ABSTRACT
The role of IL-17A+ cells remains controversial among various cancer types. This study aimed to investigate the effects of IL-17A+ cells on tumor immune contexture and clinical outcome in muscle-invasive bladder cancer (MIBC). In this study, we enrolled 141 patients from Zhongshan Hospital, 118 patients from Shanghai Cancer Center and 403 patients from TCGA cohort. In vitro studies were conducted in 32 freshly resected tumors. Survival analysis was conducted using Kaplan–Meier and Cox regression analysis. The results suggested that patients with high levels of IL-17A+ cells had prolonged overall survival and recurrence-free survival (HR = 0.268, P < .001; and HR = 0.433, P < .001). Moreover, these patients tended to be at lower risk of death and recurrence after adjuvant chemotherapy (P = .012 and P = .004). An increased number of IL-17A+ cells correlated with the infiltration of several anti-tumor immune cells into tumors. In addition, IL-17A+ cells had an influence on the recruitment, proliferation, and activation of CD8+ cells, and were positively associated with the expression of several anti-tumor effector cytokines. In conclusion, tumor-infiltrating IL17A+ cells were correlated with an elevated anti-tumor immunity in MIBC. Besides, high infiltration of IL17A+ cells can predict benefit from ACT for MIBC patients.
KEYWORDS: IL-17A+ cells, muscle-invasive bladder cancer, anti-tumor immunity, adjuvant chemotherapy
Introduction
Bladder cancer is one of the most common malignancies involving the urinary system.1 Approximately a quarter of patients with bladder cancer present with muscle invasive disease and often have a much worse prognosis.2 For these patients, radical cystectomy alone is associated with an overall cure rate ranging from 50% to 65%. However, the five-year survival rate drops to 40% or lower in patients with invasion beyond the bladder muscle.3 Besides surgical treatment, cisplatin-based chemotherapy has been proved to be effective for advanced and metastatic bladder cancer.4 There have been trials estimating the benefit of adjuvant chemotherapy for muscle-invasive bladder cancer patients, but they have been inadequately powered and prematurely closed for lack of sufficient data.4 As a result, clinicians are not yet able to identify patients who are most likely to benefit from adjuvant chemotherapy.
The efficacy of chemotherapy may not only be determined by its direct effects on cancer cells, but by off-target effect within the host immune system as well. Recent studies have been focused on the role of immune response in chemotherapy-induced cytotoxicity.5-7 Moreover, the intrinsic immune features can predict survival and potential benefit from chemotherapy in patients with bladder cancer.8 Interleukin-17A (also known as IL-17A) has attracted much attention in the past few years for its central role in immunity. It could enhance the expression of various chemokines including CXCL9 and CXCL10 and is believed to be actively involved in the recruitment of other immune cells.9-11 The IL-17A-producing cells, which are composed of CD4+ T helper (Th17) cells, cytotoxic CD8+ T (Tc17) cells and γδT (γδ-17) cells, are a fascinating cell population for their dichotomous nature in cancer.12 On the one hand, IL-17A+ cells have exhibited prominent anti-cancer ability in several types of cancer,13-15 but, on the other hand, this cell population have been reported to promote tumor growth and metastasis as well.11
In this study, we evaluated the prognostic value of IL17A+ cells in a large group of patients with muscle-invasive bladder cancer, validated its predictive significance for survival benefit from cisplatin-based chemotherapy. To our knowledge, this study is the first to reveal the function of IL-17A+ cells in bladder cancer.
Materials and methods
Ethics approval and consent to participate
Informed consent was collected from patients in TMAs and this study was approved by the Clinical Research Ethics Committee of Fudan University and each hospital. All procedures of specimen collection, handling, and application from patients are acknowledged by patients with written informed consents and were approved by institutional review board and ethics committee of Fudan University and Shanghai Jiao Tong University.
