Abstract
Recent advances in electrochemical biosensors for pathogen detection are reviewed. Electrochemical biosensors for pathogen detection are broadly reviewed in terms of transduction elements, biorecognition elements, electrochemical techniques, and biosensor performance. Transduction elements are discussed in terms of electrode material and form factor. Biorecognition elements for pathogen detection, including antibodies, aptamers, and imprinted polymers, are discussed in terms of availability, production, and immobilization approach. Emerging areas of electrochemical biosensor design are reviewed, including electrode modification and transducer integration. Measurement formats for pathogen detection are classified in terms of sample preparation and secondary binding steps. Applications of electrochemical biosensors for the detection of pathogens in food and water safety, medical diagnostics, environmental monitoring, and bio-threat applications are highlighted. Future directions and challenges of electrochemical biosensors for pathogen detection are discussed, including wearable and conformal biosensors, detection of plant pathogens, multiplexed detection, reusable biosensors for process monitoring applications, and low-cost, disposable biosensors.
Keywords: Electrochemical, Biosensors, Pathogen quantification, Food safety, Water safety, Medical diagnostics, Bio-threat, Virus detection, COVID-19
Highlights
-
•
Comprehensive review of electrochemical biosensor-based pathogen detection.
-
•
Review of emerging electrodes for transduction of pathogen binding via electrochemical methods.
-
•
Discussion of emerging electrochemical biosensor designs, including flexible and wearable form factors.
-
•
Highlight of electrochemical biosensors for coronavirus detection.
1. Introduction
Pathogens are infectious agents that cause disease. They include microorganisms, such as fungi, protozoans, and bacteria, and molecular-scale infectious agents, including viruses and prions. Foodborne, waterborne, and airborne pathogens enter the body through various modes of infection and are responsible for over 15 million deaths annually worldwide (Dye, 2014). Some of the most common pathogens include viruses, such as norovirus and influenza virus, and bacteria, such as E. coli and S. aureus. Pathogens vary in many regards, such as virulence, contagiousness, mode of transmission, and infectious dose. For example, the world is currently facing a global pandemic associated with the COVID-19 virus, for which virulence and infectious dose data are still emerging. Techniques for sensitive and rapid detection of pathogens in complex matrices, such as body fluids and aerosols, and on surfaces are critical to the treatment of infectious diseases and controlling the spread of disease.
The techniques used to identify and quantify pathogens can be broadly distinguished as immunoassays or DNA-based assays. The use of immunoassays versus DNA-based assays depends on various factors, including the stage of an infection and the availability of antibodies and DNA sequence data, such as viral DNA, toxin-producing genes, as well as species- and strain-selective genes. Immunoassays are ubiquitous across medical diagnostics and food safety applications. Pathogens can be identified through the presence of generated antibodies in an organism, which may be present both during and after an infection (i.e., after the pathogen is no longer present). In such assays, both the biorecognition element and the target are antibodies. If antibodies are available for the pathogen (e.g., anti-E. coli O157:H7), one can also directly detect the pathogen using immunoassays. The ability to indirectly and directly detect pathogens via generated antibodies and pathogen epitopes, respectively, makes immunoassays flexible techniques for pathogen detection. In cases of limited antibody availability, need for highly sensitive results, or infections that do not generate a significant level of antibody production in the organism although the pathogen is present, DNA-based assays are commonly employed. DNA-based assays require the pathogen to be present in the sample or to have been recently present. In addition to detection of pathogens using antibodies or toxin-producing genes, pathogens can also be detected based on their expression of toxins. Thus, targets associated with pathogen detection include toxins, nucleic acids, viruses, cells, and oocysts. As a result, biorecognition elements widely vary, including antibodies, aptamers, and imprinted polymers. Several comprehensive reviews have been written on pathogen detection using high-throughput, well plate-based bioanalytical techniques (Alahi and Mukhopadhyay, 2017; Lazcka et al. 2007; Zourob et al. 2008), such as enzyme-linked immunosorbent assay (ELISA) (Law et al. 2015) and polymerase chain reaction (PCR) (Klein, 2002; Malorny et al. 2003), which remain the gold standards for pathogen detection. Few reviews, however, have focused on emerging label-free biosensors for pathogen detection, which provide useful characteristics for applications in process monitoring (e.g., of biomanufacturing processes), environmental monitoring, and precision agriculture.
Bioanalytical techniques utilize a selective biorecognition element, often called a molecular probe, in combination with an analytical system, such as a plate reader or PCR analyzer, to quantify one or more components of a sample. While capable of being highly sensitive and robust, they are destructive testing methods and require the addition of reagents to the sample and extensive sample preparation steps, which increase the time-to-results (TTR). Bioanalytical techniques, such as PCR, may also encounter inhibition effects caused by background species in the sample (Justino et al. 2017; Scognamiglio et al. 2016; Sin et al. 2014), which introduce measurement bias and increase measurement uncertainty (Clark et al. 2016; Silverman et al. 2019). Considering such limitations of traditional plate-based bioanalytical techniques and the need for real-time continuous monitoring capabilities among various applications, there is a need to examine alternative bioanalytical techniques.
Over the past twenty-five years, biosensors have emerged to complement PCR and ELISA for pathogen detection. Biosensors are based on the direct integration of a selective biorecognition element and a sensitive transducer element and provide complementary platforms to PCR and ELISA for pathogen identification and quantification. According to the International Union of Pure and Applied Chemistry (IUPAC), a biosensor must contain a biorecognition element in direct spatial contact with a transduction element (Thévenot et al. 2001). In addition, a biosensor should provide quantitative or semi-quantitative analytical information and measurement without the requirement of additional processing steps or reagents. While a biosensor should also be a self-contained, integrated device, the measurement approach can vary from droplet formats to continuous flow formats that require associated fluid handling systems. Biosensors have achieved sensitive and selective real-time detection of pathogens in various environments without the need for sample preparation. For example, biosensors have enabled the detection of an abundance of pathogens in various matrices and environments, including foods, body fluids, and object surfaces. In addition to sample preparation-free protocols, biosensors are compatible with label-free protocols (Daniels and Pourmand, 2007; Rapp et al. 2010; Sang et al. 2016; Vestergaard et al. 2007). Labels, often referred to as reporters, are molecular species, such as organic dyes or quantum dots (Resch-Genger et al. 2008), that are attached to the target, either directly or through a biorecognition element, using a series of sample preparation steps or secondary binding steps to facilitate detection through the properties of the label. Thus, label-free biosensors avoid the use of a reporter species to detect the target species (Cooper, 2009; Syahir et al. 2015). Label-free assays often have fewer sample preparation steps due to the elimination of procedures associated with target labeling and lower cost than label-based assays, which are important considerations for applications in which preparation facilities or trained personnel are either limited or unavailable (Cooper, 2009; Syahir et al. 2015).
While various types of transducers have been investigated for pathogen biosensing (Lazcka et al. 2007; Singh et al. 2014; Yoo and Lee, 2016), including mechanical and optical transducers, such as cantilever biosensors or surface plasmon resonance (SPR)-based biosensors, electrochemical biosensors have been extensively applied to pathogen detection (Felix and Angnes, 2018; Pereira da Silva Neves et al. 2018; Saucedo et al. 2019). Electrochemical biosensors for pathogen detection utilize conducting and semiconducting materials as the transducer, which is commonly referred to as an electrode. The chemical energy associated with binding between target pathogens and electrode-immobilized biorecognition elements is converted into electrical energy through an electrochemical method that involves the electrode and a pathogen-containing electrolyte solution. To date, electrochemical biosensors have enabled sample preparation-free detection of pathogens in various matrices, in situ detection of pathogens on surfaces, rapid pathogen detection using low-cost platforms, multiplexed detection of pathogens in practical matrices, and detection of pathogens via wireless actuation and data acquisition formats. As a result, electrochemical biosensors for pathogen detection have been widely examined for food and water safety, medical diagnostic, environmental monitoring, and bio-threat applications (Amiri et al. 2018; Duffy and Moore, 2017; Felix and Angnes, 2018; Furst and Francis, 2019; Mishra et al. 2018; Monzó et al. 2015; Rastogi and Singh, 2019).
Here, we critically review electrochemical biosensors for pathogen detection. To gain insight into the trajectory of the field, electrochemical biosensors for pathogen detection reported since 2005 are critically reviewed and classified with respect to IUPAC-recommended definitions and classifications (Thévenot et al. 2001). Applications of electrochemical biosensors for pathogen detection are critically reviewed with respect to the target pathogen, sample matrix, biosensor design, fabrication method, measurement format, and biosensor performance. We also discuss future directions of electrochemical biosensors for pathogen detection, which includes a discussion of present technological and methodological challenges and emerging application areas.
2. Electrochemical biosensor designs for pathogen detection
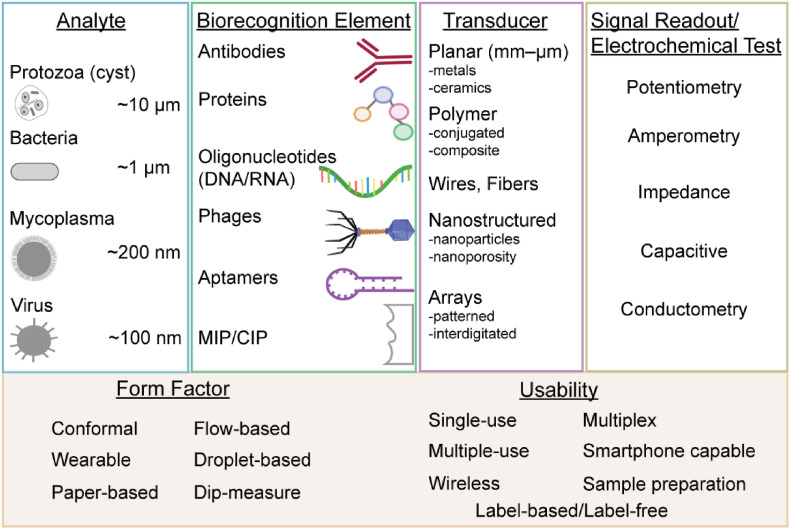
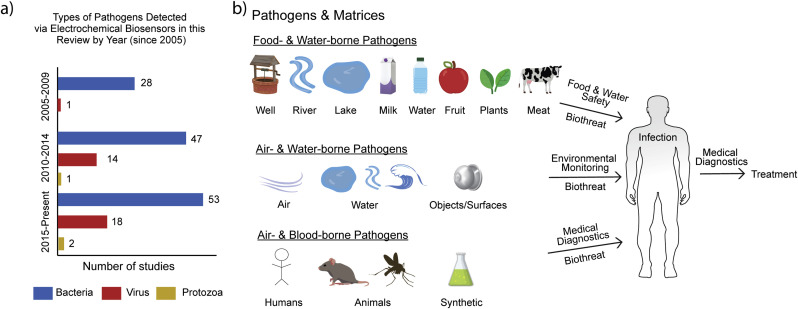
A chemical sensor is a device that transforms chemical information, such as the concentration of a specific sample component or total compositional analysis into an analytically useful signal (Thévenot et al. 2001). The electrochemical method utilized is a distinguishing aspect of an electrochemical biosensor. In addition to the electrochemical method, the sample handling approach and sensor signal readout format also provide distinguishing aspects of a biosensor-based approach for pathogen detection. Thus, we review electrochemical biosensors for pathogen detection using a framework built upon transducer elements, biorecognition elements, and measurement formats. An overview of electrochemical biosensors for pathogen detection is provided in Fig. 1 . As shown in Fig. 2 a, while the detection of bacterial pathogens remains an area of focus, the detection of viral pathogens and protozoa is an emerging area. As shown in Fig. 2b, studies have focused on pathogen detection in various matrices. We next discuss the transduction elements, biorecognition elements, and measurement formats associated with electrochemical biosensors for pathogen detection.
Fig. 1.
Components and measurement formats associated with electrochemical biosensors for pathogen detection.
Fig. 2.
a) Trend in pathogens detected by electrochemical biosensors since 2005 based on the data shown in Table 1, Table 2. b) Common matrices associated with the various pathogen detection applications.
2.1. Transduction elements
The transduction element of an electrochemical biosensor is an electrochemical cell where the main component is commonly a working electrode. A three electrode format (working, auxiliary, and reference) is commonly employed in a potentiostatic system, while a two electrode format (working and auxiliary) is often used for conductometry and electrochemical impedance spectroscopy (EIS). Electrodes can be fabricated from multiple materials and using various manufacturing processes. An electrode is an electronic conductor through which charge is transported by the movement of electrons and holes (Bard and Faulkner, 2000). Electrodes are thus fabricated from conducting and semiconducting materials, including metals, such as gold (Au), and nonmetals, such as carbon. Manufacturing processes can be used to fabricate electrodes of various sizes, including bulk structures (greater than 1 mm) and micro- and nano-structures. As a result, electrodes can be classified by type and form of material, manufacturing process, and design. Electrode designs can be classified by form factor, which includes planar, wire, nanostructured, or array-based. The material, fabrication approach, and design affect the electrode's structure and properties, which ultimately determine the biosensor's performance, including sensitivity, selectivity, limit of detection (LOD), and dynamic range. They also influence the biosensor's cost, manufacturability, disposability, and measurement capabilities.
2.1.1. Metal electrodes
Metal electrodes, such as Au and platinum (Pt), have been commonly used for pathogen detection. Thick metal electrodes are commonly fabricated from bulk structures via cutting processes. Thin-film metal electrodes are often fabricated by deposition of metals on insulating substrates through traditional microfabrication approaches, including physical vapor deposition (Hierlemann et al. 2003) and screen printing (Taleat et al. 2014). Resultant conductive components are often embedded in insulating polymer or ceramic substrates, including Teflon, polyetherkeytone (PEK), and glass, to complete fabrication of the transducer element. While not yet applied to pathogen detection applications, three-dimensional (3D) printing processes, including inkjet printing (Bhat et al. 2018; Medina-Sánchez et al. 2014; Pavinatto et al. 2015), selective laser melting (Ambrosi et al. 2016; Loo et al. 2017), and microextrusion printing (Foo et al. 2018), have also been used for the fabrication of electrochemical sensors and electrodes using a variety of metals. As shown in Table 1 , unstructured metal electrodes exhibit a range of detection limits. For example, the detection limits of electrochemical biosensors for bacteria that employ unstructured metal electrodes range from 1 to 104 CFU/mL (see Table 1).
Table 1.
Classification of label-free electrochemical biosensors for detection of pathogens in terms of: target, working electrode, biorecognition element, electrochemical method, limit of detection, and electrochemical probe. Abbreviations: quartz crystal microbalance (QCM), electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), plaque-forming unit (PFU), colony-forming unit (CFU), indium tin oxide (ITO), carbon nanotube (CNT), magnetic bead (MB), nanoparticle (NP), differential pulse voltammetry (DPV), square wave voltammetry (SWV), anodic stripping voltammetry (ASV), hemagglutination units (HAU), and median tissue culture infectious dose (TCID50).
| Target Pathogen | Working Electrode | Biorecognition Element | Electrochemical Method & Probe | Limit of Detection | Reference |
|---|---|---|---|---|---|
| E. coli | Au interdigitated microelectrode array | polyclonal anti-E.coli | EIS | 104 CFU/mL | Radke and Alocilja (2005) |
| E. coli | ITO electrode | monoclonal anti-E. coli | CV, EIS; Fe(CN)63-/4- | 4 × 103 CFU/mL | Zhang et al. (2005) |
| E. coli | chromium interdigitated microelectrode array | anti-E. coli | EIS | – | Suehiro et al. (2006) |
| S. typhimurium | ITO interdigitated microelectrode array | anti-S. typhimurium | EIS | 10 CFU/mL | Yang and Li (2006) |
| V. cholerae | carbon electrode | polyclonal anti-V. cholerae | amperometry | 8 CFU/mL | Sharma et al. (2006) |
| E. coli | Pt wire electrode | polyclonal anti-E. coli | potentiometry | 9 × 105 CFU/mL | Boehm et al. (2007) |
| E. coli | Au microelectrode | polyclonal anti-E.coli | EIS | 10 CFU/mL | Maalouf et al. (2007) |
| L. monocytogenes | TiO2 nanowires on Au electrode | monoclonal anti-L. monocytogenes | EIS | 470 CFU/mL | Wang et al. (2008) |
| E. coli | Au electrode | polyclonal anti-E. coli | CV, EIS; Fe(CN)63-/4- | 50 CFU/mL | Geng et al. (2008) |
| S. typhimurium | Au electrode | polyclonal anti-S. typhimurium | EIS; Fe(CN)63-/4- | 10 CFU/mL | Pournaras et al. (2008) |
| S. typhimurium | Au microelectrode | anti-S. typhimurium | EIS; Fe(CN)63-/4- | 500 CFU/mL | Nandakumar et al. (2008) |
| E. coli | graphite interdigitated microelectrode array | E. coli-specific bacteriophages | EIS | 104 CFU/mL | Shabani et al. (2008) |
| S. typhimurium | Au electrode | polyclonal anti-S. typhimurium | EIS | 100 CFU/mL | Mantzila et al. (2008) |
| S. typhimurium | macroporous silicon electrode | anti-S. typhimurium | EIS | 103 CFU/mL | Das et al. (2009) |
| West Nile virus (WNV) | nanostructured alumina on Pt wire electrode | monoclonal anti-WNV | AC voltammetry | 0.02 viruses/mL | Nguyen et al. (2009) |
| S. typhimurium | Au electrode | monoclonal anti-S. typhimurium | EIS; Fe(CN)63-/4- | 100 CFU/mL | La Belle et al. (2009) |
| S. typhimurium | CNTs on carbon rod electrode | anti-S. typhimurium aptamer | potentiometry | 0.2 CFU/mL | Zelada-Guillen et al. (2009) |
| E. coli | Au electrode | anti-E. coli | CV, EIS; Fe(CN)63-/4- | 3.3 CFU/mL | Escamilla-Gomez et al. (2009) |
| B. anthracis | Ag electrode | monoclonal and polyclonal anti-B. anthracis | conductometry | 420 spores/mL | Pal and Alocilja (2009) |
| E. coli | polysilicon interdigitated microelectrode array | polyclonal anti-E. coli | EIS | 300 CFU/mL | de la Rica et al. (2009) |
| E. coli | Au interdigitated microelectrode array | E. coli-specific bacteriophages | EIS | 104 CFU/mL | Mejri et al. (2010) |
| E. coli | CNTs on carbon rod electrode | anti-E. coli aptamer | potentiometry | 6 CFU/mL | Zelada-Guillen et al. (2010) |
| Campylobacter jejuni | Fe3O4 nanoparticles on carbon electrode | monoclonal anti-Flagellin A | EIS; Fe(CN)63-/4- | 103 CFU/mL | Huang et al. (2010) |
| marine pathogenic sulphate-reducing bacteria (SRB) | AuNPs on nickel foam electrode | anti-SRB | EIS | 21 CFU/mL | Wan et al. (2010) |
| E. coli | Ag nanofiber array electrode | monoclonal and polyclonal anti-E. coli | conductometry | 61 CFU/mL | Luo et al. (2010) |
| bovine viral diarrhea virus (BVDV) | Ag nanofiber array electrode | monoclonal and polyclonal anti-BVDV | conductometry | 103 CCID/mL | Luo et al. (2010) |
| E. coli | Au interdigitated microelectrode array | magainin I peptide | EIS | 103 CFU/mL | Mannoor et al. (2010) |
| E. coli | Au rod electrode | concanavalin A lectin | capacitive | 12 CFU/mL | Jantra et al. (2011) |
| rotavirus | graphene microelectrode | monoclonal anti-rotavirus | CV | 103 PFU/mL | Liu et al. (2011) |
| human influenza A virus H3N2 | Au electrode | polyclonal anti-H3N2 | EIS | 8 ng/mL | Hassen et al. (2011) |
| E. coli | Au microelectrode | polyclonal anti-E. coli | capacitive, EIS, CV; Fe(CN)63-/4- | 220 CFU/mL | Li et al. (2011) |
| Enterobacter cloacae | Au electrode | concanavalin A lectin, ricinus communis agglutinin lectin | CV, EIS; Fe(CN)63-/4- | 1 × 103 CFU/mL | Xi et al. (2011) |
| E. coli | Au electrode | concanavalin A lectin, ricinus communis agglutinin lectin | CV, EIS; Fe(CN)63-/4- | 100 CFU/mL | Xi et al. (2011) |
| B. subtilis | Au electrode | concanavalin A lectin | CV, EIS; Fe(CN)63-/4- | 1 × 104 CFU/mL | Xi et al. (2011) |
| E. coli | Pt wire electrode | anti-E. coli | EIS | 100 CFU/mL | Tan et al. (2011) |
| S. aureus | Pt wire electrode | anti-S. aureus | EIS | 100 CFU/mL | Tan et al. (2011) |
| marine pathogenic sulphate-reducing bacteria (SRB) | graphene/chitosan composite on carbon electrode | anti-SRB | CV, EIS; Fe(CN)63-/4- | 18 CFU/mL | Wan et al. (2011) |
| swine influenza virus (SIV) H1N1 | PDDA/CNT composite on Au microelectrode | anti-SIV | conductometry | 180 TCID50/mL | Lee et al. (2011) |
| E. coli | graphene microelectrode | anti-E. coli | amperometry | 10 CFU/mL | Huang et al. (2011) |
| E. coli | PEDOT:PSS electrode | anti-E. coli | amperometry | 103 CFU/mL | He et al. (2012) |
| dengue type 2 virus (DENV-2) | nanostructured alumina on Pt wire electrode | monoclonal anti-DENV-2 | DPV; Ferrocene methanol |
1 PFU/mL | Cheng et al. (2012) |
| DENV-2 | nanostructured alumina on Pt wire electrode | monoclonal anti-DENV-2 | CV, EIS; Ferrocene methanol | 1 PFU/mL | Nguyen et al. (2012) |
| human influenza A viruses H1N1 and H3N2 | silicon nanowire electrode array | anti-H1N1, anti-H3N2 | conductometry | 2.9 × 104 viruses/mL | Shen et al. (2012) |
| E. coli | AuNP/Chitosan/CNT and SiO2/thionine NP composite on Au electrode | monoclonal anti-E. coli | CV | 250 CFU/mL | Li et al. (2012) |
| E. coli | CNT/polyallylamine composite on graphite electrode | monoclonal anti-E. coli | ASV | 800 cells/mL | Viswanathan et al. (2012) |
| Campylobacter | CNT/polyallylamine composite on graphite electrode | monoclonal anti-Campylobacter | ASV | 400 cells/mL | Viswanathan et al. (2012) |
| S. typhimurium | CNT/polyallylamine composite on graphite electrode | monoclonal anti-S. typhimurium | ASV | 400 cells/mL | Viswanathan et al. (2012) |
| S. aureus | CNT electrode | anti-S. aureus aptamer | potentiometry | 800 CFU/mL | Zelada-Guillen et al. (2012) |
| E. coli | Au electrode | mannose carbohydrate ligand | EIS; Fe(CN)63-/4- | 100 CFU/mL | Guo et al. (2012) |
| S. aureus | graphene interdigitated microelectrode array | odoranin-HP peptide | conductometry | 1 × 104 cells/mL | Mannoor et al. (2012) |
| Helicobacter pylori | graphene interdigitated microelectrode array | odoranin-HP peptide | conductometry | 100 cells | Mannoor et al. (2012) |
| L. innocua | Au electrode | L. innocua-specific bacteriophage | EIS; Fe(CN)63-/4- | 1.1 × 104 CFU/mL | Tolba et al. (2012) |
| E. coli | polyaniline on Au electrode | monoclonal anti-E. coli | EIS | 100 CFU/mL | Chowdhury et al. (2012). |
| E. coli | Au interdigitated microelectrode array | anti-E. coli | EIS | 2.5 × 104 CFU/mL | Dweik et al. (2012). |
| E. coli | ultra-nanocrystalline diamond microelectrode array | anti-E. coli | EIS; Fe(CN)63-/4- | 1 × 103 CFU/mL | Siddiqui et al. (2012). |
| human influenza A virus H1N1 | Au microelectrode | phenotype-specific sialic acid-galactose moieties | EIS; Fe(CN)63-/4- | – | Wicklein et al. (2013) |
| E. coli | Au electrode | E. coli-specific bacteriophages | EIS; Fe(CN)63-/4- | 800 CFU/mL | Tlili et al. (2013) |
| DENV-2, dengue virus 3 (DENV-3) | Pt-coated nanostructured alumina membrane electrode | monoclonal anti-dengue | EIS; Fe(CN)63-/4- | 0.23 PFU/mL, 0.71 PFU/mL | Peh and Li (2013) |
| cucumber mosaic virus (CMV) | polypyrrole nanoribbons on Au microelectrode array | polyclonal anti-CMV | amperometry | 10 ng/mL | Chartuprayoon et al. (2013) |
| E. coli | Au electrode | polyclonal anti-E. coli | EIS; Fe(CN)63- | 2 CFU/mL | Barreiros dos Santos et al. (2013) |
| E. coli | AuNPs on reduced graphene oxide microelectrode | anti-E. coli | EIS; Fe(CN)63-/4- | 150 CFU/mL | Wang et al. (2013) |
| E. coli | Ag/AgCl wire electrode | anti-E. coli | EIS | 10 CFU/mL | Joung et al. (2013) |
| murine norovirus (MNV) | AuNPs on carbon electrode | anti-norovirus (MNV) aptamer | SWV, fluorescence; Fe(CN)63-/Ru(NH3)63+ | 180 viruses | Giamberardino et al. (2013) |
| rotavirus | reduced graphene oxide microelectrode | anti-rotavirus | amperometry | 100 PFU | Liu et al. (2013) |
| S. typhimurium | AuNP-functionalized poly(amidoamine)-CNT-chitosan composite on carbon electrode | anti- S. typhimurium | CV, EIS; Fe(CN)63-/4- | 500 CFU/mL | Dong et al. (2013) |
| E. coli | Au-tungsten microwire electrode | monoclonal anti-E. coli | EIS; Fe(CN)63-/4- | 5 CFU/mL | Lu et al. (2013) |
