Abstract
Aim.
Sexual dimorphisms are evident along the nephron: Females (F) exhibit higher ratios of renal distal to proximal Na+ transporters’ abundance, greater lithium clearance (CLi) more rapid natriuresis in response to saline infusion and lower plasma [K+] vs. males (M). During angiotensin II infusion hypertension (AngII-HTN) M exhibit distal Na+ transporter activation, lower proximal and medullary loop transporters, blunted natriuresis in response to saline load, and reduced plasma [K+]. This study aimed to determine whether responses of F to AngII-HTN mimicked those in M or were impacted by sexual dimorphisms evident at baseline.
Methods.
Sprague-Dawley rats and C57BL/6 mice were AngII infused via osmotic minipumps 2 and 3 wks, respectively, and assessed by metabolic cage collections, tail-cuff sphygmomanometer, semi-quantitative immunoblotting of kidney, and patch-clamp electrophysiology.
Results.
In F rats, AngII-infusion increased BP to 190 mmHg, increased phosphorylation of cortical NKCC2, NCC and cleavage of ENaC 2-3-fold, increased ENaC channel activity 3-fold and aldosterone 10-fold. K+ excretion increased and plasma [K+] decreased. Evidence of natriuresis in F included increased urine Na+ excretion and CLi, and decreased medullary NHE3, NKCC2 and Na,K-ATPase abundance. In C57BL/6 mice, AngII-HTN increased abundance of distal Na+ transporters, suppressed proximal-medullary transporters, and reduced plasma [K+] in both F and M.
Conclusion.
Despite baseline sexual dimorphisms, AngII-HTN provokes similar increases in BP, aldosterone, distal transporters, ENaC channel activation and K+ loss accompanied by similar suppression of proximal and loop Na+ transporters, natriuresis, and diuresis in females and males.
Keywords: angiotensin II, ENaC, female, potassium, proteinuria, sodium transport
INTRODUCTION
Differences between female and male nephron organization and physiological function are now quite apparent.1 Our recent exploration in kidneys from both sexes examined how different sections interact functionally in relation to their transporters and channels.2 The findings revealed significantly lower Na+ and water transporters’ abundance and activity along the proximal nephron associated with more abundant and activated transporters and channels along the distal nephron of female compared to male rats.3 Specifically, females exhibited greater phosphorylation of Na+/H+ exchanger isoform 3 (NHE3) and less bicarbonate reabsorption (both markers of less NHE3 activity), lower abundance of Na+/Pi cotransporter 2 (NaPi2), claudin-2 (cldn-2), and aquaporin 1, and higher lithium clearance (CLi), a measure of volume flow from the proximal nephron.4 Distally, females expressed higher abundance of Na+/Cl− cotransporter (NCC) both total and phosphorylated (activated) forms, claudin-7 (cldn-7), and the cleaved (activated) forms of epithelial Na+ channel (ENaC) α and γ subunits, associated with lower baseline plasma [K+]. Similar sex differences in transporter patterns were evident in C57BL/6J mice.2 Additionally, female rats excreted a saline load more rapidly, likely due to the lower fractional proximal tubule (PT) Na+ reabsorption, and exhibited lower fasting plasma [K+] than males, likely due to the higher delivery to the distal nephron coupled to the higher ENaC abundance. The lower proximal fractional reabsorption and more rapid excretion of a saline challenge suggest that female rats will maintain salt balance at lower blood pressure (BP) when faced with anti-natriuretic stimuli or injury. In fact, there is evidence for such a “female advantage,”5 for example, the pressure-natriuresis curve is shifted rightward in male vs. female rodents.6
The angiotensin II infusion model of experimental hypertension has been widely utilized in pre-clinical studies because the renin angiotensin system is a key therapeutic target in treating hypertension and AngII-HTN engages all the systems controlling cardiovascular volume and pressure.7 Importantly, the model, especially in rats, exhibits similarities with human hypertension including target organ damage, cardiac hypertrophy, vascular remodeling and chronic kidney disease.7 Na+ transporters’ responses have been studied mainly in male rodents wherein AngII activates distal renal Na+ transporters: phosphorylated Na+-K+-2 Cl− cotransporter isoform 2 (NKCC2p) in cortical thick ascending limb (cTAL), phosphorylated NCC (NCCp) in distal convoluted tubule (DCT) and cleaved epithelial Na+ channel (ENaC) in connecting tubule and collecting duct (CNT/CD). AngII also raises blood pressure associated with reduced abundance and activation of Na+ transporters from PT through medullary TAL (mTAL), presumably to normalize circulating fluid volume.8–11 Mild K+ supplementation during AngII-HTN blunts NCCp accumulation, suggesting this phenotype is secondary to K+ wasting associated with ENaC activation.12–8 The aim of the current study was to determine whether females exhibit the AngII-HTN phenotypes reported for males or whether female phenotypes are distinct, impacted by the lower proximal and higher distal transporters and lower plasma K+ at baseline.
