Abstract
Aims
The aim of this study was to evaluate the low-density lipoprotein cholesterol lowering efficacy and safety of a bempedoic acid 180 mg and ezetimibe 10 mg fixed-dose combination in patients with hypercholesterolemia and a high risk of cardiovascular disease receiving maximally tolerated statin therapy.
Methods
This phase 3, double-blind clinical trial enrolled adult patients at high risk of cardiovascular disease due to atherosclerotic cardiovascular disease, heterozygous familial hypercholesterolemia, or multiple cardiovascular disease risk factors. Patients were randomly assigned (2:2:2:1) to treatment with the fixed-dose combination, bempedoic acid 180 mg, ezetimibe 10 mg or placebo added to stable background statin therapy for 12 weeks. The primary efficacy endpoint was the percentage change from baseline to week 12 in low-density lipoprotein cholesterol.
Results
Among the 301 patients included in the primary analysis, the mean baseline low-density lipoprotein cholesterol level was 3.87 mmol/L (149.8 mg/dL). At week 12, the fixed-dose combination lowered low-density lipoprotein cholesterol (–36.2%) significantly more than placebo (1.8% (placebo-corrected difference –38.0%); P < 0.001), ezetimibe alone (–23.2%; P < 0.001) or bempedoic acid alone (–17.2%; P < 0.001). The fixed-dose combination lowered low-density lipoprotein cholesterol levels similarly across subgroups, including patients receiving high-intensity, other-intensity or no statin therapy. Improvements with the fixed-dose combination were also observed in secondary efficacy endpoints, including high-sensitivity C-reactive protein. In this trial, fixed-dose combination treatment had a generally similar safety profile compared with bempedoic acid, ezetimibe or placebo.
Conclusion
The bempedoic acid and ezetimibe fixed-dose combination significantly lowered low-density lipoprotein cholesterol versus placebo or other oral monotherapies and had a favourable safety profile when added to maximally tolerated statin therapy in patients with hypercholesterolemia and high cardiovascular disease risk.
Trial Registration
ClinicalTrials.gov identifier: NCT03337308.
Keywords: Bempedoic acid, ezetimibe, hydroxymethylglutaryl-CoA reductase inhibitors, hypercholesterolemia, hyperlipidemias, hypolipidemic agents, LDL-cholesterol, lipid-regulating agents
Introduction
For more than three decades, pharmacological lipid lowering has helped reduce cardiovascular disease (CVD) risk in patients with hypercholesterolemia.1 Nonetheless, despite the development of effective therapeutic options, including statins, ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, many patients fail to achieve adequate lowering of low-density lipoprotein (LDL) cholesterol.2–5 As a result, patients remain at elevated CVD or cardiovascular event risk due to persistent elevations in LDL-cholesterol, particularly patients with atherosclerotic cardiovascular disease (ASCVD), familial hypercholesterolemia or multiple CVD risk factors.6,7 The limitations of available therapies in terms of effectiveness as well as tolerability, adherence and access highlight the unmet need for additional therapeutic options for lipid lowering.
Bempedoic acid is a once-daily, oral, first-in-class adenosine triphosphate-citrate lyase (ACL) inhibitor. ACL is a cytosolic enzyme integral to the cholesterol synthesis pathway that acts upstream of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase.8 This mechanism of action is distinct from other lipid-lowering therapies, including statins (which target HMG-CoA reductase) and ezetimibe (an inhibitor of intestinal cholesterol absorption). By inhibiting ACL, bempedoic acid suppresses cholesterol synthesis,8 thereby triggering the upregulation of LDL receptor expression in the liver, resulting in increased clearance of LDL particles and lowering of LDL-cholesterol in the blood.6 In pivotal clinical trials, bempedoic acid as monotherapy or when added to background lipid-lowering therapy significantly lowered LDL-cholesterol as well as other relevant lipids and biomarkers.9–12
Therapeutic interventions whose lipid-lowering effects are attributed to the upregulation of LDL receptor expression (e.g. diet, statins, ezetimibe, bile acid sequestrants and ileal bypass surgery) have been shown to reduce adverse cardiovascular outcome risk commensurate with the magnitude of LDL-cholesterol reduction.1,13 As bempedoic acid and ezetimibe both lower LDL-cholesterol by the upregulation of LDL receptor expression, an effect achieved through disparate mechanisms, there is a strong rationale for the development of a fixed-dose combination (FDC) of the two agents. This study evaluated the efficacy and safety of a bempedoic acid 180 mg and ezetimibe 10 mg FDC compared with placebo, ezetimibe 10 mg alone, and bempedoic acid 180 mg alone in patients with hypercholesterolemia at high CVD risk who were receiving maximally tolerated background statin therapy.
Methods
Patients
The study (ClinicalTrials.gov identifier: NCT03337308) enrolled adults at high CVD risk due to the presence of ASCVD, heterozygous familial hypercholesterolemia (HeFH) or multiple CVD risk factors. Documented ASCVD included a history of acute myocardial infarction (MI), silent MI, unstable angina, coronary revascularisation procedures, clinically significant coronary heart disease (CHD), symptomatic peripheral arterial disease or cerebrovascular atherosclerotic disease. The presence of multiple CVD risk factors was defined as diabetes plus one other risk factor or three CVD risk factors from the following list: age (men ≥ 45 years, women ≥ 55 years); family history of CHD; smoking; hypertension; low high-density lipoprotein (HDL) cholesterol; or coronary calcium score above the 95th percentile for the patient’s age, sex, and race/ethnicity. Fasting LDL-cholesterol was required to be 2.6 mmol/L or greater (100 mg/dL; ASCVD and/or HeFH) or 3.4 mmol/L or greater (130 mg/dL; multiple CVD risk factors) while receiving stable maximally tolerated statin therapy. A patient’s maximally tolerated statin therapy was determined by the investigator using his or her medical judgement and local standards of care, and may have included statin regimens other than daily dosing or no statin therapy. Patients were excluded from the study if they had total fasting triglycerides of 5.6 mmol/L or greater (500 mg/dL), body mass index (BMI) of 40 kg/m2 or greater, recent cardiovascular or cerebrovascular event or procedure (within 3 months prior to screening), or other clinically relevant disease that would interfere with study participation. Patients were prohibited from using systemic corticosteroids, simvastatin at doses of 40 mg/day or greater, fibrates, niacin and derivatives, bile acid sequestrants, PCSK9 inhibitors, mipomersen, lomatipide, cholesteryl ester transfer protein inhibitors, or red yeast rice-containing products or undergoing apheresis during the study or within specified intervals before screening. All patients provided written informed consent.
