![]() An expanded version of this chapter is available online at ExpertConsult.
An expanded version of this chapter is available online at ExpertConsult.
Introduction
Community-acquired pneumonia (CAP) is a frequent infectious respiratory disease.1 Although many patients with CAP can be treated as outpatients, the mortality of CAP in those who do require hospitalization ranges from 5% to 15% and increases to 20% to 50% in patients who require intensive care unit (ICU) care. Hospital-acquired pneumonia (HAP) is the second most common and most frequently fatal nosocomial infection.
A clinical diagnosis of pneumonia can usually be established on the basis of signs, symptoms, and chest radiographs, although distinguishing CAP or HAP from conditions such as congestive heart failure, pulmonary embolism, and chemical aspiration pneumonia is sometimes difficult. Defining an etiologic agent is also challenging. Although early empirical therapy is necessary, it is important to identify the causative pathogen in patients who require hospitalization, both to confirm the appropriateness of therapy and to reduce unnecessary antimicrobial use.
Diagnosis and management of pneumonia has become more complex due to the growing number of aged and comorbid, debilitated, institutionalized, and immunocompromised individuals, to the diverse array of microorganisms that cause pneumonia, and to increasing antimicrobial resistance.
Pathophysiology and Pathogenesis
Aspiration of oropharyngeal or nasopharyngeal secretions is the main mechanism of contamination of lower airways by bacteria. While a person is awake, glottal reflexes prevent aspiration; during sleep, 50% of normal persons aspirate small volumes of pharyngeal secretions. Because oropharyngeal secretions may contain 107 to 1011 microorganisms per milliliter, aspiration of as little as 0.001 mL may carry more than 100,000 bacteria.
The oropharynx of healthy individuals is colonized by diverse microorganisms that vary in their potential virulence. The ability of microorganisms to colonize the oropharynx and to cause lower respiratory tract infections is determined in part by the interaction of specific microbial adhesins with cellular receptors. For example, Streptococcus pneumoniae, which contains multiple adhesions,2 binds to the receptor for platelet-activating factor on epithelial cells, and this interaction is enhanced by cigarette smoke, infection with respiratory viruses, and particulate air pollutants,3, 4, 5 all of which are linked to increased risk for pneumococcal pneumonia. Likewise, Staphylococcus aureus expresses multiple adhesins that bind host extracellular matrix proteins.6, 7 Gram-negative bacterial pathogens also possess specific adhesins, many of which form macromolecular structures, termed pili. Klebsiella pneumoniae exploits two distinct pili to adhere to epithelial cells: type 1 pili bind to diverse host target molecules with exposed mannose residues, and type 3 pili interact with extracellular matrix proteins.8
Several mechanisms in the airways prevent adherence and colonization by potential bacterial pathogens. Respiratory epithelial cells synthesize and secrete peptides, termed defensins and cathelicidins, that possess broad-spectrum antimicrobial activity.9 In the distal airways and alveoli, pulmonary surfactant proteins A and C can inhibit bacterial binding to host cells and also promote phagocytosis of selected bacteria.10, 11 The presence of complement and immunoglobulins (particularly immunoglobulin A [IgA]), also prevents colonization of the oropharynx. In addition to protection provided by host factors, the upper airway microbiota may modulate susceptibility to pathogens, as indicated by the evidence that broad-spectrum antimicrobial therapy predisposes to colonization and infection. The effects of the microbiota operate through competition for binding sites or nutritional resources, or by modulating expression of specific host defense molecules.12, 13, 14, 15 Interactions between the virulence and quantity of aspirated or inhaled microorganisms and the individual's innate and adaptive immune responses determine whether pneumonia develops.16
As an alternative to aspiration of bacteria of the upper airways, Mycoplasma pneumoniae, Chlamydophila species, Coxiella burnetii, Legionella, and Mycobacterium tuberculosis enter the lower respiratory tract by inhalation. Inhalation pneumonia is most often due to microorganisms that survive suspended in the air for prolonged periods, are present in droplet nuclei smaller than 5 µm, and are able to evade innate immune responses.
Epidemiology
Community-Acquired Pneumonia
The true incidence of CAP is uncertain because the illness is not reportable and only 20% to 50% of patients require hospitalization. Estimates of the incidence of CAP range from 2 to 15 cases per 1000 persons per year, with substantially higher rates in older adults.17
Although the severity of disease is influenced by the patient's age and by the presence and type of coexisting conditions,18, 19, 20, 21 the severity of disease is also related to the pathogen. M. pneumoniae, S. pneumoniae, Chlamydophila pneumoniae, Haemophilus influenzae, and viruses are causes of mild CAP (Table 33-1 ), whereas S. pneumoniae, M. pneumoniae, and H. influenzae can cause CAP severe enough to warrant hospitalization (Table 33-2 ).21, 22, 23 The most frequently identified pathogens causing severe CAP (i.e., CAP requiring ICU care) include S. pneumoniae, enteric gram-negative bacilli, S. aureus, Legionella pneumophila, M. pneumoniae, H. influenzae, and respiratory viruses (Table 33-3 ).21, 22, 23, 24, 25 Up to 20% of severe CAP episodes are caused by polymicrobial infection. Even if extensive diagnostic procedures are performed, the responsible pathogen is not isolated in up to 50% to 60% of patients with severe CAP.
Table 33-1.
Common Causes of Community-Acquired Pneumonia in Patients Who Do Not Require Hospitalization*
|
Organisms are listed in the general order of frequency.
Table 33-2.
Common Causes of Community-Acquired Pneumonia in Patients Who Require Hospitalization*
|
Organisms are listed in the general order of frequency.
Table 33-3.
|
Severity of disease warranting treatment in an intensive care unit.
Organisms are listed in the general order of frequency.
Gram-negative enteric bacilli, S. aureus, Legionella species, and respiratory viruses are uncommon causes of CAP, although local outbreaks can markedly increase the incidence of Legionella.26, 27 Methicillin-resistant Staphylococcus aureus (MRSA), originally a nosocomial pathogen, has appeared in the community where it is referred to as community-acquired MRSA. Community-acquired MRSA can lead to severe pulmonary infections, including necrotizing and hemorrhagic pneumonia.28 Pseudomonas aeruginosa infection is uncommon in the absence of specific risk factors (recent antibiotic treatment, acquired immunodeficiency syndrome [AIDS], and severe pulmonary comorbidity, especially bronchiectasis, cystic fibrosis, and severe chronic obstructive pulmonary disease [COPD]).21, 22, 24
The likely etiology of severe CAP varies in differing patient populations, depending on age and comorbidities, including HIV infection.22, 29, 30, 31, 31a
Age-Related Factors
Pneumonia remains one of the major causes of morbidity in children. In Europe, there are more than 2.5 million cases of childhood pneumonia yearly, which account for about 50% of hospital admissions for children. Radiographically defined pneumonia is present in 7.5% of febrile illnesses in infants up to 3 months old and in 13% of infectious illnesses during the first 2 years of life. In children younger than 2 years, S. pneumoniae and respiratory syncytial virus are the most frequent microorganisms, whereas M. pneumoniae is a leading cause of pneumonia in older children and young adults.
In adults, increased age is associated with a change in the distribution of microbial causes and an increase in the frequency and severity of pneumonia.32 The annual incidence of CAP in noninstitutionalized older adults is estimated between 18 and 44 per 1000 compared with 4.7 to 11.6 per 1000 in the general population.17, 32, 33 Although older adults are particularly at risk for pneumococcal pneumonia, they also have increased rates of pneumonia due to group B streptococci, Moraxella catarrhalis, H. influenzae, L. pneumophila, gram-negative bacilli, C. pneumoniae, and polymicrobial infections.17, 24, 34 Although the absolute rate of infection by M. pneumoniae does not decrease with age, this pathogen accounts for a smaller proportion of pneumonia in older adults than in younger populations. In patients older than 80 years, there is a higher incidence of aspiration pneumonia and lower incidence of infection with Legionella species than in younger patients.35
Personal Habits
Alcohol consumption is an important risk factor for CAP because of its potential to impair level of consciousness, thus increasing the risk for aspiration of oropharyngeal contents. In addition, diverse effects of alcoholism on innate and adaptive immunity have been reported, which may contribute to increased risk. Alcoholism has been shown to be an independent risk factor for increased rate and severity of pneumonia, especially that due to S. pneumoniae. 36, 37 This predisposition persists several months after cessation of alcohol consumption.37
Smoking is one of the most important risk factors for CAP and is associated with an increased frequency of CAP due to S. pneumoniae, L. pneumophila, and influenza.38 Smoking alters mucociliary transport and humoral and cellular defenses, affects epithelial cells, and increases adhesion of S. pneumoniae and H. influenzae to the oropharyngeal epithelium.4
Comorbidities
The most frequent comorbidity associated with CAP is COPD. Patients with COPD have an increased risk for CAP, due to alterations in mechanical and cellular defenses that allow bacterial colonization of the lower airways. Patients with severe COPD (forced expiratory volume in 1 second < 30% of predicted) and bronchiectasis have an increased risk for pneumonia caused by H. influenzae and P. aeruginosa. 38 In patients with COPD treated with oral corticosteroids for long periods, the risk for infection with Aspergillus species is increased.39
Pneumonia remains the major cause of morbidity and mortality in patients with cystic fibrosis. During the first decade of life, S. aureus and nontypeable H. influenzae are the most common pathogens, although P. aeruginosa is occasionally isolated in infants. By 18 years of age, 80% of patients with cystic fibrosis harbor P. aeruginosa and 3.5% harbor Burkholderia cepacia. 40 Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and nontuberculous mycobacteria are emerging pathogens in this population.41
Other comorbidities associated with increased rates of CAP and consequent mortality include congestive heart failure, chronic kidney or liver disease, cancer, diabetes, dementia, cerebrovascular diseases, and immunodeficiencies (e.g., neutropenia, lymphoproliferative diseases, immunoglobulin deficiencies, and human immunodeficiency virus [HIV] infection).42, 43, 44
Geographic and Occupational Considerations
Geographic factors, seasonal timing, travel history, and occupational or unusual exposures modify the risk of various microbial etiologies of CAP. For example, an increased frequency of S. pneumoniae is found in soldiers, painters, and South African gold miners. Burkholderia pseudomallei (melioidosis) is endemic in the rural tropics.45 Exposure to pet birds or work on a poultry (especially turkey) farm or processing plant increases the risk of psittacosis (Chlamydophila psittaci), while contact with horses or other large mammals including cattle, swine, sheep, goats, or deer increases exposure to Rhodococcus. Rodent contact suggests the possibility of infection with Yersinia pestis (plague) in the rural southwestern United States46 and Francisella tularensis (tularemia) in rural Arkansas or Nantucket, Massachusetts.47 Exposure to sheep, dogs, and cats should prompt evaluation for Coxiella burnetii (Q fever).48 The role of seasonal timing is illustrated by the increased incidence of lower respiratory tract infections due to S. pneumoniae and H. influenzae in winter months. Pneumonia causing the severe acute respiratory syndrome (SARS) due to a coronavirus emerged in epidemic form in Southeast Asia,49, 50 and another coronavirus causes the emerging Middle East respiratory syndrome (MERS). Finally, the infectious agents that cause anthrax, tularemia, and plague may be used for bioterrorism or biowarfare purposes and cause lower respiratory tract infections.51, 52
Hospital-Acquired (Nosocomial) Pneumonia
Early-onset HAP (<5 days of hospitalization) is most often due to microorganisms that are also associated with CAP, such as S. pneumoniae, H. influenzae, and anaerobes. Late-onset HAP (>5 days of hospitalization) is mainly caused by MRSA, enteric gram-negative bacilli, P. aeruginosa, nonfermenters such as Acinetobacter baumannii and S. maltophilia, and polymicrobial infections.53 Factors that increase the risk for HAP include antibiotic exposure, old age, severe comorbidities, underlying immunosuppression, colonization of the oropharynx by virulent microorganisms, conditions that promote pulmonary aspiration or inhibit coughing (e.g., thoracoabdominal surgery, endotracheal intubation, insertion of nasogastric tube, supine position), and exposure to contaminated respiratory equipment. A recent study suggests that multidrug-resistant microorganisms are more frequent in early-onset HAP than was initially thought54 and that risk factors for early-onset pneumonia should be reappraised.
Health Care–Associated Pneumonia
Health care now reflects a continuum with many traditional inpatient services provided in outpatient settings. Physicians often categorize new infections in such subjects as “community-acquired.” However, these health care–associated infections have a unique epidemiology more like that of hospital-acquired infections, and this has resulted in health care–acquired pneumonia (HCAP) being recognized as a separate entity by the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA).53 S. aureus (both methicillin-sensitive and methicillin-resistant) and P. aeruginosa are the most frequent associated microorganisms. Compared with CAP, HCAP patients have more severe disease, higher mortality, greater length of hospital stay, and greater cost of care.55
Clinical Presentation
Pneumonia is characterized by the presence of fever, altered general well-being, and respiratory symptoms, such as cough (90%), sputum production (66%), dyspnea (66%), pleuritic pain (50%), and hemoptysis (15%). In older and immunocompromised patients, the signs and symptoms of pulmonary infection may be muted and overshadowed by nonspecific complaints. Temperature greater than 38.5° C or accompanied by chills should never be attributed to bronchitis without examining a chest radiograph.
Occasionally, there is a “classic” history, such as that of the patient with pneumococcal infection who presents with sudden onset of rigor followed by pleuritic chest pain, dyspnea, and cough with rusty sputum. Similarly, a patient with Legionella pneumonia may complain predominantly of diarrhea, fever, headache, confusion, and myalgia. For M. pneumoniae infection, extrapulmonary manifestations such as myringitis, encephalitis, uveitis, iritis, and myocarditis may be present. However, only rarely does the clinical history clearly suggest a specific etiologic diagnosis.
Information obtained from the clinical history and physical examination is not sufficient to confirm the diagnosis of pneumonia. A definitive diagnosis requires the finding of a new opacity on the chest radiograph.
In older patients, especially those with multiple comorbidities, pneumonia may present with general weakness, decreased appetite, altered mental status, incontinence, or decompensation due to underlying disease. The presence of tachypnea may precede other signs of pneumonia by 1 to 2 days. Tachycardia is another common initial sign but is less frequent and specific than tachypnea. Fever is absent in 30% to 40% of older patients. Owing to the lack of specific symptoms, the diagnosis of CAP is frequently delayed in older adults.17, 34 Older patients with pneumonia who present with altered mental status without fever can have a delay in receiving antibiotics by more than 4 hours after arrival; this delay increases mortality.56
Typical Versus Atypical Pneumonia
The division of CAP into typical and atypical syndromes has been used to predict the likely pathogens and select appropriate empirical therapy.18, 19, 20, 21 The clinical picture of “typical” CAP is that of disease characteristically caused by bacteria such as S. pneumoniae, H. influenzae, and K. pneumoniae. The initial presentation is frequently acute, with an intense chill. Productive cough is present, and the sputum is purulent or bloody. Pleuritic pain may suggest S. pneumoniae. Physical examination reveals typical findings of pulmonary consolidation (see Chapter 16). Blood tests show leukocytosis with neutrophilia and the presence of band forms in most cases. Chest radiography shows lobar consolidation with air bronchograms (Fig. 33-1 ).
Figure 33-1.
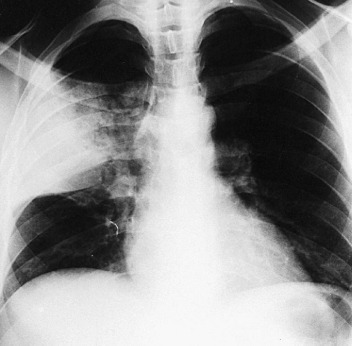
Pneumococcal pneumonia with lobar consolidation.
In contrast, the syndrome of gradual onset of fever, nonproductive cough, and a relatively normal white blood cell count in a patient without a readily demonstrable bacterial pathogen has been called “atypical pneumonia.” Frequently, systemic complaints are more prominent than the respiratory ones. The atypical syndrome is characteristic of infections by pathogens such as M. pneumoniae, Chlamydophila species, C. burnetii, and viruses. However, several studies, including one that included patients with mild CAP treated on an outpatient basis,57 have found that neither the clinical symptoms nor the radiographic manifestations are sufficiently sensitive or specific to guide pathogen-directed antibiotic treatment against “typical” versus “atypical” microorganisms.57 Therefore, current guidelines do not emphasize the use of the typical versus atypical classification to determine initial empirical antibiotic treatment for CAP.18, 19, 20, 21, 58
Patient Evaluation
Clinical Evaluation
The clinical findings that best differentiate CAP from other acute respiratory tract infections are cough, fever, tachypnea, tachycardia, and pulmonary crackles; CAP is present in 20% to 50% of persons who have all five factors.59 Specific signs of pulmonary consolidation are present in only one third of the cases that warrant hospitalization and are frequently absent in patients that are less ill. Early in the evolution of disease, pain and cough may be absent and the physical examination may be normal other than for fever. In debilitated older patients, vague clinical manifestations of pneumonia are common and the presence of fever with no apparent source, especially when accompanied by confusion or tachypnea, justifies obtaining a chest radiograph.
Clues to the etiologic diagnosis may lie outside the respiratory tract. Bradycardia in relation to the amount of fever (pulse should increase by 10 beats/min/°C of temperature elevation) has been associated with pneumonia due to Legionella, C. psittaci, Mycoplasma, or F. tularensis. M. pneumoniae infection may present with extrapulmonary manifestations including arthralgia, cervical lymphadenopathy, bullous myringitis, diarrhea, myalgia, myocarditis, hepatitis, nausea, pericarditis, and vomiting.60 Skin lesions of erythema multiforme or erythema nodosum suggest Mycoplasma infection (as well as tuberculosis and endemic fungal infection), whereas lesions of ecthyma gangrenosum are most often seen with P. aeruginosa infection. Finally, the examiner must look for the presence of complications such as pleural effusion, pericarditis, endocarditis, arthritis, and central nervous system involvement, which may necessitate further diagnostic procedures and, potentially, a change in therapy.61
Laboratory Evaluation
Once the patient is suspected to have pneumonia, laboratory studies should include blood cell counts, serum glucose and electrolyte measurements, and pulse oximetry or arterial blood gas assays.18, 19, 20, 21 These data provide a basis for making decisions regarding the need for hospitalization. The increased incidence of CAP in HIV-infected individuals provides an additional rationale for HIV testing, particularly in patients with no other risk factors for CAP.
Marked leukocytosis with a leftward shift is more often encountered with infections caused by S. pneumoniae, H. influenzae, and gram-negative bacilli than with M. pneumoniae, Chlamydophila species, Coxiella, or nonbacterial causes of pneumonia. Leukopenia may be seen with overwhelming pneumococcal or gram-negative bacillary pneumonia. The serum level of C-reactive protein and the erythrocyte sedimentation rate are increased to higher values with bacterial than with viral pneumonias. Thrombocytopenia and thrombocytosis are associated with a greater severity of pneumonia and higher mortality.
Procalcitonin (PCT), a precursor of calcitonin, is present at increased concentrations in the blood of persons with bacterial infections, and PCT assays have been used to evaluate the severity, prognosis, and evolution of pneumonia.62 Importantly, procalcitonin is used to deescalate antibiotics or to stop antibiotics when the levels decrease to a certain cutoff point.63 A randomized trial of a PCT-guided strategy compared with a guideline-based algorithm, revealed equivalent primary outcomes of treatment of lower respiratory tract infections, but the PCT-guided strategy resulted in reduced antibiotic exposure and duration, fewer adverse effects of antibiotic treatment, and shorter length of stay.64
Radiographic Evaluation
Radiographic evaluation is necessary to establish the presence of pneumonia, because there is no combination of historical data, physical findings, or laboratory results that reliably confirms the diagnosis.18, 21, 59, 65 Limitations of chest radiography include interobserver variability and suboptimal specificity, particularly in patients with the acute respiratory distress syndrome (ARDS).21 Conversely, the sensitivity of the chest radiograph is decreased in (1) patients with emphysema, bullae, or structural abnormalities of the lung, who may present with delayed or subtle radiographic changes; (2) obese patients, in whom it may be difficult to discern the existence of pneumonia; and (3) patients with very early infection, severe dehydration, or profound granulocytopenia. Computed tomography (CT) of the chest provides a more sensitive means of detecting minor radiographic abnormalities.59 However, a chest CT is not recommended for patients with suspected pneumonia who have an apparently normal chest radiograph.21
Although several radiographic patterns have been associated with pneumonia caused by specific microorganisms, the presence of a certain pattern is not a reliable method for diagnosing a specific pathogen.66, 67 Nonetheless, the presence of air bronchograms and a lobar (eFig. 33-1) or segmental pattern is more characteristic of typical than atypical causes of pneumonia. In contrast, a mixed pattern (alveolar and interstitial disease (eFig. 33-2) is more frequently observed with atypical pneumonias. Pneumonia complicating aspiration (frequently from anaerobes) (eFig. 33-3) most often involves the superior segment of the right lower lobe or posterior segment of the right upper lobe, or both, as well as the corresponding segments on the left. Infections developing from hematogenous seeding often appear as multiple rounded, small opacities, sometimes with cavities, with a basal predominance, where the distribution of blood flow is greatest. Demonstration of a lung abscess (eFig. 33-4), cavitation, or necrotizing pneumonia suggests infection by anaerobes, S. aureus, Streptococcus pyogenes, or gram-negative bacilli. Pleural effusion frequently accompanies pneumonia; the size of the pleural effusion on the chest radiograph helps determine whether thoracentesis should be performed.
eFigure 33-1.
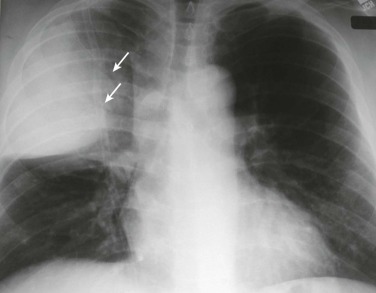
Lobar pneumonia due to pneumococcus.
Frontal chest radiograph shows homogeneous increased opacity conforming to the shape of the right upper lobe, extending to the pleural surfaces, associated with air bronchograms (arrows). These findings are typical of air space consolidation, and the pattern is consistent with lobar pneumonia, commonly seen with pneumococcal or Klebsiella pulmonary infections.
(Courtesy Michael Gotway, MD.)
eFigure 33-2.
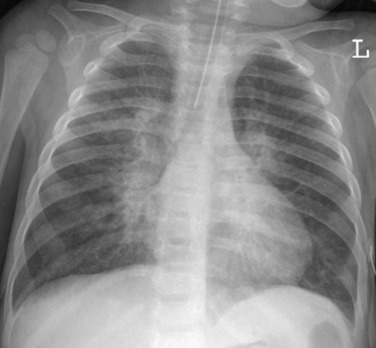
Atypical pneumonia due to adenovirus.
Frontal chest radiograph in a pediatric patient shows multifocal, bilateral central peribronchial thickening, typical of an infiltrative process involving the pulmonary interstitium, such as viral infection.
(Courtesy Michael Gotway, MD.)
eFigure 33-3.
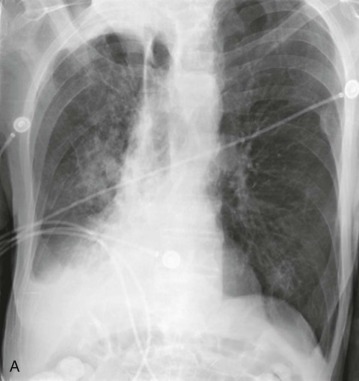
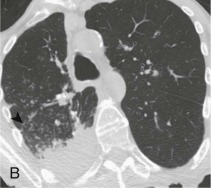
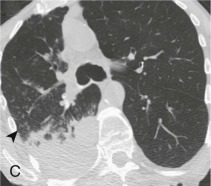
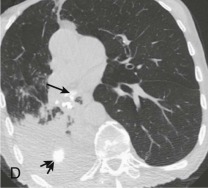
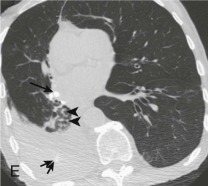
Aspiration pneumonia.
A, Frontal chest radiograph shows right lower lobe consolidation and volume loss; note shift of trachea and cardiomediastinal structures toward the right. Trace right pleural effusion is present. B–E, Axial chest CT displayed in lung windows shows right lower lobe consolidation associated with small centrilobular nodules (arrowheads) consistent with bronchopneumonia and bronchiolitis. This pattern is consistent with aspiration pneumonia, particularly when seen in dependent lung regions, but is not completely specific for aspiration; community-acquired or health care–acquired bronchopneumonia may appear similar. However, what is specific for aspiration in this circumstance is the frank aspiration of orally administered contrast (E,single arrow, oral contrast in right lower lobe bronchus; D and E,double arrows, oral contrast extending into right lower lobe pulmonary parenchyma), which flows directly into the affected regions of lung.
(Courtesy Michael Gotway, MD.)
eFigure 33-4.
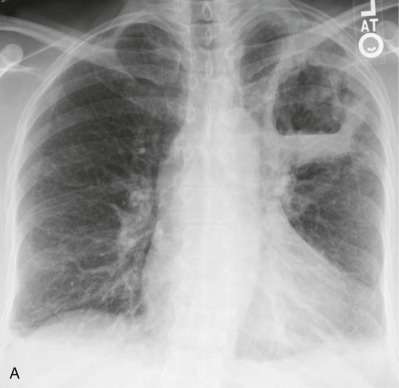
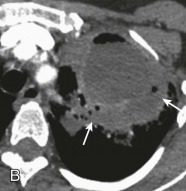
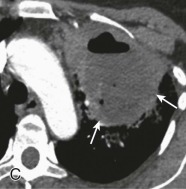
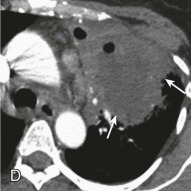
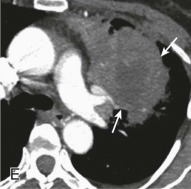
Lung abscess.
A, Frontal chest radiograph in a patient with cough and purulent sputum shows a thick-walled cavity with an irregular internal lining and air-fluid level in the left apex. B–E, Axial chest CT displayed in soft tissue windows shows a rounded area of low attenuation (arrows) in the left upper lobe, surrounded by consolidation consistent with a pulmonary abscess. The internal low attenuation is fluid density, and an air-fluid level is present, typical of pulmonary abscess. Reactive prevascular lymph node enlargement is also evident.
(Courtesy Michael Gotway, MD.)
Microbiologic Evaluation
Identification of the infecting microorganism facilitates the use of specific therapy instead of unnecessarily broad-spectrum antimicrobial agents. Although the utility of sputum examination is debated (see later), pleural fluid (if present) and two sets of blood cultures should be obtained in patients hospitalized for CAP. Optimal culture results require that specimens be obtained before initiation of antimicrobial therapy. Sputum samples must be carefully collected, transported, and processed in order to optimize the recovery of common bacterial pathogens. These recommendations are summarized in Tables 33-4 and 33-5 .
Table 33-4.
Recommended Microbiologic Evaluation in Patients with Community-Acquired Pneumonia
| PATIENTS WHO DO NOT REQUIRE HOSPITALIZATION |
| None* |
| PATIENTS WHO REQUIRE HOSPITALIZATION |
|
| ADDITIONAL TESTS FOR PATIENTS WHO REQUIRE TREATMENT IN AN ICU |
|
BAL, bronchoalveolar lavage; ICU, intensive care unit.
Gram stain and culture should be strongly considered in patients with risk factors for infection by an antimicrobial-resistant organism or unusual pathogen.
Table 33-5.
Recommended Microbiologic Evaluation in Patients with Hospital-Acquired Pneumonia
|
Gram stain and culture of valid sputum sample, endotracheal aspirate, or bronchoscopically obtained specimens using a protected specimen brush or bronchoalveolar lavage (if patient is intubated).
Sputum Examination
Microscopic examination of expectorated sputum is the easiest and most rapidly available method of evaluating the microbiology of lower respiratory tract infections. A valid expectorated sputum specimen can be obtained in about 40% of patients hospitalized with CAP. When interpreting sputum cultures, it is crucial to ensure that oropharyngeal contents do not unduly contaminate the specimens. The presence of more than 10 squamous epithelial cells per low-power field (×100 magnification) indicates excessive oropharyngeal contamination and the specimen should be discarded because it is not representative of the pulmonary milieu.18 A specimen with few or no squamous cells and many polymorphonuclear white blood cells (>25 cells/low-power field in a sample from a patient who is not granulocytopenic68) is ideal (see Fig. 33-3). Gram-stained expectorated sputum specimens of acceptable quality should be carefully examined using ×1000 magnification (oil immersion objective). Specific fluorescent antibodies are used to evaluate sputum or other respiratory tract specimens for the presence of Legionella and selected other pathogens (see Chapter 17).
Figure 33-3.
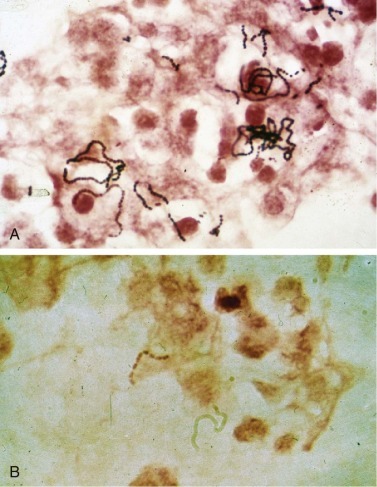
Streptococcus.
A, Group A streptococcus (Streptococcus pyogenes, β-hemolytic streptococcus). B, Group B streptococcus (Streptococcus agalactiae) are indistinguishable from Streptococcus pneumoniae on Gram stain. Both form long chains containing multiple bacteria.
