ABSTRACT
Objective: To explore the relations between liver metastases (LM) and the efficacy of the treatments with programmed cell death 1 (PD-1) or programmed cell death ligand 1 (PD-L1) inhibitors.
Method: Pubmed, Embase, American Society of Clinical Oncology and the European Society for Medical Oncology were searched to select eligible studies about PD-1 or PD-L1 inhibitors (Nivolumab, Pembrolizumab, Cemiplimab, Avelumab, Durvalumab, and Atezolizumab). We included only the original randomized controlled trials (RCTs), including the hazard ratios (HR) of death in both patients with LM and patients without LM. Then the data were extracted for the meta-analysis. Subgroup analyses of cancer types and drug types were also performed.
Results: 5293 patients [1246 (24%) patients with LM, and 4047 (76%) patients without LM] from the eight RCTs were included for the final analysis. The pooled hazard ratio (HR) of death in the patients with LM was 0.82 (95% CI, 0.71 to 0.93, P = .003) while the pooled HR in the patients without LM was 0.72 (95% CI, 0.66 to 0.79, P < .001). Additionally, no significant difference was found between the two groups (P = .137).
Conclusion: No statistically significant association of liver metastases with the efficacy of treatments with PD-1 or PD-L1 inhibitors in the treatment of advanced or metastatic cancer was found in the stratified analyses. Moreover, future studies about the safety of the PD-1 or PD-L1 inhibitors in patients with or without liver metastases are warranted.
KEYWORDS: Liver metastases, PD-1 inhibitors, PD-L1 inhibitors, overall survival
Introduction
Metastasis is the main cause of why patients with solid tumors died in the late stage.1 The liver, due to its rich dual blood supply (both the arterial and portal venous systems) and its specific immunological tolerance, is the most common site of visceral metastases.2–3 In detail, both gastrointestinal (GI) tumors and neuroendocrine tumors (NETs) showed preference to metastasize to the liver, and liver metastasis (LM) is also common in the advanced stage of lung cancer, renal cell carcinoma, urothelial carcinoma, and melanoma.4,5 Also, in some studies, the liver was regarded as the metastatic site with the worst prognosis, especially in lung cancer and also in other cancer.6–10 Surgical resection remained the only treatment for patients with liver metastases (LM) from colorectal and neuroendocrine cancers to achieve long-term survival.11 However, the surgery can be carried out in only 10% to 20% of patients with colorectal liver metastases.12 Hence, for the rest of the patients and the patients with non-colorectal and non-endocrine liver metastases (NCNELM), the standard treatment was still conventional therapy such as chemotherapy, radiotherapy and targeted therapy.13 However, these treatments played a limited role, and some patients were unable to continue chemotherapy due to liver dysfunction.14 Therefore, it is an urgent need to work out more effective treatment strategies for patients with liver metastases (LM).
Harnessing the immune system to battle cancer, immunotherapy has shown great clinical success in the fight against cancer. Among these novel treatments, the programmed cell death 1 (PD-1) or programmed cell death ligand 1 (PD-L1) blockade therapy was the most successful one.15 The ligation of the PD-1 and PD-L1 could activate the immune checkpoint, which can suppress the ability of T cell leading to cancer growth while the PD-1 or PD-L1 inhibitors can block this ligation to enhance the immune system to kill the cancer cells.16–18 Over the past years, a lot of clinical randomized controlled trials (RCTs) demonstrated that treatments with PD-1 or PD-L1 inhibitors could significantly improve the overall survival in patients with various advanced malignancies. Currently, three PD-1 inhibitors (nivolumab, pembrolizumab, and cemiplimab) and three PD-L1 inhibitors (atezolizumab, durvalumab, and avelumab) have been approved by United States Food and Drug Administration (FDA) for the treatment of different cancer.19–24 Interestingly, in a recent trial (Checkmate 214),25 a favorable hazard ratio (HR) of death (0.64 95% CI, 0.42 to 0.96) was seen in the patients with liver metastases from renal-cell carcinoma treated with nivolumab and ipilimumab, compared with the patients with sunitinib. By contrast, in another trial (IMpower 130),19 the efficacy of the treatment containing atezolizumab and chemotherapy was not significant (HR 1.04 95% CI, 0.63 to 1.72) compared with the patients treated with chemotherapy alone. Therefore, the efficacy of PD-1 or PD-L1 inhibitors remained uncertain in patients with liver metastases (LM).
A meta-analysis of the efficacy of PD-1 or PD-L1 inhibitors in the available trials recruiting patients with liver metastases (LM) might provide relevant evidence for the physicians to recommend the optimal treatment strategy for the patients with liver metastases (LM). Thus, we performed a meta-analysis to evaluate the efficacy of treatments with PD-1 or PD-L1 inhibitors both in the patients with LM and patients without LM to assess the relations of liver metastases and the effectiveness of the PD-1 or PD-L1 inhibitors.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines26 to conduct our meta-analysis. Our meta-analysis has been registered at the International Prospective Register of Systematic Reviews (PROSPERO), and the CRD code was CRD42019141792.
