Abstract
Sialic acids are important molecule with high structural diversity. They are known to occur in higher animals such as Echinoderms, Hemichordata, Cephalochorda, and Vertebrata and also in other animals such as Platyhelminthes, Cephalopoda, and Crustaceae. Plants are known to lack sialic acid. But they are reported to occur in viruses, bacteria, protozoa, and fungi. Deaminated neuraminic acid although occurs in vertebrates and bacteria, is reported to occur in abundance in the lower vertebrates. Sialic acids are mostly located in terminal ends of glycoproteins and glycolipids, capsular and tissue polysialic acids, bacterial lipooligosaccharides/polysaccharides, and in different forms that dictate their role in biology. Sialic acid play important roles in human physiology of cell-cell interaction, communication, cell-cell signaling, carbohydrate-protein interactions, cellular aggregation, development processes, immune reactions, reproduction, and in neurobiology and human diseases in enabling the infection process by bacteria and virus, tumor growth and metastasis, microbiome biology, and pathology. It enables molecular mimicry in pathogens that allows them to escape host immune responses. Recently sialic acid has found role in therapeutics. In this chapter we have highlighted the (i) diversity of sialic acid, (ii) their occurrence in the diverse life forms, (iii) sialylation and disease, and (iv) sialic acid and therapeutics.
Keywords: Theratope, Glycomimetics, Molecular mimicry, Kdn, Neu5Gc, Neu 5Ac, TACA, Globo H, Gangliosides
1. Introduction
Biomolecules including monosaccharides of carbohydrates, amino acids of proteins, fatty acid of lipids, and nucleic acids including DNA and RNA play a significant role in the growth, development, and proper function of the body. Although proteins, nucleic acids, lipids, and small molecules form the major constituents of human cell, the last decade has evidenced considerable progress in the study of glycans on human cells and their role in cell-cell interaction, signaling, host-pathogen interaction, and carbohydrates contributing to important biological functions in cells. Attached to lipids and proteins, carbohydrates comprise glycoproteins and glycolipids, respectively, and play diverse myriads of roles in development, signaling, host-parasite interaction, and immune system in different organisms [1], [2], [3], [4], [5]. The study of sialylation in bacteria is a relatively new domain which is of immense importance in understanding and targeting host-parasite interaction in infectious diseases.
Plants and insects are being exploited for the production of human recombinant glycoconjugated proteins of therapeutic importance and therefore construction of recombinant organisms capable of synthesis of glycosylated proteins as therapeutic agents in humans holds importance.
Altered glycosylation and sialylation have been associated with several diseases in humans including cancer and finds importance in disease targeting. Although with the advent of new technology, it has become possible to study the complex glycans and decipher their biological role in health and disease in greater details [1], [2], [3], [4], [5], [6], a lot remains unknown.
Sialic acids or N-acetylneuraminic acids (Neu5Ac) are a diverse group of 9‑carbon carboxylated monosaccharides synthesized in animals, present at the outermost end of N-linked and O-linked carbohydrate chains and in lipid-associated glycoconjugates (Fig. 1 , 1–6) and lack in plants [6]. Some bacterial species can de novo synthesize sialic acids while some can acquire sialic acid from host and therefore finds relevance from the point of view of host-parasite interactions revealing evolutionary relationship [7], [8]. Sialic acid-like saccharides termed as legionaminic acid have been reported to occur in Archaea [9].
Fig. 1.

The family of naturally occurring sialic acid.
(Image adapted with permission from Schauer R., Srinivasan G.V., Wipfler D., Kniep B., Schwartz-Albiez R. O-acetylated sialic acids and their role in immune defense. In: Wu A. (editor) The molecular immunology of complex carbohydrates-3. Advances in experimental medicine and biology, vol 705. 2011, Springer, Boston, MA.)
The negative charge and hydrophilic properties of sialic acid enable its role in different normal and pathological processes, acting as binding sites for various pathogens and toxins wherein pathogen-binding protein recognizes sialic acids present in specific linkages which are thought to have evolved in vertebrates through evolution. Molecular mimicry exhibited by pathogens, by decorating with sialic acids, has been known to enable them to evade the host immune system [1], [2], [3], [4], [5], [6], [10], [11], [12].
Sialic acid content of the human brain is the highest among other organisms, and may be associated with evolutionary advancement of an organism [1], [2], [3], [4], [5], [6], [13], [14], [15], [16], [17], [18]. In Neu5Gc or N-glycolylneuraminic acid, the terminal sialic acid residue is linked by the hydroxy group of the glycolic acid unit, synthesized from Neu5Ac catalyzed by CMP-N-acetylneuraminic acid (CMP-Neu5Ac) hydroxylase (CMAH) in animals including lower animals [19], [20], [21], [22]. Nue5Gc is absent in humans as they lack CMAH gene. Oxygen and reduced pyridine nucleotide play vital roles in enzyme activity together with an effective cofactor NADH and the substrate CMPNeu5Ac, and are activated by cytochrome b5. Neu5Gc is expressed in extraneural tissues but very low, absent, or reveal suppressed expression in the vertebrate brain [21].
2. Occurrence and function
Glycoconjugates are constituents of outer surface of animal cells, and their carbohydrate structures change dramatically during development. They are expressed characteristically in different stages of differentiation and are recognized by specific antibodies. In the mature organism, the expression of distinct carbohydrates is eventually restricted to specific cell types. Aberrant expression of cell surface carbohydrates in human is very often associated with malignant transformation.
Sialic acids are found in glycoconjugates of some bacteria, virus, protozoa, and fungi and in animals of the deuterostome lineage [3] constituting glycoproteins and glycolipid-like gangliosides and glycosaminoglycan in mammals and lower vertebrates [3], [5], [23], acting as ligands or receptors for cell-cell communication or host-parasite interaction (1–6, Fig. 1, Fig. 2, Fig. 3 ). Sialic acid has been reported to occur in Drosophila melanogaster (D. melanogaster) and other insects [24], [25]. N-acetylneuraminic acid is reported to occur in cicada of Philaenus spumarius (P. spumarius) [26]. Sialic acid has been reported to occur in mollusca Arion lusitanicus (A. lusitanicus) and Arion rufus (A. rufus) [27]. Sialoglycoconjugates deuterostome lineage of the echinoderms including starfish and sea urchin is an indicative of an origin of > 500 million years ago compared to higher mammals [28]. Sialic acid content in insects and molluscan gastropods is low [28], [29], [30] and lack in plants [29].
Fig. 2.

Synthesis of sialoglycoconjugate. Sialicacid (Sia, purple diamond) is synthesized from Glc through UDP-GlcNAc as a key intermediate for sialic acid metabolism. UDP-GlcNAc is changed to ManNAc 6-phosphate (ManNAc-6-P) by UDP-GlcNAc 2-epimerase/ManNAc kinase. Sia-9-phosphate synthetase then condenses ManNAc-6-P with phosphoenolpyruvate (PEP) to give Sia-9-P. After dephosphorylation by Sia-9-phosphate phosphatase, Sia is activated to CMP-Sia by CMP-Sia synthetase (CMAS) in the nucleus. CMP-Sia is transported into the Golgi apparatus and many sialyltransferases (STs), such as ST3, ST6, and ST8, transfer Sia residues onto the glycoproteins and glycolipids forming sialoglycoproteins and sialoglycolipids (gangliosides).
(Adapted with permission from Sato C, Hane M, Kitajima K. Relationship between ST8SIA2, polysialic acid and its binding molecules, and psychiatric disorders. A. Biosynthetic pathways of sialoglycoconjugates, Biochim Biophys Acta 2016,1860:1739-1752.)
Fig. 3.

Biological and pathological roles of sialic acids. The negative charge and hydrophilicity enable sialic acids to confer neural plasticity, glomerular filtration, or blood cell charge repulsion. They act as binding sites for pathogens and toxins, wherein a pathogen-binding protein or extrinsic receptor recognizes sialic acid forms in specific linkages to a defined underlying sugar chain. They also act as ligands for intrinsic receptors such as Siglecs and factor H. Sialic acids enable ‘molecular mimicry,’ by which microbial pathogens incorporate host sialic acids, thereby escaping the host immune responses. Abbreviations: L1CAM, L1 cell adhesion molecule; PILR, paired immunoglobulin-like receptor.
(Adapted with permission from Ajit Varki Sialic acids in human health and disease Trends Mol Med. 2008; 14(8): 351–360.)
In mammalian cells, the most common sialic acids are Neu5Ac and Neu5Gc but Neu5Gc is completely absent in normal human cells [30]. Gangliosides are sialylated glycolipids that act as receptors for pathogenic bacterial infection on the gut epithelial cells [3].
Acetylated and sulfated sialic acids act as receptors for viral infections while methylated sialic acids are not found to act as receptors.
2.1. Functions of sialic acid
Sialic acids affect the structure and function of glycoconjugates and act as ligands for lectins, antibodies, and enzymes. They mediate cell-cell recognition, communication, aggregation, development, carbohydrate-protein interactions, controlling the lifetimes of glycoconjugates in organisms, mediating bacterial and viral infections, tumor growth and metastasis, with role in immunology, microbiome, cell signaling, reproduction, and biology of nervous system (Fig. 3).
They play a vital role in RBC stabilization and in preventing blood component aggregation by their negative charge and hydrophilicity. Sialic acids are known to affect the stability and function of hormones and enzymes. They play a significant role in reproduction, development, and sialylation of follicle-stimulating hormone (FSH) and human chorionic gonadotropin (hCG), and contribute to their stability and function [1], [31], [32], [33].
They function as ligands for glycan-binding proteins, including animal lectins like selectins and siglecs, viral lectins like hemagglutinins (HAs), and bacterial lectins like adhesins and toxins. Sialosides interaction with lectins regulates immune response, immune cell functions, and cell growth and survival. Human influenza A viral HA preferentially binds to α2,6-linked sialic acid and avian viral HA preferentially binds to α2,3-linked sialic acid on cell surface glycoproteins thus enabling viral attachment and entry. Thus sialic acid-binding specificity of HA determines viral tropism and host specificity [34].
Bacterial lectins, from Helicobacter pylori (H. pylori) and Mycobacterium tuberculosis (M. tuberculosis) adhesins or Cholera and Tetanus toxins, can interact with host cells by their sialic acid-containing ligands during infection [35].
Sialic acids are known to play a significant role in the development of the central and peripheral nervous system by controlling neuronal cells function [23], [36]. Sialylated glycoconjugates like Lewis antigens interact with selectins, affect cell adhesion, lymphocyte homing, leukocyte migration to inflamed sites, observed in inflammation, angiogenesis, metastasis, thrombosis, and cancer [37], [38], [39]. Host cell’s sialic acids are used by microbial pathogens Trypanosoma cruzi (T. cruzi) to mask their antigenic sites and to prevent recognition and elimination by host immune cells [40], [41].
3. Structure diversity of sialic acids
Modifications of sialic acids include diverse forms differing in position 5 of an amino group of neuraminic acid derivatives or an hydroxyl group of 3-deoxy-D-glycero-D-galactononulosonic acid (Kdn), different acylations of the NH2 at position 5 (glycolyl, acetyl), and various substituent of the different hydroxyl groups including phosphate, sulfate, methyl, acetyl, etc. [3]. In the still growing family of sialic acids, > 50 different derivatives [3], [5], [42] (Table 1 ) have been reported of which the two most commonly expressed members of sialic acid family are Neu5Ac and Neu5Gc followed by KDN (2-keto-3-deoxy-nononic acid) and Neu (neuraminic acid) [43].
Table 1.
Naturally occurring sialic acid family members [5], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [83], [84], [85], [86], [87], [88]
| Name | Abbreviation |
|---|---|
| 5-N-Acetyl-4,9-di-O-acccyl-neuraminic acid 1,7-lactone | Neu4,5,9Ac3 1,7lactone |
| 1-Tauryl 5-N-acetyl-neuraminic amide | Neu5Ac1Tau |
| 5-N-Glycolyl-neuraminic acid | Neu5Gc |
| 4-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu4Ac5Gc |
| 7-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu7Ac5Gc |
| 8-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu8Ac5Gc |
| 9-O-Acetyl-5-N-glycolyl-neuraminic acid | Neu9Ac5Gc |
| 4,7-Di-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,7Ac25Gc |
| 4,9-Di-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,9Ac25Gc |
| 7,9-Di-O-acetyl-5-N-glycolyl-neuraminic acidk | Neu7,9Ac25Gc |
| 8,9-Di-O-acetyl-5-N-glycolyl-neuraminic acid | Neu8,9Ac25Gc |
| 4,7,9-Tri-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,7,9Ac35Gc |
| 7,8,9-Tri-O-acetyl-5-N- glycolyl-neuraminic acid | Neu7,8,9Ac35Gc |
| 4,7,8,9-Tetra-O-acetyl-5-N-glycolyl-neuraminic acid | Neu4,7,8,9Ac45Gc |
| 5-N-Glycolyl-9-O-lactyl-neuraminic acid | Neu5Gc9Lt |
| 4-O-Acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu4Ac5Gc9Lt |
| 7-O-Acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu7Ac5Gc9Lt |
| 8-O-Acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu8Ac5Gc9Lt |
| 4,7-Di-O-acetyl-5-N-glycobyl-9-O-lacytl-neuraminic acid | Neu4,7Ac25Gc9Lt |
| 7,8-Di-O-acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu7,8Ac25Gc9Lt |
| 5-N-Glycolyl-8-O-methyl-neuraminic acid1 | Neu5Gc8Me |
| 4-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu4Ac5Gc8Me |
| 7-O-Acetyl-5-N- glycolyl-8-O-methyl-neuraminic acid | Neu7Ac5Gc8Me |
| 9-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu9Ac5Gc8Me |
| 4,7-Di-O-aceryl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu4,7Ac25Gc8Me |
| 7,9-Di-O-acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu7,9Ac25Gc8Me |
| 5-N-Glycolyl-9-O-methyl-neuraminic acid | Neu5Gc9Me |
| 5-N-Glycolyl-8-O-sulfo-neuraminic acid | Neu5Gc8S |
| 5-N-Glycolyl-9-O-sulfo-neuraminic acid | Neu5Gc9S |
| 5-N-(O-Acetyl)glycolyl-neuraminic acid | Neu5GcAc |
| 5-N-(O-Methyl)glycolyl-neuraminic acid | Neu5GcMe |
| 2-Deoxy-2,3-didehydro-5-N-glycolyl-neuraminic acidg | Neu2en5Gc |
| 8-O-Acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu8Ac5Gc9Lt |
| 4,7-Di-O-acetyl-5-N-glycolyl-9-O-lactyl- neuraminic acid | Neu4,7Ac25Gc9Lt |
| 7,8-Di-O-acetyl-5-N-glycolyl-9-O-lactyl-neuraminic acid | Neu7,8Ac25Gc9Lt |
| 5-N-Glycolyl-8-O-methyl-neuraminic acid1 | Neu5Gc8Me |
| 4-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu4Ac5Gc8Me |
| 7-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu7Ac5Gc8Me |
| 9-O-Acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu9Ac5Gc8Me |
| 4,7-Di-O-acetyl-5-N-glycolyl-8-O-methyl-neuraminic acid | Neu 4,7Ac25Gc8Me |
| 7,9-Di-O-acetyl-5-N-glycolyl-8-O-methyl neuraminic acid | Neu7,9Ac25Gc8Me |
| 5-N-Glycolyl-9-O-methyl- neuraminic acid | Neu5Gc9Me |
| 5-N-Glycolyl-8-O-sulfo-neuraminic acid | Neu5Gc8S |
| 5-N-Glycolyl-9-O-sulfo-neuraminic acid | Neu5Gc9S |
| 5-N-(O-Acetyl)glycolyl-neuraminic acid | Neu5GcAc |
| 5-N-(O-Methyl)gIycolyl-neurammic acid | Neu5GcMe |
| 2-Deoxy-2,3-didehydro-5-N-glycolyl-neuraminic acidg | Neu2en5Gc |
| 9-O-Acetyl-2-deoxy-2,3-didehydro-5-N-glycolyl-neuraminic acidg | Neu2en9Ac5Gc |
| 2-Deoxy-2,3-didehydro-5-N-glycolyl-9-O-lactyl-neuraminic acidg | Neu2en5Gc9Lt |
| 2-Deoxy-2,3-didehydro-5-N-glycolyl-8-O-methyl neuraminic acidg | Neu2en5Gc8Me |
| 2,7-Anhydro-5-N-glycolyl-neuraminic acidg | Neu2,7an5Gc |
| 2,7-Anhydro-5-N-glycolyl-8-O-methyl-neuraminic acidg | Neu2,7an5Gc8Me |
| 4,8-Anhydro-5-N-glycolyl-neuraminic acidj | Neu4,8an5Gc |
| 5-N-Glycolyl-neuraminic acid 1,7-lactone | Neu5Gc1,7lactone |
| 9-O-Acetyl-5-N-glycolyl-neuraminic acid 1,7-lactone | Neu9Ac5Gc1,7lactone |
| 7-Acetamido-9-O-acetyl-7-deoxy-5-N-glycolyl- neuraminic acid | Neu9Ac5Gc7NAc |
| 7-Acetamido-8,9-di-O-acetyl-7-deoxy-5-N-glycolyl-neuraminic acidm | Neu8,9Ac25Gc7NAc |
| 2-Keto-3-deoxy-nononic acid | Kdn |
| 5-O-Acetyl-2-keto-3-deoxy-nononic acid | Kdn5Ac |
| 7-O-Acetyl-2-keto-3-deoxy-nonomic acid | Kdn7Ac |
| 8-O-Acetyl-2-keto-3-deoxy-nononic acid | Kdn8Ac |
| 9-O-Acetyl-2-keto-3-deoxy-nononic acid | Kdn9Ac |
| 4,5-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn4,5Ac2 |
| 4,7-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn4,7Ac2 |
| 5,9-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn5,9Ac2 |
| 7,9-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn7,9Ac2 |
| 8,9-Di-O-acetyl-2-keto-3-deoxy-nononic acid | Kdn8,9Ac2 |
| 2-Keto-3-deoxy-5-O-methyl-nononic acid | Kdn5Me |
| 2-Keto-3-deoxcy-9-O-phospho-nononic acidg,n | Kdn9P |
Adapted with permission from Schauer R, Kamerling JP. Exploration of sialic acid world Chapter 1, Advances in carbohydrate chemistry and biochemistry, vol. 75, 2018, Elsevier.
Modifications to core structures of sialic acid by O-acetylation, O-methylation, or introduction of O-lactyl groups, sulfate, or phosphate esters at positions 4, 7, 8, and/or 9, generated by enzymes, have been reported. O-acetyltransferases catalyze the synthesis of O-acetylated sialic acid derivatives.
Sialic acids can be O-acetylated at positions C-4, C-7, C-8, and C-9 of the hydroxyl groups (Table 1). The monosaccharide and its linkage to other sugars in three main configurations such as α-2,3, α-2,6, and α-2,8 contribute to the diversity. Although mono-O-acetylated forms are predominant, combinations of acetyl groups at two or more positions generate oligo-O-acetylated derivatives. O-acetylation at positions C-7, C-8, and C-9 forming N-acetyl-7, 8, and 9-O-acetyl sialic acid (O-AcSA) are most common. 9-O-acetyl sialic acid (9-O-AcSA) and 4-O-acetylated sialic acid (4 O-AcSA) function as ligands for the agglutinin of influenza virus C [78] and murine hepatitis S virus [81], respectively.
3.1. O-acetylated sialic acid
Eukaryotic sialic acid anabolism is a complex enzymatic process in the cytosol leading to the activation of free Neu5Ac by CTP in the nucleus. The resulting CMP-Neu5Ac and CMP-Neu5Gc (synthesized by a cytosolic CMAH) serve as donor substrates in various acceptor-substrate-specific ST reactions in the Golgi. Finally, sialate-O-acetylation occurs at the α-glycosidically linked sialic acid (Fig. 4 ) [82].
Fig. 4.
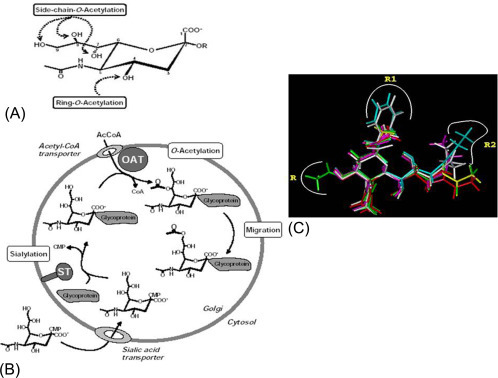
(A) Naturally occurring O-acetylated sialic acids. Sialic acid O-acetylation can take place at the positions C-4, C-7, C-8, and C-9. They can be mono-O-acetylated, oligo-O-acetylated, combined acetylation at C-7 and C-9 leading to di-O-acetylated Neu5,7,9Ac3 form. (B) Model of side-chain O-acetylation of sialic acids. Sialic acids are transported in their CMP-activated form into the Golgi serving as substrates for different sialyltransferase (ST). Acetyl-CoA (AcCoA) enters the Golgi by an AcCoA-transporter and provide it for the O-acetyltransferase (OAT), which transfers the acetyl moiety to the glycosidically bound sialic acid probably at position C-7. After a migration to position C-9, which could be enzymatically catalyzed, an additional O-acetylation reaction can take place at position C-7. (C) Disease-specific glycotope Neu5,9Ac2-GPs. expressed on PBMCALL [PBMC of childhood acute lymphoblastic leukemia (ALL)]. Superimposed three-dimensional (3D) structures of all the sialic acid derivatives which were used as inhibitors of the binding of Neu5,9Ac2-GPsALL to Achatinin-H.
(Source: (B) Reproduced from Schauer R, Schmid H, Pommerencke J, Iwersen M, Kohla G. Metabolism and role of O-acetylated sialic acids. Adv Exp Med Biol 2001;491:325-42 with permission. (C) Adapted with permission from Ghosh S, Bandyopadhyay S, Mukherjee K, Mallick A, Pal S, Mandal C, Bhattacharya DK, O-acetylation of sialic acids is required for the survival of lymphoblasts in childhood acute lymphoblastic leukemia (ALL). Glycoconj J 2007, 24:17-24.)
3.1.1. Enzymes in O-acetylated sialic acid metabolism
The metabolism of O-AcSA can be divided into two parts.
-
(i)
It is initiated by the formation of acetyl esters at different hydroxyl groups of sialic acids by the transfer of activated acetyl groups from acetyl coenzyme A (AcCoA) by O-acetyltransferases (Fig. 4). Differing in their regioselectivity, two groups of sialate-O acetyltransferases exist: (a) acetyl-CoA:sialate 7 [9]-O-acetyltransferase responsible for the frequently found O-acetylation side chain and (b) acetyl-CoA:sialate 4-O-acetyltransferase which leads to the pyranose ring-O-acetylation at C4.
-
(ii)
Several enzymes degrade free or glycosidically bound O-AcSA acids such as esterases for Neu5,9Ac2 and Neu4,5Ac2, respectively, which are found in influenza C virus, lysosomes of mammalian cells, mouse hepatitis virus, and in horse liver.
3.1.2. Functions of O-acetylated sialic acids
In the past three decades, O-acetylated sialic acids (O-AcSA) have been shown as important cell membrane components that play fundamental roles in the development, immune regulation, cancer processes, and many other biological and pathophysiological events [17], [82], [84]. The biological effect depends on the position relative to the 9‑carbon scaffold in Neu5Ac or Neu5Gc. The O-acetyl group being a more hydrophobic moiety when introduced into the sialic acid molecule the parameters like size, net charge, hydrogen bonding and conformation of glycoconjugate alters and the terminal location enables their involvement in different functions such as cell-cell adhesion, signaling, differentiation and metastasis [85], [86], [87], [88]. The extended nature of oligosaccharide chains, and possibly their negative charges, plays an important role in cell-cell and cell-matrix interactions [86]. O-acetylated sialic acids reported to occur in bacteria and parasites are known to act as receptor determinants for some viruses, well-known cancer markers in childhood acute lymphoblastic leukemia (ALL), and also regulate ganglioside-mediated apoptosis. Sialic acid-specific O-acetyltransferases and O-acetylesterases regulate their synthesis [89].
Modifications of sialic acids exhibit tissue-specific and developmentally regulated expression. O-AcSA occurrence is predominant in growing and developing tissues of neuroectodermal origin and in B and T lymphocytes [90], 9-O-Ac-GD3 distribution on the normal tissues is largely restricted to brain [91] while 9-O-acetylsialylated GT3 ganglioside [92] expression is developmentally regulated in rat embryonic cerebral cortex. Sialic acids acetylation is selectively developmentally regulated in the central and peripheral nervous system, the retina, and the medulla of kidney [93] in rat tissue.
O-acetylated GD3 on human lymphocytes [94] is reported as a marker for human CD8 + T helper cells [95]. Sialyl-Le x and Sialyl-Le a ligands for selectins are known to play a major role in the early steps of leukocyte rolling on endothelial cells [96]. 9-O-acetylations on immune cells have been reported to regulate CD22 β adhesion negatively through masking of the carbohydrate epitope. 9-O-acteyl sialoglycoconjugates (9-O-Ac-SGs) on erythrocytes in visceral leishmaniasis (VL) serve as diagnostic and prognostic markers [97], [98] and are known to activate the alternate complement pathway enhancing hemolysis accounting for anemia [99]. Sialic acids on Leishmania donovani (L. donovani) amastigotes and two 9-O-AcSGs with Mw 158,000 and 150,000 have been identified as components of amastigote cell surface [99], [100], [101]. O-acetyl GD3 is not expressed on human normal tissues [102]. 9-O-Ac-GD3 is considered as an oncofetal marker in animal and human tumors like neuronal tumors, melanoma, basalioma or breast cancer (BC), and in psoriatic lesions [88].
