Abstract
A central feature of atherosclerosis, the most prevalent chronic vascular disease and root cause of myocardial infarction and stroke, is leukocyte accumulation in the arterial wall. These crucial immune cells are produced in specialized niches in the bone marrow, where a complex cell network orchestrates their production and release. A growing body of clinical studies has documented a correlation between leukocyte numbers and cardiovascular disease risk. Understanding how leukocytes are produced and how they contribute to atherosclerosis and its complications is therefore critical to understanding and treating the disease. In this review, we focus on the key cells and products that regulate hematopoiesis under homeostatic conditions, during atherosclerosis, and after myocardial infarction.
Keywords: hematopoiesis, hematopoietic stem cell niche, leukocytes, atherosclerosis, myocardial infarction
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide 1. Despite major advances in health education, drug therapy, and interventional treatments, an estimated 17.9 million people worldwide died from CVD in 2016, representing 31% of all global deaths that year 1. In most cases the underlying disease is atherosclerosis, a chronic inflammatory process in the vessel wall characterized by progressive accumulation of lipoproteins and leukocytes. Ruptures or erosions of atherosclerotic plaques lead to fatal events such as myocardial infarction and stroke, which together account for 85% of all cardiovascular deaths 1.
Atherosclerosis has long been considered a cholesterol storage disease in which cholesterol and thrombotic material are passively deposited in the artery walls 2,3. Over the last three decades, however, it has become clear that immune cells and inflammatory processes are critical to atherosclerosis and its complications, leading to the modern understanding of atherosclerosis as a lipid-driven inflammatory disease 4–7. Various immune cells have been identified as crucial contributors to atherosclerotic lesion initiation and progression as well as the course and outcome of its major complications 4–7.
Immune cells arise from hematopoietic stem cells (HSCs) in a process called hematopoiesis. Every day, healthy adults produce ~4–5 × 1011 new blood cells to maintain homeostatic levels 8. HSCs give rise to all blood cell types and physiologically reside in a specialized bone marrow microenvironment referred to as the hematopoietic stem cell niche 9,10. As a result of a tightly controlled proliferation and differentiation process, mature leukocytes exit the bone marrow in response to various stimuli, enter the circulation, and reach their target tissues via adhesion and diapedesis. While the hematopoietic supply of inflammatory immune cells is crucial for CVD development, CVD in turn strongly affects hematopoiesis. Common cardiovascular risk factors such as hyperlipoproteinemia, arterial hypertension, and diabetes mellitus considerably alter hematopoietic processes. Likewise, atherosclerosis and myocardial infarction induce strong effects on hematopoiesis. Here, we review recent discoveries regarding the cellular composition and molecular regulation of the hematopoietic stem cell niche and hematopoietic differentiation during steady-state and cardiovascular disease.
Hematopoiesis
Hematopoiesis is the process by which a small pool of self-renewing pluripotent hematopoietic stem cells (HSCs) produce red blood cells, platelets, and all leukocyte types. Only 1 of about 10,000 bone marrow cells is an HSC 11,12 and these cells are comparatively quiescent with fewer than 5% of them being in cell cycle at any point in time. Adult humans have an estimated 3,000–10,000 HSCs with an estimated division rate between once every three months to once every three years 13. The HSC cell pool is divided into self-renewing long-term HSCs and transiently self-renewing short-term HSCs. To maintain the stem cell pool, some HSCs proliferate via asymmetric division in which one daughter cell remains an HSC and only the other differentiates into a more restricted progenitor with limited self-renewal capacity. These restricted progenitor cells proliferate robustly and give rise to most cells under steady-state conditions 14–16. To maintain homeostasis and ensure adequate blood cell production under steady state and stress conditions, the balance between HSC self-renewal and differentiation is strictly controlled by a complex network of HSC-intrinsic mechanisms, such as transcriptional regulation, epigenetic modification and metabolic adaptation, as well as by extrinsic factors 9,10 including long-distance signals from outside the bone marrow 17,18 and local signals produced in the bone marrow microenvironment. Due to its crucial role in maintaining HSCs, this local microenvironment has been termed the “hematopoietic stem cell niche”, a concept first proposed in 1978 by R. Schofield 19.
The hematopoietic stem cell niche
Location and anatomy of hematopoietic niches
During embryogenesis, hematopoiesis occurs in so-called blood islands within the yolk sac before shifting to the spleen, liver, and lymph nodes. Once the bone marrow has developed, hematopoietic stem cells seed the newly formed niche, where they begin to produce cells in a process called definitive hematopoiesis 20. In response to strong hematopoietic stress, hematopoiesis can transiently occur in extramedullary sites including the adult spleen and liver 9,21,22.
Bone surfaces are covered by the heavily vascularized and innervated periosteum 23,24. Among the innervations, sympathetic nerve terminals enter the bone marrow alongside arteries and continue down to the arterioles 17,18, where they form a structural network with neural-glial antigen- (NG2) and nestin- (Nes) positive perivascular stromal cells called the “neuro reticular complex” 25–27. While sensory nerves also innervate bone and bone marrow 18,28, the parasympathetic nervous system does not appear to broadly innervate the bone marrow 29,30. In long bones, a central artery typically enters the marrow cavity through a nutrient canal, branches into smaller ascending and descending arteries, and eventually extends into thin-walled arterioles that run near the endosteum following the long axis of the bone 25,31. These transitional vessels connect the arterioles with the extensive sinusoidal network that drains into a central venous sinus, which in turn empties into nutrient veins that leave the marrow via nutrient canals 32,33.
The perivascular HSC niche
The discovery of histological markers that unambiguously identify HSCs in tissue sections was a critical step for analyzing bone marrow niche microanatomy and cellular composition 34,35. HSCs stain positive for CD150 and negative for CD48 and CD41, enabling their identification with a two-marker protocol 34,36–38. Histological analyses of bone marrow and spleen revealed that almost all CD150+CD48–CD41–Lineage– cells reside within five cell diameters of a sinusoid and many of them are localized directly adjacent to the sinusoid 34,39.
The identification of additional highly specific genetic HSC markers, including homeo-box b5 (Hoxb5) 40 and α-catulin (Ctnnal1) 41, led to the development of α-catulin-GFP and Hoxb5mCherry knock-in mice, which further improved our understanding of the HSC niche. A marker combination of α-catulin-GFP and KIT (the receptor for stem cell factor (SCF)) has been used for deep confocal imaging of optically cleared bone marrow, allowing for 3D reconstruction of large bone marrow segments 41. This approach revealed that ~80% of dividing and non-dividing HSCs reside in close proximity to a sinusoid and nearly all HSCs are in contact with leptin receptor+ (LEPR+) and CXC chemokine ligand 12 high (CXCL12high) niche cells. The remaining HSCs are located near arterioles (10%) and transition zone vessels (10%) with only few HSCs residing adjacent to the endosteum 41. Overall, there is ample evidence that HSCs reside in perivascular niches primarily around sinusoids and that the majority of these HSCs are quiescent 42.
Identifying and characterizing niche cells
The earliest approach to functionally identify which cell types form the HSC niche was to ablate specific cell populations and analyze the effects on HSCs. Such interventions typically activated and/or depleted HSCs 42, making it difficult to distinguish between direct and indirect effects the ablated cell population had on HSCs. To overcome these issues, mouse models were engineered to specifically trace and deplete individual niche factors from defined cell populations. These models, combined with highly specific HSC markers, advanced imaging techniques, as well as functional analyses of the marrow cells by transplantation analysis, enabled the identification of cellular niche constituents, niche factors, and niche factor receptors. Among the non-hematopoietic cell types that constitute the bone marrow niche are adipocytes, neurons and glial cells, osteoblastic cells, perivascular mesenchymal stem/stromal cells (MSCs), and endothelial cells. However, hematopoietic cells, including megakaryocytes, macrophages, and T cells, can also influence hematopoiesis.
Adipocytes
Accumulating evidence suggests that adipocytes are negative regulators of HSC function. First, over the course of human life, hematopoietic red bone marrow is progressively replaced by fatty yellow marrow with far less hematopoietic activity 43. Second, the adipocyte-derived protein adiponectin reduces hematopoietic progenitor proliferation in culture 44 and hematopoietic stem and progenitor cell (HSPC) numbers negatively correlate with adipocyte content of different bones – adipocyte-free thoracic vertebrae versus adipocyte-rich tail vertebrae – in mice 45. Third, a recent study has shown that an adipocyte progenitor negatively affects hematopoiesis and bone healing 46. However, adipocytes may also be important to the niche’s protective function, as has been shown during dietary restriction 47.
Neurons and glial cells
Sensory and sympathetic nerves that innervate the bone marrow can regulate hematopoiesis 24,48,49. Sympathetic nerve terminals release noradrenaline that stimulates β3 adrenergic receptors (ADRβ3) on perivascular MSCs. The noradrenergic stimulation attenuates production of the hematopoietic retention factor CXCL12 in MSCs, leading to the release of HSCs, progenitors, and mature leukocytes from the bone marrow 50. This axis controls circadian oscillations of HSC mobilization as well as acute and chronic stress and emergency responses 27,48,50,51. Importantly, catecholamines can also activate adrenergic receptors on various hematopoietic cells, including human HSPCs, thereby regulating their migration and engraftment behaviors 52. Non-myelinating Schwann cells that encircle nerve fibers also influence hematopoiesis by inducing HSC quiescence via transforming growth factor-β (TGFβ) signaling 53.
Osteoblastic cells
Osteoblastic cells were the first non-hematopoietic cells described as direct regulators of the hematopoietic stem cell niche 54,55, although recent evidence suggests that osteoblasts’ effect on HSCs might not be direct. Specifically, imaging analyses found few HSC in contact with osteoblasts, and studies using genetic and pharmacological tools to decrease or increase osteoblasts frequently had no acute effect on HSC numbers 9,10. Furthermore, conditional deletion of osteoblast-derived CXCL12 and SCF, factors crucially required for HSC maintenance 56–61, had very limited impact on HSC function and numbers 62,63. Finally, studies have questioned the importance of the adhesion molecule N-cadherin, which was once thought to be the key mediator of HSC-osteoblast interaction. Such studies could neither confirm its expression on HSCs nor detect any effect on hematopoiesis, HSC numbers, and function following N-cadherin deletion from osteoblastic cells and HSCs 9.
Nevertheless, there are still compelling arguments that osteoblasts have an indirect role in HSC regulation. When conditional deletion of osterix prevents chondrocytes from differentiating to osteoblasts, hematopoiesis is almost completely abrogated in the HSC-rich metaphysis 64–66. Likewise, transplanted mesenchymal progenitors can create donor-derived ectopic bones that recruit host-derived blood vessels and HSCs in vivo 67,68, suggesting that mature osteoblastic cells may be important contributors of structural components required to form hematopoietic niches. Osteoblastic cells also seem to be involved in maintaining downstream hematopoietic progenitors, primarily from the lymphoid lineage 10,69.
Perivascular mesenchymal stem/stromal cells (MSCs)
The location of HSCs around blood vessels motivated extensive analyses of the stromal cells in this region. Bone marrow mesenchymal stem cells are rare stromal cells, with the ability to self-renew and differentiate into osteoblasts, chondrocytes, and adipocytes 67,68,70. Mesenchymal stromal cells, the descendants of mesenchymal stem cells, are abundant around blood vessels throughout the bone. In mice, perivascular MSCs expressing typical marker proteins such as nestin-GFP 27, leptin receptor 62, PRX-1-Cre 63, osterix-Cre 63, inducible Mx-1 Cre 71, and CXCL12-GFP 59 can regulate HSCs. An equivalent in humans are CD146-positive skeletal stem cells that synthesize the HSC niche factors CXCL12 and SCF 68. Overall, there is convincing evidence that MSCs have a crucial role as constituents of the perivascular HSC niche.
The first peri-sinusoidal MSCs shown to co-localize with HSCs were so-called CXCL12-abundant reticular (CAR) stromal cells 59. CAR cells are adipo-osteogenic progenitors that express high amounts of the HSC maintenance factors CXCL12 and SCF 72 as well as the lymphoid progenitor and B cell maintenance factor interleukin 7 (IL-7) 73. These cells’ importance for maintaining HSCs was demonstrated in studies ablating CXCL12-positive bone marrow cells, which led to HSC depletion 72. Self-renewing Nestin+ MSCs are also essential niche cells that associate with HSCs and highly express HSC maintenance genes including SCF and CXCL12 27. In the bone marrow, Nestin+ MSCs densely surround arterioles, can be found around the sinusoids, and receive direct sympathetic innervation 25,48,51,74. Advanced 3D imaging of the whole bone marrow space identified rare Nes-GFPhigh cells that are positive for the pericyte marker NG2 25 or myosin heavy chain 11 (MYH11) 75 and closely associate with arterioles and quiescent HSCs as well as Nes-GFPlow cells located around the sinusoids that overlap with LEPR+ cells and CAR cells 25.
Importantly, studies have clarified that mesenchymal stromal cell populations identified and described using markers such as CAR, LEPR-Cre, NG2-Cre, PRX1-Cre, Nes-GFP, and SCF-GFP substantially overlap (reviewed in 10).
Endothelial cells
Endothelial cells are important niche constituents and form the so-called vascular HSC niche. 34. Endothelial cells produce multiple HSC function-regulating factors including CXCL12, SCF, pleiotrophin, and Notch ligands 62,63,76–80. Different approaches to manipulating endothelial cell function using conditional deletion of proteins (e.g. SCF 62, CXCL12 63,81, gp130 82) in Tie2-Cre or Cdh5-Cre mice, knockout mice (e.g. E-selectin−/−) 80, or blocking antibodies (e.g. VEGFR2 signaling) 83 all considerably affected HSC function and numbers. In co-culture experiments endothelial cells promote HSC maintenance 84 and bone marrow sinusoidal endothelial cells even support HSC expansion 78,85.
Based on differential expression of podoplanin (PDPN) and SCA1, bone marrow endothelial cells can be divided into arteriolar endothelial cells (SCA1highPDPN−) and sinusoidal endothelial cells (SCA1+PDPN+) that fulfill distinct roles in HSC maintenance. One example is the almost exclusive production of endothelial cell-derived SCF by arteriolar endothelial cells 86.
In addition to producing crucial HSC maintenance factors, endothelial cells affect HSC function in other, more physical ways. For example, blood vessel permeability may correlate with nearby HSCs’ ROS levels, which in turn determine HSC quiescence. HSCs located around less-permeable arterioles have lower ROS levels and are therefore more quiescent, while HSCs around the leakier sinusoids show higher ROS levels promoting their differentiation and migration 87.
