Abstract
Objectives:
Despite a rising clinical and research profile, there is limited information about how frailty and sarcopenia are diagnosed and managed in clinical practice. Our objective was to build a picture of current practice by conducting a survey of UK healthcare professionals.
Methods:
We surveyed healthcare professionals in NHS organisations, using a series of four questionnaires. These focussed on the diagnosis and management of sarcopenia, and the diagnosis and management of frailty in acute medical units, community settings and surgical units.
Results:
Response rates ranged from 49/177 (28%) organisations for the sarcopenia questionnaire to 104/177 (59%) for the surgical unit questionnaire. Less than half of responding organisations identified sarcopenia; few made the diagnosis using a recognised algorithm or offered resistance training. The commonest tools used to identify frailty were the Rockwood Clinical Frailty Scale or presence of a frailty syndrome. Comprehensive Geriatric Assessment was offered by the majority of organisations, but this included exercise therapy in less than half of cases, and medication review in only one-third to two-thirds of cases.
Conclusions:
Opportunities exist to improve consistency of diagnosis and delivery of evidence-based interventions for both sarcopenia and frailty.
Keywords: Sarcopenia, Frailty, Survey, Questionnaire, Older people
Introduction
Sarcopenia and frailty have emerged as important syndromes affecting older people[1,2]. Both conditions are associated with multiple adverse outcomes, including falls, hospitalisation and longer length of hospital stay, impaired ability to live independently, an increased need for care, and earlier death[3,4]. As a result, both syndromes are now attracting attention as research topics and as important targets for diagnosis and management in clinical practice – not just within the discipline of geriatric medicine, but in other areas of clinical practice including cardiology and oncology[5,6].
As is often the case with rapidly evolving fields of clinical and research activity, several different tools and algorithms have been proposed for the diagnosis of both sarcopenia and frailty[7-10]. For both conditions, an emerging evidence base supports a limited range of interventions – resistance training has been shown to be effective in ameliorating sarcopenia[11]; outcomes for frail individuals are likely to be improved by the application of the process of Comprehensive Geriatric Assessment (CGA), based on extrapolation from the existing evidence base for CGA[12]. In addition, exercise training may be able to prevent or improve frailty[13,14].
A key challenge in improving outcomes for older people with sarcopenia or frailty is to ensure that research findings are translated into clinical practice. Conversely, programmes of research need to be designed and delivered in ways that fit with the existing landscape of clinical practice; failure to do so leads to difficulties in conducting research and implementing findings. A first step in this process is to build a picture of current practice – do organisations search for older people with sarcopenia or frailty, what tools are used to make the diagnosis, and what treatments and strategies of care are used to manage these conditions? To date, there is limited information on this topic in either a UK context or a European context[15]; this lack of information hampers the research process, and the development of a consistent approach or guidance for practice. The aim of the work described in this paper was to survey UK healthcare professionals to understand how sarcopenia and frailty are diagnosed and managed in current UK practice.
Materials and methods
We designed a series of four questionnaires focussing on: a) the diagnosis and management of sarcopenia; b) the diagnosis and management of frailty in acute medical units (AMUs)/frailty units; c) the diagnosis and management of frailty in orthopaedic and surgical services; and d) the diagnosis and management of frailty in community settings. We used the online survey tool SurveyMonkey (www.surveymonkey.com) to create the questionnaires and generated a unique link for each of the four questionnaires. These were then released in four waves, over the course of four months. During each wave the questionnaire was circulated to members of the British Geriatrics Society Sarcopenia and Frailty Research Special Interest Group (SiG) through British Geriatrics Society (BGS) media channels. In addition, the surgical questionnaire (questionnaire c) was circulated to the BGS Falls and Bone Health SiG and the community questionnaire (questionnaire d) was circulated to the BGS Community Geriatrics SiG. The questionnaires were also promoted using social media, with no limitations on who could participate.