Study population
This study was approved by the Clinical Research Ethics Committee of Fudan University and each hospital. In this study, we enrolled three independent patient cohorts, Zhongshan Hospital (ZS) cohort (N = 141), Shanghai Cancer Center (SCC) cohort (N = 118) and the Cancer Genome Atlas (TCGA) cohort (N = 403). The ZS cohort comprised of 215 patients receiving radical cystectomy between 2002 and 2014 in Zhongshan Hospital (Shanghai, China) and 73 patients were excluded for benign or non-muscle invasive disease. The SCC cohort included 178 patients receiving surgery between 2008 and 2012 in Shanghai Cancer Center (Shanghai, China). However, 41 patients with benign or non-muscle invasive disease and 18 patients without clinicopathological information were excluded. After surgery, 119 patients from the two cohorts received cisplatin-based combination chemotherapy for at least one therapeutic cycle. All these patients did not receive neoadjuvant chemotherapy or radiation treatment. During immunohistochemistry staining, one patient from each cohort was lost due to detachment. Overall survival and recurrence-free survival were calculated as interval from the date of cystectomy to death or first recurrence. The follow-up protocol was instructed by EAU guidelines.16 The follow-up period ended in July 2016. The median follow-up periods in ZS cohort and SCC cohort were 56 months and 29 months, respectively. The TCGA cohort was composed of 413 patients, among them, 407 patients were histologically diagnosed with muscle-invasive bladder cancer. Four patients were excluded for lack of IL-17A mRNA information. Two patients without overall survival time and 87 patients without recurrence data were excluded when performing survival analysis. The information of these patients was retrieved from http://www.cbioportal.org in Feb. 2018. Detailed characteristics of all three cohorts are presented in supplementary table 1. The cutoff value of IL-17A+ cells was 2 cells/HPF (*200 magnification) and the cutoff point of IL-17A mRNA was 0.2 (RNAseq Version2), which is determined by X-tile 3.6.1 (Yale University).
Immunohistochemistry (IHC)
Tissue microarray (TMA) construction and the immunohistochemistry protocol were conducted as previously described.17 For each patient, three sections of tumor tissue were taken from paraffin blocks to construct TMA. Primary antibodies (listed in supplementary table 2) were applied to sections at 4°C overnight for immunohistochemical staining. For different T helper cells, TH1 was defined as CD4+ T-bet+ cells and TH2 was defined as CD4+ GATA3+ cells. Then, slides were treated with polyperoxidase-conjugated IgG (OriGene). Staining was performed with diaminobenzidine solution (Biocare Medical) under a microscope and counterstained with hematoxylin. The primary antibody was omitted for the negative controls. The positive cells were enumerated from the representative view of the three sections in high-power field (HPF) and an average number was adopted. Two pathologists who were unaware of patient information evaluated the staining of each specimen with the assistance of Image-Pro Plus 6.0 (Media Cybernetics Inc.). In case of disagreement, the slides were reviewed and the two observers achieved a consensus.
Fresh tumor specimen and flow cytometry
All 32 fresh human specimens were obtained from patients underwent radical cystectomy in Shanghai Cancer Center, Zhongshan Hospital, and Shanghai General Hospital, respectively. Single cells were isolated from freshly resected tumor tissues using collagenase IV. About 1,000,000 cells were collected and incubated in RBC lysis buffer at 4°C for 5 min. Surface markers (listed in supplementary table 2) were stained in Cell Staining Buffer for 30 min at 4°C in dark after blocking Fc-receptors with Human TruStain FcX (Biolegend). Then, cells were fixed by Fixation Buffer (Biolegend), permeabilized by Intracellular staining permeabilization wash buffer (Biolegend) and intracellular cytokine (listed in supplementary table 2) was stained for 40 min at 4°C in dark. Specially, we performed transcription factor staining using the True-Nuclear transcription Factor Buffer set (Biolegend) according to the manufacturer’s instructions. Dead cells were excluded by Live/Dead Fixable Dead Cell StainingKit (Invitrogen). Cells were acquired on the FACSCelesta Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar).
Statistical analysis
Statistical significance was calculated by the Student t-test for comparisons between two groups or one-way ANOVA for multi-group comparisons using GraphPad Prism and SPSS software. Patient characteristics and the association with IL-17A mRNA expression/IL-17A+ cells were described statistically and evaluated by Chi-squared test, respectively. Spearman’s correlation test was used to compare differences between two continuous variables. Survival analysis was carried out by Log-rank test. P < .05 was considered statistically significant.