Megakaryocytes
Megakaryocytes promote HSC quiescence 88–91. 3D imaging of the bone marrow space revealed an HSC subset closely associated with megakaryocytes, and megakaryocyte depletion reduced HSC quiescence 88. Megakaryocytes might regulate HSC quiescence by secreting CXCL4, TGFβ, and thrombopoietin (THPO) 88–92. Indeed, TGFβ1 and CXCL4 release may be an important mechanism by which megakaryocytes control myeloid progenitor proliferation and restore HSC quiescence during emergency hematopoiesis 93. HSCs biased towards platelet formation (von Willebrand factor (vWF)-GFP+) are spatially associated with megakaryocytes that regulate their activity, suggesting the existence of a feedback loop by which megakaryocytes regulate their own production 94. Overall, megakaryocytes seem to form a second HSC quiescence-promoting niche that is distinct from the arteriolar niche that also promotes HSC quiescence 25,53.
Monocytes, Macrophages, Neutrophils, and T cells
Macrophages promote HSC retention in their bone marrow and spleen niches 95. These effects are mediated either by direct macrophage-HSC interactions or indirectly via stromal cells 96–98. In the spleen, for instance, red pulp macrophages use vascular cell adhesion molecule 1 (VCAM1) to retain HSCs 99. Furthermore, macrophages also influence the HSC niche by helping clear “aged” neutrophils (CD62lowCXCR4high) from the circulation. In this process, phagocytic macrophages modulate niche activity by liver x receptor (LXR) signaling, which regulates CXCL12 expression on MSCs and promotes the circadian release of HSPCs into the circulation 100. Besides macrophages, changes in sympathetic activity also contribute to the circadian rhythmicity of HSPC release 48,51,74. Osteoclasts, which are specialized giant cells that resorb bone, have also been implicated as niche regulators 101–103. Neutrophils likewise fulfill important roles in the bone marrow niche. Upon granulocyte colony-stimulating factor (G-CSF)-induced sympathetic stimulation, neutrophils produce prostaglandin E2, which acts on osteolineage cells to retain HSPCs in the bone marrow 104, and neutrophil-derived tumor necrosis factor α (TNFα) accelerates vascular and hematopoietic recovery of the bone marrow after irradiation 105. A recent study identified an orexin-receptor+ pre-neutrophil population in the bone marrow that produces colony stimulating factor-1 (CSF1), which accelerates myelopoiesis and atherosclerosis during sleep fragmentation due to reduced orexin signaling from the hypothalamus 106. Finally, lymphoid cells also control niche processes. FOXP3+ regulatory T cells have been shown to promote allogeneic HSC survival after transplantation by secreting interleukin 10 (IL-10) and generally seem to provide the niche with immune privilege 107–110.
Molecular regulators of the stem cell niche
Stem cell factor (SCF) is a crucial cytokine for HSC survival, proliferation, and differentiation as well as for chemotactic HSC migration and retention of HSCs and progenitors in the bone marrow 61,111–114. SCF exists in a soluble and membrane-bound forms, both of which activate the c-KIT receptor (CD117) on HSCs 115–123. Mice lacking membrane-bound SCF (Sl/Sld) have very low HSC numbers and exhibit severe anemia 60,124. In mice with chimeric spleens containing a mixture of S1/S1d and wild-type stromal cells, normal hematopoiesis was only observed in close proximity to the wild-type cells, thereby revealing the importance of locally produced SCF in forming the HSC niche 125. SCF is primarily produced by leptin receptor+ (LEPR) mesenchymal stromal cells in the perivascular space of bone marrow sinusoids as well as by sinusoidal endothelial cells, and using LEPR-Cre and Tie2-Cre to delete SCF from these cell types indeed lowered HSC levels 62,75,77,126.
Another key niche factor required for HSC maintenance and retention is the chemokine CXCL12 (SDF-1), which binds to the CXC chemokine receptor type 4 (CXCR4) (SDF-1-receptor) on hematopoietic cells 56–59,127. CXCL12 is also involved in HSC proliferation and transplanted HSC engraftment 128,129, and conditional deletion of CXCL12 or CXCR4 from niche cells strongly reduces HSC numbers in the bone marrow 59,127,130. The proliferation and retention of various hematopoietic progenitors also depends on CXCL12 56,128,131,132. Perivascular MSCs are the main sources of CXCL12, while its expression levels are 100-fold lower in endothelial cells and 1000-fold lower in osteoblasts 59,63,81,133,134.
HSC maintenance also requires thrombopoietin (THPO), which acts via myeloproliferative leukemia protein (MPL) on HSCs 135–138. In addition to its role in maintaining HSC, THPO promotes megakaryocyte and thrombocyte development 139–141. The growth factor is mainly produced in the liver and kidney, with only minor production in the bone marrow under homeostatic conditions 139,142. The relevance of locally produced versus liver- or kidney-derived THPO for HSC maintenance has not yet been evaluated.
Multiple other proteins that act locally in the bone marrow have been shown to regulate HSC function without being absolutely required for HSC maintenance. Among them, several factors that are dispensable to steady-state hematopoiesis but become important regulators of emergency hematopoiesis in response to injury or infection, including fibroblast growth factor 1 and 2, angiogenin, angiopoietin-like protein 3, interleukin 6, Notch 2, pleiotrophin, interleukin 3, and others (reviewed in 42). Among important long-range signals reaching the bone marrow niche via the circulation are corticosterone, catecholamines, and toll-like receptor (TLR) ligands.
Effect of aging on the HSC niche
Aging strongly affects the HSC niche. Characteristics of aged HSCs include reduced regenerative potential 143–146, myeloid bias upon transplantation 145–149, enhanced mobilization and reduced homing 143,150,151 as well as an altered distribution pattern within the bone marrow 152,153. These changes of HSC functions seem to mainly depend on cell intrinsic mechanisms such as DNA damage, cell polarity defects, altered metabolism and transcriptional and epigenetic profiles 143,145,146, reviewed elsewhere 154,155. However, cell extrinsic mechanisms also seem to play a role as the engraftment of transplanted young HSCs is better in young recipient than in old recipients 145,156. HSC extrinsic mechanisms include increased CCL5 production promoting myeloid bias of HSCs 156 as well as decreased osteopontin expression by bone marrow stromal cells 157.
With ageing the bone marrow space also undergoes anatomical changes including a shift from osteolineage to adipolineage differentiation of MSCs, which results in decreased bone formation and accumulation of adipocytes 158,159. Changes in the vasculature include a reduced frequency of arterioles and transitional vessel, decreased arteriolar innervation, higher leakiness and ROS levels, as well as reduced expression of HSC maintenance factors 76,153,160. The importance of bone innervation is underlined by surgical denervation experiments in young mice that recapitulated many aspects of normal HSC ageing.
The niche in context
The emergence and rapid development of single cell sequencing techniques made possible broad molecular definition and characterization of bone marrow niche cell populations. Two recent single-cell resolution studies analyzing the transcriptional landscape of the bone marrow niche revealed unexpected cellular heterogeneity and determined cellular sources of important niche factors 161,162. Tikhonova et al. performed single-cell RNA sequencing on pre-sorted vascular (VEcad-cre+), perivascular (LEPR-cre), and osteoblast (Col2.3-cre) cell populations from the mouse bone marrow in steady state and hematopoietic stress conditions (5-FU i.p.) 161. Analyses at baseline identified two endothelial, four perivascular, and three osteolineage subpopulations as well as a small cycling cell cluster and the relevant factors these subsets produce. Stress induced considerable changes in niche cell transcriptional profiles: perivascular cells were skewed adipocytic, vascular Notch delta-like ligands and E-selectin were attenuated, and proliferation of all niche subpopulations increased from 0.7% at baseline to 5.4% after chemotherapy. Using droplet-based single-cell RNA sequencing of murine bone and bone marrow stroma, Baryawno et al. identified 17 stromal cell subsets, including previously undescribed mesenchymal, pericyte, endothelial and fibroblast subpopulations that express distinct genes involved in regulating hematopoiesis 162. These analyses revealed previously unknown differentiation trajectories for osteolineage cells; production of the key niche factor CXCL12 by a fibroblast subset; a novel, distinct cluster of arterial endothelial cells with particularly high niche factor expression; and molecular distinctions among Nestin, LEPR, and NG2-expressing niche populations.
As a complement to single-cell RNA sequencing approaches, mass cytometry (CyTOF)- based single-cell protein analyses have recently identified 28 stromal cell subsets in the steady-state bone marrow, of which 14 subsets expressed factors that regulate hematopoiesis 163. While radiation conditioning induced a loss of LEPR+ and Nes+ putative niche cells and many other subsets, a CD73+NGFR+ subpopulation was preserved and supported HSPC engraftment and acute recovery of hematopoiesis. These findings raise questions about the importance of some stromal subpopulations (e.g. LEPR+ and Nes+ cells) currently though to play a dominant role, mostly based on non-inducible Cre-derived results, at least under stress conditions. Another important finding of this study is that endogenous leptin receptor and nestin production correlate poorly with reporter gene expression in the commonly used Cre and reporter lines 163. Finally, a recent study described an Apelin+ bone marrow endothelial cell population that regulates steady-state hematopoiesis and responds to signals from HSPCs to mediate regeneration of the vascular niche after irradiation injury 164.
During the last decade, our understanding of the bone marrow stem cell niche and its cellular and molecular composition has expanded rapidly (Figure 1). Genetically engineered mouse strains, advanced imaging techniques, computational analyses, and single-cell RNA sequencing approaches have shed light on the complexity and heterogeneity of stem cell niches in the bone marrow. However, important technical considerations that arose with technical progress include the specificity and recombination efficacy of the different Cre lines and the compensatory effects that might occur following deletion of a specific cell type or a factor from a specific cell type.
Figure 1: The hematopoietic stem cell niche.
HSCs mainly reside in the bone marrow, where the bulk of hematopoiesis occurs during adulthood. Bone structures that form and protect the medullary cavity are densely innervated and vascularized with the periosteum lining the outer surface and the endosteum coating the inner surface. The endosteum forms the interface between bone and bone marrow and consists of bone-lining cells including osteoblasts that form bone substance and osteoclasts that resorb it. Arteries reach the bone marrow via nutrient canals, branch into smaller arteries and eventually into thin-walled arterioles. Transitional vessels connect arterioles with an extensive sinusoidal network that drains into a central venous sinus, which in turn empties into nutrient veins that leave the marrow via nutrient canals. Sympathetic fibers follow the arteries into the bone marrow, where they form a structural network with neural-glial antigen- (NG2) and nestin- (Nes) positive perivascular stromal cells. HSCs primarily reside in perivascular niches around fenestrated sinusoids as well as around less-permeable arterioles, while lymphoid progenitors reside in an osteoblastic niche closer to the endosteum. Overall, the bone marrow niche is formed by a complex network of non-hematopoietic as well as hematopoietic cell types that produce multiple factors orchestrating maintenance, proliferation and differentiation of HSCs and their progeny. The best-studied and apparently most relevant niche cell types are endothelial cells (sinusoidal and arteriolar ECs) and perivascular mesenchymal stromal cells (Ng2-CreER+, LEPR+/CAR cells) that produce crucial HSC maintenance factors such as SCF and CXCL12. Other cell types involved in niche formation include osteoblasts, osteoclasts, sympathetic nerve fibers, non-myelinating Schwann cells, adipocytes, megakaryocytes, macrophages and regulatory T cells (Treg). Abbreviations: NG2 - neuron/glial antigen 2; HSC - hematopoietic stem cell; Treg - regulatory T cell; LEPR - leptin receptor; CAR cell - CXCL12-abundant reticular cell; EC - endothelial cell; CXCL12 - CXC chemokine ligand 12; SCF - stem cell factor; OPN - osteopontin; IL7 - interleukin-7; TGFβ - transforming growth factor-β; CXCL4 - CXC chemokine ligand 4; DARC - duffy antigen receptor for chemokines; TNF - tumor necrosis factor; vWF - von Willebrand factor; PTN - pleiotrophin.
The hematopoietic differentiation tree
Conceptual update of the tree model
The hematopoietic differentiation tree was long imagined to be strictly hierarchical. Recent studies, however, have significantly challenged this conceptualization, particularly its clear demarcation of stem and progenitor cells, the strict time points for cell fate choices, and order of differentiation steps.
The first hematopoietic tree models suggested an early separation between lymphoid and other (myeloid, erythroid, megakaryocytic) lineages, followed by several branching points so that each lineage differentiated from multipotent to bipotent to unipotent progenitors. Subsequent modifications of the tree included a longer association between myeloid and lymphoid lineages 165–167, earlier separation of the megakaryocytic lineage 168,169, and definition of multipotent progenitor subsets 170,171. In addition, several studies described heterogeneity within the HSC population 172. In recent years, new technologies provided further evidence for a dynamic system comprising heterogeneous cell populations that gradually progress from one to the other, with a high grade of flexibility to respond to changing demands.
The hematopoietic stem cell population at steady state and under stress
In the bone marrow, approximately 1 in 10,000 cells is a transplantable hematopoietic stem cell 11,12. HSCs were traditionally defined by multipotency and self-renewal capacity, while progenitors were defined by restricted lineage and lack of prolonged self-renewal capacity. While HSC can be repetitively transplanted and can recover the entire hematopoietic system, progenitors are typically lost 2–3 weeks post transplantation 165. The transcriptional program of HSCs has unique cellular and metabolic properties, including a quiescent, glycolytic state with low mitochondrial activity and low protein synthesis levels compared to other hematopoietic cells (reviewed in 172). Specific stress-response and control mechanisms render HSCs less susceptible to metabolic stress as well as to DNA and protein damage 172. In contrast to HSCs, progenitor populations strongly proliferate and show high metabolic activity requiring oxidative metabolism and mitochondrial activity.
Over the last decade, different HSC subpopulations have been identified according to their capacity for self-renewal. Using transplantation assays, HSC capable of engrafting for more than 16 weeks upon both primary transplantation and at least a second round of transplantation are defined as Long-Term (LT) HSCs 34,173–176. Multipotent cells that only transiently engraft but differ with respect to durability and graft stability are defined as Intermediate (IT) HSCs 177, Short-Term (ST) HSCs, or Multipotent Progenitors (MPP) 170,171,173.