When acceptable sputum is obtained, the specificity of the Gram stain for pneumococcal pneumonia is estimated to be greater than 80%.69 Because the fastidious nature of S. pneumoniae and H. influenzae leads to the death of these organisms, the sensitivity of sputum culture may be lower than that of sputum Gram stain examination for S. pneumoniae or H. influenzae. In contrast, S. aureus and gram-negative bacilli may dominate even if they are not the cause of the patient's illness, because these bacteria are hardier and may proliferate during sample transport and processing. True pneumonia due to S. aureus or gram-negative bacilli is doubtful if the Gram stain of a valid sputum specimen does not corroborate the presence of these bacteria. In good quality Gram-stained sputum, the presence of a single or a preponderant morphotype of bacteria (≥90%) is considered diagnostic. In the absence of an informative Gram stain, the predictive value of sputum culture is very low.
The latest IDSA/ATS guidelines58 recommend obtaining a sputum sample for Gram stain and culture in hospitalized patients with the clinical indications listed in Table 33-6 but are optional for patients without these conditions.
Table 33-6.
Clinical Indications for More Extensive Testing in Community-Acquired Pneumonia
|
UAT, urinary antigen test.
From Mandell LA, Wunderink RG, Anzueto A: Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia. Clin Infect Dis 44(Suppl 2):S27–S72, 2007.
For patients with HAP or ventilator-associated pneumonia (VAP), the range of potential pathogens is so broad and antimicrobial susceptibility patterns so diverse that vigorous diagnostic measures are justified. In ventilated patients, the equivalent of sputum is the endotracheal aspirate for which the criteria for validity are the same as those for sputum. Although the Gram stain and qualitative cultures of endotracheal aspirates have excellent sensitivity, they have poor specificity.70 Quantitative cultures of endotracheal aspirate samples may help distinguish colonization from infection. However, there has been difficulty in choosing a quantitative threshold for VAP; some have chosen to consider a range of quantitative cultures, from 103 to 106 CFU/mL, rather than a single cutoff.71
Some bacterial agents of pneumonia cannot be cultivated on conventional laboratory media. For example, Legionella requires buffered charcoal yeast extract agar for isolation, whereas recovery of Chlamydophila species and C. burnetii requires culture in mammalian cell lines. When necessary, specimens can be sent to specialized or reference laboratories for appropriate procedures. Culture of certain agents of bacterial pneumonia poses major health risks to laboratory workers (e.g., F. tularensis, Bacillus anthracis, C. burnetii). Specimens suspected to harbor one of these agents should be dealt with carefully in a biologic safety hood, and isolation of the pathogens should be reserved for specialized laboratories.
Blood and Pleural Fluid Cultures
Although the overall yield of blood cultures is less than 20% in patients hospitalized for CAP, a positive culture of blood or pleural fluid establishes the etiologic diagnosis of pneumonia.72, 73 Not surprisingly, the detected rate of bacteremia is lower in patients with mild CAP and higher in patients with severe CAP, especially those warranting ICU care. Prior antibiotic treatment decreases the yield of blood cultures.74 The latest IDSA/ATS guidelines58 recommend obtaining blood samples for culture in hospitalized patients with the clinical indications listed in Table 33-6 but are optional for patients without these conditions.
In up to 40% of CAP cases, a pleural effusion may be present. Although the specificity of pleural exudate cultures is very high, the sensitivity is low because of the low incidence of invasion of the pleura. Diagnostic thoracentesis should be performed when a significant pleural effusion is present. Gram stain of pleural fluid may produce an indication of the infecting organisms within 1 hour, while culture identification may require 24 to 48 hours.
Antigen Detection
Commercial assays can be used to detect capsular polysaccharide antigens of S. pneumoniae or L. pneumophila serogroup 1 in urine, and can require less than 1 hour.69, 74, 75 The sensitivity of these tests is little affected by prior antibiotic treatment; indeed, results may remain positive several weeks after successful treatment. For L. pneumophila serogroup 1, the sensitivity is 60% to 80%, and the specificity is greater than 95%.76 Urinary antigen testing is currently the most helpful rapid test for the diagnosis of Legionella infections. The major limitation of urinary antigen tests is that currently available tests are intended to detect L. pneumophila serogroup 1 antigen only, although this is the most common cause of Legionella infection.
The sensitivity of S. pneumoniae urinary antigen detection is 50% to 80% and the specificity is 90%.77 The degree of positivity for the S. pneumoniae urinary antigen test correlates with the Pneumonia Severity Index (PSI).78 The S. pneumoniae antigen test may also be applied on pleural fluid with a sensitivity and specificity of almost 100%. Urine specimens of children, frequent carriers of S. pneumoniae in the nasopharynx, may test positive in the absence of evidence of pneumonia, and the test should therefore be interpreted with caution in children.79 The most recent IDSA/ATS guidelines58 recommend S. pneumoniae and L. pneumophila urinary antigen detection in hospitalized patients with the clinical indications listed in Table 33-6, but are optional for patients without these conditions.
Antigens for the many common respiratory viruses, influenza virus, respiratory syncytial virus, adenovirus, and parainfluenza viruses can be detected by direct immunofluorescence or by enzyme-linked immunoassay. A rapid antigen detection test for influenza can provide an etiologic diagnosis within 15 to 30 minutes. Test performance varies according to the test used, viral strain, sample type, duration of illness, and patient age. Most show a sensitivity ranging from 50% to 70% and a specificity approaching 100% in adults (see Chapter 17).
Nucleic Acid Amplification Tests
Culture procedures for viruses and fastidious bacteria, M. pneumoniae, C. pneumoniae, L. pneumophila, and Bordetella pertussis, which normally do not colonize in the human respiratory tract, are too insensitive and too slow to be helpful in guiding therapy. These pathogens should be detected by nucleic acid amplification tests; their sensitivity is generally superior to that of the traditional procedures and some are considered as the “gold standard.”80 Real-time multiplex polymerase chain reaction assays detect respiratory viruses in both immunocompetent and immunosuppressed hosts.81 (See Chapter 17 for detailed information on nucleic acid amplification tests for respiratory pathogens.)
Serologic Evaluation
Before the development of nucleic acid amplification tests, serologic techniques were used to establish a microbiologic diagnosis for pneumonia caused by pathogens that cannot be readily cultured. Examples include common pathogens such as M. pneumoniae, C. pneumoniae, and L. pneumophila, and less common causes of pneumonia such as those caused by the agents of tularemia, brucellosis, and psittacosis, and certain viruses. Diagnosis usually requires that a convalescent specimen demonstrate a fourfold increase in immunoglobulin (Ig) G titer above that present in an acute specimen. These tests are not helpful in initial patient management but are of utility in defining the epidemiology of the pertinent infectious agents. Because IgM antibodies appear earlier than IgG antibodies, the detection of pathogen-specific IgM in serum has been used for the early serologic diagnosis of certain acute infections.
Invasive Diagnostic Techniques
Because of problems encountered with the use of expectorated sputum, it may be necessary to perform an invasive procedure to obtain suitable material for microscopy and cultures. This may be important in the management of patients with life-threatening CAP in whom diagnostic materials cannot otherwise be obtained, patients with progressive pneumonia despite seemingly appropriate antimicrobial therapy, immunocompromised patients, and patients with HAP, especially in the setting of endotracheal intubation.61, 82 Although qualitative culture of materials obtained by endotracheal suction has excellent sensitivity, the specificity of such cultures is poor; thus, overreliance on these cultures can lead to antibiotic overtreatment.71
Bronchoscopic Samples
The reliability of bronchoscopic procedures to determine the microbial etiology of pneumonia depends on the technique used and the organism sought. When compared with sputum cultures, optimally processed bronchoscopic specimens demonstrate improved sensitivity and equal specificity for the culture of pathogenic fungi and mycobacteria. However, such materials have unacceptably poor specificity for routine bacterial cultures owing to oropharyngeal contamination. Semiquantitative or quantitative cultures of materials obtained bronchoscopically with a protected sheath brush or through bronchoalveolar lavage (BAL) and by direct lung aspiration have been successfully used for aerobic and anaerobic bacterial cultures83, 84, 85 (see Chapters 17 and 22). For protected sheath brush cultures, a threshold of 103 colony-forming units (CFU)/mL has been recommended to distinguish colonization from infection. However, 14% to 40% of duplicate samples yield disparate quantitative results.
BAL fluid can be quantitatively cultured for bacteria and qualitatively cultured for fungi, mycobacteria, and viruses. A concentrate can be stained for cytochemical and fluorescence evaluation.85 In one study, the threshold of 103 CFU/mL for diagnosing bacterial pneumonia correlated well with diagnoses based on protected sheath brush results and histologic examination of the lung.86 BAL permits identification of contaminated specimens (i.e., those with greater than 1% squamous epithelial cells), the immediate diagnosis of infection (i.e., intracellular bacteria in more than 2% to 5% of examined polymorphonuclear leukocytes), and the exclusion of infection (i.e., the absence of bacterial pathogens in culture of BAL fluid, although the sensitivity is reduced by prior antibiotic administration).87, 88
In one study, the use of quantitative cultures obtained by protected sheath brush and BAL, compared with qualitative cultures of endotracheal aspirates and clinical evaluation, was associated with lower 14-day mortality rates, earlier reversal of organ dysfunction, and less antibiotic use.89 However, other randomized trials on the use of quantitative cultures of protected sheath brush and BAL specimens, rather than quantitative cultures of endotracheal aspirates, in patients with VAP have not replicated these findings.90, 91 The use of a sophisticated algorithm (i.e., Clinical Pulmonary Infection Score) increases the diagnostic accuracy of clinical judgment.92
Transthoracic Lung Aspiration
Transthoracic lung aspiration obtains specimens suitable for microbiologic and cytologic examination directly from lung parenchyma (eFig. 33-5). It is more widely used for diagnosing malignant pulmonary lesions than infectious diseases, for which, in immunocompetent hosts, the diagnostic yield by transthoracic lung aspiration is approximately 50%. Serious complications of transthoracic lung aspiration include pneumothorax and hemoptysis, even when small-gauge needles are used.
eFigure 33-5.
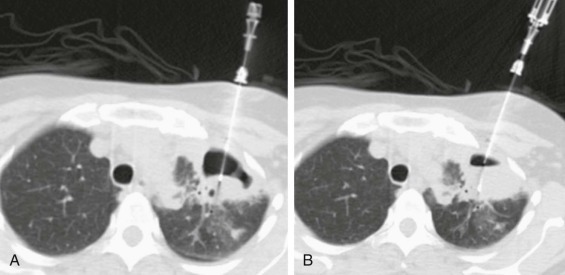
Percutaneous transthoracic sampling of a pulmonary abscess.
A and B, Axial chest CT shows placement of a needle into a left apical cavity (same patient as in eFig. 33-4). Purulent fluid was recovered, with microbiologic analysis disclosing polymicrobial infection. The lesion resolved with antibiotic therapy.
(Courtesy Michael Gotway, MD.)
Differential Diagnosis
Several diseases may present with fever and chest radiographic opacities and mimic CAP (eTable 33-1)59; such diseases should be suspected when the radiographic resolution is unusually quick or when there is a lack of response to initial or subsequent antibiotic treatments. In patients with HAP, and particularly in those with VAP, the classic signs and symptoms of pneumonia (including new radiographic changes, fever, leukocytosis or leukopenia, and purulent pulmonary secretions) are neither sufficiently sensitive nor specific to confirm the presence of a pulmonary infection. Atelectasis, pulmonary hemorrhage, ARDS, and pulmonary embolism, among others, are conditions that may mimic pneumonia. In patients with suspected HAP or VAP, the microbiologic confirmation of pneumonia is important in order to avoid unnecessary treatments and increased antibiotic resistance.
eTable 33-1.
Noninfectious Causes of Fever and Radiographic Changes That May Mimic Community-Acquired Pneumonia
| Pulmonary edema |
| Pulmonary infarction |
| Acute respiratory distress syndrome |
| Pulmonary hemorrhage |
| Lung cancer or metastatic cancer |
| Atelectasis |
| Radiation pneumonitis |
| Drug reactions involving the lung |
| Extrinsic allergic alveolitis |
| Pulmonary vasculitis |
| Pulmonary eosinophilia |
| Organizing pneumonia |
Therapeutic Approach to Pneumonia
Once the diagnosis of pneumonia is made, the clinician must decide the appropriate treatment setting: outpatient, general hospital bed, or ICU. Applying prediction rules can facilitate this decision. The second key initial decision is the selection of initial antimicrobial therapy.
Assessment of Severity
The PSI (eTable 33-2) is a scoring system derived from a retrospective analysis of a cohort of 14,199 patients with CAP and prospectively validated in a separate cohort of 38,039 patients with CAP.93 The PSI is heavily weighted by age, which means it is less useful at extremes of age and is not valid in children. Outpatient treatment is recommended for patients with a PSI score of 70 or less (class I or II). Patients with a PSI score of 71 to 90 (class III) may benefit from brief hospitalization, while inpatient care is appropriate for patients with a score greater than 90 (class IV and V). Prospective studies in both community and teaching hospitals demonstrate that the hospital admission decisions based on PSI may be safely and effectively applied in clinical practice.94, 95, 96 The PSI is complex and often needs decision support tools for efficient use in a busy emergency department.
eTable 33-2.
Scoring System for Determining Risk of Complications in Patients with Community-Acquired Pneumonia*
| Patient Characteristic | Points Assigned |
|---|---|
| DEMOGRAPHIC FACTORS | |
| Males | Age (yr) |
| Females | Age (yr) − 10 |
| Nursing home residents | Age (yr) + 10 |
| COMORBID ILLNESSES | |
| Neoplastic disease | +30 |
| Liver disease | +20 |
| Congestive heart failure | +10 |
| Cerebrovascular disease | +10 |
| Renal disease | +10 |
| PHYSICAL EXAMINATION FINDINGS | |
| Altered mental status | +20 |
| Respiratory rate 30/min or greater | +20 |
| Systolic blood pressure <90 mm Hg | +20 |
| Temperature <35° C or ≥40° C | +15 |
| Pulse 125/min or greater | +10 |
| LABORATORY FINDINGS | |
| pH <7.35 | +30 |
| BUN >10.7 mmol/L | +20 |
| Sodium <130 mEq/L | +20 |
| Glucose >13.9 mmol/L | +10 |
| Hematocrit <30% | +10 |
| Po2 <60 mm Hg or O2 saturation <90% | +10 |
| Pleural effusion | +10 |
BUN, blood urea nitrogen; Po2, oxygen pressure.
A risk score is obtained by summing the patient's age in years (age − 10 for females) and the points for each applicable patient characteristic. Patients with a score <50 are candidates for outpatient treatment, whereas those with scores >90 warrant hospitalization. Proper management of patients with scores of 70 to 90 requires careful application of clinical judgment.
Adapted from Fine MJ, Auble TE, Yealy DM, et al: A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336:243–250, 1997.
The British Thoracic Society validated the simpler CURB-65 score for admission triage decisions.25, 97 Their algorithm assigns 1 point for each of the following findings at presentation: (1) confusion; (2) urea higher than 7 mmol/L (equal to BUN more than 20 mg/dL); (3) respiratory rate of 30/min or more; (4) low systolic (<90 mm Hg) or diastolic (≤60 mm Hg) blood pressure; and (5) age 65 years or older. Outpatient treatment is recommended for 0-1 points, brief inpatient or supervised outpatient care is recommended for 2 points, and hospitalization is recommended for 3 or greater, with consideration of ICU care for patients with scores of 4 or 5.
Risk stratification for both PSI and CURB-65 was based on associated mortality. They are therefore not sensitive to logistic and social issues such as reliability of oral intake, including antibiotics, and home support.
Patients initially admitted to a general floor with subsequent transfer to the ICU have higher mortality than patients with equivalent severity of illness admitted directly to the ICU.98 Neither PSI nor CURB-65 are accurate for determining need for ICU care in patients without an obvious indication such as the need for mechanical ventilation or vasopressor support while still in the emergency department. Several scores have been developed for this critical decision.58, 99, 100, 101 These scores share many common risk factors (Table 33-7 ) and appear to be equally effective,102 and management of severe CAP per these guidelines has been associated with decreased mortality.103, 104, 105 The optimal use of these scores is to identify at-risk patients who need additional evaluation or monitoring even if not initially admitted to the ICU.
Table 33-7.
Criteria to Consider Admission to an Intensive Care Unit for Patients with Community-Acquired Pneumonia without Shock or Respiratory Failure
| Respiratory rate > 30 breaths/min*†‡§ |
| Pao2/Fio2 ratio < 250 or arterial saturation ≤90% on room air*†‡§ |
| Multilobar or bilateral radiographic involvement or pleural effusion*†‡§ |
| Confusion or disorientation*†‡ |
| Uremia (BUN level > 20 mg/dL)*†‡ |
| Leukopenia (WBC count < 4000 cells/dL) or extreme leukocytosis (>20,000 cells/dL)*§ |
| Thrombocytopenia (platelet count < 100,000 cells/dL)* |
| Hypothermia (core temperature < 36° C)* |
| Hypotension requiring aggressive fluid resuscitation* |
| Acidosis (pH < 7.30)†‡§ |
| Hypoalbuminemia (albumin < 3.5 g/dL)† |
| Hyponatremia (sodium < 130 mEq/L)§ |
| Tachycardia (>125/min)†§ |
Selection of Antimicrobial Agents
Whenever possible, treatment for pneumonia should use the antibiotic with the narrowest spectrum possible, selected on the basis of the underlying pathogen. However, pathogens are rarely identified at the time of presentation, especially when pneumonia is managed in the outpatient setting. Because optimal outcomes are associated with a rapid initiation of antibiotics, initial treatment for patients with pneumonia must be empirical. In selecting initial empirical antimicrobial therapy, physicians should consider the setting in which the pneumonia arose (e.g., community, hospital, nursing home), the severity of illness, age of the patient, presence of comorbidities and immunosuppression, recent antimicrobial therapy, and specific clinical manifestations of the illness. Geographic and facility-specific factors, such as the local prevalence of specific microorganisms (e.g., C. burnetii, L. pneumophila, endemic mycoses, and multidrug-resistant [MDR] pathogens), may also affect the initial treatment choice.
In hospitalized patients, specimens for cultures of blood, sputum, and pleural fluid (if present) should be obtained before treatment. A brief delay in starting therapy while performing diagnostic procedures is reasonable in patients who are not hypotensive. However, delays of more than 4 to 8 hours may increase the length of hospitalization and have been associated with increased mortality.106, 107
Community-Acquired Pneumonia
The standard therapy for inpatient empirical antibiotic coverage of CAP is one of two regimens: the combination of a second- or third-generation cephalosporin combined with a macrolide or one of the fluoroquinolones with efficacy against respiratory pathogens (levofloxacin, moxifloxacin, gatifloxacin).58 Either therapy should be effective against penicillin-resistant S. pneumoniae.108 The North American guidelines20, 21, 58 recommend that any empirical regimen for CAP should be active against “atypical” pathogens such as M. pneumoniae, C. pneumoniae, and L. pneumophila. Retrospective analyses of patients hospitalized with CAP indicate that regimens that cover “atypical” pathogens and those that follow recommendations made by the ATS and the IDSA are associated with improved clinical outcomes.18, 20, 96, 109, 110 In contrast, some Northern European guidelines suggest atypical coverage is not needed unless patients have clinical features more common to atypical pathogens.97 It is important to recognize that all CAP treatment guidelines are based on broad epidemiologic considerations that may vary by location. Variation from these regimens should be based on specific epidemiologic or clinical characteristics that strongly suggest one of the less common CAP pathogens such as mixed aerobic-anaerobic flora due to aspiration or presence of gram-negative Enterobacteriaceae or P. aeruginosa in patients with specified risk factors.24, 111
When tuberculosis is a possibility, fluoroquinolones should be used cautiously in CAP, because as little as 10 days of fluoroquinolone administration is sufficient to select for fluoroquinolone-resistant M. tuberculosis.112
The greatest factor to consider in the choice of regimens is a history of recent use of any of the agents.113 Widespread fluoroquinolone use, especially in subtherapeutic doses, and use of ciprofloxacin has been associated with fluoroquinolone resistance in up to 13% of S. pneumoniae isolates in Hong Kong.114 Fluoroquinolone resistance and subsequent treatment failures are reported in pneumococcal CAP,114, 115, 116 but this is less common with use of the fluoroquinolones that have improved activity against respiratory pathogens. In contrast, the frequency of macrolide resistance in S. pneumoniae is increasing, and a macrolide should not be used for monotherapy of S. pneumoniae infection unless in vitro testing confirms that the patient's strain is susceptible to macrolides.
Empirical antibiotic treatment of severe CAP (SCAP) remains controversial, predominantly due to a lack of treatment studies specifically focused on SCAP. The spectrum of etiologies is clearly greater in SCAP. Even so, penicillin-sensitive pneumococci are still the most likely etiology. Whether SCAP justifies more aggressive diagnostic testing or broader spectrum empirical treatment in all cases has not been established. Retrospective studies suggest combination therapy specifically for severe pneumococcal pneumonia and for SCAP in general are associated with lower mortality. In a large cohort of older patients with CAP needing hospitalization, antibiotic treatment including azithromycin was associated with a lower 90-day risk mortality compared with other antibiotics.116a
Summaries of the recent IDSA/ATS guideline CAP antibiotic recommendations are presented in eTable 33-3 and eTable 33-4, respectively.58
eTable 33-3.
Guidelines for Empirical Oral Outpatient Treatment of Immunocompetent Adults with Community-Acquired Pneumonia
| BRITISH THORACIC SOCIETY |
|
| AMERICAN THORACIC SOCIETY |
| INFECTIOUS DISEASES SOCIETY OF AMERICA |
|
| DRUG-RESISTANT STREPTOCOCCUS PNEUMONIAE THERAPEUTIC WORKING GROUP |
|
| CANADIAN INFECTIOUS DISEASES SOCIETY AND CANADIAN THORACIC SOCIETY |
|
COPD, chronic obstructive pulmonary disease.
American Thoracic Society comorbidities (modifying factors) include cardiopulmonary disease and age older than 65 years, receipt of a β-lactam antimicrobial within the prior 3 months, alcoholism, prior immunosuppressive therapy, multiple medical comorbidities, exposure to a child in a daycare center, residence in a nursing home, underlying cardiopulmonary disease, multiple comorbidities or recent antimicrobial therapy. Infectious Diseases Society of America comorbidities include only COPD, diabetes, renal or congestive heart failure, and malignancy.
Advanced macrolides are azithromycin and clarithromycin. Telithromycin has similar antimicrobial activity, but is associated with a higher risk of toxicity and its indications are limited.
Second-choice agent.
High-dose amoxicillin (3 to 4 g/day), high-dose amoxicillin-clavulanate (2 g amoxicillin plus 125 mg clavulanic acid every 12 hr), cefpodoxime, cefprozil, or cefuroxime.
Because of increasing macrolide resistance, erythromycin cannot be relied upon to ensure coverage of β-lactamase–producing Haemophilus influenzae. A combination of a β-lactam/β-lactamase inhibitor is preferred.
Antipneumococcal fluoroquinolones include levofloxacin, and moxifloxacin.
Levofloxacin or moxifloxacin.
Available oral second-generation cephalosporins include cefaclor, cefuroxime axetil, cefprozil, cefonocid, and loracarbef.
eTable 33-4.
Guidelines for Empirical Parenteral Inpatient Treatment of Immunocompetent Adults with Community-Acquired Pneumonia
| MILD TO MODERATE DISEASE |
|
| SEVERE DISEASE |
|
Antipneumococcal fluoroquinolones include levofloxacin, gatifloxacin, and moxifloxacin.
Advanced macrolides are azithromycin and clarithromycin.
Modifying factors include those considered to increase the risk of infection by a penicillin-resistant pneumococcus (age older than 65 years, exposure to a β-lactam antimicrobial within the prior 3 months, alcoholism, prior immunosuppressive therapy, multiple medical comorbidities, exposure to a child in a daycare center or to infection by an enteric gram-negative bacillus (residence in a nursing home, underlying cardiopulmonary disease, multiple comorbidities, or recent antimicrobial therapy).
Preferred regimen may be determined by whether the patient has received antibiotics within the prior 3 months.
Antianaerobic agents include clindamycin, metronidazole, and β-lactam/β-lactamase inhibitor combinations.
Acceptable cephalosporins include second-generation agents (e.g., cefuroxime, cefamandole), third-generation agents (cefotaxime or ceftriaxone), or fourth-generation agents (cefepime or cefpirome, neither of which is available in the United States).
Second-choice agent.
American Thoracic Society risk factors for Pseudomonas aeruginosa are structural lung disease (i.e., bronchiectasis, cystic fibrosis), corticosteroid use (>10 mg prednisone/day), broad-spectrum antibiotic therapy for more than 7 days in the past month, or malnutrition. The Infectious Diseases Society of American risk factors for P. aeruginosa include only structural lung disease or recent completion of a course of antibiotics or steroids. The Canadian risk factors include only structural lung disease, recent antibiotic therapy, or recent hospitalization in an intensive care unit.
Antipseudomonal β-lactams include ceftazidime, cefepime, imipenem, meropenem, mezlocillin, piperacillin, and piperacillin-tazobactam.
Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia
Empirical therapy for VAP is necessarily broad because the range of potential pathogens is large and mortality is increased when the responsible pathogen is resistant to the initial empirical antibiotic regimen (Table 33-8 ). Recommended empirical regimens include expanded-spectrum β-lactam agents, usually in combination with aminoglycosides and with MRSA coverage.53 The empirical β-lactam should be based on antibiotic sensitivity patterns for common gram-negative pathogens in the relevant institution or specific unit.
Table 33-8.
Guidelines for Empirical Antibiotic Treatment of Nosocomial Pneumonia*
| Setting | Core Pathogens | Antimicrobial Choices |
|---|---|---|
| 2 to 5 Days in Hospital | ||
| Mild to moderate pneumonia† Severe pneumonia “low-risk”† |
Enterobacteriaceae Streptococcus pneumoniae Haemophilus influenzae Methicillin-sensitive Staphylococcus aureus |
β-Lactam/β-lactamase inhibitor‡or ceftriaxone or fluoroquinolone§ All ± an aminoglycoside |
| ≥5 Days in Hospital | ||
| Mild to moderate pneumonia | As above | As above |
| ≥5 Days in Hospital | ||
| Severe HAP “low risk” |
Pseudomonas aeruginosa Enterobacter spp. Acinetobacter spp. |
Carbapenem or β-lactam/βl-lactamase inhibitor†or cefepime |
| All plus amikacin or fluoroquinolone§ | ||
| ≥2 Days in Hospital | ||
| Severe HAP “high risk” | As above | As above |
| Special Circumstances19, 58 | ||
| Recent abdominal surgery or witnessed aspiration | Anaerobes | As per Table 33-9 |
| Other sites of infection with MRSA or prior use of antistaphylococcal antibiotics | MRSA | As per Table 33-9 |
| Prolonged ICU stay or prior use of broad-spectrum antibiotics or structural lung disease (cystic fibrosis, bronchiectasis) | P. aeruginosa | As per Table 33-9 |
| Endemicity within facility and either impaired cell-mediated immunity or failure to respond to antibiotics | Legionella | As per Table 33-9 |
This protocol does not address the treatment of neutropenic or HIV-infected persons.
Severe pneumonia requiring care in an ICU is characterized by rapid radiographic progression, multilobar disease, or cavitation. All other cases of nosocomial pneumonia are considered mild to moderate.
HAP, hospital-acquired pneumonia; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus.
High-risk criteria include age older than 65 years, pancreatitis, chronic obstructive pulmonary disease, central nervous system dysfunction (stroke, drug overdose, coma, status epilepticus), congestive heart failure, malnutrition, diabetes mellitus, endotracheal intubation, renal failure, complicated thoracoabdominal surgery, and alcoholism. All other patients are considered to be at low risk.
Antimicrobial treatment should also be sufficient to cover core pathogens.
Ticarcillin-clavulanate and piperacillin-tazobactam are the preferred β-lactam/β-lactamase inhibitors for the treatment of nosocomial pneumonia. Ampicillin-sulbactam lacks adequate activity against many nosocomial enteric gram-negative bacilli.
Levofloxacin (IV or PO), gatifloxacin (IV or PO), moxifloxacin (IV or PO), or gemifloxacin (PO only) are preferred for Streptococcus pneumoniae. When used for severe HAP, levofloxacin should be dosed at 750 mg IV daily. Ciprofloxacin has the best in vitro activity against Pseudomonas aeruginosa.
Modified from American Thoracic Society: Hospital-acquired pneumonia in adults: Diagnosis assessment of severity, initial antimicrobial therapy, and preventative strategies. Am J Respir Crit Care Med 153:1711–1725, 1995.
Empirical antibiotics for HAP are less well studied. HAP in nonintubated patients is a mixture of CAP pathogens and the pathogens found in VAP, although the frequency of the latter is likely lower, especially in cases that present early after admission. The greatest risk for MDR pathogens in nonintubated patients with HAP is recent antibiotic therapy, and monotherapy is probably adequate for most patients without recent antibiotic exposure. Anaerobes appear to play a slightly greater role in HAP than VAP because of the risk of macroaspiration, but specific anaerobic coverage is not necessary if an appropriate β-lactam is used. Unless Legionella is known to be endemic in the institution, targeted therapy for this pathogen is seldom necessary in the empirical treatment of HAP. Efforts to identify the cause of infection are especially crucial in patients with HAP or VAP, to allow selection of optimal antimicrobial therapy and minimize the duration of empirical broad-spectrum coverage.
