Abstract
Aging is a universal and time-dependent biological decline associated with a progressive deterioration of cells, tissues, and organs. Age-related decay can eventually lead to pathologies such as cardiovascular and neurodegenerative diseases, cancer, and diabetes. A prominent molecular process underlying aging is the progressive shortening of telomeres, the structures that protect the ends of chromosomes, culminating in cellular senescence. Noncoding (nc)RNAs are emerging as major regulators of telomere length homeostasis. In this review, we describe the impact of ncRNAs on telomere function and discuss their implications in senescence and age-related diseases. We discuss emerging therapeutic strategies targeting telomere-regulatory ncRNAs in aging pathologies.
Keywords: Long noncoding RNAs, TERC, TERT, microRNA, telomerase activity, genomic instability
Noncoding RNAs In Telomere Dynamics
Only ~2% of the mammalian genome is transcribed into protein-coding RNAs, and the remaining ~98% was long considered to be inactive material (“junk DNA”). However, with the arrival of new technologies and analytical tools, noncoding (nc)RNAs have emerged as rich, abundant, and potent regulators of gene expression programs in cellular processes like proliferation, apoptosis, differentiation, and senescence [1–3]. NcRNAs broadly include all RNAs from which there is no evidence of protein translation. The functions of regulatory RNAs become particularly relevant in times of cellular stress when transcription and translation are globally suppressed or restricted to certain mRNA subsets. In addition, they offer therapeutic opportunities that cannot be achieved by targeting coding RNAs (mRNAs).
The large and heterogeneous class of ncRNAs can be divided into two main groups according to their size: long ncRNAs, typically spanning several hundred nucleotides, and short RNAs, generally in the range of 20–30 nucleotides [4]. Among a broad range of mechanisms of gene regulation, some ncRNAs have been shown to regulate transcription by interacting with chromatin-modifying complexes or by interfering with or enhancing the transcriptional machinery [5–7]. Other ncRNAs modulate key post-transcriptional steps by influencing mRNA processing, transport, storage, degradation, and translation. Many ncRNAs have been annotated thus far, but our understanding of their roles in gene regulation is in its infancy.
Telomeres are protective structures at the end of linear chromosomes comprising repeats of the tandem sequence TTAGGG and the shelterin complex (TRF1, TRF2, POT1, RAP1, TIN2, TPP1). Telomeres prevent DNA damage and end-to-end fusions of chromosomes (Figure 1) and shorten progressively with each cell division due to a defect in the DNA replicative mechanism known as the ‘end-replication problem’. Upon removal of the last RNA primer at the 3′ end of the lagging strand, the newly synthesized strand will be a few nucleotides shorter, resulting in telomere shortening. The progressive loss of telomeres causes exposure of the DNA ends, eventually triggering a DNA Damage Response (DDR) that culminates with chromosomal instability and the formation of aberrant chromosome end-to-end fusions [8, 9].
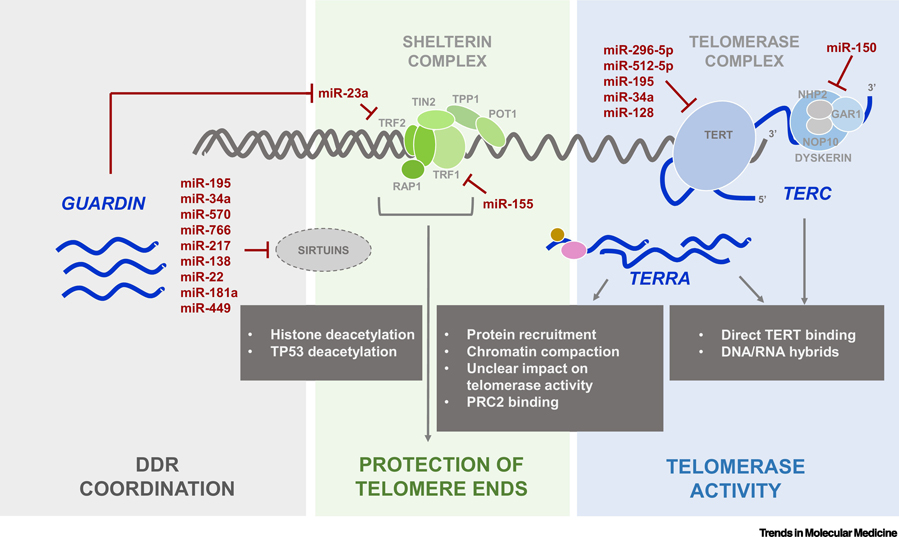
Figure 1.
Schematic indicates the major proteins that protect telomeric integrity (Shelterin complex, green) and length (Telomerase complex, blue), as well as the lncRNAs GUARDIN, TERRA and TERC (blue font) and microRNAs (red font) that modulate telomere homeostasis by integrating the DNA Damage Response (DDR, grey), protecting telomere ends, and controlling telomerase activity.
Noncoding (nc)RNAs are emerging as major regulators of telomere length homeostasis. Here, we describe the impact of ncRNAs on the telomere biology and in particular their possible implications in senescence and age-related pathologies associated with telomere dysfunctions (Figure 1). Specifically, our discussion focuses on two types of regulatory RNAs, microRNAs (miRNAs) and long noncoding RNAs (lncRNAs).
Telomeres in Senescence and Age-related Diseases
The enzyme telomerase counteracts telomere shortening by replenishing telomeric repeats. In this manner, telomerase protects the genetic information from being truncated progressively during cell division [8–10]. However, most somatic cells have no detectable telomerase and undergo telomere shortening, which eventually leads to loss of telomere capping and triggers DDR. In cells with functional checkpoints, the DDR leads to increased levels of the transcription factor TP53 (p53), which is involved in DNA repair, cell cycle arrest, and apoptosis. TP53 transcriptionally induces the production of p21 (CDKN1A), a cyclin-dependent kinase (CDK) inhibitor that can halt cell division [9, 10]. Additionally, telomere dysfunction may also increase the levels of the CDK inhibitor p16 (CDKN2A) which further suppresses cell growth. Both p21 and p16 enable the function of the retinoblastoma protein (RB) and reinforce the cell cycle arrest [11, 12]. Therefore, in cells with functional cell cycle regulators, critical telomere shortening is associated with reduced proliferative capacity. Cells then enter a state of indefinite growth arrest and become senescent, although they remain metabolically active.