| E. coli | Pt wire electrode | anti-E. coli | EIS | 10 CFU/mL | Chan et al. (2013) |
| S. aureus | reduced graphene oxide on carbon rod electrode | anti-S. aureus aptamer | potentiometry | 1 CFU/mL | Hernandez et al. (2014) |
| E. coli | PAA/PD/CNT composite on carbon electrode | anti-E. coli | ASV | 13 CFU/mL | Chen et al. (2014) |
| S. typhimurium | AuNPs on graphene oxide on carbon electrode | anti-S. typhimurium aptamer | EIS; Fe(CN)63-/4- | 3 CFU/mL | Ma et al. (2014) |
| S. aureus | AuNPs on reduced graphene oxide on carbon electrode | anti-S. aureus synthetic aptamer | EIS; Fe(CN)63-/4- | 10 CFU/mL | Jia et al. (2014) |
| E. coli | Au electrode | mannose carbohydrate ligand | CV, mass change | 1 CFU/mL | Yazgan et al. (2014) |
| L. monocytogenes | Au interdigitated microelectrode array | leucocin A antimicrobial peptide | EIS | 103 CFU/mL | Etayash et al. (2014) |
| S. typhimurium | Au interdigitated microelectrode array | monoclonal anti-S. typhimurium | EIS | 3 × 103 CFU/mL | Dastider et al. (2015) |
| S. aureus | Au electrode | polyclonal anti-S. typhimurium | EIS; Fe(CN)63-/4- | 10 CFU/mL | Bekir et al. (2015) |
| E. coli | CNTs on Au electrode | clavanin A peptide | EIS; Fe(CN)63-/4- | 100 CFU/mL | Andrade et al. (2015) |
| Klebsiella pneumoniae | CNTs on Au electrode | clavanin A peptide | EIS; Fe(CN)63-/4- | 103 CFU/mL | Andrade et al. (2015) |
| Enterococcus faecalis | CNTs on Au electrode | clavanin A peptide | EIS; Fe(CN)63-/4- | 103 CFU/mL | Andrade et al. (2015) |
| B. subtilis | CNTs on Au electrode | clavanin A peptide | EIS; Fe(CN)63-/4- | 100 CFU/mL | Andrade et al. (2015) |
| E. coli | PEI/CNT composite on carbon electrode | E. coli-specific bacteriophages | EIS; Fe(CN)63-/4- | 50 CFU/mL | Zhou and Ramasamy (2015) |
| dengue virus 1–4 | AuNPs on Au electrode | anti-DENV-1, anti-DENV-2, anti-DENV-3, anti-DENV-4 | CV, EIS; Fe(CN)63-/4- | – | Luna et al. (2015) |
| E. coli | ITO microelectrode | monoclonal anti-E. coli | EIS; Fe(CN)63-/4- | 1 CFU/mL | Barreiros dos Santos et al. (2015) |
| avian influenza virus (AIV) H5N1 | Au interdigitated microelectrode array | monoclonal anti-AIV-H5N1 | EIS; Fe(CN)63-/4- | 4 HAU/mL | Lin et al. (2015) |
| C. parvum | AuNPs on carbon electrode | anti-C. parvum aptamer | SWV; Fe(CN)63-/4- | 100 oocysts | Iqbal et al. (2015) |
| E. coli | CNT-coated Au-tungsten microwire electrodes | polyclonal anti-E. coli | amperometry | 100 CFU/mL | Yamada et al. (2016) |
| S. aureus | CNT-coated Au-tungsten microwire electrodes | polyclonal anti-S. aureus | amperometry | 100 CFU/mL | Yamada et al. (2016) |
| S. aureus | Au interdigitated microelectrode array | anti-S. aureus | EIS; Fe(CN)63-/4- | 1.3 CFU/mL | Primiceri et al. (2016) |
| L. monocytogenes | Au interdigitated microelectrode array | anti-L. monocytogenes | EIS; Fe(CN)63-/4- | 5 CFU/mL | Primiceri et al. (2016) |
| norovirus | Au microelectrode | anti-norovirus aptamer | SWV; Fe(CN)63-/Ru(NH3)63+ | 10 PFU/mL | Kitajima et al. (2016) |
| avian influenza virus (AIV) H5N1 | Au interdigitated microelectrode array | anti-AIV-H5N1 aptamer | EIS; Fe(CN)63-/4- | 4.2 HAU/mL | Callaway et al. (2016) |
| S. typhimurium | poly[pyrrole-co-3-carboxyl-pyrrole] copolymer electrode | anti-S. typhimurium aptamer | EIS | 3 CFU/mL | Sheikhzadeh et al. (2016) |
| E. coli | polysilicon interdigitated microelectrodes | polyclonal anti-E. coli | EIS | – | Mallén-Alberdi et al. (2016) |
| human influenza A virus H3N2 | Au electrode | phenotype-specific oligoethylene glycol moieties | EIS | 1.3 × 104 viruses/mL | Hushegyi et al. (2016) |
| E. coli | PEI/CNT composite on Au microwire electrode | polyclonal anti-E. coli | amperometry | 100 CFU/mL | Lee and Jun (2016) |
| V. cholerae | CeO2 nanowires on Pt microelectrode | anti-V. cholerae | EIS; Fe(CN)63-/4- | 100 CFU/mL | Tam and Thang (2016) |
| S. aureus | PEI/CNT composite on Au microwire electrode | polyclonal anti-S. aureus | amperometry | 100 CFU/mL | Lee and Jun (2016) |
| E. coli | graphene microelectrode | polyclonal anti-E. coli | amperometry | 5 × 103 CFU/mL | Wu et al. (2016) |
| E. coli | Au electrode | concanavalin A lectin | EIS; Fe(CN)63-/4- | 75 cells/mL | Yang et al. (2016b) |
| E. coli | Pt wire electrodes | anti-E. coli | EIS | 100 CFU/mL | Tian et al. (2016) |
| S. aureus | Pt wire electrodes | anti-S. aureus | EIS | 100 CFU/mL | Tian et al. (2016) |
| B. subtilis | CNTs on Au interdigitated microelectrode array | polyclonal anti-B. subtilis | conductometry | 100 CFU/mL | Yoo et al. (2017) |
| S. epidermidis | Au microelectrode | S. epidermidis-imprinted poly(3-aminophenylboronic acid) polymer film | EIS; Fe(CN)63-/4- | 103 CFU/mL | Golabi et al. (2017) |
| norovirus | graphene/AuNP composite on carbon electrode | anti-norovirus aptamer | DPV; Ferrocene | 100 pM | Chand and Neethirajan (2017) |
| norovirus | Au electrode | synthetic norovirus-specific peptide | CV, EIS; Fe(CN)63-/4- | 7.8 copies/mL | Hwang et al. (2017) |
| E. coli | CuO/cysteine/reduced graphene/Au oxide electrode | monoclonal anti-E. coli | EIS; Fe(CN)63-/4- | 3.8 CFU/mL | Pandey et al. (2017) |
| Japanese encephalitis virus (JEV) | carbon NPs on carbon electrode | monoclonal anti-JEV | CV, EIS; Fe(CN)63-/4- | 2 ng/mL | Chin et al. (2017) |
| S. aureus | CNTs on carbon electrode | polyclonal anti-S. aureus | DPV; Fe(CN)63-/4- | 13 CFU/mL | Bhardwaj et al. (2017) |
| human influenza A virus H1N1 | PEDOT film electrode | hemagglutinin-specific trisaccharide ligand | EIS, potentiometry, mass change; Fe(CN)63-/4- | 0.013 HAU | Hai et al. (2017) |
| human influenza A virus H1N1 | reduced graphene oxide on Au microelectrode | monoclonal anti-H1N1 | chrono-amperometry; Fe(CN)63-/4- | 0.5 PFU/mL | Singh et al. (2017b) |
| E. coli | Au microelectrode | E. coli-imprinted MAH/HEMA polymer film | capacitive | 70 CFU/mL | Idil et al. (2017) |
| E. coli | chitosan/polypyrrole/CNT/AuNP composite on graphite electrode | monoclonal coli | CV; Fe(CN)63-/4- | 30 CFU/mL | Güner et al. (2017) |
| S. dysenteriae | AuNPs on carbon electrode | anti-S. dysenteriae aptamer | EIS; Fe(CN)63-/4- | 1 CFU/mL | Zarei et al. (2018) |
| human influenza A virus H1N1 | PEDOT:PSS film electrode | hemagglutinin-specific trisaccharide ligand | amperometry | 0.015 HAU | Hai et al. (2018) |
| S. aureus | fluoride-doped tin oxide electrode | S. aureus-imprinted Ag–MnO2 film | DPV; Fe(CN)63-/4- | 103 CFU/mL | Divagar et al. (2019) |
| E. coli | Au microelectrode | E. coli-imprinted TEOS/MTMS sol-gel film | EIS; Fe(CN)63-/4- | 1 CFU/mL | Jafari et al. (2019) |
| norovirus | Au electrode | norovirus-specific peptide | EIS; Fe(CN)63-/4- | 1.7 copies/mL | Baek et al. (2019) |
| C. parvum | Au interdigitated microelectrode array | monoclonal anti-C. parvum | Capacitive; Fe(CN)63-/4- | 40 cells/mm2 | Luka et al. (2019) |
| E. coli | 4-(3-pyrrol) butryic acid electrode | concanavalin A lectin, Arachis hypogaea lectin | EIS | 6 × 103 CFU/mL | Saucedo et al. (2019) |
| B. subtilis | 4-(3-pyrrol) butryic acid electrode | concanavalin A lectin, Arachis hypogaea lectin | EIS | 6 × 103 CFU/mL | Saucedo et al. (2019) |
| E. coli | silica NPs on polyelectrolyte multilayer on Au electrode | polyclonal anti-E. coli | CV; Fe(CN)63-/4- | 2 × 103 CFU/mL | Mathelie-Guinlet et al. (2019) |
| E. coli | silica NPs on polyelectrolyte multilayer on Au electrode | polyclonal anti-E. coli | CV; Fe(CN)63-/4- | 2 × 103 CFU/mL | Mathelie-Guinlet et al. (2019) |
2.1.2. Ceramic electrodes
Conducting and semiconducting ceramics, including indium tin oxide (ITO), polysilicon, and titanium dioxide (TiO2) have also been examined for pathogen detection. For example, Das et al. used a silicon electrode for Salmonella typhimurium (S. typhimurium) detection (Das et al. 2009). Barreiros dos Santos et al. developed an antibody-functionalized ITO electrode for the detection of E. coliwith a dynamic range of 10–106 CFU/mL (Barreiros dos Santos et al. 2015). In addition to high conductivity, ITO is transparent, which presents various measurement advantages, including the ability to accurately correlate biosensor response with pathogen surface coverage (Aydın and Sezgintürk, 2017; Yang and Li, 2005). Transparent electrodes also enable in situ verification of target binding via microscopic techniques and offer compatibility with optical approaches, such as those based on optical stimulation (Wenzel et al. 2018). Carbon electrodes based on various allotropes of carbon, such as graphite and glass-like carbon, can also be classified as ceramic materials due to their mechanical properties (e.g., brittleness).
2.1.3. Polymer electrodes
Polymers have also been investigated as electrodes for pathogen detection. Polymers have various advantages, including tunable electrical conductivity, biocompatiblity, and environmentally stability. Polymer electrodes are also compatible with a range of biorecognition element immobilization techniques (Arshak et al. 2009; Guimard et al. 2007). Polymers also exhibit mechanical properties that enable electrode-tissue mechanical matching, an important consideration in the design of implantable and wearable biosensors. Polymer electrodes can be broadly classified as (1) conjugated polymer or (2) polymer composite.
Polyaniline and polypyrrole have been the most commonly used conjugated polymers for pathogen detection due to their high conductivity in the doped state (Kaur et al. 2015). Moreover, polypyrrole has been shown to be biocompatible and exhibit affinity for methylated nucleic acids (Arshak et al. 2009). However, polyaniline films lose electrochemical activity in solutions of pH greater than 4, which presents a measurement challenge when considering samples of varying pH (Wan, 2008). Conjugated polymer electrodes commonly exhibit thin-film form factors and are deposited onto insulating substrates via layer-by-layer approaches, spin coating, or electrochemical polymerization (Xia et al. 2010). For example, Chowdhury et al. used a polyaniline electrode for detection of E. coli over a dynamic range of 102 to 107 CFU/mL (Chowdhury et al. 2012). Hai et al. and He et al. used organic transistors based on spin-coated poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) films for detection of human influenza A virus (H1N1) and E. coli, respectively (Hai et al. 2018; He et al. 2012).
Polymer composite electrodes are often composed of a non-conducting polymer mixed with a conducting or semiconducting dispersed phase. Micro-particles and nanomaterials, such as graphite, Au nanoparticles (AuNPs), graphene, and carbon nanotubes (CNTs), have been commonly used as the dispersed phase (Dong et al. 2013; Lee et al. 2011; Lee and Jun 2016; Li et al. 2012; Viswanathan et al. 2012) in combination with various polymers, including chitosan (Güner et al. 2017), polyethylenimine (PEI) (Lee and Jun 2016), and polyallyamine (Viswanathan et al. 2012). For example, Viswanathan et al. developed a polyallylamine/CNT polymer composite electrode for the detection of E. coli, S. typhimurium, and Campylobacter via anodic stripping voltammetry over the dynamic range of 103 to 105 cells/mL (Viswanathan et al. 2012). A multicomponent polymer composite electrode of poly(amidoamine), CNTs, and chitosan layered with AuNPs enabled the detection of S. typhimurium (Dong et al. 2013). The detection limits associated with polymer composite electrodes are comparable to metallic and polymer electrodes and range from 1 to 103 CFU/mL (see Table 1). While polymer composite electrodes often contain nanomaterials, they are dispersed throughout the bulk of polymer, which is in contrast to the electrode nanostructuring techniques that occur at the electrode surface and are discussed in the following sections.
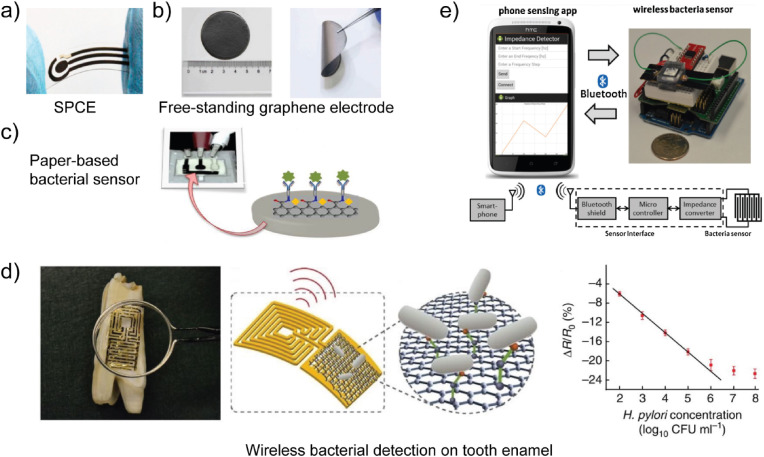
Polymer electrode development has been, in part, driven by the need for flexible biosensors. For example, free-standing film electrodes and polymer electrodes on flexible substrates, such as paper, are now being examined for biosensing applications (Xu et al. 2019). Given conjugated polymers and polymer composites are compatible with 3D printing processes (Kong et al. 2014), polymer electrodes are also emerging as attractive candidates for wearable conformal (i.e., form-fitting) biosensors. While polymer electrodes typically exhibit planar form factors, such as thin films, they can also be constructed as nanowires and nanofibers, as discussed in the following section. A comprehensive discussion of biosensor LOD and dynamic range for all electrode materials is provided in Table 1, Table 2 .
Table 2.
Classification of electrochemical biosensors employing labels for pathogen detection in terms of: target, working electrode, biorecognition element, electrochemical method, limit of detection, electrochemical probe, and label or secondary processing step. Abbreviations: quartz crystal microbalance (QCM), electrochemical impedance spectroscopy (EIS), cyclic voltommetry (CV), plaque-forming unit (PFU), colony-forming unit (CFU), indium tin oxide (ITO), carbon nanotube (CNT), magnetic bead (MB), nanoparticle (NP), differential pulse voltammetry (DPV), square wave voltammetry (SWV), anodic stripping voltammetry (ASV), hemagglutination units (HAU), and median tissue culture infectious dose (TCID50).
| Target Pathogen | Working Electrode | Biorecognition Element | Electrochemical Method & Probe | Limit of Detection | Secondary Binding Step | Reference |
|---|---|---|---|---|---|---|
| E. coli | ITO electrode | anti-E. coli | EIS; Fe(CN)63-/4- | 6 × 105 cells/mL | antibody/ALP conjugate label for amplification | Yang and Li (2005) |
| V. cholerae | carbon/polystyrene electrode | polyclonal anti-V.cholerae | chrono-amperometry | 105 cells/mL | antibody-ALP conjugate label for amplification | Rao et al. (2006) |
| E. coli | Au interdigitated microelectrode array | polyclonal anti-E. coli | EIS | 2.67 × 106 cells/mL | antibody-coated MBs for separation | Varshney et al. (2007) |
| V. parahaemolytic | carbon electrode | anti-V. parahaemolytic | CV; thionine/hydrogen peroxide | 7.37 × 104 CFU/mL | antibody/HRP conjugate label for transduction | Zhao et al. (2007) |
| E. coli | Au interdigitated microelectrode array | polyclonal anti-E. coli | EIS | 7.4 × 104 CFU/mL | antibody-coated MBs for separation and amplification | Varshney and Li (2007) |
| E. coli | AuNPs on carbon electrode | monoclonal and polyclonal anti-E. coli | CV; ferrocenedicarboxylic acid/hydrogen peroxide | 6 CFU/mL | polyclonal antibody/HRP conjugate label for amplification | Lin et al. (2008) |
| S. aureus | Au electrode | anti-S. aureus | amperometry; tetrathiafulvalene/hydrogen peroxide | 370 cells/mL | antibody/HRP conjugate label for amplification | Escamilla-Gomez et al. (2008) |
| S. typhimurium | Au electrode | monoclonal anti-S. typhimurium | chrono-amperometry; tetramethylbenzidine dihydrochloride/hydrogen peroxide | 21 CFU/mL | anti-S. typhimurium polyclonal antibody/HRP conjugate label for amplification | Salam and Tothill (2009) |
| S. typhimurium | graphite-epoxy composite electrode | polyclonal anti-S. typhimurium | amperometry | 0.1 CFU/mL | primary antibody-coated MBs for separation, secondary antibody/HRP conjugate label for amplification | Liebana et al. (2009) |
| avian influenza virus (AIV) H5N1 | Au interdigitated microelectrode array | monoclonal anti-AIV-H5 | EIS | 0.26 HAU/mL | antibody-coated MBs for separation | Wang et al. (2010) |
| Streptococcus pneumoniae | Au electrode | polyclonal anti-S. pneumoniae | amperometry; tetrathiafulvalene/hydrogen peroxide | 1.5 × 104 CFU/mL | antibody-coated MBs for separation and bacteria immobilization, antibody/HRP conjugate label for amplification | Campuzano et al. (2010) |
| E. coli | carbon-graphite electrode | monoclonal anti-E. coli | CV | 7 CFU/mL | antibody-coated MBs for separation, antibody/polyaniline label for amplification | Setterington and Alocilja (2011) |
| S. aureus | MBs on Au electrode | polyclonal anti-Protein A (S. aureus) | amperometry; tetrathiafulvalene/hydrogen peroxide | 1 CFU/mL | antibody/Protein A/HRP conjugate for amplification | Esteban-Fernandez de Avila et al. (2012) |
| avian influenza virus (AIV) H5N1 | Au interdigitated microelectrode array | monoclonal anti-AIV-H5, polyclonal anti-AIV-N1 | EIS | 103 EDI50/mL | anti-AIV-H5 monoclonal antibody- coated MBs for separation, red blood cell label for amplification | Lum et al. (2012) |
| E. coli | AuNPs/SiO2 nanocomposite on sulfhydryl chitosan/Fe(C2H5)2/C60 composite on carbon electrode | monoclonal anti-E. coli | CV; ferrocene | 15 CFU/mL | antibody/glucose oxidase/Pt nanochain conjugate label for amplification | Li et al. (2013) |
| C. parvum | polypyrrole-coated carbon electrode | polyclonal anti-C. parvum | chrono-potentiometry; o-phenylenediamine/hydrogen peroxide | 500 oocysts/mL | antibody/HRP conjugate label for amplification | Laczka et al. (2013) |
| L. monocytogenes | polymeric ion-selective membrane electrode | anti-L. monocytogenes InlA aptamer | potentiometry | 10 CFU/mL | aptamer/protamine label for transduction | Ding et al. (2014) |
| avian influenza virus (AIV) H5N1 | Au interdigitated electrode array | anti-AIVH5N1 aptamer | EIS | 0.04 HAU/mL | aptamer-coated MBs for separation, Concanavalin A/glucose oxide-coated AuNP labels for amplification | Fu et al. (2014). |
| L. monocytogenes | interdigitated microelectrode array | monoclonal and polyclonal anti-L. monocytogenes | EIS | 300 CFU/mL | monoclonal antibody-coated MBs for separation, polyclonal antibody-coated AuNP label for secondary binding amplification | Chen et al. (2015) |
| E. coli | carbon electrode | polyclonal anti-E.coli | chrono-amperometry | 148 CFU/mL | primary antibody-coated MBs for separation, secondary antibody-coated AuNPs for amplification | Hassan et al. (2015) |
| avian influenza virus (AIV) H5N1 | AuNPs on ITO microelectrode | polyclonal anti-AIVH5N1 | ASV | 10 pg/mL | antibody-coated MBs for separation and anodic stripping | Zhou et al. (2015) |
| E. coli | Au interdigitated microelectrode array | anti-E.coli | EIS; Fe(CN)63-/4- | 100 CFU/mL | wheat germ agglutinin for amplification | Li et al. (2015) |
| E. coli | carbon electrode | monoclonal and polyclonal anti-E. coli | DPV | 10 CFU/mL | monoclonal antibody-coated MBs for separation, polyclonal antibody-coated AuNP label for amplification | Wang and Alocilja (2015) |
| norovirus | nanostructured Au microelectrode | concanavalin A lectin, polyclonal anti-norovirus | CV, EIS; Fe(CN)63-/4- | 35 copies/mL | antibody-ALP conjugate label for amplification | Hong et al. (2015) |
| Legionella pneumophila | carbon electrode | polyclonal anti-L. pneumophila | amperometry; hydroquinone/hydrogen peroxide | 10 CFU/mL | primary antibody- coated MBs for separation, secondary antibody/HRP conjugate label for amplification | Martin et al. (2015) |
| S. aureus | carbon electrode | anti-S.aureus aptamer | ASV | 1 CFU/mL | primary aptamer-coated MBs for separation, secondary aptamer-coated AgNP label for anodic stripping | Abbaspour et al. (2015) |
| L. monocytogenes | Au interdigitated microelectrode array | monoclonal and polyclonal anti-L. monocytogenes | EIS | 160 CFU/mL | monoclonal antibody-coated MBs for separation, polyclonal antibody-coated AuNP label for amplification | Chen et al. (2016b) |
| E. coli | Au interdigitated microelectrode array | polyclonal anti-E. coli | CV, amperometry | 52 CFU/mL | antibody-coated, AuNP/glucose oxidase-modified MBs for separation and amplification | Xu et al. (2016a) |
| E. coli | Au interdigitated microelectrode array | anti- E. coli | EIS | 100 CFU/mL | antibody-coated MBs for separation, antibody/glucose oxidase conjugate for amplification | Xu et al. (2016b) |
| S. typhimurium | Au interdigitated microelectrode array | monoclonal anti-S. typhimurium | EIS | 100 CFU/mL | antibody-coated MBs for separation, antibody/glucose oxidase conjugate label for amplification | Xu et al. (2016b) |
| E. coli | chitosan/CNT composite on carbon electrode | polyclonal anti-E. coli | CV; thionine/hydrogen peroxide | 50 CFU/mL | secondary antibody/HRP conjugate label enzyme-assisted reduction reaction | Gayathri et al. (2016) |
| S. typhimurium | carbon electrode | polyclonal and monoclonal anti-S. typhimurium | DPV | 100 cells/mL | polyclonal antibody- coated MBs for separation, monoclonal antibody- coated AuNP label for amplification | Afonso et al. (2016) |
| E. coli | Au electrode | anti-E. coli | EIS; Fe(CN)63-/4- | 100 CFU/mL | AuNP label for amplification | Wan et al. (2016) |
| L. monocytogenes | Au interdigitated electrode array | polyclonal anti-L. monocytogenes | EIS | 1.6 × 103 CFU/mL | antibody-coated MBs for separation, antibody-coated AuNP label for amplification | Wang et al. (2017) |
| E. coli | Au microelectrode | monoclonal anti-E. coli | LSV | 39 CFU/mL | antibody-coated MBs for separation, antibody/AuNP/nucleotide/CdSNP conjugate label for amplification | Li et al. (2017) |
| V. cholerae | Au microelectrode | polyclonal anti-V. cholerae | LSV | 32 CFU/mL | antibody-coated MBs for separation, antibody/AuNP/nucleotide/PbSNP conjugate label for amplification | Li et al. (2017) |
| avian influenza virus (AIV) H5N1 | Au electrode | anti-AIVH5N1, concanavalin A lectin | CV | 0.367 HAU/mL | Concanavalin A- coated MB labels for amplification | Zhang et al. (2017) |
| human influenza A virus H9N2 | carbon electrode | polyclonal anti-influenza A virus M2 protein, fetuin A | chrono-amperometry | 16 HAU | antibody-coated MBs for separation, fetuin A-coated AuNP label for amplification | Sayhi et al. (2018) |
| human enterovirus 71 (EV71) | AuNPs on ITO electrode | monoclonal anti-EV71 | CV, EIS, colorimetry; Fe(CN)63-/4- | 10 pg/mL | antibody/HRP-coated MB labels for amplification | Hou et al. (2018) |
| E. coli | Ag interdigitated microelectrode array | melittin peptide | EIS | 1 CFU/mL | MLT-coated MBs used for separation and bacteria immobilization | Wilson et al. (2019) |
| S. typhimurium | Ag interdigitated electrode array | melittin peptide | EIS | 10 CFU/mL | MLT-coated MBs used for separation and bacteria immobilization | Wilson et al. (2019) |
| S. aureus | Ag interdigitated electrode array | melittin peptide | EIS | 110 CFU/mL | MLT-coated MBs used for separation and bacteria immobilization | Wilson et al. (2019) |
| Middle East respiratory syndrome corona virus (MERS-CoV) | AuNPs on carbon electrode | MERS-CoV antigen-antibody complex | SWV; Fe(CN)63-/4- | 400 fg/mL | MERS CoV-antibody complex | Layqah and Eissa (2019) |
2.1.4. Electrode form factor and patterning
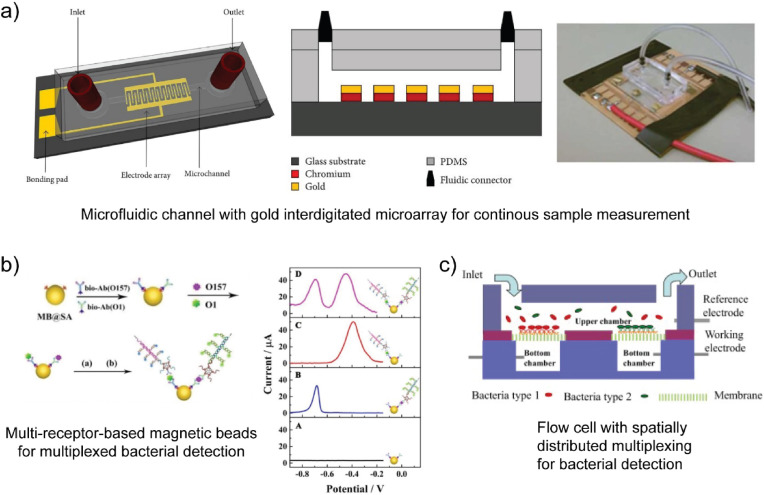
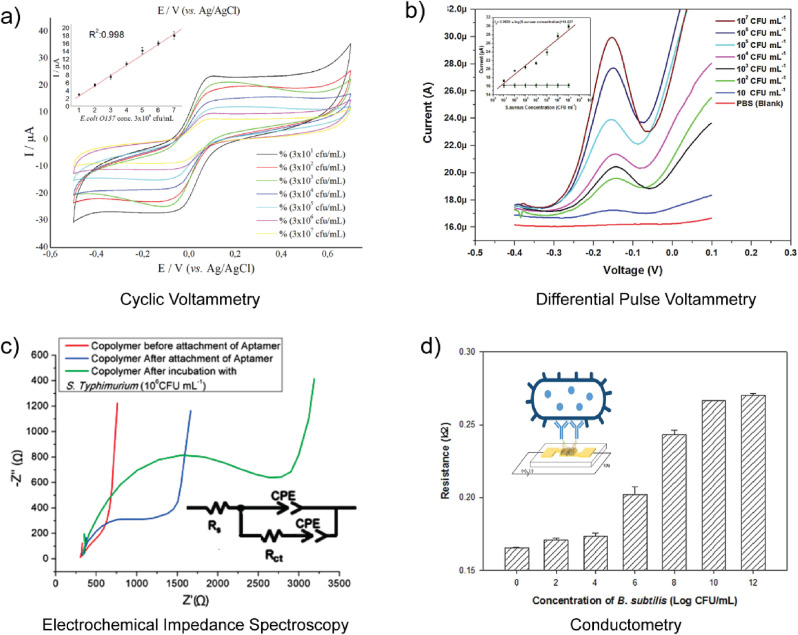
As shown in Table 1, Au electrodes of various size and form factor have been used for pathogen detection. The use of complex masks and programmable tool paths with lithographic and 3D printing processes, respectively, also enable the fabrication of complex electrode geometries (Cesewski et al. 2018; Xu et al. 2017). In addition to complex form factor, lithographic processes, 3D printing processes, and assembly operations also enable the fabrication of electrode arrays through electrode patterning (Hintsche et al. 1994). Electrode arrays, including interdigitated microelectrodes and other patterned electrodes, have been developed in an attempt to enhance the sensitivity and multiplexing capability of biosensors. Interdigitated array microelectrodes (IDAMs) consist of alternating, parallel-electrode fingers organized in an interdigitated pattern. IDAMs have been shown to exhibit rapid response and high signal-to-noise ratio (Varshney and Li, 2009). As shown in Table 1, Au interdigitated microelectrode arrays are one of the most common electrode configurations for pathogen detection. For example, Dastider et al. usedinterdigitated Au microelectrode arrays for detection of S. typhimurium via EIS (see Fig. 4a) (Dastider et al. 2015). Ceramic electrodes, such as ITO, with interdigitated array designs have also been examined for the detection of S. typhimurium (Yang and Li, 2006). Mannoor et al. also examined interdigitated carbon-based electrodes for pathogen detection (Mannoor et al. 2012). The aforementioned emerging manufacturing processes are also used to construct electrode arrays that exhibit geometries other than interdigitated designs for electrochemical sensing applications. For example, Yang et al. used aerosol jet additive manufacturing to fabricate silver (Ag) microelectrode arrays (Yang et al. 2016a).
