Abstract
Background:
The class III NAD-dependent histone deacetylase (HDAC) sirtuin 1 (SIRT1) is an important regulator of senescence, aging, and inflammation. SIRT1de-acetylates chromatin histones, thereby silencing inflammatory gene transcription. We have reported increased steroid-resistant senescent pro-inflammatory CD28nullCD8+ T cells in patients with chronic obstructive pulmonary disease (COPD). We hypothesized that SIRT1 is reduced in these cells in COPD, and that treatment with SIRT1 activators (resveratrol, curcumin) and agents preventing NAD depletion (theophylline) would upregulate SIRT1 and reduce pro-inflammatory cytokine expression in these steroid-resistant cells.
Methods:
Blood was collected from n = 10 COPD and n = 10 aged-matched controls. Expression of CD28, SIRT1, and pro-inflammatory cytokines was determined in CD8+ and CD8– T and natural killer T (NKT)-like cells cultured in the presence of ±1 µM prednisolone, ±5 mg/L theophylline, ±1 µM curcumin, ±25 µM resveratrol, using flow cytometry and immunofluorescence.
Results:
There was an increase in the percentage of CD28nullCD8+ T and NKT-like cells in COPD patients compared with controls. Decreased SIRT1 expression was identified in CD28nullCD8+T and NKT-like cells compared with CD28+ counterparts from both patients and controls (e.g. CD28null 11 ± 3% versus CD28+ 57 ± 9%). Loss of SIRT1 was associated with increased production of IFNγ and TNFα, steroid resistance, and disease severity. SIRT1 expression was upregulated in the presence of all drugs and was associated with a decrease in steroid resistance and IFNγ and TNFα production by CD28nullCD8+T and NKT-like cells. The presence of the SIRT1 inhibitor, EX-527 negated [by 92 ± 12% (median ± SEM)] the effect of the SIRT1 activator SRT720 on the percentage of CD8+ T cells producing IFNγ and TNFα.
Conclusions:
Steroid resistance in pro-inflammatory CD28nullCD8+ T and NKT-like cells is associated with decreased SIRT1 expression. Treatment with prednisolone, in combination with theophylline, curcumin or resveratrol increases SIRT1 expression, restores steroid sensitivity, and inhibits pro-inflammatory cytokine production from these cells and may reduce systemic inflammation in COPD.
The reviews of this paper are available via the supplemental material section.
Keywords: CD28nullCD8+ T and NKT-like cells, COPD, IFNγ and TNFα, lymphocyte senescence, SIRT1
Introduction
Current treatments such as corticosteroids, used to treat chronic obstructive pulmonary disease (COPD), a significant cause of death in the world, do not modify the disease.1 The exact mechanisms involved in lymphocyte resistance to corticosteroids are incompletely known.2 Our previous research has identified increased expression of pro-inflammatory cytokines and cytotoxic mediators, granzyme b and perforin, in CD8+ T lymphocytes in peripheral blood and airways of ex-current-smoker COPD patients compared with healthy smokers and subjects that have never smoked cigarettes.3,4
We have identified the lymphocyte subset/s resistant to current available treatments, and have identified several novel discoveries. Numbers of CD28nullCD8+ senescent cells are increased in the peripheral blood of both ex-smoker and current smoker COPD patients. These senescent cells express more cytotoxic mediators and pro-inflammatory cytokines than their CD8+CD28+ counterparts and are resistant to standard therapeutic dose of prednisolone.5,6 The percentages of natural killer (NK) and natural killer T (NKT)-like lymphocytes are increased in bronchoalveolar lavage (BAL) of COPD patients, and both cell types show increased cytotoxicity.7 In other inflammatory lung diseases, CD8+CD28null NKT-like cells are more cytotoxic/pro-inflammatory than CD8+CD28+ NKT-like cells.8
Recently, we showed that the glucocorticoid receptor (GCR) was reduced in CD28nullCD8+ T-cells.9 We hypothesized there may also be other mechanisms that potentiate their pathogenic influence.
Sirtuin 1 (SIRT1) is a class III NAD-dependent histone deacetylase (HDAC) and an important regulator of senescence, inflammation, and aging, as well as de-acetylating chromatin histones halting inflammatory gene transcription.10 SIRT1 is reduced in macrophages and the lungs of patients with COPD and in peripheral blood mononuclear cells11–13; however, to our knowledge, reports of SIRT1 levels in peripheral blood lymphocytes in these patients are lacking. CD8+ T cells have been reported to be central regulators of the inflammatory network in patients with COPD.14 When exposed to long-term cigarette smoke, CD8+ T-cell deficient mice showed reduced inflammation and did not develop emphysema.14
Resveratrol and curcumin are naturally occurring polyphenols have been shown to activate SIRT1 and suppress inflammation via inhibiting NF-κB signaling.15 SIRT1 was shown to physically interact with GCR, enhancing GCR-induced transcriptional activity on glucocorticoid response genes. These effects were attenuated by SIRT1 knockdown.16
We hypothesized decreased levels of SIRT1 in steroid-resistant peripheral blood pro-inflammatory CD28nullCD8+ T and NKT-like lymphocyte subsets in patients with COPD, and that treatment with SIRT1 activators would decrease pro-inflammatory cytokine production and reduce steroid resistance in these cells.
