Abstract
Introduction:
Carpal tunnel syndrome is one of the most common peripheral neuropathies. Only a few studies evaluate the efficacy of “nutraceuticals” on peripheral nerves and neuropathic pain. The aim of the present investigation is to evaluate the role of Alfa-Lipoic Acid-R (ALA-R) on clinical and functional outcomes in patients affected by mild to moderate carpal tunnel syndrome.
Material and Methods:
The present investigation is a prospective randomised controlled open label study, performed at our Hand Surgery Department (Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome) from October 2018 to March 2019. The enrolled patients were divided in two groups: Group A (ALA-R 600mg once day for 60 days) and Group B (control Group, no drug administration).
Results:
134 patients (74 F, 60 M) met the inclusion and exclusion criteria. In Group A, there was a statistically significant pain reduction compared to the control Group. Using the Boston Carpal Tunnel Questionnaire, there were no significant improvements in the other symptoms and function.
Conclusion:
ALA-R full dose administration for two months leads to positive short term results in terms of symptoms and function improvement, even if the surgical carpal tunnel release remains the treatment of choice.
Keywords: carpal tunnel syndrome, alpha-lipoic acid, ALA-R, neuroprotection, median nerve compression
Introduction
Carpal Tunnel Syndrome (CTS) is one of the most frequent and disabling peripheral nerve compression syndromes1, 2. Incidence of CTS is increasing3, and the request for treatment is increasing as well4, 5.
The increased pressure into the carpal tunnel reduces the blood flow to median nerve6, and many risk factors and comorbidities can contribute to it7. Chronic endoneural ischemia increases the local oxidative stress, resulting in degenerative changes in the nerve8. Even if the surgical carpal tunnel release can definitely reduce symptoms9, many non-surgical treatments have been proposed10–12, to address the growing request for further treatments.
Alfa-Lipoic Acid (ALA) is a well-known molecule, and its biochemical and metabolic features have been described in oxidative stress models like diabetes13, 14. The effect of ALA in CTS has been investigated15–18.
Synthetic ALA is produced as a racemic mix15, and the more active enantiomer is the Alfa-Lipoic Acid-R (ALA-R), the dextrorotatory one19. No study has investigated before the effects of ALA-R on CTS at the maximum dosage recommended (600mg per day). The aim of the present study was to investigate prospectively the role of high dosage ALA-R therapy on clinical and functional outcomes in patient affected by mild-moderate carpal tunnel syndrome, who have to undergo surgical treatment.
Materials and Methods
The present investigation is a one-centre prospective randomised controlled open label prospective study, performed at our Hand Surgery Department, Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome, from October 2018 to March 2019.
One hundred and third-four patients who were listed for open surgical carpal tunnel release, were enrolled for this study. Written informed consent, was taken from all patients before the enrolment in the study. All patients enrolled were informed that the surgical carpal tunnel release, was the necessary treatment for CTS, and that a pharmacological therapy could not replace the beneficial effects of a surgical operation to date.
Inclusion and exclusion criteria are shown in Table I. All patients who are older than 18 years old, with an electromyographically confirmed diagnosis of mild-moderate mono or bilateral CTS, according with classification of Padua et al20, with typical CTS symptoms (positive Phalen maneuvers and Tinel sign and paraesthesia in the median nerve region) were eligible for the study. The results were assessed only on the dominant hand.
Table I.
Inclusion/exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Patients who suffered from diabetes, neuromuscular disease, hepatic impairment (MELD Score > 9), moderate to severe renal impairment (creatinine clearance < 90ml/min), known psychiatric disorders, allergy or contraindication to the study drugs were excluded from the study. Pregnant and breastfeeding women were excluded too. Patients with history of trauma or previous surgery in their dominant hand side were excluded. Patients who reported concomitant use of other neuroprotective drugs or nutraceutical substances during follow-up visit were excluded from the study.
The study was in accordance with the national ethics criteria. The study was also in accordance with the Helsinki convention and Good Clinical Practice. Considering that the administration of ALA-R was already used in the preoperative management protocols at our institute, a formal ethical approval was not requested for this study.
