Biology
The families Suidae (swine) and Tayassuidae (peccaries) are nonruminating ungulates belonging to the Suina clade or suborder within the order Artiodactyla.49 Fossil records show suids appearing around 35 million years ago (upper Eocene era) in the part of the world now known as Thailand.49 Of the family Suidae, Suinae is the only extant subfamily. The characteristics of suid molars have resulted in dividing the subfamily into different tribes.49 The Hippohyini originated in Asia and have hypsodont dentition. Swine species from Africa with hypsodont dentition are in the Phacocherini tribe. The Suini tribe comprises members of the genus Sus. The Potamochoerini tribe includes the genera Hylochoerus and Potamochoerus. Molecular studies of Babyrousa spp. have yielded conflicting results, with some placing them in a separate tribe, Babirusina. Depending on the source, 14 to 19 species of suids exist (Table 58-1 ).15, 32, 35, 49 Recent references have given species designation to the subspecies of Babyrousa babirussa.15, 49 The natural range of the Suidae family spans across Europe, Africa, Asia, and East Indies. Evidence to support any wild suids having originated in the Americas is lacking. They were introduced by humans into the Americas and into Australia, New Zealand, and New Guinea. Ancestors of extant peccaries are thought to have dispersed into the New World from eastern Asia. Currently, three species of peccary range from Southwestern United States to South America (Table 58-2 ).15, 28, 35, 49
TABLE 58-1.
| Common Name | Scientific Name | Body Weight (adults, in kilograms) | Geographic Distribution | Dental Formula (incisors, canines, premolars, molars) | Longevity (years) |
|---|---|---|---|---|---|
| Buru babirusa | Babyrousa babyrussa | Up to 90 | Indonesia, Buru, and Sulu Islands | 2/3, 1/1, 2/2, 3/3 | Up to 24 |
| Bola Batu babirusa | Babyrousa bolabatuensis | Up to 90 | Indonesia, Lembeh | 2/3, 1/1, 2/2, 3/3 | Up to 24 |
| Malenge babirusa | Babyrousa togeanesis | Up to 90 | Malenge Island | 2/3, 1/1, 2/2, 3/3 | Up to 24 |
| North Sulawesi babirusa | Babyrousa celebensis | Up to 90 | Northern Sulawesi | 2/3, 1/1, 2/2, 3/3 | Up to 24 |
| Giant forest hog | Hylochoerus meinertzhageni | 130–275 | Congo basin, parts of west and east Africa | 3/3, 1/1, 4/4, 3/3 | — |
| Common warthog | Phacochoerus africanus | 50–100 | Sub-Saharan Africa | 1/3, 1/1, 3/2, 3/3 | 12–15 |
| Desert warthog | Phacochoerus aethiopicus | — | Eastern Ethiopia, northern Kenya, Somalia | — | — |
| Pygmy hog | Porcula salvanius | 6–10 | Bhutan, southern Nepal, northern India | 3/3, 1/1, 4/4, 3/3 | 10–12 |
| Red River hog | Potamochoerus porcus | 50–120 | Western Africa, Congo basin | 3/3, 1/1, 4/4, 3/3 | 10–15 |
| Bushpig | Potamochoerus larvatus | 50–120 | Eastern and southern Africa | 3/3, 1/1, 4/4, 3/3 | 10–15 |
| Wild boar | Sus scrofa | 50–200 | Europe, N. Africa, Asia; introduced into N. America, Australia, New Zealand, New Guinea | 3/3, 1/1, 4/4, 3/3 | 15–20 |
| Palawan pig | Sus ahoenobarbus | — | Philippines | — | — |
| Bearded pig | Sus barbatus | 100–200 | Malaysia, Sumatra, Borneo | 3/3, 1/1, 4/4, 3/3 | — |
| Heude pig | Sus bucculentus | — | Vietnam, Laos | — | — |
| Visayan warty pig | Sus cebifrons | — | Philippines | — | — |
| Celebes or warty pig | Sus celebensis | — | Indonesia | — | — |
| Oliver warty pig | Sus oliveri | — | Philippines | — | — |
| Philippine warty pig | Sus philippensis | — | Philippines | — | — |
| Javan warty pig | Sus verrucosus | Up to 185 | Java, Bawean | 3/3, 1/1, 4/4, 3/3 | — |
Data modified from Morris and Shima.28
TABLE 58-2.
| Common Name(s) | Scientific Name | Weight (adults, in kilograms) | Geographic Distribution | Dental Formula (incisors, canines, premolars, molars) | Chromosomes (2n) | Longevity (years) |
|---|---|---|---|---|---|---|
| Collared peccary/Javelina | Pecari tajacu | 15–35 | Southwest United States to Argentina | 2/3, 1/1, 3/3, 3/3 | 30 | 16–24 |
| White-lipped peccary | Tayassu pecari | 27–40 | Mexico to Argentina | 2/3, 1/1, 3/3, 3/3 | 26 | 15–21 |
| Chacoan peccary/Tagua | Catagonus wagneri | 30–43 | Argentina, Bolivia, Paraguay | 2/3, 1/1, 3/3, 3/3 | 20 | At least 9 |
Data modified from Morris and Shima.28
Wild suids and peccaries have poor vision but good hearing and a keen sense of smell. They are known for their rooting behavior. Despite their short limbs they are good runners and jumpers, and some are adept swimmers. Bornean bearded pigs have been recorded as swimming 45 kilometers (km), and babirusas have been observed swimming underwater.49 In general, wild suids and peccaries live in social groupings, although breeding males become solitary after the breeding season. Wild suid females and their offspring live in herds known as sounders. Suids are quite vocal, and peccaries make a characteristic clacking sound with their teeth. Wild suid and peccary populations are primarily threatened by habitat destruction through human encroachment and hunting.14, 35, 49 Isolated island populations are particularly susceptible. The International Union for the Conservation of Nature (IUCN) status of selected species is provided in Table 58-3 .18
TABLE 58-3.
IUCN Conservation Status of Selected Species
| IUCN Red List Classification | Genus and Species |
|---|---|
| Critically Endangered | Porcula salvanius, Sus cebifrons |
| Endangered | Babyrousa togeanensis, Catagonus wagneri, Sus oliveri, Sus verrucosus |
| Near Threatened | Sus celebensis, Tayassu pecari |
| Vulnerable | Sus ahoenobarbus, Sus barbatus, Sus phillipensis |
| Of Least Concern | Hylochoerus meinertzhageni, Phacochoerus aethiopicus, Phacochoerus africanus, Potamochoerus larvatus, Potamochoerus porcus, Sus scofa, Pecari tajacu |
IUCN, International Union for the Conservation of Nature.
Unique Anatomy
Suids are a diverse group ranging in size from 6 to 200 kilograms (kg). They are characterized as having a stout, barrel-shaped body, with a large head and short limbs relative to body size. The peccary has a piglike shape; however, the limbs are long and slender with small hooves. In addition, peccaries only have one dewclaw on the hindlimbs, and hindlimb dewclaws are generally lacking in Chacoan pecarries.49 Suid males are generally larger than females; however, little size dimorphism exists between sexes in peccaries.14, 32, 49 The pelage of wild suids varies from being sparse in the babirusa to being entirely covered with coarse bristly hair in the wild boar. Peccaries have a dense covering of long, coarse bristles, which makes it difficult to estimate body condition, especially during piloerection.
In the genus Sus, except for Sus scrofa, all adult males have three pairs of fleshy protuberances on the face (“warts”). Warthogs also possess these characteristic facial structures. Wild suids may possess a variety of scent glands, including preputial, anal, metacarpal, mandibular, salivary, Harderian, eyelid, genal, and preorbital glands.35, 49 Pecarries possess a unique dorsal rump scent gland located on the midline, approximately 15 centimeters (cm) cranial to base of the tail.14, 32, 49 The author has also observed a prominent papillae adjacent to the first and second maxillary molars, which are presumed to be of salivary origin in Chacoan peccaries (Figure 58-1 ).
FIGURE 58-1.
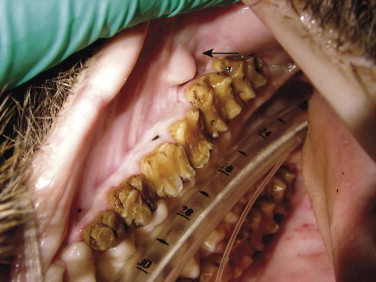
Presumed salivary papilla (arrow) in a Chacoan peccary (Catagonus wagneri).