By contrast, impairment of checkpoints and tumor suppressor pathways causes cells to continue dividing and escape from replicative senescence. Fully unprotected chromosome ends can form end-to-end fusions leading to genomic instability and a state of “crisis” associated with massive cell death. Given that the reorganization of the genome allows the generation of pre-neoplastic cells [8–10], most tumor cells have devised mechanisms to maintain telomeres of a certain minimal size by reactivating the telomerase reverse transcriptase, thus expanding their replicative potential. The enzyme telomerase is composed of two core components, the telomerase reverse transcriptase (TERT) and the telomerase RNA (TERC, TElomerase RNA Component, also known as hTR), which serves as the template for TERT in the addition of telomeric repeats. The active telomerase complex requires other ancillary components, including the binding partners dyskerin (DKC1), NOP10, NHP2, GAR1, and Telomerase Cajal body protein 1 (TCAB1) [13]. In a small percentage of telomerase-negative immortalized cells and cancer cells, alternative lengthening of telomere (ALT) is achieved through homologous recombination-dependent synthesis of new telomeric DNA [9].
Senescent cells have been detected in multiple age-related diseases and are associated with loss of tissue function during the aging process. Besides cell cycle arrest, senescent cells undergo changes in morphology, gene expression patterns, and metabolic programs. Importantly, senescent cells display a distinct trait known as the senescence-associated secretory phenotype (SASP), whereby they secrete high levels of pro-inflammatory cytokines capable of altering the microenvironment, triggering immune surveillance, enhancing angiogenesis, and remodeling the extracellular matrix, with a range of beneficial and deleterious effects on different organs [14–17]. The causes, consequences, and phenotypes of cell senescence have been reviewed in detail [17–20].
Cellular senescence may also occur independently of telomere shortening; for example, DNA damage, oxidative injury, and oncogenic signaling are able to induce cellular senescence. Stress- and oncogene-induced senescence may also incur acute damage at the telomeres and trigger DDR signaling, in turn suppressing cell proliferation [21–23]. According to this evidence, telomeres not only determine the rounds of division of a cell, but also act as sensors of intrinsic and extrinsic stresses to suppress the proliferation of cells that have accumulated significant genomic damage [10].
Several lines of evidence support the notion that short telomeres are associated with premature aging and age-related diseases [10, 24]. First, an inverse association exists between chronological age and telomere length, even though telomere length is highly heterogeneous among different tissues and different individuals of the same age [24, 25]. We note that it is unclear whether telomere length is a useful biomarker of aging and cellular senescence, as discussed elsewhere [24–28]. Second, accelerated aging conditions known as Short Telomere Syndromes (STSs) are caused by gene mutations resulting in premature telomere attrition and multisystemic diseases such as Dyskeratosis Congenita, Werner’s Syndrome, Hutchinson-Gilford Syndrome and Ataxia-telangiectasia. Patients with these syndromes often show phenotypes of accelerated aging such as diabetes, myocardial infarction, and cognitive decline [29, 30]. Third, telomere shortening has been associated with common age-related diseases including cardiovascular disease, diabetes, neurodegeneration, chronic obstructive pulmonary disease (COPD), and skin disorders [25, 31]. Fourth, many age-related diseases are exacerbated by immune cell senescence. For these reasons, the study of telomere attrition in immune cells like leukocytes is relevant for the etiology of these conditions and may serve as surrogate of telomere length in other tissues [29]. Alterations in leukocyte telomere length (LTL) is associated with increased risk of many cancer types [25, 29, 31, 32], supporting the notion that premalignant cells have lost telomere length homeostasis, prior to the activation of telomerase-mediated telomere maintenance. In sum, understanding the molecular processes underlying telomere dysfunction could help identify possible therapeutic targets in age-related diseases.
microRNAs Regulating Telomere Dynamics in Senescence and Aging
MicroRNAs (miRNAs) are a major class of short ncRNAs that regulate gene expression programs post-transcriptionally. They generally function by binding to specific mRNAs through complementary sequences and inducing degradation and/or blocking the translation of the target mRNA [33]. Many microRNAs control the expression of factors that influence telomere length dynamics (Figure 1). In this section, we focus on the senescence-associated microRNAs (SA-miRNAs) that have been shown to play a role in senescence or aging by influencing telomere maintenance (Table 1).
Table 1.
Main microRNAs identified as regulators of telomere metabolism with a role in senescence and aging.
| microRNA | Target mRNAs and pathways | Levels in senescence or aging | Ref. |
|---|---|---|---|
| miR-23a | TRF2 | ↑ | [34, 35] |
| miR-34a | PNUTS FoxM1/MYC/TERT pathway SIRT1, SIRT6 SIRT7 |
↑ | [38–43] |
| miR-195 | TERT, SIRT1 | ↑ | [44, 45] |
| miR- 126 | Inhibited telomere-TP53-p21-RB and JAK/STAT pathways VCAN (versican), proteoglycan involved in the aging process | ↓ | [46, 47] |
| miR-155 | TRF1 | ↓ | [48, 49] |
| miR-22, miR-138, miR-181a, miR-217, miR449, miR-570 | SIRT1 | ↑ | [39, 50–55] |
| miR-26a, miR-145a | SIRT6 and SIRT2 in dysfunctional telomeres | ↑ | [40] |
| miR-766 | SIRT6 | ↑ | [56] |
| miR-128 | TERT | ↑ | [57] |
| miR-296–5p, miR-512–5p | TERT | ↑ | [58, 59] |
| miR-150 | DKC1 | ↑ | [60] |
| miR-143, miR-145, miR-146 | Can be regulated by expression of ectopic telomerase | ↑ | [49] |
| miR-let-7d-5p, miR-let-7e-5p, miR-23a-3p, miR-34a-5p, miR-125a-3p, miR-125a-5p, miR-125b-5p, miR-181a-5p | Increased in senescent fibroblasts, these microRNAs reduced production of cell cycle regulatory factors | ↑ | [61] |
miR‐23a.