Study design
This phase 3, multicentre, double-blind study was conducted at 78 sites in the United States from 23 October 2017 to 3 July 2018. Patients who met the study inclusion criteria were randomly assigned 2:2:2:1 to oral, once-daily treatment with bempedoic acid 180 mg and ezetimibe 10 mg (BA + EZE FDC), bempedoic acid 180 mg, ezetimibe 10 mg or placebo for 12 weeks. Random assignment was stratified by CVD risk category (ASCVD and/or HeFH vs. multiple CVD risk factors) and baseline statin intensity (high intensity vs. other). Atorvastatin 40–80 mg/day and rosuvastatin 20–40 mg/day were considered high-intensity statin regimens; all other statin dosing regimens were categorised as ‘other intensity’ for the purposes of random assignment. The protocol and informed consent documents were reviewed and approved by the institutional review board prior to initiation of the study at each site.
Assessments
Clinical laboratory samples for the analysis of basic fasting lipids (total cholesterol, calculated LDL-cholesterol, HDL-cholesterol, non-HDL-cholesterol and triglycerides) were collected at the screening visit and before dosing on day 1 and weeks 4, 8 and 12. Samples for quantification of apolipoprotein B and high-sensitivity C-reactive protein (hsCRP) were collected before dosing on day 1 and at week 12. LDL-cholesterol concentration was calculated using the Friedewald formula; however, direct measurement was performed when triglycerides were greater than 4.5 mmol/L (400 mg/dL) or LDL-cholesterol was less than 1.3 mmol/L (50 mg/dL). All lipid measurements were conducted at a central laboratory (ICON, North Wales, Pennsylvania, USA). Blood samples for analysis of trough plasma concentrations of bempedoic acid, its active metabolite and ezetimibe were collected before dosing at weeks 4, 8 and 12.
Safety assessments included continuous monitoring of treatment-emergent adverse events (AEs) as well as clinical laboratory values, vital sign measurements, weight changes, physical examination findings and electrocardiogram readings. AEs of special interest were identified based on preclinical and clinical findings for bempedoic acid and other lipid-lowering therapies, and included metabolic acidosis, hepatic safety, muscular safety, new-onset diabetes/hyperglycemia, renal safety, cardiovascular events and neurocognitive/neurological events.
Statistical analysis
The planned sample size of 100 patients per active treatment group and 50 patients in the placebo arm was selected to provide adequate power for detecting between-group differences in the primary efficacy measure. Assuming a treatment difference of 13% (standard deviation (SD) 25%) for percentage change in LDL-cholesterol at week 12 between the BA + EZE FDC and ezetimibe or bempedoic acid, 100 patients in each of these groups would provide 95% or greater power to detect such a difference at an alpha level of 0.05 using a two-sided t-test. Similarly, 100 patients in both the BA + EZE FDC and bempedoic acid groups would provide 98% or greater power to detect a slightly larger treatment effect (15%; SD 25%). Finally, 100 patients in the BA + EZE FDC arm and 50 patients in the placebo arm would provide over 99% power to detect an estimated treatment effect of at least 33% (SD 25%). Together, a total sample size of 350 patients with a 2:2:2:1 allocation ratio to BA + EZE FDC, bempedoic acid, ezetimibe and placebo would provide an overall power of at least 92% (95% × 98% × 99%) to detect the estimated treatment differences.
Prespecified efficacy analyses were performed using the intention-to-treat (ITT) population, which included all randomly assigned patients, and prespecified safety analyses included all patients who were randomly assigned and received one or more dose of the study drug. However, following database lock and review, it was determined that an unusual number of patients (n = 51) receiving active study drug (BA + EZE FDC, bempedoic acid or ezetimibe but not placebo) who were reported to have routinely ingested the study drug had no detectable study drug in blood samples taken at week 12 for use in population pharmacokinetic modelling. These patients were evenly distributed among the three active treatment arms. Subsequent investigation revealed that most (34 of 51) of these patients were from three study sites, which were all located in the same metropolitan area and together randomly assigned 67 patients to active treatment and 14 to placebo. A root cause analysis ruled out issues with the production or distribution of study drug and the handling or analysis of pharmacokinetic samples. Inferential evidence indicated an indeterminate period of time when patients from these three sites were not taking the study drug as directed by the study protocol, which raised concerns about the integrity of any of the data from these three sites. Because of concerns that data from these three sites would not accurately reflect either the safety or efficacy of experimental therapy, data from these three sites were excluded from the additional post hoc efficacy and safety analyses reported here. Data from the ITT and safety populations that included these three sites are available in the Supplementary materials (Supplementary Tables 1–3 and Supplementary Figure 1).
The primary endpoint was the percentage change from baseline to week 12 in LDL-cholesterol. The three comparisons between the BA + EZE FDC and the other treatment arms (BA + EZE FDC vs. placebo, BA + EZE FDC vs. ezetimibe and BA + EZE FDC vs. bempedoic acid) were co-primary endpoints. Comparisons were analysed using analysis of covariance, with treatment group and random assignment stratification as factors and baseline LDL-cholesterol as a covariate. Missing values were imputed using a multiple imputation method, taking into account adherence to treatment. Each of the co-primary endpoint comparisons was conducted at a significance level of 0.05. If all three tests within the co-primary endpoint family achieved statistical significance, the hypothesis testing continued to the secondary endpoints; otherwise, all statistical comparisons for secondary endpoints were to be considered descriptive only. Least squares means, standard errors, 95% confidence intervals (CIs) and associated P values were calculated for each treatment group as well as for each treatment group comparison.
Key secondary efficacy endpoints, which included percentage change from baseline to week 12 in hsCRP, non-HDL-cholesterol, total cholesterol and apolipoprotein B, were analysed in a manner similar to the primary efficacy endpoint. The alpha allocation for secondary endpoints among the three comparison groups was: alpha = 0.01 for BA + EZE FDC versus placebo, alpha = 0.02 for BA + EZE FDC versus ezetimibe and alpha = 0.02 for BA + EZE FDC versus bempedoic acid. For hsCRP, a non-parametric analysis (Wilcoxon rank sum test) with Hodges–Lehmann estimates and CIs was performed. Changes in HDL-cholesterol and triglycerides were summarised using descriptive statistics.