To investigate this hypothesis, we determined whether peripheral blood CD28null T cells (particularly CD8+) and NKT-like cells from COPD patients express reduced levels of SIRT1 and whether loss of SIRT1 is associated with concurrent loss of GCR and increased expression of pro-inflammatory cytokines and steroid resistance. We also investigated the effect of theophylline and polyphenols resveratrol and curcumin, SIRT1 activator SRT1720 and SIRT inhibitor, EX-527, in combination with the corticosteroid, prednisolone, on SIRT1 expression and associated pro-inflammatory cytokine expression by lymphocyte subsets.17
Materials and methods
Patient and control groups
COPD volunteers were specifically recruited for the study and informed consent obtained. Patients experienced no exacerbation of COPD for 6 weeks prior to this study. Subjects with other co-existing lung disease or malignancy, or aged greater than 75 years, were excluded. Ethics approval was obtained from the Royal Adelaide Hospital Human Ethics Committee, and the experiments were conducted with the understanding and the written consent of each participant. COPD was diagnosed using the GOLD criteria with clinical correlation [mild COPD: forced expiratory volume in 1 s (FEV1)/ forced vital capacity (FVC) <70% but FEV1 ⩾80% predicted; moderate COPD FEV1 50%⩽80% predicted, severe COPD FEV1 30%⩽50% predicted, very severe COPD FEV1<30%].18 Blood was collected from 10 subjects diagnosed with COPD (Table 1). All COPD subjects were ex-smokers (at least 1 year) with an average of 39 pack years. No patients were receiving oral GCS.
Table 1.
Demographic details of the COPD and control group.
| Participants | Controls | COPD |
|---|---|---|
| No. of subjects | 10 | 10 |
| Age (years) | 56 (±9) | 58 (±16) |
| FEV1, % pred | 106.6 (±11) | 60.1 (±20)* |
| FEV1, % FVC | 97 (±11) | 58 (±15)* |
| Male/Female | 5/5 | 6/4 |
Data showing mean ± SEM.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
p < 0.05 compared with controls.
Blood was also obtained from 10 healthy aged-matched nonsmoking volunteers (Table 1) with normal lung function and no history of airways disease. All subjects underwent spirometry as part of their routine clinical assessment. Venous blood was collected into 10 U/ml preservative free sodium heparin (DBL, Sydney, Australia), and maintained at 4°C until processing. All patients were submitted to the same protocol and analysis performed retrospectively.
SIRT1 and intracellular cytokine expression in T and NKT-like cell subsets
To determine co-expression of SIRT1 and intracellular cytokine production in CD8+ and CD8– T and NKT-like cells, aliquots of blood were stimulated as previously reported,3 with phorbol myristate (25 ng/mL) (Sigma, Sydney, Australia) and ionomycin (1 µg/mL) (Sigma) in the presence of brefeldin A (1 µg/Ml) (Sigma), and the tubes incubated in a humidified 5% CO2/95% air atmosphere at 37°C. Preliminary experiments showed stimulation of cells was required for detection of SIRT1 expression in lymphocyte subsets. The addition of brefeldin A had no effect on SIRT1 expression in these experiments. At 16 h, cells were treated as previously reported,19 and appropriately diluted monoclonal antibodies to SIRT1 Alexa-Fluor 488 (Abcam ab157401, Melbourne, Australia), IFNγ PE (BD, Sydney, Australia), CD3 perCP.CY5.5 (BD), CD28 PECY7 (BD), CD56 APC (Beckman Coulter, Sydney, Australia), CD8 APC.CY7 (BD), TNFα V450 (BD) and CD45 V500 (BD) were added for 15 min in the dark at room temperature. Appropriate IgG-negative and fluorescence-minus-one control (FMO) controls were used to set all quadrant markers. Cells were washed and after decanting, cells were analyzed within 1 h on a FACSCanto II flow cytometer using FACSDiva software (BD). Samples were analyzed by gating lymphocytes using CD45 staining versus side scatter (SSC). A minimum of 3.5 × 105 low SSC events was acquired in list-mode format for analysis. T cells were identified as CD3+CD56–CD45+ and NKT-like cells identified as CD3+CD56+CD45+ low FSC/SSC events as previously reported.19
GCR and SIRT1 combined staining with intracellular cytokine expression in T and NKT-like cell subsets
To determine whether GCR and SIRT1 are associated with intracellular cytokine production in CD8+ and CD8– T and NKT-like cells, aliquots of blood were stimulated and treated as described above. Following washing of permeabilized cells, appropriately diluted GCR antibody (MCA2469, Bio-Rad, Sydney, Australia) was added to cells for 15 min. Cells were further washed and stained with anti-mouse IgG1 V450 secondary antibody for 15 min. Cells were then washed and stained with SIRT1 Alexa-Fluor 488, IFNγ PE (BD), TNFα PE (BD), CD3 perCP.CY5.5 (BD, Sydney, Australia), CD28 PECY7 (BD), CD56 APC (Beckman Coulter, Sydney, Australia), CD8 APC.CY7 (BD), and CD45 V500 (BD) for 15 min in the dark at room temperature. Appropriate controls and cells were analyzed as above.
Effect of therapies on SIRT1, GCR, and intracellular IFNγ expression in T and NKT-like cell subsets
Our aim was to investigate the effect of standard therapeutic dose of prednisolone (1 µM), theophylline (5 µg/ml) (prevents NAD+ depletion) and polyphenols,20 resveratrol (25 µM),21 curcumin (2 µM),22 SIRT activator STR720 (1 µM),23 and SIRT inhibitor, EX-527 (1 µM),17 on SIRT1, GCR expression and production of IFNγ and TNFα by CD8+ and CD8– T and NKT-like cells. Exposure to theophylline and polyphenols used concentrations previously shown not to cause significant side effects.20–23 Aliquots of blood were mixed in 10 ml sterile tubes with equal volumes of RPMI medium with 10% fetal calf serum (FCS) and incubated with treatments (and combinations) and the tubes incubated in a humidified 5% CO2/95% air atmosphere at 37°C for 24 h. Blood cultures were then stimulated as for intracellular cytokine production as described above for 16 h. SIRT1, GCR, IFNγ, and TNFα expression in blood was assessed as described above.
