Chapter 9.1. Introduction
Gerald J. Gleich, Kristin Leiferman
Overall, this section of the book summarizes our current understanding of the ability of the eosinophil to neutralize respiratory viruses and the mechanisms by which this might occur. Taken together, these three chapters highlight important information of the eosinophil’s role in both innate and adaptive immune responses.
In the beginning, the eosinophil was a mystery leukocyte, brilliantly staining but without obvious function. The earliest clues came from the application of Paul Ehrlich’s then new (in the latter part of the 19th century) technology for staining blood revealing associations with asthma and helminthiasis. By the end of Ehrlich’s life in 1915, the eosinophil had been further linked to guinea pig anaphylaxis and, subsequently, human hypersensitivity reactions. However, for a time, the quest for more discoveries seemed stuck, and anaphylaxis, asthma, and helminth infections appeared to be the major diseases in which eosinophils had a role. This view was not to last. Once eosinophil granule proteins and reagents to localize them were available, it became clear that eosinophils participate in diseases that would not be predicted based on blood counts. Along with many inflammatory disorders, a role for eosinophils in infectious diseases beyond parasitosis has emerged. This section discusses eosinophil function relating to bacterial, fungal, and viral diseases, and strong cases are made for important eosinophil contributions to some of these diseases.
Observations dating to 1893 reported that blood eosinophils were reduced during bacterial infections. Much later, in the mid-1970s, studies in murine models showed that pyelonephritis caused by Escherichia coli and early subcutaneous pneumococcal abscesses produced eosinopenia, whereas trichinosis infections were accompanied by eosinophilia.1 Remarkably, establishment of pyelonephritis or pneumococcal abscesses suppressed the eosinophilia induced by trichinosis infection.2 A factor that caused eosinopenia was identified and partially characterized, but its molecular identity was not determined.3 Eosinopenia was produced by the injection of chemotactic factors, such as complement component C5a,4 so that they could account for at least part of the eosinopenia observed during bacterial infection. Numerous studies have compared the phagocytic and bactericidal activities of eosinophils and neutrophils,5., 6., 7., 8., 9. and, overall, the eosinophil emerges as less able to ingest and kill bacteria than the neutrophil. However, experimental conditions in these studies varied, and the activation status of the cell, i.e., whether derived from a healthy, normal subject or a patient with eosinophilia, was important because cells from patients with eosinophilia were more active.10 Recognition that eosinophil granule proteins function as toxins prompted studies to determine their ability to kill bacteria, and results of the studies clearly showed that granule proteins kill both gram-negative and gram-positive bacteria.11 Nonetheless, few investigations pointed to an important role for eosinophils in bacterial disease. More recent studies indicate a mechanism by which eosinophils are able to kill bacteria, summarized in this chapter by Simon and Yousefi. They focus, in particular, on the formation of extracellular DNA traps generated by eosinophils, and the demonstration that these traps are able to bind bacteria and kill them. The traps are formed by the extrusion of mitochondrial DNA, referred to as catapult-like because of its rapidity, and by the deposition of granule proteins on the extruded DNA. Earlier studies had shown that DNA avidly binds eosinophil granule major basic protein,12 and, most probably, this complex is stable. Simon and colleagues found that eosinophil DNA traps are present in eosinophil-associated inflammatory diseases such as skin diseases and bronchial asthma. In other work, studies on eosinophil-deficient mice show reduced ability to clear Pseudomonas species from the peritoneal cavity and increased protection in the presence of added eosinophils.13 Therefore, the old literature’s teaching that eosinophils are not important in bacterial diseases must be questioned on the basis of these new findings.
Concerning fungal diseases and eosinophilia and in contrast to the comments above on the relationship between bacterial diseases and eosinophils, the literature is bereft of reports on eosinophil-fungus interactions. While numerous observations show that mucin derived from the sinus cavities of patients suffering from chronic rhinosinusitis (CRS) contains fungal elements, eosinophils, and Charcot–Leyden crystals, no studies had explored the mechanisms of eosinophil-fungus interactions. In this chapter, Kita presents a summary of the mechanisms by which the immune system responds to fungi. Extracts from numerous fungal species activate eosinophils from normal individuals with release of eosinophil-derived neurotoxin (RNase2); interestingly, Alternaria extracts do not correspondingly induce neutrophil activation. Investigation of the mechanism by which Alternaria extracts activate eosinophils concludes that the G protein-coupled protease-activated receptor is critical in the process. Eosinophils interact with living Alternaria alternata and release granule proteins onto the surface of the organism with death of the fungus. This interaction is mediated by the adherence of eosinophils through the β2 integrin, integrin alpha-M (ITAM/CD11b), possibly through recognition of β-glucans. Thus, two key factors appear important for the interactions of eosinophils with fungi, namely PAR and ITAM (CD11b). Kita then reviews the mechanisms of fungal-mediated eosinophil inflammation in vivo, and he stresses the importance of chitin and fungal proteases possibly via respiratory epithelial-derived molecules, such as thymic stromal lymphopoietin, interleukin-33, and chemokines. He discusses diseases associated with eosinophilia and fungi, including allergic bronchopulmonary Aspergillus, severe asthma with fungal sensitization, allergic fungal sinusitis, and CRS. Treatment of certain eosinophil-associated diseases with anti-fungal agents has led to clinical improvement, whereas, in others, the response has been equivocal, especially in CRS, with the caveat that antifungal medications do not penetrate well into sinus cavities. Overall, this review is a summary of heretofore lacking information on immune responses to fungi and eosinophil participation and is a valuable summary of our current knowledge.
Interest in eosinophils and viruses stems from observations that respiratory syncytial virus (RSV) infection may be associated with eosinophilia. Further attention to a relationship emerged when a clinical trial of formalin-inactivated RSV vaccine resulted in strikingly more severe disease after subsequent RSV infection, with children developing enhanced disease and showing pronounced tissue eosinophilia. This raised the question whether eosinophils were responsible for the worsened outcome in the vaccinated children. The studies pertaining to these observations are reviewed in detail by Rosenberg and colleagues. Information supporting a protective role for eosinophils comes from studies of guinea pigs sensitized by allergen administration and then virus challenged. These animals showed a reduced parainfluenza/Sendai viral content, suggesting that the eosinophil, in an interleukin-5 (IL-5)-dependent manner, neutralized virus. The mechanisms by which this might occur still remain obscure. This chapter particularly is concerned with models of primary virus challenge in mice. Although the results from these models still leaves the role of the eosinophil in doubt, a caveat here is that the murine eosinophil seems to degranulate less readily than the human eosinophil and, therefore, results in murine models may be misleading. In further exploring what is known about eosinophil-virus interactions, Rosenberg and colleagues allude to investigations with the pneumonia virus of mice (PVM), using human C-C motif chemokine 24 (CCL24/eotaxin-2)/mouse IL-5 double transgenic mice in which eosinophils in the respiratory tract demonstrate marked degranulation,14 and state that they observed accelerated PVM clearance in this model. Hence, the human CCL24 (eotaxin-2)/mouse IL-5 double transgenic mice may provide a unique insight into the potential maximal effects of the activated eosinophil. Overall, this chapter summarizes our current understanding of the ability of the eosinophil to neutralize respiratory viruses and the mechanisms by which this might occur.
Taken together, these three chapters highlight important information of the eosinophil’s role in both innate and adaptive immune responses.
References
- 1.Bass D.A. Behavior of eosinophil leukocytes in acute inflammation. I. Lack of dependence on adrenal function. J Clin Invest. 1975;55(6):1229–1236. doi: 10.1172/JCI108041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass D.A. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest. 1975;56(4):870–879. doi: 10.1172/JCI108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass D.A. Reproduction of the eosinopenia of acute infection by passive transfer of a material obtained from inflammatory exudate. Infect Immun. 1977;15(2):410–416. doi: 10.1128/iai.15.2.410-416.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass D.A. Eosinopenia of acute infection: Production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. 1980;65(6):1265–1271. doi: 10.1172/JCI109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baehner R.L., Johnston R.B., Jr. Metabolic and bactericidal activities of human eosinophils. Br J Haematol. 1971;20(3):277–285. doi: 10.1111/j.1365-2141.1971.tb07038.x. [DOI] [PubMed] [Google Scholar]
- 6.Mickenberg I.D., Root R.K., Wolff S.M. Bactericidal and metabolic properties of human eosinophils. Blood. 1972;39(1):67–80. [PubMed] [Google Scholar]
- 7.DeChatelet L.R. Comparison of intracellular bactericidal activities of human neutrophils and eosinophils. Blood. 1978;52(3):609–617. [PubMed] [Google Scholar]
- 8.Migler R., DeChatelet L.R., Bass D.A. Human eosinophilic peroxidase: role in bactericidal activity. Blood. 1978;51(3):445–456. [PubMed] [Google Scholar]
- 9.Yazdanbakhsh M. Bactericidal action of eosinophils from normal human blood. Infect Immun. 1986;53(1):192–198. doi: 10.1128/iai.53.1.192-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bass D.A. Comparison of human eosinophils from normals and patients with eosinophilia. J Clin Invest. 1980;66(6):1265–1273. doi: 10.1172/JCI109978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehrer R.I. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142(12):4428–4434. [PubMed] [Google Scholar]
- 12.Gleich G.J. Physiochemical and biological properties of the major basic protein from guinea pig eosinophil granules. J Exp Med. 1974;140(2):313–332. doi: 10.1084/jem.140.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linch S.N. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77(11):4976–4982. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochkur S.I. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178(12):7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
Chapter 9.2. Eosinophil-Mediated Antibacterial Host Defense
Although eosinophils are perceived as contributing to defense mechanisms against parasites, their exact functions in innate immunity remain unclear. Eosinophils use several strategies to eliminate invading microbes, including:1 phagocytosis and subsequent intracellular killing;2 secretion of cationic proteins; and3 formation of extracellular DNA traps. Eosinophil cationic proteins appear to be the most important antimicrobial proteins used in all three known antimicrobial strategies used by eosinophils. Eosinophil extracellular traps have recently been described and are composed of mitochondrial DNA and granule proteins. They can be generated by eosinophils activated by lipopolysaccharide from gram-negative bacteria following interleukin-5 or interferon gamma priming, and can bind and kill bacteria. Eosinophil-derived DNA traps have been identified in multiple infectious, autoimmune, and allergic diseases. Their role in innate immunity is still insufficiently understood and requires additional research.
The primary function of eosinophils has previously been related to their interactions with helminthic parasites,1 although this view has attracted some controversy. The cytoplasmic granules, which are believed to play an important role in host defense, consist of four distinct populations that can be identified by electron microscopy as primary granules, secondary granules, small granules, and lipid bodies.2 The cytotoxic cationic proteins are stored in the secondary granules that consist of a core, which contains eosinophil granule major basic protein 1 (MBP-1), and a matrix composed of eosinophil cationic protein (ECP), eosinophil-derived neurotoxin, and eosinophil peroxidase (EPO).3 MBP-1 is highly cytotoxic,4 and because of its cationic nature, it affects the charge of surface membranes resulting in disturbed permeability, and disruption and injury of cell membranes.5 Likewise, ECP can damage target cell membranes through the formation of pores or trans-membrane channels, but also has additional cytotoxic effects.6 Eosinophils have also been implicated in antiviral defense mechanisms.7., 8., 9.
Besides the antihelminthic and antiviral effects of at least some of the eosinophil granule proteins, antibacterial activities have also been demonstrated. By generating cytokines and chemokines, and by their ability to act as antigen-presenting cells, eosinophils may play different roles in antibacterial defense, although these topics are covered elsewhere in the book. In this chapter, we focus on our understanding of how eosinophils directly fight bacteria. For instance, ECP and MBP-1 can exhibit bactericidal activities by causing the permeabilization of the outer and inner membranes of Escherichia coli.10 Moreover, eosinophil-derived reactive oxygen species, in combination with EPO, are efficient in destroying E. coli,11 and eosinophil granules have also been implicated in the destruction of Pseudomonas aeruginosa.12 The antibacterial properties of eosinophils have also been demonstrated in hypereosinophilic interleukin-5 (IL-5) transgenic mice or following the adoptive transfer of eosinophils in wild-type or eosinophil-deficient mice,12., 13. showing the importance of eosinophils in clearing bacteria in vivo. These data are supported by the observation that mice with congenital eosinophil deficiency (i.e., PHIL mice) show impaired bacterial clearance in an experimental model of Pseudomonas infection.12
The killing of bacteria might take place after phagocytosis (Fig. 9.2.1 ), which eosinophils are able to perform.14 Subsequently, phagocytosis of gram-positive Staphylococcus aureus and gram-negative E. coli by eosinophils was demonstrated in vitro.15 At least in equine eosinophils, eosinophil granules discharge their contents into the phagocytic vacuole,14 providing one possible indication of how granule proteins could exhibit an antibacterial function. An alternative strategy to killing bacteria might be eosinophil degranulation16 (Fig. 9.2.1). In such a scenario, eosinophil basic proteins could be released in the vicinity of infection. While the activation of eosinophils can occur via multiple different receptors, immunoglobulin A (IgA) receptors are particularly efficient in eliciting eosinophil degranulation on cross-linking.17., 18. IgA-mediated activation of eosinophils might be particularly important in the gastrointestinal mucosa, where secretory IgA is produced in high quantities.
FIGURE 9.2.1.

Antibacterial strategies used by eosinophils.