RESULTS
Renal responses to AngII-HTN in female and male SD rats
The effects of 14 days AngII-HTN infusion (400 ng/kg/min) in female SD rats were studied by replicating a protocol used previously in male9, 12 and female13 SD rats. Systolic BP increased ~60 mmHg, similar to previously reports in both sexes,9,12,13 accompanied by a 4-fold increase in overnight urine volume (UV), 2-fold increases in urinary osmole (UosmV), Na+ (UNaV) and K+ (UKV) excretion. Plasma [Na+] fell 10% to 137 ± 2 mM and plasma [K+] fell 25% to 3.5 ± 0.2 mM with no detectable change in plasma osmolality (Posm) (Figure 1a).
Figure 1. Effect of Angiotensin II hypertension in female Sprague Dawley rats.

(a). Physiologic parameters. Rats (Protocol 1) were infused with 400 ng/kg/min AngII for 14 days (FA) or sham treated (FC); n=5/group. Systolic blood pressure (BP) was averaged over days 11-13. At day 13 urine was collected overnight (fasting with water) and terminal blood collected by cardiac puncture. Values represent individual measurements and means ± SEM are shown. UV = urine volume, UosmV = urine osmoles excreted, UNaV = urine Na+ excreted, UKV = urine K+ excreted. Regression of NCCpS71 abundance (from Figure 1b) plotted versus plasma [K+] (from Figure 1a). (b). Abundance of renal transporters determined by semi-quantitative immunoblot in homogenates from renal cortex and medulla (with m prefix). Both 1 and ½ amounts were assessed and averaged (Fig S2). Normalized (FC mean =1) values are means ± SEM. Results indicate 2-3 fold increases in abundance of sodium transporters and their activation from the cTAL to CCD, accompanied by depressed medullary transporters. *= p<0.05, **= p<0.01, ***= p<0.001. For ENaC: FL = full length and Cl = cleaved subunits. Box-Whisker plots of the non-normalized arbitrary values with p-values are summarized in Figure S3. In (a) and (b) the p-values were calculated from unpaired Student’s t-test using Graph Pad. (c). Endogenous lithium clearance and body weight were measured in rats of both sexes from Protocol 2. CLi, , a measure of volume flow from proximal tubule and TAL, was 40% higher in F than M at baseline (ns); AngII infusion increased CLi in both M and F by 50% consistent with suppressed proximal and mTAL Na+ reabsorption. UV in these rats from Protocol 2, collected for 24 hr and corrected for body weight, increased more than 2.5-fold in each sex. In (c) p-values were calculated by one-way ANOVA, corrected for multiple comparisons using Graph Pad.
Do the reported sexual dimorphisms2 affect the transporters’ responses to AngII-HTN? AngII-HTN increased the following post-macula densa transporters 1.7 to 3 fold: cortical TAL NKCC2 and NKCC2p, DCT NCC and NCCp, Cldn7 and collecting duct ENaC α and γ cleaved (activated) subunits (Figure 1b, box-whisker plots provided in Figure S3). The rise in NCCp correlated significantly with the fall in plasma K+ (Figure 1a). In contrast, AngII-HTN decreased mTAL NHE3, NKCC2, and Na,K-ATPase catalytic α1 subunit (mNKA α1) 25-50%. Endogenous CLi, a measure of volume flow from the proximal tubule was measured in rats of both sexes (Protocol 2, Figure 1c) ) in urine collected for 24 hr and plasma collected at the same time (values were corrected for body weights which were not different between control and AngII infused groups of the same sex, Figure 1c). CLi was numerically 40% higher in females than males at baseline (ns, p= 0.10); during AngII-HTN, CLi and UV increased significantly in both sexes by 1.5 fold and > 2.5-fold, respectively.
Abundance of all three ENaC subunits were increased during AngII-HTN in female SD (Figure 1b, Figure. S3), consistent with reports in males.14, 15 Direct assessment of ENaC-mediated current activity with patch-clamp (Figure 2a) shows that channel activity was equivalent in males and females at baseline, and tripled in both sexes during AngII-HTN, correlating with the increased abundance of cleaved ENaC α and γ subunits in females (Figures 1b, S3). Patch-clamp data show that AngII infusion increases both the number of functional ENaC channels on the membrane as well as ENaC open probability in rats of both sexes. Aldosterone synthesis increased 10-fold during AngII-HTN in both males and females (Figure 2b).
Figure 2. ENaC activity increases similarly in male and female Sprague Dawley rats during AngII-HTN.

