Passive immunization, passive immunity, and passive immunotherapy all refer to the transfer of antibodies to an unprotected individual for the prevention or treatment of disease. The first formal demonstration of passive immunization for successfully treating diphtheria and tetanus dates back to animal studies published in Deutsche Medizinische Wochenschrift (German Medical Journal) in 1890.1 The technique was quickly adapted to clinical use and as early as the mid-1890s, diphtheria-specific antitoxin was used successfully in the hospital setting to reduce mortality during diphtheria outbreaks.2, 3, 4 Indeed, in 1901 Emil von Behring was awarded the first Nobel Prize for Physiology or Medicine for the discovery of this important medical intervention.5 The significance of this clinical advance cannot be overstated; Behring estimated that 45,000 lives were saved each year using diphtheria-specific passive immunotherapy in Germany alone.6 In the 1890s, the mortality rate of hospitalized cases ranged from 47% to 60%,7 and the work of Emil von Behring and his colleague, Shibasaburo Kitasato, provided the only hope for diphtheria patients in the preantibiotic era.
According to Behring, the discovery of passive immunization would not have occurred if it were not for his earlier work that focused on characterizing the protective mechanisms of active immunization against diphtheria5, 8 and through the work of his collaborator, Kitasato, on the mechanisms of vaccine-mediated immunity against tetanus.1 When guinea pigs were infected with Corynebacterium diphtheriae, the animals routinely died of the disease. However, when Behring vaccinated animals and they mounted neutralizing antibodies to diphtheria toxin, he found that they were protected from a normally lethal dose of C. diphtheriae. To determine if protection was now an intrinsic property of the immune host that could be transferred to a susceptible host, he injected naïve guinea pigs with diphtheria toxin and then successfully treated them with immune serum from vaccinated animals. Likewise, injection of Clostridium tetani or purified tetanus toxin was typically lethal, but through a method developed by Paul Ehrlich,5 animals could eventually become immune to high doses of tetanus toxin by sequentially inoculating them with lower, nonlethal doses of tetanus toxin. Kitasato used this approach to demonstrate that the blood of vaccinated, tetanus-immune rabbits could be transferred to naïve mice and fully protect them from a normally lethal dose of virulent C. tetani or from filtered C. tetani culture supernatant containing tetanus toxin.1 Behring and Kitasato may have said it best in the final sentence of their landmark 1890 study, “The result of our experiments remind us forcibly of these words: Blut ist ein ganz besonderer Saft [blood is a very unusual fluid].”1
Technology has advanced substantially in the more than 125 years since Behring and Kitasato's first formal demonstration of protective passive immunotherapy.1 In those early days, it was infeasible to use human immune serum to treat diphtheria, so the first large-scale production of polyclonal diphtheria-immune serum was prepared by vaccinating dairy cows.5 To this day, commercial antisera used to treat a broad range of toxins are still produced in animals (Table 8.1 ). Passive immunotherapy with animal-derived antibody preparations should only be used under close medical supervision9 or the resulting host immune response to the foreign immunoglobulins and serum proteins may trigger serum sickness, urticaria, and/or anaphylaxis following administration. Fortunately, the advent of several innovative technologies that reduce the need for animal-derived antibodies have forged new paths in terms of safety, feasibility, and the protective efficacy afforded by passive immunization. Following the discovery of monoclonal antibody technology,10, 11 further refinements have been made, including use of various display techniques (e.g., phage display, yeast display) to screen large antibody libraries.12 Other technological advances include the development of chimeric monoclonal antibodies in which the murine antibody is “humanized” by genetically replacing the heavy chain region of the molecule with the human immunoglobulin counterpart and the use of transgenic mice in which the endogenous murine immunoglobulin genes have been replaced by human immunoglobulin genes.12 This latter approach has the advantage that hybridomas from immunized transgenic mice produce fully human monoclonal antibodies without requiring further genetic modifications. Recently, development of Epstein-Barr virus (EBV)-transformed human memory B cells for the production of monoclonal antibodies has led to yet another surge in the production of new human monoclonal antibodies with rare antigenic specificities to uncommon pathogens and these can be produced directly from immune human subjects.12, 13 Before the era of antibiotics, antibody-based therapy was the only option available for combating many bacterial diseases. Even today, there are only a handful of antiviral drugs available and no therapeutic options exist for most viral diseases. However, new antibody-based therapies are continuing to be developed with the potential to provide protection against a broad array of bacterial and viral pathogens. In this chapter, we describe the role of passive immunity in the protection of the naïve host, discuss the parameters involved with successful immunotherapy, and provide examples of protective efficacy in animal models as well as in human clinical studies.
TABLE 8.1.
Licensed U.S. Antibody Products for Passive Immunity to Infectious Diseases or Toxins
| Product | Brand Name | Manufacturer | Licensed Indicationsa |
|---|---|---|---|
| Standard Immunoglobulins (Human) | |||
| Immunoglobulin, intravenous | Bivigam Carimune Flebogamma Gammagard Gammaplex Gamunex-C Octagam Privigen |
Biotest Pharmaceuticals CSL Behring Instituto Grifols Baxter BPL Grifols Biotherapeutics Pharmazeutika Produktionsges CSL Behring |
Primary humoral immunodeficiency; multifocal motor neuropathy; chronic idiopathic thrombocytopenic purpura; Kawasaki syndrome; chronic inflammatory demyelinating polyneuropathy |
| Immunoglobulin, subcutaneous | Hizentra Hyqvia Gammagard Vivaglobin |
CSL Behring Baxter Baxter CSL Behring |
Primary humoral immunodeficiency; multifocal motor neuropathy |
| Immunoglobulin, intramuscular | GamaSTAN | Grifols Biotherapeutics | Hepatitis A; measles; varicella; rubella |
| Hyperimmunoglobulins (Human) | |||
| Anthrax immunoglobulin intravenous (human) | Anthrasil | Cangene Corporation | Treatment of inhalation anthrax |
| Botulism immunoglobulin intravenous (human) | BabyBIG | California Department of Health Services | Treatment of infant botulism (type A or type B Clostridium botulinum) |
| Cytomegalovirus immunoglobulin intravenous (human) | CytoGam | CSL Behring | Prophylaxis of cytomegalovirus (CMV) disease associated with organ transplantation |
| Hepatitis B immunoglobulin intravenous (human) | HepaGam B Nabi-HB |
Cangene Corporation Nabi Biopharmaceuticals |
Prevention and postexposure prophylaxis for hepatitis B |
| Rabies immunoglobulin (human) | HyperRab S/D | Grifols Biotherapeutics | Postexposure treatment of rabies, administered in conjunction with the rabies vaccine |
| Tetanus immunoglobulin (human) | HyperTET S/D | Grifols Biotherapeutics | Prophylactic or therapeutic treatment of tetanus |
| Vaccinia immunoglobulin intravenous (human) | N/A | Cangene Corporation | Treatment and/or modification of complications resulting from smallpox vaccination |
| Varicella zoster immunoglobulin (human) | VariZIG | Cangene Corporation | Varicella postexposure prophylaxis in high-risk groups |
| Animal-Derived Immunoglobulin Products | |||
| Antivenin (Latrodectus mactans) (equine) | Black widow spider antivenin | Merck & Co, Inc. | Treatment of bites by the black widow spider (Latrodectus mactans) |
| Botulism antitoxin bivalent (equine) types A and B | N/A | Sanofi Pasteur Ltd | Treatment of botulism (types A or type B) |
| Botulism antitoxin heptavalent (A, B, C, D, E, F, G)-(equine) | BAT | Cangene Corporation | Treatment of botulism (types A, B, C, D, E, F, or G) |
| Centruroides (scorpion) immune F(ab')2 (equine) injection | Anascorp | Rare Disease Therapeutics, Inc. | Treatment of scorpion envenomation |
| Crotalidae immune F(ab')2 (equine) | Anavip | Instituto Bioclon S.A. de C.V. | Treatment of rattlesnake envenomation |
| Crotalidae polyvalent immune Fab (ovine) | CroFab | Protherics, Inc. | Treatment of rattlesnake and cottonmouth/water moccasin envenomation |
| Digoxin immune Fab (ovine) | DigiFab | Protherics, Inc. | Treatment of digoxin toxicity or overdose |
| Diphtheria antitoxin (equine) | DAT | Instituto Butantab | Prophylactic or therapeutic treatment of diphtheria |
| Monoclonal Antibodies | |||
| Palivizumab | Synagis | MedImmune | Prevention of lower respiratory tract disease caused by respiratory syncytial virus (RSV) in high-risk children |
| Raxibacumab | N/A | Human Genome Sciences/GlaxoSmithKline | Treatment of inhalation anthrax |
N/A, not applicable.
Indications as listed by the manufacturer. Indications have been grouped for each product type.
Distributed by the Centers for Disease Control and Prevention to physicians as an Investigational New Drug.
Maternal Antibodies: The Original Passive Immunotherapy
Maternal antibodies represent a natural form of passive immunotherapy in which the immunoglobulin (Ig) G repertoire of the mother's preexisting humoral immune response is transferred to the fetus through the placenta. Acquisition of maternal antibodies varies widely among mammalian species.14 Maternal IgG is transferred in utero to the fetus of humans and monkeys through the placenta with no evidence of postnatal transport, and reaches serum concentrations that are similar between mother and infant. In contrast, there is no prenatal transport of maternal IgG in mink, cows, horses, sheep, goats, and pigs, and although the animals are born with serum that is nearly devoid of IgG, these antibodies are transferred from ingested colostrum into the bloodstream within the first 24 to 48 hours after birth across the gastrointestinal tract. Transmission of maternal IgG in mice, rats, and dogs occurs in utero as well as across the gastrointestinal tract after birth, indicating that they differ from humans and non-human primates as well as being different from mink and the ungulates. These differences also indicate that care should be taken when choosing an appropriate animal model for studying the role of maternal antibodies against infectious disease as the mechanisms may be more species-specific than typically realized.
In a comprehensive study involving the analysis of antibodies to 16 viruses using samples from 58,500 patients, the relationship between maternal immunity and infant immunity is clear (Fig. 8.1 ).15 The prevalence of antibodies to each viral antigen among infants less than 1 month old is remarkably similar to those observed in the 20- to 40-year old adults who represented the main age group of the mothers. For instance, immunity to common childhood diseases such as measles and mumps was comparable between newborns and their mothers. Immunity to less-common viral pathogens, such as influenza B, was relatively low among infants and adults in the cohorts examined in 1971–1972, but higher among those sampled in 1973–1974,1975–1976, and 1977–1978, coinciding with an influenza B epidemic that had occurred in 1974.15 This shows that the prevalence of maternal antibodies is dynamic and that recent outbreaks involving a specific pathogen will result in a higher frequency of pathogen-immune mothers and a concomitant increase in the number of infants who are likewise bestowed at least transient immunity to that particular microbe. As expected, maternal antibodies wane rapidly during the first 6 months of life and then exposure to pathogens over the following months and years results in an accumulation of different antibody specificities as children reach adulthood (see Fig. 8.1). The overall protective efficacy of maternal antibodies is perhaps most pronounced among children with genetic immunodeficiencies such as severe combined immunodeficiency (SCID), resulting in the lack of functional T and B cells or agammaglobulinemia, in which patients lack functional B cells while still having the ability to mount pathogen-specific T-cell responses. The clinical presentation of SCID is not apparent at birth but relatively uniform diagnosis occurs at a mean of 6.59 months of age,16 which is also about the age that maternal antibodies have reached their lowest levels15 (see Fig. 8.1). Likewise, agammaglobulinemic patients also begin to present with symptoms of immunodeficiency around this same age.17 Maternal antibodies represent an immunological “double-edged sword” in the sense that they are known to interfere with live attenuated virus vaccines such as the MMR (measles, mumps, rubella)18, 19, 20 and rotavirus vaccines,21, 22 whereas direct immunization of mothers in the third trimester of pregnancy can significantly increase protection of infants against common respiratory viruses such as influenza.23, 24, 25 Indeed, maternal vaccination may result in a 45% to 91% reduction in influenza-related hospitalizations among infants younger than 6 months of age.23, 24, 25 Likewise, the importance of maternal vaccination against Bordetella pertussis (i.e., whooping cough) was recognized as early as the 1930s to 1940s with studies showing higher antibacterial antibody responses and potential protection from exposure to whooping cough among infants born to vaccinated mothers.26, 27, 28, 29 Recent studies verify these earlier results, demonstrating a 90% to 91% vaccine efficacy against whooping cough among infants younger than 2 months of age who were born to mothers who received pertussis vaccination during pregnancy,30, 31 thus lending further support to the current recommendations for the vaccination of pregnant mothers against B. pertussis.32 The age limit of younger than 2 months was chosen as this is the age at which primary pediatric vaccination is recommended and analysis beyond this age might be confounded by the protective effects of direct vaccination of the child. Nevertheless, the protection afforded by maternally derived IgG against respiratory infections involving viral (e.g., influenza) or bacterial (e.g., B. pertussis) pathogens together demonstrate the broad impact that maternal vaccination and the subsequently increased transfer of maternal antibodies can have on the health of young infants.
Figure 8.1.
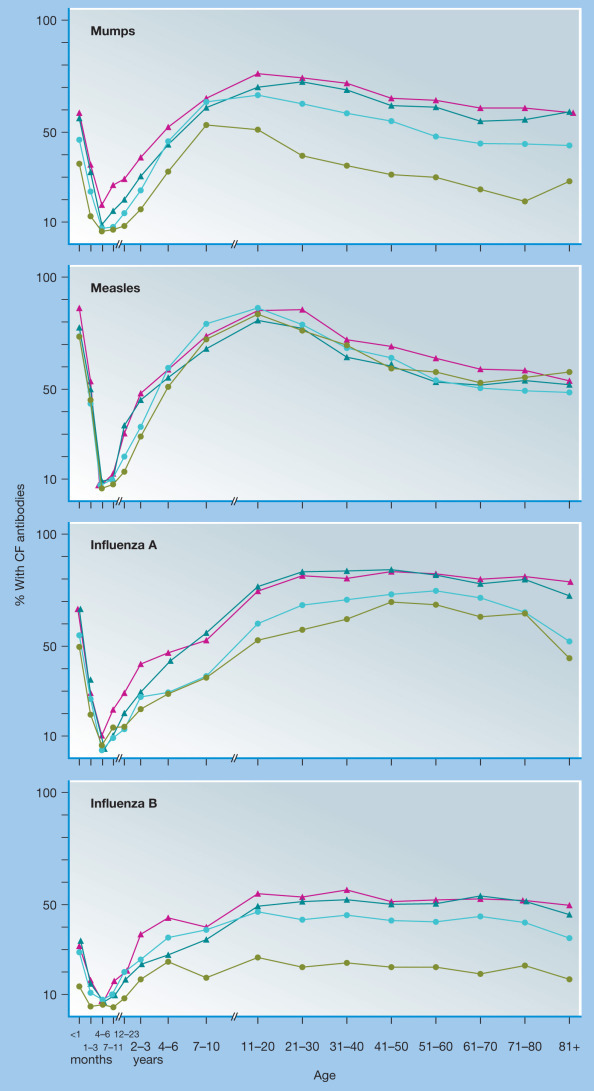
Age-specific prevalence of antibodies to mumps, measles, influenza A, and influenza B viruses in patients screened with 16 viral antigens during the years 1971–1978.
The prevalence curves represent four 2-year periods: 1971–1972 (green line), 1973–1974 (aqua line), 1975–1976 (red line), and 1977–1978 (blue line). CF, complement fixing.
(From Ukkonen P, Hovi T, von Bonsdorff C-H, Saikku P, Penttinen K. Age-specific prevalence of complement-fixing antibodies to sixteen viral antigens: A computer analysis of 58,500 patients covering a period of eight years. J Med Virol. 1984;13:131–148.)
Critical Parameters for Passive Immunotherapy
Before vaccines and antibiotics revolutionized modern medicine, antibody-based therapies represented the only effective medical treatment for many life-threatening diseases including diphtheria, scarlet fever, bacterial meningitis, and bacterial pneumonia.33, 34 Today, most commercial forms of antibody-based immunotherapy for infectious disease still rely on polyclonal antibodies of human or animal origin, with the notable exceptions of the monoclonal antibodies, palivizumab and raxibacumab (see Table 8.1). The main advantage of using polyclonal antibodies for passive immunotherapy is that this approach will include antibodies to multiple epitope specificities that may work in an additive or synergistic manner with the potential contribution of multiple immunoglobulin isotypes and subclasses that have different biological functions (Table 8.2 ).35 On the other hand, there are several potential challenges to using polyclonal antibodies for immunotherapy including low antigen-specific activity, supply limitations (especially for rare diseases), variability between manufacturing lots, and safety as well as quality control issues that are often associated with the use of human blood products. In contrast, monoclonal antibodies are, by definition, limited to a single epitope specificity but they have several advantages over polyclonal antibodies since they can be manufactured in vitro at large scale, with inherently high specificity and lot consistency (Table 8.2). For example, the combination of 0.7 mg of two tetanus-specific human monoclonal antibodies has the same neutralizing capacity observed with administration of 100 to 170 mg of polyclonal tetanus immunoglobulin.36 Likewise, administration of 0.023 mg of a vaccinia virus-specific monoclonal antibody provides the same level of protection afforded by 5 mgs of vaccinia immunoglobulin (VIG).37 Although neutralization escape mutants are a valid concern when using monoclonal antibody therapy,38, 39 this has not yet been a major problem during clinical use of palivizumab for respiratory syncytial virus (RSV). Initially, sequencing of 371 RSV isolates demonstrated that there were no mutations in the neutralizing epitope of the F protein.40 Subsequent studies identified RSV escape mutants in approximately 5% of 146 breakthrough cases, indicating that selective pressure for escape mutations is still relatively uncommon under current conditions of use.41 This suggests that monoclonal antibodies can remain effective when used clinically in the long-term, as long as they are specific for a stable epitope for that particular pathogen.
