Abstract
Drug repurposing is a strategy consisting of finding new indications for already known marketed drugs used in various clinical settings or highly characterized compounds despite they can be failed drugs. Recently, it emerges as an alternative approach for the rapid identification and development of new pharmaceuticals for various rare and complex diseases for which lack the effective drug treatments. The success rate of drugs repurposing approach accounts for approximately 30% of new FDA approved drugs and vaccines in recent years. This review focuses on the status of drugs repurposing approach for various diseases including skin diseases, infective, inflammatory, cancer, and neurodegenerative diseases. Efforts have been made to provide structural features and mode of actions of drugs.
Keywords: Cancer, Drug repositioning, Drug reprofiling, Recycling of drugs, Drug discovery, Inflammation, Neurodegenerative diseases, Skin diseases
Graphical abstract
Highlights
-
•
Repositioning is a promising approach for to explore new pharmaceuticals for rare and complex diseases.
-
•
Drug repositioning reduces the time and cost needed to reach the market.
-
•
Development of multi-target ligands by repositioning approach.
-
•
Drug repositioning leads to mutual collaborations and funding opportunities.
-
•
Successful approval for repositioned drugs is about 10% and 70% are in (pre)clinical studies.
1. Introduction
“The most fruitful basis for the discovery of a new drug is to start with an old drug.”
Sir James Whyte Black, winner of the 1988 Nobel Prize in Medicine [1].
It is reported that the Food and Drug Administration (FDA) has approved agents against about 400 human proteins [2], 90% of which comes under the classification of enzymes, transporters, G protein-coupled receptors (GPCRs), cluster of differentiation (CD) markers, voltage-gated ion channels, and nuclear receptors. It is a fact that the typical de novo drug discovery program takes 10–15 years [3,4] from the identification of lead molecule to market the drug and the probability of success rate is less than 10% [5]. Over five years, the number of new drugs approved by FDA has been around 40 per year, although billions of US dollars spent by various pharma industries on the research and development [[6], [7], [8]]. The success rate is less than 6% of new drug discovery and development, which is far away from addressing an unmet clinical need for disease treatments. Because, the effective therapeutics for complex diseases like Alzheimer’s disease (AD), Parkinson’s disease (PD), cardiovascular diseases, and neglected diseases are still lacking. This outcome strongly suggests that new strategies, approaches, and technologies are needed to accelerate drug discovery to advance the success rate of drug development.
Drug repurposing is a strategy consisting of finding new indications for already known marketed drugs used in various clinical settings or highly characterized compounds despite they can be failed drugs [9]. It is a drug discovery program, which is faster and safer to develop medications against diseases/disorders for which no potential treatment is available. In recent years, the success rate of drug repurposing approach accounts for approximately 30% of the newly FDA approved drugs, and vaccines. This is one of the main reasons for pharmaceutical companies to show their interest in drug repurposing approach. This approach does not require the initial six to 10 years typically needed for the development of new drugs. Additionally, many phases of de novo drug discovery and development can be by-passed, since clinical and pre-clinical studies of the re-purposed candidates are already being documented in the original indication(s). Thus, it reduces the time and cost needed to reach the market and risk intrinsic to any research and development program. Moreover, the risk of clinical failure is also low. As an added advantage, this approach gives a possibility to widen the market and to prolong the application of the patent life of a drug. There are mainly two ways to proceed drug-repositioning approach such as experimental strategies and computational strategies.
Experimental repurposing strategies include binding assays and phenotypic screening methods, which can be to find binding interactions of drug molecules to assay components and to identify lead compounds from a vide range of compound libraries, respectively [10]. Computational approaches are categorized into target-/mechanism based, knowledge-based, pathway- and network-based approaches. These approaches are proven economic in discovering novel therapeutic ligands. Most notably, computational methods augment the drug discovery process by effectively utilizing cheminformatics, bioinformatics, network biology and systems biology. More specifically, these methods exploit known targets, drugs, disease biomarkers or pathways to establish novel methods and accelerate the planning of crucial clinical trials [11].
In this perspective, we focused on the status of drugs repurposing approaches for various diseases including skin diseases, infective, inflammatory, cancer, and neurodegenerative diseases. Efforts have been made to provide structural features, and mode of action of drugs, which are exclusively small molecules, peptidomimetics, and macrocyclic compounds. Antibodies, vaccines, and any other biological drugs are not discussed.
Drugs repositioning for skin whitening activity. Melanin is a collection of natural pigments that primarily determine the skin, hair and hair color of the human. Melanocytes, which are found in the basal layer of the epidermis, produce melanin by the process called melanogenesis upon the skin exposure to the ultra-violet (UV)-radiation [12,13]. Although, melanin protects human skin from the radiation, continuing irradiation can result in the risk of skin damage and malignant melanoma, a cancer of melanocytes. Besides, the abnormal production of melanin leads to a serious of dermatological disorders including melasma [[13], [14], [15], [16]], freckles, age spots, and post-inflammatory melanoderma [15,17].
Melanogenesis, a process of synthesis of melanin, is a complex enzymatic and biochemical catalyzed reactions, in which tyrosinase plays a rate-limiting step: hydroxylation of l-tyrosine to L-3,4-dihydroxyphenylalanine (l-DOPA) followed by the oxidation of l-DOPA to l-dopaquinone, which serves as a substrate for the production of melanin [18]. Therefore, targeting tyrosinase has been recognized as a potential approach for controlling the abnormal production of melanin. Tyrosinase is also an important target in the food industry as inhibition of tyrosinase can prevent the enzymatic browning of fruits and vegetables. Besides, it is essential for wound healing process and immune responses in many plants, sponges and some invertebrates. Abnormal activities of tyrosinase have been linked to neurodegenerative disorders including Parkinson’s [19] and Huntington’s diseases [[20], [21], [22], [23], [24]].
Thiourea and analogs. Tyrosinase inhibitors can be broadly classified into two categories: (a) polyphenols, which are mostly natural products such as arbutin, hydroquinone and kojic acids, and b) thiourea and its derivatives. Since the 1940’s, phenylthiourea (PTU, 1) has been well known as a tyrosinase inhibitor [25,26]. Many research groups including ours have extensively studied the structure-activity relationships for PTU as tyrosinase inhibitors [27,28].
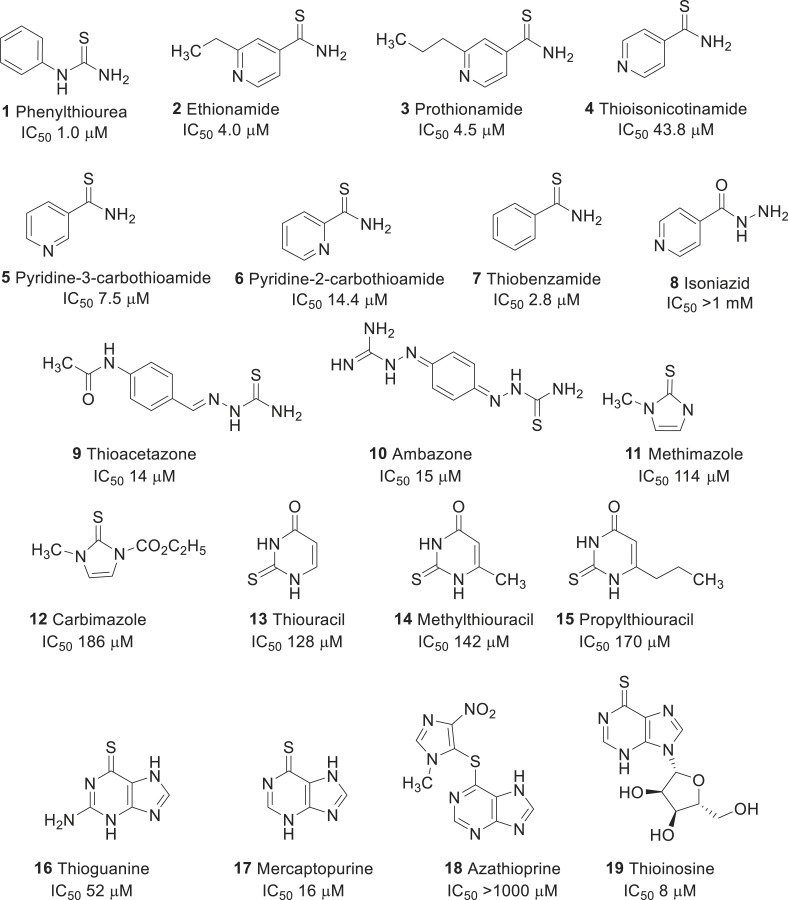
Choi et al. screened the FDA approved drug library that has close structural similarity to PTU (see Fig. 1 ) [29]. For example, ethionamide (2), a second-line antituberculosis drug used for the treatment of multi-drug resistant tuberculosis, shares a chemical similarity that led to the discovery of a new mushroom tyrosinase inhibitor with an IC50 value of 4.0 μM. Other commercially available analogs of 2 including, prothionamide (3), thioisonicotinamide (4), pyridine-3-carbothiomide (5), pyridine-2-carbothiomide (6), and thiobenzamide (7) were identified as potent tyrosinase inhibitors. In particular, compound 7 had a strong inhibitory activity than other molecules, which suggests that the pyridine ring in compound 2 can be replaced by other aromatic moieties, including a benzene ring. However, the poor inhibitory activity of isoniazid (8), a first-line antituberculosis drug, suggests that carbothiomide group was crucial for tyrosinase inhibitory activity. Moreover, comparing the inhibitory activities of drugs 2 and 3 implies that the additional aliphatic tail was not required for inhibiting the tyrosinase. Inhibitory kinetic studies suggest that drug 2, and its analogs 3–7 were reversible and non-competitive. In cellular assay, drugs 6 and 7 markedly decreased the melanin content in B16 cells to the values of 44% and 37%, respectively, without inducing any cytotoxicity up to 50 μM concentration. Further studies suggest that the drug 7 had strong inhibition of mammalian tyrosinase. However, the inhibition exhibited by drug 2, and its analogs were weaker than those obtained by PTU observed in enzyme, and melanin content assays.
Fig. 1.
Repurposing of thiourea related drugs for skin whitening activity.
The same research group continued to search for molecules that are in clinical usage, and contain thiourea moiety [30]. As a result, they could retrieve some thiourea containing drugs such as thioacetazone (9), ambazone (10), methimazole (11), carbimazole (12), thiouracil (13), methylthiouracil (14), and propylthiouracil (15). Thioacetazone (9) (also called as thiacetazone) is an anti-tuberculosis drug [31]. Ambazone (10) is an oral antiseptic drug used in Europe [32]. The other five molecules (11–15) are antithyroid drugs [33]. These drugs, except 12, exhibited remarkable inhibitory activities against mushroom tyrosinase (see IC50 values of each in Fig. 1), although they were comparatively weaker than 1 (1 μM). Kinetics studies of tyrosinase inhibition assigned these thiourea-containing drugs as non-competitive inhibitors. In cellular assay, using B16 cells, drug 10 among other thioureas, significantly decreased the melanin content by 20% without inducing any cytotoxicity up to 20 μM. Further, enzymatic studies with cell extracts of B16F10 cells confirmed that thiourea-containing drugs affected the function of mammalian tyrosinase.
In an extended study, the same group repositioned thiopurine drugs 16–19 as tyrosinase inhibitors [34]. Thioguanine (16), a drug used for the treatment of leukemia, is one of the essential medicines required for a basic health system, recommended by the world health organization (WHO). It inhibited tyrosinase activity with a Ki value of 52 μM. In addition to that, two other thiopurine drugs such as mercaptopurine (17) and azathioprine (18) were discovered as tyrosinase inhibitors, and among them, drug 17 showed stronger Ki value (Ki 52 μM) than the drug 16. Azathioprine (18) exhibited a poor tyrosinase inhibition. These results suggest that the sulfur atom possibly plays an important role in the interaction of tyrosinase. Interestingly, thioinosine (19), a metabolic product of mercaptopurine through the attachment of sugar moiety exhibited an excellent tyrosinase inhibitory activity with a Ki value of 8.0 μM. Further, kinetics studies classified the drugs 16, 17, and 19 as competitive inhibitors. In the cellular assay, these drugs inhibited the melanin content without cytotoxicity up to 50 μM. In particular, drug 16 at 50-μM concentration, remarkably reduced the melanin content by 57%, without any apparent cytotoxicity.
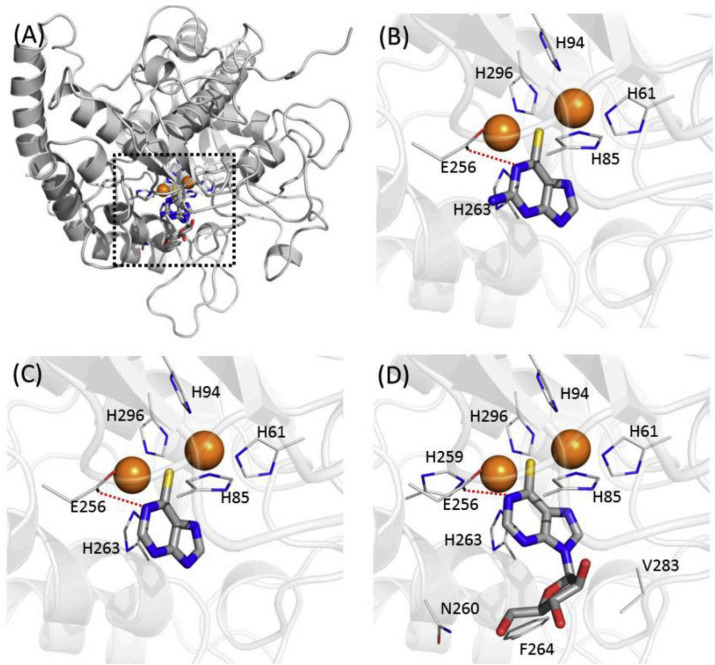
The thiopurine drugs were docked into four different crystal structures complexed with inhibitors tropolone, kojic acid, hydroquinone and PTU (Fig. 2 ) [[35], [36], [37]]. In the molecular docking study, thiopurine moieties of drugs 16, 17, and 19 exhibited an almost similar pattern of binding mode to that of PTU. Thioinsoine (19) (Fig. 2 D) made additional contacts with the side-chains of N-260, F-264, and V-283 that were thought to have the stronger inhibition. It also predicted that the intramolecular hydrophilic contacts with the residue of E-256 disappeared in mercaptopurine (Fig. 2C), whereas thioguanine (Fig. 2B) and thioinosine possess the contacts within 3.8 Å.
Fig. 2.
Binding modes of thiopurine inhibitors. (A) Overlaid poses against mushroom tyrosinase. Two copper atoms in tyrosinase are indicated as orange spheres. Predicted binding modes in (B) thioguanine (16), (C) mercaptopurine (17) and (D) thioinosine (19) are represented. This Figure was adopted from the publication of Choi, J. et al. [28]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Drugs repositioning for cancer therapy. As cancer is one of the leading cause of death worldwide [38], pharmaceutical companies invest billions of dollars in developing new anti-cancer drugs. The drug discovery and development process for the cancer treatment takes an average of 13 years at a cost of approximately $ 1.8 billion [39]. However, only 5% of the drugs that enter clinical trials are approved. This prolonged duration for the drug development and enormous cost of the (pre)clinical trials for their approval emphasizes the need for a drug repurposing approach (see Fig. 3 for drugs repurposing in cancer therapy).
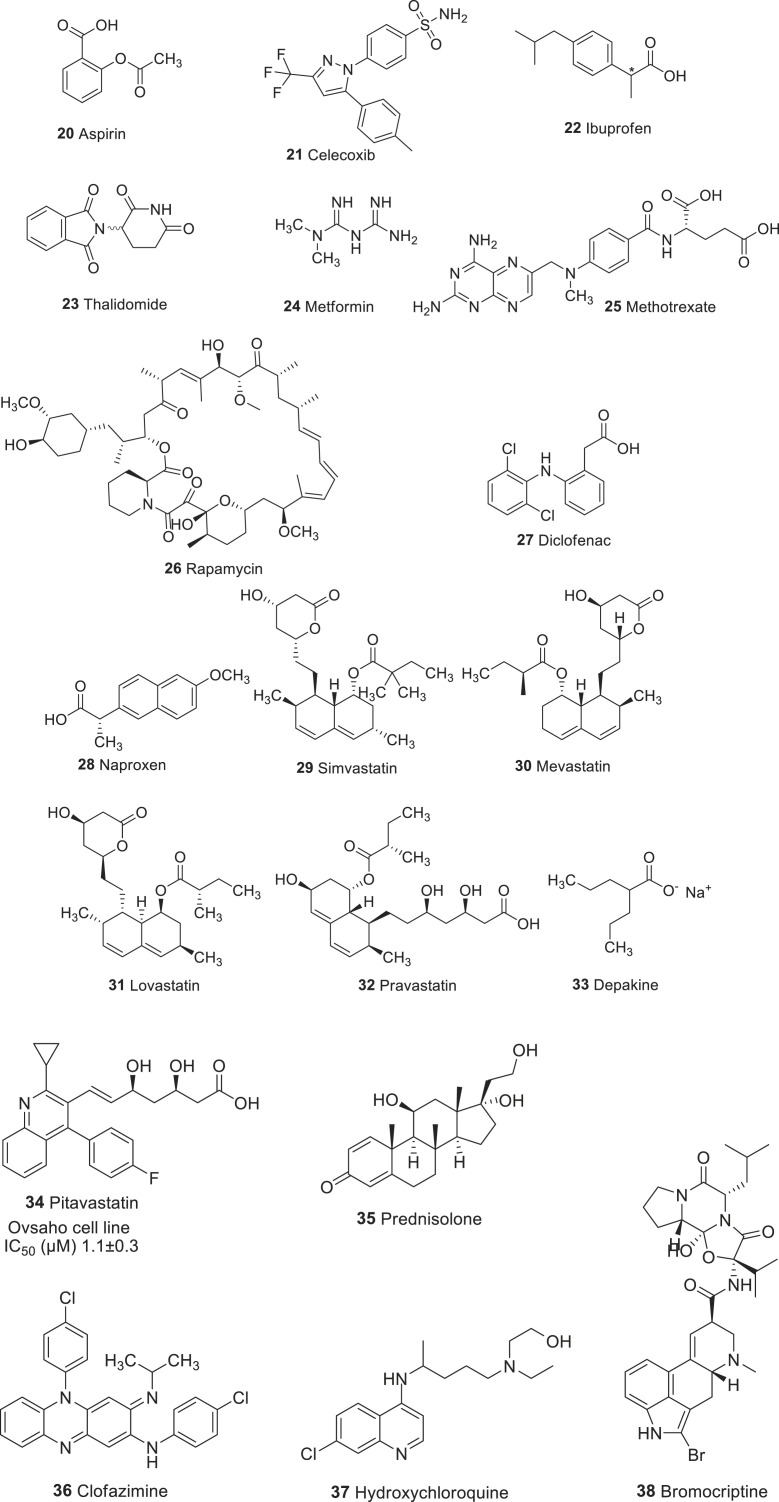
Fig. 3.