9-O-acetylated sialic acid and glycoconjugates have been reported as diagnostic and prognostic markers in childhood ALL [83], [103], [104], [105], [106], [107]. 9-O-acetylations have been reported to be upregulated in basal cell carcinoma tissues than in the surrounding skin [108].
O-acetylation of disialoganglioside GD3 by human melanoma cells has been reported to be a unique antigenic determinant [109]. BC cells have recently been reported to express b-series gangliosides GD3 and GD2, and O-acetylated GD2 (O-AcGD2) in which 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) is predominant O-acetylated sialic acid species of GD2 [110].
Two disease-specific 9-O-Ac-SGs, of molecular weight 90 and 120 kDa, have been reported on PBMC of patients from childhood acute lymphoblastic leukemia (PBMCALL) [103], [111] with diagnostic and prognostic importance [103], [111], [112], [113], [114] and biological function [83], [103], [104], [105], [106], [107], which have been demonstrated by Achatinin-H, a lectin isolated from Achatina fulica (A. fulica) snail, with 9-O-AcSAα2–6GalNAc2 as its lectinogenic epitope. 9-O-AcSA-specific IgM and IgG antibodies have been reported in ALL patients and finds importance in the detection and monitoring of patients [115], [116].
3.2. N-acetylation of sialic acids
The N-acetyl group of Neu5Ac at fifth position is known to originate from AcCoA [117], [118], [119], [120] (Fig. 5 ) during conversion of GIcNH2-6-P to GlcNAc-6-P, the precursor of UDP-GlcNAc. This then converts to ManNAc by epomerization reaction, and finally to CMP-Neu5Ac [117], [118], [119], [121]. Neu5Ac is transferred to macromolecules from the nucleotide sugar, which can later be released into the lysosomes, and exported into the cytosol [122], [123]. The N′-acetyl group can be converted to N′-glycolyl group by a specific hydroxylase [124].
Fig. 5.

Steps in N-acetylation of sialic acid.
(Adapted from Varki A. Diversity in the sialic acids. Glycobiology, 1992, 2:25–40 with permission.)
3.3. Other modifications of sialic acids
Unsaturated sialic acids occur in nature or biological fluids generated by enzymes or chemical processes such as 2,7-anhydro sialic acids released by sialidases [125], [126], [127], [128], [129], 2,3-didehydro 2,6-anhydro from mild CMP-sialic acids [130], and 4,8-anhydro compounds from release or deacetylation of 4–0-acetylated compounds [131], [132]. The phosphate group of Neu5Ac9P emerges from ManNAc-6-P.
-
(i)
Deaminated neuraminic acid (KDN) although reported to occur in vertebrates and bacteria, is predominantly expressed in lower vertebrates. KDN is linked to most glycan structures in place of Neu5Ac and is found to occur as glycoconjugates, including glycolipids, glycoproteins, and capsular polysaccharides. They exhibit linage types such as α2,3, α2,4, α2,6, and α2,8 bearing similarity to Neu5Ac. KDN de novo biosynthesis involves mannose as a precursor, activated to CMP-KDN and transferred to acceptor sugar residues. Predominant KDN expression has been reported to occur in fetal human red blood cells and ovarian tumor tissues as compared to adult RBC and normal individuals. KDNase, which cleaves KDN linkages, is reported to occur in bacteria [133]. KDN could arise from sequential deacetylation and deamination of Neu5Ac.
C8 acidic sugar 3-deoxy-d-manno-2-octulosonic acid (Kdo) is reported to occur in Gram-negative bacteria lipopolysaccharide (LPS) and plant cell pectic rhamnogalacturonan II. In the light of recent discoveries although hitherto unknown, sialic acid expression in algae Kdo has been reported. de novo biosynthesis of the deaminated sialic acid, 3-deoxy- d-glycero-d-galacto-2-nonulosonic acid (Kdn), has been reported to occur in Prymnesium parvum (P. parvum) with probable indications of role in host pathogen [134].
-
(ii)
Other types of substitutions of the hydroxyl groups arise from use of the appropriate donors such as S-adenosylmethionine for methylated sialic acids and 3′-phosphoadenosine 5′-phosphosulphate for sulfated molecules. However, not many studies have been done on other modifications like O-lactyl groups.
Sialic acid family has now been designated as subclass of the superfamily of naturally occurring non-2-ulosonic acids (NulOs), the parent molecule of the family being neuraminic acid (Neu), which is not found in free form in nature due to its immediate cyclization to form an internal Schiff base, and is a nine‑carbon-containing monosaccharide, comprising 2-keto-carboxylic acid, a deoxysugar, and an aminosugar. Other members of the NulO superfamily include 5,7-diamino-3,5,7,9-tetradeoxynon-2-ulosonic acids, with the mother molecules pseudaminic acid (Pse), legionaminic acid (Leg), 4-epi-legionaminic acid (4eLeg), 8-epilegionaminic acid (8eLeg), acinetaminic acid (Aci), and 8-epi-acinetaminic acid (8eAci), found in bacterial polysaccharides and glycoproteins [5] (Fig. 6 ).
Fig. 6.
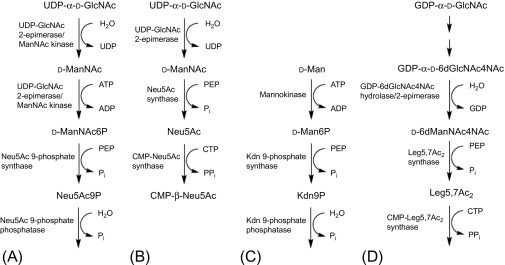
Metabolism of non-ulosonic acids: (A) Neu5Ac in vertebrates, (B) Neu5Ac in bacteria, (C) KDn in vertebrates, and (D) Leg 57Ac2 in bacteria.
(Adapted with permission from Schauer R and Kamerling JP Exploration of sialic acid world Chapter 1, Advances in carbohydrate chemistry and biochemistry, vol. 75, 2018, Elsevier.)
4. Sialic acid and the living world
4.1. Sialic acid and bacteria
Glycoconjugates occur on both prokaryotic and eukaryotic cell surfaces with important biological functions like cell-cell and small molecule-cell recognition and communication. Sialic acids are predominantly found on eukaryotic cell surfaces. Pathogens (Table 2 ) reveal property of acquiring host sialic acid on cell surfaces, thereby mimicking the host and escaping the host immune responses.
Table 2.
Pathogens expressing sialic acids on their surfaces
| Pathogen | Major disease |
|---|---|
| Sialic acid synthesized by pathogen | |
| Neisseria meningitidis B | Meningitis |
| Escherichia coli K1 | Neonatal meningitis |
| Group B Streptococcus | Neonate and infant infections |
| Campylobacter jejuni | Enteritis, Guillian-Barré syndrome |
| Host sialic acid taken up by pathogen | |
| Hemophilus influenza | Respirator infections |
| Hemophilus ducreyi | Chancroid |
| Host sialic acid transferred by trans-sialidase | |
| Trypanosoma cruzi | Chagas disease |
| Corynebacterium diphtheria | Diphtheria |
| Host CMP-sialic acid used by sialyltransferase | |
| Neisseria gonorrhoea | Gonorrhoea |
| Neisseria meningitides group A | Meningitis |
| Source of sialic acid un known | |
| Sporotrichium schenkii | Skin infection |
| Aspergillus fumigates | Opportunistic infections |
Adapted with permission from Ajit Varki Sialic acids in human health and disease Trends Mol Med. 2008; 14(8): 351–360.
Neu5Ac and its structural variants such as substitutions at carbon 5, or covalent modifications of the hydroxyl groups in sugars find importance in this context. Bacteria can either express sialic acid by de novo biosynthesis or acquire sialic acid from their environment and transport in the cell surface using transporters, thereby leading to the formation of sialic acid-acquired surfaces that can affect the host-parasite interaction. Bacteria can process them by common pathways for sialic acid metabolism and use sialic acid in different roles such colonization owing to its disease causing property.
De novo synthesis of sialic acid is reported in Escherichia coli (E. coli) K1, Neisseria meningitidis (N. meningitidis), and Campylobacter jejuni (C. jejuni) [8] wherein UDP-GlcNAc acts as sialic acid biosynthesis precursor and finds importance in cell wall biosynthesis, and NeuC and NeuB proteins enable Neu5Ac by ManNAc conversion (Fig. 7, Fig. 8 ).
Fig. 7.

Overview of the major pathways for sialic acid utilization in bacterial pathogens. Sialic acid/Neu5Ac. Black arrow: The site of interaction of factor H (fH) on the gonococcal cell surface. Monomeric O-acetylated Neu5Ac produced by E. coli NeuD is believed to enter the normal PSA biosynthetic pathway via NeuA/NeuS [164]; light-green discontinuous arrow: LPS is exposed on the outer membrane. Asterisk: O-acetylation of the disialylated LPS of C. jejuni; catalyzed by the product of the gene orf11, or sialic acid O-acetyltransferase (SOAT) [157]. IM, inner membrane; OM, outer membrane; Neu5Ac, N-acetylneuraminic or sialic acid; ManNAc, N-acetylmannosamine; GlcNAc, N-acetylglucosamine; GlcN, glucosamine; Fru, fructose; PSA, polysialic acid; PEP, phosphoenolpyruvate; Lst, Neisseria LPS sialyltransferase; NanC, E. coli Neu5Ac-specific porin; Kps, E. coli PSA capsule export system; SatABCD, H. ducreyi Neu5Ac ABC (ATP-binding cassette) transporter; SiaPQM, H. influenzae/P. multocida Neu5Ac TRAP (tripartite ATP-independent periplasmic) transporter; NanT, E. coli Neu5Ac MFS (major facilitator superfamily) transporter; SiaB and NeuA, respectively H. influenzaem and E. coli CMP-Neu5Ac synthetases; Lic3A, Lic3B: H. influenza sialyltransferases; SOAT, C. jejuni Neu5Ac O-acetyltransferase; NeuC, E. coli UDP-GlcNAc 2-epimerase; NeuB, E. coli Neu5Ac synthase; NeuS, E. coli polysialyltransferase; NeuO, E. coli PSA O-acetyltransferase; NeuD, E. coli Neu5Ac O-acetyltransferase; NanA, Neu5Ac aldolase; NanK, ManNAc kinase; NanE, ManNAc-6P epimerase; NagB, GlcNAc-6P deacetylase; and NagA, GlcN-6P deaminase (the catabolic pathway is present in several bacteria: 8).
(Image Reproduced with permission from Severi E et al. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153(Pt. 9):2817-22.)
Fig. 8.
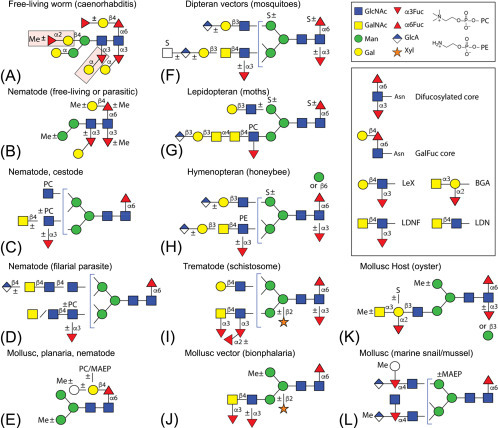
Example of N-glycans from invertebrates including parasitic or free-living organisms, hosts, or vectors for parasites. A non-exhaustive selection of core and antennal epitopes is shown in the inset: core difucosylation, core ‘GalFuc,’ Lewis X (LeX), fucosylated and non-fucosylated LacdiNAc (LDN), and blood group A.
(Image adapted with permission from Paschinger K, Wilson IBH. Comparisons of N-glycans across invertebrate phyla. Parasitology 2019, 3:1-10.)
Some bacterial pathogens acquiring sialic acid from host secrete sialidase that releases sialic acid from host [135]; but Haemophilus influenzae (H. influenzae), lacking sialidase genes [136], acquires free host sialic acid by other sialidase-expressing bacteria in the host [137], or by the action of host sialidases [138], [139], activated during disease/inflammation. Bacteria use specific transporters to take in this free sialic acid including NanT sialic acid transporter from E. coli K-12 [8] that transports Neu5Ac uptake [8]. H. influenza and Pasteurella multocida (P. multocida) use high affinity tripartite ATP-independent periplasmic (TRAP) transporter, SiaPQM [140], [141], [142] and extracytoplasmic solute receptor (ESR) protein for transport; Haemophilus ducreyi (H. ducreyi, SatABCD), causing chancroid, uses a high affinity ABC transporter [143] for the transport of sialic acid.
Neisseria gonorrhoeae (N. gonorrhoeae) uptakes activated form of sialic acid CMP-Neu5Ac by secreting the enzymes that sialylates its LPS contributing to virulence factor.
Neu5Ac-inducible porin NanC (YjhA) from E. coli K-12 has recently been studied for playing a role in growth on Neu5Ac even though lacks both OmpC and OmpF porins [144]. H. influenza and E. coli can also utilize the transported sialic acid as a carbon and nitrogen source [8], [156], the N-acetylneuraminate aldolase NanA cleaves Neu5Ac to ManNAc and pyruvate (Fig. 7). ManNAc is later converted to fructose 6-phosphate and ammonia by NanK, NanE, NagB, and NagA proteins for further metabolism [8].
After either de novo synthesis or acquiring from host, sialic acid is converted to activated form CMP-Neu5Ac by CMP-sialic acid synthetases, while linkage-specific sialyltransferases enable their addition to appropriate acceptors. N. gonorrhoeae uses outer membrane-associated sialyltransferase to scavenge CMP-Neu5Ac directly from host [145].
In E. coli, NeuA activates Neu5Ac before incorporation into the K1 and K92 capsules while the N. meningitidis ortholog enables both capsule and LPS synthesis. In E. coli, NeuS acts as polysialyltransferase adding Neu5Ac to oligosialic acid receptors to form the polysialic acid (PSA) polysaccharide capsule, exported through the Kps system [8]. After synthesis sialic acid in the PSA capsule of both N. meningitidis and E. coli can be modified by O-acetylation [146], [147], [148] like O-acetyltransferases NeuO and NeuD in E. coli can modify PSA and monomeric Neu5Ac, respectively, the latter can be deacetylated by NeuA acting as a bifunctional enzyme [148], [149], [150].
Streptococcus agalactiae (S agalactiae) is the only Gram-positive bacteria causing serious infections in newborns, and can produce sialic acid-containing capsule by using sialyltransferase (CpsK) adding terminal α-2,3-linked Neu5Ac to galactose within the capsule’s oligosaccharide repeat [151]. Neu5Ac can be modified by O-acetylation [152].
Sialylation of the LPS is mediated by linkage-specific sialyltransferases. Both N. meningitidis and N. gonorrhoeae sialylate their LPS by α-2,3 sialyltransferase L. LPS sialylation is reported to occur in Pasteurellaceae, including H. ducreyi, H. influenzae, Haemophilus somnus (H. somnus), and P. multocida [153]. Lic3A, sialyltransferase adding α-2,3-Neu5Ac [154], is associated with bacterial survival [155]. Lic3B can add mono- or disialic acid to the LPS acceptor [153]. C. jejuni possesses mono- or bifunctional LPS sialyltransferases transferring either α-2,3-Neu5Ac or disialic acid [156]. Terminal sialic acid residue in the disialylated LPS can also be modified by an O-acetyltransferase (Figs. 7) [157] as identified in C. jejuni.
Sialylated LPS and PSA capsules confer protection to the bacteria to escape host immune responses by ‘molecular mimicry’ [158] as was observed in studies on neisseria and hemophilus bacteria.
Neisseria spp., H. influenza and C. jejuni, reveal reversible on to off switching leading to variable expression and also O-acetylation of the PSA capsule of E. coli K1 [148], [159].
4.1.1. Bacterial sialylation and host immune system
The PSA capsule of N. meningitides serogroup B and E. coli K1 is poorly immunogenic and PSA reveal structural similarities to mammalian neuronal cell adhesion molecule, NCAM [160]. The sialylated capsule of S. agalactiae inhibits phagocytosis, impairs C3 deposition on the cell surface, and prevents complement alternative pathway [161] activation.
LPS sialylation inhibits the complement alternative pathway in both N. gonorrhoeae and non-typable H. influenza (NTHi) [162], [163]. Gonococcal sialylated LPS increases the binding of bacteria to factor H (fH), a complement alternative pathway inhibitor [163], thus conferring protection from C3 attack [163]. In NTHi, LPS sialylation inhibits deposition of C3 without fH binding [162].
4.2. Archaea
N-glycosylation is a posttranslational modification that occurs in all three domains. In Archaea, N-linked glycans reveal diversity which is not observed in either Eukarya or Bacteria with the lack of expression of nonulosonic acids (NulOs), sialic acids, pseudaminic acids, and legionaminic acids, in contrast to Eukarya and Bacteria. In haloarchaea Halorubrum sp. PV6 includes an N-formylated legionaminic acid and a biosynthetic pathway [9].
4.3. Virus
Viral sialic acid-recognizing lectins or HAs can agglutinate RBC. Viruses use sialic acids linked to glycoproteins and gangliosides to attach to host cells, followed by their entry, for example, corona virus, DNA tumor viruses, hepatitis virus, influenza viruses (A, B, and C), mouse polyoma virus, mumps, Newcastle disease virus (NDV), norovirus, parainfluenza viruses, rotavirus, and Sendai virus. HAs from influenza A, C, NDV, and polyoma viruses have been crystallized. Sialic acid-recognizing lectins from adenoviruses and picornaviruses have not been identified.
Some of these viruses carry neuraminidase or sialyl-O-acetyl-esterase that destroys the receptor, promotes virus release from infected cells, and removes sialic acid on host cell affecting cell surface binding of the virus. Influenza A virus enters the host by using host surface sialic acids. Influenza C virus HA-esterase specific for 9-O-acetylated sialic acids can break down 9-O-acetyl ester. HA-esterase from mouse hepatitis virus is specific to sialic acids substituted by O-acetyl group at the C-4 position (Neu4,5Ac2). HA-neuraminidase of NDV84 and parainfluenza viruses perform vital functions in infection biology [6], [165].
4.4. Fungi
Sialic acids have been reported to occur in some pathogenic fungal cells such as Candida Cryptococcus neoformans (C. neoformans), Aspergillus fumigatus (A. fumigatus), and Sporothrix schenckii (S. schenckii). Neu5Ac, Neu5Gc, and Neu5,9Ac2 were reported to have been expressed. It is hypothesized that probably fungi may have unique ways to synthesize sialic acid however they may acquire it from the environment [166], [167], [168], [169].
A. fumigatus pathogenic variety reveals greater sialic acid density as compared to that of non-pathogenic Aspergillus species [170]. Scedosporium apiospermum (S. apiospermum), Scedosporium aurantiacum (S. aurantiacum), Scedosporium minutisporum (S. minutisporum), and Lomentospora prolificans (L. prolificans) reveal lack of sialic acid [171], [172].
A. fumigatus has been known to express a sialidase termed as KDNase that prefers sialic acid substrate, 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid (KDNase) and plays an important role in maintaining cell wall integrity and virulence [173]. Core 1 O-linked glycan-specific lectin, Hericium erinaceus lecin (HeL), has been isolated from the fruiting body of the mushroom Hericium erinaceus (H. erinaceus), which acts as a natural source for a sialic acid-binding lectin (SABL) [174].
A lectin has been reported to occur in Australian indigenous mushroom Psathyrella asperospora (P. asperospora) termed as PAL with cyotoxic properties on human colon cancer HT29 and monkey kidney VERO revealing binding preference toward N-acetylglucosamine (GlcNAc) and sialic acid (Neu5Ac) [175]. Most Fusarium lectins exhibit binding affinity to d-ribose, l-fucose, d-glucose, l-arabinose, d-mannitol, d-galactosamine hydrochloride, d-galacturonic acid, N-acetyl-d-galactosamine, N-acetylneuraminic acid, 2-deoxy-d-ribose, fetuin, asialofetuin, and bovine submaxillary mucin (BSM) [176].
4.5. Plants
Plants lack sialic acid but studies on recombinant plant glycoproteins are being conducted. Neu5Ac however has been reported to occur in buckwheat using mass spectrometry. R-keto acids in plants include 3-deoxy-D-arabinoheptulosonic acid 7-phosphate (DAHP) and Kdo in cell wall polysaccharides. Arabidopsis thaliana (A. thaliana) geneome revealed lack of genes for biosynthesis, activation, or transfer of sialic acid [177], [178], [179].
4.6. Invertebrates
N-glycosylations have been reported to occur in invertebrates (Fig. 8).
Sialic acid is synthesized and expressed by different invertebrates. We discuss in brief the expression of sialic acid in invertebrate animals and its functions.
4.6.1. Protozoa
Parasitic protozoa are known to reveal sialoglycoconjugates which play an important role in their biological function (Table 3 ) and T. cruzi (Fig. 9 ), the causative agent of life-threatening Chagas disease in South America, is known to express sialic acids transferred from the host glycoconjugates to the terminal β-galactopyranosyl residues of mucin-like molecules on parasite surface by using the enzyme trans-sialidase [180]. These sialic acids might play a role in conferring protection from the recognition and response by the host immune system. Sialic acid such as Neu5Ac, Neu5Gc, Neu5,7Ac2, and Neu5,9Ac2 have been reported to occur in Dictyostelium discoideum, a trypanosome Crithidia fasciculata (C. fasciculata), piroplasmid Theileria sergenti (T. sergenti), and amoebae Entamoeba invadens (E. invadens) and Entameoba histolytica (E. histolytica) [6], [181], [182], [183], [184], [185].
Table 3.
Biological role of sialoglycoconjgates in parasitic protozoa [99], [100], [101], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207], [208], [209], [210]
| Parasite | Sialoglycoconjugates | Biological relevance |
|---|---|---|
| Trypanosoma cruzi | Acquisition of siallct acids by mucins | (a) Invasive determinant (b) Modulation of host immune response (c) Protection against cytolytic agents. |
| Trypanosoma bruœl | Acquisition of sialic acids by procyclic repetitive proteins (PARPs). | (a) Invasion of host cells (b) Survival within insect vector. |
| Entamoeba histolytica | Appearance of sialic acid during encystation and gangliosides in trophozoites. | (a) Decreases parasite adherence to target cens thereby reducing its cytolytic activity. |
| Plasmodium falciparum | Absence of Neu5Ac, instead sialic acid-binding protein EBA- 175 is present. | Ligands for EBA-175 are essential for erythrocyte invasion. |
| Trichomonas vaginalis and Trichomonas foetus | Sialic acid-specific lectin identified in parasite culture supernatant | Enhances parasite adhesion to mucosal surfaces |
| Toxoplasma gondii | Uptake of fetuin from the culture medium. | Not known |
| Leishmania donovani | Polyanionic adsorption | (a) Determinant of virulence (b) Complement mediated cell lysis |
Adapted from Chava AK, Bandyopadhyay S, Chatterjee M, Mandal C. Sialoglycans in protozoal diseases: their detection, modes of acquisition and emerging biological roles. Glycoconj J 2004a. 20:199–206 with permission.
Fig. 9.
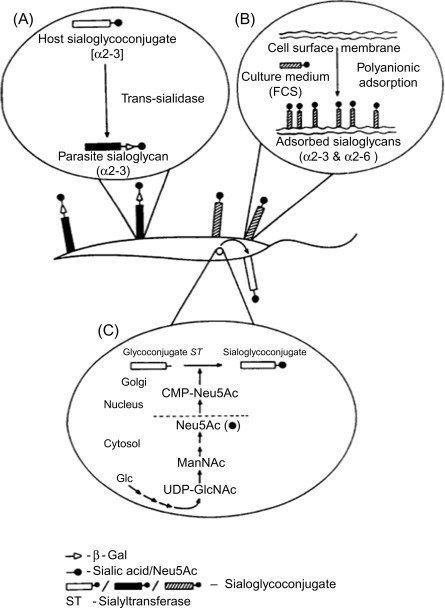
Representative profile of sialic acid acquisition by protozoa: (A) with the help of trans-sialidase in T. cruzi and Trypanosoma brucei (T. brucei), (B) polyanionic adsorption of serum sialoglycans by L. donovani promastigotes, and (C) de novo synthesis of sialic acid in E. histolytica.
(Adapted from Chava AK, Bandyopadhyay S, Chatterjee M, Mandal C. Sialoglycans in protozoal diseases: their detection, modes of acquisition and emerging biological roles. Glycoconj J 2004;20:199–206.)
4.6.2. Cnidarians
Mechanoreceptors in sea-anemone tentacles are activated on binding to acetylated sugars and proline from prey [211] while tentacles of certain sea anemones bind N-acetylneuraminic acid (NANA) predisposing contact-sensitive mechanoreceptors (CSMs) [212], [213], [214], [215] to trigger discharge upon physical contact of prey and cyclic AMP (cAMP) has been reported to be involved in NANA-sensitized nematocyst discharge [212], [216]. NnL lectin from a jellyfish Nemopilema nomurai (N. nomurai) revealed hemagglutinating activity that was inhibited by N-acetyl-D-galactosamine NANA and Neu5Gc [217].