The suid skull is unique in that it possesses an elongated flange of bone originating from the zygomatic root referred to as the prezygomatic shelf.49 This shelf of bone separates the muscles of mastication from the muscles involved in snout movement. This adaptation is thought to facilitate rooting behavior. The disklike shape of the snout, the terminally placed nostrils, which are capable of closing, and the presence of a prenasal bone within the cartilaginous disk of the snout are also adaptions for rooting behavior.32, 35, 49 The prenasal bone can be seen radiographically. Babirusas tend to root in soft, moist soils, hence their rostral bone is not as well developed as in other suids or is absent.35, 49 The rostrum of the Chacoan peccary is reported to have a more complex internal anatomy compared with other peccaries, and this is theorized to be an adaptation to living in dusty, xeric conditions.49
Another distinguishing feature of wild suids is their large canines. The orientation of canines in several species allows these teeth to grow upward and outward and thus capable of inflicting serious damage. In most species, the tusks of the males are larger than those of the females except in the warthog, in which both sexes have large tusks. The babirusa is known for its peculiar tusk arrangement. The alveoli of the upper canines rotate during development such that these teeth grow upward through the rostrum and spiral caudally.49 The lower canines also grow in a spiral shape. Canines are markedly reduced or absent in female babirusas. In peccaries, canines point straight down, which allows interlocking. This arrangement facilitates stabilization of the jaw when the animal is cracking hard seeds between the other teeth.14, 49
The dentition of suids has been used in their taxonomic classfication.49 The desert warthog differs from other suids but is similar to other ungulates in that it does not have upper incisors. Smaller species of pigs have molars with high pointed cusps; these species tend to forage on forest vegetation and fruit, whereas larger species have dentition better suited for tougher forages, with thick enamel and conical premolars.49 Similarly, because their natural diet is composed primarily of cactus, Chacoan peccaries have higher-crowned molars versus the lower-crowned molars of the other peccary species.32, 49
Except for the babirusa, suids have a simple stomach. The stomach of the babirusa is bigger and has a large diverticulum.22, 32, 35, 49 The pH in a large area of mucus-producing cardiac glands (5.3–6.4) is able to support microbial fermentation. 22, 23 Hence the babirusa is characterized as a nonruminant foregut fermenting frugivore and concentrate selector.22, 23, 24 The ultrastructure of the babirusa stomach has been extensively studied.23 Foregut fermentation is also reported in peccaries, which have a four-chambered stomach: two nonglandular blind sacs, a nonglandular gastric pouch, and a glandular hind stomach.14, 32, 49 Unlike pigs, peccaries do not have a gallbladder.14
Special Housing Requirements
The natural behaviors of wild suids and peccaries should be taken into account for appropriate exhibit specifications. The opportunity to root and dig should be provided without disrupting the structural integrity of the enclosure. These behaviors may also result in damage to indoor facilities such as padded barn floors. Animals should be given access to mud wallows, where appropriate, and kept free from fecal and urine contamination. Nesting is a common behavior, and animals should be provided bedding materials for nesting. Other considerations for housing suids and peccaries include escape potential, substrate, and intraspecific aggression. Pigs are quite capable of either jumping (both vertically and horizontally) or digging their way out of an enclosure if given the opportunity. Substrate should not be abrasive as running, pacing, or both may cause excessive hoof wear, and enclosures should be evaluated for other potential sources of foot trauma. When running along chainlink fence lines animals may traumatize the lateral dewclaws. Usually, a dominance hierarchy in social groupings exists, hence visual barriers to help divert aggression are recommended. Animals should have access to shade and, depending on the climate, protection from harsh weather.
A recent study from Brazil evaluated enclosures of three different sizes for housing collared peccaries.30 Behavioral observations were performed, and the authors concluded that a minimum of approximately 200 square meters (m2) per animal of space resulted in the least agonistic behaviors. They also found that shelter use increased with allocation of more space, which supported earlier findings regarding the importance of shelters in peccary husbandry.43
Pigs are considered fourth in intelligence of animals behind primates (human and nonhuman), dolphins, and elephants; therefore they require a stimulating environment.49 They are quick learners and have sophisticated problem-solving abilities. Environmental and behavioral enrichment should be part of any program managing suids or peccaries. Many useful resources for enrichment are available.10, 47
Feeding
In general, wild suids and peccaries, which are considered omnivores, consume such things as leaves, grasses, young saplings, seeds, roots, tubers, fruits, fungi, eggs, invertebrates, carrion, and small vertebrates.14, 32, 35, 49 In the wild, cactus also makes up a significant portion of the diet for the Chacoan peccary and, to a lesser extent, the white-lipped peccary.14, 49 The natural biology of the various species also reveals the opportunistic nature of pig foraging based on seasonal availability.24 Some authors have considered wild suids more herbivorous, with species occupying nutritional niches.8, 24 Warthogs have been classified as grazers and forest hogs as browsers. Hylochoerus, Potomachoerus sp., Sus scrofa, Sus barbatus, and possibly the warty pigs have been considered more frugivorous.8, 24 The exact nutritional requirements of wild suids are not well-defined.24 In general, diets fed to wild suids and peccaries in captivity consist of a complete pelleted herbivore ration, with varying amounts of fruits, vegetables, browse, and hay. A study evaluated the apparent digestibility of different macronutrients in warthogs, red river hogs, warty pigs, and babirusa.8 No difference in protein digestibility was observed between the species, including peccaries when comparing with prior literature. Despite differences in gastric anatomy, neither peccaries nor babirusa had more efficient fiber digestion. Hemicellulose was digested more efficiently than cellulose by red river hogs, babirusas, and peccaries, whereas warthogs were capable of efficiently digesting both hemicellulose and cellulose. Therefore, dietary items high in hemicellulose would be appropriate for most wild suids, whereas incorporating items such as grass hays that have higher cellulose content would be appropriate for warthogs.
In captivity, wild suids are prone to obesity, which may interfere with reproduction and exacerbate musculoskeletal conditions such as osteoarthritis. Routine weight monitoring is recommended. Feeding strategies should be incorporated that minimize the impact of social domination by one individual or a few and food-motivated aggression.
Restraint and Handling
In general, physical restraint is not recommended in wild suids or peccaries. Most individuals struggle violently, and no part of the body is easily held. A cornered pig may become quite aggressive, and its tusks are capable of inflicting significant injury. Attempting to restrain a nondomestic pig by the hind leg, as is done with domestic swine, is not recommended because it may result in injury to the animal. Some individual animals will remain very flighty in captivity, whereas others become quite tractable. Operant conditioning in these animals may facilitate close visual inspection and limited palpation. Some animals go into lateral recumbency when scratched with a broom or scrub brush. In animals with formidable tusks, such evaluations should be done in a protected contact situation. Piglets and infant peccaries may be manually restrained for minor procedures.
Chemical Restraint
Although immobilization in suids may be challenging, it is recommended for a thorough examination and for diagnostic procedures. Most wild suids have a thick layer of subcutaneous fat. Deposition of immobilizing agents into this fat layer may interfere with drug absorption, which would create a less than ideal immobilization. Peccaries generally do not have large subcutaneous fat reserves.14, 32 Some animals may become quite excited during immobilization. Although exotic species do not have the genetic defect that causes malignant hyperthermia in domestic swine, they are quite susceptible to hyperthermia because of their inability to sweat and the likelihood of extreme muscle exertion during an immobilization, especially in escape scenarios.27 Excessive running, lengthy inductions, and violent recoveries are also risk factors for exertional myopathy. Foot trauma may also occur with excessive running on hard surfaces. When darting an animal in a group, precautions need to be taken to avoid causing trauma to conspecifics.
In addition to remote delivery via dart systems, squeeze chutes or crates may be used for hand injections. Figure 58-2 shows a metal squeeze crate used for hand injections in small- to medium-sized animals at the author's institution. Peccaries, warty pigs, and medium-sized red river hogs are transferred into the squeeze apparatus from a transport crate. This system works well and keeps animals confined during induction.
FIGURE 58-2.

A, Metal squeeze chute or crate used for anesthetic induction of small- to medium-sized suids and peccaries. B, End-on view illustrating squeeze mechanism.
Fasting times of 12 to 24 hours have been recommended; however, hypoglycemia has been observed in some fasted pigs, especially in Potamochoerus sp. and Sus celebifrons, at the author's institution.5, 26, 28, 33, 48 Therefore, fasting times have been reduced to 3 to 6 hours for all suids and peccaries. In addition, blood glucose is monitored during immobilization and hypoglycemia treated, as needed. Problems secondary to a short fasting interval, for example, vomiting or gas distension of the gastrointestinal tract, have not been encountered.
Chemical immobilization protocols for swine and peccaries have been reviewed recently (Table 58-4 ).33 Multiple drug combinations have been used successfully. In addition, the author has added ketamine at 0.5 to 1 milligram per kilogram (mg/kg) with or without azaperone (0.25–1.3 mg/kg) to medetomidine–butorphanol–midazolam combinations. This has helped minimize some of the unpredictability in the response of wild suids to immobilizing agents. Azaperone (0.5–1.5 mg/kg, intramuscularly [IM]) has also been administered to anesthetized swine prior to recovery from anesthesia in case of concern about a possible violent recovery or excitability in the postrecovery period. Attempts to handle an animal before immobilizing agents have reached their full effect or the need for additional dosing in an animal may result in significant stimulation, which may override the drug effects and prolong induction.