SIRT1 expression in CD28+ and CD28null T cells by fluorescent microscopy
CD28+ and CD28 null T cells (1 × 103 cells) were sorted as described above, and centrifuged at 500 g for 5 min in a Cytospin 4 cytocentrifuge (ThermoFisher Scientific, Scorseby, Victoria, Australia). Slides were treated as previously reported and incubated at 4°C with 1/25 diluted SIRT1 rabbit monoclonal antibody (Serotec, Abacus ALS, Brisbane, Australia), then 1 h with AF594-conjugated donkey IgG F(ab’)2 fragment polyclonal antibody to rabbit IgG (Abcam, Sapphire Bioscience, Waterloo, NSW, Australia). 4′,6-Diamidino-2-phenylindole (DAPI) was used as a counterstain (Sigma-Aldrich). Immunofluorescence was detected and imaged with an Olympus IX73 fluorescence microscope (Olympus, Notting Hill, VIC, Australia). For quantitative analysis, cells from each cytospin were photographed under a 40× objective in eight optical fields, selected in the DAPI channel for bias prevention. The mean fluorescence intensities were then measured in the AF594 channel using ImageJ software (NIH, Bethesda, MD, USA) as described previously.9,24
Statistical analysis
Statistical analyses were performed using the Friedman test with Wilcoxon sign rank test for post hoc pairwise comparisons. For T-cell subsets CD28null/CD8+/CD3+/CD56–/CD45+/TNFα+/IFNγ+), a sample size of n = 10 allowed a power of 98–99.5% for analysis. Variance was estimated from our previous studies.3–6 Correlations were performed using Spearman Rho correlation tests. SPSS software was applied and differences between groups of p < 0.05 considered significant.
Results
Increased CD28null CD8+ T and NKT-like cells in COPD patients
There was a significant increase in CD28nullCD8+ T cells in patients with COPD compared with healthy controls, but no change in CD28nullCD8– T cells (CD28nullCD8+ T cells: COPD 57% ± 8.4% versus controls 33% ± 8.5%); CD28nullCD8– T cells: COPD 7.1% ± 3.1% versus controls 5.9% ± 4.2% (median ± SEM) consistent with our previous findings for CD28null T cells.5 CD28nullCD8+ were significantly increased in NKT-like cells from patients with COPD compared with healthy controls, but no changes were noted in CD28nullCD8– NKT-like cells (CD28nullCD8+ NKT-like cells: COPD 39% ± 5.9% versus controls 22% ± 6.1%; CD28nullCD8– T cells: COPD 8.8% ± 3.6% versus controls 7.8% ± 3.3%; median ± SEM).
SIRT1 expression by CD28+ and CD28null T and NKT-like cells
A reduced percentage of CD28nullCD8+ T and NKT-like cells expressed SIRT1 in both COPD groups and controls was noted, compared with CD28+ T and NKT-like cells (data for T cell and NKT-like cell subsets from COPD group shown in Figure 1). SIRT1 expression in CD28nullCD8+ T and NKT-like cells in controls was unchanged compared with the COPD group for example, control SIRT1+CD28nullCD8+T cells 10% ± 2.7%; SIRT1+CD28+CD8+T cells 57% ± 7.8%; SIRT1+CD28nullCD8+NKT-like cells 8% ± 2.3%; SIRT1+CD28+CD8+NKT-like cells 54% ± 6.9%.
Figure 1.
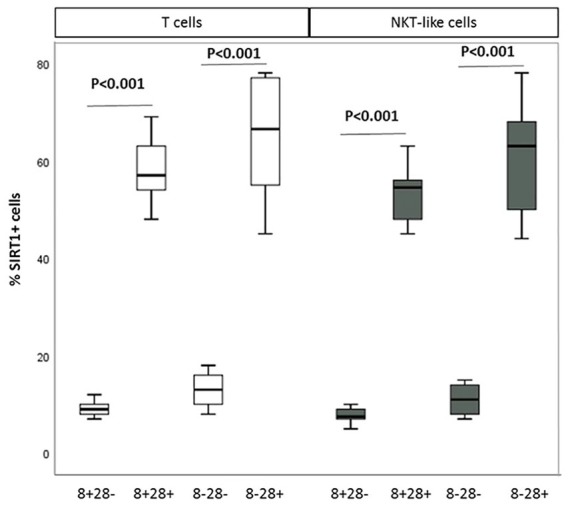
The percentage of CD28null and CD28+ CD8+ and CD8– T cells (clear bars) and NKT-like cells (grey bars) expressing SIRT1 in patients with COPD. Data presented as box plots. There was a significant decrease in the percentage of CD28null and CD28+ CD8+ and CD8– T and NKT-like cells expressing SIRT1 compared with CD28+CD8+ and CD28+CD8– T and NKT-like cells.
NKT, natural killer T; SIRT1, Sirtuin 1.
SIRT1, IFNγ, and TNFα production by CD28+, CD28null T, and NKT-like cells
A significant increase in the percentage of CD28nullCD8+ T and NKT-like cells producing IFNγ and TNFα, compared with CD28+CD8+ T and NKT-like cells, was noted in COPD patients and control groups (data for CD28null and CD28+ CD8+ and CD8– T and NKT-like cells producing IFNγ for the COPD group shown in Figure 2 and data for IFNγ and TNFα production for the control group and TNFα production by the COPD group not shown). There were no significant correlations between SIRT1 expression and the percentage of pro-inflammatory cytokine producing T cells or NKT-like cells in COPD or control groups (data not shown). A significant negative correlation was shown between loss of SIRT1 expression by CD28nullCD8+ T cells and the percentage of these cells producing IFNγ (Figure 3) and TNFα in the COPD group but not the control group (data not shown). There was a significant negative correlation between loss of SIRT1 expression by CD28nullCD8+ NKT-like cells and the percentage of these cells producing IFNγ (R = –0.627, p = 0.036) and TNFα (R = –0.541, p = 0.039) in the COPD group.