Results
Identification of tumor-infiltrating IL-17A+ cells in muscle-invasive bladder cancer
IL-17A+ cells were evaluated by immunohistochemistry on tissue microarrays (Figure 1a). Interestingly, the fraction of patients with high levels of tumor-infiltrating IL-17A+ cells and the number of IL-17A+ cells seemed to negatively correlate with TNM stage (Figure 1b,c, Supplementary table 3), indicating that the infiltration of IL-17A+ cells into tumor might associate with a better prognosis for patients. We next assessed the surface markers expressed by tumor-infiltrating IL-17A+ cells in freshly isolated clinical specimens using flow cytometry. Among all 32 fresh tumor samples, there were 6 tissues without detectable IL-17A+ cells. The majority of IL-17A+ lymphocytes expressed CD3 or CD4 (Figure 1d,e). This finding is consistent with previous studies that TH17 cells are a predominant source of IL-17A.18-20
Figure 1.
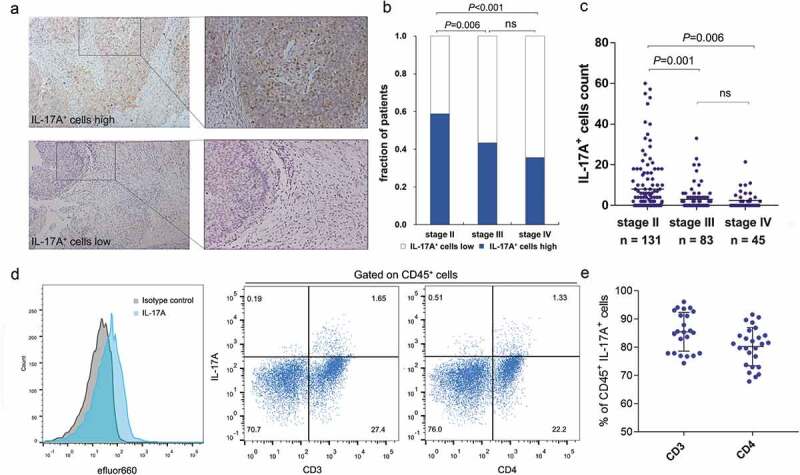
Identification of tumor-infiltrating IL-17A+ cells in muscle-invasive bladder cancer. (a) Representative immunochemistry staining of IL-17A+ cells in muscle-invasive bladder. (b) A fraction of patients with high/low IL-17A+ cells in different TNM stages. (c) The number of tumor-infiltrating IL-17A+ cells in different TNM stages. (d) Representative flow cytometry analysis of the surface markers expressed by IL-17A+ cells from fresh human muscle-invasive bladder cancer samples. (e) Quantification analysis of surface markers expressed by tumor-infiltrating IL-17A+ cells.
Prognostic value of IL17A+ cells and IL-17A mRNA
To elucidate the prognostic value of IL-17A+ cells, we performed Kaplan-Meier analysis using the data from Zhongshan Hospital (ZS), Shanghai Cancer Center (SCC) and TCGA database. We observed that patients with elevated expression of IL-17A+ cells and IL-17A mRNA tended to have prolonged overall survival in all three cohorts (P < .001, P = .003 and P = .041, respectively; Figure 2a). Moreover, high infiltration of IL-17A+ cells into tumors indicated longer recurrence-free survival in ZS cohort and SCC cohort (P < .001 and P = .012, respectively; Figure 2b). However, the IL-17A mRNA failed to predict recurrence-free survival in TCGA cohort (P = .095; Figure 2b).
Figure 2.
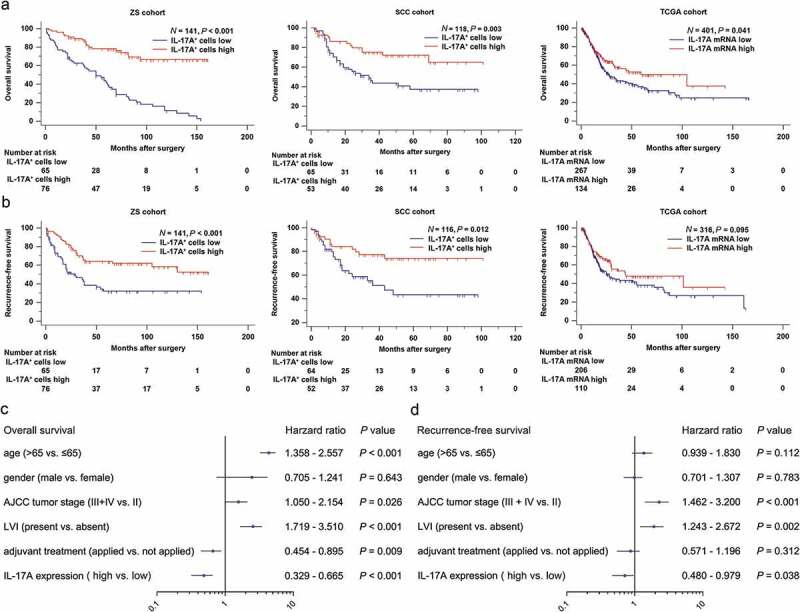
Tumor-infiltrating IL-17A+ cells and IL-17A mRNA indicate better survival for patients. (a and b) Kaplan–Meier curves comparing OS and RFS in muscle-invasive bladder cancer patients with high and low tumor-infiltrating IL-17A+ cells/IL-17A mRNA. (c and d) Multivariate cox regression analysis for IL17A expression and clinic-pathological variables.
