Abstract
This narrative review focuses on the role of strength and balance activities throughout the lifecycle to improve physical capacity and reduce all-cause mortality. The evidence suggests strong associations in middle and older age, with poor balance, poor strength or poor physical function having strong associations with mortality. Currently in the UK, the proportions of adults (69% of men and 76% of women) not meeting the strength and balance guidelines (of 2 or more sessions/week) is concerning. This report identifies specific time points in the lifecycle where specific promotion of and engagement with strength and balance activities would be most beneficial for health: 18-24y to maximize bone and muscle mass gains, 40-50y to maintain strength and reduce that downward cycle, and over 65s to preserve balance and strength and maintain independence). This review also suggests specific transition points/events in life where there may be an increase in sedentary behaviour or loss of muscle function (pregnancy, menopause, onset of on diagnosis of disease, retirement, on becoming a carer and following hospitalization), where it would be useful to initiate additional strength and balance exercises to improve future health outcomes.
Keywords: Strength, Balance, Exercise, Lifecycle, Transition period
Introduction
Chronological age is a convenient predictor of health status, disease burden and physical ability, but there is considerable inter-individual variability, with some older people having very good health and others that show accelerated onset of weakness, disability and frailty[1]. We all age differently and many events can change our behaviours such that a life course approach to ageing makes sense. However, epidemiological research that enhances our understanding of the relative importance of different stages in the life course in relation to specific disease outcomes and health capital is relatively new[2]. Certain demographics, such as socio-economic conditions help shape health and disease risk, as can social inequalities[1]. However, regardless of age, lower physical capability (in strength, balance and walking) is consistently associated with higher mortality and a dose-response relationship is evident[3].
In the context of strength and balance abilities throughout the life course, there has been a large amount of research into old age with the growth in interest in preventing falls, frailty and sarcopenia. Low performance on simple physiological tests of hand grip strength, timed chair stands speeds, usual walking speed and standing balance has been associated with higher future mortality in both community-based cohorts and patient populations. This is well described in people over the age of 70 years old[4,5] and generally follows a linear dose-response relationship. A Finnish study has shown that among 75 and 80 year olds, a low value for handgrip strength or quadriceps strength is associated with an increased risk of death during the following four to five years[6].
However, old age is not the only time through the life course during which people might be at risk of reducing their overall physical activity and MBSBA levels. During other ‘transitional’ periods of life, for example when moving from one school to the next, going to college or university, getting married, becoming a parent, and at retirement there is the potential for change in physical activity behaviour[7]. In one review, five life change categories were found: a) change in employment status, b) change in residence, c) change in physical status, d) change in relationships, and e) change in family structure[8]. Generally speaking there is a decrease in physical activity participation after a change. Participation in general in physical activity and sports at early childhood and adolescence are important predictors of adulthood participation and inactivity tends to track from youth to adulthood[9]. However, other transition points in the life course may lead to distinct changes in behaviour or changes in health and function that mean that introducing strength and balance activities may help mitigate prior periods of inactivity and the trajectory of health, for example, at diagnosis of disease or following prolonged hospitalisation.
The aim of this narrative literature review is to report on how strength and balance ability varies across the life course, whether our engagement in strength and balance activities vary across the life course, and to explore whether there are particular ages or transition points where the undertaking or initiation of additional strength and balance activities are most important.
Materials and methods
This narrative literature review aims to describe and discuss the state of the science of a specific topic or theme from a theoretical and contextual point of view. Because there is little epidemiological research that enhances our understanding of the relative importance of different stages in the life course in relation to strength and balance, we elected to perform a scoping review of the literature. We conducted a search on NCBI PubMed, Google Scholar and reviewed Position Stands and International Reviews of Physical Activity Evidence to scope out any specific information on the importance of strength or balance abilities at different ages, or at different transition points agreed on in an initial meeting with the funders. We set a broad set of MeSH terms (Medical Subject Headings) including “muscle”, “bone’, “balance” AND “function” AND “adults”, alongside specific transition points in life. A decision was made to limit the review to adults and older adults and so the potential transition points where evidence was to be reviewed were: pregnancy, menopause, becoming a carer, diagnosis of disease, hospitalisation and retirement.
Results
How does muscle strength, bone strength and balance ability vary across the life course?
Generally, muscle strength, bone strength and balance ability increases in childhood and peaks in early adulthood, eventually followed by a decline (Figure 1). The rate of decline is mostly affected by modifiable lifestyle behaviours such as levels of physical activity and exercise, smoking, alcohol consumption and diet. However, other single ‘events’, such as accidents, prolonged immobility or pregnancy may have distinct short or longer-term effects on the trajectory (Figure 1).
Figure 1.

Strength and balance ability over the life course and potential ages or events that may change the trajectory of decline with ageing.
Muscle strength
Data from several cohort studies in the UK has shown that hand grip strength (as a proxy measure for overall muscle strength) has three overall changes across the lifespan, an increase to peak in early adult life, maintenance through to mid-life and a decline from mid-life onwards[10]. At all ages, men have significantly greater muscle mass than women[10,11]. During late adolescence/early adulthood muscle strength is independently associated with long-term risk of vascular disease and arrhythmia[12] and in peak bone mass[13] suggesting that strength in young adulthood is important. It is well established that ageing is associated with a decline in muscle mass from around the age of 30y[14], but notable differences are seen by the age of 50y[11]. In those over the age of 75y, the decline of muscle mass is around 2-4 % per year, but the loss of strength can be 2-5 times faster than that because of other ageing changes to muscle quality and neural factors[15].
The age-associated loss of strength is more pronounced with advancing age[16,17]. Within the UK, in the 1990’s, the Allied Dunbar National Fitness Survey measured handgrip and quadriceps (knee extension) strength and physical function tests in a representative sample of adults (588 men and 730 women) aged 50 and over[17]. The mean handgrip strength for those aged 80+ was 27% less than that for those aged 50-54 and women were typically 30% weaker than men. Over the age cohort, the oldest were typically 22-25% weaker than the youngest in their quadriceps strength and women were typically 20% weaker than men. The same gender difference in muscle mass is seen in muscle strength, although hormonal changes associated with the menopause can further exacerbate the decline for women[18]. Across the adult lifespan, there is a decline in strength of approximately 50% between the ages of 25 to 85 years[19]. If strength declines faster than the normal ageing loss, this leads to sarcopenia[20,21].
Obesity has a part to play in muscle strength and function as well. Obese individuals show reduced muscle strength in the lower limbs when normalized to body weight, and this leads to reduced performance in motor tasks involving muscle power such as initiating gait, raising from a chair, climbing stairs[22].