Figure 2.
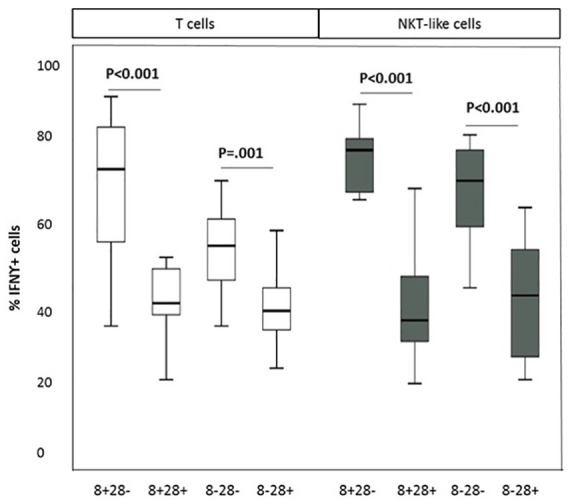
The percentage of CD28null and CD28+ CD8+ and CD8– T (clear bars) and NKT-like cells (grey bars) producing IFNγ in patients with COPD. Data presented as box plots. There was a significant decrease in the percentage of CD28null and CD28+ CD8+ and CD8– T and NKT-like cells producing IFNγ compared with CD28+CD8– and CD28+CD8+ T and NKT-like cells.
IFNγ, interferon gamma; NKT, natural killer T.
Figure 3.
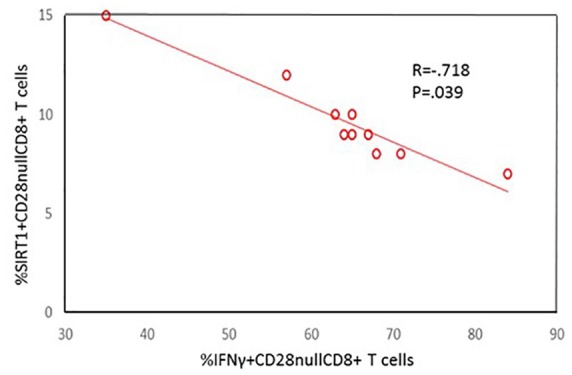
Significant negative correlation between the percentage of CD28nullCD8+ T cells expressing SIRT1 and producing IFNγ in COPD subjects.
COPD, chronic obstructive pulmonary disease; IFNγ, interferon gamma; SIRT1, Sirtuin 1.
SIRT1 expression in CD28+ and CD28null T cells by fluorescent microscopy
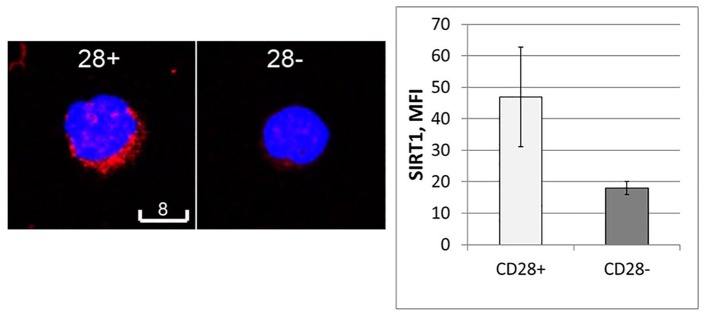
Sorted CD28+ and CD28null T cells were stained for SIRT1 expression. There was significant positive staining for SIRT1 in CD28+ T cells compared with CD28null T cells. SIRT1 staining was found to be located in the cytoplasm and cell nucleus (Figure 4).
Figure 4.
Representative laser confocal images of SIRT1 staining (red) in FACS-sorted CD28null (right) and CD28+ T cells (left). Blue is DAPI counterstaining. Scale bars are 8 µm. The bar graph shows results of quantitative analysis by Imagel. Experiments were repeated three times, showing similar results. ***p < 0.05.
DAPI, 4′,6-Diamidino-2-phenylindole; FACS, fluorescence-activated cell sorting; SIRT1, Sirtuin 1.
Correlation between SIRT1 and GCR by CD8+ T and NKT-like cells
There has been a report that SIRT1 and GCR colocalize in HeLa cells15; therefore, we investigated the possibility that these two molecules are co-expressed in CD8+ T and NKT-like cells.