Label retention studies indicate that the time an HSC spends in quiescence positively correlates with its self-renewal capacity 178–182. In mice, division frequencies vary between once monthly to twice yearly, depending on the HSC subset 13,178,180,182. A recent study estimated that LT-HSCs asynchronously undergo four symmetric self-renewal divisions during adulthood to expand their pool but irreversibly lose long-term regenerative capacity at the fifth division 182. Quiescent HSCs that have been activated by stress can return to the quiescent state 178,180.
In the steady state, a low number of HSCs are released into the circulation in a circadian pattern and some HSCs also reside in the lungs and spleen 74,183,184. So far, little is known about either potential lineage biases or differentiation and clonal expansion potentials of HSCs in these extra-medullary niches.
Various stressors, including DNA damage, inflammation, infections, metabolic stress, obesity, and psychosocial stress, directly influence HSC functions 172. Furthermore, HSC and progenitor subsets differentially react to stress. An example of subtype-specific stress responses is enhanced GMP production by MPP2 and MPP3 subsets that dynamically reorganize themselves and activate a progenitor self-renewal network under conditions of emergency myelopoiesis 46,170.
HSC heterogeneity and cell fate decisions
While HSCs are defined by their capacity to differentiate into all blood cell types, different HSC and MPP subpopulations demonstrate heterogeneous lineage output 170,171. For example, HSC subsets have varying relative myeloid and lymphoid lineage outputs 185,186, and one subset is characterized by the expression of the platelet markers CD41 and vWF 168,169 and primarily gives rise to megakaryocytes 168,169, bypassing the multipotent progenitor stages 16,168,169,187–190. Though the molecular mechanisms underlying this non-stochastic HSC heterogeneity are not well understood, micro-environmental cues and long-range signals are likely regulators.
Since cell fate decisions occur at the individual cell level, functional and biochemical assays must be conducted at single-cell resolution. In the last decade, multiple studies have used in vivo transplantation assays in mice as well as in vitro models with individual human progenitors to explore conceptual questions about lineage differentiation routes. For instance, and contrary to the classical common myeloid progenitor (CMP) – common lymphoid progenitor (CLP) model, lymphoid and myeloid lineage segregation is not the earliest fate decision; rather, lymphoid and myeloid potential are preserved until the stage of so-called lymphoid-primed multipotential progenitors (LMPPs) 166,167. Furthermore, the different branches house various additional subsets, mainly with bi- or uni-lineage potential (reviewed in 172).
Single-cell RNA sequencing has been successfully used to characterize the transcriptomes of HSC as well as diverse progenitor populations 191,192. Various algorithms have been developed to understand cells’ differentiation cascades based on gradual changes in their single-cell transcriptomes during this process 172. These analyses uncovered differentiation trajectories in which the position of each individual cell is determined by its transcriptional snapshot. For example, a recent study analyzing human HSPC using single-cell RNA sequencing with computational analyses and in vitro single-cell assays indicated early lineage restriction 193. The authors suggest HSCs continuously acquire lineage biases without passing through discrete multi- and bipotent stages, so that unipotent cells emerge directly from a “continuum of low-primed undifferentiated hematopoietic stem and progenitor cells’ (CLOUD-HSPCs)“ 193. Earlier reports of numerous unipotent progenitors within compartments that are multipotent at the population level support these data 187,191,194. That said, in contrast to these single-cell RNA sequencing-based data, HSPC can be clearly split into functionally distinct subpopulations based on surface marker combinations. There is ongoing discussion about the reasons for these seemingly incompatible results, including decoupling of mRNA and protein expression; epigenetically determined functional heterogeneity; more gradual changes in functionality, as suggested by functional assays of purified cells; and inefficacy of single-cell RNA sequencing-based methods in separating closely related cell types 172. Future research should determine the extent of continuity versus distinctness in early hematopoiesis.
To study HSC and progenitor behaviors in their physiological microenvironments, individual cells can be tagged before transplantation (reviewed in 172). Though their tagging methods vary, these studies generally support the lineage biases and restrictions demonstrated in single-cell transplant assays. In a transplantation setting, a small number of HSCs gives rise to the vast majority of differentiated cells. New technologies such as barcode labeling can track the lineage output of individual HSCs and progenitor cells during this process 14,172. Such studies indicate that during unperturbed steady-state hematopoiesis, MPPs mainly contribute to the myeloid lineage and that hematopoiesis is almost completely driven by cells from the MPP populations. Furthermore, the studies suggest that during steady-state hematopoiesis, megakaryocytes can arise independently from the other lineages and that LT-HSCs actively contribute to the megakaryocyte output 172,189.
Hematopoietic differentiation trajectories
As our understanding of stem cell heterogeneity and early hematopoiesis grows, so does our knowledge of the various differentiation trajectories cells can follow from the stem cell compartment to the mature blood cell pool. Current models indicate that the erythroid-megakaryocytic lineage separates directly from biased cells within the stem cell compartment, thus making it the earliest division in the hematopoietic tree. Three MPP populations with different progeny potentials have been identified: MPP2, MPP3, and MPP4. From the MPP stage a cell can differentiate into either a common myeloid progenitor (CMP) or a lymphoid-primed multipotential progenitor (LMPP) 166,167. LMPPs mainly differentiate into common lymphoid progenitors (CLPs), which give rise to the lymphoid lineage (B cells, T cells, natural killer (NK) cells, innate lymphoid cells (ILCs)) but still retain the potential to differentiate into granulocyte-monocyte progenitors (GMPs). Thus, the myeloid and lymphoid lineages are connected longer than previously assumed. CMPs give rise to GMPs and monocyte-dendritic cell progenitor (MDPs). GMPs further differentiate into granulocyte progenitors (GPs) and common monocyte progenitors (cMoPs) that give rise to neutrophils and monocytes, respectively. MDPs differentiate into common dendritic cell progenitors (CDPs) that give rise to dendritic cells. A recent study used fate mapping via the expression history of Ms4a3, which is specifically and transiently expressed in bone marrow GMPs, to trace monocyte-derived cells. This study found that MDPs give rise to monocytes directly, bypassing the cMoP stage, and that MDPs do not arise from GMPs 195. Altogether, these studies have enriched our understanding of hematopoiesis and the various differentiation pathways that progenitors can follow (Figure 2).
Figure 2: The hematopoietic differentiation tree.
Mature immune cells arise from hematopoietic stem cells in a process called hematopoiesis. Over the last decades, various models have been developed and continuously adapted in light of novel scientific insights to best visualize this differentiation process. Recent technical and conceptual breakthroughs that have been incorporated into the above model of the differentiation tree, include greater heterogeneity within the HSC population, less clear demarcation of stem and progenitor cell populations, earlier and more dynamic cell fate choices, as well as a much more gradual rather than stepwise differentiation of cells through the heterogeneous tree compartments with highly dynamic reactions to changing demands. The dashed line represents a trajectory of a hematopoietic cell during its differentiation process with red dots indicating transcriptional snapshots captured by single-cell RNA sequencing during this process. Overall, it is difficult to visualize all aspects of hematopoiesis in a 2-dimensional tree model and various partly controversial attempts to depict this have been published. Abbreviations: HSC - hematopoietic stem cell; LT - long term; IT - intermediate term; ST - short term; MPP - multipotent progenitors; LMPP - lymphoid-primed multipotential progenitor; CMP - common myeloid progenitor; CLP - common lymphoid progenitor; MEP - megakaryocyte-erythrocyte progenitor; EoBP - eosinophil-basophil progenitor; GMP - granulocyte-monocyte progenitor; GP - granulocyte progenitor; cMoP - common monocyte progenitors; MDP - monocyte-dendritic cell progenitor; CDP - common dendritic cell progenitors; NK - natural killer cells; ILCs - innate lymphoid cells; cDC - conventional dendritic cell
Hematopoiesis and atherosclerosis
In recent decades, we have come to understand that atherosclerosis is a multifactorial inflammatory disease that crucially depends on inflammatory leukocyte supply from the bone marrow niche and extramedullary sites 4–7. Innate immune cells such as monocytes and macrophages as well as neutrophils in particular are decisive contributors to the initiation, progression, and destabilization of atherosclerotic lesions.
The healthy arterial wall
In the healthy arterial wall, resident macrophages dwell in the adventitia where they renew via local proliferation. In mice, arterial resident macrophages mostly derive from circulating monocytes, many of which accumulate immediately after birth 196. Studies in mice with labeled CD11c+ cells indicate that dendritic cells also reside in the arterial wall, as well as in heart valves 197,198, but it is still unclear how distinct these cells are from macrophages. While arterial resident macrophages likely conduct general macrophage tasks such as tissue homeostasis and pathogen clearance, their artery-specific functions are only beginning to be appreciated 199.
Leukocytes in atherosclerosis
Macrophages are among the most abundant and functionally relevant cell types in atherosclerotic lesions, where they are responsible for plaque growth and destabilization 196,200–202. These phagocytic cells are part of the innate immune system and populate most tissues in both steady state and disease conditions. Depending on lineage, phenotype, and host tissue, macrophages fulfill various functions including disposing pathogens and foreign material, presenting antigens, regulating hematopoiesis, resorping bone, iron recycling, synaptic pruning, electric coupling, and regulating temperature, among others 203,204. Proatherogenic conditions lead to the deposition of cholesterol-rich lipoproteins, such as low-density lipoprotein (LDL), in the intima that become oxidized, triggering macrophage accumulation and inducing their differentiation programs 4–7. In an attempt to remove the oxidized lipoproteins via scavenger receptors, macrophages become lipid-laden foam cells that eventually die in the lesions, fueling the growth of the necrotic core 205, which is an area within the plaque in which lipids and dead cells accumulate. Macrophages also remove dead or dying cells in a process called efferocytosis, and insufficient efferocytosis has been implicated as an important pathomechanism in atherosclerotic lesion growth 206. As important protease producers, macrophages contribute to the destabilization of the fibrous cap that covers the necrotic core. Furthermore, macrophages attract and activate other immune cells via the production of pro-inflammatory cytokines. Intravital imaging of large arteries in mice has shown that macrophages in plaques are highly motile: in addition to entire cells migrating, stationary cells extend and retract their dendrites, an action referred to as “dancing on the spot“ 207. The importance of macrophages and their pro-inflammatory actions to atherosclerosis and its complications is undeniable, and the recent large-scale CANTOS trial has provided proof-of-concept evidence that interfering with inflammatory pathways, specifically the macrophage product interleukin 1β (IL-1β), can indeed reduce cardiovascular events 208.
Aside from monocytes and macrophages, neutrophils also play important roles in atherosclerotic lesions. Activated by chemokines (e.g. platelet-derived CCL5) neutrophils are recruited into atherosclerotic plaques, where they produce proteins such as cathelicidins, myeloperoxidase (MPO), cathepsin G and other proteases as well as reactive oxygen species (ROS), which together accelerate leukocyte recruitment along with accumulation and oxidation of LDL 209–212. The release of neutrophil extracellular traps (NETs) is another important mechanism leading to plaque progression via stimulation of plasmacytoid dendritic cells 213 and macrophages 214 as well as to plaque destabilization via the NET component cytotoxic histone H4 that perforates vascular smooth muscle cell membranes thereby inducing cell lysis 215. Metalloproteinases released by neutrophils also contribute to plaque destabilization by degrading the extracellular matrix, which eventually may trigger plaque erosions and ruptures. For further discussion we refer the reader to a recent review 216.
Monocyte recruitment into plaques
Blood monocyte numbers robustly correlate with atherosclerosis progression and cardiovascular event rate 217–222, and in animal models atherosclerosis does not develop without monocyte influx 223–225. Monocytes are divided into at least two functionally distinct subsets that can be distinguished by expression of the glycoprotein Ly6C in mice and CD14/CD16 in humans 226. Ly6Chigh monocytes (CD14++CD16− and CD14++CD16+ in humans) have a short life span and accumulate in inflamed tissues, where they can differentiate to macrophages 226,227. Ly6Clow monocytes (CD14+CD16++ in humans) have a longer life span and exhibit vascular patrolling behavior to enable early response to infections and endothelial damage 228,229. Fate mapping studies convincingly demonstrated that Ly6Clow monocytes derive from Ly6Chigh monocytes in the blood 195,230–232. Human classical monocytes (CD14++CD16–) similarly give rise to intermediate monocytes (CD14++CD16+) that further develop into non-classical monocytes (CD14+CD16++) 233.
During atherogenesis, Ly6Chigh monocytes bind to activated endothelium, migrate into the atherosclerotic lesion, and differentiate into macrophages. Monocyte recruitment depends on chemokine/chemokine receptor interaction (e.g. CCR2/MCP-1, CCR5/CCL2/CCL5, CX3CR1/fractalkine) as well as adhesion molecule expression on activated endothelial cells (e.g. VCAM-1, P-selectin etc.) reviewed in 7,234. The relevance of Ly6Clow monocytes in atherosclerosis is less clear, although we do know they can ingest oxidized lipoproteins 235, do not directly contribute to the plaque macrophage population 232,236,237, and may be important in endothelial protection 238.
As additional players in monocyte recruitment, platelets bind to activated endothelium 239, which induces production of macrophage-attracting chemokines and adhesion molecules 240. Platelet activation results in degranulation, chemokine release, increased P-selectin expression, and formation of monocyte-platelet aggregates 241–245. Neutrophils have several possible functions, including promoting monocyte accumulation in plaques by producing cathelicidin that interacts with formyl-peptide receptor 2 on monocytes, leading to integrin activation 211,212.
Plaque macrophage proliferation
In addition to monocyte recruitment, lesional macrophage numbers are determined by local macrophage proliferation within the plaque. Parabiosis and fate mapping analyses revealed that local proliferation dominates macrophage accumulation, particularly in established atherosclerotic lesions 202, while monocyte recruitment has much larger contributions to developing lesions 246,247. According to BrdU tracking experiments in ApoE−/− four-month-old mice, high turnover of lesional macrophages can renew the entire plaque macrophage population within one month 202. While little is known about factors that regulate macrophage proliferation in plaques, GM-CSF has been implicated in proliferation in early lesions 247, whereas type 1 macrophage scavenger receptor class A (Msr1) seems to play a role in advanced lesions 202.