After the enrolment in the study, patients were randomly assigned to the two groups studied with a 1:1 allocation ratio. Randomisation process was performed in blocks of 10. The randomisation scheme was generated by using the Web site Randomization.com (http://www.randomization.com) (Fig. 1).
Fig. 1:
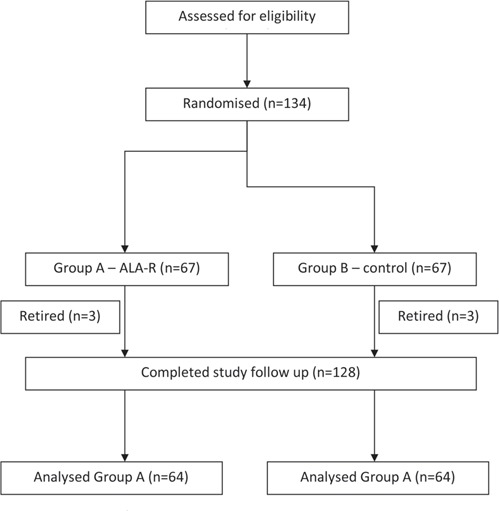
Study flow chart.
The enrolled patients were divided into two groups: Group A (Alfa Lipoic Acid-R (ALA-R) 600mg once day for 60 days) and Group B (control group, no drug administration).
During the first visit, the informed consent, demographic data (age, sex, BMI), medical history, and electromyographic data were collected. Pain was evaluated using the visual analogue scale (VAS). The patient symptoms and the functionality of the affected hand were evaluated using the Boston Carpal Tunnel Questionnaire (BCTQ).
During the second visit, two months after the enrolment, pain and functionality were evaluated again. Patients were asked about the two items: pain and functionality, both day and night.
The aim of the study was to evaluate the effect of ALA-R in the management of patients with mild-moderate carpal tunnel syndrome. The primary outcome was the evaluation of patients’ symptoms and functionality using the BCTQ. Secondary outcomes were: pain reduction measured with the VAS (both day and night), side effects, and the general satisfaction.
The target sample size was 120, with 60 patients in each group, considering an effect of 0.7 unit reduction in BCTQ value, with 80% power and 5% probability of type 1 error and assuming a standard deviation of 1.50 for this parameter. Considering a 10% possible dropout rate, 67 patients per group or 134 patients overall were recruited.
The BCTQ scores were ordinal variables used as continuous variables according to other authors21. Non-parametric tests were used. Wilcoxon signed rank test (paired analysis) was used to compare the variation of pain and BCTQ values between t0 and t1. Mann-Whitney U test was used to compare the final assessment results between group A and B (independent samples).
The significance was established for a value of p<0.05. Dedicated SPSS (version 20.0.0) statistical calculation software (SPSS Inc, Chicago, IL) was employed. Data were described using means and standard deviations for quantitative variables and numbers and percentages for qualitative variable. Only one decimal digit was reported and was rounded up.
Results
One hundred and thirty-fourth patients (74 F, 60 M) who met the inclusion and exclusion criteria were enrolled in the study. Ninety (67.2%) patients were right-handers. Group A included 67 patients (38 F, 29 M); Group B (control group) included 67 (37 F, 30 M) patients who had not received pharmacological treatments.
The mean age in Group A was 66 years old (+/-10.5), in Group B was 69 years old (+/-11.3). The mean BMI was 26.5 (+/-2.9) in Group A, 28.0 (+/-3.9) in Group B. In Group A 18 (26,9%) patients were smokers, in Group B 17 (25.4%). A summary of the characteristics of the patients enrolled in the study is reported in Table II.
Table II.