TABLE 8.2.
Comparison of Polyclonal and Monoclonal Antibody Therapy
| Polyclonal Antibodies | Monoclonal Antibodies | |
|---|---|---|
| Advantages | Polyvalent specificity Multiple isotypes with different effector functions |
High specific activity Standardized potency Unlimited availability Minimal biohazard potential |
| Disadvantages | Low specific activity Broad variation in potency Limited availability Biohazard risk of human blood products |
Monovalent specificity Single isotype Potential to select for escape mutants |
The functional characteristics of the immunoglobulins used for passive immunization is an important consideration in determining protective efficacy in vivo.35 For example, serum IgG molecules equilibrate into extravascular space whereas IgM is largely confined to intravascular space.14 IgM molecules also have a short half-life (5 days14) and are typically of low affinity, which is why IgM is not an optimal choice for passive immunotherapy. Serum IgA is monomeric and, although it also equilibrates into extravascular space,14 it has only a 6-day half-life14 and does not appear to contribute significantly to functional IgA in the lungs of mice.42, 43 Human IgG on the other hand, has an average half-life of approximately 21 days (except IgG3, which has a 7-day half-life),14, 44 is typically of high affinity, and transudation across mucosal barriers can protect against pathogens that invade through mucosal routes. Interestingly, serum IgG (and serum IgA) responses elicited in response to vaccination against Neisseria meningitidis correlate strongly with the levels of antibacterial antibodies present in the saliva at 1 month and 1 year after vaccination,45 indicating that circulating serum antibodies may be an important contributor to the antibodies released in mucosal secretions. Indeed, after intravenous administration of an HIV-specific monoclonal antibody into rhesus macaques, serum antibody titers of 690 to 725 µg/mL resulted in mucosal antibody titers of 17 to 30 µg/mL in vaginal fluids and provide complete protection against intravaginal challenge with SHIV (chimeric simian immunodeficiency virus expressing HIV envelope).46 Influenza virus is another mucosal pathogen with strict tropism to the respiratory tract, but influenza-specific serum antibody titers correlate with protection in humans.47 In mice, the relative roles of influenza-specific polymeric IgA and IgG were compared in terms of antiviral protection in the upper respiratory tract versus the lung after influenza challenge.42 When polymeric IgA was transferred 4 hours prior to influenza infection, this prevented pathology in the upper respiratory tract but was not effective in the lung, whereas transfer of IgG prevented pathology in the lung, but required higher doses to protect against infection of the upper respiratory tract. The authors concluded that different antibody isotypes may function preferentially at different anatomical sites in vivo. These results are in contrast to experimental influenza infection in humans in which inactivated influenza vaccine-derived IgG is believed to be a major contributor to protection of the nasal compartment.48 Overall, the ability of IgG to enter nonlymphoid tissues and to penetrate mucosal sites of infection is likely to explain why it is often considered the best immunoglobulin isotype for routine passive immunization and has shown clinical benefit ranging from reduced clinical symptoms to nearly complete protection from lethal infection in a number of infectious disease models (Table 8.3 ).
TABLE 8.3.
Efficacy of Passive Immunity for Infectious Diseasesa
| Animal Models | Clinical Studies | |
|---|---|---|
| Toxins | ||
| Anthrax toxin | Prophylaxis145, 146 Treatment145, 146 |
Prophylaxis: not available Treatment147, 148 |
| Botulinum toxin | Prophylaxis149, 150, 151 Treatment151 |
Prophylaxis: not available Treatment152, 153, 154, 155 |
| Diphtheria toxin | Prophylaxis156, 157 Treatment157, 158 |
Prophylaxis159 Treatment7, 49, 160, 161 |
| Ricin toxin | Prophylaxis162, 163 Treatment163 |
Prophylaxis: not available Treatment: not available |
| Tetanus toxin | Prophylaxis1, 164 Treatment157, 164, 165 |
Prophylaxis166 Treatment166, 167: not supported168 |
| Bacterial Infections | ||
| Bordetella pertussis (whooping cough) | Prophylaxis169, 170 Treatment170, 171 |
Prophylaxis30b,31b,172, 173 Treatment173, 174 |
| Borrelia spp. (Lyme disease) | Prophylaxis175, 176 Treatment175 |
Prophylaxis: not available Treatment: not available |
| Chlamydia trachomatis | Prophylaxis177, 178, 179 Treatment: not available |
Prophylaxis: not available Treatment: not available |
| Clostridium difficile | Prophylaxis180, 181 Treatment180, 181 |
Prophylaxis: not available Treatment182 |
| Escherichia coli | Prophylaxis183, 184, 185 Treatment184, 185 |
Prophylaxis110, 111, 112, 113: not supported186 Treatment: not supported187 |
| Francisella tularensis (Tularemia) | Prophylaxis119, 120 Treatment121 |
Prophylaxis: not available Treatment118, 188 |
| Haemophilus influenzae | Prophylaxis189, 190, 191 Treatment191, 192 |
Prophylaxis102 Treatment99, 101 |
| Mycobacterium tuberculosis (Tuberculosis) | Prophylaxis124, 193 Treatment124, 194, 195 |
Prophylaxis: not available Treatment128: not supported128 |
| Neisseria meningitidis (Meningococcal disease) | Prophylaxis196, 197, 198, 199 Treatment198, 199, 200 |
Prophylaxis: not available Treatment33, 201 |
| Pseudomonas aeruginosa | Prophylaxis202, 203 Treatment202, 203, 204 |
Prophylaxis: not available Treatment205, 206: not supported207 |
| Salmonella typhi (Typhoid fever) | Prophylaxis208, 209 Treatment210 |
Prophylaxis: not available Treatment211 |
| Shigella spp. | Prophylaxis212b,213b,214 Treatment: not available |
Prophylaxis215 Treatment: not supported216 |
| Staphylococcus aureus | Prophylaxis217, 218, 219 Treatment: not supported218 |
Prophylaxis: not supported220, 221, 222 Treatment: not supported223, 224 |
| Streptococcus agalactiae (Streptococcus group B) | Prophylaxis225, 226, 227, 228 Treatment225, 226 |
Prophylaxis229b,230b Treatment: not available |
| Streptococcus pneumoniae (Pneumococcal disease) | Prophylaxis231, 232, 233 Treatment234, 235, 236 |
Prophylaxis237 Treatment33, 236, 238 |
| Streptococcus pyogenes (Streptococcus group A) | Prophylaxis239, 240, 241 Treatment: not available |
Prophylaxis242, 243 Treatment243, 244, 245, 246, 247, 248 |
| Vibrio cholerae (Cholera) | Prophylaxis249, 250, 251, 251b Treatment250 |
Prophylaxis: not available Treatment252, 253 |
| Yersinia pestis (Plague) | Prophylaxis254, 255, 256, 257, 258, 259, 260 Treatment254, 255, 256, 257, 261, 262, 263 |
Prophylaxis264 Treatment257, 265 |
| Viral Infections | ||
| Chikungunya virus | Prophylaxis266, 267 Treatment266, 267 |
Prophylaxis: not available Treatment: anecdotal268c |
| Coxsackievirus | Prophylaxis269 Treatment270 |
Prophylaxis: not available Treatment271 |
| Cytomegalovirus | Prophylaxis272b,273, 274 Treatment: not available |
Prophylaxis275, 276, 277 Treatment: not available |
| Dengue virus | Prophylaxis278, 279, 280 Treatment278, 280 |
Prophylaxis: not available Treatment: not available |
| Ebola virus (Ebola hemorrhagic fever) | Prophylaxis281 Treatment281, 282, 283 |
Prophylaxis: not available Treatment: anecdotal284 |
| Epstein-Barr virus | Prophylaxis285, 286 Treatment: not available |
Prophylaxis133 Treatment: not available |
| Hantavirus (Andes virus and Sin Nombre virus) | Prophylaxis287 Treatment287, 288 |
Prophylaxis: not available Treatment289 |
| Hepatitis A virus | Prophylaxis: not available Treatment: not available |
Prophylaxis290, 291, 292, 293 Treatment: not available |
| Hepatitis B virus | Prophylaxis294 Treatment294 |
Prophylaxis295 Treatment296, 297 |
| Hepatitis C virus | Prophylaxis298 Treatment298 |
Prophylaxis299 Treatment300, 301: not supported301, 302 |
| Hepatitis E virus | Prophylaxis303 Treatment: not available |
Prophylaxis304 Treatment: not available |
| Herpes simplex virus | Prophylaxis92, 305, 306 Treatment92, 307 |
Prophylaxis308 Treatment309 |
| HIV | Prophylaxis310, 311 Treatment312, 313 |
Prophylaxis: not available Treatment140, 314, 315: not supported316, 317 |
| Human papillomavirus | Prophylaxis318, 319 Treatment: not available |
Prophylaxis: not available Treatment: not available |
| Influenza virus | Prophylaxis92, 95, 96 Treatment92, 95, 96 |
Prophylaxis23, 24, 25b Treatment97, 98, 320 |
| Japanese encephalitis virus | Prophylaxis321, 322 Treatment321, 322 |
Prophylaxis: not available Treatment: not available |
| Junin virus (Argentine hemorrhagic fever) | Prophylaxis70 Treatment70, 71, 72 |
Prophylaxis: not available Treatment68, 69 |
| Lassa virus (Lassa hemorrhagic fever) | Prophylaxis: not available Treatment76, 77 |
Prophylaxis: not available Treatment78, 79 |
| Machupo virus (Bolivian hemorrhagic fever) | Prophylaxis323 Treatment323 |
Prophylaxis: not available Treatment: not available |
| Measles virus | Prophylaxis324, 325 Treatment: not available |
Prophylaxis51, 53, 80, 81, 82, 83, 84 Treatment: not available |
| Molluscum contagiosum | Prophylaxis: not available Treatment: not available |
Prophylaxis: anecdotal308 Treatment: not available |
| Monkeypox virus | Prophylaxis326 Treatment: not available |
Prophylaxis: not available Treatment: not available |
| Mumps virus | Prophylaxis: not availabled Treatment: not available |
Prophylaxis327, 328, 329, 330 Treatment328 |
| Parvovirus B19 | Prophylaxis: not available Treatment: not available |
Prophylaxis: not available Treatment: anecdotal331, 332 |
| Poliovirus | Prophylaxis333, 334 Treatment335 |
Prophylaxis336, 337 Treatment54, 338, 339, 340 |
| Rabies virus | Prophylaxis341 Treatment342, 343, 344, 345 |
Prophylaxis346 Treatment: not supported347 |
| Respiratory syncytial virus | Prophylaxis348, 349 Treatment350, 351 |
Prophylaxis55, 56, 57, 58, 60, 352 Treatment: not supported59, 61, 62 |
| Rift Valley fever virus | Prophylaxis353, 354 Treatment: not available |
Prophylaxis: not available Treatment: not available |
| Rotavirus | Prophylaxis104, 105, 355, 356, 357, 358 Treatment104, 358 |
Prophylaxis21b,103, 104 Treatment104, 105, 106, 107, 108 |
| Rubella virus | Prophylaxis: not available Treatment: not available |
Prophylaxis359, 360, 361, 362 Treatment363 |
| Severe acute respiratory syndrome coronavirus | Prophylaxis364, 365 Treatment366 |
Prophylaxis: not available Treatment320 |
| Simian immunodeficiency virus | Prophylaxis367 Treatment368, 369: not supported367 |
Not applicable |
| Simian/human immunodeficiency virus | Prophylaxis46, 370, 371, 372, 373, 374, 375, 376 Treatment141, 374 |
Not applicable |
| Tickborne encephalitis virus | Prophylaxis377, 378 Treatment377 |
Prophylaxis379 Treatment: anecdotal380 |
| Vaccinia virus | Prophylaxis37, 381, 382, 383 Treatment37, 383, 384 |
Prophylaxis385 Treatment386, 387 |
| Varicella virus | Prophylaxis: not available Treatment: not available |
Prophylaxis388, 389, 390 Treatment: not available |
| Variola (smallpox) | Prophylaxis: not available Treatment: not available |
Prophylaxis64, 65 Treatment65, 66 |
| Venezuelan equine encephalomyelitis virus | Prophylaxis391 Treatment391 |
Prophylaxis: not available Treatment: not available |
| West Nile virus | Prophylaxis392, 393, 394 Treatment392, 394, 395 |
Prophylaxis: not available Treatment: anecdotal396, 397 |
| Yellow fever virus | Prophylaxis398, 399, 400 Treatment398, 401 |
Prophylaxis: anecdotal401 Treatment: anecdotal402, 403 |
| Parasites and Fungal Infections | ||
| Candida albicans | Prophylaxis404, 405, 406, 407 Treatment406 |
Prophylaxis308 Treatment: not available |
| Cryptococcus neoformans | Prophylaxis408, 409, 410 Treatment: not available |
Prophylaxis: not available Treatment: not supported411 |
| Cryptosporidium parvum | Prophylaxis412 Treatment413: not supported412 |
Prophylaxis: not supported414 Treatment: not available |
| Plasmodium spp. (Malaria) | Prophylaxis415, 416, 417 Treatment: not available |
Prophylaxis: not available Treatment418, 419 |
| Toxoplasma gondii (Toxoplasmosis) | Prophylaxis420, 421, 422 Treatment: not available |
Prophylaxis: not available Treatment: not available |
| General | ||
| Genetic immunodeficiency diseases | Not applicable | Prophylaxis423, 424, 425, 426, 427 |
| HIV-associated diseases | Not applicable | Prophylaxis308, 428 |
| Sepsis/septic shock | Not applicable | Treatment246, 426, 429, 430, 431, 432 |
For animal studies, prophylaxis is defined as antibody administration prior to experimental infection and treatment is defined as antibody administration after infection. For clinical studies, prophylaxis is defined as antibody administration prior to disease onset and treatment is defined as antibody administration after disease onset.
Evidence provided through maternal immunization studies.
Anecdotal results are defined as small studies that indicate passive immunization may provide clinical benefit but are too limited in scope to be conclusive.
Over the last century, it has been well established that high specific antibody titers and early timing of antibody transfer in relation to disease onset are the two most important parameters involved with determining the protective efficacy of passive immunization (Fig. 8.2 ). In one account of the early days of clinical diphtheria-specific immunotherapy developed by Behring and Ehrlich,5 initial failures in patients after treatment with weak or unstandardized diphtheria-immune serum brought Ehrlich to describe three points that he believed were important for successful immunotherapy: (a) treatment has to be initiated at the onset of disease; (b) the more the disease has progressed, the higher the serum quantities necessary for cure; and (c) depending on the severity of the case, certain minimal doses can be specified. Later studies confirmed these results: if diphtheria immunotherapy was initiated on the first day of disease, there was 0% mortality (n = 183).49 However, if therapy was delayed to 2, 3, or 4 days after disease onset, then the accompanying diphtheria case-fatality rate subsequently increased to 1.6% (n = 905), 4.4% (n = 632), and 6.9% (n = 436), respectively.49 These results are similar to those observed during antibiotic-based therapy of bacterial sepsis. In an ideal setting, it is recommended that antibiotics be administered within 1 hour of diagnosis of severe sepsis or septic shock as these drugs provide clinical benefit only if administered early in the course of disease and are generally ineffective during late-stage disease.50
Figure 8.2.
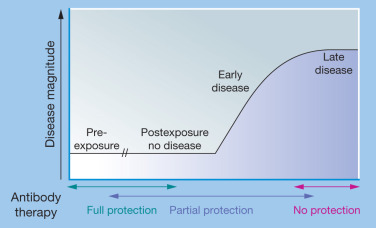
Efficacy of passive immunity decreases with disease progression.
Full protection from symptomatic disease is best achieved through prophylactic administration of antibody therapy prior to exposure or infection. However, antibody therapy may also be highly effective at early points postexposure, prior to the onset of disease symptoms. Passive immunity is generally less effective when administered after the onset of symptomatic disease, and typically shows little to no clinical benefit once severe late-stage disease has occurred.