Chemical structures of drugs repurposed for cancer therapy.
Aspirin (20), also known as acetylsalicylic acid, is one of the non-steroidal anti-inflammatory drugs (NSAIDs), which has been widely used as an analgesic and an antipyretic to prevent the heart attack and stroke. For the first time, Gasic and co-workers reported the possible role of aspirin in cancer therapy. They discovered that the antiplatelet activity of 20 in tumor-bearing mice was associated with a 50% reduction in lung metastasis [40]. A recent study also indicated that the daily intake of the drug 20 (75 mg) produced a significant beneficial effect against gastrointestinal, esophageal, pancreatic, brain, prostate, and lung cancer [41]. The mode of action aspirin is reported to modulate numerous molecules, which are associated with the tumorigenesis process [42]. Preclinical studies revealed that the anticancer activity of drug 20 has been attributed by its inhibition of cyclooxygenase (COX) enzymes that promotes carcinogenesis through the synthesis of prostaglandins (PG), including PGE2 [43]. Besides, drug 20 was reported to inhibit the activation of transcription factor NF-κB, which is critical in regulating the expression of genes involved in apoptosis [44]. In a study reported by Li Ling et al. compound 20 not only inhibited proliferations and promoted apoptosis of cancer cells, but also delayed and overcame acquired resistance to targeted therapy. The underlying mechanism could be attributed to enhanced cancer stemness and activated NF-kB signaling in acquired resistant tumors were suppressed by aspirin and rendered resistant tumors more sensitive to aspirin than parental, sensitive cells in terms of proliferation, apoptosis and cancer stemness. On the contrary, aspirin has no effects on normal lung and mammary epithelial cell proliferation at concentrations used on lung and breast cancer cells. Hence, aspirin could be a potential candidate for combination therapy for lung and breast cancers [45]. Other studies suggest that the anticancer property of aspirin (20) has been linked to the phosphatidylinositol-3-kinase (PI3K) pathway, and Ras-Raf-MEK-ERK signaling cascade. While numerous studies suggest the potent anticancer activities of drug 20, the overall benefit is limited as it is associated with serious side effects including the gastrointestinal and renal toxicities. Therefore, there is no clear recommendation to take 20 for population-wide use. On the other hand, as a primary cancer prevention tool, many reports revealed using 20 would have a greater benefit in the population at age 40–85. Thus, U.S. preventive services task force recommendation statement (USPSTF), recommended for daily intake of 20 for patients above 40 years with increased risk of cardiovascular disease and colorectal cancer.
Celecoxib (21) belongs to the family of NSAID, which has been used to treat pain and inflammation associated with rheumatoid arthritis (RA) and osteoarthritis (OA) [46]. In 1999, the FDA approved celecoxib, which is a highly selective reversible inhibitor of COX-2, a well-known inflammatory cancer target. Because of its COX-2 inhibitory activity, the antitumor activities of drug 21 have been extensively studied and shown to have chemopreventive activities against various cancer types. Other than COX-2, celecoxib targets glycogen synthase kinase (GSK) 3β, β-catenin, NF-κB, AKT8 virus oncogene cellular homolog (AKT), and B cell lymphoma (Bcl)-2 families [47]. In people with familial adenomatous polyposis (FAP), a daily dosage of drug 21 (400 mg) significantly reduced the risk of colorectal adenomas. FDA approved this compound to reduce colon and rectal polyps in people with familial adenomatous polyposis [48]. However, it was associated with some drawbacks including gastrointestinal, renal, and cardiotoxic effects.
Ibuprofen (22) is an NSAID, which has been primarily used to treat fever, pain, and inflammation. At the molecular level, ibuprofen inhibits COX, which converts arachidonic acid to prostaglandin. However, it is not selective towards any isoform of COX. The drug was marketed for the treatment of rheumatoid arthritis in the United Kingdom (1969), and in the United States (1971) as well. The anticancer activity of drug 22 has been investigated in various cancer cell types. Ibuprofen has been shown to inhibit the growth of prostate cancer cells [49]. In adenocarcinoma gastric cells, drug 22 showed antitumor effects, which have been mediated by the anti-angiogenesis, induction of apoptosis, and reduction of cell proliferation [50]. The administration of drug 22 induces apoptosis in metastatic melanoma cell lines [51]. Ibuprofen also reported to increase the chemosensitivity of cisplatin by decreasing the levels of heat shock protein 70s (Hsp70s) in lung cancer cells. Hsp70s are an important part of the cell’s machinery for protein folding, and their function was associated with resistance to apoptosis [52]. Therefore, by blocking Hsp70s, ibuprofen increased the apoptosis by increasing the sensitivity of cisplatin.
Thalidomide (23) is an immunomodulatory drug, which was originally developed as a sedative-hypnotic for the treatment of nausea during pregnancy. However, it was withdrawn due to its teratogenic effects. The drug was demonstrated whether it could be used for treating patients with refractory myeloma, because of its anti-angiogenic activity. After successful clinical evaluations, FDA approved the drug 23 for treating multiple myeloma. Additionally, molecule 23 also showed efficacy against several malignancies, including acute myeloid leukemia [53], myelodysplasia [54], and myelodysplastic syndrome [55]. Mechanistically, thalidomide binds to cereblon, which forms an E3 ubiquitin ligase complex, resulting in the rapid ubiquitination and proteasomal degradation of transcription factors, Ikaros and Aiolos [56]. These two transcription factors are transcriptional regulators of B and T cell development [57].
Metformin (24) is an orally available first-line drug and has been widely used for the treatment of type 2 diabetes. The molecular mechanism of metformin involves the activation of adenosine monophosphate (AMP)-induced protein kinase (AMPK), a key enzyme regulating cellular metabolism. Rapamycin (mTOR), a gene that is involved in the survival of cancer cells is negatively regulated by AMPK. Metformin is also able to reduce the signals of mTOR by inhibiting Rag-mediated activation of mTOR [58]. In general, diabetic patients have an increased risk of several cancer types; especially diabetic women have a 20% risk of developing breast cancer. Several studies suggested the anticancer property of drug 24. The drug 24 at the dose of 250–500 mg/day has been shown to reduce the risk of cancer as well as its daily dose reduces the incidence of gastrological cancer in patients with diabetes [59,60]. Several reviews and meta-analysis suggests that taking drug 24 was associated with reduced risk of all cancer mortality in patients with diabetes [61]. In a recent meta-analysis of several anti-diabetic drugs, it was found that the patients using drug 24 had an overall reduced risk of cancer and decreased mortality rate by 14 and 30%, respectively. Whereas, the use of insulin was associated with an increased risk of cancer and mortality. The recent phase 3 clinical trial studies using the occurrence of colorectal adenomas as a biomarker for cancer as a primary endpoint at 1 year after intervention revealed that metformin reduced both occurrence and number of adenomas/polyps in the patients at low dosage level.
Methotrexate (25) is a competitive inhibitor of dihydrofolate reductase (DHFR); a critical enzyme that involved in the synthesis of DNA, RNA, thymidylates, and proteins. The hydrofolate inhibitory activity of drug 25 was responsible for its anti-leukemia activity. Besides, drug 25 was effective against a wide range of malignancies including breast, head and neck, leukemia, lymphoma, lung, osteosarcoma, bladder, and trophoblastic neoplasms [62]. FDA approved this compound in 1988 for the treatment of osteosarcoma, breast cancer, acute lymphoblastic leukemia, and Hodgkin lymphoma. Several reports suggest that the antitumor activity of methotrexate is also due to its ability to target inflammatory pathways. For instance, methotrexate was reported to suppress the NF-κB through the release of adenosine in cancer cells [63].
Rapamycin (26), also known as sirolimus, which was originally developed as an anti-fungal agent. However, drug 26 was withdrawn due to its potent immunosuppressant and antitumor activities. Mechanistically, the drug inhibits T cells and B cells by decreasing their sensitivity to IL-2 through inhibition of mTOR, which is highly upregulated in many tumor cells [64]. In the year 1999, FDA, approved rapamycin for the prevention of allograft rejection. After that, this drug has been investigated for its anticancer properties. In recent studies, drug 26 was reported to reduce the colony formation of leukemia progenitor cells in patients with acute myeloid leukemia [64]. In addition to that, drug 26 also showed efficacious in patients with imatinib-resistant chronic myelogenous leukemia through the suppression of vascular endothelial growth factor (VEGF) mRNA levels in leukemia cells with mild side effects [65].
Diclofenac (27) is an acetic acid derivative of NSAID class, which has been used to treat pain and inflammatory diseases such as gout. Its mode of action was believed to suppress the production of prostaglandin through inhibition of both COX-1 and COX-2. The drug came into use in the year 1988 in the US. The anticancer activity of drug 27 has been reported in several cancer types including hepatoma, colon, and fibrosarcoma, pancreatic, and ovarian cancer.
In rat models of fibrosarcoma and hepatoma, the drug 27 significantly reduced the growth rate and the level of vascularization [66]. The drug 27 was found to have an anti-proliferative effect on human colon cancer cell lines [67]. The tumor inhibitory activity of drug 27 was also proved in ovarian cancer [68]. At the molecular level, diclofenac induces apoptosis through inhibition of antioxidant superoxide dismutase 2 (SOD 2), leading to increased levels of reactive oxygen species [69]. Recent Phase II clinical trial study of diclofenac for the treatment of basal cell carcinoma concluded that topical application of diclofenac was highly effective in tumor regression with 64% [70,71].
Naproxen (28) is a member of the propionic acid family of NSAID class, which has been used to treat pain and inflammatory diseases such as rheumatoid arthritis, and fever. This drug works by non-selectively inhibiting both COX-1 and COX-2 enzymes, which results in inhibition of prostaglandin synthesis. In 1976, the drug was marketed in the USA for its medical use. Recently the drug 28 has been repurposed for its anticancer activity as it was shown to inhibit cell proliferation, induce apoptosis, and suppress metastasis in various cancer cell types. In vitro studies of drug 28 in human urinary bladder cancer cell lines showed that it induces cell-cycle arrest and apoptosis through targeting PI3K [72]. The combination of atorvastatin and naproxen significantly inhibited the colonic adenocarcinomas in vivo in animals [73]. In the Phase II clinical trial, it has been investigated in combination with calcitriol for recurrent prostate cancer. The results showed that the combination was well tolerated.
Statin family of drugs are lipid-lowering agents that inhibit the rate-limiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in the cholesterol biosynthesis pathway. Statins are commonly prescribed to reduce cholesterol synthesis in patients with a high risk of cardiovascular disease. In addition to that, statins inhibit the mevalonate pathway that provides mevalonate, farnesyl, and geranyl pyrophosphate. These molecules are important for the cell cycle progression and cell proliferation, and therefore statins represent promising candidates in cancer therapeutics. In chronic myeloid leukemia cells, simvastatin (29) [74]. and other natural statins including mevastatin (30), lovastatin (31), and pravastatin (32) displayed TNF-induced apoptosis through the downregulation of NF-κB mediated anti-apoptotic gene products [75]. The anti-cancer activity of statins was also investigated in animal studies, in which statins were effective in reducing the incidence and growth of tumors [76].
Several observational studies and meta-analysis supports the positive correlation of using statins with their chemopreventive effect in humans. Meta-analyses revealed that the use of statins reduced the risk of patients with gastric cancer [77]. as well as esophageal [78], and hepatocarcinoma cancer types [79]. In a case-control study, drug 29 with a dosage of 40 mg/day for 2–5 years significantly reduced the incidence of colorectal cancer [80].
Depakine (33) or valproic acid (VPA) is a short-chain free fatty acid mainly used to treat epilepsy, bipolar disorders, and migraine. Its anticonvulsant activity has been attributed to the blockade of voltage-gated sodium channels and increased levels of gamma-aminobutyric acid (GABA) in the brain. Anti-cancer activity of drug 33 was first established in leukemia cells, in which the drug 33 was shown to inhibit histone deacetylase (HDAC) [81]. Depakine has also been found to suppress the production of cytokine and to modulate inflammatory signaling cascade. In human leukemia and human glioma cells, drug 33 was able to suppress the production of IL-6 and TNF-α [82]. In prostate cancer cells, drug 33 suppressed the IL-6 through inhibition of NF-κB activity [83]. Some of the clinical trials of drug 33 have advanced to Phase II for sarcomas, thyroid cancers, acute myelogenous leukemia, B cell lymphoma, breast cancer, melanoma, non-small, and small-cell lung cancers, prostate cancer, recurrent glioblastoma, and relapsed/refractory leukemia (www.clinicaltrials.gov.)
Recently, Abdullah et al. [84] has shown that pitavastatin (34) has potential to treat ovarian cancer if dietary geranylgeraniol is controlled. However, relatively high doses of statins are required to induce apoptosis in cancer cells, increasing the risk of myopathy, the most common adverse efect associated with statins. This makes it desirable to identify drugs which reduce the dose of pitavastatin necessary to treat cancer. A drug-repositioning strategy was employed to identify suitable candidates. Screening a custom library of 100 of-patent drugs for synergistic activity with pitavastatin identifed prednisolone (35) as the most prominent hit. Prednisolone potentiated the activity of pitavastatin in several assays measuring the growth, survival or apoptosis in several ovarian cancer cells lines. Prednisolone, alone or in some cases in combination with pitavastatin, reduced the expression of genes encoding enzymes in the mevalonate pathway, providing a mechanistic explanation for the synergy pitavastatin inhibited the growth of tested cell lines with an IC50 ranging from 1.1 to 4.8 μM. Therefore, clinical trials of prednisolone with pitavastatin in patients with ovarian cancer may be highly warranted.
In case of triple-negative breast cancer (TNBC) and several other cancers, Wnt signaling is overactivated and hence, its suppression emerges as an effective anticancer treatment. However, no drugs targeting the Wnt pathway exist on the market nor in advanced clinical trials. Very recently, Ahmed et al. have provided a comprehensive body of preclinical evidence that an anti-leprotic drug clofazimine (36) is effective against TNBC [85]. Clofazimine specifically inhibits canonical Wnt signaling in a panel of TNBC cells in vitro. For example, an IC50 of 6 μM and 7 μM was exhibited in HEK293T and BT-20 cells respectively. In several mouse xenograft models of TNBC, clofazimine efficiently suppresses tumor growth, correlating with in vivo inhibition of the Wnt pathway in the tumors. Clofazimine is well compatible with doxorubicin, exerting additive effects on tumor growth suppression, producing no adverse effects. Its excellent and well-characterized pharmacokinetics profile, lack of serious adverse effects at moderate (yet therapeutically effective) doses, its combinability with cytotoxic therapeutics, and the novel mechanistic mode of action make clofazimine a prime candidate for the repositioning clinical trials. Our work may bring forward the anti-Wnt targeted therapy, desperately needed for thousands of patients currently lacking targeted treatments.
Multiple myeloma (MM, also known as plasma cell myeloma, is a cancer of plasma cells) is an incurable hematological malignancy driven by several genetic and epigenetic alterations. This MM is associated with a variety of genetic and epigenetic events (namely aberrant DNA methylation, histone modifications including methylation, acetylation, ubiquitylation and phosphorylation, actin, and non-coding RNA expression) as well as deregulation of various signaling pathways that regulate DNA repair, RNA editing, protein homeostasis. These epigenetic events have been associated with abnormal signaling by dysregulation of critical pathways controlling cell cycle, apoptosis and drug resistance. Catalano, Raffaella, et al. recently reported anti-tumor activity of hydroxychloroquine (37), derivative of chloroquine and an anti-malarial agent also known as autophagy inhibitor). They demonstrated that 37 inhibits the allosteric binding of Polycomb repressive complex 2 (PRC2) to catalytic subunit (EED) within the lysine 27 of histone H3 (H3K27me3) binding pocket and thereby antagonizing the PRC2 catalytic activity [86].
Treatment for acute myeloid leukemia (AML) has not significantly changed in the last decades and new therapeutic approaches are needed to achieve prolonged survival rates [87].
Bromocriptine (38) is an ergoline derivative and dopamine agonist that is used in the treatment of Parkinson’s disease, acromegaly, hyperprolactinemia and galactorrhoea, and recently repositioned for diabetes mellitus [88,89].
Repurposing strategy handled by Lara-Castillo, María Carmen et al. has shown bromocriptine as a potent anti-leukemia drug that mainly targets leukemia stem cells [90]. Treatment with 38 reduced cell viability of AML cells by activation of the apoptosis program and induction of myeloid differentiation. Moreover, the LSC-enriched primitive AML cell fraction was more sensitive to the presence of bromocriptine. In fact, bromocriptine decreased the clonogenic capacity of AML cells. Interestingly, a negligible effect is observed in healthy blood cells and hematopoietic stem/progenitor cells. The results support the use of bromocriptine as an anti-AML drug in a repositioning setting and the further clinical validation.
Drugs repositioning for infectious diseases. Severe-acute respiratory syndrome (SARS) is an atypical form of pneumonia, which is caused by a human coronavirus (CoV) called SARS-CoV. The major outbreak was happened in 2003, and it affected more than 800 cases across three continents with a mortality rate of about 10% [[91], [92], [93], [94]]. In 2012, a novel human coronavirus called Middle-East respiratory syndrome coronavirus (MERS-CoV) was discovered, which was associated with severe respiratory tract infection, acute kidney injury, and coagulopathy. The major outbreak was happened in the Republic of Korea in the year 2015. As of July 2015, this virus infected around 1401 people, of which 543 (∼39%) was died [[95], [96], [97]]. Typically, the drug-discovery program to develop new potent anti-viral agents and to obtain approval for clinical use takes more than 10 years. Until now, no effective vaccines or drugs are approved treat these infections, although many are in (pre)clinical development. Hence, the drugs that have been used for the treatment of other diseases or disorders that may inhibit the replication of coronaviruses (CoVs) might be useful in an attempt to save the life of several affected patients.