We then combined the three cohorts to evaluate whether IL-17A is an independent prognostic factor for muscle-invasive bladder cancer patients. In a multivariate analysis including age, gender, tumor stage, lymphovascular invasion, and adjuvant treatment as confounding variables, the hazard ratio (HR) of disease mortality and recurrence is 0.468 (95%CI, 0.329 to 0.665; P < .001) and 0.685 (95%CI, 0.480 to 0.979; P = .038) respectively (Figure 2c,d). IL-17A proved to be an independent protective factor for patients.
Tumor-infiltrating IL-17A+ cells predict response to adjuvant chemotherapy
In the combined cohort, there was no evidence suggesting that adjuvant chemotherapy could prolong overall survival or recurrence-free survival for muscle-invasive bladder cancer patients (P = .598 and P = .891, respectively; Figure 3a,b). However, after discriminating patients with tumor-infiltrating IL-17A+ cells, adjuvant chemotherapy significantly enhanced overall survival and recurrence-free survival in IL-17A+ cells high group of patients (P = .012 and P = .004, respectively) while it failed in IL-17A+ cells low group (Figure 3cf). A test for the interaction between IL-17A+ cells and adjuvant chemotherapy further confirmed the survival benefit in terms of overall survival (P = .003, Figure 3c,e) and recurrence-free survival (P = .002, Figure 3d,f) observed in IL-17A+ cells high group. Taken together, these results suggested that tumor-infiltrating IL-17A+ cells could successfully identify patients with muscle-invasive bladder cancer who would most likely to benefit from adjuvant chemotherapy.
Figure 3.
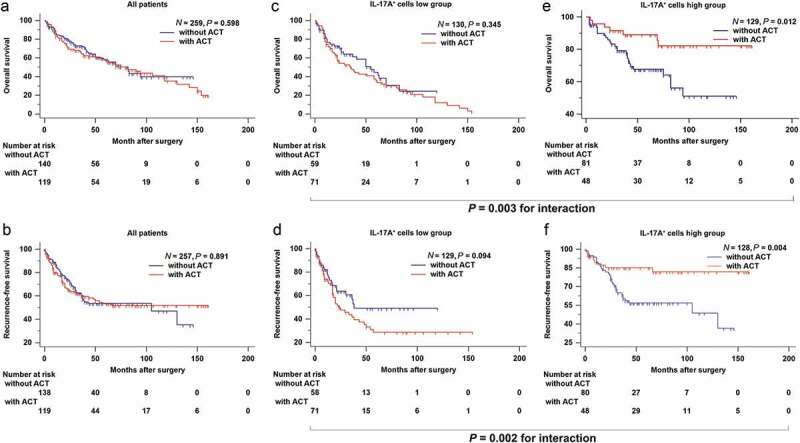
Tumor-infiltrating IL-17A+ cells indicate an improved therapeutic response to adjuvant chemotherapy. (a and b) Survival curves for the use of adjuvant chemotherapy (ACT) in all patients, (c and d) in patients with low intra-tumoral IL-17A+ cells, (e and f) and in patients with high intra-tumoral IL-17A+ cells.
The TCGA cohort was not included because over a third of patients lacked information of whether they received adjuvant pharmaceutical treatment or not. Moreover, the criteria utilized to treat patients, the regimens utilized and the duration of therapy were not clear. These variables may possibly influence outcomes.