MicroRNA miR-23a was initially found to promote cellular senescence in human fibroblasts by inhibiting the expression of TRF2, a key component of the shelterin complex (Figure 1) [34]. In a later study, Satoh et al. [35] showed that high levels of miR-23a not only induced telomere shortening, but were also associated with poor clinical outcomes in patients with coronary artery disease (CAD), an age-related condition. Furthermore, the high levels of miR-23a in the CAD group were inversely correlated to the levels of TRF2 protein [35].
miR-34a.
This microRNA was recognized as a major regulator of the levels of surtuins, a family of NAD-dependent deacetylases that perform a variety of functions in several cellular compartments, including histone modification and control of metabolic proteins. Among them, SIRT1, SIRT6 and SIRT7, may localize at chromosome ends and maintain telomere length. Previous studies have shown that the deacetylase activity of sirtuins promotes telomere integrity by maintaining chromatin condensation and by promoting the interaction between histones and telomere regulatory factors, like components of the DNA repair machinery. Moreover, sirtuins suppress the DNA damage response at the telomere by inactivating TP53 [36, 37]. MiR-34 was recognized as a major repressor of the levels of SIRT1, SIRT6 and SIRT7 [38–40].
In cardiomyocytes, miR-34a modulated telomere maintenance by reducing the abundance of protein Phosphatase 1 NUclear Targeting Subunit (PNUTS, also known as PPP1R10), which directs protein phosphatase 1 (PP1) to the nucleus. PNUTS was previously found to inhibit the DDR and to interact with TRF2, further preventing telomere shortening [41, 42]. Furthermore, miR-34a induced senescence of human hepatocellular carcinoma (HCC) cells by modulating telomerase activity. In HCC tumors, the levels of miR-34a correlated inversely with telomere length and telomerase activity, while increasing miR-34a ectopically in HCC cells triggered senescence and affected cell viability. The actions by miR-34a on telomere maintenance were attributed to the miR-34a-mediated suppression of the Forkhead Box M1 (FOXM1) and MYC pathway, required for hTERT transcription and telomerase activity [43].
miR-195.
Compared to young mesenchymal stems cells (YMSCs), in which telomerase is highly abundant and active, old mesenchymal stem cells (OMSCs) express high levels of miR-195. The 3’UTR of TERT mRNA was identified as a putative target of miR-195 and depletion of miR-195 rescued hTERT expression and cell proliferation in OMSCs. Importantly, injection of OMSCs with depleted miR-195 significantly improved the regeneration of mouse heart after infarction [44]. A further study showed that miR-195 promoted senescence in skeletal muscle cells by lowering the production of SIRT1 [45]
miR-126.
A rise in miR-126 abundance delayed the senescence of human glomerular mesangial cells (HGMCs) induced by high glucose. While high glucose led to shortened telomeres, miR-126 upregulation was associated with extended telomeres and decreased expression of DNA damage response and /or senescence markers TP53 and p21 [46]. Another study found that deletion of miR-126a promoted hepatic aging in mice and induced age-associated telomere shortening [47].
miR-155.
MiR-155 was found elevated in breast cancer specimens, together with reduced levels of TRF1, a protein component of the shelterin complex (Figure 1). Accordingly, in breast cancer cells it was shown that miR-155 interacts with the 3’UTR of the TRF1 mRNA and represses the translation of the TRF1 protein. The increased levels of miR-155 were associated with telomere and genomic instability, and with poor clinical outcome in estrogen receptor-positive breast cancer [48]. Notably, miR-155 levels are often reduced in senescent cells [49].
Other microRNAs.
Additional microRNAs (miR-570, miR-217, miR-138, miR-22, miR-181a, miR-449, mir-26, mir-145a, miR-766) were reported to suppress the expression of sirtuin family members SIRT1 and SIRT6 [39, 40, 50–56], while other microRNAs modulate the expression of telomere components TERT (miR-128, miR-296–5p, and miR-512–5p) [57–59] and DKC1 (miR-150) [60]. Interestingly, some microRNAs were themselves regulated by hTERT levels in senescent cells, although the mechanisms are not well understood (miR-143, miR-145, miR-146 [49]). Finally, the expression levels of a subset of microRNAs (let-7d-5p, let-7e-5p, miR-125a-3p, miR-125a-5p, miR-125b-5p, miR-23a-3p, miR-34a-5p, miR-181a-5p) increased upon shortening of telomeres in senescent fibroblasts, and they in turn suppressed the production of various cell cycle regulatory transcripts [61].
Taken together, microRNAs can promote or suppress telomere function by modulating the response of telomeres to DNA damage, by suppressing the levels of proteins that protect telomere ends, and by influencing the levels of telomerase components (Figure 1).
lncRNAs Impacting Telomere Maintenance in Senescence and Aging
Several lncRNAs have been associated with the integration of the DDR, the protection of telomere ends and the maintenance of telomere length (Figure 1).
TERC (hTR)
The telomerase core component hTR (human telomerase RNA) is encoded by the TERC gene, located on the chromosomal region 3q26. TERC serves as a template for TERT by providing a sequence (AAUCCC) for the insertion of the repetitive G-rich DNA sequence 5′-TTAGGG-3′ to the ends of chromosomes [62]. TERC is involved in the localization and assembly of the telomerase holoenzyme, and modifications of specific TERC residues were shown to compromise the catalytic activity of the telomerase [63]. TERC contains three major structural and functional domains: a core domain featuring the template sequence, a Stem Terminus Element (STE, featuring conserved regions CR4/CR5 domain) essential for the telomerase catalytic activity, and a 3’ terminal domain (known as H/ACA small Cajal body-specific RNA) that contains specific signals for TERC processing and localization [63]. In 1997, the development of a telomerase-deficient (TERC−/−) mouse provided a model for the study of telomere function in cancer, cardiovascular disease, and other age-related pathologies. The phenotype of late generations of TERC −/− mice included chromosome end fusions, decreased lifespan, and typical features of aging like atrophy and reduced angiogenesis [64, 65].
Genetic variations of the human TERC gene, as well as other telomere maintenance genes, can alter the stability and catalytic activity of the telomerase complex. In line with this notion, TERC mutations have been linked to telomere biology disorders [66] and to shorter human lifespan [67]. For example, mutations in TERC cause autosomal forms of dyskeratosis congenita (DC), characterized by shorter telomeres, cancer disposition, bone marrow failure, and premature aging. Although disease-associated TERC mutations are distributed throughout the RNA, the majority of them map to sequences essential for telomerase catalysis, a specific TERT-binding site, and the template region, causing reduced catalytic activity and aberrant addition of telomeric repeats [68].
