Abstract
Plants have evolved dynamic and complex networks of cell-to-cell communication to coordinate and adapt their growth and development to a variety of environmental changes. In addition to small molecules, such as metabolites and phytohormones, macromolecules such as proteins and RNAs also act as signalling agents in plants. As information molecules, RNAs can move locally between cells through plasmodesmata, and over long distances through phloem. Non-cell-autonomous RNAs may act as mobile signals to regulate plant development, nutrient allocation, gene silencing, antiviral defence, stress responses and many other physiological processes in plants. Recent work has shed light on mobile RNAs and, in some cases, uncovered their roles in intercellular and systemic signalling networks. This review summarizes the current knowledge of local and systemic RNA movement, and discusses the potential regulatory mechanisms and biological significance of RNA trafficking in plants.
Cell-to-cell communication plays a critical role in plant development, disease resistance and responses to various stresses from the external environment. As a strategy for efficient intercellular communications, plants have evolved a plant-specific symplasmic pathway mediated by plasmodesmata (PD) and phloem to transport signalling molecules between cells1. Various types of plant RNA species, including messenger RNAs (mRNAs), small interfering RNAs (siRNAs), microRNAs (miRNAs), ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs), can move from cell to cell (short-range) or systemically (long-range) to potentially regulate whole-plant physiological processes2–5. The non-cell-autonomous nature of RNA molecules suggests that RNAs may function beyond the cells in which they are synthesized. Regulatory roles of mobile RNAs in cell differentiation, organ formation and patterning, nutrient homeostasis, stress adaptations, and plant-microorganism and plant-plant interactions have been discovered, prompting further studies to understand the scope and impact of RNA trafficking in signalling networks. Recently, thanks to advances in genomics technologies, an abundance of mobile RNAs has been discovered6–10, further underscoring the question of the functional significance of mobile RNAs. Here, we review recent advances in intercellular and systemic RNA trafficking in plants and discuss the possible regulatory mechanisms and biological functions of RNA trafficking.
Routes for RNA trafficking between plant cells
Together, PD and phloem form a symplasmic pathway that links nearly all plant cells. RNAs can move cell-to-cell through PD and long-distance through phloem. A vesicle-mediated pathway is also a potential route for RNA trafficking between plant cells.
PD as intercellular micro-channels of mobile RNAs.
PD are membrane-lined micro-channels that cross the cell wall and connect neighbouring cells11. They are bordered by plasma membrane, and contain an appressed form of endoplasmic reticulum (ER), called desmotubule, in the central region (Fig. 1a). The space between these membranes forms a cytosolic sleeve that allows cellular molecules to migrate between cells. Mobile molecules may also move through the lumen or membrane of the desmotubule12–14. The key feature of PD is that they establish cytosolic and endomembrane continuity between adjacent cells, thus forming a symplasmic pathway to mediate the transportation of molecules between adjacent cells1,15. Various molecules are able to move through PD, including small molecules such as water, ions and phytohormones, as well as large molecules such as proteins and RNAs16. The transport of molecules through PD depends on their size, shape and tissue types, along with the development stages in which they are present. As highly dynamic channels, PD are tightly regulated and undergo various structural and functional changes during plant development. The numbers and size exclusion limit of PD vary among different tissues at different development stages17–19. The structure of PD ranges from simple channels to twinned and branched channels20. The size exclusion limit of PD is regulated by reversible callose (β−1,3-glucan) deposition and by certain PD-associated proteins and mobile proteins11,16,21,22. A number of mobile RNAs, such as miR390 (ref.3), miR165/166 (ref.5), sucrose transporter SUC1 mRNA (ref.23), and transcription factor KNOTTED1 mRNA (ref.24), have been reported to move through PD, indicating the importance of these channels for RNA trafficking in plants.
Fig. 1 |. Routes for RNA trafficking between plant cells.
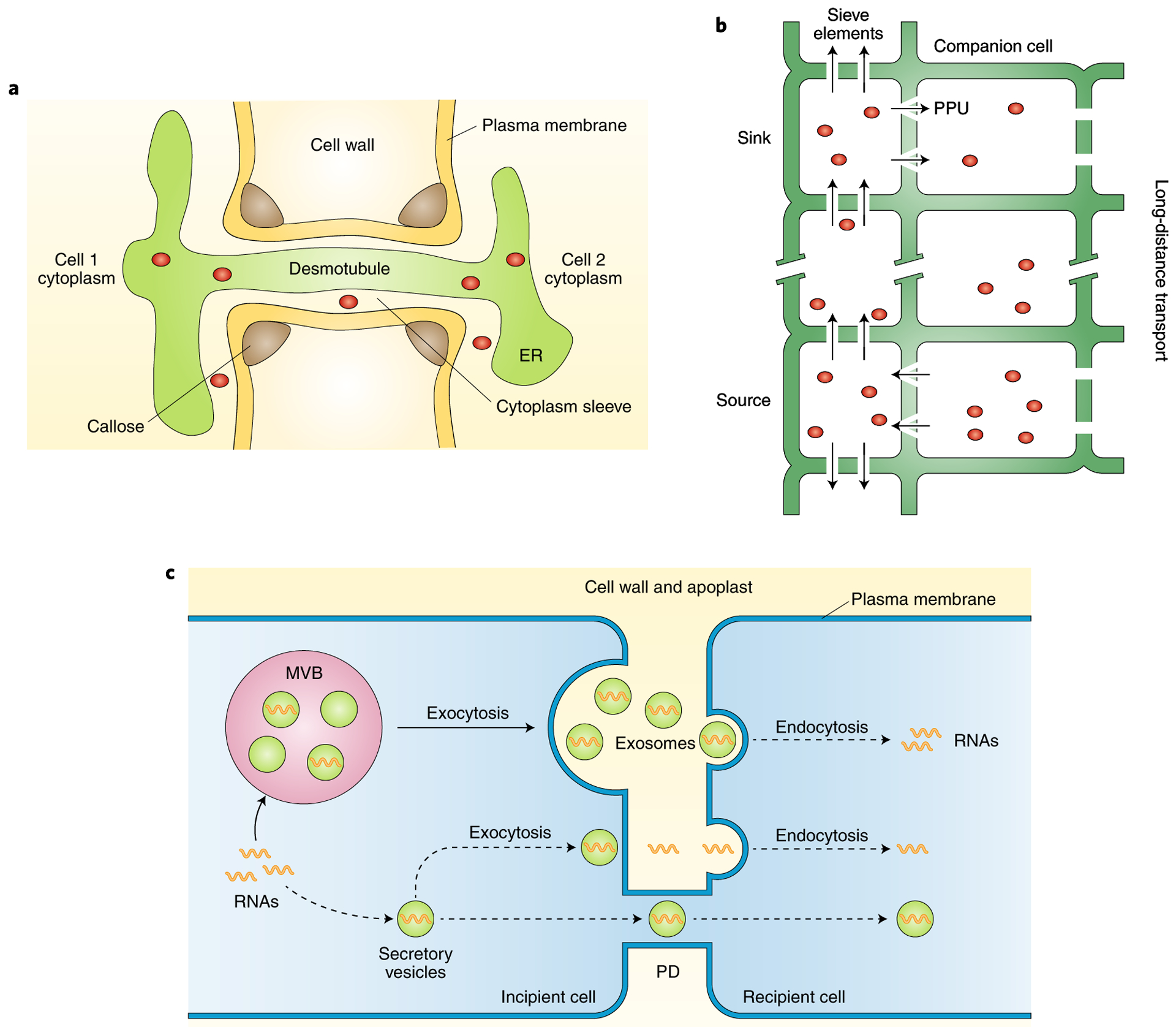
a, PD as micro-channels for cell-to-cell movement of mobile molecules. PD are membrane-lined channels that traverse the cell wall and connect neighbouring cells. Cell wall, plasma membrane, endoplasmic reticulum (ER), desmotubule and callose are indicated. Red circles represent soluble molecules capable of moving through the desmotubule or cytoplasmic sleeve of PD. b, Phloem as a conduit for long-distance movement of molecules. Phloem is composed of stacked enucleated sieve elements assisted by companion cells. Mature sieve elements are connected to adjacent companion cells by highly modified and funnel-like plasmodesmata pore units (PPUs). Mobile molecules (red circles) are primarily transported from source to sink tissues over long distances through phloem. Arrows indicate the direction of movement. Gaps in the line indicate multiple, stacked sieve elements (one cell in the diagram) mediating long-distance transport. c, Putative vesicle-mediated RNA trafficking in plants. Vesicles containing RNAs are taken into MVBs, which subsequently fuse with the plasma membrane and release their intraluminal vesicles into the extracellular space as exosomes. These exosomes fuse with the plasma membrane of the recipient cell through endocytosis and unload the cargo RNAs. Alternatively, vesicles may transport RNAs to adjacent cells, either through exocytosis/endocytosis or through the PD channels between cells. Note that these are purely hypothetical events, as indicated by dashed lines in the diagram.