(a). Male and female SD rats (Protocol 2) after 2-week infusion of vehicle or 400 ng·kg−1·min−1 AngII via osmotic mini pumps (n = 5/group). Representative continuous current traces monitoring ENaC activity in split-opened collecting ducts isolated from each group. Dashed lines indicate channel states: c denotes the closed non-conducting state; oi indicates the respective open state. Patches were held at a test potential of Vh = −Vp = −60 mV. Inward currents are downward. Summary graphs of averaged total ENaC activity (fNPo, left), functional ENaC abundance (fN, middle) and ENaC open probability (Po, right) in vehicle- and AngII-infused males and females. The numbers of individual patch recordings are shown on the respective bars. Ang II infusion significantly elevates fNPo (P=3·10−16), fN (P=2.7·10−13) and Po (P=8·10−9) with no difference between sexes (P>0.05 for all parameters), as verified by two-way ANOVA. (b). Urinary aldosterone excretion, measured by EIA assay in urine collected for 24 hr and corrected for body weight, increased 10-fold in both male and female rats during AngII-HTN; two way ANOVA. (c). Urinary proteins in male and female rats, detected by immunoblot in constant fractions of urine collected for 24 hr both before (Base) and after AngII infusion (AngII) from the same rats. 0.01% and 0.005% were assayed for albumin, angiotensinogen (A’ogen), plasminogen and furin and 0.068% and 0.034% were assayed for prostasin (only 0.01% and 0.068% amounts shown). Control lanes on the right: Ur = AngII-HTN rat urine sample, Cor = renal cortex (20 μg/lane); arrows indicate target location; molecular weights (MW) shown on the left in kD. Urinary proteins were increased during AngII-HTN in 4/5 males and 2/5 females. Prostasin was detected in cortex (see arrow) but not in urine. (d). Correlation of urinary proteins. Including all Base and AngII samples, significant correlations measured between urinary angiotensinogen and albumin, as well as urinary plasminogen and albumin (GraphPad Prism) supporting the idea that urinary excretion reflects increased glomerular filtration of all three proteins.
We investigated whether there were sexual dimorphisms in the excretion of proteins that are markers of loss of the normal glomerular filtration barrier: albumin, angiotensinogen, and plasminogen; or markers of ENaC proteolysis and activation: furin, an intracellular serine protease that can appear in the urine, filtered plasminogen which is processed to plasmin in tubular fluid where it interacts with the tubular serine protease prostasin to activate ENaCγ by cleavage.16 Urine proteins were analyzed by immunoblot in a constant fraction of 24 hr urines collected from male and female rats at baseline and after AngII-HTN (Protocol 2, Figure 2c). At baseline, albumin was below detection limit in both sexes, while angiotensinogen and plasminogen were evident at low levels in males but not females. During AngII-HTN, all three markers increased in 4/5 of the males and 2/5 of the females. Despite variability, both urinary angiotensinogen and plasminogen were significantly correlated with urinary albumin (Figure 2B). Furin was poorly detected at baseline and increased in the same 4/5 males and 2/5 females during AngII-HTN. Prostasin was below the level of detection even with analysis of maximal amount of urine (it was detected in cortical tissue control lane).
Renal responses to AngII-HTN are similar in female and male C57BL/6J mice
Using an established infusion protocol (490 ng/kg/min for 3 weeks),17, 18 female and male C57BL/6J mice responded similarly to AngII-HTN. Body weights differed between sexes but were not altered by AngII infusion (Figure 3a). Systolic BP during AngII-HTN, averaged over the last two measurement days following acclimation, increased similarly in both sexes; variability was higher in females (158±9 mmHg) vs. males (157±2 mmHg) (Figure 3a). Results from paired plasma and urine samples, collected at baseline and on termination day are shown in Figure 3b. Overnight urine provided no evidence for AngII stimulated diuresis (UV) or natriuresis (UNaV) or altered UosmV. Plasma Na+ increased in males not females during AngII-HTN. Plasma [K+] was lower in females than males at baseline and decreased during AngII infusion to 3.7 ± 0.1 mM in males and 3.4 ± 0.1 mM in females without any accompanying adaptation in UKV (Figure 3b). In response to i.p. injection of saline equivalent to 10% of the body weight, the rate of Na+ excretion, expressed as the percentage of the Na+ injected, tended to be lower in males infused with AngII-HTN vs. sham treated controls as reported,17 but AngII-infused females excreted the injected NaCl at a similar rate as in sham-treated controls (Figure. 3c).
Figure 3. Physiological responses to AngII-HTN in male (M) and female (F) C57BL/6 mice.

(a). Body weight: the impact of AngII infusion on body weight was assessed by comparing weights the day of mini-pump implantation to weight during the third week of AngII infusion (prior to saline challenge) and no difference was evident. This timepoint was chosen because mice were fasted (with water) the night before termination and the fasting lowered body weight. Systolic blood pressure was measured for a week at both baseline and during the third week of AngII infusion; values shown were averaged from last two days. Values indicate individual measurements. p values were calculated by paired Student’s t-test using Graph Pad. (b). Urine was collected overnight at baseline (M,F) and again in the same animals after infusion of 490 ng/kg/min (MA, FA) n=5-7/group (insufficient urine was collected for assay in one case). UV = urine volume, UosmV = urine osmoles excreted, UNaV = urine Na+ excreted, UKV = urine K+ excreted. Baseline blood was collected from the submandibular vein under anesthesia, and terminal blood by cardiac puncture. (c). Saline challenge. After 14 days, both sham (M,F) and AngII infused (MA, FA) were challenged with an i.p. bolus of warmed saline equivalent to 10% of their body weight and urine collected in metabolic cages hourly over 4 hrs. Data displayed as accumulated Na+ excreted over time as a fraction of the % injected (means ± SEM).