Health Care–Associated Pneumonia
The optimal approach to empirical coverage for HCAP remains controversial, due to variations in health care systems and definitions.55, 117, 118, 119 Pneumonia in nursing home and chronic care facility residents is seen in a bimodal pattern. Ambulatory patients who are able to take care of most of their activities of daily living have disease that resembles CAP,29 while in contrast, severely debilitated patients with tracheostomies, feeding tubes, frequent and recent acute care hospital admissions, and frequent exposure to antibiotics are at high risk for MDR pathogens and should be treated with VAP regimens. Culture-negative HCAP patients have equivalent or better outcomes when treated with CAP antibiotics as with broader spectrum treatment,119 but are difficult to identify at admission. If started on broader therapy, deescalation to CAP therapy after culture results return negative appears safe.120
Other Pneumonia Syndromes
On initial presentation, a variety of other infectious pulmonary syndromes may not be readily differentiated from acute bacterial pneumonia. Examples include influenza A, severe acute respiratory syndrome,49, 50 hantavirus pulmonary syndrome, and other viral pneumonias. Milder cases of viral pneumonia may be distinguished by a low PCT level and antibiotics can be safely withheld or withdrawn in these patients.121
Concerns about potential bioterrorism or biowarfare require attention to the epidemiologic, clinical, and microbiologic significance of pneumonia due to B. anthracis (anthrax),51 F. tularensis (tularemia),52 and Y. pestis (plague). These infectious agents are individually discussed later in this chapter. Further information may be obtained from organizations such as the Centers for Disease Control and Prevention (www.cdc.gov ), IDSA (www.idsociety.org), and the World Health Organization (www.who.org) (see Chapter 40).
Adjustments in Antimicrobial Therapy
If the etiologic agent of a patient's pneumonia has been identified, the initial antimicrobial regimen should be adjusted based on the results of in vitro susceptibility testing. The ideal drug for a known pathogen has the narrowest spectrum of activity and is the most efficacious, least toxic, and least costly. Pathogen-based modification of therapy is particularly important in HAP because prolonged use of broad-spectrum empirical agents promotes the emergence of MDR pathogens. Recommendations for specific drug choices for specific microorganisms are discussed under the sections devoted to individual microorganisms and are summarized in Table 33-9 . If a pathogen is not identified, reevaluation of the initial therapeutic regimen must take into account the patient's response to therapy. Change from parenteral to oral antimicrobial therapy can safely be made in hospitalized CAP patients when clinically stable and able to absorb effective oral antimicrobials122, 123; this is often achieved within 3 days. In-hospital observation after switching from intravenous to oral antibiotics for CAP patients is not needed. Because HAP pathogens are frequently resistant to available oral antimicrobials, enteral absorption is less predictable, and the severity of illness is greater, initial oral antimicrobial therapy is much less frequently appropriate.
Table 33-9.
Agents for Specific Therapy of Selected Respiratory Pathogens
| Type of Infection | Preferred Agent(s) | Alternative Agent(s) |
|---|---|---|
| COMMUNITY-ACQUIRED PNEUMONIA | ||
| Streptococcus pneumoniae | ||
| PCN-susceptible | Penicillin G, amoxicillin, clindamycin, doxycycline | Cephalosporin, macrolide,* (MIC < 2 g/mL) fluoroquinolone† |
| PCN-resistant | Agents identified using in vitro susceptibility tests, including cefotaxime, ceftriaxone, vancomycin, and fluoroquinolone† | Macrolide, if susceptible |
| Mycoplasma | Doxycycline, macrolide | Fluoroquinolone† |
| Chlamydophila pneumoniae | Doxycycline, macrolide | Fluoroquinolone† |
| Legionella | Azithromycin, fluoroquinolone (including ciprofloxacin),† erythromycin (± rifampin) | Doxycycline ± rifampin |
| Haemophilus influenzae | Second- or third-generation cephalosporin, clarithromycin, doxycycline, β-lactam/β-lactamase inhibitor, trimethoprim-sulfamethoxazole, azithromycin | Fluoroquinolone† |
| Moraxella catarrhalis | Second- or third-generation cephalosporin, trimethoprim-sulfamethoxazole, macrolide doxycycline, β-lactam/β-lactamase inhibitor | Fluoroquinolone† |
| Neisseria meningitidis | Penicillin | Ceftriaxone, cefotaxime, cefuroxime, chloramphenicol, fluoroquinolone† |
| Streptococci (other than S. pneumoniae) | Penicillin, first-generation cephalosporin | Clindamycin (susceptibility should be confirmed), vancomycin |
| Anaerobes | Clindamycin, β-lactam/β-lactamase inhibitor, β-lactam plus metronidazole | Carbapenem |
| Staphylococcus aureus | ||
| Methicillin-susceptible‡ | Oxacillin, nafcillin, cefazolin; all ± rifampin or gentamicin‡ | Cefuroxime, cefotaxime, ceftriaxone, fluoroquinolones,† clindamycin, vancomycin |
| Methicillin-resistant‡ | Vancomycin‡ ± rifampin or gentamicin | Linezolid, quinupristin-dalfopristin; trimethoprim-sulfamethoxazole, fluoroquinolones,† and tetracyclines may also show activity (in vitro testing required) |
| Klebsiella pneumoniae and other Enterobacteriaceae (excluding Enterobacter spp.) | Third-generation cephalosporin or cefepime (all ± aminoglycoside) carbapenem | Aztreonam, β-lactam/β-lactamase inhibitor,§ fluoroquinolone† |
| HOSPITAL-ACQUIRED INFECTIONS | ||
| Enterobacter spp. | Carbapenem, β-lactam/β-lactamase inhibitor, cefepime, fluoroquinolone; all + aminoglycoside in seriously ill patients | Third-generation cephalosporin + aminoglycoside |
| Pseudomonas aeruginosa | Antipseudomonal β-lactam§ + aminoglycoside, carbapenem + aminoglycoside | Ciprofloxacin + aminoglycoside, ciprofloxacin + antipseudomonal β-lactam‖ |
| Acinetobacter | Aminoglycoside + piperacillin or a carbapenem | Doxycycline, ampicillin-sulbactam, colistin |
| LESS COMMON PATHOGENS | ||
| Nocardia | Trimethoprim-sulfamethoxazole | Imipenem ± amikacin, doxycycline or minocycline, sulfonamide ± minocycline or amikacin |
| Coxiella burnetii (Q fever) | Doxycycline | Fluoroquinolone |
| Chlamydophila psittaci (psittacosis) | Doxycycline | Erythromycin, chloramphenicol |
| Eikenella corrodens | Penicillin | Tetracyclines, β-lactam/β–lactamase inhibitor, second- and third-generation cephalosporins, fluoroquinolones |
MIC, minimum inhibitory concentration.
Azithromycin (IV or PO) is the preferred macrolide; clarithromycin (PO) or erythromycin (IV or PO) may also be used.
Levofloxacin (IV or PO), gatifloxacin (IV or PO), moxifloxacin (IV or PO), or gemifloxacin (PO only) are preferred for Streptococcus pneumoniae. Ciprofloxacin has the best in vitro activity against Pseudomonas aeruginosa.
Rifampin and gentamicin should be reserved for cases of bacteremic Staphylococcus aureus pneumonia, empyema formation, or lung abscesses. Activity of rifampin and gentamicin requires laboratory confirmation for methicillin-resistant S. aureus.
Ticarcillin-clavulanate and piperacillin-tazobactam are the preferred β-lactam/β-lactamase inhibitors for the treatment of nosocomial pneumonia due to Enterobacteriaceae. Ampicillin-sulbactam lacks adequate activity against many nosocomial enteric gram-negative bacilli.
Antipseudomonal β-lactams ceftazidime, cefepime, imipenem, meropenem, mezlocillin, piperacillin, or piperacillin-tazobactam.
Modified from Bartlett JG, Dowell SF, Mandell LA, et al: Practice guidelines for the management of community-acquired pneumonia in adults: Infectious Diseases Society of America. Clin Infect Dis 31:347−382, 2000.
Common Causes of Pyogenic Pneumonia
Individual pneumonia pathogens may have unique epidemiology, diagnostic tests, and/or treatment. The sections that follow emphasize these unique aspects for selected pathogens (or groups).
Streptococcus pneumoniae (Pneumococcal Pneumonia)
Epidemiology
S. pneumoniae is the most frequent cause of CAP among patients who require hospitalization.21, 22 The overall incidence of pneumococcal pneumonia is approximately 200 cases per 100,000 persons per year, with 9 to 14 cases per 100,000 cases of bacteremia. This infection accounts for 40,000 deaths annually in the United States with most deaths in the very young and the elderly. Risk factors, particularly in adults, include cigarette smoking, HIV infection (even with preserved CD4 counts), heavy alcohol use, chronic liver disease, genetic defects in host immunity, and malnutrition.124, 125 Pneumococcal infections present predominantly in the winter and early spring and are often associated with prior infection by influenza or respiratory syncytial virus.126
Use of the conjugate pneumococcal vaccine has markedly decreased invasive pneumococcal infections in children, with a secondary reduction in adults.127, 127a This latter effect probably represents interruption of transmission by aerosolized droplets and direct physical contact, in that the conjugate vaccine is effective in blocking colonization.128 However, widespread use of the conjugate vaccine in the United States has resulted in an increase in the number and proportion of cases of invasive pneumococcal disease due to isolates with polysaccharide capsule types that are not included in the seven-valent vaccine.129 Consequently, a conjugate vaccine containing 13 capsular polysaccharide antigens was developed; this was approved by the U.S. Food and Drug Administration in 2012.
Clinical Manifestations
The classic presentation of pneumococcal pneumonia consists of a single rigor followed by sustained fever, cough, dyspnea, and production of rusty or mucoid sputum; gross hemoptysis is unusual. Severe pleuritic chest pain is common. The radiographic appearance of pneumococcal pneumonia is often either lobar consolidation (see Fig. 33-1 and eFig. 33-1) or patchy bronchopneumonia (eFig. 33-6). Although pneumococci can cause necrotizing pneumonia, cavitation rarely develops.125 Small, parapneumonic effusions are frequently found and can progress to frank empyema. Neutropenia may develop in patients with overwhelming infection.
eFigure 33-6.
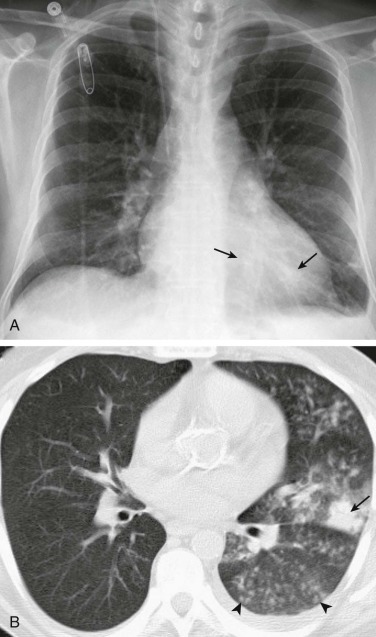
Pneumococcal bronchopneumonia.
A, Frontal chest radiograph shows patchy bronchovascular thickening (arrows) in the left lower lobe; trace blunting of the left costophrenic angle is present. B, Axial chest CT 2 days following A shows nodular lingular consolidation (arrow) and numerous small centrilobular nodules (arrowheads) consistent with bronchopneumonia. This bronchopneumonia pattern contrasts with the lobar pneumonia pattern (see Fig. 33-1 and eFig. 33-1). Both imaging patterns may be seen with pneumococcal pneumonia.
(Courtesy Michael Gotway, MD.)
Microbiologic Diagnosis
Although Gram stain of purulent sputum that reveals numerous, characteristic “lancet-shaped” diplococci with blunted ends (commonly seen in pairs and short chains) in the absence of other predominant flora is strongly suggestive of pneumococcal pneumonia (Fig. 33-2 ), a good quality sputum specimen cannot always be obtained.130 The organism is recovered from sputum culture in fewer than half of cases, and even a single dose of antibiotics can affect the yield of sputum cultures, which contributes to the discrepancy between sputum Gram stain and culture results. The frequency of positive blood cultures has fallen from 30% of hospitalized patients20 to less than 10% in many contemporary series. This decrease may reflect a greater percentage of blood cultures drawn after antibiotics because of the emphasis on timely antibiotic doses in the emergency department, deemphasis on blood cultures in CAP in general, and/or a benefit of vaccination on invasive pneumococcal disease.
Figure 33-2.
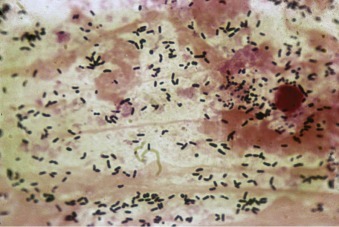
Gram stain of sputum from a patient with pneumococcal pneumonia.
The predominant organisms are gram-positive lancet-shaped diplococci.
The rapid urinary antigen S. pneumoniae test offers an alternative approach to the diagnosis of pneumococcal CAP and is becoming more widely used in diagnosis and in narrowing antibiotic therapy.74, 75, 77, 131 Despite satisfactory sensitivity and specificity, the urinary antigen test is complementary to culture methods, since it cannot provide information on antimicrobial susceptibility of the infecting organism.
Clinical Course
With an appropriate antibiotic, a clinical response is usually expected within 24 to 48 hours. The onset of suppurative complications, such as purulent pericarditis, meningitis, endocarditis, arthritis, and cellulitis after initiation of therapy is uncommon in the modern era. The exception is empyema, which appears to be increased due to serotype replacement in the vaccinated populations by serotypes more often associated with empyema.132 Pneumococcal pneumonia remains a cause of septic shock and ARDS.133
Treatment
Antimicrobial resistance complicates treatment for S. pneumoniae in much of the world, including in the United States.134 For nonmeningeal isolates of S. pneumoniae, the redefinition of full susceptibility as a minimum inhibitory concentration (MIC) of penicillin less than or equal to 2 µg/mL and high-level resistance as MIC greater than or equal to 8 µg/mL markedly changed the incidence of penicillin resistance.135 This redefinition was driven by discordance between the previous lower MIC breakpoints and clinical success rates. The rate of increase in the frequency of penicillin resistance may have stabilized, possibly as a consequence of the pneumococcal conjugate vaccine and a shift in the outpatient antibiotic prescription patterns away from β-lactams.136
Penicillin resistance in S. pneumoniae is due to alterations in penicillin-binding proteins rather than to β-lactamase production. Unlike other β-lactams, cefotaxime, ceftriaxone, and cefepime retain activity against 75% to 95% of nonmeningeal isolates of S. pneumoniae. 137 S. pneumoniae resistance rates to other antimicrobials can be as high as 30% for trimethoprim-sulfamethoxazole (TMP-SMX), 16% for tetracyclines, 26% for macrolides, and 9% for clindamycin; these rates are higher among penicillin-resistant pneumococci.134, 136 High-level macrolide resistance (MIC > 64 µg/mL) associated with the MLSB (macrolide, lincosamide, streptogramin B) phenotype is more common in Europe138 and has been associated with in vitro resistance to clindamycin.139 S. pneumoniae resistance to fluoroquinolones has also emerged with associated clinical treatment failures.113, 136
Recent exposure to an antibiotic increases the likelihood of the patient having a pneumococcal isolate resistant to that antibiotic (or class of antibiotics). Thus, it is important to avoid antibiotics that have been used in the prior 90 days when selecting a regimen for empirical treatment of a pneumococcal infection.113 Retrospective and prospective observational studies have suggested a benefit to treating severely ill patients with proven pneumococcal infections with both a β-lactam and a macrolide.76, 140, 141 Explanations that have been proposed to explain those results include nonbactericidal effects, such as inhibiting biofilm production, or an anti-inflammatory effect of the macrolide.
Other Streptococci
Epidemiology
S. pyogenes (group A β-hemolytic streptococcus) can be found in the oropharynx of more than 20% of children and a smaller percentage of adults. Carriage rates increase greatly during epidemics and in crowded conditions.128, 142 In the United States, the incidence of pneumonia due to S. pyogenes was 0.15 to 0.35 per 100,000 persons per year, but may be as high as 3.6 per 100,000 in children.143, 144 The organism is easily transferred between contacts, leading to epidemics of group A streptococcal pneumonia in military recruits, nursing homes, and other crowded settings.142 Pneumonia due to S. pyogenes most often manifests during the late winter and spring months, may follow an episode of influenza, measles, or varicella, and has been associated with increased age, alcohol abuse, diabetes mellitus, cancer, and HIV infection.143, 144 S. pyogenes can cause necrotizing pneumonia145 and is associated with pleural empyema.146
Group B (i.e., Streptococcus agalactiae) streptococci are a major cause of neonatal sepsis and pneumonia. In adults, pneumonia accounts for approximately 15% of adult infections by group B streptococci.147 Most adults with group B streptococcal pneumonia are debilitated and develop pneumonia as a consequence of aspiration.147 Diabetes, cirrhosis, stroke, decubitus ulcer, and neurogenic bladder are also risk factors.147
The Streptococcus milleri group C streptococci (which include S. intermedius, S. anginosus, and S. constellatus) have emerged as significant respiratory pathogens, predominantly causing empyema (eFig. 33-7 and Video 33-1
![]() ) and lung abscesses as well as superinfection pneumonia in severe viral pneumonia.148, 149 Infections with bacteria in this group share many of the features of anaerobic infections, including increased risk with periodontal disease and alcoholism. Other viridans and microaerophilic streptococci (α-hemolytic, nonpneumococcal) are rarely the sole pathogens in patients with pneumonia; they are more commonly found mixed with other facultative and anaerobic organisms in aspiration pneumonia.
) and lung abscesses as well as superinfection pneumonia in severe viral pneumonia.148, 149 Infections with bacteria in this group share many of the features of anaerobic infections, including increased risk with periodontal disease and alcoholism. Other viridans and microaerophilic streptococci (α-hemolytic, nonpneumococcal) are rarely the sole pathogens in patients with pneumonia; they are more commonly found mixed with other facultative and anaerobic organisms in aspiration pneumonia.
eFigure 33-7.
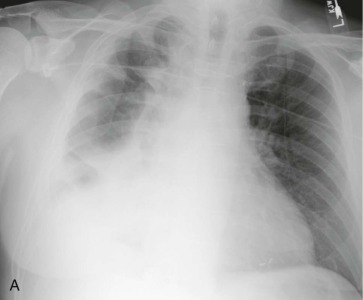
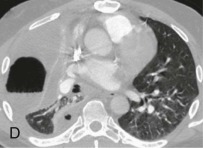
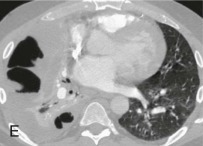
Streptococcus intermedius pneumonia and empyema.
A, Frontal chest radiograph shows right lung consolidation and volume loss. Some increased opacity adjacent to the chest wall suggests pleural disease but the findings are nonspecific. B–E, Axial chest CT displayed in an intermediate window to highlight the lung parenchyma and soft tissue features simultaneously, obtained shortly after A, shows a rounded gas collection containing fluid along the right chest wall consistent with empyema and bronchopleural fistula. S. intermedius was recovered following right thoracostomy tube placement.
(Courtesy Michael Gotway, MD.)
Clinical Manifestations
CAP from these pathogens is clinically indistinguishable from pneumococcal pneumonia. Exudative pharyngitis may be evident and unilobar involvement is common with group A streptococcal pneumonia. Pleural effusions in group A streptococcal pneumonia are frequent, may be large, accumulate rapidly, and appear early in the course of the disease, particularly in children.148 Pneumonia caused by other β-hemolytic streptococci is usually less abrupt and milder; pleural effusions are uncommon, and lung tissue necrosis is rare despite frequent bacteremia.147 S. milleri infection is predominantly associated with empyema, with or without concomitant pneumonia.148 Pneumothorax at the time of initial presentation appears to be more common with S. milleri than with other streptococcal empyemas.
Microbiologic Diagnosis
Because streptococci are common in the oropharynx, documentation of infection from these organisms requires isolation from a culture of blood, pleural fluid, or respiratory specimen obtained by means of an invasive procedure (Fig. 33-3 ). Pleural fluid cultures of children with S. pyogenes pneumonia are frequently positive. Polymerase chain reaction technology holds promise for aiding diagnosis, especially of group A streptococcal infections150 and S. milleri empyema.149
Clinical Course
Empyema and/or pericarditis are seen in 5% to 30% of patients with group A streptococcal pneumonia143; other complications include pneumothorax, mediastinitis, and bronchopleural fistula formation. The only classic nonsuppurative complication that follows S. pyogenes pneumonia is glomerulonephritis.
Treatment
Most of these streptococci are susceptible to penicillin G, ampicillin, and many cephalosporins, although α-hemolytic streptococci may require high dosage, due to the phenomenon of tolerance (growth inhibition without killing, at low and intermediate drug concentrations). Because resistance to clindamycin and erythromycin is found in up to 15% to 20% of isolates, susceptibility testing is advisable before monotherapy with a macrolide or clindamycin.151 As for empyemas caused by other pathogens, drainage of empyema fluid is an important component of therapy.
Haemophilus influenzae
Epidemiology
Invasive infection, especially pneumonia, due to H. influenzae is estimated to account for approximately 1.2 cases per 100,000 adults per year in the United States, and is one of the more common causes of pneumonia in adults requiring hospitalization. Chronic lung disease, malignancy, HIV infection, and alcoholism are among the most common predisposing conditions to Haemophilus pneumonia. Active smoking appears to particularly increase the risk of H. influenzae pneumonia.
As with S. pneumoniae, vaccination against H. influenzae type b significantly changed the epidemiology of childhood pneumonia.152 Vaccinated children are still susceptible to unencapsulated (or nontypeable) strains but the incidence of H. influenzae pneumonia has fallen dramatically. Nonbacteremic infection by unencapsulated or non–type b strains is the most common form of H. influenzae pneumonia in adults.152
Clinical Manifestations
Haemophilus pneumonia is clinically indistinguishable from other bacterial pneumonias (eFig. 33-8). On radiographs, Haemophilus pneumonia may be multilobar, patchy bronchopneumonia or have areas of frank consolidation. Spherical radiographic opacities (so-called round pneumonia) have been described, but cavitation is uncommon. Small parapneumonic effusions may occasionally progress to empyema. Bacteremia is more common in children than in adults.
eFigure 33-8.
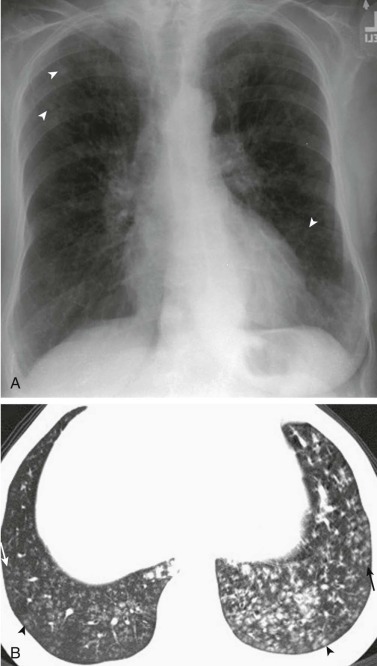
Haemophilus influenzae pneumonia: bronchiolitis.
A, Frontal chest radiograph shows several nonspecific small nodular opacities bilaterally (arrowheads). B, Axial chest CT through the lung bases shows numerous small centrilobular nodules (arrowheads), some with branching configurations (arrows), the latter consistent with “tree-in-bud” opacity, representing infectious bronchiolitis. The appearance of small centrilobular nodules with branching configurations is not specific for Haemophilus influenzae pneumonia and can be seen with other bacteria and occasionally with fungi or even viruses.
(Courtesy Michael Gotway, MD.)
Microbiologic Diagnosis
Diagnosing H. influenzae pneumonia by a Gram stain of sputum is difficult, because the small, pleomorphic coccobacilli are often overlooked. Culture of expectorated sputum reveals H. influenzae in only half of well-documented cases of pneumonia. Asymptomatic colonization with nontypeable strains in patients with COPD complicates analysis of Gram stain and sputum cultures (Fig. 33-4 ).
Figure 33-4.
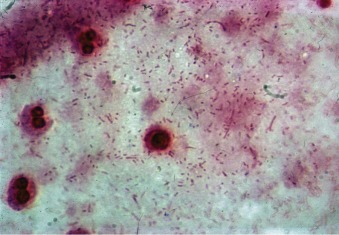
Haemophilus influenzae.
Gram stain shows small pleomorphic coccobacilli diffusely across the field that, because of their size, can be missed on sputum examination.
Clinical Course
The overall mortality rate of H. influenzae pneumonia is 5% to 7% but is higher in patients with bacteremia or extrapulmonary disease.152 Associated foci of infection, such as empyema, meningitis, arthritis, pericarditis, and epiglottitis are more common with encapsulated (type b) H. influenzae.
Treatment
H. influenzae isolates produce β-lactamase in 20% to 50% of cases and are therefore resistant to ampicillin. Increasing macrolide resistance also compromises empirical therapy with these agents. Consequently, serious H. influenzae respiratory tract infections should be treated with a second- or third-generation cephalosporin, β-lactam/β-lactamase inhibitor, or fluoroquinolone while awaiting results of susceptibility testing.
Mycoplasma pneumoniae
Epidemiology
M. pneumoniae accounts for up to 37% of CAP in persons treated as outpatients and 10% of pneumonias requiring hospitalization.20, 22 In the United States, there is an estimated 2 cases per year per 1000 individuals. Mycoplasma infections are seen throughout the year, but outbreaks are most common in the fall. Because Mycoplasma is readily transmitted from person to person via aerosolized respiratory droplets, outbreaks are common in families or closed populations.153
Clinical Manifestations
The clinical picture of M. pneumoniae pneumonia is the paradigm of atypical CAP,18, 21 as described previously. Pharyngitis, cervical adenopathy, and bullous myringitis may be encountered, although the latter is not more common with Mycoplasma pneumonia than with pneumococcal pneumonia/otitis. A wide variety of exanthems, including maculopapular eruptions, urticaria, erythema multiforme, and erythema nodosum, develop in 10% to 25% of patients. The chest radiograph usually shows an interstitial or a mixed pattern that may be more striking than expected based on chest physical findings (Fig. 33-5 and eFig. 33-9). Other chest radiographic patterns are occasionally encountered as well (eFig. 33-10).
Figure 33-5.
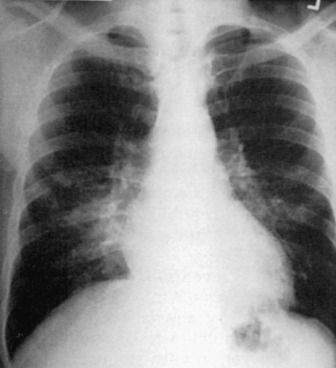
Radiographic findings in Mycoplasma pneumoniae pneumonia are nonspecific.
Bilateral bronchopneumonia is seen in this patient.
eFigure 33-9.
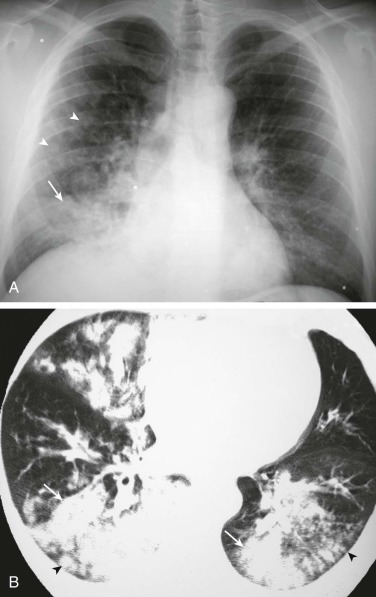
Mycoplasma pneumoniae pneumonia: multilobar pneumonia.
A, Frontal chest radiograph shows right lower lobe consolidation (arrow) associated with several small nodular opacities (arrowheads), the latter consistent with “acinar” or “air space” nodules. B, Axial chest CT through the lower lungs displayed in lung windows shows extensive bilateral consolidation (arrows) associated with small nodules (arrowheads), varying from 3 to 4 mm to 1 cm in size.
(Courtesy Michael Gotway, MD.)
eFigure 33-10.
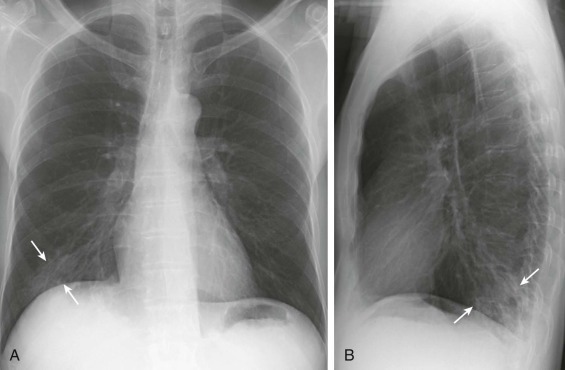
Mycoplasma pneumoniae pneumonia: unilateral bronchopneumonia.
Frontal (A) and lateral (B) chest radiographs in a patient with Mycoplasma pneumoniae pneumonia show patchy right lower lobe consolidation (arrows) consistent with bronchopneumonia, but nonspecific with regard to the specific microbiologic etiology.
(Courtesy Michael Gotway, MD.)
Microbiologic Diagnosis
When obtained, sputum generally displays moderate numbers of polymorphonuclear leukocytes without a predominant organism. Recovery of M. pneumoniae from culture of clinical specimens requires special media and takes approximately 10 days. Although acute Mycoplasma pneumonia may stimulate cold agglutinin production in a titer of 32 or greater, this nonspecific result is also found in various other infectious and noninfectious conditions including pneumonia due to Legionella, adenovirus, and influenza.18 The shortcomings of staining, culture, and serology for detection of Mycoplasma pneumoniae make this pathogen especially suitable for diagnosis by nucleic acid testing. Several nucleic acid tests for M. pneumoniae are currently available, and their role in management of CAP is being defined (see Chapter 17).