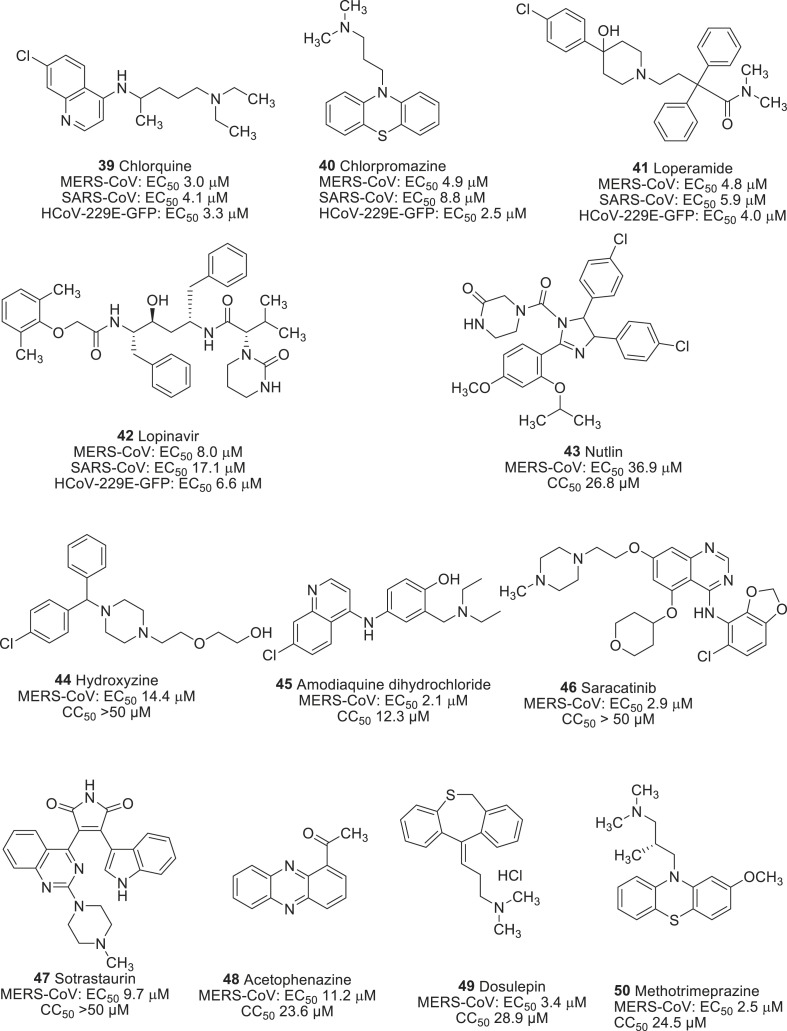
In a search of potential anti-viral agents against MERS-CoV, de Wilde et al. identified four drugs such as chloroquine (39), chlorpromazine (40), loperamide (41), and lopinavir (42) from the screening of FDA approved drugs library (Fig. 4 ) [98]. They all were able to inhibit the replication of MERS-CoV in the low micromolar range. In addition to that, all four drugs inhibited SARS-CoV as well as human coronavirus (HCoV)-229E, which suggests that they could be used for broad-spectral anti-viral activity. As a mode of action, drug 39 inhibited the replication of MERS-CoV in a dose-dependent manner with an EC50 value of 3.0 μM through the blockade of the virus at a very early stage. Chloroquine was previously reported as an effective anti-viral agent against flavivirus, influenza virus, HIV [99], Ebola virus [100], and Nipha-Hendra virus [101]. Chlorpromazine (40) was another hit compound resulted from the screening and inhibited the replication of MERS-CoV with an EC50 of 4.9 μM. It is the first antipsychotic drug developed for the treatment of schizophrenia [102], and mechanistically it inhibited the clathrin-mediated endocytosis. It has also been reported to inhibit the replication of hepatic C virus (HCV) [103], alphavirus [104], mouse hepatitis virus (MHV-2) [105], and another coronavirus SARS-CoV [106]. The mechanistic study of drug 40 on MERS-CoV indicated that it inhibited the virus at both an early and postentry stage, suggesting that an effect on clathrin-mediated endocytosis was not only the sole antiviral mechanism. Loperamide (41), an antidiarrheal opioid receptor agonist, which reduces the intestinal motility [107], inhibited the replication of MERS-CoV. Additionally, it inhibited the other two coronaviruses in low micromolar range (4–6 μM). Lopinavir (42) is an anti-HIV protease inhibitor, which also inhibited the replication of MERS-CoV with an EC50 of 8.0 μM. It was previously reported to inhibit the SARS-CoV main protease (Mpro) [108], and therefore, it was presumed that it might also target the Mpro of MERS-CoV.
Fig. 4.
Drugs repurposed on MERS-CoV and SARS-CoV infections.
Current anti-MERS-CoV agents have been primarily resulted from previous drugs used for the SARS-CoV infection. To identify potential antiviral agents against MERS-CoV, Shin et al. screened a library consisting of 2334 approved drugs and pharmaceutically active compounds [109]. This yielded a series of hit compounds, primarily categorized as, anticancer (41,42), antipsychotics (43), antidepressent (44) and antipsychotic (45) with inhibitory activity ranging from 2.1 to 14.4 μM (Fig. 4). Among them, saracatinib (46) was particularly important as it showed an excellent anti-MERS-CoV activity with an EC50 of 2.9 μM and a CC50 > 50 μM. Saracatinib (46) is an orally available small molecule drug used for the treatment of tumor malignancies through the inhibition of Src-family tyrosine kinases (SFKs). It also inhibited other coronaviruses SARS-CoV (EC50 2.4 μM) and HCoV-229E (EC50 5.1 μM) and feline infectious peritonitis (FIPV, EC50 7.0 μM) within a not-toxic range of concentration.
An in vitro study of the anti-viral effect of the drug 46 had found to inhibit the early stages of MERS-CoV life cycle in Huh-7 cells through a possible suppression of SFK signaling pathways. Interestingly, co-treatment of the drug 46 with gemcitabine, a deoxycytidine analog that is commonly used for the treatment of cancers [110,111], showed a synergistic anti-viral effect with a minimal cytotoxic effect. This supports the hypothesis of using them in a combination therapy to treat CoV diseases.
Drugs repurposed against dengue virus infection. Dengue fever is a life-threatening disease caused by four antigenically distinct dengue virus serotypes. It became a global burden, which causes approximately 390 million infections each year, of which around 10,000 to 20,000 people die. Even though a vaccine against dengue is available, its long-term protective action against each of the serotypes of dengue virus remains yet to be determined. Besides, currently, no clinically approved antiviral therapy is available to combat this virus. Among the many approaches applied to identify novel drugs for dengue fever, drug repurposing gained much attention to the scientific community.
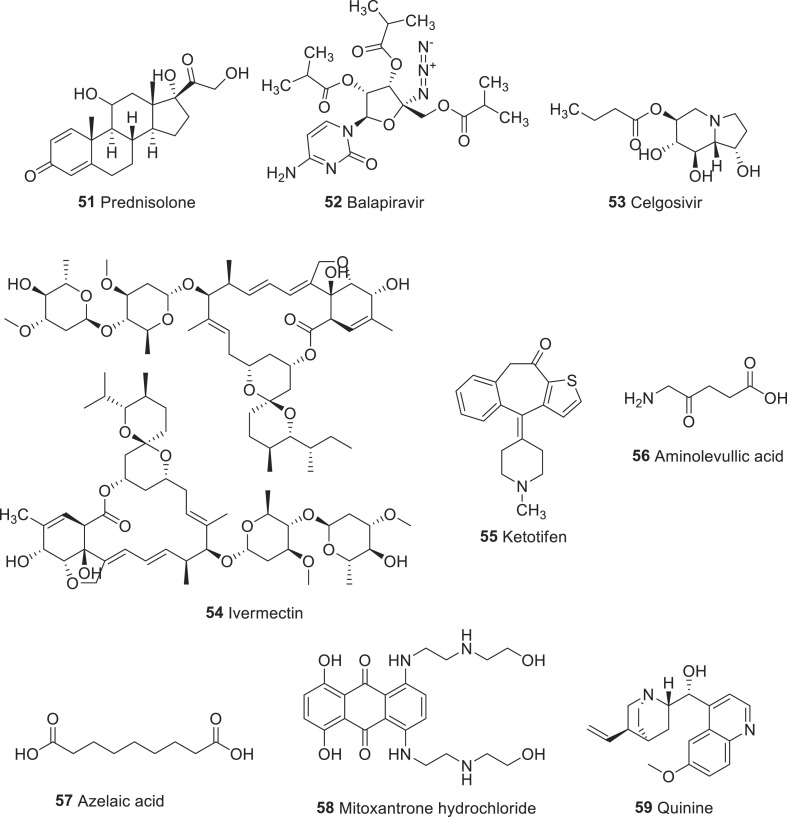
Several antiviral, antimalarial, antidiabetic, antihistamine, anticancer, antipsychotic, antiparasite, and anticholesteremic drugs have been repurposed to combat dengue virus infection. A recent publication by Botta et al. discussed each class of drugs and its repositioning in detail [112]. To identify novel and potent drug candidates, approved drugs such as lovastatin (31), chloroquine (39), prednisolone (51), balapiravir (52), and celgosivir (53) were investigated for the proof-off concept clinical trials for dengue viral infection (Fig. 5 ). Although the results showed that they were safe in patients with acute dengue, drugs failed to meet prior–defined trial endpoints [[112], [113], [114], [115], [116]]. Besides, two clinical trials conducted in Thailand and Singapore involving ivermectin (54) and ketotifen (55), the preliminary result was quite promising as phase 2 study of the drug 54 suggested a reduction in serum NS1 levels and body temperature [117].
Fig. 5.
Representative example of drugs repurposed for dengue infections.
Recently, Malakar et al. screened various classes of FDA approved drugs including aminolevulinic acid (56), azelaic acid (57), mitoxantrone hydrochloride (58), quinine sulfate (59) and tested their ability to inhibit dengue virus (DENV) replication [118,119].
Aminolevulinic acid (56), an endogenous non-proteinogenic amino acid, and azelaic acid (57) are used to treat skin diseases [120,121], while mitoxantrone hydrochloride (58), an anthracenedione antineoplastic agent is used for the treatment of leukemia [122]. Quinine sulfate (59) is a natural compound extracted from Cinchona bark, which is commercially available in 324-mg tablets under the brand name qualaquin. It has been widely used to treat chloroquine-resistant plasmodium falciparum [123]. Its ability to reduce the viral replication was also demonstrated against herpes simplex virus (HSV) [124], and influenza virus [125]. Among these four drugs investigated, the drug 59 was found to be very effective in inhibiting the replication of DENV by about 80% compared to untreated controls, while the others showed only moderate reduction of about 50%.
It was very impressive that drug 59 was able to reduce virus replication of all four serotypes of DENV in three different cell lines of human origin. At the molecular level, it inhibited DENV replication by reducing viral protein and RNA synthesis in a dose-dependent manner. Moreover, drug 59 enhanced the expression of genes related to innate immune response. These findings suggest that the efficacy of drug 59 for stimulating antiviral genes, which led to reduce DENV replication.
Repurposing on malarial infection. Malaria is a life-threatening disease caused by protozoan Plasmodium parasites. In 2017, it was estimated that approximately 219 million people were affected by malarial infections, of which 435000 died [126]. Huge efforts have been taken to prevent or cure malaria over the decades, and as a result, the mortality rates are reduced to 60%. Nevertheless, it continues to be a big problem around the world.
Although several drugs including quinine and quinoline derivatives [127,128] could moderately treat the infection, the emergence of drug-resistant strains of Plasmodium falciparum makes it more challenging in malaria treatment. Therefore, the discovery and development of new antimalarial agents with a different mechanism of action that may not only reduce mortality and morbidity of the disease, but also reduce the risk of resistance to existing antimalarial drugs are highly required.
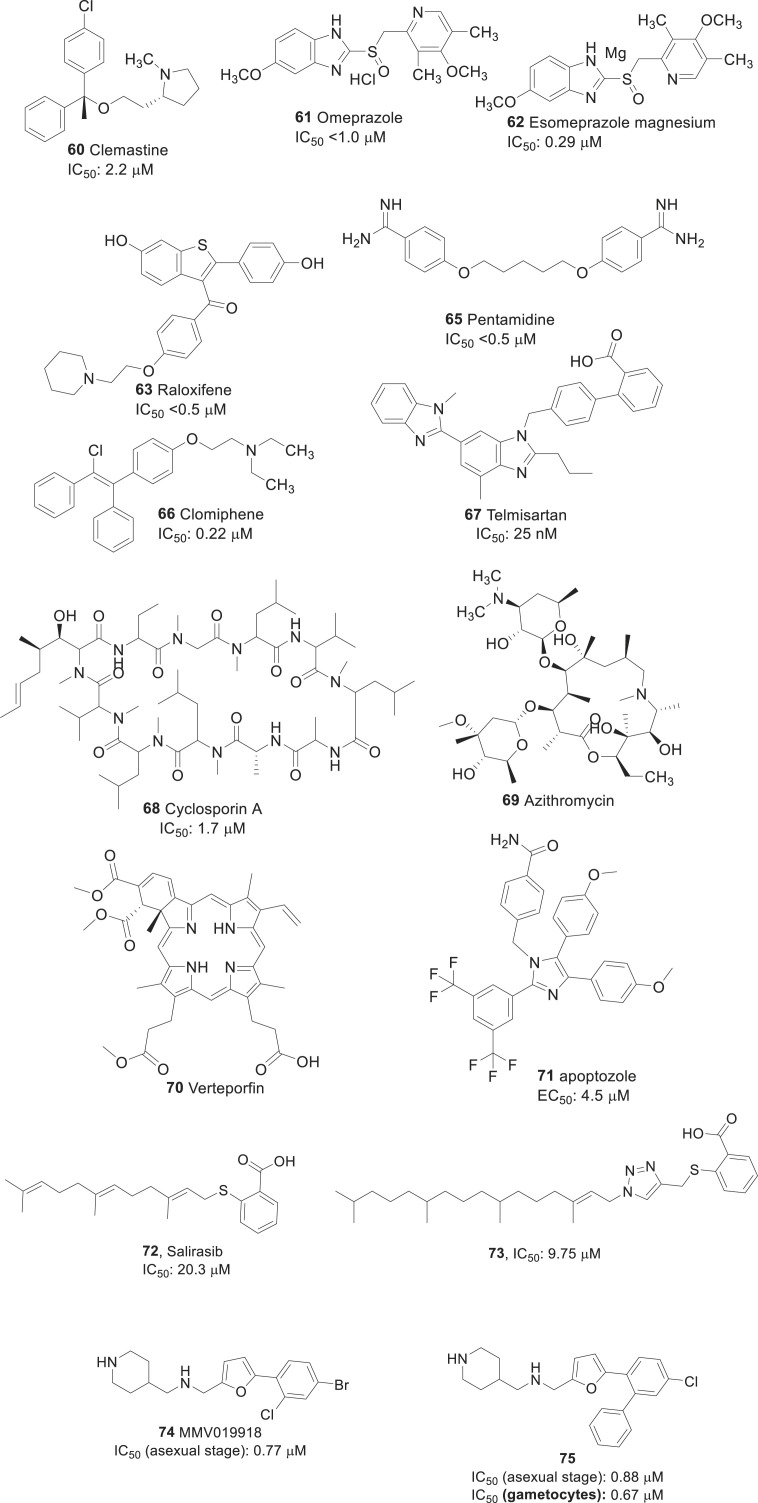
To identify potential drug candidates, two groups [[129], [130], [131]] conducted drug-repositioning approach of FDA approved drugs. Chong et al. screened around 189 drug molecules at 10 μM concentration against blood-stage P. falciparum. Derbyshire et al. analyzed 37 drugs in a liver-stage P. berghei model. Out of tested 226 drug molecules, 18 were selected as highly potent candidates with an IC50 value of ∼2.5 μM (see Fig. 6 ) [129]. It is interesting to note that out of 18 drugs, four including clemastine fumarate (60), loperamide hydrochloride (36), omeprazole (61), and esomeprazole magnesium (62) are the over-the-counter (OTC) drugs, which can be easily accessed by patients in high-risk areas, while other 14 drugs can be obtained via prescription only. Clemastine (60), an ethanolamine derivative is a first-generation H1 antagonist (antihistamine) associated with anticholinergic properties.
Fig. 6.
Repurposing of potent drug molecules for malarial infection.
This drug came into use in the year 1967 to prevent hay fever and allergy symptoms. Loperamide (41) is a medication used to decrease the frequency of diarrhea. Both drugs 41, and 59 were effective against blood-stage P.falciparum with IC50 values of 2.2 μM and 0.29 μM, respectively. Omeprazole and esomeprazole are used in the treatment of gastroesophageal reflux disease, peptic ulcer, and Zollinger-Ellison syndrome. They were tested against liver-stage P.berghei and showed IC50 values < 2 μM, make them potential lead for the development of novel anti-malarial agents. These all four OTC drugs are orally administered, which has the advantageous to develop as anti-malarial therapy.
From another set of prescribed drug candidates (63–70) were tested against blood-stage P. falciparum cultures (Fig. 6 ) and liver-stage P. berghei [129,130]. They all showed promising antimalarial activity with IC50 values ranged from 2.8 μM to 1.7 nM. Drugs raloxifene hydrochloride (63), gramicidin A (64, structure was not shown), pentamidine isethionate (65), clomiphene citrate (66), telmisartan (67) and cyclosporin A (68) showed IC50 values < 1 μM [129,130]. The rank order potency of them are as follows: 67 (25 nM) ≫66 (0.22 μM) >63 (<0.5 μM) ∼ 64 (<0.5 μM) ∼65 (<0.5 μM) >68 (1.7 μM).
Most of these prescribed drugs (11 of 14) can be injectable, oral or topically administered. Other drugs like azithromycin (69), 65, and 68 can be administered through multiple routes. Azithromycin (69), an azalide antibiotic, can be administered orally, topically or intravenously. Drug 65, an antiprotozoal agent, can be administered either as an intravenous, intramuscular injection or as an inhalable powder. Cyclosporin A, an immunosuppressant drug, can be administered orally, topically or intravenously.
Recently Chen et al. repurposed chaperone inhibitors that are anticancer agents, at the PfGRP78 as a potential drug target against P. falciparum, and identifed apoptozole (71, Fig. 6), a novel chemical scafold that inhibits chaperone function and prevents parasite growth in vitro [132]. Further, the identifcation of apoptozole as a PfGRP78 inhibitor open the possibility to use this compound as a chemical probe to study the role if this chaperone in artemisinin resistance mechanisms.
Salirasib (72, Fig. 6) is a promising cancer drug candidate inhibits isoprenylcysteine carboxyl methyltransferase (ICMT), a validated target for cancer drug development. Recently, Salirasib and its analogs with 1,2,3-triazole were repuposed for their pontential antimalarial activity [133]. In general, triazole derivatives are known to have potent antimalarial activity [134]. Compound were investigated the in vitro toxicity to P. falciparum in the asexual stages and in Vero cells. An antiplasmodial activity assay was performed using a simple, high-sensitivity methodology based on nanoluciferase (NLuc)-transfected P. falciparum parasites. The results showed that some of the analogs were active at low micromolar concentration, including Salirasib. The most potent member of the series has S-farnesyl and the 1,2,3-triazole moiety substituted with phytyl, for example compound 73 (Fig. 6. Moreover, compounds show less cytotoxicity in eukaryotic cells indicates good therapeutic indices, being starting point for the development of antimalarial drug. Vallone et al. recently discovered MMV019918 (74, 1-[5-(4-bromo-2-chlorophenyl)furan-2-yl]-N-[(piperidin-4-yl)methyl]methanamine, Fig. 6) and its analog 75 ( Fig. 6), for their dual activity against P. falciparum asexual stages and gametocytes through the standard membrane-feeding assay [135]. They both significantly prolonged atrioventricular conduction time in Langendorff-isolated rat hearts, and showed inhibitory activity of Ba2+ current through Cav1.2 channels. Compound 75 showed interesting activity against PfPMT, which could be an important starting point for the identification of more potent inhibitors active against both sexual and asexual stages of the parasite.