Phagocytosis: Bacteria are ingested (blue). Granules (red) release cationic proteins into the phagosome. Degranulation: Granules and/or granule proteins are released in the extracellular space to kill the bacteria, but can also cause widespread tissue damage. Extracellular DNA traps: The incorporation of granule proteins into DNA traps, which also bind bacteria, likely increases the local concentration of antibacterial proteins and might limit tissue damage.
Recently, a third strategy has suggested that extracellular DNA traps generated by eosinophils in the extracellular space are able to bind and kill bacteria was proposed (Fig. 9.2.1). Such mechanism might play a role in case of epithelial barrier defects of the gastrointestinal tract, avoiding the bacterial invasion of the body.13 Epithelial barrier defects may occur due to inflammatory responses. But how are DNA traps formed? Under in vitro conditions, eosinophils need to be stimulated with IL-5 or interferon gamma (IFN-γ) for 20 min before being stimulated with lipopolysaccharide (LPS). However, nonbacterial triggers, e.g., complement component 5a or eotaxin, are also able to promote efficient DNA release from eosinophils.13
Although DNA seems to be required for efficient bacterial destruction,13 it is unlikely that DNA carries out this function. Indeed, extracellular ECP and MBP-1 were detected as colocalizing with DNA, as assessed by double immunofluorescence and confocal microscopy. Thus, it is likely that bacterial death is actually mediated by granule proteins within extracellular DNA traps. Time-lapse confocal imaging allowed the analysis of the kinetics of DNA release in single cells. Strikingly, DNA release happened within 1 s. The mechanism(s) of DNA release appear(s) to differ from the secretion of granule proteins that occur either by classical exocytosis or piecemeal degranulation. Time-lapse confocal imaging revealed that DNA is released from perinuclear structures. Combined two-color DNA and mitochondrial staining suggested that IL-5-primed and LPS-stimulated eosinophils release mitochondrial DNA, which was subsequently confirmed by using molecular biological techniques.13 Release of mitochondrial DNA was independent of cell death/apoptosis.
Extracellular DNA traps can also be generated by activated neutrophils.19 The DNA here is associated with granule proteins, such as elastase or myeloperoxidase.19 However, neutrophil DNA traps may additionally contain histones.19 In contrast to eosinophils, much more information is available regarding the pathogens trapped and killed by neutrophil DNA traps; these include gram-positive and gram-negative bacteria, fungi, and parasites.20 The release of DNA can occur within minutes21., 22. or hours;23 in the latter case, cell death appears to be required.
Interestingly, eosinophil DNA traps were also seen in inflammatory skin diseases24 and in bronchial asthma.25 The primary function of these extracellular structures remains unclear under these conditions, although it is possible that eosinophils participate in anti-infection defense mechanisms in at least some of these subjects/diseases. On the other hand, the binding of released eosinophil cationic proteins to extracellular DNA may limit the collateral damage from granular contents in eosinophilic inflammatory diseases.
Taken together, there is accumulating evidence that eosinophils play a beneficial role in innate immune responses against bacteria. This suggests that therapies aiming to deplete eosinophils may cause increased susceptibility toward bacterial infections, although no such adverse effects were observed when treating patients with anti-IL-5 antibody.26., 27. Clearly, many additional questions remain. For instance:
-
•
Do eosinophils play a role in the fight against pathogens in asthma and other allergic diseases?
-
•
Under which conditions do eosinophil cationic proteins exhibit antimicrobial properties?
-
•
What are the exact molecular mechanisms of extracellular DNA release and how long do DNA traps remain in tissues?
-
•
How do DNA traps correlate with other markers of inflammation? Can they be used as biomarkers of eosinophil activation? Do they prevent exaggerated eosinophil-mediated tissue pathology?
The mechanisms of indirect protection against bacteria (e.g., promotion of epithelial repair and bridging innate and adaptive immunity) also remain largely unexplored.
Acknowledgements
The Swiss National Foundation supports the laboratory research of the authors.
References
- 1.Klion A.D., Nutman T.B. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 2.Kariyawasam H.H., Robinson D.S. The eosinophil: the cell and its weapons, the cytokines, its locations. Semin Respir Crit Care Med. 2006;27:117–127. doi: 10.1055/s-2006-939514. [DOI] [PubMed] [Google Scholar]
- 3.Peters M.S., Rodriguez M., Gleich G.J. Localization of human eosinophil granule major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin by immunoelectron microscopy. Lab Invest. 1986;54:656–662. [PubMed] [Google Scholar]
- 4.Gleich G.J., Frigas E., Loegering D.A., Wassom D.L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925–2927. [PubMed] [Google Scholar]
- 5.Kroegel C., Costabel U., Matthys H. Mechanism of membrane damage mediated by eosinophil major basic protein. Lancet. 1987;1:1380–1381. doi: 10.1016/s0140-6736(87)90686-6. [DOI] [PubMed] [Google Scholar]
- 6.Young J.D., Peterson C.G., Venge P., Cohn Z.A. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- 7.Phipps S., Lam C.E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H.F. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 8.Gleich G.J., Loegering D.A., Bell M.P., Checkel J.L., Ackerman S.J., McKean D.J. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonucleases. Proc Natl Acad Sci USA. 1986;83:3146–3150. doi: 10.1073/pnas.83.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg H.F., Domachowske J.B. Eosinophils, eosinophil ribonucleases, and their role in host defence against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 10.Lehrer R.I., Szklarek D., Barton A., Ganz T., Hammann K.J., Gleich G.J. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142:4428–4434. [PubMed] [Google Scholar]
- 11.Persson T., Andersson P., Bodelsson M., Laurell M., Malm J., Egesten A. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect Immun. 2001;69:3591–3596. doi: 10.1128/IAI.69.6.3591-3596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linch S.N., Kelly A.M., Danielson E.T., Pero R., Lee J.J., Gold J.A. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77:4976–4982. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 14.Archer G.T., Hirsch J.G. Motion picture studies on degranulation of horse eosinophils during phagocytosis. J Exp Med. 1963;118:287–294. doi: 10.1084/jem.118.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cline M.J., Hanifin J., Lehrer R.I. Phagocytosis by human eosinophils. Blood. 1968;32:922–934. [PubMed] [Google Scholar]
- 16.Melo R.C., Spencer L.A., Dvorak A.M., Weller P.F. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229–236. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Ghazaleh R.I., Fujisawa T., Mestecky J., Kyle R.A., Gleich G.J. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–2400. [PubMed] [Google Scholar]
- 18.Monteiro R.C., Hostoffer R.W., Cooper M.D., Bonner J.R., Gartland G.L., Kubagawa H. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest. 1993;92:1681–1685. doi: 10.1172/JCI116754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 20.Papayannopoulos V., Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 22.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon D., Hoesli S., Roth N., Staedler S., Yousefi S., Simon H.U. Eosinophil extracellular DNA traps in skin diseases. J Allergy Clin Immunol. 2011;127:194–199. doi: 10.1016/j.jaci.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Dworski R., Simon H.U., Hoskins A., Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260–1266. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothenberg M.E., Klion A.D., Roufosse F.E., Kahn J.E., Weller P.F., Simon H.U. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 27.Straumann A., Conus S., Grzonka P., Kita H., Kephart G., Bussmann C. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomized, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
Chapter 9.3. Interactions of Eosinophils with Respiratory Virus Pathogens
Helene F. Rosenberg, Kimberly D. Dyer, Joseph B. Domachowske
In this chapter, we review current concepts relating to eosinophils, their interactions with respiratory pathogens and contributions to the pathophysiology of respiratory virus infection. Most information on this subject focuses on the ubiquitous pediatric pathogen, human respiratory syncytial virus (RSV). A formalin-inactivated RSV vaccine used in a human trial in the 1960s elicited severe pathology in response to subsequent naturally-encountered RSV infection, including recruitment of eosinophils to the lung. Interestingly, despite assumptions to the contrary, recent evidence from mouse models suggests that eosinophils are probably not promoting the clinical symptoms or respiratory pathology characteristic of this condition. Similarly, pulmonary eosinophilia elicited in response to primary RSV infection has been observed in the youngest human infants and in neonatal mouse virus challenge models. Although pulmonary eosinophilia is nearly always perceived in a negative light, the specific roles played by virus-elicited eosinophils—negative, positive or neutral bystander—remain for the most part unclear. Lastly, we consider the role of eosinophils in promoting virus clearance and antiviral host defense, and conclude with a recent study regarding eosinophils as targets of virus infection.
Introduction
Eosinophils, granule-bearing leukocytes found in peripheral blood and tissues, are best known for their roles in asthma, allergy, and other disorders in which they are recruited in response to cytokines released by T-helper type 2 (Th2) lymphocytes. Eosinophils do not ordinarily come to mind when one thinks generally of a respiratory virus infection. However, at least for one important respiratory virus, the human respiratory syncytial virus (RSV), eosinophils and their unique secretory mediators have been detected in lung tissue in response to primary infection and as a feature of a characteristic hypersensitivity response to inactivated vaccines and vaccine components. Interestingly, eosinophil recruitment and accumulation are almost always perceived in a negative light, as it is assumed that these cells contribute to tissue damage, bronchoconstriction, and respiratory dysfunction via degranulation of their cationic secretory proteins, enzymes, and cytokines. However, recent descriptions of antiviral activity both in vitro and in vivo suggest that eosinophil function may encompass both of these functions, and present more of a double-edged sword. Clearly, we do not have a complete understanding of the role of eosinophils in disease caused by RSV; here we highlight many of the questions that remain to be explored.
Human Respiratory Syncytial Virus Disease
RSV infection is a near universal affliction of infancy and childhood, accounting for approximately 50% of all pneumonia and up to 90% of the reported cases of bronchiolitis in infancy. Of those infants infected during the first year of life, one-third develops lower respiratory tract disease and 2.5% are hospitalized, accounting for more than 90,000 children in the United States every year. In many previously healthy infants, RSV disease is a mild and self-limited infection involving the upper and lower respiratory tract, with varying degrees of peribronchiolar and interstitial inflammation. In others, disease progresses to severe bronchiolitis and pneumonia, including submucosal edema and bronchiolar obstruction requiring oxygen, and in the worst cases, mechanical ventilation. Infants at particularly high risk for severe disease include those born prematurely, infants and children with cardiac or pulmonary anomalies, and immunocompromised infants and children, although a recent study by Hall and colleagues1 noted that a substantial proportion of children with serious RSV disease had no pre-existing predisposing condition. Prophylactic monoclonal antibody therapy is available for high-risk infants only, and no vaccine has been approved for use. RSV has also recently been recognized as an important pathogen in the institutionalized elderly. The clinical features and pathology of RSV disease have been reviewed extensively, and the reader is referred to these and other excellent sources of information.2., 3.
Basic Biology of Human and Mouse Eosinophils
Eosinophils are leukocytes of the granulocyte lineage, as are neutrophils and basophils. Eosinophils differentiate in the bone marrow from CD34 antigen-positive pluripotent progenitor cells and are released into the bloodstream in a more or less completely mature state. Under normal, homeostatic conditions, very few eosinophils can be detected in peripheral blood (only approximately 2–3% of total leukocytes), as the vast majority reside in the tissues, primarily in the gastrointestinal tract. In response to as yet incompletely characterized stimuli, typically observed in allergic states, during infection with helminthic parasites, and in some idiopathic hypereosinophilic states, Th2 lymphocytes are activated, which results in the production of a specific subset of Th2 cytokines, including interleukin-5 (IL-5). IL-5 has a unique impact on the eosinophil lineage, as it induces the expansion of eosinophil progenitors in the bone marrow, it primes eosinophils in the periphery, and it prolongs eosinophil survival in the tissues. Eosinophils are capable of responding to a wide variety of other stimuli and can undergo chemotaxis in response to eotaxin (CCL11), MIP1-alpha (CCL3) and RANTES (CCL5), which are chemoattractant cytokines that interact with eosinophils via specific cell surface receptors CCR3, CCR1 and CCR5, respectively. Interestingly, despite years of research, there is still no absolute consensus on eosinophil physiology and function, even in well-characterized disease states. For example, while eosinophils and eosinophil secretory mediators can promote destruction of helminthic eggs and larvae in experiments performed in vitro, experiments performed in vivo with cytokine-deficient and eosinophil-deficient mice have yielded complex and inconsistent results. Similarly, although the weight of evidence suggests that eosinophils contribute to the pathophysiology of allergy and asthma, a chronic respiratory disease in which bronchoconstriction in response to environmental triggers is typically associated with production of Th2 cytokines and recruitment of eosinophils to the airways, asthmatic responses are obviously negative sequelae of eosinophil function that alone cannot represent a direct evolutionary advantage to the host organism. Among the more recent hypotheses, several groups have focused on eosinophils as immunomodulatory mediators, as eosinophils can interact both directly and indirectly with T cells and mast cells, and can release a wide variety of preformed cytokines and other secretory mediators, primarily from cytoplasmic granules. Other chapters in this volume provide more extensive coverage of these subjects.