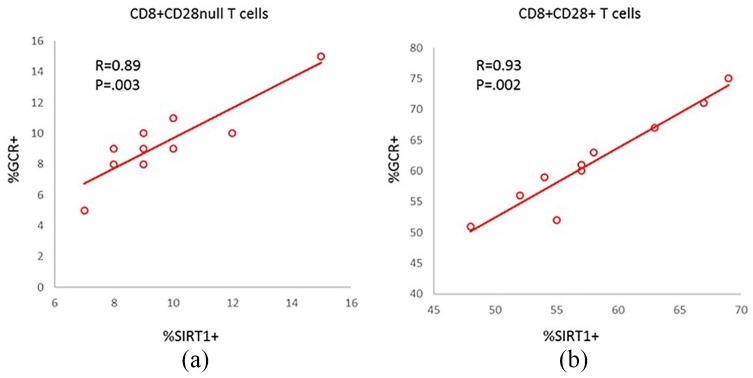
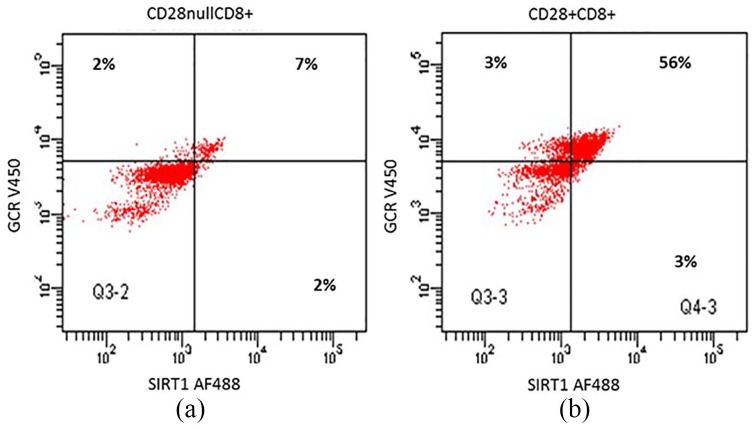
There was a correlation between SIRT1 and GCR expression by CD28nullCD8+ T and CD28+CD8+ T and NKT-like cells from both the COPD group and control group (data for COPD group are shown in Figure 5(a) and (b); data for control group not shown). Representative dot plots showing SIRT1 and GCR expression in CD28null and CD28+ CD8+ T cells from a COPD patient are shown in Figure 6.
Figure 5.
Significant correlation between SIRT1 and GCR expression by CD28nullCD8+ T (a) and CD28+CD8+ T (b) from the COPD group.
COPD, chronic obstructive pulmonary disease; GCR, glucocorticoid receptor; SIRT1, Sirtuin 1.
Figure 6.
Representative dot plots showing SIRT1 and GCR expression in CD28null (a) and CD28+ CD8+ T cells (b) from a COPD patient.
COPD, chronic obstructive pulmonary disease; GCR, glucocorticoid receptor; SIRT1, Sirtuin 1.
Correlation between SIRT1 expression by CD28nullCD8+ T cells and FEV1
There was a significant correlation between SIRT1 expression by CD28nullCD8+ T cells and FEV1 (% predicted) in COPD subjects (Figure 7). No correlations between SIRT1 expression by any other lymphocyte subset and FEV1 were observed (data not shown).
Figure 7.
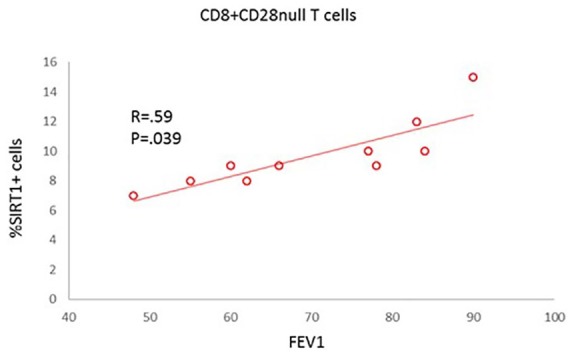
Correlation between SIRT1 expression by CD28nullCD8+ T cells and FEV1 from the COPD group.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; SIRT1, Sirtuin 1.
Effect of drugs on SIRT1, GCR, and intracellular cytokine expression by CD28null CD8+ T and NKT-like cells in COPD patients
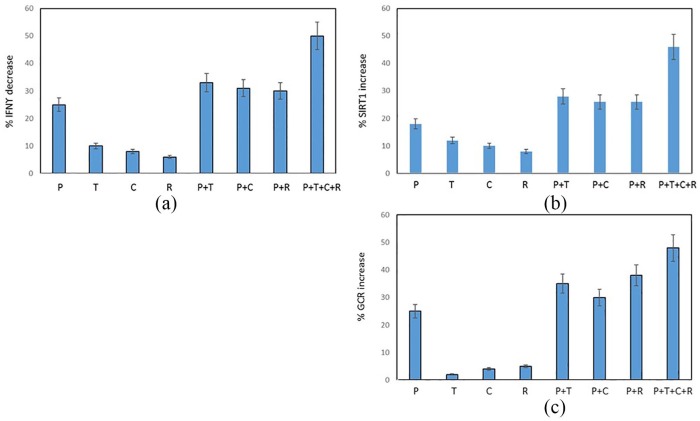
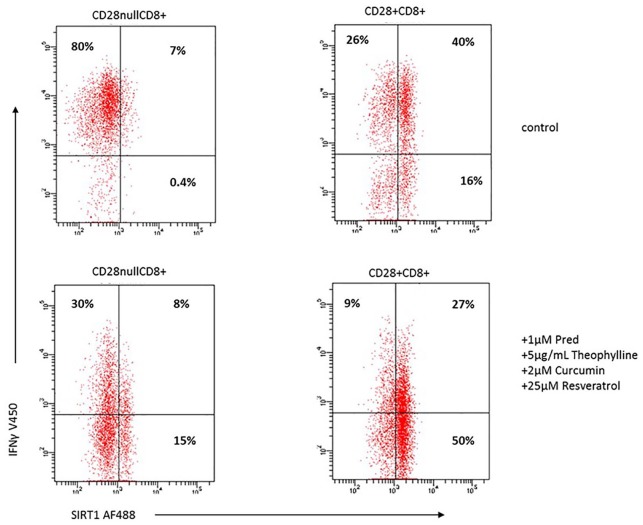
There was significant decrease in the percentage of CD28nullCD8+T cells producing IFNγ (Figure 8a), and a corresponding increase expressing SIRT1 (Figure 8b) and GCR (Figure 8c) in the presence of prednisolone, curcumin, resveratrol, or combination of drugs (p < 0.05 for all). There was an additive increase in the percentage of CD28nullCD8+T cells expressing SIRT1 and GCR in the presence of all drugs. Similar results were obtained for upregulation of SIRT1 and GCR and inhibition of IFNγ production by CD28+CD8+ and CD8– T cells and CD28+ and CD28null CD8+ and CD8– NKT-like cells (i.e. results were similar for all T and NKT-like subsets). The presence of the SIRT1 inhibitor, EX-527 (1 µM),16 negated [by 92 ± 12% (median ± SEM)] the effect of the SIRT1 activator SRT720 on the percentage of CD8+ T cells producing IFNγ and TNFα. The percentage of IFNγ+ CD8+ T cells (72 ± 9%) and TNFα (75 ± 11%); in the presence of SRT720 (IFNγ: 42 ± 5% and TNFα: 35 ± 6%) and in the presence of EX-527 (IFNγ: 70 ± 10% and TNFα: 71 ± 13%). Representative dot plots showing the combined effect of 1 µM prednisolone + 5 mg/ml theophylline + 1 µM curcumin + 25 µM resveratrol on the percentage of CD28nullCD8+ expressing SIRT1 and IFNγ compared with control (no drugs) is shown in Figure 9.