Shorter telomeres were associated with the pathogenesis and development of age-associated cognitive decline and Alzheimer’s disease (AD). In particular, TERC genetic variations in AD patients were compared with the frequencies observed in control patients. Specific combinations of single-nucleotide polymorphisms (SNPs) of the TERC gene were found to affect the age at AD onset [69].
In addition, specific TERC mutations were found to be associated with a higher risk of Idiopathic Pulmonary Fibrosis (IPF), an aging-related syndrome characterized by interstitial lung scarring, as well as premature hair greying, bone marrow failure, and liver cirrhosis. IPF is thought to arise from the impaired regeneration of damaged alveolar epithelial cells in the presence of diminished telomerase function [70–73].
A recent study identified an alternative regulatory role for TERC on senescence and aging independent of its function in the telomerase complex [74]. TERC was found to be imported into mitochondria and processed to a shorter form, TERC-53, that was exported back to the cytosol. TERC-53 accumulated in the cytosol upon cellular stress and may serve as an indicator of mitochondrial function [74, 75]. Interestingly, overexpression of TERC-53 in human fibroblasts induced cellular senescence, and overexpression of mTerc-53 in mice accelerated cognitive decline; these interventions did not affect telomerase activity, but rather caused stem cell exhaustion and proliferative decline [74].
Recent evidence indicates that the mechanisms adopted by tumor cells to activate telomerase and avoid telomere shortening include amplification of the TERC gene, besides the well-known derepression of the TERT gene. In this manner, telomerase activation contributes to the evasion of cancer cells beyond the normal limits of proliferation [76, 77].
TERRA
Despite being heterochromatic, telomeric regions are transcribed into lncRNAs collectively known as TERRA (TElomeric Repeat-containing RNA) that play crucial roles in telomere protection and maintenance. TERRA transcription starts from the subtelomeric region and then proceeds into telomere repeats. In mammals, TERRA molecules have different lengths, ranging between 100 nt and 9 kb [78, 79].
TERRA transcripts can protect chromosome ends by promoting telomere maintenance [80, 81]. The G-rich 3′ end of TERRA folds into multimeric G-quadruplex structures composed of four G rings that interact with several capping molecules located at the telomere ends, such as the shelterin components TRF1 and TRF2 [82–85]. TERRA RNAs also interact with proteins that contribute to maintaining the heterochromatic state, including the Polycomb Repressive Complex 2 (PRC2), which has histone methyltransferase activity [86, 87]. The interaction between TERRA transcripts and PRC2 enhances the deposition of heterochromatin marks like H3K9me3, H3K27me3, H4K20me3 and HP1 at chromosome ends [86, 87]. At extratelomeric sites, the interaction between TERRA and epigenetic factors can regulate chromatin remodeling and transcription [88]. Finally, TERRA transcripts form RNA-DNA hybrid structures named R-loops, which influence telomere heterochromatin assembly, replication, and homologous recombination among telomeres, and can thereby impact the onset of senescence [88–91].
TERRA RNAs have been shown to modulate the activity of the telomerase. As TERRA transcripts contain tandem G-rich sequences (UUAGGG) with strong affinity for TERC [92], they may act as direct inhibitors of telomerase activity [93]. Moreover, TERRA transcripts may bind TERT directly and modulate telomerase activity independently of TERC [93]; accordingly, TERRA-mimicking oligonucleotides inhibited telomerase activity [94], while TERRA depletion increased it [88]. However, the role of TERRA on telomerase activity remains controversial, with evidence that TERRA transcripts were capable of recruiting telomerase to critically short telomeres to promote telomere elongation [95], and that high levels of TERRA did not affect telomere elongation in human cancer cells [96].
The molecular functions of TERRA on telomere maintenance in senescence are also unclear. On one hand TERRA may protect telomere ends, but on the other it can counteract telomere elongation. Overexpression of TERRA in telomerase‐negative cells delayed the onset of senescence [91], and masked 3’ overhangs of uncapped telomeres during DNA damage‐induced senescence, thus protecting telomere ends [97]; however, in other studies high levels of TERRA triggered premature senescence by suppressing telomere elongation and DNA replication [98, 99].
The role of TERRA in aging is also poorly understood. A recent review [100] suggested that an interplay between TERRA and TERC regulates telomerase activity and the survival rate of neural stem cells during aging. Therefore, shifts in abundance or activity of these molecules might be involved in age-associated changes [100]. Other studies revealed that TERRA levels were high in blood mononuclear cells of patients with IPF, while TERRA silencing improved telomere function [70, 101].
The levels of TERRA transcripts vary widely and differ among stages of cancer progression [102]. TERRA transcripts were reduced in telomerase-positive cancer cells, where the subtelomeric translational regions are highly methylated [103], but they were elevated in ALT-positive cancers [95, 102, 103]. These differences may be related to the fact that telomeric chromatin is less compact in ALT-positive cells than in telomerase-positive cells [80, 103].
In summary, even though TERRA functions have not yet been fully elucidated, the tight regulation of TERRA levels production appears to be necessary for maintaining telomere homeostasis, with consequences on cancer, senescence, and aging.
GUARDIN
The lncRNA GUARDIN, a transcriptional target of TP53, was shown to be important for maintaining genomic integrity both in unstimulated cells and in cells responding to DNA damage. GUARDIN protected telomere ends from damage and prevented chromosome end-to-end fusion in large part by sequestering miR-23a, and thereby ensuring the production of the shelterin component TRF2. In addition, GUARDIN promoted DNA repair by acting as a scaffold to facilitate the heterodimerization of breast cancer type 1 susceptibility protein (BRCA1) and the BRCA1-associated RING domain 1 (BARD1) protein, which stabilizes BRCA1. GUARDIN silencing not only induced apoptosis and cellular senescence, but it also reduced the growth of cancer xenografts in mice and sensitized cancer cells to genotoxic drugs [104].