Search strategy and study selection
We searched Pubmed and Embase comprehensively for relevant original articles about PD-1 or PD-L1 inhibitors from inception date to June 2019 with no language restrictions. To prevent the overlook of the recent unpublished studies, we additionally reviewed the abstracts and presentations from two major conference proceedings, including the American Society of Clinical Oncology and the European Society for Medical Oncology. Two investigators independently searched the databases, and the keywords were “PD-1,” “PD-L1,” “checkpoint inhibitor,” “Nivolumab,” “Pembrolizumab,” “Cemiplimab,” “Avelumab,” “Durvalumab,” “Atezolizumab,” “cancer” “human” and “randomized controlled trial” (more detailed information can be seen in the Supplementary Table 1). The search was limited in human trials. The same two investigators also independently excluded the duplicate publications and reviewed all the studies in the databases to choose the potentially included studies for data extraction. The inclusion criteria we used were as follow: (1) phase 2 or 3 randomized controlled trials; (2) trials evaluating the relative efficacy of PD-1 or PD-L1 inhibitors alone or with other regimens compared with regimen that did not include a PD-1 or PD-L1 inhibitor in patients with advanced cancer; (3) trials reporting the HR of overall survival (OS) in patients distinctly; and (4) trials presenting HR of overall survival (OS) in both patients with LM and patients without LM. We only included the studies fulfilling all the criteria above. We excluded the studies: (1) were non-trial studies such as retrospective or prospective observational cohort studies, (2) were single-arm or non-randomized trials; (3) were review articles, letters, editorials, quality of life studies, basic science papers and cost-effectiveness analyses; and (4) did not present HR of overall survival (OS) in both subgroups of patients with LM and patients without LM. In case of trials that did not include survival subgroup analysis according to liver metastases (LM) in the main text, we also reviewed each study’s supplement in the process of at the full-text screening stage. Moreover, we only selected the most complete and updated one if the same trial appeared in different and multiple studies. Subsequently, any discrepancies in the process of study selection were discussed and resolved by a consensus formed by all investigators involved.
Risk of bias assessment and data extraction
The Cochrane Risk of Bias Tool27 was used by the same two investigators independently and subjectively to assess the quality of included studies. Every included study was evaluated by the following criteria: (1) randomized sequence generation; (2) allocation concealment; (3) blinding of participants, personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective outcome reporting, and (7) other sources of bias. According to this method, all the studies included were assessed, and each risk of bias was described as low risk, high risk, or unclear risk. From each included study, another two investigators independently collated the name, year of publication, study phase, line of therapy, type of malignancies, study drugs, number of the patients, median follow-up time, HR for death in the overall population and HR in both patients with LM and patients without LM. In the process of quality assessment and data extraction, any disagreements were discussed and resolved by the consensus formed by all investigators.
Data analysis
The HR of death and 95% confidence intervals (CI) from each included study in patients with LM and patients without LM were derived and calculated separately for the pooled HR. Cochrane’s Q statistics and I2 statistics were used to assess the heterogeneity of individual studies.27 Heterogeneity was considered low, moderate, or high according to I2 values <25%, 25–50%, and >50%, respectively. If the test showed I2 > 50% or P < .05, the data was calculated by a random-effects model. Otherwise, the fixed-effects model would be used. In this present study, we used the fixed-effects model to calculate the pooled HR because the heterogeneity was not significant in all conducted analyses. The subgroups were selected according to different diseases, lines of therapy, and drug targets. More importantly, to assess the difference between the group with LM and the group without LM in each analysis, we calculated the log HR and then assessed whether variations were different from the null using χ2 test. Besides, publication bias was assessed by three methods, including direct visual inspection of the funnel plot, Egger test, and Begg test.28–30 Three investigators performed all the statistical analyses by STATA 12.0, and all the data were expressed as the combination of HR and 95% CI. Moreover, two-tailed P < .05 was regarded as statistically significant in the two-tailed test.
Results
Literature search
Through our search strategies, a total of 5964 publications were initially identified. After duplication, 1560 articles were removed. Then the rest of the publications were screened for titles and abstracts. After the screening, 4151 articles were excluded because they did not meet our inclusion criteria. A total of 957 studies were excluded for irrelevant topics. Additionally, 2215 studies of review or meta-analysis, 323 studies of case report, 71 basic or animal studies, 551 non-trial studies, and 74 non-randomized trials were all excluded. Subsequently, 253 potentially eligible studies were screened for full-text review.In consequence, we excluded 41 phase-1 studies as well as 66 single-arm studies. Besides, 74 studies were excluded for including PD-1 or PD-L1 inhibitors in both arms. A total of 64 studies with insufficient data were excluded because, in these studies, patients were not classified according to the presence of the LM, or the HRs of overall survival in both the group with LM or the group without LM were not given. Finally, eight randomized controlled trials fulfilled our inclusion criteria and were chosen for the final analysis. Figure 1 shows the flowchart diagram of our study selection process.
Figure 1.