After 3 wk. AngII-HTN, Na+ transporter abundance tended to be higher along the distal nephron in both sexes of mice (Figure 4), reaching significance in cortical NKCC2p, NCC, and NCCp in both females and males, and Cldn7 in males. While decreases in full-length ENaC γ and increases in cleaved ENaC γ did not reach significance, the ratio of cleaved/full-length within samples, an indicator of activation, was significantly increased (Figure 4b). Na+ transporter abundance tended to be lower along proximal nephron and medullary thick ascending limb in both sexes, reaching significance with NHE3p (males), NaPi2 (females), and medullary (m) transporters: mNKCC2 and mNKA α1 (males and females), mNHE3, mNHE3p and mNKCC2p (males).
Figure 4. Effect of Angiotensin II hypertension in male and female C57BL/6 mice.

Mice were infused with 490 ng/kg/min AngII for 21 days (MA, FA) or sham treated (M, F); n=6/group. Abundance of renal transporters determined by semi-quantitative immunoblot in homogenates from renal cortex and medulla (m prefix). Samples from all 4 groups, run at 1 and ½ amounts, were assessed on the same blot and averaged as in Fig S2) To account for differences in baseline abundance, while also assessing AngII regulation, results are displayed two ways: (a). Immunoblots and mean density values normalized to mean of sham males = 1; (b). Box-Whisker plots of arbitrary values with p-values assessing impact of sex and AngII. For ENaC, FL = full length and Cl = cleaved subunits. p values calculated by one-way ANOVA with correction for multiple comparisons.
Urinary albumin, assessed in Coomassie stained gels and by EIA, was detected at low levels at baseline in all mice, and increased in 3/6 males and 2/6 females during AngII-HTN (Figure S4).
DISCUSSION
The aim of the current study was to determine the impact of AngII-HTN on Na+ transporters’ regulation along the nephron in females. The distinct renal Na+ transporter profiles in females vs. males at baseline result in lower fractional reabsorption of the filtered load along the proximal nephron and greater fractional reabsorption past the macula densa,2 confirmed by our recent mathematical simulations of the female nephron.3, 19 Interestingly, males respond to AngII-HTN with a pattern that resemble the female profile: transporter activation past the macula densa and reduced transport along the PT and mTAL (Figure 5). Given these findings, one cannot intuit the responses of females to AngII-HTN. We discovered that females respond to AngII-HTN in a manner that amplifies the female profile: accumulating activated distal Na+ transporters (NKCC2, NKCC2p, NCC, NCCp, Cldn-7, ENaC cleavage), decreasing transporters along the mTAL (NHE3, NKCC2, Na,K-ATPase), raising CLi, and lowering plasma [K+]. Nevertheless, Figure 5 illustrates that the relative accumulation of