Tumor-infiltrating IL-17A+ cells associate with enhanced anti-tumor immunity
The immune contexture, which is determined by the composition, density, and functional state of the tumor-infiltrating leukocytes, can yield information relevant to prognosis, prediction of a treatment response and a variety of other pharmacodynamic parameters.21 According to previous reports, IL-17A happened to be a chef orchestrator of immunity.22 Thus, we decided to looked into the differences of immune cells infiltration between two groups of patients in order to understand the possible mechanism underlying the phenomena mentioned above. We performed semiquantitative immunohistochemistry and calculated the correlation of IL-17A+ cells with several types of immune cells infiltrating into the tumor (Figure 4a, Supplementary figure 1A). IL-17A+ cells were positively correlated with CD8+ T cells, dendritic cells, B cells, neutrophils, and macrophages (Figure 4b, Supplementary figure 1B). When regarding tumor-infiltrating IL-17A+ cells as a dichotomous data, the number of B cells, dendritic cells, and CD8+ T cell was significantly higher in IL-17A+ cells high group than in IL-17A+ cells low group. This was in line with our previous finding that B cells were a protective factor for muscle-invasive bladder cancer patients.23 With consecutive tissue sections, we observed the distribution of IL-17A+ cells was close to CD8+ cells, together with increased expression of IFN-γ and GZMB, the interaction between these cells deserves to be further investigated (Supplementary figure 1 C).
Figure 4.
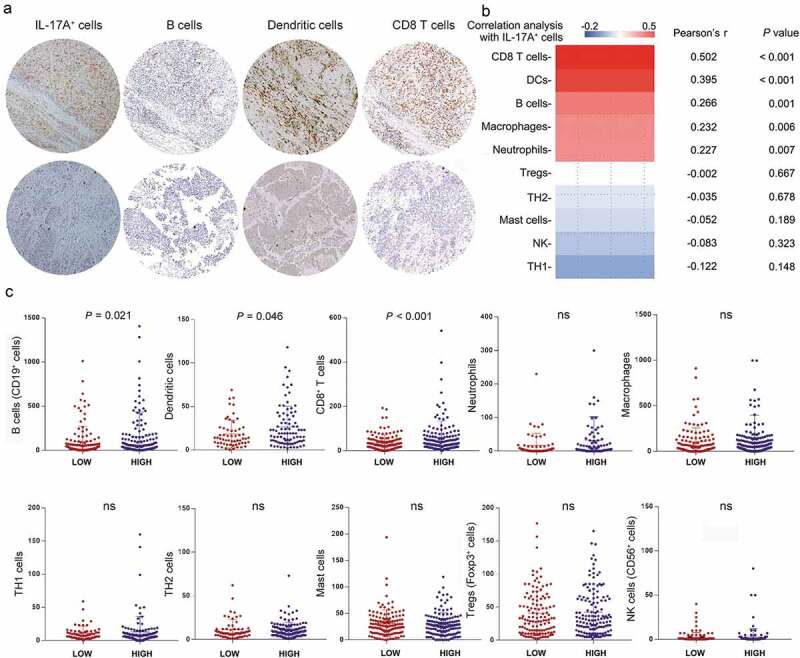
Association of IL-17A+ cells and various tumor-infiltrating immune cells. (a) Representative immunochemistry staining of B cells, Dendritic cells and CD8+ T cells in IL-17A+ cells high tumors and in IL-17A+ cells low tumors. (b) Pearson correlation analysis of IL-17A+ cells with other tumor-infiltrating lymphocytes. (c) Differences in tumor-infiltrating lymphocytes between IL-17A+ cells high tumors and IL-17A+ cells low tumors.
Recent studies have revealed that CD8+ T cells were necessary for therapeutic activity5 and have determined the importance of cytokines in promoting CD8/TH1 response to chemotherapy.6,24,25 Thus, we compared the different functional states of CD8+ T cells between two groups of patients. The percentage of CD8+ T cells expressing GZMB, IFN-γ and PRF1 was significantly higher in IL-17A+ cells high tumors, as compared to IL-17A+ cells low tumors (Figure 5a, Supplementary figure 2A). The proportion of GZMB+ CD8+ T cells, IFN-γ+ CD8+ T cells, and PRF1+ CD8+ T cells was also increased among CD45+ cells in IL-17A+ cells high tumors (Figure 5b). In addition, CD8+ T cells in IL-17A+ cells high group exhibited more proliferative ability than their counterparts in IL-17A+ cells low tumors (Figure 5a,b). This could be one of the reasons that there were more CD8+ T cells detected in IL-17A+ cells present group of patients (Figure 4c). However, the checkpoint expressed by CD8+ T cells exhibited no difference between IL-17A+ cells high and IL-17A+ cells low tumors, except for TIGIT (Figure 5c, Supplementary figure 2B). Among CD45+ cells, the CTLA-4+ CD8+ cells and LAG-3+ CD8+ cells also significantly increased (Figure 5d) in IL-17A+ cells high tumors.