Clinical Course
Mycoplasma pneumonia is usually a benign, often self-limited infection with an excellent prognosis for complete recovery. ARDS and death have been reported but are rare. A unique aspect of M. pneumoniae infection is the frequency of associated autoimmune disorders including fulminant autoimmune hemolytic anemia, Stevens-Johnson syndrome, aseptic meningitis, meningoencephalitis, pericarditis, and myocarditis.154
Treatment
Antimicrobial therapy with a tetracycline, macrolide, or fluoroquinolone shortens the course of clinical symptoms and hastens resolution of radiographic abnormalities. To prevent clinical relapse, 2 weeks is the minimum recommended duration for treatment.18 Respiratory isolation can limit transmission, and azithromycin prophylaxis can prevent infection in close contacts of patients.155
Chlamydophila pneumoniae
Epidemiology
C. pneumoniae (formerly Chlamydia pneumoniae) accounts for 5% to 15% of cases of CAP.22 Seroepidemiologic studies suggest that C. pneumoniae eventually causes infection in 40% to 50% of the general population.
Clinical Manifestations
Primary infection by C. pneumoniae is usually asymptomatic: an acute, mild respiratory tract infection is observed in only 10% of infected adolescents and young adults.156 There may be bronchitis, sinusitis, laryngitis, tonsillitis, or exacerbations of asthma, with or without associated pneumonia. Sore throat with hoarseness is often severe and may precede pneumonia by up to a week and resolve before pneumonia onset, resulting in a biphasic illness. The erythrocyte sedimentation rate is elevated, but leukocytosis or elevated PCT may be absent.
Microbiologic Diagnosis
C. pneumoniae cannot be visualized by Gram stain, and tissue culture is required to grow the pathogen. Although direct fluorescent antibody detection of C. pneumoniae is available, nucleic acid testing is emerging as a rapid, sensitive mode of detection that yields results in a time frame useful for clinical management (see Chapter 17).
Clinical Course and Treatment
Complete recovery following C. pneumoniae infection is the rule; fatalities are principally seen in patients with mixed infection and preexisting illness.156, 157 When associated with an exacerbation of asthma, C. pneumoniae can require a prolonged time for recovery. Two-week treatment with a macrolide, tetracycline, doxycycline, or fluoroquinolone is recommended.18, 157 Older adults can be reinfected, often with severe symptoms.
Staphylococcus aureus
Epidemiology
S. aureus accounts for less than 10% of cases of CAP,21 but is the second or third most common etiology in patients with CAP requiring ICU admission. S. aureus, especially MRSA, accounts for up to 30% of nosocomial pneumonias.29 Nasal colonization is the major source for pneumonia and other invasive S. aureus infections: 30% to 50% of healthy adults carry the organism transiently in the anterior nares. Health care workers may have even higher carriage rates. Although the organism is easily transferred from person to person by direct hand contact, 67% of patients in whom MRSA pneumonia develops have nasal colonization on admission, indicating that most cases of S. aureus HAP are not due to S. aureus transmission in the hospital.158
A community-acquired strain of MRSA (CA-MRSA) has become an important CAP pathogen.159, 160, 161, 162 In addition to antibiotic resistance, the DNA cassette containing the mecA gene that confers methicillin resistance to this strain includes other virulence factors. The combination of antibiotic resistance and multiple virulence factors is associated with significantly higher mortality.163 Typical hospital-acquired strains of MRSA also cause CAP but usually in patients with risk factors for HCAP.164 The ability to differentiate clinically between hospital- and community-acquired cases is increasingly difficult because risk factors, such as prior antibiotic therapy, often overlap.
Factors that predispose patients to acquire staphylococcal pneumonia include underlying pulmonary disease (e.g., COPD, carcinoma, cystic fibrosis), chronic illness (e.g., diabetes mellitus, renal failure), or viral infection (e.g., influenza, measles).165 S. aureus, including CA-MRSA, is second in frequency to S. pneumoniae as a cause of postinfluenza bacterial pneumonia. Postinfluenza CAP due to CA-MRSA is associated with a high frequency of complications and mortality. Pneumonia due to hematogenous spread of S. aureus is a unique type of pneumonia, usually a consequence of intravenous drug use or septic embolization from endocarditis or an infected vascular site.
Clinical Manifestations
CA-MRSA pneumonia can be seen in young patients without underlying illnesses. The clinical presentation in severe cases includes high fever, hypotension, and hemoptysis with rapidly progressive deterioration and septic shock. Leukopenia, rather than leukocytosis, is observed in a substantial fraction of cases and is associated with poor outcomes.159, 161, 165 The radiographic features of CA-MRSA pneumonia include multilobar opacities and/or cavitary lesions (eFig. 33-11).
eFigure 33-11.
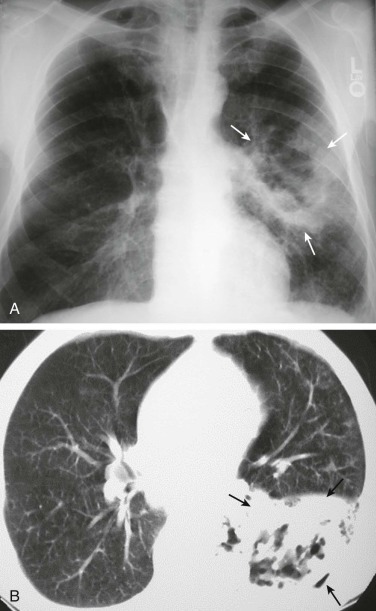
Methicillin-resistant Staphylococcus aureus pneumonia.
A, Frontal chest radiograph in a patient subsequently proven to have methicillin-resistant Staphylococcus aureus (MRSA) pneumonia shows left lung consolidation (arrows) with internal lucency consistent with necrosis and cavitation. B, Axial chest CT displayed in lung windows shows left lower lobe consolidation with internal lucency (arrows) consistent with a necrotic pneumonia. The patient recovered following antibiotic therapy.
(Courtesy Michael Gotway, MD.)
In cases acquired hematogenously such as in endocarditis or other endovascular infection, signs and symptoms related to the underlying endovascular infection predominate; if pulmonary infarction results from a septic embolism, pleuritic chest pain and hemoptysis are often noted. Otherwise, respiratory tract symptoms are mild or absent. The chest radiograph in patients with hematogenous staphylococcal pneumonia often reveals multiple, discrete, and often cavitary shadows with a predilection for the lower lobes (Fig. 33-6 ).166
Figure 33-6.
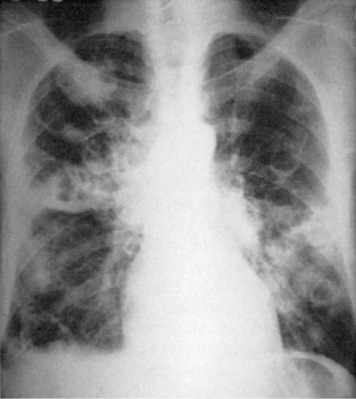
Chest radiograph shows hematogenous staphylococcal pneumonia associated with bacterial endocarditis.
The pneumonia is characterized by many cavities.
Microbiologic Diagnosis
Purulent sputum with multiple clusters of large gram-positive cocci, particularly if intracellular, is strongly suggestive of S. aureus pneumonia (Fig. 33-7 ). The organism is easily recovered from sputum cultures. Absence of MRSA on culture, even after several doses of antibiotics, is strong evidence that MRSA is not the causative pathogen. Fewer than 15% of pneumonias due to aspiration are associated with positive blood cultures. In contrast, hematogenous staphylococcal pneumonia usually yields multiple positive blood cultures. CA-MRSA pleural effusions are often exudative rather than grossly purulent but are still high yield on culture. An important clue to CA-MRSA is the presence of skin lesions,167 which are often positive on Gram stain as well.
Figure 33-7.
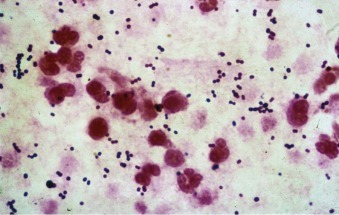
Staphylococcus.
Gram stain of sputum from a patient with staphylococcal pneumonia shows abundant large, round gram-positive cocci in clusters.
Clinical Course
Even with appropriate antibiotics, the duration of fever and need for ICU care is often prolonged for S. aureus pneumonia, particularly CA-MRSA. Local complications of staphylococcal pneumonia include empyema and abscess formation. Infection can spread hematogenously to the central nervous system, bones, joints, skin, and kidneys. Cavities and necrotic tissue may prevent adequate local antibiotic penetration, whereas unrecognized or incompletely drained empyema may prolong fever. Most of these manifestations are due to strains secreting Panton-Valentine leukocidin or one of the other exotoxins produced by S. aureus. Pleuroscopy or decortication is required in a large percentage of cases with empyema.
The mortality of S. aureus CAP is generally higher than most etiologies, with the mortality in methicillin-sensitive strains about 30%. S. aureus CAP following influenza has a reported mortality of greater than 60%, even if not methicillin-resistant.16
Treatment
The treatment of choice for methicillin-susceptible S. aureus pneumonia is a penicillinase-resistant penicillin (e.g., oxacillin 8 to 12 g/day) or a first-generation cephalosporin. Therapy for 7 to 10 days is adequate in uncomplicated cases, but 4 to 6 weeks of treatment is recommended for patients with bacteremia or cavitation. In the penicillin-allergic patient, clindamycin or linezolid can be used.
Treatment of MRSA pneumonia is more challenging, and the incidence of CAP caused by both health care–associated MRSA and CA-MRSA strains is increasing. Although resistance to vancomycin is still rare, the MIC has been shifting upward and MICs greater than 1 µg/mL have been associated with clinical failure. In higher-risk patients, such as those with VAP or with underlying renal insufficiency, linezolid has been found to have better clinical response rates than vancomycin, although differences in patient survival have been variable.168, 169, 170 For CA-MRSA, vancomycin therapy alone has been associated with a significant failure rate.160, 163 Addition of clindamycin or use of linezolid has been associated with improved outcomes in small case series. Unlike in MRSA skin infections, clindamycin, fluoroquinolones, and TMP-SMX are unreliable in severe CA-MRSA cases. Daptomycin is ineffective for treatment of pneumonia, because it binds to and is inactivated by pulmonary surfactant.171
Gram-Negative Bacillary Pneumonia
The term gram-negative bacillary pneumonia refers to infections caused by members of two groups, the Enterobacteriaceae and Pseudomonadaceae and other aerobic gram-negative bacilli. Infections caused by Haemophilus, Legionella, and anaerobes are usually excluded from this categorization.
Enterobacteriaceae
Epidemiology.
While they are more common as causes of HAP, gram-negative bacilli may cause up to 5% to 10% of CAP.21, 24, 111 CAP due to gram-negative bacilli is often severe and frequently requires ICU care. Patients in an ICU, especially those undergoing mechanical ventilation, have the highest risk for development of gram-negative bacillary pneumonia.
The Enterobacteriaceae normally colonize the digestive tract, and pneumonia usually results from aspiration of oropharyngeal flora. Although uncommon in healthy, nonhospitalized individuals, oropharyngeal colonization by gram-negative bacilli is greatly increased by hospitalization and antimicrobial use; the risk for aspiration is increased by comorbidities, such as cerebrovascular accidents, seizures, or anesthesia.24 Occasionally, contaminated home respiratory therapy equipment directly introduces gram-negative rods into the respiratory tract. Finally, Enterobacteriaceae pneumonia may result from hematogenous seeding from infection at other anatomic sites.
Among the Enterobacteriaceae, Escherichia coli is the single most frequent cause of CAP.24 The classic cause of community-acquired gram-negative bacillary pneumonia, K. pneumoniae (Friedlander pneumonia), causes fewer than 10% of CAPs, but more than 20% of nosocomial pneumonias. Alcohol abuse is the most common underlying condition for community-acquired K. pneumoniae pneumonia. Other underlying conditions that predispose to Klebsiella infections are diabetes mellitus and COPD.
Clinical Manifestations.
In Klebsiella CAP, a syndrome of pleuritic chest pain, hemoptysis, and bloody sputum (occasionally with a “currant jelly” appearance) is classic but rarely seen. The clinical manifestations of Enterobacteriaceae pneumonias are not sufficiently unique to distinguish these infections from pneumonias due to other causes.24
Most laboratory abnormalities are nonspecific, but neutropenia is associated with a poor prognosis. Chest radiographs often demonstrate lower lobe bronchopneumonia (eFig. 33-12), which is often bilateral. A classic radiographic appearance of Klebsiella pneumonia is upper lobe consolidation (especially on the right) (Fig. 33-8 ) with a bulging or bowed fissure. This manifestation is now uncommon. Klebsiella can also cause lung abscess (eFig. 33-13) in patients with HCAP.
eFigure 33-12.
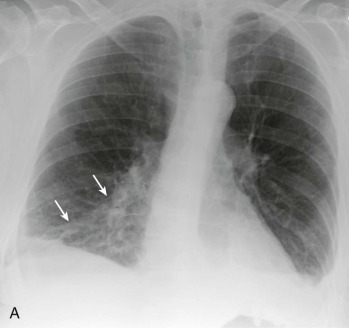
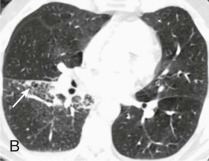
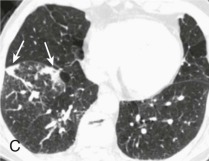
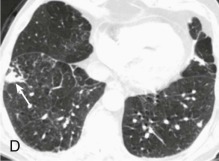
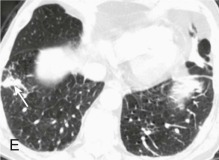
Klebsiella pneumoniae bronchopneumonia.
A, Frontal chest radiograph shows patchy right lower lobe opacity (arrows). B–E, Axial chest CT through the lower lobes displayed in lung windows shows patchy nodular opacities (arrows) consistent with bronchopneumonia but not specific for a microbial etiology.
(Courtesy Michael Gotway, MD.)
Figure 33-8.
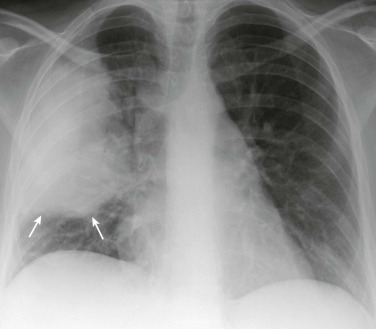
Klebsiella pneumoniae lobar pneumonia with “bulging” fissure.
Frontal chest radiograph shows dense right upper lobe air-space opacity, which bows the right minor fissure inferiorly (arrows). The bulging fissure sign at chest radiography is traditionally associated with Klebsiella pneumoniae infection, but is actually nonspecific, and can be seen with other pulmonary infectious and noninfectious pulmonary processes as well.
(Courtesy Michael Gotway, MD.)
eFigure 33-13.
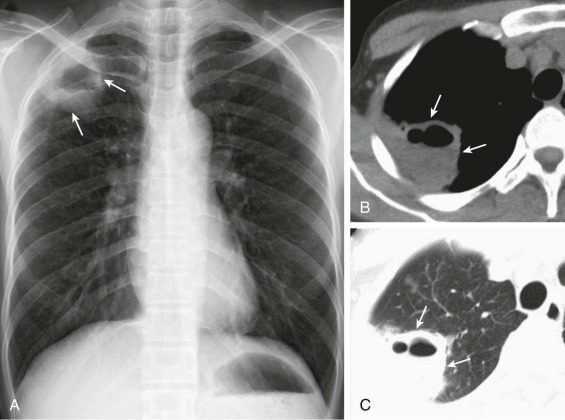
Klebsiella pneumoniae lung abscess.
A, Frontal chest radiograph shows a subpleural right apical cavity (arrows) with an air-fluid level consistent with a pulmonary abscess. Axial chest CT displayed in soft tissue (B) and lung (C) windows shows a nonspecific cavity (arrows) consistent with a pulmonary abscess but not specific for a specific microbiologic etiology.
(Courtesy Michael Gotway, MD.)
Microbiologic Diagnosis.
Enterobacteriaceae pneumonia should be suspected when sputum Gram stain reveals large numbers of uniform-appearing gram-negative rods (Fig. 33-9 ). Sputum culture alone is nonspecific for Enterobacteriaceae in either nonintubated or intubated patients because of oropharyngeal colonization and is one of the major reasons for interest in quantitative cultures as an approach to distinguishing colonization from infection.
Figure 33-9.
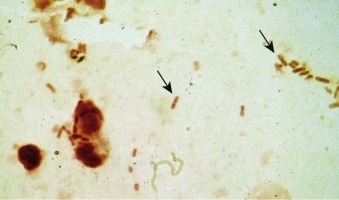
Klebsiella pneumoniae.
Large gram-negative rods in the sputum of a patient with Klebsiella pneumoniae pneumonia (arrows).
Clinical Course.
Enterobacteriaceae pneumonia fatality rates are 25% to 50%.24 Bacteremia, neutropenia, and advanced age contribute to a poor prognosis. Destruction of pulmonary alveolar septae may lead to cavitation.
Treatment.
Treatment of serious infections due to Enterobacteriaceae is complicated by widespread antimicrobial resistance. Extended-spectrum β-lactamase, carbapenemase, fluoroquinolone, and aminoglycoside resistance are all common in patients infected with Enterobacteraceae.172, 173 Due to regional and institutional variations in the frequency of resistance to specific drugs and classes of drugs, initial therapy of possible Enterobacteriaceae pneumonia must be selected using knowledge of local antibiotic resistance patterns. In patients with serious infection, a two-drug regimen of an aminoglycoside with a broad-spectrum β-lactam or carbapenem is recommended for treatment until susceptibility results are known. Monotherapy may be reasonable for immunocompetent patients with mild to moderate disease who are infected with susceptible strains of Proteus, Morganella, K. pneumoniae, or E. coli. Recommendations for empirical therapy for HAP are listed in Table 33-8, although local resistance patterns must be taken into account.
Pseudomonas aeruginosa and Related Organisms
Epidemiology.
P. aeruginosa is an uncommon cause of CAP except in specific risk groups. Although one large study in Spain found that 7% of CAP was due to P. aeruginosa, most studies have found substantially lower rates.24, 111 One major risk factor for Pseudomonas CAP is structural lung disease, such as cystic fibrosis, bronchiectasis, and severe COPD (forced expiratory volume in 1 second <30%). Another risk factor is HIV infection, especially with a marked deficiency of CD4+ T cells.174, 175, 176 Pseudomonas pneumonia in AIDS patients can be severe, with mortality rates as high as 50%, and be associated with cavitation, even when the patient has a profound CD4+ T-cell deficiency. The incidence of Pseudomonas pneumonia has decreased with widespread availability of combination antiretroviral therapy. Pseudomonas pneumonia is rare in normal hosts, but it can develop after exposure to aerosols of contaminated water such as in hot tubs.21, 24, 40, 68, 177, 178
P. aeruginosa is a leading cause of nosocomial pneumonia and a particularly frequent cause of VAP.179 Prolonged endotracheal intubation and prior antibiotic therapy, especially with broad-spectrum antibiotics, are major risk factors for Pseudomonas VAP. Other nonfermenters, such as S. maltophilia and B. cepacia, cause pneumonia in patients after prolonged broad-spectrum antibiotic therapy, and are associated with a high mortality rate. B. cepacia is also found in outpatients with cystic fibrosis.
Clinical Manifestations.
The clinical picture of pneumonia due to P. aeruginosa (eFig. 33-14) is indistinguishable from that of the Enterobacteriaceae. Bacteremia is slightly more common than for other gram-negative pathogens; physical examination may reveal ecthyma gangrenosum, and leukopenia is common. Its propensity to invade vascular tissue makes Pseudomonas the most common cause of cavitary pneumonia (eFigs. 33-15 and 33-16) in hospitalized or immunocompromised patients, and empyema can develop.
eFigure 33-14.
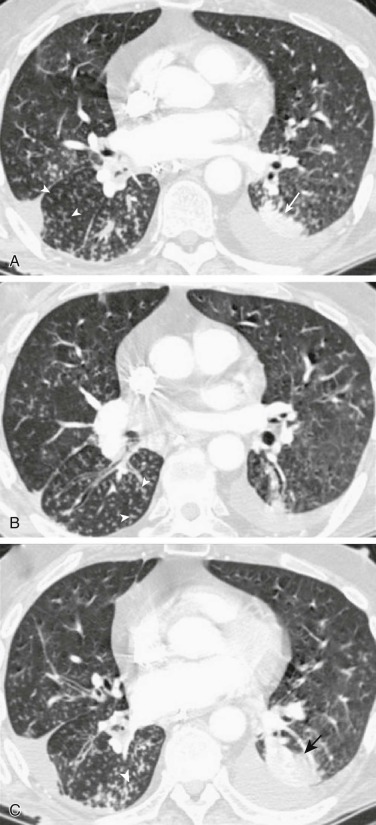
Pseudomonas aeruginosa bronchopneumonia.
A–C, Axial chest CT through the lower lobes displayed in lung windows shows nodular consolidation (A and C,arrows) and numerous small centrilobular nodules with branching morphologies consistent with “tree-in-bud” opacities (arrowheads).
(Courtesy Michael Gotway, MD.)
eFigure 33-15.
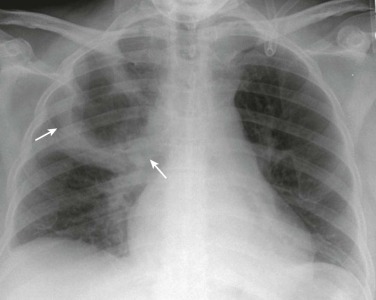
Pseudomonas aeruginosa cavitary pneumonia.
Frontal chest radiograph in a patient with P. aeruginosa pneumonia shows a large right upper lobe thick-walled cavity (arrows).
(Courtesy Michael Gotway, MD.)
eFigure 33-16.
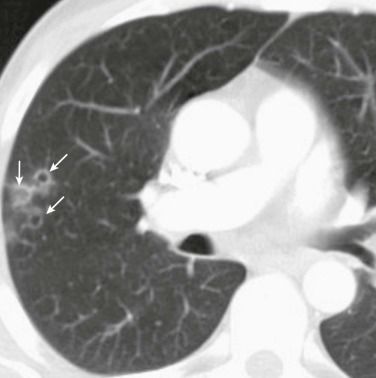
Pseudomonas aeruginosa cavitary pneumonia.
Axial chest CT displayed in lung windows of a patient with P. aeruginosa pneumonia shows several small, relatively thin-walled peripheral right upper lobe cavities (arrows).
(Courtesy Michael Gotway, MD.)
Microbiologic Diagnosis.
Gram-stained sputum from patients with Pseudomonas pneumonia typically shows many slender, gram-negative bacilli (Fig. 33-10 ); neutrophils are commonly abundant in the sputum except in neutropenic patients. Since Pseudomonas and other gram-negative bacilli colonize the oropharynx in hospitalized or debilitated patients, Gram stain results in these patients can be misleading. In endotracheally intubated patients, the absence of Pseudomonas on culture is strong evidence against Pseudomonas as the cause of the patient's pneumonia, because the organism is typically easy to recover.
Figure 33-10.
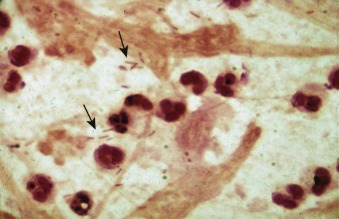
Pseudomonas.
Gram stain of sputum showing slender gram-negative bacilli (arrows).
Clinical Course.
Mortality from community-acquired P. aeruginosa pneumonia can exceed 25%,24 and in persons with VAP due to P. aeruginosa, mortality rates are 40% to 70%. The prognosis in neutropenic patients with P. aeruginosa pneumonia is particularly poor.180 P. aeruginosa VAP can recur in 25% to 50% of cases, approximately half due to a new strain.
Treatment.
P. aeruginosa pneumonia should initially be treated with two antimicrobial agents expected to be active against isolates in the region, such as an aminoglycoside and an antipseudomonal β-lactam antibiotic; this is especially true for bacteremic or neutropenic patients.180 Amikacin is the most reliably active aminoglycoside in most regions. The β-lactam antibiotics, in descending order of probable activity against P. aeruginosa, are the carbapenems (imipenem and meropenem), the acylureidopenicillins (e.g., piperacillin), cefepime, and ceftazidime.181 Although fluoroquinolones (particularly ciprofloxacin) initially possessed good intrinsic activity against P. aeruginosa, resistance is now common, making empirical fluoroquinolone monotherapy hazardous. Resistance can emerge in Pseudomonas during the course of fluoroquinolone monotherapy.
S. maltophilia is inherently resistant to most standard antibiotics.182 TMP-SMX is the most reliable agent, while fluoroquinolones or ticarcillin-clavulanate have activity against some strains.180 Isolates of S. maltophilia may become resistant in the face of seemingly effective therapy. B. cepacia may be susceptible to acylureidopenicillins, ceftazidime, TMP-SMX, fluoroquinolones, minocycline, and chloramphenicol. Resistance rates are higher in isolates from patients with cystic fibrosis.
Acinetobacter baumannii
Epidemiology.
A. baumannii may cause either CAP or HAP/VAP. Acinetobacter causes CAP in hot climates, both dry183 and humid,45, 184 and has become one of the most common causes of CAP in southeast Asia. In the United States, Acinetobacter CAP is most commonly seen in male alcoholics. The risk of Acinetobacter VAP varies widely by region and by health care facility. Nosocomial infections caused by Acinetobacter show seasonal variation, peaking in late summer, similar to patterns in CAP.
Clinical Manifestations.
Patients with Acinetobacter CAP often present acutely ill and may have leukopenia, pleural effusions, and empyema.185 Nosocomial Acinetobacter pneumonia has a less dramatic presentation, similar to those of other hospital-acquired gram-negative pneumonias.45
Microbiologic Diagnosis.
Examination of expectorated sputum, which is usually purulent, may reveal a predominance of paired gram-negative coccobacilli that resemble Neisseria, Haemophilus, and Moraxella species. Bacteremia complicates community-acquired more often than nosocomial Acinetobacter pneumonia.
Clinical Course.
The mortality rate of community-acquired Acinetobacter pneumonia approaches 50%.45 Patients at greatest risk of death are those with leukopenia or empyema. The fatality rate for nosocomial Acinetobacter pneumonia is determined by the severity of underlying disease.
Treatment.
Community isolates of A. baumannii can be susceptible to amikacin, tobramycin, ceftazidime, carbapenems, and doxycycline.186 Nosocomial Acinetobacter species are resistant to most β-lactams and aminoglycosides and therefore are most reliably treated with carbapenems, but poor outcomes are common.187 Some investigators have reported successful treatment of highly resistant isolates with ampicillin-sulbactam or colistin.188, 189 Resistance to β-lactam antimicrobials, carbapenems, aminoglycosides, fluoroquinolones, and even polymyxin B and colistin189 is increasingly common among nosocomial isolates.
Legionella
Epidemiology
L. pneumophila causes both epidemic and sporadic infections; both patterns may be seen either in the community or in hospitals. Outbreaks have been linked to contaminated potable water systems, ultrasonic mist devices, whirlpool baths, air-conditioning condensates, and water-evaporative systems.190
Legionella is acquired through inhalation of contaminated aerosols or aspiration. Sporadic cases of L. pneumophila pneumonia (50% to 80% of which are due to serogroup 1) account for 2% to 6% of CAPs in immunocompetent hosts.21 L. pneumophila is one of the most common causes of severe CAP in certain communities. Risk factors include exposure to contaminated water, immunosuppression, cigarette use, diabetes, cancer, end-stage renal disease, and alcohol use. Infection with L. pneumophila is more common in specific geographic regions, such as the Mediterranean or the northeastern United States.
In addition to L. pneumophila, 40 other Legionella species have been identified. Many, such as Legionella micdadei and Legionella longbeachae, produce a pneumonic illness indistinguishable from that of L. pneumophila. Much less is known about the epidemiology of non-pneumophila Legionella infections, but they also appear to be from water or soil-related sources. Immunosuppression appears to be the major host risk for these species.
Clinical Manifestations
The incubation period for Legionella pneumonia is 2 to 10 days. Lethargy, headache, fever, recurring rigors, anorexia, and myalgias are frequent early symptoms. After several days, cough becomes more pronounced; occasionally, watery or purulent sputum develops. Dyspnea is prominent in half of cases, and one third of patients complain of pleuritic chest pain. Extrapulmonary manifestations may overshadow respiratory complaints; gastrointestinal (watery diarrhea, nausea, vomiting, abdominal pain) and neurologic symptoms and signs (headache, confusion, obtundation, seizures, hallucinations) are particularly noteworthy. Patients may appear acutely ill. Temperatures reach 40.5° C in one third of patients, are typically sustained, and may be accompanied by relative bradycardia. Physical findings are usually limited to the chest, including pleural friction rubs, but generalized abdominal tenderness, hepatomegaly, splenomegaly, cutaneous rash, nuchal rigidity, and focal neurologic deficits have all been described.
Hyponatremia and hypophosphatemia are present in more than half of severe cases. Mild elevations of serum creatinine, creatine phosphokinase, and liver enzymes are also common, as are hematuria and proteinuria and occasionally frank rhabdomyolysis. There may be leukopenia and thrombocytopenia especially in severely ill patients. Cold agglutinins may be present, as in infection due to M. pneumoniae.