Therapies Directed at Telomere-Regulatory ncRNAs: Progress and Prospects
The past decade has seen an escalation in the development of therapeutic approaches exploiting noncoding RNAs, mainly microRNAs and lncRNAs [105, 106]. Some interventions directed at microRNAs may be advantageous because a microRNA may jointly suppress multiple proteins implicated in a given phenotype, or it may suppress proteins selectively expressed in a specific cell type. Current therapeutic strategies to overexpress a specific miRNA involves the delivery of synthetic oligoribonucleotides that mimic the native miRNA, often bearing modifications and complexed molecules for stability and efficacy. Conversely, suppression of microRNA actions often relies on the delivery of antisense oligonucleotides that neutralize the endogenous microRNA and block its activity [107, 108].
Similarly, lncRNAs represent a class of attractive drug targets, given their dysregulation in disease and their tissue-specific expression. For example, many lncRNAs interact with the repressive PRC2 machinery and thus may have a broad impact on gene expression patterns. In this regard, different oligonucleotides have been developed to induce cleavage of a specific lncRNA or to compete with PRC2 for the association with a target lncRNA [106, 109].
Major challenges in RNA-directed oligonucleotide therapeutics are the toxicity at the therapeutic dose, the binding to unintended targets, the stability of the oligonucleotide, and the delivery to the intended tissue. Nanotechnology-based delivery systems have improved greatly the stability and target tissue specificity of therapeutic RNAs, while chemical modifications have improved the pharmacokinetic properties of the oligonucleotides and have lessened their toxicity and off-target actions [105, 106].
Several RNA-based therapeutics have been developed to treat age-related pathologies like cardiovascular disease, diabetes, neurodegeneration, and cancer [105]. Interestingly, some of the ncRNAs targeted therapeutically influence telomere maintenance. For example, in preclinical studies, the miR-34a mimic MRX34 reduced the growth of non-small cell lung cancer in mice and prolonged survival [110]. However, the phase 1 clinical trial of MRX34 was terminated due to adverse immune complications in some patients (NCT01829971)I . Anti-miR-155 molecules are being investigated for the treatment of cutaneous T cell lymphoma (CTCL) and have successfully passed through phase 1 of clinical trials (NCT03837457) II . MiR-155 inhibitors are currently investigated also for the treatment of neuroinflammatory and neurodegenerative diseases [111].
LncRNAs linked to telomere function are also being evaluated as therapeutic targets. Given earlier evidence that inhibiting telomerase could be an effective therapeutic approach in various cancers, oligonucleotides or small molecules specifically directed at TERRA and TERC are being developed with the hope of modulating telomerase activity and telomere length. For example, the oligonucleotide Imetelstat binds with high affinity the template region of TERC, directly inhibiting telomerase activity. Phase 1 trials were successfully completed and clinical phase 2 trials are underway (NCT02598661) III . Strategies directed at TERC inhibition include the design of hammerhead ribozymes, RNA molecules able to cleave the target RNA in a site-specific manner, resulting in a reduced telomerase activity [112, 113].
TERRA RNAs may negatively regulate the telomerase activity and are often dysregulated in cancer and age-related diseases. Sinha et al. [102] recently reviewed several small molecules, like BRACO-19, which possess anti-cancer potential and are able to stabilize the telomeric DNA G-quadruplexes [114]. Given that ligand-induced telomeric G-quadruplex stabilization was shown to inhibit the telomerase activity, it will be interesting to investigate whether TERRA G-quadruplexes might be involved in the proposed mechanism and could be therapeutic targets.
In closing, the development of oligonucleotides, small molecules and other strategies targeting ncRNAs that modulate telomere function, together with the advancements in delivery systems, will further help in the development of new treatments against cancer and other age-related diseases (see Clinician’s Corner).
CLINICIAN’S CORNER.
An important feature of aging is the progressive accumulation of senescent cells in some tissues, with negative consequences on organ homeostasis and function.
Senescent cells are characterized by many different hallmarks including a state of indefinite cell cycle arrest and dysregulation of telomere maintenance. Cancer cells are able to overcome replicative senescence by reactivating the telomerase enzyme, thus acquiring aberrant cell proliferation.
The molecular processes underlying age-associated diseases involve a multitude of noncoding RNAs with crucial roles in cellular processes. New interventions directed at enhancing or suppressing noncoding RNAs are being developed, some of which have already entered human clinical trials. There is mounting interest in RNA-centered strategies to intervene in age-related pathologies such as cancer and neurodegeneration.
Therapeutic approaches targeting ncRNAs include RNA interference, antisense oligonucleotides and small molecules. Chemical modifications and innovative delivery systems are being developed to improve the stability and effectiveness of these molecules.
Concluding Remarks
NcRNAs are major regulators of telomere dynamics, which are well-known for playing an essential role in cellular senescence, aging and cancer. We have described the broad, pleiotropic action of miRNAs in several pathways involved in telomere homeostasis. We have also reported the role of specific lncRNAs in chromosome ends protection and regulation of the telomerase activity. However, despite progresses made in RNA studies, several questions still need to be addressed (see Outstanding Questions). Besides chromosome ends protection and DNA damage sensing, telomeres play an important role in other biological processes. An area of telomere research which has been unexplored for long time is the chromosomal looping between telomeres and distant genic regions. This long-distance interaction was shown to regulate gene expression and was shown to modulate, among other transcripts, the expression of ncRNAs [115]. Whether ncRNAs might in turn regulate the process of telomere looping in long-distance interactions remains to be determined.
OUTSTANDING QUESTIONS BOX.
What other cellular processes are regulated by telomeres?
Telomeres are more than just a clock, shortening after each cell division. They protect the genetic information from being truncated during DNA replication, prevent the fusion of chromosomes, and suppress the proliferation of cells with genomic damage. Telomeres may also regulate gene expression through long-distance interaction with other DNA regions. Whether or not ncRNAs are implicated in these trans-acting functions of telomeres awaits investigation.
Are there ubiquitous and tissue-restricted telomeric ncRNAs?
We have limited knowledge of the molecular mechanisms by which ncRNAs regulate telomere homeostasis in different cell types. A comprehensive understanding of the molecules and pathways whereby ncRNAs control telomere homeostasis constitutively and in specific tissues will enable more precise interventions.
What disease processes are amenable to therapies targeting telomere-related ncRNAs?