Clinical and demographic features
| Demographics | Group A | Group B |
|---|---|---|
| Number of patients | 67 | 67 |
| Gender | 38 F, 29 M | 37 F, 30 M |
| Age (years) | 66.1 (+/-10,5) | 69.0 (+/-11.3) |
| BMI | 26.5 (+/-2,9) | 28.0 (+/-3,9) |
| Symptom duration (months) | 18.4 +/- 5,0 | 20.0 +/- 4,5 |
| Smokers | 18 (26,9%) | 17 (25,4%) |
| History of chronic alcohol consumption | 3 (4,5%) | 2 (2,9%) |
| Comorbidities with impact on peripheral nervous system* | 10 (14.9%) | 11 (16.4%) |
| Other comorbidities** | 22 (32,8%) | 22 (32,8%) |
*cervical spine disease, rheumatoid arthritis
**hypercholesterolemia, hypertension, glaucoma, history of cardiac infarction
Three patients (4.5%) in Group A did not complete the therapy cycle because of side effects (one patient reported headache, two reported nausea) and they were excluded from the study. There were no patients lost at follow-up in Group A. Three patients were lost (4.5%) in Group B. Concerning BTCQ, after two months, scores improved from 3.5 (+/-1.3) to 3.0 (+/-1.0) (p=0.072) in Group A. In Group B did not change significantly, from 3.8 (+/-1.4) to 3.9 (+/-1.5) (p=0.270). Comparing the final assessment results between group A and B (t1), Mann Whitney U-test showed no statistically significant difference (p=0.194) (Table III).
Table III.
The results. Data represent the mean +/- SD
| ALA-R (Group A) | Control (Group B) | t1 | |||||
|---|---|---|---|---|---|---|---|
| t0 | t1 | p value | t0 | t1 | p value | (Group A vs Group B) | |
| BTCQ score | 3.5 (+/-1.3) | 3.0 (+/-1.0) | 0.072 | 3.8 (+/-1.4) | 3.9 (+/-1.5) | 0.270 | 0.194 |
| Pain night (VAS score) | 6.0 (+/- 1.5) | 2.9 (+/-1.3) | <0.0001 | 6.3 (+/- 0.8) | 6.5 (+/-1.3) | 0.232 | <0.0001 |
| Pain day (VAS score) | 5.3 (+/- 1.4) | 1.9 (+/-1.3) | <0.0001 | 6.5 (+/- 1.2) | 6.6 (+/-1.3) | 0.200 | <0.0001 |
Pain at night; in Group A the mean VAS score at t0 was 6.0 (+/- 1.5), decreased to 2.9 (+/-1.3) at two months, (p<0.0001). In Group B the mean VAS score at t0 was 6.3 (+/- 0.8), and increased to 6.5 (+/-1.3) at two months (p=0.232). Comparing the final assessment results between group A and B (t1), Mann Whitney U-test showed statistically significant differences (p<0.0001) (Table III).
Regarding pain during the day, in Group A the mean VAS score at t0 was 5.3 (+/- 1.4), decreased to 1.9 (+/-1.3) at two months, (p<0.0001). In Group B the mean VAS score at t0 was 6.5 (+/- 1.2), and increased to 6.6 (+/-1.3) at two months (p=0.200). Comparing the final assessment results between group A and B (t1), Mann Whitney U-test showed statistically significant differences (p<0.0001) (Table III).
After two months (the last follow-up), at the question “how are you with your disease (CTS)?”, in Group A, 9 patients (14.0% of the patients who completed the follow-up) declared that they do not need further treatment at the moment, because of the satisfying relief of symptoms. In Group B all patients asked to be treated.
Discussion
Other studies had already investigated the efficacy of nonsurgical treatment of CTS, sometimes with promising short term results22, 23. However, even if the results supporting the conservative treatment were not straightforward24, conservative treatment could be effective as well, and is therefore recommended for patients with very low grade CTS17, 25. The efficacy of ALA in CTS has been already investigated. Pajardi et al prospectively investigated the efficacy of a mix of natural substances including ALA administered from three months before surgery for CTS, followed up to three months after surgery. They observed that taking therapy both before and after surgery improved nocturnal symptoms and Phalen test result. Differently from what we did, they evaluated patients only after surgery. Furthermore, they did not use exclusively ALA-R, and they administered ALA 300mg twice a day. Hence, the positive results they obtained came from different factors15.