4.6.3. Helminths
Lymphatic filariasis reveals altered IgG glycosylation, and while decreased galactosylation bears relation with inflammation, increased sialylation is associated with anti-inflammatory responses [218]. Mucins of Haemonchus contortus (H. contortus) or Teladorsagia circumcincta (T. circumcincta) parasites in sheep revealed fucose, glucosamine, galactose, and galactosamine and minute amounts of sialic acids [219]. Schistosoma bovis (S. bovis) a parasite of wild and domestic ruminants lack sialic acid expression but expressed complex-type N-glycans and immunogenic GalNAcβ1–4GlcNAc (LDN) terminate antennae on excretory-secretory (ES) glycoproteins [220]. Dogs infected with adult tapeworms of Echinococcus granulosus (E. granulosus) release fecal antigens (coproantigens) constituting α-D-mannose and/or α-d-glucose, β-galactose and N-acetyl-β-glucosamine, N-acetyl-β-glucosamine, and sialic acid residues [221] and antigenic properties occur in cyst-derived glycilipids [222]. Sialic acids were identified in the acidic fraction of glycolipids of E. granulosus metacestode tissue [221]. The altered glycosylation of intestinal mucins of mouse infected with Nippostrongylus brasiliensis (N. brasiliensis) revealed a transferase adding a terminal GalNAc to sialic acid-containing epitope in rat [223]. The sialylation of mucins during a 13-day infectious cycle in Sprague-Dawley rats infected with N. brasiliensis parasite revealed a relative decrease in Neu5Gc compared with Neu5Ac by decreased expression of a CMAH hydroxylase [224]. The removal of adult worms of parasite N. brasiliensis from the small intestinal goblet cell mucins of mice is hypothesized to be possibly associated with terminal GalNAc and sialic acid residues of the small intestinal goblet cell mucins prior to infection [225].
Recently Schistosoma mansoni (S. mansoni), causing human schistosomiasis, has been reported to be rich in fucose, containing terminal beta-GalNAc residues but lack sialic acid [226], [227]. The expressed fucosylated glycans containing Lewis x (Le(x)) antigen common to human leukocytes and other tissues produce autoantibodies, thereby probably playing a role in affecting lymphocyte functions. Triantennary- and biantennary-like complex-type asparagine-linked glycoproteins with the expression of mannose, fucose, N-acetylglucosamine, and N-acetylgalactosamine in S. mansoni have been associated with a role in the host immune response to infection [228]. A cysticercus membrane glycoprotein antigen has been identified with hexoses and sialic acids [229].
4.6.4. Annedida
Very few studies have reported the observation of sialic acid in earthworm. Glycolipid fraction of earthworm Lumbricus terrestris (L. terrestris) has been reported to include cerebrosides and sulfatides containing glucose and galactose, and gangliosides containing glucosamine and sialic acid [230].
4.6.5. Arthropoda
Sialylation pathway in Drosophila reveals similarities in the initial steps to the mammalian sialylation pathways indicating a probable common evolutionary origin [231], [232]. N-glycan processing in insects reveal similari9*ty at early steps with differences in subsequent steps to mammalian N-glycan synthesis with the insect cell lines not processed to terminally sialylated complex-type structures but modified to paucimannosidic or oligomannose structures thus differing from the mammalian cells due to the lack of enzymes including glycosyltransferases involved in generating complex-type structures and appropriate sugar nucleotides [233]. The baculovirus-insect cell expression system has been used to produce recombinant therapeutic glycoproteins [234]. Neu5Ac has been reported to occur in larvae of the cicada Philaenus spumarius (P. spumarius) [26].
4.6.6. Mollusca
Sialic acid-binding lectins (SABLs) SgSABL-1 and SgSABL-2 of Solen grandis (S. grandis) have been revealed to have functions like pattern-recognition receptor (PRRs) and hypothesized to be involved in the innate immune response of S. grandis [235], [236]. Siglec gene or CgSiglec-1 has been characterized from the Pacific oyster, Crassostrea gigas (C. gigas) [237].
Gastropod Haliotis tuberculata (H. tuberculata) foot epithelium reveal N-glycoproteins rich in fucose and mannose while secretory cells reveal expressions of acidic sulfated glycoconjugates such as glycosaminoglycans and mucins, enriched with galactose, N-acetylgalactosamine, and N-acetylglucosamine but foot epithelium lack sialic acid [238]. Lectins were known to participate in immune recognition and host defense and Ch-salectin, a novel sialic acid-binding lectin, was reported to occur in Crassostrea hongkongensis (C. hongkongensis) with a role in immune recognition and host defense against bacterial infection caused by C. hongkongensis [239]. SABL from Manila clam Venerupis philippinarum, VpSABL [240], has been reported. A novel sialic acid-specific lectin (MCsialec) was detected from Manila clam hemocytes infected with Perkinsus olseni that plays a vital role during pathogenic infection [241]. Scalarin from the eggs of Pomacea canaliculata (P. canaliculata, Lamarck, 1822) and Pomacea scalaris (P. scalaris, d’Orbigny, 1835) revealed expression of terminal sialic acid residues possibly resistant to neuraminidase and O-linked residues derived from the T and Tn antigens [242]. N-linked oligosaccharides are expressed in the nacreous layer of Japanese pearl oyster Pinctada fucata (P. fucata) [243]. A novel α/β-galactoside α-2,3-sialyltransferase was expressed by luminous marine bacterium, Photobacterium phosphoreum JT-ISH-467, isolated from the Japanese common squid (Todarodes pacificus, T. pacificus) [244]. Garden snail Cepaea hortensis (C. hortensis) [245], [246] has been reported to express sialic acid-specific lectin. C. hortensis agglutinin-I (CHA—I) lectin binds to O-linked sialic acids [247]. Newly hatched Hawaiian squid Euprymna scolopes (E. scolopes) rapidly become colonized by the bioluminescent marine bacterium Vibrio fischeri which exhibited the unusual ability to migrate to nucleosides, nucleotides, and sialic acid, a component of squid mucus [248]. Neu5Ac and Neu5Gc have been reported to occur in slug Arion lusitanicus (Gastropoda) revealing specificity toward MAA [249]. Eye lenses of common squid (T. pacificus) and Pacific octopus (Octopus vulgaris, O vulgaris) has been known to express gangliosides including gangliotetraose species and c-series gangliosides [250]. Acidic lipids from T. pacificus and O. vulgaris revealed expression of Neu5Ac. Lipid-bound sialic acid in cerebral ganglia were significantly lower as compared to the expression in hepatopancreatic tissues indicative of ganglioside expression in protostomia [167].
Achatinin, a 9-O-acetyl sialic acid (9-O-AcSA) binding lectin, has been isolated from A. fulica snails with sugar specificity toward 9-O-AcSAα2 → 6GalNAc [251]. Sialic acids have been reported to occur in two marine bivalves, the Pacific oyster C. gigas and the horse mussel Modiolus modiolus (M. modiolus) [252]. A heterogeneous SABL with affinity toward bacterial LPS was expressed in hemolymph of M. modiolus [253]. The M. modiolus SABL is reported to agglutinate erythrocytes and bacterial LPS and react with sialoconjugates [254]. A cDNA library of Limax flavus (L. flavus) was constructed and screened for sialic acid-specific lectin [255]. A total of 16 lectins have been reported to be expressed in the digestive gland of the bivalve mollusc Mytilus galloprovincialis (M. galloprovincialis) [256]. Salmonella djakarta (S. djakarta) and Salmonella isaszeg (S. isaszeg) LPS were investigated for neuraminic acid expression [257]. Neu5Gc-specific lectin (AFL) has been isolated from the foot muscles of the marine clam Anadara granosa (A. granosa) [258]. A Neu5Gc-specific lectin (PAL) has been isolated from apple snail, Pila globosa (P. globosa) [259]. A sialic acid lectin is expressed in slug Limax [260]. Sialic acid-containing substrates as intracellular calcium receptors have been reported to be involved in transmitter release [261].
4.6.7. Echinoderms
In echinoderms including starfish and sea urchin, expression of Neu5Gc is predominant [50], [59], [60], [64]. Di-sialoglycoconjugates have additional Neu5Gc, O-methyl-Neu5Gc, N-acetyl-O-methylneuraminic acid, and N-glycoloyl-O-methyl neuraminic acid [262], [263]. Starfish Asterias rubens (A. rubens) reveals expression of 8-O-methyl-5-Neu5Gc (Neu5Gc8Me) [264] with Neu5Ac, Neu5Gc, and their O-acetylated derivatives, while starfish additionally possess 8-O-methylated sialic acids [264], [265]. Neu5Gc is formed by CMP-Neu5Ac hydroxylase found in gonads of starfish A. rubens revealing similarities to the mammals. However, the echinoderm hydroxylase reveals differences from the mammalian counterpart in membrane association and a requirement for high ionic strength for optimal activity [266], [267] which has been cloned [266].
4.6.8. Vertebrates
Intestinal glycoconjugates of the blunthead pufferfish Sphoeroides pachygaster (S. pachygaster) and gray triggerfish Balistes capriscus (B. capriscus) reveal expression of GalNAc and GlcNAc residuals with GalNAc residuals in S. pachygaster subterminal to sialic acid [268].
Zebra fish Danio rerio (D. rerio) has been studied extensively for glycosylation [269], [270], [271] and development of vertebrates and reported the expression of protein and lipid-associated alpha2–8-linked oligosialic acid motifs in the early development [272]. CMP-Sia synthetase (CMAS) has been reported to occur in D. rerio (dreCmas) [273]. PSA action has been reported during axon growth and pathfinding in the developing zebra fish CNS [274].
Sialic acid acetylesterase (SIAE) removes acetyl moieties from the carbon 9 and 4 hydroxyl groups of sialic acid and adult fish reveal expression of siae mRNA in heart, eye, muscle, liver, brain, kidney, and ovary revealing their role in immune system function and the development of central nervous system [270].
The epidermis of sea lamprey Petromyzon marinus (P. marinus) reveals expression of glycoconjugates including sulfated glycosaminoglycans (N-acetylglucosamine and N-acetylgalactosamine) and N-glycoproteins rich in mannose in the mucous. The skin cells, a unique cell of lampreys, reveal expression of l-fucose and sialic acid residues that is lost with metamorphosis [275].
Sialidase removes sialic acids from glycoconjugates and medaka sialidase Neu1 has been reported to exhibit desialylation of α2–3 sialic acid linkage [276]. Gangliosides has been reported to occur in fish containing only N-acetylneuraminic acid/sialic acid, while beef, chicken, and pork contained GD1a/b species that incorporated both Neu5Ac and Neu5Gc and hydroxylated fatty acids [277]. Mucin O-glycosylation of five freshwater acclimated Atlantic salmon have been reported to contain sialylated intestinal mucins, Neu5Ac and sialylated core 5 was the most dominant structure with a probable role in host-pathogen interactions [278]. Fish skin mucus reveals NANA/sialic acid, glucose, N-acetylglucosamine, N-acetylgalactosamine, galactose, and fucose residues [278]. Trisialyllactosylceramide, GT3, containing an O-acetylated sialic acid has been reported to occur in cod fish brain [279]. In fish neu4 sialidase, neu4 gene has been reported and was cloned from medaka brain mRNA with a probable role in embryonic development [280]. Polysialic acid (PSA) linked to neural cell adhesion molecule NCAM1 forms PSA-NCAM1 by polysialyltransferases STX with functions during the development of vertebrate nervous systems including axon extension and fasciculation. NCAM1 and NCAM2 have been reported in tetrapods and fishes [281].
PSA-NCAM plays a vital role in neuronal differentiation, maintenance, plasticity, and regeneration. In zebra fish homologues of STX (St8sia2) and PST (St8sia4) have been studied and found that PSA-NCAM regulates motility for cerebellar neuronal progenitors [282] and NANS-mediated synthesis of sialic acid was essential for the development of brain and skeleton [283]. Sialyltransferase gene found in the tunicate Ciona intestinalis (C. intestinalis) reveals a possible ortholog of the common ancestor of galactose α2,3-sialyltransferases and ST3Gal II gene from the bony fish Takifugu rubripes (T. rubripes) [284]. Polysialoglycoprotein (PSGP) in salmonid fish egg is a unique glycoprotein bearing α2,8-linked PSA on its O-linked glycans and two α2,8-polysialyltransferases (α2,8-polySTs), PST (ST8Sia IV) and STX (ST8Sia II), have been reported for PSA biosynthesis on N-glycans of glycoproteins in mammal [285].
Unambiguous orthologs of mammalian siglec-4, exclusively expressed in the nervous system, has been identified from fugu and zebra fish. As in mammals, fish siglec-4 is expressed by nervous tissue. Fish Siglec-4 recombinant protein, fish siglec-4 has been reported to bind to sialic acids with a specificity similar to the mammalian orthologs indicating that siglec occurs in the nervous system of all vertebrates [286]. Infectious salmon anemia virus (ISAV) causes infections in farmed Atlantic salmon. Purified ISAV hydrolyzed free 5-N-acetyl-4-O-acetyl neuraminic acid and the enzymatic activity of the HA-esterase of ISAV revealed similarities to sialate-4-O-esterases of murine coronaviruses and related group 2 coronaviruses [287]. PSA-NCAM facilitates axon growth. In lizard, retinal ganglion cell axons have been reported to be transiently PSA-NCAM positive, whereas in goldfish retinal ganglion cell (RGC) axons are PSA-NCAM negative. PSA-NCAM is negative both in normal animals and throughout regeneration with the exception of a PSA-NCAM-positive fascicle arising from newly generated RGCs [288].
Epidermal, branchial, and digestive mucous cells, and the gastric glands of larvae/postlarvae of three fish species (two teleostean and a chondrostean) revealed negative Con A lectin staining, but oesophageal mucous cell of sturgeon revealed expression of mannose -Man- and/or glucose -Glc-, L-fucose -Fuc-, N-acetyl-D-galactosamine -GalNAc-, N-acetyl-D-glucosamine, -GlcNAc-, and/or sialic acid-NANA-residues [289]. Fish type III antifreeze protein is homologous to the C-terminal region of mammalian sialic acid synthase [290]. In all 18 gangliosides were isolated from dogfish Squalus acanthias (S. acanthias) brain, including GM2, GQ1c, GP1c, and GD2 [291]. CRPs in Labeo rohita (L. rohita) reveal microheterogeneity [292]. Cichlid fish and rat brains also contained GM1b-, GT1b-, and GQ1c-synthase and sialyltransferase activities [293].
α2 → 8-Linked PSA chains terminate O-linked oligosaccharide chains on Salmonidae fish egg polysialoglycoproteins (PSGPs) the expression of which are developmentally regulated [294]. Gangliosides expression is reported to occur in electric organ of Torpedo marmorata: synaptosomes, presynaptic membranes, postsynaptic membranes, and synaptic vesicle membranes [295]. A 9-O-actetylated GT2 has been reported to occur in cod fish brain [296]. A trisialyllactosylceramide GT3 was found in cod fish brain with a chemical structure of II3(9-O-Ac-NeuAc2–8NeuAc2–8NeuAc2–3)Lac Cer [297]. Trout liver has been reported to express sialate cytidylyltransferase activity. The sialic acid fraction of trout liver after hydrolysis is composed of N-acetylneuraminic acid, N-acetyl-9-O-acetylneuraminic acid, and N-acetyl-9-O-lactoylneuraminic acid [298].
The luminal surface of the saccular macula in the rainbow trout revealed expression of a glycoconjugate constituting glucose, galactose, fucose, mannose, N-acetylglucosamine, N-acetylneuraminic acid, and N-acetylgalactosamine [299].
Carbohydrate-rich sialoglycopolyprotein was isolated from the fertilized eggs of the Medaka fish species, Oryzias melastigma (O. melastigma), as a member of the L-hyosophorin family [300].
The mucin-like keratan sulfate glycopolymer has been reported to occur in ampullae of lorenzini [301]. Amphibians have been studied for molecules with anticancer properties. Onconase, amphinase, cSBL from Rana catesbeiana (R. catesbeiana) eggs, and jSBL from Rana japonica (R. japonica) eggs, which belong to the RNase A family, were purified from the oocyte cells and eggs of three amphibians, and they were found to induce cytotoxicity by degrading cellular RNA [302]. Xenopus embryos revealed expression of polysialo-ganglioside including asialo-GMI as the core structure of the ganglioside XI and palmitic and oleic acid as the fatty acids of the ceramide moiety [303].
N-glycan structures have been identified from snake venoms [304]. Four different sialidase forms are known in vertebrates: the lysosomal NEU1, the cytosolic NEU2 and the membrane-associated NEU3 and NEU4. Sialidase orthologs from 21 different organisms have been identified and shown distribution in the evolutionary tree including Metazoa relative, marine choanoflagellate Monosiga brevicollis (M. brevicollis), early Deuterostomia, precursor of Chordata, and Vertebrata (teleost fishes, amphibians, reptiles, avians and early and recent mammals [305]. Mucins in the alimentary tract of the grass snake, Natrix natrix (N. natrix), has revealed expression of mannosylated sialosulfomucins and fundic mucosa of stomach reveal expression of sialomucins with terminal sialic acid linked to galactose, with neck cells expressing sialomucins with mannose, N-acetylglucosamine, galactose, N-acetylgalactosamine, and fucose-α- (1,2)-linked residues [306]. A serine protease with thrombin-like activity (TLBan) expressed by Bothrops andianus (B. andianus) commonly called Andean Lancehead has been reported to contain both N-linked carbohydrates and sialic acid [307]. Asian pit viper venom reveals expression of partially O-acetylated NeuAcα2–8NeuAcα2–3Galβ1-4GlcNAcβ1-terminal epitope [308]. Platypus revealed expression of Neu5Ac. Among nine reptiles and one turtle, Neu5Gc was expressed in the egg and an adult basilisk, belonging to the lizard family. BLAST analysis of platypus, chicken, and zebra finch genomes did not reveal similarity to CMAH structure. Monotremes including Platypus and Sauropsids including birds and reptiles lacked Neu5Gc synthesis machinery. Neu5Gc found in eggs may probably have been acquired from diet or by an alternative pathway [309]. Carbohydrate components of the ductus epididymis epithelium of a lizard revealed location differences [310]. DM43, an opossum serum protein inhibitor of snake venom metalloproteinases, revealed constitution of N-acetylglucosamine, mannose, galactose, and sialic acid forming four biantennary N-linked chains [311]. The expression of PNA-binding glycoproteins has been reported to occur in lizard lymphocytes [312]. A heavily glycosylated protein fraction was isolated from cobra venom containing both O– and N-linked oligosaccharides; 1 N-linked chain for every 8–10 O-linked oligosaccharides and the O-linked chains revealed expression of fucose, galactose, and N-acetylglucosamine more than N-acetylgalactosamine; with minute level of sialic acid lacking sulfate esters [313]. N-CAM has been shown to undergo decrease in sialic acid content during embryonic to adult conversion with an increase in binding efficacy thus regulating morphogenesis [314].
Sialic acids are found to be constituents of milk oligosaccharides, components of glycoproteins in blood, sera, or plasma of mammals. Human sera reveal expression of Neu5Ac and Neu5Ac9Lt (Lt = lactoyl) and minute quantities of O-acetylated sialic acid derivatives, Neu5,9Ac2 [89]. Sialic acid derivatives in serum glycoproteins reveal differences in expression in mammalian species and level and position of O-acetylation and Neu5Gc expression [2]. Sialic acid and its acetylated derivatives have been reported to occur in epithelial and mucous glycoproteins revealing the presence of Neu5Ac, Neu5,9Ac2, and Neu5,7,9Ac3 [2]. Gangliosides have also been reported. Pathogens are known to bind to sialic acid on human cell surfaces (Table 4 ).
Table 4.
Pathogens that bind to sialic acids on human cell surfaces
| Pathogen | Binding protein | Known target sialylated sequence |
|---|---|---|
| Human Influenza A | Hemagglutinin | Siaα2–6Gal(NAc) |
| Avian Influenza A | Hemagglutinin | Siaα2–3Galβ1- |
| Human Influenza C | Hemagglutinin-esterase | 9-O-Ac-Siaα2- |
| Vibrio cholera | Toxin | Galβ1–3GalNAcβ1,4(Siaα2–3)Lac-Cer |
| Plasmodium falciparum | EBA-175 | Siaα2–3Galβ1–3(Siaα2–6)GalNAc-O- |
| Clostridium botulinum | Toxin | Polysialogangliosides |
| Helicobacter pylori | SabA | Siaα2–3Gal on gangliosides |
Table adapted with permission from Ajit Varki Sialic acids in human health and disease Trends Mol Med. 2008; 14(8): 351–360.
Sialic acid-binding protein
Sialic acid components of oligosaccharide side chains in glycoconjugates occur in most higher animals and a few microorganisms act as ligands in glycobiological interactions on binding to a specific sialic acid-binding protein acting as receptors [315].
Siglecs
Siglecs are sialic acid-binding immunoglobulin (Ig)-type lectins which are the members of the immunoglobulin superfamily that act as transmembrane cell surface immune regulatory receptors predominantly found on hematopoietic cells containing an N terminal V-set Ig-like domain with sialic acid-binding sites that recognizes different sialylated glycoconjugates, leading to the activation or inhibition of the immune response. Siglecs include (a) CD33-related Siglecs and (b) Siglec-1 (Sialoadhesin), Siglec-2 (CD22), Siglec-4 (myelin-associated glycoprotein, MAG), and Siglec-15. Phylogenetic studies in higher vertebrates including fishes, amphibians, birds, reptiles and mammals have revealed that Siglecs are conserved in evolution [316]. A loss of Siglec genes in rodents have been reported.
The cytoplasmic domain of most Siglecs contain immune receptor tyrosine-based inhibitory motifs (ITIMs) that recruit tyrosine phosphatases SHP-1 and SHP-2 and function as inhibiting receptors, inhibiting signal transduction. Siglec-14, Siglec-15, and Siglec-16 associate with tyrosine-based activation motif (ITAM) adaptor DAP12 and act as activating receptors by recruiting SYK kinase. Siglecs are known to play a vital role in immune regulation in host-pathogen interaction in infectious diseases, inflammation, neurodegeneration, autoimmune diseases, and cancer [317]. The sialic acid-Siglec axis has been recently reported to be exploited by tumors and pathogens for the induction of immune tolerance [318].
Sialic acid-binding lectins
SABLs are lectins that specifically recognize sialic acid residues [319]. They have been reported to occur in plants and animal sources with diverse specificity and are being exploited for analytical properties (Table 5 ).
Table 5.
Vertebrate sialic acid-specific lectins [18], [320], [321], [322], [323], [324], [325], [326], [327], [328], [329], [330], [331], [332], [333], [334], [335], [336], [337], [338], [339], [340], [341], [342], [343], [344], [345], [346], [347], [348]
| Names (synonyms) | Expression (source)a | Binding specificity |
|---|---|---|
| E-selectin (ELAM-1, CD62E) | Activated endothelium | sialyl Lex, sialyl Lea |
| L-selectin (MEL 14 antigen, CD62L) | Leucocyte | 6′-sulfo sialyl Lex, heparan sulfate |
| P-sclectin (GMP 140, PADGEM. CD62P) | Activated endothelium, platelet | sialyl Lex, sialyl Lea, heparan sulfate (HS) |
| Siglecs | ||
| Siglec-1 (sialoadhesin) | Macrophage | Neu5Acα2–3Gal > Neu5Acα2 – 6Cal |
| Siglec-2 (CD22) | B-cell | Siaα2–6Gal |
| Siglec-3 (CD33) | Myeloid precursor, Mobc | Siaα2–6Gal ≥ 2 Siaα2–3Gal |
| Siglec-4 (MAG) | Glial cells | Neu5Acα2–3Gal on complex gangliosides |
| Siglec-5 | Mo, Gr | Siaα2–6Gal ≈ Sioα2–3Gal, Neu5Acα2–8 |
| Siglec-6 (OB-BP1) | Placenta, B-cell | Siaα2–6Gal NAc |
| Siglec-7 (AIRM-1) | NK cells, Mo, Gr | Siaα2–6Gal ≈ Siaα2–3Cal (3-Ig isoform), Siaα2–6Gal (2-Ig isoform) |
| Siglec-8 | Eosinophil, basophil | Siaα2–3Gal ≥ Siaα2–6Gal |
| Siglec-9 | Mo, Gr | Siaα2–6Gal ≈ Siaα2–3Gal |
| Siglec-10 | B-cell | Siaα2–6Gal ≈ Siaα2–3Gal |
| Siglec-11 | Fibroblasts | Neu5Acα2–8Neu5Ac |
| Others | ||
| Complement factor H | Blood | Sia; C7–C9 side chain is a part of epitope |
| CD83 | Dendritic cell | Sia |
| LI | Mouse neuron | Neu5Acα2–3 (on CD24) |
| Interleukin-1α | Blood | Neu5Acα2–3Galβ1–4GlcNAc, biantennary |
| Interleukin-1β | Blood | Neu5Acα2–3Galβ1-Cer (GM4) |
| lnterleukin-2 | Blood | GD1b |
| Interleukin-4 | Blood | Neu5Ac1,7lactone |
| Interleukin-7 | Blood | Neu5Acα2–6GalNAc |
| Laminin | Extracellular matrix | Neu5Acα2–3Galβ1–4GlcNAc (α2–3 > α2–6) |
| Sialic acid-binding protein | Endometrium | Neu5Gc > Neu5Ac |
| Sarcolectin | Placenta | Neu5Ac, Neu5Gc |
| Calreticulin | Ovine placenta | Neu5Gc > Neu5Ac, prefer O-acetyl |
| Sialic acid-binding protein | Rat uterus | Sia |
| Calcyclin | Bovine heart | Neu5Gc |
| Sialic acid-binding protein | Frog egg | Sia |
| Ganglioside binding protein⁎ | Rat brain myelin | Gangliosides (CT1b, CQ1b, GD1b) |
| Hemagglutinin⁎ | Rat brain | Neu5Ac, Neu5Gc |
Expression (source) is in human, unless otherwise stated.