Figure 5.

Tumor-infiltrating IL-17A+ cells indicate enhanced anti-tumor immunity. (a) Difference in effector cytokines and proliferation marker expressed by CD8+ T cells in IL-17A+ cells low tumors and IL-17A+ cells high tumors. (b) Proportion of CD8+ T cells expressing effector cytokines and proliferation marker in CD45+ cells. (c) Difference in immunosuppressive molecules expressed by CD8+ T cells. (d) Proportion of CD8+ T cells expressing immunosuppressive molecules in CD45+ cells. (e and f) Effector cytokines and immunosuppressive molecules expressed by CD45+ cells in IL-17A+ cells low tumors and IL-17A+ cells high tumors.
In addition to cytotoxic T cells, the percentage of CD45+ T cells expressing immune effector molecules all increased in IL-17A+ cells high tumors (Figure 5e). However, we also observed an elevated expression of PD-1 by CD45+ cells (figure 5f). Results from TCGA database further confirmed our findings. GSEA analysis using the Hallmark gene sets26 identified inflammatory response and IFN-γ response as two of the most significantly up-regulated pathways in IL-17A mRNA high tumors (Supplementary figure 3A). In IL-17A mRNA high group, expression of chemokines that could recruit CD8+ T cells was significantly higher (Supplementary figure 3B). This could be another explanation for more CD8 + T cells observed in IL-17A+ cells high group of patients. Genes mediating cytotoxic functions27,28 were also positively correlated with IL-17A mRNA expression (Supplementary figure 3 C). Taken together, these results suggested that tumor-infiltrating IL-17A+ cells were associated with enhanced anti-tumor immunity.
Discussion
It has been well established that IL-17A and IL-17A+ cells are important in promoting inflammation and linked to many immune/autoimmune-related diseases.29,30 However, their role in cancer remained fascinating and required further investigation.20,31 Our study revealed that IL-17A+ cells were correlated with prolonged survival and response to adjuvant chemotherapy. The underlying mechanism was found associated with the recruitment of various anti-tumor immune cells and activation of CD8+ T cells.
Patients are of great heterogeneity in clinical outcomes even with identical clinicopathological features. Thus, integrating multiple biomarkers, especially the intrinsic immune features, has aroused much interest and been proved to provide a more accurate prediction.32,33 As a chief orchestrator of immunity, IL-17A has the ability to induce the expression of important proinflammatory cytokines and recruit a variety of immune cells.33 Thus, IL-17A+ cells may indicate the immune contexture of hosts. Our study demonstrated that tumor-infiltrating IL-17A+ cell was indeed an ideal biomarker to differentiate patients suitable for more active surveillance and adjuvant chemotherapy. Of interest, almost half of the tumor tissues were with low expression of IL-17A+ cell or IL-17A mRNA. This suggested that tumors, particularly with advanced stage, could prevent the infiltration of IL-17A+ cells through some sort of unknown mechanism. This deserves further investigation and may be a potential target to enhance anti-tumor immunity.