Long-distance movement of RNAs through phloem.
While PD mediate the cell-to-cell movement of cellular molecules, the vascular system-consisting of xylem and phloem-facilitates long-distance trafficking in plants. Water and mineral nutrients are transported from roots to the aerial parts of plants through the xylem. In contrast, phloem supports the movement of photosynthates and macromolecules from source to sink tissues25. The phloem is composed of living enucleated sieve elements assisted by companion cells (Fig. 1b). Mature sieve-elements are connected to adjacent companyion cells by highly modified, funnel-like PD called plasmodesmata pore units (PPUs). These specialized PPUs are structurally distinct from regular PD. They consist of multiple channels on the companion-cell side, which merge into a single pore on the sieve-element side26. The sieve element cells stack together to form the sieve tube, which allows for rapid flow of molecules over long distances in plants27. Macromolecules, including proteins, RNAs and ribonucleoprotein complexes, have been found in the phloem stream4,28,29. Plant phloem appears to be an ideal route for RNA trafficking because no RNase activity is detectable in phloem sap30,31. As discussed in detail in the section ‘Mobile RNAs in plants’, many lines of evidence support that various RNA species are transported through the phloem to distant tissues6,8,32.
Potential RNA trafficking routes mediated by vesicles.
In eukaryotes, proteins are generally secreted into the extracellular space through the classic ER-Golgi route. However, cytosolic proteins lacking signal peptides are also found outside of cells, indicating the existence of unconventional protein secretion pathways33. In animals, one such secretion pathway involves the release of exosomes to the extracellular compartment. Exosomes are membrane-bound vesicles 30–100 nm in diameter and derived from multivesicular bodies (MVBs) that fuse with the plasma membrane, resulting in the release of their intraluminal vesicles as exosomes34. Interestingly, in addition to proteins, various RNAs including mRNAs, miRNAs and other non-coding RNAs, have also been identified in exosomes and have been shown to be critical for intercellular communication between animal cells34. The secretion of exosomes appears to be an evolutionarily conserved process, and accumulating evidence indicates that exosome-like vesicles also exist in plants. Structures similar to exosomes have been isolated from the apoplastic fluids of sunflower (Helianthus annuus) seeds, olive (Olea europaea) pollen grains and Arabidopsis leaves, and these exosome-like vesicles are enriched with leaderless secretory proteins35–37. Ultrastructural data has shown that barley leaves release vesicles resembling exosomes under pathogenic fungal attack38. When invaded by filamentous oomycetes pathogens, a series of structural and biochemical responses takes place in the host plant cells, including the re-organization of subcellular structures. Numerous organelles accumulate in the vicinity of haustorium infected sites, particularly MVBs, Golgi stacks, ER and secretory vesicles, indicating that the secretion process of the host plant cell may be activated at the penetration site to defend against pathogenic infection39,40. Animal exosomes can mediate RNA trafficking for cell-to-cell communication and affect the phenotypes of recipient cells34. It was recently revealed that plants also employ exosome-like vesicles to transport small RNAs to a fungal pathogen41. It is unknown whether exosomes or other vesicles are used in RNA trafficking between plant cells, as plant cells are separated by cell walls, unlike the highly specialized haustoria at fungal infection sites. However, given that exosomes can be detected from plant tissues or organs, it is a formal possibility that RNA trafficking between neighbouring cells occurs through exosomes. Figure 1c depicts imaginary scenarios whereby exosomes or vesicles may transport RNAs intercellularly, either through exocytosis/endocytosis or through the PD channels between cells. Vesicle-mediated RNA trafficking deserves attention in future research.
Mobile RN As in plants
Various types of plant RNA species, including mRNAs, small RNAs, ribosomal RNAs and transfer RNAs, have been found to travel beyond the cells in which they are synthesized.
Mobile mRNAs.
Intercellular trafficking of plant mRNAs through PD was first shown in microinjection experiments with the maize KNOTTED1 (KN1) transcription factor24. Fluorescently labelled sense kn1 RNA and KN1 protein were coinjected into tobacco mesophyll cells, and the fluorescent probes were observed to move rapidly from the injected cell into neighbouring cells, indicating that the transcript of KN1 can be transported through PD with the assistance of KN1 protein24. Another strong evidence for mRNA being mobile is from localization studies of potato sucrose transporter suc1 mRNA (ref.23). Suc1 mRNA was transcribed in companion cells, however in situ hybridization revealed the presence of the transcript in both companion cells and sieve elements. Because enucleated sieve elements lack the transcription machinery, the presence of suc1 mRNA was attributed to cell-to-cell movement of the mRNA from companion cells to adjacent sieve elements, probably through PD (ref.23). Subsequent studies investigated the distribution of RNAs in the phloem sap of various plant species, including Arabidopsis42, rice31, barley30,43, pumpkin44, melon45, Ricinus communis46, Lupinus albus47, watermelon and cucumber32. These studies identified numerous phloem transcripts encoding various types of proteins, such as transcription factors, phytohormone regulators, stress response factors and proteins involved in a wide range of plant developmental processes. Although the presence in phloem sap alone was insufficient evidence of RNA systemic movement or functionality, the mobility of some phloem mRNAs was subsequently confirmed by grafting studies44,48–52 and host-parasite interaction analysis8,9,53,54.
Grafting connects two or more living tissues from different plants into one single plant55. Grafting has been widely used to test the translocation of molecules across the graft junctions and has proven to be a useful tool for characterizing long-distance mobile RNAs. Examples of mRNAs whose mobility was demonstrated by grafting experiments (Table 1) include Arabidopsis FT (ref.56) and Aux/IAA (ref.48), pumpkin PP16 (ref.49) and NACP (ref.44), potato BEL5 (ref.50) and POTH1 (ref.51), apple SLR/IAA14 (ref.52), and tomato PFP-T6 (ref.2) and PS (ref.57). Some recent studies have identified large numbers of graft-transmissible mRNAs using high-throughput sequencing. From the heterograft of Arabidopsis and tobacco, 138 transcripts from the stock of Arabidopsis were found to move into the tobacco scion58. A separate study reported a total number of 2,006 mobile RNAs by grafting shoots and roots of different Arabidopsis ecotypes8. The difference in the numbers of mobile RNAs identified in these two studies may be attributable to differences in sequencing coverage and plant growth conditions. Indeed, even 2,006 mobile RNAs may be an underestimate, given that mobile RNA detection is dependent on the availability of single nucleotide polymorphisms (SNPs), mRNA stability, sequencing depth, materials sampled and other biological factors. In addition to the model plant Arabidopsis, high-throughput sequencing has identified mobile RNAs across graft junctions in agricultural crops, including grape10 and cucumber7 from which the numbers of identified mobile transcripts were 3,333 and 3,546, respectively.
Table | 1.