TABLE 58-4.
Reference Range for Hematologic Parameters (Adults) in Selected Species
| Parameter | Red River Hog (Potamochoerus porcus)* | S. African Warthog (Phacochoerus africanus)† | Babirusa (Babyrousa babyrussa)† | Visayan Warty Pig (Sus celebrifons)* | Collared Peccary (Tayassu tajacua)† |
|---|---|---|---|---|---|
| Erythrocytes × 106/microliter (µL) | 5.08–8.86 | 4.73–10 | 4.56–10.5 | 4.8–9.7 | 4.89–9.58 |
| Packed cell volume (%) | 31.3–56.5 | 27.4–60 | 28.8–54.3 | 30.9–53.2 | 26–53 |
| Hemoglobin, gram per deciliter (g/dL) | 1.36–16.2 | 9.4–16.2 | 9.1–17.6 | 9.9–15.9 | 9.4–18 |
| Mean corpuscular volume, (dL) | 17.5–69.9 | 39.4–71.7 | 51.5–93.8 | 25.9–69.6 | 43.8–92 |
| Mean corpuscular hemoglobin, picogram (pg) | 16.3–23.3 | 10.2–23.9 | 15.7–28.1 | 14.2–22.3 | 16–23.4 |
| Mean corpuscular hemoglobin concentration, (g/dL) | 28.6–36.8 | 25.8–36.8 | 26.3–35.7 | 0.6–33.2 | 21.5–36.4 |
| Leukocytes/µL | 4500–1800 | 4.1–15,000 | 3500–17,800 | 5900–23,300 | 2900–22,600 |
| Neutrophils/µL | 2745–12060 | 614–10,300 | 714–12,100 | 2599–18,407 | 1800–15,100 |
| Band neutrophils/µL | 87–424 | 86–801 | 57–595 | 83 | 44–5420 |
| Lymphocytes/µL | 391–5610 | 224–7840 | 755–9870 | 1328–7520 | 414–8100 |
| Eosinophils/µL | 45–1761 | 23–1357 | 44–1176 | 59–352 | 33–528 |
| Monocytes/µL | 213–1980 | 47–1053 | 38–1780 | 112–1066 | 33–1668 |
| Basophils/µL | 67–200 | 0–340 | 11–540 | 54–264 | 44–225 |
| Platelets ×103/µL | 0.176–499 | 120–732 | 36–784 | 0.194–296 | 106–255 |
| Plasma protein (g/dL) | 5.7–9.5 | ND | ND | ND | ND |
| Fibrinogen, milligram per deciliter (mg/dL) | 200–500 | ND | 0–700 | 100–600 | 200–300 |
ND, No data.
Zoological Society of San Diego, San Diego Zoo Department of Pathology, Clinical Pathology Laboratory.
International Species Information System, August 2002.
For neurolepsis, the author's institution has used a protocol of diazepam and amitriptyline (0.5 mg/kg each, orally [PO], twice daily [BID]) to help reduce excitability during shipment or other relocation events. Dosages may be adjusted upward or downward based on the individual responses. Azaperone (1–2 mg/kg, IM) has been used to facilitate animal introductions.
Anesthesia and Surgery
General anesthesia is indicated for prolonged or invasive procedures. The most common conditions that require surgical intervention in nondomestic suids and peccaries are trauma and dystocias. These are managed with standard veterinary techniques.
Intubation may be difficult in suids. Laryngeal access is problematic because of their inability to open their mouths widely, their long rostrum, and the narrow oropharyngeal space.33, 48 In addition, a straight path from the epiglottis to the trachea does not exist because of the laryngeal anatomy (Figure 58-3 ).33, 48 A long laryngoscope blade and stylet facilitate intubation. If a stylet is used, precautions need to be taken so to avoid trauma to the larynx. Once within the larynx, the endotracheal tube should be rotated 180 degrees to reach the tracheal opening. Threading an endoscope through the endotracheal tube may facilitate passage into the trachea. Laryngospasm may also complicate intubation. The use of benzodiazepines has been noted to significantly reduce jaw tone, which may facilitate easier intubation. In peccaries, however, intubation is generally not difficult, as the mouth can be opened widely to gain adequate access to the larynx and a more direct path exists from the epiglottis into the trachea. Intubation may be done in lateral, sternal, or dorsal recumbency, but sternal recumbency is generally recommended.
FIGURE 58-3.
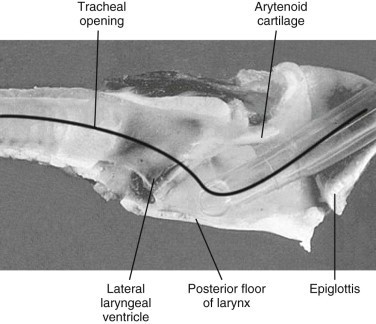
Laryngeal anatomy of a domestic pig.
(From Thurmon, JC, Tranquilli WJ, Benson GJ: Lumb and Jones veterinary anesthesia, 3rd ed. Baltimore, MD, 1996, Williams & Wilkins.)
Anesthesia monitoring is similar as for other ungulates. Inhalant or injectable agents may be used to maintain anesthesia. If venous access is available, propofol is an option for the use of injectable anesthetics.
Diagnostics
Anesthesia is generally required for most diagnostic procedures. Standard techniques used in domestic swine and other ungulates may be applied in wild suids and peccaries. Similar to domestic swine venipuncture may be difficult in exotic species. Vascular access sites include the saphenous vein, femoral vein, cephalic vein, coccygeal vein, and aural vein, as well as the cranial vena cava, which is used in the traditional technique. The cranial vena cava is, however, not routinely used at the author's institution. A technique for jugular venipuncture has been described.33 A needle greater than 1.5 inches in length is inserted cranially and slightly medially into jugular furrow. Ultrasonography may facilitate localization of jugular veins, which are buried within the musculature of the neck. The saphenous vein in peccaries is prominent as it courses over the cranial aspect of the proximal tibia and is easily accessed.25 For low-volume infusions, the aural vein, if present, may be catheterized. Catheters may also be placed in the cephalic and saphenous veins; however, a blind stick may be necessary, as the veins are not always visible. Hematology and serum biochemistry data for selected species are summarized in Tables 58-5 and 58-6 .
TABLE 58-5.
Reference Ranges for Serum Biochemical Parameters for Selected Species
| Parameter | Red River Hog (Potamochoerus porcus)* | S. African Warthog (Phacochoerus africanus)† | Babirusa (Babyrousa babyrussa)† | Visayan Warty Pig (Sus celebrifons)* | Collared Peccary (Tayassu tajacua)† |
|---|---|---|---|---|---|
| Total protein (g/dL) | 5.2–9 | 5.2–7.0 | 6.0–9.1 | 4.7–7.2 | 5.8–8.9 |
| Albumin (g/dL) | 2–5 | 3.3–4.1 | 3.3–5.7 | 4–5.7 | 3.1–4.7 |
| Calcium (mg/dL) | 7.5–10.60 | 9.0–14.4 | 8.8–11.9 | 7.6–10.2 | 8.0–12.2 |
| Phosphorus (mg/dL) | 3.1–8 | 3.4–11.8 | 3.2–8.3 | 3.5–8.4 | 3.5–8.3 |
| Sodium (mEq/L) | 130–150 | 131–154 | 134–154 | 134–144 | 134–160 |
| Potassium (mEq/L) | 2.7–6.0 | 3.4–6.9 | 3.5–5.5 | 3.2–4.9 | 3.5–5.5 |
| Chloride (mEq/L) | 92–115 | 90–110 | 94–112 | 99–109 | 93–115 |
| Creatinine (mg/dL) | 0.5–1.6 | 1.2–3.9 | 0.7–2.1 | 0.9–1.6 | 1.0–2.3 |
| Urea nitrogen (mg/dL) | 8.5–22.9 | 5–8 | 6–31 | 8–22.6 | 11–23 |
| Cholesterol (mg/dL) | 57–151 | 48–340 | 25–136 | 16–89 | 53–166 |
| Glucose (mg/dL) | 50–171 | 39–250 | 24–192 | 63–154 | 76–258 |
| Total carbon dioxide (mmol/L) | 8–40 | 18–36 | 13–45 | 21–41 | 8–40 |
| ALP (IU/L) | 2.2–186 | 11–1283 | 12–209 | 20–299 | 7–293 |
| ALT (IU/L) | 23–349 | 12–231 | 19–64 | 41–189 | 11–104 |
| AST (IU/L) | 6–296 | 7–156 | 4–60 | 7–76 | 18–82 |
| GGT (IU/L) | 7–123 | 25–145 | 39–137 | 30–65 | 0–21 |
| Amylase (U/L) | 9.2–3052 | 23–1898 | 60–914 | 778–1994 | 25–158 |
| CPK (IU/L) | 179–1344 | 227–3356 | 127–656 | 251–1893 | 99–2447 |
| Uric acid (mg/dL) | 0.1–3.2 | 0–0.3 | 0–1.3 | 0.1–1.4 | 0.1–0.4 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; g/dL, gram per deciliter; GGT, gamma glutamyltransferase; IU/L, international unit per liter; mEq/L, milliequivalent per liter; mg/dL, milligram per deciliter; mmol/L, millimole per liter.
