous findings, which showed that a treatment of mixed isoflavones had no significant effect on measures of bone health in OZR, despite the relative low bone mass observed in the phenotype. Our results further support the hypothesis that the detrimental effects of obesity on bone health may be more attributable to oxidative stress and leptin resistance, both hallmarks of the OZR model, rather than a deficiency in estrogen like other models of osteoporosis. Therefore, it may be more appropriate in future research to explore treatments, which attenuate the effects of oxidative stress and inflammation when studying obesity-associated osteoporosis rather than traditional methods, which focus on hormone replacement.
Obese white adipose tissue contains an abundance of pro-inflammatory cytokines that can stimulate the differentiation and proliferation of osteoclasts through activation of RANKL and osteoprotogerin31–33. Furthermore, superoxide-driven oxidative stress, a common factor in obese adipose tissue, has been shown to stimulate osteoclastogenesis, bone matrix degradation and bone resorption34–36. Additionally, white adipose tissue-derived leptin, is increased with obesity and has been implicated in obesity associated osteoporosis, although results from in vivo studies are mixed.37 A previous publication from this study revealed no difference in serum leptin between low and high daidzein treated OZR.29 The lack of differences in body weight between groups in the present study may explain why we did not observe differences in measures of bone density and osteogenesis.
The current study is not without several limitations: 1) The intervention was relatively short in length. Perhaps we would have observed differences with a longer term daidzein intervention; 2) We may have seen differences with a higher dose of daidzein over the 8-week intervention period. However, the high daidzein group received ~ 10 μg/g body weight/day of daidzein, which would equate to 700 mg/day for a 70kg human, which is far beyond the dose (100–200 mg/d)21,22 of other isoflavones that have shown to be protective against bone loss. Thus, increasing the dose may reduce the translational potential of the intervention; 3) Although the rats consuming the diet with a low level of daidzein are considered a control since we added less than 0.01 g daidzein/kg (low level of daidzein was chosen to match the amount of daidzein found in isoflavone-free or low-isoflavone soy protein isolate) this design did allow us to isolate the independent affects of obesity on bone loss, which may have allowed a greater chance of detecting measurable differences; 4) Moreover, the specific phenolic metabolites of daidzein were not quantified in urine or other tissues, which could provide insight into the lack of response observed in the current study.
In summary, results of the present study indicate that there is not a dose-dependent response between low and high daidzein treatment on bone density and markers of osteogenesis in female obese Zucker rats. Importantly, the mechanisms by which bone health is negatively affected in obese populations, remains to be fully elucidated, and may dictate treatments distinct from those used in hormone deficient osteoporosis.
Acknowledgements:
This study was supported in-part by The College of Medicine’s University Medical Group (RH), the ACRI Student and Clinical Staff Intramural Grant (AB), and the Arkansas Biosciences Institute (RH). ZSC is currently supported by the National Institutes of Health award T32 DK007135–44.
Footnotes
Disclosure Statement: No competing financial interests exist.
