Although there is no formal definition, financial toxicity (FT) refers to the detrimental effects of the excess financial strain caused by the diagnosis of cancer on the well-being of patients, their families and society. With continued escalation in the costs of cancer treatment, FT has become an important consideration in recent cancer care.
In this article, we propose a four-step approach addressing the issue of FT in patients with cancer: first, acknowledging and understanding the problem; second, quantifying the problem; third, engaging key stakeholders and fostering communication and fourth, implementing solutions at various levels of the healthcare system.
Acknowledging and Understanding the Problem – That cancer diagnosis comes with a substantial financial burden to the individual and society is no news. The total expenditure for cancer care in the US is projected to increase by 39% from $125 billion in 2010 to $173 billion in 2020 [1]. Average out of pocket (OOP) costs for patients increased dramatically averaging from $1800 to $2900 in the month of diagnosis alone [2]. This problem will only worsen as estimated expenditure on cancer medicine continues to escalate while the relative median household income has stagnated. Indeed, newer advances in cancer care become meaningless if patients can't afford them or if the patients’ prognoses continue to be determined by where they live or how good their insurance is.
Certain subgroups of the population are at higher risk of FT. Younger patients and those with lower household income were found to be predisposed to greater financial burden. Other sociodemographic characteristics, including type of insurance, race, marital status, education, geographic location, and comorbidity, have also been associated with FT in studies [3]. Apart from cancer drug prices and rising cost of health insurance, increased non-drug expenditure such as travel and lodging cost, hospital costs and supportive care play a significant role.
The pattern of FT also differs among countries based on their health care system. Unlike countries with public health insurance and a fixed OOP cost model, financial planning constitutes an important part of treatment planning and goal-setting in low- and middle-income countries where the entire treatment costs are borne by the patients. United States has a unique model in which for most patients, the cost of treatment is not known until the medical bill arrives. Thus, medical bills can take many patients by surprise and without prior planning, this can lead to FT. Nevertheless, public health insurance system does not necessarily protect against FT in individual cancer patients as highlighted by studies from Italy and Japan [4,5].
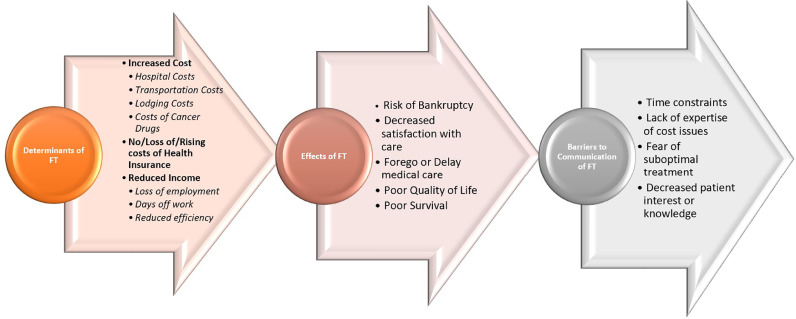
Although there have been plenty studies on cost of cancer drugs and policy issues relating to curbing these increasing costs, studies on addressing FT in individual patients are still lacking. We need increased funding and incentives to encourage research to understand the most actionable and effective domain to intervene in the pathway of FT (Fig. 1).
Fig. 1.
Determinants, effects and barriers for FT.
Measuring the Problem – Various tools and definitions have been used to measure FT in cancer patients, but there is no uniform consensus [6]. Some studies have assessed FT as a percentage of income spent OOP while others have asked straightforward if the patient felt financial burden due to treatment. To our knowledge, the only validated tool so far is the “COmprehensive Score for financial Toxicity (COST)” tool to assess FT in US patients with cancer. COST has been validated as an adequate measure of FT with correlation with health related quality of life which makes it a clinically relevant patient-centered outcome [7]. In Japan, researchers have translated the COST tool into Japanese to assess FT among Japanese patients with cancer and discovered that a significant percentage of Japanese cancer patients do experience meaningful FT despite public health insurance system [4]. COST shows potential to provide us with an objective measure of FT but more research is needed to explore whether these tools correlate with clinical outcomes, and whether country specific tools are needed.
Engaging stakeholders and fostering communication – Given that FT has been shown to affect patients’ satisfaction of cancer care, leading to delay or foregoing cancer care, bankruptcy, poor quality of life and poor survival [5,8], it is important to acknowledge that FT is not an abstract socio-political policy issue, but a clinical issue impacting patients every day. For a patient on chemotherapy, physicians proactively ask for physical toxicities such as nausea or neuropathy. Similarly, we should foster an environment where we proactively enquire patients regarding their financial concerns and have proper mitigating measures in place for those who do.
Various clinician, patient, and institutional barriers such as concerns about time, a lack of expertise or knowledge relating to cost issues, fear of providing or receiving suboptimal treatment, self-consciousness or unwillingness on the part of the patients to provide financial details can impede these conversations. Proper policy on how to incentivize such discussions and consults are needed. Training, recruitment and appropriate involvement of financial navigators and social workers is indispensable. Cancer economics could be included in the formal oncology training and oncologists get trained to detect and address FT in patients. Ultimately, FT and its impact must be communicated to key stakeholders, including policymakers and patients, to build awareness of the problem and support appropriate policy level actions.