The sarcopenia element (questionnaire a) included questions on the identification of sarcopenia, the diagnostic criteria used, and the interventions offered to those found to have sarcopenia, with a maximum of 10 questions to be answered in this questionnaire. The frailty questionnaires focussed on the tools used to identify frailty, the professionals involved in identifying frailty, and the interventions offered to those found to have frailty. There were a maximum of 43 questions to be answered over the three frailty questionnaires (questionnaires b, c and d; 21 questions on acute medical units/frailty units, 12 questions on orthopaedic and surgical services, 10 questions on primary care and community services). Each questionnaire was designed to take no longer than 3 minutes to answer. Respondents were questioned on current practice within their organisation, rather than at the individual level.
Raw data from SurveyMonkey were downloaded as Microsoft Excel files and analysed using SPSS v22 (IBM, New York, USA). For each questionnaire, responses were analysed at the level of the responding organisation. Non-UK institutions were excluded from analysis. Where more than one response was received from an organisation, responses were combined and a liberal approach to responses was adopted – e.g. if one respondent said that a tool was used to diagnose frailty, and another respondent said that the tool was not used, we recorded this as ‘tool used’. Descriptive statistics were generated for each questionnaire.
Results
Sarcopenia diagnosis and management
Sixty-one people completed the sarcopenia questionnaire (22 consultant geriatricians, 2 general practitioners (GPs), 3 trainee geriatricians, 7 specialist nurses, 13 Allied Health Professionals (AHPs) and 14 others), representing 49 organisations from a total of 177 hospital-based NHS organisations or health boards in the UK (28%)[16]. 26/49 (53%) reported that their organisation identified sarcopenia; 23/49 (47%) in inpatients, 22/49 (45%) in outpatients.
19/26 (73%) of organisations who identified sarcopenia reported using any tools to do so; the tools used are shown in Table 1. Two organisations reported using the European Working Group on Sarcopenia in Older People (EWGSOP) diagnostic criteria[17], one reported using the Foundation for the National Institutes of Health (FNIH) criteria[18], and 16/19 (84%) reported not using a diagnostic algorithm for sarcopenia. Only one organisation out of the 16 responding, included and coded sarcopenia as a diagnosis on clinic letters or discharge summaries.
Table 1.
Tools used to identify sarcopenia (n=19 organisations).
| Muscle mass (%) | Bioimpedance assessment | 2 (11) |
| Dual X-ray absorptiometry | 3 (16) | |
| Computed tomography | 2 (11) | |
| Magnetic resonance imaging | 1 (5) | |
| Observation or anthropometry (%) | 7 (37) | |
| Muscle function (%) | Walk speed | 16 (84) |
| Grip strength | 10 (53) | |
| Questionnaire / history (%) | 3 (16) | |
| Number measuring muscle mass (%) | 4 (21) | |
| Number measuring muscle function (%) | 18 (95) | |
| Number measuring muscle mass AND muscle function (%) | 4 (21) | |
Resistance training was offered to those with sarcopenia by 9/19 (47%) of organisations; 15/19 (79%) offered functional exercise training, and 3/19 (16%) offered other types of exercise training. 11/19 (58%) offered vitamin D, 5/19 (26%) offered protein supplementation, and 5/19 (26%) offered other nutritional interventions. 2/19 (11%) offered drug interventions, although these were not specified.
Frailty diagnosis and management
There were 98 responses to the AMU questionnaire, representing 71 organisations (40% of 177 possible); 178 responses to the surgical and orthopaedic questionnaire, representing 104 organisations (59% of 177 possible), and 117 responses to the community questionnaire, representing 80 organisations (of 194 possible when community NHS trust organisations are included; 41%). The professions of respondents for each questionnaire are given in Supplementary Table 1. 45/50 (90%) of respondent organisations using an integrated AMU model reported identifying people with frailty on the AMU; 16/17 (94%) of organisations running a dedicated AMU for older people did so, and 10/17 (59%) of organisations running an AMU for younger people did so. 77/104 (74%) of organisations reported identifying people with frailty on orthopaedic wards. 41/90 (46%) of organisations reported identifying people with frailty on non-orthopaedic surgical wards.
Supplementary Table 1.