This loss of muscle strength is the primary limiting factor for functional independence[18], rather than aerobic physical activity. The decrease in muscle strength can play a detrimental role in physical function impairments, such as rising from a chair, walking speed, climbing stairs, and the capacity to maintain balance if we trip. As women have lower muscle strength than men, they fall below important thresholds of strength needed for independence earlier[17,24]. People need a quadriceps strength equivalent to 35%[25] of their body weight to be confident of getting up from a low chair without using their arms[17]. [Table 1] shows that among 50-74 year olds, 2% of men and 14% of women had knee extension strength less than this, rising to a quarter of women aged 70-74y.
Table 1.
Prevalence of risk of being unable to rise out of a low chair without difficulty according to age*.
| At risk being unable to rise out of a low chair | 50-69y | 70-74y |
|---|---|---|
| Men | 2% | 7% |
| Women | 14% | 25% |
Prediction based on measurements of isometric knee extension strength
In the functional tests, men aged 75+ had little difficulty in actually rising from a low chair without using their arms, but among women aged 75+ the proportion unable to rise from a low stool increased from 8% of those aged 75-79 to 42% of those aged 85+ years[17]. Explosive power (a measure of the speed with which a person can generate strength), shows a larger decline with age, where the oldest had a 33% reduction in power/weight ratio compared to the youngest cohort and women had power/weight ratios of about two thirds that of men of the same age[17]. Low levels of muscle strength and balance capability increase the risk of falling and sustaining a related injury and can lead to disability, and frailty[26,27], all of which have implications for the individual, their carers, and the health services that support them[28]. Strength training, even in advanced ages can preserve muscle function[29].
Bone Strength
Bone mineral density (BMD) measured by dual X-ray absorptiometry (DXA) is reported to account for 60% to 70% of the variation in bone strength[30]. Bone mineral density peaks at around the age of 25 years, at around the age of 30 the balance between bone formation and bone resorption is altered, so that resorption begins to exceed deposition, and by the age of 50 progressive losses of calcium and deterioration in the organic matrix of bone occur[31]. Bone mass peaks between the ages of 20 and 40 years, with men achieving a greater peak bone mass[13]. After the age of 40, bone mass declines at a rate of 0.5-1% per year with an accelerated period of loss in women for 5-10 years after the menopause[31]. This accelerated bone loss occurs because of the decrease in the levels of the sex hormone oestrogen, which protects the female skeleton from excessive bone resorption[32]. In total, women lose about 25-30% of the cortical bone and 35-50% of the trabecular bone over a lifetime; men lose at about two-thirds this rate[33]. Most bone loss is cortical, not trabecular, and occurs after the age of 65 years[34].
Osteoporosis is a condition resulting in an increased risk of skeletal fractures due to a reduction in the density of bone tissue[35]. Osteoporosis is increasingly prevalent in the UK, and it is estimated that over half of women, and one-fifth of men aged 50, will sustain a fragility fracture[36]. Prospective studies have documented that the lifetime risk of an osteoporotic-related fracture increases 1.5 to 3 times with each standard deviation (SD) decrease in bone density[37]. The two ultimate determinants of fracture are bone strength and propensity to trauma. Although measurements of BMD contribute to the prediction of fracture risk they cannot identify individuals who will have a fracture[38] as many fractures, particularly in older populations, are as a result of a fall[26]. Bone strength depends not only upon bone mass but also upon a variety of qualitative aspects of bone structure[39]. These include its architecture, the amount of fatigue damage it has sustained, and changes in its bulk material properties, indices that are collectively subsumed into the term ‘bone quality’[39].
Bone requires a variety of stimuli to increase production, not just strengthening activities, and engagement in moderate to vigorous activity has strong correlations, particularly in adolescence in the building of peak bone mass[40]. It has also long been recognised that long periods of enforced inactivity, reduced weight bearing and muscle loading, such as bed rest, change the bone turnover and mineral homeostasis[41]. Recent studies on sedentary behaviour suggest that TV/screen time and prolonged sitting is detrimental to growth of bone across the age range, likely as a result of a lack of muscle activation and unloading of bone structure[42,43]. Strength training and multimodal exercise including balance, improves BMD if at sufficient exercise intensity and duration[44].
Balance
Good balance and mobility are essential to the successful performance of most activities of daily living as well as being able to take part in recreational activity. Balance is a complex automatic integration of several body systems. The task being undertaken and the environment in which it is taking place both affect an individual’s ability to control balance, by altering the biomechanical and information processing needs[45]. With age and inactivity these conscious processes may not integrate as well or as quickly as they did when a person was younger[46]. Static balance testing using the modified Romberg Test (one leg stand) shows a decline from the age of 40 onwards and once below a threshold level of 20 seconds, there is a threefold increase in the odds of falling[47].
Preserving balance and postural control during walking requires the maintenance of the centre of mass within the base of support[48,49]. When gait stability is challenged, common gait adaptations associated with maintaining stability are an increase in step width, decrease in step length, or even a change in step frequency[50,51]. Age is associated with a loss of neural function, slower complex reaction times and slower central processing[52]. Other age related effects also affect balance such as the sensitivity of skin receptors, poor co-ordination and eyesight, pain and instability in joints, and vestibular dysfunction[46]. Balance capability is a key risk factor for maintenance of mobility and prevention of disability[53], indeed those with compromised balance are often weaker in key independence muscles as they avoid activity[46]. Poor balance is also a marker or predictor for many other outcomes, for example, poor one leg stance time predicts a higher rate of cognitive decline[54] and poor standing balance predicts higher all-cause mortality[4]. Loss of the ability to balance is associated with a higher risk of falling and subsequent injury, which in turn can lead to loss of independence, illness, and premature mortality[26,55].
Again, obesity can affect an individual’s balance. Obese individuals display great excursions in the medio-lateral direction, longer anticipatory postural adjustments and a reduced centre of pressure in the antero-posterior direction[56] compared to non-obese individuals, which increases the risk of a fall[57].
Balance enhancing activities have been shown to be a critical part of an effective falls prevention programme[17,26,27]. Indeed, strength and balance training has an important role to play in fear of falling, which often limits activity and social engagement[58] and balance can be improved with a variety of balance challenging activities well into old age[55]. These activities include structured exercises to improve balance as well as balance challenging physical activities such as Tai Chi.