Fig. 4.
Measurement settings associated with electrochemical biosensor-based multiplexed pathogen detection. a) Microfluidic device with an interdigitated Au microelectrode array for continuous measurement of S. typhimurium (Dastider et al. 2015). b) Conjugated nanoparticles with two different biorecognition elements for E. coli and V. cholerae detection via voltammetry using Fe(CN)63-/4- (Li et al. 2017). c) Schematic of a microfluidic device with two separate spatial regions of biorecognition elements for E. coli and S. aureus (Tian et al. 2016).
2.1.5. Electrode nanostructuring
Transducers with physical dimensions comparable to the target species have been widely investigated as a means of creating sensitive biosensors (Gupta et al. 2004; Pumera et al. 2007; Singh et al. 2010; Wei et al. 2009). Thus, electrodes ranging from micrometers to nanometers have been investigated for pathogen detection. While nanoscale planar electrodes are among the most commonly examined for pathogen detection (Hong et al. 2015; Peh and Li, 2013), the fabrication of nanoscale structures of conducting and semiconducting materials using a wide range of bottom-up and top-down nanomanufacturing processes, such as nanowires, has led to the investigation of nanostructured electrodes for pathogen detection (Patolsky and Lieber, 2005). Nanostructuring can be performed simultaneously with bottom-up electrode fabrication processes or as a post-processing step with top-down electrode fabrication processes.
Nanowire-based electrodes have been fabricated using a variety of engineering materials using both bottom-up and top-down nanomanufacturing processes (Hu et al. 1999; Yogeswaran and Chen, 2008). A detailed review of nanomanufacturing processes for nanowire fabrication can be found elsewhere (Hu et al. 1999). Nanowires can exhibit circular, hexagonal, and even triangular cross-sections. The nanowire aspect ratio, defined as the ratio of the length to width, often ranges from 1 to greater than 10 (Hu et al. 1999; Vaseashta and Dimova-Malinovska, 2005; Wanekaya et al. 2006).
As shown in Table 1, metallic and ceramic microwire- and nanowire-based electrodes have been examined for pathogen detection. For example, Wang et al. used nanowire-bundled TiO2 electrodes synthesized using a bottom-up wet chemistry process for the detection of Listeria monocytogenes (L. monocytogenes) (Wang et al. 2008). Shen et al. fabricated silicon nanowire-based electrodes using a chemical vapor deposition process for the rapid detection of human influenza A virus in an array-based format (Shen et al. 2012).
Although polymer nanowires have been relatively more applied to the detection of non-pathogenic species (Travas-Sejdic et al. 2014), there appears to be potential for their application to pathogen detection. Polymer nanowires are also synthesized via bottom-up and top-down nanomanufacturing processes, including hard template methods, soft template methods, or physical approaches, but efficient, large-scale synthesis remains a challenge (Xia et al. 2010). A comprehensive summary of studies using micro- and nano-wire electrodes for pathogen detection is shown in Table 1. For example, Chartuprayoon et al. used Au microelectrode arrays modified with polypyrrole nanoribbons to detect cucumber mosaic virus (Chartuprayoon et al. 2013).
The topographical modification of electrode surfaces with micro- and nano-structured features beyond wire-like structures has also been investigated for pathogen detection. Electrode nanostructuring increases the electrode surface area without significantly increasing the electrode volume, thereby increasing the ratio of electrode surface area to fluid volume analyzed (Soleymani et al. 2009). Topographical modification of electrodes can also affect their mechanical and electrical properties. For example, electrochemical deposition of PEDOT on silicon electrodes reduces the electrode electrical impedance across a wide frequency range, which offers measurement advantages for neural monitoring and recording applications (Ludwig et al. 2006).
Electrode nanostructuring for pathogen detection beyond the fabrication of nanowire-based electrodes has been accomplished primarily using bottom-up wet chemistry approaches and electrochemical methods. Among the wet chemistry approaches for electrode nanostructuring (Eftekhari et al. 2008), nanostructured electrodes are often fabricated by the deposition or coupling of nanoparticles to planar electrodes. For example, AuNPs are commonly deposited on planar electrodes to provide a nanostructured surface for biorecognition element immobilization. In such studies, the particles are bound to the planar electrode via physical adsorption processes (Attar et al. 2016) or chemical methods (Wang et al. 2013). In addition to AuNPs, CNTs have also been extensively investigated as potentially useful nanomaterials for electrode nanostructuring (see Table 1).
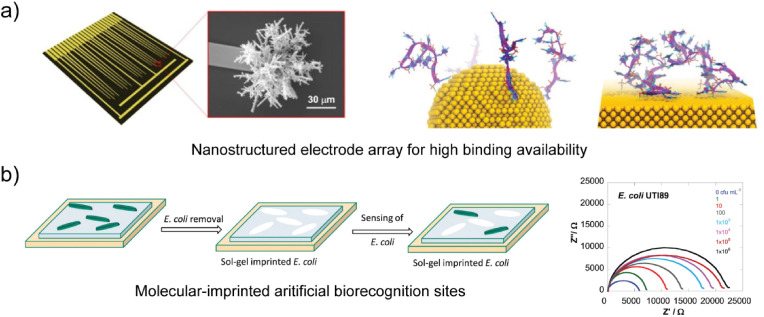
De Luna et al. found that high-curvature nanostructured Au microelectrodes exhibited a reduced extent of biorecognition element aggregation relative to that found on planar electrodes in DNA sensing studies using a combination of experimental studies and molecular dynamics simulations (see Fig. 3 a) (De Luna et al. 2017; Mahshid et al. 2016). A study by Chin et al. found that nanostructuring of carbon electrodes with carbon nanoparticles enhanced the electron transfer kinetics and current intensity of the electrode by 63% for the detection of Japanese encephalitis virus (Chin et al. 2017).
Fig. 3.
Emerging transduction approaches associated with electrochemical biosensors for pathogen detection. a) A nanostructured Au microelectrode array with high curvature (De Luna et al. 2017). b) Cell-imprinted polymer (CIP) with ‘artificial’ biorecognition elements for detection of E. coli using electrochemical impedance spectroscopy (EIS) and the Fe(CN)63-/4- redox probe (Jafari et al. 2019).
In addition to fabricating nanostructured electrodes by coupling already processed nanomaterials to planar electrodes, electrochemical methods are also commonly used for bottom-up electrode nanostructuring processes and have been leveraged to fabricate nanostructured electrodes for pathogen detection. For example, Hong et al. fabricated a nanostructured Au electrode via electrochemical deposition of gold (III) chloride hydrates for the detection of norovirus in lettuce extracts (Hong et al. 2015). While the physical or chemical deposition of materials on planar electrodes provides a useful nanostructuring approach, introducing porosity to the electrode, such as nanoporosity, also enables electrode nanostructuring. For example, Nguyen et al. utilized nanoporous alumina-coated Pt microwires for the detection of West Nile virus (Nguyen et al. 2009).
While studies have reported improved biosensor performance using electrode nanostructuring, such as improved sensitivity and LOD, it is prudent to consider the effect of nanostructuring on biorecognition element immobilization and target binding. For example, nanostructured electrodes that exhibit high-aspect-ratio structures and other three-dimensional structures have also been shown to enhance biomolecular steric hindrance effects, which may have implications for pathogen detection applications (Hong et al. 2015; Lam et al. 2012; Mahshid et al. 2017). There also remains a need to understand device-to-device and batch-to-batch variation in electrode nanostructuring quality. For example, it is presently unclear how the structure (e.g., topography, crystal structure) and material properties (e.g., electrical properties) of nanostructured surfaces vary among mass-produced electrodes. It is also unclear how such variance in nanostructuring quality affects the repeatability of biosensor performance.
2.1.6. Integration of complementary transduction elements
Given the need for rapid and reliable measurements, biosensors that contain integrated electrodes and complementary transducers have also been examined for pathogen detection applications. For example, electrodes have been integrated with transducers that enable simultaneous fluid mixing and monitoring of molecular binding events (Choi et al. 2011). Biosensors composed of multiple transducers, referred to as hybrid biosensors, also offer unique opportunities for in situ verification of target binding as well as complementary analytical measurements (i.e., dual detection).
Hybrid electrochemical biosensors for pathogen detection have been developed by integrating electrodes with optical and mechanical transducers. Electrochemical-optical waveguide light mode spectroscopy (EC-OWLS) combines evanescent-field optical sensing with electrochemical sensing (Bearinger et al. 2003). EC-OWLS optically monitors changes and growth at the electrode surface to provide complementary information on surface reactions. EC-OWLS has been used to monitor the growth of bacteria (Nemeth et al. 2007) and could potentially be applied to selective detection of pathogens. Electrochemical-surface plasmon resonance (EC-SPR) combines SPR sensing capability based on binding-induced refractive index changes at the electrode-electrolyte interface with electrochemical sensing capability on the same electrode (Hu et al. 2008). This approach has been used for monitoring molecular binding events (Juan-Colas et al. 2017) and could potentially be applied to selective detection of pathogens.
In addition to their combination with optical transducers, hybrid electrochemical biosensors have also been combined with mechanical transducers. Mechanical transducers have included shear-mode resonators, such as the quartz crystal microbalance (QCM) and cantilever biosensors. Electrochemical-QCMs (E-QCMs) integrate mass-change and electrochemical sensing capabilities into a single platform. For example, Li et al. used an antibody-functionalized E-QCM for the detection of E. coli, which provided complementary cyclic voltammetry, EIS, and capacitive sensing measurements associated with the detection response (Li et al. 2011). Serra et al. used a lectin-modified E-QCM to detect E. coli using the biosensor's mass-change response (Serra et al. 2008).
Besides providing complementary responses for verification of binding events (Johnson and Mutharasan, 2012, 2013a), hybrid biosensors for pathogen detection can also generate fluid and particle mixing at the electrode-electrolyte interface and in the bulk solution via acoustic streaming or primary radiation effects of mechanical transducers (Cesewski et al. 2018). Thus, secondary transducers can apply force to bound species, such as nonspecifically adsorbed background species or captured target species. For example, various studies have reported the removal of surface-bound biomolecules using mechanical transducers, such as shear-mode resonators or cantilever biosensors (Johnson and Mutharasan, 2014; Yeh et al. 2007). While the impediment or removal of nonspecifically adsorbed background species is a vital biosensor characteristic in pathogen detection applications that involve complex matrices, the regeneration of biosensor surfaces that contain specifically bound target species is essential for applications involving high-throughput characterization or process monitoring (e.g., bioprocesses or biomanufacturing processes) (Goode et al. 2015). Hybrid designs may also be useful for electrodes that exhibit a high extent of biofouling.
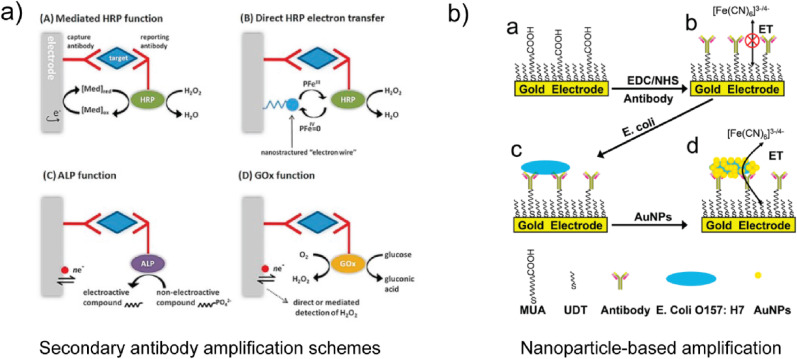
In addition to hybrid biosensor designs composed of combinations of electrodes with other transducers, hybrid biosensor-based assays for pathogen detection based on the combination of an electrochemical biosensor with a traditional bioanalytical technique have also been utilized. For example, electrochemical-colorimetric (EC-C) biosensing combines an electrochemical method and a colorimetric, fluorescent, or luminescent detection method. The electrode detects the presence of a target species, while the colorimetric transduction pathway enables quantification of the products associated with the reaction between the target and an active species (Hou et al. 2018). For example, Hou et al. used an EC-C approach based on a monoclonal antibody-functionalized AuNP-modified ITO electrode and dual-labeled magnetic beads for the detection of human enterovirus 71 (Hou et al. 2018). In that study, antibody- and horseradish peroxidase (HRP)-labeled magnetic nanobeads were introduced as a secondary binding step following exposure of the electrode to enterovirus-containing samples. Following the secondary binding step, the HRP-nanobead conjugates enabled colorimetric detection via monitoring of oxidative products produced by HRP-catalyzed redox reactions, while the functionalized electrode enabled electrochemical detection via chronoamperometry. Various techniques often rely on the use of optically-active labels for colorimetric, fluorescent, or luminescent sensing. The optical labels used in pathogen detection applications commonly include biological fluorophores, such as green fluorescent protein, non-protein organic fluorophores, such as fluorescein and rhodamine, and nanoparticles, such as quantum dots, including CdS, CdSe, and GaAs, among others (Mungroo and Neethirajan 2016; Pires et al. 2014). The use of such additional reagents to detect the target species is discussed further in the following sections.
2.2. Biorecognition elements
The previous section discussed the transduction elements associated with pathogen detection using electrochemical biosensors. Given a biosensor is a device composed of integrated transducer and biorecognition elements, we next discuss the biorecognition elements used for selective detection of pathogens and corresponding immobilization techniques for their coupling to electrodes.
Biorecognition elements for electrochemical biosensors can be defined as (1) biocatalytic or (2) biocomplexing. In the case of biocatalytic biorecognition elements, the biosensor response is based on a reaction catalyzed by macromolecules. Enzymes, whole cells, and tissues are the most commonly used biocatalytic biorecognition element. While enzyzmes provide biorecognition elements in various chemical sensing applications, they are often used as labels for pathogen detection applications and most commonly introduced via secondary binding steps. In the case of biocomplexing biorecognition elements, the biosensor response is based on the interaction of analytes with macromolecules or organized molecular assemblies. As shown in Table 1, Table 2, antibodies, peptides, and phages are the most commonly used biocomplexing biorecognition elements for pathogen detection. In addition to biomacromolecules, imprinted polymers have also been examined as biocomplexing biorecognition elements for pathogen detection using electrochemical biosensors.
2.2.1. Antibodies and antibody fragments
Antibodies and antibody fragments are among the most commonly utilized biorecognition elements for pathogen detection using electrochemical biosensors. Biosensors employing antibody-based biorecognition elements are commonly referred to as immunosensors. Given antibodies exhibit high selectivity and binding affinity for target species and can be generated for a wide range of infectious agents, antibodies are the gold-standard biorecognition element for pathogen detection. Antibodies contain recognition sites that selectively bind to antigens through a specific region of the antigen, referred to as an epitope (Patris et al. 2016). Antibodies can be labeled with fluorescent or enzymatic tags, which leads to the designation of the approach as label-based. While label-based approaches present measurement constraints associated with the use of additional reagents and processing steps (Cooper, 2009; Sang et al. 2016), antibody labeling may also alter the binding affinity to the antigen, which could affect the biosensor's selectivity. A detailed discussion of label-based biosensing approaches for pathogen detection has been reported elsewhere (Ahmed et al. 2014; Alahi and Mukhopadhyay, 2017; Bozal-Palabiyik et al. 2018; Leonard et al. 2003). A list of recent label-based approaches for pathogen detection using electrochemical biosensors, however, is provided in Table 2.
While both monoclonal and polyclonal antibodies enable the selective detection of pathogens (Patris et al. 2016), they vary in terms of production method, selectivity, and binding affinity. Monoclonal antibodies are produced by hybridoma technology (Birch and Racher, 2006; James and Bell, 1987). Thus, monoclonal antibodies are highly selective and bind to a single epitope, making them less vulnerable to cross-reactivity. While monoclonal antibodies tend to have a higher degree of selectivity, they are more expensive and take longer to develop than polyclonal antibodies. Polyclonal antibodies are produced by separation of immunoglobulin proteins from the blood of an infected host (Birch and Racher, 2006). Polyclonal antibodies target different epitopes on a single antigen. While polyclonal antibodies exhibit increased variability between batches, they are relatively less expensive to produce than monoclonal antibodies and facilitate robust measurements in various settings (Byrne et al. 2009). Drawbacks to antibody use include high cost and stability challenges, such as the need for low-temperature storage. As shown in Table 1, Table 2, both monoclonal and polyclonal antibodies are used as biorecognition elements for pathogen detection. For assays involving secondary binding steps, monoclonal antibodies typically serve as the primary biorecognition element and are immobilized on the electrode, while polyclonal antibodies serve as the secondary biorecognition element and often facilitate target labeling. For assays that do not require secondary binding steps, polyclonal antibodies are also commonly used as immobilized biorecognition elements for pathogen detection. For example, Pandey et al. immobilized monoclonal anti-E. coli on a composite nanostructured electrode to detect E. coli across a wide dynamic range of 10 to 108 CFU/mL with a LOD of 3.8 CFU/mL (Pandey et al. 2017). Wu et al. used polyclonal anti-E. coli for detection of E. coli via amperometry that exhibited a LOD of 5 × 103 CFU/mL (Wu et al. 2016). Lin et al. used monoclonal antibodies for detection of avian influenza virus H5N1 in chicken swabs across a dynamic range of 2- 1 to 24 hemagglutination units (HAU)/50 μL using EIS and the ferri/ferrocyanide (Fe(CN)6 3 - /4-) couple as a redox probe (Lin et al. 2015). Luka et al. detected Cryptosporidium parvum (C. parvum) with a LOD of 40 cells/mm2 via capacitive sensing and Fe(CN)6 3 - /4- (Luka et al. 2019).
Antibody fragments, such as single-chain variable fragments (scFvs), offer selectivity similar to antibodies, but they have the advantage of achieving relatively higher packing densities on electrode surfaces due to their relatively smaller size. For example, half-antibody fragments have been shown to improve biosensor sensitivity without the loss of selectivity, which warrants further investigation of reduced antibodies as biorecognition elements for pathogen detection applications (Sharma and Mutharasan, 2013). In addition to scFvs, Fabs, re-engineered IgGs, and dimers can also potentially be used as biorecognition elements for pathogen detection (Byrne et al. 2009).
2.2.2. Carbohydrate-binding proteins
Carbohydrate-binding proteins, such as lectins, also provide selective biorecognition elements for pathogen detection based on their ability to selectively bind ligands on target species. Peptide-based biorecognition elements are relatively low-cost, can be produced with high yield automated synthesis processes, and are modifiable (Pavan and Berti, 2012). For example, lectins have been investigated as biorecognition elements for pathogen detection through their ability to selectively bind glycosylated proteins on the surfaces of viruses and cells (Reina et al. 2008). Concanavalin A (ConA) lectin has been extensively investigated for E. coli detection (see Table 1) (Jantra et al. 2011; Saucedo et al. 2019; Xi et al. 2011; Yang et al. 2016b). While not yet widely investigated for pathogen detection using electrochemical biosensors, Etayash et al. recently showed that oligopeptides also provide attractive biorecognition elements for real-time biosensor-based detection of breast cancer cells (Etayash et al. 2015).
2.2.3. Oligosaccharides
Trisaccharides are carbohydrates that can selectively bind carbohydrate-specific receptors on pathogens. Thus, trisaccharide ligands have been used as biorecognition elements for pathogen detection using electrochemical biosensors. For example, Hai et al. used a hybrid E-QCM biosensor coated with hemagglutinin-specific trisaccharide ligands for the detection of human influenza A virus (H1N1) (Hai et al. 2017). The use of carbohydrates as biorecognition elements is limited in part due to the weak affinity of carbohydrate-protein interactions and low selectivity, which are currently mitigated through secondary interactions (Zeng et al. 2012).
2.2.4. Oligonucleotides
Single-stranded DNA (ssDNA) is a useful biorecognition element for the detection of pathogens. While ssDNA is commonly used as a biorecognition element for DNA-based assays, ssDNA aptamers are commonly used for pathogen detection using electrochemical biosensors. Aptamers are single-stranded oligonucleotides capable of binding various molecules with high affinity and selectivity (Lakhin et al. 2013; Reverdatto et al. 2015). Aptamers are isolated from a large random sequence pool through a selection process that utilizes systematic evolution of ligands by exponential enrichment, also known as SELEX (Stoltenburg et al. 2007). Suitable binding sequences can be isolated from a large random oligonucleotide sequence pool and subsequently amplified for use. Thus, aptamers can exhibit high selectivity to target species (Stoltenburg et al. 2007). Aptamers can also be produced at a lower cost than alternative biorecognition elements, such as antibodies. Giamberardino et al. used SELEX to discover an aptamer for norovirus detection, which showed a million-fold higher binding affinity for the target than a random DNA strand that served as a negative control (Giamberardino et al. 2013). Iqbal et al. performed 10 rounds of SELEX to discover 14 aptamer clones with high affinities for C. parvum for detection in fruit samples (Iqbal et al. 2015). However, the use of aptamers as biorecognition elements has not yet replaced traditional biorecognition elements, such as antibodies, because of several challenges, such as aptamer stability, degradation, cross-reactivity, and reproducibility using alternative processing approaches (Lakhin et al. 2013).
2.2.5. Phages
Phages, also referred to as bacteriophages, are viruses that infect and replicate in bacteria through selective binding via tail-spike proteins (Haq et al. 2012). Thus, they have been examined as biorecognition elements for pathogen detection using electrochemical biosensors (Kutter and Sulakvelidze, 2004). Bacteriophages exhibit varying morphologies and are thus classified by selectivity and structure. A variety of bacteriophage-based electrochemical biosensors for pathogen detection can be found in Table 1. For example, Shabani et al. used E. coli-specific T4 bacteriophages for selective impedimetric detection studies (Shabani et al. 2008). Mejri et al. compared the use of bacteriophages to antibodies as biorecognition elements for E. coli detection (Mejri et al. 2010). In that study, they found that bacteriophages improved the water stability of the biosensor and increased the sensitivity by approximately a factor of four relative to the response obtained with antibodies based on EIS measurements (Mejri et al. 2010). In another study, Tolba et al. utilized immobilized bacteriophage-encoded peptidoglycan hydrolases on Au screen-printed electrodes for detection of L. innocua in pure milk with a LOD of 105 CFU/mL (Tolba et al. 2012). These results suggest that bacteriophages are potentially attractive biorecognition elements for water safety and environmental monitoring applications that require chronic monitoring of liquids.
2.2.6. Cell- and molecularly-imprinted polymers
Given traditional biorecognition elements used in biosensing exhibit stability concerns, such as antibodies or aptamers, as discussed in Sections 2.2.1–2.2.4, there have been efforts to create engineered molecular biorecognition elements, such as scFvs. In contrast, materials-based biorecognition elements exploit the principle of target-specific morphology for selective capture (Pan et al. 2018; Zhou et al. 2019). The most common approach in materials-based biorecognition is based on cell- and molecularly-imprinted polymers (CIPs and MIPs, respectively) (Gui et al. 2018). CIPs and MIPs have been created using various processes, including bacteria-mediated lithography, micro-contact stamping, and colloid imprints (Chen et al. 2016a; Pan et al. 2018).
As shown in Fig. 3b, Jafari et al. used imprinted organosilica sol-gel films of tetraethoxysilane and (3-mercaptopropyl)trimethoxysilane (MPTS) for selective detection of E. coli using an impedimetric method (Jafari et al. 2019). Similarly, Golabi et al. used imprinted poly(3-aminophenylboronic acid) films for detection of Staphylococcus epidermidis (S. epidermidis) (Golabi et al. 2017). Despite the absence of a highly selective molecular biorecognition element, CIPs and MIPs exhibit selectivity when exposed to samples that contain multiple analytes (i.e., non-target species) (Golabi et al. 2017; Jafari et al. 2019; Qi et al. 2013). MIPs and CIPs are also of interest with regard to opportunities in biosensor regeneration. Common adverse effects of regeneration on biosensors that employ molecular biorecognition elements, such as irreversible changes in structure, are less likely to affect MIPs and CIPs. However, it is generally accepted that current CIPs and MIPs exhibit lower selectivity to target species than antibodies and aptamers due to reduction of available chemical selectivity (Cheong et al. 2013; Kryscio and Peppas, 2012; Yáñez-Sedeño et al. 2017).
2.3. Immobilization and surface passivation
Given biosensors are self-contained devices composed of integrated transducer-biorecognition elements, the immobilization of biorecognition elements on electrodes is central to the design, fabrication, and performance of electrochemical biosensors for pathogen detection. The goal of immobilization is to achieve a stable, irreversible bond between the biorecognition element and the electrode with suitable packing density and orientation that maintains high accessibility and binding affinity to target species. Electrochemical biosensors for pathogen detection have typically used established techniques for preparation of the biorecognition layer. A detailed discussion of immobilization and surface passivation techniques is provided in Supporting Information.