Based on fate mapping experiments, it has been suggested that macrophage-like cells in the plaque may derive from smooth muscle cells. An estimated 18% of macrophages in human plaques seem to originate from smooth muscle cells, but the functions of these macrophages remains unclear 248. The relevance of vascular smooth muscle cell transdifferentiation to macrophage-like cells is still under discussion 248,249. Overall, monocyte production from hematopoietic progenitors in medullary and extramedullary sites, monocyte recruitment into atherosclerotic lesions, and local proliferation of macrophages all contribute to the progression of atherosclerotic lesions 250–252 (Figure 3). Whether and how monocyte-derived macrophages functionally differ from locally produced macrophages requires further study.
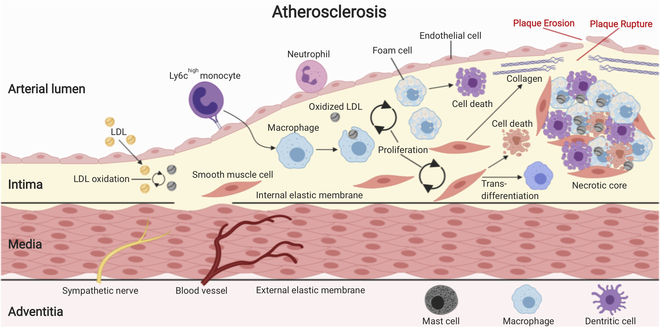
Figure 3: Atherosclerosis.
Atherosclerotic plaque formation begins with the accumulation of low-density lipoprotein (LDL) particles in the intima of large arterial blood vessels. Common atherosclerotic risk factors accelerate this process. Within the intima, LDL particles are oxidatively modified, which renders them immunogenic and triggers an early inflammatory response, including endothelial cell activation. Upregulation of endothelial adhesion molecules and release of pro-inflammatory chemokines leads to the recruitment of inflammatory monocytes. Monocytes in the intima differentiate into macrophages that take up oxidized LDL via scavenger receptors and eventually become lipid-laden foam cells. The proinflammatory milieu in the intima also triggers the migration of quiescent smooth muscle cells (SMCs) from the media into the intima, where they proliferate and produce excessive extracellular matrix (ECM), including proteoglycans and collagens. Some SMCs may even trans-differentiate into macrophage-like cells. Cell recruitment, cell proliferation, and ECM production fuel atherosclerotic plaque growth. Ongoing cell death and impaired efferocytosis (removal of dead/dying cells) lead to the formation of an ever-growing lipid-rich necrotic core that sustains inflammation. Necrotic core growth and production of proteases, such as matrix metalloproteinases (MMPs) by activated macrophages that degrade collagens in the fibrous cap set the stage for plaque ruptures and plaque erosions that are responsible for the life-threatening complications of atherosclerosis.
Fate of plaque macrophages
Mechanisms that reduce plaque macrophage numbers may include macrophage death and exit from plaques as well as reduced monocyte recruitment 6,253,254. Methods that can image cells in the mouse arterial wall in vivo were an important technical breakthrough to address cell motility and cell-cell interactions 207,255. Such methods may enable visual analyses of macrophages fates in plaques (death versus exit) and the pathways they might take (blood versus lymph). Although macrophage departure from atherosclerotic lesions has not be visually confirmed, there is evidence that dendritic cells exit the aortic wall of healthy mice in response to infectious stimuli 197. Whether lipid-laden macrophages depart similarly and whether this is a plaque regression mechanism still remains to be imaged.
Atherosclerosis accelerates hematopoiesis
Over the course of atherosclerosis progression, monocyte levels in the blood progressively increase 224. These circulating monocytes accelerate atherosclerosis, which in turn reinforces immune cell production and release from the bone marrow, thus fueling a feedforward cycle. The involved pathways include catecholaminergic signaling on bone marrow niche cells as well as hematopoietic stem and progenitors cells, all of which express adrenergic receptors. Elevated catecholamine release from the adrenal glands as well as elevated noradrenaline release from sympathetic terminals in the bone marrow contribute to this process 74,50,256. Other danger signals such as TLR ligands also regulate HSC functions via different transcription factors, including PU.1, Egr-1, Irf8, Klf4, Mafb, and C/EBPα 257, which can induce myeloid bias of HSC, leading to increased myeloid cell production.
Myeloid cell release into the circulation is tightly controlled by several bone marrow intrinsic and extrinsic signals. Chemokines/chemokine receptor interactions are an important regulatory mechanism. C-C chemokine receptor type 2 (CCR2) blockade in mouse models of atherosclerosis, for instance, reduces monocyte release from the bone marrow and strongly decreases plaque burden 258. Major mechanisms involved in neutrophil release include interactions between CXCL12 and CXCR4, as well as between CXCL1/2 and CXCR2 259.
Cardiovascular risk factors also directly influence the stem cell niche to induce increased HSPC proliferation, myeloid-biased differentiation, release into the circulation, and extramedullary hematopoiesis 260. Various mechanisms control HSC proliferation and differentiation to myeloid cells. For example, increased plasma lipid levels impair reverse cholesterol transport in HSPCs, leading to higher myeloid cell production 224,225,261,262. Proteoglycan-bound ApoE at the cell surface of HSPCs promotes adenosine triphosphate-binding cassette (ABC) transporter ABCA1/ABCG1-mediated cholesterol efflux, which reduces proliferation, blood monocytosis, and atherosclerosis. In ApoE-deficient HSPCs, cholesterol efflux pathways are disrupted and the accumulating cholesterol raises the expression of the common beta subunit (CBS) of the interleukin 3/granulocyte-macrophage colony-stimulating factor (IL-3/GM-CSF) receptor, thereby rendering the cells hypersensitive to IL3/GM-CSF signaling, which in turn induces phosphorylation of ERK1/2 and STAT5. Overall, this HSPC-intrinsic mechanism supports proliferation and leukocytosis, consequently accelerating atherosclerosis 22,224,261,262.
While we are beginning to identify pathways through which risk factors accelerate leukocyte production in CVD, many questions remain to be answered before these insights can be translated into novel therapies. Although several significant CVD risk factors, such as arterial hypertension, smoking, and aging, are all associated with considerable leukocytosis, the underlying mechanisms remain unknown. Acute cardiovascular events like MI or stroke also have major impacts on hematopoiesis via sympathetic activation, glucocorticoid release, and production of various proinflammatory molecules (IL-1β, TLR ligands, chemokines) 7,48,256,263. The overall hematopoietic response can be summarized as a massive expansion of myelopoiesis driven by catecholamines, inflammatory cytokines, damage-associated molecular patterns (DAMPs), and TLR ligands, at the expense of a strongly suppressed lymphopoiesis, which is mainly induced via corticosterone signaling on lymphocyte progenitors.
Formation of extramedullary hematopoietic niches
Under healthy steady state conditions, only a few hematopoietic progenitors are detectable outside the bone marrow 264,265. In inflammatory diseases such as atherosclerosis, however, myeloid cell production can partly shift to other lymphoid organs, particularly the splenic red pulp 22,256,262,266,267. Within this extramedullary niche myeloid-biased HSCs give rise to significant numbers of monocytes and neutrophils that can be released into the circulation to eventually enter atherosclerotic lesions 22.
Little is known about factors that influence the extramedullary retention of HSPCs and the formation of niches outside the bone marrow that maintain HSC proliferation and differentiation, but sphingosine 1-phosphate (S1P) signaling might be involved 265,268. HSPC-extrinsic factors that potentially accelerate splenic myelopoiesis include GM-CSF-producing innate response activator (IRA) B cells, which develop in the spleen in response to infections or atherosclerosis 269–271, and high-fat diet-induced interleukin 23 (IL-23), which triggers production of the HSC-mobilizing factor G-CSF 272. Atherosclerotic risk factors and disease-promoting lifestyle factors, such as stress, lack of exercise, or poor sleep, also influence HSC functions 50,106,273 and are reviewed in detail elsewhere 274. Finally, recent studies have documented the importance of clonal hematopoiesis in cardiovascular risk 260,275–277.
Hematopoiesis and myocardial infarction
Myocardial infarction (MI) and post-MI heart failure are accompanied by profound inflammatory immune activation that shapes and is shaped by the course of the disease. Here we discuss our growing knowledge of the immune system’s role in the healthy heart as well as in myocardial infarction and ischemic heart failure.
Leukocytes in the healthy heart
Healthy mouse heart tissue is populated by at least an order of magnitude more leukocytes than regular skeletal muscle; among the leukocytes, macrophages represent the biggest fractions 204,278–281. Macrophages are also present in the healthy human myocardium 282, forming a dense network between the cardiomyocytes 204,280,281,283,284.
Cardiac macrophages are heterogeneous. While all macrophages are CD45+CD11b+F4/80+ CD64+MERTK+, their relative expression of MHCII, CCR2, and Ly6C differs. CCR2– macrophages can be divided into MHCIIhigh and MHCIIlow subsets while only a minor population of CCR2+ macrophages expresses MHCII. The smallest subset is a Ly6C-expressing macrophage population 204,280,281. Available fate mapping evidence suggests that CCR2– and Ly6C+ macrophages arise from cells that enter the heart during embryogenesis and self-renew postnatally to sustain these populations without monocytic input 280,283. CCR2– cells are found throughout the myocardium adjacent to developing arteries. In contrast, the smaller CCR2+ subset in adult mice most likely derives from recruited monocytes and resides near the endocardium. Although several studies challenged these seemingly clear ontogenic demarcations, the weight of evidence suggests that only a minority of cardiac macrophages in the healthy heart derive from circulating monocytes 196,285,286. Additional leukocyte sources, under specific conditions, include pericardial fluid and pericardial adipose tissue 287,288.
We are only beginning to understand macrophages’ tissue-specific functions in the heart, though they likely ingest invading pathogens, dying cells, and matrix components 280,283. Determining cardiac macrophages’ functions may be particularly important for diseases in which resident cardiac macrophages die and are replaced by monocyte-derived macrophages that might not necessarily acquire tissue-specific tasks from the resident cells. In addition, cardiac resident macrophages themselves may neglect tissue-specific tasks when exposed to an inflammatory milieu. Cardiac resident macrophages may be involved in heart development, coronary maturation 289, adaptation to increased tissue strain, training and pregnancy-induced cardiac remodeling, and regulation of autonomic innervation 290. A recent study showed that macrophages form connexin 43-containing gap junctions with cardiomyocytes in the conduction system of the heart 282. These gap junctions electrically couple cardiomyocytes with macrophages and influence conduction, especially via the AV node where macrophages reside in high numbers. Indeed, macrophage depletion in CD11b DTR mice induced progressive AV block as well as milder conduction abnormalities in atria and ventricles 282.
During aging, the cardiac leukocyte population undergoes significant changes, including lower overall macrophage numbers, potentially higher percentage of monocyte-derived macrophages, and fewer granulocytes 278,285,291. In older mice, phenotypic changes in macrophages such as increased IL-10 production 291,292 contribute to myocardial fibrosis and diastolic dysfunction. However, the implications of these changes for cardiac diseases remain largely unclear. Further details on leukocyte functions and intercellular communication pathways in the steady-state heart have recently been reviewed 281,290.
Leukocytes and myocardial infarction
Atherosclerotic lesions develop over decades and progressively destabilize as dying myeloid and foam cells fuel necrotic core growth and proteases thin out the fibrous cap. Eventually, atherosclerotic plaques may either rupture, meaning a break in the fibrous cap exposes highly thrombogenic necrotic core material to the blood stream, or erode, as a loss of plaque endothelial cells uncovers thrombogenic subendothelial proteins, such as collagens. In the coronary arteries, the resulting thrombus may acutely occlude the vessel and cause downstream ischemia. Epidemiological data suggest that acute stressful events trigger plaque ruptures and erosions. Mechanistically, stress-induced elevations of blood pressure, heart rate, and coronary flow elevate shear stress at the plaque cap. The immediate contribution of immune cells to either rupture or erosion are not known, but extracellular matrix-destabilizing proteases synthesized by neutrophils and macrophages drive the destabilization process that precedes the actual event. The resulting myocardial infarction is a life-threatening complication that can, depending on infarct size and location, lead to severe arrhythmias, heart failure, or even cardiac rupture 4–7,293.
Ischemic injury rapidly triggers a series of immune responses that begin within the resident cardiac leukocyte population, including mast cell degranulation 294, production of inflammatory factors (IL-1, IL-6, TNF, CCL2) by cardiomyocytes and macrophages 295–297, GM-CSF release from fibroblasts 298, and endothelial cell activation. In response to these inflammatory mediators and the progressive cell death of cardiomyocytes and resident cardiac macrophages, the bone marrow and extramedullary sites produce and release large numbers of neutrophils and inflammatory monocytes that enter the circulation and reach the infarcted myocardium 7,21,267. Strong clinical evidence exists for the occurrence of post-MI leukocytosis 222,299 and a robust association between leukocytosis and cardiovascular morbidity and mortality 300,301.
The early phase after MI is dominated by the recruitment of millions of neutrophils and inflammatory monocytes (Ly6Chigh in mice and CD16low in humans) that differentiate into macrophages with high proteolytic and phagocytic activity 232,302–304. During the first several days, these cells scavenge dead and dying cells along with debris 305,306 and fuel inflammation by producing IL-1, TNF, and IL-6 307. Neutrophil numbers decrease after 3 days, and after 1 week they have largely disappeared from the infarcted myocardium. Experimentally depleting neutrophils, which are important for cardiac healing, elevates cardiac fibrosis, impaired function, and heart failure 308. Unlike neutrophils, monocytes migrate to the heart for several days. Comparatively low numbers of non-inflammatory monocytes also enter the heart.
Around day 4, the initial inflammatory clean-up phase transitions into a reparative healing phase with rapidly decreasing neutrophil numbers and a phenotypic macrophage switch towards reparative Ly6Clow macrophages that mainly contribute to angiogenesis and fibrosis by producing factors such as IL-10, TGFβ and vascular endothelial growth factor (VEGF) 232,302. The reasons why resident macrophages die and are replaced by monocyte-derived macrophages are not well understood but may include a need for high numbers of macrophages with alternative phenotypes.
Macrophage subsets in the heart were generally identified based on the surface expression of CCR2, Ly6C, and MHCII. With the emergence of single-cell RNA sequencing, these cell population definitions are being re-evaluated. Indeed, single-cell RNA sequencing analyses of sorted CD45+ infarct cells have identified up to seven transcriptionally distinct subsets. On day 4 after MI in mice, one macrophage subset is activated via the interferon regulatory factor 3(IRF3)-type 1 interferon pathway, which induces the transcription of genes that typically respond to viral infections 306. Interferon-based activation of these macrophages that were termed “IFNICs” seems to be detrimental in MI, as pathway blockade via global IRF3 or type 1 interferon receptor knockout improves recovery and survival after MI in mice 306. However, IFNICs may have other beneficial functions during MI healing. While the other six subsets’ functional distinctiveness remains to be investigated, these single-cell RNA sequencing data provide additional evidence for the complexity of cardiovascular macrophage biology and the insufficiency of the M1/M2 model 260,309. Single-cell approaches, including CyTOF and RNA sequencing, are very effective in identifying new leukocyte subsets in cardiovascular tissues, but it is equally important to evaluate their functional differences 200,201,310.