Mo, monocyte; Gr, granulocyte.
Most of the proteins are cloned and/or purified to homogeneity, except for the entities marked with an asterisk (*).
Adapted with permission and updation from Angata T, Varki A. Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem Rev 2002;102:439–469.
Selectins
Selectins are a diverse group of calcium-dependent, type I transmembrane molecules that bind to sialylated, fucosylated carbohydrates, function in vascular adhesion, and play a significant role in inflammation, immunity, hemostasis, and cancer progression. Selectin ligand reveal overexpression of tumor cells leading to increased metastasis, poor prognosis, mediate tumor cell adhesion, extravasation during metastasis, and activate signaling cascade in tumor.
Selectins play a vital role in leukocyte homing. L-Selectin binding to ligands on leukocytes activates leukocytes. Selectins enable interactions with platelets and endothelial cells. The interaction between selectin ligand P-selectin glycoprotein ligand 1 PSGL-1 on leukocytes and P-selectin on platelet or E-selectin on endothelial cells triggers intracellular signaling in leukocytes.
Selectins and their ligands play an important role in the human implantation. L-selectin on interaction with its ligands plays a critical role in the adhesion of the blastocyst to the endometrium at the maternal-fetal interface. P-selectin and E-selectin play a vital role in human implantation [349]. PSGL-1 is now known as a major participant in inflammation, thrombus, along with cancer [350]. Due to their role in cancer immune system modulation they are being studied as possible targets for controlling tumor immunity [351].
4.6.9. Sialylation and disease
Although sialic acids are found to occupy the terminal position of glycans of all cell types, in disease states, like cancer or immunological disorders, the sialylation profile of cells in affected tissues manifest altered downregulation or overexpression—or neoexpression of certain glycan structures [352].
Aberrant sialylation of oligosaccharide branches of N-glycans (β1,6-GlcNAc branching) or affected terminal glycan sequences, like premature sialylation of truncated saccharide units, are reported to occur due to affected or altered enzyme expression [352]. In rheumatoid arthritis, an incomplete IgG glycosylation with galactose and sialic acid is observed to lead to immune disorder [353].
But upregulated expression of sialyltransferases like ST3Gal-I is observed in human BC [354] and upregulated ST6Gal-I is reported in human BC and colon cancer. Ganglioside overexpression in cancer has been reported such as CNS-specific GD3s from tumor tissues [355]. The overexpression of 9-O-acetylated sialic acid in BC [356], childhood ALL [83], [103], [104], [105], [106], [107] are reported.
Altered cell sialylation in cancer affects tumor cell interactions with other cells affecting cell adhesion, migration, and metastasis [357], [358], [359] Sialylation affects natural killer (NK) cell cytotoxicity [360]. Tumor cell hypersialylation has been observed to enable tumor cell to evade recognition by NK cells, thus escaping the immune responses. Hypersialylation of tumor cells enables it to escape the immune surveillance [358].
Sialic acid ligand-protein interaction is being exploited for design and development of therapeutics and anticancer therapies containing sialoside that targets siglecs [361]. Nanoparticle formulations are being tested to deliver potential therapeutic agents to the target cell [317].
4.6.10. Tumor-associated carbohydrate antigens
Upregulated sialyltransferases and fucosyltransferases lead to the overexpression of tumor-associated carbohydrate antigens (TACA), being mucin related to Thomsen nouvelle (Tn) antigen (Tn), the sialyl-Thomsen nouvelle (sTn) antigen, Thomsen-Friedenreich antigen (TF-Ag), the blood group-related Thomsen-Lewis antigens LewisY, Sialyl LewisX and Sialyl LewisA, and LewisX (or specific embryonic antigen-1, SSEA-1), the glycosphingolipids Globo H, stage-specific embryonic antigen-3 (SSEA-3), sialic acid-containing glycosphingolipids, the gangliosides GD2, GD3, GM2, fucosyl GM1, and Neu5GcGM3, and PSA [362] that have been implicated in tumor, metastasis, and poor prognosis. Some TACAs are also expressed in fetal tissue, and termed as oncofetal antigens [362].
TF-Ags increased the expression in pancarcinoma and carcinomas of the breast, colon, bladder, prostate, liver, and stomach as compared to normal cells and its role in metastasis renders it as an important tumor target [362].
The overexpression of LewisY in ovarian, breast, prostate, colon, and epithelial cell lung cancers than in the normal cells makes them potential targets. The overexpression of SLeX in breast, ovarian, melanoma, colon, liver, lung, and prostate cancers and SLeA in breast, colon, and pancreas cancers, and in melanomas due to the upregulated expression of ST3Gal-III and FucT-III enzymes, synthesizing sLea and ST3Gal-IV, ST3Gal-VI, and FucTVII, catalyzing the synthesis of sLex, or due to deficiency in the enzymes responsible for sulfation, and their overexpression acting as E-selectin ligands contribute to metastasis and poor prognosis makes them potential targets. The treatment of these antigens with antibodies inhibited metastasis in pancreatic tumor mouse models. PSA is overexpressed in small cell lung cancer (SCLC), rhabdomyosarcoma, Wilms tumor, and neuroblastoma and PSA-NCAM is involved in increased tumor growth and metastasis with decreased patient survival. Endoneuraminidase N, cleaving PSA could stop cell growth in rhabdomyosarcoma and neuroblastoma cells [362].
Downregulated sialyltransferases and fucosyltransferases lead to reduced cell surface sialylation and reduced TACA expression, decreasing adhesion and migration potency and metastatic activity [363].
sTn antigen generated by sialylation of Tn antigen [364] by ST6GalNAc I occurs rarely in healthy tissues but has been observed in epithelial cancer cells and breast tumors [365].
4.6.11. Sialic acids and therapeutics: Where we stand
Strategies like enzyme inhibitors for disease-associated carbohydrates are being studied, designed, and developed. Inhibitors for the carbohydrate-binding receptors are also being conceived as a strategy [366]. X-ray crystallographic structures of mammalian sialyltransferases (STs) complexed with their ligands are enabling us to better understand the substrate specificities and inhibitor design [367].
Inhibition of STs, glycosyltransferases, is a strategy to reduce cell surface sialylation particularly in cancer [368]. Lack of ST3Gal-IV and ST3Gal-VI reduces sialic acid containing ligands for selectin interactions, thus affecting leukocyte homing and leukocyte recruitment at inflammation sites [369]. Streptococcus pneumonia leads to septicemia, meningitis, and community-acquired pneumonia in human. Although prophylactic vaccines are the strategies available for disease targeting, Streptococcus pneumonia (S. pneumonia) sialidases, including NanA and NanB, implicated in the pathogenesis of S. pneumonia and design of novel NanB-selective inhibitors are being tested for drug targets [370].
Binding with selectin antagonists, like bimosiamose (TBC-1269) and GSC-150, sialoside-selectin interactions can be blocked; selectin antagonists also find application in the treatment of inflammatory disorders, autoimmune diseases and metastatic cancers [371]. 11a sLex-derived inhibitor of E-selectin, CGP69669A, with affinity higher than natural ligand sLex has been designed [372]. Modified sLex-containing glycopeptide analogues for E-selectin inhibition has been designed [373]. P-selectin antagonist, GMI-1070, is in phase II clinical trials for the treatment of sickle cell crisis, associated with sickle cell disease (SCD) [374]. Bimosiamose (TBC-1269), a P-selectin antagonist, has been reported to have successfully completed phase II clinical trials for psoriasis and chronic obstructive pulmonary disease (COPD) [375].
N-acetylglucosamine 2-epimerase and N-acetylneuraminic acid aldolase allow conversion of simple and sugars into different sialic acid-related compounds from whole cell extracts enabling large scale and economical synthesis of sialic acid and sialyloligosaccharides [376].
Low molecular weight antagonists, targeting sialoside interaction with the lectin MAG, have been designed as a therapeutic strategy to promote axon regeneration after neuronal injury [377]. Zanamivir (Relenza) and oseltamivir (Tamiflu) are designed as sialic acid-based inhibitors of the viral enzyme neuraminidase for targeting influenza infections [378].
But design of carbohydrate-derived drug molecules faces challenges such as pharmacokinetic profiles, poor bioavailability, requirement for active transport through membranes, short plasma half-life, poor metabolic stability, and are rapidly excreted [379], [380]. However, this area of biology is developing with studies or approaches to improve their pharmacokinetic profiles like systematic structural modifications such as the replacement of certain moieties, the introduction of hydrophobic moieties, and/or the incorporation of a prodrug strategy leading to compounds that mimic the biological activity of their carbohydrate precursors called as ‘glycomimetics’ like neuraminidase inhibitors zanamivir and oseltamivir [381], [382].
Drugs targeting glycan-lectin interactions need to be designed by synthetic introduction of moieties that increase the affinity. Inhibition of enzymes in the synthesis of tumor-associated glycans is another strategy [383].
Liposomal nanoparticle are being exploited in selective delivery of drugs and therapeutics like chemotherapeutic doxorubicin in B cell lymphoma, wherein high-affinity ligand, 9- N-biphenylcarboxyl (BPC)-Neu5Ac-α2,6-Gal-β1,4-GlcNAc, on the liposomal surface confers the selectivity being recognized by CD22 (Siglec-2), exclusively expressed on B cells and trisaccharide derivative BPC-Neu5Ac-α2,6-Gal-β1,4-GlcNAc conjugated to pegylated lipid is used in vesicle formation with encapsulated doxorubicin thereby selectively targeting CD22-expressing cells, including B cell lymphoma [384], [385], [386].
Selective ligands targeting the lectins CD22 and CD33 are being designed [387], [388], [389]. 4-Cyclohexyl-1,2,3-triazole residues at position 5 of sialic acid in combination with meta-substituted moieties at position 9 have been reported to improve affinity and selectivity profile [390].
Therapeutic antibodies
Therapeutic antibodies find application in leukemia and lymphoma. CD33 in leukemic cells and CD22 in B-cell lymphoma are being exploited for targeting. Anti-siglec-monoclonal antibodies conjugated to toxins or chemotherapeutic agents are being designed like gemtuzumab (Mylotarg), an anti-CD33-antibody in acute myeloid leukemia (AML), approved by the FDA in 2000 but faces challenges of safety issues [390], [391] and epratuzumab, an anti-CD22- antibody, is being designed for targeting non-Hodgkin lymphoma (NHL) and the autoimmune disorder systemic lupus erythematosus (SLE) [392].
Carbohydrate-based vaccines
Carbohydrate-based vaccines designed based on altered cell surface glycans in disease as compared to normal cells are the latest strategy for targeting cancer, developed over the last two decades, and are used after conventional therapeutic modes of surgery, radiation, and chemotherapy [390], [392].
TACA are being exploited as vaccine targets for their elimination. However, they suffer challenges of being poorly immunogenic self-antigens and strategies to improve antigenicity like conjugating to a carrier protein, for example, keyhole limpet hemocyanin (KLH), and attachment sites for carbohydrate antigen conjugation [390], [393]. Vaccine constructs need to be designed to activate both cell miated and humoral immune responses against specific carbohydrate epitopes of tumor cells [390], [394], [395]. Theratope, a sTn-KLH conjugate, is in clinical practice since 2002 for metastatic BC and colorectal cancer, however, is still in phase III clinical trials since 2003 [390], [396], [397].
Globo H, cancer vaccine against a carbohydrate epitope is unique to target prostate and ovarian cancer cells, such as TACA, including Globo H, sTn, Tn, TF, and Ley antigens attached to a MUC1 glycopeptide backbone conjugated to KLH [362], [390], [394], [398], [399] (Fig. 10 ). Mimicking of the tumor cell’s natural carbohydrate epitope composition is hypothesized for a more effective immune response.
Fig. 10.

Structures of the mucin carbohydrate antigens Tn, Sialyl Tn, and TF, and the Lewis blood group-related antigens. (A) LewisY, Sialyl LewisX and Sialyl LewisA, and LewisX. (B) Structures of the glycosphingolipids, the globo series Globo H and SSEA-3, and the gangliosides GM2, GD2, GD3, and Fucosyl GM1. (C) Structures of the additional sialic acid-containing compounds. NeuGcGM3 and polysialic acid.
(Adapted with permission from Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K, Cancer vaccines and carbohydrate epitopes. Vaccine 2011;29:8802–26.)
Sialic acid containing SSEA3 (Gb5) and SSEA4 detected on BC cells and other types of cancer [390], [400], [401], [402] finds application in anticancer vaccine development. Hypersialylation has been observed to increase their serum half-lives with increased stability of luteinizing hormone (LH) and erythropoietin (EPO) [390], [403]. Ligand for Siglec-7 is being exploited in anticancer therapy.
Efficiency of Gp120 immunogens (MN- and A244-rgp120) used in the RV144 trial conferring protection against HIV were improved by enriching the sialic acid content and lacking N-linked glycosylation sites needed for binding by bN-mAbs [404].
Thus sialic acid is offering advantages in designing therapeutics and in targeting diseases like cancer and other immunological disorders.
5. Discussions
Sialic biology, with huge diversity in forms, structures, expression, metabolism, and functions in the diverse living world, is a fascinating science and our knowledge in this domain is growing day by day. While the pathogens primarily use sialic acid to mimic the host and escape immune responses, it has different functions in mediating cell-cell interaction, signaling, immune reactions, development and function in animals, and completely lacks in plants. Glycoengineering is enabling modification of plant glycoslation pathways toward the synthesis of mammalian glycoproteins which we have discussed in subsequent chapters. Insect sialylation revealing differences from the mammalian sialylation pathway has been discussed in the subsequent chapters.
In the cancer cells, overexpression of sialylation and enzymes involved is reported and forms an interesting area in cancer targeting. Glycomimetics is another such developing domain in the designing of sialic acid-related therapeutics. Nanobiotechnology and its application in sialic acid biology is an emerging domain and has been discussed in subsequent chapters.
At this point although a lot of studies has been done, the scope of applications of sialic acid in therapy is an emerging science which seems to be just the tip of the iceberg for the immensely important potential of sialic acid biology.
References
- 1.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14(8):351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varki A., Schauer R. Sialic acids. In: Varki A., Cummings R.D., Esko J.D., editors. Essentials of glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. Chapter 14. [Google Scholar]
- 3.Schauer R., editor. Sialic acids chemistry, metabolism, and function. Springer; 1982. [DOI] [PubMed] [Google Scholar]
- 4.Carter A., Martin N.H. Serum sialic acid levels in health and disease. J Clin Pathol. 1962;15:69–72. doi: 10.1136/jcp.15.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauer R., Kamerling J.P. Advances in carbohydrate chemistry and biochemistry. vol. 75. Elsevier; 2018. Exploration of sialic acid world. Chapter 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angata T., Varki A. Chemical diversity in the sialic acids and related r-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 7.Haines-Menges B.L., Whitaker W.B., Lubin J.B., Boyd E.F. Host sialic acids: a delicacy for the pathogen with discerning taste. Microbiol Spectr. 2015;3(4) doi: 10.1128/microbiolspec.MBP-0005-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vimr E.R., Kalivoda K.A., Deszo E.L., Steenbergen S.M. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68(1):132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaretsky M., Roine E., Eichler J. Sialic acid-like sugars in archaea: legionaminic acid biosynthesis in the halophile Halorubrum sp. PV6. Front Microbiol. 2018;9:2133. doi: 10.3389/fmicb.2018.02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varki A., Schnaar R.L., Schauer R. Sialic acids and other nonulosonic acids. In: Varki A., Cummings R.D., Esko J.D., editors. Essentials of glycobiology [Internet] 3rd ed. vol. 2015-2017. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2017. Chapter 15. [Google Scholar]
- 11.Mahajan V.S., Pillai S. Sialic acids and autoimmune disease. Immunol Rev. 2016;269(1):145–161. doi: 10.1111/imr.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowthaman U, Eswarakumar VP. Molecular mimicry: good artists copy, great artists steal. Virulence.;4(6):433–434. [DOI] [PMC free article] [PubMed]
- 13.Sprenger N., Duncan P.I. Sialic acid utilization. Adv Nutr. 2012;3(3):392S–397S. doi: 10.3945/an.111.001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rademacher T.W. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- 15.Varki A. 1st ed. Cold Spring Harbor Press; 1999. Essentials of glycobiology. [Google Scholar]
- 16.Drickamer K., Taylor M. 2nd ed. Oxford University Press; 2006. Introduction to glycobiology. [Google Scholar]
- 17.Schauer R. Achievements and challenges of sialic acid research. Glycoconj J. 2000;17:485–499. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angata T., Varki A. Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 19.Bergfeld A.K., Pearce O.M., Diaz S.L., Pham T., Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J Biol Chem. 2012;287:28865–28881. doi: 10.1074/jbc.M112.363549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. 2001;33(Suppl):54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies L.R., Varki A. Why is N-glycolylneuraminic acid rare in the vertebrate brain? Top Curr Chem. 2015;366:31–54. doi: 10.1007/128_2013_419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman M.O., Gagneux P. Absence of Neu5Gc and presence of anti-Neu5Gc antibodies in humans—an evolutionary perspective. Front Immunol. 2019;10:789. doi: 10.3389/fimmu.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnaar R.L., Gerardy-Schahn R., Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth J., Kempf A., Reuter G., Schauer R., Gehring W.J. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992;256:673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S. Sialylation and sialyltransferase in insects. Glycoconj J. 2018;35(5):433–441. doi: 10.1007/s10719-018-9835-6. [DOI] [PubMed] [Google Scholar]
- 26.Malykh Y.N., Krisch B., Gerardy-Schahn R., Lapina E.B., Shaw L. The presence of N-acetylneuraminic acid in Malpighian tubules of larvae of the cicada Philaenus spumarius. Glycoconj J. 1999;16:731–739. doi: 10.1023/a:1007115627708. [DOI] [PubMed] [Google Scholar]
- 27.Staudacher E., Buergmayr S., Grabher-Meier H., Halama T. N-glycans of Arion lusitanicus and Arion rufus contain sialic acid residues. Glycoconj J. 1999;16 [Google Scholar]
- 28.Zhukova I.G., Bogdanovskaia T.A., Smirnova G.P., Chekareva N.V., Kochetkov N.K. Structure of sialoglycolipid from the digestive gland of the starfish Distolasterias nipon. Biochim Biophys Acta. 1973;208:981–984. [PubMed] [Google Scholar]
- 29.Kim C.H. Sialic acid (N-acetylneuraminic acid) as the functional molecule for differentiation between animal and plant kingdom. J Glycomics Lipidomics. 2014;4 [Google Scholar]
- 30.Chou H.H. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tecle E., Gagneux P. Sugar-coated sperm: unraveling the functions of the mammalian sperm glycocalyx. Mol Reprod Dev. 2015;82:635–650. doi: 10.1002/mrd.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung H.S., Li O.T., Chan R.W., Chan M.C., Nicholls J.M., Poon L.L. Entry of influenza A Virus with a α2,6-linked sialic acid binding preference requires host fibronectin. J Virol. 2012;86:10704–10713. doi: 10.1128/JVI.01166-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nizet V., Varki A., Aebi M. Microbial lectins: hemagglutinins, adhesins, and toxins. In: Varki A., Cummings R.D., Esko J.D., editors. Essentials of glycobiology [Internet] 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2017. 2015–2017; Chapter 37. [Google Scholar]
- 34.Yu R.K., Tsai Y.T., Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res. 2012;37:1230–1244. doi: 10.1007/s11064-012-0744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barthel S.R., Gavino J.D., Descheny L., Dimitroff C.J. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva M., Videira P.A., Sackstein R. E-selectin ligands in the human mononuclear phagocyte system: implications for infection, inflammation, and immunotherapy. Front Immunol. 2018;8:1878. doi: 10.3389/fimmu.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrias E.S., de Carvalho T.M., De Souza W. Trypanosoma cruzi: entry into mammalian host cells and parasitophorous vacuole formation. Front Immunol. 2013;4:186. doi: 10.3389/fimmu.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca L.M., da Costa K.M., Chaves V.S., Freire-de-Lima C.G., Morrot A., Mendonça-Previato L., Previato J.O., Freire-de-Lima L. Theft and reception of host cell's sialic acid: dynamics of Trypanosoma Cruzi Trans-sialidases and mucin-like molecules on chagas' disease immunomodulation. Front Immunol. 2019;10:164. doi: 10.3389/fimmu.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schauer R. Sialic acids: fascinating sugars in higher animals and man. Zoology (Jena) 2004;107(1):49–64. doi: 10.1016/j.zool.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Zanetta J.-P., Pons A., Iwersen M., Mariller C., Leroy Y., Timmerman P., Schauer R. Diversity of sialic acids revealed using gas chromatography/mass spectrometry of heptafluorobutyrate derivatives. Glycobiology. 2001;11:663–676. doi: 10.1093/glycob/11.8.663. [DOI] [PubMed] [Google Scholar]
- 42.Li Y.-T., Maskos K., Chou C.-W., Cole R.B., Li S.-C. Presence of an unusual GM2 derivative, taurine-conjugated GM2, in Tay–Sachs brain. J Biol Chem. 2003;278:35286–35291. doi: 10.1074/jbc.M306126200. [DOI] [PubMed] [Google Scholar]
- 43.Schauer R., Kamerling J.P. Chemistry, biochemistry and biology of sialic acids. In: Montreuil J., Vliegenthart J.F.G., Schachter H., editors; Neuberger A., van Deenen L.L.M., editors. Glycoproteins II. vol. 29b. Elsevier Science B.V; Amsterdam, The Netherlands: 1997. pp. 243–402. (New comprehensive biochemistry). [Google Scholar]
- 44.Blix G., Lindberg E., Odin L., Werner I. Studies on sialic acids. Acta Soc Med Ups. 1956;61:1–25. [PubMed] [Google Scholar]
- 45.Kamerling J.P., Vliegenthart J.F.G., Vink J. Mass spectrometry of pertrimethylsilyl neuraminic acid derivatives. Carbohydr Res. 1974;33:297–306. [Google Scholar]