Subgroup analyses for the change from baseline in LDL-cholesterol were performed for the following groups: sex, age (<65 vs. ≥ 65 years), baseline CVD risk category, baseline statin intensity (high intensity, other intensity, no statin), race (white vs. other), baseline LDL-cholesterol category (<3.4 mmol/L vs. ≥ 3.4 to < 4.1 mmol/L vs. ≥ 4.1 mmol/L), history of diabetes and BMI (<25 kg/m2, 25 to < 30 kg/m2, ≥ 30 kg/m2). The percentage change from baseline was analysed using analysis of covariance, with treatment group, subgroup, and treatment by subgroup interaction as factors and baseline LDL-cholesterol as a covariate. No imputation was performed for missing data in subgroup analyses. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
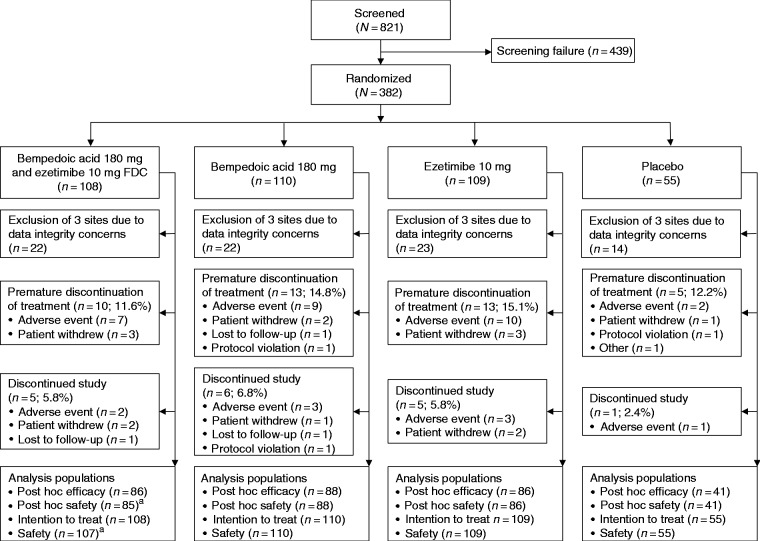
Of the 821 patients screened, 382 were randomly assigned to treatment with BA + EZE FDC (n = 108), bempedoic acid (n = 110), ezetimibe (n = 109) or placebo (n = 55) (Figure 1). A total of 338 (88.5% of randomly assigned) patients completed study treatment; the proportion who discontinued treatment was similar among the treatment groups. The exclusion of three study sites in the same metropolitan area because of data integrity concerns (see explanation in the Methods section) affected 81 patients, who were distributed across the treatment groups. The post hoc efficacy population therefore comprised 301 patients. One patient who was randomly assigned to BA + EZE FDC did not receive any dose of study drug and was excluded from the safety analyses. Data reported below are for the post hoc population unless otherwise specified.
Figure 1.
Patient disposition. aOne patient randomly assigned to the bempedoic acid and ezetimibe fixed-dose combination (FDC) treatment group did not receive any dose of study drug and was therefore excluded from the safety analyses.
Adherence to study drug, as assessed by pill count, was 80% or more for most patients in all treatment groups (BA + EZE FDC, 92.9%; bempedoic acid, 90.9%; ezetimibe, 95.3%; placebo, 95.1%). Median study drug exposure was 84 days in all active treatment groups and 85 days in the placebo group.
The mean age of the study population was 64.3 (SD 9.5) years and 50.5% of patients were women (Table 1). The majority (62.5%) of patients had ASCVD and/or HeFH, and comorbid hypertension (>80%) and diabetes (>40%) were prevalent. Most patients had a baseline mean LDL-cholesterol of 3.4 mmol/L or greater (130 mg/dL) despite treatment with maximally tolerated statin therapy, which consisted of a high-intensity statin (34.6%), other-intensity statin (30.2%) or no statin (35.2%).
Table 1.
Baseline patient demographics and characteristics, post hoc population.
| Characteristic | BA + EZE FDC (n = 86) | Bempedoic acid 180 mg (n = 88) | Ezetimibe 10 mg (n = 86) | Placebo (n = 41) |
|---|---|---|---|---|
| Age, years | 62.2 ± 9.5 | 65.0 ± 9.8 | 65.1 ± 8.4 | 65.4 ± 10.8 |
| Women, n (%) | 44 (51.2) | 48 (54.5) | 43 (50.0) | 17 (41.5) |
| Race, n (%) | ||||
| White | 67 (77.9) | 70 (79.5) | 72 (83.7) | 34 (82.9) |
| Black or African American | 16 (18.6) | 17 (19.3) | 12 (14.0) | 7 (17.1) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 10 (11.6) | 11 (12.5) | 9 (10.5) | 6 (14.6) |
| Not Hispanic or Latino | 76 (88.4) | 77 (87.5) | 77 (89.5) | 35 (85.4) |
| CV risk category, n (%) | ||||
| ASCVD and/or HeFH | 53 (61.6) | 55 (62.5) | 54 (62.8) | 26 (63.4) |
| Multiple CV risk factors | 33 (38.4) | 33 (37.5) | 32 (37.2) | 15 (36.6) |
| History of diabetes, n (%) | 35 (40.7) | 45 (51.1) | 43 (50.0) | 17 (41.5) |
| History of hypertension, n (%) | 74 (86.0) | 77 (87.5) | 71 (82.6) | 35 (85.4) |
| Body mass index, kg/m2 | 31.1 ± 6.3 | 30.6 ± 5.5 | 29.9 ± 4.4 | 30.7 ± 4.2 |
| eGFR category, n (%) | ||||
| ≥90 mL/min/1.73 m2 | 30 (34.9) | 27 (30.7) | 29 (33.7) | 19 (46.3) |
| 60 to < 90 mL/min/1.73 m2 | 40 (46.5) | 41 (46.6) | 43 (50.0) | 14 (34.1) |
| <60 mL/min/1.73 m2 | 16 (18.6) | 20 (22.7) | 14 (16.3) | 8 (19.5) |
| Baseline statin intensity, n (%) | ||||
| High intensity | 31 (36.0) | 29 (33.0) | 28 (32.6) | 16 (39.0) |