Given that senescent cells have been implicated in many declines and pathologies of aging, interventions aimed at stabilizing or exposing telomeres could prove to be valuable therapeutic tools. With an expanding number of ncRNAs successfully targeted in disease conditions, telomeric ncRNAs are becoming attractive therapeutic targets when senescent cells contribute to disease pathogenesis.
Most studies have focused on the role of telomeres in tumor suppressor mechanisms and cellular senescence. For this reason, an area of particular interest is the development of therapeutic strategies targeting telomeric ncRNAs in cancer and age-related diseases. Notably, some of these compounds have already passed the first phase of clinical trials. Future research will probably identify tissue-specific ncRNAs with a role in telomere dynamics, allowing a more precise intervention in the context of therapeutic strategies.
HIGHLIGHTS.
Senescent cells accumulating in aging tissues display indefinite cell cycle arrest, shortened telomeres, and enhanced secretion of pro-inflammatory factors.
Non-coding RNAs critically regulate gene expression programs in many physiological and pathological processes, including those associated with aging.
Non-coding RNAs regulate telomere homeostasis, either directly or indirectly, and thereby coordinate molecular processes inherent to cellular senescence and aging.
ACKNOWLEDGEMENTS
This work was supported by the NIA IRP, NIH. We appreciate the advice of Dr. Yie Liu (NIA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
RESOURCES
REFERENCES
- 1.Birney E, et al. , Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature, 2007. 447(7146): p. 799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taft RJ, Pheasant M, and Mattick JS, The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays, 2007. 29(3): p. 288–99. [DOI] [PubMed] [Google Scholar]
- 3.de Andres-Pablo A, Morillon A, and Wery M, LncRNAs, lost in translation or licence to regulate? Curr Genet, 2017. 63(1): p. 29–33. [DOI] [PubMed] [Google Scholar]
- 4.Clancy S, RNA Functions, in Nature Education. 2008 [Google Scholar]
- 5.Clemson CM, et al. , An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell, 2009. 33(6): p. 717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasanth KV, et al. , Regulating gene expression through RNA nuclear retention. Cell, 2005. 123(2): p. 249–63. [DOI] [PubMed] [Google Scholar]
- 7.Sunwoo H, et al. , MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res, 2009. 19(3): p. 347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shay JW, Telomeres and aging. Curr Opin Cell Biol, 2018. 52: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Bernal A and Tusell L, Telomeres: Implications for Cancer Development. Int J Mol Sci, 2018. 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victorelli S and Passos JF, Telomeres and Cell Senescence - Size Matters Not. EBioMedicine, 2017. 21: p. 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JJ and de Lange T, Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol, 2004. 14(24): p. 2302–8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, et al. , Loss of p16(Ink4a) function rescues cellular senescence induced by telomere dysfunction. Int J Mol Sci, 2012. 13(5): p. 5866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venteicher AS, et al. , A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science, 2009. 323(5914): p. 644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta JC, et al. , A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol, 2013. 15(8): p. 978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppé JP, et al. , Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol, 2008. 6(12): p. 2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demaria M, et al. , Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov, 2017. 7(2): p. 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Deursen JM, The role of senescent cells in ageing. Nature, 2014. 509(7501): p. 439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuilman T, et al. , The essence of senescence. Genes Dev, 2010. 24(22): p. 2463–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz-Espin D and Serrano M, Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol, 2014. 15(7): p. 482–96. [DOI] [PubMed] [Google Scholar]
- 20.Salama R, et al. , Cellular senescence and its effector programs. Genes Dev, 2014. 28(2): p. 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fumagalli M, et al. , Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol, 2012. 14(4): p. 355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaul Z, et al. , Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep, 2011. 13(1): p. 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suram A, et al. , Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J, 2012. 31(13): p. 2839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernadotte A, Mikhelson VM, and Spivak IM, Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY), 2016. 8(1): p. 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chilton W, O’Brien B, and Charchar F, Telomeres, Aging and Exercise: Guilty by Association? Int J Mol Sci, 2017. 18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fasching CL, Telomere length measurement as a clinical biomarker of aging and disease. Crit Rev Clin Lab Sci, 2018. 55(7): p. 443–465. [DOI] [PubMed] [Google Scholar]
- 27.Mather KA, et al. , Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci, 2011. 66(2): p. 202–13. [DOI] [PubMed] [Google Scholar]
- 28.Sanders JL and Newman AB, Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev, 2013. 35: p. 112–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackburn EH, Epel ES, and Lin J, Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science, 2015. 350(6265): p. 1193–8. [DOI] [PubMed] [Google Scholar]
- 30.Opresko PL and Shay JW, Telomere-associated aging disorders. Ageing Res Rev, 2017. 33: p. 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arsenis NC, et al. , Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget, 2017. 8(27): p. 45008–45019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shay JW, Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov, 2016. 6(6): p. 584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartel DP, MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004. 116(2): p. 281–97. [DOI] [PubMed] [Google Scholar]
- 34.Luo Z, et al. , Mir-23a induces telomere dysfunction and cellular senescence by inhibiting TRF2 expression. Aging Cell, 2015. 14(3): p. 391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh M, et al. , Expression of miR-23a induces telomere shortening and is associated with poor clinical outcomes in patients with coronary artery disease. Clin Sci (Lond), 2017. 131(15): p. 2007–2017. [DOI] [PubMed] [Google Scholar]
- 36.Amano H and Sahin E, Telomeres and sirtuins: at the end we meet again. Mol Cell Oncol, 2019. 6(5): p. e1632613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SH, et al. , Sirtuin signaling in cellular senescence and aging. BMB Rep, 2019. 52(1): p. 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker JR, et al. , Oxidative stress dependent microRNA-34a activation via PI3Kalpha reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci Rep, 2016. 6: p. 35871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes PJ, Baker J, and Donnelly LE, Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am J Respir Crit Care Med, 2019. [DOI] [PubMed]