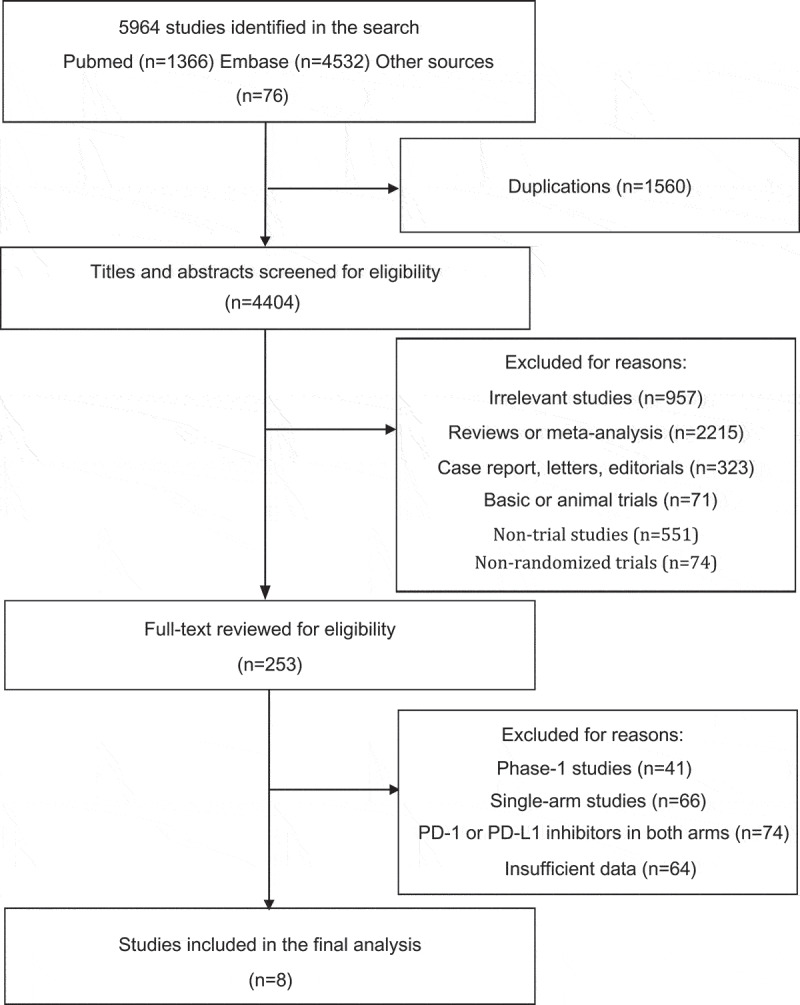
Flowchart diagram of study selection. A total of eight RCTs were included in the final analysis.
Study characteristics
Table 1 shows the main characteristic of the eight included studies. All the eight eligible studies were international multicenter phase 3 randomized controlled trials (RCTs).19–21,25,31–34 A total of 5293 subjects were included in our study, of which liver metastases (LM) were present in 1246 (24%) patients and absent in 4047 (76%) patients. Patients who underwent surgery for cancer or metastatic sites were excluded in all eight trials. All the eligible studies were performed in the patients with advanced or metastatic malignancies, and the specific disease conditions and histological features of each study are described in Table 2. Among them, three studies were conducted in the patients with lung cancer,19,31,34 two studies in renal cell carcinoma,25,32 two studies in urothelial carcinoma20,33 and 1 study21 in gastric or gastro-esophageal junction cancer. Moreover, nivolumab was studied in 3 studies,21,25,32 atezolizumab19,31,33,34 in 4 studies and pembrolizumab in only one study.20 The PD-1 or PD-L1 inhibitors were combined with other treatments as intervention groups in 4 studies, while in the other four studies, patients received PD-1 or PD-L1 inhibitors alone. Besides, half of the trials (4 trials) evaluated the PD-1 or PD-L1 inhibitors after the failure of the previous systemic therapy. In contrast, the other half assessed the efficacy of OS in the first-line setting. After the assessment of the risks of bias, the quality of the studies was moderate to good in general. However, the lack of blinding was found in most of the included studies since most of them were open-label, and only IMpower13334 and ATTRACTION-221 were double-blinded. Also, the blinding of outcome assessment was unclear in all included studies since the relevant information in the articles or protocols was not found. The supplementary Table 2 shows the risk of bias assessment.
Table 1.
The basic characteristics and main outcomes of the 8 included randomized controlled trials.
| Study ID | Phase | Tumor Type | Treatment groups |
Line | Number of patients |
Median Follow-up (month) | Overall Survival |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Total | With LM (%) |
Without LM (%) |
HR (95% CI) for Patients with LM | HR (95% CI) for patients without LM | |||||
| IMpower130 | 3 | Non-squamous Non-small-cell Lung Cancer | Atezolizumab + Carboplatin + Nab-paclitaxel | Carboplatin + Nab-paclitaxel | 1 | 679 | 100(15%) | 579(85%) | 18.5 vs 18.8 | 1.04 (0.63 ~ 1.72) | 0.73 (0.57 ~ 0.92) |
| IMvigor211 | 3 | Urothelial Carcinoma | Atezolizumab | *Investigator’s choice Chemotherapy | >1 | 931 | 268(29%) | 663(71%) | 17.3 | 0.84(0.64 ~ 1.09) | 0.83(0.69 ~ 1.0) |
| IMpower133 | 3 | Small-Cell Lung Cancer | Atezolizumab + Carboplatin + Etoposide | Placebo + Carboplatin + Etoposide |
1 | 403 | 149 (37%) | 254 (63%) | 13.9 | 0.81 (0.55 ~ 1.20) | 0.64 (0.45 ~ 0.90) |
| KEYNOTE-045 | 3 | Urothelial Carcinoma | Pembrolizumab | *Investigator’s choice Chemotherapy | >1 | 541 | 186(34%) | 355(66%) | 14.1 | 0.85 (0.61 ~ 1.20) | 0.67 (0.50 ~ 0.89) |
| ATTRACTION-2 | 3 | Gastric or Gastro-esophageal Junction Cancer | Nivolumab | placebo | >1 | 493 | 106(22%) | 387(78%) | 8.87 vs 8.59 | 0.67 (0.42 ~ 1.07) | 0.64 (0.50 ~ 0.81) |
| CheckMate025 | 3 | Renal-Cell Carcinoma | Nivolumab | Everolimus | >1 | 821 | 187 (23%) | 634(77%) | 22 | 0.81 (0.55–1.18) | 0.73 (0.58–0.92) |
| Checkmate214 | 3 | Renal-Cell Carcinoma | Nivolumab + Ipilimumab | Sunitinib | 1 | 847 | 177(21%) | 670(79%) | 25.2 | 0.64 (0.42–0.96) | 0.66 (0.51–0.85) |
| IMpower132 | 3 | Non-squamous Non-small-cell Lung Cancer | Atezolizumab + Carboplatin+ Cisplatin/Pemetrexed | Carboplatin + Cisplatin/Pemetrexed | 1 | 578 | 73(13%) | 505(87%) | 14.8 | 0.99 (0.57–1.70) | 0.76 (0.59–0.98) |
PD-1: programmed cell death 1; PD-L1: programmed cell death ligand 1; LM: liver metastases; HR: hazard ratios; CI: confidence interval: *Investigator’s choice chemotherapy: paclitaxel, docetaxel, or vinflunine.