| Other intensity | 22 (25.6) | 32 (36.4) | 26 (30.2) | 11 (26.8) |
| No statin | 33 (38.4) | 27 (30.7) | 32 (37.2) | 14 (34.1) |
| Total cholesterol, mmol/La | 6.14 ± 1.26 | 5.83 ± 1.12 | 5.98 ± 1.31 | 5.98 ± 1.30 |
| Non-HDL-C, mmol/La | 4.87 ± 1.21 | 4.54 ± 1.05 | 4.66 ± 1.22 | 4.68 ± 1.29 |
| LDL-C, mmol/La | 3.98 ± 1.05 | 3.75 ± 0.99 | 3.85 ± 1.08 | 3.95 ± 1.21 |
| LDL-C category, n (%) | ||||
| <3.4 mmol/L | 30 (34.9) | 40 (45.5) | 31 (36.0) | 13 (31.7) |
| ≥3.4 to < 4.1 mmol/L | 24 (27.9) | 23 (26.1) | 30 (34.9) | 10 (24.4) |
| ≥4.1 mmol/L | 32 (37.2) | 25 (28.4) | 25 (29.1) | 18 (43.9) |
| HDL-C, mmol/La | 1.27 ± 0.38 | 1.29 ± 0.32 | 1.33 ± 0.41 | 1.30 ± 0.36 |
| Triglycerides, mmol/Lb | 1.77 (1.20, 2.36) | 1.59 (1.22, 2.15) | 1.62 (1.24, 2.40) | 1.57 (1.18, 1.90) |
| Apolipoprotein B, mg/dLa | 121.1 ± 30.9 | 113.4 ± 26.4 | 115.5 ± 31.3 | 115.1 ± 32.5 |
| hsCRP, mg/Lb | 3.1 (1.7, 6.2) | 2.9 (1.4, 5.0) | 2.8 (1.3, 5.9) | 3.0 (1.3, 5.5) |
ASCVD: atherosclerotic cardiovascular disease; BA + EZE FDC: bempedoic acid and ezetimibe fixed-dose combination; CV: cardiovascular; eGFR: estimated glomerular filtration rate; HDL-C: high-density lipoprotein cholesterol; HeFH: heterozygous familial hypercholesterolemia; hsCRP: high-sensitivity C-reactive protein; LDL-C: low-density lipoprotein cholesterol.
Data are means ± standard deviations.
Data are medians (interquartile ranges).
Efficacy
At week 12, LDL-cholesterol lowering with BA + EZE FDC was significantly greater than that for the placebo, ezetimibe, or bempedoic acid groups (P < 0.001 for all comparisons; Figure 2(a)), with BA + EZ FDC providing a reduction of 38.0% compared with placebo. A significantly greater proportion of patients had achieved LDL-cholesterol less than 2.6 mmol/L (100 mg/dL) or less than 1.8 mmol/L (70 mg/dL) at week 12 in the BA + EZE FDC treatment group (67.5% and 31.3%, respectively) compared with the placebo (17.5% and 0%; P < 0.001), ezetimibe (42.5% and 10.0%; P ≤ 0.002) or bempedoic acid groups (43.9% and 6.1%; P ≤ 0.003). At week 12, 33.7% of patients in the BA + EZE FDC group had an LDL-cholesterol reduction from baseline of 50% or greater versus 0%, 5.0% and 3.7% of patients in the placebo, ezetimibe and bempedoic acid groups, respectively (P < 0.001 for all comparisons). LDL-cholesterol lowering with BA + EZE FDC was generally consistent in subgroup analyses (Supplementary Figure 2). Although the study was not powered to assess between-group differences in the subgroup analyses, LDL-cholesterol lowering with BA + EZE FDC was greater than placebo in all subgroups (P ≤ 0.001). Moreover, BA + EZE FDC lowered LDL-cholesterol in all statin intensity groups.
Figure 2.
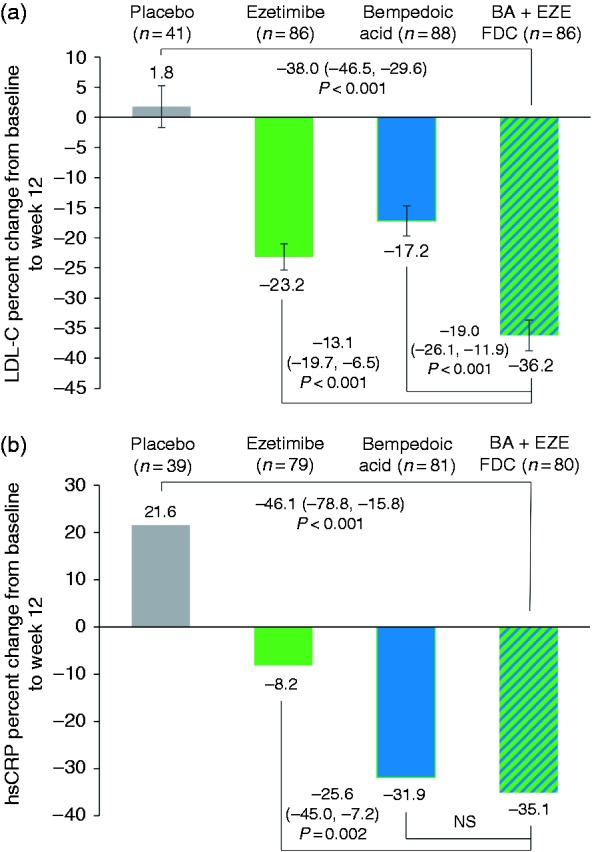
Change from baseline to week 12 in low-density lipoprotein (LDL) cholesterol and high-sensitivity C-reactive protein (hsCRP), post hoc population. (a) Percentage change from baseline in LDL-cholesterol was analysed using analysis of covariance with treatment group and random assignment stratification as factors and baseline LDL-cholesterol as a covariate. Baseline was defined as the mean of the values from week –2 and pre-dose on day 1. Missing values were imputed using a multiple imputation method, taking into account adherence to treatment. Bars represent least-squares means ± standard errors. (b) Percentage change from baseline in hsCRP was analysed using a non-parametric (Wilcoxon rank sum test) analysis with Hodges–Lehmann estimates and confidence intervals. Baseline was defined as the value recorded pre-dose on day 1. Bars represent medians. Data are as observed, without imputation for missing values. Between-group differences are shown with 95% confidence intervals for LDL-cholesterol and (1 – alpha) percentage confidence intervals for hsCRP. The BA + EZE FDC versus placebo comparison used alpha = 0.01, and the BA + EZE FDC versus ezetimibe and BA + EZE FDC versus bempedoic acid comparisons used alpha = 0.02. BA + EZE FDC: bempedoic acid and ezetimibe fixed-dose combination; NS: not significant.