Physical Function as a result of poor strength and balance
In older people, using the Short Physical Performance Battery (SPPB) as a marker for physical function, there is a linear relationship with mortality[59] with a threshold SPPB score of less than 10 predictive of all-cause mortality. Studies in those under the age of 70 are less frequent but perhaps suggest that the association is weaker in younger adults[4] and that there may be a threshold effect, with only those below certain levels of ability experiencing an increased risk of mortality rather than the linear relationship seen in older people[60-62]. Younger adults are likely to have lower levels of co-morbidity than older adults, an important potential confounding factor in physical capability-mortality associations. Recently a UK based study looked at participants in a large cohort study (EPIC-Norfolk) utilizing data from 8477 men and women aged 48-92 years who had physical capacity data recorded[3]. Participants in lower sex-specific physical capability categories were more likely to die in the follow up period (approx. 8 years) than those in the highest categories, irrespective of the physical capability measure used. Keevil’s work suggests that the association of physical function with mortality is visible as young as mid life[3] and concurs with others work on walking speed and mortality in 50-73 year olds[63] and grip strength and mortality in 35-74 year olds[64]. However, there is still little work in those under the age of 40 years.
Lui et al. have shown in their meta-analysis that resistance exercise and multimodal exercise improves strength, balance and physical function in those with reduced physical capacity[65]. Progressive resistance strength exercise is effective in improving muscle strength of the lower extremity and static standing balance. Multimodal exercise was almost exclusively strength and balance training and was effective in improving strength of the lower extremity, dynamic standing balance, gait speed and chair stand, as well as reducing falls[65].
Are there particular events or transitions where strength and balance activity engagement is important?
As chronological age is not the only factor affecting maintenance of good strength and balance, a number of transition points or life events have been considered. These include: during pregnancy during the menopause, on diagnosis or following disease ‘events’, at retirement, becoming a carer, and following hospitalization.
During pregnancy
There is nearly always a decline in physical activity during pregnancy[66]. Women during pregnancy are more predisposed to falls than those not pregnant[67,68]. Balance and postural control change during pregnancy, as a consequence of increased pelvic width, anterior pelvic tilt and changes in centre of mass due to baby weight gain[69]. As pregnancy progresses there are changes in step width, lateral trunk lean, and the medio-lateral deviations in centre of pressure[69]. Pregnant women demonstrate greater hip flexion, more extended knees and lower ankle plantar flexion, which could explain the development of musculoskeletal discomfort[70]. In particular, the musculoskeletal system suffers several soft tissue, joint and postural adaptations, resulting in discomfort and pain of the lower back, pelvis, hips, knees and feet. Guidelines on physical activity or exercise and pregnancy encourage pregnant women to continue or adopt an active lifestyle during and following pregnancy[71]. Strength training or balance training only studies are rare, but where they exist there are benefits to physical function and rare adverse events are musculoskeletal, not any effects on the pregnancy per se[72,73]. Studies and reviews demonstrate the importance of antenatal exercise programmes that focus on core strength, balance and pelvic stability that may improve mobility, reduce lower back pain and potentially mitigate the risk of falling during walking[69,70].
During Menopause
Women undergoing menopause face many changes that may lead to loss of health-related fitness, especially if sedentary. A good body of evidence supports that the decline in muscle mass follows the decrease in oestrogen that occurs during the menopause[74]. This decrease in oestrogen contributes to the loss of bone mineral density, reduction in muscle mass and quality, the redistribution of subcutaneous fat to the visceral area, the increased risk of cardiovascular disease and the decrease in quality of life[74]. Quality of life in women entering menopause is associated with upper body strength and static balance abilities[75]. However, a number of studies have shown the positive effects of progressive strength training on muscle strength, waist circumference, blood glucose concentration, resting heart rate and blood pressure[76,77]. Multicomponent exercise, including strength, balance and aerobic activities can increase or prevent muscle and bone mass loss during the menopause[78]. Lower volume resistance training confers benefits to strength, but higher volume (more sets/reps) resistance training additionally confers benefits to adiposity, lipid metabolism and inflammation[77]. Balance training has also been shown to improve balance, tactile sensation, ankle flexibility and muscle strength and on a longer term follow up, a more active lifestyle[79]. These studies, amongst others, demonstrate the importance of strength and balance activities to help reduce the loss of strength and balance skills that occur over the menopause[80].
On diagnosis of disease or following disease ‘events’
Studies have shown varying changes of behaviour depending on the disease diagnosis, whether there was a significant hospitalization and of course, any and subsequent treatment. For example, studies in women with cancer revealed no change[81], but colon cancer survivors reported an increase in physical activity participation over a 2-year period of follow-up[82]. However, stroke patients, even up to 3 years post stroke, spend 66-94% of their day sitting[83,84], increasing their risk of a future stroke. As a result of a diagnosis of osteoporosis, nearly 7 out of 10 patients reduced their activity, mostly as a result of fear of falls and fractures but also of pain perceived as a result of their osteoporosis and half have given up any sport or exercise they used to do[85,86]. The avoidance of activity, and therefore the consequent decline in strength and balance that will result, will likely be detrimental to these patient’s health and future fall and fracture risk.
On Retirement
Slingerland et al. (2007) examined the effect of retirement on changes in physical activity in the GLOBEStudy (Netherlands) and found that physical activity associated with work related transportation greatly decreased and that the reduction was not compensated by increased sports participation or other leisure-time physical activity[87]. Other studies, in the US, have found that physical activity decreased with retirement from a physically demanding job, but increased with retirement from a sedentary job[88]. Another found that greater social disadvantage led to more sedentary behaviour in retirement in the UK[89]. However, physical activity participation after retirement may be different according to culture, as Touvier et al. (2010) found the opposite in French retirees, amongst whom leisure-time physical activity increased by about 2 h/wk, mainly related to an increase in activities of moderate intensity, such as walking[90]. However, most studies suggest that on retirement people sit more, and sedentary behaviour is strongly associated with poor muscle quality and reduced strength[91]. Retirement commonly occurs around the same time as studies have shown an increase in loss of muscle and bone, so this is an important transition point to instigate strength and balance activities.