Mature eosinophils from all species are readily recognized by their eccentric bilobed nuclei and their characteristic red-staining cytoplasmic granules. As noted, human eosinophil granules are storage sites for cationic secretory mediators, including a unique eosinophil peroxidase, the eosinophil granule major basic protein 1 (MBP-1), eosinophil cationic protein (ECP/ribonuclease 3), eosinophil-derived neurotoxin (EDN/ribonuclease 2), and numerous enzymes and cytokines. Despite similar morphology, human and mouse eosinophils differ from one another and cannot be presumed to function identically in all circumstances. For instance, while there are several reports describing a high-affinity immunoglobulin E (IgE) receptor [high affinity immunoglobulin epsilon receptor subunit beta (FcεRI)] in human eosinophils from parasite-infected and asthmatic subjects,4., 5., 6. this finding and its functional significance has been questioned,7 and FcεRI has never been detected on eosinophils isolated from mice. Similarly, sialic acid-binding Ig-like lectin 8 (Siglec-8) can be detected on the surface of human eosinophils, while mouse eosinophils express the highly divergent functional orthologue, Siglec-F (Siglec-5). The mouse eosinophil ribonucleases are highly divergent orthologues of human ECP and EDN8 and Charcot–Leyden crystal protein, a major human eosinophil component, cannot even be identified in the mouse genome. Mouse eosinophils also display a profoundly reduced propensity to degranulate and undergo differential chemotaxis to known exogenous stimuli (reviewed in9). As such, eosinophils from humans and mice may look similar to one another, but they may not be formally identical to one another in their actions and in their capacity to cause, to ameliorate, or even to serve as biomarkers for disease.
A number of recent studies have associated eosinophils and eosinophil degranulation products with various aspects of RSV infection in both human disease and in parallel mouse models, which need to be understood with the aforementioned caveats in mind. Here, we review some of these findings with an eye toward understanding what is and what is not known regarding the role of eosinophils, their role in vaccine-induced pathology, their interactions with respiratory virus pathogens, and the outcome of severe respiratory virus disease.
Hypersensitivity Responses to Formalin-Inactivated Respiratory Syncytial Virus Vaccine—Are the Eosinophils at Fault?
In the early 1960s, a number of children were enrolled in a clinical trial of a formalin-inactivated RSV vaccine. The negative outcomes of this trial, including records of the detailed responses of the vaccinated children who encountered natural RSV infection sometime thereafter, have been documented and reviewed.10., 11. Briefly, children immunized with the formalin-inactivated virus, upon encountering a natural RSV challenge, developed a hypersensitivity response, characterized by bronchoconstriction and severe pneumonia; this has been attributed in part to the development of non-neutralizing antibodies. Lung histology from two children that ultimately died as a result of this trial revealed the deposition of antibody–virus complexes and a pronounced tissue eosinophilia.12 One or more of these features, which have collectively been termed enhanced disease, have been replicated and modeled with formalin-inactivated RSV in multiple species, including other primates, ferrets, cotton rats, and mice as well as with formalin-inactivated bovine RSV in cows.13 Interestingly, hypersensitivity responses of this nature are not unique to formalin-inactivated RSV; there are a limited number of reports describing aberrant responses to formalin-inactivated measles vaccine.14 This phenomenon has also been replicated experimentally to varying extents with formalin-inactivated versions of human metapneumovirus15 and parainfluenza virus,16 and in some reports even to carrier antigens.17 There is also a recent report of a severe hypersensitivity reaction, including Th2 cytokine-mediated eosinophil infiltration into the lung tissue, in BALB/c mice immunized with a vaccinia virus construct expressing the severe acute respiratory syndrome (SARS) coronavirus (CoV) N nucleocapsid protein.18 Our group has demonstrated that immunization of mice with formalin-inactivated pneumonia virus of mice (PVM) followed by intranasal virus challenge likewise results in pulmonary hypereosinophilia in the absence of a serum-neutralizing antibody response19 (Fig. 9.3.1 ). PVM is a natural rodent pneumovirus pathogen that is related to RSV; PVM replicates extensively in mouse bronchiolar epithelial cells tissue, eliciting a profound and potentially lethal inflammatory response, similar to the more severe forms of RSV disease.20
FIGURE 9.3.1.

Hypersensitivity responses in mice vaccinated with formalin-fixed pneumovirus antigens.
(A) Lung tissue from a mouse vaccinated with formalin-inactivated pneumonia virus of mice (PVM; a mouse pneumovirus related to human RSV) and then challenged intranasally with actively replicating virus. (B) Eosinophils detected in the bronchoalveolar lavage (BAL) fluid from the mouse described in (A). (C) Percentage eosinophils detected in the BAL fluid of mice vaccinated with formalin-inactivated PVM vs. control antigen. Ag, antigen; BAL, bronchoalveolar lavage; RSV, respiratory syncytial virus.
Reprinted from19.
Gene-deletion and cytokine depletion mouse model studies all point to Th2 cytokines [interleukin-4 (IL-4), IL-5, and interleukin-13 (IL-13)] as crucial to eliciting pulmonary eosinophilia in response to formalin-inactivated RSV.21., 22. A recent study by Moghaddam and colleagues23 suggested that the oxidation of RSV antigens resulting from formalin exposure elicits a Th2 response in vivo. Delgado and colleagues24 and Cyr and colleagues25 have both reported that independent toll-like receptor (TLR) stimulation in conjunction with RSV antigens results in a rebalancing of the Th1/Th2 cytokine responses, thereby reducing pathology. In initial studies aimed at exploring the molecular mechanism of Th2-mediated immunopathology, pulmonary eosinophilia was observed in mice immunized with recombinant vaccinia virus expressing RSV-G protein followed by live RSV challenge in most, but not all published trials (reviewed in11), results which initially suggested that the pathology related to formalin-inactivation might be attributed mechanistically to aberrant reactivity to this one virus protein alone. Interestingly, although the end point—pulmonary eosinophilia—looks more or less the same, recent analysis indicates that the pulmonary eosinophilia that develops in response to RSV-G protein and the eosinophilia that develops in response to formalin-inactivated RSV proceed via different molecular mechanisms. Among other distinctions, eosinophilia in response to RSV-G protein is not dependent on IL-4 and requires the actions of RSV-G protein-specific Vbeta14+ T cells; in contrast, eosinophilia in response to formalin-inactivated RSV antigens is IL-4 dependent, while not dependent on RSV-G protein-specific Vbeta14+ T cells (reviewed in11).
Are Eosinophils Contributing to the Pathophysiology?
Much of the focus of the enhanced disease/hypersensitivity studies has been on the presence of eosinophils and the mechanisms of eosinophil recruitment to the lung tissue, yet it was never really clear whether or not eosinophils were directly responsible for the pathophysiological responses. In other words, it was unclear whether the vaccinated children became ill because of pulmonary eosinophilia, whether the eosinophils were engaged in altering future responses to virus infection, or whether eosinophilia was a neutral, secondary finding. These questions have been explored to some extent in mouse models using wild-type and eosinophil-deficient mice (including ΔdblGATA mice) immunized with vaccinia virus vectors expressing RSV-G and RSV-F proteins,11., 26. but, as noted above, these experimental systems are now recognized as mechanistically unrelated to the pathology induced by formalin-inactivated RSV antigens. As such, although findings address a role for eosinophils, and they likewise suggest that eosinophils actually may not be contributing to systemic disease—specifically, that clinical symptoms, weight loss, and respiratory dysfunction measurements may be unrelated to the presence of absence of pulmonary eosinophilia—these conclusions may not be directly relevant to the way in which eosinophils contribute to pathology in the setting of formalin-inactivated RSV antigens. The role of eosinophils in modulating the pathology induced by formalin-inactivated vaccine antigens has not been explored and might be addressed in mouse models of explicit eosinophil deficiency with formalin-inactivated RSV antigens.
Thus, although our long-standing prejudices might make it easy to conclude that eosinophils contributed directly to the lung and systemic pathology observed in the initial vaccine trials and in the subsequent mouse modeling experiments, it is important to recognize that the presence of eosinophils in the lung may or may not lead to these outcomes. The data from mouse models are inconclusive on this point. Furthermore, the presence of eosinophils alone, even in human conditions, does not necessarily imply severe respiratory pathology. For example, in eosinophilic bronchiolitis, patients complain of only minimal respiratory symptomatology despite pronounced pulmonary eosinophilia.27 Thus, at current writing, while eosinophils may serve as important biomarkers for aberrant hypersensitivity reactions, we can reach no conclusions regarding their contributions to pathophysiology from the published experimental data.
Eosinophil Recruitment in Response to Primary Respiratory Syncytial Virus Infection—A Cause for Alarm?
Although respiratory virus infections are not among the diseases typically associated with Th2 lymphocyte activation and profound pulmonary eosinophilia, eosinophils and/or eosinophil granule secretory proteins have been detected in lung washings or systemically in infants in need of supplemental oxygen secondary to severe RSV infection.28., 29., 30. As mentioned earlier, it is not at all certain whether pulmonary eosinophilia is uniquely related to the RSV pathogen, or whether eosinophilia is observed in response to RSV because it is the predominant severe respiratory pathogen among very young infants. Although not reported as frequently, the eosinophil granule protein ECP has been detected in nasopharyngeal secretions in response to other respiratory virus infections, including influenza and parainfluenza.31., 32.
A number of recent studies have suggested that the age at which the individual experiences a first RSV infection has a profound impact on the nature of the primary response. In general, Th2 cytokines (IL-4, IL-5) and evidence of eosinophilia (cells and/or degranulation products) are detected more readily in younger infants, although results are not completely consistent in all studies. For example, Kristjansson and colleagues32 examined the responses of infants diagnosed with RSV and found that those who were less than 3 months of age at the time of first infection had higher levels of IL-4 in their nasopharyngeal secretions than children who were older, although no differences were observed in analogous levels of ECP. Likewise, Sung and colleagues33 documented elevated levels of both IL-4 and IL-5 in serum samples of RSV-infected infants who were less than 18 months old at the time of primary infection than in older infants. Similarly, Kim and colleagues34 examined eosinophils in the bronchoalveolar lavage fluid (BALF) from RSV-infected infants (ages 0.4–1.8 years) and found that the number of eosinophils detected in BALF correlated closely with IL-5 concentration, although interestingly, the age range of the group in which eosinophils were detected was not significantly different from the age range of the group in which eosinophils were absent.
Nasal eosinophilia has been detected in response to respiratory viruses other than RSV (including rhinoviruses and CoVs), although the circumstances tend to be limited and highly specific, such as in patients with pre-existing respiratory allergies.35 Of particular interest, several groups have reported that influenza infection stimulates the production of the eosinophil chemoattractants eotaxin (CCL11) and CCL5 (RANTES) in normal nasal and airway epithelial cells in culture, which suggests the possibility of eosinophil recruitment.36., 37., 38., 39. The role of eosinophils in acute SARS-CoV remains completely unexplored, but the eosinophil secretory ribonuclease, eosinophil-derived neurotoxin (RNASE2/EDN), was among the 52 signature genes that discriminated between individuals recovering from severe SARS-CoV infection and healthy controls.40
Several large clinical studies have led to the consistent conclusion that infants who have recovered from severe RSV bronchiolitis are at significantly increased risk for both recurrent wheezing and childhood asthma.41., 42., 43. Given the presumed role of eosinophils in the pathogenesis of acute allergic asthma, it seems reasonable to ask whether the eosinophils recruited to the lungs during severe primary RSV bronchiolitis might cause, or at least predict, the progression to wheezing. Causation is of course difficult to ascertain in human subjects; however, a prospective study by Pifferi and colleagues44 demonstrated that infants who were less than 1 year old and who had elevated serum ECP levels during a primary RSV infection were nearly 10 times more likely to have developed symptoms of wheezing in later childhood than older children and children without elevated levels of serum ECP. However, Sigurs and colleagues,45 following much the same methodology, found that serum ECP was not predictive of progression to wheezing. In a more recent prospective study, Castro and colleagues46 examined the outcomes of RSV-infected, <1-year-old infants; in this study, 48% of those enrolled went on to develop allergic symptomatology by age 6, with a significantly higher prevalence of asthma developing among the children who were infected with RSV at a younger age (below 6 months). Among those developing asthma, there were no differences in peripheral blood eosinophil counts at the time of acute RSV infection, nor were the cytokine profiles (as determined by phorbol myristate acetate stimulation of isolated mononuclear cells) of children who developed allergic disease different from those who did not.
What Can We Learn from Mouse Models of Primary Virus Challenge?
Given the complexities of natural disease, and the fact that there is no human condition in which an individual is uniquely devoid of eosinophils, it is helpful (if not crucial) to have appropriate animal models to explore questions of association and causation. Inbred mice have been used extensively to study responses to RSV, although it is important to recognize that RSV inoculation of mice is formally a challenge-clearance model rather than an infection model, as RSV undergoes little if any replication in mouse lung tissue.