Figure 8.
Graphs showing the effect of ±1 µM prednisolone (P), ±5 mg/l theophylline (T), ±1 µM curcumin (C), ±25 µM resveratrol (R) on (a) the inhibition of IFNγ production by CD28nullCD8+ T cells and (b) upregulation of SIRT1. There was significant increase in the percentage of CD28nullCD8+T cells expressing SIRT1 and a decrease in IFNγ production in the presence of prednisolone, theophylline, curcumin, resveratrol, or combination of drugs (p < 0.05 for all). There was an additive increase in the percentage of CD28nullCD8+T cells expressing SIRT1 in the presence of all drugs.
IFNγ, interferon gamma; SIRT1, Sirtuin 1.
Figure 9.
Representative dot plots showing the combined effect of 1 µM prednisolone + 5 mg/ml theophylline + 1 µM curcumin + 25 µM resveratrol on the percentage of CD28nullCD8+ and CD28+CD8+ T cells expressing SIRT1 and IFNγ from a patient with COPD. Note the significant increase in SIRT1 expression and decrease in IFNγ production in both CD28null and CD28+ CD8+ T cells in the presence of the combination of drugs compared with control (no drugs).
COPD, chronic obstructive pulmonary disease; IFNγ, interferon gamma; SIRT1, Sirtuin 1.
Discussion
Loss of SIRT1 from senescent CD8+CD28null T and NKT-like cells has not previously been reported in the literature. Loss of SIRT1 correlated with the potential pro-inflammatory ability of these cells, and, importantly, the severity of the disease in these patients.
COPD is associated with significant chronic inflammation, and we have frequently reported increased CD8+ pro-inflammatory T lymphocytes in lungs and peripheral blood,3 an increase in NK and NKT-like lymphocytes in the lungs,6 and increased CD28nullCD8+ cells producing increased pro-inflammatory cytokines (TNFα and IFNγ) in ex-smoker and current COPD subjects.5 Chronic inflammation and cellular senescence are intertwined with disease-related premature aging, and are considered important contributing factors driving the pathogenesis of COPD.2
SIRT1 is a key modulator of inflammation, with anti-inflammatory effects including inhibiting the activity of transcription factors such as NF-κB and AP-1 that are involved in the production of inflammatory cytokines.25 There has been a report of reduced SIRT1 in macrophages and the lungs of patients with COPD11; however, to our knowledge, there have been no reports of SIRT1 levels in CD8+ peripheral blood lymphocytes in patients with COPD. Our findings of reduced expression of SIRT1 by these pro-inflammatory lymphocytes is therefore likely to be of clinical significance in the control of inflammatory responses in COPD. We and others have also shown that a further potential contributor to the chronic inflammation in COPD is the resistance of pro-inflammatory lymphocytes to the effects of corticosteroids.9,24 In this regard, we further reported reduced GCR expression by CD8+CD28null T and NKT-like lymphocyte subsets in COPD.9 SIRT1 acts as a transcriptional enhancer of the GCR, possibly by functioning as a scaffold for the transcriptional complex.13 Further to this, SIRT1 and GCR colocalize in HeLa cells.15 Our new findings of a loss of SIRT1 and GCR in the same cell therefore suggest that therapeutics aimed at increasing both SIRT1 and GCR may be required to improve steroid sensitivity in these pro-inflammatory lymphocytes in COPD. Interestingly, prednisolone increased both GCR and SIRT1 expression,9 suggesting a previously unidentified important mode of action for this commonly used corticosteroid.
We showed a reduction in a further mediator linked to steroid resistance, histone deacetylase, HDAC2, in senescent CD8+ T and NKT-like cells.24 Prednisolone upregulated HDAC2 in these cells, suggesting yet another mode of action of this drug.24 The upregulation was synergistically enhanced when prednisolone was used in combination with theophylline. The combination also decreased pro-inflammatory cytokine production by these steroid-resistant lymphocyte subsets. Importantly, these effects were noted at a low dose of theophylline shown to have no adverse side effects in patients.25
Taken together with other studies showing that theophylline can restore HDAC2 in lung macrophages, a further major cell type found increased in the lungs of patients with COPD, our findings suggest that theophylline may restore multiple deficiencies found in several cell types in COPD.25 The involvement of specific cell types in cellular senescence and their regulation by SIRT1 in the lung is still relatively unclear.26
In addition to the synergistic effects of theophylline with prednisolone, the present study found an additive increase in the percentage of CD28nullCD8+T cells expressing SIRT1 in the presence of curcumin or resveratrol. The effects of these well-studied anti-inflammatory agents with regard to steroid resistance in lymphocytes have not been previously studied. The phytoalexin resveratrol is found in wine, grapes, and other plant products, and its anti-inflammatory, antioxidant and anti-tumour activities have been reported.23 Resveratrol has been shown to inhibit several aspects of cell function (cell proliferation, cell-mediated cytotoxicity and cytokine production) partially via inhibiting NF-κB activation,23 and inhibits CD4+ T cell activation by increasing SIRT1 activity.27 At higher doses, resveratrol has toxic side effects28; however, we used doses that were not associated with any adverse side effects that were previously identified for human studies.21 Curcumin is a naturally occurring polyphenolic phytochemical, and has previously been shown to inhibit COPD-like airway inflammation in mice.29 Our results indicate combined treatment with prednisolone, theophylline, curcumin, and resveratrol may significantly upregulate SIRT1 and reduce inflammation.