Similarly, another more recent prospective study had investigated the effect of ALA before and after surgery in CTS, they only assessed post-surgical improvement18. Luchetti et al reported short term results (two months follow-up) from an observational Italian multicentric study about conservative treatment of CTS. They studied the effects of conservative treatment in patients with a CTS diagnosis (from low to extremely severe). At the end of follow-up, less than 50% of patients were scheduled for surgery. Their results suggested a use of a conservative therapy based on neurotrophic agents, especially ALA, in order to control symptoms (both nocturnal and diurnal pain) and improve function17. Other studies demonstrated the efficacy of a combination of ALA and gamma-linolenic acid in up to severe CTS16, 18. All the above mentioned studies15-18 investigated specifically ALA use in CTS, but they did not distinguish between the ALA-R and racemic preparations.
Conversely, we considered this distinction to be important. Only the ALA-R exists in nature and it works as a cofactor in many chemical reactions19. Currently, ALA-R is considered the eutomer26, and different preparations of the drug have been described27, 28. Both enantiomers are equally absorbed in proportion to the dose administered from 50 to 600mg (Time of Maximum Concentration: from 30 to 60 minutes)29, but they are poorly bioavailable (less than 30%)30. Food intake further decrease bioavailability31. In view of all this, evidence to date indicates how to optimise the use of ALA-R14, and it should not be overlooked.
Concerning our study, although the BCTQ score (primary outcome) was reduced in Group A compared to the control group, the difference between the two groups was not statistically significant. Furthermore, there was no functional improvement comparing t0 and t1 in each group. However, our data show that the use of ALA-R had a favorable impact on pain, both night and day.
Therefore the administration of ALA-R full dose could be effective in reduction of pain in patients who are waiting for surgical treatment. None had prospectively evaluated before how a full “one-shot” dose of the eutomer (ALA-R) could impact in terms of symptoms improvement in CTS. To the best of our knowledge, the biochemical activity of ALA could not stop the process of nerve degeneration of the progressing disease14, 15, 32. Similarly, evidence to date suggested that conservative treatment alone did not result in “complete recovery”24. In our institution, the waiting list for surgery could be more than 10 months long, therefore the patients could benefit from the administration of the ALA-R pending surgery. Furthermore, patients who could not undergo surgery (because of major health problems) or who had to postpone surgery, or who did not undergo surgery for personal convictions could benefit from our non-surgical treatment.
This study had the limitation of a small follow-up. It was possible that patients could relapse with symptoms again after treatment. Nevertheless it was equally possible that a second period of treatment could relieve symptoms again. Further studies should be done. Longer follow-up should be considered to evaluate the maintenance of beneficial effects, and to assess the possibility to repeat the drug therapy.
Conclusion
ALA-R is a safe drug. No major side effects were observed in our study. Compliance results were positive, probably because therapy was simply taken once a day. ALA-R full dose administration for two months led to positive short term results in terms of pain improvement. Even if the surgical carpal tunnel release is the treatment of choice, all patients with a clear diagnosis of CTS should be treated with ALA-R if not contraindicated. Patients should be correctly informed about the uncertain and non-durable outcome of this treatment.