Schwarze and colleagues47., 48. have explored RSV challenge in mouse models; in these studies, the authors describe Th2 cytokine-dependent recruitment of eosinophils and associated airways hyperreactivity, a finding that has implicated eosinophils in the pathophysiological mechanism. Eosinophilia was also noted in a mouse model of secondary RSV challenge; Culley and colleagues49 detected eosinophil recruitment to the airways on secondary RSV challenge among mice undergoing primary challenge at 1 day of age; eosinophil recruitment declined dramatically if primary challenge was delayed until mice were 1 week old, although the authors found no statistically significant difference in systemic disease, measured as weight loss, between these two sets of challenged mice. Dakhama and colleagues50 likewise found that the extent of airway hyperresponsiveness induced by a secondary challenge was directly dependent on the age of first virus challenge, similarly associated with augmented eosinophil recruitment when the primary challenge occurred in mice <1 week of age. Tasker and colleagues51 performed a similar study, and identified Th2 cytokine responses in neonatally primed mice that were associated with diminished virus replication in lung tissue. Finally, also noteworthy is the study by Harker and colleagues52 in which mice were primed with recombinant RSV (rRSV) expressing Th1 [interferon gamma (IFN-γ)] or Th2 (IL-4) cytokines prior to RSV challenge. In contrast to mice primed with rRSV/IFN-γ, mice primed with rRSV/IL-4 sustained airway eosinophilia in response to subsequent RSV challenge. Although IL-4 clearly functions to suppress antiviral CD8+ T cell function in other experimental settings,53., 54. challenge with RSV/IL-4 had no impact on the number of CD8+ T cells recruited to the lung nor on the fraction producing IFN-γ when compared to mice challenged with wild-type RSV alone. The RSV/IL-4-primed mice had reduced lung virus titer and were protected against weight loss, a finding that correlated with the recruitment of eosinophils. As is clear from these findings, the precise role of eosinophils remains uncertain, but one thing that is clear is the fact that eosinophils do not universally provoke lung pathology and systemic disease.
The role of RSV and PVM in enhancing asthmatic-type responses via an interplay with known allergens has also been explored;55., 56., 57., 58., 59. while the weight of evidence suggests that eosinophils play crucial roles in mouse models of asthma, what that precise role might be (promoting acute airways hyperreactivity vs. more chronic remodeling) is a complex and controversial issue that has been considered extensively by others and is beyond the scope of this chapter.
Do Eosinophils Promote Antiviral Host Defense?
One of the more curious aspects of eosinophil biology is, as discussed thus far in this review, that once they are detected, particularly in lung tissue, eosinophils are almost always considered as contributing in some negative way to the pathophysiology of disease. This is most intriguing, given our understanding of the role of their sister cell, the neutrophil, and the concept of the double-edged sword.60 In other words, we know that neutrophils are recruited in response to bacterial and fungal infection, and serve to promote host defense against these invasive pathogens. However, if signals go awry, if neutrophil clearance does not proceed, and/or if neutrophil activation persists, pathology ensues. We were among the first groups to suggest that eosinophil function might encompass a positive, host-defense aspect as part of perhaps a more subtle double-edged sword,61., 62. and to consider the possibility that eosinophils may be recruited in part to promote primary antiviral host defense, perhaps in situations in which acquired immune responses are less than immediately effective.63 Interestingly, eosinophilia has been reported in association with T cell dysfunction in human immunodeficiency virus (HIV) infection;64., 65., 66. this finding may in turn be related to the propensity for hypersensitivity reactions observed among HIV-infected patients.67 However, correlations between eosinophilia and disease pathogenesis are often difficult to ascertain. In a primary study, Tietz and colleagues64 found that elevated eosinophil counts among HIV-infected patients correlated with progression of disease and declining CD4+ T cell counts, while Chorba and colleagues68 found no correlation between HIV viral loads, CD4+ T cell and eosinophil counts among more than 600 HIV-infected patients in sub-Saharan Africa, although concurrent helminthic parasite infection was clearly a confounding variable.
The first indication that eosinophils might have the means to function in promoting antiviral host defense came from a series of studies we performed in the late 1990s. In this work, we determined that eosinophils, acting at least in part via their secretory mediators, could reduce the infectivity of RSV for target epithelial cells in vitro;69 Soukup and Becker70 likewise demonstrated that eosinophils inhibit RSV infection in tissue culture. Shortly thereafter, Adamko and colleagues71 demonstrated that eosinophils elicited by allergen sensitization served to limit virus replication and/or promote virus clearance in guinea pigs challenged with parainfluenza/Sendai virus. In a more recent study, Phipps and colleagues72 demonstrated accelerated clearance of RSV from the lungs of the eosinophil-enriched IL-5 transgenic mice, and furthermore found that full antiviral activity was dependent on intact TLR signaling in eosinophils introduced exogenously (Fig. 9.3.2 ).
FIGURE 9.3.2.

Eosinophils promote antiviral host defense.
(A) Eosinophils reduce the infectivity of RSV for target epithelial cells in vitro. (B) Eosinophil-enriched interleukin-5 transgenic mice promote accelerated RSV clearance compared to wild-type mice, with reduced virus titers detected at all time points examined. IL-5, interleukin-5; RSV, respiratory syncytial virus; Wt, wild-type.
As discussed earlier, we have explored the responses of mice immunized with formalin-inactivated PVM followed by live virus challenge. In experiments using eosinophil-deficient ΔdblGATA mice, we found that eosinophils were not a crucial component of the (limited) protection resulting from this immunization strategy,19 a finding perhaps related to the virulence of this pathogen in inbred strains of mice, as well as its ability to infect eosinophils.73 Nonetheless, we have recently observed accelerated clearance of PVM in eotaxin-2/IL-5 double-transgenic mice74 in which eosinophils are undergoing profound and extensive degranulation (Percopo et al., unpublished data)
Are Eosinophils Among the Direct Targets of Respiratory Virus Infection?
Our studies with PVM75 and the examination of pathology specimens from RSV patients76., 77. demonstrate that pneumovirus replication in vivo takes place primarily in respiratory epithelial cells. However, it is clear that other cells, including human monocytes, support the replication of RSV and PVM in culture, and release proinflammatory cytokines in response to virus infection.78., 79., 80. Given the questions regarding the role of eosinophils and their interactions with respiratory viruses, we set out to determine whether pneumoviruses could infect and replicate within eosinophils, and to determine what the outcome of this infection might be. Kimpen and colleagues81., 82. originally demonstrated that RSV could be taken up by purified human eosinophils, and virions were identified in phagolysosomal compartments, but virus replication was not examined. To explore PVM replication in mouse eosinophils, we used our recently described method for generating sustained cultures of >95% pure mature eosinophils from unselected bone marrow progenitors.83 With eosinophils generated by this culture method, we demonstrated a dramatic increase in virus titer within 7 days of inoculation (Fig. 9.3.3 ), associated with the replication-dependent release of infectious virions accompanied by the cytokine interleukin-6. Among others who have explored the direct interactions of pneumoviruses with eosinophils, Davoine and colleagues84 determined that human eosinophils were unable to release granule proteins in response to RSV challenge without coincident exposure of virus to CD4+ T cells and antigen-presenting cells. Among the questions left to be explored are:
-
•
How often are infected eosinophils detected in vivo?
-
•
At what point during an acute infection are they detected and under what specific circumstances?
-
•
Does virus infection and intracellular replication induce eosinophil apoptosis or disable eosinophils in some other, more subtle way, and thereby reduce their ability to promote virus clearance and antiviral host defense?
Answers to these questions may shed some light on the differential responses observed in the aforementioned experiments.
FIGURE 9.3.3.

Pneumovirus replication in eosinophils.
(A–C) Electron micrographs of cultured eosinophils derived from unselected mouse bone marrow; the symbol N denotes the characteristic bilobed nucleus, and sg (at arrows) denotes the cytoplasmic-specific granules. (D) Replication of PVM in cultured mouse eosinophils; filled symbols, replication-competent PVM; open symbols, heat-inactivated PVM. PVM is detected by quantitative RT-PCR targeting the virus small hydrophobic (SH) gene. (E) Detection of PVM replication in cultured mouse eosinophils with anti-PVM N protein-specific antibody. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PVM, pneumonia virus of mice; RSV, respiratory syncytial virus; RT-PCR, reverse transcription polymerase chain reaction.
Reprinted from73.
Conclusions and Future Perspectives
The manner in which eosinophils respond to and participate in respiratory virus infection is very far from clear. While pulmonary eosinophilia is a hallmark, or biomarker, of the aberrant hypersensitivity response to formalin-inactivated RSV, there is no clear indication that eosinophils actually contribute to the negative sequelae of disease. Likewise, while severe primary RSV is associated with pulmonary eosinophilia and progression to asthma, these two features have not been linked clearly to one another mechanistically or pathophysiologically. Finally, several groups have shown that eosinophils can promote virus clearance, but this interesting and positive feature of eosinophil function is not observed in all circumstances or in all situations. Among the possibilities that have yet to be explored, eosinophil function may be less dependent on numbers elicited, and may be more closely related to the quality and extent of activation, to the unique nature of the cytokines eliciting recruitment, and/or to the strength of the signals sustaining viability in situ. These are all issues that are worthy of consideration as we attempt to improve our understanding of the true nature of the eosinophilic leukocyte and strive to achieve some clarification and sense of balance between their perceived negative and their incompletely characterized positive contributions to homeostasis and host defense.
Acknowledgements
Research in Dr. Rosenberg’s laboratory is supported by the Division of Intramural Research (DIR AI000941, AI000942, and AI000943) of the National Institute of Allergy and Infectious Diseases. Research in Dr. Domachowske’s laboratory is supported by the Children’s Miracle Network of Greater New York. This chapter is a revised version of a previously published review article.85
References
- 1.Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens W.W., Falsey A.R., Braciale T.J. RSV 2007: recent advances in respiratory syncytial virus research. Viral Immunol. 2008;21:133–140. doi: 10.1089/vim.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyairi I., DeVincenzo J.P. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gounni A.S., Lamkhioued B., Ochiai K., Tanaka Y., Delaporte E., Capron A. High affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 5.Barata L., Ying S., Humbert M., Barkans J., Meng Q., Durham S.R. Allergen-induced recruitment of FcεRI+ eosinophils in human atopic skin. Eur J Immunol. 1997;27:1236–1241. doi: 10.1002/eji.1830270527. [DOI] [PubMed] [Google Scholar]
- 6.Rajakulasingam K., Till S., Ying S., Humbert M., Barkans J., Sullivan M. Increased expression of high affinity IgE (FcepsilonRI) receptor-alpha chain mRNA and protein-bearing eosinophils in human allergen-induced atopic asthma. Am J Respir Crit Care Med. 1998;158:233–240. doi: 10.1164/ajrccm.158.1.9708106. [DOI] [PubMed] [Google Scholar]
- 7.Kita H., Kaneko M., Bartemes K.R., Weiler D.A., Schimming A.W., Reed C.E. Does IgE bind to and activate eosinophils from patients with allergy? J Immunol. 1999;162:6901–6911. [PubMed] [Google Scholar]
- 8.Larson K.A., Olson E.V., Madden B.J., Gleich G.J., Lee N.A., Lee J.J. Two highly homologous ribonuclease genes expressed in mouse eosinophils identify a larger subgroup of the mammalian ribonuclease superfamily. Proc Natl Acad Sci USA. 1996;93:12370–12375. doi: 10.1073/pnas.93.22.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J.J., Lee N.A. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 10.Castilow E.M., Varga S.M. Overcoming T cell-mediated immunopathology to achieve safe RSV vaccination. Future Virol. 2008;2008(3):445–454. doi: 10.2217/17460794.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castilow E.M., Olson M.R., Varga S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res. 2007;39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 13.Antonis A.F., Schrijver R.S., Daus F., Steverink P.J., Stockhofe N., Hensen E.J. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: exploring the parameters of pathogenesis. J Virol. 2003;77:12067–12073. doi: 10.1128/JVI.77.22.12067-12073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin D.E., Pan C.H., Moss W.J. Measles vaccines. Front Biosci. 2008;13:1352–1370. doi: 10.2741/2767. [DOI] [PubMed] [Google Scholar]
- 15.de Swart R.L., van den Hoogen B.G., Kuiken T., Herfst S., van Amerongen G., Yüksel S. Immunization of macaques with formalin-inactivated human metapneumovirus induces hypersensitivity to hMPV infection. Vaccine. 2007;25:8518–8528. doi: 10.1016/j.vaccine.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Ottolini M.G., Porter D.D., Hemming V.G., Prince G.A. Enhanced pulmonary pathology in cotton rats upon challenge after immunization with inactivated parainfluenza virus 3 vaccines. Viral Immunol. 2000;13:231–236. doi: 10.1089/vim.2000.13.231. [DOI] [PubMed] [Google Scholar]
- 17.Piedra P.A., Wyde P.R., Castleman W.L., Ambrose M.W., Jewell A.M., Speelman D.J. Enhanced pulmonary pathology associated with the use of formalin-inactivated respiratory syncytial virus vaccine in cotton rats is not a unique viral phenomenon. Vaccine. 1993;11:1415–1423. doi: 10.1016/0264-410x(93)90170-3. [DOI] [PubMed] [Google Scholar]