- 46.Kamerling J.P., Vliegenthart J.F.G., Versluis C., Schauer R. Identification of O-acetylated N-acylneuraminic acids by mass spectrometry. Carbohydr Res. 1975;41:7–17. doi: 10.1016/s0008-6215(00)87002-0. [DOI] [PubMed] [Google Scholar]
- 47.Haverkamp J., van Halbeek H., Dorland L., Vliegenthart J.F.G., Pfeil R., Schauer R. High-resolution 1H-NMR spectroscopy of free and glycosidically linked O-acetylated sialic acids. Eur J Biochem. 1982;122:305–311. doi: 10.1111/j.1432-1033.1982.tb05881.x. [DOI] [PubMed] [Google Scholar]
- 48.Kamerling J.P., Vliegenthart J.F.G. Gas-liquid chromatography and mass spectrometry of sialic acids. In: Schauer R., editor. Sialic acids—chemistry, metabolism and function. vol. 10. Springer-Verlag; Vienna, Austria: 1982. pp. 95–125. (Cell biology monographs). [Google Scholar]
- 49.Manzi A.E., Dell A., Azadi P., Varki A. Studies of naturally occurring modifications of sialic acids by fast-atom bombardment-mass spectrometry—analysis of positional isomers by periodate cleavage. J Biol Chem. 1990;265:8094–8107. [PubMed] [Google Scholar]
- 50.Klein A., Diaz S., Ferreira I., Lamblin G., Roussel P., Manzi A.E. New sialic acids from biological sources identified by a comprehensive and sensitive approach: liquid chromatography–electrospray ionization-mass spectrometry (LC-ESI-MS) of SIA quinoxalinones. Glycobiology. 1997;7:421–432. doi: 10.1093/glycob/7.3.421. [DOI] [PubMed] [Google Scholar]
- 51.Kamerling J.P., Gerwig G.J. Structural analysis of naturally occurring sialic acids. In: Brockhausen I., editor. Glycobiology protocols. vol. 347. Humana Press Inc.; Totowa, NJ: 2006. pp. 69–91. Methods Mol. Biol. [DOI] [PubMed] [Google Scholar]
- 52.Klenk E., Uhlenbruck G. Uber die Abspaltung von N-Glykolyl-neuramins€aure (P-Sialins€aure) aus dem Schweine-Submaxillarismucin durch das “Receptor Destroying Enzyme.”. Hoppe Seylers Z Physiol Chem. 1957;307:266–271. [PubMed] [Google Scholar]
- 53.Handa S., Yamakawa T. The chemistry of lipids of posthemolytic residue or stroma of erythrocytes. XII chemical structure and chromatographic behaviour of hematosides obtained from equine and dog erythrocytes. Jpn J Exp Med. 1964;34:293–304. [PubMed] [Google Scholar]
- 54.Buscher H.-P., Casals-Stenzel J., Schauer R. New sialic acids—identification of N-glycoloyl-O-acetylneuraminic acids and N-acetyl-O-glycoloylneuraminic acids by improved methods for detection of N-acyl and O-acyl groups and by gas–liquid chromatography. Eur J Biochem. 1974;50:71–82. doi: 10.1111/j.1432-1033.1974.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 55.Reuter G., Pfeil R., Stoll S., Schauer R., Kamerling J.P., Versluis C., Vliegenthart J.F.G. Identification of new sialic acids derived from glycoprotein of bovine submandibular gland. Eur J Biochem. 1983;134:139–143. doi: 10.1111/j.1432-1033.1983.tb07542.x. [DOI] [PubMed] [Google Scholar]
- 56.Schauer R., Schroder C., Shukla A.K. New techniques for the investigation of structure and metabolism of sialic acids. Adv Exp Med Biol. 1984;174:75–86. doi: 10.1007/978-1-4684-1200-0_7. [DOI] [PubMed] [Google Scholar]
- 57.Bergwerff A.A., Hulleman S.H.D., Kamerling J.P., Vliegenthart J.F.G., Shaw L., Reuter G., Schauer R. Nature and biosynthesis of sialic acids in the starfish asterias rubens—identification of sialo-oligomers and detection of S-adenosyl-L-methionine: N-acylneuraminate 8-O-methyltransferase and CPM-N-acetylneuraminate monooxygenase activities. Biochimie. 1992;74:25–38. doi: 10.1016/0300-9084(92)90181-d. [DOI] [PubMed] [Google Scholar]
- 58.Warren L. N-glycolyl-8-O-methylneuraminic acid—a new form of sialic acid in the starfish Asterias forbesi. Biochim Biophys Acta. 1964;83:129–132. doi: 10.1016/0926-6526(64)90062-x. [DOI] [PubMed] [Google Scholar]
- 59.Smirnova G.P., Kochetkov N.K., Sadovskaya V.L. Gangliosides of the starfish Aphelasterias japonica—evidence for a new linkage between two N-glycolylneuraminic acid residues through the hydroxy group of the glycolic acid residue. Biochim Biophys Acta. 1987;920:47–55. doi: 10.1016/0005-2760(87)90309-2. [DOI] [PubMed] [Google Scholar]
- 60.Zanetta J.-P., Srinivasan V., Schauer R. Analysis of monosaccharides, fatty constituents and rare O-acetylated sialic acids from gonads of the starfish Asterias rubens. Biochimie. 2006;88:171–178. doi: 10.1016/j.biochi.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Inagaki M., Shiizaki M., Hiwatashi T., Miyamoto T., Higuchi R. Constituents of crinoidea—5. isolation and structure of a new glycosyl inositolphosphoceramide- type ganglioside from the feather star Comanthina schlegeli. Chem Pharm Bull. 2007;55:1649–1651. doi: 10.1248/cpb.55.1649. [DOI] [PubMed] [Google Scholar]
- 62.Arao K., Inagaki M., Higuchi R. Constituents of crinoidea, 2—isolation and structure of the novel type gangliosides from the feather star Comanthus japonica. Chem Pharm Bull. 2001;49:695–698. doi: 10.1248/cpb.49.695. [DOI] [PubMed] [Google Scholar]
- 63.Kochetkov N.K., Smirnova G.P., Chekareva N.V. Isolation and structural studies of a sulfated sialosphingolipid from the sea urchin Echinocardium cordatum. Biochim Biophys Acta. 1976;424:274–283. doi: 10.1016/0005-2760(76)90195-8. [DOI] [PubMed] [Google Scholar]
- 64.Kubo H., Irie A., Inagaki F., Hoshi M. Gangliosides from the eggs of the sea urchin, Anthocidaris crassispina. J Biochem. 1990;108:185–192. doi: 10.1093/oxfordjournals.jbchem.a123179. [DOI] [PubMed] [Google Scholar]
- 65.Kitazume S., Kitajima K., Inoue S., Haslam S.M., Morris H.R., Dell A., Lennarz W.J., Inoue Y. The occurrence of novel 9-O-sulfated N-glycolylneuraminic acid-capped α2!5-O-glycolyl-linked oligo/PolyNeu5Gc chains in sea urchin egg cell surface glycoprotein—identification of a new chain termination signal for Polysialyltransferase. J Biol Chem. 1996;271:6694–6701. doi: 10.1074/jbc.271.12.6694. [DOI] [PubMed] [Google Scholar]
- 66.Kluge A., Reuter G., Lee H., Ruch-Heeger B., Schauer R. Interaction of rat peritoneal macrophages with homologous sialidase-treated thrombocytes in vitro: biochemical and morphological studies—detection of N-(O-Acetyl) glycoloylneuraminic acid. Eur J Cell Biol. 1992;59:12–20. [PubMed] [Google Scholar]
- 67.Higuchi R., Inukai K., Jhou J.X., Honda M., Komori T., Tsuji S., Nagai Y. Biologically active glycosides from asteroidea—XXXI. glycosphingolipids from the starfish Asterias amurensis versicolor sladen, 2—structure and biological activity of ganglioside molecular species. Liebigs Ann Chem. 1993;1993:359–366. [Google Scholar]
- 68.Shukla A.K., Schroder C., Nohle U., Schauer R. Natural occurrence and preparation of O-acylated 2,3-unsaturated sialic acids. Carbohydr Res. 1987;168:199–209. doi: 10.1016/0008-6215(87)80026-5. [DOI] [PubMed] [Google Scholar]
- 69.Nohle U., Shukla A.K., Schroder C., Reuter G., Schauer R., Kamerling J.P., Vliegenthart J.F.G. Structural parameters and natural occurrence of 2-deoxy- 2,3-didehydro-N- glycoloylneuraminic. Acid Eur J Biochem. 1985;152:459–463. doi: 10.1111/j.1432-1033.1985.tb09219.x. [DOI] [PubMed] [Google Scholar]
- 70.Nadano D., Iwasaki M., Endo S., Kitajima K., Inoue S., Inoue Y. A naturally occurring deaminated neuraminic acid, 3-deoxy-D-glycero-D-galacto-nonulosonic Acid (KDN)—its unique occurrence at the nonreducing ends of oligosialyl chains in polysialoglycoprotein of rainbow trout eggs. J Biol Chem. 1986;261:11550–11557. [PubMed] [Google Scholar]
- 71.Knirel Y.A., Kocharova N.A., Shashkov A.S., Kochetkov N.K., Mamontova V.A., Solov’eva T.F. Structure of the capsular polysaccharide of Klebsiella ozaenae serotype K4 containing 3-deoxy-D-glycero-D-galacto-nonulosonic acid. Carbohydr Res. 1989;188:145–155. doi: 10.1016/0008-6215(89)84067-4. [DOI] [PubMed] [Google Scholar]
- 72.Strecker G., Wieruszeski J.-M., Michalski J.-C., Alonso C., Boilly B., Montreuil J. Characterization of lex, ley and ley antigen determinants in KDN-containing O-linked glycan chains from pleurodeles waltlii jelly coat eggs. FEBS Lett. 1992;298:39–43. doi: 10.1016/0014-5793(92)80018-c. [DOI] [PubMed] [Google Scholar]
- 73.Iwasaki M., Inoue S., Troy F.A. A new sialic acid analogue, 9-O-acetyl-deaminated neuraminic acid, and α-2,8-linked O-acetylated poly(N-glycolylneuraminyl) chains in a novel polysialoglycoprotein from salmon eggs. J Biol Chem. 1990;265:2596–2602. [PubMed] [Google Scholar]
- 74.Plancke Y., Wieruszeski J.-M., Boilly B., Strecker G. Primary structure of seven new acidic oligosaccharide-alditols from the egg jelly coats of Axolotl mexicanum and Pleurodeles waltl. Ci Cult J Braz Assoc Adv Sci. 1994;46:273–279. [Google Scholar]
- 75.Gil-Serrano A.M., Rodríguez-Carvajal M.A., Tejero-Mateo P., Espartero J.L., Thomas-Oates J., Ruiz-Sainz J.E., Buendía-Clavería A.M. Structural determination of a 5-O-methyl-deaminated neuraminic acid (Kdn)-containing polysaccharide isolated from Sinorhizobium fredii. Biochem J. 1998;334:585–594. doi: 10.1042/bj3340585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angata T., Nakata D., Matsuda T., Kitajima K., Troy F.A., II Biosynthesis of KDN (2-Keto-3-deoxy-D-glycero-D-galacto-nononic acid)—identification and characterization of a KDN-9-phosphate synthetase activity from trout testis. J Biol Chem. 1999;274:22949–22956. doi: 10.1074/jbc.274.33.22949. [DOI] [PubMed] [Google Scholar]
- 77.Varki A., Cummings R., Esko J. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 1999. Essentials of glycobiology. Chapter 15, Sialic Acids. [PubMed] [Google Scholar]
- 78.Roy R., Andersson F.O., Harms G., Kelm S., Schauer R. Synthesis of esterase-resistant 9-O-acetylated polysialoside as inhibitor of influenza-C virus hemagglutinin. Angew Chem Int Ed Engl. 1992;31:1478–1481. [Google Scholar]
- 79.Schauer R., Srinivasan G.V., Wipfler D., Kniep B., Schwartz-Albiez R. O-acetylated sialic acids and their role in immune defense. In: Wu A., editor. The molecular immunology of complex carbohydrates-3. vol 705. Springer; Boston, MA: 2011. (Advances in experimental medicine and biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato C., Hane M., Kitajima K. Relationship between ST8SIA2, polysialic acid and its binding molecules, and psychiatric disorders. A. Biosynthetic pathways of sialoglycoconjugates. Biochim Biophys Acta. 2016;1860:1739–1752. doi: 10.1016/j.bbagen.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Regl G., Kaser A., Iwersen M., Schmid H., Kohla G. The hemagglutinin-esterase of mouse hepatitis virus strain S is a sialate-4-O-acetylesterase. J Virol. 1999;73:4721–4727. doi: 10.1128/jvi.73.6.4721-4727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schauer R., Schmid H., Pommerencke J., Iwersen M., Kohla G. Metabolism and role of O-acetylated sialic acids. Adv Exp Med Biol. 2001;491:325–342. doi: 10.1007/978-1-4615-1267-7_21. [DOI] [PubMed] [Google Scholar]
- 83.Ghosh S., Bandyopadhyay S., Mukherjee K., Mallick A., Pal S., Mandal C., Bhattacharya D.K. O-acetylation of sialic acids is required for the survival of lymphoblasts in childhood acute lymphoblastic leukemia (ALL) Glycoconj J. 2007;24:17–24. doi: 10.1007/s10719-006-9007-y. [DOI] [PubMed] [Google Scholar]
- 84.Shen Y., Kohla G., Lrhorfi A.L., Sipos B., Kalthoff H., Gerwig G.J., Kamerling J.P., Schauer R., Tiralongo J. O-acetylation and de-O-acetylation of sialic acids in human colorectal carcinoma. Eur J Biochem. 2004;271:281–290. doi: 10.1046/j.1432-1033.2003.03927.x. [DOI] [PubMed] [Google Scholar]
- 85.Shi W.X., Chammas R., Varki N.M., Powell L., Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem. 1996;271(49):31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 86.Kelm S., Schauer R. Sialic acids in molecular and cellular interactions. Int Rev Cytol. 1997;175:137–240. doi: 10.1016/S0074-7696(08)62127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corfield A.P., Myerscough N., Warren B.F., Durdey P., Paraskeva C., Schauer R. Reduction of sialic acid O-acetylation in human colonic mucins in the adenoma-carcinoma sequence. Glycoconj J. 1999;16(6):307–317. doi: 10.1023/a:1007026314792. [DOI] [PubMed] [Google Scholar]
- 88.Corfield A.P., Myerscough N., Warren B.F., Durdey P., Paraskeva C., Schauer R., Kohla G., Stockfleth E. Gangliosides with O-acetylated sialic acids in tumors of neuroectodermal origin. Neurochem Res. 2002;27:583–592. doi: 10.1023/a:1020211714104. [DOI] [PubMed] [Google Scholar]
- 89.Mandal C., Schwartz-Albiez R., Vlasak R. Functions and biosynthesis of O-acetylated sialic acids. Top Curr Chem. 2015;366:1–30. doi: 10.1007/128_2011_310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmer G., Suguri T., Reuter G., Yu R.K., Schauer R., Herrler G. Modification of sialic acids by 9-O-acetylation is detected in human leucocytes using the lectin property of influenza C virus. Glycobiology. 1994;4(3):343–349. doi: 10.1093/glycob/4.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamilton W.B., Helling F., Lloyd K.O., Livingston P.O. Gangliosides expression on human malignant melanoma assessed by quantitative immune thin layer chromatography. Int J Cancer. 1993;53:1. doi: 10.1002/ijc.2910530407. [DOI] [PubMed] [Google Scholar]
- 92.Hirabayashi Y., Hirota M., Suzuki Y., Matsumoto M., Obata K., Ando S. Developmentally expressed O-acetyl ganglioside GT3 in fetal rat cerebral cortex. Neurosci Lett. 1989;106:193–198. doi: 10.1016/0304-3940(89)90225-5. [DOI] [PubMed] [Google Scholar]
- 93.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kniep B., Flegel W.A., Northoff H., Rieber E.P. CDw60 glycolipid antigens of human leukocytes: Structural characterization and cellular distribution. Blood. 1993;82:1776–1786. [PubMed] [Google Scholar]
- 95.Rieber E.P., Rank G. CDw60: a marker for human CD8 + T helper cells. J Exp Med. 1994;179:1385–1390. doi: 10.1084/jem.179.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tedder R.S., Zuckerman M.A., Goldstone A.H., Hawkins A.E., Fielding A., Briggs E.M., Irwin D., Blair S., Gorman A.M., Patterson K.G., Linch D.C., Heptonstall J., Brink N.S. Hepatitis B transmission from a contaminated cryopreservation tank. Lancet. 1995;346:137–140. doi: 10.1016/s0140-6736(95)91207-x. [DOI] [PubMed] [Google Scholar]
- 97.Chava A.K., Chatterjee M., Sundar S., Mandal C. Development of an assay for quantification of linkage-specific O-acetylated sialoglycans on erythrocytes; its application in Indian visceral leishmaniasis. J Immunol Methods. 2002;270:1–10. doi: 10.1016/s0022-1759(02)00216-8. [DOI] [PubMed] [Google Scholar]
- 98.Sharma V., Chatterjee M., Mandal C., Sen S., Basu D. Rapid diagnosis of visceral leishmaniasis using Achatinin-H, a 9-O-acetylated sialic acid binding lectin. Am J Trop Med Hyg. 1998;58:551–554. doi: 10.4269/ajtmh.1998.58.551. [DOI] [PubMed] [Google Scholar]
- 99.Chava A.K., Bandyopadhyay S., Chatterjee M., Mandal C. Sialoglycans in protozoal diseases: their detection, modes of acquisition and emerging biological roles. Glycoconj J. 2004;20:199–206. doi: 10.1023/B:GLYC.0000024251.30100.08. [DOI] [PubMed] [Google Scholar]
- 100.Chava A.K., Chatterjee M., Gerwig G.J., Kamerling J.P., Mandal C. Identification of sialic acids on Leishmania donovani amastigotes. Biol Chem. 2004;385:59–66. doi: 10.1515/BC.2004.008. [DOI] [PubMed] [Google Scholar]
- 101.Chava A.K., Chatterjee M., Sharma V., Sundar S., Mandal C. Variable degree of alternative complement pathway-mediated hemolysis in Indian visceral leishmaniasis induced by differential expression of 9-O-acetylated sialoglycans. J Infect Dis. 2004;189:1257–1264. doi: 10.1086/382752. [DOI] [PubMed] [Google Scholar]
- 102.Ravindranaths M.H., Paulson J.C., Irie R.F. Human melanoma antigen O-acetylated ganglioside GD3 is recognized by cancer antennarius lectin. J Biol Chem. 1988;263:2079–2086. [PubMed] [Google Scholar]
- 103.Pal S., Ghosh S., Mandal C., Kohla G., Brossmer R., Isecke R., Merling A., Schauer R., Schwartz-Albiez R., Bhattacharya D.K., Mandal C. Purification and characterization of 9-O-acetylated sialoglycoproteins from leukemic cells and their potential as immunological tool for monitoring childhood acute lymphoblastic leukemia. Glycobiology. 2004;14(10):859–870. doi: 10.1093/glycob/cwh111. [DOI] [PubMed] [Google Scholar]
- 104.Pal S., Ghosh S., Bandyopadhyay S., Mandal C., Bandhyopadhyay S., Kumar Bhattacharya D. Mandal C differential expression of 9-O-acetylated sialoglycoconjugates on leukemic blasts: a potential tool for long-term monitoring of children with acute lymphoblastic leukemia. Int J Cancer. 2004;111:270–277. doi: 10.1002/ijc.20246. [DOI] [PubMed] [Google Scholar]
- 105.Ghosh S., Bandyopadhyay S., Bhattacharya D.K. Mandal C altered erythrocyte membrane characteristics during anemia in childhood acute lymphoblastic leukemia. Ann Hematol. 2005;84:76–84. doi: 10.1007/s00277-004-0933-0. [DOI] [PubMed] [Google Scholar]
- 106.Ghosh S., Bandyopadhyay S., Pal S., Das B., Bhattacharya D.K., Mandal C. Increased interferon gamma production by peripheral blood mononuclear cells in response to stimulation of overexpressed disease-specific 9-O-acetylated sialoglycoconjugates in children suffering from acute lymphoblastic leukaemia. Br J Haematol. 2005;128:35–41. doi: 10.1111/j.1365-2141.2004.05256.x. [DOI] [PubMed] [Google Scholar]
- 107.Ghosh S., Bandyopadhyay S., Mallick A., Pal S., Vlasak R., Bhattacharya D.K., Mandal C. Interferon gamma promotes survival of lymphoblasts overexpressing 9-O-acetylated sialoglycoconjugates in childhood acute lymphoblastic leukaemia (ALL) J Cell Biochem. 2005;95:206–216. doi: 10.1002/jcb.20382. [DOI] [PubMed] [Google Scholar]
- 108.Fahr C., Schauer R. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J Invest Dermatol. 2001;116:254–260. doi: 10.1046/j.1523-1747.2001.01237.x. [DOI] [PubMed] [Google Scholar]
- 109.Cheresh D.A., Varki A.P., Varki N.M., Stallcup W.B., Levine J., Reisfeld R.A. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984;259:7453–7459. [PubMed] [Google Scholar]
- 110.Cavdarli S., Dewald J.H., Yamakawa N., Guérardel Y., Terme M., Le Doussal J.M., Delannoy P. Groux-Degroote S identification of 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) as main O-acetylated sialic acidspecies of GD2 in breast cancer cells. Glycoconj J. 2019;36:79–90. doi: 10.1007/s10719-018-09856-w. [DOI] [PubMed] [Google Scholar]
- 111.Sinha D., Mandal C., Bhattacharya D.K. Identification of 9-O acetyl sialoglycoconjugates (9-OAcSGs) as biomarkers in childhood acute lymphoblastic leukemia using a lectin, AchatininH, as a probe. Leukemia. 1999;13:119–125. doi: 10.1038/sj.leu.2401239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sinha D., Mandal C., Bhattacharya D.K. A novel method for prognostic evaluation of childhood acute lymphoblastic leukemia. Leukemia. 1999;13:309–312. doi: 10.1038/sj.leu.2401312. [DOI] [PubMed] [Google Scholar]
- 113.Sinha D., Mandal C., Bhattacharya D.K. Development of a simple, blood based lymphoproliferation assay to assess the clinical status of patients acute lymphoblastic leukemia. Leuk Res. 1999;23:433–439. doi: 10.1016/s0145-2126(98)00184-2. [DOI] [PubMed] [Google Scholar]
- 114.Sinha D., Mandal C., Bhattacharya D.K. A colorimetric assay to evaluate the chemotherapeutic response of children with acute lymphoblastic leukemia (ALL) employing Achatinin-H: a 9-O acetyl sialic acid binding lectin. Leuk Res. 1999;23:803–809. doi: 10.1016/s0145-2126(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 115.Pal S., Chatterjee M., Bhattacharya D.K., Bandhyopadhyay S., Mandal C. Identification and purification of cytolytic antibodies directed against O-acetylated sialic acid in childhood acute lymphoblastic leukemia. Glycobiology. 2000;10:539–549. doi: 10.1093/glycob/10.6.539. [DOI] [PubMed] [Google Scholar]
- 116.Pal S., Chatterjee M., Bhattacharya D.K., Bandhyopadhyay S., Mandal C., Mandal C. O-acetyl sialic acid specific IgM in childhood acute lymphoblastic leukaemia. Glycoconj J. 2001;18:529–537. doi: 10.1023/a:1019692329568. [DOI] [PubMed] [Google Scholar]
- 117.Warren L., Felsenfeld H. The biosynthesis of sialic acids. J Biol Chem. 1962;237:1421–1431. [PubMed] [Google Scholar]
- 118.Komfeld S., Kornfeld R., Neufeld E.F., O'Brien P.J. The feedback control of sugar nucleotide biosynthesis in liver. Proc Natl Acad Sci U S A. 1964;52:371–379. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roseman S. The synthesis of carbohydrates by muluglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970;5:270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- 120.Neufeld E.J., Pastan I. A mutant fibroblast cell line defective in glycoprotein synthesis due to a deficiency of glucosamine phosphate acetyltransferase. Arch Biochem Biophys. 1978;188:323–327. doi: 10.1016/s0003-9861(78)80016-2. [DOI] [PubMed] [Google Scholar]
- 121.Schauer R. vol. 10. Springer-Verlag; New York: 1982. Sialic acids: chemistry, metabolism and function, cell biology monographs. [Google Scholar]
- 122.Hildreth J., IV, Sacks L., Hancock L.W. N-acetyl-neuraminic acid accumulation in a buoyant lysosomal fraction of cultured fibroblasts from patients with infantile generalized N-acetyl-neuraminic acid storage disease. Biochem Biophys Res Common. 1986;139:838–844. doi: 10.1016/s0006-291x(86)80066-3. [DOI] [PubMed] [Google Scholar]
- 123.Renlund M., Tietze F., Gahl W.A. Defective sialic acid egress from isolated fibroblast lysosomes of patients with Salla disease. Science. 1986;232:759–762. doi: 10.1126/science.3961501. [DOI] [PubMed] [Google Scholar]
- 124.Schcop H.J., Schauer R., Faillard H. On the biosynthesis of A'-glycolyneuraminic acid. Oxidative formation of A'-glycolylneuraminic acid from A'-acetylneuraminic acid. Hoppe Seylers Z Physiol Chem. 1969;350:155–162. [PubMed] [Google Scholar]
- 125.Li Y.-T., Nakagawa H., Ross S.A., Hansson G.C., Li S.-C. A novel sialidase which releases 2,7-anhydro-a-/V-acetylneuraminic acid from sialoglycoconjugates. J Biol Chem. 1990;1990(265):21629–21633. [PubMed] [Google Scholar]
- 126.Saito M., Rosenberg A. Identification and characterization of Af-acetyl-2,3-didehydro-2-deoxyneuraminic acid as a metabolite in mammalian brain. Biochemistry. 1984;23:3784–3788. doi: 10.1021/bi00311a034. [DOI] [PubMed] [Google Scholar]
- 127.Nohle U., Shukla A.K., Schroder C., Reuter G., Schauer R., Kamerling J.P., Vliegenthart J.F.G. Structural parameters and natural occurrence of 2-deoxy-2,3-didehydro-A'-glycoloylneuraminic acid. Eur J Biochem. 1985;152:459–463. doi: 10.1111/j.1432-1033.1985.tb09219.x. [DOI] [PubMed] [Google Scholar]
- 128.Shukla A.K., Schroder C., Nohle U., Schauer R. Natural occurrence and preparation of O-acetylated 2,3Hinsaturated sialic acids. Carbohydr Res. 1987;168:199–209. doi: 10.1016/0008-6215(87)80026-5. [DOI] [PubMed] [Google Scholar]
- 129.Suzuki M., Suzuki A., Yamakawa T., Matsunaga E. Characterization of 2,7-anhydro-A'-acetylneuraminic acid in human wet cerumen. J Biochem (Tokyo) 1985;97:509–515. doi: 10.1093/oxfordjournals.jbchem.a135085. [DOI] [PubMed] [Google Scholar]
- 130.Beau J.M., Schauer R., Haverkamp J., Kamerling J.P., Dorland L., Vliegenthart J.F.G. Chemical behaviour of cytidine 5'-monophospho- ZV-acetyl-beta-D-neuramiruc acid under neutral and alkaline conditions. Eur J Biochem. 1984;140:203–208. doi: 10.1111/j.1432-1033.1984.tb08087.x. [DOI] [PubMed] [Google Scholar]
- 131.Pozsgay V., Jennings H.J., Kasper D.L. 4,8-anhydro-jV-acetylneuraminjc acid; isolation from edible bird's nest and structure determination. Eur J Biochem. 1987;162:445–450. doi: 10.1111/j.1432-1033.1987.tb10622.x. [DOI] [PubMed] [Google Scholar]