These findings may have clinical relevance for treating and monitoring COPD. A reduction in adverse side effects of GCS, for example, may be achieved by reducing GCS dose due to the additive anti-inflammatory effects of the other agents.30 SIRT1/GCR deficient lymphocytes enumerated using our ex vivo assays may identify COPD patients that may benefit from these drug combinations and post-therapy lymphocyte phenotyping could identify therapeutic effectiveness. The very recent finding that reduced levels of SIRT1 destabilize the expression and function of transcription factor FoxO1 to enhance the pro-inflammatory and cytotoxic capacity of CD28nullCD8+ T cells, thereby contributing to immune dysfunctions of this cell type, may explain its pathophysiological role in age-related chronic inflammatory diseases.31
Interestingly, we also observe reduced expression of SIRT1 by CD28nullCD8+ T and NKT-like cells from healthy control subjects, although not to the same extent as found in COPD patients. Lymphocyte senescence and resistance to GC occur in several other inflammatory conditions, including cardiovascular disease,32 autoimmune disease,33 arthritis,34 IBD,35 aging,36 and inflammaging in COPD.37 Hence it could speculated that the CD28null lymphocytes may be the precursors to these other inflammatory diseases.
Important forward studies will be to determine whether levels of SIRT1 are changed in lymphocyte subsets in smokers who have not yet developed COPD, and to determine other correlations such as pack years of smokers and SIRT1 expression in COPD patients. Interestingly, these findings may seem counterintuitive to the evidence in the literature that SIRT1 and AMP-activated protein kinase (AMPK) both regulate each other and have similar effects on diverse processes such as inflammation and aging,38 although reports suggesting SIRT1 abundance and AMPK activation diminish in some mammalian tissues with aging are consistent with the findings of this current study.38
In conclusion, lymphocyte senescence in COPD is associated with loss of SIRT1 in CD28nullCD8+ T and NKT-like cells. Loss of SIRT1 is related to disease severity in COPD, and, hence, therapeutics aimed at increasing SIRT1 expression in pro-inflammatory senescent lymphocytes may reduce inflammaging reported in patients with COPD.10
Supplemental Material
Supplemental material, Author_response_1 for Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes by Greg Hodge, Hai B. Tran, Paul N. Reynolds, Hubertus Jersmann and Sandra Hodge in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes by Greg Hodge, Hai B. Tran, Paul N. Reynolds, Hubertus Jersmann and Sandra Hodge in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes by Greg Hodge, Hai B. Tran, Paul N. Reynolds, Hubertus Jersmann and Sandra Hodge in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors would like to thank Rhys Hamon for expert technical assistance.
Footnotes
Author contributions: GH performed the concept and design of experiments, analysis, and interpretation of data and manuscript preparation; HJ supplied and characterized patient specimens and helped draft the manuscript; HT performed immunofluorescence staining and helped draft the manuscript; ER performed western blots and helped draft the manuscript; PNR supplied and characterized patient specimens and helped draft the manuscript; SH helped with study design, statistical analysis, and helped draft the manuscript. All authors read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: this study was funded by a Lung Foundation Australia/Boehringer Ingelheim (GH) and COPD Research Fellowship (SH).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Greg Hodge  https://orcid.org/0000-0002-8689-963X
https://orcid.org/0000-0002-8689-963X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Greg Hodge, Lung Research, Department of Thoracic Medicine, Royal Adelaide Hospital, AHMS building, North Terrace, Adelaide, South Australia 5000, Australia; Department of Medicine, University of Adelaide, Adelaide, South Australia, Australia.
Hai B. Tran, Lung Research, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South Australia, Australia
Paul N. Reynolds, Lung Research, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South Australia, Australia Department of Medicine, University of Adelaide, Adelaide, South Australia, Australia.
Hubertus Jersmann, Lung Research, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South Australia, Australia; Department of Medicine, University of Adelaide, Adelaide, South Australia, Australia.
Sandra Hodge, Lung Research, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South Australia, Australia; Department of Medicine, University of Adelaide, Adelaide, South Australia, Australia.