Drug repurposed on Neurodegenerative diseases. In general, neurodegenerative diseases including amyotrophic lateral sclerosis, Parkinson’s disease (PD), Alzheimer’s disease (AD), and Huntington’s disease (HD) occurs because of several neurodegenerative processes.
Drug repositioned on Alzheimer’s disease (AD). Dementia is a general term for a decline in mental ability severe enough to interfere with daily life. Memory loss is an example. Alzheimer’s is the most common type of dementia. It affects around 5% of people over the age of 65, 20% over the age of 80, and more than a third of those over the age of 90 [136]. In the year 2015, there were approximately 29.8 million people worldwide with AD of which dementia resulted in about 1.9 million deaths. It is estimated that there will be more than 115 million people with dementia worldwide by 2050. Therefore, AD represents a major and rising public health concern, and there is an urgent need to develop more therapies that are effective.
AD refers to a devastating condition leading to progressive cognitive decline, functional impairment, and loss of independence. Accumulation of amyloid-β peptide (Aβ)-enriched neuritic plaques, and neurofibrillary tangles, synaptic, and neuronal dysfunction, as well as loss in combination with the associated neurochemical changes in the brain, are the crucial pathological event for AD [137]. Recent work suggests that higher levels of total tau may potentiate the toxic effects of Aβ [138]. Other factors such as inflammatory processes and mitochondrial function are also likely to have an important role [139].
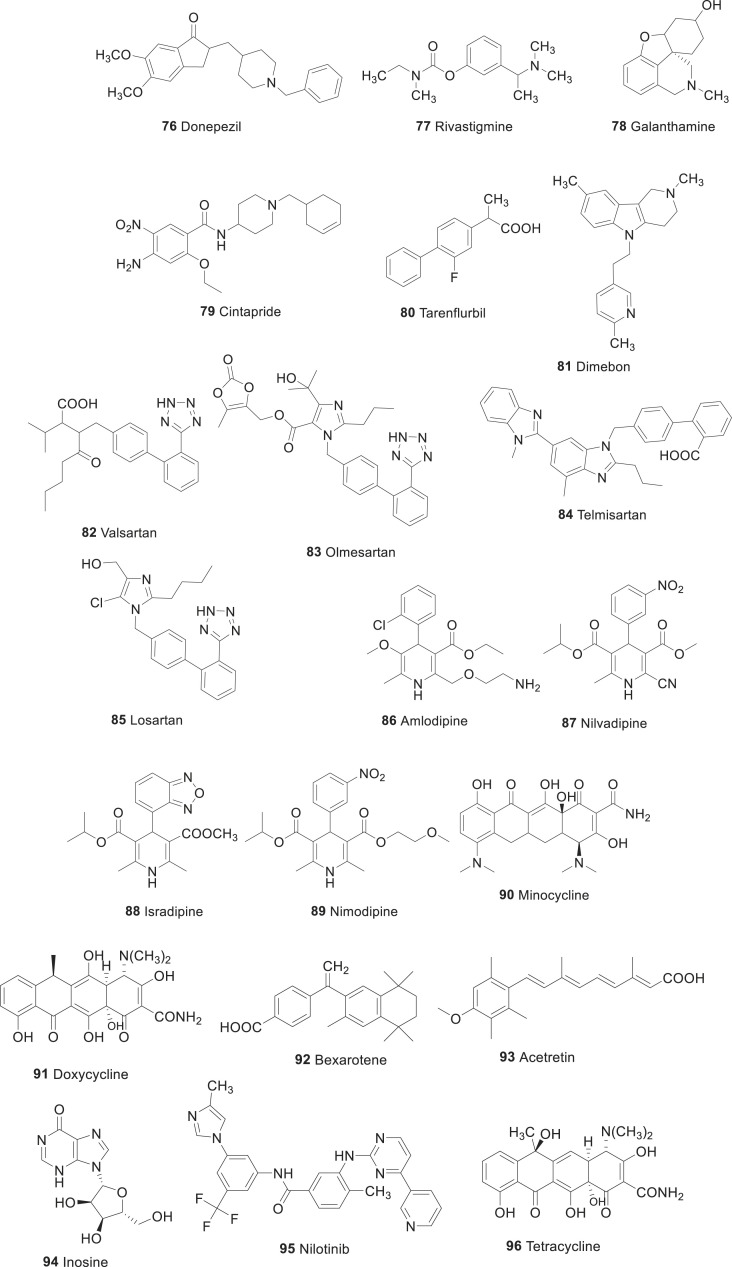
Three acetylcholinesterase inhibitors including donepezil (76), rivastigmine (77) and galanthamine (78, Fig. 7 ) are approved for the treatment of patients with mild to moderate AD. Memantine, an NMDA (N-methyl-d-aspartate) receptor antagonist, is approved for the treatment of patients with moderate to severe AD. Meta-analyses and cost-effectiveness evaluations have demonstrated that this repurposing confer moderate symptomatic benefits and are cost-effective [140]. For example, acetylcholinesterase inhibitors improve cognition to above pre-treatment performance for ∼6–12 months. The availability of these drugs has substantially advanced the treatment of patients with AD, but there is a persistent need to build on our increasing understanding of disease pathogenesis to develop more effective symptomatic treatments and disease-modifying therapies.
Fig. 7.
Drugs repurposed for Alzheimer’s disease.
Current study carried out by Hassan et al. [141] utilized both computational and enzyme inhibitory experimental kinetic approaches to evaluate the repositioning of known FDA approved drugs for AD. The computational shaped-based virtual screening results showed that from 1516 FDA approved drugs, 36 displayed good structural similarity with standard template donepezil. Moreover, docking profile and pharmacogenomics evaluations depicted that out of 36 only five drugs were most active and showed good results compared to other drugs. The MD simulation results exposed that these five drugs (Risperidone, Domperidone, Verapamil, Tamsulosin and Cinitapride) showed better profiles with respect to their RMSD, RMSF, SASA and Rg evaluations graphs and steady stable behavior in all docking complexes. In-vitro AChE inhibition assay of the above best-screened drugs were performed by spectrophotometric method using acetylthiocholine iodide as substrate. The enzyme inhibition and kinetic mechanism of these drugs showed that Cinitapride (79) has good therapeutic potential with respect to standard and other drugs. It is well known that, Cintapride (79) is a gastroprokinetic agent and antiulcer agent of the benzamide class [142]. It is an agonist of 5-HT1 and 5-HT4 receptors and as an antagonist of the 5-HT2 receptors [143]. Based on aforementioned results, it is justified that Cinitapride has better repositioning profile which may be used in the treatment of AD after clinical assessment.
Efforts to develop more effective therapies have so far been unsuccessful with several high-profile clinical trial fails to demonstrate the benefit. The reasons for this are probably multifactorial. The majority of putative disease-modifying therapies that have been evaluated have targeted amyloid pathology. This lack of breadth in treatment approaches has been criticized, and some commentators have argued that a more sophisticated knowledge of disease pathways is needed before we can develop more effective candidate therapies.
For example, Phase II trial of tarenflurbil (80, Fig. 7) only provided a suggestion of benefit in a posthoc subgroup analysis [144,145]; the putative mechanism of action via γ-secretase modulation and its related impact on amyloid pathology was never confirmed in patients with AD. Subsequent Phase III trial had negative outcomes. Although, the results of a Phase II trial of dimebon (81) were much more favorable than of analog 80 (Fig. 7). It seems that the significant benefit seen in the treatment group was driven by a larger-than-expected deterioration in the group receiving placebo treatment, and the mechanism of action was not well characterized.
In the central nervous system (CNS), angiotensin II mediates key processes including, the release of inflammatory mediators, vasoconstriction, mitochondrial dysfunction, and inhibition of acetylcholine release at central synapses. All of which are proposed to be relevant to AD and are potential targets for therapeutic intervention [146,147]. Based on this background, it has been proposed that angiotensin receptor blockers (ARBs) may confer symptomatic benefits on cognition.
A large-scale screen of 55 antihypertensive drugs by Want et al. [148], identified the ARB valsartan (82, Fig. 7) as the only compound that was able to reduce Aβ accumulation in cultured neurons and inhibit Aβ aggregation in vitro. The same group was demonstrated that the reduced plaque burden as well as the improved learning and memory in cognitive tests (including the Morris water maze task) following 5 months of treatment with valsartan in 6-month-old Tg2576 transgenic mice. As a result, the greatest benefits were seen at a dose of 40 mg/kg/day, which is equivalent to 1.5 times the maximum recommended dose for treating patients with hypertension.
Using another ARB, olmesartan (83, Fig. 7) by Takeda et al. [149], demonstrated that daily treatment of young APP23 mice for one month improved cerebral blood flow without affecting Aβ1-40 and Aβ1-42 levels. In the Aβ1-40-injected mouse model, in which Aβ fragments are injected intracranially to generate deficits, pre-treatment with telmisartan (84, Fig. 7) increased cerebral blood flow and inhibited the plaque deposition [150]. However, the physiological relevance of this model is unclear. Perhaps, the most striking preclinical evidence comes from a study in which losartan (85, Fig. 7) was administered intranasally to APP/PSEN1 mice, at a dose much lower than that mediating hypotensive effects; drug 80 led to a 3.7-fold reduction in Aβ plaques compared to vehicle-treated mice but also reduced the levels of pro-inflammatory mediators and increased the levels of anti-inflammatory mediator IL-10 in the serum of these animals [151,152]. Overall, there was significant reduction in the incidence of dementia in patients taking ARBs compared to those taking the comparator cardiovascular drugs (hazard ratio: 0.76; 95% confidence interval: 0.69–0.84) and those taking angiotensin-converting enzyme (ACE) inhibitor lisinopril (hazard ratio: 0.81; 95% confidence interval: 0.73–0.90).
Although the evidence for the potential of ARBs in AD is conflicting, and difficult to interpret, in our view, there is a sufficient indication of potential benefit to merit further in vivo work to clarify the relative importance of different mechanisms, the optimal dose, and the optimal agent, which could lead to proof-of-concept study in patients with AD. The best evidence from in vitro and in vivo studies points to either drug 85 or 84 as a preferred ARB, with some animal studies also highlighting drug 83 as a potential candidate. However, there is an unfortunate disconnect between in vivo and clinical studies, as no formal studies to provide direct clinical evidence have so far been conducted on any of the most promising candidates.
Calcium channel blockers (CCBs) of the dihydropyridine class are widely used to treat hypertension and angina through their vasodilatory activity on smooth muscle vasculature. Most of the drugs in this class have good blood-brain barrier penetration and induces cerebral vasodilatation, increased cerebral blood flow in animals and humans [153]. In vitro studies have revealed that certain CCBs reduce Aβ production, oligomerization, and accumulation, rescue Aβ-induced neurotoxicity, and improve cell survival in the presence of Aβ [[154], [155], [156]]. CCBs have also been shown to reduce glutamate-induced cell death and levels of intracellular calcium [157]. The ability of CCBs to prevent Aβ1-40 and Aβ1-42 production was investigated in Chinese hamster ovary cells (CHO) by Paris et al. and Iwasaki et al. In that study, amlodipine (86) and nilvadipine (87) (Fig. 7) were identified as the only agents that inhibited Aβ production [158]. However, the concentrations studied were several-fold higher than can be achieved therapeutically.
Isradipine (88, Fig. 7) was shown to have a neuroprotective effect against Aβ-induced apoptosis in neuroblastoma MG65 cell lines. A protective effect in a Drosophila melanogaster model of Aβ-induced neurotoxicity and it’s brain-penetrant in the 3xTg-AD mouse model of AD was also reported [156,159]. The differential effects of CCBs indicate that their potential benefits in AD are probably independent of their anti-hypertensive activity and may be specific to individual drugs within this class. Dihydropyridines seem to be more effective than compounds with different chemical structures (such as verapamil or diltiazem), with evidence from preclinical studies that highlighted nilvadipine (87) as the best therapeutic candidate.
There was some clinical evidence regarding the potential benefit of the CCB nimodipine (89, Fig. 7) in patients with clinically significant dementia. The evidence has been summarized in a Cochrane review 51 of 15 nimodipine trials in more than 3000 patients with dementia. The review reported that the treatment showed efficacy in improving cognition, but not activities of daily living, at doses of 90 mg/day [160]. However, the evidence was limited by small size and duration (mostly 12 weeks) of trials as well as the lack of operational diagnostic criteria for AD or vascular dementia.
Tetracycline antibiotics are widely used to treat bacterial infections and are well tolerated in older people, but most of the treatment data pertain to short-term periods of exposure. Studies related to AD have predominantly focused on minocycline (90, Fig. 7) because it is the most lipophilic tetracycline, with greater blood-brain barrier penetration than other agents in this class. Concerning preclinical studies, the drug 90 has been shown to reduce Aβ1-42 aggregation, and promote disassembly of pre-formed fibrils in vitro studies.186 Various groups have independently shown that drug 90 reduces the levels of proinflammatory mediators and microglial activation in a range of mouse models of AD [[161], [162], [163], [164], [165]]. Besides, two of the three studies using transgenic mouse models of AD for 28 days or more of treatment have shown a significant decrease in cerebral Aβ accumulation and improvements in behavioral outcomes as well [165]. One of these studies used 8-month-old 3xTg-AD mice and reported reductions in cortical amyloid levels following 4 months of minocycline treatment, but no changes in tau pathology was observed.190 The third study reported that no benefit concerning amyloid accumulation or behavior over a treatment period of 12 months.194 An additional study in a rat model of diabetes demonstrated a reduction in Aβ1-40 and Aβ1-42 levels and associated improvements in behavioral outcomes over 8 weeks of treatment [165].
Doxycycline (91) is a second generation antibiotic of the tetracycline class that are promising drugs tested in many clinical trials for a number of different pathologies. Compound 91 is endowed with antiamyloidogenic properties and better crosses the blood-brain barrier, but its efficacy has never been tested in AD mice. Balducci, Claudia, et al. showed that 15- to 16-month-old APP/PS1dE9 (APP/PS1) AD mice receiving 96 under different treatment regimens recovered their memory without plaque reduction. An acute 96 treatment was, also, sufficient to improve APP/PS1 mouse memory, suggesting an action against soluble AbOs. This was confirmed in an AbO-induced mouse model, where the AbO-mediated memory impairment was abolished by a its pretreatment. Although AbOs induce memory impairment through glial activation, assessing the anti-inflammatory action of 96, we found that in both the AbO-treated and APP/PS1 mice, the memory recovery was associated with a lower neuroinflammation. Our data promote 96 as a hopeful repositioned drug counteracting crucial neuropathological AD targets [166].
Drugs that activate retinoic acid receptors (RARs) are used to treat several skin-related conditions such as acne and psoriasis. Retinoic acid is also vital for normal nerve function and repair. There is genomic and epidemiological evidence suggest that impaired retinoic acid signaling may contribute to the etiology of AD [167]. Chronic deprivation of retinoic acid in rats leads to deposition of Aβ in the vasculature [168] and dysregulation of amyloid processing in the cortex [169]. It has been shown that treatment with retinoid X receptor (RXR) agonist bexarotene (92, Fig. 7), which is approved for the treatment of cutaneous T cell lymphoma, leads to pathological and behavioral improvements in transgenic mouse models of AD. Acute treatment with drug 92 (lasting less than 14 days) caused a rapid reduction (25%) in Aβ1-40 and Aβ1-42 levels and Aβ plaque burden in both young and old mice [170]. Chronic 90-day treatment resulted in a sustained reduction (30%) insoluble Aβ levels.
Mechanistically, drug 93 resulted in the upregulation of components of the high-density lipoprotein (HDL) pathway such as apolipoprotein E (APOE), which promotes the proteolytic degradation of Aβ [171]. Further, potential mechanism of action of retinoids may include the upregulation of enzymes involved in amyloid clearance such as insulin-degrading enzyme [172] and components of the APOE pathway [170]. Retinoids may also induce potentially beneficial changes related to insulin signaling and increased neurogenesis and promote neuronal differentiation of progenitor cells [173,174]. Retinoids also act as antioxidants (by regulating SOD and inhibiting glutathione depletion to reduce mitochondrial damage) as well as anti-inflammatory agents (by reducing the production of IL-6) in vitro. Both of these activities have potential importance in AD pathology [175,176]. Therefore, there was a strong mechanistic rationale for the potential benefit of retinoid therapies beyond amyloid modulation, but there is a need to further clarify the impact of treatment on these pathways through in vivo studies. Overall, studies in the literature indicate that retinoids have strong potential mechanistic plausibility as therapies for AD owing to their effects on APP processing, Aβ clearance, insulin signaling, and neurogenesis. Out of the approved drugs, data for bexarotene have provided proof of concept as potential candidate for the treatment of Alzheimer’s disease as noted above, whereas acitretin (93, Fig. 7), which is known to penetrate tissues including brain may also be a promising candidate for AD [177].
Repurposing on Parkinson’s disease (PD). It is a long-term degenerative disorder of the CNS, which mainly affects the motor system. As of 2015, PD affected 6.2 million individuals, of which 117400 people died. It usually occurs people over the age of 60, of whom about one percent are affected. In addition to the classic motor symptoms caused by the death of dopaminergic neurons, Parkinson’s disease encompasses a wide range of nonmotor symptoms. Although novel disease-modifying medications that slow or stop Parkinson’s disease progression are being developed, drug repurposing, which is the use of existing drugs that have passed numerous toxicity and clinical safety tests for new indications, can be used to identify treatment compounds. This strategy has revealed that tetracyclines (96) are promising candidates for the treatment of Parkinson’s disease [178]. Tetracyclines, which are neuroprotective, inhibit proinflammatory molecule production, matrix metalloproteinase activity, mitochondrial dysfunction, protein misfolding/aggregation, and microglial activation. Two commonly used semisynthetic second-generation tetracycline derivatives, minocycline (90) and doxycycline (91), exhibit effective neuroprotective activity in experimental models of neurodegenerative/neuropsychiatric diseases and no substantial toxicity. Moreover, novel synthetic tetracyclines with different biological properties due to chemical tuning are now available. In this review, we discuss the multiple effects and clinical properties of tetracyclines and their potential use in Parkinson’s disease treatment. In addition, we examine the hypothesis that the anti-inflammatory activities of tetracyclines regulate inflammasome signaling. Based on their excellent safety profiles in humans from their use for over 50 years as antibiotics, we propose the repurposing of tetracyclines, a multitarget antibiotic, to treat Parkinson’s disease.