The importance of high-dose immunotherapy given at the earliest sign of disease is not unique to bacterial anti-toxin therapy. The same rules apply to preventing or treating viral infections as well. During a measles epidemic in 1931–1932, 72% of exposed individuals (n = 32) who received no passive immunization contracted measles. If convalescent serum was administered within 10 days of exposure, then the attack rate was reduced to 16% (n = 219) whereas if therapy was not initiated until 12 to 16 days postexposure, approximately 80% of contacts subsequently contracted measles (n = 5).51 In a study published in 1945 involving 1024 cases of measles exposure, 36% of the individuals who received immunotherapy within 0 to 2 days of exposure contracted measles compared to 48% for those whose treatment was delayed to 6 to 8 days postexposure.52 The dose used in these studies was also critical: 67% of patients who received 0.01 mL/kg of gammaglobulin contracted measles whereas only 16% of patients who received 0.06 mL/kg of gammaglobulin contracted the disease. The titer of virus-specific antibodies will often differ between lots of polyclonal immunoglobulin preparations (see Table 8.2). In another study, when the measles-specific titer of gammaglobulin from different lots decreased from 33 IU/mL to 16 IU/mL, the postexposure incidence of measles increased from 17% to 57% despite either lot being administered within 5 days of exposure.53 Likewise, the timing of passive immunotherapy is also important for enteric (e.g., polio) and respiratory pathogens (e.g., RSV). An outbreak in 1934 involving 2992 polio patients showed that if convalescent serum was administered within 0 to 2 days of meningitis, then paralysis was reported in 5.4% of patients (n = 2367). If treatment was delayed until 3 to 6 days after meningeal disease onset, 15.5% reported paralysis (n = 536), and if treatment was delayed for more than 6 days, then paralysis was noted in 30.3% of polio patients (n = 89).54 For RSV, polyclonal RSV-immunoglobulin reduced the incidence of RSV-associated hospitalization by 41% among children with a history of prematurity or bronchopulmonary dysplasia.55 Prophylactic administration of a neutralizing monoclonal antibody, palivizumab, was shown to significantly improve clinical outcome by reducing RSV-associated hospitalizations of children with congenital heart disease by 45%.56 Among premature infants or those with bronchopulmonary dysplasia, RSV-associated hospitalizations were reduced by 55%.57 A third palivizumab study confirmed these results by showing a 70% reduction in hospitalizations among premature infants and infants with chronic lung disease.58 Another monoclonal antibody, motavizumab,59 demonstrated a further 26% relative reduction in RSV hospitalizations compared with patients receiving palivizumab-based prophylaxis.60 In contrast, once RSV infection has been established, the use of palivizumab,61 motavizumab,59 or RSV-immunoglobulin62 shows no clinical benefit, although RSV-immunoglobulin may provide limited protection in the most severe cases.62
Passive immunotherapy can be highly successful for severe, even life-threatening human diseases such as smallpox, or hemorrhagic fever caused by arenaviruses including Junin or Lassa fever virus (see Table 8.3). Successful intervention, however, typically requires initiating treatment before or very shortly after symptom onset. When convalescent serum from smallpox survivors was administered to smallpox patients during the late stages of confluent or hemorrhagic smallpox, there was no clinical benefit observed in comparison to untreated controls (80% vs. 72% mortality, respectively).63 When vaccinia-immune gammaglobulin (VIG) was administered to smallpox contacts prior to disease onset in addition to postexposure vaccination (i.e., standard of care), the number of smallpox cases was reduced by 70% compared to contacts who received postexposure smallpox vaccination alone.64 Likewise, administration of vaccinia-immune serum of animal origin along with postexposure vaccination resulted in 0 of 13 cases (0%) of smallpox among close contacts compared to 13 of 29 cases (45%) among controls who received smallpox vaccination alone.65 During a smallpox outbreak in 1941, 3 of 10 patients (30%) died while undergoing standard clinical care.66 To determine if addition of passive immunotherapy would reduce mortality after smallpox diagnosis, 250 cases of smallpox were treated with convalescent serum or blood, with no smallpox-associated deaths reported (0 of 250). Approximately 75 patients were described as having severe or hemorrhagic smallpox at the time of treatment and yet all survived. This appears to be the result of using convalescent serum obtained at the peak of the humoral immune response shortly after recovery from smallpox and the use of an optimized dosing schedule with higher doses administered to patients with the more severe disease manifestations.66
Argentine hemorrhagic fever is caused by infection with the Junin virus and untreated cases result in 15% to 40% mortality.67, 68, 69 Convalescent serum is protective in animal models of Junin infection70, 71, 72 and when administered within 8 days of symptom onset, the mortality rate among human cases drops to 1% to 3%.68, 69 Likewise, in 35% to 50% of hospitalized cases, Lassa fever virus causes severe disease including diffuse capillary leakage and hemorrhagic diathesis.73 Prophylactic administration of immune serum protects guinea pigs74, 75 and nonhuman primates76, 77 from subsequent lethal challenge, indicating that antibodies play a clear role in protection against this virulent viral pathogen. In one small clinical study, if passive immunotherapy was administered within 0 to 5 days after admission to the hospital, 4 of 4 (100%) patients survived whereas if immunotherapy was initiated 7 to 9 days after hospitalization, 0 of 3 (0%) patients survived.78 In another study,79 patients with virologically confirmed Lassa fever who received immune serum within 10 days of hospitalization survived (4 of 4; 100%). However, if treatment was not initiated until more than 10 days after hospitalization, then only 1 of 4 (25%) patients survived, similar to the untreated group in which only 1 of 5 (20%) patients with virologically confirmed Lassa fever survived.
Passive Immunity Against Respiratory and Enteric Pathogens
Although passive immunity against toxins and systemic infections such as measles51, 53, 80, 81, 82, 83, 84 and smallpox64, 65, 66 is well established, the impact of this approach for the prevention or amelioration of disease caused by respiratory and enteric pathogens may not be as well recognized. However, several studies support the role of passive immunity against mucosal pathogens (see Table 8.3), including examples such as influenza (respiratory virus), Haemophilus influenzae (respiratory bacterium), rotavirus (enteric virus), and Escherichia coli (enteric bacterium). Influenza is a significant cause of morbidity and mortality throughout the world, including both seasonal transmission and pandemic outbreaks.85, 86, 87, 88 The clinical correlation between homotypic influenza immunity and vaccine-associated protection was recognized early in the development of the influenza vaccine.89, 90, 91 Early animal studies confirmed this result, with passive transfer of antibodies (both systemic and mucosal delivery) able to protect naïve animals against subsequent challenge, or provide therapeutic benefit when administered postexposure.92, 93, 94 More recent animal studies with defined monoclonal antibodies continue to support and extend these earlier results.95, 96 Passive immunization against influenza in humans has also been successful. In a comprehensive retrospective metaanalysis of eight passive immunization studies performed during the Spanish Influenza outbreak (1918–1925), a significant 21% decrease in mortality (95% confidence interval [CI], 15–27%; P < .001) was observed.97 Subset analysis of studies that recorded early (treatment initiated within 4 days of pneumonia complications) versus late intervention (>4 days) showed a significant advantage for early treatment, with mortality decreasing from 59% (49 of 83) to 19% (28 of 148) with earlier intervention, consistent with general considerations for effective passive immunity against infectious diseases (see Fig. 8.2). In a recent double-blinded, randomized controlled study during the 2009 influenza pandemic, the use of hyperimmune intravenous immunoglobulin (IVIG) (from recovered convalescent donors) was compared to normal IVIG in the treatment of severe infection in 34 subjects.98 The hyperimmune treated group (n = 17) demonstrated more rapid viral clearance than the control group (n = 17), with a greater than 90% drop in viral loads by day 5 posttreatment. In those patients receiving immunoglobulin within 5 days of symptom onset (n = 22), all 12 who received hyperimmune IVIG survived (12 of 12), whereas only 60% of patients receiving normal IVIG survived (6/10, P = .02).
H. influenzae type b (Hib) is an extracellular gram-negative bacterium that initially infects the host via the respiratory tract and represents another important human pathogen that can be controlled through passive immunization. Several early reports described the use of concentrated rabbit immune serum as a successful adjunct therapy to sulfonamide treatment for patients suffering from Hib meningitis.99, 100, 101 Indeed, a full course of serum therapy (in addition to antibiotics) was able to reduce mortality to 14% (3 of 19) when compared to 78% mortality rate (7 of 9) in those patients only receiving sulfonamides.101 A more recent study established the prophylactic use of human immunoglobulin in at-risk populations.102 Santosham and colleagues administered hyperimmunoglobulin (n = 353), or saline placebo (n = 350) to infants at 2, 6, and 10 months of age and examined the rates of invasive Hib. For the first 90 days following the passive immunization protocol, none of the treated infants experienced invasive Hib (0% incidence), compared to 7 of 350 placebo-treated children (2.0% incidence, P = .007).102
Rotavirus represents an enteric viral pathogen wherein protective passive immunotherapy has been demonstrated.103, 104, 105, 106, 107, 108 In one example of postexposure treatment in infants, oral administration of hyperimmune antibody (in addition to standard supportive care) was able to efficiently reduce rotavirus shedding compared to placebo controls; treated patients (n = 26) exhibited no evidence of viral shedding by day 8 posttreatment as compared to 25% of controls (n = 26).105 In a separate study, prophylactic passive immunity using orally administered bovine colostrum from immunized animals was tested in a blinded and randomized trial among infant children (3–15 months old) admitted to a hospital, typically for respiratory conditions.103 Following admission, infants were given a 10-day course of the bovine colostrum or placebo. Infants who received placebo contracted symptomatic rotavirus at a rate of 14% (9 of 65) whereas no symptomatic rotavirus disease was observed in the colostrum-treated infants (0 of 55; P < .001). Analysis of rotavirus vaccine failures also indicates that maternally derived antibodies play a role in passive immunity to rotavirus infection. In a study involving 177 vaccinated infants, a strong inverse correlation was observed between maternally derived rotavirus antibodies and the ability of infants to seroconvert following vaccination with a live rotavirus vaccine.21 This is an important demonstration not only of passive immunity to an enteric pathogen, but also has broader implications on the timing of vaccine administration, especially in developing countries where preexisting immunity is relatively high, and rotavirus vaccine immunogenicity appears impaired.109
E. coli is a significant enteric pathogen wherein prophylaxis through passive immunity has been demonstrated in several clinical studies.110, 111, 112, 113 Tacket and colleagues were able to passively protect human subjects against experimentally induced E. coli diarrhea with specific bovine antibody.110 Using heat-inactivated or glutaraldehyde-inactivated E. coli for vaccination, pregnant cows were hyperimmunized with a large number of enterotoxigenic O serogroups. Milk collected during the first 10 days of lactation was purified, concentrated, lyophilized, and formulated for oral administration. As a control, a similar preparation was made using rotavirus as the immunizing antigen. Subjects received daily treatment (3 times daily) for 7 days, with E. coli challenge administered 3 days into the treatment regimen. Of the 10 subjects who received the E. coli antibody prophylaxis, all remained disease-free following challenge, compared with clinical diarrhea in 9 of 10 placebo subjects (P < .0001). Using a closely related clinical protocol, Otto and colleagues also demonstrated good efficacy with hyperimmune bovine colostrum tablets.111 In the first study conducted in this trial, 11 of 15 (73%) of placebo subjects contracted diarrhea following challenge, but this was reduced to only 1 of 15 (7%) in treated subjects (P = .0005). In a second study investigating the impact of omitting buffer to the oral prophylaxis, the authors also examined dose sparing. In these studies, the standard dose still conferred significant protection with 3 of 15 (20%) treated subjects contracting diarrhea, compared with 12 of 14 (86%) of controls. Interestingly, if the dose was reduced by one-half then disease incidence increased to 5 of 14 subjects (36%), indicating a key role played by treatment dose in achieving successful passive immunotherapy.
Passive Immunization: A Paradigm Shift in Progress?
With any new scientific advance, there is controversy. In 1890, when Behring demonstrated that immune serum therapy could protect against diphtheria, it went against the current dogma at that time in which the cellular theory of phagocytosis was believed to be the primary mechanism of host protection.5 There were also skeptics who, as early as 1896, discussed why antibody immunotherapy would not work.114 However, the science not only prevailed but today a number of passive immunotherapy products are in clinical use (see Table 8.1) and an ever-increasing number of human diseases benefit from the use of this technology (see Table 8.3). Some believe that antibody plays a more important role in protection against cytopathic viruses and extracellular bacteria, but that T cells must be required for protection against infection by noncytopathic viruses and other intracellular pathogens.115 Although this is partially refuted by the protective efficacy of maternal antibodies and IVIG therapy in SCID patients who do not have functioning T cells, it is important to bear in mind that antibody-mediated protection by passive immunotherapy in immunocompetent individuals does not function in isolation, but instead works best in conjunction with other immune defenses, including host T cells, B cells, natural killer (NK) cells, etc. Although the role of antibody-mediated protection against intracellular bacteria and chronic viral infections was thought to be relatively minor, there are examples in each of these instances in which passive immunity provides substantial clinical benefit.
As noted previously, prior to the advent of antibiotics, passive immunotherapy was the only option for clinical treatment of most bacterial infections including Francisella tularensis, a facultative intracellular bacterium that causes tularemia, a severe disease associated with up to 30% mortality in untreated cases.116, 117 When streptomycin became available, a comparative study in 1946 was performed with 542 tularemia patients who received only symptomatic treatment, 832 who received immune equine serum, 60 who received hyperimmune equine serum, and 9 who received streptomycin.118 The untreated tularemia cases required an average of 3.78 months to recover and only three modes of therapy showed substantial improvement—treatment with immune serum within 9 days of disease onset (2.41 months until recovery), treatment with hyperimmune serum (2.15 months until recovery), and treatment with streptomycin (2.40 months until recovery). Two clinical cases were extensively described, with the following summary: “The clinical responses to each agent [i.e., immune serum, and streptomycin] were similar, prompt amelioration of the symptoms of intoxication–headache, mental dullness or lethargy, sense of prostration and severe malaise; reduction of fever and of the sizes of the buboes, acceleration in the healing of ulcers and in the resolution of pulmonary exudates.” In other words, passive immunotherapy appeared in many ways to mimic antibiotic therapy in terms of protective efficacy. However, it was noted that treatment with equine serum caused serum sickness in 51% of the patients and had a more variable outcome than the antibiotic approach, leading to the recommendation that streptomycin would be the agent of choice for future treatment of this disease.118 With the recent development of polyclonal and monoclonal antibodies that show protective efficacy against tularemia in animal models,119, 120, 121 it may be possible to incorporate both passive immunotherapy and antibiotic treatment into clinical practice not only for tularemia, but for other bacterial diseases, especially in cases in which antibiotic resistance is becoming more widespread.122, 123
Mycobacterium tuberculosis is another intracellular bacterium that, despite the availability of antibiotics, remains one of the most common human diseases and it is estimated to infect up to one-third of the world's population.124 The development of strains of extensively drug-resistant (XDR) tuberculosis (TB),125 some of which are resistant to all current antibiotic therapies,126, 127 is also a growing concern, especially as there are few antibiotic drugs in the pipeline.122, 123 There is considerable debate over the role of antibodies in controlling TB, with many believing that antibody plays little or no role in protective immunity (reviewed in references 124 and 128). In a comprehensive historical review by Glatman-Freedman and Casadevall,128 the clinical benefit of antibody-mediated immunotherapy, albeit quite variable, provides evidence to suggest that antibody plays a role in protection against TB. In studies reported by Paquin in 1895, a group of patients with pulmonary TB confirmed by the presence of bacterium in their sputum showed clinical benefit. After 2 months of passive immunotherapy, 82% of patients showed reduced cough, reduction in bacterial load in sputum, clearance of pulmonary infiltrates, reduction in hemoptysis, improved appetite, and weight gain.128, 129 At 6 months after initiating treatment, all the treated patients were alive and more than half were discharged from the hospital. In contrast, more than 30 untreated TB patients from another ward in the hospital had died within 4 months of starting the study. Experimental proof of antibody-mediated protection against TB was also published in 1897 by Fisch.128, 130 After lethal TB challenge of guinea pigs, administration of immune serum was performed on days 4, 7, and 10, with further doses administered every other day for 4 weeks and once a week after that. Fisch reported that 16 of 18 treated animals were alive after 2.5 months (89% survival). If treatment was delayed until day 14 postchallenge, then 2 of 3 (66%) animals survived but showed signs of illness. If no antibody treatment was performed, then 0 of 3 (0%) of the animals survived past day 28. The same approach was used to treat 50 patients with pulmonary TB.131 All of the 19 patients treated at the earliest stages of disease improved rapidly after passive immunotherapy and were tuberculin negative at the end of the study. Of the 11 patients treated at the “incipient” stage of disease, 36% no longer had bacilli in their sputum and were considered cured and 64% showed substantial improvement in disease symptoms. The 20 patients with advanced TB showed only modest or no improvement after therapy and it was concluded that immune serum was only beneficial in early but not advanced cases of disease.131
EBV is a common human pathogen that causes a chronic infection and is a leading cause of posttransplant non-Hodgkin lymphoma resulting from the uncontrolled proliferation of EBV-infected B lymphocytes in patients undergoing immunosuppressive therapies.132 In a large retrospective study involving 44,828 kidney transplant patients, the effect of prophylactic treatment for cytomegalovirus (CMV) on posttransplant incidence of non-Hodgkin lymphomas was examined.133 The standardized incidence ratio (SIR) for non-Hodgkin lymphoma was expressed as the number of lymphoma cases per 100,000 persons and calculated after normalizing for age, sex, and geographical origin. The 30,255 patients who did not receive CMV prophylaxis had a SIR = 26.4, which remained unchanged (SIR = 24.2, P = .62) among the 12,470 patients who received antiviral drugs (acyclovir or ganciclovir). In striking contrast, the 2103 patients who received anti-CMV immunotherapy showed a complete absence of lymphomas during the first year after transplantation (SIR = 0, P = .016 vs. antiviral treatment). The most common anti-CMV immunoglobulin products were shown to contain antibodies against EBV and it is believed that this is the mechanism of action for the protection afforded during the first year posttransplantation.133 In the subsequent 5 years of follow-up, new cases of lymphoma developed at similar rates among all three groups (P = .97). However, because administration of immunoglobulin is typically only performed during the first 4 months after transplantation and antiviral antibody half-life is estimated to be approximately 25 days,134 it is not surprising that the protective effects of passive immunotherapy were only maintained through the first year. Nevertheless, the inadvertent discovery of the protective role of antibodies in preventing EBV-induced non-Hodgkin lymphoma represents a potential breakthrough in clinical management of this vulnerable patient population.