Chest radiographic findings typically lag behind the early clinical illness (Fig. 33-11 ). Small pleural effusions develop in 50% and may precede the parenchymal process. Multilobar opacities are commonly seen (eFig. 33-17), particularly on chest CT. Frank cavitation rarely is seen.66
Figure 33-11.
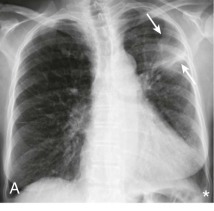
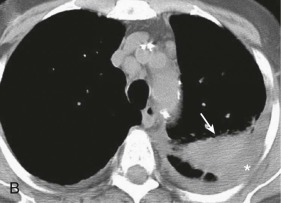
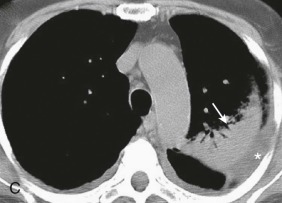
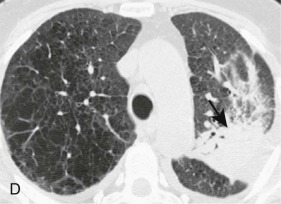
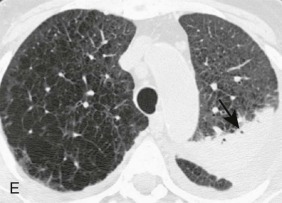
Legionella pneumophila pneumonia: unilateral disease.
A, Frontal chest radiograph shows subpleural left upper lobe consolidation (arrows). B–E, Axial chest CT displayed in soft tissue (B and C) and lung (D and E) windows confirms left upper lobe consolidation (arrows). A small left pleural effusion (*) is present.
(Courtesy Michael Gotway, MD.)
eFigure 33-17.
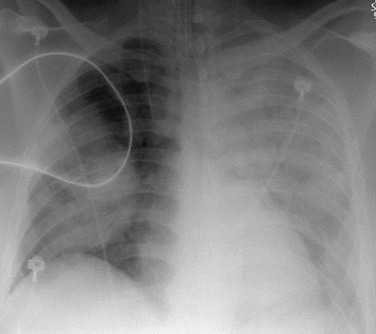
Legionella pneumophila pneumonia: multilobar pneumonia.
Frontal chest radiograph in a patient with Legionella pneumonia and respiratory failure shows left-greater-than-right multilobar consolidation.
(Courtesy Michael Gotway, MD.)
Microbiologic Diagnosis
Legionella are obligatory aerobic, fastidious, gram-negative bacilli that stain poorly with Gram stain (see Chapter 17, Figure 17-4) and grow poorly on conventional media. L. micdadei and some other Legionella species may stain weakly acid-fast. Early in the infection, sputum Gram stain from patients with Legionella pneumonia contains few or no polymorphonuclear leukocytes. The sensitivity of cultures of respiratory specimens is as low as 10%, despite use of appropriate media. Culture diagnosis of L. pneumophila infection often requires invasive procedures because at least 25% of patients with Legionella infection do not produce sputum.76 Rarely, Legionella has been recovered from blood, pleural fluid, and other extrapulmonary sites.
The Legionella urinary antigen test is currently the most commonly used method of diagnosis. It has a sensitivity of 60% to 80% and specificity greater than 95% for L. pneumophila serogroup 1, but the test is limited to this single species and serogroup.76 The sensitivity of direct fluorescent antibody assay for sputum ranges from 33% to 68%, with specificity greater than 95%,76 but its use is hindered by difficulty of obtaining sputum in some patients, the expertise required for interpretation, and the requirement for specific antibodies to the multiplicity of Legionella species and serogroups. Polymerase chain reaction–based assays for sputum have greater than 80% sensitivity and greater than 90% specificity, and their availability is likely to become more widespread.76
Clinical Course
A clinical response to appropriate antibiotic therapy is usually observed within the first 48 hours. In contrast, radiographic findings may temporarily continue to progress despite observed clinical improvement and ultimately take months to resolve.66 Acute renal failure and oliguria, often independent of shock and myoglobinuria, may develop in approximately 10% of patients and dialysis may be required. Many patients note lingering fatigue and weakness for months following Legionella pneumonia.
The mortality of community-acquired Legionella pneumonia is approximately 15%. Poor clinical outcomes are associated with immunodeficiencies, comorbidities, delayed initiation of appropriate therapy, and the need for ventilatory support or dialysis.
Treatment
Azithromycin and fluoroquinolones are superior to erythromycin or clarithromycin for treatment of Legionella infections. Although never used alone, the addition of rifampin is advised for patients who are severely ill or immunocompromised.18 Antibiotics should be continued for 10 to 21 days in immunocompetent patients to decrease the rate of relapse.18
Anaerobic Bacteria
Epidemiology
Mixed aerobic and anaerobic infection is usually a complication of macroaspiration of oropharyngeal contents. Rare causes include rupture of the esophagus and extension of intra-abdominal abscesses. Underlying pulmonary conditions such as malignancy and pulmonary infarction are present in 20% of patients who have an anaerobic lung infection. Although acute complications of macroaspiration are largely due to a chemical injury pneumonitis (Mendelson syndrome) and/or infection by pathogenic aerobes in the oral flora, many of these episodes later result in the emergence of mixed aerobic and anaerobic pneumonia.
Clinical Manifestations
Anaerobic infections present as four different syndromes: chemical pneumonitis, aspiration pneumonia, anaerobic pleuropneumonia, or primary anaerobic empyema.
Chemical pneumonitis can precede anaerobic pneumonia, and is characterized by the acute onset of hypoxemia, fever, cough (often dry), dyspnea, and pleuritic pain. The foul sputum and hemoptysis characteristic of anaerobic lung abscess are absent at this stage. The risk of infection is dependent on the nature of the inoculum; many cases of aspiration pneumonitis are inflammatory alone and not infectious. Imaging may demonstrate bronchopneumonic opacities, but usually not lobar consolidation, in the aspiration-prone segments of the lung (e.g., posterior segment of the right upper lobe and superior segment of the right lower lobe; see eFig. 33-3). ARDS is a common complication of aspiration of low pH gastric fluid.
Aspiration pneumonia is indistinguishable from either CAP or HAP (eFig. 33-18), with the exception that it is seen in patients with risk factors for macroaspiration. Rapid development of pulmonary opacities over a short period of time may suggest the diagnosis of aspiration pneumonia (Fig. 33-12 and eFig. 33-19). High concentrations of amylase or pepsinogen in BAL fluid are very suggestive of this entity.191 Localization of an opacity in a dependent lung segment has less discriminating value.
eFigure 33-18.
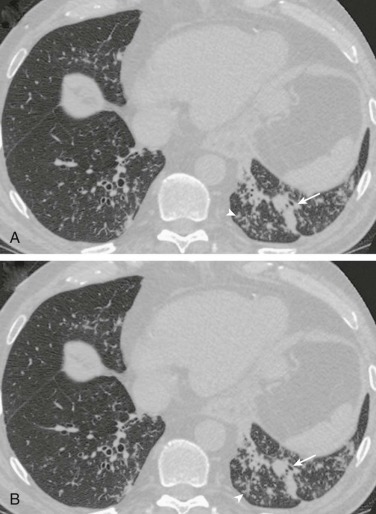
Aspiration pneumonia: bronchopneumonia/bronchiolitis appearance at chest CT.
A and B, Axial chest CT through the lower lobes displayed in lung windows in a patient with swallowing dysfunction shows numerous small centrilobular nodules, some with branching morphologies (arrowheads), and peribronchial consolidation (arrows). The imaging appearance is consistent with bronchopneumonia, but not specific for aspiration. Note resemblance of this CT appearance with P. aeruginosa pneumonia (see eFig. 33-14), Haemophilus influenzae pneumonia (see eFig. 33-8B), and pneumococcal bronchopneumonia (see eFig. 33-6).
(Courtesy Michael Gotway, MD.)
Figure 33-12.
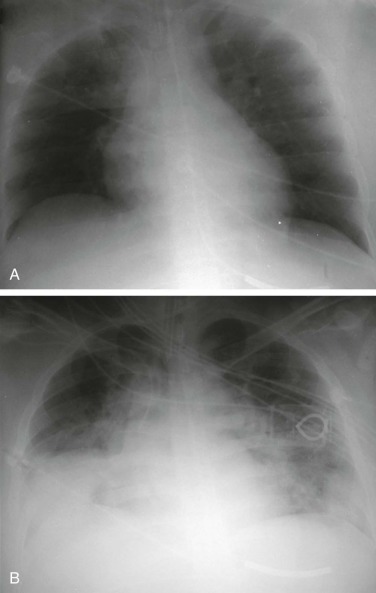
Aspiration pneumonitis: rapid interval development of pulmonary opacities.
A, Frontal chest radiograph performed for central venous catheter placement shows a right subclavian central venous catheter without pneumothorax; the lungs are clear. B, Repeat frontal chest radiograph obtained following an episode of altered level of consciousness and vomiting only a few hours after A shows interval development of extensive mid and lower lung consolidation. Such rapid development of extensive pulmonary opacities in the context of mental status changes accompanied by vomiting is characteristic of aspiration. The differential diagnosis of rapid interval appearance of extensive pulmonary opacities also includes increased pressure edema, noncardiac edema injury, and hemorrhage.
(Courtesy Michael Gotway, MD.)
eFigure 33-19.
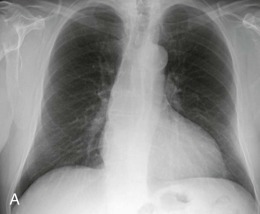
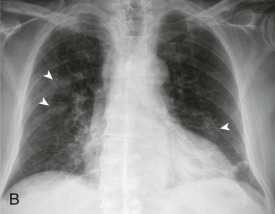
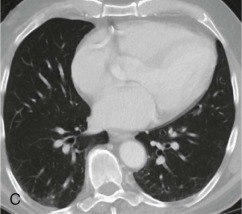
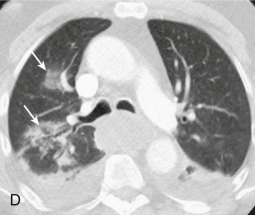
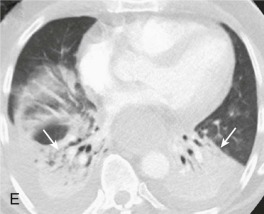
Aspiration bronchopneumonia: rapid appearance of new lung opacity at imaging and dependent lung involvement.
Frontal chest radiograph performed at admission (A) shows clear lungs; several days later, following a witnessed aspiration event, the radiograph (B) shows development of multifocal peribronchial nodular foci (arrowheads). C, Lower thoracic images from an abdominal CT scan several weeks before A and B shows only minimal basal atelectasis. D and E, Axial chest CT through the lower lobes performed immediately following B shows peribronchial consolidation (D,arrows) and extensive lower lobe, dependent consolidation (E,arrows). Note volume loss, evidenced by posterior displacement of the major fissures.
(Courtesy Michael Gotway, MD.)
Anaerobic pleuropneumonia is characterized by necrosis and suppuration of lung parenchyma. Early in the course, imaging may demonstrate dense segmental opacification with multiple small lucent areas of lung necrosis (<2 cm in diameter), usually without air-fluid levels (eFig. 33-20). In the absence of appropriate treatment, these lesions may evolve into a primary lung abscess (eFig. 33-21) and empyema. Patients commonly present with fatigue, low-grade fever, weight loss, and productive cough for several weeks after an episode of loss of consciousness. Approximately half describe putrid sputum, and some may have hemoptysis. Patients appear chronically ill and toxic, with temperatures up to 39° C. In some patients, a single lung abscess greater than 2 cm in diameter is detected in a dependent lung segment on radiography (Fig. 33-13 ). The abscess may be multilocular; occasionally, multiple abscesses are located in different lung segments.
eFigure 33-20.
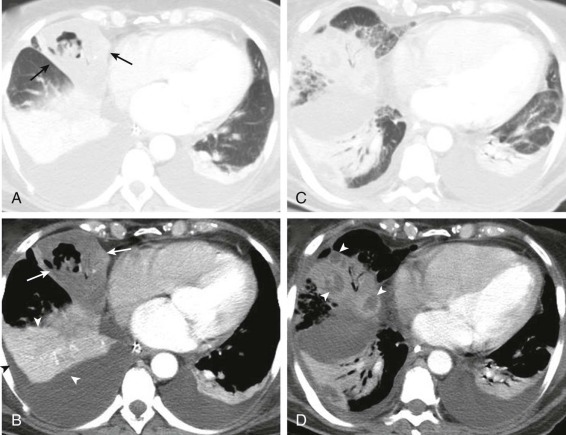
Anaerobic pneumonia.
A–D, Axial chest CT in a patient with polymicrobial anaerobic pneumonia shows multifocal consolidation in the right middle lobe (A and B,arrows) and bilateral lower lobes. The soft tissue window images show the right middle lobe consolidation to be hypovascular; compare attenuation characteristics of the right middle lobe opacity (arrows, B) with the appearance of the lower lobe consolidation (arrowheads,B). After several days, the poorly defined hypovascular right middle lobe consolidation (B,arrows,) evolved into discrete abscesses (D,arrowheads).
(Courtesy Michael Gotway, MD.)
eFigure 33-21.
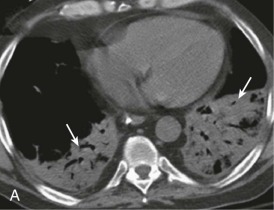
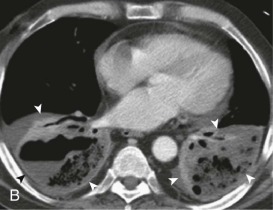
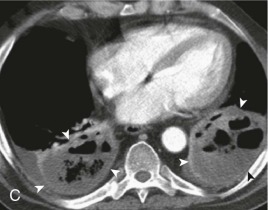
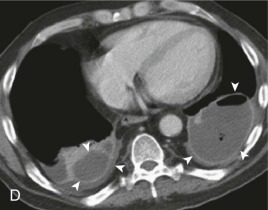
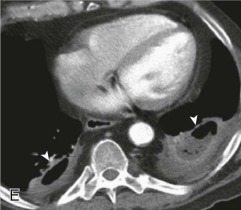
Evolution of pulmonary aspiration into lung abscess.
A, Axial chest CT displayed in soft tissue windows performed shortly following a witnessed aspiration event shows extensive bilateral lower lobe consolidation (arrows). B, Contrast-enhanced chest CT performed several days following A shows developing necrosis and cavitation (arrowheads) within the lower lobe consolidation. C and D, Repeat contrast-enhanced chest CT performed over the ensuing week following A and B shows maturation of frank bilateral lower lobe pulmonary abscesses (arrowheads); note well-defined, enhancing walls surrounding these gas and fluid collections. E, Axial enhanced chest CT following 3 weeks of antibiotic therapy shows partial resolution of the bilateral lower lobe pulmonary abscesses (arrowheads).
(Courtesy Michael Gotway, MD.)
Figure 33-13.
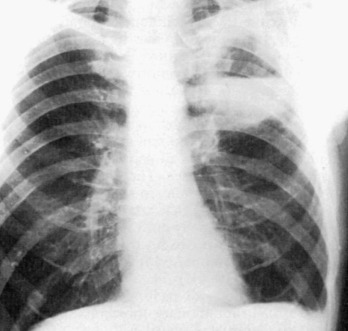
A single parenchymal cavity with an air-fluid level typifies an anaerobic lung abscess.
Most often, these lesions are located in the dependent, aspiration-prone lung segments (superior segment of the right lower lobe and left lower lobe and posterior segments of the upper lobes). This patient also has a small left empyema.
Primary anaerobic empyemas are usually due to S. milleri/intermedius (see eFig. 33-7) rather than anaerobes. 149 However, anaerobes still play a significant role. In these cases, the pleural manifestations may dominate, with less evidence of pneumonia. Anaerobic empyema can also be seen in the absence of parenchymal lung infection when empyema develops in association with esophageal rupture or from subphrenic or other intra-abdominal abscesses. More information on pleural empyema is in Chapter 80.
Microbiologic Diagnosis
Gram stain of sputum or examination of a bronchoscopically obtained specimen from a patient with anaerobic pneumonia reveals numerous polymorphonuclear leukocytes with an abundance of intracellular and extracellular bacteria. Typically, a mixture of Gram stain reactions and morphologies are seen, including pale-staining gram-negative rods with tapered ends (suggestive of Fusobacterium nucleatum), small, pale-staining gram-negative coccobacilli, and chains of tiny gram-positive cocci.
Because the endogenous flora of the upper respiratory tract predominantly consists of anaerobic bacteria, cultures of expectorated sputum are not appropriate for diagnosis of anaerobic infections. With careful technique, recovery on average of 3.2 bacterial isolates, of which 80% are anaerobes, is possible in a case of mixed aerobic/anaerobic pneumonia or empyema. The most common anaerobes in pleuropulmonary infections include F. nucleatum, Prevotella, Porphyromonas, Peptostreptococcus, and microaerophilic Streptococcus. The major aerobic and facultative organisms recovered in conjunction with anaerobes are Streptococcus species. Although S. aureus, various enteric gram-negative bacilli, and Pseudomonas may also be isolated, their significance is often questionable. Molecular techniques can often identify anaerobes in culture negative cases.149
Clinical Course
Uncomplicated aspiration pneumonia generally responds promptly to appropriate antibiotics. Fever resolves within a few days, and the chest radiograph normalizes within 3 weeks. Fever resolves more slowly in anaerobic pleuropulmonary infection. Closure of abscess cavities (see eFig. 33-21) and resorption of empyema collections may require months. Fatality rates are low in adequately treated patients, except those with necrotizing pneumonia, in which mortality approaches 20%.149 Chronic lung abscess has been complicated by brain abscess, other metastatic abscess, secondary amyloidosis, life-threatening hemoptysis, bronchopleural fistula or empyema necessitans (rupture through the chest wall), but these complications are currently rare.
Treatment
Emergence of β-lactamase–mediated resistance mandates that penicillin G and ampicillin are no longer the drugs of choice for treatment of patients with serious anaerobic pleuropulmonary infection. There is resistance not only among Bacteroides species but also among Prevotella and some F. nucleatum strains. Empirical treatment for serious anaerobic pleuropulmonary infection requires the use of a β-lactam/β-lactamase inhibitor (e.g., ampicillin-sulbactam, ticarcillin-clavulanate, or piperacillin-tazobactam) or clindamycin. Because of the frequent simultaneous presence of aerobes, metronidazole monotherapy is not adequate for suspected anaerobic pneumonia. Occasional pulmonary isolates are resistant to one or more of these agents. For example, Eikenella corrodens is resistant to clindamycin. Carbapenem monotherapy is also effective but generally provides unnecessarily broad coverage.
Ten days of total treatment is usually adequate for uncomplicated pneumonitis. Necrotizing pneumonia, abscess, and empyema require prolonged parenteral therapy to achieve clinical improvement, and extended courses of oral therapy, often requiring several months, may be required for cure.
Drainage of empyema fluid is required. Surgical resection of anaerobic lung abscess is almost never indicated. Bronchoscopy is useful for excluding an underlying malignancy in patients without other risk factors (i.e., edentulous patients).
Less Common Causes of Pneumonia
A variety of pathogens are less common causes of pneumonia. These agents may be suspected in the presence of unique risk factors or presentations, or may be diagnosed by results of cultures.
Actinomycosis
A variety of species within the two genera of the Actinomycetaceae family (Actinomyces and Propionibacterium), which are normally harmless commensals in the oropharynx, can cause subacute to chronic pulmonary infections that are virtually indistinguishable. Pulmonary actinomycosis follows aspiration of oropharyngeal material. Periodontitis and other dental disease increase the risk of cervicofacial invasion and of pneumonia. Most patients are 30 to 60 years old; men outnumber women by a ratio of 4:1.
Patients with actinomycosis appear chronically ill but not toxic. Constitutional symptoms, including fatigue, weight loss, and low-grade fever, may be present for weeks to months before diagnosis and often mimic the presentation of chronic fungal infection, tuberculosis, or malignancy. Fever may be absent. Most patients gradually develop productive cough and pleuritic chest pain, but hemoptysis and putrid sputum are unusual. Cervicofacial involvement is rarely observed in patients with thoracic involvement.
The imaging finding classic for actinomycosis is direct extension of a cavity or mass through an interlobar fissure. More commonly, changes are confined to a single lobe with one or more small cavitary lesions (eFig. 33-22). In advanced cases, the findings may be more distinctive, with penetration through the chest wall and/or destruction of adjacent bone tissue (eFig. 33-23).
eFigure 33-22.
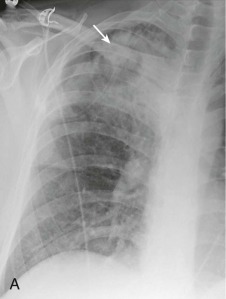
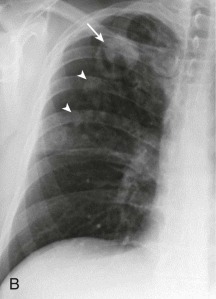
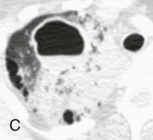
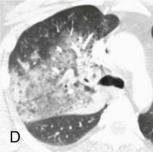
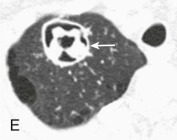
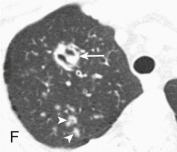
Actinomycosis: cavitary nodule.
A, Frontal chest radiograph shows right upper lobe consolidation and a poorly defined nodular opacity (arrow). B, Frontal chest radiograph 2 weeks following A shows resolution of the right upper lobe consolidation, now exposing a dominant cavitary right apical nodule with internal opacity (arrow), and small surrounding nodules (arrowheads). C and D, Axial chest CT displayed in lung windows performed within 1 day of the presenting chest radiograph (A) shows the right apical opacity as a cavitary nodule with an internal air-fluid level; surrounding ground-glass opacity and consolidation are present, as seen on the chest radiograph (A). E and F, Chest CT displayed in lung windows performed the same day as B shows the dominant right apical opacity with complex internal architecture (arrows) and confirms small surrounding nodules. Biopsy of this lesion recovered Actinomyces israelii.
(Courtesy Michael Gotway, MD.)
eFigure 33-23.
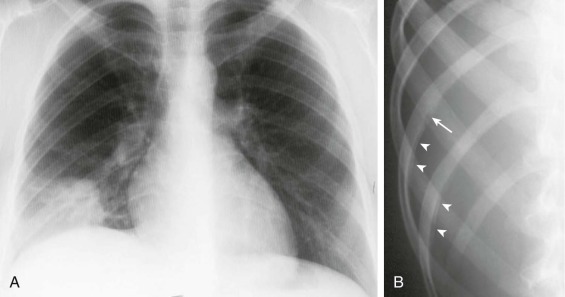
Actinomycosis: chest wall involvement.
A, Frontal chest radiograph shows a right lower lung mass. This finding is nonspecific. B, Rib detail radiograph shows subtle periosteal reaction (arrowheads) with erosion of the inferior rib cortex (arrow) consistent with chest wall invasion. Biopsy of the right lung mass recovered Actinomyces israelii.
(Courtesy Michael Gotway, MD.)
Members of the Actinomyces and Propionibacterium genera are gram-positive, diphtheroidal or filamentous, branching bacilli. Most strains grow best in anaerobic conditions, although some also grow aerobically. In patients with a cutaneous chest wall sinus, the best means of establishing a diagnosis of actinomycosis is by detection of “sulfur granules” that, when crushed and stained, form a characteristic pattern of gram-positive, branching filaments (eFig. 33-24). The organism usually can be recovered from culture of this material, provided anaerobic conditions are maintained for the specimen. Diagnosis of an actinomycotic parenchymal lesion is more difficult. Sulfur granules are rarely present in sputum, and recovery of the organism in sputum cultures is unreliable because the organism may colonize without invading any mucosal surface. Definitive diagnosis depends on demonstrating the characteristic histopathology and culture from a sterile body fluid or tissue biopsy. Other organisms are commonly identified, including Haemophilus (Actinobacillus) actinomycetemcomitans and Prevotella, in addition to the Actinomyces or Propionibacterium.
eFigure 33-24.
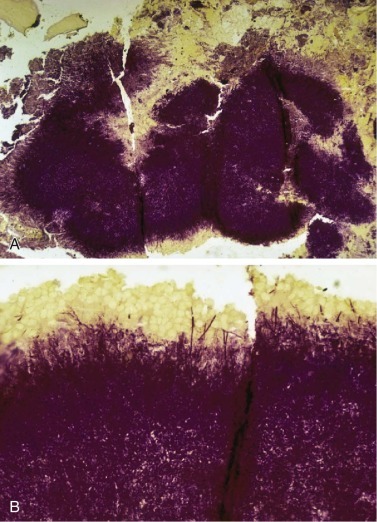
Gram stain of an actinomycotic sulfur granule.
A, Original magnification ×100; B, original magnification ×400.
Complications of pulmonary actinomycosis relate to its ability to invade across anatomic barriers. Pleural empyema, cutaneous thoracic sinuses, mediastinitis, pericarditis, and vertebral osteomyelitis are not infrequent. Metastatic infection, including brain, skin, and bone, is more commonly seen with pulmonary actinomycosis than with other variants (e.g., cervicofacial disease) but is still unusual. With adequate therapy, death from actinomycosis is rare.
Prolonged antibiotic treatment is the key to curing actinomycosis. Actinomyces are universally susceptible to penicillin G, which should be given in intravenous doses of 12 to 20 million units daily for 4 to 6 weeks, followed by at least 6 months of oral penicillin V or ampicillin. Multiple other drugs have also been successfully used; prolonged therapy is essential. With polymicrobial infection, the presence of the other organisms may require modification of therapy. If β-lactamase–producing anaerobes are present, treatment choices as described for anaerobic pneumonia are recommended. On occasion, clindamycin fails because of concomitant infection by H. actinomycetemcomitans. Rarely, surgical resection is required for cure.
Chlamydophila psittaci (Formerly Chlamydia psittaci)—Psittacosis
Exposure to birds is the classic risk factor for psittacosis. Multiple species harbor the organism, but most cases have been acquired from canaries, parakeets, cockatiels, parrots, and pigeons. Although infected birds are typically ill, there can be asymptomatic fecal carriage. C. psittaci can be detected in blood, tissues, feathers, and feces of infected birds. Human acquisition is typically via inhalation of contaminated bird excreta. Although approximately 50% of cases are reported in owners of infected pet birds, there may be sporadic cases and occasional outbreaks without a history of known bird exposure. History of exposure is more likely with more severe disease.192
The symptoms of psittacosis may develop abruptly, with high fever and chills, or they may evolve slowly. Headache, arthralgia, and painful myalgia (especially in the head and neck) are prominent features. A severe cough develops that may be either dry and hacking or productive of mucoid sputum. Chest pain and dyspnea are present with extensive pulmonary involvement. Temperatures are 38° C to 40° C and are frequently accompanied by relative bradycardia. Splenomegaly or occasionally a pale macular rash (Horder spots) can be seen. Hematologic and blood chemistry findings are nonspecific, except occasionally for findings consistent with granulomatous hepatitis.
C. psittaci is an obligate intracellular parasite that does not stain by the Gram method but can be seen as large intracytoplasmic inclusions in infected cells when stained with Giemsa stain. Cultivation of the organism, which requires tissue culture, poses a threat to laboratory personnel and should be performed only in specialized facilities. Nucleic acid testing offers a rapid alternative method for diagnosis, with higher sensitivity than cultures.193 In the absence of available nucleic acid testing, diagnosis of psittacosis can be made by demonstrating a fourfold rise in complement-fixing antibodies in paired acute and convalescent serum. A single titer of 16 or greater can be considered presumptive evidence of infection in a patient with a compatible illness. The antibody can cross-react with C. burnetii or Brucella.
Treatment with doxycycline is recommended, but the clinical response may be slow. Because of the risk of relapse, therapy should be given for a minimum of 2 weeks after fever has resolved. Chloramphenicol, erythromycin, azithromycin, and moxifloxacin also have in vitro activity, but clinical experience with use of these drugs to treat C. psittaci is limited. The case-fatality rate is about 1% with antimicrobial therapy. Unusual complications include respiratory failure, encephalitis, hepatitis, disseminated intravascular coagulation, renal failure, and endocarditis.
Coxiella burnetii—Q Fever
Worldwide, Q fever is a particular problem in farming communities in Europe, North America, and Australia. C. burnetii asymptomatically infects a wide variety of domestic and wild animals, as well as ticks. Transmission to humans is primarily via exposure to the urine, feces, placenta, or unpasteurized milk of an infected animal: cows, sheep, and goats are most common. Outbreaks have happened in tanneries, dairies, and wool-rendering plants, among laboratory personnel, and in household members exposed to an infected cat or dog during parturition.194, 195
Following an incubation period of 2 to 4 weeks, an atypical pneumonia syndrome develops in 10% to 20% of infected persons.194 High temperature (>40° C), relative bradycardia, conjunctivitis, hepatosplenomegaly, and chest crackles may be detected; a rash is typically absent.195
C. burnetii is a small, obligate intracellular bacterium that cannot be cultured on standard media or visualized with the Gram stain. Because of the high infectivity of the organism, cultures should be attempted only by experienced personnel in Biosafety Level-3 laboratories. At present, the diagnosis usually relies on a fourfold rise in antibody titer from acute to convalescent serum samples. Nucleic acid testing is likely to provide a more rapid diagnosis, which would be useful in guiding treatment decisions.