activated transporters from cortical TAL to CCD and the reduction in medullary TAL transporters is quite similar in females to what has been reported in male rats and mice.9, 10, 12,14, 20 Notable differences in responses of female mice versus rats to AngII include greater lowering of PT NHE3 in mice and greater stimulation of ENaC cleavage in rats. Whether similar sexual dimorphisms and responses to elevated AngII and hypertension exist in humans remains to be determined.
Figure 5. Comparison of renal transporter profiles of male and female rats and mice during AngII-HTN, normalized to baseline.
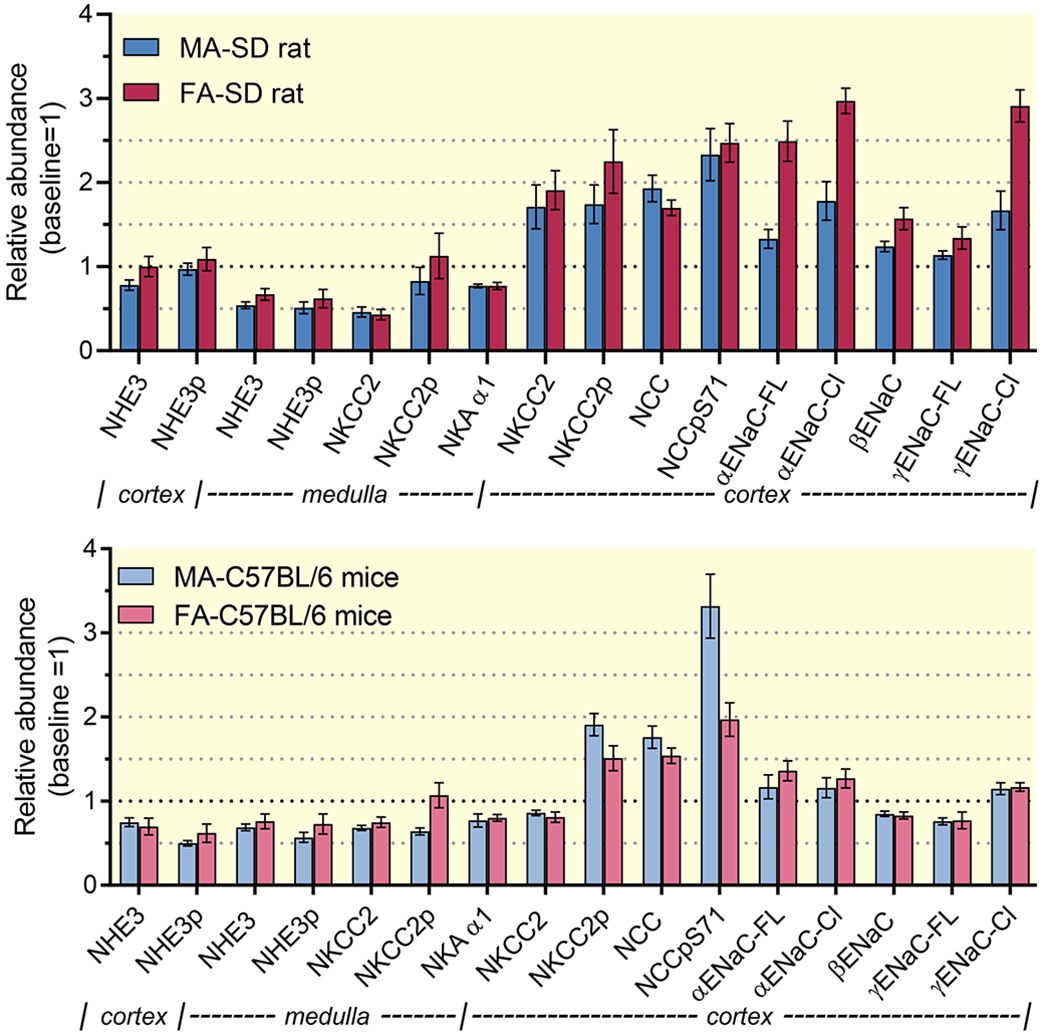
In both sets, relative abundance of AngII measurements were normalized to baseline relative abundance within sexes, defined as 1. (a). In Sprague Dawley, data from females taken from Fig. 1 and Fig. S3; data from males (same protocol) taken from Nguyen et al.9 (b). In C57BL/6 mice, data are from Figure 4.
Can we attribute the natriuresis, diuresis and elevated CLi during AngII to pre-macula densa transporter suppression, or does AngII stimulate changes in intake? We did not measure water and food intake but the excretion data (Figure 1) suggest both were increased in female rats: Uosm fell from 750 to 250 mOsm, while Posm was unchanged consistent with the dipsogenic effect of AngII;21 UosmV, UNaV and UKV all doubled and body weight did not fall (new steady state), consistent with increased dietary intake that contributes to the natriuresis and diuresis. In contrast, evidence for increased intake in AngII infused mice is lacking: UosmV, UNaV, UKV and body weight were unchanged by AngII and P[Na+] did not fall (Figure 3).
2 week-AngII-HTN reduced both mNHE3 and mNKCC2 abundance by ~ 50% and Na,K-ATPase α subunit by 23% in female rats and, in contrast, doubled cTAL NKCC2 and NKCC2p (Figures 1,4). This pronounced differential regulation of NKCC2 along the medullary-cortical TAL axis during AngII-HTN suggests that the rise in BP (or a related factor) suppresses mTAL transporters.9 Our recent mathematical simulations of AngII-HTN in male rats point to the importance of this NHE3 and mNKCC2 inhibition to reducing Na+ reabsorption along the nephron for maintaining electrolyte homeostasis,8 and these new data indicate this inference can be extended to females.
Lithium clearance results, which provides evidence for increased volume flow from the proximal nephron during AngII are not without significant caveats. At baseline, reabsorption of endogenous Li+ beyond the pars recta is < 10% of the filtered load at baseline,4 yet depression of potential Li+ reabsorption routes in the mTAL (mNHE3, mNKCC2) during AngII-HTN may contribute to the increased CLi.22 Thus, a more prudent interpretation is that the rise in CLi during AngII-HTN reflects changes in volume flow emanating from the mTAL.