Representative examples of mobile RNAs in plants
| RNA | Function | Plant Species | References |
|---|---|---|---|
| mRNA | |||
| SUC1 | Sucrose transport | Potato | 23 |
| FT1 | Flowering induction | Arabidopsis | 56 |
| Aux/IAA | Root development | Arabidopsis | 48 |
| PP16 | RNA transport | Pumpkin | 49 |
| NACP | Meristem maintenance | Pumpkin | 44 |
| BEL5 | Tuber formation | Potato | 50 |
| POTH1 | Leaf development | Potato | 51 |
| SLR/IAA14 | Lateral root formation | Apple | 52 |
| PFP-T6 | Leaf development | Tomato | 2 |
| PS | Pathogen resistance | Tomato | 57 |
| GAI | Leaf development | Tomato | 53,54 |
| PFP | Leaf development | Tomato | 54 |
| miRNA | |||
| miR165/166 | Root development | Arabidopsis | 5 |
| miR390 | Leaf polarity | Arabidopsis | 3 |
| miR394 | Shoot meristem formation | Arabidopsis | 82 |
| miR395 | Sulfate homeostasis | Arabidopsis | 73 |
| miR399 | Phosphate homeostasis | Arabidopsis | 73, 83,84 |
| siRNA | |||
| ta-siRNA | Leaf polarity | Arabidopsis | 3 |
| hc-siRNA | DNA methylation | Arabidopsis, N. tabacum, N. benthamiana | 6,75–79 |
| rRNA | |||
| 5S rRNA | Translation | B. napus, pumpkin | 4,72 |
| 5.8S rRNA | Translation | B. napus, pumpkin | 4,72 |
| 18S rRNA | Translation | B. napus, pumpkin | 4,72 |
| 25S rRNA | Translation | B. napus, pumpkin | 4,72 |
| tRNAa | Translation | Pumpkin | 4 |
Not all tRNA species are detected in the phloem sap, and some tRNA are present in truncated forms or as tRNA halves.
Besides the grafting approach, host-parasite interactions analyses have also provided strong evidence for RNA movement. In host-parasite interactions, mRNAs can move from host plants to parasitic plants across the parasite’s haustorium. Examples of mRNAs capable of moving from host plants to their parasitic plants (Table 1) include tomato GAI and PFP (refs53,54). Genome-wide analysis of mRNAs exchanged between host and parasitic plants revealed thousands of mobile transcripts8,9,59. In an Arabidopsis-Cuscuta host-parasite system, nearly half of the expressed transcriptome of the Arabidopsis host was found to move into the Cuscuta parasite9.
These mobile RNAomics data reveal a large number of mobile transcripts and raise the possibility of RNA-based systemic signalling in plants. Future studies are needed to identify the patterns of RNA movement in plants, the underlying regulatory mechanisms and the functional impacts of mobile RNAs.
Mobile small RNAs: siRNAs and miRNAs.
Small RNAs are a population of 21 to 24 nucleotide (nt) RNAs that guide RNA silencing. They can be classified into two major categories in plants, siRNAs and miRNAs, based on their biogenesis and molecular features60. miRNA genes are transcribed by RNA polymerase II (Pol II), generating imperfectly paired hairpin precursors that are cleaved by DICER-LIKE1 (DCL1) to produce mature miRNAs. These mature miRNAs are incorporated into ARGONAUTE 1 (AGO1) to form the RNA-induced silencing complex (RISC), which catalyses the cleavage or translational repression of target RNAs61. SiRNAs are further classified as trans-acting siRNAs (ta-siRNAs) and heterochromatic siRNAs (hc-siRNAs). These siRNAs are generated from long double-stranded RNAs (dsRNAs) produced from single-stranded RNAs by RNA-dependent RNA polymerases (RDRs) or formed from the transcription of inverted-repeat (IR) sequences. In Arabidopsis, the dsRNAs are mainly processed by DCL2, DCL3 or DCL4 and generate 21-, 22- or 24-nt siRNAs, which are sorted into AGO1, AGO2, AGO3, AGO4, AGO6 or AGO9 RISCs to silence target genes62. Silencing mediated by small RNAs can be classified as transcriptional gene silencing (TGS) or post-transcriptional gene silencing (PTGS). TGS regulates transposable elements and repetitive DNA sequences through DNA methylation or histone modifications in the nucleus, whereas PTGS functions primarily to eliminate exogenous invading RNAs or to regulate endogenous genes through RNA cleavage or translation inhibition in the cytoplasm63.
Over the past few decades, extensive studies have uncovered non-cell-autonomous features of RNA silencing. Systemic spreading of silencing was initially shown in tobacco plants using grafting experiments, in which a scion containing a non-silenced transgene was grafted onto a stock containing a silenced transgene64. In a parallel study, green fluorescent protein (GFP) silencing was observed in the upper leaves of tobacco plants stably expressing a GFP transgene after the induction of GFP silencing by transient Agrobacterium infiltration of the lower leaves65. Subsequent studies further confirmed the systemic nature of RNA silencing and indicated the existence of mobile sequence-specific signals66–68. It was hypothesized that small RNAs or their precursors act as mobile signalling molecules that mediate the spreading of RNA silencing. To further investigate the non-cell-autonomous nature of RNA silencing, two artificial siRNA reporter systems, SUC-SUL and SUC-PDS, were developed. In both systems, long inverted-repeat dsRNAs are expressed in phloem companion cells but target the chlorophyll biosynthesis gene SULPHUR (SUL) or the carotenoid biosynthesis gene PHYTOENE DESATURASE (PDS) in leaf mesophyll cells69,70. Processing of the inverted repeat dsRNAs generates siRNAs and results in non-cell-autonomous RNA silencing spreading from the vasculature to the surrounding 10 to 15 neighbouring cells, reflecting local cell-to-cell transmission of RNA silencing. Genetic screens based on these two reporter systems were performed, leading to identification of a number of genes that affect RNA silencing, including AGO1, HEN1, CLSY1, DCL1, DCL4, NRPD1A, RDR2 and HPR1; these findings have been well reviewed in ref.68.
Both siRNAs and miRNAs have been observed in phloem exudates from various plant species, including pumpkin71, oilseed rape (Brassica napus)72,73, apple74 and white lupin (Lupinus albus)47, further supporting the hypothesis that small RNAs act as long-distance transmitters of RNA silencing in plants.
The mobility of many siRNAs has been documented (Table 1). The first case of endogenous mobile siRNAs was that of the low-abundance, conserved group of TAS3-derived ta-siRNAs, termed tasiR-ARFs, which target auxin response factors. The biogenesis of tasiR-ARFs is restricted to the upper adaxial side of leaves by the localized expression of AGO7 and TAS3A. However, in situ hybridization shows that tasiR-ARFs accumulate outside this defined domain of biogenesis and form an adaxial-to-abaxial gradient across leaves. This gradient shapes the expression pattern of the abaxial determinant AUXIN RESPONSE FACTOR3 (ARF3). Thus, tasiR-ARFs function as a mobile signal in the establishment of adaxial-abaxial leaf polarity3. Transposable-element-derived siRNAs could be transported from the pollen vegetative cell to sperm cells to inhibit transposable element activity and stabilize the genome during reproduction75,76. Recently, elegant grafting experiments have demonstrated that both transgene-derived siRNAs and endogenous siRNAs can move long distances6,77–79. By grafting roots from the siRNA biogenesis-defective Dicer triple mutant dc12 dc13 dc14 to wild-type Arabidopsis shoots, a substantial population of mobile siRNAs was identified using high-throughput sequencing77. Moreover, these mobile siRNAs were found to target thousands of genomic loci, predominately transposons located in euchromatic regions, for DNA methylation6.
In contrast to siRNAs, miRNAs are thought to be relatively less mobile as the sites of their transcription and function are well correlated80,81. However, a few miRNAs have been reported to act non-cell-autonomously (Table 1). For example, the miR390 precursor localizes to the vasculature and pith region below the shoot apical meristem but not in the meristem or youngest leaf primordia, based on in situ hybridization. However, the accumulation of mature miR390 throughout the vegetative apex, including the meristem and the youngest leaf primordia, strongly suggests that miR390 is capable of cell-to-cell short-distance movement3. miR165/166 species are probably mobile as well; the gene promoters are active in the single-cell-layer root endodermis, while the mature miRNAs accumulate across all radial layers of the root in Arabidopsis5. Similarly, miR394 was found to spread from the site of biogenesis in the epidermal layer to internal cells in the shoot apical meristem82. In addition to short-range movement, long-distance movement of miRNAs has been demonstrated by grafting experiments. Using Arabidopsis overexpressing miR399 as the scion and wild type as the rootstock, miR399 was found to exhibit long-range movement from shoot to root to degrade its target transcript PHOSPHATE 2 (PHO2) and to regulate plant phosphate homeostasis83,84. The mobility of miR399 was confirmed in an independent grafting experiment using wild-type Arabidopsis as the scion and the miRNA-deficient mutant hen1 as the rootstock. The same study also revealed that miR395 could traverse graft junctions to target the APS gene in the root73. Using the same approach of wild-type-hen1 micrografting, another study further showed that, in addition to miR399, the corresponding near-complementary miR399* species was graft-transmissible between shoots and roots. The analysis also revealed that miR827 and miR2111 were capable of long-distance movement, while their respective miRNA* species were not, indicating that the mobility of miRNA species is selective85. miR2111 in the legume Lotus japonicus has also been found to undergo shoot-to-root long-distance translocation to regulate rhizobial infection. While miR2111:GUS-expressing lines showed that miR2111 was synthesized in shoots, mature miR2111 was detected in both shoots and roots. In addition, the identification of miR2111 from phloem sap further supports the long-distance transport of this miRNA (ref.86).