Leukocytes and ischemic heart failure
The balanced sequence of inflammatory and reparative actions by various contributing immune cell populations determines the mechanical outcomes of the myocardial infarct, including scar size, thickness, and stability 290,311,312. Only after several weeks do cardiac leukocyte populations return to baseline levels. While the myocardial tissue that died during the infarct turns into a scar, the surrounding myocardium undergoes intense remodeling that is critical for the development of ischemic heart failure with reduced ejection fraction (HFrEF). In addition to stromal cells, leukocytes are also involved in this process. Different leukocyte classes are recruited to the remote myocardium starting shortly after MI and peaking around day 10 post-MI 313–316. In the surviving areas, macrophage pools expand due to resident macrophage proliferation and monocyte recruitment 267,315. If heart failure develops, the increased sympathetic tone fuels myeloid cell production in the bone marrow via altered expression of CXCL12 and other niche factors and leads to leukocytosis in the blood 220,315. During remodeling, macrophages in the remote myocardium also change their expression of key enzymes and factors, including reduced production of MPPs and increased production of IL-1β, TNF, as well as VEGF 315. Overall, recruited macrophages seem to negatively influence the post-MI remodeling process 315. Recent reviews provide detailed information about the roles played by different leukocyte populations in myocardial infarct healing and remodeling 260,290,317.
Myocardial infarction accelerates inflammatory hematopoiesis
If the patient survives, a myocardial infarction still has dramatic consequences. Within the first year after an index MI, the frequency of stroke, secondary MI, or death is around 8–12% despite optimal medical treatment 318–321 and the rate of other ischemic events is even higher 322–324. Understanding the reasons for this phenomenon is crucial for developing successful secondary prophylaxis strategies. While ongoing exposure to risk factors is one important aspect, another is MI-induced long-lasting changes in the immune system. Given the short lifespan and rapid turnover of leukocytes in inflamed tissues, a continuous supply of newly produced immune cells is crucial for cardiovascular disease progression. Several different pathways have been suggested to directly accelerate inflammatory hematopoiesis in MI.
Dead and dying cells in the infarcted myocardium release various inflammatory mediators, including IL-1, IL-6, TNF, CCL2 295–297,325, and GM-CSF 298, that act on their respective receptors on hematopoietic and niche cells in the bone marrow to promote hematopoiesis and leukocyte release. The infarcted myocardium also releases damage-associated molecular patterns (DAMPs), including DNA, S100A8-S100A9, high-mobility group protein B1 (HMGB1), and ATP, that circulate and activate toll-like receptors on HSPCs, thereby triggering production of innate immune cells 260,290,326. Such external alarm signals activate intracellular signal cascades leading to the production of multiple transcription factors, including C/EBPα, Egr-1, Irf8, Klf4, Mafb, and PU.1, that activate HSC proliferation and/or induce myeloid bias 257.
Another important mechanism that accelerates hematopoiesis in response to MI is the attenuation of quiescence-promoting niche factors, such as CXCL12 (SDF-1), in the bone marrow. Similar to other stressors, MI mobilizes the sympathetic nervous system, which activates β3 adrenergic receptors on niche cells leading to decreased CXCL12 production 256. The extent to which MI-induced sympathetic activity directly influences HSPCs via their adrenergic receptors is not known. We do know a population of lineage− c-kit+ sca-1+ CD150+ CD48− HSCs, which are referred to as SLAM HSCs 34, that increase proliferation within 48h after myocardial infarction 326. A subset of SLAM HSCs that express the chemokine receptor CCR2, which is regulated by the co-transcription factor Mtg16, show the highest proliferation rate 326. CCR2+ HSCs are myeloid biased, migrate to extramedullary sites, and give rise to MDPs and CMoPs, which eventually become monocytes 264 that leave the bone marrow in response to the chemokines CCL2 327 and CCL7 328. Bone marrow mesenchymal stem cells and peripheral B cells contribute to CCL2 and CCL7 production 296,327–329. In addition to mature leukocytes, the bone marrow also releases HSPCs, which seed into extramedullary sites like the spleen, where they initiate extramedullary hematopoiesis 267. In response to myocardial infarction, the spleen also releases huge numbers of inflammatory leukocytes and overall contributes as much as 50% of all myeloid cells that enter the ischemic myocardium within the first day 21. Splenic monocyte release depends on angiotensin-2 signaling 21,330.
After a few days, HSPCs released from the bone marrow in response to increased sympathetic activity 256,331 seed the spleen, where they help form an extramedullary hematopoietic niche 267. Splenic mechanisms supporting extramedullary niche formation might include CD169+ macrophages that retain HSPCs via VCAM-1 99. While experimentally proven in mice with atherosclerosis and MI 22,256,262, splenic hematopoiesis in humans has so far only been indirectly shown via increased 18F-FDG uptake in PET imaging after acute MI 332,333. Overall, heightened systemic inflammation after MI increases monocyte production in the bone marrow and spleen. In ApoE–/– mice, the resulting blood monocytosis leads to increased recruitment not only to the ischemic myocardium but also to preexisting atherosclerotic plaques. Via this mechanism, a first myocardial infarct accelerates atherosclerosis and might increase the likelihood of secondary ischemic events 256.
In agreement with these studies, clinical data indicate higher levels of innate immune system activity in patients with recurrent cardiovascular events 334. Translational evidence to confirm experimental animal observations in humans mostly derives from blood analyses and imaging, due to the limited availability of in vivo hematopoietic organ samples. Clinical studies revealed patients with acute MI have more circulating HSPCs in their blood, very similar to the observations in mice post MI 256,331,335. Imaging studies described higher 18F-FDG-uptake in non-culprit plaques and hematopoietic tissue activation after myocardial infarction 332,336,337. Similar to MI-induced acceleration of atherosclerosis in mice 256, coronary imaging in humans also showed accelerated lesion growth post MI 338. Furthermore, a retrospective study reported metabolic activity in bone marrow and spleen, measured via 18F-FDG uptake, positively correlated with subsequent cardiovascular events 332. While these studies provide first insights, larger prospective studies focusing on immune pathways are necessary to conclusively test translatability and relevance of mouse findings in patients with myocardial infarction. Nevertheless, despite important insights (Figure 4), both the signaling pathways that expand myelopoiesis after MI and the pathways that limit the hematopoietic response to restore steady state conditions after MI remain key areas of research.
Figure 4: Myocardial infarction.
Leukocytes play important roles in the steady-state myocardium as well as during and after myocardial infarction (MI). The healthy myocardium contains various mature immune cell subsets, with macrophages being the most abundant population fulfilling important maintenance tasks that we are only beginning to understand. In the event of acute myocardial ischemia, a massive reaction of basically all cell types in the infarcted area occurs within minutes, including TNF release by degranulating mast cells, cytokine-, growth factor- and chemokine production (e.g. CCL2 and IL-1β) by activated and dying macrophages and fibroblasts, as well as release of damage-associated molecular patterns (DAMPs) by dead and dying cardiomyocytes, all of which lead to a strong expression of adhesion molecules, such as VCAM1 and selectins on endothelial cells. In consequence, the first day after an MI is characterized by a massive influx of neutrophils and monocytes from the blood stream into the myocardium. These innate immune cells secrete matrix metalloproteinases (MMPs) and are phagocytically active to clear dead cells and debris, and they also release inflammatory cytokines that sustain the inflammation. Around day 4, the inflammatory clean-up phase passes over to a reparative phase with disappearance of neutrophils and appearance of reparative macrophages that produce IL10, TGFβ, and VEGF to promote regression of inflammation, angiogenesis, fibrosis, and scar formation. Abbreviations: TNF - tumor necrosis factor; DAMPs - damage-associated molecular patterns; VEGF - vascular endothelial growth factor; TGFβ - transforming growth factor-β; IL10 - interleukin 10
Conclusions and Future Directions
Research conducted in recent decades has demonstrated that atherosclerosis, from its early initiation to its major complications, crucially depends on inflammatory leukocyte supply from the bone marrow niche. Technical and conceptual breakthroughs, including identification of highly-specific HSC markers, genetically engineered mouse strains for cell fate tracking and cell type-specific manipulations, advanced imaging techniques, and single-cell multiomics have dramatically expanded our knowledge of the cellular and molecular composition and interactions of leukocyte niche cell networks in the bone marrow, as well as in atherosclerotic plaques and infarcted myocardium. While we can now appreciate the complexity of such processes at unprecedented detail, we are still far from fully understanding such pathways, much less successfully targeting them therapeutically.
Future studies will need to focus on further unraveling the heterogeneity of bone marrow niche populations, identifying and characterizing additional niche factors, determining the timing of cell fate decisions, and tracking cells’ precise differentiation trajectories and relative contributions to mature leukocyte production. In the context of atherosclerosis, further work is needed to understand cell behavior and fate in atherosclerotic lesions as well as ischemic and remodeling myocardium, uncover MI-induced epigenetic changes in HSPCs, identify additional mechanisms through which cardiovascular risk factors accelerate atherosclerosis, and determine tissue-specific macrophage functions. This is an exciting area for the hematology of cardiovascular disease, with many discoveries yet to come.
Acknowledgments
Sources of funding
This work was supported in part by a postdoctoral research fellowship by the German Research Foundation to W.C.P. (DFG 398190272) and NIH grants R35 HL135752, P01HL131478, P01 HL142494 (to F.K.S.), R35HL139598 (to M.N.)
Non-standard Abbreviations and Acronyms
- ADRβ3
β3 adrenergic receptor
- ApoE
apolipoprotein E
- BM
bone marrow
- CAR
cellCXCL12-abundant reticular cell
- CCR
C-C chemokine receptor
- CDP
common dendritic cell progenitor
- CLP
common lymphoid progenitor
- cMoP
common monocyte progenitor
- CMP
common myeloid progenitor
- CSF1
colony stimulating factor-1
- CVD
cardiovascular disease
- CXCL
CXC chemokine ligand
- CXCR
CXC chemokine receptor
- DAMP
damage-associated molecular pattern
- G-CSF
granulocyte colony-stimulating factor
- GMP
granulocyte-monocyte progenitor
- GP
granulocyte progenitors
- HFrEF
heart failure with reduced ejection fraction
- HSC
hematopoietic stem cells
- HSPC
hematopoietic stem and progenitor cell
- IL
interleukin
- ILC
innate lymphoid cell
- IT-HSC
intermediate-term hematopoietic stem cell
- LDL
low density lipoprotein
- LEPR
leptin receptor
- LMPP
lymphoid-primed multipotential progenitor
- LT-HSC
long-term hematopoietic stem cell
- MDP
monocyte-dendritic cell progenitor
- MPO
myeloperoxidase
- MPP
multipotent progenitor
- Nes
Nestin
- NG2
neural-glial antigen 2
- NK cell
natural killer cell
- MI
myocardial infarction
- MSC
mesenchymal stem/stromal cell
- ROS
reactive oxygen species
- SCF
stem cell factor (SCF)
- ST-HSC
short-term hematopoietic stem cell
- TGFβ
transforming growth factor-β
- THPO
thrombopoietin
- TLR
toll-like receptor
- TNFα
tumor necrosis factor α
- VCAM1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
Disclosures: Dr. Swirski has received consultant fees from Verseau Therapuetics, Partner Therapeutics, and Novartis. Dr. Nahrendorf received consulting fees from Verseau Therapeutics and IFM Therapeutics.