What is the evidence that changes in transporters’ covalent modification predict changes in transport activity? Physiological assessments in vivo have utilized diuretics that inhibit specific transporters: In mice, the NCC inhibitor hydrochlorothiazide or the NKCC2 inhibitor furosemide provoked significant natriuretic responses at baseline that were significantly increased during AngII-HTN, paralleling the increases in abundance of NKCC2p and NCCp.10 In the cyp1a1-Ren2 rat (that produces elevated AngII), thiazide and amiloride diuretic tests were similarly used to establish correlations between changes in urine Na+ excretion and changes in NCC and ENaC transporters’ abundance. In this current study, we provide a gold standard validation of the apparent activation of ENaC by AngII-HTN: direct measurement of ENaC activity by patch-clamp in split open collecting ducts (Figure 2). The activation, reported for the first time in the female rat during AngII-HTN, is evidenced as increases in open probability (Po), functional ENaC abundance (N) and total ENaC activity (NPo) that closely parallel the 3-fold increases in ENaC cleavage detection by immunoblot. These results complement and confirm the previous results reported for regulation of ENaC in males during AngII-HTN.14, 23
What signal(s) activate ENaC and NCC during AngII-HTN? Candidate mediators include aldosterone, plasma [K+] and tubular flow in addition to direct activation by AngII. Although aldosterone increases more than 10-fold during AngII-infusion in rats (Figure 2), this steroid has been ruled out as a direct regulator of NCC or ENaC during AngII hypertension in males.14, 23, 24 Evidence supports direct activation of ENaC by AngII in isolated tubules by both acute and chronic exposure to AngII.14, 15, 23 The pronounced activation of ENaC during AngII-HTN provides a proximal signal that can contribute to the kaliuresis and drop in plasma [K+] to 3.5 mM (Figure 1a). In turn, lower plasma [K+] can directly stimulate accumulation of NCCp in the DCT 25, 26 raising the question of the role of lowered plasma K+ as a signal during AngII-HTN. We previously reported in male rat that dietary K+ supplementation during AngII-HTN significantly blunts the accumulation of NCC and NCCp,12 supporting plasma K+ itself as the signal that drives the NCC and NCCp accumulation in the DCT, perhaps independent of signaling through AT1R in DCT. Our recent simulation of AngII-HTN indicates that lower activity of pre-macula densa transporters will also increase distal flow and delivery of NaCl, which increase the driving force for ENaC activity and K+ secretion. These simulations support the idea that accumulation of active NCCp is important to minimize Na+ delivery to ENaC to counteract the urinary K+ loss.8 In the female mice, plasma [K+] also falls during AngII-HTN, but there was no evidence for a kaliuresis nor for anti-kaliuresis as would be predicted from lower plasma [K+] (Figure 3). A recent study of AT1R knockout mice with reduced PT NHE3 and increased NCC abundance implicates the increased distal flow and NaCl delivery in raising NCC in this model in both sexes.27 In summary, evidence provided are consistent with the following chain of events in female rats during chronic AngII-HTN: increased flow from PT and mTAL (CLi and UV) stimulate ENaC activity along the CNT/CCD which drives K+ secretion via apical channels located in this region, decreasing plasma [K+] which activates NCC and NCCp accumulation in the DCT to minimize Na+ delivery to the ENaCs downstream.
Experimental reports indicate that females are less susceptible to hypertension, renal injury and cardiovascular disease than males.28–30 In this study, BP increased in females to a level equivalent to that measured in males using very similar AngII infusion dose and time.9, 13 Urinary protein excretion can provide a measure of renal injury. We measured proteins in a constant fraction of urine collected before and after AngII-HTN and the results indicate less proteinuria in female rats (Figure 1c), and no female advantage in female C57BL/6J mice (Figure S4) a strain reported to be resistant to glomerular damage.31, 32 Specifically, in rats we observed: 1) female rats at baseline have undetectable albumin, angiotensinogen or plasminogen while males have low but detectable angiotensinogen and plasminogen; 2) urinary proteins increase during AngII-HTN with stark animal-to-animal variation (increases in 4/5 males, 2/5 females) supporting a relative advantage in females. Supporting elevated filtration across the glomerulus as a common mechanism for proteins in the urine, the correlation of urine angiotensinogen or plasminogen versus urine albumin were highly significant (Figure 2d). A previous study in rats also measured less urine and renal angiotensinogen in female rats, before and after AngII infusion.13
In summary, despite differences in transporter profiles and physiological responses at baseline between females and males, the regulatory responses during AngII infusion, likely driven by AngII per se, fall in plasma K+ and/or rise in tubular flow, were similar between females and males. However, sexual dimorphisms in response to AngII may emerge if AngII is infused at lower doses or rates, or if different animals are considered. We anticipate these findings will provide a useful resource for future investigations in kidneys across sexes.
METHODS
Additional method details are provided in Supplemental Materials available online.
Animal Experiments.
All animal procedures were approved by the Institutional Animal Care and Use Committees of the Keck School of Medicine of the University of Southern California (Protocol 20181) and the Medical College of Georgia at Augusta University (AUP 2017-0844) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Protocol 1. Female Sprague Dawley (SD) rats (150-175 g body weight, Harlan Laboratories, San Diego, CA) were fed grain-based chow (Rodent diet 5001, LabDiet), and water ad libitum. As described for males,9, 12 under anesthesia (40 mg/kg ketamine (Phoenix Pharmaceuticals, St. Joseph, MO) and 8 mg/kg xylazine (Lloyd Laboratories, Shenandoah, IA), intramuscular (IM) injection), osmotic minipumps (Alzet, Model 2002) were implanted subcutaneously to deliver AngII (Sigma-Aldrich, A9525) at 400 ng/kg/min for 2 weeks; controls underwent sham surgery (n=5/group). Blood pressures were measured by tail cuff (Visitech BP2000, Apex, NC) mid-day (11 am- 2 pm) during wk 2, after 1 wk acclimation.33 After acclimation to metabolic cages, rats were placed overnight for 16-hour urine collection (with water, no food) at day 13. At termination day 14 (between 10 AM-12 PM), rats were anesthetized with a 1:1 volume ratio of ketamine (80 mg/kg) and xylazine (8 mg/kg) IM. Kidneys were removed, decapsulated dissected into cortex and medulla and immediately homogenized. Terminal blood was collected by cardiac puncture. There was no animal attrition during the protocol.