Small RNAs can traffic between parasites and host plants. The parasitic plant Cuscuta was found to induce many miRNAs at the haustorium when it parasitizes Arabidopsis and tobacco. The majority of these miRNAs are 22-nt long and they can hijack the silencing machinery of the host cells to produce secondary siRNAs, resulting in the degradation of host mRNAs87. In addition, small RNAs were observed to move in a trans-kingdom manner from host plants to parasitic pathogens. miR166 and miR159 were found to be exported from host cotton plants to a fungal pathogen and were shown to down-regulate their fungal target genes Clp-1 and HiC-15, which are essential for fungal virulence88. Using the Arabidopsis-Botrytis cinerea pathosystem, another study41 profiled small RNAs from pathogen B. cinerea protoplasts and extracellular vesicles of infected Arabidopsis leaves. A number of siRNAs and miRNAs have been identified to transfer from Arabidopsis to B. cinerea through extracellular vesicles to silence fungal virulence-related genes and contribute to host immunity.
The findings described here have helped establish that siRNAs and miRNAs can serve as mobile agents that function in recipient cells (Fig. 2). Thus, non-cell-autonomous RNA silencing may be a signalling mechanism that coordinates developmental and physiological processes in plants.
Fig. 2 |. Schematic drawing of non-cell-autonomous RNA silencing in plants.
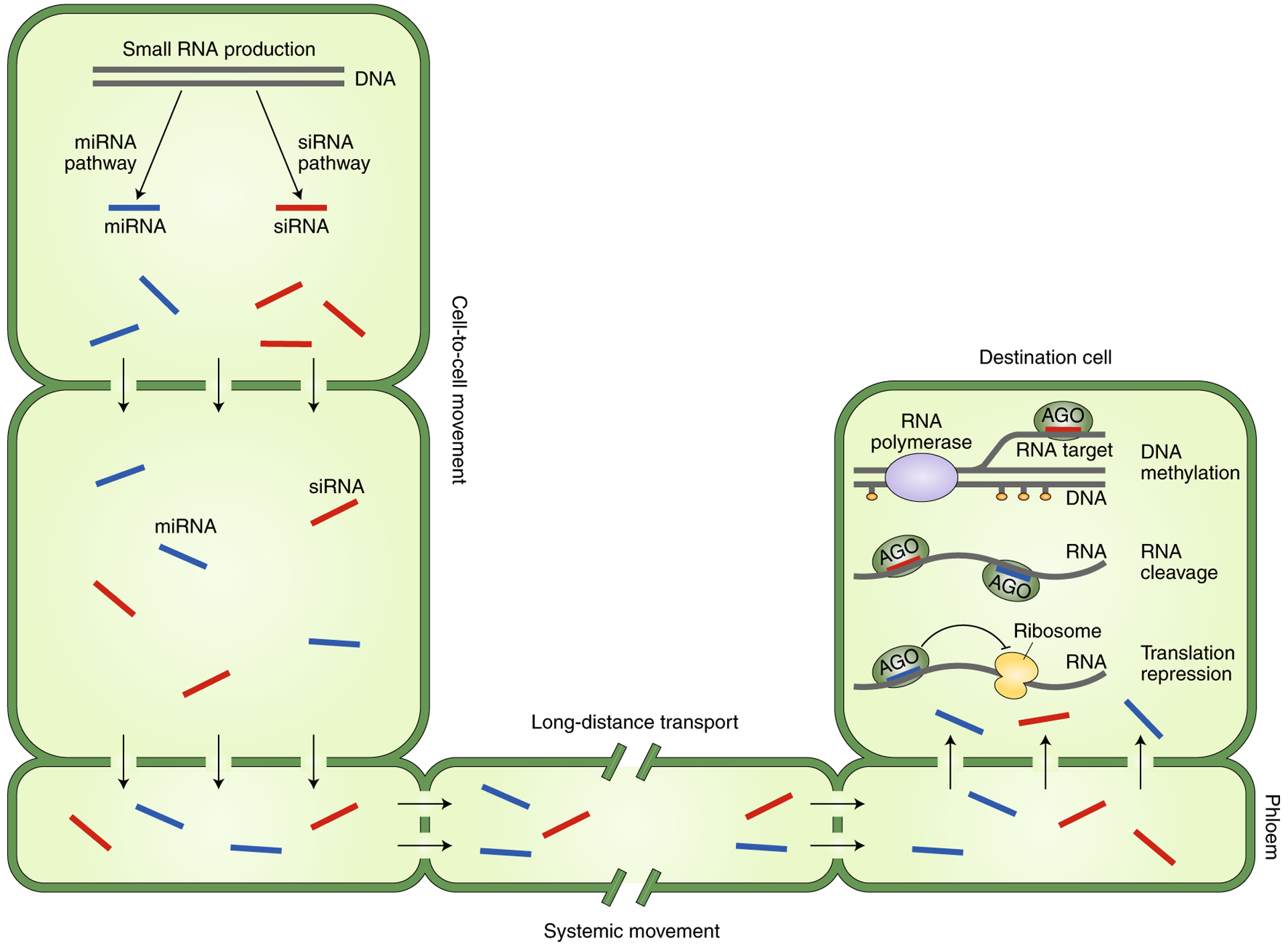
SiRNAs (red) and miRNAs (blue) can act as mobile signals and move from cell to cell or over long distances to mediate non-cell-autonomous RNA silencing in plants. In the destination cells, siRNAs mediate RNA-directed DNA methylation or guide the cleavage of target RNAs, whereas miRNAs guide the cleavage or translational repression of target mRNAs. Filled orange circles indicate methyl groups on DNA or histones.
Other mobile RNAs: rRNAs and tRNAs.
Transcriptome analyses have also revealed the presence of rRNAs and tRNAs in phloem sap (Table 1). All plant rRNA species, including 5S, 5.8S, 18S, and 25S rRNAs, were found in the phloem exudate of B. napus and pumpkin4,28,72. Large quantities of tRNAs have been identified from an RNA pool derived from pumpkin phloem, and high-throughput sequencing revealed that tRNA species distributed non-equally in the phloem sap4,89. For example, while tRNA-Asp and tRNA-Lys were highly abundant, tRNA-Ile and tRNA-Thr were barely detected. Moreover, a considerable fraction of the phloem-sap-derived tRNAs was in truncated forms, or tRNA halves, derived from cleavage of tRNAs in the anticodon loop. In vitro assays showed that these phloem-specific tRNA halves effectively inhibited translation in a non-specific manner4. In addition to rRNAs and tRNAs, several ribosomal proteins and ribonucleoprotein complexes were also identified in the phloem sap, however, some important ribosomal proteins or translation factors essential for ribosomal function are missing28. According to these observations, it was suggested that mRNA translation cannot take place in phloem. Further strong evidence supporting this notion is that in a hypocotyl-grafted GUS:tRNAMet/wild-type (Col-0) Arabidopsis plant, the mobile GUS:tRNAMet fusion can move to the root tip of wild-type plants and show GUS activity there, but GUS activity could not be detected in the phloem of the root close to the graft junction, indicating that mobile transcripts are translated after transport90. Other non-coding RNA species identified in phloem sap include small nuclear RNAs (snRNAs), mitochondrial and chloroplastic rRNAs and tRNAs, signal recognition particle RNAs and RNAs with unknown function, but it is unclear whether they may unload from the phloem to serve functions elsewhere4.