References
- 1.World Health Organization. Cardiovascular diseases (CVDs) Fact Sheet. 2017. [Google Scholar]
- 2.Ross R & Glomset JA The pathogenesis of atherosclerosis (second of two parts). N Engl J Med 295, 420–425, doi: 10.1056/NEJM197608192950805 (1976). [DOI] [PubMed] [Google Scholar]
- 3.Ross R & Glomset JA The pathogenesis of atherosclerosis (first of two parts). N Engl J Med 295, 369–377, doi: 10.1056/NEJM197608122950707 (1976). [DOI] [PubMed] [Google Scholar]
- 4.Libby P Inflammation in atherosclerosis. Nature 420, 868–874, doi: 10.1038/nature01323 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Randolph GJ The fate of monocytes in atherosclerosis. J Thromb Haemost 7 Suppl 1, 28–30, doi: 10.1111/j.1538-7836.2009.03423.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KJ & Tabas I Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355, doi: 10.1016/j.cell.2011.04.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swirski FK & Nahrendorf M Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166, doi: 10.1126/science.1230719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushansky K Lineage-specific hematopoietic growth factors. N Engl J Med 354, 2034–2045, doi: 10.1056/NEJMra052706 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Morrison SJ & Scadden DT The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334, doi: 10.1038/nature12984 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinho S & Frenette PS Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 20, 303–320, doi: 10.1038/s41580-019-0103-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Till JE & Mc CE A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14, 213–222 (1961). [PubMed] [Google Scholar]
- 12.Spangrude GJ, Heimfeld S & Weissman IL Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62, doi: 10.1126/science.2898810 (1988). [DOI] [PubMed] [Google Scholar]
- 13.Catlin SN, Busque L, Gale RE, Guttorp P & Abkowitz JL The replication rate of human hematopoietic stem cells in vivo. Blood 117, 4460–4466, doi: 10.1182/blood-2010-08-303537 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J et al. Clonal dynamics of native haematopoiesis. Nature 514, 322–327, doi: 10.1038/nature13824 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busch K et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518, 542–546, doi: 10.1038/nature14242 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Sawai CM et al. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity 45, 597–609, doi: 10.1016/j.immuni.2016.08.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellinger DL, Lorton D, Felten SY & Felten DL Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol 14, 329–344, doi: 10.1016/0192-0561(92)90162-e (1992). [DOI] [PubMed] [Google Scholar]
- 18.Mach DB et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113, 155–166, doi: 10.1016/s0306-4522(02)00165-3 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978). [PubMed] [Google Scholar]
- 20.Birbrair A & Frenette PS Niche heterogeneity in the bone marrow. Ann N Y Acad Sci 1370, 82–96, doi: 10.1111/nyas.13016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swirski FK et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616, doi: 10.1126/science.1175202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins CS et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374, doi: 10.1161/CIRCULATIONAHA.111.061986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chanavaz M Anatomy and histophysiology of the periosteum: quantification of the periosteal blood supply to the adjacent bone with 85Sr and gamma spectrometry. J Oral Implantol 21, 214–219 (1995). [PubMed] [Google Scholar]
- 24.Maryanovich M, Takeishi S & Frenette PS Neural Regulation of Bone and Bone Marrow. Cold Spring Harb Perspect Med 8, doi: 10.1101/cshperspect.a031344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunisaki Y et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643, doi: 10.1038/nature12612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki K & Allen TD Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the “neuro-reticular complex”. Am J Anat 187, 261–276, doi: 10.1002/aja.1001870306 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Mendez-Ferrer S et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834, doi: 10.1038/nature09262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjurholm A, Kreicbergs A, Brodin E & Schultzberg M Substance P- and CGRP-immunoreactive nerves in bone. Peptides 9, 165–171, doi: 10.1016/0196-9781(88)90023-x (1988). [DOI] [PubMed] [Google Scholar]
- 29.Bajayo A et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci U S A 109, 15455–15460, doi: 10.1073/pnas.1206061109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artico M et al. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med 10, 77–80 (2002). [PubMed] [Google Scholar]
- 31.Mysorekar VR Diaphysial nutrient foramina in human long bones. J Anat 101, 813–822 (1967). [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasamy SK et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun 7, 13601, doi: 10.1038/ncomms13601 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson EC & Adams RH Biology of Bone: The Vasculature of the Skeletal System. Cold Spring Harb Perspect Med 8, doi: 10.1101/cshperspect.a031559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiel MJ et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121, doi: 10.1016/j.cell.2005.05.026 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Morrison SJ & Spradling AC Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611, doi: 10.1016/j.cell.2008.01.038 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiel MJ et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature 449, 238–242, doi: 10.1038/nature06115 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita Y, Ema H & Nakauchi H Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med 207, 1173–1182, doi: 10.1084/jem.20091318 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yilmaz OH, Kiel MJ & Morrison SJ SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood 107, 924–930, doi: 10.1182/blood-2005-05-2140 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiel MJ, Radice GL & Morrison SJ Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 1, 204–217, doi: 10.1016/j.stem.2007.06.001 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Chen JY et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530, 223–227, doi: 10.1038/nature16943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acar M et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130, doi: 10.1038/nature15250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane GM, Jeffery E & Morrison SJ Adult haematopoietic stem cell niches. Nat Rev Immunol 17, 573–590, doi: 10.1038/nri.2017.53 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Kricun ME Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol 14, 10–19, doi: 10.1007/bf00361188 (1985). [DOI] [PubMed] [Google Scholar]
- 44.Yokota T et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96, 1723–1732 (2000). [PubMed] [Google Scholar]
- 45.Naveiras O et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263, doi: 10.1038/nature08099 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambrosi TH et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 20, 771–784 e776, doi: 10.1016/j.stem.2017.02.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins N et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell 178, 1088–1101 e1015, doi: 10.1016/j.cell.2019.07.049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katayama Y et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421, doi: 10.1016/j.cell.2005.10.041 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Calvo W The innervation of the bone marrow in laboratory animals. Am J Anat 123, 315–328, doi: 10.1002/aja.1001230206 (1968). [DOI] [PubMed] [Google Scholar]
- 50.Heidt T et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 20, 754–758, doi: 10.1038/nm.3589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucas D, Battista M, Shi PA, Isola L & Frenette PS Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 3, 364–366, doi: 10.1016/j.stem.2008.09.004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiegel A et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol 8, 1123–1131, doi: 10.1038/ni1509 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki S et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158, doi: 10.1016/j.cell.2011.09.053 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Calvi LM et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846, doi: 10.1038/nature02040 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Zhang J et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841, doi: 10.1038/nature02041 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Nagasawa T et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638, doi: 10.1038/382635a0 (1996). [DOI] [PubMed] [Google Scholar]
- 57.Petit I et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol 3, 687–694, doi: 10.1038/ni813 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Ara T et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267, doi: 10.1016/s1074-7613(03)00201-2 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Sugiyama T, Kohara H, Noda M & Nagasawa T Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988, doi: 10.1016/j.immuni.2006.10.016 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Barker JE Sl/Sld hematopoietic progenitors are deficient in situ. Exp Hematol 22, 174–177 (1994). [PubMed] [Google Scholar]
- 61.Ogawa M et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med 174, 63–71, doi: 10.1084/jem.174.1.63 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding L, Saunders TL, Enikolopov G & Morrison SJ Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462, doi: 10.1038/nature10783 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenbaum A et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230, doi: 10.1038/nature11926 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nombela-Arrieta C et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 15, 533–543, doi: 10.1038/ncb2730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guezguez B et al. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell 13, 175–189, doi: 10.1016/j.stem.2013.06.015 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Zhou X et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci U S A 107, 12919–12924, doi: 10.1073/pnas.0912855107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan CK et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494, doi: 10.1038/nature07547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacchetti B et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336, doi: 10.1016/j.cell.2007.08.025 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Wei Q & Frenette PS Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 48, 632–648, doi: 10.1016/j.immuni.2018.03.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frenette PS, Pinho S, Lucas D & Scheiermann C Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol 31, 285–316, doi: 10.1146/annurev-immunol-032712-095919 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Park D et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272, doi: 10.1016/j.stem.2012.02.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Omatsu Y et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399, doi: 10.1016/j.immuni.2010.08.017 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Cordeiro Gomes A et al. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity 45, 1219–1231, doi: 10.1016/j.immuni.2016.11.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendez-Ferrer S, Lucas D, Battista M & Frenette PS Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447, doi: 10.1038/nature06685 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Asada N et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19, 214–223, doi: 10.1038/ncb3475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kusumbe AP et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384, doi: 10.1038/nature17638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Himburg HA et al. Distinct Bone Marrow Sources of Pleiotrophin Control Hematopoietic Stem Cell Maintenance and Regeneration. Cell Stem Cell 23, 370–381 e375, doi: 10.1016/j.stem.2018.07.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler JM et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264, doi: 10.1016/j.stem.2010.02.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Himburg HA et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med 16, 475–482, doi: 10.1038/nm.2119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winkler IG et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med 18, 1651–1657, doi: 10.1038/nm.2969 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Ding L & Morrison SJ Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235, doi: 10.1038/nature11885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao L, Yokota T, Xia L, Kincade PW & McEver RP Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood 106, 4093–4101, doi: 10.1182/blood-2005-02-0671 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hooper AT et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274, doi: 10.1016/j.stem.2009.01.006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W, Johnson SA, Shelley WC & Yoder MC Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol 32, 1226–1237, doi: 10.1016/j.exphem.2004.09.001 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi H et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol 12, 1046–1056, doi: 10.1038/ncb2108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu C et al. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat Commun 9, 2449, doi: 10.1038/s41467-018-04726-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Itkin T et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328, doi: 10.1038/nature17624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruns I et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20, 1315–1320, doi: 10.1038/nm.3707 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao M et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20, 1321–1326, doi: 10.1038/nm.3706 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Nakamura-Ishizu A, Takubo K, Fujioka M & Suda T Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun 454, 353–357, doi: 10.1016/j.bbrc.2014.10.095 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K & Suda T CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med 212, 2133–2146, doi: 10.1084/jem.20150057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang L et al. SHP-1 regulates hematopoietic stem cell quiescence by coordinating TGF-beta signaling. J Exp Med 215, 1337–1347, doi: 10.1084/jem.20171477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herault A et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature 544, 53–58, doi: 10.1038/nature21693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pinho S et al. Lineage-Biased Hematopoietic Stem Cells Are Regulated by Distinct Niches. Dev Cell 44, 634–641 e634, doi: 10.1016/j.devcel.2018.01.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winkler IG et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828, doi: 10.1182/blood-2009-11-253534 (2010). [DOI] [PubMed] [Google Scholar]
- 96.Ludin A et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol 13, 1072–1082, doi: 10.1038/ni.2408 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Hur J et al. CD82/KAI1 Maintains the Dormancy of Long-Term Hematopoietic Stem Cells through Interaction with DARC-Expressing Macrophages. Cell Stem Cell 18, 508–521, doi: 10.1016/j.stem.2016.01.013 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Chow A et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208, 261–271, doi: 10.1084/jem.20101688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dutta P et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 212, 497–512, doi: 10.1084/jem.20141642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casanova-Acebes M et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035, doi: 10.1016/j.cell.2013.04.040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams GB et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603, doi: 10.1038/nature04247 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Kollet O et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 12, 657–664, doi: 10.1038/nm1417 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Mansour A et al. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med 209, 537–549, doi: 10.1084/jem.20110994 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawano Y et al. G-CSF-induced sympathetic tone provokes fever and primes antimobilizing functions of neutrophils via PGE2. Blood 129, 587–597, doi: 10.1182/blood-2016-07-725754 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Bowers E et al. Granulocyte-derived TNFalpha promotes vascular and hematopoietic regeneration in the bone marrow. Nat Med 24, 95–102, doi: 10.1038/nm.4448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McAlpine CS et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387, doi: 10.1038/s41586-019-0948-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gandy KL, Domen J, Aguila H & Weissman IL CD8+TCR+ and CD8+TCR- cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity 11, 579–590, doi: 10.1016/s1074-7613(00)80133-8 (1999). [DOI] [PubMed] [Google Scholar]
- 108.Kaufman CL et al. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood 84, 2436–2446 (1994). [PubMed] [Google Scholar]
- 109.Fujisaki J et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216–219, doi: 10.1038/nature10160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirata Y et al. CD150(high) Bone Marrow Tregs Maintain Hematopoietic Stem Cell Quiescence and Immune Privilege via Adenosine. Cell Stem Cell 22, 445–453 e445, doi: 10.1016/j.stem.2018.01.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Broudy VC Stem cell factor and hematopoiesis. Blood 90, 1345–1364 (1997). [PubMed] [Google Scholar]
- 112.Czechowicz A, Kraft D, Weissman IL & Bhattacharya D Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 318, 1296–1299, doi: 10.1126/science.1149726 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Russell ES Hereditary anemias of the mouse: a review for geneticists. Adv Genet 20, 357–459 (1979). [PubMed] [Google Scholar]
- 114.Heissig B et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109, 625–637, doi: 10.1016/s0092-8674(02)00754-7 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams DE et al. Identification of a ligand for the c-kit proto-oncogene. Cell 63, 167–174, doi: 10.1016/0092-8674(90)90297-r (1990). [DOI] [PubMed] [Google Scholar]
- 116.Copeland NG et al. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell 63, 175–183, doi: 10.1016/0092-8674(90)90298-s (1990). [DOI] [PubMed] [Google Scholar]
- 117.Flanagan JG & Leder P The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell 63, 185–194, doi: 10.1016/0092-8674(90)90299-t (1990). [DOI] [PubMed] [Google Scholar]