Zoological Society of San Diego, San Diego Zoo Department of Pathology, Clinical Pathology Laboratory.
International Species Information System, August 2002.
TABLE 58-6.
Common Immobilization Protocols Used in Nondomestic Suids and Peccaries33
| Drug Combination | Dose (mg/kg) | Species Documented | Comments | References |
|---|---|---|---|---|
| Ketamine (K) | 20 (K) | Collared peccaries | Not recommended as first choice for immobilization Recoveries may be prolonged and violent Fatality associated with ambient temperature |
16 |
| Ketamine (K)/tiletamine–zolazepam (TZ)/medetomidine (M) | 3.9(K)/0.63(TZ)/0.03(M) | Chacoan peccaries | Prolonged recoveries despite medetomidine reversal with atipamezole, residual ataxia | 45 |
| Tiletamine–zolazepam (TZ) |
2–5 (TZ) | Multiple species | Smooth induction, poor muscle relaxation, prolonged recoveries might be rough Duration of recovery is dose dependent |
1, 5 |
| 2.18 (TZ) | Chacoan peccaries | Prolonged recoveries, poor relaxation | ||
| Tiletamine–zolazepam (TZ)/xylazine (X) |
2.35 (TZ)/2.35 (X) | Collared peccaries | Prolonged recoveries, but study did not antagonize xylazine Fatality associated with double dose |
13 |
| 1.23 (TZ)/1.23 (X) | White-lipped peccaries | Dose of 1.51 TZ and 1.51 X not successful in collared peccaries | 39 | |
| 1.2–2.1(X)/1.8–3.3 (TZ) | Babirusa | (X) administered as premedicant, followed by TZ 20 min later Antagonism with 0.14 mg/kg yohimbine and 1 mg flumazenil for every 20 mg zolazepam Bradycardia seen in some cases |
19 | |
| 3 (TZ)/0.5 (X) | Warthogs | Recoveries > 90 min | 42 | |
| 3.3 (TZ)/1.6 (X) | Feral pigs | 46 | ||
| Tiletamine–zolazepam (TZ)/romifidine (R) | 3–6 (TZ)/0.1 (R) | Wild pigs | — | 40 |
| Tiletamine–zolazepam (TZ)/butorphanol (B) |
1.46 (TZ)/0.14 (B) | White-lipped peccaries | Similar doses ineffective for collared peccaries | 39 |
| 1.26 (TZ)/0.36 (B) | Babirusa | Reverse with naltrexone, poor overall relaxation | 34 | |
| Medetomidine (M)/butorphanol (B)/midazolam (Mz) | 0.04–0.07 (M)/0.15–0.3 (B)/0.08–0.3 (Mz) | Multiple species | Bradycardia, hypoxemia reported Antagonize with atipamezole, naloxone or naltrexone, and flumazenil Lower dose range used in calm, captive individuals |
28 |
| Detomidine (D)/butorphanol (B)/midazolam (Mz) | 0.12 (D)/0.3 (B)/0.3 (Mz) | — | — | 29 |
| Xylazine (X)/butorphanol (B)/midazolam (Mz) | 2–3 (X)/0.3–0.4 (B)/0.3–0.4 (Mz) | — | — | 29 |
| Detomidine (D)/butorphanol (B)/tiletamine–zolazepam (TZ) | 0.12 (D)/0.3 (B)/0.6 (TZ) | — | — | 29 |
Diseases
Infectious Disease
Nondomestic suids and peccaries are susceptible to many of the same diseases as domestic swine and ungulates. These include leptospirosis, pasteurellosis, rabies, salmonellosis, and tuberculosis. Diagnosis and treatment are similar as for other species. A thorough review of infectious and parasitic diseases was previously compiled and is presented in Tables 58-7 and 58-8 .28
TABLE 58-7.
| Disease | Etiology | Epizootiology | Signs | Diagnosis | Management |
|---|---|---|---|---|---|
| African swine fever | Asfivirus (Asfarviridae) | Wild boar, feral swine Giant forest hog, bushpig, warthog have inapparent infections Peccaries are not susceptible |
Acute form: inappetence, fever, leukopenia, hemorrhagic syndrome Chronic form: respiratory signs, abortion |
CF, ELISA, TEM, immunoperoxidase staining, DNA-hybridization Most useful is direct immunofluorescence and hemagglutination |
Vaccines are experimental Test and slaughter is the accepted management method Avoid swill (garbage) feeding any swine |
| Classical swine fever (hog cholera) | Pestivirus (Togaviridae) | All swine species affected Peccaries are mildly affected |
Fever, rapid death, hemorrhagic syndrome in highly susceptible species Infertility, abortions mummified fetuses with mild viral strains |
During outbreaks, direct FA for viral antigen and using conserved epitopes on frozen pharyngeal tonsil is the method of choice Virus isolation, ELISA, virus neutralization are available |
Vaccination limits the use of diagnostics Vaccines are available for domestic swine C strain vaccine in widest use for domestic swine Test and slaughter of affected animals is widely practiced for control of outbreaks Avoid swill feeding any swine |
| Canine distemper virus | Morbillivirus Paramyxoviridae | Collared peccaries | Encephalitis | Serum neutralization | Thought to be enzootic in free-ranging collared peccaries in southern Arizona |
| Encephalomyocarditis | Cardiovirus (Picornaviridae) | Wild boar and domestic swine affected Unknown effects in other swine species Rats and mice thought to be primary reservoirs |
Reproductive failure, high preweaning mortality, fever, malaise, dyspnea, inappetence, syncope, rapid death | Histopathology of heart lesions is suggestive Virus isolation from heart muscle of young/newborn swine Antibody titers are most useful from fetal serum |
Inactivated vaccine is available for domestic swine use in the United States Active vermin control program in endemic areas |
| Foot-and-mouth disease (FMD) | Aphthovirus (Picornaviridae) | Feral pig, wild boar, giant forest hog, bushpig, warthog, babirusa affected Pecarries mildly affected Highly contagious in aerosols, fomites direct contact |
Vesicles on lips, oral cavity, coronary bands, soreness, inappetence, malaise | Virus isolation CF, antigen capture, animal inoculation, from vesicles; serology for precipitating antibodies, neutralizing antibodies Vaccinates may be differentiated from wild type infections with ELISA by using baculovirus-expressed FMD nonstructural proteins 3D, 2C |
Test and slaughter is currently or historically used to eradicate the disease Inactivated vaccine available |
| Pseudorabies | Alphaherpesvirus (Herpesviridae) | Feral swine, wild boar, giant forest hog, bushpig, warthog, peccary susceptible | Peripheral neuritis, pruritus, encephalitis, convulsions, especially in young animals Older animals generally show respiratory signs often mistaken for influenza Causes abortion in sows Signs are species and viral strain dependent, varying from inapparent infection to high mortality |
Direct FA detection in tonsillar tissue or virus isolation of brain, spleen, or lung are methods of choice Serodiagnosis by serum neutralization, latex agglutination, ELISA, CF, immunodiffusion, indirect immunofluorescence have all been used but have limited value in diagnosis |
Modified-live, inactivated, and gene-modified vaccines are available and are effective in domestic swine |
| Rinderpest | Morbillivirus (Paramyxoviridae) | All swine and peccaries are susceptible Asian swine (Sus scrofa) are especially sensitive, followed by African species European species (Sus scrofa) are more resistant Some swine species have subclinical infections Peccaries are also susceptible to canine distemper virus |
Enteritis, fever, malaise, vomiting, epistaxis, oral, perineal vesicles, hemorrhagic diarrhea, in severe cases Inapparent signs, abortion, mild dermatitis, malaise in milder cases |
Electron microscopy, immunofluorescence staining of tissues, immunoperoxidase staining of tissues, PCR, ELISA, agar-gel immunodiffusion, counterimmunoelectrophoresis, passive hemagglutination | Vaccination of endemic nidus areas with perimeter serosurveillance zones is recommended in the Global Rinderpest Eradication Program (GREP) |
| Swine vesicular disease | Enterovirus (Picornaviridae) | Feral swine and wild boar are affected No data on African swine or peccaries Virus is long-lived in the environment |
Oral and foot vesicles indistinguishable from FMD | Virus isolation, test animal inoculation, ELISA, serum neutralization, antigen precipitation, and CF are available | Prevention is primarily through banning of swill feeding |
| Swine influenza | Type A influenza virus (Orthomyx oviridae) | Feral swine and wild boar affected No data on African swine or peccaries |
Pneumonia, conjunctivitis, rhinitis, inappetence, weight loss High morbidity, low mortality in domestic swine |
Virus isolation or viral antigen (serum ELISA), immunofluorescent staining of lung or nasal preps, PCR, enzyme immunoassay membrane test are applicable | Good biosecurity practices offer the best prevention |
| Tranmissible gastroenteritis (TGE) | Coronavirus (Coronaviridae) | Feral swine, wild boar are affected No data on other swine or peccaries |
Vomiting, diarrhea, weight loss, dehydration High morbidity and mortality in very young swine Coronavirus was seen in EM of diarrhea samples from red river hog piglets (Potamochoerus porcus) |
Most common method is immunofluorescent staining or immunoperoxidase staining of intestinal mucosal scrapings, ELISA of cell culture media inoculated with gut samples from affected animals; fecal nucleic acid hybridization probe detection, TEM, virus isolation | Vaccination with modified-live and inactivated vaccines in young swine has been used to help prevent TGE in domestic swine |