Becoming a carer
Evidence regarding physically demanding activities that place physical strain on professional caregivers is widely available. Transfers, lifts and patient repositioning are associated with musculoskeletal injuries in professional caregivers (e.g. nurses; rehabilitation personnel)[92,93]. However, in 2000, it is estimated that there were around 5.8 million carers in England, of whom between 3.4 and 4 million were providing care to those aged 65 and over[94]. A recent review considering the most physically demanding caregiving activities for informal caregivers found high levels of physical strain and musculoskeletal discomfort related to transferring and mobilising patients and helping during self-care[95]. Indeed, 30% of carer had accidents during their caregiving and the majority were falls[96]. The age and health of the caregiver will obviously affect their ability to care for someone, as often caring involves lifting and transferring, or supporting people with poor balance. Indeed, often the informal carers of frailer older people are older themselves and have their own medical conditions, which limit their physical activity and mean they are deconditioned[97]. Indeed, a quarter of those interviewed had injured themselves whilst lifting and handling their dependents[97]. One in five had been unable to continue caring as a result of the injuries they sustained. However, not having enough strength, or good enough balance, to safely support or transfer their dependent meant that a significant number of patients had also received injuries while being moved by their caregiver[97]. Only half had ever received training in manual handling. Having optimal strength and balance for a carer therefore seems to have importance not just for the health of the carer but also of the patient they are caring for[95].
Following hospitalization
Prolonged bed rest, for example during hospitalization, can lead to losses of muscle strength at all ages but its effects are particularly noticeable in frailer older adults. It is known that a few weeks of immobilization or disuse has a detrimental effect on muscle mass, muscle strength and power[98]. The decrease in muscle strength is greatest, 3-4% per day, during the first week of immobilization and up to a 40% decrease in isokinetic muscle strength has been seen after 3 weeks of immobilization[98]. Even in healthy older adults, 10 days of bed rest leads to a 13% decline in quadriceps strength[99]. Hospital admission in the past 12 months is the single most predictive risk for functional decline in community dwelling older people[100]. Thirty to 60% of older patients experience functional decline after hospitalisation, resulting in a decline in health-related quality of life and autonomy[101]. Frailer older people (those who require a walker), those who report unsteadiness at hospital admission, and those with cognitive impairment are significantly more likely to suffer functional decline whilst in hospital[102,103]. This hospitalisation-associated decline in function is associated with increased risk of readmission, nursing home placement and mortality[104-106]. Patients in hospital are particularly sedentary, on average, patients in a rehabilitation ward were in an upright position for only 70 (± 50) min. per day, with 70% of this time spent in standing or walking epochs of less than 5 minutes[107]. So the first opportunity to get the message across is in the hospital setting and with patients in rehabilitation. Sadly, one year after hospitalisation, older people, irrespective of health changes, have had further significant losses of function and reduced physical activity[108]. A failure to regain function 3 months after acute hospitalisation is a strong predictor of nursing home admission within a year[109].
Barriers to increasing physical activity in older people post-acute hospitalization include a lack of energy, health problems that they perceive as limiting movement and that few had been advised to be active so it was not important[110]. Older patients views after hospitalization report fatigue, apathy, unsteadiness while standing, and fear of falling, all perceived as effects of the hospital stay itself[111]. This highlights consistent messaging about activity and regular mobilization (through regular sit to stands and walking to help maintain levels of strength and balance) in this population are important. Functional exercise involving transfer training (sit to stands) and balance activities in a subacute hospital setting showed a reduction in falls within the hospital setting[112].
How does engagement in muscle strength, bone strength and balance activities vary across the life course?
Historically, health surveys have surveyed the population for activities that are moderate or vigorous intensity in order to gain a record of those meeting the physical activity guidelines. It is only recently, since the UK’s Chief Medical Officers updated the UK Physical Activity Guidelines[113], that surveys attempted to distinguish whether the newer guidelines on strength (for adults and older adults)[114,115], balance and co-ordination (for older adults)[115], and sedentary behaviour (adults and older adults) were being met[116].
Strength activities
Activities that improve strength are recommended twice a week for adults and older adults, with no bout length recommendation[113]. Across adulthood, 31% of men and 24% of women self-reported performing strength activities at least twice a week, approximately half that of the figures reported for aerobic physical activity[115].
The proportions that self-reported meeting the strength guidelines in Scotland[115] are similar to those reported in England (34% of men and 24% of women)[114] but are higher than Northern Ireland (25% of men and 14% of women)[117]. In the US, the National Health Interview Survey asks about how often they do leisure time activities designed to strengthen their muscles, with 28% of men and 20% of women reporting to engage in balance and co-ordination activities 2 or more times a week[118]. Older age groups reported lower levels of engagement (9% of men and 4% of women over 75) and more men met the guidelines than women in all age groups, with the largest difference amongst 16-24 year olds (55% men compared with 40% women)[115] (Figure 2). Participation in muscle strengthening activities differed by gender, with men more likely to go to a gym for strengthening activities (18%) and swimming (as aquatic exercise was not differentiated from swimming lengths) was the most likely strengthening activity for women (15%), followed closely by ‘exercises’ (11%)[115].
Figure 2.
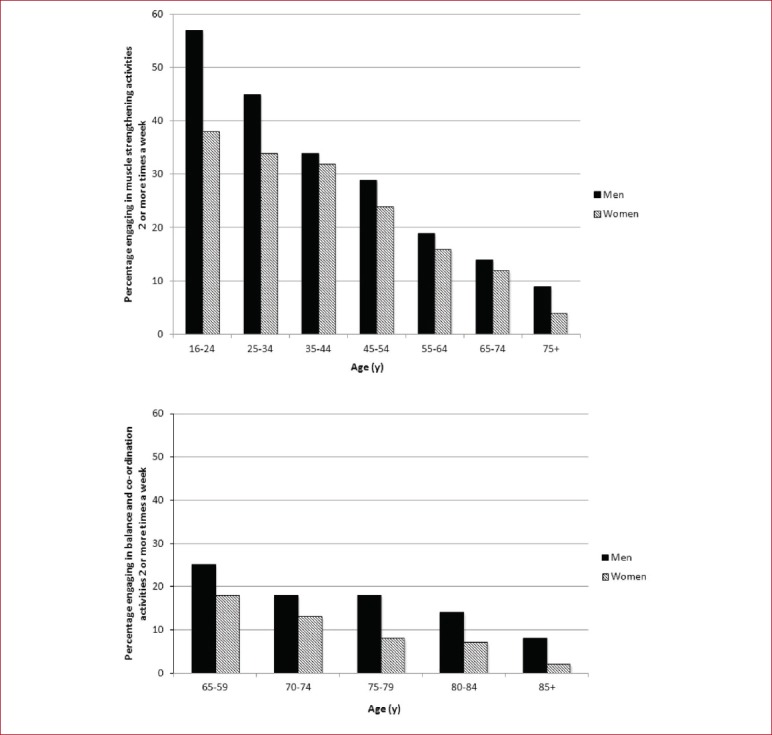
Percentage of men and women meeting the strength and balance/co-ordination guidelines (≥2 times per week).