- 118.Zsebo KM et al. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver--conditioned medium. Cell 63, 195–201, doi: 10.1016/0092-8674(90)90300-4 (1990). [DOI] [PubMed] [Google Scholar]
- 119.Martin FH et al. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell 63, 203–211, doi: 10.1016/0092-8674(90)90301-t (1990). [DOI] [PubMed] [Google Scholar]
- 120.Zsebo KM et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63, 213–224, doi: 10.1016/0092-8674(90)90302-u (1990). [DOI] [PubMed] [Google Scholar]
- 121.Huang E et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell 63, 225–233, doi: 10.1016/0092-8674(90)90303-v (1990). [DOI] [PubMed] [Google Scholar]
- 122.Anderson DM et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 63, 235–243, doi: 10.1016/0092-8674(90)90304-w (1990). [DOI] [PubMed] [Google Scholar]
- 123.Ikuta K & Weissman IL Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A 89, 1502–1506, doi: 10.1073/pnas.89.4.1502 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barker JE Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Exp Hematol 25, 542–547 (1997). [PubMed] [Google Scholar]
- 125.Wolf NS Dissecting the hematopoietic microenvironment. III. Evidence for a positive short range stimulus for cellular proliferation. Cell Tissue Kinet 11, 335–345 (1978). [PubMed] [Google Scholar]
- 126.Yue R, Zhou BO, Shimada IS, Zhao Z & Morrison SJ Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 18, 782–796, doi: 10.1016/j.stem.2016.02.015 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Tzeng YS et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 117, 429–439, doi: 10.1182/blood-2010-01-266833 (2011). [DOI] [PubMed] [Google Scholar]
- 128.McDermott DH et al. Chromothriptic cure of WHIM syndrome. Cell 160, 686–699, doi: 10.1016/j.cell.2015.01.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lai CY et al. Stage-specific roles for CXCR4 signaling in murine hematopoietic stem/progenitor cells in the process of bone marrow repopulation. Stem Cells 32, 1929–1942, doi: 10.1002/stem.1670 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Zou YR, Kottmann AH, Kuroda M, Taniuchi I & Littman DR Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599, doi: 10.1038/31269 (1998). [DOI] [PubMed] [Google Scholar]
- 131.Nagasawa T, Kikutani H & Kishimoto T Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A 91, 2305–2309, doi: 10.1073/pnas.91.6.2305 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nie Y, Han YC & Zou YR CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med 205, 777–783, doi: 10.1084/jem.20072513 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ponomaryov T et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest 106, 1331–1339, doi: 10.1172/JCI10329 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dar A et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol 6, 1038–1046, doi: 10.1038/ni1251 (2005). [DOI] [PubMed] [Google Scholar]
- 135.Kimura S, Roberts AW, Metcalf D & Alexander WS Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A 95, 1195–1200, doi: 10.1073/pnas.95.3.1195 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qian H et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1, 671–684, doi: 10.1016/j.stem.2007.10.008 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Yoshihara H et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685–697, doi: 10.1016/j.stem.2007.10.020 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Sitnicka E et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood 87, 4998–5005 (1996). [PubMed] [Google Scholar]
- 139.de Sauvage FJ et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 369, 533–538, doi: 10.1038/369533a0 (1994). [DOI] [PubMed] [Google Scholar]
- 140.Kaushansky K et al. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci U S A 92, 3234–3238, doi: 10.1073/pnas.92.8.3234 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaushansky K et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 369, 568–571, doi: 10.1038/369568a0 (1994). [DOI] [PubMed] [Google Scholar]
- 142.Sungaran R, Markovic B & Chong BH Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood 89, 101–107 (1997). [PubMed] [Google Scholar]
- 143.Morrison SJ, Wandycz AM, Akashi K, Globerson A & Weissman IL The aging of hematopoietic stem cells. Nat Med 2, 1011–1016, doi: 10.1038/nm0996-1011 (1996). [DOI] [PubMed] [Google Scholar]
- 144.de Haan G, Nijhof W & Van Zant G Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood 89, 1543–1550 (1997). [PubMed] [Google Scholar]
- 145.Rossi DJ et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 102, 9194–9199, doi: 10.1073/pnas.0503280102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dykstra B, Olthof S, Schreuder J, Ritsema M & de Haan G Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med 208, 2691–2703, doi: 10.1084/jem.20111490 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sudo K, Ema H, Morita Y & Nakauchi H Age-associated characteristics of murine hematopoietic stem cells. J Exp Med 192, 1273–1280, doi: 10.1084/jem.192.9.1273 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cho RH, Sieburg HB & Muller-Sieburg CE A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111, 5553–5561, doi: 10.1182/blood-2007-11-123547 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beerman I et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A 107, 5465–5470, doi: 10.1073/pnas.1000834107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liang Y, Van Zant G & Szilvassy SJ Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1487, doi: 10.1182/blood-2004-11-4282 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xing Z et al. Increased hematopoietic stem cell mobilization in aged mice. Blood 108, 2190–2197, doi: 10.1182/blood-2005-12-010272 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Florian MC et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell 10, 520–530, doi: 10.1016/j.stem.2012.04.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maryanovich M et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med 24, 782–791, doi: 10.1038/s41591-018-0030-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beerman I & Rossi DJ Epigenetic regulation of hematopoietic stem cell aging. Exp Cell Res 329, 192–199, doi: 10.1016/j.yexcr.2014.09.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Haan G & Lazare SS Aging of hematopoietic stem cells. Blood 131, 479–487, doi: 10.1182/blood-2017-06-746412 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Ergen AV, Boles NC & Goodell MA Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 119, 2500–2509, doi: 10.1182/blood-2011-11-391730 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guidi N et al. Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J 36, 1463, doi: 10.15252/embj.201796968 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nishikawa K et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest 120, 3455–3465, doi: 10.1172/JCI42528 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh L et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 85, 29–36, doi: 10.1016/j.bone.2016.01.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Poulos MG et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J Clin Invest 127, 4163–4178, doi: 10.1172/JCI93940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tikhonova AN et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228, doi: 10.1038/s41586-019-1104-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Baryawno N et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 177, 1915–1932 e1916, doi: 10.1016/j.cell.2019.04.040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Severe N et al. Stress-Induced Changes in Bone Marrow Stromal Cell Populations Revealed through Single-Cell Protein Expression Mapping. Cell Stem Cell 25, 570–583 e577, doi: 10.1016/j.stem.2019.06.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen Q et al. Apelin(+) Endothelial Niche Cells Control Hematopoiesis and Mediate Vascular Regeneration after Myeloablative Injury. Cell Stem Cell 25, 768–783 e766, doi: 10.1016/j.stem.2019.10.006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Doulatov S et al. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol 11, 585–593, doi: 10.1038/ni.1889 (2010). [DOI] [PubMed] [Google Scholar]
- 166.Adolfsson J et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121, 295–306, doi: 10.1016/j.cell.2005.02.013 (2005). [DOI] [PubMed] [Google Scholar]
- 167.Goardon N et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 19, 138–152, doi: 10.1016/j.ccr.2010.12.012 (2011). [DOI] [PubMed] [Google Scholar]
- 168.Sanjuan-Pla A et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 502, 232–236, doi: 10.1038/nature12495 (2013). [DOI] [PubMed] [Google Scholar]
- 169.Yamamoto R et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154, 1112–1126, doi: 10.1016/j.cell.2013.08.007 (2013). [DOI] [PubMed] [Google Scholar]
- 170.Pietras EM et al. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell 17, 35–46, doi: 10.1016/j.stem.2015.05.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cabezas-Wallscheid N et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell 15, 507–522, doi: 10.1016/j.stem.2014.07.005 (2014). [DOI] [PubMed] [Google Scholar]
- 172.Laurenti E & Gottgens B From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426, doi: 10.1038/nature25022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oguro H, Ding L & Morrison SJ SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116, doi: 10.1016/j.stem.2013.05.014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kent DG et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood 113, 6342–6350, doi: 10.1182/blood-2008-12-192054 (2009). [DOI] [PubMed] [Google Scholar]
- 175.Majeti R, Park CY & Weissman IL Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1, 635–645, doi: 10.1016/j.stem.2007.10.001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Notta F et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333, 218–221, doi: 10.1126/science.1201219 (2011). [DOI] [PubMed] [Google Scholar]
- 177.Benveniste P et al. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell 6, 48–58, doi: 10.1016/j.stem.2009.11.014 (2010). [DOI] [PubMed] [Google Scholar]
- 178.Wilson A et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129, doi: 10.1016/j.cell.2008.10.048 (2008). [DOI] [PubMed] [Google Scholar]
- 179.Foudi A et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 27, 84–90, doi: 10.1038/nbt.1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S & Manz MG Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med 208, 273–284, doi: 10.1084/jem.20101643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Qiu J, Papatsenko D, Niu X, Schaniel C & Moore K Divisional history and hematopoietic stem cell function during homeostasis. Stem Cell Reports 2, 473–490, doi: 10.1016/j.stemcr.2014.01.016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bernitz JM, Kim HS, MacArthur B, Sieburg H & Moore K Hematopoietic Stem Cells Count and Remember Self-Renewal Divisions. Cell 167, 1296–1309 e1210, doi: 10.1016/j.cell.2016.10.022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Baryawno N, Severe N & Scadden DT Hematopoiesis: Reconciling Historic Controversies about the Niche. Cell Stem Cell 20, 590–592, doi: 10.1016/j.stem.2017.03.025 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Inra CN et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 527, 466–471, doi: 10.1038/nature15530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dykstra B et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1, 218–229, doi: 10.1016/j.stem.2007.05.015 (2007). [DOI] [PubMed] [Google Scholar]
- 186.Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF & Sieburg HB Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood 103, 4111–4118, doi: 10.1182/blood-2003-10-3448 (2004). [DOI] [PubMed] [Google Scholar]
- 187.Notta F et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351, aab2116, doi: 10.1126/science.aab2116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grover A et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun 7, 11075, doi: 10.1038/ncomms11075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rodriguez-Fraticelli AE et al. Clonal analysis of lineage fate in native haematopoiesis. Nature 553, 212–216, doi: 10.1038/nature25168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Carrelha J et al. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature 554, 106–111, doi: 10.1038/nature25455 (2018). [DOI] [PubMed] [Google Scholar]
- 191.Paul F et al. Transcriptional Heterogeneity and Lineage Commitment in Myeloid Progenitors. Cell 163, 1663–1677, doi: 10.1016/j.cell.2015.11.013 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Nestorowa S et al. A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation. Blood 128, e20–31, doi: 10.1182/blood-2016-05-716480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Velten L et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol 19, 271–281, doi: 10.1038/ncb3493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Grun D et al. De Novo Prediction of Stem Cell Identity using Single-Cell Transcriptome Data. Cell Stem Cell 19, 266–277, doi: 10.1016/j.stem.2016.05.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu Z et al. Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells. Cell 178, 1509–1525 e1519, doi: 10.1016/j.cell.2019.08.009 (2019). [DOI] [PubMed] [Google Scholar]
- 196.Ensan S et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol 17, 159–168, doi: 10.1038/ni.3343 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Roufaiel M et al. CCL19-CCR7-dependent reverse transendothelial migration of myeloid cells clears Chlamydia muridarum from the arterial intima. Nat Immunol 17, 1263–1272, doi: 10.1038/ni.3564 (2016). [DOI] [PubMed] [Google Scholar]
- 198.Choi JH et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med 206, 497–505, doi: 10.1084/jem.20082129 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lim HY et al. Hyaluronan Receptor LYVE-1-Expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity 49, 326–341 e327, doi: 10.1016/j.immuni.2018.06.008 (2018). [DOI] [PubMed] [Google Scholar]
- 200.Cochain C et al. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res 122, 1661–1674, doi: 10.1161/CIRCRESAHA.117.312509 (2018). [DOI] [PubMed] [Google Scholar]
- 201.Winkels H et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res 122, 1675–1688, doi: 10.1161/CIRCRESAHA.117.312513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Robbins CS et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19, 1166–1172, doi: 10.1038/nm.3258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ginhoux F & Jung S Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14, 392–404, doi: 10.1038/nri3671 (2014). [DOI] [PubMed] [Google Scholar]
- 204.Epelman S, Lavine KJ & Randolph GJ Origin and functions of tissue macrophages. Immunity 41, 21–35, doi: 10.1016/j.immuni.2014.06.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hansson GK & Libby P The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6, 508–519, doi: 10.1038/nri1882 (2006). [DOI] [PubMed] [Google Scholar]
- 206.Wang Y et al. Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell 171, 331–345 e322, doi: 10.1016/j.cell.2017.08.041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McArdle S, Chodaczek G, Ray N & Ley K Intravital live cell triggered imaging system reveals monocyte patrolling and macrophage migration in atherosclerotic arteries. J Biomed Opt 20, 26005, doi: 10.1117/1.JBO.20.2.026005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ridker PM et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377, 1119–1131, doi: 10.1056/NEJMoa1707914 (2017). [DOI] [PubMed] [Google Scholar]
- 209.Ortega-Gomez A et al. Cathepsin G Controls Arterial But Not Venular Myeloid Cell Recruitment. Circulation 134, 1176–1188, doi: 10.1161/CIRCULATIONAHA.116.024790 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Drechsler M, Megens RT, van Zandvoort M, Weber C & Soehnlein O Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845, doi: 10.1161/CIRCULATIONAHA.110.961714 (2010). [DOI] [PubMed] [Google Scholar]
- 211.Doring Y et al. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res 110, 1052–1056, doi: 10.1161/CIRCRESAHA.112.265868 (2012). [DOI] [PubMed] [Google Scholar]
- 212.Wantha S et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res 112, 792–801, doi: 10.1161/CIRCRESAHA.112.300666 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Doring Y et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125, 1673–1683, doi: 10.1161/CIRCULATIONAHA.111.046755 (2012). [DOI] [PubMed] [Google Scholar]
- 214.Warnatsch A, Ioannou M, Wang Q & Papayannopoulos V Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320, doi: 10.1126/science.aaa8064 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Silvestre-Roig C et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569, 236–240, doi: 10.1038/s41586-019-1167-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Silvestre-Roig C, Braster Q, Ortega-Gomez A & Soehnlein O Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol, doi: 10.1038/s41569-019-0326-7 (2020). [DOI] [PubMed] [Google Scholar]
- 217.Rogacev KS et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J 32, 84–92, doi: 10.1093/eurheartj/ehq371 (2011). [DOI] [PubMed] [Google Scholar]
- 218.Berg KE et al. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet 5, 122–131, doi: 10.1161/CIRCGENETICS.111.960385 (2012). [DOI] [PubMed] [Google Scholar]
- 219.Ernst E, Hammerschmidt DE, Bagge U, Matrai A & Dormandy JA Leukocytes and the risk of ischemic diseases. JAMA 257, 2318–2324 (1987). [PubMed] [Google Scholar]
- 220.Engstrom G, Melander O & Hedblad B Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail 2, 217–222, doi: 10.1161/CIRCHEARTFAILURE.108.827071 (2009). [DOI] [PubMed] [Google Scholar]
- 221.Maekawa Y et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol 39, 241–246, doi: 10.1016/s0735-1097(01)01721-1 (2002). [DOI] [PubMed] [Google Scholar]
- 222.Tsujioka H et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol 54, 130–138, doi: 10.1016/j.jacc.2009.04.021 (2009). [DOI] [PubMed] [Google Scholar]
- 223.Swirski FK et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A 103, 10340–10345, doi: 10.1073/pnas.0604260103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Swirski FK et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117, 195–205, doi: 10.1172/JCI29950 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tacke F et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117, 185–194, doi: 10.1172/JCI28549 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Geissmann F, Jung S & Littman DR Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82, doi: 10.1016/s1074-7613(03)00174-2 (2003). [DOI] [PubMed] [Google Scholar]
- 227.Jakubzick C et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610, doi: 10.1016/j.immuni.2013.08.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Auffray C et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670, doi: 10.1126/science.1142883 (2007). [DOI] [PubMed] [Google Scholar]
- 229.Carlin LM et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375, doi: 10.1016/j.cell.2013.03.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sunderkotter C et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172, 4410–4417, doi: 10.4049/jimmunol.172.7.4410 (2004). [DOI] [PubMed] [Google Scholar]