| Vesicular exanthema | Calicivirus (Caliciviridae) | Feral swine, wild boar, and peccaries reported susceptible No data for African swine species Typically virus is spread through swill feeding in domestic species |
Oral and foot vesicles indistinguishable from FMD | Virus isolation, serum neutralization | Avoid swill feeding Practice good biosecurity with new additions |
| Vesicular stomatitis | Vesiculovirus (Rhabdoviridae) | Feral swine, wild boar, and peccaries susceptible Transmission by sandflies (Lutzomyia shanoni) and black flies also (Simulium sp.) is reported as the primary route of transmission |
Oral and foot vesicles indistinguishable from FMD | Virus isolation, serum neutralization, ELISA CF; ELISA on epithelial and vesicular fluid or tissue samples | Vaccine is generally not used in domestic swine Avoidance of infected domestic and wild animals is reported as the default preventive measure |
| Brucellosis | Brucella suis | Feral swine and wild boar affected Biovar 1 most significant Biovars 2 and 3 are reported as significant regionally Spread is mainly venereal |
Abortion, infertility, posterior paralysis, lameness, undulating pyrexia | Lymph node culture is best diagnostic Serology (plate agglutination) to detect antibodies is most practical |
For domestic swine, whole herd slaughter and replacement with SPF animals has been the most successful practice Testing and removing affected animals from herds has been the least effective strategy |
| Colibacillosis | Escherichia coli | Associated with severe diarrhea/death in red river hog piglets Reported in feral swine, wild boar, African swine, peccaries |
Enterotoxemia, diarrhea, dehydration, acidosis, metabolic crisis, death | Culture of feces and characterization of colonies for enterotoxigenic properties Sensitivity to antimicrobials aids in treatment |
Fluids, antibacterials, supportive therapy for affected cases Colostral E.coli vaccination of dams appears to be protective |
| Erysipelas | Erysipelothrix rhusiopathiae | Feral swine, wild boar, African swine species affected No data for peccaries Many wild animals are reservoirs |
Septicemia, fever, malaise, diamond skin lesions Acute, subacute, and chronic forms are described in domestic swine |
Culture of blood, joint fluids, and other body fluids | Penicillin is historic drug of choice Tetracycline, chlortetracycline, lincomycin, and tylosin are effective Hyperimmune serum treatment Prevention with attenuated bacterial vaccines and bacterins are available Concomitant attenuated vaccine with antibiotic therapy is not recommended |
CF, Complement fixation; DNA, deoxyribonucleic acid; ELISA, enzyme-linked immunosorbent assay; FA, fluorescent antibody; PCR, polymerase chain reaction; TEM, transmission electron microsocpy.
Data modified from Morris and Shima.28
TABLE 58-8.
| Location in Host |
|||||
|---|---|---|---|---|---|
| Disease | Etiology | Adult | Immature | Diagnosis | Management |
| Nasal bots | Rhinoestrus sp. | Nasopharynx | Nasopharynx Life cycle is known only for the horse |
Morphology of bots and larvae | Organophosphates or macrocyclic lactones reported to be effective |
| Sarcoptic mange |
Sarcoptes scabei Transmission is via direct contact of affected skin, and indirect contact with mites in environment |
Skin | Skin | Microscopic inspection of skin scrapings | Biosecurity protection of large herds from exposure is invaluable Treatment of individual cases with avermectins at weekly intervals will resolve active cases Ivermectin at 500 µg/kg, PO, q7d, resolved cases of sarcoptic mange in red river hogs over a 6-month period |
| Sucking lice |
Hematopinus suis Sucking louse of feral and domestic swine Pecaroecus javalii Sucking louse of peccaries Direct life cycle May cause decreased activity, retarded development, and hematologic changes in young swine |
Skin | Skin | Morphology | Topical acaricides Doramectin (pour-on) is reported effective against many parasites in domestic swine |
| Biting lice | Pecaroecus javalii (collared peccary) | Skin | Skin | ||
| Ticks |
Amblyomma inoratum, Dermacentor halli, Haemaphysalis leporispalustris (peccaries) Ixodes scapularis, Amblyomma americanum, cajennense, maculatum; Dermacentor albipictus, D. andersoni, D. varibilis Ornithodoros coriaceus, truicata, puertoricensis, tahale, dugesi are potential vectors of African swine fever Ornithodoros moubata is the primary vector of African swine fever in Africa |
Skin, multifocal dermatitis | Skin | Morphology | Destruction of animal bedding or nesting materials where adults breed, topical insecticides, systemic or topical avermectins |
| Ascarids | Ascaris suum | Small intestines; usually no disease results | Direct life cycle Hepatotracheal larval migration |
Egg or adult morphology | Elimination of adults through anthelminthic treatment of new acquisitions in quarantine (FBZ, AVE, PIP, PRT, LVM, DCV, TBZ) Periodic anthelminthic treatment of infested herds |
| Strongyloidiasis (threadworm) | Strongyloides ransomi | Small intestine, producing diarrhea, dehydration |
Eggs hatch into infective larvae and/or free-living adults that mate, thereby giving rise to more infective larvae Larvae mature into adults in small intestine Percutaneous larval and transcolostral infestation occur |
Egg or adult morphology | Anthelminthic treatment (LVM, TBZ, AVE) |
| Nodular worms | Oesophagostomum sp. | Cecum or colon, producing parasitic mucosal nodules | Eggs hatch in the environment, developing to infective, ensheathed L3, which may survive in the environment for up to 12 months | Egg or adult morphology, parasitic nodules in cecum, colon, rectum | Anthelminthic treatment (FBZ, AVE, DCV, LVM, PRT, PIP), housing on fallow substrate |
| Lungworms | Metastrongylus sp. | Lungs; adults and eggs found in the bronchioles mainly of diaphragmatic lobes | Earthworms are intermediate hosts for L1-L3 | Egg or adult morphology, verminous pneumonia in severe cases | Anthelminthic treatment (FBZ, AVE, LVM) |
| Trichinosis | Trichinella sp. | Larval cysts within skeletal muscle, adults in small intestine | Ingestion of infested meat containing encysted larvae | Adult and larval morphology | Public health significance Avoidance of uncooked skeletal muscle Trichinosis is a cosmopolitan concern |
| Stomach worms | Hyostrongylus sp. | Stomach, usually incidental Heavy infestation may result in gastritis |
Stomach | Egg or adult morphology | Anthelminthic treatment (DCV, FBZ, TBZ, PRT, AVE) |
| Thick stomach worm | Ascarops sp. Physocephalus sp. | Stomach | Dung beetles are intermediate hosts | Egg or adult morphology | Anthelminthic treatment (FBZ, DCV) |
| Thorny-headed worm | Macrocanthorhynchus hirudinaceus | Ileum, producing parasitic mucosal nodules, but rarely results in disease Mucosal perforation and peritonitis occasionally results |
Intermediate hosts are beetle grubs Infestation via ingestion of grubs carrying larval stages |
Egg or adult morphology | |
| Kidney worm | Stephanurus dentatus | Kidneys primarily, also perirenal fat, ureters, lumbar muscles, spinal cord, lungs | Eggs passed in urine primarily in the morning Eggs hatch and develop in environment to L3 Infestation occurs via ingestion of larvae, earthworms harboring infective larvae, or by percutaneous penetration by larvae |
Egg or adult morphology | Avoidance of infective larvae is essential Affected animals are housed on concrete that is cleaned regularly to remove voided eggs. PRT, FBZ effective |
| Cysticercosis | Echinococcus granulosus | Intestines (canids) | Multiple organ cysts | Morphology of cysticerci | Albendazole and/or praziquantel may be of some benefit |
| Taenia acinonyxi | Intestines (cheetah, leopard) | African swine are intermediate hosts | |||
| Taenia multiceps | Intestines (canids) | Sus scrofa is intermediate host | |||
| Taenia regis | Intestines (lion, leopard) | Warthogs, bushpigs are intermediate hosts | |||
| Taenia solium | Intestines (human) | Sus scrofa is an intermediate host | |||
| Trematodiasis | Fascioloides magna | Liver Variable lesions from mild fibrosis of migratory tracts to severe cirrhosis |
Snails | The diagnosis typically is made at necropsy | Treatment of larvae with rafoxanide or triclabendazole Treatment of adults with oxyclozanide or triclabendazole |
| Lancet fluke | Dicroceolium dendriticum | Wild hogs Causes liver damage, cirrhosis, cholangitis, regional cholestasis, and anemia |
Two intermediate hosts Snails eat eggs; cercariae develop in the lungs Expelled cercariae eaten by ants and develop into metacercariae Ants ingested by definitive host, adults develop in the liver |
Morphology of eggs or adults | Praziquantel and benzimidazoles may be helpful to treat liver flukes |
AVE, Avermectins; DCV, dicloros; FBZ, fenbendazole; LVM, levamisole; PIP, piperazine; PRT, pyrantel; TBZ, thiabendazole.