- 18.Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 19.Percopo C.M., Phipps S., Foster P.S., Domachowske J.B., Rosenberg H.F. 2009. Pulmonary eosinophils and their role in immunopathology associated with formalin-inactivated pneumovirus vaccination, in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg H.F., Domachowske J.B. Pneumonia virus of mice: severe respiratory infection in a natural host. Immunol Lett. 2008;118:6–12. doi: 10.1016/j.imlet.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors M., Giese N.A., Kulkarni A.B., Firestone C.Y., Morse H.C., 3rd, Murphy B.R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castilow E.M., Meyerholz D.K., Varga S.M. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol. 2008;180:2376–2384. doi: 10.4049/jimmunol.180.4.2376. [DOI] [PubMed] [Google Scholar]
- 23.Moghaddam A., Olszewska W., Wang B., Tregoning J.S., Helson R., Sattentau Q.J. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 24.Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cyr S.L., Angers I., Guillot L., Stoica-Popescu I., Lussier M., Qureshi S. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine. 2009;27:421–430. doi: 10.1016/j.vaccine.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 26.Castilow E.M., Legge K.L., Varga S.M. Cutting edge: Eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J Immunol. 2008;181:6692–6696. doi: 10.4049/jimmunol.181.10.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott K.A., Wardlaw A.J. Eosinophilic airway disorders. Semin Respir Crit Care Med. 2006;27:128–133. doi: 10.1055/s-2006-939515. [DOI] [PubMed] [Google Scholar]
- 28.Kristjánsson S., Wennergren D., Eriksson B., Thórarinsdóttir H., Wennergren G. U-EPX levels and wheezing in infants and young children with and without RSV bronchiolitis. Respir Med. 2006;100:878–883. doi: 10.1016/j.rmed.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Harrison A.M., Bonville C.A., Rosenberg H.F., Domachowske J.B. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med. 1999;159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 30.Garofalo R., Kimpen J.L., Welliver R.C., Ogra P.L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 31.Colocho Zelaya E.A., Orvell C., Strannegård O. Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatr Allergy Immunol. 1994;5:100–106. doi: 10.1111/j.1399-3038.1994.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 32.Kristjansson S., Bjarnarson S.P., Wennergren G., Palsdottir A.H., Arnadottir T., Haraldsson A. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116:805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Sung R.Y., Hui S.H., Wong C.K., Lam C.W., Yin J. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur J Pediatr. 2001;160:117–122. doi: 10.1007/s004310000676. [DOI] [PubMed] [Google Scholar]
- 34.Kim C.K., Kim S.W., Park C.S., Kim B.I., Kang H., Koh Y.Y. Bronchoalveolar lavage cytokine profiles in acute asthma and acute bronchiolitis. J Allergy Clin Immunol. 2003;112:64–71. doi: 10.1067/mai.2003.1618. [DOI] [PubMed] [Google Scholar]
- 35.van Benten I.J., KleinJan A., Neijens H.J., Osterhaus A.D., Fokkens W.J. Prolonged nasal eosinophilia in allergic patients after common cold. Allergy. 2001;56:949–956. doi: 10.1034/j.1398-9995.2001.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawaguchi M., Kokubu F., Kuga H., Tomita T., Matsukura S., Kadokura M. Expression of eotaxin by normal airway epithelial cells after influenza virus A infection. Int Arch Allergy Immunol. 2000;122(Suppl. 1):44–49. doi: 10.1159/000053632. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi M., Kokubu F., Kuga H., Tomita T., Matsukura S., Suzaki H. Influenza virus A stimulates expression of eotaxin by nasal epithelial cells. Clin Exp Allergy. 2001;31:873–880. doi: 10.1046/j.1365-2222.2001.01103.x. [DOI] [PubMed] [Google Scholar]
- 38.Matsukura S., Kokubu F., Noda H., Tokunaga H., Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98(6 Pt 1):1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 39.Matsukura S., Kokubu F., Kubo H., Tomita T., Tokunaga H., Kadokura M. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol. 1998;18:255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y.S., Chen C.H., Chao A., Chen E.S., Wei M.L., Chen L.K. Molecular signature of clinical severity in recovering patients with severe acute respiratory syndrome coronavirus (SARS-CoV) BMC Genomics. 2005;6:132. doi: 10.1186/1471-2164-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohapatra S.S., Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev. 2008;21:495–504. doi: 10.1128/CMR.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez-Yarza E.G., Moreno A., Lázaro P., Mejías A., Ramilo O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr Infect Dis J. 2007;26:733–739. doi: 10.1097/INF.0b013e3180618c42. [DOI] [PubMed] [Google Scholar]
- 43.Dakhama A., Lee Y.M., Gelfand E.W. Virus-induced airway dysfunction: pathogenesis and biomechanisms. Pediatr Infect Dis J. 2005;24(11 Suppl):S159–S169. doi: 10.1097/01.inf.0000188155.46381.15. [DOI] [PubMed] [Google Scholar]
- 44.Pifferi M., Ragazzo V., Caramella D., Baldini G. Eosinophil cationic protein in infants with respiratory syncytial virus bronchiolitis: predictive value for subsequent development of persistent wheezing. Pediatr Pulmonol. 2001;31:419–424. doi: 10.1002/ppul.1069. [DOI] [PubMed] [Google Scholar]
- 45.Sigurs N., Bjarnason R., Sigurbergsson F. Eosinophil cationic protein in nasal secretion and in serum and myeloperoxidase in serum in respiratory syncytial virus bronchiolitis: relation to asthma and atopy. Acta Paediatr. 1994;83:1151–1155. doi: 10.1111/j.1651-2227.1994.tb18269.x. [DOI] [PubMed] [Google Scholar]
- 46.Castro M., Schweiger T., Yin-Declue H., Ramkumar T.P., Christie C., Zheng J. Cytokine response after severe respiratory syncytial virus bronchiolitis in early life. J Allergy Clin Immunol. 2008;122:726–733. doi: 10.1016/j.jaci.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarze J., Cieslewicz G., Hamelmann E., Joetham A., Shultz L.D., Lamers M.C. IL-5 and eosinophils are essential for the development of airway hyperresponsiveness following acute respiratory syncytial virus infection. J Immunol. 1999;162:2997–3004. [PubMed] [Google Scholar]
- 48.Schwarze J., Cieslewicz G., Joetham A., Ikemura T., Mäkelä M.J., Dakhama A. Critical roles for interleukin-4 and interleukin-5 during respiratory syncytial virus infection in the development of airway hyperresponsiveness after airway sensitization. Am J Respir Crit Care Med. 2000;162(2 Pt 1):380–386. doi: 10.1164/ajrccm.162.2.9903057. [DOI] [PubMed] [Google Scholar]
- 49.Culley F.J., Pollott J., Openshaw P.J. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196:1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dakhama A., Park J.W., Taube C., Joetham A., Balhorn A., Miyahara N. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol. 2005;175:1876–1883. doi: 10.4049/jimmunol.175.3.1876. [DOI] [PubMed] [Google Scholar]
- 51.Tasker L., Lindsay R.W., Clarke B.T., Cochrane D.W., Hou S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin Exp Immunol. 2008;153:277–288. doi: 10.1111/j.1365-2249.2008.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harker J, Bukreyev, A, Collins PL, Wang B, Openshaw PJ, Tregoning JS. Virally delivered cytokines alter the immune response to future lung infections. J Virol; 81:13105–11 [DOI] [PMC free article] [PubMed]
- 53.Villacres M.C., Bergmann C.C. Enhanced cytotoxic T cell activity in IL-4-deficient mice. J Immunol. 1999;162:2663–2670. [PubMed] [Google Scholar]
- 54.Bot A., Holz A., Christen U., Wolfe T., Temann A., Flavell R. Local IL-4 expression in the lung reduces pulmonary influenza-virus-specific secondary cytotoxic T cell responses. Virology. 2000;269:66–77. doi: 10.1006/viro.2000.0187. [DOI] [PubMed] [Google Scholar]
- 55.Schwarze J., Hamelmann E., Bradley K.L., Takeda K., Gelfand E.W. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997;100:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barends M., de Rond L.G., Dormans J., van Oosten M., Boelen A., Neijens H.J. Respiratory syncytial virus, pneumonia virus of mice, and influenza A virus differently affect respiratory allergy in mice. Clin Exp Allergy. 2004;34:488–496. doi: 10.1111/j.1365-2222.2004.01906.x. [DOI] [PubMed] [Google Scholar]
- 57.Siegle J.S., Hansbro N., Herbert C., Rosenberg H.F., Asquith K.L., Foster P.S. Early life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir Res. 2010;11:14. doi: 10.1186/1465-9921-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mäkelä M.J., Tripp R., Dakhama A., Park J.W., Ikemura T., Joetham A. Prior airway exposure to allergen increases virus-induced airway hyperresponsiveness. J Allergy Clin Immunol. 2003;112:861–869. doi: 10.1016/s0091-6749(03)02020-7. [DOI] [PubMed] [Google Scholar]
- 59.Barends M., Van Oosten M., De Rond C.G., Dormans J.A., Osterhaus A.D., Neijens H.J. Timing of infection and prior immunization with respiratory syncytial virus (RSV) in RSV-enhanced allergic inflammation. J Infect Dis. 2004;189:1866–1872. doi: 10.1086/386341. [DOI] [PubMed] [Google Scholar]
- 60.Smith J.A. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg H.F., Domachowske J.B. Eosinophils, ribonucleases and host defense: solving the puzzle. Immunol Res. 1999;20:261–274. doi: 10.1007/BF02790409. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg H.F., Domachowske J.B. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 63.Milner J.D., Ward J.M., Keane-Myers A., Paul W.E. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc Natl Acad Sci USA. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tietz A., Sponagel L., Erb P., Bucher H., Battegay M., Zimmerli W. Eosinophilia in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1997;16:675–677. doi: 10.1007/BF01708558. [DOI] [PubMed] [Google Scholar]
- 65.Cohen A.J., Steigbigel R.T. Eosinophilia in patients infected with human immunodeficiency virus. J Infect Dis. 1996;174:615–618. doi: 10.1093/infdis/174.3.615. [DOI] [PubMed] [Google Scholar]
- 66.Drabick J.J., Magill A.J., Smith K.J., Nutman T.B., Benson P.M. Hypereosinophilic syndrome associated with HIV infection. Military Medical Consortium for Applied Retroviral Research. South Med J. 1994;87:525–529. doi: 10.1097/00007611-199404000-00021. [DOI] [PubMed] [Google Scholar]
- 67.Phillips E., Mallal S. Drug hypersensitivity in HIV. Curr Opin Allergy Clin Immunol. 2007;7:324–330. doi: 10.1097/ACI.0b013e32825ea68a. [DOI] [PubMed] [Google Scholar]
- 68.Chorba T.L., Nkengasong J., Roels T.H., Monga B., Maurice C., Maran M. Assessing eosinophil count as a marker of immune activation among human immunodeficiency virus-infected persons in sub-Saharan Africa. Clin Infect Dis. 2002;34:1264–1266. doi: 10.1086/339940. [DOI] [PubMed] [Google Scholar]
- 69.Domachowske J.B., Dyer K.D., Bonville C.A., Rosenberg H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 70.Soukup J.M., Becker S. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin Immunol. 2003;107:178–185. doi: 10.1016/s1521-6616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 71.Adamko D.J., Yost B.L., Gleich G.J., Fryer A.D., Jacoby D.B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phipps S., Lam C.E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H.F. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 73.Dyer K.D., Percopo C.M., Fischer E.R., Gabryszewski S.J., Rosenberg H.F. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ochkur S.I., Jacobsen E.A., Protheroe C.A., Biechele T.L., Pero R.S., McGarry M.P. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 75.Bonville C.A., Bennett N.J., Koehnlein M., Haines D.M., Ellis J.A., DelVecchio A.M. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology. 2006;349:87–95. doi: 10.1016/j.virol.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Welliver T.P., Garofalo R.P., Hosakote Y., Hintz K.H., Avendano L., Sanchez K. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson J.E., Gonzales R.A., Olson S.J., Wright P.F., Graham B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 78.Barr F.E., Pedigo H., Johnson T.R., Shepherd V.L. Surfactant protein-A enhances uptake of respiratory syncytial virus by monocytes and U937 macrophages. Am J Respir Cell Mol Biol. 2000;23:586–592. doi: 10.1165/ajrcmb.23.5.3771. [DOI] [PubMed] [Google Scholar]
- 79.Dyer K.D., Schellens I.M., Bonville C.A., Martin B.V., Domachowske J.B., Rosenberg H.F. Efficient replication of pneumonia virus of mice (PVM) in a mouse macrophage cell line. Virol J. 2007;4:48. doi: 10.1186/1743-422X-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krilov L.R., McCloskey T.W., Harkness S.H., Pontrelli L., Pahwa S. Alterations in apoptosis of cord and adult peripheral blood mononuclear cells induced by in vitro infection with respiratory syncytial virus. J Infect Dis. 2000;181:349–353. doi: 10.1086/315203. [DOI] [PubMed] [Google Scholar]
- 81.Kimpen J.L., Garofalo R., Welliver R.C., Fujihara K., Ogra P.L. An ultrastructural study of the interaction of human eosinophils with respiratory syncytial virus. Pediatr Allergy Immunol. 1996;7:48–53. doi: 10.1111/j.1399-3038.1996.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 82.Kimpen J.L., Garofalo R., Welliver R.C., Ogra P.L. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr Res. 1992;32:160–164. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Dyer K.D., Moser J.M., Czapiga M., Siegel S.J., Percopo C.M., Rosenberg H.F. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davoine F., Cao M., Wu Y., Ajamian F., Ilarraza R., Kokaji A.I. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122:69–77. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 85.Rosenberg H.F., Dyer K.D., Domachowske J.B. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 2009;83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapter 9.4. Antifungal Immunity by Eosinophils: Mechanisms and Implications in Human Diseases
Eosinophils are considered multifunctional leukocytes involved in tissue homeostasis, modulation of adaptive immune responses, and innate immunity to certain microbes. In particular, human eosinophils have proinflammatory and destructive capabilities and appear to defend against large, nonphagocytosable organisms, most notably the multicellular helminth parasites and fungi. During immune responses to fungi, host innate immune cells recognize several fungus-derived biological molecules, such as chitin, β-glucan, and proteases, and recruit eosinophils to the affected tissues through T cell-dependent and -independent mechanisms. Eosinophils produce proinflammatory mediators and kill fungal organisms by responding to fungal β-glucan and proteases. Protease-activated receptors and β2 integrin likely play pivotal roles in eosinophil interaction with fungi. Dysregulation of such antifungal immune response may play roles in pathophysiology of certain human diseases, such as severe bronchial asthma and chronic rhinosinusitis. This chapter summarizes the emerging concept of the antifungal immunobiology of eosinophils and discusses the roles of eosinophils in health and disease.