BA + EZE FDC reduced hsCRP by 35.1% compared with an increase of 21.6% in the placebo group (P < 0.001) and a reduction of 8.2% in the ezetimibe group (P = 0.002; Figure 2(b)). The comparison of BA + EZE FDC versus bempedoic acid was not statistically significant, probably due to the considerable hsCRP lowering observed in the bempedoic acid treatment group (–31.9%). For the other key secondary endpoints, BA + EZE FDC reduced non-HDL-cholesterol, total cholesterol and apolipoprotein B more than placebo (P < 0.001), ezetimibe (P ≤ 0.003) or bempedoic acid (P < 0.001; Table 2). The magnitude of reduction for the primary and key secondary endpoint comparisons was greater in the post hoc analysis compared with the ITT analysis (Supplementary Figure 1 and Supplementary Table 2). Changes from baseline in HDL-cholesterol and triglycerides were modest (<10%) in all treatment groups.
Table 2.
Percentage changes in key secondary endpoints from baseline to week 12, post hoc population.
| Parameter treatment | n | LS mean ± SE | Difference (confidence interval)a | P value |
|---|---|---|---|---|
| Non-HDL-C | ||||
| BA + EZE FDC vs. | 86 | –31.9 ± 2.2 | ||
| Placebo | 41 | 1.8 ± 3.3 | –33.7 (–43.9, –23.4) | <0.001c |
| Ezetimibe | 86 | –19.9 ± 2.1 | –12.1 (–19.1, –5.0) | <0.001d |
| Bempedoic acid | 88 | –14.1 ± 2.2 | –17.8 (–25.1, –10.5) | <0.001d |
| Total cholesterol | ||||
| BA + EZE FDC vs. | 86 | –26.4 ± 1.9 | ||
| Placebo | 41 | 0.7 ± 2.5 | –27.1 (–35.1, –19.1) | <0.001c |
| Ezetimibe | 86 | –16.0 ± 1.6 | –10.4 (–16.1, –4.6) | <0.001d |
| Bempedoic acid | 88 | –12.1 ± 1.8 | –14.2 (–20.4, –8.1) | <0.001d |
| Apolipoprotein Bb | ||||
| BA + EZE FDC vs. | 82 | –24.6 ± 2.4 | ||
| Placebo | 38 | 5.5 ± 3.0 | –30.1 (–39.9, –20.3) | <0.001c |
| Ezetimibe | 84 | –15.3 ± 2.0 | –9.3 (–16.5, –2.1) | 0.003d |
| Bempedoic acid | 85 | –11.8 ± 2.2 | –12.8 (–20.3, –5.3) | <0.001d |
BA + EZE FDC: bempedoic acid and ezetimibe fixed-dose combination; LDL-C: low-density lipoprotein cholesterol; LS: least-squares; non-HDL-C: non-high-density lipoprotein cholesterol; SE: standard error.
The percentage change from baseline was analysed using analysis of covariance with treatment group and random assignment stratification as factors and baseline value as a covariate. Missing values were imputed using a multiple imputation method, taking into account adherence to treatment. Baseline for non-HDL-cholesterol and total cholesterol was defined as the mean of the values from week –2 and pre-dose on day 1.
Confidence intervals are (1 – alpha) percentage.
Baseline for apolipoprotein B was defined as the value recorded pre-dose on day 1. Baseline data for apolipoprotein B were available for 82, 38, 84 and 85 patients in the FDC, placebo, ezetimibe and bempedoic acid groups, respectively.
BA + EZE FDC versus placebo comparison used alpha = 0.01.
BA + EZE FDC versus ezetimibe and BA + EZE FDC versus bempedoic acid comparisons used alpha = 0.02.
Safety
Treatment-emergent AEs were reported by 176 (58.7%) patients overall, and were more frequent in the BA + EZE FDC and bempedoic acid groups than in the ezetimibe or placebo groups (Table 3). Rates of individual AEs were low (affecting < 7% of patients per treatment group), and were generally mild or moderate in intensity. No treatment-related serious AEs or fatal AEs occurred during the study. The most common treatment-related AEs in the BA + EZE FDC group were blood uric acid increase, constipation, fatigue, muscle spasms and oral discomfort, each reported by two (2.4%) patients. Rates of AEs leading to treatment discontinuation were similar in the active treatment groups (BA + EZE FDC, 8.2%; bempedoic acid, 10.2%; ezetimibe, 11.6%). Myalgia led to treatment discontinuation for three (3.4%) patients in the bempedoic acid group, one (1.2%) patient in the ezetimibe group and no patients in the BA + EZE FDC group. Other events that led to discontinuation in more than one patient were oral discomfort (BA + EZE FDC, two patients (2.4%)) and fatigue (BA + EZE FDC, one patient (1.2%); bempedoic acid, one patient (1.1%)).
Table 3.