- 132.Manzi A.E., Dell A., Azadi P., Varki A. Studies of naturally occurring modifications of sialic acids by fast-atom bombardment-mass spectrometry. Analysis of positional isomers by penodate cleavage. J Biol Chem. 1990;265:8094–8107. [PubMed] [Google Scholar]
- 133.Inoue S., Kitajima K. KDN (deaminated neuraminic acid): dreamful past and exciting future of the newest member of the sialic acid family. Glycoconj J. 2006;23:277–290. doi: 10.1007/s10719-006-6484-y. [DOI] [PubMed] [Google Scholar]
- 134.Wagstaff B.A., Rejzek M., Field R.A. Identification of a Kdn biosynthesis pathway in the haptophyte Prymnesium parvum suggests widespread sialic acid biosynthesis among microalgae. J Biol Chem. 2018;293:16277–16290. doi: 10.1074/jbc.RA118.004921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corfield T. Bacterial sialidases—roles in pathogenicity and nutrition. Glycobiology. 1992;2:509–521. doi: 10.1093/glycob/2.6.509. [DOI] [PubMed] [Google Scholar]
- 136.Bouchet V., Hood D.W., Li J., Brisson J.R., Randle G.A., Martin A., Li Z., Goldstein R., Schweda E.K., Pelton S.I., Richards J.C., Moxon E.R. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A. 2003;100:8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shakhnovich E., King S., Wiser J.N. Neuraminidase expressed by Streptococcus pneumoniaedesialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect Immun. 2002;70:7161–7164. doi: 10.1128/IAI.70.12.7161-7164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sohanpal B.K., El Labany S., Lahooti M., Plumbridge J.A., Blomfield I.C. Integrated regulatory responses of fimB to Nacetylneuraminic (sialic) acid and GlcNAc in Escherichia coli K-12. Proc Natl Acad Sci U S A. 2004;101:16322–16327. doi: 10.1073/pnas.0405821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sohanpal B.K., Friar S., Roobol J., Plumbridge J.A., Blomfield I.C. Multiple co-regulatory elements and IHF are necessary for the control of fimB expression in response to sialic acid and N-acetylglucosamine in Escherichia coli K-12. Mol Microbiol. 2007;63:1223–1236. doi: 10.1111/j.1365-2958.2006.05583.x. [DOI] [PubMed] [Google Scholar]
- 140.Steenbergen S.M., Lichtensteiger C.A., Caughlan R., Garfinkle J., Fuller T.E., Vimr E.R. Sialic acid metabolism and systemic pasteurellosis. Infect Immun. 2005;73:1284–1294. doi: 10.1128/IAI.73.3.1284-1294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stafford G., Roy S., Honma K., Sharma A. Sialic acid, periodontal pathogens and Tannerella forsythia: stick around and enjoy the feast! Mol Oral Microbiol. 2012;27:11–22. doi: 10.1111/j.2041-1014.2011.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Severi E., Randle G., Kivlin P., Whitfield K., Young R., Moxon R., Kelly D., Hood D., Thomas G.H. Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol Microbiol. 2005;58:1173–1185. doi: 10.1111/j.1365-2958.2005.04901.x. [DOI] [PubMed] [Google Scholar]
- 143.Post D.M., Mungur R., Gibson B.W., Munson R.S., Jr. Identification of a novel sialic acid transporter in Haemophilus ducreyi. Infect Immun. 2005;73:6727–6735. doi: 10.1128/IAI.73.10.6727-6735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Condemine G., Berrier C., Plumbridge J., Ghazi A. Function and expression of an N-acetylneuraminic acid-inducible outer membrane channel in Escherichia coli. J Bacteriol. 2005;187:1959–1965. doi: 10.1128/JB.187.6.1959-1965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shell D.M., Chiles L., Judd R.C., Seal S., Rest R.F. The Neisseria lipooligosaccharide-specific alpha-2,3-sialyltransferase is a surface-exposed outer membrane protein. Infect Immun. 2002;70:3744–3751. doi: 10.1128/IAI.70.7.3744-3751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Claus H., Maiden M.C., Maag R. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology. 2002;148:1813–1819. doi: 10.1099/00221287-148-6-1813. [DOI] [PubMed] [Google Scholar]
- 147.Steenbergen S.M., Wrona T.J., Vimr E.R. Functional analysis of the sialyltransferase complexes in Escherichia coli K1 and K92. J Bacteriol. 1992;174:1099–1108. doi: 10.1128/jb.174.4.1099-1108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deszo E.L., Steenbergen S.M., Freedberg D.I., Vimr E.R. Escherichia coli K1 polysialic acid O-acetyltransferase gene, neuO, and the mechanism of capsule form variation involving a mobile contingency locus. Proc Natl Acad Sci U S A. 2005;102:5564–5569. doi: 10.1073/pnas.0407428102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Steenbergen S.M., Lee Y.C., Vann W.F., Vionnet J., Wright L.F., Vimr E.R. Separate pathways for O acetylation of polymeric and monomeric sialic acids and identification of sialyl O-acetyl esterase in Escherichia coli K1. J Bacteriol. 2006;188:6195–6206. doi: 10.1128/JB.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bergfeld A.K., Claus H., Lorenzen N.K., Spielmann F., Vogel U. The polysialic acid-specific O-acetyltransferase OatC from Neisseria meningitidis serogroup C evolved apart from other bacterial sialate O-acetyltransferases. J Biol Chem. 2009;284:6–16. doi: 10.1074/jbc.M807518200. [DOI] [PubMed] [Google Scholar]
- 151.Chaffin D.O., McKinnon K., Rubens C.E. CpsK of Streptococcus agalactiae exhibits alpha2,3-sialyltransferase activity in Haemophilus ducreyi. Mol Microbiol. 2002;45(1):109–122. doi: 10.1046/j.1365-2958.2002.02988.x. [DOI] [PubMed] [Google Scholar]
- 152.Lewis A.L., Hensler M.E., Varki A., Nizet V. The group B streptococcal sialic acid O-acetyltransferase is encoded by neuD, a conserved component of bacterial sialic acid biosynthetic gene clusters. J Biol Chem. 2006;281:11186–11192. doi: 10.1074/jbc.M513772200. [DOI] [PubMed] [Google Scholar]
- 153.Fox K.L., Cox A.D., Gilbert M., Wakarchuk W.W., Li J., Makepeace K., Richards J.C., Moxon E.R., Hood D.W. Identification of a bifunctional lipopolysaccharide sialyltransferase in Haemophilus influenzae: Incorporation of disialic acid. J Biol Chem. 2006;281:40024–40032. doi: 10.1074/jbc.M602314200. [DOI] [PubMed] [Google Scholar]
- 154.Hood D.W., Cox A.D., Gilbert M., Makepeace K., Walsh S., Deadman M.E., Cody A., Martin A., Månsson M., Schweda E.K., Brisson J.R., Richards J.C., Moxon E.R., Wakarchuk W.W. Identification of a lipopolysaccharide alpha-2,3-sialyltransferase from Haemophilus influenzae. Mol Microbiol. 2001;39:341–350. doi: 10.1046/j.1365-2958.2001.02204.x. [DOI] [PubMed] [Google Scholar]
- 155.Bouchet V., Hood D.W., Li J., Brisson J.-R., Randle G.A., Martin A., Li Z., Goldstein R., Schweda E.K., Pelton S.I., Richards J.C., Moxon E.R. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A. 2003;100:8890–8898. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gilbert M., Brisson J.R., Karwaski M.F., Michniewicz J., Cunningham A.M., Wu Y., Young N.M., Wakarchuk W.W. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-MHz (1)H and (13)C NMR analysis. J Biol Chem. 2000;275:3896–3906. doi: 10.1074/jbc.275.6.3896. [DOI] [PubMed] [Google Scholar]
- 157.Houliston R.S., Endtz H.P., Yuki N., Li J., Jarrell H.C., Koga M., van Belkum A., Karwaski M.F., Wakarchuk W.W., Gilbert M. Identification of a sialate O-acetyltransferase from Campylobacter jejuni: demonstration of direct transfer to the C-9 position of terminalalpha-2, 8-linked sialic acid. J Biol Chem. 2006;281:11480–11486. doi: 10.1074/jbc.M512183200. [DOI] [PubMed] [Google Scholar]
- 158.Harvey H.A., Swords W.E., Apicella M.A. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic neisseria and haemophilus. J Autoimmun. 2001;16:257–262. doi: 10.1006/jaut.2000.0477. [DOI] [PubMed] [Google Scholar]
- 159.Bergfeld A.K., Claus H., Vogel U., Mühlenhoff M. Biochemical characterization of the polysialic acid-specific O-acetyltransferase NeuO of Escherichia coli K1. J Biol Chem. 2007;282:22217–22227. doi: 10.1074/jbc.M703044200. [DOI] [PubMed] [Google Scholar]
- 160.Vimr E., Lichtensteiger C. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 2002;10:254–257. doi: 10.1016/s0966-842x(02)02361-2. [DOI] [PubMed] [Google Scholar]
- 161.Marques M.B., Kasper D.L., Pangburn M.K., Wessels M.R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun. 1992;60:3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Figueira M.A., Ram S., Goldstein R. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun. 2007;75:325–333. doi: 10.1128/IAI.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ram S., Lewis L.A., Rice P.A. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Severi E., Hood D.W., Thomas G.H. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153(Pt. 9):2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 165.Matrosovich M., Herrler G., Klenk H.D. Sialic acid receptors of viruses. Top Curr Chem. 2015;367:1–28. doi: 10.1007/128_2013_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Soares R.M.A., Soares R.M.D., Alviano D.S., Angluster J., Alviano C.S., Travassos L.R. Identification of sialic acids on the cell surface of Candida albicans. Biochim Biophys Acta Gen Subj. 2000;1474:262–268. doi: 10.1016/s0304-4165(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 167.Saito M., Kitamura H., Sugiyama K. Occurrence of gangliosides in the common squid and pacific octopus among protostomia. Biochim Biophys Acta Biomembr. 2001;1511:271–280. doi: 10.1016/s0005-2736(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 168.Rodrigues M.L., Rozental S., Couceiro J.N., Angluster J., Alviano C.S., Travassos L.R. Identification of N-acetylneuraminic acid and its 9-O-acetylated derivative on the cell surface of Cryptococcus neoformans: influence on fungal phagocytosis. Infect Immun. 1997;65:4937–4942. doi: 10.1128/iai.65.12.4937-4942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deshpande N., Wilkins M.R., Packer N., Nevalainen H. Protein glycosylation pathways in filamentous fungi. Glycobiology. 2008;18:626–637. doi: 10.1093/glycob/cwn044. [DOI] [PubMed] [Google Scholar]
- 170.Wasylnka J.A., Simmer M.I., Moore M.M. Differences in sialic acid density in pathogenic and non-pathogenic Aspergillus species. Microbiology. 2001;147(Pt. 4):869–877. doi: 10.1099/00221287-147-4-869. [DOI] [PubMed] [Google Scholar]
- 171.Mello T.P., Oliveira S.S.C., Frasés S., Branquinha M.H., ALS S. Surface properties, adhesion and biofilm formation on different surfaces by Scedosporium spp. and Lomentospora prolificans. Biofouling. 2018;24:1–15. doi: 10.1080/08927014.2018.1503652. [DOI] [PubMed] [Google Scholar]
- 172.Mello T.P., Aor A.C., Gonçalves D.S., Seabra S.H., Branquinha M.H., Santos A.L.S.D. Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans: a comparative study of surface molecules produced by conidial and germinated conidial cells. Mem Inst Oswaldo Cruz. 2018;113(6) doi: 10.1590/0074-02760180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nesbitt J.R., Steves E.Y., Schonhofer C.R., Cait A., Manku S.S., Yeung J.H.F., Bennet A.J., McNagny K.M., Choy J.C., Hughes M.R., Moore M.M. The Aspergillus fumigatus Sialidase (Kdnase) contributes to cell wall integrity and virulence in amphotericin b-treated mice. Front Microbiol. 2018;8:2706. doi: 10.3389/fmicb.2017.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim S. A novel core 1 O-linked glycan-specific binding lectin from the fruiting body of Hericium erinaceus. Int J Biol Macromol. 2018;107(Pt. B):1528–1537. doi: 10.1016/j.ijbiomac.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 175.Ribeiro J.P., Ali Abol Hassan M., Rouf R., Tiralongo E., May T.W., Day C.J., Imberty A., Tiralongo J., Varrot A. Biophysical characterization and structural determination of the potent cytotoxic Psathyrella asperospora lectin. Proteins. 2017;85:969–975. doi: 10.1002/prot.25265. [DOI] [PubMed] [Google Scholar]
- 176.Bhari R., Kaur B., Singh R.S. Lectin activity in mycelial extracts of Fusarium species. Braz J Microbiol. 2016;47:775–780. doi: 10.1016/j.bjm.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matsumoto S., Ikura K., Ueda M., Sasaki R. Characterization of a human glycoprotein (erythropoietin) produced in cultured tobacco cells. Plant Mol Biol. 1995;27:1163–1172. doi: 10.1007/BF00020889. [DOI] [PubMed] [Google Scholar]
- 178.Lerouge P., Cabanes-Macheteau M., Rayon C., Fischette-Laine A.C., Gomord V., Faye L. N-Glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol Biol. 1998;38:31–48. [PubMed] [Google Scholar]
- 179.Bourbouze R., Akiki C., Chardon-Loriaux I., Percheron F. Mise en evidence de derives de l’acide neuraminique dans des glycoprotéines végétales. Carbohydr Res. 1982;106:21–30. [Google Scholar]
- 180.Freire-de-Lima L., Fonseca L.M., Oeltmann T., Mendonça-Previato L., Previato J.O. The trans-sialidase, the major Trypanosoma cruzi virulence factor: three decades of studies. Glycobiology. 2015;25:1142–1149. doi: 10.1093/glycob/cwv057. [DOI] [PubMed] [Google Scholar]
- 181.Yoshida M., Fuse G., Matsui T., Ouchi S. Identification of sialic acids in cell adhesion molecule, contact site A from Dictyostelium discoideum. Biochem Biophys Res Commun. 1992;188:794–798. doi: 10.1016/0006-291x(92)91126-b. [DOI] [PubMed] [Google Scholar]
- 182.Matta M.A., Aleksitch V., Angluster J., Alviano C.S., De Souza W., Andrade A.F., Esteves M. Occurrence of N-acetyl- and N-O-diacetyl-neuraminic acid derivatives in wild and mutant Crithidia fasciculata. J Parasitol Res. 1995;81:426–433. doi: 10.1007/BF00931505. [DOI] [PubMed] [Google Scholar]
- 183.Watarai S., Sugimoto C., Hosotani-Kaihara K., Kobayashi K., Onuma M., Lee J.T., Kushi Y., Handa S., Yasuda T. Isolation and characterisation of gangliosides from Theileria sergenti. J Vet Med Sci. 1996;58:1099–1105. doi: 10.1292/jvms.58.11_1099. [DOI] [PubMed] [Google Scholar]
- 184.Avron B., Chayen A., Stolarsky T., Schauer R., Reuter G., Mirelman D. A stage-specific sialoglycoprotein in encysting cells of Entamoeba invadens Mol. Mol Biochem Parasitol. 1987;25:257–266. doi: 10.1016/0166-6851(87)90089-2. [DOI] [PubMed] [Google Scholar]
- 185.Sorice M., Griggi T., Nicodemo G., Garofalo T., Marangi M., Sanguigni S., Becker S.I., Mirelman D. Evidence for the existence of ganglioside molecules in the antigen of Entamoeba histolytica. Parasite Immunol. 1996;18:133–137. doi: 10.1046/j.1365-3024.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- 186.Buschiazzo A., Amaya M.F., Cremona M.L., Frasch A.C., Alzari P.M. The crystal structure and mode of action of trans-sialidase, a key enzyme in Trypanosoma cruzi pathogenesis. Mol Cell. 2002;10:757–768. doi: 10.1016/s1097-2765(02)00680-9. [DOI] [PubMed] [Google Scholar]
- 187.Schenkman S., Jiang M.S., Hart G.W., Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stagespecific epitope required for invasion of mammalian cells. Cell. 1991;65:1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- 188.Schenkman S., Kurosaki T., Ravetch J.V., Nussenzweig V. Evidence for the participation of the Ssp-3 antigen in the invasion of nonphagocytic mammalian cells by Trypanosoma cruzi. J Exp Med. 1992;175:1635–1641. doi: 10.1084/jem.175.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Frevert U., Schenkman S., Nussenzweig V. Stage-specific expression and intracellular shedding of the cell surface trans-sialidase of Trypanosoma cruzi. Infect Immun. 1992;60:2349–2360. doi: 10.1128/iai.60.6.2349-2360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pereira-Chioccola V.L., Acosta-Serrano A., Correia de Almeida I., Ferguson M.A., Souto-Padron T., Rodrigues M.M., Travassos L.R., Schenkman S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J Cell Sci. 2000;113:1299–1307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 191.Costa F., Franchin G., Pereira-Chioccola V.L., Ribeirao M., Schenkman S., Rodrigues M.M. Immunization with a plasmidDNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine. 1998;16:768–774. doi: 10.1016/s0264-410x(97)00277-6. [DOI] [PubMed] [Google Scholar]
- 192.Belen Carrillo M., GaoW H.M., Alroy J., Moore J.B., Beverley S.M., Pereira M.A. Heterologous expression of Trypanosoma cruzi trans-sialidase in Leishmania major enhances virulence. Infect Immun. 2000;68:2728–2734. doi: 10.1128/iai.68.5.2728-2734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Leguizamon M.S., Mocetti E., Garcia Rivello H., Argibay P., Campetella O. Trans-sialidase from Trypanosoma cruzi induces apoptosis in cells from the immune system in vivo. J Infect Dis. 1999;180:1398–1402. doi: 10.1086/315001. [DOI] [PubMed] [Google Scholar]
- 194.Todeschini A.R., Girard M.F., Wieruszeski J.M., Nunes M.P., DosReis G.A., Mendonca-Previato L., Previato J.O. Trans-Sialidase from Trypanosoma cruzi binds hostT-lymphocytes in a lectin manner. J Biol Chem. 2002;16:45962–45968. doi: 10.1074/jbc.M203185200. [DOI] [PubMed] [Google Scholar]
- 195.Argibay P.F., Di Noia J.M., Hidalgo A., Mocetti E., Barbich M., Lorenti A.S., Bustos D., Tambutti M., Hyon S.H., Frasch A.C., Sanchez D.O. Trypanosoma cruzi surface mucin TcMuc-e2 expressed on higher eukaryotic cells induces human T cell anergy, which is reversible. Glycobiology. 2002;12:25–32. doi: 10.1093/glycob/12.1.25. [DOI] [PubMed] [Google Scholar]
- 196.Ferguson M.A. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. Review. [DOI] [PubMed] [Google Scholar]
- 197.Mehlert A., Zitzmann N., Richardson J.M., Treumann A., Ferguson M.A. The glycosylation of the variant surface glycoproteins and procyclic acidic repetitive proteins of Trypanosoma brucei. Mol Biochem Parasitol. 1998;91:145–152. doi: 10.1016/s0166-6851(97)00187-4. [DOI] [PubMed] [Google Scholar]
- 198.Ferguson M.A., Murray P., Rutherford H., McConville M.J. A simple purification of procyclic acidic repetitive protein and demonstration of a sialylated glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1993;291:51–55. doi: 10.1042/bj2910051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Engstler M., Reuter G., Schauer R. The developmentally regulated trans-sialidase from Trypanosoma brucei sialylates the procyclic acidic repetitive protein. Mol Biochem Parasitol. 1993;61:1–13. doi: 10.1016/0166-6851(93)90153-o. [DOI] [PubMed] [Google Scholar]
- 200.Chayen A., Avron B., Nuchamowitz Y., Mirelman D. Appearance of sialoglycoproteins in encysting cells of Entamoeba histolytica. Infect Immun. 1988;56:673–681. doi: 10.1128/iai.56.3.673-681.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leitch G.J., Dickey A.D., Udezulu I.A., Bailey G.B. Entamoeba histolytica trophozoites in the lumen and mucus blanket of rat colons studied in vivo. Infect Immun. 1985;47:68–73. doi: 10.1128/iai.47.1.68-73.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Orlandi P.A., Klotz F.W., Haynes J.D. Amalaria invasion receptor, the 175-kilodalton erythrocyte-binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac (alpha 2-3)Gal-sequences of glycophorin A. J Cell Biol. 1992;116:901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Klotz F.W., Orlandi P.A., Reuter G., Cohen S.J., Haynes J.D., Schauer R., Howard R.J., Palese P., Miller L.H. Binding of Plasmodium falciparum 175-kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic acid but not its O-acetylated form. Mol Biochem Parasitol. 1992;51:49–54. doi: 10.1016/0166-6851(92)90199-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dias Filho B.P., Alviano C.S., de Souza W., Angluster J. Polysaccharide and glycolipid composition in Tritrichomonas foetus. Int J Biochem. 1988;20:329–335. doi: 10.1016/0020-711x(88)90360-6. [DOI] [PubMed] [Google Scholar]
- 205.Babal P., Pindak F.F., Russell L.C., Gardner W.A., Jr. Sialic acid-specific lectin from Tritrichomonas foetus. Biochim Biophys Acta. 1999;1428:106–116. doi: 10.1016/s0304-4165(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 206.Dias Filho B.P., Andrade A.F., de Souza W., Esteves M.J., Angluster J. Cell surface saccharide differences in drug-susceptible and drugresistant strains of Trichomonas vaginalis. Microbios. 1992;71:55–64. [PubMed] [Google Scholar]
- 207.Kneipp L.F., Andrade A.F., de Souza W., Angluster J., Alviano C.S., Travassos L.R. Trichomonas vaginalis and Tritrichomonas foetus: expression of chitin at the cell surface. Exp Parasitol. 1998;89:195–204. doi: 10.1006/expr.1998.4290. [DOI] [PubMed] [Google Scholar]
- 208.Babal P., Russell L.C. Sialic acid-specific lectin-mediated adhesion of Tritrichomonas foetus and Tritrichomonas mobilensis. J Parasitol. 1999;85:33–40. [PubMed] [Google Scholar]
- 209.Gross U., Hambach C., Windeck T., Heesemann J. Toxoplasma gondii: uptake of fetuin and identification of a 15-kDa fetuinbinding protein. Parasitol Res. 1993;79:191–194. doi: 10.1007/BF00931891. [DOI] [PubMed] [Google Scholar]
- 210.Chatterjee M., Chava A.K., Kohla G., Pal S., Merling A., Hinderlich S., Unger U., Strasser P., Gerwig G.J., Kamerling J.P., Vlasak R., Crocker P.R., Schauer R., Schwartz-Albiez R., Mandal C. Identification and characterization of adsorbed serum sialoglycans on Leishmania donovani promastigotes. Glycobiology. 2003;13:351–361. doi: 10.1093/glycob/cwg027. [DOI] [PubMed] [Google Scholar]
- 211.Allaire K.M., Watson G.M. Rho participates in chemoreceptor-induced changes in morphology to hair bundle mechanoreceptors of the sea anemone, Nematostella vectensis. Comp Biochem Physiol A Mol Integr Physiol. 2013;165:139–148. doi: 10.1016/j.cbpa.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 212.Ozacmak V.H., Thorington G.U., Fletcher W.H. Hessinger DAN-acetylneuraminic acid (NANA) stimulates in situ cyclic AMP production in tentacles of sea anemone (Aiptasia pallida): possible role in chemosensitization of nematocyst discharge. J Exp Biol. 2001;204(Pt. 11):2011–2020. doi: 10.1242/jeb.204.11.2011. [DOI] [PubMed] [Google Scholar]
- 213.Watson G.M., Mire P. Hudson RRFrequency specificity of vibration dependent discharge of nematocysts in sea anemones. J Exp Zool. 1998;281:582–593. [PubMed] [Google Scholar]
- 214.Watson G.M. Roberts J Chemoreceptor-mediated polymerization and depolymerization of actin in hair bundles of sea anemones. Cell Motil Cytoskeleton. 1995;30:208–220. doi: 10.1002/cm.970300305. [DOI] [PubMed] [Google Scholar]
- 215.Mire-Thibodeaux P., Watson G.M. Morphodynamic hair bundles arising from sensory cell/supporting cell complexes frequency-tune nematocyst discharge in sea anemones. J Exp Zool. 1994;268:282–292. doi: 10.1002/jez.1402680404. [DOI] [PubMed] [Google Scholar]
- 216.Watson G.M., Hessinger D.A. Receptors for N-acetylated sugars may stimulate adenylate cyclase to sensitize and tune mechanoreceptors involved in triggering nematocyst discharge. Exp Cell Res. 1992;198:8–16. doi: 10.1016/0014-4827(92)90142-u. [DOI] [PubMed] [Google Scholar]
- 217.Imamichi Y., Yokoyama Y. Purification, characterization and cDNA cloning of a novel lectin from the jellyfish Nemopilema nomurai. Comp Biochem Physiol B Biochem Mol Biol. 2010;156:12–18. doi: 10.1016/j.cbpb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 218.O'Regan N.L., Steinfelder S., Schwedler C., Rao G.B., Srikantam A., Blanchard V., Hartmann S. Filariasis asymptomatically infected donors have lower levels of disialylated IgG compared to endemic normals. Parasite Immunol. 2014;36(12):713–720. doi: 10.1111/pim.12137. [DOI] [PubMed] [Google Scholar]