Regulatory mechanisms of RNA movement
Although a large number of RNAs have been found to be mobile in the past few decades, the mechanisms that regulate their movement have only recently begun to emerge. Based on current knowledge, here we discuss the possible mechanisms of RNA trafficking in plants.
RNA sequence, length, abundance and stability are possible factors impacting mobility.
The identification of numerous mobile RNAs raises questions about the mechanisms that determine their mobility. A recent study investigated the potential link between mRNA abundance and mobility using a computational diffusion-based model and concluded that mRNA abundance is a key determinant of mobility. The statistical analyses also indicated that mRNA stability and transcript length might contribute to mobility-mRNAs with predicted longer half-lives and smaller size seem to be more mobile91. These findings might lead to the assumption that mRNAs traffic in a passive, non-selective manner. However, evidence exists for an active, selective mechanism. For example, GUS-YFP transcripts from the strong 35S promoter do not move to distant plant tissues, suggesting that high levels of expression do not necessarily induce mobility of RNAs8,90. Some highly expressed GFP protein fusions have been shown to be graft-mobile, but their mRNAs are not92. Besides, it was observed that mobile transcripts can move from shoot to root (source to sink), or from root to shoot against the source-sink gradient, and certain mobile mRNAs may be transported preferentially to specific tissues, such as flowers or leaves8,93. Specific motifs have also been found to affect RNA mobility. In potato, the 5’ and 3’ untranslated regions (UTRs) of the BEL1-like transcription factor BEL5 are required for its long-distance transport into roots, where BEL5 regulates tuber formation94. Some low-abundance mobile transcripts were found to be enriched for three sequence motifs, indicating a sequence-specific selective mechanism for the movement of this set of transcripts91. In addition, fusion of the phloem enriched tRNA-like structures (TLSs) with immobile RNAs can make the fused transcripts mobile across graft junctions, while deletion of the TLS motif from a dicistronic mRNA-tRNA transcript made it immobile, indicating that TLS motifs can trigger the mobility of mRNAs90. By analysing the graft-mobile transcriptome data from Arabidopsis8 and grape10, it has been revealed that a large number of mobile transcripts harbour a TLS motif in the coding sequences or UTRs, or are transcribed from genes that are in close proximity to annotated tRNA genes, which further supports that TLSs are necessary for RNA mobility and indicates the existence of a selective mRNA delivery mechanism90. This evidence indicates two possible transport pathways for mRNAs in plants: a passive, nonselective pathway and an active, selective pathway.
RNA trafficking mediated by non-cell-autonomous proteins with RNA-binding activity.
The existence of specific motifs associated with mobile RNAs implies that RNA-binding proteins may interact with RNAs and modulate their trafficking. Phloem exudate analyses have shown the presence of many RNA-binding proteins29,95,96. Some of these proteins were found to associate with RNAs to form ribonucleoprotein complexes and might be involved in phloem-mediated RNA movement. Microinjection assays in tobacco revealed that fluorescein-labelled maize KN1 (ref.24) and pumpkin PP16 (ref.49) interact with their own RNAs and specifically facilitate their cell-to-cell or long-distance translocation by regulating the size-exclusion limit of PD. Pumpkin RBP50, a polypyrimidine tract binding (PTB) protein, acts as the core of a ribonucleoprotein (RNP) complex that moves in the phloem translocation stream, as shown by heterografting assays. Co-immunoprecipitation showed that RBP50 associates with six mRNAs containing PTB motifs and incorporates them into the RNP complex to mediate their long-range transport in the phloem97. Potato PTB proteins PTB1 and PTB6 have also been found to bind the 3’ UTR of the transcript of BEL5 (ref.98). Overexpression of PTB1/6 resulted in both enhanced stability and long-distance movement of the BEL5 mRNA, whereas suppression of PTB1/6 led to decreased stability and movement98. Small RNAs may also require RNA-binding proteins for their mobility. The pumpkin phloem protein PSRP1 was found to preferentially bind 25-nt single-stranded RNA (ssRNA) species by gel mobility-shift assays. Co-injection of PSRP1 and fluorescein-labelled 25-nt ssRNAs in tobacco showed that PSRP1 could mediate the trafficking of ssRNAs to neighbouring cells71. PSRP1 interacts with a specific set of proteins and forms a complex99. In vivo co-immunoprecipitation showed that the PSRP1-based complex contains endogenous 24-nt small RNAs. Dephosphorylation of PSRP1 results in disassembly of this small RNA-protein complex, which presumably needs to happen to release small RNAs in target cells99. However, orthologues of PSRP1 have yet to be characterized in other plant species.
Control of RNA trafficking by regulating PD permeability.
PD permeability can be regulated to facilitate or block the trafficking of RNA and other molecules during plant development and stress responses, and PD apertures may be temporarily dilated by mobile proteins, such as the aforementioned KN1 and PP16 (refs24,49,100). Many phloem sap proteins were reported to interact with PD to increase their size exclusion limit, whereas isoforms of the proteins absent in phloem were incapable of such interactions101,102. Reversible callose deposition is another mechanism employed by plants to regulate PD permeability in response to various environmental stresses, such as pathogen invasion, wounding and nutrient deficiency17,100. A gain-of-function mutation in Arabidopsis CALLOSE SYNTHASE 3 (CALS3) abolishes the intercellular movement of miR165/166, indicating that the regulation of PD permeability is an effective way to control small RNA trafficking103. However, research on PD is challenging as mutants lacking PD are lethal and biochemical isolation of PD is difficult11,104.
Specific mechanisms for small RNA mobility.
Given that the biogenesis machinery is different for the different classes of small RNAs, it is possible that the biogenesis pathway affects the mobility of small RNAs. To investigate the movement capability of different types of small RNAs, de Felippes et al.105 generated artificial miRNA and ta-siRNA reporter lines, in which a small RNA was expressed in phloem companion cells but exerted its silencing effect in leaf mesophyll cells. When the miRNAs and ta-siRNAs were designed to have identical sequences, ta-siRNA-based silencing spread into a much broader area than miRNA-based silencing105, indicating an influence of small RNA biogenesis on the non-cell-autonomous effects of RNA silencing.
A more recent study revealed that miRNA mobility is regulated through a gating mechanism different from that regulating mobile proteins106. By using a miR-GFP sensor system, in which the artificial miRNA miR-GFP driven by tissue specific promoters silences a constitutively expressed GFP reporter, miRNA movement was shown to be directional across specific cell-cell interfaces106. This unidirectional movement generates selectivity in long-distance shoot-to-root trafficking and leads to domain-autonomous behaviours within plant stem cell niches. It should be noted that the two studies105,106 both employed artificial miRNAs. Further research is necessary to determine if endogenous miRNAs behave similarly.
Biological functions of RNA trafficking
Against the backdrop of a large set of mobile RNAs, only a relatively small number of them have been demonstrated to function non-cell-autonomously. In these cases, the mobile RNAs play roles in a broad range of physiological processes in plants. For example, long-distance trafficking of tomato PFP-T6 (ref.2) and pumpkin GAI (ref.107) transcripts was found to affect leaf morphology. miR166 and tasiR-ARFs act non-cell-autonomously in a concentration-dependent manner to generate sharp developmental boundaries to pattern leaf polarity108. Through grafting experiments, phloem-mobile FT was shown to function in systemic floral signalling to regulate flowering time in Arabidopsis56. RNA detection methods and heterografting experiments demonstrated that potato transcription factor BEL5 transcripts could be transported to stolon tips and induce tuber formation50,94. The transcripts of two other BEL1-like genes, BEL11 and BEL29, are also phloem-mobile and function antagonistically to BEL5 to fine-tune the development processes of potato tuberization109. In Arabidopsis, mobile AUX/IAA transcripts (ref.48) and miR165/166 (ref.5) regulate root development. Under stress conditions, miR395 (ref.73) and miR399 (refs73,83,84) act as long-distance signals to regulate sulfate and phosphate homeostasis, respectively. In addition, mobile siRNAs travel systemically to direct DNA methylation of transposable elements in target tissues, including meristematic and meiotically active cells6,76,77,79, which may contribute to epigenetic memory. The non-cell-autonomous nature of RNA silencing also facilitates plant defence against pathogens. On viral infection, siRNAs may act as mobile signals and move in advance of the spread of infection to prime antiviral silencing in uninfected cells to restrict viral spreading110,111. Certain types of small RNAs can even be transferred from plants to pathogens to induce cross-kingdom gene silencing to inhibit virulence gene expression41,88,112. These accumulating pieces of evidence indicate that RNA trafficking has a role in intercellular and systemic information communication that regulates fundamental biological processes in plants (Fig. 3).