Isradipine (88) is an L-type calcium channel blocker of the dihydropyridine class, which has been widely used for the treatment of high blood pressure to reduce the risk of heart attack and stroke. This drug 88 came into medical use in the year 1989. Epidemiological data support that calcium channel blockers may have the potential to reduce the risk of developing PD. Among dihydropyridines, drug 88 has attracted very much as it inhibits the subtype Cav1 Ca2+ channels Cav1.2 and Cav1.3, which are most likely mediate the risk in PD. Moreover, the good brain bioavailability of drug 88 has made it the most promising candidate for repurposing [178,179]. Studies have shown that drug 88 dose repentantly protect the dopaminergic neurons from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine MPTP) and 6-hydroxydopamine (6-OHDA)-induced toxicity by reverting dopaminergic neurons to a latent juvenile pacemaking mechanism independent of calcium [180,181]. An open-label, dose-escalation study assessing safety (STEADY-PD) of drug 88 controlled release 5–20 mg/day in patients with early PD suggested that the acceptable tolerability at doses of ≤10 mg per day; however at higher doses caused leg edema and dizziness [182]. Isradipine (88) with 10 mg being the highest dosage was again confirmed in another STEADY-PD-II, a randomized, double-blinded trial, which was undertaken in 99 subjects with early PD not requiring dopaminergic therapy [183]. Results suggest that the most common adverse effect was peripheral edema, which occurred in 34% of patients receiving drug 88 (10 mg). Placebo-controlled phase III clinical study to assess the efficacy of drug 88 (10 mg daily) as a disease-modifying agent in early PD was completed in November 2018 [184]. The study recruited 336 patients with early PD not requiring dopaminergic therapy and followed them prospectively for 36 months, with the primary outcome designated as a change in the unified Parkinson Disease Rating Scale (UPDRS) Part I-III score as measured in the ON state. The results are expected in the middle of 2019. In animal models, drug 88 reported with neuroprotection effect in a dose-dependent manner, higher doses confirmed better protection.
Inosine (94, Fig. 7) is a purine nucleoside, which has been used as a dietary supplement by athletes for improving aerobic performance. It has been shown to have neuroprotective roles by elevating the level of serum urate, a natural antioxidant and peroxynitrite scavenging property with potential benefits to patients with multiple sclerosis. Many studies suggest that individuals with increased levels of urate in serum have a reduced risk of developing PD, as well as in patients with PD are associated with a reduced rate of disease progression. Moreover, in toxin-based models of PD, increased urate levels have conferred protection against dopaminergic cell death stimulated by MPTP, 6-OHDA, and rotenone. Akt-GSK-3B signaling and nuclear factor (erythroid-derived-2)-like 2 (Nrf2) were thought to involve in these effects. Therefore, given the data supporting a neuroprotective role of urate, inosine has been repurposed in the pathogenesis of PD. The ability to raise urate level of drug 88 in serum was demonstrated in SURE-PD, a randomized, double blind, placebo-controlled in 75 patients with early PD not yet requiring any medication. The results showed that inosine raised the mild urate level (6.1–7.0 mg/dl) or moderate urate elevation (7.1–8.0 mg/dl) after 25 months and well-tolerated with favorable progression rate in UPDRS score, which amounted to ∼1 point per year on the total UPDRS scale. On the other hand, the elevated level of urate in serum have the risk of hypertension, coronary heart disease, and stroke over the long term. These side effects are potentially limiting its utility in older patients with PD. However, in patients of Asian origin with PD, inosine elevated urate levels (6.1–7.0 mg/dl) without side effects after 1 year of treatment was reported. The multicenter SURE-PD3 trial, which involves a large number of patients (240 patients), is currently underway intending to elevate the urate level to 7.1–8.0 mg/dl. The result of the study will be expected in the year 2020.
Simvastatin (29), Since statins are also known to modulate various biological processes relevant to the pathogenesis of PD [185,186]. Simvastatin has been repurposed for treating this disease. Pretreatment with simvastatin preserved dopaminergic cells and motor behavior in rodents treated with 6-hydroxydopamine (6-OHDA), promoting antioxidant protein expression or via modulation of NMDA receptor and pro-inflammatory cytokine expression [[187], [188], [189]]. Similarly, in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) models, pretreatment with simvastatin suppressed activation of NF-κB, protected dopaminergic neurons, and improved the motor function [190,191]. Although, stains, in general, have given encouraging preclinical studies, epidemiological data regarding the association between usage of statins and the risk of PD are unclear. Moreover, the modest protective effect of statins that disappeared when adjusted for cholesterol level [192]. However, due to the promising biochemical and pharmacological properties of simvastatin, it is currently being examined with 235 patients having moderate-stage PD in phase II double-blind, randomized, controlled, multicenter trial.
Nilotinib (95, Fig. 7) is a selective c-Abl tyrosine kinase inhibitor approved for the treatment of imatinib-resistant chronic myelogenous leukemia (CML). Accumulating evidence suggests that c-Abl activation has been linked in the pathogenesis of PD and other synucleinopathies. Activated (phosphorylated) c-Abl has been found in a high level in post-mortem studies of patients with PD [193,194]. It was also reported that activation of c-Abl in mice induces neurodegeneration in the hippocampal and striatal brain areas [195]. Continuous work has demonstrated that c-Abl phosphorylation occurs as a result of mitochondrial dysfunction, and oxidative stress [196], which can promote the accumulation of α-synuclein in through effects on autophagy mechanisms [195] and can further promote phosphorylation of parkin, causing inhibition of its ubiquitin E3 ligase activity, inducing mitochondrial dysfunction and dopaminergic neuronal death [194]. All the above evidences propose that c-Abl may be a promising therapeutic target in the management of PD. The CNS penetration of nilotinib over other Abl inhibitors has favored it to fetch more data in PD related studies. Indeed, in preclinical models of PD, drug 92 has been shown to cross the blood-brain barrier and reduces c-Abl activity, ameliorating autophagic clearance of α-synuclein in transgenic and lentiviral gene-transfer models [195]. More importantly, these effects were seen at doses far lower (1–10 mg/kg/day) than those used to treat CML, which is considered as the key characteristics for potential drug repurposing. Furthermore, nilotinib prevented dopaminergic cell loss and motor impairments induced by MPTP in mice, which were associated with inhibition of parkin phosphorylation and reduced accumulation of parkin substrate PARIS, thus hinting at another potential mechanism of action [197,198]. Based on these preclinical data, a small, open-label proof-of-concept study was recently conducted to evaluate the safety and tolerability of nilotinib in 12 patients with PD dementia or dementia with Lewy bodies followed-up for 24 weeks, followed by a final assessment 12 weeks later [199]. The necessary lowest choice of dose for the clinical study was taken as 150–300 mg. The authors reported that nilotinib was well tolerated, though one patient receiving 300 mg was diagnosed with myocardial infarction, and two had transient QTc prolongations. There was also evidence of CNS penetration with nilotinib CSF plasma ratio of 12 and 15% with 300 and 150 mg, respectively. Due to numerous methodological limitations, these findings should be interpreted with caution [200]. Due to unwanted off-target nonselective tyrosine kinase inhibition; side effects of nilotinib at doses used to treat CML include cardiac conduction abnormalities. Therefore, claims of tolerability should be interpreted with caution. Indeed, the effects on efficacy was also highly impossible to be concluded. Besides, it was also reported in the CSF, none of the markers used were validated as biomarkers in PD; they can also vary greatly between patients and track poorly with disease stage and progression. This situation again raises questions about the optimum dose of nilotinib, its brain penetrance, assessments of cardiovascular effects in patients.
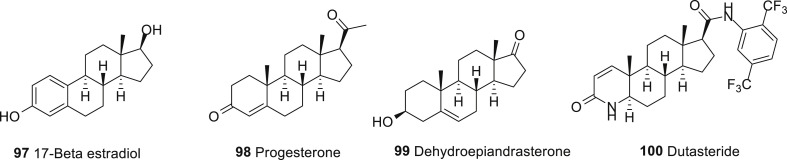
Parkinson’s disease (PD) is a neurodegenerative disorder for which a greater prevalence and incidence is described in men. This suggests a protective effect of sex hormones in the brain. Sex steroids and related drugs are already used in clinic for treatment of menopause symptoms, cancer and use for contraception; thus they could be repositioned for PD in both men and women. Steroids are modulators of neurotransmitters system, for this reason their effects may help to control PD symptoms and side effect of DA medication. Animal studies provide good support for a protective effect of 17β-estradiol (97, Fig. 8 ), progesterone (98, Fig. 8), raloxifene (99, Fig. 8), DHEA and dutasteride (100, Fig. 8) on dopaminergic system. Sex specific treatment options may thus be proposed, with non-feminizing agents (raloxifene, DHEA, progesterone, dutasteride) for men. Indeed, the SERMs, 5α-reductase inhibitors and DHEA are alternatives to estrogens and could be used for both men and women. In the current trend to develop personalized medicine, estrogens could be used in priority for women. Because a variety of estrogens and progestins are available and clinically used, additional studies are needed to optimize the formulation of these agents, timing of initiation and duration of treatment to provide a beneficial therapeutic approach on PD risk and symptoms management [201].
Fig. 8.
Drugs repurposed for Parkinson’s disease.
Advantageous and limitations of drug re-purposing/re-positioning. Drug re-purposing has gained significance attention among scientists and pharmaceutical companies involved in the identification of new therapeutic uses/indications for discontinued during the clinical development phase because of efficacy or commercial reasons. Recently, this approach has accounted for ∼30% of all newly approved drugs by the FDA. This means the success rate of drug approval is so high when compared to the traditional methods [202]. During the year 2012–2017 [203], almost 170 re-purposed drugs entered the drug development pipeline. Currently, these 170 drugs are at different stages of development. Around 72% are in Phase II clinical development, 7% are in proof of concept clinical studies, 8% are in pre-clinical stages, and regulatory bodies have approved 3% in research and development and 10%. Almost 70% of the Phase I and II trials for re-purposed drugs were sponsored by academia and 30% by industry.
Besides, drug-repositioning approach increases the fruitful collaborations and funding opportunities. During the year 2012–2017, there were almost 40 collaborations relating to drug repurposing. Among these, around 50% of collaborations were between two industries mainly focused on rare diseases. Almost, 20% between an industry and a Non-profit Organization (NPO) and 9% between industry and academia. Regarding funding opportunities, there was a substantial increase in funds (US$1 million to US$100 million) for drug repurposing projects by various funding agencies like National Center for Advancing Translational Sciences (NCATS), Cures within Reach (CWR, an NPO), the Canadian Institutes of Health Research (CIHR), Findacure.
Investigational New Chemical Entities (NCEs) that failed to show efficacy/potency and lack of safety for a pre-intended indication typically provides a good start for their revival against new indications by repurposing. This can be very much useful in cases of rare diseases such as autoimmune disorders, bacterial infections, neglected diseases and rare cancers such as pediatric cancers where unmet medical need owing to non-availability of standard therapies, worsening clinical outcomes and more difficult to treat. Unlike NCEs, all re-purposed molecules possess all required safety, preclinical and efficacy data enables the investigator to make an informed decision at much earlier stage of drug development substantially reduces the costs, time and efforts required for successfully bringing a repositioned drug to the market. Drug repositioning also offers significant potential for out-licensing as well, because of availability of potential buyers. Drug re-purposing also provides an opportunity to broaden our knowledge without limiting the existing information.
On the other hand, this approach has some limitations due to lack of a clear exclusivity path/well-defined regulatory guidelines. It is still a big hurdle/uphill task in utilizing repurposed drug candidates for human use. In general, a repurposing candidate carries a potential time risk because it has failed previously for a pre-intended indication. Hence, it is advisable to design a branched development program, in which the lead compound is evaluated for several indications simultaneously. Drug repurposing requires thorough comprehension of biological and molecular pathways that a drug can modulate as well as its interactions with endogenous bio-molecules in order for a successful repurposing of that drug molecule. When repurposing a drug for rare and neglected diseases, there is no assurance that the economic returns will be substantial as expected for a pharmaceutical company. Lack of financial incentives and research funding is another challenge for pharmaceutical companies. Problems in clinical trials: chance of failure of proof of studies for new indications as well as limited availability of volunteers for large clinical studies in case of rare diseases due to scarcity of patients. Intellectual property related issues hinder the commercialization of repositioned molecule.
2. Conclusions and perspectives
In conclusion, repurposing of drugs has the potential to find and improve treatments for various diseases. The drugs listed above are only a few of plenty drugs that can be repurposed in various therapies. The drugs discussed in the perspective are summarized in Table 1 and compared to the previous uses of each drug with the repurposed use. The opportunities for drug repurposing are diverse, but a lot still has to be done for its exploration. Drug repurposing or drug repositioning offers a new strategy to academic centers, research council programs, and not-for-profit organizations, as well as pharmaceutical and biotechnology companies for drug development. The main advantage of drug repurposing is the established safety of the known candidate compounds when compared to that of the development of novel therapeutic compounds. The time and cost required to advance a candidate into clinical trials can be substantially reduced because in vitro and in vivo screening, chemical optimization, toxicity studies, bulk manufacturing, and formulation development have already been completed in many cases, and can, therefore, be bypassed. Moreover, these drugs have been on the market for many years, and consequently, the side effects are already known, and safety is therefore very high. For medicinal chemists, the repurposing of drugs on cancer is a rewarding job for the global healthcare system and possibly a step forward for people to get cheaper and safer medicines rather than afford for cancer therapy. However, time and minimum investment have to be spent to conduct further studies to improve the safety and success of the repurposed drugs.
Table 1.
List of approved drugs repurposed on various diseases.
| Drug | Previous use(s) | Repurposed use(s) |
|---|---|---|
| Ethionamide (2) | Treatment of multi-drug resistant tuberculosis | Anti-melanogenesis (Tyrosinase inhibitor) |
| Prothionamide (3) | Antituberculosis | Anti-melanogenesis (Tyrosinase inhibitor) |
| Isoniazid (8) | Antibiotic used for the treatment of tuberculosis | Anti-melanogenesis (Tyrosinase inhibitor) |
| Thioacetazone (9) | Antibiotic used for the treatment of tuberculosis | Anti-melanogenesis (Tyrosinase inhibitor) |
| Ambazone (10) | Antiseptic | Anti-melanogenesis (Tyrosinase inhibitor) |
| Methimazole (11) | Antithyroid | Anti-melanogenesis (Tyrosinase inhibitor) |
| Antithyroid | Antithyroid | Anti-melanogenesis (Tyrosinase inhibitor) |
| Thiouracil (13) | Antithyroid | Anti-melanogenesis (Tyrosinase inhibitor) |
| Methylthiouracil (14) | Antithyroid | Anti-melanogenesis (Tyrosinase inhibitor) |
| Propylthiouracil (15) | Antithyroid | Anti-melanogenesis (Tyrosinase inhibitor) |
| Thioguanine (16) | Anti-cancer (treatment of leukemia) | Anti-melanogenesis (Tyrosinase inhibitor) |
| Mercaptopurine (17) | Cancer and autoimmune diseases | Anti-melanogenesis (Tyrosinase inhibitor) |
| Azathioprine (18) | Immunosuppressive agent for rheumatoid arthritis, Crohn’s disease, ulcerative colitis and in kidney transplants to prevent rejection | Anti-melanogenesis (Tyrosinase inhibitor) |
| Aspirin (20) | Analgesic drug for treatment of pain, fever or inflammation | Anticancer (COX-inhibitor) |
| Thalidomide (21) | Immunomodulatory drug- used as sedative and hypnotic for the treatment of nausea during pregnancy | Anticancer (NF-κB inhibitor) |
| Metformin (22) | Treatment of type 2 diabetes | Anticancer (Activator of AMP-induced protein kinase) |
| Methotrexate (23) | Osteosarcoma, breast cancer, acute lymphoblastic leukemia and Hodgkin lymphoma | Anticancer (inhibitor of dihydrofolate reductase) |
| Rapamycin (24) | Anti-fungal agent | Anticancer (Inhibitor of T cells and B cells) |
| Celecoxib (25) | Pain and inflammation associated with rheumatoid arthritis (RA) and osteoarthritis | Anticancer (inhibitor of COX-2) |
| Ibuprofen (26) | Treatment of pain and inflammation | Anticancer (inhibitor of COX) |
| Diclofenac (27) | Pain and inflammatory diseases such as gout | Anticancer (inhibitor of COX-1 and 2) |
| Naproxen (28) | Pain and inflammatory diseases such as rheumatoid arthritis and fever | Anticancer (inhibitor of COX-1 and 2) |
| Statins (29–32, 80–82) | Lipid lowering agents that reduce illness and mortality in those who are at high risk of cardiovascular disease | Anticancer, Schistosomiasis (Inhibition of adult and immature S. mansoniworms), Treatment of dengue virus infection and Parkinson’s disease |
| Depakine (33) | Epilepsy and bipolar disorder as well as migraines | Anticancer (suppress the production of IL-6 and TNF-α) |
| Chloroquine (39) | Anti-malarial | Anti-infective (SARS-and MERS-CoV inhibition) |
| Chlorpromazine (40) | Anti-psychotic drug (for the treatment of schizophrenia) | Anti-infective (SARS-and MERS-CoV inhibition) |
| Loperamide (41) | Diarrhea, gastroenteritis, inflammatory bowel disease and short bowel syndrome | Anti-infective (SARS-and MERS-CoV inhibition), Anti-malarial (P. falciparum inhibition) |
| Lopinavir (42) | Anti-HIV agent (protease inhibitor) | Anti-infective (SARS-and MERS-CoV inhibition) |
| Saracatinib (46) | Tumor malignancies (Src-family of tyrosine kinases (SFKs) inhibition) | Anti-infective (MERS-CoV inhibition) |
| Chloroquine (39) | Anti-malarial | Dengue virus infection |
| Prednisolone (51) | Allergies, inflammatory conditions, autoimmune disorders and cancers | Dengue virus infection |
| Balapiravir (52) | HCV (polymerase inhibitor) | Dengue virus infection |
| Celgosivir (53) | HCV (alpha-glucosidase-I inhibitor) | Dengue virus infection |
| Ivermectin (54) | Parasite infestation (head lice, scabies, river blindness, strongyloidiasis, trichuriasis and lymphatic filariasis) | Dengue virus infection |
| Ketotifen (55) | Anti-allergic (noncompetitive H1-antihistamine and mast cell stabilizer) | Dengue virus infection |
| Aminolevulinic acid (56) | Skin diseaes also used in photodynamic detection and surgery of cancer | Dengue virus infection |
| Azelaic acid (57) | Skin diseaes and being used as a component in hair and skin conditioners | Dengue virus infection |
| Mitoxantrone hydrochloride (58) | Anthracenedione antineoplastic agent (metastatic breast cancer, acute myeloid leukemia and non-Hodgkin’s lymphoma) | Dengue virus infection |
| Quinine sulfate (59) | Anti-malarial (used to treat chloroquine-resistant falciparum) | Dengue virus infection |
| Clemastine fumarate (60) | Hay fever and allergy symptoms (Antihistamine with anticholinergic properties and sedative side effects) | Anti-malarial (P. falciparum inhibitor) |
| Raloxifene hydrochloride (63) | Prevention and treatment of osteoporosis in postmenopausal women and those on glucocorticoids | Anti-malaria (P. falciparum inhibitor) |
| Gramicidin A (64) | Anti-bacterial (against gram-positive bacteria) | Anti-malarial (P. falciparum inhibitor) |
| Pentamidine isethionate (65) | Antimicrobial (to treat African trypanosomiasis, leishmaniasis, babesiosis) | Anti-malarial (P. falciparum inhibitor) |
| Clomiphene citrate (66) | Infertility in women who do not ovulate | Anti-malarial (P. falciparum inhibitor) |
| Telmisartan (67) | High blood pressure, heart failure and diabetic kidney disease | Anti-malaria (P. falciparum inhibitor) |
| cyclosporin A (68) | Immunosuppressant agent for rheumatoid arthritis, psoriasis, Crohn’s disease, nephrotic syndrome and in organ transplants to prevent rejection | Anti-malaria (P. falciparum inhibitor |
| Donepezil (76) | Acetylcholinesterase inhibitor | Alzheimer’s disease |
| Rivastigmine (77) | Acetylcholinesterase inhibitor | Alzheimer’s disease and Parkinson’s disease |
| Galanthamine (78) | Acetylcholinesterase inhibitor | Alzheimer’s disease |
| Dimebon (81) | Antihistamine drug | Alzheimer’s disease |
| Valsartan (82) | High blood pressure, heart failure and diabetic kidney disease | Alzheimer’s disease |
| Olmesartan (83) | High blood pressure, heart failure and diabetic kidney disease | Alzheimer’s disease |
| Telmisartan (84) | High blood pressure, heart failure and diabetic kidney disease | Alzheimer’s disease |
| Losartan (85) | High blood pressure, heart failure and diabetic kidney disease | Alzheimer’s disease |
| Amlodipine (86) | High blood pressure and coronary artery disease | Alzheimer’s disease |
| Nilvadipine (87) | Calcium channel blocker for the treatment of hypertension and chronic major cerebral artery occlusion | Alzheimer’s disease |
| Isradipine (88) | Calcium channel blocker to reduce the risk of stroke and heart attack | Alzheimer’s disease and Parkinson’s disease |
| Nimodipine (89) | Calcium channel blocker for the treatment of high blood pressure | Alzheimer’s disease |
| Minocycline (90) | Antibiotic used to treat a number of bacterial infections such as pneumonia | Alzheimer’s disease |
| Bexarotene (92) | Anticancer (treatment for cutaneous T cell lymphoma) | Alzheimer’s disease |
| Acitretin (93) | Treatment of severe resistant psoriasis | Alzheimer’s disease |
Drug repurposing also offers the possibility to develop multi-target drugs that can interact with more than one pathway/protein at the same time. For complex diseases such as (neuro) inflammatory and degenerative disorders, the development of multi-target drugs will be an emerging area for the treatment having multiple advantages such as i) synergistic effect ii) reduced drug-resistance iii) better compliance iv) simplified pharmacokinetic and pharmacodynamic profile and a reduced risk of drug-drug interactions.