Balance and coordination activities
Activities that improve balance and co-ordination are recommended twice a week for older adults (≥65 years) at risk of falls. Nineteen percent of older men and 12% of older women met the balance & co-ordination guidelines, with a decline in engagement across the age range, to only 8% of men and 2% of women over the age of 85[115] (Figure 2). Older men tended to engage in golf (11%) and women in aerobics/Gymnastics (including keep fit and dance for fitness, 6%)[115].
Discussion
There is very little research on whether specific points on the life curve are important to initiate strength and balance activities to preserve or improve muscle and bone strength and balance. However, there are time points in the life curve where there are specific changes to strength and balance that would benefit from initiation of strength and balance activities to improve health and function and reduce the risk of falls and fractures. We offer the following recommendations for people over the age of 18 years, pertaining to periods at which muscle and bone strengthening and balance activities (MBSBA) are most important, and which transition points or events in adult life may also be important to consider initiating strength and balance activities. The data presented here have originated from a mixture of cross-sectional and RCT studies, which in turn has an effect on the predictive quality of the evidence presented in this paper. Not all people over the age of 65 are the same however, and practical resources for interpreting the strength and balance guidelines for the ‘Actives’, ‘Those in transition’ and ‘Frailer older people’ have been produced[119].
Recommendations for age/transition points when MBSBA are most important during adulthood:
18-24 year olds, as bone and muscle mass peak in early adulthood and strengthening activities will help maximize this. This is particularly important in women who are less likely to be meeting the strengthening activity guidelines, have lower levels of muscle and bone strength than men, and this building of peak bone mass will help reduce the chances of osteoporosis and dependence later in life.
40-50 year olds, as maintenance of muscle and bone strength at this stage of life will help reduce the downward cycle of muscle and bone loss, preventing future frailty and risk of osteoporosis, falls and fractures. Although the physical activity guidelines only introduce balance challenging activities for the over 65s at risk of falls, balance is deteriorating by this stage and poor balance predicts a reduction in physical activity and consequent loss of function and increased falls risk.
Over 65 year olds, as preservation of balance and of muscle and bone strength is key to maintaining independence, preventing the risk of falls, fractures, dependence and frailty.
Recommendations for transition points/events where MBSBA are important to instigate:
Pregnancy, to improve mobility, reduce musculoskeletal pain and reduce falls risk.
Menopause, to offset the future risk of osteoporosis, falls and fractures, dependence and cardiovascular risk.
On diagnosis of disease or after a disease event, particularly in those diagnosed with osteoporosis or following a stroke, to reduce muscle and bone loss associated with inactivity.
Retirement, when strength and balance activities would help preserve function and health associated with a reduction in activity often seen at retirement.
Becoming a carer, strength and balance activities will help reduce care related musculoskeletal injuries, reduce accidents in dependents and allow continued caring over time.
Following hospitalisation, to regain lost strength, bone density, balance ability and function seen during hospital stays, particularly in older people.
Conclusion
Evidence suggests that it is important to initiate strength and balance activities to improve physical capacity and reduce all-cause mortality. The proportions of adults and older adults not meeting the strength and balance guidelines in the UK is concerning and specific age points in which specific promotion of and engagement with strength and balance activities are those aged 18-24, those aged 40-50 and those over the age of 65. Although the associations are very clear in middle and older age, poor balance, poor strength or poor physical function have strong associations with mortality and therefore, irrespective of age, poor strength and balance performance is a sign to start strength and balance exercise programmes or activities. This review suggests specific transition points in life that evidence suggests that having better strength and balance would improve future health outcomes, including pregnancy, menopause, onset of on diagnosis of disease, retirement, on becoming a carer and following hospitalization.
Acknowledgements
This research was funded by a grant from The Centre for Ageing Better. DS and AM wrote and reviewed the manuscript. DS is a Director of a not for profit training company, Later Life Training, which provides training to health and leisure professionals to deliver strength and balance training.
References
- 1.World Health Organisation. The implications for training of embracing A Life Course Approach to Health. Geneva. 2000 [Google Scholar]
- 2.World Health Organization and ExpandNET. World Health Organization, ExpandNet Nine Steps for Developing a Scaling-up Strategy. Geneva. 2010 [Google Scholar]
- 3.Keevil VL, Luben R, Hayat S, Sayer AA, Wareham NJ, Khaw KT. Physical capability predicts mortality in late mid-life as well as in old age:Findings from a large British cohort study. Arch Gerontol Geriatr. 2018;74:77–82. doi: 10.1016/j.archger.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper R, Kuh D, Hardy R, et al. Objectively measured physical capability levels and mortality:systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75-84-year-old people. Age Ageing. 1995;24(6):468–73. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- 7.Corder K, Ogilvie D, van Sluijs EM. Invited commentary:Physical activity over the life course--whose behavior changes, when, and why? Am J Epidemiol. 2009;170(9):1078–81. doi: 10.1093/aje/kwp273. discussion 82-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allender S, Hutchinson L, Foster C. Life-change events and participation in physical activity:a systematic review. Health Promot Int. 2008;23(2):160–72. doi: 10.1093/heapro/dan012. [DOI] [PubMed] [Google Scholar]
- 9.Hirvensalo M, Lintunen T. Life-course perspective for physical activity and sports participation. European Review of Aging and Physical Activity. 2011;8(1):13–22. [Google Scholar]