Although patients may be acutely ill on presentation, the disease is rarely fatal and generally runs its course in 1 to 2 weeks.194 Some patients, particularly older adults, have a very prolonged illness. The most concerning aspect of Q fever is the potential for chronic vascular complications including endocarditis, vascular graft infections, and infected aortic aneurysms. Acute Q fever pneumonia during pregnancy is often associated with fetal loss.
Tetracyclines, especially doxycycline, are first-line therapy for Q fever. Quinolones have excellent in vitro activity and may be advantageous in the treatment of meningoencephalitis.195
Nocardiosis
Nocardia asteroides is the etiologic agent in more than 80% of pulmonary or disseminated cases of nocardiosis, although several other species (e.g., Nocardia brasiliensis) have also been associated with human infection. The organisms are widespread in nature, primarily in soil. The respiratory tract, skin, and gastrointestinal tract are portals of infection. Dysfunction of cell-mediated immunity and, to a much lesser extent, immunoglobulin defects predispose to infection. Thus, the infection rate is increased in patients who have lymphoma or leukemia, Cushing disease, or AIDS, or who are receiving immunosuppressive medications. The most recent cases have been reported in lung, heart, or stem-cell transplant recipients. Persons with pulmonary alveolar proteinosis are also at increased risk. Nonetheless, approximately half of the patients in whom nocardiosis develops have no known underlying medical disorder.
Although nocardiosis and actinomycosis are clinically similar infections of the lower respiratory tract, nocardiosis can be distinguished by less proclivity for sinus tract formation and a greater tendency for hematogenous dissemination in both healthy and impaired hosts. Dissemination may involve almost every organ system, but the central nervous system and skin are most common.196 Many patients with pulmonary nocardiosis have low-grade fever, fatigue, weight loss, productive cough, and pleuritic chest pain for weeks before seeking medical attention. However, some immunosuppressed patients present with acute, fulminant pneumonia. Physical examination is nonspecific unless sites of dissemination are obvious. Neurologic signs of a mass lesion may be present. Cutaneous dissemination appears as multiple subcutaneous abscesses with or without sinus tracts. Imaging commonly demonstrates localized bronchopneumonia or lobar consolidation (eFig. 33-25), but there may also be solitary, multiple (eFig. 33-26), or miliary nodules, and abscesses (eFig. 33-27). Pleural effusion develops in up to one third of cases.
eFigure 33-25.
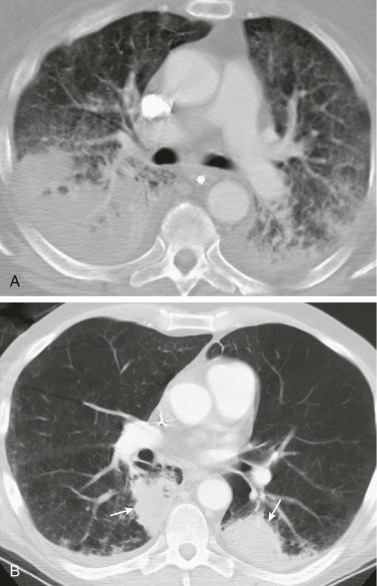
Nocardia asteroides pulmonary infection: consolidation.
A, Axial chest CT shows extensive bilateral lower lobe consolidation and more anteriorly located interstitial thickening and ground-glass opacity. The right lower lobe consolidation was cavitary at a more inferior level. B, Axial chest CT in a different patient shows bilateral lower lobe masslike opacities (arrows); biopsy of the left lower lobe lesion recovered Nocardia asteroides.
(Courtesy Michael Gotway, MD.)
eFigure 33-26.
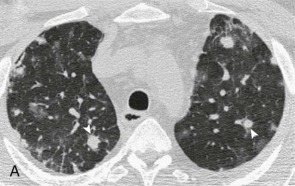
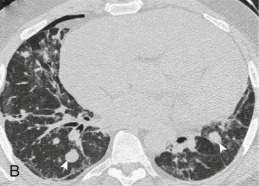
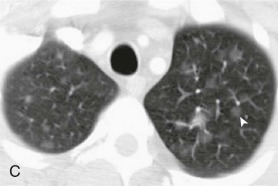
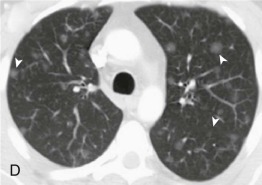
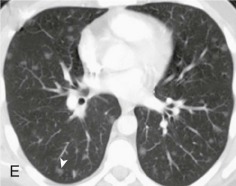
Nocardia asteroides pulmonary infection: multiple nodules.
A and B, Axial chest CT in an immunosuppressed renal transplant recipient shows multiple bilateral nodules, many of which are solid-appearing (arrowheads), due to N. asteroides.C–E, Axial chest CT in another immunosuppressed renal transplant recipient shows ground-glass opacity nodules (arrowheads), also shown to be due to N. asteroides.
(Courtesy Michael Gotway, MD.)
eFigure 33-27.
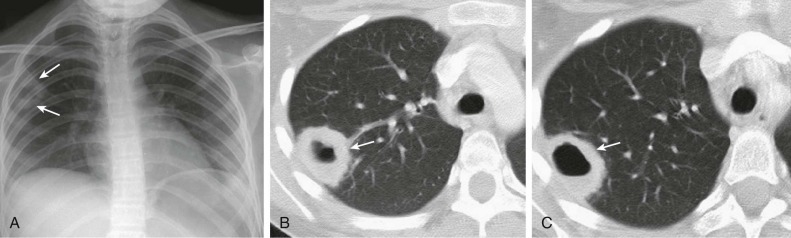
Nocardia asteroides pulmonary infection: abscess.
A, Frontal chest radiograph shows a poorly defined opacity (arrows) in the subpleural right upper lobe. B and C, Axial chest CT displayed in lung windows shows a subpleural right upper lobe cavity (arrows) containing an air-fluid level. This lesion is nonspecific in appearance and could be the result of a number of infections, but N. asteroides was recovered at biopsy.
(Courtesy Michael Gotway, MD.)
Nocardia species are gram-positive bacilli that appear as beaded, branching filaments. Unlike the anaerobic Actinomycetaceae, Nocardia requires aerobic growth conditions and is usually weakly acid-fast when stained by the modified Ziehl-Neelsen method (eFig. 33-28). Nocardia can be cultivated on conventional blood agar or Sabouraud medium but growth may not be apparent for 3 to 21 days. Although an occasional colonizer of the upper respiratory tract, recovery of Nocardia from a culture of sputum or invasively obtained material is highly suggestive of the diagnosis.
eFigure 33-28.
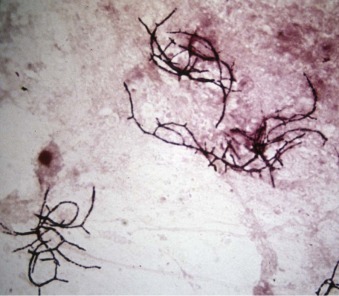
Modified acid-fast stain of sputum containing Nocardia asteroides shows filamentous branching organisms.
Mortality approaches 50% in those with central nervous system lesions but is less than 10% in those with only pulmonary disease.196 Because of in vitro synergy, TMP-SMX has become the standard treatment.196 In case of a sulfa allergy or a resistant organism, minocycline, amikacin, cefotaxime, imipenem, or linezolid may be useful, but choices should always be guided by the results of susceptibility testing.196 Prolonged therapy is needed to prevent relapse. Adequate drainage or excision of abscesses and empyema is a crucial adjunct to antimicrobial therapy.
Melioidosis (Burkholderia pseudomallei)
In endemic areas such as Southeast Asia and northern Australia, melioidosis may be the most common cause of severe CAP.197 B. pseudomallei is found in soil, vegetation, and water throughout tropical regions between latitudes 20 degrees N and 20 degrees S.48, 198 Acquisition of the organism is through cutaneous inoculation or inhalation in patients with regular contact with water and soil.48 Risk factors for disease include diabetes, alcoholism, and renal disease. Typhoons or episodic heavy rain may increase the risk of acute fulminant pneumonia; more than 75% of cases happen during the rainy season.198
Melioidosis can produce either acute fulminant pneumonia or indolent, cavitary disease. Clinical manifestations of acute melioidosis include high fever, prostration, dyspnea, pleuritic chest pain, purulent sputum, and hemoptysis. Concomitant bacteremia is common.197 The chest radiograph typically shows diffuse miliary nodules, which may expand and cavitate. Subacute or chronic B. pseudomallei pneumonia is milder and often manifests after a period of latency. Patients may be entirely asymptomatic (i.e., abnormal radiograph only), or present with an illness clinically and radiographically indistinguishable from tuberculosis.
B. pseudomallei is an aerobic gram-negative bacillus that grows readily on routine culture media. Despite improvements in recognition and treatment, melioidosis is still associated with high morbidity and mortality.197 B. pseudomallei is not sensitive to the usual CAP antibiotics but is usually susceptible to carbapenems, ceftazidime, and TMP-SMX. Optimal treatment for disseminated or life-threatening melioidosis requires initial intensive therapy with a carbapenem or ceftazidime followed by 3 months of TMP-SMX.48, 197, 199
Rhodococcus equi
Rhodococcus equi may cause lung abscess and pneumonia. Most cases are seen in the setting of impaired cell-mediated immunity (e.g., high doses of corticosteroids, HIV infection, solid organ transplantation)200 and in persons with a history of animal exposure. Illness develops subacutely, mimicking mycobacterial or fungal infection. Chest radiographs often show upper lobe nodules that gradually cavitate.
The organism is an intracellular gram-positive bacillus. It may stain weakly acid-fast but is much smaller than mycobacteria. The most effective regimens appear to be prolonged courses of vancomycin or erythromycin. Addition of rifampin may be useful.
Pulmonary Anthrax (Bacillus anthracis) (see Chapter 40)
Although B. anthracis is detected in many agricultural regions, anthrax is a rare infection in the developed world. Spores, the transmissible agent of infection, reside in soil, water, and vegetation and primarily infect large herbivorous animals (e.g., cows, sheep, and horses). Humans are infected by spores via contact with contaminated animals or animal products (e.g., animal hides and wools).33 B. anthracis is a proven agent of bioterrorism.201, 202, 203
The manifestations of anthrax are cutaneous, gastrointestinal, and inhalational (woolsorter's disease); inhalational anthrax is the most severe. Disease results from germination of B. anthracis spores in the lungs or draining lymph nodes, followed by growth of the vegetative forms of the bacteria and production of edema toxin and lethal toxin. Clinical illness begins insidiously with fever, malaise, nonproductive cough, and precordial pain. This stage is followed by rapid pulmonary deterioration with dyspnea, stridor, chest pain, tachypnea, cyanosis, nausea, vomiting, and drenching night sweats. Diffuse edema of the neck and anterior chest may be evident, due to the action of edema toxin. Meningitis is a common complication.
Radiographically, the lung parenchyma is initially clear. A widened mediastinum and bilateral pleural effusions are clues to inhalational anthrax. Mediastinal widening is seen in 100% of inhalational anthrax cases,204 although a chest CT scan may be required to define these characteristics.51 Later findings include vascular engorgement and lung parenchymal opacities.
B. anthracis is a large, facultative, gram-positive rod that forms spores. The organism grows readily on routine culture media and can be rapidly recovered from cultures of blood, sputum, and pleural fluid. In advanced disease, the organism may be demonstrated by Gram stain of peripheral blood.51 B. anthracis can be detected in nasal swabs of persons exposed to anthrax spores: the predictive value of this test for diagnosing clinical disease is ill-defined.51
Treatment recommendations for inhalational anthrax may be affected by resistance and the potential need to treat mass casualties. Current recommendations for inhalational anthrax call for initial treatment with ciprofloxacin plus one or two additional antibiotics with in vitro activity, such as clindamycin, vancomycin, imipenem, meropenem, chloramphenicol, penicillin, ampicillin, rifampin, and clarithromycin.205 If the isolate is susceptible, therapy can be changed from the fluoroquinolone to high-dose penicillin G or doxycycline. Intravenous therapy may be converted to oral therapy once the patient's condition stabilizes. Since pulmonary and systemic anthrax are associated with high mortality rates, therapy with an FDA-approved humanized monoclonal antibody (Raxibacumab) to anthrax lethal toxin is recommended as an adjunct to antimicrobial therapy.51a Treatment for 60 days is recommended, to eliminate spores that could be the source of relapse. Although the reported mortality rate has been as high as 90%, 6 of 11 patients with inhalational anthrax survived during the 2001 anthrax attacks in the United States.51
Tularemia (Francisella tularensis) (see Chapter 40)
Although F. tularensis has been recovered from numerous insects and species of wild or domestic mammals throughout the temperate zones of the Northern Hemisphere, fewer than 200 cases of tularemia per year are reported in the United States.52 Humans acquire infection following direct contact with tissues of an infected animal (as when skinning or eating an infected animal), through the bite of an infected tick or deerfly, or by inhalation of contaminated aerosols.47, 52, 206 Persons engaged in landscaping or agricultural activities that generate aerosols in endemic areas are at particular risk for development of pneumonic tularaemia.47 Because of the efficiency of aerosol transmission, F. tularensis is regarded as a potential agent of bioterrorism.52
Pneumonia develops from inhalation of contaminated aerosols or as a complication of bacteremia. Clinical manifestations typically begin abruptly with fever, chills, malaise, and headache. Shortly thereafter, dyspnea, cough, and chest pain may develop.52 Chest radiographs are usually normal at the onset (3 to 5 days following aerosol exposure) but ultimately show diffuse bronchopneumonia, often with hilar adenopathy.52 Pleural effusion is common and may be seen without parenchymal involvement.52
F. tularensis is a fastidious, pleomorphic, gram-negative bacillus rarely visualized on Gram stain of sputum and requires specially enriched media for optimal recovery by culture. Because of the hazardous nature of the organism, culture is best undertaken by reference laboratories. The organism can also be rapidly identified in tissues, secretions, and exudates by use of immunohistochemical techniques.52 Retrospective diagnosis can be accomplished by demonstrating a fourfold rise in agglutinating titers. A single titer of 160 or greater is compatible with either past or current infection.
Mortality can be as high as 60% if pneumonic tularemia is not suspected and treated appropriately. Although gentamicin has been used successfully, streptomycin remains the preferred therapy.52 Ciprofloxacin is an acceptable alternative agent; doxycycline and chloramphenicol are associated with higher relapse rates and require a longer duration of therapy.52 Ceftriaxone is unsatisfactory despite in vitro activity. After a known aerosol exposure to F. tularensis, prophylaxis with doxycycline or ciprofloxacin for 14 days is recommended.52
Plague (Yersinia pestis) (see Chapter 40)
Plague is typically associated with ground squirrels, rabbits, prairie dogs, rats, and other small ground animals. Rodent fleas are responsible for transmission of the organism between animal hosts. Humans become infected when bitten by an infected rodent flea, by handling an infected animal carcass, or by inhaling an aerosol from a human or animal with pulmonary involvement. In the United States, most cases of plague are in rural New Mexico, Arizona, and California.46 Because of the disease severity and potential for aerosol transmission, Y. pestis is also regarded as a potential bioterrorism weapon.207
Three clinical forms of infection exist: pneumonic, bubonic, and septicemic. Although the pneumonic form presents as a primary pneumonia, the lung may also be involved in bubonic and septicemic infections. Plague pneumonia may develop 2 to 7 days after the initial exposure. Early in the course of the illness, patients experience fever and toxicity, followed by chest pain, productive cough, dyspnea, and hemoptysis. The presence of hemoptysis can cause confusion with the hantavirus pulmonary syndrome, whose geographic distribution overlaps with plague in the United States. If pulmonary disease complicates bubonic plague, painful adenopathy is also noted. In the septicemic form, the patient may show only signs of septic shock. Chest radiographs in cases of pneumonic plague commonly reveal bilateral lower lobe alveolar opacities, but there may also be nodules, adenopathy, and pleural effusions.
Y. pestis is a short, nonmotile, gram-negative rod. Most patients with pneumonic plague have positive blood cultures. In addition, the organism can be recovered from sputum and lymph node aspirates by routine bacteriologic techniques. Fluorescent antibody staining of sputum and tissues facilitates the rapid diagnosis of plague but is available only in specialized laboratories.
Because of the potential for person-to-person transmission, all patients with plague pneumonia should be isolated. Recommended treatment consists of gentamicin or doxycycline.207, 208 Alternative treatments include fluoroquinolones, streptomycin, or chloramphenicol. For all regimens, the duration of treatment is a minimum of 10 days.
Moraxella catarrhalis
M. catarrhalis causes pneumonia, acute exacerbations of COPD, otitis media, and maxillary sinusitis.209 Pneumonia typically is seen in patients with underlying COPD, although alcoholism, malnutrition, increased age, congestive heart failure, and malignancy are also risks.209
Because M. catarrhalis is part of the normal upper respiratory tract flora, only adequately screened sputum samples provide useful diagnostic information. A purulent specimen that contains many intracellular gram-negative diplococci and yields heavy growth of M. catarrhalis is highly suggestive of true pneumonia. Blood cultures are rarely positive.209
The mortality of M. catarrhalis is approximately 10%, primarily due to exacerbations of severe underlying pulmonary disease.209 Effective agents include TMP-SMX, cephalosporin, macrolide, tetracycline, quinolone, or β-lactam/β-lactamase inhibitor combination. Virtually all isolates are resistant to penicillin and ampicillin because of β-lactamase production.
Neisseria meningitidis
N. meningitidis pneumonia is often a surprising culture diagnosis because clinical manifestations of meningococcal pneumonia resemble those of pneumococcal pneumonia. The estimated incidence of sporadic primary meningococcal pneumonia is 0.4 cases per 100,000 adults per year; pneumonia also complicates 5% to 15% of invasive meningococcal infections.210 Asymptomatic carriage rates vary according to the season and are increased under conditions of crowding.210 The organism is transmitted from person-to-person largely through droplet aerosols. Nosocomial clusters of meningococcal pneumonia are well described.
N. meningitidis is a gram-negative diplococcus; its appearance in sputum is similar to that of Moraxella and Acinetobacter. Rates of isolation of the organism from blood, cerebrospinal fluid, and pleural fluid from patients with meningococcal pneumonia are highly variable.
Aqueous penicillin G for 10 days, in daily doses of 4 to 6 million units, is adequate therapy for isolated pneumonia. Coexistence of septicemia or meningitis warrants increasing the dose to 18 to 24 million units per day. Isolates with decreased susceptibility to penicillin are not yet a significant problem in the United States, but have been reported in Europe and Africa.211
Because meningococci can be transmitted from patients with pneumonia to susceptible contacts, respiratory droplet isolation should be implemented during the initial days of treatment. Prophylaxis with ceftriaxone, ciprofloxacin, or rifampin is advised for household and other intimate contacts of the patient, including health care providers who have been exposed to respiratory secretions.
Pasteurella multocida
Pasteurella multocida is part of the normal oral flora of many domestic and wild mammals. Although skin and soft tissue infections following a cat or dog bite are the more common manifestations of human infection, sporadic cases of pneumonia, lung abscess, and empyema are seen in patients with chronic respiratory diseases, including COPD, carcinoma, and bronchiectasis. Most patients recall prior exposure to animals.
Pasteurella pneumonia is indistinguishable from other etiologies of CAP, although empyema may be more frequent. The organism is a small, gram-negative coccobacillus indistinguishable from other gram-negative rods by Gram stain. Identification of the organism by culture of sputum, blood, and pleural fluid is easily accomplished.
The treatment of choice for P. multocida pneumonia is penicillin G, 4 to 12 million units daily for 10 to 14 days. Tetracycline, amoxicillin-clavulanate, second- and third-generation cephalosporins, TMP-SMX, fluoroquinolones, and chloramphenicol are also active. P. multocida is resistant to clindamycin and macrolides.
Nonresponding Pneumonia/Treatment Failure
Two different clinical patterns of treatment failure in pneumonia have been described212: progressive pneumonia with clinical deterioration including respiratory failure or septic shock; and nonresponding pneumonia, in which clinical improvement is not achieved (fever and clinical symptoms persist). In those treated as outpatients or inpatients, evaluation for response should be undertaken after 72 hours of antibiotic treatment, as this represents the median time required to achieve clinical improvement.212 In addition to clinical evaluation, reduction of procalcitonin (PCT) levels after 3 to 4 days of treatment correlates with clinical responses.213 Levels of certain biomarkers, mainly PCT and C-reactive protein, have also been found useful for predicting inadequate response. Initial higher levels of PCT or C-reactive protein represent a risk factor for inadequate response (odds ratio, 2.6),213 whereas low levels are associated with responses to therapy. A recently described biomarker, MR-proadrenomedullin, has shown a greater association with severity assessment, and levels greater than 1.8 were associated with subsequent deterioration and ICU admission.214
The causes of nonresponding pneumonia are classified as infectious, noninfectious, and of unknown origin.212 eTable 33-5 lists common infectious and noninfectious causes.
eTable 33-5.
Causes of Nonresponding Pneumonia
| INFECTIOUS |
|
| NONINFECTIOUS |
|
Infectious Causes
In patients hospitalized for CAP, specific infections are responsible for 40% of nonresponding cases. The most frequent microorganisms found are S. pneumoniae, Legionella, P. aeruginosa, and S. aureus.
Patients with CAP, HAP, or VAP may fail to respond because of resistance to the empirical antibiotic regimen selected. P. aeruginosa, which is not covered by empirical therapy for CAP, causes about 10% of cases of nonresponding CAP.212 Up to 50% of episodes of nonresponding VAP are caused by multiresistant microorganisms; the most frequent causes are MRSA, P. aeruginosa, carbapenemase-producing Klebsiella, and Acinetobacter species.215
More unusual microorganisms in nonresponding CAP212 include Mycobacteria, Nocardia species, anaerobes, fungi, Pneumocystis jirovecii, and other organisms requiring antibiotics other than those recommended for CAP or HAP (eFig. 33-29). Investigation of the etiology of some of these microorganisms requires intensified microbiologic diagnostic testing as well as exhaustive review to search for risk factors, including epidemiology (travel, professional, leisure, or animal exposures), personal habits, and environmental factors.
eFigure 33-29.
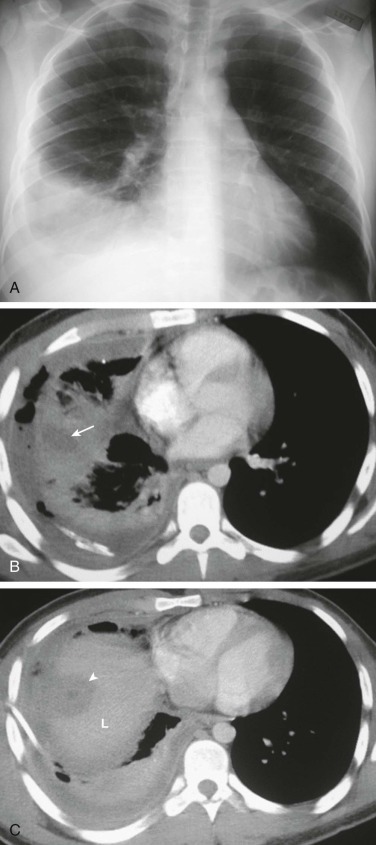
An infectious cause of “nonresponding” pneumonia: amebic pleuropulmonary infection.
A, Frontal chest radiograph in a 23-year-old man with fever and chest pain shows right lower lobe consolidation and a small-to-moderate right pleural effusion, presumed to represent pneumonia and parapneumonic effusion. B and C, Axial chest CT after the patient failed to respond to therapy for community-acquired pneumonia was performed to assess right pleural fluid drainage following thoracostomy tube placement (the thoracostomy tube is visible posteriorly in B). Chest CT performed through the right lower lung (B) shows consolidation with central low-attenuation (arrow), the latter consistent with a pulmonary abscess or area of necrosis. Chest CT through the extreme lung base and upper abdomen (C) shows a low attenuation focus (arrowhead) in the cranial liver (L), also consistent with an abscess. The liver and lung lesions are in close proximity to one another, suggesting that the liver lesion may have extended through the diaphragm to produce the lung findings; such behavior is typical of an amebic abscess. Further evaluation revealed that the patient recently immigrated to the United States from Mexico, and stool analysis recovered Entamoeba histolytica trophozoites.
(Courtesy Michael Gotway, MD.)
Local or metastatic infectious complications also contribute to treatment failure. Empyema (see eFig. 33-7) is one of the most frequent complications in pneumonia and is thus a cause of nonresponse that must be evaluated with thoracentesis when a pleural effusion is present. Other causes of treatment failure are abscess formation (see eFig. 33-4) and necrotizing pneumonia (see eFigs. 33-20 and 33-21). Metastatic infections such as endocarditis, arthritis, pericarditis, meningitis, or peritonitis can contribute to treatment failure and are more common in bacteremic pneumonia. In approximately 30% of the cases, no specific cause for lack of response can be identified despite adequate antibiotic treatment. This may be due to the presence of comorbidities or to an exaggerated or diminished inflammatory response.215a
Noninfectious Causes
Noninfectious diseases with acute involvement of the pulmonary parenchyma may simulate pneumonia. These include pulmonary infarction, pulmonary hemorrhage, organizing pneumonia, eosinophilic pneumonia, hypersensitivity pneumonitis, drug-induced lung disease, and neoplasms. Alveolar cell lung cancer (eFig. 33-30 and Video 33-2
![]() ) may be particularly difficult to distinguish radiographically from pyogenic pneumonia. The frequency of noninfectious etiologies has been reported to be 22% in CAP212 and 19% in nosocomial pneumonia.215
eTable 33-5 summarizes infectious and noninfectious causes of nonresponding pneumonia.
) may be particularly difficult to distinguish radiographically from pyogenic pneumonia. The frequency of noninfectious etiologies has been reported to be 22% in CAP212 and 19% in nosocomial pneumonia.215
eTable 33-5 summarizes infectious and noninfectious causes of nonresponding pneumonia.
eFigure 33-30.
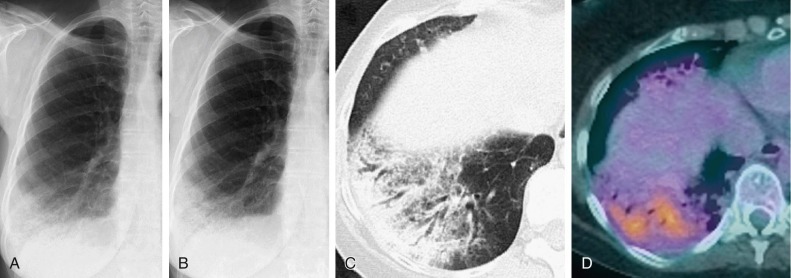
Noninfectious “nonresponding” pneumonia: invasive mucinous adenocarcinoma (formerly referred to as mucinous bronchioloalveolar carcinoma).
A, Frontal chest radiograph in a patient with persistent shortness of breath shows right lower lobe consolidation. The patient was treated with broad-spectrum antibiotics for presumed community-acquired pneumonia. B, Repeat frontal chest radiograph 3 months following A shows no change in the appearance of the right lower lobe opacity. C, Axial chest CT through the right lung base shown in lung windows reveals relatively nonspecific subpleural consolidation with air bronchograms and reticulation associated with ground-glass opacity. D, Fused FDG-PET image shows the right lower lobe opacity to be hypermetabolic. Bronchoscopic evaluation did not disclose a specific diagnosis for the persistent right lower lobe opacity. Resection of the right lower lobe proved invasive mucinous adenocarcinoma.
(Courtesy Michael Gotway, MD.)
Diagnostic Evaluation
The diagnostic approach to treatment failure requires a complete reevaluation of the history, physical examination, and laboratory studies including factors that may be related to delayed response.212, 216 Reconsideration of the initial diagnosis is also an important component of the diagnostic approach. Important epidemiologic clues may suggest unusual microorganisms along with unexpected resistance to antimicrobials, or underlying immunodeficiency such as HIV infection.
Microbiologic Studies
The microbiologic investigation of treatment failures requires comprehensive reexamination of initial microbiologic results, together with obtaining new samples for culture and other assays (eTable 33-6). Invasive techniques (i.e., bronchoscopy) for microbiologic samples and local evaluation of airways are recommended if they are not contraindicated. Both protected sheath brush and BAL sampling should be done during the same procedure for bacterial cultures, direct fluorescent antibody staining, and nucleic acid testing. Although culture results may be altered by the prior administration of antibiotics, the sensitivity of brush or BAL cultures approaches 40% in nonresponding CAP. In patients undergoing mechanical ventilation, the tracheal aspirate can provide diagnostic information. Gram stain of cytocentrifuged BAL fluid can rapidly identify intracellular microorganisms88 and may guide decisions regarding changes in antimicrobial therapy. Comprehensive microbiologic studies should also be performed on samples from nonrespiratory sites (eTable 33-7). When present, pleural fluid should be obtained for culture, direct fluorescent antibody, and nucleic acid testing for likely pathogens. The role of transbronchial biopsy is not established, and its indication depends on the possible alternative diagnosis suspected.
eTable 33-6.
Recommended Microbiologic Evaluation in Patients with Nonresolving Pneumonia
| BLOOD CULTURES (TWO SETS) |
|---|
| URINE |
| Antigen test for detection of Legionella pneumophila |
| SPUTUM |
|
| BRONCHOSCOPY SPECIMENS (USING PSB OR BAL)* |
|
| PLEURAL FLUID |
|
BAL, bronchoalveolar lavage; PSB, protected specimen brush.
Quantitative criteria for the interpretation of PSB and BAL specimens are described in the text.
eTable 33-7.