Safety summary, post hoc population.
| BA + EZE FDC (n = 85) | Bempedoic acid (n = 88) | Ezetimibe (n = 86) | Placebo (n = 41) | |
|---|---|---|---|---|
| Overview of AEs, number of patients (%) | ||||
| Any treatment-emergent AE | 53 (62.4) | 58 (65.9) | 47 (54.7) | 18 (43.9) |
| Serious AEs | 8 (9.4) | 7 (8.0) | 9 (10.5) | 1 (2.4) |
| Study drug-related AEs | 13 (15.3) | 12 (13.6) | 9 (10.5) | 4 (9.8) |
| AEs leading to treatment discontinuation | 7 (8.2) | 9 (10.2) | 10 (11.6) | 2 (4.9) |
| Fatal AEs | 0 | 0 | 0 | 0 |
| Common AEs, number of patients (%) | ||||
| Urinary tract infection | 5 (5.9) | 3 (3.4) | 2 (2.3) | 1 (2.4) |
| Nasopharyngitis | 4 (4.7) | 6 (6.8) | 4 (4.7) | 0 |
| Constipation | 4 (4.7) | 0 | 2 (2.3) | 0 |
| Back pain | 3 (3.5) | 3 (3.4) | 2 (2.3) | 2 (4.9) |
| Fatigue | 3 (3.5) | 2 (2.3) | 1 (1.2) | 0 |
| Upper respiratory tract infection | 3 (3.5) | 1 (1.1) | 0 | 0 |
| Blood creatinine increased | 3 (3.5) | 1 (1.1) | 0 | 0 |
| Blood uric acid increased | 3 (3.5) | 1 (1.1) | 0 | 0 |
| Bronchitis | 3 (3.5) | 0 | 3 (3.5) | 0 |
| Headache | 2 (2.4) | 3 (3.4) | 1 (1.2) | 1 (2.4) |
| Arthralgia | 1 (1.2) | 4 (4.5) | 3 (3.5) | 1 (2.4) |
| Hypertension | 1 (1.2) | 3 (3.4) | 2 (2.3) | 0 |
| Acute myocardial infarction | 1 (1.2) | 2 (2.3) | 3 (3.5) | 0 |
| Dyspnoea | 0 | 0 | 1 (1.2) | 2 (4.9) |
| Muscular disorders, number of patients (%) | 6 (7.1) | 7 (8.0) | 7 (8.1) | 3 (7.3) |
| Muscle spasms | 2 (2.4) | 1 (1.1) | 4 (4.7) | 0 |
| Muscular weakness | 0 | 0 | 0 | 1 (2.4) |
| Myalgia | 2 (2.4) | 5 (5.7) | 2 (2.3) | 1 (2.4) |
| Pain in extremity | 2 (2.4) | 2 (2.3) | 1 (1.2) | 1 (2.4) |
| Laboratory results | ||||
| ALT or AST > 3 × ULN, n (%)c | 1 (1.2) | 0 | 0 | 0 |
| Creatine kinase > 5 × ULN, n (%)c | 0 | 0 | 0 | 0 |
| Change in creatinine, µmol/Ld | 2.0 ± 10.2 | 5.6 ± 11.7 | 2.7 ± 17.4 | –0.9 ± 8.3 |
| Change in uric acid, µmol/Ld | 36.9 ± 67.2 | 51.7 ± 65.6 | 3.0 ± 46.0 | –8.9 ± 47.6 |
AE: adverse event; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BA + EZE FDC: bempedoic acid and ezetimibe fixed-dose combination; ULN: upper limit of normal.
Treatment-emergent AEs occurring in 3% or more of patients in any treatment group, excluding muscle-related AEs.
Muscular disorders were predefined as: muscular weakness, muscle necrosis, muscle spasms, myalgia, myoglobin blood increased, myoglobin blood present, myoglobin urine present, myoglobinemia, myoglobinuria, myopathy, myopathy toxic, necrotising myositis, pain in extremity and rhabdomyolysis.
Patients with repeated and confirmed aminotransferase or creatine kinase elevations.
Data are means ± standard deviations for change from baseline to week 12.
Analysis of AEs of special interest did not reveal any new safety signals with BA + EZE FDC versus bempedoic acid or ezetimibe. The incidence of AEs in the muscular disorders category was similar among treatment groups (Table 3). No patient had a repeated and confirmed creatine kinase elevation more than 5 × the upper limit of normal (ULN), and one patient in the BA + EZE FDC group had a repeated and confirmed aspartate aminotransferase elevation greater than 3 × ULN (aspartate aminotransferase levels were also > 3 × ULN at random assignment for this patient). A modest increase in mean uric acid levels was observed in the BA + EZE FDC (11.8%) and bempedoic acid (16.1%) treatment groups, but no patient in any treatment group reported an AE of gout. Safety findings in the post hoc population were consistent with those observed in the overall safety population (Supplementary Table 3).
Discussion
In this study of patients with high CVD risk and hypercholesterolemia despite treatment with maximally tolerated statin therapy, treatment with BA + EZE FDC resulted in statistically significant LDL-cholesterol lowering compared with placebo, ezetimibe and bempedoic acid. The extent of lipid lowering in the BA + EZE FDC treatment group suggests an additive effect of bempedoic acid and ezetimibe, wherein lipid reductions with the combination were greater than those seen with the individual components alone. Additive effects are consistent with the known differences in mechanisms of action of bempedoic acid8 and ezetimibe14 and with previous clinical data.9,15 In a phase 2 study, combination therapy with bempedoic acid 180 mg and ezetimibe 10 mg in patients not receiving other lipid-modifying therapies lowered LDL-cholesterol by 48%. In the monotherapy arms, LDL-cholesterol lowering was 30% with bempedoic acid 180 mg and 21% with ezetimibe 10 mg.15 The addition of bempedoic acid 180 mg to stable ezetimibe therapy with or without other lipid-modifying therapies (including statins) in a phase 3 study yielded an additional 24% LDL-cholesterol lowering.9 Together, these results indicate a complementary effect on LDL-cholesterol lowering when combining bempedoic acid and ezetimibe.
BA + EZE FDC lowered LDL-cholesterol to a degree generally consistent across demographic and clinical subgroups, including patients receiving various intensities of background statin therapy. The use of a high-intensity statin regimen was reported by more than one-third of patients enrolled in the study. Within this subgroup, treatment with BA + EZE FDC lowered LDL-cholesterol by 38.9% (beyond any statin-mediated LDL-cholesterol lowering). The study population also included a large segment (31–38%) of patients who were not receiving a statin due to statin intolerance. Within this subgroup, treatment with BA + EZE FDC lowered LDL-cholesterol by 38.8%. These findings support the potential for substantial LDL-cholesterol lowering with BA + EZE FDC in diverse patient populations, independent of baseline statin use.