Table 2.
Clinicopathological characteristics of the eligible studies.
| Study | Cancer type | Cancer condition |
|---|---|---|
| IMpower130 | Non-squamous Non-small-cell Lung Cancer (NSNSCLC) | Histologically or cytologically confirmed stage IV NSNSCLC, no previous chemotherapy |
| IMvigor211 | Urothelial carcinoma | Locally advanced (T4b, any N; or any T, N 2–3) or metastatic (M1, Stage IV) urothelial carcinoma, progression during or following one or more platinum-containing regimens for metastatic urothelial carcinoma |
| IMpower133 | Small-Cell Lung Cancer | Histologically or cytologically confirmed extensive-stage small-cell lung cancer, no previous systemic treatment for extensive-stage small-cell lung cancer |
| KEYNOTE-045 | Urothelial Carcinoma | Histologically or cytologically confirmed locally advanced (unresectable) or metastatic urothelial carcinoma with predominant transitional-cell features, progression or recurrence after two or fewer lines of systemic chemotherapy |
| ATTRACTION-2 | Gastric or Gastro-esophageal Junction Cancer | Unresectable advanced or recurrent gastric or gastro-esophageal junction cancer histologically confirmed to be adenocarcinoma, refractory to or intolerant of standard therapy including two or more previous chemotherapy regimens |
| CheckMate025 | Renal-Cell Carcinoma (clear-cell) | Histologic confirmation of advanced or metastatic renal-cell carcinoma with a clear-cell component, disease progression after or during no more than three total previous regimens of systemic therapy |
| Checkmate214 | Renal-Cell Carcinoma (clear-cell) | Advanced (not amenable to curative surgery or radiation therapy) or metastatic (*AJCC Stage IV) renal cell carcinoma, no prior systemic therapy |
| Impower 132 | Non-squamous Non-small-cell Lung Cancer (NSNSCLC) | Histologically or cytologically confirmed stage IV non-squamous NSCLC, no prior treatment |
*AJCC: American Joint Committee on cancer.
Efficacy of PD-1 or PD-L1 inhibitors and liver metastases
In our study, we found that the patients with LM treated with PD-1 or PD-L1 inhibitors had a significantly lower risk of death compared with the patients in the control groups which did not include a PD-1 or PD-L1 inhibitor (pooled HR 0.82 95% CI, 0.71 to 0.93, P = .003) (see Figure 2). Since no significant heterogeneity between studies was detected in the heterogeneity assessment (I2 = 0.0%, P = .836), the fixed-effects model was deployed in the data analysis. In the patients without LM, the benefits obtained from PD-1 or PD-L1 inhibitors compared with controlled treatment were also significant (HR 0.72 95% CI, 0.66 to 0.79, P < .001) (see Figure 2). The fixed-effects model was used again, as no substantial heterogeneity presented between individual studies. Interestingly, it should be noted that we found no statistical difference in OS advantage between patients with LM and patients without LM (P = .137).
Figure 2.
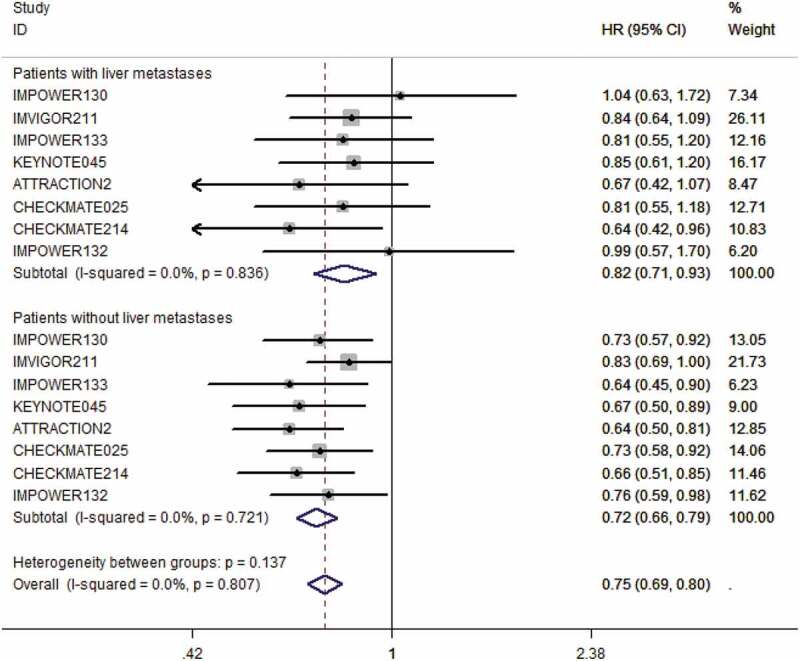
The forest plot of the hazard ratios and 95% CI of death in both groups of patients with LM and without LM assigned to intervention treatment, compared with those in the control groups. The pooled HR was calculated by fixed-effects model since no heterogeneity was found in both groups. The difference between these two groups was not significant (P = .137).