Profession of respondents to the three frailty questionnaires.
| Acute medical unit questionnaire | Community questionnaire | Orthopaedic and surgical questionnaire | |
|---|---|---|---|
| Consultant geriatrician | 35 | 53 | 103 |
| General practitioner | 0 | 14 | 2 |
| Non-consultant career grade | 1 | 6 | 12 |
| Trainee geriatrician | 14 | 4 | 15 |
| Nurse practitioner (older people specialist) | 20 | 15 | 0 |
| Specialist nurse (surgical) | - | - | 6 |
| District nurse | - | 1 | - |
| Allied health professional | 16 | 18 | 23 |
| Manager | 3 | 2 | 3 |
| Consultant surgeon | - | - | 2 |
| Consultant anaesthetist | - | - | 4 |
| Consultant acute physician | 1 | - | - |
| Other | 8 | 3 | 3 |
| Not stated | 0 | 1 | 5 |
At the time of the community questionnaire, targets for identification of people living with frailty had been introduced in England, but not in the devolved nations of Scotland, Wales or Northern Ireland. In England, 45/63 (71%) of organisations reported that their unit identified frailty as part of community team work; this was true for 14/17 (82%) of respondent organisations in the devolved nations.
A wide variety of tools were reported as being in use for case-finding across the different areas of clinical activity (Table 2). The Rockwood Clinical Frailty Scale (CFS)[19] was commonly used, as was the presence of a frailty syndrome (falls, delirium, incontinence or immobility)[8]. In community settings, the timed up and go test[20] and the electronic Frailty Index[21] were also commonly used. Case finding was performed by a wide range of staff, with multiple professions involved in case finding in most organisations (Table 3). In the community questionnaire, GPs were the commonest professional group undertaking case finding (33/80 organisations) followed by specialist nurses (29/80), geriatricians (22/80), AHPs (17/80), district nurses (11/80) and practice nurses (10/80). A variety of other professional groups also identified frailty, including social workers, paramedics, mental health professionals and care coordinators.
Table 2.
Tools currently being used to identify frailty.
| Integrated AMU (n=45) | Geriatric AMU (n=16) | General AMU (n=10) | Community (n=67) | Orthopaedic units (n=77) | Other surgical units (n=41) | |
|---|---|---|---|---|---|---|
| Electronic frailty index | 6 (13) | 1 (6) | 0 (0) | 21 (31) | 5 (6) | 2 (5) |
| Rockwood CFS | 15 (33) | 7 (44) | 3 (30) | 31 (46) | 23 (30) | 17 (41) |
| Timed up and go | 2 (4) | 2 (13) | 1 (10) | 16 (24) | 9 (12) | 5 (12) |
| PRISMA-7 | 1 (2) | 1 (6) | 0 (0) | 7 (10) | 2 (3) | 1 (2) |
| Edmonton Frail Scale | 4 (9) | 0 (0) | 0 (0) | 8 (12) | 9 (12) | 6 (15) |
| One or more frailty syndromes | 15 (33) | 8 (50) | 3 (30) | 19 (28) | 31 (40) | 12 (29) |
| Gait speed | 2 (4) | 1 (6) | 0 (0) | 11 (16) | 1 (1) | 1 (2) |
| Frailty index from CGA | 0 (0) | 0 (0) | 0 (0) | 8 (12) | 1 (1) | 0 (0) |
| Grip strength | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 4 (5) | 3 (7) |
| ISAR tool | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| No tool | 5 (11) | 2 (13) | 0 (0) | 2 (3) | 22 (29) | 9 (22) |
| Fried frailty score | 1 (2) | 1 (6) | 0 (0) | 4 (6) | 3 (4) | 2 (5) |
| Other | 8 (18) | 6 (38) | 3 (30) | 1 (1) | 4 (5) | 5 (12) |
| Don’t know | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (2) |
N=number of organisations responding. CGA: Comprehensive Geriatric Assessment. CFS: Clinical Frailty Scale. ISAR: Identification of Seniors at Risk. PRISMA: Program of Research on Integrations of Services for the Maintenance of Autonomy.
Table 3.