Introduction
Eosinophils are implicated in the pathophysiology of allergic diseases, such as bronchial asthma and atopic dermatitis, and in host immunity to helminth infections.1 During such inflammatory reactions, soluble mediators released by immune cells induce eosinophil recruitment from the bloodstream into the sites of inflammation, where as yet unknown stimuli trigger the release of proinflammatory mediators.2 Marked extracellular deposition of released eosinophil granule proteins has been detected in specimens from patients with asthma, chronic rhinosinusitis (CRS), and atopic dermatitis.3., 4., 5. However, the presence of eosinophils per se, as in normal intestinal mucosa,6 does not lead to disease pathology. Thus, a fundamental and important question still remains: what triggers eosinophil activation and proinflammatory mediator release in human disease? The occurrence of eosinophilic inflammation in such disparate conditions as parasitic infections (presumably for the benefit of the human host) and hypersensitivity diseases (perhaps to the detriment of the patient) has become better understood as a consequence of recently reported information.
Fungi are ubiquitous in the environment, and as saprophytes or commensals, they may coexist without effect in the host with normal cellular immunity.7 Nonetheless, these airborne fungi and their products may contribute to the development and exacerbation of human airway diseases. For example, fungal products induce immunological and inflammatory reactions, resulting in a T-helper type 2 (Th2)-like cytokine response and the destruction of mucosal barrier functions.8., 9. Clinically, an association between fungal exposure and asthma has been widely recognized.10 Therefore, antifungal immunity may provide a valuable clue toward improved understanding of eosinophil biology and the mechanisms of human diseases. This chapter describes the recent development of our understanding of the biological and immunological properties of eosinophils and the mechanisms of eosinophilic inflammation with specific focus on antifungal immune responses. The potential roles of eosinophils and antifungal immune responses in human diseases are discussed.
Overview of Antifungal Immune Responses
The host immune mechanisms against fungi range from protective mechanisms that were present early in the evolution of multicellular organisms (innate immunity) to adaptive mechanisms, which are specifically induced during infection and disease (adaptive immunity).11 The life cycle of fungi starts with conidia (often called spores) that germinate and produce filaments (hyphae); these grow and branch in all directions to form a mass, collectively called the mycelium or colony. Colony formation only occurs if there are sufficient nutrients and moisture in the substrate. When the mycelium is mature, specialized hyphae called conidiophores are formed. On the conidiophores, thousands of conidia are produced, which can easily become airborne because of their small size and buoyancy.
Fungi are multicellular organisms and express various biological molecules during different stages of their lifecycle. The fungal cell wall is composed of several components, including a fibrillar layer, mannoprotein, chitin, and β-glucan.12 Chitin is a (β1-4)-linked polymer of N-acetyl-D-glucosamine (NAG), and produced in the cytosol by chitin synthase. Glucan has a large group of D-glucose polymers having glycosidic bonds; of these, the most common glucans composing the fungal cell wall have the α-configuration, such as the (β1-3)- and (β1-6)-linked glucosyl units. Up to 60% of the dry weight of the cell wall of fungi consists of glucans.13 Fungi also produce many proteases that are required for their germination, growth, and survival.14
Fungal cell wall components are recognized by cognate receptors, such as the soluble receptor, pentraxin-related protein PTX3 (PTX3),15 and membrane-bound receptors, such as Toll-like receptors (TLRs). PTX3 binds to the yeast cells of Paracoccidioides brasiliensis, but not to Candida albicans, suggesting that PTX3 recognizes specific fungal species and morphological forms.16 In Aspergillus-induced asthma, mannan-binding lectin A (MBL-A) appears to enhance the Th2-type immune responses, suggesting that this opsonin enhances fungus-specific T-cell responses.17 Fungal killing and host immunity often depend on multiple TLR- and non-TLR-mediated pathways.18 Because the composition of the fungal cell wall is complex, the cell wall constituents can activate more than one TLR-dependent signaling pathway. For example, macrophage tumor necrosis factor (TNF-α) and interferon gamma (IFN-γ) secretion triggered by C. albicans yeast cells depend on TLR2 and TLR4, whereas hyphal cells trigger only TLR2-dependent responses.19
The receptors for β-glucans are expressed by several immune cells including macrophages20 and neutrophils.21 The nonopsonic recognition of β-glucans by these cells has been ascribed to multiple receptors, including dectin-1,22 lactosylceramide,23 and CD11b/CD18 (integrin beta-2/CR3).24 Dectin-1 possesses a single nonclassical C-type carbohydrate recognition domain (CRD) connected to the transmembrane region by a stalk, and a cytoplasmic tail, possessing an immunoreceptor tyrosine-based activation (ITAM) motif, which induces the production of reactive oxygen species and inflammatory cytokines.25 Lactosylceramide (CDw17) is a glycosphingolipid found in the plasma membranes of many cells and was identified as a β-glucan receptor from the biochemical analyses of the interactions between poly-1-6--D-glucopyranosyl-1-3--D-glucopyranose glucan (PGG glucan) and isolated human leukocyte membrane components.23 CD11b/CD18 (CR3) is a heterodimeric integrin, consisting of the αM (CD11b) and β2 (CD18) chains.26 CD11b is an adhesion molecule but it also serves as a phagocytic receptor for iC3b-opsonized particles, including opsonized particulate glucans,27 through its I-domain. Importantly, CD11b also possesses a lectin domain, which maps to a site C-terminus to the I-domain. The lectin domain recognizes selected monosaccharides and a variety of β-glucans, including zymosan, although the polymeric ligand with the highest affinity contained very little β-glucan and consisted mostly of mannose.28
Eosinophil Responses to Fungi and Fungal Products In Vitro
Activated human eosinophils defend against large, nonphagocytosable organisms, most notably the multicellular helminthic parasites. Therefore, one could reasonably speculate that eosinophils may be involved in host defense and/or immune responses to other organisms with a large surface, for example filamentous fungi. We incubated human eosinophils with extracts from several environmental airborne fungi (Alternaria alternata, Aspergillus versicolor, Bipolaris sorokiniana, C. albicans, Cladosporium herbarum, Curvularia spicifera, and Penicillium chrysogenum).29 Alternaria and Penicillium induced calcium-dependent exocytosis [e.g., eosinophil-derived neurotoxin (EDN) release] in eosinophils from normal individuals (Fig. 9.4.1 ). Alternaria also strongly induced other activation events in eosinophils, including increases in intracellular calcium concentration, cell surface expression of CD63 and CD11b, and production of interleukin-8 (IL-8). Interestingly, Alternaria did not induce neutrophil activation.
FIGURE 9.4.1.
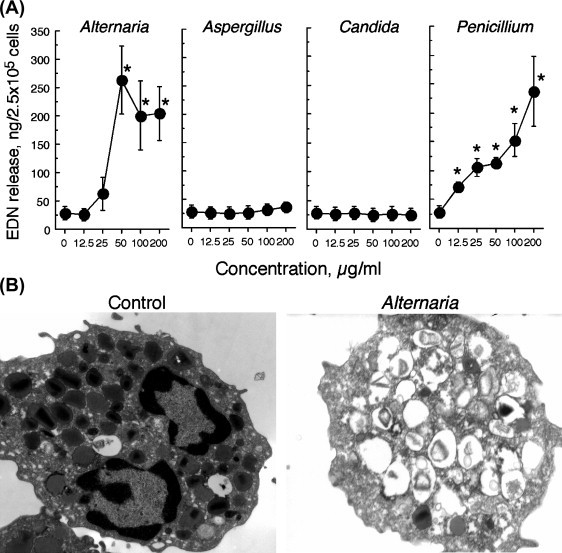
Eosinophil degranulation induced by fungal extracts.
(A) Eosinophils were incubated with fungal extracts and the concentrations of eosinophil-derived neurotoxin (EDN) in the supernatants were measured by immunoassay. (B) Photoelectron micrographs of eosinophils stimulated with medium alone (control) or Alternaria extract. Note the granule fusions and electron lucent granules in eosinophils stimulated with Alternaria.
How do eosinophils recognize and respond to fungal products within the Alternaria extracts? An extensive analysis of eosinophil expression of TLRs and their responses to TLR ligands showed that eosinophils constitutively expressed TLR1, TLR4, TLR7, TLR9, and TLR10 mRNAs and that the TLR7 ligand, R848, can activate eosinophils.2 However, eosinophil response to Alternaria is unlikely to be dependent on TLRs and their ligands.30 Rather, the eosinophil-stimulating activity in Alternaria extract was highly heat-labile and had a molecular mass of about 60 kDa, suggesting the involvement of proteinaceous molecules and their receptors.
Proteases, especially serine proteases, activate hematological and interstitial cells through a family of G protein-coupled proteinase-activated receptors (PARs) and induce the production of several proinflammatory mediators.31 Four members of this receptor family have been cloned and are designated PAR-1, PAR-2, PAR-3, and PAR-4. Protease cleavage of these receptors creates a neo-NH2 terminus, which acts as a tethered ligand and activates the seven transmembrane segments of the PAR. Human eosinophils constitutively transcribe mRNA for PAR-2 and PAR-3, but not for PAR-1 and PAR-4.32 Trypsin, an authentic agonist for PAR-2, induces superoxide anion production and degranulation of eosinophils; 5 nM trypsin induces responses that are 50–70% of those induced by 100 nM PAF, a positive control. Similarly, a cysteine protease, papain, induces isolated human eosinophils to degranulate and to produce superoxide anion.33 A. alternata produces aspartate protease(s) and recognition of this protease(s) by PAR-2 is likely responsible for the activation of eosinophils in response to Alternaria extracts. Indeed, when stimulated with Alternaria extracts, eosinophils show an increased intracellular calcium concentration that is desensitized by peptide and protease ligands for PAR-2 and inhibited by PAR-2 antagonistic peptide(s). Alternaria-derived aspartate protease(s) cleaves PAR-2 to expose neo-ligands; these neo-ligands bind to the seven transmembrane segments of PAR-2 and activate eosinophil degranulation (Fig. 9.4.2 ). Treatment of Alternaria extract with aspartate protease inhibitors, which are conventionally used for human immunodeficiency virus 1 (HIV-1) and other microorganisms, attenuates the eosinophils’ responses to Alternaria. Thus, fungal aspartate protease and eosinophil PAR-2 appear critical for the eosinophils’ innate immune response to certain fungi, suggesting a novel mechanism for eosinophil activation, for pathological inflammation in asthma and for host–pathogen interaction.
FIGURE 9.4.2.
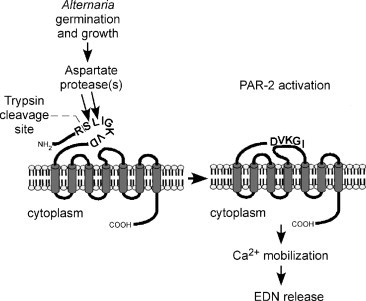
Mechanisms of PAR-2 activation and eosinophil EDN release in response to Alternaria extract.
EDN, eosinophil-derived neurotoxin.
Eosinophils respond not only to fungal extracts but also to living fungal organisms. Human eosinophils react vigorously to A. alternata grown in tissue culture dishes34 (Fig. 9.4.3 ). Eosinophils release their cytotoxic granule proteins, such as EDN and eosinophil granule major basic protein 1 (MBP-1), into the extracellular milieu and onto the surface of fungal organisms and kill the fungus. While human eosinophils do not express common fungal receptors such as dectin-1 and lactosylceramide as described earlier, eosinophils do express and use their integrin β2 molecule, CD11b, to adhere to a major fungus cell wall component, β-glucan. More specifically, the I-domain of CD11b is distinctively involved in how eosinophils interact with β-glucan. It has been known that eosinophils express CD11b constitutively and that the level of expression is increased by incubating eosinophils with various agonists, such as fMet-Leu-Phe (fMLP), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-5 (IL-5), or platelet-activating factor (PAF).1 Furthermore, compared with neutrophils, eosinophils have a larger total pool of CD11b within the cells.35 Historically, receptor ligands immobilized to relatively large surfaces [such as immunoglobulin G (IgG)-coated sepharose beads and parasites], though not particulate ligands (such as aggregated IgG and bacteria), are effective stimuli for eosinophil degranulation.1 Eosinophil CD11b plays a pivotal role in eosinophil adhesion to these large surfaces and degranulation.36 Thus, eosinophils likely use their versatile CD11b molecules to react to microorganisms (e.g., helminths, fungi) as well as other cellular targets with large surfaces.
FIGURE 9.4.3.

Interaction of eosinophils with living Alternaria organisms.
Isolated human eosinophils were incubated with germinating Alternaria for 24 h. The culture mix was analyzed by the following: (A) inverted light microscopy, (B) anti-MBP-1 staining and fluorescence microscopy, (C) scanning electron microscopy, and (D) transmission electron microscopy. Note that fungal hyphae are covered with eosinophils and their cellular components in (C). In (D), eosinophils adhere tightly to fungal hyphae and their granules are lost in >50% of the cells.