Several subgroup analyses were performed according to disease, line of therapy, and drug target. No statistically significant difference in the efficacy of PD-1 or PD-L1 inhibitors were found between the patients with LM and the patients without LM in any of these analyses. In all the subgroup analyses, the patients without LM can benefit from the PD-1 or PD-L1 inhibitors. For patients with LM, the significant efficacy can be seen in the subgroups of renal cell carcinoma, subsequent line, and PD-1 inhibitors. In the rest of the subgroups, including non-small-cell lung cancer (NSNSCLC), urothelial carcinoma, PD-L1 inhibitors, and first-line therapy, the efficacy seemed marginal. (see Table 3)
Table 3.
Differences in efficacy of PD-1 or PD-L1 inhibitors in LM (+) and LM (-) by subgroups.
| Number of patients (N) |
Pooled HR (95% CI) |
Test for difference |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Number of trials | LM (+) | LM (-) | LM (+) | LM (-) | χ2 | *P |
| Overall | 8 | 1246 | 4047 | 0.82 (0.71 ~ 0.93) | 0.72 (0.66 ~ 0.79) | 2.21 | 0.14 |
| Disease | |||||||
| Renal-Cell Carcinoma | 2 | 364 | 1304 | 0.73 (0.55 ~ 0.96) | 0.70 (0.59 ~ 0.83) | 0.06 | 0.81 |
| Urothelial Carcinoma | 2 | 454 | 1018 | 0.84 (0.68 ~ 1.04) | 0.78 (0.67 ~ 0.91) | 0.35 | 0.55 |
| NSNSCLC | 2 | 173 | 1084 | 1.02 (0.70 ~ 1.47) | 0.74 (0.63 ~ 0.89) | 2.24 | 0.13 |
| Line of therapy | |||||||
| First line | 4 | 499 | 2008 | 0.82 (0.66 ~ 1.03) | 0.70 (0.62 ~ 0.80) | 1.33 | 0.25 |
| Subsequent line | 4 | 747 | 2039 | 0.81 (0.68 ~ 0.96) | 0.73 (0.66 ~ 0.82) | 0.92 | 0.34 |
| Drug target | |||||||
| PD-1 | 4 | 656 | 2046 | 0.76 (0.62 ~ 0.92) | 0.68 (0.60 ~ 0.77) | 0.86 | 0.35 |
| PD-L1 | 4 | 590 | 2001 | 0.88 (0.72 ~ 1.06) | 0.76 (0.68 ~ 0.86) | 1.41 | 0.24 |
LM (+): Patients with liver metastases; LM (-): Patients without liver metastases; *P: P value for difference on HR between patients with liver metastases and patients without liver metastases.
Publication bias
The assessment of the publication bias was performed in the group with LM, the group without LM, and their combination. No significant publication bias was found in the arm of patients with LM after three methods, including the visual inspection of the funnel plot, the Begg rank correlation test, and the Egger linear regression test (see supplementary material). Similarly, no substantial publication bias was detected in the combination of both arms (see Figure 3). As for the arm of patients without LM, the funnel plot all showed slight asymmetry, but the Begg test indicated no significant publication bias (P = .174). Interestingly, the Egger test showed significant publication bias (P = .027). When the number of the included studies was small, assessment of the publication bias should be based on the Egger test. Therefore, we considered that the publication bias in the arm of patients without LM existed. Despite the existence of publication bias, sensitivity analysis of the Trim and Fill Method35 showed the result was reliable and stable, as no trimming or filling data was performed (see supplementary material).
Figure 3.
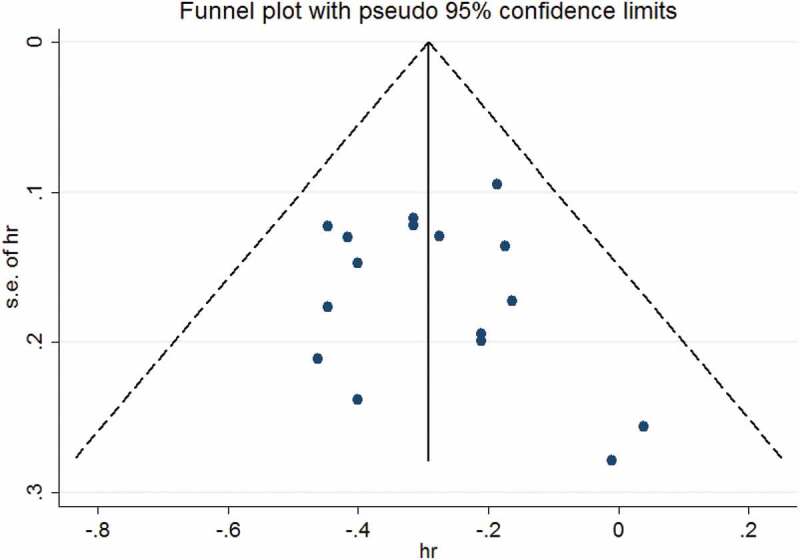
The funnel plot of the overall survival from both arms from the included 8 RCTs for the visual detection of systematic publication bias and small study effect. The visual inspection of the funnel plot was symmetric.