2.4. Thermodynamics of pathogen-biorecognition element binding reactions
While the rate of biosensor response is typically governed by a mass transfer-limited heterogeneous reaction between the immobilized biorecognition element and target species, the net change in the biosensor response is dependent on the reaction thermodynamics. The binding affinity between a biorecognition element and target species, such as an antibody and antigen, is often reported in terms of a dissociation constant (K D), which has units of M. While the value of K D, solution = 1 nM provides a reasonable estimate for biosensor design considerations, such as understanding the mass transfer limitations associated with biosensor response (Squires et al. 2008), the binding affinity of antibodies can vary by orders of magnitude depending on the pathogen of interest and the clonality of the antibody. One important consideration when immobilizing biorecognition elements is potential effects of immobilization on binding affinity to the target. Traditionally, K D is obtained from a kinetic or thermodynamic analysis. Kinetic analyses measure association and dissociation rate constants (k a and k d, respectively) and enable calculation of K D as k d/k a. Thermodynamic analyses, such as calorimetric techniques, measure the binding enthalpy and entropy, which in turn provides the standard Gibbs free energy of the reaction (ΔG°), and thus, K A = K D −1 though the expression K A = exp(-ΔG°/RT), where R is the gas constant and T is the temperature. A detailed discussion of the kinetics and thermodynamics of biorecognition element-target binding reactions for solution- and surface-based biosensors is provided in Supporting Information.
3. Measurement formats for pathogen detection
In addition to a physical device composed of an integrated transduction element and biorecognition element, an electrochemical biosensor-based assay for pathogen detection potentially involves processing steps associated with sample preparation and complementary physical systems for biosensor housing and sample handling. The associated protocols for sample preparation and sample handling are often referred to as the measurement format. Several important considerations regarding the measurement format for pathogen detection applications can be considered and vary based on the assay design, the biosensor performance (e.g., sensitivity and LOD), the volume, material properties, and composition of the pathogen-containing sample, and the application. For example, the use of DNA-based assays for pathogen detection typically requires sample preparation steps associated with the extraction of genetic material. Similarly, the use of a label-based biosensing approach requires sample preparation steps associated with target labeling. In cases where the concentration of target species in the sample is below the biosensor's LOD, pre-concentration steps may be required. Applications to process monitoring, such in bioreactor or tissue-chip monitoring, may require flow-based sample handling formats. We next discuss the measurement formats associated with pathogen detection in terms of sample preparation and sample handling.
3.1. Sample preparation: Filtration and pre-concentration
Sample preparation steps have various purposes, including concentrating or amplifying the target species through separation and growth processes, reducing the concentration of background inhibitory species, and reducing the heterogeneity of the sample's composition and properties (Zourob et al. 2008). We next discuss sample filtration and pre-concentration techniques.
3.1.1. Sample filtration
Generally, sample filtration relies on the principle of size discrepancy between the target pathogen and background species. Membranes, fibers, and channels have been used in size-selective sample filtration processes for biosensing applications. Biorecognition elements are commonly used to assist the separation process when the target species exhibits similar properties to background species or the matrix. For example, biorecognition elements that exhibit affinity to a broad group of pathogens, such as lectins, have been used in pre-concentration steps for pathogen detection (Zourob et al. 2008). Bacteria typically exhibit a net negative charge at physiological pH (7.4) because of an abundance of lipopolysaccharides or teichoic acids on the cell membrane (Gram-negative bacteria and Gram-positive bacteria, respectively) (Silhavy et al. 2010). This physical property of cell-based pathogens is leveraged in biofiltration processes, for example, using electropositive filters (Altintas et al. 2015). While the majority of the aforementioned separation processes involve manual handling steps, sample filtration processes are now being integrated with microfluidic-based biosensing platforms (Song et al. 2013). For example, Chand and Neethirajan incorporated an integrated sample filtration technique using silica microbeads for the detection of norovirus in spiked blood samples (Chand and Neethirajan 2017).
3.1.2. Centrifugal separation
Centrifugation can be used as a density gradient-based separation principle for concentrating target pathogens within a sample. In cases where the target species exhibits similar density to background species, the approach is often implemented with antibody-functionalized beads. This technique is commonly employed in applications requiring pathogen detection in complex matrices (e.g., body fluids). Centrifugation-based separation techniques can also potentially be applied to microfluidic-based biosensing platforms. For example, Lee et al. utilized centrifugal microfluidics to process a whole blood sample for subsequent analysis using ELISA (Lee et al. 2009), suggesting that this approach could be extended to electrochemical biosensor-based assays for pathogen detection.
3.1.3. Broth enrichment
Broth enrichment is a technique used to increase the concentration of target species in the sample through growth or replication of target species prior to measurement, thereby increasing the number present for detection. The technique is commonly used in food safety applications. For example, Liebana et al. enriched S. typhimurium-spiked milk samples in Luria broth (LB) for 8 h to improve the assay LOD from 7.5 × 103 CFU/mL for the 50-min enriched sample to 0.108 CFU/mL (Liebana et al. 2009). Salam et al. enriched fresh chicken samples in enrichment buffer peptone for 18–24 h to recover injured S. typhimurium cells for detection via chronoamperometry (Salam and Tothill, 2009). While enrichment can be a useful sample preparation step when the target concentration is below the biosensor's LOD, it is inherently limited to viable and cultural organisms. Further, analysis of the results obtained from multiple samples should consider potential differences in the growth rate of bacteria across different samples. It is important to note that the need for sample enrichment significantly increases the TTR and impedes rapid and real-time detection.
3.1.4. Magnetic separation
The separation of the target species from a sample using magnetic beads has become a commonly used sample preparation approach in pathogen detection applications. Target pre-concentration via magnetic bead-based separation processes typically involves the binding of antibody-functionalized magnetic beads to the target species. The bead-target complexes are subsequently separated from the solution by externally-applied magnetic fields. Magnetic-assisted separation processes are useful when the target species exhibits similar properties to other analytes or background species in the sample, such as those with similar size, density, or chemical properties (Chen et al. 2017). The bead-target complexes are then introduced directly to the biosensor to enable quantification of the target pathogen that was present in the initial sample. As shown in Table 2, magnetic bead-based separation processes have been extensively used for pathogen detection as well as general substrates for traditional immunoassays. Such assays have been used to detect a variety of pathogens, including bacteria, such as E. coli (Chan et al. 2013 ) and Bacillus anthracis (B. anthracis) (Pal and Alocilja, 2009), and viruses, such as bovine viral diarrhea virus (Luo et al. 2010) and human influenza A virus (Shen et al. 2012). In addition to serving as a separation agent, magnetic beads also serve as labels.
3.2. Sample handling formats
The sample handling format is highly influenced by the biosensor application. As discussed in further detail in the following sections, pathogens are present in liquid and solid matrices and on surfaces (e.g., of biomedical devices). In addition, pathogens can be aerosolized, which is a significant mode of disease transmission associated with viral pathogens (e.g., influenza and COVID-19). Sample handling formats can be generally classified as droplet-, flow-, or surface-based.
Droplet formats involve sampling from a larger volume of potentially pathogen-containing material or fluid. The sample droplet is subsequently analyzed by deposition on a functionalized transducer or transferred to a fluidic delivery system. For example, Cheng et al. created an electrochemical biosensor based on a nanoporous alumina electrode tip capable of analyzing 5 μL of dengue virus-containing solutions (Cheng et al. 2012). Droplet formats are simplistic sample handling formats and have the advantage of being performed by unskilled users. While dropletformats have been extensively used with colorimetric biosensors, they have also been adapted for electrochemical biosensors. For example, commercially-available blood glucose meters use a droplet format (Vashist et al. 2011). Examples of low-cost, paper-based, or disposable electrochemical biosensors for pathogen detection that utilize droplet formats are provided in Table 1. For example, Zhao et al. created a screen-printed graphite-based electrode for electrochemical detection of Vibrio parahaemolyticus (V. parahaemolyticus) based on 5 μL samples (Zhao et al. 2007). However, while droplet formats minimize the technical and methodological barriers to measurement, such as eliminating the need for physical systems associated with biosensor housing and sample handling, they can exhibit measurement challenges associated with mass transport and target sampling limitations.
One of the most critical considerations associated with application of droplet formats to pathogen detection is sampling, specifically if sufficient sampling has been performed on the system for which bioanalytical information is desired (e.g., a human, a food source, or source of drinking water). For example, the rationale that the bioanalytical characteristics of a droplet represent that of the bulk system is sound only in a well-mixed system, specifically, a system that exhibits a uniform spatial distribution of species (i.e., concentration profile). We note that while this is typically the case for samples acquired from closed, convective systems, such as body fluids, it should be challenged when considering open systems that exhibit complex flow profiles or regions of static fluid. For example, groundwater systems (e.g., aquifers), rivers, and lakes have been reported to have complex flow profiles (Ji, 2017; Zhang et al. 1996). Thus, the sampling approach should be considered when examining droplet formats for food and water safety applications. In addition to a consideration of system mixing, one should also consider the potential measurement pitfalls when analyzing samples that contain dilute levels of highly infectious pathogens, such as the potential for false-negative results.
Flow formats involve the detection of target species in the presence of flow fields. Flow formats include continuously-stirred systems (e.g., continuously-stirred tank bioreactors), flow cells, and microfluidics. Flow formats have the advantage of exposing the biosensor to target-containing samples in a controlled and repeatable fashion and the benefit of driving exposure of the functionalized biosensor to target species via convective mass transfer mechanisms. Flow formatsare also typically compatible with large sample volumes (liters). Flow cells are typically fabricated via milling and extrusion processes using materials such as Teflon or Plexiglas. They have the advantage of accommodating a variety of biosensor form factors, such as rigid three-dimensional biosensors. In addition to flow cells, flow formats are commonly achieved using microfluidic devices. While microfluidic devices are typically used with biosensors that exhibit thin two-dimensional form factors, such as planar electrodes, they offer various measurement advantages. Unlike flow cells, which are typically fabricated from machinable polymers, microfluidics are typically fabricated using polydimethylsiloxane (PDMS) and polymethyl methacrylate (PMMA) given their low cost and compatibility with microfabrication approaches. One advantage of microfluidic devices is their ability to perform integrated sample preparation steps, which eliminates the need for additional steps in the sample-to-result process (Sin et al. 2014). For example, microfluidic formats for pathogen detection using electrochemical biosensors have demonstrated fluid pumping, valving, and mixing of small sample volumes (Rivet et al. 2011). An example of a microfluidic format created by Dastider et al. for detection of S. typhimurium is shown in Fig. 4a (Dastider et al. 2015).
Detection in the presence of flow fields requires high stability of immobilized biorecognition elements (Bard and Faulkner, 2000). The effect of flow characteristics on biosensor collection rates is an important consideration, especially when considering micro- and nano-scale transducers with microfluidic formats (Squires et al. 2008). For example, emerging nanostructured electrodes, such as functionalized nanoporous membranes, have been shown to achieve high stability in microfluidic devices (Joung et al. 2013; Tan et al. 2011). A detailed discussion on the relationship between device dimensions, flow characteristics, achievable target collection rates, and equilibrium measurement times has been provided elsewhere (Squires et al. 2008). It is paramount for interpreting biosensor response that users understand the interplay between mass transport of target molecules (both diffusive and convective mechanisms) and reaction at the biosensor surface (i.e., binding of target species to immobilized biorecognition elements). Such fundamental understanding can also be employed in biosensor and experiment design to create improved assay outcomes, such as reducing TTR or improving measurement confidence.
While the presence of pathogens on the surfaces of objects can be analyzed using droplet- and flow-based sample handling formats using material transfer processes, such as swabbing, in situ pathogen detection on the object surfaces is a vital measurement capability for medical diagnostic, infection control, and food safety applications. Surface-based measurement formats typically require biosensors with flexible or conforming (i.e., form-fitting) form factors. For example, Mannoor et al. detected the presence of pathogenic species directly on teeth using a flexible graphene-based biosensor (Mannoor et al. 2012). Further discussion of surface-based pathogen detection applications are provided in the following sections.
The sample handling format often provides insight into the biosensor's reusability. Biosensors within the aforementioned measurement formats can be broadly classified as single- or multi-use biosensors. Single-use biosensors are unable to monitor the analyte concentration continuously or upon regeneration, while multiple-use biosensors can be repeatedly recalibrated (Thévenot et al. 2001). For example, droplet-based low-cost, disposable biosensors for water safety are typically single-use, while biosensors for process monitoring applications can be recalibrated to characterize multiple samples and facilitate continuous monitoring. The ability to regenerate biosensor surfaces following pathogen detection (i.e., remove selectively-bound pathogens) is a significant technical barrier limiting progress in multiple-use biosensors, and industrial applications thereof, and is discussed further in the following sections.
3.3. Electrochemical methods for pathogen detection using electrochemical biosensors
Various electrochemical methods can be performed using functionalized electrodes to enable pathogen detection (Bard and Faulkner, 2000). These methods differ in electrode configuration, applied signals, measured signals, mass transport regimes, binding information provided (Thévenot et al. 2001), and target size-selectivity (Amiri et al. 2018). Electrochemical methods used for pathogen detection can be classified as potentiometric, amperometric, conductometric, impedimetric, or ion-charge/field-effect, which often signify the measured signal (Thévenot et al. 2001). The applied signals may be constant or time-varying. The result of the electrochemical method may require analysis of the output signal's transient response, steady-steady response, or a combination of both. A detailed discussion of the aforementioned electrochemical methods has been provided elsewhere (Bard and Faulkner, 2000). Here, we briefly review the most recent methods employed for pathogen detection using electrochemical biosensors.
3.3.1. Potentiometry
Potentiometric methods, also referred to as controlled-current methods, are those in which an electrical potential is measured in response to an applied current (Bard and Faulkner, 2000). The applied current is typically low amplitude. An advantage of controlled-current methods is the ability to use low-cost measurement instrumentation relative to that required for controlled-potential methods.
Hai et al. used potentiometry with a conductive polymer-based biosensor to detect human influenza A virus (H1N1) at a LOD of 0.013 HAU (Hai et al. 2017). Hernandez et al. used potentiometry with a carbon-rod modified electrode that contained reduced graphene oxide to detect S. aureus at a single CFU/mL (Hernandez et al. 2014). Boehm et al. detected E. coli via potentiometry utilizing a Pt wire electrode (Boehm et al. 2007). Further studies utilizing potentiometric sensing approaches are listed in Table 1, Table 2.
3.3.2. Voltammetry
Voltammetric methods, also referred to as controlled-potential methods, are those in which a current is measured in response to an applied electrical potential that drives redox reactions (Bard and Faulkner, 2000). The measured current is indicative of electron transfer within the sample and at the electrode surface, and thus, the concentration of the analyte. In chronoamperometry, the electrical potential at the working electrode is applied in steps, and the resulting current is measured as a function of time. The applied electrical potential can also be held constant or varied with time as the current is measured.
Although various types of biosensors are compatible with voltammetry-based measurements, field-effect transistor (FET)-based biosensors often utilize amperometric-based methods for pathogen detection (Huang et al. 2011; Liu et al. 2013). FET biosensors detect pathogens via measured changes in source-drain channel conductivity that arise from the electric field of the sample environment. This is achieved by immobilizing biorecognition elements on the metal or polymer gate electrode of the device. He et al. showed that FETs based on PEDOT:PSS organic electrochemical transistor electrodes enabled the detection of E. coli in KCl solutions using Pt and Ag/AgCl gate electrodes (He et al. 2012). Wu et al. used a graphene-based FET to detect E. coli in nutrient broth diluted with phosphate buffered saline solution with amperometry using a Ag/AgCl gate electrode (Wu et al. 2016).
Further examples of amperometric sensing include the detection of human influenza A virus by Singh et al. using a reduced graphene oxide-based electrode and chronoamperometry using Fe(CN)6 3 - /4- at a LOD of 0.5 plaque-forming units (PFU)/mL (Singh et al. 2017b). Lee and Jun utilized wire-based electrodes for amperometric detection of E. coli and S. aureus (Lee and Jun 2016). A detailed list of studies that utilize amperometric methods for pathogen detection is provided in Table 1, Table 2
3.3.2.1. Linear sweep and cyclic voltammetry
Linear sweep voltammetry (LSV) methods are those in which a current is measured in response to an applied electrical potential that is swept at a constant rate across a range of electrical potentials (Bard and Faulkner, 2000). Cyclic voltammetry (CV) is a commonly used linear-sweep method in which the electrical potential is swept in both the forward and reverse directions in partial cycles, full cycles, or a series of cycles. CV is one of the most widely used voltammetric methods for pathogen detection.
Hong et al. used sweep voltammetry to detect norovirus in a sample solution with Fe(CN)6 3 - /4- extracted from lettuce (Hong et al. 2015). A typical CV response using Fe(CN)6 3 - /4- associated with pathogen detection is shown in Fig. 5 a for various concentrations of E. coli binding to a polymer composite electrode (Güner et al. 2017). A detailed overview of pathogen detection studies based on CV is provided in Table 1, Table 2.
Fig. 5.
Typical responses associated with the common electrochemical methods used for pathogen detection. a) Cyclic voltammetry (CV) data using Fe(CN)63-/4- for varying concentrations of E. coli (Güner et al. 2017). b) Differential pulse voltammetry (DPV) data using Fe(CN)63-/4- for varying concentrations of S. aureus (Bhardwaj et al. 2017). c) Electrochemical impedance spectroscopy (EIS) in 100 mM LiClO4 solution in the form of a Nyquist plot and corresponding equivalent circuit model associated with biorecognition element immobilization and detection of S. typhimurium (Sheikhzadeh et al. 2016). d) Conductometry data for varying concentrations of B. subtilis (Yoo et al. 2017).
3.3.2.2. Pulse voltammetry
Pulse voltammetry is a type of voltammetry in which the electrical potential is applied in pulses. The technique has the advantage of improved speed and sensitivity relative to traditional voltammetric techniques (Bard and Faulkner, 2000; Molina and González, 2016). In staircase voltammetry, the electrical potential is pulsed in a series of stair steps and the current is measured following each step change, which reduces the effect of capacitive charging on the current signal. Square wave voltammetry (SWV) is a type of staircase voltammetry that applies a symmetric square-wave pulse superimposed on a staircase potential waveform. The forward pulse of the waveform coincides with the staircase step. In differential pulse voltammetry (DPV), the electrical potential is scanned with a series of fixed amplitude pulses and superimposed on a changing base potential. The current is measured before the pulse application and again at the end of the pulse, which allows for the decay of the nonfaradaic current (Scott, 2016).
For example, Iqbal et al. used SWV with AuNP-modified carbon electrodes for detection of C. parvum in samples taken from fruit (Iqbal et al. 2015). Kitajima et al. also used SWV with Au microelectrodes to detect norovirus at a LOD of 10 PFU/mL (Kitajima et al. 2016). Cheng et al. used DPV and a nanostructured alumina electrode for detection of dengue type 2 virus with a LOD of 1 PFU/mL (Cheng et al. 2012). As shown in Fig. 5b, Bhardwaj et al. used DPV with a carbon-based electrode to detect S. aureus (Bhardwaj et al. 2017). Additional studies that utilize pulse voltammetry methods forpathogen detection are listed in Table 1, Table 2.
3.3.2.3. Stripping voltammetry
Many of the previously described voltammetric methods can be modified to include a step that pre-concentrates the target on the electrode surface. Subsequently, the pre-concentrated target is stripped from the surface by application of an electrical potential. In anodic stripping voltammetry (ASV), a negative potential is used to pre-concentrate metal ions onto the electrode surface. These ions are then stripped from the surface by applied positive potentials. Although most commonly used to detect trace amounts of metals, this method has been adapted for pathogen detection by electrocatalytically coating metallic labels on bound targets for oxidative stripping and subsequently measuring the current response (Abbaspour et al. 2015).
Chen et al. used stripping voltammetry with a polymer-CNT composite-based electrode to detect E. coli at a LOD of 13 CFU/mL (Chen et al. 2014). In that study, the biosensor was first incubated with E. coli. Silica-coated Ag nanoparticles conjugated with anti-E.coli were subsequently introduced to the system, inducing a binding reaction between the bacteria and the nanoparticles. After rinsing non-specifically bound particles, acid was introduced to dissolve Ag(s), and the resulting Ag+-rich solution was characterized using DPV. Viswanathan et al. used ASV with screen-printed composite electrodes for multiplexed detection of Campylobacter, S. typhimurium, and E. coli with a LOD of 400 cells/mL, 400 cells/mL, and 800 cells/mL, respectively (Viswanathan et al. 2012). In that study, antibody-functionalized nanocrystalline bioconjugates were first introduced to biosensor-bound bacteria, the specifically bound particles were dissolved with acid, and the ions were then stripped using a square-wave voltammetric waveform. Additional studies using stripping voltammetry for electrochemical detection of pathogens can be found in Table 1, Table 2.
3.3.3. Electrochemical impedance spectroscopy
The aforementioned electrochemical methods involved responses based on step changes or continuous sweeps in the applied current or voltage that drove the electrode to a condition far from equilibrium. Alternatively, frequency response methods, often referred to as impedance-based or impedimetric methods, are based on frequency response analysis (i.e., the response of the system to periodic applied current or potential waveforms at either a fixed frequency or over a range of frequencies) (Bard and Faulkner, 2000). This provides several advantages, including measurement over a wide range of times and frequencies and high precision in time-averaged responses. We next discuss impedance-based electrochemical methods for detection of pathogens using electrochemical biosensors.
In EIS the impedance and phase angle of the system are measured as a function of the frequency of the applied electrical potential. EIS is a diverse electrochemical method, which can be done as a faradaic or non-faradaic process, and enables the study of intrinsic material properties, experiment-specific processes, or biorecognition events at the electrode surface. EIS is often performed using an applied low-amplitude sinusoidal electrical potential and a three-electrode configuration. Equivalent circuit models are commonly fit to experimental impedance and phase angle data to interpret the electrochemical process in terms of passive circuit elements, such as resistors and capacitors. For example, the electric double layer is typically modeled as a capacitive element, while the resistance to faradaic charge transfer at the electrode-electrolyte interface is represented as a resistor, often referred to as the charge transfer resistance. Additional circuit elements, such as constant-phase or Warburg elements, can also be included to represent other features of the electrochemical cell and process, such transport characteristics of the species at the electrode-electrolyte interface. The Randles model is a commonly used equivalent circuit for interpretation of biosensor EIS data. The circuit consists of an electrolyte resistance in series with a parallel combination of the double-layer capacitance with the charge transfer resistance and the Warburg impedance element (Randles, 1947). Variations of this model have been formulated for a variety of biosensing studies. For example, the equivalent circuit model and associated Nyquist plot for electrochemical detection of S. typhimurium using EIS with a poly(pyrrole-co-3-carboxyl-pyrrole) copolymer supported aptamer can be found in Fig. 5c (Sheikhzadeh et al. 2016). The equivalent circuit model consists of the solution resistance, charge transfer resistance at the copolymer-aptamer/electrolyte interface, and constant phase element for the charge capacitance at the copolymer-aptamer/electrolyte interface (Sheikhzadeh et al. 2016).
While the impedance can be measured across a range of frequencies and interpreted using equivalent circuit models that describe impedance response over a wide frequency range, fixed-frequency measurements are also useful for biosensing applications. Fixed-frequency measurements are typically based on the identification of single frequencies or small frequency ranges in the impedance spectra that are most sensitive to molecular binding events. Fixed-frequency approaches have the advantage of increasing the sampling frequency of the biosensor. As a result, impedance-based electrochemical methods generate biosensor responses in terms of changes in the measured physical quantities (e.g., changes in impedance) or calculated equivalent circuit elements (e.g., double-layer capacitance or charge-transfer resistance).
As shown in Table 1, Table 2, EIS is one of the most commonly used methods for electrochemical detection of pathogens. For example, Zarei et al. used EIS with an Au nanoparticle-modified carbon-based electrode for detection of Shigella dysenteriae (S. dysenteriae) at a LOD of 1 CFU/mL (Zarei et al. 2018). Primiceri et al. used EIS with Au interdigitated microelectrode arrays and Fe(CN)6 3 - /4- to detect L. monocytogenes at a LOD of 5 CFU/mL (Primiceri et al. 2016). Andrade et al. used EIS with a CNT-based electrode for multiplexed detection of E. coli, B. subtilis, and Enterococcus faecalis (Andrade et al. 2015).
Redox reactions at the electrode-electrolyte interface are typically established using a redox probe. Owing to its high reversibility, the Fe(CN)6 3 - /4- redox couple has been widely investigated as an electrochemical probe for biosensing applications and is regarded as a standard model for highly reversible electrochemical reactions (Daum and Enke, 1969). While useful electrochemical probes, redox reactions may also affect the electrode and immobilized biorecognition elements. For example, redox reactions associated with the Fe(CN)6 3 - /4- probe can cause etching of Au electrodes due to the presence of CN− ions when using the redox couple for EIS measurements (Vogt et al. 2016). This observation warrants further investigation, particularly in the context of establishing the effects on biosensor repeatability and reusability. The use of alternative redox probes or electrode materials may mitigate such effects. For example, ferrocene and ferrocenemethanol have also been used as redox probes for pathogen detection. Ruthenium(III)/ruthenium(II) (Schrattenecker et al. 2019) and immobilized quinone pairs (Piro et al. 2013) are also potentially useful alternatives.
Biosensors that use impedance-based methods and whose impedance response can be modeled using equivalent circuit models can be used to calculate the capacitance of the electric double layer. The double-layer capacitance is recognized to be sensitive to the structure of the electrode, the characteristics and concentration of analytes at the electrode surface and in the electrolyte, and the characteristics of the electrolyte (Lisdat and Schäfer, 2008). As a capacitor, the double-layer is not only dependent on the dielectric material but also the thickness of the dielectric layer. Importantly, both characteristics could be affected by molecular binding events on an electrode. For example, when a target analyte binds to an immobilized biorecognition element, counter ions around the electrode surface are displaced, leading to a change in the capacitance (Berggren et al. 2001). The capacitance can be determined from the reactive component of the impedance or by fitting of an equivalent circuit model (Barsoukov and Macdonald, 2018).
Idil et al. used the capacitive response of a MIP electrode for the detection of E. coli (Idil et al. 2017). Jantra et al. similarly used the capacitive response of an Au rod electrode for the detection of E. coli (Jantra et al. 2011). Luka et al. used the capacitive response of an Au interdigitated microelectrode array based on equivalent circuit analysis for the detection of C. parvum (Luka et al. 2019). See Table 1, Table 2 for a detailed list of studies that have used the capacitive response of an electrochemical biosensor for pathogen detection.
3.3.4. Conductometry
Conductometry methods are those in which the conductivity of the sample solution is monitored using a low-amplitude alternating electrical potential (Dzyadevych and Jaffrezic-Renault, 2014). The principle relies on conductivity change in the sample via the production or consumption of charged species. The measurement has the advantage of not requiring a reference electrode and can be used to detect both electroactive and electroinactive analytes (Jaffrezic-Renault and Dzyadevych, 2008; Narayan, 2016). Given the method can be performed using a two-electrode configuration, conductometric biosensors can be easily miniaturized. In addition, they are less vulnerable to many types of interference due to their differential measurement mode (Jaffrezic-Renault and Dzyadevych, 2008).
As shown in Fig. 5d, Yoo et al. used a conductometric biosensor with CNT-based electrodes for the detection of B. subtilis (Yoo et al. 2017). Mannoor et al. used a previously described conductometric biosensor to detect S. aureus and Helicobacter pylori on tooth enamel (Mannoor et al. 2012). Shen et al. detected two strains of human influenza A virus (H1N1 and H3N2) using conductometry with a silicon nanowire array at a LOD of 29 viruses/μL (Shen et al. 2012). Additional studies that have examined the use of conductometric biosensors for pathogen detection can be found in Table 1, Table 2.