Fig. 3 |. Dynamic network of intercellular communication.
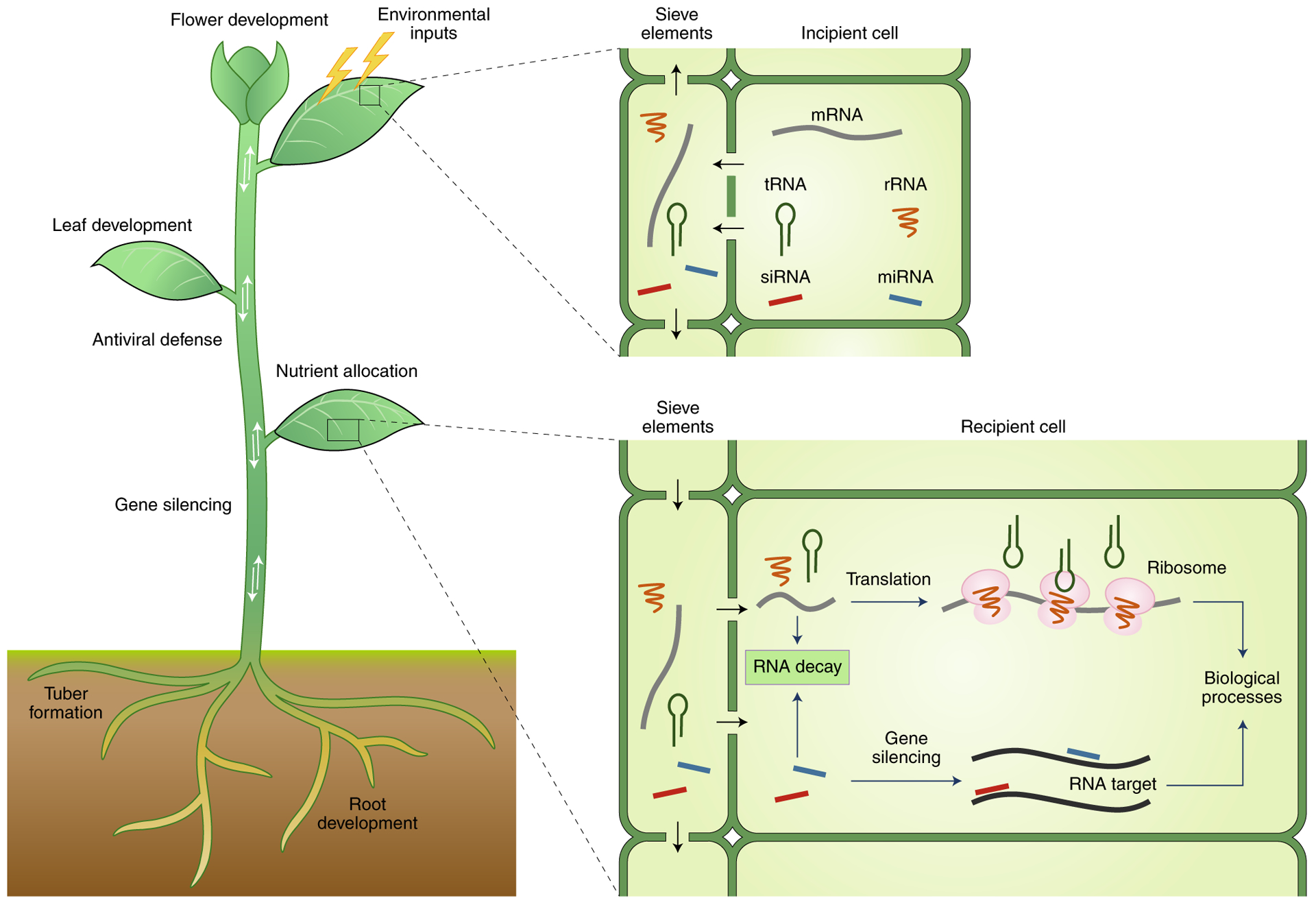
A hypothetical model of how RNA trafficking influences plant development and physiological processes. Internal or external stimuli may trigger the movement of plant RNAs, including mRNAs, siRNAs, miRNAs, rRNAs and tRNAs, from their synthesis sites (incipient cells) to distant tissues (recipient cells). These mobile RNAs may act non-cell-autonoumously. In the recipient cells, mRNAs, rRNAs and tRNAs may participate in translation, while miRNAs and siRNAs may mediate the silencing and regulation of genes. These mobile RNAs may therefore act as signalling molecules for intercellular information exchange to regulate plant development, nutrient allocation, stress responses and many other physiological processes in plants.
Grafting is routinely used in the cultivation of horticultural crops113. The large genomic-scale RNA exchange between scions and stocks raises the possibility that RNA trafficking benefits graft performance. The combination of two genetically different graft partners would increase the diversity of the RNA pool accessible to both scion and stock, and this merged RNA pool may provide plants with more genetic resources to achieve better traits, such as enhanced quality or yield. However, it is also possible that mobile RNAs underlie genetic incompatability leading to graft failure. It has been demonstrated that siRNAs can move into germ-line tissues to methylate target loci75,76,79, so DNA methylation patterns may be altered by grafting and changes could be transmitted to future progeny. Transcriptome analysis of parasitic plants and their hosts revealed that a large population of mRNAs moves in a bidirectional manner across species8,9,59. The host-parasite interaction has been compared to perfect heterografts, since both of them connect separate plants to form a chimaera. The movement of RNAs between hosts and parasites may function as a means of communication to coordinate their mutual development. The ability for translocation of RNAs from hosts to parasites raises the possibility of using RNA silencing as a strategy to control parasitic weeds114.
Concluding remarks
There have been many advances in documenting and understanding the non-cell autonomy of RNAs in recent years. Thousands of mobile RNAs have been identified through genomics approaches. The recently released database PlaMOM (Plant Mobile Macromolecules) collects and organizes published data on mobile RNAs115. A fluorescence-based RNA labelling system based on the bacteriophage coat protein MS2 has been recently optimized in plants and may potentially serve as a powerful approach for visualizing the real-time trafficking of specific mobile RNAs in living cells116. The discovery of RNAs functioning as mobile molecules is an exciting revelation in plant biology, since RNAs were traditionally believed to function in the same cell in which they are synthesized. With the spatial uncoupling of RNA synthesis and action, at least for some RNAs, the site of action of a given RNA cannot be definitively determined from promoter assays alone.
Despite these advances, many challenges remain. A considerable number of RNAs, but still a tiny portion of the mobile RNAome, have been shown to act as active, mobile signals with roles in coordinating plant growth, development and stress responses6,8–10. Although it has been confirmed that some mobile mRNAs can be translated into functional proteins after transport90, it remains unclear to what extent translation of mobile RNAs occurs in recipient tissues. Proteomic data on grafted plants revealed the presence of heterologous protein products that are perhaps encoded by mobile mRNAs8, however, the identified proteins are few in number due to their low abundance in recipient tissues. It is also difficult to distinguish between the proteins translated by mobile RNAs and proteins themselves that are mobile. Moving forward, many questions need to be addressed, such as: What are the underlying mechanisms of RNA trafficking? How are RNAs selected for export and import, and do mobile RNAs have other unknown common features? What are the factors that mediate RNA transport and how do they function? How do plants control the trafficking of RNAs into specific cells or tissues, and what is the fate of mobile RNAs in those recipient tissues? Are they translated and degraded in the same manner as local RNAs in recipient tissues? Are mobile small RNAs transported in single-or double-stranded forms, and are their precursors mobile? Are mobile RNAs conserved in different plant species? Is RNA movement a widespread and functional phenomenon in plant-parasite relationships? Answers to these questions will have broad impacts on our understanding of RNA trafficking and its role in intercellular signalling in plants.