BA + EZE FDC also reduced circulating concentrations of key secondary endpoints, including hsCRP, non-HDL-cholesterol, total cholesterol and apolipoprotein B. Reductions in hsCRP with BA + EZE FDC and bempedoic acid were –35.1% and –31.9%, respectively. These reductions were achieved on top of stable background statin therapy, which is also known to reduce hsCRP levels.16 The comparatively lower reduction in hsCRP with ezetimibe observed in the current study (–8.2%) is consistent with previous reports of a 9% to 10% reduction in hsCRP when ezetimibe is added to a statin.17,18 Hence, the overall reduction in hsCRP with BA + EZE FDC appeared to be largely driven by the effects of bempedoic acid. Additive effects of BA + EZE FDC were observed in lowering of non-HDL-cholesterol, total cholesterol and apolipoprotein B. Apolipoprotein B lowering was concordant with that of LDL-cholesterol, although reduction of the former was slightly less than that of LDL-cholesterol in a manner that has been described with statins and ezetimibe.19–21 These improvements coupled with substantial LDL-cholesterol lowering support a positive role for BA + EZE FDC in ameliorating hyperlipidemia and reducing inflammation.
BA + EZE FDC had a favourable safety profile and was well tolerated when added to background maximally tolerated statin therapy. Safety and tolerability findings were consistent with expectations based on previous bempedoic acid clinical trials,9–12 and no new safety concerns were detected with BA + EZE FDC treatment compared with bempedoic acid, ezetimibe or placebo. The incidences of AEs and serious AEs were similar in the BA + EZE FDC group compared with the other active treatment groups, as were the rates of discontinuations due to AEs. No differences in the safety profile of BA + EZE FDC were observed in demographic or clinical subgroups, including patients who were receiving background high-intensity statin therapy.
From a clinical standpoint, BA + EZE FDC has several potential advantages. LDL-cholesterol goal achievement in clinical practice remains an elusive aspiration, particularly among those at high or very high cardiovascular risk.22,23 Statin therapy alone may be insufficient to achieve LDL-cholesterol goals,6 and a single, oral, once-daily, add-on therapy with demonstrated lipid-lowering efficacy and statin compatibility is an attractive option. BA + EZE FDC may also be a viable alternative for patients with statin intolerance, for whom dyslipidemia management guidelines currently recommend ezetimibe and/or bile acid sequestrants.6 Bempedoic acid acts through the same pathway as statins, yet due to its selective activation in the liver but not skeletal muscle,24 bempedoic acid is not associated with muscle-related side effects, even among patients with a history of statin intolerance.9,11 Finally, although there are not cardiovascular outcomes data for bempedoic acid at this time, its mechanism (LDL-cholesterol lowering through upregulation of LDL receptor expression)1,13 and Mendelian randomisation analysis of genetic variants whose effects are akin to endogenous inhibition of ACL25 – the target of bempedoic acid – support its potential for reducing cardiovascular outcome risks.
The limitations of this study include its relatively short duration (12 weeks) and the occurrence of slight imbalances in baseline demographics and patient characteristics among treatment groups. In addition, as noted in the Methods section, 81 patients from three study sites were excluded due to data integrity concerns (see explanation in the Methods section). Finally, this study was not designed to evaluate CVD outcomes; rather, the intent was limited to assessment of 12-week lipid-altering efficacy and safety of BA + EZE FDC relative to monotherapy with the component agents or placebo. The influence of bempedoic acid on CVD event risk is being evaluated in an ongoing CVD outcomes trial (CLEAR Outcomes; ClinicalTrials.gov identifier: NCT02993406). Although CLEAR Outcomes is not a BA + EZE FDC study, because it is enrolling high-risk patients with statin intolerance, the concomitant use of bempedoic acid with ezetimibe is likely.
Conclusions
The addition of BA + EZE FDC to maximally tolerated statin therapy provides significant atherogenic lipid lowering compared with either agent alone or placebo. BA + EZE FDC lowered LDL-cholesterol in patients who were receiving high-intensity statin therapy as well as patients who were statin intolerant. Clinical guidelines and practice are moving towards the achievement of lower LDL-cholesterol levels and more stringent treatment targets. BA + EZE FDC may help provide a potent and convenient therapy complementary to existing lipid-modifying therapy regimens.
Supplemental Material
Supplemental Material for Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy by Christie M Ballantyne, Ulrich Laufs, Kausik K Ray, Lawrence A Leiter, Harold E Bays, Anne C Goldberg, Erik SG Stroes, Diane MacDougall, Xin Zhao and Alberico L Catapano in European Journal of Preventive Cardiology
Acknowledgements
The authors are appreciative of study investigators, clinical site staff and study participants, and acknowledge the role of ICON in oversight of study management and monitoring, statistical analysis and programming. The authors also thank Crystal Murcia, PhD, of JB Ashtin, who developed a first draft of the manuscript based on an author-approved outline and assisted in implementing revisions based on the authors’ input and direction. KKR acknowledges support from the Imperial NIHR Biomedical Research Centre.
Data from this study have been presented at the 2019 European Atherosclerosis Society Congress.