- 10.Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course:normative data from twelve British studies. PLoS One. 2014;9(12):e113637. doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89(1):81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 12.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundstrom J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men:cohort study. BMJ. 2015;351:h4543. doi: 10.1136/bmj.h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 14.Silva AM, Shen W, Heo M, et al. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22(1):76–82. doi: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength;a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994;23(5):371–7. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 17.Skelton D, Dinan S. Exercise for falls management:Rationale for an exercise programme aimed at reducing postural instability. Physiotherapy Theory &Practice. 1999;15:105–20. [Google Scholar]
- 18.Montero-Fernandez N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. 2013;49(1):131–43. [PubMed] [Google Scholar]
- 19.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility:an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95(5):1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 20.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137(4):231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 21.Shaw SC, Dennison EM, Cooper C. Epidemiology of Sarcopenia:Determinants Throughout the Lifecourse. Calcif Tissue Int. 2017;101(3):229–47. doi: 10.1007/s00223-017-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capodaglio P, Vismara L, Menegoni F, Baccalaro G, Galli M, Grugni G. Strength characterization of knee flexor and extensor muscles in Prader-Willi and obese patients. BMC Musculoskelet Disord. 2009;10:47. doi: 10.1186/1471-2474-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper R, Hardy R, Bann D, et al. Body mass index from age 15 years onwards and muscle mass, strength, and quality in early old age:findings from the MRC National Survey of Health and Development. J Gerontol A Biol Sci Med Sci. 2014;69(10):1253–9. doi: 10.1093/gerona/glu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young A, Skelton DA. Applied physiology of strength and power in old age. Int J Sports Med. 1994;15(3):149–51. doi: 10.1055/s-2007-1021037. [DOI] [PubMed] [Google Scholar]
- 25.Levy DI, Young A, Skelton DA, Yeo A. Strength, power and functional ability. In: Passeri M, editor. Geriatrics '94 International Association of Gerontology (I.A.G.) European Region Clinical Section Congress. Rome, Italy: CIC Edizioni Internazionali; 1994. pp. 85–93. [Google Scholar]
- 26.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherrington C, Tiedemann A, Fairhall N, Close JC, Lord SR. Exercise to prevent falls in older adults:an updated meta-analysis and best practice recommendations. N S W Public Health Bull. 2011;22(3-4):78–83. doi: 10.1071/NB10056. [DOI] [PubMed] [Google Scholar]
- 28.Public Health England. Falls and fractures:consensus statement and resources pack. London. 2017 [Google Scholar]
- 29.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3:CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13–8. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 31.Morgenthal AP, Shephard RJ. Physiological aspects of ageing. In: Jones CJ, Rose D, editors. Physical activity instruction of older adults. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 32.Eastell R, Riggs BL. Diagnostic evaluation of osteoporosis. Endocrinol Metab Clin North Am. 1988;17(3):547–71. [PubMed] [Google Scholar]
- 33.Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton LJ., 3rd Differential changes in bone mineral density of the appendicular and axial skeleton with aging:relationship to spinal osteoporosis. J Clin Invest. 1981;67(2):328–35. doi: 10.1172/JCI110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women:a cross-sectional study. Lancet. 2010;375(9727):1729–36. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 35.Centre for Disease Control. Consensus development conference:prophylaxis and treatment of osteoporosis. Osteoporos Int. 1991;1(2):114–7. [PubMed] [Google Scholar]
- 36.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–22. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 37.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341(8837):72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 38.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper C. The epidemiology of fragility fractures:is there a role for bone quality? Calcif Tissue Int. 1993;53(Suppl 1):S23–6. doi: 10.1007/BF01673397. [DOI] [PubMed] [Google Scholar]
- 40.Bielemann RM, Martinez-Mesa J, Gigante DP. Physical activity during life course and bone mass:a systematic review of methods and findings from cohort studies with young adults. BMC Musculoskelet Disord. 2013;14:77. doi: 10.1186/1471-2474-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog:a review. J Musculoskelet Neuronal Interact. 2007;7(1):33–47. [PubMed] [Google Scholar]
- 42.Chastin SF, Mandrichenko O, Helbostadt JL, Skelton DA. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone. 2014;64:254–62. doi: 10.1016/j.bone.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Gracia-Marco L, Rey-Lopez JP, Santaliestra-Pasias AM, et al. Sedentary behaviours and its association with bone mass in adolescents:the HELENA Cross-Sectional Study. BMC Public Health. 2012;12:971. doi: 10.1186/1471-2458-12-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333. doi: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Huxham FE, Goldie PA, Patla AE. Theoretical considerations in balance assessment. Aust J Physiother. 2001;47(2):89–100. doi: 10.1016/s0004-9514(14)60300-7. [DOI] [PubMed] [Google Scholar]
- 46.Skelton DA. Effects of physical activity on postural stability. Age Ageing. 2001;30(Suppl 4):33–9. doi: 10.1093/ageing/30.suppl_4.33. [DOI] [PubMed] [Google Scholar]
- 47.Agrawal Y, Carey JP, Hoffman HJ, Sklare DA, Schubert MC. The modified Romberg Balance Test:normative data in U.S. adults. Otol Neurotol. 2011;32(8):1309–11. doi: 10.1097/MAO.0b013e31822e5bee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38(1):1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 49.Winter DA. Human balance and posture control during standing and walking. Gait &Posture. 1995;3:193–214. [Google Scholar]
- 50.Hak L, Houdijk H, Steenbrink F, et al. Speeding up or slowing down?:Gait adaptations to preserve gait stability in response to balance perturbations. Gait Posture. 2012;36(2):260–4. doi: 10.1016/j.gaitpost.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Hak L, Houdijk H, Steenbrink F, et al. Stepping strategies for regulating gait adaptability and stability. J Biomech. 2013;46(5):905–11. doi: 10.1016/j.jbiomech.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt RA, Lee TD. Motor control and learning:a behavioural emphasis. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- 53.Judge JO. Balance training to maintain mobility and prevent disability. Am J Prev Med. 2003;25(3):150–6. doi: 10.1016/s0749-3797(03)00178-8. [DOI] [PubMed] [Google Scholar]
- 54.Rolland Y, Abellan van Kan G, Nourhashemi F, et al. An abnormal «one-leg balance»test predicts cognitive decline during Alzheimer's disease. J Alzheimers Dis. 2009;16(3):525–31. doi: 10.3233/JAD-2009-0987. [DOI] [PubMed] [Google Scholar]