Discussion
To our knowledge, this is the first meta-analysis to study the relations between liver metastases (LM) and the long-term efficacy of PD-1 or PD-L1 inhibitors in patients with advanced cancers. In our pooled study, the treatments with PD-1 or PD-L1 inhibitors, compared with the regimen that did not include a PD-1 or PD-L1 inhibitor, can significantly reduce the risk of death by 18% in the patients with LM and by 28% in the patients without LM with no significant difference (P = .137). Similarly, in our subgroup analyses, no statistically significant differences were detected in any analyses, although the magnitude of the efficacy of PD-1 or PD-L1 inhibitors was greater for patients without LM than for the patients with LM. Our finding was consistent with previous studies, including one RCT and one case report.36,37 In this case report,37 a 77-year-old man with multiple liver metastases from gastric cancer was treated with nivolumab (PD-1 inhibitor). After 12 cycles of systemic treatment using nivolumab, the liver metastases in this patient completely disappeared, and no adverse reaction was observed. In another study, the efficacy of nivolumab in the patients with LM from advanced non-small-cell lung cancer was evaluated, and the researchers found that compared with docetaxel, nivolumab can significantly decrease the risk of death in the patients with LM (HR 0.68 95% CI, 0.50 to 0.91) which was consistent with findings from the overall study population (HR 0.70 95% CI, 0.61 to 0.81). This trial only included 96 patients with LM from non-small-cell lung cancer, while our meta-analysis comprised 1246 patients with LM from various diseases. Our findings suggested that the presence or absence of liver metastases should not be the adequate criteria to select the patients in the routine clinical practice of PD-1 or PD-L1 blockade treatment.
In subgroup analyses, for patients without LM, treatments with PD-1 or PD-L1 inhibitors can significantly improve the overall survival in any analyses. For patients with LM, the efficacy of PD-1 and PD-L1 inhibitors was marginal for patients with LM in urothelial carcinoma (HR: 0.84 95% CI, 0.68 to 1.04) and NSNSCLC (HR: 1.02 95% CI, 0.70 to 1.47). Notably, for subgroups of the two diseases mentioned above, data were available from only two trials. Besides, the subgroup of NSNSCLC only included 173 patients. Partly owing to the limited number of the included trials and included patients, the subgroup analyses were not sufficiently powered to deny the efficacy of PD-1 or PD-L1 inhibitors in the patients with LM from NSNSCLC and urothelial carcinoma. Therefore, more future studies were urgently needed to assess the efficacy of PD-1 blockade therapy in patients with LM from various cancer. Besides, for patients with LM, the efficacy of the PD-1 or PD-L1 inhibitors was significant in the subsequent-line setting (HR: 0.81 95% CI, 0.68 to 0.96), but it is marginal in the first-line setting (HR: 0.82, 95% CI, 0.66 to 1.03). In terms of the first-line therapy, we have conducted the appropriate test to compare the estimates between the group with LM (HR: 0.82, 95% CI, 0.66 to 1.03) and the group without LM (HR: 0.70 95% CI, 0.62 to 0.80) and the difference was not significant (P = .25). Therefore, the non-statistical significance can be explained by chance, and we cannot deny the efficacy of the PD-1 or PD-L1 inhibitors as a first-line setting so far.38 For the same reason, the efficacy of PD-L1 inhibitors in patients with LM cannot be denied, either. Interestingly, the biological difference between PD-1 inhibitors and PD-L1 inhibitors did exist since PD-1 inhibitors can have more significant antitumor effects because they blocked the binding between PD-1 receptors and two ligands (PD-L1 and PD-L2) simultaneously and blocking the binding of PD-L2 can also relieve the immune suppression induced by cancer cells while PD-L1 inhibitors can only block the PD-L1.39 Besides, in a previous meta-analysis, the researchers performed a comparison between nivolumab, pembrolizumab, and atezolizumab. They found that patients with lung cancer treated with nivolumab or pembrolizumab had a significantly higher objective response rate (ORR) than the patients treated with atezolizumab.40 However, there was no available study specifically comparing the efficacy in the patients with LM among different PD-1 or PD-L1 inhibitors at present. Therefore, it was hard to tell which PD-1 or PD-L1 inhibitors were more appropriate for patients with LM so far. Thus, large RCTs were needed to evaluate the relative efficacy of different PD-1 or PD-L1 inhibitors in patients with LM to choose the optimal PD-1 or PD-L1 for these patients.