- 219.Hoang V.C., Williams M.A., Simpson H.V. Monosaccharide composition of fundic and duodenal mucins in sheep infected with Haemonchus contortus or Teladorsagia circumcincta. Vet Parasitol. 2010;170:253–261. doi: 10.1016/j.vetpar.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 220.Ramajo-Hernández A., Oleaga A., Ramajo-Martín V., Pérez-Sánchez R. Carbohydrate profiling and protein identification of tegumental and excreted/secreted glycoproteins of adult Schistosoma bovis worms. Vet Parasitol. 2007;144(1–2):45–60. doi: 10.1016/j.vetpar.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 221.Elayoubi F.A., Craig P.S. Echinococcus granulosus coproantigens: chromatographic fractionation and characterization. Parasitology. 2004;128(Pt. 4):455–465. doi: 10.1017/s0031182003004712. [DOI] [PubMed] [Google Scholar]
- 222.Dennis R.D., Baumeister S., Irmer G., Gasser R.B. Geyer E Chromatographic and antigenic properties of Echinococcus granulosus hydatid cyst-derived glycolipids. Parasite Immunol. 1993;15:669–681. doi: 10.1111/j.1365-3024.1993.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 223.Holmén J.M., Olson F.J., Karlsson H., Hansson G.C. Two glycosylation alterations of mouse intestinal mucins due to infection caused by the parasite Nippostrongylus brasiliensis. Glycoconj J. 2002;19:67–75. doi: 10.1023/a:1022589015687. [DOI] [PubMed] [Google Scholar]
- 224.Karlsson N.G., Olson F.J., Jovall P.A., Andersch Y., Enerbäck L., Hansson G.C. Identification of transient glycosylation alterations of sialylated mucin oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem J. 2000;350(Pt 3):805–814. [PMC free article] [PubMed] [Google Scholar]
- 225.Ishikawa N. Histochemical characteristics of the goblet cell mucins and their role in defence mechanisms against Nippostrongylus brasiliensis infection in the small intestine of mice. Parasite Immunol Parasite Immunol. 1994;16:649–654. doi: 10.1111/j.1365-3024.1994.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 226.Cummings R.D., Nyame A.K. Glycobiology of schistosomiasis. FASEB J. 1996;10:838–848. doi: 10.1096/fasebj.10.8.8666160. [DOI] [PubMed] [Google Scholar]
- 227.Makaaru C.K., Damian R.T., Smith D.F. Cummings RDThe human blood fluke Schistosoma mansoni synthesizes a novel type of glycosphingolipid. J Biol Chem. 1992;267:2251–2257. [PubMed] [Google Scholar]
- 228.Nyame K., Smith D.F., Damian R.T., Cummings R.D. Complex-type asparagine-linked oligosaccharides in glycoproteins synthesized by Schistosoma mansoni adult males contain terminal beta-linked N-acetylgalactosamine. J Biol Chem. 1989;264:3235–3243. [PubMed] [Google Scholar]
- 229.Khan N.A., Sotelo J. Presentation of a membrane cysticercus antigen and its homology with excretory--secretory antigen. Acta Leiden. 1989;57:123–129. [PubMed] [Google Scholar]
- 230.Albro P.W., Schroeder J.L., Corbett J.T. Lipids of the earthworm Lumbricus terrestris. Lipids. 1992;27:136–143. doi: 10.1007/BF02535813. [DOI] [PubMed] [Google Scholar]
- 231.Koles K., Repnikova E., Pavlova G., Korochkin L.I., Panin V.M. Sialylation in protostomes: a perspective from Drosophila genetics and biochemistry. Glycoconj J. 2009;26:313–324. doi: 10.1007/s10719-008-9154-4. [DOI] [PubMed] [Google Scholar]
- 232.Islam R., Nakamura M., Scott H., Repnikova E., Carnahan M., Pandey D., Caster C., Khan S., Zimmermann T., Zoran M.J., Panin V.M. The role of Drosophila cytidine monophosphate-sialic acid synthetase in the nervous system. J Neurosci. 2013;33:12306–12315. doi: 10.1523/JNEUROSCI.5220-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tomiya N., Narang S., Lee Y.C., Betenbaugh M.J. Comparing N-glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj J. 2004;21:343–360. doi: 10.1023/B:GLYC.0000046275.28315.87. [DOI] [PubMed] [Google Scholar]
- 234.Jarvis D.L. Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology. 2003;310:1–7. doi: 10.1016/s0042-6822(03)00120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wei X., Yang D., Li H., Jiang H., Liu X., Zhang Q., Yang J. Sialic acid-binding lectins (SABLs) from Solen grandis function as PRRs ensuring immune recognition and bacterial clearance. Fish Shellfish Immunol. 2018;72:477–483. doi: 10.1016/j.fsi.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 236.Ghosh S. Sialic acid binding lectins (SABL) from molluscs, a review and insilico study of SABL from Solen grandis and Limax flavus. J Entomol Zool Stud. 2017;5:1563–1572. [Google Scholar]
- 237.Liu C., Jiang S., Wang M., Wang L., Chen H., Xu J., Lv Z., Song L. A novel siglec (CgSiglec-1) from the Pacific oyster (Crassostrea gigas) with broad recognition spectrum and inhibitory activity to apoptosis, phagocytosis and cytokine release. Dev Comp Immunol. 2016;61:136–144. doi: 10.1016/j.dci.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 238.Bravo Portela I., Martinez-Zorzano V.S., Molist-Perez I., Molist G.P. Ultrastructure and glycoconjugate pattern of the foot epithelium of the abalone Haliotis tuberculata (Linnaeus, 1758) (Gastropoda, Haliotidae) ScientificWorldJournal. 2012;2012:960159. doi: 10.1100/2012/960159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.He X., Zhang Y., Yu F., Yu Z. A novel sialic acid binding lectin with anti-bacterial activity from the Hong Kong oyster (Crassostrea hongkongensis) Fish Shellfish Immunol. 2011;31:1247–1250. doi: 10.1016/j.fsi.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 240.Li C., Yu S., Zhao J., Su X., Li T. Cloning and characterization of a sialic acid binding lectins (SABL) from Manila clam Venerupis philippinarum. Fish Shellfish Immunol. 2011;30:1202–1206. doi: 10.1016/j.fsi.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 241.Adhya M., Choi K.S., Yu Y., Cho M. Expression and localization of MCsialec, a sialic acid-specific lectin in the marine bivalve Manila clam, Ruditapes philppinarum. J Fish Dis. 2010;33:889–899. doi: 10.1111/j.1365-2761.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- 242.Ituarte S., Dreon M.S., Pasquevich M.Y., Fernández P.E., Heras H. Carbohydrates and glycoforms of the major egg perivitellins from Pomacea apple snails (Architaenioglossa: Ampullariidae) Comp Biochem Physiol B Biochem Mol Biol. 2010;157:66–72. doi: 10.1016/j.cbpb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 243.Takakura D., Norizuki M., Ishikawa F., Samata T. Isolation and characterization of the N-linked oligosaccharides in nacrein from Pinctada fucata. Mar Biotechnol (NY) 2008;10:290–296. doi: 10.1007/s10126-007-9063-8. [DOI] [PubMed] [Google Scholar]
- 244.Tsukamoto H., Takakura Y., Yamamoto T. Purification, cloning, and expression of an alpha/beta-galactoside alpha-2,3-sialyltransferase from a luminous marine bacterium, Photobacterium phosphoreum. J Biol Chem. 2007;282:29794–29802. doi: 10.1074/jbc.M701907200. [DOI] [PubMed] [Google Scholar]
- 245.Gerlach D., Schlott B., Schmidt K.H. Cloning and expression of a sialic acid-binding lectin from the snail Cepaea hortensis. FEMS Immunol Med Microbiol. 2004;40:215–221. doi: 10.1016/S0928-8244(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 246.Gerlach D., Wagner M., Schlott B., Zähringer U., Schmidt K.H. Chemical and physicochemical characterization of the sialic acid-specific lectin from Cepaea hortensis. FEMS Microbiol Lett. 2002;214:61–68. doi: 10.1111/j.1574-6968.2002.tb11325.x. [DOI] [PubMed] [Google Scholar]
- 247.Fischer E., Wagner M., Bertsch T. Cepaea hortensis agglutinin-I, specific for O-glycosidically linked sialic acids, selectively labels endothelial cells of distinct vascular beds. Histochem J. 2000;32:105–109. doi: 10.1023/a:1004066212317. [DOI] [PubMed] [Google Scholar]
- 248.DeLoney-Marino C.R., Wolfe A.J., Visick K.L. Chemoattraction of Vibrio fischeri to serine, nucleosides, and N-acetylneuraminic acid, a component of squid light-organ mucus. Appl Environ Microbiol. 2003;69:7527–7530. doi: 10.1128/AEM.69.12.7527-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bürgmayr S., Grabher-Meier H., Staudacher E. Sialic acids in gastropods. FEBS Lett. 2001;508:95–98. doi: 10.1016/s0014-5793(01)03024-1. [DOI] [PubMed] [Google Scholar]
- 250.Saito M., Sugiyama K. Major and c-series gangliosides in lenticular tissues: mammals to molluscs. Comp Biochem Physiol B Biochem Mol Biol. 2001;130:313–321. doi: 10.1016/s1096-4959(01)00433-x. [DOI] [PubMed] [Google Scholar]
- 251.Biswas C., Sinha D., Mandal C. Investigation on interaction of Achatinin, a 9-O-acetyl sialic acid-binding lectin, with lipopolysaccharide in the innate immunity of Achatina fulica snails. Mol Immunol. 2000;37:745–754. doi: 10.1016/s0161-5890(00)00096-1. [DOI] [PubMed] [Google Scholar]
- 252.Tunkijjanukij S., Giaever H., Chin C.C., Olafsen J.A., Tunkijjanukij S., Giaever H., Chin C.C., Olafsen J.A. Sialic acid in hemolymph and affinity purified lectins from two marine bivalves. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:705–713. doi: 10.1016/s0305-0491(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 253.Tunkijjanukij S., Olafsen J.A. Sialic acid-binding lectin with antibacterial activity from the horse mussel: further characterization and immunolocalization. Dev Comp Immunol. 1998;22:139–150. doi: 10.1016/s0145-305x(98)00017-2. [DOI] [PubMed] [Google Scholar]
- 254.Tunkijjanukij S., Mikkelsen H.V., Olafsen J.A. A heterogeneous sialic acid-binding lectin with affinity for bacterial LPS from horse mussel (Modiolus modiolus) hemolymph. Comp Biochem Physiol B Biochem Mol Biol. 1997;117:273–286. doi: 10.1016/s0305-0491(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 255.Kurachi S., Song Z., Takagaki M., Yang Q., Winter H.C., Kurachi K., Goldstein I.J. Sialic-acid-binding lectin from the slug Limax flavus--cloning, expression of the polypeptide, and tissue localization. Eur J Biochem. 1998;254:217–222. doi: 10.1046/j.1432-1327.1998.2540217.x. [DOI] [PubMed] [Google Scholar]
- 256.Robledo Y., Madrid J.F., Leis O., Cajaraville M.P. Analysis of the distribution of glycoconjugates in the digestive gland of the bivalve mollusc Mytilus galloprovincialis by conventional and lectin histochemistry. Cell Tissue Res. 1997;288:591–602. doi: 10.1007/s004410050845. [DOI] [PubMed] [Google Scholar]
- 257.Basu S., Schlecht S., Wagner M., Mayer H.L., Basu S., Schlecht S., Wagner M., Mayer H.L. The sialic acid-containing lipopolysaccharides of Salmonella djakarta and Salmonella isaszeg (serogroup O: 48): chemical characterization and reactivity with a sialic acid-binding lectin from Cepaea hortensis. FEMS Immunol Med Microbiol. 1994;9:189–197. doi: 10.1111/j.1574-695X.1994.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 258.Dam T.K., Sarkar M., Ghosal J., Choudhury A. Purification and partial characterization of a N-glycolylneuraminic acidspecific lectin from the clam Anadara granosa (L) Biochem Biophys Res Commun. 1993;196:422–429. doi: 10.1006/bbrc.1993.2266. [DOI] [PubMed] [Google Scholar]
- 259.Swarnakar S., Chowdhury P.S., Sarkar M. N-glycolylneuraminic acid specific lectin from Pila globosa snail. Biochem Biophys Res Commun. 1991;178:85–94. doi: 10.1016/0006-291x(91)91783-9. [DOI] [PubMed] [Google Scholar]
- 260.Miller R.L., Collawn J.F., Jr., Fish W.W. Purification and macromolecular properties of a sialic acid-specific lectin from the slug Limax flavus. J Biol Chem. 1982;257:7574–7580. [PubMed] [Google Scholar]
- 261.Simonneau M., Baux G., Tauc L. Sialic acid containing substrates as intracellular calcium receptors involved in transmitter release. J Physiol Paris. 1980;76:427–433. [PubMed] [Google Scholar]
- 262.Muralikrishna G., Reuter G., Peter-KataliniA J., Egge H., Hanisch F.G. Identification of a new ganglioside from the starfish Asterias rubens. Carbohydr Res. 1992;236:321–326. doi: 10.1016/0008-6215(92)85025-u. [DOI] [PubMed] [Google Scholar]
- 263.Warren L. N-Glycolyl-8-O-methylneuraminin acid, a new form of sialic acid in the starfish Asteriasforbesi. Biochim Biophys Acta. 1964;83:129–132. doi: 10.1016/0926-6526(64)90062-x. [DOI] [PubMed] [Google Scholar]
- 264.Bergwerff A.A., Hulleman S.H., Kamerling J.P., Vliegenthart J.F., Shaw L. Nature and biosynthesis of sialic acids in the starfish Asterias rubens. Identification of sialo-oligomers and detection of S-adenosyl-L-methionine: N-acylneuraminate 8-O-methyltransferase and CMP-N-acetylneuraminate monooxygenase activities. Biochimie. 1992;74:25–37. doi: 10.1016/0300-9084(92)90181-d. [DOI] [PubMed] [Google Scholar]
- 265.Kelm A., Shaw L., Schauer R., Reuter G. The biosynthesis of 8-O-methylated sialic acids in the starfish Asterias rubens—isolation and characterisation of S-adenosyl-L-methionine:sialate-8-O-methyltransferase. Eur J Biochem. 1998;251:874–884. doi: 10.1046/j.1432-1327.1998.2510874.x. [DOI] [PubMed] [Google Scholar]
- 266.Martensen I., Schauer R., Shaw L. Cloning and expression of a membrane-bound CMP-N-acetylneuraminic acid hydroxylase from the starfish Asterias rubens. Eur J Biochem. 2001;268:5157–5166. doi: 10.1046/j.0014-2956.2001.02446.x. [DOI] [PubMed] [Google Scholar]
- 267.Gollub M., Schauer R., Shaw L. Cytidine monophosphate-N-acetylneuraminate hydroxylase in the starfish Asterias rubens and other echinoderms. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:605–615. doi: 10.1016/s0305-0491(98)10058-5. [DOI] [PubMed] [Google Scholar]
- 268.Carlucci R., Mentino D., Semeraro D., Ricci P., Sion L., Scillitani G. Comparative histochemical analysis of intestinal glycoconjugates in the blunthead pufferfish Sphoeroides pachygaster and grey triggerfish Balistes capriscus (Teleostei: Tetraodontiformes) J Fish Biol. 2019;94:122–131. doi: 10.1111/jfb.13871. [DOI] [PubMed] [Google Scholar]
- 269.Yamakawa N., Vanbeselaere J., Chang L.Y., Yu S.Y., Ducrocq L., Harduin-Lepers A., Kurata J., Aoki-Kinoshita K.F., Sato C., Khoo K.H., Kitajima K., Guerardel Y. Systems glycomics of adult zebrafish identifies organ-specific sialylation and glycosylation patterns. Nat Commun. 2018;9:4647. doi: 10.1038/s41467-018-06950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ravasio V., Damiati E., Zizioli D., Orizio F., Giacopuzzi E., Manzoni M., Bresciani R., Borsani G., Monti E. Genomic and biochemical characterization of sialic acid acetylesterase (siae) in zebrafish. Glycobiology. 2017;27:938–946. doi: 10.1093/glycob/cwx068. [DOI] [PubMed] [Google Scholar]
- 271.Guérardel Y., Chang L.Y., Maes E., Huang C.J., Khoo K.H. Glycomic survey mapping of zebrafish identifies unique sialylation pattern. Glycobiology. 2006;16:244–257. doi: 10.1093/glycob/cwj062. [DOI] [PubMed] [Google Scholar]
- 272.Chang L.Y., Harduin-Lepers A., Kitajima K., Sato C., Huang C.J., Khoo K.H., Guérardel Y. Developmental regulation of oligosialylation in zebrafish. Glycoconj J. 2009;26:247–261. doi: 10.1007/s10719-008-9161-5. [DOI] [PubMed] [Google Scholar]
- 273.Schaper W., Bentrop J., Ustinova J., Blume L., Kats E., Tiralongo J., Weinhold B., Bastmeyer M. Münster-Kühnel AKIdentification and biochemical characterization of two functional CMP-sialic acid synthetases in Danio rerio. J Biol Chem. 2012;287:13239–13248. doi: 10.1074/jbc.M111.327544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Marx M., Rutishauser U., Bastmeyer M. Dual function of polysialic acid during zebrafish central nervous system development. Development. 2001;128:4949–4958. doi: 10.1242/dev.128.24.4949. [DOI] [PubMed] [Google Scholar]
- 275.Rodríguez-Alonso R., Megías M., Pombal M.A., Molist P. Morphological and functional aspects of the epidermis of the sea lamprey Petromyzon marinus throughout development. J Fish Biol. 2017;91:80–100. doi: 10.1111/jfb.13330. [DOI] [PubMed] [Google Scholar]
- 276.Ryuzono S., Takase R., Kamada Y., Ikenaga T., Chigwechokha P.K., Komatsu M., Shiozaki K. Suppression of Neu1 sialidase delays the absorption of yolk sac in medaka (Oryzias latipes) accompanied with the accumulation of α2-3 sialo-glycoproteins. Biochimie. 2017;135:63–71. doi: 10.1016/j.biochi.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 277.Fong B.Y., Ma L., Khor G.L., van der Does Y., Rowan A., McJarrow P., MacGibbon A.K. Ganglioside composition in beef, chicken, pork, and fish determined using liquid chromatography-high-resolution mass spectrometry. J Agric Food Chem. 2016;64:6295–6305. doi: 10.1021/acs.jafc.6b02200. [DOI] [PubMed] [Google Scholar]
- 278.Datta S., Datta S.C. Purification and characterization of fish surface mucin. Ital J Biochem. 1987;36:143–152. [PubMed] [Google Scholar]
- 279.Guardiola F.A., Cuesta A., Abellán E., Meseguer J. Esteban MA Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014;40:24–31. doi: 10.1016/j.fsi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 280.Shiozaki K., Ryuzono S., Matsushita N., Ikeda A., Takeshita K., Chigwechokha P.K., Komatsu M., Miyagi T. Molecular cloning and biochemical characterization of medaka (Oryzias latipes) lysosomal neu4 sialidase. Fish Physiol Biochem. 2014;40:1461–1472. doi: 10.1007/s10695-014-9940-9. [DOI] [PubMed] [Google Scholar]
- 281.Langhauser M., Ustinova J., Rivera-Milla E., Ivannikov D., Seidl C., Slomka C., Finne J., Yoshihara Y., Bastmeyer M., Bentrop J. Ncam1a and Ncam1b: two carriers of polysialic acid with different functions in the developing zebrafish nervous system. Glycobiology. 2012;22:196–209. doi: 10.1093/glycob/cwr129. [DOI] [PubMed] [Google Scholar]
- 282.Rieger S., Volkmann K., Köster R.W. Polysialyltransferase expression is linked to neuronal migration in the developing and adult zebrafish. Dev Dyn. 2008;237:276–285. doi: 10.1002/dvdy.21410. [DOI] [PubMed] [Google Scholar]
- 283.van Karnebeek C.D., Bonafé L., Wen X.Y., Tarailo-Graovac M., Balzano S., Royer-Bertrand B., Ashikov A., Garavelli L., Mammi I., Turolla L., Breen C., Donnai D., Cormier-Daire V., Heron D., Nishimura G., Uchikawa S., Campos-Xavier B., Rossi A., Hennet T., Brand-Arzamendi K., Rozmus J., Harshman K., Stevenson B.J., Girardi E., Superti-Furga G., Dewan T., Collingridge A., Halparin J., Ross C.J., Van Allen M.I., Rossi A., Engelke U.F., Kluijtmans L.A., van der Heeft E., Renkema H., de Brouwer A., Huijben K., Zijlstra F., Heise T., Boltje T., Wasserman W.W., Rivolta C., Unger S., Lefeber D.J., Wevers R.A., Superti-Furga A. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat Genet. 2016;48:777–784. doi: 10.1038/ng.3578. [DOI] [PubMed] [Google Scholar]
- 284.Lehmann F., Kelm S., Dietz F., von Itzstein M., Tiralongo J. The evolution of galactose alpha2,3-sialyltransferase: Ciona intestinalis ST3GAL I/II and Takifugu rubripes ST3GAL II sialylate Galbeta1,3GalNAc structures on glycoproteins but not glycolipids. Glycoconj J. 2008;25:323–334. doi: 10.1007/s10719-007-9078-4. [DOI] [PubMed] [Google Scholar]
- 285.Asahina S., Sato C., Matsuno M., Matsuda T., Colley K., Kitajima K. Involvement of the alpha2,8-polysialyltransferases II/STX and IV/PST in the biosynthesis of polysialic acid chains on the O-linked glycoproteins in rainbow trout ovary. J Biochem. 2006;140:687–701. doi: 10.1093/jb/mvj200. [DOI] [PubMed] [Google Scholar]
- 286.Lehmann F., Gäthje H., Kelm S., Dietz F. Evolution of sialic acid-binding proteins: molecular cloning and expression of fish siglec-4. Glycobiology. 2004;14:959–968. doi: 10.1093/glycob/cwh120. [DOI] [PubMed] [Google Scholar]
- 287.Hellebø A., Vilas U., Falk K., Vlasak R. Infectious salmon anemia virus specifically binds to and hydrolyzes 4-O-acetylated sialic acids. J Virol. 2004;78:3055–3062. doi: 10.1128/JVI.78.6.3055-3062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Harman A.M., Rodger J., Ahmat A., Thomas C., Bartlett C., Chen P., Dunlop S.A., Beazley L.D. PSA-NCAM is up-regulated during optic nerve regeneration in lizard but not in goldfish. Exp Neurol. 2003;182:180–185. doi: 10.1016/s0014-4886(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 289.Sarasquete C., Gisbert E., Ribeiro L., Vieira L., Dinis M.T. Glyconjugates in epidermal, branchial and digestive mucous cells and gastric glands of gilthead sea bream, Sparus aurata, Senegal sole, Solea senegalensis and Siberian sturgeon, Acipenser baeri development. Eur J Histochem. 2001;45:267–278. doi: 10.4081/1637. [DOI] [PubMed] [Google Scholar]
- 290.Baardsnes J., Davies P.L. Sialic acid synthase: the origin of fish type III antifreeze protein? Trends Biochem Sci. 2001;26:468–469. doi: 10.1016/s0968-0004(01)01879-5. [DOI] [PubMed] [Google Scholar]
- 291.Nakamura K., Tamai Y., Kasama T. Gangliosides of dogfish (Squalus acanthias) brain. Neurochem Int. 1997;30:593–604. doi: 10.1016/s0197-0186(96)00096-4. [DOI] [PubMed] [Google Scholar]
- 292.Sinha S., Mandal C. Microheterogeneity of C-reactive protein in the sera of fish Labeo rohita induced by metal pollutants. Biochem Biophys Res Commun. 1996;226:681–687. doi: 10.1006/bbrc.1996.1414. [DOI] [PubMed] [Google Scholar]
- 293.Freischütz B., Saito M., Rahmann H. Yu RK Characterization of sialyltransferase-IV activity and its involvement in the c-pathway of brain ganglioside metabolism. J Neurochem. 1995;64:385–393. doi: 10.1046/j.1471-4159.1995.64010385.x. [DOI] [PubMed] [Google Scholar]
- 294.Sato C., Kitajima K., Tazawa I., Inoue Y., Inoue S., Troy F.A., 2nd. Structural diversity in the alpha 2-->8-linked polysialic acid chains in salmonid fish egg glycoproteins. Occurrence of poly(Neu5Ac), poly(Neu5Gc), poly(Neu5Ac, Neu5Gc), poly(KDN), and their partially acetylated forms. J Biol Chem. 1993;268:23675–23684. [PubMed] [Google Scholar]
- 295.Ledeen R.W., Diebler M.F., Wu G., Lu Z.H., Varoqui H. Ganglioside composition of subcellular fractions, including pre- and postsynaptic membranes, from Torpedo electric organ. Neurochem Res. 1993;18:1151–1155. doi: 10.1007/BF00978366. [DOI] [PubMed] [Google Scholar]
- 296.Jin C., Padra J.T., Sundell K., Sundh H., Karlsson N.G., Lindén S.K. Atlantic salmon carries a range of novel O-glycan structures differentially localized on skin and intestinal mucins. J Proteome Res. 2015;14:3239–3251. doi: 10.1021/acs.jproteome.5b00232. [DOI] [PubMed] [Google Scholar]
- 297.Waki H., Murata A., Kon K., Maruyama K., Kimura S., Ogura H., Ando S. Isolation and characterization of a trisialyllactosylceramide, GT3, containing an O-acetylated sialic acid in cod fish brain. J Biochem. 1993;113:502–507. doi: 10.1093/oxfordjournals.jbchem.a124073. [DOI] [PubMed] [Google Scholar]
- 298.Schmelter T., Ivanov S., Wember M., Stangier P., Thiem J., Schauer R. Partial purification and characterization of cytidine-5'-monophosphosialate synthase from rainbow trout liver. Biol Chem Hoppe Seyler. 1993;374:337–342. doi: 10.1515/bchm3.1993.374.1-6.337. [DOI] [PubMed] [Google Scholar]
- 299.Khan K.M., Hatfield J.S., Drescher D.G. Carbohydrates associated with the cell coat surrounding cells of the rainbow trout saccular macula as revealed by lectin probes. Hear Res. 1991;53:223–239. doi: 10.1016/0378-5955(91)90056-f. [DOI] [PubMed] [Google Scholar]
- 300.Taguchi T., Seko A., Kitajima K., Inoue S., Iwamatsu T., Khoo K.H., Morris H.R., Dell A., Inoue Y. Structural studies of a novel type of tetraantennary sialoglycan unit in a carbohydrate-rich glycopeptide isolated from the fertilized eggs of Indian Medaka fish, Oryzias melastigma. J Biol Chem. 1993;268:2353–2362. [PubMed] [Google Scholar]
- 301.Melrose J. Mucin-like glycopolymer gels in electrosensory tissues generate cues which direct electrolocation in amphibians and neuronal activation in mammals. Neural Regen Res. 2019;14:1191–1195. doi: 10.4103/1673-5374.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lu C.X., Nan K.J., Lei Y. Agents from amphibians with anticancer properties. Anticancer Drugs. 2008;19:931–939. doi: 10.1097/CAD.0b013e3283139100. [DOI] [PubMed] [Google Scholar]