- 55.Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;11:CD004963. doi: 10.1002/14651858.CD004963.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cau N, Cimolin V, Galli M, et al. Center of pressure displacements during gait initiation in individuals with obesity. J Neuroeng Rehabil. 2014;11:82. doi: 10.1186/1743-0003-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menegoni F, Galli M, Tacchini E, Vismara L, Cavigioli M, Capodaglio P. Gender-specific effect of obesity on balance. Obesity (Silver Spring) 2009;17(10):1951–6. doi: 10.1038/oby.2009.82. [DOI] [PubMed] [Google Scholar]
- 58.Kendrick D, Kumar A, Carpenter H, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev. 2014;11:CD009848. doi: 10.1002/14651858.CD009848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality:systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper R, Strand BH, Hardy R, Patel KV, Kuh D. Physical capability in mid-life and survival over 13 years of follow-up:British birth cohort study. BMJ. 2014;348:g2219. doi: 10.1136/bmj.g2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katzmarzyk PT, Craig CL. Musculoskeletal fitness and risk of mortality. Med Sci Sports Exerc. 2002;34(5):740–4. doi: 10.1097/00005768-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death:cohort study of one million participants. BMJ. 2012;345:e7279. doi: 10.1136/bmj.e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elbaz A, Sabia S, Brunner E, et al. Association of walking speed in late midlife with mortality:results from the Whitehall II cohort study. Age (Dordr) 2013;35(5):943–52. doi: 10.1007/s11357-012-9387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120(4):337–42. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 65.Liu CJ, Chang WP, Araujo de Carvalho I, Savage KEL, Radford LW, Amuthavalli Thiyagarajan J. Effects of physical exercise in older adults with reduced physical capacity:meta-analysis of resistance exercise and multimodal exercise. Int J Rehabil Res. 2017;40(4):303–14. doi: 10.1097/MRR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 66.Owe KM, Nystad W, Skjaerven R, Stigum H, Bo K. Exercise during pregnancy and the gestational age distribution:a cohort study. Med Sci Sports Exerc. 2012;44(6):1067–74. doi: 10.1249/MSS.0b013e3182442fc9. [DOI] [PubMed] [Google Scholar]
- 67.Inanir A, Cakmak B, Hisim Y, Demirturk F. Evaluation of postural equilibrium and fall risk during pregnancy. Gait Posture. 2014;39(4):1122–5. doi: 10.1016/j.gaitpost.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 68.McCrory JL, Chambers AJ, Daftary A, Redfern MS. Dynamic postural stability during advancing pregnancy. J Biomech. 2010;43(12):2434–9. doi: 10.1016/j.jbiomech.2009.09.058. [DOI] [PubMed] [Google Scholar]
- 69.Krkeljas Z. Changes in gait and posture as factors of dynamic stability during walking in pregnancy. Hum Mov Sci. 2017 Dec 15; doi: 10.1016/j.humov.2017.12.011. pii:S0167-9457(17)30750-9. [DOI] [PubMed] [Google Scholar]
- 70.Ribeiro AP, Joao SM, Sacco IC. Static and dynamic biomechanical adaptations of the lower limbs and gait pattern changes during pregnancy. Womens Health (Lond) 2013;9(1):99–108. doi: 10.2217/whe.12.59. [DOI] [PubMed] [Google Scholar]
- 71.ACOG Committee. Opinion No. 650:Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126(6):e135–42. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 72.Babbar S, Shyken J. Yoga in Pregnancy. Clin Obstet Gynecol. 2016;59(3):600–12. doi: 10.1097/GRF.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 73.Barakat R, Perales M. Resistance Exercise in Pregnancy and Outcome. Clin Obstet Gynecol. 2016;59(3):591–9. doi: 10.1097/GRF.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 74.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9(4):186–97. [PubMed] [Google Scholar]
- 75.Moratalla-Cecilia N, Soriano-Maldonado A, Ruiz-Cabello P, et al. Association of physical fitness with health-related quality of life in early postmenopause. Qual Life Res. 2016;25(10):2675–81. doi: 10.1007/s11136-016-1294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw BS, Gouveia M, McIntyre S, Shaw I. Anthropometric and cardiovascular responses to hypertrophic resistance training in postmenopausal women. Menopause. 2016;23(11):1176–81. doi: 10.1097/GME.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 77.Nunes PR, Barcelos LC, Oliveira AA, et al. Effect of resistance training on muscular strength and indicators of abdominal adiposity, metabolic risk, and inflammation in postmenopausal women:controlled and randomized clinical trial of efficacy of training volume. Age (Dordr) 2016;38(2):40. doi: 10.1007/s11357-016-9901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marin-Cascales E, Alcaraz PE, Ramos-Campo DJ, Rubio-Arias JA. Effects of multicomponent training on lean and bone mass in postmenopausal and older women:a systematic review. Menopause. 2017 Aug 14; doi: 10.1097/GME.0000000000000975. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 79.Fu S, Choy NL, Nitz J. Controlling balance decline across the menopause using a balance-strategy training program:a randomized, controlled trial. Climacteric. 2009;12(2):165–76. doi: 10.1080/13697130802506614. [DOI] [PubMed] [Google Scholar]
- 80.Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women:a systematic review of randomised controlled trials. Sports Med. 2004;34(11):753–78. doi: 10.2165/00007256-200434110-00004. [DOI] [PubMed] [Google Scholar]
- 81.Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer:trends and effects on mood and quality of life. Psychooncology. 2002;11(5):389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 82.Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev. 2004;13(6):1022–31. [PubMed] [Google Scholar]
- 83.Kunkel D, Fitton C, Burnett M, Ashburn A. Physical inactivity post-stroke:a 3-year longitudinal study. Disabil Rehabil. 2015;37(4):304–10. doi: 10.3109/09638288.2014.918190. [DOI] [PubMed] [Google Scholar]
- 84.English C, Manns PJ, Tucak C, Bernhardt J. Physical activity and sedentary behaviors in people with stroke living in the community:a systematic review. Phys Ther. 2014;94(2):185–96. doi: 10.2522/ptj.20130175. [DOI] [PubMed] [Google Scholar]
- 85.Lewczuk E, Bialoszewski D. The level of physical activity in patients with osteoporosis in relation to the risk and prevention of falls. Ortop Traumatol Rehabil. 2006;8(4):412–21. [PubMed] [Google Scholar]
- 86.National Osteoporosis Society. Life with Osteoporosis:the untold story. Bath. 2014 [Google Scholar]
- 87.Slingerland AS, van Lenthe FJ, Jukema JW, et al. Aging, retirement, and changes in physical activity:prospective cohort findings from the GLOBE study. Am J Epidemiol. 2007;165(12):1356–63. doi: 10.1093/aje/kwm053. [DOI] [PubMed] [Google Scholar]