Author contribution
CMB, UL, KKR, LAL, HEB, ESGS, DM, XZ and ALC contributed to the conception and/or design of the study. CMB and DM were involved in data acquisition as well as analysis of the data. LAL, HEB and XZ contributed to data analysis. All authors were involved in the interpretation of the study data, critically revised the manuscript, gave final approval for publication, and agree to be accountable for all aspects of the work.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CMB has received research grant(s)/support from Abbott Diagnostic, Akcea, Amarin, Amgen, Esperion, Ionis, Novartis, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, NIH, AHA and ADA (all paid to institution, not individual). He has also served as a consultant for Abbott Diagnostics, Amarin, Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Esperion, Intercept, Ionis, Matinas BioPharma Inc., Merck, Novartis, Novo Nordisk, Regeneron, Roche Diagnostic and Sanofi-Synthelabo. UL has served as a consultant for Amgen, Esperion and Sanofi. KKR has received research grant(s)/support from Amgen, MSD, Pfizer, Regeneron and Sanofi (all paid to institution, not individual), and has served as a consultant for/received honoraria from AbbVie, Akcea, Algorithm, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, Cipla, Dr Reddy’s Laboratories, Eli Lilly, Esperion, Kowa, Medco, MSD, Novo Nordisk, Pfizer, Regeneron, Resverlogix, Sanofi, Takeda and Zuellig Pharma. LAL has received research grant(s)/support from Astra Zeneca, Amgen, Kowa, Merck, The Medicines Company and Sanofi/Regeneron. He has also served as a consultant for Astra Zeneca, Amgen, Esperion, Kowa, Merck, The Medicines Company and Sanofi/Regeneron. HEB has received research grant(s)/support from Amgen, LIB Therapeutics, Merck, Regeneron and Sanofi, and has served as a consultant for/received honoraria from Aegerion, Amgen, Regeneron and Sanofi. ACG has received research grant(s)/support from Amgen, Amarin, Regeneron, Sanofi, Pfizer and Ionis Pharmaceuticals (all paid to institution, not individual), and has served as a consultant for Novartis, Sanofi/Regeneron, Akcea and Esperion, and has received honoraria from Merck. ESGS has received research grant(s)/support to his institution from Amgen, Sanofi, Resverlogix and Athera, and has served as a consultant for Amgen, Sanofi, Esperion, Novartis and Ionis Pharmaceuticals. DM is an employee of Esperion Therapeutics, Inc. XZ is a past employee of Esperion Therapeutics, Inc. ALC has received research grant(s)/support from Sanofi, Sanofi Regeneron, Amgen, Mylan and Menarini, and has served as a consultant for Amgen, Sanofi, Esperion, Novartis, Ionis Pharmaceuticals, Mylan, Menarini, Recordati and Sankyo.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was funded by Esperion Therapeutics Inc. Medical writing/editorial support for preparation of this article was provided to JB Ashtin by Esperion Therapeutics Inc.
References
- 1.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016; 316: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 2.Menzin J, Aggarwal J, Boatman B, et al. Ezetimibe use and LDL-C goal achievement: a retrospective database analysis of patients with clinical atherosclerotic cardiovascular disease or probable heterozygous familial hypercholesterolemia. J Manag Care Spec Pharm 2017; 23: 1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arca M, Ansell D, Averna M, et al. Statin utilization and lipid goal attainment in high or very-high cardiovascular risk patients: insights from Italian general practice. Atherosclerosis 2018; 271: 120–127. [DOI] [PubMed] [Google Scholar]
- 4.Ferrieres J, Gorcyca K, Iorga SR, et al. Lipid-lowering therapy and goal achievement in high-risk patients from French general practice. Clin Ther 2018; 40: 1484–1495; e22. [DOI] [PubMed] [Google Scholar]
- 5.Razek O, Cermakova L, Armani H, et al. Attainment of recommended lipid targets in patients with familial hypercholesterolemia: real-world experience with PCSK9 inhibitors. Can J Cardiol 2018; 34: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 6.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016; 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 73: e285–e350. [DOI] [PubMed] [Google Scholar]
- 8.Pinkosky SL, Filippov S, Srivastava RA, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res 2013; 54: 134–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018; 277: 195–203. [DOI] [PubMed] [Google Scholar]
- 10.Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019; 380: 1022–1032. [DOI] [PubMed] [Google Scholar]
- 11.Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019; 8: e011662–e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg AC, Leiter LA, Stroes ESG, et al. Efficacy and safety of bempedoic acid added to maximally tolerated statins in patients with hypercholesterolemia and high cardiovascular risk: the CLEAR Wisdom trial. In: American College of Cardiology Annual Scientific Session, New Orleans, LA, USA, 2019.
- 13.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018; 319: 1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Calvo M, Lisnock J, Bull HG, et al. The target of ezetimibe is Niemann–Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci USA 2005; 102: 8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson PD, MacDougall DE, Newton RS, et al. Treatment with ETC-1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol 2016; 10: 556–567. [DOI] [PubMed] [Google Scholar]
- 16.Genser B, Grammer TB, Stojakovic T, et al. Effect of HMG CoA reductase inhibitors on low-density lipoprotein cholesterol and C-reactive protein: systematic review and meta-analysis. Int J Clin Pharmacol Ther 2008; 46: 497–510. [DOI] [PubMed] [Google Scholar]
- 17.Pearson TA, Ballantyne CM, Veltri E, et al. Pooled analyses of effects on C-reactive protein and low density lipoprotein cholesterol in placebo-controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am J Cardiol 2009; 103: 369–374. [DOI] [PubMed] [Google Scholar]
- 18.Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012; 223: 251–261. [DOI] [PubMed] [Google Scholar]
- 19.Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol targets in high-risk patients: the MERCURY II (Measuring Effective Reductions in Cholesterol Using Rosuvastatin) trial. J Am Coll Cardiol 2008; 52: 626–632. [DOI] [PubMed] [Google Scholar]
- 20.Ballantyne CM, Pitt B, Loscalzo J, et al. Alteration of relation of atherogenic lipoprotein cholesterol to apolipoprotein B by intensive statin therapy in patients with acute coronary syndrome (from the Limiting UNdertreatment of lipids in ACS With Rosuvastatin [LUNAR] Trial). Am J Cardiol 2013; 111: 506–509. [DOI] [PubMed] [Google Scholar]
- 21.Farnier M, Guyton JR, Jensen E, et al. Effects of ezetimibe, simvastatin and ezetimibe/simvastatin on correlations between apolipoprotein B, LDL cholesterol and non-HDL cholesterol in patients with primary hypercholesterolemia. Atherosclerosis 2013; 229: 415–422. [DOI] [PubMed] [Google Scholar]
- 22.Ferrieres J, De Ferrari GM, Hermans MP, et al. Predictors of LDL-cholesterol target value attainment differ in acute and chronic coronary heart disease patients: results from DYSIS II Europe. Eur J Prev Cardiol 2018; 25: 1966–1976. [DOI] [PubMed] [Google Scholar]
- 23.Danchin N, Almahmeed W, Al-Rasadi K, et al. Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: the International ChoLesterol management Practice Study (ICLPS). Eur J Prev Cardiol 2018; 25: 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinkosky SL, Newton RS, Day EA, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun 2016; 7: 13457–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ference BA, Ray KK, Catapano AL, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med 2019; 380: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy by Christie M Ballantyne, Ulrich Laufs, Kausik K Ray, Lawrence A Leiter, Harold E Bays, Anne C Goldberg, Erik SG Stroes, Diane MacDougall, Xin Zhao and Alberico L Catapano in European Journal of Preventive Cardiology