Data from Morris and Shima.28
Necrotizing enteritis caused by Clostridium perfringens was reported in collared peccaries and white-lipped peccaries from a facility in Brazil.6 Lethargy and inappetence were followed by death within 24 hours in seven animals. Crowded housing conditions were thought to have played a role in the disease outbreak.
Feral swine have been a concern for the domestic swine industry because of the risk of disease transmission.36, 37, 51 Similarly, local feral swine may also act as a source of disease for exotic suids located in rural areas. Brucellosis was diagnosed in a group of red river hogs at a facility in Florida. Through testing of serum banked from the originating institution and genotyping of the Brucella isolate, it was determined that local feral swine were the likely source of the infection (Janssen, personal communication, 2012).
Postweaning multisystemic wasting syndrome (PMWS) emerged in the domestic swine industry in the mid-1970s, and porcine circovirus 2 (PCV2) was discovered to be associated with the syndrome.50 An illness in a 10-month old red river hog in a facility in England fitted the criteria for PMWS. The hog died following a course of profuse diarrhea and weight loss. An enterophathogenic Escherichia coli and PCV2 were isolated from the hog. The source of the PCV2 was not identified.
In recent years, research has focused on influenza viruses. Domestic swine are of interest because of their ability for gene reassortment with the avian, human, and swine influenza viruses. They may also be infected by various influenza viruses and the risk exists for influenza viruses to be transmitted among exotic swine species, their caretakers, and the visiting public.
Neoplasia
Reports of neoplasia in exotic swine are sparse. Neoplasia is generally seen in older animals. The following neoplasms were reported at necropsy in exotic suids at the author's institution: intestinal lymphosarcoma and intestinal carcinoma in two Bornean bearded pigs, each 11 years of age; squamous cell carcinoma involving the prepuce and multiple carcinomas in warthogs aged 10 and 13 years, respectively; reproductive neoplasia in four wild boars, ages 12 to 17 years: (1) testicular malignant seminoma, (2) uterine leiomyosarcoma, (3) uterine adenocarcinoma, and (4) uterine sarcoma; pharyngeal squamous cell carcinoma in an 18-year-old babirusa; and carotid body tumor in a 10-year-old red river hog.
Dentistry
Dental problems are not uncommon in wild suids and peccaries: fractured or avulsed canines, periodontal disease, and dental abscesses are seen. Figure 58-4 shows a Visayan warty pig with a mandibular canine fractured at the gumline. An endodontic procedure consisting of a vital root canal was performed, and the canine continued to grow normally. A nylon screw was used to successfully cap a tooth defect in a babirusa.38 Sites of tusk avulsions generally granulate with wound care and antimicrobial therapy. Some animals may need periodic tusk trimming. Dental attrition and uneven wear are expected findings in aging suids and peccaries (Figure 58-5 ). Some species in the wild have been noted to only have canines and caudal molars.32 Floating dental points is performed, as needed. Periodontal disease is managed through regular prophylaxis, dental extractions, as needed, and antimicrobial therapy. Pulsatile low-dose doxycycline (0.3 mg/kg, PO, BID, for 1–3 months, rotating on and off therapy) has been used for adjunct management of periodontal disease in suids and tassysuids.4 Diets high in simple sugars may predispose these animals to periodontal disease. Routine dental care should be a part of suid and peccary preventive health care.
FIGURE 58-4.
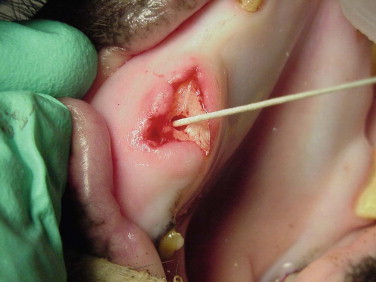
Fractured upper canine in a Visayan warty pig.
FIGURE 58-5.
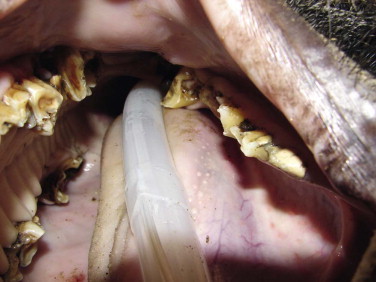
Dental attrition and wear in a wild boar.
Disorders of the Feet
Trauma, especially to the feet, is common in exotic swine and peccaries.41 The delicate nature of the suid and peccary hoofs compared with those of other ungulates makes them more susceptible to abrasive wear. Some animals may need periodic hoof trims, depending on the substrate used and activity levels (Figure 58-6 ). Excessive sole wear and hoof trauma may occur from digging, running, or pacing on hard or abrasive surfaces (Figure 58-7, A and B ). Any lame suid or peccary should be carefully monitored and examined if lameness does not resolve within a few days. If left untreated, sole erosions and hoof defects may progress to infection of soft tissues and osteomyelitis. Hoof acrylics may be used to repair defects once the tissue has become healthy (Figure 58-8 ). Vettec hoof care products (Vettec Hoof Care Products, Oxnard, CA, www.vettec.com) are commonly used at the author's institution. Hoof acrylics are also used prophylactically to create protective toe caps when the animals are likely to pace excessively (e.g., during relocations). These products have also been used on the bottoms of bandages to extend bandage wear. Behavioral conditioning to facilitate inspection of feet is helpful to monitor hoof conditions.
FIGURE 58-6.
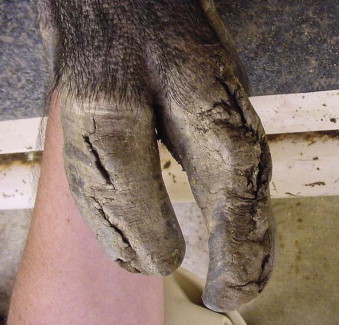
Overgrown hooves in a Bornean bearded pig.
FIGURE 58-7.
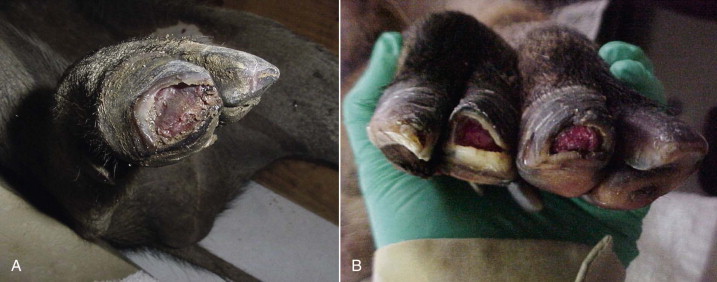
A, Toe tip trauma in a Visayan warty pig. B, Toe tip defects in a red river hog.
FIGURE 58-8.
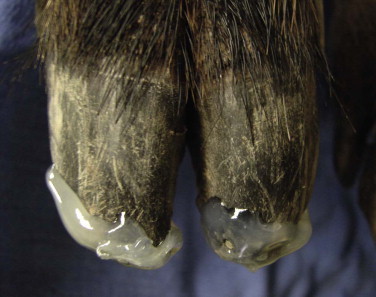
Toe tip defects covered with hoof acrylic.
Miscellaneous Conditions
Although pigs are quite hardy, they are still susceptible to hyperthermia, exertional myopathy, or both. Possible scenarios include stormy anesthetic induction or recovery, escape, excessive chasing by enclosure mates, and conspecific aggression. In addition, suids are prone to gastric ulceration. Prophylactic treatment with gastroprotectants is recommended when animals are stressed such as by relocation, hospitalization, anorexia, or social aggression.