Protocol 2 was the same as Protocol 1 with the following exceptions: both males (280-395 gm) and females (205-245), bred at Augusta Univ., were included; metabolic cage collections occurred over 24 hrs with access to food and water; serum rather than plasma was collected. There was no attrition of these animals during the protocol. In these samples, clearance of endogenous lithium (CLi), was calculated conventionally as previously described to provide an indirect estimate of volume flow from the PT-mTAL. 4,34,22 Urinary aldosterone was measured by EIA (Cayman Chemical).
Protocol 3. Male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were fed grain-based chow (Mouse Diet 20 – 5062, LabDiet) and water ad libitum. A total of 30 mice were studied (MC:7, MA:8, FC:9, FA:6); 3 FC were excluded due to aversion to urinating in metabolic cages and 1 MA died of unknown cause. After acclimating to metabolic cages, baseline 18-hour overnight urine was collected (with water, no food), and baseline blood collected from the submandibular vein under isoflurane (1.5%, 1.5 L/min inhaled) anesthesia. Osmotic minipumps (Alzet, Model 2004) were implanted subcutaneously for AngII (Sigma-Aldrich, A2900) infusion at 490 ng/kg/min for 3 weeks; controls were sham-operated, all under isoflurane (1.5%, 1.5 L/min inhaled) anesthesia. Blood pressures were measured after acclimation both at baseline and during 3-week AngII infusion mid-day as for rats. Final 18-hr overnight urine was collected in metabolic cages (with water, no food). At termination on day 21 (between 10 AM-12 PM), mice were anesthetized with ketamine-xylazine, kidneys rapidly removed, frozen and stored at −80˚C. Terminal blood was collected by cardiac puncture. Data from 3 independent series were combined for total n=6/group. In mice, urinary albumin was assessed in a constant fraction of the overnight urine by Coomassie staining after SDS-PAGE resolution, as well as with a commercial kit for mouse albumin (AlbuwellM, Exocell, Philadelphia, PA).
Saline challenge was performed in mice (Protocol 3) after 2 weeks of AngII or sham treatment.2 In brief (see Supplement), mice were weighed and injected i.p. with a volume of saline equivalent to 10% of their body weight. Urine was collected and weighed hourly for 4 hours. Results are plotted as the accumulated urinary Na+ excreted each hour normalized to the initial volume injected (a function of body weight).
ENaC activity in SD rats.
ENaC activity was assessed using previously published procedures,14, 20, 35 detailed in Supplement. Female and male SD rats, bred and studied at Medical College of Georgia at Augusta University, were fed grain-based vivarium chow (Teklad Irradiated Rodent Diet 8904) and studied under baseline conditions and after infusion of AngII as described in Protocol 1. Collecting ducts from a minimum of 5 rats were analyzed per group.
Quantitative immunoblotting.
As described,12 and expanded in Supplement: Uniform protein loading was verified by densitometry of Coomassie blue stained gels (Figure S1). We assessed the linearity of the immunodetection system by running ½ and 1 amounts of each sample on each immunoblot then measured whether the signal density increased by 2 ± 0.3 fold (Figure S2); if out of this range, protein loading was re-evaluated and adjusted to meet this metric before abundance was determined.36 Table S1 outlines the amounts assayed, incubation times and antibody information.
Statistical Analyses.
Data are presented as individual measurements along with mean ± SEM. Female Protocol 1 data were analyzed by Student’s t-test. One-way ANOVA followed by a Tukey’s multiple comparison post-test was used to analyze differences between multiple groups. Systolic blood pressures were analyzed by two-way ANOVA, and the mean values summarized for individuals were analyzed by one-way ANOVA, both followed by a Tukey’s multiple comparison post-test. For saline test, two-way ANOVA followed by a post-test was used to analyze differences. Statistical tests were calculated using GraphPad Prism 6.0 (San Diego, CA). Differences are reported as p values. Transporter profile data were not corrected for the compilation of the multiple transporters because each transporter was assessed separately, blots were not stripped and re-probed, and only one transporter per blot was assessed as previously discussed.2
Supplementary Material
Acknowledgments
FUNDING INFORMATION: AHA 15GRNT23160003 to AAMcD, NIH NIDDK 2R01DK083785 To AAMcD, 1 R01 DK098382 to Susan B. Gurley, AHA 15SDG25550150 to MM.
Footnotes
CONFLICT OF INTEREST: All authors declared no competing interests.