Altogether, eosinophil response to filamentous fungi is mediated by at least two major pathways, recognition of fungal proteases by PARs, and cellular adherence to the cell wall β-glucan by integrin β2 integrin, CD11b/CD18. This two-pronged approach may have important implications in the mechanisms of antifungal immunity. Drosophila detects fungal infections by recognizing pathogen-associated molecular patterns (PAMPs) and by monitoring the effects of fungal virulence factors. Specifically, the receptor gram-negative bacteria-binding protein 3 on Drosophila recognizes β-glucan, and the secreted fungal virulence factor PR1 protease cleaves Drosophila serine protease Persephone, activating the downstream immune response to the fungi.37 Similarly, eosinophil CD11b may recognize fungal structures by monitoring the presence of β-glucan. Eosinophil PARs may act like sensors to monitor fungal protease activities or putative virulence factors and provide a cue to immune and inflammatory systems once the activities reach a certain threshold. Therefore, recognition of fungi by both their structures and activities may be a conserved trait during the evolution of immunity. Such immune responses by eosinophils to fungi may benefit the host, but in turn, it may also play a role in the development and/or exacerbation of eosinophil-related human airway diseases, such as asthma.
Mechanisms Involved in Fungus-Mediated Eosinophilic Inflammation In Vivo
Recently, major progress has been made regarding the mechanisms involved in eosinophilic inflammation in response to fungi in vivo. As described earlier, chitin is one of the major cell wall components and provides structural rigidity to fungi, as well as to crustaceans, helminths, and insects. Airway administration of chitin induces the accumulation of interleukin-4-expressing innate immune cells, including eosinophils and basophils.38 Tissue infiltration of eosinophils is unaffected by the absence of TLRs but is reduced when the injected chitin is pre-treated with mammalian chitinase, acidic mammalian chitinase (AMCase), or when the chitin is injected into mice overexpressing AMCase. Chitin induces macrophage activation and the production of leukotriene B4 (LTB4), which is required for optimal recruitment of eosinophils. Similarly, cell wall preparations of Aspergillus isolated from house dust induce the recruitment of eosinophils into mouse lungs, and the effects are attenuated by enzymatic degradation of cell wall chitin and β-glucans.39 Thus, chitin and likely β-glucan are recognized by innate immune cells in the airway and are implicated in eosinophil infiltration induced by fungal exposure.
Fungal proteases also likely play critical roles in eosinophilic airway inflammation induced by fungi. The protease activities in Aspergillus extracts correlate with their activities to induce immunoglobulin E (IgE) response and eosinophilic inflammation when they are administered to mouse lungs.9 Furthermore, active proteases are present in almost all houses and many of these activities are derived from fungi, especially Aspergillus niger.40 The conidia of A. niger readily establish airway mucosal infection, eosinophilic inflammation, and IgE antibody production, while protease-deficient A. niger has reduced ability to promote airway eosinophilia. Importantly, in mice infected with A. niger, IL-5 and interleukin-13 are required for optimal clearance of lung infection in vivo, suggesting Th2-type airway inflammation and/or that eosinophils are protective against fungal infection in vivo.
The immunological pathways that link airway fungal exposure and development of Th2-type eosinophilic inflammation have been an active area of research for many years. The airway epithelium is no longer just a structural barrier;41 it can respond to environmental insults, such as allergens, microorganisms, cigarette smoke, and pollution, by secreting inflammatory mediators and antimicrobial peptides, and by recruiting immune cells.42 Our current understanding emphasizes the importance of epithelial-derived cytokines, particularly the C-C chemokine receptor family, interleukin-25 and -33, and thymic stromal lymphopoietin (TSLP), in initiating Th2-type airway inflammation.43., 44.
TSLP is an interleukin-7-like cytokine that is produced by epithelial cells in the lungs, gut, and skin.45 Expression of TSLP in the airways of patients with asthma correlates with the severity of the disease, suggesting that it is involved in the development of allergic airway inflammation.46 TSLP activates dendritic cells to polarize naive T cells toward the Th2 cells that produce Th2-type cytokines as well as TNF-α.47 Mice expressing TSLP in the lungs develop a spontaneous airway inflammation with characteristics similar to human asthma.48 Conversely, mice deficient in the TSLP receptor show decreased airway inflammation when they are challenged with allergens.49 TSLP mRNA and protein are induced when a human airway epithelial cell line, BEAS-2B, is exposed to prototypic proteases, namely trypsin and papain. TSLP induction by trypsin requires intact protease activity and PAR-2. Importantly, when BEAS-2B cells or normal human bronchial epithelial cells are exposed to Alternaria extract, TSLP is also induced. The TSLP-inducing activity of Alternaria is partially blocked by treating the extract with a cysteine protease inhibitor, E-64, or by infecting BEAS-2B cells with small interfering RNA for PAR-2, suggesting roles for fungal protease(s) and their receptor, PAR-2.
Among the most potent molecules of the innate immune system are the interleukin-1 (IL-1) family members;50 these cytokines, such as IL-1, interleukin-18, and interleukin-33 (IL-33), are evolutionarily ancient and are involved in regulating both innate and adaptive immune responses. IL-33 was first described as a nuclear factor abundantly expressed in the nucleus of endothelial and epithelial cells.51 In vivo systemic administration of IL-33 to mice induces lung and gastrointestinal eosinophilia and increased levels of serum IgE and immunoglobulin A (IgA).52 IL-33 drives the production of cytokines and chemokines by Th2 cells, mast cells, basophils, eosinophils, natural killer T (NKT) cells, and natural killer (NK) cells.52 Airway exposure of naive mice to Alternaria extracts induces the rapid release of IL-33 into the airway lumen, followed by innate Th2-type responses.53 Biologically active IL-33 is constitutively stored in the nuclei of human airway epithelial cells, and exposing these cells to Alternaria releases IL-33 extracellularly. Pharmacological inhibitors of purinergic receptors or deficiency in the purinergic receptor P2Y, G-protein coupled, 2 (P2Y2) gene abrogate IL-33 release and Th2-type responses in the Alternaria-induced airway inflammation, suggesting essential roles for adenosine triphosphate and the P2Y purinoceptor 2 receptor in epithelial responses to airborne fungi. Altogether, these findings suggest that airway exposure to fungi or their products activate the innate immune cells (e.g., macrophages, epithelial cells) and facilitate their production of immunological molecules that are involved in eosinophilic inflammation, such as IL-33, LTB4, and TSLP. Fungal chitin and proteases likely are key components in triggering the immune response.
Potential Roles for Eosinophil Antifungal Immunity in Human Diseases
Information regarding the roles of eosinophils in protecting against fungal infection in humans is scarce. Coccidioidomycosis, caused by the fungus Coccidioides immitis, may be accompanied by an increase in peripheral blood eosinophils.54 Marked deposition of MBP-1 is observed in lesions of patients with paracoccidioidomycosis.55 A number of studies suggest potential detrimental roles for eosinophils and fungi in Th2-mediated airway diseases, such as allergic bronchopulmonary aspergillosis (ABPA), severe asthma associated with fungal sensitivity (SAFS), and CRS.
Allergic asthma is generally considered a chronic inflammatory disorder of the airways mediated by Th2 cells, which drives the cardinal features of asthma, including airway eosinophilia, airway hyperreactivity, and excessive production of mucus. These pathological immune responses have long been recognized to mirror the beneficial immune responses to helminthic infection. Although the etiology of asthma could be complex, a number of epidemiological studies suggest that fungi are involved in asthma. For example, direct associations between increased fungal exposure and worsening of asthma have been demonstrated repeatedly.56 Fungal sensitivity, particularly to Aspergillus, Alternaria, and Cladosporium, is associated with asthma.57., 58. In a large survey of US housing, exposure to A. alternata antigens was correlated with active asthma symptoms.59 Sensitization to Alternaria at age 6 correlated with chronic asthma at age 22,60 and sensitization to Alternaria and other species has been associated with severe and potentially fatal episodes of asthma.56 In addition, epidemics of asthma caused by increased airborne fungal spores that occur during thunderstorms further illustrate the association between fungal exposure and worsening of asthma symptoms.61
ABPA is a Th2 hypersensitivity lung disease caused by bronchial colonization of A. fumigatus and is characterized by exacerbations of asthma, recurrent transient chest radiographic infiltrates, and peripheral and pulmonary eosinophilia, especially during exacerbation.62 The minimal criteria required for the diagnosis of ABPA are:
-
•
asthma or cystic fibrosis with deterioration of lung function;
-
•
immediate Aspergillus skin test reactivity;
-
•
total serum IgE ≥ 1000 ng/ml (416 IU/ml);
-
•
elevated Aspergillus-specific IgE and IgG antibodies; and
-
•
chest radiographic infiltrates.
ABPA is likely the most common form of allergic bronchopulmonary mycosis (ABPM). Other fungi, including Candida, Penicillium and Curvularia, are occasionally responsible for a syndrome similar to ABPA.63 The characteristics of ABPM include severe asthma, blood and pulmonary eosinophilia, markedly elevated IgE and specific IgE, bronchiectasis, and mold colonization of the airways.
The term SAFS has been coined to illustrate the high rate of fungal sensitivity in patients with severe asthma.56 It is possible that ABPA represents one manifestation of a spectrum of fungus-associated airway diseases. From a fungal perspective, the human lung is not sterile. The conidia of A. fumigatus, Penicillium and Cladosporium, and presumably other fungi, are often present, and they induce an immune response when germination is initiated.64 Excess mucus and airway architecture distortion may allow fungal germination and escape from host defense. In approximately 50% of patients with severe asthma, fungal cultures are positive without evidence of IgE fungal sensitization, suggesting that fungal colonization of the airways is common even in the absence of an allergic component.65 There is a significant association between A. fumigatus IgE sensitization, colonization, and impaired postbronchodilator forced expiratory volume in one second (FEV1).66 Patients with SAFS respond to oral antifungal therapy, implicating fungi in the pathophysiology of asthma.67
CRS is chronic inflammation of the upper airway and is often associated with nasal polyps. CRS is defined as symptoms and signs of sinus inflammation persisting for more than 8–12 weeks.68 In the past, a number of studies have attempted to elucidate the mechanisms of CRS. Several hypotheses concerning its pathogenesis have been proposed and tested, including chronic bacterial infection, inhalant or food allergies, and T-cell disturbance caused by aerodynamic factors.69 Although its cause is still unclear, there are several distinctive features in CRS. For example, the histological hallmark of CRS is marked tissue eosinophilia, which is seen in almost all patients.70., 71. These eosinophils likely play a major role in the pathophysiology of CRS via the release of their cytotoxic granule proteins, such as MBP-1.72 In fact, a highly significant correlation was noted between the extent of disease, as examined by computed tomography (CT) scan, and the peripheral blood eosinophil count.73 Consequently, MBP-1 levels in mucus exceeding the concentrations that cause damage to the upper airway respiratory epithelium are detected in sinus mucus from patients with CRS.4
In 1981, Millar and colleagues first described sinus specimens from five patients with CRS that showed histological similarities to ABPA.74 Since then the majority of reports have shown non-Aspergillus fungi, such as Curvularia, Alternaria, and Bipolaris, causing similar findings, promoting a change to the more general term, allergic fungal sinusitis (AFS). The diagnostic histological feature of AFS is the presence of allergic mucin, characterized by sloughed respiratory epithelial cells, accumulations of intact and degenerating eosinophils, Charcot–Leyden crystals, and cellular debris containing eosinophils and their products; noninvasive fungal hyphae are found within the allergic mucin and are best demonstrated with impregnated silver stain. AFS prevalence ranges from 4% to 7%75 of all the CRS cases; however, it is also speculated that AFS is underdiagnosed because of confusion concerning the criteria for diagnosis76 and less than adequate techniques to detect fungi. Indeed, fungi are ubiquitous and found in the mucus of a majority of patients with CRS as well as in healthy individuals.77., 78. Furthermore, in some patients with CRS, eosinophils sometimes form clusters around fungal organisms in the mucus (Fig. 9.4.4 ), reminiscent of the accumulation of eosinophils around helminth parasites. These findings suggest that the eosinophilic immune response to fungal organisms may be implicated in the pathophysiology of CRS.77., 78. Interestingly, systemic antifungal treatment in patients with SAFS not only improved asthma symptoms but also rhinological symptoms.67 Two other randomized controlled trials of CRS patients with antifungal agents have demonstrated a benefit while other trials have not.79 The controversy over antifungal therapy for CRS and the involvement of fungi in CRS continues, requiring further investigations.
FIGURE 9.4.4.

Eosinophilic inflammation and fungal localization in the mucus of a CRS patient.
A surgical tissue specimen from a patient with CRS was stained by hematoxylin and eosin stain (A), Gomori–Grocott Methenamine Silver GMS stain (B), anti-MBP-1 antibody (C), or anti-Alternaria antibody (D). Note several clusters of eosinophils within the mucus (A and C). Fungi were not visualized well by GMS stain (B) but were clearly shown by the antibody (D). CRS, chronic rhinosinusitis; MBP-1, eosinophil granule major basic protein 1.