A pheochromocytoma was found at necropsy in a 14-year-old male warthog that had been evaluated for epistaxis.9 No lesions were present in the nasal cavity. On the basis of blood pressure readings and epinephrine norepinephrine ratios, the epistaxis was presumed secondary to the pheochromocytoma.
Reproduction
Compared with other ungulate species, gestation is shorter in wild suids, and they are considered the only true multiparous group of the artiodactylids.35 Because of this, the young are smaller relative to the size of the dam. Males actively court females. Females develop vulvar swelling and urinate frequently when in estrus. The uterine horns of the warthog and babirusa are reported to be smaller than those in other suid species, and this is attributed to be the reason for their smaller litters.49 Peccary uterine horns are shorter than those of suids.14 The penis is curved in a sigmoid shape, and the ends have a corkscrew appearance, correlating with the shape of the cervix.49 A summary of reproductive parameters for select species are summarized in Table 58-9 .
TABLE 58-9.
| Parameter | Red River Hog (Potamochoerus Porcus) | Babirusa (Babyrousa Babyrussa) | Bearded Pig (Sus Barbatus) |
Common Warthog (Phacochoerus Africanus) | Collared Peccary (Tayassu Tajacu) | Chacoan Peccary (Catagonus Wagneri) |
|---|---|---|---|---|---|---|
| Karyotype (2n) | 34 | 38 | 36–40 | 34 | 30 | 20 |
| Age at sexual maturity | 3 years | In captivity as early as 5–10 months | 10–20 months | 18–20 months | 8–10 months | 1–2 years |
| Details of estrous cycle | Seasonal? | Polyestrous, may have two litters per year; urinary hormones. 28–42 day estrous cycle | Breed through year, two litters per year | Seasonal polyestrous; estrus lasts 72 hours approximately every 6 weeks | Polyestrous; 27–28 day estrous cycle; 1- to 5-day estrus (5.7 day mean) | Breed through year |
| Gestation | 127 days | 155–175 days (163 day mean) | 160 days | 160–170 days | 140–150 days (142 day mean) | ∼150 days |
| Litter size | 1–8 (usually 3–4) | 1–3 (usually 2) | 8–10 | 1–8 (usually 2–3) | 1–3 (usually 2) | 1–4 (usually 2) |
| Number of mammae | 3 pairs | 2 pairs | 6 pairs | 2 pairs | 2 pairs | 4 pairs |
| Weaning | — | 26–32 weeks | 5–6 weeks | 24–25 weeks | 6 weeks | — |
Data modified from Morris and Shima.28
Fecal hormones to characterize reproductive biology were evaluated in red river hogs, babirusas, and warthogs.3 Researchers found that estrus cycles could be monitored using 20 α-OH- and 20-oxo-pregnane assays. In contrast, estrogens and androgens were not useful in characterizing follicular activity during estrus. The duration of the estrus cycle was approximately 35 days for all three species in this study. During the second half of pregnancy, estrogens and 17-oxo-androstanes were elevated.
Use of ultrasonography to diagnose and monitor pregnancy in both awake and anesthetized babirusa has been reported.17 Uterine changes were noted at 28 to 32 days of gestation. Ventral midline transabdominal ultrasonography was superior to rectal ultrasonography in visualizing fetal structures. During weekly ultrasonographic examinations, fetuses were detected at 38 days of gestation. Cranial measurements, done weekly, revealed a linear growth pattern. The authors documented resorption of one of three fetuses in each of three pregnancies.
Suids also differ from other artiodactylids in that they show nesting behavior prior to parturition. They generally do not clean their young after birth. Except for the Babirusa sp., Phacochoerus sp., and Hylochoerus sp., piglets are born with horizontal stripes.32, 35, 49 Similar to other artiodactylids, placentation is epitheliochorial, so neonates are dependent on nursing for transfer of maternal immunity. At the author's institution, pregnant suids are vaccinated, where feasible, against colibacillosis. In captivity, promiscuous suckling has been observed in white-lipped peccaries but not in collared peccaries.49
Most newborn piglets are not able to fully thermoregulate, so protection from the elements and supplemental heat are needed, depending on weather circumstances. Infanticide and cannibalism of young have been reported in pigs and peccaries and are not uncommon in exotic swine species in zoos as well. Neonatal mortality comprises a large proportion of swine and peccary mortality at the author's institution. Trauma accounts for a majority of cases, followed by infectious etiologies. When the young start to sample solids, creep feeders may have to be provided to allow the young access to food without interference from adult animals.
Melengesterol acetate implants, gonadotropin-releasing hormone (GnRH) inhibitors, and medroxyprogesterone injections have been used in females for contraception. Information on the most current contraceptive recommendations for swine and peccaries may be found through the Association of Zoos and Aquariums' Wildlife Contraception Center.2
Acknowledgment
The author wishes to recognize Dr. Pat Morris for his valuable contributions to the field of exotic swine medicine. The author also thanks Dr. Pat Morris for his input into this chapter, as well as the San Diego Zoo Global library staff for their assistance with references.
References
- 1.Allen JL. Immobilization of Chacoan peccaries (Catagonus wagneri) with a tiletamine hydrochloride/zolazepam hydrochloride combination. J Wildl Dis. 1992;28:499–501. doi: 10.7589/0090-3558-28.3.499. [DOI] [PubMed] [Google Scholar]
- 2.Association of Zoos and Aquariums, Contraceptive Advisory Group http://www.stlzoo.org/animals/scienceresearch/contraceptioncenter/ Accessed 2/1/2013.
- 3.Berger EM, Leus K, Vercammen P. Fecal steroid metabolites for non-invasive assessment of reproduction in common warthogs (Phacochoerus africanus), red river hogs (Potamochoerus porcus) and babirusa (Babyrousa babyrussa) Anim Reprod Sci. 2005;91:155–171. doi: 10.1016/j.anireprosci.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Bicknese BJ, Fagan DA, Lamberski N. Proceedings of the American Association of Zoo Veterinarians. 2008. “Cyclic” regime of low-dose doxycycline to treat periodontal disease in a Chacoan peccary (Catagonus wagneri), red pandas (Ailurus fulgens), and bat-eared foxes (Otocyon megalotis megalotis) Los Angeles, CA. [Google Scholar]
- 5.Calle PP, Morris PJ. Anesthesia for nondomestic suids. In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine. ed 4. Saunders; Philadelphia, PA: 1999. [Google Scholar]
- 6.Carvalho MAG, Santos PS, Nogueira SSC. Necrotic enteritis in collared (Pecari tajacu) and white-lipped (Tayassu pecari) peccaries. J Zoo Wildl Med. 2011;42:732–734. doi: 10.1638/2010-0212.1. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri M, Carrasco P, Kalk P. Urinary oestrogen excretion during oestrus and pregnancy in the Babirusa. Int Zoo Yb. 1990;29:188–192. [Google Scholar]
- 8.Clauss M, Nijboer J, Loermans JH. Comparative digestion studies in wild suids at Rotterdam Zoo. Zoo Biol. 2008;27:305–319. doi: 10.1002/zoo.20191. [DOI] [PubMed] [Google Scholar]
- 9.Cole G, Suedmeyer WK, Johnson G. Pheochromocytoma in an African warthog (Phacochoerus aethiopicus) J Zoo Wildl Med. 2008;39:663–666. doi: 10.1638/2008-0009.1. [DOI] [PubMed] [Google Scholar]
- 10.Disney's Animal Kingdom www.animalenrichment.org Accessed 2/1/2013.
- 11.Durden LA. Lice. In: Samuel WM, Pybus MF, Kocan AA, editors. Parasitic diseases of wild mammals. ed 2. Iowa State University Press; Ames, IA: 2001. [Google Scholar]
- 12.Fowler ME. Husbandry and diseases of captive wild swine and peccaries. Rev Vet Tech Off Int Epizootiol. 1996;15:141–154. doi: 10.20506/rst.15.1.913. [DOI] [PubMed] [Google Scholar]
- 13.Gabor TM, Hellgren EC, Silvy JH. Immobilization of collared peccaries (Tayassu tajacu) and feral hogs (Sus scrofa) with Telazol® and xylazine. J Wildl Dis. 1997;33:161–164. doi: 10.7589/0090-3558-33.1.161. [DOI] [PubMed] [Google Scholar]
- 14.Gottdenker N, Bodmer R. Peccaries. In: Hutchins M, editor. ed 2. vol 16. Gale; Detroit, MI: 2004. (Grzimek's animal life encyclopedia). [Google Scholar]
- 15.Grubb P. Artiodactyla. In: Wilson DE, Reeder DM, editors. Mammal species of the world: A taxonomic and geographical reference. ed 3. The John Hopkins University Press; Baltimore, MD: 2005. [Google Scholar]
- 16.Hellgren EC, Lochmiller RL, Amoss MS. Endocrine and metabolic responses of the collared peccary (Tayassu tajacu) to immobilization with ketamine hydrochloride. J Wildl Dis. 1985;21:417–425. doi: 10.7589/0090-3558-21.4.417. [DOI] [PubMed] [Google Scholar]
- 17.Houston EW, Hagberg PK, Fisher MT. Monitoring pregnancy in babirusa (Babyrousa babyrussa) with transabdominal ultrasonography. J Zoo Wildl Med. 2001;32:366–372. doi: 10.1638/1042-7260(2001)032[0366:MPIBBB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.International Union of Conserving Nation Red List. http://www.iucnredlist.org Accessed 2/1/2013.