In current clinical practice, adjuvant chemotherapy is applied to patients who are at great risk of disease relapse (pT3 + pT4 or N+), but the debate is still ongoing for it has not exhibited obvious benefit for muscle-invasive bladder cancer patients.34 In our study, patients with high tumor-infiltrating IL-17A+ cells have improved response to adjuvant chemotherapy. According to previous researches, immune cell/cytokine pathways involving CD8+ T cells were responsible for mediating response to chemotherapy.6 Moreover, there have been clinical trials proving that the usage of immune checkpoint inhibitor following treatment with platinum-based chemotherapy was effective in patients with locally advanced or metastatic urothelial carcinoma.27,28 The efficacy seemed to be underlying genomic, molecular, and immunological factors. Based on these researches, we hypothesized that the efficacy of adjuvant chemotherapy in IL-17A+ cells high group of patients was due to the activation of cytotoxic immune cells. Actually, we have observed enhanced expression of anti-cancer cytokines by CD8+ T cells in IL-17A+ cells high tumors. A confusing point is that intratumoral IL-17A+ cells were actually removed by surgery, how these cells contribute to the therapeutic outcome remains unknown. Our previous study revealed that immunophenotype, which is an important feature of cancer, could predict benefit from adjuvant chemotherapy in bladder cancer.8 IL-17 is reported to be a chief orchestrator of immunity.22 Thus, high infiltration of IL-17A+ cells could represent an immune-activated phenotype of bladder cancer and serve as a predictive biomarker for adjuvant chemotherapy. In addition, it is reported that the immune composition of metastatic site is close to that of the primary tumor.35 Patients with a high frequency of intratumoral IL-17A+ cells might also have high infiltration of this cell population in their metastatic sites if the disease relapsed. It could be a possible reason for how these cells contribute to the therapeutic outcome but need to be confirmed in further study.
Interestingly, we also observed the elevated expression of immunosuppressive markers by lymphocytes in IL-17A+ cells high tumors, including PD-1. IL-17A mRNA was also positively correlated with several immunosuppressive genes (supplementary Figure 4d). This intrinsic correlation needs further investigation to be explained. We assumed that it might be a counterbalance mechanism in response to hyper-activated anti-tumor immunity status in IL-17A+ high tumors. This finding suggested that patients with high intratumoral IL-17A+ cells might remarkably benefit from immune checkpoint blockade therapy. However, we lacked sufficient clinical data to carry out a further investigation at present.
There were several limitations to be addressed in this study. It was a retrospective study. Therefore, a prospective study with a larger cohort is needed in the future. In addition, the data we obtained from TCGA database were the mRNA information of IL-17A; its correlation with tumor-infiltrating IL-17A+ cells remained unclear. Also, we have not uncovered how IL-17A+ cells influenced the downstream cells. Interventional and functional studies are required to fully understand the underlying mechanisms.
In conclusion, this is the first study identified IL-17A+ cells as favorable factors for muscle-invasive bladder cancer patients in three independent cohorts. Furthermore, high infiltration of IL-17A+ cells into tumors is associated with an anti-cancer immune contexture and could predict therapeutic response to adjuvant chemotherapy.
Funding Statement
This study was funded by grants from National Natural Science Foundation of China (31770851, 81702496, 81702497, 81702805, 81772696, 81872082, 81902556, 81902563, 81902898, 81974393), National Key R&D Program of China (2017YFC0114303), Shanghai Municipal Natural Science Foundation (16ZR1406500, 17ZR1405100, 19ZR1431800), Guide Project of Science and Technology Commission of Shanghai Municipality (17411963100), Shanghai Sailing Program (18YF1404500, 19YF1407900, 19YF1427200), Shanghai Municipal Commission of Health and Family Planning Program (20174Y0042, 201840168, 20184Y0151), Fudan University Shanghai Cancer Center for Outstanding Youth Scholars Foundation (YJYQ201802) and Shanghai Cancer Research Charity Center. All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their excellent pathological technology help. The results published here are in part based upon data generated by The Cancer Genome Atlas managed by the NCI and NHGRI. Information about TCGA can be found at http://cancergenome.nih.gov.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Author contributions
Z. Wang, Q. Zhou, H. Zeng, and H. Zhang for acquisition of data, analysis, and interpretation of data, statistical analysis, and drafting of the manuscript; Z. Liu, Q. Huang, Y. Xiong, J. Wang, Y. Chang, Q. Bai, Y. Xia, Y. Wang, Y. Zhu, L. Xu and B. Dai for technical and material support; L. Liu, J. Guo and J. Xu for study concept and design, analysis, and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
References