Staff involved in frailty case-finding.
| Integrated AMU (n=45) | Geriatric AMU (n=17) | General AMU (n=10) | Orthopaedic units (n=77) | Other surgical units (n=41) | |
|---|---|---|---|---|---|
| Consultant Geriatricians | 21 (47) | 11 (65) | 2 (20) | 51 (66) | 25 (61) |
| Specialty Consultants* | 17 (38) | 1 (6) | 1 (10) | 5 (6) | 3 (7) |
| Non-consultant career grade | 5 (11) | 1 (6) | 1 (10) | 18 (24) | 7 (17) |
| Junior doctors | 15 (33) | 8 (47) | 2 (20) | 19 (25) | 14 (34) |
| Specialist nurses in geriatrics | 16 (36) | 9 (53) | 2 (20) | 9 (12) | 14 (34) |
| Advanced nurse practitioners | 14 (31) | 6 (35) | 3 (30) | 10 (13) | 8 (20) |
| AMU nursing staff | 15 (33) | 3 (18) | 2 (20) | 17 (22) | 6 (15) |
| Physiotherapy | 18 (40) | 8 (47) | 5 (50) | 20 (26) | 16 (39) |
| Occupational therapy | 18 (40) | 9 (53) | 5 (50) | 19 (25) | 16 (39) |
| Therapy assistants | 7 (16) | 0 (0) | 0 (0) | 7 (10) | 5 (12) |
| Dietitians | 0 (0) | 1 (6) | 1 (10) | 1 (1) | 3 (7) |
| Care assistants | 2 (4) | 1 (6) | 0 (0) | 2 (3) | 0 (0) |
| Not sure | 1 (2) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Other | 4 (9) | 2 (12) | 1 (10) | 4 (5) | 2 (5) |
Acute physicians on AMU; surgeons on orthopaedic or non-orthopaedic surgical wards. AMU: Acute medical unit.
Figure 1 depicts interventions offered to patients with frailty. Although CGA was offered to a majority of patients, key components of CGA were offered less often; medication reviews were offered by only a third to two-thirds of units, and exercise or physiotherapy programmes by less than half of units. In the community questionnaire, geriatricians and non-geriatricians gave similar responses to the type of interventions offered, with the exception of primary care review (11/62 geriatricians vs 20/54 non-geriatricians; p=0.02); falls prevention programmes were reported by 13/62 geriatricians and 19/54 non-geriatricians (p=0.09)
Figure 1.
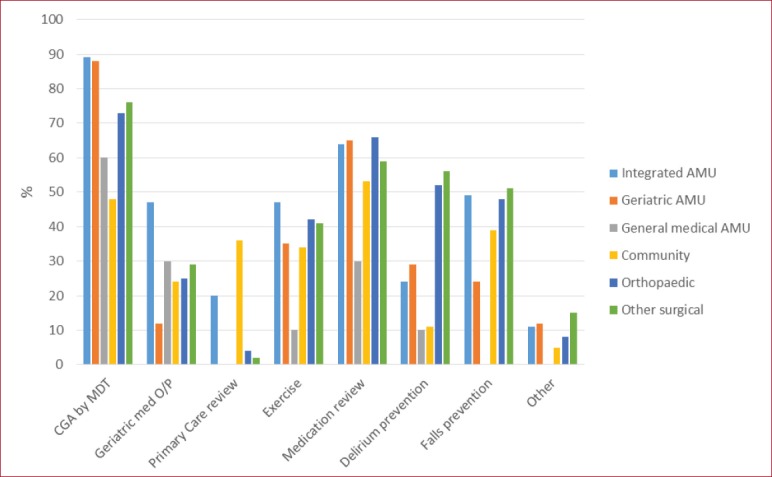
Interventions for people found to have frailty. (CGA: Comprehensive geriatric assessment. O/P: Outpatients. AMU: Acute medical unit).
Discussion
Main findings
From the frailty perspective, a high proportion of respondents from hospital settings reported they identify frailty on both integrated AMUs and those dedicated to the care of older people. A broad range of staff members are involved in identifying frailty, and similar tools were popular across the inpatient areas surveyed. We found that frailty identification on general medical AMUs and surgical areas was reported to be less common. Many respondents reported that CGA is offered as an intervention. When considering the components of CGA offered, rates of exercise interventions, medication review and delirium prevention were all lower than would be expected for a fully comprehensive approach, suggesting that the process of CGA may not be optimal in all cases.