References
- 1.Hales CM, Carroll MD, Fryar CD and Ogden CL, Prevalence of obesity among adults and youth: United States, 2015–2016, NCHS data brief, 2017, 288, 1–8. [PubMed] [Google Scholar]
- 2.Kopelman P, Health risks associated with overweight and obesity, Obes. Rev, 2007, 8, 13–17. [DOI] [PubMed] [Google Scholar]
- 3.Castro JP, Joseph LA, Shin JJ, Arora SK, Nicasio J, Shatzkes J, Raklyar I, Erlikh I, Pantone V, Bahtiyar G, Chandler L, Pabon L, Choudhry S, Ghadiri N, Gosukonda P, Muniyappa R, von-Gicyzki H. and McFarlane SI, Differential effect of obesity on bone mineral density in White, Hispanic and African American women: a cross sectional study, Nutr. Metab, 2005, 2, 9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Da Silva HGV, Mendonca LMC, Conceicao FL, Zahar SEV and Farias MLF, Influence of obesity on bone density in postmenopausal women, Arq. Bras. Endocrinol. Metabol, 2007, 51, 943–949. [DOI] [PubMed] [Google Scholar]
- 5.Arimatsu M, Kitano T, Kitano N, Inomoto T, Shono M. and Futatsuka M, Correlation between forearm bone mineral density and body composition in Japanese females aged 18–40 years, Environ. Health Prev. Med, 2005, 10, 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki N, Yano T, Nakazawa N, Yoshikawa H. and Taketani Y, A possible role of estrone produced in adipose tissues in modulating postmenopausal bone-density, Maturitas, 1995, 22, 9–12. [DOI] [PubMed] [Google Scholar]
- 7.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B. and Riggs BL, Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes, Endocrinology, 1999, 140, 1630–1638. [DOI] [PubMed] [Google Scholar]
- 8.Shapses SA, Pop LC and Wang Y, Obesity is a concern for bone health with aging, Nutr. Res, 2017, 39, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L-J, Liu Y-J, Liu P-Y, Hamilton J, Recker RR and Deng H-W, Relationship of obesity with osteoporosis, J. Clin. Endocrinol. Metab, 2007, 92, 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, Pfeilschifter J, Silverman S, Diez-Perez A, Lindsay R, Saag KG, Netelenbos JC, Gehlbach S, Hooven FH, Flahive J, Adachi JD, Rossini M, Lacroix AZ, Roux C, Sambrook PN and Siris ES, Obesity is not protective against fracture in postmenopausal women: GLOW, Am. J. Med, 2011, 124, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ and Wallace RB, Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials, JAMA, J. Am. Med. Assoc, 2013, 310, 1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawton B, Rose S, McLeod D. and Dowell A, Changes in use of hormone replacement therapy after the report from the Women’s Health Initiative: cross sectional survey of users, Br. Med. J, 2003, 327, 845–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC, Mamdani MM, Tu K. and Jaakkimainen L, Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women’s Health Initiative study, JAMA, J. Am. Med. Assoc, 2003, 289, 3241–3242. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM and Ockene J, Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial, JAMA, J. Am. Med. Assoc, 2002, 288, 321–333. [DOI] [PubMed] [Google Scholar]
- 15.Messina M, Soy foods, isoflavones, and the health of postmenopausal women, Am. J. Clin. Nutr, 2014, 100, 423S–430S. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B. and J.-A. k. Gustafsson, Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β, Endocrinology, 1998, 139, 4252–4263. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Shu XO, Li H, Yang G, Li Q, Gao YT and Zheng W, Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women, Arch. Intern. Med, 2005, 165, 1890–1895. [DOI] [PubMed] [Google Scholar]
- 18.Koh WP, Wu AH, Wang R, Ang LW, Heng D, Yuan JM and Yu MC, Gender-specific associations between soy and risk of hip fracture in the Singapore Chinese Health Study, Am. J. Epidemiol, 2009, 170, 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somekawa Y, Chiguchi M, Ishibashi T. and Aso T, Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women, Obstet. Gynecol, 2001, 97, 109–115. [DOI] [PubMed] [Google Scholar]