Acknowledgements
We apologize for not being able to discuss or cite many studies due to limited space. Research in the Liu and Chen labs is supported by National Key Research and Development Program of China (91740202), National Institutes of Health (GM061146), Guangdong Innovation Research Team Fund (2014ZT05S078), National Science Foundation (IOS-1340001) and National Natural Science Foundation of China (31600982).
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reference
- 1.Otero S, Helariutta Y & Benitez-Alfonso Y Symplastic communication in organ formation and tissue patterning. Curr. Opin. Plant Biol 29, 21–28 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Kim M, Canio W, Kessler S & Sinha N Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293, 287–289 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Chitwood DH et al. Pattern formation via small RNA mobility. Genes Dev. 23, 549–554 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Sun L & Kragler F The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant Physiol. 150, 378–387 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsbecker A et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465, 316–321 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewsey MG et al. Mobile small RNAs regulate genome-wide DNA methylation. Proc. Nat1 Acad. Sci. USA 113, E801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z et al. Vascular-mediated signalling involved in early phosphate stress response in plants. Nat. Plants 2, 16033 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Thieme CJ et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat. Plants 1, 15025 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Kim G, LeBlanc ML, Wafula EK, dePamphilis CW & Westwood JH Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 345, 808–811 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Yang Y et al. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol. 15, 251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunkard JO, Runkel AM & Zambryski PC The cytosol must flow: intercellular transport through plasmodesmata. Curr. Opin. Cell Biol 35, 13–20 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Cantrill LC, Overall RL & Goodwin PB Cell-to-cell communication via plant endomembranes. Cell Biol. Int 23, 653–661 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Barton DA et al. Cell-to-cell transport via the lumen of the endoplasmic reticulum. Plant J. 66, 806–817 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Grabski S, De Feijter AW & Schindler M Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. Plant Cell 5, 25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson D Plasmodesmata spread their influence. F1000 Prime Rep. 7, 25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY Plasmodesmata: a signaling hub at the cellular boundary. Curr. Opin. Plant Biol 27, 133–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sager R & Lee JY Plasmodesmata in integrated cell signalling: insights from development and environmental signals and stresses. J. Exp. Bot 65, 6337–6358 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oparka KJ et al. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Crawford KM & Zambryski PC Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125, 1802–1812 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burch-Smith TM, Stonebloom S, Xu M & Zambryski PC Plasmodesmata during development: re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 248, 61–74 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haywood V, Kragler F & Lucas WJ Plasmodesmata: pathways for protein and ribonucleoprotein signaling. Plant Cell 14, S303–325 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy A, Erlanger M, Rosenthal M & Epel BL A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J 49, 669–682 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Kuhn C, Franceschi VR, Schulz A, Lemoine R & Frommer WB Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275, 1298–1300 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Lucas WJ et al. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270, 1980–1983 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Notaguchi M & Okamoto S Dynamics of long-distance signaling via plant vascular tissues. Front. Plant Sci 6, 161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Bel AJ Interaction between sieve element and companion cell and the consequences for photoassimilate distribution. Two structural hardware frames with associated physiological software packages in dicotyledons? J. Exp. Botany 47, 1129–1140 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Heo JO, Roszak P, Furuta KM & Helariutta Y Phloem development: current knowledge and future perspectives. Am. J. Bot 101, 1393–1402 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Ostendorp A et al. Functional analysis of Brassica napus phloem protein and ribonucleoprotein complexes. New Phytol. 214, 1188–1197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giavalisco P, Kapitza K, Kolasa A, Buhtz A & Kehr J Towards the proteome of Brassica napus phloem sap. Proteomics 6, 896–909 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Doering-Saad C, Newbury HJ, Bale JS & Pritchard J Use of aphid stylectomy and RT-PCR for the detection of transporter mRNAs in sieve elements. J. Exp. Bot 53, 631–637 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Sasaki T, Chino M, Hayashi H & Fujiwara T Detection of several mRNA species in rice phloem sap. Plant Cell Physiol. 39, 895–897 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Guo S et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet 45, 51–58 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Rabouille C Pathways of unconventional protein secretion. Trends Cell Biol. 27, 230–240 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Colombo M, Raposo G & Thery C Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol 30, 255–289 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Regente M et al. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 583, 3363–3366 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Prado N et al. Nanovesicles are secreted during pollen germination and pollen tube growth: a possible role in fertilization. Mol. Plant 7, 573–577 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Rutter BD & Innes RW Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 173, 728–741 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An Q, Huckelhoven R, Kogel KH & van Bel AJ Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell. Microbiol 8, 1009–1019 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Takemoto D, Jones DA & Hardham AR GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J. 33, 775–792 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Lu YJ et al. Patterns of plant subcellular responses to successful oomycete infections reveal differences in host cell reprogramming and endocytic trafficking. Cell. Microbiol 14, 682–697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai Q et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 8, 1126–1129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeken R et al. Identification of Arabidopsis thaliana phloem RNAs provides a search criterion for phloem-based transcripts hidden in complex datasets of microarray experiments. Plant J. 55, 746–759 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Gaupels F et al. Adaptation of aphid stylectomy for analyses of proteins and mRNAs in barley phloem sap. J. Exp. Bot 59, 3297–3306 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Medrano R, Xoconostle-Cazares B & Lucas WJ Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development 126, 4405–4419 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Omid A, Keilin T, Glass A, Leshkowitz D & Wolf S Characterization of phloem-sap transcription profile in melon plants. J. Exp. Bot 58, 3645–3656 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Doering-Saad C, Newbury HJ, Couldridge CE, Bale JS & Pritchard J A phloem-enriched cDNA library from Ricinus: insights into phloem function. J. Exp. Bot 57, 3183–3193 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Medina C, Atkins CA, Mann AJ, Jordan ME & Smith PM Macromolecular composition of phloem exudate from white lupin (Lupinus albus L.). BMC Plant Biol. 11, 36 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Notaguchi M, Wolf S & Lucas WJ Phloem-mobile Aux/IAA transcripts target to the root tip and modify root architecture. J. Integr. Plant Biol 54, 760–772 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Xoconostle-Cazares B et al. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283, 94–98 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Banerjee AK et al. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 18, 3443–3457 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahajan A, Bhogale S, Kang IH, Hannapel DJ & Banerjee AK The mRNA of a Knotted1-like transcription factor of potato is phloem mobile. Plant Mol. Biol 79, 595–608 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Kanehira A et al. Apple phloem cells contain some mRNAs transported over long distances. Tree Genet. Genom 6, 635–642 (2010). [Google Scholar]
- 53.Roney JK, Khatibi PA & Westwood JH Cross-species translocation of mRNA from host plants into the parasitic plant dodder. Plant Physiol. 143, 1037–1043 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David-Schwartz R, Runo S, Townsley B, Machuka J & Sinha N Long-distance transport of mRNA via parenchyma cells and phloem across the host-parasite junction in. Cuscuta. New Phytol 179, 1133–1141 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Goldschmidt EE Plant grafting: new mechanisms, evolutionary implications. Front. Plant Sci 5, 727 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu KJ, Huang NC, Liu YS, Lu CA & Yu TS Long-distance movement of Arabidopsis FLOWERING LOCUS T RNA participates in systemic floral regulation. RNA Biol. 9, 653–662 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Zhang H et al. Expression of tomato prosystemin gene in Arabidopsis reveals systemic translocation of its mRNA and confers necrotrophic fungal resistance. New Phytol. 217, 799–812 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Notaguchi M, Higashiyama T & Suzuki T Identification of mRNAs that move over long distances using an RNA-Seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant Cell Physiol. 56, 311–321 (2015). [DOI] [PubMed] [Google Scholar]