On the other hand, the limitations of drug repurposing should also be considered. They involve a) technical challenges and legal requirements such as intellectual property rights which could hamper the whole process and are often hard to overcome b) serious problem on the development of resistant germs on account of consuming a drug for a variety of diseases c) owing to target selectivity, it is difficult to identify a drug that can cure or treat two different diseases on its own. Nevertheless, drug repurposing is only a part of the solution for the sinking numbers of new diseases and its treatment. However, it is quite hard to replace the common way of identifying new drug candidates.
Further, this approach could be improved in many differents ways: Integrating data about repositioning drugs which are available in many public platforms, such as PubChem), CheEMBL, DrugBank, and DrugCentral. Various informations including bioactivities, combined with their chemical structure, physical properties, and clinical indications, have been recorded in these databases. Integrating these approaches can extend the domain of applicability of each method and provide novel information. Combining in silico prediction and in vitro validation may lead to new therapeutic ligands
High throughput screening of chemicals using in vitro and/or in vivo systems can also strongly drive drug repositioning. However, most in vitro systems currently used for high throughput screening are two-dimensional monolayer cultures that differ from physiological conditions.
Notes
The authors declare no competing financial interest.
CRediT authorship contribution statement
Thanigaimalai Pillaiyar: Writing - original draft. Sangeetha Meenakshisundaram: Data curation. Manoj Manickam: Data curation. Murugesan Sankaranarayanan: Data curation.
Acknowledgments
M.M thank PSG Management for their financial support and thank Dr. M. Alagar, Visiting Research Consultant, PSG Institute of Technology and Applied Research for his moral support.
Abbreviations used
- NSAID
nonsteroidal anti-inflammatory drugs
- COX
cyclooxygenase
- PG
prostaglandins
- PI3K
phoshatidylinositol-3-kinase
- USPSTF
preventive services task force recommendation statement
- AMP
adenosine monophosphate
- DNA
deoxyribonucleic acid
- RNA
ribonucleic acid
- IL
interleukin
- VEGF
vascular endothelial growth factor
- Bcl
B cell lymphoma
- FDA
food and drug administration
- SOD
superoxide dismutase
- PSADT
prostate specific antigen doubling time;
- TNF
tumor necrosis factor
- GABA
gamma-aminobutyric acid
- VPA
valproic acid
- HDAC
histone deacetylase
- WHO
world health organization
- MERS
middle-east respiratory syndrome
- SARS
severe acute respirator syndrome
- CoV
coronavirus
- HCoV
human coronavirus
- CQ
chloroquine,
- HIV
human immunodeficiency virus
- DENV
dengue virus
- HCV
hepatic C virus
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- MHV
mouse hepatitis virus
- FIPV
feline infectious peritonitis
- HSV
herpes simplex virus
- DNDi
drugs for neglected disease initiative;
- ROS
reactive oxygen species
- PZQ
praziquantel
- HMG-CoA
3-Hydroxy-3-methyl-glutaryl-coenzyme A
- Abl-TKs
Abelson tyrosine kinases
- AMPA
amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AML
acute myeloid leukemia
- ALL
acute lymphocytic leukemia
- CML
chronic myeloid leukemia
- GVBD assay
competitive germinal vesicle breakdown assay
- HAT
human African trypanosomiasis
- VL
visceral leishmaniosis
- PUVA
psoralen and ultraviolet A
- IBD
inflammatory bowel disease
- UC
ulcerative colitis
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine;
- 6-OHDA
6-hydroxydopamine.
References
- 1.Raju T.N. The Nobel chronicles. 1988: James Whyte Black, (b 1924), Gertrude elion (1918-99), and George H Hitchings (1905-98) Lancet. 2000;355:1022. doi: 10.1016/s0140-6736(05)74775-9. [DOI] [PubMed] [Google Scholar]
- 2.Santos R., Ursu O., Gaulton A., Bento A.P., Donadi R.S., Bologa C.G., Karlsson A., Al-Lazikani B., Hersey A., Oprea T.I., Overington J.P. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017;16:19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichert J.M. Trends in development and approval times for new therapeutics in the United States. Nat. Rev. Drug Discov. 2003;2:695–702. doi: 10.1038/nrd1178. [DOI] [PubMed] [Google Scholar]
- 4.Ashburn T.T., Thor K.B. Drug repositioning identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert J., Henske P., Singh A. Rebuilding big pharma’s business model, in vivo. The Business Med. Rep. 2003;21:73–80. [Google Scholar]
- 6.Mullard A. FDA drug approvals. Nat. Rev. Drug Discov. 2014;14(2015):77–81. doi: 10.1038/nrd4545. [DOI] [PubMed] [Google Scholar]
- 7.Genetic and rare diseases information centre. https://rarediseases.info.nih.gov
- 8.http://www.fda.gov/Drugs/DevelopmentApprovalProcess/
- 9.Barratt M.J., Frail D.E. John Wiley & Sons.; 2012. Drug Repositioning: Bringing New Life to Shelved Assets and Existing Drugs; pp. 389–431. [Google Scholar]
- 10.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 11.Park K. A review of computational drug repurposing. Transl Clin. Pharmacol. 2019;27:59–63. doi: 10.12793/tcp.2019.27.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonaventure J., Domingues M.J., Larue L. Cellular and molecular mechanisms controlling the migration of melanocytes and melanoma cells. Pigm. Cell Melanoma Res. 2013;26:316–325. doi: 10.1111/pcmr.12080. [DOI] [PubMed] [Google Scholar]
- 13.Riley P.A., Borovansky J., Wiley I. Wiley Blackwell; Weinheim, Germany: 2011. Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions; pp. 343–381. [Google Scholar]
- 14.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32:403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Akihisa T., Kurita M., Kikuchi T., Zhu W.F., Ye F., Dong Z.H., Liu W.H., Feng F., Xu J. Melanogenesis-inhibitory and cytotoxic activities of triterpene glycoside constituents from the bark of Albiziaprocera. J. Nat. Prod. 2018;81:2612–2620. doi: 10.1021/acs.jnatprod.8b00167. [DOI] [PubMed] [Google Scholar]
- 16.Pillaiyar T., Manickam M., Jung S.H. Inhibitors of melanogenesis: a patent review (2009 - 2014) Expert Opin. Ther. Pat. 2015;25:775–788. doi: 10.1517/13543776.2015.1039985. [DOI] [PubMed] [Google Scholar]
- 17.Ali S.A., Naaz I., Zaidi K.U., Ali A.S. Recent updates in melanocyte function: the use of promising bioactive compounds for the treatment of hypopigmentary disorders. Mini Rev. Med. Chem. 2017;17:785–798. doi: 10.2174/1389557516666161223153953. [DOI] [PubMed] [Google Scholar]
- 18.Pillaiyar T., Namasivayam V., Manickam M., Jung S.H. Inhibitors of melanogenesis: an updated review. J. Med. Chem. 2018;61:7395–7418. doi: 10.1021/acs.jmedchem.7b00967. [DOI] [PubMed] [Google Scholar]
- 19.Carballo-Carbajal I., Laguna A., Romero-Giménez J., Cuadros T., Bove J., Martinez-Vicente M., Parent A., Gonzalez-Sepulveda M., Peñuelas N., Torra A., Rodríguez-Galván B., Ballabio A., Hasegawa T., Bortolozzi A., Gelpi E., Vila M. Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat. Commun. 2019;7:973. doi: 10.1038/s41467-019-08858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z., Wang Y., Guo Z., Tan T., Zhang Y. Novel tyrosinase inhibitory peptide with free radical scavenging ability. J. Enzym. Inhib. Med. Chem. 2019;34:1633–1640. doi: 10.1080/14756366.2019.1661401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa T. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson’s disease. Int. J. Mol. Sci. 2010;11:1082–1089. doi: 10.3390/ijms11031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tessari I., Bisaglia M., Valle F., Samori B., Bergantino E., Mammi S., Bubacco L. The reaction of alpha-synuclein with tyrosinase: possible implications for Parkinson disease. J. Biol. Chem. 2008;283:16808–16817. doi: 10.1074/jbc.M709014200. [DOI] [PubMed] [Google Scholar]
- 23.Vontzalidou A., Zoidis G., Chaita E., Makropoulou M., Aligiannis N., Lambrinidis G., Mikros E., Skaltsounis A.L. Design, synthesis and molecular simulation studies of dihydrostilbene derivatives as potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2012;22:5523–5526. doi: 10.1016/j.bmcl.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Greggio E., Bergantino E., Carter D., Ahmad R., Costin G.E., Hearing V.J., Clarimon J., Singleton A., Eerola J., Hellstrom O., Tienari P.J., Miller D.W., Beilina A., Bubacco L., Cookson M.R. Tyrosinase exacerbates dopamine toxicity but is not genetically associated with Parkinson’s disease. J. Neurochem. 2005;93:246–256. doi: 10.1111/j.1471-4159.2005.03019.x. [DOI] [PubMed] [Google Scholar]
- 25.Hall A.M., Orlow S.J. Degradation of tyrosinase induced by phenylthiourea occurs following Golgi maturation. Pigm. Cell Res. 2005;18:122–129. doi: 10.1111/j.1600-0749.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- 26.Poma A., Bianchini S., Miranda M. Inhibition of L-tyrosine-induced micronuclei production by phenylthiourea in human melanoma cells. Mutat. Res. 1999;446:143–148. doi: 10.1016/s1383-5718(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 27.Thanigaimalai P., Lee K.C., Sharma V.K., Joo C., Cho W.J., Roh E., Kim Y., Jung S.H. Structural requirement of phenylthiourea analogs for their inhibitory activity of melanogenesis and tyrosinase. Bioorg. Med. Chem. Lett. 2011;21:6824–6828. doi: 10.1016/j.bmcl.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Thanigaimalai P., Hoang T.A.L., Lee K.C., Bang S.C., Sharma V.K., Yun C.Y., Roh E., Hwang B.Y., Kim Y., Jung S.H. Structural requirement(s) of N-phenylthioureas and benzaldehyde thiosemicarbazones as inhibitors of melanogenesis in melanoma B 16 cells, Bioorg. Med. Chem. Lett. 2010;20:2991–2993. doi: 10.1016/j.bmcl.2010.02.067. [DOI] [PubMed] [Google Scholar]
- 29.Choi J., Park S.J., Jee J.G. Analogues of ethionamide, a drug used for multi drug resistant tuberculosis, exhibit potent inhibition of tyrosinase. Eur. J. Med. Chem. 2015;106:157–166. doi: 10.1016/j.ejmech.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Choi J., Jee J.G. Repositioning of thiourea-containing drugs as tyrosinase inhibitors. Int. J. Mol. Sci. 2015;16:28534–28548. doi: 10.3390/ijms161226114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falzon D., Hill G., Pal S.N., Suwankesawong W., Jaramillo E. Pharmacovigilance and tuberculosis: applying the lessons of thioacetazone. Bull. World Health Organ. 2014;92:918–919. doi: 10.2471/BLT.14.142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.https://www.chemblink.com/products/539-21-9.htm
- 33.Cooper D.S. Antithyroid drugs, N. Engl. J. Med. 2005;352:905–917. doi: 10.1056/NEJMra042972. [DOI] [PubMed] [Google Scholar]
- 34.Choi J., Lee Y.M., Jee J.G. Thiopurine drugs repositioned as tyrosinase inhibitors. Int. J. Mol. Sci. 2017;19:77–92. doi: 10.3390/ijms19010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ismaya W.T., Rozeboom H.J., Weijn A., Mes J.J., Fusetti F., Wichers H.J., Dijkstra B.W. Crystal structure of agaricusbisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry. 2011;50:5477–5486. doi: 10.1021/bi200395t. [DOI] [PubMed] [Google Scholar]
- 36.Deri B., Kanteev M., Goldfeder M., Lecina D., Guallar V., Adir N., Fishman A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016;6:34993. doi: 10.1038/srep34993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buitrago E., Vuillamy A., Boumendjel A., Yi W., Gellon G., Hardré R., Philouze C., Serratrice G., Jamet H., Réglier M., Belle C. Exploring the interaction of N/S compounds with a dicopper center: tyrosinase inhibition and model studies. Inorg. Chem. 2014;53:12848–12858. doi: 10.1021/ic501829s. [DOI] [PubMed] [Google Scholar]
- 38.United States cancer statistics Incidence and mortality web based report. Atlanta, US: 2014. 1999-2011. https://smhs.gwu.edu/cancercontroltap/resources/united-states-cancer-statistics-1999%E2%80%932011-incidence-and-mortality-web-based-report
- 39.Gupta S.C., Sung B., Prasad S., Webb L.J., Aggarwal B.B. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol. Sci. 2013;34:508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Elwood P.C., Pickering J.E., Morgan G., Galante J., Weightman A.L., Morris D., Longley M., Mason M., Adams R., Dolwani S., Chia W.K.J., Lanas A. Systematic review update of observational studies further supports aspirin role in cancer treatment: time to share evidence and decision-making with patients? PLoS One. 2018;13 doi: 10.1371/journal.pone.0203957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothwell P.M., Fowkes F.G., Belch J.F., Ogawa H., Warlow C.P., Meade T.W. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 42.Elwood P.C., Gallahar A.M., Duthie G.G., Mur L.A.J., Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 43.Simmons D.L., Botting R.M., Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 44.Takada Y., Bhardwaj A., PravinPotdar P., Aggarwal B.B. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappa B activation, inhibition of expression of cyclooxygenase-2 and cyclin D1 and abrogation of tumor cell proliferation. Oncogene. 2004;23:9247–9258. doi: 10.1038/sj.onc.1208169. [DOI] [PubMed] [Google Scholar]
- 45.Li L., Hu M., Wang T., Chen H., Xu L. Repositioning aspirin to treat lung and breast cancers and overcome acquired resistance to targeted therapy. Front. Oncol. 2020;9:1503. doi: 10.3389/fonc.2019.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celecoxib Monograph for Professionals "Drugs.Com. American society of health-system pharmacists; 15 April 2019. Retrieved. [Google Scholar]
- 47.Jendrossek V. Targeting apoptosis pathways by celecoxib in cancer. Canc. Lett. 2011;332:313–324. doi: 10.1016/j.canlet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Lynch P.M., Burke C.A., Phillips R., Morris J.S., Slack R., Wang X., Liu J., Patterson S., Sinicrope F.A., Rodriguez-Bigas M.A., Half E., Bulow S., Latchford A., Clark S., Ross W.A., Malone B., Hasson H., Richmond E., Hawk E. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut. 2016;65:286–295. doi: 10.1136/gutjnl-2014-307235. [DOI] [PubMed] [Google Scholar]
- 49.Costea T., Nagy P., Ganea C., Szöllősi J., Mocanu M.M. Molecular mechanisms and bioavailability of polyphenols in prostate cancer. Int. J. Mol. Sci. 2019;20:1–39. doi: 10.3390/ijms20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akrami H., Aminzadeh S., Fallahi H. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumour Biol. 2015;36:3237–3243. doi: 10.1007/s13277-014-2952-3. [DOI] [PubMed] [Google Scholar]
- 51.Redpath M., Marques C.M., Dibden C., Waddon A., Lalla R., Macneil S. Ibuprofen and hydrogel-released ibuprofen in the reduction of inflammation induced migration in melanoma cells. Br. J. Dermatol. 2009;161:25–33. doi: 10.1111/j.1365-2133.2009.09220.x. [DOI] [PubMed] [Google Scholar]
- 52.Endo H., Yano M., Okumura Y., Kido H. Ibuprofen enhances the anticancer activity of cisplatin in lung cancer cells by inhibiting the heat shock protein 70. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fehniger T.A., Byrd J.C., Marcucci G., Abboud C.N., Kefauver C., Payton J.E., Vij R., Blum W. Single-agent lenalidomide induces complete remission of acute myeloid leukemia in patients with isolated trisomy13. Blood. 2009;113:1002–1005. doi: 10.1182/blood-2008-04-152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiramatsu T., Yoshizawa J., Miyaguni K., Sugihara T., Harada A., Kaji S., Uchida G., Kanamori D., Baba Y., Ashizuka S., Ohki T. Thalidomide potentiates etoposide-induced apoptosis in murine neuroblastoma through suppression of NF-κB activation. Pediatr. Surg. Int. 2018;34:443–450. doi: 10.1007/s00383-018-4234-4. [DOI] [PubMed] [Google Scholar]
- 55.Wei W., Fan Z., Yizi Z., Lieping G., Haotian S., Jian H. A combination of thalidomide and arsenic trioxideis effective and well tolerated in patients with myelodysplastic syndromes. Leuk. Res. 2012;36:715–719. doi: 10.1016/j.leukres.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 56.Stewart A.K. Medicine. How thalidomide works against cancer. Science. 2014;343:256–257. doi: 10.1126/science.1249543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazzurana L., Forkel M., Rao A., Van Acker A., Kokkinou E., Ichiya T., Almer S., Höög C., Friberg D., Mjösberg J. Suppression of Aiolos and Ikaros expression by lenalidomide reduces human ILC3-ILC1/NK cell transdifferentiation. Eur. J. Immunol. 2019;49:1344–1355. doi: 10.1002/eji.201848075. [DOI] [PubMed] [Google Scholar]
- 58.Kalender A., Selvaraj A., Kim S.Y., Gulati P., Brûlé S., Viollet B., Kemp B.E., Bardeesy N., Dennis P., Schlager J.J., Marette A., Kozma S.C., Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metabol. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee M.S., Hsu C.C., Wahlqvist M.L., Tsai H.N., Chang Y.H., Huang Y.C. Type 2 diabetes increases and metformin reducestotal, colorectal, liver and pancreatic cancer incidences in taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Canc. 2011;11:20–30. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu H., Zhong X., Gao P., Shi J., Wu Z., Guo Z., Wang Z., Song Y. The potential effect of metformin on cancer: an umbrella review. Front. Endocrinol. 2019;10:617. doi: 10.3389/fendo.2019.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noto H., Goto A., Tsujimoto T., Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang D., Li Y., Sun P. miR-770-5p modulates resistance to methotrexate in human colorectal adenocarcinoma cells by downregulating HIPK1. Exp. Ther. Med. 2020;19:339–346. doi: 10.3892/etm.2019.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabas I., Glass C.K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvarado Y., Mita M.M., Vemulapalli S., Mahalingam D., Mita A.C. Clinical activity of mammalian target of rapamycin inhibitors in solid tumors. Targeted Oncol. 2011;6:69–94. doi: 10.1007/s11523-011-0178-5. [DOI] [PubMed] [Google Scholar]