- 1.Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström P-U, Choi W, Guo CC, Lotan Y, Kassouf W.. Bladder cancer. Lancet. 2016;388(10061):2796–8. doi: 10.1016/s0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 2.Compérat E, Larré S, Roupret M, Neuzillet Y, Pignot G, Quintens H, Houéde N, Roy C, Durand X, Varinot J, et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Archiv. 2015;466(5):589–594. doi: 10.1007/s00428-015-1739-2. [DOI] [PubMed] [Google Scholar]
- 3.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng A-C, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D, et al. ICUD-EAU international consultation on bladder cancer 2012: chemotherapy for urothelial carcinoma—neoadjuvant and adjuvant settings. Eur Urol. 2013;63(1):58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 5.de Mingo Pulido Á, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, Coussens LM, Ruffell B. TIM-3 regulates CD103+ dendritic cell function and response to chemotherapy in breast cancer. Cancer Cell. 2018. e66;33(1):60–74. doi: 10.1016/j.ccell.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CT, Pryer N, Daniel D, Hwang E, Rugo H, Coussens L, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krantz D, Hartana CA, Winerdal ME, Johansson M, Alamdari F, Jakubczyk T, Huge Y, Aljabery F, Palmqvist K, Zirakzadeh AA, et al. Neoadjuvant chemotherapy reinforces antitumour T cell response in urothelial urinary bladder cancer. Eur Urol. 2018. [DOI] [PubMed] [Google Scholar]
- 8.Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Xie H, Fu Q, Dai B, Ye D, Xu J. Identification and validation of stromal immunotype predict survival and benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Clin Cancer Res 2017;2018. clincanres. 2687. [DOI] [PubMed] [Google Scholar]
- 9.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 2012;33(7):343–349. doi: 10.1016/j.it.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Romagnoli PA, Sheridan BS, Pham Q-M, Lefrançois L, Khanna KM. IL-17A–producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc Natl Acad Sci USA. 2016;113(30):8502–8507. doi: 10.1073/pnas.1600713113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114(3):596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14(11):3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oosterlinck W, Lobel B, Jakse G, Malmström P-U, Stöckle M, Sternberg C. Guidelines on bladder cancer. Eur Urol. 2002;41(2):105–112. doi: 10.1016/S0302-2838(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Zhu Y, An H, Liu Y, Lin Z, Wang G, Xu J. Clinical significance of tumor-derived IL-1β and IL-18 in localized renal cell carcinoma: associations with recurrence and survival. Paper presented at: Urologic Oncology: Seminars and Original Investigations; 2015. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector T H 17 and regulatory T cells. Nature. 2006;441(7090):235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 20.Amicarella F, Muraro M, Hirt C, Cremonesi E, Padovan E, Mele V, Governa V, Han J, Huber X, Droeser RA, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. 2017;66(4):692–704. doi: 10.1136/gutjnl-2015-310016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fridman WH, Zitvogel L, Sautès–Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 22.Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol. 2017;18(6):612. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Q, Fu Q, Chang Y, Liu Z, Zhang J, Xu L, Zhu Y, Wang Y, Zhang W, Xu J. CD19+ tumor-infiltrating B-cells prime CD4+ T-cell immunity and predict platinum-based chemotherapy efficacy in muscle-invasive bladder cancer. Cancer Immunol Immunother. 2018;112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastenmüller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013;38(3):502–513. doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 26.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database hallmark gene set collection. Cell Syst. 2015;1(6):417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg JE, Hoffman-Censits J, Powles T, Van Der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and-17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi M, Kokubu F, Fujita J, S-K H, Hizawa N. Role of interleukin-17F in asthma. Inflammation Allergy-Drug Targets (Form Current Drug Targets-Inflammation Allergy). 2009;8:383–389. [DOI] [PubMed] [Google Scholar]
- 31.Dahal LN. The dichotomy of T helper 17 cells in cancer. Nat Rev Immunol. 2017;17(9):592. doi: 10.1038/nri.2017.93. [DOI] [PubMed] [Google Scholar]
- 32.Fridman WH, Pagès F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):nrc3245. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J-X, Song W, Chen Z-H, Wei J-H, Liao Y-J, Lei J, Hu M, Chen G-Z, Liao B, Lu J, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14(13):1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 34.Sternberg CN, Donat SM, Bellmunt J, Millikan RE, Stadler W, De Mulder P, Sherif A, von der Maase H, Tsukamoto T, Soloway MS, et al. Chemotherapy for bladder cancer: treatment guidelines for neoadjuvant chemotherapy, bladder preservation, adjuvant chemotherapy, and metastatic cancer. Urology. 2007;69(1):62–79. doi: 10.1016/j.urology.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 35.Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kashiwagi S, Tanaka H, Hirakawa K, Ohira M. A comparison of the local immune status between the primary and metastatic tumor in colorectal cancer: a retrospective study. BMC Cancer. 2018;18(1):371. doi: 10.1186/s12885-018-4276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