Despite decades of research aimed at finding a vaccine or a cure for HIV infection, this virus remains a scourge of global proportions. Early attempts at passive immunotherapy using first-generation HIV-specific monoclonal antibodies were not highly effective135, 136, 137 and this approach was not further pursued until a new generation of highly potent and broadly neutralizing antibodies were identified.138, 139 In particular, a recent Phase I clinical trial140 involving a single administration of a broadly neutralizing antibody, 3BNC117, has renewed interest in the study of passive immunotherapy for HIV prevention and therapeutic intervention. 3BNC117 is an anti-CD4 binding site antibody that neutralizes 195 of 237 HIV strains comprising six different clades and was tested in a dose-escalation study among HIV-positive patients with different levels of viremia. At a dose of 10 or 30 mg/kg, patient viral load was reduced by up to 2.5 log10 (average decline: 1.48 log10) in 10 of 11 individuals. The subject that did not respond to antibody treatment at 10 mg/kg was infected with a resistant strain of HIV. Although the effect of antibody therapy on viremia was mainly transient after a single administration, the viral load remained lower than their preexisting set point in 3 of 10 patients at 56 days and one subject exhibited viremia levels that remained near the limits of detection throughout the 56-day study. It is currently unclear if HIV viremia in these patients will eventually rebound to their original levels. Similar results were observed during antibody-based therapy of SHIV-infected rhesus macaques in which most animals showed a rebound in viral replication after the transferred monoclonal antibodies declined to undetectable levels but a subset of animals maintained virological control in the absence of further infusions.141 Combinations of antiretroviral drugs are currently the standard of care for treatment of HIV infection and it is unlikely that one dose of a single monoclonal antibody will be sufficient to have a long-term clinical benefit among a broad patient base. However, there is growing optimism that combining a cocktail of potent, broadly neutralizing monoclonal antibodies with antiretroviral drugs and/or agents that activate latent virus reservoirs could theoretically provide long-term reduction in viral load and reduce the rates of transmission.
Future of Passive Immunization
With substantial advances in monoclonal antibody technologies and an increasing appreciation for the role of antibodies in the control of infectious disease, the development of sophisticated new passive immunotherapies is likely to continue at an accelerated pace. Antibiotic resistance among clinically relevant bacteria including multidrug-resistant (MDR) and XDR M. tuberculosis, methicillin-resistant Staphylococcus aureus (MRSA), and dominant strains of antibiotic-resistant Salmonella typhi and other gram-negative bacterial species is a growing concern.122, 125, 142, 143, 144 This, coupled with the knowledge that fewer new antibiotics are moving through the drug pipeline,122, 123 may further motivate research into the development of antibody-based therapies to overcome these challenges to clinical intervention against microbial disease. One drawback to passive immunization is that antibody half-life in vivo often provides only transient protection unless repeated administrations are performed. This may change as new technologies that increase the half-life of monoclonal antibodies are employed. For example, the Fc region of an anti-RSV monoclonal antibody, motavizumab, was mutated to increase its binding to the neonatal Fc receptor (FcRn), resulting in serum antibody pharmacokinetics in human subjects that increased from a typical 19- to 34-day half-life to up to a 100-day half-life while still retaining virus-specific neutralizing activity.144a Nevertheless, while passive immunization may be sufficient for protection or therapeutic intervention of acute or remittent disease, active immunization through improved vaccine design may still be needed to train the host immune system to maintain long-term levels of protective immunity. Importantly, examples of successful passive immunization approaches may provide a useful framework for developing new and improved vaccines that elicit the most protective antibody responses.
![]() References for this chapter are available at ExpertConsult.com.
References for this chapter are available at ExpertConsult.com.
References
- 1.von Behring E, Kitasato S. Ueber das zustandekommen der diphtherie-immunitat und der tetanus-immunitat bei thieren (On the realization of immunity in diphtheria and tetanus in animals) Dtsch Med Wochenschr. 1890;16:1113–1114. [Google Scholar]
- 2.Molz G. Variations in child mortality in the past 100 years. Helv Paediatr Acta. 1970;25:1–12. in German. [PubMed] [Google Scholar]
- 3.Kossel H. Ueber die Behandlung diphtheriekranker Kinder mit “Diphtherieheilserum” (Concerning the treatment of children suffering from diphtheria with “diphtheria serum”) Dtsch Med Wochenschr. 1893;19:392–393. [Google Scholar]
- 4.Grundbacher FJ. Behring's discovery of diphtheria and tetanus antitoxins. Immunol Today. 1992;13(5):188–190. doi: 10.1016/0167-5699(92)90125-Q. [DOI] [PubMed] [Google Scholar]
- 5.Winau F, Winau R. Emil von Behring and serum therapy. Microbes Infect. 2002;4(2):185–188. doi: 10.1016/s1286-4579(01)01526-x. [DOI] [PubMed] [Google Scholar]
- 6.Obituary on Emil von Behring. Lancet. 1917;890 [Google Scholar]
- 7.Linton DS. American Philosophical Society; Philadelphia: 2005. Emil von Behring: Infectious Disease, Immunology, Serum Therapy. [Google Scholar]
- 8.von Behring E. Thieme; Leipzig: 1893. Gesammelte abhandlungen zur Atiologischen Therapie der Ansteckenden Krankheiten (Collected Treaties of Aetiologic Therapy of Infectious Diseases) [Google Scholar]
- 9.Stiehm ER, Keller MA. Passive Immunization. In: Feigin RD, Cherry JD, Demmler-Harrison GJ, editors. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. 6th ed. Saunders Elsevier; Philadelphia: 2009. pp. 3401–3446. [Google Scholar]
- 10.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 11.Alkan SS. Monoclonal antibodies: the story of a discovery that revolutionized science and medicine. Nat Rev Immunol. 2004;4(2):153–156. doi: 10.1038/nri1265. [DOI] [PubMed] [Google Scholar]
- 12.Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25(12):1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traggiai E, Becker S, Subbarao K. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- 15.Ukkonen P, Hovi T, von Bonsdorff C-H, Saikku P, Penttinen K. Age-specific prevalence of complement-fixing antibodies to sixteen viral antigens: A computer analysis of 58,500 patients covering a period of eight years. J Med Virol. 1984;13:131–148. doi: 10.1002/jmv.1890130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer A. Severe combined immunodeficiencies (SCID) Clin Exp Immunol. 2000;122(2):143–149. doi: 10.1046/j.1365-2249.2000.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederman HM, Winkelstein JA. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 1985;64(3):145–156. [PubMed] [Google Scholar]
- 18.Albrecht P, Ennis FA, Saltzman EJ, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91(5):715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 19.Hayden GF. Measles vaccine failure. A survey of causes and means of prevention. Clin Pediatr (Phila) 1979;18(3):155–156. doi: 10.1177/000992287901800308. 161–153,167. [DOI] [PubMed] [Google Scholar]
- 20.Orenstein WA, Markowitz L, Preblud SR. Appropriate age for measles vaccination in the United States. Dev Biol Stand. 1986;65:13–21. [PubMed] [Google Scholar]
- 21.Appaiahgari MB, Glass R, Singh S. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine. 2014;32(6):651–656. doi: 10.1016/j.vaccine.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Becker-Dreps S, Vilchez S, Velasquez D. Rotavirus-specific IgG antibodies from mothers' serum may inhibit infant immune responses to the pentavalent rotavirus vaccine. Pediatr Infect Dis J. 2015;34(1):115–116. doi: 10.1097/INF.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaman K, Roy E, Arifeen SE. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355–1361. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poehling KA, Szilagyi PG, Staat MA. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol. 2011;204(6 suppl 1):S141–S148. doi: 10.1016/j.ajog.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Rie A, Wendelboe AM, Englund JA. Role of maternal pertussis antibodies in infants. Pediatr Infect Dis J. 2005;24:S62–S65. doi: 10.1097/01.inf.0000160915.93979.8f. [DOI] [PubMed] [Google Scholar]
- 27.Kendrick P, Thompson M, Eldering G. Immunity response of mothers and babies to injections of pertussis vaccine during pregnancy. Am J Dis Child. 1945;70(1):25–28. [Google Scholar]
- 28.Lichty JA, Slavin B, Bradford WL. An attempt to increase resistance to pertussis in newborn infants by immunizing their mothers during pregnancy. J Clin Invest. 1938;17(5):613–621. doi: 10.1172/JCI100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen P, Scadron SJ. The placental transmission of protective antibodies against whooping cough by inoculation of the pregnant mother. JAMA. 1943;121(9):656–662. [Google Scholar]
- 30.Amirthalingam G, Andrews N, Campbell H. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 31.Dabrera G, Amirthalingam G, Andrews N. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis. 2015;60:333–337. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 32.Swamy GK, Wheeler SM. Neonatal pertussis, cocooning and maternal immunization. Expert Rev Vaccines. 2014;13(9):1107–1114. doi: 10.1586/14760584.2014.944509. [DOI] [PubMed] [Google Scholar]
- 33.Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38(8):1695–1702. doi: 10.1128/aac.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21(1):150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeitlin L, Cone RA, Moench TR, Whaley KJ. Preventing infectious disease with passive immunization. Microbes Infect. 2000;2(6):701–708. doi: 10.1016/s1286-4579(00)00355-5. [DOI] [PubMed] [Google Scholar]
- 36.Lang AB, Cryz SJ, Jr, Schurch U, Ganss MT, Bruderer U. Immunotherapy with human monoclonal antibodies. Fragment A specificity of polyclonal and monoclonal antibodies is crucial for full protection against tetanus toxin. J Immunol. 1993;151(1):466–472. [PubMed] [Google Scholar]
- 37.Chen Z, Earl P, Americo J. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci USA. 2006;103:1882–1887. doi: 10.1073/pnas.0510598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Chen FP, Megaw AG, Sullender WM. Variable resistance to palivizumab in cotton rats by respiratory syncytial virus mutants. J Infect Dis. 2004;190(11):1941–1946. doi: 10.1086/425515. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Chen FP, Sullender WM. Respiratory syncytial virus escape mutant derived in vitro resists palivizumab prophylaxis in cotton rats. Virology. 2004;318(2):608–612. doi: 10.1016/j.virol.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 40.DeVincenzo JP, Hall CB, Kimberlin DW. Surveillance of clinical isolates of respiratory syncytial virus for palivizumab (Synagis)-resistant mutants. J Infect Dis. 2004;190(5):975–978. doi: 10.1086/423213. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Q, McAuliffe JM, Patel NK. Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J Infect Dis. 2011;203(5):674–682. doi: 10.1093/infdis/jiq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 43.Lemaitre-Coelho I, Yamakido M, Montgomery PC, Langendries AE, Vaerman JP. Selective excretion of IgA in rat bronchial secretions: lack of significant contribution from plasma IgA. Immunol Commun. 1982;11(6):441–453. doi: 10.3109/08820138209050741. [DOI] [PubMed] [Google Scholar]
- 44.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49(4):673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoof SP, van der Klis FR, van Rooijen DM. Salivary antibody levels in adolescents in response to a meningococcal serogroup C conjugate booster vaccination nine years after priming: systemically induced local immunity and saliva as potential surveillance tool. Vaccine. 2015;33(32):3933–3939. doi: 10.1016/j.vaccine.2015.06.055. [DOI] [PubMed] [Google Scholar]
- 46.Parren PW, Marx PA, Hessell AJ. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75(17):8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dowdle WR, Coleman MT, Mostow SR, Kaye HS, Schoenbaum SC. Inactivated influenza vaccines. 2. Laboratory indices of protection. Postgrad Med J. 1973;49(569):159–163. doi: 10.1136/pgmj.49.569.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24(1):157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tasman A, Lansberg HP. Problems concerning the prophylaxis, pathogenesis and therapy of diphtheria. Bull World Health Organ. 1957;16(5):939–973. [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen J, Vincent JL, Adhikari NK. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(5):581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 51.Hunter TM. Prevention of Measles by Convalescent Serum. Br Med J. 1933;1(3762):217–219. doi: 10.1136/bmj.1.3762.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janeway C. Use of concentrated human serum g-globulin in the prevention and attenuation of measles. Bull N Y Acad Med. 1945;21:202–222. [PMC free article] [PubMed] [Google Scholar]
- 53.Endo A, Izumi H, Miyashita M. Current efficacy of postexposure prophylaxis against measles with immunoglobulin. J Pediatr. 2001;138(6):926–928. doi: 10.1067/mpd.2001.113710. [DOI] [PubMed] [Google Scholar]
- 54.Meyer KF. The therapeutic use of convalescent serum in poliomyelitis. Cal West Med. 1936;44(4):254. [PMC free article] [PubMed] [Google Scholar]
- 55.Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99(1):93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 56.Feltes TF, Cabalka AK, Meissner HC. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 57.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3):531–537. [PubMed] [Google Scholar]
- 58.Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J, Group IS. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J. 2003;22(9):823–827. doi: 10.1097/01.inf.0000086403.50417.7c. [DOI] [PubMed] [Google Scholar]
- 59.Ramilo O, Lagos R, Saez-Llorens X. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatr Infect Dis J. 2014;33(7):703–709. doi: 10.1097/INF.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 60.Carbonell-Estrany X, Simoes EA, Dagan R. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 61.Saez-Llorens X, Moreno MT, Ramilo O. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23(8):707–712. doi: 10.1097/01.inf.0000133165.85909.08. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez WJ, Gruber WC, Groothuis JR. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics. 1997;100(6):937–942. doi: 10.1542/peds.100.6.937. [DOI] [PubMed] [Google Scholar]
- 63.Patel TB, Naidu BPB. Smallpox and sulphonamide. Ind Med Gaz. 1940;75:730–732. [PMC free article] [PubMed] [Google Scholar]
- 64.Kempe CH, Bowles C, Meiklejohn G. The use of vaccinia hyperimmune gammaglobulin in the prophylaxis of smallpox. Bull World Health Organ. 1961;25:41–48. [PMC free article] [PubMed] [Google Scholar]
- 65.Marennikova SS. The use of hyperimmune antivaccinia gamma-globulin for the prevention and treatment of smallpox. Bull World Health Organ. 1962;27:325–330. [PMC free article] [PubMed] [Google Scholar]
- 66.Couzi G, Kircher JP. Immunotherapie de la Variole. Bulletin de l'Institut d'hygiène. 1941;1:59–68. [Google Scholar]
- 67.Enria DA, Briggiler AM, Sanchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res. 2008;78(1):132–139. doi: 10.1016/j.antiviral.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2(8154):1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 69.Ruggiero HA, Perez Isquierdo F, Milani HA. [Treatment of Argentine hemorrhagic fever with convalescent's plasma. 4433 cases] Presse Med. 1986;15(45):2239–2242. [PubMed] [Google Scholar]
- 70.Weissenbacher M, De Guerrero LB, Parodi AS. Acción de los inmunosueros en la fiebre hemorrágica experimental. Medicina (Kaunas) 1968;28(2):53–58. [PubMed] [Google Scholar]
- 71.Kenyon RH, Green DE, Eddy GA, Peters CJ. Treatment of junin virus-infected guinea pigs with immune serum: development of late neurological disease. J Med Virol. 1986;20(3):207–218. doi: 10.1002/jmv.1890200303. [DOI] [PubMed] [Google Scholar]
- 72.Kenyon RH, Condie RM, Jahrling PB, Peters CJ. Protection of guinea pigs against experimental Argentine hemorrhagic fever by purified human IgG: importance of elimination of infected cells. Microb Pathog. 1990;9:219–226. doi: 10.1016/0882-4010(90)90010-n. [DOI] [PubMed] [Google Scholar]
- 73.Monath TP, Casals J. Diagnosis of Lassa fever and the isolation and management of patients. Bull World Health Organ. 1975;52(4-6):707–715. [PMC free article] [PubMed] [Google Scholar]
- 74.Jahrling PB, Frame JD, Rhoderick JB, Monson MH. Endemic Lassa fever in Liberia. IV. Selection of optimally effective plasma for treatment by passive immunization. Trans R Soc Trop Med Hyg. 1985;79(3):380–384. doi: 10.1016/0035-9203(85)90388-8. [DOI] [PubMed] [Google Scholar]
- 75.Jahrling PB. Protection of Lassa virus-infected guinea pigs with Lassa-immune plasma of guinea pig, primate, and human origin. J Med Virol. 1983;12(2):93–102. doi: 10.1002/jmv.1890120203. [DOI] [PubMed] [Google Scholar]
- 76.Jahrling PB, Peters CJ. Passive antibody therapy of Lassa fever in cynomolgus monkeys: importance of neutralizing antibody and Lassa virus strain. Infect Immun. 1984;44(2):528–533. doi: 10.1128/iai.44.2.528-533.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jahrling PB, Peters CJ, Stephen EL. Enhanced treatment of Lassa fever by immune plasma combined with ribavirin in cynomolgus monkeys. J Infect Dis. 1984;149(3):420–427. doi: 10.1093/infdis/149.3.420. [DOI] [PubMed] [Google Scholar]
- 78.Clayton AJ. Lassa immune serum. Bull World Health Organ. 1977;55(4):435–439. [PMC free article] [PubMed] [Google Scholar]