3.4. Secondary binding approaches
Electrochemical biosensors would ideally produce sensitive and selective results using label-free protocols. However, secondary binding reactions are sometimes required to facilitate the robust detection of pathogens that lack initial labels depending on the biosensor characteristics and measurement demands. Secondary binding steps can facilitate target labeling, biosensor signal amplification, and verification of target binding. Secondary binding steps provide useful in situ controls and can increase sensitivity, LOD, dynamic range, and measurement confidence (e.g., verification of target binding). Secondary binding steps also provide opportunities for acquiring additional bioanalytical information about the target species.
Here, we classify assays that use secondary binding steps as labeled approaches in Table 1, Table 2 regardless of if the primary binding step produced a response. There is, however, a more subtle distinction if binding of the secondary species is used for amplification or verification purposes as previously discussed. Labels often include a biorecognition element-enzyme or -nanoparticle conjugate. In electrochemical biosensing applications, such labels often serve the purpose of altering the material properties or transport processes of the electrode-electrolyte interface, often by inducing a secondary reaction. Secondary binding of optically-active nanomaterials to captured targets can also enable the use of optical transducers for simultaneous detection or bioanalysis. Enzymes are among the most commonly used secondary binding species for label-based pathogen detection. As shown in Table 2, electrochemical biosensors for pathogen detection that employ enzymes are commonly performed as a sandwich assay format. A schematic of secondary binding steps for biosensor amplification based on the binding of HRP-antibody conjugates is shown in Fig. 6 a (Kokkinos et al. 2016). Hong et al. used HRP-labeled secondary antibodies to amplify the CV and EIS responses of a concanavalin A-functionalized nanostructured Au electrode to detect norovirus (Hong et al. 2015). Gayathri et al. used an HRP-antibody conjugate to induce an enzyme-assisted reduction reaction with an immobilized thionine-antibody receptor in an H2O2 system for detection of E. coli down to 50 CFU/mL using a sandwich assay format (Gayathri et al. 2016). Xu et al. used glucose oxidase and monoclonal anti-S. typhimurium to functionalize magnetic bead labels for separation and detection of S. typhimurium on an Au IDAM using EIS and glucose to catalyze the reaction that exhibited a linear working range of 102 to 106 CFU/mL (Xu et al. 2016b).
Fig. 6.
Highlight of secondary binding and signal amplification approaches utilized in electrochemical biosensor-based pathogen detection. a) Four amplification approaches associated with the secondary binding of enzyme-labeled secondary antibodies: (A) electron transfer mediation; (B) nanostructuring of surface for increased rate of charge transfer kinetics; (C) conversion of electrochemically inactive substrate into a detectable electroactive product; (D) catalysis of oxidation of glucose for production of hydrogen peroxide for electrochemical detection (Kokkinos et al. 2016). b) Signal amplification via non-selective binding of AuNPs to bound bacterial target (E. coli) (Wan et al. 2016).
In addition to enzymes, secondary binding of nanoparticles has also been used for pathogen detection. As shown in Fig. 6b, Wan et al. utilized non-functionalized AuNPs to amplify the EIS response of an antibody-immobilized planar Au electrode to E. coli detection (Wan et al. 2016). A detailed overview of studies that employ enzymes and nanoparticles is provided in Table 2. We remind the reader that while secondary binding steps are useful techniques, assays that avoid secondary binding steps have advantages for bioprocess monitoring and control applications, as they avoid the addition of reagents to a process that may compromise product quality).
4. Applications to pathogen detection
As identified in the previous sections, the application influences the biosensor design and measurement format associated with a given electrochemical biosensor-based assay for pathogen detection. We next review applications of electrochemical biosensors for pathogen detection in food and water safety, environmental monitoring and infection control, medical diagnostics, and bio-threat defense.
4.1. Food and water safety applications
Detection of foodborne and waterborne pathogens is an essential aspect of public healthcare. Foodborne and waterborne pathogens originate from a variety of sources and matrices and typically infect humans through the consumption of contaminated food and water. Waterborne pathogens are responsible for about 2.2 million deaths annually worldwide (Pandey et al. 2014), and contaminated food-related deaths amount to around 420,000 annually (WHO, 2015). In 2019, the United States suffered an outbreak of multidrug-resistant S. typhimurium in turkey products caused 358 infections across 42 states, demonstrating the importance of detecting pathogens in food sources (CDC, 2019).
While biosensors for pathogen detection are critical to water and food safety in developed regions, biosensors are particularly important aspects of public healthcare in remote and under-developed regions due to relatively reduced infrastructure and resources for food and water quality analysis. For example, in 2014, a cholera outbreak linked to V. cholerae in Ghana, which has been associated with poor environmental water management and sanitation issues, infected over 20,000 individuals (Ohene-Adjei et al. 2017). The selective detection of pathogens in food and water remains a global healthcare challenge. Several comprehensive reviews have been written on biosensors for food and water safety (Baeumner, 2003; Bozal-Palabiyik et al. 2018; Leonard et al. 2003; Ye et al., 2019). Here, we describe the most common foodborne and waterborne pathogens. Common foodborne and waterborne pathogens include protozoa, such as C. parvum and G. lamblia, bacteria, such as E. coli, L. monocytogenes, S. typhimurium, S. aureus, and Campylobacter, and viruses, such as norovirus and rotavirus (Beuchat et al. 2013; Cabral, 2010).
The infectious dose of foodborne and waterborne pathogens can vary by 4–6 orders of magnitude, from a single cell or oocyst to greater than one million cells. For example, the infectious dose of S. dysenteriae is 200 CFU (Greig and Todd, 2010), while that of S. aureus is 100,000 CFU (Schmid-Hempel and Frank, 2007). Given the extensive use of immunoassays in food and water safety, such as ELISA, it is possible to obtain commercially-available monoclonal and polyclonal antibodies for a large number of foodborne and waterborne pathogens.
Biosensor applications associated with process monitoring applications may require biosensor designs and measurement formats that facilitate high-throughput analysis, continuous monitoring capability, and biosensor reusability. Alternatively, those for water safety applications in under-developed regions may require biosensor designs and measurement formats that facilitate field use, such as sample preparation-free protocols. Pathogens can also enter food and water through processing, packaging, distribution, and storage processes (e.g., via workers and pests) (Beuchat et al. 2013; Mehrotra, 2016; Ye et al., 2019). As a result, biosensors for food and water safety applications should facilitate pathogen detection at various stages of the processing operation. Recent advances in electrochemical biosensors for food and water safety applications have established new low-cost biosensor designs, portable measurement formats, and flexible form-factors and are discussed further in the following sections.
4.2. Environmental monitoring and infection control applications
In addition to foodborne and waterborne pathogens, the detection of environmental pathogens is also an important aspect of healthcare. For example, diseases associated with environmental pathogens are one of the leading causes of death in low-income economies (WHO, 2018a). For example, malaria was reported to cause an estimated 435,000 deaths in 2017 (WHO, 2018b). Environmental pathogens are microorganisms that typically spend a substantial part of their lifecycle outside human hosts, but when introduced to humans through contact or inhalation cause disease with measurable frequency. Thus, environmental pathogens are often targets in medical diagnostics applications. However, here, we choose to distinguish environmental monitoring applications, which require pathogen detection in the environment (e.g., in air or on surfaces), from medical diagnostics applications, which require detection in body fluids. Thus, the distinction is based on the matrix in which the pathogen is present. Similar to food and water safety applications, which require biosensors capable of analyzing pathogen-containing complex matrices, such as a water or food matrix, environmental pathogens are present in multiple types of matrices. While environmental pathogens can enter the body through direct physical contact, they can also be transmitted through aerosols or interaction with organisms that serve as vectors for the infectious agent, such as mosquitos in the case of Plasmodium falciparum (the infectious agent associated with malaria). Thus, the detection of environmental pathogens often requires analysis of matrices, such as air, and objects, such as the surfaces of biomedical devices or objects within healthcare facilities, that are present in the human environment (Lai et al. 2009).
Several comprehensive reviews have been provided on the detection of environmental pathogens (Baeumner, 2003; Justino et al. 2017). Here, we describe the most common environmental pathogens found both in and outside of clinical settings. Common environmental pathogens in a non-clinical setting include Legionella spp., which cause Legionnellosis, Mycobacterium tuberculosis, which causes tuberculosis, and Naegleria fowleri, which causes amoebic meningitis. In addition to bacteria and protozoa, fungi, nematodes, and insects are also environmental pathogens. Common environmental pathogens in clinical settings associated with healthcare-acquired infections include drug-resistant and multi-drug resistant (MDR) pathogens, such as Clostridium difficile (CD) (Hookman and Barkin, 2009), which causes CD-associated diarrhea and antibiotic-induced colitis, and methicillin-resistant S. aureus (MRSA), which causes severe infections in various parts of the body, including the urinary tract (Gordon and Lowy, 2008).
The infectious dose of environmental pathogens also varies by orders of magnitude depending on the pathogen as well as age and health of the individual. For example, the infectious dose of CD is less than 10 spores, while that of MRSA is greater than 100,000 organisms (Schmid-Hempel and Frank, 2007). While it is possible to obtain antibodies for foodborne and waterborne pathogens, it can be challenging to obtain antibodies for various environmental pathogens, including protozoa and nematodes. Thus, traditional bioanalytical techniques, such as PCR, are often utilized for the detection of environmental pathogens.
Similar to food and water safety applications, biosensor-based assays for environmental pathogen detection applications also utilize measurement formats that facilitate the analysis of liquids. However, they also require measurement formats for the detection of aerosolized pathogens. In addition to airborne transmission, environmental pathogens are transmitted by direct surface contact (similar to many foodborne pathogens), which is a significant mode of transmission in healthcare settings (e.g., of healthcare-acquired infections). Standardized guidelines for disinfecting and sterilizing the surfaces of medical equipment, assistive technologies, counters, and doors, among other surfaces, have emerged as an important aspect of infection control in modern healthcare facilities (Fraise et al. 2008). Thus, the detection of pathogens on the surfaces of biomedical devices and objects present in healthcare facilities is an important research area (Kramer et al. 2006; Weber et al. 2010). For example, bacterial contamination of inanimate surfaces and equipment has been examined as a source of intensive care unit-acquired infections, a global healthcare challenge, especially when caused by MDR pathogens (Russotto et al. 2015). Hospital-acquired infections are prevalent causes of morbidity in patients (Orsi et al. 2002). This problem has only been exasperated by the rise of MDR CD, as well as drug-resistant strains of Campylobacter, Enterococcus, Salmonella, S. aureus, and S. dysenteriae (Ventola, 2015). In addition to clinical pathogens, it is also of interest to detect pathogens in non-clinical settings (Faucher and Charette, 2015). Toxin-producing algae, such as cyanobacteria and sulphate-reducing bacteria, are also important targets for electrochemical biosensors associated with the prevention of water-based diseases.
4.3. Medical diagnostic applications
The field of medical diagnostics heavily relies on the identification and quantification of pathogens found in body fluids, including whole blood, stool, urine, mucus, saliva, or sputum. Diagnostic assays based on traditional bioanalytical techniques for detection of pathogens in body fluids are the gold standard and serve an essential role in healthcare by enabling the diagnosis and treatment of various diseases. Biosensors offer a complementary diagnostic platform that enable rapid and cost-effective measurements, high sensitivity, and the ability to make measurements in complex matrices that pose challenges to traditional bioanalytical techniques. Studies suggest that rapid diagnostic testing can potentially reduce the chance of hospitalization, duration of hospitalization and antimicrobial use, and mortality rates (Barenfanger et al. 2000; Beekmann et al. 2003; Dierkes et al. 2009; Rappo et al. 2016). For example, repeated rapid screening programs for human immunodeficiency virus (HIV) detection is recommended as a means of increasing quality-adjusted life years of health for citizens in the United States (Paltiel et al. 2006). Additionally, the need for rapid antibody screening has been identified as an important aspect of mitigating the ongoing COVID-19 pandemic.
Several comprehensive reviews have been published on traditional bioanalytical assays and biosensor-based assays for pathogen detection in medical diagnostics applications (Ahmed et al., 2014; da Silva et al., 2017; Singh et al., 2014). Common pathogens include the aforementioned foodborne, waterborne, and environmental pathogens (e.g., Mycobacterium and Plasmodium spp.), as well as additional airborne and bloodborne pathogens. Pathogens such as Mycobacterium, HIV, and Plasmodium falciparum, represent some of the top causes of death from infectious diseases worldwide (WHO, 2018a). Other common pathogens associated with medical diagnostics applications include those that cause respiratory infections, urinary tract infections, and diarrheal diseases, such as CD and MRSA, which can be life-threatening to the children, elderly and individuals with compromised immune systems. Other airborne and bloodborne pathogens of interest include the influenza virus, COVID-19, hepatitis virus, rabies virus, and bacteria such as Mycoplasma pneumonia and Bordetella pertussis.
The infectious dose of airborne and bloodborne pathogens also varies by orders of magnitude depending on the pathogen, the method of contraction, and the age and health of the individual. For example, the infectious dose of influenza is between 100–1000 particles (Gürtler, 2006), while the median infectious dose of HIV can vary, for example, from two RNA copies to 65,000 depending on the strain and source (Reid and Juma, 2009).
The diagnostically-relevant concentration of pathogens in each type of matrix must be considered when designing a biosensor for pathogen detection. For example, the detection of bacteria in blood versus urine exhibit different diagnostic thresholds (Kelley, 2017). Such knowledge can inform the need for sample preparation steps.
4.4. Biological defense and bio-threat applications
The potential for the weaponization of pathogens drives the need for rapid and sensitive biosensors for biological defense applications. Biosensor applications to biological defense and bio-threat are related to the aforementioned applications in food and water safety, environmental monitoring, and medical diagnostics but consider weaponized pathogens. However, while pathogens found in environmental monitoring applications are often native and endogenous agents, pathogens found in biological defense and bio-threat applications are often exogenous agents, which may have been weaponized and intentionally dispersed. For example, pathogen-based bio-threat situations typically involve the overt or covert introduction of an exogenous pathogen into either the food or water supply or environments which with humans closely interact (Cirino et al. 2004; Mirski et al. 2014; Shah and Wilkins, 2003).
The reader is directed to various comprehensive reviews on biosensor-based assays for the detection of biowarfare agents (Christopher et al. 1997; Shah and Wilkins, 2003). Common targets include the aforementioned airborne pathogens. In addition to the aforementioned naturally-occurring pathogens, pathogens for bio-threat may include engineered pathogens, such as genetically-modified viruses that can be transmitted via airborne pathways. B. anthracis (Anthrax), yersinia pestis (plague), and vaccinia virus are among several pathogens that have been utilized or suggested as biowarfare agents (Christopher et al. 1997; Shah and Wilkins, 2003).
While pathogen-based bio-threats may be introduced to the water and food supply, the detection of pathogen-based bio-threats in air is particularly critical to biowarfare defense, as they may be introduced into the battlefield in the form of aerosols. Further, the dispersal of pathogen-based bio-threats by air in facilities (e.g., via air-handling systems) represents a significant domestic bioterrorism concern. Thus, biosensor-based assays for bio-threat applications should be low-cost and portable to enable integration with existing physical systems (e.g., facilities) and movement with the warfighter or drones on the battlefield. Having discussed transduction elements, biorecognition elements, electrochemical methods, measurement formats, and pathogen detection applications, we next discuss the present challenges and future directions in the field of electrochemical biosensor-based pathogen detection.
5. Present challenges and future directions for pathogen detection using electrochemical biosensors
Here, we discuss the present challenges and future directions associated with pathogen detection using electrochemical biosensors to identify future research opportunities and emerging areas in the field.
5.1. Emerging electrode materials, fabrication processes, and form factors
The ability to create robust, low-cost biosensors for pathogen detection is a significant challenge in the field. One of the primary methods of reducing cost is decreasing the material cost per device. Carbon-based electrodes (e.g., graphite, graphene, CNTs), such as those shown in Fig. 7 a (Afonso et al. 2016) and 7b (Wang et al. 2013), are now being examined as potential alternatives to relatively more expensive metallic or ceramic electrodes. Many of these carbon-based materials are also nanoscale in structure, and thus offer advantages regarding nanostructuring. Similarly, polymer-based electrodes have also been examined as low-cost alternatives to metal electrodes as described in Section 2.1.3. For example, Afonso et al. used a home craft cutter printer as a highly accessible means of fabricating high quantities of disposable carbon-based sensors (Afonso et al. 2016).
Fig. 7.
State-of-the-art developments in electrochemical biosensors for pathogens. a) Low-cost, flexible, disposable screen-printed carbon electrodes (Afonso et al. 2016). b) Free-standing graphene electrodes (Wang et al. 2013). c) Paper-based substrates for pathogen detection using electrochemical methods (Bhardwaj et al. 2017). d) Wearable wireless bacterial biosensor for tooth enamel (Mannoor et al. 2012). e) Smartphone-enabled signal processing for field-based environmental monitoring (Jiang et al. 2014).
In addition to reducing the material cost per device, efforts to reduce the manufacturing cost of biosensors have also been examined. 3D printing processes have emerged as popular methods for biosensor fabrication. For example, 3D printing is compatible with flexible and curved substrates. 3D printing has also been used for the fabrication of various components of electrochemical biosensors, such as electrodes, substrates, fluid handling components, or device packaging. In particular, 3D printing has emerged as a useful fabrication platform for microfluidic-based analytical platforms (Waheed et al. 2016). For example, to date, 3D printing has enabled the fabrication of electrode-integrated microfluidics (Erkal et al. 2014), 3D microfluidics, organ-conforming microfluidics (Singh et al. 2017a), and transducer-integrated microfluidics (Cesewski et al. 2018). Thus, 3D printing may serve as an important fabrication platform for the creation of wearable microfluidic-based electrochemical biosensors for pathogen detection.
The ability to quantify the level of pathogens on the surfaces of objects (e.g., skin, food, and medical equipment) remains a present challenge in the biosensing field. Wearable biomedical devices have emerged as promising tools for point-of-care (POC) diagnostics and health monitoring. The application constraints of wearable devices require them to be lightweight and simple to operate. Wearable devices can provide continuous monitoring of body fluids, such as blood and sweat, allowing patients to obtain real-time bioanalytical information without the inconvenience of facility-based screening. To date, biosensors have been incorporated into a variety of wearable devices, including contact lenses, clothing, bandages, rings, and tattoos (Bandodkar and Wang, 2014). This is a rapidly emerging area linked to smartphone technology for biosensor actuation and monitoring. The rise of flexible electronics has also contributed to the success of incorporating electrochemical biosensors into flexible textiles, which has enhanced their wearability (Rim et al. 2016). Although most wearable electrochemical biosensors are used to detect small molecules, such as lactate, glucose, or electrolytes, there is increasing interest in their application to pathogen detection. Challenges include biocompatibility (e.g., reduction of skin irritation), device power consumption, and biosensor-tissue mechanical and geometric matching. Because of the small sample size of body fluid secretions and the need to transport the sample to the electrode surface, microfluidic formats are now emerging for wearable bioanalytical systems (Singh et al. 2017a).
5.2. Detection of protozoa
Importantly, the size of the pathogen may have a significant impact on a given electrochemical biosensor's performance based on the type of electrochemical method used. For example, pathogens can range greater than three orders of magnitude in size. For example, the diameter of norovirus was estimated at 27 nm (Robilotti et al. 2015), while the diameter of G. lamblia oocysts is ~14 μm (Adam, 2001). Electrochemical biosensors for the detection of protozoa-based pathogens is an area requiring further attention. Protozoa, as large pathogens, achieve relatively less coverage of the electrode than small pathogens, thereby having a relatively smaller effect on charge transfer at the electrode-electrolyte interface. C. parvum is at present the most commonly detected protozoa using electrochemical biosensors (see Table 1) (Iqbal et al. 2015) (Luka et al. 2019).
5.3. Detection of plant pathogens
While the majority of infectious agents detected using electrochemical biosensors are human pathogens, emerging agricultural applications of electrochemical biosensors, such as in smart agriculture, suggest the need for biosensors capable of detecting plant pathogens (Khater et al., 2017). For example, crop yield losses associated with plant pathogens range from 8.1 to 41.1% based on global production of wheat, rice, maize, potato, and soybean (Savary et al., 2019). Common plant pathogens include viruses, viroids, bacteria, fungi, and oomycetes. Chartuprayoon et al. recently established a polypyrrole nanoribbon-based chemiresistive immunosensor for detection of viral plant pathogens (Chartuprayoon et al., 2013).
5.4. Multiplexed detection
Multiplexed detection of pathogens has emerged as a technique for phenotype identification and identification of multiple pathogenic threats. Multiplexing can be achieved via various approaches, but typically involves the use of multiple transducers that exhibit different biorecognition elements. For example, a strategy for multiplexed bacterial detection by Li et al. via immobilization of anti-E. coli and anti-V. cholerae on AuNPs is shown in Fig. 4b (Li et al. 2017). Spatially-distributed biorecognition elements on a single electrode or multiple electrodes can also provide multiplexing capability. For example, a strategy based on the immobilization of anti-E. coli and anti-S. aureus within a microfluidic chamber created by Tian et al. is shown in Fig. 4c (Tian et al. 2016).
5.5. Saturation-free continuous monitoring formats
The inability to regenerate biosensors is a major hindrance to biosensor-based process monitoring and control applications. While various biosensors must be disposed of after a single use, the regeneration of biosensor surfaces using chemical approaches has been leveraged as an approach for creating multiple-use biosensors. Biosensor regeneration approaches typically involve chemically-mediated dissociation of the target from the immobilized biorecognition element or removal of the biorecognition element altogether. This can be accomplished through acid-base mediated regeneration, detergents, glycine, and urea as well as achieved by thermal regeneration, plasma cleaning, or even direct electrochemical desorption (Goode et al. 2015; Huang et al. 2010; Zelada-Guillen et al. 2010). For example, Dweik et al. used a combination of organic (acetone) and plasma cleaning protocols to regenerate an Au interdigitated microelectrode array after detection of E. coli to use devices five times each (Dweik et al. 2012). Johnson and Mutharasan used a liquid-phase hydrogen peroxide-mediated UV-photooxidation process for regeneration of biosensor surfaces as an alternative to aggressive chemical treatments, such as those based on the use of high- or low-pH solutions (Johnson and Mutharasan, 2013b). We note that an ideal biosensor regeneration (i.e., cleaning) approach for process monitoring applications would remove the captured target in situ using a chemical-free approach and preserve the biorecognition layer for subsequent measurements.
5.6. Low-cost, single-use portable biosensors
The creation of environmentally-friendly disposable substrates is a present challenge for low-cost single-use biosensors. Paper-based substrates have recently emerged as attractive alternatives to costlier ceramic substrates (Martinez et al. 2009). Paper-based substrates can also eliminate the need for supporting fluid handling components through capillary effects. For example, paper substrates can be patterned with hydrophobic and hydrophilic regions to direct fluid flow (Carrilho et al. 2009). Paper-based devices are also relatively environmentally friendly in terms of material sourcing, disposal, and degradation. However, the potential toxicity of materials that may have been deposited on paper substrates, such as nanomaterials, should still be considered when assessing the environmental impact of a disposable single-use biosensing platform. For example, the long-term environmental and health impacts of nanomaterials remain active areas of research (Colvin, 2003; Klaine et al. 2008; Lead et al. 2018). Although paper-based devices have historically been most commonly used with colorimetric sensing techniques, they have been increasingly investigated for electrochemical biosensing (Ahmed et al. 2016; Meredith et al. 2016). A highlight of paper-based substrates is provided in Fig. 7c.
The need for water safety and medical diagnostics in remote and under-developed regions has led to the demand for low-cost portable biosensing platforms. One of the major challenges in creating portable biosensors for field use is the need to establish sample preparation-free protocols (Johnson and Mutharasan, 2012) and miniaturize components for actuation, data acquisition, and readout. However, device miniaturization also presents measurement challenges, such as increasing the biosensor signal-to-noise ratio (Wei et al. 2009). Further, portable biosensing platforms should exhibit biorecognition elements that remain stable for extended periods and at a variety of temperatures and humidity levels. The measurement robustness associated with the analysis of small sample volumes also requires further attention with the use of emerging low-cost materials, fabrication approaches, and transduction methods (Kumar et al. 2013; Luppa et al. 2016; Narayan, 2016; Wan et al. 2013).
The elimination of sample preparation steps from biosensor-based assays represents a significant advantage relative to traditional bioanalytical techniques (Johnson and Mutharasan, 2012) and is an important advantage and consideration for single-use biosensors and remote biosensing applications based on portable low-cost platforms. Sample preparation-free protocols can improve measurement confidence, repeatability, and reduce TTR, which are important aspects of healthcare decision-making. For example, it has been shown that a reduction in turnaround time for diagnostic assays could have a positive effect on clinical treatment outcomes (Davenport et al. 2017; Sin et al. 2014). When sample preparation is required, integrated alternatives to manual techniques, such as microfluidic processes, may provide a new path toward achieving rapid and robust pathogen detection. For example, separation and pre-concentration steps have been increasingly examined for integration with microfluidic-based biosensor platforms to reduce the number of steps, materials needed, and required technical personnel, and thus TTR (Bunyakul and Baeumner, 2014).
5.7. Wireless transduction approaches
The examination of wireless transduction and monitoring approaches has an important role in creating portable and wearable biosensing platforms for pathogen detection and distributed sensing systems for infection control and process monitoring (Ghafar-Zadeh, 2015). Wireless biosensing platforms are also essential to the creation of implantable and integrated biosensors for pathogen detection, including those for medical diagnostics. For example, as previously referenced, Mannoor et al. fabricated a conformal biosensor for bacteria detection on tooth enamel based on a radiofrequency (RF) link approach (Mannoor et al. 2012) (see Fig. 7d). Wireless transduction approaches remains an emerging area for pathogen detection. An example of smartphone-enabled wireless signal processing for detection of E. coli can be found in Fig. 7e (Jiang et al. 2014).