Conclusion and Future Perspectives
Evidence exists that eosinophils respond to fungal organisms and their products, resulting in the production of proinflammatory mediators and killing of fungi. Immune cells in the airways recognize carbohydrate molecules and proteases that are produced by fungal organisms and initiate Th2 immune responses and eosinophilic inflammation. There are a number of epidemiological findings that link the exposure or sensitivity to fungi, eosinophilic airway inflammation, and human airway diseases. However, several major questions still remain. For example: are eosinophils protective against fungal colonization or infection in humans? How do human eosinophils become activated in diseased tissues or at the site of the immune response, resulting in tissue damage? Which molecules in fungi and host receptors play a pivotal role in antifungal immune responses? In SAFS or CRS, do antifungal immune responses and the aberrant activation of eosinophils play major roles in disease pathophysiology? Careful analysis of recent genetically engineered eosinophil-deficient mice in vivo and further characterization of immunobiology of eosinophils in vitro will be necessary to answer these critical questions. Well-designed clinical studies and pharmacological trials in patients with SAFS and CRS will be necessary to dissect the roles for eosinophils and fungi in human diseases. Although these are challenging tasks, future studies have a promise to reveal the true importance of eosinophils in human health and disease.
References
- 1.Gleich G.J., Adolphson C.R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 2.Kita H., Adolphson C.R., Gleich G.J. Biology of eosinophils. In: Adkinson, Jr. N.F., Bochner B.S., Yunginger J.W., Holgate S.T., Busse W.W., Simons F.E.R., editors. 6th ed. Vol. 1. Mosby; St. Louis: 2003. pp. 305–332. (Middleton’s Allergy: Principles and Practice). [Google Scholar]
- 3.Filley W.V., Holley K.E., Kephart G.M., Gleich G.J. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982;2:11–16. doi: 10.1016/s0140-6736(82)91152-7. [DOI] [PubMed] [Google Scholar]
- 4.Ponikau J.U., Sherris D.A., Kephart G.M., Kern E.B., Congdon D.J., Adolphson C.R. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362–369. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Leiferman K.M., Ackerman S.J., Sampson H.A., Haugen H.S., Venencie P.Y., Gleich G.J. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med. 1985;313:282–285. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- 6.Matthews A.N., Friend D.S., Zimmermann N., Sarafi M.N., Luster A.D., Pearlman E. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Burik J.A., Magee P.T. Aspects of fungal pathogenesis in humans. Annu Rev Microbiol. 2001;55:743–772. doi: 10.1146/annurev.micro.55.1.743. [DOI] [PubMed] [Google Scholar]
- 8.Kauffman H.F., Tomee J.F., van der Werf T.S., de Monchy J.G., Koeter G.K. Review of fungus-induced asthmatic reactions. Am J Respir Crit Care Med. 1995;151:2109–2115. doi: 10.1164/ajrccm.151.6.7767565. discussion 2116. [DOI] [PubMed] [Google Scholar]
- 9.Kheradmand F., Kiss A., Xu J., Lee S.H., Kolattukudy P.E., Corry D.B. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 10.Bush R.K., Prochnau J.J. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 12.Latge J.P. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 13.Douwes J. (1-->3)-Beta-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air. 2005;15:160–169. doi: 10.1111/j.1600-0668.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 14.Reed C.E. Inflammatory effect of environmental proteases on airway mucosa. Curr Allergy Asthma Rep. 2007;7:368–374. doi: 10.1007/s11882-007-0056-5. [DOI] [PubMed] [Google Scholar]
- 15.Diniz S.N., Nomizo R., Cisalpino P.S., Teixeira M.M., Brown G.D., Mantovani A. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 16.Garlanda C., Hirsch E., Bozza S., Salustri A., De Acetis M., Nota R. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 17.Kaur S., Thiel S., Sarma P.U., Madan T. Mannan-binding lectin in asthma and allergy. Curr Allergy Asthma Rep. 2006;6:377–383. doi: 10.1007/s11882-996-0007-6. [DOI] [PubMed] [Google Scholar]
- 18.Roeder A., Kirschning C.J., Rupec R.A., Schaller M., Korting H.C. Toll-like receptors and innate antifungal responses. Trends Microbiol. 2004;12:44–49. doi: 10.1016/j.tim.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.van der Graaf C.A., Netea M.G., Verschueren I., van der Meer J.W., Kullberg B.J. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–7464. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav M., Schorey J.S. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor P.R., Brown G.D., Reid D.M., Willment J.A., Martinez-Pomares L., Gordon S. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 22.Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn P.Y., Evans S.E., Kottom T.J., Standing J.E., Pagano R.E., Limper A.H. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J Biol Chem. 2003;278:2043–2050. doi: 10.1074/jbc.M209715200. [DOI] [PubMed] [Google Scholar]
- 24.Di Renzo L., Yefenof E., Klein E. The function of human NK cells is enhanced by beta-glucan, a ligand of CR3 (CD11b/CD18) Eur J Immunol. 1991;21:1755–1758. doi: 10.1002/eji.1830210726. [DOI] [PubMed] [Google Scholar]
- 25.Herre J., Marshall A.S., Caron E., Edwards A.D., Williams D.L., Schweighoffer E. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 26.Vetvicka V., Thornton B.P., Ross G.D. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross G.D. Role of the lectin domain of Mac-1/CR3 (CD11b/CD18) in regulating intercellular adhesion. Immunol Res. 2002;25:219–227. doi: 10.1385/IR:25:3:219. [DOI] [PubMed] [Google Scholar]
- 28.Xia Y., Borland G., Huang J., Mizukami I.F., Petty H.R., Todd R.F., 3rd Function of the lectin domain of Mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion. J Immunol. 2002;169:6417–6426. doi: 10.4049/jimmunol.169.11.6417. [DOI] [PubMed] [Google Scholar]
- 29.Inoue Y., Matsuwaki Y., Shin S.H., Ponikau J.U., Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 30.Matsuwaki Y., Wada K., White T.A., Benson L.M., Charlesworth M.C., Checkel J.L. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J Immunol. 2009;183:6708–6716. doi: 10.4049/jimmunol.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shpacovitch V., Feld M., Bunnett N.W., Steinhoff M. Protease-activated receptors: novel PARtners in innate immunity. Trends Immunol. 2007;28:541–550. doi: 10.1016/j.it.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Miike S., McWilliam A.S., Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- 33.Miike S., Kita H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol. 2003;111:704–713. doi: 10.1067/mai.2003.1332. [DOI] [PubMed] [Google Scholar]
- 34.Yoon J., Ponikau J.U., Lawrence C.B., Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundahl J., Hallden G., Hed J. Differences in intracellular pool and receptor-dependent mobilization of the adhesion-promoting glycoprotein Mac-1 between eosinophils and neutrophils. J Leukoc Biol. 1993;53:336–341. doi: 10.1002/jlb.53.3.336. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko M., Horie S., Kato M., Gleich G.J., Kita H. A crucial role for beta 2 integrin in the activation of eosinophils stimulated by IgG. J Immunol. 1995;155:2631–2641. [PubMed] [Google Scholar]
- 37.Gottar M., Gobert V., Matskevich A.A., Reichhart J.M., Wang C., Butt T.M. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reese T.A., Liang H.E., Tager A.M., Luster A.D., Van Rooijen N., Voehringer D. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dyken S.J., Garcia D., Porter P., Huang X., Quinlan P.J., Blanc P.D. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol. 2011;187:2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter P., Susarla S.C., Polikepahad S., Qian Y., Hampton J., Kiss A. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam A., Wadsworth S., Dorscheid D., Man S.F., Sin D.D. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5:255–273. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- 42.Parker D., Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saenz S.A., Taylor B.C., Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Locksley R.M. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammad H., Lambrecht B.N. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 46.Ying S., O’Connor B., Ratoff J., Meng Q., Mallett K., Cousins D. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 47.Ito T., Wang Y.H., Duramad O., Hori T., Delespesse G.J., Watanabe N. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou B., Comeau M.R., De Smedt T., Liggitt H.D., Dahl M.E., Lewis D.B. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 49.Al-Shami A., Spolski R., Kelly J., Keane-Myers A., Leonard W.J. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sims J.E., Smith D.E. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 51.Moussion C., Ortega N., Girard J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3 doi: 10.1371/journal.pone.0003331. e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Kouzaki H., Iijima K., Kobayashi T., O’Grady S.M., Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drutz D.J., Catanzaro A. Coccidioidomycosis. Am Rev Respir Dis. 1978;117:727–771. doi: 10.1164/arrd.1978.117.4.727. Part II. [DOI] [PubMed] [Google Scholar]
- 55.Wagner J.M., Franco M., Kephart G.M., Gleich G.J. Localization of eosinophil granule major basic protein in paracoccidioidomycosis lesions. Am J Trop Med Hyg. 1998;59:66–72. doi: 10.4269/ajtmh.1998.59.66. [DOI] [PubMed] [Google Scholar]
- 56.Denning D.W., O’Driscoll B.R., Hogaboam C.M., Bowyer P., Niven R.M. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 57.Arbes S.J., Jr., Gergen P.J., Vaughn B., Zeldin D.C. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2007;120:1139–1145. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaakkola M.S., Ieromnimon A., Jaakkola J.J. Are atopy and specific IgE to mites and molds important for adult asthma? J Allergy Clin Immunol. 2006;117:642–648. doi: 10.1016/j.jaci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Salo P.M., Arbes S.J., Jr., Sever M., Jaramillo R., Cohn R.D., London S.J. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stern D.A., Morgan W.J., Halonen M., Wright A.L., Martinez F.D. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pulimood T.B., Corden J.M., Bryden C., Sharples L., Nasser S.M. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007;120:610–617. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 62.Greenberger P.A. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002;110:685–692. doi: 10.1067/mai.2002.130179. [DOI] [PubMed] [Google Scholar]
- 63.Lotvall J., Akdis C.A., Bacharier L.B., Bjermer L., Casale T.B., Custovic A. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 64.Aimanianda V., Bayry J., Bozza S., Kniemeyer O., Perruccio K., Elluru S.R. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 65.Denning D.W., Park S., Lass-Florl C., Fraczek M.G., Kirwan M., Gore R. High-frequency triazole resistance found In nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52:1123–1129. doi: 10.1093/cid/cir179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fairs A., Agbetile J., Hargadon B., Bourne M., Monteiro W.R., Brightling C.E. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182:1362–1368. doi: 10.1164/rccm.201001-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denning D.W., O’Driscoll B.R., Powell G., Chew F., Atherton G.T., Vyas A. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 68.Spector S.L., Bernstein I.L., Li J.T., Berger W.E., Kaliner M.A., Schuller D.E. Parameters for the diagnosis and management of sinusitis. J Allergy Clin Immunol. 1998;102:S107–S144. doi: 10.1016/s0091-6749(98)70045-4. [DOI] [PubMed] [Google Scholar]
- 69.Tomassen P., Van Zele T., Zhang N., Perez-Novo C., Van Bruaene N., Gevaert P. Pathophysiology of chronic rhinosinusitis. Proc Am Thorac Soc. 2011;8:115–120. doi: 10.1513/pats.201005-036RN. [DOI] [PubMed] [Google Scholar]
- 70.Harlin S.L., Ansel D.G., Lane S.R., Myers J., Kephart G.M., Gleich G.J. A clinical and pathologic study of chronic sinusitis: the role of the eosinophil. J Allergy Clin Immunol. 1988;81:867–875. doi: 10.1016/0091-6749(88)90944-x. [DOI] [PubMed] [Google Scholar]
- 71.Stoop A.E., van der Heijden H.A., Biewenga J., van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol. 1993;91:616–622. doi: 10.1016/0091-6749(93)90267-j. [DOI] [PubMed] [Google Scholar]
- 72.Motojima S., Frigas E., Loegering D.A., Gleich G.J. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989;139:801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- 73.Hoover G.E., Newman L.J., Platts-Mills T.A., Phillips C.D., Gross C.W., Wheatley L.M. Chronic sinusitis: risk factors for extensive disease. J Allergy Clin Immunol. 1997;100:185–191. doi: 10.1016/s0091-6749(97)70223-9. [DOI] [PubMed] [Google Scholar]
- 74.Millar J.W., Johnston D., L. A., D., L Allergic aspergillosis of the maxillary sinuses. Thorax. 1981;36:710. (Abstract) [Google Scholar]
- 75.Corey J.P., Delsupehe K.G., Ferguson B.J. Allergic fungal sinusitis: allergic, infectious, or both? Otolaryngol Head Neck Surg. 1995;113:110–119. doi: 10.1016/s0194-5998(95)70153-2. [DOI] [PubMed] [Google Scholar]
- 76.deShazo R.D., Swain R.E. Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol. 1995;96:24–35. doi: 10.1016/s0091-6749(95)70029-3. [DOI] [PubMed] [Google Scholar]
- 77.Ponikau J.U., Sherris D.A., Kern E.B., Homburger H.A., Frigas E., Gaffey T.A. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 78.Braun H., Buzina W., Freudenschuss K., Beham A., Stammberger H. ‘Eosinophilic fungal rhinosinusitis’: a common disorder in Europe? Laryngoscope. 2003;113:264–269. doi: 10.1097/00005537-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 79.Rank M.A., Adolphson C.R., Kita H. Antifungal therapy for chronic rhinosinusitis: the controversy persists. Curr Opin Allergy Clin Immunol. 2009;9:67–72. doi: 10.1097/ACI.0b013e328320d279. [DOI] [PMC free article] [PubMed] [Google Scholar]