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Gelfman R, Melton LJ 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72(1):33–41. doi: 10.1212/01.wnl.0000338533.88960.b9. doi:10.1212/01.wnl.0000338533.88960.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalimar A, Nor-Hazla MH, Arifaizad A, Jamari S. Splinting after Carpal Tunnel Release: Does it really Matter?. Malaysian Orthop J. 2015;9(2):41–6. doi: 10.5704/MOJ.1507.011. doi:10.5704/MOJ.1507.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atroshi I, Englund M, Turkiewicz A, Tagil M, Petersson IF. Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Arch Intern Med. 2011;171(10):943–4. doi: 10.1001/archinternmed.2011.203. doi:10.1001/archinternmed.2011.203. [DOI] [PubMed] [Google Scholar]
- 4.Pourmemari MH, Heliovaara M, Viikari-Juntura E, Shiri R. Carpal tunnel release: Lifetime prevalence, annual incidence, and risk factors. Muscle Nerve. 2018;58(4):497–502. doi: 10.1002/mus.26145. doi:10.1002/mus.26145. [DOI] [PubMed] [Google Scholar]
- 5.Tadjerbashi K, Akesson A, Atroshi I. Incidence of referred carpal tunnel syndrome and carpal tunnel release surgery in the general population: Increase over time and regional variations. J Orthop Surg (Hong Kong). 2019;27(1):2309499019825572. doi: 10.1177/2309499019825572. doi:10.1177/2309499019825572. [DOI] [PubMed] [Google Scholar]
- 6.Wahab KW, Sanya EO, Adebayo PB, Babalola MO, Ibraheem HG. Carpal Tunnel Syndrome and Other Entrapment Neuropathies. Oman Med J. 2017;32(6):449–54. doi: 10.5001/omj.2017.87. doi:10.5001/omj.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharief F, Kanmani J, Kumar S. Risk factors, symptom severity and functional status among patients with carpel tunnel syndrome. Neurol India. 2018;66(3):743–6. doi: 10.4103/0028-3886.232351. doi:10.4103/0028-3886.232351. [DOI] [PubMed] [Google Scholar]
- 8.Werner RA, Andary M. Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol. 2002;113(9):1373–81. doi: 10.1016/s1388-2457(02)00169-4. [DOI] [PubMed] [Google Scholar]
- 9.Verdugo RJ, Salinas RA, Castillo JL, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane database Syst Rev. 2008;(4):CD001552. doi: 10.1002/14651858.CD001552.pub2. doi:10.1002/14651858.CD001552.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MJ, O’Connor D, Pitt V, Massy-Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane database Syst Rev. 2012;(6):CD009899. doi: 10.1002/14651858.CD009899. doi:10.1002/14651858.CD009899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, Massy-Westropp N, O’Connor D, Pitt V. Splinting for carpal tunnel syndrome. Cochrane database Syst Rev. 2012;(7):CD010003. doi: 10.1002/14651858.CD010003. doi:10.1002/14651858.CD010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, O’Connor D, Pitt V, Massy-Westropp N. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane database Syst Rev. 2013;(3):CD009601. doi: 10.1002/14651858.CD009601.pub2. doi:10.1002/14651858.CD009601.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochette L, Ghibu S, Muresan A, Vergely C. Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can J Physiol Pharmacol. 2015;93(12):1021–7. doi: 10.1139/cjpp-2014-0353. doi:10.1139/cjpp-2014-0353. [DOI] [PubMed] [Google Scholar]
- 14.Mrakic-Sposta S, Vezzoli A, Maderna L, Gregorini F, Montorsi M, Moretti S et al. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxid Med Cell Longev. 2018;2018:1767265. doi: 10.1155/2018/1767265. doi:10.1155/2018/1767265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pajardi G, Bortot P, Ponti V, Novelli C. Clinical usefulness of oral supplementation with alpha-lipoic Acid, curcumin phytosome, and B-group vitamins in patients with carpal tunnel syndrome undergoing surgical treatment. Evid Based Complement Alternat Med. 2014;2014:891310. doi: 10.1155/2014/891310. doi:10.1155/2014/891310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Geronimo G, Caccese AF, Caruso L, Soldati A, Passaretti U. Treatment of carpal tunnel syndrome with alpha-lipoic acid. Eur Rev Med Pharmacol Sci. 2009;13(2):133–9. [PubMed] [Google Scholar]