6. Conclusions
Here, we provided a critical review of electrochemical biosensors for pathogen detection. Biosensor transduction elements and biorecognition elements for electrochemical pathogen detection were reviewed. Bacteria remain the most commonly detected pathogens using electrochemical biosensors, though the detection of viruses and protozoa have been increasingly examined over the past five years. Electrochemical biosensors now exhibit LODs as low as a single plaque-forming unit (PFU)/mL and colony-forming unit (CFU)/mL and dynamic ranges that span multiple orders of magnitude. While planar Au electrodes remain the most commonly utilized working electrode, nanostructured electrodes derived from a variety of engineering materials, including polymers and composites, have been increasingly examined. Present challenges and future directions in the field were discussed, including a need for further low-cost, reusable, and wearable biosensors. Electrochemical biosensors offer great potential as resources for improving global healthcare, such as preventing the spread of highly contagious diseases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments:
The authors are grateful for the generous support of the United States National Science Foundation (CBET-1650601 and CMMI-1739318), which provided funding for the reported work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bios.2020.112214.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abbaspour A., Norouz-Sarvestani F., Noori A., Soltani N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens. Bioelectron. 2015;68:149–155. doi: 10.1016/j.bios.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Adam R.D. Biology of giardia lamblia. Clin. Microbiol. Rev. 2001;14(3):447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso A.S., Uliana C.V., Martucci D.H., Faria R.C. Simple and rapid fabrication of disposable carbon-based electrochemical cells using an electronic craft cutter for sensor and biosensor applications. Talanta. 2016;146:381–387. doi: 10.1016/j.talanta.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Ahmed A., Rushworth J.V., Hirst N.A., Millner P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014;27(3):631–646. doi: 10.1128/CMR.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Bui M.P., Abbas A. Paper-based chemical and biological sensors: engineering aspects. Biosens. Bioelectron. 2016;77:249–263. doi: 10.1016/j.bios.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Alahi E.E.M., Mukhopadhyay C.S. Detection methodologies for pathogen and toxins: a review. Sensors. 2017;17(8) doi: 10.3390/s17081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintas Z., Gittens M., Pocock J., Tothill I.E. Biosensors for waterborne viruses: detection and removal. Biochimie. 2015;115:144–154. doi: 10.1016/j.biochi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Ambrosi A., Moo J.G.S., Pumera M. Helical 3D-printed metal electrodes as custom-shaped 3D platform for electrochemical devices. Adv. Funct. Mater. 2016;26(5):698–703. [Google Scholar]
- Amiri M., Bezaatpour A., Jafari H., Boukherroub R., Szunerits S. Electrochemical methodologies for the detection of pathogens. ACS Sens. 2018;3(6):1069–1086. doi: 10.1021/acssensors.8b00239. [DOI] [PubMed] [Google Scholar]
- Andrade C.A., Nascimento J.M., Oliveira I.S., de Oliveira C.V., de Melo C.P., Franco O.L., Oliveira M.D. Nanostructured sensor based on carbon nanotubes and clavanin A for bacterial detection. Colloids Surf. B Biointerfaces. 2015;135:833–839. doi: 10.1016/j.colsurfb.2015.03.037. [DOI] [PubMed] [Google Scholar]
- Arshak K., Velusamy V., Korostynska O., Oliwa-Stasiak K., Adley C. Conducting polymers and their applications to biosensors: emphasizing on foodborne pathogen detection. IEEE Sensor. J. 2009;9(12):1942–1951. [Google Scholar]
- Attar A., Mandli J., Ennaji M.M., Amine A. Label-free electrochemical impedance detection of rotavirus based on immobilized antibodies on gold sononanoparticles. Electroanalysis. 2016;28(8):1839–1846. [Google Scholar]
- Aydın E.B., Sezgintürk M.K. Indium tin oxide (ITO): a promising material in biosensing technology. Trac. Trends Anal. Chem. 2017;97:309–315. [Google Scholar]
- Baek S.H., Kim M.W., Park C.Y., Choi C.S., Kailasa S.K., Park J.P., Park T.J. Development of a rapid and sensitive electrochemical biosensor for detection of human norovirus via novel specific binding peptides. Biosens. Bioelectron. 2019;123:223–229. doi: 10.1016/j.bios.2018.08.064. [DOI] [PubMed] [Google Scholar]
- Baeumner A.J. Biosensors for environmental pollutants and food contaminants. Anal. Bioanal. Chem. 2003;377(3):434–445. doi: 10.1007/s00216-003-2158-9. [DOI] [PubMed] [Google Scholar]
- Bandodkar A.J., Wang J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 2014;32(7):363–371. doi: 10.1016/j.tibtech.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Bard A.J., Faulkner L.R. second ed. Wiley; New York: 2000. Electrochemical Methods: Fundamentals and Applications. [Google Scholar]
- Barenfanger J., Drake C., Leon N., Mueller T., Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J. Clin. Microbiol. 2000;38(8):2824. doi: 10.1128/jcm.38.8.2824-2828.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiros dos Santos M., Agusil J.P., Prieto-Simon B., Sporer C., Teixeira V., Samitier J. Highly sensitive detection of pathogen Escherichia coli O157:H7 by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2013;45:174–180. doi: 10.1016/j.bios.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Barreiros dos Santos M., Azevedo S., Agusil J.P., Prieto-Simon B., Sporer C., Torrents E., Juarez A., Teixeira V., Samitier J. Label-free ITO-based immunosensor for the detection of very low concentrations of pathogenic bacteria. Bioelectrochemistry. 2015;101:146–152. doi: 10.1016/j.bioelechem.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Barsoukov E., Macdonald J.R. John Wiley & Sons; 2018. Impedance Spectroscopy: Theory, Experiment, and Applications. [Google Scholar]
- Bearinger J., Vörös J., Hubbell J., Textor M. Electrochemical optical waveguide lightmode spectroscopy (EC‐OWLS): a pilot study using evanescent‐field optical sensing under voltage control to monitor polycationic polymer adsorption onto indium tin oxide (ITO)‐coated waveguide chips. Biotechnol. Bioeng. 2003;82(4):465–473. doi: 10.1002/bit.10591. [DOI] [PubMed] [Google Scholar]
- Beekmann S.E., Diekema D.J., Chapin K.C., Doern G.V. Effects of rapid detection of bloodstream infections on length of hospitalization and hospital charges. J. Clin. Microbiol. 2003;41(7):3119. doi: 10.1128/JCM.41.7.3119-3125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekir K., Barhoumi H., Braiek M., Chrouda A., Zine N., Abid N., Maaref A., Bakhrouf A., Ouada H.B., Jaffrezic-Renault N., Mansour H.B. Electrochemical impedance immunosensor for rapid detection of stressed pathogenic Staphylococcus aureus bacteria. Environ. Sci. Pollut. Control Ser. 2015;22(20):15796–15803. doi: 10.1007/s11356-015-4761-7. [DOI] [PubMed] [Google Scholar]
- Berggren C., Bjarnason B., Johansson G. Capacitive biosensors. Electroanalysis. 2001;13(3):173–180. doi: 10.1016/s0956-5663(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Beuchat L.R., Komitopoulou E., Beckers H., Betts R.P., Bourdichon F., Fanning S., Joosten H.M., Ter Kuile B.H. Low-water activity foods: increased concern as vehicles of foodborne pathogens. J. Food Protect. 2013;76(1):150–172. doi: 10.4315/0362-028X.JFP-12-211. [DOI] [PubMed] [Google Scholar]
- Bhardwaj J., Devarakonda S., Kumar S., Jang J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sensor. Actuator. B Chem. 2017;253:115–123. [Google Scholar]
- Bhat K.S., Ahmad R., Yoo J.-Y., Hahn Y.-B. Fully nozzle-jet printed non-enzymatic electrode for biosensing application. J. Colloid Interface Sci. 2018;512:480–488. doi: 10.1016/j.jcis.2017.10.088. [DOI] [PubMed] [Google Scholar]
- Birch J.R., Racher A.J. Antibody production. Adv. Drug Deliv. Rev. 2006;58(5):671–685. doi: 10.1016/j.addr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Boehm D.A., Gottlieb P.A., Hua S.Z. On-chip microfluidic biosensor for bacterial detection and identification. Sensor. Actuator. B Chem. 2007;126(2):508–514. [Google Scholar]
- Bozal-Palabiyik B., Gumustas A., Ozkan S.A., Uslu B. Biosensor-based methods for the determination of foodborne pathogens. In: Holban A.M., Grumezescu A.M., editors. Foodborne Diseases. Academic Press; 2018. pp. 379–420. [Google Scholar]
- Bunyakul N., Baeumner A.J. Combining electrochemical sensors with miniaturized sample preparation for rapid detection in clinical samples. Sensors. 2014;15(1):547–564. doi: 10.3390/s150100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B., Stack E., Gilmartin N., O'Kennedy R. Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors. 2009;9(6):4407–4445. doi: 10.3390/s90604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral J.P. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Publ. Health. 2010;7(10):3657–3703. doi: 10.3390/ijerph7103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway Z., Wang Y., Zhang B., Zhang T., Costello T.A., Slavik M.F., Li Y. A portable impedance biosensing system for rapid detection of avian influenza virus. Trans. Asabe. 2016;59(2):421–428. [Google Scholar]
- Campuzano S., de Avila B.E., Yuste J., Pedrero M., Garcia J.L., Garcia P., Garcia E., Pingarron J.M. Disposable amperometric magnetoimmunosensors for the specific detection of Streptococcus pneumoniae. Biosens. Bioelectron. 2010;26(4):1225–1230. doi: 10.1016/j.bios.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Carrilho E., Martinez A.W., Whitesides G.M. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009;81(16):7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2019. Outbreak of Multidrug-Resistant Salmonella Infections Linked to Raw Turkey Products. [Google Scholar]
- Cesewski E., Haring A.P., Tong Y., Singh M., Thakur R., Laheri S., Read K.A., Powell M.D., Oestreich K.J., Johnson B.N. Additive manufacturing of three-dimensional (3D) microfluidic-based microelectromechanical systems (MEMS) for acoustofluidic applications. Lab Chip. 2018;18(14):2087–2098. doi: 10.1039/c8lc00427g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.Y., Ye W.W., Zhang Y., Xiao L.D., Leung P.H., Li Y., Yang M. Ultrasensitive detection of E. coli O157:H7 with biofunctional magnetic bead concentration via nanoporous membrane based electrochemical immunosensor. Biosens. Bioelectron. 2013;41:532–537. doi: 10.1016/j.bios.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Chand R., Neethirajan S. Microfluidic platform integrated with graphene-gold nano-composite aptasensor for one-step detection of norovirus. Biosens. Bioelectron. 2017;98:47–53. doi: 10.1016/j.bios.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Chartuprayoon N., Rheem Y., Ng J.C.K., Nam J., Chen W., Myung N.V. Polypyrrole nanoribbon based chemiresistive immunosensors for viral plant pathogen detection. Anal. Methods. 2013;5(14):3497–3502. [Google Scholar]
- Chen G.Z., Yin Z.Z., Lou J.F. Electrochemical immunoassay of Escherichia coli O157:H7 using Ag@SiO2 nanoparticles as labels. J. Anal. Methods Chem. 2014;2014:247034. doi: 10.1155/2014/247034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang X., Lu W., Wu X., Li J. Molecular imprinting: perspectives and applications. Chem. Soc. Rev. 2016;45(8):2137–2211. doi: 10.1039/c6cs00061d. [DOI] [PubMed] [Google Scholar]
- Chen Q., Lin J., Gan C., Wang Y., Wang D., Xiong Y., Lai W., Li Y., Wang M. A sensitive impedance biosensor based on immunomagnetic separation and urease catalysis for rapid detection of Listeria monocytogenes using an immobilization-free interdigitated array microelectrode. Biosens. Bioelectron. 2015;74:504–511. doi: 10.1016/j.bios.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Chen Q., Wang D., Cai G., Xiong Y., Li Y., Wang M., Huo H., Lin J. Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens. Bioelectron. 2016;86:770–776. doi: 10.1016/j.bios.2016.07.071. [DOI] [PubMed] [Google Scholar]
- Chen Y.T., Kolhatkar A.G., Zenasni O., Xu S., Lee T.R. Biosensing using magnetic particle detection techniques. Sensors. 2017;17(10):2300. doi: 10.3390/s17102300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.S., Ho J.S., Tan C.H., Wong J.P., Ng L.C., Toh C.S. Development of an electrochemical membrane-based nanobiosensor for ultrasensitive detection of dengue virus. Anal. Chim. Acta. 2012;725:74–80. doi: 10.1016/j.aca.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Cheong W.J., Yang S.H., Ali F. Molecular imprinted polymers for separation science: a review of reviews. J. Separ. Sci. 2013;36(3):609–628. doi: 10.1002/jssc.201200784. [DOI] [PubMed] [Google Scholar]
- Chin S.F., Lim L.S., Pang S.C., Sum M.S.H., Perera D. Carbon nanoparticle modified screen printed carbon electrode as a disposable electrochemical immunosensor strip for the detection of Japanese encephalitis virus. Microchimica Acta. 2017;184(2):491–497. [Google Scholar]
- Choi S., Goryll M., Sin L.Y.M., Wong P.K., Chae J. Microfluidic-based biosensors toward point-of-care detection of nucleic acids and proteins. Microfluid. Nanofluidics. 2011;10(2):231–247. doi: 10.1007/s10404-010-0638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A.D., De A., Chaudhuri C.R., Bandyopadhyay K., Sen P. Label free polyaniline based impedimetric biosensor for detection of E. coli O157:H7 Bacteria. Sensor. Actuator. B Chem. 2012;171:916–923. [Google Scholar]
- Christopher G.W., Cieslak T.J., Pavlin J.A., Eitzen E.M., Jr. Biological warfare. A historical perspective. J. Am. Med. Assoc. 1997;278(5):412–417. [PubMed] [Google Scholar]
- Cirino N.M., Musser K.A., Egan C. Multiplex diagnostic platforms for detection of biothreat agents. Expert Rev. Mol. Diagn. 2004;4(6):841–857. doi: 10.1586/14737159.4.6.841. [DOI] [PubMed] [Google Scholar]
- Clark K.D., Zhang C., Anderson J.L. Sample preparation for bioanalytical and pharmaceutical analysis. Anal. Chem. 2016;88(23):11262–11270. doi: 10.1021/acs.analchem.6b02935. [DOI] [PubMed] [Google Scholar]
- Colvin V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003;21(10):1166. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- Cooper M.A. Cambridge University Press; 2009. Label-free Biosensors: Techniques and Applications. [Google Scholar]
- da Silva E.T., Souto D.E., Barragan J.T., de F., Giarola J., de Moraes A.C., Kubota L.T. Electrochemical biosensors in point‐of‐care devices: recent advances and future trends. ChemElectroChem. 2017;4(4):778–794. [Google Scholar]
- Daniels J.S., Pourmand N. Label-free impedance biosensors: opportunities and challenges. Electroanalysis. 2007;19(12):1239–1257. doi: 10.1002/elan.200603855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R.D., RoyChaudhuri C., Maji S., Das S., Saha H. Macroporous silicon based simple and efficient trapping platform for electrical detection of Salmonella typhimurium pathogens. Biosens. Bioelectron. 2009;24(11):3215–3222. doi: 10.1016/j.bios.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Dastider S.G., Barizuddin S., Yuksek N.S., Dweik M., Almasri M.F. Efficient and rapid detection of Salmonella using microfluidic impedance based sensing. J. Sensors. 2015;8 2015. [Google Scholar]
- Daum P.H., Enke C.G. Electrochemical kinetics of the ferri-ferrocyanide couple on platinum. Anal. Chem. 1969;41(4):653–656. [Google Scholar]
- Davenport M., Mach K.E., Shortliffe L.M.D., Banaei N., Wang T.H., Liao J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 2017;14(5):296–310. doi: 10.1038/nrurol.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rica R., Baldi A., Fernandez-Sanchez C., Matsui H. Selective detection of live pathogens via surface-confined electric field perturbation on interdigitated silicon transducers. Anal. Chem. 2009;81(10):3830–3835. doi: 10.1021/ac9001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luna P., Mahshid S.S., Das J., Luan B., Sargent E.H., Kelley S.O., Zhou R. High-curvature nanostructuring enhances probe display for biomolecular detection. Nano Lett. 2017;17(2):1289–1295. doi: 10.1021/acs.nanolett.6b05153. [DOI] [PubMed] [Google Scholar]
- Dierkes C., Ehrenstein B., Siebig S., Linde H.-J., Reischl U., Salzberger B. Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis. BMC Infect. Dis. 2009;9(1):126. doi: 10.1186/1471-2334-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Lei J., Ma X., Gong J., Qin W. Potentiometric aptasensing of Listeria monocytogenes using protamine as an indicator. Anal. Chem. 2014;86(19):9412–9416. doi: 10.1021/ac502335g. [DOI] [PubMed] [Google Scholar]
- Divagar M., Sriramprabha R., Sornambikai S., Ponpandian N., Viswanathan C. Surface imprinted Ag decorated MnO2 thin film electrodes for the synergic electrochemical detection of bacterial pathogens. J. Electrochem. Soc. 2019;166(2):G1–G9. [Google Scholar]
- Dong J., Zhao H., Xu M., Ma Q., Ai S. A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem. 2013;141(3):1980–1986. doi: 10.1016/j.foodchem.2013.04.098. [DOI] [PubMed] [Google Scholar]
- Duffy G., Moore E. Electrochemical immunosensors for food analysis: a review of recent developments. Anal. Lett. 2017;50(1):1–32. [Google Scholar]
- Dweik M., Stringer R.C., Dastider S.G., Wu Y., Almasri M., Barizuddin S. Specific and targeted detection of viable Escherichia coli O157:H7 using a sensitive and reusable impedance biosensor with dose and time response studies. Talanta. 2012;94:84–89. doi: 10.1016/j.talanta.2012.02.056. [DOI] [PubMed] [Google Scholar]
- Dye C. After 2015: infectious diseases in a new era of health and development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1645) doi: 10.1098/rstb.2013.0426. 20130426-20130426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzyadevych S., Jaffrezic-Renault N. Conductometric biosensors. In: Schaudies R.P., editor. Biological Identification. Woodhead Publishing; 2014. pp. 153–193. [Google Scholar]
- Eftekhari A., Alkire R.C., Gogotsi Y., Simon P. Wiley; 2008. Nanostructured Materials in Electrochemistry. [Google Scholar]
- Erkal J.L., Selimovic A., Gross B.C., Lockwood S.Y., Walton E.L., McNamara S., Martin R.S., Spence D.M. 3D printed microfluidic devices with integrated versatile and reusable electrodes. Lab Chip. 2014;14(12):2023–2032. doi: 10.1039/c4lc00171k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla-Gomez V., Campuzano S., Pedrero M., Pingarron J.M. Electrochemical immunosensor designs for the determination of Staphylococcus aureus using 3,3-dithiodipropionic acid di(N-succinimidyl ester)-modified gold electrodes. Talanta. 2008;77(2):876–881. [Google Scholar]
- Escamilla-Gomez V., Campuzano S., Pedrero M., Pingarron J.M. Gold screen-printed-based impedimetric immunobiosensors for direct and sensitive Escherichia coli quantisation. Biosens. Bioelectron. 2009;24(11):3365–3371. doi: 10.1016/j.bios.2009.04.047. [DOI] [PubMed] [Google Scholar]
- Esteban-Fernandez de Avila B., Pedrero M., Campuzano S., Escamilla-Gomez V., Pingarron J.M. Sensitive and rapid amperometric magnetoimmunosensor for the determination of Staphylococcus aureus. Anal. Bioanal. Chem. 2012;403(4):917–925. doi: 10.1007/s00216-012-5738-8. [DOI] [PubMed] [Google Scholar]
- Etayash H., Jiang K., Azmi S., Thundat T., Kaur K. Real-time detection of breast cancer cells using peptide-functionalized microcantilever arrays. Sci. Rep. 2015;5:13967. doi: 10.1038/srep13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etayash H., Jiang K., Thundat T., Kaur K. Impedimetric detection of pathogenic Gram-positive bacteria using an antimicrobial peptide from class IIa bacteriocins. Anal. Chem. 2014;86(3):1693–1700. doi: 10.1021/ac4034938. [DOI] [PubMed] [Google Scholar]
- Faucher S.P., Charette S.J. Editorial on: bacterial pathogens in the non-clinical environment. Front. Microbiol. 2015;6(331):331. doi: 10.3389/fmicb.2015.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix F.S., Angnes L. Electrochemical immunosensors - a powerful tool for analytical applications. Biosens. Bioelectron. 2018;102:470–478. doi: 10.1016/j.bios.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Foo C.Y., Lim H.N., Mahdi M.A., Wahid M.H., Huang N.M. Three-dimensional printed electrode and its novel applications in electronic devices. Sci. Rep. 2018;8(1):7399. doi: 10.1038/s41598-018-25861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraise A.P., Lambert P.A., Maillard J.-Y. John Wiley & Sons; 2008. Russell, Hugo & Ayliffe's Principles and Practice of Disinfection, Preservation and Sterilization. [Google Scholar]
- Fu Y., Callaway Z., Lum J., Wang R., Lin J., Li Y. Exploiting enzyme catalysis in ultra-low ion strength media for impedance biosensing of avian influenza virus using a bare interdigitated electrode. Anal. Chem. 2014;86(4):1965–1971. doi: 10.1021/ac402550f. [DOI] [PubMed] [Google Scholar]
- Furst A.L., Francis M.B. Impedance-based detection of bacteria. Chem. Rev. 2019;119(1):700–726. doi: 10.1021/acs.chemrev.8b00381. [DOI] [PubMed] [Google Scholar]
- Gayathri C.H., Mayuri P., Sankaran K., Kumar A.S. An electrochemical immunosensor for efficient detection of uropathogenic E. coli based on thionine dye immobilized chitosan/functionalized-MWCNT modified electrode. Biosens. Bioelectron. 2016;82:71–77. doi: 10.1016/j.bios.2016.03.062. [DOI] [PubMed] [Google Scholar]
- Geng P., Zhang X.N., Meng W.W., Wang Q.J., Zhang W., Jin L.T., Feng Z., Wu Z.R. Self-assembled monolayers-based immunosensor for detection of Escherichia coli using electrochemical impedance spectroscopy. Electrochim. Acta. 2008;53(14):4663–4668. [Google Scholar]
- Ghafar-Zadeh E. Wireless integrated biosensors for point-of-care diagnostic applications. Sensors. 2015;15(2):3236–3261. doi: 10.3390/s150203236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamberardino A., Labib M., Hassan E.M., Tetro J.A., Springthorpe S., Sattar S.A., Berezovski M.V., DeRosa M.C. Ultrasensitive norovirus detection using DNA aptasensor technology. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0079087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golabi M., Kuralay F., Jager E.W.H., Beni V., Turner A.P.F. Electrochemical bacterial detection using poly(3-aminophenylboronic acid)-based imprinted polymer. Biosens. Bioelectron. 2017;93:87–93. doi: 10.1016/j.bios.2016.09.088. [DOI] [PubMed] [Google Scholar]
- Goode J.A., Rushworth J.V., Millner P.A. Biosensor regeneration: a review of common techniques and outcomes. Langmuir. 2015;31(23):6267–6276. doi: 10.1021/la503533g. [DOI] [PubMed] [Google Scholar]
- Gordon R.J., Lowy F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008;46(Suppl. 5):S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig J.D., Todd E.C. Food Safety Education Conference; Atlanta, Georgia: 2010. Infective Doses and Pathogen Carriage. 2010. [Google Scholar]
- Gui R., Jin H., Guo H., Wang Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018;100:56–70. doi: 10.1016/j.bios.2017.08.058. [DOI] [PubMed] [Google Scholar]
- Guimard N.K., Gomez N., Schmidt C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007;32(8–9):876–921. [Google Scholar]
- Güner A., Cevik E., Senel M., Alpsoy L. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem. 2017;229:358–365. doi: 10.1016/j.foodchem.2017.02.083. [DOI] [PubMed] [Google Scholar]
- Guo X., Kulkarni A., Doepke A., Halsall H.B., Iyer S., Heineman W.R. Carbohydrate-based label-free detection of Escherichia coli ORN 178 using electrochemical impedance spectroscopy. Anal. Chem. 2012;84(1):241–246. doi: 10.1021/ac202419u. [DOI] [PubMed] [Google Scholar]
- Gupta A., Akin D., Bashir R. Single virus particle mass detection using microresonators with nanoscale thickness. Appl. Phys. Lett. 2004;84(11):1976–1978. [Google Scholar]
- Gürtler L. In: Virology of Human Influenza. Influenza Report. Kamps B.S., Hoffmann C., Preiser W., editors. Flying Publisher; Wuppertal: 2006. [Google Scholar]
- Hai W., Goda T., Takeuchi H., Yamaoka S., Horiguchi Y., Matsumoto A., Miyahara Y. Specific recognition of human influenza virus with PEDOT bearing sialic acid-terminated trisaccharides. ASC Appl. Mater. Interfaces. 2017;9(16):14162–14170. doi: 10.1021/acsami.7b02523. [DOI] [PubMed] [Google Scholar]
- Hai W., Goda T., Takeuchi H., Yamaoka S., Horiguchi Y., Matsumoto A., Miyahara Y. Human influenza virus detection using sialyllactose-functionalized organic electrochemical transistors. Sensor. Actuator. B Chem. 2018;260:635–641. [Google Scholar]
- Haq I.U., Chaudhry W.N., Akhtar M.N., Andleeb S., Qadri I. Bacteriophages and their implications on future biotechnology: a review. Virol. J. 2012;9 doi: 10.1186/1743-422X-9-9. 9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.R., de la Escosura-Muniz A., Merkoci A. Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags. Biosens. Bioelectron. 2015;67:511–515. doi: 10.1016/j.bios.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Hassen W.M., Duplan V., Frost E., Dubowski J.J. Quantitation of influenza A virus in the presence of extraneous protein using electrochemical impedance spectroscopy. Electrochim. Acta. 2011;56(24):8325–8328. [Google Scholar]
- He R.-X., Zhang M., Tan F., Leung P.H.M., Zhao X.-Z., Chan H.L.W., Yang M., Yan F. Detection of bacteria with organic electrochemical transistors. J. Mater. Chem. 2012;22(41):22072–22076. [Google Scholar]
- Hernandez R., Valles C., Benito A.M., Maser W.K., Rius F.X., Riu J. Graphene-based potentiometric biosensor for the immediate detection of living bacteria. Biosens. Bioelectron. 2014;54:553–557. doi: 10.1016/j.bios.2013.11.053. [DOI] [PubMed] [Google Scholar]
- Hierlemann A., Brand O., Hagleitner C., Baltes H. Microfabrication techniques for chemical/biosensors. Proc. IEEE. 2003;91(6):839–863. [Google Scholar]
- Hintsche R., Paeschke M., Wollenberger U., Schnakenberg U., Wagner B., Lisec T. Microelectrode arrays and application to biosensing devices. Biosens. Bioelectron. 1994;9(9–10):697–705. [Google Scholar]