- 65.Sillaber C., Mayerhofer M., Böhm A., Vales A., Aruze A., Aichberger K.J., Esterbauer H., Pfeilstöcker M., Sperr W.R., Pickl W.F., Haas O.A., Valent P. Evaluation of antileukaemic effects of rapamycin in patients with imatinib-resistant chronic myeloid leukaemia. Eur. J. Clin. Invest. 2008;38:43–52. doi: 10.1111/j.1365-2362.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 66.Pantziarka P., Sukhatme V., Bouche G., Meheus L., Sukhatme V.P. Repurposing drugs in oncology (ReDO)-diclofenac as an anti-cancer agent. Ecancermed. Sci. 2016;10:610. doi: 10.3332/ecancer.2016.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arisan E.D., Ergül Z., Bozdağ G., Rencüzoğulları Ö., Çoker-Gürkan A., Obakan-Yerlikaya P., Coşkun D., Palavan-Ünsal N. Diclofenac induced apoptosis via altering PI3K/Akt/MAPK signaling axis in HCT 116 more efficiently compared to SW480 colon cancer cells. Mol. Biol. Rep. 2018;45:2175–2184. doi: 10.1007/s11033-018-4378-2. [DOI] [PubMed] [Google Scholar]
- 68.Valle B.L., D’Souza T., Becker K.G., Wood W.H., Zhang Y., Wersto R.P. Non-steroidal anti-inflammatory drugs decrease E2F1 expression and inhibit cellgrowth in ovarian cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cecere F., Iuliano A., Albano F., Zappelli C., Castellano I., Grimaldi P. Diclofenac-induced apoptosis in the neuroblastoma cell line SH-SY5Y: possible involvement of the mitochondrial superoxide dismutase. J. Biomed. Biotechnol. 2010:801726. doi: 10.1155/2010/801726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brinkhuizen T., Frencken K.J., Nelemans P.J., Hoff M.L., Kelleners-Smeets N.W., ZurHausen A. The effect of topical diclofenac 3% and calcitriol 3 mug/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): a phase II, randomized controlled trial. J. Am. Acad. Dermatol. 2016;75:126–134. doi: 10.1016/j.jaad.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 71.Janowska A., Dini V., Oranges T., Colombo G., Bruno G., Di Matteo S., Romanelli M. The relapse rate in patients with actinic keratosis treated with diclofenac sodium 3% gel. Int. J. Med. Sci. Clin. Invent. 2019;6:4313–4317. [Google Scholar]
- 72.Kim M.S., Kim J.E., Lim D.Y., Huang Z., Chen H., Langfald A. Naproxen induces cell cycle arrest and apoptosis in human urinary bladder cancer cell lines and chemically induced cancers by targeting PI3K. Canc. Prev. Res. 2014;7:236–245. doi: 10.1158/1940-6207.CAPR-13-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suh N., Reddy B.S., DeCastro A., Paul S., Lee H.J., Smolarek A.K. Combination of atorvastatin with sulindac or naproxen profoundly inhibits colonic adenocarcinomas by suppressing the p65/beta-catenin/cyclin D1signaling pathway in rats. Canc. Prev. Res. 2011;4:1895–1902. doi: 10.1158/1940-6207.CAPR-11-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahn K.S., Sethi G., Aggarwal B.B. Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: role of IkappaBalpha kinase and TGFbeta-activated kinase-1. J. Immunol. 2007;178:2507–2516. doi: 10.4049/jimmunol.178.4.2507. [DOI] [PubMed] [Google Scholar]
- 75.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K., Varol M., Jain A., Khan M.A., Sethi G. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9:E735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y., He X., Ding Y., Chen H., Sun L. Statin uses and mortality in colorectal cancer patients: an updated systematic review and meta-analysis. Cancer Med. 2019;8:3305–3313. doi: 10.1002/cam4.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Broughton T., Sington J., Beales I.L.B. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterol. 2012;12:36–44. doi: 10.1186/1471-230X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ibáñez-Sanz G., Guinó E., Pontes C., Quijada-Manuitt M.Á., de la Peña-Negro L.C., Aragón M., Domínguez M., Rodríguez-Alonso L., Blasco A., García-Rodríguez A., Morros R., Moreno V. Statin use and the risk of colorectal cancer in a population-based electronic health records study. Sci. Rep. 2019;9:13560. doi: 10.1038/s41598-019-49877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sehdev A., Shih Y.C., Huo D., Vekhter B., Lyttle C., Polite B. The role of statins for primary prevention in non-elderly colorectal cancer patients. Anticancer Res. 2014;34:5043–5050. [PubMed] [Google Scholar]
- 80.Broughton T., Sington J., Beales I.L.B. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterol. 2012;12:36–44. doi: 10.1186/1471-230X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rocha M.A., Veronezi G.M.B., Felisbino M.B., Gatti M.S.V., Tamashiro W.M.S.C., Mello M.L.S. Sodium valproate and 5-aza-2’-deoxycytidine differentially modulate DNA demethylation in G1 phase-arrested and proliferative HeLa cells. Sci. Rep. 2019;9:18236. doi: 10.1038/s41598-019-54848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soria-Castro R., Schcolnik-Cabrera A., Rodríguez-López G., Campillo-Navarro M., Puebla-Osorio N., Estrada-Parra S., Estrada-García I., Chacón-Salinas R., Chávez-Blanco A.D. Exploring the drug repurposing versatility of valproic acid as a multifunctional regulator of innate and adaptive immune cells. J. Immunol. Res. 2019;14:9678098. doi: 10.1155/2019/9678098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin F., Ufodiama C., Watt I., Bland M., Brackenbury W.J. Therapeutic value of voltage-gated sodium channel inhibitors in breast, colorectal, and prostate cancer: a systematic review. Front. Pharmacol. 2015;6:273. doi: 10.3389/fphar.2015.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdullah M.I., Abed M.N., Khanim F., Richardson A. Screening a library of approved drugs reveals that prednisolone synergizes with pitavastatin to induce ovarian cancer cell death. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-46102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmed K., Koval A., Xu J., Katanaev V.L. Towards the first targeted therapy for triple-negative breast cancer: repositioning of clofazimine as a chemotherapy-compatible selective Wnt pathway inhibitor. Canc. Lett. 2019;449:45–55. doi: 10.1016/j.canlet.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 86.Catalano R., Rocca R., Juli G., Costa G., Maruca A., Artese A., Caracciolo D., Tagliaferri P., Alcaro S., Tassone P., Amodio N. A drug repurposing screening reveals a novel epigenetic activity of hydroxychloroquine. Eur. J. Med. Chem. 2019;183:111715. doi: 10.1016/j.ejmech.2019.111715. [DOI] [PubMed] [Google Scholar]
- 87.Ferrara F., Schiffer C.A. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 88.Murteira S., Ghezaiel Z., Karray S., Lamure M. Drug reformulations and repositioning in pharmaceutical industry and its impact on market access: reassessment of nomenclature. J Mark Access Health Policy. 2013;1:21131. doi: 10.3402/jmahp.v1i0.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Millan M.J., Maiofiss L., Cussac D., Audinot V., Boutin J.A., Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of mono- aminergic receptor I. a multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Therapeut. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- 90.Lara-Castillo M.C., Cornet-Masana J.M., Etxabe A., Banús-Mulet A., Torrente M.Á., Nomdedeu M., Díaz-Beyá M., Esteve J., Risueño R.M. Repositioning of bromocriptine for treatment of acute myeloid leukemia. J. Transl. Med. 2016;14:261. doi: 10.1186/s12967-016-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 92.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.H. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016;59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pillaiyar T., Manickam M., Jung S.H. Middle East respiratory syndrome-coronavirus (MERS-CoV): an updated overview and pharmacotherapeutics. Med. Chem. 2015;5:361–372. [Google Scholar]
- 95.Mers . 2015. South Korea Closes 700 Schools after Third Death. [Google Scholar]
- 96.Severe Respiratory Disease Associated with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) 2015. [Google Scholar]
- 97.Pan X., Wang L., Grundemann D., Sweet D.H. Interaction of ethambutol with human organic cation transporters of the SLC22 family indicates potential for drug-drug interactions during antituberculosis therapy. Antimicrob. Agents Chemother. 2013;57:5053–5059. doi: 10.1128/AAC.01255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome corona virus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Madrid P.B., Chopra S., Manger I.D., Gilfillan L., Keepers T.R., Shurtleff A.C., Green C.E., Iyer L.V., Dilks H.H., Davey R.A., Kolokoltsov A.A., Carrion R., Patterson J.L., Jr., Bavari S., Panchal R.G., Warren T.K., Wells J.B., Moos W.H., Burke R.L., Tanga M.J. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One. 2003;8 doi: 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Porotto M., Orefice G., Yokoyama C.C., Mungall B.A., Realubit R., Sganga M.L., Aljofan M., Whitt M., Glickman F., Moscona A. Simulating henipa virus multi cycle replication in a screening assay leads to identification of a promising candidate for therapy. J. Virol. 2009;83:5148–5155. doi: 10.1128/JVI.00164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyamoto S., Miyake N., Jarskog L.F., Fleischhacker W.W., Lieberman J.A. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatr. 2012;17:1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 103.Benedicto I., Gondar V., Molina-Jiménez F., García-Buey L., López-Cabrera M., Gastaminza P., Majano P.L. Clathrin mediates infectious hepatitis C virus particle egress. J. Virol. 2015;89:4180–4190. doi: 10.1128/JVI.03620-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pohjala L., Utt A., Varjak M., Lulla A., Merits A., Ahola T., Tammela P. Inhibitors of alpha virus entry and replication identified with as table Chikungunya replicon cell line and virus-based assays. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pu Y., Zhang X. Mouse hepatitis virus type 2 enters cells through a clathrin-mediated endocytic pathway independent of Eps15. J. Virol. 2008;82:8112–8123. doi: 10.1128/JVI.00837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Regnard C., Twycross R., Mihalyo M., Wilcock A., Loperamide J. vol. 42. 2011. Pain Symptom Manage; pp. 319–323. [DOI] [PubMed] [Google Scholar]
- 108.Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., Hsu H.H., Huang H.C., Wu D., Brik A., Liang F.S., Liu R.S., Fang J.M., Chen S.T., Liang P.H., Wong C.H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shin J.S., Jung E., Kim M., Baric R.S., Go Y.Y. Saracatinib inhibits Middle East respiratory syndrome-coronavirus replication invitro. Viruses. 2018;10:283–302. doi: 10.3390/v10060283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gesto D.S., Cerqueira N.M., Fernandes P.A., Ramos M.J. Gemcitabine: a critical nucleoside for cancer therapy. Curr. Med. Chem. 2012;19:1076–1087. doi: 10.2174/092986712799320682. [DOI] [PubMed] [Google Scholar]
- 111.Kang H., Kim C., Kim D.E., Song J.H., Choi M., Choi K., Kang M., Lee K., Kim H.S., Shin J.S., Kim J., Han S.B., Lee M.Y., Lee S.U., Lee C.K., Kim M., Ko H.J., van Kuppeveld F.J., Cho S. Synergistic antiviral activity of gemcitabine and ribavirin against enteroviruses. Antivir. Res. 2015;124:1–10. doi: 10.1016/j.antiviral.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 112.Botta L., Rivara M., Zuliani V., Radi M. Drug repurposing approaches to fight Dengue virus infection and related diseases. Front. Biosci. Landmark. 2018;23:997–1019. doi: 10.2741/4630. [DOI] [PubMed] [Google Scholar]
- 113.Amemiya T., Gromiha M.M., Horimoto K., Fukui K. Drug repositioning for dengue haemorrhagic fever by integrating multiple omics analyses. Sci. Rep. 2019;9:523. doi: 10.1038/s41598-018-36636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tam D.T., Ngoc T.V., Tien N.T., Kieu N.T., Thuy T.T., Thanh L.T., Tam C.T., Truong N.T., Dung N.T., Qui P.T., Hien T.T., Farrar J.J., Simmons C.P., Wolbers M., Wills B.A. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin. Infect. Dis. 2012;55:1216–1224. doi: 10.1093/cid/cis655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Low J.G., Sung C., Wijaya L., Wei Y., Rathore A.P.S., Watanabe S., Tan B.H., Toh L., Chua L.T., Hou Y., Chow A., Howe S., Chan W.K., Tan K.H., Chung J.S., Cherng B.P., Lye D.C., Tambayah P.A., Ng L.C., Connolly J., Hibberd M.L., Leo Y.S., Cheung Y.B., Ooi E.E., Vasudevan S.G. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014;14:706–715. doi: 10.1016/S1473-3099(14)70730-3. [DOI] [PubMed] [Google Scholar]
- 116.Whitehorn J., Nguyen C.V.V., Khanh L.P., Kien D.T.H., Quyen N.T.H., Tran N.T.T., Hang N.T., Truong N.T., Hue-Tai L.T., Cam-Huong N.T., Nhon V.T., Van-Tram T., Farrar J., Wolbers M., Simmons C.P., Wills B. Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2016;62:468–476. doi: 10.1093/cid/civ949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Avirutnan P. Presented at 26th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands. 2016. Ivermectin: a promising anti-dengue replication treatment [abstract S634] pp. 9–12. (April) [Google Scholar]
- 118.Jenny G.H.L., Eng E.O., Subhash G.V. Current status of dengue therapeutics research and development. J. Infect. Dis. 2017;215:S96–S102. doi: 10.1093/infdis/jiw423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Catherine H. Repurposing approved drugs on the pathway tonovel therapies. Schein, Med. Res. Rev. 2020;40:586–605. doi: 10.1002/med.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Del Rosso J.Q., Kircik L.H. Update on the management of rosacea: a status report on the current role and new horizons with topical azelaic acid. J. Drugs Dermatol. JDD. 2014;12:s101–s107. [PubMed] [Google Scholar]
- 121.Yin G., Zhang Y., Geng M., Cai B., Zheng Y. Cure of condylomaacuminata covering the glans penis using aminolevulinic acid/photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020:101658. doi: 10.1016/j.pdpdt.2020.101658. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 122.Cahill K.E., Karimi Y.H., Karrison T.G., Jain N., Green M., Weiner H., Fulton N., Kadri S., Godley L.A., Artz A.S., Liu H., Thirman M.J., Le Beau M.M., McNerney M.E., Segal J., Larson R.A., Stock W., Odenike O. A phase 1 study of azacitidine with high-dose cytarabine and mitoxantrone in high-risk acute myeloid leukemia. Blood Adv. 2020;4:599–606. doi: 10.1182/bloodadvances.2019000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Achan J., Talisuna A.O., Erhart A., Yeka A., Tibenderana J.K., Baliraine F.N., Rosenthal P.J., D’Alessandro U. Quinine an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar. J. 2011;10:144–156. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baroni A., Paoletti I., Ruocco E., Ayala F., Corrado F., Wolf R., Tufano M.A., Donnarumma G. Antiviral effects of quinine sulfate on HSV-1 HaCat cells infected: analysis of the molecular mechanisms involved. J. Dermatol. Sci. 2007;47:253–255. doi: 10.1016/j.jdermsci.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 125.Marois I., Cloutier A., Meunier I., Weingartl H.M., Cantin A.M., Richter M.V. Inhibition of influenza virus replication by targeting broad host cell pathways. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.https://www.who.int/news-room/fact-sheets/detail/malaria
- 127.Hu Y.Q., Gao C., Zhang S., Xu L., Xu Z., Feng L.S., Wu X., Zhao F. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2017;139:22–47. doi: 10.1016/j.ejmech.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 128.Kum ar R., Sharma R., Kumar I., Upadhyay P., Dhiman A.K., Kumar R., Purohit R. Kumar R., Sahal D., Sharma U. Evaluation of antiplasmodial potential of C2 and C8 modified quinolines: in vitro and in silico Study. Med. Chem. 2019;15:790–800. doi: 10.2174/1573406414666181015144413. [DOI] [PubMed] [Google Scholar]
- 129.Tian J., Vandermosten L., Peigneur S., Moreels L., Rozenski J., Tytgat J., Herdewijn P., Van den Steen P.E., De Jonghe S. Astemizole analogues with reduced hERG inhibition as potent antimalarial compounds. Bioorg. Med. Chem. 2017;25:6332–6344. doi: 10.1016/j.bmc.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 130.Derbyshire E.R., Prudêncio M., Mota M.M., Clardy J. Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2012;109:8511–8851. doi: 10.1073/pnas.1118370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pazhayam N.M., Chhibber-Goel J., Sharma A. New leads for drug repurposing against malaria. Drug Discov. Today. 2019;24:263–271. doi: 10.1016/j.drudis.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y., Murillo-Solano C., Kirkpatrick M.G., Antoshchenko T., Park H.-W., Pizarro J.C. Repurposing drugs to target the malaria parasite unfolding protein response. Sci. Rep. 2018;8:10333. doi: 10.1038/s41598-018-28608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Porta E.O.J., Bofill Verdaguer I., Perez C., Banchio C., Ferreira de Azevedo M., Katzin A.M., Labadie G.R. Repositioning Salirasib as a new antimalarial agent. Med. Chem. Comm. 2019;10:1599–1605. doi: 10.1039/c9md00298g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chu X.M., Wang C., Wang W.L., Liang L.L., Li W., Gong K.K., Sun K.L. Triazole derivatives and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2019;15:206–223. doi: 10.1016/j.ejmech.2019.01.047. [DOI] [PubMed] [Google Scholar]