- 19.James SB, Cook RA, Raphael BL. Immobilization of babirusa (Babyrousa babyrussa) with xylazine and tiletamine/zolazepam and reversal with yohimbine and flumazenil. J Zoo Wildl Med. 1999;30:521–525. [PubMed] [Google Scholar]
- 20.Karesh WB, Uhart MM. Proceedings of the American Association of Zoo Veterinarians. 1998. Health evaluation of white lipped peccary populations in Bolivia. Omaha, NB. [Google Scholar]
- 21.Kemp Y. Growth and development in captive Bornean bearded pigs, Sus barbatus, at the San Diego Zoo. Zool Garden NF. 2000;70:73–92. [Google Scholar]
- 22.Leus K, Goodall GP, Macdonald AA. Anatomy and histology of the babirusa (Babyrousa babyrussa) stomach. C R Acad Sci Paris. 1999;233:102. doi: 10.1016/s0764-4469(99)00107-9. Ser III. [DOI] [PubMed] [Google Scholar]
- 23.Leus K, Macdonald AA, Goddall G. Light and scanning microscopy of the cardiac gland region of the stomach of the Babirusa (Babyrousa babyrussa—Suidae, Mammalia) C R Biologies. 2004;327:735–743. doi: 10.1016/j.crvi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Leus K, Macdonald AA. From babirusa (Babyrousa babyrussa) to domestic pig: The nutrition of swine. Proc Nutr Soc. 1997;56:1001–1012. doi: 10.1079/pns19970105. [DOI] [PubMed] [Google Scholar]
- 25.Lochmiller R, Hellgren E, Robinson R, Grant W. Techniques for collecting blood from collared peccaries, Dicotyles tajacu. J Wildl Dis. 1984;20:47–50. doi: 10.7589/0090-3558-20.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Mercado JA, Morris PJ. Proceedings of the American Association of Zoo Veterinarians. 2006. Comparative serum glucose levels in sedated Suidae and Tayassuidae. Los Angeles, CA. [Google Scholar]
- 27.Moon PF, Smith LJ. General anesthetic techniques in swine. Vet Clin North Am Food Anim Pract. 1996;12:663–691. doi: 10.1016/s0749-0720(15)30392-3. [DOI] [PubMed] [Google Scholar]
- 28.Morris P, Shima A. Suidae and Tayassuidae. In: Fowler M, Miller E, editors. Zoo and wildlife medicine. ed 5. Saunders; St. Louis, MO: 2003. [Google Scholar]
- 29.Morris PJ, Bicknese EL, Janssen DL. Proceedings of the American Association of Zoo Veterinarians. 1999. Chemical immobilization of exotic swine at the San Diego Zoo. Columbus, OH. [Google Scholar]
- 30.Nogueira SS, Silva MG, Dias CT. Social behaviour of collared peccaries (Pecari tajacu) under three space allowances. Anim Welfare. 2010;19:243–248. [Google Scholar]
- 31.Noon TH, Heffelfinger JR, Olding RJ. Serologic survey of antibodies to canine distemper virus in collared peccary (Tayassu tajacu) populations in Arizona. J Wild Dis. 2003;39:221–223. doi: 10.7589/0090-3558-39.1.221. [DOI] [PubMed] [Google Scholar]
- 32.Nowak R. ed 6. vol 2. Johns Hopkins University Press; Baltimore, MD: 1999. (Walker's mammals of the world). [Google Scholar]
- 33.Padilla LR, Ko JCH. Non-domestic suids. In: West G, Heard D, Caulkett N, editors. Zoo animal and wildlife immobilization and anesthesia. Blackwell Publishing; Ames, IA: 2007. [Google Scholar]
- 34.Padilla LR. Proceedings of the American Association of Zoo Veterinarians. 2004. Immobilization of babirusa (Babyrousa babyroussa) using a butorphanol-tiletamine-zolazepam combination. San Diego, CA. [Google Scholar]
- 35.Powell DM. Pigs. In: Hutchins M, editor. ed 2. vol 16. Gale; Detroit, MI: 2004. (Grzimek's animal life encyclopedia). [Google Scholar]
- 36.Roberts DC. Disease transfer from wild to domestic pigs. In: Leman AD, Straw BE, Mengeling WL, editors. Diseases of swine. ed 7. Iowa State University Press; Ames, IA: 1992. [Google Scholar]
- 37.Sandfoss MR, DePerno CS, Bets CW. A serosurvey for Brucella suis, classical swine fever, porcine circovirus type 2, and pseudorabies virus in feral swine (Sus scrofa) of Eastern North Carolina. J Wildl Dis. 2012;48:462–466. doi: 10.7589/0090-3558-48.2.462. [DOI] [PubMed] [Google Scholar]
- 38.Schaftenaar W. Treatment of a fractured tusk in a male babirusa (Babyrousa babyrussa) using a polyoxymethylene bolt. J Zoo Wildl Med. 1991;22(3):364–366. [Google Scholar]
- 39.Selmi AL, Mendes GM, Figueiredo JP. Chemical restraint of peccaries with tiletamine/zolazepam and xylazine or tiletamine/zolazepam and butorphanol. Vet Anesth Analg. 2003;30:24–29. doi: 10.1046/j.1467-2995.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 40.Siemon A, Siesner H, vin Hegel G. Die Verwendung von Tiletamine/Zolazepam/Romifidine zur Distansimmobilization von Wildschweinen. Tierarztl Prax. 1992;20:55–58. [PubMed] [Google Scholar]
- 41.Singleton C, Morris P. Proceedings of the American Association of Zoo Veterinarians. 2006. Survey of foot problems in a collection of captive exotic swine. Tampa, FL. [Google Scholar]
- 42.Sonntag S, Hackenbroich C, Boer M. Proceedings European Association of Zoo and Wildlife Veterinarians. 2004. Tiletamine-zolazepam-xylazine immobilization in warthogs (Phacochoerus aethiopicus) Ebeltoft, Denmark. [Google Scholar]
- 43.Sowls LK. ed 2. University of Arizona Press; Tucson, AZ: 1997. Javelinas and other peccaries: Their biology, management, and use. [Google Scholar]
- 44.Straw BE, Allaire SD, Mengeling WL, Taylor DJ, editors. Diseases of wild swine. ed 8. Iowa State University Press; Ames, IA: 1999. [Google Scholar]
- 45.Sutherland-Smith M, Campos JM, Cramer C. Immobilization of Chacoan peccaries (Catagonus wagneri) using medetomidine, Telazol®, and ketamine. J Wildl Dis. 2004;40:731–736. doi: 10.7589/0090-3558-40.4.731. [DOI] [PubMed] [Google Scholar]
- 46.Sweitzer RA, Ghneim GS, Garnder AI. Immobilization and physiological parameters associated with chemical restraint of wild pigs with Telazol® and xylazine hydrochloride. J Wildl Dis. 1997;33:198–205. doi: 10.7589/0090-3558-33.2.198. [DOI] [PubMed] [Google Scholar]
- 47.The shape of enrichment. www.enrichment.org Accessed 2/1/2013.
- 48.Thurmon JC, Tranquilli WJ, Benson GJ. ed 3. Williams & Wilkins; Baltimore, MD: 1996. Lumb and Jones veterinary anesthesia. [Google Scholar]
- 49.Wilson DE, Mittermeir RA, editors. vol 2. Lynx Edicions; Barcelona, Spain: 2011. (Handbook of mammals of the world). [Google Scholar]
- 50.Woodger NGA, Hosegood OM. PMWS associated with diarrhoea and ill thrift in a captive red river hog (Potamochoerus porcus) Vet Rec. 2011;168:512. doi: 10.1136/vr.c7303. [DOI] [PubMed] [Google Scholar]
- 51.Wu N, Abril C, Thomann A. Risk factors for contacts between wild boar and outdoor pigs in Switzerland and investigations on potential Brucella suis spill-over. BMC Vet Res. 2012;8:1–12. doi: 10.1186/1746-6148-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]