- Hong S.A., Kwon J., Kim D., Yang S. A rapid, sensitive and selective electrochemical biosensor with concanavalin A for the preemptive detection of norovirus. Biosens. Bioelectron. 2015;64:338–344. doi: 10.1016/j.bios.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Hookman P., Barkin J.S. Clostridium difficile associated infection, diarrhea and colitis. World J. Gastroenterol. 2009;15(13):1554–1580. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.H., Wang J.J., Jiang Y.Z., Lv C., Xia L., Hong S.L., Lin M., Lin Y., Zhang Z.L., Pang D.W. A colorimetric and electrochemical immunosensor for point-of-care detection of enterovirus 71. Biosens. Bioelectron. 2018;99:186–192. doi: 10.1016/j.bios.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Hu J., Odom T.W., Lieber C.M. Chemistry and physics in one dimension: synthesis and properties of nanowires and nanotubes. Acc. Chem. Res. 1999;32(5):435–445. [Google Scholar]
- Hu W., Li C.M., Dong H. Poly(pyrrole-co-pyrrole propylic acid) film and its application in label-free surface plasmon resonance immunosensors. Anal. Chim. Acta. 2008;630(1):67–74. doi: 10.1016/j.aca.2008.09.059. [DOI] [PubMed] [Google Scholar]
- Huang J., Yang G., Meng W., Wu L., Zhu A., Jiao X. An electrochemical impedimetric immunosensor for label-free detection of Campylobacter jejuni in diarrhea patients' stool based on O-carboxymethylchitosan surface modified Fe3O4 nanoparticles. Biosens. Bioelectron. 2010;25(5):1204–1211. doi: 10.1016/j.bios.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Huang Y.X., Dong X.C., Liu Y.X., Li L.J., Chen P. Graphene-based biosensors for detection of bacteria and their metabolic activities. J. Mater. Chem. 2011;21(33):12358–12362. [Google Scholar]
- Hushegyi A., Pihikova D., Bertok T., Adam V., Kizek R., Tkac J. Ultrasensitive detection of influenza viruses with a glycan-based impedimetric biosensor. Biosens. Bioelectron. 2016;79:644–649. doi: 10.1016/j.bios.2015.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H.J., Ryu M.Y., Park C.Y., Ahn J., Park H.G., Choi C., Ha S.D., Park T.J., Park J.P. High sensitive and selective electrochemical biosensor: label-free detection of human norovirus using affinity peptide as molecular binder. Biosens. Bioelectron. 2017;87:164–170. doi: 10.1016/j.bios.2016.08.031. [DOI] [PubMed] [Google Scholar]
- Idil N., Hedstrom M., Denizli A., Mattiasson B. Whole cell based microcontact imprinted capacitive biosensor for the detection of Escherichia coli. Biosens. Bioelectron. 2017;87:807–815. doi: 10.1016/j.bios.2016.08.096. [DOI] [PubMed] [Google Scholar]
- Iqbal A., Labib M., Muharemagic D., Sattar S., Dixon B.R., Berezovski M.V. Detection of Cryptosporidium parvum oocysts on fresh produce using DNA aptamers. PloS One. 2015;10(9) doi: 10.1371/journal.pone.0137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari H., Amiri M., Abdi E., Navid S.L., Bouckaert J., Jijie R., Boukherroub R., Szunerits S. Entrapment of uropathogenic E. coli cells into ultra-thin sol-gel matrices on gold thin films: a low cost alternative for impedimetric bacteria sensing. Biosens. Bioelectron. 2019;124–125:161–166. doi: 10.1016/j.bios.2018.10.029. [DOI] [PubMed] [Google Scholar]
- Jaffrezic-Renault N., Dzyadevych S.V. Conductometric microbiosensors for environmental monitoring. Sensors. 2008;8(4):2569–2588. doi: 10.3390/s8042569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K., Bell G.T. Human monoclonal antibody production: current status and future prospects. J. Immunol. Methods. 1987;100(1):5–40. doi: 10.1016/0022-1759(87)90170-0. [DOI] [PubMed] [Google Scholar]
- Jantra J., Kanatharana P., Asawatreratanakul P., Hedstrom M., Mattiasson B., Thavarungkul P. Real-time label-free affinity biosensors for enumeration of total bacteria based on immobilized concanavalin A. J. Environ. Sci. Health - Part A Toxic/Hazard. Subst. Environ. Eng. 2011;46(13):1450–1460. doi: 10.1080/10934529.2011.609022. [DOI] [PubMed] [Google Scholar]
- Ji Z.-G. John Wiley & Sons; 2017. Hydrodynamics and Water Quality: Modeling Rivers, Lakes, and Estuaries. [Google Scholar]
- Jia F., Duan N., Wu S.J., Ma X.Y., Xia Y., Wang Z.P., Wei X.L. Impedimetric aptasensor for Staphylococcus aureus based on nanocomposite prepared from reduced graphene oxide and gold nanoparticles. Microchimica Acta. 2014;181(9–10):967–974. [Google Scholar]
- Jiang J., Wang X.H., Chao R., Ren Y.K., Hu C.P., Xu Z.D., Liu G.L. Smartphone based portable bacteria pre-concentrating microfluidic sensor and impedance sensing system. Sensor. Actuator. B Chem. 2014;193:653–659. [Google Scholar]
- Johnson B.N., Mutharasan R. Sample preparation-free, real-time detection of microRNA in human serum using piezoelectric cantilever biosensors at attomole level. Anal. Chem. 2012;84(23):10426–10436. doi: 10.1021/ac303055c. [DOI] [PubMed] [Google Scholar]
- Johnson B.N., Mutharasan R. Electrochemical piezoelectric-excited millimeter-sized cantilever (ePEMC) for simultaneous dual transduction biosensing. Analyst. 2013;138(21):6365–6371. doi: 10.1039/c3an01353g. [DOI] [PubMed] [Google Scholar]
- Johnson B.N., Mutharasan R. Regeneration of gold surfaces covered by adsorbed thiols and proteins using liquid-phase hydrogen peroxide-mediated UV-photooxidation. J. Phys. Chem. C. 2013;117(3):1335–1341. [Google Scholar]
- Johnson B.N., Mutharasan R. Reduction of nonspecific protein adsorption on cantilever biosensors caused by transverse resonant mode vibration. Analyst. 2014;139(5):1112–1120. doi: 10.1039/c3an01675g. [DOI] [PubMed] [Google Scholar]
- Joung C.K., Kim H.N., Lim M.C., Jeon T.J., Kim H.Y., Kim Y.R. A nanoporous membrane-based impedimetric immunosensor for label-free detection of pathogenic bacteria in whole milk. Biosens. Bioelectron. 2013;44:210–215. doi: 10.1016/j.bios.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Juan-Colas J., Johnson S., Krauss T.F. Dual-mode electro-optical techniques for biosensing applications: a review. Sensors. 2017;17(9) doi: 10.3390/s17092047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justino C.I.L., Duarte A.C., Rocha-Santos T.A.P. Recent progress in biosensors for environmental monitoring: a review. Sensors. 2017;17(12) doi: 10.3390/s17122918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Adhikari R., Cass P., Bown M., Gunatillake P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015;5(47):37553–37567. [Google Scholar]
- Kelley S.O. What are clinically relevant levels of cellular and biomolecular analytes? ACS Sens. 2017;2(2):193–197. doi: 10.1021/acssensors.6b00691. [DOI] [PubMed] [Google Scholar]
- Khater M., de la Escosura-Muñiz A., Merkoçi A. Biosensors for plant pathogen detection. Biosens. Bioelectron. 2017;93:72–86. doi: 10.1016/j.bios.2016.09.091. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Wang N., Tay M.Q.X., Miao J.M., Whittle A.J. Development of a MEMS-based electrochemical aptasensor for norovirus detection. Micro & Nano Lett. 2016;11(10):582–585. [Google Scholar]
- Klaine S.J., Alvarez P.J., Batley G.E., Fernandes T.F., Handy R.D., Lyon D.Y., Mahendra S., McLaughlin M.J., Lead J.R. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008;27(9):1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Klein D. Quantification using real-time PCR technology: applications and limitations. Trends Mol. Med. 2002;8(6):257–260. doi: 10.1016/s1471-4914(02)02355-9. [DOI] [PubMed] [Google Scholar]
- Kokkinos C., Economou A., Prodromidis M.I. Electrochemical immunosensors: critical survey of different architectures and transduction strategies. Trac. Trends Anal. Chem. 2016;79:88–105. [Google Scholar]
- Kong Y.L., Tamargo I.A., Kim H., Johnson B.N., Gupta M.K., Koh T.W., Chin H.A., Steingart D.A., Rand B.P., McAlpine M.C. 3D printed quantum dot light-emitting diodes. Nano Lett. 2014;14(12):7017–7023. doi: 10.1021/nl5033292. [DOI] [PubMed] [Google Scholar]
- Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006;6(1):130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio D.R., Peppas N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012;8(2):461–473. doi: 10.1016/j.actbio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kumar S., Ali M.A., Anand P., Agrawal V.V., John R., Maji S., Malhotra B.D. Microfluidic-integrated biosensors: prospects for point-of-care diagnostics. Biotechnol. J. 2013;8(11):1267–1279. doi: 10.1002/biot.201200386. [DOI] [PubMed] [Google Scholar]
- Kutter E., Sulakvelidze A. CRC Press; 2004. Bacteriophages: Biology and Applications. [Google Scholar]
- La Belle J.T., Shah M., Reed J., Nandakumar V., Alford T.L., Wilson J.W., Nickerson C.A., Joshi L. Label-free and ultra-low level detection of Salmonella enterica serovar typhimurium using electrochemical impedance spectroscopy. Electroanalysis. 2009;21(20):2267–2271. [Google Scholar]
- Laczka O., Skillman L., Ditcham W.G., Hamdorf B., Wong D.K., Bergquist P., Sunna A. Application of an ELISA-type screen printed electrode-based potentiometric assay to the detection of Cryptosporidium parvum oocysts. J. Microbiol. Methods. 2013;95(2):182–185. doi: 10.1016/j.mimet.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Lai K., Emberlin J., Colbeck I. Outdoor environments and human pathogens in air. Environ. Health. 2009;8(Suppl. 1):S15. doi: 10.1186/1476-069X-8-S1-S15. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhin A.V., Tarantul V.Z., Gening L.V. Aptamers: problems, solutions and prospects. Acta Naturae. 2013;5(4):34–43. [PMC free article] [PubMed] [Google Scholar]
- Lam B., Fang Z., Sargent E.H., Kelley S.O. Polymerase chain reaction-free, sample-to-answer bacterial detection in 30 minutes with integrated cell lysis. Anal. Chem. 2012;84(1):21–25. doi: 10.1021/ac202599b. [DOI] [PubMed] [Google Scholar]
- Law J.W.-F., Ab Mutalib N.-S., Chan K.-G., Lee L.-H. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front. Microbiol. 2015;5:770. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchimica Acta. 2019;186(4):224. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcka O., Del Campo F.J., Munoz F.X. Pathogen detection: a perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007;22(7):1205–1217. doi: 10.1016/j.bios.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Lead J.R., Batley G.E., Alvarez P.J., Croteau M.N., Handy R.D., McLaughlin M.J., Judy J.D., Schirmer K. Nanomaterials in the environment: behavior, fate, bioavailability, and effects—an updated review. Environ. Toxicol. Chem. 2018;37(8):2029–2063. doi: 10.1002/etc.4147. [DOI] [PubMed] [Google Scholar]
- Lee B.S., Lee J.N., Park J.M., Lee J.G., Kim S., Cho Y.K., Ko C. A fully automated immunoassay from whole blood on a disc. Lab Chip. 2009;9(11):1548–1555. doi: 10.1039/b820321k. [DOI] [PubMed] [Google Scholar]
- Lee D., Chander Y., Goyal S.M., Cui T. Carbon nanotube electric immunoassay for the detection of swine influenza virus H1N1. Biosens. Bioelectron. 2011;26(8):3482–3487. doi: 10.1016/j.bios.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Jun S. Simultaneous detection of E. coli K12 and S. aureus using a continuous flow multijunction biosensor. J. Food Sci. 2016;81(6):N1530–N1536. doi: 10.1111/1750-3841.13307. [DOI] [PubMed] [Google Scholar]
- Leonard P., Hearty S., Brennan J., Dunne L., Quinn J., Chakraborty T., O'Kennedy R. Advances in biosensors for detection of pathogens in food and water. Enzym. Microb. Technol. 2003;32(1):3–13. [Google Scholar]
- Li D., Feng Y., Zhou L., Ye Z., Wang J., Ying Y., Ruan C., Wang R., Li Y. Label-free capacitive immunosensor based on quartz crystal Au electrode for rapid and sensitive detection of Escherichia coli O157:H7. Anal. Chim. Acta. 2011;687(1):89–96. doi: 10.1016/j.aca.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Li Y., Cheng P., Gong J., Fang L., Deng J., Liang W., Zheng J. Amperometric immunosensor for the detection of Escherichia coli O157:H7 in food specimens. Anal. Biochem. 2012;421(1):227–233. doi: 10.1016/j.ab.2011.10.049. [DOI] [PubMed] [Google Scholar]
- Li Y., Fang L., Cheng P., Deng J., Jiang L., Huang H., Zheng J. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 using C60 based biocompatible platform and enzyme functionalized Pt nanochains tracing tag. Biosens. Bioelectron. 2013;49:485–491. doi: 10.1016/j.bios.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Li Y., Xiong Y., Fang L., Jiang L., Huang H., Deng J., Liang W., Zheng J. An electrochemical strategy using multifunctional nanoconjugates for efficient simultaneous detection of Escherichia coli O157: H7 and Vibrio cholerae O1. Theranostics. 2017;7(4):935–944. doi: 10.7150/thno.17544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Fu Y., Fang W., Li Y. Electrochemical impedance immunosensor based on self-assembled monolayers for rapid detection of Escherichia coli O157:H7 with signal amplification using lectin. Sensors. 2015;15(8):19212–19224. doi: 10.3390/s150819212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebana S., Lermo A., Campoy S., Cortes M.P., Alegret S., Pividori M.I. Rapid detection of Salmonella in milk by electrochemical magneto-immunosensing. Biosens. Bioelectron. 2009;25(2):510–513. doi: 10.1016/j.bios.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Lin J., Wang R., Jiao P., Li Y., Li Y., Liao M., Yu Y., Wang M. An impedance immunosensor based on low-cost microelectrodes and specific monoclonal antibodies for rapid detection of avian influenza virus H5N1 in chicken swabs. Biosens. Bioelectron. 2015;67:546–552. doi: 10.1016/j.bios.2014.09.037. [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Chen S.H., Chuang Y.C., Lu Y.C., Shen T.Y., Chang C.A., Lin C.S. Disposable amperometric immunosensing strips fabricated by Au nanoparticles-modified screen-printed carbon electrodes for the detection of foodborne pathogen Escherichia coli O157:H7. Biosens. Bioelectron. 2008;23(12):1832–1837. doi: 10.1016/j.bios.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Lisdat F., Schäfer D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008;391(5):1555. doi: 10.1007/s00216-008-1970-7. [DOI] [PubMed] [Google Scholar]
- Liu F., Choi K.S., Park T.J., Lee S.Y., Seo T.S. Graphene-based electrochemical biosensor for pathogenic virus detection. Biochip J. 2011;5(2):123–128. [Google Scholar]
- Liu F., Kim Y.H., Cheon D.S., Seo T.S. Micropatterned reduced graphene oxide based field-effect transistor for real-time virus detection. Sensor. Actuator. B Chem. 2013;186:252–257. [Google Scholar]
- Loo A.H., Chua C.K., Pumera M. DNA biosensing with 3D printing technology. Analyst. 2017;142(2):279–283. doi: 10.1039/c6an02038k. [DOI] [PubMed] [Google Scholar]
- Lu L., Chee G., Yamada K., Jun S. Electrochemical impedance spectroscopic technique with a functionalized microwire sensor for rapid detection of foodborne pathogens. Biosens. Bioelectron. 2013;42:492–495. doi: 10.1016/j.bios.2012.10.060. [DOI] [PubMed] [Google Scholar]
- Ludwig K.A., Uram J.D., Yang J., Martin D.C., Kipke D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly (3, 4-ethylenedioxythiophene)(PEDOT) film. J. Neural. Eng. 2006;3(1):59. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- Luka G., Samiei E., Dehghani S., Johnson T., Najjaran H., Hoorfar M. Label-free capacitive biosensor for detection of Cryptosporidium. Sensors. 2019;19(2) doi: 10.3390/s19020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J., Wang R., Lassiter K., Srinivasan B., Abi-Ghanem D., Berghman L., Hargis B., Tung S., Lu H., Li Y. Rapid detection of avian influenza H5N1 virus using impedance measurement of immuno-reaction coupled with RBC amplification. Biosens. Bioelectron. 2012;38(1):67–73. doi: 10.1016/j.bios.2012.04.047. [DOI] [PubMed] [Google Scholar]
- Luna D.M.N., Avelino K.Y.P.S., Cordeiro M.T., Andrade C.A.S., Oliveira M.D.L. Electrochemical immunosensor for dengue virus serotypes based on 4-mercaptobenzoic acid modified gold nanoparticles on self-assembled cysteine monolayers. Sensor. Actuator. B Chem. 2015;220:565–572. [Google Scholar]
- Luo Y., Nartker S., Miller H., Hochhalter D., Wiederoder M., Wiederoder S., Setterington E., Drzal L.T., Alocilja E.C. Surface functionalization of electrospun nanofibers for detecting E. coli O157:H7 and BVDV cells in a direct-charge transfer biosensor. Biosens. Bioelectron. 2010;26(4):1612–1617. doi: 10.1016/j.bios.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Luppa P.B., Bietenbeck A., Beaudoin C., Giannetti A. Clinically relevant analytical techniques, organizational concepts for application and future perspectives of point-of-care testing. Biotechnol. Adv. 2016;34(3):139–160. doi: 10.1016/j.biotechadv.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Ma X., Jiang Y., Jia F., Yu Y., Chen J., Wang Z. An aptamer-based electrochemical biosensor for the detection of Salmonella. J. Microbiol. Methods. 2014;98:94–98. doi: 10.1016/j.mimet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Maalouf R., Fournier-Wirth C., Coste J., Chebib H., Saikali Y., Vittori O., Errachid A., Cloarec J.P., Martelet C., Jaffrezic-Renault N. Label-free detection of bacteria by electrochemical impedance spectroscopy: comparison to surface plasmon resonance. Anal. Chem. 2007;79(13):4879–4886. doi: 10.1021/ac070085n. [DOI] [PubMed] [Google Scholar]
- Mahshid S., Mepham A.H., Mahshid S.S., Burgess I.B., Safaei T.S., Sargent E.H., Kelley S.O. Mechanistic control of the growth of three-dimensional gold sensors. J. Phys. Chem. C. 2016;120(37):21123–21132. [Google Scholar]
- Mahshid S.S., Vallee-Belisle A., Kelley S.O. Biomolecular steric hindrance effects are enhanced on nanostructured microelectrodes. Anal. Chem. 2017;89(18):9751–9757. doi: 10.1021/acs.analchem.7b01595. [DOI] [PubMed] [Google Scholar]
- Mallén-Alberdi M., Vigués N., Mas J., Fernández-Sánchez C., Baldi A. Impedance spectral fingerprint of E. coli cells on interdigitated electrodes: a new approach for label free and selective detection. Sens. Bio-Sens. Res. 2016;7:100–106. [Google Scholar]
- Malorny B., Tassios P.T., Rådström P., Cook N., Wagner M., Hoorfar J. Standardization of diagnostic PCR for the detection of foodborne pathogens. Int. J. Food Microbiol. 2003;83(1):39–48. doi: 10.1016/s0168-1605(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Mannoor M.S., Tao H., Clayton J.D., Sengupta A., Kaplan D.L., Naik R.R., Verma N., Omenetto F.G., McAlpine M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012;3:763. doi: 10.1038/ncomms1767. [DOI] [PubMed] [Google Scholar]
- Mannoor M.S., Zhang S., Link A.J., McAlpine M.C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 2010;107(45):19207–19212. doi: 10.1073/pnas.1008768107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzila A.G., Maipa V., Prodromidis M.I. Development of a faradic impedimetric immunosensor for the detection of Salmonella typhimurium in milk. Anal. Chem. 2008;80(4):1169–1175. doi: 10.1021/ac071570l. [DOI] [PubMed] [Google Scholar]
- Martin M., Salazar P., Jimenez C., Lecuona M., Ramos M.J., Ode J., Alcoba J., Roche R., Villalonga R., Campuzano S., Pingarron J.M., Gonzalez-Mora J.L. Rapid Legionella pneumophila determination based on a disposable core-shell Fe(3)O(4)@poly(dopamine) magnetic nanoparticles immunoplatform. Anal. Chim. Acta. 2015;887:51–58. doi: 10.1016/j.aca.2015.05.048. [DOI] [PubMed] [Google Scholar]
- Martinez A.W., Phillips S.T., Whitesides G.M., Carrilho E. ACS Publications; 2009. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. [DOI] [PubMed] [Google Scholar]
- Mathelie-Guinlet M., Cohen-Bouhacina T., Gammoudi I., Martin A., Beven L., Delville M.H., Grauby-Heywang C. Silica nanoparticles-assisted electrochemical biosensor for the rapid, sensitive and specific detection of Escherichia coli. Sensor. Actuator. B Chem. 2019;292:314–320. [Google Scholar]
- Medina-Sánchez M., Martínez-Domingo C., Ramon E., Merkoçi A. An inkjet-printed field-effect transistor for label-free biosensing. Adv. Funct. Mater. 2014;24(40):6291–6302. [Google Scholar]
- Mehrotra P. Biosensors and their applications - a review. J. Oral. Biol. Craniofac. Res. 2016;6(2):153–159. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejri M.B., Baccar H., Baldrich E., Del Campo F.J., Helali S., Ktari T., Simonian A., Aouni M., Abdelghani A. Impedance biosensing using phages for bacteria detection: generation of dual signals as the clue for in-chip assay confirmation. Biosens. Bioelectron. 2010;26(4):1261–1267. doi: 10.1016/j.bios.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Meredith N.A., Quinn C., Cate D.M., Reilly T.H., 3rd, Volckens J., Henry C.S. Paper-based analytical devices for environmental analysis. Analyst. 2016;141(6):1874–1887. doi: 10.1039/c5an02572a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirski T., Bartoszcze M., Bielawska-Drózd A., Cieslik P., Michalski A.J., Niemcewicz M., Kocik J., Chomiczewski K. Review of methods used for identification of biothreat agents in environmental protection and human health aspects. Ann. Agric. Environ. Med. 2014;21(2) doi: 10.5604/1232-1966.1108581. [DOI] [PubMed] [Google Scholar]
- Mishra G., Sharma V., Mishra R. Electrochemical aptasensors for food and environmental safeguarding: a review. Biosensors. 2018;8(2):28. doi: 10.3390/bios8020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A., González J. Springer International Publishing; Switzerland: 2016. Pulse Voltammetry in Physical Electrochemistry and Electroanalysis, Monographs in Electrochemistry. [DOI] [Google Scholar]
- Monzó J., Insua I., Fernandez-Trillo F., Rodriguez P. Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst. 2015;140(21):7116–7128. doi: 10.1039/c5an01330e. [DOI] [PubMed] [Google Scholar]
- Mungroo N., Neethirajan S. 2016. Optical Biosensors for the Detection of Food Borne Pathogens; pp. 179–206. [Google Scholar]
- Nandakumar V., La Belle J.T., Reed J., Shah M., Cochran D., Joshi L., Alford T.L. A methodology for rapid detection of Salmonella typhimurium using label-free electrochemical impedance spectroscopy. Biosens. Bioelectron. 2008;24(4):1045–1048. doi: 10.1016/j.bios.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Narayan R.J. Woodhead Publishing; 2016. Medical Biosensors for Point of Care (POC) Applications. [Google Scholar]
- Nemeth E., Adanyi N., Halasz A., Varadi M., Szendro I. Real-time study of the effect of different stress factors on lactic acid bacteria by electrochemical optical waveguide lightmode spectroscopy. Biomol. Eng. 2007;24(6):631–637. doi: 10.1016/j.bioeng.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Nguyen B.T., Koh G., Lim H.S., Chua A.J., Ng M.M., Toh C.S. Membrane-based electrochemical nanobiosensor for the detection of virus. Anal. Chem. 2009;81(17):7226–7234. doi: 10.1021/ac900761a. [DOI] [PubMed] [Google Scholar]
- Nguyen B.T., Peh A.E., Chee C.Y., Fink K., Chow V.T., Ng M.M., Toh C.S. Electrochemical impedance spectroscopy characterization of nanoporous alumina dengue virus biosensor. Bioelectrochemistry. 2012;88:15–21. doi: 10.1016/j.bioelechem.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Ohene-Adjei K., Kenu E., Bandoh D.A., Addo P.N.O., Noora C.L., Nortey P., Afari E.A. Epidemiological link of a major cholera outbreak in Greater Accra region of Ghana, 2014. BMC Publ. Health. 2017;17(1):801. doi: 10.1186/s12889-017-4803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi G.B., Di Stefano L., Noah N. Hospital-acquired, laboratory-confirmed bloodstream infection: increased hospital stay and direct costs. Infect. Control Hosp. Epidemiol. 2002;23(4):190–197. doi: 10.1086/502034. [DOI] [PubMed] [Google Scholar]
- Pal S., Alocilja E.C. Electrically active polyaniline coated magnetic (EAPM) nanoparticle as novel transducer in biosensor for detection of Bacillus anthracis spores in food samples. Biosens. Bioelectron. 2009;24(5):1437–1444. doi: 10.1016/j.bios.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Paltiel A.D., Walensky R.P., Schackman B.R., Seage G.R., Mercincavage L.M., Weinstein M.C., Freedberg K.A. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann. Intern. Med. 2006;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- Pan J., Chen W., Ma Y., Pan G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018;47(15):5574–5587. doi: 10.1039/c7cs00854f. [DOI] [PubMed] [Google Scholar]
- Pandey C.M., Tiwari I., Singh V.N., Sood K.N., Sumana G., Malhotra B.D. Highly sensitive electrochemical immunosensor based on graphene-wrapped copper oxide-cysteine hierarchical structure for detection of pathogenic bacteria. Sensor. Actuator. B Chem. 2017;238:1060–1069. [Google Scholar]
- Pandey P.K., Kass P.H., Soupir M.L., Biswas S., Singh V.P. Contamination of water resources by pathogenic bacteria. Amb. Express. 2014;4:51. doi: 10.1186/s13568-014-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patolsky F., Lieber C.M. Nanowire nanosensors. Mater. Today. 2005;8(4):20–28. [Google Scholar]
- Patris S., Vandeput M., Kauffmann J.M. Antibodies as target for affinity biosensors. Trac. Trends Anal. Chem. 2016;79:239–246. [Google Scholar]
- Pavan S., Berti F. Short peptides as biosensor transducers. Anal. Bioanal. Chem. 2012;402(10):3055–3070. doi: 10.1007/s00216-011-5589-8. [DOI] [PubMed] [Google Scholar]
- Pavinatto F.J., Paschoal C.W.A., Arias A.C. Printed and flexible biosensor for antioxidants using interdigitated ink-jetted electrodes and gravure-deposited active layer. Biosens. Bioelectron. 2015;67:553–559. doi: 10.1016/j.bios.2014.09.039. [DOI] [PubMed] [Google Scholar]
- Peh A.E., Li S.F. Dengue virus detection using impedance measured across nanoporous alumina membrane. Biosens. Bioelectron. 2013;42:391–396. doi: 10.1016/j.bios.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Pereira da Silva Neves M.M., González-García M.B., Hernández-Santos D., Fanjul-Bolado P. Future trends in the market for electrochemical biosensing. Curr. Opin. Electrochem. 2018;10:107–111. [Google Scholar]
- Pires N.M., Dong T., Hanke U., Hoivik N. Recent developments in optical detection technologies in lab-on-a-chip devices for biosensing applications. Sensors. 2014;14(8):15458–15479. doi: 10.3390/s140815458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piro B., Reisberg S., Anquetin G., Duc H.-T., Pham M.-C. Quinone-based polymers for label-free and reagentless electrochemical immunosensors: application to proteins, antibodies and pesticides detection. Biosensors. 2013;3(1):58–76. doi: 10.3390/bios3010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournaras A.V., Koraki T., Prodromidis M.I. Development of an impedimetric immunosensor based on electropolymerized polytyramine films for the direct detection of Salmonella typhimurium in pure cultures of type strains and inoculated real samples. Anal. Chim. Acta. 2008;624(2):301–307. doi: 10.1016/j.aca.2008.06.043. [DOI] [PubMed] [Google Scholar]