- 303.Bernardini G., Rizzo A.M., Gornati R., Rossi F., Berra B. Tissue and developmental specificity of a polysialo-ganglioside species in the amphibian Xenopus. Cell Biol Int. 1996;20:667–672. doi: 10.1006/cbir.1996.0088. [DOI] [PubMed] [Google Scholar]
- 304.Andrade-Silva D., Ashline D., Tran T., Lopes A.S., Travaglia Cardoso S.R., Reis M.D.S., Zelanis A., Serrano S.M.T., Reinhold V. Structures of N-glycans of bothrops venoms revealed as molecular signatures that contribute to venom phenotype in viperid snakes. Mol Cell Proteomics. 2018;17:1261–1284. doi: 10.1074/mcp.RA118.000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Giacopuzzi E., Bresciani R., Schauer R., Monti E., Borsani G. New insights on the sialidase protein family revealed by a phylogenetic analysis in metazoa. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Scillitani G., Mentino D., Liquori G.E., Ferri D. Histochemical characterization of the mucins of the alimentary tract of the grass snake, Natrix natrix (Colubridae) Tissue Cell. 2012;44:288–295. doi: 10.1016/j.tice.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 307.Valeriano-Zapana J.A., Segovia-Cruz F.S., Rojas-Hualpa J.M., Martins-de-Souza D., Ponce-Soto L.A., Marangoni S. Functional and structural characterization of a new serine protease with thrombin-like activity TLBan from Bothrops andianus (Andean Lancehead) snake venom. Toxicon. 2012;59:231–240. doi: 10.1016/j.toxicon.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 308.Lin C.W., Chen J.M., Wang Y.M., Wu S.W., Tsai I.H., Khoo K.H. Terminal disialylated multiantennary complex-type N-glycans carried on acutobin define the glycosylation characteristics of the Deinagkistrodon acutus venom. Glycobiology. 2011;21:530–542. doi: 10.1093/glycob/cwq195. [DOI] [PubMed] [Google Scholar]
- 309.Schauer R., Srinivasan G.V., Coddeville B., Zanetta J.P., Guérardel Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr Res. 2009;344:1494–1500. doi: 10.1016/j.carres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 310.Desantis S., Labate M., Labate G.M., Cirillo F. Evidence of regional differences in the lectin histochemistry along the ductus epididymis of the lizard, Podarcis sicula Raf. Histochem J. 2002;34:123–130. doi: 10.1023/a:1020986313281. [DOI] [PubMed] [Google Scholar]
- 311.Neves-Ferreira A.G., Perales J., Fox J.W., Shannon J.D., Makino D.L., Garratt R.C., Domont G.B. Structural and functional analyses of DM43, a snake venom metalloproteinase inhibitor from Didelphis marsupialis serum. J Biol Chem. 2002;277:13129–13137. doi: 10.1074/jbc.M200589200. [DOI] [PubMed] [Google Scholar]
- 312.Mansour M.H., Negm H.I., Saad A.H., Zahran A.Y., Badir N. Identification of peanut agglutinin-binding glycoproteins on lizard lymphocytes. Zoolog Sci. 1995;12:79–85. doi: 10.2108/zsj.12.79. [DOI] [PubMed] [Google Scholar]
- 313.Gowda D.C. Davidson EA Isolation and characterization of novel mucin-like glycoproteins from cobra venom. J Biol Chem. 1994;269:20031–20039. [PubMed] [Google Scholar]
- 314.Hoffman S., Chuong C.M. Edelman GMEvolutionary conservation of key structures and binding functions of neural cell adhesion molecules. Proc Natl Acad Sci U S A. 1984;81:6881–6885. doi: 10.1073/pnas.81.21.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zeng F.Y., Gabius H.J. Sialic acid-binding proteins: characterization, biological function and application. Z Naturforsch C. 1992;47:641–653. doi: 10.1515/znc-1992-9-1001. [DOI] [PubMed] [Google Scholar]
- 316.Bornhöfft K.F., Goldammer T., Rebl A., Galuska S.P. Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins. Dev Comp Immunol. 2018;86:219–231. doi: 10.1016/j.dci.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 317.Macauley M.S., Crocker P.R., Paulson J.C. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lübbers J., Rodríguez E., van Kooyk Y. Modulation of immune tolerance via siglec-sialic acid interactions. Front Immunol. 2018;9:2807. doi: 10.3389/fimmu.2018.02807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Lehmann F., Tiralongo E., Tiralongo J. Sialic acid-specific lectins: occurrence, specificity and function. Cell Mol Life Sci. 2006;63:1331–1354. doi: 10.1007/s00018-005-5589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lasky L.A. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 321.Varki A. Selectin ligands: will the real ones please stand up? J Clin Invest. 1997;99(2):158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Brinkman-van der Linden E.C.M., Varki A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. J Biol Chem. 2000;275:8625–8632. doi: 10.1074/jbc.275.12.8625. [DOI] [PubMed] [Google Scholar]
- 323.Crocker P.R., Mucklow S., Bouckson V., McWilliam A., Willis A.C., Gordon S., Milon G., Kelm S., Bradfield P. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 1994;13:4490–4503. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Powell L.D., Varki A. The oligosaccharide binding specificities of CD22 beta, a sialic acid-specific lectin of B cells. J Biol Chem. 1994;269:10628–10636. [PubMed] [Google Scholar]
- 325.Powell L.D., Jain R.K., Matta K.L., Sabesan S., Varki A. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22. Selective recognition of immunoglobulin M and haptoglobin. J Biol Chem. 1995;270:7523–7532. doi: 10.1074/jbc.270.13.7543. [DOI] [PubMed] [Google Scholar]
- 326.Freeman S.D., Kelm S., Barber E.K., Crocker P.R. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–2012. [PubMed] [Google Scholar]
- 327.Collins B.E., Yang L.J.S., Mukhopadhyay G., Filbin M.T., Kiso M., Hasegawa A., Schnaar R.L. Sialic acid specificity of myelin-associated glycoprotein binding. J Biol Chem. 1997;272:1248–1255. doi: 10.1074/jbc.272.2.1248. [DOI] [PubMed] [Google Scholar]
- 328.Collins B.E., Ito H., Sawada N., Ishida H., Kiso M., Schnaar R.L. Enhanced binding of the neural siglecs, myelin-associated glycoprotein and Schwann cell myelin protein, to Chol-1 (alpha-series) gangliosides and novel sulfated Chol-1 analogs. J Biol Chem. 1999;274:27893–27899. doi: 10.1074/jbc.274.53.37637. [DOI] [PubMed] [Google Scholar]
- 329.Cornish A.L., Freeman S., Forbes G., Ni J., Zhang M., Cepeda M., Gentz R., Augustus M., Carter K.C., Crocker P.R. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123–2132. [PubMed] [Google Scholar]
- 330.Patel N., Brinkman-van der Linden E.C.M., Altmann S.W., Gish K., Balasubramanian S., Timans J.C., Peterson D., Bell M.P., Bazan J.F., Varki A., Kastelein R.A. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274:22729–22738. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- 331.Nicoll G., Ni J., Liu D., Klenerman P., Munday J., Dubock S., Mattei M.G., Crocker P.R. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 332.Angata T., Varki A. Siglec-7: a sialic acid-binding lectin of the immunoglobulin superfamily. Glycobiology. 2000;10:431–438. doi: 10.1093/glycob/10.4.431. [DOI] [PubMed] [Google Scholar]
- 333.Floyd H., Ni J., Cornish A.L., Zeng Z.Z., Liu D., Carter K.C., Steel J., Crocker P.R. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 334.Angata T., Varki A. Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J Biol Chem. 2000;275:22127–22135. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- 335.Zhang J.Q., Nicoll G., Jones C., Crocker P.R. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275:22121–22126. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 336.Munday J., Kerr S., Ni J., Cornish A.L., Zhang J.Q., Nicoll G., Floyd H., Mattei M.G., Moore P., Liu D., Crocker P.R. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355:489–497. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Fearon D.T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978;75:1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Pangburn M.K., Muller-Eberhard H. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978;75:2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Scholler N., Hayden-Ledbetter M., Hellström K.E., Hellström I., Ledbetter J.A. CD83 is an I-type lectin adhesion receptor that binds monocytes and a subset of activated CD8 + T cells. J Immunol. 2001;166:3865–3872. doi: 10.4049/jimmunol.166.6.3865. [DOI] [PubMed] [Google Scholar]
- 340.Kleene R., Yang H., Kutsche M., Schachner M. The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J Biol Chem. 2001;276:21656–21663. doi: 10.1074/jbc.M101790200. [DOI] [PubMed] [Google Scholar]
- 341.Cebo C., Dambrouck T., Maes E., Laden C., Strecker G., Michalski J.C., Zanetta J.P. Function and molecular modeling of the interaction between human interleukin 6 and its HNK-1 oligosaccharide ligands. J Biol Chem. 2001;276:5685–5691. doi: 10.1074/jbc.M106816200. [DOI] [PubMed] [Google Scholar]
- 342.Ravindranath M.H., Gonzales A., Soh D., Nishimoto K., Tam W.Y., Bilchik A., Morton D.L., O’Day S. Interleukin-2 binds to ganglioside GD(1b) Biochem Biophys Res Commun. 2001;283:369–373. doi: 10.1006/bbrc.2001.4797. [DOI] [PubMed] [Google Scholar]
- 343.Banerjee M., Chowdhury M. Purification and characterization of a sperm-binding glycoprotein from human endometrium. Hum Reprod. 1994;9:1497–1504. doi: 10.1093/oxfordjournals.humrep.a138737. [DOI] [PubMed] [Google Scholar]
- 344.Chakraborty I., Mandal C., Chowdhury M. Modulation of sialic acid-binding proteins of rat uterus in response to changing hormonal milieu. Mol Cell Biochem. 1993;126:77–86. doi: 10.1007/BF01772210. [DOI] [PubMed] [Google Scholar]
- 345.Nitta K., Takayanagi G., Kawauchi H., Hakomori S. Isolation and characterization of Rana catesbeiana lectin and demonstration of the lectin-binding glycoprotein of rodent and human tumor cell membranes. Cancer Res. 1987;47:4877–4883. [PubMed] [Google Scholar]
- 346.Tiemeyer M., Yasuda Y., Schnaar R.L. Ganglioside-specific binding protein on rat brain membranes. J Biol Chem. 1989;264:1671–1681. [PubMed] [Google Scholar]
- 347.Tiemeyer M., Swank-Hill P., Schnaar R.L.J. A membrane receptor for gangliosides is associated with central nervous system myelin. Biol Chem. 1990;265:11990–11999. [PubMed] [Google Scholar]
- 348.Popoli M., Mengano A. A hemagglutinin specific for sialic acids in a rat brain synaptic vesicle-enriched fraction. Neurochem Res. 1988;13:63–67. doi: 10.1007/BF00971856. [DOI] [PubMed] [Google Scholar]
- 349.Feng Y., Ma X., Deng L., Yao B., Xiong Y., Wu Y., Wang L., Ma Q., Ma F. Role of selectins and their ligands in human implantation stage. Glycobiology. 2017;27:385–391. doi: 10.1093/glycob/cwx009. [DOI] [PubMed] [Google Scholar]
- 350.Kappelmayer J., Nagy B., Jr. The interaction of selectins and PSGL-1 as a key component in thrombus formation and cancer progression. Biomed Res Int. 2017;2017:6138145. doi: 10.1155/2017/6138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Borsig L. Selectins in cancer immunity. Glycobiology. 2018;28:648–655. doi: 10.1093/glycob/cwx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Varki A., Kannagi R., Toole B.P. Glycosylation changes in cancer. In: Varki A., Cummings R.D., Esko J.D., editors. Essentials of glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. Chapter 44. [Google Scholar]
- 353.Dekkers G., Rispens T., Vidarsson G. Novel concepts of altered immunoglobulin g galactosylation in autoimmune diseases. Front Immunol. 2018;9:553. doi: 10.3389/fimmu.2018.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Picco G., Julien S., Brockhausen I. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology. 2010;20:1241–1250. doi: 10.1093/glycob/cwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Groux-Degroote S., Guérardel Y., Delannoy P. Gangliosides: structures, biosynthesis, analysis, and roles in cancer. Chembiochem. 2017;18:1146–1154. doi: 10.1002/cbic.201600705. [DOI] [PubMed] [Google Scholar]
- 356.Cavdarli S., Dewald J.H., Yamakawa N., Guérardel Y., Terme M., Le Doussal J.M., Delannoy P., Groux-Degroote S. Identification of 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) as main O-acetylated sialic acid species of GD2 in breast cancer cells. Glycoconj J. 2019;36:79–90. doi: 10.1007/s10719-018-09856-w. [DOI] [PubMed] [Google Scholar]
- 357.Häuselmann I., Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Rodrigues E., Macauley M.S. Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities. Cancers (Basel) 2018;10:207. doi: 10.3390/cancers10060207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang Z., Wuhrer M., Holst S. Serum sialylation changes in cancer. Glycoconj J. 2018;35:139–160. doi: 10.1007/s10719-018-9820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Van Rinsum J., Smets L.A., Van Rooy H., Van den Eijnden D.H., Van Rinsum J., Smets L.A., Van Rooy H., Van den Eijnden D.H. Specific inhibition of human natural killer cell-mediated cytotoxicity by sialic acid and sialo-oligosaccharides. Int J Cancer. 1986;38:915–922. doi: 10.1002/ijc.2910380620. [DOI] [PubMed] [Google Scholar]
- 361.Angata T., Nycholat C.M., Macauley M.S. Therapeutic targeting of siglecs using antibody- and glycan-based approaches. Trends Pharmacol Sci. 2015;36:645–660. doi: 10.1016/j.tips.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Heimburg-Molinaro J., Lum M., Vijay G., Jain M., Almogren A., Rittenhouse-Olson K. Cancer vaccines and carbohydrate epitopes. Vaccine. 2011;29:8802–8826. doi: 10.1016/j.vaccine.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Glavey S.V., Manier S., Natoni A. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood. 2014;124:1765–1776. doi: 10.1182/blood-2014-03-560862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Munkley J. The role of sialyl-Tn in cancer. Int J Mol Sci. 2016;17:275. doi: 10.3390/ijms17030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ju T., Aryal R.P., Kudelka M.R., Wang Y., Cummings R.D. The cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014;14:63–81. doi: 10.3233/CBM-130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kumar V., Turnbull W.B. Carbohydrate inhibitors of cholera toxin. Beilstein J Org Chem. 2018;14:484–498. doi: 10.3762/bjoc.14.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Rao F.V., Rich J.R., Rakić B., Buddai S., Schwartz M.F., Johnson K., Bowe C., Wakarchuk W.W., Defrees S., Withers S.G., Strynadka N.C. Structural insight into mammalian sialyltransferases. Nat Struct Mol Biol. 2009;16:1186–1188. doi: 10.1038/nsmb.1685. [DOI] [PubMed] [Google Scholar]
- 368.Vasconcelos-Dos-Santos A., Oliveira I.A., Lucena M.C. Biosynthetic machinery involved in aberrant glycosylation: promising targets for developing of drugs against cancer. Front Oncol. 2015;5:138. doi: 10.3389/fonc.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Yang W.H., Nussbaum C., Grewal P.K., Marth J.D., Sperandio M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood. 2012;120:1015–1026. doi: 10.1182/blood-2012-04-424366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Brear P., Telford J., Taylor G.L., Westwood N.J. Synthesis and structural characterisation of selective non-carbohydrate-based inhibitors of bacterial sialidases. Chembiochem. 2012;13:2374–2383. doi: 10.1002/cbic.201200433. [DOI] [PubMed] [Google Scholar]
- 371.Schön M.P. Inhibitors of selectin functions in the treatment of inflammatory skin disorders. Ther Clin Risk Manag. 2005;1:201–208. [PMC free article] [PubMed] [Google Scholar]
- 372.Norman K.E., Anderson G.P., Kolb H.C., Ley K., Ernst B. Sialyl Lewis(x) (sLe(x)) and an sLe(x) mimetic, CGP69669A, disrupt E-selectin-dependent leukocyte rolling in vivo. Blood. 1998;91:475–483. [PubMed] [Google Scholar]
- 373.Stahn R., Schäfer H., Kernchen F., Schreiber J. Multivalent sialyl Lewis x ligands of definite structures as inhibitors of E-selectin mediated cell adhesion. Glycobiology. 1998;8:311–319. doi: 10.1093/glycob/8.4.311. [DOI] [PubMed] [Google Scholar]
- 374.Telen M.J., Wun T., McCavit T.L., De Castro L.M., Krishnamurti L., Lanzkron S., Hsu L.L., Smith W.R., Rhee S., Magnani J.L., Thackray H. Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood. 2015;125:2656–2664. doi: 10.1182/blood-2014-06-583351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Page C. New drugs and targets for asthma and chronic obstructive pulmonary disease (CDPD) Br J Clin Pharmacol. 2011;71:969. [Google Scholar]
- 376.Lv X., Cao H., Lin B., Wang W., Zhang W., Duan Q., Tao Y., Liu X.W. Li X synthesis of sialic acids, their derivatives, and analogs by using a whole-cell catalyst. Chemistry. 2017;23:15143–15149. doi: 10.1002/chem.201703083. [DOI] [PubMed] [Google Scholar]
- 377.Vyas A.A., Blixt O., Paulson J.C., Schnaar R.L. Potent glycan inhibitors of myelin-associated glycoprotein enhance axon outgrowth in vitro. J Biol Chem. 2005;280:16305–16310. doi: 10.1074/jbc.M500250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Hata K., Koseki K., Yamaguchi K., Moriya S., Suzuki Y., Yingsakmongkon S., Hirai G., Sodeoka M., von Itzstein M., Miyagi T. Limited inhibitory effects of oseltamivir and zanamivir on human sialidases. Antimicrob Agents Chemother. 2008;52:3484–3491. doi: 10.1128/AAC.00344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Gao S., Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev Med Chem. 2010;10:550–567. doi: 10.2174/138955710791384081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Masimirembwa C.M., Bredberg U., Andersson T.B. Metabolic stability for drug discovery and development: pharmacokinetic and biochemical challenges. Clin Pharmacokinet. 2003;42:515–528. doi: 10.2165/00003088-200342060-00002. [DOI] [PubMed] [Google Scholar]
- 381.Hsu P.H., Chiu D.C., Wu K.L., Lee P.S., Jan J.T., Cheng Y.E., Tsai K.C., Cheng T.J., Fang J.M. Acylguanidine derivatives of zanamivir and oseltamivir: potential orally available prodrugs against influenza viruses. Eur J Med Chem. 2018;154:314–323. doi: 10.1016/j.ejmech.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 382.Hsu K.C., Hung H.C., HuangFu W.C. Identification of neuraminidase inhibitors against dual H274Y/I222R mutant strains. Sci Rep. 2017;7:12336. doi: 10.1038/s41598-017-12101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Hevey R, Strategies for the development of glycomimetic drug candidates. Pharmaceuticals. 2019; 12(2). pii: E55. [DOI] [PMC free article] [PubMed]
- 384.Sriraman S.K., Salzano G., Sarisozen C., Torchilin V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur J Pharm Biopharm. 2016;105:40–49. doi: 10.1016/j.ejpb.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Filser C., Kowalczyk D., Jones C. Synthetic glycopeptides from the e-selectin ligand 1 with varied sialyl lewis(x) structure as cell-adhesion inhibitors of e-selectin. Angew Chem Int Ed. 2007;46:2108–2111. doi: 10.1002/anie.200604442. [DOI] [PubMed] [Google Scholar]
- 386.Chen W.C., Sigal D.S., Saven A., Paulson J.C. Targeting b lymphoma with nanoparticles bearing glycan ligands of cd22. Leuk Lymphoma. 2012;53:208–210. doi: 10.3109/10428194.2011.604755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Rillahan C.D., Macauley M.S., Schwartz E. Disubstituted sialic acid ligands targeting siglecs CD33 and CD22 associated with myeloid leukaemias and b cell lymphomas. Chem Sci. 2014;5:2398–2406. doi: 10.1039/C4SC00451E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Anonymous Deal watch: Pfizer deal for selectin inhibitor highlights potential of glycomimetic drugs. Nat Rev Drug Discov. 2011;10(12):890. doi: 10.1038/nrd3622. [DOI] [PubMed] [Google Scholar]
- 389.http://www.clinicaltrials.gov/
- 390.Bauer J., Osborn H.M. Sialic acids in biological and therapeutic processes: opportunities and challenges. Future Med Chem. 2015;7:2285–2299. doi: 10.4155/fmc.15.135. [DOI] [PubMed] [Google Scholar]
- 391.http://www.fda.gov/
- 392.O'Reilly M.K., Paulson J.C. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wu J., Guo Z. Improving the antigenicity of sTn antigen by modification of its sialic acid residue for development of glycoconjugate cancer vaccines. Bioconjug Chem. 2006;17:1537–1544. doi: 10.1021/bc060103s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Zhu J., Warren J.D., Danishefsky S.J. Synthetic carbohydrate-based anticancer vaccines: the memorial sloan-kettering experience. Expert Rev Vaccines. 2009;8:1399–1413. doi: 10.1586/erv.09.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Astronomo R.D., Burton D.R. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zeichner S.B. The failed theratope vaccine: 10 years later. J Am Osteopath Assoc. 2012;112:482–483. [PubMed] [Google Scholar]
- 397.Adis International Ltd Cancer vaccine THERATOPE- Biomira. Drugs R&D. 2003;4(4):236–240. doi: 10.2165/00126839-200304040-00004. [DOI] [PubMed] [Google Scholar]
- 398.Buskas T., Thompson P., Boons G.J. Immunotherapy for cancer: synthetic carbohydrate-based vaccines. Chem Commun (Camb) 2009;36:5335–5349. doi: 10.1039/b908664c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zhou Z., Liao G., Mandal S.S., Suryawanshi S., Guo Z. A fully synthetic self-adjuvanting globo H-based vaccine elicited strong T cell-mediated antitumor immunity. Chem Sci. 2015;6:7112–7121. doi: 10.1039/c5sc01402f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Lou Y.W., Wang P.Y., Yeh S.C. Stage-specific embryonic antigen-4 as a potential therapeutic target in glioblastoma multiforme and other cancers. Proc Natl Acad Sci U S A. 2014;111:2482–2487. doi: 10.1073/pnas.1400283111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Danishefsky S.J., Shue Y.-K., Chang C.-H. Development of globo-H cancer vaccine. Acc Chem Res. 2015;48:643–652. doi: 10.1021/ar5004187. [DOI] [PubMed] [Google Scholar]
- 402.Cheung S.K., Chuang P.K., Huang H.W. Stage-specific embryonic antigen-3 (SSEA-3) and β3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc Natl Acad Sci U S A. 2016;13:960–965. doi: 10.1073/pnas.1522602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Byrne B., Donohoe G.G., O'Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discov Today. 2007;12:319–326. doi: 10.1016/j.drudis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 404.Doran R.C., Tatsuno G.P., O'Rourke S.M., Yu B., Alexander D.L., Mesa K.A., Berman P.W., Doran R.C., Tatsuno G.P., O'Rourke S.M., Yu B., Alexander D.L., Mesa K.A., Berman P.W. Glycan modifications to the gp120 immunogens used in the RV144 vaccine trial improve binding to broadly neutralizing antibodies. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0196370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 405.Campo S., Andreone L., Ambao V., Urrutia M., Calandra R.S., Rulli S.B. Hormonal regulation of follicle-stimulating hormone glycosylation in males. Front Endocrinol. 2019;10:17. doi: 10.3389/fendo.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Velásquez J.G., Canovas S., Barajas P., Marcos J., Jiménez-Movilla M., Gallego R.G., Ballesta J., Avilés M., Coy P. Role of sialic acid in bovine sperm-zona pellucida binding. Mol Reprod Dev. 2007;74:617–628. doi: 10.1002/mrd.20619. [DOI] [PubMed] [Google Scholar]
- 407.Paschinger K., Wilson I.B.H. Comparisons of N-glycans across invertebrate phyla. Parasitology. 2019;3:1–10. doi: 10.1017/S0031182019000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Zhu J., Wan Q., Ragupathi G., George C.M., Livingston P.O., Danishefsky S.J. Biologics through chemistry: total synthesis of a proposed dual-acting vaccine targeting ovarian cancer orchestration of oligosaccharide and polypeptide domains. J Am Chem Soc. 2009;131:4151–4158. doi: 10.1021/ja810147j. [DOI] [PMC free article] [PubMed] [Google Scholar]