Nowadays, when liver metastases were detected in patients with advanced cancer, the surgery was still the first choice, but most of the patients with LM at diagnosis were not appropriate for surgery.1 Therefore, a novel treatment strategy was needed for patients with LM. Our study suggested that patients with liver metastases (LM) should not be excluded in the clinical practice of PD-1 or PD-L1 blockade therapy. Treatment with PD-1 or PD-L1 inhibitors might provide a novel treatment strategy for patients with LM. Our study might promote the future use of PD-1 or PD-L1 in patients with LM. However, we should not exaggerate the efficacy of PD-1 or PD-L1 inhibitors in patients with LM. In some studies, even after the treatment of PD-1 or PD-L1 inhibitors, the presence of liver metastases was still associated with poor outcomes.36,41,42 In a retrospective study,41 the median overall survival of the patients with LM treated with durvalumab (PD-L1 inhibitor) was only 5.5 months, which was significantly lower than that in the patients without LM (16.7 months). Also, in another study,42 61 samples from the patients with metastatic melanoma before the treatment of pembrolizumab (PD-1 inhibitor) were obtained for immunohistochemical staining. Then the outcome indicated that the CD8+ T cells count in the metastatic sites from the patients with LM was significantly lower compared with the patients without LM. CD8+ T cells, including cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, were significant cellular proportions associated with the response to the PD-1 or PD-L1 inhibitors. The researchers explained the decreased CD8+ T cells in patients with LM by liver tolerance. Liver tolerance was introduced to explain the liver’s unique ability to suppress the immune responses to maintain its homeostasis since this well-supplied lymphoid organ was exposed to the massive antigenic life-sustaining dietary products from the gastrointestinal tract via portal vein system.2,43 Also, some investigators believed that the liver tolerance might be responsible for the chronic infection of hepatitis B virus and hepatitis C virus in the liver, the better acceptance of liver transplantation and the establishment of the metastatic tumors in the liver.43,44 In brief, for patients with LM, the ability of PD-1 or PD-L1 inhibitors to enhance the immune system might be partly counteracted by their relatively strong liver tolerance. Therefore, future development of the combination strategies has been an urgent need to improve PD‐1 blockade efficacy. For example, in a case report, the physicians combined the nivolumab with chimeric antigen receptor (CAR) T cells 19 in a patient with refractory follicular lymphoma and achieved an exciting outcome.45 Since CAR T cells can promote the infiltration of the T cell in the tumors while PD-1 or PD-L1 inhibitors can enhance the function of T cells, the combination of these two treatments can work together to counteract the immunosuppression.46 Currently, there are several ongoing larger clinical trials exploring combination therapy with CAR T Cells and PD-1 or PD-L1 inhibitors on different types of advanced solid tumors such as neuroblastoma (NCT01822652) and glioblastoma (NCT03726515). Moreover, the combinations with PD-1 or PD-L1 inhibitors and other different checkpoint inhibitors might also be promising. The combination of nivolumab and ipilimumab [cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors] significantly improved the overall survival in patients with metastatic melanoma compared with ipilimumab alone in a phase-3 RCT.47 FDA also approved this combination for the management of unresectable melanoma in 2016. It was thought that the combination of CTLA-4 and PD-1 blockade would act synergistically to promote the activation of the T cells and NK cells in multiple pathways to enhance the immune system to fight cancer.48 Besides CTLA-4, some other clinical trials of the combination therapy of PD-1 blockade with the inhibition of other negative co-receptors such as lymphocyte activation gene 3 (NCT02658981) are also ongoing. In addition, our study only discussed the efficacy of different medications while some radiologic managements such as stereotactic body radiotherapy (SBRT), transarterial embolization (TAE), transarterial chemoembolization (TACE) also played an important role in the patients with LM.11,49,50 In one study, the PD-1 inhibitors were combined with SBRT for three patients (one patient with nivolumab and two patients with pembrolizumab) with intrahepatic cholangiocarcinoma and the decrease of the liver lesions was observed in all of the patients.51 Besides, one of the patients even achieved progression-free and maintained a complete response for 11 months. The radiation therapy has shown immunomodulating effects such as reversing immunosuppressive barriers within the tumor microenvironment, augmented the generation of the CTL, and increasing PD-1 tumor expression, all of which have the potential to enhance the efficacy of PD-1 blockade therapy.52–54 More importantly, the combination of PD-1 blockade and radiotherapy can boost the abscopal effect leading to regression of metastatic cancer at distant sites.55
There were also several limitations. Firstly, our study only studied the efficacy of PD-1 or PD-L1 inhibitors. An optimal treatment strategy needed to maximize the benefit as well as minimize the risk of toxicities. However, in our study, the information regarding adverse events from the included patients with or without liver metastases was unavailable. Currently, there is no publication studying the safety of the PD-1 or PD-L1 inhibitors specifically in the patients with LM, although several previous meta-analyses indicated that compared with controlled regimens such as chemotherapy and targeted therapy, the PD-1 or PD-L1 inhibitors seemed to have better safety profile in patients with cancer.56,57 Future studies should focus on the safety of PD-1 or PD-L1 inhibitors in patients with LM. Secondly, since our study only extracted data in a trial level rather than an individual level, other variables other than liver metastases could affect the response of PD-1 or PD-L1 inhibitors such as age, gender, region, and PD-L1 expression status. Thirdly, there was a publication bias in the group of patients without LM, despite sensitivity analysis of the Trim and Fill Method was used to confirm the stability and reliability of our results.