- 17.Luchetti R, Tognon S, Cacciavillani M, Ronco S, Buzzelli N, Lanni G. Observational multicentric survey on carpal tunnel syndrome: demographic and clinical data from 34 Italian centers. Eur Rev Med Pharmacol Sci. 2017;21(3):460–9. [PubMed] [Google Scholar]
- 18.Monroy-Guizar EA, Garcia-Benavides L, Ambriz-Plascencia AR, Pascoe-Gonzalez S, Totsuka-Sutto SE, Cardona-Munoz EG et al. Effect of Alpha-Lipoic Acid on Clinical and Neurophysiologic Recovery of Carpal Tunnel Syndrome: A Double-Blind, Randomized Clinical Trial. J Med Food. 2018;21(5):521–6. doi: 10.1089/jmf.2017.0056. doi:10.1089/jmf.2017.0056. [DOI] [PubMed] [Google Scholar]
- 19.Raddatz G, Bisswanger H. Receptor site and stereospecifity of dihydrolipoamide dehydrogenase for R- and S-lipoamide: a molecular modeling study. J Biotechnol. 1997;58(2):89–100. doi: 10.1016/s0168-1656(97)00135-1. [DOI] [PubMed] [Google Scholar]
- 20.Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997;96(4):211–7. doi: 10.1111/j.1600-0404.1997.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 21.Boriani F, Granchi D, Roatti G, Merlini L, Sabattini T, Baldini N. Alpha-lipoic Acid After Median Nerve Decompression at the Carpal Tunnel: A Randomized Controlled Trial. J Hand Surg Am. 2017;42(4):236–42. doi: 10.1016/j.jhsa.2017.01.011. doi:10.1016/j.jhsa.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Chen PC, Chuang CH, Tu YK, Bai CH, Chen CF, Liaw MY. A Bayesian network meta-analysis: Comparing the clinical effectiveness of local corticosteroid injections using different treatment strategies for carpal tunnel syndrome. BMC Musculoskelet Disord. 2015;16:363. doi: 10.1186/s12891-015-0815-8. doi:10.1186/s12891-015-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;(1):CD003219. doi: 10.1002/14651858.CD003219. doi:10.1002/14651858.CD003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton CL, Chesterton LS, Chen Y, van der Windt DA. Clinical Course and Prognostic Factors in Conservatively Managed Carpal Tunnel Syndrome: A Systematic Review. Arch Phys Med Rehabil. 2016;97(5):836–52.e1. doi: 10.1016/j.apmr.2015.09.013. doi:10.1016/j.apmr.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Huisstede BM, Friden J, Coert JH, Hoogvliet P. Carpal tunnel syndrome: hand surgeons, hand therapists, and physical medicine and rehabilitation physicians agree on a multidisciplinary treatment guideline-results from the European HANDGUIDE Study. Arch Phys Med Rehabil. 2014;95(12):2253–63. doi: 10.1016/j.apmr.2014.06.022. doi:10.1016/j.apmr.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17(10):888–95. doi: 10.1016/s0899-9007(01)00658-x. [DOI] [PubMed] [Google Scholar]
- 27.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12(4):343–51. [PubMed] [Google Scholar]
- 28.Amenta F, Traini E, Tomassoni D, Mignini F. Pharmacokinetics of different formulations of tioctic (alpha-lipoic) acid in healthy volunteers. Clin Exp Hypertens. 2008;30(8):767–75. doi: 10.1080/10641960802563568. doi:10.1080/10641960802563568. [DOI] [PubMed] [Google Scholar]
- 29.Breithaupt-Grogler K, Niebch G, Schneider E, Erb K, Hermann R, Blume HH et al. Dose-proportionality of oral thioctic acid-coincidence of assessments via pooled plasma and individual data. Eur J Pharm Sci. 1999;8(1):57–65. doi: 10.1016/s0928-0987(98)00061-x. [DOI] [PubMed] [Google Scholar]
- 30.Teichert J, Kern J, Tritschler HJ, Ulrich H, Preiss R. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36(12):625–8. [PubMed] [Google Scholar]
- 31.Gleiter CH, Schug BS, Hermann R, Elze M, Blume HH, Gundert-Remy U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur J Clin Pharmacol. 1996;50(6):513–514. doi: 10.1007/s002280050151. [DOI] [PubMed] [Google Scholar]
- 32.Keir PJ, Rempel DM. Pathomechanics of peripheral nerve loading. Evidence in carpal tunnel syndrome. J Hand Ther. 2005;18(2):259–269. doi: 10.1197/j.jht.2005.02.001. doi:10.1197/j.jht.2005.02.001. [DOI] [PubMed] [Google Scholar]