- Primiceri E., Chiriaco M.S., de Feo F., Santovito E., Fusco V., Maruccio G. A multipurpose biochip for food pathogen detection. Anal. Methods. 2016;8(15):3055–3060. [Google Scholar]
- Pumera M., Sánchez S., Ichinose I., Tang J. Electrochemical nanobiosensors. Sensor. Actuator. B Chem. 2007;123(2):1195–1205. [Google Scholar]
- Qi P., Wan Y., Zhang D. Impedimetric biosensor based on cell-mediated bioimprinted films for bacterial detection. Biosens. Bioelectron. 2013;39(1):282–288. doi: 10.1016/j.bios.2012.07.078. [DOI] [PubMed] [Google Scholar]
- Radke S.M., Alocilja E.C. A high density microelectrode array biosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 2005;20(8):1662–1667. doi: 10.1016/j.bios.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Randles J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947;1:11–19. [Google Scholar]
- Rao V.K., Sharma M.K., Goel A.K., Singh L., Sekhar K. Amperometric immunosensor for the detection of Vibrio cholerae O1 using disposable screen-printed electrodes. Anal. Sci. 2006;22(9):1207–1211. doi: 10.2116/analsci.22.1207. [DOI] [PubMed] [Google Scholar]
- Rapp B.E., Gruhl F.J., Länge K. Biosensors with label-free detection designed for diagnostic applications. Anal. Bioanal. Chem. 2010;398(6):2403–2412. doi: 10.1007/s00216-010-3906-2. [DOI] [PubMed] [Google Scholar]
- Rappo U., Schuetz A.N., Jenkins S.G., Calfee D.P., Walsh T.J., Wells M.T., Hollenberg J.P., Glesby M.J. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J. Clin. Microbiol. 2016;54(8):2096. doi: 10.1128/JCM.00549-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M., Singh S.K. Advances in molecular diagnostic approaches for biothreat agents. In: Singh S.K., Kuhn J.H., editors. Defense against Biological Attacks: Volume II. Springer International Publishing; Cham: 2019. pp. 281–310. [Google Scholar]
- Reid S., Juma O.A. Minimum infective dose of HIV for parenteral dosimetry. Int. J. STD AIDS. 2009;20(12):828–833. doi: 10.1258/ijsa.2009.009284. [DOI] [PubMed] [Google Scholar]
- Reina J.J., Díaz I., Nieto P.M., Campillo N.E., Páez J.A., Tabarani G., Fieschi F., Rojo J. Docking, synthesis, and NMR studies of mannosyl trisaccharide ligands for DC-SIGN lectin. Org. Biomol. Chem. 2008;6(15):2743–2754. doi: 10.1039/b802144a. [DOI] [PubMed] [Google Scholar]
- Resch-Genger U., Grabolle M., Cavaliere-Jaricot S., Nitschke R., Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods. 2008;5(9):763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- Reverdatto S., Burz D.S., Shekhtman A. Peptide aptamers: development and applications. Curr. Top. Med. Chem. 2015;15(12):1082–1101. doi: 10.2174/1568026615666150413153143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim Y.S., Bae S.H., Chen H., De Marco N., Yang Y. Recent progress in materials and devices toward printable and flexible sensors. Adv. Mater. 2016;28(22):4415–4440. doi: 10.1002/adma.201505118. [DOI] [PubMed] [Google Scholar]
- Rivet C., Lee H., Hirsch A., Hamilton S., Lu H. Microfluidics for medical diagnostics and biosensors. Chem. Eng. Sci. 2011;66(7):1490–1507. [Google Scholar]
- Robilotti E., Deresinski S., Pinsky B.A. Norovirus. Clin. Microbiol. Rev. 2015;28(1):134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russotto V., Cortegiani A., Raineri S.M., Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care. 2015;3:54. doi: 10.1186/s40560-015-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam F., Tothill I.E. Detection of Salmonella typhimurium using an electrochemical immunosensor. Biosens. Bioelectron. 2009;24(8):2630–2636. doi: 10.1016/j.bios.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Sang S., Wang Y., Feng Q., Wei Y., Ji J., Zhang W. Progress of new label-free techniques for biosensors: a review. Crit. Rev. Biotechnol. 2016;36(3):465–481. doi: 10.3109/07388551.2014.991270. [DOI] [PubMed] [Google Scholar]
- Saucedo N.M., Srinives S., Mulchandani A. Electrochemical biosensor for rapid detection of viable bacteria and antibiotic screening. J. Anal. Testing. 2019;3(1):117–122. [Google Scholar]
- Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3(3):430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- Sayhi M., Ouerghi O., Belgacem K., Arbi M., Tepeli Y., Ghram A., Anik U., Osterlund L., Laouini D., Diouani M.F. Electrochemical detection of influenza virus H9N2 based on both immunomagnetic extraction and gold catalysis using an immobilization-free screen printed carbon microelectrode. Biosens. Bioelectron. 2018;107:170–177. doi: 10.1016/j.bios.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P., Frank S.A. Pathogenesis, virulence, and infective dose. PLoS Pathog. 2007;3(10):1372–1373. doi: 10.1371/journal.ppat.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrattenecker J.D., Heer R., Melnik E., Maier T., Fafilek G., Hainberger R. Hexaammineruthenium (II)/(III) as alternative redox-probe to Hexacyanoferrat (II)/(III) for stable impedimetric biosensing with gold electrodes. Biosens. Bioelectron. 2019;127:25–30. doi: 10.1016/j.bios.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Scognamiglio V., Rea G., Arduini F., Palleschi G. Elsevier Science; 2016. Biosensors for Sustainable Food - New Opportunities and Technical Challenges. [Google Scholar]
- Scott K. 2 - electrochemical principles and characterization of bioelectrochemical systems. In: Scott K., Yu E.H., editors. Microbial Electrochemical and Fuel Cells. Woodhead Publishing; Boston: 2016. pp. 29–66. [Google Scholar]
- Serra B., Gamella M., Reviejo A.J., Pingarron J.M. Lectin-modified piezoelectric biosensors for bacteria recognition and quantification. Anal. Bioanal. Chem. 2008;391(5):1853–1860. doi: 10.1007/s00216-008-2141-6. [DOI] [PubMed] [Google Scholar]
- Setterington E.B., Alocilja E.C. Rapid electrochemical detection of polyaniline-labeled Escherichia coli O157:H7. Biosens. Bioelectron. 2011;26(5):2208–2214. doi: 10.1016/j.bios.2010.09.036. [DOI] [PubMed] [Google Scholar]
- Shabani A., Zourob M., Allain B., Marquette C.A., Lawrence M.F., Mandeville R. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal. Chem. 2008;80(24):9475–9482. doi: 10.1021/ac801607w. [DOI] [PubMed] [Google Scholar]
- Shah J., Wilkins E. Electrochemical biosensors for detection of biological warfare agents. Electroanalysis. 2003;15(3):157–167. [Google Scholar]
- Sharma H., Mutharasan R. Half antibody fragments improve biosensor sensitivity without loss of selectivity. Anal. Chem. 2013;85(4):2472–2477. doi: 10.1021/ac3035426. [DOI] [PubMed] [Google Scholar]
- Sharma M.K., Goel A.K., Singh L., Rao V.K. Immunological biosensor for detection of Vibrio cholerae O1in environmental water samples. World J. Microbiol. Biotechnol. 2006;22(11):1155–1159. [Google Scholar]
- Sheikhzadeh E., Chamsaz M., Turner A.P.F., Jager E.W.H., Beni V. Label-free impedimetric biosensor for Salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens. Bioelectron. 2016;80:194–200. doi: 10.1016/j.bios.2016.01.057. [DOI] [PubMed] [Google Scholar]
- Shen F., Wang J., Xu Z., Wu Y., Chen Q., Li X., Jie X., Li L., Yao M., Guo X., Zhu T. Rapid flu diagnosis using silicon nanowire sensor. Nano Lett. 2012;12(7):3722–3730. doi: 10.1021/nl301516z. [DOI] [PubMed] [Google Scholar]
- Siddiqui S., Dai Z., Stavis C.J., Zeng H., Moldovan N., Hamers R.J., Carlisle J.A., Arumugam P.U. A quantitative study of detection mechanism of a label-free impedance biosensor using ultrananocrystalline diamond microelectrode array. Biosens. Bioelectron. 2012;35(1):284–290. doi: 10.1016/j.bios.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harbor Perspect. Biol. 2010;2(5):a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J.D., Bloom R.J., Jiang S., Durand H.K., Mukherjee S., David L.A. Measuring and mitigating PCR bias in microbiome data. bioRxiv. 2019:604025. doi: 10.1371/journal.pcbi.1009113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin M.L., Mach K.E., Wong P.K., Liao J.C. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Rev. Mol. Diagn. 2014;14(2):225–244. doi: 10.1586/14737159.2014.888313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.V., Whited A.M., Ragineni Y., Barrett T.W., King J., Solanki R. 3D nanogap interdigitated electrode array biosensors. Anal. Bioanal. Chem. 2010;397(4):1493–1502. doi: 10.1007/s00216-010-3682-z. [DOI] [PubMed] [Google Scholar]
- Singh M., Tong Y., Webster K., Cesewski E., Haring A.P., Laheri S., Carswell B., O'Brien T.J., Aardema C.H., Senger R.S., Robertson J.L., Johnson B.N. 3D printed conformal microfluidics for isolation and profiling of biomarkers from whole organs. Lab Chip. 2017;17(15):2561–2571. doi: 10.1039/c7lc00468k. [DOI] [PubMed] [Google Scholar]
- Singh R., Das Mukherjee M., Sumana G., Gupta R.K., Sood S., Malhotra B.D. Biosensors for pathogen detection: a smart approach towards clinical diagnosis. Sensor. Actuator. B Chem. 2014;197:385–404. [Google Scholar]
- Singh R., Hong S., Jang J. Label-free detection of influenza viruses using a reduced graphene oxide-based electrochemical immunosensor integrated with a microfluidic platform. Sci. Rep. 2017;7:42771. doi: 10.1038/srep42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleymani L., Fang Z., Sargent E.H., Kelley S.O. Programming the detection limits of biosensors through controlled nanostructuring. Nat. Nanotechnol. 2009;4(12):844–848. doi: 10.1038/nnano.2009.276. [DOI] [PubMed] [Google Scholar]
- Song Y.-A., Jianping f., Wang Y.-C., Han J. 2013. Biosample Preparation by Lab-On-A-Chip Devices; pp. 1–19. [Google Scholar]
- Squires T.M., Messinger R.J., Manalis S.R. Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 2008;26:417. doi: 10.1038/nbt1388. [DOI] [PubMed] [Google Scholar]
- Stoltenburg R., Reinemann C., Strehlitz B. SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007;24(4):381–403. doi: 10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Suehiro J., Ohtsubo A., Hatano T., Hara M. Selective detection of bacteria by a dielectrophoretic impedance measurement method using an antibody-immobilized electrode chip. Sensor. Actuator. B Chem. 2006;119(1):319–326. [Google Scholar]
- Syahir A., Usui K., Tomizaki K.Y., Kajikawa K., Mihara H. Label and label-free detection techniques for protein microarrays. Microarrays. 2015;4(2):228–244. doi: 10.3390/microarrays4020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleat Z., Khoshroo A., Mazloum-Ardakani M. Screen-printed electrodes for biosensing: a review (2008–2013) Microchimica Acta. 2014;181(9–10):865–891. [Google Scholar]
- Tam P.D., Thang C.X. Label-free electrochemical immunosensor based on cerium oxide nanowires for Vibrio cholerae O1 detection. Mater. Sci. Eng. C. 2016;58:953–959. doi: 10.1016/j.msec.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Tan F., Leung P.H.M., Liu Z.B., Zhang Y., Xiao L.D., Ye W.W., Zhang X., Yi L., Yang M. A PDMS microfluidic impedance immunosensor for E. coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sensor. Actuator. B Chem. 2011;159(1):328–335. [Google Scholar]
- Thévenot D.R., Toth K., Durst R.A., Wilson G.S. Electrochemical biosensors: recommended definitions and classification1International union of pure and applied chemistry: physical chemistry division, commission I.7 (biophysical chemistry); analytical chemistry division, commission V.5 (electroanalytical Chemistry).1. Biosens. Bioelectron. 2001;16(1–2):121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- Tian F., Lyu J., Shi J.Y., Tan F., Yang M. A polymeric microfluidic device integrated with nanoporous alumina membranes for simultaneous detection of multiple foodborne pathogens. Sensor. Actuator. B Chem. 2016;225:312–318. [Google Scholar]
- Tlili C., Sokullu E., Safavieh M., Tolba M., Ahmed M.U., Zourob M. Bacteria screening, viability, and confirmation assays using bacteriophage-impedimetric/loop-mediated isothermal amplification dual-response biosensors. Anal. Chem. 2013;85(10):4893–4901. doi: 10.1021/ac302699x. [DOI] [PubMed] [Google Scholar]
- Tolba M., Ahmed M.U., Tlili C., Eichenseher F., Loessner M.J., Zourob M. A bacteriophage endolysin-based electrochemical impedance biosensor for the rapid detection of Listeria cells. Analyst. 2012;137(24):5749–5756. doi: 10.1039/c2an35988j. [DOI] [PubMed] [Google Scholar]
- Travas-Sejdic J., Aydemir N., Kannan B., Williams D.E., Malmstrom J. Intrinsically conducting polymer nanowires for biosensing. J. Mater. Chem. B. 2014;2(29):4593–4609. doi: 10.1039/c4tb00598h. [DOI] [PubMed] [Google Scholar]
- Varshney M., Li Y. Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle-antibody conjugates for detection of Escherichia coli O157:H7 in food samples. Biosens. Bioelectron. 2007;22(11):2408–2414. doi: 10.1016/j.bios.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Varshney M., Li Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens. Bioelectron. 2009;24(10):2951–2960. doi: 10.1016/j.bios.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Varshney M., Li Y.B., Srinivasan B., Tung S. A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157 : H7 in food samples. Sensor. Actuator. B Chem. 2007;128(1):99–107. [Google Scholar]
- Vaseashta A., Dimova-Malinovska D. Nanostructured and nanoscale devices, sensors and detectors. Sci. Technol. Adv. Mater. 2005;6(3–4):312–318. [Google Scholar]
- Vashist S.K., Zheng D., Al-Rubeaan K., Luong J.H., Sheu F.-S. Technology behind commercial devices for blood glucose monitoring in diabetes management: a review. Anal. Chim. Acta. 2011;703(2):124–136. doi: 10.1016/j.aca.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- Vestergaard M., Kerman K., Tamiya E. An overview of label-free electrochemical protein sensors. Sensors. 2007;7(12):3442–3458. doi: 10.3390/s7123442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S., Rani C., Ho J.A. Electrochemical immunosensor for multiplexed detection of food-borne pathogens using nanocrystal bioconjugates and MWCNT screen-printed electrode. Talanta. 2012;94:315–319. doi: 10.1016/j.talanta.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Vogt S., Su Q., Gutiérrez-Sánchez C., Nöll G. Critical view on electrochemical impedance spectroscopy using the ferri/ferrocyanide redox couple at gold electrodes. Anal. Chem. 2016;88(8):4383–4390. doi: 10.1021/acs.analchem.5b04814. [DOI] [PubMed] [Google Scholar]
- Waheed S., Cabot J.M., Macdonald N.P., Lewis T., Guijt R.M., Paull B., Breadmore M.C. 3D printed microfluidic devices: enablers and barriers. Lab Chip. 2016;16(11):1993–2013. doi: 10.1039/c6lc00284f. [DOI] [PubMed] [Google Scholar]
- Wan J., Ai J., Zhang Y., Geng X., Gao Q., Cheng Z. Signal-off impedimetric immunosensor for the detection of Escherichia coli O157:H7. Sci. Rep. 2016;6:19806. doi: 10.1038/srep19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M. Springer; 2008. Conducting Polymers with Micro or Nanometer Structure. [Google Scholar]
- Wan Y., Lin Z., Zhang D., Wang Y., Hou B. Impedimetric immunosensor doped with reduced graphene sheets fabricated by controllable electrodeposition for the non-labelled detection of bacteria. Biosens. Bioelectron. 2011;26(5):1959–1964. doi: 10.1016/j.bios.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wan Y., Su Y., Zhu X., Liu G., Fan C. Development of electrochemical immunosensors towards point of care diagnostics. Biosens. Bioelectron. 2013;47:1–11. doi: 10.1016/j.bios.2013.02.045. [DOI] [PubMed] [Google Scholar]
- Wan Y., Zhang D., Wang Y., Hou B. A 3D-impedimetric immunosensor based on foam Ni for detection of sulfate-reducing bacteria. Electrochem. Commun. 2010;12(2):288–291. [Google Scholar]
- Wanekaya A.K., Chen W., Myung N.V., Mulchandani A. Nanowire‐based electrochemical biosensors. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis. 2006;18(6):533–550. [Google Scholar]
- Wang D., Chen Q., Huo H.L., Bai S.S., Cai G.Z., Lai W.H., Lin J.H. Efficient separation and quantitative detection of Listeria monocytogenes based on screen-printed interdigitated electrode, urease and magnetic nanoparticles. Food Contr. 2017;73:555–561. [Google Scholar]
- Wang R., Dong W., Ruan C., Kanayeva D., Tian R., Lassiter K., Li Y. TiO2 nanowire bundle microelectrode based impedance immunosensor for rapid and sensitive detection of Listeria monocytogenes. Nano Lett. 2008;8(9):2625–2631. doi: 10.1021/nl080366q. [DOI] [PubMed] [Google Scholar]
- Wang R., Li Y., Mao X., Huang T., Lu H. IEEE International Conference on Nano/Molecular Medicine and Engineering; 2010. Magnetic Bio-Nanobeads and Nanoelectrode Based Impedance Biosensor for Detection of Avian Influenza Virus; pp. 214–217. 2010. [Google Scholar]
- Wang Y., Alocilja E.C. Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J. Biol. Eng. 2015;9(1):16. doi: 10.1186/s13036-015-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ping J., Ye Z., Wu J., Ying Y. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2013;49:492–498. doi: 10.1016/j.bios.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Weber D.J., Rutala W.A., Miller M.B., Huslage K., Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Contr. 2010;38(5 Suppl. 1):S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- Wei D., Bailey M.J.A., Andrew P., Ryhänen T. Electrochemical biosensors at the nanoscale. Lab Chip. 2009;9(15):2123–2131. doi: 10.1039/b903118a. [DOI] [PubMed] [Google Scholar]
- Wenzel T., Härtter D., Bombelli P., Howe C.J., Steiner U. Porous translucent electrodes enhance current generation from photosynthetic biofilms. Nat. Commun. 2018;9(1):1299. doi: 10.1038/s41467-018-03320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2015. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007-2015. [Google Scholar]
- WHO . World Health Organization; Geneva: 2018. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and Region, 2000-2016. [Google Scholar]
- WHO . World Health Organization; Geneva: 2018. World Malaria Report 2018. [Google Scholar]
- Wicklein B., del Burgo M.A.M., Yuste M., Carregal-Romero E., Llobera A., Darder M., Aranda P., Ortin J., del Real G., Fernandez-Sanchez C., Ruiz-Hitzky E. Biomimetic architectures for the impedimetric discrimination of influenza virus phenotypes. Adv. Funct. Mater. 2013;23(2):254–262. [Google Scholar]
- Wilson D., Materon E.M., Ibanez-Redin G., Faria R.C., Correa D.S., Oliveira O.N., Jr. Electrical detection of pathogenic bacteria in food samples using information visualization methods with a sensor based on magnetic nanoparticles functionalized with antimicrobial peptides. Talanta. 2019;194:611–618. doi: 10.1016/j.talanta.2018.10.089. [DOI] [PubMed] [Google Scholar]
- Wu G., Meyyappan M., Lai K.W.C. 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO) IEEE; 2016. Graphene field-effect transistors-based biosensors for Escherichia coli detection; pp. 22–25. [Google Scholar]
- Xi F.N., Gao J.Q., Wang J.N., Wang Z.X. Discrimination and detection of bacteria with a label-free impedimetric biosensor based on self-assembled lectin monolayer. J. Electroanal. Chem. 2011;656(1–2):252–257. [Google Scholar]
- Xia L., Wei Z., Wan M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010;341(1):1–11. doi: 10.1016/j.jcis.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Xu M., Obodo D., Yadavalli V.K. The design, fabrication, and applications of flexible biosensing devices. Biosens. Bioelectron. 2019;124–125:96–114. doi: 10.1016/j.bios.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Wang R., Li Y. An electrochemical biosensor for rapid detection of E. coli O157:H7 with highly efficient bi-functional glucose oxidase-polydopamine nanocomposites and Prussian blue modified screen-printed interdigitated electrodes. Analyst. 2016;141(18):5441–5449. doi: 10.1039/c6an00873a. [DOI] [PubMed] [Google Scholar]
- Xu M., Wang R., Li Y. Rapid detection of Escherichia coli O157:H7 and Salmonella Typhimurium in foods using an electrochemical immunosensor based on screen-printed interdigitated microelectrode and immunomagnetic separation. Talanta. 2016;148:200–208. doi: 10.1016/j.talanta.2015.10.082. [DOI] [PubMed] [Google Scholar]
- Xu Y., Wu X., Guo X., Kong B., Zhang M., Qian X., Mi S., Sun W. The boom in 3D-printed sensor technology. Sensors. 2017;17(5):1166. doi: 10.3390/s17051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Choi W., Lee I., Cho B.K., Jun S. Rapid detection of multiple foodborne pathogens using a nanoparticle-functionalized multi-junction biosensor. Biosens. Bioelectron. 2016;77:137–143. doi: 10.1016/j.bios.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Yáñez-Sedeño P., Campuzano S., Pingarrón J.M. Electrochemical sensors based on magnetic molecularly imprinted polymers: a review. Anal. Chim. Acta. 2017;960:1–17. doi: 10.1016/j.aca.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Yang H., Rahman M.T., Du D., Panat R., Lin Y. 3-D printed adjustable microelectrode arrays for electrochemical sensing and biosensing. Sensor. Actuator. B Chem. 2016;230:600–606. doi: 10.1016/j.snb.2016.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.Y., Zhou H.F., Hao H.Y., Gong Q.J., Nie K. Detection of Escherichia coli with a label-free impedimetric biosensor based on lectin functionalized mixed self-assembled monolayer. Sensor. Actuator. B Chem. 2016;229:297–304. [Google Scholar]
- Yang L., Li Y. AFM and impedance spectroscopy characterization of the immobilization of antibodies on indium-tin oxide electrode through self-assembled monolayer of epoxysilane and their capture of Escherichia coli O157:H7. Biosens. Bioelectron. 2005;20(7):1407–1416. doi: 10.1016/j.bios.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Yang L., Li Y. Detection of viable Salmonella using microelectrode-based capacitance measurement coupled with immunomagnetic separation. J. Microbiol. Methods. 2006;64(1):9–16. doi: 10.1016/j.mimet.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Yazgan I., Noah N.M., Toure O., Zhang S., Sadik O.A. Biosensor for selective detection of E. coli in spinach using the strong affinity of derivatized mannose with fimbrial lectin. Biosens. Bioelectron. 2014;61:266–273. doi: 10.1016/j.bios.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Ye Y., Guo H., Sun X. Recent progress on cell-based biosensors for analysis of food safety and quality control. Biosens. Bioelectron. 2019;126:389–404. doi: 10.1016/j.bios.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Yeh P.-Y.J., Kizhakkedathu J.N., Madden J.D., Chiao M. Electric field and vibration-assisted nanomolecule desorption and anti-biofouling for biosensor applications. Colloids Surf. B Biointerfaces. 2007;59(1):67–73. doi: 10.1016/j.colsurfb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Yogeswaran U., Chen S.M. A review on the electrochemical sensors and biosensors composed of nanowires as sensing. Material. Sensors (Basel) 2008;8(1):290–313. doi: 10.3390/s8010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo M.S., Shin M., Kim Y., Jang M., Choi Y.E., Park S.J., Choi J., Lee J., Park C. Development of electrochemical biosensor for detection of pathogenic microorganism in Asian dust events. Chemosphere. 2017;175:269–274. doi: 10.1016/j.chemosphere.2017.02.060. [DOI] [PubMed] [Google Scholar]
- Yoo S.M., Lee S.Y. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol. 2016;34(1):7–25. doi: 10.1016/j.tibtech.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Zarei S.S., Soleimanian-Zad S., Ensafi A.A. An impedimetric aptasensor for Shigella dysenteriae using a gold nanoparticle-modified glassy carbon electrode. Mikrochim. Acta. 2018;185(12):538. doi: 10.1007/s00604-018-3075-0. [DOI] [PubMed] [Google Scholar]
- Zelada-Guillen G.A., Bhosale S.V., Riu J., Rius F.X. Real-time potentiometric detection of bacteria in complex samples. Anal. Chem. 2010;82(22):9254–9260. doi: 10.1021/ac101739b. [DOI] [PubMed] [Google Scholar]
- Zelada-Guillen G.A., Riu J., Duzgun A., Rius F.X. Immediate detection of living bacteria at ultralow concentrations using a carbon nanotube based potentiometric aptasensor. Angew Chem. Int. Ed. Engl. 2009;48(40):7334–7337. doi: 10.1002/anie.200902090. [DOI] [PubMed] [Google Scholar]
- Zelada-Guillen G.A., Sebastian-Avila J.L., Blondeau P., Riu J., Rius F.X. Label-free detection of Staphylococcus aureus in skin using real-time potentiometric biosensors based on carbon nanotubes and aptamers. Biosens. Bioelectron. 2012;31(1):226–232. doi: 10.1016/j.bios.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Zeng X., Andrade C.A.S., Oliveira M.D.L., Sun X.-L. Carbohydrate–protein interactions and their biosensing applications. Anal. Bioanal. Chem. 2012;402(10):3161–3176. doi: 10.1007/s00216-011-5594-y. [DOI] [PubMed] [Google Scholar]
- Zhang D., Chen S., Qin L., Li R., Wang P., Li Y. 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference. 2005. The novel immunobiosensors for detection of Escherichia coli O157:H7 using electrochemical impedance spectroscopy; pp. 7111–7113. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Li L., Qiao Z., Lei C., Fu Y., Xie Q., Yao S., Li Y., Ying Y. Electrochemical conversion of Fe3O4 magnetic nanoparticles to electroactive prussian blue analogues for self-sacrificial label biosensing of avian influenza virus H5N1. Anal. Chem. 2017;89(22):12145–12151. doi: 10.1021/acs.analchem.7b02784. [DOI] [PubMed] [Google Scholar]
- Zhang R., Hamerlinck J., Gloss P., S, Munn L. Determination of nonpoint-source pollution using GIS and numerical models. J. Environ. Qual. 1996;25:411–418. [Google Scholar]
- Zhao G.Y., Xing F.F., Deng S.P. A disposable amperometric enzyme immunosensor for rapid detection of Vibrio parahaemolyticus in food based on agarose/Nano-Au membrane and screen-printed electrode. Electrochem. Commun. 2007;9(6):1263–1268. [Google Scholar]
- Zhou C.H., Wu Z., Chen J.J., Xiong C., Chen Z., Pang D.W., Zhang Z.L. Biometallization-based electrochemical magnetoimmunosensing strategy for avian influenza A (H7N9) virus particle detection. Chem. Asian J. 2015;10(6):1387–1393. doi: 10.1002/asia.201500105. [DOI] [PubMed] [Google Scholar]
- Zhou T., Ding L., Che G., Jiang W., Sang L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: preparation and application in sample pretreatment. Trac. Trends Anal. Chem. 2019;114:11–28. [Google Scholar]
- Zhou Y., Ramasamy R.P. Phage-based electrochemical biosensors for detection of pathogenic bacteria. ECS Transactions. 2015;69(38):1–8. [Google Scholar]
- Zourob M., Elwary S., Turner A.P. Springer Science & Business Media; 2008. Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.