Implementing solution strategies: International Societies/Guidelines – some steps are now being taken by global cancer societies to raise voice against the high cost of drugs and discourage low value care. Some notable examples are the ASCO value framework, Choosing Wisely initiative and the ESMO Magnitude of Clinical Benefit Scale (MCBS). These frameworks take clinical benefit, side effects, and improvement in patient symptoms or quality of life into account and provide relative rankings for decision making. The MCBS tool also helps policymakers to make assessments of clinical benefits for drug approvals or reimbursement decisions. The National Comprehensive Cancer Network guidelines also now incorporate evidence blocks, including affordability as one of the five domains.
Such societies can lead with example and a strong stance against low-value interventions will go a long way to dissuade the use of expensive but futile treatments. In 2017, the NCCN decided to remove the FDA-approved drug necitumumab for non-small cell lung cancer from its guidelines based on marginal benefits and high costs. Although this is a welcomed step, there are other oncology drugs in use that have escaped scrutiny.
National level – Policy changes at the national level to reduce the cost of cancer treatment and discourage the use of low-value cancer interventions can ease financial strain on patients while improving quality care. Such interventions include lowering cost of drugs by price negotiations, value-aligned pricing strategies, aligning price based on quality of evidence, policies separating physician reimbursements from the cost of interventions, and supporting trials testing cheaper alternatives to expensive treatment strategies [9]. The regulatory agencies should take a stronger, regulatory role in this context by discouraging the approval of low-value drugs which show minimal to no clinical benefit, or at least ask that the cost be tied to the level of evidence. Indeed, U.S spent more than $500 million in 3 years on a cancer drug olaratumab that ultimately failed, with the society-but not the industry-bearing the full financial burden of the failure.
Hospital level – cost transparency, availability of financial counsellors in hospital, and elimination of low value practices [10] are strong measures that hospitals can employ to address FT. Appropriate facilities for referral to discuss financial issues should be available at cancer centres. Information on the costs of the interventions should be made available to the physicians and the patients beforehand. Increased cost discrepancy across hospitals also needs to be addressed.
Physicians and Patient level – Increased awareness of low-value practices and FT of cancer treatment is necessary both among physicians and patients. FT of new interventions should be reported and published. Although this may not be feasible for new molecules, for trials testing approved molecules in a new disease, cost is already known, and cost-effectiveness analyses should be reported when the efficacy data are reported.
Patients can seek guidance on financial issues using online resources such as CancerCare.org. Some hospitals and disease specific charities also provide support for patients in need. In some countries, the government also provides financial assistance. Such information should be available upon contacting the patient advocacy organizations. Indeed, patient advocacy groups should make helping ease FT in patients with cancer one of their top priorities. Sadly, we have seen over recent years that some patients need to start their own online funding campaigns to afford the cancer treatment.
In conclusion, FT is not just a policy issue, but a real clinical problem with adverse consequences for the patient and family. Objective measurement, recognition and discussion of FT is an important step. Avoiding low-value practices in clinic is the strategy we as clinicians have at our disposal. Patient support groups have a big role by providing appropriate support and information online and via other resources to address financial toxicity. Cancer societies should take their responsibility more seriously in eliminating low-value practice, as should the regulatory agencies. The oncology community may not be able to prevent or cure cancers completely, but we can certainly prevent FT with appropriate steps.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Mariotto A.B., Robin Yabroff K., Shao Y., Feuer E.J., Brown M.L. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieguez G., Ferro C., Pyenson B. Milliman Inc; Seattle, Washington USA: 2017. A multi-year look at the cost burden of cancer care. [Google Scholar]
- 3.Zafar S.Y., Abernethy A.P. Financial toxicity, part I: a new name for a growing problem. Oncology. 2013;27(2):80–149. [PMC free article] [PubMed] [Google Scholar]
- 4.Honda K., Gyawali B., Ando M. Prospective survey of financial toxicity measured by the comprehensive score for financial toxicity in japanese patients with cancer. J Global Oncol. 2019;5:1–8. doi: 10.1200/JGO.19.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrone F., Jommi C., Di Maio M. The association of financial difficulties with clinical outcomes in cancer patients: secondary analysis of 16 academic prospective clinical trials conducted in Italy. Ann Oncol. 2016:mdw433. doi: 10.1093/annonc/mdw433. [DOI] [PubMed] [Google Scholar]
- 6.Witte J., Mehlis K., Surmann B. Methods for measuring financial toxicity after cancer diagnosis and treatment: a systematic review and its implications. Ann Oncol. 2019;30(7):1061–1070. doi: 10.1093/annonc/mdz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Souza J.A., Yap B.J., Wroblewski K. Measuring financial toxicity as a clinically relevant patient‐reported outcome: the validation of the COmprehensive score for financial toxicity (COST) Cancer. 2017;123(3):476–484. doi: 10.1002/cncr.30369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zafar S.Y. Financial toxicity of cancer care: it's time to intervene. J Natl Cancer Inst. 2016;108(5) doi: 10.1093/jnci/djv370. [DOI] [PubMed] [Google Scholar]
- 9.Ratain M.J., Goldstein D.A., Lichter A.S. Interventional pharmacoeconomics—a new discipline for a cost-constrained environment. JAMA Oncol. 2019;5(8):1097–1098. doi: 10.1001/jamaoncol.2019.1341. [DOI] [PubMed] [Google Scholar]
- 10.Gyawali B. Low-value practices in oncology contributing to financial toxicity. Ecancermedicalscience. 2017;11 doi: 10.3332/ecancer.2017.727. [DOI] [PMC free article] [PubMed] [Google Scholar]