The community frailty element of the questionnaire revealed that a wide range of individuals are involved in case finding, using a variety of tools. Again, the majority reported offering CGA, but we found that key components, such as medication review, were often missing suggesting a less than comprehensive approach.
Less than half of those responding reported that they identified sarcopenia in their organisation; it is also probable that respondents are more likely to diagnose sarcopenia given the nature of the group targeted by the survey. Despite this, very few respondents made the diagnosis of sarcopenia using a recognised algorithm, and some respondents relied on questions or history rather than objective measures of muscle mass and function. These findings suggest that the label of sarcopenia is being applied without an accurate diagnostic workup. The responses also indicated that resistance-based exercise programmes - the only intervention with proven efficacy in sarcopenia – were not routinely offered, suggesting that current therapeutic approaches are not aligned with the available evidence.
Two surveys of practice performed recently merit comparison with our results; both allude to the management of frailty rather than sarcopenia. The UK-based Hospital Wide Comprehensive Geriatric Assessment (HoW-CGA) project included a survey of frailty identification that was sent to all acute NHS Trusts and health boards in the UK[14]. 60/175 organisations returned a questionnaire; a similar variety of tools was used to identify people with frailty, with the Edmonton Frail Scale and timed-up-and-go test being the most commonly reported. Components of assessment performed for those found to be frail were variable; cognitive assessment, mobility and falls risk, pain, medication record and skin integrity were assessed in >90% of services, but sensory loss and depression were sought in <70% of services.
An international survey of 388 clinicians (88% of whom were geriatricians, mostly from Europe) found that similar to our results, gait speed, the Rockwood CFS, the short physical performance battery, and the Fried phenotypic criteria were the most commonly used diagnostic tools for frailty, being used by >25% of respondents in each case.15 Reasons given for assessing frailty included prognostic purposes, to aid decision-making, and because it was recommended by guidelines, with a combination of these factors being the most common reason. Lack of time and lack of appropriate tools were cited as barriers by respondents. The survey did not attempt to collect information on what aspects of management were offered to people with frailty.
Strengths and limitations
Our survey had several strengths. Our enquiries into current practice in the diagnosis and management of sarcopenia are novel, and our results shed important light on the management of frailty in the UK across several different clinical environments. The multidisciplinary nature of the responses to our survey is also a strength. A number of limitations also require comment. Survey response rates are never optimal, and so cannot provide us with a complete picture of practice across the UK. In particular, response rates to the sarcopenia element of the survey were low, perhaps in keeping with the lower clinical profile that sarcopenia currently enjoys compared to frailty. Additionally, the responses received will be subject to responder bias i.e. organisations responding to the survey will be those that are more likely to identify sarcopenia and frailty.
In terms of hospital practice, we received responses from between one half and one quarter of NHS trusts and NHS Boards in the UK. Within these responses, we have relied on individuals’ knowledge and perspective of the service provided within their organisation, and that perspective may differ between individuals in the same organisation. In the community part of the survey, the respondents were mainly geriatricians. As the vast majority of clinical activity around frailty in the community setting will be led by GPs, it is unlikely that we have gained a full picture of how frailty is identified and managed in the community setting, and practice is likely to be different in areas without geriatrician involvement. Response rates to the sarcopenia questionnaire were lower than for the frailty questionnaires, and this may reflect the lower profile of sarcopenia in clinical practice compared to frailty.
Implications for clinical practice and research
Several lessons for practice and research can be drawn from these results. Firstly, it is reassuring to see that frailty identification has engaged a wide range of professional groups – this is to be celebrated and augurs well for the delivery of responsive, accessible services. Less optimistically, a very wide range of tools are being used to diagnose frailty, and this is a barrier to comparisons across or within organisations. Use of a standard tool to screen for, and to diagnose frailty, would facilitate training, communication between teams, audit and benchmarking, but would also enable research studies to be designed using the same tool as was used in clinical practice – thus enabling findings to be translated more easily into practice. Similar issues surround the identification of sarcopenia; attention needs to be paid to promotion of standard diagnostic criteria, provision of equipment and training to allow measurement of muscle mass in routine practice. The recently revised EWGSOP criteria for diagnosing sarcopenia have been simplified somewhat, in acknowledgement of the challenges of making the diagnosis in clinical practice; whilst muscle mass measurement is still recommended, a diagnosis of ‘probable sarcopenia’ can now be made on measures of strength alone[22].