- 20.Mei J, Yeung SSC and Kung AWC, High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women, J. Clin. Endocrinol. Metab, 2001, 86, 5217–5221. [DOI] [PubMed] [Google Scholar]
- 21.Huang HY, Yang HP, Yang HT, Yang TC, Shieh MJ and Huang SY, One-year soy isoflavone supplementation prevents early postmenopausal bone loss but without a dose-dependent effect, J. Nutr. Biochem, 2006, 17, 509–517. [DOI] [PubMed] [Google Scholar]
- 22.Chi X-X and Zhang T, The effects of soy isoflavone on bone density in north region of climacteric Chinese women, J. Clin. Biochem. Nutr, 2013, 53, 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arjmandi BH, Birnbaum R, Goyal NV, Getlinger MJ, Juma S, Alekel L, Hasler CM, Drum ML, Hollis BW and Kukreja SC, Bone-sparing effect of soy protein in ovarian hormone-deficient rats is related to its isoflavone content, Am. J. Clin. Nutr, 1998, 68, 1364S–1368S. [DOI] [PubMed] [Google Scholar]
- 24.Picherit C, Horcajada MN, Mathey J, Chanteranne B, Puel C, Lebecque P, Davicco MJ, Coxam V. and Barlet JP, Isoflavone consumption does not increase the bone mass in osteopenic obese female Zucker rats, Ann. Nutr. Metab, 2003, 47, 70–77. [DOI] [PubMed] [Google Scholar]
- 25.Picherit C, Coxam V, Bennetau-Pelissero C, Kati-Coulibaly S, Davicco MJ, Lebecque P. and Barlet JP, Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats, J. Nutr, 2000, 130, 1675–1681. [DOI] [PubMed] [Google Scholar]
- 26.Somjen D, Katzburg S, Kohen F, Gayer B. and Livne E, Daidzein but not other phytoestrogens preserves bone architecture in ovariectomized female rats in vivo, J. Cell. Biochem , 2008, 103, 1826–1832. [DOI] [PubMed] [Google Scholar]
- 27.Ishida H, Uesugi T, Hirai K, Toda T, Nukaya H, Yokotsuka K. and Tsuji K, Preventive effects of the plant isoflavones, daidzin and denistin, on bone loss in ovariectomized rats fed a calcium-deficient diet, Biol. Pharm. Bull, 1998, 21, 62–66. [DOI] [PubMed] [Google Scholar]
- 28.Fujioka M, Uehara M, Wu J, Adlercreutz H, Suzuki K, Kanazawa K, Takeda K, Yamada K. and Ishimi Y, Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice, J. Nutr, 2004, 134, 2623–2627. [DOI] [PubMed] [Google Scholar]
- 29.Bell A, Korourian S, Zeng H, Phelps J. and Hakkak R, A diet containing a high- versus low-daidzein level does not protect against liver steatosis in the obese Zucker rat model, Food Funct, 2017, 8, 1293–1298. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Sun WJ, Li ZY, Li L, Wang Y, Zhao Y, Wang C, Yu LR, Li LZ and Zhang YL, Daidzein increases OPG/RANKL ratio and suppresses IL-6 in MG-63 osteoblast cells, Int. Immunopharmacol, 2016, 40, 32–40. [DOI] [PubMed] [Google Scholar]
- 31.Horowitz MC, Xi Y, Wilson K. and Kacena MA, Control of osteoclastogenesis and bone resorption by members of the TNF family of receptors and ligands, Cytokine Growth Factor Rev, 2001, 12, 9–18. [DOI] [PubMed] [Google Scholar]
- 32.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH and Meydani SN, Aging up-regulates expression of inflammatory mediators in mouse adipose tissue, J. Immunol, 2007, 179, 4829–4839. [DOI] [PubMed] [Google Scholar]
- 33.Boyle WJ, Simonet WS and Lacey DL, Osteoclast differentiation and activation, Nature, 2003, 423, 337–342. [DOI] [PubMed] [Google Scholar]
- 34.Key LL Jr., Wolf WC, Gundberg CM and Ries WL, Superoxide and bone resorption, Bone, 1994, 15, 431–436. [DOI] [PubMed] [Google Scholar]
- 35.Wauquier F, Leotoing L, Coxam V, Guicheux J. and Wittrant Y, Oxidative stress in bone remodelling and disease, Trends Mol. Med, 2009, 15, 468–477. [DOI] [PubMed] [Google Scholar]
- 36.Halade GV, Rahman MM, Williams PJ and Fernandes G, High fat diet-induced animal model of age-associated obesity and osteoporosis, J. Nutr. Biochem, 2010, 21, 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upadhyay J, Farr OM and Mantzoros CS, The role of leptin in regulating bone metabolism, Metab., Clin. Exp, 2015, 64, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]