REFERENCES
- 1.Layton AT, Sullivan JC: Recent advances in sex differences in kidney function. Am J Physiol Renal Physiol, 316: F328–F331, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA: Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J Am Soc Nephrol, 28: 3504–3517, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu R, McDonough AA, Layton AT: Functional implications of the sex differences in transporters’ abundance along the rat nephron: modeling and analysis. Am J Physiol Renal Physiol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomsen K, Shirley DG: The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron, 77: 125–138, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Maranon R, Reckelhoff JF: Sex and gender differences in control of blood pressure. Clin Sci (Lond), 125: 311–318, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM: Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol, 307: F901–907, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, Reckelhoff JF, Iadecola C, Coffman TM: Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension, 73: e87–e120, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards A, McDonough AA: Impact of Angiotensin II-mediated stimulation of sodium transporters in the nephron assessed by computational modelling. Am J Physiol Renal Physiol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen MT, Lee DH, Delpire E, McDonough AA: Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol, 305: F510–519, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA: The absence of intrarenal ACE protects against hypertension. J Clin Invest, 123: 2011–2023, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab, 13: 469–475, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veiras LC, Han J, Ralph DL, McDonough AA: Potassium Supplementation Prevents Sodium Chloride Cotransporter Stimulation During Angiotensin II Hypertension. Hypertension, 68: 904–912, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rands VF, Seth DM, Kobori H, Prieto MC: Sexual dimorphism in urinary angiotensinogen excretion during chronic angiotensin II-salt hypertension. Gender medicine, 9: 207–218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O: Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension, 62: 1111–1122, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peti-Peterdi J, Warnock DG, Bell PD: Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol, 13: 1131–1135, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Svenningsen P, Friis UG, Versland JB, Buhl KB, Moller Frederiksen B, Andersen H, Zachar RM, Bistrup C, Skott O, Jorgensen JS, Andersen RF, Jensen BL: Mechanisms of renal NaCl retention in proteinuric disease. Acta physiologica (Oxford, England), 207: 536–545, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA: Renal Transporter Activation During Angiotensin-II Hypertension is Blunted in Interferon-gamma−/− and Interleukin-17A−/− Mice. Hypertension, 65: 569–576, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS: Interleukin-17A Regulates Renal Sodium Transporters and Renal Injury in Angiotensin II-Induced Hypertension. Hypertension, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, McDonough AA, Layton HE, Layton AT: Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am J Physiol Renal Physiol, 315: F692–F700, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O: Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem, 287: 660–671, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skott O: Body sodium and volume homeostasis. Am J Physiol Regul Integr Comp Physiol, 285: R14–18, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Boer WH, Fransen R, Shirley DG, Walter SJ, Boer P, Koomans HA: Evaluation of the lithium clearance method: direct analysis of tubular lithium handling by micropuncture. Kidney Int, 47: 1023–1030, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Zaika O, Mamenko M, Staruschenko A, Pochynyuk O: Direct activation of ENaC by angiotensin II: recent advances and new insights. Current hypertension reports, 15: 17–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashek A, Menzies RI, Mullins LJ, Bellamy CO, Harmar AJ, Kenyon CJ, Flatman PW, Mullins JJ, Bailey MA: Activation of thiazide-sensitive co-transport by angiotensin II in the cyp1a1-Ren2 hypertensive rat. PLoS One, 7: e36311, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su XT, Ellison DH, Wang WH: Kir4.1/Kir5.1 in the DCT plays a role in the regulation of renal K(+) excretion. Am J Physiol Renal Physiol, 316: F582–F586, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonough AA, Youn JH: Potassium Homeostasis: The Knowns, the Unknowns, and the Health Benefits. Physiology (Bethesda), 32: 100–111, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Hatano R, Xu S, Wan L, Yang L, Weinstein AM, Palmer LG, Wang T: Gender Difference In Electrolyte Transport I: Role of AT1a Receptor in Thiazide Sensitive Na-Cl Cotransporter Activity and Expression in Male and Female Mice. Am J Physiol Renal Physiol: ajprenal 00087 02017, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Gomez I, Wangensteen R, Perez-Abud R, Quesada A, Del Moral RG, Osuna A, O’Valle F, de Dios Luna J, Vargas F: Long-term consequences of uninephrectomy in male and female rats. Hypertension, 60: 1458–1463, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Aufhauser DD Jr., Wang Z, Murken DR, Bhatti TR, Wang Y, Ge G, Redfield RR 3rd, Abt PL, Wang L, Svoronos N, Thomasson A, Reese PP, Hancock WW, Levine MH: Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest, 126: 1968–1977, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maric-Bilkan C, Arnold AP, Taylor DA, Dwinell M, Howlett SE, Wenger N, Reckelhoff JF, Sandberg K, Churchill G, Levin E, Lundberg MS: Report of the National Heart, Lung, and Blood Institute Working Group on Sex Differences Research in Cardiovascular Disease: Scientific Questions and Challenges. Hypertension, 67: 802–807, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM: Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol, 290: F214–222, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF: Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transplant, 18: 1999–2004, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM: Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A, 92: 3521–3525, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA: Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J Am Soc Nephrol, 28: 3504–3517, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamenko M, Zaika O, Tomilin V, Jensen VB, Pochynyuk O: Compromised regulation of the collecting duct ENaC activity in mice lacking AT1a receptor. J Cell Physiol, 233: 7217–7225, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonough AA, Veiras LC, Minas JN, Ralph DL: Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol, 308: C426–433, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