- 40.Amano H, et al. , Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metab, 2019. 29(6): p. 1274–1290 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boon RA, et al. , MicroRNA-34a regulates cardiac ageing and function. Nature, 2013. 495(7439): p. 107–10. [DOI] [PubMed] [Google Scholar]
- 42.Marques FZ, et al. , Telomere dynamics during aging in polygenic left ventricular hypertrophy. Physiol Genomics, 2016. 48(1): p. 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, et al. , miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget, 2015. 6(6): p. 3988–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada M, et al. , Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells, 2016. 34(1): p. 148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondo H, et al. , Blockade of senescence-associated microRNA-195 in aged skeletal muscle cells facilitates reprogramming to produce induced pluripotent stem cells. Aging Cell, 2016. 15(1): p. 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao DW, et al. , Upregulation of MiR-126 Delays the Senescence of Human Glomerular Mesangial Cells Induced by High Glucose via Telomere-p53-p21-Rb Signaling Pathway. Curr Med Sci, 2018. 38(5): p. 758–764. [DOI] [PubMed] [Google Scholar]
- 47.Yan Y, et al. , Deletion of miR-126a Promotes Hepatic Aging and Inflammation in a Mouse Model of Cholestasis. Mol Ther Nucleic Acids, 2019. 16: p. 494–504. [DOI] [PMC free article] [PubMed]
- 48.Dinami R, et al. , miR-155 drives telomere fragility in human breast cancer by targeting TRF1. Cancer Res, 2014. 74(15): p. 4145–56. [DOI] [PubMed] [Google Scholar]
- 49.Bonifacio LN and Jarstfer MB, MiRNA profile associated with replicative senescence, extended cell culture, and ectopic telomerase expression in human foreskin fibroblasts. PLoS One, 2010. 5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menghini R, et al. , MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation, 2009. 120(15): p. 1524–32. [DOI] [PubMed] [Google Scholar]
- 51.Rivetti di Val Cervo P, et al. , p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A, 2012. 109(4): p. 1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu D, et al. , miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol, 2011. 193(2): p. 409–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jazbutyte V, et al. , MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age (Dordr), 2013. 35(3): p. 747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, et al. , Protection of CD4+ T cells from hepatitis C virus infection-associated senescence via DeltaNp63-miR-181a-Sirt1 pathway. J Leukoc Biol, 2016. 100(5): p. 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bou Kheir T, et al. , miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol Cancer, 2011. 10: p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma A, et al. , The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem, 2013. 288(25): p. 18439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guzman H, et al. , miR-128 inhibits telomerase activity by targeting TERT mRNA. Oncotarget, 2018. 9(17): p. 13244–13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dinami R, et al. , Epigenetic silencing of miR-296 and miR-512 ensures hTERT dependent apoptosis protection and telomere maintenance in basal-type breast cancer cells. Oncotarget, 2017. 8(56): p. 95674–95691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, et al. , miR-512–5p suppresses tumor growth by targeting hTERT in telomerase positive head and neck squamous cell carcinoma in vitro and in vivo. PLoS One, 2015. 10(8): p. e0135265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe A, et al. , The role of microRNA-150 as a tumor suppressor in malignant lymphoma. Leukemia, 2011. 25(8): p. 1324–34. [DOI] [PubMed] [Google Scholar]
- 61.Markopoulos GS, et al. , Senescence-associated microRNAs target cell cycle regulatory genes in normal human lung fibroblasts. Exp Gerontol, 2017. 96: p. 110–122. [DOI] [PubMed] [Google Scholar]
- 62.Jafri MA, et al. , Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med, 2016. 8(1): p. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Q, Kim NK, and Feigon J, Architecture of human telomerase RNA. Proc Natl Acad Sci U S A, 2011. 108(51): p. 20325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blasco MA, et al. , Telomere shortening and tumor formation by mouse cells lacking telomerase RNA, in Cell. 1997: United States: p. 25–34. [DOI] [PubMed] [Google Scholar]
- 65.Wong LS, et al. , Telomere biology in cardiovascular disease: the TERC−/− mouse as a model for heart failure and ageing, in Cardiovasc Res. 2009: England: p. 244–52. [DOI] [PubMed] [Google Scholar]
- 66.Sarek G, et al. , Molecular basis of telomere dysfunction in human genetic diseases. Nat Struct Mol Biol, 2015. 22(11): p. 867–74. [DOI] [PubMed] [Google Scholar]
- 67.Scarabino D, et al. , Analysis of the Association Between TERC and TERT Genetic Variation and Leukocyte Telomere Length and Human Lifespan-A Follow-Up Study. Genes (Basel), 2019. 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson ND and Bertuch AA, Dyskeratosis congenita as a disorder of telomere maintenance. Mutat Res, 2012. 730(1–2): p. 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scarabino D, et al. , Common variants of human TERT and TERC genes and susceptibility to sporadic Alzheimers disease. Exp Gerontol, 2017. 88: p. 19–24. [DOI] [PubMed] [Google Scholar]
- 70.Arish N, Petukhov D, and Wallach-Dayan SB, The Role of Telomerase and Telomeres in Interstitial Lung Diseases: From Molecules to Clinical Implications. Int J Mol Sci, 2019. 20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armanios MY, et al. , Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med, 2007. 356(13): p. 1317–26. [DOI] [PubMed] [Google Scholar]
- 72.Borie R, et al. , Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J, 2016. 48(6): p. 1721–1731. [DOI] [PubMed] [Google Scholar]
- 73.Tsakiri KD, et al. , Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A, 2007. 104(18): p. 7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng Q, et al. , Mitochondrion-processed TERC regulates senescence without affecting telomerase activities. Protein Cell, 2019. [DOI] [PMC free article] [PubMed]
- 75.Cheng Y, et al. , Mitochondrial Trafficking and Processing of Telomerase RNA TERC. Cell Rep, 2018. 24(10): p. 2589–2595. [DOI] [PubMed] [Google Scholar]
- 76.Cao Y, Bryan TM, and Reddel RR, Increased copy number of the TERT and TERC telomerase subunit genes in cancer cells. Cancer Sci, 2008. 99(6): p. 1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang J, et al. , Detection of TERC amplification in cervical epithelial cells for the diagnosis of high-grade cervical lesions and invasive cancer: a multicenter study in China, in J Mol Diagn. 2010. p. 808–17. [DOI] [PMC free article] [PubMed]