- 59.LeBlanc M, Kim G, Patel B, Stromberg V & Westwood J Quantification of tomato and Arabidopsis mobile RNAs trafficking into the parasitic plant Cuscuta pentagona. New Phytol. 200, 1225–1233 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Bologna NG & Voinnet O The diversity, biogenesis, and activities of endogenous silencing small RNAs in. Arabidopsis. Annu. Rev. Plant Biol 65, 473–503 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Rogers K & Chen X Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 25, 2383–2399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molnar A, Melnyk C & Baulcombe DC Silencing signals in plants: a long journey for small RNAs. Genome Biol. 12, 215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim YJ, Maizel A & Chen X Traffic into silence: endomembranes and post-transcriptional RNA silencing. EMBO J. 33, 968–980 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palauqui JC, Elmayan T, Pollien JM & Vaucheret H Systemic acquired silencing: transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 16, 4738–4745 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voinnet O & Baulcombe DC Systemic signalling in gene silencing. Nature 389, 553 (1997). [DOI] [PubMed] [Google Scholar]
- 66.Voinnet O, Vain P, Angell S & Baulcombe DC Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95, 177–187 (1998). [DOI] [PubMed] [Google Scholar]
- 67.Palauqui JC & Balzergue S Activation of systemic acquired silencing by localised introduction of DNA. Curr. Biol 9, 59–66 (1999). [DOI] [PubMed] [Google Scholar]
- 68.Melnyk CW, Molnar A & Baulcombe DC Intercellular and systemic movement of RNA silencing signals. EMBO J. 30, 3553–3563 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunoyer P, Himber C, Ruiz-Ferrer V, Alioua A & Voinnet O Intra- and intercellular RNA interference in Arabidopsis thaliana requires components of the microRNA and heterochromatic silencing pathways. Nat. Genet 39, 848–856 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Smith LM et al. An SNF2 protein associated with nuclear RNA silencing and the spread of a silencing signal between cells in Arabidopsis. Plant Cell 19, 1507–1521 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo BC et al. A systemic small RNA signaling system in plants. Plant Cell 16, 1979–2000 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buhtz A, Springer F, Chappell L, Baulcombe DC & Kehr J Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J. 53, 739–749 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Buhtz A, Pieritz J, Springer F & Kehr J Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol. 10, 64 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varkonyi-Gasic E, Gould N, Sandanayaka M, Sutherland P & MacDiarmid RM Characterisation of microRNAs from apple (Malus domestica ‘Royal Gala’) vascular tissue and phloem sap. BMC Plant Biol. 10, 159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slotkin RK et al. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136, 461–472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinez G, Panda K, Kohler C & Slotkin RK Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell. Nat. Plants 2, 16030 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Molnar A et al. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 328, 872–875 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Melnyk CW, Molnar A, Bassett A & Baulcombe DC Mobile 24 nt small RNAs direct transcriptional gene silencing in the root meristems of Arabidopsis thaliana. Curr. Biol 21, 1678–1683 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Zhang W et al. Graft-transmissible movement of inverted-repeat-induced siRNA signals into flowers. Plant J. 80, 106–121 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Parizotto EA, Dunoyer P, Rahm N, Himber C & Voinnet O In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev. 18, 2237–2242 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alvarez JP et al. Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell 18, 1134–1151 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knauer S et al. A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev. Cell 24, 125–132 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Pant BD, Buhtz A, Kehr J & Scheible WR MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J. 53, 731–738 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin SI et al. Regulatory network of microRNA399 and PHO2 by systemic signalling. Plant Physiol. 147, 732–746 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huen AK, Rodriguez-Medina C, Ho AY, Atkins CA & Smith PM Long-distance movement of phosphate starvation-responsive microRNAs in. Arabidopsis. Plant Biol 19, 643–649 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Tsikou D et al. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science http://doi.org/ct72. (2018) [DOI] [PubMed] [Google Scholar]
- 87.Shahid S et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 553, 82–85 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Zhang T et al. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2, 16153 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Kragler F RNA in the phloem: A crisis or a return on investment? Plant Sci. 178, 99–104 (2010). [Google Scholar]
- 90.Zhang W et al. tRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell 28, 1237–1249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calderwood A, Kopriva S & Morris RJ Transcript abundance explains mRNA mobility data in Arabidopsis thaliana. Plant Cell 28, 610–615 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paultre DS, Gustin MP, Molnar A & Oparka KJ Lost in transit: long-distance trafficking and phloem unloading of protein signals in Arabidopsis homografts. Plant Cell 28, 2016–2025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Westwood JH RNA transport: Delivering the message. Nat. Plants 1, 15038 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Banerjee AK, Lin T & Hannapel DJ Untranslated regions of a mobile transcript mediate RNA metabolism. Plant Physiol. 151, 1831–1843 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carella P, Wilson DC, Kempthorne CJ & Cameron RK Vascular sap proteomics: Providing insight into long-distance signaling during stress. Front. Plant Sci 7, 651 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gomez G, Torres H & Pallas V Identification of translocatable RNA-binding phloem proteins from melon, potential components of the long-distance RNA transport system. Plant J. 41, 107–116 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Ham BK et al. A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 21, 197–215 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho SK et al. Polypyrimidine tract-binding proteins of potato mediate tuberization through an interaction with StBEL5 RNA. J. Exp. Bot 66, 6835–6847 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ham BK, Li G, Jia W, Leary JA & Lucas WJ Systemic delivery of siRNA in pumpkin by a plant PHLOEM SMALL RNA-BINDING PROTEIN 1-ribonucleoprotein complex. Plant J. 80, 683–694 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Sevilem I, Yadav SR & Helariutta Y Plasmodesmata: channels for intercellular signaling during plant growth and development. Methods Mol. Biol 1217, 3–24 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Balachandran S, Xiang Y, Schobert C, Thompson GA & Lucas WJ Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc. Natl Acad. Sci. USA 94, 14150–14155 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aoki K, Kragler F, Xoconostle-Cazares B & Lucas WJ A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc. Natl Acad. Sci. USA 99, 16342–16347 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vaten A et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144–1155 (2011). [DOI] [PubMed] [Google Scholar]
- 104.Grison MS, Fernandez-Calvino L, Mongrand S & Bayer EM Isolation of plasmodesmata from Arabidopsis suspension culture cells. Methods Mol. Biol 1217, 83–93 (2015). [DOI] [PubMed] [Google Scholar]
- 105.de Felippes FF, Ott F & Weigel D Comparative analysis of non-autonomous effects of tasiRNAs and miRNAs in Arabidopsis thaliana. Nucleic Acids Res. 39, 2880–2889 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skopelitis DS et al. Gating of miRNA movement at defined cell-cell interfaces governs their impact as positional signals. Nat. Commun 9, 3107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haywood V, Yu TS, Huang NC & Lucas WJ Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 42, 49–68 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Skopelitis DS, Benkovics AH, Husbands AY & Timmermans MCP Boundary formation through a direct threshold-based readout of mobile small RNA gradients. Dev. Cell 43, 265–273 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Ghate TH, Sharma P, Kondhare KR, Hannapel DJ & Banerjee AK The mobile RNAs, StBEL11 and StBEL29, suppress growth of tubers in potato. Plant Mol. Biol 93, 563–578 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Dunoyer P, Melnyk C, Molnar A & Slotkin RK Plant mobile small RNAs. Cold Spring Harb. Perspect. Biol 5, a017897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sarkies P & Miska EA Small RNAs break out: the molecular cell biology of mobile small RNAs. Nat. Rev. Mol. Cell Biol 15, 525–535 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Weiberg A & Jin H Small RNAs-the secret agents in the plant-pathogen interactions. Curr. Opin. Plant Biol 26, 87–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Melnyk CW & Meyerowitz EM Plant grafting. Curr. Biol 25, 183–188 (2015). [DOI] [PubMed] [Google Scholar]
- 114.Yoder JI, Gunathilake P, Wu B, Tomilova N & Tomilov AA Engineering host resistance against parasitic weeds with RNA interference. Pest Manag. Sci 65, 460–466 (2009). [DOI] [PubMed] [Google Scholar]
- 115.Guan D et al. PlaMoM: a comprehensive database compiles plant mobile macromolecules. Nucleic Acids Res. 45, 1021–1028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luo KR, Huang NC & Yu TS Selective targeting of mobile mRNAs to plasmodesmata for cell-to-cell movement. Plant Physiol. http://doi.org/gdrsfz. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