Possible Diseases Depending on Differential Cell Count in Bronchoalveolar Lavage Fluid
| PREDOMINANCE OF POLYMORPHONUCLEAR LEUKOCYTES |
|
| PREDOMINANCE OF LYMPHOCYTES |
|
| HEMOSIDERIN-LADEN MACROPHAGES |
| Alveolar hemorrhage |
| EOSINOPHILS |
|
Imaging Studies
Chest radiographs may demonstrate complications such as pleural effusion, cavitation, or new opacities. Chest CT scans provide a more detailed study of the parenchyma, interstitium, pleura, and mediastinum, potentially suggesting specific microorganisms (see eFig. 33-29) or alternative diagnoses. In a patient with applicable risk factors, the appearance of nodular images with the halo sign (i.e., a nodule surrounded by a halo of ground-glass attenuation, especially near the pleura) on CT scan is suggestive of pulmonary aspergillosis (see Chapter 91 and eFigs. 91-8A and 91-10) or mucormycosis (see Chapters 38, 91, and 95Chapter 38Chapter 91Chapter 95 and eFig. 91-9).217, 218 Nodules of similar appearance have also been described in cytomegalovirus infection (see Chapter 91 and eFig. 91-2), granulomatosis with polyangiitis (formerly Wegener granulomatosis), Kaposi sarcoma, and metastases with necrosis and/or hemorrhage. Ground-glass opacities consistent with interstitial pneumonia suggest P. jirovecii pneumonia. Nodules or multiple masses with or without cavitation are compatible with Nocardia species, M. tuberculosis, or Q fever. Diffuse or mixed interstitial and alveolar opacities may be due to viral infections or M. pneumoniae. Other imaging studies, such as chest CT pulmonary angiography should be considered to evaluate the possibility of pulmonary emboli.
Therapeutic Management
Correction of Host Abnormalities
Defects related to the host's immune system may impede recovery from pneumonia. Immunodeficiency may arise as a complication of cancer chemotherapy, immunosuppressive agents, or corticosteroid use; or it may result from a congenital (e.g., agammaglobulinemia) or acquired (e.g., HIV infection) immune defect. Many of these immune deficiencies are not remediable; however, drug-related immunosuppression may be improved by discontinuing the offending agent or reducing the dose. Although reduction of immunosuppression may promote recovery from the active infection, it can also be complicated by enhanced inflammation due to immune reconstitution.219, 220
Granulocytopenia (absolute granulocyte count less than 500 cells/mm3) has been associated with fulminant, antibiotic-unresponsive pneumonia, and administration of granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) is effective in increasing the number of circulating neutrophils. Despite this effect on neutrophils, routine administration of G-CSF or GM-CSF has not been found to improve survival from infections.221 Because pneumonia is the infection most frequently associated with a poor clinical outcome in profoundly neutropenic patients, the use of G-CSF or GM-CSF in these patients may be justified, even though a benefit has not been demonstrated.222 Corticosteroid treatment has been investigated because of its suppressant effect on inflammatory responses; studies have yielded discordant findings. A recent meta-analysis found evidence for a positive effect on survival in severe cases of CAP.223 In contrast, a recent randomized trial showed no benefit, although the number of patients with severe CAP may have been insufficient to reveal a difference in this select group.224
Antimicrobial Adjustment
The optimum therapeutic approach to nonresolving pneumonia requires close monitoring, transfer to a higher level of care, and optimization of the antibiotic regimen, including dosing.212 The optimal time to make these changes is not defined, although it has been suggested that one should wait until 72 hours after the initiation of treatment except in the presence of severe clinical deterioration or dramatic progression as determined by chest radiograph. Before initiating a change in antibiotics, new samples should be obtained for microbiologic studies.
In nonresponding CAP, strong consideration should be given to extending the antibacterial spectrum to ensure coverage of resistant S. pneumoniae, P. aeruginosa, S. aureus, and anaerobes. Such broad-spectrum therapy should be undertaken after all abscesses or empyemas have been drained, the results of all previous cultures are reviewed and, whenever possible, vigorous new efforts have been made to identify the responsible microorganisms. The specific antimicrobial regimen chosen depends on patient risk factors, disease severity, and the local epidemiology of antimicrobial resistance. In community-acquired MRSA, antimicrobial treatments may include linezolid or clindamycin plus vancomycin, depending on results of susceptibility testing.21, 163
In nonresponding nosocomial pneumonia, combinations of up to three antibiotics may be necessary to cover P. aeruginosa, MRSA, and the endemic flora of each hospital, such as Acinetobacter species or other microorganisms.215 The increasing spread of virulent carbapenemase-producing K. pneumoniae also necessitates vigilance for these organisms and consideration of combinations of polymyxin B or E, tigecycline, and/or ampicillin-sulbactam.225 Occasionally, empirical coverage against Aspergillus species should be considered (i.e., severe COPD, significant immunosuppressive therapy, corticosteroid treatments), especially if supported by clinical, radiologic,217 or laboratory data. The recommended approach is to cover empirically all likely causal microorganisms while awaiting the results of repeated respiratory samples and then adjust and deescalate antibiotics accordingly.
Lung Abscess
Lung abscesses are pus-containing necrotic lesions of the lung parenchyma that result from aspiration of bacteria-laden secretions and show an air-fluid level (see Fig. 33-13). Lung abscesses are distinct from, and may follow, necrotizing pneumonia, in which multiple small cavities develop in contiguous areas of the lung.226, 227 Lung abscesses must be distinguished from septic pulmonary emboli, which are often multiple and bilateral, involve the lower lobes (see Fig. 33-6), and are secondary to an endovascular infection.
Unlike most other respiratory infections that are caused by single pathogens, lung abscesses are caused by mixed populations of bacteria. The most common components of the mixed bacterial populations in lung abscesses are anaerobic bacteria (principally Peptostreptococcus species (now termed Finegoldia magna), F. nucleatum, and Prevotella melaninogenica (formerly Bacteroides melaninogenicus). Microaerophilic streptococci and viridans streptococci are also frequently isolated and can contribute to treatment failure if appropriate antibiotics are not included.228 Lung abscess may also be associated with pyogenic bacteria, mycobacteria, fungi, and parasites such as Paragonimus, Entamoeba, and Echinococcus (see Chapter 39). Secondary lung abscesses develop from congenital lung abnormalities, obstructing neoplasms, foreign bodies, and bronchiectasis. Lung abscess may also complicate pulmonary infarction, primary lung cancer (central carcinoma with necrosis), metastatic malignancies, and the necrotic conglomerate lesions of silicosis and coal miners' pneumoconiosis. Lesions in diseases such as granulomatosis with polyangiitis (formerly termed Wegener granulomatosis) and rheumatoid arthritis with rheumatoid nodules may also mimic lung abscess.
The clinical manifestations of lung abscesses are distinct from those of CAP, because they are usually prolonged in time (2 weeks to 3 months or more) and include fever, night sweats, cough with foul-smelling sputum, fatigue, weight loss, and sometimes hemoptysis.
The typical appearance of a lung abscess on a chest radiograph is a thick-walled cavity with an air-fluid level (see Fig. 33-13 and eFigure 33-4, eFigure 33-13, eFigure 33-15). A contrast-enhanced CT is occasionally necessary to differentiate lung abscess from other conditions, and bronchoscopy may be needed to distinguish lung abscess from endobronchial carcinoma.
Antibiotics with activity against anaerobic and aerobic bacteria and that are unaffected by the β-lactamases produced by anaerobes are the mainstay of treatment for lung abscesses.228 Clindamycin has been widely used and is superior to penicillin alone, undoubtedly because of the increasing prevalence of β-lactamase production by the anaerobes that cause lung abscesses. More recently, β-lactam/β-lactamase inhibitor combinations (amoxicillin-clavulanate or ampicillin-sulbactam) have been found to provide cure rates indistinguishable from those with clindamycin; moxifloxacin and carbapenems have also been used successfully.228 Metronidazole alone is not recommended, because it lacks sufficient activity against microaerophilic streptococci and viridans streptococci that are often part of the mixed microbial flora in lung abscesses. If metronidazole is used, penicillin should be added to cover streptococci. The optimal duration of antibiotic treatment has not been determined, although treatment for 6 to 8 weeks is commonly employed.
Failure to respond to antibiotics within 7 to 10 days warrants investigation for alternative diagnoses or complications. Antibiotic treatment may fail if the patient has immunodeficiency, if the cavity is large (>8 cm), or if the abscess is due to pyogenic bacteria such as P. aeruginosa or S. aureus. CT-guided percutaneous transthoracic tube drainage229 or endoscopic drainage230 are alternatives to surgical resection; the reported success rates with both of these procedures are high, although no prospective controlled trials have been reported. Complications of CT-guided tube drainage include pneumothorax, pyopneumothorax, and bronchopleural fistula. After drainage, patients show clinical improvement usually in 48 hours. Persistent fever can also be seen if there is a secondary pleural empyema that requires drainage.
Prevention of Pneumonia
Vaccines
Prevention of pneumonia may be achieved by administering the influenza and pneumococcal vaccines. Recommendations for administration of influenza231 and pneumococcal232 vaccines are presented in eTable 33-8 and eTable 33-9, respectively.
eTable 33-8.
Recommendations for Administration of Influenza Vaccine*
| Inactivated vaccine: All persons aged 6 months and older including pregnant women |
| Live attenuated vaccine†: Healthy, nonpregnant women aged 2 to 49 years without high-risk medical conditions |
Avoid giving vaccine to patients with anaphylactic allergy to eggs or to other influenza vaccine components. The optimal period for vaccination is October to November. However, it is acceptable to provide vaccine from September to early March.
Health care personnel who care for severe immunocompromised persons should receive inactivated rather than live vaccine.
Modified from Recommended Immunization schedule for adults aged 19 years and older. MMWR 62:Suppl, 2013.
eTable 33-9.
Recommendations for PPSV23 and PCV13 by Risk Group for Adults 19 Years and Older*
| Risk Group | PCV13 Recommended | PPSV23 Recommended | PPSV23 Revaccination 5 Years after First Dose |
|---|---|---|---|
| Immunocompetent |
|
||
| Asplenia |
|
|
|
| Immunocompromised |
|
|
|
All adults aged 65 years and older should receive PVC13 and PPSV23, with the sequence and interval depending on previous history of vaccination with pneumococcal vaccine.
Including congestive heart failure and cardiomyopathies.
COPD, emphysema, and asthma.
Including long-term systemic corticosteroids and radiation therapy.
Modified from Centers for Disease Control and Prevention: Tomczyk S, Bennett NM, Stoecker C, et al: Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 63(37):822–825, 2014.
Inactivated influenza vaccination is recommended annually for all persons aged 6 months and older, including pregnant women. For those averse to injections, a live attenuated influenza vaccine can be given by intranasal administration to healthy persons 5 to 49 years old. The live attenuated vaccine must be avoided in pregnancy, in high-risk persons with chronic underlying diseases or immunodeficiencies, and in health care staff taking care of immunosuppressed patients; it is not approved for use in those older than 49 years. While annual influenza vaccination is widely recommended, the immunogenicity and efficacy of the currently available vaccine is lower in individuals older than 65 years of age, and breakthrough infections are frequent.232
Two pneumococcal vaccines are currently available. The purified polysaccharide vaccine (PPSV23) contains capsular antigens isolated from 23 of the most prevalent capsule types and is immunogenic in adults, although antibody levels decrease to prevaccination levels after 4 to 7 years. The pneumococcal conjugate vaccine (PCV13) contains the polysaccharide antigens from 13 of the most prevalent capsule types, conjugated to a nontoxic mutant of diphtheria toxin protein, which generates T-cell help and long lived memory B cells specific for the pneumococcal antigens. PPSV23 should be administered to all individuals 65 years of age and older, as well as those 19 to 64 years of age with chronic conditions that increase the risk of invasive pneumococcal infection (e.g., diabetes mellitus, chronic lung, heart, or liver disease; cigarette smoking or alcoholism). Patients aged 19 years and older with immunodeficiencies or other conditions that impose an especially high risk of invasive pneumococcal infection (asplenia, HIV or other congenital or acquired immunodeficiencies, myeloma, lymphoma, leukemia, chronic renal failure) should receive an initial dose of PCV13 followed 8 weeks or longer later by PPSV23. eTable 33-9 lists the conditions and indications for which administration of each of the pneumococcal vaccines is currently recommended.233 In 2014, the updated ACIP recommendations are that both PCV13 and PPSV23 should be administered in series to all adults 65 years of age and older. A dose of PCV13 should be received first followed by a dose of PPSV23 6 to 12 months later. Individuals previously vaccinated with PPSV23 should be given a dose of PCV13 after approximately 1 year.234, 235
Smoking Cessation
Not only is smoking a risk factor for pneumococcal disease, quitting smoking reduces the risk.236 The IDSA/ATS recommend smoking cessation counseling as well as pneumococcal vaccination for smokers who are hospitalized with pneumonia.18
Key Points.
-
▪
All patients with suspected pneumonia should have a chest radiograph. Gram stains of sputum and cultures of blood, sputum, and other sites should be obtained in hospitalized patients before treatment. S. pneumoniae and L. pneumophila urinary antigens can make an etiologic diagnosis with reasonable sensitivity and specificity.
-
▪
Aspiration is the cause of infection with S. pneumoniae, H. influenzae, gram-negative bacilli, and other organisms, whereas aerosolization is the route of infection with intracellular bacteria such as M. pneumoniae, Chlamydophila species, and C. burnetii. Aside from inhalational pneumonia due to Legionella or contaminated medical aerosols, aspiration is the cause of hospital-acquired pneumonia, especially in intubated patients.
-
▪
Nucleic acid amplification tests should be used increasingly to diagnose viruses and fastidious bacteria, M. pneumoniae, C. pneumoniae, L. pneumophila, and B. pertussis because culture procedures are too insensitive and too slow to be relevant therapeutically.
-
▪
In older and immunocompromised patients, the signs and symptoms of pneumonia may be muted and overshadowed by nonspecific complaints. Temperature greater than 38.5° C or accompanied by chills should never be attributed to bronchitis without examining a chest radiograph. Older patients with pneumonia who present with altered mental status without fever often have a delay in receiving antibiotics; this delay can increase mortality.
-
▪
The treatment for pneumonia should be pathogen-directed, but definitive identification of the patient's causal pathogen may be difficult. Therefore, the setting in which the patient resides (e.g., community, hospital, nursing home), the severity of the disease, the age of the patient, the presence of comorbidities and immunosuppression, previous antimicrobial therapy, and specific clinical and radiologic manifestations of the illness are used to select initial empirical antimicrobial therapy.
-
▪
If the etiologic agent has been identified, the antimicrobial regimen should be adjusted based on the results of in vitro susceptibility testing. The ideal drug for a known pathogen has the narrowest spectrum of activity and is the most efficacious, least toxic, and least costly.
Complete reference list available at ExpertConsult.
eFigure Image Gallery
Key Readings
- Aliberti S, Blasi F. Clinical stability versus clinical failure in patients with community-acquired pneumonia. Semin Respir Crit Care. 2012;33:284–291. doi: 10.1055/s-0032-1315640. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society and Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2008;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- Charles PG, Wolfe R, Whitby M. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375–384. doi: 10.1086/589754. [DOI] [PubMed] [Google Scholar]
- Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371(17):1619–1628. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- Fagon JY, Chastre J, Wolff M. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132:621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- Mandell LA, Wunderink RG, Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Wateska AR, Nowalk MP. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. J Am Med Assoc. 2012;307:804–812. doi: 10.1001/jama.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Torres A, Nathwani P. Clinical and economical burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- Whitney CG, Farley MM, Hadler J. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- Woodhead M, Blasi F, Ewig S. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 2.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374(9700):1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 3.Golda A, Malek N, Dudek B. Infection with human coronavirus NL63 enhances streptococcal adherence to epithelial cells. J Gen Virol. 2011;92(Pt 6):1358–1368. doi: 10.1099/vir.0.028381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grigg J, Walters H, Sohal SS. Cigarette smoke and platelet-activating factor receptor dependent adhesion of Streptococcus pneumoniae to lower airway cells. Thorax. 2012;67(10):908–913. doi: 10.1136/thoraxjnl-2011-200835. [DOI] [PubMed] [Google Scholar]
- 5.Mushtaq N, Ezzati M, Hall L. Adhesion of Streptococcus pneumoniae to human airway epithelial cells exposed to urban particulate matter. J Allergy Clin Immunol. 2011;127(5):1236–1242. doi: 10.1016/j.jaci.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Heilmann C. Adhesion mechanisms of staphylococci. Adv Exp Med Biol. 2011;715:105–123. doi: 10.1007/978-94-007-0940-9_7. [DOI] [PubMed] [Google Scholar]
- 7.Parker D, Prince A. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin Immunopathol. 2012;34(2):281–297. doi: 10.1007/s00281-011-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcántar-Curiel MD, Blackburn D, Saldaña Z. Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence. 2013;4(2):129–138. doi: 10.4161/viru.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33(10):537–542. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- 10.Awasthi S. Surfactant protein (SP)-A and SP-D as antimicrobial and immunotherapeutic agents. Recent Pat Antiinfect Drug Discov. 2010;5(2):115–123. doi: 10.2174/157489110791233559. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd VL, Lopez JP. The role of surfactant-associated protein A in pulmonary host defense. Immunol Res. 2001;23(2–3):111–120. doi: 10.1385/IR:23:2-3:111. [DOI] [PubMed] [Google Scholar]
- 12.Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012;33(9):459–466. doi: 10.1016/j.it.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarchum I, Pamer EG. Regulation of innate and adaptive immunity by the commensal microbiota. Curr Opin Immunol. 2011;23(3):353–360. doi: 10.1016/j.coi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ubeda C, Taur Y, Jenq RR. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandl K, Plitas G, Mihu CN. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcón A, Fábregas N, Torres A. Pathophysiology of pneumonia. Clin Chest Med. 2005;26(1):39–46. doi: 10.1016/j.ccm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Marrie T. Community-acquired pneumonia in the elderly. Clin Infect Dis. 2000;31:1066–1078. doi: 10.1086/318124. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett JG, Dowell SF, Mandell LA. Practice guidelines for the management of community-acquired pneumonia. Clin Infect Dis. 2000;31:347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandell LA, Bartlett JG, Dowell SF. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37:1405–1433. doi: 10.1086/380488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederman M, Mandell L, Anzueto A. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 21.Mandell LA, Marrie TJ, Grossman RF. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community-Acquired Pneumonia Working Group. Clin Infect Dis. 2000;31:383–421. doi: 10.1086/313959. [DOI] [PubMed] [Google Scholar]
- 22.Cillóniz C, Ewig S, Polverino E. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax. 2011;66(4):340–346. doi: 10.1136/thx.2010.143982. [DOI] [PubMed] [Google Scholar]
- 23.File TM, Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130–134. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 24.Arancibia F, Bauer T, Ewig S. Community acquired pneumonia due to Gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk and prognosis. Arch Intern Med. 2002;162:1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 25.Lim W, van der Eerden M, Laing R. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugosson A, Hjorth M, Bernander S. A community outbreak of Legionnaires’ disease from an industrial cooling tower: assessment of clinical features and diagnostic procedures. Scand J Infect Dis. 2007;39(3):217–224. doi: 10.1080/00365540601001930. [DOI] [PubMed] [Google Scholar]
- 27.Sabria M, Alvarez J, Dominguez A. A community outbreak of Legionnaires’ disease: evidence of a cooling tower as the source. Clin Microbiol Infect. 2006;12(7):642–647. doi: 10.1111/j.1469-0691.2006.01447.x. [DOI] [PubMed] [Google Scholar]
- 28.Hyvernat H, Pulcini C, Carles D. Fatal Staphylococcus aureus hemorrhagic pneumonia producing Panton-Valentine leucocidin. Scand J Infect Dis. 2007;39:183–185. doi: 10.1080/00365540600818003. [DOI] [PubMed] [Google Scholar]
- 29.El Solh A, Sikka P, Ramadan F. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001;163:645–651. doi: 10.1164/ajrccm.163.3.2005075. [DOI] [PubMed] [Google Scholar]
- 30.Cordero E, Pachon J, Rivero A. Community-acquired bacterial pneumonia in human immunodeficiency virus-infected patients: validation of severity criteria. Am J Respir Crit Care Med. 2000;162:2063–2068. doi: 10.1164/ajrccm.162.6.9910104. [DOI] [PubMed] [Google Scholar]
- 31.Park D, Sherbin V, Goodman M. The etiology of community-acquired pneumonia at an urban public hospital: influence of human immunodeficiency virus infection and initial severity of illness. J Infect Dis. 2001;184:268–277. doi: 10.1086/322040. [DOI] [PubMed] [Google Scholar]
- Cilloniz C, Torres A, Polverino E. Community-acquired lung respiratory infections in HIV-infected patients: microbial aetiology and outcome. Eur Respir J. 2014;43(6):1698–1708. doi: 10.1183/09031936.00155813. [DOI] [PubMed] [Google Scholar]
- 32.Ewig S, Birkner N, Strauss R. New perspectives on community-acquired pneumonia in 388,406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64(12):1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan V, Angus DC, Griffin MF. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 34.Zalacain R, Torres A, Celis R. Community-acquired pneumonia in the elderly: Spanish multicenter study. Eur Respir J. 2003;21:294–302. doi: 10.1183/09031936.03.00064102. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Sabe N, Carratala J, Roson B. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore) 2003;82:159–169. doi: 10.1097/01.md.0000076005.64510.87. [DOI] [PubMed] [Google Scholar]
- 36.Feldman C. The role of alcohol in severe pneumonia and acute lung injury. In: Rello J, Leeper K, editors. Severe community acquired pneumonia. Kluwer Academic; Boston: 2001. pp. 139–152. [Google Scholar]
- 37.de Roux A, Cavalcanti M, Marcos MA. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129(5):1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- 38.Ferrer M, Liapikou A, Valencia M. Validation of the American Thoracic Society–Infectious Diseases Society of America guidelines for hospital-acquired pneumonia in the intensive care unit. Clin Infect Dis. 2010;50(7):945–952. doi: 10.1086/651075. [DOI] [PubMed] [Google Scholar]
- 39.Agusti C, Rano A, Filella X. Pulmonary infiltrates in patients receiving long-term glucocorticoid treatment: etiology, prognostic factors, and associated inflammatory response. Chest. 2003;123:488–498. doi: 10.1378/chest.123.2.488. [DOI] [PubMed] [Google Scholar]
- 40.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol. 2003;24(5 Suppl):S6–S52. doi: 10.1086/503485. [DOI] [PubMed] [Google Scholar]
- 41.Schidlow D, Fiel S. Cystic fibrosis. In: Albert R, Spiro S, Jett J, editors. Comprehensive respiratory medicine. Elsevier; Philadelphia: 2008. pp. 42.1–42.14. [Google Scholar]
- 42.Sullivan RJ, Jr, Dowdle WR, Marine WM, Hierholzer JC. Adult pneumonia in a general hospital. Etiology and host risk factors. Arch Intern Med. 1972;129(6):935–942. [PubMed] [Google Scholar]
- 43.Feldman C, Anderson R. HIV-associated bacterial pneumonia. Clin Chest Med. 2013;34(2):205–216. doi: 10.1016/j.ccm.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Murray JF. Epidemiology of human immunodeficiency virus-associated pulmonary disease. Clin Chest Med. 2013;34(2):165–179. doi: 10.1016/j.ccm.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Chen MZ, Hsueh PR, Lee LN. Severe community-acquired pneumonia due to Acinetobacter baumannii. Chest. 2001;120:1072–1077. doi: 10.1378/chest.120.4.1072. [DOI] [PubMed] [Google Scholar]
- 46.Imported plague—New York City, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:725–728. [PubMed] [Google Scholar]
- 47.Feldman KA, Stiles-Enos D, Julian K. Tularemia on Martha's Vineyard: seroprevalence and occupational risk. Emerg Infect Dis. 2003;9:350–354. doi: 10.3201/eid0903.020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 49.Poutanen SM, Low DE, Henry B. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 50.Tsang KW, Ho PL, Ooi GC. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 51.Inglesby TV, O'Toole T, Henderson DA. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- Hendricks KA, Wright ME, Shadomy SV. Workgroup on anthrax clinical guidelines. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis. 2014;20(2) doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dennis DT, Inglesby TV, Henderson DA. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 53.American Thoracic Society Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2008;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 54.Liapikou A, Polverino E, Ewig S. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. Eur Respir J. 2012;39(4):855–861. doi: 10.1183/09031936.00067111. [DOI] [PubMed] [Google Scholar]
- 55.Kollef MH, Shorr A, Tabak YP. Epidemiology and outcomes of health-care–associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 56.Waterer GW, Kessler LA, Wunderink RG. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest. 2006;130:11–15. doi: 10.1378/chest.130.1.11. [DOI] [PubMed] [Google Scholar]
- 57.Bochud PY, Moser F, Erard P. Community-acquired pneumonia. A prospective outpatient study. Medicine (Baltimore) 2001;80:75–87. doi: 10.1097/00005792-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Mandell LA, Wunderink RG, Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metlay JP, Fine MJ. Testing strategies in the initial management of patients with community-acquired pneumonia. Ann Intern Med. 2003;138:109–118. doi: 10.7326/0003-4819-138-2-200301210-00012. [DOI] [PubMed] [Google Scholar]
- 60.Blasi F, Tarsia P, Aliberti S. Chlamydia pneumoniae and Mycoplasma pneumoniae. Semin Respir Crit Care Med. 2005;26:617–624. doi: 10.1055/s-2005-925525. [DOI] [PubMed] [Google Scholar]
- 61.Menendez R, Perpiña M, Torres A. Evaluation of nonresolving and progressive pneumonia. Semin Respir Infect. 2003;18:103–111. [PubMed] [Google Scholar]
- 62.Menendez R, Cavalcanti M, Reyes S. Markers of treatment failure in hospitalised community acquired pneumonia. Thorax. 2008;63:447–452. doi: 10.1136/thx.2007.086785. [DOI] [PubMed] [Google Scholar]
- 63.Bouadma L, Luyt CE, Tubach F, PRORATA trial group Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 64.Schuetz P, Christ-Crain M, Thomann R. ProHOSP Study Group: Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 65.American Thoracic Society Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 66.Tan MJ, Tan JS, Hamor RH. The radiologic manifestations of Legionnaire's disease. The Ohio Community-Based Pneumonia Incidence Study Group. Chest. 2000;117:398–403. doi: 10.1378/chest.117.2.398. [DOI] [PubMed] [Google Scholar]
- 67.Virkki R, Juven T, Rikalainen H. Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57:438–441. doi: 10.1136/thorax.57.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garau J, Gomez L. Pseudomonas aeruginosa pneumonia. Curr Opin Infect Dis. 2003;16:135–143. doi: 10.1097/00001432-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Roson B, Carratala J, Verdaguer R. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis. 2000;31:869–874. doi: 10.1086/318151. [DOI] [PubMed] [Google Scholar]
- 70.Blot F, Raynard B, Chachaty E. Value of Gram stain examination of lower respiratory tract secretions for early diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 2000;162:1731–1737. doi: 10.1164/ajrccm.162.5.9908088. [DOI] [PubMed] [Google Scholar]
- 71.Cook D, Mandell L. Endotracheal aspiration in the diagnosis of ventilator-associated pneumonia. Chest. 2000;117:195S–197S. doi: 10.1378/chest.117.4_suppl_2.195s. [DOI] [PubMed] [Google Scholar]
- 72.Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 73.Falguera M, Trujillano J, Caro S, NAC-CALIDAD (Proyecto Integrado de Investigación de la Sociedad Española de Patología del Aparato Respiratorio sobre Infecciones Respiratorias de Vías Bajas) Study Group A prediction rule for estimating the risk of bacteremia in patients with community-acquired pneumonia. Clin Infect Dis. 2009;49(3):409–416. doi: 10.1086/600291. [DOI] [PubMed] [Google Scholar]
- 74.Marcos M, Jimenez de Anta M, Puig de la Bellacasa J. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003;21:209–214. doi: 10.1183/09031936.03.00058802. [DOI] [PubMed] [Google Scholar]
- 75.Murdoch DR, Laing RT, Cook JM. The NOW S. pneumoniae urinary antigen test positivity rate 6 weeks after pneumonia onset and among patients with COPD. Clin Infect Dis. 2003;37:153–154. doi: 10.1086/375610. [DOI] [PubMed] [Google Scholar]