- 78.Azzalin CM, et al. , Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends, in Science. 2007: United States: p. 798–801. [DOI] [PubMed] [Google Scholar]
- 79.Feretzaki M, Nunes P. Renck, and Lingner J, Expression and differential regulation of human TERRA at several chromosome ends. RNA, 2019. 25(11): p. 1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bettin N, Oss Pegorar C, and Cusanelli E, The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability. Cells, 2019. 8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lopez de Silanes I, et al. , Identification of TERRA locus unveils a telomere protection role through association to nearly all chromosomes, in Nat Commun. 2014: England: p. 4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collie GW, et al. , A crystallographic and modelling study of a human telomeric RNA (TERRA) quadruplex. Nucleic Acids Res, 2010. 38(16): p. 5569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Randall A and Griffith JD, Structure of long telomeric RNA transcripts: the G-rich RNA forms a compact repeating structure containing G-quartets. J Biol Chem, 2009. 284(21): p. 13980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Biffi G, Tannahill D, and Balasubramanian S, An intramolecular G-quadruplex structure is required for binding of telomeric repeat-containing RNA to the telomeric protein TRF2. J Am Chem Soc, 2012. 134(29): p. 11974–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng Z, et al. , TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell, 2009. 35(4): p. 403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montero JJ, et al. , TERRA recruitment of polycomb to telomeres is essential for histone trymethylation marks at telomeric heterochromatin. Nat Commun, 2018. 9(1): p. 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, et al. , Targeting of Polycomb Repressive Complex 2 to RNA by Short Repeats of Consecutive Guanines, in Mol Cell. 2017, 2017 Elsevier Inc: United States: p. 1056–1067 e5. [DOI] [PubMed] [Google Scholar]
- 88.Chu HP, et al. , TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell, 2017. 170(1): p. 86–101 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balk B, et al. , Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence, in Nat Struct Mol Biol. 2013: United States: p. 1199–205. [DOI] [PubMed] [Google Scholar]
- 90.Balk B, et al. , The differential processing of telomeres in response to increased telomeric transcription and RNA-DNA hybrid accumulation. RNA Biol, 2014. 11(2): p. 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu TY, Kao YW, and Lin JJ, Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc Natl Acad Sci U S A, 2014. 111(9): p. 3377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porro A, et al. , Molecular dissection of telomeric repeat-containing RNA biogenesis unveils the presence of distinct and multiple regulatory pathways. Mol Cell Biol, 2010. 30(20): p. 4808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Redon S, Reichenbach P, and Lingner J, The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res, 2010. 38(17): p. 5797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schoeftner S and Blasco MA, Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II, in Nat Cell Biol. 2008: England: p. 228–36. [DOI] [PubMed] [Google Scholar]
- 95.Cusanelli E, Romero CA, and Chartrand P, Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol Cell, 2013. 51(6): p. 780–91. [DOI] [PubMed] [Google Scholar]
- 96.Farnung BO, et al. , Telomerase efficiently elongates highly transcribing telomeres in human cancer cells. PLoS One, 2012. 7(4): p. e35714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porro A, Feuerhahn S, and Lingner J, TERRA-reinforced association of LSD1 with MRE11 promotes processing of uncapped telomeres, in Cell Rep. 2014, 2014 The Authors. Published by Elsevier Inc: United States: p. 765–76. [DOI] [PubMed] [Google Scholar]
- 98.Deng Z, Campbell AE, and Lieberman PM, TERRA, CpG methylation and telomere heterochromatin: lessons from ICF syndrome cells. Cell Cycle, 2010. 9(1): p. 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maicher A, et al. , Deregulated telomere transcription causes replication-dependent telomere shortening and promotes cellular senescence. Nucleic Acids Res, 2012. 40(14): p. 6649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pereira Fernandes D, et al. , Long Non-Coding RNAs in Neuronal Aging. Noncoding RNA, 2018. 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao Y, et al. , Regulation of TERRA on telomeric and mitochondrial functions in IPF pathogenesis. BMC Pulm Med, 2017. 17(1): p. 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sinha S, et al. , Telomeric Repeat Containing RNA (TERRA): Aging and Cancer, in CNS Neurol Disord Drug Targets. 2015: United Arab Emirates: p. 936–46. [DOI] [PubMed] [Google Scholar]
- 103.Ng LJ, et al. , Telomerase activity is associated with an increase in DNA methylation at the proximal subtelomere and a reduction in telomeric transcription. Nucleic Acids Res, 2009. 37(4): p. 1152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu WL, et al. , GUARDIN is a p53-responsive long non-coding RNA that is essential for genomic stability, in Nat Cell Biol. 2018: England: p. 492–502. [DOI] [PubMed] [Google Scholar]
- 105.BD A, et al. , Targeting noncoding RNAs in disease. 2017. p. 761–771. [DOI] [PMC free article] [PubMed]
- 106.Matsui M and Corey DR, Non-coding RNAs as drug targets. Nat Rev Drug Discov, 2017. 16(3): p. 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Z and Rana TM, Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov, 2014. 13(8): p. 622–38. [DOI] [PubMed] [Google Scholar]
- 108.Ling H, Fabbri M, and Calin GA, MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov, 2013. 12(11): p. 847–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sarma K, et al. , Locked nucleic acids (LNAs) reveal sequence requirements and kinetics of Xist RNA localization to the X chromosome. Proc Natl Acad Sci U S A, 2010. 107(51): p. 22196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kasinski AL, et al. , A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene, 2015. 34(27): p. 3547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pena-Philippides JC, et al. , In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation, 2016. 13(1): 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nosrati M, et al. , Antitumor activity of systemically delivered ribozymes targeting murine telomerase RNA. Clin Cancer Res, 2004. 10(15): p. 4983–90. [DOI] [PubMed] [Google Scholar]
- 113.Yeo M, et al. , Attenuation of telomerase activity by hammerhead ribozyme targeting human telomerase RNA induces growth retardation and apoptosis in human breast tumor cells. Int J Cancer, 2005. 114(3): p. 484–9. [DOI] [PubMed] [Google Scholar]
- 114.Burger AM, et al. , The G-quadruplex-interactive molecule BRACO-19 inhibits tumor growth, consistent with telomere targeting and interference with telomerase function. Cancer Res, 2005. 65(4): p. 1489–96. [DOI] [PubMed] [Google Scholar]
- 115.Robin JD, et al. , Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Dev, 2014. 28(22): p. 2464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]