- 79.Frame JD, Verbrugge GP, Gill RG, Pinneo L. The use of Lassa fever convalescent plasma in Nigeria. Trans R Soc Trop Med Hyg. 1984;78(3):319–324. doi: 10.1016/0035-9203(84)90107-x. [DOI] [PubMed] [Google Scholar]
- 80.Zingher A. Convalescent whole blood plasma and serum in prophylaxis of measles. JAMA. 1924;82(15):1180–1187. doi: 10.1002/rmv.480. [DOI] [PubMed] [Google Scholar]
- 81.Gunn W. The serum prophylaxis of measles: (section of epidemiology and state medicine) Proc R Soc Med. 1938;31(7):828–840. doi: 10.1177/003591573803100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4) doi: 10.1002/14651858.CD010056.pub2. CD010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ordman CW, Jennings CG, Janeway CA. Chemical, clinical, and immunological studies on the products of human plasma fractionation. Xii. The use of concentrated normal human serum gamma globulin (human immune serum globulin) in the prevention and attenuation of measles. J Clin Invest. 1944;23(4):541–549. doi: 10.1172/JCI101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKhann CF. The prevention and modification of measles. JAMA. 1937;109(25):2034–2038. [Google Scholar]
- 85.Simonsen L, Spreeuwenberg P, Lustig R. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013;10:e1001558. doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 87.Nair H, Brooks WA, Katz M. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 88.Thompson WW, Shay DK, Weintraub E. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 89.Stuart-Harris CH. Immunity to influenza. J Infect Dis. 1972;126(4):466–468. doi: 10.1093/infdis/126.4.466. [DOI] [PubMed] [Google Scholar]
- 90.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70(4):767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morris JA, Kasel JA, Saglam M, Knight V, Loda FA. Immunity to influenza as related to antibody levels. N Engl J Med. 1966;274(10):527–535. doi: 10.1056/NEJM196603102741001. [DOI] [PubMed] [Google Scholar]
- 92.Akerfeldt S, Geijer S, Holubars E, Fuchs G, Brundin M. Prophylactic and therapeutic antiviral effect of human gamma globulin. Biochem Pharmacol. 1972;21:503–509. doi: 10.1016/0006-2952(72)90323-1. [DOI] [PubMed] [Google Scholar]
- 93.Laidlaw PP, Smith W, Andrews CH, Dunkin GW. Influenza: The preparation of immune sera in horses. Br J Exp Pathol. 1935;16(3):275–290. [Google Scholar]
- 94.Henle W, Stokes JJ, Shaw DR. Passive immunization of mice against human influenza virus by the intranasal route. J Immunol. 1941;40:201–212. [Google Scholar]
- 95.Itoh Y, Yoshida R, Shichinohe S. Protective efficacy of passive immunization with monoclonal antibodies in animal models of H5N1 highly pathogenic avian influenza virus infection. PLoS Pathog. 2014;10:e1004192. doi: 10.1371/journal.ppat.1004192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corti D, Voss J, Gamblin SJ. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 97.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 98.Hung IF, To KK, Lee CK. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144:464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 99.Alexander HE. Treatment of haemophilus influenzae infections and of meningococcic and pneumococcic meningitis. Am J Dis Child. 1943;66(2):172–187. [Google Scholar]
- 100.Edmonds AM, Neter E. Appraisal of treatment of Hemophilus influenzae type B meningitis with specific rabbit serum and sulfonamides; based on observation of 60 cases. J Pediatr. 1946;28:462–470. doi: 10.1016/s0022-3476(46)80029-5. [DOI] [PubMed] [Google Scholar]
- 101.Beck KH, Janney FR. Alexander's rabbit serum in the treatment of influenzal meningitis; an evaluation of its use in conjunction with sulfonamide compounds. JAMA. 1947;73(3):317–325. doi: 10.1001/archpedi.1947.02020380062004. [DOI] [PubMed] [Google Scholar]
- 102.Santosham M, Reid R, Letson GW, Wolff MC, Siber G. Passive immunization for infection with Haemophilus influenzae type b. Pediatrics. 1990;85(4 Pt 2):662–666. [PubMed] [Google Scholar]
- 103.Davidson GP, Whyte PB, Daniels E. Passive immunisation of children with bovine colostrum containing antibodies to human rotavirus. Lancet. 1989;2:709–712. doi: 10.1016/s0140-6736(89)90771-x. [DOI] [PubMed] [Google Scholar]
- 104.Hammarstrom L. Passive immunity against rotavirus in infants. Acta Paediatr. 1999;88(430):127–132. doi: 10.1111/j.1651-2227.1999.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 105.Rahman S, Higo-Moriguchi K, Htun KW. Randomized placebo-controlled clinical trial of immunoglobulin Y as adjunct to standard supportive therapy for rotavirus-associated diarrhea among pediatric patients. Vaccine. 2012;30:4661–4669. doi: 10.1016/j.vaccine.2012.04.091. [DOI] [PubMed] [Google Scholar]
- 106.Mitra AK, Mahalanabis D, Ashraf H. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: a double-blind, controlled clinical trial. Acta Paediatr. 1995;84(9):996–1001. doi: 10.1111/j.1651-2227.1995.tb13814.x. [DOI] [PubMed] [Google Scholar]
- 107.Sarker SA, Casswall TH, Mahalanabis D. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatr Infect Dis J. 1998;17(12):1149–1154. doi: 10.1097/00006454-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 108.Sarker SA, Casswall TH, Juneja LR. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J Pediatr Gastroenterol Nutr. 2001;32(1):19–25. doi: 10.1097/00005176-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 109.Patel M, Shane AL, Parashar UD. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200(suppl 1):S39–S48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tacket CO, Losonsky G, Link H. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N Engl J Med. 1988;318(19):1240–1243. doi: 10.1056/NEJM198805123181904. [DOI] [PubMed] [Google Scholar]
- 111.Otto W, Najnigier B, Stelmasiak T, Robins-Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand J Gastroenterol. 2011;46(7-8):862–868. doi: 10.3109/00365521.2011.574726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tawfeek HI, Najim NH, Al-Mashikhi S. Efficacy of an infant formula containing anti-Escherichia coli colostral antibodies from hyperimmunized cows in preventing diarrhea in infants and children: a field trial. Int J Infect Dis. 2003;7:120–128. doi: 10.1016/s1201-9712(03)90007-5. [DOI] [PubMed] [Google Scholar]
- 113.Freedman DJ, Tacket CO, Delehanty A. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis. 1998;177(3):662–667. doi: 10.1086/514227. [DOI] [PubMed] [Google Scholar]
- 114.Strueh C. Once more on antitoxin. JAMA. 1896;26:957–964. [Google Scholar]
- 115.Zinkernagel RM, Bachmann MF, Kundig TE. On immunological memory. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 116.Sjostedt A. Virulence determinants and protective antigens of Francisella tularensis. Curr Opin Microbiol. 2003;6(1):66–71. doi: 10.1016/s1369-5274(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 117.McLendon MK, Apicella MA, Allen LA. Francisella tularensis: taxonomy, genetics, and Immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Foshay L. A comparative study of the treatment of tularemia with immune serum, hyperimmune serum and streptomycin. Am J Med. 1946;1:180–188. doi: 10.1016/0002-9343(46)90036-8. [DOI] [PubMed] [Google Scholar]
- 119.Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308(2):83–87. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 120.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19(31):4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 121.Lu Z, Roche MI, Hui JH. Generation and characterization of hybridoma antibodies for immunotherapy of tularemia. Immunol Lett. 2007;112(2):92–103. doi: 10.1016/j.imlet.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brooks BD, Brooks AE. Therapeutic strategies to combat antibiotic resistance. Adv Drug Deliv Rev. 2014;78:14–27. doi: 10.1016/j.addr.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 123.Servick K. The drug push. Science. 2015;348:850–853. doi: 10.1126/science.348.6237.850. [DOI] [PubMed] [Google Scholar]
- 124.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe. 2013;13(3):250–262. doi: 10.1016/j.chom.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gandhi NR, Nunn P, Dheda K. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 126.Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill. 2007;12(5):E070517.1. doi: 10.2807/esw.12.20.03194-en. [DOI] [PubMed] [Google Scholar]
- 127.Velayati AA, Masjedi MR, Farnia P. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest. 2009;136(2):420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 128.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11(3):514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paquin P. The treatment of tuberculosis by injections of immunized blood serum. JAMA. 1895;29:842–845. [Google Scholar]
- 130.Fisch C. The antitoxic and bactericidal properties of the serum of horses treated with Koch's new tuberculin. JAMA. 1897;29:882–889. [Google Scholar]
- 131.Holmes AM. A further report on the use of “anti-phthisic serum T.R.” (Fisch) in tuberculosis. JAMA. 1899;33:886–888. [Google Scholar]
- 132.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1(1):106–112. [PMC free article] [PubMed] [Google Scholar]
- 133.Opelz G, Daniel V, Naujokat C, Fickenscher H, Dohler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol. 2007;8(3):212–218. doi: 10.1016/S1470-2045(07)70040-2. [DOI] [PubMed] [Google Scholar]
- 134.Thurmann PA, Sonnenburg C, Valentova K. Pharmacokinetics of viral antibodies after administration of intravenous immunoglobulin in patients with chronic lymphocytic leukaemia or multiple myeloma. Eur J Clin Pharmacol. 2001;57(3):235–241. doi: 10.1007/s002280100305. [DOI] [PubMed] [Google Scholar]
- 135.Armbruster C, Stiegler GM, Vcelar BA. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J Antimicrob Chemother. 2004;54(5):915–920. doi: 10.1093/jac/dkh428. [DOI] [PubMed] [Google Scholar]
- 136.Trkola A, Kuster H, Rusert P. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 137.Mehandru S, Vcelar B, Wrin T. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81(20):11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klein F, Mouquet H, Dosenovic P. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341(6151):1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.West AP, Jr, Scharf L, Scheid JF. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156(4):633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Caskey M, Klein F, Lorenzi JC. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015 doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barouch DH, Whitney JB, Moldt B. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wong VK, Baker S, Pickard DJ. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet. 2015 doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tarai B, Das P, Kumar D. Recurrent challenges for clinicians: emergence of methicillin-resistant Staphylococcus aureus, vancomycin resistance, and current treatment options. J Lab Physicians. 2013;5(2):71–78. doi: 10.4103/0974-2727.119843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poulakou G, Bassetti M, Righi E, Dimopoulos G. Current and future treatment options for infections caused by multidrug-resistant gram-negative pathogens. Future Microbiol. 2014;9(9):1053–1069. doi: 10.2217/fmb.14.58. [DOI] [PubMed] [Google Scholar]
- Robbie GJ, Criste R, Dall'acqua WF. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother. 2013;57:6147–6153. doi: 10.1128/AAC.01285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Z, Moayeri M, Purcell R. Monoclonal antibody therapies against anthrax. Toxins (Basel) 2011;3:1004–1019. doi: 10.3390/toxins3081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Migone TS, Subramanian GM, Zhong J. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 147.Symmers D. The serum treatment of anthrax septicaemia. Ann Surg. 1922;75(6):663–667. doi: 10.1097/00000658-192206000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Regan JC. The local and general serum treatment of cutaneous anthrax. JAMA. 1921;77(25):1944–1948. [Google Scholar]
- 149.Gelzleichter TR, Myers MA, Menton RG. Protection against botulinum toxins provided by passive immunization with botulinum human immune globulin: evaluation using an inhalation model. J Appl Toxicol. 1999;19(suppl 1):S35–S38. doi: 10.1002/(sici)1099-1263(199912)19:1+<s35::aid-jat612>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 150.Shearer JD, Vassar ML, Swiderski W. Botulinum neurotoxin neutralizing activity of immune globulin (IG) purified from clinical volunteers vaccinated with recombinant botulinum vaccine (rBV A/B) Vaccine. 2010;28:7313–7318. doi: 10.1016/j.vaccine.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 151.Cheng LW, Stanker LH, Henderson TD, 2nd, Lou J, Marks JD. Antibody protection against botulinum neurotoxin intoxication in mice. Infect Immun. 2009;77:4305–4313. doi: 10.1128/IAI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tacket CO, Shandera WX, Mann JM, Hargrett NT, Blake PA. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med. 1984;76:794–798. doi: 10.1016/0002-9343(84)90988-4. [DOI] [PubMed] [Google Scholar]
- 153.Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med. 2006;354:462–471. doi: 10.1056/NEJMoa051926. [DOI] [PubMed] [Google Scholar]
- 154.Chang GY, Ganguly G. Early antitoxin treatment in wound botulism results in better outcome. Eur Neurol. 2003;49:151–153. doi: 10.1159/000069073. [DOI] [PubMed] [Google Scholar]
- 155.Underwood K, Rubin S, Deakers T, Newth C. Infant botulism: a 30-year experience spanning the introduction of botulism immune globulin intravenous in the intensive care unit at Childrens Hospital Los Angeles. Pediatrics. 2007;120:e1380–e1385. doi: 10.1542/peds.2006-3276. [DOI] [PubMed] [Google Scholar]
- 156.Sevigny LM, Booth BJ, Rowley KJ. Identification of a human monoclonal antibody to replace equine diphtheria antitoxin for treatment of diphtheria intoxication. Infect Immun. 2013;81:3992–4000. doi: 10.1128/IAI.00462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smith JW, Schallibaum EM. The therapeutic effect of homologous and heterologous antitoxins in experimental diphtheria and tetanus. Br J Exp Pathol. 1970;51(1):73–80. [PMC free article] [PubMed] [Google Scholar]
- 158.Tasman A, Minkenhof JE, Vink HH, Brandwijk AC, Smith L. Importance of intravenous injection of diphtheria antiserum. Lancet. 1958;1(7034):1299–1304. doi: 10.1016/s0140-6736(58)92061-0. [DOI] [PubMed] [Google Scholar]
- 159.Ford WW. The recent epidemic of diphtheria in the Johns Hopkins hospital and medical school: General procedures adopted. Johns Hopkins Med J. 1911;22(248):357–361. [Google Scholar]
- 160.Park WH. Use of diphtheria antitoxin in the treatment and prevention of diphtheria. JAMA. 1900;34(15):902–904. [Google Scholar]
- 161.Logina I, Donaghy M. Diphtheritic polyneuropathy: a clinical study and comparison with Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 1999;67(4):433–438. doi: 10.1136/jnnp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lemley PV, Wright DC. Mice are actively immunized after passive monoclonal antibody prophylaxis and ricin toxin challenge. Immunology. 1992;76(3):511–513. [PMC free article] [PubMed] [Google Scholar]
- 163.Hu WG, Yin J, Chau D. Conformation-dependent high-affinity potent ricin-neutralizing monoclonal antibodies. Biomed Res Int. 2013;2013:471346. doi: 10.1155/2013/471346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matsuda M, Kamei M, Sugimoto N, Ma Y, Hashizume S. Characteristics of toxin-neutralization by anti-tetanus human monoclonal antibodies directed against the three functional domains [A], [B] and [C] of the tetanus toxin molecule and a reliable method for evaluating the protective effects of monoclonal antibodies. Eur J Epidemiol. 1992;8(1):1–8. doi: 10.1007/BF02427384. [DOI] [PubMed] [Google Scholar]
- 165.Sherrington CS. Observations with antitetanus serum in the monkey. Lancet. 1917;190(4922):964–966. [Google Scholar]
- 166.Bruce D. Tetanus: Analysis of 1458 cases, which occurred in home military hospitals during the years 1914–1918. J Hyg (Lond) 1920;19(1):1–32. doi: 10.1017/s0022172400007671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kabura L, Ilibagiza D, Menten J, Van den Ende J. Intrathecal vs. intramuscular administration of human antitetanus immunoglobulin or equine tetanus antitoxin in the treatment of tetanus: a meta-analysis. Trop Med Int Health. 2006;11:1075–1081. doi: 10.1111/j.1365-3156.2006.01659.x. [DOI] [PubMed] [Google Scholar]
- 168.Huntington RW, Thompson WR, Gordon HH. The treatment of tetanus with antitoxin: An analysis of the outcome in six-hundred forty-two cases. Ann Surg. 1937;105(1):93–96. doi: 10.1097/00000658-193701000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sato H, Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990;58(10):3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bruss JB, Siber GR. Protective effects of pertussis immunoglobulin (P-IGIV) in the aerosol challenge model. Clin Diagn Lab Immunol. 1999;6(4):464–470. doi: 10.1128/cdli.6.4.464-470.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bradford WL, Brooks AM, Katsampes CP. The therapeutic effect of sulfadiazine and immune rabbit serum in experimental murine pertussis. Yale J Biol Med. 1944;16(5):435–442. [PMC free article] [PubMed] [Google Scholar]
- 172.Meader FM. Prophylaxis of whooping cough. Am J Dis Child. 1937;53(3):760–767. [Google Scholar]
- 173.Bradford WL. Use of convalescent blood in whooping cough with a review of the literature. Am J Dis Child. 1935;50(4):918–928. [Google Scholar]
- 174.Granstrom M, Olinder-Nielsen AM, Holmblad P, Mark A, Hanngren K. Specific immunoglobulin for treatment of whooping cough. Lancet. 1991;338(8777):1230–1233. doi: 10.1016/0140-6736(91)92101-7. [DOI] [PubMed] [Google Scholar]