- 135.Vallone A., D’Alessandro S., Brogi S., Brindisi M., Chemi G., Alfano Lamponi S., Lee S.G., Jez J.M., Koolen K.J.M., Dechering K.J., Saponara S., Fusi F., Gorelli B., Taramelli D., Parapini S., Caldelari R., Campiani G., Gemma S., Butini S. Antimalarial agents against both sexual and asexual parasites stages: structure-activity relationships and biological studies of the Malaria Box compound 1-[5-(4-bromo-2-chlorophenyl)furan-2-yl]-N-[(piperidin-4-yl)methyl]methanamine (MMV019918) and analogues. Eur. J. Med. Chem. 2018;150:698–718. doi: 10.1016/j.ejmech.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wimo A., Prince M. Alzheimer’s disease international; 2011. World Alzheimer Report 2010: the Global Economic Impact of Dementia. [Google Scholar]
- 137.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 138.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A., Eckert A., Staufenbiel M., Hardeman E., Götz J. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 139.Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D., Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 140.Ballard C., Corbett A., Sharp S. Aligning the evidence with practice: NICE guidelines for drug treatment of Alzheimer’s disease. Expert Rev. Neurother. 2011;11:327–329. doi: 10.1586/ern.11.13. [DOI] [PubMed] [Google Scholar]
- 141.Hassan M., Raza H., Abbasi M.A., Moustafa A.A. The exploration of novel Alzheimer’s therapeutic agents from the pool of FDA approved medicines using drug repositioning, enzyme inhibition and kinetic mechanism approaches. Biomed. Pharmacother. 2019;109:2513–2526. doi: 10.1016/j.biopha.2018.11.115. [DOI] [PubMed] [Google Scholar]
- 142.Robert M., Salvà M., Segarra R., Pavesi M., Esbri R., Roberts D., Golor G. The prokinetic cinitapride has no clinically relevant pharmacokinetic interaction and effect on qt during coadministration with ketoconazole. Drug Metabol. Dispos. 2007;35:1149–1156. doi: 10.1124/dmd.106.010835. [DOI] [PubMed] [Google Scholar]
- 143.Alarcón-de-la-Lastra Romero C., López A., Martín M.J., la Casa C., Motilva V. Cinitapride protects against ethanol-induced gastric mucosal injury in rats: role of 5-hydroxytryptamine, prostaglandins and sulfhydryl compounds. Pharmacology. 1997;54:193–202. doi: 10.1159/000139487. [DOI] [PubMed] [Google Scholar]
- 144.Nikseresht Ai Bush S., Ayton S. Treating Alzheimer’s disease by targeting iron. Br. J. Pharmacol. 2019;176:3622–3635. doi: 10.1111/bph.14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Green R.C., Schneider L.S., Amato D.A., Beelen A.P., Wilcock G., Swabb E.A., Zavitz K.H. Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. J. Am. Med. Assoc. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koronyo-Hamaoui M., Sheyn J., Hayden E.Y., Li S., Fuchs D.T., Regis G.C., Lopes D.H., Black K.L., Bernstein K.E., Teplow D.B., Fuchs S., Koronyo Y., Rentsendorj A. Peripherally derived angiotensin converting enzyme-enhanced macrophages alleviate Alzheimer-related disease. Brain. 2020;143:336–358. doi: 10.1093/brain/awz364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kehoe P.G., Passmore P.A. The renin–angiotensin system and antihypertensive drugs in Alzheimer’s disease: current standing of the angiotensin hypothesis? J. Alzheimers Dis. 2012;30:S251–S269. doi: 10.3233/JAD-2012-111376. [DOI] [PubMed] [Google Scholar]
- 148.Wang J., Ho L., Chen L., Zhao Z., Zhao W., Qian X., Humala N., Seror I., Bartholomew S., Rosendorff C., Pasinetti G.M. Valsartan lowers brain β-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Takeda S., Sato N., Takeuchi D., Kurinami H., Shinohara M., Niisato K., Kano M., Ogihara T., Rakugi H., Morishita R. Angiotensin receptor blocker prevented β-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- 150.Mogi M., Li J.M., Tsukuda K., Iwanami J., Min L.J., Sakata A., Fujita T., Iwai M., Horiuchi M. Telmisartan prevented cognitive decline partly due to PPAR-γ activation. Biochem. Biophys. Res. Commun. 2008;375:446–449. doi: 10.1016/j.bbrc.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 151.Danielyan L., Klein R., Hanson L.R., Buadze M., Schwab M., Gleiter C.H., Frey W.H. Protective effects of intranasal losartan in the APP/PS1 transgenic mouse model of Alzheimer disease. Rejuvenation Res. 2010;13:195–201. doi: 10.1089/rej.2009.0944. [DOI] [PubMed] [Google Scholar]
- 152.Li N.C., Lee A., Whitmer R.A., Kivipelto M., Lawler E., Kazis L.E., Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. Br. Med. J. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hanyu H., Hirao K., Shimizu S., Iwamoto T., Koizumi K., Abe K. Favourable effects of nilvadipine on cognitive function and regional cerebral blood flow on SPECT in hypertensive patients with mild cognitive impairment. Nucl. Med. Commun. 2007;28:281–287. doi: 10.1097/MNM.0b013e32804c58aa. [DOI] [PubMed] [Google Scholar]
- 154.Zhao W., Wang J., Ho L., Ono K., Teplow D.B., Pasinetti G.M. Identification of antihypertensive drugs, which inhibit amyloid-β protein oligomerization. J. Alzheimers Dis. 2009;16:49–57. doi: 10.3233/JAD-2009-0925. [DOI] [PubMed] [Google Scholar]
- 155.Bachmeier C., Beaulieu-Abdelahad D., Mullan M., Paris D. Selective dihydropyiridine compounds facilitate the clearance of β-amyloid across the blood–brain barrier. Eur. J. Pharmacol. 2011;659:124–129. doi: 10.1016/j.ejphar.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 156.Anekonda T.S., Quinn J.F., Harris C., Frahler K., Wadsworth T.L., Woltjer R.L. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 2011;41:62–70. doi: 10.1016/j.nbd.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li N., Liu B., Dluzen D.E., Jin Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007;111:458–463. doi: 10.1016/j.jep.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 158.Paris D., Bachmeier C., Patel N., Quadros A., Volmar C.H., Laporte V., Ganey J., Beaulieu-Abdelahad D., Ait-Ghezala G., Crawford F., Mullan M.J. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood–brain barrier. Mol. Med. 2011;17:149–162. doi: 10.2119/molmed.2010.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Copenhaver P.F., Anekonda T.S., Musashe D., Robinson K.M., Ramaker J.M., Swanson T.L., Wadsworth T.L., Kretzschmar D., Woltjer R.L., Quinn J.F. A translational continuum of model systems for evaluating treatment strategies in Alzheimer’s disease: isradipine as a candidate drug. Dis. Model. Mech. 2011;4:634–648. doi: 10.1242/dmm.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lopez-Arrieta J.M., Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst. Rev. 2002:CD000147. doi: 10.1002/14651858.CD000147. [DOI] [PubMed] [Google Scholar]
- 161.Fan R., Xu F., Previti M.L., Davis J., Grande A.M., Robinson J.K., Van Nostrand W.E. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J. Neurosci. 2007;27:3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garcia-Alloza M., Prada C., Lattarulo C., Fine S., Borrelli L.A., Betensky R., Greenberg S.M., Frosch M.P., Bacskai B.J. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. J. Neurochem. 2009;109:1636–1647. doi: 10.1111/j.1471-4159.2009.06096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Noble W., Garwood C.J., Hanger D.P. Minocycline as a potential therapeutic agent in neurodegenerative disorders characterised by protein misfolding. Prion. 2009;3:78–83. doi: 10.4161/pri.3.2.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parachikova A., Vasilevko V., Cribbs D.H., LaFerla F.M., Green K.N. Reductions in amyloid-β-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. J. Alzheimers Dis. 2010;21:527–542. doi: 10.3233/JAD-2010-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cai Z.Y., Zhao Y., Yao S.T., Zhao B. Increases in β-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-κB pathway activation. Pharmacol. Rep. 2011;63:381–391. doi: 10.1016/s1734-1140(11)70504-7. [DOI] [PubMed] [Google Scholar]
- 166.Balducci C., Santamaria G., La Vitola P., Brandi E. Doxycycline counteracts neuroinflammation restoring memory in Alzheimer’s disease mouse models. Neurobiol. Aging. 2018;70:128–139. doi: 10.1016/j.neurobiolaging.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 167.Goodman A.B., Pardee A.B. Evidence for defective retinoid transport and function in late onset Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2901–2905. doi: 10.1073/pnas.0437937100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Corcoran J.P.T., So P.L., Maden M. Disruption of the retinoid signalling pathway causes a deposition of amyloid β in the adult rat brain. Eur. J. Neurosci. 2004;20:896–902. doi: 10.1111/j.1460-9568.2004.03563.x. [DOI] [PubMed] [Google Scholar]
- 169.Husson M., Enderlin V., Delacourte A., Ghenimi N., Alfos S., Pallet V., Higueret P. Retinoic acid normalizes nuclear receptor mediated hypo-expression of proteins involved in β-amyloid deposits in the cerebral cortex of vitamin A deprived rats. Neurobiol. Dis. 2006;23:1–10. doi: 10.1016/j.nbd.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 170.Cramer P.E., Cirrito J.R., Wesson D.W., Lee C.Y., Karlo J.C., Zinn A.E., Casali B.T., Restivo J.L., Goebel W.D., James M.J., Brunden K.R., Wilson D.A., Landreth G.E. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Castellano J.M., Kim J., Stewart F.R., Jiang H., DeMattos R.B., Patterson B.W., Fagan A.M., Morris J.C., Mawuenyega K.G., Cruchaga C., Goate A.M., Bales K.R., Paul S.M., Bateman R.J., Holtzman D.M. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002156. 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Melino G., Draoui M., Bernardini S., Bellincampi L., Reichert U., Cohen P. Regulation by retinoic acid of insulin degrading enzyme and of a related endoprotease in human neuroblastoma cell lines. Cell Growth Differ. 1996;7:787–796. [PubMed] [Google Scholar]
- 173.So P.L., Yip P.K., Bunting S., Wong L.F., Mazarakis N.D., Hall S., McMahon S., Maden M., Corcoran J.P. Interactions between retinoic acid, nerve growth factor and sonic hedgehog signalling pathways in neurite outgrowth. Dev. Biol. 2006;298:167–175. doi: 10.1016/j.ydbio.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 174.Goncalves M.B., Agudo M., Connor S., McMahon S., Minger S.L., Maden M., Corcoran J.P. Sequential RARβ and α signalling in vivo can induce adult forebrain neural progenitor cells to differentiate into neurons through Shh and FGF signalling pathways. Dev. Biol. 2009;326:305–313. doi: 10.1016/j.ydbio.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 175.Lee H.P., Casadesus G., Zhu X., Lee H.G., Perry G., Smith M.A., Gustaw-Rothenberg K., Lerner A. All-trans retinoic acid as a novel therapeutic strategy for Alzheimer’s disease. Expert Rev. Neurother. 2009;9:1615–1621. doi: 10.1586/ern.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shudo K., Fukasawa H., Nakagomi M., Yamagata N. Towards retinoid therapy for Alzheimer’s disease. Curr. Alzheimer Res. 2009;6:302–311. doi: 10.2174/156720509788486581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eisenhardt E.U., Bickel M.H. Kinetics of tissue distribution and elimination of retinoid drugs in the rat. I. Acitretin. Drug Metab. Dispos. 1994;22:26–30. [PubMed] [Google Scholar]
- 178.Bortolanza M., Nascimento G.C., Socias S.B., Ploper D., Chehín R.N., Raisman-Vozari R., Del-Bel E. Tetracycline repurposing in neurodegeneration: focus on Parkinson’s disease. J. Neural. Transm. 2018;125:1403–1415. doi: 10.1007/s00702-018-1913-1. [DOI] [PubMed] [Google Scholar]
- 179.Sinnegger-Brauns M.J., Huber I.G., Koschak A., Wild C., Obermair G.J., Einzinger U., Hoda J.C., Sartori S.B., Striessnig J. Expression and 1,4-dihydropyridine binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol. 2009;75 doi: 10.1124/mol.108.049981. 407-144. [DOI] [PubMed] [Google Scholar]
- 180.Chan C.S., Guzman J.N., Ilijic E., Mercer J.N., Rick C., Tkatch T., Meredith G.E., Surmeier D.J. Rejuvenation protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 181.Ilijic E., Guzman J.N., Surmeier D.J. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2011;43:364–371. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Simuni T., Borushko E., Avram M.J., Miskevics S., Martel A., Zadikoff C., Videnovic A., Weaver F.M., Williams K., Surmeier D.J. Tolerability of isradipine in early Parkinson’s disease: a pilot dose escalation study. Mov. Disord. 2010;25:2863–2866. doi: 10.1002/mds.23308. [DOI] [PubMed] [Google Scholar]
- 183.Parkinson Study Group Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD) Mov. Disord. 2013;28:1823–1831. doi: 10.1002/mds.25639. [DOI] [PubMed] [Google Scholar]
- 184.https://content.iospress.com/articles/journal-of-parkinsons-disease/jpd190002
- 185.Carroll C.B., Wyse R.K.H. Simvastatin as a potential disease modifying therapy for patients with Parkinson’s disease: rationale for clinical trial, and current progress. J. Parkinsons Dis. 2017;7:545–568. doi: 10.3233/JPD-171203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roy A., Pahan K. Prospects of statins in Parkinson disease. Neuroscience. 2011;17:244–255. doi: 10.1177/1073858410385006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tong H., Zhang X., Meng X., Lu L., Mai D., Qu S. Simvastatin inhibits activation of NADPH oxidase/p38 MAPK pathway and enhances expression of antioxidant protein in Parkinson disease models. Front. Mol. Neurosci. 2018;11:165–181. doi: 10.3389/fnmol.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yan J., Sun J., Huang L., Fu Q., Du G. Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J. Neurosci. Res. 2014;92:634–640. doi: 10.1002/jnr.23329. [DOI] [PubMed] [Google Scholar]
- 189.Kumar A., Sharma N., Gupta A., Kalonia H., Mishra J. Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res. 2012;1471:13–22. doi: 10.1016/j.brainres.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 190.Ghosh A., Roy A., Matras J., Brahmachari S., Gendelman H.E., Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neurosci. 2009;29:13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Selley M.L. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005;1037:1–6. doi: 10.1016/j.brainres.2004.02.083. [DOI] [PubMed] [Google Scholar]
- 192.Bykov K., Yoshida K., Weisskopf M.G., Gagne J.J. Confounding of the association between statins and Parkinson disease: systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2017;26:294–300. doi: 10.1002/pds.4079. [DOI] [PubMed] [Google Scholar]
- 193.Imam S.Z., Zhou Q., Yamamoto A., Valente A.J., Ali S.F., Bains M., Roberts J.L., Kahle P.J., Clark R.A., Li S. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson’s disease. J. Neurosci. 2011;31:157–163. doi: 10.1523/JNEUROSCI.1833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ko H.S., Lee Y., Shin J.H., Karuppagounder S.S., Gadad B.S., Koleske A.J., Pletnikova O., Troncoso J.C., Dawson V.L., Dawson T.M. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107:16691–16696. doi: 10.1073/pnas.1006083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hebron M.L., Lonskaya I., Moussa C.E.H. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of -synuclein in Parkinson’s disease models. Hum. Mol. Genet. 2013;22:3315–3328. doi: 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hantschel O., Superti-Furga G. Regulation of the c-Abl and BcrAbl tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 197.Tanabe A., Yamamura Y., Kasahara J., Morigaki R., Kaji R., Goto S. A novel tyrosine kinase inhibitor AMN107 (nilotinib) normalizes striatal motor behaviors in a mouse model of Parkinson’s disease. Front. Cell. Neurosci. 2014;8:50. doi: 10.3389/fncel.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Karuppagounder S.S., Brahmachari S., Lee Y., Dawson V.L., Dawson T.M., Ko H.S. The c-Abl inhibitor, Nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease. Sci. Rep. 2015;4:4874. doi: 10.1038/srep04874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pagan F., Hebron M., Valadez E.H., Torres-Yaghi Y., Huang X., Mills R.R., Wilmarth B.M., Howard H., Dunn C., Carlson A., Lawler A., Rogers S.L., Falconer R.A., Ahn J., Li Z., Moussa C. vol. 6. IOS Press; 2016. pp. 503–517. (Nilotinib Effects in Parkinson’s Disease and Dementia with Lewy Bodies, J. Parkinsons Dis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wyse R.K., Brundin P., Sherer T.B. Nilotinib-differentiating the hope from the hype. J. Parkinsons Dis. 2016;6:519–522. doi: 10.3233/JPD-160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bourque M., Morissette M., Di Paolo T. Repurposing sex steroids and related drugs as potential treatment for Parkinson’s disease. Neuropharmacology. 2019;147:37–54. doi: 10.1016/j.neuropharm.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 202.Parvathaneni V., Kulkarni N.S., Muth A., Gupta V. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov. Today. 2019;24:2076–2085. doi: 10.1016/j.drudis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Polamreddy P., Gattu N. The drug repurposing landscape from 2012 to 2017: evolution, challenges, and possible solutions. Drug Discov. Today. 2019;24:789–795. doi: 10.1016/j.drudis.2018.11.022. [DOI] [PubMed] [Google Scholar]