- 76.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837–1842. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 77.Gutierrez F, Masia M, Rodriguez JC. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis. 2003;36:286–292. doi: 10.1086/345852. [DOI] [PubMed] [Google Scholar]
- 78.Ortega L, Sierra M, Domínguez J. Utility of a pneumonia severity index in the optimization of the diagnostic and therapeutic effort for community-acquired pneumonia. Scand J Infect Dis. 2005;37:657–663. doi: 10.1080/00365540510027174. [DOI] [PubMed] [Google Scholar]
- 79.Esposito S, Bosis S, Colombo R. Evaluation of rapid assay for detection of Streptococcus pneumoniae urinary antigen among infants and young children with possible invasive pneumococcal disease. Pediatr Infect Dis J. 2004;23:365–367. doi: 10.1097/00006454-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 80.Weinberg GA, Erdman DD, Edwards KM. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 81.Templeton KE, Scheltinga SA, van den Eeden WC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rano A, Agusti C, Jimenez P. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax. 2001;56:379–387. doi: 10.1136/thorax.56.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baughman R. Protected-specimen brush technique in the diagnosis of ventilator-associated pneumonia. Chest. 2000;117:203S–206S. doi: 10.1378/chest.117.4_suppl_2.203s. [DOI] [PubMed] [Google Scholar]
- 84.Grossman R, Fein A. Evidence based assessment of diagnostic tests for ventilator-associated pneumonia. Executive summary. Chest. 2000;117:177S–181S. doi: 10.1378/chest.117.4_suppl_2.177s. [DOI] [PubMed] [Google Scholar]
- 85.Torres A, El-Ebiary M. Bronchoscopic BAL in the diagnosis of ventilator-associated pneumonial. Chest. 2000;117:198S–202S. doi: 10.1378/chest.117.4_suppl_2.198s. [DOI] [PubMed] [Google Scholar]
- 86.Torres A, Fabregas N, Ewig S. Sampling methods for ventilator-associated pneumonia: validation using different histologic and microbiological references. Crit Care Med. 2000;28:2799–2804. doi: 10.1097/00003246-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 87.Hubmayr R, Burchardi H, Elliot M. Statement of the 4th International Consensus Conference in Critical Care on ICU Acquired Pneumonia. Intensive Care Med. 2002;28:1521–1536. doi: 10.1007/s00134-002-1514-0. [DOI] [PubMed] [Google Scholar]
- 88.Sirvent JM, Vidaur L, Gonzalez S. Microscopic examination of intracellular organisms in protected bronchoalveolar mini-lavage fluid for the diagnosis of ventilator-associated pneumonia. Chest. 2003;123:518–523. doi: 10.1378/chest.123.2.518. [DOI] [PubMed] [Google Scholar]
- 89.Fagon JY, Chastre J, Wolff M. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132(8):621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 90.Ruiz M, Torres A, Ewig S. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med. 2000;162(1):119–125. doi: 10.1164/ajrccm.162.1.9907090. [DOI] [PubMed] [Google Scholar]
- 91.The Canadian Critical Care Trial Group A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630. doi: 10.1056/NEJMoa052904. [DOI] [PubMed] [Google Scholar]
- 92.Singh N, Rogers P, Atwood C. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 93.Fine MJ, Auble TE, Yealy DM. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 94.Dean NC, Suchyta MR, Bateman KA. Implementation of admission decision support for community-acquired pneumonia. Chest. 2000;117:1368–1377. doi: 10.1378/chest.117.5.1368. [DOI] [PubMed] [Google Scholar]
- 95.Chan SS, Yuen EH, Kew J. Community-acquired pneumonia—implementation of a prediction rule to guide selection of patients for outpatient treatment. Eur J Emerg Med. 2001;8:279–286. doi: 10.1097/00063110-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 96.Marrie TJ, Lau CY, Wheeler SL. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283:749–755. doi: 10.1001/jama.283.6.749. [DOI] [PubMed] [Google Scholar]
- 97.Woodhead M, Blasi F, Ewig S, Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases Guidelines for the management of adult lower respiratory tract infections–full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Renaud B, Santin A, Coma E. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med. 2009;37:2867–2874. doi: 10.1097/CCM.0b013e3181b02dbb. [DOI] [PubMed] [Google Scholar]
- 99.Charles PG, Wolfe R, Whitby M, Australian Community-Acquired Pneumonia Study Collaboration SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375–384. doi: 10.1086/589754. [DOI] [PubMed] [Google Scholar]
- 100.España PP, Capelastegui A, Gorordo I. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249–1256. doi: 10.1164/rccm.200602-177OC. [DOI] [PubMed] [Google Scholar]
- 101.Renaud B, Labarère J, Coma E. Risk stratification of early admission to the intensive care unit of patients with no major criteria of severe community-acquired pneumonia: development of an international prediction rule. Crit Care. 2009;13:R54. doi: 10.1186/cc7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Labarère J, Schuetz P, Renaud B. Validation of a clinical prediction model for early admission to the intensive care unit of patients with pneumonia. Acad Emerg Med. 2012;19:993–1003. doi: 10.1111/j.1553-2712.2012.01424.x. [DOI] [PubMed] [Google Scholar]
- 103.Brown SM, Jones JP, Aronsky D. Relationships among initial hospital triage, disease progression and mortality in community-acquired pneumonia. Respirology. 2012;17:1207–1213. doi: 10.1111/j.1440-1843.2012.02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Phua J, Ngerng WJ, Lim TK. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J. 2010;36(4):826–833. doi: 10.1183/09031936.00154209. [DOI] [PubMed] [Google Scholar]
- 105.Menendez R, Ferrando D, Valles JM, Vallterra J. Influence of deviation from guidelines on the outcome of community-acquired pneumonia. Chest. 2002;122:612–617. doi: 10.1378/chest.122.2.612. [DOI] [PubMed] [Google Scholar]
- 106.Battleman DS, Callahan M, Thaler HT. Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community-acquired pneumonia: link between quality of care and resource utilization. Arch Intern Med. 2002;162:682–688. doi: 10.1001/archinte.162.6.682. [DOI] [PubMed] [Google Scholar]
- 107.Houck PM, Bratzler DW, Nsa W. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637–644. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 108.Heffelfinger JD, Dowell SF, Jorgensen JH. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med. 2000;160:1399–1408. doi: 10.1001/archinte.160.10.1399. [DOI] [PubMed] [Google Scholar]
-
109.Feldman RB, Rhew DC, Wong JY. Azithromycin monotherapy for patients hospitalized with community-acquired pneumonia: a
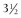 -year experience from a Veterans Affairs hospital. Arch Intern Med. 2003;163:1718–1726. doi: 10.1001/archinte.163.14.1718. [DOI] [PubMed] [Google Scholar]
-year experience from a Veterans Affairs hospital. Arch Intern Med. 2003;163:1718–1726. doi: 10.1001/archinte.163.14.1718. [DOI] [PubMed] [Google Scholar] - 110.Houck PM, MacLehose RF, Niederman MS, Lowery JK. Empiric antibiotic therapy and mortality among Medicare pneumonia inpatients in 10 western states: 1993, 1995, and 1997. Chest. 2001;119:1420–1426. doi: 10.1378/chest.119.5.1420. [DOI] [PubMed] [Google Scholar]
- 111.von Baum H, Welte T, Marre R, CAPNETZ study group Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- 112.Devasia RA, Blackman A, Gebretsadik T. Fluoroquinolone resistance in Mycobacterium tuberculosis: the effect of duration and timing of fluoroquinolone exposure. Am J Respir Crit Care Med. 2009;180(4):365–370. doi: 10.1164/rccm.200901-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vanderkooi OG, Low DE, Green K, Toronto Invasive Bacterial Disease Network Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis. 2005;40:1288–1297. doi: 10.1086/429242. [DOI] [PubMed] [Google Scholar]
- 114.Ho PL, Yung RW, Tsang DN. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J Antimicrob Chemother. 2001;48:659–665. doi: 10.1093/jac/48.5.659. [DOI] [PubMed] [Google Scholar]
- 115.Davidson R, Cavalcanti R, Brunton JL. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:747–750. doi: 10.1056/NEJMoa012122. [DOI] [PubMed] [Google Scholar]
- 116.Anderson KB, Tan JS, File TM., Jr Emergence of levofloxacin-resistant pneumococci in immunocompromised adults after therapy for community-acquired pneumonia. Clin Infect Dis. 2003;37:376–381. doi: 10.1086/376642. [DOI] [PubMed] [Google Scholar]
- Mortensen EM, Halm EA, Pugh MJ. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311(21):2199–2208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Micek ST, Kollef KE, Reichley RM. Healthcare-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carratalà J, Mykietiuk A, Fernández-Sabé N. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med. 2007;167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 119.Labelle AJ, Arnold H, Reichley RM. A comparison of culture-positive and culture-negative health-care-associated pneumonia. Chest. 2010;137:1130–1137. doi: 10.1378/chest.09-1652. [DOI] [PubMed] [Google Scholar]
- 120.Kollef MH, Kollef KE. Antibiotic utilization and outcomes for patients with clinically suspected ventilator-associated pneumonia and negative quantitative BAL culture results. Chest. 2005;128:2706–2713. doi: 10.1378/chest.128.4.2706. [DOI] [PubMed] [Google Scholar]
- 121.Christ-Crain M, Stolz D, Bingisser R. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 122.Rhew DC, Tu GS, Ofman J. Early switch and early discharge strategies in patients with community-acquired pneumonia: a meta-analysis. Arch Intern Med. 2001;161:722–727. doi: 10.1001/archinte.161.5.722. [DOI] [PubMed] [Google Scholar]
- 123.Castro-Guardiola A, Viejo-Rodriguez AL, Soler-Simon S. Efficacy and safety of oral and early-switch therapy for community-acquired pneumonia: a randomized controlled trial. Am J Med. 2001;111:367–374. doi: 10.1016/s0002-9343(01)00868-3. [DOI] [PubMed] [Google Scholar]
- 124.Nuorti JP, Butler JC, Farley MM. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 125.Musher DM, Alexandraki I, Graviss EA. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore) 2000;79:210–221. doi: 10.1097/00005792-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 126.Dowell SF, Whitney CG, Wright C. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9:573–579. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Whitney CG, Farley MM, Hadler J, Active Bacterial Core Surveillance of the Emerging Infections Program Network decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- Pilishvili T, Lexau C, Farley MM. Active bacterial core surveillance/emerging infections program network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 128.Simell B, Auranen K, Käyhty H, Pneumococcal Carriage Group The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 129.Hicks LA, Harrison LH, Flannery B. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196(9):1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 130.Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004;39:165–169. doi: 10.1086/421497. [DOI] [PubMed] [Google Scholar]
- 131.Sorde R, Falco V, Lowak M. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Int Med. 2011;171(2):166–172. doi: 10.1001/archinternmed.2010.347. [DOI] [PubMed] [Google Scholar]
- 132.Bender JM, Ampofo K, Korgenski K. Pneumococcal necrotizing pneumonia in Utah: does serotype matter? Clin Infect Dis. 2008;46:1346–1352. doi: 10.1086/586747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wunderink RG, Laterre PF, Francois B, CAPTIVATE Trial Group Recombinant tissue factor pathway inhibitor in severe community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2011;183:1561–1568. doi: 10.1164/rccm.201007-1167OC. [DOI] [PubMed] [Google Scholar]
- 134.Whitney CG, Farley MM, Hadler J. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 135.National Committee for Clinical Laboratory Standards . NCCLS; Wayne, PA: 2002. Twelfth informational supplement. M100-S12. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 136.Doern GV, Heilmann KP, Huynh HK. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob Agents Chemother. 2001;45:1721–1729. doi: 10.1128/AAC.45.6.1721-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sahm DF, Thornsberry C, Mayfield DC. In vitro activities of broad-spectrum cephalosporins against nonmeningeal isolates of Streptococcus pneumoniae: MIC interpretation using NCCLS M100-S12 recommendations. J Clin Microbiol. 2002;40:669–674. doi: 10.1128/JCM.40.2.669-674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lonks JR, Garau J, Gomez L. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resist-ant Streptococcus pneumoniae. Clin Infect Dis. 2002;35:556–564. doi: 10.1086/341978. [DOI] [PubMed] [Google Scholar]
- 139.Musher DM, Dowell ME, Shortridge VD. Emergence of macrolide resistance during treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:630–631. doi: 10.1056/NEJM200202213460820. [DOI] [PubMed] [Google Scholar]
- 140.Martinez JA, Horcajada JP, Almela M. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2003;36:389–395. doi: 10.1086/367541. [DOI] [PubMed] [Google Scholar]
- 141.Brown RB, Iannini P, Gross P, Kunkel M. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: analysis of a hospital claims-made database. Chest. 2003;123:1503–1511. doi: 10.1378/chest.123.5.1503. [DOI] [PubMed] [Google Scholar]
- 142.Outbreak of group A streptococcal pneumonia among Marine Corps recruits—California, November 1-December 20, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:106–109. [PubMed] [Google Scholar]
- 143.O’Brien KL, Beall B, Barrett NL. Epidemiology of invasive group A streptococcus disease in the United States, 1995–1999. Clin Infect Dis. 2002;35:268–276. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- 144.Stockmann C, Ampofo K, Hersh AL. Evolving epidemiologic characteristics of invasive group A streptococcal disease in Utah, 2002–2010. Clin Infect Dis. 2012;55:479–487. doi: 10.1093/cid/cis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kalima P, Riordan T, Berrisford RG, Sarsfield PT. Necrotizing pneumonia associated with group A streptococcal bacteraemia. Eur J Clin Microbiol Infect Dis. 1998;17(4):296–298. doi: 10.1007/BF01699993. [DOI] [PubMed] [Google Scholar]
- 146.Barnham M, Weightman N, Anderson A. Review of 17 cases of pneumonia caused by Streptococcus pyogenes. Eur J Clin Microbiol Infect Dis. 1999;18(7):506–509. doi: 10.1007/s100960050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis. 2001;33:556–561. doi: 10.1086/322696. [DOI] [PubMed] [Google Scholar]
- 148.Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006;119:877–883. doi: 10.1016/j.amjmed.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 149.Maskell NA, Batt S, Hedley EL. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med. 2006;174:817–823. doi: 10.1164/rccm.200601-074OC. [DOI] [PubMed] [Google Scholar]
- 150.Morozumi M, Nakayama E, Iwata S. Simultaneous detection of pathogens in clinical samples from patients with community-acquired pneumonia by real-time PCR with pathogen-specific molecular beacon probes. J Clin Microbiol. 2006;44:1440–1446. doi: 10.1128/JCM.44.4.1440-1446.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alos JI, Aracil B, Oteo J, Gomez-Garces JL. Significant increase in the prevalence of erythromycin-resistant, clindamycin- and miocamycin-susceptible (M phenotype) Streptococcus pyogenes in Spain. J Antimicrob Chemother. 2003;51:333–337. doi: 10.1093/jac/dkg100. [DOI] [PubMed] [Google Scholar]
- 152.Livorsi DJ, Macneil JR, Cohn AC. Invasive Haemophilus influenzae in the United States, 1999–2008: epidemiology and outcomes. J Infect. 2012;65:496–504. doi: 10.1016/j.jinf.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hammerschlag MR. Mycoplasma pneumoniae infections. Curr Opin Infect Dis. 2001;14:181–186. doi: 10.1097/00001432-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 154.Smith R, Eviatar L. Neurologic manifestations of Mycoplasma pneumoniae infections: diverse spectrum of diseases. A report of six cases and review of the literature. Clin Pediatr (Phila) 2000;39:195–201. doi: 10.1177/000992280003900401. [DOI] [PubMed] [Google Scholar]
- 155.Hyde TB, Gilbert M, Schwartz SB. Azithromycin prophylaxis during a hospital outbreak of Mycoplasma pneumoniae pneumonia. J Infect Dis. 2001;183:907–912. doi: 10.1086/319258. [DOI] [PubMed] [Google Scholar]
- 156.Blasi F, Tarsia P, Aliberti S. Chlamydophila pneumoniae. Clin Microbiol Infect. 2009;15:29–35. doi: 10.1111/j.1469-0691.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 157.Monno R, De Vito D, Losito G. Chlamydia pneumoniae in community-acquired pneumonia: seven years of experience. J Infect. 2002;45:135–138. doi: 10.1016/s0163-4453(02)91036-4. [DOI] [PubMed] [Google Scholar]
- 158.Robicsek A, Suseno M, Beaumont JL. Prediction of methicillin-resistant Staphylococcus aureus involvement in disease sites by concomitant nasal sampling. J Clin Microbiol. 2008;46:588–592. doi: 10.1128/JCM.01746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hidron AI, Low CE, Honig EG, Blumberg HM. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis. 2009;9(6):384–392. doi: 10.1016/S1473-3099(09)70133-1. [DOI] [PubMed] [Google Scholar]
- 160.Lobo LJ, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest. 2010;138(1):130–136. doi: 10.1378/chest.09-1562. [DOI] [PubMed] [Google Scholar]
- 161.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wunderink RG. How important is methicillin-resistant Staphylococcus aureus as a cause of community-acquired pneumonia and what is best antimicrobial therapy? Infect Dis Clin North Am. 2013;27(1):177–188. doi: 10.1016/j.idc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 163.Micek ST, Dunne M, Kollef MH. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus: importance of treatment with antimicrobials inhibiting exotoxin production. Chest. 2005;128:2732–2738. doi: 10.1378/chest.128.4.2732. [DOI] [PubMed] [Google Scholar]
- 164.Micek ST, Kollef KE, Reichley RM. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vardakas KZ, Matthaiou DK, Falagas ME. Incidence, characteristics and outcomes of patients with severe community acquired-MRSA pneumonia. Eur Respir J. 2009;34(5):1148–1158. doi: 10.1183/09031936.00041009. [DOI] [PubMed] [Google Scholar]
- 166.DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gillet Y, Vanhems P, Lina G. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis. 2007;45:315–321. doi: 10.1086/519263. [DOI] [PubMed] [Google Scholar]
- 168.Wunderink RG, Niederman MS, Kollef MH. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 169.Kollef MH, Rello J, Cammarata SK. Clinical cure and survival in gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 2004;30(3):388–394. doi: 10.1007/s00134-003-2088-1. Epub 2004; January 9. [DOI] [PubMed] [Google Scholar]
- 170.Wunderink RG, Rello J, Cammarata SK. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124(5):1789–1797. [PubMed] [Google Scholar]
- 171.Silverman JA, Mortin LI, Vanpraagh AD. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191(12):2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 172.Savard P, Perl TM. A call for action: managing the emergence of multidrug-resistant Enterobacteriaceae in the acute care settings. Curr Opin Infect Dis. 2012;25(4):371–377. doi: 10.1097/QCO.0b013e3283558c17. [DOI] [PubMed] [Google Scholar]
- 173.Bush K, Fisher JF. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu Rev Microbiol. 2011;65:455–478. doi: 10.1146/annurev-micro-090110-102911. [DOI] [PubMed] [Google Scholar]
- 174.Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa. Part 1. Epidemiology, clinical diagnosis, and source. Chest. 2011;139(4):909–919. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 175.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171(11):1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Allen SH, Brennan-Benson P, Nelson M. Pneumonia due to antibiotic resistant Streptococcus pneumoniae and Pseudomonas aeruginosa in the HAART era. Postgrad Med J. 2003;79(938):691–694. [PMC free article] [PubMed] [Google Scholar]
- 177.Hatchette TF, Gupta R, Marrie TJ. Pseudomonas aeruginosa community-acquired pneumonia in previously healthy adults: case report and review of the literature. Clin Infect Dis. 2000;31:1349–1356. doi: 10.1086/317486. [DOI] [PubMed] [Google Scholar]
- 178.Crnich CJ, Gordon B, Andes D. Hot tub-associated necrotizing pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis. 2003;36:e55–e57. doi: 10.1086/345851. [DOI] [PubMed] [Google Scholar]
- 179.Chastre J, Fagon J. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 180.Chatzinikolaou I, Abi-Said D, Bodey GP. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: retrospective analysis of 245 episodes. Arch Intern Med. 2000;160:501–509. doi: 10.1001/archinte.160.4.501. [DOI] [PubMed] [Google Scholar]
- 181.Giamarellou H, Antoniadou A. Antipseudomonal antibiotics. Med Clin North Am. 2001;85:19–42. doi: 10.1016/s0025-7125(05)70303-5. v. [DOI] [PubMed] [Google Scholar]
- 182.Valdezate S, Vindel A, Loza E. Antimicrobial susceptibilities of unique Stenotrophomonas maltophilia clinical strains. Antimicrob Agents Chemother. 2001;45:1581–1584. doi: 10.1128/AAC.45.5.1581-1584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Scott P, Deye G, Srinivasan A. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 184.Leung WS, Chu CM, Tsang KY. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest. 2006;129(1):102–109. doi: 10.1378/chest.129.1.102. [DOI] [PubMed] [Google Scholar]
- 185.Ong CW, Lye DC, Khoo KL. Severe community-acquired Acinetobacter baumannii pneumonia: an emerging highly lethal infectious disease in the Asia-Pacific. Respirology. 2009;14(8):1200–1205. doi: 10.1111/j.1440-1843.2009.01630.x. [DOI] [PubMed] [Google Scholar]
- 186.Wisplinghoff H, Edmond MB, Pfaller MA. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000;31:690–697. doi: 10.1086/314040. [DOI] [PubMed] [Google Scholar]
- 187.Garnacho-Montero J, Ortiz-Leyba C, Fernandez-Hinojosa E. Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med. 2005;31:649–655. doi: 10.1007/s00134-005-2598-0. [DOI] [PubMed] [Google Scholar]
- 188.Urban C, Segal-Maurer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003;36:1268–1274. doi: 10.1086/374847. [DOI] [PubMed] [Google Scholar]
- 189.Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36:1111–1118. doi: 10.1086/374337. [DOI] [PubMed] [Google Scholar]
- 190.Pedro-Botet ML, Stout JE, Yu VL. Legionnaires’ disease contracted from patient homes: the coming of the third plague? Eur J Clin Microbiol Infect Dis. 2002;21:699–705. doi: 10.1007/s10096-002-0813-2. [DOI] [PubMed] [Google Scholar]
- 191.Weiss CH, Moazed F, DiBardino D. Bronchoalveolar lavage amylase is associated with risk factors for aspiration and predicts bacterial pneumonia. Crit Care Med. 2013;41:765–773. doi: 10.1097/CCM.0b013e31827417bc. [DOI] [PubMed] [Google Scholar]
- 192.Gacouin A, Revest M, Letheulle J. Distinctive features between community-acquired pneumonia (CAP) due to Chlamydophila psittaci and CAP due to Legionella pneumophila admitted to the intensive care unit (ICU) Eur J Clin Microbiol Infect Dis. 2012;31:2713–2718. doi: 10.1007/s10096-012-1618-6. [DOI] [PubMed] [Google Scholar]
- 193.Messmer TO, Skelton SK, Moroney JF. Application of a nested, multiplex PCR to psittacosis outbreaks. J Clin Microbiol. 1997;35(8):2043–2046. doi: 10.1128/jcm.35.8.2043-2046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Raoult D, Tissot-Dupont H, Foucault C. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000;79:109–123. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 195.Marrie TJ, Raoult D. Update on Q fever, including Q fever endocarditis. Curr Clin Top Infect Dis. 2002;22:97–124. [PubMed] [Google Scholar]
- 196.Torres HA, Reddy BT, Raad II. Nocardiosis in cancer patients. Medicine (Baltimore) 2002;81:388–397. doi: 10.1097/00005792-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 197.Currie BJ, Fisher DA, Howard DM. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis. 2000;31:981–986. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 198.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 199.Cheng AC, Hanna JN, Norton R. Melioidosis in northern Australia, 2001–02. Commun Dis Intell. 2003;27:272–277. doi: 10.33321/cdi.2003.27.52. [DOI] [PubMed] [Google Scholar]
- 200.Yamshchikov AV, Schuetz A, Lyon GM. Rhodococcus equi infection. Lancet ID. 2010;10:350–359. doi: 10.1016/S1473-3099(10)70068-2. [DOI] [PubMed] [Google Scholar]
- 201.Bush LM, Abrams BH, Beall A, Johnson CC. Index case of fatal inhalational anthrax due to bioterrorism in the United States. N Engl J Med. 2001;345:1607–1610. doi: 10.1056/NEJMoa012948. [DOI] [PubMed] [Google Scholar]
- 202.Borio L, Frank D, Mani V. Death due to bioterrorism-related inhalational anthrax: report of 2 patients. JAMA. 2001;286:2554–2559. doi: 10.1001/jama.286.20.2554. [DOI] [PubMed] [Google Scholar]
- 203.Mayer TA, Bersoff-Matcha S, Murphy C. Clinical presentation of inhalational anthrax following bioterrorism exposure: report of 2 surviving patients. JAMA. 2001;286:2549–2553. doi: 10.1001/jama.286.20.2549. [DOI] [PubMed] [Google Scholar]
- 204.Kyriacou DN, Yarnold PR, Stein AC. Discriminating inhalational anthrax from community-acquired pneumonia using chest radiograph findings and a clinical algorithm. Chest. 2007;131:489–496. doi: 10.1378/chest.06-1687. [DOI] [PubMed] [Google Scholar]
- 205.Bartlett JG, Inglesby TV, Jr, Borio L. Management of anthrax. Clin Infect Dis. 2002;35:851–858. doi: 10.1086/341902. [DOI] [PubMed] [Google Scholar]
- 206.Feldman KA, Enscore RE, Lathrop SL. An outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med. 2001;345:1601–1606. doi: 10.1056/NEJMoa011374. [DOI] [PubMed] [Google Scholar]
- 207.Inglesby TV, Dennis DT, Henderson DA. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 208.Mwengee W, Butler T, Mgema S. Treatment of plague with gentamicin or doxycycline in a randomized clinical trial in Tanzania. Clin Infect Dis. 2006;42:614–621. doi: 10.1086/500137. [DOI] [PubMed] [Google Scholar]
- 209.Verduin CM, Hol C, Fleer A. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev. 2002;15:125–144. doi: 10.1128/CMR.15.1.125-144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rosenstein NE, Perkins BA, Stephens DS. Meningococcal disease. N Engl J Med. 2001;344:1378–1388. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 211.Richter SS, Gordon KA, Rhomberg PR. Neisseria meningitidis with decreased susceptibility to penicillin: report from the SENTRY antimicrobial surveillance program, North America, 1998–99. Diagn Microbiol Infect Dis. 2001;41:83–88. doi: 10.1016/s0732-8893(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 212.Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest. 2007;132:1348–1355. doi: 10.1378/chest.06-1995. [DOI] [PubMed] [Google Scholar]
- 213.Menendez R, Cavalcanti M, Reyes S. Markers of treatment failure in hospitalizad community-acquired pneumonia. Thorax. 2008;63:447–452. doi: 10.1136/thx.2007.086785. [DOI] [PubMed] [Google Scholar]
- 214.Renaud B, Schuets P, Claessens YE. ProAdrenomedullin improves risk of early admission to ICU score for predicting early severe community-acquired pneumonia. Chest. 2012;142:1447–1454. doi: 10.1378/chest.11-2574. [DOI] [PubMed] [Google Scholar]
- 215.Ferrer M, Ioanas M, Torres A. The evaluation of the non-responding patients with ventilator-associated pneumonia. Clin Pulm Med. 2001;8:290–295. [Google Scholar]
- Martin-Loeches I, Valles X, Menendez R. Predicting treatment failure in patients with community acquired pneumonia: a case-control study. Respir Res. 2014;15:75. doi: 10.1186/1465-9921-15-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sialer R, Liapikou A, Torres A. What is the best approach to the nonresponding patient with CAP. Infect Dis Clin N Am. 2013;27:189–203. doi: 10.1016/j.idc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 217.Yeghen T, Kibbler CC, Prentice HG. Management of invasive pulmonary aspergillosis in hematology patients: a review of 87 consecutive cases at a single institution. Clin Infect Dis. 2000;31:859–868. doi: 10.1086/318133. [DOI] [PubMed] [Google Scholar]
- 218.Franquet T. Imaging of pneumonia: trends and algorithms. Eur Respir J. 2001;18:196–208. doi: 10.1183/09031936.01.00213501. [DOI] [PubMed] [Google Scholar]
- 219.Sun HY, Singh N. Opportunistic infection-associated immune reconstitution syndrome in transplant recipients. Clin Infect Dis. 2011;53(2):168–176. doi: 10.1093/cid/cir276. [DOI] [PubMed] [Google Scholar]
- 220.Sun HY, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. 2009;22(4):394–402. doi: 10.1097/QCO.0b013e32832d7aff. [DOI] [PubMed] [Google Scholar]
- 221.Freifeld AG, Bow EJ, Sepkowitz KA. Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 222.Ozer H, Armitage JO, Bennett CL. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol. 2000;18:3558–3585. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 223.Nie W, Zhang Y, Cheng J, Xiu Q. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. Plos One. 2012;7:E 47926. doi: 10.1371/journal.pone.0047926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Snijders D, Daniels JM, de Graaff CS. Efficacy of corticosteroids in community-acquired pneumonia. Am J Respir Crit Care Med. 2010;181(9):975–982. doi: 10.1164/rccm.200905-0808OC. [DOI] [PubMed] [Google Scholar]
- 225.Tzouvelekis LS, Markogiannakis A, Psichogiou M. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yu H. Management of pleural effusion, empyema and lung abscess. Semin Interv Radiol. 2011;28:75–86. doi: 10.1055/s-0031-1273942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chapman SJ, Gary Lee YC, Davies RJO. Empyema lung abscess and necrotizing pneumonia. In: Torres A, Ewig S, Mandell L, Woodhead M, editors. Respiratory infections. Hodder Arnold; London: 2006. pp. 385–400. [Google Scholar]
- 228.Bartlett JG. Anaerobic bacterial infection of the lung. Anaerobe. 2012;18(2):235–239. doi: 10.1016/j.anaerobe.2011.12.004. Epub 2011; December 27. Review. [DOI] [PubMed] [Google Scholar]
- 229.Wali SO. An update on the drainage of pyogenic lung abscesses. Ann Thorac Med. 2012;7(1):3–7. doi: 10.4103/1817-1737.91552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Herth F, Ernst A, Becker HD. Endoscopic drainage of lung abscesses: technique and outcome. Chest. 2005;127(4):1378–1381. doi: 10.1378/chest.127.4.1378. [DOI] [PubMed] [Google Scholar]
- 231.Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices (ACIP). ACIP Adult Immunization Work Group ACIP recommended immunization schedule for adults aged 19 years or older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(5):110–112. [PMC free article] [PubMed] [Google Scholar]
- 232.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 233.Centers for Disease Control and Prevention Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–819. [PubMed] [Google Scholar]
- 234.Tomczyk S, Bennett NM, Stoecker C. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63(37):822–825. [PMC free article] [PubMed] [Google Scholar]
- 235.Greenberg RN, Gurtman A, Frenck RW. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364–2374. doi: 10.1016/j.vaccine.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 236.Nuorti JP, Butler JC, Farley MM. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]