- 175.Hanson MS, Cassatt DR, Guo BP. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66(5):2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Johnson RC, Kodner C, Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986;53(3):713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang YX, Stewart SJ, Caldwell HD. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–582. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 179.Rank RG, Batteiger BE. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun. 1989;57(1):299–301. doi: 10.1128/iai.57.1.299-301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Babcock GJ, Broering TJ, Hernandez HJ. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun. 2006;74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang Z, Ramsey J, Hamza T. Mechanisms of protection against Clostridium difficile infection by the monoclonal antitoxin antibodies actoxumab and bezlotoxumab. Infect Immun. 2015;83:822–831. doi: 10.1128/IAI.02897-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lowy I, Molrine DC, Leav BA. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 183.Imberechts H, Deprez P, Van Driessche E, Pohl P. Chicken egg yolk antibodies against F18ab fimbriae of Escherichia coli inhibit shedding of F18 positive E. coli by experimentally infected pigs. Vet Microbiol. 1997;54:329–341. doi: 10.1016/s0378-1135(96)01293-x. [DOI] [PubMed] [Google Scholar]
- 184.Kim KS, Cross AS, Zollinger W, Sadoff J. Prevention and therapy of experimental Escherichia coli infection with monoclonal antibody. Infect Immun. 1985;50(3):734–737. doi: 10.1128/iai.50.3.734-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sheoran AS, Chapman S, Singh P, Donohue-Rolfe A, Tzipori S. Stx2-specific human monoclonal antibodies protect mice against lethal infection with Escherichia coli expressing Stx2 variants. Infect Immun. 2003;71(6):3125–3130. doi: 10.1128/IAI.71.6.3125-3130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brunser O, Espinoza J, Figueroa G. Field trial of an infant formula containing anti-rotavirus and anti-Escherichia coli milk antibodies from hyperimmunized cows. J Pediatr Gastroenterol Nutr. 1992;15(1):63–72. doi: 10.1097/00005176-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 187.Casswall TH, Sarker SA, Faruque SM. Treatment of enterotoxigenic and enteropathogenic Escherichia coli-induced diarrhoea in children with bovine immunoglobulin milk concentrate from hyperimmunized cows: a double-blind, placebo-controlled, clinical trial. Scand J Gastroenterol. 2000;35(7):711–718. doi: 10.1080/003655200750023372. [DOI] [PubMed] [Google Scholar]
- 188.Foshay L. Tularemia: A summary of certain aspects of the disease including methods for early diagnosis and the results of serum treatment in 600 patients. Medicine (Baltimore) 1940;19(1):1–84. [Google Scholar]
- 189.Ambrosino D, Schreiber JR, Daum RS, Siber GR. Efficacy of human hyperimmune globulin in prevention of Haemophilus influenzae type b disease in infant rats. Infect Immun. 1983;39(2):709–714. doi: 10.1128/iai.39.2.709-714.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Borrelli S, Diab A, Lindberg A, Svanborg C. Monoclonal anti-LPS inner core antibodies protect against experimental hematogenous Haemophilus influenzae type b meningitis. Microb Pathog. 2000;28:1–8. doi: 10.1006/mpat.1999.0318. [DOI] [PubMed] [Google Scholar]
- 191.Gigliotti F, Insel RA. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- 192.Wollstein M. Serum treatment of influenzal meningitis. J Exp Med. 1911;14(1):73–82. doi: 10.1084/jem.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hamasur B, Haile M, Pawlowski A. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roy E, Stavropoulos E, Brennan J. Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice. Infect Immun. 2005;73:6101–6109. doi: 10.1128/IAI.73.9.6101-6109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guirado E, Amat I, Gil O. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–1259. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 196.Schmidt S, Zhu D, Barniak V. Passive immunization with Neisseria meningitidis PorA specific immune sera reduces nasopharyngeal colonization of group B meningococcus in an infant rat nasal challenge model. Vaccine. 2001;19:4851–4858. doi: 10.1016/s0264-410x(01)00229-8. [DOI] [PubMed] [Google Scholar]
- 197.Saukkonen K, Abdillahi H, Poolman JT, Leinonen M. Protective efficacy of monoclonal antibodies to class 1 and class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model: new prospects for vaccine development. Microb Pathog. 1987;3:261–267. doi: 10.1016/0882-4010(87)90059-3. [DOI] [PubMed] [Google Scholar]
- 198.Miller CP, Castles R. Experimental meningococcal infection in the mouse. J Infect Dis. 1936;58(3):263–279. [Google Scholar]
- 199.Flexner S. Experimental cerebro-spinal meningitis and its serum treatment. JAMA. 1906;47(8):560–566. [Google Scholar]
- 200.Brodeur BR, Larose Y, Tsang P. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985;50(2):510–516. doi: 10.1128/iai.50.2.510-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Flexner S. The results of the serum treatment in thirteen hundred cases of epidemic meningitis. J Exp Med. 1913;17(5):553–576. doi: 10.1084/jem.17.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Warrener P, Varkey R, Bonnell JC. A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models. Antimicrob Agents Chemother. 2014;58:4384–4391. doi: 10.1128/AAC.02643-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.DiGiandomenico A, Keller AE, Gao C. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci Transl Med. 2014;6:262ra155. doi: 10.1126/scitranslmed.3009655. [DOI] [PubMed] [Google Scholar]
- 204.Adawi A, Bisignano C, Genovese T. In vitro and in vivo properties of a fully human IgG1 monoclonal antibody that combats multidrug resistant Pseudomonas aeruginosa. Int J Mol Med. 2012;30(3):455–464. doi: 10.3892/ijmm.2012.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Van Wye JE, Collins MS, Baylor M. Pseudomonas hyperimmune globulin passive immunotherapy for pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 1990;9(1):7–18. doi: 10.1002/ppul.1950090104. [DOI] [PubMed] [Google Scholar]
- 206.Winnie GB, Cowan RG, Wade NA. Intravenous immune globulin treatment of pulmonary exacerbations in cystic fibrosis. J Pediatr. 1989;114(2):309–314. doi: 10.1016/s0022-3476(89)80804-2. [DOI] [PubMed] [Google Scholar]
- 207.Francois B, Luyt CE, Dugard A. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized,double-blind, placebo-controlled trial. Crit Care Med. 2012;40(8):2320–2326. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- 208.Singh SP, Williams YU, Benjamin WH, Klebba PE, Boyd D. Immunoprotection by monoclonal antibodies to the porins and lipopolysaccharide of Salmonella typhimurium. Microb Pathog. 1996;21:249–263. doi: 10.1006/mpat.1996.0059. [DOI] [PubMed] [Google Scholar]
- 209.Felix A, Pitt RM. A new antigen of B. typhosus: Its relation to virulence and to active and passive immunisation. Lancet. 1917;224(5787):186–191. [Google Scholar]
- 210.Yokoyama H, Umeda K, Peralta RC. Oral passive immunization against experimental salmonellosis in mice using chicken egg yolk antibodies specific for Salmonella enteritidis and S. typhimurium. Vaccine. 1998;16:388–393. doi: 10.1016/s0264-410x(97)80916-4. [DOI] [PubMed] [Google Scholar]
- 211.Felix A. Clinical trials with a new antitiyphoid serum. Lancet. 1935;225(5823):799–802. [Google Scholar]
- 212.Mitra S, Chakrabarti MK, Koley H. Multi-serotype outer membrane vesicles of Shigellae confer passive protection to the neonatal mice against shigellosis. Vaccine. 2013;31(31):3163–3173. doi: 10.1016/j.vaccine.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 213.Barman S, Koley H, Nag D. Passive immunity with multi-serotype heat-killed Shigellae in neonatal mice. Microbiol Immunol. 2014;58(8):463–466. doi: 10.1111/1348-0421.12164. [DOI] [PubMed] [Google Scholar]
- 214.Adamus G, Mulczyk M, Witkowska D, Romanowska E. Protection against keratoconjunctivitis shigellosa induced by immunization with outer membrane proteins of Shigella spp. Infect Immun. 1980;30(2):321–324. doi: 10.1128/iai.30.2.321-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tacket CO, Binion SB, Bostwick E. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47(3):276–283. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- 216.Ashraf H, Mahalanabis D, Mitra AK, Tzipori S, Fuchs GJ. Hyperimmune bovine colostrum in the treatment of shigellosis in children: a double-blind, randomized, controlled trial. Acta Paediatr. 2001;90(12):1373–1378. doi: 10.1080/08035250152708743. [DOI] [PubMed] [Google Scholar]
- 217.Thammavongsa V, Rauch S, Kim HK, Missiakas DM, Schneewind O. Protein A-neutralizing monoclonal antibody protects neonatal mice against Staphylococcus aureus. Vaccine. 2015;33(4):523–526. doi: 10.1016/j.vaccine.2014.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.van den Berg S, Bonarius HP, van Kessel KP. A human monoclonal antibody targeting the conserved staphylococcal antigen IsaA protects mice against Staphylococcus aureus bacteremia. Int J Med Microbiol. 2015;305(1):55–64. doi: 10.1016/j.ijmm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 219.Lorenz U, Lorenz B, Schmitter T. Functional antibodies targeting IsaA of Staphylococcus aureus augment host immune response and open new perspectives for antibacterial therapy. Antimicrob Agents Chemother. 2011;55:165–173. doi: 10.1128/AAC.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Benjamin DK, Schelonka R, White R. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol. 2006;26:290–295. doi: 10.1038/sj.jp.7211496. [DOI] [PubMed] [Google Scholar]
- 221.Shah PS, Kaufman DA. Antistaphylococcal immunoglobulins to prevent staphylococcal infection in very low birth weight infants. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD006449.pub2. CD006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bloom B, Schelonka R, Kueser T. Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low birth weight infants. Pediatr Infect Dis J. 2005;24:858–866. doi: 10.1097/01.inf.0000180504.66437.1f. [DOI] [PubMed] [Google Scholar]
- 223.Rupp ME, Holley HP, Jr, Lutz J. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51:4249–4254. doi: 10.1128/AAC.00570-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Weems JJ, Jr, Steinberg JP, Filler S. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:2751–2755. doi: 10.1128/AAC.00096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Raff HV, Siscoe PJ, Wolff EA, Maloney G, Shuford W. Human monoclonal antibodies to group B streptococcus. Reactivity and in vivo protection against multiple serotypes. J Exp Med. 1988;168(3):905–917. doi: 10.1084/jem.168.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hill HR, Kelsey DK, Gonzales LA, Raff HV. Monoclonal antibodies in the therapy of experimental neonatal group B streptococcal disease. Clin Immunol Immunopathol. 1992;62(1 Pt 2):S87–S91. doi: 10.1016/0090-1229(92)90046-q. [DOI] [PubMed] [Google Scholar]
- 227.Senn BM, Visram Z, Meinke AL. Monoclonal antibodies targeting different cell wall antigens of group B streptococcus mediate protection in both Fc-dependent and independent manner. Vaccine. 2011;29:4116–4124. doi: 10.1016/j.vaccine.2011.03.100. [DOI] [PubMed] [Google Scholar]
- 228.Meinke AL, Senn BM, Visram Z. Immunological fingerprinting of group B streptococci: from circulating human antibodies to protective antigens. Vaccine. 2010;28:6997–7008. doi: 10.1016/j.vaccine.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 229.Baker CJ, Carey VJ, Rench MA. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. J Infect Dis. 2014;209:781–788. doi: 10.1093/infdis/jit549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 231.Garcia-Suarez Mdel M, Cima-Cabal MD, Florez N. Protection against pneumococcal pneumonia in mice by monoclonal antibodies to pneumolysin. Infect Immun. 2004;72:4534–4540. doi: 10.1128/IAI.72.8.4534-4540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kaur R, Surendran N, Ochs M, Pichichero ME. Human antibodies to PhtD, PcpA, and Ply reduce adherence to human lung epithelial cells and murine nasopharyngeal colonization by Streptococcus pneumoniae. Infect Immun. 2014;82:5069–5075. doi: 10.1128/IAI.02124-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect Immun. 2011;79:238–245. doi: 10.1128/IAI.00378-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.De Hennezel L, Ramisse F, Binder P, Marchal G, Alonso JM. Effective combination therapy for invasive pneumococcal pneumonia with ampicillin and intravenous immunoglobulins in a mouse model. Antimicrob Agents Chemother. 2001;45(1):316–318. doi: 10.1128/AAC.45.1.316-318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cecil RL, Blake FG. Studies on experimental pneumonia: VII. Treatment of experimental pneumococcus Type I pneumonia in monkeys with Type I antipneumococcus serum. J Exp Med. 1920;32(1):1–18. doi: 10.1084/jem.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cecil RL, Sutliff WD. The treatment of lobar pneumonia with concentrated anti-pneumococcus serum. JAMA. 1928;91(26):2035–2042. [Google Scholar]
- 237.Shurin PA, Rehmus JM, Johnson CE. Bacterial polysaccharide immune globulin for prophylaxis of acute otitis media in high-risk children. J Pediatr. 1993;123(5):801–810. doi: 10.1016/s0022-3476(05)80865-0. [DOI] [PubMed] [Google Scholar]
- 238.Finland M. The serum treatment of lobar pneumonia. N Engl J Med. 1930;202:1244–1247. [Google Scholar]
- 239.Huang YS, Fisher M, Nasrawi Z, Eichenbaum Z. Defense from the Group A Streptococcus by active and passive vaccination with the streptococcal hemoprotein receptor. J Infect Dis. 2011;203:1595–1601. doi: 10.1093/infdis/jir149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liu M, Zhu H, Zhang J, Lei B. Active and passive immunizations with the streptococcal esterase Sse protect mice against subcutaneous infection with group A streptococci. Infect Immun. 2007;75:3651–3657. doi: 10.1128/IAI.00038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pandey M, Batzloff MR, Good MF. Mechanism of protection induced by group A Streptococcus vaccine candidate J8-DT: contribution of B and T-cells towards protection. PLoS ONE. 2009;4(4):e5147. doi: 10.1371/journal.pone.0005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Meader FM. Scarlet fever prophylaxis: Use of blood serum from persons who have recovered from scarlet fever. JAMA. 1930;94(9):622–625. [Google Scholar]
- 243.Dick GF, Dick GH. The prevention of scarlet fever. JAMA. 1924;83(2):84–86. [Google Scholar]
- 244.Darenberg J, Ihendyane N, Sjolin J. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003;37:333–340. doi: 10.1086/376630. [DOI] [PubMed] [Google Scholar]
- 245.Linner A, Darenberg J, Sjolin J, Henriques-Normark B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis. 2014;59:851–857. doi: 10.1093/cid/ciu449. [DOI] [PubMed] [Google Scholar]
- 246.Kaul R, McGeer A, Norrby-Teglund A. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28(4):800–807. doi: 10.1086/515199. [DOI] [PubMed] [Google Scholar]
- 247.Lucchesi PF, Bowman JE. Antitoxin versus no antitoxin in scarlet fever. JAMA. 1934;103(14):1049–1051. [Google Scholar]
- 248.Symmers D, Lewis KM. The antitoxin treatment of erysipelas. JAMA. 1932;99(13):1082–1084. [Google Scholar]
- 249.Panse MV, Jhala HI, Dutta NK. Passive immunity in experimental cholera. J Infect Dis. 1964;114:26–30. doi: 10.1093/infdis/114.1.26. [DOI] [PubMed] [Google Scholar]
- 250.Hirai K, Arimitsu H, Umeda K. Passive oral immunization by egg yolk immunoglobulin (IgY) to Vibrio cholerae effectively prevents cholera. Acta Med Okayama. 2010;64(3):163–170. doi: 10.18926/AMO/40008. [DOI] [PubMed] [Google Scholar]
- 251.Chaicumpa W, Rowley D. Experimental cholera in infant mice: protective effects of antibody. J Infect Dis. 1972;125(5):480–485. doi: 10.1093/infdis/125.5.480. [DOI] [PubMed] [Google Scholar]
- 252.Ghosh H. Further investigation of a new anti-cholera serum. Br Med J. 1936;1(3931):936–938. doi: 10.1136/bmj.1.3931.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ghosh H. Treatment of cholera with a new anti-cholera serum: preliminary note. Br Med J. 1935;1(3862):56–57. doi: 10.1136/bmj.1.3862.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xiao X, Zhu Z, Dankmeyer JL. Human anti-plague monoclonal antibodies protect mice from Yersinia pestis in a bubonic plague model. PLoS ONE. 2010;5(10):e13047. doi: 10.1371/journal.pone.0013047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hill J, Copse C, Leary S. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersinia pestis. Infect Immun. 2003;71(4):2234–2238. doi: 10.1128/IAI.71.4.2234-2238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yersin A, Calmette A, Borrel A. La peste bubonique (duexiéme note) Ann Inst Pasteur (Paris) 1895;9:589–592. [Google Scholar]
- 257.Anisimov AP, Amoako KK. Treatment of plague: promising alternatives to antibiotics. J Med Microbiol. 2006;55:1461–1475. doi: 10.1099/jmm.0.46697-0. [DOI] [PubMed] [Google Scholar]
- 258.Anderson GW, Jr, Worsham PL, Bolt CR. Protection of mice from fatal bubonic and pneumonic plague by passive immunization with monoclonal antibodies against the F1 protein of Yersinia pestis. Am J Trop Med Hyg. 1997;56(4):471–473. doi: 10.4269/ajtmh.1997.56.471. [DOI] [PubMed] [Google Scholar]
- 259.Green M, Rogers D, Russell P. The SCID/Beige mouse as a model to investigate protection against Yersinia pestis. FEMS Immunol Med Microbiol. 1999;23:107–113. doi: 10.1111/j.1574-695X.1999.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 260.Williamson ED, Flick-Smith HC, Lebutt C. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun. 2005;73:3598–3608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hill J, Eyles JE, Elvin SJ. Administration of antibody to the lung protects mice against pneumonic plague. Infect Immun. 2006;74:3068–3070. doi: 10.1128/IAI.74.5.3068-3070.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Motin VL, Nakajima R, Smirnov GB, Brubaker RR. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect Immun. 1994;62(10):4192–4201. doi: 10.1128/iai.62.10.4192-4201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Une T, Brubaker RR. Roles of V antigen in promoting virulence and immunity in yersiniae. J Immunol. 1984;133(4):2226–2230. [PubMed] [Google Scholar]
- 264.Yersin A, Simond P-L. Les épidémies de peste en Extrême-Orient. XIIIe Congrès international de médecine, Aug 1900;1901; Paris.