- 231.Yona S et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91, doi: 10.1016/j.immuni.2012.12.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hilgendorf I et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 114, 1611–1622, doi: 10.1161/CIRCRESAHA.114.303204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Korkosz M, Bukowska-Strakova K, Sadis S, Grodzicki T & Siedlar M Monoclonal antibodies against macrophage colony-stimulating factor diminish the number of circulating intermediate and nonclassical (CD14(++)CD16(+)/CD14(+)CD16(++)) monocytes in rheumatoid arthritis patient. Blood 119, 5329–5330, doi: 10.1182/blood-2012-02-412551 (2012). [DOI] [PubMed] [Google Scholar]
- 234.Hilgendorf I, Swirski FK & Robbins CS Monocyte fate in atherosclerosis. Arterioscler Thromb Vasc Biol 35, 272–279, doi: 10.1161/ATVBAHA.114.303565 (2015). [DOI] [PubMed] [Google Scholar]
- 235.Wu H et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol 30, 186–192, doi: 10.1161/ATVBAHA.109.198044 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hanna RN et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res 110, 416–427, doi: 10.1161/CIRCRESAHA.111.253377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hamers AA et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res 110, 428–438, doi: 10.1161/CIRCRESAHA.111.260760 (2012). [DOI] [PubMed] [Google Scholar]
- 238.Quintar A et al. Endothelial Protective Monocyte Patrolling in Large Arteries Intensified by Western Diet and Atherosclerosis. Circ Res 120, 1789–1799, doi: 10.1161/CIRCRESAHA.117.310739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Massberg S et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med 196, 887–896, doi: 10.1084/jem.20012044 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.van Gils JM, Zwaginga JJ & Hordijk PL Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol 85, 195–204, doi: 10.1189/jlb.0708400 (2009). [DOI] [PubMed] [Google Scholar]
- 241.An G et al. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation 117, 3227–3237, doi: 10.1161/CIRCULATIONAHA.108.771048 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Duerschmied D, Bode C & Ahrens I Immune functions of platelets. Thromb Haemost 112, 678–691, doi: 10.1160/TH14-02-0146 (2014). [DOI] [PubMed] [Google Scholar]
- 243.von Hundelshausen P & Weber C Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res 100, 27–40, doi: 10.1161/01.RES.0000252802.25497.b7 (2007). [DOI] [PubMed] [Google Scholar]
- 244.Barrett TJ et al. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci Transl Med 11, doi: 10.1126/scitranslmed.aax0481 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Swirski FK Platelets have a dangerous hold over immune cells in cardiovascular disease. Nature News and Views, doi:doi: 10.1038/d41586-019-03732-9 (2019). [DOI] [PubMed] [Google Scholar]
- 246.Libby P, Ridker PM & Hansson GK Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325, doi: 10.1038/nature10146 (2011). [DOI] [PubMed] [Google Scholar]
- 247.Zhu SN, Chen M, Jongstra-Bilen J & Cybulsky MI GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med 206, 2141–2149, doi: 10.1084/jem.20090866 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shankman LS et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21, 628–637, doi: 10.1038/nm.3866 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Feil S et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115, 662–667, doi: 10.1161/CIRCRESAHA.115.304634 (2014). [DOI] [PubMed] [Google Scholar]
- 250.Murphy AJ & Tall AR Proliferating macrophages populate established atherosclerotic lesions. Circ Res 114, 236–238, doi: 10.1161/CIRCRESAHA.113.302813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Parks BW & Lusis AJ Macrophage accumulation in atherosclerosis. N Engl J Med 369, 2352–2353, doi: 10.1056/NEJMcibr1312709 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Randolph GJ Proliferating macrophages prevail in atherosclerosis. Nat Med 19, 1094–1095, doi: 10.1038/nm.3316 (2013). [DOI] [PubMed] [Google Scholar]
- 253.Potteaux S et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest 121, 2025–2036, doi: 10.1172/JCI43802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Moore KJ, Sheedy FJ & Fisher EA Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13, 709–721, doi: 10.1038/nri3520 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.McArdle S, Mikulski Z & Ley K Live cell imaging to understand monocyte, macrophage, and dendritic cell function in atherosclerosis. J Exp Med 213, 1117–1131, doi: 10.1084/jem.20151885 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dutta P et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329, doi: 10.1038/nature11260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pittet MJ, Nahrendorf M & Swirski FK The journey from stem cell to macrophage. Ann N Y Acad Sci 1319, 1–18, doi: 10.1111/nyas.12393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Boring L, Gosling J, Cleary M & Charo IF Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897, doi: 10.1038/29788 (1998). [DOI] [PubMed] [Google Scholar]
- 259.Eash KJ, Greenbaum AM, Gopalan PK & Link DC CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest 120, 2423–2431, doi: 10.1172/JCI41649 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nahrendorf M Myeloid cell contributions to cardiovascular health and disease. Nat Med 24, 711–720, doi: 10.1038/s41591-018-0064-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yvan-Charvet L et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693, doi: 10.1126/science.1189731 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Murphy AJ et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 121, 4138–4149, doi: 10.1172/JCI57559 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Courties G et al. Glucocorticoids Regulate Bone Marrow B Lymphopoiesis After Stroke. Circ Res 124, 1372–1385, doi: 10.1161/CIRCRESAHA.118.314518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Geissmann F et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661, doi: 10.1126/science.1178331 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Massberg S et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008, doi: 10.1016/j.cell.2007.09.047 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cortez-Retamozo V et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A 109, 2491–2496, doi: 10.1073/pnas.1113744109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Leuschner F et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 209, 123–137, doi: 10.1084/jem.20111009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cortez-Retamozo V et al. Angiotensin II drives the production of tumor-promoting macrophages. Immunity 38, 296–308, doi: 10.1016/j.immuni.2012.10.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hilgendorf I et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 129, 1677–1687, doi: 10.1161/CIRCULATIONAHA.113.006381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rauch PJ et al. Innate response activator B cells protect against microbial sepsis. Science 335, 597–601, doi: 10.1126/science.1215173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang M et al. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency. Arterioscler Thromb Vasc Biol 34, 976–984, doi: 10.1161/ATVBAHA.113.303097 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Westerterp M et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 11, 195–206, doi: 10.1016/j.stem.2012.04.024 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Frodermann V et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med 25, 1761–1771, doi: 10.1038/s41591-019-0633-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nahrendorf M & Swirski FK Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res 116, 884–894, doi: 10.1161/CIRCRESAHA.116.303550 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jaiswal S & Ebert BL Clonal hematopoiesis in human aging and disease. Science 366, doi: 10.1126/science.aan4673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jaiswal S & Libby P Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol, doi: 10.1038/s41569-019-0247-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Libby P et al. Clonal Hematopoiesis: Crossroads of Aging, Cardiovascular Disease, and Cancer: JACC Review Topic of the Week. J Am Coll Cardiol 74, 567–577, doi: 10.1016/j.jacc.2019.06.007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ramos GC et al. Myocardial aging as a T-cell-mediated phenomenon. Proc Natl Acad Sci U S A 114, E2420–E2429, doi: 10.1073/pnas.1621047114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pinto AR et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7, e36814, doi: 10.1371/journal.pone.0036814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Epelman S et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104, doi: 10.1016/j.immuni.2013.11.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Epelman S, Liu PP & Mann DL Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 15, 117–129, doi: 10.1038/nri3800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hulsmans M et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 169, 510–522 e520, doi: 10.1016/j.cell.2017.03.050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Heidt T et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115, 284–295, doi: 10.1161/CIRCRESAHA.115.303567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pinto AR et al. Revisiting Cardiac Cellular Composition. Circ Res 118, 400–409, doi: 10.1161/CIRCRESAHA.115.307778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Molawi K et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 211, 2151–2158, doi: 10.1084/jem.20140639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bajpai G et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24, 1234–1245, doi: 10.1038/s41591-018-0059-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Butts B et al. Increased Inflammation in Pericardial Fluid Persists 48 Hours After Cardiac Surgery. Circulation 136, 2284–2286, doi: 10.1161/CIRCULATIONAHA.117.029589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Horckmans M et al. Pericardial Adipose Tissue Regulates Granulopoiesis, Fibrosis, and Cardiac Function After Myocardial Infarction. Circulation 137, 948–960, doi: 10.1161/CIRCULATIONAHA.117.028833 (2018). [DOI] [PubMed] [Google Scholar]
- 289.Leid J et al. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ Res 118, 1498–1511, doi: 10.1161/CIRCRESAHA.115.308270 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Swirski FK & Nahrendorf M Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 18, 733–744, doi: 10.1038/s41577-018-0065-8 (2018). [DOI] [PubMed] [Google Scholar]
- 291.Hulsmans M et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med 215, 423–440, doi: 10.1084/jem.20171274 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pinto AR et al. Age-related changes in tissue macrophages precede cardiac functional impairment. Aging (Albany NY) 6, 399–413, doi: 10.18632/aging.100669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nabel EG & Braunwald E A tale of coronary artery disease and myocardial infarction. N Engl J Med 366, 54–63, doi: 10.1056/NEJMra1112570 (2012). [DOI] [PubMed] [Google Scholar]
- 294.Frangogiannis NG et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98, 699–710, doi: 10.1161/01.cir.98.7.699 (1998). [DOI] [PubMed] [Google Scholar]
- 295.Gwechenberger M et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 99, 546–551, doi: 10.1161/01.cir.99.4.546 (1999). [DOI] [PubMed] [Google Scholar]
- 296.Frangogiannis NG Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res 53, 585–595, doi: 10.1007/s00011-004-1298-5 (2004). [DOI] [PubMed] [Google Scholar]
- 297.Frangogiannis NG et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115, 584–592, doi: 10.1161/CIRCULATIONAHA.106.646091 (2007). [DOI] [PubMed] [Google Scholar]
- 298.Anzai A et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med 214, 3293–3310, doi: 10.1084/jem.20170689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B & Lip GY The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Haemost 10, 1231–1241, doi: 10.1111/j.1538-7836.2011.04603.x (2012). [DOI] [PubMed] [Google Scholar]
- 300.van der Laan AM et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J 163, 57–65 e52, doi: 10.1016/j.ahj.2011.09.002 (2012). [DOI] [PubMed] [Google Scholar]
- 301.Montange D et al. The number of circulating CD14(+) cells is related to infarct size and postinfarct volumes in ST segment elevation myocardial infarction but not non-ST segment elevation myocardial infarction. Exp Clin Cardiol 17, 131–135 (2012). [PMC free article] [PubMed] [Google Scholar]
- 302.Nahrendorf M et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204, 3037–3047, doi: 10.1084/jem.20070885 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.van der Laan AM et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J 35, 376–385, doi: 10.1093/eurheartj/eht331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yan X et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62, 24–35, doi: 10.1016/j.yjmcc.2013.04.023 (2013). [DOI] [PubMed] [Google Scholar]
- 305.Wan E et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113, 1004–1012, doi: 10.1161/CIRCRESAHA.113.301198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.King KR et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med 23, 1481–1487, doi: 10.1038/nm.4428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Frangogiannis NG, Smith CW & Entman ML The inflammatory response in myocardial infarction. Cardiovasc Res 53, 31–47, doi: 10.1016/s0008-6363(01)00434-5 (2002). [DOI] [PubMed] [Google Scholar]
- 308.Horckmans M et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 38, 187–197, doi: 10.1093/eurheartj/ehw002 (2017). [DOI] [PubMed] [Google Scholar]
- 309.Nahrendorf M & Swirski FK Abandoning M1/M2 for a Network Model of Macrophage Function. Circ Res 119, 414–417, doi: 10.1161/CIRCRESAHA.116.309194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Thomas GD et al. Human Blood Monocyte Subsets: A New Gating Strategy Defined Using Cell Surface Markers Identified by Mass Cytometry. Arterioscler Thromb Vasc Biol 37, 1548–1558, doi: 10.1161/ATVBAHA.117.309145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Panizzi P et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol 55, 1629–1638, doi: 10.1016/j.jacc.2009.08.089 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH & van Luyn MJ Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170, 818–829, doi: 10.2353/ajpath.2007.060547 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lee WW et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol 59, 153–163, doi: 10.1016/j.jacc.2011.08.066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ismahil MA et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 114, 266–282, doi: 10.1161/CIRCRESAHA.113.301720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sager HB et al. Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circ Res 119, 853–864, doi: 10.1161/CIRCRESAHA.116.309001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bansal SS et al. Activated T Lymphocytes are Essential Drivers of Pathological Remodeling in Ischemic Heart Failure. Circ Heart Fail 10, e003688, doi: 10.1161/CIRCHEARTFAILURE.116.003688 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nahrendorf M & Swirski FK Innate immune cells in ischaemic heart disease: does myocardial infarction beget myocardial infarction? Eur Heart J 37, 868–872, doi: 10.1093/eurheartj/ehv453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Roe MT & Ohman EM A new era in secondary prevention after acute coronary syndrome. N Engl J Med 366, 85–87, doi: 10.1056/NEJMe1112770 (2012). [DOI] [PubMed] [Google Scholar]
- 319.Alexander JH et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 365, 699–708, doi: 10.1056/NEJMoa1105819 (2011). [DOI] [PubMed] [Google Scholar]
- 320.Mega JL et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 366, 9–19, doi: 10.1056/NEJMoa1112277 (2012). [DOI] [PubMed] [Google Scholar]
- 321.Wallentin L et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361, 1045–1057, doi: 10.1056/NEJMoa0904327 (2009). [DOI] [PubMed] [Google Scholar]
- 322.Goldstein JA et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med 343, 915–922, doi: 10.1056/NEJM200009283431303 (2000). [DOI] [PubMed] [Google Scholar]
- 323.Jernberg T et al. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 36, 1163–1170, doi: 10.1093/eurheartj/ehu505 (2015). [DOI] [PubMed] [Google Scholar]
- 324.Fox KA et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J 31, 2755–2764, doi: 10.1093/eurheartj/ehq326 (2010). [DOI] [PubMed] [Google Scholar]
- 325.Sager HB et al. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation 132, 1880–1890, doi: 10.1161/CIRCULATIONAHA.115.016160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Dutta P et al. Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 16, 477–487, doi: 10.1016/j.stem.2015.04.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shi C et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 34, 590–601, doi: 10.1016/j.immuni.2011.02.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zouggari Y et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19, 1273–1280, doi: 10.1038/nm.3284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Dewald O et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96, 881–889, doi: 10.1161/01.RES.0000163017.13772.3a (2005). [DOI] [PubMed] [Google Scholar]
- 330.Leuschner F et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 107, 1364–1373, doi: 10.1161/CIRCRESAHA.110.227454 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Massa M et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 105, 199–206, doi: 10.1182/blood-2004-05-1831 (2005). [DOI] [PubMed] [Google Scholar]
- 332.Emami H et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 8, 121–130, doi: 10.1016/j.jcmg.2014.10.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Tawakol A et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389, 834–845, doi: 10.1016/S0140-6736(16)31714-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Biasucci LM et al. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation 99, 855–860, doi: 10.1161/01.cir.99.7.855 (1999). [DOI] [PubMed] [Google Scholar]
- 335.Assmus B et al. Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur Heart J 33, 1911–1919, doi: 10.1093/eurheartj/ehr388 (2012). [DOI] [PubMed] [Google Scholar]
- 336.Kim EJ, Kim S, Kang DO & Seo HS Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ Cardiovasc Imaging 7, 454–460, doi: 10.1161/CIRCIMAGING.113.001093 (2014). [DOI] [PubMed] [Google Scholar]
- 337.Wollenweber T et al. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ Cardiovasc Imaging 7, 811–818, doi: 10.1161/CIRCIMAGING.114.001689 (2014). [DOI] [PubMed] [Google Scholar]
- 338.Han Y et al. ST elevation acute myocardial infarction accelerates non-culprit coronary lesion atherosclerosis. Int J Cardiovasc Imaging 30, 253–261, doi: 10.1007/s10554-013-0354-z (2014). [DOI] [PubMed] [Google Scholar]