A ‘know-do’ gap exists in the management of frailty and sarcopenia. For both conditions, exercise training is known to be effective, and yet patients with frailty or sarcopenia are not being offered such programmes. There is a need to develop specific, rigorously tested programmes (particularly based on resistance training) in a form that can be delivered at scale to those found to have sarcopenia or frailty. Although CGA is purportedly offered to patients living with frailty by most respondent organisations, the intervention appears to be less than comprehensive in many cases. Care is needed to ensure that patients receive all the ingredients of this complex intervention; an approach that pays lip service to the concept of CGA without full delivery is unlikely to yield benefits for patients living with frailty.
Nevertheless, the fact that such a wide range of UK healthcare professionals are engaged in finding patients with frailty, and that a growing number are paying attention to sarcopenia, are encouraging developments. Research and clinical practice in these fields continues to evolve rapidly, and these findings exhort us to promote standardised approaches diagnostic tools and management algorithms that will further facilitate clinical activity in this field.
Acknowledgements
With thanks to Jo Gough at the British Geriatrics Society for her assistance in distributing the questionnaires.
Footnotes
Edited by: Yannis Dionyssiotis
References
- 1.Dodds R, Sayer AA. Sarcopenia and frailty:new challenges for clinical practice. Clin Med (Lond) 2016;16:455–8. doi: 10.7861/clinmedicine.16-5-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft A. Sarcopenia –the last organ insufficiency. Eur Geriatr Med. 2016;7:195–6. [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health Outcomes of Sarcopenia:A Systematic Review and Meta-Analysis. PLoS One. 2017;12:e0169548. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients:a systematic review. Ann Oncol. 2015;26:1091–101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 6.Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond:update 2017. ESC Heart Fail. 2017;4:492–8. doi: 10.1002/ehf2.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locquet M, Beaudart C, Reginster JY, Petermans J, Bruyère O. Comparison of the performance of five screening methods for sarcopenia. Clin Epidemiol. 2017;10:71–82. doi: 10.2147/CLEP.S148638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaudart C, Reginster JY, Slomian J, et al. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol. 2015;61:31–7. doi: 10.1016/j.exger.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Turner G, Clegg A, British Geriatrics Society;Age UK;Royal College of General Practitioners Best practice guidelines for the management of frailty:a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43:744–7. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 10.Mayhew AJ, Amog K, Phillips S, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions:a systematic review and meta-analyses. Age Ageing. 2018 doi: 10.1093/ageing/afy106. epub ahead of print. doi:10.1093/ageing/afy106. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults:a systematic review Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–59. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis G, Gardner M, Tsiachristas A, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9:CD006211. doi: 10.1002/14651858.CD006211.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care:a randomised controlled trial. Age Ageing. 2017;46:401–7. doi: 10.1093/ageing/afw242. [DOI] [PubMed] [Google Scholar]
- 14.Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults:a systematic review. JBI Database System Rev Implement Rep. 2018;16:140–232. doi: 10.11124/JBISRIR-2017-003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruyère O, Buckinx F, Beaudart C, et al. How clinical practitioners assess frailty in their daily practice:an international survey. Aging Clin Exp Res. 2017;29:905–12. doi: 10.1007/s40520-017-0806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NHS Confederation. NHS Facts and Statistics. [Accessed 6th August 2018]. http://www.nhsconfed.org/resources/key-statistics-on-the-nhs .
- 17.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia:European consensus on definition and diagnosis:Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project:rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–58. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S. The timed Up &Go:a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 21.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–60. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia:revised European consensus on definition and diagnosis. Age Ageing. 2018 Oct 12; doi: 10.1093/ageing/afz046. Doi:10.1093/ageing/afy169 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