- 265.Yersin A. Sur la peste bubonique (sérothérapie) Ann Inst Pasteur (Paris) 1897;11:81–93. [Google Scholar]
- 266.Couderc T, Khandoudi N, Grandadam M. Prophylaxis and therapy for Chikungunya virus infection. J Infect Dis. 2009;200(4):516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pal P, Fox JM, Hawman DW. Chikungunya viruses that escape monoclonal antibody therapy are clinically attenuated, stable, and not purified in mosquitoes. J Virol. 2014;88:8213–8226. doi: 10.1128/JVI.01032-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chusri S, Siripaitoon P, Hirunpat S, Silpapojakul K. Case reports of neuro-Chikungunya in southern Thailand. Am J Trop Med Hyg. 2011;85:386–389. doi: 10.4269/ajtmh.2011.10-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Buttinelli G, Donati V, Ruggeri FM. Antigenic sites of coxsackie A9 virus inducing neutralizing monoclonal antibodies protective in mice. Virology. 2003;312:74–83. doi: 10.1016/s0042-6822(03)00182-x. [DOI] [PubMed] [Google Scholar]
- 270.Liu Q, Shi J, Huang X. A murine model of coxsackievirus A16 infection for anti-viral evaluation. Antiviral Res. 2014;105:26–31. doi: 10.1016/j.antiviral.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 271.Yen MH, Huang YC, Chen MC. Effect of intravenous immunoglobulin for neonates with severe enteroviral infections with emphasis on the timing of administration. J Clin Virol. 2015;64:92–96. doi: 10.1016/j.jcv.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 272.Bia FJ, Griffith BP, Tarsio M, Hsiung GD. Vaccination for the prevention of maternal and fetal infection with guinea pig cytomegalovirus. J Infect Dis. 1980;142(5):732–738. doi: 10.1093/infdis/142.5.732. [DOI] [PubMed] [Google Scholar]
- 273.Streblow DN, Hwee YK, Kreklywich CN. Rat cytomegalovirus vaccine prevents accelerated chronic rejection in CMV-naive recipients of infected donor allograft hearts. Am J Transplant. 2015;15(7):1805–1816. doi: 10.1111/ajt.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang H, Huang C, Dong J. Complete protection of mice against lethal murine cytomegalovirus challenge by immunization with DNA vaccines encoding envelope glycoprotein complex III antigens gH, gL and gO. PLoS ONE. 2015;10:e0119964. doi: 10.1371/journal.pone.0119964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 276.Conti DJ, Freed BM, Gruber SA, Lempert N. Prophylaxis of primary cytomegalovirus disease in renal transplant recipients. A trial of ganciclovir vs immunoglobulin. Arch Surg. 1994;129(4):443–447. doi: 10.1001/archsurg.1994.01420280121016. [DOI] [PubMed] [Google Scholar]
- 277.Bonaros N, Mayer B, Schachner T, Laufer G, Kocher A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant. 2008;22:89–97. doi: 10.1111/j.1399-0012.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 278.Shrestha B, Brien JD, Sukupolvi-Petty S. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 2010;6(4):e1000823. doi: 10.1371/journal.ppat.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lai CJ, Goncalvez AP, Men R. Epitope determinants of a chimpanzee dengue virus type 4 (DENV-4)-neutralizing antibody and protection against DENV-4 challenge in mice and rhesus monkeys by passively transferred humanized antibody. J Virol. 2007;81:12766–12774. doi: 10.1128/JVI.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li PC, Liao MY, Cheng PC. Development of a humanized antibody with high therapeutic potential against dengue virus type 2. PLoS Negl Trop Dis. 2012;6:e1636. doi: 10.1371/journal.pntd.0001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Qiu X, Fernando L, Melito PL. Ebola GP-specific monoclonal antibodies protect mice and guinea pigs from lethal Ebola virus infection. PLoS Negl Trop Dis. 2012;6:e1575. doi: 10.1371/journal.pntd.0001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Qiu X, Audet J, Wong G. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012;4:138ra181. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 283.Pettitt J, Zeitlin L, Kim do H. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med. 2013;5:199ra113. doi: 10.1126/scitranslmed.3006608. [DOI] [PubMed] [Google Scholar]
- 284.Mupapa K, Massamba M, Kibadi K. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 285.Abedi MR, Linde A, Christensson B. Preventive effect of IgG from EBV-seropositive donors on the development of human lympho-proliferative disease in SCID mice. Int J Cancer. 1997;71:624–629. doi: 10.1002/(sici)1097-0215(19970516)71:4<624::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 286.Haque T, Johannessen I, Dombagoda D. A mouse monoclonal antibody against Epstein-Barr virus envelope glycoprotein 350 prevents infection both in vitro and in vivo. J Infect Dis. 2006;194:584–587. doi: 10.1086/505912. [DOI] [PubMed] [Google Scholar]
- 287.Custer DM, Thompson E, Schmaljohn CS, Ksiazek TG, Hooper JW. Active and passive vaccination against hantavirus pulmonary syndrome with Andes virus M genome segment-based DNA vaccine. J Virol. 2003;77(18):9894–9905. doi: 10.1128/JVI.77.18.9894-9905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hooper JW, Brocato RL, Kwilas SA. DNA vaccine-derived human IgG produced in transchromosomal bovines protect in lethal models of hantavirus pulmonary syndrome. Sci Transl Med. 2014;6:264ra162. doi: 10.1126/scitranslmed.3010082. [DOI] [PubMed] [Google Scholar]
- 289.Vial PA, Valdivieso F, Calvo M. A non-randomized multicentre trial of human immune plasma for treatment of hantavirus cardiopulmonary syndrome by ANDV. Antivir Ther. 2015;20(4):377–386. doi: 10.3851/IMP2875. [DOI] [PubMed] [Google Scholar]
- 290.Gellis SS, Stokes J, Jr, Brother GM. The use of human immune serum globulin (gamma globulin) in infectious (epidemic) hepatitis in the Mediterranean theater of operations I. Studies on prophylaxis in two epidemics of infectious hepatitis. JAMA. 1945;128(15):1062–1063. [Google Scholar]
- 291.Havens WP, Jr, Paul JR. Prevention of infectious hepatitis with gamma globulin. JAMA. 1945;129(4):270–272. [Google Scholar]
- 292.Stokes J, Jr, Neefe JR. The prevention and attenuation of infectious hepatitis by gamma globulin. JAMA. 1945;127(3):144–145. [Google Scholar]
- 293.Hsia DY, Lonsway M, Jr, Gellis SS. Gamma globulin in the prevention of infectious hepatitis; studies on the use of small doses in family outbreaks. N Engl J Med. 1954;250(10):417–419. doi: 10.1056/NEJM195403112501004. [DOI] [PubMed] [Google Scholar]
- 294.Eren R, Ilan E, Nussbaum O. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology. 2000;32:588–596. doi: 10.1053/jhep.2000.9632. [DOI] [PubMed] [Google Scholar]
- 295.Redeker AG, Mosley JW, Gocke DJ, McKee AP, Pollack W. Hepatitis B immune globulin as a prophylactic measure for spouses exposed to acute type B hepatitis. N Engl J Med. 1975;293(21):1055–1059. doi: 10.1056/NEJM197511202932101. [DOI] [PubMed] [Google Scholar]
- 296.Grazi GL, Mazziotti A, Sama C. Liver transplantation in HBsAg-positive HBV-DNA—negative cirrhotics: immunoprophylaxis and long-term outcome. Liver Transpl Surg. 1996;2:418–425. doi: 10.1002/lt.500020603. [DOI] [PubMed] [Google Scholar]
- 297.Muller R, Gubernatis G, Farle M. Liver transplantation in HBs antigen (HBsAg) carriers. Prevention of hepatitis B virus (HBV) recurrence by passive immunization. J Hepatol. 1991;13(1):90–96. doi: 10.1016/0168-8278(91)90869-d. [DOI] [PubMed] [Google Scholar]
- 298.Morin TJ, Broering TJ, Leav BA. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012;8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Piazza M, Sagliocca L, Tosone G. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157(14):1537–1544. [PubMed] [Google Scholar]
- 300.Chung RT, Gordon FD, Curry MP. Human monoclonal antibody MBL-HCV1 delays HCV viral rebound following liver transplantation: a randomized controlled study. Am J Transplant. 2013;13(4):1047–1054. doi: 10.1111/ajt.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Angus AG, Patel AH. Immunotherapeutic potential of neutralizing antibodies targeting conserved regions of the HCV envelope glycoprotein E2. Future Microbiol. 2011;6(3):279–294. doi: 10.2217/fmb.11.9. [DOI] [PubMed] [Google Scholar]
- 302.Galun E, Terrault NA, Eren R. Clinical evaluation (Phase I) of a human monoclonal antibody against hepatitis C virus: safety and antiviral activity. J Hepatol. 2007;46:37–44. doi: 10.1016/j.jhep.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 303.Tsarev SA, Tsareva TS, Emerson SU. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci USA. 1994;91(21):10198–10202. doi: 10.1073/pnas.91.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Arankalle VA, Chadha MS, Dama BM. Role of immune serum globulins in pregnant women during an epidemic of hepatitis E. J Viral Hepat. 1998;5(3):199–204. doi: 10.1046/j.1365-2893.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- 305.Kumel G, Kaerner HC, Levine M, Schroder CH, Glorioso JC. Passive immune protection by herpes simplex virus-specific monoclonal antibodies and monoclonal antibody-resistant mutants altered in pathogenicity. J Virol. 1985;56(3):930–937. doi: 10.1128/jvi.56.3.930-937.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kohl S, Strynadka NC, Hodges RS, Pereira L. Analysis of the role of antibody-dependent cellular cytotoxic antibody activity in murine neonatal herpes simplex virus infection with antibodies to synthetic peptides of glycoprotein D and monoclonal antibodies to glycoprotein B. J Clin Invest. 1990;86(1):273–278. doi: 10.1172/JCI114695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bravo FJ, Bourne N, Harrison CJ. Effect of antibody alone and combined with acyclovir on neonatal herpes simplex virus infection in guinea pigs. J Infect Dis. 1996;173(1):1–6. doi: 10.1093/infdis/173.1.1. [DOI] [PubMed] [Google Scholar]
- 308.Mofenson LM, Moye J, Jr, Bethel J. Prophylactic intravenous immunoglobulin in HIV-infected children with CD4+ counts of 0.20 x 10(9)/L or more. Effect on viral, opportunistic, and bacterial infections. The National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. JAMA. 1992;268(4):483–488. doi: 10.1001/jama.268.4.483. [DOI] [PubMed] [Google Scholar]
- 309.Masci S, De Simone C, Famularo G. Intravenous immunoglobulins suppress the recurrences of genital herpes simplex virus: a clinical and immunological study. Immunopharmacol Immunotoxicol. 1995;17(1):33–47. doi: 10.3109/08923979509052718. [DOI] [PubMed] [Google Scholar]
- 310.Gruell H, Bournazos S, Ravetch JV. Antibody and antiretroviral preexposure prophylaxis prevent cervicovaginal HIV-1 infection in a transgenic mouse model. J Virol. 2013;87:8535–8544. doi: 10.1128/JVI.00868-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Balazs AB, Chen J, Hong CM. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Klein F, Halper-Stromberg A, Horwitz JA. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Horwitz JA, Halper-Stromberg A, Mouquet H. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci USA. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Vittecoq D, Chevret S, Morand-Joubert L. Passive immunotherapy in AIDS: a double-blind randomized study based on transfusions of plasma rich in anti-human immunodeficiency virus 1 antibodies vs. transfusions of seronegative plasma. Proc Natl Acad Sci USA. 1995;92(4):1195–1199. doi: 10.1073/pnas.92.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Trkola A, Kuster H, Rusert P. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 316.Onyango-Makumbi C, Omer SB, Mubiru M. Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY) J Acquir Immune Defic Syndr. 2011;58(4):399–407. doi: 10.1097/QAI.0b013e31822f8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jacobson JM, Colman N, Ostrow NA. Passive immunotherapy in the treatment of advanced human immunodeficiency virus infection. J Infect Dis. 1993;168(2):298–305. doi: 10.1093/infdis/168.2.298. [DOI] [PubMed] [Google Scholar]
- 318.Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85:13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Day PM, Kines RC, Thompson CD. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8:260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Mair-Jenkins J, Saavedra-Campos M, Baillie JK. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kimura-Kuroda J, Yasui K. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J Immunol. 1988;141(10):3606–3610. [PubMed] [Google Scholar]
- 322.Zhang MJ, Wang MJ, Jiang SZ, Ma WY. Passive protection of mice, goats, and monkeys against Japanese encephalitis with monoclonal antibodies. J Med Virol. 1989;29(2):133–138. doi: 10.1002/jmv.1890290211. [DOI] [PubMed] [Google Scholar]
- 323.Eddy GA, Wagner FS, Scott SK, Mahlandt BJ. Protection of monkeys against Machupo virus by the passive administration of Bolivian haemorrhagic fever immunoglobulin (human origin) Bull World Health Organ. 1975;52(4–6):723–727. [PMC free article] [PubMed] [Google Scholar]
- 324.Ziegler D, Fournier P, Berbers GA. Protection against measles virus encephalitis by monoclonal antibodies binding to a cystine loop domain of the H protein mimicked by peptides which are not recognized by maternal antibodies. J Gen Virol. 1996;77(Pt 10):2479–2489. doi: 10.1099/0022-1317-77-10-2479. [DOI] [PubMed] [Google Scholar]
- 325.Giraudon P, Wild TF. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985;144(1):46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- 326.Edghill-Smith Y, Golding H, Manischewitz J. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 327.Hess AF. A protective therapy for mumps. Am J Dis Child. 1915;10(2):99–103. [Google Scholar]
- 328.Rambar AC. Mumps; use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child. 1946;71:1–13. [PubMed] [Google Scholar]
- 329.Regan JC. Serum Prophylaxis of epidemic parotitis. JAMA. 1925;84(4):279–280. [Google Scholar]
- 330.Barenberg LH, Ostroff J. Use of human blood in protection against mumps. Am J Dis Child. 1931;42(5):1109–1113. [Google Scholar]
- 331.Crabol Y, Terrier B, Rozenberg F. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis. 2013;56:968–977. doi: 10.1093/cid/cis1046. [DOI] [PubMed] [Google Scholar]
- 332.Anderson D, Ali K, Blanchette V. Guidelines on the use of intravenous immune globulin for hematologic conditions. Transfus Med Rev. 2007;21:S9–S56. doi: 10.1016/j.tmrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 333.Flexner S, Amoss HL. The relation of the meninges and choroid plexus to poliomyelitic infection. J Exp Med. 1917;25(4):525–537. doi: 10.1084/jem.25.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rhodes AJ, Shimada FT, Clark EM, Wood W, Ritchie RC. Passive immunity in poliomyelitis. IV. Protection of rhesus monkeys against cerebral challenge. Proc Soc Exp Biol Med. 1952;79(3):421–424. doi: 10.3181/00379727-79-19400. [DOI] [PubMed] [Google Scholar]
- 335.Flexner S, Lewis PA. Experimental poliomyelitis in monkeys Seventh note: Active immunization and passive serum protection. JAMA. 1910;54(22):1780–1782. [Google Scholar]
- 336.Hammon WM, Coriell LL, Wehrle PF, Stokes J., Jr Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. IV. Final report of results based on clinical diagnoses. JAMA. 1953;151(15):1272–1285. [PubMed] [Google Scholar]
- 337.Hammon WM, Coriell LL, Ludwig EH. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. 5. Reanalysis of results based on laboratory-confirmed cases. JAMA. 1954;156(1):21–27. doi: 10.1001/jama.1954.02950010023009. [DOI] [PubMed] [Google Scholar]
- 338.McEachern JM, Chown B, Bell LG, McKenzie M. Resume of the results of therapy with convalescent serum in poliomyelitis. Can Med Assoc J. 1929;20(4):369–371. [PMC free article] [PubMed] [Google Scholar]
- 339.Shaw EB, Thelander HE. Intramuscular use of convalescent serum in treatment of poliomyelitis. JAMA. 1928;90(24):1923–1927. [Google Scholar]
- 340.Aycock W, Luther EH. Preparalytic poliomyelitis: Observations in one hundred and six cases in which convalescent serum was used. JAMA. 1928;91(6):387–394. [Google Scholar]
- 341.Babès V, Lepp M. Recherches sur la vaccination antirabique. Ann Inst Pasteur (Paris) 1889;3:384–390. [Google Scholar]
- 342.Winkler WG, Schmidt RC, Sikes RK. Evaluation of human rabies immune globulin and homologous and heterologous antibody. J Immunol. 1969;102(5):1314–1321. [PubMed] [Google Scholar]
- 343.Habel K. Antiserum in the prophylaxis of rabies. Bull World Health Organ. 1954;10(5):781–788. [PMC free article] [PubMed] [Google Scholar]
- 344.Goudsmit J, Marissen WE, Weldon WC. Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal anti-rabies immune globulin. J Infect Dis. 2006;193:796–801. doi: 10.1086/500470. [DOI] [PubMed] [Google Scholar]
- 345.Koprowski H, Van Der Scheer J, Black J. Use of hyperimmune anti-rabies serum concentrates in experimental rabies. Am J Med. 1950;8(4):412–420. doi: 10.1016/0002-9343(50)90224-5. [DOI] [PubMed] [Google Scholar]
- 346.Habel K, Koprowski H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull World Health Organ. 1955;13(5):773–779. [PMC free article] [PubMed] [Google Scholar]