References
- 1. Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet 2009; 373: 1905–1917. [DOI] [PubMed] [Google Scholar]
- 2. Barnes PJ, Shapiro SD, Pauwells RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003; 22: 672–688. [DOI] [PubMed] [Google Scholar]
- 3. Hodge G, Nairn J, Holmes M, et al. Increased intracellular Th1 pro-inflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelieal T cells of COPD subjects. Clin Exp Immunol 2007; 150: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodge S, Hodge G, Nairn J, et al. Increased airway granzyme b and perforin in current and ex-smoking COPD subjects. COPD 2006; 4: 179–187. [DOI] [PubMed] [Google Scholar]
- 5. Hodge G, Mukaro V, Reynolds P, et al. Role of increased CD8/CD28null T cells and alternative co-stimulatory molecules in chronic obstructive pulmonary disease. Clin Exp Immunol 2011; 166: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodge G, Holmes M, Jersamnn H, et al. The drug efflux pump Pgp1 in pro-inflammatory lymphocytes is a target for novel treatment strategies in COPD. Respir Res 2013; 14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hodge G, Mukaro V, Holmes M, et al. Enhanced cytotoxic function of natural killer and natural killer T-like cells with associated decreased CD94 (Kp43) in the chronic obstructive pulmonary disease airway. Respirology 2013; 18: 369–376. [DOI] [PubMed] [Google Scholar]
- 8. Arosa FA. CD8+CD28– T cells: certainties and uncertainties of a prevalent human T-cell subset. Immunol Cell Biol 2002; 80: 1–13. [DOI] [PubMed] [Google Scholar]
- 9. Hodge G, Jersmann H, Tran HB, et al. Lymphocyte senescence in COPD is associated with loss of glucocorticoid receptor expression by pro-inflammatory/cytotoxic lymphocytes. Respir Res 2015; 16: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol 2012; 84: 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajendrasozhan S, Yang SR, Kinnula VL, et al. SIRT1, an anti-inflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conti V, Corbi G, Manzo V, et al. SIRT1 activity in peripheral blood mononuclear cells correlates with altered lung function in patients with chronic obstructive pulmonary disease. Oxid Med Cell Long 2018; 2018: 9391261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao H, Sundar IK, Gerloff J, et al. Disruption of sirtuin-1 mediated control of circadian molecular clock and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 53: 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maeno T, Houghton AM, Quintero PA, et al. CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 2007; 178: 8090–8096. [DOI] [PubMed] [Google Scholar]
- 15. Chung S, Yao H, Caito S, et al. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 2010; 50: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki S, Iben JR, Kino T. SIRT1 is a transcriptional enhancer of the glucocorticoid receptor acting independently to its deacetylase activity. Mol Cell Endocrinol 2018; 461: 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vetterli L, Brun T, Giovannoni L, et al. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1Eβ-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem 2011; 286: 6049–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Cri Care Med 2001; 163: 1256–1276. [DOI] [PubMed] [Google Scholar]
- 19. Hodge G, Hodge S. Steroid resistant CD8+CD28null NKT-like pro-inflammatory cytotoxic cells in chronic obstructive pulmonary disease. Front Immunol 2016; 7: 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moonen HJ, Geraets L, Vaarhorst A, et al. Theophylline prevents NAD+ depletion via PARP-1 inhibition in human pulmonary epithelial cells. Biochem Biophys Res Commun 2005; 338: 1 805–1810. [DOI] [PubMed] [Google Scholar]
- 21. Gao X, Xu YX, Janakiraman N, et al. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol 2001; 9: 1299–1308. [DOI] [PubMed] [Google Scholar]
- 22. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 2009; 14: 141–153. [PubMed] [Google Scholar]
- 23. Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT720, SRT2183, SRT1460 and resveratrol are not direct activators of SIRT1. J Biol Chem 2010; 285: 8340–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodge G, Jersmann H, Tran HB, et al. Lymphocyte senescence in COPD is associated with decreased histone deacetylse 2 expression by proinflammatory lymphocytes. Respir Res 2015; 16: 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cosio BG, Tsaprouni L, Kazuhiro I, et al. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med 2004; 200: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kong S, McBurney MW, Fang D. Sirtuin 1 in immune regulation and autoimmunity. Immunol Cell Biol 2012; 90: 6–13. [DOI] [PubMed] [Google Scholar]
- 27. Zou T, Yang Y, Xia F, et al. Resveratrol inhibits CD4+ T cell activation by enhancing the expression and activation of SIRT1. PLoS One 2013; 8: e75139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukherjee S, Dudley JI, Das DK. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010; 8: 478–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moghaddam SJ, Barta P, Mirabolfathinejad SG, et al. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis 2009; 30: 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Volgelmeier CF. Systemic steroids in COPD- the beauty and the beast. Resp Res 2014; 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeng MY, Hull PA, Fei M, et al. Metabolic reprogramming of human CD8+ memory T cells through loss of SIRT1. J Exp Med 2018; 251: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teo FH, de Oliveira RT, Mamoni RL, et al. Characterisation of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for artherosclerosis. Cell Immunol 2013; 281: 11–19. [DOI] [PubMed] [Google Scholar]
- 33. Thewissen M, Somers V, Hellings N, et al. CD4+CD28null T cells in autoimmune disease: pathologenic features and decreased susceptibility to immunoregulation. J Immunol 2007; 179: 6514–6523. [DOI] [PubMed] [Google Scholar]
- 34. Fasth AE, Snir O, Johansson AA, et al. Skewed distribution of pro-inflammatory CD4+CD28null T cells in rheumatoid arthritis. Arthritis Res Ther 2007; 9: R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yokoyama Y, Fukunaga K, Ikeuchi H, et al. The CD4+CD28null and the regulatory CD4+CD25High T-cell phenotypes in patients with ulcerative colitis during active and quiescent disease, following colectomy. Cytokine 2011; 56: 466–470. [DOI] [PubMed] [Google Scholar]
- 36. Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev 2005; 205: 158–169. [DOI] [PubMed] [Google Scholar]
- 37. Yao H, Rahman I. Role of histone deacetylase 2 in epigenetics and cellular senescence: implications in lung inflammaging and COPD. Am J Physiol Lung Cell Mol Physiol 2012; 303: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruderman NB, Xu XJ, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 2010; 298: E751–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_response_1 for Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes by Greg Hodge, Hai B. Tran, Paul N. Reynolds, Hubertus Jersmann and Sandra Hodge in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes by Greg Hodge, Hai B. Tran, Paul N. Reynolds, Hubertus Jersmann and Sandra Hodge in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes by Greg Hodge, Hai B. Tran, Paul N. Reynolds, Hubertus Jersmann and Sandra Hodge in Therapeutic Advances in Respiratory Disease