- 88.Chung S, Domino ME, Stearns SC, Popkin BM. Retirement and physical activity:analyses by occupation and wealth. Am J Prev Med. 2009;36(5):422–8. doi: 10.1016/j.amepre.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 89.Shaw RJ, Cukic I, Deary IJ, et al. Relationships between socioeconomic position and objectively measured sedentary behaviour in older adults in three prospective cohorts. BMJ Open. 2017;7:e016436. doi: 10.1136/bmjopen-2017-016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Touvier M, Bertrais S, Charreire H, Vergnaud AC, Hercberg S, Oppert JM. Changes in leisure-time physical activity and sedentary behaviour at retirement:a prospective study in middle-aged French subjects. Int J Behav Nutr Phys Act. 2010;7:14. doi: 10.1186/1479-5868-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chastin SF, Ferriolli E, Stephens NA, Fearon KC, Greig C. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing. 2012;41(1):111–4. doi: 10.1093/ageing/afr075. [DOI] [PubMed] [Google Scholar]
- 92.Hui L, Ng GY, Yeung SS, Hui-Chan CW. Evaluation of physiological work demands and low back neuromuscular fatigue on nurses working in geriatric wards. Appl Ergon. 2001;32(5):479–83. doi: 10.1016/s0003-6870(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 93.Campo M, Weiser S, Koenig KL, Nordin M. Work-related musculoskeletal disorders in physical therapists:a prospective cohort study with 1-year follow-up. Phys Ther. 2008;88(5):608–19. doi: 10.2522/ptj.20070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beesley L. Informal Care in England:Wanless Social Care Review. London. 2006 [Google Scholar]
- 95.Darragh AR, Sommerich CM, Lavender SA, Tanner KJ, Vogel K, Campo M. Musculoskeletal Discomfort, Physical Demand, and Caregiving Activities in Informal Caregivers. J Appl Gerontol. 2015;34(6):734–60. doi: 10.1177/0733464813496464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hartke RJ, King RB, Heinemann AW, Semik P. Accidents in older caregivers of persons surviving stroke and their relation to caregiver stress. Rehabilitation Psychology. 2006;51(2):150–6. [Google Scholar]
- 97.Brown AR, Mulley GP. Injuries sustained by caregivers of disabled elderly people. Age Ageing. 1997;26(1):21–3. doi: 10.1093/ageing/26.1.21. [DOI] [PubMed] [Google Scholar]
- 98.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29(2):197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Kortebein P, Symons TB, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(10):1076–81. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- 100.Arnau A, Espaulella J, Serrarols M, Canudas J, Formiga F, Ferrer M. Risk factors for functional decline in a population aged 75 years and older without total dependence:A one-year follow-up. Arch Gerontol Geriatr. 2016;65:239–47. doi: 10.1016/j.archger.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women:the Women's Health and Aging Study I. J Am Geriatr Soc. 2009;57(10):1757–66. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lindenberger EC, Landefeld CS, Sands LP, et al. Unsteadiness reported by older hospitalized patients predicts functional decline. J Am Geriatr Soc. 2003;51(5):621–6. doi: 10.1034/j.1600-0579.2003.00205.x. [DOI] [PubMed] [Google Scholar]
- 103.Sands LP, Yaffe K, Covinsky K, et al. Cognitive screening predicts magnitude of functional recovery from admission to 3 months after discharge in hospitalized elders. J Gerontol A Biol Sci Med Sci. 2003;58(1):37–45. doi: 10.1093/gerona/58.1.m37. [DOI] [PubMed] [Google Scholar]
- 104.Fortinsky RH, Covinsky KE, Palmer RM, Landefeld CS. Effects of functional status changes before and during hospitalization on nursing home admission of older adults. J Gerontol A Biol Sci Med Sci. 1999;54(10):M521–6. doi: 10.1093/gerona/54.10.m521. [DOI] [PubMed] [Google Scholar]
- 105.Rudberg MA, Sager MA, Zhang J. Risk factors for nursing home use after hospitalization for medical illness. J Gerontol A Biol Sci Med Sci. 1996;51(5):M189–94. doi: 10.1093/gerona/51a.5.m189. [DOI] [PubMed] [Google Scholar]
- 106.Covinsky KE, Justice AC, Rosenthal GE, Palmer RM, Landefeld CS. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med. 1997;12(4):203–8. doi: 10.1046/j.1525-1497.1997.012004203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grant PM, Granat MH, Thow MK, Maclaren WM. Analyzing free-living physical activity of older adults in different environments using body-worn activity monitors. J Aging Phys Act. 2010;18(2):171–84. doi: 10.1123/japa.18.2.171. [DOI] [PubMed] [Google Scholar]
- 108.Helvik AS, Selbaek G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57(3):305–10. doi: 10.1016/j.archger.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 109.Portegijs E, Buurman BM, Essink-Bot ML, Zwinderman AH, de Rooij SE. Failure to regain function at 3 months after acute hospital admission predicts institutionalization within 12 months in older patients. J Am Med Dir Assoc. 2012;13(6):569.e1–7. doi: 10.1016/j.jamda.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 110.Buttery AK, Martin FC. Knowledge, attitudes and intentions about participation in physical activity of older post-acute hospital inpatients. Physiotherapy. 2009;95(3):192–8. doi: 10.1016/j.physio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 111.van Seben R, Reichardt LA, Essink DR, van Munster BC, Bosch JA, Buurman BM. «I Feel Worn Out, as if I Neglected Myself»:Older Patients'Perspectives on Post-hospital Symptoms After Acute Hospitalization. Gerontologist. 2018 Jan 3; doi: 10.1093/geront/gnx192. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 112.Haines TP, Hill KD, Bennell KL, Osborne RH. Additional exercise for older subacute hospital inpatients to prevent falls:benefits and barriers to implementation and evaluation. Clin Rehabil. 2007;21(8):742–53. doi: 10.1177/0269215507079842. [DOI] [PubMed] [Google Scholar]
- 113.Department of Health. Start Active, Stay Active:Physical activity recommendations for health. London. 2011 [Google Scholar]
- 114.Scholes S, Mindell J. Craig R, Mindell J, editors. Chapter 2:Physical activity in adults. Health Survey for England 2012 Volume 1:Health, social care and lifestyles. Leeds:Health and Social Care Information Centre. 2013 [Google Scholar]
- 115.Strain T, Fitzsimons C, Kelly P, Mutrie N. The forgotten guidelines:cross-sectional analysis of participation in muscle strengthening and balance &co-ordination activities by adults and older adults in Scotland. BMC Public Health. 2016;16(1):1108. doi: 10.1186/s12889-016-3774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Strain T, Kelly P, Mutrie N, Fitzsimons C. Differences by age and sex in the sedentary time of adults in Scotland. J Sports Sci. 2018;36(7):732–41. doi: 10.1080/02640414.2017.1339904. [DOI] [PubMed] [Google Scholar]
- 117.Walker H, Scarlett M, Williams B. Health Survey Northern Ireland:First results 13/14. Public Health Information &Research Branch, Information Analysis Directorate. Belfast. 2014 [Google Scholar]
- 118.Office of Disease Prevention and Health Promotion, Healthy People 2020. [Accessed Aug 2014 by Strain et al. 2016]. Data details. https://www.healthypeople.gov/node/5071/data_details .
- 119.School of Sport Health and Exercise Science (SSHES) Interpreting the UK physical activity guidelines for older adults (65+):Guidance for those who work with older adults described as actives, those in transition and frailer older adults 2012. [[accessed Jan 2018]]. http://www.ssehsactive.org.uk/older-adults-resources-and-publications-results/39/index.html .