- 347.Jackson AC, Warrell MJ, Rupprecht CE. Management of rabies in humans. Clin Infect Dis. 2003;36:60–63. doi: 10.1086/344905. [DOI] [PubMed] [Google Scholar]
- 348.Johnson S, Oliver C, Prince GA. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176(5):1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 349.Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hemming VG, Prince GA, Horswood RL. Studies of passive immunotherapy for infections of respiratory syncytial virus in the respiratory tract of a primate model. J Infect Dis. 1985;152(5):1083–1087. doi: 10.1093/infdis/152.5.1083. [DOI] [PubMed] [Google Scholar]
- 351.Crowe JE, Jr, Gilmour PS, Murphy BR. Isolation of a second recombinant human respiratory syncytial virus monoclonal antibody fragment (Fab RSVF2–5) that exhibits therapeutic efficacy in vivo. J Infect Dis. 1998;177(4):1073–1076. doi: 10.1086/517397. [DOI] [PubMed] [Google Scholar]
- 352.Simoes EA, Groothuis JR, Tristram DA. Respiratory syncytial virus-enriched globulin for the prevention of acute otitis media in high risk children. J Pediatr. 1996;129:214–219. doi: 10.1016/s0022-3476(96)70245-7. [DOI] [PubMed] [Google Scholar]
- 353.Niklasson BS, Meadors GF, Peters CJ. Active and passive immunization against Rift Valley fever virus infection in Syrian hamsters. Acta Pathol Microbiol Immunol Scand [C] 1984;92(4):197–200. doi: 10.1111/j.1699-0463.1984.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 354.Besselaar TG, Blackburn NK. Topological mapping of antigenic sites on the Rift Valley fever virus envelope glycoproteins using monoclonal antibodies. Arch Virol. 1991;121(1–4):111–124. doi: 10.1007/BF01316748. [DOI] [PubMed] [Google Scholar]
- 355.Offit PA, Shaw RD, Greenberg HB. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Westerman LE, McClure HM, Jiang B, Almond JW, Glass RI. Serum IgG mediates mucosal immunity against rotavirus infection. Proc Natl Acad Sci USA. 2005;102:7268–7273. doi: 10.1073/pnas.0502437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Knipping K, McNeal MM, Crienen A. A gastrointestinal rotavirus infection mouse model for immune modulation studies. Virol J. 2011;8:109. doi: 10.1186/1743-422X-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tokuhara D, Alvarez B, Mejima M. Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J Clin Invest. 2013;123(9):3829–3838. doi: 10.1172/JCI70266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Korns RF. Prophylaxis of German measles with immune serum globulin. J Infect Dis. 1952;90(2):183–189. doi: 10.1093/infdis/90.2.183. [DOI] [PubMed] [Google Scholar]
- 360.Brody JA, Sever JL, Schiff GM. Prevention of rubella by gamma globulin during an epidemic in Barrow, Alaska, in 1964. N Engl J Med. 1965;272:127–129. doi: 10.1056/NEJM196501212720304. [DOI] [PubMed] [Google Scholar]
- 361.Urquhart GE, Crawford RJ, Wallace J. Trial of high-titre human rubella immunoglobulin. Br Med J. 1978;2(6148):1331–1332. doi: 10.1136/bmj.2.6148.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sever JL, Nelson KB, Gilkeson MR. Rubella epidemic, 1964: effect on 6,000 pregnancies. Am J Dis Child. 1965;110(4):395–407. doi: 10.1001/archpedi.1965.02090030415009. [DOI] [PubMed] [Google Scholar]
- 363.Neumann-Haefelin D, Neumann-Haefelin C, Petersen EE, Luthardt T, Hass R. [Passive immunization against rubella: studies on the effectiveness of rubella-immunoglobulin after intranasal infection with rubella vaccination virus] Dtsch Med Wochenschr. 1975;100(5):177–181. doi: 10.1055/s-0028-1106191. [DOI] [PubMed] [Google Scholar]
- 364.ter Meulen J, Bakker AB, van den Brink EN. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363:2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Miyoshi-Akiyama T, Ishida I, Fukushi M. Fully human monoclonal antibody directed to proteolytic cleavage site in severe acute respiratory syndrome (SARS) coronavirus S protein neutralizes the virus in a rhesus macaque SARS model. J Infect Dis. 2011;203:1574–1581. doi: 10.1093/infdis/jir084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Roberts A, Thomas WD, Guarner J. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Van Rompay KK, Berardi CJ, Dillard-Telm S. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177(5):1247–1259. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]
- 368.Haigwood NL, Watson A, Sutton WF. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 369.Haigwood NL, Montefiori DC, Sutton WF. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J Virol. 2004;78:5983–5995. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Hessell AJ, Hangartner L, Hunter M. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 371.Mascola JR, Lewis MG, Stiegler G. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73(5):4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Nishimura Y, Igarashi T, Haigwood N. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76(5):2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Moldt B, Rakasz EG, Schultz N. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Shingai M, Nishimura Y, Klein F. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Shingai M, Donau OK, Plishka RJ. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Shibata R, Igarashi T, Haigwood N. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5(2):204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 377.Kreil TR, Eibl MM. Pre- and postexposure protection by passive immunoglobulin but no enhancement of infection with a flavivirus in a mouse model. J Virol. 1997;71(4):2921–2927. doi: 10.1128/jvi.71.4.2921-2927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Phillpotts RJ, Stephenson JR, Porterfield JS. Passive immunization of mice with monoclonal antibodies raised against tick-borne encephalitis virus. Brief report. Arch Virol. 1987;93(3-4):295–301. doi: 10.1007/BF01310983. [DOI] [PubMed] [Google Scholar]
- 379.Pen'evskaia NA, Rudakov NV. [Efficiency of use of immunoglobulin preparations for the postexposure prevention of tick-borne encephalitis in Russia (a review of semi-centennial experience)] Med Parazitol (Mosk) 2010;(1):53–59. [PubMed] [Google Scholar]
- 380.Kleiter I, Jilg W, Bogdahn U, Steinbrecher A. Delayed humoral immunity in a patient with severe tick-borne encephalitis after complete active vaccination. Infection. 2007;35(1):26–29. doi: 10.1007/s15010-006-6614-2. [DOI] [PubMed] [Google Scholar]
- 381.McCausland MM, Benhnia MR, Crickard L. Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model. Antivir Ther. 2010;15(4):661–675. doi: 10.3851/IMP1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Fogg CN, Americo JL, Earl PL. Disparity between levels of in vitro neutralization of vaccinia virus by antibody to the A27 protein and protection of mice against intranasal challenge. J Virol. 2008;82:8022–8029. doi: 10.1128/JVI.00568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Crickard L, Babas T, Seth S. Protection of rabbits and immunodeficient mice against lethal poxvirus infections by human monoclonal antibodies. PLoS ONE. 2012;7:e48706. doi: 10.1371/journal.pone.0048706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Fisher RW, Reed JL, Snoy PJ. Postexposure prevention of progressive vaccinia in SCID mice treated with vaccinia immune globulin. Clin Vaccine Immunol. 2011;18:67–74. doi: 10.1128/CVI.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Nanning W. Prophylactic effect of antivaccinia gamma-globulin against post-vaccinal encephalitis. Bull World Health Organ. 1962;27:317–324. [PMC free article] [PubMed] [Google Scholar]
- 386.Sharp JC, Fletcher WB. Experience of anti-vaccinia immunoglobulin in the United Kingdom. Lancet. 1973;1:656–659. doi: 10.1016/s0140-6736(73)92215-0. [DOI] [PubMed] [Google Scholar]
- 387.Sussman S, Grossman M. Complications of smallpox vaccination. Effects of vaccinia immune globulin therapy. J Pediatr. 1965;67(7):1168–1173. [Google Scholar]
- 388.Brunell PA, Ross A, Miller LH, Kuo B. Prevention of varicella by zoster immune globulin. N Engl J Med. 1969;280(22):1191–1194. doi: 10.1056/NEJM196905292802201. [DOI] [PubMed] [Google Scholar]
- 389.Funkhouser WL. The use of serum gamma globulin antibodies to control chicken pox in a convalescent hospital for children. J Pediatr. 1948;32(3):257–259. doi: 10.1016/s0022-3476(48)80028-4. [DOI] [PubMed] [Google Scholar]
- 390.Cohen A, Moschopoulos P, Stiehm RE, Koren G. Congenital varicella syndrome: the evidence for secondary prevention with varicella-zoster immune globulin. CMAJ. 2011;183:204–208. doi: 10.1503/cmaj.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Hunt AR, Frederickson S, Hinkel C, Bowdish KS, Roehrig JT. A humanized murine monoclonal antibody protects mice either before or after challenge with virulent Venezuelan equine encephalomyelitis virus. J Gen Virol. 2006;87:2467–2476. doi: 10.1099/vir.0.81925-0. [DOI] [PubMed] [Google Scholar]
- 392.Engle MJ, Diamond MS. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J Virol. 2003;77(24):12941–12949. doi: 10.1128/JVI.77.24.12941-12949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Tesh RB, Arroyo J, Travassos Da Rosa AP. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg Infect Dis. 2002;8(12):1392–1397. doi: 10.3201/eid0812.020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Ben-Nathan D, Gershoni-Yahalom O, Samina I. Using high titer West Nile intravenous immunoglobulin from selected Israeli donors for treatment of West Nile virus infection. BMC Infect Dis. 2009;9:18. doi: 10.1186/1471-2334-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Oliphant T, Engle M, Nybakken GE. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11(5):522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Hamdan A, Green P, Mendelson E. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl Infect Dis. 2002;4:160–162. doi: 10.1034/j.1399-3062.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 397.Shimoni Z, Niven MJ, Pitlick S, Bulvik S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg Infect Dis. 2001;7(4):759. doi: 10.3201/eid0704.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Brandriss MW, Schlesinger JJ, Walsh EE, Briselli M. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol. 1986;67(Pt 2):229–234. doi: 10.1099/0022-1317-67-2-229. [DOI] [PubMed] [Google Scholar]
- 399.Bauer JH. The duration of passive immunity in Yellow Fever. Am J Trop Med Hyg. 1931;11(6):451–457. [Google Scholar]
- 400.Sawyer WA. Persistence of yellow fever immunity. J Prev Med. 1931;5:413–428. [Google Scholar]
- 401.Davis NC. On the use of immune serum at various intervals after the inoculation of yellow fever virus into rhesus monkeys. J Immunol. 1934;26(5):361–390. [Google Scholar]
- 402.Burke AW, Davis NC. Notes on laboratory infections with yellow fever. Am J Trop Med Hyg. 1930;s1-10(6):419–426. [Google Scholar]
- 403.Low GC, Fairley NH. Observations on laboratory and hospital infections with yellow fever in England. Br Med J. 1931;1(3655):125–128. doi: 10.1136/bmj.1.3655.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Tavares D, Ferreira P, Vilanova M, Videira A, Arala-Chaves M. Immunoprotection against systemic candidiasis in mice. Int Immunol. 1995;7(5):785–796. doi: 10.1093/intimm/7.5.785. [DOI] [PubMed] [Google Scholar]
- 405.Cassone A, Boccanera M, Adriani D, Santoni G, De Bernardis F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun. 1995;63(7):2619–2624. doi: 10.1128/iai.63.7.2619-2624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Han Y, Morrison RP, Cutler JE. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun. 1998;66(12):5771–5776. doi: 10.1128/iai.66.12.5771-5776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Xin H, Cutler JE. Vaccine and monoclonal antibody that enhance mouse resistance to candidiasis. Clin Vaccine Immunol. 2011;18:1656–1667. doi: 10.1128/CVI.05215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Nussbaum G, Yuan R, Casadevall A, Scharff MD. Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans. J Exp Med. 1996;183(4):1905–1909. doi: 10.1084/jem.183.4.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Beenhouwer DO, Yoo EM, Lai CW, Rocha MA, Morrison SL. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect Immun. 2007;75:1424–1435. doi: 10.1128/IAI.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J Immunol. 2003;170(7):3621–3630. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 411.Larsen RA, Pappas PG, Perfect J. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother. 2005;49:952–958. doi: 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Kuhls TL, Orlicek SL, Mosier DA. Enteral human serum immunoglobulin treatment of cryptosporidiosis in mice with severe combined immunodeficiency. Infect Immun. 1995;63(9):3582–3586. doi: 10.1128/iai.63.9.3582-3586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Riggs MW, Schaefer DA, Kapil SJ, Barley-Maloney L, Perryman LE. Efficacy of monoclonal antibodies against defined antigens for passive immunotherapy of chronic gastrointestinal cryptosporidiosis. Antimicrob Agents Chemother. 2002;46(2):275–282. doi: 10.1128/AAC.46.2.275-282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Okhuysen PC, Chappell CL, Crabb J. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis. 1998;26(6):1324–1329. doi: 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- 415.Coggeshall LT, Kumm HW. Demonstration of passive immunity in experimental monkey malaria. J Exp Med. 1937;66(2):177–190. doi: 10.1084/jem.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Sack BK, Miller JL, Vaughan AM. Model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect Immun. 2014;82:808–817. doi: 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.McIntosh RS, Shi J, Jennings RM. The importance of human FcgammaRI in mediating protection to malaria. PLoS Pathog. 2007;3:e72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 419.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172(6):1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Johnson AM, McDonald PJ, Neoh SH. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from toxoplasmosis. J Protozool. 1983;30(2):351–356. doi: 10.1111/j.1550-7408.1983.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 421.Fu YF, Feng M, Ohnishi K. Generation of a neutralizing human monoclonal antibody Fab fragment to surface antigen 1 of Toxoplasma gondii tachyzoites. Infect Immun. 2011;79:512–517. doi: 10.1128/IAI.00969-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Pavia CS. Protection against experimental toxoplasmosis by adoptive immunotherapy. J Immunol. 1986;137(9):2985–2990. [PubMed] [Google Scholar]
- 423.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin Immunol. 2010;137:21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 424.Liese JG, Wintergerst U, Tympner KD, Belohradsky BH. High- vs low-dose immunoglobulin therapy in the long-term treatment of X-linked agammaglobulinemia. Am J Dis Child. 1992;146(3):335–339. doi: 10.1001/archpedi.1992.02160150075025. [DOI] [PubMed] [Google Scholar]
- 425.Eibl MM. History of immunoglobulin replacement. Immunol Allergy Clin North Am. 2008;28(4):737–764. doi: 10.1016/j.iac.2008.06.004. viii. [DOI] [PubMed] [Google Scholar]
- 426.Orange JS, Hossny EM, Weiler CR. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 427.Silk HJ, Ambrosino D, Geha RS. Effect of intravenous gammaglobulin therapy in IgG2 deficient and IgG2 sufficient children with recurrent infections and poor response to immunization with Hemophilus influenzae type b capsular polysaccharide antigen. Ann Allergy. 1990;64(1):21–25. [PubMed] [Google Scholar]
- 428.Intravenous immune globulin for the prevention of bacterial infections in children with symptomatic human immunodeficiency virus infection. The National Institute of Child Health and Human Developments Intravenous Immunoglobulin Study Group. N Engl J Med. 1991;325(2):73–80. doi: 10.1056/NEJM199107113250201. [DOI] [PubMed] [Google Scholar]
- 429.Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med. 2007;35(12):2686–2692. [PubMed] [Google Scholar]
- 430.Werdan K, Pilz G. Supplemental immune globulins in sepsis: a critical appraisal. Clin Exp Immunol. 1996;104(suppl 1):83–90. [PubMed] [Google Scholar]
- 431.Douzinas EE, Pitaridis MT, Louris G. Prevention of infection in multiple trauma patients by high-dose intravenous immunoglobulins. Crit Care Med. 2000;28(1):8–15. doi: 10.1097/00003246-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 432.Jenson HB, Pollock BH. The role of intravenous immunoglobulin for the prevention and treatment of neonatal sepsis. Semin Perinatol. 1998;22(1):50–63. doi: 10.1016/s0146-0005(98)80007-4. [DOI] [PubMed] [Google Scholar]
- 433.Enders JF, Kane LW, Cohen S, Levens JH. Immunity in mumps: I. Experiments with monkeys (macacus mulatta). The development of complement-fixing antibody following infection and experiments on immunization by means of inactivated virus and convalescent human serum. J Exp Med. 1945;81(1):93–117. doi: 10.1084/jem.81.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Wollstein M. An experimental study of parotitis. JAMA. 1918;71(8):639–644. [Google Scholar]