Conclusion
No difference was found in the efficacy of treatment with PD-1 or PD-L1 inhibitors between patients with LM and patients without LM. Patients with liver metastases (LM) should not be excluded in the clinical practice of PD-1 or PD-L1 blockade therapy. Future studies are needed to assess the safety of PD-1 or PD-L1 inhibitors and to seek optimal multimodality therapy, including PD-1 or PD-L1 inhibitors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Informed consent
For the type of study, formal consent is not required.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Gupta GP, Massagué J.. Cancer metastasis: building a framework. Cell. 2006;127(4):679–10. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Moris D, Lu L, Qian S. Mechanisms of liver-induced tolerance. Curr Opin Organ Transplant. 2017;22(1):71–78. doi: 10.1097/MOT.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567(7747):249–252. doi: 10.1038/s41586-019-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016;139(12):2679–2686. doi: 10.1002/ijc.30400. [DOI] [PubMed] [Google Scholar]
- 5.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–1633. doi: 10.1002/()1097-0142. [DOI] [PubMed] [Google Scholar]
- 6.Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, HIZAWA N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3(1):217–221. doi: 10.3892/mco.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, HIZAWA N. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4(4):617–620. doi: 10.3892/ol.2012.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, Tangen CM, Rosenthal M, Petrylak DP, Hussain M, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong F, Shen Y, Gao F, Xu T, Wang X, Zhang X, Zhong S, Zhang M, Chen S, Shen Z, et al. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: a population-based study. Cancer Manage Res. 2017;9:611–626. doi: 10.2147/CMAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouri BE, Abrams RA, Al-Refaie WB, Azad N, Farrell J, Gaba RC, Gervais DA, Gipson MG, Kolbeck KJ, Marshalleck FE, et al. ACR appropriateness criteria radiologic management of hepatic malignancy. J Am Coll Radiol. 2016;13(3):265–273. doi: 10.1016/j.jacr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Akgül Ö, Çetinkaya E, Ersöz Ş, Tez M. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20(20):6113–6122. doi: 10.3748/wjg.v20.i20.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, Jaeck D, Saric J, Le Treut YP, Belghiti J, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244(4):524–535. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, Kameyama K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur Jcardio-thoracic Surg. 2008;34(3):499–504. doi: 10.1016/j.ejcts.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11(11):3887–3895. doi: 10.1002/embj.1992.11.issue-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribas A. Releasing the brakes on cancer immunotherapy. N Engl J Med. 2015;373(16):1490–1492. doi: 10.1056/NEJMp1510079. [DOI] [PubMed] [Google Scholar]
- 19.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp H-G, Daniel D, McCune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 20.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 22.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 23.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed). 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical Research Ed). 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 31.Barlesi F, Nishio M, Cobo M, Steele N, Paramonov V, Parente B, Dear R, Berard H, Peled N, Seneviratne LC, et al. LBA54IMpower132: efficacy of atezolizumab (atezo) + carboplatin (carbo)/cisplatin (cis) + pemetrexed (pem) as 1L treatment in key subgroups with stage IV non-squamous non-small cell lung cancer (NSCLC). Ann Oncol. 2018;29(suppl_8). doi: 10.1093/annonc/mdy424.066. [DOI] [Google Scholar]
- 32.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (London, England). 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 34.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 35.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 36.Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, Aren Frontera O, Gettinger S, Holgado E, Spigel D, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- 37.Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J, Munekage M, Uemura S, Tsujii S, Maeda H, et al. Successful treatment of liver metastases arising from early gastric cancer achieved clinical complete response by nivolumab. Surgical Case Reports. 2018;4(1):71. doi: 10.1186/s40792-018-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA. 2014;311(4):405–411. doi: 10.1001/jama.2013.285063. [DOI] [PubMed] [Google Scholar]
- 39.Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112(9):1421–1427. doi: 10.1038/bjc.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passiglia F, Galvano A, Rizzo S, Incorvaia L, Listì A, Bazan V, Russo A. Looking for the best immune-checkpoint inhibitor in pre-treated NSCLC patients: an indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int J Cancer. 2018;142(6):1277–1284. doi: 10.1002/ijc.v142.6. [DOI] [PubMed] [Google Scholar]
- 41.Sridhar S, Paz-Ares L, Liu H, Shen K, Morehouse C, Rizvi N, Segal NH, Jin X, Zheng Y, Narwal R, et al. Prognostic significance of liver metastasis in durvalumab-treated lung cancer patients. Clin Lung Cancer. 2019;20(6):e601–e8. doi: 10.1016/j.cllc.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N, et al. Liver metastasis and treatment outcome with Anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13(2):88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 44.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DAL. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223(5205):472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Deng Q, Jiang YY, Zhang R, Zhu HB, Meng JX, Li, YM. CAR-T 19 combined with reduced-dose PD-1 blockade therapy for treatment of refractory follicular lymphoma: a case report. Oncol Lett. 2019;18(5):4415–4420. doi: 10.3892/ol.2019.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471–482. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF.. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 48.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, D’Ambrosio D, Sharma S, Perry D, Kolker J, Davis J, et al. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch® patient registry. Radiat Oncol. 2018;13(1):26. doi: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bale R, Putzer D, Schullian P. Local treatment of breast cancer liver metastasis. Cancers. 2019;11:9. doi: 10.3390/cancers11091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Yao J, Song L, Zhang S, Huang T, Li Y. Local and abscopal responses in advanced intrahepatic cholangiocarcinoma with low TMB, MSS, pMMR and negative PD-L1 expression following combined therapy of SBRT with PD-1 blockade. J Immuno Cancer. 2019;7(1):204. doi: 10.1186/s40425-019-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70(7):2697–2706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 54.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu Y-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist. 2017;22(4):470–479. doi: 10.1634/theoncologist.2016-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis of published data. Int J Cancer. 2017;141(5):1018–1028. doi: 10.1002/ijc.v141.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


